Abstract
Photoperiod in plants is perceived by leaves and in many species influences the transition to reproductive growth through long-distance signaling. CONSTANS (CO) is implicated as a mediator between photoperiod perception and the transition to flowering in Arabidopsis. To test the role of CO in long-distance signaling, CO was expressed from a promoter specific to the companion cells of the smallest veins of mature leaves. This expression in tissues at the inception of the phloem translocation stream was sufficient to accelerate flowering at the apical meristem under noninductive (short-day) conditions. Grafts that conjoined the vegetative stems of plants with different flower-timing phenotypes demonstrated that minor-vein expression of CO is able to substitute for photoperiod in generating a mobile flowering signal. Our results suggest that a CO-derived signal(s), or possibly CO itself, fits the definition of the hypothetical flowering stimulant, florigen.
Many plant species rely on environmental stimuli to time the reproductive phase of their life cycle. Prominent among these stimuli is light, from which plants perceive the duration of day/night cycles and entrain their circadian rhythms (McClung, 2001; Mouradov et al., 2002; Kevei and Nagy, 2003). In elegant, and now classic, studies it was shown that the duration of the day/night cycles is perceived by leaves, which in turn produce a signal that promotes the transition to flowering at the shoot apical meristem (Zeevaart, 1976). This signal is graft transmissible and functionally conserved among closely related species, as demonstrated in interspecific grafting experiments. The signal migrates with radiolabeled products of photosynthesis, and graft transmission requires intact vascular strands, indicating that transport is via the phloem (Zeevaart, 1976). The molecular identity of the signal—commonly called florigen—remains elusive, and its existence as a single entity is questioned by some (Colasanti and Sundaresan, 2000).
Arabidopsis is a facultative long-day plant: The transition to flowering is accelerated under long days, but the plants will eventually flower under short-day conditions. (Although conventional terminology refers to the daylength, it is the dark period that is perceived.) Plants carrying mutant alleles of the CONSTANS gene (CO) are late flowering in long days but flower at the normal time under short days (Putterill et al., 1995). Consistent with a role in promoting flowering in long days, CO mRNA accumulates under long-day conditions, relative to short days (Putterill et al., 1995), and oscillates in response to circadian rhythms (Suarez-Lopez et al., 2001). Under long-day conditions, CO mRNA begins to accumulate during the light period, whereas in short days, accumulation begins during the night period. These patterns of CO accumulation in relation to light stimuli are proposed to be a mechanism by which Arabidopsis coordinates flowering with daylength (Suarez-Lopez et al., 2001). As may be expected for a gene that controls development in response to transient stimuli, both mRNA and protein accumulate to only very low levels (Putterill et al., 1995; Suarez-Lopez et al., 2001). In long-day, inductive conditions, CO mRNA was identified in leaves and stems as early as the four-leaf stage by reverse transcription (RT)-PCR (Putterill et al., 1995) as well as in the vegetative meristem and leaf initials by in situ hybridization (Simon et al., 1996).
At present, it is not clear whether CO, which encodes a transcription factor, promotes flowering by acting locally in the meristem or over long distances by generating a phloem-mobile signal. The latter was recently supported by Takada and Goto (2003), who fused the uidA reporter gene to the CO promoter and observed β-glucuronidase (GUS) activity in vascular tissues.
If CO participates in generating a phloem-mobile signal that accelerates the transition to flowering, CO expression should be sufficient to induce flowering even if limited to tissues at the inception of the translocation stream—the minor veins of mature leaves (Oparka and Turgeon, 1999). To test this hypothesis, we used a galactinol synthase (GAS) promoter from melon (Cucumis melo). This promoter is active in the companion cells of minor veins in mature leaves but not in larger vascular bundles nor in apices (Haritatos et al., 2000) and therefore provides a valuable genetic tool for studying leaf-derived signals that are phloem mobile and act in distant tissues (Ayre et al., 2003).
We report here that CO expression from the GAS promoter resulted in early flowering. CO fusions with the uidA reporter gene confirmed minor vein-limited expression. In grafting experiments, scions homozygous for the co-1 allele flowered early when grafted to CO-expressing stocks, demonstrating that CO participates in generating a phloem-mobile floral stimulant rather than responding to one.
RESULTS
CO Expression in Minor Veins Accelerates Flowering
A CO cDNA was expressed from the GAS promoter, alone or with an 11-amino acid T7 epitope fused in frame to the amino terminus of the protein (T7CO). Arabidopsis plants were transformed by the floral dip procedure (Clough and Bent, 1998), and T1 plants were grown from seed under noninductive, short-day conditions (10 h light/14 h dark). A range of flowering-time phenotypes was observed: Some plants had visible floral buds within 20 d postgermination and initiated the transition to flowering when the plants were at the four-true-leaf stage. Most plants flowered by the 9- or 10-leaf stage (Fig. 1A; Table I), and there was no difference in the range observed among GAS:CO (9.3 ± 1.0 leaves [se]; n = 7) and GAS:T7CO (8.5 ± 0.5 leaves; n = 10 plants). By contrast, wild-type plants, or plants transformed with a vector lacking the CO cDNA, did not begin to flower until 80 d postgermination and had 28 or more leaves, consistent with previous findings (Corbesier et al., 1996). These results demonstrate that CO expression from a minor vein-specific promoter is sufficient to induce a switch from vegetative to reproductive development at the apical meristem.
