Abstract
Quorum sensing triggers virulence factor expression in medically important bacterial pathogens in response to a density-dependent increase in one or more autoinducing pheromones. Here, we show that phagocyte-derived oxidants target these autoinducers for inactivation as an innate defense mechanism of the host. In a skin infection model, expression of phagocyte NADPH oxidase, myeloperoxidase, or inducible nitric oxide synthase was critical for defense against a quorum-sensing pathogen, Staphylococcus aureus, but not for defense against a quorum sensing-deficient mutant. A virulence-inducing peptide of S. aureus was inactivated in vitro and in vivo by reactive oxygen and nitrogen intermediates, including HOCl and ONOO–. Inactivation of the autoinducer prevented both the up-regulation of virulence gene expression and the downstream sequelae. MS analysis of the inactivated peptide demonstrated that oxidation of the C-terminal methionine was primarily responsible for loss of activity. Treatment of WT but not NADPH oxidase-deficient mice with N-acetyl methionine to scavenge the inhibitory oxidants increased in vivo quorum sensing independently of the bacterial burden at the site of infection. Thus, oxidant-mediated inactivation of an autoinducing peptide from S. aureus is a critical innate defense mechanism against infection with this pathogen.
Bacteria sense each other and their numbers by means of a cell-to-cell communication mechanism, called quorum sensing, that is mediated by secretion of small, diffusible pheromones or autoinducers (1, 2). At a concentration threshold that reflects a sufficient quorum of bacteria, the autoinducers trigger behaviors that are primarily effective at high population densities, including the secretion of virulence factors (1–6). Whereas the genetic systems that regulate quorum sensing in many bacterial pathogens are undergoing intensive investigation, specific effector mechanisms in mammalian hosts that limit density-dependent virulence have not been defined. We speculated that an important contribution of innate immunity in defense against bacterial infection would be to inhibit autoinducer signaling of virulence gene expression.
Staphylococcus aureus is a medically important bacterial pathogen that uses quorum sensing to regulate virulence in several experimental models of infection (4, 7–9). The agr operon, which is responsible in part for this regulation, combines secretion of an autoinducing thiolactone peptide (AIP) with a two-component regulatory pathway to generate a regulatory RNA transcript, RNAIII, that is the effector of the operon (10–12). S. aureus strains can be categorized into four groups based on the amino acid sequence of the AIP produced (12). Whereas clinical isolates are enriched for the type I AIP (13, 14), all groups are represented in human disease (14). Under conditions of high AIP concentration, i.e., high bacterial density, RNAIII down-regulates gene expression encoding for surface adhesins while up-regulating those encoding for capsule production, secreted toxins, proteases, and metabolic pathways (10, 12, 15). This conversion in phenotype from adhesive and colonizing to tissue-damaging and invasive is thought to contribute to the pathogenesis of systemic infection (10, 12, 16).
The phagocyte NADPH oxidase is an important innate effector against this pathogen, and humans and mice that lack this enzyme are more susceptible to staphylococcal infection (17–19). Activation of the NADPH oxidase could contribute to host defense against S. aureus in many ways, from direct (20) and indirect (21) bacterial killing to regulating gene transcription of inflammatory mediators (22). We hypothesized that phagocyte-derived oxidants act additionally as a barrier against density-dependent virulence by inactivating the pheromone autoinducers. Here, we show that phagocyte-derived oxidants contribute to innate immunity by inhibiting pathogenic bacterial communication.
Materials and Methods
Mice. C57BL/6 mice (8–16 wk, ≈20–25 g) from Charles River Breeding Laboratories, NADPH oxidase (gp91phox)–/– and inducible NO synthase (INOS)–/–mice from The Jackson Laboratory, and myeloperoxidase (MPO)–/– mice (23) on the C57BL/6 background were gender- and age-matched. The appropriate institutional committees approved all experiments.
Bacteria. AIP group I WT RN6390, its agr deleted mutant RN6911, and AIP group I methicillin-resistant S. aureus (MRSA) COL strains containing plasmids encoding the promoter for RNAIII or α hemolysin (hla) driving expression of GFP were generated as described (24). Early exponential phase, nonfluorescent bacteria with a mean channel of fluorescence (MCF) of 10–20 by flow cytometry (FACSCalibur, Becton Dickinson) were prepared as described (7). Culture supernatants from WT and agr– bacteria were prepared as described (7).
Air Pouch Infection Model. Pouches created by the injection of air (7) were infected with 100 μl of early exponential phase WT or agr– bacteria. After 4–28 h, the pouch was lavaged (5 ml) and sonicated, and the kidneys (a measure of blood stream metastasis) were homogenized and sonicated before colony-forming unit (cfu) evaluation (7). The intracellular viable cfu (25) in the pouch lavage was determined after killing the extracellular bacteria by incubating the lavage with 1 μg/ml recombinant lysostaphin (Sigma) for 30 min at room temperature before washing and sonication in PBS/0.1% Triton X-100. This procedure reduced 107 WT bacteria per ml of buffer by >5 logs in the presence or absence of 106 uninfected murine macrophages. In vivo promoter activation was measured as the percentage of bacteria with an MCF of >20 (GFP+) and the average fluorescence of the GFP+ bacteria (MCF) (7). Lavage neutrophil percentage and number were determined as described (25). Morbidity was measured as weight loss after infection (7). Injections directly into the pouch to modify in vivo quorum sensing included the following: 100 μl of either 1 μM AIP or oxidized AIP at the time of infection for collection at 4 h and 100 μl of either 100 mM N-acetyl methionine (NAM) (Sigma) or vehicle control (sterile, endotoxin-free saline or PBS) at 24 h for collection at 28 h.
