Abstract
Purpose
Monocytes and their progeny are abundant constituents of the tumor microenvironment in lymphoproliferative disorders, including chronic lymphocytic leukemia (CLL). Monocyte-derived cells, including nurse-like cells (NLC) in CLL, promote lymphocyte proliferation and survival, confer resistance to chemotherapy, and are associated with more rapid disease progression. Colony-stimulating factor-1 receptor (CSF-1R) regulates the homeostatic survival of tissue-resident macrophages. Therefore, we sought to determine whether CSF-1R is similarly required for NLC survival.
Experimental Design
CSF-1R expression by NLC was examined by flow cytometry and immunohistochemistry. CSF-1R blocking studies were performed using an antagonistic monoclonal antibody to examine its role in NLC generation and in CLL survival. A rational search strategy was performed to identify a novel tyrosine kinase inhibitor (TKI) targeting CSF-1R. The influence of TKI-mediated CSF-1R inhibition on NLC and CLL viability was examined.
Results
We demonstrated that the generation and survival of NLC in CLL is dependent upon CSF-1R signaling. CSF-1R blockade is associated with significant depletion of NLC and consequently inhibits CLL B-cell survival. We found that the JAK2/FLT3 inhibitor pacritinib suppresses CSF-1R signaling, thereby preventing the generation and survival of NLC and impairs CLL B-cell viability.
Conclusions
CSF-1R is a novel therapeutic target that may be exploited in lymphoproliferative disorders, like CLL, that are dependent upon lymphoma-associated macrophages.
Keywords: CSF-1R, Macrophage, Lymphoma, Pacritinib, Nurse-like cell
Introduction
Tumor-associated macrophages (TAM) are abundant constituents of the tumor microenvironment (TME) and are key instigators of cancer-related inflammation, now recognized as a “hallmark” of cancer (1). TAM promote angiogenesis and suppress host anti-tumor immunity, and are thus, when abundant, an adverse prognostic factor in most tumors (1), including both Hodgkin and non-Hodgkin lymphomas. Results obtained from large gene-expression profiling studies in follicular lymphoma further demonstrate that heterogeneity within the TME, including that which may be attributed to lymphoma-associated macrophages (LAM), may explain the variable natural history associated with many B-cell lymphoproliferative disorders. Macrophages provide cell-surface ligands and cytokines that regulate humoral immunity, including B-cell growth and survival. Therefore, LAM may directly promote the growth and survival of malignant B cells, and confer resistance to chemotherapy.
The contribution of LAM to B-cell lymphomagenesis is arguably best appreciated in chronic lymphocytic leukemia (CLL). CLL represents the clonal expansion of mature B cells with a characteristic immunophenotype. CLL B-cells are resistant to apoptosis and accumulate in the peripheral blood, secondary lymphoid organs and bone marrow. Despite their resistance to chemotherapy and longevity in vivo, the establishment of long-term CLL cell lines has been challenging, as CLL cells frequently undergo spontaneous apoptosis during in vitro culture (2). Therefore, resistance to apoptosis in CLL cells is not entirely explained by an intrinsic resistance to apoptosis, but is likely explained by the provision of extrinsic growth and survival signals by nonneoplastic cells present within the TME. Monocytes and macrophages have been shown to support the survival of CLL cells during in vitro culture (3), are specifically recruited to the TME by malignant B cells (4, 5), and their depletion in Eμ-TCL1 mice delays CLL progression (4, 6). Furthermore, many of the factors previously implicated in supporting the growth and survival of CLL cells, including stromal cell-derived factor-1 (SDF-1) and B-lymphocyte stimulator (BLyS)/B-cell activating factor (BAFF), are produced by monocytes and their progeny, including LAM (7). Collectively, these findings may explain the clinical observation that CLL patients presenting with a monocytosis in peripheral blood experience more rapid disease progression (8, 9). Consistent with this notion, a population of monocyte-derived cells, or nurse-like cells (NLC), can be generated in bulk PBMC, or in cocultures of malignant B cells with either autologous or allogeneic monocytes (10–12). These NLC, resembling LAM (13), promote the growth and survival of CLL cells and confer resistance to chemotherapy. Gene expression profiling (13, 14) and immunophenotypic analysis (13, 15) demonstrate that NLC most closely resemble alternatively polarized macrophages. For example, NLC are CD163bright and produce abundant IL-10, characteristics which are classically associated with alternatively (M2) polarized macrophages (13, 14), The positive influence of monocytes and their progeny on cell growth and survival has been similarly observed in other chronic lymphoproliferative disorders (16). Collectively, this data suggests that monocytes and their progeny within the tumor microenvironment (TME) are attractive therapeutic targets in CLL.
Two therapeutic approaches to target NLC may be imagined. The first approach would qualitatively inhibit the pro-tumoral functions of NLC, by impairing their functional polarization (17). The second approach involves targeting their Achilles’ heel in order to inhibit NLC survival and thus deplete them from the TME. This is an attractive strategy, as TAM depletion has been associated with impaired tumor growth, metastasis, and angiogenesis in experimental models, including CLL (6).
While a number of tumor-derived factors have been implicated in the recruitment and survival of LAM, colony-stimulating factor-1 (CSF-1, or M-CSF) is required for normal macrophage homeostasis and viability. Mice lacking functional CSF-1 or nullizygous for CSF-1 receptor (CSF-1R, c-fms, CD115) have a marked decrease in tissue resident macrophages (18–21). Therefore, we sought to determine whether NLC generation and survival in CLL is similarly CSF-1R dependent. If CSF-1R is an “Achilles heel” for NLC, then therapeutic strategies targeting this protein tyrosine kinase may be therapeutically exploited in CLL and other lymphoproliferative disorders.
Materials and Methods
Cell isolation and NLC generation
Monocytes were positively selected from peripheral blood mononuclear cells (PBMC) obtained from healthy donors using CD14 magnetic beads (Miltenyi Biotec, San Diego, CA), as previously described (16), and were cultured in complete RPMI 1640, as indicated. All cells were maintained at 37°C in 5% CO2. Recombinant cytokines were obtained from Miltenyi Biotec. All studies were performed in accordance with the Declaration of Helsinki and were approved by the Institutional Review Board of the University of Michigan. NLC were generated as previously described (10). Briefly, freshly isolated PBMC from CLL patients who were not actively receiving therapy (86% treatment naïve) were resuspended in complete (10% FBS) RPMI at a density of 3–5 × 106 cells/mL and plated in 24 well plates for 7–10 days, and their characteristic morphology and immunophenotype confirmed (Supplementary Fig. S1). The characteristics of these patients are described in Supplementary Table 1. Cells were treated with ruxolitinib, fedratinib, pacritinib (Selleckchem, Houston, TX) or vehicle control (DMSO) at the concentrations shown. Fedratinib concentrations within the 1–5 μM range are clinically achievable (22). Therefore, 5 μM of ruxolitinib or fedratinib was selected for the experiments shown, except where indicated. Pacritinib, due to a lower IC50, was used at 1 μM, except where indicated. NLC, identified by morphology, were counted in each of five high-power fields. Slides were viewed with an Olympus BX41 microscope (20× objective) and pictures taken with an Olympus DP72 camera. Olympus cellSens Dimension software was used for image acquisition and storage. Alternatively, NLC were identified by their distinct forward- and side-scatter characteristics (i.e. high forward- and side-scatter) by flow cytometry (as shown in Supplementary Figure 1), using a CyAn ADP Analyzer (Beckman Coulter, Indianapolis, IN), and analyzed using Summit 5.3 software.
