Abstract
Studies on the Caenorhabditis elegans nucleus have provided fascinating insight to the organization and activities of eukaryotic cells. Being the organelle that holds the genetic blueprint of the cell, the nucleus is critical for basically every aspect of cell biology. The stereotypical development of C. elegans from a one cell-stage embryo to a fertile hermaphrodite with 959 somatic nuclei has allowed the identification of mutants with specific alterations in gene expression programs, nuclear morphology, or nuclear positioning. Moreover, the early C. elegans embryo is an excellent model to dissect the mitotic processes of nuclear disassembly and reformation with high spatiotemporal resolution. We review here several features of the C. elegans nucleus, including its composition, structure, and dynamics. We also discuss the spatial organization of chromatin and regulation of gene expression and how this depends on tight control of nucleocytoplasmic transport. Finally, the extensive connections of the nucleus with the cytoskeleton and their implications during development are described. Most processes of the C. elegans nucleus are evolutionarily conserved, highlighting the relevance of this powerful and versatile model organism to human biology.
Keywords: Caenorhabditis elegans, chromatin, gene expression, lamin, LEM-domain proteins, LINC, P granule, nuclear pore complex, nuclear envelope, nucleocytoplasmic transport, nucleolus, WormBook
THE term “nucleus” originates from Latin and means “little nut” or “kernel.” Although it was unknown when the term was first introduced in 1831 by botanist Robert Brown (Mazzarello 1999), just like a kernel that contains all the properties to develop into an adult organism, so does the nucleus; it contains the genetic blueprint to regulate cell function and development. The nucleus is a fascinating organelle that stores the chromosomes but also regulates their duplication, segregation, repair, and expression through a series of tightly controlled and dynamic processes. Most of these functions are widely conserved among eukaryotes, although several structural and dynamic aspects are specific to animals vs. plants, fungi, etc. In this text we will discuss observations made in Caenorhabditis elegans that have provided important insight to understanding the role of the nucleus.
C. elegans as a model system to study cell biology of the nucleus
The transparency of C. elegans facilitates observation of individual nuclei in living embryos, larvae, and adults with simple differential interference contrast (DIC) microscopy, a characteristic that was key to establishing the complete cell lineage of this popular model organism (Sulston and Horvitz 1977; Sulston et al. 1983). The application of green fluorescent protein (GFP) to study gene expression in vivo was first demonstrated in C. elegans (Chalfie et al. 1994), and the development of color variants has enabled detailed spatiotemporal analysis of multiple reporters simultaneously (Figure 1). C. elegans nuclei contain six pairs of chromosomes with the notable exception of haploid gametes and polyploidy intestinal cells. Most nuclei contain prominent nucleoli, whereas others have a uniform appearance by DIC microscopy. Smaller nuclear bodies, such as nuclear speckles, stress granule-like structures, and polycomb bodies are also present in C. elegans (Loria et al. 2003; Zhang et al. 2004; Morton and Lamitina 2013). The nucleus is enclosed by the nuclear envelope (NE), which consists of two nuclear membranes: the inner nuclear membrane (INM) and outer nuclear membrane (ONM). Transport across the two nuclear membranes occurs through nuclear pore complexes (NPCs). Bridging the two nuclear membranes is the linker of nucleoskeleton and cytoskeleton (LINC) complex (Starr and Fridolfsson 2010), which is important for transducing signals across the NE and for nuclear positioning in several tissues. Underlying the INM is the nuclear lamina which contributes to nuclear shape, nuclear rigidity, and chromosome organization. The components of the nucleus, as well as the nucleus itself, are highly dynamic throughout the cell cycle and during development. In fact, the dynamic nature of the nucleus is critical for proper nuclear function. Before we begin our discussion on how the nucleus functions, it is useful to consider the various structural components of the nucleus in more detail.
Figure 1.
The transparency of C. elegans makes it very suitable for observation of nuclear processes in living animals. In this example, chromatin in a young adult hermaphrodite is visualized by expression of mCherry-tagged HIS-58/HisH2B from a MosSCI single-copy transgene (A; magenta in C and E), whereas GFP was inserted into the mel-28 locus by CRISPR to label NEs (B; green in C and E). The proteins are expressed in all tissues, but not at equal levels: MEL-28 is particularly abundant in germ line nuclei. Shown are maximal projections of seven confocal sections from hypodermal cells toward the body center (A–C and E) and DIC images (D–E). Strain from Gomez-Saldivar et al. (2016). Bar, 100 µm. mCh, mCherry.
Structural components of the nucleus
Components of the NE:
The INM and ONM:
The INM and ONM are part of the endoplasmic reticulum (ER) (Figure 2A). However, while most of the C. elegans ER is made of an intricate network of tubules (at least in the embryos, see Poteryaev et al. 2005), the NE is a giant membrane sheet. The ONM is continuous with the ER and contains many of the same proteins, and the lumen between the INM and ONM is continuous with the ER lumen. The INM connects to the ONM around NPCs, which are embedded within both membranes. The INM contains a unique set of proteins, including LEM-domain proteins, that interact with chromatin and the nuclear lamina. As will be described in more detail below, the NE disassembles during mitosis and reassembles once chromosome segregation is complete.
Figure 2.
Overview of the C. elegans NE. (A) The NE is an essential structure that regulates many nuclear processes. It is composed of an ONM and an INM, the nuclear lamina, and NPCs. The ONM is continuous with the ER and many macromolecules, including ribosomes, are associated with both membrane structures. In contrast, several NE transmembrane proteins (NETs) are found specifically in the INM, such as LEM-domain proteins EMR-1 and LEM-2, which both bind the chromatin factor BAF-1. Other proteins at the INM include CEC-4, HPL-1/2, and LEM-4 as well as SUN-domain proteins that interact with KASH-domain proteins in the lumen between the INM and the ONM to connect the nucleus to the cytoskeleton. See text for further details. (B) NPCs are composed of ∼30 nuclear pore proteins (NPPs, nups), each present in multiple copies, bringing the total number of proteins close to 1000. Many nups form stable NPC subcomplexes, such as the NPP-5/NUP107 and NPP-13/NUP93 complexes that form the outer (cytoplasmic and nucleoplasmic) and inner rings, respectively. Transmembrane nups are involved in anchoring of the NPC in the NE, whereas nups in the central channel and peripheral cytoplasmic and nucleoplasmic structures are responsible for translocation of substrates through the NPCs. The relative positions of C. elegans nups are predictions based on EM data from yeast and vertebrate cells.
NPCs:
NPCs provide a selective gateway between the nucleoplasm and cytoplasm (Figure 2A, for more on this process see section Nucleocytoplasmic transport as a regulatory process below). NPCs are large ∼100-MDa structures formed by ∼30 different nuclear pore proteins (NPPs, also known as nucleoporins or nups), each present in 8–32 copies (Figure 2B). NPCs disassemble along with the NE during every mitosis, and reassemble once chromosome segregation is completed (Dultz et al. 2008). Two mechanisms have been proposed for NPC incorporation into the nuclear membrane: at the end of mitosis NPCs begin to assemble on chromatin and are subsequently surrounded by the NE, while during interphase NPCs are inserted, through an unknown mechanism, into an intact NE (for review, see Wandke and Kutay 2013). The mitotic mechanism for NPC assembly will be further discussed below.
LMN-1:
The single C. elegans lamin is a B-type lamin, but phylogenetically it is closer to B-type lamins from other organisms with a single lamin gene (e.g., Hydra) than to its vertebrate counterparts (Riemer et al. 1993; Liu et al. 2000; Dittmer and Misteli 2011). LMN-1 is the main component of the C. elegans nuclear lamina (Figure 2A). The vertebrate family of lamin proteins includes lamin A; a derivative of lamin A, called lamin C, which arises through alternative splicing; and two lamin-B genes (for a review on lamins see Gruenbaum and Foisner 2015). While vertebrate lamin B is expressed in all cell types, lamins A/C are expressed mainly in differentiated cells. Lamin A and B are farnesylated at their C-termini although the C-terminal of lamin A is later cleaved, resulting in loss of farnesylation. Nonetheless, lamin A remains associated with the NE via interactions with other NE proteins. The worm LMN-1 likely serves both lamin A and lamin B functions. Lamins are type-V intermediate filaments. The building block of the C. elegans LMN-1 filament is a dimer composed of a ∼55-nm-long rod-shaped domain flanked by an N-terminal head domain and a globular C-terminal domain. The lamin dimer assembles with other units head to tail as well as laterally to form a 10-nm-thick filament (Karabinos et al. 2003; Strelkov et al. 2004; Ben-Harush et al. 2009). Mutations in lamin genes, as well as genes of other NE protein such as emerin (see below), result in a variety of diseases that are collectively called laminopathies. These include Emery–Dreifuss muscular dystrophy and the premature aging syndrome Hutchison–Gilford progeria syndrome (for review see Dobrzynska et al. 2016). Interestingly, although lamins are present in all tissues, different lamin mutations result in distinct tissue-specific defects. In C. elegans, downregulation of LMN-1 leads to severe abnormalities in nuclear morphology and the mislocalization of many NE proteins (Liu et al. 2000, and see below). In an effort to understand the underlying mechanism of various laminopathies, a number of disease-related lamin mutations have been introduced into C. elegans. Many of these mutations disrupted C. elegans LMN-1 oligomerization in vitro and lamin function in vivo (Wiesel et al. 2008; Bank et al. 2011, 2012; Mattout et al. 2011; Zuela et al. 2016). For example, LMN-1∆K46, which corresponds to the deletion of lysine 32 in the rod domain of lamin A/C in Emery–Dreifuss muscular dystrophy patients, causes defects in the lateral assembly of LMN-1 dimers and results in abnormal muscle structure and motility in the worm (Bank et al. 2011). Other lmn-1 mutations associated with Emery–Dreifuss muscular dystrophy affect muscle-specific gene expression (Mattout et al. 2011) and have tissue-specific effects on mechanical properties of the nucleus (Zuela et al. 2016). The Zuela et al. study is especially significant because it examined the mechanical properties of the nucleus in an intact organism, unlike previous studies that were done using tissue culture.
EMR-1 and LEM-2:
These are two of the LEM-domain proteins in C. elegans (Figure 2A). LEM-2 is also referred to in the literature as Ce-MAN1. Like their mammalian counterparts, emerin and LEMD2, respectively, EMR-1 and LEM-2 have transmembrane domains and are integral INM proteins (Lee et al. 2000; for a general review on LEM-domain proteins see Barton et al. 2015). EMR-1 and LEM-2 are expressed in all C. elegans cells and are thought to have overlapping functions, as downregulation of both genes by RNA interference (RNAi), but not each gene alone, results in embryonic lethality (Liu et al. 2003). EMR-1 and LEM-2 are also needed later in development: animals depleted for both proteins show defects in muscle function (Barkan et al. 2012). The localization of both to the nuclear periphery is dependent on LMN-1 (Lee et al. 2000; Liu et al. 2003) and the small chromatin-binding protein barrier to autointegration factor 1 (BAF-1) (Margalit et al. 2007, and see below). Both proteins bind to BAF-1 through their LEM domain and to LMN-1 through a separate domain. The binding to BAF-1 likely mediates the association of these two LEM-domain proteins with chromatin. Importantly, the two proteins are not functionally identical: deletion of LEM-2, but not EMR-1, leads to reduced life span (Barkan et al. 2012); while depletion of EMR-1, but not LEM-2, leads to neuromuscular junction defects (Gonzalez-Aguilera et al. 2014). In addition, the relative amounts of EMR-1 and LEM-2 vary between tissues, with EMR-1 being more abundant than LEM-2 except in embryos, germ line, and intestine (Morales-Martinez et al. 2015).
BAF:
BAF, or BANF1 in mammals, was originally discovered as a protein that prevented viral autointegration (Lee and Craigie 1998). The C. elegans homolog of BAF is BAF-1 (Figure 2A). BAF is a conserved small (∼10 kDa) DNA binding protein with roles in numerous nuclear processes (Jamin and Wiebe 2015). As in other organisms, in C. elegans BAF-1 associates with the lamin protein LMN-1 and the LEM-domain proteins EMR-1 and LEM-2. BAF-1 depends on these LEM-domain proteins as well as lamin for its localization (Liu et al. 2003); conversely, lamin and LEM-domain proteins depend on BAF for their NE localization (Haraguchi et al. 2001; Margalit et al. 2007). BAF-1 is phosphorylated by the VRK-1 kinase (Gorjanacz et al. 2007) and this reduces its affinity to DNA. This phosphorylation is reversed by the PP2A phosphatase (Asencio et al. 2012).
The LINC complexes:
These complexes consist of Sad1 and UNC-84 (SUN)-domain INM proteins that interact with Klarsicht, ANC-1 and Syne/Nesprin homology (KASH)-domain ONM proteins in the NE lumen (Starr and Fridolfsson 2010) (Figure 2A). Owing to this structure, which bridges between the cytoplasm and nucleoplasm, LINC complexes can transduce mechanical signals from the cytoplasm to the nucleus. As such, LINC complexes contribute to meiotic chromosome pairing, nuclear movement, and mechanotransduction. C. elegans has two SUN-domain proteins (SUN-1 and UNC-84) and four KASH domain proteins (ANC-1, KDP-1, UNC-83, and ZYG-12) (Zhou and Hanna-Rose 2010). We will revisit the role of LINC complexes in the aforementioned processes toward the end of this chapter.
The C. elegans chromosomes:
The size of the C. elegans genome is 97 Mb, distributed on six chromosomes: five autosomes, numbered I–V, and an X chromosome. Each somatic cell contains a pair of autosomes and either two X chromosomes (in hermaphrodites) or a single X chromosome (in males). The functional organization of a C. elegans chromosome is somewhat different from that of chromosomes in vertebrate cells. First, while in vertebrate cells chromosomes typically have a single centromere (the site of kinetochore assembly, through which chromosomes associate with microtubules of the mitotic spindle), C. elegans chromosomes are holocentric, meaning that they have centromeres along their entire length (Figure 3) (Maddox et al. 2004). These centromeres are discrete point centromeres distributed throughout the chromosomes, each containing a single nucleosome with the centromere-specific histone H3 variant, HCP-3 (a CENP-A homolog, also known as CenH3) (Steiner and Henikoff 2014). Furthermore, C. elegans centromeres tend to associate with regions of low transcriptional activity, possibly due to exclusion of HCP-3 by high histone turnover in regions with active transcription (Gassmann et al. 2012; Steiner and Henikoff 2014). Second, the distribution of active genes along C. elegans autosomes is nonrandom, such that the density of active genes is higher in the center than along the arms (Figure 3). Whole genome analysis of histone modifications through the modENCODE project revealed sharp boundaries of repressive and activating histone modification between the center region and the arms of autosomes, although repressive histone marks are present within the overall active region, and vice versa (Gerstein et al. 2010). On the other hand, chromatin marks are more uniformly distributed on the X chromosome (Gerstein et al. 2010).
Figure 3.
Comparison of global chromosome patterning in humans and C. elegans. Centromeric histone variant (in blue) CENP-A is confined to a single region in the human chromosome, whereas the C. elegans ortholog HCP-3 is present throughout the chromosome, reflecting the monocentric and holocentric chromosome structures, respectively. Large domains enriched for either active chromatin marks (here represented by H3K36me3, in green) or repressive marks (H3K27me3 and H3K9me3, in red) are also more widespread on C. elegans autosomes, with a higher density of active marks in the center and repressive marks on the arms. Adapted from Ho et al. (2014).
Nuclear bodies:
Nuclear bodies include Cajal bodies [involved in premessenger RNA (pre-mRNA) and ribosomal RNA (rRNA) processing and telomere maintenance], speckles and paraspeckles (splicing), histone locus bodies (histone mRNA processing), PML bodies (DNA repair, transcription regulation, and more), and nucleoli (ribosome biogenesis) (Dundr 2012). The assembly of nuclear bodies can occur at a specific genomic locus and often starts with a specific nucleating RNA or protein. For example, nucleoli are associated with ribosomal DNA (rDNA) repeats [also known as nucleolar organizer (NOR)] and are seeded by rRNA; while histone locus bodies associated with histone genes are nucleated by histone pre-mRNA. Nuclear bodies appear to self-organize, meaning that their size is determined by the concentration of their components. The best-studied nuclear body in C. elegans is the nucleolus, which will be discussed in more detail in a later section.
The Dynamic Nature of the Nucleus
Changes to nuclear structure during the cell cycle: an overview
As in most organisms, nuclei of C. elegans cells are oval or spherical, especially in embryos and in the germ line (Figure 1 and Figure 4). In the adult worm, most cells do not divide and thus changes to nuclear shape occur predominantly due to aging, as is the case in other metazoans (see below). In contrast, after fertilization and in dividing cells, such as embryonic cells and certain larval tissues, the nucleus is remarkably dynamic. First, during interphase, the nucleus grows in size (see, for example, Cowan and Hyman 2006; Meyerzon et al. 2009b). Several studies have shown that the increase in nuclear size is dependent on nuclear import of certain nuclear components, including lamin proteins (Levy and Heald 2010; Jevtic et al. 2015). Moreover, nuclear size scales with cell size but the mechanism behind this process is poorly understood (Vukovic et al. 2016). Second, as cells enter mitosis, the NE, which separates the chromosomes from cytoplasmic proteins and structures such as centrosomes, has to break down to allow centrosome-nucleated microtubules to access chromosomes. Finally, once chromosome segregation is completed, the NE must reassemble and expand to the appropriate size. The changes to nuclear shape and integrity during mitosis have been well documented, although much remains to be learned about the mechanisms that drive them.
Figure 4.
Two (left), four (middle), and eight (right) cell-stage C. elegans embryos, visualized using an NPC protein, NPP-1, fused to GFP (top row). The bottom row shows a schematic view of the same embryos. Note that as the embryos progress through early development, their cells and nuclei become smaller. Adapted from Rahman et al. (2015). Bar, 10 µm.
Considering all the components that make up and associate with the NE, the process of NE breakdown and reassembly is a massive undertaking (for reviews see Guttinger et al. 2009 and Vietri et al. 2016): chromosomes must detach from the NE, the lamina and NPCs must disassemble, and the nuclear membrane must retract into the ER. Following NPC disassembly, certain NPC subunits associate with kinetochores and assist in chromosome segregation. Intranuclear structures, such as the nucleolus, also disassemble. This whole process has to then be reversed to form NEs around the segregated chromosomes and reorganize the chromosomes in the appropriate chromosomal locations. These changes in nuclear structure are driven, at least in part, by protein kinases and phosphatases. The disassembly of the NE is triggered by a series of phosphorylation events, largely driven by the mitotic cell cycle kinase, CDK1 (CDK-1/NCC-1 in C. elegans), working in conjunction with cyclin B (CYB-1, CBY-2.1, CBY-2.2, or CBY-3). CDK1/cyclin B has a large number of mitotic substrates, including lamins, various lamina-associated proteins, and subunits of the NPC (for review see Guttinger et al. 2009). Other relevant kinases are polo-like kinase 1 (PLK-1), which has multiple targets in mitosis, including subunits of the NPC (Grosstessner-Hain et al. 2011; Laurell et al. 2011); VRK1 (VRK-1), which phosphorylated BAF (Nichols et al. 2006; Gorjanacz et al. 2007); and the AIR-1 aurora kinase (Portier et al. 2007). Counteracting the activity of these kinases are a number of phosphatases, including protein phosphatase 1 (PP1) and PP2A (Wurzenberger and Gerlich 2011). In the sections below we discuss the various steps of NE breakdown and reassembly, with emphasis on the process in C. elegans.
NE breakdown
As cells enter mitosis, the NE must somehow be breached to allow the centrosome-nucleated microtubules to make contact with chromosomes. In most animal cells, the NE disassembles completely in prometaphase, meaning that at this stage, components of the NE are largely undetected. In the C. elegans early embryo, however, components of the NE are present up until metaphase (Lee et al. 2000, and see below). This does not mean that the permeability barrier of the nucleus has not been breached. Indeed, soluble cytoplasmic proteins are detected in the vicinity of chromosomes well before metaphase.
In mammalian cells, prior to NE breakdown, the NE begins to form indentations and invaginations. The first structures to disassemble during mitosis are the NPCs in a process that is driven, at least in part, by Cdk1 phosphorylation (Macaulay et al. 1995; Laurell et al. 2011). This destroys the permeability barrier function of the NE, allowing cytoplasmic proteins to enter the nucleus. The integrity of the NE of vertebrate cells is further disrupted by the physical tearing of the NE in a process that involves microtubules (Beaudouin et al. 2002; Salina et al. 2002). These NE breaks occur at regions that are remote from centrosomes in a microtubule-dependent manner. The detachment of chromatin from the NE is likely regulated by phosphorylation events, including the phosphorylation of BAF by VRK1, which reduces the affinity between BAF and chromatin (Nichols et al. 2006; Molitor and Traktman 2014). Components of the nuclear lamina are phosphorylated by Cdk1 and possibly other kinases (Heald and McKeon 1990; Peter et al. 1990), leading to their dissociation from the NE.
