Abstract
Growth of elongating primary roots of maize (Zea mays) seedlings was approximately 50% inhibited after 48 h in aerated nutrient solution under water deficit induced by polyethylene glycol 6000 at −0.5 MPa water potential. Proton flux along the root elongation zone was assayed by high resolution analyses of images of acid diffusion around roots contacted for 5 min with pH indicator gel. Profiles of root segmental elongation correlated qualitatively and quantitatively (r2 = 0.74) with proton flux along the surface of the elongation zone from water-deficit and control treatments. Proton flux and segmental elongation in roots under water deficit were remarkably well maintained in the region 0 to 3 mm behind the root tip and were inhibited from 3 to 10 mm behind the tip. Associated changes in apoplastic pH inside epidermal cell walls were measured in three defined regions along the root elongation zone by confocal laser scanning microscopy using a ratiometric method. Finally, external acidification of roots was shown to specifically induce a partial reversal of growth inhibition by water deficit in the central region of the elongation zone. These new findings, plus evidence in the literature concerning increases induced by acid pH in wall-extensibility parameters, lead us to propose that the apparently adaptive maintenance of growth 0 to 3 mm behind the tip in maize primary roots under water deficit and the associated inhibition of growth further behind the tip are related to spatially variable changes in proton pumping into expanding cell walls.
Root elongation is dependent on massive elongation of daughter cells produced by the division of meristematic cells in the root tip and is essential for continuing uptake of mineral nutrients and water from the rhizosphere. Under water-deficit conditions plants become water stressed, for example, external water availability falls below that required for maximum rates of growth and transpiration. Root and leaf growth are then typically inhibited (Neumann, 1995). Segmental analysis of growth distribution profiles in maize (Zea mays) seedling primary roots has revealed a region of cell elongation behind the tip that can be divided into a zone of accelerating growth rate adjacent to the tip, followed by a transition zone in which growth rate peaks and a zone of decelerating growth rate. These growth profiles can be altered in a spatially selective manner by a variety of environmental stresses (Silk, 1992). For example, while growth in the accelerating zone of maize roots was remarkably maintained under water deficits, growth in adjacent zones further behind the tip was inhibited (Sharp et al., 1988; Pritchard, 1994). The possible involvement of pH as a key regulatory process involved in the respective maintenance and inhibition of cell elongation in adjacent root segments responding to the same water-deficit conditions is the subject of this article.
Processes regulating the wall loosening, water uptake, and solute uptake that are involved in maintaining cell expansion have each been associated with pH regulation in cell walls and/or cytoplasm (Rayle and Cleland, 1992; Taylor and Bloom, 1998; Peters and Felle, 1999; Tournaire-Roux et al., 2003). For example, according to the acid growth hypothesis of Rayle and Cleland (1992), maintenance of sufficiently acid pH in the cell wall facilitates turgor-driven expansion by directly loosening the polysaccharide matrix or by optimizing the activity of cell wall proteins and enzymes associated with loosening (Cosgrove, 1997; Tanimoto et al., 2000; Wu and Cosgrove, 2000). Recent findings that cell wall acidification is associated with auxin effects on seed embryo growth (Rober-Kleber et al., 2003) and with early growth responses to gravistimulation of root cells (Taylor et al., 1996; Fasano et al., 2001) support the existence of a close relationship between wall acidification and cell expansion. Similarly, the spatial profile of growth rate along the elongation zone of maize roots has been shown to coincide with the spatial profile of root-surface acidification (Pilet et al., 1983; Peters and Felle, 1999). Changes in cell wall acidification profiles could result from regulated changes in the activity of outward proton pumping H+ ATPase in the plasma membrane. They could also be influenced by wall accumulation of respiratory carbon dioxide, proton exchanges involved in solute transport, and/or enzyme regulated changes in the methylation state of cell wall pectins. The possibility that the intriguing maintenance and inhibition of growth in adjacent tissues of roots responding to water deficit might be regulated by changes in profiles of wall acidification does not appear to have been previously investigated.
Interestingly, the inhibition of maize leaf growth under water deficit has been related to an inhibition of cell wall acidification (Van Volkenburgh and Boyer, 1985; Bogoslavsky and Neumann, 1998). By contrast, acidification capacity did not change significantly during inhibition of leaf or root growth by salinity (Zidan et al., 1990; Neves-Piestun and Bernstein, 2001). However, the resolution in these studies did not allow any evaluation of the spatial distribution of wall acidification, and sodium-proton exchange processes may have affected the findings. Imposition of water deficits by nonionic osmoticum should minimize such complications. Indeed, water deficit caused by addition of 100 mm mannitol for 2 h resulted in decreases in root-surface acidification in the maize root elongation zone, as shown by measurements of proton flux at a single point with a noninvasive vibrating probe technique (Shabala and Newman, 1998). These changes may have been associated with initial adaptation to water stress. By contrast, exposure of maize roots to polyethylene glycol (PEG) for up to 48 h resulted in a small degree of membrane hyperpolarization, as assayed by puncturing individual cells in the elongation zone with microelectrodes. This suggested a possible increase in the activity of proton-pumping ATPase (Ober and Sharp, 2003). Growth inhibition under water deficit therefore appeared to be associated with an inhibition of wall acidification in some cases and maintenance in others. Comparisons of the effects of water deficit on profiles of elongation and acidification in roots of whole plants might throw additional light on mechanisms of root growth regulation under water deficit. The specific aims of this investigation were the following: (1) to determine the effects of 48 h of growth-inhibitory water deficit on profiles of segmental elongation, proton flux, and apoplastic pH in the developing primary roots of whole maize seedlings; (2) to investigate possible correlations between spatial effects on proton flux and segmental elongation rates; and (3) to determine effects of exogenous acidification on the growth profiles of roots under water deficit.
RESULTS
Spatially Variable Growth Responses to Water Deficit
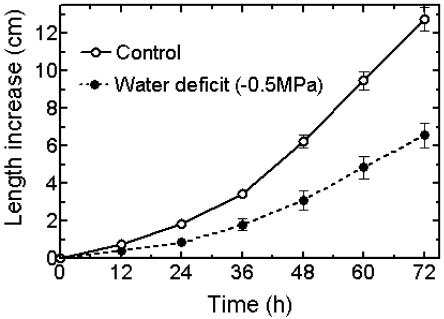
The imposition of water deficit by nonpenetrating PEG at −0.5 MPa water potential reduced the elongation of the primary roots of germinating maize seedlings, although they retained a white and healthy appearance. Exponential length increases over time for seedling roots in control and water-deficit treatments became quasi linear after 48 h (Fig. 1). Roots were therefore used at around this time for assays of relative segmental elongation rates (RSERs) and acidification.
