Abstract
Using a Non-invasive Micro-test Technique, flux profiles of Cd2+, Ca2+, and H+ were investigated in axenically grown cultures of two strains of Paxillus involutus (MAJ and NAU), ectomycorrhizae formed by these fungi with the woody Cd2+-hyperaccumulator, Populus × canescens, and non-mycorrhizal (NM) roots. The influx of Cd2+ increased in fungal mycelia, NM and ectomycorrhizal (EM) roots upon a 40-min shock, after short-term (ST, 24 h), or long-term (LT, 7 days) exposure to a hydroponic environment of 50 μM CdCl2. Cd2+ treatments (shock, ST, and LT) decreased Ca2+ influx in NM and EM roots but led to an enhanced influx of Ca2+ in axenically grown EM cultures of the two P. involutus isolates. The susceptibility of Cd2+ flux to typical Ca2+ channel blockers (LaCl3, GdCl3, verapamil, and TEA) in fungal mycelia and poplar roots indicated that the Cd2+ entry occurred mainly through Ca2+-permeable channels in the plasma membrane (PM). Cd2+ treatment resulted in H2O2 production. H2O2 exposure accelerated the entry of Cd2+ and Ca2+ in NM and EM roots. Cd2+ further stimulated H+ pumping activity benefiting NM and EM roots to maintain an acidic environment, which favored the entry of Cd2+ across the PM. A scavenger of reactive oxygen species, DMTU, and an inhibitor of PM H+-ATPase, orthovanadate, decreased Ca2+ and Cd2+ influx in NM and EM roots, suggesting that the entry of Cd2+ through Ca2+-permeable channels is stimulated by H2O2 and H+ pumps. Compared to NM roots, EM roots exhibited higher Cd2+-fluxes under shock, ST, and LT Cd2+ treatments. We conclude that ectomycorrhizal P. × canescens roots retained a pronounced H2O2 production and a high H+-pumping activity, which activated PM Ca2+ channels and thus facilitated a high influx of Cd2+ under Cd2+ stress.
Keywords: ectomycorrhizal fungi, Paxillus involutus, MAJ, NAU, Cd2+-hyperaccumulator, poplar, NMT
Introduction
The presence of highly toxic cadmium (Cd2+) in the environment is a serious threat to human health as heavy metals can be enriched in plants and eventually enter the human body through the food chain (Nawrot et al., 2006; Kaplan et al., 2011). The genus Populus spp. is of particular interest for phytoremediation of Cd2+ pollution (Sell et al., 2005; Krpata et al., 2008, 2009; Kieffer et al., 2009; He et al., 2011, 2013, 2015; Ma Y. et al., 2014), due to its widespread distribution, rapid growth, and genotypic differences in response to ion-specific stress (Chen and Polle, 2010; Polle et al., 2013; Chen et al., 2014; Polle and Chen, 2015). Populus tremula (Kieffer et al., 2009) and Populus × canescens (He et al., 2011) have been recently identified as woody Cd2+-hyperaccumulators. Cd2+ enrichment in these poplars (Kieffer et al., 2009; He et al., 2011; Ma Y. et al., 2014) exceed the threshold of 100 μg Cd2+ g-1 DW that has commonly been defined for hyperaccumulation (Milner and Kochian, 2008; Krämer, 2010). He et al. (2013) demonstrated that P. × canescens could detoxify Cd2+ by its sequestration in the bark.
In nature, poplar roots form symbioses with mycorrhizal fungi (Danielsen et al., 2012, 2013). For example, colonization of P. × canescens roots with the ectomycorrhizal fungus Paxillus involutus improves growth, primes for increased stress tolerance, increases nutrition, and regulates the ion balance under salt stress (Schützendübel and Polle, 2002; Gafur et al., 2004; Langenfeld-Heyser et al., 2007; Luo et al., 2009, 2011; Li J. et al., 2012; Ma X. et al., 2014). A notable finding was that Paxillus involutus ectomycorrhizas enhance both Cd2+ uptake and tolerance in P. × canescens (Ma Y. et al., 2014). Thus, ectomycorrhizal poplar plants offer a great potential for phytoremediation of Cd2+-polluted soils (Sell et al., 2005; Krpata et al., 2008, 2009; Luo et al., 2014; Ma Y. et al., 2014).
Cd2+ is generally believed to enter plant cells through high affinity transporters responsible for the uptake of divalent cations (Cu2+, Co2+, Fe2+, Ca2+, Mn2+, and Zn2+; Liu et al., 1997; Clemens et al., 1998; Cohen et al., 1998; Hirschi et al., 2000; Thomine et al., 2000; Zhao et al., 2002; Cosio et al., 2004; Clemens, 2006; Roth et al., 2006). Cd2+ can even induce nutrient deficiencies by competing with the uptake of essential elements (Zhao et al., 2006; Papoyan et al., 2007; DalCorso et al., 2008; Gallego et al., 2012; Baliardini et al., 2015). On the other hand, elevated Ca2+ levels suppress Cd2+ uptake in different ecotypes of Sedum alfredii also supporting competition of Cd2+ uptake with nutrient cations (Lu et al., 2010). Transcript levels of the transporters involved in Cd2+ uptake and transport have been investigated in herbaceous and woody species (Kim et al., 2006; Plaza et al., 2007; Krämer, 2010; Migeon et al., 2010; Mendoza-Cózatl et al., 2011; Lin and Aarts, 2012). In poplar plants, a variety of heavy metal transporters, such as ZRT-IRT-like proteins (ZIP2, ZIP6.2), natural resistance associated macrophage proteins (NRAMP1.1, NRAMP1.3), ATP-binding cassette transporter C1 (ABCC1), heavy metal ATPase 4 (HMA4), ATP-binding cassette transporter in mitochondria (ATM3), have been suggested to play pivotal roles in Cd2+ transport and detoxification (Ma Y. et al., 2014; He et al., 2015). In addition to these heavy metal transporters, ion channels in the plasma membrane (PM) that are permeable to Cd2+ contribute the Cd2+ uptake (Li et al., 2012a; Sun et al., 2013a,b; He et al., 2015). High external Cd2+ concentrations establish a large electrochemical gradient facilitating the rapid movement of Cd2+ ions through Cd2+-permeable channels. Perfus-Barbeoch et al. (2002) suggested that Cd2+ enters root cells via plasma membrane (PM) Ca2+ channels.
Ca2+ channels in the PM have been characterized by electrophysiological measurements involving incorporation of plasma-membrane vesicles into planar lipid bilayers (PLB, White, 2000) and patch clamping (Perfus-Barbeoch et al., 2002). According to their electrophysiological properties, the channels can be divided into depolarisation-, hyperpolarisation-, elicitor-activated, and voltage-insensitive channels (Thuleau et al., 1998; White, 2000). These channels display different sensitivities to typical inhibitors of Ca2+ channels, such as La3+, Gd3+, TEA, and verapamil. Specifically, verapamil and TEA inhibit depolarisation-activated Ca2+ channels, such as the wheat root channel rca (Piñeros and Tester, 1997; White, 1998), and rye root voltage-dependent cation channel 2, VDCC2 (White, 1998). La3+ shares a high similarity to another trivalent cation, Gd3+. Both cations are able to inhibit three distinct classes of Ca2+ channels, including depolarisation-activated Ca2+ channels, rca (Piñeros and Tester, 1997; White, 1998), hyperpolarisation-activated Ca2+ channels (HACCs) in onion bulb epidermis (Pickard and Ding, 1993), voltage-insensitive channels such as Arabidopsis root epidermal non-selective cation channels (NSCCs; Demidchik et al., 2002), and large-conductance elicitor-activated channel (LEAC) in parsley cell suspension (Zimmermann et al., 1997). Ca2+ channels in the PM are permeable to divalent (including Ca2+, Mg2+, Ba2+, Sr2+, Co2+, Zn2+, Mn2+, Ni2+, Cu2+; Cosgrove and Hedrich, 1991; Ping et al., 1992; Pickard and Ding, 1993; Thuleau et al., 1994a,b; Gelli and Blumwald, 1997; Zimmermann et al., 1997; White, 1998; Grabov and Blatt, 1998, 1999) and monovalent cations (Na+, K+, Cs+, Li+, Rb+; Cosgrove and Hedrich, 1991; Pickard and Ding, 1993; Zimmermann et al., 1997; Piñeros and Tester, 1997; White, 1998). In accordance with the suggestion that Cd2+ ions can be transported into cells through Ca2+ channels (Perfus-Barbeoch et al., 2002; Gallego et al., 2012; Li et al., 2012b) the permeability for Cd2+ through wheat VDCC2 was detected when the plasma membrane derived from root cells was incorporated into PLB (White, 1998). Using the whole-cell patch-clamp technique, Perfus-Barbeoch et al. (2002) confirmed that Cd2+ permeates through the PM Ca2+ channels in Arabidopsis guard cells. The Cd2+ influx was effectively blocked by Ca2+ channel blockers, e.g., LaCl3 and verapamil in Suaeda salsa (Li et al., 2012a), Populus euphratica (Sun et al., 2013b), and P. tremula × P. alba (He et al., 2015), further indicating that Cd2+ ions penetrate into plant cells through Ca2+-permeable channels.
It is possible that hydrogen peroxide (H2O2) stimulates the entry of Cd2+ through PM Ca2+ channels as the activity of these channels has been shown to be stimulated by H2O2. Pei et al. (2000) found that H2O2 activates the PM Ca2+ channels, leading to a subsequent rise of cytosolic Ca2+ in Arabidopsis guard cells. Demidchik et al. (2007) observed a transient increase of Ca2+ influx in the root epidermis when exogenous H2O2 was applied to Arabidopsis thaliana. In NaCl-stressed P. euphratica cells, Ca2+ influx through Ca2+ channels was activated by H2O2 (Sun et al., 2010). Recently, H2O2 was shown to accelerate Cd2+ influx in P. euphratica cells, while the H2O2-stimulated Cd2+ influx was blocked by LaCl3 (Sun et al., 2013b; Han et al., 2016). Moreover, the application of a H2O2 scavenger, catalase, lowered the Cd2+ influx across the PM in Cd2+-stressed P. euphratica cells (Sun et al., 2013b). In Cd2+-treated P. euphratica cells, hydrogen sulfide was found to reduce Cd2+ influx through down-regulation of H2O2-stimulated Cd2+ transport across the PM Ca2+ channels (Sun et al., 2013b). H2O2 is not only produced in Cd2+-stressed poplar cells (Sun et al., 2013b; Han et al., 2016) and roots (Ma Y. et al., 2014; He et al., 2015), but is also massively enriched in Populus × canescens–Paxillus involutus ectomycorrhizal associations (Gafur et al., 2004; Langenfeld-Heyser et al., 2007). Thus, it can be speculated that the fungal-elicited H2O2 accelerates the entry of Cd2+ through PM Ca2+ channels. However, this hypothesis needs to be clarified by further electrophysiological investigations.
In addition to H2O2, the PM H+-ATPase plays a crucial role in accelerating Cd2+ transport in poplar roots (Ma Y. et al., 2014; He et al., 2015). He et al. (2015) demonstrated that the net Cd2+ influx was pH-dependent in poplar roots and effectively blocked by inhibitors of H+-pumps. Ma Y. et al. (2014) showed that the active PM H+-ATPase-driven Cd2+ uptake is a major factor for increased Cd2+ accumulation in ectomycorrhizal (EM) poplar plants. They suggested that the EM-induced transcripts of HA2.1 and AHA10.1 genes, encoding PM H+-ATPases in P. × canescens, may result in H+-pump-stimulated Cd2+ enrichment (Ma Y. et al., 2014). In agreement with this suggestion transgenic poplars that were more Cd2+ tolerant by overexpression of γ-glutamylcysteine synthetase, showed upregulated transcript levels of VHA1.1, HA2.1 and AHA10.1 and a high Cd2+ uptake rate (He et al., 2015). The PM H+-ATPases maintain a H+ gradient across the membrane to promote active transport of essential elements across the PM (Beritognolo et al., 2007; Ma et al., 2010; Sun et al., 2010; Luo et al., 2013). Increased H+-pumping activities have been well characterized in arbuscular mycorrhizal associations (Ramos et al., 2005; Rosewarne et al., 2007) and in ectomycorrhizal associations formed by Paxillus involutus (strains MAJ and NAU) with Populus × canescens (Li J. et al., 2012). We have previously shown that the upregulated H+-pumping activities in Paxillus involutus-Populus × canescens symbiosis resulted in enhanced Ca2+ uptake and enrichment (Li J. et al., 2012). Demidchik et al. (2002) proposed that voltage modulation of the co-existing NSCC/HACC by PM H+-ATPase would be a potent regulator for Ca2+ entry to the root cell cytoplasm. The high H+-pumping activity leads to hyperpolarization of the PM and, thus, may increase Cd2+ influx through hyperpolarisation-activated Ca2+ channels. However, it is unknown whether the PM H+-ATPases could stimulate the entry of Cd2+ through Ca2+-permeable channels in ectomycorrhizal plants.
The two P. involutus strains, MAJ and NAU, form different colonization structures with P. × canescens roots (Gafur et al., 2004). Strain MAJ forms a typical hyphal mantle and Hartig net with roots of P. × canescens, while NAU is unable to intrude between the host cells and forms only a hyphal mantle ensheathing the root tips (Gafur et al., 2004). The colonization of P. × canescens roots with the competent strain MAJ results in enriched Cd2+ levels under Cd2+ stress (Ma Y. et al., 2014). Whether the incompatible fungal isolate NAU also affects the Cd2+ entry into P. × canescens host plants needs to be clarified.
In this study, we used a non-invasive micro-test technique (NMT) to measure fluxes of Cd2+, Ca2+ and H+ in Cd2+-stressed roots of non-mycorrhizal (NM) and ectomycorrhizal P. × canescens plants colonized with Paxillus involutus strains, MAJ and NAU. The aim was to elucidate whether the Cd2+ influx through Ca2+-permeable channels is stimulated by H2O2 and H+-ATPase in ectomycorrhizal roots since the ectomycorrhizas exhibit enhanced H2O2 production and upregulated H+-pumping activity. NMT microelectrodes measure the ion fluxes on the surface of the tissues, which are either the plant root cells for the NM plants or the fungal hyphae forming the mantle structure ensheathing the roots. To discriminate between potentially different Cd2+ effects on fungus and plant roots, fluxes of Cd2+, Ca2+ and H+ were examined for pure fungal mycelia of the two P. involutus isolates, MAJ and NAU, in addition to flux recordings on NM and EM roots. Furthermore, flux profiles of Cd2+ and Ca2+ were recorded in P. involutus-inoculated roots after 7 days of co-culture. The aim was to determine whether flux profiles of mature EM associations resemble the pattern of those from host roots at early stages of fungal colonization when the host is known to activate transient defense responses in contrast to the mature ectomycorrhizal symbioses (Duplessis et al., 2005).
Materials and Methods
Fungus and Plant Cultures for EM Colonization
The Paxillus involutus isolates MAJ and NAU, obtained from the Büsgen Institute: Institute of Forest Botany and Tree Physiology (Göttingen University, Germany), were grown on 2% modified Melin Norkrans (MMN) agar medium (g⋅L-1): KH2PO4 0.5, (NH4)2SO4 0.25, MgSO4⋅7H2O 0.15, CaCl2⋅2H2O 0.05, NaCl 0.025, FeCl3⋅6H2O 0.01, thiamine HCl 0.0001, glucose 10, malt extract 3, pH 5.2 (Gafur et al., 2004; Li J. et al., 2012). Prior to the colonization, the fungi were pre-grown on the agar culture medium for 1 week in petri dishes (diameter 90 mm) and kept in darkness at 23°C.