Monoclonal antibodies, Western blot, Immunohistochemistry
The CSF-1R specific monoclonal antibody (rat IgG1), clone 2-4A5 (LS Bio, Seattle, WA), is a high-affinity CSF-1R antibody that inhibits CSF-1 binding (23). Functional grade, neutralizing IL-10 (clone JES3-9D7), IL-6 (clone MQ2-13A5), and appropriate isotype controls were obtained from eBioscience (San Diego, CA). Monoclonal antibodies and isotype controls utilized for flow cytometry were obtained from BD Biosciences (San Jose, CA), R&D Systems (Minneapolis, MN), or eBioscience. For Western blot, freshly isolated monocytes were cultured overnight. Monocytes were treated with recombinant human CSF-1 (10 ng/mL) in the presence of a vehicle control (DMSO), ruxolitinib, fedratinib or pacritinib for five minutes. Total cell extracts were prepared in 50 mM Tris (pH 7.5), 5 mM EDTA, 150 mM NaCl, 1% NP-40, 0,5% Deoxycholate, 0.1% SDS and Protease/phosphatase inhibitor cocktail (Boston BioProducts, Boston, MA). Protein extracts were subsequently separated by SDS-PAGE on 5–12% gradient gels (Bio-Rad, Hercules, CA) and blotted on PVDF membranes. The blot was incubated in blocking solution (5% nonfat dry milk in 0.1% TBST buffer) for 1 hour. The primary antibodies against total AKT (11E7), phospho (T308)-AKT (C31E5E), phospho (S473)-AKT (D9E), total ERK 1/2 (rabbit polyclonal), phospho (T202/Y204)-ERK 1/2 (rabbit polyclonal) were obtained from Cell Signaling (Danvers, MA). An enhanced chemiluminescence system was used for detection (Thermo Scientific). Immunohistochemistry was performed, as previously described (15). Briefly, formalin-fixed, paraffin-embedded tissue microarrays were cut at 5 microns and rehydrated in water. Heat induced epitope retrieval was performed with the FLEX target retrieval system (high pH) for 20 minutes (Dako, Carpinteria, CA). After peroxidase blocking, a rabbit polyclonal (H-300, Santa Cruz Biotechnology, Dallas, TX) antibody was used at a 1:200 dilution using the DAKO AutoStainer. The FLEX EnVision detection system was used for detection and DAB chromogen applied prior to Harris Hematoxylin counterstaining.
ELISA
CSF-1R phosphorylation was analyzed by sandwich ELISA, as per the manufacturer’s instructions (Cell Signalling Technologies). Briefly, monocytes were treated, as indicated, and cell lysates generated. Both total and phosphorylated CSF-1R were quantified in parallel. Results were reported as the ratio of phosphorylated to total CSF-1R.
Cell viability
Cell viability was determined after the indicated treatments in a standard MTT assay, as per the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO). Apoptosis was determined by Annexin V and propidium iodide staining, per the manufacturer’s instructions (BioLegend, San Diego, CA), after gating on CD5+CD19+ CLL B-cells. Loss of the mitochondrial membrane potential in apoptotic cells was determined by 3,3′-dihexyloxacarbocyanine iodide (DiOC6, Life Technologies, Grand Island, NY) staining. Briefly, cells were loaded with 40 nM DiOC6 15 minutes prior to flow cytometric analysis. Viable, non-apoptotic cells were identified as DiOC6high. Cell and nuclear morphology following the indicated treatments was visualized by phalloidin and DAPI labeling. Briefly, macrophages or NLC cells were harvested by vigorous pipetting and fixed in 4% paraformaldehyde. Fixed cells were spun at 2000 rpm for 3 minutes using a Cytopro cytocentrifuge (Wescor, Logan, UT) unto Colorfirst Plus microscope slides (Fisherbrand, Pittsburgh, PA). Cells were labeled with phalloidin (1:200;) and DAPI, and preserved with ProLong Gold (Life Technologies) anti-fading reagent prior to visualization by immunoflourescent microscopy, using an Olympus microscope (200x).
Kinase assay
In order to determine pacritinib’s selectivity, an in vitro kinase assay was performed, as previously described (24), using 429 recombinant human protein kinases in a radiometric assay that directly measures kinase activity (Reaction Biology, Malvern, PA). Kinase targets identified in the initial screen with an IC50 <50 nM were confirmed and pacritinib subsequently titrated (range: 1–1000 nM) for determination of the IC50.
Bioinformatics
To examine mRNA expression levels of CSF-1R and its ligands (CSF-1 and IL-34) in CLL, we utilized a publicly available microarray dataset (GSE10138) obtained from patients with stable and progressive CLL (25). Normalized data was used for comparison.
Statistics
Paired t-tests were conducted using GraphPad Prism and p-values <0.05 considered statistically significant.
