Abstract
Clinical outcomes for patients undergoing allogeneic hematopoietic stem cell transplantation continue to improve, but chronic graft-versus-host disease (GVHD) remains a common toxicity and major cause of nonrelapse morbidity and mortality. Treatment of chronic GVHD has previously relied primarily on corticosteroids and other broadly immune suppressive agents. However, conventional immune suppressive agents have limited clinical efficacy in chronic GVHD, and prolonged immune suppressive treatments result in additional toxicities that further limit clinical recovery from transplant and return to normal daily function. Recent advances in our understanding of the immune pathology of chronic GVHD offer the possibility that new therapeutic approaches can be directed in more precise ways to target specific immunologic mechanisms and pathways. In this review, we briefly summarize current standard treatment options and present new therapeutic approaches that are supported by preclinical studies and early-phase clinical trials suggesting that these approaches may have clinical utility for treatment or prevention of chronic GVHD. Further evaluation of these new therapeutic options in well-designed prospective multicenter trials are needed to identify the most effective new agents and improve outcomes for patients with chronic GVHD.
Introduction
In all patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT), engrafting lymphocyte progenitors and mature donor lymphocytes encounter a broad array of recipient antigens that are highly immunogenic. Immune responses directed against these alloantigens result in the clinical syndromes of acute and chronic graft-versus-host disease (GVHD). Eventually, a successful transplant outcome requires that the donor immune system develop tolerance to these alloantigens while maintaining the ability to detect and respond effectively to an even broader array of foreign antigens. In patients with hematologic malignancies, donor immune responses directed against tumor cells (graft versus tumor) also help prevent relapse. Many studies in preclinical model systems and in patients undergoing allogeneic HSCT have enhanced our understanding of the various immunologic mechanisms that play key roles in the development of acute and chronic GVHD and graft-versus-tumor responses. While acute GVHD appears to be mediated primarily by mature donor T cells in the allogeneic stem cell product, chronic GVHD is now known to be a more complex immune reaction. Donor-derived effector T cells and B cells both contribute to the immune pathology of chronic GVHD, and regulatory elements within the T- and B-cell lineages play important roles in the development and maintenance of immune tolerance after HSCT. In this context, the immunologic mechanisms that result in chronic GVHD share many features with common autoimmune diseases, where defects in immune tolerance combine with adaptive immune responses targeting autoantigens to produce chronic tissue damage.
Studies in preclinical models have provided important insights into the complex interactions between subsets of effector T cells and B cells and abnormalities of immune regulation that contribute to tissue destruction and fibrosis in chronic GVHD.1,2 Although targeting a single noneffector cell type for the prevention or management of chronic GVHD may seem counterintuitive, the complex nature of these interactions offers many opportunities for specific interventions that have the potential to interrupt critical pathways needed to initiate and maintain these responses. Nonetheless, it is likely that interventions in individual pathways will not benefit all patients, and we do not yet have diagnostic biomarkers that can predict which pathway may represent the most appropriate target for intervention in any given patient. This review will briefly summarize current standard therapies for chronic GVHD and then focus on new therapeutic approaches that are supported by preclinical data and results of early-phase clinical trials. Many of these new approaches target specific immunologic pathways now known to play a role in chronic GVHD.
Standard therapy for chronic GVHD
Despite advances in the understanding of the pathobiology of chronic GVHD, there have been relatively few advances in the clinical management of this disease. To this day, corticosteroids and meticulous organ-specific care remain the mainstay of therapy.3,4 Corticosteroids are profoundly anti-inflammatory and broadly immune suppressive, affecting both innate and adaptive immunity. The addition of a calcineurin inhibitor to corticosteroids does not increase the response rate, but it allows for a reduction in steroid dosing that can reduce the sequelae of chronic corticosteroid therapy. Alternate-day dosing of corticosteroids similarly can reduce toxicity, but this approach is rarely used in clinical practice. The administration of other agents in combination with corticosteroids has not been proven to be beneficial when examined in prospective randomized trials.5 Therapy of corticosteroid-resistant chronic GVHD has relied primarily on broadly immune suppressive agents and specific inhibitors of T-cell signaling (such as tacrolimus and calcineurin inhibitors). Beyond those agents used in the initial therapy of chronic GVHD, the efficacy of second-line agents is limited, with response rates of ∼30% regardless of the agent that is chosen.3 There are no standard second-line therapies for advanced chronic GVHD, and enrollment in clinical trials designed to evaluate the safety and efficacy of new agents and/or combinations is often recommended in this setting.6 Ultimately, an effective approach to the prevention of chronic GVHD will be more useful than strategies to treat established disease.