Plantlets of Populus × canescens (a hybrid of Populus tremula × Populus alba) were propagated by micropropagation as described by Leple et al. (1992). Regenerated P. × canescens plants were grown for 3–4 weeks on Murashige and Skoog (MS) medium (Murashige and Skoog, 1962). Uniform plants with sufficient roots were used for ectomycorrhization. The colonization of P. × canescens with Paxillus involutus strains MAJ and NAU was followed the procedures described by Gafur et al. (2004). In brief, rooted plantlets from sterile culture were placed on the MMN agar medium in the presence or absence of EM mycelium. After fungal inoculation, the petri dishes were sealed with Parafilm and covered with aluminum foil to keep the roots in darkness. During the period of incubation, the temperature in the climate chamber was maintained at 23°C with a light period of 16 h (6:00 AM–22:00 PM). Photosynthetic active radiation (PAR) of 200 μmol m-2 s-1 was supplied by cool white fluorescent lamps. After 1 month of inoculation, EM and NM root tips for anatomical investigations were embedded, stained, and photographed as described previously (Gafur et al., 2004). EM and NM plants with similar height and growth performance were used for CdCl2 treatment.
Liquid Culture of Fungi
Liquid culture of P. involutus was grown as previously described (Ott et al., 2002; Langenfeld-Heyser et al., 2007; Li J. et al., 2012). In brief, mycelium from the agar plate was homogenized, transferred into 100 mL of liquid medium (pH 4.8) in flasks, and incubated on a rotary shaker in darkness (150 rpm, 23°C). P. involutus in submerged culture grew in the form of compact spherical masses of mycelium (pellets). For Cd2+ shock treatment, sterile filtered CdCl2 solutions were added to achieve final concentrations of 50 μM. After ST (24 h) or LT (7 days) treatment, axenic cultures of MAJ and NAU were used for steady flux measurements of Cd2+, H+, and Ca2+.
Cadmium Treatment
Ectomycorrhizal and non-mycorrhizal plants were carefully removed from MMN agar medium. Rooted plantlets were cultivated in individual pots containing hydroponic MS nutrient solution (MS medium without agar and sucrose) (Murashige and Skoog, 1962). Plants were covered with plastic bags to reduce the rapid water loss in a growth room. NM and EM plantlets were subjected to 50 μM CdCl2 for a short-term (ST) exposure, 24 h or a long-term (LT) exposure for 7 days. The required amount of CdCl2 was added to the MS nutrient solution. Control plants were treated in the same manner without the addition of CdCl2. The plants were maintained at 23°C with a light period of 16 h (6:00 AM–22:00 PM) and PAR was 200 μmol m-2 s-1. Plants were continuously aerated by passing air to hydroponic MS nutrient solution, which was regularly renewed. Steady fluxes of Cd2+, Ca2+ and H+ in NM and EM roots were examined after 24 h and 7 days of CdCl2 treatment. In addition, ST-induced alterations of Cd2+ and Ca2+ fluxes were also examined in non-inoculated and P. involutus-inoculated roots after 7 days of co-culture.
Measurements of Net Cd2+, Ca2+, and H+ Fluxes
Preparations of Ion-Selective Microelectrodes
Non-invasive Micro-test Technique (NMT-YG-100, Younger USA LLC, Amherst, MA01002, USA) with ASET 2.0 (Sciencewares, Falmouth, MA 02540, USA) and iFluxes 1.0 Software (Younger USA, LLC, Amherst, MA 01002, USA) was used to monitor fluxes of Cd2+, Ca2+ and H+ in EM and NM roots (Sun et al., 2009a,b; Sun et al., 2013a,b; Ma X. et al., 2014). Ion-selective electrodes were prepared as described in Sun et al. (2009a, 2013a) and Ma X. et al. (2014). Briefly, pre-pulled and silanized glass micropipettes (diameter 4–5 μm, XY-DJ-01; Xuyue (Beijing) Science and Technology Co. Ltd., Beijing, China) were back-filled with backfilling solution [Cd2+ microelectrodes: 10 mM Cd(NO3)2 and 0.1 mM KCl; Ca2+ microelectrodes: 100 mM CaCl2; H+ microelectrodes: 40 mM KH2PO4 and 15 mM NaCl, pH 7.0] to a length of 1.0 cm from the tip. Then the micropipettes were front-filled with 15 μm columns of selective liquid ion exchange cocktails (LIXs) (Cd: Fluka 20909, Sigma–Aldrich, St Louis, MO, USA; Ca: Fluka 21048; H: Fluka 95293 Fluka Chemie GmbH, Buchs, Switzerland). An Ag/AgCl wire electrode holder (XYEH01-1; Xuyue Sci. and Tech. Co., Ltd.) was inserted in the back of the electrode to create an electrical contact with the electrolyte solution. DRIREF-2 (World Precision Instruments, Inc., Sarasota, FL, USA) was used as the reference electrode (CMC-4). Prior to the measurements, ion-selective microelectrodes for the target ions were calibrated by the following standard solution:
-
simple (1)
Cd2+: 0.01, 0.05, 0.1 mM (Cd2+ concentration was 0.05 mM in the measuring solution);
-
simple (2)
Ca2+: 0.1, 0.5, 1.0 mM (Ca2+ was 0.2 mM in the measuring buffer);
-
simple (3)
H+: pH 4.2, 5.2, 6.2 (pH of the measuring solution was adjusted to 5.2 with KOH and HCl for root samples).
Electrodes were used when the Nernstian slopes in ranges of 29 ± 3 mV/decade (Cd2+, Ca2+) and 58 ± 5 mV/decade (H+). The flux rate was calculated on the basis of Fick’s law of diffusion:
J = -D (dc/dx),
where J is the ion flux in the x direction, D is the ion diffusion coefficient in a particular medium, dc represents the ion concentration difference, dx is the microelectrode movement between two positions, and dc/dx represents the ion concentration gradient. As part of the NMT system, ASET software [Science Wares (East Falmouth, MA, USA) and Applicable Electronics], was used for data and image acquisition, preliminary processing, control of three-dimensional electrode positioner and stepper-motor-controlled fine focus of the microscope stage.
Experimental Protocols for Steady-State Flux Measurements
Cd2+, Ca2+, and H+ fluxes were non-invasively measured by moving the ion-selective microelectrode between two positions close to the materials in a preset excursion (30 μm for excised roots and fungal mycelia) at a programmable frequency in the range of 0.3–0.5 Hz. P. involutus mycelia, EM and NM roots from the ST and LT CdCl2 treatments were rinsed with re-distilled water for 2–3 times, and then incubated in the basic measuring solution to equilibrate for 25 min. The concentration gradients of Cd2+, Ca2+, and H+ were measured as previously described (Li J. et al., 2012; Lu et al., 2013; Sun et al., 2013a,b).
-
simple (1)
Cd2+ measuring solutions: 0.1 mM KCl, 0.1 mM MgCl2, 0.05 mM CaCl2 and 0.05 mM CdCl2, pH was adjusted to 5.2 with KOH and HCl;
-
simple (2)
Ca2+ measuring solutions: 0.1 mM NaCl, 0.1 mM MgCl2, 0.1 mM KCl, and 0.2 mM CaCl2, pH was adjusted to 5.2 with KOH and HCl;
-
simple (3)
H+ measuring solutions: 0.1 mM NaCl, 0.1 mM MgCl2, 0.1 mM CaCl2 and 0.5 mM KCl, pH 5.2 was adjusted with KOH and HCl.
The steady fluxes of roots were then recorded 100 μm from the apex and conducted along the root axis until 2300 μm at intervals of 200–300 μm. The fluxes of each measuring point in apical regions were continuously recorded for 6–8 min. For P. involutus mycelia, Cd2+, Ca2+, and H+ fluxes were measured around the surface of pelleted hyphae over a recording period of 30 min.
Transient Flux Recording
Paxillus involutus fungal mycelia and roots sampled from EM and NM plants were immobilized in the measuring solutions of Cd2+ (0.1 mM KCl, 0.1 mM MgCl2, 0.05 mM CaCl2, pH 5.2); Ca2+ (0.1 mM NaCl, 0.1 mM MgCl2, 0.1 mM KCl, and 0.2 mM CaCl2, pH 5.2) and H+ (0.1 mM NaCl, 0.1 mM MgCl2, 0.1 mM CaCl2 and 0.5 mM KCl, pH 5.2) for 25 min equilibration. Then the steady-state fluxes in fungal mycelia and the root apical region (100 μm from the root apex) were continuously recorded for 5 min prior to the CdCl2 shock. CdCl2 stock (100 μM) was slowly added to the measuring solution using a pipette until the final Cd2+ concentration reached 50 μM. Afterward, transient kinetics of Cd2+, Ca2+, and H+ were restarted and continued for 40 min. The data measured during the first 1–2 min was discarded, due to the effects of the diffusing stock solution. The high flux of Cd2+, Ca2+, and H+ during the following 2 min was defined as peaking values.
Effects of H2O2 on CdCl2-altered transient kinetics of Cd2+ and Ca2+ were also examined in NM and EM roots. Following the CdCl2 shock (50 μM) as described above, H2O2 (1.0 mM) was introduced to the measuring solution and transient kinetics of Cd2+ and Ca2+ were recorded for 20 min.
Fungal mycelia were exposed to 50 μM CdCl2 to induce a shock. Cd2+, Ca2+, and H+ fluxes were monitored over a continuous recording period of 40 min. For transient flux kinetics, the data measured during the first 1–2 min were discarded due to the diffusion effects of stock addition.
Effects of Ca2+ on Sensitivity of Cd2+ Electrodes
To determine whether Ca2+ ions compete with Cd2+ to penetrate across PM Ca2+-permeable channels, the effects of additional Ca2+ ions on Cd2+ electrodes was examined. Cd2+ calibrating solutions were added with 0, 0.01, 0.025, 0.05, 0.1, 0.2, 0.5, 1.0, or 2.0 mM Ca2+. Then Cd2+ microelectrodes were calibrated in Ca2+-supplemented solutions as described above. Moreover, the Nernst slope and intercept of the Cd2+ electrodes were calibrated in the measuring solution containing 0.1 mM KCl, 0.1 mM MgCl2, and 0.05 mM CaCl2.
Flux Oscillations
Oscillations in membrane-transport activity are ubiquitous in plant response to salinity, temperature, osmotic, hypoxia, and pH stresses (Shabala et al., 2006). In our study, rhythmic (ultradian) flux oscillations in NM and EM P. × canescens roots were not noticeable as that observed in herbaceous species (Shabala et al., 1997, 2003, 2006; Shabala and Knowles, 2002). This finding is presumably due to a lower growth rate of woody roots compared with crop species (Li J. et al., 2012). The flux oscillations of the measured ions, e.g., H+, Ca2+, and Cd2+, were more like fluctuations as previously reported in poplar roots (e.g., Na+, K+, H+, and Ca2+; Li J. et al., 2012). In this study, H+, Ca2+, and Cd2+ fluxes were recorded for 6–8 min at each point, which is long enough to cover oscillatory periods of measured ions.
Inhibitor and Stimulator Treatment
In this study, the effects of Ca2+, pH, H2O2, and PM transporter and channel inhibitors on Cd2+-altered ion flux profiles were examined in fungal mycelia and roots (NM and EM). Briefly,
Series 1: Ca2+ channel inhibitors. NM and EM roots were pre-treated with or without LaCl3 (5 mM; Sun et al., 2010; Li et al., 2012b), GdCl3 (500 μM, Demidchik et al., 2007, 2009; Sun et al., 2012), TEA (50 μM, White, 1998; Li J. et al., 2012), or verapamil (20 μM, Li et al., 2012a; He et al., 2015) for 24 h in the presence and absence of 50 μM CdCl2. Fungal mycelia of the two P. involutus isolates, MAJ and NAU, were subjected to 0 or 5 mM LaCl3 treatment for 24 h supplemented with or without 50 μM CdCl2.
Series 2: Ca2+. After being subjected to Cd2+ stress (CdCl2, 50 μM) for 24 h, NM and EM roots were then exposed to 25, 50, or 100 μM CaCl2 for flux recordings in the presence of CdCl2.
Cd2+ and Ca2+ fluxes in Series 1 and 2 were measured along root axes, 100–2,300 μm from the apex, at intervals of 200–300 μm. In P. involutus mycelia, Cd2+ and Ca2+ fluxes were continuously measured around the surface of pelleted hyphae over a recording period of 30 min.
Series 3: Hydrogen peroxide. NM and EM roots were sampled and immobilized in Cd2+ or Ca2+ measuring solutions for transient flux recordings in the apical region (100 μm from the root apex). The steady-state fluxes were continuously recorded for 10–20 min prior to the CdCl2 shock. CdCl2 stock (100 μM) was slowly added to the measuring solution until the final Cd2+ concentration reached 50 μM and transient kinetics of Cd2+ and Ca2+ were continuously for 20–30 min. Afterward, H2O2 (1.0 mM) was slowly added to the measuring solution and transient kinetics of Cd2+ and Ca2+ were restarted and continued for 20 min.
Series 4: ROS scavenger. NM and EM roots were pre-treated with or without 1, 3-Dimethyl-2-thiourea (DMTU, 5 mM, Chung et al., 2008; Sun et al., 2010) for 24 h in the presence and absence of 50 μM CdCl2. Then Cd2+ and Ca2+ fluxes were measured along root axes, 100–2,300 μm from the apex, at intervals of 200–300 μm.
Series 5: External pH. NM and EM roots were pre-treated with 50 μM CdCl2 for 24 h prior to flux measurements. Cd2+ and Ca2+ fluxes along root axes (100–2,300 μm from the apex) were recorded in Cd2+ or Ca2+ measuring solutions at pH 5.2, 6.2, or 7.2, respectively.
Series 6: PM H+-ATPase inhibitor. NM and EM roots were pre-treated with or without sodium orthovanadate (500 μM, Sun et al., 2010; Lu et al., 2013) for 24 h in the presence and absence of 50 μM CdCl2. Then H+, Cd2+, and Ca2+ fluxes were measured along root axes, 100–2,300 μm from the apex, at intervals of 200–300 μm. P. involutus isolates, MAJ and NAU, were exposed to 0 or 500 μM sodium orthovanadate for 24 h prior to a 30-min of continuous recording of H+ flux.
Measurements of Net H2O2 Fluxes
An H2O2-sensititive microelectrode [tip diameter 2–3 μm, XY-DJ-502, Xuyue (Beijing) Science and Technology Co. Ltd., Beijing, China] was used to monitor H2O2 fluxes in EM and NM roots. H2O2 microelectrodes were prepared according to the method described by Twig et al. (2001). Before the measurement, H2O2 microelectrode was polarized at +0.60 V against an Ag/AgCl reference electrode. Thereafter, the microelectrodes were calibrated by the standard solution: 0.01, 0.1 and 1 mM H2O2. Roots sampled from control and CdCl2 (50 μM,30 min)-treated EM and NM plants were immobilized in the measuring solution (0.1 mM NaCl, 0.1 mM MgCl2, 0.1 mM CaCl2 and 0.5 mM KCl, pH was adjusted to 5.2 with KOH and HCl) and equilibrated for 25 min. The fluxes were recorded 100 μm from the apex and conducted along the root axis until 2300 μm, at intervals of 200–300 μm, and then calculated.
Data Analysis
Ionic fluxes were calculated using the program JCal V3.2.1, a free MS Excel spreadsheet, which was developed by the Yue Xu1. The experimental data were subjected to SPSS (SPSS Statistics 17.0, 2008) for statistical tests and analyses. Unless otherwise stated, P < 0.05 was considered as significant.
