SUMMARY
Parvovirus B19 (B19V) and human bocavirus 1 (HBoV1), members of the large Parvoviridae family, are human pathogens responsible for a variety of diseases. For B19V in particular, host features determine disease manifestations. These viruses are prevalent worldwide and are culturable in vitro, and serological and molecular assays are available but require careful interpretation of results. Additional human parvoviruses, including HBoV2 to -4, human parvovirus 4 (PARV4), and human bufavirus (BuV) are also reviewed. The full spectrum of parvovirus disease in humans has yet to be established. Candidate recombinant B19V vaccines have been developed but may not be commercially feasible. We review relevant features of the molecular and cellular biology of these viruses, and the human immune response that they elicit, which have allowed a deep understanding of pathophysiology.
KEYWORDS: B19 virus, human bocavirus, parvovirus
INTRODUCTION
Parvovirus, a word derived from the Latin word “parvus,” meaning small, is the name for a family of small (∼25-nm), nonenveloped viruses. Parvoviruses have a linear and single-stranded DNA (ssDNA) genome of 5 to 6 kb, which is flanked by two terminal hairpin structures (1, 2). The first parvoviruses identified in humans were adeno-associated viruses (AAVs), which are nonpathogenic (1, 3). Later, two pathogenic parvoviruses were identified, human parvovirus B19 (B19V) and human bocavirus 1 (HBoV1). B19V was discovered in 1975 by Cossart and colleagues during screening for hepatitis B virus. The serum sample, which contained parvovirus-like particles, was coded as panel B and number 19 and hence named “parvovirus B19” (4). B19V is highly infectious and causes a wide range of pathological conditions: fifth disease in children, persistent anemia in immunocompromised patients, transient aplastic crises, hydrops fetalis in pregnant women, and arthropathy (5–7) (see “Diseases Caused by B19V Infection,” below). It should be emphasized that many B19V infections are likely asymptomatic without apparent illness after seroconversion (8). HBoV1 was first identified in respiratory nasopharyngeal aspirates of children with lower respiratory tract infections (9). HBoV1 is an important cause of acute respiratory tract infections, with wheezing being the most common symptom (10) (see “Diseases Associated with HBoV Infection,” below). Several other parvoviruses, including HBoV2 (11), HBoV3 (12), HBoV4 (13), parvovirus 4 (PARV4) (14), and human bufavirus (BuV) (15), are emerging viruses associated with human diseases of unclear clinical significance.
VIRUS TAXONOMY
Based on the type of infected host, the family Parvoviridae is divided into two subfamilies, Parvovirinae and Densovirinae, which infect vertebrates and invertebrates, respectively. A revised taxonomy of the family Parvoviridae was proposed by the International Committee on Taxonomy of Viruses (ICTV) in 2014 (16). In the revised taxonomy, parvoviruses are classified based on phylogenetic analysis of the amino acid sequence of the large nonstructural protein NS1 (16). Notably, data from sequence analyses of core capsid proteins are overall in conformity with the NS1-based classification. All the viruses in a given genus should be monophyletic, with >30% of the amino acid sequences of the NS1 proteins being identical to each other or <30% of the amino acid sequences being identical to those of the NS1 proteins of parvoviruses in other genera. Within a given species, >85% identity of the NS1 proteins is required. Based on this principle, the Parvovirinae subfamily has been divided into 8 genera: Protoparvovirus, Amdoparvovirus, Aveparvovirus, Bocaparvovirus, Dependoparvovirus, Erythroparvovirus, Copiparvovirus, and Tetraparvovirus. Parvoviruses that infect humans, discussed in this review, are B19V, HBoVs, BuV, and PARV4, which belong to the Erythroparvovirus, Bocaparvovirus, Protoparvovirus, and Tetraparvovirus genera, respectively (Table 1).
TABLE 1.
Human parvoviruses of the family Parvoviridae, subfamily Parvovirinae, discussed in this review
| Genus | Species | Member(s) |
|---|---|---|
| Bocaparvovirus | Primate bocaparvovirus 1 | HBoV1, HBoV3 |
| Primate bocaparvovirus 2 | HBoV2, HBoV4 | |
| Erythroparvovirus | Primate erythroparvovirus 1 | B19V |
| Primate erythroparvovirus 2 | SPV | |
| Protoparvovirus | Primate protoparvovirus 1 | BuV |
| Tetraparvovirus | Primate tetraparvovirus 1 | PARV4 |
BASIC VIROLOGY
Virus Structure
The parvovirus capsid comprises 60 copies of the capsid (VP) proteins that assemble into a T=1 icosahedral symmetry, with the larger protein, VP1, as a minor constituent (see “B19V entry and the role of B19V VP1u in virus entry,” below). The 3-dimensional structures of recombinant B19V VP2 and HBoV1 VP3 capsids as well as B19V native virions have been resolved by X-ray crystallography and cryoreconstruction to 3.5- to 8-Å resolutions, and they are quite similar (17–21).
The cores of the capsids are structurally similar among all parvoviruses, formed by subunits of an α-helix and an eight-stranded antiparallel β-barrel motif (21). Large insertions between the β-strands form long loops shaping the surface of the capsid. Contrary to the highly conserved core, the surface is highly variable among parvovirus species. The capsid surface is involved in many functions in the virus life cycle: specific binding to cellular receptors, intracellular trafficking with its phospholipase A2 (PLA2) activity, nuclear entry and exit, genome encapsidation, and recognition and avoidance of the host immune response (21–24).
At the capsid 5-fold axis, five β-barrels arrange to form a cylindrical structure with a wide surrounding canyon, which is less pronounced in HBoV1 (20). In HBoV1, as in many other parvoviruses, this cylinder creates an open channel that has been proposed to be a portal for genome packaging and VP1-unique region (VP1u) externalization, but this portal seems to be closed in B19V (17–20, 23), instead possibly presenting a flexible cap that may be opened upon receptor attachment (18, 25). There is a preserved depression at the 2-fold axis among the parvoviruses, whereas the 3-fold structure varies widely. Many parvoviruses contain prominent 3-fold protrusions, which in HBoV1 are much less pronounced and in B19V are depressed centrally to render smooth capsid surface topologies (18–21, 23). The B19V capsid recognizes its cellular receptor, globoside, in the region of the 3-fold depression (23), which, along with VP1u, is a major immunodominant area. Four antigenic epitopes have been mapped in the HBoV1 capsid: three HBoV1-specific epitopes have been mapped to the 3-fold protrusions and the wall between the 2- and 5-fold axes, and one HBoV1- to HBoV-4-cross-reacting epitope has been mapped to the 5-fold axis (26).
The N terminus of B19V VP1 remains unresolved, reflecting unordered differential conformations and small amounts of VP1u in the capsid. Nevertheless, from measuring the binding of neutralizing monoclonal B19V VP1u antibodies and the VP1u-associated PLA2 activity of native virions, VP1u alters its conformation after receptor attachment to become more exposed on the capsid surface (25), although this has not been structurally confirmed (17). The very tip of the VP2 N terminus is localized on the capsid surface (17, 27). The N terminus of HBoV1 VP1 has not been visualized, but contrary to VP1 of B19V, it does not contain immunodominant epitopes (28).
B19V Genome Organization and Expression
B19V genome and infectious clones.
B19V contains a linear ssDNA genome that is 5,596 nucleotides (nt) long (J35 strain [GenBank accession no. AY386330]) (29). The central coding region is flanked on both sides by identical inverted terminal repeats (ITRs) (Fig. 1A) (30). B19V is a homotelomeric parvovirus capable of packaging an equal number of minus and plus strands of the ssDNA genome in separate virus particles. The ITR is 401 nt long with an imperfect palindromic sequence that folds into a hairpin-like structure (31–33). The ITR exists in two equal configurations named “flip” and “flop,” with one being the inverted complement of the other. ITRs carry the origin of replication (Ori) and form active replication origins in double-stranded (replicative-form [RF]) DNA during viral DNA replication (34). The B19V RF DNA genome harbors a single promoter at map unit 6 (P6), with a transcription start site at nt 531 (Fig. 1B) (35, 36). A number of enhancer elements (nt 180 to 490) upstream of the P6 promoter bind the cellular transcription factors CREBP, C-Ets, GATA, YY1, and Oct-1, which strengthen P6 promoter activity (37–40). B19V NS1 binds NS1-binding elements (NSBEs) (5′-CCGGCGGC-3′) at nt 337 to 354 (41), which are located within the ITR and transactivate the P6 promoter.
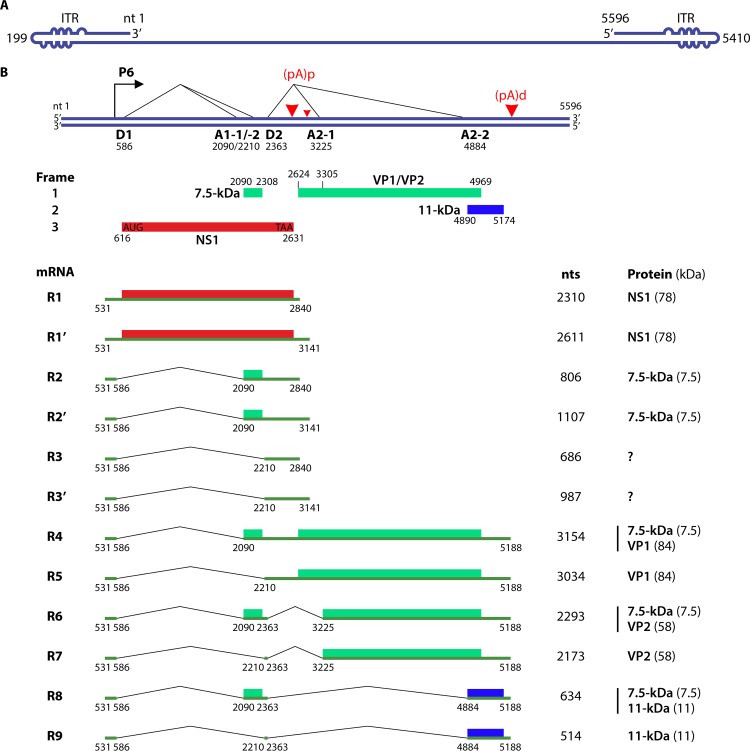
FIG 1.
B19V transcription map. (A) B19V packages a linear ssDNA genome of either positive or negative polarity. The ssDNA genome of B19V is shown in negative polarity. Two ITRs (nt 1 to 383 and nt 5214 to 5596) are diagrammed at two ends of the genome with unpaired or mismatched bases in the palindromes represented by “bulges” or “bubbles,” respectively. (B) Schematic diagram of the duplex replicative form (RF) of the B19V genome. It is capable of expressing viral genes, replicating, and producing progeny virions. The P6 promoter, the RNA initiation site, splice donor sites (D1 and D2), splice acceptor sites (A1-1, A1-2, A2-1, and A2-2), and proximal/distal polyadenylation sites [(pA)p/(pA)d] are indicated, along with the nine major mRNAs (R1 to R9) that are polyadenylated at nt 2840 of the major (pA)p site and three minor mRNAs (R1′ to R3′) that are polyadenylated at nt 3141 of the minor (pA)p site. The numbering of nucleotides is according to the numbering for the B19V J35 isolate (GenBank accession no. AY386330). Proteins encoded by each mRNA are shown on the right with detected molecular masses in kilodaltons. The ITRs and the NS1- and VP1-encoding regions are not to scale. The coding capabilities of R3 and R3′ mRNAs are unknown. The size of each mRNA is shown in nucleotides without inclusion of the poly(A) tail. Different ORFs are depicted in different-colored boxes.
A number of B19V variants, which have >11% genome sequence divergence from previously characterized B19V isolates, have been reported (42–46). B19V is now classified into three distinct genotypes, genotypes 1, 2, and 3 (see “Genotypes and Molecular Epidemiology,” below). Biological properties, at least in vitro, of the three B19V genotypes are similar (47, 48). However, genotype 2 (based on the A6 isolate) has two unique features: it uses only one splice acceptor, A1-1, to remove the first intron (Fig. 1B), and the prototype B19V ITR did not support replication of the A6 genome (49). The NS1 protein has a divergence of ∼6% between genotype 1 and genotypes 2 and 3 (48). Genotype 2 also has variations in the ITR (50). However, at present, no ITR sequence of genotype 3 has been reported. The clinical spectrum associated with genotype 2 or 3 infection is similar to that observed for genotype 1 B19V infection (42). The NS1 proteins of both genotypes 2 and 3 are potent inducers of apoptosis in B19V-permissive cells (49).
The first molecular clone of B19V was constructed without the two ITRs (51). A full-length B19V genome (J35 isolate) named plasmid pB19-M20 has been successfully cloned with the two ITRs (29). pB19-M20 replicated and produced infectious virions in B19V-semipermissive UT7/Epo-S1 cells (29) as well as in human embryonic kidney 293 (HEK293) cells when an adenoviral helper plasmid that expresses the adenoviral E2, E4orf6, and VA genes was provided (52, 53). The production of infectious progeny virions from pB19-M20-transfected UT7/Epo-S1 cells was significantly improved when cells were cultured under hypoxia (1% O2) (54), as hypoxia has been shown to enhance B19V replication (55, 56). Several full-length genomes of B19V have been sequenced (57, 58), confirming the sequence of the ITR. In addition, two full-length B19V genome clones, pB19-FL (NAN isolate) and pB19-HG1 (HV isolate), were constructed and could replicate in UT7/Epo-S1 cells (57). However, a point mutation in the VP1 PLA2 motif of pB19-FL inhibits the production of infectious progeny virions, even though the mutation was present in the wild-type virus in viremic blood.
B19V transcription.
The B19V genome consists of the large nonstructural protein (NS1) and the capsid protein (VP1/2) genes at the left and right sides, respectively. In addition, the B19V genome contains two genes that encode small nonstructural proteins, a 7.5-kDa protein in the middle and a 11-kDa protein at the right end. B19V transcription uses the single promoter P6 to transcribe a single precursor mRNA (pre-mRNA). In total, 12 mature mRNA transcripts are generated from alternative splicing and polyadenylation of the single pre-mRNA (59–61) (Fig. 1). There are two polyadenylation sites, proximal and distal [(pA)p and (pA)d, respectively]. (pA)p consists of the (pA)p1 and (pA)p2 sites, which account for internal polyadenylation of 90% and 10%, respectively (Fig. 1B, large and small arrowheads) (60). B19V pre-mRNA harbors two introns with alternative splice acceptor sites. Both the unspliced mRNAs and the mRNA transcripts that are spliced only at the A1-1 acceptor, which are polyadenylated at (pA)p, encode the large nonstructural protein NS1 and a small nonstructural protein of 7.5 kDa, respectively (62) (R1/R1′ and R2/R2′) (Fig. 1B). The first intron is spliced out from all the mRNA transcripts that are polyadenylated at (pA)d. The mRNAs that are polyadenylated at (pA)d and splice out the first intron encode capsid protein VP1 (R4 and R5) (Fig. 1B). The mRNAs that are polyadenylated at (pA)d and excise both the first intron and the second small intron (D2 to A2-1) encode VP2 (R6 and R7) (Fig. 1B), and the mRNAs that are polyadenylated at (pA)d and excise both the first and the second large introns (D2 to A2-2) encode the 11-kDa protein (R8 and R9) (Fig. 1B) (63). The coding capability and function of the small viral mRNAs spliced at the D1-to-A1-2 intron are unknown (R3/R3′) (Fig. 1B). Notably, the majority of viral mRNAs generated during B19V infection comprise the small 0.6- to 1.2-kb mRNAs (R2/2′, R3/R3′, R8, and R9) (Fig. 1B) (59, 64).
B19V gene expression regulation.
B19V RNA transcripts are alternatively spliced from a single pre-mRNA transcript and alternatively polyadenylated (59, 60) (Fig. 1), and therefore, the relative abundance of processed mRNA transcripts varies considerably and depends on the efficiency of splicing and polyadenylation.
(i) Internal polyadenylation controls production of VP- and 11-kDa protein-encoding mRNAs.
Differential expression of VP- and NS1-encoding RNAs has been observed in B19V-permissive and -nonpermissive cells. In nonpermissive cells, all the mRNAs are polyadenylated at (pA)p, and thus, mRNAs encoding capsid proteins are limited. However, in permissive cells, most B19V mRNAs read through the (pA)p sites and produce VP- and 11-kDa protein-encoding mRNAs (65, 66). Early blockade of the production of full-length B19V mRNA transcripts has been identified as a mechanism of B19V tropism (65). In permissive cells, a block in the production of the full-length mRNA transcripts is overcome by the replication of the viral genome (66). A careful quantitation of different species of viral mRNAs in B19V-infected primary CD36+ human erythroid progenitor cells (EPCs) revealed two distinct patterns in the viral mRNA profile with regulation using mRNA processing signals: at an early phase of infection, a block at (pA)p leads to relatively higher-level production of NS1-encoding mRNAs, and at a late phase, readthrough of (pA)p is more efficient, which leads to the abundant generation of VP- and 11-kDa protein-encoding mRNAs (67). How precisely viral DNA replication overcomes the transcription block in B19V-permissive cells is not known.
(ii) Multiple splicing enhancers function to define the central exon of B19V pre-mRNA splicing.
B19V pre-mRNA has two splice donor sites (D1 and D2) and four acceptor sites (A1-1, A1-2, A2-1, and A2-2) (59). Alternative splicing is tightly regulated in B19V-infected cells to maintain appropriate levels of virus-encoded proteins. All the D2-spliced (pA)p readthrough transcripts, which are VP1- and 11-kDa protein-encoding mRNAs, contain a 55-nt-long leader sequence and a central exon (exon 2) spanning from the A1-1/A1-2 site to the D2 site (R4-9) (Fig. 1B). Serine-arginine (SR) protein-binding GAA motifs have been found in exon 2 (68). The GAA motif in the region between A1-1 and A1-2, also called exon-splicing enhancer 1 (ESE1), facilitates splicing at the A1-1 acceptor site and is also required to define exon 2. The 5′ end of exon 2 serves as ESE2 and facilitates splicing at the A1-2 acceptor site, while ESE3 at the 3′ end of exon 2 plays a role in recognizing the D2 donor site. The G/GU-rich region adjacent to the D2 site acts as an intron-splicing enhancer (ISE2). The definition of exon 2 is a consequence of the weak splice donor site D2, ISE1/2, and ESE1/2/3 (68).
Alternative splicing coordinates alternate polyadenylation to generate VP- and 11-kDa protein-encoding B19V mRNA transcripts (69). Efficient splicing of the pre-mRNA within the first intron (D1-A1) stimulates polyadenylation at the (pA)p site, and splicing of the second intron (D2-A2) promotes polyadenylation at the (pA)d site. Splicing of the second intron competes with polyadenylation at the (pA)p site. U1 small nuclear RNA, which binds to the 5′ splice donor site of the second intron, inhibits polyadenylation at the (pA)p site (69).
B19V proteins.
The large B19V nonstructural protein NS1, of 671 amino acids (aa), has a molecular mass of ∼78 kDa (59, 70, 71). NS1 localizes predominantly to the nucleus (71) and contains a nuclear localization signal (NLS) at aa 177 to 180 (KKPR) (72, 73). NS1 is essential for viral DNA replication (74) and has an origin DNA-binding/endonuclease domain at the N terminus (41), an ATPase- and nucleoside triphosphate (NTP)-binding motif of 160 aa in the central region (75), and a putative transactivation domain (TAD) at the C terminus (76). NS1, with the help of the transcription factor Sp1/Sp3, binds the P6 promoter of the virus to regulate viral gene expression (77, 78). Apart from acting on the P6 promoter, NS1 has been shown to transactivate several other host genes, including tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and p21 (79–81). NS1 induces apoptosis, which involves caspase-3 and -9, in B19V-semipermissive erythroid-lineage K562 and UT7/Epo cells (82) and nonpermissive HepG2 cells (83, 84). The NTP-binding motif at aa 328 to 335 (Walker box A) of NS1 has been implicated as a key motif in NS1-induced apoptosis (75, 82, 83). NS1 also induces cell cycle arrest (73, 85, 86) and the DNA damage response (DDR) (76, 87), which are discussed below (see “Cellular Response to Productive B19V Infection”).
The minor capsid protein VP1, of 781 aa, has a molecular mass of 84 kDa. The major capsid protein VP2, of 554 aa, has a molecular mass of 58 kDa (71). VP1 is poorly translated from the VP1-encoded mRNA that has multiple AUG codons upstream of the VP1 initiation site (88) and has an additional 227 aa at the N terminus, known as VP1u, compared with VP2 (59, 71). VP1 and VP2, together at a ratio of ∼1:20 (71), assemble to form a B19V capsid of a T=1 icosahedral structure, which consists of 60 proteins, with 3 proteins on each face of the capsid (17). VP2 alone can assemble virus-like particles (VLPs) (89), which resemble the icosahedral structure of the B19V capsid (17–19). VP2 harbors a nonconventional NLS motif (KLGPRKATGRW) at the C terminus (90), which localizes VP1 and VP2 proteins to the nucleus to assemble into empty capsids (Fig. 2, steps 12a and 13). The N terminus (aa 1 to 100) of VP1u plays a crucial role in virion binding and internalization during B19V entry into cells (91), whereas the central portion of VP1u (aa 128 to 160) contains a motif with PLA2 activity (22, 92, 93). The PLA2 motif is possibly utilized during intracellular trafficking to escape late endosomes for nuclear entry (Fig. 2, step 4), as for other parvoviruses (94, 95).
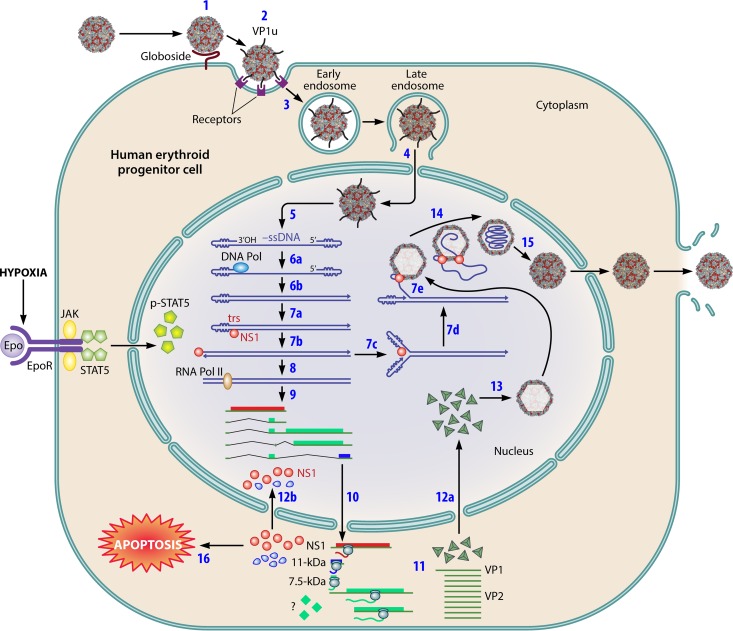
FIG 2.
B19V infection of human erythroid progenitor cells (B19V life cycle). When human erythroid progenitor cells are infected with B19V, the virus initially interacts with P antigen (globoside) (step 1), the primary low-affinity attachment sugar molecule on the cell surface. This interaction confers a conformational change of VP1, extruding itself on the surface of the virion instead of remaining embedded inside the virion (step 2). The VP1u region on the virion then binds a putative cellular surface receptor (VP1u-interacting protein), which mediates internalization of the virion inside the cells, presumably by endocytosis (step 3). Virions undergo several steps of intracellular trafficking in the endosome, are eventually released from the endosome through a function of the PLA2 motif of VP1u, and enter the nucleus (step 4). In the nucleus, the virion is uncoated and releases either a positive-sense ssDNA or a negative-sense ssDNA (−ssDNA) genome (step 5). The ssDNA genome, shown as negative-sense ssDNA, is first converted into dsDNA that is primed by the 3′ OH of the left-hand ITR (step 6), a process that requires cellular DNA polymerase (DNA Pol) and other DNA replication factors. Phosphorylated STAT5 (p-STAT5), which is activated through the Epo/Epo-R/Jak2/STAT5 pathway, is required for viral DNA replication. After dsDNA conversion, NS1 binds NSBEs and nicks one of the strands at the trs (step 7a). This event creates a new 3′ OH to lead DNA synthesis following melting of the hairpinned ITR, which subsequently repairs the ITR and results in an open-ended duplex replicative intermediate (step 7b). The repaired ITR is then denatured (step 7c), which likely requires the helicase activity of NS1, and is reannealed, in a process termed reinitiation, to form a double-hairpinned intermediate, which creates a new 3′ primer (3′ OH) to initiate a round of strand displacement synthesis (step 7d). The RF DNA is capable of transcription of viral mRNA by cellular RNA polymerase II (RNA Pol II). Various B19V mRNAs are generated through alternative processing of the pre-mRNA (step 9) and are exported to the cytoplasm (step 10). Capsid proteins; VP1 and VP2; and the NS1, 11-kDa, and 7.5-kDa nonstructural proteins are translated in the cytoplasm (step 11). VP1 and VP2 are assembled as trimers (capsid precursors), which are transported into the nucleus (step 12a) to assemble empty capsids (step 13). A viral ssDNA genome is presumably produced through a process called strand displacement (step 7e), when an empty capsid is available, through a putative NS1-mediated packaging mechanism (step 14). NS1 is transported to the nucleus (step 12b) and is essential for viral DNA replication. NS1 also induces cell cycle arrest at G2 phase. Finally, apoptosis is induced, in which both NS1 and the 11-kDa protein play important roles (step 16). Apoptosis releases the matured virion from infected cells through the broken nuclear membrane (step 15). As noted, steps 6, 7, and 12 to 14 are partially hypothetical.
In addition, B19V expresses two small nonstructural proteins of 11 kDa and 7.5 kDa (62, 96). The 11-kDa protein is abundantly expressed in B19V-infected CD36+ EPCs (97). It is localized more in the cytoplasm than in the nucleus, and its protein expression level in the cytoplasm of these cells is at least 100 times higher than that of nuclear NS1 (63, 97). The 11-kDa protein contains three proline-rich regions and binds in vitro to the SH3 domain-containing protein Grb2 (98). The 11-kDa protein is a more potent inducer of apoptosis, as it is abundantly expressed during infection, which involves caspase-10 in B19V-infected CD36+ EPCs (97). A role for the 11-kDa protein in VP2 production and cellular distribution has also been suggested (74). However, the 11-kDa and 7.5-kDa proteins are not required for DNA replication of the infectious clone pB19-M20 in UT7/Epo-S1 cells (74). Currently, nothing is known about the function of the 7.5-kDa protein during B19V infection.
An open reading frame (ORF) in the VP1-unique region is predicted to encode a third small nonstructural protein (X protein) of 9 kDa (72). An X protein knockout B19V infectious clone did not show any differences between the wild type and the knockout mutant with respect to viral DNA replication (74). Furthermore, it has not been demonstrated to be expressed during either transfection of a B19V clone or B19V infection.
B19V Tropism and Entry
B19V cell culture.
In patients, productive B19V infection is highly restricted to erythroid progenitor cells of the bone marrow (99). B19V was first demonstrated to infect cultured erythroid progenitor cells isolated from human bone marrow cells (100). More primitive erythroid progenitors, at stages of burst-forming unit–erythroid (BFU-E) and CFU-erythroid (CFU-E), were permissive to B19V infection (100, 101). Various sources, including human bone marrow (100–103), umbilical cord blood (104, 105), peripheral blood (106, 107), and fetal liver (108, 109), were used to propagate erythroid progenitor cells for in vitro infection by B19V. Target cells of B19V infection are in various stages of erythroid differentiation, from BFU-E to proerythroblasts, with susceptibility to the virus increasing with differentiation (110). A pure population of CD36+ EPCs, which are expanded ex vivo and derived from hematopoietic stem cells (HSCs) isolated from either human bone marrow or peripheral blood mononuclear cells (PBMCs), are permissive to B19V (111), and they are widely used for B19V infection and neutralization antibody tests (54, 73, 112–114). Hypoxic conditions, about 1% O2, significantly increase B19V infectivity in CD36+ EPCs (54). Although CD36+ EPCs and hypoxia facilitate B19V infection, the production of infectious progeny virions may be limited due to a failure of genome encapsidation (115).
Megakaryocyte-erythroid lineage cell lines have been tested for B19V infection. MB-02, UT7/Epo, and UT7/Epo-S1 cells are megakaryoblastoid cell lines (116–119) prone to B19V infection. Two erythroid leukemia cell lines, JK-1 and KU812Ep6, have also been documented to support B19V infection (120, 121). Based on the expression of the viral NS1 protein and viral DNA replication, UT7/Epo-S1 cells appear to be most permissive, but they are not as efficient as CD36+ EPCs for virus propagation, even under hypoxia (54, 85).
B19V receptor and coreceptors.
Globoside or P antigen is the primary cell surface receptor for B19V infection (122). Both the purified soluble P antigen and a monoclonal antibody to P antigen prevent B19V infection of human erythroid progenitors (122). B19V VP1- and VP2-containing VLPs also bind to P antigen in vitro (123), confirming the role of globoside as a receptor for B19V. P antigen is expressed largely on the cell surface of human erythroid progenitors (111, 112). However, not all P-antigen-expressing cells are permissive to infection by recombinant B19V, indicating that P antigen is necessary for but not sufficient in mediating recombinant B19V infection (124). Therefore, individuals who lack P antigen are resistant to B19V infection (125). Mature human red blood cells (RBCs), despite expressing P antigen, are not permissive to virus entry (126); viral particles remain attached to the surface of human RBCs during the course of virus infection, with P antigen aiding in systemic dissemination (126). Two potential coreceptors for B19V, integrin α5β1 (127) and Ku80 (128), have been proposed. However, the expression of Ku80 on the surface of CD36+ EPCs does not correlate with high infectivity of B19V (112). As B19V VP1u plays a key role in the binding and internalization of B19V virions, a VP1u-interacting protein, which has not yet been identified, has been hypothesized to function as a coreceptor (91, 129).
In nonerythroid cells such as endothelial cells, despite similar expression levels of P antigen, Ku80, and α5β1 on the cell surface, internalization of B19V is inefficient (130). An alternative route for B19V internalization in endothelial cells might be mediated by the C1q receptor CD93 and B19V-antibody complexes (130, 131).
B19V entry and the role of B19V VP1u in virus entry.
VP1 has an unique N-terminal VP1u of 227 aa in comparison to VP2 (70, 71). VP1u displays PLA2 activity during the transport of virus to the nucleus via the endosomal pathway (132–135). In many parvoviruses, VP1u is hidden inside the capsid and not accessible during virus binding to cells, while in some other parvoviruses, it is exposed on the surface. Despite its low proportion in the virion, B19V VP1u represents a dominant antigenic target for neutralizing antibodies (89, 136, 137), implying that it must be exposed to the extracellular milieu prior to B19V internalization (138–140). B19V VP1u becomes accessible to neutralizing antibodies upon the interaction of the capsid with the P antigen on the cell surface (25, 126, 141) (Fig. 2, step 1). During B19V uptake, the VP2 capsid predominantly attaches to P antigen of target cells (126), which in turn induces structural changes in the capsid that lead to the exposure of VP1u (141). The N-terminal 100 aa of the exposed VP1u then binds on the cell surface, leading to the internalization of the capsid (91, 141) (Fig. 2, step 2). It is hypothesized that the interaction of the B19V virion with host cells may require a VP1u-interacting protein on the cell surface to accomplish the binding and internalization of B19V virions. Further study has shown that N-terminal aa 5 to 80 of VP1u are necessary and sufficient for cellular binding and internalization, representing the VP1u-interacting protein-binding domain required for B19V uptake (142). Little is known about B19V intracellular trafficking. One study has shown that B19V was internalized by clathrin-dependent endocytosis and traffics rapidly throughout the endosomal compartment to the lysosomal compartment (143). The virus is supposed to escape the late endosome; otherwise, it may be degraded in the lysosome, as observed for other parvoviruses (132).
B19V Replication
Elements involved in B19V DNA replication both in cis and in trans.
The B19V minimum Ori is located at nt 5214 to 5280 (67 nt) and contains 4 repeats of the NSBE. NSBE1 and NSBE2 are 8-bp-long identical motifs separated by 2 bp, while NSBE3 and NSBE4 are degenerate sequences (41). NSBE1 to -3 are essential for viral DNA replication, and NSBE4 further enhances replication (52). The Ori also harbors a terminal resolution site (trs), where NS1 presumably nicks ssDNA to generate a free 3′-OH end that primes the DNA extension (Fig. 2, step 7b) (34). NS1 specifically binds NSBE1 and -2 in vitro (41).
Cellular control of B19V DNA replication.
The remarkable erythroid tropism of B19V partly depends on the expression of the virus receptor and coreceptors on erythroid progenitor cells (122, 127, 128); however, it is also dependent on erythroid-lineage-specific host factors. B19V has been shown to alter various cell signaling pathways (e.g., erythropoietin [Epo] signaling, the DDR, and cell cycle arrest) for efficient viral DNA replication (54, 76, 85, 112).
(i) S-phase-dependent viral DNA replication.
B19V, without a viral DNA polymerase (Pol), relies solely on the host DNA replication machinery. B19V induces cell cycle arrest of infected cells in “G2” phase with a 4N DNA content, which is assessed only by 4′,6-diamidino-2-phenylindole (DAPI) staining for DNA content (118, 144). When assessed by both BrdU (5′-bromo-2-deoxyuridine) incorporation and DAPI staining, B19V infection induces cell cycle arrest in late S phase with both BrdU incorporation and a 4N DNA content (85). Several S-phase factors, such as DNA Pol δ, proliferating cell nuclear antigen (PCNA), replication factor complex 1 (RFC1), cyclin A, and minichromosome maintenance complex (MCM), colocalize in B19V replication centers (85). B19V exploits a prolonged S phase and utilizes S-phase cellular factors for viral DNA replication.
(ii) Epo-dependent B19V DNA replication.
Epo is essential for the differentiation of erythroid progenitor cells, and both CD36+ EPCs and B19V-semipermissive cell lines, e.g., UT7/Epo-S1, depend on Epo for cell proliferation and survival. Epo, a hormone produced by human renal interstitial fibroblasts in response to local partial oxygen pressure, precisely regulates erythropoiesis. The earlier stages of differentiation to BFU-E are Epo independent but rely on stem cell factor (SCF), IL-6, and IL-3. The later stage of differentiation from BFU-E to CFU-E requires Epo. CFU-E progenitors and proerythroblasts are highly susceptible to B19V infection (110, 112). The permissivity of these cells to B19V infection depends on Epo: the Epo/Epo receptor (Epo-R)/Jak2 signaling pathway plays a direct role in B19V replication (112). CD36+ EPCs differentiated from CD34+ HSCs in the absence of Epo are not permissive to B19V infection, and the B19V genome replicates in CD36+ EPCs only in the presence of Epo. The activation of Epo-R activates three major pathways, MEK/extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K), and JAK2-STAT5A, but only the phosphorylation of STAT5A is essential for B19V replication, the MEK/ERK pathway has a negative effect, and the PI3K pathway is dispensable for B19V replication (54).
(iii) Hypoxia-facilitated B19V replication.
Propagation of B19V in ex vivo-expanded CD36+ EPCs requires a multiplicity of infection (MOI) of >1,000 viral genome copies (vgc)/cell and produces infectious progeny virions at a low level, even under hypoxia (54, 115). The plasma of B19V-infected patients may contain virions at levels as high as 1013 to 1014 vgc/ml (145, 146). Thus, ex vivo propagation of B19V is not as efficient as that in human bone marrow of B19V-infected patients. Areas of bone marrow are at low O2 tension (1.3%) (147), and lower oxygen pressure favors erythroid cell development in culture (148). CD36+ EPCs under hypoxia (1%) have enhanced B19V gene expression, viral replication, and virus production (55). Hypoxia also enables B19V-infected KU812Ep6 and UT7/Epo-S1 cells to yield a higher level of progeny virions than under normoxia (54, 149). Hypoxia regulates the Epo/Epo-R signaling pathway through the upregulation of STAT5A activation and the downregulation of MEK activation, enhancing B19V DNA replication in both B19V-infected CD36+ EPCs and pB19-M20-transfected UT7/Epo-S1 cells (54).
HBoV1 Biology
HBoV1 genome and infectious clone.
Only one full-length HBoV1 genome of 5,543 nt has been sequenced and cloned (150); the source of viral DNA was a nasopharyngeal aspirate from a child with community-acquired pneumonia in Salvador, Brazil, who had acute viral infection (seroconversion, viremia, and >108 vgc per ml of aspirate) (151). The sequence of the Salvador isolate has been deposited in GenBank (accession no. JQ923422). Ninety-five percent of bocaparvoviruses contain a negative-sense genome, and only 5% of bocaparvoviruses have a positive-sense genome (152, 153). The HBoV1 negative-sense genome has an imperfect “rabbit-ear-type” palindromic hairpin structure of 140 nt at the left-end hairpin (LEH) and a perfect palindromic structure of 190 nt at the right-end hairpin (REH) of the genome (150). A plasmid DNA clone of pIHBoV1, which contains the full-length HBoV1 genome of the Salvador isolate, replicates and produces progeny virions in HEK293 cells. HBoV1 virions generated from this production system exhibit a typical icosahedral structure of ∼26 nm in diameter and are capable of productively infecting polarized primary human airway epithelial cells cultured at the air-liquid interface (HAE-ALI cultures) (150).
The HBoV1 genome has heterogeneous terminal repeats, as is characteristic of the parvoviruses of the genus Protoparvovirus. The REH contains the terminal resolution site, which plays a role in the replication of viral replicative-form DNA, whereas the LEH is critical for junction resolution, which generates the ssDNA genome from the replicative-form DNA for encapsidation into capsids (34, 154). The HBoV1 LEH and REH do not share conserved NS1-binding sequences, and the REH is a perfectly paired palindromic sequence (150, 154). HBoV1 ssDNA genome replication in HAE-ALI cultures generates intermediates of double-replicative forms (dRFs) and monoreplicative forms (mRFs) (155).
HBoV1 gene transcription and regulation.
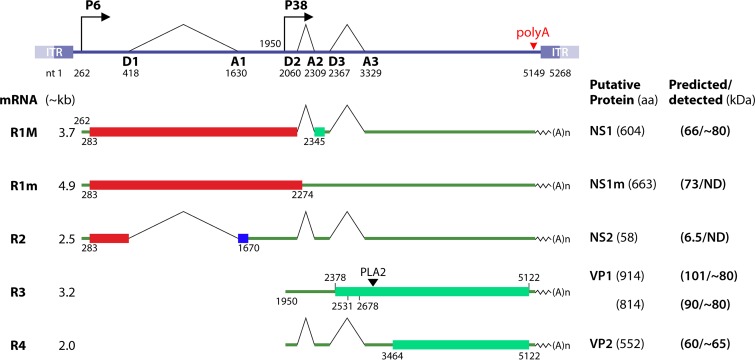
HBoV1 transcription uses one promoter, P5, at nt 282, to transcribe a single pre-mRNA, which is both alternatively spliced and polyadenylated at (pA)p and (pA)d, respectively, to generate at least 12 mature mRNA transcripts for encoding viral NS and structural proteins (156–159) (Fig. 3). The HBoV1 genome consists of the nonstructural protein (NS1 to -4), NP1, and capsid protein (VP1 to -3) genes at the left, middle, and the right sides, respectively. NS1 is encoded by R1 mRNA transcripts that are spliced at the internal small intron (D3-A3) and are alternatively polyadenylated (R1) (Fig. 3). A small NS1 protein, NS1-70, is expressed from an unspliced R1 mRNA. Additionally, three small NS proteins, NS2, NS3, and NS4, are expressed from R2, R3, and R4 mRNA transcripts, respectively, which are alternatively spliced at the D1-A1′, D1′-A1, or both introns (R3-4) (Fig. 3). Unique to bocaparvoviruses is an ORF located in the middle of the viral genome. A small NS protein, NP1, is encoded by R5 mRNA transcripts that are spliced at the D1-A1 and D2-A2 introns (R5) (Fig. 3). The R6 mRNA transcript, which is spliced at all three major introns (D1-A1, D2-A2, and D3-A3) and polyadenylated at the (pA)p site, encodes the capsid proteins VP1, VP2, and VP3 (Fig. 3).
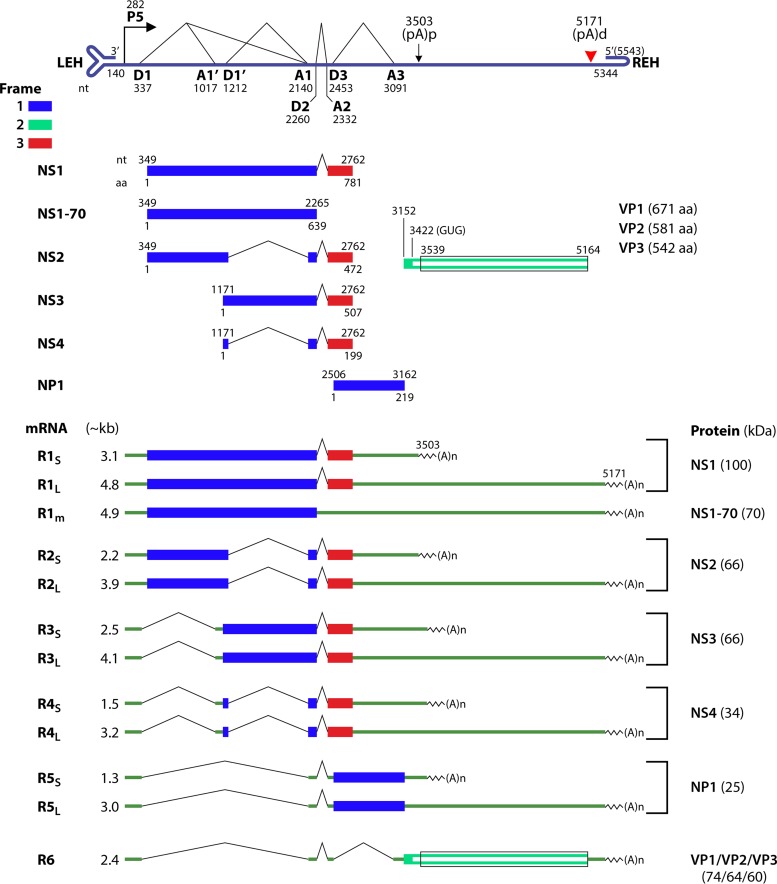
FIG 3.
HBoV1 transcription map. The ssDNA genome of HBoV1 is shown in negative polarity. The transcription and posttranscriptional units are depicted to scale, including the P5 promoter, 5′ splice donor sites (D1, D1′, D2, and D3), 3′ splice acceptor sites (A1, A1′, A2, and A3), the internal proximal polyadenylation site [(pA)p], and the distal polyadenylation site [(pA)d], which are functional when the ssDNA genome is converted to a dsDNA form. The left- and right-end hairpin structures of the genome (LEH and REH, respectively) are diagrammed. Six groups of HBoV1 mRNA transcripts detected during infection, which have either a long form of mRNA (RxL) that reads through the (pA)p site or a short form of mRNA (RxS) that is polyadenylated at the (pA)p site, are shown below the diagrammed genome. R1 mRNA has a minor species (R1m) that is unspliced at the central small intron (D3-A3). Major ORFs are depicted as colored boxes with the nucleotide and amino acid of the start codon and the stop codon indicated. Proteins expressed from each mRNA species are indicated beside their respective mRNAs with molecular masses in kilodaltons detected during infection.
The HBoV1 nonstructural proteins NS1, of 781 aa; NS2, of 472 aa; NS3, of 507 aa; and NS4, of 199 aa, have detected molecular masses of ∼100, ∼66, ∼69, and ∼34 kDa, respectively. They share a C terminus of aa 639 to 781 of the NS1 protein (Fig. 3, red) (158). NS1, which has a putative DNA origin-binding/endonuclease domain (OBD), a helicase activity domain, and a TAD at the N terminus, middle, and C terminus, respectively, is essential for viral DNA replication (150). The OBD structure is canonical for the histidine-hydrophobic histidine superfamily of nucleases, combining two distinct DNA-binding sites: (i) a positively charged region mediated by a surface hairpin (aa 190 to 198) that is responsible for the recognition of the viral DNA Ori and (ii) the endonuclease active site that performs strand-specific cleavage at the Ori (160). NS2 contains the entire OBD and TAD of NS1, while NS3 contains the helicase domain and TAD of NS1, and NS4 has only the TAD. NS2 to -4 are not required for viral DNA replication of the pIHBoV1 infectious clone in HEK293 cells; NS2 plays an important role in virus replication in HAE-ALI cultures (158). The functions of NS3 and NS4 are currently unknown.
HBoV1 NP1, of 219 aa, has a molecular mass of 25 kDa. NP1, which is unique to bocaparvoviruses, plays an important role not only in viral DNA replication (150, 152) but also in viral pre-mRNA processing (161). It is required for viral mRNA splicing at the A3 splice site and readthrough of the viral mRNA from the (pA)p site (159). Therefore, NP1 is essential for generating VP-encoding mRNA (R6) (Fig. 3) and for the production of viral capsid proteins. Of note, HBoV1 NP1 colocalizes with autonomous parvovirus-associated replication (APAR) bodies and complements some functions of minute virus of mice (MVM) NS2 during early-phase infection (162).
Unlike B19V, HBoV1 expresses three capsid proteins, VP1, VP2, and VP3, during HBoV1 infection, at a ratio of ∼1:1:10 (158, 159), similar to that of AAV (3). Like AAV VP1 (3), HBoV1 VP1 has a VP1u of 90 aa, which is shorter than that of B19V (227 aa). A motif of aa 11 to 66 of VP1u exhibits PLA2 activity (163). VP2 is translated from a noncanonical GUG translation initiation codon at nt 3422 of the HBoV1 genome (159, 164) (Fig. 3). VP3, the major capsid protein, assembles into VLPs with a typical T=1 parvovirus icosahedral structure (20, 164–166). The VLP capsid formed by HBoV1 VP3 contains putative epitopes of neutralization/receptor binding, which are recognized by anti-HBoV1 monoclonal antibodies 4C2, 9G12, and 12C1 and anti-HBoV1, -2, and -4 monoclonal antibody 15C6 (26).
HBoV1 cell culture.
HBoV1 infects only well-differentiated or polarized primary human airway epithelial cells (150, 156, 167, 168). An HAE culture is generated by growing isolated human airway (tracheobronchial) epithelial cells on collagen-coated, semipermeable membrane inserts, and cells differentiate at the ALI for 3 to 4 weeks (150, 155). HBoV1 infects primary HAE-ALI cultures efficiently (167). Immortalized human airway epithelial cells of the CuFi-8 cell line, which were originally derived from a cystic fibrosis patient (169), have been polarized successfully to produce ALI cultures, which support HBoV1 infection but at a 1-log-lower level of apical virus release than that of infected primary HAE-ALI cultures (150). Although two commercially available primary HAE-ALI cultures, EpiAirway and MucilAir HAE-ALI, can be infected with HBoV1, infectivity was much poorer than that in in-house-made (primary and CuFi-8) HAE-ALI cultures (168). However, the infection was also persistent, releasing virions for as long as 50 days (168). HBoV1 infects HAE-ALI cultures from both the basolateral and apical sides of the ALI, and infected HAE-ALI cultures release virions from both sides. The amount of virus released from the apical side is 1 to 2 logs larger than that released from the basolateral side (150, 167).
Monolayer-cultured primary airway epithelial cells or airway epithelial cell lines do not support HBoV1 infection or replication of infectious DNA (pIHBoV1) (150). The steps in virus infection or viral DNA replication that are blocked during HBoV1 infection of monolayer-cultured cells is not known; the limiting step is likely at the stage of viral DNA replication.
Cellular control of HBoV1 DNA replication.
In contrast to S-phase-dependent B19V DNA replication, HBoV1 infects and replicates in terminally differentiated or nondividing airway epithelial cells of HAE-ALI cultures. Therefore, HBoV1 is independent of the cell cycle (155). HBoV1 infection of nondividing epithelial cells employs the cellular DNA damage and repair machinery in order to amplify the viral genome. HBoV1 infection activates all three PI3K-related kinases, ataxia telangiectasia mutated kinase (ATM), ATM- and rad3-related kinase (ATR), and DNA-dependent protein kinase (DNA-PKcs), at serine 1981 on ATM, threonine 1989 on ATR, and serine 2056 on DNA-PKcs, which are functionally required to transduce DDR signaling. The Y-family DNA polymerases Pol η and Pol κ function in HBoV1 genome amplification. Thus, HBoV1 replication is cell cycle independent, and the DNA repair process recruits cellular DNA repair DNA polymerases in viral DNA replication centers (155).
HOST CELL RESPONSE AND PATHOGENESIS
Cellular Response to Productive B19V Infection
B19V infection-induced DNA damage response.
B19V infection induces a DDR by activating the ATR, ATM, and DNA-PKcs kinases of the PI3Ks (87). Activation of ATR and DNA-PKcs is essentially required for efficient B19V DNA replication, and DDR effectors (e.g., Chk1 and Ku70/80) associate with replicating viral DNA (76, 87). None of the virus-encoded proteins (NS1, VP1, VP2, 11-kDa, or 7.5-kDa protein) are responsible for the phosphorylation of RPA32 and H2AX, typical of the DDR, in CD36+ EPCs (76). The B19V infectious clone pB19-M20, but not its replication-defective mutant, induces a DDR in transfected UT7/Epo-S1 cells, implying that viral DNA replication, and not merely the expression of the viral genome, is required to induce a DDR (76). NS1 is essential for DNA replication and is required for inducing the DDR during B19V infection. Viral DNA replication is a prerequisite for a B19V-induced DDR, and DNA replication intermediates could be potential inducers of the DDR. How the DDR facilitates the replication of the B19V genome is unclear; probably, the linear DNA genome initially recruits the DDR machinery for repair and exploits it to accomplish second-strand DNA synthesis prior to viral DNA replication (Fig. 2, step 6).
B19V infection-induced cell cycle arrest.
B19V infection of CD36+ EPCs and UT7/Epo-S1 cells induces arrest in G2 phase, a cell cycle status with 4N DNA content (118, 144). Upon G2 arrest with 4N DNA content, there is also incorporation of BrdU, so infection-induced arrest occurs in late S phase (85). In these studies, there was a gradual switch of the arrested CD36+ EPCs from late S phase (50% of infected cells) during early infection to G2 phase (60% of infected cells) later. However, the expression of only B19V NS1 induces true G2-phase arrest, with the majority of the cells having 4N DNA content and no BrdU uptake (85). B19V infection of UT7/Epo-S1 cells also induced 4N cell cycle arrest, and prevention of the nuclear import of the activated cdc2/cyclin B1 complex was observed (118). In CD36+ EPCs, NS1 induces stable G2 arrest by interacting with repressive E2F transcription factors (E2F4 or E2F5) and facilitating their nuclear import (73). The predicted transactivation domain 2 (TAD2) at the C terminus of NS1 is required for NS1-induced G2-phase arrest (76). Nevertheless, the mechanism underlying NS1-induced G2-phase arrest requires further validation. In addition to G2-phase arrest, B19V infection of UT7/Epo-S1 cells has been reported to induce G1-phase arrest, and G1-phase arrest was confirmed to be induced by NS1 in NS1-transfected UT7/Epo-S1 cells (86). However, G1-phase arrest has not been observed in either B19V-infected or NS1-expressing CD36+ EPCs (73, 76, 85).
Of note, a 5′-GTTTTGT-3′ sequence in the P6 promoter, a CpG oligodeoxynucleotide-2006 (containing the CpG motif 5′-GTCGTT-3′) analog that is a ligand of Toll-like receptor 9 (TLR9), was shown to inhibit the growth of BFU-E progenitors by arresting cells at the S and G2/M phases (113). Thus, the viral genome is also capable of inducing S- and G2/M-phase arrest.
Taken together, these findings show that the viral genome and/or its replication is capable of inducing S-phase arrest, while NS1 per se induces G2-phase arrest. Therefore, B19V infection-induced late-S-phase arrest is a compromised outcome of genome replication-induced S-phase arrest and NS1-induced G2-phase arrest (85). While S-phase arrest enriches S-phase factors that favor viral DNA replication, G2 arrest halts erythropoiesis of erythroid progenitors and eventually kills the cells.
B19V infection-induced erythroid cell death.
B19V infection of human erythroid progenitors in bone marrow and fetal tissues ultimately leads to cell death, which results in transient aplastic crisis (99). B19V specifically infects BFU-E and CFU-E progenitors, thereby arresting erythropoiesis (101, 102, 170). The mechanism of B19V-induced cell death of infected erythroid progenitors was apoptotic (171): hydrops fetalis tissue infected with B19V had characteristics of apoptosis (172), and fetal erythroid progenitors infected by B19V revealed ultrastructural features of apoptotic cell death (109).
Examination of B19V-mediated cytotoxicity in CD36+ EPCs and UT7/Epo-S1 cells revealed that both B19V infection and NS1 transfection induced apoptotic cell death, which involved caspase-3, -6, and -8 activation and DNA fragmentation (82, 144). B19V induced extrinsic apoptosis pathway activation, which involved the TNF-α pathway, in both infected CD36+ EPCs and NS1-expressing UT7/Epo cells (144). Furthermore, it was found that the virus-encoded 11-kDa protein played a role in B19V-induced apoptosis of CD36+ EPCs (97). The 11-kDa protein was shown to be a more potent inducer of apoptosis, due to its high expression level (∼100 times higher than that of NS1) and localization (cytoplasmic), and involved the activation of caspase-10 (97). In conclusion, upon B19V infection of erythroid progenitor cells, viral NS1 and 11-kDa proteins, possibly with other unidentified viral factors, synergistically act to induce the apoptosis of erythroid progenitor cells upon B19V infection.
B19V infection of CD36+ EPCs has been reported to coincide with the downregulation of thyroid, retinoid, and estrogen hormone receptors (173), which is of unknown consequence.
Cellular Response to Unproductive B19V Infection
The presence of B19V, as detected by viral DNA or viral proteins, has been associated with clinical diseases such as acute and chronic inflammatory cardiomyopathies (174–177), rheumatoid arthritis (178–181), vasculitis (182, 183), meningoencephalitis (184, 185), hepatitis (186, 187), and thyroid diseases (173, 188–190) (see “Diseases Caused by B19V Infection,” below). However, it has not been established that nonerythroid cells/tissues support productive viral DNA replication and the release of progeny virions (191).
B19V infection of endothelial cells.
B19V DNA is highly prevalent in endothelial cells of the myocardium during acute and chronic inflammatory cardiomyopathies (192). In one study, B19V DNA was frequently detected in patients with normal coronary anatomy that clinically mimicked acute myocardial infarction (193). However, another study also showed that B19V DNA was highly prevalent in myocardial autopsy specimens from subjects without myocarditis or dilative cardiomyopathy (194). B19V was reported to infect fetal capillary endothelia in placental villi and to express viral proteins (195). These findings suggest the potential role of B19V in cardiomyopathies.
Although primary endothelial cells from the pulmonary artery, umbilical vein, and aorta express the B19V receptor/coreceptors and bind virus similarly to UT7/Epo-S1 cells (130), B19V uses an alternative route, antibody-mediated endocytosis, to enter endothelial cells. The B19V-antibody complex could interact with the complement factor C1q and use the C1q receptor (CD93) for cell entry via endocytosis (130). Antibody-dependent virus entry might explain the frequent prevalence of B19V in various endothelial cells. B19V was also reported to infect U937 cells by exploiting antibody-dependent enhancement of entry, although this infection was abortive (131). NS1 expression in transfected and immobilized human endothelial cells (HMEC-1) activates STAT3/PIAS3 signaling, which upregulates immune response genes (IFNAR1 and IL-2) and downregulates genes associated with antiviral defense (OAS1 and TYK2) (196). In mice, anti-B19 VP1u IgG aggravates cardiac injury by the induction of inflammation (197). Recent studies of B19V infection of bone marrow-derived circulating angiogenic cells (CACs) and CD34+ KDR+ endothelial progenitor cells from patients who had chronic B19V-associated cardiomyopathy highlight the potential for B19V to be a pathogen in microvascular disease and cardiomyopathy (198, 199). B19V DNA replicative intermediates and mRNA transcripts were detected in nearly one-half of patients as well as in B19V-infected epithelial progenitor cells in vitro (198). VP1 was identified as a novel inducer of apoptosis with the activation of caspase-8 and caspase-10, through the activation of death receptor signaling. B19V causally impaired endothelial regeneration and spread in epithelial progenitor cell-xenografted SCID Beige mice (198). These observations are evidence that B19V infection can damage CACs and results in dysfunctional endogenous vascular repair.
Nevertheless, no studies reported to date show progeny virion production during B19V infection of endothelial cells or CACs in vitro. Possibly, B19V enters such cells and undergoes only one step of double-stranded DNA (dsDNA) conversion, expresses NS1 and VP proteins, and induces apoptosis or an inflammatory immune response in host cells and tissues.
B19V infection in other tissues.
The presence of viral DNA in a wide range of tissues in both healthy and diseased subjects reveals a lifelong persistence of B19V infection (200, 201). Persistence of viral DNA has been detected in up to 50% of biopsy specimens of the spleen, lymph nodes, tonsils, liver, heart, synovial tissues, skin, brain, and testes, for decades after infection (58, 178, 191, 200–204). Persistence may be partly maintained by silencing of viral gene expression by CpG DNA methylation (205). Increased NF-κB, COX2, and IL-6 expression levels in thyroid, colon, synoviocytes, and lymphoid tissues was correlated with the expression of B19V capsid proteins (206–209). The PLA2 activity of VP1u is responsible for the inflammatory response in synoviocytes (208). NS1 ectopic expression in the hepatocyte cell line HepG2 induces apoptosis, involving caspase-3 and -9 but not caspase-8 activation (83, 210).
In summary, B19V may enter and persist in various non-erythroid-lineage tissues, but there is no clear evidence that infection is productive, and it does not seem to cause disease.
Airway Epithelium Damage Caused by HBoV1 Infection
From in vitro modeling of HBoV1 infection of HAE-ALI cultures, HBoV1 infection appears to disrupt the epithelial barrier and shows hallmarks of lung airway tract injury, including disruption of the tight-junction barrier, loss of cilia, and epithelial cell hypertrophy (150, 167, 168). Infected HAE-ALI cultures manifest a clear dissociation of the tight junctions as the transepithelial electrical resistance of infected HAE-ALI drops significantly after infection. HBoV1 infection abolishes cilia on the apical side of the airway epithelium. Infected HAE cultures have a discernibly thinner epithelium and show nuclear enlargement at late stages of infection. Infected HAE cultures undergo gradual thinning of the epithelium and loss of epithelial cells (150, 167), suggesting that HBoV1 infection eventually kills HAE cells. The infected airway epithelium is regenerated by epithelial cells produced from airway stem cells, which maintains both its integrity and apical virus release for at least 50 days postinfection in vitro (168). HBoV1 infection causes a DDR with the hallmark phosphorylation of H2AX and RPA32 (155). The DDR could induce programmed cell death, for example, through reactive oxygen species (ROS) (211, 212). However, the nature of HBoV1 infection-induced airway epithelial cell death and maintenance of the integrity of infected epithelia is not known.
In bronchoalveolar lavage fluids of HBoV1-infected individuals, the cytokines epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), CCL-17, TNF-α, TNF-β, and TIMP-1 are upregulated. These cytokines were also detected in apical wash specimens of HBoV1-infected CuFi HAE-ALI cultures (213). HBoV1-induced airway damage might be mediated by HBoV1-induced cytokine expression.
In acute respiratory tract infections due to HBoV1, wheezing is a common symptom, and HBoV1 pathogenesis is at least partially due to airway epithelium damage, as seen in in vitro-infected HAE-ALI cultures.
EPIDEMIOLOGY
Virus Prevalence and Transmission
B19V.
B19V infection is common worldwide, showing regional epidemiological differences, with generally over one-half of the adult population having been exposed. The prevalence of B19V-specific antibodies in the population is age dependent, increasing from 2 to 20% in children <5 years old to 15 to 40% in children 5 to 18 years old and to 40 to 80% in the adult population, depending on both the assays used and the population (214–225). Seroprevalence, however, is much lower in some isolated areas, such as the Rodriguez Islands and among some Brazilian tribes, with adult seroprevalences of only 2 to 10% (226, 227). Prevalence can also be much higher, such as the 85% seroprevalence reported for 9-year-olds in Papua New Guinea (228). The typical age when an individual contracts B19V infection is 5 to 15 years, but susceptible adults may also be infected. Infection induces an immune response, which confers lifelong protection against reinfections. Neutralizing IgG is formed about 2 weeks after infection and is very effective in eradicating the virus from the bloodstream.
B19V is transmitted mainly by the respiratory route, but prodromal symptoms are fever, malaise, headache, and myalgia rather than respiratory symptoms (229). It is currently unknown how B19V overcomes the airway epithelium barrier to eventually reach bone marrow for infection. The virus can also be transmitted via blood or pooled-blood products, from a pregnant mother to her fetus, and possibly even from tattooing (230). Higher seroprevalences than those among controls have been detected among patients receiving blood products and women having experienced abortions but not in people with tattoos (218, 231, 232).
Droplet transmission was evident after intranasal inoculation of volunteers, as B19V was shown to be able to infect subjects and cause disease (229, 233). Furthermore, during the prodrome, viral DNA can be detected in the upper airways (229, 234–236). Detectable DNA also coincides with a transient high-titer viremia of >1010 vgc/ml, which rapidly declines to a low level that can persist for many months or even years (236–242). The viral load in the acute phase, however, does not correlate with disease severity (229). In patients with different chronic pathological backgrounds, B19V DNA has also been detected at a low frequency in the lower respiratory tract (243).