In C. elegans early embryos, some subunits of the NPC are still visible at the NE even by metaphase (Figure 5A; Lee et al. 2000), while in older embryos (>30 cells) NPCs disassemble in prometaphase (Lee et al. 2000). LMN-1, EMR-1, and LEM-2 are also present at the nuclear periphery at metaphase of both early and late embryos (Figure 5B and Lee et al. 2000). Since chromosome movement is mediated by microtubules emanating from cytoplasmic centrosomes, this means that although in prometaphase and metaphase components of the NE are still present, the NE itself is no longer intact. Consistent with deterioration of the NE in the vicinity of centrosomes, at metaphase the presence of the NPC subunit NPP-1 and LMN-1 are less pronounced at the region of the NE that is adjacent to the spindle poles (on the axis perpendicular to the metaphase plate, time point 4 min in Figure 5, and Lee et al. 2000). Moreover, in the one-cell embryo during prometaphase, the NPC subunit NPP-3 and other subunits in the same subcomplex are selectively removed from the NE that is adjacent to centrosomes, preceding the dissociation of other NPC subunits and lamina disassembly (Hachet et al. 2012). This selective loss is dependent on both proximity to a centrosome and on the AIR-1 kinase. Thus, unlike in vertebrate cells where the initial breaks in the NE occur away from centrosomes, in C. elegans there is likely a mechanism that breaches the NE specifically near centrosomes. Whether this mechanism also exists in vertebrates, and the extent to which this occurs after the C. elegans one-cell stage, remains to be determined.
Figure 5.
Time-lapse images of two C. elegans embryos, starting at the two-cell stage, showing NE breakdown as it occurs during mitosis. The chromosomes are visualized using histone H2B fused to mCherry and the NE is visualized using (A) the NPC subunit NPP-1 fused to GFP or (B) LMN-1 fused to GFP. The images shown were taken 2 min apart. At the two-cell stage, the AB cell (situated at the bottom half of the embryos in these images) enters mitosis before the P1 cells (at the top half of these embryos). Note that both NPP-1 and LMN-1 are still visible in metaphase. The bright red spots at the bottom edge of the AB cell are the polar bodies. Adapted from Rahman et al. (2015). Bar, 10 µm.
An important step in NE breakdown in C. elegans is the detachment of the NE from chromatin. As noted above, LEM-domain proteins are linked to chromatin through their attachment to BAF. In mitosis, BAF-1 is phosphorylated by VRK-1, leading to its detachment from chromatin (Gorjanacz et al. 2007). When VRK-1 is downregulated by RNAi, both BAF-1 and LEM-domain proteins remain associated with chromatin throughout mitosis, interfering with chromosome segregation and the reassembly of the NE at the end of mitosis (Gorjanacz et al. 2007 and see below).
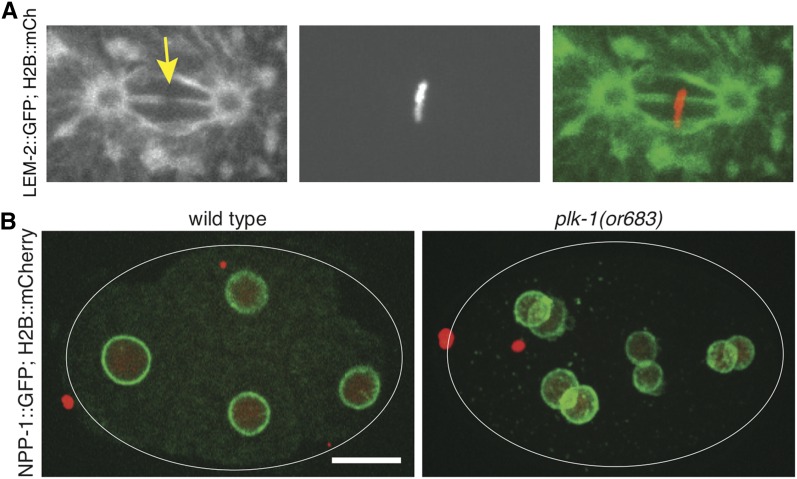
A distinct feature of NE breakdown in C. elegans is that it is, in fact, incomplete. As mitosis progresses past metaphase, remnant NE remains as a “shell” around the general region of the segregating chromosomes (Figure 6A, 2 min; and Figure 6B, 6 min). This remnant NE contains integral nuclear membrane proteins such as LEM-2 (Figure 6A) but not LMN-1 or NPCs, which have already disassembled at metaphase/early anaphase. The remnant NE is clearly permeable to cytoplasmic proteins, but it may prevent organelles from interfering with spindle assembly, elongation, and/or chromosome segregation, as was described in other systems (Smyth et al. 2012; Schlaitz et al. 2013; Schweizer et al. 2015). Moreover, the remnant NE in C. elegans encapsulates a region in which certain proteins, such as tubulin, can accumulate despite the absence of a permeability barrier (Hayashi et al. 2012). How this compartmentalization is achieved is not known, but one could imagine that a distinct environment may be sustained via signals emanating from the chromosomes, as is the case for the RanGTP gradient (Kalab et al. 2002). Alternatively, or in addition, there may exist a mechanism that restricts the movement of certain proteins to inside the remnant NE based on size exclusion (Schweizer et al. 2015), affinity capture by a “spindle matrix”-like structure, or even phase transition, such as the recently discovered vertebrate BuGZ, which accumulates in the area of spindle formation and promotes spindle assembly (Jiang et al. 2015). The remnant NE eventually collapses as new NEs form around the segregated chromosomes (Figure 6A, 6 min; and Figure 6B, 8 min).
Figure 6.
Time-lapse images of early C. elegans embryonic divisions as seen by following the ER/NE. The ER/NE is visualized using (A) the NE protein LEM-2 fused to GFP or (B) the ER marker SP12 fused to GFP. Chromosomes in both panels are visualized using histone H2B fused to mCherry. The embryo in (A) is at the one-cell stage, and the time-lapse begins shortly after fertilization and pronuclear meeting (time −2 min). At time 0 the chromosomes are aligned at the metaphase plate and the two pronuclei have rotated such that the spindle (not shown) is aligned with the long embryo axis. Note that the NE/ER forms a “shell” around the region occupied by the chromosomes and spindle. This remnant NE/ER elongates during anaphase (2 and 4 min) and eventually collapses, as new NEs form around the segregated chromosomes (6 min). The two ER/NE circles indicated by arrows (time 0) surround the centrosomes. The membrane configuration and role of these membranous structures is not known. (B) Shows the ER/NE in a dividing two-cell embryo, where a similar behavior of the NE/ER is observed. The membranous structures around centrosomes are indicated by arrows. Adapted from Rahman et al. (2015). Bar, 10 µm.
A special case of NE breakdown is that which occurs immediately after fertilization (see more below on nuclear structure during fertilization). The chromosomes contributed by the oocyte and sperm are initially encapsulated by separate NEs, forming the maternal and paternal pronuclei, respectively. After pronuclear meeting, NPCs and the nuclear lamina largely disassemble, but the nuclear membranes between the two pronuclei still separate the maternal and paternal genomes (Figure 6A, time 0; Audhya et al. 2007; Rahman et al. 2015). Mixing of parental genomes is initiated by the formation of a membrane gap (Figure 7A). The gap then expands during anaphase, and eventually new NEs form around the segregated chromosomes (Figure 6). A failure to form this gap can result in the formation of paired nuclei (also referred to as “twinned nuclei”) in subsequent cell divisions, with one nucleus containing the maternal genome and the other containing the paternal one (Figure 7B). This phenotype is observed when lipid metabolism and ER structure are disrupted (Audhya et al. 2007; Golden et al. 2009; Gorjanacz and Mattaj 2009; Bahmanyar et al. 2014), when NPP-12/gp210 is downregulated (Audhya et al. 2007; Galy et al. 2008), or when PLK-1 is partially inactivated (Rahman et al. 2015). The formation of the membrane gap between the two pronuclei coincides with metaphase chromosome alignment; conditions that perturb alignment also abrogate the formation of the membrane gap (Rahman et al. 2015). The exact structure of this membrane gap and the mechanism of its formation are unknown.
Figure 7.
(A) The nuclear membranes between the two pronuclei in the one-cell C. elegans embryo are breached (arrow) only when chromosomes in both pronuclei are aligned on their respective metaphase plates, and the two metaphase plates are aligned relative to each other. The configuration of the membrane at this membrane gap, the mechanism of its formation, and the link between chromosome alignment and membrane gap formation are not known. In these images, the NE/ER and chromosomes are visualized using LEM-2 fused to GFP and histone H2B fused to mCherry, respectively. Adapted from Rahman et al. (2015). (B) Four-cell embryos from a wild type (left) and plk-1ts mutant, both growth at 23°C, the semipermissive temperature for this plk-1 temperature-sensitive allele. NE is detected with the nuclear pore subunit NPP-1 fused to GFP (in green) and the chromosomes are highlighted with histone H2B fused to mCherry (red). Bright red spots outside the nuclei are polar bodies. Adapted from Rahman et al. (2015). Bar, 10 µm.
NE reassembly
Once chromosome segregation is completed, the NE must reassemble around all the chromosomes in each of the daughter cells. This largely entails the reversal of the processes that took place during NE breakdown, although not necessarily in the exact reversed order.
The nuclear membrane of the reforming NE originates from the ER, but there had been some debate as to the exact origin of this membrane: studies showing that membrane vesicles are the source of the nuclear membrane gave way in recent years to studies showing that intact ER membrane, in the form of either sheets or tubules, associates with chromosomes to form the two membranes of the NE (Anderson and Hetzer 2007; Lu et al. 2009). Regardless of the exact mechanism, almost all models predict that a membrane-sealing event will ultimately be needed to form an intact NE. Recent studies in human cells identified ESCRT-III as being responsible for this process (Olmos et al. 2015; Vietri et al. 2015). ESCRT proteins promote membrane fission, in particular in membrane necks (such as at the base of a budding vesicle), and they are involved in a variety of cellular processes (Hurley 2015). At the NE, it is thought that the annuli left after ER-membrane flattening are similar to membrane necks, and it was proposed that they are sealed by ESCRT-III in a manner similar to ESCRT activity in other membrane necks (Vietri et al. 2016). The involvement of ESCRT-III in NE reformation in C. elegans has yet to be examined.
How do membranes form stable contacts with chromosomes? In animal cells, the NE protein lamin B receptor (LBR) was shown to be one of the first proteins to interact with chromatin, and specifically with histone H3/H4. C. elegans, however, does not have an LBR homolog. Several NPC subunits, such as the NPP-5/NUP107 subcomplex and MEL-28/ELYS, are highly abundant on the C. elegans holocentric chromosome during mitosis (Franz et al. 2005; Fernandez and Piano 2006; Galy et al. 2006; Rodenas et al. 2012). C. elegans studies demonstrated that the chromatin-binding activity of MEL-28/ELYS is conferred by a domain at the C-terminal of the protein (Gomez-Saldivar et al. 2016), but it does not depend on the protein’s two AT-hook domains, contrary to previous conclusions (Rasala et al. 2008). Once bound to chromatin, MEL-28/ELYS recruits the NPP-5/NUP107 subcomplex, ultimately leading to the formation of NPCs embedded in the newly formed NE (Franz et al. 2007). In the absence of MEL-28/ELYS, the integrity of the newly formed NEs is compromised and they are depleted of both NPCs and nuclear lamina proteins (Fernandez and Piano 2006; Galy et al. 2006). The formation of pore-less NEs was also observed after depletion of the NUP107 subcomplex (Harel et al. 2003; Walther et al. 2003a). Thus, while MEL-28/ELYS is needed for NPC formation, the association of the nuclear membrane with chromosomes is independent of MEL-28/ELYS activity.
As noted above, NE breakdown is associated with phosphorylation of a multitude of NE-associated proteins. As such, NE reformation involves the activity of phosphatases such as PP1 and PP2A (Wurzenberger and Gerlich 2011), which counteract the activity of these kinases. As it turns out, the association of the nuclear membrane with chromatin during NE reassembly is dependent on this phosphorylation/dephosphorylation cycle. To promote NE detachment from chromatin, BAF-1 is phosphorylated by VRK-1 (Gorjanacz et al. 2007). To overcome this VRK-1-dependent phosphorylation of BAF-1, LEM-4, a protein homologous to the human LEM4/ANKLE2, recruits the PP2A phosphatase, which dephosphorylates BAF-1 to allow NE reassembly (Asencio et al. 2012). LEM-4 also inhibits VRK-1’s kinase activity (Asencio et al. 2012). Of note, unlike its human homolog, LEM-4 has neither a LEM domain nor a transmembrane domain, and hence it is considered a LEM4-like protein. Antibodies against LEM-4 revealed that it is enriched at the NE (Asencio et al. 2012). What regulates LEM-4 so that it desphosphorylates BAF-1 only when chromosome segregation is completed is an interesting and unresolved question. Other dephosphorylation events are also likely to contribute to NE reassembly at the end of mitosis. For instance, it was recently reported that MEL-28 recruits the PP1 catalytic subunit GSP-2 to meiotic chromosomes to disassemble kinetochores and promote nuclear assembly (Hattersley et al. 2016).
Nuclear morphology during fertilization and early embryogenesis
The very first nuclei to form during the worm’s life cycle are the male and female pronuclei, which form in the one-cell embryo following fertilization. At this stage, the two pronuclei are positioned at opposite poles of the embryos. The two pronuclei then migrate toward each other, powered by microtubules and their associated motor proteins, with the maternal pronucleus moving significantly faster than the paternal pronucleus. Once the pronuclei meet, they rotate such that the interface between the two pronuclei is parallel to the embryo’s long axis, and NE breakdown begins. The four membranes between the two pronuclei (two from each pronucleus) are breached only after chromosome alignment at metaphase (Rahman et al. 2015), allowing mixing of the parental genomes, followed by chromosome segregation. At this point the NEs reassemble to form the first zygotic nuclei of the two-cell embryo.
In the C. elegans embryo, cell divisions occur within a confined volume. As a result, cell size decreases with each cell division, as does nuclear size (Figure 4). How do cellular organelles, and in particular the nucleus, adjust to this continual reduction in cell size? This process is not unique to C. elegans, as early embryonic divisions in many organisms occur without a change in the overall size of the embryo. There appears to be a universal adherence to a constant nuclear:cell volume ratio, the mechanism of which is not known (Vukovic et al. 2016). In Xenopus embryos, nuclear volume is regulated by nuclear import factors, lamin concentration and protein kinase C, and there are indications that the same holds true in human cells (reviewed in Vukovic et al. 2016). Not only must nuclear volume decrease to match cell size, the compaction of chromosomes must also increase to avoid getting “clipped” during cytokinesis in cells with an ever-decreasing size. Hara and colleagues found that in C. elegans, chromosome size depends on chromosome density (namely, an increase in chromosome number results in more compact chromosomes), and that chromosome size scales with nuclear size (Hara et al. 2013). But as nuclear size scales with cell size, the determining factor for chromosome size, namely the nucleus or the cell, was not known. To decouple nuclear size from cell size, Ladouceur et al. (2015) manipulated nuclear size in C. elegans embryos by downregulating the levels of RAN-1GTP, which is needed for nuclear size increase by promoting nuclear import. Under these conditions nuclei were smaller than expected based on cell size, and chromosomes scaled with nuclear size. However, it is possible that both chromosome size and nuclear size depend, independently of each other, on nuclear import.
Nuclear morphology during aging
Studies in human cells have shown that nuclear shape is altered during normal aging (Scaffidi and Misteli 2006). These alterations have been attributed to changes in the nuclear lamina, and in particular the accumulation of an aberrant splicing form of lamin A mRNA that causes the prelamin A to be permanently farnesylated and hence constitutively membrane associated (Eriksson et al. 2003). In cells from patients with Hutchinson–Gilford progeria syndrome and in a mouse progeria model, correcting this splicing defect reversed these premature aging phenotypes (Scaffidi and Misteli 2005; Fong et al. 2006), but whether this would also be the case during normal human aging has been more difficult to address. Worms also display nuclear phenotypes associated with aging, including changes to nuclear shape, the appearance of blebs, invaginations, fragmentation, and loss of peripheral heterochromatin (Haithcock et al. 2005). Interestingly, nuclei of neuronal cells do not change shape as worms age. Consistent with premature aging due to defects in the nuclear lamina seen in humans, downregulation of the worm lamin, LMN-1, or EMR-1 and LEM-2 simultaneously, leads to a shortened life span (Haithcock et al. 2005). Lamin levels, however, do not change during worm aging, suggesting that if the deterioration in nuclear morphology is due to lamin dysfunction, the problem may lie in lamin-associated proteins and/or age-dependent changes in lamin post-translational modifications. Systemic inhibition of farnesylation with a chemical inhibitor or through a mutation in the farnesylating enzyme improves nuclear shape in aging worm cells (Bar et al. 2009; Bar and Gruenbaum 2010). However, it is not clear whether the effect of farnesylation inhibition on nuclear morphology in the worm is through inhibiting LMN-1 farnesylation specifically. Interestingly, restoring nuclear morphology in aged worms by inhibiting farnesylation did not increase life span (Bar et al. 2009; Bar and Gruenbaum 2010). This suggests that altered nuclear morphology and longevity can be uncoupled, and that changes in nuclear shape are not the main driver of aging. A similar conclusion was reached by Pérez-Jiménez et al. (2014), who found that in daf-2 mutant worms grown at 20°, which have increased longevity, irregular nuclear morphology occurred at the same age as in wild-type worms.
Nevertheless, in aging worms, as in humans, there is a gradual deterioration in nuclear shape as well as relocalization of heterochromatin. Thus it is likely that gene expression in cells of old worms is altered, and while this may not affect longevity under laboratory conditions, it may affect the fitness of the animal in the wild. Interestingly, NPCs deteriorate and become “leaky” as cells age (D’Angelo et al. 2009, and see below), but whether and how this contributes to altered nuclear shape is not known.
Nucleocytoplasmic Transport
Nucleocytoplasmic transport as a regulatory process
The compartmentalization of eukaryotic cells, with RNA transcription taking place in the nucleus and protein translation occurring in the cytoplasm, provides several points of regulation of gene expression. In particular, nuclear import of transcription factors and nuclear export of RNA molecules play critical roles (Adam 2009). The mechanisms of nucleocytoplasmic transport are highly conserved and here we will focus on examples from C. elegans. As discussed above, translocation of macromolecules from the cytoplasm to the nucleus or vice versa occurs through NPCs. So far, 28 C. elegans orthologs of vertebrate NPPs have been identified (Table 1) (Galy et al. 2003; Askjaer et al. 2014b) and it is likely that the worm genome encodes a few additional NPPs whose primary structure is so divergent that they have escaped detection. Although the openings in the nuclear membranes at NPCs are ∼80 nm wide, a tight meshwork of NPPs in the central part of the NPC effectively prevents macromolecules larger than ∼40 kDa from free diffusion in and out of the nucleus. Instead, nucleocytoplasmic transport relies on association of substrates with either import or export transport receptors that are specialized for particular substrate classes (Figure 8). The transport receptors have affinity for phenylalanine-glycine (FG) dipeptide repeats found in many NPPs. The FG domains form a permeability barrier in the central channel of NPCs and mediate translocation of cargo-bound transport receptors through the NPC. Import and export receptors are structurally related and are encoded by importin β (imb) and exportin (xpo) genes. They bind their substrates either directly, or, in the case of substrates with classical nuclear localization signals (NLSs), via importin α (IMA) proteins (Geles and Adam 2001). Directionality of transport is achieved by promoting either association or disassociation of substrate-receptor complexes in a spatial manner. In particular, the asymmetric distribution of the GTP- and GDP-bound conformations of the small GTPase RAN-1 across the NE serves as a gradient in many transport processes (Cautain et al. 2015). RAN-1’s guanine nucleotide exchange factor RAN-3/RCC1 is nuclear, whereas its GTPase activating protein RAN-2/RanGAP is predicted to be cytoplasmic, leading to accumulation of RAN-1GTP in the nucleus and RAN-1GDP in the cytoplasm (Askjaer et al. 2002; Bamba et al. 2002). Nuclear RAN-1GTP stimulates disassembly of nuclear import complexes and assembly of nuclear export complexes, which, in turn, are disassociated by hydrolysis of the RAN-1-bound GTP when they encounter RAN-2 in the cytoplasm. Interestingly, the effect of RAN-1GTP on disassembly of cargoes from import receptors, in particular IMB-1, is also relevant in mitosis to induce mitotic spindle assembly and NE reformation, (Askjaer et al. 2002; Bamba et al. 2002; Walther et al. 2003b), although the exact role of RAN-1 in reassembling the NE is not known.