Results
Cd2+-Altered Ion Flux Profiles in Paxillus involutus, and Roots of NM and EM Poplar
Cd2+ Fluxes
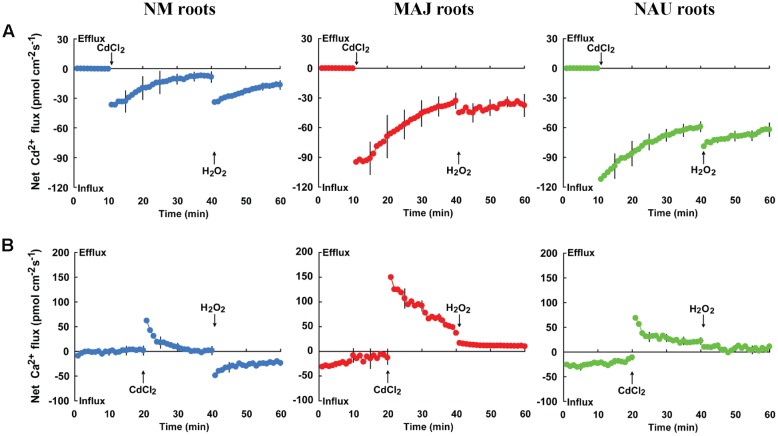
We recorded transient Cd2+ kinetics upon Cd2+ shock at the root apex (100 μm from the root tip; Figure 1A), where a vigorous ion flux (e.g., Na+, K+, Ca2+, Cd2+, Cl-) is usually observed in woody and herbaceous plants (Sun et al., 2009a,b; Li J. et al., 2012; Lu et al., 2013; Han et al., 2014). The addition of CdCl2 (50 μM) caused an immediate Cd2+ influx in both EM and NM roots which declined with increasing duration of Cd2+ exposure (40 min; Figure 1A). The peak and mean flux rate of Cd2+ in EM roots with MAJ were significantly (13.5 and 38.8%) higher than in NM roots or NAU-colonized roots (Figure 1A). Similar to the Cd2+ kinetics in EM roots, an instantaneous increase in the Cd2+ influx was detected in pure P. involutus mycelia after CdCl2 exposure (50 μM; Figure 1A). However, the fungal Cd2+ influx remained constant over the recording period (40 min; Figure 1A) with significantly higher flux rates in MAJ (75.4 pmol cm-2 s-1) than in NAU (25.9 pmol cm-2 s-1).
FIGURE 1.
Effects of CdCl2 on transient kinetics of Cd2+, Ca2+, and H+ in Populus × canescens roots and Paxillus involutus strains MAJ and NAU. Cd2+ (A), Ca2+ (B), and H+ (C) kinetics were recorded before and after the required amount of 50 μM CdCl2 was introduced into the measuring chamber. Prior to the CdCl2 shock, steady-state fluxes of Cd2+, Ca2+, and H+ in ectomycorrhizal (MAJ and NAU) and non-mycorrhizal (NM) P. × canescens roots (measuring site was ca. 100 μm from the root tip) and P. involutus isolates were monitored for approximately 5 min. Transient kinetics of Cd2+, Ca2+, and H+ were recorded after the required amount of 50 μM CdCl2 was introduced into the measuring solution. Inserted sections show the peaking and/or mean values of Cd2+, Ca2+, and H+ flux before (-Cd) and after (+Cd) the addition of CdCl2. Columns represent the mean of four to five individual plants or axenic EM cultures (pelleted hyphae), and bars represent the standard error of the mean. Different letters, a, b, c, d, e, and f, indicate significant difference at P < 0.05 between treatments.
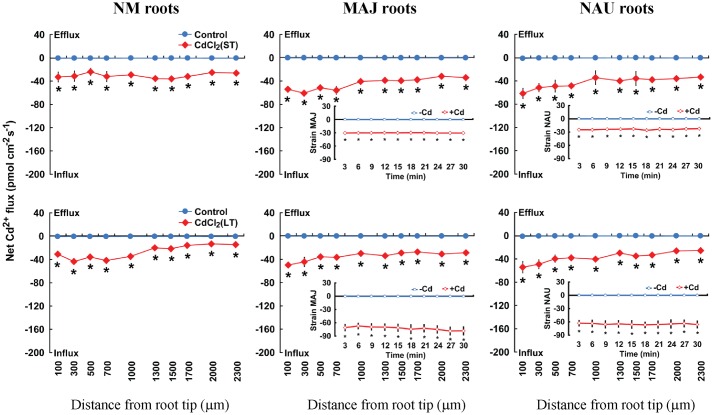
After ST (24 h) or LT (7 days) exposure to 50 μM CdCl2 in hydroponic conditions, steady-state Cd2+ flux was recorded along root axis (100–2,300 μm from the apex) at intervals of 200–300 μm (Figure 2). In NM roots, ST and LT stress caused a net Cd2+ influx with an overall mean of 28.9 pmol cm-2 s-1 along the whole measured distance; LT treatment resulted in a higher flux rate at the region 100–1,000 μm from the apex than at more distant root positions (Figure 2). A similar trend was observed in the Cd2+-stressed EM roots, though mean Cd2+ fluxes in MAJ- and NAU-ectomycorrhizal roots were 43.1 and 32.0% higher than those of the NM roots under ST and LT stress (Figure 2). The mycelia of the two P. involutus strains, MAJ and NAU, exhibited a stable Cd2+ influx under ST and LT stress, although the CdCl2-induced Cd2+ influx was typically higher under LT conditions, 68.9 pmol cm-2 s-1, compared with ST treatment, 27.1 pmol cm-2 s-1 (Figure 2). Cd2+-induced alterations of Cd2+ flux were also examined in non-inoculated and P. involutus-inoculated roots after 7 days of co-culture. NAU- and MAJ-colonized roots showed larger flux rates than non-inoculated roots after ST Cd2+ stress (Supplementary Figure S1A).
FIGURE 2.
Effects of CdCl2 on steady Cd2+ fluxes in Populus × canescens roots and Paxillus involutus strains MAJ and NAU. P. involutus isolates, ectomycorrhizal (MAJ and NAU) and non-mycorrhizal (NM) P. × canescens plants were subjected to short-term (ST, 24 h) and long-term (LT, 7 d) exposure to 50 μM CdCl2, respectively. Control roots and axenic mycelia were well fertilized but treated without CdCl2. Cd2+ fluxes in poplar roots were measured along root axis, 100–2,300 μm from the apex, at intervals of 200–300 μm. Cd2+ fluxes of P. involutus isolates MAJ and NAU were measured along the surface of pelleted hyphae over a recording period of 30 min. Inserted sections show the Cd2+ fluxes in P. involutus isolates after short-term (ST, 24 h) or long-term (LT, 7 days) CdCl2 treatment. Each point is the mean of 4–5 individual plants or axenic EM cultures (pelleted hyphae), and bars represent the standard error of the mean. Asterisks denote significant difference at P < 0.05 between treatments.
Our data show that P. involutus mycelia and EM roots both exhibited an enhanced Cd2+ uptake upon Cd2+ shock, ST, or LT treatment (Figures 1A and 2). Unexpectedly, the Cd2+ influx in EM roots did not show a high correlation to the flux rate of Cd2+ in fungal hyphae under various treatments (shock, ST, or LT, Supplementary Figure S2). However, a relatively high correlation between EM and NM roots was observed especially in response to Cd2+ shock (Supplementary Figure S2). This result supports that in the ectomycorrhizal symbioses the continuous Cd2+ entry detected by NMT microelectrodes depends on the uptake capacity of inner root cells and that in the plant–fungal interaction divergent regulation of fungal Cd2+ transport compared with pure mycelium must take place.
Ca2+ Fluxes
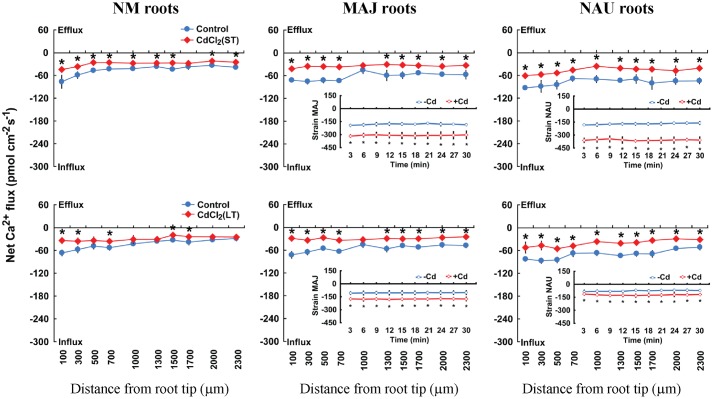
In the absence of Cd2+ stress, poplar roots exhibited a net Ca2+ influx, with a greater flux rate in MAJ- and NAU-ectomycorrhizal roots, 26.9 pmol cm-2 s-1, than in NM roots, 9.6 pmol cm-2 s-1 (Figure 1B). Similarly, the mycelia of the two strains exhibited a stable and steady influx of Ca2+ (162.3 pmol cm-2 s-1), which is ca. 6.0-fold higher than that detected in EM roots (Figure 1B). CdCl2 shock (50 μM) caused a transient Ca2+ efflux in NM and EM roots with maximum values ranging from 10.9 to 14.8 pmol cm-2 s-1 (Figure 1B). Thereafter, the direction shifted toward an influx and the mean flux over the recording period then declined in EM roots, or displayed a net efflux in NM roots (Figure 1B). In contrast to NM and EM roots, Cd2+ addition markedly increased the Ca2+ influx in the hyphae of pure mycelium, typically with higher flux rates in strain NAU than in MAJ in the first 20 min of Cd2+ application (Figure 1B). Under ST and LT treatment, Cd2+ stress caused a marked decline of Ca2+ influx along the root axis (Figure 3). MAJ- and NAU-ectomycorrhizal roots maintained 40.5 and 20.6% higher Ca2+ fluxes than NM roots under ST and LT stress (Figure 3). In the hyphae of the two fungal strains, the Ca2+ influx was enhanced by ST and LT treatments (Figure 3), similar to the shock treatment (Figure 1B). We observed that the flux rate in the two strains declined with increasing duration of hydroponic culture regardless of control and Cd2+ treatments (Figure 3). Non-inoculated P. × canescens roots exhibited a net Ca2+ influx under unstressed control conditions and the Ca2+ influx was stimulated by 7 days inoculation with MAJ and NAU (Supplementary Figure S1B). ST-treated P. involutus-inoculated roots retained higher Ca2+ influx than non-inoculated roots although the Ca2+ influx in poplar roots was lowered by Cd2+ stress (Supplementary Figure S1B).
FIGURE 3.
Effects of CdCl2 on steady Ca2+ fluxes in Populus × canescens roots and Paxillus involutus strains MAJ and NAU. P. involutus isolates, ectomycorrhizal (MAJ and NAU) and non-mycorrhizal (NM) P. × canescens plants were subjected to short-term (ST, 24 h) and long-term (LT, 7 days) exposure to 50 μM CdCl2, respectively. Control roots and axenic mycelia were well fertilized but treated without CdCl2. Ca2+ fluxes in poplar roots were measured along root axis, 100–2,300 μm from the apex, at intervals of 200–300 μm. Ca2+ fluxes of P. involutus isolates MAJ and NAU were measured along the surface of pelleted hyphae over a recording period of 30 min. Inserted sections show the Ca2+ fluxes in P. involutus isolates after short-term (ST, 24 h) or long-term (LT, 7 days) CdCl2 treatment. Each point is the mean of 4–5 individual plants or axenic EM cultures (pelleted hyphae), and bars represent the standard error of the mean. Asterisks denote significant difference at P < 0.05 between treatments.
It has been suggested that the Ca2+ enrichment in EM roots was associated with the P. involutus fungal hyphae exhibiting a high capacity for Ca2+ uptake (Figures 1B and 3; Li J. et al., 2012; Ma X. et al., 2014). However, the Ca2+ influx in EM roots was not evidently correlated to the flux rate of Ca2+ in fungal hyphae under Cd2+ shock, ST, or LT (Supplementary Figure S3). Unexpectedly, the Ca2+ flux in EM roots was even negatively correlated to the flux rate of Ca2+ in fungal hyphae after a shock treatment (Supplementary Figure S3). The observed correlation of Ca2+ fluxes between EM roots and NM roots (Supplementary Figure S3) supports that the Ca2+ flow was mainly the consequence of host roots in the Cd2+-stressed ectomycorrhizal symbioses.
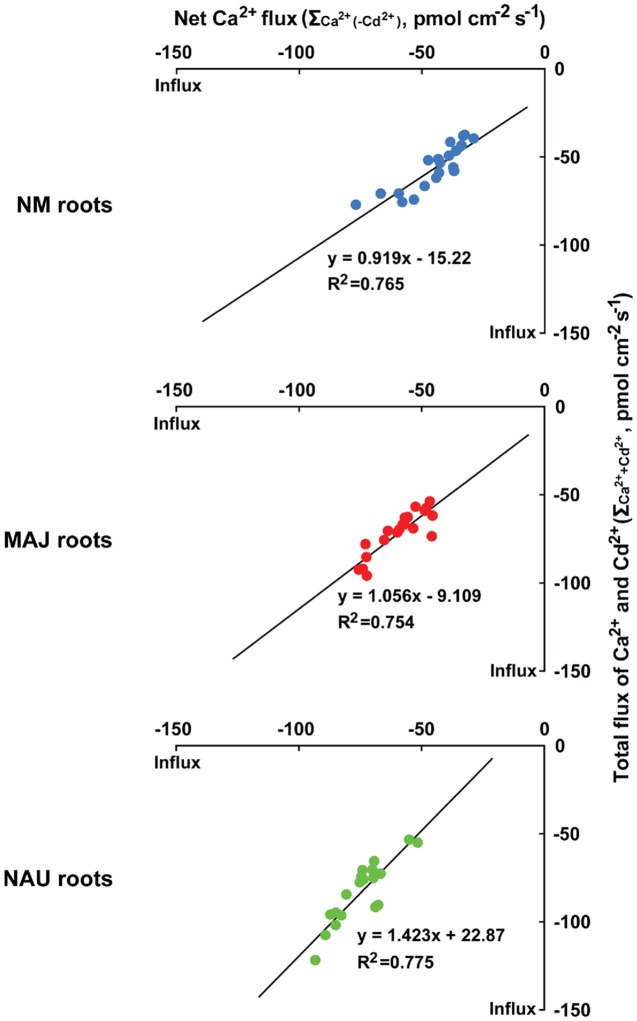
Correlations between Cd2+ and Ca2+ Fluxes
We analyzed the correlation between Cd2+ and Ca2+ fluxes as NM and EM roots took up these elements with a similar flux rate (Figures 1A,B, 2 and 3). Under ST and LT stress conditions, the total flux rates of Cd2+ and Ca2+ in the presence of Cd2+ (=ΣCa2++Cd2+ with a molar ratio of Cd2+ to Ca2+ of 1:1) were 37.8–77.4 (NM), 54.1–96.2 (MAJ), and 53.7–122.1 pmol cm-2 s-1 (NAU), as calculated on the basis of Figures 2 and 3 (Supplementary Figure S4). The relationships between ΣCa2++Cd2+ and Ca2+ flux in the absence of CdCl2 [ΣCa2+(-Cd2+)] were highly significant and close to 1 for NM and MAJ colonized roots and slightly increased to 1.4 for NAU colonized roots (Figure 4). These suggest that the entry of Cd2+ and Ca2+ is mainly through the same pathway in NM and EM roots, mostly likely through Ca2+-permeable channels in the PM (see below).
FIGURE 4.
The correlation between total fluxes of Ca2+ and Cd2+ (ΣCa2++Cd2+) in the presence of CdCl2 (50 μM, +Cd) and Ca2+ flux [ΣCa2+(-Cd2+)] in the absence of CdCl2 (-Cd) in roots of ectomycorrhizal (MAJ and NAU) and non-mycorrhizal (NM) Populus × canescens. Ectomycorrhizal (MAJ and NAU) and NM P. × canescens plants were subjected to short-term (ST, 24 h) and long-term (LT, 7 days) exposure to 50 μM CdCl2, respectively. Control roots were well fertilized but treated without CdCl2. Ca2+ and Cd2+ fluxes were measured along root axis, 100–2,300 μm from the apex, at intervals of 200–300 μm. Each point is the mean of 4–5 individual plants.