Results
NLC generation is CSF-1R dependent
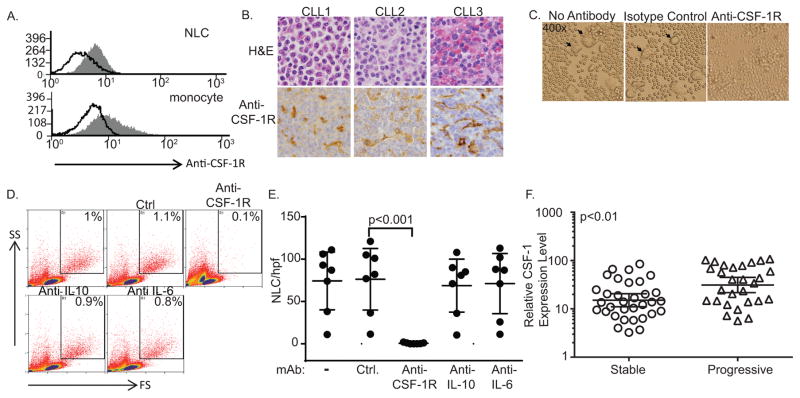
The differentiation and survival of tissue resident macrophages is largely dependent upon colony-stimulating factor-1 receptor (CSF-1R) signaling. Mice that are either homozygous for an inactivating mutation of the CSF-1 gene or nullizygous for its receptor have a dramatic reduction in tissue macrophages (18–21), as do mice treated with an antagonistic CSF-1R monoclonal antibody (26). Whether NLC express CSF-1R and are dependent upon its activation for survival are unknown. Therefore, CSF-1R expression was examined in NLC. CSF-1R expression was demonstrated by flow cytometry on in vitro generated NLC (Figure 1A), and was comparable to that observed on cultured monocytes. Similarly, CSF-1R expression was identified by immunohistochemistry in CLL/SLL lymph nodes (Figure 1B). In order to determine the extent to which NLC survival is dependent upon CSF-1R activation, PBMC obtained from CLL patients were cultured with either an isotype control or antagonistic CSF-1R monoclonal antibody and NLC were quantified 7–10 days later. Upon CSF-1R neutralization, NLC were virtually undetectable in these cultures (Figure 1C, D, E). In contrast, neutralization of the cytokines IL-10 and IL-6, which have been previously implicated in CLL and/or NLC biology (15, 27–30), did not significantly inhibit NLC generation. CSF-1 was at or below the limit of detection (by ELISA) in culture supernatants obtained from NLC cultures (data not shown). As CSF-1 is not available at saturating concentrations in plasma, likely due to macrophage-mediated clearance (31), we were not able to detect a significant difference inplasma CSF-1 between CLL patients and healthy donors (data not shown). Therefore, we examined the relative abundance of CSF-1 transcripts in a previously described cohort of treatment-naïve CLL patients. These patients were stratified into asymptomatic (“stable”) and symptomatic (“progressive”) cohorts (25). Full-length CSF-1 transcripts were significantly more abundant in progressive CLL when compared with asymptomatic CLL (Figure 1F). No significant difference in CSF-1R or IL-34 transcripts were observed (data not shown).
Figure 1.
CSF-1R is required for nurse-like cell survival. (A) Cultured PBMC from CLL patients were stained with an isotype control (open histogram) or anti-CSF-1R (gray histogram). NLC, identified by forward and side scatter, were gated and CSF-1R expression determined by flow cytometry. A representative example is shown (n=3). (B) Immunohistochemical staining for CSF-1R was performed in paraffin-embedded CLL (n=43). Staining of CLL-associated macrophages is appreciated in the representative lymph nodes (CLL1, CLL2) and spleen (CLL3) shown. (Original magnification 400x) (C–E) PBMC from CLL patients (n=7) were cultured in the presence or absence of an isotype control or antagonistic anti-CSF-1R, anti-IL-10, or anti-IL-6 monoclonal antibody. NLC were identified by light microscopy (arrowheads in C) or flow cytometry (gates shown in D). The number of NLC per high-power field (±95% confidence interval) for paired samples is shown (E). (F) Normalized fold-change in full-length CSF-1 transcripts in CLL patients with stable and progressive disease is shown. Line and error bars represent the geometric mean with 95% confidence interval.
CSF-1R blockade and NLC depletion impairs CLL viability
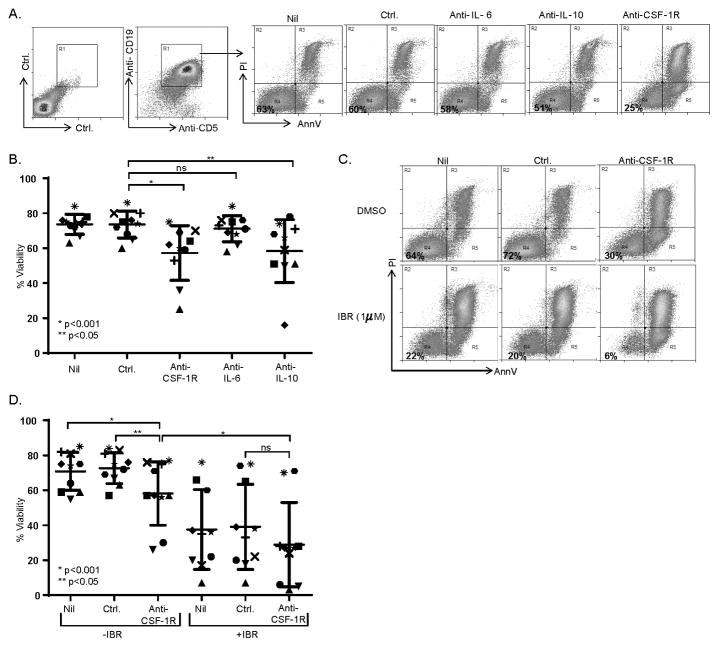
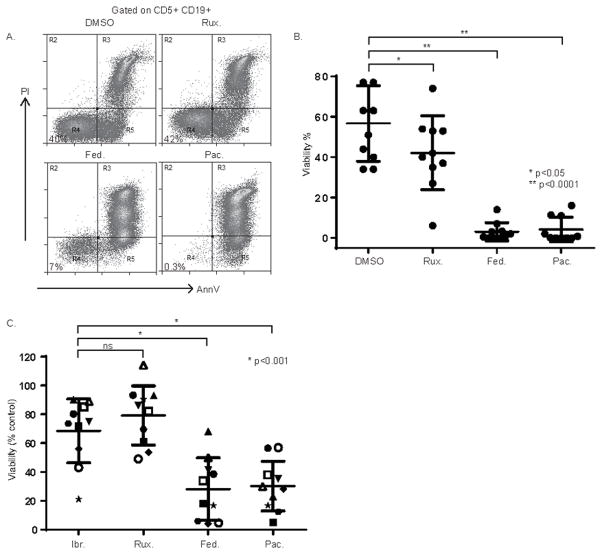
In order to investigate the potential of CSF-1R blockade as a novel therapeutic strategy in CLL, the viability of CLL B-cells was examined in paired NLC cultures to which an isotype control or anti-CSF-1R, anti-IL-10 or anti-IL-6 antibodies had been included. After 7–10 days in culture, the viability of CD5+CD19+ CLL B-cells (gates shown in Fig. 2A) was examined by Annexin V and propidium iodide staining (Fig. 2A). The majority of CLL B-cells (≈75%) remained viable in the presence of NLC. However, a significant reduction in CD5+CD19+ CLL viability (16.3% absolute decrease in mean viability, and 22.1% relative decrease, compared with isotype control) was observed upon CSF-1R blockade and NLC depletion (Fig. 2A, B), although susceptibility to NLC depletion was variable. This heterogeneity mirrored the variable NLC densities observed in culture and suggested to us that CLL samples with a higher NLC density may be more susceptible to their depletion, although additional cell-autonomous mechanisms certainly cannot be excluded. While conducting these experiments, we observed that apoptotic (Annexin V+/PI+) and viable (Annexin V−/PI−) CLL B-cells were readily distinguishable by their respective forward and side-scatter characteristics (Supplementary Fig. S2). Therefore, this strategy was utilized to further examine the extent to which NLC depletion impairs CLL viability in additional independent samples. Concordant with our initial observation, a significant reduction in CLL cell viability was observed upon CSF-1R blockade (Supplementary Fig. S2). Ibrutinib, an irreversible inhibitor of Bruton’s tyrosine kinase (BTK), inhibits BTK-dependent antigen-receptor signaling in CLL and has had a dramatic impact in CLL management (32, 33). In addition to impairing B-cell receptor signaling, ibrutinib inhibits cross-talk between malignant B cells and constituents of the tumor microenvironment, including NLC/LAM (34, 35). Therefore, we sought to examine the extent to which NLC depletion may augment (or inhibit) responsiveness to ibrutinib. A significant reduction in CLL viability was observed following either NLC depletion or treatment with ibrutinib (Fig. 2C, D). Compared with ibrutinib or anti-CSF-1R treatments alone, a further decrease in CLL viability was observed when ibrutinib and anti-CSF-1R were combined, although this difference did not reach statistical significance. No evidence for antagonism was observed.