Table 1. NPPs in the yeast Saccharomyces cerevisiae, C. elegans, and humans.
| Positiona | S. cerevisiae | C. elegans | H. sapiens |
|---|---|---|---|
| Cytoplasmic region | — | NPP-9 | NUP358 |
| Nup159 | NPP-14 | NUP214 | |
| Gle2 | NPP-17/ RAE-1 | RAE1 | |
| Gle1 | Y17G9B.1 | GLE1 | |
| Nup82 | ZK177.4 | NUP88 | |
| — | — | AAAS/ALADIN | |
| Nup42 | — | NUPL2/hCG1 | |
| Cytoplasmic and nucleoplasmic rings | Nup85 | NPP-2 | NUP85 |
| Nup84 | NPP-5 | NUP107 | |
| Nup120 | NPP-6 | NUP160 | |
| Nup145C | NPP-10C | NUP96 | |
| Nup133 | NPP-15 | NUP133 | |
| Seh1 | NPP-18 | SEH1 | |
| Sec13 | NPP-20 | SEC13R | |
| — | NPP-23 | NUP43 | |
| — | MEL-28 | ELYS/AHCTF1 | |
| — | — | NUP37 | |
| Inner ring | Nup192 | NPP-3 | NUP205 |
| Nup157/170 | NPP-8 | NUP155 | |
| Nup145N | NPP-10N | NUP98 | |
| Nic96 | NPP-13 | NUP93 | |
| Nup53/59 | NPP-19 | NUP35 | |
| Nup188 | — | NUP188 | |
| Central channel | Nup57 | NPP-1 | NUP54 |
| Nup49 | NPP-4 | NUPL1 | |
| Nsp1 | NPP-11 | NUP62 | |
| Transmembrane nups | — | NPP-12 | NUP210 |
| Ndc1 | NPP-22/NDC-1 | NDC1/TMEM48 | |
| Pom33 | Y37D8A.17 | TMEM33 | |
| — | — | POM121 | |
| Pom34 | — | — | |
| Pom152 | — | — | |
| Nuclear basket | — | NPP-7 | NUP153 |
| Nup2 | NPP-16 | NUP50 | |
| Mlp1/2 | NPP-21 | TPR | |
| Nup1 | — | — | |
| Nup60 | — | — |
Some nucleoporins are reported to localize to several positions within the NPC but for simplicity each protein is only listed once.
Figure 8.
Nuclear export and import of macromolecules are regulated by transport receptors and the RAN-1 GTPase cycle. Nuclear export (left) of substrates containing NESs involves the formation of a trimeric complex with an XPO transport receptor and RAN-1GTP. XPO mediates the translocation through the NPC via interactions with NPPs. When the export complex reaches the cytoplasm, RAN-2/RanGAP and RAN-5/RanBP1 stimulate the GTPase activity of RAN-1, which causes release of the NES cargo from XPO. Nuclear import (right) is also regulated by the RAN-1 GTPase cycle, but in this case IMB-like transport receptors associate either directly or via IMA adaptors with substrates harboring NLSs in the cytoplasm. After translocation through the NPC, RAN-1GTP binds IMB, which induces release of the NLS cargo and nuclear export of the RAN-1GTP-IMB complex. RAN-1 is reimported in its GDP-bound form by RAN-4/NTF2. Directionality of transport is determined by a high concentration of RAN-1GTP in the nucleus vs. a low concentration in the cytoplasm: The gradient of RAN-1GTP is established by the asymmetric distribution of RAN-2, RAN-5, and the guanine nucleotide exchange factor RAN-3/RCC1.
Nuclear localization and nuclear export signals
Arguably the most studied nuclear import substrate in C. elegans is the FOXO-like transcription factor DAF-16, which accumulates in the nucleus in response to various stress stimuli and regulates life span (Henderson and Johnson 2001; Lee et al. 2001; Lin et al. 2001). The precise mechanisms controlling DAF-16 nuclear import are still unclear but a so-called PY-NLS has been suggested in the central part of DAF-16 (Putker et al. 2013). Moreover, DAF-16 interacts directly with IMB-2, the C. elegans ortholog of transportin-1, and knockdown of IMB-2 inhibits heat and redox stress-induced nuclear accumulation of DAF-16 (Putker et al. 2013). In addition to DAF-16, regulated nucleocytoplasmic transport of other longevity factors is presumably also involved in life span determination. Recently, the small intracellular fatty acid binding protein, LBP-8, was reported to mediate signaling from lysosomes to the nucleus and thereby activate genes that act positively in longevity (Folick et al. 2015). LBP-8 contains a predicted NLS, and a relative large deletion that removes this sequence (but potentially also other functions of LBP-8) abolishes LBP-8’s capacity to increase life span (Folick et al. 2015). Many transcriptional reporter genes designed for analyzing C. elegans promoter activities rely on one or several copies of the classical NLS from the simian virus 40 (SV40) large T antigen (see the “Fire Lab C. elegans Vector Kits” at https://www.addgene.org/Andrew_Fire/). Although a single SV40 NLS is sufficient to target GFP to the nucleus, stronger accumulation of the fusion protein is obtained by addition of the N-terminal 25 amino acid residues of EGL-13, which resemble a bipartite NLS (Lyssenko et al. 2007). Another example of a bipartite NLS was identified in OCR-2, a cation channel expressed in sensory cilia. Residues 841–856 of OCR-2 confer nuclear import activity to an inert substrate, and, importantly, substitution of six basic residues within this fragment for alanines abolishes NLS activity (Ezak and Ferkey 2011). Based on observations on calcium channel proteins in mammalian cells, the authors suggested that upon activation of OCR-2, its NLS-containing C-terminal might translocate to the nucleus to activate target genes (Ezak and Ferkey 2011).
Many proteins with active NLSs also contain nuclear export signals (NESs) that enable shuttling between the nucleus and the cytoplasm, although only few cases have been experimentally demonstrated in C. elegans. One such example is the cell cycle-related phosphatase CDC-14 for which site-directed mutagenesis revealed a basic NLS (366KRNVRR371) juxtaposed to a hydrophobic NES (372LVNQVDDINL381) (Roy et al. 2011). This class of NESs is recognized and exported by XPO-1 (also called IMB-4), and depletion of XPO-1 causes nuclear retention of CDC-14 in V cells of L1 larvae. Interestingly, CDC-14 also accumulates in the nuclei of cyd-1/cyclin D mutants but the role of CYD-1 in nuclear export of CDC-14 remains to be determined (Roy et al. 2011). The export activity of XPO-1 is also required during early embryogenesis where it contributes to Wnt signaling-induced asymmetric cell division. In four cell-stage embryos, the posterior P2 blastomere affects the asymmetry of the adjacent EMS cell when it divides to form posterior endodermal E and anterior mesodermal MS precursor cells. E and MS cells both inherit the TCF/LEF transcription factor POP-1 from EMS but nuclear levels of POP-1, a component of the Wnt-signaling pathway, are higher in MS due to XPO-1-mediated nuclear export in E (Lo et al. 2004). The rate of POP-1 nuclear export is regulated by phosphorylation of POP-1 by the MAPK LIT-1 as well as the polarity factor PAR-5 (Lo et al. 2004). The higher POP-1 export rate in E compared to MS depends on MOM-2/Wnt signaling from P2 to E as well as increased nuclear levels of LIT-1 and WRM-1/β-catenin in E, which in turn depends on XPO-1-mediated nuclear export of WRM-1 in EMS (Lo et al. 2004; Nakamura et al. 2005). These reciprocal differences in nuclear accumulation of Wnt-related factors are repetitively employed later in embryogenesis to generate asymmetry along the anterior-posterior axis.
From the examples above, it is clear that regulation of protein localization to either the nuclear or cytoplasmic compartments can have profound effects on cellular functions. Recently, optogenetic tools were developed to experimentally control the nucleocytoplasmic distribution of proteins in living nematodes using light pulses. Taking advantage of the reversible conformational changes in the light oxygen voltage (AsLOV2) domain of phototropin 1 from Avena sativa, a tag was designed that has a constitutive NES and a light-inducible NLS (Yumerefendi et al. 2015). When this tag, termed light-activated nuclear shuttle (LANS), is fused to a fluorescent protein, nuclear import and export kinetics can be determined (Figure 9). The import and export receptors for LANS in C. elegans are presumably IMB-1 (together with IMA-1, IMA-2, or IMA-3) and XPO-1, respectively, although this needs to be addressed in future experiments, which potentially could also include tests of other NLS and NES sequences to cover a broader repertoire of transport pathways. Importantly, inserting the LANS tag into the lin-1 locus demonstrated that this technique can also be used to affect cell fate. The transcription factor LIN-1 is an inhibitor of the primary vulval cell fate and when animals were grown in the dark to exclude LIN-1::LANS from the nucleus, a multivulval phenotype was observed (Yumerefendi et al. 2015). Conversely, exposing the animals to blue light during development induced a weak vulvaless phenotype, successfully mimicking a lin-1 gain-of-function allele (Yumerefendi et al. 2015).
Figure 9.
The LANS tag allows spatiotemporal control of nuclear accumulation by illumination. (A) In the dark, the LOV2 protein adopts a conformation that makes the NLS inaccessible, whereas the NES mediates active exclusion from the nucleus. Upon illumination, LOV2 changes conformation and the NLS is recognized by IMB, leading to nuclear accumulation. (B and C) Expression of the LANS tag fused to mCherry allows (B) visualization and (C) quantification of nuclear import and export dynamics. A small region of an early embryo was observed by live confocal scanning microscopy using a 561-nm laser. Nuclear import was induced by illumination with a 488-nm laser in the three indicated intervals (blue lightning bolts). Time in micrographs is indicated in seconds. For more information on the LANS tag, see Yumerefendi et al. (2015). Nuc, nucleus; cyt, cytoplasm.
Nuclear export of RNA
Nuclear export of most mRNAs relies on NXF-1/TAP and HEL-1/UAP56 (Tan et al. 2000; MacMorris et al. 2003), whereas XPO-1 is responsible for export of other RNA polymerase II-transcribed genes, such as small nuclear RNAs, premicro RNAs (pre-miRNAs), and certain mRNAs. The best-characterized miRNA in C. elegans is let-7, which controls developmental timing. Mutations in let-7 block the transition from late larval to adult cell fates, in particular by fusion of seam cells, and animals often die from a bursting vulvae phenotype (Bussing et al. 2010). Knockdown of XPO-1, or inhibition of the cap-binding complex (consisting of NCBP-1 and NCBP-2), which acts as an adaptor for XPO-1, causes similar developmental defects as well as a ∼50% reduction in mature let-7 miRNA, arguing that XPO-1 is likely the transport receptor of pre-miRNAs in C. elegans (Bussing et al. 2010). There are, however, examples where XPO-1 affects mRNA export. Sex determination in C. elegans involves a series of proteins that act differently in XX hermaphrodites and XO males. One of these is the transcription factor TRA-1, which is required for sex-specific development of somatic tissues in hermaphrodites. TRA-1 binds to and stimulates export of the tra-2 mRNA in a manner that is sensitive to the XPO-1 inhibitor leptomycin B: nuclear levels of TRA-1 increase in males treated with leptomycin B and production of yolk proteins and oocytes is induced (Segal et al. 2001). Nuclear export of TRA-1 protein and tra-2 mRNA also depends on the factors NXF-2, ALY-1, and ALY-2 (Kuersten et al. 2004), which all seem dispensable for bulk mRNA export (MacMorris et al. 2003).
NPPs and NPC function
Concordantly with their contribution to NPC structure, and extensively explored in other genetic systems, NPPs are required for correct nucleocytoplasmic distribution of numerous macromolecules. Knockdown of several npp genes, including FG repeat-containing npp-1, npp-4, npp-11, as well as npp-2, npp-3, and npp-13 causes a reduction in nuclear size, which typically reflects diminished nuclear import (Galy et al. 2003). More direct evidence for a role in nuclear protein import has been established for NPP-19/NUP35 because nuclear levels of PCN-1/PCNA and PIE-1 are strongly reduced in npp-19(RNAi) embryos (Rodenas et al. 2009). Presumably, additional NPPs are involved in nucleocytoplasmic transport, but due to the pronounced block in postmitotic NE assembly further experiments are required to address this (Galy et al. 2003, 2006; Franz et al. 2005; Fernandez and Piano 2006). Furthermore, recent analysis of the C. elegans torsinA homolog OOC-5 revealed that several NPPs (NPP-1, NPP-9, NPP-19, and NPP-22) are mislocalized and nuclear import of PCN-1 and PIE-1 is delayed in ooc-5 mutants (VanGompel et al. 2015). The exact role of OOC-5 in NPC function is unclear, but NE alterations are also observed upon TorsinA inhibition in mammals and flies, suggesting that OOC-5/TorsinA functions might be conserved (Laudermilch and Schlieker 2016).
Accumulation of RNA and proteins in either the nucleus or the cytoplasm requires that NPCs are efficient transport channels for receptor-associated cargoes as discussed above but also impermeable to “free” cargoes and other macromolecules. NPC permeability can be analyzed with inert fluorescent molecules, such as dye-conjugated dextrans: ∼70-kDa dextrans are unable to cross the NPC in wild-type embryos, but can diffuse into nuclei of npp-3(RNAi) and npp-13(RNAi) embryos (Galy et al. 2003). Interestingly, about one third of nuclei purified from old nematodes are leaky to ∼70-kDa dextrans, suggesting that impaired NPC function might be related to aging (D’Angelo et al. 2009). In support of this, the permeability barrier of nuclei from long-lived daf-2 mutants is more effective than in similar-aged wild-type animals, whereas exposure to oxidative stress can exacerbate the leakiness (D’Angelo et al. 2009). The mechanism underlying these effects is unclear, but might involve oxidative damage of NPPs. Expression analysis comparing embryos, larvae, and adults revealed that several NPPs are not transcribed in postmitotic cells (D’Angelo et al. 2009). Instead, these NPPs have an extremely low protein turnover, both in C. elegans and in mammals, which might make them more susceptible to accumulation of oxidative damage (D’Angelo et al. 2009). Changes in NE permeability are not restricted to aging cells but also occur during apoptosis through cleavage of NPPs. Ca2+ overload is toxic to neurons and relates to ischemic cell death. This can be mimicked in C. elegans by a gain of function of the acetylcholine receptor DEG-3. In deg-3(u662) mutant larvae, NPP-1 levels are strongly reduced at the NE of dying neurons and mixing of nuclear and cytoplasmic proteins is observed (Bano et al. 2010).
The link between NPCs and P granules
In many animal species, germ cells are characterized by the presence of large ribonucleotide protein (RNP) particles, which in C. elegans are known as P granules (Strome 2005). In the distal (mitotic) and middle (meiotic pachytene) part of the gonad in hermaphrodites as well as in the P4 blastomere in embryos, P granules form large perinuclear structures, which, at least in gonads, are adjacent to clusters of NPCs (Figure 10) (Pitt et al. 2000). Within perinuclear P granules, electron-dense material accumulates in the immediate vicinity of NPCs, whereas cytoplasmic P granules (e.g., in oocytes) do not contain such structures, suggesting they might represent transport cargo associated with NPCs (Pitt et al. 2000). Interestingly, several P-granule proteins, such as GLH-1, GLH-2, GLH-3, and GLH-4 contain FG repeats, suggesting they may facilitate the passage of transport receptors and their cargoes from NPCs into P granules (Schisa et al. 2001). In support of this, extensive colocalization of NXF-1 and DDX-19, an FG repeat-containing RNA helicase, with perinuclear P granules was observed in the pachytene region (Sheth et al. 2010). Moreover, inhibition of either transcription through injection of α-amanitin or mRNA export by RNAi against nxf-1 leads to loss of perinuclear accumulation of P granule component PGL-1 (Sheth et al. 2010). Further evidence for a functional relation between NPCs and P granules was provided by a genome-wide RNAi screen for factors that affect P granule formation and/or localization. Of the 173 genes retrieved in the screen, five encode NPC components (npp-6, npp-7, npp-9, npp-10, and npp-20) whereas another four genes encode transport factors (ran-1, ran-4, xpo-1, and xpo-2) (Updike and Strome 2009). RNAi against any of these nine genes caused detachment of P granules from the NE in embryonic germ line blastomeres and diffuse PGL-1 staining in the cytoplasm. Testing of additional NPC components found that depleting NPP-1, NPP-3, NPP-8, or NPP-19 caused similar defects (Updike and Strome 2009). These observations were confirmed in an independent RNAi study, which reported strong PGL-1 dispersal after deletion of NPP-7, NPP-8, NPP-9, or NPP-10N and milder phenotypes in embryos in which npp-1, npp-2, npp-3, npp-5, npp-6, or npp-13 were knocked down (Voronina and Seydoux 2010). Note that, similarly to the situation in yeast and mammals, NPP-10N/NUP98 and NPP-10C/NUP96 are produced from a single protein precursor, which implies that a given RNAi phenotype will generally reflect the combined effect of depleting both proteins. P granule phenotypes are, however, specific to NPP-10N depletion. Importantly, the severity of PGL-1 delocalization did not correlate with inhibition of gene expression as visualized with a pes-10 GFP reporter, arguing that the identified NPPs have a specific role in P granule organization beyond a general role in nucleocytoplasmic transport (Voronina and Seydoux 2010).
Figure 10.
P granules interact with and share characteristics with NPCs. (A) Transmission electron micrograph showing a P granule associated with a germ cell nucleus. Note that NPCs (indicated by arrows) cluster in the NE underneath the P granule. Nuc, nucleus; cyt, cytoplasm. (B) Many NPPs have cohesive FG dipeptide repeats (blue ○) that function to establish a permeability barrier of the NPC. FG repeats are also found in several P granule proteins, extending the exclusion barrier. (C) Small 10-kDa fluorescent dextran molecules (red) can diffuse freely across the NE and into P granules (visualized with GFP::PGL-1; green), whereas larger 155-kDa dextrans are excluded from nuclei and P granules. Bars, 10 μm (top) and 5 μm (bottom). (A) Courtesy of James Priess, whereas (B and C) are reproduced from Updike et al. (2011) with permission.
Much like NPCs, P granules also establish a size-exclusion barrier by hydrophobic interactions that might regulate access of cytoplasmic RNA processing and translation factors to RNA molecules inside the P granules (Figure 10) (Updike et al. 2011). Ectopic expression of multimerized FG domains from either NPP-4 or GLH-1 produced aggregates, suggesting similar biophysical properties of the domains and potentially also similarity between the interior of nuclear pores and P granules (Updike et al. 2011). Depletion of GLH-1 abolishes perinuclear positioning of P granules but GLH-1 is not sufficient to anchor P granules at NPCs, implying that additional molecules must be involved (Updike et al. 2011). PGL-1 and PGL-3 are required for nucleation of P granules, but they are presumably not responsible for their perinuclear localization (Hanazawa et al. 2011; Updike et al. 2011). NPP-9 and NPP-10N (and perhaps also NPP-8) localize to P granules, suggesting they might be the physical link between NPCs and P granules (Sheth et al. 2010; Voronina and Seydoux 2010). NUP358, the vertebrate homolog of NPP-9, forms part of the NPC cytoplasmic fibrils where it is involved in disassembly of export complexes. NPP-9 is therefore a particularly relevant candidate, but NPP-9 is considerably smaller than NUP358 (93 vs. 358 kDa) and lacks several domains found in NUP358, so future experiments are required to address this. The biophysical properties of P granules are similar to liquid droplets whose assembly and disassembly depend on condensation and dissolution, respectively (also known as phase transition; see also Nuclear bodies below) (Brangwynne et al. 2009). Cytoplasmic polarity factors such has MEX-5 and PAR-1 have been implicated in regulating condensation (Brangwynne et al. 2009; Saha et al. 2016) and we speculate that NPCs might also act as condensation points. Finally, it is interesting to note that Mutator RNP foci related to small interfering RNA-mediated RNA silencing also accumulate at NPCs in germ line nuclei, adjacent to P granules (Phillips et al. 2012), and depletion of NPP-1, NPP-4, NPP-9, or NPP-16 affects RNAi efficiency and/or transposon silencing (Vastenhouw et al. 2003; Kim et al. 2005).
