Abstract
During the transition from darkness to light, the rate of hypocotyl elongation is determined from the integration of light signals sensed through the phototropin, cryptochrome, and phytochrome signaling pathways. In all light conditions studied, from UV to far-red, early hypocotyl growth is rapidly and robustly suppressed within minutes of illumination in a manner dependent upon light quality and quantity. In this study, it is shown that green light (GL) irradiation leads to a rapid increase in the growth rate of etiolated Arabidopsis seedlings. GL-mediated growth promotion was detected in response to constant irradiation or a short, single pulse of light with a similar time course. The response has a threshold between 10−1 and 100 μmol m−2, is saturated before 102 μmol m−2 and obeys reciprocity. Genetic analyses indicate that the cryptochrome or phototropin photoreceptors do not participate in the response. The major phytochrome receptors influence the normal amplitude and timing of the GL response, yet the GL response is normal in seedlings grown for hours under constant dim-red light. Therefore, phytochrome activation enhances, but is not required for, the GL response. Seedlings grown under green, red, and blue light together are longer than those grown under red and blue alone. These data indicate that a novel GL-activated light sensor promotes early stem elongation that antagonizes growth inhibition.
The first sensing of light transitions the etiolated seedling into a developmental program that prepares the plant for autotrophy. This process, photomorphogenesis, is typified by changes at the biochemical, molecular, and physiological levels that guide early plant morphology during establishment. One of the most conspicuous changes to occur during photomorphogenic development is an inhibition of hypocotyl (stem) growth rate. Ultraviolet, blue, red, and far-red light each rapidly inhibit stem growth within minutes of irradiation (Meijer, 1968; Gaba et al., 1984; Spalding and Cosgrove, 1989), making this rapid response an excellent reporter of light sensing and signal integration.
High-resolution imaging techniques have allowed monitoring of the growth inhibition process with high temporal resolution in the miniscule Arabidopsis seedling. These methods facilitated genetic tests to describe two critical parameters of the growth inhibition response: first, which photosensors mediate early growth inhibition, and second, precisely when specific photosensors contribute to this rapid response. These studies demonstrated that growth inhibition is dependent upon contributions from phytochromes, phototropins, and cryptochromes, often acting in a sequential and orchestrated manner (Parks et al., 2001a). In red light, growth inhibition is first imparted through phytochrome A (phyA) activation for 3 h before phytochrome B (phyB) exerts its influence and the effect of phyA wanes (Parks and Spalding, 1999). In response to blue light, inhibition occurs in at least two distinct phases that can be separated genetically, as well as by time course and fluence response (Folta et al., 2003b). The primary phase of growth inhibition is mediated by phototropin 1 (phot1; Folta and Spalding, 2001a), the autophosphorylating Ser-Thr kinase that mediates phototropism (Huala et al., 1997; Christie et al., 1998). The second phase of growth inhibition requires cryptochrome 1 (cry1) and cryptochrome 2 (cry2) as well as phyA and initiates after 30 min of continuous irradiation of 100 μmol m−2 s−1 (Parks et al., 1998; Folta and Spalding, 2001a, 2001b). In all cases studied, irradiation with monochromatic light induces growth inhibition. The timing of inhibition coincides closely with the translocation of phyA and phyB to the nucleus in red or far-red light (Hisada et al., 2000; Kircher et al., 2002), phototropin and cryptochrome phosphorylation in blue light (Reymond et al., 1992; Shalitin et al., 2002), as well as alterations in the global gene expression (Ma et al., 2001; Tepperman et al., 2001; Folta et al., 2003a).
Monochromatic green light (GL) has been shown to act as a signal in regulating specific facets of plant physiology, inhibiting seedling mass, plant cell culture growth, and light-induced gravitropic root elongation (Klein, 1992). Recently it has been shown that GL can reverse blue light-induced stomatal opening (Frechilla et al., 2000; Talbott et al., 2002, 2003; Eisinger et al., 2003). The GL response is mediated through a yet-to-be-defined photosensor, and genetic analyses suggest the response to be zeaxanthin based (Frechilla et al., 1999; Zeiger, 2000). Plant responses to GL may be initiated through known light sensors. Phytochromes and cryptochromes absorb GL and possibly influence light-induced events (Mandoli and Briggs, 1981; Lin et al., 1995b; Liscum and Briggs, 1995; Swartz et al., 2001). However, the action/response spectra for GL-induced responses exhibit a peak between 540 to 550 nm (Klein, 1964, 1979; Steinitz et al., 1985; Reymond et al., 1992; Frechilla et al., 2000) and thus are incongruous with the absorption spectra for phytochromes, cryptochromes, and phototropins and the action spectra for the responses they govern (Christie et al., 1998; Ahmad et al., 2002). GL signals may also be a consequence of low-level coactivation of multiple sensory systems that together guide atypical physiological outcomes (Pepper et al., 2001).
In this report, high-resolution analyses of early growth kinetics have identified that GL irradiation causes a rapid increase in early stem elongation rate, a response that is contrary to that induced by all other light conditions studied. The transient growth promotion is evident within 15 min of irradiation, its magnitude is regulated in a dose-dependent manner, and it cannot be completely attributed genetically or photophysiologically to the described action of known photoreceptors. This report presents photophysiological and genetic characterization of a novel response to narrow-bandwidth GL.
RESULTS
Time Course of GL-Induced Growth Promotion
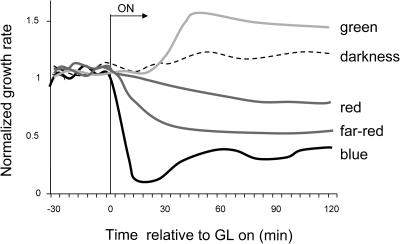
Figure 1 shows the mean normalized growth kinetics of 24 individual seedlings grown under 1 h of constant dim GL (2 × 10−1 μmol m−2 s−1) compared to idealized growth kinetics of plants responding to saturating blue, red, and far-red light (Parks et al., 2001a). Whereas irradiation with all other light qualities studied results in a decrease in stem elongation rate, GL treatment causes the hypocotyl to grow more rapidly. The response becomes evident within 15 min of light onset and peaks after 30 min at 144% (±8.9%) of the dark growth rate. These data indicate that GL-treated seedlings elongate significantly faster than dark-grown seedlings and are growing 3 to 4 times the rate of seedlings receiving high-fluence rate blue light.
Figure 1.