Depletion of alloreactive T cells for the prevention of chronic GVHD
Mature donor-derived effector T cells are critical mediators of tissue damage in both acute and chronic GVHD. Various approaches that deplete donor T cells from hematopoietic stem cell products reduce the subsequent incidence and severity of chronic GVHD. These include positive selection of CD34 stem cells as well as methods to deplete T cells that express αβ T cell receptors, pan–T-cell antigens such as CD6 or naive T cells.7-11 T-cell depletion in vivo with antithymocyte globulin (ATG) administered in the peritransplant period to promote engraftment and prevent acute GVHD also reduces the incidence of chronic GVHD.12,13 Methods have also been developed to selectively deplete alloreactive T cells in the early posttransplant period. For example, the administration of posttransplant cyclophosphamide (pTCy) selectively depletes alloantigen-activated T cells in vivo and allows engraftment of hematopoietic stem cells from HLA-mismatched donors. Similarly, in vitro treatment of alloantigen-activated donor T cells with a photosensitizer (TH9402) enables selective photodepletion of these cells and allows the transfusion of donor T cells that can enhance T-cell immune reconstitution. When effective depletion of alloreactive T cells is achieved, these approaches appear to significantly reduce the incidence of both acute and chronic GVHD.14-18
B-cell depletion for established chronic GVHD
Studies in preclinical models and in patients after HSCT have suggested a role for donor B cells in the immune pathology of chronic GVHD.19,20 Ratanatharathorn et al21 were the first to note clinical improvement of chronic GVHD symptoms in a patient who received rituximab for immune-mediated thrombocytopenia that occurred after allogeneic HSCT. This provided a strong rationale for the evaluation of B-cell depletion strategies for the treatment or prevention of chronic GVHD, and subsequent reports have documented the effectiveness of this approach as therapy for steroid-refractory chronic GVHD.22-24 In a prospective study, we demonstrated a response rate of 70%, clinical safety, a dramatic steroid-sparing effect, and a meaningful reduction in chronic GVHD symptom score after a single 4-week course of rituximab. Clinical responses were also associated with reductions in titers of antibodies directed against Y-encoded minor histocompatibility antigens.25 In several published studies, there appeared to be a higher response rate in patients with sclerodermatous chronic GVHD. This led to a prospective randomized crossover trial that compared rituximab with imatinib in subjects previously treated with corticosteroids. However, significant clinical responses were noted in only 27% of subjects initially randomized to rituximab. In this study, clinical response to rituximab was predicted by higher levels of CD27+ activated B cells at trial entry.26
Two studies have examined the role of B-cell depletion combined with corticosteroids as initial therapy for chronic GVHD. The Stanford group treated 35 subjects with moderate to severe chronic GVHD using rituximab plus corticosteroids, demonstrating an overall response rate of 77% at 6 months and a complete response rate of 34%. However, by 24 months, the majority of subjects required additional therapy or succumbed to chronic GVHD.27 In contrast to other studies, clinical response was predicted by a naive (CD19+CD38−IgD+CD27−) B-cell phenotype. Pidala et al28 used the second-generation humanized monoclonal antibody ofatumumab, in conjunction with corticosteroids, in a phase 1 study that enrolled 12 subjects. Treatment was well tolerated, but in this small study, only 4 of 12 patients had complete responses after 6 months of therapy.
B-cell depletion for the prevention of chronic GVHD
Several studies have incorporated monoclonal anti–B-cell therapy as part of the conditioning regimen or given in the peritransplant period with the intent of reducing relapse of B-cell malignancies. In the BMT CTN 0701 trial, 65 subjects were given 4 doses of rituximab (1000 mg/m2) in the week prior and following a reduced-intensity HSCT.29 Despite very good depletion of CD20+ B cells and measurable levels of rituximab in the circulation that persisted beyond 6 months, the rate of chronic GVHD was >40% at 1 year and 60% at 2 years. A slightly larger study performed by the German High-Grade Lymphoma Study group prospectively randomized subjects to receive rituximab or no additional therapy after allogeneic HSCT.30 In this study, subjects received two 4-week courses of rituximab, beginning 21 and 175 days after HSCT. Results from this trial are difficult to interpret, because nearly half of the enrolled subjects received ATG. Although there was a reduction in the rate of chronic GVHD in patients randomized to the rituximab arm (33% vs 41%, P = .28), this was not statistically significant.
Two trials have employed B-cell depletion specifically with the goal of preventing chronic GVHD. The Stanford group administered rituximab 2 to 3 months after total lymphoid irradiation-ATG–based HSCT in a study that included 35 patients with B-cell malignancies.31 The cumulative incidence of chronic GVHD was only 20%, with an expected rate using this preparative regimen of ∼30%. Notably, male patients who receive stem cell transplants from female donors frequently develop antibodies to HY antigens, but this did not occur in patients that received rituximab for the prevention of chronic GVHD. In a phase 2 trial at the Dana-Farber Cancer Institute, 65 patients received single doses of rituximab (375 mg/m2) at 3-month intervals for 1 year after peripheral blood HSCT from related or unrelated donors.32 B-cell depletion was very effective and reconstitution of donor B cells was markedly delayed. The overall incidence of chronic GVHD was 48% at 2 years, however the rate of corticosteroid-requiring chronic GVHD was only 31% at 2 years, and was as low as 23% for patients with related donors. Comparison with a contemporaneous cohort of subjects who did not receive rituximab demonstrated a statistically significant decrease in the rate of steroid-requiring chronic GVHD. Non-relapse mortality was reduced, and overall survival improved, suggesting prevention of severe chronic GVHD can improve long-term survival. Given the promising activity of B-cell depletion for the prevention of chronic GVHD, a randomized trial using a second-generation monoclonal agent, obinutuzumab, is about to begin accrual.