Figure 1.
Kinetics of root growth inhibition under water deficit. Maize seedlings with primary roots approximately 1.5 cm in length were transferred at 0 h to aerated nutrient solution in an environmentally controlled growth chamber with a 12-h photoperiod. Water deficit was imposed by stepwise increases in PEG 6000 concentration to a final water potential of −0.5 MPa after 5 h. Means ± se, n = 17 for each treatment.
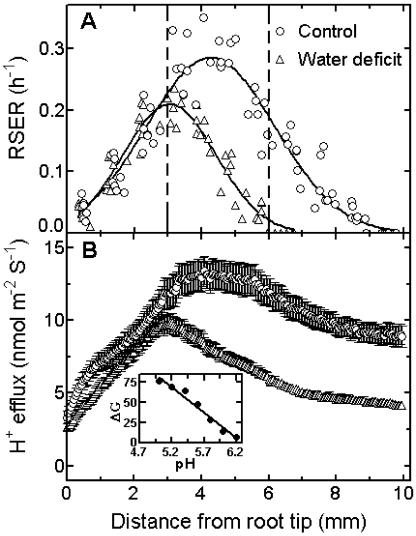
RSERs were determined along the zone of elongation at the root apex. In control roots, RSERs increased progressively in the zone of accelerating elongation situated adjacent to the tip and reached a maximum at approximately 4 mm from the tip (Fig. 2A). The zone 3 to 6 mm from the apex represented a zone of transition from accelerating to decelerating elongation. Rates of elongation then decreased progressively from 6 to 10 mm after which they became negligible. The overall length of the root elongation zone was therefore around 10 mm. The overall length of the elongation zone was shortened from 10 mm to 7 mm, and the maximum growth rate (which was comparatively reduced) occurred at 3 mm behind the root tip in plants held under water deficit for 48 h. Moreover, relative elongation rates in the segments located between 3 mm and 9 mm from the tip were all reduced as compared with control roots. Note, however, that RSERs in the 0 to 3 mm zone immediately behind the root tip of plants under water deficit were equivalent to control values.
Figure 2.
Spatial variation in effects of water deficit on RSER and proton efflux along the root elongation zone. A, Roots grown ±PEG 6000 at −0.5 MPa water potential for 2 d were marked, equilibrated, and then photographed with a 30-min interval. The RSER in each marked segment was determined as RSER = (lnLt − lnL0) L0−1 t−1. L0 is the initial distance between marks and Lt the final distance after time t (n = 8 to 9 roots). The lines represent fitted curves for Gaussian distribution (R2 = 0.90 for control and R2 = 0.91 for water-deficit treatment). The vertical dashed lines separate zones of accelerating, transition, and decelerating growth in control roots. B, Roots from control and 2-d water-deficit treatments were embedded into pH indicator gel for 5 min. H+ efflux was calculated using grayscale images of gel pH profiles, as in “Materials and Methods.” Values are means ± se (n = 15). Inset shows the linear calibration for gel pH attained using buffers ranging from pH 5.0 to 6.2. The calibration equation is pH = 6.2826 − 0.0154511 × ΔG (R2 = 0.97, n = 4). ΔG represents the difference between the grayscale values measured using indicator gel before and after buffer addition.
Profiles of Acidification
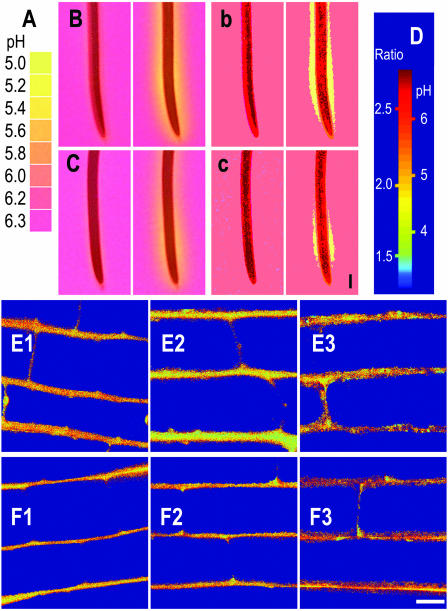
We next determined whether the different growth responses to water stress of adjacent regions in the root elongation zone could be related to differences in capacity to acidify the expanding cell walls. Spatial distribution of root surface acidification in control and water-stressed roots was measured by contacting the roots for 5 min with agar gel containing bromocresol purple as a pH indicator. The gel was initially at pH 6.3, and acid secretion from the root surface caused the gel to turn yellow. Figure 3, B and C, shows the typical zone of acidification that developed in roots from control and water-deficit treatments, respectively, after contact with the indicator gel. Similar images were obtained when roots under water deficit were transferred to indicator gel containing mannitol at −0.5 MPa (data not shown). The adjacent sections (Fig. 3, b and c) show posterized versions of the original images. Posterization is a process by which pixels in an image are grouped according to intensity. The inhibitory effect of water stress on the length and width of the intense yellow zone of acidification was clearly revealed after posterization (compare Fig. 3, b and c). Similar patterns of acidification were observed if roots were held vertical rather than horizontal during the 5-min gel assay. In addition, short-term exposures to PEG or air drying also resulted in inhibition of acidification (data not shown).
Figure 3.
Effect of water stress on distribution of acidification along root elongation zones. A, Color bar based on images of indicator gel with buffers at indicated pH. B, Images of root tips from control seedlings 0 and 5 min after contact with agar gel containing bromocresol purple pH indicator at pH 6.3. Yellow zones indicate acidification. b, Digital posterization of original root tip images reveals regions of intense acidification. C and c, Equivalent images before and after posterization for root tips of seedlings after 2-d water-deficit treatment. Vertical scale bar in c represents 1 mm. D, Color bar shows the range of pixel ratios in relationship to pH as determined by confocal laser scanning microscopy. E1, E2, and E3 show pseudo color images of pH distribution in epidermal cell walls of control plants at 2 to 3, 4 to 5, and 7 to 8 mm from the root tips. F1, F2, and F3 represent equivalent images from roots after water deficit for 2 d. Horizontal bar in F3 = 10 μm.