Oxidant Treatment of AIP. The type I AIP was synthesized (26) and stored at 100 μM in DMSO at –70°C. H2O2 and HOCl (Sigma) and purified human MPO (Calbiochem) or bovine lactoperoxidase (Sigma) were diluted with PBS or chloride-free phosphate buffer (pH 7.4) and incubated with AIP at 37°C for 30 min. HOCl concentration was determined by its absorbance at 292 nm (pH 12, ε292 = 350 M–1·cm–1) (27). S-nitrosoglutathione (GSNO) was synthesized as described (28). Reagent peroxynitrite (ONOO–) was synthesized in a quenched flow reactor (29) and stored at –70°C in 1.5 M NaOH, and its concentration was calculated by its absorbance at 302 nm (ε302 = 1,670 M–1·cm–1). The reactions were carried out in sterile microfuge tubes in 40-μl volumes and contained 2.5 μM AIP or a control peptide of similar size, 2.5 μM H2O2, 25 nM MPO, 2.5 μM HOCl in the appropriate buffer, 1 μM–10 mM GSNO, or 50–500 μM ONOO– diluted into 0.3 M NaOH. To neutralize the base concentration necessary to maintain the activity of ONOO– (29), an equivalent volume of an acidic phosphate buffer was included to bring the pH to 7.4 during the AIP treatment. AIP exposed to the acid/base only was included as a control. NAM at 0.25–250 μM was included to demonstrate protection from oxidation. Residual oxidants were scavenged by the addition of 100 μl of 100 μM NAM and/or catalase (780 units, Worthington) before addition to the bacterial reporter assay or injection into the air pouch.
In Vitro RNAIII Promoter Activation. Early exponential-phase bacteria (107 cfu) containing the RNAIII promoter-GFP construct were cultured as described (7) in the presence or absence of the following: 100 nM AIP or oxidant-treated AIP (1:25 final dilution), 100 nM control peptide, 20% WT supernatant, or 20% agr– supernatant in 1 ml of broth. After 3 h, the bacteria were processed for flow cytometry. RNAIII promoter activation was measured as the mean fluorescence of the entire population (MCF) (7). Colony counts were performed to control for residual effects of the reagents used to oxidize the AIP and quench the oxidants. A direct effect of oxidants on GFP fluorescence was determined by incubation of fluorescent bacteria with 10 μM HOCl or H2O2 for 30 min at 37°C before flow cytometry. To control for direct effects of NAM on RNAIII promoter activation, 100 μM NAM was included during broth culture (3 h and overnight), and the magnitude of RNAIII promoter activation was determined by flow cytometry.
MS. For quantitative measurements, 10.0 μl of AIP samples generated as described above were analyzed by using a QSTAR Pulsar I mass spectrometer (Applied Biosystems/MDS Sciex) coupled with LC Packings Ultimate microcapillary LC system (Dionex) by using a PepMap C18 column (3 μm, 100 Å, 75 μm i.d., 15-cm length). A 10-μm i.d. PicoTip nanospray emitter (New Objective, Woburn, MA) was used with a spray voltage set between 1,800–2,100 V. AIP tandem MS fragmentation (30) sequencing of ions 961 and 977 was performed by using the QSTAR oMALDI source with a laser power of 5%, multichannel panel of 2,225, and a collision active dissociation gas of 55. Peptide samples for oMALDI analysis were further purified and plated by using C18 ZipTips (Millipore) and eluted with 4.0 μl of 50% 0.1% trifluoroacetic acid/50% acetonitrile solution saturated with recrystallized α-cyano-4-hydroxycinnamic acid (10 mg/ml).
Statistical Evaluation. Data are depicted as the mean ± SEM, and statistical significance was determined by the Mann–Whitney U test for nonparametrics.
Results
Phagocyte-Derived Oxidants Are Essential for Control of Quorum Sensing-Dependent but Not Quorum Sensing-Independent S. aureus Infection. To determine whether quorum sensing is targeted by phagocyte-derived oxidants as a defense mechanism against S. aureus infection, we infected NADPH oxidase-, MPO-, and INOS-deficient mice with either quorum sensing-sufficient WT bacteria or an agr mutant deficient in quorum sensing. We used an air pouch model of skin infection that mimics systemic staphylococcal infection in humans initiated as a cutaneous or s.c. abscess (31) and in which bacteremia and metastatic infection follow agr-dependent RNAIII production (7). We quantified quorum sensing in vivo by infecting with early exponential phase bacteria containing an RNAIII promoter-GFP construct and measuring induction of both the percentage of fluorescent bacteria and their magnitude of fluorescence over time (7, 8, 24).