Figure 1.
Accelerated flowering and graft transmission of floral stimulant resulting from CO expression confined to the companion cells of minor veins in mature leaves. A, T1 generation plants (Columbia ecotype) transformed with an empty-vector control (left) and GAS:CO-containing vector (right) grown for 41 d with 10-h-light/14-h-dark cycles. B, Vegetative stem section of grafted plant 10 d after grafting. Arrow points to callus tissue formed between the scion (top) and stock (bottom); scale bar = 0.5 mm. C, Scion homozygous for the co-1 allele showing floral buds 20 d after grafting to a GAS:CO stock plant. D, Scion homozygous for the co-1 allele showing a vegetative apex 20 d after grafting to a homozygous co-1 allele stock plant. Flowers were not visible until 43 d after grafting. Scale bar for C and D = 2 mm.
Table I.
Accelerated transition to flowering in Arabidopsis plants expressing CO in minor veins from the GAS promoter
| Transgene | Background | No. of Leaves at the Transition to Flowering |
|---|---|---|
| Wild type | Columbia | >28 (n > 12)a |
| GAS:CO | Columbia | 9.3 ± 1.0b (n = 7) |
| GAS:T7CO | Columbia | 8.5 ± 0.5 (n = 10) |
| GAS:T7CO | co-1 Columbia | 8.7 ± 0.7 (n = 6) |
| GAS:T7CO | Ei-2 | 25.6 ± 2.3 (n = 11) |
Not all wild-type plants were flowering when assayed 80 d after germination.
Variation is expressed as se.
The effect of CO expression from the GAS promoter on flowering time was also tested in different genetic backgrounds. Homozygous co-1 plants (ecotype Columbia), which flower late and are insensitive to daylength (Putterill et al., 1995), exhibited precocious flowering when transformed with GAS:T7CO (8.7 ± 0.7 leaves; n = 6), comparable to wild-type plants transformed with the same gene (Table I). This demonstrates that expression in the minor veins is sufficient to overcome the absence of endogenous, functional CO. This contrasts with the late-flowering ecotype Ei-2, in which expressing T7CO from the GAS promoter did not accelerate the transition to flowering to the same extent, if at all (25.6 ± 2.3 leaves; n = 11). The switch to reproductive development in Ei-2 is therefore regulated by additional factors acting most likely downstream of CO.
CO-Derived Signal Is Graft Transmissible
Graft transmissibility is a recognized property of the leaf-derived, phloem-mobile signal that controls flowering in response to photoperiod (Zeevaart, 1976), and previous work demonstrates that CO expression responds to photoperiod (Suarez-Lopez et al., 2001). To determine if CO expression in the minor veins is able to substitute for photoperiod in initiating a graft-transmissible signal, rosettes of different flowering genotypes were conjoined as stock and scion (Fig. 1B). All plants were grown and maintained under noninductive (short-day) conditions, and grafting was carried out when co-1 plants were between 32 and 56 d old. Under standard conditions, co-1 and wild-type plants remain vegetative for more than 80 d.
Approximately 120 grafts were initiated, including controls. A total of 13 scions became established and resumed healthy growth (Fig. 1B). The mean time between grafting and the appearance of flower buds (Table II) was 17.6 ± 1.4 (n = 5) d when co-1 scions were grafted to GAS:CO stocks (Fig. 1C). In control experiments, in which co-1 and wild-type plants were used as stocks and scions in different combinations, the appearance of flower buds occurred 42.3 ± 5.2 (n = 8) d after grafting (Fig. 1D; Table II).
Table II.
Accelerated transition to flowering in co-1 scions after grafting to GAS:CO stock plants
| Stock | Scion | Days from Grafting to the Appearance of Flower Buds | Days from the Resumption of Growth to the Appearance of Flower Buds |
|---|---|---|---|
| GAS:CO | co-1 | 17.6 ± 1.4a (n = 5) | 0.8 ± 1.3 |
| co-1 or wild type | co-1 or wild type | 42.3 ± 5.2 (n = 8) | 27.6 ± 5.5 |
Variation is expressed as se.
In these experiments, the time required for graft healing is variable and difficult to assess. It is therefore more informative to compare the time between resumption of visible growth (14–23 d) and flowering (Table II). In four out of the five cases in which GAS:CO plants were used as stocks, co-1 scion flower buds were noted on the same day that visible scion growth resumed, implying that the morphogenic events leading to flowering occurred quickly after graft healing. The fifth grafted plant flowered 4 d after resumption of visible growth. In sharp contrast, flower buds were seen 27.6 ± 5.5 d (range 11–55 d) following the resumption of visible scion growth among the control graft unions in which co-1 and wild-type plants were used in different combinations. Importantly, control grafts using co-1 or wild-type plants as either the stock or scion flowered concurrently with ungrafted plants of the same age, demonstrating that grafting and subsequent manipulations did not trigger one of the Arabidopsis flowering pathways. Our experiments strongly implicate CO in generating the phloem-mobile signal.