Chromosome Organization and its Relationship to Gene Expression
Chromatin organization: an overview
In interphase nuclei of animal cells, chromosomes exist in distinct domains, known as chromosome territories (Figure 11; for review, see Cremer and Cremer 2010; for examination of territories in C. elegans, see Lau et al. 2014). The chromatin fiber itself is organized into topologically associated domains (TADs) (Figure 11); for review, see Dixon et al. (2016). A TAD represents a higher-order structure of the chromatin; DNA regions within a certain TAD are more likely to be in close proximity with each other than with DNA regions outside the TAD. TADs vary in length from 100s of kilobases to several megabases, they are stable through many cell divisions, and they tend to be invariant between cell types in a given organism. Moreover, the transcriptional activity of genes within a certain TAD tends to be the same—either active or repressed—and neighboring TADs can have different levels of transcriptional activity. Active and repressed TADs also differ in their timing of DNA replication, with active TADs replicating earlier than repressed TADs. How TADs are formed is still a mystery, but they are often flanked by binding sites for architectural proteins such as CTCF, a DNA binding protein associated with insulator elements; and cohesin, a complex involved in, among other things, sister chromatid cohesion. Deletion of TAD boundaries affects gene expression in the neighboring TADs (Lupianez et al. 2015). Interestingly, C. elegans does not have a CTCF homolog (Heger et al. 2009) and worm TADs are less pronounced than those of other organisms, except on the X chromosome (Crane et al. 2015, see below).
Figure 11.
Chromosomes occupy separate territories and are organized in TADs. Chromosome territories are characterized by intrachromosomal contacts being much more frequent than interchromosomal contacts. Each territory consists of A and B compartments, which generally correlate with open and closed chromatin states. At the megabase scale, chromatin forms TADs consisting of closely spaced loops.
Active and repressed TADs are thought to exist in two different compartments, called A and B, which roughly coincide with the more historical classification of chromatin into euchromatin, which includes chromatin regions containing transcribed genes; and heterochromatin, containing chromatin regions that are transcriptionally repressed (Solovei et al. 2016). These two chromatin states, which are also referred to as open and closed chromatin, respectively, can be distinguished not only by their staining intensity and histone post-translational modifications, but also by their intranuclear localization: in differentiated cells, heterochromatin tends to reside at the nuclear periphery and surrounding nucleoli, while euchromatin tends to be more intranuclear (although notable exceptions exist; see for example chromatin organization in rod photoreceptors of nocturnal animals in Solovei et al. 2009).
The association of a particular chromatin region with the nuclear periphery is often assessed using assays that detect the proximity between chromatin and components of the nuclear lamina [e.g., Dam-mediated identification (DamID), chromatin immunoprecipitation (ChIP); see below for more details on these methods], but whether and how components of the nuclear lamina associate with chromatin is largely unknown. Nonetheless, chromatin domains that interact via these assays with components of the nuclear lamina are called lamina-associated domains (LADs), and they typically include genes that are either repressed or expressed at a low level (Pickersgill et al. 2006; Guelen et al. 2008).
Euchromatic regions can also be found at the nuclear periphery, and in particular near NPCs (reviewed in Burns and Wente 2014). This led to the hypothesis that NPCs affect gene expression more directly, beyond just mediating RNA export. Indeed, in budding yeast, induced genes move upon induction to the NPC. This NPC association allows not only more robust gene expression, but also serves as a memory function for subsequent inductions, after the initial induction has subsided (Light et al. 2010). This type of peripheral localization requires cis-acting DNA elements, proteins involved in transcriptional activation and subunits of the NPC. In Drosophila and human cells, NPC subunits also interact with transcriptionally active genes, but, at least in some cases, this interaction occurs away from the nuclear periphery, suggesting that NPC subunits may be “moonlighting” in transcriptional activation (Capelson et al. 2010; Kalverda et al. 2010; Franks et al. 2016). In mammalian cells, developmentally regulated genes shift from a peripheral localization where they are inactive to a nuclear interior localization upon differentiation (for example, see Yao et al. 2011). Thus, in different organisms peripheral localization and NPCs may play different roles in regulating gene expression. The situation in C. elegans is discussed in the sections below.
Methodologies for studying chromosome organization in C. elegans
Is the organization of C. elegans chromosomes the same as in other animal cells? To answer this question, let us first consider some of the experimental approaches currently available to C. elegans researchers. Traditionally, chromosome organization was examined relative to nuclear landmarks using microscopy. To localize specific chromosomes or a region within a chromosome, investigators can employ fluorescence in situ hybridization (FISH) using a fluorescently-labeled, single-stranded DNA probe against one or more loci of interest. While FISH can examine the localization of very large chromosomal domains (such as when looking at chromosome territories) or at a specific chromosomal domain relative to a nuclear landmark such as the NE, it cannot determine how the localization of these domains changes over time. For chromosome localization in live samples one can use a system that takes advantage of a fluorescently-tagged bacterial or phage repressor that binds to an array of its cognate DNA binding sites, or operators, integrated into a specific chromosomal locus. Examples include the LacI/lacO or the TetR/tetO repressor/operator pairs, with only the former currently being used in C. elegans (see Gonzalez-Serricchio and Sternberg 2006 for the original development of this system in C. elegans). Using this system, one can not only follow the behavior of a particular chromosomal locus over time, but also identify lineage or tissue-specific differences within the worm. This kind of cell type-specific analysis is not possible using approaches that involve DNA preparation from entire worms or embryos, such as for the methods described below. For additional uses of this experimental approach see Askjaer et al. (2014a).
A higher resolution examination of chromosome localization relative to a nuclear protein or structure can be obtained using ChIP or DamID (for protocols in C. elegans see Askjaer et al. 2014a). ChIP employs protein-DNA cross-linking, followed by shearing the DNA and immunoprecipitation with antibodies against a protein of interest. The cross-links are then reversed and the associated DNA is identified, often by sequencing. This can be done either genome-wide or for a specific chromosomal domain. In DamID, the protein of interest is fused to the Escherichia coli Dam DNA adenine methyltrasferase. The Dam methyltransferase will methylate the adenine of GATC sequences on DNA in the vicinity of the tagged protein. The DNA is then purified, digested with the restriction enzyme DpnI that recognizes methylated GATC sequences [present at very low frequency in worms (Greer et al. 2015)], ligated to linkers, and sequenced. Unlike ChIP, which provides a snapshot of protein-DNA interactions at the time of cross-linking, DamID can uncover DNA regions that were in the vicinity of a given protein during a prolonged time window, provided that the cell did not undergo DNA replication. Both methods have been used to identify chromosome regions in the vicinity of the NE, as will be discussed below.
As noted above, the analysis of chromosome folding into higher order structures can be done using various chromosome conformation capture methods (for review see Fraser et al. 2015). These methods rely on cross-linking, followed by DNA fragmentation, restriction enzymes digestion, ligation, and sequencing of ligation junctions. The closer two DNA sequences are to each other, the higher the likelihood that they will be ligated to each other. DNA segments that are right next to each other on the chromosome show the highest probability of interaction. However, this method can also detect interactions between DNA segments that are further apart: DNA regions that are next to each other due to chromosome folding, such as in a particular TAD, have a higher chance of ligating to each other than DNA regions that are, for example, in another TAD or on another chromosome. In this way it is possible to examine the proximity of either selected sequences or all chromosomal sequences relative to all other sequences. Two recent C. elegans studies, one employing Hi-C and the other a variation of tethered chromatin capture, revealed unique features of the C. elegans chromosome organization (Crane et al. 2015; Gabdank et al. 2016).
Features of C. elegans chromosome organization
In C. elegans, TAD boundaries are barely detectable on autosomes (Figure 12). It should be kept in mind that these chromosome conformation capture analyses are nearly always done on a population of cells. Therefore, it is formally possible that worm cells have well-defined TADs, but they differ from cell to cell (in embryos, Crane et al. 2015) or from tissue to tissue (Gabdank et al. 2016). However, this would still be different from classical TADs, which are largely conserved within all cells of an organism. Moreover, since genes within a TAD have the same type of activity, and neighboring TADs can have opposite activities, it is difficult to imagine how these putative nonsequence-specific worm TADs might function in regulating gene expression. Thus, the significance, if any, of these ill-defined autosomal TADs in regulating autosome function in C. elegans remains to be determined. The X chromosome, on the other hand, does have defined TADs (Crane et al. 2015; Gabdank et al. 2016; see below).
Figure 12.
Spatial organization of C. elegans chromosomes. Examples of X chromosome (top) and chromosome I (bottom) are shown. Chromosome conformation capture (Hi-C) analysis was carried out on chromosomes from C. elegans embryos. The data from Hi-C analyses are typically depicted on a matrix where all chromosomal loci (in this case binned in 50-kb resolution) are on both the x- and y-axes, and the frequency of interaction (as reflected by the number of reads that span two loci) is color coded, with darker colors indicating a higher incidence of interaction (or more reads). Interactions will obviously be the greatest between two adjacent loci on the same chromosome, generating a very dark diagonal. The panels shown in this figure focus on a few megabases to each side of this diagonal. Diamond-shaped structures, such as the ones seen along the diagonal for the X chromosome, reflect TADs and indicate that there is a higher level of interaction between distant sites within the coordinates of the TAD than outside the TAD. This analysis revealed that C. elegans chromosomes are organized in megabase-sized TADs separated by boundaries (green lines; darker green indicates stronger boundary). The organization in TADs is more pronounced for the X chromosome than for autosomes (chromosome I shown as example). Figure courtesy of Barbara Meyer; data from Crane et al. (2015).
Analysis of the chromatin association with the nuclear periphery in C. elegans embryos using a variety of methods (e.g., ChIP and DamID with the NE proteins LEM-2, LMN-1 and EMR-1) revealed that the distal ∼4 Mb of both chromosome arms of all autosomes tend to associate with the nuclear periphery, while the center part of the autosomes appears to be largely looped away from the periphery (Figure 13) (Ikegami et al. 2010; Gonzalez-Aguilera et al. 2014). Consistent with this interpretation, analysis of telomere positioning by FISH confirmed that the distal-most ends of chromosomes associate with the nuclear periphery (Ferreira et al. 2013). These lamina-associated chromatin regions are the worm’s version of LADs, and they are inherent to the DNA sequence rather than to the chromosomal location, because translocation of an arm region to the center of a chromosome retained the region’s peripheral association (Ikegami et al. 2010). Moreover, the regions that are associated with the NE are conserved between embryos and adults (Gonzalez-Aguilera et al. 2014). However, not all lamina proteins associate with the same chromosomal regions. For example, chromosomal domains that associate preferentially with EMR-1 are enriched in genes involved muscle and neuronal functions (Gonzalez-Aguilera et al. 2014). When EMR-1 is absent, these same regions can associate with LMN-1, suggesting that when both are present, EMR-1 blocks the access of LMN-1 to a subset of EMR-1-associated sites. In contrast to the autosomes, the X chromosome tends to associate with the nuclear periphery only at one end of the chromosome, at the left arm (Figure 13) (Ikegami et al. 2010). The chromosomal regions associated with the periphery are enriched in repressive histone modifications, such as trimethylation of lysine 9 of histone H3 (H3K9me3) or trimethylation for lysine 27 on histone H3 (H3K27me3) (Ho et al. 2014), but it should be noted that within these distal chromosome arm regions there are also active genes in between the repressed ones (Ikegami et al. 2010).
Figure 13.
LMN-1 and EMR-1 associate with chromosome arms. Genome-wide chromatin association profiles for LMN-1 and EMR-1 in adult worms were obtained by DamID. Both NE proteins interact extensively with both chromosome arms of autosomes (chromosome V is shown as representative example) and the left arm of the X chromosome, whereas chromosome centers are largely devoid of contacts. From Gonzalez-Aguilera et al. (2014). MA2C, model-based analysis of two-color arrays.
How does chromatin associate with the nuclear periphery? On the NE side, the C. elegans nuclear lamina proteins LMN-1, EMR-1, and LEM-2 are all involved in tethering chromatin to the nuclear periphery (Mattout et al. 2011), as is the case in mammalian cells. Downregulation of LMN-1 or codownregulation of EMR-1 and LEM-2 leads a detachment of large heterochromatin arrays from the nuclear periphery (Mattout et al. 2011). On the chromatin side, peripheral association of chromatin in the embryo (but not in larvae or adults) depends, in part, on the presence of H3K9 (mono-, di-, and tri-) methylation by MET-2 and SET-25 (Towbin et al. 2012). This dependence is dramatic when examining repetitive gene arrays but is also seen with native chromosome arms: in the absence of H3K9 methylation, the association of chromosome arms with the nuclear periphery in embryos is reduced, but not eliminated (Towbin et al. 2012). Moreover, the peripheral localization of chromosome arms that are H3K9-methylation poor (such as the right arm of chromosome IV and the left arm of chromosome V) and that of the X chromosome were not affected by mutations that reduced H3K9 methylation (Towbin et al. 2012). Thus, there is an additional, still-unknown mechanism for tethering chromatin to the nuclear periphery that is independent of H3K9 methylation.
But what links H3K9 methylated chromatin to the nuclear periphery? Using an RNAi screen against proteins that contain a motif that recognizes methylated histones, Gonzalez-Sandoval et al. (2015) searched for a protein that, when inactivated, leads to mislocalization of peripheral chromatin in embryos. This led to the identification of a chromodomain protein, CEC-4, that fits the bill (Figure 14): CEC-4 localizes to the NE, binds specifically methylated H3K9 through its chromodomain, and in its absence a marked heterochromatic domain fails to maintain its peripheral localization (Gonzalez-Sandoval et al. 2015). However, unlike the inactivation of both MET-2 and SET-25, which results in both derepression and detachment of chromatin from the nuclear periphery (Towbin et al. 2012), the absence of CEC-4 resulted in detachment of heterochromatin from the nuclear periphery without activation of transcription. There are also examples where chromatin can be derepressed while maintaining its peripheral localization (Towbin et al. 2012). Thus, gene expression and localization can be decoupled. Finally, while CEC-4 is needed for peripheral chromatin localization in the embryo, its function is either redundant with a yet-unknown protein(s) or not needed in later developmental stages (Gonzalez-Sandoval et al. 2015) as mutants lacking CEC-4 develop normally, despite changes to chromatin organization in the embryo. However, cec-4 null mutants did show developmental defects in a system where muscle tissue was ectopically induced (see below). CEC-4 does not appear to have homologs outside nematodes, but there may be proteins with analogous functions.
Figure 14.
The chromodomain protein CEC-4 is involved in perinuclear anchoring of heterochomatin. CEC-4 localizes to the NE where it is engaged in binding of nucleosomes containing methylated H3K9. Loss of CEC-4 leads to detachment of perinuclear heterochromatin.
The role of chromatin association with the NE in regulating gene expression
The relationship between nuclear localization and gene expression appears to be complex. In the set-25; met-2 and cec-4 mutants described above, detachment from the nuclear periphery did generally not result in activation of repressed genes (Towbin et al. 2012; Gonzalez-Sandoval et al. 2015). In contrast, inactivation of LMN-1, which also results in detachment of chromatin from the nuclear periphery, does lead to gene derepression, at least when it comes to a promoter present on a gene array (Mattout et al. 2011). However, given that LMN-1 inactivation affects many nuclear functions, the effect on gene expression may not be direct. Concomitant downregulation of both EMR-1 and LEM-2 also increased gene expression and led to the detachment of genes from the NE, but the two were not always linked: in some cases gene expression increased without detachment from the NE (Gonzalez-Aguilera et al. 2014). Thus, the relationship between association with the NE and gene expression may involve different processes for different genes.
There are, however, examples in C. elegans where NE components have a direct effect on gene expression. For example, components of the NPC play a role in processing certain transcripts produced by RNA polymerase III (Ikegami and Lieb 2013). Specifically, NPP-13 (homologous to human NUP93) is an integral NPC subunit that, at least by immunofluorescence, is present exclusively at the NE. Depletion of NPP-13 led to the accumulation of precursors of small nucleolar RNAs and of at least two transfer RNAs that are transcribed by RNA polymerase III, while no effect was seen on processing of RNA polymerase II-dependent transcripts. ChIP analyses revealed that NPP-13 and its interacting partner NPP-3 associate with 223 chromosomal loci, nearly all of which are near noncoding genes transcribed by RNA polymerase III (note, however, that not all genes transcribed by RNA polymerase III were found to interact with NPP-13). Assuming that NPP-13 really carries out its function at the NPC, these observations suggest that RNA polymerase III genes are recruited to the NE for their transcript to be properly processed at the NPC.
An association of specific genes with the NPC has also been shown to occur under certain stress or nutritional conditions. In yeast, for example, several inducible genes relocate to the nuclear periphery, promoting both a more robust induction and serving as a memory function for more efficient future inductions (Ibarra and Hetzer 2015). An analogous situation occurs in C. elegans for the promoter of the heat shock gene hsp-16.2 (Rohner et al. 2013). Because of the chromosomal location of this gene, which is 1.8 Mb from the telomere of the left arm of chromosome V, even before heat shock the gene tended to be close to the NE. Nonetheless, upon heat shock there was a significant increase in the number of cells in the embryo with the hsp-16.2 locus at the NE. This increase was dependent on HSF-1, a conserved heat shock transcription factor, and on proteins essential for transcription. Importantly, the hsp-16.2 locus moved specifically to the vicinity of the NPC. The association with the NPC could facilitate rapid induction, but could also promote efficient degradation of the transcribed mRNA once the inducing signal is extinguished. Thus, in C. elegans, as in other organisms, recruitment to the NE can serve in either a repressive capacity through an association with the nuclear lamina, or in an activating capacity, through the association with NPCs.
Another type of stress is the complete depletion of oxygen (anoxia), which in worms and other organisms leads to a state called suspended animation: in this state cell-cycle progression and developmental programs “freeze,” and energy usage decreases. This state can persist for several days and is reversible, meaning that worms that have experienced anoxia can fully recover (Padilla and Ladage 2012). The mechanism behind suspended animation is poorly understood. Interestingly, under these conditions, chromosomes appear condensed (Padilla et al. 2002) and they accumulate at the nuclear periphery (Hajeri et al. 2005). An alteration in DNA distribution in response to anoxia was also observed in zebrafish (Padilla and Roth 2001) and flies (Foe and Alberts 1985). Live imaging of C. elegans embryos revealed that in prophase, cell’s chromosomes move to the nuclear periphery within 8.5 min of oxygen depletion and move away from the periphery within 4 sec of reexposure to air (Hajeri et al. 2010), raising the intriguing question of what is responsible for this movement. Interestingly, depletion of NPP-16/NUP50 prevents anoxia-induced prophase arrest, suggesting that NPCs might be involved in peripheral chromosome condensation (Hajeri et al. 2010). Although anoxia is known to induce changes in gene expression in the fly (Ragland et al. 2010), whether and how the anoxia-induced peripheral chromosome localization affects gene expression in C. elegans are not known.
Chromosome organization and dosage compensation of the X chromosome
As noted above, in most organisms chromosomes are organized into TADs, which are thought to be important for coregulation of gene expression (Solovei et al. 2016). While C. elegans autosomes have ill-defined TADs, the situation is different with the X chromosome, in which TADs are more pronounced (Figure 12). However, whether X-chromosome TADs affect gene expression locally or are a consequence of the mechanism that ensures the special expression pattern of the X chromosome globally is still unclear. To equalize the expression of the X chromosome between hermaphrodites (which have two X chromosomes) and males (which have only a single X chromosome), the expression of genes on the X chromosomes in hermaphrodites is reduced by twofold in a process known as dosage compensation (for review, see Meyer 2010). This is in contrast to dosage compensation in mammals where in cells carrying two X chromosomes, one of the X chromosomes is inactivated while dosage-sensitive genes in the other are upregulated (Deng et al. 2011). A key component of the dosage-compensation machinery in C. elegans is the dosage compensation complex (DCC), which is structurally similar to the condensin complex that can induce structural changes to DNA (Sharma et al. 2014; Lau and Csankovszki 2015). Both FISH and Hi-C data suggest that the DCC leads to compaction of chromosome X in XX animals. Interestingly, while C. elegans lacks the CTCF protein that is necessary for TAD formation in other organisms, TADs on the C. elegans X chromosome are dependent on the DCC (Crane et al. 2015). Since inactivation of the DCC decreases or abolishes TADs (with the exception of TADs in the LADs, on the left end of the chromosome) and increases the expression of genes on the X chromosome, it is possible that TADs create a chromosome-wide repressive environment that lowers gene expression.
Another potential contributing factor to expression from the X chromosome is its nuclear localization. Sharma et al. (2014) examined the nuclear localization of several X-chromosome loci by FISH and live imaging and found that in hermaphrodites, where the two X chromosomes are dosage compensated, there is no preferential nuclear localization. In contrast, in males the single X chromosome is preferentially localized at the nuclear periphery in a DCC-dependent manner. Sharma et al. further observed, using DamID, that when compared with the two hermaphroditic X chromosomes, the male X chromosome shows a higher degree of interaction with the MEL-28 NPC subunit. A similar interaction of the male X chromosome with NPPs has been reported in Drosophila (reviewed in Sharma and Meister 2016). However, the preferential localization of the male X chromosome at the nuclear periphery was recently contested by Wheeler et al. (2016) who found no difference in the nuclear localization of the X chromosome(s) in either sex. While Wheeler et al. did not examine the association of the X chromosomes with MEL-28, it is possible that this interaction occurs away from the NE, as is the case for several moonlighting NPC subunits in other organisms, which have functions independent of their NPC roles (Capelson et al. 2010; Kalverda et al. 2010). The reason for the discrepancy between the two studies is not clear.