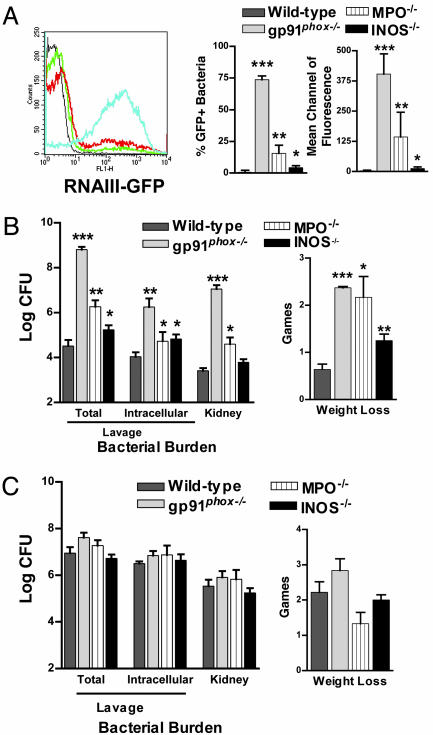
At a time after infection (24 h) and dose of WT bacteria (2 × 107) that result in negligible RNAIII promoter activation in WT mice (Fig. 1A), bacteria isolated from the pouch of gp91phox–/– mice that lack functional NADPH oxidase (18) demonstrated significant RNAIII promoter activation (Fig. 1 A). Activation of the promoter for α hemolysin, a downstream target of RNAIII (15), also increased in infected gp91phox–/– mice, demonstrating that elevation of RNAIII levels up-regulated virulence factor gene expression (Fig. 6, which is published as supporting information on the PNAS web site). No RNAIII promoter activation was demonstrable in mice infected with agr-deficient bacteria (7) (data not shown). Consistent with this difference, both the bacterial burden (total and intracellular viable cfu per ml of lavage and both kidneys) and morbidity (weight loss) were increased in gp91phox–/– (Fig. 1B). The appearance and behavior of the mice also differed; the gp91phox–/– mice demonstrated clinical signs of systemic infection, including lethargy, piloerection, inappetance, and impaired ambulation whereas the WT mice ate, drank, and groomed normally.
Fig. 1.
Comparison of WT, gp91phox–/–, MPO–/–, and INOS–/– mice for in vivo RNAIII promoter activation, bacterial burden, and morbidity 24 h after infection with either quorum sensing-sufficient or -deficient S. aureus.(A and B)WT (n = 29), gp91phox–/– (n = 10), MPO–/– (n = 8), and INOS–/– (n = 8) mice infected with 2 × 107 WT[RNAIII-GFP] were compared for the magnitude of RNAIII promoter activation expressed by bacteria isolated from the pouch lavage (WT = black line; gp91phox–/– = blue line; MPO–/– = red line; INOS–/– = green line) (A) and for the total bacterial burden in the lavage, the intracellular viable bacteria in the lavage, the bacterial burden in the kidney, and weight loss (B). (C) WT (n = 24), gp91phox–/– (n = 8), MPO–/– (n = 7), and INOS–/– (n = 8) mice infected with 0.7–1.0 × 108 agr–[RNAIII-GFP] were compared for the total bacterial burden and the intracellular viable bacteria per ml of lavage, the bacterial burden in the kidney, and weight loss. In this model, there is no RNAIII promoter activation in the absence of agr (7). ***, P < 0.001; **, P < 0.01; *, P < 0.05.
MPO in phagosomes and secreted extracellularly uses a product of the NADPH oxidase, H2O2, in the presence of chloride to generate HOCl, the oxidant primarily implicated in host defense against S. aureus infection (20). MPO–/– mice were more susceptible to infection with WT bacteria as demonstrated by increased RNAIII promoter activation, bacterial burden, and morbidity (Fig. 1 A and B). However, the magnitude of the defect was reduced compared with gp91phox–/– mice. The primary product of the oxidase, superoxide anion, combines with NO• generated by INOS to create peroxynitrite (ONOO–), an unstable, short-lived powerful oxidant speculated to compensate for HOCl in MPO deficiency (20). INOS–/– mice were more susceptible to WT infection (Fig. 1B), but the defect was minimal compared with either the gp91phox–/– or the MPO–/– mice. At a higher dose (3.3 × 107), we observed greater differences between WT and INOS–/– mice (data not shown). These data indicate that products of all of these phagocytic enzymes are important at this time point for control of infection with quorum sensing-sufficient S. aureus and suggest that activation of the oxidase in the absence of either MPO or INOS generates partially protective oxidants for control of quorum sensing-dependent infection.
If products of these enzymes are primarily important for controlling infection by suppressing quorum sensing at this early time point, mice that lack these enzymes should have equivalent host defense against agr-deficient S. aureus as WT mice. Although agr-deletion mutants are less virulent in several models, they cause serious infection and death when given at increased inocula (7, 16). We infected mice with a dose (0.7–1 × 108 cfu) that caused moderate morbidity in WT mice (7). Neither the bacterial burden nor the amount of weight lost was significantly increased in either gp91phox–/–, MPO–/– mice, or INOS–/– mice (Fig. 1C). In fact, the MPO–/–and INOS–/– mice lost less weight than WT mice. The similarity of the response was also observed in the appearance and behavior of the mice. These data indicate that expression of NADPH oxidase, MPO, or INOS is critical for control of infection with quorum-sensing but not quorum sensing-deficient S. aureus early in the course of infection.
