Abstract
A polymorphic multigene family (p44) of Anaplasma phagocytophilum encodes the immunodominant 44-kDa major outer membrane proteins. With p44-specific PCR and gene-specific probes, p44-1 was found in all human isolates from New York State but not in isolates from Minnesota, whereas p44-18 and two other p44 species were found in isolates from both regions. We therefore sequenced the genomic locus corresponding to the p44-1/p44-18 tandem locus of A. phagocytophilum HZ in 14 other geographically divergent strains from various hosts. The locus was found in all 14 strains, and p44-18 was conserved among all 13 United States isolates studied. In all nine northeastern strains, p44-1 was conserved. However, in three of the Minnesota strains and in one California strain, p44-1 was replaced at this genomic locus by the novel gene p44-61 (p44-61/18), whose hypervariable region (hv) was a chimera of p44-20hv and p44-23hv. The conserved base sequence within the hv region linked the two segments. In contrast, in the Old Sourhope strain isolated from sheep in the United Kingdom, only a single and distinct p44, p44-OS, was found in this locus. This suggests different rates of evolution of p44-1 and p44-18 at this locus and conservation of the locus within strains isolated from the same geographic region. Locus-specific reverse transcription-PCR revealed expression of p44-1 by New York and p44-61 by Minnesota strains at this locus. These p44 loci provide insight into the molecular evolution and functional divergence of p44 paralogs and may serve as markers for typing strains from different geographic regions.
Anaplasma phagocytophilum, formerly designated Ehrlichia (Cytoecetes) phagocytophila, is an obligate intracellular bacterium that invades the granulocytes of various mammals, including humans, sheep, goats, horses, dogs, cattle, llamas, deer, and rodents. The bacterium is widely maintained among wild mammals through biological transmission by Ixodes species of ticks. Spillover infection of domestic animals and humans has been reported in the United States, Europe, and other parts of the globe. In its mammalian hosts it often causes an acute and/or chronic febrile disease characterized by hematological abnormalities and immunosuppression; its human manifestation, human granulocytic anaplasmosis, is acute and potentially fatal (6).
Gene duplications underlie the diversification of genes, often resulting in the development of novel gene functions or pseudogenes that may help the organism adapt to new environments (28). The p44 multigene family of A. phagocytophilum consists of more than 100 nonidentical polymorphic paralogs, which encode P44s, 44-kDa major outer membrane proteins that are exposed on the cell surface (41). Anti-P44 antibodies can protect mice from experimental infection with A. phagocytophilum (16), and a recombinant P44 protein induces proinflammatory cytokines in human leukocytes in vitro (17); thus, P44s are likely to be important in the pathogenesis of A. phagocytophilum in its mammalian hosts. The diversity of paralogous p44 genes and the surface proteins they encode among strains may reflect differences among geographic and host-specific variants. Thus, several studies attempted to use p44 for typing A. phagocytophilum strains (4). However, due to the presence of a large number of diverse p44 paralogs per genome, it has been difficult to delineate strain-specific variations. Further characterization of this gene and protein family may help to clarify some of the mechanisms of functional divergence under the selection pressures of different environments and correlate A. phagocytophilum strains.
All p44 genes contain a central hypervariable region (hv) of approximately 280 bp, flanked by conserved 5′- and 3′-terminal sequences of 100 to 500 bp. Most p44 paralogs lack the translational start codon, ATG. The hv has four short conserved segments that divide it into three extremely diverse domains (20). Among several strains isolated from New York and Massachusetts, the hv sequences of each p44 ortholog are conserved across strains (12, 19, 20). Therefore, each individual p44 paralog or ortholog can be identified by its signature hv nucleotide sequence (1, 19, 20, 41). Similarly, the genomic loci of several p44 paralogs are conserved in several human isolates from New York State, as demonstrated by Southern blot analysis (19, 20, 41). Published data show that diverse p44 paralogs are expressed by the same strain or by different strains growing in different hosts (human, mouse, horse, and ticks) and under different environmental conditions (1, 12, 14, 19, 20, 40, 42). A. phagocytophilum has a unique polymorphic p44 expression locus (1, 19), and it has been proposed that the expression of all p44 paralogs takes place in the same locus by segmental gene conversion (1). However, it has also been shown that some p44s can be expressed at their own genomic loci (40, 41).
In the present study, we examined conservation of p44s among A. phagocytophilum strains. Since the genomic locus consisting of two tandem p44s, p44-1 and p44-18, was found to be strain polymorphic, we further examined the structure, geographic polymorphism, and transcription of this genomic locus. At this genomic locus the HZ strain (isolated from a human patient in New York State in 1995) expresses the p44-18 pseudogene but not the full-length gene p44-1 when cultivated in HL-60 cells at 37°C. The same expression pattern, which utilizes a novel RNA splicing mechanism, also occurs in vivo in infected horses (40). Here we studied the sequence of the p44-1/18 locus in 14 strains isolated from different geographical areas and from different mammalian and tick hosts and the p44 transcription at this locus in several strains.
MATERIALS AND METHODS
A. phagocytophilum strains.
The geographical origins and source species of the various A. phagocytophilum strains used in this study are summarized in Table 1 (20, 36, 43). All A. phagocytophilum strains except the Scottish ovine strain Old Sourhope (OS) were cultivated in HL-60 cells at 37°C in 5% CO2 and 95% air, with RPMI 1640 medium (Invitrogen, Carlsbad, Calif.) supplemented with 5 to 10% heat-inactivated fetal bovine serum (U.S. Bio-Technologies, Parker Ford, Pa.). Infection rates were checked daily by Diff-Quik staining (Baxter Scientific Products, Obetz, Ohio). The infected cells were harvested when the infection level reached 80%. A blood sample containing the A. phagocytophilum OS strain (13) was obtained from an experimentally infected sheep in Liverpool, United Kingdom. Forty-five Ixodes scapularis nymphs collected in Westchester County, New York, by drag sampling were also used as sources of DNA (11).
TABLE 1.