H+ Fluxes
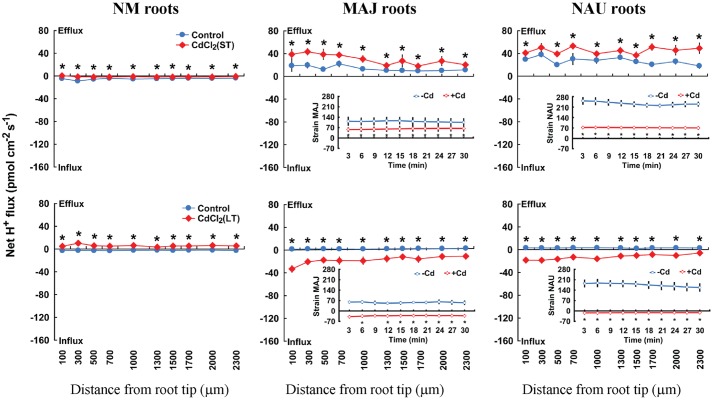
In the absence of CdCl2, EM roots showed a typical H+ efflux at the apex, which was 7.6-fold higher than that in NM roots (Figure 1C). CdCl2 (50 μM) shock stimulated H+ efflux in both NM and EM plants with a stronger response in EM than in NM roots (Figure 1C). Pure MAJ and NAU mycelia exhibited a net H+ efflux under control conditions similar to that observed for MAJ- and NAU-colonizing roots (Figure 1C). However, in pure mycelia the fluxes were 4.8-fold higher than in EM roots (Figure 1C). After exposure to CdCl2 (50 μM), hyphae exhibited a transient increase in the H+ efflux, which then remained constant during the period of recording (40 min; Figure 1C). Compared with strain MAJ, strain NAU exhibited higher H+ efflux irrespective of control or CdCl2 shock treatments (Figure 1C).
Steady-state recordings on EM roots showed that the pattern of H+ flux in ST-stressed roots (50 μM CdCl2, 24 h) differed from those subjected to LT Cd2+ exposure (50 μM CdCl2, 7 days). Under ST conditions, CdCl2 (50 μM) stimulated H+ efflux in EM plants, whereas under LT conditions, EM roots showed a pronounced H+ influx (Figure 5). In NM roots, CdCl2 (50 μM) decreased H+ influx upon ST exposure or shifted it to a net H+ efflux under LT stress conditions (Figure 5). The pattern of H+ flux in the fungal mycelia differed from that in EM roots under ST stress (Figure 5). ST treatment reduced the efflux of H+ from the two fungal strains, which is contrast to EM roots where an enhanced H+ efflux was observed (Figure 5). LT stress caused a pronounced shift of H+ efflux to influx into pure mycelia of the two strains, similar to the finding in LT-stressed EM roots (Figure 5).
FIGURE 5.
Effects of CdCl2 on steady H+ fluxes in Populus × canescens roots and Paxillus involutus strains MAJ and NAU. P. involutus isolates, ectomycorrhizal (MAJ and NAU) and non-mycorrhizal (NM) P. × canescens plants were subjected to short-term (ST, 24 h) and long-term (LT, 7 days) exposure to 50 μM CdCl2, respectively. Control roots and axenic mycelia were well fertilized but treated without CdCl2. H+ fluxes in poplar roots were measured along root axis, 100–2,300 μm from the apex, at intervals of 200–300 μm. H+ fluxes of P. involutus isolates MAJ and NAU were measured along the surface of pelleted hyphae over a recording period of 30 min. Inserted sections show the H+ fluxes in P. involutus isolates after short-term (ST, 24 h) or long-term (LT, 7 days) CdCl2 treatment. Each point is the mean of 4–5 individual plants or axenic EM cultures (pelleted hyphae), and bars represent the standard error of the mean. Asterisks denote significant difference at P < 0.05 between treatments.
Cd2+-Altered Flux Profiles of H2O2 in EM Roots
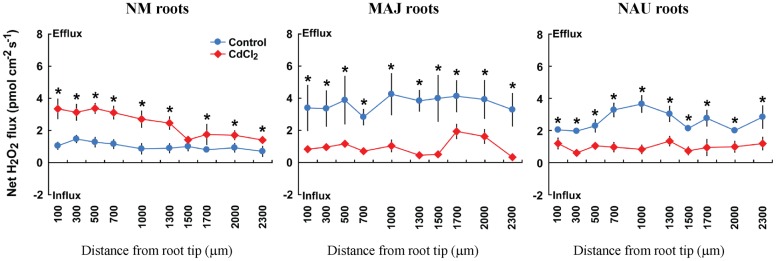
H2O2-sensitive microprobes were used to detect the H2O2 response to Cd2+ exposure in NM and EM roots. In the absence of Cd2+, NM roots exhibited a stable H2O2 efflux (0.7–1.5 pmol cm-2 s-1) along the root axis; the mean flux rate increased 2.4-fold in response to Cd2+ treatment (50 μM CdCl2, 30 min, Figure 6). Ectomycorrhization of poplar roots with P. involutus stains, MAJ and NAU, resulted in a significant increase of H2O2 efflux along the roots (Figure 6). However, upon CdCl2 exposure EM roots displayed decreased H2O2 efflux in contrast to NM roots (Figure 6).
FIGURE 6.
Effects of CdCl2 on steady H2O2 flux in roots of ectomycorrhizal (MAJ and NAU) and non-mycorrhizal (NM) Populus × canescens plants. Ectomycorrhizal (MAJ and NAU) and NM P. × canescens plants were subjected to 50 μM CdCl2 for 30 min. Control roots were well fertilized but treated without CdCl2. H2O2 flux was measured along root axis, 100–2,300 μm from the apex, at intervals of 200–300 μm. Each point is the mean of 4–5 individual plants, and bars represent the standard error of the mean. Asterisks denote significant difference at P < 0.05 between treatments.
Effects of Ca2+, H2O2, pH, and PM Transporter and Channel Inhibitors on Cd2+-Altered Ion Flux Profiles in EM Roots
Ca2+ and Ca2+ Channel Inhibitors
Here, pharmacological experiments were carried out to test whether putative Ca2+ channels inhibitors could inhibit Cd2+ influx in poplar roots. Four typical Ca2+ channels inhibitors, LaCl3, GdCl3, verapamil, and TEA effectively inhibited Ca2+ influx in NM and EM roots, regardless of Cd2+ treatments (Figure 7A, Supplementary Figures S5A, S6A, and S7A). LaCl3 restricted Cd2+ influx in CdCl2-treated NM and EM roots (Figure 7B). This suggests that Cd2+ is taken up through Ca2+-permeable channels because La3+ is able to block various types of Ca2+-permeable channels, including depolarisation-, hyperpolarisation-, elicitor-activated, and voltage-insensitive channels (Weiss, 1974; Pickard and Ding, 1993; Gelli and Blumwald, 1997; Piñeros and Tester, 1997; Zimmermann et al., 1997; White, 1998, 2000). Moreover, the other three Ca2+-permeable channel inhibitors, GdCl3, verapamil, and TEA, diminished Cd2+ influx to a similar extent as LaCl3-treated plants (Figure 7B, Supplementary Figures S5B, S6B, and S7B). Similarly, in the pure P. involutus mycelia, LaCl3 also effectively restricted influx of Ca2+ and Cd2+ or induced net efflux (Supplementary Figure S8).
FIGURE 7.
Effects of LaCl3 and external Ca2+ on steady Cd2+ and/or Ca2+ fluxes in roots of ectomycorrhizal (MAJ and NAU) and non-mycorrhizal (NM) Populus × canescens plants under Cd2+ stress. (A,B) Ectomycorrhizal (MAJ and NAU) and NM P. × canescens plants were subjected to 50 μM CdCl2 for 24 h in the presence and absence of 5 mM LaCl3. Control roots were well fertilized but treated without CdCl2 or LaCl3. (C) Ectomycorrhizal (MAJ and NAU) and NM P. × canescens plants were subjected to 50 μM CdCl2 for 24 h prior to Cd2+ flux recordings in the presence of CaCl2 (25 μM, 50 μM, or 100 μM; the ratio of Ca2+:Cd2+ was 1:2; 1:1, and 2:1). Ca2+ (A) and Cd2+ (B,C) fluxes were measured along root axes, 100–2,300 μm from the apex, at intervals of 200–300 μm. Each point is the mean of 4–5 individual plants and bars represent the standard error of the mean. Inserted sections show the mean flux rates and different letters, a, b, c, and d, indicate significant difference at P < 0.05 between treatments.
Additionally, a co-application of Cd2+ and Ca2+ suppressed the entry of Cd2+ in NM and EM roots, and the restriction increased with the increasing fraction of Ca2+ in the mixture (Ca2+: Cd2+ = 1:2, 1:1, 2:1; Figure 7C). The mean Cd2+ flux decreased by 95.7% (NM), 72.1% (MAJ), and 45.5% (NAU) at a ratio of Ca2+:Cd2+ = 2:1, compared to a those with a higher Cd2+ fraction, Ca2+:Cd2+ = 1:2 (Figure 7C). These results suggest that the divalent cations, Cd2+ and Ca2+, competitively permeated the plasma membrane through Ca2+ channels. The lower reduction in Cd2+ influx in EM than in NM roots in the presence of Ca2+ (Figure 7C) reflects the high flow of Cd2+ through the activated Ca2+ channels.
We observed that the presence of Ca2+ in the measuring solution marginally lowered the Cd2+ signals (14.7–26.0%) detected by the Cd2+ microelectrodes filled with Cd2+ liquid ion exchanger (LIX) (Supplementary Table S1). In the absence of Ca2+, the working voltage of microelectrodes and the detected Cd2+ signals in Cd2+-treated roots were unstable and fluctuated greatly during the period of recording (data not shown). This behavior is presumably caused by the plant response to nutrient deficiency in the root medium (Li J. et al., 2012). In our study, Cd2+ electrodes exhibited higher sensitivity at 0.05 mM Ca2+ in the absence and presence of 0.1 mM K+ and 0.1 mM Mg2+ (Supplementary Table S1). The presence of nutrients, K+, Ca2+, and Mg2+, did not affect the accuracy of our conclusions relating to Cd2+ fluxes in NM and EM roots.
H2O2 and ROS Scavenger
To investigate whether Cd2+ entry through Ca2+-permeable channels is activated by H2O2, we examined the effects of hydrogen peroxide and the ROS (reactive oxygen species) scavenger DMTU on Cd2+ and Ca2+ fluxes. Transient kinetic recordings showed that Cd2+ shock caused an immediate increase of Cd2+ influx but enhanced Ca2+ efflux in NM and EM roots (Figure 8). The flux rates of Cd2+ and Ca2+ decreased with prolonged exposure time (Figure 8). Notably, Cd2+ influx markedly increased upon H2O2 shock (1.0 mM) in both NM and EM roots (Figure 8A). However, the Cd2+-elicited Ca2+ efflux was reduced by H2O2 in EM roots or shifted to a net influx in NM roots (Figure 8B). These results suggest that H2O2 stimulated the entry of Cd2+ and Ca2+, presumably through the plasma membrane Ca2+ channels of the roots.
FIGURE 8.
Effects of CdCl2 and H2O2 on transient kinetics of Cd2+ and Ca2+ in roots of ectomycorrhizal (MAJ and NAU) and non-mycorrhizal (NM) Populus × canescens. Cd2+ (A) and Ca2+ (B) kinetics were recorded before and after the required amount of 50 μM CdCl2 or 1.0 mM H2O2was introduced into the measuring chamber. Prior to the CdCl2 shock, steady-state fluxes of Cd2+ and Ca2+ were monitored at the apex (measuring site was ca. 100 μm from the root tip) for approximately 10–20 min. Transient kinetics of Cd2+ and Ca2+ were recorded after the required amount of 50 μM CdCl2 was introduced into the measuring solution. After 20–30 min continuous recording of Cd2+ and Ca2+ fluxes, Cd2+ and Ca2+ kinetics were recorded for 20 min after 1.0 mM H2O2 was introduced into the measuring solution. Each point represents the mean of 4–5 individual plants and bars represent the standard error of the mean.
Ca2+ influx in NM and EM roots were suppressed by the ROS scavenger, DMTU (5 mM), irrespective of the presence and absence of Cd2+ (Figure 9A). Similarly, the supplement of DMTU significantly reduced the influx of Cd2+ in NM and EM roots (Figure 9B). These data indicated that H2O2 play a crucial role in accelerating the influx of Ca2+ and Cd2+, which is accordance to the results obtained by direct H2O2 applications (Figure 8).
FIGURE 9.
Effects of DMTU on steady Ca2+ and Cd2+ fluxes in roots of ectomycorrhizal (MAJ and NAU) and non-mycorrhizal (NM) Populus × canescens under Cd2+ stress. Ectomycorrhizal (MAJ and NAU) and NM P. × canescens plants were subjected to 0 or 50 μM CdCl2 for 24 h in the presence or absence of 5 mM DMTU. Ca2+ (A) and Cd2+ (B) fluxes were measured along root axes, 100–2,300 μm from the apex, at intervals of 200–300 μm. Each point is the mean of 4–5 individual plants and bars represent the standard error of the mean. Inserted sections show the mean flux rates and different letters, a, b, c, and d, indicate significant difference at P < 0.05 between treatments.
External pH and H+-ATPase Inhibitor
Fluxes of Cd2+ and Ca2+ depend on external pH. An acidic environment accelerated Cd2+ and Ca2+ influxes in both NM and EM roots with the strongest influx at pH 5.2 and the lowest at pH 6.2 or a neutral pH, 7.2 (Figure 10). Moreover, we noticed that the pH effects on fluxes of Cd2+ and Ca2+ were more pronounced in NM roots than in EM roots (Figure 10). Compared to an acidic environment (pH 5.2), the mean flux rate of the divalent cations decreased by 45.8% (Ca2+) and 38.8% (Cd2+) in EM roots under pH 6.2–7.2 (Figure 10). In NM roots, the increasing pH lowered Cd2+ influxes by 56.5% or even reversed the rectifications of Ca2+ (influx → efflux) at a neutral pH, 7.2 (Figure 10). The less reduced influx of Ca2+ and Cd2+ in EM roots at pH 6.2 or 7.2 was due to the high H+-pumping activity in the PM (see below).
FIGURE 10.
Effects of pH on steady Cd2+ and Ca2+fluxes in roots of ectomycorrhizal (MAJ and NAU) and non-mycorrhizal (NM) Populus × canescens under Cd2+ stress. Ectomycorrhizal (MAJ and NAU) and NM P. × canescens plants were subjected to 50 μM CdCl2 for 24 h prior to flux recordings at pH 5.2, 6.2 or 7.2. Cd2+ (A) and Ca2+ (B) fluxes were measured along root axes, 100–2,300 μm from the apex, at intervals of 200–300 μm. Each point is the mean of four to five individual plants and bars represent the standard error of the mean. Inserted sections show the mean flux rates and different letters, a, b, and c, indicate significant difference at P < 0.05 between treatments.