Due to the relative ease of spread of the virus, outbreaks of B19V-induced childhood rash (erythema infectiosum) are most common in schools and day care centers, affecting up to one-half of schoolchildren and one-fifth of susceptible staff (244–246). B19V outbreaks occur mostly in the winter and spring, with major epidemics occurring every few years. The high-risk period for spread is early in the acute phase of infection, before rash or arthralgia appears, when the viral loads are at their highest. A convalescent child, even with recurring episodes of rash, is no longer infectious and may attend school. In patients with an underlying hemolytic disorder who suffer from B19V-induced aplastic crisis, titers as high as 1014 vgc/ml can be observed (146). In contrast to erythema infectiosum patients, these patients are at the time of disease extremely contagious, so to hinder nosocomial spread, aplastic crisis patients should be isolated. Among both hospital staff and patients, the risk of nosocomial spread of the disease, acquired from close contact or environmental surfaces, is quite high, with reported attack rates of 50% (247–249). Control measures such as handwashing, closure of the ward, utilization of B19V-immune staff, and B19V education likely are crucial to contain transmission. To avoid contagion, standard and droplet precautions and isolation should be implemented (250).
The timing of viremia before rash symptoms, high viral load, persistence, and resistance of this nonenveloped virus to most virus inactivation procedures used in the manufacturing of blood products create a risk of transmission through blood or blood products such as plasma, blood cells, and clotting factors (251–264) as well as through bone marrow and solid-organ transplantations (265–270). Comparisons of subjects with and those without blood transfusions have revealed a significantly higher seropositivity rate in individuals who have received blood transfusions (218, 231). Even if symptomatic transfusion-transmitted B19V infections are generally rare (259, 271), among eight patients with transfusion-transmitted B19V infection, five became ill with anemia, pure red blood cell aplasia (PRCA), or pancytopenia, all of whom had an underlying hematological disorder, whereas recipients without such disorders exhibited only moderate symptoms (264). Among solid-organ transplant recipients, most seronegative pediatric kidney transplant recipients of B19V DNA-positive organs became infected within 1 month (with four exhibiting anemia) (265). Patients at high risk of severe complications due to B19V infection from contaminated blood products are immunocompromised individuals (AIDS patients, patients with congenital immunodeficiencies, transplant recipients, and other immunosuppressed patients), individuals who are hematopoietically deficient, and pregnant women. In 2004, the U.S. Food and Drug Administration (FDA) implemented the regulation that B19V DNA levels in plasma pools used for manufacturing of blood products must not exceed 104 IU/ml (see http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm). Similar regulations apply in Europe (European Pharmacopoeia Commission, Council of Europe, European Directorate for the Quality of Medicines). This DNA limit is intended to ensure the safety of blood products (259, 261). Most probably, the existence of neutralizing antibodies in the donor is protective for the recipient if the viral load is low and the recipient has no underlying hemolytic diseases (238).
B19V can also be transmitted from an infected pregnant mother to the fetus. Although the normal outcome of intrauterine infection is delivery of a healthy baby, miscarriage and fetal death can also result if the mother is infected before her 20th week of pregnancy (272–275) (see “Erythema infectiosum,” below). The rate of transmission from mother to fetus has been estimated to be 25 to 50%, and the incidence of fetal loss in B19V-infected mothers has been estimated to be 1.7 to 12.5% (276–280). Seroprevalence has been shown to be higher in pregnant women who have experienced abortion than in those who have not (232). The risk of B19V infection during pregnancy is greatest among susceptible day care workers and schoolteachers (281), but as the risk is also high for pregnant women who are not in these professions, it has been debated whether a policy of excluding women from high-risk workplaces should be recommended (246, 277, 281–284). The increased risks of B19V infection among day care employees compared with those of socioeconomically similar health care professionals was recently estimated by using proportional-hazard regression. The relative risks were estimated to be 2.63 (95% confidence interval [CI], 1.27 to 5.46) among all women and, eliminating the effect of a woman's own children, 5.59 (95% CI, 1.40 to 22.4) among nulliparous subjects (281).
HBoV.
The other human-pathogenic parvovirus, HBoV1, is most likely also transmitted by the respiratory route; it causes respiratory illness, and it can be detected in very high loads in the airways during the acute phase, after which it may persist at low viral loads for months (10, 285–292). The general lack of the other HBoVs, HBoV2 to -4, in airway samples and their presence in feces suggest that these viruses are transmitted by the fecal-oral route (11–13, 293–301).
The HBoVs have not been shown to be transmitted by blood products or vertically (302), nor have they been shown even to be present in blood products (146, 303, 304), with the exception of one recent study from China (305). The most common method of detection of bocavirus infections is PCR, by which HBoV1 has been found globally and throughout the year in about 2 to 20% of airway samples, mainly from children aged 6 months to 5 years with upper or lower respiratory tract illness (306–310). In adults and the elderly, detection is infrequent (310–314). In stool, the most prevalent bocavirus is HBoV2 followed by HBoV1, HBoV3, and HBoV4. Besides airway and stool samples, HBoVs have also been detected worldwide by PCR in serum, tonsils, saliva, urine, gut, and cerebrospinal fluid (CSF) (28, 291, 315–327) as well as in river and sewage water (328–331).
Like B19V, HBoVs also cause systemic infections leading to viremia and an immune response (9, 28, 322, 323, 332–334). However, viremias seem to be more rare and short-lived and/or of lower titers in infections by the enteric HBoVs than in infections by HBoV1 (334, 335). Likewise, the corresponding IgG responses are generally weaker and more prone to waning (335, 336). In a follow-up study of children from birth to adolescence, the median age for HBoV1 infection was 2 years, whereas the median age for both HBoV2 and -3 infections was slightly lower (335). HBoV1 is the most common HBoV in the population, with a seroprevalence of 80% in 6-year-olds, while the seroprevalences of the enteric HBoVs for the same age group are 50% for HBoV2 and 10% for HBoV3 (335). HBoV4 is too rare to make any conclusions regarding transmission or seroprevalence. Due to serological cross-reactivity leading to overestimation and the immunological phenomenon of “original antigenic sin” (337) leading to underestimation (see “HBoV Laboratory Diagnosis,” below), the true frequency of exposure to these closely related viruses is difficult to determine (334, 335, 338).
In a study of saliva samples from 87 infants monitored from birth to 18 months, 76% had a primary HBoV1 infection. Based on the detection of single-nucleotide polymorphisms (SNPs), 12 of these infants had HBoV1 DNA demonstrating multiple variants over time, suggesting reinfection with different HBoV1 strains (291). However, high mutation rates of a persistent virus or contamination from other infants was not excluded, and secondary infections were not confirmed by, e.g., detected increases in IgG levels (336). Moreover, another follow-up study based on identical virus sequences suggested that prolonged DNA positivity could be the result of reactivation of a latent virus (292); if reactivations were to occur, the rate of HBoV1 detection would be expected to be high in elderly individuals, which is not the case (314).
Genotypes and Molecular Epidemiology
B19V.
For many years, the sequence divergence of B19V isolates was considered minimal, <2% in the whole genome (339–342). However, in the late 1990s, a new isolate, V9, was identified and shown to diverge by >11% from the prototypical B19V isolates (44, 45). Later, in 2002, yet another B19V variant emerged, represented by isolates LaLi and A6 (42, 43, 46). The three variants were named genotype 1, for the prototypical B19V isolates; genotype 2, for LaLi- and A6-like isolates; and genotype 3, for the V9- and D91.1-like isolates (42). Of the three B19V genotypes, by far the most common genotype currently circulating in the population worldwide is genotype 1 (343). Since the 1960s, genotype 2 has been seldom found in acute infections or in blood (58, 258, 343–348). However, due to the ability of the B19V genome to persist in tissues for decades after infection (58, 200–204) (see “B19V infection in other tissues,” above), it can frequently be detected within various soft tissues of subjects born before 1972 (58). Recently, B19V DNA was detected in 45% of 106 old bones of Finnish victims from World War II, and all sequences were of genotype 2, except for two, which surprisingly were of genotype 3 (349). Genotype 3 is currently circulating endemically, mainly in some geographical regions such as Ghana, but it has sporadically been encountered in Europe, Brazil, India, and South Africa (42, 343, 344, 350–355). All three genotypes appear to have similar biological, pathogenic, and antigenic properties and make up a single serotype (42, 48, 356, 357). Although rare, it is possible for more than one genotype to infect the same host (354, 358). Recombination between B19V genotypes has also been documented (354, 359). Of the three B19V genotypes, genotype 3 seems to be the most diverse, which might indicate that it has a longer evolutionary history than the other two genotypes (343, 357).
Considering that B19V is a DNA virus and the level of sequence divergence among the prototypical B19V isolates is low, the evolutionary speed is strikingly high, with substitution rates of up to 4 × 10−4 substitutions per site per year, which is within the range for RNA viruses (357, 360–362). Such high evolutionary rates have also been observed for canine parvovirus and other ssDNA viruses that use the host cellular DNA polymerase(s) for their replication (360, 362, 363).
HBoV.
Four differing HBoV variants, HBoV1 to -4, have been identified (9, 11–13). Of these, HBoV1 is the only respiratory virus; the others are more often detected in stool and therefore seem to be enteric. HBoVs have been shown to have a close evolutionary relationship to bocaviruses found in great apes (364–367). HBoV1 and -3 as well as gorilla and chimpanzee bocaviruses are, based on the NS1 protein sequence, members of the same species, Primate bocaparvovirus 1, whereas HBoV2 and -4 belong to the Primate bocaparvovirus 2 species (Table 1) (16, 367). It has been postulated that HBoV1 diverged from the ancestor common to chimpanzee bocavirus ∼60 to 80 years ago, whereas HBoV4 separated from great ape bocaviruses ∼200 to 300 years ago (366). More data are needed to confirm this theory. Furthermore, it seems that extensive inter- and intraspecies recombination has occurred among primate bocaparvoviruses (364, 365, 367–370).
IMMUNE RESPONSE
Adaptive Immune Response
Humoral immune response to B19V infection.
Following either natural (371) or experimental (229, 233) infection by B19V, a strong humoral response is elicited (372). IgM antibody is initially produced at 8 to 12 days postinfection, clears viremia, and lasts for 3 to 6 months (215, 229). The production of IgG antibody follows IgM a few days later. During the following weeks and months, IgM antibody wanes to an undetectable level, whereas IgG prevails. IgA antibody has also been detected and probably protects nasopharyngeal mucosa (373).
The IgM response, as well as the IgG response, is directed against both the VP1 and VP2 proteins (372, 374–376). Several epitopes have been identified on VP2 (377–380) and VP1 (93, 140). Most of the neutralizing epitopes of VP1 are localized in the VP1u or the VP1-VP2 junction region, eliciting a stronger response than VP2 epitopes (136, 139). Neutralization epitopes on the VP1u region are mainly linear and have been mapped to the N-terminal 80 aa (139), which also contain two epitopes for two neutralizing monoclonal antibodies generated from human peripheral blood mononuclear cells (381). B19V-specific B cell memory has been shown to be well established and maintained against conformational epitopes of VP2 and linear epitopes of VP1 but not linear epitopes of VP2 (382, 383).
During both acute and persistent B19V infections, the presence of antibodies against viral nonstructural proteins has also been documented. Anti-NS1 IgG has been proposed to be associated with persistent and complicated infection (384), while anti-NS1 IgM may also appear in acute infections (385) (see “B19V Serology,” below). Detection of antibodies against the two nonstructural 11-kDa and 7.5-kDa proteins has not been reported, although the 11-kDa protein was expressed at a level 100 times higher than that of NS1 during infection (97).
Cellular immunity to B19V infection.
In PBMCs from healthy individuals naturally infected with B19V, B19V-specific T cell-mediated responses are directed against the VP1 and VP2 proteins and presented to CD4+ T cells by HLA class II molecules (386). B19V-specific T helper cell proliferation can be detected in infected patients, and B cells, which recognize the VP1/2 capsids, receive class II-restricted help from CD4+ T cells (387).
Striking CD8+ T cell responses can be observed in patients acutely infected with B19V, which are sustained over a period of months, even after viremia clears (388). Ex vivo measurement of B19V-specific CD8+ T cell responses confirmed that the HLA-B35-restricted peptide derived from the NS1 protein is highly immunogenic in B19V-seropositive donors (389). In contrast, persistently infected individuals show more cellular immune responses to VP1 and VP2 than to NS1 (390). Both the VP1/2 and VP2-only capsids stimulate T helper cells to release gamma interferon (IFN-γ) and IL-10, suggesting that VP2 provides the major target for B19V-specific T helper cells years after virus infection (387). In disagreement, a study reported that the VP1/2 capsid did not promote positive responses for the production of TNF-α and IL-1α from a human monocytic cell line, THP-1, exposed to the B19V capsid (391). The VP1u-specific IFN-γ response is predominant in recently infected subjects, while VP1u-specific IFN-γ and IL-10 responses are absent in remotely infected patients despite the presence of B cell immunity against VP1u (392). Examination of cytokine responses to B19V infection shows that they are of the Th1 type, with IL-2, IL-12, and IL-15 being detected in acutely infected patients, correlating with the sustained CD8+ T cell response (393). There is no imbalance of cytokine patterns in persistent infection, except for an elevated IFN-γ response.
Overall, B19V-specific cellular immunity develops, which is directed against not only the capsid proteins VP1 and VP2 but also the nonstructural protein NS1. The CD8+ T cell response may play a prominent role in the control of acute B19V infection.
Humoral and cellular immune response to HBoV1 infection.
(i) Humoral immune response.
HBoV1 has been shown to induce a strong and long-lasting, albeit often fluctuating, antibody response (336). In contrast to systemic HBoV1 infections, infections by the enteric HBoVs have been hypothesized to be more local and result in weak immune responses (334). However, HBoV2 and -3 also cause systemic infections, including both viremia and antibodies; nevertheless, the IgG responses are generally weaker and more prone to waning than those to HBoV1 (335).
HBoV1 to -4 are structurally similar (26), differing within the major capsid protein VP3 by only 10 to 20%. The high similarity in virion capsid structures and capsid protein sequences among HBoV1 to -4 results in considerable cross-reactivity of IgG antibodies in VLP-based enzyme immunoassays (EIAs) (334, 394). Serological cross-reactivity partially accounts for the high HBoV1 seroprevalences reported previously (164–166). Exclusion of cross-reactivity, by competition with heterotypic VLPs, is a prerequisite for the detection of IgG toward specific epitopes of HBoV1, especially in past immunity (334, 335, 394). In addition to cross-reactivity, it has been shown that interactions between consecutive diverse HBoV infections affect HBoV immunity via a phenomenon called original antigenic sin (337). This was detected by observing that preexisting HBoV2 immunity in a subsequent HBoV1 infection resulted in low-level or nonexistent HBoV1-specific antibody responses (335). Instead, a vigorous recall response against the first HBoV2 strain appeared. Noncompetition HBoV1 and HBoV2 EIAs, however, showed the IgG responses to both consecutive virus types. This original antigenic sin was further characterized in a more controlled noninfectious setting in 10 sequentially VLP-inoculated rabbit pairs, 5 of which exhibited immune responses of various degrees, in line with this phenomenon (338). Based on the newly established HBoV competition EIA, the median age of HBoV1 IgG seroconversion is 1.9 years (range, 0.5 to 8.0 years). The HBoV1-specific IgG seroprevalence in children aged 6 years is 80% (335).
(ii) Cellular immune response.
The presence of HBoV1-specific CD4+ T cell immune responses in adults is strong and age dependent (395). In cultures of PBMCs isolated from healthy adults with HBoV1-specific IgG, CD4+ T helper cell responses specifically against HBoV1 VP3 VLPs include the release of IFN-γ, IL-10, and IL-13 (395, 396), and there is no cross-reactivity with responses against B19V VP2 VLPs (396). In HBoV1-infected individuals, levels of the cytokines TNF-α, IL-2, IL-5, and IL-8 are increased in sera (397). In nasopharyngeal aspirates of HBoV1-infected patients, levels of Th1/2 cytokines, especially IFN-γ, IL-2, and IL-4, are increased in children with HBoV1-related bronchiolitis (398).
Innate Immunity
Innate immunity to B19V infection.
The innate immune response during B19V infection has not been well studied. TLR9 can recognize the CpG oligodeoxynucleotide-2006 analog (5′-GTTTTGT-3′) with a phosphodiester backbone, localized in the P6 promoter region of the B19V genome (113). CpG oligonucleotide-2006 selectively inhibited the growth of BFU-E progenitors in a sequence-specific manner and stalled cells in S and G2/M phases (113). Transfection of B19V NS1 and VP2 in nonpermissive COS7 cells significantly increased the expression levels of defensins and also regulated the expression of TLR4, -5, -7, and -9 (399). These findings need to be validated in B19V-permissive cells and during the natural course of B19V infection. SNPs associated with acute symptomatic B19V infection are present in the SKIP, MACF1, SPAG7, FLOT1, c6orf48, and RASSF5 genes, and these genes and their products might have a role in parvovirus infections (400).
Innate immunity to HBoV1 infection.
HBoV1 VP3 has been reported to modulate the IFN pathway by targeting ring finger protein 125 (RNF125), a negative regulator of type I IFN signaling. RNF125 conjugated Lys48-linked ubiquitination to RIG-I and subsequently led to the proteasome-dependent degradation of RIG-I. VP3 not only upregulated IFN-β promoter activity but also enhanced IFN-β production at both the mRNA and protein levels. VP3 interacted with RNF125, which resulted in the reduction of RNF125-mediated ubiquitination and proteasome-dependent degradation of RIG-I (401). In contrast, NP1 blocked IFN-β activation. NP1 interacted with IFN regulatory factor 3 (IRF-3) through the DNA-binding domain of IRF-3, preventing associations between IRF-3 and the IFN-β promoter (402). These studies were performed by using Sendai virus-infected HEK293 cells, which were transfected with HBoV1 VP3 or NP1. Whether or not HBoV1 modulates the innate immune response through the IFN-β pathway during virus infection of human airway epithelia awaits further investigation.
CLINICAL MANIFESTATIONS
Diseases Caused by B19V Infection
In several diverse diseases, B19V is the etiological agent (Table 2). Their pathophysiology has been established by studies of B19V outbreaks, series of well-defined cases, and intensive investigations of small numbers of patients. Furthermore, intranasal inoculation of B19V into healthy volunteers produced symptoms, signs, and laboratory findings later identified in ill patients, particularly cutaneous eruption, arthralgia, and depression of blood counts (229) with associated pathognomonic bone marrow morphology (233). Fifth disease and transient aplastic crisis are typical features of B19V infection in normal and hematologically stressed individuals; hydrops fetalis can follow in utero infection, and B19V can persist in marrow, causing pure red cell aplasia (PRCA) in an immunocompromised host. Many of the historical complications of clinical fifth disease have been observed in acute B19V infection and can be assumed to be unusual presentations of infection. In other syndromes, the evidence is less firm, often based on single, invalidated, marginally positive, or misinterpreted test results. As a result, some of the literature is in conflict, and initial reports may not be confirmed by more rigorous and better-controlled studies. Hepatitis, myocarditis, autoimmunity, and chronic fatigue syndrome have been linked to B19V infection, and there are mechanisms to explain these peculiar manifestations of the virus in each disease. It should be stressed that most B19V infections are likely asymptomatic (8): seroconversion occurs without apparent illness.
TABLE 2.
Major diseases caused by B19V infection
| Disease(s) | Progression | Host(s) |
|---|---|---|
| Erythema infectiosum | Acute | Healthy children |
| Arthropathy | Acute or chronic | Healthy adults |
| Hydrops fetalis/fetal loss | Acute or chronic | Fetus |
| Transient aplastic crisis | Acute | Patients with a high rate of red blood cell turnover, e.g., sickle cell disease patients |
| Persistent infection and pure red cell aplasia | Chronic | Immunocompromised patients, e.g., HIV/AIDS or postchemotherapy patients |
Erythema infectiosum.
Erythema infectiosum or fifth disease is more accurately designated acute B19V infection. “Slapped cheek” disease was categorized in the 19th century as fifth among a series of childhood rash illnesses and, as their etiologies were uncovered, fifth disease also was suspected of having a viral origin, perhaps as a rubella variant. Shortly following the discovery of B19V, this virus was established as the etiological agent of fifth disease.
Historically, fifth disease was described as occurring in seasonal outbreaks in patterns similar to those of rubella, being most prevalent in winter and spring and in 3-year cycles, with a brief period of incubation, a high infectivity rate, and likely droplet spread (403–406). Clinically, prodromal symptoms did not usually precede the rash, which appeared suddenly in otherwise well children. The rash appeared in stages, first as facial erythema (the “slapped cheek”) and then as erythematous maculopapular eruptions over the trunk and proximal extremities, followed by fluctuating and evanescent exanthematous eruptions lasting for weeks or longer. Arthralgia and myalgia were seen in more affected adults. Arthritis and encephalitis (407–409) were noted as rare complications of fifth disease.
Following the discovery of B19V, its candidacy as a disease agent was rapidly established by investigations of epidemics of fifth disease: B19V-specific IgM and seroconversion of IgG appeared in the blood of affected children (234). Numerous confirmatory studies of fifth-disease epidemics throughout the world have been reported (214, 410, 411). Variable presentations of infection, even within a family (412); a high proportion of asymptomatic seroconversion (8); and the prevalence of rheumatic symptoms in adults, especially women (8, 413), were corollary results. B19V was detected in skin biopsy specimens (414, 415). When parvovirus is epidemic in a community, it causes a mild rash illness in the normal pediatric population and a severe but purely hematological syndrome in susceptible sickle cell disease patients (235) (see below).
Variants in the stereotypical pattern of cutaneous eruption (416–419) have attracted the attention of dermatologists, usually as “glove-and-socks” syndromes (420, 421). The characteristic distribution of glove and socks can be seen with papular, petechial, pustular, and bullous morphologies (422–428), even desquamation (429); concurrently in family members (430); and with oral lesions (431), lymphangitis (432), and vasculitic features (see below). Lymphadenopathy is present in some children with fifth disease (433–435), and presentation with enlarged lymph nodes and hepatosplenomegaly can mimic mononucleosis (436, 437).
B19V arthropathy.
About one-third of adults with fifth disease have acute joint symptoms (8), more frequently than in children (438, 439), as predicted from earlier observations of fifth disease (406). However, chronic prolonged rheumatic symptoms can occur in children as well as adults (440, 441). Arthralgia and arthritis can persist for months or years after parvovirus infection and may be debilitating, but there is no joint erosion (442). The arthropathy resembles that of rheumatoid arthritis, but B19V is not the etiological agent of that disease. The relationship of parvovirus infection to other rheumatic syndromes is controversial (180, 443–449) as discussed below.
Arthropathy following B19V infection was described soon after the virus was linked to fifth disease (450, 451). In patients with serological confirmation of infection, many will have recently experienced a rash (450–457). Arthritis—swelling and redness—as well as pain mimic rheumatoid arthritis in joint distribution, but in most cases, resolution occurs within a few weeks of symptoms and signs (451), and upon long-term follow-up, patients are without symptoms (458). Of patients identified in a chronic arthropathy clinic, about 15% of adults had had a recent parvovirus infection (450); in rheumatic diseases of childhood, 20 to 35% of patients may show serological evidence of recent B19V infection (459, 460). In a German series, about one-quarter of synovial biopsy specimens from arthritis cases contained B19V DNA by PCR, and these cases were typically diagnosed as undifferentiated mono- and oligoarthritis (461). In more recent surveys, serological evidence of recent B19V infection was present in only 9/813 patients with persistent joint swelling and possible rheumatoid arthritis (462).
Rheumatoid factor (409) and other autoantibodies (463–468) may be detected, transiently, in B19V arthropathy, as in viral arthropathy in general (469). There are many reports of B19V DNA and occasionally of capsid protein (suggesting viral replication) in joints (446, 461, 470–475). However, in other studies, B19V infection has been infrequently determined to precede the onset of arthritis (476, 477). Conversely, B19V DNA also appears in control samples, either in joint fluid from patients without inflammation (473, 476, 478) or in healthy individuals who have undergone arthrocentesis for trauma (202, 476). That synovial tissue may be positive for multiple virus types suggests that inflammatory sites may harbor cells containing viruses (475).
The treatment of patients suffering from chronic parvovirus arthropathy is unclear. Most patients respond to nonsteroidal anti-inflammatory drugs or short courses of corticosteroids. Eventually, symptoms should resolve without residual joint disease (with exceedingly rare exceptions) (479). There are sporadic, unique reports of immunological abnormalities of uncertain clinical significance: histocompatibility antigen overrepresentation and low complement levels (480), low levels of proinflammatory cytokines and chemokines (481), and anti-NS1 antibodies (482). As B19V does not circulate in arthropathy patients, and the significance of detection of viral DNA in joint fluid or tissue is unclear, there is no obvious justification for immunoglobulin therapy, and the benefit of its occasional use (483, 484) is uncertain due to polypharmacy and/or natural resolution of the inflammatory process.
Hydrops fetalis.
Maternal B19V infection is a serious complication of pregnancy; it can cause both miscarriage (death before week 22) and intrauterine fetal death (IUFD) thereafter. Hydrops fetalis, massive edema in the fetus associated with death in utero or at birth, is best characterized, and B19V is important in the consideration of the differential diagnosis of nonimmune hydrops. Hydrops follows most commonly midtrimester infection of the mother but may occasionally occur with earlier- and later-trimester transmission (485–487). B19V exposure in early pregnancy may increase the rate of spontaneous abortion. Fetal demise was recognized early and has been extensively reviewed (488–498). From seroepidemiology, an annual loss of 120 fetuses was calculated for Japan (499), and about twice this number might be extrapolated for the United States. Congenital malformations due to B19V are rare, but occasionally neonates who survive in utero infection may have hematological abnormalities similar to those of persistent B19V infection (see below). Hydrops late in pregnancy and spontaneous fetal loss are the typical clinical sequelae of B19V, but other reported complications of infection have been isolated fetal effusions (rather than generalized edema) (500), mirror syndrome (edema concurrently in mother and fetus) (501), and preeclampsia and eclampsia (502).
(i) Pregnancy risks.
About 50% of women of childbearing age are seronegative for antibodies to B19V, but the proportion may be higher in developing countries (503–505). Seroconversion rates are variable during nonepidemic and epidemic periods, over which they may range 10-fold, from 1.5 to 13% (282). In early studies from the Public Health Laboratory in the United Kingdom (277) and the U.S. Centers for Disease Control and Prevention (506, 507), fetal transmission occurred in about 30% of women who became infected with B19V. The excess risk of fetal death is 3% to 11% when maternal infection occurs before 20 weeks of gestation, but the risk is very low thereafter (487, 508). In a smaller series of 39 B19V-infected pregnant women, no cases of hydrops were detected, but the overall fetal loss rate was 5% (278). In a long-term retrospective survey from Pittsburgh, PA, numbers of cases of B19V infection in pregnancy appeared to vary from year to year, usually without typical fifth-disease symptoms; hydrops occurred in 12% and intrauterine death occurred in 16% of 25 pregnancies (509). In another study of 43 intrauterine B19V infections (determined by fetal/infant PCR or IgM), none of the cases developed hydrops or died; the incidence of intrauterine B19V infection was 48% in the first half and 56% in the second half of pregnancy (280). In one prospective study of third-trimester fetal death, 7.5% of placentas contained B19V DNA (510). The variability in transmission from mother to fetus is illustrated by instances of infection and death of only a single twin (511, 512).
Fetal loss early in pregnancy has been associated with first-trimester B19V infections. In a population-based Danish study, the presence of anti-B19V IgM almost doubled the risk of fetal loss, but only 0.1% of the losses were attributable to the virus (513). In a cross-sectional study of fixed tissue from fetal loss, 6% contained B19V DNA, all in first-trimester spontaneous abortions (514). Some (515, 516) but not all (517) other cross-sectional studies have been confirmatory in implicating B19V in spontaneous abortion in early pregnancy.
Retrospectively, B19V was found in some series of tissues from nonimmune hydrops in 8 to 17.5% of cases, perhaps due to sampling during epidemics (518–521), but others have not shown risks of fetal loss and hydrops associated with serological evidence of recent B19V infection (522). One meta-analysis yielded a real but relatively low risk of fetal demise with maternal B19V infection, 10% overall and 12.5% for infection during the first 20 weeks of pregnancy (523). In a large British cohort study, the fetal risk was similar, 9%, and confined to the first 20 weeks of pregnancy (508). Risks of both infection and a poor fetal outcome have been sufficiently low that some experts have argued against the exclusion of women from occupations with exposure to children and monitoring during pregnancy (524, 525).
Children are the likely source of infection for most adults, and thus, multiparous women (with children at home) as well as teachers and health care workers may be at special risk of infection during pregnancy (246, 281, 526, 527) (see “Cellular response to productive B19V infection,” above).
It has been argued that lack of surveillance and virological investigations led to inaccurate estimates of fetal loss and associated risk factors such as maternal environmental contacts (528, 529). Of note, B19V infection may be without symptoms in an expectant mother, and fetal loss secondary to B19V might not be suspected except by the interested specialist (487, 530, 531).
(ii) Pathogenesis.
Hydrops is characterized by gross and global edema of fetus or newborn, often with visceral organomegaly. Severe anemia is typical, often with leukoerythroblastic reactions in the blood and evidence of inflammation, especially in the liver, where erythropoiesis is located. While anemia is the dominant hematological feature of B19V hydrops, fetal thrombocytopenia occurs and may be common (532, 533). Cytopathic effects of B19V infection include typical eosinophilic intranuclear inclusions and “balloon” cells, especially in fetal liver. Many postmortem pathological studies have documented B19V infection in fetal tissues by a variety of methods, including PCR, in situ hybridization, electron microscopy, and histochemistry (274, 489, 534–547). A few brief reports focus on myocardial infection and its consequences, such as heart failure and myocardial necrosis (548–550).
Maternal levels of antibody to B19V are higher in the mother than in the fetus, while viral DNA levels have been higher in fetal blood than in maternal blood (551).
(iii) Diagnosis.
Hydrops in utero is detected by ultrasound (552). Other diagnostic tests include specific evidence of cardiac failure in the fetus (553), maternal serum alpha-fetoprotein (554, 555), and detection of virus by amniocentesis (556) and cordocentesis (557). The presence of anti-NS1 antibodies may signal recent infection of a pregnant woman (558, 559).
(iv) Treatment.
There are many case reports of successful treatment of hydrops by intrauterine red blood cell transfusion (560–567), but this procedure may fail to rescue the fetus (568). The mortality rate in one series of even aggressively transfused fetuses was still high, with a survival rate of 70% overall (569). In 10 South Korean pregnancies, transfusion was associated with better outcomes, but the overall survival rate was only 60% for the entire cohort (570). Other therapeutic strategies have included immunoglobulin and digitalis (571). The efficacy of interventions is difficult to estimate, not only because transfusion is not completely effective but also because hydrops due to B19V can resolve spontaneously (572–576).
(v) Congenital infection.
Infants usually survive without any clinical consequence of uterine exposure to B19V. IgM and even on occasion viral DNA can be detected in infants months after birth (577); however, in other follow-up studies, evidence of chronic infection among exposed infants was lacking (578). Surveys of infants with congenital malformations have failed to implicate B19V (508, 579). In a large Danish cohort of >1,000 children whose mothers tested positive for B19V infection during pregnancy, there was no increase in rates of mortality and morbidity for children monitored for a median of 9 years after birth (580).
Pure red cell aplasia is the best-described and perhaps only congenital lesion following fetal infection. Anemia with a deficiency of erythroid precursors is indistinguishable from Diamond-Blackfan syndrome. In one small series from Denmark, B19V was considered the etiological agent in 3/11 children with Diamond-Blackfan syndrome, in all of whom anemia resolved (581). Anemia due to infection with B19V in utero may be fatal or resolve as the infant produces appropriate anti-B19V antibodies (582–584). Immunoglobulin infusions for persistent infection diminished viremia and led to apparent remission (585, 586) but in other instances have not been effective (529, 587, 588). Other infants resolved anemia and infection with blood transfusion support alone (584).
Congenital anemia may be accompanied by other hematological abnormalities such as thrombocytopenia (589) and transient leukoerythroblastosis (590). A few instances of fetal liver disease with B19V hydrops have been described (548, 591). Neurological symptoms, mainly developmental delays but also seizures and hydrocephalus, may be direct sequelae of viral infection or indirectly related to severe anemia in utero and at birth (587, 592, 593). Other diverse associations include ascites (594–596), bone lesions (597), and corneal opacification (598). Embryonic malformations have been seen in incomplete embryos after uterine infection (599). However, infants who survive hydrops do not have other congenital abnormalities, nor is B19V considered a major etiological agent of congenital malformations.
Transient aplastic crisis (TAC).
When parvovirus is epidemic in the general population, some patients in hematology clinics will coincidentally suffer a specific complication of B19V, transient aplastic crisis (235). Transient aplastic crisis was recognized as a life-threatening acute event in sickle cell disease long before its viral etiology was known (600–602). Its sudden appearance in families, apparently brief incubation period, and transiency were suggestive of an infection (603); early findings in marrow giant pronormoblasts are now recognized as a viral signature of infection. The target cell of B19V is the erythroid progenitor, always infected during parvovirus illness. As demonstrated in healthy volunteers infected with B19V, blood counts fall and there is an absence of reticulocytes (the young circulating erythrocytes) (229). Anemia does not develop in healthy volunteers and in otherwise healthy individuals, due to the brief cessation of erythropoiesis and the long life span of erythrocytes in the circulation. Under conditions of hematopoietic stress, usually in patients with sickle cell disease, in which there is increased red cell destruction due to chronic hemolytic anemia, B19V infection leads to a precipitous worsening of anemia. Red blood cell transfusion is adequate to treat transient aplastic crisis, but anemia can be severe and fatal if transfusions are not available or not administered urgently.
Transient aplastic crisis was the first human illness associated with B19V, and it was discovered during routine immunoelectrophoresis for testing for antibodies to B19V and antigen in young children (604). Antigenemia, signifying viremia, is common in cases of aplastic crisis but almost never observed in fifth disease; in healthy volunteers, marrow suppression occurs with viremia and fifth-disease symptoms with antibody production and immune complex formation (229) (occasional case reports document this sequence [605]). Levels of circulating virus in cases of aplastic crisis can be extraordinary, 108 to 1012 vgc/ml (146, 371). IgM is usually detectable in serum (371, 606, 607).
Transient aplastic crisis due to B19V infection has been well characterized by studies of outbreaks (371, 608, 609) and many large case series (606, 607, 610–615). B19V explains virtually all community-acquired cases of transient aplastic crisis. Parvovirus infection is the presumptive diagnosis when anemia worsens acutely in sickle cell disease, especially if the usually elevated reticulocyte count is low. Reticulocytopenia is diagnostic, and bone marrow examination is not required. Aplastic crisis in cases of sickle cell disease is serious and life-threatening. Patients are acutely ill, febrile, and often in pain; not only is the anemia usually extremely severe, but there also may be accompanying infarction events, acute chest syndrome, and splenic sequestration. Recovery within a week or two is typical, associated with the appearance of IgM and IgG antibodies specific to B19V; rarely, anemia may be prolonged (616). Particularly severe B19V aplastic crisis in cases of hemolytic anemia can produce marrow necrosis (617–619) and infarction (620), hemophagocytosis (621, 622), fat embolism (623, 624), and splenic sequestration (625, 626). Conversely, and as in the general population, B19V infection may be subclinical in sickle cell disease, inferred from seroconversion without a history of aplastic crisis (610, 627).
Siblings and other family members are affected at a high rate, consistent with the known rate of transmission of B19V, and the incubation period is brief, usually just a few days. Seroconversion occurs early in childhood and continues throughout adult life, as in the general population; in a well-studied cohort in Philadelphia, PA, the incidence was estimated to be 11/100 patient years (611). About one-half of sickle cell patients enter adulthood seronegative and thus at risk of infection. Transient aplastic crisis appears to be a unique event in the course of sickle cell disease (611, 613): from lack of recurrence, lifelong immunity to B19V has been inferred.
Transient aplastic crisis occurs with other hematological syndromes (628). It can be the first evidence of hereditary spherocytosis, in which hemolysis is well compensated and baseline anemia is mild (606, 607, 629–636). B19V aplastic crisis has been documented in cases of hereditary somatocytosis (637), thalassemia (638, 639), hemoglobin C disease (640), red cell enzyme deficiencies (641–645), iron deficiency anemia (646), acquired immune hemolytic anemias (621, 647–649), Diamond-Blackfan anemia (650), hereditary erythrocyte multinuclearity (651), human immunodeficiency syndrome (652), myelofibrosis (653) and complicating or mimicking myelodysplastic syndrome (628, 654–657), and acute (658) and chronic (659) lymphocytic leukemia.
Transient cessation of erythropoiesis likely occurs in all individuals infected with B19V, as first observed among inoculated normal volunteers (229) and later in laboratory personnel who were accidently infected (659). Erythroblastopenia immediately precedes fifth disease when early blood counts are obtained (660). Acute anemia with parvovirus infection in apparently normal children has been reported occasionally (651, 660–664), but B19V infection is not the cause of transient erythroblastopenia of childhood (665–667), although recent B19V infection has been reported in a few instances (661, 663, 668).
Persistent infection and pure red blood cell aplasia.
B19V infection can persist in individuals with compromised immune systems. As infection causes acute anemia when the patient's bone marrow is susceptible because of underlying erythroid stress, anemia is chronic when the immune system fails to mount a neutralizing antibody response. The marrow morphologies in acute and chronic B19 infections are the same, with a lack of erythroid precursors and with the presence of scattered giant pronormoblasts and anemia accompanied by a virtual absence of reticulocytes. Other blood counts are normal. As with transient aplastic crisis, in pure red cell aplasia due to the persistence of B19V, there is little clinical evidence of viral infections. These symptoms and signs—fever, malaise, cutaneous eruption, and joint pain—are mediated by immune complexes, which do not form in the absence of antibodies to the virus. Because persistent infection does not manifest as a typical viral illness and lacks the typical features of fifth disease, the diagnosis can be obscure. Recognition of B19V persistence is important because antibody therapy is effective in ameliorating or curing disease manifestations. However, an inaccurate diagnosis subjects the patient to unnecessary toxicity from immunoglobulin infusions, which are costly (669).
Typically, in cases of red cell aplasia due to persistent infection, titers of B19V in the circulation are extremely elevated. Viral DNA can be measured readily by direct hybridization methods. Low levels of B19V, detectable by gene amplification, may be present in the blood of normal persons for months or longer after an acute infection, unassociated with symptoms or blood count changes (373, 670–672). The virus may persist in many visceral tissues as well, complicating attempts to associate B19V infection with disease (see below).
In the first described case (137), the patient had had a diagnosis of pure red cell aplasia for a decade; his brother had developed the same syndrome at the same time and succumbed to it. The presence of giant pronormoblasts in marrow was the clue to a viral etiology, and B19V DNA was found at very high concentrations in the living patient and in his and his brother's pathological specimens. The proband was ultimately diagnosed with Nezelof's immunodeficiency, and as a result, he lacked reactivity to the VP1-unique region of the B19V capsid protein and neutralizing antibodies to the virus. In general, for persistent infection, serological testing is uninformative: either antibodies are not produced or there is weak reactivity of anti-B19V IgG on capture assays or enzyme-liked immunosorbent assays (ELISAs). DNA testing, preferably by direct hybridization and quantitative PCR (qPCR), should show abundant viral genetic material. The initial patient was treated with normal donor immunoglobulins to restore antibody activity, with prompt reticulocytosis and complete correction of his long-standing transfusion-dependent anemia. Identification of persistent parvovirus infection causing anemia is important, as effective therapy is available and easily applied; also, persistently infected patients remain highly infectious to others.
B19V persistence occurs with immunodeficiencies of different origins: congenitally (673–675); secondary to chemotherapy and with immunosuppression, especially posttransplantation; and in patients infected with human immunodeficiency virus (HIV). Children are particularly susceptible because of a lack of prior immunity to B19V. In a survey of cases of pediatric red cell aplasia, 7/33 children had persistent B19V infection (676). B19V is a particular complication in cases of acute lymphocytic leukemia, the major leukemia of childhood (137, 677–685), and other pediatric cancers (620, 686, 687). Adults with acute and chronic lymphocytic leukemia and lymphoproliferative disease can be affected; they have often been treated with lymphocyte-depleting drugs and monoclonal antibodies (620, 688–690). Iatrogenic immunosuppression is required after organ replacement, and B19V DNA, like DNAs of other viruses, may be found in the circulation in this context, without clinical manifestations (691–693). B19V anemia should be accompanied by reticulocytopenia and viremia (267). PRCA due to B19V occurs in post-hematopoietic stem cell transplant (692, 694–702) and post-solid-organ transplant (703–722) patients. In post-hematopoietic stem cell transplant patients, persistent B19V infection was associated with severe lymphopenia (723). B19V persistence can cause PRCA in HIV-infected patients, sometimes as the presenting syndrome and prior to the development of AIDS (652, 724–734). B19V is not prevalent in HIV-infected individuals, however, and it is likely that a very-high-titer infection is required for the development of PRCA (735, 736). B19V has been detected in marrow specimens from AIDS patients with PRCA, with accompanying giant pronormoblasts (733, 737).
Persistent B19V infection causing PRCA has been described rarely for immunologically normal persons (738–740) and patients without an apparent immunological defect (741, 742). Nevertheless, about 30% of patients admitted to the hospital with chronic anemia in one serological screen showed DNA or IgM evidence of B19V infection (628).
Anemia due to chronic B19V infection is responsive to immunoglobulin administration, but repeated courses may be necessary (724, 743, 744). HIV patients also clear B19V with immune reconstitution upon highly active antiretroviral therapy (744–746).
B19V infection and malaria.
Of relevance to potential vaccine development, B19V occurs in areas where malaria is endemic (747), and serological surveys have suggested worse chronic anemia in children who are seropositive for B19V (IgG) or have evidence of recent infection (IgM and viral DNA) (748–751). Acute B19V infection may occur concurrently with malaria (752, 753). Among pregnant Sudanese women, seropositivity for IgG to B19V was associated with lower hemoglobin levels (754).
Other hematological syndromes linked to B19V.
B19V has been linked to other blood disorders by serological evidence of recent infection and sometimes detection of the virus in blood and marrow, but an etiological role of B19V in these diseases is less defined than for transient aplastic crisis. Coincidental infection usually cannot be excluded, and the causative role suggested in individual case reports has often not been validated when sera from larger numbers of patients have been systematically examined (6, 755).
Hemophagocytosis is generalized, usually severe, and frequently fatal marrow failure and pancytopenia, with accompanying fever and a marked elevation of levels of inflammatory markers and cytokines. Hemophagocytosis, especially in children, is triggered by viral infections, most often herpesviruses and less commonly B19V (621, 729, 756–762). Some cases are fatal. Hemophagocytosis may be the manifestation of B19V infection during pregnancy (763). Hemophagocytosis can be observed in marrow of patients with transient aplastic crisis (764, 765).
Although anemia is the hallmark of symptomatic hematological disease due to B19V infection, thrombocytopenia, usually moderate, can accompany transient aplastic crisis (605, 663, 766), as may neutropenia (767–769), or both, producing transient pancytopenia (770). Mild decreases in blood counts have been noted rarely in cases of fifth disease, without underlying hematological disorders (771), but they may be more frequent if actively ascertained (772). Cytopenias without manifest fifth disease but with serological evidence of acute B19V infection may occur (773). B19V protein has been detected in granulocytes of an affected patient (702).
Thrombocytopenia occurs alone in B19V infection, which may lead to a diagnosis of idiopathic thrombocytopenic purpura (ITP) (in which immune destruction of platelets is the pathophysiology) (774–776) and occasionally megakaryocytic thrombocytopenic purpura (in which marrow does not produce platelets) (777). For ITP, systematic surveys suggest that a minority of otherwise typical cases might have a B19V origin (701, 778). The absence of megakaryocytes upon bone marrow examination in a few cases (663) implies a direct effect of B19V on platelet production.
Neutropenia may also result from immune-mediated peripheral destruction of granulocytes or a failure of the marrow to produce white blood cells, termed agranulocytosis. As described above, a modest decrease in neutrophils can be observed with B19V infection. Rare instances of isolated neutropenia have been blamed on B19V in children (779) and adults (780, 781). A claim of B19V as the etiological agent of chronic neutropenia in childhood (782) could not be confirmed (783). In a single convincing case, recurrent agranulocytosis was associated with persistent B19V infection (784). In a newborn with apparent congenital neutropenia, or Schwachman-Diamond syndrome, delivery had been proceeded by maternal-fetal B19V transmission and infection (785).
Aplastic anemia, pancytopenia with marrow hypocellularity, is not due to B19V in general; rare instances with associated seroconversion at presentation may be coincident with B19V infection rather than evidence of causation (786–793).
B19V infection can occur in patients with leukemia, especially during or after chemotherapy, producing the expected reticulocytopenia (794–797). Its significance in other respects, especially for pathogenesis, is unclear (6, 798). Viral infection preceding presentation with acute lymphoblastic leukemia has been observed (799, 800). Giant pronormoblasts can mimic the appearance of myelo- and lymphoblasts and therefore complicate the interpretation of blood and marrow histology (415).
Encephalitis and other neurological syndromes.
Fifth-disease patients were occasionally recognized to suffer from neurological symptoms, and diverse neurological complications have been associated with parvovirus infection, including encephalitis, meningitis, stroke, and neuropathy (407, 408, 801–804).
Neurological complications of B19V may be more frequent in immunocompromised hosts who have underlying diseases, in patients undergoing chemotherapy or immunosuppression, and in the setting of hemolytic anemia (805), but they can occur in immunocompetent, apparently normal children and adults and in the context of acute or chronic parvovirus infection. Pediatric cases include diagnoses of encephalitis (184, 805–810), meningitis (811), status epilepticus (812) and seizures (813), stroke (814, 815), amyotrophy (816), Guillain-Barré syndrome (817), ocular disease (813, 818), transverse myelitis (819), peripheral neuropathy (820), cerebellar ataxia (821), and others. Chorea and ataxia may be features of B19V encephalitis (184, 805, 822, 823). Fetal brain infection has occurred after in utero infection in hydropic births (824, 825). B19V DNA has been detected by gene amplification in brains at autopsy of patients with psychiatric disorders (826, 827), likely due to either laboratory contamination or a persistence of low levels of viral DNA in these tissues (828, 829), as in many others (see below). B19V DNA was detected by gene amplification in about 5% of cerebrospinal fluid samples from patients with encephalitis (184, 830).
A specific genotype of B19V in the blood of encephalitis cases was described (831). Associations with histocompatibility antigens and serum cytokines have been inferred to support an immune pathophysiology (822).
Intravenous immunoglobulin (IVIG) has been employed therapeutically for neurological syndromes linked to B19V (805, 808, 824), but efficacy is difficult to judge, as many patients improve spontaneously.
Myocarditis and other heart diseases.
B19V infection can be followed by serious cardiac disease, especially pediatric myocarditis. Other viruses are more frequently associated clinically or found by molecular methods in endocardial biopsy specimens (832). The clinical course of B19V myocarditis can be severe, with significant morbidity and mortality, including the need for a transplant.
B19V infection, detected most often by serological evidence of recent infection and sometimes by the detection of viral genomes in cardiac tissue, has been reported in myocarditis in the fetus (see the section on hydrops below), in infancy, during childhood, and among young adults (174, 176, 832–840). In one center's series of 19 pediatric patients, the presenting symptoms were upper respiratory and gastrointestinal symptoms; all infected children had ventricular dysfunction, 2 died, and 3 required heart transplantation (176). B19V infection has been cited as a cause of cardiac inflammation after heart transplantation (175, 841–845) and as a possible etiological agent of adverse events after coronary artery stenting (846). In some series, B19V myocarditis was distinguished clinically by presentation with infarction-type pain but with a better prognosis for recovery (847). In children with acute heart failure, the presence of B19V DNA has been associated with improvement of cardiac function over time and a better long-term prognosis (848). Dilated cardiomyopathy in a few Chinese systemic lupus patients was associated with serological evidence of B19V infection and immune cytokine levels (849).
As with other tissues, the prevalence of B19V DNA in the hearts of individuals not suspected of having a B19V syndrome (essentially, controls) is high (193, 203, 850, 851). In an autopsy study, B19V was detected by gene amplification in one-third of postmortem hearts (viral DNA was also detected in about 10% of livers, 20 to 30% of marrow, and 10% of kidneys, not correlated with serological status) (852). Therefore, it is not surprising that investigators have detected B19V DNA in various percentages of patients with myocarditis (192, 853, 854), pediatric heart transplant recipients (855), and cases of dilated cardiomyopathy (853, 856). The presence of replicative intermediate B19V DNA forms (857–859) and B19V capsid protein in biopsy specimens has been reported (860), but B19V viral loads have not correlated with outcomes (861). Myositis affecting muscles other than the heart is rarely associated with B19V (798).
A virus-triggered immune pathophysiology has been inferred from NS1-specific cytotoxic lymphocytes in cases of inflammatory cardiomyopathy (862). T cell infiltrates in the heart and elevated circulating concentrations of cytokines may accompany putative viral infection (174, 863) and immunomodulatory therapies applied to eliminate the virus (864).
Hepatitis and other liver diseases.
B19V has been reported to be associated with a range of liver findings (865), from a mild elevation of levels of hepatic transaminases (866, 867) to viral-like acute hepatitis (186, 793, 868–876) and acute or fulminant liver failure (765, 877–882). As in other tissues, livers at autopsy or sampled by biopsy can show persistence of B19V DNA (883, 884), confounding the interpretation of data from case reports. Systematic studies of sera from patients with non-A, non-B, non-C hepatitis have not shown B19V infection (885–888).
B19V and autoimmune/immune-mediated diseases.
Similarities between B19V arthropathy—specific clinical and serological features—suggested that parvovirus infection might be etiological or associated with rheumatoid arthritis, especially as an infectious etiology for rheumatoid arthritis for many years, and earlier work even suggested a candidate parvovirus, RA-1 (889) (likely a cell culture contaminant, in retrospect). Viruses have been suspected of triggering or causing a wide variety of immune-mediated and autoimmune diseases, with elegant mechanisms and models proposed for B19V as well (179, 890–893).
Some investigators claimed evidence of a strong link between B19V infection, most often serological but including detection of DNA in joint tissue, and classic rheumatoid arthritis (181, 894–897), including possible interactions with histocompatibility antigen (898). However, these data have not been confirmed (446, 899–901). The relationship of B19V to juvenile rheumatoid arthritis (Still's disease) followed a similar pattern of enthusiastic claims of causation (902–905) but had a lack of reproducibility of results (202, 449, 906). A higher prevalence of antibodies to NS1 was present in children with a variety of rheumatic diseases, interpreted as evidence of persistent infection (907).
Facial (malar) rash and joint pain and swelling, even angioedema, are features of systemic lupus erythematosus (SLE), and B19V may mimic the clinical presentation of SLE (908–912). In a few cases, B19V infection seemed to be followed by the development of SLE (913), although misdiagnosis of B19V arthropathy with the more serious autoimmune disease seems possible in retrospect (824, 913–915). Interesting speculation has hypothesized a mechanistic link of B19V to SLE (891, 916, 917). However, B19V could not be serologically linked to SLE (918). B19V DNA was first detected in a high proportion of marrow specimens and later in skin (920) from cases of systemic sclerosis, a severe multiorgan fibrotic disease (919); the same group found viral DNA localized to endothelial and perivascular inflammatory cells in skin biopsy specimens by in situ hybridization, with negative controls (921). However, the virus has also been detected in skin samples from controls in other studies (922).
Necrosis of skin lesions and sometimes visceral organ involvement suggestive of vasculitis have been reported in cases of fifth disease (923–929). As a group, various vasculitis syndromes have been linked to B19V infection, usually by single case reports or small case series, including leukocytoclastic vasculitis (930), Kawasaki disease (931), Henoch-Schonlein purpura (932, 933), polyarteritis nodosa (183), Wegener's granulomatosis (183), and giant cell arthritis (934). In some of these reports, documentation of acute infection and characteristic vasculitis pathology is convincing, and patients may even have responded to immunoglobulin therapy after failing immunosuppression (183, 935), suggesting an etiological rather than coincidental role for parvovirus. Anti-NS1 antibodies were prevalent in one series of Henoch-Schonlein disease cases (936), as in cases of childhood arthritis (see above). However, systematic attempts to relate B19V infection to a range of vasculitis syndromes have often yielded negative results (356, 937–941).
B19V and kidney disease.
B19V infection has been linked to kidney diseases (942). The most common disease is red cell aplasia in immunosuppressed allograft recipients (943). There are case reports of B19V and glomerulonephritis (944–946), nephrotic syndrome (947), thrombotic microangiopathy (948, 949), and other post-renal-transplant complications. B19V DNA may be present in tissue obtained at biopsy but, as with other organs, may represent only viral persistence long after infection.
Other associations.
Chronic fatigue syndrome may be a debilitating consequence of apparent viral infection. B19V has been reported to be the trigger in individual cases (950–952) and case series (952). Psychological stress has been suggested to worsen symptomatology (953). Viremia has been present in some series (952) and absent in others (954). A few patients appeared to have improved after immunoglobulin administration (955), but in one Italian patient, immunoglobulin infusion worsened symptoms and was associated with the appearance of circulating viral DNA (956). Fibromyalgia has also followed B19V infection (957).
Pneumonia, usually in the setting of other apparent organ involvements, has been observed in B19V-infected patients (436, 870, 958–962).
Other syndromes for which a causative role for B19V is unclear include generalized edema (963–966), myositis (967), the onset of diabetes (968) and inflammatory bowel disease (969), uveitis (970), retinal detachment (971), retinal pigment epitheliopathy (972), Rosai-Dorfman histiocytic proliferation (973), laryngitis (974), inner ear disease (975), and testicular tumors (976). B19V DNA persistence in the thyroid after previous infection may account for associations with thyroid cancer (191) and autoimmune thyroiditis (977).
Diseases Associated with HBoV Infection
Respiratory tract infections.
Although Koch's postulates cannot be fulfilled for HBoV1 by using animal models, there is increasing evidence suggesting that HBoV1 is indeed an etiological pathogen, rather than an innocent bystander, in both upper and lower respiratory tract infections, especially in children under 5 years of age.
HBoV1 DNA has been found in nasopharyngeal samples worldwide in ∼2 to 20% of patients with upper or lower respiratory disease (10). In early studies of respiratory tract infections, and unfortunately also in current laboratory diagnostics, nonquantitative PCR detection of HBoV1 DNA is overwhelmingly used for the detection of HBoV1 infections (9, 307, 308, 310, 311, 317, 978–996) (see “HBoV Laboratory Diagnosis,” below). A high coinfection rate (up to 83%) is generally detected in respiratory specimens, and the virus has also been detected in asymptomatic children. The etiological role of HBoV1 in respiratory diseases has consequently been questioned. However, the ubiquitous presence of HBoV1 is actually due to the persistence of the virus in the nasopharynx for several weeks to up to a year after infection (285–289, 291). To determine the causative role of HBoV1, other diagnostic markers are needed beyond mere PCR positivity in airway samples. These markers include high viral loads, mRNA or antigen detection in respiratory samples, viremia, serodiagnosis, and monoinfection (28, 316, 322, 323, 332, 333, 997–1001). Careful clinical studies applying more accurate markers have been done, providing accumulating evidence that HBoV1 is an important respiratory pathogen in children (28, 151, 285, 286, 288, 290, 310, 316, 322, 323, 332, 333, 1001–1010). Pneumonia, bronchiolitis, acute otitis media, the common cold, and exacerbations of asthma are the most common clinical manifestations of HBoV1 respiratory tract infections, with symptoms of cough, fever, rhinitis, wheezing, and diarrhea (151, 287, 306–308, 310, 311, 316, 317, 322, 979, 990, 1002, 1005–1007, 1011–1020). In one study, seven children with severe acute respiratory tract illness in a pediatric intensive care unit had HBoV1 as the sole pathogen, as verified by next-generation sequencing (NGS) (1010).
A low virus load of less than 104 to 106 vgc/ml, depending on the study, detected by quantitative PCR in respiratory specimens should not be used as a diagnostic marker for acute HBoV1 infection, as prolonged/persistent infection/reinfection by HBoV1 in the respiratory tract also results in low viral loads (285, 287, 288, 291, 316, 1012, 1021–1025). Considering viral loads below this threshold as being diagnostic leads to high rates of detection in asymptomatic subjects or patients coinfected with other respiratory pathogens.
HBoV1 monodetection, high viral loads of >104 to 108 vgc/ml in respiratory specimens (151, 285, 290, 316, 322, 323, 999, 1009, 1026–1031) and detection of HBoV1-specific IgM or a 4-fold increase in the levels of HBoV1-specific IgG antibodies (28, 151, 332, 1006) have been clearly linked to acute respiratory infections. In some studies, high viral loads during acute infection have further been shown to correlate with increased symptom severity or duration (290, 1026, 1029), while other studies found no such differences (285, 1028). Lower respiratory tract infection has been correlated with the detection of HBoV1 spliced mRNA, an indirect marker of active HBoV1 replication, in nasopharyngeal aspirates (997, 1001, 1032) and the detection of viral DNA in serum (viremia) (1009, 1033). In a clinical assessment of HBoV1 infection among 258 wheezing children (332), 96% of the children with HBoV1 loads of >104 vgc/ml in nasopharyngeal aspirates also had a serodiagnosis (IgM and often also IgG conversion or a ≥4-fold increase in the IgG level), compared with only 38% of the children with low viral DNA loads. Of 49 children with HBoV1 viremia, 92% exhibited a serodiagnosis. Furthermore, among 39 HBoV1 PCR-positive children with codetection of other respiratory viruses, 64% had true serologically proven acute HBoV1 infections, i.e., had IgM and often also seroconversion or a ≥4-fold increase in IgG levels.
Life-threatening HBoV1 infections in pediatric patients, detected by high virus loads or diagnostic HBoV1-specific antibodies, have been reported (1007–1009, 1030, 1034). In a case of severe airway constriction, a 4-year-old girl who had a history of wheezing had bronchiolitis, leading to severe respiratory failure and the need for extracorporeal membrane oxygenation (1009). The patient was negative for both bacterial and viral respiratory pathogens but positive for HBoV1 infection, with virus loads of up to 109 vgc/ml in nasopharyngeal aspirates and of up to 104 vgc/ml in serum, and she was serum IgM positive. In another case, a 20-month-old child had acute bronchiolitis that developed into a severe airway constriction course characterized by pneumothorax, pneumomediastinum, and respiratory failure with air leak syndrome. HBoV1 infection was verified by electron microscopy and HBoV1 genome monitoring in respiratory secretions and plasma (1013). In addition, an immunosuppressed adult patient who died of respiratory complications was diagnosed with HBoV1 infection with very high virus loads in respiratory secretions (>4.3 × 109 vgc/ml) and in serum (1.5 × 104 vgc/ml) but without HBoV1-specific IgG/IgM. No other pathogens were detected at high titers, suggesting that HBoV1 may indeed have been the causative agent of the respiratory complications that led to the death of this patient (1031). Besides being a respiratory pathogen, HBoV1 has also been detected as the sole pathogen in cerebrospinal fluid samples of five patients with encephalitis, one of whom, a malnourished child from Bangladesh, died (326, 327).
In conclusion, primary acute HBoV1 infection should be diagnosed only when high virus loads are detected, active virus is found replicating in respiratory secretions, or the patient is IgM positive and/or has a seroconversion or a ≥4-fold increase in IgG titers or viremia. Primary acute HBoV1 infection causes both upper and lower respiratory tract infections, with manifestations ranging from the mild symptoms of the common cold to severe pneumonia and bronchiolitis, which may be life-threatening.
Diseases associated with HBoV2 to HBoV4.
HBoV2 to -4 are detected primarily in stool; however, the role of HBoV2 to -4 in gastrointestinal infections is unclear. HBoV2 was present in 20% of symptomatic and asymptomatic patients, whereas lower prevalence rates have been found for HBoV3 and HBoV4 (295, 298, 300, 1035–1037). Although some reports show a difference in the prevalences of HBoV2 in stool specimens between patients with gastroenteritis and healthy or nondiarrheal controls (11, 1036), other reports have found no such associations. Furthermore, HBoV2 has often been codetected with other viral pathogens, especially rotavirus and norovirus (300, 1036, 1038). In addition, HBoV2 infection does not appear to exacerbate clinical symptoms of gastroenteritis (1036). In most studies, however, there were no associations of HBoVs with gastroenteritis (300, 1035, 1038, 1039). Consequently, there is no clear evidence yet to support a causative role of HBoV2 in gastroenteritis. HBoV2 has been involved in two fatal cases, one of myocarditis and one of encephalitis, as the sole finding in a range of tissues, blood, or CSF (326, 1040). HBoV3 and -4 are too rare for clinical associations, although HBoV3 has been detected as the sole agent in the CSF of a child with encephalitis (327).
LABORATORY DIAGNOSTICS
Antibody detection in serum is the cornerstone for the diagnosis of B19V infection, while nucleic acid tests give further valuable aid (1041, 1042). Antigen detection is infrequently used, and B19V culture is not included in the routine diagnostic laboratory. Among immunocompromised patients, detection of B19V DNA may be required due to a deficiency in antibody production. For pregnant women, it is also recommended that levels of both antibodies and DNA be measured in serum. Past infection is determined by the detection of IgG antibodies, while the time of acute infection is possible to deduce by determining the presence of IgM and the quality of IgG or an increase of the IgG quantity, as discussed below.