Differences in measured pixel intensity of grayscale conversions of the original root images at 0 and 5 min after contact with indicator gel were used to quantify proton efflux along the surface of the root elongation zone. The patterns of mean proton flux in the region of accelerating elongation up to 3 mm from the root apex increased similarly in control roots and roots under water deficit, although flux at 0 to 1 mm from the tip was somewhat reduced (Fig. 2B). Proton flux along the 3 to 9 mm region of roots under water deficit was clearly reduced by comparison with control roots. The correlation coefficient determined for the regression line between RSERs and proton fluxes 0 to 9 mm from the root tip of control plants or 0 to 6 mm from the tip of plants under water deficit was r2 = 0.74 in each case.
To test whether changes in proton efflux from root surfaces after 2 d of water deficit coincided with pH changes inside expanding epidermal cell walls, we used confocal laser scanning microscopy to estimate apoplastic pH. The pH was measured 9 μm below the wall surface in epidermal cells at positions 2 to 3 mm, 4 to 5 mm, and 7 to 8 mm from the root apex. These positions approximated to accelerating, transition, and decelerating zones of elongation in control roots (Fig. 2A).
Figure 4 shows in vitro and in situ calibrations of fluorescence ratios against buffered pH. In situ calibrations were performed prior to each set of in vivo determinations of apoplastic pH. Figure 3D shows a pseudo color scale for cell wall pH based on in situ calibration. The images in Fig. 3, E1 to E3, are in vivo pH color maps of epidermal cell walls at 2 to 3, 4 to 5, and 7 to 8 mm from the root tip. In Figure 3, F1 to F3 represent equivalent images from a root assayed after water deficit for 2 d. The color ranges in E1 and F1 do not appear to differ by much. However, the cell walls in F2 and F3 from further behind the tip of a root after water deficit show more brown coloration (i.e. appear to be less acidic) than the control cell walls in E2 and E3. This is confirmed in Table I, which shows the derived values of mean wall pH at indicated positions along the root elongation zones of several roots from control and water-deficit treatments. Thus, the pH of 5.14 at 2 to 3 mm from the tip of roots under water deficit coincided with pH 5.15 in the equivalent region of control roots. Cell wall pH was therefore well maintained, along with proton flux and growth in the accelerating region of roots under water deficit. By contrast, increases in wall pH of roots under water deficit were measured 4 to 5 mm from the tip (+0.25 pH units), and larger increases were measured 7 to 8 mm from the tip (+0.39 pH units). Thus, wall pH in epidermal cells of roots under water deficit became less acid in the regions of the elongation zone where proton flux and segmental elongation were also reduced.
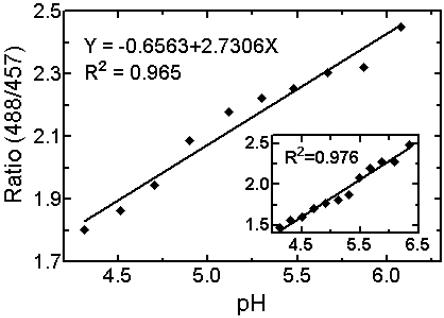
Figure 4.
In situ calibration of pH in root-tip cell walls. Root segments were incubated for 30 min with 10 μm DM-NERF in 100 mm succinic acid-NaOH buffers at pHs ranging from 3.9 to 6.3. Solutions also contained 1 mm KCN and 0.5 mm sodium orthovanadate. The means of the measured pixel ratios (488 nm/457 nm) in the whole area of epidermal cell walls, as seen under confocal laser scanning microscopy, were then plotted against the pH of the buffers. For prior in vitro calibration (inset), filter paper strips were saturated with 10 μm DM-NERF in succinic acid-NaOH buffers at appropriate pH. Similar in situ and in vitro calibrations were obtained in five independent experiments.
Table I.
Water deficit results in spatially variable increases in apoplastic pH in epidermal cell walls along the root elongation zone
| Distance from Root Tip (mm)
|
|||
|---|---|---|---|
| 2 to 3 | 4 to 5 | 7 to 8 | |
| Apoplastic pH | |||
| Control | 5.15 ± 0.10 | 4.90 ± 0.12 | 5.10 ± 0.12 |
| Water deficit | 5.14 ± 0.05 | 5.15 ± 0.07 | 5.49 ± 0.10 |
| ΔpH | 0.01 | +0.25 | +0.39 |
The pH was measured by confocal laser scanning microscopy at 9 μm below the surface of epidermal cell walls loaded with DM-NERF fluorescent dye. Means ± se, n = 6.
Effects of Acidification on Root Growth
Since increases in pH and decreases in proton flux correlated well with profiles of root growth inhibition under water deficit, we investigated the possibility that exogenous acidification might partially restore the inhibited growth of roots under water deficit. Preliminary findings indicated that additions to the root medium of 10 μm fusicoccin or 2 mm succinate buffer at pH 4.5 could dramatically accelerate the elongation of roots under water deficit for about 15 min (data not shown).
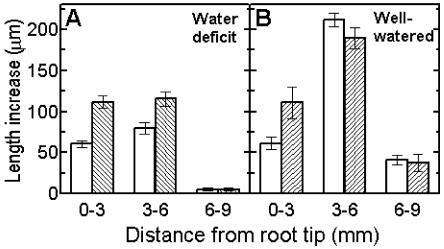
A more detailed study showed that addition of pH 4.5 buffer accelerated overall elongation of roots under water stress from 145 ± 9 μm 15 min−1 to 232 ± 10 μm 15 min−1 (means ± se, n = 20). A smaller growth response to acid, from 314 ± 12 to 338 ± 25 μm 15 min−1, was measured in the faster-growing roots of well-watered plants. The spatial distribution of these effects was also investigated (Fig. 5). The region of accelerating growth at 0 to 3 mm behind the root tip (compare with Fig. 2A) initially elongated at similar rates in roots under water deficit or in well-watered roots, and elongation rates were additionally accelerated to the same extent by acidification to pH 4.5. Clearly, the expansion of relatively unexpanded young cells in this region is unaffected by moderate water deficits, and they have reserves of growth potential that are expressed during exogenous acidification. By contrast, acidification did not further accelerate the already rapid elongation in the region 3 to 6 mm behind the tip of well-watered roots (Fig. 5B). However, acidification did accelerate the inhibited growth of the same 3 to 6 mm region in roots under water deficit (Fig. 5A). Thus, local acidification acted specifically to partially reverse the inhibition of elongation in the region 3 to 6 mm behind the tips of roots under water deficit. Exogenous acidification did not accelerate growth in tissues 6 to 9 mm behind the tips of either well-watered or stressed roots. Thus, the potential for acid-accelerated growth appears to be lost in this region where the root tissues approach full elongation. Additional control experiments indicated that equivalent transfers of well-watered roots or roots under water deficit to solutions buffered to pH 5.5 had little or no effect on elongation (data not shown).