Idealized early responses to different light qualities. GL treatment induces an increase in early stem elongation rate, whereas blue, red, and far-red light generate growth inhibition. GL data represent light treatment with constant light applied starting at time 0 (for GL 2 × 10−1 μmol m−2 s−1 as in Figure 5A).
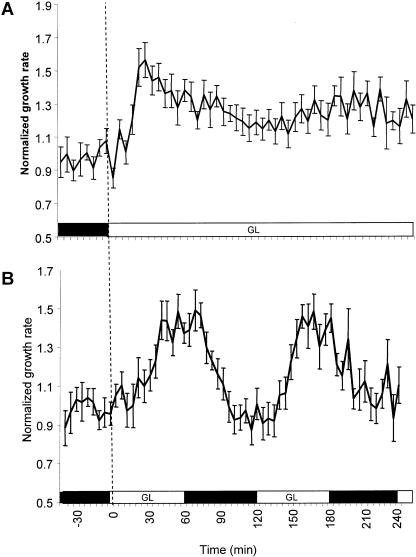
The high amplitude of growth induction is transient. Dark-grown seedlings were irradiated with 2 × 10−1 μmol m−2 s−1 GL, and their growth rates were assessed over 4 h. The results are presented in Figure 2A. Robust growth promotion is evident for the first hour then proceeds at a growth rate that exceeds the dark rate for the remainder of the experiment.
Figure 2.
The GL response is transient but can be reset by dark acclimation. GL-induced growth promotion was measured over a 4-h period. Seedlings were grown and imaged as described in “Materials and Methods.” A, Growth under constant GL (2 × 10−1 μmol m−2 s−1; n = 20). B, Growth kinetics under alternating 1-h periods of GL (2 × 10−1 μmol m−2 s−1) and darkness (n = 16). The black box along the abscissa indicates time in darkness. The white box indicates time under the defined GL treatment. The dark rate was determined as the average growth rate 30 min prior to the first light pulse. Error bars represent se of the mean.
Dark/Green Light Intervals Actuate the Rapid Growth Response
Seedlings grown for days under GL are typically shorter than dark-grown seedlings (Lin et al., 1995a; Liscum and Briggs, 1995), indicating a low level of growth inhibition and/or the inability to sustain GL-induced growth promotion for days of continuous light. The results from Figure 2A show that after robust initial growth promotion, the rate decreases within hours. Since GL is minimally activating other photosensory systems, it may be possible to observe the GL response, its decay, and reactivation by treating the seedlings with alternating intervals of GL and darkness. With this approach, the activation and decay kinetics of the GL response can be measured without activating systems that generate growth inhibition.
The results in Figure 2B indicate that GL pulses can actuate the rapid growth response. Dark-grown seedlings were given the initial GL pulse to induce rapid growth. After 1 h, the seedlings were imaged in darkness for 1 h. The rapidly growing seedlings return to the dark growth rate within 30 min after the GL signal is removed. When seedlings are irradiated again with a GL pulse, they resume the rapid growth rate.
Fluence Response and Reciprocity
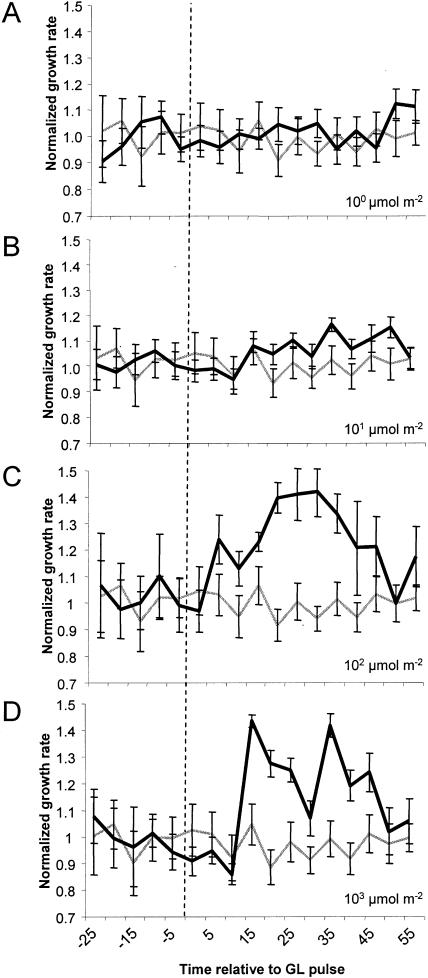
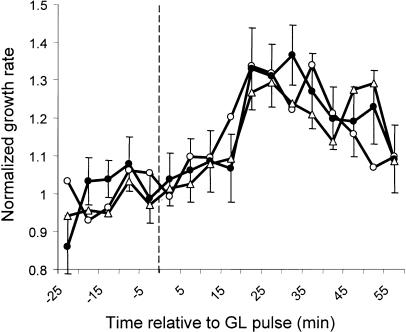
Since the major increase in growth rate occurs within the first hour following the GL pulse, fluence-response relationship between a GL pulse and growth promotion was tested in this time frame. Seedling growth kinetics were monitored after treatment with a short, single pulse of GL with a duration between 0.5 and 500 s, ranging in fluence between 10−1 and 103 μmol m−2 (Fig. 3). Growth promotion was induced during this time course with a threshold between 100 and 101 μmol m−2 and saturation above 102 μmol m−2. The growth rate of seedlings pulsed with 100 μmol m−2 is only slightly higher than dark controls, although statistically significant elongation becomes evident after 50 min. Higher fluences of GL hasten the timing and increase the magnitude of growth promotion. A 103 pulse leads to strong growth promotion by 20 min followed by transient and reproducible growth inhibition at 35 min. The consistent decline in growth rate is presumably due to activation of additional photosensory systems that inhibit growth. This speculation is later confirmed in Figure 6.
Figure 3.
The fluence-response characteristics of stem growth promotion by GL. Individual seedlings were grown in darkness then were imaged for measurement in 1 h of darkness to establish a dark-growth rate. A short, single pulse of GL of varying fluence was applied, and the growth kinetics were measured for 1 h. The averaged normalized response of wild-type (Col-0) seedlings is presented following a 100 μmol m−2 (n = 19; A), 101 μmol m−2 (n = 22; B), 102 μmol m−2 (n = 21; C), or 103 μmol m−2 (n = 20; D) GL pulse of short duration (1–50 s). The gray line represents the mean growth rate of many dark-grown seedlings subjected to a mock pulse. The vertical dashed line represents the point when the pulse was delivered. The data were normalized to the dark rate (1), derived from the average growth rate 30 min prior to the light pulse. Error bars represent se of the mean.