Figure 2.
CSF-1R blockade impairs CLL B-cell survival. (A) PBMC from CLL patients (n=10) were cultured in the presence or absence of an isotype control or antagonistic anti-CSF-1R, anti-IL-10, or anti-IL-6 monoclonal antibody. Viability of CD5+CD19+ CLL cells (gates shown) was determined by Annexin V/PI staining. A representative example is shown (A) and the data summarized in B. (C, D) CLL PBMC were cultured in the presence of ibrutinib (1 μM) or vehicle control (DMSO). An isotype control or anti-CSF-1R antibody was included, as indicated. Viability of CD5+CD19+ CLL B cells was determined by Annexin V/PI staining (representative example shown in C). Data obtained from paired samples (n=10) is summarized in D.
Pacritinib inhibits CSF-1R
While pharmacologic strategies to inhibit CSF-1R are in clinical development, macrophage depletion with these approaches is frequently partial and dependent upon the tissue compartment examined (36–38), possibly due to the availability of cytokines that promote LAM survival in a CSF-1R independent, but JAK/STAT-dependent, manner (15, 39). Therefore, we sought to identify a dual CSF-1R and JAK/STAT inhibitor. CSF-1R is a type III protein tyrosine kinase that is highly homologous to FMS-related tyrosine kinase 3 (FLT3), a frequent “off target” for many TKI’s, including “JAK inhibitors” (40). Therefore, we sought to determine whether JAK/FLT3 inhibitors similarly inhibit CSF-1R. Two JAK inhibitors – ruxolitinib and fedratinib – also inhibit FLT3 and have been shown to inhibit CSF-1R in vitro, but with IC50’s of ≈ 1 uM and >1uM, respectively (40). In contrast, the JAK2/FLT3 inhibitor pacritinib inhibits FLT3 at nanomolar concentrations. Therefore, we performed an in vitro kinase assay with 429 recombinant human protein tyrosine kinases (24). Pacritinib inhibited 13 kinases with an IC50 <50 nM, including CSF-1R (Supplementary Table 2). Therefore, we examined each of these tyrosine kinase inhibitors (TKI) for their ability to inhibit CSF-1R signaling in cultured monocytes exposed to CSF-1.
Upon engagement by either of its two ligands (CSF-1 or IL-34), several tyrosine residues within the CSF-1R intracytoplasmic domain are phosphorylated, culminating in the activation of a number of pathways regulating macrophage differentiation, motility, survival and proliferation, including Ras-Raf-MEK-ERK- and PI3-kinase-dependent pathways [reviewed in (41)].
Total CSF-1R tyrosine phosphorylation was determined by ELISA in CSF-1 treated monocytes exposed to ruxolitinib, fedratinib, or pacritinib. Fedratinib (5 μM) and pacritinib (1 μM) significantly reduced total CSF-1R phosphorylation (Fig. 3A) in response to recombinant CSF-1. In addition, downstream phosphorylation of ERK and AKT was significantly impaired in pacritinib-treated monocytes (Fig. 3B, C).
Figure 3.
Pacritinib inhibits CSF-1R. (A) Monocytes were treated (5 minutes) with recombinant human CSF-1 (10 ng/mL) in the presence of ruxolitinib (5 μM), fedratinib (5 μM), pacritinib (1 μM) or vehicle control (DMSO). Total and phosphorylated CSF-1R was detected in cell lysates by sandwich ELISA (in triplicate). The ratio of phosphorylated:total CSF-1R is shown. (B,C) Phosphorylation of the CSF-1R targets indicated was examined by immunoblotting (B) or flow cytometry (C).
Pacritinib impairs macrophage viability
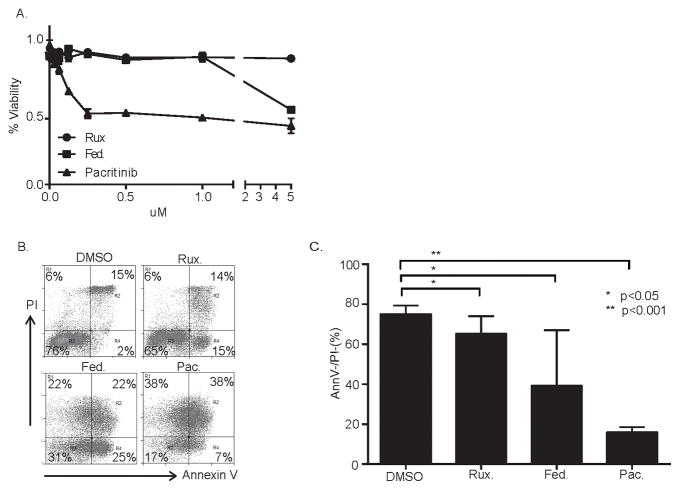
Macrophage survival is CSF-1R dependent, and pacritinib is a potent inhibitor of CSF-1R activity. Therefore, we examined the viability of CSF-1-treated monocytes cultured with ruxolitinib, fedratinib, and pacritinib. In contrast to ruxolitinib and fedratinib, pacritinib significantly impaired monocyte and monocyte-derived macrophage viability at clinically achievable concentrations (Figure 4A, B, C). Pacritinib culminated in caspase 3 activation, loss of mitochondrial membrane potential, and changes in cell morphology, all consistent with apoptotic cell death (Supplementary Fig. S3).
Figure 4.