Changes in chromosome organization during development
Relocation of tissue-specific genes from the nuclear periphery to the nuclear interior has been observed in mammalian hematopoietic cells (see for example Brown et al. 2001) and in in vitro differentiating embryonic stem cells (Peric-Hupkes et al. 2010). However, examining the relationship between nuclear positioning and gene expression in C. elegans affords the unique opportunity of following a particular genomic locus from embryo to adulthood in an intact organism. Using both the LacI/lacO system and FISH, in addition to several distinct transgenes carrying tissue-specific promoters, Meister et al. showed that in contrast to embryos, where the tested promoters were distributed randomly throughout the nucleus, in first larval-stage worms (L1), nuclear positioning correlated with gene expression: transgenes were at the nuclear periphery in tissues where their associated promoters were silent, but they shifted to a more internal nuclear localization is tissues where the promoters were expressed. Thus, chromatin organization is developmentally regulated (Meister et al. 2010). It is important to note, however, that gene expression drives nuclear localization rather than localization determining gene expression: when a muscle-specific promoter was ectopically induced to be expressed in the “wrong” cell type, the intranuclear localization of the promoter locus changed in these cells from peripheral to intranuclear (Meister et al. 2010). Moreover, expression of genes at the nuclear periphery can be genetically derepressed without resulting in a change in their localization (Towbin et al. 2012).
Still, there is a strong correlation between expression and nuclear localization, suggesting that there ought to be some functional significance to the position of genes within the nucleus. The challenge of addressing this question is that inactivating factors required for this localization, such as lamins or histone modifying genes, will likely have pleiotropic effects. However, a unique opportunity came about with the discovery of CEC-4, which seems to be specific for interactions of H3K9me3 with the periphery and yet has no effect on gene expression (Gonzalez-Sandoval et al. 2015). To address whether nuclear positioning may have an effect on differentiation, Gonzalez-Sandoval et al. induced a pulse of HLH-1, a master regulator of muscle differentiation, throughout the entire embryo in either wild-type or cec-4 null-mutant embryos. While all wild-type embryos turned into lumps of muscle-like cells, as reported previously (Fukushige and Krause 2005), 25% of the cec-4 mutant embryos continued to develop and even hatched (Gonzalez-Sandoval et al. 2015), despite expressing the same levels of HLH-1 as in wild-type embryos. This suggests that proper intranuclear localization is important for commitment to a differentiated state, perhaps by repressing other developmental programs.
Does the nuclear lamina itself affect tissue-specific expression? Laminopathies are a group of diseases caused by mutations in nuclear lamina proteins (Dobrzynska et al. 2016). Interestingly, many of these lamina-associated mutations affect only a subset of tissues, despite the ubiquitous expression of lamina proteins throughout the organism. Importantly, in most cases the disease causing mechanism in not known. While defects in the nuclear lamina could affect the cell’s ability to deal with mechanical pressure, it is also possible that there are mutations in which the disease phenotype is caused by abnormal gene expression due to altered chromatin-NE associations. To address this question, Mattout et al. expressed in worms an LMN-1 variant carrying the autosomal-dominant Emery–Dreifuss muscular dystrophy mutation, Y59C. These worms also contained a heterochromatin-inducing gene array that also contains a muscle-specific promoter (Mattout et al. 2011). Normally during differentiation, tissue-specific genes move from the nuclear periphery into the nuclear interior, correlating with the activation of their expression. However, in animals expressing LMN-1Y59C, movement of the muscle-specific promoter within the gene array was perturbed and was instead retained at the nuclear periphery. This correlated with reduced expression of the gene array promoter (although the expression of the same promoter at its endogenous locus was unaltered) and resulted in animals with decreased muscle function and altered muscle organization, suggesting that the expression of additional muscle-specific genes was also altered. It is of course possible that altered lamina structure led to mechanical damage, which, in turn, led to the muscle phenotypes. Regardless, the localization data suggest that one of the properties of lamin is that it assists in sequestering inactive genes to the nuclear periphery but allows their release upon gene activation. Whether gene activation was reduced because of the sequestration to the nuclear periphery, or whether a defective activation of gene expression resulted in the inability to detach from the periphery is still an open question.
Emery–Dreifuss muscular dystrophy can also be caused by mutations in the NE protein emerin, which, like lamin, is ubiquitously expressed. While in C. elegans both proteins are present throughout the NE, some chromosomal regions bind to one but not the other (Gonzalez-Aguilera et al. 2014). In particular, chromosomal regions that associate with EMR-1 but not LMN-1 are enriched for genes involved in muscle and neuronal functions. Worms lacking EMR-1 are viable and fertile, likely due to its overlapping functions with LEM-2. Nonetheless, in the absence of EMR-1, adult worms display altered neuromuscular-junction activity (Gonzalez-Aguilera et al. 2014). This is consistent with a role for EMR-1 in regulating muscle and neuronal genes, and could explain the tissue-specific defects in Emery–Dreifuss muscular dystrophy due to emerin mutations, although this still remains to be tested.
Finally, we must consider changes in nuclear organization during aging. An RNAi screen for genes that affect C. elegans longevity uncovered several chromatin-modifying enzymes, the downregulation of which led to longevity extension (Hamilton et al. 2005). Moreover, the repressive H3K27me3 modification is lost as cells age (Jin et al. 2011). As discussed above, the nucleus of aging worms undergoes morphological changes reminiscent of aging cells in mammals, although the two phenomena can be genetically uncoupled (Pérez-Jiménez et al. 2014). The link in C. elegans between nuclear morphology in aging cells and gene expression is not known: does altered nuclear morphology affect the ability of chromatin to associate with the nuclear periphery, and if so, does that lead to changes in gene expression that affect the aging process? In mammals, the effect of aging on gene expression is often studied using a mutation in nuclear lamina components which leads to progeroid syndromes. The worm’s rapid life cycle, however, provides a system in which the relationship between nuclear structure and nuclear function during aging can be studied in a normal physiological setting.
Nuclear Bodies
Nuclear bodies—general
Besides chromosomes, the nucleus contains a number of nonmembrane-bound structures that are collectively referred to as nuclear bodies (Sleeman and Trinkle-Mulcahy 2014). Nuclear bodies serve to compartmentalize the nucleus, providing a local high concentration of the factors involved, mostly, in specific RNA processing events. In recent years, the idea that subnuclear compartments (as well as P granules described above) are liquid-phase droplets that form through phase separation has come into favor (Aumiller et al. 2014). These compartments tend to be highly dynamic, meaning that their components can readily exchange with the surrounding milieu (Phair and Misteli 2000). Moreover, proteins within these phase-separated compartments tend to have intrinsically disordered, low complexity sequence domains (Brangwynne 2013). One of the roles of segregating proteins into phase-separated compartments could be to increase the local concentration of relevant enzymes and their substrates, thereby enhancing enzymatic activity (Aumiller et al. 2014). In this way, the nucleus can have numerous distinct, dynamic compartments, each performing its specialized function(s). Conversely, regulation of nuclear volume may be important for maintaining these types of subnuclear compartments.
Few studies have been devoted to nuclear bodies in C. elegans, with the exception of the nucleolus. Indeed, studies in C. elegans were among the first to describe the regulation of nucleolar size and the biophysical properties of nucleolar assembly, as described below.
Nucleolar function and organization
The main function of the nucleolus is rRNA synthesis and ribosome subunit assembly. The nucleolus is frequently surrounded by heterochromatin and is also a hub for proteins involved in various other processes, such as cell-cycle regulation, stress response, growth control, virus replication, and—in C. elegans—innate immunity (Fuhrman et al. 2009; Pederson 2011). Unlike various autonomously existing cellular structures, the nucleolus exists by virtue of rRNA synthesis. In C. elegans there are ∼55 tandem repeats of an array containing the 18S, 5.8S, and 26S ribosomal genes at the end of chromosome I, while the 5S RNA is on chromosome V (C. elegans Sequencing Consortium 1998). When the rDNA is expressed, the nucleolus assembles around these tandem repeats such that three distinct domains can be visualized: the fibrillar center, which is surrounded by the dense fibrillar component, which, in turn, is embedded in the granular component. Recent studies in Xenopus and other systems showed that these nucleolar subdomains are themselves phase separated within the nucleolus (Feric et al. 2016). The organization of the nucleolus reflects a functional hierarchy with transcription occurring in the fibrillar center, early rRNA processing in the dense fibrillar component, and late rRNA processing in the granular component. However, the precise structure of the rDNA genes within these three domains is still uncertain (for review, see Dundr 2012). In mitosis, the rDNA is not expressed and the rRNA is not processed, causing the nucleolus to disassemble in an ordered fashion (Hernandez-Verdun 2011). Disassembly is regulated by Cdk1-cyclin B inhibitory phosphorylation of RNA polymerase I and components of the rRNA processing machinery; nucleolar reassembly at the end of mitosis is facilitated by dephosphorylation mediated by the PP1 and PP2A phosphatases (Hernandez-Verdun 2011). During gametogenesis, the nucleolus is absent from the transcriptionally silent oocyte and sperm, and it reforms in the embryo once zygotic expression starts. In C. elegans, the nucleolus disappears in mature oocytes and reappears at the six- to eight-cell stage, typically two nucleoli per nucleus corresponding to the two rDNA clusters in diploid cells (Korcekova et al. 2012).
Regulation of nucleolar size
The regulation of nucleolar size is an interesting question, not only because of the fundamental aspect of organelle/structure scaling relative to cell size, but because the nucleolus directly affects cell size through its involvement in protein translation, and there is a direct correlation between nucleolar size and translational activity. A mutation that causes nucleolar size increase in C. elegans was first identified by Hedgecock and Herman (1995). The mutation resided in the ncl-1 gene, which was later found to be a repressor of ribosome synthesis (Frank and Roth 1998). Downregulation of ncl-1 results not only in enlarged nucleoli but also in enlarged cell and body size (Frank and Roth 1998), suggesting that NCL-1 may be a key regulator in determining cell and organismal growth. The expression of ncl-1 is regulated by the let-7 miRNA (Yi et al. 2015), a heterochronic gene encoding a 21-base miRNA needed for correct temporal regulation of development (Reinhart et al. 2000, reviewed in Moss 2007). NCL-1, in turn, regulates the C. elegans fib-1, which codes for the C. elegans homolog of fibrillarin involved in pre-rRNA processing. The levels of FIB-1 affect nucleolar size (Yi et al. 2015). Thus, in C. elegans, nucleolar size appears to be regulated, at least in part, through pathways that oversee the temporal execution of development. In addition, nutrient signaling through the C. elegans TOR pathway also affects nucleolar size, likely as a way for regulating protein synthesis and cell growth (for example, see Sheaffer et al. 2008).
In the developing C. elegans embryo, nucleolar size scales with cell and nuclear size: as cells divide and become smaller, so do their nuclei and nucleoli (Weber and Brangwynne 2015). In intestinal cells of the developing C. elegans larvae, nucleolar size, but not nuclear size, scales with cell size (Uppaluri et al. 2016). The mechanism(s) behind this scaling process is not known. Interestingly, if cell size is manipulated in early embryos such that cells are smaller, nucleoli become bigger than in normally sized cells. It has been postulated that this inverse correlation is because early embryos have a finite pool of nucleolar components deposited by the mother; in embryos with smaller cells the concentration of these components is higher, leading to a greater likelihood of being incorporated into the nucleolus (Weber and Brangwynne 2015). This type of behavior fits structures whose assembly depends on phase separation, as described above. Indeed, studies in both Xenopus and C. elegans showed that the nucleolus has properties of a phase-separated compartment (Brangwynne et al. 2011; Weber and Brangwynne 2015).
External Forces that Affect Nuclear Function
Components of the NE involved in transducing force
Many animal cells are characterized by a nonrandom location of their nucleus, and several pathologies affecting muscles and the nervous system are linked to aberrant nuclear positioning (Starr and Fridolfsson 2010). Also, activities inside the nucleus, such as chromosome movement and signaling cascades, are influenced by events in the cytoplasm, and NE proteins serve to transduce signals and force between the two compartments. Genetic screens in C. elegans were instrumental in identifying the first regulators of nuclear positioning (Sulston and Horvitz 1981; Hedgecock and Thomson 1982), and a bridging model with a LINC complex between ONM and INM proteins was proposed early on in this organism (Figure 15) (Starr et al. 2001). Below, we discuss three situations where LINC complexes play important roles.
Figure 15.
LINC complexes formed by SUN-domain proteins (SUN-1, UNC-84) in the INM and KASH domain proteins (ANC-1, KDP-1, UNC-83, ZYG-12) in the ONM mediate extensive contacts to the cytoskeleton. ANC-1, a homolog of mammalian nesprin 2, associates with actin and microtubules; whereas dynein and kinesin mediate the connection of UNC-83 and ZYG-12 to microtubules. Examples of proteins functionally involved in these contacts are depicted. A ZYG-12 isoform without transmembrane domain localizes to centrosomes and interacts with ZYG-12 at the NE. In humans, LINC complexes involve trimeric SUN and KASH proteins (Sosa et al. 2012). LEM-2 is also involved in tethering of centrosomes to the NE, but the mechanism is unknown. Presumably, many of these interactions occur only in specific cell types. KDP-1 interacts with SUN-1 and perhaps also UNC-84 and regulates cell cycle progression. In addition to controlling nuclear positioning, the LINC complexes also mediate mechanosignaling to the genome via the nuclear lamina, and SUN-1 serves as anchoring point for chromosome PCs during meiosis and for telomeres. ZYG-12/SUN-1 complexes colocalize with PC at the NE, whereas KDP-1 and POT-1/telomeres are arbitrarily connected to the same SUN-1 molecules for simplicity.
Pairing of meiotic chromosomes
During meiosis, homologous chromosomes need to pair to ensure accurate chromosome segregation. In C. elegans, each chromosome contains a pairing center (PC) region, which during meiosis is bound by the C2H2 proteins HIM-8 (chromosome X), ZIM-1 (chromosomes II and III), ZIM-2 (chromosome V), or ZIM-3 (chromosomes I and IV) (Phillips et al. 2005; Phillips and Dernburg 2006). These four zinc-finger proteins are paralogs expressed from a single operon and they all localize to the NE, which might increase the pairing efficiency by reducing the mobility space of PCs from three dimensional to two dimensional (Phillips et al. 2005). Several mutations in him-8 that prevent recruitment of X-chromosome PCs to the nuclear periphery also cause defects in X-chromosome pairing and segregation. However, these defects are also observed in him-8(me4) mutants, in which PCs of the X chromosomes are recruited to the NE, arguing that tethering to the nuclear periphery is not sufficient to ensure correct pairing and segregation (Phillips et al. 2005). In the meiotic part of the gonad, HIM-8, ZIM-1, -2, and -3 colocalize with patches of LINC proteins SUN-1 and ZYG-12 (SUN and KASH domain proteins, respectively; see Figure 15); whereas the LINC proteins are more uniformly distributed in the NE in the premeiotic part of the gonad (Penkner et al. 2007; Penkner et al. 2009; Sato et al. 2009). Colocalization of HIM-8 with SUN-1 and ZYG-12 is abolished in him-8(me4) mutants, which indicates that the interaction of HIM-8 (or the ZIM proteins on autosomes) with the LINC complex is required for correct chromosome pairing (Sato et al. 2009). In support of this, reduction of SUN-1 and ZYG-12 activity prevents pairing of homologous chromosomes, whereas promiscuous pairing of nonhomologous chromosomes and increased apoptosis are observed (Penkner et al. 2007; Sato et al. 2009). ZYG-12 interacts with dynein, while the ZYG-12A isoform, which has no transmembrane domain, is enriched at centrosomes (Malone et al. 2003). Furthermore, chemical inhibition of microtubules or depletion of dynein interferes with meiotic chromosome movement and synapsis formation (Sato et al. 2009; Labrador et al. 2013). ZYG-12-mediated recruitment of dynein to the NE is also required for proper organization of microtubules and nuclear positioning in the syncytial gonad. Accumulation of ZYG-12 at the ONM requires ZYG-12’s KASH domain and SUN-1, which together form a relatively immobile complex in the NE (Minn et al. 2009). Moreover, oligomerization of SUN-1 (Figure 15; see also Sosa et al. 2012) via its central coiled-coil domains contribute to the retention of SUN-1 in the INM, although SUN-1 lacking the coiled-coil domains still form functional LINC complexes (Daryabeigi et al. 2016).
PCs have been proposed to share analogy with centromeres, and recent experiments found that mitotic spindle assembly checkpoint (SAC) proteins MDF-1/Mad1, MDF-2/Mad2, and BUB-3 are part of a meiotic surveillance mechanism to ensure that synapsis formation occurs between homologous chromosomes (Bohr et al. 2015). SAC proteins accumulate at the NE in gonads and embryos although the tethers presumably differ (Rodenas et al. 2012; Bohr et al. 2015). The mechanism by which MDF-1 and MDF-2 prevent nonhomologous chromosomes from pairing is unclear, but it might involve interactions with SUN-1 and dynein based on co-immunoprecipitation experiments and genetic interactions, respectively (Bohr et al. 2015). Interestingly, MDF-1 and MDF-2 are also involved in DNA damage repair and accumulate at the NE in response to ionizing irradiation or hydroxyurea (Lawrence et al. 2015). This behavior depends on both the centromeric protein HCP-3/CENP-A and the DNA damage-response kinase ATL-1/ATR, and indicates that SAC proteins have wider roles in genome stability than initially recognized. HTP-1, which is predicted to have structural similarity with Mad2, also regulates timing of homology searches between chromosomes and SUN-1 dynamics in the NE (Baudrimont et al. 2010; Silva et al. 2014), suggesting that multiple pathways to ensure correct chromosome pairing might exist.
Nuclear migration and positioning
During C. elegans development, nuclei actively migrate in several cell types, and migration is already critical in the one cell-stage embryo to juxtapose the two pronuclei in the center of the cell (Starr and Fridolfsson 2010). A mutagenesis screen designed to identify genes with roles in early embryos identified nine loci involved in pronuclear migration (pnm) and pronuclear/nuclear position (nup) (Gonczy et al. 1999b). The gene identities remain unknown, except for lis-1 (pnm-1), which encodes a homolog of the human lissencephaly-related protein LIS1 (Cockell et al. 2004). LIS-1 localizes throughout the cytoplasm and accumulates at the NE. In lis-1 mutants or in embryos from lis-1 RNAi treated worms, the male pronucleus does not move away from the cell cortex and the female pronucleus only migrates partially toward the posterior part of the embryos (Cockell et al. 2004). Moreover, the centrosomes do not separate and, consequently, a mitotic spindle is not formed. The same phenotypes are produced in embryos depleted for dynein components and regulators, such as DHC-1, DNC-1, and DNC-2, which suggests that these proteins act together to ensure nuclear movement along microtubules (Gonczy et al. 1999a). As mentioned above, dynein interacts with ZYG-12, presumably via the dynein subunit DLI-1, and knockdown or mutation of ZYG-12 or SUN-1 causes centrosomes to detach from pronuclei (Malone et al. 2003). In contrast to the situation when LIS-1 or dynein is absent, bipolar spindles are still formed in ZYG-12-depleted embryos, indicating that the SUN-1/ZYG-12 complex acts specifically to anchor nuclei to centrosomes (Malone et al. 2003). Moreover, reduction of the surface area of the NE or prevention of NE assembly causes centrosomes to separate prematurely, further demonstrating the importance of the NE in centrosome positioning (Askjaer et al. 2002; Meyerzon et al. 2009b). SUN-1/ZYG-12 activity is also required for nuclear tethering to centrosomes in embryos beyond the one-cell stage (Penkner et al. 2007) and for connection of syncytial germ line nuclei to the microtubule cytoskeleton (Zhou et al. 2009). Recently, delay in nuclear separation and centrosome detachment was also observed in lem-2-mutant two to four cell-stage embryos (Figure 16A) (Morales-Martinez et al. 2015), but it remains to be analyzed if this phenotype reflects an interaction of LEM-2 with the LINC complex or an alternative anchoring mechanism.
Figure 16.