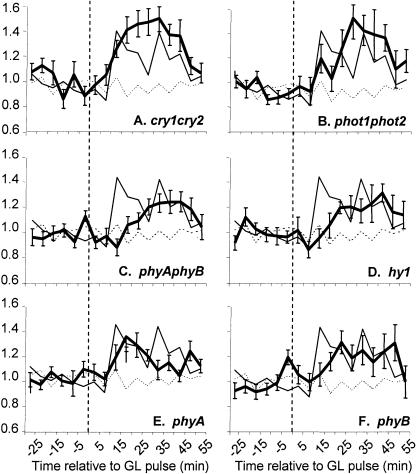
Figure 6.
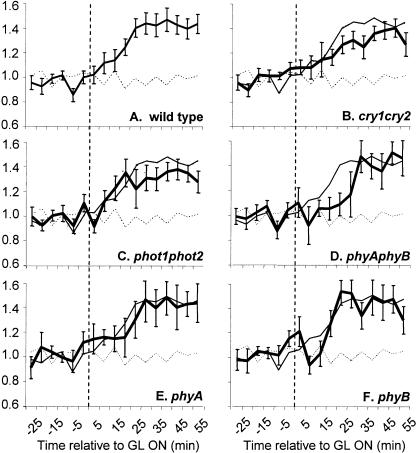
Genetic analysis of growth promotion in response to a short, single pulse of GL. Dark-grown seedlings were grown, imaged, and analyzed as described in “Materials and Methods.” The average growth kinetics of individual seedlings were monitored in response to a single 103 μmol m−2 GL pulse delivered at 0 min (vertical dashed line). The mean growth response of many individual seedlings (15–30 per genotype) is shown for cry1cry2 (A), phot1phot2 (B), phyAphyB (C), hy1 (D), phyA (E), and phyB mutants (F), represented by the thick-black line. Wild-type growth kinetics induced by the same treatment are represented by a thin-black line in all panels. The growth patterns of dark-grown seedlings are represented as a dashed line. Error bars represent the se of the mean.
The amplitude of growth promotion for any given fluence was independent of duration of the pulse (Fig. 4). Dark-grown seedlings were irradiated with 102 μmol m−2 GL delivered over 5, 50, or 500 s. The growth rate was monitored as described for Figure 3. The results indicate that a 102 μmol m−2 pulse induces a response with similar magnitude and time course. Seedlings treated with a 101 μmol m−2 pulse of GL delivered over various durations also show a similar increase in growth rate under all conditions tested (data not shown). These data indicate that the response obeys the Bunsen-Roscoe law of reciprocity and therefore is activated by first-order photochemistry.
Figure 4.
The response to GL obeys the Bunsen-Roscoe law of reciprocity. Etiolated seedling growth was monitored in response to a 102 μmol m−2 GL pulse delivered over 5 (white circles, n = 21), 50 (black circles, n = 20), or 500 (triangles, n = 21) seconds. Growth rates were monitored in darkness for 1 h and then for 1 h after the onset of the pulse. The vertical dashed line indicates the start of the pulse. Error bars represent se of the mean and are presented only for the 50-s pulse for clarity. se of the mean is similar between data sets.
Genetic Analyses
The GL sources used in this study stimulate known photoreceptors to some degree. The flavin chromophore of cryptochromes may exist as a flavin-semiquinone that can absorb in the green portion of the spectrum (Lin et al., 1995a, 1995b; Liscum and Briggs, 1995; Swartz et al., 2001), the absorption spectrum of phototropins indicates minimal sensitivity to GL (Christie et al., 1998; Ahmad et al., 2002), while phytochromes readily absorb GL, generating a phytochrome photoequilibrium under constant GL irradiation (Hanke et al., 1969).
Indirect tests, such as fluence-response analyses, suggest that phototropins are not involved in this response, as even minor excitation of phototropins (<1 μmol m−2 blue light) will induce 50% growth inhibition within 15 min (Folta et al., 2003b). Excitation of phytochromes by GL with even extremely dim red light results in a decrease in stem elongation rate (Mandoli and Briggs, 1981; Gaba et al., 1991; Kigel and Cosgrove, 1991). Examination of the action spectra for stem growth elongation in Arabidopsis indicates that GL (525 nm) treatment leads to a slightly longer hypocotyl (Goto et al., 1993) that is unaffected by the hy2 mutation. This also indicates that phytochromes are not likely mediating growth promotion.
Although this evidence implies that known photoreceptors are not likely mediating this response, this conclusion can be directly tested by monitoring growth kinetics in photoreceptor mutant backgrounds. Mutant seedlings were planted, germinated, and tested in a manner identical to wild-type seedlings. The effect of mutations on the GL response was measured for 1 h under one of two conditions: in response to low-fluence rate constant GL (2 × 10−1 μmol m−2 s−1; Fig. 5) or in response to a high-fluence single GL pulse (103 μmol m−2; Fig. 6).
Figure 5.
Genetic analysis of growth promotion in response to constant dim GL. Dark-grown seedlings were prepared, imaged, and analyzed as described in “Materials and Methods.” The growth kinetics of photoreceptor mutant seedlings were monitored in response to continuous GL delivered at 2 × 10−1 μmol m−2 s−1 after 0 min (vertical dashed line). The mean growth response is shown for wild-type seedlings (A), cry1cry2 (B), phot1phot2 (C), phyAphyB (D), phyA (E), and phyB (F) mutants, represented by the thick-black line. For comparison, wild-type growth kinetics induced by the same treatment are represented by a thin-black line in all panels. A dark control is shown as a dashed line. Between 15 and 25 individual seedlings were imaged per mutant line per light treatment. Error bars represent the se of the mean.
Under constant low-fluence GL irradiation, mutant seedlings responded essentially as wild type. The exception was that phyAphyB mutants exhibited a slight (10–15 min) delay in the onset of growth promotion (Fig. 5D). To further test the role, if any, of phytochromes in the GL response, the growth kinetics of single phyA and phyB mutants were assessed under constant low-fluence rate GL. The mean growth kinetic profiles of many individual seedlings are shown in Figure 5, E and F. Both phyA and phyB exhibited only minor timing deviations, indicating that either of the major phytochromes is sufficient to allow the full response to dim GL.