Pacritinib inhibits monocyte-derived macrophage viability. (A) Monocytes were plated in triplicate wells of a flat-bottom 96-well plate supplemented with CSF-1 to generate monocyte-derived macrophages. Groups were treated with ruxolitinib (rux), fedratinib (fed) or pacritinib (pac) at the concentrations shown and cell viability (as a percent of vehicle-treated controls) determined 48 hours later by MTT assay. (B, C) Monocyte-derived macrophages were treated with ruxolitinib (5 μM), fedratinib (5 μM), pacritinib (1 μM) or vehicle control (DMSO) and apoptosis determined by Annexin V/propidium iodide (PI) staining 24 hours later. Values in C represent the percentage of total cells that were Annexin V−/PI− (n=3).
Pacritinib inhibits NLC survival
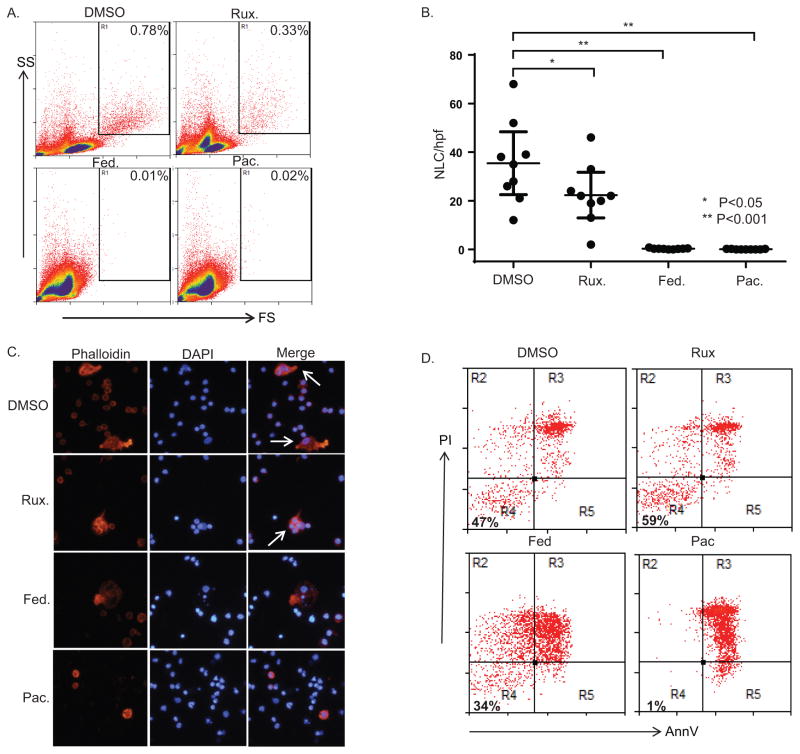
In order to determine whether pacritinib prevents NLC survival, PBMC from CLL patients were cultured for 7–10 days, as before. NLC cultures were treated with ruxolitinib, fedratinib, pacritinib, or vehicle control. Whereas inhibition of NLC survival was modest with ruxolitinib, a significant, and near total, loss of NLC was observed in cultures containing either fedratinib or pacritinib (Fig. 5A, B). Morphologic changes similar to those observed in pacritinib-treated macrophages were observed in established NLC cultures treated for 24 hours with either fedratinib or pacritinib (Figure 5C). NLC emperipolesis of CLL cells in culture has been previously described, and is readily observed in DMSO- or ruxolitinib-treated cultures (Figure 5C). In contrast, intact NLC were rarely observed in cultures treated with fedratinib or pacritinib, and when observed were pyknotic and appeared morphologically similar to pacritinib-treated macrophages. As NLC and CLL viability are possibly interdependent, pacritinib-dependent cytotoxicity was directly examined by gently removing CLL cells from established NLC cultures prior to treatment with pacritinib (Fig. 5D). After exposure to pacritinib, viable NLC were no longer identified by annexin V/PI staining (Fig. 5D) or by morphologic assessment (Supplementary Fig. S4). NLC depletion upon CSF-1R blockade, or its pharmacologic inhibition, is unlikely to be CLL-specific, as inhibition of CSF-1R similarly impaired LAM survival in multiple myeloma (Supplementary Fig. S6).
Figure 5.
Pacritinib inhibits nurse-like cell viability. (A, B) PBMC from CLL patients were treated with ruxolitinib (5 μM), fedratinib (5 μM), pacritinib (1 μM) or vehicle control (DMSO) and NLC identified by flow cytometry (gate shown in A). A representative example is shown. The number of NLC per high-power field (± 95% confidence interval) is shown in B (n=9). (C) NLC were generated from CLL patient PBMC and treated with ruxolitinib (5 μM), fedratinib (5 μM), pacritinib (1 μM) or vehicle control (DMSO). NLC were visualized by fluorescent microscopy 24 hours later after phalloidin and DAPI staining. (D) CLL cells were removed from NLC cultures and NLC treated with ruxolitinib (5 μM), fedratinib (5 μM), pacritinib (1 μM) or vehicle control (DMSO). NLC viability was determined 24 hours later by Annexin V and PI staining (representative example is shown, n=3)
Pacritinib inhibits CLL viability
The CSF-1R blocking studies demonstrate that the long-term survival of CLL B-cells in vitro is NLC-dependent. Therefore, we examined CLL viability in long-term cultures treated with ruxolitinib, fedratinib, or pacritinib. A modest, and statistically significant, decrease in CLL viability was observed in ruxolitinib-treated cultures. In contrast, both fedratinib and pacritinib significantly reduced CLL viability (Figure 6A, B). Pre-clinical studies with ibrutinib demonstrated that the induction of apoptosis in primary CLL samples exposed to ibrutinib in vitro is highly variable, with the majority of samples largely resistant to the cell-autonomous effects of ibrutinib at concentrations approximating those that are clinically achievable [e.g. Figure 2D, (42)]. In order to examine the extent to which pacritinib inhibits viability in such cases, paired CLL samples were treated with ibrutinib (1 uM) or pacritinib (1 uM) and cell viability determined (Figure 6C). The induction of apoptosis in ibrutinib-treated cultures was modest. In contrast, a significant increase in apoptosis was observed in pacritinib-treated samples, including those harboring a 17p deletion/p53 mutation.
Figure 6.
Pacritinib impairs CLL B-cell survival. (A, B) NLC and CLL cocultures were generated from CLL patient PBMC (n=10) and were treated with ruxolitinib (5 μM), fedratinib (5 μM), pacritinib (1 μM) or vehicle control (DMSO). Viable CD5+CD19+ CLL cells were identified by Annexin V and PI staining (representative example is shown in A, and the data summarized in B). (C) In similarly performed experiments, CLL patient PBMC (n=10) were treated with ibrutinib (1 uM), ruxolitinib (5 μM), fedratinib (5 μM), pacritinib (1 μM) or vehicle control (DMSO) and viability of CD5+CD19+ CLL cells determined by Annexin V and PI staining. Viability was normalized to the paired DMSO control. High-risk CLL with a 17p deletion identified by FISH (n=3) are designated by an open symbol.