One concern over the routine use of B-cell depletion in the post-HSCT setting is the persistent and profound hypogammaglobulinemia that is anticipated. In all studies, treatment with anti-CD20 antibodies results in profound and prolonged B-cell depletion.33 However, despite delayed reconstitution of B-cell immunity after HSCT, excess infections have generally not been noted when immunoglobulin repletion is routinely administered following American Society for Blood and Marrow Transplantation guidelines.34
Targeting the B-cell receptor signaling pathway
Spleen tyrosine kinase (SYK) and Bruton’s tyrosine kinase (BTK) play essential roles in B-cell receptor signaling. Upon engagement of B-cell receptor, SYK is activated and phosphorylates BTK. Both phosphorylated SYK and BTK can activate the downstream phospholipase C–γ2 pathway, whereas SYK can also activate the B-cell linker BLNK.35,36 Inhibition of B-cell signaling by targeting either SYK or BTK has become possible with the development of small-molecule inhibitors for these tyrosine kinases. While BTK is expressed primarily in B cells and other hematopoietic tissues, SYK has a broader expression pattern, including both epithelial and endothelial cells.35 Therefore, targeting these signaling pathways may be associated with broad on-target effects and potential toxicities, such as increased susceptibility to infections (for the SYK inhibitors) and cytopenias (for the BTK inhibitors). Nevertheless, studies in preclinical models suggest that targeting either SYK or BTK may be promising strategies for chronic GVHD therapy.36-38
Fostamatinib, the first SYK inhibitor to undergo advanced-stage clinical evaluation, has been tested extensively in rheumatologic diseases and is currently being tested in established chronic GVHD (#NCT02611063). Preliminary studies of the BTK inhibitor ibrutinib have also been reported.39 In a study of 28 subjects with steroid-refractory chronic GVHD, ibrutinib was given for a median of 4 months. Of 22 evaluable subjects, 11 had a partial response and 1 had a complete response, and the median corticosteroid dose reduction was 35%. Given these encouraging findings, a randomized trial is being planned, and this agent has been granted breakthrough status by the US Food and Drug Administration. The prospective use of ibrutinib in the post-HSCT setting to prevent relapse of chronic lymphocytic leukemia or other B-cell malignancies will additionally inform the role of ibrutinib for the prevention of chronic GVHD.
Although BTK is selectively expressed in B cells, ibrutinib also inhibits the closely related interleukin-2 (IL-2)–inducible kinase (ITK), which is highly expressed in T cells and ordinarily drives a TH2 response. In murine models, inhibition of both BTK and ITK by ibrutinib contributed to the prevention of chronic GVHD.40 In a recent clinical experience with ibrutinib, T cells were noted to be less activated.39 Inhibition of ITK may lead to a theoretical increase in CD8+-mediated graft-versus-leukemia (GVL), but broad inhibition of T cell activation may inhibit GVL as well as GVHD. Close monitoring of both T- and B-cell function as well as relapse will be needed to better understand the immunologic effects of ibrutinib in patients with chronic GVHD.
Prevention of B-cell development
Rather than depletion or inhibition of B-cell activity, preventing the development of pathogenic B cells may also be a useful strategy for the prevention or treatment of chronic GVHD. T follicular helper (TFH) cells migrate to germinal centers, where they promote differentiation of mature B cells and secretion of high-affinity immunoglobulin G antibodies.41 We have demonstrated that levels of circulating TFH cells are decreased in patients with active chronic GVHD, but these TFH cells are activated and have increased functional ability to promote B-cell maturation and immunoglobulin secretion.42 Strategies to inhibit or prevent migration of these cells to lymphoid organs have been effective in preclinical models and may prove useful for chronic GVHD prevention or therapy in the future.43
Inhibition of cytokine receptor–mediated signaling
Ruxolitinib, a selective inhibitor of Janus kinase 1 (JAK1) and JAK2, is an approved agent for the treatment of myelofibrosis.44,45 JAK1 and JAK2 mediate receptor-mediated signaling for a variety of proinflammatory cytokines, including interferon-γ and IL-6, and inhibition of this pathway has been shown to suppress activation and differentiation of dendritic cells as well as T cells. Proinflammatory cytokines are characteristically elevated in both acute and chronic GVHD, and inhibition of JAK1/JAK2 signaling with ruxolitinib has been shown to reduce GVHD in murine models.46-48 This novel approach has been tested in patients with steroid-refractory acute and chronic GVHD with encouraging results in both settings.46,49
CD4+CD25+Foxp3+ regulatory T cells (Tregs) are critical mediators of immune tolerance and are required to prevent fatal autoimmunity in healthy individuals. While Treg comprise only ∼5% to 10% of circulating CD4+ T cells, these cells use a variety of mechanisms to dominantly suppress autoreactivity and control innate and adaptive immune responses.50-55 Treg impairment is associated with loss of tolerance, autoimmunity, and chronic GVHD.56-58 In preclinical models of allogeneic HSCT, adoptive transfer of Tregs can ameliorate GVHD without impairing therapeutic GVL responses.59-61 In clinical studies, adult recipients of HLA-matched T-cell–replete grafts often experience poor reconstitution of Tregs.56,62 Impaired Treg reconstitution appears predictive for subsequent chronic GVHD in some, but not all, studies.57,62-65 Analysis of Treg homeostasis after HSCT has shown that Treg deficiency after HSCT appears to result primarily from decreased thymic production of Tregs. Although there is a compensatory increased homeostatic proliferation of circulating memory Tregs, this is ultimately insufficient to prevent Treg deficiency, due in part to proliferation-induced telomere shortening,66 enhanced death signaling, and apoptosis of rapidly dividing Treg.57,67 Treatment strategies attempting to enhance Treg number and function are therefore attractive for chronic GVHD therapy, offering the possibility of therapeutic immune modulation without generalized immunosuppression.