Figure 5.
Spatially variable acceleration of root elongation by acid buffer. A, Profiles of elongation response of roots under water deficit (white columns) to pH 4.5 buffer (gray columns, means ± se, n = 20). B, Responses of well-watered roots to pH 4.5 buffer (means ± se, n = 12).
DISCUSSION
The quasi steady-state inhibition of maize root growth under water deficit was shown to be caused by a selective inhibition of segmental elongation rates along the zone situated 3 to 10 mm behind the root tip. By remarkable contrast, segmental elongation rates in the zone situated 0 to 3 mm behind the tips of roots under water deficit were maintained at control rates. This capacity to maintain some root growth under water-deficit conditions may represent a useful stress-acclimation mechanism. The water deficits in our growth experiments were imposed by gradually adding PEG 6000 to the aerated root medium, and the resultant alterations in root growth profiles were essentially similar to those found in previous investigations in which water deficits were imposed by other means (Sharp et al., 1988; Pritchard, 1994).
Because it does not penetrate plant cell walls and because of its osmotic and mass flow effects on aqueous solutions, PEG 6000 is widely utilized to inhibit water uptake by plants in general and roots in particular (compare with Chazen et al., 1995, and references therein). Verslues et al. (1998) found that supplying extra oxygen partly stimulated the inhibited growth of roots in solutions of PEG 8000 at up to −1.6 MPa water potential. This suggested that their roots were oxygen limited, and we cannot rule out the possibility that the PEG 6000 had a similar effect. However, serious oxygen limitations seem unlikely for several reasons. First, oxygen status measured inside elongating root tip tissues by Verslues et al. (1998) was not reduced by PEG 8000. Second, Mexal et al. (1975), who first suggested that roots in PEG might become oxygen limited, also found that the diffusion coefficient of oxygen was similar in water or PEG 6000 solutions. Third, any physiologically significant oxygen deficiency under water deficit would be expected to inhibit respiration-dependent growth in the metabolically active cells 0 to 3 mm behind the root tip in the same way as in other regions of the elongation zone, but this did not occur. Finally, the accelerating growth kinetics of our roots during 48 h in PEG 6000 at −0.5 MPa water potential (Fig. 1) plus their white, slime-free, and healthy appearance indicated that hypoxia was not a problem.
The main thrust of this investigation was to determine whether the spatially variable profiles of root growth inhibition under water deficit could be related to differences in cell wall acidification. The new findings presented here and several lines of previous evidence appear to support the existence of close and possibly causal relationships between wall acidification and root growth responses to water deficit.
Root Segmental Elongation and Acidification Show Qualitative and Quantitative Correlations
Qualitative correlations between acidification and root growth were indicated by the finding that the length of the zone of intense acidification extending behind the root tip was shortened under water deficit. Because of its relative availability and convenience, the high-resolution method used by us to visualize zones of acidification could prove useful in other aspects of root research. The quantitative correlations between growth and calculated proton flux are dependent on the reliability of the indicator gel-image analysis approach that we used. A comparison of the profiles of surface proton flux determined by us with pH profiles determined by carefully moving microelectrodes along the surface of the maize root elongation zone (Peters and Felle, 1999) reveals close similarities. In both cases, acidification peaked at around 4 mm from the root apex and then declined; in both cases, there appeared to be a small subsidiary peak of enhanced acidification centered at around 1 mm from the apex. In addition, our values for proton flux from the root surface of control plants are of the same order of magnitude as those determined for the elongation zone of maize seedling roots by Shabala and Newman (1998), using the vibrating probe technique. The indicator gel-image analysis approach for determining acidification profiles along the root surface therefore gives results that are consistent with those obtained by other approaches.
Although several recent findings with roots indicate an association between surface acidification and the zone of elongation, at least one early report, using bromocresol purple indicator gel adjusted to pH 5.0, revealed a relative alkalinization of the barley root elongation zone (Wiesenseel et al., 1979). At this starting pH, the gel had a yellow color, and roots placed on the surface of the gel caused a surrounding zone of color change from yellow to red or purple. However, the actual pH values at the surface of the elongation zone, found with pH-sensitive microelectrodes, ranged from pH 5.5 to pH 6.0 (i.e. the root surface still had an acidic pH). The greater degree of acidification observed in maize may be species related but may also reflect technical differences. Thus, relatively fragile barley roots were assayed by Wiesenseel et al. (1979) for 10 min on the surface of indicator gel. In our approach, relatively robust maize roots within indicator gel at pH 6.3 were assayed for only 5 min. Air drying maize roots for 10 min before assay caused reductions in acidification (data not shown), and perhaps the barley roots were affected in this way.
Another technical caveat is that changes in surface distribution of acid mucilages from the root cap could have influenced the measured profiles of surface acidification, and such changes would be unrelated to root growth regulation. However, pH changes induced by water deficit were measured at points within the epidermal cell walls of the root elongation zone using confocal laser scanning microscopy (see below). Moreover, additions of 1 mm KCN, in order to inhibit oxidative phosphorylation, inhibited wall acidification measured in this way within 15 min, even though it would not be expected to affect the pH of mucilage at the root surface (data not shown). Similarly, Peters and Felle (1999) showed that cyanide rapidly dissipated acidification measured along the surface of the maize root elongation zone with pH-sensitive microelectrodes and also inhibited growth. Such findings suggest that wall acidification is an actively ongoing process.
Apoplastic pH Is Altered by Water Deficit
Confocal laser scanning microscope measurements of apoplastic pH in epidermal cells along the root elongation zone revealed profiles of pH change that provided independent support for the observed relationships between proton flux and growth rate distribution. We do not know whether profiles of acidification in subtending cortical cells responded to water deficit in the same way, but changes in epidermal growth would need to be somehow coordinated with similar changes in underlying tissues.