The NE is critical for nuclear positioning. (A) During early embryonic cell divisions, centrosomes (arrows) are not properly anchored to the NE in lem-2 mutants. From Morales-Martinez et al. (2015). (B and C) ∼5 hr after fertilization, (B) cells of the intestinal E lineage are polarized and nuclei (asterisks) align at the midline in the interior of the embryo whereas (C) hypodermal cells in dorsal part of the embryos intercalate and nuclei migrate transversally (note relative position of nuclei labeled 3–5; nuclei 1–2 have not yet migrated). (D) As a consequence of abnormal nuclear migration in hypodermal cells during embryogenesis, nuclei are present in the dorsal side of unc-84 larvae (arrows). Bars, 10 µm.
Two features made C. elegans very suitable for the early identification and characterization of genes required for nuclear positioning in differentiating embryos and larvae. First, nuclei can easily be observed throughout C. elegans development by live DIC microscopy. Second, despite having clearly visible phenotypes, many mutants with mispositioned nuclei develop to adulthood and are fertile. The genes encoding UNC-84, a founding member of SUN domain proteins, and KASH domain protein UNC-83 were cloned based on nuclear migration phenotypes in several cell types (Malone et al. 1999; Starr et al. 2001). Normally, embryonic hypodermal hyp7 nuclei migrate transversally from left to right or vice versa following dorsal cell intercalation. However, in multiple alleles of unc-83 and unc-84, several nuclei only migrate partially, resulting in abnormal appearance of nuclei in the dorsal side of larvae (Figure 16, C and D). Moreover, ventral migration of P cells and P-cell nuclei often fail in unc-83 and unc-84 mutants, which leads to P cell death, and, consequently, uncoordinated (Unc) and egg-laying (Egl) phenotypes (Sulston and Horvitz 1981). Finally, mutations of unc-83 or unc-84 abolish alignment of E-cell nuclei during proliferation and specification of the intestine (Figure 16B) (Starr et al. 2001). The unc-83 and unc-84 genes both encode different protein isoforms (3 and 2, respectively), and, whereas UNC-84 is ubiquitously expressed, the expression pattern of UNC-83 is more restricted, potentially limited to cells with migrating nuclei (Malone et al. 1999; Starr et al. 2001). The SUN domain of UNC-84 is required for NE accumulation of UNC-83, whereas UNC-84 localization depends on LMN-1 in the nuclear lamina, leading to the model of an NE-spanning anchor consisting of LMN-1, UNC-84, and UNC-83 (Starr et al. 2001; Lee et al. 2002). Moreover, the KASH domain of UNC-83 is necessary for targeting to the NE and nuclear migration of hyp7 cells (McGee et al. 2006). Interestingly, nuclei in the adult syncytial seam cells are irregularly spaced in many unc-84 mutants but not in unc-83 mutants, suggesting that nuclear migration and anchoring may involve separate LINC complexes (Figure 15) (Malone et al. 1999). The nuclear anchoring phenotype of unc-84 is shared with anc-1 (Hedgecock and Thomson 1982), which encodes a giant transmembrane protein (∼950 kDa) with an N-terminal actin-binding domain and a C-terminal KASH domain (Figure 15) (Starr and Han 2002). Accumulation of ANC-1 at the NE depends on UNC-84, presumably reflecting a direct interaction between their respective KASH and SUN domains in the NE lumen (Starr and Han 2002). In the cytoplasm, ANC-1 interacts with AMPH-1, an ortholog of human amphiphysin 2, mutations in which are linked to centronuclear myopathies and myotonic dystrophies (D’Alessandro et al. 2015). Interestingly, AMPH-1 interacts with actin as well as the microtubule-binding protein CLIP-170, thus linking ANC-1 to both cytoskeletal structures. Moreover, seam cell nuclei are not correctly anchored in amph-1 mutants, and altered nuclear shape is observed in ∼10–30% of animals mutant for anc-1, amph-1, or clip-1 (D’Alessandro et al. 2015).
Centrosome attachment to nuclei is normal in unc-83 or unc-84 mutants (Starr et al. 2001; Lee et al. 2002). Instead, the mechanism underlying the nuclear migration phenotypes is linked to NE-associated molecular motors. UNC-83 interacts with the kinesin 1 light chain KLC-2 and the dynein regulators BICD-1, NUD-2, and DLC-1 (Meyerzon et al. 2009a; Fridolfsson et al. 2010). Inhibition of kinesin 1 (klc-2, unc-116) or dynein (dhc-1, dlc-1, bicd-1, lis-1, nud-2) activity causes defects in hyp7 nuclear migration, whereas artificially tethering KLC-2 to the NE can rescue unc-83 nuclear migration defects, arguing that a major role of UNC-83 is to serve as a nuclear anchor for kinesin and dynein motors (Meyerzon et al. 2009a; Fridolfsson et al. 2010). Based on detailed time-lapse microscopy including analysis of microtubule dynamics, it has been proposed that the involvement of two different molecular motors increases the efficiency of nuclear migration by accommodating for bidirectional movements to bypass “roadblocks” (Fridolfsson and Starr 2010). Most studies on nuclear migration have focused on the UNC-84/UNC-83 and UNC-84/ANC-1 LINC complexes, but it is interesting to note that a screen for enhancers of unc-84 phenotypes uncovered a potential parallel pathway involving the conserved protein TOCA-1 (Chang et al. 2013). TOCA-1 presumably interacts with actin but it remains unclear how it regulates nuclear migration. Further analysis of this and other enhancers retrieved in the screen is likely to provide novel insight to nuclear migration.
Mechanotransduction
Tension-mediated signaling from the cytoplasm is thought to have a significant impact on gene expression, both in healthy tissues and upon alteration of NE composition (Dobrzynska et al. 2016). In addition to amphiphysin 2/AMPH-1 and nesprin/ANC-1 described in the previous section, mutations in LMN-1 and EMR-1 cause muscular dystrophy. The physical strain that muscle cells are exposed to makes them particularly vulnerable to loss of NE integrity and perturbed mechanosignaling. In recent years, C. elegans has been implemented as an attractive model to study the interaction of LINC complexes with the nuclear lamina and their role in mechanotransduction.
Although UNC-84 is the only SUN-domain protein expressed in somatic cells, hypodermal and pharyngeal nuclei of unc-84 mutant embryos and L1 larvae have normal morphology and spacing between the INM and ONM (Cain et al. 2014). However, in muscles of unc-84 larvae, the distance between the two nuclear membranes is increased, presumably because tension acting on the NE is higher in these cells. Moreover, unc-84 mutants placed in liquid medium have reduced locomotion (Cain et al. 2014), although tissue-specific rescue experiments are needed to determine if the two observations are functionally linked. The normal morphology of hypodermal nuclei in unc-84 L1 larvae is in contrast with the situation in seam cells of adult anc-1 mutants described above (D’Alessandro et al. 2015). Because UNC-84 is needed to recruit ANC-1 to the ONM (and ANC-1 is therefore likely to be mislocalized in unc-84 L1 larvae), this suggests that nuclear shape is either independent of ANC-1 localization to the NE or depends on the UNC-84/ANC-1 complex only at certain stages of development. The fact that muscular dystrophies caused by mutations in human lamin, emerin, or nesprin-encoding genes appear several years after birth suggests that it might be relevant to revisit nuclear morphology in older unc-84 mutant animals.
Mutations that affect the nucleoplasmic part of UNC-84 cause milder hypodermal nuclear migration defects than complete null alleles (Malone et al. 1999; Bone et al. 2014). Nevertheless, a single amino acid substitution in UNC-84 (P91S; e1411), which reduces its interaction with LMN-1 in the yeast two-hybrid assay, or depletion of LMN-1 by RNAi, induces a significant migration defect, highlighting the relevance of the nuclear lamina in LINC activity (Bone et al. 2014). In parallel to LMN-1, the conserved transmembrane NE protein SAMP-1 also contributes to nuclear migration, but by unknown mechanisms (Bone et al. 2014). Interestingly, a recent study measured mechanical responses to external physical tension and found a significant increase in hypodermal nuclear stiffness in animals depleted for UNC-84 (Zuela et al. 2016). Ectopic expression of LMN-1 carrying a L535P substitution, which mimics an Emery–Dreifuss muscular dystrophy mutation in the human LMNA gene, extends the effects of UNC-84 depletion on nuclear stiffness to include muscles (Zuela et al. 2016).
Neurons are subjected to physical stress during muscle contraction and absence of dystroglycan DGN-1 causes frequent displacement of cell bodies of several neurons in the lumbar ganglion, in particular the PVQ neuron (Johnson and Kramer 2012). A mutagenesis screen for other genes implicated in maintenance of cell body position retrieved 10 alleles, of which 6 corresponded to anc-1. Epistasis experiments suggested that ANC-1 might act on the extracellular matrix, although analysis of unc-83 and unc-84 mutants also revealed PVQ displacements, arguing that nuclear anchoring might have a prominent role in neural maintenance (Johnson and Kramer 2012). Another interesting role of ANC-1 in the nervous system was discovered in a screen for binding partners of regulator of presynaptic morphology 1 (RPM-1) (Tulgren et al. 2014). Mutation of anc-1 or unc-84 enhances the synapse formation and axon termination defects of rpm-1(+/−) and fsn-1 mutant animals, probably by altered β-catenin/BAR-1 signaling (Tulgren et al. 2014). Interestingly, human emerin regulates β-catenin by sequestering it at the NE (Dobrzynska et al. 2016), whereas loss of EMR-1 suppresses the axon termination phenotypes of anc-1 and anc-1; fsn-1 mutants (Tulgren et al. 2014) and affects neuromuscular-junction activity (Gonzalez-Aguilera et al. 2014). Further analysis will hopefully help to elucidate the mechanisms underlying these genetic interactions.
Whereas ANC-1, UNC-83, and ZYG-12 are being intensively studied, fewer details have emerged on KDP-1, a fourth C. elegans KASH domain protein. KDP-1 was identified as a ubiquitously expressed NE protein in a yeast two-hybrid screen using UNC-84 as bait (McGee et al. 2009). In the germ line and young embryos, KDP-1 localization is SUN-1 dependent, and depletion of KDP-1 inhibits both meiotic and mitotic cell cycle progression as well as pronuclear migration, but the molecular components remain to be discovered (McGee et al. 2009; Xiong et al. 2011).
Future Perspectives: Opportunities for Discovery
You can observe a lot by watching (L. P. “Yogi” Berra, American baseball player)
For nearly 200 years, researchers have been watching the nucleus by various means, from light microscopy, through electron and super-resolution microscopy, to molecular methods such as Hi-C and DamID. Indeed, a lot has been learned about the inner workings of the nucleus, and in particular the structures that make up the nucleus and reside within it. These studies have led to the notion that the nucleus is less like a well-oiled machine and more like a village, with many processes occurring in parallel, components coming and going as needed, and the occasional dismantling of the entire enterprise, only to start all over. However, to gain mechanistic insight that is critical for understanding the basis of disease one needs to go beyond just watching. In more recent years, biochemical and genetic studies have led the way in deciphering molecular mechanisms of processes that occur within the nucleus, with model organisms leading the way. The combination of sophisticated genetic tools and exquisite cytology has made C. elegans especially suitable for exploring nuclear processes. And while there is no tissue culture system in C. elegans that could have allowed biochemical analyses on a uniform population of cells, the ability to examine and genetically manipulate molecular processes in a developmental context is unmatched.
And yet, much remains to be learned. The answers to fundamental questions such as how nuclear size is determined; what controls NE expansion; what goes on inside P granules; and what the relationship is between nuclear structure and function, such as during aging; are just beginning to emerge. Moreover, even in processes for which we have a general understanding, the fine details of questions such as what drives and controls the assembly of nuclear bodies, how the selectivity of NPC actually works, and what TADs really do, remain to be addressed. Newly developed methodologies, such as optogenetics and CRISPR/Cas9-tailored mutations can be used in C. elegans to address these and other questions. The molecular function of many NE proteins is still unknown, and a significant number of NE proteins for which a function has been attributed may have additional roles in nuclear processes. Look no further than components of the NPC, which are also found on chromosomes and are needed for NE breakdown and P granule function in ways that are poorly understood. To date, studies of the C. elegans nucleus have mostly concentrated on the early embryo, but as is elegantly demonstrated in nuclear migration and mechanotransduction studies, there is a treasure trove of discoveries in nuclei of differentiated cells that awaits a new generation of C. elegans geneticists/cell biologists.
Acknowledgments
We thank Daniel A. Starr for discussions on LINC function and Thomas Schwartz for identifying potential C. elegans orthologs of NUP88 and GLE1. We also wish to thank Victor Carranco for help with figures. We apologize to our colleagues whose excellent work has not been discussed here owing to space limitation. Work in the O.C.-F. laboratory is funded by an intramural grant from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD. Work in the P.A. laboratory is funded by the Spanish Ministry of Economy and Competitiveness (BFU2013-42709P) and the European Regional Development Fund.
Glossary
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- INM
inner nuclear membrane
- LAD
lamina associated domain
- LINC
LInker of Nucleoskeleton and Cytoskeleton
- NE
nuclear envelope
- NES
nuclear export signal
- NLS
nuclear localization signal
- NPC
nuclear pore complex
- ONM
outer nuclear membrane
- PC
pairing center
- SAC
spindle assembly checkpoint
- TAD
topologically associated domain
Footnotes
Communicating editor: B. Grant
Literature Cited
- Adam S. A., 2009. The nuclear transport machinery in Caenorhabditis elegans: a central role in morphogenesis. Semin. Cell Dev. Biol. 20: 576–581. [DOI] [PubMed] [Google Scholar]
- Anderson D. J., Hetzer M. W., 2007. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat. Cell Biol. 9: 1160–1166. [DOI] [PubMed] [Google Scholar]
- Asencio C., Davidson I. F., Santarella-Mellwig R., Ly-Hartig T. B., Mall M., et al. , 2012. Coordination of kinase and phosphatase activities by Lem4 enables nuclear envelope reassembly during mitosis. Cell 150: 122–135. [DOI] [PubMed] [Google Scholar]
- Askjaer P., Ercan S., Meister P., 2014a Modern techniques for the analysis of chromatin and nuclear organization in C. elegans (April 2, 2014), WormBook, ed. The C. elegans Research Community WormBook, 10.1895/wormbook.1.169.1, http://wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askjaer P., Galy V., Meister P., 2014b Modern tools to study nuclear pore complexes and nucleocytoplasmic transport in Caenorhabditis elegans. Methods Cell Biol. 122: 277–310. [DOI] [PubMed] [Google Scholar]
- Askjaer P., Galy V., Hannak E., Mattaj I. W., 2002. Ran GTPase cycle and importins alpha and beta are essential for spindle formation and nuclear envelope assembly in living Caenorhabditis elegans embryos. Mol. Biol. Cell 13: 4355–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A., Desai A., Oegema K., 2007. A role for Rab5 in structuring the endoplasmic reticulum. J. Cell Biol. 178: 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumiller W. M., Jr, Davis B. W., Keating C. D., 2014. Phase separation as a possible means of nuclear compartmentalization. Int. Rev. Cell Mol. Biol. 307: 109–149. [DOI] [PubMed] [Google Scholar]
- Bahmanyar S., Biggs R., Schuh A. L., Desai A., Muller-Reichert T., et al. , 2014. Spatial control of phospholipid flux restricts endoplasmic reticulum sheet formation to allow nuclear envelope breakdown. Genes Dev. 28: 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamba C., Bobinnec Y., Fukuda M., Nishida E., 2002. The GTPase Ran regulates chromosome positioning and nuclear envelope assembly in vivo. Curr. Biol. 12: 503–507. [DOI] [PubMed] [Google Scholar]
- Bank E. M., Ben-Harush K., Wiesel-Motiuk N., Barkan R., Feinstein N., et al. , 2011. A laminopathic mutation disrupting lamin filament assembly causes disease-like phenotypes in Caenorhabditis elegans. Mol. Biol. Cell 22: 2716–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank E. M., Ben-Harush K., Feinstein N., Medalia O., Gruenbaum Y., 2012. Structural and physiological phenotypes of disease-linked lamin mutations in C. elegans. J. Struct. Biol. 177: 106–112. [DOI] [PubMed] [Google Scholar]
- Bano D., Dinsdale D., Cabrera-Socorro A., Maida S., Lambacher N., et al. , 2010. Alteration of the nuclear pore complex in Ca(2+)-mediated cell death. Cell Death Differ. 17: 119–133. [DOI] [PubMed] [Google Scholar]
- Bar D. Z., Gruenbaum Y., 2010. Reversal of age-dependent nuclear morphology by inhibition of prenylation does not affect lifespan in Caenorhabditis elegans. Nucleus 1: 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar D. Z., Neufeld E., Feinstein N., Gruenbaum Y., 2009. Gliotoxin reverses age-dependent nuclear morphology phenotypes, ameliorates motility, but fails to affect lifespan of adult Caenorhabditis elegans. Cell Motil. Cytoskeleton 66: 791–797. [DOI] [PubMed] [Google Scholar]
- Barkan R., Zahand A. J., Sharabi K., Lamm A. T., Feinstein N., et al. , 2012. Ce-emerin and LEM-2: essential roles in Caenorhabditis elegans development, muscle function, and mitosis. Mol. Biol. Cell 23: 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton L. J., Soshnev A. A., Geyer P. K., 2015. Networking in the nucleus: a spotlight on LEM-domain proteins. Curr. Opin. Cell Biol. 34: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudrimont A., Penkner A., Woglar A., Machacek T., Wegrostek C., et al. , 2010. Leptotene/zygotene chromosome movement via the SUN/KASH protein bridge in Caenorhabditis elegans. PLoS Genet. 6: e1001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudouin J., Gerlich D., Daigle N., Eils R., Ellenberg J., 2002. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell 108: 83–96. [DOI] [PubMed] [Google Scholar]
- Ben-Harush K., Wiesel N., Frenkiel-Krispin D., Moeller D., Soreq E., et al. , 2009. The supramolecular organization of the C. elegans nuclear lamin filament. J. Mol. Biol. 386: 1392–1402. [DOI] [PubMed] [Google Scholar]
- Bohr T., Nelson C. R., Klee E., Bhalla N., 2015. Spindle assembly checkpoint proteins regulate and monitor meiotic synapsis in C. elegans. J. Cell Biol. 211: 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone C. R., Tapley E. C., Gorjanacz M., Starr D. A., 2014. The Caenorhabditis elegans SUN protein UNC-84 interacts with lamin to transfer forces from the cytoplasm to the nucleoskeleton during nuclear migration. Mol. Biol. Cell 25: 2853–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne C. P., 2013. Phase transitions and size scaling of membrane-less organelles. J. Cell Biol. 203: 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne C. P., Eckmann C. R., Courson D. S., Rybarska A., Hoege C., et al. , 2009. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324: 1729–1732. [DOI] [PubMed] [Google Scholar]
- Brangwynne C. P., Mitchison T. J., Hyman A. A., 2011. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. USA 108: 4334–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. E., Amoils S., Horn J. M., Buckle V. J., Higgs D. R., et al. , 2001. Expression of alpha- and beta-globin genes occurs within different nuclear domains in haemopoietic cells. Nat. Cell Biol. 3: 602–606. [DOI] [PubMed] [Google Scholar]
- Burns L. T., Wente S. R., 2014. From hypothesis to mechanism: uncovering nuclear pore complex links to gene expression. Mol. Cell. Biol. 34: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussing I., Yang J. S., Lai E. C., Grosshans H., 2010. The nuclear export receptor XPO-1 supports primary miRNA processing in C. elegans and Drosophila. EMBO J. 29: 1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain N. E., Tapley E. C., McDonald K. L., Cain B. M., Starr D. A., 2014. The SUN protein UNC-84 is required only in force-bearing cells to maintain nuclear envelope architecture. J. Cell Biol. 206: 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelson M., Liang Y., Schulte R., Mair W., Wagner U., et al. , 2010. Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell 140: 372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cautain B., Hill R., de Pedro N., Link W., 2015. Components and regulation of nuclear transport processes. FEBS J. 282: 445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C. elegans Sequencing Consortium , 1998. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282: 2012–2018. [DOI] [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C., 1994. Green fluorescent protein as a marker for gene expression. Science 263: 802–805. [DOI] [PubMed] [Google Scholar]
- Chang Y. T., Dranow D., Kuhn J., Meyerzon M., Ngo M., et al. , 2013. toca-1 is in a novel pathway that functions in parallel with a SUN-KASH nuclear envelope bridge to move nuclei in Caenorhabditis elegans. Genetics 193: 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockell M. M., Baumer K., Gonczy P., 2004. lis-1 is required for dynein-dependent cell division processes in C. elegans embryos. J. Cell Sci. 117: 4571–4582. [DOI] [PubMed] [Google Scholar]