Discussion
Various subsets of myeloid cells have been convincingly implicated with processes, including angiogenesis, suppression of host immunity, and the creation of a microenvironment favorable for metastatic spread, that are required for the growth, survival and metastatic potential of malignant cells (1). Gene-expression profiling and immunohistochemistry-based studies highlight the pathogenic role of monocytes and their progeny, including LAM, in Hodgkin and non-Hodgkin lymphomas (NHL) (43). Burger et. al. identified a subset of mononuclear cells in the peripheral blood of CLL patients which, during in vitro culture, differentiated into adherent cells resembling macrophages. Malignant B cells were observed to form clusters around these so-called “nurse-like” cells, conferring resistance to spontaneous apoptosis (10, 12). Subsequent studies support the conclusion that NLC are monocyte-derived macrophages that are alternatively polarized and further highlight their role in promoting the survival and expansion of malignant B cells (3, 11, 12). The lack of in vivo data is a significant limitation of this study, but may be alleviated by previous studies demonstrating that NLC depletion in CLL mouse models delays CLL progression and reverses, at least partially, CLL-associated immunologic incompetence (4, 6). Ibrutinib, an irreversible BTK inhibitor, inhibits signaling downstream from various receptors, including the antigen receptor, and disrupts interactions between CLL cells and NLC (34, 35, 44). Collectively, this data, including the extraordinary success achieved with Ibrutinib in CLL, provides a compelling argument for the therapeutic targeting of the TME, particularly NLC, in chronic lymphoproliferative disorders like CLL.
Macrophages are terminally differentiated cells and are resistant to most conventional chemotherapeutic agents. Therefore, with possibly a few exceptions (45), conventional chemotherapeutic agents ineffectively target LAM. An alternative strategy is to prevent the functional (or “alternative”) polarization that is observed in tumor-associated macrophages (15). A quantitative approach that depletes LAM outright represents an attractive alternative. As the homeostatic survival of tissue resident macrophages is dependent upon CSF-1R, antibody-mediated blockade of this tyrosine kinase receptor is one strategy. In fact, this approach has been adopted to successfully deplete macrophages (26, 46–48). Alternatively, CSF-1R kinase inhibitors may have a number of advantages, including the ability to block autocrine CSF-1R signaling [reviewed in (49)]. While a number of CSF-1R kinase inhibitors have been developed, to the best of our knowledge none of these have been clinically developed for use in lymphoproliferative disorders (49). Macrophages, depending upon the tissue compartment in which they reside, may differ in their susceptibility to CSF-1R inhibition. Perhaps most relevant to this study, macrophages present in the lymph node may be less dependent upon CSF-1 (18). Alternatively, additional cytokines, including those that are likely abundant within the TME, may act in concert with CSF-1 (39, 50). Therefore, macrophage depletion with highly selective CSF-1R inhibitors may be incomplete. In contrast, pacritinib is a potent CSF-1R inhibitor with an IC50 in the low nanomolar range, and is well tolerated in patients with lymphoma (51). In addition, inhibition of the JAK2/STAT pathway by pacritinib may be anticipated to inhibit alternative macrophage polarization (52). Therefore, dual inhibition of both JAK2/STAT and CSF-1R dependent pathways by pacritinib may qualitatively and quantitatively target NLC in CLL. Further studies are warranted to investigate pacritinib’s potential cell-autonomous effects on CLL cell viability, presumably mediated by JAK/STAT inhibition, as CLL cells do not express CSF-1R, but IL-10- and IL-4-dependent JAK/STAT signalinghas been previously implicated in CLL (53–55). Importantly, pacritinib is clinically available and well tolerated, and is the subject of ongoing investigations in various hematologic malignancies [reviewed in (56)]. Finally, pacritinib has demonstrated clinical activity in various NHL, including CLL, although the extent to which this activity is CSF-1R- or TME-dependent will require further study (51). Notably, inhibition of CLL viability was more pronounced with pacritinib than with ibrutinib (Fig. 2C cf. Fig. 6B; Fig. 6C), and may be due to concomitant inhibition of JAK2/STAT3 signaling in CLL B cells in conjunction with NLC depletion (53). Furthermore, whether “low-” and “high-risk” (e.g. IGHV unmutated, 17p-/p53 mutated) CLL are equally sensitive to NLC depletion and pacritinib was not addressed, although the induction of apoptosis in 17p− CLL (Fig. 6C) with pacritinib may be noteworthy. Nonetheless, future studies will be needed to address these questions..
In conclusion, CSF-1R is an Achilles’ heel for NLC and LAM, as the generation and survival of these important constituents of the TME are dependent upon this receptor tyrosine kinase. Pacritinib is a potent CSF-1R inhibitor that depletes NLC in vitro and significantly impairs CLL viability. Preliminary experience with pacritinib demonstrates its clinical activity in various lymphomas. Collectively, these findings shed new light on a potentially important mechanism of action for pacritinib that can be therapeutically exploited to target tumor-associated macrophages.
Supplementary Material
Translational Relevance.
Colony-stimulating factor-1 receptor (CSF-1R) regulates the homeostatic survival of tissue resident macrophages, which are abundant constituents of the tumor microenvironment in B-cell lymphomas. Lymphoma-associated macrophages (LAM) promote the growth and survival of malignant B cells. Consequently, their depletion is a rational therapeutic strategy in these lymphomas, including CLL, but has not yet been achieved. Here, we demonstrate that CSF-1R is required for the generation of CLL-associated macrophages (“nurse-like cells”), and that the JAK2/FLT3 inhibitor pacritinib is a potent CSF-1R inhibitor that depletes CLL-associated macrophages and impairs CLL cell viability. Pacritinib is clinically available and well tolerated in phase I studies. Collectively, these findings suggest that LAM depletion may now be clinically achievable, and clinical trials to address this question are planned.
Acknowledgments
This work was supported in part by grants from the Leukemia & Lymphoma Society (6270-13); the Melanoma Research Foundation; the Claude D. Pepper Older Americans Independence Center, the Michigan Institute for Clinical and Health Research, the University of Michigan Geriatrics Center (AG-024824); and the NIH-NCI (K08CA172215, and P30CA046592).