Detection of HBoVs has been achieved primarily with PCR-based assays (1043). However, to accurately diagnose acute infections, other means such as serology, qPCR, antigen detection, or viral mRNA detection by reverse transcription (RT)-PCR are needed to overcome the problem of viral DNA persistence in both the respiratory tract and stool.
B19V Serology
Detection of IgM and a 4-fold increase or seroconversion of IgG in paired serum samples are the most reliable markers for acute B19V infection. IgG persists for life and is thus a marker of past infection. Furthermore, low epitope-type specificity (ETS); low IgG avidity; and the presence of IgG3, IgA, or IgE as well as NS1 antibodies can be helpful for a definitive diagnosis, as discussed below.
The first antibody assays for B19V used native virus as an antigen. The assays were in the formats of immunoelectron microscopy (IEM), IgM antibody capture radioimmunoassays (MACRIAs), IgG antibody capture radioimmunoassays (GACRIAs), enzyme immunoassays (EIAs), immunofluorescence assays (IFAs), and hemadherence tests (HATs), followed by chemiluminescent immunoassays (CLIAs) and Luminex-based singleplex and multiplex microsphere suspension immunoassays (SIAs) applying recombinant structural antigens (215, 1044–1057).
Recombinant empty capsids (VP2- or both VP1- and VP2-assembled VLPs) expressed in insect or mammalian cells are suitable sources of antigen for serological assays (89, 373, 1053, 1055, 1058–1063). Insect cell-produced VP1 or prokaryotically produced VP1 or VP2 has also been used successfully (375, 1061, 1064–1069), in addition to synthetic peptides (1070–1072). There are many in-house as well as commercial antibody detection assays of variable quality (1056, 1073–1078).
VLPs are antigenically analogous to the native viral capsid and, if treated and immobilized gently, exhibit conformational epitopes, whereas prokaryotically expressed proteins are denatured, exhibiting linear epitopes. Especially for VP2 as the antigen, it is of the utmost importance to maintain conformational epitopes (383, 1079). IgG antibodies to linear VP2 epitopes are detected mostly during acute infections and early convalescence, whereafter they disappear. In contrast, IgG antibodies to conformational epitopes persist for life (383). The kinetic difference between or the ratio of the antibody responses of linear and conformational epitopes has been exploited to develop unique serological assays, named ETS EIAs, to differentiate past from recent B19V infections (383, 1072, 1080). In ETS EIAs, either VP1 versus VP2, linear denatured VP2 versus conformational VP2, or a linear acute-phase-specific peptide versus conformational VP2 may be employed. Another means of distinguishing between past and recent infections is by measuring IgG avidity or functional affinity, the binding force between the antigen and the antibody (1081). The avidity of specific IgG antibodies is initially low after primary antigenic challenge but, upon clonal selection of B cells in germinal centers, increases during subsequent weeks and up to 6 months (1082, 1083). The avidity of IgG is measured by comparing the antibody titration curves from a normal EIA with those from a protein-denaturing EIA, where weakly binding antibodies are detached and washed away by a denaturing agent, whereas high-affinity antibodies remain antigen bound. For B19V, the antigen of the IgG avidity EIA should be VP1, because VP2 is not recognized by past-immunity IgG after denaturing treatment (376, 383). Both ETS and IgG avidity EIAs can be reliably performed on a single IgG-positive serum sample and are therefore important for confirmation of occasional unreliable IgM results. While this ETS phenomenon seems to be specific for parvoviruses, IgG avidity measurements have been used successfully for the detection of a wide variety of microbial infections (1081, 1084), including those by B19V and HBoV1 (374, 376, 1085). ETS and IgG avidity EIAs have been shown in different settings to increase the accuracy of the diagnosis of B19V infections when applied together with an IgM EIA (1042, 1080, 1086, 1087).
The levels and response kinetics of B19V-specific IgG subclasses to the VP1 and VP2 proteins have also been elucidated (1088). For both antigens, the predominant IgG subclass at all times is IgG1, whereas IgG2 levels remain very low and IgG3 is associated with the acute phase of infection. In contrast, IgG4 is detected exclusively against VP1 and only after 5 to 6 months postinfection, suggesting long-term immune stimulation. The different kinetics of IgG3 and IgG4 were further utilized for a diagnostic test for recent B19V infection, with good sensitivity and specificity (1088). The past-immunity differential responses of IgG1 to -3 to B19V were later confirmed and further shown to be similar to those for mycoplasmal but different from those for chlamydial protein antigens (1089).
Besides the more common IgM and IgG measurements, B19V-specific IgA has also been detected in human serum, but the specificity in cases of acute infections does not seem to be high enough for diagnostic purposes (373, 1061). B19V-specific IgE is present in human sera, but its potential for diagnostic use has not been evaluated (1090, 1091). B19V-specific IgE has also been detected by line blot assays of breast milk and infant sera, perhaps providing further antiviral protection in nursing children (1092). Besides breast milk, B19V antibodies have also been detected in saliva and thumb prick blood samples, suggesting that these samples may be convenient alternatives to serum for the serodiagnosis of B19V infection (1093–1095).
Although the structural proteins VP1 and VP2 are the most important antigens, the diagnostic value of the nonstructural protein NS1 has been assessed (384, 385, 559, 1096–1102). B19V NS1-specific IgG has been proposed to be a serological marker for patients with severe arthritis and chronic infections but not for those without complications, suggesting a potential involvement of NS1 or anti-NS1 antibodies in pathogenesis (384, 1096, 1099, 1102). However, this association has been disputed (1098, 1100).
B19V Nucleic Acid Testing
B19V DNA is detected in the respiratory tract and blood a week after infection, and high-titer viremia, detectable by dot blot hybridization, is present for a few days to a week (1103), whereafter viremia persists at lower levels (236–240). Nucleic acid detection in blood, serum, or plasma is important for both B19V diagnostics and screening of blood products. B19V PCR may have diagnostic utility in a very early phase of infection, before the appearance of antibody, and also in the late convalescence phase (1041). PCR may be the only diagnostic method for immunodeficiency and is of valuable help for the detection of B19V infection in pregnant women and fetuses. However, due to virus persistence, detection of B19V DNA in tissue specimens does not indicate acute infection (203). The possible persistence of B19V in blood (viremia) also among immunocompetent subjects greatly complicates the interpretation of PCR positivity. A threshold of 104 vgc/ml has been suggested as a diagnostic criterion (1042).
The first nucleic acid tests for B19V were based on dot blot hybridization (1103). They were later replaced by more sensitive qualitative PCRs (542, 1104, 1104–1106) and qPCRs (1107, 1108). There are many in-house and commercial qPCR assays for genotype-specific as well as pan-B19V amplification for diagnostic and blood screening purposes (242, 1042, 1109–1114). Many PCR methods show poor sensitivity or fail to detect all three genotypes. For validation of B19V PCR-based assays, a reference panel for B19V DNAs of different genotypes is available from the World Health Organization Expert Committee on Biological Standardization (ECBS) (1115). In addition to PCR, in situ hybridization is used to detect B19V DNA in cells and tissues (1116, 1117).
B19V Antigen Assays
B19V antigen in serum can be detected with monoclonal antibodies in EIAs, radioimmunoassays, or immunoblot assays, but these methods are relatively insensitive and not reliable for the detection of acute infections, except perhaps for patients with aplastic crisis (215, 535, 1046, 1118, 1119). Antigen detection by an EIA has been used to screen blood donors (1120). Antigen detection by immunohistology or detection of virus particles by electron microscopy (543, 728, 860, 1121–1123) can be useful for localizing the virus in individual host cells in tissue sections. DNA hybridization or amplification assays are preferred for more sensitivity of virus detection.
HBoV Laboratory Diagnosis
The diagnosis of respiratory infections traditionally has included immunofluorescence antigen detection and virus culture but increasingly is based on PCR with respiratory samples (1124). Several commercial and in-house multiplex respiratory virus panel PCR assays have been developed, but many do not include HBoV1, and some are not very sensitive for HBoV1 (1125–1128). It is also possible to apply a metagenomics approach for the detection of respiratory pathogens, with fairly good sensitivity (1129). However, the long persistence of HBoV1 in the nasopharynx complicates the interpretation of positive DNA amplification test results (285–292), and the clinical significance of low viral loads is doubtful. PCR positivity in the airways is therefore not a diagnostic marker of primary infection (28, 332, 335). The same consideration applies to enteric HBoVs in tissues and stool (325, 335). Detection of anti-HBoV1 antibodies or viral DNA in serum or of HBoV1 spliced mRNA, high copies of viral DNA, or antigen in airway samples is a more reliable tool to detect primary infection (28, 316, 322, 323, 332, 333, 997–1001, 1032).
Probe-based qPCR assays for HBoV2 (1130, 1131), HBoV3, and HBoV4 (295, 298), in a singleplex or multiplex format, have been developed for the detection of HBoV2 to -4 genomes in clinical specimens. These qPCR assays are highly specific for each type of HBoV and have a limit of detection of ∼10 vgc per reaction (298, 1131).
HBoV1 capsid proteins produced in Escherichia coli, baculovirus-infected insect cells, or yeast have been used as antigens in serological assays, mostly as VLPs (28, 165, 166, 322, 333, 394, 1132, 1133). In contrast to B19V serology, VP1u is not a good antigen for HBoV serology (28). Serological markers for HBoV diagnosis are the presence of IgM, seroconversion or a ≥4-fold increase in the IgG titer in paired sera, and low IgG avidity, the latter of which can stage a primary infection by a single serum sample (1085). Serodiagnosis of HBoV1 infection is not simple due to the existence of HBoV2 to -4 complicating the immune response. The HBoVs are closely related and structurally very similar (26), with VP3 protein sequence divergences of 20% between HBoV1 and the enteric viruses and only 10% among the enteric HBoVs (13). This similarity generates both serological cross-reactivity and an immunological phenomenon called original antigenic sin, familiar in dengue and influenza virus research (337). Such factors need to be taken into consideration in the design of and interpretation of results from serological assays (334, 335, 338, 394). For the detection of HBoV1-specific antibodies, a competition EIA has been developed, in which the immobilized VLP antigen of HBoV1 is competed with soluble VLPs of the other heterologous HBoVs for patient antibodies, thus blocking the antibodies against the shared epitopes and leaving only those that react with the unique epitopes of the HBoV1 VLP antigen (334, 394). In a recent study of constitutionally healthy children who were monitored by serology for many years, several PCR-confirmed heterotypic secondary HBoV infections with inefficient or no specific IgG responses to the unique epitopes of the second virus type were observed. Instead, a strong recall response against the first virus type appeared (335). Because of original antigenic sin and/or cross protection, HBoV1 infections in individuals with preexisting HBoV2 or -3 IgG may be difficult to detect serologically. HBoV1 serological assays are not yet commercially available.
Recently, HBoV1 was added to the platform of an innovative multiplex point-of-care antigen test for respiratory tract infections, which is based on separation-free two-photon excitation fluorometry (1000, 1134, 1135). The HBoV1 antigen test was subsequently used to estimate the period of active HBoV1 infection to about 1 week, which coincided with the decrease in the severity of clinical symptoms (1000).
TREATMENT AND PREVENTION
IVIG is effective in treating certain disease conditions triggered by B19V infection. High-dose IVIG is currently administered to patients with B19V-associated chronic anemia and PRCA (137, 1136, 1137). Symptoms can recur when IVIG treatment is interrupted (1138–1141). The administered IVIG may be contaminated with B19V or other viruses (e.g., hepatitis A virus) (700, 1138). There are no known effective antiviral drugs for the treatment of diseases caused by B19V, especially for transient aplastic crisis due to B19V.
In certain B19V-infected patients, e.g., pregnant women and sickle cell disease patients, B19V vaccination would provide substantial benefit by preventing fetal loss, aplastic crisis, and blood dyscrasias. A B19V vaccine might also prevent other B19V-associated diseases, such as myocarditis.
An early study showed that antisera resulting from immunization with baculovirus-derived B19V VP1-containing VLP capsids neutralized B19V infection of human erythroid progenitor cells, highlighting that VP1-containing VLPs are applicable as a human vaccine for preventing B19V infection (89, 1142). A B19V vaccine candidate, which was produced as B19V VLPs from insect cells expressing wild-type VP1 and VP2, has been tested in phase I clinical trials using the MF59 adjuvant (1143, 1144). Insect cells were coinfected with two baculoviruses at different MOIs at a VP1-to-VP2 ratio of ∼1:3. After receiving at least two doses of the vaccine intramuscularly, all vaccinated volunteers seroconverted to become positive for anti-B19V antibody, as determined by EIAs and neutralization assays (1143, 1144). B19V-neutralizing antibodies were sustained for at least 6 months after the third dose (1143). In the first clinical trial, safety evaluations revealed mostly injection site reactions that were mild to moderate (1143). However, the second clinical trial was halted because skin rashes near the infection site occurred (1144). Insect cell contaminants in the vaccine preparations and/or the PLA2 activity of the VLPs might cause reactogenicity.
For the production of the B19V VLP vaccine, the relative amounts of VP1 and VP2 expressed were adjusted by manipulating the MOIs of the two baculoviruses, which created process reproducibility and logistical challenges. B19V PLA2-mutated VLPs have been produced by expressing PLA2-mutated VP1 and VP2 in a consistent ratio of 1:5 from Saccharomyces cerevisiae transfected with a bicistronic plasmid (1145). Immunization of BALB/c mice demonstrated that mouse PLA2-mutated VLPs (mPLA2-VLPs) did not exhibit PLA2 activity and elicited a neutralizing response in the presence of the MF59 adjuvant as strong as that of the wild-type VLP counterpart.
The MF59-adjuvanted vaccine candidates elicited B19V-neutralizing antibody levels similar to those following infection in humans (1143, 1144). However, it is unknown whether vaccine-induced neutralizing antibody titers are sufficient for protection against B19V-associated diseases. High-risk groups with diseases caused by B19V are children with sickle cell disease, who have significant morbidity following B19V infection, and pregnant women who have not previously been infected with B19V. Targeted immunization of these groups will potentially reduce the risk of life-threatening B19V-associated diseases. In addition to the prevention of B19V-associated diseases, the demonstration of an effective B19V vaccine might attract development and application utilizing B19V VLPs as antigen carriers for the presentation of antigenic determinants of other infectious agents, such as dengue 2 virus and anthrax (1146, 1147).
OTHER EMERGING HUMAN PARVOVIRUSES
Human Parvovirus 4
Human parvovirus 4 (PARV4) was first discovered in 2005 in a hepatitis B virus-infected injection drug user (14). The virus has been detected in plasma samples worldwide (1148). PARV4 and PARV4-like viruses have been classified as members of a new genus, Tetraparvovirus, in the family Parvoviridae (Table 1) (16). The full-length genome of PARV4 has not been sequenced, lacking information on the ITR (14).
PARV4 genomes have been detected in human plasma pools (14, 1149), in the livers of both hepatitis C virus (HCV)-positive individuals (1150) and healthy individuals (1150), and in the bone marrow of HIV-positive individuals (828, 1151). PARV4 DNA was also found in cerebrospinal fluid of two children with encephalitis of an unknown etiology (1152). A few cases of acute PARV4 infections have been demonstrated but with no clear clinical manifestations (1153, 1154). There are currently three known genotypes of PARV4 (1155), of which genotypes 1 and 2 are widespread in Europe, Asia, and North America and, as suggested by the high prevalence of PARV4 in hemophiliacs, injection drug users, and HIV- or HCV-infected subjects, seem to be transmitted parenterally by contaminated needles or other blood contact (828, 1156, 1157). Genotype 3, in contrast, has been detected in non-drug users in sub-Saharan Africa, where viral DNA has been found in nasal and stool samples of children, indicating foodborne, respiratory, or contact spread (1158–1161). PARV4 DNA has been detected in plasma during acute infection but often with a low viral load (<3 × 104 vgc/ml) (1153, 1162, 1163). The highest PARV4 load reported for acute viremia so far is 1010 vgc/ml (1153). An 8.6% prevalence of asymptomatic viremia due to PARV4 in infants in Africa has been found (1159). Viremia can last for various time periods, from 30 days to 7 months (1153, 1164). In most cases, PARV4 viremia appears to be self-limiting and asymptomatic (1159), and PARV4 infection does not seem to increase the severity of disease in coinfections with other blood-borne viruses (1165). Overall, the clinical significance of PARV4 infection remains unclear. Nevertheless, due to its blood-borne nature, PARV4 infection may manifest clinical symptoms, particularly in immunocompromised patients.
Little is known about the biology of PARV4. PARV4 has not been cultured in vitro. It is predicted that PARV4 has two identical termini at the ends. The gene expression profile of PARV4 has been studied only by transfection of an incomplete PARV4 genome (1166) (Fig. 4). Two promoters, P6 and P38, are used to transcribe NS-encoding and VP-encoding mRNAs from the left and right sides of the genome, respectively. A spliced form of R2 mRNA, which is transcribed from the P6 promoter and spliced in the NS1-encoding region, likely encodes a small NS2 protein, although it was not detected by transfection (1166). During transfection, the R4 mRNA, which encodes VP2, was abundantly expressed, ∼80% in total viral mRNA. However, the R1M mRNA, which encodes NS1, was expressed at a level of ∼10% in total. Other species of viral mRNAs were minimally expressed, at <5%. NS1 and VP2 were clearly detected at molecular masses of ∼80 and ∼65 kDa, respectively. However, which start codon VP1 actually uses to translate mRNA is not clear (Fig. 4). PARV4 VP1u should be long, at least 261 aa, based on the location of a putative PLA2 motif, although VP1u did not exhibit PLA2 activity in vitro (1166). NS1 exhibited cytopathic activity in ex vivo-generated HSCs (1166).
FIG 4.
Transcription map of PARV4. The incomplete PARV4 ssDNA genome (GenBank accession no. NC_007018) is shown to scale in negative sense with predicted inverted terminal repeats (ITRs). Transcription units, including promoters, the polyadenylation site [poly(A)], and splice donor (D) and acceptor (A) sites, are indicated with their respective nucleotide positions. Major detected mRNA species are diagrammed to display their identities and respective sizes in the absence of the poly(A) tail. The encoding ORFs are diagrammed in colored boxes, and the predicted molecular masses (kilodaltons) of translated proteins and detected molecular masses upon transfection are indicated at the right. Potential VP1 start sites are speculated to be located at nt 2378 for a VP1 protein of 914 aa and at nt 2678 for a VP1 protein of 814 aa, as shown. ND, not detected.
For PARV4 DNA detection, there is a qPCR with a probe targeting the NS-encoding region with a limit of detection of 50 vgc/reaction (1167). Multiplex PCR can identify and quantify levels of PARV4 genotypes 1 and 2 (303) and genotype 3 (1159). A two-step qPCR assay for quantitation and genotyping of all three PARV4 genotypes has been further established (1163); it first applies a single panprobe for screening for and quantitation of the levels of PARV4 and then, for positive samples, employs multiple genotype-specific probes for genotyping. This novel PARV4 genotyping qPCR has high sensitivity and specificity.
PARV4 VP2-generated VLPs have been used to establish an IgG EIA and a μ-capture-format IgM EIA for serodiagnosis (325, 1154, 1157). In the general population, the seroprevalence of PARV4 varies by genotype and geographically, from 20 to 37% in sub-Saharan Africa (1168) to 9.4% for a low-risk population in Lithuania (1169), 4.76% for blood donors in the United Kingdom (1170), and 0% in healthy subjects in Nordic countries (1154, 1171). Additionally, PARV4 infection induced strong, broad, and persistent T cell responses (1164).
Human Bufavirus
BuV was first identified in 2012 in feces from children <5 years of age with acute diarrhea by metagenomics analysis (15). A prevalence of 4% among the rotavirus antigen-negative cases of childhood diarrhea was found (15). The NS1 protein of BuV shares 38% identity with those of the other protoparvoviruses, barely including it in this genus (15). BuV has three genotypes (15, 1172), belongs to a new species called Primate protoparvovirus 1 (Table 1), and is the first human parvovirus in the genus Protoparvovirus (16). NS1 shares >95% identity among all bufaviruses, but capsid proteins share only 72% identity (15). The longest genome of BuV1 strain BF.7 (GenBank accession no. JX027295) reported so far has a size of 4,822 nt, which includes NS1, a middle ORF protein, and capsid protein genes, from the left, middle, and right sides of the genome, respectively. It does not contain the left- and right-end hairpins and therefore is an incomplete genome.
BuV DNA has been detected worldwide in feces of children and adult patients with gastroenteritis but with a low detection rate (<1.4%) and a low viral load (103 to 104 vgc/ml of the supernatant of feces) (1172–1177). BuV DNA has not been detected in stool samples of healthy individuals (1174, 1176). These studies suggest that bufavirus may, albeit infrequently, cause gastroenteritis, but further studies are needed to confirm causality. Assays for serodiagnosis are being developed (M. Söderlund-Venermo, unpublished observations).
Another protoparvovirus, tusavirus, has been detected in the stool of one Tunisian child with unexplained diarrhea, but whether this rodent parvovirus-like virus is a true human virus is still unconfirmed (1178).
FUTURE DIRECTIONS
Disease Validation and Animal Models
An animal model of B19V infection would greatly contribute to our understanding of the pathogenesis of infection in humans and to test antiviral drugs. The remarkable similarities between simian parvovirus (SPV) and B19V suggest that experimentally SPV-infected cynomolgus monkeys may serve as a useful animal model of B19V infection (1179). However, SPV has not been cultured in vitro and is difficult to obtain in large quantities from sick animals. B19V replicates in cynomolgus bone marrow (1180), and thus, cynomolgus monkeys may be a suitable animal model for pathogenesis studies of B19V infection (1181).
HBoV1 infection is highly restricted to human airway epithelia. No animal models of HBoV1 infection have been reported. An open-ended human bronchial xenograft nude mouse model (1182–1184) could be used as an alternative in vivo model. This model, which develops a fully differentiated mucociliary epithelium and mimics human airways, supports productive HBoV1 infection (J. Qiu, unpublished observations). In addition, animal viruses of the same genus as HBoVs or BuV may give further information on diseases and pathogenesis, some of which may also apply to humans (1185–1187).
Antiviral Drug Development
No specific antiviral drugs have been developed for the treatment of B19V infection. High-dose IVIG is administered to patients with B19V chronic anemia due to PRCA (137, 1136, 1137), but symptoms can recur when IVIG treatment is halted (1138–1141). Repeated applications of IVIG and maintenance therapy (744, 1136, 1188–1193) may be cost-prohibitive. Anti-B19V drugs to prevent virus entry or to inhibit B19V replication would be an effective approach to the treatment of bone marrow failure due to B19V infection. Cidofovir (CDV), an acyclic nucleoside phosphonate, has shown activities against five families of human dsDNA viruses (1194) and relevant anti-B19V activity in UT7/Epo-S1 cells as well as in ex vivo-expanded CD36+ EPCs (1195, 1196).
The neutralization activity of anti-B19V antibody is directed to linear epitopes of VP1u (136, 139). An human monoclonal antibody against a peptide (aa 30 to 42) of VP1u showed strong neutralization of B19V infection in vitro (381). An anti-VP1u human monoclonal antibody would be ideal to treat B19V infection of human bone marrow.
An inhibitor of PI3K kinases significantly decreased HBoV1 replication in HAE-ALI culture (155); these inhibitors are in development for cancer treatments and might be repurposed to treat HBoV1-associated diseases.
ACKNOWLEDGMENTS
We acknowledge support from PHS grants AI105543, AI112803, and AI105543 from the National Institute of Allergy and Infectious Diseases; a subaward of P30 GM103326 from the Centers of Biomedical Research Excellence (COBRE) Program of the National Institute of General Medical Sciences, National Institutes of Health (to J.Q.); the Finnish Medical Foundation; the Sigrid Jusélius Foundation; the Medical Association Liv och Hälsa; the Helsinki University 375-year celebration grant (to M.S.-V.); and the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health (to N.S.Y.).
The funders had no role in data collection and interpretation or the decision to submit the work for publication.
We are indebted to Safder Saieed for his early input on the review. We thank Martha Montello and Heather McNeill at the COBRE Writing Core and Elizabeth Jenkins for editing the review.
Biographies

Jianming Qiu obtained his Veterinary Medicine (B.S.) and Biochemistry (M.S.) degrees from Zhejiang University, China, and his Ph.D. in Virology from the National Institute for Viral Disease Control and Prevention, China CDC, Beijing, China. He acquired postdoctoral training in parvovirology at the Hematology Branch, National Institutes of Health, Bethesda, MD, and at the Department of Molecular Microbiology and Immunology, University of Missouri—Columbia, Columbia, MO. He is currently Professor of the Department of Microbiology, Molecular Genetics and Immunology, University of Kansas Medical Center, Kansas City, KS. His current research interests have focused on the molecular biology and pathogenesis of human parvoviruses B19 and HBoV and the use of parvoviruses as vectors for human gene therapy. He is a member of the ICTV Parvoviridae Study Group.

Maria Söderlund-Venermo obtained her M.Sc. at the University of Helsinki, Department of General Microbiology, and her Ph.D. at the Department of Virology, Helsinki, Finland. She was a visiting scientist at the University College London, London, UK, and did her postdoctoral training at the Departments of Molecular Microbiology and Immunology and Veterinary Pathobiology, University of Missouri—Columbia, Columbia, MO, USA. She is a docent, specialist in clinical microbiology, and senior lecturer at the University of Helsinki Medical Faculty, Department of Virology. Her research interests include both clinical and molecular virology, with the main focus on B19V, HBoV, and the emerging human parvoviruses. She is furthermore a member of the ICTV Parvoviridae Study Group.

Neal S. Young received an A.B. from Harvard College and an M.D. from the Johns Hopkins School of Medicine. His internal medicine residency was at Massachusetts General Hospital. He completed a clinical fellowship in the Hematology-Oncology Division at Barnes Hospital, Washington University, St. Louis, MO. His entire career has been in the Intramural Research Program of the National Institutes of Health in Bethesda, MD. He is currently Chief of the Hematology Branch of the National Heart, Lung, and Blood Institute. He is primarily known for work in the pathophysiology and treatment of aplastic anemia and is also known for his contributions to the pathophysiology of B19V infection.
REFERENCES
- 1.Berns KI, Parrish CR. 2015. Parvoviridae, p 1768–1791. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Cotmore SF, Tattersall P. 2005. Structure and organization of the viral genome, p 73–94. In Kerr J, Cotmore SF, Bloom ME, Linden RM, Parrish CR (ed), Parvoviruses. Hodder Arnold, London, United Kingdom. [Google Scholar]
- 3.Samulski RJ, Muzyczka N. 2014. AAV-mediated gene therapy for research and therapeutic purposes. Annu Rev Virol 1:427–451. doi: 10.1146/annurev-virology-031413-085355. [DOI] [PubMed] [Google Scholar]
- 4.Cossart YE, Field AM, Cant B, Widdows D. 1975. Parvovirus-like particles in human sera. Lancet i:72–73. [DOI] [PubMed] [Google Scholar]
- 5.Heegaard ED, Brown KE. 2002. Human parvovirus B19. Clin Microbiol Rev 15:485–505. doi: 10.1128/CMR.15.3.485-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young NS, Brown KE. 2004. Parvovirus B19. N Engl J Med 350:586–597. doi: 10.1056/NEJMra030840. [DOI] [PubMed] [Google Scholar]
- 7.Brown KE, Young NS. 1997. Parvovirus B19 in human disease. Annu Rev Med 48:59–67. doi: 10.1146/annurev.med.48.1.59. [DOI] [PubMed] [Google Scholar]
- 8.Woolf AD, Campion GV, Chishick A, Wise S, Cohen BJ, Klouda PT, Caul O, Dieppe PA. 1989. Clinical manifestations of human parvovirus B19 in adults. Arch Intern Med 149:1153–1156. doi: 10.1001/archinte.1989.00390050111022. [DOI] [PubMed] [Google Scholar]
- 9.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A 102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jartti T, Hedman K, Jartti L, Ruuskanen O, Allander T, Söderlund-Venermo M. 2012. Human bocavirus—the first 5 years. Rev Med Virol 22:46–64. doi: 10.1002/rmv.720. [DOI] [PubMed] [Google Scholar]
- 11.Arthur JL, Higgins GD, Davidson GP, Givney RC, Ratcliff RM. 2009. A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog 5:e1000391. doi: 10.1371/journal.ppat.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapoor A, Slikas E, Simmonds P, Chieochansin T, Naeem A, Shaukat S, Alam MM, Sharif S, Angez M, Zaidi S, Delwart E. 2009. A newly identified bocavirus species in human stool. J Infect Dis 199:196–200. doi: 10.1086/595831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapoor A, Simmonds P, Slikas E, Li L, Bodhidatta L, Sethabutr O, Triki H, Bahri O, Oderinde BS, Baba MM, Bukbuk DN, Besser J, Bartkus J, Delwart E. 2010. Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. J Infect Dis 201:1633–1643. doi: 10.1086/652416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones MS, Kapoor A, Lukashov VV, Simmonds P, Hecht F, Delwart E. 2005. New DNA viruses identified in patients with acute viral infection syndrome. J Virol 79:8230–8236. doi: 10.1128/JVI.79.13.8230-8236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phan TG, Vo NP, Bonkoungou IJ, Kapoor A, Barro N, O'Ryan M, Kapusinszky B, Wang C, Delwart E. 2012. Acute diarrhea in West African children: diverse enteric viruses and a novel parvovirus genus. J Virol 86:11024–11030. doi: 10.1128/JVI.01427-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotmore SF, Agbandje-McKenna M, Chiorini JA, Mukha DV, Pintel DJ, Qiu J, Söderlund-Venermo M, Tattersall P, Tijssen P, Gatherer D, Davison AJ. 2014. The family Parvoviridae. Arch Virol 159:1239–1247. doi: 10.1007/s00705-013-1914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufmann B, Chipman PR, Kostyuchenko VA, Modrow S, Rossmann MG. 2008. Visualization of the externalized VP2 N termini of infectious human parvovirus B19. J Virol 82:7306–7312. doi: 10.1128/JVI.00512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufmann B, Simpson AA, Rossmann MG. 2004. The structure of human parvovirus B19. Proc Natl Acad Sci U S A 101:11628–11633. doi: 10.1073/pnas.0402992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agbandje M, Kajigaya S, McKenna R, Young NS, Rossmann MG. 1994. The structure of human parvovirus B19 at 8 A resolution. Virology 203:106–115. doi: 10.1006/viro.1994.1460. [DOI] [PubMed] [Google Scholar]
- 20.Gurda BL, Parent KN, Bladek H, Sinkovits RS, DiMattia MA, Rence C, Castro A, McKenna R, Olson N, Brown K, Baker TS, Agbandje-McKenna M. 2010. Human bocavirus capsid structure: insights into the structural repertoire of the Parvoviridae. J Virol 84:5880–5889. doi: 10.1128/JVI.02719-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halder S, Ng R, Agbandje-McKenna M. 2012. Parvoviruses: structure and infection. Future Virol 7:253–278. doi: 10.2217/fvl.12.12. [DOI] [Google Scholar]
- 22.Zádori Z, Szelei J, Lacoste MC, Li Y, Gariépy S, Raymond P, Allaire M, Nabi IR, Tijssen P. 2001. A viral phospholipase A2 is required for parvovirus infectivity. Dev Cell 1:291–302. doi: 10.1016/S1534-5807(01)00031-4. [DOI] [PubMed] [Google Scholar]
- 23.Chipman PR, Agbandje-McKenna M, Kajigaya S, Brown KE, Young NS, Baker TS, Rossmann MG. 1996. Cryo-electron microscopy studies of empty capsids of human parvovirus B19 complexed with its cellular receptor. Proc Natl Acad Sci U S A 93:7502–7506. doi: 10.1073/pnas.93.15.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agbandje-McKenna M, Chapman MS. 2005. Correlating structure with function in the viral capsid, p 125–139. In Kerr J, Cotmore SF, Bloom ME, Linden RM, Parrish CR (ed), Parvoviruses. Hodder Arnold, London, United Kingdom. [Google Scholar]
- 25.Ros C, Gerber M, Kempf C. 2006. Conformational changes in the VP1-unique region of native human parvovirus B19 lead to exposure of internal sequences that play a role in virus neutralization and infectivity. J Virol 80:12017–12024. doi: 10.1128/JVI.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kailasan S, Garrison J, Ilyas M, Chipman P, McKenna R, Kantola K, Söderlund-Venermo M, Kucinskaite-Kodze I, Žvirbliene A, Agbandje-McKenna M. 2016. Mapping antigenic epitopes on the human bocavirus capsid. J Virol 90:4670–4680. doi: 10.1128/JVI.02998-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michel PO, Makela AR, Korhonen E, Toivola J, Hedman L, Soderlund-Venermo M, Hedman K, Oker-Blom C. 2008. Purification and analysis of polyhistidine-tagged human parvovirus B19 VP1 and VP2 expressed in insect cells. J Virol Methods 152:1–5. doi: 10.1016/j.jviromet.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Kantola K, Hedman L, Allander T, Jartti T, Lehtinen P, Ruuskanen O, Hedman K, Söderlund-Venermo M. 2008. Serodiagnosis of human bocavirus infection. Clin Infect Dis 46:540–546. doi: 10.1086/526532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhi N, Zadori Z, Brown KE, Tijssen P. 2004. Construction and sequencing of an infectious clone of the human parvovirus B19. Virology 318:142–152. doi: 10.1016/j.virol.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Deiss V, Tratschin JD, Weitz M, Siegl G. 1990. Cloning of the human parvovirus B19 genome and structural analysis of its palindromic termini. Virology 175:247–254. doi: 10.1016/0042-6822(90)90205-6. [DOI] [PubMed] [Google Scholar]
- 31.Astell CR, Blundell MC. 1989. Sequence of the right hand terminal palindrome of the human B19 parvovirus genome has the potential to form a ‘stem plus arms' structure. Nucleic Acids Res 17:5857. doi: 10.1093/nar/17.14.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori J, Beattie P, Melton DW, Cohen BJ, Clewley JP. 1987. Structure and mapping of the DNA of human parvovirus B19. J Gen Virol 68:2797–2806. doi: 10.1099/0022-1317-68-11-2797. [DOI] [PubMed] [Google Scholar]
- 33.Luo Y, Qiu J. 2015. Human parvovirus B19: a mechanistic overview of infection and DNA replication. Future Virol 10:155–167. doi: 10.2217/fvl.14.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cotmore SF, Tattersall P. 2014. Parvoviruses: small does not mean simple. Annu Rev Virol 1:517–537. doi: 10.1146/annurev-virology-031413-085444. [DOI] [PubMed] [Google Scholar]
- 35.Blundell MC, Beard C, Astell CR. 1987. In vitro identification of a B19 parvovirus promoter. Virology 157:534–538. doi: 10.1016/0042-6822(87)90296-0. [DOI] [PubMed] [Google Scholar]
- 36.Doerig C, Beard P, Hirt B. 1987. A transcriptional promoter of the human parvovirus B19 active in vitro and in vivo. Virology 157:539–542. doi: 10.1016/0042-6822(87)90297-2. [DOI] [PubMed] [Google Scholar]
- 37.Momoeda M, Kawase M, Jane SM, Miyamura K, Young NS, Kajigaya S. 1994. The transcriptional regulator YY1 binds to the 5′-terminal region of B19 parvovirus and regulates P6 promoter activity. J Virol 68:7159–7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blundell MC, Astell CR. 1989. A GC-box motif upstream of the B19 parvovirus unique promoter is important for in vitro transcription. J Virol 63:4814–4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raab U, Bauer B, Gigler A, Beckenlehner K, Wolf H, Modrow S. 2001. Cellular transcription factors that interact with p6 promoter elements of parvovirus B19. J Gen Virol 82:1473–1480. doi: 10.1099/0022-1317-82-6-1473. [DOI] [PubMed] [Google Scholar]
- 40.Liu JM, Fujii H, Green SW, Komatsu N, Young NS, Shimada T. 1991. Indiscriminate activity from the B19 parvovirus p6 promoter in nonpermissive cells. Virology 182:361–364. doi: 10.1016/0042-6822(91)90682-2. [DOI] [PubMed] [Google Scholar]
- 41.Tewary SK, Zhao H, Deng X, Qiu J, Tang L. 2014. The human parvovirus B19 non-structural protein 1 N-terminal domain specifically binds to the origin of replication in the viral DNA. Virology 449:297–303. doi: 10.1016/j.virol.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Servant A, Laperche S, Lallemand F, Marinho V, De Saint MG, Meritet JF, Garbarg-Chenon A. 2002. Genetic diversity within human erythroviruses: identification of three genotypes. J Virol 76:9124–9134. doi: 10.1128/JVI.76.18.9124-9134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hokynar K, Söderlund-Venermo M, Pesonen M, Ranki A, Kiviluoto O, Partio EK, Hedman K. 2002. A new parvovirus genotype persistent in human skin. Virology 302:224–228. doi: 10.1006/viro.2002.1673. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen QT, Sifer C, Schneider V, Allaume X, Servant A, Bernaudin F, Auguste V, Garbarg-Chenon A. 1999. Novel human erythrovirus associated with transient aplastic anemia. J Clin Microbiol 37:2483–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen QT, Sifer C, Schneider V, Bernaudin F, Auguste V, Garbarg-Chenon A. 1998. Detection of an erythrovirus sequence distinct from B19 in a child with acute anaemia. Lancet 352:1524. doi: 10.1016/S0140-6736(05)60330-3. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen QT, Wong S, Heegaard ED, Brown KE. 2002. Identification and characterization of a second novel human erythrovirus variant, A6. Virology 301:374–380. doi: 10.1006/viro.2002.1585. [DOI] [PubMed] [Google Scholar]
- 47.Blümel M, Eis-Hübinger AM, Stühler A, Bönsch C, Gessner M, Löwer J. 2005. Characterization of parvovirus B19 genotype 2 in KU812Ep6 cells. J Virol 79:14197–14206. doi: 10.1128/JVI.79.22.14197-14206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ekman A, Hokynar K, Kakkola L, Kantola K, Hedman L, Bonden H, Gessner M, Aberham C, Norja P, Miettinen S, Hedman K, Söderlund-Venermo M. 2007. Biological and immunological relations among human parvovirus B19 genotypes 1 to 3. J Virol 81:6927–6935. doi: 10.1128/JVI.02713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Z, Guan W, Cheng F, Chen AY, Qiu J. 2009. Molecular characterization of human parvovirus B19 genotypes 2 and 3. Virology 394:276–285. doi: 10.1016/j.virol.2009.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsujikawa M, Nishigaki H, Yoshikawa M, Furuki R, Takahashi K, Adan-Kubo J, Shimamura Y, Urayama T, Hattori S, Sakai K, Yunoki M, Ikuta K. 2012. Variability of parvovirus B19 genotype 2 in plasma products with different compositions in the inactivation sensitivity by liquid-heating. Vox Sang 102:93–99. doi: 10.1111/j.1423-0410.2011.01523.x. [DOI] [PubMed] [Google Scholar]
- 51.Cotmore SF, Tattersall P. 1984. Characterization and molecular cloning of a human parvovirus genome. Science 226:1161–1165. doi: 10.1126/science.6095448. [DOI] [PubMed] [Google Scholar]
- 52.Guan W, Wong S, Zhi N, Qiu J. 2009. The genome of human parvovirus B19 virus can replicate in nonpermissive cells with the help of adenovirus genes and produces infectious virus. J Virol 83:9541–9553. doi: 10.1128/JVI.00702-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winter K, von Kietzell K, Heilbronn R, Pozzuto T, Fechner H, Weger S. 2012. Roles of E4orf6 and VA I RNA in adenovirus-mediated stimulation of human parvovirus B19 DNA replication and structural gene expression. J Virol 86:5099–5109. doi: 10.1128/JVI.06991-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen AY, Kleiboeker S, Qiu J. 2011. Productive parvovirus b19 infection of primary human erythroid progenitor cells at hypoxia is regulated by STAT5A and MEK signaling but not HIF alpha. PLoS Pathog 7:e1002088. doi: 10.1371/journal.ppat.1002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pillet S, Le Guyader N, Hofer T, NguyenKhac F, Koken M, Aubin JT, Fichelson S, Gassmann M, Morinet F. 2004. Hypoxia enhances human B19 erythrovirus gene expression in primary erythroid cells. Virology 327:1–7. doi: 10.1016/j.virol.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 56.Morinet F, Parent M, Bergeron C, Pillet S, Capron C. 2015. Oxygen and viruses: a breathing story. J Gen Virol 96:1979–1982. doi: 10.1099/vir.0.000172. [DOI] [PubMed] [Google Scholar]
- 57.Filippone C, Zhi N, Wong S, Lu J, Kajigaya S, Gallinella G, Kakkola L, Söderlund-Venermo M, Young NS, Brown KE. 2008. VP1u phospholipase activity is critical for infectivity of full-length parvovirus B19 genomic clones. Virology 374:444–452. doi: 10.1016/j.virol.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Norja P, Hokynar K, Aaltonen LM, Chen R, Ranki A, Partio EK, Kiviluoto O, Davidkin I, Leivo T, Eis-Hubinger AM, Schneider B, Fischer HP, Tolba R, Vapalahti O, Vaheri A, Söderlund-Venermo M, Hedman K. 2006. Bioportfolio: lifelong persistence of variant and prototypic erythrovirus DNA genomes in human tissue. Proc Natl Acad Sci U S A 103:7450–7453. doi: 10.1073/pnas.0602259103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozawa K, Ayub J, Hao YS, Kurtzman G, Shimada T, Young N. 1987. Novel transcription map for the B19 (human) pathogenic parvovirus. J Virol 61:2395–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoto Y, Qiu J, Pintel DJ. 2006. Identification and characterization of two internal cleavage and polyadenylation sites of parvovirus B19 RNA. J Virol 80:1604–1609. doi: 10.1128/JVI.80.3.1604-1609.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beard C, St Amand J, Astell CR. 1989. Transient expression of B19 parvovirus gene products in COS-7 cells transfected with B19-SV40 hybrid vectors. Virology 172:659–664. doi: 10.1016/0042-6822(89)90211-0. [DOI] [PubMed] [Google Scholar]
- 62.Luo W, Astell CR. 1993. A novel protein encoded by small RNAs of parvovirus B19. Virology 195:448–455. doi: 10.1006/viro.1993.1395. [DOI] [PubMed] [Google Scholar]
- 63.St Amand J, Astell CR. 1993. Identification and characterization of a family of 11-kDa proteins encoded by the human parvovirus B19. Virology 192:121–131. doi: 10.1006/viro.1993.1014. [DOI] [PubMed] [Google Scholar]
- 64.Servant-Delmas A, Lefrere JJ, Morinet F, Pillet S. 2010. Advances in human B19 erythrovirus biology. J Virol 84:9658–9665. doi: 10.1128/JVI.00684-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu JM, Green SW, Shimada T, Young NS. 1992. A block in full-length transcript maturation in cells nonpermissive for B19 parvovirus. J Virol 66:4686–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guan W, Cheng F, Yoto Y, Kleiboeker S, Wong S, Zhi N, Pintel DJ, Qiu J. 2008. Block to the production of full-length B19 virus transcripts by internal polyadenylation is overcome by replication of the viral genome. J Virol 82:9951–9963. doi: 10.1128/JVI.01162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bua G, Manaresi E, Bonvicini F, Gallinella G. 2016. Parvovirus B19 replication and expression in differentiating erythroid progenitor cells. PLoS One 11:e0148547. doi: 10.1371/journal.pone.0148547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guan W, Cheng F, Huang Q, Qiu J. 2011. Inclusion of the central exon of parvovirus B19 precursor mRNA is determined by multiple splicing enhancers both in the exon and the downstream intron. J Virol 85:2463–2468. doi: 10.1128/JVI.01708-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guan W, Huang Q, Cheng F, Qiu J. 2011. Internal polyadenylation of the parvovirus B19 precursor mRNA is regulated by alternative splicing. J Biol Chem 286:24793–24805. doi: 10.1074/jbc.M111.227439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cotmore SF, McKie VC, Anderson LJ, Astell CR, Tattersall P. 1986. Identification of the major structural and nonstructural proteins encoded by human parvovirus B19 and mapping of their genes by procaryotic expression of isolated genomic fragments. J Virol 60:548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ozawa K, Young N. 1987. Characterization of capsid and noncapsid proteins of B19 parvovirus propagated in human erythroid bone marrow cell cultures. J Virol 61:2627–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown KE. 2005. The genus Erythrovirus, p 25–45. In Kerr J, Cotmore SF, Bloom ME, Linden RM, Parrish CR (ed), Parvoviruses. Hodder Arnold, London, United Kingdom. [Google Scholar]
- 73.Wan Z, Zhi N, Wong S, Keyvanfar K, Liu D, Raghavachari N, Munson PJ, Su S, Malide D, Kajigaya S, Young NS. 2010. Human parvovirus B19 causes cell cycle arrest of human erythroid progenitors via deregulation of the E2F family of transcription factors. J Clin Invest 120:3530–3544. doi: 10.1172/JCI41805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhi N, Mills IP, Lu J, Wong S, Filippone C, Brown KE. 2006. Molecular and functional analyses of a human parvovirus B19 infectious clone demonstrates essential roles for NS1, VP1, and the 11-kilodalton protein in virus replication and infectivity. J Virol 80:5941–5950. doi: 10.1128/JVI.02430-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Momoeda M, Wong S, Kawase M, Young NS, Kajigaya S. 1994. A putative nucleoside triphosphate-binding domain in the nonstructural protein of B19 parvovirus is required for cytotoxicity. J Virol 68:8443–8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lou S, Luo Y, Cheng F, Huang Q, Shen W, Kleiboeker S, Tisdale JF, Liu Z, Qiu J. 2012. Human parvovirus B19 DNA replication induces a DNA damage response that is dispensable for cell cycle arrest at G2/M phase. J Virol 86:10748–10758. doi: 10.1128/JVI.01007-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raab U, Beckenlehner K, Lowin T, Niller HH, Doyle S, Modrow S. 2002. NS1 protein of parvovirus B19 interacts directly with DNA sequences of the p6 promoter and with the cellular transcription factors Sp1/Sp3. Virology 293:86–93. doi: 10.1006/viro.2001.1285. [DOI] [PubMed] [Google Scholar]
- 78.Gareus R, Gigler A, Hemauer A, Leruez-Ville M, Morinet F, Wolf H, Modrow S. 1998. Characterization of cis-acting and NS1 protein-responsive elements in the p6 promoter of parvovirus B19. J Virol 72:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fu Y, Ishii KK, Munakata Y, Saitoh T, Kaku M, Sasaki T. 2002. Regulation of tumor necrosis factor alpha promoter by human parvovirus B19 NS1 through activation of AP-1 and AP-2. J Virol 76:5395–5403. doi: 10.1128/JVI.76.11.5395-5403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moffatt S, Tanaka N, Tada K, Nose M, Nakamura M, Muraoka O, Hirano T, Sugamura K. 1996. A cytotoxic nonstructural protein, NS1, of human parvovirus B19 induces activation of interleukin-6 gene expression. J Virol 70:8485–8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakashima A, Morita E, Saito S, Sugamura K. 2004. Human parvovirus B19 nonstructural protein transactivates the p21/WAF1 through Sp1. Virology 329:493–504. doi: 10.1016/j.virol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 82.Moffatt S, Yaegashi N, Tada K, Tanaka N, Sugamura K. 1998. Human parvovirus B19 nonstructural (NS1) protein induces apoptosis in erythroid lineage cells. J Virol 72:3018–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poole BD, Zhou J, Grote A, Schiffenbauer A, Naides SJ. 2006. Apoptosis of liver-derived cells induced by parvovirus B19 nonstructural protein. J Virol 80:4114–4121. doi: 10.1128/JVI.80.8.4114-4121.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thammasri K, Rauhamäki S, Wang L, Filippou A, Kivovich V, Marjomaki V, Naides SJ, Gilbert L. 2013. Human parvovirus B19 induced apoptotic bodies contain altered self-antigens that are phagocytosed by antigen presenting cells. PLoS One 8:e67179. doi: 10.1371/journal.pone.0067179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo Y, Kleiboeker S, Deng X, Qiu J. 2013. Human parvovirus B19 infection causes cell cycle arrest of human erythroid progenitors at late S phase that favors viral DNA replication. J Virol 87:12766–12775. doi: 10.1128/JVI.02333-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morita E, Nakashima A, Asao H, Sato H, Sugamura K. 2003. Human parvovirus B19 nonstructural protein (NS1) induces cell cycle arrest at G1 phase. J Virol 77:2915–2921. doi: 10.1128/JVI.77.5.2915-2921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luo Y, Lou S, Deng X, Liu Z, Li Y, Kleiboeker S, Qiu J. 2011. Parvovirus B19 infection of human primary erythroid progenitor cells triggers ATR-Chk1 signaling, which promotes B19 virus replication. J Virol 85:8046–8055. doi: 10.1128/JVI.00831-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ozawa K, Ayub J, Young N. 1988. Translational regulation of B19 parvovirus capsid protein production by multiple upstream AUG triplets. J Biol Chem 263:10922–10926. [PubMed] [Google Scholar]
- 89.Kajigaya S, Fujii H, Field A, Anderson S, Rosenfeld S, Anderson LJ, Shimada T, Young NS. 1991. Self-assembled B19 parvovirus capsids, produced in a baculovirus system, are antigenically and immunogenically similar to native virions. Proc Natl Acad Sci U S A 88:4646–4650. doi: 10.1073/pnas.88.11.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pillet S, Annan Z, Fichelson S, Morinet F. 2003. Identification of a nonconventional motif necessary for the nuclear import of the human parvovirus B19 major capsid protein (VP2). Virology 306:25–32. doi: 10.1016/S0042-6822(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 91.Leisi R, Ruprecht N, Kempf C, Ros C. 2013. Parvovirus B19 uptake is a highly selective process controlled by VP1u, a novel determinant of viral tropism. J Virol 87:13161–13167. doi: 10.1128/JVI.02548-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dorsch S, Liebisch G, Kaufmann B, von Landenberg P, Hoffmann JH, Drobnik W, Modrow S. 2002. The VP1 unique region of parvovirus B19 and its constituent phospholipase A2-like activity. J Virol 76:2014–2018. doi: 10.1128/JVI.76.4.2014-2018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dorsch S, Kaufmann B, Schaible U, Prohaska E, Wolf H, Modrow S. 2001. The VP1-unique region of parvovirus B19: amino acid variability and antigenic stability. J Gen Virol 82:191–199. doi: 10.1099/0022-1317-82-1-191. [DOI] [PubMed] [Google Scholar]
- 94.Bleker S, Sonntag F, Kleinschmidt JA. 2005. Mutational analysis of narrow pores at the fivefold symmetry axes of adeno-associated virus type 2 capsids reveals a dual role in genome packaging and activation of phospholipase A2 activity. J Virol 79:2528–2540. doi: 10.1128/JVI.79.4.2528-2540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Farr GA, Zhang LG, Tattersall P. 2005. Parvoviral virions deploy a capsid-tethered lipolytic enzyme to breach the endosomal membrane during cell entry. Proc Natl Acad Sci U S A 102:17148–17153. doi: 10.1073/pnas.0508477102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.St Amand J, Beard C, Humphries K, Astell CR. 1991. Analysis of splice junctions and in vitro and in vivo translation potential of the small, abundant B19 parvovirus RNAs. Virology 183:133–142. doi: 10.1016/0042-6822(91)90126-V. [DOI] [PubMed] [Google Scholar]
- 97.Chen AY, Zhang EY, Guan W, Cheng F, Kleiboeker S, Yankee TM, Qiu J. 2010. The small 11kDa non-structural protein of human parvovirus B19 plays a key role in inducing apoptosis during B19 virus infection of primary erythroid progenitor cells. Blood 115:1070–1080. doi: 10.1182/blood-2009-04-215756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fan MM, Tamburic L, Shippam-Brett C, Zagrodney DB, Astell CR. 2001. The small 11-kDa protein from B19 parvovirus binds growth factor receptor-binding protein 2 in vitro in a Src homology 3 domain/ligand-dependent manner. Virology 291:285–291. doi: 10.1006/viro.2001.1217. [DOI] [PubMed] [Google Scholar]