A. phagocytophilum isolates
| Isolatea | Geographic origin, source species, and yr of isolation | Reference |
|---|---|---|
| HZ | New York State, human, 1995 | 36 |
| BJ | New York State, human, 1996 | 43 |
| BS | New York State, human, 1996 | 43 |
| LL | New York State, human, 1996 | 43 |
| NY-31 | New York State, human, 2000 | 20 |
| NY-36 | New York State, human, 2000 | 20 |
| NY-37 | New York State, human, 2000 | 20 |
| MN-1 | Minnesota, human | |
| MN-2 | Minnesota, human | |
| MN-9 | Minnesota, human | |
| Trustom | Rhode Island, tick | |
| AVK-HLPA1 | Pennsylvania, tick | |
| Gaillard | Connecticut, tick | |
| MRK | California, horse | |
| OS | Scotland, sheep |
Cultured A. phagocytophilum Minnesota isolates MN-1, MN-2, and MN-9 in HL-60 cells were supplied by J. Goodman, University of Minnesota, St. Paul, Minn. Strains Trustom, AVK-HLPA1, Gaillard, MRK, and OS are discussed by Lin et al. (20a).
DNA purification.
Genomic DNAs of A. phagocytophilum strains HZ, LL, NY-31, NY-36, and NY-37 were isolated from purified organisms by Sephacryl S-1000 column chromatography (Amersham Pharmacia Biotech, Piscataway, N.J.) with the QIAamp DNA mini kit (Qiagen, Valencia, Calif.) (35). Total DNA of strains BS, BJ, MN-1, MN-2, MN-9, Trustom, AVK-HLPA1, Gaillard, and MRK was extracted from 5 × 106 A. phagocytophilum-infected HL-60 cells with the QIAamp DNA mini kit (Qiagen). OS strain DNA was extracted from the blood obtained from experimentally infected sheep during the peak period of bacteremia.
Total DNA from nymphal Ixodes scapularis was purified as follows. Each tick was homogenized in a sterile glass depression slide with a glass rod. The homogenate was placed in a 1.5-ml tube with 75 μl of 10 mM Tris-EDTA buffer, pH 8.0, and 25 μl of proteinase K solution (20 mg/ml) was added. The mixture was incubated at 56°C for 30 min. To inactivate proteinase K, the sample was heated to 100°C for 60 min. After centrifuging at 2,000 × g for 10 min, the supernatant was transferred to a new 1.5-ml tube, and StrataClean resin was added following the manufacturer's instructions to separate DNA from proteins, including restriction enzymes and some modification enzymes (Stratagene, La Jolla, Calif.).
Reverse transcription-PCR and DNA-PCR.
Reverse transcription-PCR was performed as described previously (20). Total RNA was extracted from 5 × 106 A. phagocytophilum-infected HL-60 cells (90 to 100% infection) with the RNeasy mini kit (Qiagen). After DNase I treatment, the isolated RNA (5 μg) was heated to 70°C for 10 min. Samples were then reverse transcribed at 42°C for 50 min in a 20-μl reaction mixture containing a 0.5 mM concentration of each deoxynucleoside triphosphate, 200 U of SuperScript II reverse transcriptase (Invitrogen), 200 ng of random hexamers, and 3 mM MgCl2. PCR was performed in a 50-μl reaction mixture containing 4 μl of the cDNA product or 5 μl of DNAs purified as above, 10 pmol of each primer, a 0.2 mM concentration of each deoxynucleoside triphosphate, 5 U of Taq DNA polymerase, and 1.5 mM MgCl2.
PCR conditions included 3 min of denaturation at 94°C, followed by 35 cycles consisting of 30 s of denaturation at 94°C, 1 min of annealing at 54°C, and 1 min of extension at 72°C. Gene-specific primers 13 to 15 are listed in Table 2. A BLAST search of the GenBank and A. phagocytophilum HZ strain databases showed that no other locus in the A. phagocytophilum genome anneals with these primer pairs. DNA PCR products were purified from the gel and cloned into a pCRII vector (Invitrogen). Twenty cDNA clones were randomly selected from the transformants and sequenced on an ABI 373XL Stretch DNA sequencer, with the ABI Prism BigDye terminator cycle sequencing reaction kit.
TABLE 2.
Oligonucleotide primers for PCR and Southern blot analysis
| Primer | Target | 5′ primer | 3′ primer | Purpose |
|---|---|---|---|---|
| 1 | p44s | 5′-CTGCTCTKGCCAARACCTC-3′ | 5′-CAATAGTYTTAGCTAGTAACC-3′ | DNA-PCR |
| 2 | p44-1hv | 5′-GAAGGTTTGTAGTGGAAAGCA-3′ | 5′-GTGCTCCAACTACAATGCTAT-3′ | Southern blotting |
| 3 | p44-2hv | 5′-TACTGGTAGCCATGCTGACC-3′ | 5′-AATCACCCGTCTTCAGTGATG-3′ | Southern blotting |
| 4 | p44-18hv | 5′-GGAGATTTCTAATTCCGGTAT-3′ | 5′-TGTCGTTATTCGATTTAGACG-3′ | Southern blotting |
| 5 | p44-28hv | 5′-GGACGAAGCGGAAGGCTGGT-3′ | 5′-TTTGTGTTAGTATTTCCCGATTTCT-3′ | Southern blotting |
| 6 | p44-1/p44-18 or p44-61/18 locus | 5′-GAGTACACCACCACTCTTAAC-3′ | 5′-TACAGACCCGCTCTGTATTTGT-3′ | DNA-PCR |
| 7 | p44-1/p44-18 or p44-61/18 locus | 5′-AGCCTTTTCTTTAGGAAGCGT-3′ | 5′-TACAGACCCGCTCTGTATTTGT-3′ | DNA-PCR |
| 8 | p44-20hv | 5′-CTGCTCTKGCCAARACCTC-3′ | 5′-TTCATCACGATTAAGACCTAAT-3′ | DNA-PCR |
| 9 | p44-20hv | 5′-TAACTAAGGCTAAGGGGAAGA-3′ | 5′-CAATAGTYTTAGCTAGTAACC-3′ | DNA-PCR |
| 10 | p44-23hv | 5′-AAAGGCTACGAAGGGAAAAGCA-3′ | 5′-CAATAGTYTTAGCTAGTAACC-3′ | DNA-PCR |
| 11 | p44-23hv | 5′-CTGCTCTKGCCAARACCTC-3′ | 5′-TCAGAGGTGAGCTTTGTGAG-3′ | DNA-PCR |
| 12 | p44-61hv | 5′-TAAGGCGGTGGAGATCTCTCAT-3′ | 5′-CTCGCCCTCTAGGCCAGTTT-3′ | DNA-PCR |
| 13 | p44-1 in p44-1/18 locus | 5′-CATTTTCTTTAAAAGGCAGAC-3′ | 5′-GTGCTCCAACTACAATGCTAT-3′ | RT-PCR, DNA-PCR |
| 14 | p44-61 in p44-61/18 locus | 5′-CATTTTCTTTAAAAGGCAGAC-3′ | 5′-CTCGCCCTCTAGGCCAGTTT-3′ | RT-PCR, DNA-PCR |
| 15 | p44-18 in p44-1/18 locus | 5′-CATTTTCTTTAAAAGGCAGAC-3′ | 5′-TGTCGTTATTCGATTTAGACG-3′ | RT-PCR, DNA-PCR |
PCR hybridization.