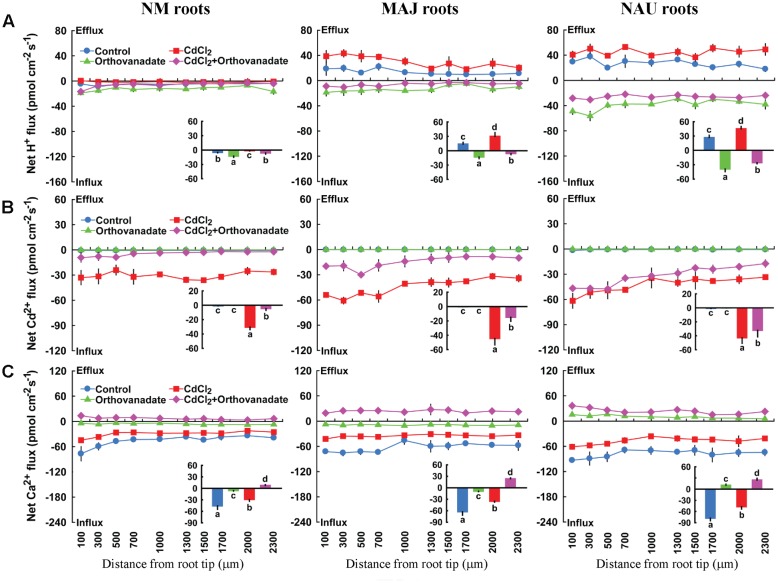
Sodium orthovanadate (500 μM), the specific inhibitor of PM H+-ATPase, increased the H+ influx in NM roots slightly, but caused a drastic shift from H+ efflux toward influx in both EM roots and P. involutus mycelia, irrespective of Cd2+ treatment (Figure 11A, Supplementary Figure S9). Sodium orthovanadate significantly reduced the Cd2+ influx along the roots in Cd2+-treated NM and EM plants (Figure 11B). In the absence of Cd2+, the PM H+-ATPase inhibitor reduced Ca2+ influx in NM and MAJ-ectomycorrhizal roots or shifted to efflux in NAU-ectomycorrhizal roots (Figure 11C). The inhibition of Ca2+ influx by sodium orthovanadate was more pronounced in the presence of Cd2+: the H+-pump inhibitor reversed the rectifications of Ca2+ from influx to efflux in NM and EM roots (Figure 11C).
FIGURE 11.
Effects of sodium orthovanadate on steady H+, Cd2+, and Ca2+ fluxes in roots of ectomycorrhizal (MAJ and NAU) and non-mycorrhizal (NM) Populus × canescens under Cd2+ stress. Ectomycorrhizal (MAJ and NAU) and NM P. × canescens plants were subjected to 0 or 50 μM CdCl2 for 24 h in the presence and absence of 500 μM sodium orthovanadate. H+ (A), Cd2+ (B), and Ca2+ (C) fluxes were measured along root axes, 100–2,300 μm from the apex, at intervals of 200–300 μm. Each point is the mean of 4–5 individual plants and bars represent the standard error of the mean. Inserted sections show the mean flux rates and different letters, a, b, c, and d, indicate significant difference at P < 0.05 between treatments.
Discussion
Colonization of P. × canescens Roots with Paxillus involutus Stimulates Cd2+ Uptake under Cd2+ Stress
The woody Cd2+-hyperaccumulator P. × canescens exhibited a vigorous Cd2+ uptake after a 50 μM CdCl2 shock (40 min), ST (24 h), and LT (7 days) treatment (Figures 1A and 2). The result is consistent with previous findings where P. × canescens roots exhibited a high Cd2+ uptake after 40 days of CdSO4 exposure (50 μM, Ma Y. et al., 2014). Similarly, a high entry of Cd2+ was recorded in hyperaccumulating ecotypes of Sedum alfredii (Lu et al., 2010; Sun et al., 2013a) and Suaeda salsa under Cd2+ stress (Li et al., 2012a). An important result was that EM roots exhibited higher Cd2+ influx than NM roots irrespective of Cd2+ stress conditions, shock, ST, and LT (Figures 1A and 2). Substantial evidence indicates that Cd2+ can be enriched in ectomycorrhizal plants (Sell et al., 2005; Baum et al., 2006; Krpata et al., 2008, 2009; Sousa et al., 2012; Ma Y. et al., 2014). The enhanced Cd2+ uptake in EM roots is partly due to the capacity of the fungus to take up Cd2+ because CdCl2 shock resulted in a net Cd2+ influx in the mycelia of the two P. involutus strains and the flux rate increased with the prolonged duration of CdCl2 treatment from 24 h to 7 days (Figures 1A and 2). In liquid cultures, P. involutus cultures also showed high capacities for Cd2+ accumulation (Ott et al., 2002). P. involutus could bind Cd2+ onto the cell walls or accumulate the metal in the vacuolar compartment (Blaudez et al., 2000; Ott et al., 2002). Moreover, the ectomycorrhizal fungus appears to detoxify high concentrations of Cd2+ by (i) the chelation of metal ions in the cytosol with thiol-containing compounds, e.g., glutathione, phytochelatins, or metallothioneins (Courbot et al., 2004; Jacob et al., 2004), and (ii) activation of antioxidative defense system (Jacob et al., 2001; Ott et al., 2002). Our pharmacological data revealed that Cd2+ entered the fungal hyphae mainly through PM Ca2+ channels because the influx was suppressed by LaCl3, a Ca2+ channel blocker (Supplementary Figure S8B). Therefore, Cd2+ enriched by ectomycorrhizal hyphae is thought to be transferred to the host roots, probably through the apoplastic space during the period of Cd2+ stress.
There were marked differences between the two strains in Cd2+ uptake given the shock treatment (Figure 1A). Pure fungal mycelium of MAJ accumulated Cd2+ with a higher rate than NAU (Figure 1A). In the P. involutus-ectomycorrhizal symbioses, the incompatible fungal isolate NAU is unable to induce a functional ectomycorrhizae while MAJ forms a typical Hartig net with the roots of P. × canescens (Gafur et al., 2004). Thus, in MAJ-colonized roots the host cells might have been more accessible to Cd2+. In accordance, MAJ roots exhibited a higher influx than NAU roots after the onset of CdCl2 shock (Figure 1A). However, Cd2+ influx into NAU-colonized roots was similar to that of MAJ-colonized roots during ST or LT Cd2+ treatment (Figure 2). This was likely due to (i) similar capacities for Cd2+ uptake of MAJ and NAU hyphae during a 24-h or 7-days of Cd2+ exposure (Figure 2), or (ii) similar uptake capacity of the fungus-ensheathed inner root cells (Figure 2). The observed correlation between EM and NM roots showed that the continuous Cd2+ flow was mainly the consequence of host roots in the Cd2+-stressed ectomycorrhizal symbioses during a prolonged period of Cd2+ exposure (24 h to 7 days; Supplementary Figure S2).
Paxillus involutus-Ectomycorrhizas Enhance Cd2+ Influx through Ca2+-Permeable Channels in the Plasma Membrane
Our data revealed that the entry of Cd2+ is likely mediated through PM Ca2+ channels in the fungal hyphae and poplar roots, and P. involutus-ectomycorrhizas facilitated the channel-mediated Cd2+ influx under Cd2+ stress. The experimental evidence for these conclusions is briefly listed below.
-
simple (1)
The addition of Cd2+ resulted in an immediate influx of Cd2+ in NM roots, and the flux was more pronounced in EM roots (Figure 1A). Rapid entry of Cd2+ is generally through PM ion channels that are permeable to Cd2+ (The first 1–2 min flux recordings were discarded to diminish the diffusion effect of stock addition in roots and fungal mycelia). Our pharmacological data revealed that the net Cd2+ influx in CdCl2-stressed NM and EM roots was strongly suppressed by typical Ca2+ channel blockers, such as LaCl3, GdCl3, verapamil, and TEA (Figure 7B, Supplementary Figures S5B, S6B, and S7B). Moreover, in P. involutus mycelium the CdCl2-elicited influx of Cd2+ was also inhibited by LaCl3 (Supplementary Figure S8B). These results suggest that under CdCl2 stress Cd2+ enters fungal and root tissues through PM Ca2+ channels.
-
simple (2)
Cd2+ treatments (shock, ST, and LT) affected the uptake of Ca2+ in poplar roots (Figures 1B and 3), while the influx of Cd2+ declined with increasing the concentration of Ca2+ when NM and EM roots were subjected to the concomitant application of Cd2+ and Ca2+ (Figure 7C). Similarly, the Cd2+ influx was affected by the presence of Ca2+ in two contrasting (hyperaccumulating and non-hyperaccumulating) Sedum alfredii ecotypes (Lu et al., 2010). It was suggested that Ca2+ and Cd2+ ions compete for the binding sites of transporters (Gussarsson et al., 1996; Rodríguez-Serrano et al., 2009). Our transient kinetics showed that Cd2+ exposure blocked the Ca2+ influx and caused an immediate change in the rectification of Ca2+ from influx to efflux (Figures 1B and 8B). This suggests that Cd2+ ions competed with Ca2+ to penetrate across PM Ca2+ channels that are permeable to divalent cations (Perfus-Barbeoch et al., 2002).
-
simple (3)
In ST- and LT-stressed NM and EM roots, the total flux rates of Cd2+ and Ca2+ in the presence of Cd2+ (ΣCa2++Cd2+) were nearly equal to the flux rate of Ca2+ in the absence of Cd2+ stress (ΣCa2+(-Cd2+); Supplementary Figure S4). Moreover, the correlations between ΣCa2++Cd2+ and ΣCa2+(-Cd2+) (Figure 4) suggest that Cd2+ ions enter NM and EM roots mainly through Ca2+-permeable channels in the PM.
Collectively, under CdCl2 stress Cd2+ ions could penetrate the PM Ca2+ channels in fungal hyphae and in P. × canescens roots. At present we cannot exclude the possibility that Cd2+ penetrated the PM through transporters for Cd2+ (Ma Y. et al., 2014; He et al., 2015) or other nutritional ions (Gussarsson et al., 1996; Cohen et al., 1998; Zhao et al., 2002; Cosio et al., 2004; Clemens, 2006), because (1) the four types of Ca2+ channel inhibitors applied here were not able to fully block the Cd2+ influx in NM and EM roots (Figure 7B, Supplementary Figures S5B, S6B and S7B), and (2) the total flux of Cd2+ and Ca2+ (ΣCa2++Cd2+, molar ratio of Cd2+ to Ca2+ is 1:1) under ST and LT Cd2+ stress was 10.9–27.7% higher than the flux rate of Ca2+ under non-Cd2+ conditions (Figure 4, Supplementary Figure S4). This implies that a small fraction of Cd2+ ions penetrated the PM through other channels and transporters.
Plasma membrane Ca2+ channels in P. involutus hyphae maybe more permeable to Cd2+ compared to the channels in P. × canescens roots as the fungal mycelium displayed a typical higher Ca2+ influx than poplar roots under control and Cd2+-stress conditions (Figures 1B and 3). We cannot discriminate between the channels of the fungus and those of the plant in the ectomycorrhizal symbiosis, but the Cd2+ and Ca2+ fluxes in EM roots appear to mainly reflect the response of the host plants to Cd2+ stress because (1) EM roots exhibited a different pattern from the P. involutus mycelia in enhancing Ca2+ and Cd2+ uptake under hydroponic Cd2+ conditions. Cd2+-shocked MAJ and NAU fungal strains usually displayed a stable Cd2+ influx with the exception of an initial transient increase (Figure 1A). However, EM roots showed a declined Cd2+ influx over the duration of Cd2+ exposure, similar to the Cd2+ kinetics in NM roots (Figure 1A). Moreover, the Cd2+ influx in the mycelia of the two P. involutus strains increased with the prolonged CdCl2 exposure from 24 h to 7 days (from 23.9 to 72.7 pmol cm-2 s-1; Figure 2). In contrast, the Cd2+ fluxes in EM roots were relatively stable under ST (43.7 ± 8.4 pmol cm-2 s-1) and LT treatments (35.9 ± 6.0 pmol cm-2 s-1; Figure 2). ST, LT, and Cd2+ shock increased the Ca2+ influx in P. involutus mycelia, while the Ca2+ influx in EM roots was declined by these Cd2+ treatments (Figures 1B and 3). (2) NMT data showed that ion fluxes in mature P. × canescens–P. involutus symbiotic associations bear a striking resemblance to the ST inoculated roots (Supplementary Figure S1). Similar findings have been previously reported in a salt stress study where P. × canescens roots were inoculated with P. involutus for 10 and 20 days (Ma X. et al., 2014). At early stages of fungal co-culture the Cd2+ and Ca2+ influx is mostly the result of host properties. Therefore, the Cd2+ and Ca2+ stimulation in P. involutus-ectomycorrhizal roots reflects the enhanced root uptake ability. (3) The correlation analyses revealed that Cd2+ and Ca2+ influxes in EM roots show a significant relationship with NM roots but not with fungal mycelia under various Cd2+ treatments (shock, ST, and LT; Supplementary Figures S2 and S3). Taken together, these data suggest that the continuous flow of Cd2+ and Ca2+ in EM roots detected by NMT microelectrodes was largely driven by the host and that the fungal partner enhanced fluxes leading to enriched Cd2+ and Ca2+ concentrations.
The observed patterns of Cd2+ and Ca2+ fluxes upon Cd2+ exposure could be explained by channel-mediated ion fluxes. NMT data show that the Ca2+ flux in EM roots was negatively correlated with the Ca2+ influx in fungal hyphae upon Cd2+ shock treatment (Supplementary Figure S3). This is presumably the result of Cd2+-Ca2+ competition across the Ca2+ channels in the root PM. After being exposed to Cd2+ shock, Ca2+ entry was enhanced in the hyphae (Figure 1B). However, the fungal hyphae which were enriched in Ca2+ ions, were unable to deliver Ca2+ to the root cells because the Cd2+ ions competitively inhibited the entry of Ca2+ through the PM channels. As a result, the high influx of Ca2+ through fungal hyphae led to an apparently greater Ca2+ efflux in Cd2+-exposed EM roots (Figures 1B and 8B).
Paxillus involutus colonization enhanced the uptake of Cd2+ under shock, ST, and LT stress, compared to NM roots (Figures 1A and 2). The increased entry of Cd2+ is likely due to the activation of PM Ca2+ channels in the ectomycorrhizas. The stimulated Ca2+ influx by P. involutus inoculation revealed the activation of PM Ca2+ channels since the ectomycorrhiza-enhanced entry of Ca2+ was suppressed by Ca2+ channel blockers (LaCl3, GdCl3, verapamil, or TEA; Figure 7A, Supplementary Figures S5A, S6A, and S7A). The activated PM Ca2+ channels allowed the entry of Cd2+ in addition to Ca2+ under Cd2+ stress (Figures 1A and 2).
Hydrogen Peroxide Induced by CdCl2 and Fungal Colonization Stimulates Cd2+ Influx through PM Ca2+ Channels
After being subjected to CdCl2 exposure, NM roots displayed an increased H2O2 efflux along the root axis (Figure 6). It is well documented that Cd2+ induced accumulation of H2O2 in pine roots (Schützendübel et al., 2001), P × canescens roots (Schützendübel et al., 2002), and in suspension cultures of tobacco (Piqueras et al., 1999) and P. euphratica (Sun et al., 2013b; Han et al., 2016). H2O2 efflux was evident in EM roots irrespective of the presence or absence of Cd2+ treatments (Figure 6). Our results suggest that the Cd2+ influx through PM Ca2+ channels is stimulated by H2O2 in NM and EM roots. The experimental evidence and explanations are briefly listed here.
-
simple (1)
H2O2 (1.0 mM) exhibited an enhancement on Ca2+ influx in NM and EM roots (Figure 8B). Pei et al. (2000) showed that H2O2 (0.05–5.0 mM) activates Ca2+ currents through PM Ca2+ channels of Arabidopsis thaliana guard cells. Moreover, H2O2 increased Ca2+ influx across the PM in P. euphratica cells (Sun et al., 2010), roots of A. thaliana (Demidchik et al., 2007) and mangroves (Lu et al., 2013). Furthermore, the H2O2-stimulated entry of Ca2+ in P. euphratica cells was inhibited by LaCl3 (Sun et al., 2010). In this study, Cd2+ influx in NM and EM roots was significantly enhanced after exposure to 1.0 mM H2O2 (Figure 8A). This finding is in agreement with Sun et al. (2013b) and Han et al. (2016), who found that H2O2 (3.0 mM) stimulated entry of Cd2+ into P. euphratica cells. In addition, the Cd2+ influx was blocked by LaCl3 in CdCl2-stressed roots (Figure 7B). These results suggest that H2O2 stimulates the influx of Cd2+ and Ca2+ through Ca2+-permeable channels in the PM.