- 99.Young NS, Mortimer PP, Moore JG, Humphries RK. 1984. Characterization of a virus that causes transient aplastic crisis. J Clin Invest 73:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Young N, Harrison M, Moore J, Mortimer P, Humphries RK. 1984. Direct demonstration of the human parvovirus in erythroid progenitor cells infected in vitro. J Clin Invest 74:2024–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Srivastava A, Lu L. 1988. Replication of B19 parvovirus in highly enriched hematopoietic progenitor cells from normal human bone marrow. J Virol 62:3059–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ozawa K, Kurtzman G, Young N. 1986. Replication of the B19 parvovirus in human bone marrow cell cultures. Science 233:883–886. doi: 10.1126/science.3738514. [DOI] [PubMed] [Google Scholar]
- 103.Ozawa K, Kurtzman G, Young N. 1987. Productive infection by B19 parvovirus of human erythroid bone marrow cells in vitro. Blood 70:384–391. [PubMed] [Google Scholar]
- 104.Sosa CE, Mahony JB, Luinstra KE, Sternbach M, Chernesky MA. 1992. Replication and cytopathology of human parvovirus B19 in human umbilical cord blood erythroid progenitor cells. J Med Virol 36:125–130. [DOI] [PubMed] [Google Scholar]
- 105.Srivastava CH, Zhou S, Munshi NC, Srivastava A. 1992. Parvovirus B19 replication in human umbilical cord blood cells. Virology 189:456–461. doi: 10.1016/0042-6822(92)90569-B. [DOI] [PubMed] [Google Scholar]
- 106.Serke S, Schwarz TF, Baurmann H, Kirsch A, Hottentrager B, Von BA, Roggendorf M, Huhn D, Deinhardt F. 1991. Productive infection of in vitro generated haemopoietic progenitor cells from normal human adult peripheral blood with parvovirus B19: studies by morphology, immunocytochemistry, flow-cytometry and DNA-hybridization. Br J Haematol 79:6–13. doi: 10.1111/j.1365-2141.1991.tb07999.x. [DOI] [PubMed] [Google Scholar]
- 107.Schwarz TF, Serke S, Hottentrager B, Von Brunn A, Baurmann H, Kirsch A, Stolz W, Huhn D, Deinhardt F, Roggendorf M. 1992. Replication of parvovirus B19 in hematopoietic progenitor cells generated in vitro from normal human peripheral blood. J Virol 66:1273–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yaegashi N, Shiraishi H, Takeshita T, Nakamura M, Yajima A, Sugamura K. 1989. Propagation of human parvovirus B19 in primary culture of erythroid lineage cells derived from fetal liver. J Virol 63:2422–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morey AL, Fleming KA. 1992. Immunophenotyping of fetal haemopoietic cells permissive for human parvovirus B19 replication in vitro. Br J Haematol 82:302–309. doi: 10.1111/j.1365-2141.1992.tb06422.x. [DOI] [PubMed] [Google Scholar]
- 110.Takahashi T, Ozawa K, Takahashi K, Asano S, Takaku F. 1990. Susceptibility of human erythropoietic cells to B19 parvovirus in vitro increases with differentiation. Blood 75:603–610. [PubMed] [Google Scholar]
- 111.Wong S, Zhi N, Filippone C, Keyvanfar K, Kajigaya S, Brown KE, Young NS. 2008. Ex vivo-generated CD36+ erythroid progenitors are highly permissive to human parvovirus B19 replication. J Virol 82:2470–2476. doi: 10.1128/JVI.02247-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen AY, Guan W, Lou S, Liu Z, Kleiboeker S, Qiu J. 2010. Role of erythropoietin receptor signaling in parvovirus B19 replication in human erythroid progenitor cells. J Virol 84:12385–12396. doi: 10.1128/JVI.01229-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guo YM, Ishii K, Hirokawa M, Tagawa H, Ohyagi H, Michishita Y, Ubukawa K, Yamashita J, Ohteki T, Onai N, Kawakami K, Xiao W, Sawada K. 2010. CpG-ODN 2006 and human parvovirus B19 genome consensus sequences selectively inhibit growth and development of erythroid progenitor cells. Blood 115:4569–4579. doi: 10.1182/blood-2009-08-239202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Filippone C, Franssila R, Kumar A, Saikko L, Kovanen PE, Söderlund-Venermo M, Hedman K. 2010. Erythroid progenitor cells expanded from peripheral blood without mobilization or preselection: molecular characteristics and functional competence. PLoS One 5:e9496. doi: 10.1371/journal.pone.0009496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wolfisberg R, Ruprecht N, Kempf C, Ros C. 2013. Impaired genome encapsidation restricts the in vitro propagation of human parvovirus B19. J Virol Methods 193:215–225. doi: 10.1016/j.jviromet.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 116.Munshi NC, Zhou S, Woody MJ, Morgan DA, Srivastava A. 1993. Successful replication of parvovirus B19 in the human megakaryocytic leukemia cell line MB-02. J Virol 67:562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shimomura S, Komatsu N, Frickhofen N, Anderson S, Kajigaya S, Young NS. 1992. First continuous propagation of B19 parvovirus in a cell line. Blood 79:18–24. [PubMed] [Google Scholar]
- 118.Morita E, Tada K, Chisaka H, Asao H, Sato H, Yaegashi N, Sugamura K. 2001. Human parvovirus B19 induces cell cycle arrest at G2 phase with accumulation of mitotic cyclins. J Virol 75:7555–7563. doi: 10.1128/JVI.75.16.7555-7563.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bonvicini F, Filippone C, Manaresi E, Zerbini M, Musiani M, Gallinella G. 2008. Functional analysis and quantitative determination of the expression profile of human parvovirus B19. Virology 381:168–177. doi: 10.1016/j.virol.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 120.Takahashi T, Ozawa K, Takahashi K, Okuno Y, Takahashi T, Muto Y, Takaku F, Asano S. 1993. DNA replication of parvovirus B 19 in a human erythroid leukemia cell line (JK-1) in vitro. Arch Virol 131:201–208. [DOI] [PubMed] [Google Scholar]
- 121.Miyagawa E, Yoshida T, Takahashi H, Yamaguchi K, Nagano T, Kiriyama Y, Okochi K, Sato H. 1999. Infection of the erythroid cell line, KU812Ep6 with human parvovirus B19 and its application to titration of B19 infectivity. J Virol Methods 83:45–54. [DOI] [PubMed] [Google Scholar]
- 122.Brown KE, Anderson SM, Young NS. 1993. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science 262:114–117. doi: 10.1126/science.8211117. [DOI] [PubMed] [Google Scholar]
- 123.Nasir W, Nilsson J, Olofsson S, Bally M, Rydell GE. 2014. Parvovirus B19 VLP recognizes globoside in supported lipid bilayers. Virology 456–457:364–369. doi: 10.1016/j.virol.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 124.Weigel-Kelley KA, Yoder MC, Srivastava A. 2001. Recombinant human parvovirus B19 vectors: erythrocyte P antigen is necessary but not sufficient for successful transduction of human hematopoietic cells. J Virol 75:4110–4116. doi: 10.1128/JVI.75.9.4110-4116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brown KE, Hibbs JR, Gallinella G, Anderson SM, Lehman ED, McCarthy P, Young NS. 1994. Resistance to parvovirus B19 infection due to lack of virus receptor (erythrocyte P antigen). N Engl J Med 330:1192–1196. doi: 10.1056/NEJM199404283301704. [DOI] [PubMed] [Google Scholar]
- 126.Bonsch C, Kempf C, Ros C. 2008. Interaction of parvovirus B19 with human erythrocytes alters virus structure and cell membrane integrity. J Virol 82:11784–11791. doi: 10.1128/JVI.01399-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Weigel-Kelley KA, Yoder MC, Srivastava A. 2003. Alpha5beta1 integrin as a cellular coreceptor for human parvovirus B19: requirement of functional activation of beta1 integrin for viral entry. Blood 102:3927–3933. doi: 10.1182/blood-2003-05-1522. [DOI] [PubMed] [Google Scholar]
- 128.Munakata Y, Saito-Ito T, Kumura-Ishii K, Huang J, Kodera T, Ishii T, Hirabayashi Y, Koyanagi Y, Sasaki T. 2005. Ku80 autoantigen as a cellular coreceptor for human parvovirus B19 infection. Blood 106:3449–3456. doi: 10.1182/blood-2005-02-0536. [DOI] [PubMed] [Google Scholar]
- 129.Leisi R, von Nordheim M, Kempf C, Ros C. 2015. Specific targeting of proerythroblasts and erythroleukemic cells by the VP1u region of parvovirus B19. Bioconjug Chem 26:1923–1930. doi: 10.1021/acs.bioconjchem.5b00321. [DOI] [PubMed] [Google Scholar]
- 130.von Kietzell K, Pozzuto T, Heilbronn R, Grossl T, Fechner H, Weger S. 2014. Antibody-mediated enhancement of parvovirus B19 uptake into endothelial cells mediated by a receptor for complement factor C1q. J Virol 88:8102–8115. doi: 10.1128/JVI.00649-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Munakata Y, Kato I, Saito T, Kodera T, Ishii KK, Sasaki T. 2006. Human parvovirus B19 infection of monocytic cell line U937 and antibody-dependent enhancement. Virology 345:251–257. doi: 10.1016/j.virol.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 132.Harbison CE, Chiorini JA, Parrish CR. 2008. The parvovirus capsid odyssey: from the cell surface to the nucleus. Trends Microbiol 16:208–214. doi: 10.1016/j.tim.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 133.Mani B, Baltzer C, Valle N, Almendral JM, Kempf C, Ros C. 2006. Low pH-dependent endosomal processing of the incoming parvovirus minute virus of mice virion leads to externalization of the VP1 N-terminal sequence (N-VP1), N-VP2 cleavage, and uncoating of the full-length genome. J Virol 80:1015–1024. doi: 10.1128/JVI.80.2.1015-1024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sonntag F, Bleker S, Leuchs B, Fischer R, Kleinschmidt JA. 2006. Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J Virol 80:11040–11054. doi: 10.1128/JVI.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vihinen-Ranta M, Wang D, Weichert WS, Parrish CR. 2002. The VP1 N-terminal sequence of canine parvovirus affects nuclear transport of capsids and efficient cell infection. J Virol 76:1884–1891. doi: 10.1128/JVI.76.4.1884-1891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saikawa T, Anderson S, Momoeda M, Kajigaya S, Young NS. 1993. Neutralizing linear epitopes of B19 parvovirus cluster in the VP1 unique and VP1-VP2 junction regions. J Virol 67:3004–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kurtzman G, Frickhofen N, Kimball J, Jenkins DW, Nienhuis AW, Young NS. 1989. Pure red-cell aplasia of 10 years' duration due to persistent parvovirus B19 infection and its cure with immunoglobulin therapy. N Engl J Med 321:519–523. doi: 10.1056/NEJM198908243210807. [DOI] [PubMed] [Google Scholar]
- 138.Kawase M, Momoeda M, Young NS, Kajigaya S. 1995. Most of the VP1 unique region of B19 parvovirus is on the capsid surface. Virology 211:359–366. doi: 10.1006/viro.1995.1418. [DOI] [PubMed] [Google Scholar]
- 139.Anderson S, Momoeda M, Kawase M, Kajigaya S, Young NS. 1995. Peptides derived from the unique region of B19 parvovirus minor capsid protein elicit neutralizing antibodies in rabbits. Virology 206:626–632. doi: 10.1016/S0042-6822(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 140.Rosenfeld SJ, Yoshimoto K, Kajigaya S, Anderson S, Young NS, Field A, Warrener P, Bansal G, Collett MS. 1992. Unique region of the minor capsid protein of human parvovirus B19 is exposed on the virion surface. J Clin Invest 89:2023–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bonsch C, Zuercher C, Lieby P, Kempf C, Ros C. 2010. The globoside receptor triggers structural changes in the B19 virus capsid that facilitate virus internalization. J Virol 84:11737–11746. doi: 10.1128/JVI.01143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Leisi R, Di Tommaso C, Kempf C, Ros C. 2016. The receptor-binding domain in the VP1u region of parvovirus B19. Viruses 8:61. doi: 10.3390/v8030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Quattrocchi S, Ruprecht N, Bonsch C, Bieli S, Zurcher C, Boller K, Kempf C, Ros C. 2012. Characterization of the early steps of human parvovirus B19 infection. J Virol 86:9274–9284. doi: 10.1128/JVI.01004-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sol N, Le Junter J, Vassias I, Freyssinier JM, Thomas A, Prigent AF, Rudkin BB, Fichelson S, Morinet F. 1999. Possible interactions between the NS-1 protein and tumor necrosis factor alpha pathways in erythroid cell apoptosis induced by human parvovirus B19. J Virol 73:8762–8770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wong S, Brown KE. 2006. Development of an improved method of detection of infectious parvovirus B19. J Clin Virol 35:407–413. doi: 10.1016/j.jcv.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 146.Takano T, Yamada K. 2007. Quantitation of human parvovirus B19 DNA by real-time polymerase chain reaction. Pediatr Int 49:459–462. doi: 10.1111/j.1442-200X.2007.02388.x. [DOI] [PubMed] [Google Scholar]
- 147.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, Yusuf R, Cote D, Vinogradov SA, Scadden DT, Lin CP. 2014. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508:269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Koller MR, Bender JG, Miller WM, Papoutsakis ET. 1992. Reduced oxygen tension increases hematopoiesis in long-term culture of human stem and progenitor cells from cord blood and bone marrow. Exp Hematol 20:264–270. [PubMed] [Google Scholar]
- 149.Caillet-Fauquet P, Draps ML, Di Giambattista M, de Launoit Y, Laub R. 2004. Hypoxia enables B19 erythrovirus to yield abundant infectious progeny in a pluripotent erythroid cell line. J Virol Methods 121:145–153. doi: 10.1016/j.jviromet.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 150.Huang Q, Deng X, Yan Z, Cheng F, Luo Y, Shen W, Lei-Butters DC, Chen AY, Li Y, Tang L, Söderlund-Venermo M, Engelhardt JF, Qiu J. 2012. Establishment of a reverse genetics system for studying human bocavirus in human airway epithelia. PLoS Pathog 8:e1002899. doi: 10.1371/journal.ppat.1002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nascimento-Carvalho CM, Cardoso MR, Meriluoto M, Kemppainen K, Kantola K, Ruuskanen O, Hedman K, Söderlund-Venermo M. 2012. Human bocavirus infection diagnosed serologically among children admitted to hospital with community-acquired pneumonia in a tropical region. J Med Virol 84:253–258. doi: 10.1002/jmv.22268. [DOI] [PubMed] [Google Scholar]
- 152.Sun Y, Chen AY, Cheng F, Guan W, Johnson FB, Qiu J. 2009. Molecular characterization of infectious clones of the minute virus of canines reveals unique features of bocaviruses. J Virol 83:3956–3967. doi: 10.1128/JVI.02569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bohmer A, Schildgen V, Lusebrink J, Ziegler S, Tillmann RL, Kleines M, Schildgen O. 2009. Novel application for isothermal nucleic acid sequence-based amplification (NASBA). J Virol Methods 158:199–201. doi: 10.1016/j.jviromet.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 154.Shen W, Deng X, Zou W, Engelhardt JF, Yan Z, Qiu J. 2016. Analysis of the cis and trans requirements for DNA replication at the right-end hairpin of the human bocavirus 1 genome. J Virol 90:7761–7777. doi: 10.1128/JVI.00708-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Deng X, Yan Z, Cheng F, Engelhardt JF, Qiu J. 2016. Replication of an autonomous human parvovirus in non-dividing human airway epithelium is facilitated through the DNA damage and repair pathways. PLoS Pathog 12:e1005399. doi: 10.1371/journal.ppat.1005399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dijkman R, Koekkoek SM, Molenkamp R, Schildgen O, van der Hoek L. 2009. Human bocavirus can be cultured in differentiated human airway epithelial cells. J Virol 83:7739–7748. doi: 10.1128/JVI.00614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen AY, Cheng F, Lou S, Luo Y, Liu Z, Delwart E, Pintel D, Qiu J. 2010. Characterization of the gene expression profile of human bocavirus. Virology 403:145–154. doi: 10.1016/j.virol.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shen W, Deng X, Zou W, Cheng F, Engelhardt JF, Yan Z, Qiu J. 2015. Identification and functional analysis of novel nonstructural proteins of human bocavirus 1. J Virol 89:10097–10109. doi: 10.1128/JVI.01374-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zou W, Cheng F, Shen W, Engelhardt JF, Yan Z, Qiu J. 2016. Nonstructural protein NP1 of human bocavirus 1 plays a critical role in the expression of viral capsid proteins. J Virol 90:4658–4669. doi: 10.1128/JVI.02964-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tewary SK, Zhao H, Shen W, Qiu J, Tang L. 2013. Structure of the NS1 protein N-terminal origin recognition/nickase domain from the emerging human bocavirus. J Virol 87:11487–11494. doi: 10.1128/JVI.01770-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fasina OO, Dong Y, Pintel DJ. 2016. NP1 protein of the bocaparvovirus minute virus of canines controls access to the viral capsid genes via its role in RNA processing. J Virol 90:1718–1728. doi: 10.1128/JVI.02618-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mihaylov IS, Cotmore SF, Tattersall P. 2014. Complementation for an essential ancillary non-structural protein function across parvovirus genera. Virology 468–470:226–237. doi: 10.1016/j.virol.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qu XW, Liu WP, Qi ZY, Duan ZJ, Zheng LS, Kuang ZZ, Zhang WJ, Hou YD. 2008. Phospholipase A2-like activity of human bocavirus VP1 unique region. Biochem Biophys Res Commun 365:158–163. doi: 10.1016/j.bbrc.2007.10.164. [DOI] [PubMed] [Google Scholar]
- 164.Cecchini S, Negrete A, Virag T, Graham BS, Cohen JI, Kotin RM. 2009. Evidence of prior exposure to human bocavirus as determined by a retrospective serological study of 404 serum samples from adults in the United States. Clin Vaccine Immunol 16:597–604. doi: 10.1128/CVI.00470-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lin F, Guan W, Cheng F, Yang N, Pintel D, Qiu J. 2008. ELISAs using human bocavirus VP2 virus-like particles for detection of antibodies against HBoV. J Virol Methods 149:110–117. doi: 10.1016/j.jviromet.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kahn JS, Kesebir D, Cotmore SF, D'Abramo A Jr, Cosby C, Weibel C, Tattersall P. 2008. Seroepidemiology of human bocavirus defined using recombinant virus-like particles. J Infect Dis 198:41–50. doi: 10.1086/588674. [DOI] [PubMed] [Google Scholar]
- 167.Deng X, Yan Z, Luo Y, Xu J, Cheng Y, Li Y, Engelhardt J, Qiu J. 2013. In vitro modeling of human bocavirus 1 infection of polarized primary human airway epithelia. J Virol 87:4097–4102. doi: 10.1128/JVI.03132-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Deng X, Li Y, Qiu J. 2014. Human bocavirus 1 infects commercially available primary human airway epithelium cultures productively. J Virol Methods 195:112–119. doi: 10.1016/j.jviromet.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zabner J, Karp P, Seiler M, Phillips SL, Mitchell CJ, Saavedra M, Welsh M, Klingelhutz AJ. 2003. Development of cystic fibrosis and noncystic fibrosis airway cell lines. Am J Physiol Lung Cell Mol Physiol 284:L844–L854. doi: 10.1152/ajplung.00355.2002. [DOI] [PubMed] [Google Scholar]
- 170.Mortimer PP, Humphries RK, Moore JG, Purcell RH, Young NS. 1983. A human parvovirus-like virus inhibits haematopoietic colony formation in vitro. Nature 302:426–429. doi: 10.1038/302426a0. [DOI] [PubMed] [Google Scholar]
- 171.Chen AY, Qiu J. 2010. Parvovirus infection-induced cell death and cell cycle arrest. Future Virol 5:731–741. doi: 10.2217/fvl.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yaegashi N, Niinuma T, Chisaka H, Uehara S, Moffatt S, Tada K, Iwabuchi M, Matsunaga Y, Nakayama M, Yutani C, Osamura Y, Hirayama E, Okamura K, Sugamura K, Yajima A. 1999. Parvovirus B19 infection induces apoptosis of erythroid cells in vitro and in vivo. J Infect 39:68–76. doi: 10.1016/S0163-4453(99)90105-6. [DOI] [PubMed] [Google Scholar]
- 173.Ignatovich IV, Hobbs JA. 2013. Human parvovirus B19 infection leads to downregulation of thyroid, estrogen, and retinoid hormone receptor expression. Virology 446:173–179. doi: 10.1016/j.virol.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 174.Nigro G, Bastianon V, Colloridi V, Ventriglia F, Gallo P, D'Amati G, Koch WC, Adler SP. 2000. Human parvovirus B19 infection in infancy associated with acute and chronic lymphocytic myocarditis and high cytokine levels: report of 3 cases and review. Clin Infect Dis 31:65–69. doi: 10.1086/313929. [DOI] [PubMed] [Google Scholar]
- 175.Schowengerdt KO, Ni J, Denfield SW, Gajarski RJ, Bowles NE, Rosenthal G, Kearney DL, Price JK, Rogers BB, Schauer GM, Chinnock RE, Towbin JA. 1997. Association of parvovirus B19 genome in children with myocarditis and cardiac allograft rejection: diagnosis using the polymerase chain reaction. Circulation 96:3549–3554. doi: 10.1161/01.CIR.96.10.3549. [DOI] [PubMed] [Google Scholar]
- 176.Molina KM, Garcia X, Denfield SW, Fan Y, Morrow WR, Towbin JA, Frazier EA, Nelson DP. 2013. Parvovirus B19 myocarditis causes significant morbidity and mortality in children. Pediatr Cardiol 34:390–397. doi: 10.1007/s00246-012-0468-4. [DOI] [PubMed] [Google Scholar]
- 177.Bock CT, Klingel K, Kandolf R. 2010. Human parvovirus B19-associated myocarditis. N Engl J Med 362:1248–1249. doi: 10.1056/NEJMc0911362. [DOI] [PubMed] [Google Scholar]
- 178.Kerr JR. 2000. Pathogenesis of human parvovirus B19 in rheumatic disease. Ann Rheum Dis 59:672–683. doi: 10.1136/ard.59.9.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kerr JR. 2016. The role of parvovirus B19 in the pathogenesis of autoimmunity and autoimmune disease. J Clin Pathol 69:279–291. doi: 10.1136/jclinpath-2015-203455. [DOI] [PubMed] [Google Scholar]
- 180.Moore TL. 2000. Parvovirus-associated arthritis. Curr Opin Rheumatol 12:289–294. doi: 10.1097/00002281-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 181.Kozireva SV, Zestkova JV, Mikazane HJ, Kadisa AL, Kakurina NA, Lejnieks AA, Danilane IN, Murovska MF. 2008. Incidence and clinical significance of parvovirus B19 infection in patients with rheumatoid arthritis. J Rheumatol 35:1265–1270. [PubMed] [Google Scholar]
- 182.Dingli D, Pfizenmaier DH, Arromdee E, Wennberg P, Spittell PC, Chang-Miller A, Clarke BL. 2000. Severe digital arterial occlusive disease and acute parvovirus B19 infection. Lancet 356:312–314. doi: 10.1016/S0140-6736(00)02512-5. [DOI] [PubMed] [Google Scholar]
- 183.Finkel TH, Torok TJ, Ferguson PJ, Durigon EL, Zaki SR, Leung DY, Harbeck RJ, Gelfand EW, Saulsbury FT, Hollister JR. 1994. Chronic parvovirus B19 infection and systemic necrotising vasculitis: opportunistic infection or aetiological agent? Lancet 343:1255–1258. doi: 10.1016/S0140-6736(94)92152-0. [DOI] [PubMed] [Google Scholar]
- 184.Barah F, Vallely PJ, Chiswick ML, Cleator GM, Kerr JR. 2001. Association of human parvovirus B19 infection with acute meningoencephalitis. Lancet 358:729–730. doi: 10.1016/S0140-6736(01)05905-0. [DOI] [PubMed] [Google Scholar]
- 185.Yoto Y, Kudoh T, Haseyama K, Tsutsumi H. 2001. Human parvovirus B19 and meningoencephalitis. Lancet 358:2168. doi: 10.1016/S0140-6736(01)07199-9. [DOI] [PubMed] [Google Scholar]
- 186.Drago F, Semino M, Rampini P, Rebora A. 1999. Parvovirus B19 infection associated with acute hepatitis and a purpuric exanthem. Br J Dermatol 141:160–161. doi: 10.1046/j.1365-2133.1999.02943.x. [DOI] [PubMed] [Google Scholar]
- 187.Yoto Y, Kudoh T, Haseyama K, Suzuki N, Chiba S. 1996. Human parvovirus B19 infection associated with acute hepatitis. Lancet 347:868–869. doi: 10.1016/S0140-6736(96)91348-3. [DOI] [PubMed] [Google Scholar]
- 188.Hobbs JA, Adamson-Small LA. 2015. Parvovirus and thyroid cancer. Semin Oncol 42:304–308. doi: 10.1053/j.seminoncol.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 189.Adamson LA, Fowler LJ, Ewald AS, Clare-Salzler MJ, Hobbs JA. 2014. Infection and persistence of erythrovirus B19 in benign and cancerous thyroid tissues. J Med Virol 86:1614–1620. doi: 10.1002/jmv.23852. [DOI] [PubMed] [Google Scholar]
- 190.Adamson LA, Fowler LJ, Clare-Salzler MJ, Hobbs JA. 2011. Parvovirus B19 infection in Hashimoto's thyroiditis, papillary thyroid carcinoma, and anaplastic thyroid carcinoma. Thyroid 21:411–417. doi: 10.1089/thy.2010.0307. [DOI] [PubMed] [Google Scholar]
- 191.Adamson-Small LA, Ignatovich IV, Laemmerhirt MG, Hobbs JA. 2014. Persistent parvovirus B19 infection in non-erythroid tissues: possible role in the inflammatory and disease process. Virus Res 190:8–16. doi: 10.1016/j.virusres.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 192.Klingel K, Sauter M, Bock CT, Szalay G, Schnorr JJ, Kandolf R. 2004. Molecular pathology of inflammatory cardiomyopathy. Med Microbiol Immunol 193:101–107. doi: 10.1007/s00430-003-0190-1. [DOI] [PubMed] [Google Scholar]
- 193.Kuhl U, Pauschinger M, Bock T, Klingel K, Schwimmbeck CP, Seeberg B, Krautwurm L, Poller W, Schultheiss HP, Kandolf R. 2003. Parvovirus B19 infection mimicking acute myocardial infarction. Circulation 108:945–950. doi: 10.1161/01.CIR.0000085168.02782.2C. [DOI] [PubMed] [Google Scholar]
- 194.Schenk T, Enders M, Pollak S, Hahn R, Huzly D. 2009. High prevalence of human parvovirus B19 DNA in myocardial autopsy samples from subjects without myocarditis or dilative cardiomyopathy. J Clin Microbiol 47:106–110. doi: 10.1128/JCM.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pasquinelli G, Bonvicini F, Foroni L, Salfi N, Gallinella G. 2009. Placental endothelial cells can be productively infected by parvovirus B19. J Clin Virol 44:33–38. doi: 10.1016/j.jcv.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 196.Duechting A, Tschope C, Kaiser H, Lamkemeyer T, Tanaka N, Aberle S, Lang F, Torresi J, Kandolf R, Bock CT. 2008. Human parvovirus B19 NS1 protein modulates inflammatory signaling by activation of STAT3/PIAS3 in human endothelial cells. J Virol 82:7942–7952. doi: 10.1128/JVI.00891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tzang BS, Lin TM, Tsai CC, Hsu JD, Yang LC, Hsu TC. 2011. Increased cardiac injury in NZB/W F1 mice received antibody against human parvovirus B19 VP1 unique region protein. Mol Immunol 48:1518–1524. doi: 10.1016/j.molimm.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 198.Schmidt-Lucke C, Zobel T, Schrepfer S, Kuhl U, Wang D, Klingel K, Becher PM, Fechner H, Pozzuto T, Van Linthout S, Lassner D, Spillmann F, Escher F, Holinski S, Volk HD, Schultheiss HP, Tschope C. 2015. Impaired endothelial regeneration through human parvovirus B19-infected circulating angiogenic cells in patients with cardiomyopathy. J Infect Dis 212:1070–1081. doi: 10.1093/infdis/jiv178. [DOI] [PubMed] [Google Scholar]
- 199.Schmidt-Lucke C, Spillmann F, Bock T, Kuhl U, Van Linthout S, Schultheiss HP, Tschope C. 2010. Interferon beta modulates endothelial damage in patients with cardiac persistence of human parvovirus B19 infection. J Infect Dis 201:936–945. doi: 10.1086/650700. [DOI] [PubMed] [Google Scholar]
- 200.Corcioli F, Zakrzewska K, Rinieri A, Fanci R, Innocenti M, Civinini R, De Giorgi V, Di Lollo S, Azzi A. 2008. Tissue persistence of parvovirus B19 genotypes in asymptomatic persons. J Med Virol 80:2005–2011. doi: 10.1002/jmv.21289. [DOI] [PubMed] [Google Scholar]
- 201.Cassinotti P, Burtonboy G, Fopp M, Siegl G. 1997. Evidence for persistence of human parvovirus B19 DNA in bone marrow. J Med Virol 53:229–232. [PubMed] [Google Scholar]
- 202.Söderlund M, von Essen R, Haapasaari J, Kiistala U, Kiviluoto O, Hedman K. 1997. Persistence of parvovirus B19 DNA in synovial membranes of young patients with and without chronic arthropathy. Lancet 349:1063–1065. doi: 10.1016/S0140-6736(96)09110-6. [DOI] [PubMed] [Google Scholar]
- 203.Söderlund-Venermo M, Hokynar K, Nieminen J, Rautakorpi H, Hedman K. 2002. Persistence of human parvovirus B19 in human tissues. Pathol Biol (Paris) 50:307–316. doi: 10.1016/S0369-8114(02)00307-3. [DOI] [PubMed] [Google Scholar]
- 204.Hokynar K, Norja P, Hedman K, Söderlund-Venermo M. 2007. Tissue persistence and prevalence of B19 virus types 1-3. Future Virol 2:377–388. doi: 10.2217/17460794.2.4.377. [DOI] [Google Scholar]
- 205.Bonvicini F, Manaresi E, Di Furio F, De Falco L, Gallinella G. 2012. Parvovirus b19 DNA CpG dinucleotide methylation and epigenetic regulation of viral expression. PLoS One 7:e33316. doi: 10.1371/journal.pone.0033316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang JH, Zhang WP, Liu HX, Wang D, Li YF, Wang WQ, Wang L, He FR, Wang Z, Yan QG, Chen LW, Huang GS. 2008. Detection of human parvovirus B19 in papillary thyroid carcinoma. Br J Cancer 98:611–618. doi: 10.1038/sj.bjc.6604196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li Y, Wang J, Zhu G, Zhang X, Zhai H, Zhang W, Wang W, Huang G. 2007. Detection of parvovirus B19 nucleic acids and expression of viral VP1/VP2 antigen in human colon carcinoma. Am J Gastroenterol 102:1489–1498. doi: 10.1111/j.1572-0241.2007.01240.x. [DOI] [PubMed] [Google Scholar]
- 208.Lu J, Zhi N, Wong S, Brown KE. 2006. Activation of synoviocytes by the secreted phospholipase A2 motif in the VP1-unique region of parvovirus B19 minor capsid protein. J Infect Dis 193:582–590. doi: 10.1086/499599. [DOI] [PubMed] [Google Scholar]
- 209.Polcz ME, Adamson LA, Lu X, Chang MN, Fowler LJ, Hobbs JA. 2013. Increased IL-6 detection in adult and pediatric lymphoid tissue harboring parvovirus B19. J Clin Virol 57:233–238. doi: 10.1016/j.jcv.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 210.Poole BD, Karetnyi YV, Naides SJ. 2004. Parvovirus B19-induced apoptosis of hepatocytes. J Virol 78:7775–7783. doi: 10.1128/JVI.78.14.7775-7783.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hristov G, Kramer M, Li J, El-Andaloussi N, Mora R, Daeffler L, Zentgraf H, Rommelaere J, Marchini A. 2010. Through its nonstructural protein NS1, parvovirus H-1 induces apoptosis via accumulation of reactive oxygen species. J Virol 84:5909–5922. doi: 10.1128/JVI.01797-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Luo Y, Qiu J. 2013. Parvovirus infection induced DNA damage response. Future Virol 8:245–257. doi: 10.2217/fvl.13.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Khalfaoui S, Eichhorn V, Karagiannidis C, Bayh I, Brockmann M, Pieper M, Windisch W, Schildgen O, Schildgen V. 2016. Lung infection by human bocavirus induces the release of profibrotic mediator cytokines in vivo and in vitro. PLoS One 11:e0147010. doi: 10.1371/journal.pone.0147010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nunoue T, Okochi K, Mortimer PP, Cohen BJ. 1985. Human parvovirus (B19) and erythema infectiosum. J Pediatr 107:38–40. doi: 10.1016/S0022-3476(85)80610-7. [DOI] [PubMed] [Google Scholar]
- 215.Anderson LJ, Tsou C, Parker RA, Chorba TL, Wulff H, Tattersall P, Mortimer PP. 1986. Detection of antibodies and antigens of human parvovirus B19 by enzyme-linked immunosorbent assay. J Clin Microbiol 24:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cohen BJ, Buckley MM. 1988. The prevalence of antibody to human parvovirus B19 in England and Wales. J Med Microbiol 25:151–153. doi: 10.1099/00222615-25-2-151. [DOI] [PubMed] [Google Scholar]
- 217.Koch WC, Adler SP. 1989. Human parvovirus B19 infections in women of childbearing age and within families. Pediatr Infect Dis J 8:83–87. [PubMed] [Google Scholar]
- 218.Lin KH, You SL, Chen CJ, Wang CF, Yang CS, Yamazaki S. 1999. Seroepidemiology of human parvovirus B19 in Taiwan. J Med Virol 57:169–173. [PubMed] [Google Scholar]
- 219.Tolfvenstam T, Enbom M, Ghebrekidan H, Ruden U, Linde A, Grandien M, Wahren B. 2000. Seroprevalence of viral childhood infections in Eritrea. J Clin Virol 16:49–54. doi: 10.1016/S1386-6532(99)00070-0. [DOI] [PubMed] [Google Scholar]
- 220.Rohrer C, Gartner B, Sauerbrei A, Bohm S, Hottentrager B, Raab U, Thierfelder W, Wutzler P, Modrow S. 2008. Seroprevalence of parvovirus B19 in the German population. Epidemiol Infect 136:1564–1575. doi: 10.1017/S0950268807009958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mossong J, Hens N, Friederichs V, Davidkin I, Broman M, Litwinska B, Siennicka J, Trzcinska A, Van Damme P, Beutels P, Vyse A, Shkedy Z, Aerts M, Massari M, Gabutti G. 2008. Parvovirus B19 infection in five European countries: seroepidemiology, force of infection and maternal risk of infection. Epidemiol Infect 136:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ihara T, Furusyo N, Hayashi T, Toyoda K, Murata M, Hayashi J. 2013. A population-based epidemiological survey of human parvovirus B19 infection: a project of the Kyushu and Okinawa Population Study (KOPS). Arch Virol 158:2465–2472. doi: 10.1007/s00705-013-1746-z. [DOI] [PubMed] [Google Scholar]
- 223.Adam O, Makkawi T, Reber U, Kirberg H, Eis-Hübinger AM. 2015. The seroprevalence of parvovirus B19 infection in pregnant women in Sudan. Epidemiol Infect 143:242–248. doi: 10.1017/S0950268814000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang L, Cai C, Pan F, Hong L, Luo X, Hu S, Xu J, Chen Z. 2016. Epidemiologic study of human parvovirus B19 infection in East China. J Med Virol 88:1113–1119. doi: 10.1002/jmv.24459. [DOI] [PubMed] [Google Scholar]
- 225.Mor O, Ofir I, Pavel R, Bassal R, Kra-Oz Z, Cohen D, Shohat T, Mendelson E. 2016. Parvovirus B19V infection in Israel: prevalence and occurrence of acute infection between 2008 and 2013. Epidemiol Infect 144:207–214. doi: 10.1017/S0950268815000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Schwarz TF, Gurtler LG, Zoulek G, Deinhardt F, Roggendorf M. 1989. Seroprevalence of human parvovirus B19 infection in Sao Tome and Principe, Malawi and Mascarene Islands. Zentralbl Bakteriol 271:231–236. doi: 10.1016/S0934-8840(89)80077-5. [DOI] [PubMed] [Google Scholar]
- 227.de Freitas RB, Wong D, Boswell F, de Miranda MF, Linhares AC, Shirley J, Desselberger U. 1990. Prevalence of human parvovirus (B19) and rubella virus infections in urban and remote rural areas in northern Brazil. J Med Virol 32:203–208. [DOI] [PubMed] [Google Scholar]
- 228.Wildig J, Mueller I, Kiniboro B, Maraga S, Siba P, Cossart Y. 2007. Seroprevalence of antibodies to parvovirus B19 among children in Papua New Guinea. Am J Trop Med Hyg 77:354–357. [PubMed] [Google Scholar]
- 229.Anderson MJ, Higgins PG, Davis LR, Willman JS, Jones SE, Kidd IM, Pattison JR, Tyrrell DA. 1985. Experimental parvoviral infection in humans. J Infect Dis 152:257–265. doi: 10.1093/infdis/152.2.257. [DOI] [PubMed] [Google Scholar]
- 230.Shneerson JM, Mortimer PP, Vandervelde EM. 1980. Febrile illness due to a parvovirus. Br Med J 280:1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Slavov SN, Kashima S, Rocha-Junior MC, Oliveira LC, Silva-Pinto AC, Yamamoto AY, Covas DT. 2014. Frequent human parvovirus B19 DNA occurrence and high seroprevalence in haemophilic patients from a non-metropolitan blood centre, Brazil. Transfus Med 24:130–132. doi: 10.1111/tme.12113. [DOI] [PubMed] [Google Scholar]
- 232.Khameneh ZR, Hanifian H, Barzegari R, Sepehrvand N. 2014. Human parvovirus B19 in Iranian pregnant women: a serologic survey. Indian J Pathol Microbiol 57:442–444. doi: 10.4103/0377-4929.138748. [DOI] [PubMed] [Google Scholar]
- 233.Potter CG, Potter AC, Hatton CS, Chapel HM, Anderson MJ, Pattison JR, Tyrrell DA, Higgins PG, Willman JS, Parry HF. 1987. Variation of erythroid and myeloid precursors in the marrow and peripheral blood of volunteer subjects infected with human parvovirus (B19). J Clin Invest 79:1486–1492. doi: 10.1172/JCI112978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Plummer FA, Hammond GW, Forward K, Sekla L, Thompson LM, Jones SE, Kidd IM, Anderson MJ. 1985. An erythema infectiosum-like illness caused by human parvovirus infection. N Engl J Med 313:74–79. doi: 10.1056/NEJM198507113130203. [DOI] [PubMed] [Google Scholar]
- 235.Chorba T, Coccia P, Holman RC, Tattersall P, Anderson LJ, Sudman J, Young NS, Kurczynski E, Saarinen UM, Moir R. 1986. The role of parvovirus B19 in aplastic crisis and erythema infectiosum (fifth disease). J Infect Dis 154:383–393. doi: 10.1093/infdis/154.3.383. [DOI] [PubMed] [Google Scholar]
- 236.Patou G, Pillay D, Myint S, Pattison J. 1993. Characterization of a nested polymerase chain reaction assay for detection of parvovirus B19. J Clin Microbiol 31:540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Musiani M, Zerbini M, Gentilomi G, Plazzi M, Gallinella G, Venturoli S. 1995. Parvovirus B19 clearance from peripheral blood after acute infection. J Infect Dis 172:1360–1363. doi: 10.1093/infdis/172.5.1360. [DOI] [PubMed] [Google Scholar]
- 238.Cassinotti P, Siegl G. 2000. Quantitative evidence for persistence of human parvovirus B19 DNA in an immunocompetent individual. Eur J Clin Microbiol Infect Dis 19:886–887. doi: 10.1007/s100960000384. [DOI] [PubMed] [Google Scholar]
- 239.Brown KE. 2004. Detection and quantitation of parvovirus B19. J Clin Virol 31:1–4. doi: 10.1016/j.jcv.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 240.Lefrere JJ, Servant-Delmas A, Candotti D, Mariotti M, Thomas I, Brossard Y, Lefrere F, Girot R, Allain JP, Laperche S. 2005. Persistent B19 infection in immunocompetent individuals: implications for transfusion safety. Blood 106:2890–2895. doi: 10.1182/blood-2005-03-1053. [DOI] [PubMed] [Google Scholar]
- 241.Kleinman SH, Glynn SA, Lee TH, Tobler L, Montalvo L, Todd D, Kiss JE, Shyamala V, Busch MP. 2007. Prevalence and quantitation of parvovirus B19 DNA levels in blood donors with a sensitive polymerase chain reaction screening assay. Transfusion 47:1756–1764. doi: 10.1111/j.1537-2995.2007.01341.x. [DOI] [PubMed] [Google Scholar]
- 242.Hokynar K, Norja P, Laitinen H, Palomaki P, Garbarg-Chenon A, Ranki A, Hedman K, Söderlund-Venermo M. 2004. Detection and differentiation of human parvovirus variants by commercial quantitative real-time PCR tests. J Clin Microbiol 42:2013–2019. doi: 10.1128/JCM.42.5.2013-2019.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Costa C, Terlizzi ME, Solidoro P, Libertucci D, Bergallo M, Cavallo R. 2009. Detection of parvovirus B19 in the lower respiratory tract. J Clin Virol 46:150–153. doi: 10.1016/j.jcv.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tuckerman JG, Brown T, Cohen BJ. 1986. Erythema infectiosum in a village primary school: clinical and virological studies. J R Coll Gen Pract 36:267–270. [PMC free article] [PubMed] [Google Scholar]
- 245.Gillespie SM, Cartter ML, Asch S, Rokos JB, Gary GW, Tsou CJ, Hall DB, Anderson LJ, Hurwitz ES. 1990. Occupational risk of human parvovirus B19 infection for school and day-care personnel during an outbreak of erythema infectiosum. JAMA 263:2061–2065. doi: 10.1001/jama.1990.03440150069028. [DOI] [PubMed] [Google Scholar]
- 246.Cartter ML, Farley TA, Rosengren S, Quinn DL, Gillespie SM, Gary GW, Hadler JL. 1991. Occupational risk factors for infection with parvovirus B19 among pregnant women. J Infect Dis 163:282–285. doi: 10.1093/infdis/163.2.282. [DOI] [PubMed] [Google Scholar]
- 247.Seng C, Watkins P, Morse D, Barrett SP, Zambon M, Andrews N, Atkins M, Hall S, Lau YK, Cohen BJ. 1994. Parvovirus B19 outbreak on an adult ward. Epidemiol Infect 113:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Dowell SF, Torok TJ, Thorp JA, Hedrick J, Erdman DD, Zaki SR, Hinkle CJ, Bayer WL, Anderson LJ. 1995. Parvovirus B19 infection in hospital workers: community or hospital acquisition? J Infect Dis 172:1076–1079. doi: 10.1093/infdis/172.4.1076. [DOI] [PubMed] [Google Scholar]
- 249.Miyamoto K, Ogami M, Takahashi Y, Mori T, Akimoto S, Terashita H, Terashita T. 2000. Outbreak of human parvovirus B19 in hospital workers. J Hosp Infect 45:238–241. doi: 10.1053/jhin.2000.0771. [DOI] [PubMed] [Google Scholar]
- 250.Garner JS, Hospital Infection Control Practices Advisory Committee. 1996. Guideline for isolation precautions in hospitals. Infect Control Hosp Epidemiol 17:53–80. doi: 10.2307/30142367. [DOI] [PubMed] [Google Scholar]
- 251.Zakrzewska K, Azzi A, Patou G, Morfini M, Rafanelli D, Pattison JR. 1992. Human parvovirus B19 in clotting factor concentrates: B19 DNA detection by the nested polymerase chain reaction. Br J Haematol 81:407–412. doi: 10.1111/j.1365-2141.1992.tb08248.x. [DOI] [PubMed] [Google Scholar]
- 252.Lefrere JJ, Mariotti M, Thauvin M. 1994. B19 parvovirus DNA in solvent/detergent-treated anti-haemophilia concentrates. Lancet 343:211–212. doi: 10.1016/S0140-6736(94)90993-8. [DOI] [PubMed] [Google Scholar]
- 253.Koenigbauer UF, Eastlund T, Day JW. 2000. Clinical illness due to parvovirus B19 infection after infusion of solvent/detergent-treated pooled plasma. Transfusion 40:1203–1206. doi: 10.1046/j.1537-2995.2000.40101203.x. [DOI] [PubMed] [Google Scholar]
- 254.Schmidt I, Blumel J, Seitz H, Willkommen H, Löwer J. 2001. Parvovirus B19 DNA in plasma pools and plasma derivatives. Vox Sang 81:228–235. doi: 10.1046/j.1423-0410.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 255.Blümel J, Schmidt I, Effenberger W, Seitz H, Willkommen H, Brackmann HH, Löwer J, Eis-Hübinger AM. 2002. Parvovirus B19 transmission by heat-treated clotting factor concentrates. Transfusion 42:1473–1481. doi: 10.1046/j.1537-2995.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- 256.Gallinella G, Moretti E, Nardi G, Zuffi E, Bonvicini F, Bucci E, Musiani M, Zerbini M. 2002. Analysis of B19 virus contamination in plasma pools for manufacturing, by using a competitive polymerase chain reaction assay. Vox Sang 83:324–331. doi: 10.1046/j.1423-0410.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- 257.Schneider B, Becker M, Brackmann HH, Eis-Hübinger AM. 2004. Contamination of coagulation factor concentrates with human parvovirus B19 genotype 1 and 2. Thromb Haemost 92:838–845. [DOI] [PubMed] [Google Scholar]
- 258.Plentz A, Hahn J, Knoll A, Holler E, Jilg W, Modrow S. 2005. Exposure of hematologic patients to parvovirus B19 as a contaminant of blood cell preparations and blood products. Transfusion 45:1811–1815. doi: 10.1111/j.1537-2995.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- 259.Kleinman SH, Glynn SA, Lee TH, Tobler LH, Schlumpf KS, Todd DS, Qiao H, Yu MY, Busch MP. 2009. A linked donor-recipient study to evaluate parvovirus B19 transmission by blood component transfusion. Blood 114:3677–3683. doi: 10.1182/blood-2009-06-225706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Marano G, Vaglio S, Pupella S, Facco G, Calizzani G, Candura F, Liumbruno GM, Grazzini G. 2015. Human parvovirus B19 and blood product safety: a tale of twenty years of improvements. Blood Transfus 13:184–196. doi: 10.2450/2014.0174.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Juhl D, Ozdemir M, Dreier J, Gorg S, Hennig H. 2015. Look-back study on recipients of parvovirus B19 (B19V) DNA-positive blood components. Vox Sang 109:305–311. doi: 10.1111/vox.12295. [DOI] [PubMed] [Google Scholar]
- 262.Williams MD, Cohen BJ, Beddall AC, Pasi KJ, Mortimer PP, Hill FG. 1990. Transmission of human parvovirus B19 by coagulation factor concentrates. Vox Sang 58:177–181. doi: 10.1111/j.1423-0410.1990.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 263.Mortimer PP, Luban NL, Kelleher JF, Cohen BJ. 1983. Transmission of serum parvovirus-like virus by clotting-factor concentrates. Lancet ii:482–484. [DOI] [PubMed] [Google Scholar]
- 264.Satake M, Hoshi Y, Taira R, Momose SY, Hino S, Tadokoro K. 2011. Symptomatic parvovirus B19 infection caused by blood component transfusion. Transfusion 51:1887–1895. doi: 10.1111/j.1537-2995.2010.03047.x. [DOI] [PubMed] [Google Scholar]
- 265.Barzon L, Murer L, Pacenti M, Biasolo MA, Vella MD, Ghirardo G, Gamba PG, De Arias AE, Zanon GF, Palu G. 2009. Detection of viral DNA in kidney graft preservation and washing solutions is predictive of posttransplant infections in pediatric recipients. J Infect Dis 200:1425–1433. doi: 10.1086/644504. [DOI] [PubMed] [Google Scholar]
- 266.Heegaard ED, Laub PB. 2000. Parvovirus B19 transmitted by bone marrow. Br J Haematol 111:659–661. doi: 10.1046/j.1365-2141.2000.02407.x. [DOI] [PubMed] [Google Scholar]
- 267.Eid AJ, Chen SF, AST Infectious Diseases Community of Practice. 2013. Human parvovirus B19 in solid organ transplantation. Am J Transplant 13(Suppl 4):201–205. doi: 10.1111/ajt.12111. [DOI] [PubMed] [Google Scholar]
- 268.Norja P, Lassila R, Makris M. 2012. Parvovirus transmission by blood products—a cause for concern? Br J Haematol 159:385–393. doi: 10.1111/bjh.12060. [DOI] [PubMed] [Google Scholar]
- 269.Söderlund M, Ruutu P, Ruutu T, Asikainen K, Franssila R, Hedman K. 1997. Primary and secondary infections by human parvovirus B19 following bone marrow transplantation: characterization by PCR and B-cell molecular immunology. Scand J Infect Dis 29:129–135. doi: 10.3109/00365549709035872. [DOI] [PubMed] [Google Scholar]
- 270.Eid AJ, Brown RA, Patel R, Razonable RR. 2006. Parvovirus B19 infection after transplantation: a review of 98 cases. Clin Infect Dis 43:40–48. doi: 10.1086/504812. [DOI] [PubMed] [Google Scholar]
- 271.Yu MY, Alter HJ, Virata-Theimer ML, Geng Y, Ma L, Schechterly CA, Colvin CA, Luban NL. 2010. Parvovirus B19 infection transmitted by transfusion of red blood cells confirmed by molecular analysis of linked donor and recipient samples. Transfusion 50:1712–1721. doi: 10.1111/j.1537-2995.2010.02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Knott PD, Welply GA, Anderson MJ. 1984. Serologically proved intrauterine infection with parvovirus. Br Med J (Clin Res Ed) 289:1660. doi: 10.1136/bmj.289.6459.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Brown T, Anand A, Ritchie LD, Clewley JP, Reid TM. 1984. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet ii:1033–1034. [DOI] [PubMed] [Google Scholar]
- 274.Anand A, Gray ES, Brown T, Clewley JP, Cohen BJ. 1987. Human parvovirus infection in pregnancy and hydrops fetalis. N Engl J Med 316:183–186. doi: 10.1056/NEJM198701223160403. [DOI] [PubMed] [Google Scholar]
- 275.Enders M, Klingel K, Weidner A, Baisch C, Kandolf R, Schalasta G, Enders G. 2010. Risk of fetal hydrops and non-hydropic late intrauterine fetal death after gestational parvovirus B19 infection. J Clin Virol 49:163–168. doi: 10.1016/j.jcv.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 276.Kerr JR, O'Neill HJ, Coyle PV, Thompson W. 1994. An outbreak of parvovirus B19 infection; a study of clinical manifestations and the incidence of fetal loss. Ir J Med Sci 163:65–67. doi: 10.1007/BF02943018. [DOI] [PubMed] [Google Scholar]
- 277.Public Health Laboratory Service Working Party on Fifth Disease. 1990. Prospective study of human parvovirus (B19) infection in pregnancy. BMJ 300:1166–1170. doi: 10.1136/bmj.300.6733.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Rodis JF, Quinn DL, Gary GW Jr, Anderson LJ, Rosengren S, Cartter ML, Campbell WA, Vintzileos AM. 1990. Management and outcomes of pregnancies complicated by human B19 parvovirus infection: a prospective study. Am J Obstet Gynecol 163:1168–1171. doi: 10.1016/0002-9378(90)90681-V. [DOI] [PubMed] [Google Scholar]
- 279.Gratacos E, Torres PJ, Vidal J, Antolin E, Costa J, Jimenez de Anta MT, Cararach V, Alonso PL, Fortuny A. 1995. The incidence of human parvovirus B19 infection during pregnancy and its impact on perinatal outcome. J Infect Dis 171:1360–1363. doi: 10.1093/infdis/171.5.1360. [DOI] [PubMed] [Google Scholar]
- 280.Koch WC, Harger JH, Barnstein B, Adler SP. 1998. Serologic and virologic evidence for frequent intrauterine transmission of human parvovirus B19 with a primary maternal infection during pregnancy. Pediatr Infect Dis J 17:489–494. doi: 10.1097/00006454-199806000-00011. [DOI] [PubMed] [Google Scholar]
- 281.Riipinen A, Sallmén M, Hedman L, Ojajärvi A, Lindbohm ML, Meriluoto M, Surcel HM, Taskinen H, Nuutila M, Karikoski R, Hedman K, Söderlund-Venermo M. 2014. Increased risk of human parvovirus B19 infection in day-care employees: a cohort study among pregnant workers during an epidemic in Finland. Occup Environ Med 71:836–841. doi: 10.1136/oemed-2014-102217. [DOI] [PubMed] [Google Scholar]
- 282.Valeur-Jensen AK, Pedersen CB, Westergaard T, Jensen IP, Lebech M, Andersen PK, Aaby P, Pedersen BN, Melbye M. 1999. Risk factors for parvovirus B19 infection in pregnancy. JAMA 281:1099–1105. doi: 10.1001/jama.281.12.1099. [DOI] [PubMed] [Google Scholar]
- 283.Crowcroft NS, Roth CE, Cohen BJ, Miller E. 1999. Guidance for control of parvovirus B19 infection in healthcare settings and the community. J Public Health Med 21:439–446. doi: 10.1093/pubmed/21.4.439. [DOI] [PubMed] [Google Scholar]
- 284.Centers for Disease Control. 1989. Risks associated with human parvovirus B19 infection. MMWR Morb Mortal Wkly Rep 38:81–87. [PubMed] [Google Scholar]
- 285.Brieu N, Guyon G, Rodiere M, Segondy M, Foulongne V. 2008. Human bocavirus infection in children with respiratory tract disease. Pediatr Infect Dis J 27:969–973. doi: 10.1097/INF.0b013e31817acfaa. [DOI] [PubMed] [Google Scholar]
- 286.von Linstow ML, Hogh M, Høgh B. 2008. Clinical and epidemiologic characteristics of human bocavirus in Danish infants: results from a prospective birth cohort study. Pediatr Infect Dis J 27:897–902. doi: 10.1097/INF.0b013e3181757b16. [DOI] [PubMed] [Google Scholar]
- 287.Blessing K, Neske F, Herre U, Kreth HW, Weissbrich B. 2009. Prolonged detection of human bocavirus DNA in nasopharyngeal aspirates of children with respiratory tract disease. Pediatr Infect Dis J 28:1018–1019. doi: 10.1097/INF.0b013e3181a854ae. [DOI] [PubMed] [Google Scholar]
- 288.Martin ET, Fairchok MP, Kuypers J, Magaret A, Zerr DM, Wald A, Englund JA. 2010. Frequent and prolonged shedding of bocavirus in young children attending daycare. J Infect Dis 201:1625–1632. doi: 10.1086/652405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lehtoranta L, Söderlund-Venermo M, Nokso-Koivisto J, Toivola H, Blomgren K, Hatakka K, Poussa T, Korpela R, Pitkäranta A. 2012. Human bocavirus in the nasopharynx of otitis-prone children. Int J Pediatr Otorhinolaryngol 76:206–211. doi: 10.1016/j.ijporl.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Deng Y, Gu X, Zhao X, Luo J, Luo Z, Wang L, Fu Z, Yang X, Liu E. 2012. High viral load of human bocavirus correlates with duration of wheezing in children with severe lower respiratory tract infection. PLoS One 7:e34353. doi: 10.1371/journal.pone.0034353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Martin ET, Kuypers J, McRoberts JP, Englund JA, Zerr DM. 2015. Human bocavirus-1 primary infection and shedding in infants. J Infect Dis 212:516–524. doi: 10.1093/infdis/jiv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Wagner JC, Pyles RB, Miller AL, Nokso-Koivisto J, Loeffelholz MJ, Chonmaitree T. 2016. Determining persistence of bocavirus DNA in the respiratory tract of children by pyrosequencing. Pediatr Infect Dis J 35:471–476. doi: 10.1097/INF.0000000000001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Chieochansin T, Kapoor A, Delwart E, Poovorawan Y, Simmonds P. 2009. Absence of detectable replication of human bocavirus species 2 in respiratory tract. Emerg Infect Dis 15:1503–1505. doi: 10.3201/eid1509.090394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Han TH, Kim CH, Park SH, Kim EJ, Chung JY, Hwang ES. 2009. Detection of human bocavirus-2 in children with acute gastroenteritis in South Korea. Arch Virol 154:1923–1927. doi: 10.1007/s00705-009-0533-3. [DOI] [PubMed] [Google Scholar]
- 295.Kantola K, Sadeghi M, Antikainen J, Kirveskari J, Delwart E, Hedman K, Söderlund-Venermo M. 2010. Real-time quantitative PCR detection of four human bocaviruses. J Clin Microbiol 48:4044–4050. doi: 10.1128/JCM.00686-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Chow BD, Ou Z, Esper FP. 2010. Newly recognized bocaviruses (HBoV, HBoV2) in children and adults with gastrointestinal illness in the United States. J Clin Virol 47:143–147. doi: 10.1016/j.jcv.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 297.Song JR, Jin Y, Xie ZP, Gao HC, Xiao NG, Chen WX, Xu ZQ, Yan KL, Zhao Y, Hou YD, Duan ZJ. 2010. Novel human bocavirus in children with acute respiratory tract infection. Emerg Infect Dis 16:324–327. doi: 10.3201/eid1602.090553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Santos N, Peret TC, Humphrey CD, Albuquerque MC, Silva RC, Benati FJ, Lu X, Erdman DD. 2010. Human bocavirus species 2 and 3 in Brazil. J Clin Virol 48:127–130. doi: 10.1016/j.jcv.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 299.Cheng W, Chen J, Xu Z, Yu J, Huang C, Jin M, Li H, Zhang M, Jin Y, Duan ZJ. 2011. Phylogenetic and recombination analysis of human bocavirus 2. BMC Infect Dis 11:50. doi: 10.1186/1471-2334-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Paloniemi M, Lappalainen S, Salminen M, Kätkä M, Kantola K, Hedman L, Hedman K, Söderlund-Venermo M, Vesikari T. 2014. Human bocaviruses are commonly found in stools of hospitalized children without causal association to acute gastroenteritis. Eur J Pediatr 173:1051–1057. doi: 10.1007/s00431-014-2290-x. [DOI] [PubMed] [Google Scholar]
- 301.Zhao M, Zhu R, Qian Y, Deng J, Wang F, Sun Y, Dong H, Liu L, Jia L, Zhao L. 2014. Prevalence analysis of different human bocavirus genotypes in pediatric patients revealed intra-genotype recombination. Infect Genet Evol 27:382–388. doi: 10.1016/j.meegid.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 302.Riipinen A, Väisänen E, Lahtinen A, Karikoski R, Nuutila M, Surcel HM, Taskinen H, Hedman K, Söderlund-Venermo M. 2010. Absence of human bocavirus from deceased fetuses and their mothers. J Clin Virol 47:186–188. doi: 10.1016/j.jcv.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 303.Fryer JF, Delwart E, Hecht FM, Bernardin F, Jones MS, Shah N, Baylis SA. 2007. Frequent detection of the parvoviruses, PARV4 and PARV5, in plasma from blood donors and symptomatic individuals. Transfusion 47:1054–1061. doi: 10.1111/j.1537-2995.2007.01235.x. [DOI] [PubMed] [Google Scholar]
- 304.Modrow S, Wenzel JJ, Schimanski S, Schwarzbeck J, Rothe U, Oldenburg J, Jilg W, Eis-Hübinger AM. 2011. Prevalence of nucleic acid sequences specific for human parvoviruses, hepatitis A and hepatitis E viruses in coagulation factor concentrates. Vox Sang 100:351–358. doi: 10.1111/j.1423-0410.2010.01445.x. [DOI] [PubMed] [Google Scholar]
- 305.Li H, He M, Zeng P, Gao Z, Bian G, Yang C, Li W. 2015. The genomic and seroprevalence of human bocavirus in healthy Chinese plasma donors and plasma derivatives. Transfusion 55:154–163. doi: 10.1111/trf.12785. [DOI] [PubMed] [Google Scholar]
- 306.Arnold JC, Singh KK, Spector SA, Sawyer MH. 2006. Human bocavirus: prevalence and clinical spectrum at a children's hospital. Clin Infect Dis 43:283–288. doi: 10.1086/505399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kesebir D, Vazquez M, Weibel C, Shapiro ED, Ferguson D, Landry ML, Kahn JS. 2006. Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis 194:1276–1282. doi: 10.1086/508213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Manning A, Russell V, Eastick K, Leadbetter GH, Hallam N, Templeton K, Simmonds P. 2006. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J Infect Dis 194:1283–1290. doi: 10.1086/508219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Chung JY, Han TH, Kim CK, Kim SW. 2006. Bocavirus infection in hospitalized children, South Korea. Emerg Infect Dis 12:1254–1256. doi: 10.3201/eid1208.060261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Fry AM, Lu X, Chittaganpitch M, Peret T, Fischer J, Dowell SF, Anderson LJ, Erdman D, Olsen SJ. 2007. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis 195:1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Longtin J, Bastien M, Gilca R, Leblanc E, de Serres G, Bergeron MG, Boivin G. 2008. Human bocavirus infections in hospitalized children and adults. Emerg Infect Dis 14:217–221. doi: 10.3201/eid1402.070851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Garbino J, Soccal PM, Aubert JD, Rochat T, Meylan P, Thomas Y, Tapparel C, Bridevaux PO, Kaiser L. 2009. Respiratory viruses in bronchoalveolar lavage: a hospital-based cohort study in adults. Thorax 64:399–404. doi: 10.1136/thx.2008.105155. [DOI] [PubMed] [Google Scholar]