PCR products were amplified with primer sets 2 to 5 (Table 2) with strain HZ genomic DNA and labeled with [α-32P]dCTP with Ready-To-Go DNA labeling beads (Amersham Pharmacia Biotech) to make various p44 probes. Genomic DNA PCR products from various strains of A. phagocytophilum were subjected to electrophoresis on a 1% agarose gel and transferred to a positively charged nylon membrane (Amersham Pharmacia Biotech). Hybridization was carried out under high-stringency conditions (65°C) in rapid hybridization buffer (Amersham Pharmacia Biotech) as described elsewhere (20). After being washed, the membrane was exposed to Hyperfilm (Amersham Pharmacia Biotech) to visualize the locations of the hybridized bands.
Nucleotide sequence accession numbers.
The sequences identified in this study have been assigned the following GenBank accession numbers: p44-1/p44-18 locus of strain HZ, AY151054; p44-61/p44-18 locus of strain MN-1, AY319831; p44-61/p44-18 locus of strain MN-2, AY299122; and the p44-OS locus of strain OS, AY541008. The genome of A. phagocytophilum HZ was sequenced at the Institute for Genomic Research, and the sequences are available at www.tigr.org.
RESULTS
Analysis of p44-1, p44-18, and other p44s in A. phagocytophilum strains with p44 paralog-specific probes.
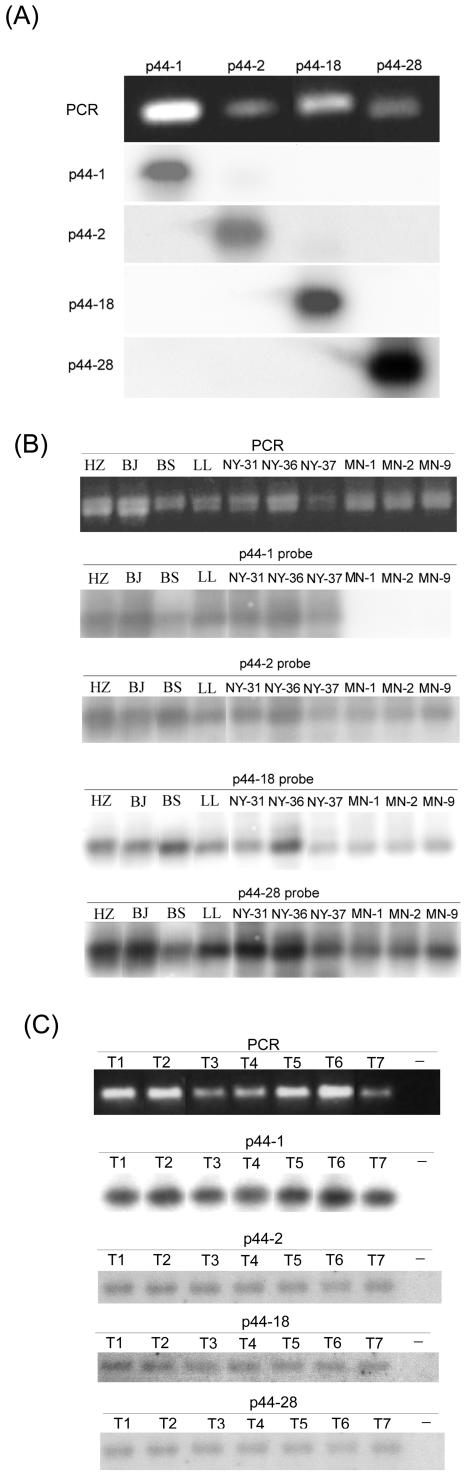
Conventional Southern blotting of restriction enzyme digestion of whole genomic DNA (19, 20, 41) requires a relatively large amount of A. phagocytophilum genomic DNA purified from host cells. To facilitate detection of p44 species in field specimens without the need of culture, we developed a more sensitive method by combining p44 PCR and p44-specific probe hybridization assays. p44s were amplified with primer pair 1 (Table 2); these primers anneal to the 5′- and 3′-terminal conserved regions of all p44 genes present in the A. phagocytophilum HZ genome. This PCR assay could detect a minimum of approximately 10 copies of p44 genes in 5 μl of DNA samples, and the additional p44 hybridization was approximately 10 times more sensitive than PCR alone (data not shown). The specificity of p44 hybridization was tested by probing four different p44 amplicons with each specific p44 probe. p44-1, p44-2, p44-18, and p44-28 were amplified from the A. phagocytophilum HZ genome by p44-specific primers (sets 2 to 5 in Table 2). Four paralog-specific probes, p44-1, p44-2, p44-18, and p44-28, were used for hybridization. Each p44 species probe specifically hybridized only with the corresponding p44 amplicon (Fig. 1A).
FIG. 1.
