Abstract
The dysregulation of apoptosis contributes in a variety of ways to the malignant phenotype. It is increasingly recognized that the alteration of pro-apoptotic and anti-apoptotic molecules determines not only escape from mechanisms that control cell cycle and DNA damage, but also endows the cancer cells with the capacity to survive in the presence of a metabolically adverse milieu, to resist the attack of the immune system, to locally invade and survive despite a lack of tissue anchorage, and to evade the otherwise lethal insults induced by drugs and radiotherapy. A multitude of apoptosis mediators has been identified in the past decade, and the roles of several of them in breast cancer have been delineated by studying the clinical correlates of pathologically documented abnormalities. Using this information, attempts are being made to correct the fundamental anomalies at the genetic level. Fundamental to this end are the design of more efficient and selective gene transfer systems, and the employment of complex interventions that are tailored to breast cancer and that are aimed concomitantly towards different components of the redundant regulatory pathways. The combination of such genetic modifications is most likely to be effective when combined with conventional treatments, thus robustly activating several pro-apoptotic pathways.
Keywords: breast cancer, gene therapy, apoptosis
Introduction
The highly orchestrated form of cell death known as apoptosis goes awry to some extent in most cancers. Increasingly, a general theme in cancer pathophysiology is the development of a defect in the function of pro-apoptotic molecules, such as p53, that commonly prepare the cell for apoptosis whenever cell proliferation or DNA damage is induced; lack of these molecules therefore deprives the cell of a critical safety mechanism [1]. Alternatively, a functional excess of anti-apoptotic molecules, such as Bcl-2, may also occur in tumors. In each case, the result is an imbalance that favors the inappropriate survival of tumor cells. The mechanisms involved and their components are attractive therapeutic targets because the tumor cell is totally dependent on them for its survival, and appears typically to have a higher sensitivity to the induction of apoptosis than normal tissues [1,2]. In addition, restoring or enhancing the capacity to undergo apoptosis may, in some cases, be a crucial event that renders tumors sensitive to classical anticancer agents, such as those used in chemotherapy [3,4**] and radiotherapy [5,6]. The present review discusses the most common genetic alterations that alter apoptosis regulation in breast cancer, and the initial gene-based approaches that have been explored to overcome them with therapeutic intent.
Dysregulated apoptosis contributes to cancer
There is a multitude of critical steps during the pathogenesis of cancer in which avoidance of programmed cell death assures the progression, and maintenance, of the malignant phenotype (Fig. 1) [7,8*,9,10]. Early during tumor progression, defects in apoptosis allow the survival of the cancer cell despite the existence of DNA damage and cell-cycle dysregulation. This severe breaking of a basic DNA housekeeping action contributes to the genetic instability that characterizes cancer, and thus initiates and sustains a spiral of further and further genetic aberrations that endow tumor cells with extraordinary capacities and adaptability. In fact, a reduction in apoptosis has been observed in patients with both carcinomas and 'normal' epithelium in the breast that is associated with fibrocystic changes. This is in contrast with breasts with benign fibroadenomas, which have normal levels of apoptosis and are not associated with malignant transformation [11*]. Further evidence has been obtained in transgenic mice that express the oncogenic simian virus 40 (SV40) large T antigen. These animals show a dramatic increase in apoptosis during the development of preneoplastic mammary lesions, which is associated with a significant elevation in expression of the pro-apoptotic Bax. In double-transgenic mice that are engineered to additionally carry a mutated bax, a marked reduction in apoptosis occurs in the preneoplastic lesions and the animals show a subsequent increase in the number, size, and rate of growth of breast tumors [12*]. Thus, disruption of apoptosis mediators clearly contributes to mammary tumor progression.
Figure 1.
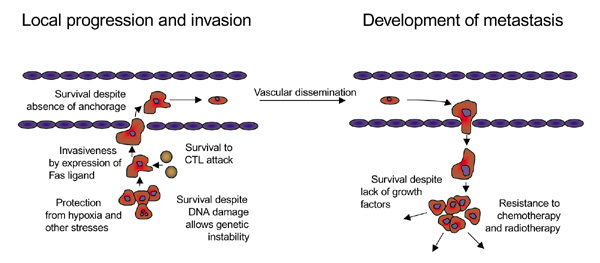
Dysregulation of apoptosis contributes to the pathophysiology of cancer. A variety of defects in the apoptotic machinery contribute to avoidance of apoptosis by tumor cells throughout the entire carcinogenic process. The enhanced capacity of tumor cells to survive allows them to overcome (in analogy to decathlon athletes) numerous challenges encountered, not only in the primary tumor location, but also during their vascular distribution and at their multiple final destinations. The universal dependence of tumor cells on mechanisms to avoid apoptosis suggests a 'window of homogeneity' that could be exploited therapeutically. CTL, cytotoxic T lymphocyte.
As the tumor mass grows, the demands for oxygen, basic nutrients, and growth factors are increasingly unmet. This imbalance, which would otherwise induce cell death, is not translated into effective death signals by a disturbance in the apoptotic machinery, however. Furthermore, the accumulating mutations of the tumor cell give rise to new cell surface mutated proteins, or epitopes. These potential tumor antigens, however, do not usually generate a strong immune response, or the response is not efficacious. Such 'sheltering' from cytotoxic T lymphocytes (CTLs) and other immune effectors has been associated with a variety of changes in the control of apoptosis that effectively protect the cancer cell from otherwise lethal insults mediated by CTLs and other immune mediators [13].
Later in the natural history of malignant tumors, metastases eventually develop, which determines in most cases an ominous change in the prognosis of the disease. For this event to occur the cell needs to acquire the capacity to disseminate and survive despite its lacking tissue anchorage, which normally would induce a type of apoptosis called 'anoikis'. Recently, the involvement of cellular receptors of death signals in anoikis has been described [14**], and the disturbance of these receptors and their related transductional apparatus in cancer cells has been suggested. Lastly, the tumor regression induced by chemotherapy, radiotherapy, and hormone therapy depends to a large extent on the induction of apoptosis [7,15,16,17]. In breast cancer, the administration of chemotherapy immediately before surgery has allowed analysis of the induction of apoptosis in the remaining tumor within the resected specimen. Interestingly, a positive correlation has been found between the apoptotic index, the clinical response, and patient survival [18**]. Tumor cells frequently emerge, however, that resist the apoptosis-inducing effects of those maneuvers, thus essentially escaping from current therapeutic interventions [19,20,21]. On aggregate, the consequences of dysregulation of apoptosis are pervasive throughout the natural history of cancer, and have been established as a universal component of the malignant phenotype.
Mechanisms of apoptosis avoidance in breast cancer
In cancer, cellular proliferation goes on unchecked and cellular death does not occur to the extent that it should, despite several otherwise potent death stimuli being conspicuously present. These two aspects of tumor pathophysiology have been analyzed in breast cancer, and consistently found to be disturbed [22,23,24]. In fact, expression levels of several cell growth regulators and pro-apoptotic and anti-apoptotic proteins are usually perturbed in breast cancer, and their alteration has been associated with prognosis and with the response to conventional anticancer treatments (see below).
Growth factors and their receptors
Many growth factors and their receptors influence the growth and proliferation of breast cancer cells [25]. Typically, normal versions of human epidermal growth factor receptor-related gene (HER)-2/neu (c-erbB-2), epidermal growth factor receptor (or c-erbB-1), and insulin-like growth factor (IGF)-1 receptor are overexpressed. Interestingly, the aberrant control of cell proliferation by growth factor receptors is mechanistically related to an inhibition of apoptosis. For instance, overexpression of HER-2 upregulates anti-apoptotic bcl-2 and bcl-xL, and thus inhibits in vitro tamoxifen-induced apoptosis [26]. A similar effect of HER-2 has been found on taxol-induced apoptosis. As another example, IGF-1 protects breast cancer cells from apoptosis that is induced by chemotherapeutic drugs [27]. Thus, mechanisms known to alter tumor cell proliferation may also directly contribute to the avoidance of apoptosis in breast cancer cells. The relevant molecular pathology, and the potential for modulating these molecules in the context of gene therapy, has been reviewed elsewhere [28,29].
Genes that regulate apoptosis
In addition to factors that are involved in controlling cell proliferation, abnormalities have been identified in breast cancers in many genes that regulate the apoptotic cascade, including p53, bcl-2, bax, c-myc, p21WAF/CIP1, and many others (Table 1). As a consequence, tumor cells express several proteins that render them resistant to apoptosis. Blocking cell death promotes neoplastic transformation [10].