- 313.Costa C, Bergallo M, Cavallo R. 2009. Detection of human bocavirus in bronchoalveolar lavage from Italian adult patients. J Clin Virol 45:81–82. doi: 10.1016/j.jcv.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 314.Aronen M, Viikari L, Vuorinen T, Langen H, Söderlund-Venermo M, Hameenaho M, Sadeghi M, Viitanen M, Jartti T. 2016. Virus etiology of airway illness in elderly adults. J Am Geriatr Soc 64:1358–1360. doi: 10.1111/jgs.14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Lin F, Zeng A, Yang N, Lin H, Yang E, Wang S, Pintel D, Qiu J. 2007. Quantification of human bocavirus in lower respiratory tract infections in China. Infect Agent Cancer 2:3. doi: 10.1186/1750-9378-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Allander T, Jartti T, Gupta S, Niesters HG, Lehtinen P, Osterback R, Vuorinen T, Waris M, Bjerkner A, Tiveljung-Lindell A, van den Hoogen BG, Hyypiä T, Ruuskanen O. 2007. Human bocavirus and acute wheezing in children. Clin Infect Dis 44:904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Pozo F, Garcia-Garcia ML, Calvo C, Cuesta I, Perez-Brena P, Casas I. 2007. High incidence of human bocavirus infection in children in Spain. J Clin Virol 40:224–228. doi: 10.1016/j.jcv.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Lu X, Gooding LR, Erdman DD. 2008. Human bocavirus in tonsillar lymphocytes. Emerg Infect Dis 14:1332–1334. doi: 10.3201/eid1408.080300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Clement N, Battaglioli G, Jensen RL, Schnepp BC, Johnson PR, St George K, Linden RM. 2009. Prevalence of human bocavirus in human tonsils and adenoids. Emerg Infect Dis 15:1149–1150. doi: 10.3201/eid1507.090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Tozer SJ, Lambert SB, Whiley DM, Bialasiewicz S, Lyon MJ, Nissen MD, Sloots TP. 2009. Detection of human bocavirus in respiratory, fecal, and blood samples by real-time PCR. J Med Virol 81:488–493. doi: 10.1002/jmv.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Martin ET, Taylor J, Kuypers J, Magaret A, Wald A, Zerr D, Englund JA. 2009. Detection of bocavirus in saliva of children with and without respiratory illness. J Clin Microbiol 47:4131–4132. doi: 10.1128/JCM.01508-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Wang K, Wang W, Yan H, Ren P, Zhang J, Shen J, Deubel V. 2010. Correlation between bocavirus infection and humoral response, and co-infection with other respiratory viruses in children with acute respiratory infection. J Clin Virol 47:148–155. doi: 10.1016/j.jcv.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Christensen A, Nordbø SA, Krokstad S, Rognlien AG, Døllner H. 2010. Human bocavirus in children: mono-detection, high viral load and viraemia are associated with respiratory tract infection. J Clin Virol 49:158–162. doi: 10.1016/j.jcv.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Kapoor A, Hornig M, Asokan A, Williams B, Henriquez JA, Lipkin WI. 2011. Bocavirus episome in infected human tissue contains non-identical termini. PLoS One 6:e21362. doi: 10.1371/journal.pone.0021362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Norja P, Hedman L, Kantola K, Kemppainen K, Suvilehto J, Pitkaranta A, Aaltonen LM, Seppänen M, Hedman K, Söderlund-Venermo M. 2012. Occurrence of human bocaviruses and parvovirus 4 in solid tissues. J Med Virol 84:1267–1273. doi: 10.1002/jmv.23335. [DOI] [PubMed] [Google Scholar]
- 326.Mitui MT, Tabib SM, Matsumoto T, Khanam W, Ahmed S, Mori D, Akhter N, Yamada K, Kabir L, Nishizono A, Söderlund-Venermo M, Ahmed K. 2012. Detection of human bocavirus in the cerebrospinal fluid of children with encephalitis. Clin Infect Dis 54:964–967. doi: 10.1093/cid/cir957. [DOI] [PubMed] [Google Scholar]
- 327.Mori D, Ranawaka U, Yamada K, Rajindrajith S, Miya K, Perera HK, Matsumoto T, Dassanayake M, Mitui MT, Mori H, Nishizono A, Söderlund-Venermo M, Ahmed K. 2013. Human bocavirus in patients with encephalitis, Sri Lanka, 2009-2010. Emerg Infect Dis 19:1859–1862. doi: 10.3201/eid1911.121548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Blinkova O, Rosario K, Li L, Kapoor A, Slikas B, Bernardin F, Breitbart M, Delwart E. 2009. Frequent detection of highly diverse variants of cardiovirus, cosavirus, bocavirus, and circovirus in sewage samples collected in the United States. J Clin Microbiol 47:3507–3513. doi: 10.1128/JCM.01062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Hamza IA, Jurzik L, Wilhelm M, Uberla K. 2009. Detection and quantification of human bocavirus in river water. J Gen Virol 90:2634–2637. doi: 10.1099/vir.0.013557-0. [DOI] [PubMed] [Google Scholar]
- 330.Rasanen S, Lappalainen S, Kaikkonen S, Hämäläinen M, Salminen M, Vesikari T. 2010. Mixed viral infections causing acute gastroenteritis in children in a waterborne outbreak. Epidemiol Infect 138:1227–1234. doi: 10.1017/S0950268809991671. [DOI] [PubMed] [Google Scholar]
- 331.Myrmel M, Lange H, Rimstad E. 2015. A 1-year quantitative survey of noro-, adeno-, human boca-, and hepatitis E viruses in raw and secondarily treated sewage from two plants in Norway. Food Environ Virol 7:213–223. doi: 10.1007/s12560-015-9200-x. [DOI] [PubMed] [Google Scholar]
- 332.Söderlund-Venermo M, Lahtinen A, Jartti T, Hedman L, Kemppainen K, Lehtinen P, Allander T, Ruuskanen O, Hedman K. 2009. Clinical assessment and improved diagnosis of bocavirus-induced wheezing in children, Finland. Emerg Infect Dis 15:1423–1430. doi: 10.3201/eid1509.090204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Karalar L, Lindner J, Schimanski S, Kertai M, Segerer H, Modrow S. 2010. Prevalence and clinical aspects of human bocavirus infection in children. Clin Microbiol Infect 16:633–639. doi: 10.1111/j.1469-0691.2009.02889.x. [DOI] [PubMed] [Google Scholar]
- 334.Kantola K, Hedman L, Arthur J, Alibeto A, Delwart E, Jartti T, Ruuskanen O, Hedman K, Söderlund-Venermo M. 2011. Seroepidemiology of human bocaviruses 1-4. J Infect Dis 204:1403–1412. doi: 10.1093/infdis/jir525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Kantola K, Hedman L, Tanner L, Simell V, Makinen M, Partanen J, Sadeghi M, Veijola R, Knip M, Ilonen J, Hyöty H, Toppari J, Simell O, Hedman K, Söderlund-Venermo M. 2015. B-cell responses to human bocaviruses 1-4: new insights from a childhood follow-up study. PLoS One 10:e0139096. doi: 10.1371/journal.pone.0139096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Meriluoto M, Hedman L, Tanner L, Simell V, Mäkinen M, Simell S, Mykkänen J, Korpelainen J, Ruuskanen O, Ilonen J, Knip M, Simell O, Hedman K, Söderlund-Venermo M. 2012. Association of human bocavirus 1 infection with respiratory disease in childhood follow-up study, Finland. Emerg Infect Dis 18:264–271. doi: 10.3201/eid1802.111293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Francis TJ. 1960. On the doctrine of original antigenic sin. Proc Am Philos Soc 104:572–578. [Google Scholar]
- 338.Li X, Kantola K, Hedman L, Arku B, Hedman K, Söderlund-Venermo M. 2015. Original antigenic sin with human bocaviruses 1-4. J Gen Virol 96:3099–3108. doi: 10.1099/jgv.0.000253. [DOI] [PubMed] [Google Scholar]
- 339.Umene K, Nunoue T. 1993. Partial nucleotide sequencing and characterization of human parvovirus B19 genome DNAs from damaged human fetuses and from patients with leukemia. J Med Virol 39:333–339. doi: 10.1002/jmv.1890390413. [DOI] [PubMed] [Google Scholar]
- 340.Gallinella G, Venturoli S, Gentilomi G, Musiani M, Zerbini M. 1995. Extent of sequence variability in a genomic region coding for capsid proteins of B19 parvovirus. Arch Virol 140:1119–1125. doi: 10.1007/BF01315420. [DOI] [PubMed] [Google Scholar]
- 341.Erdman DD, Durigon EL, Wang QY, Anderson LJ. 1996. Genetic diversity of human parvovirus B19: sequence analysis of the VP1/VP2 gene from multiple isolates. J Gen Virol 77:2767–2774. doi: 10.1099/0022-1317-77-11-2767. [DOI] [PubMed] [Google Scholar]
- 342.Hemauer A, von Poblotzki A, Gigler A, Cassinotti P, Siegl G, Wolf H, Modrow S. 1996. Sequence variability among different parvovirus B19 isolates. J Gen Virol 77:1781–1785. doi: 10.1099/0022-1317-77-8-1781. [DOI] [PubMed] [Google Scholar]
- 343.Hubschen JM, Mihneva Z, Mentis AF, Schneider F, Aboudy Y, Grossman Z, Rudich H, Kasymbekova K, Sarv I, Nedeljkovic J, Tahita MC, Tarnagda Z, Ouedraogo JB, Gerasimova AG, Moskaleva TN, Tikhonova NT, Chitadze N, Forbi JC, Faneye AO, Otegbayo JA, Charpentier E, Muller CP. 2009. Phylogenetic analysis of human parvovirus B19 sequences from eleven different countries confirms the predominance of genotype 1 and suggests the spread of genotype 3b. J Clin Microbiol 47:3735–3738. doi: 10.1128/JCM.01201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Liefeldt L, Plentz A, Klempa B, Kershaw O, Endres AS, Raab U, Neumayer HH, Meisel H, Modrow S. 2005. Recurrent high level parvovirus B19/genotype 2 viremia in a renal transplant recipient analyzed by real-time PCR for simultaneous detection of genotypes 1 to 3. J Med Virol 75:161–169. doi: 10.1002/jmv.20251. [DOI] [PubMed] [Google Scholar]
- 345.Toan NL, Duechting A, Kremsner PG, Song LH, Ebinger M, Aberle S, Binh VQ, Duy DN, Torresi J, Kandolf R, Bock CT. 2006. Phylogenetic analysis of human parvovirus B19, indicating two subgroups of genotype 1 in Vietnamese patients. J Gen Virol 87:2941–2949. doi: 10.1099/vir.0.82037-0. [DOI] [PubMed] [Google Scholar]
- 346.Grabarczyk P, Kalinska A, Kara M, Wieczorek R, Ejduk A, Sulkowska E, Golebiowska-Staroszczyk S, Matysiak M, Baylis SA, Brojer E. 2011. Identification and characterization of acute infection with parvovirus B19 genotype 2 in immunocompromised patients in Poland. J Med Virol 83:142–149. doi: 10.1002/jmv.21947. [DOI] [PubMed] [Google Scholar]
- 347.Ivanova SK, Mihneva ZG, Toshev AK, Kovaleva VP, Andonova LG, Muller CP, Hubschen JM. 2016. Insights into epidemiology of human parvovirus B19 and detection of an unusual genotype 2 variant, Bulgaria, 2004 to 2013. Euro Surveill 21:pii=30116. doi: 10.2807/1560-7917.ES.2016.21.4.30116. [DOI] [PubMed]
- 348.Eis-Hübinger AM, Reber U, Edelmann A, Kalus U, Hofmann J. 2014. Parvovirus B19 genotype 2 in blood donations. Transfusion 54:1682–1684. doi: 10.1111/trf.12591. [DOI] [PubMed] [Google Scholar]
- 349.Toppinen M, Perdomo MF, Palo JU, Simmonds P, Lycett SJ, Soderlund-Venermo M, Sajantila A, Hedman K. 2015. Bones hold the key to DNA virus history and epidemiology. Sci Rep 5:17226. doi: 10.1038/srep17226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Candotti D, Etiz N, Parsyan A, Allain JP. 2004. Identification and characterization of persistent human erythrovirus infection in blood donor samples. J Virol 78:12169–12178. doi: 10.1128/JVI.78.22.12169-12178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sanabani S, Neto WK, Pereira J, Sabino EC. 2006. Sequence variability of human erythroviruses present in bone marrow of Brazilian patients with various parvovirus B19-related hematological symptoms. J Clin Microbiol 44:604–606. doi: 10.1128/JCM.44.2.604-606.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Freitas RB, Melo FL, Oliveira DS, Romano CM, Freitas MR, Macedo O, Linhares AC, Zanotto PMDA, Durigon EL. 2008. Molecular characterization of human erythrovirus B19 strains obtained from patients with several clinical presentations in the Amazon region of Brazil. J Clin Virol 43:60–65. doi: 10.1016/j.jcv.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 353.Corcoran C, Hardie D, Yeats J, Smuts H. 2010. Genetic variants of human parvovirus B19 in South Africa: cocirculation of three genotypes and identification of a novel subtype of genotype 1. J Clin Microbiol 48:137–142. doi: 10.1128/JCM.00610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.da Costa AC, Bendit I, de Oliveira AC, Kallas EG, Sabino EC, Sanabani SS. 2013. Investigation of human parvovirus B19 occurrence and genetic variability in different leukaemia entities. Clin Microbiol Infect 19:E31–E43. doi: 10.1111/1469-0691.12058. [DOI] [PubMed] [Google Scholar]
- 355.Jain P, Jain A, Prakash S, Khan DN, Singh DD, Kumar A, Moulik NR, Chandra T. 2015. Prevalence and genotypic characterization of human parvovirus B19 in children with hemato-oncological disorders in North India. J Med Virol 87:303–309. doi: 10.1002/jmv.24028. [DOI] [PubMed] [Google Scholar]
- 356.Heegaard ED, Taaning EB. 2002. Parvovirus B19 and parvovirus V9 are not associated with Henoch-Schonlein purpura in children. Pediatr Infect Dis J 21:31–34. doi: 10.1097/00006454-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 357.Parsyan A, Szmaragd C, Allain JP, Candotti D. 2007. Identification and genetic diversity of two human parvovirus B19 genotype 3 subtypes. J Gen Virol 88:428–431. doi: 10.1099/vir.0.82496-0. [DOI] [PubMed] [Google Scholar]
- 358.Schneider B, Höne A, Tolba RH, Fischer HP, Blumel J, Eis-Hübinger AM. 2008. Simultaneous persistence of multiple genome variants of human parvovirus B19. J Gen Virol 89:164–176. doi: 10.1099/vir.0.83053-0. [DOI] [PubMed] [Google Scholar]
- 359.Hongxing S, Zhang W, Wang H, Shao S. 3 February 2016. Identification of recombination in the NS1 and VPs genes of parvovirus B19. J Med Virol doi: 10.1002/jmv.24471. [DOI] [PubMed] [Google Scholar]
- 360.Shackelton LA, Holmes EC. 2006. Phylogenetic evidence for the rapid evolution of human B19 erythrovirus. J Virol 80:3666–3669. doi: 10.1128/JVI.80.7.3666-3669.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Norja P, Eis-Hübinger AM, Söderlund-Venermo M, Hedman K, Simmonds P. 2008. Rapid sequence change and geographical spread of human parvovirus B19: comparison of B19 virus evolution in acute and persistent infections. J Virol 82:6427–6433. doi: 10.1128/JVI.00471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Duffy S, Shackelton LA, Holmes EC. 2008. Rates of evolutionary change in viruses: patterns and determinants. Nat Rev Genet 9:267–276. doi: 10.1038/nrg2323. [DOI] [PubMed] [Google Scholar]
- 363.Shackelton LA, Parrish CR, Truyen U, Holmes EC. 2005. High rate of viral evolution associated with the emergence of carnivore parvovirus. Proc Natl Acad Sci U S A 102:379–384. doi: 10.1073/pnas.0406765102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Sharp CP, LeBreton M, Kantola K, Nana A, Diffo JD, Djoko CF, Tamoufe U, Kiyang JA, Babila TG, Ngole EM, Pybus OG, Delwart E, Delaporte E, Peeters M, Söderlund-Venermo M, Hedman K, Wolfe ND, Simmonds P. 2010. Widespread infection with homologues of human parvoviruses B19, PARV4, and human bocavirus of chimpanzees and gorillas in the wild. J Virol 84:10289–10296. doi: 10.1128/JVI.01304-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Kapoor A, Mehta N, Esper F, Poljsak-Prijatelj M, Quan PL, Qaisar N, Delwart E, Lipkin WI. 2010. Identification and characterization of a new bocavirus species in gorillas. PLoS One 5:e11948. doi: 10.1371/journal.pone.0011948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Babkin IV, Tyumentsev AI, Tikunov AY, Kurilshikov AM, Ryabchikova EI, Zhirakovskaya EV, Netesov SV, Tikunova NV. 2013. Evolutionary time-scale of primate bocaviruses. Infect Genet Evol 14:265–274. doi: 10.1016/j.meegid.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 367.Brozova K, Hrazdilova K, Slaninkova E, Modry D, Cerny J, Celer V. 2016. Genetic and phylogenetic characterization of novel bocaparvovirus infecting chimpanzee. Infect Genet Evol 37:231–236. doi: 10.1016/j.meegid.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 368.Fu X, Wang X, Ni B, Shen H, Wang H, Zhang X, Chen S, Shao S, Zhang W. 2011. Recombination analysis based on the complete genome of bocavirus. Virol J 8:182–188. doi: 10.1186/1743-422X-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Khamrin P, Okitsu S, Ushijima H, Maneekarn N. 2013. Complete genome sequence analysis of novel human bocavirus reveals genetic recombination between human bocavirus 2 and human bocavirus 4. Infect Genet Evol 17:132–136. doi: 10.1016/j.meegid.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 370.Tyumentsev AI, Tikunova NV, Tikunov AY, Babkin IV. 2014. Recombination in the evolution of human bocavirus. Infect Genet Evol 28:11–14. doi: 10.1016/j.meegid.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 371.Saarinen UM, Chorba TL, Tattersall P, Young NS, Anderson LJ, Palmer E, Coccia PF. 1986. Human parvovirus B19-induced epidemic acute red cell aplasia in patients with hereditary hemolytic anemia. Blood 67:1411–1417. [PubMed] [Google Scholar]
- 372.Kurtzman GJ, Cohen BJ, Field AM, Oseas R, Blaese RM, Young NS. 1989. Immune response to B19 parvovirus and an antibody defect in persistent viral infection. J Clin Invest 84:1114–1123. doi: 10.1172/JCI114274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Erdman DD, Usher MJ, Tsou C, Caul EO, Gary GW, Kajigaya S, Young NS, Anderson LJ. 1991. Human parvovirus B19 specific IgG, IgA, and IgM antibodies and DNA in serum specimens from persons with erythema infectiosum. J Med Virol 35:110–115. doi: 10.1002/jmv.1890350207. [DOI] [PubMed] [Google Scholar]
- 374.Gray JJ, Cohen BJ, Desselberger U. 1993. Detection of human parvovirus B19-specific IgM and IgG antibodies using a recombinant viral VP1 antigen expressed in insect cells and estimation of time of infection by testing for antibody avidity. J Virol Methods 44:11–23. doi: 10.1016/0166-0934(93)90003-A. [DOI] [PubMed] [Google Scholar]
- 375.Söderlund M, Brown KE, Meurman O, Hedman K. 1992. Prokaryotic expression of a VP1 polypeptide antigen for diagnosis by a human parvovirus B19 antibody enzyme immunoassay. J Clin Microbiol 30:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Söderlund M, Brown CS, Cohen BJ, Hedman K. 1995. Accurate serodiagnosis of B19 parvovirus infections by measurement of IgG avidity. J Infect Dis 171:710–713. doi: 10.1093/infdis/171.3.710. [DOI] [PubMed] [Google Scholar]
- 377.Brown CS, Jensen T, Meloen RH, Puijk W, Sugamura K, Sato H, Spaan WJ. 1992. Localization of an immunodominant domain on baculovirus-produced parvovirus B19 capsids: correlation to a major surface region on the native virus particle. J Virol 66:6989–6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Sato H, Hirata J, Kuroda N, Shiraki H, Maeda Y, Okochi K. 1991. Identification and mapping of neutralizing epitopes of human parvovirus B19 by using human antibodies. J Virol 65:5485–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Sato H, Hirata J, Furukawa M, Kuroda N, Shiraki H, Maeda Y, Okochi K. 1991. Identification of the region including the epitope for a monoclonal antibody which can neutralize human parvovirus B19. J Virol 65:1667–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Yoshimoto K, Rosenfeld S, Frickhofen N, Kennedy D, Hills R, Kajigaya S, Young NS. 1991. A second neutralizing epitope of B19 parvovirus implicates the spike region in the immune response. J Virol 65:7056–7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Gigler A, Dorsch S, Hemauer A, Williams C, Kim S, Young NS, Zolla-Pazner S, Wolf H, Gorny MK, Modrow S. 1999. Generation of neutralizing human monoclonal antibodies against parvovirus B19 proteins. J Virol 73:1974–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Corcoran A, Mahon BP, Doyle S. 2004. B cell memory is directed toward conformational epitopes of parvovirus B19 capsid proteins and the unique region of VP1. J Infect Dis 189:1873–1880. doi: 10.1086/382963. [DOI] [PubMed] [Google Scholar]
- 383.Söderlund M, Brown CS, Spaan WJ, Hedman L, Hedman K. 1995. Epitope type-specific IgG responses to capsid proteins VP1 and VP2 of human parvovirus B19. J Infect Dis 172:1431–1436. doi: 10.1093/infdis/172.6.1431. [DOI] [PubMed] [Google Scholar]
- 384.von Poblotzki A, Hemauer A, Gigler A, Puchhammer-Stockl E, Heinz FX, Pont J, Laczika K, Wolf H, Modrow S. 1995. Antibodies to the nonstructural protein of parvovirus B19 in persistently infected patients: implications for pathogenesis. J Infect Dis 172:1356–1359. doi: 10.1093/infdis/172.5.1356. [DOI] [PubMed] [Google Scholar]
- 385.Ennis O, Corcoran A, Kavanagh K, Mahon BP, Doyle S. 2001. Baculovirus expression of parvovirus B19 (B19V) NS1: utility in confirming recent infection. J Clin Virol 22:55–60. doi: 10.1016/S1386-6532(01)00168-8. [DOI] [PubMed] [Google Scholar]
- 386.von Poblotzki A, Gerdes C, Reischl U, Wolf H, Modrow S. 1996. Lymphoproliferative responses after infection with human parvovirus B19. J Virol 70:7327–7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Franssila R, Hedman K. 2004. T-helper cell-mediated interferon-gamma, interleukin-10 and proliferation responses to a candidate recombinant vaccine for human parvovirus B19. Vaccine 22:3809–3815. doi: 10.1016/j.vaccine.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 388.Norbeck O, Isa A, Pohlmann C, Broliden K, Kasprowicz V, Bowness P, Klenerman P, Tolfvenstam T. 2005. Sustained CD8+ T-cell responses induced after acute parvovirus B19 infection in humans. J Virol 79:12117–12121. doi: 10.1128/JVI.79.18.12117-12121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Tolfvenstam T, Oxenius A, Price DA, Shacklett BL, Spiegel HM, Hedman K, Norbeck O, Levi M, Olsen K, Kantzanou M, Nixon DF, Broliden K, Klenerman P. 2001. Direct ex vivo measurement of CD8+ T-lymphocyte responses to human parvovirus B19. J Virol 75:540–543. doi: 10.1128/JVI.75.1.540-543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Isa A, Norbeck O, Hirbod T, Lundqvist A, Kasprowicz V, Bowness P, Klenerman P, Broliden K, Tolfvenstam T. 2006. Aberrant cellular immune responses in humans infected persistently with parvovirus B19. J Med Virol 78:129–133. doi: 10.1002/jmv.20514. [DOI] [PubMed] [Google Scholar]
- 391.Fujita T, Ikejima H, Yamagata N, Kudo Y, Hoshi K. 2007. In vitro response of immunoregulatory cytokine expression in human monocytic cells to human parvovirus B19 capsid. Biol Pharm Bull 30:2027–2030. doi: 10.1248/bpb.30.2027. [DOI] [PubMed] [Google Scholar]
- 392.Franssila R, Auramo J, Modrow S, Möbs M, Oker-Blom C, Käpylä P, Söderlund-Venermo M, Hedman K. 2005. T helper cell-mediated interferon-gamma expression after human parvovirus B19 infection: persisting VP2-specific and transient VP1u-specific activity. Clin Exp Immunol 142:53–61. doi: 10.1111/j.1365-2249.2005.02886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Isa A, Lundqvist A, Lindblom A, Tolfvenstam T, Broliden K. 2007. Cytokine responses in acute and persistent human parvovirus B19 infection. Clin Exp Immunol 147:419–425. doi: 10.1111/j.1365-2249.2006.03286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Guo L, Wang Y, Zhou H, Wu C, Song J, Li J, Paranhos-Baccala G, Vernet G, Wang J, Hung T. 2012. Differential seroprevalence of human bocavirus species 1-4 in Beijing, China. PLoS One 7:e39644. doi: 10.1371/journal.pone.0039644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Lindner J, Zehentmeier S, Franssila R, Barabas S, Schroeder J, Deml L, Modrow S. 2008. CD4+ T helper cell responses against human bocavirus viral protein 2 viruslike particles in healthy adults. J Infect Dis 198:1677–1684. doi: 10.1086/592985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Kumar A, Filippone C, Lahtinen A, Hedman L, Söderlund-Venermo M, Hedman K, Franssila R. 2011. Comparison of Th-cell immunity against human bocavirus and parvovirus B19: proliferation and cytokine responses are similar in magnitude but more closely interrelated with human bocavirus. Scand J Immunol 73:135–140. doi: 10.1111/j.1365-3083.2010.02483.x. [DOI] [PubMed] [Google Scholar]
- 397.Hirose Y, Hamada H, Wakui T, Ogawa T, Terai M. 2014. Characteristic systemic cytokine responses in children with human bocavirus-positive lower respiratory tract infection. Microbiol Immunol 58:215–218. doi: 10.1111/1348-0421.12132. [DOI] [PubMed] [Google Scholar]
- 398.Chung JY, Han TH, Kim JS, Kim SW, Park CG, Hwang ES. 2008. Th1 and Th2 cytokine levels in nasopharyngeal aspirates from children with human bocavirus bronchiolitis. J Clin Virol 43:223–225. doi: 10.1016/j.jcv.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 399.Hsu GJ, Tzang BS, Tsai CC, Chiu CC, Huang CY, Hsu TC. 2011. Effects of human parvovirus B19 on expression of defensins and Toll-like receptors. Chin J Physiol 54:367–376. [PubMed] [Google Scholar]
- 400.Kerr JR, Kaushik N, Fear D, Baldwin DA, Nuwaysir EF, Adcock IM. 2005. Single-nucleotide polymorphisms associated with symptomatic infection and differential human gene expression in healthy seropositive persons each implicate the cytoskeleton, integrin signaling, and oncosuppression in the pathogenesis of human parvovirus B19 infection. J Infect Dis 192:276–286. doi: 10.1086/430950. [DOI] [PubMed] [Google Scholar]
- 401.Luo H, Zhang Z, Zheng Z, Ke X, Zhang X, Li Q, Liu Y, Bai B, Mao P, Hu Q, Wang H. 2013. Human bocavirus VP2 upregulates IFN-beta pathway by inhibiting ring finger protein 125-mediated ubiquitination of retinoic acid-inducible gene-I. J Immunol 191:660–669. doi: 10.4049/jimmunol.1202933. [DOI] [PubMed] [Google Scholar]
- 402.Zhang Z, Zheng Z, Luo H, Meng J, Li H, Li Q, Zhang X, Ke X, Bai B, Mao P, Hu Q, Wang H. 2012. Human bocavirus NP1 inhibits IFN-beta production by blocking association of IFN regulatory factor 3 with IFNB promoter. J Immunol 189:1144–1153. doi: 10.4049/jimmunol.1200096. [DOI] [PubMed] [Google Scholar]
- 403.Cherry JD. 2016. Diseases of probable viral etiology, p 1401–1404. In Feigin RD, Cherry JD (ed), Feigin and Cherry's textbook of pediatric infectious diseases. WB Saunders Company, Jacksonville, FL. [Google Scholar]
- 404.Balfour HH., Jr 1969. Erythema infectiosum (fifth disease). Clinical review and description of 91 cases seen in an epidemic. Clin Pediatr (Phila) 8:721–727. [DOI] [PubMed] [Google Scholar]
- 405.Lauer BA, MacCormack JN, Wilfert C. 1976. Erythema infectiosum. An elementary school outbreak. Am J Dis Child 130:252–254. [DOI] [PubMed] [Google Scholar]
- 406.Ager EA, Chin TD, Poland JD. 1966. Epidemic erythema infectiosum. N Engl J Med 275:1326–1331. doi: 10.1056/NEJM196612152752402. [DOI] [PubMed] [Google Scholar]
- 407.Breese C, Horner FA. 1977. Encephalopathy with erythema infectiosum. Am J Dis Child 131:65–67. [PubMed] [Google Scholar]
- 408.Balfour HH Jr, Schiff GM, Bloom JE. 1970. Encephalitis associated with erythema infectiosum. J Pediatr 77:133–136. doi: 10.1016/S0022-3476(70)80059-2. [DOI] [PubMed] [Google Scholar]
- 409.Naides SJ, Field EH. 1988. Transient rheumatoid factor positivity in acute human parvovirus B19 infection. Arch Intern Med 148:2587–2589. doi: 10.1001/archinte.1988.00380120051010. [DOI] [PubMed] [Google Scholar]
- 410.Cramp HE, Armstrong BD. 1976. Erythema infectiosum: an outbreak of “slapped cheek” disease in north Devon. Br Med J i:885–886. doi: 10.1136/bmj.1.6014.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Brass C, Elliott LM, Stevens DA. 1982. Academy rash. A probable epidemic of erythema infectiosum ('fifth disease'). JAMA 248:568–572. [DOI] [PubMed] [Google Scholar]
- 412.Leahy ST, Marshman G. 1998. Variable presentation of parvovirus B19 in a family. Australas J Dermatol 39:112–115. doi: 10.1111/j.1440-0960.1998.tb01261.x. [DOI] [PubMed] [Google Scholar]
- 413.Oiwa H, Shimada T, Hashimoto M, Kawaguchi A, Ueda T, Sugiyama E, Kamiya T. 2011. Clinical findings in parvovirus B19 infection in 30 adult patients in Kyoto. Mod Rheumatol 21:24–31. doi: 10.3109/s10165-010-0338-y. [DOI] [PubMed] [Google Scholar]
- 414.Schwarz TF, Wiersbitzky S, Pambor M. 1994. Case report: detection of parvovirus B19 in a skin biopsy of a patient with erythema infectiosum. J Med Virol 43:171–174. doi: 10.1002/jmv.1890430214. [DOI] [PubMed] [Google Scholar]
- 415.Fruhauf J, Massone C, Mullegger RR. 2009. Bullous papular-purpuric gloves and socks syndrome in a 42-year-old female: molecular detection of parvovirus B19 DNA in lesional skin. J Am Acad Dermatol 60:691–695. doi: 10.1016/j.jaad.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 416.Feldmann R, Harms M, Saurat JH. 1994. Papular-purpuric ‘gloves and socks' syndrome: not only parvovirus B19. Dermatology 188:85–87. doi: 10.1159/000247106. [DOI] [PubMed] [Google Scholar]
- 417.Mage V, Lipsker D, Barbarot S, Bessis D, Chosidow O, Del Giudice P, Aractingi S, Avouac J, Bernier C, Descamps V, Dupin N. 2014. Different patterns of skin manifestations associated with parvovirus B19 primary infection in adults. J Am Acad Dermatol 71:62–69. doi: 10.1016/j.jaad.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 418.Magro CM, Dawood MR, Crowson AN. 2000. The cutaneous manifestations of human parvovirus B19 infection. Hum Pathol 31:488–497. doi: 10.1053/hp.2000.6714. [DOI] [PubMed] [Google Scholar]
- 419.Lefrere JJ, Meyer O, Menkes CJ, Beaulieu MJ, Courouce AM. 1985. Human parvovirus and rheumatoid arthritis. Lancet i:982. [DOI] [PubMed] [Google Scholar]
- 420.Drago F, Ciccarese G, Broccolo F, Javor S, Parodi A. 2015. Atypical exanthems associated with parvovirus B19 (B19V) infection in children and adults. J Med Virol 87:1981–1984. doi: 10.1002/jmv.24246. [DOI] [PubMed] [Google Scholar]
- 421.Smith SB, Libow LF, Elston DM, Bernert RA, Warschaw KE. 2002. Gloves and socks syndrome: early and late histopathologic features. J Am Acad Dermatol 47:749–754. doi: 10.1067/mjd.2002.124612. [DOI] [PubMed] [Google Scholar]
- 422.Tuccio A, Zanelli G, Rodriguez DC, Tataranno ML, Vascotto M, Balestri P. 2014. Petechial rash associated with parvovirus B19 in children: case report and literature review. Infez Med 22:250–254. [PubMed] [Google Scholar]
- 423.Segura Saint-Gerons R, Ceballos SA, Gutierrez TP, Gonzalez RA, Gavilan FI, Martinez-Sahuquillo MA. 2007. Papular purpuric gloves and socks syndrome. Presentation of a clinical case. Med Oral Patol Oral Cir Bucal 12:E4–E6. [PubMed] [Google Scholar]
- 424.Naides SJ, Piette W, Veach LA, Argenyi Z. 1988. Human parvovirus B19-induced vesiculopustular skin eruption. Am J Med 84:968–972. doi: 10.1016/0002-9343(88)90081-2. [DOI] [PubMed] [Google Scholar]
- 425.Halasz CL, Cormier D, Den M. 1992. Petechial glove and sock syndrome caused by parvovirus B19. J Am Acad Dermatol 27:835–838. doi: 10.1016/0190-9622(92)70260-M. [DOI] [PubMed] [Google Scholar]
- 426.Carrascosa JM, Just M, Ribera M, Ferrandiz C. 1998. Papular acrodermatitis of childhood related to poxvirus and parvovirus B19 infection. Cutis 61:265–267. [PubMed] [Google Scholar]
- 427.Lee D, Kang JN, Hwang SH, Lee YS, Kim H, Seo JK, Sung HS. 2014. Acute generalized exanthematous pustulosis induced by parvovirus b19 infection. Ann Dermatol 26:399–400. doi: 10.5021/ad.2014.26.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Rajendran A, Trehan A. 2014. Fever and rash in an immunocompromised child. Lancet Infect Dis 14:256. doi: 10.1016/S1473-3099(13)70215-9. [DOI] [PubMed] [Google Scholar]
- 429.Dinerman JL, Corman LC. 1990. Human parvovirus B19 arthropathy associated with desquamation. Am J Med 89:826–828. doi: 10.1016/0002-9343(90)90235-6. [DOI] [PubMed] [Google Scholar]
- 430.Alfadley A, Aljubran A, Hainau B, Alhokail A. 2003. Papular-purpuric “gloves and socks” syndrome in a mother and daughter. J Am Acad Dermatol 48:941–944. doi: 10.1067/mjd.2003.91. [DOI] [PubMed] [Google Scholar]
- 431.Sklavounou-Andrikopoulou A, Iakovou M, Paikos S, Papanikolaou V, Loukeris D, Voulgarelis M. 2004. Oral manifestations of papular-purpuric ‘gloves and socks' syndrome due to parvovirus B19 infection: the first case presented in Greece and review of the literature. Oral Dis 10:118–122. doi: 10.1046/j.1354-523X.2003.00986.x. [DOI] [PubMed] [Google Scholar]
- 432.Vanden Eijnden S, Carlier F, Van Beers D, Dangoisse C, De Laet C. 2003. Gloves and socks lymphangitis associated with acute parvovirus B19 infection. Pediatr Dermatol 20:184–186. doi: 10.1046/j.1525-1470.2003.20221_4.x. [DOI] [PubMed] [Google Scholar]
- 433.Sadahira Y, Yoshimoto S, Manabe T. 1998. Parvovirus B19-associated transient pure red cell aplasia with lymphadenopathy: a case report. Pathol Int 48:829–833. doi: 10.1111/j.1440-1827.1998.tb03845.x. [DOI] [PubMed] [Google Scholar]
- 434.Savasan S, Ozdemir O, Ovali F, Zulfikar B, Yilmaz K. 1996. Various associations of human parvovirus B19 infection. J Pak Med Assoc 46:235–239. [PubMed] [Google Scholar]
- 435.Prcic S, Gajinov Z, Zrnic B, Radulovic A, Matic M, Djuran V. 2013. Epidemiological and clinical features of erythema infectiosum in children in Novi Sad from 2000 to 2009. Vojnosanit Pregl 70:1081–1084. doi: 10.2298/VSP110607026P. [DOI] [PubMed] [Google Scholar]
- 436.Zerbini M, Musiani M, Venturoli S, Gallinella G, Gibellini D, Gentilomi G, La Placa M. 1992. Different syndromes associated with B19 parvovirus viraemia in paediatric patients: report of four cases. Eur J Pediatr 151:815–817. doi: 10.1007/BF01957931. [DOI] [PubMed] [Google Scholar]
- 437.Munoz-Gomez S, Cunha BA. 2013. Parvovirus B19 mimicking Epstein-Barr virus infectious mononucleosis in an adult. Am J Med 126:e7–e8. doi: 10.1016/j.amjmed.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 438.Joseph PR. 1986. Fifth disease: the frequency of joint involvement in adults. N Y State J Med 86:560–563. [PubMed] [Google Scholar]
- 439.Hosszu E, Sallai A. 1997. Human parvovirus B19 infection in a child suffering from chronic arthritis. Orv Hetil 138:611–613. (In Hungarian.) [PubMed] [Google Scholar]
- 440.Nocton JJ, Miller LC, Tucker LB, Schaller JG. 1993. Human parvovirus B19-associated arthritis in children. J Pediatr 122:186–190. doi: 10.1016/S0022-3476(06)80111-3. [DOI] [PubMed] [Google Scholar]
- 441.Rivier G, Gerster JC, Terrier P, Cheseaux JJ. 1995. Parvovirus B19 associated monoarthritis in a 5-year-old boy. J Rheumatol 22:766–767. [PubMed] [Google Scholar]
- 442.Naides SJ. 1998. Rheumatic manifestations of parvovirus B19 infection. Rheum Dis Clin North Am 24:375–401. doi: 10.1016/S0889-857X(05)70014-4. [DOI] [PubMed] [Google Scholar]
- 443.Phillips PE. 1997. Viral arthritis. Curr Opin Rheumatol 9:337–344. [DOI] [PubMed] [Google Scholar]
- 444.Schnitzer TJ, Penmetcha M. 1996. Viral arthritis. Curr Opin Rheumatol 8:341–345. doi: 10.1097/00002281-199607000-00011. [DOI] [PubMed] [Google Scholar]
- 445.Caramaschi P, Zeminian S, Carletto A, Biasi D, Marino A, Bambara LM. 1996. Parvovirus B19 infection and rheumatic diseases. Rev Rhum Engl Ed 63:846–853. [PubMed] [Google Scholar]
- 446.Nikkari S, Roivainen A, Hannonen P, Möttönen Y, Luukkainen R, Yli-Jama T, Toivanen P. 1995. Persistence of parvovirus B19 in synovial fluid and bone marrow. Ann Rheum Dis 54:597–600. doi: 10.1136/ard.54.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Wendling D, Lorge JF, Kremer P. 1995. Rheumatic manifestations of parvovirus B19 infection. Presse Med 24:233–236. (In French.) [PubMed] [Google Scholar]
- 448.Mangi RJ. 1995. Viral arthritis: the lessons of parvovirus B19. Hosp Pract (1995) 30:65–72. doi: 10.1080/21548331.1995.11443289. [DOI] [PubMed] [Google Scholar]
- 449.Maciejewski JP, Sloand EM, Nunez O, Boss C, Young NS. 2003. Recombinant humanized anti-IL-2 receptor antibody (daclizumab) produces responses in patients with moderate aplastic anemia. Blood 102:3584–3586. doi: 10.1182/blood-2003-04-1032. [DOI] [PubMed] [Google Scholar]
- 450.White DG, Woolf AD, Mortimer PP, Cohen BJ, Blake DR, Bacon PA. 1985. Human parvovirus arthropathy. Lancet i:419–421. [DOI] [PubMed] [Google Scholar]
- 451.Reid DM, Reid TM, Brown T, Rennie JA, Eastmond CJ. 1985. Human parvovirus-associated arthritis: a clinical and laboratory description. Lancet i:422–425. [DOI] [PubMed] [Google Scholar]
- 452.Semble EL, Agudelo CA, Pegram PS. 1987. Human parvovirus B19 arthropathy in two adults after contact with childhood erythema infectiosum. Am J Med 83:560–562. doi: 10.1016/0002-9343(87)90771-6. [DOI] [PubMed] [Google Scholar]
- 453.Biasi D, Zeminian S, Caramaschi P, Carletto A, Manzo T, Bambara LM. 1996. A case of parvovirus-B19 adult acute arthritis with some allergic disease clinical features. Clin Rheumatol 15:508–510. doi: 10.1007/BF02229653. [DOI] [PubMed] [Google Scholar]
- 454.Suris X, Collado A, del Olmo JA, Alsina M, Vidal J, Munoz-Gomez J. 1995. Arthropathy associated with infection by parvovirus B19. Description of 4 cases. Med Clin (Barc) 104:22–24. (In Spanish.) [PubMed] [Google Scholar]
- 455.Kaufmann J, Buccola JM, Stead W, Rowley C, Wong M, Bates CK. 2007. Secondary symptomatic parvovirus B19 infection in a healthy adult. J Gen Intern Med 22:877–878. doi: 10.1007/s11606-007-0173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Drago F, Ciccarese G, Agnoletti AF, Cogorno L, Muda A, Cozzani E, Parodi A. 2015. Remitting seronegative symmetrical synovitis with pitting edema associated with parvovirus B19 infection: two new cases and review of the comorbidities. Int J Dermatol 54:e389–e393. doi: 10.1111/ijd.12854. [DOI] [PubMed] [Google Scholar]
- 457.Dougados M, Amor B, Lefrere JJ, Courouce AM. 1986. Human parvovirus arthropathy. Arthritis Rheum 29:575–576. doi: 10.1002/art.1780290422. [DOI] [PubMed] [Google Scholar]
- 458.Speyer I, Breedveld FC, Dijkmans BA. 1998. Human parvovirus B19 infection is not followed by inflammatory joint disease during long term follow-up. A retrospective study of 54 patients. Clin Exp Rheumatol 16:576–578. [PubMed] [Google Scholar]
- 459.Lehmann HW, von Landenberg P, Modrow S. 2003. Parvovirus B19 infection and autoimmune disease. Autoimmun Rev 2:218–223. doi: 10.1016/S1568-9972(03)00014-4. [DOI] [PubMed] [Google Scholar]
- 460.Gonzalez B, Larranaga C, Leon O, Diaz P, Miranda M, Barria M, Gaggero A. 2007. Parvovirus B19 may have a role in the pathogenesis of juvenile idiopathic arthritis. J Rheumatol 34:1336–1340. [PubMed] [Google Scholar]
- 461.Stahl HD, Seidl B, Hubner B, Altrichter S, Pfeiffer R, Pustowoit B, Liebert UG, Hofmann J, von Salis-Soglio G, Emmrich F. 2000. High incidence of parvovirus B19 DNA in synovial tissue of patients with undifferentiated mono- and oligoarthritis. Clin Rheumatol 19:281–286. doi: 10.1007/s100670070046. [DOI] [PubMed] [Google Scholar]
- 462.Varache S, Narbonne V, Jousse-Joulin S, Guennoc X, Dougados M, Daures JP, Devauchelle-Pensec V, Saraux A. 2011. Is routine viral screening useful in patients with recent-onset polyarthritis of a duration of at least 6 weeks? Results from a nationwide longitudinal prospective cohort study. Arthritis Care Res (Hoboken) 63:1565–1570. doi: 10.1002/acr.20576. [DOI] [PubMed] [Google Scholar]
- 463.Knight B, Isenberg DA. 1990. Autoantibodies in sera from patients with parvovirus B19 infection. J Rheumatol 17:416–417. [PubMed] [Google Scholar]
- 464.von Landenberg P, Lehmann HW, Knoll A, Dorsch S, Modrow S. 2003. Antiphospholipid antibodies in pediatric and adult patients with rheumatic disease are associated with parvovirus B19 infection. Arthritis Rheum 48:1939–1947. doi: 10.1002/art.11038. [DOI] [PubMed] [Google Scholar]
- 465.Hermann J, Demel U, Stunzner D, Daghofer E, Tilz G, Graninger W. 2005. Clinical interpretation of antineutrophil cytoplasmic antibodies: parvovirus B19 infection as a pitfall. Ann Rheum Dis 64:641–643. doi: 10.1136/ard.2004.024877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Canpolat N, Topal N, Civilibal M, Caliskan S, Sever L, Kasapcopur O, Baserer T, Arisoy N. 2008. A case of catastrophic antiphospholipid syndrome in an adolescent girl with parvovirus B19 infection. Clin Pediatr (Phila) 47:593–597. doi: 10.1177/0009922808315216. [DOI] [PubMed] [Google Scholar]
- 467.Uthman IW, Gharavi AE. 2002. Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum 31:256–263. doi: 10.1053/sarh.2002.28303. [DOI] [PubMed] [Google Scholar]
- 468.Gross O, Tschernatsch M, Brau ME, Hempelmann G, Birklein F, Kaps M, Madlener K, Blaes F. 2007. Increased seroprevalence of parvovirus B 19 IgG in complex regional pain syndrome is not associated with antiendothelial autoimmunity. Eur J Pain 11:237–240. doi: 10.1016/j.ejpain.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 469.Hansen KE, Arnason J, Bridges AJ. 1998. Autoantibodies and common viral illnesses. Semin Arthritis Rheum 27:263–271. doi: 10.1016/S0049-0172(98)80047-4. [DOI] [PubMed] [Google Scholar]
- 470.Kandolf R, Kirschner P, Hofschneider PH, Vischer TL. 1989. Detection of parvovirus in a patient with “reactive arthritis” by in situ hybridization. Clin Rheumatol 8:398–401. doi: 10.1007/BF02030355. [DOI] [PubMed] [Google Scholar]
- 471.Stierle G, Brown KA, Rainsford SG, Smith CA, Hamerman D, Stierle HE, Dumonde DC. 1987. Parvovirus associated antigen in the synovial membrane of patients with rheumatoid arthritis. Ann Rheum Dis 46:219–223. doi: 10.1136/ard.46.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Saal JG, Steidle M, Einsele H, Muller CA, Fritz P, Zacher J. 1992. Persistence of B19 parvovirus in synovial membranes of patients with rheumatoid arthritis. Rheumatol Int 12:147–151. doi: 10.1007/BF00274934. [DOI] [PubMed] [Google Scholar]
- 473.Cassinotti P, Siegl G, Michel BA, Bruhlmann P. 1998. Presence and significance of human parvovirus B19 DNA in synovial membranes and bone marrow from patients with arthritis of unknown origin. J Med Virol 56:199–204. [PubMed] [Google Scholar]
- 474.Mehraein Y, Lennerz C, Ehlhardt S, Venzke T, Ojak A, Remberger K, Zang KD. 2003. Detection of parvovirus B19 capsid proteins in lymphocytic cells in synovial tissue of autoimmune chronic arthritis. Mod Pathol 16:811–817. doi: 10.1097/01.MP.0000083145.68333.9B. [DOI] [PubMed] [Google Scholar]
- 475.Stahl HD, Hubner B, Seidl B, Liebert UG, van der Heijden IM, Wilbrink B, Kraan MC, Emmrich F, Tak PP. 2000. Detection of multiple viral DNA species in synovial tissue and fluid of patients with early arthritis. Ann Rheum Dis 59:342–346. doi: 10.1136/ard.59.5.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Nikkari S, Luukkainen R, Möttönen T, Meurman O, Hannonen P, Skurnik M, Toivanen P. 1994. Does parvovirus B19 have a role in rheumatoid arthritis? Ann Rheum Dis 53:106–111. doi: 10.1136/ard.53.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Soderlin MK, Kautiainen H, Puolakkainen M, Hedman K, Söderlund-Venermo M, Skogh T, Leirisalo-Repo M. 2003. Infections preceding early arthritis in southern Sweden: a prospective population-based study. J Rheumatol 30:459–464. [PubMed] [Google Scholar]
- 478.Kerr JR, Ferguson WP, Mcmillan SA, Bruce IN, Bell AL. 1996. Parvovirus B19 and acute joint swelling in rheumatoid arthritis patients. Ann Rheum Dis 55:648–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Tyndall A, Jelk W, Hirsch HH. 1994. Parvovirus B19 and erosive polyarthritis. Lancet 343:480–481. doi: 10.1016/S0140-6736(94)92725-1. [DOI] [PubMed] [Google Scholar]
- 480.Gendi NS, Gibson K, Wordsworth BP. 1996. Effect of HLA type and hypocomplementaemia on the expression of parvovirus arthritis: one year follow up of an outbreak. Ann Rheum Dis 55:63–65. doi: 10.1136/ard.55.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Kerr JR, Cunniffe VS, Kelleher P, Coats AJ, Mattey DL. 2004. Circulating cytokines and chemokines in acute symptomatic parvovirus B19 infection: negative association between levels of pro-inflammatory cytokines and development of B19-associated arthritis. J Med Virol 74:147–155. doi: 10.1002/jmv.20158. [DOI] [PubMed] [Google Scholar]
- 482.Azzi A, Manaresi E, Zakrzewska K, DeSantis R, Musiani M, Zerbini M. 2004. Antibody response to B19 parvovirus VP1 and VP2 linear epitopes in patients with haemophilic arthritis. J Med Virol 72:679–682. doi: 10.1002/jmv.20031. [DOI] [PubMed] [Google Scholar]
- 483.Stahl HD, Pfeiffer R, Emmrich F. 2000. Intravenous treatment with immunoglobulins may improve chronic undifferentiated mono- and oligoarthritis. Clin Exp Rheumatol 18:515–517. [PubMed] [Google Scholar]
- 484.Lehmann HW, Plentz A, von Landenberg P, Muller-Godeffroy E, Modrow S. 2004. Intravenous immunoglobulin treatment of four patients with juvenile polyarticular arthritis associated with persistent parvovirus B19 infection and antiphospholipid antibodies. Arthritis Res Ther 6:R1–R6. doi: 10.1186/ar1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Riipinen A, Vaisanen E, Nuutila M, Sallmen M, Karikoski R, Lindbohm ML, Hedman K, Taskinen H, Söderlund-Venermo M. 2008. Parvovirus b19 infection in fetal deaths. Clin Infect Dis 47:1519–1525. doi: 10.1086/593190. [DOI] [PubMed] [Google Scholar]
- 486.Chalouhi GE, Benedetti S, Alby C, Benzina N, Ville Y. 2014. Cause of fetal demise in first-trimester parvovirus infection: anemia, placentitis or myocarditis? Ultrasound Obstet Gynecol 44:618–619. doi: 10.1002/uog.13416. [DOI] [PubMed] [Google Scholar]
- 487.Enders M, Weidner A, Zoellner I, Searle K, Enders G. 2004. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases. Prenat Diagn 24:513–518. doi: 10.1002/pd.940. [DOI] [PubMed] [Google Scholar]
- 488.Anderson LJ, Hurwitz ES. 1988. Human parvovirus B19 and pregnancy. Clin Perinatol 15:273–286. [PubMed] [Google Scholar]
- 489.Anderson MJ, Khousam MN, Maxwell DJ, Gould SJ, Happerfield LC, Smith WJ. 1988. Human parvovirus B19 and hydrops fetalis. Lancet i:535. [DOI] [PubMed] [Google Scholar]
- 490.Hall CJ. 1994. Parvovirus B19 infection in pregnancy. Arch Dis Child Fetal Neonatal Ed 71:F4–F5. doi: 10.1136/fn.71.1.F4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.American Academy of Pediatrics Committee on Infectious Diseases. 1990. American Academy of Pediatrics Committee on Infectious Diseases: parvovirus, erythema infectiosum, and pregnancy. Pediatrics 85:131–133. [PubMed] [Google Scholar]
- 492.Alger LS. 1997. Toxoplasmosis and parvovirus B19. Infect Dis Clin North Am 11:55–75. doi: 10.1016/S0891-5520(05)70341-X. [DOI] [PubMed] [Google Scholar]
- 493.Levy R, Weissman A, Blomberg G, Hagay ZJ. 1997. Infection by parvovirus B 19 during pregnancy: a review. Obstet Gynecol Surv 52:254–259. [DOI] [PubMed] [Google Scholar]
- 494.Markenson GR, Yancey MK. 1998. Parvovirus B19 infections in pregnancy. Semin Perinatol 22:309–317. [DOI] [PubMed] [Google Scholar]
- 495.Ergaz Z, Ornoy A. 2006. Parvovirus B19 in pregnancy. Reprod Toxicol 21:421–435. doi: 10.1016/j.reprotox.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 496.de Jong EP, de Haan TR, Kroes AC, Beersma MF, Oepkes D, Walther FJ. 2006. Parvovirus B19 infection in pregnancy. J Clin Virol 36:1–7. doi: 10.1016/j.jcv.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 497.Plentz A, Modrow S. 2011. Diagnosis, management and possibilities to prevent parvovirus B19 infection in pregnancy. Future Virol 6:1435–1450. doi: 10.2217/fvl.11.120. [DOI] [Google Scholar]
- 498.Dijkmans AC, de Jong EP, Dijkmans BA, Lopriore E, Vossen A, Walther FJ, Oepkes D. 2012. Parvovirus B19 in pregnancy: prenatal diagnosis and management of fetal complications. Curr Opin Obstet Gynecol 24:95–101. doi: 10.1097/GCO.0b013e3283505a9d. [DOI] [PubMed] [Google Scholar]
- 499.Nabae K, Satoh H, Nishiura H, Tanaka-Taya K, Okabe N, Oishi K, Matsumoto K, Hasegawa T. 2014. Estimating the risk of parvovirus B19 infection in blood donors and pregnant women in Japan. PLoS One 9:e92519. doi: 10.1371/journal.pone.0092519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Parilla BV, Tamura RK, Ginsberg NA. 1997. Association of parvovirus infection with isolated fetal effusions. Am J Perinatol 14:357–358. doi: 10.1055/s-2007-994160. [DOI] [PubMed] [Google Scholar]
- 501.Zhao Y, Liu G, Wang J, Yang J, Shen D, Zhang X. 2013. Mirror syndrome in a Chinese hospital: diverse causes and maternal fetal features. J Matern Fetal Neonatal Med 26:254–258. doi: 10.3109/14767058.2012.733765. [DOI] [PubMed] [Google Scholar]
- 502.Yeh SP, Chiu CF, Lee CC, Peng CT, Kuan CY, Chow KC. 2004. Evidence of parvovirus B19 infection in patients of pre-eclampsia and eclampsia with dyserythropoietic anaemia. Br J Haematol 126:428–433. doi: 10.1111/j.1365-2141.2004.05043.x. [DOI] [PubMed] [Google Scholar]
- 503.Kyriazopoulou V, Simitsopoulou M, Bondis J, Diza E, Athanasiadis A, Frantzidou F, Souliou E. 1997. Human parvovirus B19: immunity of Greek females and prenatal investigation of hydrops fetalis. Eur J Obstet Gynecol Reprod Biol 74:157–160. doi: 10.1016/S0301-2115(97)00107-3. [DOI] [PubMed] [Google Scholar]
- 504.Maksheed M, Pacsa AS, Essa SS, Ahmed MA, Monem RA, Surkouh M. 1999. The prevalence of antibody to human parvovirus B19 in pregnant women in Kuwait. Acta Trop 73:225–229. doi: 10.1016/S0001-706X(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 505.Abiodun I, Opaleye OO, Ojurongbe O, Fagbami AH. 2013. Seroprevalence of parvovirus B19 IgG and IgM antibodies among pregnant women in Oyo State, Nigeria. J Infect Dev Ctries 7:946–950. doi: 10.3855/jidc.3157. [DOI] [PubMed] [Google Scholar]
- 506.Alter BP, Potter NU, Li FP. 1978. Classification and aetiology of the aplastic anaemias. Clin Haematol 7:431–465. [PubMed] [Google Scholar]
- 507.Kinney JS, Anderson LJ, Farrar J, Strikas RA, Kumar ML, Kliegman RM, Sever JL, Hurwitz ES, Sikes RK. 1988. Risk of adverse outcomes of pregnancy after human parvovirus B19 infection. J Infect Dis 157:663–667. doi: 10.1093/infdis/157.4.663. [DOI] [PubMed] [Google Scholar]
- 508.Miller E, Fairley CK, Cohen BJ, Seng C. 1998. Immediate and long term outcome of human parvovirus B19 infection in pregnancy. Br J Obstet Gynaecol 105:174–178. [DOI] [PubMed] [Google Scholar]
- 509.Beigi RH, Wiesenfeld HC, Landers DV, Simhan HN. 2008. High rate of severe fetal outcomes associated with maternal parvovirus b19 infection in pregnancy. Infect Dis Obstet Gynecol 2008:524601. doi: 10.1155/2008/524601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Skjoldebrand-Sparre L, Tolfvenstam T, Papadogiannakis N, Wahren B, Broliden K, Nyman M. 2000. Parvovirus B19 infection: association with third-trimester intrauterine fetal death. BJOG 107:476–480. doi: 10.1111/j.1471-0528.2000.tb13265.x. [DOI] [PubMed] [Google Scholar]
- 511.Schiesser M, Sergi C, Enders M, Maul H, Schnitzler P. 2009. Discordant outcomes in a case of parvovirus b19 transmission into both dichorionic twins. Twin Res Hum Genet 12:175–179. doi: 10.1375/twin.12.2.175. [DOI] [PubMed] [Google Scholar]
- 512.Foster RT Sr, Allen SR. 2004. Differential transmission of parvovirus B19 in a twin gestation: a case report. Twin Res 7:412–414. doi: 10.1375/1369052042335205. [DOI] [PubMed] [Google Scholar]
- 513.Lassen J, Jensen AK, Bager P, Pedersen CB, Panum I, Norgaard-Pedersen B, Aaby P, Wohlfahrt J, Melbye M. 2012. Parvovirus B19 infection in the first trimester of pregnancy and risk of fetal loss: a population-based case-control study. Am J Epidemiol 176:803–807. doi: 10.1093/aje/kws177. [DOI] [PubMed] [Google Scholar]
- 514.Shabani Z, Esghaei M, Keyvani H, Shabani F, Sarmadi F, Mollaie H, Monavari SH. 2015. Relation between parvovirus B19 infection and fetal mortality and spontaneous abortion. Med J Islam Repub Iran 29:197. [PMC free article] [PubMed] [Google Scholar]
- 515.Rahbar N, Vali Zadeh S, Ghorbani R, Kheradmand P. 2015. Prevalence of parvovirus B19 specific antibody in pregnant women with spontaneous abortion. Acta Med Iran 53:168–172. [PubMed] [Google Scholar]
- 516.Brkic S, Bogavac MA, Simin N, Hrnjakovic-Cvetkovic I, Milosevic V, Maric D. 2011. Unusual high rate of asymptomatic maternal parvovirus B19 infection associated with severe fetal outcome. J Matern Fetal Neonatal Med 24:647–649. doi: 10.3109/14767058.2010.511330. [DOI] [PubMed] [Google Scholar]
- 517.Zhou Y, Bian G, Zhou Q, Gao Z, Liao P, Liu Y, He M. 2015. Detection of cytomegalovirus, human parvovirus B19, and herpes simplex virus-1/2 in women with first-trimester spontaneous abortions. J Med Virol 87:1749–1753. doi: 10.1002/jmv.24218. [DOI] [PubMed] [Google Scholar]
- 518.Yaegashi N, Niinuma T, Chisaka H, Uehara S, Okamura K, Shinkawa O, Tsunoda A, Moffatt S, Sugamura K, Yajima A. 1999. Serologic study of human parvovirus B19 infection in pregnancy in Japan. J Infect 38:30–35. doi: 10.1016/S0163-4453(99)90026-9. [DOI] [PubMed] [Google Scholar]
- 519.Swain S, Cameron AD. 1997. Establishing the cause of nonimmune hydrops. Am J Obstet Gynecol 176:951. doi: 10.1016/S0002-9378(97)70630-6. [DOI] [PubMed] [Google Scholar]
- 520.Skjoldebrand-Sparre L, Nyman M, Broliden K, Wahren B. 1999. All cases of intrauterine fetal death should be evaluated for parvovirus B19 viral deoxyribonucleic acid. Am J Obstet Gynecol 180:1595–1596. doi: 10.1016/S0002-9378(99)70059-1. [DOI] [PubMed] [Google Scholar]
- 521.Poeschmann RP, Verheijen RH, Van Dongen PW. 1991. Differential diagnosis and causes of nonimmunological hydrops fetalis: a review. Obstet Gynecol Surv 46:223–231. doi: 10.1097/00006254-199104000-00015. [DOI] [PubMed] [Google Scholar]
- 522.Sarfraz AA, Samuelsen SO, Bruu AL, Jenum PA, Eskild A. 2009. Maternal human parvovirus B19 infection and the risk of fetal death and low birthweight: a case-control study within 35 940 pregnant women. BJOG 116:1492–1498. doi: 10.1111/j.1471-0528.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- 523.Yaegashi N, Niinuma T, Chisaka H, Watanabe T, Uehara S, Okamura K, Moffatt S, Sugamura K, Yajima A. 1998. The incidence of, and factors leading to, parvovirus B19-related hydrops fetalis following maternal infection; report of 10 cases and meta-analysis. J Infect 37:28–35. doi: 10.1016/S0163-4453(98)90346-2. [DOI] [PubMed] [Google Scholar]
- 524.Guidozzi F, Ballot D, Rothberg AD. 1994. Human B19 parvovirus infection in an obstetric population. A prospective study determining fetal outcome. J Reprod Med 39:36–38. [PubMed] [Google Scholar]
- 525.Harger JH, Adler SP, Koch WC, Harger GF. 1998. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol 91:413–420. doi: 10.1016/S0029-7844(97)00701-1. [DOI] [PubMed] [Google Scholar]
- 526.Daniilidis A, Sidiropoulos K, Panna ZD, Hatzipantelis E, Loufopoulos A, Dinas K. 2014. Association of fetal loss with recent parvovirus infection and other demographic prognostic risk factors. J Obstet Gynaecol 34:40–44. doi: 10.3109/01443615.2013.820269. [DOI] [PubMed] [Google Scholar]