PCR and hybridization analysis of p44s in different strains. (A) Specificity of p44 PCR followed by p44 species-specific hybridization. p44-1, p44-2, p44-18, and p44-28 were amplified by gene-specific primer sets 2 to 5 (Table 2). The amplified products were resolved on 1% agarose gels containing ethidium bromide. After agarose gel electrophoresis, the amplicons were transferred to a nylon membrane and hybridized with 32P-labeled p44-1, p44-2, p44-18, and p44-28 probes. (B) p44 PCR and hybridization of different A. phagocytophilum human isolates from New York and Minnesota with four gene-specific probes. The strain names and probes used are shown above each panel. (C) p44 PCR of DNA from seven individual nymphal ticks from New York State and hybridization with four gene-specific probes.
We used this method to determine whether p44-1, p44-18, and other p44s were conserved among A. phagocytophilum strains. The genomic DNAs of the 10 different strains were used as templates for PCR amplifications with p44-specific primers (set 1 in Table 2). The p44-1-specific probe hybridized with all seven New York strains; it did not recognize any Minnesota (MN) strains (Fig. 1B). Specific probes for p44-2, p44-18, and p44-28 hybridized with all 10 strains (Fig. 1B). These results indicated that p44-1 was missing or divergent in the three Minnesota strains, whereas p44-2, p44-18, and p44-28 were conserved in all 10 strains. Therefore, geographic diversity levels may vary among p44 genomic loci, and the p44-1 locus may represent one of the hot spots of p44 strain variations.
DNA specimens (5 μl) from 7 of 45 nymphal ticks collected in Westchester County, N.Y., were p44-PCR positive with a p44 gene-specific primer pair (set 1 in Table 2). Since there are more than 100 copies of p44 per A. phagocytophilum genome (www.tigr.org) (21) and this PCR could detect ≈10 copies of p44, most likely PCR-negative ticks were not infected with A. phagocytophilum. All A. phagocytophilum genomes from the seven individual ticks had all four p44 species (Fig. 1C). Thus, our method was sensitive enough to detect p44 species present in A. phagocytophilum genomes from individual field-collected nymphal ticks. The A. phagocytophilum strains present in these ticks contained the same p44 species as human isolates from New York State, perhaps reflecting a geographic type.
Characterization of the p44-1/18 locus in northeastern United States isolates.
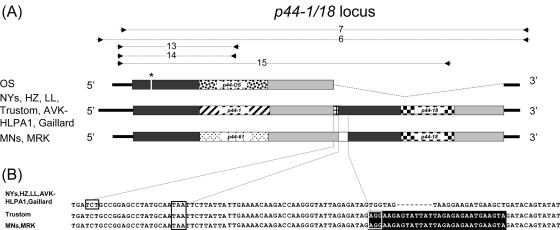
In strain HZ, p44-1 is a single and complete gene that is 1,242 bp long. It occurs in tandem with a downstream p44-18 pseudogene (762 bp), which overlaps p44-1 by 22 bp with a frame shift of −1 bp (40). This tandem locus is referred to as p44-1/18. Since both p44-1 and p44-18 were detectable in all New York human and tick strains but only p44-18 was detectable in Minnesota human isolates, we analyzed the p44-1/18 genomic locus in various strains. In addition to the HZ strain, this locus had previously been identified by Southern blotting in strains NY-31, NY-36, and NY-37 (20). We designed a p44-1/18 locus-specific primer pair (set 6 in Table 2 and Fig. 2A) based on the region of the strain HZ p44-1/18 locus, starting 133 bp upstream of p44-1 and ending 77 bp downstream of the p44-18 pseudogene. The p44-1/18 locus was amplified with locus-specific primer pairs and sequenced for LL, a 1996 New York State isolate, and NY-31, NY-36, and NY-37, isolated in 2000. Sequencing results (2,230 bp) showed that the p44-1/18 loci of these four New York isolates were 100% identical to that of strain HZ isolated in 1995 (Fig. 2A). These results suggest that the p44-1/18 genomic locus has been conserved among the New York human isolates for at least 5 years.
FIG. 2.
Comparison of p44-1/18 genomic loci in geographically different isolates. (A) Schematic diagram of p44-1/18 genomic loci. p44-1, p44-18, p44-61, p44-MRK, and p44-OS hv regions are shown by different fill patterns. Flanking conserved regions are shown as gray bars. The p44-1 and p44-18 overlap region in northeastern isolates is shown as a small grid. p44-61 and p44-18 are separated by a 37-bp intergenic space in the p44-61/18 locus in Minnesota strains (white box). Primer set 6 was used to amplify the p44-1/18 locus in all northeastern strains; primer set 7 was used to amplify the p44-1/18 locus equivalents in the Minnesota, MRK, and OS strains. Primer sets 13 to 15 were used in transcriptional analysis of this locus. Amplicons are shown above the genetic map; the in-frame stop codon in p44-OS is shown by an asterisk. (B) Sequence comparison of the p44-1 (p44-61) and p44-18 junction in different United States isolates. Nucleotides shown on a black background are identical in the Minnesota, MRK, and Trustom strains. The boxed nucleotides (TCT) shown are the first in-frame codon of p44-18. The boxed nucleotides TAA are the stop codon for p44-1 in northeastern strains and for p44-61 in Minnesota and MRK strains. The boxed nucleotides with black background indicate the first in-frame codon (AGG) of p44-18. Gaps are shown by dashes.
Since p44-1 and p44-18 were detectable in all A. phagocytophilum-infected I. scapularis tick specimens from New York State, we analyzed the p44-1/18 genomic locus in A. phagocytophilum isolated from ticks from northeastern states. We could amplify and sequence the p44-1/18 locus in I. scapularis tick isolates from three states, Rhode Island (Trustom), Pennsylvania (AVK-HLPA1), and Connecticut (Gaillard) (primer set 6 in Table 2). In these three isolates, p44-1/18 loci (≈2,230 bp) were conserved and showed an average of 99.6% identity to the locus in strain HZ (Fig. 2). In the Gaillard and AVK-HLPA1 isolates, p44-1 and p44-18 overlapped by 22 bp, as in strain HZ. The p44-1 and p44-18 junctional genomic region of the Trustom isolate differed from those of the other six strains by 30 bp, including an 8-bp insertion (AGTATTAT) (Fig. 2B).