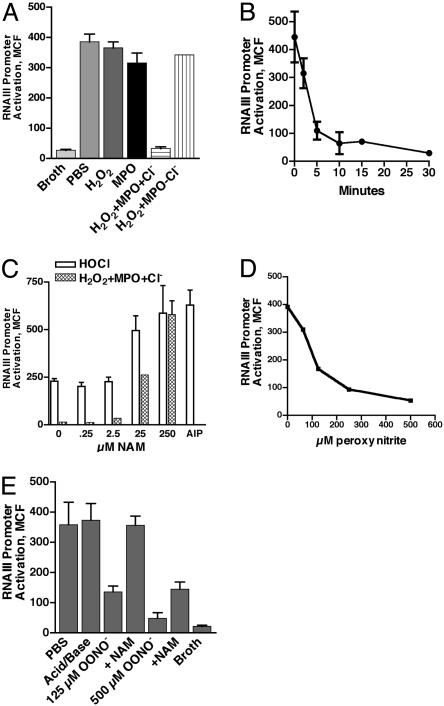
H2O2-MPO-Halide System and ONOO– Inhibit AIP-Induced RNAIII Promoter Activation in Vitro. We investigated products of these phagocytic enzymes for their ability to inactivate the octapeptide thiolactone virulence autoinducer. Incubation of early exponential phase, nonfluorescent WT bacteria with either 100 nM AIP, but not a control peptide, or supernatant from WT bacteria, but not supernatant from agr-deficient bacteria, induced significant RNAIII promoter activation by 3 h (data not shown). Qualitatively similar data were obtained with the type I AIP methicillinresistant S. aureus strain COL that contained the RNAIII-GFP (24) construct, verifying that the ability of this AIP to activate RNAIII was not exclusive to strain RN6390 (data not shown). We exposed AIP to various oxidants vs. PBS before inclusion in the reporter assay. Neither MPO nor H2O2 affected the function of the AIP. However, inclusion of both in the presence of chloride but not in its absence completely ablated AIP biologic activity (Fig. 2A) by 30 min (Fig. 2B). Salivary lactoperoxidase was substituted for MPO with equivalent results (data not shown). These data indicate that peroxidase production of HOCl is sufficient for inactivation of AIP.
Fig. 2.
Oxidant inhibition of AIP-induced RNAIII promoter activation in vitro. Type I AIP was incubated with either H2O2-MPO-Cl– generated oxidants (A, B, and C) or ONOO– (D and E) as compared with buffer controls to determine effect on biologic activity (A and E), kinetics of inactivation (B), dose–response of inactivation (D), and the ability of NAM to protect against the loss of biologic activity (C and E, respectively). RNAIII promoter activity was measured at 3 h (MCF of entire population) after incubation of early exponential phase, nonfluorescent WT[RNAIII-GFP] bacteria with 100 nM native or modified AIP. Data represent a minimum of three determinations.
Because methionine is a target of HOCl oxidation (32) and this AIP contains a methionine (4), we included the HOCL scavenger, NAM, during inactivation of AIP with equimolar HOCl or H2O2 plus MPO and chloride. Both oxidants reduced AIP activity with enzymatic oxidant generation, resulting in a complete loss of activity (Fig. 2C). Higher concentrations of HOCl totally inactivated AIP activity. AIP activity was protected by including NAM at a 10–100 fold excess (Fig. 2C). The oxidants did not affect the fluorescence of GFP because incubation of fluorescent bacteria with HOCl or H2O2 did not inhibit either the percentage or the magnitude of fluorescence (data not shown). In addition, the results could not be explained by differences in cfu during the 3-h incubation because the log cfus ranged from 8.47 to 8.52 under all conditions, demonstrating equivalent bacterial growth independent of AIP treatment (Fig. 7, which is published as supporting information on the PNAS web site). These data indicate that products of the MPO-H2O2-halide system inactivate the biologic activity of the secreted autoinducer necessary for quorum sensing in S. aureus.
The partial protection of the MPO–/– mice as compared with the gp91phox–/– mice suggested that an oxidant other than HOCl could compensate for inactivation of the autoinducer (20). Inclusion of the NO• donor, S-nitrosoglutathione, from 1 μM to 10 mM in the reaction had no effect on AIP-induced RNAIII promoter activation (data not shown). In contrast, the highly reactive oxidant ONOO– reduced AIP activity in a dose-dependent fashion (Fig. 2D) that was prevented by the inclusion of 250 μM NAM (Fig. 2E). As with the MPO-H2O2-halide system, inactivation occurred at neutral pH, consistent with known oxidation of methionine sulfhydryls by ONOO– (29), and had no effect on bacterial growth in the reporter assay after scavenging residual oxidant. These data indicate that HOCl and ONOO– can inhibit AIP biologic function.