- 527.Jensen IP, Thorsen P, Jeune B, Moller BR, Vestergaard BF. 2000. An epidemic of parvovirus B19 in a population of 3,596 pregnant women: a study of sociodemographic and medical risk factors. BJOG 107:637–643. doi: 10.1111/j.1471-0528.2000.tb13306.x. [DOI] [PubMed] [Google Scholar]
- 528.Wong SF, Chan FY, Cincotta RB, Tilse M. 2002. Human parvovirus B19 infection in pregnancy: should screening be offered to the low-risk population? Aust N Z J Obstet Gynaecol 42:347–351. doi: 10.1111/j.0004-8666.2002.00347.x. [DOI] [PubMed] [Google Scholar]
- 529.Watt AP, Brown M, Pathiraja M, Anbazhagan A, Coyle PV. 2013. The lack of routine surveillance of parvovirus B19 infection in pregnancy prevents an accurate understanding of this regular cause of fetal loss and the risks posed by occupational exposure. J Med Microbiol 62:86–92. doi: 10.1099/jmm.0.046714-0. [DOI] [PubMed] [Google Scholar]
- 530.Lowden E, Weinstein L. 1997. Unexpected second trimester pregnancy loss due to maternal parvovirus B19 infection. South Med J 90:702–704. doi: 10.1097/00007611-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 531.Lefrere JJ, Dumez Y, Courouce A-M, Deschene G. 1986. Letter. Lancet 327:449. [Google Scholar]
- 532.Forestier F, Tissot JD, Vial Y, Daffos F, Hohlfeld P. 1999. Haematological parameters of parvovirus B19 infection in 13 fetuses with hydrops foetalis. Br J Haematol 104:925–927. doi: 10.1046/j.1365-2141.1999.01241.x. [DOI] [PubMed] [Google Scholar]
- 533.Wright IM, Williams ML, Cohen BJ. 1991. Congenital parvovirus infection. Arch Dis Child 66:253–254. doi: 10.1136/adc.66.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Woernle CH, Anderson LJ, Tattersall P, Davison JM. 1987. Human parvovirus B19 infection during pregnancy. J Infect Dis 156:17–20. doi: 10.1093/infdis/156.1.17. [DOI] [PubMed] [Google Scholar]
- 535.Clewley JP, Cohen BJ, Field AM. 1987. Detection of parvovirus B19 DNA, antigen, and particles in the human fetus. J Med Virol 23:367–376. doi: 10.1002/jmv.1890230409. [DOI] [PubMed] [Google Scholar]
- 536.Knisely AS, O'Shea PA, McMillan P, Singer DB, Magid MS. 1988. Electron microscopic identification of parvovirus virions in erythroid-line cells in fatal hydrops fetalis. Pediatr Pathol 8:163–170. doi: 10.3109/15513818809022293. [DOI] [PubMed] [Google Scholar]
- 537.Schwarz TF, Nerlich A, Hottentrager B, Jager G, Wiest I, Kantimm S, Roggendorf H, Schultz M, Gloning KP, Schramm T, Holzgreve W, Roggendorf M. 1991. Parvovirus B19 infection of the fetus. Histology and in situ hybridization. Am J Clin Pathol 96:121–126. [DOI] [PubMed] [Google Scholar]
- 538.Rodis JF, Hovick TJ Jr, Quinn DL, Rosengren SS, Tattersall P. 1988. Human parvovirus infection in pregnancy. Obstet Gynecol 72:733–738. [PubMed] [Google Scholar]
- 539.Morey AL, Keeling JW, Porter HJ, Fleming KA. 1992. Clinical and histopathological features of parvovirus B19 infection in the human fetus. Br J Obstet Gynaecol 99:566–574. doi: 10.1111/j.1471-0528.1992.tb13822.x. [DOI] [PubMed] [Google Scholar]
- 540.Panero C, Azzi A, Carbone C, Pezzati M, Mainardi G, Di Lollo S. 1994. Fetoneonatal hydrops from human parvovirus B19. Case report. J Perinat Med 22:257–264. doi: 10.1515/jpme.1994.22.3.257. [DOI] [PubMed] [Google Scholar]
- 541.Wattre P, Dewilde A, Subtil D, Andreoletti L, Thirion V. 1998. A clinical and epidemiological study of human parvovirus B19 infection in fetal hydrops using PCR Southern blot hybridization and chemiluminescence detection. J Med Virol 54:140–144. doi:. [DOI] [PubMed] [Google Scholar]
- 542.Salimans MM, van de Rijke FM, Raap AK, Van Elsacker-Niele AM. 1989. Detection of parvovirus B19 DNA in fetal tissues by in situ hybridisation and polymerase chain reaction. J Clin Pathol 42:525–530. doi: 10.1136/jcp.42.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Caul EO, Usher MJ, Burton PA. 1988. Intrauterine infection with human parvovirus B19: a light and electron microscopy study. J Med Virol 24:55–66. [DOI] [PubMed] [Google Scholar]
- 544.Walters C, Powe DG, Padfield CJ, Fagan DG. 1997. Detection of parvovirus B19 in macerated fetal tissue using in situ hybridisation. J Clin Pathol 50:749–754. doi: 10.1136/jcp.50.9.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Essary LR, Vnencak-Jones CL, Manning SS, Olson SJ, Johnson JE. 1998. Frequency of parvovirus B19 infection in nonimmune hydrops fetalis and utility of three diagnostic methods. Hum Pathol 29:696–701. doi: 10.1016/S0046-8177(98)90278-7. [DOI] [PubMed] [Google Scholar]
- 546.Sohan K, Carroll S, Byrne D, Ashworth M, Soothill P. 2000. Parvovirus as a differential diagnosis of hydrops fetalis in the first trimester. Fetal Diagn Ther 15:234–236. doi: 10.1159/000021013. [DOI] [PubMed] [Google Scholar]
- 547.Al-Khan A, Caligiuri A, Apuzzio J. 2003. Parvovirus B-19 infection during pregnancy. Infect Dis Obstet Gynecol 11:175–179. doi: 10.1080/10647440300025518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Metzman R, Anand A, DeGiulio PA, Knisely AS. 1989. Hepatic disease associated with intrauterine parvovirus B19 infection in a newborn premature infant. J Pediatr Gastroenterol Nutr 9:112–114. doi: 10.1097/00005176-198909010-00021. [DOI] [PubMed] [Google Scholar]
- 549.Lambot MA, Noel JC, Peny MO, Rodesch F, Haot J. 1999. Fetal parvovirus B19 infection associated with myocardial necrosis. Prenat Diagn 19:389. [PubMed] [Google Scholar]
- 550.Hichijo A, Morine M. 2014. A case of fetal parvovirus b19 myocarditis that caused terminal heart failure. Case Rep Obstet Gynecol 2014:463571. doi: 10.1155/2014/463571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.de Haan TR, Beersma MF, Oepkes D, de Jong EP, Kroes AC, Walther FJ. 2007. Parvovirus B19 infection in pregnancy: maternal and fetal viral load measurements related to clinical parameters. Prenat Diagn 27:46–50. doi: 10.1002/pd.1619. [DOI] [PubMed] [Google Scholar]
- 552.Suchet I, Ens W, Suchet R. 2000. Parvovirus B19 infection in utero—natural history and spectrum of sonographic manifestations in 7 cases. Can Assoc Radiol J 51:198–204. [PubMed] [Google Scholar]
- 553.Carraca T, Matias A, Brandao O, Montenegro N. 2011. Early signs of cardiac failure: a clue for parvovirus infection screening in the first trimester? Fetal Diagn Ther 30:150–152. doi: 10.1159/000323590. [DOI] [PubMed] [Google Scholar]
- 554.Komischke K, Searle K, Enders G. 1997. Maternal serum alpha-fetoprotein and human chorionic gonadotropin in pregnant women with acute parvovirus B19 infection with and without fetal complications. Prenat Diagn 17:1039–1046. [PubMed] [Google Scholar]
- 555.Carrington D, Gilmore DH, Whittle MJ, Aitken D, Gibson AA, Patrick WJ, Brown T, Caul EO, Field AM, Clewley JP, Cohen BJ. 1987. Maternal serum alpha-fetoprotein—a marker of fetal aplastic crisis during intrauterine human parvovirus infection. Lancet i:433–435. [DOI] [PubMed] [Google Scholar]
- 556.Gentilomi G, Zerbini M, Gallinella G, Venturoli S, Manaresi E, Morandi R, Musiani M. 1998. B19 parvovirus induced fetal hydrops: rapid and simple diagnosis by detection of B19 antigens in amniotic fluids. Prenat Diagn 18:363–368. [PubMed] [Google Scholar]
- 557.Peters MT, Nicolaides KH. 1990. Cordocentesis for the diagnosis and treatment of human fetal parvovirus infection. Obstet Gynecol 75:501–504. [PubMed] [Google Scholar]
- 558.Searle K, Schalasta G, Enders G. 1998. Development of antibodies to the nonstructural protein NS1 of parvovirus B19 during acute symptomatic and subclinical infection in pregnancy: implications for pathogenesis doubtful. J Med Virol 56:192–198. doi:. [DOI] [PubMed] [Google Scholar]
- 559.Hemauer A, Gigler A, Searle K, Beckenlehner K, Raab U, Broliden K, Wolf H, Enders G, Modrow S. 2000. Seroprevalence of parvovirus B19 NS1-specific IgG in B19-infected and uninfected individuals and in infected pregnant women. J Med Virol 60:48–55. doi:. [DOI] [PubMed] [Google Scholar]
- 560.Soothill P. 1990. Intrauterine blood transfusion for non-immune hydrops fetalis due to parvovirus B19 infection. Lancet 336:121–122. doi: 10.1016/0140-6736(90)91642-N. [DOI] [PubMed] [Google Scholar]
- 561.Gloning KP, Schramm T, Brusis E, Schwarz T, Roggendorf M. 1990. Successful intrauterine treatment of fetal hydrops caused by parvovirus B19 infection. Behring Inst Mitt 1990:79–85. [PubMed] [Google Scholar]
- 562.Mielke G, Enders G. 1997. Late onset of hydrops fetalis following intrauterine parvovirus B19 infection. Fetal Diagn Ther 12:40–42. doi: 10.1159/000264424. [DOI] [PubMed] [Google Scholar]
- 563.Fairley CK, Smoleniec JS, Caul OE, Miller E. 1995. Observational study of effect of intrauterine transfusions on outcome of fetal hydrops after parvovirus B19 infection. Lancet 346:1335–1337. doi: 10.1016/S0140-6736(95)92346-2. [DOI] [PubMed] [Google Scholar]
- 564.Goodear M, Hayward C, Crowther C. 1998. Foetal intracardiac transfusion for the treatment of severe anaemia due to human parvovirus B-19 infection. Australas Radiol 42:275–277. doi: 10.1111/j.1440-1673.1998.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 565.Xu J, Raff TC, Muallem NS, Neubert AG. 2003. Hydrops fetalis secondary to parvovirus B19 infections. J Am Board Fam Pract 16:63–68. doi: 10.3122/jabfm.16.1.63. [DOI] [PubMed] [Google Scholar]
- 566.Odibo AO, Campbell WA, Feldman D, Ling PY, Leo MV, Borgida AF, Rodis JF. 1998. Resolution of human parvovirus B19-induced nonimmune hydrops after intrauterine transfusion. J Ultrasound Med 17:547–550. [DOI] [PubMed] [Google Scholar]
- 567.Rugolotto S, Padovani EM, Sanna A, Chiaffoni GP, Marradi PL, Borgna-Pignatti C. 1999. Intrauterine anemia due to parvovirus B19: successful treatment with intravenous immunoglobulins. Haematologicaae 84:668–669. [PubMed] [Google Scholar]
- 568.Cameron AD, Swain S, Patrick WJ. 1997. Human parvovirus B19 infection associated with hydrops fetalis. Aust N Z J Obstet Gynaecol 37:316–319. doi: 10.1111/j.1479-828X.1997.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 569.Mace G, Sauvan M, Castaigne V, Moutard ML, Cortey A, Maisonneuve E, Garel C, Dhombres F, Boujenah J, Mailloux A, Carbonne B. 2014. Clinical presentation and outcome of 20 fetuses with parvovirus B19 infection complicated by severe anemia and/or fetal hydrops. Prenat Diagn 34:1023–1030. doi: 10.1002/pd.4413. [DOI] [PubMed] [Google Scholar]
- 570.Kyeong KS, Won HS, Lee MY, Shim JY, Lee PR, Kim A. 2015. Clinical features of 10 fetuses with prenatally diagnosed parvovirus b19 infection and fetal hydrops. Fetal Pediatr Pathol 34:49–56. doi: 10.3109/15513815.2014.962197. [DOI] [PubMed] [Google Scholar]
- 571.Naides SJ, Weiner CP. 1989. Antenatal diagnosis and palliative treatment of non-immune hydrops fetalis secondary to fetal parvovirus B19 infection. Prenat Diagn 9:105–114. doi: 10.1002/pd.1970090205. [DOI] [PubMed] [Google Scholar]
- 572.Bekhit MT, Greenwood PA, Warren R, Aarons E, Jauniaux E. 2009. In utero treatment of severe fetal anaemia due to parvovirus B19 in one fetus in a twin pregnancy—a case report and literature review. Fetal Diagn Ther 25:153–157. doi: 10.1159/000209200. [DOI] [PubMed] [Google Scholar]
- 573.Morey AL, Nicolini U, Welch CR, Economides D, Chamberlain PF, Cohen BJ. 1991. Parvovirus B19 infection and transient fetal hydrops. Lancet 337:496. doi: 10.1016/0140-6736(91)93435-C. [DOI] [PubMed] [Google Scholar]
- 574.Faure JM, Giacalone PL, Deschamps F, Boulot P. 1997. Nonimmune hydrops fetalis caused by intrauterine human parvovirus B19 infection: a case of spontaneous reversal in utero. Fetal Diagn Ther 12:66–67. doi: 10.1159/000264432. [DOI] [PubMed] [Google Scholar]
- 575.Ovali F, Samanci N, Ozdemir O, Dagoglu T. 1996. Human parvovirus B19 associated non-immune hydrops fetalis. J Pak Med Assoc 46:88–90. [PubMed] [Google Scholar]
- 576.Kelly T, Mathers A. 1998. Early presentation and spontaneous resolution of hydrops fetalis, secondary to parvovirus B19 infection. J Obstet Gynaecol 18:190–191. doi: 10.1080/01443619868055. [DOI] [PubMed] [Google Scholar]
- 577.Koch WC, Adler SP, Harger J. 1993. Intrauterine parvovirus B19 infection may cause an asymptomatic or recurrent postnatal infection. Pediatr Infect Dis J 12:747–750. [DOI] [PubMed] [Google Scholar]
- 578.Dembinski J, Eis-Hubinger AM, Maar J, Schild R, Bartmann P. 2003. Long term follow up of serostatus after maternofetal parvovirus B19 infection. Arch Dis Child 88:219–221. doi: 10.1136/adc.88.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Mortimer PP, Cohen BJ, Buckley MM, Cradock-Watson JE, Ridehalgh MK, Burkhardt F, Schilt U. 1985. Human parvovirus and the fetus. Lancet ii:1012. [DOI] [PubMed] [Google Scholar]
- 580.Lassen J, Bager P, Wohlfahrt J, Bottiger B, Melbye M. 2013. Parvovirus B19 infection in pregnancy and subsequent morbidity and mortality in offspring. Int J Epidemiol 42:1070–1076. doi: 10.1093/ije/dyt117. [DOI] [PubMed] [Google Scholar]
- 581.Heegaard ED, Hasle H, Clausen N, Hornsleth A, Kerndrup GB. 1996. Parvovirus B19 infection and Diamond-Blackfan anaemia. Acta Paediatr 85:299–302. doi: 10.1111/j.1651-2227.1996.tb14020.x. [DOI] [PubMed] [Google Scholar]
- 582.Brown KE, Green SW, Antunez de Mayolo J, Bellanti JA, Smith SD, Smith TJ, Young NS. 1994. Congenital anaemia after transplacental B19 parvovirus infection. Lancet 343:895–896. doi: 10.1016/S0140-6736(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 583.Hudson AC, Montegudo AE, Steele RW. 2015. Congenital human parvovirus b19 infection with persistent viremia. Clin Pediatr (Phila) 54:409–413. doi: 10.1177/0009922814533412. [DOI] [PubMed] [Google Scholar]
- 584.Donders GG, Van Lierde S, Van Elsacker-Niele AM, Moerman P, Goubau P, Vandenberghe K. 1994. Survival after intrauterine parvovirus B19 infection with persistence in early infancy: a two-year follow-up. Pediatr Infect Dis J 13:234–236. doi: 10.1097/00006454-199403000-00016. [DOI] [PubMed] [Google Scholar]
- 585.Heegaard ED, Hasle H, Skibsted L, Bock J, Brown KE. 2000. Congenital anemia caused by parvovirus B19 infection. Pediatr Infect Dis J 19:1216–1218. doi: 10.1097/00006454-200012000-00024. [DOI] [PubMed] [Google Scholar]
- 586.Lejeune A, Cremer M, von Bernuth H, Edelmann A, Modrow S, Buhrer C. 2014. Persistent pure red cell aplasia in dicygotic twins with persistent congenital parvovirus B19 infection-remission following high dose intravenous immunoglobulin. Eur J Pediatr 173:1723–1726. doi: 10.1007/s00431-014-2420-5. [DOI] [PubMed] [Google Scholar]
- 587.Nigro G, D'Eufemia P, Zerbini M, Krzysztofiak A, Finocchiaro R, Giardini O. 1994. Parvovirus B19 infection in a hypogammaglobulinemic infant with neurologic disorders and anemia: successful immunoglobulin therapy. Pediatr Infect Dis J 13:1019–1021. doi: 10.1097/00006454-199411000-00022. [DOI] [PubMed] [Google Scholar]
- 588.Mackie FL, Pretlove SJ, Martin WL, Donovan V, Kilby MD. 2015. Fetal intracardiac transfusions in hydropic fetuses with severe anemia. Fetal Diagn Ther 38:61–64. doi: 10.1159/000369798. [DOI] [PubMed] [Google Scholar]
- 589.Vogel H, Kornman M, Ledet SC, Rajagopalan L, Taber L, McClain K. 1997. Congenital parvovirus infection. Pediatr Pathol Lab Med 17:903–912. doi: 10.1080/15513819709168754. [DOI] [PubMed] [Google Scholar]
- 590.Duran R, Vatansever U, Acunas B, Orhaner B, Demir M. 2009. Transient leukoerythroblastosis in a very low birth weight infant with parvovirus B19 infection. Int J Infect Dis 13:e473–e475. doi: 10.1016/j.ijid.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 591.White FV, Jordan J, Dickman PS, Knisely AS. 1995. Fetal parvovirus B19 infection and liver disease of antenatal onset in an infant with Ebstein's anomaly. Pediatr Pathol Lab Med 15:121–129. doi: 10.3109/15513819509026944. [DOI] [PubMed] [Google Scholar]
- 592.Wiersbitzky SK, Beyersdorff E, Mueller C, Bruns R, Eberhard B, Burtzlaff C, Wiersbitzky H. 1997. Perinatal parvovirus B19 infections: what are the clinical consequences? Pediatr Hematol Oncol 14:589–592. doi: 10.3109/08880019709030917. [DOI] [PubMed] [Google Scholar]
- 593.Katz VL, McCoy MC, Kuller JA, Hansen WF. 1996. An association between fetal parvovirus B19 infection and fetal anomalies: a report of two cases. Am J Perinatol 13:43–45. doi: 10.1055/s-2007-994201. [DOI] [PubMed] [Google Scholar]
- 594.Travan L, Naviglio S, Cont G, Brovedani P, Davanzo R, Demarini S. 2016. Isolated hypoplasia of abdominal wall muscles associated with fetal ascites. Congenit Anom (Kyoto) 56:184–186. doi: 10.1111/cga.12156. [DOI] [PubMed] [Google Scholar]
- 595.Sarafidis K, Drossou-Agakidou V, Evdoridou I, Petridou S, Hatzisevastou-Loukidou H, Dadamojas C, Roilides E. 2008. Hydrothorax as a sole manifestation of congenital parvovirus B19 infection. Am J Perinatol 25:551–554. doi: 10.1055/s-0028-1085621. [DOI] [PubMed] [Google Scholar]
- 596.Savarese I, De Carolis MP, Costa S, De Rosa G, De Carolis S, Lacerenza S, Romagnoli C. 2008. Atypical manifestations of congenital parvovirus B19 infection. Eur J Pediatr 167:1463–1466. doi: 10.1007/s00431-008-0688-z. [DOI] [PubMed] [Google Scholar]
- 597.Cantey JB, Pritchard MA, Sanchez PJ. 2013. Bone lesions in an infant with congenital parvovirus b19 infection. Pediatrics 131:e1659–e1663. doi: 10.1542/peds.2012-0898. [DOI] [PubMed] [Google Scholar]
- 598.Plachouras N, Stefanidis K, Andronikou S, Lolis D. 1999. Severe nonimmune hydrops fetalis and congenital corneal opacification secondary to human parvovirus B19 infection. A case report. J Reprod Med 44:377–380. [PubMed] [Google Scholar]
- 599.Hartwig NG, Vermeij-Keers C, Van Elsacker-Niele AM, Fleuren GJ. 1989. Embryonic malformations in a case of intrauterine parvovirus B19 infection. Teratology 39:295–302. doi: 10.1002/tera.1420390311. [DOI] [PubMed] [Google Scholar]
- 600.Owren PA. 1948. Congenital hemolytic jaundice; the pathogenesis of the hemolytic crisis. Blood 3:231–248. [PubMed] [Google Scholar]
- 601.Conklin GT, George JN, Sears DA. 1974. Transient erythroid aplasia in hemolytic anemia: a review of the literature with two case reports. Tex Rep Biol Med 32:391–411. [PubMed] [Google Scholar]
- 602.Horne JL, Lederer H, Kirkpatrick HJR, Leys DG. 1945. Familial crises in congenital haemolytic disease. Lancet ii:33. [Google Scholar]
- 603.Mortimer PP. 1983. Hypothesis: the aplastic crisis of hereditary spherocytosis is due to a single transmissible agent. J Clin Pathol 36:445–448. doi: 10.1136/jcp.36.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Pattison JR, Jones SE, Hodgson J, Davis LR, White JM, Stroud CE, Murtaza L. 1981. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet i:664–665. [DOI] [PubMed] [Google Scholar]
- 605.Inoue S, Kinra NK, Mukkamala SR, Gordon R. 1991. Parvovirus B-19 infection: aplastic crisis, erythema infectiosum and idiopathic thrombocytopenic purpura. Pediatr Infect Dis J 10:251–253. doi: 10.1097/00006454-199103000-00018. [DOI] [PubMed] [Google Scholar]
- 606.Lefrere JJ, Courouce AM, Bertrand Y, Girot R, Soulier JP. 1986. Human parvovirus and aplastic crisis in chronic hemolytic anemias: a study of 24 observations. Am J Hematol 23:271–275. doi: 10.1002/ajh.2830230311. [DOI] [PubMed] [Google Scholar]
- 607.Smith JC, Megason GC, Iyer RV, Andrew ME, Pullen DJ. 1994. Clinical characteristics of children with hereditary hemolytic anemias and aplastic crisis: a 7-year review. South Med J 87:702–708. doi: 10.1097/00007611-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 608.Serjeant GR, Topley JM, Mason K, Serjeant BE, Pattison JR, Jones SE, Mohamed R. 1981. Outbreak of aplastic crises in sickle cell anaemia associated with parvovirus-like agent. Lancet ii:595–597. [DOI] [PubMed] [Google Scholar]
- 609.Mallouh AA, Qudah A. 1995. An epidemic of aplastic crisis caused by human parvovirus B19. Pediatr Infect Dis J 14:31–34. doi: 10.1097/00006454-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 610.Serjeant GR, Serjeant BE, Thomas PW, Anderson MJ, Patou G, Pattison JR. 1993. Human parvovirus infection in homozygous sickle cell disease. Lancet 341:1237–1240. doi: 10.1016/0140-6736(93)91145-C. [DOI] [PubMed] [Google Scholar]
- 611.Smith-Whitley K, Zhao H, Hodinka RL, Kwiatkowski J, Cecil R, Cecil T, Cnaan A, Ohene-Frempong K. 2004. Epidemiology of human parvovirus B19 in children with sickle cell disease. Blood 103:422–427. doi: 10.1182/blood-2003-01-0069. [DOI] [PubMed] [Google Scholar]
- 612.Serjeant BE, Hambleton IR, Kerr S, Kilty CG, Serjeant GR. 2001. Haematological response to parvovirus B19 infection in homozygous sickle-cell disease. Lancet 358:1779–1780. doi: 10.1016/S0140-6736(01)06807-6. [DOI] [PubMed] [Google Scholar]
- 613.Goldstein AR, Anderson MJ, Serjeant GR. 1987. Parvovirus associated aplastic crisis in homozygous sickle cell disease. Arch Dis Child 62:585–588. doi: 10.1136/adc.62.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Kelleher JF Jr, Luban NL, Cohen BJ, Mortimer PP. 1984. Human serum parvovirus as the cause of aplastic crisis in sickle cell disease. Am J Dis Child 138:401–403. [DOI] [PubMed] [Google Scholar]
- 615.Regaya F, Oussaief L, Bejaoui M, Karoui M, Zili M, Khelifa R. 2007. Parvovirus B19 infection in Tunisian patients with sickle-cell anemia and acute erythroblastopenia. BMC Infect Dis 7:123. doi: 10.1186/1471-2334-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Win N, Lee E, Needs M, Homeida S, Stasi R. 2014. Profound sustained reticulocytopenia and anaemia in an adult patient with sickle cell disease. Transfus Med 24:418–420. doi: 10.1111/tme.12168. [DOI] [PubMed] [Google Scholar]
- 617.Pardoll DM, Rodeheffer RJ, Smith RR, Charache S. 1982. Aplastic crisis due to extensive bone marrow necrosis in sickle cell disease. Arch Intern Med 142:2223–2225. [PubMed] [Google Scholar]
- 618.Conrad ME, Studdard H, Anderson LJ. 1988. Aplastic crisis in sickle cell disorders: bone marrow necrosis and human parvovirus infection. Am J Med Sci 295:212–215. doi: 10.1097/00000441-198803000-00009. [DOI] [PubMed] [Google Scholar]
- 619.Godeau B, Galacteros F, Schaeffer A, Morinet F, Bachir D, Rosa J, Portos JL. 1991. Aplastic crisis due to extensive bone marrow necrosis and human parvovirus infection in sickle cell disease. Am J Med 91:557–558. doi: 10.1016/0002-9343(91)90198-7. [DOI] [PubMed] [Google Scholar]
- 620.Rao SP, Desai N, Miller ST. 1996. B19 parvovirus infection and transient aplastic crisis in a child with sickle cell anemia. J Pediatr Hematol Oncol 18:175–177. doi: 10.1097/00043426-199605000-00016. [DOI] [PubMed] [Google Scholar]
- 621.Uike N, Miyamura T, Obama K, Takahira H, Sato H, Kozuru M. 1993. Parvovirus B19-associated haemophagocytosis in Evans syndrome: aplastic crisis accompanied by severe thrombocytopenia. Br J Haematol 84:530–532. doi: 10.1111/j.1365-2141.1993.tb03113.x. [DOI] [PubMed] [Google Scholar]
- 622.Muir K, Todd WT, Watson WH, Fitzsimons E. 1992. Viral-associated haemophagocytosis with parvovirus-B19-related pancytopenia. Lancet 339:1139–1140. doi: 10.1016/0140-6736(92)90735-L. [DOI] [PubMed] [Google Scholar]
- 623.Kolquist KA, Vnencak-Jones CL, Swift L, Page DL, Johnson JE, Denison MR. 1996. Fatal fat embolism syndrome in a child with undiagnosed hemoglobin S/beta+ thalassemia: a complication of acute parvovirus B19 infection. Pediatr Pathol Lab Med 16:71–82. doi: 10.1080/15513819609168662. [DOI] [PubMed] [Google Scholar]
- 624.Tsitsikas DA, Gallinella G, Patel S, Seligman H, Greaves P, Amos RJ. 2014. Bone marrow necrosis and fat embolism syndrome in sickle cell disease: increased susceptibility of patients with non-SS genotypes and a possible association with human parvovirus B19 infection. Blood Rev 28:23–30. doi: 10.1016/j.blre.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 625.Mallouh AA, Qudah A. 1993. Acute splenic sequestration together with aplastic crisis caused by human parvovirus B19 in patients with sickle cell disease. J Pediatr 122:593–595. doi: 10.1016/S0022-3476(05)83542-5. [DOI] [PubMed] [Google Scholar]
- 626.Yates AM, Hankins JS, Mortier NA, Aygun B, Ware RE. 2009. Simultaneous acute splenic sequestration and transient aplastic crisis in children with sickle cell disease. Pediatr Blood Cancer 53:479–481. doi: 10.1002/pbc.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Zimmerman SA, Davis JS, Schultz WH, Ware RE. 2003. Subclinical parvovirus B19 infection in children with sickle cell anemia. J Pediatr Hematol Oncol 25:387–389. doi: 10.1097/00043426-200305000-00007. [DOI] [PubMed] [Google Scholar]
- 628.Heegaard ED, Myhre J, Hornsleth A, Gundestrup M, Boye H. 1997. Parvovirus B19 infections in patients with chronic anemia. Haematologicaae 82:402–405. [PubMed] [Google Scholar]
- 629.Kelleher JF, Luban NL, Mortimer PP, Kamimura T. 1983. Human serum “parvovirus”: a specific cause of aplastic crisis in children with hereditary spherocytosis. J Pediatr 102:720–722. doi: 10.1016/S0022-3476(83)80243-1. [DOI] [PubMed] [Google Scholar]
- 630.Davidson RJ, Brown T, Wiseman D. 1984. Human parvovirus infection and aplastic crisis in hereditary spherocytosis. J Infect 9:298–300. doi: 10.1016/S0163-4453(84)90750-3. [DOI] [PubMed] [Google Scholar]
- 631.Nunoue T, Koike T, Koike R, Sanada M, Tsukada T, Mortimer PP, Cohen BJ. 1987. Infection with human parvovirus (B19), aplasia of the bone marrow and a rash in hereditary spherocytosis. J Infect 14:67–70. doi: 10.1016/S0163-4453(87)90886-3. [DOI] [PubMed] [Google Scholar]
- 632.McLellan NJ, Rutter N. 1987. Hereditary spherocytosis in sisters unmasked by parvovirus infection. Postgrad Med J 63:49–50. doi: 10.1136/pgmj.63.735.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Cohen H, Walker H, Delhanty JD, Lucas SB, Huehns ER. 1991. Congenital spherocytosis, B19 parvovirus infection and inherited interstitial deletion of the short arm of chromosome 8. Br J Haematol 78:251–257. doi: 10.1111/j.1365-2141.1991.tb04425.x. [DOI] [PubMed] [Google Scholar]
- 634.Cutlip AC, Gross KM, Lewis MJ. 1991. Occult hereditary spherocytosis and human parvovirus infection. J Am Board Fam Pract 4:461–464. [PubMed] [Google Scholar]
- 635.Cubel RC, Valadao MC, Pereira WV, Magalhaes MC, Nascimento JP. 1992. Aplastic crisis due to human parvovirus B19 infection in hereditary hemolytic anaemia. Rev Inst Med Trop Sao Paulo 34:479–482. doi: 10.1590/S0036-46651992000500017. [DOI] [PubMed] [Google Scholar]
- 636.Kataoka A, Doi S, Suemori S, Nakanishi H, Jonen D, Mori M, Mizushima Y, Wakazono Y. 2014. Varied clinical course of aplastic crisis in hereditary spherocytosis. Pediatr Int 56:100–102. doi: 10.1111/ped.12153. [DOI] [PubMed] [Google Scholar]
- 637.Mabin DC, Chowdhury V. 1990. Aplastic crisis caused by human parvovirus in two patients with hereditary stomatocytosis. Br J Haematol 76:153–154. doi: 10.1111/j.1365-2141.1990.tb07855.x. [DOI] [PubMed] [Google Scholar]
- 638.Brownell AI, McSwiggan DA, Cubitt WD, Anderson MJ. 1986. Aplastic and hypoplastic episodes in sickle cell disease and thalassaemia intermedia. J Clin Pathol 39:121–124. doi: 10.1136/jcp.39.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639.Singh S, Chand G, Charan S, Arora S, Singh P. 2014. Recurrent severe anaemia: a rare presentation of parvovirus b19 infection. J Clin Diagn Res 8:MD01–MD02. doi: 10.7860/JCDR/2014/7840.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.De Caluwe JP, Gilbert L, Alexander M. 1993. Transient aplasia of the red progenitor cells manifest in a child with double heterozygote Hb SC carriership: the role of human parvovirus B19 (HPV-B19). Rev Med Brux 14:141–144. (In French.) [PubMed] [Google Scholar]
- 641.Rechavi G, Vonsover A, Manor Y, Mileguir F, Shpilberg O, Kende G, Brok-Simoni F, Mandel M, Gotlieb-Stematski T, Ben-Bassat I, Ramot B. 1989. Aplastic crisis due to human B19 parvovirus infection in red cell pyrimidine-5′-nucleotidase deficiency. Acta Haematol 82:46–49. [DOI] [PubMed] [Google Scholar]
- 642.Kojima S, Matsuyama K, Ishii E. 1988. High serum iron in human parvovirus-induced aplastic crisis in iron deficiency anemia. Acta Haematol 80:171–172. [DOI] [PubMed] [Google Scholar]
- 643.Nibu K, Matsumoto I, Yanai F, Nunoue T. 1989. Aplastic crisis due to human parvovirus B19 infection in glucose-6-phosphate dehydrogenase deficiency. Nihon Ketsueki Gakkai Zasshi 52:1117–1121. (In Japanese.) [PubMed] [Google Scholar]
- 644.Schaefer HE. 1992. Aplastic crisis in haemolytic anaemia due to infection parvovirus B19. Pathol Res Pract 188:817–823. doi: 10.1016/S0344-0338(11)80190-1. [DOI] [PubMed] [Google Scholar]
- 645.Duncan JR, Potter CB, Cappellini MD, Kurtz JB, Anderson MJ, Weatherall DJ. 1983. Aplastic crisis due to parvovirus infection in pyruvate kinase deficiency. Lancet ii:14–16. [DOI] [PubMed] [Google Scholar]
- 646.Lefrere JJ, Bourgeois H. 1986. Human parvovirus associated with erythroblastopenia in iron deficiency anaemia. J Clin Pathol 39:1277–1278. doi: 10.1136/jcp.39.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 647.Chitnavis VN, Patou G, Makar YF, Kendra JR. 1990. B19 parvovirus induced red cell aplasia complicating acute cold antibody mediated haemolytic anaemia. Br J Haematol 76:433–434. doi: 10.1111/j.1365-2141.1990.tb06380.x. [DOI] [PubMed] [Google Scholar]
- 648.Smith MA, Shah NS, Lobel JS. 1989. Parvovirus B19 infection associated with reticulocytopenia and chronic autoimmune hemolytic anemia. Am J Pediatr Hematol Oncol 11:167–169. [PubMed] [Google Scholar]
- 649.Ganzel C, Constantin R. 2015. Parvovirus B19 diagnosed by bone marrow biopsy. Blood 125:3351. doi: 10.1182/blood-2015-02-628008. [DOI] [PubMed] [Google Scholar]
- 650.Tchernia G, Morinet F, Congard B, Croisille L. 1993. Diamond Blackfan anaemia: apparent relapse due to B19 parvovirus. Eur J Pediatr 152:209–210. doi: 10.1007/BF01956146. [DOI] [PubMed] [Google Scholar]
- 651.West NC, Meigh RE, Mackie M, Anderson MJ. 1986. Parvovirus infection associated with aplastic crisis in a patient with HEMPAS. J Clin Pathol 39:1019–1020. doi: 10.1136/jcp.39.9.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Nigro G, Gattinara GC, Mattia S, Caniglia M, Fridell E. 1992. Parvovirus-B19-related pancytopenia in children with HIV infection. Lancet 340:115. doi: 10.1016/0140-6736(92)90436-7. [DOI] [PubMed] [Google Scholar]
- 653.Soutar RL, Birnie DH, Bennett B. 1993. Parvovirus B19 induced red cell aplasia in myelofibrosis. Br J Haematol 85:623–624. [DOI] [PubMed] [Google Scholar]
- 654.Baurmann H, Schwarz TF, Oertel J, Serke S, Roggendorf M, Huhn D. 1992. Acute parvovirus B19 infection mimicking myelodysplastic syndrome of the bone marrow. Ann Hematol 64:43–45. doi: 10.1007/BF01811471. [DOI] [PubMed] [Google Scholar]
- 655.Yetgin S, Cetin M, Yenicesu I, Ozaltin F, Uckan D. 2000. Acute parvovirus B19 infection mimicking juvenile myelomonocytic leukemia. Eur J Haematol 65:276–278. doi: 10.1034/j.1600-0609.2000.065004276.x. [DOI] [PubMed] [Google Scholar]
- 656.Yarali N, Duru F, Sipahi T, Kara A, Tezic T. 2000. Parvovirus B19 infection reminiscent of myelodysplastic syndrome in three children with chronic hemolytic anemia. Pediatr Hematol Oncol 17:475–482. doi: 10.1080/08880010050120836. [DOI] [PubMed] [Google Scholar]
- 657.Kishore J, Mukhopadhyay C. 2004. Persistence of parvovirus B19 IgM antibodies and DNA in pure red cell aplasia resulting in myelodysplasia—a case report. Indian J Pathol Microbiol 47:78–81. [PubMed] [Google Scholar]
- 658.Mihal V, Dusek J, Hajduch M, Cohen BJ, Fingerova H, Vesely J. 1996. Transient aplastic crisis in a leukemic child caused by parvovirus B19 infection. Pediatr Hematol Oncol 13:173–177. doi: 10.3109/08880019609030809. [DOI] [PubMed] [Google Scholar]
- 659.Hitchins R, Sloots TP. 1993. Another parvovirus B19 infection of a chronic lymphatic leukaemia patient. Aust N Z J Med 23:217–218. doi: 10.1111/j.1445-5994.1993.tb01821.x. [DOI] [PubMed] [Google Scholar]
- 660.Miyata H, Yagi K, Takemura T, Maki S. 1994. Transient erythroblastopenia due to human parvovirus B19 infection: a case report of a boy suffering from purpura. Acta Paediatr Jpn 36:217–219. doi: 10.1111/j.1442-200X.1994.tb03165.x. [DOI] [PubMed] [Google Scholar]
- 661.Wodzinski MA, Lilleyman JS. 1989. Transient erythroblastopenia of childhood due to human parvovirus B19 infection. Br J Haematol 73:127–128. doi: 10.1111/j.1365-2141.1989.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 662.Guillot M, Lefrere JJ, Ravenet N, Leveque E, Girot R. 1987. Acute anaemia and aplastic crisis without haemolysis in human parvovirus infection. J Clin Pathol 40:1264–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663.Nagai K, Morohoshi T, Kudoh T, Yoto Y, Suzuki N, Matsunaga Y. 1992. Transient erythroblastopenia of childhood with megakaryocytopenia associated with human parvovirus B19 infection. Br J Haematol 80:131–132. doi: 10.1111/j.1365-2141.1992.tb06416.x. [DOI] [PubMed] [Google Scholar]
- 664.Prassouli A, Papadakis V, Tsakris A, Stefanaki K, Garoufi A, Haidas S, Dracou C. 2005. Classic transient erythroblastopenia of childhood with human parvovirus B19 genome detection in the blood and bone marrow. J Pediatr Hematol Oncol 27:333–336. doi: 10.1097/01.mph.0000169249.72858.8c. [DOI] [PubMed] [Google Scholar]
- 665.Rogers BB, Rogers ZR, Timmons CF. 1996. Polymerase chain reaction amplification of archival material for parvovirus B19 in children with transient erythroblastopenia of childhood. Pediatr Pathol Lab Med 16:471–478. doi: 10.1080/15513819609168684. [DOI] [PubMed] [Google Scholar]
- 666.Skeppner G, Kreuger A, Elinder G. 2002. Transient erythroblastopenia of childhood: prospective study of 10 patients with special reference to viral infections. J Pediatr Hematol Oncol 24:294–298. doi: 10.1097/00043426-200205000-00015. [DOI] [PubMed] [Google Scholar]
- 667.Bhambhani K, Inoue S, Sarnaik SA, Merline J. 1986. Transient erythroblastopenia of childhood not associated with human parvovirus infection. Lancet i:509. [DOI] [PubMed] [Google Scholar]
- 668.Nikkari S, Meurman O, Wanne O. 1993. Parvovirus B19 and transient erythroblastopenia of childhood. Br J Haematol 83:679. doi: 10.1111/j.1365-2141.1993.tb04716.x. [DOI] [PubMed] [Google Scholar]
- 669.Mouthon L, Michel M, Gandre C, Montagnier-Petrissans C, Chevreul K. 2015. Costs of intravenous immunoglobulin therapy in patients with unconfirmed parvovirus b19 pure red cell aplasia. Clin Infect Dis 60:488. doi: 10.1093/cid/ciu828. [DOI] [PubMed] [Google Scholar]
- 670.Bonjoch X, Obispo F, Alemany C, Pacha A, Rodriguez E, Xairo D. 2015. Characterization of markers of the progression of human parvovirus B19 infection in virus DNA-positive plasma samples. Transfus Med Hemother 42:233–238. doi: 10.1159/000381979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Juhl D, Gorg S, Hennig H. 2014. Persistence of parvovirus B19 (B19V) DNA and humoral immune response in B19V-infected blood donors. Vox Sang 107:226–232. doi: 10.1111/vox.12162. [DOI] [PubMed] [Google Scholar]
- 672.Juhl D, Steppat D, Gorg S, Hennig H. 2014. Parvovirus b19 infections and blood counts in blood donors. Transfus Med Hemother 41:52–59. doi: 10.1159/000357650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Tang ML, Kemp AS, Moaven LD. 1994. Parvovirus B19-associated red blood cell aplasia in combined immunodeficiency with normal immunoglobulins. Pediatr Infect Dis J 13:539–542. doi: 10.1097/00006454-199406000-00015. [DOI] [PubMed] [Google Scholar]
- 674.Tavil B, Sanal O, Turul T, Yel L, Gurgey A, Gumruk F. 2009. Parvovirus B19-induced persistent pure red cell aplasia in a child with T-cell immunodeficiency. Pediatr Hematol Oncol 26:63–68. doi: 10.1080/08880010902754735. [DOI] [PubMed] [Google Scholar]
- 675.Seyama K, Kobayashi R, Hasle H, Apter AJ, Rutledge JC, Rosen D, Ochs HD. 1998. Parvovirus B19-induced anemia as the presenting manifestation of X-linked hyper-IgM syndrome. J Infect Dis 178:318–324. doi: 10.1086/515633. [DOI] [PubMed] [Google Scholar]
- 676.Kynaston JA, West NC, Reid MM. 1993. A regional experience of red cell aplasia. Eur J Pediatr 152:306–308. doi: 10.1007/BF01956739. [DOI] [PubMed] [Google Scholar]
- 677.Smith MA, Shah NR, Lobel JS, Cera PJ, Gary GW, Anderson LJ. 1988. Severe anemia caused by human parvovirus in a leukemia patient on maintenance chemotherapy. Clin Pediatr (Phila) 27:383–386. [DOI] [PubMed] [Google Scholar]
- 678.Sallan SE, Buchanan GR. 1977. Selective erythroid aplasia during therapy for acute lymphoblastic leukemia. Pediatrics 59:895–898. [PubMed] [Google Scholar]
- 679.Fisher D, Spencer D, Iland H, Brammah S, Cossart Y. 1992. Red cell aplasia caused by human parvovirus B19 in acute leukaemia. Aust N Z J Med 22:303–304. doi: 10.1111/j.1445-5994.1992.tb02128.x. [DOI] [PubMed] [Google Scholar]
- 680.Rao SP, Miller ST, Cohen BJ. 1990. Severe anemia due to B19 parvovirus infection in children with acute leukemia in remission. Am J Pediatr Hematol Oncol 12:194–197. doi: 10.1097/00043426-199022000-00013. [DOI] [PubMed] [Google Scholar]
- 681.Azzi A, Macchia PA, Favre C, Nardi M, Zakrzewska K, Corsi OB. 1989. Aplastic crisis caused by B19 virus in a child during induction therapy for acute lymphoblastic leukemia. Haematologicaae 74:191–194. [PubMed] [Google Scholar]
- 682.Coulombel L, Morinet F, Mielot F, Tchernia G. 1989. Parvovirus infection, leukaemia, and immunodeficiency. Lancet i:101–102. [Google Scholar]
- 683.Carstensen H, Ornvold K, Cohen BJ. 1989. Human parvovirus B19 infection associated with prolonged erythroblastopenia in a leukemic child. Pediatr Infect Dis J 8:56. doi: 10.1097/00006454-198901000-00016. [DOI] [PubMed] [Google Scholar]
- 684.McNall RY, Head DR, Pui CH, Razzouk BI. 2001. Parvovirus B19 infection in a child with acute lymphoblastic leukemia during induction therapy. J Pediatr Hematol Oncol 23:309–311. doi: 10.1097/00043426-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 685.Fritch Lilla SA, Burgett SE, McGann KA, Wechsler DS. 2015. Persistent and prolonged parvovirus B19 viremia in a pediatric patient with acute lymphoblastic leukemia. J Pediatr Infect Dis Soc 4:e38–e40. doi: 10.1093/jpids/piu112. [DOI] [PubMed] [Google Scholar]
- 686.Graeve JL, de Alarcon PA, Naides SJ. 1989. Parvovirus B19 infection in patients receiving cancer chemotherapy: the expanding spectrum of disease. Am J Pediatr Hematol Oncol 11:441–444. [PubMed] [Google Scholar]
- 687.Shaw PJ, Eden T, Cohen BJ. 1993. Parvovirus B19 as a cause of chronic anemia in rhabdomyosarcoma. Cancer 72:945–949. doi:. [DOI] [PubMed] [Google Scholar]
- 688.Crowley B, Woodcock B. 2002. Red cell aplasia due to parvovirus b19 in a patient treated with alemtuzumab. Br J Haematol 119:279–280. doi: 10.1046/j.1365-2141.2002.37668.x. [DOI] [PubMed] [Google Scholar]
- 689.Herbert KE, Prince HM, Westerman DA. 2003. Pure red-cell aplasia due to parvovirus B19 infection in a patient treated with alemtuzumab. Blood 101:1654. doi: 10.1182/blood-2002-09-2923. [DOI] [PubMed] [Google Scholar]
- 690.De Renzo A, Azzi A, Zakrzewska K, Cicoira L, Notaro R, Rotoli B. 1994. Cytopenia caused by parvovirus in an adult ALL patient. Haematologicaae 79:259–261. [PubMed] [Google Scholar]
- 691.Zolnourian ZR, Curran MD, Rima BK, Coyle PV, O'Neill HJ, Middleton D. 2000. Parvovirus B19 in kidney transplant patients. Transplantation 69:2198–2202. doi: 10.1097/00007890-200005270-00043. [DOI] [PubMed] [Google Scholar]
- 692.Rahiala J, Koskenvuo M, Norja P, Meriluoto M, Toppinen M, Lahtinen A, Vaisanen E, Waris M, Vuorinen T, Saarinen-Pihkala U, Lappalainen M, Allander T, Ruuskanen O, Hedman K, Soderlund-Venermo M, Vettenranta K. 2013. Human parvoviruses B19, PARV4 and bocavirus in pediatric patients with allogeneic hematopoietic SCT. Bone Marrow Transplant 48:1308–1312. doi: 10.1038/bmt.2013.63. [DOI] [PubMed] [Google Scholar]
- 693.Porignaux R, Vuiblet V, Barbe C, Nguyen Y, Lavaud S, Toupance O, Andreoletti L, Rieu P, Leveque N. 2013. Frequent occurrence of parvovirus B19 DNAemia in the first year after kidney transplantation. J Med Virol 85:1115–1121. doi: 10.1002/jmv.23557. [DOI] [PubMed] [Google Scholar]
- 694.Niitsu H, Takatsu H, Miura I, Chubachi A, Ito T, Hirokawa M, Endo Y, Miura A, Fukuda M, Sasaki T. 1990. Pure red cell aplasia induced by B19 parvovirus during allogeneic bone marrow transplantation. Rinsho Ketsueki 31:1566–1571. (In Japanese.) [PubMed] [Google Scholar]
- 695.Solano C, Juan O, Gimeno C, Garcia-Conde J. 1996. Engraftment failure associated with peripheral blood stem cell transplantation after B19 parvovirus infection. Blood 88:1515–1517. [PubMed] [Google Scholar]
- 696.Itala M, Kotilainen P, Nikkari S, Remes K, Nikoskelainen J. 1997. Pure red cell aplasia caused by B19 parvovirus infection after autologous blood stem cell transplantation in a patient with chronic lymphocytic leukemia. Leukemia 11:171. doi: 10.1038/sj.leu.2400527. [DOI] [PubMed] [Google Scholar]
- 697.Bertoni E, Rosati A, Zanazzi M, Azzi A, Zakrewska K, Guidi S, Salvadori M. 1997. Unusual incidence of aplastic anaemia due to B-19 parvovirus infection in renal transplant recipients. Transplant Proc 29:818–819. doi: 10.1016/S0041-1345(96)00147-9. [DOI] [PubMed] [Google Scholar]
- 698.Cohen BJ, Beard S, Knowles WA, Ellis JS, Joske D, Goldman JM, Hewitt P, Ward KN. 1997. Chronic anemia due to parvovirus B19 infection in a bone marrow transplant patient after platelet transfusion. Transfusion 37:947–952. doi: 10.1046/j.1537-2995.1997.37997454023.x. [DOI] [PubMed] [Google Scholar]
- 699.Hayes-Lattin B, Seipel TJ, Gatter K, Heinrich MC, Maziarz RT. 2004. Pure red cell aplasia associated with parvovirus B19 infection occurring late after allogeneic bone marrow transplantation. Am J Hematol 75:142–145. doi: 10.1002/ajh.10474. [DOI] [PubMed] [Google Scholar]
- 700.Plentz A, Hahn J, Holler E, Jilg W, Modrow S. 2004. Long-term parvovirus B19 viraemia associated with pure red cell aplasia after allogeneic bone marrow transplantation. J Clin Virol 31:16–19. doi: 10.1016/j.jcv.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 701.Klumpen HJ, Petersen EJ, Verdonck LF. 2004. Severe multiorgan failure after parvovirus B19 infection in an allogeneic stem cell transplant recipient. Bone Marrow Transplant 34:469–470. doi: 10.1038/sj.bmt.1704612. [DOI] [PubMed] [Google Scholar]
- 702.Kobayashi S, Maruta A, Yamamoto T, Katayama N, Higuchi R, Sakano Y, Fujita H, Koharazawa H, Tomita N, Taguchi J, Kodama F, Nakamura Y, Shimizu A. 1998. Human parvovirus B19 capsid antigen in granulocytes in parvovirus-B19-induced pancytopenia after bone marrow transplantation. Acta Haematol 100:195–199. [DOI] [PubMed] [Google Scholar]
- 703.Neild G, Anderson M, Hawes S, Colvin BT. 1986. Parvovirus infection after renal transplant. Lancet ii:1226–1227. [DOI] [PubMed] [Google Scholar]
- 704.Nour B, Green M, Michaels M, Reyes J, Tzakis A, Gartner JC, McLoughlin L, Starzl TE. 1993. Parvovirus B19 infection in pediatric transplant patients. Transplantation 56:835–838. doi: 10.1097/00007890-199310000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Calvet A, Pujol MO, Bertocchi M, Bastien O, Boissonnat P, Mornex JF. 1999. Parvovirus B19 infection in thoracic organ transplant recipients. J Clin Virol 13:37–42. doi: 10.1016/S1386-6532(99)00012-8. [DOI] [PubMed] [Google Scholar]
- 706.Ahsan N, Holman MJ, Gocke CD, Groff JA, Yang HC. 1997. Pure red cell aplasia due to parvovirus B19 infection in solid organ transplantation. Clin Transplant 11:265–270. [PubMed] [Google Scholar]
- 707.Wicki J, Samii K, Cassinotti P, Voegeli J, Rochat T, Beris P. 1997. Parovirus [sic] B19-induced red cell aplasia in solid-organ transplant recipients. Two case reports and review of the literature. Hematol Cell Ther 39:199–204. [DOI] [PubMed] [Google Scholar]
- 708.Geetha D, Zachary JB, Baldado HM, Kronz JD, Kraus ES. 2000. Pure red cell aplasia caused by parvovirus B19 infection in solid organ transplant recipients: a case report and review of literature. Clin Transplant 14:586–591. doi: 10.1034/j.1399-0012.2000.140612.x. [DOI] [PubMed] [Google Scholar]
- 709.Wong TY, Chan PK, Leung CB, Szeto CC, Tam JS, Li PK. 1999. Parvovirus B19 infection causing red cell aplasia in renal transplantation on tacrolimus. Am J Kidney Dis 34:1132–1136. doi: 10.1016/S0272-6386(99)70021-1. [DOI] [PubMed] [Google Scholar]
- 710.Bertoni E, Rosati A, Zanazzi M, Azzi A, Zakrzewska K, Guidi S, Fanci R, Salvadori M. 1997. Aplastic anemia due to B19 parvovirus infection in cadaveric renal transplant recipients: an underestimated infectious disease in the immunocompromised host. J Nephrol 10:152–156. [PubMed] [Google Scholar]
- 711.Mathias RS. 1997. Chronic anemia as a complication of parvovirus B19 infection in a pediatric kidney transplant patient. Pediatr Nephrol 11:355–357. doi: 10.1007/s004670050296. [DOI] [PubMed] [Google Scholar]
- 712.Amiot L, Langanay T, Drenou B, Lelong B, Le Prise PY, Logeais Y, Colimon R, Fauchet R. 1998. Spontaneous recovery from severe parvovirus B19 pure red cell aplasia, in a heart transplant recipient, as demonstrated by marrow culture. Hematol Cell Ther 40:71–73. [PubMed] [Google Scholar]
- 713.Pamidi S, Friedman K, Kampalath B, Eshoa C, Hariharan S. 2000. Human parvovirus B19 infection presenting as persistent anemia in renal transplant recipients. Transplantation 69:2666–2669. doi: 10.1097/00007890-200006270-00030. [DOI] [PubMed] [Google Scholar]
- 714.Ndimbie OK, Frezza E, Jordan JA, Koch W, van Thiel DH. 1996. Parvovirus B19 in anemic liver transplant recipients. Clin Diagn Lab Immunol 3:756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Zhang M, Zhong X, Zhang W, Xu J, Zhang M, Shen Y, Wang W, Zheng S. 2015. Human parvovirus B19 infection induced pure red cell aplasia in liver transplant recipients. Int J Clin Pract Suppl 2015:29–34. doi: 10.1111/ijcp.12664. [DOI] [PubMed] [Google Scholar]
- 716.Assy N, Rosenthal E, Hazani A, Etzioni A, Baruch Y. 1997. Human parvovirus B19 infection associated with idiopathic thrombocytopenic purpura in a child following liver transplantation. J Hepatol 27:934–936. doi: 10.1016/S0168-8278(97)80334-0. [DOI] [PubMed] [Google Scholar]
- 717.Shekar K, Hopkins PM, Kermeen FD, Dunning JJ, McNeil KD. 2008. Unexplained chronic anemia and leukopenia in lung transplant recipients secondary to parvovirus B19 infection. J Heart Lung Transplant 27:808–811. doi: 10.1016/j.healun.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 718.Bergen GA, Sakalosky PE, Sinnott JT. 1996. Transient aplastic anemia caused by parvovirus B19 infection in a heart transplant recipient. J Heart Lung Transplant 15:843–845. [PubMed] [Google Scholar]
- 719.Kariyawasam HH, Gyi KM, Hodson ME, Cohen BJ. 2000. Anaemia in lung transplant patient caused by parvovirus B19. Thorax 55:619–620. doi: 10.1136/thorax.55.7.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 720.Garewal G, Ahluwalia J, Varma N, Das R, Sakhuja VK. 2004. Parvovirus B19 infection-associated red-cell aplasia in renal-transplant recipients: clues from the bone marrow. Transplantation 77:320–321. doi: 10.1097/01.TP.0000092957.49621.37. [DOI] [PubMed] [Google Scholar]
- 721.Beckhoff A, Steffen I, Sandoz P, Hirsch HH, Schaub S. 2007. Relapsing severe anaemia due to primary parvovirus B19 infection after renal transplantation: a case report and review of the literature. Nephrol Dial Transplant 22:3660–3663. doi: 10.1093/ndt/gfm531. [DOI] [PubMed] [Google Scholar]
- 722.Malarme M, Vandervelde D, Brasseur M. 1989. Parvovirus infection, leukaemia, and immunodeficiency. Lancet i:1457. [DOI] [PubMed] [Google Scholar]
- 723.Suzuki M, Ito Y, Shimada A, Saito M, Muramatsu H, Hama A, Takahashi Y, Kimura H, Kojima S. 2014. Long-term parvovirus B19 infections with genetic drift after cord blood transplantation complicated by persistent CD4+ lymphocytopenia. J Pediatr Hematol Oncol 36:e65–e68. doi: 10.1097/MPH.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 724.Frickhofen N, Abkowitz JL, Safford M, Berry JM, Antunez-de-Mayolo J, Astrow A, Cohen R, Halperin I, King L, Mintzer D, Cohen B, Young NS. 1990. Persistent B19 parvovirus infection in patients infected with human immunodeficiency virus type 1 (HIV-1): a treatable cause of anemia in AIDS. Ann Intern Med 113:926–933. doi: 10.7326/0003-4819-113-12-926. [DOI] [PubMed] [Google Scholar]
- 725.de Mayolo JA, Temple JD. 1990. Pure red cell aplasia due to parvovirus B19 infection in a man with HIV infection. South Med J 83:1480–1481. doi: 10.1097/00007611-199012000-00028. [DOI] [PubMed] [Google Scholar]
- 726.Mitchell SA, Welch JM, Weston-Smith S, Nicholson F, Bradbeer CS. 1990. Parvovirus infection and anaemia in a patient with AIDS: case report. Genitourin Med 66:95–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Heller HM, Dzieczkowski JS. 1993. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 36-1993. A 28-year-old man with AIDS, persistent pancytopenia, and lymphoma. N Engl J Med 329:792–799. [DOI] [PubMed] [Google Scholar]
- 728.Chrystie IL, Almeida JD, Welch J. 1990. Electron microscopic detection of human parvovirus (B19) in a patient with HIV infection. J Med Virol 30:249–252. [DOI] [PubMed] [Google Scholar]
- 729.Naides SJ, Howard EJ, Swack NS, True CA, Stapleton JT. 1993. Parvovirus B19 infection in human immunodeficiency virus type 1-infected persons failing or intolerant to zidovudine therapy. J Infect Dis 168:101–105. doi: 10.1093/infdis/168.1.101. [DOI] [PubMed] [Google Scholar]
- 730.Gyllensten K, Sonnerborg A, Jorup-Ronstrom C, Halvarsson M, Yun Z. 1994. Parvovirus B19 infection in HIV-1 infected patients with anemia. Infection 22:356–358. doi: 10.1007/BF01715548. [DOI] [PubMed] [Google Scholar]
- 731.Zuckerman MA, Williams I, Bremner J, Cohen B, Miller RF. 1994. Persistent anaemia in HIV-infected individuals due to parvovirus B19 infection. AIDS 8:1191–1192. doi: 10.1097/00002030-199408000-00029. [DOI] [PubMed] [Google Scholar]
- 732.Higashi Y, Sakai K, Tada S, Miyase S, Nakamura T, Kamio T, Haraguchi O. 1996. Case report: agranulocytosis induced by interferon-alpha therapy for chronic hepatitis C. J Gastroenterol Hepatol 11:1012–1015. [DOI] [PubMed] [Google Scholar]
- 733.Liu W, Ittmann M, Liu J, Schoentag R, Tierno P, Greco MA, Sidhu G, Nierodzik M, Wieczorek R. 1997. Human parvovirus B19 in bone marrows from adults with acquired immunodeficiency syndrome: a comparative study using in situ hybridization and immunohistochemistry. Hum Pathol 28:760–766. doi: 10.1016/S0046-8177(97)90146-5. [DOI] [PubMed] [Google Scholar]
- 734.Gryfe CI. 1976. Letter: agranulocytosis and aplastic anemia possibly due to ibuprofen. Can Med Assoc J 114:877. [PMC free article] [PubMed] [Google Scholar]
- 735.Raguin G, Leruez-Ville M, Gregoire V, Deplanche M, Leport C, Morinet F, Vilde JL. 1997. Low prevalence of active parvovirus B19 infection in HIV-infected patients. Eur J Clin Microbiol Infect Dis 16:760–762. doi: 10.1007/BF01709261. [DOI] [PubMed] [Google Scholar]
- 736.Abkowitz JL, Brown KE, Wood RW, Kovach NL, Green SW, Young NS. 1997. Clinical relevance of parvovirus B19 as a cause of anemia in patients with human immunodeficiency virus infection. J Infect Dis 176:269–273. doi: 10.1086/517264. [DOI] [PubMed] [Google Scholar]
- 737.Vadlamudi G, Rezuke WN, Ross JW, Cartun RW, Ackroyd R, Knibbs DR, Tsongalis GJ. 1999. The use of monoclonal antibody R92F6 and polymerase chain reaction to confirm the presence of parvovirus B19 in bone marrow specimens of patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med 123:768–773. [DOI] [PubMed] [Google Scholar]
- 738.Faden H, Gary GW Jr, Anderson LJ. 1992. Chronic parvovirus infection in a presumably immunologically healthy woman. Clin Infect Dis 15:595–597. doi: 10.1093/clind/15.4.595. [DOI] [PubMed] [Google Scholar]
- 739.Sasaki T, Murai C, Muryoi T, Takahashi Y, Munakata Y, Sugamura K, Abe K. 1995. Persistent infection of human parvovirus B19 in a normal subject. Lancet 346:851. doi: 10.1016/S0140-6736(95)91673-3. [DOI] [PubMed] [Google Scholar]
- 740.Biesma DH, Nieuwenhuis HK. 1997. Life-threatening anaemia caused by B19 parvovirus infection in a non-immunocompromised patient. Neth J Med 50:81–84. doi: 10.1016/S0300-2977(96)00076-9. [DOI] [PubMed] [Google Scholar]
- 741.Isomoto H, Fukuda Y, Bando Y, Machida I, Machida H, Omagari K, Mizuta Y, Murase K, Fukushima T, Murata I, Kohno S. 2003. Pure red cell aplasia associated with parvovirus B19 infection in a patient with ulcerative colitis. Dig Dis Sci 48:2104–2107. doi: 10.1023/A:1026163530455. [DOI] [PubMed] [Google Scholar]
- 742.Pitchaipillai S, Kelsey P, Haeney M. 2006. Persistent pure red cell aplasia due to parvovirus B19 infection in a patient with Turner's syndrome. Clin Lab Haematol 28:347–350. doi: 10.1111/j.1365-2257.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 743.Gosset C, Viglietti D, Hue K, Antoine C, Glotz D, Pillebout E. 2012. How many times can parvovirus B19-related anemia recur in solid organ transplant recipients? Transpl Infect Dis 14:E64–E70. doi: 10.1111/j.1399-3062.2012.00773.x. [DOI] [PubMed] [Google Scholar]
- 744.Ramratnam B, Gollerkeri A, Schiffman FJ, Rintels P, Flanigan TP. 1995. Management of persistent B19 parvovirus infection in AIDS. Br J Haematol 91:90–92. doi: 10.1111/j.1365-2141.1995.tb05250.x. [DOI] [PubMed] [Google Scholar]
- 745.Chen MY, Hung CC, Fang CT, Hsieh SM. 2001. Reconstituted immunity against persistent parvovirus B19 infection in a patient with acquired immunodeficiency syndrome after highly active antiretroviral therapy. Clin Infect Dis 32:1361–1365. doi: 10.1086/319988. [DOI] [PubMed] [Google Scholar]
- 746.Morelli P, Bestetti G, Longhi E, Parravicini C, Corbellino M, Meroni L. 2007. Persistent parvovirus B19-induced anemia in an HIV-infected patient under HAART. Case report and review of literature. Eur J Clin Microbiol Infect Dis 26:833–837. doi: 10.1007/s10096-007-0360-y. [DOI] [PubMed] [Google Scholar]
- 747.Lortholary O, Eliaszewicz M, Dupont B, Courouce AM. 1992. Parvovirus B19 infection during acute Plasmodium falciparum malaria. Eur J Haematol 49:219. [DOI] [PubMed] [Google Scholar]
- 748.Wildig J, Michon P, Siba P, Mellombo M, Ura A, Mueller I, Cossart Y. 2006. Parvovirus B19 infection contributes to severe anemia in young children in Papua New Guinea. J Infect Dis 194:146–153. doi: 10.1086/505082. [DOI] [PubMed] [Google Scholar]
- 749.Gupta R, Singh T. 2005. Parvovirus B19 co-infection with falciparum malaria: a cause of severe anemia. Haematologicaae 90:ECR41. [PubMed] [Google Scholar]