Characterization of the p44-1/18 genomic locus in Minnesota strains and a California horse isolate, MRK.
This locus also existed in all three Minnesota strains, MN-1, MN-2, and MN-9 (19), as detected by p44-1/18 locus-specific PCR (primer set 7 in Table 2) and DNA sequencing. The locus (≈2,000 bp) showed 99.7 to 99.9% identity among strains MN-1, MN-2, and MN-9. Notably in this locus, p44-1 was replaced by a new p44 species, p44-61, in all Minnesota strains, but p44-18 was conserved (Fig. 2 and 3). p44-61 was 1,167 bp long, with an ATG start codon and a TAA stop codon (Fig. 3). p44-61 and p44-1 showed only 83% nucleotide and amino acid identity. This explains why p44-1 was undetectable in the Minnesota strains but p44-18 was detectable by PCR hybridization analysis (Fig. 1B). The p44-18 ortholog in the Minnesota strains was 708 bp long and was located 37 bp downstream from p44-61 (Fig. 2) rather than overlapping the upstream cistron, as in HZ (40) and most other northeastern strains. There was an additional polymorphism in this locus. The three Minnesota strains had 30 bp, including an 8-bp insertion fragment, that were 100% identical to that of the Trustom (Rhode Island) strain (Fig. 2B). The p44-18 orthologs were completely conserved (base sequences 100% identical) between MN-2 and MN-9 and 99.9% identical between MN-1 and MN-2 and between MN-1 and MN-9. The p44-18 of the three Minnesota strains had 96% base sequence identity with HZ p44-18.
FIG. 3.
Sequence comparison of p44-61hv, p44-20hv, and p44-23hv in the Minnesota and MRK strains corresponding to the p44-61 region of Fig. 2. The chimeric regions are marked by gray and black backgrounds. The underlined sequence was conserved among the three p44s. Arrows indicate the gene-specific primers used to amplify the p44-20 hv and p44-23 hv. The locations of primer sets 8 and 9 (p3761-p44-20R and p44-20F-p4183) (Table 2), used to amplify the p44-20 hv, and primer sets 10 and 11 (p3761-p44-23R and p44-23F-p4183) (Table 2), used to amplify the p44-23 hv, are shown.
A. phagocytophilum California strain MRK (previously Ehrlichia equi) is an agent of equine granulocytic anaplasmosis (previously called equine ehrlichiosis). The p44-1/18 locus also existed in strain MRK. However, p44-1 was undetectable in strain MRK by p44-1 gene-specific PCR (data not shown). Sequencing showed that p44-1 was also replaced by p44-61 at this genomic locus as in the Minnesota strains (Fig. 2 and 3). The p44-61 gene newly detected in this study was not found in the database of the strain HZ genome (www.tigr.org). Thus, p44-61 appeared to be a novel p44 species specific to the Minnesota and MRK strains. p44-61 DNA sequence identity between the Minnesota and MRK strains was 99.4%; the downstream p44-18 showed 99.8% DNA sequence identity with strain HZ p44-18. The 3′ primer of primer set 7 was located 77 bp downstream from the p44-18 pseudogene (Fig. 2). After removing the primer sequence, the downstream 55-bp sequences were 100% identical between HZ and the three Minnesota and MRK strains.
Alignment of p44-61 with previously identified p44 homologs suggested that this p44 is derived from two other p44 family members, p44-23 and p44-20, which were originally identified in all New York State strains, including HZ, LL, NY-31, NY-36, and NY-37 (20, 36, 43). To determine whether p44-20 and p44-23 exist in the Minnesota and MRK strains, these two p44 species were amplified with gene-specific primer sets 8 to 11 (Table 2 and Fig. 3) in the Minnesota and MRK strains and sequenced. The p44-20hv and p44-23hv sequences were present and identical between the Minnesota and MRK strains and had 95 to 98% identity to p44-20 and p44-23 of strain HZ at the nucleotide level. Sequence comparison showed that the hv region of p44-61 was a chimera of partially duplicated p44-20 and p44-23 present within the same genome of each strain. Specifically, in both the Minnesota and MRK strains, nucleotides 543 to 739 of p44-61 were identical to those of p44-20, and nucleotides 724 to 843 of p44-61 were identical to those of p44-23. In each strain, p44-61, p44-20, and p44-23 showed 17 bp of absolutely conserved sequence within the hv regions (nucleotides 724 to 740, GGTAAAAACTGGCCTAG; amino acid sequence GKNWP) (Fig. 3). This sequence included the absolutely conserved GCCTAG (amino acid sequence WP) in one of the four highly conserved regions within the hv region in p44 homologs (20).
p44-1/18 genomic locus in an ovine strain from the United Kingdom.
A. phagocytophilum (previously known as Ehrlichia [Cytoecetes] phagocytophila) causes tick-borne fever in sheep and cattle and is transmitted by Ixodes ricinus ticks in the United Kingdom and mainland Europe (13). The p44-1/18 locus was also amplified in strain OS with locus-specific primer set 7 (Table 2). The downstream 55-bp sequences upstream of the 3′ primer of primer set 7 were 100% identical between HZ and all 14 U.S. and Scotland OS strains (Fig. 2). The upstream primer of pair 6 (Table 2) did not amplify the p44-OS locus, indicating that its 5′-flanking region was different from those of the U.S. strains. The PCR product was a single band of approximately 1,200 bp. Sequencing revealed only one p44, p44-OS, in this genomic locus in strain OS. p44-OS was a truncated p44 paralog with an in-frame stop codon at 233 bp. p44-OS was 1,068 bp long, including the stop codon; it had 74.7% identity to strain HZ p44-1 at both the nucleotide and amino acid levels. p44-OS was not found by a BLAST search of the A. phagocytophilum HZ genome database (www.tigr.org) or the GenBank database. p44-1 and p44-18 were undetectable in strain OS by PCR (primer sets 2 and 4 in Table 2).