Table 1.
Apoptosis avoidance in breast cancer and therapeutic approaches
| Mechanism of apoptosis avoidance | Example | Normal function | Therapeutic strategy | References |
| Increased activity of growth factor receptors | IGF-1 receptors are overexpressed and IGF-1 binding protein 3 is decreased | IGF-1 binding protein-3 binds to IGF-1, thus blocking the effect of this factor essential for tumor growth; IGF-1 binding protein 3 secretion is induced by p53 | Achieve high concentrations of IGF-1 binding protein 3 by intratumoral gene transfer | [27,112] |
| Decreased activity of death receptors | TRAIL family | Binding of TRAIL to its receptors TRAIL-R1 and TRAIL-R2 induces cell death, whereas TRAIL-R3 and TRAIL-R4 behave as regulatory decoys | Achieve high concentrations of TRAIL by intratumoral gene transfer; normal expression of TRAIL-R3 and TRAIL-R4 in normal cells determines a good therapeutic index | |
| TNF receptor 1 | Mediator in inflammatory and immune responses; it delivers a potent pro-apoptotic signal to the nucleus that is inhibited by NF-κB | Achieve high concentrations of TNF by intratumoral gene transfer; the problem is the extreme toxicity on normal cells; more attractive is to inhibit NF-κB (eg by gene delivery of IκB) | [129] | |
| Fas is downregulated | Determines sensitivity to CTLs; Fas is induced by p53; Fas ligand expression correlates with tumor grade, possibly contributes to local deletion of lymphocytes, and has a role in tumor invasion through Fas+ stroma | Achieve high concentrations of Fas ligand by intratumoral gene transfer; the problems are the toxicity on normal cells and the risk of endowing the tumor cell with more invasiveness and resistance to the immune system | [130] | |
| Increased activity of survival proteins | HER-2/neu | Overexpression of this growth factor receptor contributes to the malignant phenotype | Inhibit exogenous survival signals by a single chain antibody: scFv anti-erbB-2 | [131] |
| Bcl-2 and functional analogs | Blocks apoptosis triggered by several factors. Early in tumor development, Bcl-2 may rescue cells undergoing lethal mutations, and thus favor the accumulation of further genetic damage. Later, when other oncogenes are activated and Bax is lost, the loss of Bcl-2 may confer an additional growth advantage | Block by antisense or intracellular single chain antibodies: scFv anti-Bcl-2 | [132] | |
| NF-κB | Activates transcription of IAPs and of its own activators | Inhibit this inhibitor of exogenous death signals (eg by gene delivery of IκB) | [129] | |
| Survivin | Member of the IAP family; overexpressed in most tumors; inhibits apoptosis by binding to caspases | Block by antisense, intracellular single-chain antibodies, etc | [133] | |
| HSP-70 | Inhibits apoptosis by binding to activated caspases; prognostic factor in breast cancer; correlates with shorter disease-free survival, increased cell | Block by antisense proliferation, and poor differentiation, as well as with lymph node metastasis | [134] | |
| Decreased activity of apoptosis executioners | Bax | Effects apoptosis; acts by inducing opening of mitochondrial permeability transition pore, and cytochromec release; active even in the presence of Bcl-2 and independently of p53 | Achieve high concentrations of Bax by intratumoral gene transfer [eg Ad/Bax (proof-of-principle in ovarian cancer)]; induce Bax by upstream positive regulators, such as mda-7 | [126,135,136] |
| Bcl-Xs | Induces apoptosis probably without requiring dimerization and even in the presence of Bcl-2 | Achieve high concentrations of Bcl-Xs by intratumoral gene transfer (eg Ad/Bcl-Xs) | [3,137,138,139] | |
| Caspase-7 | Member of the caspase family of proteins with an effector role in the activation cascade | Achieve high concentrations of caspase by intratumoral gene transfer (proof of principle shown in prostate cancer) | ||
| Apoptin | This is a chicken anemia virus-derived protein that induces apoptosis in transformed cells, but not in normal, diploid cells | Induce intratumoral or systemic levels of apoptin (eg with Ad/apoptin) | [140,141] | |
| Deranged activity of checkpoint controls and DNA repair | p53 | Induces cell cycle stop or apoptosis when DNA damage is detected; frequently mutated in breast cancer; increases the function of Bcl-2 and FasL | Restore levels of p53 (eg by Ad/p53); limited by inactivation of wild-type p53 and dependence on multiple cofactors | [84,85,86,87,88,89,90] |
| BRCA1 | Involved in DNA damage checkpoints; possibly has a pivotal role in maintaining stability of the genome; BRCA1 induces apoptosis | Gene transfer of BRCA1; limitation of lacking a means for local amplification of effect | [142] | |
| PML | The promyelocytic leukemia gene (PML) is a growth and transformation suppressor | Delivery of PML via an adenoviral vector has shown induction of massive apoptotic death in in vivo animal models of breast cancer | [143] | |
| Combination treatment | Various | Association with chemotherapy or radiotherapy; blocks for apoptosis are removed, allowing the conventional treatment to | [4**,6,144] |
Ad, adenovirus; CTL, cytotoxic T lymphocyte; HER, human epidermal growth factor receptor-related gene; HSP, heat shock protein; IAP, inhibitor of apoptosis protein; IGF, insulin-like growth factor; NF-κB, nuclear factor-κB; TNF, tumor necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand.
p53
Mutations in the p53 gene are a common molecular abnormality in breast cancer [30,31]. A consequence of the lack of normal function of p53 may be the failure to induce apoptosis in cells with damaged DNA [32], and it can also possibly impair a full apoptotic response to the administration of hormonal or chemotherapeutic interventions. Furthermore, it can contribute to genomic instability [33], and thus increase the probability of appearance of additional mutations that are advantageous for survival of the tumor cell. p53 stops the cell cycle and induces apoptosis through stimulation of p21WAF/CIP1, an inhibitor of cyclin-dependent kinases. In effect, experimental overexpression of p21WAF/CIP1 in human breast cancer cell lines suppresses growth, and induces apoptosis [34].
Bcl-2 family
The genes of the bcl-2 family have emerged as key regulators of apoptosis, and appear to be dysregulated in a number of tumors, including breast cancers [23,35,36]. Several members of the Bcl-2 family, including bcl-2, Bcl-XL, Mcl-1, and A1/Bfl-1, suppress apoptosis; whereas others, including Bax, Bak, Bok/Mtd, Bad, Bik, Bid, Bim/Bod, and HrK, induce apoptosis. The extent of apoptosis is inversely associated with Bcl-2 expression in pre-malignant and malignant breast lesions [37,38,39,40]. Paradoxically, Bcl-2 expression correlates with favorable clinicopathologic features, as well as with improved disease-free and overall survival [41,42,43,44,45,46,47]. Furthermore, patients with elevated Bcl-2 levels appear to derive the greatest benefit from endocrine therapy [48,49,50]. Only in a subset of well differentiated and progesterone receptor-positive tumors has Bcl-2 been reported to enhance disease progression [35]. As an explanation for the apparent paradox of decreasing levels of anti-apoptotic Bcl-2 levels with increasing tumor grade, it has been proposed that Bcl-2 has an early role within the tumor by rescuing cells with otherwise lethal mutations. After additional oncogene activation, some cells would acquire additional ways to protect themselves against apoptosis [51]. At that point, loss of Bcl-2 might confer a growth advantage. In fact, Bcl-2 is known to restrain cell proliferation [10]. Thus, expression of Bcl-2 would change from high levels in early or low-grade tumors, characterized by low apoptotic indices, to low levels in advanced or high-grade tumors, characterized by high apoptotic indices.
Bax
The levels of expression of Bax or the Bax:Bcl-2 ratio, as determined by immunostaining, directly correlate in breast tumors with longer patient survival [46,47] and/or better response to therapy in some [52] but not all studies [53]. In this regard, bax is considered a tumor suppressor gene, and is itself a direct transcriptional target of p53. Interestingly, haploid loss of bax leads to accelerated mammary tumor development in SV40 large T antigen double trans-genic mice [12*], which suggest that the protective effect of bax is dose-dependent. In addition, successful induction of apoptosis by chemotherapy and radiation is associated with augmented expression of Bax [54]. Furthermore, induced overexpression of Bax renders tumor cells more sensitive to drugs [55], whereas ablation of Bax reduces drug-induced apoptosis [56]. Mechanistically, Bax promotes cell death by directly binding and antagonizing pro-survival Bcl-2 [57], and it also induces apoptosis by itself through its direct channel-forming activity in mitochondria and activation of caspase pathway [58*,59**]. The correlations mentioned above and the described mechanism of action of Bax demonstrates its importance not only as a modulator of the response to treatments, but also as a direct mediator of their cytotoxicity.