The root cell wall pH values measured by us were similar to those previously measured by others (compare with Taylor et al., 1996; Fasano et al., 2001; Yu et al., 2001). However, a possible artifact that needs to be considered is that our measurements may also have reflected parallel pH responses to gravistimulation. These could be caused by transferring roots to a horizontal position during the assay. For example, pH in the Arabidopsis root cap decreased from 5.5 to 4.6 within 1 min of gravistimulation. However, walls of expanding cells in the upper side (but not the lower side) of the central elongation zone of Arabidopsis only began to show significant decreases in pH about 10 min after gravistimulation (Fasano et al., 2001). Similarly, apoplastic pH in the upper side of the elongation zone of maize seedling roots decreased by around 0.4 pH units after approximately 60 min of gravistimulation (Taylor et al., 1996). In our study, apoplastic pH values measured on the upper side of the elongation zone were increased by water deficit (compare with Table I). Moreover, in order to minimize gravity induced changes in pH, both control and water-stressed maize roots were held horizontal for less than 10 min during the confocal laser assay. The observed inhibitory effects of water deficit on root acidification therefore appear to have occurred independently of gravitropic effects.
Acid pH Accelerates Growth of Roots under Water Deficit
Several reports indicate that pH changes can rapidly cause transient changes in root growth. For example, Edwards and Scott (1974) measured growth responses of excised maize root segments to a range of external pH values. Their data indicated that growth in nonbuffered solutions was stimulated for about 60 min by incubation at pH 5.2 and lower. Similarly, Winch and Pritchard (1999) found that transfer of well-watered maize primary roots from pH 7 to pH 3.4 caused transient increases in local growth rates. The increases occurred primarily in the accelerating region of the elongation zone situated 0 to 4 mm behind the tip. Since profiles of cell turgor pressure were not changed by acid pH treatment but growth was, the authors concluded that the growth stimulatory effect of pH 3.4 buffer resulted from its wall loosening effect. By contrast, Walter et al. (2000) reported that longer term growth of well-watered maize seedling roots in sandy soils was unaffected by external pH values ranging from 4.2 to 8.6. However, the root medium had a low buffer capacity, which may not have sufficiently affected pH inside the cell walls and wall pH may have undergone gradual feedback regulation to reset higher pH values (compare with Felle, 1998). In addition, increases in root growth induced by additional acidification of expanding cell walls may not be sustainable over time because necessary accompanying changes in cellular metabolism, e.g. those required for turgor maintenance or accelerated synthesis of new cell wall and cell membrane material, are not induced.
Our finding that acidification to pH 4.5 accelerated growth in the 0 to 3 mm region behind the tip of well-watered roots is in line with the previous report of Winch and Pritchard (1999). Both endogenous wall acidification and growth in this region of young, rapidly expanding cells were relatively unaffected by water deficit, and additional acidification to pH 4.5 similarly accelerated growth 0 to 3 mm behind the tip of the stressed roots. More importantly, our results show, apparently for the first time, that external acidification to pH 4.5 could partially reverse the specific inhibition by water deficit of elongation in the region 3 to 6 mm behind the tip. Note that under water-deficit conditions, endogenous proton flux in this region was reduced and wall pH became less acid (compare with Fig. 3 and Table I). A causal link between growth inhibition by water deficit and wall acidification is therefore indicated.
The fact that growth in the region 6 to 9 mm behind the root tip of well-watered roots or roots under water deficit was not accelerated by acid pH suggests that the maturing cells in this region lose their ability to respond to additional acidification, possibly as a result of mechanical stiffening by wall polymer cross-linking. Thus, varying degrees of acidification and varying responsiveness of growing cell walls to acidification may be involved in regulating root growth responses to water deficit.
Changes in cell wall pH are most likely to produce rapid changes in root growth rate by directly affecting the mechanical extensibility of wall polysaccharides and/or the activity of wall-loosening proteins such as expansins. For example, Tanimoto et al. (2000) found that viscosity increased and mechanical extensibility decreased as pH in 1-mm cell wall segments from along the elongation zone of pea roots was increased from pH 4 to pH 6 and beyond. The in vivo increases in average cell wall pH measured by us in the regions 4 to 5 mm and 7 to 8 mm behind the tip of roots under water deficit might therefore contribute to decreases in wall extensibility. Conversely, the maintenance of pH 5.14 in the region 0 to 3 mm behind the tip of roots under water deficit could help maintain wall extensibility. In another investigation, Wu et al. (1996) found that a prior 48-h water deficit increased the extensibility of cell walls in the 0 to 5 mm apical region of the maize root elongation zone that were responding to acidification with pH 4.5 buffer. Importantly, the extensibility of more basal tissues at 5 to 10 mm from the tip but treated in the same way was comparatively decreased as a result of water deficit. The responsiveness of cell walls in the apical zone to exogenous expansin proteins was also increased by water deficits, whereas the more basal walls became essentially unresponsive. It therefore seems that in addition to alterations in cell wall responsiveness to pH, alterations in wall responsiveness to expansin activity may also be involved in the regulation of root growth by water deficits.
Of course many additional factors can be involved in the complex regulation of root growth without or with water deficit. Some examples are (1) source-sink regulation of water and solute distribution via the phloem (Pritchard, 1994; Nerd and Neumann, 2004), (2) molecular changes (e.g. Pritchard et al., 1993; Wu and Cosgrove, 2000; Rodríguez et al., 2002), (3) changes in plant hormones such as abscisic acid or ethylene (e.g. Bacon et al., 1998; Sharp, 2002), and (4) cell wall-structural changes (e.g. Wu et al., 1996; Carpita et al., 2001; Takeda et al., 2002; Fry, 2004) and genetic changes (e.g. Wagner and Kohorn, 2001; Foreman et al., 2003). Nevertheless, changes in rates of proton pumping into expanding cell walls could offer a convenient and spatially selective response mechanism for rapidly regulating wall extensibility, solute uptake, and growth. Moreover, inhibitors of proton pumping, such as cyanide, orthovanadate, and erythrosin B, have been shown to rapidly inhibit growth in expanding roots and leaves (e.g. Bogoslavsky and Neumann, 1998; Peters and Felle, 1999) In this context, recent supportive evidence indicates that a gene encoding a modulator of plasma membrane proton-pumping ATPase activity, which binds to a site different from the 14-3-3 binding site, is preferentially expressed in the accelerating growth region of the maize root elongation zone under control and water-deficit conditions (M. Bassani, P.M. Neumann, and S. Gepstein, unpublished data).