Footnotes
Contributions: A.P., Y.L, P.S.B, and T.W. assisted with study design, performed research, analyzed data, and assisted with manuscript preparation; N.G.B. and M.S.L. provided patient material and assisted with data interpretation and manuscript preparation; J.W.S. provided data (supplementary table 3) and assisted with manuscript preparation; E.S. and S.M. abstracted and/or analyzed clinical data and assisted with manuscript preparation; R.A.W. conceived the study, designed and performed research, analyzed data, and wrote the manuscript. All study authors approved the final manuscript.
Conflict-of-interest disclosure: Jack W. Singer is employed by CTI Biopharma. The remaining authors declare no competing financial interests.
References
- 1.Wilcox RA. Cancer-associated myeloproliferation: old association, new therapeutic target. Mayo Clin Proc. 2010;85:656–63. doi: 10.4065/mcp.2010.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coscia M, Pantaleoni F, Riganti C, Vitale C, Rigoni M, Peola S, et al. IGHV unmutated CLL B cells are more prone to spontaneous apoptosis and subject to environmental prosurvival signals than mutated CLL B cells. Leukemia. 2011;25:828–37. doi: 10.1038/leu.2011.12. [DOI] [PubMed] [Google Scholar]
- 3.Seiffert M, Schulz A, Ohl S, Dohner H, Stilgenbauer S, Lichter P. Soluble CD14 is a novel monocyte-derived survival factor for chronic lymphocytic leukemia cells, which is induced by CLL cells in vitro and present at abnormally high levels in vivo. Blood. 2010;116:4223–30. doi: 10.1182/blood-2010-05-284505. [DOI] [PubMed] [Google Scholar]
- 4.Reinart N, Nguyen PH, Boucas J, Rosen N, Kvasnicka HM, Heukamp L, et al. Delayed development of chronic lymphocytic leukemia in the absence of macrophage migration inhibitory factor. Blood. 2013;121:812–21. doi: 10.1182/blood-2012-05-431452. [DOI] [PubMed] [Google Scholar]
- 5.Zucchetto A, Benedetti D, Tripodo C, Bomben R, Dal Bo M, Marconi D, et al. CD38/CD31, the CCL3 and CCL4 chemokines, and CD49d/vascular cell adhesion molecule-1 are interchained by sequential events sustaining chronic lymphocytic leukemia cell survival. Cancer Res. 2009;69:4001–9. doi: 10.1158/0008-5472.CAN-08-4173. [DOI] [PubMed] [Google Scholar]
- 6.Hanna BS, McClanahan F, Yazdanparast H, Zaborsky N, Kalter V, Rossner P, et al. Depletion of CLL-associated patrolling monocytes and macrophages controls disease development and repairs immune dysfunction in vivo. Leukemia. 2015 doi: 10.1038/leu.2015.305. [DOI] [PubMed] [Google Scholar]
- 7.Mueller CG, Boix C, Kwan WH, Daussy C, Fournier E, Fridman WH, et al. Critical role of monocytes to support normal B cell and diffuse large B cell lymphoma survival and proliferation. J Leukoc Biol. 2007;82:567–75. doi: 10.1189/jlb.0706481. [DOI] [PubMed] [Google Scholar]
- 8.Friedman DR, Sibley AB, Owzar K, Chaffee KG, Slager S, Kay NE, et al. Relationship of blood monocytes with chronic lymphocytic leukemia aggressiveness and outcomes: A multi-institutional study. Am J Hematol. 2016 doi: 10.1002/ajh.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herishanu Y, Kay S, Sarid N, Kohan P, Braunstein R, Rotman R, et al. Absolute monocyte count trichotomizes chronic lymphocytic leukemia into high risk patients with immune dysregulation, disease progression and poor survival. Leuk Res. 2013;37:1222–8. doi: 10.1016/j.leukres.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell’Aquila M, Kipps TJ. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–63. [PubMed] [Google Scholar]
- 11.Bhattacharya N, Diener S, Idler IS, Rauen J, Habe S, Busch H, et al. Nurse-like cells show deregulated expression of genes involved in immunocompetence. Br J Haematol. 2011;154:349–56. doi: 10.1111/j.1365-2141.2011.08747.x. [DOI] [PubMed] [Google Scholar]
- 12.Tsukada N, Burger JA, Zvaifler NJ, Kipps TJ. Distinctive features of “nurselike” cells that differentiate in the context of chronic lymphocytic leukemia. Blood. 2002;99:1030–7. doi: 10.1182/blood.v99.3.1030. [DOI] [PubMed] [Google Scholar]
- 13.Ysebaert L, Fournie JJ. Genomic and phenotypic characterization of nurse-like cells that promote drug resistance in chronic lymphocytic leukemia. Leuk Lymphoma. 2011;52:1404–6. doi: 10.3109/10428194.2011.568078. [DOI] [PubMed] [Google Scholar]
- 14.Filip AA, Cisel B, Koczkodaj D, Wasik-Szczepanek E, Piersiak T, Dmoszynska A. Circulating microenvironment of CLL: are nurse-like cells related to tumor-associated macrophages? Blood cells, molecules & diseases. 2013;50:263–70. doi: 10.1016/j.bcmd.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Wang T, Feldman AL, Wada DA, Lu Y, Polk A, Briski R, et al. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood. 2014;123:3007–15. doi: 10.1182/blood-2013-12-544809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcox RA, Wada DA, Ziesmer SC, Elsawa SF, Comfere NI, Dietz AB, et al. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood. 2009;114:2936–44. doi: 10.1182/blood-2009-05-220111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T, Feldman AL, Wada DA, Lu Y, Polk A, Briski R, et al. GATA-3 Expression Promotes IL-10 Production, Alternative Macrophage Polarization, and Identifies a Subset of High-risk PTCL, NOS. Blood (ASH Annual Meeting Abstracts) 2013;122:841. [Google Scholar]
- 18.Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, et al. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–72. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 19.Marks SC, Jr, Lane PW. Osteopetrosis, a new recessive skeletal mutation on chromosome 12 of the mouse. The Journal of heredity. 1976;67:11–8. doi: 10.1093/oxfordjournals.jhered.a108657. [DOI] [PubMed] [Google Scholar]
- 20.Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Jr, Ahmed-Ansari A, Sell KW, Pollard JW, et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990;87:4828–32. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–4. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Xu CR, Shamiyeh E, Liu F, Yin J, von Moltke LL, et al. A randomized, placebo-controlled study of the pharmacokinetics, pharmacodynamics, and tolerability of the oral JAK2 inhibitor fedratinib (SAR302503) in healthy volunteers. Journal of clinical pharmacology. 2013 doi: 10.1002/jcph.218. [DOI] [PubMed] [Google Scholar]