Caspases
Caspases are the essential effectors of apoptotic cell death [60]. Disturbance of their activation is therefore an obvious mechanism whereby breast cancer cells might avoid apoptosis. Two distinct starting points for the activation of caspases have been characterized [10] as follows.
First, activation may originate at so-called death receptors. The ligation of CD95 (Fas) or related members of the tumor necrosis factor (TNF) receptor family leads to assembly of a 'death-inducing signaling complex' at the plasma membrane. Within the death-inducing signaling complex, the adaptor protein fas-associating death domain-containing protein/mediator of receptor induced toxicity 1 (FADD/MORT1) recruits cytosolic caspase-8, which promotes its autocatalytic activation and release into the cytosol. This event leads to the subsequent activation of downstream caspases (ie caspase-3, -6 and -7), which serve as the final effectors of apoptosis [61]. Additionally, the TNF/CD95 receptor pathway has been found to activate apoptosis also via the mitochondria, which possibly allows the amplification of the pro-apoptotic signal. In effect, caspase-8 can cleave cytosolic Bcl-2 homology domain 3 interacting domain death agonist (BID), a pro-apoptotic member of the Bcl-2 superfamily [62**,63]. The truncated BID translocates into the mitochondria, where it binds to Bax and leads to the release of cytochrome c into the cytosol. Once released, cytochrome c combines with apoptotic protease activating factor-1 and procaspase-9 in the complex termed apoptosome, leading to the formation of caspase-9, and to the subsequent activation of the caspase cascade, including caspase-3. Binding to the apoptosome of Bcl-2 or Bcl-XL can inhibit this effector pathway. Of interest, Bcl-2 and Bcl-XL can also interfere with the function of translocated BID [62**,64], thus blocking the mitochondria-dependent signaling arm that originates in the death receptors.
Second, apoptosis can be triggered by DNA damage and independently of FADD/MORT1 and caspase-8, via a similar mitochondrial step that culminates in the release of cytochrome c from mitochondria into the cytosol. DNA damage and other stimuli act through both p53-dependent and -independent pathways that are actively being characterized (for a summary see [20]).
In breast cancer, disturbance of death receptor pathways may be important for several reasons. First, it may help to avoid killing by CTLs, which usually act by expression of CD95L (Fas ligand) to trigger apoptosis of target cells. Second, it may conversely allow expression of CD95 ligand by tumor cells and thus determine the killing of activated lymphocytes, which usually express Fas. Finally, expression of Fas ligand may contribute to invasiveness by clearing stromal cells that express Fas. Recently, all of these hypotheses have been elegantly confirmed in tumor specimens from patients [65]. In effect, expression of Fas ligand has been positively correlated and expression of CD95 inversely correlated with the histopathologic grading of the analyzed breast tumors. In addition, tumor-infiltrating lymphocytes and stromal cells in close proximity to CD95L-expressing breast cancer cells underwent apoptosis. Furthermore, CD95+ target cells cultured on breast cancer tissue sections underwent apoptosis, but could be rescued when CD95 ligand was specifically blocked by a CD95-Fc fusion molecule [65]. Thus, alterations in CD95/death receptor signaling may allow breast tumor cells to avoid apoptosis and also contributes to their escape from the immune system and to their invasive phenotype.
Inhibitor of apoptosis proteins
The signaling pathways that promote apoptosis are exquisitely counterbalanced by several families of proteins, each of which render the cell resistant to particular pro-apoptotic stimuli and effectors. Not surprisingly, increased activity of such protective proteins may result in aggressive tumor growth and resistance to cytotoxic treatment [66]. In addition to anti-apoptotic Bcl-2 and analogs, other relevant proteins are the inhibitor of apoptosis protein (IAP) family and the heat shock proteins (HSPs). The IAP family members directly bind and inhibit certain caspases [67,68]. Given the central role of caspases in apoptosis, and the role of apoptosis in the cytotoxicity of chemotherapy and radio-therapy, it follows that IAPs protect tumor cells from several drugs and other therapies that induce apoptosis [68]. Information about the expression of most IAP members in human tumors, including breast cancer, is scarce. One exception is the IAP named survivin, which is known to be highly overexpressed in breast and most other cancers [69**,70], thus supporting the concept that increased activity of IAPs contributes to breast tumorigenesis. Interestingly, survivin may counteract a default induction of apoptosis in G2/M phase [71]. Its overexpression may therefore overcome an apoptosis-related cell cycle checkpoint and favor aberrant progression of transformed cells through mitosis. Of note, transcriptional activation of IAPs is dependent on nuclear factor-κB (NF-κB). In turn, IAPs can activate NF-κB and thus create a positive feedback loop, enhancing survival signals. This loop may in part explain the potent anti-apoptotic activity of NF-κB [66], and also suggests a possible target for therapeutic intervention.
Heat shock proteins
HSPs are involved in cellular resistance to stress. They are among the most conserved proteins in phylogeny, indicating their central role in supporting cell survival not only after heat shock, but also most other apoptotic stimuli [66]. Clinicopathologic studies [72,73,74,75] have shown that expression of HSP-70 occurs in high-grade tumors and correlates in breast cancer with shorter disease-free survival, increased cell proliferation, and poor differentiation, as well as lymph node involvement. In addition, expression of HSP-70 inversely correlates with the response to combination chemotherapy, radiotherapy, and hyperthermia, whereas no correlation has been found with the response to tamoxifen [75,76,77]. Interestingly, HSP-70 can rescue cells from apoptosis induced by TNF, even after the activation of effector caspases, which indicates that it has a markedly downstream point of action in the apoptosis pathway and is therefore a particularly attractive therapeutic target.
Gene therapeutics for modulation of apoptosis
The consequences of dysregulation of apoptosis on tumor pathophysiology are extremely diverse (see above and Fig. 1). The core machinery of cell death, however, seems to be formed by a discreet number of protein families (Fig. 2). Modulation of a limited number of targets by delivery of genes that encode apoptosis-related proteins would therefore have multiple beneficial effects on the malignant phenotype. Not only would tumor cell proliferation be properly balanced by increased cell death, but also barriers to the immune response would fall, tumor invasiveness would be crippled, and sensitivity to the cytotoxicity induced by drugs and radiation would be restored. Importantly, the need that tumor cells have for mechanisms that allow them to avoid apoptosis is universal, and shared by all tumor localizations in the body. This avoidance represents a 'window of homogeneity' that can be targeted in the context of the formidable tumor heterogeneity. With the increasing recognition of the molecular basis of the apoptotic pathway [1,60,78,79,80], and the description of several of its components acting as oncogenes or tumor suppressor genes, gene therapy has thus emerged as a rational strategy for the modulation of apoptosis [20,81,82,83,84]. The following is a brief summary of approaches that have already been clinically explored in the context of breast cancer. A discussion of the requirements for successful exploitation of the apoptotic machinery using gene transfer and some of the solutions being developed is then provided.
Figure 2.
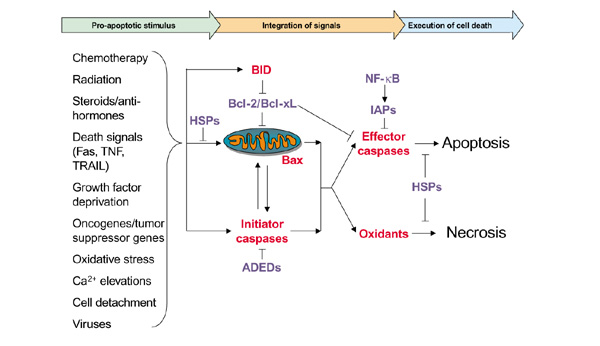
Regulation of cell death. Apoptosis involves a sensor that detects pro-apoptotic stimuli, a signal transduction network, and execution machinery. Despite the complexity of its regulation, execution of programmed cell death is effected by the well-defined family of caspases. Upstream, at least one family of proteins exist at each level of response to pro-apoptotic stimuli that is able to block a deadly signal, including the heat shock proteins (HSPs), the anti-apoptotic death effector domain proteins (ADEDs), several members of the Bcl-2 family, the inhibitors of apoptosis proteins (IAPs), and the nuclear factor-κB (NF-κB) family of transcription factors. Conversely, executioners of apoptosis such as Bax may be not functional, inclining the balance towards inappropriate survival of the tumor cell. In breast cancer, many of these proteins are dysregulated. BID, Bcl-2 homology domain 3 interacting domain death agonist; TNF, tumor necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand.