In conclusion, convenient indicator gel-image analysis methods with high resolution were developed and applied to the investigation of mechanisms underlying steady-state root growth inhibition by water deficit. Profiles of proton flux and RSER in the elongation zone of whole maize seedling roots, with or without water deficit, were shown to be well correlated. In addition, changes in pH induced by water deficit within root epidermal cell walls were assayed by confocal laser scanning microscopy and provided independent confirmation of changes in profiles of surface acidification. Finally, external acidification of roots was shown to specifically induce a partial reversal of growth inhibition by water deficit in the central region of the elongation zone. These new findings, plus evidence in the literature concerning increases induced by acid pH in wall-extensibility parameters, lead us to propose that the apparently adaptive maintenance of growth 0 to 3 mm behind the tip in maize primary roots under water deficit and the associated inhibition of growth further behind the tip are related to spatially variable changes in proton pumping into expanding cell walls.
MATERIALS AND METHODS
Plant Growth
Maize seeds (Zea mays cv 647) supplied by Galilee Seeds (Acco, Israel), were germinated on moistened filter paper for 4 d and then transferred to hydroponic culture with roots in well-aerated 0.1-strength nutrient solutions under a controlled environment, as by Bogoslavsky and Neumann (1998). Water deficit was imposed by sequential transfer at 1-h intervals to root media containing freshly prepared nutrient solution with the nonpenetrating osmolyte PEG 6000 plus 0.5 mm CaCl2, at water potentials of −0.1 MPa, −0.2 MPa, −0.3 MPa, −0.4 MPa, and finally −0.5 MPa. Increases in root length were measured with a ruler.
Profiles of Root Elongation
RSER along the root elongation zone was measured by digital photography (Sharp et al., 1988). The surface of the root elongation zone of a whole seedling was gently blotted and marked with Indian ink using a device consisting of 12 silk threads (0.06 mm diameter) spaced at equal distances of about 1 mm. Marking can affect root growth. Moreover, recently developed methods can give more detailed spatial resolution without the need to mark the roots (Walter et al., 2002; van der Weele et al., 2003). However, in our experiments, the marked roots were first allowed to equilibrate for 60 min in 150 mL of aerated solution. In the following 30 min, during which RSERs were determined, elongation did not differ significantly from the elongation of unmarked control or water stressed roots (data not shown). A series of digital images of the marked roots were taken at 0.5-h intervals using a digital camera (Olympus [Tokyo] C-5050). The resolution was 92 pixels per mm at 3 cm from the root. The RSER was determined as (lnLt−lnL0) L0−1 t−1, where L0 is the initial distance between marks and Lt is the final distance after time t. Distance between marks and elongation profiles were obtained from the images with a customized program in Matlab (high-performance numeric computation and visualization software, version 6.5). This fitted a vertical line to all the marks and determined the midpoints of the marks, thus avoiding human error. The distances between the marks were then calculated from pixel counts before and after a 30-min interval. The increases in root length during 30 min comprised less than 20% of the length of the elongation zone in water-deficit and control treatments, and possible distortions of the growth profile were therefore minimal (compare with Schnyder et al., 1987; Peters and Felle, 1999). Measurement error, based on equivalent assays of millimeter markings on a thin steel ruler, was less than 1%.
A similar approach was used to determine the distribution of root elongation responses to acidification of the root medium with 2 mm succinate buffer at pH 4.5. The distance between marks was increased to 3 mm, and the interval between image capture was reduced to 15 min. Total elongation of each 3-mm segment in well-watered roots and roots under water deficit (with basal elongation rates ≥0.4 mm h−1) was assayed for 15 min both before and after acidification.
Root Surface Acidification
A pH indicator agar gel (0.5%, w/v) containing bromocresol purple (0.01%, w/v) in 0.1-strength nutrient solution at pH 6.3 was used to visualize root surface pH via color changes (Römheld et al., 1984). A solution (30 mL) of freshly prepared gel was poured into a glass petri dish with an internal diameter of 11 cm to produce an even layer of pH indicator gel. For the assay, roots of control seedlings were rinsed with 0.5 mm CaCl2 solution, and the water-stressed roots were rinsed with PEG solution (at −0.5 MPa) containing 0.5 mm CaCl2. Roots were then lightly blotted to remove excess solution and gently guided to the bottom of the petri dish along an incision in the gel.
A uniform diffusive light source was used to weakly illuminate the gel from below and achieve high image quality (Jaillard et al., 1996). A 13 W double “U” long-life fluorescence lamp was anchored at the bottom of a light-sealed box (21 cm high). A layer of white translucent plastic was used to cover a 14 cm diameter window at the top of the box, and the petri dish was inverted onto the plastic. The root and gel were photographed from above through the base of the petri dish using an Olympus digital camera (C-5050 ZOOM) preset in manual mode (F 4.0, 0.5 s, white balance set for room light, ISO-64, no flash). The space between the petri dish and camera (15 cm) was sealed from external light. With the whole petri dish in focus, resolution was 23 pixels per mm. The initial image was taken within 10 s of root contact with the gel, and a second image was taken 5 min later. The stored images were posterized to level 3 using the Posterize command in Photoshop version 6.0 (Adobe Systems, Mountain View, CA). This specifies the number of tonal levels (or brightness values) for each channel in an image and then maps pixels to the closest matching level.
For quantitative evaluations of proton flux from the root surface, a calibration between color and known pH of buffered indicator gel was first established. Droplets (20 μL) of 50 mm succinic acid-NaOH buffers ranging from pH 4.8 to pH 6.2 in 0.2 unit steps were injected into the gel at separate locations and imaged after 20 min. The color images were converted to grayscale, and the intensity was measured using image processing tools in Matlab. The grayscale intensity of the untreated gel served as background and was subtracted from the intensity values for buffer-treated gel. A linear calibration relating grayscale intensity to pH was derived (Fig. 2B, inset). The same approach was used to automatically calculate pH values at pixel resolution along a 10 mm length of the root surface (Pilet et al., 1986; Rao et al., 2000). In brief, the original color images of roots in indicator gel were converted to grayscale. The gray level value along each file of pixels extending at 90 degrees from the surface of the root whose color was changed by proton efflux was assayed and converted into equivalent pH values. The calculated pH adjacent to the root surface was >pH 5.2. The total volume of gel below each pixel area and the total number of pixels in each file showing pH-associated changes in grayscale intensity were used to calculate the number of protons that diffused from a specified area of root surface to the gel during the 5-min assay. A customized Matlab program calculated and displayed proton flux per unit root surface area and time along the length of the root elongation zone.