The timing and amplitude of growth promotion are not significantly affected in cry1cry2 or phot1phot2 mutants in response to a high-fluence pulse of GL (103 μmol m−2 pulse; Fig. 6). The kinetics match those of wild-type plants but are slightly higher in amplitude and do not exhibit the inhibition observed between 20 to 35 min. These data indicate that the GL pulse is sufficient to induce transient growth inhibition, imparted through at least a cryptochrome and a phototropin receptor. The inhibition likely arises from blue light (<1% of light from the LED source is between 493–500 nm) activation of phot1 and/or cry1/cry2, or possibly GL activation of these flavin-based receptors in their semiquinone state. After a single 103 μmol m−2 GL pulse phyAphyB mutants still exhibit growth promotion, although the onset is delayed (Fig. 6C). To determine which phytochrome receptor is required for the response, the response was measured in phyA and phyB single mutants. Both phyA and phyB mutants display GL-induced growth promotion, with a 10- to 15-min delay in the onset of the response. These data suggest that a subset of the five Arabidopsis phytochromes may act redundantly in generating GL growth promotion. This possibility may be tested using the hy1 mutant, which contains a lesion in a heme oxygenase gene required for chromophore synthesis (Davis et al., 1999; Muramoto et al., 1999). This mutation results in a drastic, but not complete, reduction in the pool of convertible Pr (Chory et al., 1989; Parks and Quail, 1991). Figure 6D illustrates that the hy1 mutant response is almost identical to that of phyAphyB double mutants, showing that the minor phytochromes likely have little role in regulating the onset of GL-induced growth promotion. These data indicate that phytochromes do not solely modulate the response to GL yet influence its onset and enhance its magnitude.
The GL Response Persists under Dim-Red Light
Although phyA, phyB, and hy1 mutants maintain the general response to GL, it remains a formal possibility that minor phytochromes may redundantly contribute to the response. The light conditions tested provide ample energy to activate accumulation of phyA-induced nuclear transcripts (Tepperman et al., 2001), a series of which increase in response to a 102 μmol m−2 GL pulse, but not in a phyA mutant (K. Folta, unpublished data). Photophysiological methods can be implemented to formally dismiss phytochromes as the central receptors driving the GL response. Far-red light could be used to photoconvert phytochrome back to an inactive state, but this approach would also be inconclusive, as phytochrome VLF responses are not photoreversible (Mandoli and Briggs, 1981). The definitive experiment tests the GL response under conditions where a phytochrome equilibrium has been established with a background of red light. If the GL response persists in the presence of Pfr phytochrome, then the response cannot be attributed to low-level phytochrome excitation and must involve additional photoreceptors. Such an approach was used to dissect photosynthesis-dependent stomatal responses from those mediated specifically by blue light (Ogawa, 1981; Talbott et al., 2003).
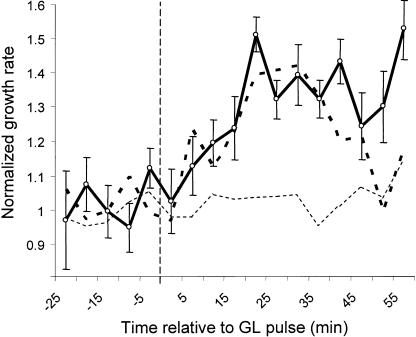
Two-day-old, etiolated seedlings were grown between 2 to 5 h under continuous dim-red light (2.0–3.0 × 10−2 μmol m−2 s−1). Seedlings exhibited stable growth, elongating between 90% and 100% of their absolute dark-growth rate. Seedlings were then imaged for 1 h under constant red light conditions and then were treated with a single 1.0 × 102 μmol m−2 pulse of GL. The results are shown in Figure 7. The data indicate that the GL response persists normally in a background of dim-red light, even when phytochrome is exerting slight growth inhibition. This finding shows that although phytochromes are necessary for normal growth promotion by GL, activation of phytochrome alone generates growth inhibition, and growth promotion is dependent upon activation of an additional GL sensor.
Figure 7.
The GL response persists in a background of dim-red light. Dark-grown seedlings were transferred to dim-red light (2–3 × 10−2 μmol m−2 s−1) for 2 to 5 h to establish phytochrome equilibrium and a consistent growth rate. Growth kinetics were measured starting 1 h prior to a 5-s GL pulse (1 × 102 μmol m−2) delivered at 0 min (vertical dashed line) and for 1 h thereafter. Dim-red light alone is represented by the thin dashed line (n = 15). The averaged growth kinetics of 13 individual seedlings given the same red-light treatment followed by a pulse of GL-treated are shown as the dark line. The thick dashed line represents data from dark-grown seedlings given equivalent GL treatment (Fig. 3C) and is provided for comparison. Error bars represent se of the mean.
Supplemental GL Antagonizes Long-Term Blue and Red Light Effects on Stem Growth
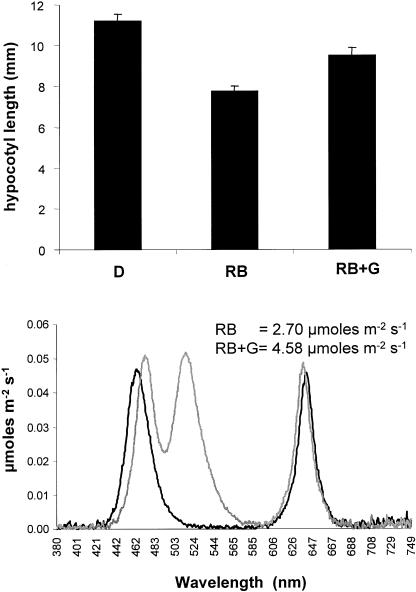
Enhanced growth under monochromatic GL suggests that a system is in place to counter the effects of inhibitory wavebands. Growth of seedlings under growth-inhibitory conditions (blue and red light) supplemented with GL may allow separation of the GL-mediated growth promotion from the inhibitory influence mediated through other photoreceptors. If GL-supplemented seedlings grew taller than those grown under blue and red alone, this would provide additional evidence against GL growth promotion being mediated through partial activation of known photoreceptors or low-level coaction between blue and red systems. Two-day-old, dark-grown seedlings were transferred to custom LED chambers containing blue and red light (2.70 μmol m−2 s−1) or identical blue and red conditions supplemented with GL (4.58 μmol m−2 s−1). A foil-wrapped set of seedlings was included to assess dark-growth rate and verify growth inhibition. Transfer of dark-grown seedlings to the experimental light conditions was performed to ensure that different light conditions did not cause variation in germination that could be misinterpreted as differences in end-point length. Seedling height was recorded at 96 h (poststratification). The results are presented in Figure 8. Figure 8A displays the mean hypocotyl length of many (>40) individual seedlings grown under each of the three conditions. The data indicate that the red and blue treatment decreases end-point hypocotyl length by 30.7% (±3.1%) relative to dark-grown controls. Addition of GL opposes blue and red light-mediated inhibition, as seedlings are only 15.1% (±4.0%) shorter than dark controls, despite the fact that the photon fluence rate was higher under GL-supplemented conditions. Figure 8B shows the actual light spectra measured in each chamber.