Clinically explored strategies
Preliminary attempts to explore the therapeutic modulation of apoptosis against cancer by gene transfer have been started, driven by encouraging preclinical data in animal models. Clinical trials are currently ongoing to evaluate the value of pro-apoptotic p53 and adenoviral E1A, and a growing number of other candidate genes are being considered and tested preclinically (see [20,81,82] and Table 1).
Supplementation or restoration of p53
Direct induction of apoptosis has been attempted by replacing the tumor suppressor gene p53. Several factors made this gene an attractive candidate, including its frequent inactivation in human tumors, the observed lack of toxicity of wild-type p53 itself, the control that it exerts in multiple other genes implicated in apoptosis and cell cycle regulation, and some experimental evidence for a bystander effect. Both in vitro and in vivo animal studies [84,85,86,87] have indeed shown induction of apoptosis and suppression of tumorigenicity in human breast cancer models after p53 gene delivery via recombinant adenoviral vectors. Of interest, this result has also been observed in tumor cell sublines selected for resistance to drugs that are commonly used in the treatment of breast cancer [88] and in tumor cells expressing wild-type p53 [89]. Furthermore, adenovirus-mediated delivery of p53 augments the cytotoxic effect of the chemotherapeutic drug paclitaxel [90]. These preclinical studies were followed by two clinical trials in breast cancer in which p53 is currently being administered intralesionally, or incubated ex vivo with bone marrow for purging of contaminating breast cancer cells [91]. Potential obstacles for the successful clinical exploitation of p53, however, have arisen with the observation that wild-type p53 can be inactivated in human breast cancer cells that express mutant p53 [92], and with the low efficiency in vivo of current gene delivery vectors.
Adenoviral E1A
In addition to restoration of p53, the other pro-apoptotic approach currently being clinically tested is based on liposome-mediated delivery of the adenoviral gene E1A [91,93]. Gene transfer of E1A inhibits transcription of the human HER-2/neu promoter, and suppresses the tumorigenicity and metastatic potential of the HER-2/neu oncogene in cells that overexpress this oncogene [94]. An analogous effect of E1A in anchorage-independent growth and tumorigenicity has been shown in a HER-2/neu-independent manner [95]. Finally, E1A sensitizes mammalian cells to immune-mediated apoptosis in a p53-independent manner [96]. Thus, delivery of E1A could have an effect in vivo that exceeds the limitations imposed by the heterogeneity in the HER-2/neu or p53 status within the tumor.
Requirements for pro-apoptotic gene therapy
A variety of additional interventions and targets have been proposed for the gene-based therapeutic induction of apoptosis (see Table 1, and the references therein). Here, comments are limited to theoretical aspects that need to be considered for designing successful therapeutic interventions for breast cancer based on the genetic modulation of apoptosis. Specifically, the gene transfer systems and knowledge of the genetic pathophysiology of the disease as the most critical aspects are considered.
High levels of gene transfer
Gene transfer vectors are needed that can trigger apopto-sis in most malignant cells in any given tumor. Most frequently, current vector systems have been employed for delivery of therapeutic nucleic acids to relevant target cells in loco-regional contexts. In general, a fundamental recognition in most of these studies has been the disparity noted between the in vitro and in vivo gene transfer efficiencies of these vectors, with a generally suboptimal tumor transduction [97]. For disseminated disease, employment of available vectors is not presently feasible. This restriction is based on their limited capacity to accomplish efficient gene transfer to widely disseminated tumor targets in vivo. Implicit in this limitation is the recognition of three requirements for any candidate vector system for this purpose: the ability to accomplish highly efficient and nontoxic gene delivery after direct in vivo delivery via the intravascular route; the ability to accomplish targeted, specific gene delivery to a selected cellular subset; and the ability to escape the innate and adaptive immune responses.
With regard to the first requirement, only two presently available vector systems, cationic liposomes and recombinant adenoviral vectors, have been reported to transduce various end organs after in vivo gene delivery [98,99,100,101]. However, low levels of gene expression [102,103], or promiscuous tropism and entrapment of the vector by the reticuloendothelial system, mostly in the liver [104], respectively, have undermined the utility of the aforementioned vector systems for accomplishing transduction of normal breast tissue and disseminated breast cancer [105]. This fundamental limit has hindered achieving the second requirement (ie the evaluation of tissue-specific or tumor-specific promoters for transcriptional targeting of therapeutic genes in the context of in vivo models of breast cancer) [105]. With regard to the effects of the immune response, viral vectors display foreign antigens that can induce a strong cellular and humoral immune response in humans [106,107,108]. This can ultimately determine immune-mediated clearance of the vector and of the virally infected target cells [109], and precludes efficient readministration of the vector.
Thus, important limitations of current approaches used for implementation of pro-apoptotic gene therapy for disseminated cancers, including breast cancer, have been noted. Although many potentially effective strategies exist to achieve the molecular treatment of breast cancer, gene delivery issues have limited definitive evaluation of these methods in clinically relevant models.
In this regard, understanding of the determinants of vector efficacy at a cellular level has recently allowed vectors to be designed that achieve cell-specific gene delivery in the loco-regional context. For instance, altering the tropism of adenoviral vectors by bifunctional, antibody-based conjugates and by genetic engineering of the binding sites of the virus dramatically enhances the infectivity of otherwise refractory tumor cells [110]. This occurs by allowing the cellular entry of the virus through heterologous pathways, thus overcoming the paucity of its receptor, coxsackie and adenovirus receptor (CAR), which characterizes human primary tumors. Here the mandate for a high level of gene transfer may be stricter, given the lack of firm evidence for a useful bystander effect mediated by pro-apoptotic interventions, perhaps with the exception of replacement of p53 [111]. In this case, a better definition of the basis of bystander effects observed after gene delivery of p53 should lead to the exploitation of similar schemas by designing ad hoc therapeutic payloads. Examples would be secretory molecules such as the IGF binding protein 3 [112], which is induced by p53 and inhibits the powerful growth factor IGF-1; and translocating molecules, such as fusion proteins derived from the herpes simplex virus protein VP22 [113] or from the Tat protein of human immunodeficiency virus type 1 [114,115,116,117]. Further advances are also needed to understand the determinants of the biodistribution of viral vector after systemic intravascular administration. To this end, novel molecular and imaging technologies [118] have been developed that allow the amount of vector in relevant tissues to be quantified [119] and gene expression to be followed noninvasively in vivo [120*], both of which should accelerate these fundamental studies.
High therapeutic index
Given the ubiquity of the numerous cellular proteins involved in apoptotic pathways, highly selective activation in cancer cells of lethal processes may also be a critical requirement of therapeutic maneuvers. The lower threshold for undergoing apoptosis that characterizes tumor cells [1,2] could, however, offer an advantageous therapeutic window that makes this requirement less stringent. Certain pro-apoptotic interventions might still require a more stringent level of specificity. In such cases, vector targeting could provide the required restriction in the expression of the pro-apoptotic molecules.
Targeted gene therapy for breast cancer can be accomplished at different levels [121]. In one approach, the tumor cell can be targeted at the level of transduction to achieve the selective delivery of the therapeutic gene. This involves the derivation of a vector that binds selectively to the target breast cancer cell. Alternatively, the therapeutic gene can be placed under the control of breast tumor-specific transcriptional regulatory sequences that are activated in tumor cells, but not in normal cells, and therefore target expression selectively to the tumor cell (for a review see [28]). In addition, targeted gene therapy for cancer can exploit the unique physiology of solid tumors, such as hypoxia [122]. To date, targeted gene therapy has been attempted by employing either transductional targeting or transcriptional targeting alone. Again, enhancement of the overall level of specificity by combining the complementary approaches of transductional and transcriptional targeting may be required, each of which might be imperfect or 'leaky' by itself [121]. Perhaps more importantly, vector targeting may confer, in addition to selectivity, a significantly higher level of gene transfer, as mentioned above.