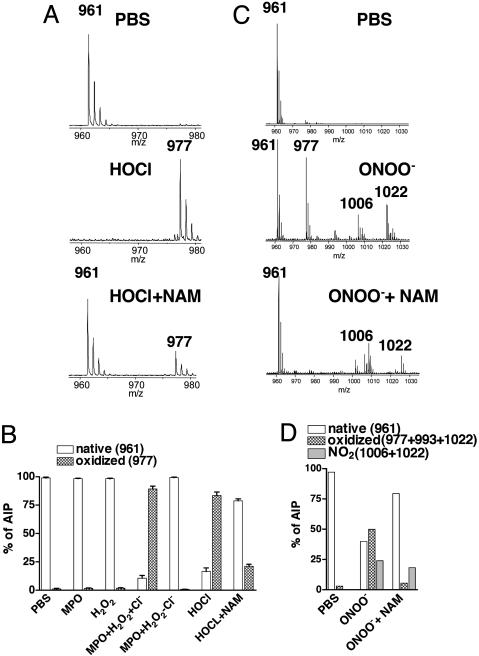
Oxidation of the C-Terminal Methionine Inactivates AIP, and Inclusion of NAM Protects It from Oxidation. We determined potential modifications in mass by MS analysis of AIP treated with oxidants in the presence and absence of NAM. Native functional AIP had a mass/charge ratio (m/z) of 961 that increased to 977 after incubation with equimolar HOCl (Fig. 3A), a treatment that reduced AIP activity (Fig. 2C). The 16 m/z increase was prevented in the presence of NAM (Fig. 3A), a condition that retained activity (Fig. 2C). When we evaluated the percentage of AIP in either the native or oxidized form over multiple experiments, incubation of AIP with MPO plus H2O2 in the presence of chloride but not in its absence resulted in a significant increase in the oxidized form (977) (Fig. 3B). An equivalent result was obtained with HOCl and was prevented by the inclusion of NAM (Fig. 3B). Treatment of native AIP with OONO– at a concentration that reduced activity by ≈60%, generated major species at 961 (native) and 977 (one oxygen atom) (Fig. 3C). Minor species at 993 (two oxygen atoms), 1,006 (NO2 only), and 1,022 (O plus NO2) were also observed. Inclusion of NAM at a concentration that protected AIP activity (Fig. 2E) prevented generation of peaks at 977, 993, and 1,022 but not the peak at 1,006 (Fig. 3C). Quantification of the percentage of AIP containing either oxygen or NO2 modifications demonstrated that NAM primarily affected the generation of oxygen-modified species without an effect on the addition of NO2 (Fig. 3D). These data indicate that the addition of oxygen by the MPO-H2O2-Cl– system or by ONOO– to the type I AIP inhibits its activity.
Fig. 3.
MS analysis of AIP after inhibition by reactive oxidants. The m/z of AIP modified by either HOCl with or without NAM (A) or ONOO– with or without NAM (C) are depicted. The percentage of native AIP, oxidized AIP, or nitrated AIP in the presence and absence of NAM is shown for HOCl (B) and ONOO– (D). Data represent a minimum of three determinations.
Because NAM prevented oxidant inactivation of AIP activity and protected against oxidation, oxidation of the C-terminal methionine was most likely responsible for the loss in function (32). To assign the site of AIP oxidation, we subjected the native (961) and oxidized (977) forms to tandem MS fragmentation (y1–y7) (30). The 16 m/z increase was observed only in the 977 fragments that contained the methionine residue (e.g., fragment y5) whereas the same fragments in the 961 spectra were 16 m/z less (Fig. 8, which is published as supporting information on the PNAS web site), indicating that oxidation of the C-terminal methionine is responsible for the loss in biologic activity.
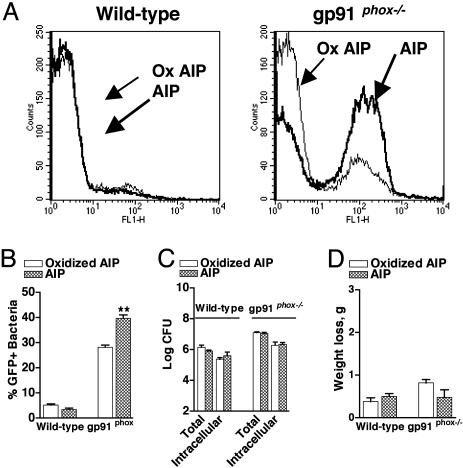
Exogenous Native AIP but Not Oxidized AIP Enhances RNAIII Promoter Activation Independently of Bacterial Burden in gp91phox–/– but Not WT Mice. RNAIII promoter activation should be increased by the exogenous administration of native AIP but not oxidized AIP in NADPH oxidase-deficient mice. We infected WT and gp91phox–/– mice with WT bacteria in the presence of either native AIP or AIP inactivated in vitro by the MPO-H2O2-Cl– system, and, after 4 h, the percentage of fluorescent bacteria in the pouch lavage from gp91phox–/– mice treated with native AIP increased significantly (Fig. 4 A and B). No effect was observed in the WT mice. Neither native AIP nor oxidized AIP affected either the bacterial burden or the minimal morbidity (weight loss) (Fig. 4 C and D). These data demonstrate that, in the absence of NADPH oxidase, exogenous administration of biologically functional AIP can increase the percentage of S. aureus undergoing quorum sensing independently of any effect on bacterial burden at the site of infection.