Facilitating Treg reconstitution for the prevention of chronic GVHD
GVHD prophylaxis regimens after transplantation with unmodified bone marrow or peripheral blood stem cell grafts often use short-term methotrexate plus a calcineurin inhibitor (CNI) such as cyclosporine A or tacrolimus (Tac). Continuous CNI therapy is often maintained for 6 months after transplant. While effective for acute GVHD prophylaxis, such regimens are suboptimal for chronic GVHD prevention, owing partly to the inhibition of Treg during CNI therapy.68 In animal models the use of alternative immune suppressive agents such as mTOR inhibitors (sirolimus/Sir) and mycophenolate mofetil (MMF) are more permissive of Treg reconstitution.68,69 However, when compared with standard of care Tac/methotrexate in a prospective phase 3 randomized trial, Tac/Sir resulted in equivalent incidence of acute and chronic GVHD, survival, and relapse.70 Similarly, the use of MMF plus steroids in the initial treatment of chronic GVHD was also evaluated in a phase 3 trial and did not result in clinical benefit, with a potentially increased mortality in the double-therapy arm.5
Novel acute GVHD prophylaxis regimens can also preferentially facilitate Treg recovery after HSCT. Posttransplantation cyclophosphamide (pTCy) is frequently used in the context of haploidentical bone marrow transplantation and is now being prospectively evaluated in patients with HLA-matched donors. pTCy selectively depletes activated alloreactive T cells while sparing resting T cells and hematopoietic stem cells. Interestingly, the GVHD protective effect of pTCy appears to be Treg dependent due to the differential expression of aldehyde dehydrogenase in Tregs compared with conventional CD4 T cells (Tcon). This difference protects Tregs and facilitates their subsequent expansion.71,72 Similarly, Treg-sparing effects and GVHD prevention have been reported for other novel regimens, including ruxolitinib,46 hypomethylating agents (azacytidine),73 and proteasome inhibitors (bortezomib).74-77 Bortezomib in conjunction with corticosteroids has also been evaluated as primary therapy for chronic GVHD in a phase 2 trial, with high response rates.78 All novel approaches, however, require confirmation of benefit in prospective randomized trials.
Extracorporeal photopheresis (ECP) is an established immunomodulatory therapy with a documented safety record in steroid-refractory chronic GVHD. The procedure involves leukapheresis of patient peripheral blood mononuclear cells followed by exposure of the cellular product to UV-A light in the presence of 8-methoxypsoralen. Ex vivo–treated cells are subsequently reinfused to complete the procedure. While the mechanisms by which ECP results in clinical improvement are not completely known, it is thought that ECP can suppress alloimmune responses in part by altering the maturation and function of dendritic cells and through the induction of Tregs.79 Regardless of the mechanism, responses in chronic GVHD patients have been associated with increased Treg cell counts, increased cellular activation, and inhibition of Tcon.80,81 ECP has been evaluated in a phase 2 prospective randomized trial, with demonstrated efficacy in sclerodermatous chronic GVHD, including a reduction in steroid dose, meaningful decrease in skin sclerosis, and nonblinded investigator assessment of skin complete or partial responses. However, this study did not meet its prespecified primary end point.82
Innovations in ECP are being developed to enhance its immunologic and clinical efficacy. One example is the use of photodepletion with a dibromorhodamine (TH9402) photosensitizer in lieu of 8-methoxypsoralen, which resulted in selective eradication of endogenous proliferating Tcon with concomitant sparing and expansion of Treg. This resulted in a higher level of circulating Tregs in patients receiving TH9402-based phototherapy.14 Further clinical evaluation of this approach is eagerly awaited, but even conventional ECP remains a useful option for patients with steroid-refractory chronic GVHD, especially for patients with severe cutaneous manifestations of disease who have geographic access to this therapy.
Adoptive Treg-cell therapy
Adoptive transfer of highly purified CD4 Tregs is the most direct method for Treg enhancement in vivo. This approach has been investigated by several groups, with and without ex vivo Treg expansion. In patients receiving stem cell transplants from haploidentical donors, Di Ianni et al infused immunomagnetic bead–purified donor Tregs without ex vivo expansion.83 This study evaluated the ability of donor Tregs to permit infusion of relatively large numbers of donor Tcon without invoking excess GVHD. With Treg infusion, the incidence of GVHD was low and immune recovery was enhanced. In patients receiving umbilical cord blood (UCB) transplants, Blazar and colleagues infused ex vivo–expanded third-party UCB Tregs in adult patients receiving double UCB transplants.84,85 These studies demonstrated the technical feasibility of purifying and selectively expanding Tregs from UCB products, and patients who received expanded Tregs had lower GVHD rates than historical controls. Additional innovations currently being developed include Treg fucosylation to improve engraftment and homing of Tregs,86 selective expansion of minor histocompatibility antigen–specific Tregs,87 and the genetic modification of Tregs with alloantigen-specific chimeric antigen receptors.88 These strategies exemplify different approaches that can be applied to generate highly functional Tregs for adoptive therapy, but each approach will need further clinical evaluation. Since Tregs represent a relatively small fraction of circulating lymphocytes, complex methods for cell purification and expansion are needed to manufacture sufficient numbers of Tregs for each patient and ensure that the final product does not also contain effector T cells that may cause GVHD. Additional theoretical concerns include the functional stability of transferred Tregs that may convert to an effector phenotype. Finally, the persistence of adoptively transferred Tregs may be limited if environmental conditions in vivo will not support their survival or further expansion.
Enhancement of CD4 Tregs in vivo
High-dose IL-2 (aldesleukin) is approved for the treatment of advanced malignant melanoma and renal cell carcinoma. However, at low physiologic concentrations, IL-2 is a critical homeostatic cytokine that is required for normal Treg development, expansion, activity, and survival.89-91 In a phase 1 clinical trial, daily administration of IL-2 at a dose of 1 × 106 IU/m2 per day was demonstrated to be safe and well tolerated in patients with steroid-refractory chronic GVHD. Notably, daily IL-2 treatment at this low dose selectively enhanced CD4+ Tregs in vivo and led to clinical responses in 52% of patients.92,93 In a phase 2 study, 35 adult patients with steroid-refractory chronic GVHD received daily IL-2 (1 × 106 IU/m2 per day) for 12 weeks, with a 61% clinical response rate and improvement at sites of chronic GVHD that included liver, skin, gastrointestinal tract, lung, and joint/muscle/fascia.94 As in the phase 1 study, Tregs and natural killer cell counts rose significantly, without change in conventional CD4 Tcon or CD8 T-cell counts. Notably, high Treg:Tcon ratios at baseline and at week 1 were predictive of IL-2 clinical response. Twenty-three patients with clinical benefit continued to receive daily IL-2 for 2 years. Clinical and Treg immune responses persisted, while the Tcon count and Treg:Tcon ratio gradually normalized. In patients with advanced tissue damage, very few complete responses have been observed and partial response rates are only in the 50% to 60% range, despite all patients experiencing increased number of circulating Tregs. The need for daily subcutaneous injections for prolonged periods is also a limitation. Nonetheless, these encouraging results provide a basis for further evaluation of low-dose IL-2 as primary therapy for chronic GVHD, and prospective comparative trials are currently being planned.