Multiple interventions and modalities
The signaling circuits that control apoptosis are complex and redundant, and tumors are formidably heterogeneous. Therefore, concomitant modulation of several components of the apoptotic pathways may be needed to provoke cell death in a robust and consistent manner. In this regard, the complex phenotype of the caspase knockout mice revealed that multiple and redundant mechanisms of caspase activation operate in parallel [123]. Recent high-throughput studies (for instance employing complementary DNA microarrays and serial analysis of gene expression) allow identification of patterns of gene expression and their variation under appropriate stimuli, which will contribute to the dissection of the relevant pathways in breast cancer [124**,125]. Interventions downstream in the circuits might also be preferable for avoiding regulatory counterbalances that may dissipate the effect of the intervention. For instance, multiple genes act downstream of p53 that alter the response to p53 restoration. In contrast, Bax has a more downstream role in effecting apoptosis, which perhaps explains its more predictable cytotoxicity on tumor cells [126*]. Finally, the magnitude of cell death after most pro-apoptotic interventions in vitro is cell line-dependent, with some tumor cells typically showing refractoriness to the induction of apoptosis. This has prompted the evaluation of combining a variety of pro-apoptotic interventions, and their association with chemotherapy [4**], radiotherapy [6,127], and other biologicals [128]. These multimodality treatments have consistently showed that higher levels of cell death are obtained when different pathways are modulated and modalities are combined, clearly indicating that the targeted pathways do not totally overlap.
Conclusion
The contribution of a dysregulation of apoptosis to the malignant phenotype is increasingly being recognized. In that context, novel technologies for high throughput analysis of the expression profile of breast tissues, normal and malignant, under a variety of experimental and treatment conditions, are becoming widespread. The information thus collected will allow the apoptotic machinery and its dysfunction to be defined with extraordinary breadth and precision. At the same time, more efficient and selective gene transfer systems are being designed, which will allow the implementation of complex genetic interventions tailored to breast cancer and aimed at different components of the redundant regulatory pathways. The combination of such genetic modifications is most likely to be effective when combined with conventional treatments, thus robustly activating several pro-apoptotic pathways, and paving the way for the therapeutically beneficial application of pro-apoptotic gene therapy for cancer of the breast.
Figures and Table
References
- Evan G, Littlewood T. A matter of life and cell death. . Science. 1998;281:1317–1322. doi: 10.1126/science.281.5381.1317. [DOI] [PubMed] [Google Scholar]
- Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- Sumantran VN, Ealovega MW, Nunez G, Clarke MF, Wicha MS. Overexpression of Bcl-XS sensitizes MCF-7 cells to chemotherapy-induced apoptosis. Cancer Res. 1995;55:2507–2510. [PubMed] [Google Scholar]
- Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. . Cancer Res. 1999;59:734–741. [PubMed] [Google Scholar]
- Muschel RJ, Soto DE, McKenna WG, Bernhard EJ. Radiosensitization and apoptosis. Oncogene. 1998;17:3359–3363. doi: 10.1038/sj.onc.1202580. [DOI] [PubMed] [Google Scholar]
- Sheard MA, Krammer PH, Zaloudik J. Fractionated gamma-irradiation renders tumour cells more responsive to apoptotic signals through CD95. . Br J Cancer. 1999;80:1689–1696. doi: 10.1038/sj.bjc.6690585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Williams GT. Programmed cell death: apoptosis and oncogenesis. Cell. 1991;65:1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- Strasser A. Dr. Josef Steiner Cancer Research Prize Lecture: the role of physiological cell death in neoplastic transformation and in anti-cancer therapy. Int J Cancer. 1999;81:505–511. doi: 10.1002/(sici)1097-0215(19990517)81:4<505::aid-ijc1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Allan DJ, Howell A, Roberts SA, et al. Reduction in apoptosis relative to mitosis in histologically normal epithelium accompanies fibrocystic change and carcinoma of the premenopausal human `breast. . J Pathol. 1992;167:25–32. doi: 10.1002/path.1711670106. [DOI] [PubMed] [Google Scholar]
- Shibata MA, Liu ML, Knudson MC, et al. Haploid loss of bax leads to accelerated mammary tumor development in C3(1)/SV40-TAg transgenic mice: reduction in protective apoptotic response at the preneoplastic stage. . EMBO J. 1999;18:2692–2701. doi: 10.1093/emboj/18.10.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaattela M, Benedict M, Tewari M, Shayman JA, Dixit VM. Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene. 1995;10:2297–2305. [PubMed] [Google Scholar]
- Frisch SM. Evidence for a function of death-receptor-related, death-domain-containing proteins in anoikis. Curr Biol . 1999;9:1047–1049. doi: 10.1016/s0960-9822(99)80455-2. [DOI] [PubMed] [Google Scholar]
- Hannun YA. Apoptosis and the dilemma of cancer chemotherapy. Blood. 1997;89:1845–1853. [PubMed] [Google Scholar]
- Haimovitz-Friedman A, Kan CC, Ehleiter D, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GJ, Kimijima I, Onda M, et al. Tamoxifen-induced apoptosis in breast cancer cells relates to down-regulation of bcl-2, but not bax and bcl-X(L), without alteration of p53 protein levels. . Clin Cancer Res. 1999;5:2971–2977. [PubMed] [Google Scholar]
- Shao ZM, Li J, Wu J, et al. Neo-adjuvant chemotherapy for operable breast cancer induces apoptosis. Breast Cancer Res Treat. 1999;53:263–269. doi: 10.1023/a:1006194921139. [DOI] [PubMed] [Google Scholar]
- Reed JC. Bcl-2: prevention of apoptosis as a mechanism of drug resistance. Hematol Oncol Clin North Am. 1995;9:451–473. [PubMed] [Google Scholar]
- Schmitt CA, Lowe SW. Apoptosis and therapy. J Pathol . 1999;187:127–137. doi: 10.1002/(SICI)1096-9896(199901)187:1<127::AID-PATH251>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Petit P, Zamzami N, Vayssiere JL, Mignotte B. The biochemistry of programmed cell death. FASEB J. 1995;9:1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- Walker RA, Jones JL, Chappell S, Walsh T, Shaw JA. Molecular pathology of breast cancer and its application to clinical management. . Cancer Metastasis Rev. 1997;16:5–27. doi: 10.1023/a:1005740222307. [DOI] [PubMed] [Google Scholar]
- Wu J. Apoptosis and angiogenesis: two promising tumor markers in breast cancer. Anticancer Res. 1996;16:2233–2239. [PubMed] [Google Scholar]
- Brenner AJ, Aldaz CM. The genetics of sporadic breast cancer. Prog Clin Biol Res. 1997;396:63–82. [PubMed] [Google Scholar]
- Dickson RB, Lippman ME. Growth factors in breast cancer. . Endocr Rev. 1995;16:559–589. doi: 10.1210/edrv-16-5-559. [DOI] [PubMed] [Google Scholar]
- Kumar R, Mandal M, Lipton A, Harvey H, Thompson CB. Overexpression of HER2 modulates bcl-2, bcl-XL, and tamoxifen-induced apoptosis in human MCF-7 breast cancer cells. . Clin Cancer Res. 1996;2:1215–1219. [PubMed] [Google Scholar]
- Gooch JL, Van Den Berg CL, Yee D. Insulin-like growth factor (IGF)-1 rescues breast cancer cells from chemotherapy-induced cell death: proliferative and anti-apoptotic effects. Breast Cancer Res Treat . 1999;56:1–10. doi: 10.1023/a:1006208721167. [DOI] [PubMed] [Google Scholar]
- Ruppert JM, Wright M, Rosenfeld M, et al. Gene therapy strategies for carcinoma of the breast. Breast Cancer Res Treat . 1997;44:93–114. doi: 10.1023/a:1005761723853. [DOI] [PubMed] [Google Scholar]