Transcription at the p44-1/18 genomic locus varies among strains.
p44-1/18 in strain HZ is a unique genomic locus that can undergo posttranscriptional splicing (40). Only the p44-18 transcript is expressed at this genomic locus in strain HZ-infected HL-60 cells at 37°C because transcribed RNA is spliced to remove a part of p44-1; at 24°C, on the other hand, primarily p44-1 is expressed (40). We studied transcription from the p44-1/18 locus in the three New York strains from 2000, because we did not find p44-18 expression in these strains in our previous study (20) despite the presence of an identical p44-1/18 locus between New York strains and strain HZ.
By reverse transcription-PCR with primer pairs specific to this locus and the individual genes within it (primer sets 13 to 15 in Table 2) (40), p44-1 was expressed by all three New York strains growing in HL-60 cells at 37°C (Fig. 4A). The p44-18 transcript was undetectable in these three isolates with both locus-specific and p44-generic primers (primer sets 1 and 15 in Table 2; Fig. 2, Fig. 4A). p44-61 was expressed at this genomic locus in the three Minnesota strains cultured in HL-60 cells at 37°C (Fig. 4B). These results suggest that transcription of p44-1/18 varies among strains. The results were in agreement with our previous observation (40) that p44s can be expressed in genomic loci distinct from the polycistronic p44 expression locus (1, 19).
FIG. 4.
Reverse transcription-PCR analysis of p44 expression in the p44-1/18 and p44-61/18 loci. (A) p44-1 and p44-18 expression in the p44-1/18 locus of strains NY-31, NY-36, and NY-37. (B) p44-61 and p44-18 expression in the p44-61/18 locus of strains MN-1, MN-2, and MN-9. The DNA template control included 0.1 ng of genomic DNA from each strain. RT+ and RT− indicate the presence and absence of reverse transcriptase, respectively.
DISCUSSION
In the United States, the majority of human granulocytic anaplasmosis cases have originated in the northeastern and upper midwestern states—Massachusetts, Connecticut, New York, Minnesota, and Wisconsin (26), whereas most cases of equine granulocytic anaplasmosis have been reported in California (21). White-footed mice, wild deer, and other wild animals were found infected with A. phagocytophilum strains. A. phagocytophilum is transmitted to humans or animals by I. scapularis ticks in the northeast and upper midwest and by I. pacificus ticks in the western states (29, 34, 38). In Europe, I. ricinus is considered the main vector, but other ticks have also been implicated (27), and A. phagocytophilum occurs primarily in sheep and cattle and less frequently in humans than in the United States (2, 7, 10, 13, 15, 30-32, 39).
So far, the 16S rRNA gene sequences of A. phagocytophilum strains infecting humans have been identical. However, divergent (98.8%) 16S rRNA gene sequences were found in ticks and animals (25, 27, 33). Generally, human isolates can infect laboratory mice, but Massung et al. (24) reported that strains isolated from wild deer cannot infect laboratory mice. Analysis of the p44 profiles of these wild deer isolates would facilitate better understanding of the functional divergence of p44 genes. The diversity in host mammalian species adaptation suggests genetic heterogeneity among strains. Our present study clearly demonstrates diversity among A. phagocytophilum strains at the p44-1/18 genomic locus. Intraspecies variation of p44 genes may contribute to our understanding of the role of p44s in immunoprotection, pathogenesis, and perhaps the mammalian host specificity of A. phagocytophilum.
The classic model for the evolution of a multigene family involves gene duplication followed by divergence. One mechanism of p44 molecular evolution discovered in this study was recombination among conserved hv sequences with a short conserved sequence within the hv region. The identical sequence found within the same genome suggests recombination within the same genome after the emergence of p44-20 and p44-23. Although it appears that p44-1 and p44-18 are under the same transcriptional control (operon), the present study indicated that each p44 paralog may have evolved at different rates, suggesting that each p44 may be under different selective pressure. For example, p44-1 appeared to be evolving more rapidly than p44-18 and perhaps p44-2, p44-28, p44-20, and p44-23. p44-18 was conserved at this genomic locus in all United States strains. The shared p44-61/18 in the Minnesota and MRK strains indicates common ancestry of the human and equine strains in the western and northwestern United States. Although the corresponding genomic locus was found in the European sheep isolate OS, neither p44-1 nor p44-18 was detected. Thus, the OS and U.S. strains may have diverged much earlier, before the divergence of the western and midwestern strains from the northeastern strains in the United States These results suggest that the p44-1/18 locus exemplifies independent evolution of p44 genes in geographically separate A. phagocytophilum strains.
The unique p44-1/18 genomic loci may thus be useful as markers to distinguish strains from different geographic areas. Other methods used to identify genetic strains have proven problematic. Although the 16S rRNA, groESL, and ank genes show sequence variability among isolates from humans, ticks, wild animals, horses, sheep, and goats (5, 8, 15, 18, 22, 23, 37), pulsed-field gel electrophoresis failed to distinguish seven North American strains from diverse states and host species (9). Restriction fragment length polymorphism analysis of PCR-amplified p44 genes has shown p44 polymorphism among different isolates; however, multiple p44s per genome generate overlapping banding patterns that might make regional strain typing difficult (3). Our results suggest that p44-1/18 and corresponding genomic loci differ significantly among geographically separated strains and are conserved within each region; thus, p44-1/18 has the requisite properties of a geographic strain marker for A. phagocytophilum.
The expression of p44 at an additional genomic locus different from the polymorphic p44 expression locus (1, 19) may result in more flexible control of transcription than a single expression site. The present study further confirmed p44 transcription at the p44-1/18 genomic locus in six additional strains. The p44-1/18 locus is self-expressing, suggesting that it is under selective pressure different from that of paralogs expressed from the common expression locus. Further comparative analysis of other self-expressing p44s may provide insights into differential p44 evolution.
Acknowledgments
This research was supported by grant R01AI47407 from the National Institutes of Health. The sequencing project was supported by National Institutes of Health grant R01 AI47885 to Y.R.