Figure 8.
A, Supplemental GL antagonizes blue and red light inhibition of hypocotyl elongation. Seedlings were planted and grown in darkness as described in “Materials and Methods.” Etiolated seedlings (36–40 h old) were irradiated under LED banks generating approximately equal amounts of blue and red light (RB) or the same RB treatment supplemented with an equal quanta of GL (RBG). A significant difference was observed 96 h postgermination. Dark-grown seedlings were measured for comparison. At least 40 individual seedlings were measured in each of two completely independent replicates. B, The emission spectra of red and blue (RB; black trace), and red and blue with supplemental green (RB + G; gray trace) used to obtain the results from A are presented.
DISCUSSION
The most salient feature of the seedling's etiolated growth program is the rapidly elongating hypocotyl. It has always been assumed that this rate defined a default state—a seedling growing as rapidly as possible to move through soil in search of light. However, this study shows that seedlings sense GL to direct an increase in their growth rate, at times approaching 150% the rate observed in the dark-grown seedling. The larger implication is that this is not just a curious artifact of the monochromatic laboratory environment but instead is a biologically relevant response possibly mediated through an uncharacterized photomorphogenic system that shapes plant form during the first hours of light sensing and seedling establishment. Regardless of mechanism, it is clear that the effects of GL must be considered for a complete understanding of light signaling and integration.
The fact that blue, red, and far-red signals inhibit stem growth, yet GL promotes stem growth, reiterates the concept that seedling growth rate in light is a compromise between multiple systems that simultaneously suppress and promote expansion of hypocotyl cells (Parks et al., 2001a). In red light, phyA and phyB suppress growth (Parks and Spalding, 1999), while a system involving SPA1 actively promotes growth (Parks et al., 2001b). In blue light, phyA, cry1, and cry2 are required for growth rate suppression after 30 min of irradiation, while phyB is required to induce rapid elongation during this time frame (Folta and Spalding, 2001a, 2001b). The concurrent activation of two systems that positively and negatively influence a common physiological output allows for greater agility in adjustment to new conditions—an advantage to the sessile seedling. This study indicates that GL-mediated growth promotion is an active response that complements the effects of other systems that inhibit growth, tailoring the ultimate stem expansion rate of the juvenile plant.
Promotion of stem growth by GL has been observed in studies of the action spectra of stem growth responses. Goto et al. (1993) indicate that growth promotion is detectable under green or low-fluence red light, yet is masked by inhibition imparted through the phytochromes in red light. Treatment of 2-d-old, dark-grown, wild-type seedlings with low-fluence rate (between 10−1 and 101 μmol m−2 s−1) GL for 8 h followed by 16 h of darkness leads to a significant difference in stem length compared to dark controls (Goto et al., 1993). GL-induced growth promotion was not observed in a separate study that defined the action spectrum of stem growth inhibition (Young et al., 1992). Although growth promotion is detected early (Figs. 2–7), persists for at least 4 h (Fig. 2A), and is detectable in the presence of blue/red growth inhibition, it is difficult to reconcile why GL-grown seedlings are not significantly taller than dark-grown seedlings. One interpretation is that the seedling reaches a terminal expansion for its developmental state, and GL-grown seedlings attain this point earlier than dark-grown seedlings, leading to similar lengths at a 24-h endpoint. In the Col-0 ecotype there is a significant difference in hypocotyl length detected between dark and GL-grown seedlings after 4, 8, and 16 h of GL treatment (10−1 to 101 μmol m−2 s−1; data not shown). The growth rate of the GL-treated seedling growth must therefore slow between 16 and 24 h. When coirradiated with blue and red light, the GL-induced expansion can be visualized because these seedlings possess a greater potential for expansion. Another potential explanation is that growth promotion may eventually be balanced by slight inhibition imparted through the cryptochromes, which absorb GL and generate cryptochrome-mediated growth inhibition (Lin et al., 1995a, 1995b). However, the GL-treated cry1cry2 mutant is not significantly longer than wild type under GL or dark conditions (data not shown).
The antagonistic role of GL signals is reflected in the literature. It has been observed in tomato (Went, 1957), marigold, and corn (Klein et al., 1965) that vegetative plant growth was enhanced by removing the green component of white light with filters. Later, it was demonstrated that GL could inhibit light-stimulated root gravitropism. Response spectra indicated a peak activity at 546 nm that could be negated by irradiation with 620 nm light (Klein, 1979). Recently, several reports have detailed the GL reversibility of blue light-induced stomatal opening. Guard cells exhibit several genetically and photophysiologically separable responses to light. A specific blue light induced stomatal opening response can be reversed by a single pulse of GL delivered after the blue pulse. Blue-green reversibility persists normally in a background of red, is far-red insensitive, is fluence-rate dependent, has an action spectrum that peaks at 540 nm, is absent in npq1 mutants, and cannot be attributed to any of the current suite of photoreceptors (Frechilla et al., 2000; Talbott et al., 2003). The promotion of stem elongation observed in this report is another example where a light-mediated response is antagonized by GL.
Genetic analyses indicate that GL-stimulated stem elongation also cannot be completely attributed to any known photoreceptors. Mutations in the cryptochromes, phototropins, and the major phytochromes do not eliminate the GL response. To the contrary, high-fluence GL treatment led to a transient inhibition of growth rate that was detectable between 20 to 35 min (Fig. 3D). The observed inhibition was shown to be the effect of a subset (or all) of the phototropin and cryptochrome receptors (Fig. 6, A and B). A recent study has shown that phot1 mediates a transient decrease in stem elongation rate following low-fluence blue light irradiation (Folta et al., 2003b). It is likely that the high-fluence GL treatments contain a sufficient quanta of blue light energies to excite the phototropins (probably phot1), and this accounts for the transient inhibition.
Genetic analyses indicate that phyA and phyB participate in the GL response. Under constant dim GL, both phyA and phyB act redundantly in regulating the normal timing of the GL response (Figs. 5D and 6C). The response is delayed in phyB, phyAphyB, and hy1 mutants. Growth promotion, usually apparent at 10 to 15 min, is not clear until 20 to 25 min and only reaches on average approximately 75% of the normal amplitude. The participation of phyA and phyB in this response precedes translocation of phyA and phyB into the nucleus (Hisada et al., 2000; Kircher et al., 2002) and the onset of phyA- or phyB-mediated growth inhibition (Parks and Spalding, 1999). It is possible that the GL response defines an extranuclear role for the phytochromes. Other phytochrome-interacting factors have been shown to be cytosolic, such as pKS1 (Fankhauser et al., 1999), and they may participate in the GL-growth promotion response.