Considering the important role of IL-2 in the development and maintenance of Tregs, many efforts are underway to enhance the efficacy and utility of IL-2 therapy to promote immune tolerance. Approaches being evaluated at the Dana-Farber Cancer Institute include combining daily low-dose IL-2 with infusions of highly purified donor-derived Tregs (#NCT01937468). This combined therapy provides healthy donor Tregs to augment endogenous Tregs that may be dysfunctional in patients with chronic GVHD and also provides low-dose IL-2 as a growth factor to promote the proliferation and persistence of adoptively transferred Tregs. Another approach in pediatric and adult patients with chronic GVHD is evaluating whether individual IL-2 dose-escalation schedules can better sustain Treg cell activation in vivo (#NCT02318082). Alternatively, low-dose IL-2 can be combined with immune suppressive agents that facilitate Tregs, such as sirolimus, MMF, or ECP (#NCT02340676). Because of aldesleukin’s short half-life, current GVHD regimens employ daily dosing schedules that are inconvenient and cumbersome for prolonged therapy. Previous reports have described long-acting IL-2 formulations such as IL-2/albumin fusion proteins95 or IL-2/monoclonal antibody combinations96 and genetically engineered IL-2 variants that have greater selectivity for individual components of the trimeric IL-2 receptor expressed on different cell types.97,98 Use of modified IL-2 agents may enhance the ability of patients to continue therapy for prolonged periods and increase the ability of these agents to induce and maintain immune tolerance.
Ultra-low-dose IL-2 has also been tested in healthy volunteers and early after haploidentical (#NCT02226861), related, and unrelated donor transplantation.99,100 IL-2 has been safe and well tolerated and also promotes Tregs despite short periods of therapy in these studies. These observations suggest that low-dose IL-2 may also be useful for the prevention of chronic GVHD in different settings. With early posttransplant therapy as well as with prolonged treatment late after transplant, further studies will be necessary to determine whether GVL responses and pathogen responses are compromised. While early-phase clinical results and preclinical data are promising, additional trials are necessary before these options can be considered for routine clinical use.
Conclusions
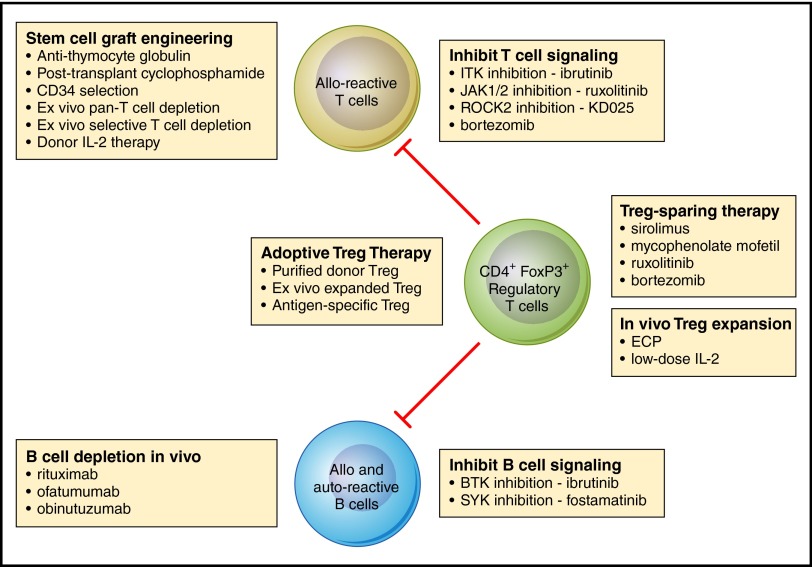
With improved understanding of the immunologic mechanisms that lead to the inability to establish immune tolerance and subsequent development of chronic GVHD, it is now possible to conceive of new therapeutic approaches that target specific immunologic mechanisms. These new approaches have been tested in preclinical models, and several promising therapies have also been evaluated in early-phase clinical trials. Figure 1 provides an overview of current therapies and new agents and identifies the immunologic cells and pathways targeted by different approaches. While results of recent clinical studies are promising, further studies in larger multicenter cohorts of patients are needed to identify the most effective and least toxic regimens. As these agents undergo clinical evaluation, by themselves and in combination, it seems likely that more effective and less toxic therapies will soon be available for the prevention and treatment of chronic GVHD.
Figure 1.
Mechanistic interventions for the prevention or treatment of chronic GVHD. Current and new approaches for the prevention or treatment of chronic GVHD primarily target alloreactive donor T cells, allo- and autoreactive B cells, or CD4+FoxP3+ regulatory T cells. As advances in our understanding of the role of each of these cell types in the development of chronic GVHD has advanced in recent years, it is now possible to develop and select for clinical testing a variety of therapeutic interventions that focus on specific mechanistic pathways. Professional illustration by Patrick Lane, ScEYEnce Studios.
Acknowledgments
This study was supported by National Institutes of Health, National Cancer Institute grants P01CA142106, R01CA183559, and R01CA183560.
Authorship
Contribution: All authors wrote and edited the manuscript.
Conflict-of-interest disclosure: J.K. receives research support from Prometheus Labs, Inc. J.R. is a member of the Delinia Inc. scientific advisory board. C.S.C. declares no competing financial interests.