- Cowan C. R., Hyman A. A., 2006. Cyclin E-Cdk2 temporally regulates centrosome assembly and establishment of polarity in Caenorhabditis elegans embryos. Nat. Cell Biol. 8: 1441–1447. [DOI] [PubMed] [Google Scholar]
- Crane E., Bian Q., McCord R. P., Lajoie B. R., Wheeler B. S., et al. , 2015. Condensin-driven remodelling of X chromosome topology during dosage compensation. Nature 523: 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer T., Cremer M., 2010. Chromosome territories. Cold Spring Harb. Perspect. Biol. 2: a003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessandro M., Hnia K., Gache V., Koch C., Gavriilidis C., et al. , 2015. Amphiphysin 2 orchestrates nucleus positioning and shape by linking the nuclear envelope to the actin and microtubule cytoskeleton. Dev. Cell 35: 186–198. [DOI] [PubMed] [Google Scholar]
- D’Angelo M. A., Raices M., Panowski S. H., Hetzer M. W., 2009. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell 136: 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daryabeigi A., Woglar A., Baudrimont A., Silva N., Paouneskou D., et al. , 2016. Nuclear envelope retention of LINC complexes is promoted by SUN-1 oligomerization in the Caenorhabditis elegans germ line. Genetics 203: 733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Hiatt J. B., Nguyen D. K., Ercan S., Sturgill D., et al. , 2011. Evidence for compensatory upregulation of expressed X-linked genes in mammals, Caenorhabditis elegans and Drosophila melanogaster. Nat. Genet. 43: 1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer T. A., Misteli T., 2011. The lamin protein family. Genome Biol. 12: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J. R., Gorkin D. U., Ren B., 2016. Chromatin domains: the unit of chromosome organization. Mol. Cell 62: 668–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzynska A., Gonzalo S., Shanahan C., Askjaer P., 2016. The nuclear lamina in health and disease. Nucleus 7: 233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dultz E., Zanin E., Wurzenberger C., Braun M., Rabut G., et al. , 2008. Systematic kinetic analysis of mitotic dis- and reassembly of the nuclear pore in living cells. J. Cell Biol. 180: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundr M., 2012. Nuclear bodies: multifunctional companions of the genome. Curr. Opin. Cell Biol. 24: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M., Brown W. T., Gordon L. B., Glynn M. W., Singer J., et al. , 2003. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423: 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezak M. J., Ferkey D. M., 2011. A functional nuclear localization sequence in the C. elegans TRPV channel OCR-2. PLoS One 6: e25047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feric M., Vaidya N., Harmon T. S., Mitrea D. M., Zhu L., et al. , 2016. Coexisting liquid phases underlie nucleolar subcompartments. Cell 165: 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A. G., Piano F., 2006. MEL-28 is downstream of the Ran cycle and is required for nuclear-envelope function and chromatin maintenance. Curr. Biol. 16: 1757–1763. [DOI] [PubMed] [Google Scholar]
- Ferreira H. C., Towbin B. D., Jegou T., Gasser S. M., 2013. The shelterin protein POT-1 anchors Caenorhabditis elegans telomeres through SUN-1 at the nuclear periphery. J. Cell Biol. 203: 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe V. E., Alberts B. M., 1985. Reversible chromosome condensation induced in Drosophila embryos by anoxia: visualization of interphase nuclear organization. J. Cell Biol. 100: 1623–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folick A., Oakley H. D., Yu Y., Armstrong E. H., Kumari M., et al. , 2015. Aging. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science 347: 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong L. G., Frost D., Meta M., Qiao X., Yang S. H., et al. , 2006. A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science 311: 1621–1623. [DOI] [PubMed] [Google Scholar]
- Frank D. J., Roth M. B., 1998. ncl-1 is required for the regulation of cell size and ribosomal RNA synthesis in Caenorhabditis elegans. J. Cell Biol. 140: 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks T. M., Benner C., Narvaiza I., Marchetto M. C., Young J. M., et al. , 2016. Evolution of a transcriptional regulator from a transmembrane nucleoporin. Genes Dev. 30: 1155–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz C., Askjaer P., Antonin W., Iglesias C. L., Haselmann U., et al. , 2005. Nup155 regulates nuclear envelope and nuclear pore complex formation in nematodes and vertebrates. EMBO J. 24: 3519–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz C., Walczak R., Yavuz S., Santarella R., Gentzel M., et al. , 2007. MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. EMBO Rep. 8: 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J., Williamson I., Bickmore W. A., Dostie J., 2015. An overview of genome organization and how we got there: from FISH to Hi-C. Microbiol. Mol. Biol. Rev. 79: 347–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridolfsson H. N., Starr D. A., 2010. Kinesin-1 and dynein at the nuclear envelope mediate the bidirectional migrations of nuclei. J. Cell Biol. 191: 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridolfsson H. N., Ly N., Meyerzon M., Starr D. A., 2010. UNC-83 coordinates kinesin-1 and dynein activities at the nuclear envelope during nuclear migration. Dev. Biol. 338: 237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman L. E., Goel A. K., Smith J., Shianna K. V., Aballay A., 2009. Nucleolar proteins suppress Caenorhabditis elegans innate immunity by inhibiting p53/CEP-1. PLoS Genet. 5: e1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushige T., Krause M., 2005. The myogenic potency of HLH-1 reveals wide-spread developmental plasticity in early C. elegans embryos. Development 132: 1795–1805. [DOI] [PubMed] [Google Scholar]
- Gabdank I., Ramakrishnan S., Villeneuve A. M., Fire A. Z., 2016. A streamlined tethered chromosome conformation capture protocol. BMC Genomics 17: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy V., Mattaj I. W., Askjaer P., 2003. Caenorhabditis elegans nucleoporins Nup93 and Nup205 determine the limit of nuclear pore complex size exclusion in vivo. Mol. Biol. Cell 14: 5104–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy V., Askjaer P., Franz C., Lopez-Iglesias C., Mattaj I. W., 2006. MEL-28, a novel nuclear-envelope and kinetochore protein essential for zygotic nuclear-envelope assembly in C. elegans. Curr. Biol. 16: 1748–1756. [DOI] [PubMed] [Google Scholar]
- Galy V., Antonin W., Jaedicke A., Sachse M., Santarella R., et al. , 2008. A role for gp210 in mitotic nuclear-envelope breakdown. J. Cell Sci. 121: 317–328. [DOI] [PubMed] [Google Scholar]
- Gassmann R., Rechtsteiner A., Yuen K. W., Muroyama A., Egelhofer T., et al. , 2012. An inverse relationship to germline transcription defines centromeric chromatin in C. elegans. Nature 484: 534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geles K. G., Adam S. A., 2001. Germline and developmental roles of the nuclear transport factor importin alpha3 in C. elegans. Development 128: 1817–1830. [DOI] [PubMed] [Google Scholar]
- Gerstein M. B., Lu Z. J., Van Nostrand E. L., Cheng C., Arshinoff B. I., et al. , 2010. Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science 330: 1775–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Liu J., Cohen-Fix O., 2009. Inactivation of the C. elegans lipin homolog leads to ER disorganization and to defects in the breakdown and reassembly of the nuclear envelope. J. Cell Sci. 122: 1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Saldivar G., Fernandez A., Hirano Y., Mauro M., Lai A., et al. , 2016. Identification of conserved MEL-28/ELYS domains with essential roles in nuclear assembly and chromosome segregation. PLoS Genet. 12: e1006131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P., Pichler S., Kirkham M., Hyman A. A., 1999a Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J. Cell Biol. 147: 135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P., Schnabel H., Kaletta T., Amores A. D., Hyman T., et al. , 1999b Dissection of cell division processes in the one cell stage Caenorhabditis elegans embryo by mutational analysis. J. Cell Biol. 144: 927–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Aguilera C., Ikegami K., Ayuso C., de Luis A., Iniguez M., et al. , 2014. Genome-wide analysis links emerin to neuromuscular junction activity in Caenorhabditis elegans. Genome Biol. 15: R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sandoval A., Towbin B. D., Kalck V., Cabianca D. S., Gaidatzis D., et al. , 2015. Perinuclear anchoring of H3K9-methylated chromatin stabilizes induced cell fate in C. elegans embryos. Cell 163: 1333–1347. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Serricchio A. S., Sternberg P. W., 2006. Visualization of C. elegans transgenic arrays by GFP. BMC Genet. 7: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorjanacz M., Mattaj I. W., 2009. Lipin is required for efficient breakdown of the nuclear envelope in Caenorhabditis elegans. J. Cell Sci. 122: 1963–1969. [DOI] [PubMed] [Google Scholar]
- Gorjanacz M., Klerkx E. P., Galy V., Santarella R., Lopez-Iglesias C., et al. , 2007. Caenorhabditis elegans BAF-1 and its kinase VRK-1 participate directly in post-mitotic nuclear envelope assembly. EMBO J. 26: 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer E. L., Blanco M. A., Gu L., Sendinc E., Liu J., et al. , 2015. DNA Methylation on N6-adenine in C. elegans. Cell 161: 868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosstessner-Hain K., Hegemann B., Novatchkova M., Rameseder J., Joughin B. A., et al. , 2011. Quantitative phospho-proteomics to investigate the polo-like kinase 1-dependent phospho-proteome. Mol Cell Proteomics 10: M111.008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y., Foisner R., 2015. Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu. Rev. Biochem. 84: 131–164. [DOI] [PubMed] [Google Scholar]
- Guelen L., Pagie L., Brasset E., Meuleman W., Faza M. B., et al. , 2008. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 453: 948–951. [DOI] [PubMed] [Google Scholar]
- Guttinger S., Laurell E., Kutay U., 2009. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat. Rev. Mol. Cell Biol. 10: 178–191. [DOI] [PubMed] [Google Scholar]
- Hachet V., Busso C., Toya M., Sugimoto A., Askjaer P., et al. , 2012. The nucleoporin Nup205/NPP-3 is lost near centrosomes at mitotic onset and can modulate the timing of this process in Caenorhabditis elegans embryos. Mol. Biol. Cell 23: 3111–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haithcock E., Dayani Y., Neufeld E., Zahand A. J., Feinstein N., et al. , 2005. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 102: 16690–16695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajeri V. A., Trejo J., Padilla P. A., 2005. Characterization of sub-nuclear changes in Caenorhabditis elegans embryos exposed to brief, intermediate and long-term anoxia to analyze anoxia-induced cell cycle arrest. BMC Cell Biol. 6: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajeri V. A., Little B. A., Ladage M. L., Padilla P. A., 2010. NPP-16/Nup50 function and CDK-1 inactivation are associated with anoxia-induced prophase arrest in Caenorhabditis elegans. Mol. Biol. Cell 21: 712–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton B., Dong Y., Shindo M., Liu W., Odell I., et al. , 2005. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 19: 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa M., Yonetani M., Sugimoto A., 2011. PGL proteins self associate and bind RNPs to mediate germ granule assembly in C. elegans. J. Cell Biol. 192: 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y., Iwabuchi M., Ohsumi K., Kimura A., 2013. Intranuclear DNA density affects chromosome condensation in metazoans. Mol. Biol. Cell 24: 2442–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi T., Koujin T., Segura-Totten M., Lee K. K., Matsuoka Y., et al. , 2001. BAF is required for emerin assembly into the reforming nuclear envelope. J. Cell Sci. 114: 4575–4585. [DOI] [PubMed] [Google Scholar]
- Harel A., Orjalo A. V., Vincent T., Lachish-Zalait A., Vasu S., et al. , 2003. Removal of a single pore subcomplex results in vertebrate nuclei devoid of nuclear pores. Mol. Cell 11: 853–864. [DOI] [PubMed] [Google Scholar]
- Hattersley N., Cheerambathur D., Moyle M., Stefanutti M., Richardson A., et al. , 2016. A nucleoporin docks protein phosphatase 1 to direct meiotic chromosome segregation and nuclear assembly. Dev. Cell 38: 463–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Kimura K., Kimura A., 2012. Localized accumulation of tubulin during semi-open mitosis in the Caenorhabditis elegans embryo. Mol. Biol. Cell 23: 1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., McKeon F., 1990. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell 61: 579–589. [DOI] [PubMed] [Google Scholar]
- Hedgecock E. M., Herman R. K., 1995. The ncl-1 gene and genetic mosaics of Caenorhabditis elegans. Genetics 141: 989–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock E. M., Thomson J. N., 1982. A gene required for nuclear and mitochondrial attachment in the nematode Caenorhabditis elegans. Cell 30: 321–330. [DOI] [PubMed] [Google Scholar]
- Heger P., Marin B., Schierenberg E., 2009. Loss of the insulator protein CTCF during nematode evolution. BMC Mol. Biol. 10: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S. T., Johnson T. E., 2001. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 11: 1975–1980. [DOI] [PubMed] [Google Scholar]
- Hernandez-Verdun D., 2011. Assembly and disassembly of the nucleolus during the cell cycle. Nucleus 2: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J. W., Jung Y. L., Liu T., Alver B. H., Lee S., et al. , 2014. Comparative analysis of metazoan chromatin organization. Nature 512: 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. H., 2015. ESCRTs are everywhere. EMBO J. 34: 2398–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra A., Hetzer M. W., 2015. Nuclear pore proteins and the control of genome functions. Genes Dev. 29: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K., Lieb J. D., 2013. Integral nuclear pore proteins bind to Pol III-transcribed genes and are required for Pol III transcript processing in C. elegans. Mol. Cell 51: 840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K., Egelhofer T. A., Strome S., Lieb J. D., 2010. Caenorhabditis elegans chromosome arms are anchored to the nuclear membrane via discontinuous association with LEM-2. Genome Biol. 11: R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamin A., Wiebe M. S., 2015. Barrier to Autointegration Factor (BANF1): interwoven roles in nuclear structure, genome integrity, innate immunity, stress responses and progeria. Curr. Opin. Cell Biol. 34: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtic P., Edens L. J., Li X., Nguyen T., Chen P., et al. , 2015. Concentration-dependent effects of nuclear lamins on nuclear size in Xenopus and Mammalian cells. J. Biol. Chem. 290: 27557–27571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Wang S., Huang Y., He X., Cui H., et al. , 2015. Phase transition of spindle-associated protein regulate spindle apparatus assembly. Cell 163: 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Li J., Green C. D., Yu X., Tang X., et al. , 2011. Histone demethylase UTX-1 regulates C. elegans life span by targeting the insulin/IGF-1 signaling pathway. Cell Metab. 14: 161–172. [DOI] [PubMed] [Google Scholar]
- Johnson R. P., Kramer J. M., 2012. Neural maintenance roles for the matrix receptor dystroglycan and the nuclear anchorage complex in Caenorhabditis elegans. Genetics 190: 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalab P., Weis K., Heald R., 2002. Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science 295: 2452–2456. [DOI] [PubMed] [Google Scholar]
- Kalverda B., Pickersgill H., Shloma V. V., Fornerod M., 2010. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell 140: 360–371. [DOI] [PubMed] [Google Scholar]
- Karabinos A., Schunemann J., Meyer M., Aebi U., Weber K., 2003. The single nuclear lamin of Caenorhabditis elegans forms in vitro stable intermediate filaments and paracrystals with a reduced axial periodicity. J. Mol. Biol. 325: 241–247. [DOI] [PubMed] [Google Scholar]
- Kim J. K., Gabel H. W., Kamath R. S., Tewari M., Pasquinelli A., et al. , 2005. Functional genomic analysis of RNA interference in C. elegans. Science 308: 1164–1167. [DOI] [PubMed] [Google Scholar]
- Korcekova D., Gombitova A., Raska I., Cmarko D., Lanctot C., 2012. Nucleologenesis in the Caenorhabditis elegans embryo. PLoS One 7: e40290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuersten S., Segal S. P., Verheyden J., LaMartina S. M., Goodwin E. B., 2004. NXF-2, REF-1, and REF-2 affect the choice of nuclear export pathway for tra-2 mRNA in C. elegans. Mol. Cell 14: 599–610. [DOI] [PubMed] [Google Scholar]
- Labrador L., Barroso C., Lightfoot J., Muller-Reichert T., Flibotte S., et al. , 2013. Chromosome movements promoted by the mitochondrial protein SPD-3 are required for homology search during Caenorhabditis elegans meiosis. PLoS Genet. 9: e1003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur A. M., Dorn J. F., Maddox P. S., 2015. Mitotic chromosome length scales in response to both cell and nuclear size. J. Cell Biol. 209: 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A. C., Csankovszki G., 2015. Balancing up and downregulation of the C. elegans X chromosomes. Curr. Opin. Genet. Dev. 31: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A. C., Nabeshima K., Csankovszki G., 2014. The C. elegans dosage compensation complex mediates interphase X chromosome compaction. Epigenetics Chromatin 7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudermilch E., Schlieker C., 2016. Torsin ATPases: structural insights and functional perspectives. Curr. Opin. Cell Biol. 40: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell E., Beck K., Krupina K., Theerthagiri G., Bodenmiller B., et al. , 2011. Phosphorylation of Nup98 by multiple kinases is crucial for NPC disassembly during mitotic entry. Cell 144: 539–550. [DOI] [PubMed] [Google Scholar]
- Lawrence K. S., Chau T., Engebrecht J., 2015. DNA damage response and spindle assembly checkpoint function throughout the cell cycle to ensure genomic integrity. PLoS Genet. 11: e1005150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. K., Gruenbaum Y., Spann P., Liu J., Wilson K. L., 2000. C. elegans nuclear envelope proteins emerin, MAN1, lamin, and nucleoporins reveal unique timing of nuclear envelope breakdown during mitosis. Mol. Biol. Cell 11: 3089–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. K., Starr D., Cohen M., Liu J., Han M., et al. , 2002. Lamin-dependent localization of UNC-84, a protein required for nuclear migration in Caenorhabditis elegans. Mol. Biol. Cell 13: 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. S., Craigie R., 1998. A previously unidentified host protein protects retroviral DNA from autointegration. Proc. Natl. Acad. Sci. USA 95: 1528–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. Y., Hench J., Ruvkun G., 2001. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr. Biol. 11: 1950–1957. [DOI] [PubMed] [Google Scholar]
- Levy D. L., Heald R., 2010. Nuclear size is regulated by importin alpha and Ntf2 in Xenopus. Cell 143: 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light W. H., Brickner D. G., Brand V. R., Brickner J. H., 2010. Interaction of a DNA zip code with the nuclear pore complex promotes H2A.Z incorporation and INO1 transcriptional memory. Mol. Cell 40: 112–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Rolef Ben-Shahar T., Riemer D., Treinin M., Spann P., et al. , 2000. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol. Biol. Cell 11: 3937–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Lee K. K., Segura-Totten M., Neufeld E., Wilson K. L., et al. , 2003. MAN1 and emerin have overlapping function(s) essential for chromosome segregation and cell division in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 100: 4598–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K., Hsin H., Libina N., Kenyon C., 2001. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 28: 139–145. [DOI] [PubMed] [Google Scholar]
- Lo M. C., Gay F., Odom R., Shi Y., Lin R., 2004. Phosphorylation by the beta-catenin/MAPK complex promotes 14–3-3-mediated nuclear export of TCF/POP-1 in signal-responsive cells in C. elegans. Cell 117: 95–106. [DOI] [PubMed] [Google Scholar]
- Loria P. M., Duke A., Rand J. B., Hobert O., 2003. Two neuronal, nuclear-localized RNA binding proteins involved in synaptic transmission. Curr. Biol. 13: 1317–1323. [DOI] [PubMed] [Google Scholar]
- Lu L., Ladinsky M. S., Kirchhausen T., 2009. Cisternal organization of the endoplasmic reticulum during mitosis. Mol. Biol. Cell 20: 3471–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupianez D. G., Kraft K., Heinrich V., Krawitz P., Brancati F., et al. , 2015. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell 161: 1012–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssenko N. N., Hanna-Rose W., Schlegel R. A., 2007. Cognate putative nuclear localization signal effects strong nuclear localization of a GFP reporter and facilitates gene expression studies in Caenorhabditis elegans. Biotechniques 43: 596, 598,, 560. [DOI] [PubMed] [Google Scholar]