-
simple (2)
The H2O2 induction of Cd2+ resembles the pattern of Ca2+ kinetics in response to H2O2 (Figure 8). Moreover, Cd2+ and Ca2+ influx in NM and EM roots were both suppressed by the ROS scavenger, DMTU (Figure 9). Similarly, Sun et al. (2013b) showed that the entry of Cd2+ into P. euphratica cells was reduced when a H2O2 scavenger, catalase, was applied.
Taken together, these results suggest that Cd2+ and Ca2+ ions enter NM and EM roots by the same pathway involving PM Ca2+ channels that are activated by Cd2+-elicited H2O2.
The high Cd2+ influx in EM roots resulted from the pronounced activation of PM Ca2+ channels that were stimulated, at least in part, by the fungal-elicited H2O2. Compared to NM roots, MAJ- and NAU-ectomycorrhizal roots displayed a significant higher H2O2 efflux in the absence of Cd2+ stress (Figure 6), suggesting that the inoculation with P. involutus caused a strong production of H2O2 in EM roots. This finding agrees with Gafur et al. (2004) and Langenfeld-Heyser et al. (2007), who detected strong H2O2 accumulation in the outer hyphae mantle of compatible (MAJ) and incompatible (NAU) interactions. H2O2 production in the hyphae is suggested to regulate host’s root growth, defense against other invading microbes, and increasing plant-innate immunity (Salzer et al., 1999; Gafur et al., 2004). In our study, H2O2 produced in the ectomycorrhizae accelerated the influx of Ca2+ in the absence of Cd2+, whereas it increased entry of Cd2+ in the presence of high external Cd2+ (Figure 8). ROS scavenging by DMTU simultaneously decreased Ca2+ and Cd2+ influxes along the root axis of EM plants (Figure 9). These observations suggest that H2O2 produced in compatible (MAJ) and incompetent (NAU) ectomycorrhizal associations activated Ca2+ permeable channels, which allowed the entry of Cd2+ under Cd2+ stress.
We noticed that the H2O2 efflux in MAJ and NAU-ectomycorrhizal roots was lowered by Cd2+ stress (Figure 6). This reduction may have resulted from the activation of antioxidant enzymes and increased amounts of ROS scavengers produced as a defense response. It has been repeatedly shown that the antioxidant enzyme activities are activated under heavy metal stresses (Schützendübel et al., 2001, 2002; Rozpądek et al., 2014; Chen et al., 2015; Tan et al., 2015). The enhanced activities of superoxide dismutase, peroxidase, catalase, and ascorbate peroxidase play an important role in scavenging the Cd2+-elicited H2O2 in plants (Garg and Aggarwal, 2012; Anjum et al., 2015; Tan et al., 2015). To combat Cd2+-induced superoxide and H2O2, P. × canescens plants were found to rely mainly on antioxidant enzymes and the formation of the potential radical scavenging molecules, such as proline, sugar alcohols and soluble phenolics (He et al., 2011). However, the lowered H2O2 efflux in EM roots (Figure 6) did not reduce the Cd2+-elicited entry of Cd2+, because (i) the fungal-elicited H2O2 had already activated the Ca2+-channels before the Cd2+ addition, and/or (ii) the H2O2 level is still high enough to activate the channels under Cd2+ stress. The observation that stressed EM roots still contain high concentrations of H2O2 in the hyphae (Langenfeld-Heyser et al., 2007) supports these speculations.
PM H+-ATPase Activated by Cd2+ and Fungal Colonization Stimulates Cd2+ Influx through PM Ca2+ Channels
In addition to H2O2, PM H+-ATPase activated by Cd2+ and enhanced by fungal colonization also accelerated Cd2+ influx through PM Ca2+ channels in NM and EM roots. PM H+-ATPases pump protons into the external medium to maintain an electrochemical H+ gradient across the PM (Blumwald et al., 2000; Zhu, 2003). Krämer (2010) suggested that H+-ATPases play an important role in adaptation of plants to heavy metal stress. The finding that the net H+ efflux in fungal mycelia and EM roots was markedly reduced by a specific inhibitor of PM H+-ATPase (sodium orthovanadate) in the presence and absence of Cd2+ stress (Figure 11A, Supplementary Figure S9) supports that the vigorous H+ efflux is the consequence of H+-ATPase activity. Accordingly, the increased H+ efflux upon Cd2+ shock (NM, MAJ and NAU roots; Figure 1C), ST (MAJ and NAU roots; Figure 5) and LT stress (NM roots; Figure 5) indicates the activated H+-pumping activity. In NM and EM roots, Cd2+ exposure led to a marked upregulation of HA2.1 and AHA10.1, two important genes encoding PM H+-ATPases (Ma Y. et al., 2014). The activation of PM H+-ATPase by Cd2+ is likely associated with the Cd2+-elicited H2O2, since (i) H2O2 increased H+ pumping activity in P. euphratica callus cells (Sun et al., 2010), in roots of P. euphratica (Sun et al., 2010) and secretor and non-secretor mangrove species (Lu et al., 2013; Lang et al., 2014), and (ii) the expression of genes encoding PM H+-ATPase are stimulated by H2O2 in Cucumis sativus roots (Janicka-Russak and Kabala, 2012; Janicka-Russak et al., 2012).
The activated PM H+-ATPase enabled NM and EM roots to maintain an acidic environment, which favors the entry of Cd2+ across the PM (Figure 10A). Similarly, He et al. (2015) showed that pH 5.5 accelerates Cd2+ influx into poplar roots compared to pH 4.0 or pH 7.0. Moreover, the Cd2+ influx was markedly suppressed by the application of sodium vanadate, an inhibitor of PM H+-ATPase (Figure 11B). These results indicate that the PM H+-pumps play a crucial role in enhancing the entry of Cd2+ (Ma Y. et al., 2014). Accordingly, NMT profiles of NM and EM roots showed that the maximum influx of Ca2+ was observed at pH 5.2 (Figure 10B), and that Ca2+ influx was blocked by sodium vanadate (Figure 11C). Therefore, we infer that Cd2+ activated H+-pumping in the PM, which led to hyperpolarization of the PM and increased Cd2+ influx through hyperpolarization-activated Ca2+ channels (HACCs). However, at present we cannot exclude the possibility that Cd2+ ions also penetrated through depolarization-activated (DACCs) and voltage-independent Ca2+ channels (VICCs), because the inhibitor of PM H+-ATPase, sodium vanadate, could not fully block the Cd2+ influx in NM and EM roots (Figure 11B). It has been shown that NSCCs co-exist with HACCs in the root cell plasma membrane to mediate the entry of Ca2+, but the two Ca2+ influx routes differ in their voltage sensitivity (Demidchik et al., 2002).
Ectomycorrhizal Populus × canescens show highly activated H+-pumping activity in the PM, which favors the Cd2+ influx through HACCs. Our NMT data showed that colonization of P. × canescens with P. involutus caused a marked H+ efflux (Figures 1C, 5, and 11A), suggesting that the fungal colonization could activate the PM H+-ATPase in ectomycorrhizas. This is consistent to our previous studies (Li J. et al., 2012; Ma X. et al., 2014). It has been documented that some host PM H+-ATPase isoforms show high activity in arbuscular mycorrhizal associations (Ramos et al., 2005; Rosewarne et al., 2007). Obviously, H+-pumping activity was activated by Cd2+ shock and ST exposure, as the H+ efflux in MAJ- and NAU-ectomycorrhizal roots were significantly higher than the NM roots (Figures 1C and 5). Increased abundance of HA2.1 and AHA10.1 encoding PM H+-ATPase in ectomycorrhizas compared to NM roots of P. × canescens were suggested to lead to higher activities of PM H+-ATPases (Ma Y. et al., 2014). The highly activated PM H+-ATPase, on the one hand maintains a more suitable acidic environment to promote the Cd2+ and Ca2+ influx across the PM (Figure 10) and on the other hand, provides an electrochemical H+ gradient for PM hyperpolarization, thus increasing Cd2+ influx via HACCs. Accordingly, the Cd2+-stimulated Cd2+ and Ca2+ in the P. involutus mycelia (Figures 1–3) was associated with the activated H+ pumps since Cd2+ treatment markedly upregulated the transcription of PM H+-ATPase 1 (Jacob et al., 2004).
Importantly, the H2O2 produced in the ectomycorrhizal associations may accelerate the Cd2+ through the PM H+-ATPase-mediated HACCs. Whole-cell patch clamp recordings of Arabidopsis guard cells showed that the PM hyperpolarization only activates Ca2+ currents in the presence of H2O2 (50 μM to 5 mM), and the Ca2+ current amplitudes increase with increasing H2O2 concentrations (Pei et al., 2000). Demidchik et al. (2007) showed that application of H2O2 (10 mM) to the external PM face of elongation zone epidermal protoplasts resulted in the appearance of a hyperpolarization-activated Ca2+ permeable conductance. In mature epidermal protoplasts, PM HACCs were activated only when H2O2 was present at the intracellular membrane face, and channel opening probability increased with intracellular H2O2 concentrations at hyperpolarized voltages (Demidchik et al., 2007). A massive presence of H2O2 was demonstrated in the outer hyphae mantle of P. involutus symbiosis (Gafur et al., 2004; Langenfeld-Heyser et al., 2007) and obviously could be released from the hyphae into the surrounding medium (Figure 6). Therefore, we suppose that in ectomycorrhizal P. × canescens, H2O2 elicited by fungal colonization stimulated Cd2+ influx through the HACCs that had been activated by P. involutus colonization. In addition, we found that Cd2+ influx in NAU-roots was less restricted than in MAJ-roots by DMTU and sodium orthvanadate (Figures 9B and 11B). The difference in the sensitivity to antagonists of H2O2 and PM H+-ATPase indicates the involvement of voltage-independent Ca2+ channels (VICCs) in the mediation of Cd2+ uptake in NAU-roots, in addition to the dominant Cd2+ entry through HACCs.
We noticed that LT stress in hydroponic conditions caused a pronounced shift of H+ efflux toward an influx in EM roots (Figure 5). LT-stressed P. involutus mycelia exhibited a trend similar to that in EM roots (Figure 5). These results imply that ectomycorrhization activated an H+/Cd2+ antiport to reduce excessive Cd2+ uptake and accumulation under prolonged stress conditions (Sun et al., 2013b). Similarly, we have previously shown that NaCl-treated P. euphratica roots retain an active PM Na+/H+ antiport to avoid the excessive buildup of Na+ when exposed to LT salinity (Sun et al., 2009a,b). Here, the rate of H+/Cd2+ antiport could not be determined, because our NMT data only show the net flux of the target element across the PM, instead of an unidirectional flux. In addition, EM roots were able to avoid the ROS burst in Cd2+ environments (Figure 6), probably because these roots were characterized by elevated H2O2 production (Gafur et al., 2004). Therefore, EM roots are likely to control the Cd2+ influx through the H2O2-activated PM Ca2+ channels, thus avoiding an excessive accumulation of the heavy metal ions under prolonged period of Cd2+ stress.
Conclusion
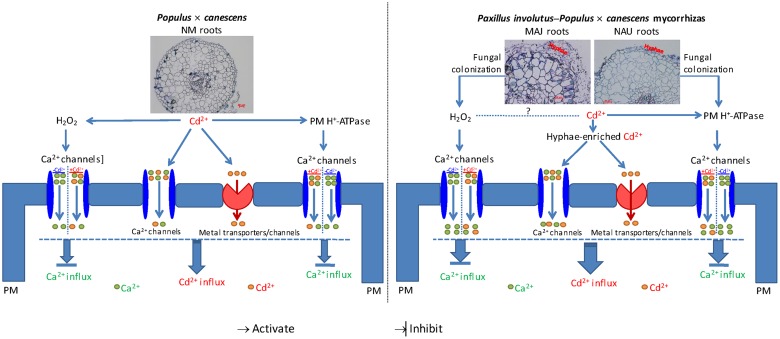
High external Cd2+ facilitates the rapid movement of Cd2+ along its electrochemical gradient into fungal and plant cells. Based on pharmacological evidence, we conclude that Cd2+ ions mainly penetrated the ectomycorrhizal fungal hyphae and poplar roots through PM Ca2+ channels. Because the entry of Cd2+ could not be fully blocked by various Ca2+ channel inhibitors (LaCl3, GdCl3, verapamil, and TEA), our results indicate that Cd2+ ions also entered the root and fungal cells through other metal transporters or channels. Our flux measurements show that the Cd2+-permeable Ca2+ channels were activated by H2O2 and H+-pumping activity. Altogether based on the current and literature data, we propose a signaling pathway that triggers Ca2+-channel-mediated Cd2+ influx in NM P. × canescens roots and explains the pronounced Cd2+ stimulation in ectomycorrhizal associations under Cd2+ stress. As shown in Figure 12, the Cd2+-elicited H2O2 and active H+-pumps favored the Cd2+ influx through Ca2+ channels in NM roots and P. involutus-ectomycorrhiza, while these channels mediate Ca2+ influx in the absence of Cd2+ stress. In ectomycorrhizas, Cd2+ enriched in hyphae is thought to be delivered to the host roots. Moreover, the colonization of P. × canescens roots with the fungal strains MAJ and NAU stimulates H2O2 production and increases H+-pumping activity, and thus accelerates Cd2+ entry through Ca2+ channels, in particular through HACCs, under excessive Cd2+. Cd2+ ions competitively enter Ca2+ channels, and thus diminish the entry of Ca2+, leading to a marked Cd2+ enrichment in ectomycorrhizal roots under Cd2+ stress.
FIGURE 12.
Schematic models showing Cd2+ influx through plasma membrane (PM) Ca2+ channels that stimulated by H2O2 and H+-ATPase in Paxillus involutus-ectomycorrhizal (MAJ and NAU) and non-mycorrhizal (NM) Populus × canescens roots under Cd2+ stress. High external Cd2+ facilitates the rapid movement of Cd2+ along its electrochemical gradient into fungal and plant cells. Cd2+ ions penetrated the ectomycorrhizal fungal hyphae and poplar roots through PM Ca2+ channels and other metal transporters or channels. The PM Ca2+ channels mediate the entry of Ca2+ in the absence of Cd2+ (-Cd) while allow the entry of Cd2+ in the presence of Cd2+ ions (+Cd). The Cd2+-permeable Ca2+ channels were activated by H2O2 and H+-pumping activity. Thus the Cd2+-elicited H2O2 and active H+-pumps favored the Cd2+ influx through Ca2+ channels in NM roots and P. involutus-ectomycorrhizas. In ectomycorrhizas, Cd2+ enriched in hyphae is thought to be delivered to the host roots. Moreover, the colonization of P. × canescens roots with the fungal strains MAJ and NAU stimulates H2O2 production and increases H+-pumping activity, and thus accelerates Cd2+ entry through Ca2+ channels under excessive Cd2+. Cd2+ ions competitively enter Ca2+ channels, and thus diminish the entry of Ca2+, leading to a marked Cd2+ enrichment in ectomycorrhizal roots under Cd2+ stress.
Author Contributions
YhZ and SC conceived the original screening and research plans; SC supervised the experiments; YhZ, GS, YnZ, ZZ, and NL performed most of the experiments; SD, JS, JL, JY, NZ, RZ, and XM provided technical assistance to YhZ, GS and YnZ; YhZ designed the experiments and analyzed the data; YhZ conceived the project and wrote the article with contributions of all the authors; SC and AP supervised and complemented the writing. All authors have read and approved the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Ms. Christine Kettner (Georg-August Universität Göttingen) and Dr. Ulrike Lipka (Georg-August Universität Göttingen) for their excellent technical assistance.