Correspondence: Jerome Ritz, Dana-Farber Cancer Institute, 450 Brookline Ave, M530, Boston, MA 02215; e-mail: jerome_ritz@dfci.harvard.edu.
References
- 1.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124(3):374-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flowers ME, Martin PJ. How we treat chronic graft-versus-host disease. Blood. 2015;125(4):606-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpenter PA, Kitko CL, Elad S, et al. . National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2015;21(7):1167-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin PJ, Storer BE, Rowley SD, et al. . Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009;113(21):5074-5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff D, Schleuning M, von Harsdorf S, et al. . Consensus Conference on Clinical Practice in Chronic GVHD: Second-Line Treatment of Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2011;17(1):1-17. [DOI] [PubMed] [Google Scholar]
- 7.Soiffer RJ, Fairclough D, Robertson M, et al. . CD6-depleted allogeneic bone marrow transplantation for acute leukemia in first complete remission. Blood. 1997;89(8):3039-3047. [PubMed] [Google Scholar]
- 8.Soiffer RJ, Weller E, Alyea EP, et al. . CD6+ donor marrow T-cell depletion as the sole form of graft-versus-host disease prophylaxis in patients undergoing allogeneic bone marrow transplant from unrelated donors. J Clin Oncol. 2001;19(4):1152-1159. [DOI] [PubMed] [Google Scholar]
- 9.Pasquini MC, Devine S, Mendizabal A, et al. . Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30(26):3194-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertaina A, Merli P, Rutella S, et al. . HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood. 2014;124(5):822-826. [DOI] [PubMed] [Google Scholar]
- 11.Bleakley M, Heimfeld S, Loeb KR, et al. . Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest. 2015;125(7):2677-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Socié G, Schmoor C, Bethge WA, et al. ; ATG-Fresenius Trial Group. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117(23):6375-6382. [DOI] [PubMed] [Google Scholar]
- 13.Kröger N, Solano C, Wolschke C, et al. . Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med. 2016;374(1):43-53. [DOI] [PubMed] [Google Scholar]
- 14.Bastien JP, Krosl G, Therien C, et al. . Photodepletion differentially affects CD4+ Tregs versus CD4+ effector T cells from patients with chronic graft-versus-host disease. Blood. 2010;116(23):4859-4869. [DOI] [PubMed] [Google Scholar]
- 15.Bastien JP, Roy J, Roy DC. Selective T-cell depletion for haplotype-mismatched allogeneic stem cell transplantation. Semin Oncol. 2012;39(6):674-682. [DOI] [PubMed] [Google Scholar]
- 16.Devillier R, Granata A, Fürst S, et al. . Low incidence of chronic GVHD after HLA-haploidentical peripheral blood stem cell transplantation with post transplantation cyclophosphamide in older patients. Br J Haematol. 2016;22(3):S369-S370. [DOI] [PubMed] [Google Scholar]
- 17.Robinson TM, O’Donnell PV, Fuchs EJ, Luznik L. Haploidentical bone marrow and stem cell transplantation: experience with post-transplantation cyclophosphamide. Semin Hematol. 2016;53(2):90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bashey A, Zhang X, Jackson K, et al. . Comparison of outcomes of hematopoietic cell transplants from T-replete haploidentical donors using post-transplantation cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allele-matched unrelated donors and HLA-identical sibling donors: A multivariable analysis including disease risk index. Biol Blood Marrow Transplant. 2016;22(1):125-133. [DOI] [PubMed] [Google Scholar]
- 19.Sarantopoulos S, Blazar BR, Cutler C, Ritz J. B cells in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(1):16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarantopoulos S, Ritz J. Aberrant B-cell homeostasis in chronic GVHD. Blood. 2015;125(11):1703-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratanatharathorn V, Carson E, Reynolds C, et al. . Anti-CD20 chimeric monoclonal antibody treatment of refractory immune-mediated thrombocytopenia in a patient with chronic graft-versus-host disease. Ann Intern Med. 2000;133(4):275-279. [DOI] [PubMed] [Google Scholar]
- 22.Ratanatharathorn V, Ayash L, Reynolds C, et al. . Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant. 2003;9(8):505-511. [DOI] [PubMed] [Google Scholar]
- 23.Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, Cutler C, Mohty M, Kumar A. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2009;15(9):1005-1013. [DOI] [PubMed] [Google Scholar]
- 24.Kharfan-Dabaja MA, Cutler CS. Rituximab for prevention and treatment of graft-versus-host disease. Int J Hematol. 2011;93(5):578-585. [DOI] [PubMed] [Google Scholar]
- 25.Cutler C, Miklos D, Kim HT, et al. . Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arai S, Pidala J, Pusic I, et al. . A randomized phase II crossover study of imatinib or rituximab for cutaneous sclerosis after hematopoietic cell transplantation. Clin Cancer Res. 2016;22(2):319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahaf B, Arai S, Otani J, Schoenrock K, Logan A, Miklos D. Rituximab provides steroid-sparing therapy in new-onset chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19:S140. [Google Scholar]
- 28.Pidala J, Kim J, Betts BC, et al. . Ofatumumab in combination with glucocorticoids for primary therapy of chronic graft-versus-host disease: phase I trial results. Biol Blood Marrow Transplant. 2015;21(6):1074-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laport GG, Wu J, Logan B, et al. ; Blood and Marrow Transplant Clinical Trials Network. Reduced-intensity conditioning with fludarabine, cyclophosphamide, and high-dose rituximab for allogeneic hematopoietic cell transplantation for follicular lymphoma: A phase two multicenter trial from the Blood and Marrow Transplant Clinical Trials Network. Biol Blood Marrow Transplant. 2016;22(8):1440-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glass B, Hasenkamp J, Wulf G, et al. ; German High-Grade Lymphoma Study Group. Rituximab after lymphoma-directed conditioning and allogeneic stem-cell transplantation for relapsed and refractory aggressive non-Hodgkin lymphoma (DSHNHL R3): an open-label, randomised, phase 2 trial. Lancet Oncol. 2014;15(7):757-766. [DOI] [PubMed] [Google Scholar]