Determination of Apoplastic pH by Confocal Laser Scanning Microscopy
A fluorescent pH-sensitive probe DM-NERF (pK = 5.4) was purchased from Molecular Probes (Eugene, OR). DM-NERF was dissolved in distilled water to make 1 mm stock solution (Amtmann et al., 1999; Yu et al., 2001). Preliminary tests showed that prior incubation in DM-NERF did not inhibit the subsequent growth of roots over 24 h. The dye was diluted to 10 μm in 100 mm succinic acid-NaOH buffer (pK1 4.2, pK2 5.6) at pHs ranging from 3.9 to 6.3, and for in vitro calibration filter paper strips were saturated with the buffered dye prior to assay under a glass coverslip. For in situ calibrations 1-cm-long root tip segments were incubated for 30 min with 10 μm dye solutions in 100 mm succinic acid-NaOH buffers containing 1 mm KCN and 0.5 mm sodium orthovanadate to inhibit proton pumping.
Images were captured by using a Laser Scanning Confocal Imaging System (model MRC 1024; Bio-Rad, Cambridge, MA). A ×60 (1.4 numerical aperture) oil-immersion objective and a ×2.6 electronic zoom were used. Fluorescence images were acquired sequentially using the 488-nm and 457-nm excitation lines of an argon laser at 10% intensity. Emission was collected at >515 nm (long pass). The emission intensity following excitation of DM-NERF at 488 nm is pH responsive, whereas the emission intensity after excitation at 457 nm is relatively insensitive so that this probe is suitable for ratiometric pH measurements (Lin et al., 1999). The pinhole size and gain setting were fixed after adjustments to fluorescence intensity so that equivalent gray levels in viewed images ranged from 60 to 200 (values appropriate to the pH range tested). Under these conditions, measured autofluorescence was less than 10% of total emission intensity in the presence of DM-NERF and did not vary along the elongation zone.
For image processing, the non-cell wall area in the 488-nm image was first manually deleted using the magic wand tool in Photoshop to eliminate fluorescence from underlying cell walls (Rober-Kleber et al., 2003). The image pairs were then transferred to Matlab, in which a custom program divided each pixel value in the 488-nm image of the walls by the equivalent pixel value in the 457-nm image in order to generate pixel ratios. All of the pixel ratios in the cell wall areas were then between >0 and <3. For in situ calibration, the means of the pixel ratios in the whole area of cell walls were plotted against the pH of the buffers in which the roots were previously incubated. Independent in situ calibrations were performed prior to each set of in vivo pH measurements, and microscope settings were then fixed.
For in vivo measurements of epidermal cell wall pH, roots of intact seedlings from control or water-deficit treatment were incubated vertically in aqueous 10 μm DM-NERF solution for 5 min. After dye loading, the seedlings were attached to slides. The root elongation zone with a fresh drop of dye solution was covered with a supported coverslip, and the remaining exposed root was covered with damp tissue to prevent drying. The mean of the pixel ratios in the cell wall area was used to determine a mean pH value. Pseudo color images of cell walls were then generated by fitting each pixel ratio in the cell walls to a corresponding color by matching with the ratio range/pH color index.
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
This work was supported in part by the German Israeli Project Cooperation (DIP; grant no. DIP4.3).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.041426.
References
- Amtmann A, Jelitto TC, Sanders D (1999) K+-selective inward-rectifying channels and apoplastic pH in barley roots. Plant Physiol 120: 331–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon MA, Wilkinson S, Davies WJ (1998) pH-regulated leaf cell expansion in droughted plants is abscisic acid dependent. Plant Physiol 118: 1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoslavsky L, Neumann PM (1998) Rapid regulation by acid-pH of cell-wall adjustment and leaf growth in intact maize plants responding to reversal of water stress. Plant Physiol 118: 701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita NC, Defernez M, Findlay K, Wells B, Shoue DA, Catchpole G, Wilson RH, McCann MC (2001) Cell wall architecture of the elongating maize coleoptile. Plant Physiol 127: 551–565 [PMC free article] [PubMed] [Google Scholar]
- Chazen O, Hartung W, Neumann PM (1995) The different effects of PEG 6000 and NaCl on leaf development are associated with differential inhibition of root water transport. Plant Cell Environ 18: 727–735 [Google Scholar]
- Cosgrove DJ (1997) Assembly and enlargement of the primary cell wall in plants. Annu Rev Cell Dev Biol 13: 171–201 [DOI] [PubMed] [Google Scholar]
- Edwards KL, Scott TK (1974) Rapid growth responses of corn root segments: effect of pH on elongation. Planta 119: 27–37 [DOI] [PubMed] [Google Scholar]
- Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao T, Gilroy S (2001) Changes in root cap pH are required for the gravity response of the Arabidopsis root. Plant Cell 13: 907–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H (1998) The apoplastic pH of the Zea mays root cortex as measured with pH-sensitive microelectrodes: aspects of regulation. J Exp Bot 49: 987–995 [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Fry SC (2004) Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytol 161: 641–675 [DOI] [PubMed] [Google Scholar]
- Jaillard B, Ruiz L, Arvieu JC (1996) pH mapping in transparent gel using color indicator videodensitometry. Plant Soil 183: 85–95 [Google Scholar]
- Lin HJ, Szmacinski H, Lakowicz JR (1999) Lifetime-based pH sensors: indicators for acidic environments. Anal Biochem 269: 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mexal J, Fisher JT, Osteryoung J, Reid CPP (1975) Oxygen availability in polyethylene glycol solutions and its implications in plant-water relations. Plant Physiol 55: 20–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerd A, Neumann PM (2004) Phloem water transport maintains stem growth in a drought stressed crop cactus (Hylocerus undatus). J Amer Soc Hort Sci 129: 486–490 [Google Scholar]
- Neumann PM (1995) The role of cell wall adjustment in plant resistance to water deficits. Crop Sci 35: 1258–1266 [Google Scholar]