Genetic tests establish that phytochromes influence the response to GL, but are they redundantly mediating the response? It is unclear how GL may activate phytochrome to respond in a manner completely at opposition to its well-documented effects on growth inhibition (Mandoli and Briggs, 1981; Gaba et al., 1984; Kigel and Cosgrove, 1991). The definitive evidence that GL-induced growth promotion is not a phytochrome response is presented in Figure 7. Here, a phytochrome photoequilibrium is established by transferring etiolated seedlings to constant dim-red irradiation for hours before GL treatment. Confirmation of the activation of phytochromes is verified by a slight decrease in average absolute elongation rate (data not shown). When treated with GL, the red light-grown seedlings respond by increasing their growth rate in a response that is similar to that of dark-grown seedlings (Fig. 7) only with longer duration. The longer duration is consistent with genetic data indicating that phytochrome activation appears to enhance the response.
The question of phytochrome redundancy (as well as coaction with blue light sensors) in the GL response can also be tested in end-point experiments that measure the effect of GL on stem growth in the presence of saturating fluences of red and blue light. Figure 8 presents the results that indicate that supplemental GL antagonizes the inhibitory effects of red and blue light. Under these conditions the phytochromes, cryptochromes, and phototropins are activated by their optimal wavebands, yet the effect of GL is still observed. It is formally possible that GL reverses an active photoreceptor through stimulation of a blue light-generated flavin-semiquinone chromophore akin to the photoreversibility observed in phytochrome responses. This outcome is unlikely as overexpression of CRY1 has been shown to enhance growth inhibition in response to blue and green light (Lin et al., 1995a), and growth promotion was not observed. Figure 8 indicates that the effect of GL is not due to a GL-induced VLF response of phytochrome or low-level coaction between multiple blue-red sensing systems by GL. It is remarkable that a significantly higher fluence rate fails to further enhance, and actually reverses, growth inhibition. It is possible that the additional photon flux from GL aids photosynthesis in the developing seedling, allowing it to grow faster. However, early growth promotion occurs in seedlings lacking chlorophyll and chloroplasts in response to a short single pulse of GL, so if the long-term response is an extension of the acute response, photosynthesis is not likely contributing.
It is exciting to speculate that a yet-uncharacterized photoreceptor generates the response to GL. Zeiger and colleagues have proposed that the blue light-mediated stomatal opening involves a carotenoid photoreceptor (Zeiger, 2000; Talbott et al., 2003). This hypothesis is based on the finding that Arabidopsis npq1 mutants (representing a lesion in zeaxanthin de-epoxidase) fail to accumulate zeaxanthin and fail to exhibit GL reversal of blue light-induced stomatal opening (Frechilla et al., 2000; Talbott et al., 2002, 2003; Eisinger et al., 2003). It has been suggested that a zeaxanthin chromophore may undergo cis-trans-isomerization, altering its absorptive properties in a photoreversible toggle similar in analogy to phytochrome (Zeiger, 2000). Similar tests will be performed to test the npq1 mutant for an effect on GL-induced growth promotion.
Practical by-products of this work directly apply to plant research and propagation. This work certainly reiterates that extreme care be exercised in the use of green safelights in photobiological studies. A brief 102 μmol m−2 GL pulse is capable of generating a significant change in plant growth rate. Since growth is affected it is likely that gene expression patterns have changed as well, either as a direct consequence of the GL-signaling pathway or as an indirect product in response to the mechanical signals generated by elongating cells. Of equal importance, the addition or depletion of GL may be a means to manipulate seedling establishment, growth, and stature in artificial lighting environments. Fluorescent bulbs emit three principle wavebands, approximately equal fluences of blue, red, and green light that are perceived by the human brain as white. Filtering or supplementing GL may be a useful tool to affect plant growth in general or regulate progression through key developmental stages. These concepts, as well as tests of interaction between GL and other photosensory systems, are ongoing and will determine the biologically relevant effects of GL on controlling plant development through the transition to the light environment.
MATERIALS AND METHODS
Plant Materials
All genotypes tested are identical to those previously assessed for blue light responses: cry1-304 (Mockler et al., 1999), cry2-1 (Guo et al., 1998), phot1-5 (nph1-5; Huala et al., 1997), phot2-1 (Kagawa et al., 2001), phyB-5 (Reed et al., 1993), and phyA-201 (Nagatani et al., 1993). The phytochrome mutants are in the Landsberg erecta ecotype, and all others are in the Col-0 background. A phyA allele in the Col background was obtained from Salk SIGNAL T-DNA pools (SALK_014575), identified by a long hypocotyl under far-red light. Both parental wild-type ecotypes exhibited comparable responses (data not shown). Surface-sterile Arabidopsis seeds were planted singly on media composed of 1 mm KCl and 1 mm CaCl2 solidified with 1% Difco agar (Beckton, Dickinson and Company, Sparks, MD). The seeds were stratified in absolute darkness for 48 h then received a 1-h pulse of fluorescent white light (16 μmol m−2 s−1) to synchronize germination. The seeds were then moved back to absolute darkness at 23°C for 30 to 36 h, until seedlings reached an appropriate stage for growth assessment (approximately 2–4 mm tall with a tightly closed apical hook).
Kinetic Growth Measurement
High-resolution image capture and analysis were performed as described (Parks and Spalding, 1999; Folta and Spalding, 2001a). The exception was that the time interval between the transfer of seedlings and the initiation of image capture was varied randomly between 0 and 180 min to separate potential posttransfer growth rate deviations from those specifically induced by light treatment. Dark trials were performed under identical conditions without activation of the light source. All data were normalized to the mean growth rate over the 30 min before light treatment.
GL Sources and Treatments
Actinic GL was supplied by one of two sources, each producing similar results. The first was a single Philips (Eindhoven, The Netherlands) Cool Home Light fluorescent bulb wrapped with three layers of green cellulose-acetate theatrical gel (M-124; Cinemills, Burbank, CA), producing peak emission at 534 with an approximate 20 nm half-bandwidth (used for Fig. 2). The second was a green LED array (S10-30 or R30-123; Ledtronics, Torrance, CA) passed through a transparent green plastic filter resulting in a peak emission at 525 nm and a 16 nm half-bandwidth and was used for both low- and high-fluence rate experiments (Figs. 3–8). Attenuation of fluence rate was accomplished using neutral density filters (layers of M-209 and/or M-211; Cinemills, Burbank, CA). The emission spectra of light sources used in these experiments are viewable online at www.arabidopsisthaliana.com/lightsources. Fluence rates were measured with a LI-COR LI-250 photometer using a PAR sensor (LI-COR, Lincoln, NE). Light qualities were assessed using a StellarNet EPP2000 spectroradiometer (Apogee Instruments, Logan, UT). The minimal use of a dim-green or dim-red safelight during seedling transfer did not affect the outcome of light or dark experimental treatments (data not shown). Light intervals were controlled using a GrayLab 655 intervalometer (Dimco-Gray, Centerville, OH).
Growth Kinetics over 24 Hours
Wild-type (Col-0) and mutant (cry1cry2) seeds were stratified for 48 h at 4°C and then were treated with a single 1-h pulse of florescent white light (16 μmol m−2 s−1). Seedlings began to germinate after approximately 40 h in darkness. At this point the emerging seedlings (2–3 mm with a tightly closed hook) were transferred to vertical agar plates and were imaged as described (Parks and Spalding, 1999). Seedlings were measured using UTHSCA Image Tool software (version 3.0) calibrated to known standards.
GL Supplementation Experiments
The data in Figure 7 were derived from analysis of 2-d-old, dark-grown seedlings transferred to dim-red light (2–3 × 10−2 μmol m−2 s−1) for 2 to 5 h prior to the GL pulse. Red light was generated from a Quantum Devices (Barneveld, WI) Q-Beam LED array. The data in Figure 8 were obtained from 2-d-old, dark-grown seedlings grown on 0.5× Murashige and Skoog media (pH 5.8) with 1.5 mm MES and 1% Suc, solidified with 1% phytagar (RPI, Mt. Prospect, IL). Custom LED arrays were built from commercially available electronics parts and allowed precise control of individual blue (470 nm), red (630 nm), and green (525 nm) fluence rates. Diagrams of the arrays, method of construction, and their spectral ranges are presented online at www.arabidopsisthaliana.com/lightboxes.
Acknowledgments
I thank Dustin Kenitz for assistance in design and assembly of the custom LED arrays and Dawn Bies for critical reading of this manuscript.
This work was supported by the Florida Agricultural Experiment Station, initiated with support from the U.S. Department of Agriculture (postdoctoral award 2001–35304–10851), completed with support from NASA-SABRE (NAG10–316), and approved for publication as Journal Series Number R–10303.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.038893.
References
- Ahmad M, Grancher N, Heil M, Black RC, Giovani B, Galland P, Lardemer D (2002) Action spectrum for cryptochrome-dependent hypocotyl growth inhibition in Arabidopsis. Plant Physiol 129: 774–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J, Peto CA, Ashbaugh M, Saganich R, Pratt L, Ausubel F (1989) Different roles for phytochrome in etiolated and green plants deduced from characterization of Arabidopsis thaliana mutants. Plant Cell 1: 867–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR (1998) Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science 282: 1698–1701 [DOI] [PubMed] [Google Scholar]
- Davis SJ, Kurepa J, Vierstra RD (1999) The Arabidopsis thaliana HY1 locus, required for phytochrome-chromophore biosynthesis, encodes a protein related to heme oxygenases. Proc Natl Acad Sci USA 96: 6541–6546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger WR, Bogomolni RA, Taiz L (2003) Interactions between a blue-green reversible photoreceptor and a separate UV-B receptor in stomatal guard cells. Am J Bot 90: 1560–1566 [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Yeh KC, Lagarias JC, Zhang H, Elich TD, Chory J (1999) PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 284: 1539–1541 [DOI] [PubMed] [Google Scholar]
- Folta KM, Leig EJ, Durham T, Spalding EP (2003. b) Primary inhibition of hypocotyl growth and phototropism depend differently on phototropin-mediated increases in cytoplasmic calcium induced by blue light. Plant Physiol 133: 1464–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta KM, Pontin MA, Karlin-Neumann G, Bottini R, Spalding EP (2003. a) Genomic and physiological studies demonstrate roles for auxin and gibberellin in the early phase of cryptochrome 1 action in blue light. Plant J 36: 203–214 [DOI] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001. b) Opposing roles of phytochrome A and phytochrome B in early cryptochrome-mediated growth inhibition. Plant J 28: 333–340 [DOI] [PubMed] [Google Scholar]
- Folta KM, Spalding EP (2001. a) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Frechilla S, Talbott LD, Bogomolni RA, Zeiger E (2000) Reversal of blue light-stimulated stomatal opening by green light. Plant Cell Physiol 41: 171–176 [DOI] [PubMed] [Google Scholar]
- Frechilla S, Zhu J, Talbott LD, Zeiger E (1999) Stomata from npq1, a zeaxanthin-less Arabidopsis mutant, lack a specific response to blue light. Plant Cell Physiol 40: 949–954 [DOI] [PubMed] [Google Scholar]
- Gaba V, Black M, Attridge TH (1984) Photocontrol of hypocotyl elongation in de-etiolated Cucumis sativus L. Long-term fluence rate-dependent responses to blue light. Plant Physiol 74: 897–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaba V, Black M, Canaani O, Attridge TH (1991) Photocontrol of hypocotyl elongation in light-grown Cucumis-sativus L. photosynthetic requirement for a fluence rate dependent phytochrome response. Photochem Photobiol 53: 399–405 [Google Scholar]
- Goto N, Yamamoto KT, Watanabe M (1993) Action spectra for inhibition of hypocotyl growth of wild-type plants and of the hy2 long-hypocotyl mutant of Arabidopsis-thaliana L. Photochem Photobiol 57: 867–871 [Google Scholar]
- Guo H, Yang H, Mockler TC, Lin C (1998) Regulation of flowering time by Arabidopsis photoreceptors. Science 279: 1360–1363 [DOI] [PubMed] [Google Scholar]
- Hanke J, Hartmann KM, Mohr H (1969) Effects of night breaks on flowering of Sinapis alba L. Planta 86: 235–249. [DOI] [PubMed] [Google Scholar]
- Hisada A, Hanzawa H, Weller JL, Nagatani A, Reid JB, Furuya M (2000) Light-induced nuclear translocation of endogenous pea phytochrome A visualized by immunocytochemical procedures. Plant Cell 12: 1063–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huala E, Oeller PW, Liscum E, Han IS, Larsen E, Briggs WR (1997) Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science 278: 2120–2123 [DOI] [PubMed] [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kigel J, Cosgrove DJ (1991) Photoinhibition of stem elongation by blue and red light: effects on hydraulic and cell wall properties. Plant Physiol 95: 1049–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher S, Gil P, Kozma-Bognar L, Fejes E, Speth V, Husselstein-Muller T, Bauer D, Adam E, Schafer E, Nagy F (2002) Nucleocytoplasmic partitioning of the plant photoreceptors phytochrome A, B, C, D, and E is regulated differentially by light and exhibits a diurnal rhythm. Plant Cell 14: 1541–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RM (1964) Repression of tissue culture growth by visible and near visible radiation. Plant Physiol 39: 536–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RM (1979) Reversible effects of green and orange-red radiation on plant cell elongation. Plant Physiol 63: 114–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RM (1992) Effects of green light on biological systems. Biol Rev Camb Philos Soc 67: 199–284 [DOI] [PubMed] [Google Scholar]
- Klein RM, Edsall PC, Gentile AC (1965) Effects of near ultraviolet and green radiations on plant growth. Plant Physiol 40: 903–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Ahmad M, Gordon D, Cashmore AR (1995. a) Expression of an Arabidopsis cryptochrome gene in transgenic tobacco results in hypersensitivity to blue, UV-A, and green light. Proc Natl Acad Sci USA 92: 8423–8427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Robertson DE, Ahmad M, Raibekas AA, Jorns MS, Dutton PL, Cashmore AR (1995. b) Association of flavin adenine dinucleotide with the Arabidopsis blue light receptor CRY1. Science 269: 968–970 [DOI] [PubMed] [Google Scholar]
- Liscum E, Briggs WR (1995) Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7: 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Li J, Qu L, Hager J, Chen Z, Zhao H, Deng XW (2001) Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13: 2589–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandoli DF, Briggs WR (1981) Phytochrome control of 2 low-irradiance responses in etiolated oat seedlings. Plant Physiol 67: 733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer G (1968) Rapid growth inhibition of gherkin hypocotyls in blue light. Acta Bot Neerl 17: 9–14 [Google Scholar]
- Mockler TC, Guo H, Yang H, Duong H, Lin C (1999) Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development 126: 2073–2082 [DOI] [PubMed] [Google Scholar]
- Muramoto T, Kohchi T, Yokota A, Hwang I, Goodman HM (1999) The Arabidopsis photomorphogenic mutant hy1 is deficient in phytochrome chromophore biosynthesis as a result of a mutation in a plastid heme oxygenase. Plant Cell 11: 335–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani A, Reed JW, Chory J (1993) Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol 102: 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T (1981) Blue light response of stomata with starch-containing (Vicia faba) and starch-deficient (Allium cepa) guard cells under background illumination with red light. Plant Science Letters 22: 103–108< [Google Scholar]
- Parks BM, Cho MH, Spalding EP (1998) Two genetically separable phases of growth inhibition induced by blue light in Arabidopsis seedlings. Plant Physiol 118: 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Folta KM, Spalding EP (2001. a) Photocontrol of stem growth. Curr Opin Plant Biol 4: 436–440 [DOI] [PubMed] [Google Scholar]
- Parks BM, Hoecker U, Spalding EP (2001. b) Light-induced growth promotion by SPA1 counteracts phytochrome-mediated growth inhibition during de-etiolation. Plant Physiol 126: 1291–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Quail PH (1991) Phytochrome-deficient hy1 and hy2 long hypocotyl mutants of Arabidopsis are defective in phytochrome chromophore biosynthesis. Plant Cell 3: 1177–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Spalding EP (1999) Sequential and coordinated action of phytochromes A and B during Arabidopsis stem growth revealed by kinetic analysis. Proc Natl Acad Sci USA 96: 14142–14146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper AE, Seong-Kim M, Hebst SM, Ivey KN, Kwak SJ, Broyles DE (2001) shl, a new set of Arabidopsis mutants with exaggerated developmental responses to available red, far-red, and blue light. Plant Physiol 127: 295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Short TW, Briggs WR, Poff KL (1992) Light-induced phosphorylation of a membrane protein plays an early role in signal transduction for phototropism in Arabidopsis thaliana. Proc Natl Acad Sci USA 89: 4718–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalitin D, Yang H, Mockler TC, Maymon M, Guo H, Whitelam GC, Lin C (2002) Regulation of Arabidopsis cryptochrome 2 by blue-light-dependent phosphorylation. Nature 417: 763–767 [DOI] [PubMed] [Google Scholar]
- Spalding EP, Cosgrove DJ (1989) Large plasma-membrane depolarization precedes rapid blue-light-induced growth inhibition in cucumber. Planta 178: 407–410 [PubMed] [Google Scholar]
- Steinitz B, Ren ZL, Poff KL (1985) Blue and green light-induced phototropism in Arabidopsis-thaliana and Lactuca-sativa L. seedlings Plant Physiol 77: 248–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz TE, Corchnoy SB, Christie JM, Lewis JW, Szundi I, Briggs WR, Bogomolni RA (2001) The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J Biol Chem 276: 36493–36500 [DOI] [PubMed] [Google Scholar]
- Talbott LD, Nikolova G, Ortiz A, Shmayevich I, Zeiger E (2002) Green light reversal of blue-light-stimulated stomatal opening is found in a diversity of plant species. Am J Bot 89: 366–368 [DOI] [PubMed] [Google Scholar]
- Talbott LD, Shmayevich IJ, Chung Y, Hammad JW, Zeiger E (2003) Blue light and phytochrome-mediated stomatal opening in the npq1 and phot1 phot2 mutants of Arabidopsis. Plant Physiol 133: 1522–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman JM, Zhu T, Chang HS, Wang X, Quail PH (2001) Multiple transcription-factor genes are early targets of phytochrome A signaling. Proc Natl Acad Sci USA 98: 9437–9442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went FW (1957) The Experimental Control of Plant Growth. Chronica Botanica, Waltham, MA
- Young JC, Liscum E, Hangarter RP (1992) Spectral-dependence of light-inhibited hypocotyl elongation in photomorphogenic mutants of Arabidopsis: evidence for a UV-a photosensor. Planta 188: 106–114 [DOI] [PubMed] [Google Scholar]
- Zeiger E (2000) Sensory transduction of blue light in guard cells. Trends Plant Sci 5: 183–185 [DOI] [PubMed] [Google Scholar]