- 750.Manning L, Laman M, Rosanas-Urgell A, Michon P, Aipit S, Bona C, Siba P, Mueller I, Davis TM. 2012. Severe anemia in Papua New Guinean children from a malaria-endemic area: a case-control etiologic study. PLoS Negl Trop Dis 6:e1972. doi: 10.1371/journal.pntd.0001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 751.Duedu KO, Sagoe KW, Ayeh-Kumi PF, Affrim RB, Adiku T. 2013. The effects of co-infection with human parvovirus B19 and Plasmodium falciparum on type and degree of anaemia in Ghanaian children. Asian Pac J Trop Biomed 3:129–139. doi: 10.1016/S2221-1691(13)60037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Urganci N, Arapoglu M, Kayaalp N. 2003. Plasmodium falciparum malaria with coexisting parvovirus B19 infection. Indian Pediatr 40:369–370. [PubMed] [Google Scholar]
- 753.Wildig J, Cossart Y, Peshu N, Gicheru N, Tuju J, Williams TN, Newton CR. 2010. Parvovirus B19 infection and severe anaemia in Kenyan children: a retrospective case control study. BMC Infect Dis 10:88. doi: 10.1186/1471-2334-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Gasim GI, Eltayeb R, Elhassan EM, Haggaz AD, Rayis DA, Adam I. 2016. Human parvovirus B19 and low hemoglobin levels in pregnant Sudanese women. Int J Gynaecol Obstet 132:318–320. doi: 10.1016/j.ijgo.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 755.Kerr JR. 2015. A review of blood diseases and cytopenias associated with human parvovirus B19 infection. Rev Med Virol 25:224–240. doi: 10.1002/rmv.1839. [DOI] [PubMed] [Google Scholar]
- 756.Hoang MP, Dawson DB, Rogers ZR, Scheuermann RH, Rogers BB. 1998. Polymerase chain reaction amplification of archival material for Epstein-Barr virus, cytomegalovirus, human herpesvirus 6, and parvovirus B19 in children with bone marrow hemophagocytosis. Hum Pathol 29:1074–1077. doi: 10.1016/S0046-8177(98)90416-6. [DOI] [PubMed] [Google Scholar]
- 757.Boruchoff SE, Woda BA, Pihan GA, Durbin WA, Burstein D, Blacklow NR. 1990. Parvovirus B19-associated hemophagocytic syndrome. Arch Intern Med 150:897–899. [PubMed] [Google Scholar]
- 758.Shirono K, Tsuda H. 1995. Parvovirus B19-associated haemophagocytic syndrome in healthy adults. Br J Haematol 89:923–926. [DOI] [PubMed] [Google Scholar]
- 759.Yufu Y, Matsumoto M, Miyamura T, Nishimura J, Nawata H, Ohshima K. 1997. Parvovirus B19-associated haemophagocytic syndrome with lymphadenopathy resembling histiocytic necrotizing lymphadenitis (Kikuchi's disease). Br J Haematol 96:868–871. doi: 10.1046/j.1365-2141.1997.d01-2099.x. [DOI] [PubMed] [Google Scholar]
- 760.Larroche C, Scieux C, Honderlick P, Piette AM, Morinet F, Bletry O. 2002. Spontaneous resolution of hemophagocytic syndrome associated with acute parvovirus B19 infection and concomitant Epstein-Barr virus reactivation in an otherwise healthy adult. Eur J Clin Microbiol Infect Dis 21:739–742. doi: 10.1007/s10096-002-0793-2. [DOI] [PubMed] [Google Scholar]
- 761.Dutta U, Mittal S, Ratho RK, Das A. 2005. Acute liver failure and severe hemophagocytosis secondary to parvovirus B19 infection. Indian J Gastroenterol 24:118–119. [PubMed] [Google Scholar]
- 762.Pedrosa AF, Mota A, Morais P, Nogueira A, Brochado M, Fonseca E, Azevedo F. 2014. Haemophagocytic syndrome with a fatal outcome triggered by parvovirus B19 infection in the skin. Clin Exp Dermatol 39:222–223. doi: 10.1111/ced.12208. [DOI] [PubMed] [Google Scholar]
- 763.Mayama M, Yoshihara M, Kokabu T, Oguchi H. 2014. Hemophagocytic lymphohistiocytosis associated with a parvovirus B19 infection during pregnancy. Obstet Gynecol 124:438–441. doi: 10.1097/AOG.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 764.Yilmaz S, Oren H, Demircioglu F, Firinci F, Korkmaz A, Irken G. 2006. Parvovirus B19: a cause for aplastic crisis and hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 47:861. doi: 10.1002/pbc.20807. [DOI] [PubMed] [Google Scholar]
- 765.Drexler B, Holbro A. 2014. Unexpected bone marrow finding in a patient with pancytopenia after hematopoietic stem cell transplantation. Blood 124:678. doi: 10.1182/blood-2014-05-576769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Yaguchi D, Marui N, Matsuo M. 2015. Three adult cases of HPV-b19 infection with concomitant leukopenia and low platelet counts. Clin Med Insights Case Rep 8:19–22. doi: 10.4137/CCRep.S18085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 767.Doran HM, Teall AJ. 1988. Neutropenia accompanying erythroid aplasia in human parvovirus infection. Br J Haematol 69:287–288. doi: 10.1111/j.1365-2141.1988.tb07636.x. [DOI] [PubMed] [Google Scholar]
- 768.Elian JC, Frappaz D, Pozzetto B, Taimi A, Jacquemard R, Freycon F. 1991. Érythroblastopénie et neutropénie transitoires révélatrices d'une infection à parvovirus humain B19. Pediatrie 46:673–675. [PubMed] [Google Scholar]
- 769.Kamper AM, Malbrain M, Zachee P, Chew SL. 1994. Parvovirus infection causing red cell aplasia and leukopenia in rheumatoid arthritis. Clin Rheumatol 13:129–131. doi: 10.1007/BF02229883. [DOI] [PubMed] [Google Scholar]
- 770.Hanada T, Koike K, Takeya T, Nagasawa T, Matsunaga Y, Takita H. 1988. Human parvovirus B19-induced transient pancytopenia in a child with hereditary spherocytosis. Br J Haematol 70:113–115. doi: 10.1111/j.1365-2141.1988.tb02442.x. [DOI] [PubMed] [Google Scholar]
- 771.Kara TT, Ozdemir H, Ciftci E, Ince E. 2016. Petechial-purpuric rash, leukopenia and thromboytopenia associated parvovirus B19. J Clin Anal Med 7:414–416. [Google Scholar]
- 772.Wolfromm A, Rodriguez C, Michel M, Habibi A, Audard V, Benayoun E, Rogier O, Challine D, Chosidow O, Lelievre JD, Chevalier X, Le Bras F, Pautas C, Imbert M, Pawlotsky JM, Wagner-Ballon O. 2015. Spectrum of adult parvovirus B19 infection according to the underlying predisposing condition and proposals for clinical practice. Br J Haematol 170:192–199. doi: 10.1111/bjh.13421. [DOI] [PubMed] [Google Scholar]
- 773.Barlow GD, McKendrick MW. 2000. Parvovirus B19 causing leucopenia and neutropenia in a healthy adult. J Infect 40:192–195. doi: 10.1016/S0163-4453(00)80018-3. [DOI] [PubMed] [Google Scholar]
- 774.Foreman NK, Oakhill A, Caul EO. 1988. Parvovirus-associated thrombocytopenic purpura. Lancet ii:1426–1427. [DOI] [PubMed] [Google Scholar]
- 775.Lefrere JJ, Got D. 1987. Peripheral thrombocytopenia in human parvovirus infection. J Clin Pathol 40:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.Scheurlen W, Ramasubbu K, Wachowski O, Hemauer A, Modrow S. 2001. Chronic autoimmune thrombopenia/neutropenia in a boy with persistent parvovirus B19 infection. J Clin Virol 20:173–178. doi: 10.1016/S1386-6532(00)00179-7. [DOI] [PubMed] [Google Scholar]
- 777.Bhattacharyya J, Kumar R, Tyagi S, Kishore J, Mahapatra M, Choudhry VP. 2005. Human parvovirus B19-induced acquired pure amegakaryocytic thrombocytopenia. Br J Haematol 128:128–129. doi: 10.1111/j.1365-2141.2004.05252.x. [DOI] [PubMed] [Google Scholar]
- 778.Heegaard ED, Rosthoj S, Petersen BL, Nielsen S, Karup PF, Hornsleth A. 1999. Role of parvovirus B19 infection in childhood idiopathic thrombocytopenic purpura. Acta Paediatr 88:614–617. doi: 10.1111/j.1651-2227.1999.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 779.Murray JC, Morad AB. 1994. Childhood autoimmune neutropenia and human parvovirus B19. Am J Hematol 47:336. doi: 10.1002/ajh.2830470424. [DOI] [PubMed] [Google Scholar]
- 780.Gautier E, Bourhis JH, Bayle C, Cartron J, Pico JL, Tchernia G. 1997. Parvovirus B19 associated neutropenia. Treatment with Rh G-CSF. Hematol Cell Ther 39:85–87. [DOI] [PubMed] [Google Scholar]
- 781.Honda K, Ishiko O, Tsujimura A, Hino M, Hirai K, Itoh F, Tanaka T, Ogita S. 2000. Neutropenia accompanying parvovirus B19 infection after gynecologic surgery. Acta Haematol 103:186–190. doi: 10.1159/000041047. [DOI] [PubMed] [Google Scholar]
- 782.McClain K, Estrov Z, Chen H, Mahoney DH Jr. 1993. Chronic neutropenia of childhood: frequent association with parvovirus infection and correlations with bone marrow culture studies. Br J Haematol 85:57–62. doi: 10.1111/j.1365-2141.1993.tb08645.x. [DOI] [PubMed] [Google Scholar]
- 783.Hartman KR, Brown KE, Green SW, Young NS. 1994. Lack of evidence for parvovirus B19 viraemia in children with chronic neutropenia. Br J Haematol 88:895–896. doi: 10.1111/j.1365-2141.1994.tb05136.x. [DOI] [PubMed] [Google Scholar]
- 784.Pont J, Puchhammer-Stockl E, Chott A, Popow-Kraupp T, Kienzer H, Postner G, Honetz N. 1992. Recurrent granulocytic aplasia as clinical presentation of a persistent parvovirus B19 infection. Br J Haematol 80:160–165. doi: 10.1111/j.1365-2141.1992.tb08894.x. [DOI] [PubMed] [Google Scholar]
- 785.Miniero R, Dalponte S, Linari A, Saracco P, Testa A, Musiani M. 1996. Severe Shwachman-Diamond syndrome and invasive parvovirus B19 infection. Pediatr Hematol Oncol 13:555–561. doi: 10.3109/08880019609030872. [DOI] [PubMed] [Google Scholar]
- 786.Hamon MD, Newland AC, Anderson MJ. 1988. Severe aplastic anaemia after parvovirus infection in the absence of underlying haemolytic anaemia. J Clin Pathol 41:1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787.Frickhofen N, Raghavachar A, Heit W, Heimpel H, Cohen BJ. 1986. Human parvovirus infection. N Engl J Med 314:645–647. doi: 10.1056/NEJM198603063141012. [DOI] [PubMed] [Google Scholar]
- 788.Osaki M, Matsubara K, Iwasaki T, Kurata T, Nigami H, Harigaya H, Baba K. 1999. Severe aplastic anemia associated with human parvovirus B19 infection in a patient without underlying disease. Ann Hematol 78:83–86. doi: 10.1007/s002770050477. [DOI] [PubMed] [Google Scholar]
- 789.Qian XH, Zhang GC, Jiao XY, Zheng YJ, Cao YH, Xu DL, Chen CS. 2002. Aplastic anaemia associated with parvovirus B19 infection. Arch Dis Child 87:436–437. doi: 10.1136/adc.87.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 790.Yetgin S, Cetin M, Ozyurek E, Aslan D, Uckan D. 2004. Parvovirus B19 infection associated with severe aplastic anemia in an immunocompetent patient. Pediatr Hematol Oncol 21:223–226. doi: 10.1080/08880010490276935. [DOI] [PubMed] [Google Scholar]
- 791.Heegaard ED, Jensen L, Hornsleth A, Schmiegelow K. 1999. The role of parvovirus B19 infection in childhood acute lymphoblastic leukemia. Pediatr Hematol Oncol 16:329–334. doi: 10.1080/088800199277155. [DOI] [PubMed] [Google Scholar]
- 792.Kawakami C, Kono Y, Inoue A, Takitani K, Ikemoto T, Tamai H. 2012. Severe bone marrow failure associated with human parvovirus B19 infection in a case with no underlying disorder. Int J Hematol 96:820–821. doi: 10.1007/s12185-012-1214-7. [DOI] [PubMed] [Google Scholar]
- 793.Dame C, Hasan C, Bode U, Eis-Hubinger AM. 2002. Acute liver disease and aplastic anemia associated with the persistence of B19 DNA in liver and bone marrow. Pediatr Pathol Mol Med 21:25–29. doi: 10.1080/pdp.21.1.25.29. [DOI] [PubMed] [Google Scholar]
- 794.Lee YM, Tsai WH, You JY, Ing-Tiau KB, Liao PT, Ho CK, Hsu HC. 2003. Parvovirus B19 infection in Taiwanese patients with hematological disorders. J Med Virol 71:605–609. doi: 10.1002/jmv.10517. [DOI] [PubMed] [Google Scholar]
- 795.Soliman O, Abd El-Aal Hegazi Hasan M, El-Ashry R, Zaghloul MH, Kora B. 2009. Parvovirus B19 infection in pediatric oncology patients: diagnostic value of clinical and serologic parameters compared with nested PCR. J Pediatr Hematol Oncol 31:173–176. doi: 10.1097/MPH.0b013e3181983b2d. [DOI] [PubMed] [Google Scholar]
- 796.Zaki ME, Ashray RE. 2010. Clinical and hematological study for parvovirus b19 infection in children with acute leukemia. Int J Lab Hematol 32:159–166. doi: 10.1111/j.1751-553X.2009.01150.x. [DOI] [PubMed] [Google Scholar]
- 797.Yoto Y, Kudoh T, Suzuki N, Matsunaga Y, Chiba S. 1993. Retrospective study on the influence of human parvovirus B19 infection among children with malignant diseases. Acta Haematol 90:8–12. [DOI] [PubMed] [Google Scholar]
- 798.Cakirca M, Karatoprak C, Ugurlu S, Zorlu M, Kiskac M, Cetin G. 2015. Parvovirus B19 infection as a cause of acute myositis in an adult. Rev Bras Reumatol 55:185–188. doi: 10.1016/j.rbr.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 799.Heegaard ED, Madsen HO, Schmiegelow K. 2001. Transient pancytopenia preceding acute lymphoblastic leukaemia (pre-ALL) precipitated by parvovirus B19. Br J Haematol 114:810–813. doi: 10.1046/j.1365-2141.2001.03021.x. [DOI] [PubMed] [Google Scholar]
- 800.Kerr JR, Barah F, Cunniffe VS, Smith J, Vallely PJ, Will AM, Wynn RF, Stevens RF, Taylor GM, Cleator GM, Eden OB. 2003. Association of acute parvovirus B19 infection with new onset of acute lymphoblastic and myeloblastic leukaemia. J Clin Pathol 56:873–875. doi: 10.1136/jcp.56.11.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 801.Douvoyiannis M, Litman N, Goldman DL. 2009. Neurologic manifestations associated with parvovirus B19 infection. Clin Infect Dis 48:1713–1723. doi: 10.1086/599042. [DOI] [PubMed] [Google Scholar]
- 802.Barah F, Vallely PJ, Cleator GM, Kerr JR. 2003. Neurological manifestations of human parvovirus B19 infection. Rev Med Virol 13:185–199. doi: 10.1002/rmv.388. [DOI] [PubMed] [Google Scholar]
- 803.Sane F, Sauter P, Fronval S, Goffard A, Dewilde A, Hober D. 2008. Fruit of the emergence of an enterovirus: acute haemorrhagic conjunctivitis. Ann Biol Clin (Paris) 66:485–492. (In French.) doi: 10.1684/abc.2008.0257. [DOI] [PubMed] [Google Scholar]
- 804.Barah F, Whiteside S, Batista S, Morris J. 2014. Neurological aspects of human parvovirus B19 infection: a systematic review. Rev Med Virol 24:154–168. doi: 10.1002/rmv.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805.Watanabe T, Kawashima H. 2015. Acute encephalitis and encephalopathy associated with human parvovirus B19 infection in children. World J Clin Pediatr 4:126–134. doi: 10.5409/wjcp.v4.i4.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 806.Bakhshi S, Sarnaik SA, Becker C, Shurney WW, Nigro M, Savasan S. 2002. Acute encephalopathy with parvovirus B19 infection in sickle cell disease. Arch Dis Child 87:541–542. doi: 10.1136/adc.87.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Nolan RC, Chidlow G, French MA. 2003. Parvovirus B19 encephalitis presenting as immune restoration disease after highly active antiretroviral therapy for human immunodeficiency virus infection. Clin Infect Dis 36:1191–1194. doi: 10.1086/374603. [DOI] [PubMed] [Google Scholar]
- 808.Tonnellier M, Bessereau J, Carbonnell N, Guidet B, Meritet JF, Kerr JR, Monnier-Cholley L, Offenstadt G, Maury E. 2007. A possible parvovirus B19 encephalitis in an immunocompetent adult patient. J Clin Virol 38:186–187. doi: 10.1016/j.jcv.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 809.Bonvicini F, Marinacci G, Pajno MC, Gallinella G, Musiani M, Zerbini M. 2008. Meningoencephalitis with persistent parvovirus B19 infection in an apparently healthy woman. Clin Infect Dis 47:385–387. doi: 10.1086/589863. [DOI] [PubMed] [Google Scholar]
- 810.Oshima K, Kikuchi A, Mochizuki S, Arai T, Oishi T, Hanada R. 2008. Acute encephalopathy with human parvovirus B19 infection in hereditary spherocytosis. Pediatr Infect Dis J 27:651–652. doi: 10.1097/INF.0b013e3181694fcf. [DOI] [PubMed] [Google Scholar]
- 811.Cassinotti P, Schultze D, Schlageter P, Chevili S, Siegl G. 1993. Persistent human parvovirus B19 infection following an acute infection with meningitis in an immunocompetent patient. Eur J Clin Microbiol Infect Dis 12:701–704. doi: 10.1007/BF02009384. [DOI] [PubMed] [Google Scholar]
- 812.Takasawa K, Takeda S, Nishioka M, Sakuma H, Morio T, Shimohira M. 2016. Steroid-responsive status epilepticus caused by human parvovirus B19 encephalitis. Pediatr Infect Dis J 35:227–228. doi: 10.1097/INF.0000000000000979. [DOI] [PubMed] [Google Scholar]
- 813.Hsu D, Sandborg C, Hahn JS. 2004. Frontal lobe seizures and uveitis associated with acute human parvovirus B19 infection. J Child Neurol 19:304–306. doi: 10.1177/088307380401900413. [DOI] [PubMed] [Google Scholar]
- 814.Guidi B, Bergonzini P, Crisi G, Frigieri G, Portolani M. 2003. Case of stroke in a 7-year-old male after parvovirus B19 infection. Pediatr Neurol 28:69–71. doi: 10.1016/S0887-8994(02)00504-0. [DOI] [PubMed] [Google Scholar]
- 815.Mandrioli J, Portolani M, Cortelli P, Sola P. 2004. Middle cerebral artery thrombosis in course of parvovirus B19 infection in a young adult: a new risk factor for stroke? J Neurovirol 10:71–74. doi: 10.1080/13550280490261752. [DOI] [PubMed] [Google Scholar]
- 816.Denning DW, Amos A, Rudge P, Cohen BJ. 1987. Neuralgic amyotrophy due to parvovirus infection. J Neurol Neurosurg Psychiatry 50:641–642. doi: 10.1136/jnnp.50.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 817.Minohara Y, Koitabashi Y, Kato T, Nakajima N, Murakami H, Masaki H, Ishiko H. 1998. A case of Guillain-Barre syndrome associated with human parvovirus B19 infection. J Infect 36:327–328. doi: 10.1016/S0163-4453(98)94531-5. [DOI] [PubMed] [Google Scholar]
- 818.Corridan PG, Laws DE, Morrell AJ, Murray PI. 1991. Tonic pupils and human parvovirus (B19) infection. J Clin Neuroophthalmol 11:109–110. [PubMed] [Google Scholar]
- 819.Suzuki M, Yoto Y, Ishikawa A, Asakura H, Tsutsumi H. 2014. Acute transverse myelitis associated with human parvovirus b19 infection. J Child Neurol 29:280–282. doi: 10.1177/0883073813499824. [DOI] [PubMed] [Google Scholar]
- 820.Faden H, Gary GW Jr, Korman M. 1990. Numbness and tingling of fingers associated with parvovirus B19 infection. J Infect Dis 161:354–355. doi: 10.1093/infdis/161.2.354. [DOI] [PubMed] [Google Scholar]
- 821.Shroff S, Kamiya-Matsuoka C, Woodman K. 2014. An unusual cause of cerebellar ataxia in an immunocompromised elderly patient. J Neurol Sci 340:218–220. doi: 10.1016/j.jns.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 822.Kerr JR, Barah F, Chiswick ML, McDonnell GV, Smith J, Chapman MD, Bingham JB, Kelleher P, Sheppard MN. 2002. Evidence for the role of demyelination, HLA-DR alleles, and cytokines in the pathogenesis of parvovirus B19 meningoencephalitis and its sequelae. J Neurol Neurosurg Psychiatry 73:739–746. doi: 10.1136/jnnp.73.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 823.Greco F, Barbagallo ML, Chiodo DC, Guglielmino R, Sorge G. 2008. Severe ataxia as a complication of human parvovirus B19 acute encephalitis in a child. J Child Neurol 23:1078–1080. doi: 10.1177/0883073808315420. [DOI] [PubMed] [Google Scholar]
- 824.Nigro G, Piazze J, Taliani G, Mazzocco M, Cassinotti P, Cosmi EV. 1997. Postpartum lupus erythematosus associated with parvovirus B19 infection. J Rheumatol 24:968–970. [PubMed] [Google Scholar]
- 825.Isumi H, Nunoue T, Nishida A, Takashima S. 1999. Fetal brain infection with human parvovirus B19. Pediatr Neurol 21:661–663. doi: 10.1016/S0887-8994(99)00055-7. [DOI] [PubMed] [Google Scholar]
- 826.Hammond CJ, Hobbs JA. 2007. Parvovirus B19 infection of brain: possible role of gender in determining mental illness and autoimmune thyroid disorders. Med Hypotheses 69:113–116. doi: 10.1016/j.mehy.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 827.Druschky K, Walloch J, Heckmann J, Schmidt B, Stefan H, Neundorfer B. 2000. Chronic parvovirus B-19 meningoencephalitis with additional detection of Epstein-Barr virus DNA in the cerebrospinal fluid of an immunocompetent patient. J Neurovirol 6:418–422. doi: 10.3109/13550280009018306. [DOI] [PubMed] [Google Scholar]
- 828.Manning A, Willey SJ, Bell JE, Simmonds P. 2007. Comparison of tissue distribution, persistence, and molecular epidemiology of parvovirus B19 and novel human parvoviruses PARV4 and human bocavirus. J Infect Dis 195:1345–1352. doi: 10.1086/513280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 829.Hobbs JA. 2006. Detection of adeno-associated virus 2 and parvovirus B19 in the human dorsolateral prefrontal cortex. J Neurovirol 12:190–199. doi: 10.1080/13550280600827351. [DOI] [PubMed] [Google Scholar]
- 830.Haseyama K, Kudoh T, Yoto Y, Suzuki N, Chiba S. 1997. Detection of human parvovirus B19 DNA in cerebrospinal fluid. Pediatr Infect Dis J 16:324–326. doi: 10.1097/00006454-199703000-00013. [DOI] [PubMed] [Google Scholar]
- 831.Umene K, Nunoue T. 1995. A new genome type of human parvovirus B19 present in sera of patients with encephalopathy. J Gen Virol 76:2645–2651. doi: 10.1099/0022-1317-76-11-2645. [DOI] [PubMed] [Google Scholar]
- 832.Simpson KE, Storch GA, Lee CK, Ward KE, Danon S, Simon CM, Delaney JW, Tong A, Canter CE. 2016. High frequency of detection by PCR of viral nucleic acid in the blood of infants presenting with clinical myocarditis. Pediatr Cardiol 37:399–404. doi: 10.1007/s00246-015-1290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833.Saint-Martin J, Choulot JJ, Bonnaud E, Morinet F. 1990. Myocarditis caused by parvovirus. J Pediatr 116:1007–1008. doi: 10.1016/S0022-3476(05)80677-8. [DOI] [PubMed] [Google Scholar]
- 834.Beghetti M, Gervaix A, Haenggeli CA, Berner M, Rimensberger PC. 2000. Myocarditis associated with parvovirus B19 infection in two siblings with merosin-deficient congenital muscular dystrophy. Eur J Pediatr 159:135–136. doi: 10.1007/s004310050034. [DOI] [PubMed] [Google Scholar]
- 835.Dettmeyer R, Kandolf R, Baasner A, Banaschak S, Eis-Hubinger AM, Madea B. 2003. Fatal parvovirus B19 myocarditis in an 8-year-old boy. J Forensic Sci 48:183–186. [PubMed] [Google Scholar]
- 836.Buob A, Siaplaouras S, Janzen I, Schwaab B, Hammer B, Schneider G, Kandolf R, Bohm M, Jung J. 2003. Focal parvovirus B19 myocarditis in a patient with Brugada syndrome. Cardiol Rev 11:45–49. doi: 10.1097/00045415-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 837.Dina J, Villedieu F, Labombarda F, Freymuth F, de la Gastine G, Jokic M, Vabret A. 2011. Childhood myocarditis and parvovirus B19 genotypes. J Clin Virol 50:61–64. doi: 10.1016/j.jcv.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 838.Vigneswaran TV, Brown JR, Breuer J, Burch M. 2016. Parvovirus B19 myocarditis in children: an observational study. Arch Dis Child 101:177–180. doi: 10.1136/archdischild-2014-308080. [DOI] [PubMed] [Google Scholar]
- 839.Frasure SE, Siadecki SD, Saul T, Lewiss RE. 2014. Viral myocarditis leading to acute heart failure in a young adult. J Emerg Med 46:e75–e77. doi: 10.1016/j.jemermed.2013.08.092. [DOI] [PubMed] [Google Scholar]
- 840.Butin M, Mekki Y, Phan A, Billaud G, Di Filippo S, Javouhey E, Cochat P, Belot A. 2013. Successful immunotherapy in life-threatening parvovirus B19 infection in a child. Pediatr Infect Dis J 32:789–792. doi: 10.1097/INF.0b013e31828df4d1. [DOI] [PubMed] [Google Scholar]
- 841.Orth T, Herr W, Spahn T, Voigtlander T, Michel D, Mertens T, Mayet WJ, Dippold W, Meyer zum Buschenfelde KH. 1997. Human parvovirus B19 infection associated with severe acute perimyocarditis in a 34-year-old man. Eur Heart J 18:524–525. doi: 10.1093/oxfordjournals.eurheartj.a015275. [DOI] [PubMed] [Google Scholar]
- 842.Bultmann BD, Klingel K, Sotlar K, Bock CT, Baba HA, Sauter M, Kandolf R. 2003. Fatal parvovirus B19-associated myocarditis clinically mimicking ischemic heart disease: an endothelial cell-mediated disease. Hum Pathol 34:92–95. doi: 10.1053/hupa.2003.48. [DOI] [PubMed] [Google Scholar]
- 843.Heegaard ED, Eiskjaer H, Baandrup U, Hornsleth A. 1998. Parvovirus B19 infection associated with myocarditis following adult cardiac transplantation. Scand J Infect Dis 30:607–610. doi: 10.1080/00365549850161188. [DOI] [PubMed] [Google Scholar]
- 844.Lamparter S, Schoppet M, Pankuweit S, Maisch B. 2003. Acute parvovirus B19 infection associated with myocarditis in an immunocompetent adult. Hum Pathol 34:725–728. doi: 10.1016/S0046-8177(03)00235-1. [DOI] [PubMed] [Google Scholar]
- 845.Bal A, Mishra B, Singh N, Das A, Jindal SK. 2009. Fulminant parvovirus B19-associated pancarditis with haemophagocytic lympho-histiocytosis in an immunocompetent adult. APMIS 117:773–777. doi: 10.1111/j.1600-0463.2009.02528.x. [DOI] [PubMed] [Google Scholar]
- 846.Niccoli G, Severino A, Pieroni M, Cosentino N, Ventrone MA, Conte M, Roberto M, Gallinella G, Liuzzo G, Leone AM, Porto I, Burzotta F, Trani C, Crea F. 2014. Parvovirus B19 at the culprit coronary stenosis predicts outcome after stenting. Eur J Clin Invest 44:209–218. doi: 10.1111/eci.12223. [DOI] [PubMed] [Google Scholar]
- 847.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. 2006. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 114:1581–1590. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 848.Gagliardi MG, Fierabracci A, Pilati M, Chinali M, Bassano C, Saura F, Giovannoni I, Francalanci P. 2016. The impact of specific viruses on clinical outcome in children presenting with acute heart failure. Int J Mol Sci 17:486. doi: 10.3390/ijms17040486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 849.Chen DY, Chen YM, Tzang BS, Lan JL, Hsu TC. 2014. Th17-related cytokines in systemic lupus erythematosus patients with dilated cardiomyopathies: a possible linkage to parvovirus B19 infection. PLoS One 9:e113889. doi: 10.1371/journal.pone.0113889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 850.Kuethe F, Lindner J, Matschke K, Wenzel JJ, Norja P, Ploetze K, Schaal S, Kamvissi V, Bornstein SR, Schwanebeck U, Modrow S. 2009. Prevalence of parvovirus B19 and human bocavirus DNA in the heart of patients with no evidence of dilated cardiomyopathy or myocarditis. Clin Infect Dis 49:1660–1666. doi: 10.1086/648074. [DOI] [PubMed] [Google Scholar]
- 851.Pankuweit S, Stein A, Karatolios K, Richter A, Ruppert V, Maisch B. 2013. Viral genomes in the pericardial fluid and in peri- and epicardial biopsies from a German cohort of patients with large to moderate pericardial effusions. Heart Fail Rev 18:329–336. doi: 10.1007/s10741-013-9375-x. [DOI] [PubMed] [Google Scholar]
- 852.Aravindh R, Saikia UN, Mishra B, Kumari V, Sarkar S, Sharma M, Ratho RK, Joshi K. 2014. Persistence of human parvovirus B19 in tissues from adult individuals: a comparison with serostatus and its clinical utility. Arch Virol 159:2371–2376. doi: 10.1007/s00705-014-2065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 853.Pankuweit S, Moll R, Baandrup U, Portig I, Hufnagel G, Maisch B. 2003. Prevalence of the parvovirus B19 genome in endomyocardial biopsy specimens. Hum Pathol 34:497–503. doi: 10.1016/S0046-8177(03)00078-9. [DOI] [PubMed] [Google Scholar]
- 854.Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss HP, McCarthy R, Hare J, Bricker JT, Bowles KR, Towbin JA. 2003. Detection of viruses in myocardial tissues by polymerase chain reaction. Evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol 42:466–472. doi: 10.1016/S0735-1097(03)00648-X. [DOI] [PubMed] [Google Scholar]
- 855.Schowengerdt KO, Ni J, Denfield SW, Gajarski RJ, Radovancevic B, Frazier HO, Demmler GJ, Kearney D, Bricker JT, Towbin JA. 1996. Diagnosis, surveillance, and epidemiologic evaluation of viral infections in pediatric cardiac transplant recipients with the use of the polymerase chain reaction. J Heart Lung Transplant 15:111–123. [PubMed] [Google Scholar]
- 856.Nguyen Y, Renois F, Leveque N, Giusti D, Picard-Maureau M, Bruneval P, Fornes P, Andreoletti L. 2013. Virus detection and semiquantitation in explanted heart tissues of idiopathic dilated cardiomyopathy adult patients by use of PCR coupled with mass spectrometry analysis. J Clin Microbiol 51:2288–2294. doi: 10.1128/JCM.00820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 857.Bock CT, Duchting A, Utta F, Brunner E, Sy BT, Klingel K, Lang F, Gawaz M, Felix SB, Kandolf R. 2014. Molecular phenotypes of human parvovirus B19 in patients with myocarditis. World J Cardiol 6:183–195. doi: 10.4330/wjc.v6.i4.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 858.Kuhl U, Lassner D, Dorner A, Rohde M, Escher F, Seeberg B, Hertel E, Tschope C, Skurk C, Gross UM, Schultheiss HP, Poller W. 2013. A distinct subgroup of cardiomyopathy patients characterized by transcriptionally active cardiotropic erythrovirus and altered cardiac gene expression. Basic Res Cardiol 108:372. doi: 10.1007/s00395-013-0372-y. [DOI] [PubMed] [Google Scholar]
- 859.Jain P, Jain A, Khan DN, Kumar M. 5 August 2013. Human parvovirus B19 associated dilated cardiomyopathy. BMJ Case Rep doi: 10.1136/bcr-2013-010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 860.Escher F, Kuhl U, Sabi T, Suckau L, Lassner D, Poller W, Schultheiss HP, Noutsias M. 2008. Immunohistological detection of parvovirus B19 capsid proteins in endomyocardial biopsies from dilated cardiomyopathy patients. Med Sci Monit 14:CR333–CR338. [PubMed] [Google Scholar]
- 861.Greulich S, Kindermann I, Schumm J, Perne A, Birkmeier S, Grun S, Ong P, Schaufele T, Klingel K, Schneider S, Kandolf R, Bohm M, Sechtem U, Mahrholdt H. 2016. Predictors of outcome in patients with parvovirus B19 positive endomyocardial biopsy. Clin Res Cardiol 105:37–52. doi: 10.1007/s00392-015-0884-6. [DOI] [PubMed] [Google Scholar]
- 862.Streitz M, Noutsias M, Volkmer R, Rohde M, Brestrich G, Block A, Klippert K, Kotsch K, Ay B, Hummel M, Kuhl U, Lassner D, Schultheiss HP, Volk HD, Kern F. 2008. NS1 specific CD8+ T-cells with effector function and TRBV11 dominance in a patient with parvovirus B19 associated inflammatory cardiomyopathy. PLoS One 3:e2361. doi: 10.1371/journal.pone.0002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 863.Tavora F, Gonzalez-Cuyar LF, Dalal JS, O'Malley MT, Zhao R, Peng HQ, Burke AP. 2008. Fatal parvoviral myocarditis: a case report and review of literature. Diagn Pathol 3:21–23. doi: 10.1186/1746-1596-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 864.Kuhl U, Pauschinger M, Schwimmbeck PL, Seeberg B, Lober C, Noutsias M, Poller W, Schultheiss HP. 2003. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation 107:2793–2798. doi: 10.1161/01.CIR.0000072766.67150.51. [DOI] [PubMed] [Google Scholar]
- 865.Bihari C, Rastogi A, Saxena P, Rangegowda D, Chowdhury A, Gupta N, Sarin SK. 2013. Parvovirus b19 associated hepatitis. Hepat Res Treat 2013:472027. doi: 10.1155/2013/472027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 866.Lee PC, Hung CJ, Lin YJ, Wang JR, Jan MS, Lei HY. 2002. A role for chronic parvovirus B19 infection in liver dysfunction in renal transplant recipients? Transplantation 73:1635–1639. doi: 10.1097/00007890-200205270-00019. [DOI] [PubMed] [Google Scholar]
- 867.Diaz F, Collazos J. 2000. Hepatic dysfunction due to parvovirus B19 infection. J Infect Chemother 6:63–64. doi: 10.1007/s101560050052. [DOI] [PubMed] [Google Scholar]
- 868.Langnas AN, Markin RS, Cattral MS, Naides SJ. 1995. Parvovirus B19 as a possible causative agent of fulminant liver failure and associated aplastic anemia. Hepatology 22:1661–1665. doi: 10.1016/0270-9139(95)90188-4. [DOI] [PubMed] [Google Scholar]
- 869.Wiggers H, Rasmussen LH, Moller A. 1995. Parvovirus B19 infection as the cause of hepatitis and neutrophil granulocytosis in a 20-year old woman. Ugeskr Laeger 157:5994–5995. (In Danish.) [PubMed] [Google Scholar]
- 870.Bousvaros A, Sundel R, Thorne GM, McIntosh K, Cohen M, Erdman DD, Perez-Atayde A, Finkel TH, Colin AA. 1998. Parvovirus B19-associated interstitial lung disease, hepatitis, and myositis. Pediatr Pulmonol 26:365–369. [DOI] [PubMed] [Google Scholar]
- 871.Bernuau J, Durand F, Valla D. 1999. Parvovirus B19 infection and fulminant hepatitis. Lancet 353:754–755. doi: 10.1016/S0140-6736(05)76124-9. [DOI] [PubMed] [Google Scholar]
- 872.Hillingso JG, Jensen IP, Tom-Petersen L. 1998. Parvovirus B19 and acute hepatitis in adults. Lancet 351:955–956. doi: 10.1016/S0140-6736(05)60609-5. [DOI] [PubMed] [Google Scholar]
- 873.Pinho JR, Alves VA, Vieira AF, Moralez MO, Fonseca LE, Guz B, Wakamatsu A, Cancado EL, Carrilho FJ, da Silva LC, Bernardini AP, Durigon EL. 2001. Detection of human parvovirus B19 in a patient with hepatitis. Braz J Med Biol Res 34:1131–1138. [DOI] [PubMed] [Google Scholar]
- 874.Alliot C, Barrios M, Taib J, Brunel M. 2001. Parovirus B19 infection in an HIV-infected patient with febrile pancytopenia and acute hepatitis. Eur J Clin Microbiol Infect Dis 20:43–45. doi: 10.1007/s100960000418. [DOI] [PubMed] [Google Scholar]
- 875.Koliou M, Karaoli E, Soteriades ES, Pavlides S, Bashiardes S, Christodoulou C. 2014. Acute hepatitis and myositis associated with erythema infectiosum by parvovirus B19 in an adolescent. BMC Pediatr 14:6. doi: 10.1186/1471-2431-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 876.Bihari C, Rastogi A, Rangegowda D, Chowdhury A, Saxena P, Garg H, Sarin SK. 2014. Parvovirus B19 associated acute hepatitis and hepatosplenomegaly. Clin Res Hepatol Gastroenterol 38:e9–e10. doi: 10.1016/j.clinre.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 877.Sokal EM, Melchior M, Cornu C, Vandenbroucke AT, Buts JP, Cohen BJ, Burtonboy G. 1998. Acute parvovirus B19 infection associated with fulminant hepatitis of favourable prognosis in young children. Lancet 352:1739–1741. doi: 10.1016/S0140-6736(98)06165-0. [DOI] [PubMed] [Google Scholar]
- 878.Ho JK, Tha SP, Coupland R, Dalal BI, Bowie WR, Sreenivasan GM, Krajden M, Yoshida EM. 2005. Parvovirus B19 in an immunocompetent adult patient with acute liver failure: an underdiagnosed cause of acute non-A-E viral hepatitis. Can J Gastroenterol 19:161–162. doi: 10.1155/2005/853947. [DOI] [PubMed] [Google Scholar]
- 879.Huang RJ, Varr BC, Triadafilopoulos G. 2012. Acute fulminant hepatic failure associated with parvovirus B19 infection in an immunocompetent adult. Dig Dis Sci 57:2811–2813. doi: 10.1007/s10620-012-2110-y. [DOI] [PubMed] [Google Scholar]
- 880.Shan YS, Lee PC, Wang JR, Tsai HP, Sung CM, Jin YT. 2001. Fibrosing cholestatic hepatitis possibly related to persistent parvovirus B19 infection in a renal transplant recipient. Nephrol Dial Transplant 16:2420–2422. doi: 10.1093/ndt/16.12.2420. [DOI] [PubMed] [Google Scholar]
- 881.Mogensen TH, Jensen JM, Hamilton-Dutoit S, Larsen CS. 2010. Chronic hepatitis caused by persistent parvovirus B19 infection. BMC Infect Dis 10:246. doi: 10.1186/1471-2334-10-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 882.Hatakka A, Klein J, He R, Piper J, Tam E, Walkty A. 2011. Acute hepatitis as a manifestation of parvovirus B19 infection. J Clin Microbiol 49:3422–3424. doi: 10.1128/JCM.00575-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 883.Naides SJ, Karetnyi YV, Cooling LL, Mark RS, Langnas AN. 1996. Human parvovirus B19 infection and hepatitis. Lancet 347:1563–1564. doi: 10.1016/S0140-6736(96)90720-5. [DOI] [PubMed] [Google Scholar]
- 884.Wang C, Heim A, Schlaphoff V, Suneetha PV, Stegmann KA, Jiang H, Krueger M, Fytili P, Schulz T, Cornberg M, Kandolf R, Manns MP, Bock CT, Wedemeyer H. 2009. Intrahepatic long-term persistence of parvovirus B19 and its role in chronic viral hepatitis. J Med Virol 81:2079–2088. doi: 10.1002/jmv.21638. [DOI] [PubMed] [Google Scholar]
- 885.He Z, Zhuang H, Wang X, Song S, Dong Q, Yan J, Buehring GC, Luo G. 2003. Retrospective analysis of non-A-E hepatitis: possible role of hepatitis B and C virus infection. J Med Virol 69:59–65. doi: 10.1002/jmv.10248. [DOI] [PubMed] [Google Scholar]
- 886.Lee WM, Brown KE, Young NS, Dawson GJ, Schlauder GG, Gutierrez RA, Fontana R, Rossaro L, Davern T, Lalani E. 2006. Brief report: no evidence for parvovirus B19 or hepatitis E virus as a cause of acute liver failure. Dig Dis Sci 51:1712–1715. doi: 10.1007/s10620-005-9061-5. [DOI] [PubMed] [Google Scholar]
- 887.Notari EP, Orton SL, Cable RG, Grindon AJ, Lenes BA, Williams AE, McMillan KM, Trouern-Trend JJ, Wolf-Nugent JS, Xu YL, Dodd RY. 2001. Seroprevalence of known and putative hepatitis markers in United States blood donors with ALT levels at least 120 IU per L. Transfusion 41:751–755. doi: 10.1046/j.1537-2995.2001.41060751.x. [DOI] [PubMed] [Google Scholar]
- 888.Arista S, De Grazia S, Di Marco V, Di Stefano R, Craxi A. 2003. Parvovirus B19 and “cryptogenic” chronic hepatitis. J Hepatol 38:375–376. doi: 10.1016/s0168-8278(02)00416-6. [DOI] [PubMed] [Google Scholar]
- 889.Simpson RW, McGinty L, Simon L, Smith CA, Godzeski CW, Boyd RJ. 1984. Association of parvoviruses with rheumatoid arthritis of humans. Science 223:1425–1428. doi: 10.1126/science.6701529. [DOI] [PubMed] [Google Scholar]
- 890.Barash J, Dushnitzki D, Barak Y, Miron S, Hahn T. 2003. Tumor necrosis factor (TNF)alpha and its soluble receptor (sTNFR) p75 during acute human parvovirus B19 infection in children. Immunol Lett 88:109–112. doi: 10.1016/S0165-2478(03)00075-0. [DOI] [PubMed] [Google Scholar]
- 891.Pavlovic M, Kats A, Cavallo M, Shoenfeld Y. 2010. Clinical and molecular evidence for association of SLE with parvovirus B19. Lupus 19:783–792. doi: 10.1177/0961203310365715. [DOI] [PubMed] [Google Scholar]
- 892.Lunardi C, Tinazzi E, Bason C, Dolcino M, Corrocher R, Puccetti A. 2008. Human parvovirus B19 infection and autoimmunity. Autoimmun Rev 8:116–120. doi: 10.1016/j.autrev.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 893.Page C, Francois C, Goeb V, Duverlie G. 2015. Human parvovirus B19 and autoimmune diseases. Review of the literature and pathophysiological hypotheses. J Clin Virol 72:69–74. doi: 10.1016/j.jcv.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 894.Takahashi Y, Murai C, Shibata S, Munakata Y, Ishii T, Ishii K, Saitoh T, Sawai T, Sugamura K, Sasaki T. 1998. Human parvovirus B19 as a causative agent for rheumatoid arthritis. Proc Natl Acad Sci U S A 95:8227–8232. doi: 10.1073/pnas.95.14.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 895.Jobanputra P, Davidson F, Graham S, O'Neill H, Simmonds P, Yap PL. 1995. High frequency of parvovirus B19 in patients tested for rheumatoid factor. BMJ 311:1542. doi: 10.1136/bmj.311.7019.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 896.Murai C, Munakata Y, Takahashi Y, Ishii T, Shibata S, Muryoi T, Funato T, Nakamura M, Sugamura K, Sasaki T. 1999. Rheumatoid arthritis after human parvovirus B19 infection. Ann Rheum Dis 58:130–132. doi: 10.1136/ard.58.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 897.Chua PK, Nerurkar VR, Yu Q, Woodward CL, Melish ME, Yanagihara R. 2000. Lack of association between Kawasaki syndrome and infection with parvovirus B19, human herpesvirus 8, TT virus, GB virus C/hepatitis G virus or Chlamydia pneumoniae. Pediatr Infect Dis J 19:477–479. doi: 10.1097/00006454-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 898.Chen YS, Chou PH, Li SN, Tsai WC, Lin KH, Tsai KB, Yen JH, Liu HW. 2006. Parvovirus B19 infection in patients with rheumatoid arthritis in Taiwan. J Rheumatol 33:887–891. [PubMed] [Google Scholar]
- 899.Kerr JR, Cartron JP, Curran MD, Moore JE, Elliott JR, Mollan RA. 1995. A study of the role of parvovirus B19 in rheumatoid arthritis. Br J Rheumatol 34:809–813. doi: 10.1093/rheumatology/34.9.809. [DOI] [PubMed] [Google Scholar]
- 900.Hajeer AH, MacGregor AJ, Rigby AS, Ollier WE, Carthy D, Silman AJ. 1994. Influence of previous exposure to human parvovirus B19 infection in explaining susceptibility to rheumatoid arthritis: an analysis of disease discordant twin pairs. Ann Rheum Dis 53:137–139. doi: 10.1136/ard.53.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 901.Harrison B, Silman A, Barrett E, Symmons D. 1998. Low frequency of recent parvovirus infection in a population-based cohort of patients with early inflammatory polyarthritis. Ann Rheum Dis 57:375–377. doi: 10.1136/ard.57.6.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 902.Longo G, Luppi M, Bertesi M, Ferrara L, Torelli G, Emilia G. 1998. Still's disease, severe thrombocytopenia, and acute hepatitis associated with acute parvovirus B19 infection. Clin Infect Dis 26:994–995. doi: 10.1086/517644. [DOI] [PubMed] [Google Scholar]
- 903.Kishore J, Misra R, Gupta D, Ayyagari A. 1998. Raised IgM antibodies to parvovirus B19 in juvenile rheumatoid arthritis. Indian J Med Res 107:15–18. [PubMed] [Google Scholar]
- 904.Rodrigo de Liria C, Mendez M, Olive A, Junca J, Prats J, Tena X. 1996. Parvovirus infection mimicking a systematic onset of juvenile chronic arthritis (Still's disease). Clin Exp Rheumatol 14:105–106. [PubMed] [Google Scholar]
- 905.Blidi M, Gatfosse M, Barjonet G. 1996. Adult-onset Still disease associated with acute parvovirus B19 infection in pregnancy. Ann Med Interne (Paris) 147:518–519. (In French.) [PubMed] [Google Scholar]
- 906.Godeau B, Palazzo E, Morinet F, Deplanche M, Deforge L, Schaeffer A, Kahn MF. 1995. Is Still's disease associated with parvovirus B19 infection? Lancet 345:59–60. doi: 10.1016/S0140-6736(95)91182-0. [DOI] [PubMed] [Google Scholar]
- 907.Lehmann HW, Kuhner L, Beckenlehner K, Muller-Godeffroy E, Heide KG, Kuster RM, Modrow S. 2002. Chronic human parvovirus B19 infection in rheumatic disease of childhood and adolescence. J Clin Virol 25:135–143. doi: 10.1016/S1386-6532(01)00247-5. [DOI] [PubMed] [Google Scholar]
- 908.Kalish RA, Knopf AN, Gary GW, Canoso JJ. 1992. Lupus-like presentation of human parvovirus B19 infection. J Rheumatol 19:169–171. [PubMed] [Google Scholar]
- 909.Moore TL, Bandlamudi R, Alam SM, Nesher G. 1999. Parvovirus infection mimicking systemic lupus erythematosus in a pediatric population. Semin Arthritis Rheum 28:314–318. doi: 10.1016/S0049-0172(99)80015-8. [DOI] [PubMed] [Google Scholar]
- 910.Tanaka A, Sugawara A, Sawai K, Kuwahara T. 1998. Human parvovirus B19 infection resembling systemic lupus erythematosus. Intern Med 37:708–710. doi: 10.2169/internalmedicine.37.708. [DOI] [PubMed] [Google Scholar]
- 911.Trapani S, Ermini M, Falcini F. 1999. Human parvovirus B19 infection: its relationship with systemic lupus erythematosus. Semin Arthritis Rheum 28:319–325. doi: 10.1016/S0049-0172(99)80016-X. [DOI] [PubMed] [Google Scholar]
- 912.Cooray M, Manolakos JJ, Wright DS, Haider S, Patel A. 2013. Parvovirus infection mimicking systemic lupus erythematosus. CMAJ 185:1342–1344. doi: 10.1503/cmaj.121565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 913.Fawaz-Estrup F. 1996. Human parvovirus infection: rheumatic manifestations, angioedema, C1 esterase inhibitor deficiency, ANA positivity, and possible onset of systemic lupus erythematosus. J Rheumatol 23:1180–1185. [PubMed] [Google Scholar]
- 914.Cope AP, Jones A, Brozovic M, Shafi MS, Maini RN. 1992. Possible induction of systemic lupus erythematosus by human parvovirus. Ann Rheum Dis 51:803–804. doi: 10.1136/ard.51.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 915.Chassagne P, Mejjad O, Gourmelen O, Moore N, Le Loet X, Deshayes P. 1993. Exacerbation of systemic lupus erythematosus during human parvovirus B19 infection. Br J Rheumatol 32:158–159. doi: 10.1093/rheumatology/32.2.158. [DOI] [PubMed] [Google Scholar]
- 916.Meyer O. 2003. Parvovirus B19 and autoimmune diseases. Joint Bone Spine 70:6–11. doi: 10.1016/S1297-319X(02)00004-0. [DOI] [PubMed] [Google Scholar]
- 917.Aslanidis S, Pyrpasopoulou A, Kontotasios K, Doumas S, Zamboulis C. 2008. Parvovirus B19 infection and systemic lupus erythematosus: activation of an aberrant pathway? Eur J Intern Med 19:314–318. doi: 10.1016/j.ejim.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 918.Bengtsson A, Widell A, Elmstahl S, Sturfelt G. 2000. No serological indications that systemic lupus erythematosus is linked with exposure to human parvovirus B19. Ann Rheum Dis 59:64–66. doi: 10.1136/ard.59.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 919.Ferri C, Zakrzewska K, Longombardo G, Giuggioli D, Storino FA, Pasero G, Azzi A. 1999. Parvovirus B19 infection of bone marrow in systemic sclerosis patients. Clin Exp Rheumatol 17:718–720. [PubMed] [Google Scholar]
- 920.Ohtsuka T, Yamazaki S. 2004. Increased prevalence of human parvovirus B19 DNA in systemic sclerosis skin. Br J Dermatol 150:1091–1095. doi: 10.1111/j.0007-0963.2004.05930.x. [DOI] [PubMed] [Google Scholar]
- 921.Magro CM, Crowson AN, Dawood M, Nuovo GJ. 2002. Parvoviral infection of endothelial cells and its possible role in vasculitis and autoimmune diseases. J Rheumatol 29:1227–1235. [PubMed] [Google Scholar]
- 922.Zakrzewska K, Corcioli F, Carlsen KM, Giuggioli D, Fanci R, Rinieri A, Ferri C, Azzi A. 2009. Human parvovirus B19 (B19V) infection in systemic sclerosis patients. Intervirology 52:279–282. doi: 10.1159/000232945. [DOI] [PubMed] [Google Scholar]
- 923.Li Loong TC, Coyle PV, Anderson MJ, Allen GE, Connolly JH. 1986. Human serum parvovirus associated vasculitis. Postgrad Med J 62:493–494. doi: 10.1136/pgmj.62.728.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 924.Martinelli C, Azzi A, Buffini G, Comin CE, Leoncini F. 1997. Cutaneous vasculitis due to human parvovirus B19 in an HIV-infected patient: report of a case. AIDS 11:1891–1893. doi: 10.1097/00002030-199715000-00020. [DOI] [PubMed] [Google Scholar]
- 925.Engel F, Maradeix S, Braun-Parvez L, Lipsker D, Cribier B. 2007. Leukocytoclastic vasculitis with severe renal involvement following parvovirus B19 primary infection. Ann Dermatol Venereol 134:160–163 (In French.) doi: 10.1016/S0151-9638(07)91610-5. [DOI] [PubMed] [Google Scholar]
- 926.Dyrsen ME, Iwenofu OH, Nuovo G, Magro CM. 2008. Parvovirus B19-associated catastrophic endothelialitis with a Degos-like presentation. J Cutan Pathol 35(Suppl 1):20–25. doi: 10.1111/j.1600-0560.2007.00974.x. [DOI] [PubMed] [Google Scholar]
- 927.Durst R, Goldschmidt N, Ben YA. 2002. Parvovirus B19 infection associated with myelosuppression and cutaneous polyarteritis nodosa. Rheumatology (Oxford) 41:1210–1212. doi: 10.1093/rheumatology/41.10.1210. [DOI] [PubMed] [Google Scholar]
- 928.Dass R, Ramesh P, Ratho RK, Saxena AK, Singh S. 2005. Parvovirus B19-induced multisystem disease simulating systemic vasculitis in a young child. Rheumatol Int 25:125–129. doi: 10.1007/s00296-004-0465-x. [DOI] [PubMed] [Google Scholar]
- 929.Johnson LB, Pasumarthy A, Saravolatz LD. 2003. Parvovirus B19 infection presenting with necrotizing lymphadenitis. Am J Med 114:340–341. doi: 10.1016/S0002-9343(02)01496-1. [DOI] [PubMed] [Google Scholar]
- 930.Cooper CL, Choudhri SH. 1998. Photo quiz II. Leukocytoclastic vasculitis secondary to parvovirus B19 infection. Clin Infect Dis 26:849, 989. [DOI] [PubMed] [Google Scholar]
- 931.Schennach H, Mayersbach P, Schonitzer D, Fuchs D, Wachter H, Reibnegger G. 1994. Increased prevalence of IgM antibodies to Epstein-Barr virus and parvovirus B19 in blood donations with above-normal neopterin. Clin Chem 40:2104–2105. [PubMed] [Google Scholar]
- 932.Ferguson PJ, Saulsbury FT, Dowell SF, Torok TJ, Erdman DD, Anderson LJ. 1996. Prevalence of human parvovirus B19 infection in children with Henoch-Schonlein purpura. Arthritis Rheum 39:880–881. doi: 10.1002/art.1780390523. [DOI] [PubMed] [Google Scholar]
- 933.Veraldi S, Mancuso R, Rizzitelli G, Gianotti R, Ferrante P. 1999. Henoch-Schonlein syndrome associated with human parvovirus B19 primary infection. Eur J Dermatol 9:232–233. [PubMed] [Google Scholar]
- 934.Gabriel SE, Espy M, Erdman DD, Bjornsson J, Smith TF, Hunder GG. 1999. The role of parvovirus B19 in the pathogenesis of giant cell arteritis: a preliminary evaluation. Arthritis Rheum 42:1255–1258. doi:. [DOI] [PubMed] [Google Scholar]
- 935.Viguier M, Guillevin L, Laroche L. 2001. Treatment of parvovirus B19-associated polyarteritis nodosa with intravenous immune globulin. N Engl J Med 344:1481–1482. doi: 10.1056/NEJM200105103441919. [DOI] [PubMed] [Google Scholar]
- 936.Ohtsuka T, Yamazaki S. 2005. Prevalence of human parvovirus B19 component NS1 gene in patients with Henoch-Schonlein purpura and hypersensitivity vasculitis. Br J Dermatol 152:1080–1081. doi: 10.1111/j.1365-2133.2005.06566.x. [DOI] [PubMed] [Google Scholar]
- 937.Rowley AH, Wolinsky SM, Relman DA, Sambol SP, Sullivan J, Terai M, Shulman ST. 1994. Search for highly conserved viral and bacterial nucleic acid sequences corresponding to an etiologic agent of Kawasaki disease. Pediatr Res 36:567–571. doi: 10.1203/00006450-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 938.Crowson AN, Magro CM, Dawood MR. 2000. A causal role for parvovirus B19 infection in adult dermatomyositis and other autoimmune syndromes. J Cutan Pathol 27:505–515. doi: 10.1034/j.1600-0560.2000.027010505.x. [DOI] [PubMed] [Google Scholar]
- 939.Eden A, Mahr A, Servant A, Radjef N, Amard S, Mouthon L, Garbarg-Chenon A, Guillevin L. 2003. Lack of association between B19 or V9 erythrovirus infection and ANCA-positive vasculitides: a case-control study. Rheumatology (Oxford) 42:660–664. doi: 10.1093/rheumatology/keg206. [DOI] [PubMed] [Google Scholar]
- 940.Salvarani C, Farnetti E, Casali B, Nicoli D, Wenlan L, Bajocchi G, Macchioni P, Lo Scocco G, Grazia Catanoso M, Boiardi L, Cantini F. 2002. Detection of parvovirus B19 DNA by polymerase chain reaction in giant cell arteritis: a case-control study. Arthritis Rheum 46:3099–3101. doi: 10.1002/art.10580. [DOI] [PubMed] [Google Scholar]
- 941.Alvarez-Lafuente R, Fernandez-Gutierrez B, Jover JA, Judez E, Loza E, Clemente D, Garcia-Asenjo JA, Lamas JR. 2005. Human parvovirus B19, varicella zoster virus, and human herpes virus 6 in temporal artery biopsy specimens of patients with giant cell arteritis: analysis with quantitative real time polymerase chain reaction. Ann Rheum Dis 64:780–782. doi: 10.1136/ard.2004.025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 942.Waldman M, Kopp JB. 2007. Parvovirus B19 and the kidney. Clin J Am Soc Nephrol 2(Suppl 1):S47–S56. doi: 10.2215/CJN.01060307. [DOI] [PubMed] [Google Scholar]
- 943.Waldman M, Kopp JB. 2007. Parvovirus-B19-associated complications in renal transplant recipients. Nat Clin Pract Nephrol 3:540–550. doi: 10.1038/ncpneph0609. [DOI] [PubMed] [Google Scholar]
- 944.Tanawattanacharoen S, Falk RJ, Jennette JC, Kopp JB. 2000. Parvovirus B19 DNA in kidney tissue of patients with focal segmental glomerulosclerosis. Am J Kidney Dis 35:1166–1174. doi: 10.1016/S0272-6386(00)70055-2. [DOI] [PubMed] [Google Scholar]
- 945.Bleumink GS, Halma C, van Vliet AC, de Jong GM, van Bommel EF. 2000. Human parvovirus B19 and renal disease? Neth J Med 56:163–165. doi: 10.1016/S0300-2977(00)00006-1. [DOI] [PubMed] [Google Scholar]
- 946.Watanabe T. 2003. Renal involvement in human parvovirus B19 infection. Pediatr Nephrol 18:966–967. doi: 10.1007/s00467-003-1232-z. [DOI] [PubMed] [Google Scholar]
- 947.Ohtomo Y, Kawamura R, Kaneko K, Yamashiro Y, Kiyokawa N, Taguchi T, Mimori K, Fujimoto J. 2003. Nephrotic syndrome associated with human parvovirus B19 infection. Pediatr Nephrol 18:280–282. [DOI] [PubMed] [Google Scholar]
- 948.Murer L, Zacchello G, Bianchi D, Dall'Amico R, Montini G, Andreetta B, Perini M, Dossi EC, Zanon G, Zacchello F. 2000. Thrombotic microangiopathy associated with parvovirus B 19 infection after renal transplantation. J Am Soc Nephrol 11:1132–1137. [DOI] [PubMed] [Google Scholar]
- 949.Ardalan MR, Shoja MM, Tubbs RS, Esmaili H, Keyvani H. 2008. Postrenal transplant hemophagocytic lymphohistiocytosis and thrombotic microangiopathy associated with parvovirus b19 infection. Am J Transplant 8:1340–1344. doi: 10.1111/j.1600-6143.2008.02244.x. [DOI] [PubMed] [Google Scholar]
- 950.Matano S, Kinoshita H, Tanigawa K, Terahata S, Sugimoto T. 2003. Acute parvovirus B19 infection mimicking chronic fatigue syndrome. Intern Med 42:903–905. doi: 10.2169/internalmedicine.42.903. [DOI] [PubMed] [Google Scholar]
- 951.Jacobson SK, Daly JS, Thorne GM, McIntosh K. 1997. Chronic parvovirus B19 infection resulting in chronic fatigue syndrome: case history and review. Clin Infect Dis 24:1048–1051. doi: 10.1086/513627. [DOI] [PubMed] [Google Scholar]
- 952.Kerr JR, Bracewell J, Laing I, Mattey DL, Bernstein RM, Bruce IN, Tyrrell DA. 2002. Chronic fatigue syndrome and arthralgia following parvovirus B19 infection. J Rheumatol 29:595–602. [PubMed] [Google Scholar]
- 953.Kerr JR, Mattey DL. 2008. Preexisting psychological stress predicts acute and chronic fatigue and arthritis following symptomatic parvovirus B19 infection. Clin Infect Dis 46:e83–e87. doi: 10.1086/533471. [DOI] [PubMed] [Google Scholar]
- 954.Ilaria RL Jr, Komaroff AL, Fagioli LR, Moloney WC, True CA, Naides SJ. 1995. Absence of parvovirus B19 infection in chronic fatigue syndrome. Arthritis Rheum 38:638–641. doi: 10.1002/art.1780380510. [DOI] [PubMed] [Google Scholar]
- 955.Kerr JR, Cunniffe VS, Kelleher P, Bernstein RM, Bruce IN. 2003. Successful intravenous immunoglobulin therapy in 3 cases of parvovirus B19-associated chronic fatigue syndrome. Clin Infect Dis 36:e100–e106. doi: 10.1086/374666. [DOI] [PubMed] [Google Scholar]
- 956.Attard L, Bonvicini F, Gelsomino F, Manfredi R, Cascavilla A, Viale P, Varani S, Gallinella G. 2015. Paradoxical response to intravenous immunoglobulin in a case of parvovirus B19-associated chronic fatigue syndrome. J Clin Virol 62:54–57. doi: 10.1016/j.jcv.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 957.Leventhal LJ, Naides SJ, Freundlich B. 1991. Fibromyalgia and parvovirus infection. Arthritis Rheum 34:1319–1324. doi: 10.1002/art.1780341018. [DOI] [PubMed] [Google Scholar]
- 958.Nikkari S, Lappalainen H, Saario R, Lammintausta K, Kotilainen P. 1996. Detection of parvovirus B19 in skin biopsy, serum, and bone marrow of a patient with fever, rash, and polyarthritis followed by pneumonia, pericardial effusion, and hepatitis. Eur J Clin Microbiol Infect Dis 15:954–957. doi: 10.1007/BF01690517. [DOI] [PubMed] [Google Scholar]
- 959.Morris CN, Smilack JD. 1998. Parvovirus B19 infection associated with respiratory distress. Clin Infect Dis 27:900–901. doi: 10.1086/517164. [DOI] [PubMed] [Google Scholar]
- 960.Wardeh A, Marik P. 1998. Acute lung injury due to parvovirus pneumonia. J Intern Med 244:257–260. doi: 10.1046/j.1365-2796.1998.00364.x. [DOI] [PubMed] [Google Scholar]
- 961.Wilson ML. 2015. Decreasing inappropriate laboratory test utilization: controlling costs and improving quality of care. Am J Clin Pathol 143:614–616. doi: 10.1309/AJCPHQODM9XYWLZ9. [DOI] [PubMed] [Google Scholar]
- 962.Beske F, Modrow S, Sorensen J, Schmidt H, Kriener S, Allwinn R, Klingebiel T, Schwabe D, Lehrnbecher T. 2007. Parvovirus B19 pneumonia in a child undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 40:89–91. doi: 10.1038/sj.bmt.1705693. [DOI] [PubMed] [Google Scholar]
- 963.Sotto A, Bessis D, Jourdan J. 1997. Gloves and socks edema disclosing parvovirus B19 infection. Presse Med 26:421 (In French.) [PubMed] [Google Scholar]
- 964.Nakazawa T, Machi T, Kitagawa S, Miyamori H, Saitoh Y. 1995. Parvovirus infection and generalized edema in adults. Intern Med 34:163–165. doi: 10.2169/internalmedicine.34.163. [DOI] [PubMed] [Google Scholar]
- 965.Wiggli B, Imhof E, Meier CA, Laifer G. 2013. Water, water, everywhere. Acute parvovirus B19 infection. Lancet 381:776. doi: 10.1016/S0140-6736(12)61894-7. [DOI] [PubMed] [Google Scholar]
- 966.Vlaar PJ, Mithoe G, Janssen WM. 2014. Generalized edema associated with parvovirus B19 infection. Int J Infect Dis 29:40–41. doi: 10.1016/j.ijid.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 967.Oliver ND, Millar A, Pendleton A. 10 December 2012. A case report on parvovirus b19 associated myositis. Case Rep Rheumatol doi: 10.1155/2012/250537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 968.Kasuga A, Harada R, Saruta T. 1996. Insulin-dependent diabetes mellitus associated with parvovirus B19 infection. Ann Intern Med 125:700–701. doi: 10.7326/0003-4819-125-8-199610150-00030. [DOI] [PubMed] [Google Scholar]