- Neves-Piestun BG, Bernstein N (2001) Salinity-induced inhibition of leaf elongation in maize is not mediated by changes in cell wall acidification capacity. Plant Physiol 125: 1419–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober ES, Sharp RE (2003) Electrophysiological responses of maize roots to low water potentials: relationship to growth and ABA accumulation. J Exp Bot 54: 813–824 [DOI] [PubMed] [Google Scholar]
- Peters WS, Felle HH (1999) The correlation of profiles of surface pH and elongation growth in maize roots. Plant Physiol 121: 905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilet PE, Versel JM, Mayor G (1983) Growth distribution and surface pH patterns along maize roots. Planta 158: 398–402 [DOI] [PubMed] [Google Scholar]
- Pritchard J (1994) The control of cell expansion in roots. New Phytol 127: 3–26 [DOI] [PubMed] [Google Scholar]
- Pritchard J, Hetherington PR, Fry SC, Tomos AD (1993) Xyloglucan endotransglycosylase activity, microfibril orientation and the profiles of cell wall properties along growing regions of maize roots. J Exp Bot 44: 1281–1289 [Google Scholar]
- Rao TP, Yano K, Yamauchi A, Tatsumi J (2000) A simple method for quantitative estimation of rhizosphere pH along root axes through visualization. Plant Prod Sci 3: 94–100 [Google Scholar]
- Rayle DL, Cleland RE (1992) The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol 99: 1271–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rober-Kleber N, Albrechtová JTP, Fleig S, Huck N, Michalke W, Wagner E, Speth V, Neuhaus G, Fischer-Iglesias C (2003) Plasma membrane H+-ATPase is involved in auxin-mediated cell elongation during wheat embryo development. Plant Physiol 131: 1302–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez AA, Grunberg KA, Taleisnik EL (2002) Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol 129: 1627–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V, Müller C, Marschner H (1984) Localization and capacity of proton pumps in roots of intact sunflower plants. Plant Physiol 76: 603–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder H, Nelson CJ, Coutts JH (1987) Assessment of spatial distribution of growth in the elongation zone of grass leaf blades. Plant Physiol 85: 290–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala SN, Newman IA (1998) Osmotic sensitivity of Ca2+ and H+ transporters in corn roots: effects on fluxes and their oscillations in the elongation region. J Membr Biol 161: 45–54 [DOI] [PubMed] [Google Scholar]
- Sharp RE (2002) Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ 25: 211–222 [DOI] [PubMed] [Google Scholar]
- Sharp RE, Silk WK, Hsaio TC (1988) Growth of the maize primary root at low water potentials. 1. Spatial distribution of expansive growth. Plant Physiol 87: 50–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk WK (1992) Steady form from changing cells. Int J Plant Sci 153: S49–S58 [Google Scholar]
- Takeda T, Furuta Y, Awano T, Mizuno K, Mitsuishi Y, Hayashi T (2002) Suppression and acceleration of cell elongation by integration of xyloglucans in pea stem segments. Proc Natl Acad Sci USA 99: 9055–9060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto E, Fujii S, Yamamoto R, Inanaga S (2000) Measurement of viscoelastic properties of root cell walls affected by low pH in lateral roots of Pisum sativum L. Plant Soil 226: 21–28 [Google Scholar]
- Taylor AR, Bloom AJ (1998) Ammonium, nitrate, and proton fluxes along the maize root. Plant Cell Environ 21: 1255–1267 [Google Scholar]
- Taylor DP, Slattery J, Leopold AC (1996) Apoplastic pH in corn root gravitropism: a laser scanning confocal microscopy measurement. Physiol Plant 97: 35–38 [PubMed] [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu DT, Bligny R, Maurel C (2003) Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425: 393–397 [DOI] [PubMed] [Google Scholar]
- van der Weele CM, Jiang HS, Palaniappan KK, Ivanov VB, Palaniappan K, Baskin TI (2003) A new algorithm for computational image analysis of deformable motion at high spatial and temporal resolution applied to root growth. Roughly uniform elongation in the meristem and also, after an abrupt acceleration, in the elongation zone. Plant Physiol 132: 1138–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Volkenburgh E, Boyer JS (1985) Inhibitory effects of water deficit on maize leaf elongation. Plant Physiol 77: 190–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE, Ober ES, Sharp RE (1998) Root growth and oxygen relations at low water potentials. Impact of oxygen availability in polyethylene glycol solutions. Plant Physiol 116: 1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner TA, Kohorn BD (2001) Wall-associated kinases are expressed throughout plant development and are required for cell expansion. Plant Cell 13: 303–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Silk WK, Schurr U (2000) Effect of soil pH on growth and cation deposition in the root tip of Zea mays. J Plant Growth Regul 19: 65–76 [DOI] [PubMed] [Google Scholar]
- Walter A, Spies H, Terjung S, Küsters R, Kirchgeßner N, Schurr U (2002) Spatio-temporal dynamics of expansion growth in roots: automatic quantification of diurnal course and temperature response by digital image sequence processing. J Exp Bot 53: 689–698 [DOI] [PubMed] [Google Scholar]
- Wiesenseel MH, Dorn A, Jaffe LF (1979) Natural H+ currents traverse growing roots and root hairs of Barley (Hordeum vulgare L.) Plant Physiol 64: 512–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winch S, Prichard J (1999) Acid induced wall loosening is confined to the accelerating region of the root growing zone. J Exp Bot 50: 1481–1487 [Google Scholar]
- Wu Y, Sharp RE, Durachko DM, Cosgrove DJ (1996) Growth maintenance of the maize primary root at low water potentials involves increases in cell wall extension properties, expansin activity and wall susceptibility to expansins. Plant Physiol 111: 765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YJ, Cosgrove DJ (2000) Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. J Exp Bot 51: 1543–1553 [DOI] [PubMed] [Google Scholar]
- Yu Q, Kuo J, Tang C (2001) Using confocal laser scanning microscopy to measure apoplastic pH change in roots of Lupinus angustifolius L. in response to high pH. Ann Bot (Lond) 87: 47–52 [Google Scholar]
- Zidan I, Azaizeh H, Neumann PM (1990) Does salinity reduce growth in maize root epidermal cells by inhibiting their capacity for cell wall acidification? Plant Physiol 93: 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]