- 23.Haegel H, Thioudellet C, Hallet R, Geist M, Menguy T, Le Pogam F, et al. A unique anti-CD115 monoclonal antibody which inhibits osteolysis and skews human monocyte differentiation from M2-polarized macrophages toward dendritic cells. mAbs. 2013;5:736–47. doi: 10.4161/mabs.25743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1039–45. doi: 10.1038/nbt.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman DR, Weinberg JB, Barry WT, Goodman BK, Volkheimer AD, Bond KM, et al. A genomic approach to improve prognosis and predict therapeutic response in chronic lymphocytic leukemia. Clin Cancer Res. 2009;15:6947–55. doi: 10.1158/1078-0432.CCR-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–63. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- 27.DiLillo DJ, Weinberg JB, Yoshizaki A, Horikawa M, Bryant JM, Iwata Y, et al. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia. 2013;27:170–82. doi: 10.1038/leu.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai R, O’Brien S, Maushouri T, Rogers A, Kantarjian H, Keating M, et al. Prognostic value of plasma interleukin-6 levels in patients with chronic lymphocytic leukemia. Cancer. 2002;95:1071–5. doi: 10.1002/cncr.10772. [DOI] [PubMed] [Google Scholar]
- 29.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–4. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 30.Saulep-Easton D, Vincent FB, Quah PS, Wei A, Ting SB, Croce CM, et al. The BAFF receptor TACI controls IL-10 production by regulatory B cells and CLL B cells. Leukemia. 2015 doi: 10.1038/leu.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartocci A, Mastrogiannis DS, Migliorati G, Stockert RJ, Wolkoff AW, Stanley ER. Macrophages specifically regulate the concentration of their own growth factor in the circulation. Proc Natl Acad Sci U S A. 1987;84:6179–83. doi: 10.1073/pnas.84.17.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrd JC, O’Brien S, James DF. Ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:1278–9. doi: 10.1056/NEJMc1309710. [DOI] [PubMed] [Google Scholar]
- 34.ten Hacken E, Burger JA. Molecular pathways: targeting the microenvironment in chronic lymphocytic leukemia--focus on the B-cell receptor. Clin Cancer Res. 2014;20:548–56. doi: 10.1158/1078-0432.CCR-13-0226. [DOI] [PubMed] [Google Scholar]
- 35.Niemann CU, Herman SE, Maric I, Gomez-Rodriguez J, Biancotto A, Chang BY, et al. Disruption of in vivo chronic lymphocytic leukemia tumor-microenvironment interactions by ibrutinib - findings from an investigator initiated phase 2 study. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mok S, Koya RC, Tsui C, Xu J, Robert L, Wu L, et al. Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer Res. 2014;74:153–61. doi: 10.1158/0008-5472.CAN-13-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–69. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–8. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou T, Georgeon S, Moser R, Moore DJ, Caflisch A, Hantschel O. Specificity and mechanism-of-action of the JAK2 tyrosine kinase inhibitors ruxolitinib and SAR302503 (TG101348) Leukemia. 2014;28:404–7. doi: 10.1038/leu.2013.205. [DOI] [PubMed] [Google Scholar]
- 41.Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends in cell biology. 2004;14:628–38. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 42.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilcox RA, Ristow K, Habermann TM, Inwards DJ, Micallef IN, Johnston PB, et al. The absolute monocyte and lymphocyte prognostic score predicts survival and identifies high-risk patients in diffuse large-B-cell lymphoma. Leukemia. 2011;25:1502–9. doi: 10.1038/leu.2011.112. [DOI] [PubMed] [Google Scholar]
- 44.Burger JA. Targeting the microenvironment in chronic lymphocytic leukemia is changing the therapeutic landscape. Current opinion in oncology. 2012;24:643–9. doi: 10.1097/CCO.0b013e3283589950. [DOI] [PubMed] [Google Scholar]
- 45.Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–62. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Radi ZA, Koza-Taylor PH, Bell RR, Obert LA, Runnels HA, Beebe JS, et al. Increased serum enzyme levels associated with kupffer cell reduction with no signs of hepatic or skeletal muscle injury. Am J Pathol. 2011;179:240–7. doi: 10.1016/j.ajpath.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cassier PA, Italiano A, Gomez-Roca CA, Le Tourneau C, Toulmonde M, Cannarile MA, et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. The Lancet Oncology. 2015;16:949–56. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- 48.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–59. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 49.Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810–20. doi: 10.1182/blood-2011-09-379214. [DOI] [PubMed] [Google Scholar]
- 50.Breen FN, Hume DA, Weidemann MJ. Interactions among granulocyte-macrophage colony-stimulating factor, macrophage colony-stimulating factor, and IFN-gamma lead to enhanced proliferation of murine macrophage progenitor cells. J Immunol. 1991;147:1542–7. [PubMed] [Google Scholar]
- 51.Younes A, Romaguera J, Fanale M, McLaughlin P, Hagemeister F, Copeland A, et al. Phase I study of a novel oral Janus kinase 2 inhibitor, SB1518, in patients with relapsed lymphoma: evidence of clinical and biologic activity in multiple lymphoma subtypes. J Clin Oncol. 2012;30:4161–7. doi: 10.1200/JCO.2012.42.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilcox RA, Feldman AL, Ziesmer SC, Novak AJ, Witzig TE, Ansell SM. GATA-3 Promotes IL-10 Production and Is An Adverse Prognostic Factor in Peripheral T-Cell Lymphoma Unspecified (PTCL-U) Blood (ASH Annual Meeting Abstracts) 2009;114:3929. [Google Scholar]
- 53.Rozovski U, Wu JY, Harris DM, Liu Z, Li P, Hazan-Halevy I, et al. Stimulation of the B-cell receptor activates the JAK2/STAT3 signaling pathway in chronic lymphocytic leukemia cells. Blood. 2014;123:3797–802. doi: 10.1182/blood-2013-10-534073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitabayashi A, Hirokawa M, Miura AB. The role of interleukin-10 (IL-10) in chronic B-lymphocytic leukemia: IL-10 prevents leukemic cells from apoptotic cell death. Int J Hematol. 1995;62:99–106. doi: 10.1016/0925-5710(95)00395-9. [DOI] [PubMed] [Google Scholar]
- 55.Steele AJ, Prentice AG, Cwynarski K, Hoffbrand AV, Hart SM, Lowdell MW, et al. The JAK3-selective inhibitor PF-956980 reverses the resistance to cytotoxic agents induced by interleukin-4 treatment of chronic lymphocytic leukemia cells: potential for reversal of cytoprotection by the microenvironment. Blood. 2010;116:4569–77. doi: 10.1182/blood-2009-09-245811. [DOI] [PubMed] [Google Scholar]
- 56.Hatzimichael E, Tsolas E, Briasoulis E. Profile of pacritinib and its potential in the treatment of hematologic disorders. Journal of blood medicine. 2014;5:143–52. doi: 10.2147/JBM.S51253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.