Fig. 4.
Effect of exogenous AIP as compared with oxidized AIP on in vivo RNAIII promoter activation, bacterial burden, and morbidity in WT and gp91phox–/– mice 4 h after infection with quorum sensing-sufficient S. aureus. WT and gp91phox–/– mice were infected with 2 × 107 WT[RNAIII-GFP] in the presence of either native (n = 8) or oxidant-modified, biologically inactive AIP (n = 8). Representative flow cytometry profiles of bacteria isolated from the pouch lavage (A), the percentage of GFP+ bacteria (B), the total and intracellular bacterial burden per ml of lavage (C), and weight loss (D) are depicted. **, P < 0.01.
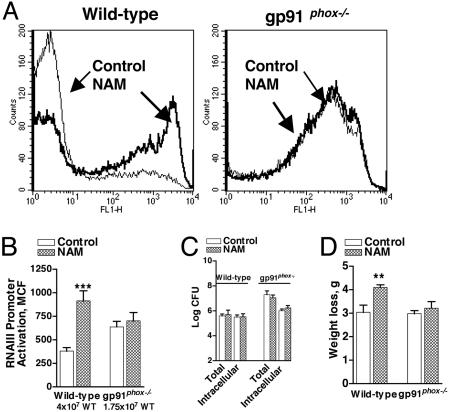
Exogenous NAM Enhances RNAIII Promoter Activation in Vivo Independently of Bacterial Burden in WT but Not gp91phox–/– Mice. Because NAM protected AIP from oxidant inactivation, it should increase quorum sensing in vivo in WT mice but not in oxidase-deficient mice. We injected NAM into the pouch 24 h after infection with a dose of WT S. aureus that results in submaximal RNAIII promoter activation and concomitant morbidity in each mouse strain (7) (Fig. 1 A and B). After 4 h, the bacteria isolated from the NAM-treated WT, but not the NAM-treated gp91phox–/– mice, had significantly increased RNAIII promoter activation compared to the vehicle control (Fig. 5 A and B). The increased bacterial quorum sensing in WT mice did not derive from an increase in bacterial burden in the lavage (Fig. 5C). However, weight loss was significantly increased by NAM, indicating that RNAIII-up-regulated virulence factor expression-enhanced morbidity in WT mice independently of an increase in bacterial burden at the site of infection (Fig. 5D). Consistent with the partial protection of MPO–/– and INOS–/– mice for defense against quorum-sensing bacteria (Fig. 1 A and B), NAM treatment of these mice enhanced the magnitude of quorum sensing (data not shown). These data indicate that NAM is able to quench the oxidants that suppress in vivo quorum sensing. Moreover, they demonstrate that phagocyte-derived oxidants contribute to innate immunity early in infection by inhibiting density-dependent virulence.
Fig. 5.
Effect of NAM on in vivo RNAIII promoter activation, bacterial burden, and morbidity in WT and gp91phox–/– mice 28 h after infection with quorum sensing-sufficient S. aureus. WT mice infected with 4 × 107 WT[RNAIII-GFP] were given PBS (n = 12) or NAM (n = 12) and gp91phox–/– mice infected with 1.75 × 107 WT[RNAIII-GFP] were given PBS (n = 11) or NAM (n = 7) 24 h after infection. Representative flow cytometry profiles of bacteria isolated from the pouch lavage (A), the MCF of the GFP+ bacteria (B), the total and intracellular bacterial burden per ml of lavage (C), and weight loss (D) are depicted. ***, P < 0.001; **, P < 0.01.
Discussion
The quorum-sensing communication system in S. aureus uses a peptide pheromone to trigger a virulence transcriptome (10, 12, 15) that contributes to morbidity and mortality. We show here that the type I peptide is inactivated by oxidants, including HOCl and ONOO–, whose production depends on the phagocyte NADPH oxidase (20). The inactivation is caused by oxidation of the C-terminal methionine in this peptide and is prevented both in vitro and in vivo by the scavenger NAM. ONOO– exposure also generates nitrated species (Fig. 3C), and the biologic significance of this modification in the absence of oxidation is presently unknown. Even though the methionine is not essential for the function of this AIP (33), our data confirm the loss of biologic activity observed with the methionine sulfoxide form of this peptide purified from culture supernatants (34). This finding suggests that oxidation may alter either the conformation or polarity of the peptide so its interaction with the bacterial receptor agrC is impaired. Consistent with this possibility, oxidized AIP is not a competitive antagonist of native AIP in our RNAIII promoter assay (J.M.R. and H.D.G., unpublished observations).
Phagocyte-derived oxidants are important effectors of innate immunity and could contribute to host defense against S. aureus infection by numerous mechanisms. Our data demonstrate that a major role of neutrophil-derived oxidants in early host defense against S. aureus infection is the suppression of quorum sensing and its contribution to virulence independently of an effect on bacterial burden (Figs. 1, 4, and 5). This finding suggests that the known role of oxidants in bacterial killing and clearance is less demonstrable at a point where quorum sensing is optimal. However, this may not be the case later in the infection after quorum sensing has occurred, as suggested by the impaired clearance of S. aureus from peritoneal abscesses in gp91phox–/– mice 7 days after infection (18). In addition, the equivalent host defense against non-quorum-sensing infection between WT and gp91phox–/– mice (Fig. 1C) suggests that compensatory inflammatory mechanisms up-regulated in NADPH oxidase-deficient neutrophils (22) can compensate for infection control in the absence of the virulence factors up-regulated by RNAIII (15).
Whereas our present data apply only to the type I AIP, examination of the sequences and structures of other bacterial autoinducers, including peptides, acyl homoserine lactones, and the furanosyl borate diester (AI-2), reveals multiple potential targets for oxidation and nitration, including residues within two of the other three AIPs of S. aureus (2, 3). Therefore, the mechanism we have described here for innate control over bacterial communication could apply broadly to other bacterial infections that depend on quorum sensing for optimal virulence. Furthermore, the recent description of enzymatic degradation of a Pseudomonas aeruginosa autoinducer by human airway epithelia (35) suggests that multiple effectors of innate immunity may contribute to control of pathogenic bacterial communication.
S. aureus is primarily a commensal colonizing a third of the population at any time (31). Our data suggest that oxidantmediated suppression of quorum sensing contributes to the high level of resistance of colonized hosts to infection with this pathogen and thus provides a barrier against quorum sensing-dependent virulence. This possibility has several implications for human infection. First, infection may occur primarily in hosts with a defect in this barrier. This result is likely for hospitalacquired infections that develop in patients with severe underlying diseases (36) where S. aureus behaves as an opportunistic pathogen. Second, S. aureus could subvert inactivation of the autoinducer either by producing an AIP that is resistant to oxidant attack or by gaining access to a host niche where phagocyte-derived oxidants cannot penetrate. For example, the amino acid sequence of the type 3 AIP (33) suggests that it may be fairly resistant to oxidant inhibition. This resistance could help explain the emergence in immunocompetent hosts of severe community-acquired infections caused primarily by agr group 3 strains (37). Thus, S. aureus may be evolving to subvert oxidant inactivation of quorum sensing. In addition, we have shown that RNAIII promoter activation is enhanced in fibrin(ogen)clumped bacteria in WT mice (7), suggesting that, in this niche, the bacteria undergoing quorum sensing are protected from phagocyte-derived oxidants. Finally, S. aureus growth on implanted catheters and in some anatomic sites does not lead to detectable RNAIII production (7, 9), suggesting that human infection could occur in the absence of the agr effector.
Here we have described a previously unrecognized role for the NADPH oxidase in innate immunity. Because quorum quenching is being pursued as a therapeutic goal (4, 38), understanding the contribution of innate immunity to quorum quenching and how pathogens avoid it could augment drug design that targets virulence pheromones for inactivation.
Supplementary Material
Acknowledgments
We thank Anny Alsup and Susan Alexander for technical support and Rick Brown and Vojo Deretic for advice. This work was supported by National Institutes of Health Grants AI46615 (to H.D.G.), AI47441 (to A.L.C.), and HL30568 (to A.J.L.), the Department of Veterans Affairs (to H.D.G.), and the Cystic Fibrosis Foundation (to G.S.T.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AIP, autoinducing thiolactone peptide; cfu, colony-forming unit; INOS, inducible NO synthase; MPO, myeloperoxidase; NAM, N-acetyl methionine; MCF, mean channel of fluorescence.
References
- 1.Greenberg, E. P. (2003) J. Clin. Invest. 112, 1288–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Federle, M. & Bassler, B. L. (2003) J. Clin. Invest. 112, 1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller, M. B. & Bassler, B. L. (2001) Annu. Rev. Microbiol. 55, 165–199. [DOI] [PubMed] [Google Scholar]