- 31.Arai S, Sahaf B, Narasimhan B, et al. . Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119(25):6145-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cutler C, Kim HT, Bindra B, et al. . Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122(8):1510-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarantopoulos S, Stevenson KE, Kim HT, et al. . Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood. 2011;117(7):2275-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majhail NS, Rizzo JD, Lee SJ, et al. ; Center for International Blood and Marrow Transplant Research (CIBMTR); American Society for Blood and Marrow Transplantation (ASBMT); European Group for Blood and Marrow Transplantation (EBMT); Asia-Pacific Blood and Marrow Transplantation Group (APBMT); Bone Marrow Transplant Society of Australia and New Zealand (BMTSANZ); East Mediterranean Blood and Marrow Transplantation Group (EMBMT); Sociedade Brasileira de Transplante de Medula Ossea (SBTMO). Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(3):348-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan SL, Liao C, Lucas MC, Stevenson C, DeMartino JA. Targeting the SYK-BTK axis for the treatment of immunological and hematological disorders: recent progress and therapeutic perspectives. Pharmacol Ther. 2013;138(2):294-309. [DOI] [PubMed] [Google Scholar]
- 36.Allen JL, Tata PV, Fore MS, et al. . Increased BCR responsiveness in B cells from patients with chronic GVHD. Blood. 2014;123(13):2108-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubovsky JA, Flynn R, Du J, et al. . Ibrutinib treatment ameliorates murine chronic graft-versus-host disease. J Clin Invest. 2014;124(11):4867-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flynn R, Allen JL, Luznik L, et al. . Targeting Syk-activated B cells in murine and human chronic graft-versus-host disease. Blood. 2015;125(26):4085-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miklos D, Cutler C, Arora M, et al. . Multicenter open-label phase 1b/2 study of ibrutinib in steroid-dependent/refractory chronic graft versus host disease (cGVHD) [abstract]. Bone Marrow Transplant. 2016;51(suppl S1):S176-S177. [Google Scholar]
- 40.Schutt SD, Fu J, Nguyen H, et al. . Inhibition of BTK and ITK with ibrutinib Is effective in the prevention of chronic graft-versus-host disease in mice. PLoS One. 2015;10(9):e0137641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flynn R, Du J, Veenstra RG, et al. . Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123(25):3988-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forcade E, Kim HT, Cutler C, et al. . Circulating T follicular helper cells with increased function during chronic graft-versus-host disease. Blood. 2016;127(20):2489-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flynn R, Paz K, Du J, et al. . Targeted Rho-associated kinase 2 inhibition suppresses murine and human chronic GVHD through a Stat3-dependent mechanism. Blood. 2016;127(17):2144-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrison C, Kiladjian JJ, Al-Ali HK, et al. . JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787-798. [DOI] [PubMed] [Google Scholar]
- 45.Verstovsek S, Mesa RA, Gotlib J, et al. . A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spoerl S, Mathew NR, Bscheider M, et al. . Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123(24):3832-3842. [DOI] [PubMed] [Google Scholar]
- 47.Choi J, Cooper ML, Alahmari B, et al. . Pharmacologic blockade of JAK1/JAK2 reduces GvHD and preserves the graft-versus-leukemia effect. PLoS One. 2014;9(10):e109799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carniti C, Gimondi S, Vendramin A, et al. . Pharmacologic inhibition of JAK1/JAK2 signaling reduces experimental murine acute GVHD while preserving GVT effects. Clin Cancer Res. 2015;21(16):3740-3749. [DOI] [PubMed] [Google Scholar]
- 49.Zeiser R, Burchert A, Lengerke C, et al. . Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188(2):287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30(6):1538-1543. [DOI] [PubMed] [Google Scholar]
- 52.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16(2):81-88. [DOI] [PubMed] [Google Scholar]
- 53.Fehérvari Z, Sakaguchi S. Development and function of CD25+CD4+ regulatory T cells. Curr Opin Immunol. 2004;16(2):203-208. [DOI] [PubMed] [Google Scholar]
- 54.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med. 2003;197(1):111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janssens W, Carlier V, Wu B, VanderElst L, Jacquemin MG, Saint-Remy JM. CD4+CD25+ T cells lyse antigen-presenting B cells by Fas-Fas ligand interaction in an epitope-specific manner. J Immunol. 2003;171(9):4604-4612. [DOI] [PubMed] [Google Scholar]
- 56.Zorn E, Kim HT, Lee SJ, et al. . Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuoka K, Kim HT, McDonough S, et al. . Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120(5):1479-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10(12):849-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99(10):3493-3499. [DOI] [PubMed] [Google Scholar]
- 61.Edinger M, Hoffmann P, Ermann J, et al. . CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144-1150. [DOI] [PubMed] [Google Scholar]
- 62.Miura Y, Thoburn CJ, Bright EC, et al. . Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104(7):2187-2193. [DOI] [PubMed] [Google Scholar]
- 63.Ukena SN, Grosse J, Mischak-Weissinger E, et al. . Acute but not chronic graft-versus-host disease is associated with a reduction of circulating CD4(+)CD25 (high)CD127 (low/-) regulatory T cells. Ann Hematol. 2011;90(2):213-218. [DOI] [PubMed] [Google Scholar]
- 64.Xhaard A, Moins-Teisserenc H, Busson M, et al. . Reconstitution of regulatory T-cell subsets after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2014;49(8):1089-1092. [DOI] [PubMed] [Google Scholar]