RNA and Protein Movement
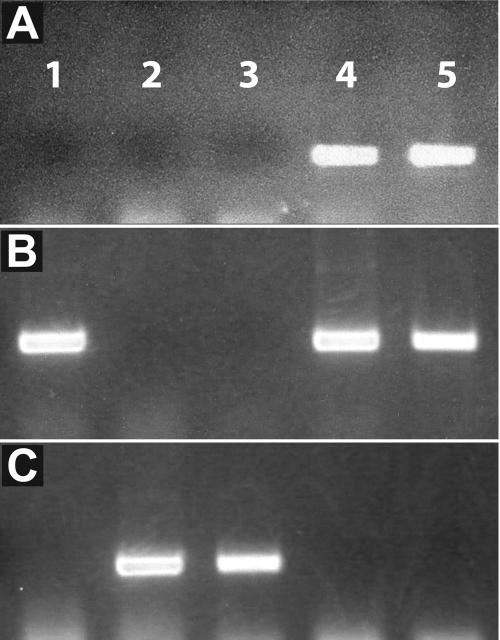
Could CO itself, as RNA or protein, be the phloem-mobile floral stimulant? The presence of RNA and peptides in the phloem sap is well documented (Fisher et al., 1992; Kuhn et al., 1997; Marentes and Grusak, 1998; Xoconostle-Cazares et al., 1999; Kim et al., 2001; Hoffmann-Benning et al., 2002). In tomato (Lycopersicon esculentum), RNA encoding a transcription factor that promotes compound leaf development was identified in tomato scions coincidently with graft transmission of the compound leaf morphology, suggesting that the RNA itself may be the mobile signal (Kim et al., 2001). To test whether CO RNA from the GAS:CO gene was similarly entering the co-1 scion, RNA was extracted from vegetative and inflorescence tissues of both a GAS:CO stock and its co-1 scion. RT-PCR analysis with oligonucleotide primers specific for GAS:CO transcripts did not produce a band from co-1 scion tissues but gave a robust signal from mature leaf tissue of the GAS:CO stock (Fig. 2A). A robust signal was also obtained from inflorescence tissue from an axillary shoot of the stock plant since the GAS promoter is active in the vascular elements of sepals and petals (Haritatos et al., 2000). RT-PCR analysis with oligonucleotides specific for wild-type CO demonstrated the presence of wild-type transcripts only in stock portions (Fig. 2B), and oligonucleotides specific to the co-1 allele demonstrated the presence of mutant transcripts only in scion portions (Fig. 2C). Since the grafted plants were grown under short-day conditions, these findings demonstrate the RT-PCR assay was sensitive enough to detect CO transcript present at levels insufficient to trigger the transition to flowering. Overall, our results suggest that neither GAS:CO nor CO transcripts migrated from stock to scion. However, we cannot exclude the possibility that transcripts transiently passed from stock to scion immediately after the graft junction healed and were subsequently degraded after the morphogenetic events of flowering were initiated.
Figure 2.
RT-PCR analysis of CO mRNA distribution. Total RNA was extracted from a wild-type plant (lane 1) and different tissues of a co-1 scion grafted to a GAS:CO stock plant. Lane 2, Homozygous co-1 scion inflorescence; lane 3, homozygous co-1 scion mature leaf; lane 4, GAS:CO stock axillary inflorescence; lane 5, GAS:CO stock mature leaf. A, RT-PCR with a primer pair specific for GAS:CO transcripts. B, RT-PCR with a primer pair specific for wild-type CO transcripts. C, RT-PCR with a primer pair specific for co-1 allele transcripts.
Proteins can also move cell-to-cell via plasmodesmata and traffic long distances in the phloem (Fisher et al., 1992; Kuhn et al., 1997; Marentes and Grusak, 1998; Hoffmann-Benning et al., 2002). Several transcription factors, despite predominant nuclear localization, are present in the cytoplasm and can move between cells through plasmodesmata (Sessions et al., 2000; Nakajima et al., 2001; Kim et al., 2002; Wu et al., 2003). These proteins may gate plasmodesmata since they exceed the generally accepted upper size exclusion limit of 50 kD for plasmodesmata (Oparka et al., 1999; Crawford and Zambryski, 2000).
Attempts to detect CO and monitor potential movement by virtue of the amino-terminal T7 epitope or a carboxy-terminal green fluorescent protein (GFP) tag were not successful, presumably due to the previously established minimal accumulation of protein (Suarez-Lopez et al., 2001). Notwithstanding, plants expressing GAS:T7CO-GFP demonstrated accelerated flowering equivalent to GAS:CO and GAS:T7CO plants (9.6 ± 1.6 leaves; n = 10), demonstrating that these CO fusion proteins were functional.
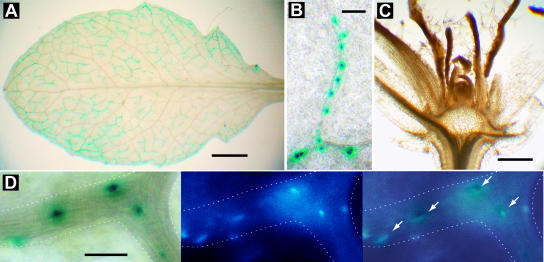
In an alternative approach to detect CO, the GUS enzyme (encoded by uidA) was fused to the T7CO carboxy terminus. When this fusion gene was expressed from the GAS promoter, GUS activity was limited to the companion cells of minor veins and localized predominantly to nuclei (Fig. 3, A and B) as expected and demonstrated in epidermal cells expressing CO fusions from a strong double cauliflower mosaic virus (CaMV) 35S promoter (Robson et al., 2001). No GUS activity was detected at the vegetative apex (Fig. 3C), indicating that the fusion protein did not enter the translocation stream and that there was no spurious expression from the GAS promoter. These expression patterns were observed in 15 independent transformants.
Figure 3.
X-glcA staining in GAS:T7CO-GUS plants demonstrating promoter specificity. A, Expression is confined to the minor veins of mature leaves. Note absence of staining in the midrib and petiole. X-glcA staining was for 24 h in the presence of 0.1 mm of each potassium ferricyanide and potassium ferrocyanide; scale bar = 0.5 mm. B, T7CO-GUS fusion protein localized to the nuclei of companion cells in minor veins. Pictured is a blind-ending vein. X-glcA staining was for 24 h in the presence of 2.0 mm of each potassium ferricyanide and potassium ferrocyanide; scale bar = 0.05 mm. C, Absence of X-glcA staining in the vegetative body and apical meristem, demonstrating the absence of gene expression from GAS promoter in these regions; scale bar = 1 mm. B and C are from the same stained plant, while A is a sibling grown in the same pot. These staining patterns are characteristic of those observed in 15 independent transformants. D, Overlay (right) of X-glcA staining (left) with DAPI-stained nuclei (center) of a branched minor vein (outlined); arrows indicate overlapping X-glcA and DAPI stain. X-glcA staining was for 24 h in the presence of 5.0 mm of each potassium ferricyanide and potassium ferrocyanide; scale bar = 0.02 mm.
In contrast to plants expressing T7CO-GFP fusions in the minor veins, plants expressing the T7CO-GUS fusion did not demonstrate accelerated flowering. Similarly, the T7CO-GUS fusion did not promote accelerated flowering when expressed constitutively from the CaMV 35S promoter. One interpretation of these results is that the GUS fusion protein prevented CO activity. However, CO is tolerant of both amino- and carboxy-terminal fusions as evident from fusions with the T7 epitope and GFP, and GUS activity was clearly retained and localized to nuclei as expected for fusions with transcription factors. Another possible explanation for CO inactivation is that the 112-kD T7CO-GUS fusion protein was too large to gate plasmodesmata between companion cells and sieve elements of the phloem (Itaya et al., 2002), thereby blocking an obligatory transport step.
DISCUSSION
Numerous environmental stimuli influence the transition to reproductive growth in plants (Mouradov et al., 2002). Prominent among these is photoperiod. Leaves perceive photoperiod and respond by generating a phloem-mobile, graft-transmissible signal that Mikhail Chailakyan called florigen in the 1930s (Colasanti and Sundaresan, 2000). Despite decades of effort, the identity of this signal(s) remains elusive. Arabidopsis is a facultative long-day plant, in that flowering is accelerated in long-day photoperiods but will eventually flower under noninductive, short-day conditions. CO stimulates the transition to flowering and is implicated in mediating between the circadian clock and photoperiod perception. Based on previous localization studies (Putterill et al., 1995; Simon et al., 1996), it has not been clear if CO stimulates flowering by acting in meristems where the transition to reproductive growth occurs, or if it acts in the leaves where photoperiod is perceived. Recent fusions between the CO promoter and the uidA reporter gene suggest that CO is expressed in vascular tissues (Takada and Goto, 2003) and support the latter hypothesis.
The objective of this study was to test directly if CO participates in generating a phloem-mobile stimulus or if it instead responds to it. A GAS promoter isolated from C. melo is strongly active in the minor veins of mature leaves, but is not active in larger vascular bundles of leaves, stems, and roots or in vegetative apices (Haritatos et al., 2000). This expression pattern reflects the precept that GAS functions in phloem loading specifically, and not the broader functions of phloem transport (Haritatos et al., 2000), and makes the GAS promoter an ideal tool for analyzing long-distance transport out of leaves to recipient tissues (Ayre et al., 2003). Expressing CO from the GAS promoter resulted in accelerated flowering under short-day, noninductive conditions in the Columbia background and similarly in Columbia plants homozygous for the co-1 allele. This latter finding is significant, as grafting experiments suggest that the phloem-mobile signal may autoinduce in a positive feedback loop (Zeevaart, 1976). Phloem-limited expression in a co-1 background eliminates the possibility that a leaf-derived signal induces CO expression in meristems to trigger the transition from vegetative to reproductive growth. These results indicate strongly that CO participates in generating a phloem-mobile floral stimulant rather than responding to one.
In addition to phloem mobility, graft transmission is a recognized property of florigen. Vegetative scions of co-1 homozygous plants clearly underwent the transition to reproductive growth shortly after grafting to plants expressing CO from the GAS promoter, as evident from the appearance of floral buds coinciding with the clear resumption of scion growth. The grafting procedure itself did not influence flower timing since control grafts between wild-type and co-1 plants averaged an additional 27.6 d of vegetative growth and flowered concurrently with ungrafted plants of the same age. These grafting experiments further demonstrate that CO gene expression in the meristem is not required for floral induction and places CO firmly between photoperiod perception and the phloem-mobile signal that promotes flowering.
CO encodes a zinc-finger transcription factor that directly induces other genes involved in floral induction, such as SUPPRESSOR OF OVEREXPRESSION OF CO 1 and FLOWERING LOCUS T (Kardailsky et al., 1999; Kobayashi et al., 1999; Samach et al., 2000; Takada and Goto, 2003). The presence of RNA and proteins in the phloem is now well documented (Fisher et al., 1992; Marentes and Grusak, 1998; Oparka and Turgeon, 1999; Xoconostle-Cazares et al., 1999; Kim et al., 2001; Hoffmann-Benning et al., 2002), as is the traffic of transcription factors through plasmodesmata, despite significant nuclear localization (Crawford and Zambryski, 2000; Sessions et al., 2000; Nakajima et al., 2001; Kim et al., 2002; Wu et al., 2003). However, after grafting, CO RNA derived from transgenic stock tissues was not detected in co-1 scions when probed by a sensitive PCR technique, suggesting that CO mRNA does not migrate through the phloem.
Attempts to monitor movement of the CO protein by virtue of an amino-terminal T7 epitope or a carboxy-terminal GFP fusion were not successful; however, both accelerated the transition to flowering under noninductive conditions when expressed from the GAS promoter. By contrast, a T7CO-GUS fusion was detected in nuclei of minor vein companion cells when the corresponding gene was expressed from the GAS promoter but precocious flowering did not occur. Similarly, this fusion did not stimulate flowering when expressed constitutively from the CaMV 35S promoter. One explanation for this is that the CO portion of the fusion protein was inactive, either due to aberrant folding or an inability to interact with cellular targets, even though CO is tolerant of both amino- and carboxy-terminal fusions and nuclear localization was not abrogated.
An alternative explanation for the failure of T7CO-GUS to promote flowering is that CO transport is an obligatory part of the photoperiod-controlled signal transduction pathway and that the GUS fusion (112 kD) blocked this transport step by exceeding the size exclusion limit for plasmodesmata between companion cells and sieve elements. Although CO-GFP fusion proteins appear cell autonomous in onion epidermal cells (Robson et al., 2001), the larger size exclusion limit between companion cells and sieve elements (Oparka and Turgeon, 1999) may permit passage of T7CO-GFP while excluding T7CO-GUS. The phloem contains proteins as large as 200 kD, but they are thought to enter in an unfolded state (Balachandran et al., 1997; Kragler et al., 1998), and GUS passage through plasmodesmata is very limited unless fused to a viral movement protein (Waigmann and Zambryski, 1995; Itaya et al., 1997). Creating CO chimeras with other high molecular mass proteins will help resolve if T7CO-GUS fails to induce flowering because of blocked transport or misfolding.
Support for an obligatory transport step is provided by experiments in potato (Solanum tuberosum), where CO regulates the transition to reproductive growth by inhibiting tuberization rather than inducing flowering. When Arabidopsis CO is expressed from the CaMV 35S promoter in scions of grafted potatoes, it inhibits tuberization in stocks, indicating that CO, or a CO-derived signal, is transported to stolons. Remarkably, CO expression directly in the stock does not similarly inhibit tuberization, indicating that the CO-induced signaling must originate in aerial tissues, presumably leaves (Martinez-Garcia et al., 2002). One interpretation of these results, when considered with our findings that neither GAS:T7CO-GUS or 35S:T7CO-GUS accelerated flowering, is that CO itself, rather than a low molecular mass compound, is normally transported and is modified to an active state during this transport process.
MATERIALS AND METHODS
Constructs and Plant Growth
Total RNA was isolated from Arabidopsis ecotype Columbia (Col-1) using Trizol (Invitrogen, Carlsbad, CA) and reverse transcribed using an oligo(dT) primer and Superscript RT II (Invitrogen) according to the manufacturer's instructions. CO cDNA was PCR amplified with LA Taq (TaKaRa; distributed by Fisher Scientific, Pittsburgh) as described by the manufacturer, with forward oligonucleotide 5′-ATGAAGGTACCAACAATGGTTAAACAAGAGAGTAACGAC-3′ and reverse oligonucleotide 5′-GTACTCGAGCTCAGAATGAAGGAACAATC-3′. This clone was then used to create T7CO cDNA using PCR with forward oligonucleotide 5′-ATGAAGGTACCAACAATGGCTAGCATGACTGGTGGACAGCAAATGGGTATGTTGAAACAAGAGAGTAACGAC-3′ and reverse oligonucleotide 5′-CAATTGAGCTCAAGCCCGGGCGAATGAAGGAACAATCCCATATCC-3′. T7CO encodes the 11-amino acid T7 epitope (Novagen, Madison, WI) upstream of the CO open reading frame and has an XmaI site upstream and a SacI site downstream of the stop codon, such that it can be cloned with or without a fused gene. To create a GFP fusion, a sGFP(S65T) (Chiu et al., 1996) cDNA was amplified with forward primer 5′-TCATCCCCGGGCTGCTGCAGCTGCCGCGGCTGCCATGGTGAGCAAGGGCGAGG-3′ and reverse primer 5′-TTCTAGAGCTCACTTGTACAGCTCGTCCATGCC-3′ to introduce sequences encoding eight Ala residues and an appropriately spaced XmaI site for in-frame fusions with T7CO. The GAS promoter used in these studies was previously described (Ayre et al., 2003), and the CaMV 35S promoter was PCR amplified from the CaMV 35S promoter cassette vector of the pGreen series (Hellens et al., 2000) with forward oligonucleotide 5′-CGATACTCTAGACCTACTCCAAAAATGTCAAAGATAC-3′ and reverse oligonucleotide 5′-TAGAAAGGTACCGCTGTCCTCTCCAAATGAAATG-3′. Expression cassettes were assembled in a pUC19 intermediate: promoters as XbaI-KpnI fragments, CO and T7CO as KpnI-SacI fragments, and 8xAla-sGFP as an XmaI-SacI fragment. Cassettes were then subcloned into pGPTV-BAR (Becker et al., 1992) as XbaI-SacI fragments, or as XbaI-XmaI fragments to create in-frame fusions with uidA (encoding the GUS enzyme). Transgenic Arabidopsis plants were generated by the floral dip procedure (Clough and Bent, 1998) with Agrobacterium tumefaciens strain GV3101 mp90 and selected on potting media by misting with glufosinate ammonium (10 mg/L). Plants were germinated and grown under cool-white fluorescent bulbs delivering 80 μmol photons m−2 s−1 at potting mixture surface for 10 h per 24 h cycle. Ei-2 plants (Nottingham Arabidopsis Stock Centre no. N1124) were a gift from Mikhail Nasrallah and were illuminated for 14 h per day.
GUS enzyme activity was localized by vacuum infiltrating tissues with X-glcA solution (1 mm 5-Bromo-4-chloro-3-indolyl β-d-glucuronide cyclohexylamine salt, 50 mm potassium phosphate, pH 7.0, 0.1% Triton X-100), incubating at 37°C for 24 h, and clearing with 70% ethanol. X-glcA solution was supplemented with potassium ferricyanide and potassium ferrocyanide as indicated. Fifty-micrometer sections through the vegetative body of X-glcA-stained plants were obtained with a McIlwain Tissue Chopper (Brinkman, Westbury, NY). X-glcA staining and GFP fluorescence were observed with a Nikon (Lewisville, TX) epifluorescent microscope equipped with a Sony (Park Ridge, NJ) DSC-F707 camera. For DAPI (4′,6-diamidino-2-phenyl-indole; Sigma, St. Louis) staining of nuclei previously stained with X-glcA, we found it necessary to first remove the precipitated X-glcA product by incubating overnight in dimethyl formamide (Sigma). Tissues were then stained with DAPI (1 μg/mL) and visualized under UV light using UV-2E/C filter from Nikon.
Grafting and RNA Analysis
For the GAS:CO stocks, a single T1 generation GAS:CO plant that flowered at the nine-leaf stage was allowed to self-pollinate, and the segregating T2 population plants harboring the transgene were selected with glufosinate ammonium. Of these transgenic plants, some flowered very early, presumably because they were homozygous (Putterill et al., 1995), and were discarded because they were too small to graft. Wild-type, homozygous co-1 and hemizygous GAS:CO plants 32 to 56 d old were used for grafting. The girth of the rosette stem was equivalent among plants at this age, which was important for successful grafting. The corner of a double-edged razor blade was cut with heavy paper shears to create a wedge-shaped blade that was inserted into an X-Acto knife handle (Hunt, Philadelphia). Cutting the blade produced a nearly 90° curl from base to tip that facilitated making perpendicular cuts through the vegetative stem while the plants were in pots. To prepare the scion, the plant was cut just below the rosette, and all leaves except those less than 6 mm long were excised as close as possible to the stem. A fresh cut was then made through the stem, approximately halfway along its length. The blunt end of a fine stainless steel pin (10 mm × 0.1 mm; Fine Science Tools, Foster City, CA) was bent to form a 2-mm hook, and the sharp end was driven through the scion from the top, avoiding the youngest tissues, to emerge near the center of the cut surface. The prepared scion was placed in distilled water to prevent drying. For the stock, a fresh blade was prepared and the stem of a second plant was cut with a single smooth stroke approximately halfway along its length, leaving behind approximately six mature true leaves (range four to eight). The scion was then secured to the stock by driving the pin through both until the hook was firmly seated against the scion with tight contact between the cut surfaces and alignment of the vascular strands. The grafted plants were placed in a tightly sealed, clear plastic bag and returned to the growth chamber. Axillary shoots were pinched off with Dumont 5 forceps (Fine Science Tools) every 3 d to maintain scion apical dominance. Due to the humid, protected environment, scions frequently remained healthy, and leaf expansion continued among unsuccessful grafts. After 10 d, plants were hardened off, at which point successful grafts became evident, and unsuccessfully fused scions rapidly deteriorated. Plants were subsequently scored for visible flowers daily.
Total RNA was isolated from 30 mg of mature leaves and inflorescences of both GAS:CO stocks and co-1 scions, treated with RQ1 DNAse (Promega, Madison, WI) according to the manufacturer's instructions, and reverse transcribed as described above. Oligonucleotide GAS5UTRF (5′-GTTGTTTCAAAAGCACCAAAGTCAATAG-3′) is specific to the GAS-derived 5′ untranslated region of GAS:CO, CO5UTRF (5′-GCTCCCACACCATCAAACTTACTACATCTG-3′) is specific to the CO 5′ untranslated region, CO285R (5′-CTCGCTGATGGCGTCTAGCAAG-3′) is a reverse oligonucleotide initiating 307 nucleotides downstream of the CO start codon and is specific to the wild-type allele, and co1283R (5′-TTGGAACTCGCTGATGGCGTGG-3′) is a reverse oligonucleotide specific to the co-1 allele, which contains a nine-nucleotide deletion (Putterill et al., 1995). GAS:CO transcripts were detected with GAS5UTRF and CO285R, and co-1 transcripts were detected with CO5UTRF and co1283R, with 45 cycles of melting at 94°C for 15 s, annealing at 65°C for 15 s, and extension at 72°C for 30 s. CO transcripts were detected with CO5UTRF and CO285R with 45 cycles of melting at 94°C for 15 s and combined annealing and extension at 67°C for 30 s. Oligonucleotides were used at 100 nm; TaKaRa supplied ExTaq polymerase, dNTP mix, and buffer.
Acknowledgments
We thank Kent D. Chapman, Rebecca Dickstein, and Harris D. Schwark for use of equipment; Mikhail Nasrallah for the gift of ecotype Ei-2 seeds; and Roisin C. McGarry and for helpful discussions.
This work was supported by the U.S. Department of Agriculture/Cooperative State Research, Education and Extension Service/National Research Initiative Competitive Grants Program (USDA/CSREES/NRICGP; grant no. 2001–35318–10893 to R.T.) and by the National Science Foundation (grant nos. IBN–0110638 to R.T. and IBN–0344088 to B.G.A.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.040592.
References
- Ayre BG, Keller F, Turgeon R (2003) Symplastic continuity between companion cells and the translocation stream: long-distance transport is controlled by retention and retrieval mechanisms in the phloem. Plant Physiol 131: 1518–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran S, Xiang Y, Schobert C, Thompson GA, Lucas WJ (1997) Phloem sap proteins from Cucurbita maxima and Ricinus communis have the capacity to traffic cell to cell through plasmodesmata. Proc Natl Acad Sci USA 94: 14150–14155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D, Kemper E, Schell J, Masterson R (1992) New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol Biol 20: 1195–1197 [DOI] [PubMed] [Google Scholar]
- Chiu WL, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6: 325–330 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Colasanti J, Sundaresan V (2000) ‘Florigen’ enters the molecular age: long-distance signals that cause plants to flower. Trends Biochem Sci 25: 236–240 [DOI] [PubMed] [Google Scholar]
- Corbesier L, Gadisseur I, Silvestre G, Jacqmard A, Bernier G (1996) Design in Arabidopsis thaliana of a synchronous system of floral induction by one long day. Plant J 9: 947–952 [DOI] [PubMed] [Google Scholar]
- Crawford KM, Zambryski PC (2000) Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr Biol 10: 1032–1040 [DOI] [PubMed] [Google Scholar]
- Fisher DB, Wu Y, Ku MSB (1992) Turnover of soluble-proteins in the wheat sieve tube. Plant Physiol 100: 1433–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haritatos E, Ayre BG, Turgeon R (2000) Identification of phloem involved in assimilate loading in leaves by the activity of the galactinol synthase promoter. Plant Physiol 123: 929–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42: 819–832 [DOI] [PubMed] [Google Scholar]
- Hoffmann-Benning S, Gage DA, McIntosh L, Kende H, Zeevaart JA (2002) Comparison of peptides in the phloem sap of flowering and non-flowering Perilla and lupine plants using microbore HPLC followed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Planta 216: 140–147 [DOI] [PubMed] [Google Scholar]
- Itaya A, Hickman H, Bao Y, Nelson R, Ding B (1997) Cell-to-cell trafficking of cucumber mosaic virus movement protein:green fluorescent protein fusion produced by biolistic gene bombardment in tobacco. Plant J 12: 1223–1230 [Google Scholar]
- Itaya A, Ma F, Qi Y, Matsuda Y, Zhu Y, Liang G, Ding B (2002) Plasmodesma-mediated selective protein traffic between “symplasmically isolated” cells probed by a viral movement protein. Plant Cell 14: 2071–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D (1999) Activation tagging of the floral inducer FT. Science 286: 1962–1965 [DOI] [PubMed] [Google Scholar]
- Kevei E, Nagy F (2003) Phytochrome controlled signalling cascades in higher plants. Physiol Plant 117: 305–313 [DOI] [PubMed] [Google Scholar]
- Kim JY, Yuan Z, Cilia M, Khalfan-Jagani Z, Jackson D (2002) Intercellular trafficking of a KNOTTED1 green fluorescent protein fusion in the leaf and shoot meristem of Arabidopsis. Proc Natl Acad Sci USA 99: 4103–4108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Canio W, Kessler S, Sinha N (2001) Developmental changes due to long-distance movement of a homeobox fusion transcript in tomato. Science 293: 287–289 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962 [DOI] [PubMed] [Google Scholar]
- Kragler F, Monzer J, Shash K, Xoconostle-Cazares B, Lucas WJ (1998) Cell-to-cell transport of proteins: requirement for unfolding and characterization of binding to a putative plasmodesmal receptor. Plant J 15: 367–381 [Google Scholar]
- Kuhn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB (1997) Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275: 1298–1300 [DOI] [PubMed] [Google Scholar]
- Marentes E, Grusak MA (1998) Mass determination of low-molecular-weight proteins in phloem sap using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Exp Bot 49: 903–911 [Google Scholar]
- Martinez-Garcia JF, Virgos-Soler A, Prat S (2002) Control of photoperiod-regulated tuberization in potato by the Arabidopsis flowering-time gene CONSTANS. Proc Natl Acad Sci USA 99: 15211–15216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CR (2001) Circadian rhythms in plants. Annu Rev Plant Physiol Plant Mol Biol 52: 139–162 [DOI] [PubMed] [Google Scholar]
- Mouradov A, Cremer F, Coupland G (2002) Control of flowering time: interacting pathways as a basis for diversity. Plant Cell (Suppl) 14: S111–S130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Sena G, Nawy T, Benfey PN (2001) Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413: 307–311 [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Roberts AG, Boevink P, Santa Cruz S, Roberts I, Pradel KS, Imlau A, Kotlizky G, Sauer N, Epel B (1999) Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 97: 743–754 [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Turgeon R (1999) Sieve elements and companion cells—traffic control centers of the phloem. Plant Cell 11: 739–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G (1995) The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80: 847–857 [DOI] [PubMed] [Google Scholar]
- Robson F, Costa MM, Hepworth SR, Vizir I, Pineiro M, Reeves PH, Putterill J, Coupland G (2001) Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J 28: 619–631 [DOI] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288: 1613–1616 [DOI] [PubMed] [Google Scholar]
- Sessions A, Yanofsky MF, Weigel D (2000) Cell-cell signaling and movement by the floral transcription factors LEAFY and APETALA1. Science 289: 779–782 [DOI] [PubMed] [Google Scholar]
- Simon R, Igeno MI, Coupland G (1996) Activation of floral meristem identity genes in Arabidopsis. Nature 384: 59–62 [DOI] [PubMed] [Google Scholar]
- Suarez-Lopez P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410: 1116–1120 [DOI] [PubMed] [Google Scholar]
- Takada S, Goto K (2003) TERMINAL FLOWER2, an Arabidopsis homolog of HETEROCHROMATIN PROTEIN1, counteracts the activation of FLOWERING LOCUS T by CONSTANS in the vascular tissues of leaves to regulate flowering time. Plant Cell 15: 2856–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waigmann E, Zambryski P (1995) Tobacco mosaic virus movement protein-mediated protein transport between trichome cells. Plant Cell 7: 2069–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Dinneny JR, Crawford KM, Rhee Y, Citovsky V, Zambryski PC, Weigel D (2003) Modes of intercellular transcription factor movement in the Arabidopsis apex. Development 130: 3735–3745 [DOI] [PubMed] [Google Scholar]
- Xoconostle-Cazares B, Yu X, Ruiz-Medrano R, Wang HL, Monzer J, Yoo BC, McFarland KC, Franceschi VR, Lucas WJ (1999) Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science 283: 94–98 [DOI] [PubMed] [Google Scholar]
- Zeevaart JAD (1976) Physiology of flower formation. Annu Rev Plant Physiol 27: 321–348 [Google Scholar]