- 4.Mayville, P., Ji, G., Beavis, R., Yang, H., Goger, M., Novick, R. & Muir, T. W. (1999) Proc. Natl. Acad. Sci. USA 96, 1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swift, S., Downie, J., Whitehead, N., Barnard, A., Salmond, G. & Williams, P. (2001) Adv. Microb. Physiol. 45, 199–270. [DOI] [PubMed] [Google Scholar]
- 6.Sturme, M., Kleerebezem, M., Nakayama, J., Akkermans, A., Vaughn, E. & de Vos, W. (2002) Antonie Leeuwenhoek 81, 233–243. [DOI] [PubMed] [Google Scholar]
- 7.Rothfork, J., Dessus-Babus, S., Van Wamel, W., Cheung, A. & Gresham, H. (2003) J. Immunol. 171, 5389–5395. [DOI] [PubMed] [Google Scholar]
- 8.Xiong, Y., Van Wamel, W., Nast, C., Yeaman, M., Cheung, A. & Bayer, A. (2002) J. Infect. Dis. 186, 668–678. [DOI] [PubMed] [Google Scholar]
- 9.Yarwood, J. & Schlievert, P. (2003) J. Clin. Invest. 112, 1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung, A., Bayer, A., Zhang, G., Gresham, H. & Xiong, Y. Q. (2004) FEMS Immunol. Med. Microbiol. 40, 1–9. [DOI] [PubMed] [Google Scholar]
- 11.Korem, M., Sheoran, A., Gov, Y., Tzipori, S., Borovok, I. & Balaban, N. (2003) FEMS Microbiol. Lett. 223, 167–175. [DOI] [PubMed] [Google Scholar]
- 12.Novick, R. P. (2003) Mol. Microbiol. 48, 1429–1449. [DOI] [PubMed] [Google Scholar]
- 13.Vuong, C., Saenz, H., Gotz, F. & Otto, M. (2000) J. Infect. Dis. 182, 1688–1693. [DOI] [PubMed] [Google Scholar]
- 14.Jarraud, S., Mougel, C., Thioulouse, J., Lina, G., Meugnier, H., Forey, F., Nesme, X., Etienne, J. & Vandenesch, F. (2002) Infect. Immun. 70, 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunman, P., Murphy, E., Haney, S., Palacios, D., Tucker-Kellogg, G., Wu, S., Brown, E., Zagursky, R., Shlaes, D. & Projan, S. (2001) J. Bacteriol. 183, 7341–7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyer, G., Saba, S., Adamo, R., Rush, W., Soong, G., Cheung, A & Prince, A. (2002) Infect. Immun. 70, 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guide, S., Stock, F., Gill, V., Anderson, V., Malech, H., Gallin, J. & Holland, S. (2003) J. Infect. Dis. 187, 845–853. [DOI] [PubMed] [Google Scholar]
- 18.Pollock, J., Williams, D., Gifford, M., Li, L., Du, X., Fisherman, J., Orkin, S., Doerschuk, C. & Dinauer, M. (1995) Nat. Genet. 9, 202–209. [DOI] [PubMed] [Google Scholar]
- 19.Jackson, S., Gallin, J. & Holland, S. (1995) J. Exp. Med. 182, 751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hampton, M., Kettle, A. & Winterbourn, C. (1998) Blood 92, 3007–3017. [PubMed] [Google Scholar]
- 21.Ahluwalia, J., Tinker, A., Clapp, L., Duchen, M., Abramov, A., Pope, S., Nobles, M. & Segal, A. (2004) Nature 427, 853–858. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Kobayashi, S., Voyich, J., Braughton, K., Whitney, A., Nauseef, W., Malech, H. & DeLeo, F. (2004) J. Immunol. 172, 636–643. [DOI] [PubMed] [Google Scholar]
- 23.Brennan, M., Anderson, M., Shih, D., Qu, X., Wang, X., Mehta, A., Lim, L., Shi, W., Hazen, S., Jacob, J., et al. (2001) J. Clin. Invest. 107, 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kupferwasser, L., Yeaman, M., Nast, C., Kupferwasser, D., Xiong, Y., Palma, M., Cheung, A.L. & Bayer, A. (2003) J. Clin. Invest. 112, 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gresham, H., Lowrance, J., Caver, T., Wilson, B., Cheung, A. & Lindberg, F. (2000) J. Immunol. 164, 3713–3722. [DOI] [PubMed] [Google Scholar]
- 26.Otto, M., Echner H., Voelter, W. & Gotz, F. (2001) Infect. Immun. 69, 1957–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris, J. C. (1966) J. Phys. Chem. 70, 3798–3805. [Google Scholar]
- 28.Hart, T. W. (1985) Tetrahedron Lett. 29, 2013–2016. [Google Scholar]
- 29.Rai, R., Beckman, J., Bush, K. & Freeman, B. (1991) J. Biol. Chem. 266, 4244–4250. [PubMed] [Google Scholar]
- 30.Kalkum, M., Lyon, G. & Chait, B. (2003) Proc. Natl. Acad. Sci. USA 100, 2795–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowy, F. D. (1998) N. Engl. J. Med. 339, 520–532. [DOI] [PubMed] [Google Scholar]
- 32.Hawkins, C., Pattison, D. & Davies, M. (2003) Amino Acids 3–4, 259–274. [DOI] [PubMed] [Google Scholar]
- 33.Lyon, G., Wright, J., Muir, T. & Novick, R. (2002) Biochemistry 41, 10095– 10104. [DOI] [PubMed] [Google Scholar]
- 34.McDowell, P., Affas, Z., Reynolds, C., Holden, M., Wood, S., Saint, S., Cockayne, A., Hill, P., Dodd, C., Bycroft, B., et al. (2001) Mol. Microbiol. 41, 503–512. [DOI] [PubMed] [Google Scholar]
- 35.Chun, C., Ozer, E., Welsh, M., Zabner, J. & Greenberg, E. (2004) Proc. Natl. Acad. Sci. USA 101, 3587–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Safdar, N. & Maki, D. (2002) Ann. Intern. Med. 136, 834–845. [DOI] [PubMed] [Google Scholar]
- 37.Vandenesch, F., Naimi, T., Enright, M., Lina, G., Nimmo, G., Heffernan, H., Liassine, N., Bes, T., Reverdy, M. & Etienne, J. (2003) Emerg. Infect. Dis. 9, 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hentzer, M. & Givskov, M. (2003) J. Clin. Invest. 112, 1300–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.