- 65.Alho AC, Kim HT, Chammas MJ, et al. . Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood. 2016;127(5):646-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawano Y, Kim HT, Matsuoka K, et al. . Low telomerase activity in CD4+ regulatory T cells in patients with severe chronic GVHD after hematopoietic stem cell transplantation. Blood. 2011;118(18):5021-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murase K, Kim HT, Bascug OR, et al. . Increased mitochondrial apoptotic priming of human regulatory T cells after allogeneic hematopoietic stem cell transplantation. Haematologica. 2014;99(9):1499-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeiser R, Nguyen VH, Beilhack A, et al. . Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108(1):390-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. . Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111(1):453-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cutler C, Logan B, Nakamura R, et al. . Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kanakry CG, Ganguly S, Zahurak M, et al. . Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5(211):211ra157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ganguly S, Ross DB, Panoskaltsis-Mortari A, et al. . Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood. 2014;124(13):2131-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goodyear OC, Dennis M, Jilani NY, et al. . Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood. 2012;119(14):3361-3369. [DOI] [PubMed] [Google Scholar]
- 74.Kim JS, Lee JI, Shin JY, et al. . Bortezomib can suppress activation of rapamycin-resistant memory T cells without affecting regulatory T-cell viability in non-human primates. Transplantation. 2009;88(12):1349-1359. [DOI] [PubMed] [Google Scholar]
- 75.Blanco B, Pérez-Simón JA, Sánchez-Abarca LI, et al. . Treatment with bortezomib of human CD4+ T cells preserves natural regulatory T cells and allows the emergence of a distinct suppressor T-cell population. Haematologica. 2009;94(7):975-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koreth J, Stevenson KE, Kim HT, et al. . Bortezomib-based graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor transplantation. J Clin Oncol. 2012;30(26):3202-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koreth J, Kim HT, Lange PB, et al. . A bortezomib-based regimen offers promising survival and graft-versus-host disease prophylaxis in myeloablative HLA-mismatched and unrelated donor transplantation: A phase II trial. Biol Blood Marrow Transplant. 2015;21(11):1907-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herrera AF, Kim HT, Bindra B, et al. . A phase II study of bortezomib plus prednisone for initial therapy of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(11):1737-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bruserud Ø, Tvedt TH, Paulsen PQ, et al. . Extracorporeal photopheresis (photochemotherapy) in the treatment of acute and chronic graft versus host disease: immunological mechanisms and the results from clinical studies. Cancer Immunol Immunother. 2014;63(8):757-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biagi E, Di Biaso I, Leoni V, et al. . Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+CD25+GITR+Foxp3+CD62L+ functional regulatory T-cells in patients with graft-versus-host disease. Transplantation. 2007;84(1):31-39. [DOI] [PubMed] [Google Scholar]
- 81.Di Biaso I, Di Maio L, Bugarin C, et al. . Regulatory T cells and extracorporeal photochemotherapy: correlation with clinical response and decreased frequency of proinflammatory T cells. Transplantation. 2009;87(9):1422-1425. [DOI] [PubMed] [Google Scholar]
- 82.Flowers ME, Apperley JF, van Besien K, et al. . A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112(7):2667-2674. [DOI] [PubMed] [Google Scholar]
- 83.Di Ianni M, Falzetti F, Carotti A, et al. . Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921-3928. [DOI] [PubMed] [Google Scholar]
- 84.Brunstein CG, Miller JS, Cao Q, et al. . Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brunstein CG, Miller JS, McKenna DH, et al. . Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood. 2016;127(8):1044-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parmar S, Liu X, Najjar A, et al. . Ex vivo fucosylation of third-party human regulatory T cells enhances anti-graft-versus-host disease potency in vivo. Blood. 2015;125(9):1502-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Veerapathran A, Pidala J, Beato F, et al. . Human regulatory T cells against minor histocompatibility antigens: ex vivo expansion for prevention of graft-versus-host disease. Blood. 2013;122(13):2251-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.MacDonald KG, Hoeppli RE, Huang Q, et al. . Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest. 2016;126(4):1413-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4(9):665-674. [DOI] [PubMed] [Google Scholar]
- 90.Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172(7):3983-3988. [DOI] [PubMed] [Google Scholar]
- 91.Chinen T, Kannan AK, Levine AG, et al. . An essential role for the IL-2 receptor in Treg cell function. Nat Immunol. 2016;17(11):1322-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koreth J, Matsuoka K, Kim HT, et al. . Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matsuoka K, Koreth J, Kim HT, et al. . Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5(179):179ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koreth J, Kim HT, Jones KT, et al. . Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood. 2016;128(1):130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Melder RJ, Osborn BL, Riccobene T, et al. . Pharmacokinetics and in vitro and in vivo anti-tumor response of an interleukin-2-human serum albumin fusion protein in mice. Cancer Immunol Immunother. 2005;54(6):535-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180-190. [DOI] [PubMed] [Google Scholar]
- 97.Shanafelt AB, Lin Y, Shanafelt MC, et al. . A T-cell-selective interleukin 2 mutein exhibits potent antitumor activity and is well tolerated in vivo. Nat Biotechnol. 2000;18(11):1197-1202. [DOI] [PubMed] [Google Scholar]
- 98.Mitra S, Ring AM, Amarnath S, et al. . Interleukin-2 activity can be fine tuned with engineered receptor signaling clamps. Immunity. 2015;42(5):826-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ito S, Bollard CM, Carlsten M, et al. . Ultra-low dose interleukin-2 promotes immune-modulating function of regulatory T cells and natural killer cells in healthy volunteers. Mol Ther. 2014;22(7):1388-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kennedy-Nasser AA, Ku S, Castillo-Caro P, et al. . Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res. 2014;20(8):2215-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]