Editor: V. J. DiRita
REFERENCES
- 1.Barbet, A. F., P. F. M. Meeus, M. Belanger, M. V. Bowie, J. Yi, A. M. Lundgren, A. R. Alleman, S. J. Wong, F. K. Chu, U. G. Munderloh, and S. D. Jauron. 2003. Expression of multiple outer membrane protein sequence variants from a single genomic locus of Anaplasma phagocytophilum. Infect. Immun. 71:1706-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanco, J. R., and J. A. Oteo. 2002. Human granulocytic ehrlichiosis in Europe. Clin. Microbiol. Infect. 8:763-772. [DOI] [PubMed] [Google Scholar]
- 3.Carter, S. E., M. D. Ravyn, Y. Xu, and R. C. Johnson. 2001. Molecular typing of the etiologic agent of human granulocytic ehrlichiosis. J. Clin. Microbiol. 39:3398-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey, A. N., R. J. Birtles, A. D. Radford, K. J. Bown, N. P. French, Z. Woldehiwet, and N. H. Ogden. 2004. Groupings of highly similar major surface protein (p44)-encoding paralogues: a potential index of genetic diversity amongst isolates of Anaplasma phagocytophilum. Microbiology 150:727-734. [DOI] [PubMed] [Google Scholar]
- 5.Chae, J. S., J. E. Foley, J. S. Dumler, and J. E. Madigan. 2000. Comparison of the nucleotide sequences of 16S rRNA, 444 Ep-ank, and groESL heat shock operon genes in naturally occurring Ehrlichia equi and human granulocytic ehrlichiosis agent isolates from northern California. J. Clin. Microbiol. 38:1364-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christova, I., J. Van De Pol, S. Yazar, E. Velo, and L. Schouls. 2003. Identification of Borrelia burgdorferi sensu lato, Anaplasma and Ehrlichia species, and spotted fever group Rickettsiae in ticks from Southeastern Europe. Eur. J. Clin. Microbiol. Infect. Dis. 22:535-542. [DOI] [PubMed] [Google Scholar]
- 8.Courtney, J. W., R. L. Dryden, J. Montgomery, B. S. Schneider, G. Smith, and R. F. Massung. 2003. Molecular characterization of Anaplasma phagocytophilum and Borrelia burgdorferi in Ixodes scapularis ticks from Pennsylvania. J. Clin. Microbiol. 41:1569-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumler, J. S., K. M. Asanovich, and J. S. Bakken. 2003. Analysis of genetic identity of North American Anaplasma phagocytophilum strains by pulsed-field gel electrophoresis. J. Clin. Microbiol. 41:3392-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egenvall, A., A. Bjoersdorff, I. Lilliehook, E. Olsson Engvall, E. Karlstam, K. Artursson, A. Hedhammar, and A. Gunnarsson. 1998. Early manifestations of granulocytic ehrlichiosis in dogs inoculated experimentally with a Swedish Ehrlichia species isolate. Vet. Rec. 143:412-417. [DOI] [PubMed] [Google Scholar]
- 11.Falco, R. C., D. F. McKenna, T. J. Daniels, R. B. Nadelman, J. Nowakowski, D. Fish, and G. P. Wormser. 1999. Temporal relation between Ixodes scapularis abundance and risk for Lyme disease associated with erythema migrans. Am. J. Epidemiol. 149:771-776. [DOI] [PubMed] [Google Scholar]
- 12.Felek, S., S. Telford III, R. C. Falco, and Y. Rikihisa. 2004. Sequence analysis of p44 homologs expressed by Anaplasma phagocytophilum in infected ticks feeding on naive hosts and in mice infected by tick attachment. Infect. Immun. 72:659-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster, W. N., and A. E. Cameron. 1970. Observations on ovine strains of tick-borne fever. J. Comp. Pathol. 80:429-436. [DOI] [PubMed] [Google Scholar]
- 14.Ijdo, J. W., C. Wu, S. R. Telford III, and E. Fikrig. 2002. Differential expression of the p44 gene family in the agent of human granulocytic ehrlichiosis. Infect. Immun. 70:5295-5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson, K. E., B. Pettersson, M. Uhlen, A. Gunnarsson, M. Malmqvist, and E. Olsson. 1995. Identification of the causative agent of granulocytic ehrlichiosis in Swedish dogs and horses by direct solid phase sequencing of PCR products from the 16S rRNA gene. Res. Vet. Sci. 58:109-112. [DOI] [PubMed] [Google Scholar]
- 16.Kim, H. Y., and Y. Rikihisa. 1998. Characterization of monoclonal antibodies to the 44-kilodalton major outer membrane protein of the human granulocytic ehrlichiosis agent. J. Clin. Microbiol. 36:3278-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, H.-Y., and Y. Rikihisa. 2000. Expression of interleukin-1β, tumor necrosis factor alpha, and interleukin-6 in human peripheral blood leukocytes exposed to human granulocytic ehrlichiosis agent or recombinant major surface protein P44. Infect. Immun. 68:3394-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin, M. L., W. L. Nicholson, R. F. Massung, J. W. Sumner, and D. Fish. 2002. Comparison of the reservoir competence of medium-sized mammals and Peromyscus leucopus for Anaplasma phagocytophilum in Connecticut. Vector Borne Zoonotic Dis. 2:125-136. [DOI] [PubMed] [Google Scholar]
- 19.Lin, Q., Y. Rikihisa, N. Ohashi, and N. Zhi. 2003. Mechanisms of variable p44 expression by Anaplasma phagocytophilum. Infect. Immun. 71:5650-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, Q., N. Zhi, N. Ohashi, H. W. Horowitz, M. E. Aguero-Rosenfeld, J. Raffalli, G. P. Wormser, and Y. Rikihisa. 2002. Analysis of sequences and loci of p44 homologs expressed by Anaplasma phagocytophila in acutely infected patients. J. Clin. Microbiol. 40:2981-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Lin, Q., Y. Rikihisa, S. Felek, X. Wang, R. F. Massung, and Z. Woldehiwet. 2004. Anaplasma phagocytophilum has a functional msp2 gene that is distinct from p44. Infect. Immun. 72:3883-3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madigan, J. E., and D. Gribble. 1987. Equine ehrlichiosis in northern California: 49 cases (1968-1981). J. Am. Vet. Med. Assoc. 190:445-448. [PubMed] [Google Scholar]
- 22.Massung, R. F., M. J. Mauel, J. H. Owens, N. Allan, J. W. Courtney, K. C. Stafford III, and T. N. Mather. 2002. Genetic variants of Ehrlichia phagocytophila, Rhode Island and Connecticut. Emerg. Infect. Dis. 8:467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massung, R. F., J. H. Owens, D. Ross, K. D. Reed, M. Petrovec, A. Bjoersdorff, R. T. Coughlin, G. A. Beltz, and C. I. Murphy. 2000. Sequence analysis of the ank gene of granulocytic ehrlichiae. J. Clin. Microbiol. 38:2917-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massung, R. F., R. A. Priestley, N. J. Miller, T. N. Mather, and M. L. Levin. 2003. Inability of a variant strain of Anaplasma phagocytophilum to infect mice. J. Infect. Dis. 188:1757-1763. [DOI] [PubMed] [Google Scholar]
- 25.Massung, R. F., K. Slater, J. H. Owens, W. L. Nicholson, T. N. Mather, V. B. Solberg, and J. G. Olson. 1998. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 36:1090-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McQuiston, J. H., C. D. Paddock, R. C. Holman, and J. E. Childs. 1999. The human ehrlichioses in the United States. Emerg. Infect. Dis. 5:635-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogden, N. H., K. Bown, B. K. Horrocks, Z. Woldehiwet, and M. Bennett. 1998. Granulocytic Ehrlichia infection in ixodid ticks and mammals in woodlands and uplands of the U.K. Med. Vet. Entomol. 12:423-429. [DOI] [PubMed] [Google Scholar]
- 28.Ohno, S. 1970. Evolution by gene duplication. Springer-Verlag, Heidelberg, Germany.