Funding. The research was supported jointly by the Research Project of the Chinese Ministry of Education (grant no. 113013A), the German Science Foundation (DFG) to AP, the Guest Lecturer Scheme of Georg-August Universität Göttingen (Germany), the Alexander von Humboldt-Stiftung (Germany), travel grants by the Bundesministerium für Ernährung, Landwirtschaft und Verbraucherschutz (BMELV), the National Natural Science Foundation of China (grant nos. 31570587, 31270654), the key project for Oversea Scholars by the Ministry of Human Resources and Social Security of PR China (grant no. 2012001), the Program for Changjiang Scholars and Innovative Research Teams in University (grant no. IRT13047), the Program of Introducing Talents of Discipline to Universities (111 Project, grant no. B13007), and Basic and Frontier Research Plan of Henan Province (No. 132300410399).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01975/full#supplementary-material
References
- Anjum S. A., Tanveer M., Hussain S., Bao M., Wang L., Khan I., et al. (2015). Cadmium toxicity in Maize (Zea mays L.): consequences on antioxidative systems, reactive oxygen species and cadmium accumulation. Environ. Sci. Pollut. Res. 22 17022–17030. 10.1007/s11356-015-4882-z [DOI] [PubMed] [Google Scholar]
- Baliardini C., Meyer C. L., Salis P., Saumitou-Laprade P., Verbruggen N. (2015). CATION EXCHANGER1 cosegregates with cadmium tolerance in the metal hyperaccumulator Arabidopsis halleri and plays a role in limiting oxidative stress in Arabidopsis Spp. Plant Physiol. 169 549–559. 10.1104/pp.15.01037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum C., Hrynkiewicz K., Leinweber P., Meißner R. (2006). Heavy-metal mobilization and uptake by mycorrhizal and nonmycorrhizal willows (Salix × dasyclados). J. Plant. Nutr. Soil Sci. 169 516–522. 10.1002/jpln.200521925 [DOI] [Google Scholar]
- Beritognolo I., Piazzai M., Benucci S., Kuzminsky E., Sabatti M., Mugnozza G. S., et al. (2007). Functional characterisation of three Italian Populus alba L. genotypes under salinity stress. Trees 21 465–477. 10.1007/s00468-007-0139-x [DOI] [Google Scholar]
- Blaudez D., Botton B., Chalot M. (2000). Cadmium uptake and subcellular compartmentation in the ectomycorrhizal fungus Paxillus involutus. Microbiology 146 1109–1117. 10.1099/00221287-146-5-1109 [DOI] [PubMed] [Google Scholar]
- Blumwald E., Aharon G. S., Apse M. P. (2000). Sodium transport in plant cells. Biochim. Biophys. Acta 1465 140–151. 10.1016/S0005-2736(00)00135-8 [DOI] [PubMed] [Google Scholar]
- Chen L., Hu X., Yang W., Xu Z., Zhang D., Gao S. (2015). The effects of arbuscular mycorrhizal fungi on sex-specific responses to Pb pollution in Populus cathayana. Ecotoxicol. Environ. Saf. 113 460–468. 10.1016/j.ecoenv.2014.12.033 [DOI] [PubMed] [Google Scholar]
- Chen S., Hawighorst P., Sun J., Polle A. (2014). Salt tolerance in Populus: significance of stress signaling networks, mycorrhization, and soil amendments for cellular and whole-plant nutrition. Environ. Exp. Bot. 107 113–124. 10.1016/j.envexpbot.2014.06.001 [DOI] [Google Scholar]
- Chen S., Polle A. (2010). Salinity tolerance of Populus. Plant Biol. 12 317–333. 10.1111/j.1438-8677.2009.00301.x [DOI] [PubMed] [Google Scholar]
- Chung J. S., Zhu J. K., Bressan R. A., Hasegawa P. M., Shi H. (2008). Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. Plant J. 53 554–565. 10.1111/j.1365-313X.2007.03364.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S. (2006). Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88 1707–1719. 10.1016/j.biochi.2006.07.003 [DOI] [PubMed] [Google Scholar]
- Clemens S., Antosiewicz D. M., Ward J. M., Schachtman D. P., Schroeder J. I. (1998). The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proc. Natl. Acad. Sci. U.S.A. 95 12043–12048. 10.1073/pnas.95.20.12043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. K., Fox T. C., Garvin D. F., Kochian L. V. (1998). The role of iron-deficiency stress responses in stimulating heavy-metal transport in plants. Plant Physiol. 116 1063–1072. 10.1104/pp.116.3.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. J., Hedrich R. (1991). Stretch-activated chloride, potassium, and calcium channels coexisting in plasma membranes of guard cells of Vicia faba L. Planta 186 143–153. 10.1007/BF00201510 [DOI] [PubMed] [Google Scholar]
- Cosio C., Martinoia E., Keller C. (2004). Hyperaccumulation of cadmium and zinc in Thlaspi caerulescens and Arabidopsis halleri at the leaf cellular level. Plant Physiol. 134 716–725. 10.1104/pp.103.031948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courbot M., Diez L., Ruotolo R., Chalot M., Leroy P. (2004). Cadmium-responsive thiols in the ectomycorrhizal fungus Paxillus involutus. Appl. Environ. Microb. 70 7413–7417. 10.1128/AEM.70.12.7413-7417.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DalCorso G., Farinati S., Maistri S., Furini A. (2008). How plants cope with cadmium: staking all on metabolism and gene expression. J. Integr. Plant Biol. 50 1268–1280. 10.1111/j.1744-7909.2008.00737.x [DOI] [PubMed] [Google Scholar]
- Danielsen L., Lohaus G., Sirrenberg A., Karlovsky P., Bastien C., Pilate G., et al. (2013). Ectomycorrhizal colonization and diversity in relation to tree biomass and nutrition in a plantation of transgenic poplars with modified lignin biosynthesis. PLoS ONE 8:e59207 10.1371/journal.pone.0059207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen L., Thürmer A., Meinicke P., Buee M., Morin E., Martin F., et al. (2012). Fungal soil communities in a young transgenic poplar plantation form a rich reservoir for fungal root communities. Ecol. Evol. 2 1935–1948. 10.1002/ece3.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V., Bowen H. C., Maathuis F. J., Shabala S. N., Tester M. A., White P. J., et al. (2002). Arabidopsis thaliana root non-selective cation channels mediate calcium uptake and are involved in growth. Plant J. 32 799–808. 10.1046/j.1365-313X.2002.01467.x [DOI] [PubMed] [Google Scholar]
- Demidchik V., Shabala S. N., Davies J. M. (2007). Spatial variation in H2O2 response of Arabidopsis thaliana root epidermal Ca2+ flux and plasma membrane Ca2+ channels. Plant J. 49 377–386. 10.1111/j.1365-313X.2006.02971.x [DOI] [PubMed] [Google Scholar]
- Demidchik V., Shang Z., Shin R., Thompson E., Rubio L., Laohavisit A., et al. (2009). Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J. 58 903–913. 10.1111/j.1365-313X.2009.03830.x [DOI] [PubMed] [Google Scholar]
- Duplessis S., Courty P. E., Tagu D., Martin F. (2005). Transcript patterns associated with ectomycorrhiza development in Eucalyptus globulus and Pisolithus microcarpus. New Phytol. 165 599–611. 10.1111/j.1469-8137.2004.01248.x [DOI] [PubMed] [Google Scholar]
- Gafur A., Schützendübel A., Langenfeld-Heyser R., Fritz E., Polle A. (2004). Compatible and incompetent Paxillus involutus isolates for ectomycorrhiza formation in vitro with poplar (Populus × canescens) differ in H2O2 production. Plant Biol. 6 91–99. 10.1055/s-2003-44718 [DOI] [PubMed] [Google Scholar]
- Gallego S. M., Pena L. B., Barcia R. A., Azpilicueta C. E., Iannone M. F., Rosales E. P., et al. (2012). Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ. Exp. Bot. 83 33–46. 10.1016/j.envexpbot.2012.04.006 [DOI] [Google Scholar]
- Garg N., Aggarwal N. (2012). Effect of mycorrhizal inoculations on heavy metal uptake and stress alleviation of Cajanus cajan (L.) Millsp. genotypes grown in cadmium and lead contaminated soils. Plant Growth Regul. 66 9–26. 10.1007/s10725-011-9624-8 [DOI] [Google Scholar]
- Gelli A., Blumwald E. (1997). Hyperpolarization-activated Ca2+-permeable channels in the plasma membrane of tomato cells. J. Membr. Biol. 155 35–45. 10.1007/s002329900156 [DOI] [PubMed] [Google Scholar]
- Grabov A., Blatt M. R. (1998). Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc. Natl. Acad. Sci. U.S.A. 95 4778–4783. 10.1007/s002329900156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A., Blatt M. R. (1999). A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol. 119 277–288. 10.1104/pp.119.1.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussarsson M., Asp H., Adalsteinsson S., Jensén P. (1996). Enhancement of cadmium effects on growth and nutrient composition of birch (Betula pendula) by buthionine sulphoximine (BSO). J. Exp. Bot. 47 211–215. 10.1093/jxb/47.2.211 [DOI] [Google Scholar]
- Han Y., Sa G., Sun J., Shen Z., Zhao R., Ding M., et al. (2014). Overexpression of Populus euphratica xyloglucan endotransglucosylase/hydrolase gene confers enhanced cadmium tolerance by the restriction of root cadmium uptake in transgenic tobacco. Environ. Exp. Bot. 100 74–83. 10.1016/j.envexpbot.2013.12.021 [DOI] [Google Scholar]
- Han Y., Wang S., Zhao N., Deng S., Zhao C., Li N., et al. (2016). Exogenous abscisic acid alleviates cadmium toxicity by restricting Cd2+ influx in Populus euphratica cells. J. Plant Growth Regul. 35 827–837. 10.1007/s00344-016-9585-2 [DOI] [Google Scholar]
- He J., Li H., Luo J., Ma C., Li S., Qu L., et al. (2013). A transcriptomic network underlies microstructural and physiological responses to cadmium in Populus × canescens. Plant Physiol. 162 424–439. 10.1104/pp.113.215681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Li H., Ma C., Zhang Y., Polle A., Rennenberg H., et al. (2015). Overexpression of bacterial γ-glutamylcysteine synthetase mediates changes in cadmium influx, allocation and detoxification in poplar. New Phytol. 205 240–254. 10.1111/nph.13013 [DOI] [PubMed] [Google Scholar]
- He J., Qin J., Long L., Ma Y., Li H., Li K., et al. (2011). Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus × canescens. Physiol. Plant. 143 50–63. 10.1111/j.1399-3054.2011.01487.x [DOI] [PubMed] [Google Scholar]
- Hirschi K. D., Korenkov V. D., Wilganowski N. L., Wagner G. J. (2000). Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation and increased manganese tolerance. Plant Physiol. 124 125–134. 10.1104/pp.124.1.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C., Courbot M., Brun A., Steinman H. M., Jacquot J. P., Botton B., et al. (2001). Molecular cloning, characterization and regulation by cadmium of a superoxide dismutase from the ectomycorrhizal fungus Paxillus involutus. Eur. J. Biochem. 268 3223–3232. 10.1046/j.1432-1327.2001.02216.x [DOI] [PubMed] [Google Scholar]
- Jacob C., Courbot M., Martin F., Brun A., Chalot M. (2004). Transcriptomic responses to cadmium in the ectomycorrhizal fungus Paxillus involutus. FEBS Lett. 576 423–427. 10.1016/j.febslet.2004.09.028 [DOI] [PubMed] [Google Scholar]
- Janicka-Russak M., Kabala K. (2012). Abscisic acid and hydrogen peroxide induce modification of plasma membrane H+-ATPase from Cucumis sativus L. roots under heat shock. J. Plant Physiol. 169 1607–1614. 10.1016/j.jplph.2012.05.013 [DOI] [PubMed] [Google Scholar]
- Janicka-Russak M., Kabala K., Wdowikowska A., Klobus G. (2012). Response of plasma membrane H+-ATPase to low temperature in cucumber roots. J. Plant Res. 125 291–300. 10.1007/s10265-011-0438-6 [DOI] [PubMed] [Google Scholar]
- Kaplan O., Ince M., Yaman M. (2011). Sequential extraction of cadmium in different soil phases and plant parts from a former industrialized area. Environ. Chem. Lett. 9 397–404. 10.1007/s10311-010-0292-0 [DOI] [Google Scholar]
- Kieffer P., Schröder P., Dommes J., Hoffmann L., Renaut J., Hausman J. F. (2009). Proteomic and enzymatic response of poplar to cadmium stress. J. Proteomics 72 379–396. 10.1016/j.jprot.2009.01.014 [DOI] [PubMed] [Google Scholar]
- Kim D. Y., Bovet L., Kushnir S., Noh E. W., Martinoia E., Lee Y. (2006). AtATM3 is involved in heavy metal resistance in Arabidopsis. Plant Physiol. 140 922–932. 10.1104/pp.105.074146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer U. (2010). Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 61 517–534. 10.1146/annurev-arplant-042809-112156 [DOI] [PubMed] [Google Scholar]
- Krpata D., Fitz W., Peintner U., Langer I., Schweiger P. (2009). Bioconcentration of zinc and cadmium in ectomycorrhizal fungi and associated aspen trees as affected by level of pollution. Environ. Pollut. 157 280–286. 10.1016/j.envpol.2008.06.038 [DOI] [PubMed] [Google Scholar]
- Krpata D., Peintner U., Langer I., Fitz W. J., Schweiger P. (2008). Ectomycorrhizal communities associated with Populus tremula growing on a heavy metal contaminated site. Mycol. Res. 112 1069–1079. 10.1016/j.mycres.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Lang T., Sun H., Li N., Lu Y., Shen Z., Jing X., et al. (2014). Multiple signaling networks of extracellular ATP, hydrogen peroxide, calcium, and nitric oxide in the mediation of root ion fluxes in secretor and non-secretor mangroves under salt stress. Aquat. Bot. 119 33–43. 10.1016/j.aquabot.2014.06.009 [DOI] [Google Scholar]
- Langenfeld-Heyser R., Gao J., Ducic T., Tachd P., Lu C. F., Fritz E., et al. (2007). Paxillus involutus mycorrhiza attenuate NaCl-stress responses in the salt-sensitive hybrid poplar Populus × canescens. Mycorrhiza 17 121–131. 10.1007/s00572-006-0084-3 [DOI] [PubMed] [Google Scholar]
- Leple J. C., Brasileiro A. C. M., Michel M. F., Delmotte F., Jouanin L. (1992). Transgenic poplars: expression of chimeric genes using four different constructs. Plant Cell Rep. 11 137–141. 10.1007/BF00232166 [DOI] [PubMed] [Google Scholar]
- Li J., Bao S., Zhang Y., Ma X., Mishra-Knyrim M., Sun J., et al. (2012). Paxillus involutus strains MAJ and NAU mediate K+/Na+ homeostasis in ectomycorrhizal Populus × canescens under sodium chloride stress. Plant Physiol. 159 1771–1786. 10.1104/pp.112.195370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Liu X., Peijnenburg W. J., Zhao J., Chen X., Yu J., et al. (2012a). Pathways of cadmium fluxes in the root of the halophyte Suaeda salsa. Ecotoxicol. Environ. Saf. 75 1–7. 10.1016/j.ecoenv.2011.09.007 [DOI] [PubMed] [Google Scholar]
- Li L., Liu X., You L., Zhang L., Zhao J., Wu H. (2012b). Uptake pathways and subcellular fractionation of Cd in the polychaete Nereis diversicolor. Ecotoxicology 21 104–110. 10.1007/s10646-011-0770-6 [DOI] [PubMed] [Google Scholar]