- Boxhorn HK, Eck SL. Gene therapy for breast cancer. . Hematol Oncol Clin North Am. 1998;12:665–675. doi: 10.1016/s0889-8588(05)70014-9. [DOI] [PubMed] [Google Scholar]
- Elledge RM, Allred DC. The p53 tumor suppressor gene in breast cancer. Breast Cancer Res Treat. 1994;32:39–47. doi: 10.1007/BF00666204. [DOI] [PubMed] [Google Scholar]
- Berns EM, De Witte HH, Klijn JG, et al. Prognostic value of TP53 protein accumulation in human primary breast cancer: an analysis by luminometric immunoassay on 1491 tumor cytosols. . Anticancer Res. 1997;17:3003–3006. [PubMed] [Google Scholar]
- White E. Tumour biology. p53, guardian of Rb. Nature . 1994;371:21–22. doi: 10.1038/371021a0. [DOI] [PubMed] [Google Scholar]
- Livingstone LR, White A, Sprouse J, et al. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992;70:923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Sheikh MS, Rochefort H, Garcia M. Overexpression of p21WAF1/CIP1 induces growth arrest, giant cell formation and apoptosis in human breast carcinoma cell lines. Oncogene. 1995;11:1899–1905. [PubMed] [Google Scholar]
- Sierra A, Lloveras B, Castellsague X, et al. Bcl-2 expression is associated with lymph node metastasis in human ductal breast carcinoma. Int J Cancer. 1995;60:54–60. doi: 10.1002/ijc.2910600108. [DOI] [PubMed] [Google Scholar]
- Eissa S, Labib R, Khalifa A, et al. Regulators of apoptosis in human breast cancer. Clin Biochem. 1999;32:321–326. doi: 10.1016/s0009-9120(99)00025-9. [DOI] [PubMed] [Google Scholar]
- Mustonen M, Raunio H, Paakko P, Soini Y. The extent of apoptosis is inversely associated with bcl-2 expression in premalignant and malignant breast lesions. Histopathology. 1997;31:347–354. doi: 10.1046/j.1365-2559.1997.2710877.x. [DOI] [PubMed] [Google Scholar]
- Holmqvist P, Lundstrom M, Stal O. Apoptosis and Bcl-2 expression in relation to age, tumor characteristics and prognosis in breast cancer. South-East Sweden Breast Cancer Group. Int J Biol Markers. 1999;14:84–91. doi: 10.1177/172460089901400205. [DOI] [PubMed] [Google Scholar]
- Sierra A, Castellsague X, Coll T, et al. Expression of death-related genes and their relationship to loss of apoptosis in T1 ductal breast carcinomas. Int J Cancer. 1998;79:103–110. doi: 10.1002/(sici)1097-0215(19980417)79:2<103::aid-ijc1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Sierra A, Castellsague X, Coll T, et al. Expression of death-related genes and their relationship to loss of apoptosis in T1 ductal breast carcinomas. Int J Cancer. 1998;79:103–110. doi: 10.1002/(sici)1097-0215(19980417)79:2<103::aid-ijc1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Leek RD, Kaklamanis L, Pezzella F, Gatter KC, Harris AL. bcl-2 in normal human breast and carcinoma, association with oestrogen receptor-positive, epidermal growth factor receptor-negative tumours and in situ cancer. Br J Cancer. 1994;69:135–139. doi: 10.1038/bjc.1994.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensuu H, Pylkkanen L, Toikkanen S. Bcl-2 protein expression and long-term survival in breast cancer. Am J Pathol. 1994;145:1191–1198. [PMC free article] [PubMed] [Google Scholar]
- Silvestrini R, Veneroni S, Daidone MG, et al. The Bcl-2 protein: a prognostic indicator strongly related to p53 protein in lymph node-negative breast cancer patients. J Natl Cancer Inst. 1994;86:499–504. doi: 10.1093/jnci/86.7.499. [DOI] [PubMed] [Google Scholar]
- Zhang GJ, Kimijima I, Abe R, et al. Apoptotic index correlates to bcl-2 and p53 protein expression, histological grade and prognosis in invasive breast cancers. Anticancer Res. 1998;18:1989–1998. [PubMed] [Google Scholar]
- van Slooten HJ, van de Vijver MJ, van de Velde CJ, van Dierendonck JH. Loss of Bcl-2 in invasive breast cancer is associated with high rates of cell death, but also with increased proliferative activity. . Br J Cancer. 1998;77:789–796. doi: 10.1038/bjc.1998.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix P, Krajewski S, Reed JC, et al. In vivo patterns of Bcl-2 family protein expression in breast carcinomas in relation to apoptosis. J Pathol. 1999;187:410–415. doi: 10.1002/(SICI)1096-9896(199903)187:4<410::AID-PATH266>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Vakkala M, Lahteenmaki K, Raunio H, Paakko P, Soini Y. Apoptosis during breast carcinoma progression. Clin Cancer Res. 1999;5:319–324. [PubMed] [Google Scholar]
- Hurlimann J, Larrinaga B, Vala DL. bcl-2 protein in invasive ductal breast carcinomas. Virchows Arch. 1995;426:163–168. doi: 10.1007/BF00192638. [DOI] [PubMed] [Google Scholar]
- Gee JM, Robertson JF, Ellis IO, et al. Immunocytochemical localization of BCL-2 protein in human breast cancers and its relationship to a series of prognostic markers and response to endocrine therapy. Int J Cancer. 1994;59:619–628. doi: 10.1002/ijc.2910590508. [DOI] [PubMed] [Google Scholar]
- Gasparini G, Barbareschi M, Doglioni C, et al. Expression of bcl-2 protein predicts efficacy of adjuvant treatments in operable node-positive breast cancer. Clin Cancer Res. 1995;1:189–198. [PubMed] [Google Scholar]
- Olopade OI, Adeyanju MO, Safa AR, et al. Overexpression of BCL-x protein in primary breast cancer is associated with high tumor grade and nodal metastases. Cancer J Sci Am. 1997;3:230–237. [PubMed] [Google Scholar]
- Krajewski S, Blomqvist C, Franssila K, et al. Reduced expression of proapoptotic gene BAX is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res. 1995;55:4471–4478. [PubMed] [Google Scholar]
- Veronese S, Mauri FA, Caffo O, et al. Bax immunohistochemical expression in breast carcinoma: a study with long term follow-up. Int J Cancer. 1998;79:13–18. doi: 10.1002/(sici)1097-0215(19980220)79:1<13::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Zhan Q, Fan S, Bae I, et al. Induction of bax by genotoxic stress in human cells correlates with normal p53 status and apoptosis. Oncogene . 1994;9:3743–3751. [PubMed] [Google Scholar]
- Bargou RC, Wagener C, Bommert K, et al. Overexpression of the death-promoting gene bax-alpha which is downregulated in breast cancer restores sensitivity to different apoptotic stimuli and reduces tumor growth in SCID mice. J Clin Invest. 1996;97:2651–2659. doi: 10.1172/JCI118715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nature Med . 1997;3:1228–1232. doi: 10.1038/nm1197-1228. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. . Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Xiang J, Chao DT, Korsmeyer SJ. BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. . Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo I, Brenner C, Zamzami N, et al. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. . Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Wallach D, Varfolomeev EE, Malinin NL, et al. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. . Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- Gross A, Yin XM, Wang K, et al. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- Muschen M, Moers C, Warskulat U, et al. CD95 ligand expression in dedifferentiated breast cancer. J Pathol. 1999;189:378–386. doi: 10.1002/(SICI)1096-9896(199911)189:3<378::AID-PATH439>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Jaattela M. Escaping cell death: survival proteins in cancer. . Exp Cell Res. 1999;248:30–43. doi: 10.1006/excr.1999.4455. [DOI] [PubMed] [Google Scholar]
- LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247–3259. doi: 10.1038/sj.onc.1202569. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Reed JC. IAP family proteins: suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, surviving, expressed in cancer and lymphoma. Nature Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- Tamm I, Wang Y, Sausville E, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- Li F, Ambrosini G, Chu EY, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Clark GM, Tandon AK, et al. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J Natl Cancer Inst. 1993;85:570–574. doi: 10.1093/jnci/85.7.570. [DOI] [PubMed] [Google Scholar]