- 29.Pancholi, P., C. P. Kolbert, P. D. Mitchell, K. D. Reed, Jr., J. S. Dumler, J. S. Bakken, S. R. Telford III, and D. H. Persing. 1995. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect. Dis. 172:1007-1012. [DOI] [PubMed] [Google Scholar]
- 30.Petrovec, M., S. L. Furlan, T. A. Zupanc, F. Strle, P. Brouqui, V. Roux, and J. S. Dumler. 1997. Human disease in Europe caused by a granulocytic Ehrlichia species. J. Clin. Microbiol. 35:1556-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pusterla, N., J. Huder, C. Wolfensberger, B. Litschi, A. Parvis, and H. Lutz. 1997. Granulocytic ehrlichiosis in two dogs in Switzerland. J. Clin. Microbiol. 35:2307-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pusterla, N., J. B. Huder, K. Feige, and H. Lutz. 1998. Identification of a granulocytic Ehrlichia strain isolated from a horse in Switzerland and comparison with other rickettsiae of the Ehrlichia phagocytophila genogroup. J. Clin. Microbiol. 36:2035-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reubel, G. H., R. B. Kimsey, J. E. Barlough, and J. E. Madigan. 1998. Experimental transmission of Ehrlichia equi to horses through naturally infected ticks (Ixodes pacificus) from northern California. J. Clin. Microbiol. 36:2131-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richter, P. J., Jr., R. B. Kimsey, J. E. Madigan, J. E. Barlough, J. S. Dumler, and D. L. Brooks. 1996. Ixodes pacificus (Acari: Ixodidae) as a vector of Ehrlichia equi (Rickettsiales: Ehrlichieae). J. Med. Entomol. 33:1-5. [DOI] [PubMed] [Google Scholar]
- 35.Rikihisa, Y., S. A. Ewing, J. C. Fox, A. G. Siregar, F. H. Pasaribu, and M. B. Malole. 1992. Analyses of Ehrlichia canis and a canine granulocytic Ehrlichia infection. J. Clin. Microbiol. 30:143-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rikihisa, Y., N. Zhi, G. P. Wormser, B. Wen, H. W. Horowitz, and K. E. Hechemy. 1997. Ultrastructural and antigenic characterization of a granulocytic ehrlichiosis agent directly isolated and stably cultivated from a patient in New York state. J. Infect. Dis. 175:210-213. [DOI] [PubMed] [Google Scholar]
- 37.Sumner, J. W., W. L. Nicholson, and R. F. Massung. 1997. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J. Clin. Microbiol. 35:2087-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Telford, S. R., III, T. J. Lepore, P. Snow, C. K. Warner, and J. E. Dawson. 1995. Human granulocytic ehrlichiosis in Massachusetts. Ann. Intern. Med. 123:277-279. [DOI] [PubMed] [Google Scholar]
- 39.Von Loewenich, F. D., G. Stumpf, B. U. Baumgarten, M. Rollinghoff, J. S. Dumler, and C. Bogdan. 2003. A case of equine granulocytic ehrlichiosis provides molecular evidence for the presence of pathogenic Anaplasma phagocytophilum (HGE agent) in Germany. Eur. J. Clin. Microbiol. Infect. Dis. 22:303-305. [DOI] [PubMed] [Google Scholar]
- 40.Zhi, N., N. Ohashi, and Y. Rikihisa. 2002. Activation of a p44 pseudogene in Anaplasma phagocytophila by bacterial RNA splicing: a novel mechanism for post-transcriptional regulation of a multigene family encoding immunodominant major outer membrane proteins. Mol. Microbiol. 46:135-145. [DOI] [PubMed] [Google Scholar]
- 41.Zhi, N., N. Ohashi, and Y. Rikihisa. 1999. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J. Biol. Chem. 274:17828-17836. [DOI] [PubMed] [Google Scholar]
- 42.Zhi, N., N. Ohashi, T. Tajima, J. Mott, R. W. Stich, D. Grover, S. R. Telford III, Q. Lin, and Y. Rikihisa. 2002. Transcript heterogeneity of the p44 multigene family in a human granulocytic ehrlichiosis agent transmitted by ticks. Infect. Immun. 70:1175-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhi, N., Y. Rikihisa, H. Y. Kim, G. P. Wormser, and H. W. Horowitz. 1997. Comparison of major antigenic proteins of six strains of the human granulocytic ehrlichiosis agent by Western immunoblot analysis. J. Clin. Microbiol. 35:2606-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]