- Lin Y. F., Aarts M. G. (2012). The molecular mechanism of zinc and cadmium stress response in plants. Cell Mol. Life. Sci. 69 3187–3206. 10.1007/s00018-012-1089-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. F., Supek F., Nelson N., Culotta V. C. (1997). Negative control of heavy metal uptake by the Saccharomyces cerevisiae BSD2 gene. J. Biol. Chem. 272 11763–11769. 10.1074/jbc.272.18.11763 [DOI] [PubMed] [Google Scholar]
- Lu L., Tian S., Zhang M., Zhang J., Yang X., Jiang H. (2010). The role of Ca pathway in Cd uptake and translocation by the hyperaccumulator Sedum alfredii. J. Hazard. Mater. 183 22–28. 10.1016/j.jhazmat.2010.06.036 [DOI] [PubMed] [Google Scholar]
- Lu Y., Li N., Sun J., Hou P., Jing X., Zhu H., et al. (2013). Exogenous hydrogen peroxide, nitric oxide and calcium mediate root ion fluxes in two non-secretor mangrove species subjected to NaCl stress. Tree Physiol. 33 81–95. 10.1093/treephys/tps119 [DOI] [PubMed] [Google Scholar]
- Luo J., Qin J., He F., Li H., Liu T., Polle A., et al. (2013). Net fluxes of ammonium and nitrate in association with H+ fluxes in fine roots of Populus popularis. Planta 237 919–931. 10.1007/s00425-012-1807-7 [DOI] [PubMed] [Google Scholar]
- Luo Z. B., Janz D., Jiang X., Göbel C., Wildhagen H., Tan Y., et al. (2009). Upgrading root physiology for stress tolerance by ectomycorrhizas: insights from metabolite and transcriptional profiling into reprogramming for stress anticipation. Plant Physiol. 151 1902–1917. 10.1104/pp.109.143735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z. B., Li K., Gai Y., Göbel C., Wildhagen H., Jiang X., et al. (2011). The ectomycorrhizal fungus (Paxillus involutus) modulates leaf physiology of poplar towards improved salt tolerance. Environ. Exp. Bot. 72 304–311. 10.1016/j.envexpbot.2011.04.008 [DOI] [Google Scholar]
- Luo Z. B., Wu C., Zhang C., Li H., Lipka U., Polle A. (2014). The role of ectomycorrhizas in heavy metal stress tolerance of host plants. Environ. Exp. Bot. 108 47–62. 10.1016/j.envexpbot.2013.10.018 [DOI] [Google Scholar]
- Ma X., Deng L., Li J., Zhou X., Li N., Zhang D., et al. (2010). Effect of NaCl on leaf H+-ATPase and the relevance to salt tolerance in two contrasting poplar species. Trees 24 597–607. 10.1007/s00468-010-0430-0 [DOI] [Google Scholar]
- Ma X., Sun M., Sa G., Zhang Y., Li J., Sun J., et al. (2014). Ion fluxes in Paxillus involutus-inoculated roots of Populus × canescens under saline stress. Environ. Exp. Bot. 108 99–108. 10.1016/j.envexpbot.2013.11.016 [DOI] [Google Scholar]
- Ma Y., He J., Ma C., Luo J., Li H., Liu T., et al. (2014). Ectomycorrhizas with Paxillus involutus enhance cadmium uptake and tolerance in Populus × canescens. Plant Cell Environ. 37 627–642. 10.1111/pce.12183 [DOI] [PubMed] [Google Scholar]
- Mendoza-Cózatl D. G., Jobe T. O., Hauser F., Schroeder J. I. (2011). Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr. Opin. Plant Biol. 14 554–562. 10.1016/j.pbi.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon A., Blaudez D., Wilkins O., Montanini B., Campbell M. M., Richaud P., et al. (2010). Genome-wide analysis of plant metal transporters, with an emphasis on poplar. Cell. Mol. Life Sci. 67 3763–3784. 10.1007/s00018-010-0445-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner M. J., Kochian L. V. (2008). Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann. Bot 102 3–13. 10.1093/aob/mcn063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15 473–497. 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- Nawrot T., Plusquin M., Hogervorst J., Roels H. A., Celis H., Thijs L., et al. (2006). Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncol. 7 119–126. 10.1016/S1470-2045(06)70545-9 [DOI] [PubMed] [Google Scholar]
- Ott T., Fritz E., Polle A., Schützendübel A. (2002). Characterisation of antioxidative systems in the ectomycorrhiza-building basidiomycete Paxillus involutus (Bartsch) Fr. and its reaction to cadmium. FEMS Microbiol. Ecol. 42 359–366. 10.1111/j.1574-6941.2002.tb01025.x [DOI] [PubMed] [Google Scholar]
- Papoyan A., Piñeros M., Kochian L. V. (2007). Plant Cd2+ and Zn2+ status effects on root and shoot heavy metal accumulation in Thlaspi caerulescens. New Phytol. 175 51–58. 10.1111/j.1469-8137.2007.02073.x [DOI] [PubMed] [Google Scholar]
- Pei Z. M., Murata Y., Benning G., Thomine S., Klüsener B., Allen G. J., et al. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406 731–734. 10.1038/35021067 [DOI] [PubMed] [Google Scholar]
- Perfus-Barbeoch L., Leonhardt N., Vavasseur A., Forestier C. (2002). Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 32 539–548. 10.1046/j.1365-313X.2002.01442.x [DOI] [PubMed] [Google Scholar]
- Pickard B. G., Ding J. P. (1993). The mechanosensory calcium-selective ion channel: key component of a plasmalemmal control centre? Aust. J. Plant Physiol. 20 439–459. 10.1071/PP9930439 [DOI] [PubMed] [Google Scholar]
- Piñeros M., Tester M. (1997). Calcium channels in higher plant cells: selectivity, regulation and pharmacology. J. Exp. Bot. 48 551–577. 10.1093/jxb/48 [DOI] [PubMed] [Google Scholar]
- Ping Z., Yabe I., Muto S. (1992). Voltage-dependent Ca2+ channels in the plasma membrane and the vacuolar membrane of Arabidopsis thaliana. Biochim. Biophys Acta 1112 287–290. 10.1016/0005-2736(92)90404-A [DOI] [PubMed] [Google Scholar]
- Piqueras A., Olmos E., Martinez-Solano J. R., Hellin E. (1999). Cd-induced oxidative burst in tobacco BY2 cells: time course, subcellular location and antioxidant response. Free Radic. Res. 31 S33–S38. 10.1080/10715769900301291 [DOI] [PubMed] [Google Scholar]
- Plaza S., Tearall K. L., Zhao F. J., Buchner P., McGrath S. P., Hawkesford M. J. (2007). Expression and functional analysis of metal transporter genes in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. J. Exp. Bot. 58 1717–1728. 10.1093/jxb/erm025 [DOI] [PubMed] [Google Scholar]
- Polle A., Chen S. (2015). On the salty side of life: molecular, physiological and anatomical adaptation and acclimation of trees to extreme habitats. Plant Cell Environ. 38 1794–1816. 10.1111/pce.12440 [DOI] [PubMed] [Google Scholar]
- Polle A., Klein T., Kettner C. (2013). Impact of cadmium on young plants of Populus euphratica and P. × canescens, two poplar species that differ in stress tolerance. New For. 44 13–22. 10.1007/s11056-011-9301-9 [DOI] [Google Scholar]
- Ramos A. C., Martins M. A., Façanha A. R. (2005). ATPase and pyrophosphatase activities in corn root microsomes colonized with arbuscular mycorrhizal fungi. Braz. J. Plant Physiol. 29 207–213. 10.1590/S0100-06832005000200006 [DOI] [Google Scholar]
- Rodríguez-Serrano M., Romero-Puertas M. C., Pazmiño D. M., Testillano P. S., Risueno M. C., Río L. A., et al. (2009). Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 150 229–243. 10.1104/pp.108.131524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosewarne G. M., Smith F. A., Schachtman D. P., Smith S. E. (2007). Localization of proton-ATPase genes expressed in arbuscular mycorrhizal tomato plants. Mycorrhiza 17 249–258. 10.1007/s00572-006-0101-6 [DOI] [PubMed] [Google Scholar]
- Roth U., von Roepenack-Lahaye E., Clemens S. (2006). Proteome changes in Arabidopsis thaliana roots upon exposure to Cd2+. J. Exp. Bot. 57 4003–4013. 10.1093/jxb/erl170 [DOI] [PubMed] [Google Scholar]
- Rozpądek P., Wężowicz K., Stojakowska A., Malarz J., Surówka E., Sobczyk L., et al. (2014). Mycorrhizal fungi modulate phytochemical production and antioxidant activity of Cichorium intybus L. (Asteraceae) under metal toxicity. Chemosphere 112 217–224. 10.1016/j.chemosphere.2014.04.023 [DOI] [PubMed] [Google Scholar]
- Salzer P., Corbière H., Boller T. (1999). Hydrogen peroxide accumulation in Medicago truncatula roots colonized by the arbuscular mycorrhiza-forming fungus Glomus intraradices. Planta 208 319–325. 10.1007/s004250050565 [DOI] [Google Scholar]
- Schützendübel A., Nikolova P., Rudolf C., Polle A. (2002). Cadmium and H2O2-induced oxidative stress in Populus × canescens roots. Plant Physiol. Biochem. 40 577–584. 10.1016/S0981-9428(02)01411-0 [DOI] [Google Scholar]
- Schützendübel A., Polle A. (2002). Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 53 1351–1365. 10.1093/jexbot/53.372.1351 [DOI] [PubMed] [Google Scholar]
- Schützendübel A., Schwanz P., Teichmann T., Gross K., Langenfeld-Heyser R., Godbold D. L., et al. (2001). Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in scots pine roots. Plant Physiol. 127 887–898. 10.1104/pp.010318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell J., Kayser A., Schulin R., Brunner I. (2005). Contribution of ectomycorrhizal fungi to cadmium uptake of poplars and willows from a heavily polluted soil. Plant Soil 277 245–253. 10.1007/s11104-005-7084-5 [DOI] [Google Scholar]
- Shabala S., Knowles A. (2002). Rhythmic patterns of nutrient acquisition by wheat roots. Funct. Plant Biol. 29 595–605. 10.1071/PP01130 [DOI] [PubMed] [Google Scholar]
- Shabala S., Shabala L., Gradmann D., Chen Z., Newman I., Mancuso S. (2006). Oscillations in plant membrane transport: model predictions, experimental validation, and physiological implications. J. Exp. Bot. 57 171–184. 10.1093/jxb/erj022 [DOI] [PubMed] [Google Scholar]
- Shabala S., Shabala L., Van Volkenburgh E. (2003). Effect of calcium on root development and root ion fluxes in salinised barley seedlings. Funct. Plant Biol. 30 507–514. 10.1071/FP03016 [DOI] [PubMed] [Google Scholar]
- Shabala S. N., Newman I. A., Morris J. (1997). Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol. 113 111–118. 10.1104/pp.113.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N. R., Ramos M. A., Marques A. P., Castro P. M. (2012). The effect of ectomycorrhizal fungi forming symbiosis with Pinus pinaster seedlings exposed to cadmium. Sci. Total Environ. 414 63–67. 10.1016/j.scitotenv.2011.10.053 [DOI] [PubMed] [Google Scholar]
- Sun J., Chen S., Dai S., Wang R., Li N., Shen X., et al. (2009a). NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol. 149 1141–1153. 10.1104/pp.108.129494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Dai S., Wang R., Chen S., Li N., Zhou X., et al. (2009b). Calcium mediates root K+/Na+ homeostasis in poplar species differing in salt tolerance. Tree Physiol. 29 1175–1186. 10.1093/treephys/tpp048 [DOI] [PubMed] [Google Scholar]
- Sun J., Wang M. J., Ding M. Q., Deng S. R., Liu M. Q., Lu C. F., et al. (2010). H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant Cell Environ. 33 943–958. 10.1111/j.1365-3040.2010.02118.x [DOI] [PubMed] [Google Scholar]
- Sun J., Wang R., Liu Z., Ding Y., Li T. (2013a). Non-invasive microelectrode cadmium flux measurements reveal the spatial characteristics and real-time kinetics of cadmium transport in hyperaccumulator and nonhyperaccumulator ecotypes of Sedum alfredii. J. Plant Physiol. 170 355–359. 10.1016/j.jplph.2012.10.014 [DOI] [PubMed] [Google Scholar]
- Sun J., Wang R., Zhang X., Yu Y., Zhao R., Li Z., et al. (2013b). Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes in Populus euphratica cells. Plant Physiol. Biochem. 65 67–74. 10.1016/j.plaphy.2013.01.003 [DOI] [PubMed] [Google Scholar]
- Sun J., Zhang C. L., Deng S. R., Lu C. F., Shen X., Zhou X. Y., et al. (2012). An ATP signalling pathway in plant cells: extracellular ATP triggers programmed cell death in Populus euphratica. Plant Cell Environ. 35 893–916. 10.1111/j.1365-3040.2011.02461.x [DOI] [PubMed] [Google Scholar]
- Tan S. Y., Jiang Q. Y., Zhuo F., Liu H., Wang Y. T., Li S. S., et al. (2015). Effect of inoculation with Glomus versiforme on cadmium accumulation, antioxidant activities and phytochelatins of Solanum photeinocarpum. PLoS ONE 10:e0132347 10.1371/journal.pone.0132347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomine S., Wang R., Ward J. M., Crawford N. M., Schroeder J. I. (2000). Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc. Natl. Acad. Sci. U.S.A. 97 4991–4996. 10.1073/pnas.97.9.4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuleau P., Moreau M., Schroeder J. I., Ranjeva R. (1994a). Recruitment of plasma membrane voltage-dependent calcium-permeable channels in carrot cells. EMBO J. 13 5843–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuleau P., Schroeder J. I., Ranjeva R. (1998). Recent advances in the regulation of plant calcium channels: evidence for regulation by G-proteins, the cytoskeleton and second messengers. Curr. Opin. Plant Biol 1 424–427. 10.1016/S1369-5266(98)80267-7 [DOI] [PubMed] [Google Scholar]
- Thuleau P., Ward J. M., Ranjeva R., Schroeder J. I. (1994b). Voltage-dependent calcium-permeable channels in the plasma membrane of a higher plant cell. EMBO J. 13 2970–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G., Jung S. K., Messerli M. A., Smith P. J., Shirihai O. S. (2001). Real-time detection of reactive oxygen intermediates from single microglial cells. Biol. Bull. 201 261–262. 10.2307/1543355 [DOI] [PubMed] [Google Scholar]
- Weiss G. B. (1974). Cellular pharmacology of lanthanum. Annu. Rev. Pharmacol. 14 343–354. 10.1146/annurev.pa.14.040174.002015 [DOI] [Google Scholar]
- White P. J. (1998). Calcium channels in the plasma membrane of root cells. Ann. Bot. 81 173–183. 10.1006/anbo.1997.0554 [DOI] [Google Scholar]
- White P. J. (2000). Calcium channels in higher plants. Biochim. Biophys. Acta 1465 171–189. 10.1016/S0005-2736(00)00137-1 [DOI] [PubMed] [Google Scholar]
- Zhao F. J., Hamon R. E., Lombi E., McLaughlin M. J., McGrath S. P. (2002). Characteristics of cadmium uptake in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. J. Exp. Bot. 53 535–543. 10.1093/jexbot/53.368.535 [DOI] [PubMed] [Google Scholar]
- Zhao F. J., Jiang R. F., Dunham S. J., McGrath S. P. (2006). Cadmium uptake, translocation and tolerance in the hyperaccumulator Arabidopsis halleri. New Phytol. 172 646–654. 10.1111/j.1469-8137.2006.01867.x [DOI] [PubMed] [Google Scholar]
- Zhu J. K. (2003). Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 6 441–445. 10.1016/S1369-5266(03)00085-2 [DOI] [PubMed] [Google Scholar]
- Zimmermann S., Nürnberger T., Frachisse J. M., Wirtz W., Guern J., Hedrich R., et al. (1997). Receptor-mediated activation of a plant Ca2+-permeable ion channel involved in pathogen defense. Proc. Natl. Acad. Sci. U.S.A. 94 2751–2755. 10.1073/pnas.94.6.2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.