- Lazaris AC, Chatzigianni EB, Panoussopoulos D, et al. Proliferating cell nuclear antigen and heat shock protein 70 immunolocalization in invasive ductal breast cancer not otherwise specified. Breast Cancer Res Treat. 1997;43:43–51. doi: 10.1023/a:1005706110275. [DOI] [PubMed] [Google Scholar]
- Vargas-Roig LM, Fanelli MA, Lopez LA, et al. Heat shock proteins and cell proliferation in human breast cancer biopsy samples. Cancer Detect Prev. 1997;21:441–451. [PubMed] [Google Scholar]
- Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer. 1998;79:468–475. doi: 10.1002/(sici)1097-0215(19981023)79:5<468::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Liu FF, Miller N, Levin W, et al. The potential role of HSP70 as an indicator of response to radiation and hyperthermia treatments for recurrent breast cancer. Int J Hyperthermia. 1996;12:197–208. doi: 10.3109/02656739609022508. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Green S, Elledge RM, et al. Heat shock proteins hsp27 and hsp70: lack of correlation with response to tamoxifen and clinical course of disease in estrogen receptor-positive metastatic breast cancer (a Southwest Oncology Group Study). Clin Cancer Res. 1998;4:1263–1266. [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science . 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. . Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Favrot M, Coll JL, Louis N, Negoescu A. Cell death and cancer: replacement of apoptotic genes and inactivation of death suppressor genes in therapy. Gene Ther. 1998;5:728–739. doi: 10.1038/sj.gt.3300661. [DOI] [PubMed] [Google Scholar]
- Reed JC. Apoptosis as a goal of cancer gene therapy. The Development of Human Gene Therapy. Edited by Friedmann T. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. 1999;:545–572. [Google Scholar]
- Neubauer A, Thiede C, Huhn D, Wittig B. P53 and induction of apoptosis as a target for anticancer therapy [Review]. Leukemia. 1996;10 (suppl 3):S2–S4. [PubMed] [Google Scholar]
- Nielsen LL, Maneval DC. P53 tumor suppressor gene therapy for cancer. Cancer Gene Ther. 1998;5:52–63. [PubMed] [Google Scholar]
- Seth P, Brinkmann U, Schwartz GN, et al. Adenovirus-mediated gene transfer to human breast tumor cells: an approach for cancer gene therapy and bone marrow purging. . Cancer Res. 1996;56:1346–1351. [PubMed] [Google Scholar]
- Nielsen LL, Dell J, Maxwell E, et al. Efficacy of p53 adenovirus-mediated gene therapy against human breast cancer xenografts. Cancer Gene Ther. 1997;4:129–138. [PubMed] [Google Scholar]
- Nielsen LL, Gurnani M, Syed J, et al. Recombinant E1-deleted adenovirus-mediated gene therapy for cancer: efficacy studies with p53 tumor suppressor gene and liver histology in tumor xenograft models. Hum Gene Ther. 1998;9:681–694. doi: 10.1089/hum.1998.9.5-681. [DOI] [PubMed] [Google Scholar]
- Seth P, Katayose D, Li Z, et al. A recombinant adenovirus expressing wild type p53 induces apoptosis in drug-resistant human breast cancer cells: a gene therapy approach for drug-resistant cancers. Cancer Gene Ther. 1997;4:383–390. [PubMed] [Google Scholar]
- Li P, Bui T, Gray D, Klamut HJ. Therapeutic potential of recombinant p53 overexpression in breast cancer cells expressing endogenous wild-type p53. Breast Cancer Res Treat. 1998;48:273–286. doi: 10.1023/a:1005961705860. [DOI] [PubMed] [Google Scholar]
- Nielsen LL, Lipari P, Dell J, Gurnani M, Hajian G. Adenovirus-mediated p53 gene therapy and paclitaxel have synergistic efficacy in models of human head and neck, ovarian, prostate, and breast cancer. Clin Cancer Res. 1998;4:835–846. [PubMed] [Google Scholar]
- Office of Recombinant DNA Activities. 1998. http://www.nih.gov/od/orda/docs.pdf
- Vinyals A, Peinado MA, Gonzalez-Garrigues M, et al. Failure of wild-type p53 gene therapy in human cancer cells expressing a mutant p53 protein. Gene Ther. 1999;6:22–33. doi: 10.1038/sj.gt.3300786. [DOI] [PubMed] [Google Scholar]
- Hortobagyi GN, Hung MC, Lopez-Berestein G. A Phase I multicenter study of E1A gene therapy for patients with metastatic breast cancer and epithelial ovarian cancer that overexpresses HER-2/neu or epithelial ovarian cancer. Hum Gene Ther. 1998;9:1775–1798. doi: 10.1089/hum.1998.9.12-1775. [DOI] [PubMed] [Google Scholar]
- Chang JY, Xia W, Shao R, et al. The tumor suppression activity of E1A in HER-2/neu-overexpressing breast cancer. Oncogene . 1997;14:561–568. doi: 10.1038/sj.onc.1200861. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Dolter KE. Adenovirus E1a-mediated tumor suppression by a c-erbB-2/neu-independent mechanism. Cancer Res. 1995;55:5551–5555. [PubMed] [Google Scholar]
- Cook JL, Routes BA, Leu CY, Walker TA, Colvin KL. E1A oncogene-induced cellular sensitization to immune-mediated apoptosis is independent of p53 and resistant to blockade by E1B 19 kDa protein. . Exp Cell Res. 1999;252:199–210. doi: 10.1006/excr.1999.4617. [DOI] [PubMed] [Google Scholar]
- Takamiya Y, Short MP, Ezzeddine ZD, et al. Gene therapy of malignant brain tumors: a rat glioma line bearing the herpes simplex virus type 1-thymidine kinase gene and wild type retrovirus kills other tumor cells. J Neurosci Res. 1992;33:493–503. doi: 10.1002/jnr.490330316. [DOI] [PubMed] [Google Scholar]
- Debs RJ, Heath TD, Papahadjopoulos D. Targeting of anti-Thy 1.1 monoclonal antibody conjugated liposomes in Thy 1.1 mice after intravenous administration. Biochim Biophys Acta. 1987;901:183–190. doi: 10.1016/0005-2736(87)90114-3. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet LD, Briand P, Perricaudet M. Feasibility of adenovirus-mediated gene transfer in vivo. . Bone Marrow Transplant. 1992;9 (suppl 1):151–152. [PubMed] [Google Scholar]
- Brody SL, Crystal RG. Adenovirus-mediated in vivo gene transfer. Ann N Y Acad Sci. 1994;716:90–101. doi: 10.1111/j.1749-6632.1994.tb21705.x. [DOI] [PubMed] [Google Scholar]
- Heise C, Sampson-Johannes A, Williams A, et al. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nature Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- Lesoon-Wood LA, Kim WH, Kleinman HK, Weintraub BD, Mixson AJ. Systemic gene therapy with p53 reduces growth and metastases of a malignant human breast cancer in nude mice. Hum Gene Ther. 1995;6:395–405. doi: 10.1089/hum.1995.6.4-395. [DOI] [PubMed] [Google Scholar]
- Chen QR, Mixson JA. Systemic gene therapy with p53 inhibits breast cancer: recent advances and therapeutic implications. Front Biosci. 1998;3:D997–D1004. doi: 10.2741/a340. [DOI] [PubMed] [Google Scholar]
- Vrancken PM, Perkins AL, Kay MA. Method for multiple portal vein infusions in mice: quantitation of adenovirus-mediated hepatic gene transfer. Biotechniques. 1996;20:278–285. doi: 10.2144/96202rr05. [DOI] [PubMed] [Google Scholar]
- Anderson LM, Swaminathan S, Zackon I, et al. Adenovirus-mediated tissue-targeted expression of the HSVtk gene for the treatment of breast cancer. Gene Ther. 1999;6:854–864. doi: 10.1038/sj.gt.3300909. [DOI] [PubMed] [Google Scholar]
- Molnar-Kimber KL, Sterman DH, Chang M, et al. Impact of preexisting and induced humoral and cellular immune responses in an adenovirus-based gene therapy phase I clinical trial for localized mesothelioma. Hum Gene Ther. 1998;9:2121–2133. doi: 10.1089/hum.1998.9.14-2121. [DOI] [PubMed] [Google Scholar]
