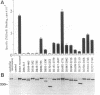
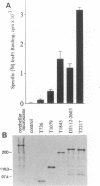
Abstract
The homotetrameric complex of inositol 1,4,5-triphosphate (InsP3) receptors displays a Ca2+ release activity in response to InsP3 molecules. Structure-function relationships of the mouse cerebellar InsP3 receptor have been studied by analyses of a series of internal deletion or C-terminal truncation mutant proteins expressed in NG108-15 cells. Within the large cytoplasmic portion of the InsP3 receptor, approximately 650 N-terminal amino acids are highly conserved between mouse and Drosophila, and this region has the critical sequences for InsP3 binding that probably form the three-dimensionally restricted binding site. The N-terminal region of each InsP3 receptor subunit also binds one InsP3 molecule. Cross-linking experiments have revealed that InsP3 receptors are intermolecularly associated at the transmembrane domains and/or the successive C termini. The interaction between the receptor subunit and InsP3 may cause a conformational change in the tetrameric complex, resulting in the opening of Ca2+ channels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Chadwick C. C., Saito A., Fleischer S. Isolation and characterization of the inositol trisphosphate receptor from smooth muscle. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2132–2136. doi: 10.1073/pnas.87.6.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. Y. Binding of heparin to human antithrombin III activates selective chemical modification at lysine 236. Lys-107, Lys-125, and Lys-136 are situated within the heparin-binding site of antithrombin III. J Biol Chem. 1989 Feb 25;264(6):3111–3115. [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Snyder S. H. Calcium flux mediated by purified inositol 1,4,5-trisphosphate receptor in reconstituted lipid vesicles is allosterically regulated by adenine nucleotides. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2147–2151. doi: 10.1073/pnas.87.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Supattapone S., Snyder S. H. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989 Nov 2;342(6245):87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989 Nov 2;342(6245):32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Gill D. L., Ghosh T. K., Mullaney J. M. Calcium signalling mechanisms in endoplasmic reticulum activated by inositol 1,4,5-trisphosphate and GTP. Cell Calcium. 1989 Jul;10(5):363–374. doi: 10.1016/0143-4160(89)90062-6. [DOI] [PubMed] [Google Scholar]
- Lochrie M. A., Simon M. I. G protein multiplicity in eukaryotic signal transduction systems. Biochemistry. 1988 Jul 12;27(14):4957–4965. doi: 10.1021/bi00414a001. [DOI] [PubMed] [Google Scholar]
- Maeda N., Kawasaki T., Nakade S., Yokota N., Taguchi T., Kasai M., Mikoshiba K. Structural and functional characterization of inositol 1,4,5-trisphosphate receptor channel from mouse cerebellum. J Biol Chem. 1991 Jan 15;266(2):1109–1116. [PubMed] [Google Scholar]
- Maeda N., Niinobe M., Mikoshiba K. A cerebellar Purkinje cell marker P400 protein is an inositol 1,4,5-trisphosphate (InsP3) receptor protein. Purification and characterization of InsP3 receptor complex. EMBO J. 1990 Jan;9(1):61–67. doi: 10.1002/j.1460-2075.1990.tb08080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery G. A., Newton C. L., Archer B. T., 3rd, Südhof T. C. Structure and expression of the rat inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1990 Jul 25;265(21):12679–12685. [PubMed] [Google Scholar]
- Mignery G. A., Südhof T. C. The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. EMBO J. 1990 Dec;9(12):3893–3898. doi: 10.1002/j.1460-2075.1990.tb07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A., Furuichi T., Maeda N., Mikoshiba K. Expressed cerebellar-type inositol 1,4,5-trisphosphate receptor, P400, has calcium release activity in a fibroblast L cell line. Neuron. 1990 Jul;5(1):11–18. doi: 10.1016/0896-6273(90)90029-f. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R., Potter B. V. Molecular recognition of inositol polyphosphates by intracellular receptors and metabolic enzymes. Trends Pharmacol Sci. 1989 Apr;10(4):139–144. doi: 10.1016/0165-6147(89)90165-x. [DOI] [PubMed] [Google Scholar]
- O'Rourke F., Feinstein M. B. The inositol 1,4,5-trisphosphate receptor binding sites of platelet membranes. pH-dependency, inhibition by polymeric sulphates, and the possible presence of arginine at the binding site. Biochem J. 1990 Apr 15;267(2):297–302. doi: 10.1042/bj2670297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supattapone S., Danoff S. K., Theibert A., Joseph S. K., Steiner J., Snyder S. H. Cyclic AMP-dependent phosphorylation of a brain inositol trisphosphate receptor decreases its release of calcium. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8747–8750. doi: 10.1073/pnas.85.22.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]