- Macaulay C., Meier E., Forbes D. J., 1995. Differential mitotic phosphorylation of proteins of the nuclear pore complex. J. Biol. Chem. 270: 254–262. [DOI] [PubMed] [Google Scholar]
- MacMorris M., Brocker C., Blumenthal T., 2003. UAP56 levels affect viability and mRNA export in Caenorhabditis elegans. RNA 9: 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox P. S., Oegema K., Desai A., Cheeseman I. M., 2004. “Holo”er than thou: chromosome segregation and kinetochore function in C. elegans. Chromosome Res. 12: 641–653. [DOI] [PubMed] [Google Scholar]
- Malone C. J., Fixsen W. D., Horvitz H. R., Han M., 1999. UNC-84 localizes to the nuclear envelope and is required for nuclear migration and anchoring during C. elegans development. Development 126: 3171–3181. [DOI] [PubMed] [Google Scholar]
- Malone C. J., Misner L., Le Bot N., Tsai M. C., Campbell J. M., et al. , 2003. The C. elegans hook protein, ZYG-12, mediates the essential attachment between the centrosome and nucleus. Cell 115: 825–836. [DOI] [PubMed] [Google Scholar]
- Margalit A., Neufeld E., Feinstein N., Wilson K. L., Podbilewicz B., et al. , 2007. Barrier to autointegration factor blocks premature cell fusion and maintains adult muscle integrity in C. elegans. J. Cell Biol. 178: 661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattout A., Pike B. L., Towbin B. D., Bank E. M., Gonzalez-Sandoval A., et al. , 2011. An EDMD mutation in C. elegans lamin blocks muscle-specific gene relocation and compromises muscle integrity. Curr. Biol. 21: 1603–1614. [DOI] [PubMed] [Google Scholar]
- Mazzarello P., 1999. A unifying concept: the history of cell theory. Nat. Cell Biol. 1: E13–E15. [DOI] [PubMed] [Google Scholar]
- McGee M. D., Rillo R., Anderson A. S., Starr D. A., 2006. UNC-83 IS a KASH protein required for nuclear migration and is recruited to the outer nuclear membrane by a physical interaction with the SUN protein UNC-84. Mol. Biol. Cell 17: 1790–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee M. D., Stagljar I., Starr D. A., 2009. KDP-1 is a nuclear envelope KASH protein required for cell-cycle progression. J. Cell Sci. 122: 2895–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister P., Towbin B. D., Pike B. L., Ponti A., Gasser S. M., 2010. The spatial dynamics of tissue-specific promoters during C. elegans development. Genes Dev. 24: 766–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B. J., 2010. Targeting X chromosomes for repression. Curr. Opin. Genet. Dev. 20: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerzon M., Fridolfsson H. N., Ly N., McNally F. J., Starr D. A., 2009a UNC-83 is a nuclear-specific cargo adaptor for kinesin-1-mediated nuclear migration. Development 136: 2725–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerzon M., Gao Z., Liu J., Wu J. C., Malone C. J., et al. , 2009b Centrosome attachment to the C. elegans male pronucleus is dependent on the surface area of the nuclear envelope. Dev. Biol. 327: 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn I. L., Rolls M. M., Hanna-Rose W., Malone C. J., 2009. SUN-1 and ZYG-12, mediators of centrosome-nucleus attachment, are a functional SUN/KASH pair in Caenorhabditis elegans. Mol. Biol. Cell 20: 4586–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor T. P., Traktman P., 2014. Depletion of the protein kinase VRK1 disrupts nuclear envelope morphology and leads to BAF retention on mitotic chromosomes. Mol. Biol. Cell 25: 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Martinez A., Dobrzynska A., Askjaer P., 2015. Inner nuclear membrane protein LEM-2 is required for correct nuclear separation and morphology in C. elegans. J. Cell Sci. 128: 1090–1096. [DOI] [PubMed] [Google Scholar]
- Morton E. A., Lamitina T., 2013. Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell 12: 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss E. G., 2007. Heterochronic genes and the nature of developmental time. Curr. Biol. 17: R425–R434. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Kim S., Ishidate T., Bei Y., Pang K., et al. , 2005. Wnt signaling drives WRM-1/beta-catenin asymmetries in early C. elegans embryos. Genes Dev. 19: 1749–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols R. J., Wiebe M. S., Traktman P., 2006. The vaccinia-related kinases phosphorylate the N′ terminus of BAF, regulating its interaction with DNA and its retention in the nucleus. Mol. Biol. Cell 17: 2451–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos Y., Hodgson L., Mantell J., Verkade P., Carlton J. G., 2015. ESCRT-III controls nuclear envelope reformation. Nature 522: 236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla P. A., Ladage M. L., 2012. Suspended animation, diapause and quiescence: arresting the cell cycle in C. elegans. Cell Cycle 11: 1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla P. A., Roth M. B., 2001. Oxygen deprivation causes suspended animation in the zebrafish embryo. Proc. Natl. Acad. Sci. USA 98: 7331–7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla P. A., Nystul T. G., Zager R. A., Johnson A. C., Roth M. B., 2002. Dephosphorylation of cell cycle-regulated proteins correlates with anoxia-induced suspended animation in Caenorhabditis elegans. Mol. Biol. Cell 13: 1473–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson T., 2011. The nucleolus. Cold Spring Harb. Perspect. Biol. 3: a000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penkner A., Tang L., Novatchkova M., Ladurner M., Fridkin A., et al. , 2007. The nuclear envelope protein Matefin/SUN-1 is required for homologous pairing in C. elegans meiosis. Dev. Cell 12: 873–885. [DOI] [PubMed] [Google Scholar]
- Penkner A. M., Fridkin A., Gloggnitzer J., Baudrimont A., Machacek T., et al. , 2009. Meiotic chromosome homology search involves modifications of the nuclear envelope protein Matefin/SUN-1. Cell 139: 920–933. [DOI] [PubMed] [Google Scholar]
- Pérez-Jiménez M. M., Rodriguez-Palero M. J., Rodenas E., Askjaer P., Munoz M. J., 2014. Age-dependent changes of nuclear morphology are uncoupled from longevity in Caenorhabditis elegans IGF/insulin receptor daf-2 mutants. Biogerontology 15: 279–288. [DOI] [PubMed] [Google Scholar]
- Peric-Hupkes D., Meuleman W., Pagie L., Bruggeman S. W., Solovei I., et al. , 2010. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol. Cell 38: 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M., Nakagawa J., Doree M., Labbe J. C., Nigg E. A., 1990. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell 61: 591–602. [DOI] [PubMed] [Google Scholar]
- Phair R. D., Misteli T., 2000. High mobility of proteins in the mammalian cell nucleus. Nature 404: 604–609. [DOI] [PubMed] [Google Scholar]
- Phillips C. M., Dernburg A. F., 2006. A family of zinc-finger proteins is required for chromosome-specific pairing and synapsis during meiosis in C. elegans. Dev. Cell 11: 817–829. [DOI] [PubMed] [Google Scholar]
- Phillips C. M., Wong C., Bhalla N., Carlton P. M., Weiser P., et al. , 2005. HIM-8 binds to the X chromosome pairing center and mediates chromosome-specific meiotic synapsis. Cell 123: 1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C. M., Montgomery T. A., Breen P. C., Ruvkun G., 2012. MUT-16 promotes formation of perinuclear mutator foci required for RNA silencing in the C. elegans germline. Genes Dev. 26: 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickersgill H., Kalverda B., de Wit E., Talhout W., Fornerod M., et al. , 2006. Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nat. Genet. 38: 1005–1014. [DOI] [PubMed] [Google Scholar]
- Pitt J. N., Schisa J. A., Priess J. R., 2000. P granules in the germ cells of Caenorhabditis elegans adults are associated with clusters of nuclear pores and contain RNA. Dev. Biol. 219: 315–333. [DOI] [PubMed] [Google Scholar]
- Portier N., Audhya A., Maddox P. S., Green R. A., Dammermann A., et al. , 2007. A microtubule-independent role for centrosomes and aurora a in nuclear envelope breakdown. Dev. Cell 12: 515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteryaev D., Squirrell J. M., Campbell J. M., White J. G., Spang A., 2005. Involvement of the actin cytoskeleton and homotypic membrane fusion in ER dynamics in Caenorhabditis elegans. Mol. Biol. Cell 16: 2139–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putker M., Madl T., Vos H. R., de Ruiter H., Visscher M., et al. , 2013. Redox-dependent control of FOXO/DAF-16 by transportin-1. Mol. Cell 49: 730–742. [DOI] [PubMed] [Google Scholar]
- Ragland G. J., Denlinger D. L., Hahn D. A., 2010. Mechanisms of suspended animation are revealed by transcript profiling of diapause in the flesh fly. Proc. Natl. Acad. Sci. USA 107: 14909–14914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. M., Munzig M., Kaneshiro K., Lee B., Strome S., et al. , 2015. Caenorhabditis elegans polo-like kinase PLK-1 is required for merging parental genomes into a single nucleus. Mol. Biol. Cell 26: 4718–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasala B. A., Ramos C., Harel A., Forbes D. J., 2008. Capture of AT-rich chromatin by ELYS recruits POM121 and NDC1 to initiate nuclear pore assembly. Mol. Biol. Cell 19: 3982–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart B. J., Slack F. J., Basson M., Pasquinelli A. E., Bettinger J. C., et al. , 2000. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403: 901–906. [DOI] [PubMed] [Google Scholar]
- Riemer D., Dodemont H., Weber K., 1993. A nuclear lamin of the nematode Caenorhabditis elegans with unusual structural features; cDNA cloning and gene organization. Eur. J. Cell Biol. 62: 214–223. [PubMed] [Google Scholar]
- Rodenas E., Klerkx E. P., Ayuso C., Audhya A., Askjaer P., 2009. Early embryonic requirement for nucleoporin Nup35/NPP-19 in nuclear assembly. Dev. Biol. 327: 399–409. [DOI] [PubMed] [Google Scholar]
- Rodenas E., Gonzalez-Aguilera C., Ayuso C., Askjaer P., 2012. Dissection of the NUP107 nuclear pore subcomplex reveals a novel interaction with spindle assembly checkpoint protein MAD1 in Caenorhabditis elegans. Mol. Biol. Cell 23: 930–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner S., Kalck V., Wang X., Ikegami K., Lieb J. D., et al. , 2013. Promoter- and RNA polymerase II-dependent hsp-16 gene association with nuclear pores in Caenorhabditis elegans. J. Cell Biol. 200: 589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. H., Clayton J. E., Holmen J., Beltz E., Saito R. M., 2011. Control of Cdc14 activity coordinates cell cycle and development in Caenorhabditis elegans. Mech. Dev. 128: 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Weber C. A., Nousch M., Adame-Arana O., Hoege C., et al. , 2016. Polar positioning of phase-separated liquid compartments in cells regulated by an mRNA competition mechanism. Cell 166: 1572–1584.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salina D., Bodoor K., Eckley D. M., Schroer T. A., Rattner J. B., et al. , 2002. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell 108: 97–107. [DOI] [PubMed] [Google Scholar]
- Sato A., Isaac B., Phillips C. M., Rillo R., Carlton P. M., et al. , 2009. Cytoskeletal forces span the nuclear envelope to coordinate meiotic chromosome pairing and synapsis. Cell 139: 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P., Misteli T., 2005. Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat. Med. 11: 440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P., Misteli T., 2006. Lamin A-dependent nuclear defects in human aging. Science 312: 1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisa J. A., Pitt J. N., Priess J. R., 2001. Analysis of RNA associated with P granules in germ cells of C. elegans adults. Development 128: 1287–1298. [DOI] [PubMed] [Google Scholar]
- Schlaitz A. L., Thompson J., Wong C. C., Yates J. R., III, Heald R., 2013. REEP3/4 ensure endoplasmic reticulum clearance from metaphase chromatin and proper nuclear envelope architecture. Dev. Cell 26: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer N., Pawar N., Weiss M., Maiato H., 2015. An organelle-exclusion envelope assists mitosis and underlies distinct molecular crowding in the spindle region. J. Cell Biol. 210: 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S. P., Graves L. E., Verheyden J., Goodwin E. B., 2001. RNA-Regulated TRA-1 nuclear export controls sexual fate. Dev. Cell 1: 539–551. [DOI] [PubMed] [Google Scholar]
- Sharma R., Meister P., 2016. Dosage compensation and nuclear organization: cluster to control chromosome-wide gene expression. Curr. Opin. Genet. Dev. 37: 9–16. [DOI] [PubMed] [Google Scholar]
- Sharma R., Jost D., Kind J., Gomez-Saldivar G., van Steensel B., et al. , 2014. Differential spatial and structural organization of the X chromosome underlies dosage compensation in C. elegans. Genes Dev. 28: 2591–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaffer K. L., Updike D. L., Mango S. E., 2008. The target of Rapamycin pathway antagonizes pha-4/FoxA to control development and aging. Curr. Biol. 18: 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth U., Pitt J., Dennis S., Priess J. R., 2010. Perinuclear P granules are the principal sites of mRNA export in adult C. elegans germ cells. Development 137: 1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva N., Ferrandiz N., Barroso C., Tognetti S., Lightfoot J., et al. , 2014. The fidelity of synaptonemal complex assembly is regulated by a signaling mechanism that controls early meiotic progression. Dev. Cell 31: 503–511. [DOI] [PubMed] [Google Scholar]
- Sleeman J. E., Trinkle-Mulcahy L., 2014. Nuclear bodies: new insights into assembly/dynamics and disease relevance. Curr. Opin. Cell Biol. 28: 76–83. [DOI] [PubMed] [Google Scholar]
- Smyth J. T., Beg A. M., Wu S., Putney J. W., Jr, Rusan N. M., 2012. Phosphoregulation of STIM1 leads to exclusion of the endoplasmic reticulum from the mitotic spindle. Curr. Biol. 22: 1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovei I., Kreysing M., Lanctot C., Kosem S., Peichl L., et al. , 2009. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell 137: 356–368. [DOI] [PubMed] [Google Scholar]
- Solovei I., Thanisch K., Feodorova Y., 2016. How to rule the nucleus: divide et impera. Curr. Opin. Cell Biol. 40: 47–59. [DOI] [PubMed] [Google Scholar]
- Sosa B. A., Rothballer A., Kutay U., Schwartz T. U., 2012. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell 149: 1035–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D. A., Fridolfsson H. N., 2010. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu. Rev. Cell Dev. Biol. 26: 421–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D. A., Han M., 2002. Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science 298: 406–409. [DOI] [PubMed] [Google Scholar]
- Starr D. A., Hermann G. J., Malone C. J., Fixsen W., Priess J. R., et al. , 2001. unc-83 encodes a novel component of the nuclear envelope and is essential for proper nuclear migration. Development 128: 5039–5050. [DOI] [PubMed] [Google Scholar]
- Steiner F. A., Henikoff S., 2014. Holocentromeres are dispersed point centromeres localized at transcription factor hotspots. eLife 3: e02025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelkov S. V., Schumacher J., Burkhard P., Aebi U., Herrmann H., 2004. Crystal structure of the human lamin A coil 2B dimer: implications for the head-to-tail association of nuclear lamins. J. Mol. Biol. 343: 1067–1080. [DOI] [PubMed] [Google Scholar]
- Strome S., 2005. Specification of the germ line (July 28, 2005), Wormbook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.9.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., Horvitz H. R., 1977. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56: 110–156. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Horvitz H. R., 1981. Abnormal cell lineages in mutants of the nematode Caenorhabditis elegans. Dev. Biol. 82: 41–55. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N., 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100: 64–119. [DOI] [PubMed] [Google Scholar]
- Tan W., Zolotukhin A. S., Bear J., Patenaude D. J., Felber B. K., 2000. The mRNA export in Caenorhabditis elegans is mediated by Ce-NXF-1, an ortholog of human TAP/NXF and Saccharomyces cerevisiae Mex67p. RNA 6: 1762–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin B. D., Gonzalez-Aguilera C., Sack R., Gaidatzis D., Kalck V., et al. , 2012. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell 150: 934–947. [DOI] [PubMed] [Google Scholar]
- Tulgren E. D., Turgeon S. M., Opperman K. J., Grill B., 2014. The Nesprin family member ANC-1 regulates synapse formation and axon termination by functioning in a pathway with RPM-1 and beta-Catenin. PLoS Genet. 10: e1004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike D. L., Strome S., 2009. A genomewide RNAi screen for genes that affect the stability, distribution and function of P granules in Caenorhabditis elegans. Genetics 183: 1397–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updike D. L., Hachey S. J., Kreher J., Strome S., 2011. P granules extend the nuclear pore complex environment in the C. elegans germ line. J. Cell Biol. 192: 939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppaluri S., Weber S. C., Brangwynne C. P., 2016. Hierarchical size scaling during multicellular growth and development. Cell Reports 17: 345–352. [DOI] [PubMed] [Google Scholar]
- VanGompel M. J., Nguyen K. C., Hall D. H., Dauer W. T., Rose L. S., 2015. A novel function for the Caenorhabditis elegans torsin OOC-5 in nucleoporin localization and nuclear import. Mol. Biol. Cell 26: 1752–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastenhouw N. L., Fischer S. E., Robert V. J., Thijssen K. L., Fraser A. G., et al. , 2003. A genome-wide screen identifies 27 genes involved in transposon silencing in C. elegans. Curr. Biol. 13: 1311–1316. [DOI] [PubMed] [Google Scholar]
- Vietri M., Schink K. O., Campsteijn C., Wegner C. S., Schultz S. W., et al. , 2015. Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature 522: 231–235. [DOI] [PubMed] [Google Scholar]
- Vietri M., Stenmark H., Campsteijn C., 2016. Closing a gap in the nuclear envelope. Curr. Opin. Cell Biol. 40: 90–97. [DOI] [PubMed] [Google Scholar]
- Voronina E., Seydoux G., 2010. The C. elegans homolog of nucleoporin Nup98 is required for the integrity and function of germline P granules. Development 137: 1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic L. D., Jevtic P., Edens L. J., Levy D. L., 2016. New insights into mechanisms and functions of nuclear size regulation. Int. Rev. Cell Mol. Biol. 322: 1–59. [DOI] [PubMed] [Google Scholar]
- Walther T. C., Alves A., Pickersgill H., Loiodice I., Hetzer M., et al. , 2003a The conserved Nup107–160 complex is critical for nuclear pore complex assembly. Cell 113: 195–206. [DOI] [PubMed] [Google Scholar]
- Walther T. C., Askjaer P., Gentzel M., Habermann A., Griffiths G., et al. , 2003b RanGTP mediates nuclear pore complex assembly. Nature 424: 689–694. [DOI] [PubMed] [Google Scholar]
- Wandke C., Kutay U., 2013. Enclosing chromatin: reassembly of the nucleus after open mitosis. Cell 152: 1222–1225. [DOI] [PubMed] [Google Scholar]
- Weber S. C., Brangwynne C. P., 2015. Inverse size scaling of the nucleolus by a concentration-dependent phase transition. Curr. Biol. 25: 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler B. S., Anderson E., Frøkjær-Jensen C., Bian Q., Jorgensen E., et al. , 2016. Chromosome-wide mechanisms to decouple gene expression from gene dose during sex-chromosome evolution. eLife 5: e17365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel N., Mattout A., Melcer S., Melamed-Book N., Herrmann H., et al. , 2008. Laminopathic mutations interfere with the assembly, localization, and dynamics of nuclear lamins. Proc. Natl. Acad. Sci. USA 105: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurzenberger C., Gerlich D. W., 2011. Phosphatases: providing safe passage through mitotic exit. Nat. Rev. Mol. Cell Biol. 12: 469–482. [DOI] [PubMed] [Google Scholar]
- Xiong H., Mohler W. A., Soto M. C., 2011. The branched actin nucleator Arp2/3 promotes nuclear migrations and cell polarity in the C. elegans zygote. Dev. Biol. 357: 356–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., Fetter R. D., Hu P., Betzig E., Tjian R., 2011. Subnuclear segregation of genes and core promoter factors in myogenesis. Genes Dev. 25: 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y. H., Ma T. H., Lee L. W., Chiou P. T., Chen P. H., et al. , 2015. A genetic cascade of let-7-ncl-1-fib-1 modulates nucleolar size and rRNA pool in Caenorhabditis elegans. PLoS Genet. 11: e1005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumerefendi H., Dickinson D. J., Wang H., Zimmerman S. P., Bear J. E., et al. , 2015. Control of protein activity and cell fate specification via light-mediated nuclear translocation. PLoS One 10: e0128443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Smolen G. A., Palmer R., Christoforou A., van den Heuvel S., et al. , 2004. SUMO modification is required for in vivo Hox gene regulation by the Caenorhabditis elegans Polycomb group protein SOP-2. Nat. Genet. 36: 507–511. [DOI] [PubMed] [Google Scholar]
- Zhou K., Hanna-Rose W., 2010. Movers and shakers or anchored: Caenorhabditis elegans nuclei achieve it with KASH/SUN. Dev. Dyn. 239: 1352–1364. [DOI] [PubMed] [Google Scholar]
- Zhou K., Rolls M. M., Hall D. H., Malone C. J., Hanna-Rose W., 2009. A ZYG-12-dynein interaction at the nuclear envelope defines cytoskeletal architecture in the C. elegans gonad. J. Cell Biol. 186: 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuela N., Zwerger M., Levin T., Medalia O., Gruenbaum Y., 2016. Impaired mechanical response of an EDMD mutation leads to motility phenotypes that are repaired by loss of prenylation. J. Cell Sci. 129: 1781–1791. [DOI] [PubMed] [Google Scholar]