- Gahery-Segard H, Farace F, Godfrin D, et al. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J Virol. 1998;72:2388–2397. doi: 10.1128/jvi.72.3.2388-2397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon KB, Liu CC, Gall JG, et al. Tumor necrosis factor alpha plays a central role in immune-mediated clearance of adenoviral vectors. Proc Natl Acad Sci USA. 1997;94:9814–9819. doi: 10.1073/pnas.94.18.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafri T, Morgan D, Krahl T, et al. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc Natl Acad Sci USA. 1998;95:11377–11382. doi: 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds PN, Curiel DT. Strategies to adapt adenoviral vectors for gene therapy applications: targeting and integration. . The Development of Human Gene Therapy. Edited by Friedmann T. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. 1999;:111–130. [Google Scholar]
- Bouvet M, Ellis LM, Nishizaki M, et al. Adenovirus-mediated wild-type p53 gene transfer down-regulates vascular endothelial growth factor expression and inhibits angiogenesis in human colon cancer. Cancer Res. 1998;58:2288–2292. [PubMed] [Google Scholar]
- Buckbinder L, Talbott R, Velasco-Miguel S, et al. Induction of the growth inhibitor IGF-binding protein 3 by p53. . Nature. 1995;377:646–649. doi: 10.1038/377646a0. [DOI] [PubMed] [Google Scholar]
- Dilber MS, Phelan A, Aints A, et al. Intercellular delivery of thymidine kinase prodrug activating enzyme by the herpes simplex virus protein, VP22. Gene Ther. 1999;6:12–21. doi: 10.1038/sj.gt.3300838. [DOI] [PubMed] [Google Scholar]
- Fawell S, Seery J, Daikh Y, et al. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. . Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Phelan A, Elliott G, O'Hare P. Intercellular delivery of functional p53 by the herpesvirus protein VP22. Nature Biotechnol . 1998;16:440–443. doi: 10.1038/nbt0598-440. [DOI] [PubMed] [Google Scholar]
- Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- Gambhir SS, Barrio JR, Herschman HR, Phelps ME. Assays for non-invasive imaging of reporter gene expression. Nucl Med Biol. 1999;26:481–490. doi: 10.1016/s0969-8051(99)00021-9. [DOI] [PubMed] [Google Scholar]
- Becker K, Pan D, Whitley CB. Real-time quantitative polymerase chain reaction to assess gene transfer. Hum Gene Ther. 1999;10:2559–2566. doi: 10.1089/10430349950016898. [DOI] [PubMed] [Google Scholar]
- Rogers BE, McLean SF, Kirkman RL, et al. In vivo localization of [(111)In]-DTPA-D-Phe1-octreotide to human ovarian tumor xenografts induced to express the somatostatin receptor subtype 2 using an adenoviral vector. Clin Cancer Res. 1999;5:383–393. [PubMed] [Google Scholar]
- Douglas JT, Curiel DT. Targeted gene therapy. Tumor Targeting. 1995;2:67–84. [Google Scholar]
- Binley K, Iqball S, Kingsman A, Kingsman S, Naylor S. An adenoviral vector regulated by hypoxia for the treatment of ischaemic disease and cancer. Gene Ther. 1999;6:1721–1727. doi: 10.1038/sj.gt.3301001. [DOI] [PubMed] [Google Scholar]
- Li H, Yuan J. Deciphering the pathways of life and death. . Curr Opin Cell Biol. 1999;11:261–266. doi: 10.1016/s0955-0674(99)80035-0. [DOI] [PubMed] [Google Scholar]
- Perou CM, Jeffrey SS, van de RM, et al. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci USA. 1999;96:9212–9217. doi: 10.1073/pnas.96.16.9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacht M, Ferguson AT, Zhang W, et al. Combining serial analysis of gene expression and array technologies to identify genes differentially expressed in breast cancer. Cancer Res. 1999;59:5464–5470. [PubMed] [Google Scholar]
- Xiang J, Piche A, Rancourt C, et al. Adenoviral vector-mediated expression of Bax selectively induces apoptosis in ovarian cancer cells. Tumor Targeting. 1999;4:84–91. [Google Scholar]
- Zhivotovsky B, Joseph B, Orrenius S. Tumor radiosensitivity and apoptosis. Exp Cell Res. 1999;248:10–17. doi: 10.1006/excr.1999.4452. [DOI] [PubMed] [Google Scholar]
- Putzer BM, Bramson JL, Addison CL, et al. Combination therapy with interleukin-2 and wild-type p53 expressed by adenoviral vectors potentiates tumor regression in a murine model of breast cancer. . Hum Gene Ther. 1998;9:707–718. doi: 10.1089/hum.1998.9.5-707. [DOI] [PubMed] [Google Scholar]
- Paillard F. Induction of apoptosis with I-kappaB, the inhibitor of NF-kappaB. Hum Gene Ther. 1999;10:1–3. doi: 10.1089/10430349950019138. [DOI] [PubMed] [Google Scholar]
- Rakkar AN, Katayose Y, Kim M, et al. A novel adenoviral vector expressing human Fas/CD95/APO-1 enhances p53-mediated apoptosis. Cell Death Differ. 1999;6:326–333. doi: 10.1038/sj.cdd.4400498. [DOI] [PubMed] [Google Scholar]
- Wright M, Grim J, Deshane J, et al. An intracellular anti-erbB-2 single-chain antibody is specifically cytotoxic to human breast carcinoma cells overexpressing erbB-2. Gene Ther. 1997;4:317–322. doi: 10.1038/sj.gt.3300372. [DOI] [PubMed] [Google Scholar]
- Piche A, Grim J, Rancourt C, et al. Modulation of Bcl-2 protein levels by an intracellular anti-Bcl-2 single-chain antibody increases drug-induced cytotoxicity in the breast cancer cell line MCF-7. . Cancer Res. 1998;58:2134–2140. [PubMed] [Google Scholar]
- Ambrosini G, Adida C, Sirugo G, Altieri DC. Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. . J Biol Chem. 1998;273:11177–11182. doi: 10.1074/jbc.273.18.11177. [DOI] [PubMed] [Google Scholar]
- Wei Y, Zhao X, Kariya Y, Teshigawara K, Uchida A. Inhibition of proliferation and induction of apoptosis by abrogation of heat-shock protein (HSP) expression in tumor cells. Cancer Immunol Immunother. 1995;40:73–78. doi: 10.1007/BF01520287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Madireddi MT, Lin JJ, et al. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci USA. 1998;95:14400–14405. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YT, Strobel T, Kufe D, Cannistra SA. In vivo cytotoxicity of ovarian cancer cells through tumor-selective expression of the BAX gene. . Cancer Res. 1999;59:2121–2126. [PubMed] [Google Scholar]
- Clarke MF, Apel IJ, Benedict MA, et al. A recombinant bcl-xs adenovirus selectively induces apoptosis in cancer cells but not in normal bone marrow cells. Proc Natl Acad Sci USA. 1995;92:11024–11028. doi: 10.1073/pnas.92.24.11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelli M, Cunningham GR, Walkup M, et al. Signaling pathway activated during apoptosis of the prostate cancer cell line LNCaP: overexpression of caspase-7 as a new gene therapy strategy for prostate cancer. Cancer Res. 1999;59:382–390. [PubMed] [Google Scholar]
- Ealovega MW, McGinnis PK, Sumantran VN, Clarke MF, Wicha MS. bcl-xs gene therapy induces apoptosis of human mammary tumors in nude mice. Cancer Res. 1996;56:1965–1969. [PubMed] [Google Scholar]
- Danen-Van Oorschot AA, Fischer DF, Grimbergen JM, et al. Apoptin induces apoptosis in human transformed and malignant cells but not in normal cells. Proc Natl Acad Sci USA. 1997;94:5843–5847. doi: 10.1073/pnas.94.11.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersen AM, van der Eb MM, Rademaker HJ, et al. Specific tumor-cell killing with adenovirus vectors containing the apoptin gene. Gene Ther. 1999;6:882–892. doi: 10.1038/sj.gt.3300876. [DOI] [PubMed] [Google Scholar]
- Shao N, Chai YL, Shyam E, Reddy P, Rao VN. Induction of apoptosis by the tumor suppressor protein BRCA1. Oncogene. 1996;13:1–7. [PubMed] [Google Scholar]
- Le XF, Vallian S, Mu ZM, Hung MC, Chang KS. Recombinant PML adenovirus suppresses growth and tumorigenicity of human breast cancer cells by inducing G1 cell cycle arrest and apoptosis. Oncogene. 1998;16:1839–1849. doi: 10.1038/sj.onc.1201705. [DOI] [PubMed] [Google Scholar]
- Putzer BM, Bramson JL, Addison CL, et al. Combination therapy with interleukin-2 and wild-type p53 expressed by adenoviral vectors potentiates tumor regression in a murine model of breast cancer. . Hum Gene Ther. 1998;9:707–718. doi: 10.1089/hum.1998.9.5-707. [DOI] [PubMed] [Google Scholar]