- 969.Pironi L, Bonvicini F, Gionchetti P, D'Errico A, Rizzello F, Corsini C, Foroni L, Gallinella G. 2009. Parvovirus b19 infection localized in the intestinal mucosa and associated with severe inflammatory bowel disease. J Clin Microbiol 47:1591–1595. doi: 10.1128/JCM.00706-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 970.Maini R, Edelsten C. 1999. Uveitis associated with parvovirus infection. Br J Ophthalmol 83:1403–1404. doi: 10.1136/bjo.83.12.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 971.Suzuki J, Goto H, Usui M, Sakai J. 2007. Serous retinal detachment in a patient with aplastic anemia associated with parvovirus B19 infection. Graefes Arch Clin Exp Ophthalmol 245:324–326. doi: 10.1007/s00417-006-0315-5. [DOI] [PubMed] [Google Scholar]
- 972.Grand MG, Storch GA. 2000. Presumed parvovirus B19-associated retinal pigment epitheliopathy. Retina 20:199–202. doi: 10.1097/00006982-200002000-00015. [DOI] [PubMed] [Google Scholar]
- 973.Mehraein Y, Wagner M, Remberger K, Fuzesi L, Middel P, Kaptur S, Schmitt K, Meese E. 2006. Parvovirus B19 detected in Rosai-Dorfman disease in nodal and extranodal manifestations. J Clin Pathol 59:1320–1326. doi: 10.1136/jcp.2005.029850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 974.Ozbek OY, Onay OS, Kinik ST, Ozbek N. 2007. Laryngitis and neutropenia from parvovirus-B19. Indian J Pediatr 74:950–952. doi: 10.1007/s12098-007-0176-x. [DOI] [PubMed] [Google Scholar]
- 975.Cotter CS, Singleton GT, Corman LC. 1994. Immune-mediated inner ear disease and parvovirus B19. Laryngoscope 104:1235–1239. [DOI] [PubMed] [Google Scholar]
- 976.Diss TC, Pan LX, Du MQ, Peng HZ, Kerr JR. 1999. Parvovirus B19 is associated with benign testes as well as testicular germ cell tumours. Mol Pathol 52:349–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 977.Page C, Duverlie G, Sevestre H, Desailloud R. 2015. Erythrovirus B19 and autoimmune thyroid diseases. Review of the literature and pathophysiological hypotheses. J Med Virol 87:162–169. doi: 10.1002/jmv.23963. [DOI] [PubMed] [Google Scholar]
- 978.Bastien N, Brandt K, Dust K, Ward D, Li Y. 2006. Human bocavirus infection, Canada. Emerg Infect Dis 12:848–850. doi: 10.3201/eid1205.051424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 979.Choi EH, Lee HJ, Kim SJ, Eun BW, Kim NH, Lee JA, Lee JH, Song EK, Kim SH, Park JY, Sung JY. 2006. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000-2005. Clin Infect Dis 43:585–592. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 980.Foulongne V, Olejnik Y, Perez V, Elaerts S, Rodiere M, Segondy M. 2006. Human bocavirus in French children. Emerg Infect Dis 12:1251–1253. doi: 10.3201/eid1708.060213.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 981.Weissbrich B, Neske F, Schubert J, Tollmann F, Blath K, Blessing K, Kreth HW. 2006. Frequent detection of bocavirus DNA in German children with respiratory tract infections. BMC Infect Dis 6:109. doi: 10.1186/1471-2334-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 982.Lau SK, Yip CC, Que TL, Lee RA, Au-Yeung RK, Zhou B, So LY, Lau YL, Chan KH, Woo PC, Yuen KY. 2007. Clinical and molecular epidemiology of human bocavirus in respiratory and fecal samples from children in Hong Kong. J Infect Dis 196:986–993. doi: 10.1086/521310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 983.Ma X, Endo R, Ishiguro N, Ebihara T, Ishiko H, Ariga T, Kikuta H. 2006. Detection of human bocavirus in Japanese children with lower respiratory tract infections. J Clin Microbiol 44:1132–1134. doi: 10.1128/JCM.44.3.1132-1134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 984.Vicente D, Cilla G, Montes M, Perez-Yarza EG, Perez-Trallero E. 2007. Human bocavirus, a respiratory and enteric virus. Emerg Infect Dis 13:636–637. doi: 10.3201/eid1304.061501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 985.Volz S, Schildgen O, Klinkenberg D, Ditt V, Muller A, Tillmann RL, Kupfer B, Bode U, Lentze MJ, Simon A. 2007. Prospective study of human bocavirus (HBoV) infection in a pediatric university hospital in Germany 2005/2006. J Clin Virol 40:229–235. doi: 10.1016/j.jcv.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 986.Esposito S, Bosis S, Niesters HG, Tremolati E, Sabatini C, Porta A, Fossali E, Osterhaus AD, Principi N. 2008. Impact of human bocavirus on children and their families. J Clin Microbiol 46:1337–1342. doi: 10.1128/JCM.02160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 987.Chieochansin T, Samransamruajkit R, Chutinimitkul S, Payungporn S, Hiranras T, Theamboonlers A, Poovorawan Y. 2008. Human bocavirus (HBoV) in Thailand: clinical manifestations in a hospitalized pediatric patient and molecular virus characterization. J Infect 56:137–142. doi: 10.1016/j.jinf.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 988.Regamey N, Frey U, Deffernez C, Latzin P, Kaiser L. 2007. Isolation of human bocavirus from Swiss infants with respiratory infections. Pediatr Infect Dis J 26:177–179. doi: 10.1097/01.inf.0000250623.43107.bc. [DOI] [PubMed] [Google Scholar]
- 989.Margaret IP, Nelson EA, Cheuk ES, Leung E, Sung R, Chan PK. 2008. Pediatric hospitalization of acute respiratory tract infections with human bocavirus in Hong Kong. J Clin Virol 42:72–74. doi: 10.1016/j.jcv.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 990.Calvo C, Garcia-Garcia ML, Pozo F, Carvajal O, Perez-Brena P, Casas I. 2008. Clinical characteristics of human bocavirus infections compared with other respiratory viruses in Spanish children. Pediatr Infect Dis J 27:677–680. doi: 10.1097/INF.0b013e31816be052. [DOI] [PubMed] [Google Scholar]
- 991.Garcia-Garcia ML, Calvo C, Pozo F, Perez-Brena P, Quevedo S, Bracamonte T, Casas I. 2008. Human bocavirus detection in nasopharyngeal aspirates of children without clinical symptoms of respiratory infection. Pediatr Infect Dis J 27:358–360. doi: 10.1097/INF.0b013e3181626d2a. [DOI] [PubMed] [Google Scholar]
- 992.Pierangeli A, Scagnolari C, Trombetti S, Grossi R, Battaglia M, Moretti C, Midulla F, Antonelli G. 2008. Human bocavirus infection in hospitalized children in Italy. Influenza Other Respir Viruses 2:175–179. doi: 10.1111/j.1750-2659.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 993.Dina J, Vabret A, Gouarin S, Petitjean J, Lecoq J, Brouard J, Arion A, Lafay-Delaire F, Freymuth F. 2009. Detection of human bocavirus in hospitalised children. J Paediatr Child Health 45:149–153. doi: 10.1111/j.1440-1754.2008.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 994.Yoshida LM, Suzuki M, Yamamoto T, Nguyen HA, Nguyen CD, Nguyen AT, Oishi K, Vu TD, Le TH, Le MQ, Yanai H, Kilgore PE, Dang DA, Ariyoshi K. 2010. Viral pathogens associated with acute respiratory infections in central Vietnamese children. Pediatr Infect Dis J 29:75–77. doi: 10.1097/INF.0b013e3181af61e9. [DOI] [PubMed] [Google Scholar]
- 995.Calvo C, Pozo F, Garcia-Garcia ML, Sanchez M, Lopez-Valero M, Perez-Brena P, Casas I. 2010. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three-year prospective study. Acta Paediatr 99:883–887. doi: 10.1111/j.1651-2227.2010.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 996.Miron D, Srugo I, Kra-Oz Z, Keness Y, Wolf D, Amirav I, Kassis I. 2010. Sole pathogen in acute bronchiolitis: is there a role for other organisms apart from respiratory syncytial virus? Pediatr Infect Dis J 29:e7–e10. doi: 10.1097/INF.0b013e3181c2a212. [DOI] [PubMed] [Google Scholar]
- 997.Proenca-Modena JL, Gagliardi TB, de Paula FE, Iwamoto MA, Criado MF, Camara AA, Acrani GO, Cintra OA, Cervi MC, de Paula Arruda LK, Arruda E. 2011. Detection of human bocavirus mRNA in respiratory secretions correlates with high viral load and concurrent diarrhea. PLoS One 6:e21083. doi: 10.1371/journal.pone.0021083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 998.Lindner J, Karalar L, Zehentmeier S, Plentz A, Pfister H, Struff W, Kertai M, Segerer H, Modrow S. 2008. Humoral immune response against human bocavirus VP2 virus-like particles. Viral Immunol 21:443–449. doi: 10.1089/vim.2008.0045. [DOI] [PubMed] [Google Scholar]
- 999.Zhao B, Yu X, Wang C, Teng Z, Wang C, Shen J, Gao Y, Zhu Z, Wang J, Yuan Z, Wu F, Zhang X, Ghildyal R. 2013. High human bocavirus viral load is associated with disease severity in children under five years of age. PLoS One 8:e62318. doi: 10.1371/journal.pone.0062318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1000.Bruning AHL, Susi P, Toivola H, Christensen A, Söderlund-Venermo M, Hedman K, Aatola H, Zvirbliene A, Koskinen JO. 2016. Detection and monitoring of human bocavirus 1 infection by a new rapid antigen test. New Microbes New Infect 11:17–19. doi: 10.1016/j.nmni.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1001.Christensen A, Døllner H, Shanke LH, Krokstad S, Moe N, Nordbø SA. 2013. Detection of spliced mRNA from human bocavirus 1 in clinical samples from children with respiratory tract infections. Emerg Infect Dis 19:574–580. doi: 10.3201/eid1904.121775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1002.Lu X, Chittaganpitch M, Olsen SJ, Mackay IM, Sloots TP, Fry AM, Erdman DD. 2006. Real-time PCR assays for detection of bocavirus in human specimens. J Clin Microbiol 44:3231–3235. doi: 10.1128/JCM.00889-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1003.Monteny M, Niesters HG, Moll HA, Berger MY. 2007. Human bocavirus in febrile children, The Netherlands. Emerg Infect Dis 13:180–182. doi: 10.3201/eid1301.060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1004.Christensen A, Nordbo SA, Krokstad S, Rognlien AG, Dollner H. 2008. Human bocavirus commonly involved in multiple viral airway infections. J Clin Virol 41:34–37. doi: 10.1016/j.jcv.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1005.Schildgen O, Muller A, Allander T, Mackay IM, Volz S, Kupfer B, Simon A. 2008. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev 21:291–304. doi: 10.1128/CMR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1006.Don M, Söderlund-Venermo M, Valent F, Lahtinen A, Hedman L, Canciani M, Hedman K, Korppi M. 2010. Serologically verified human bocavirus pneumonia in children. Pediatr Pulmonol 45:120–126. doi: 10.1002/ppul.21151. [DOI] [PubMed] [Google Scholar]
- 1007.Ursic T, Steyer A, Kopriva S, Kalan G, Krivec U, Petrovec M. 2011. Human bocavirus as the cause of a life-threatening infection. J Clin Microbiol 49:1179–1181. doi: 10.1128/JCM.02362-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1008.Korner RW, Soderlund-Venermo M, van Koningsbruggen-Rietschel S, Kaiser R, Malecki M, Schildgen O. 2011. Severe human bocavirus infection, Germany. Emerg Infect Dis 17:2303–2305. doi: 10.3201/eid1712.110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1009.Edner N, Castillo-Rodas P, Falk L, Hedman K, Soderlund-Venermo M, Allander T. 2012. Life-threatening respiratory tract disease with human bocavirus-1 infection in a 4-year-old child. J Clin Microbiol 50:531–532. doi: 10.1128/JCM.05706-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1010.Moesker FM, van Kampen JJ, van der Eijk AA, van Rossum AM, de Hoog M, Schutten M, Smits SL, Bodewes R, Osterhaus AD, Fraaij PL. 2015. Human bocavirus infection as a cause of severe acute respiratory tract infection in children. Clin Microbiol Infect 21:964.e1–964.e8. doi: 10.1016/j.cmi.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1011.Schildgen O. 2010. Human bocavirus: increasing evidence for virulence. Pediatr Pulmonol 45:118–119. doi: 10.1002/ppul.21159. [DOI] [PubMed] [Google Scholar]
- 1012.Don M, Söderlund-Venermo M, Hedman K, Ruuskanen O, Allander T, Korppi M. 2011. Don't forget serum in the diagnosis of human bocavirus infection. J Infect Dis 203:1031–1032. doi: 10.1093/infdis/jiq157. [DOI] [PubMed] [Google Scholar]
- 1013.Ursic T, Jevsnik M, Zigon N, Krivec U, Beden AB, Praprotnik M, Petrovec M. 2012. Human bocavirus and other respiratory viral infections in a 2-year cohort of hospitalized children. J Med Virol 84:99–108. doi: 10.1002/jmv.22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1014.Terrosi C, Fabbiani M, Cellesi C, Cusi MG. 2007. Human bocavirus detection in an atopic child affected by pneumonia associated with wheezing. J Clin Virol 40:43–45. doi: 10.1016/j.jcv.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1015.Neske F, Blessing K, Tollmann F, Schubert J, Rethwilm A, Kreth HW, Weissbrich B. 2007. Real-time PCR for diagnosis of human bocavirus infections and phylogenetic analysis. J Clin Microbiol 45:2116–2122. doi: 10.1128/JCM.00027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1016.Moriyama Y, Hamada H, Okada M, Tsuchiya N, Maru H, Shirato Y, Maeda Y, Hirose Y, Yoshida M, Omura Y, Honda T, Muto A, Hayashi K, Terai M. 2010. Distinctive clinical features of human bocavirus in children younger than 2 years. Eur J Pediatr 169:1087–1092. doi: 10.1007/s00431-010-1183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1017.Ruohola A, Waris M, Allander T, Ziegler T, Heikkinen T, Ruuskanen O. 2009. Viral etiology of common cold in children, Finland. Emerg Infect Dis 15:344–346. doi: 10.3201/eid1502.081468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1018.Del Rosal T, Garcia-Garcia ML, Calvo C, Gozalo F, Pozo F, Casas I. 4 December 2015. Recurrent wheezing and asthma after bocavirus bronchiolitis. Allergol Immunopathol (Madr) doi: 10.1016/j.aller.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 1019.do Amaral de Leon C, Amantea SL, Pilger DA, Cantarelli V. 2013. Clinical and epidemiologic profile of lower respiratory tract infections associated with human bocavirus. Pediatr Pulmonol 48:1112–1118. doi: 10.1002/ppul.22732. [DOI] [PubMed] [Google Scholar]
- 1020.Rezes S, Soderlund-Venermo M, Roivainen M, Kemppainen K, Szabo Z, Sziklai I, Pitkaranta A. 2009. Human bocavirus and rhino-enteroviruses in childhood otitis media with effusion. J Clin Virol 46:234–237. doi: 10.1016/j.jcv.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1021.Maggi F, Andreoli E, Pifferi M, Meschi S, Rocchi J, Bendinelli M. 2007. Human bocavirus in Italian patients with respiratory diseases. J Clin Virol 38:321–325. doi: 10.1016/j.jcv.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 1022.Gerna G, Piralla A, Campanini G, Marchi A, Stronati M, Rovida F. 2007. The human bocavirus role in acute respiratory tract infections of pediatric patients as defined by viral load quantification. New Microbiol 30:383–392. [PubMed] [Google Scholar]
- 1023.Jartti T, Jartti L, Peltola V, Waris M, Ruuskanen O. 2008. Identification of respiratory viruses in asymptomatic subjects: asymptomatic respiratory viral infections. Pediatr Infect Dis J 27:1103–1107. doi: 10.1097/INF.0b013e31817e695d. [DOI] [PubMed] [Google Scholar]
- 1024.Zheng LS, Yuan XH, Xie ZP, Jin Y, Gao HC, Song JR, Zhang RF, Xu ZQ, Hou YD, Duan ZJ. 2010. Human bocavirus infection in young children with acute respiratory tract infection in Lanzhou, China. J Med Virol 82:282–288. doi: 10.1002/jmv.21689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1025.Chow BD, Esper FP. 2009. The human bocaviruses: a review and discussion of their role in infection. Clin Lab Med 29:695–713. doi: 10.1016/j.cll.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1026.Zhou L, Zheng S, Xiao Q, Ren L, Xie X, Luo J, Wang L, Huang A, Liu W, Liu E. 2014. Single detection of human bocavirus 1 with a high viral load in severe respiratory tract infections in previously healthy children. BMC Infect Dis 14:424. doi: 10.1186/1471-2334-14-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1027.Jiang W, Yin F, Zhou W, Yan Y, Ji W. 1 February 2016. Clinical significance of different virus load of human bocavirus in patients with lower respiratory tract infection. Sci Rep doi: 10.1038/srep20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1028.Ghietto LM, Majul D, Ferreyra SP, Baumeister E, Avaro M, Insfran C, Mosca L, Camara A, Moreno LB, Adamo MP. 2015. Comorbidity and high viral load linked to clinical presentation of respiratory human bocavirus infection. Arch Virol 160:117–127. doi: 10.1007/s00705-014-2238-5. [DOI] [PubMed] [Google Scholar]
- 1029.Ricart S, Garcia-Garcia JJ, Anton A, Pumarola T, Pons M, Munoz-Almagro C, Marcos MA. 2013. Analysis of human metapneumovirus and human bocavirus viral load. Pediatr Infect Dis J 32:1032–1034. doi: 10.1097/INF.0b013e3182932f4f. [DOI] [PubMed] [Google Scholar]
- 1030.Jula A, Waris M, Kantola K, Peltola V, Söderlund-Venerm M, Hedman K, Ruuskanen O. 2013. Primary and secondary human bocavirus 1 infections in a family, Finland. Emerg Infect Dis 19:1328–1331. doi: 10.3201/eid1908.130074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1031.Sadeghi M, Kantola K, Finnegan DP, McCaughey C, Hedman L, Söderlund-Venermo M, Hedman K. 2013. Possible involvement of human bocavirus-1 in the death of a middle-aged immunosuppressed patient. J Clin Microbiol 51:3461–3463. doi: 10.1128/JCM.01157-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1032.Moreno B, Abrego L, Carrera JP, Franco D, Gaitan M, Castillo J, Pascale JM, Arbiza J. 2016. Detection of human bocavirus type 1 infection in Panamanian children with respiratory illness. J Med Virol 88:389–394. doi: 10.1002/jmv.24346. [DOI] [PubMed] [Google Scholar]
- 1033.Bubshait DK, Albuali WH, Yousef AA, Obeid OE, Alkharsah KR, Hassan MI, Vatte C, Alzahrani AJ, Bukhari H. 2015. Clinical description of human bocavirus viremia in children with LRTI, Eastern Province, Saudi Arabia. Ann Thorac Med 10:146–149. doi: 10.4103/1817-1737.151437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1034.Ursic T, Krivec U, Kalan G, Petrovec M. 2015. Fatal human bocavirus infection in an 18-month-old child with chronic lung disease of prematurity. Pediatr Infect Dis J 34:111–112. doi: 10.1097/INF.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 1035.Ong DS, Schuurman R, Heikens E. 2016. Human bocavirus in stool: a true pathogen or an innocent bystander? J Clin Virol 74:45–49. doi: 10.1016/j.jcv.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 1036.Jin Y, Cheng WX, Xu ZQ, Liu N, Yu JM, Li HY, Jin M, Li DD, Zhang Q, Duan ZJ. 2011. High prevalence of human bocavirus 2 and its role in childhood acute gastroenteritis in China. J Clin Virol 52:251–253. doi: 10.1016/j.jcv.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 1037.Wang Y, Gonzalez R, Zhou H, Li J, Li Y, Paranhos-Baccala G, Vernet G, Guo L, Wang J. 2011. Detection of human bocavirus 3 in China. Eur J Clin Microbiol Infect Dis 30:799–805. doi: 10.1007/s10096-011-1159-4. [DOI] [PubMed] [Google Scholar]
- 1038.Tymentsev A, Tikunov A, Zhirakovskaia E, Kurilschikov A, Babkin I, Klemesheva V, Netesov S, Tikunova N. 2016. Human bocavirus in hospitalized children with acute gastroenteritis in Russia from 2010 to 2012. Infect Genet Evol 37:143–149. doi: 10.1016/j.meegid.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 1039.Risku M, Katka M, Lappalainen S, Rasanen S, Vesikari T. 2012. Human bocavirus types 1, 2 and 3 in acute gastroenteritis of childhood. Acta Paediatr 101:e405–e410. doi: 10.1111/j.1651-2227.2012.02727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1040.Brebion A, Vanlieferinghen P, Dechelotte P, Boutry M, Peigue-Lafeuille H, Henquell C. 2014. Fatal subacute myocarditis associated with human bocavirus 2 in a 13-month-old child. J Clin Microbiol 52:1006–1008. doi: 10.1128/JCM.03013-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1041.Gallinella G, Zuffi E, Gentilomi G, Manaresi E, Venturoli S, Bonvicini F, Cricca M, Zerbini M, Musiani M. 2003. Relevance of B19 markers in serum samples for a diagnosis of parvovirus B19-correlated diseases. J Med Virol 71:135–139. doi: 10.1002/jmv.10452. [DOI] [PubMed] [Google Scholar]
- 1042.Maple PA, Hedman L, Dhanilall P, Kantola K, Nurmi V, Söderlund-Venermo M, Brown KE, Hedman K. 2014. Identification of past and recent parvovirus B19 infection in immunocompetent individuals by quantitative PCR and enzyme immunoassays: a dual-laboratory study. J Clin Microbiol 52:947–956. doi: 10.1128/JCM.02613-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1043.Jartti T, Jartti L, Ruuskanen O, Söderlund-Venermo M. 2012. New respiratory viral infections. Curr Opin Pulm Med 18:271–278. doi: 10.1097/MCP.0b013e328351f8d4. [DOI] [PubMed] [Google Scholar]
- 1044.Paver WK, Clarke SK. 1976. Comparison of human fecal and serum parvo-like viruses. J Clin Microbiol 4:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1045.Anderson MJ, Davis LR, Jones SE, Pattison JR, Serjeant GR. 1982. The development and use of an antibody capture radioimmunoassay for specific IgM to a human parvovirus-like agent. J Hyg (Lond) 88:309–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1046.Cohen BJ, Mortimer PP, Pereira MS. 1983. Diagnostic assays with monoclonal antibodies for the human serum parvovirus-like virus (SPLV). J Hyg (Lond) 91:113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1047.Okabe N, Koboyashi S, Tatsuzawa O, Mortimer PP. 1984. Detection of antibodies to human parvovirus in erythema infectiosum (fifth disease). Arch Dis Child 59:1016–1019. doi: 10.1136/adc.59.11.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1048.Shiraishi H, Wong D, Purcell RH, Shirachi R, Kumasaka E, Numazaki Y. 1985. Antibody to human parvovirus in outbreak of erythema infectiosum in Japan. Lancet i:982–983. [DOI] [PubMed] [Google Scholar]
- 1049.Schwarz TF, Roggendorf M, Deinhardt F. 1988. Human parvovirus B19: ELISA and immunoblot assays. J Virol Methods 20:155–168. doi: 10.1016/0166-0934(88)90149-8. [DOI] [PubMed] [Google Scholar]
- 1050.Brown KE, Buckley MM, Cohen BJ, Samuel D. 1989. An amplified ELISA for the detection of parvovirus B19 IgM using monoclonal antibody to FITC. J Virol Methods 26:189–198. doi: 10.1016/0166-0934(89)90148-1. [DOI] [PubMed] [Google Scholar]
- 1051.Yaegashi N, Shiraishi H, Tada K, Yajima A, Sugamura K. 1989. Enzyme-linked immunosorbent assay for IgG and IgM antibodies against human parvovirus B19: use of monoclonal antibodies and viral antigen propagated in vitro. J Virol Methods 26:171–181. doi: 10.1016/0166-0934(89)90146-8. [DOI] [PubMed] [Google Scholar]
- 1052.Brown CS, van Bussel MJ, Wassenaar AL, Van Elsacker-Niele AM, Weiland HT, Salimans MM. 1990. An immunofluorescence assay for the detection of parvovirus B19 IgG and IgM antibodies based on recombinant viral antigen. J Virol Methods 29:53–62. doi: 10.1016/0166-0934(90)90007-3. [DOI] [PubMed] [Google Scholar]
- 1053.Cubie HA, Leslie EE, Smith S, O'Neill HJ, Hart H, Cohen BJ, Inglis JM. 1993. Use of recombinant human parvovirus B19 antigens in serological assays. J Clin Pathol 46:840–845. doi: 10.1136/jcp.46.9.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1054.Cubel RC, Alferes AC, Cohen BJ, Nascimento JP. 1994. Application to immunoglobulin M capture hemadherence assays of hemagglutination of monkey erythrocytes by native and recombinant human parvovirus B19 antigens. J Clin Microbiol 32:1997–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1055.Wang QY, Erdman DD. 1995. Development and evaluation of capture immunoglobulin G and M hemadherence assays by using human type O erythrocytes and recombinant parvovirus B19 antigen. J Clin Microbiol 33:2466–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1056.de Ory F, Minguito T, Echevarria JE, Del Mar MM, Fuertes A. 2014. Comparative evaluation of tests for detection of parvovirus B19 IgG and IgM. APMIS 122:223–229. doi: 10.1111/apm.12127. [DOI] [PubMed] [Google Scholar]
- 1057.Wang Y, Hedman L, Perdomo MF, Elfaitouri A, Bolin-Wiener A, Kumar A, Lappalainen M, Soderlund-Venermo M, Blomberg J, Hedman K. 2016. Microsphere-based antibody assays for human parvovirus B19V, CMV and T. gondii. BMC Infect Dis 16:8. doi: 10.1186/s12879-015-1194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1058.Kajigaya S, Shimada T, Fujita S, Young NS. 1989. A genetically engineered cell line that produces empty capsids of B19 (human) parvovirus. Proc Natl Acad Sci U S A 86:7601–7605. doi: 10.1073/pnas.86.19.7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1059.Brown CS, Salimans MM, Noteborn MH, Weiland HT. 1990. Antigenic parvovirus B19 coat proteins VP1 and VP2 produced in large quantities in a baculovirus expression system. Virus Res 15:197–211. doi: 10.1016/0168-1702(90)90028-A. [DOI] [PubMed] [Google Scholar]
- 1060.Salimans MM, van Bussel MJ, Brown CS, Spaan WJ. 1992. Recombinant parvovirus B19 capsids as a new substrate for detection of B19-specific IgG and IgM antibodies by an enzyme-linked immunosorbent assay. J Virol Methods 39:247–258. doi: 10.1016/0166-0934(92)90098-X. [DOI] [PubMed] [Google Scholar]
- 1061.Gray JJ, Roth C, Swygart C, Desselberger U. 1994. Human parvovirus B19 serology with recombinant VP1 and VP2 antigens: diagnosis of acute infections by detecting B19-specific IgM and IgA antibodies. Clin Diagn Virol 2:331–341. doi: 10.1016/0928-0197(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 1062.Cohen BJ, Field AM, Mori J, Brown KE, Clewley JP, St Amand J, Astell CR. 1995. Morphology and antigenicity of recombinant B19 parvovirus capsids expressed in transfected COS-7 cells. J Gen Virol 76:1233–1237. doi: 10.1099/0022-1317-76-5-1233. [DOI] [PubMed] [Google Scholar]
- 1063.O'Neill HJ, Venugopal K, Coyle PV, Gould EA. 1995. Development of an IgM capture assay for the diagnosis of B19 parvovirus infection using recombinant baculoviruses expressing VP1 or VP2 antigens. Clin Diagn Virol 3:181–190. [DOI] [PubMed] [Google Scholar]
- 1064.Sisk WP, Berman ML. 1987. Expression of human parvovirus B19 structural protein in E. coli and detection of antiviral antibodies in human serum. Nat Biotechnol 5:1077–1080. doi: 10.1038/nbt1087-1077. [DOI] [Google Scholar]
- 1065.Morinet F, Courouce AM, Galibert F, Perol Y. 1990. Development of an IgM antibody capture test using labelled fusion protein as antigen for diagnosis of B19 human parvovirus infections. Behring Inst Mitt 1990:28–34. [PubMed] [Google Scholar]
- 1066.Rayment FB, Crosdale E, Morris DJ, Pattison JR, Talbot P, Clare JJ. 1990. The production of human parvovirus capsid proteins in Escherichia coli and their potential as diagnostic antigens. J Gen Virol 71:2665–2672. doi: 10.1099/0022-1317-71-11-2665. [DOI] [PubMed] [Google Scholar]
- 1067.Schwarz TF, Modrow S, Hottentrager B, Hoflacher B, Jager G, Scharti W, Sumazakl R, Wolf H, Middeldorp J, Roggendorf M, Deinhardt F. 1991. New oligopeptide immunoglobulin G test for human parvovirus B19 antibodies. J Clin Microbiol 29:431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1068.Schwarz TF, Jager G. 1994. A recombinant immunoblot and ELISA for detection of acute parvovirus B19 infection. Zentralbl Bakteriol 280:526–533. doi: 10.1016/S0934-8840(11)80513-X. [DOI] [PubMed] [Google Scholar]
- 1069.Yaegashi N, Okamura K, Tsunoda A, Nakamura M, Sugamura K, Yajima A. 1995. A study by means of a new assay of the relationship between an outbreak of erythema infectiosum and non-immune hydrops fetalis caused by human parvovirus B19. J Infect 31:195–200. doi: 10.1016/S0163-4453(95)80026-3. [DOI] [PubMed] [Google Scholar]
- 1070.Fridell E, Trojnar J, Wahren B. 1989. A new peptide for human parvovirus B19 antibody detection. Scand J Infect Dis 21:597–603. doi: 10.3109/00365548909021686. [DOI] [PubMed] [Google Scholar]
- 1071.Patou G, Ayliffe U. 1991. Evaluation of commercial enzyme linked immunosorbent assay for detection of B19 parvovirus IgM and IgG. J Clin Pathol 44:831–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1072.Kaikkonen L, Lankinen H, Harjunpää I, Hokynar K, Söderlund-Venermo M, Oker-Blom C, Hedman L, Hedman K. 1999. Acute-phase-specific heptapeptide epitope for diagnosis of parvovirus B19 infection. J Clin Microbiol 37:3952–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1073.Bruu AL, Nordbo SA. 1995. Evaluation of five commercial tests for detection of immunoglobulin M antibodies to human parvovirus B19. J Clin Microbiol 33:1363–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1074.Sloots T, Devine PL. 1996. Evaluation of four commercial enzyme immunoassays for detection of immunoglobulin M antibodies to human parvovirus B19. Eur J Clin Microbiol Infect Dis 15:758–761. doi: 10.1007/BF01691968. [DOI] [PubMed] [Google Scholar]
- 1075.Schwarz TF, Jager G, Gilch S. 1997. Comparison of seven commercial tests for the detection of parvovirus B19-specific IgM. Zentralbl Bakteriol 285:525–530. doi: 10.1016/S0934-8840(97)80114-4. [DOI] [PubMed] [Google Scholar]
- 1076.Butchko AR, Jordan JA. 2004. Comparison of three commercially available serologic assays used to detect human parvovirus B19-specific immunoglobulin M (IgM) and IgG antibodies in sera of pregnant women. J Clin Microbiol 42:3191–3195. doi: 10.1128/JCM.42.7.3191-3195.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1077.Enders M, Helbig S, Hunjet A, Pfister H, Reichhuber C, Motz M. 2007. Comparative evaluation of two commercial enzyme immunoassays for serodiagnosis of human parvovirus B19 infection. J Virol Methods 146:409–413. doi: 10.1016/j.jviromet.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 1078.Siennicka J, Trzcinska A. 2010. Comparison of three enzyme immunoassays used to detect human parvovirus B19-specific IgM antibodies in sera of people suspected of measles. Med Sci Monit 16:BR154–BR159. [PubMed] [Google Scholar]
- 1079.Kerr S, O'Keeffe G, Kilty C, Doyle S. 1999. Undenatured parvovirus B19 antigens are essential for the accurate detection of parvovirus B19 IgG. J Med Virol 57:179–185. doi:. [DOI] [PubMed] [Google Scholar]
- 1080.Kaikkonen L, Söderlund-Venermo M, Brunstein J, Schou O, Panum JI, Rousseau S, Caul EO, Cohen B, Valle M, Hedman L, Hedman K. 2001. Diagnosis of human parvovirus B19 infections by detection of epitope-type-specific VP2 IgG. J Med Virol 64:360–365. doi: 10.1002/jmv.1059. [DOI] [PubMed] [Google Scholar]
- 1081.Hedman K, Lappalainen M, Söderlund-Venermo M, Hedman L. 1993. Avidity of IgG in serodiagnosis of infectious diseases. Rev Med Microbiol 4:123–129. doi: 10.1097/00013542-199307000-00001. [DOI] [Google Scholar]
- 1082.Inouye S, Hasegawa A, Matsuno S, Katow S. 1984. Changes in antibody avidity after virus infections: detection by an immunosorbent assay in which a mild protein-denaturing agent is employed. J Clin Microbiol 20:525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1083.Nossal GJ. 1992. The molecular and cellular basis of affinity maturation in the antibody response. Cell 68:1–2. doi: 10.1016/0092-8674(92)90198-L. [DOI] [PubMed] [Google Scholar]
- 1084.Gutierrez J, Maroto C. 1996. Are IgG antibody avidity assays useful in the diagnosis of infectious diseases? A review. Microbios 87:113–121. [PubMed] [Google Scholar]
- 1085.Hedman L, Söderlund-Venermo M, Jartti T, Ruuskanen O, Hedman K. 2010. Dating of human bocavirus infection with protein-denaturing IgG-avidity assays—secondary immune activations are ubiquitous in immunocompetent adults. J Clin Virol 48:44–48. doi: 10.1016/j.jcv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 1086.Enders M, Schalasta G, Baisch C, Weidner A, Pukkila L, Kaikkonen L, Lankinen H, Hedman L, Söderlund-Venermo M, Hedman K. 2006. Human parvovirus B19 infection during pregnancy—value of modern molecular and serological diagnostics. J Clin Virol 35:400–406. doi: 10.1016/j.jcv.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 1087.Enders M, Weidner A, Rosenthal T, Baisch C, Hedman L, Söderlund-Venermo M, Hedman K. 2008. Improved diagnosis of gestational parvovirus B19 infection at the time of nonimmune fetal hydrops. J Infect Dis 197:58–62. doi: 10.1086/524302. [DOI] [PubMed] [Google Scholar]
- 1088.Franssila R, Söderlund M, Brown CS, Spaan WJ, Seppala I, Hedman K. 1996. IgG subclass response to human parvovirus B19 infection. Clin Diagn Virol 6:41–49. doi: 10.1016/0928-0197(96)00156-0. [DOI] [PubMed] [Google Scholar]
- 1089.Hjelholt A, Christiansen G, Sorensen US, Birkelund S. 2013. IgG subclass profiles in normal human sera of antibodies specific to five kinds of microbial antigens. Pathog Dis 67:206–213. doi: 10.1111/2049-632X.12034. [DOI] [PubMed] [Google Scholar]
- 1090.Bluth MH, Norowitz KB, Chice S, Shah VN, Nowakowski M, Durkin HG, Smith-Norowitz TA. 2005. IgE, CD8+ CD60+ T cells and IFN-alpha in human immunity to parvovirus B19 in selective IgA deficiency. Hum Immunol 66:1029–1038. doi: 10.1016/j.humimm.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 1091.Bluth MH, Norowitz KB, Chice S, Shah VN, Nowakowski M, Josephson AS, Durkin HG, Smith-Norowitz TA. 2003. Detection of IgE anti-parvovirus B19 and increased CD23+ B cells in parvovirus B19 infection: relation to Th2 cytokines. Clin Immunol 108:152–158. doi: 10.1016/S1521-6616(03)00098-6. [DOI] [PubMed] [Google Scholar]
- 1092.Smith-Norowitz TA, Drew H, Norowitz HM, Nowakowski M, Bluth EF, Durkin HG, Bluth MH. 2008. Detection of IgE anti-parvovirus antibodies in human breast milk. Ann Clin Lab Sci 38:168–173. [PubMed] [Google Scholar]
- 1093.Cubel RC, Oliveira SA, Brown DW, Cohen BJ, Nascimento JP. 1996. Diagnosis of parvovirus B19 infection by detection of specific immunoglobulin M antibody in saliva. J Clin Microbiol 34:205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1094.Rice PS, Cohen BJ. 1996. A school outbreak of parvovirus B19 infection investigated using salivary antibody assays. Epidemiol Infect 116:331–338. doi: 10.1017/S0950268800052651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1095.Hoebe CJ, Claas EC, Steenbergen JE, Kroes AC. 2002. Confirmation of an outbreak of parvovirus B19 in a primary school using IgM ELISA and PCR on thumb prick blood samples. J Clin Virol 25:303–307. doi: 10.1016/S1386-6532(02)00021-5. [DOI] [PubMed] [Google Scholar]
- 1096.von Poblotzki A, Gigler A, Lang B, Wolf H, Modrow S. 1995. Antibodies to parvovirus B19 NS-1 protein in infected individuals. J Gen Virol 76:519–527. doi: 10.1099/0022-1317-76-3-519. [DOI] [PubMed] [Google Scholar]
- 1097.Venturoli S, Gallinella G, Manaresi E, Gentilomi G, Musiani M, Zerbini M. 1998. IgG response to the immunoreactive region of parvovirus B19 nonstructural protein by immunoblot assay with a recombinant antigen. J Infect Dis 178:1826–1829. doi: 10.1086/314500. [DOI] [PubMed] [Google Scholar]
- 1098.Jones LP, Erdman DD, Anderson LJ. 1999. Prevalence of antibodies to human parvovirus B19 nonstructural protein in persons with various clinical outcomes following B19 infection. J Infect Dis 180:500–504. doi: 10.1086/314894. [DOI] [PubMed] [Google Scholar]
- 1099.Kerr JR, Cunniffe VS. 2000. Antibodies to parvovirus B19 non-structural protein are associated with chronic but not acute arthritis following B19 infection. Rheumatology (Oxford) 39:903–908. doi: 10.1093/rheumatology/39.8.903. [DOI] [PubMed] [Google Scholar]
- 1100.Heegaard ED, Rasksen CJ, Christensen J. 2002. Detection of parvovirus B19 NS1-specific antibodies by ELISA and Western blotting employing recombinant NS1 protein as antigen. J Med Virol 67:375–383. doi: 10.1002/jmv.10079. [DOI] [PubMed] [Google Scholar]
- 1101.Tzang BS, Tsai CC, Tsay GJ, Wang M, Sun YS, Hsu TC. 2009. Anti-human parvovirus B19 nonstructural protein antibodies in patients with rheumatoid arthritis. Clin Chim Acta 405:76–82. doi: 10.1016/j.cca.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 1102.Kerr JR, Gough J, Richards SC, Main J, Enlander D, McCreary M, Komaroff AL, Chia JK. 2010. Antibody to parvovirus B19 nonstructural protein is associated with chronic arthralgia in patients with chronic fatigue syndrome/myalgic encephalomyelitis. J Gen Virol 91:893–897. doi: 10.1099/vir.0.017590-0. [DOI] [PubMed] [Google Scholar]
- 1103.Anderson MJ, Jones SE, Minson AC. 1985. Diagnosis of human parvovirus infection by dot-blot hybridization using cloned viral DNA. J Med Virol 15:163–172. doi: 10.1002/jmv.1890150209. [DOI] [PubMed] [Google Scholar]
- 1104.Salimans MM, Holsappel S, van de Rijke FM, Jiwa NM, Raap AK, Weiland HT. 1989. Rapid detection of human parvovirus B19 DNA by dot-hybridization and the polymerase chain reaction. J Virol Methods 23:19–28. doi: 10.1016/0166-0934(89)90085-2. [DOI] [PubMed] [Google Scholar]
- 1105.Clewley JP. 1989. Polymerase chain reaction assay of parvovirus B19 DNA in clinical specimens. J Clin Microbiol 27:2647–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1106.Koch WC, Adler SP. 1990. Detection of human parvovirus B19 DNA by using the polymerase chain reaction. J Clin Microbiol 28:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1107.Gallinella G, Zerbini M, Musiani M, Venturoli S, Gentilomi G, Manaresi E. 1997. Quantitation of parvovirus B19 DNA sequences by competitive PCR: differential hybridization of the amplicons and immunoenzymatic detection on microplate. Mol Cell Probes 11:127–133. doi: 10.1006/mcpr.1996.0095. [DOI] [PubMed] [Google Scholar]
- 1108.Gruber F, Falkner FG, Dorner F, Hammerle T. 1998. Precise quantitation of human parvovirus B19 DNA in biological samples by PCR. Biologicals 26:213–216. doi: 10.1006/biol.1998.0129. [DOI] [PubMed] [Google Scholar]
- 1109.Baylis SA, Shah N, Minor PD. 2004. Evaluation of different assays for the detection of parvovirus B19 DNA in human plasma. J Virol Methods 121:7–16. doi: 10.1016/j.jviromet.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 1110.Koppelman MH, Rood IG, Fryer JF, Baylis SA, Cuypers HT. 2007. Parvovirus B19 genotypes 1 and 2 detection with real-time polymerase chain reaction assays. Vox Sang 93:208–215. doi: 10.1111/j.1423-0410.2007.00957.x. [DOI] [PubMed] [Google Scholar]
- 1111.Ke L, He M, Li C, Liu Y, Gao L, Yao F, Li J, Bi X, Lv Y, Wang J, Hirsch ML, Li W. 2011. The prevalence of human parvovirus B19 DNA and antibodies in blood donors from four Chinese blood centers. Transfusion 51:1909–1918. doi: 10.1111/j.1537-2995.2011.03067.x. [DOI] [PubMed] [Google Scholar]
- 1112.Koppelman MH, Cuijpers HT, Wessberg S, Valkeajarvi A, Pichl L, Schottstedt V, Saldanha J. 2012. Multicenter evaluation of a commercial multiplex polymerase chain reaction test for screening plasma donations for parvovirus B19 DNA and hepatitis A virus RNA. Transfusion 52:1498–1508. doi: 10.1111/j.1537-2995.2012.03705.x. [DOI] [PubMed] [Google Scholar]
- 1113.Bonvicini F, Manaresi E, Bua G, Venturoli S, Gallinella G. 2013. Keeping pace with parvovirus B19 genetic variability: a multiplex genotype-specific quantitative PCR assay. J Clin Microbiol 51:3753–3759. doi: 10.1128/JCM.01970-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1114.Toppinen M, Norja P, Aaltonen LM, Wessberg S, Hedman L, Soderlund-Venermo M, Hedman K. 2015. A new quantitative PCR for human parvovirus B19 genotypes. J Virol Methods 218:40–45. doi: 10.1016/j.jviromet.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 1115.Baylis SA, Ma L, Padley DJ, Heath AB, Yu MW. 2012. Collaborative study to establish a World Health Organization international genotype panel for parvovirus B19 DNA nucleic acid amplification technology (NAT)-based assays. Vox Sang 102:204–211. doi: 10.1111/j.1423-0410.2011.01541.x. [DOI] [PubMed] [Google Scholar]
- 1116.Morey AL, Ferguson DJ, Leslie KO, Taatjes DJ, Fleming KA. 1993. Intracellular localization of parvovirus B19 nucleic acid at the ultrastructural level by in situ hybridization with digoxigenin-labelled probes. Histochem J 25:421–429. doi: 10.1007/BF00157806. [DOI] [PubMed] [Google Scholar]
- 1117.Morey AL, Porter HJ, Keeling JW, Fleming KA. 1992. Non-isotopic in situ hybridisation and immunophenotyping of infected cells in the investigation of human fetal parvovirus infection. J Clin Pathol 45:673–678. doi: 10.1136/jcp.45.8.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1118.Loughrey AC, O'Neill HJ, Coyle PV, DeLeys R. 1993. Identification and use of a neutralising epitope of parvovirus B19 for the rapid detection of virus infection. J Med Virol 39:97–100. doi: 10.1002/jmv.1890390204. [DOI] [PubMed] [Google Scholar]
- 1119.Corcoran A, Kerr S, Elliott G, Koppelman M, Doyle S. 2007. Improved detection of acute parvovirus B19 infection by immunoglobulin M EIA in combination with a novel antigen EIA. Vox Sang 93:216–222. doi: 10.1111/j.1423-0410.2007.00956.x. [DOI] [PubMed] [Google Scholar]
- 1120.Sakata H, Matsubayashi K, Ihara H, Sato S, Kato T, Wakisaka A, Tadokoro K, Yu MY, Baylis SA, Ikeda H, Takamoto S. 2013. Impact of chemiluminescent enzyme immunoassay screening for human parvovirus B19 antigen in Japanese blood donors. Transfusion 53:2556–2566. doi: 10.1111/j.1537-2995.2012.03949.x. [DOI] [PubMed] [Google Scholar]
- 1121.Field AM, Cohen BJ, Brown KE, Mori J, Clewley JP, Nascimento JP, Hallam NF. 1991. Detection of B19 parvovirus in human fetal tissues by electron microscopy. J Med Virol 35:85–95. doi: 10.1002/jmv.1890350204. [DOI] [PubMed] [Google Scholar]
- 1122.Morey AL, O'Neill HJ, Coyle PV, Fleming KA. 1992. Immunohistological detection of human parvovirus B19 in formalin-fixed, paraffin-embedded tissues. J Pathol 166:105–108. doi: 10.1002/path.1711660204. [DOI] [PubMed] [Google Scholar]
- 1123.Morey AL, Ferguson DJ, Fleming KA. 1993. Ultrastructural features of fetal erythroid precursors infected with parvovirus B19 in vitro: evidence of cell death by apoptosis. J Pathol 169:213–220. doi: 10.1002/path.1711690207. [DOI] [PubMed] [Google Scholar]
- 1124.Jartti T, Söderlund-Venermo M, Hedman K, Ruuskanen O, Makela MJ. 2013. New molecular virus detection methods and their clinical value in lower respiratory tract infections in children. Paediatr Respir Rev 14:38–45. doi: 10.1016/j.prrv.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1125.Gadsby NJ, Hardie A, Claas EC, Templeton KE. 2010. Comparison of the Luminex Respiratory Virus Panel fast assay with in-house real-time PCR for respiratory viral infection diagnosis. J Clin Microbiol 48:2213–2216. doi: 10.1128/JCM.02446-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1126.Gharabaghi F, Hawan A, Drews SJ, Richardson SE. 2011. Evaluation of multiple commercial molecular and conventional diagnostic assays for the detection of respiratory viruses in children. Clin Microbiol Infect 17:1900–1906. doi: 10.1111/j.1469-0691.2011.03529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1127.Dabisch-Ruthe M, Vollmer T, Adams O, Knabbe C, Dreier J. 2012. Comparison of three multiplex PCR assays for the detection of respiratory viral infections: evaluation of xTAG respiratory virus panel fast assay, RespiFinder 19 assay and RespiFinder SMART 22 assay. BMC Infect Dis 12:163. doi: 10.1186/1471-2334-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1128.Ellis C, Misir A, Hui C, Jabbour M, Barrowman N, Langill J, Bowes J, Slinger R. 2016. Detection of respiratory viruses and bacteria in children using a twenty-two target reverse-transcription real-time PCR (RT-qPCR) panel. World J Pediatr 12:183–189. doi: 10.1007/s12519-015-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1129.Graf EH, Simmon KE, Tardif KD, Hymas W, Flygare S, Eilbeck K, Yandell M, Schlaberg R. 2016. Unbiased detection of respiratory viruses by use of RNA sequencing-based metagenomics: a systematic comparison to a commercial PCR panel. J Clin Microbiol 54:1000–1007. doi: 10.1128/JCM.03060-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1130.Zhao H, Zhao L, Sun Y, Qian Y, Liu L, Jia L, Zhang Y, Dong H. 2012. Detection of a bocavirus circular genome in fecal specimens from children with acute diarrhea in Beijing, China. PLoS One 7:e48980. doi: 10.1371/journal.pone.0048980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1131.Xu ZQ, Cheng WX, Li BW, Li J, Lan B, Duan ZJ. 2011. Development of a real-time PCR assay for detecting and quantifying human bocavirus 2. J Clin Microbiol 49:1537–1541. doi: 10.1128/JCM.00196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1132.Endo R, Ishiguro N, Kikuta H, Teramoto S, Shirkoohi R, Ma X, Ebihara T, Ishiko H, Ariga T. 2007. Seroepidemiology of human bocavirus in Hokkaido prefecture, Japan. J Clin Microbiol 45:3218–3223. doi: 10.1128/JCM.02140-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1133.Tamosiunas PL, Petraityte-Burneikiene R, Bulavaite A, Marcinkeviciute K, Simutis K, Lasickiene R, Firantiene R, Emuzyte R, Zvirbliene A, Sasnauskas K. 2016. Yeast-generated virus-like particles as antigens for detection of human bocavirus 1-4 specific antibodies in human serum. Appl Microbiol Biotechnol 100:4935–4946. doi: 10.1007/s00253-016-7336-8. [DOI] [PubMed] [Google Scholar]
- 1134.Koskinen JO, Vainionpaa R, Meltola NJ, Soukka J, Hanninen PE, Soini AE. 2007. Rapid method for detection of influenza A and B virus antigens by use of a two-photon excitation assay technique and dry-chemistry reagents. J Clin Microbiol 45:3581–3588. doi: 10.1128/JCM.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1135.Gunell M, Antikainen P, Porjo N, Irjala K, Vakkila J, Hotakainen K, Kaukoranta SS, Hirvonen JJ, Saha K, Manninen R, Forsblom B, Rantakokko-Jalava K, Peltola V, Koskinen JO, Huovinen P. 2016. Comprehensive real-time epidemiological data from respiratory infections in Finland between 2010 and 2014 obtained from an automated and multianalyte mariPOC respiratory pathogen test. Eur J Clin Microbiol Infect Dis 35:405–413. doi: 10.1007/s10096-015-2553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1136.Mouthon L, Guillevin L, Tellier Z. 2005. Intravenous immunoglobulins in autoimmune- or parvovirus B19-mediated pure red-cell aplasia. Autoimmun Rev 4:264–269. doi: 10.1016/j.autrev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 1137.Young NS. 1996. Parvovirus infection and its treatment. Clin Exp Immunol 104(Suppl 1):S26–S30. [PubMed] [Google Scholar]
- 1138.Hayakawa F, Imada K, Towatari M, Saito H. 2002. Life-threatening human parvovirus B19 infection transmitted by intravenous immune globulin. Br J Haematol 118:1187–1189. doi: 10.1046/j.1365-2141.2002.03741.x. [DOI] [PubMed] [Google Scholar]
- 1139.Egbuna O, Zand MS, Arbini A, Menegus M, Taylor J. 2006. A cluster of parvovirus B19 infections in renal transplant recipients: a prospective case series and review of the literature. Am J Transplant 6:225–231. doi: 10.1111/j.1600-6143.2005.01139.x. [DOI] [PubMed] [Google Scholar]
- 1140.Renoult E, Bachelet C, Krier-Coudert MJ, Diarrassouba A, Andre JL, Kessler M. 2006. Recurrent anemia in kidney transplant recipients with parvovirus B19 infection. Transplant Proc 38:2321–2323. doi: 10.1016/j.transproceed.2006.06.116. [DOI] [PubMed] [Google Scholar]
- 1141.Ogawa E, Otaguro S, Murata M, Kainuma M, Sawayama Y, Furusyo N, Hayashi J. 2008. Intravenous immunoglobulin therapy for severe arthritis associated with human parvovirus B19 infection. J Infect Chemother 14:377–382. doi: 10.1007/s10156-008-0636-X. [DOI] [PubMed] [Google Scholar]
- 1142.Bansal GP, Hatfield JA, Dunn FE, Kramer AA, Brady F, Riggin CH, Collett MS, Yoshimoto K, Kajigaya S, Young NS. 1993. Candidate recombinant vaccine for human B19 parvovirus. J Infect Dis 167:1034–1044. doi: 10.1093/infdis/167.5.1034. [DOI] [PubMed] [Google Scholar]
- 1143.Ballou WR, Reed JL, Noble W, Young NS, Koenig S. 2003. Safety and immunogenicity of a recombinant parvovirus B19 vaccine formulated with MF59C.1. J Infect Dis 187:675–678. doi: 10.1086/368382. [DOI] [PubMed] [Google Scholar]
- 1144.Bernstein DI, El Sahly HM, Keitel WA, Wolff M, Simone G, Segawa C, Wong S, Shelly D, Young NS, Dempsey W. 2011. Safety and immunogenicity of a candidate parvovirus B19 vaccine. Vaccine 29:7357–7363. doi: 10.1016/j.vaccine.2011.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1145.Chandramouli S, Medina-Selby A, Coit D, Schaefer M, Spencer T, Brito LA, Zhang P, Otten G, Mandl CW, Mason PW, Dormitzer PR, Settembre EC. 2013. Generation of a parvovirus B19 vaccine candidate. Vaccine 31:3872–3878. doi: 10.1016/j.vaccine.2013.06.062. [DOI] [PubMed] [Google Scholar]
- 1146.Amexis G, Young NS. 2006. Parvovirus B19 empty capsids as antigen carriers for presentation of antigenic determinants of dengue 2 virus. J Infect Dis 194:790–794. doi: 10.1086/506361. [DOI] [PubMed] [Google Scholar]
- 1147.Ogasawara Y, Amexis G, Yamaguchi H, Kajigaya S, Leppla SH, Young NS. 2006. Recombinant viral-like particles of parvovirus B19 as antigen carriers of anthrax protective antigen. In Vivo 20:319–324. [PubMed] [Google Scholar]
- 1148.Matthews PC, Malik A, Simmons R, Sharp C, Simmonds P, Klenerman P. 2014. PARV4: an emerging tetraparvovirus. PLoS Pathog 10:e1004036. doi: 10.1371/journal.ppat.1004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1149.Schneider B, Fryer JF, Oldenburg J, Brackmann HH, Baylis SA, Eis-Hübinger AM. 2008. Frequency of contamination of coagulation factor concentrates with novel human parvovirus PARV4. Haemophilia 14:978–986. doi: 10.1111/j.1365-2516.2008.01800.x. [DOI] [PubMed] [Google Scholar]
- 1150.Schneider B, Fryer JF, Reber U, Fischer HP, Tolba RH, Baylis SA, Eis-Hübinger AM. 2008. Persistence of novel human parvovirus PARV4 in liver tissue of adults. J Med Virol 80:345–351. doi: 10.1002/jmv.21069. [DOI] [PubMed] [Google Scholar]
- 1151.Longhi E, Bestetti G, Acquaviva V, Foschi A, Piolini R, Meroni L, Magni C, Antinori S, Parravicini C, Corbellino M. 2007. Human parvovirus 4 in the bone marrow of Italian patients with AIDS. AIDS 21:1481–1483. doi: 10.1097/QAD.0b013e3281e38558. [DOI] [PubMed] [Google Scholar]
- 1152.Benjamin LA, Lewthwaite P, Vasanthapuram R, Zhao G, Sharp C, Simmonds P, Wang D, Solomon T. 2011. Human parvovirus 4 as potential cause of encephalitis in children, India. Emerg Infect Dis 17:1484–1487. doi: 10.3201/eid1708.110165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1153.Sharp CP, Lail A, Donfield S, Gomperts ED, Simmonds P. 2012. Virologic and clinical features of primary infection with human parvovirus 4 in subjects with hemophilia: frequent transmission by virally inactivated clotting factor concentrates. Transfusion 52:1482–1489. doi: 10.1111/j.1537-2995.2011.03420.x. [DOI] [PubMed] [Google Scholar]
- 1154.Lahtinen A, Kivela P, Hedman L, Kumar A, Kantele A, Lappalainen M, Liitsola K, Ristola M, Delwart E, Sharp C, Simmonds P, Soderlund-Venermo M, Hedman K. 2011. Serodiagnosis of primary infections with human parvovirus 4, Finland. Emerg Infect Dis 17:79–82. doi: 10.3201/eid1701.100750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1155.Fryer JF, Delwart E, Bernardin F, Tuke PW, Lukashov VV, Baylis SA. 2007. Analysis of two human parvovirus PARV4 genotypes identified in human plasma for fractionation. J Gen Virol 88:2162–2167. doi: 10.1099/vir.0.82620-0. [DOI] [PubMed] [Google Scholar]
- 1156.Simmonds P, Manning A, Kenneil R, Carnie FW, Bell JE. 2007. Parenteral transmission of the novel human parvovirus PARV4. Emerg Infect Dis 13:1386–1388. doi: 10.3201/eid1309.070428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1157.Sharp CP, Lail A, Donfield S, Simmons R, Leen C, Klenerman P, Delwart E, Gomperts ED, Simmonds P. 2009. High frequencies of exposure to the novel human parvovirus PARV4 in hemophiliacs and injection drug users, as detected by a serological assay for PARV4 antibodies. J Infect Dis 200:1119–1125. doi: 10.1086/605646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1158.Simmonds P, Douglas J, Bestetti G, Longhi E, Antinori S, Parravicini C, Corbellino M. 2008. A third genotype of the human parvovirus PARV4 in sub-Saharan Africa. J Gen Virol 89:2299–2302. doi: 10.1099/vir.0.2008/001180-0. [DOI] [PubMed] [Google Scholar]
- 1159.Panning M, Kobbe R, Vollbach S, Drexler JF, Adjei S, Adjei O, Drosten C, May J, Eis-Hubinger AM. 2010. Novel human parvovirus 4 genotype 3 in infants, Ghana. Emerg Infect Dis 16:1143–1146. doi: 10.3201/eid1607.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1160.Drexler JF, Reber U, Muth D, Herzog P, Annan A, Ebach F, Sarpong N, Acquah S, Adlkofer J, Adu-Sarkodie Y, Panning M, Tannich E, May J, Drosten C, Eis-Hubinger AM. 2012. Human parvovirus 4 in nasal and fecal specimens from children, Ghana. Emerg Infect Dis 18:1650–1653. doi: 10.3201/eid1810.111373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1161.Matthews PC, Sharp CP, Malik A, Gregory WF, Adland E, Jooste P, Goulder PJ, Simmonds P, Klenerman P. 2015. Human parvovirus 4 infection among mothers and children in South Africa. Emerg Infect Dis 21:713–715. doi: 10.3201/eid2104.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1162.May J, Drexler JF, Reber U, Sarpong N, Adjei O, Panning M, Drosten C, Eis-Hubinger AM. 2012. Human parvovirus 4 viremia in young children, Ghana. Emerg Infect Dis 18:1690–1692. doi: 10.3201/eid1810.111836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1163.Vaisanen E, Lahtinen A, Eis-Hubinger AM, Lappalainen M, Hedman K, Söderlund-Venermo M. 2014. A two-step real-time PCR assay for quantitation and genotyping of human parvovirus 4. J Virol Methods 195:106–111. doi: 10.1016/j.jviromet.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 1164.Simmons R, Sharp C, Levine J, Bowness P, Simmonds P, Cox A, Klenerman P. 2013. Evolution of CD8+ T cell responses after acute PARV4 infection. J Virol 87:3087–3096. doi: 10.1128/JVI.02793-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1165.Yu X, Zhang J, Hong L, Wang J, Yuan Z, Zhang X, Ghildyal R. 2012. High prevalence of human parvovirus 4 infection in HBV and HCV infected individuals in Shanghai. PLoS One 7:e29474. doi: 10.1371/journal.pone.0029474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1166.Lou S, Xu B, Huang Q, Zhi N, Cheng F, Wong S, Brown K, Delwart E, Liu Z, Qiu J. 2012. Molecular characterization of the newly identified human parvovirus 4 in the family Parvoviridae. Virology 422:59–69. doi: 10.1016/j.virol.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1167.Tuke PW, Parry RP, Appleton H. 2010. Parvovirus PARV4 visualization and detection. J Gen Virol 91:541–544. doi: 10.1099/vir.0.014852-0. [DOI] [PubMed] [Google Scholar]
- 1168.Sharp CP, Vermeulen M, Nebie Y, Djoko CF, LeBreton M, Tamoufe U, Rimoin AW, Kayembe PK, Carr JK, Servant-Delmas A, Laperche S, Harrison GL, Pybus OG, Delwart E, Wolfe ND, Saville A, Lefrere JJ, Simmonds P. 2010. Changing epidemiology of human parvovirus 4 infection in sub-Saharan Africa. Emerg Infect Dis 16:1605–1607. doi: 10.3201/eid1610.101001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1169.Tamosiunas PL, Simutis K, Kodze I, Firantiene R, Emuzyte R, