Abstract
Photosynthetic acclimation to CO2-limiting stress is associated with control of genetic and physiological responses through a signal transduction pathway, followed by integrated monitoring of the environmental changes. Although several CO2-responsive genes have been previously isolated, genome-wide analysis has not been applied to the isolation of CO2-responsive genes that may function as part of a carbon-concentrating mechanism (CCM) in photosynthetic eukaryotes. By comparing expression profiles of cells grown under CO2-rich conditions with those of cells grown under CO2-limiting conditions using a cDNA membrane array containing 10,368 expressed sequence tags, 51 low-CO2 inducible genes and 32 genes repressed by low CO2 whose mRNA levels were changed more than 2.5-fold in Chlamydomonas reinhardtii Dangeard were detected. The fact that the induction of almost all low-CO2 inducible genes was impaired in the ccm1 mutant suggests that CCM1 is a master regulator of CCM through putative low-CO2 signal transduction pathways. Among low-CO2 inducible genes, two novel genes, LciA and LciB, were identified, which may be involved in inorganic carbon transport. Possible functions of low-CO2 inducible and/or CCM1-regulated genes are discussed in relation to the CCM.
Since acclimation to changing environmental conditions is crucial for cell growth and survival, organisms have developed sensing and signaling mechanisms for acclimation to stress conditions. By sensing the CO2 availability, a number of aquatic photosynthetic organisms induce a carbon-concentrating mechanism (CCM) to concentrate inorganic carbon (Ci) intracellularly, resulting in increased photosynthetic affinity for Ci and accumulation of Ci in close proximity to Rubisco despite the low affinity and low selectivity of Rubisco for CO2 (Kaplan and Reinhold, 1999; Badger and Spalding, 2000). This induction is controlled by the transcriptional regulator CCM1 (CIA5) in the eukaryotic alga Chlamydomonas reinhardtii through a CO2-signal transduction pathway (Fukuzawa et al., 2001; Xiang et al., 2001). This CO2-signaling is a highly regulated process not only in microorganisms but also in higher plants whose stomatal numbers are tightly controlled by atmospheric CO2 partial pressure (Stretton and Goodman, 1998; Lake et al., 2002). During this process of acclimation to CO2-limiting conditions, several genes are induced in Chlamydomonas cells, such as Cah1 encoding a periplasmic carbonic anhydrase (CA; Fukuzawa et al., 1990), Mca coding for a mitochondrial CA (Eriksson et al., 1996), Ccp for a chloroplast envelope protein LIP-36 (Chen et al., 1997), Aat1 for Ala-α-ketoglutarate aminotransferase (Chen et al., 1996), and Pgp1 for phosphoglycolate phosphatase (Mamedov et al., 2001). Cah3, encoding a chloroplast CA, is slightly induced under CO2-limiting conditions and is essential for the CCM because cia3, which is impaired in the expression of chloroplastic CA, shows a severe high-CO2 requiring phenotype (Karlsson et al., 1998). Although these low CO2-inducible genes have been characterized, other components of the CCM are still poorly understood.
Photorespiration has been shown to be necessary for acclimation to CO2-limiting stress using the high-CO2 requiring mutant, pgp1-1 (Suzuki et al., 1990). The necessity of the Ci-uptake system has also been clearly shown by isolation and characterization of a high-CO2 requiring mutant, pmp1-1, defective in bicarbonate transport and Ci accumulation (Spalding et al., 1983). In the CCM-containing cyanobacteria, four Ci-uptake systems are reported: two bicarbonate transport systems encoded by CmpABCD (Omata et al., 1999) and SbtA (Shibata et al., 2002b), and two CO2 uptake systems consisting of the NdhD3 (Ohkawa et al., 2000a) and NdhD4 types (Shibata et al., 2001).
It is not yet clear how many genes are induced or repressed during acclimation to low-CO2 conditions. The roles of proteins encoded by low-CO2 inducible genes in the acclimation to CO2-limiting conditions are not fully understood in photosynthetic eukaryotes. Quantitative and global analysis of expression profiles by means of cDNA array analyses have been used to improve our overall understanding of the molecular basis of various mechanisms (Lockhart and Winzeler, 2000). In this study, we have identified CO2-responsive genes by analyzing the transcript profiles of wild-type cells and CCM1-regulated genes by comparing expression of genes in a mutant with that in wild-type cells. Since the ccm1 mutant C16 exhibits a high-CO2 requiring phenotype (Fukuzawa et al., 1998), it is possible to identify genes essential to the CCM by analyzing the expression profile of the C16 mutant. Based on transcriptome analyses, the role of the Ccm1 gene in the induction of the CCM and possible functions of CO2-inducible and CCM1-regulated genes are discussed in relation to the CCM.
RESULTS
Identification of Differentially Expressed Genes during Changes in CO2 Levels
We generated a cDNA macroarray using expressed sequence tag (EST) clones collected from wild-type Chlamydomonas cells to allow global analyses of gene expression. By assembling the 5′-end EST sequences of 50,832 clones (Asamizu et al., 1999, 2000; E. Asamizu, Y. Nakamura, K. Miura, H. Fukuzawa, S. Fujiwara, M. Hirono, K. Iwamoto, Y. Matsuda, J. Minagawa, K. Shimogawara, Y. Takahashi, and S. Tabata, unpublished data), 10,368 nonredundant EST clones were selected to construct a membrane array for simultaneous analysis of gene expression. The cDNA inserts from the selected EST clones were amplified by PCR and spotted on membranes in duplicate. The resulting array comprised 6 membranes containing 20,736 spots consist of 10,368 PCR fragments derived from EST clones.
To identify differentially expressed genes during the low-CO2 acclimation process, two independent hybridization experiments were carried out using different preparations of RNA samples. The array membranes were hybridized with 32P-labeled target cDNA samples produced from poly(A)+ RNA isolated from wild-type cells. To maintain high-CO2 conditions, air enriched with 5% CO2 was bubbled through the medium. For low-CO2 conditions, cells grown in high-CO2 conditions were transferred to conditions where ordinary air containing 0.04% CO2 was bubbled through the medium for 1 h. The induction period of 1 h was used because previously identified low-CO2 inducible genes such as Cah1 and Mca are induced within 1 h of exposure to low CO2 (Fukuzawa et al., 1990; Eriksson et al., 1996). After hybridization, the image data of the hybridization patterns were converted into digital data and the signal intensities of the spots were adjusted with mean-normalization. Each expression ratio of the signal intensity of the clone in low-CO2 cells to that in high-CO2 cells was calculated as described in “Materials and Methods” (Fig. 1A; Tables I and II). Because the macroarray carries duplicates of each EST clone, there were 4 expression ratios for each EST clone. If 3 expression ratios out of 4 exceeded 2.5-fold and the average of the expression ratios was more than 2.5, then the EST was selected as a differentially expressed clone. In this initial step, 122 induced clones and 55 repressed clones were identified under CO2-limiting conditions. Based on the 3′-end sequences of these EST clones, chimerical and redundant clones were removed. In total, 51 low-CO2 inducible and 32 low-CO2 repressed transcripts were identified after analysis of the macroarray results.
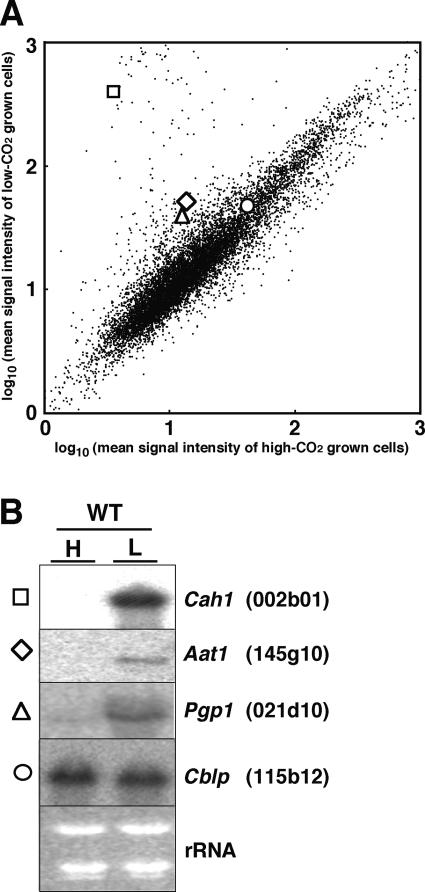
Figure 1.
Scatter plot of hybridization signals in the cDNA array (A) and northern-blot analyses of representative low-CO2 inducible genes (B). Mean signal intensities in hybridization with probes from high-CO2 grown wild-type cells and low-CO2 grown wild-type cells were plotted along the x and y axes, respectively. The signal intensities of Cah1 (clone ID 002b01, square), Aat1 (clone ID 145g10, diamond), Pgp1 (clone ID 021d10, triangle), and Cblp (clone ID 115b12, circle) are highlighted. B, Ten micrograms of total RNA from either high-CO2 grown (H) or 1-h low-CO2 adapted wild-type cells (L) were hybridized with 32P-labeled Cah1, Aat1, Pgp1, and Cblp gene-specific probes.
Table I.
Genes induced under low-CO2 conditions and/or CCM1-dependent genes revealed by cDNA array analyses
| Array IDa | Clone IDb | WT-LC/ WT-HC | WT-LC/ C16-LC | Genec | Putative Functionc | Organismc | GenBank Hitd | Motif(pfam)e |
|---|---|---|---|---|---|---|---|---|
| 135h02 (28) | AV620271 | 110(±55) | 150(±99) | Mca | Mitochondrial carbonic anhydrase | C. reinhardtii | U41190 | – |
| 002b01 (6) | AV388733 | 57(±27) | 77(±49) | Cah1 | Periplasmic carbonic anhydrase | C. reinhardtii | P20507 | – |
| 019d11 (3) | AV619824 | 57(±38) | 79(±57) | Lci1 | Low-CO2 inducible membrane protein | C. reinhardtii | U31976 | – |
| 018h08 (4) | AV619223 | 56(±18) | 52(±15) | LciA | Nitrite transporter homolog (this study) | C. reinhardtii | AF149737 | – |
| 022b07 | AV623489 | 39(±14) | 50(±25) | Ccp1 | Chloroplast envelope protein LIP-36 | C. reinhardtii | U75345 | – |
| 012g07 | AV637344 | 25(±7.1) | 17(±6.8) | LciB | Putative chloroplast protein (this study) | – | – | Dynamin family (pfam00350) |
| 150h12 | AV629279 | 22(±10) | 12(±5.7) | – | – | – | – | No hit |
| 013d05 | AV638622 | 20(±0.6) | 11(±3.3) | – | Unknown (AT3g61320) | Arabidopsis | NP_191691 | No hit |
| 028e01 (4) | AV630016 | 18(±5.5) | 18(±3.4) | LciC | Putative chloroplast protein (this study) | – | – | Dynamin family (pfam00350) |
| 019b05 (7) | AV619622 | 13(±7.1) | 15(±9.5) | LI818 | Chlorophyll a/b-binding protein-like | C. reinhardtii | X95326 | – |
| 146f04 | AV627166 | 12(±4.5) | 16(±3.7) | – | – | – | – | No hit |
| 019g12 | AV620297 | 12(±5.6) | 14(±2.2) | – | – | – | – | No hit |
| 142b11 | AV624994 | 11(±4.8) | 3.6(±1.7) | Sag29 | Senescence-associated membrane protein | Arabidopsis | NP_196821 | – |
| 024b04 (2) | AV626358 | 11(±2.8) | 13(±3.8) | – | – | – | – | CBM_20, Starch binding domain (pfam00686) |
| 149b12 | AV628400 | 9.7(±3.0) | 7.2(±4.1) | – | – | – | – | Interferon induced GTPase(pfam05049) |
| 154c05 | AV630876 | 9.0(±1.2) | 21(±14) | – | – | – | – | No hit |
| 028f03 (2) | AV630146 | 8.9(±4.2) | 14 (±7.0) | – | Unknown (At2g45870) | Arabidopsis | NP_182111 | No hit |
| 164b10 | BP087144 | 7.7(±1.2) | 6.5(±2.8) | – | – | – | – | No hit |
| 135f03 | AV620059 | 7.6(±4.8) | 7.8(±4.4) | – | – | – | – | No hit |
| 025f01 (3) | AV627578 | 7.5(±3.3) | 8.4(±3.9) | Mmp | Putative mitochondrial matrix protein | C. reinhardtii | Y11586 | – |
| 021c08 | AV622166 | 6.3(±1.2) | 3.3(±0.4) | Sgat | Serine-glyoxylate aminotransferase | Methylobacterium extorquens | P55819 | – |
| 137c09 | AV386723 | 5.7(±1.4) | 14 (±0.8) | Ggps | Geranylgeranyl pyrophosphate synthase | Tagetes erecta | AAG10424 | – |
| 134g10 (3) | AV619535 | 5.2(±1.5) | 8.3(±2.0) | – | – | – | – | No hit |
| 102f05 | AV627999 | 4.9(±1.4) | 3.4(±1.3) | – | – | – | – | No hit |
| 169e06 | BP097010 | 4.8(±1.4) | 6.3(±1.9) | Fhs | 10-Formyltetrahydrofolate synthetase | Arabidopsis | NP_564571 | – |
| 022g04 (2) | AV624187 | 4.8(±1.6) | 3.2(±0.6) | – | Unknown (At5g57040) | Arabidopsis | NC_003076 | Glyoxalase (pfam00903) |
| 151c11 | AV629419 | 4.4(±1.3) | 5.9(±1.8) | Mcp | Mitochondrial carrier protein | Schizosaccharomyces pombe | NP_593701 | – |
| 024a11 (3) | AV626334 | 4.4(±0.6) | 3.7(±0.5) | Gdh1 | Putative glycolate dehydrogenase | Vibrio cholerae | NP_233369 | – |
| 020c02 | AV620842 | 4.2(±1.1) | 4.6(±0.7) | – | – | – | – | No hit |
| 138e10 | AV622332 | 3.8(±0.6) | 4.4(±0.7) | – | – | – | – | No hit |
| 138c12 | AV622214 | 3.8(±0.4) | 4.4(±1.0) | – | – | – | – | No hit |
| 021d10 (2) | AV635342 | 3.6(±1.1) | 3.9(±1.4) | Pgp1 | Phosphoglycolate phosphatase | C. reinhardtii | AB052169 | – |
| 145g10 | AV626859 | 3.4(±0.6) | 6.0(±1.7) | Aat1 | Alanine aminotransferase | C. reinhardtii | U31975 | – |
| 011e02 (2) | AV634930 | 3.4(±1.0) | 4.0(±1.5) | Lci5 | Low-CO2 inducible protein | C. reinhardtii | AAK77552 | No hit |
| 022f03 (2) | AV624023 | 3.4(±0.7) | 2.8(±0.1) | Shmt | Serine hydroxymethyltransferase | C. reinhardtii | AF442558 | – |
| 029g12 (3) | AV631115 | 3.3(±1.1) | 3.7(±0.7) | Sta2 | Granule-bound starch synthase I | C. reinhardtii | AF026420 | – |
| 123h02 | BP098683 | 3.3(±0.8) | 3.6(±0.9) | Sta3 | Soluble starch synthase | C. reinhardtii | AF026422 | – |
| 030h09 | BP086273 | 3.3(±1.0) | 2.8(±0.6) | – | – | – | – | No hit |
| 005c07 | AV387887 | 3.2(±0.8) | 3.5(±0.9) | – | – | – | – | No hit |
| 022b10 | AV623502 | 3.0(±0.5) | 4.7(±0.6) | – | Unknown (At4g36720) | Arabidopsis | NP_195390 | TB2/DP1, HVA22 family (pfam03134) |
| 023d09 | AV624492 | 2.9(±0.3) | 3.0(±0.4) | – | – | – | – | No hit |
| 148a10 | AV627826 | 2.9(±0.5) | 2.8(±0.8) | – | – | – | – | Chromate transporter (pfam02417) |
| 022h10 (2) | AV624435 | 2.8(±0.4) | 2.7(±0.3) | – | – | – | – | No hit |
| 170b12 | BP097305 | 2.8(±0.2) | 2.6(±0.3) | Ndh | Mitochondrial type 2 NADH dehydrogenase | Arabidopsis | NP_193880 | – |
| 167b10 | BP095941 | 2.7(±0.5) | 2.9(±0.3) | CarB | Carbamoyl phosphate synthetase | Arabidopsis | BAB90007 | – |
| 011d04 | AV634814 | 2.6(±0.7) | 2.6(±0.2) | Trxf1 | Thioredoxin f1 | C. reinhardtii | AY184800 | – |
| 003b05 (2) | AV396054 | 2.5(±0.3) | 4.1(±0.9) | GcsP | Glycine cleavage system P-protein | Arabidopsis | O49850 | – |
| 171b12 | BP097792 | 4.7(±2.7) | 2.1 (±0.9) | – | – | – | – | No hit |
| 021d12 | AV622387 | 2.9(±0.3) | 2.4 (±0.2) | – | – | – | – | No hit |
| 010b02 | AV632213 | 2.8(±0.5) | 2.3 (±0.3) | – | Unknown (At1g65230) | Arabidopsis | NP_176702 | No hit |
| 160c11 | BP088539 | 2.6(±0.2) | 2.0 (±0.1) | – | – | – | – | No hit |
| 021e03 | AV622398 | 1.8 (±0.4) | 7.3(±2.4) | – | – | – | – | No hit |
| 023c07 | AV624911 | 1.9 (±0.2) | 4.6(±0.9) | Hspg | Putative heparan sulfate proteoglycan | Ovis aries | AAD01973 | – |
| 139e12 | AV623221 | 2.4 (±0.6) | 4.0(±0.5) | – | – | – | – | Reverse transcriptase (pfam00078) |
| 019h09 (2) | AV620492 | 1.9 (±0.5) | 3.4(±0.4) | Ptp | Putative tyrosine phosphatase | O. sativa | AAF81798 | – |
| 022a05 | AV392559 | 2.1 (±0.6) | 3.2(±0.6) | Vdac | Voltage-dependent anion channel | O. sativa | CAB82853 | – |
| 142e05 | AU301246 | 2.2 (±0.4) | 3.1(±0.3) | – | – | – | – | No hit |
| 024c01 | AV626401 | 2.1 (±0.4) | 3.1(±0.8) | Cah3 | Chloroplast carbonic anhydrase | C. reinhardtii | U40871 | – |
| 022c08 | AV623614 | 2.4 (±0.4) | 2.9(±0.2) | – | Unknown (PA0315) | Pseudomonas aeruginosa | NP_249006 | No hit |
| 148c05 | AV627926 | 2.3 (±0.1) | 2.8(±0.4) | Mdh1 | NADP-malate dehydrogenase | C. reinhardtii | AJ277281 | – |
| 138h02 | AV622607 | 1.8 (±0.1) | 2.7(±0.2) | Cry | Crystallin J1C | T. cystophora | C46745 | – |
| 150d03 | AV628957 | 2.2 (±0.2) | 2.5(±0.4) | – | Unknown (At2g42750) | Arabidopsis | AAM19907 | DnaJ domain (pfam00226) |
Genes whose expression ratios were in excess of 2.5-fold are underlined. Dashes indicate not applicable.
Representative array IDs are shown in the first column. Numbers in parentheses indicate numbers of array IDs grouped into the same contigs, after sequencing the 3′ end of the cDNA.
GenBank accession numbers for the EST clones.
Genes with e-values <e−10 were annotated.
Best or most informative GenBank sequence alignments.
Motifs were annotated by the conserved domain database using RPS-BLAST (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
Table II.
Genes repressed under low-CO2 conditions and/or CCM1-dependent genes revealed by cDNA array analyses
| IDa | Clone IDb | WT-HC/ WT-LC | C16-LC/ WT-LC | Genec | Putative Functionc | Organismc | GenBank Hitd | Motif(pfam)e |
|---|---|---|---|---|---|---|---|---|
| 033b11 | BP094164 | 9.9(±5.9) | 4.0(±2.3) | Gsa | Glutamate-1-semialdehyde aminomutase | C. reinhardtii | U03632 | – |
| 156h09 (2) | BP086423 | 6.2(±2.4) | 4.3(±1.7) | HemC | Porphobilinogen deaminase | Pisum sativum | Q43082 | – |
| 019c02 (2) | AV619723 | 5.4(±2.8) | 7.2(±3.7) | Lil3 | Light-harvesting-like protein 3 | Arabidopsis | T52310 | – |
| 007h05 (2) | AV390641 | 5.1(±2.7) | 3.4(±1.0) | Cpx1 | Coproporphyrinogen III oxidase | C. reinhardtii | AF133672 | – |
| 028b04 | AV629643 | 3.8(±1.5) | 3.4(±0.5) | Crt | Calreticulin, Calcium-binding protein | C. reinhardtii | AJ000765 | – |
| 169b10 | BP096864 | 3.8(±1.0) | 2.7(±0.5) | – | – | – | – | No hit |
| 165d02 | AV644167 | 3.7(±1.4) | 4.1(±1.7) | – | – | – | – | No hit |
| 122h07 (2) | AV391593 | 3.6(±1.7) | 2.8(±0.8) | Psb3 | Oxgen evolving enhancer protein 3 | C. reinhardtii | X13832 | – |
| 031b05 | BP086554 | 3.5(±2.2) | 5.7(±2.3) | H43 | High-CO2 inducible periplasmic protein | C. reinhardtii | AB042098 | – |
| 033e05 | BP094463 | 3.4(±1.2) | 4.3(±2.6) | Ipmdh | 3-Isopropylmalate dehydratase, small subunit | Arabidopsis | NP_181837 | – |
| 126f08 (2) | BP098729 | 3.2(±0.8) | 3.2(±1.2) | Lhcbm9 | Light-harvesting chlorophyll a/b-binding protein | C. reinhardtii | AF479778 | – |
| 002g07 (5) | AV397762 | 3.0(±1.2) | 3.0(±1.3) | LhcII-3 | Light-harvesting chlorophyll a/b-binding protein | C. reinhardtii | AB051209 | – |
| 107c05 | AV386724 | 3.0(±0.1) | 2.9(±0.7) | Ubc1 | Ubiquitin-conjugating enzyme UBC1 | Arabidopsis | NP_563951 | – |
| 157h02 | BP087178 | 2.9(±0.3) | 2.7(±0.7) | – | – | – | – | No hit |
| 011f10 | AV635306 | 2.8(±0.7) | 2.7(±0.3) | Pgk | Phosphoglycerate kinase | C. reinhardtii | U14912 | – |
| 160e07 | BP088719 | 5.0(±0.9) | 1.5 (±0.1) | Crd1 | Copper response defect 1 protein | C. reinhardtii | AF237671 | – |
| 022c06 (2) | AV623576 | 4.4(±2.0) | 2.3 (±1.2) | Ggh | Geranylgeranyl hydrogenase | Mesembryanthemum crystallinum | AAD28640 | – |
| 029e10 | AV630927 | 4.2(±1.4) | 2.3 (±1.4) | Por1 | NADPH:protochlorophyllide oxidoreductase | C. reinhardtii | Q39617 | – |
| 170d07 | BP097411 | 3.9(±0.7) | 2.1 (±1.0) | ChlH | Magnesium chelatase H-subunit | C. reinhardtii | AJ307054 | – |
| 016e07 (2) | AV642279 | 3.8(±1.8) | 1.6 (±0.5) | Sar | SAR DNA binding protein | P. sativum | T06377 | – |
| 152c03 | AV642602 | 3.6(±1.8) | 2.1 (±0.7) | Hsp70 | 70 kD heat shock protein | C. reinhardtii | P25840 | – |
| 149a01 | AV628277 | 3.5(±1.0) | 2.3 (±0.3) | Hsp81-2 | Heat shock cognate protein 80 | Lycopersicon esculentum | P36181 | – |
| 008g12 | AV391709 | 3.5(±1.3) | 2.0 (±0.5) | Lhcb4 | Light-harvesting chlorophyll a/b-binding protein | C. reinhardtii | AB051211 | – |
| 159g09 | BP088102 | 3.4(±0.5) | 2.4 (±1.0) | Uo2 | Urate oxidase II | C. reinhardtii | AF195795 | – |
| 032a05 | AV636296 | 3.3(±1.4) | 2.5 (±2.0)f | Lhcb2 | Light-harvesting chlorophyll a/b-binding protein | C. reinhardtii | AF104630 | – |
| 031c08 | BP086706 | 3.3(±0.7) | 1.8 (±1.0) | – | – | – | – | No hit |
| 010c10 | AV632566 | 3.1(±0.7) | 1.8 (±0.2) | Rpl3 | 50S ribosomal protein L3 | Arabidopsis | NP_181831 | – |
| 035a04 (2) | BP096510 | 3.1(±1.0) | 1.8 (±0.3) | Cpn60 α | Chaperonin 60 alpha chain | C. reinhardtii | L27472 | – |
| 110a08 | AV388533 | 3.0(±0.6) | 2.0 (±0.1) | – | – | – | – | No hit |
| 007f10 | AV638595 | 2.8(±1.1) | 1.9 (±0.7) | Psb2 | Oxygen-evolving enhancer protein 2 | C. reinhardtii | M15187 | – |
| 159g03 | BP088024 | 2.7(±0.1) | 2.1 (±0.7) | Alad | Delta-aminolevulinic acid dehydratase | C. reinhardtii | U19876 | – |
| 002b05 | AV395046 | 2.6(±0.3) | 0.4 (±0.1) | MetC | Cobalamin-independent methionine synthase | C. reinhardtii | U36197 | – |
| 019f08 | AV620136 | 0.8 (±0.2) | 22(±7.7) | – | – | – | – | No hit |
| 136a01 (2) | AV620380 | 1.0 (±0.1) | 5.5(±1.6) | H3-I | Histone H3 | C. reinhardtii | S59581 | – |
| 167g10 | AV619315 | 1.8 (±0.2) | 5.4(±2.4) | Gbp1 | G-strand telomere binding protein 1 | C. reinhardtii | S46234 | – |
| 036b09 (2) | BP097895 | 1.7 (±0.5) | 5.2(±1.7) | – | – | – | – | No hit |
| 139d05 | AV623043 | 0.4 (±0.2) | 4.4(±2.5) | – | – | – | – | No hit |
| 029h01 | AV631148 | 1.2 (±0.2) | 4.2(±0.8) | Ftr1 | Plasma membrane iron transporter | C. reinhardtii | AAM45938 | – |
| 115d09 | AV392433 | 1.5 (±0.5) | 3.9(±1.4) | Bip | Luminal binding protein, BiP | Scherffelia dubia | CAC37635 | – |
| 019b08 (4) | AV619634 | 1.4 (±0.4) | 3.9(±1.1) | Heph | Hephaestin; multicopper ferroxidase | Mus musculus | NP_034547 | – |
| 034h03 | BP096342 | 1.2 (±0.5) | 3.9(±1.0) | – | – | – | – | No hit |
| 030f10 | BP086097 | 1.8 (±0.7) | 3.8(±1.1) | – | – | – | – | No hit |
| 114b09 | AV391439 | 1.7 (±0.8) | 3.8(±0.9) | DnaK | DnaK-type molecular chaperone | O. sativa | T03581 | – |
| 143f07 | AV625849 | 0.8 (±0.1) | 3.8(±0.7) | – | – | – | – | No hit |
| 004f08 (2) | AV387133 | 1.7 (±0.4) | 3.7(±0.9) | – | – | – | – | No hit |
| 033g07 (5) | BP094894 | 1.2 (±0.1) | 3.7(±1.4) | Hsp22 | 22 kD Heat shock protein | C. reinhardtii | P12811 | – |
| 033b07 | BP094124 | 2.2 (±1.2) | 3.6(±1.5) | – | – | – | – | No hit |
| 159h04 | BP088169 | 2.1 (±0.3) | 3.2(±0.4) | – | – | – | – | No hit |
| 101a04 | AV393298 | 1.0 (±0.2) | 3.1(±0.8) | – | – | – | – | No hit |
| 106g04 | AV386548 | 1.0 (±0.2) | 3.1(±0.8) | – | – | – | – | No hit |
| 017a08 (2) | AV642841 | 1.3 (±0.1) | 3.0(±0.5) | – | – | – | – | No hit |
| 140d07 | AV623827 | 1.8 (±1.0) | 2.9(±0.5) | – | – | – | – | No hit |
| 028f11 | AV630205 | 1.6 (±0.3) | 2.9(±0.3) | Mbf1 | Multiprotein bridging factor 1 | Solanum tuberosum | AAF81108 | – |
| 158h12 | BP087717 | 1.4 (±0.1) | 2.8(±0.3) | Thi1 | Thiazole (thiamine) biosynthetic enzyme | Citrus sinensis | O23787 | – |
| 120g09 | AV636430 | 2.3 (±0.8) | 2.5(±0.2) | PetO | Cytochrome b6/f-associated phosphoprotein | C. reinhardtii | AF222893 | – |
Genes whose expression ratios were in excess of 2.5-fold are underlined. Dashes indicate not applicable.
Representative array IDs are shown in the first column. Numbers in parentheses indicate numbers of array IDs grouped into the same contigs, after sequencing the 3′ end of the cDNA.
GenBank accession numbers for the EST clones.
Genes with e-values <e−10 were annotated.
Best or most informative GenBank sequence alignments.
Motifs were annotated by the conserved domain database using RPS-BLAST (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
Two expression ratios out of 4 did not exceed 2.5-fold.
To annotate the putative biological functions of these 83 differentially expressed genes, consensus sequences of the EST contigs were obtained by using the GenBank EST-sequence databases (Asamizu et al., 1999, 2000; Shrager et al., 2003). Then these sequences were annotated by using the programs BLASTX and RPS-BLAST (Tables I and II). As expected, 7 previously known low-CO2 inducible genes, Mca, Cah1, Lci1, Ccp1, Pgp1, Aat1, and Lci5, were found to be part of the group of 51 genes detected as low-CO2 inducible in our array analysis. To compare the ratios of the expression levels in high-CO2 conditions to that in low-CO2 conditions, mRNA levels corresponding to 3 previously known low-CO2 inducible genes, Cah1, Aat1, and Pgp1, were measured by northern-blot analyses using the same RNA samples used in the array analysis (Fig. 1). Indeed, the induction ratio of Cah1 was 57 (low-CO2/high-CO2) in the array analysis, which is comparable to the ratio of 57 measured in the northern blot analysis. Similarly, the ratio of Aat1 was 3.4 in the array and 3.4 in the northern blot, while the ratio of Pgp1 was 3.6 in the array and 3.3 in the northern (Fig. 1). The similarity in ratios between the array and northern-blot analyses indicates that the cDNA membrane array is useful for identification of differentially expressed genes among the 10,378 EST clones.
Up-Regulation of Genes during Acclimation to CO2-Limiting Stresses
Seven of the 51 low-CO2 inducible genes have significant sequence similarities with photorespiration-related genes such as Aat1, Pgp1, Sgat-coding for Ser-glyoxylate aminotransferase, Fhs-coding for formyltetrahydrofolate synthetase, Gdh1-coding for glycolate dehydrogenase, Shmt-coding for Ser hydroxymethyltransferase, and GscP-coding for the Gly cleavage system P-protein. Three genes, Aat1, Pgp1, and Gdh1, are low-CO2 inducible as shown previously (Fig. 1; Miura et al., 2002). Given that the CarB protein, a carbamoyl phosphate synthetase, plays a role in assimilation of ammonium, it seems likely that the CarB homolog in Chlamydomonas functions to assimilate ammonium resulting from Gly cleavage in the photorespiratory cycle.
Two genes involved in starch synthesis, Sta2 and Sta3, were also induced under low-CO2 conditions. The Sta2 gene, encoding a granule-bound starch synthase I, and the Sta3 gene, which codes for a soluble starch synthase (ADP-Glc: α-1, 4-d-glucan-4-α-d-glucosyltransferase), play roles in elongation of starch (Ball, 1998). Since another low-CO2 inducible gene, 024b04, contains the starch-binding domain of CBM_20 (pfam00686), this gene may be involved in starch metabolism. Because Chlamydomonas cells develop pyrenoids under low-CO2 conditions, which are surrounded by a typical starch sheath (Ramazanov et al., 1995), these starch-related genes may function in pyrenoid development during acclimation to low-CO2 conditions.
Although four genes encoding chlorophyll a/b-binding proteins were repressed under low-CO2 conditions (Table II), LI818 encoding an unknown protein similar to chlorophyll a/b-binding proteins was highly induced under low-CO2 conditions (Richard et al., 2000). The gene 142b11, with low level but significant sequence similarity to Arabidopsis Sag29, which encodes a senescence-associated membrane protein, is also induced. Since thioredoxin plays a role in redox signaling in many photosynthetic organisms (Huppe et al., 1990), it is noteworthy that Trxf1, which codes for thioredoxin f1, was also induced under CO2-limiting conditions. These genes may have some regulatory roles in controlling enzyme activities or expression of other genes. In the low-CO2 induced genes, a gene with sequence similarity to Arabidopsis Ndh encoding a type-2 NADH dehydrogenase, a gene similar to Tagetes Ggps coding for geranylgeranyl pyrophosphate synthase, and Chlamydomonas Mmp encoding a mitochondrial matrix protein, were included. Additionally, three of the low-CO2 inducible genes encode proteins containing several domains related with gene regulation; e.g. 149b12 contains the interferon-induced GTPase domain (pfam05049), 022g04 harbors the glyoxalase domain (pfam00903), and 022b10 has a TB2/DP1, HVA22 domain (pfam03134).
Down-Regulated Genes during Acclimation to CO2-Limiting Conditions
During acclimation to low-CO2 stress, 32 genes were repressed within 1 h (Table II). Several photosynthetic genes were among these 32 genes such as genes encoding light harvesting chlorophyll-a/b binding proteins (Lhcb2, Lhcb4, Lhcbm9, and LhcII-3), a light-harvesting-like protein (019c02, similar to Arabidopsis Lil3), proteins for chlorophyll synthesis and chlorophyll assembly (Gsa coding for Glu-1-semialdehyde aminomutase, 156h09 similar to pea HemC coding for porphobilinogen deaminase, Cpx1 coding for coproporphyrinogen III oxidase, ChlH coding for magnesium chelatase H subunit, Ggh coding for geranylgeranyl hydrogenase, Por1 coding for NADPH: protochlorophyllide oxidoreductase, and Alad coding for δ-aminolevulinic acid dehydratase), and components for the oxygen-evolving complex (Psb2 and Psb3). LIL3 is a counterpart of the cyanobacterial high light inducible proteins (Jannson, 1999). These chlorophyll-related genes are presumably repressed for photoprotection during CO2-limiting stress conditions, because light energy is in excess under low-CO2 conditions. The Crd1 gene encoding a putative di-iron enzyme was also repressed in low-CO2 conditions. CRD1 is required for accumulation of photosystem I and light harvesting chlorophyll complex-I under copper-deficient conditions, and the crd1 mutant exhibits copper-deficiency-conditional chlorosis (Moseley et al., 2000).
A high-CO2 induced gene, H43, coding for a periplasmic protein (Shiraiwa and Kobayashi, 1999), was repressed in low-CO2 conditions, although this gene is induced under cadmium- and iron-stress conditions (Rubinelli et al., 2002). Several nutrient-related metabolic genes such as Pgk coding for phosphoglycerate kinase, 033e05 similar to Ipmdh encoding 3-isopropylmalate dehydrogenase, Uo2 coding for urate oxidase, and MetC coding for the Met synthase were also repressed in low-CO2 conditions. Three genes, Hsp70, Hsp81-2, and Cpn60a encoding molecular chaperons, and 016e07 similar to pea Sar, which codes for a DNA-binding protein, were repressed under low-CO2 conditions. In contrast, three other chaperon-related genes, Chlamydomonas Hsp22, 115d09 similar to Scherffelia Bip, and 114b09 similar to rice DnaK, were not repressed in the ccm1 mutant C16 but repressed in wild-type irrespective to CO2 levels.
Induction of Most Genes under Low-CO2 Conditions Was Impaired by the ccm1 Mutation
Furthermore, to evaluate the role of Ccm1, which encodes a protein containing a zinc-finger motif, in the regulatory network for acclimation to low-CO2 stress conditions, the DNA array was used to obtain expression profiles of a ccm1 mutant, C16, which does not induce the CCM and its related genes during acclimation to low-CO2 stress (Fukuzawa et al., 2001). After culturing the C16 cells in high-CO2 conditions, cells were transferred into low-CO2 conditions for 1 h. This change in CO2 levels does not lead to increased photosynthetic affinity for inorganic carbon since the ccm1 mutant C16 does not induce the CCM (Fukuzawa et al., 2001). The poly(A)+-RNA was isolated from the C16 cells and used for hybridization with the membrane arrays to monitor global gene expression after 1 h of low-CO2 stress conditions. Genes, whose expression failed to be induced in the ccm1 mutant C16 under low-CO2 conditions, were selected and grouped by the same strategy as for selecting low-CO2 inducible genes described above. Of 51 low-CO2 inducible genes, the expression ratios of 47 genes were significantly reduced in the ccm1 mutant. Since expression levels of the other 4 genes, 171b12 (2.1-fold), 021d12 (2.4-fold), 010b02 (2.3-fold), and 160c11 (2.0-fold), decreased relatively by the ccm1 mutation (Table I), it was shown that CCM1 controls almost all the low-CO2 inducible genes in wild-type cells. In addition, eleven genes, which were slightly induced under low-CO2 conditions in the wild-type cells, were not induced by the ccm1 mutation (Table I). Of these genes, Cah3, coding for a chloroplast CA, which is essential to the CCM, was slightly up-regulated in low-CO2 conditions (Karlsson et al., 1998) and is controlled by CCM1 as shown previously (Fukuzawa et al., 2001). Some of the EST clones showed significant sequence similarities to known genes; e.g., 023c07 product was similar to a putative heparan sulfate proteoglycan of Ovis aies, 019h09 to a putative Tyr phophatase gene from Oryza sativa, 022a05 to the voltage-dependent anion channel gene from O. sativa, 138h02 to a putative crystalline J1C of Tripedalia cystophora. Chlamydomonas Mdh1, coding for NADP-malate dehydrogenase, which plays a role in the malate valve, was not induced in the ccm1 mutant.
Suppression of Expression of Several Genes under Low-CO2 Conditions Was Impaired by the ccm1 Mutation
Suppression of 15 genes including Gsa, HemC, Lil3, Cpx1, Crt coding for Calreticulin, Psb3, H43, lpmdh, Lhchm9, LhcII-3, Ubc1 for ubiquitin-conjugating enzyme, and Pgk, was impaired by the ccm1 mutation under low-CO2 conditions. In addition, 23 genes were expressed irrespective to CO2 levels in the wild type, but expression levels of these genes were higher in the ccm1 mutant compared with those in wild type. H3-I coding for the histone H3 and Gbp1 coding for a telomere-binding protein, possibly involved in changes of the chromosomal organization, and PetO coding for a cytochrome b6/f-associated phosphoprotein were not repressed in the ccm1 mutant C16. Expression levels of 4 other genes, Chlamydomonas Ftr1 coding for a plasma membrane iron transporter, 019b08 similar to Mus Heph for multicopper ferroxidase, 028f11 similar to Solanum Mbf1 for a multiprotein-bridging factor, and 158h12 similar to Citrrus Thi1 for thiamin biosynthetic enzyme were also higher in the ccm1 mutant under low-CO2 conditions. Whether these 23 genes are actually regulated by CCM1 or by a secondary effect is not clear; some of these genes may play roles in the regulation of CO2-responsive genes. Interestingly, expression of housekeeping genes such as H3-1, Gbp1, and 114b09 for a DnaK-homolog was repressed under CO2-limiting conditions, possibly by the secondary effect of the growth arrest of the ccm1 mutant in the low-CO2 environment.
Putative Transporters Encoded by Low-CO2 Induced Genes
Although CO2 uptake systems have not been elucidated in eukaryotes including C. reinhardtii, several candidate genes responsible for Ci uptake and Ci-transport systems were included in low-CO2 inducible genes. One of them is Ccp, encoding LIP-36, which has sequence similarity with the mitochondrial-carrier-protein superfamily. Since this protein has six transmembrane domains and is located in the chloroplast envelope (Chen et al., 1997), it is assumed that Ccp is involved in Ci-transport systems. Other genes related to transporters are Mcp, which is similar to the mitochondrial carrier protein, and 148a10, which is similar to the chromate resistance protein. Although 022a05 was not induced under low-CO2 conditions, this gene shows similarity with voltage-dependent anion channels, termed porins, of several plant species (Elekeles et al., 1995).
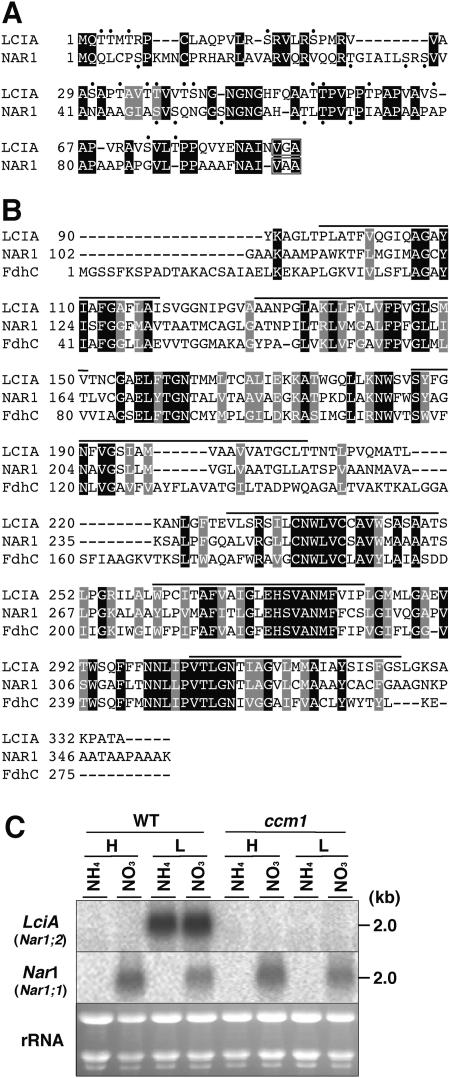
Of other low-CO2 inducible genes, expression ratios in low- to high-CO2 conditions of the two unknown genes, 018h08 and 012g07, designated as LciA and LciB, respectively, were typically high among the low-CO2 inducible genes. To predict the possible functions of these gene products, amino acid sequences of LciA and LciB were deduced from the cDNA sequences (Figs. 2 and 3). By comparison with the protein sequence databases, LciA is predicted to encode a putative polypeptide of 336 amino acid residues with significant similarities with 2 anion transporters, the chloroplast nitrite transporter NAR1 in Chlamydomonas (CrNAR1;1) (Rexach et al., 2000) and the formate transporter, FdhC, from Methanothermobacter thermautotrophicus (Nölling and Reeve, 1997; Fig. 2B).
Figure 2.
Amino acid sequence comparison of LCIA (AB168092) with anion-transporters and mRNA expression of the LciA gene. A, Amino acid sequence alignment of the putative chloroplast transit peptide of LCIA with that of NAR1 (AF149738). Artificially introduced gaps to allow optimal alignment are indicated by hyphens. Hydroxylated amino acid residues are depicted by dots. The putative signal cleavage sequences (V/I-X-A) are boxed. B, Sequence alignment of LCIA with the mature NAR1 protein and the formate transporter, FdhC (Q50568), from Methanothermobacter thermautotrophicus. Bars indicate transmembrane regions. Identical and similar amino acids in the three proteins are indicated by black and gray boxes, respectively. C, Northern-blot analyses of LciA and Nar1 in response to different carbon and nitrogen sources. Cells were cultured in media containing ammonium salts (NH4) or nitrate (NO3) as a nitrogen source. Total RNA samples isolated from wild-type and the ccm1 mutant C16 cells grown under high-CO2 (H) or acclimated to low-CO2 for 2 h (L) were hybridized with 32P-labeled gene specific probes for LciA and Nar1. To show that identical amounts of RNA were loaded, an image of the ethidium bromide-stained gel is shown.
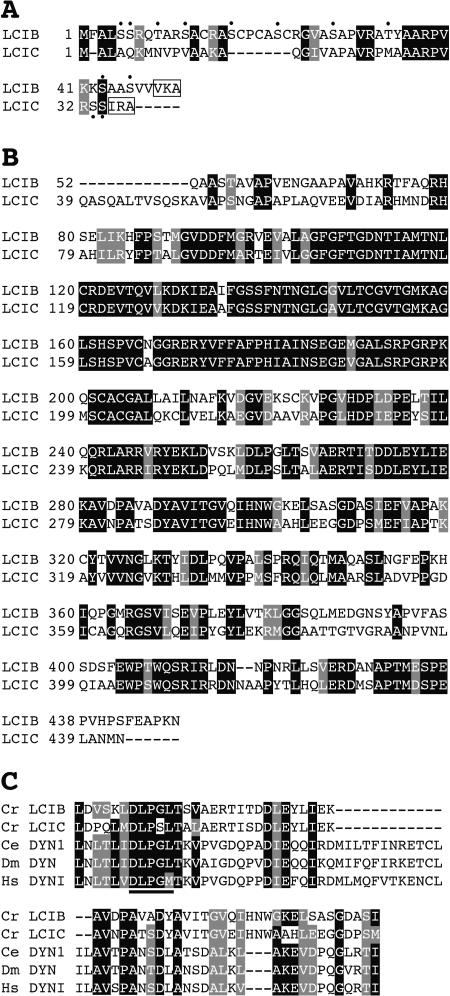
Figure 3.
Amino acid sequence alignment of the Chlamydomonas LCIB (AB168093) and LCIC (AB168094). A, Putative chloroplast transit peptides of LCIB and LCIC. Hydroxylated amino acid residues are depicted by dots. The putative signal peptide cleavage sequences (V/I-X-A) are boxed. B, Sequence alignment of the mature LCIB and LCIC proteins. C, Sequence comparison of a portion of the dynamin domains (pfam00350): Chlamydomonas reinhardtii LCIB and LCIC, Caenorhabditis elegans DYN1 (P39055), Drosophila melanogaster DYN (P27619), and Homo sapiens DYNI (Q0519). The GTP-binding domain is underlined. Identical and conserved amino acids are indicated by black and gray boxes, respectively.
To examine the regulation of LciA with respect to the previously identified Nar1, cells cultured in high CO2 conditions were transferred into low-CO2 conditions in medium containing ammonia or nitrate as a sole nitrogen source (Fig. 2C). The mRNA transcript of Nar1 was detected only when cells were grown in nitrate-containing culture medium but not when grown in ammonium-containing medium, as reported previously (Rexach et al., 2000). The Nar1 expression was largely unaffected by the change in CO2 levels and by the ccm1 mutation (Fig. 2C). In contrast, the expression of LciA corresponding to Nar1;2 (Galván et al., 2002) was induced in low-CO2 conditions irrespective of the nitrogen source, and the ccm1 mutation completely abolished LciA expression (Fig. 2C). The computer programs PSORT (http://psort.nibb.ac.jp/form.html) and SOSUI (http://sosui.proteome.bio.tuat.ac.jp/sosuiframe0.html) predicted that LCIA would localize in the thylakoid or chloroplast envelope membranes, since LCIA has a putative chloroplast transit peptide containing hydroxylated amino acid residues, which tend to be high in many chloroplast transit peptides (Heijne et al., 1989; Fig. 2A). The mature polypeptide of LCIA has 6 transmembrane domains. These results strongly suggest that LCIA functions under low-CO2 stress conditions but not for nitrite transport. On the other hand, the LCIB protein, consisting of 448 amino acid residues, did not show any sequence similarities over the entire sequence except with 028e01, designated as LCIC, which is one of the low-CO2 induced genes (Table I; Fig. 3). The amino acid sequence of LCIC was 59.9% identical to that of LCIB. By using the RPS-BLAST program, a portion of LCIB (positions 252–312) was shown to have significant sequence similarity with the dynamin families (pfam00350), e.g. DYN1 derived from Caenorhabditis elegans, DYN from Drosophila melanogaster, and DYNI from Homo sapiens (Fig. 3C). Although dynamin proteins have 3 GTPase domains, LCIB has only 1 putative GTP-binding domain. The signature sequence of the GTPase domain, DLPGL, was conserved in the LICB protein, but it was replaced by the sequence DLPSL in the LCIC protein (Fig. 3C). LCIB and LCIC were predicted to localize in the chloroplast stroma, and any transmembrane domains were not detected by the SOSUI program. These transit peptides also have several hydroxylated amino acid residues (Fig. 3A) as found in LCIA.
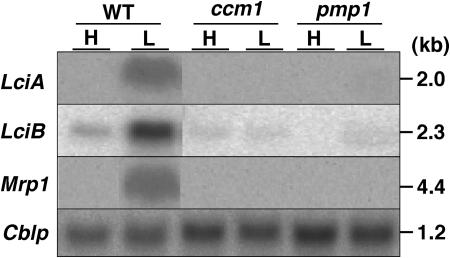
A high-CO2 requiring mutant, pmp1-1, defective in the Ci transport and accumulation, has been isolated (Spalding et al., 1983). The lesion in this strain may be associated with a gene involved in Ci uptake. To determine whether these two genes are involved in Pmp1 function, we examined LciA and LciB mRNA levels in the pmp1 mutant background. The mRNA accumulation of LciA and LciB was not detected in a high-CO2 requiring mutant pmp1 as well as in the ccm1 mutant (Fig. 4). In addition, one of the putative ATP-binding cassette-type transporters, Mrp1 (=Hla3), shows low-CO2 inducibility and Ccm1 dependency. Although significant induction of the Mrp1 was not detected in the array analysis due to the low level of signal intensity using our criteria (normalized value of SA > 25; see “Materials and Methods”), the mRNA levels of the Mrp1 reached significant levels in wild-type cells 2 h after transfer to low-CO2 conditions. This induction of the Mrp1 was impaired by both the ccm1 and pmp1 mutations.
Figure 4.
Northern-blot analyses of low-CO2-inducible genes, LciA, LciB, and Mrp1. For detection of LciA and LciB mRNA, 30 μgs of total RNA isolated from wild-type (WT), ccm1, or pmp1-1 cells grown under high-CO2 (H) or 1 h of low-CO2 (L) were hybridized with gene specific 32P-labeled probes. For detection of Mrp1 mRNA, 50 μgs of total RNA from cells grown in high-CO2 (H) or 2 h of low-CO2 (L) conditions. A 32P-labeled cDNA probe for Cblp encoding the G-protein β-subunit, which is expressed constitutively, was used as a loading control.
DISCUSSION
In this study, we have identified 83 CO2-responsive genes including 51 low-CO2 inducible genes and 32 genes repressed under low-CO2 conditions by global expression analyses using a cDNA macroarray in the eukaryotic photosynthetic alga, C. reinhardtii. Previously known CO2-responsive genes in Chlamydomonas—CA genes (Mca and Cah1), a chloroplast membrane protein gene (Ccp1), unknown low-CO2 inducible genes (Lci1 and Lci5), and photorespiratory genes (Aat1 and Pgp1)—were identified as CO2-responsive using the macroarray with the exception of a few such as Mrp1, encoding a putative ATP-binding cassette-type transporter (Fig. 4). Since we used stringent criteria to identify low-CO2 responsive genes and used cells only adapted for 1 h to low-CO2 conditions, several CO2-responsive genes have not been identified by this macroarray analysis. Indeed, Mrp1 was significantly induced after 2 h exposure to low-CO2 stress conditions (Fig. 4).
In addition, CCM1-regulated genes were selected by comparing the transcript profiles of the wild-type cells and the ccm1 mutant C16 cultured under CO2-limiting conditions. Since the ccm1 mutant C16 exhibits a high-CO2 requiring phenotype (Fukuzawa et al., 1998), some of the genes whose expression was impaired in the C16 mutant should also be essential to the operation of the CCM, in addition to the Ccm1 gene itself. Since almost all genes induced by low-CO2 conditions (47 out of 51) failed to be induced in the ccm1 mutant C16 and the other 4 genes were affected 2.0-fold to 2.4-fold in C16 (Table I), CCM1 is probably a master regulatory factor of low-CO2 inducible genes (Fig. 5). Furthermore, expression profiles of approximately one-half of the genes repressed by low-CO2 conditions (15 out of 32) were also highly altered in the ccm1 mutant C16. Therefore, it is likely that CCM1 is at the higher position in both the high-CO2- and the low-CO2-signal transduction cascades but preferentially functions as a positive regulator. Considering that CCM1 regulates the expression of genes for CA isozymes, chlorophyll-related proteins, putative Ci uptake, photorespiratory activity, and development of the pyrenoid structure, CCM1 must play a substantial role not only in the regulation of the CCM but also in the modulation of photosynthesis. Some of the low-CO2 inducible genes may be induced by secondary effects of the induction of regulatory genes in low-CO2. By the array analyses, 34 genes were shown to be impaired in the ccm1 mutant, which were not so affected by changes in CO2 levels (11 genes in Table I and 23 in Table II). Some of 34 CCM1-dependent genes may not be regulated by CCM1, because differences in the expression ratios might be caused by differences in genetic background between the wild-type strain C9 and the ccm1 mutant C16.
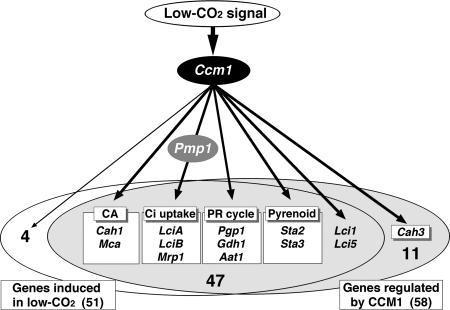
Figure 5.
Schematic illustration of a model for the relationship among Ccm1, Pmp1, 51 low-CO2 inducible genes, and 58 Ccm1-regulated genes. Numbers of the genes are indicated in each group. Of these genes, Cah1 and Mca encoding carbonic anhydrases (CA); LciA, LciB, and Mrp1 possibly involved in Ci uptake; Pgp1, Gdh1, and Aat1 for the photorespiratory cycle (PR cycle); Sta2 and Sta3 possibly involved in the pyrenoid development, are highlighted. Previously known low-CO2 inducible genes (Lci1 and Lci5) and chloroplast CA gene (Cah3) are also indicated.
The photorespiratory carbon oxidation cycle of C. reinhardtii is different from that of higher plants, because these microalgae have no peroxisomes (Spalding, 1989). The photorespiratory cycle of C. reinhardtii is thought to exist in the mitochondria. The CCM elevates the internal levels of dissolved inorganic carbon and facilitates the carboxylation reaction of Rubisco over the competitive oxygenation reaction (Kaplan and Reinhold, 1999). Therefore, it has been suggested that photorespiratory activity is greatly suppressed by the CCM in air-adapted cells even under CO2-limiting conditions. However, our macroarray analysis indicated that seven genes encoding enzymes, which function in the photorespiratory cycle, were induced by lowering CO2 concentration. In fact, activities of photorespiratory enzymes such as phosphoglycolate phosphatase and glycolate dehydrogenase were increased in air-adapted cells (Marek and Spalding, 1991), and a pgp1-1 mutant, impaired in the phosphoglycolate phosphatase activity, showed a high-CO2 requiring phenotype (Suzuki et al., 1990). Considering the fact that mRNA levels of other photorespiratory genes such as Sgat and GcsP were induced under low-CO2 conditions, it is possible that the photorespiratory cycle is transcriptionally activated during or before induction of the CCM in acclimation steps to CO2-limiting conditions via regulation by CCM1. In addition, one of the photorespiratory genes, Shmt, is induced also under high light conditions (Im and Grossman, 2002). Considering that other photorespiratory genes are not induced under high light conditions (Im et al., 2003), it is possible that Shmt has additional functions besides photorespiration.
In a cyanobacterium Synechocystis sp. PCC6803, two types of functionally distinct NDH-1 complexes have been identified with the aid of mutants impaired in one or more subunits of NDH-1 (Ohkawa et al., 2000a). One is for photosystem-I cyclic electron flow, another is for CO2, not bicarbonate, uptake (Ohkawa et al., 2000b; Shibata et al., 2001, 2002a). Although the Ndh gene encoding a type-2 NADH dehydrogenase was detected as a low-CO2 inducible gene in our array analysis, it is not known whether this type of NADH dehydrogenase functions in Ci-uptake systems. Taken with the fact that at least four systems for Ci acquisition are present in cyanobacteria (Shibata et al., 2002a), it is likely that there could be several Ci-uptake systems in the eukaryotic alga Chlamydomonas. Although the necessity of the Ci-uptake system in the CCM is clear and the system is assigned to localize in chloroplast envelope (Kaplan and Reinhold, 1999; Badger and Spalding, 2000), no molecular components for Ci-uptake systems in photosynthetic eukaryotes have been identified yet. Taken with the fact that the pmp1-1 and the ccm1 mutants show high-CO2 requiring phenotypes (Spalding et al., 1983; Fukuzawa et al., 1998), expression levels of genes involved in the Ci-uptake system should be decreased in these mutants. By the global expression analyses using DNA arrays and northern-blot analyses, two new candidates, LciA and LciB, for the inorganic carbon transporters were isolated in this study. LciA shows sequence similarity with Nar1, and five Nar1-related genes, Nar1;1 to Nar1;5, were identified in C. reinhardtii (Galván et al., 2002). Of these genes, Nar1;1 corresponds to Nar1 encoding a nitrite transporter, and Nar1;2 is identical to LciA. The fact that a bicarbonate transporter shows homology to a nitrate transporter in cyanobacteria (Omata et al., 1999) supports our assumption that LCIA functions as an anion transporter to take up bicarbonate into chloroplasts. Our array data showed that the other three Nar1-related genes were not induced in low-CO2 conditions (Nar1;3 [array ID, 028e05; expression ratio of signal intensity in wild type cells (WT) under low-CO2 (LC) to that in WT under high-CO2 (HC), 1.1 ± 0.3], Nar1;4 [149a12, 0.8 ± 0.2], and Nar1;5 [127g03, 0.8 ± 0.0]), suggesting that low-CO2 inducible LciA (=Nar1;2) may encode a bicarbonate-transporter. This prediction is supported by a finding that LCIA has a putative chloroplast-targeting sequence in its N-terminal region, since Ci is accumulated in the intact chloroplasts isolated from Chlamydomonas grown in low-CO2 conditions but not in high-CO2 conditions (Moroney et al., 1987). LciB failed to be induced in the ccm1 and pmp1-1 mutants. This gene product does not have any sequence similarities with known proteins except for LCIC (Fig. 3). LCIB is probably localized in chloroplast stroma because of a lack of transmembrane domains. Previous work indicates two soluble proteins with molecular masses of 46 and 44 kD are not induced in the pmp1-1 mutant (Manuel and Moroney, 1988). Since the molecular mass of mature LCIB is predicted to be 44 kD, this 44-kD protein missing in the pmp1-1 mutant could be the LICB protein. Motif searches show that LCIB has a GTP-binding domain. We also identified an LciB-related gene, LciC, which failed to be induced in the ccm1 mutant C16. Since the signature sequence of GTPase domains (DLPGL) was not conserved in LCIC (Fig. 3C), this protein may not have GTPase activity. Determination of subcellular localization and further biochemical analyses would reveal the functions of LCIA, LCIB, and LCIC.
The Chlamydomonas pmp1-1 mutant was isolated as lacking an active Ci-uptake system (Spalding et al., 1983). This mutation in Pmp1 caused a severe requirement of high levels of CO2 for its photosynthetic growth in various pH ranges (Van and Spalding, 1999). In cyanobacteria, four Ci-uptake systems exist and a deletion mutant of one system does not show severe a high-CO2 requiring phenotype (Shibata et al., 2002a). Considering the above two facts, it is more likely that Pmp1 is involved in regulation of several Ci-uptake systems. Our analyses revealed that Pmp1 regulates LciA, LciB, and Mrp1, but not Cah1, transcriptionally (Fig. 4; data not shown). Since the ccm1 mutant is impaired in Ci accumulation (Fukuzawa et al., 2001), it is possible that some genes for Ci-uptake systems other than LciA, LciB, and Mrp1 could also be regulated by Pmp1 (Fig. 5).
Large-scale analyses in this study extend our knowledge of acclimation to CO2-limiting conditions and CO2-signal transduction through CCM1. Since photosynthetic electron flow is required for the CCM (Badger and Spalding, 2000), expression of some genes would be regulated by not only CO2 availability but also light intensities as in the case of Shmt (Im and Grossman, 2002; Im et al., 2003). For the control of these genes, it is likely that the redox state of the plastoquinone pool is involved. Accumulation of further genome-wide transcriptome information on various acclimation processes and conditions should enable us to identify the genes involved in many adaptation processes to different environmental stress conditions, which would lead to a better understanding of the complicated network of regulatory interactions (Kucho et al., 2003; Yoshioka et al., 2004). Identification of the functions of CO2-responsive genes and/or CCM1-regulated genes would enhance our understanding of the molecular mechanisms of acclimation to CO2-limiting conditions.
MATERIALS AND METHODS
Cells and Growth Conditions
Chlamydomonas reinhardtii Dangeard wild-type cells C9 (mt-) strain and the high-CO2 requiring mutant, C16 (ccm1), have been described previously (Fukuzawa et al., 1998, 2001) and another high-CO2 requiring mutant, pmp1-1, was kindly provided by Dr. J.V. Moroney. Cells were cultured in buffered high salt (HS) medium supplemented with 20 mm MOPS (pH 7.2) under aeration with ordinary air containing 0.04% CO2 (low-CO2) or air enriched with 5% CO2 (high-CO2) as described previously (Kucho et al., 1999).
Preparation of DNA Macroarrays
To construct the Chlamydomonas cDNA membrane array, three groups of cDNA libraries derived from cells in photoautotrophic growth (Asamizu et al., 1999), from high-CO2 and low-CO2 grown cells (Asamizu et al., 2000), and from cells grown under 23 different kinds of stress conditions to generate 12,842 5′-ESTs (E. Asamizu, Y. Nakamura, K. Miura, H. Fukuzawa, S. Fujiwara, M. Hirono, K. Iwamoto, Y. Matsuda, J. Minagawa, K. Shimogawara, Y. Takahashi, and S. Tabata, unpublished data). A total of 50,832 EST sequences were clustered by BLAST followed by PHRAP programs as described previously (Asamizu et al., 1999). Esherichia coli cells containing EST clones were cultured and used for PCR in the mixture containing 1× buffer, 0.2 mm each of dATP, dCTP, dGTP, and dTTP, 5% (v/v) dimethyl sulfoxide, 1.75 units Ex Taq DNA polymerase (Takara, Kyoto) and the primers T7-25-kyoto (5′-CGCGTAATACGACTCACTATAG-GGC-3′) and T3-25-kyoto (5′-AGCGCGCAATTAACCCTCACTAAAG-3′). After confirmation of PCR amplification, one-sixth volume of dye solution containing 10 mm Tris-HCl (pH 8.0), 1 mm EDTA, 0.25% (w/v) bromo-phenol blue, and 60% (v/v) glycerol was added to the PCR products. These PCR products were spotted on Biodyne-A nylon membranes (0.2 μm, Pall Biosupport, Port Washington, NY) using a spotting device, Biomek2000 (Beckman Coulter, Roissy, France). One membrane can contain 1,728 cDNAs spotted in duplicate. To spot 10,368 cDNAs, we used 6 membranes per set. The spotted membranes were treated twice with UV light using a UV Stratalinker 2400 (Stratagene, La Jolla, CA), and baked at 80°C for 2 h.
Preparation of Probes and Hybridization
The 32P-labeled target DNA samples were prepared from poly(A)+ RNAs by incorporation of [α-32P]dCTP during first-strand cDNA synthesis. Each reaction consisted of 1 μg of poly(A)+ RNAs, 2 μg random primers pd (N)9, 1 mm each of dATP, dGTP, and dTTP, 15 μm dCTP, 0.1mCi [α-32P]dCTP (6000 Ci/mmol, NEN Life Science Products, Boston), 60 units RNase inhibitor (RNaseOUT, Invitrogen, Carlsbad, CA), 20 mm Tris-HCl (pH 8.4), 50 mm KCl, 2.5 mm MgCl2, 10 mm dithiothreitol, 0.6 m trehalose, and 400 units Superscript II reverse transcriptase (Invitrogen). The reverse transcription reaction was performed at 37°C for 15 min and at 50°C for 5 min followed by incubation for 90 min at 60°C. The labeled cDNA products were used for hybridization with ExpressHyb Hybridization Solution (CLONTECH, Palo Alto, CA) at 68°C for 12 to 16 h. The membranes were washed with 0.1× SSC and 0.5% SDS, and with 0.08× SSC and 0.5% SDS, and then exposed to imaging plates (Fuji Photo Film, Tokyo).
Data Analysis
Radioactive images were obtained using a scanner, FLA-2000 (Fuji Photo Film), and quantification of the signal intensity was performed using the program ArrayVision (Amersham Pharmacia Biotech, Little Chalfont, UK). Raw values were measured as the volume of pixels within a circle encompassing the spot. The background for each membrane was calculated as follows: 40 sample values, which were located at nonspotted areas in each membrane, were quantified. Average and sd of the background were calculated by using 36 sample values, ignoring the top 5% and bottom 5% of background data values. The average background value was subtracted from the value of each spot on the membrane to give the sample value (c). To reduce area-specific effects, mean normalization was adapted. A trimmed mean (μmem) was calculated for each membrane by using 80% of the data points, ignoring the top 10% and the bottom 10% of the data points to prevent the normalization from skewing. Then the sample value was normalized. After calculating normalized values S = (c/μmem) × 18.08 (a correction factor), the relative signal intensity was calculated as the ratio of 2 normalized values. This estimated relative signal intensity is called the expression ratio (SA/SB). The expression ratios (WT-LC/WT-HC or WT-LC/C16-LC) of the duplicated spots were averaged. Data were obtained from 2 independent cultures and hybridizations for each experiment. If the correlation coefficient between these two experimental data was more than 0.90, these were used for further analyses. Only ESTs whose averaged expression ratios were more than 2.5 and normalized values of numerators (SA) were more than 25, corresponding to 0.1% of the total signal, were assigned to be significantly expressed in the cells. It was confirmed that normalized values of numerators (SA) were at least 2-fold higher than the average background (plus 2 × sd of background). Using 4 expression ratio data per EST clone, the means and their sd were calculated. Since each EST clone has 4 expression ratios, if 3 of 4 expression ratios were more than 2.5-fold, the EST clone was selected as a significantly differentially expressed gene for further analysis. After grouping, the mean and sd of the expression ratios of each gene were calculated as the arithmetic average by using values from the 4 signal intensities of each EST clone.
Northern-Blot Analyses
Northern-blot analyses were performed as described previously (Kucho et al., 1999) using probes generated by PCR in the presence of [α-32P]dCTP with primers T7-25- kyoto and T3-25-kyoto and EST clones as follows: CM035d02 for Cah1, LCL016g10 for Aat1, LC048g05 for Pgp1, CM085d07 for Cblp, LC010h12 for LciB, and HCL059f06 for Mrp1. To detect the LciA gene, the EST clone, LC055f02, was amplified with the primers LciA-f (5′-GCTCTAGAGCTAGCATGCAGACCACTATGA-3′) and ds-LciA-r (5′-CTAGATCTATGCATGCGGAAACAGCGACGG-3′). To detect the Nar1 gene, wild-type genomic DNA was used as a template for amplification with the primers Nar1;1_5UTR-f (5′-GAAACGGGTTGGTTGAAGAGAATTCAACCT-3′) and Nar1;1_5UTR-r (5′-TTTCATCCCTTGTGGCGCGATGATG-3′).
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purpose.
Sequence data from this article have been deposited with the DDBJ/EMBL/GenBank data libraries under accession numbers AB168092 (LCIA), AB168093 (LCIB), and AB168094 (LCIC).
Acknowledgments
We thank Drs. Ayumi Tanaka, Jun Minagawa, Yoshihiro Shiraiwa, Koji Iwamoto, Kan Tanaka, Mikio Tsuzuki, Shoko Fujiwara, Norihiro Sato, Masahiro Ishiura, Junichi Obokata, Nobuyoshi Mochizuki, Yoshihiro Matsuda, Tatsuaki Saito, and their colleagues for construction of cDNA macroarray.
This work was supported by the Japanese Ministry of Education, Science and Culture (grant nos. 14656136 and 15380071), by the Japan Society for the Promotion of Science (grant no. JSPS–RFTF97R16001 to H.F.), and by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists (grant no. 3117 to K.M.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.041400.
References
- Asamizu E, Miura K, Kucho K, Inoue Y, Fukuzawa H, Ohyama K, Nakamura Y, Tabata S (2000) Generation of expressed sequence tags from low-CO2 and high-CO2 adapted cells of Chlamydomonas reinhardtii. DNA Res 7: 305–307 [DOI] [PubMed] [Google Scholar]
- Asamizu E, Nakamura Y, Sato S, Fukuzawa H, Tabata S (1999) A large scale structural analysis of cDNAs in a unicellular green alga, Chlamydomonas reinhardtii. I. Generation of 3433 non-redundant expressed sequence tags. DNA Res 6: 369–373 [DOI] [PubMed] [Google Scholar]
- Badger MR, Spalding MH (2000) CO2 acquisition, concentration and fixation in cyanobacteria and algae. In RC Leegood, TD Sharkey, S von Caemmerer, eds, Photosynthesis: Physiology and Metabolism. Kluwer Academic Publishers, The Netherlands, pp 369–397
- Ball SG (1998) Regulation of starch biosynthesis. In JD Rochaix, M Goldschmidt-Clermont, S Merchant, eds, The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 549–567
- Chen Z-Y, Burow MD, Mason CB, Moroney JV (1996) A low-CO2-inducible gene encoding an alanine: α-ketoglutarate aminotransferase in Chlamydomonas reinhardtii. Plant Physiol 112: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-Y, Lavigne LL, Mason CB, Moroney JV (1997) Cloning and overexpression of two cDNAs encoding the low-CO2-inducible chloroplast envelope protein LIP-36 from Chlamydomonas reinhardtii. Plant Physiol 114: 265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elekeles A, Devos KM, Graur D, Zizi M, Breiman A (1995) Multiple cDNAs of wheat voltage-dependent anion channels (VDAC): isolation, differential expression, mapping and evolution. Plant Mol Biol 29: 109–124 [DOI] [PubMed] [Google Scholar]
- Eriksson M, Karlsson J, Ramazanov Z, Gardeström P, Samuelsson G (1996) Discovery of an algal mitochondrial carbonic anhydrase: molecular cloning and characterization of a low-CO2-induced polypeptide in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 93: 12031–12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa H, Fujiwara S, Yamamoto Y, Dionisio-Sese ML, Miyachi S (1990) cDNA cloning, sequence, and expression of carbonic anhydrase in Chlamydomonas reinhardtii: regulation by environmental CO2 concentration. Proc Natl Acad Sci USA 87: 4383–4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzawa H, Ishizaki K, Miura K, Matsueda S, Ino-ue T, Kucho K, Ohyama K (1998) Isolation and characterization of high-CO2 requiring mutants from Chlamydomonas reinhardtii by gene tagging. Can J Bot 76: 1092–1097 [Google Scholar]
- Fukuzawa H, Miura K, Ishizaki K, Kucho K, Saito T, Kohinata T, Ohyama K (2001) Ccm1, a regulatory gene controlling the induction of a carbon-concentrating mechanism in Chlamydomonas reinhardtii by sensing CO2 availability. Proc Natl Acad Sci USA 98: 5347–5352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A, Rexach J, Mariscal V, Fernández E (2002) Nitrite transport to the chloroplast in Chlamydomonas reinhardtii: molecular evidence for a regulated process. J Exp Bot 53: 845–853 [DOI] [PubMed] [Google Scholar]
- Huppe HC, de Lamotte-Guery F, Jacquot J-P, Buchanan BB (1990) The ferredoxin-thioredoxin system of a green alga, Chlamydomonas reinhardtii. Planta 180: 341–351 [PubMed] [Google Scholar]
- Im C-S, Grossman AR (2002) Identification and regulation of high light-induced genes in Chlamydomonas reinhardtii. Plant J 30: 301–313 [DOI] [PubMed] [Google Scholar]
- Im C-S, Zhang Z, Shrager J, Chang C-W, Grossman AR (2003) Analysis of light and CO2 regulation in Chlamydomonas reinhardtii using genome-wide approaches. Photosynth Res 75: 111–125 [DOI] [PubMed] [Google Scholar]
- Jannson S (1999) A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci 4: 236–240 [DOI] [PubMed] [Google Scholar]
- Kaplan A, Reinhold L (1999) CO2 concentrating mechanisms in photosynthetic microorganisms. Annu Rev Plant Physiol Plant Mol Biol 50: 539–570 [DOI] [PubMed] [Google Scholar]
- Karlsson J, Clarke AK, Chen Z-Y, Hugghins SY, Park Y-I, Husic HD, Moroney JV, Samuelsson G (1998) A novel α-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J 17: 1208–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucho K, Ohyama K, Fukuzawa H (1999) CO2-responsive transcriptional regulation of CAH1 encoding carbonic anhydrase is mediated by enhancer and silencer regions in Chlamydomonas reinhardtii. Plant Physiol 121: 1329–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucho K, Yoshioka S, Taniguchi F, Ohyama K, Fukuzawa H (2003) Cis-acting elements and DNA-binding proteins involved in CO2-responsive transcriptional activation of Cah1 encoding a periplasmic carbonic anhydrase in Chlamydomonas reinhardtii. Plant Physiol 133: 783–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JA, Woodward FI, Quick WP (2002) Long-distance CO2 signaling in plants. J Exp Bot 53: 183–193 [DOI] [PubMed] [Google Scholar]
- Lockhart DJ, Winzeler EA (2000) Genomics, gene expression and DNA arrays. Nature 405: 827–836 [DOI] [PubMed] [Google Scholar]
- Mamedov TG, Suzuki K, Miura K, Kucho K, Fukuzawa H (2001) Characteristics and sequence of phosphoglycerate phosphatase from an eukaryotic green algae Chlamydomonas reinhardtii. J Biol Chem 276: 45573–45579 [DOI] [PubMed] [Google Scholar]
- Manuel LJ, Moroney JV (1988) Inorganic carbon accumulation by Chlamydomonas reinhardtii. Plant Physiol 88: 491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek LF, Spalding MH (1991) Changes in photorespiratory enzyme activity in response to limiting CO2 in Chlamydomonas reinhardtii. Plant Physiol 97: 420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Kohinata T, Yoshioka S, Ohyama K, Fukuzawa H (2002) Regulation of a carbon concentrating mechanism through CCM1 in Chlamydomonas reinhardtii. Funct Plant Biol 29: 211–219 [DOI] [PubMed] [Google Scholar]
- Moroney JV, Kitayama M, Togasaki RK, Tolbert NE (1987) Evidence for inorganic carbon transport by intact chloroplasts of Chlamydomonas reinhardtii. Plant Physiol 83: 460–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley J, Quinn J, Eriksson M, Merchant S (2000) The Crd1 gene encodes a putative di-iron enzyme required for photosystem I accumulation in copper deficiency and hypoxia in Chlamydomonas reinhardtii. EMBO J 19: 2139–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nölling J, Reeve JN (1997) Growth- and substrate-dependent transcription of the formate dehydrogenase (fdhCAB) operon in Methanobacterium thermoformicicum Z-245. J Bacteriol 179: 899–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Pakrasi HB, Ogawa T (2000. a) Two types of functionally distinct NAD(P)H dehydrogenase in Synechocystis sp. strain PCC6803. J Biol Chem 275: 31630–31634 [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Price GD, Badger MR, Ogawa T (2000. b) Mutation of ndh genes leads to inhibition of CO2 uptake rather than HCO3− uptake in Synechocystis sp. strain PCC 6803. J Bacteriol 182: 2591–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T, Price GD, Badger MR, Okamura M, Gohta S, Ogawa T (1999) Identification of an ATP-binding cassette transporter involved in bicarbonate uptake in the cyanobacterium Synechococcus sp. strain PCC7942. Proc Natl Acad Sci USA 96: 13571–13576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramazanov Z, Rawat M, Henk MC, Mason CB, Matthews SW, Moroney JV (1995) The induction of the CO2-concentrating mechanism is correlated with the formation of the starch sheath around the pyrenoid of Chlamydomonas reinhardtii. Planta 195: 210–216 [Google Scholar]
- Rexach J, Fernandez E, Gálvan A (2000) The Chlamydomonas reinhardtii Nar1 gene encodes a chloroplast membrane protein involved in nitrite transport. Plant Cell 12: 1441–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard C, Ouellet H, Guertin M (2000) Characterization of the LI818 polypeptide from the green unicellular alga Chlamydomonas reinhardtii. Plant Mol Biol 42: 303–316 [DOI] [PubMed] [Google Scholar]
- Rubinelli P, Siripornadulsil S, Gao-Rubinelli F, Sayre RT (2002) Cadmium- and iron-stress-inducible gene expression in the green alga Chlamydomonas reinhardtii: evidence for H43 protein function in iron assimilation. Planta 215: 1–13 [DOI] [PubMed] [Google Scholar]
- Shibata M, Katoh H, Sonoda M, Ohkawa H, Shimoyama M, Fukuzawa H, Kaplan A, Ogawa T (2002. b) Genes essential to sodium-dependent bicarbonate transport in cyanobacteria: function and phylogenetic analysis. J Biol Chem 277: 18658–18664 [DOI] [PubMed] [Google Scholar]
- Shibata M, Ohkawa H, Kaneko T, Fukuzawa H, Tabata S, Kaplan A, Ogawa T (2001) Distinct constitutive and low-CO2-induced CO2 uptake systems in cyanobacteria: Genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc Natl Acad Sci USA 98: 11789–11794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Ohkawa H, Katoh H, Shimoyama M, Ogawa T (2002. a) Two CO2 uptake systems in cyanobacteria: four systems for inorganic carbon acquisition in Synechocystis sp. strain PCC6803. Funct Plant Biol 29: 123–129 [DOI] [PubMed] [Google Scholar]
- Shiraiwa Y, Kobayashi H (1999) Characterization of a high-CO2-inducible 43-kDa-polypeptide in Chlamydomonas reinhardtii. Plant Cell Physiol 40: s123 [Google Scholar]
- Shrager J, Hauser C, Chang C-W, Harris EH, Davies J, McDermott J, Tamse R, Zhang Z, Grossman AR (2003) Chlamydomonas reinhardtii genome project. A guide to the generation and use of the cDNA information. Plant Physiol 131: 401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding MH (1989) Photosynthesis and photorespiration in freshwater green algae. Aquat Botany 34: 181–209 [Google Scholar]
- Spalding MH, Spreitzer RJ, Ogren WL (1983) Reduced inorganic carbon transport in a CO2-requiring mutant of Chlamydomonas reinhardtii. Plant Physiol 73: 273–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stretton S, Goodman AE (1998) Carbon dioxide as a regulator of gene expression in microorganisms. Antonie Van Leeuwenhoek 73: 79–85 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Marek LF, Spalding MH (1990) A photorespiratory mutant of Chlamydomonas reinhardtii. Plant Physiol 93: 231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van K, Spalding MH (1999) Periplasmic carbonic anhydrase structural gene (Cah1) mutant in Chlamydomonas reinhardtii. Plant Physiol 120: 757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G, Steppuhn J, Herman SG (1989) Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem 180: 535–545 [DOI] [PubMed] [Google Scholar]
- Xiang Y, Zhang J, Weeks DP (2001) The Cia5 gene controls formation of the carbon concentrating mechanisms in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 98: 5341–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka S, Taniguchi F, Miura K, Inoue T, Yamano T, Fukuzawa H (2004) A novel Myb transcription factor LCR1 regulates the CO2-responsive gene Cah1 encoding a periplasmic carbonic anhydrase in Chlamydomonas reinhardtii. Plant Cell (in press) [DOI] [PMC free article] [PubMed]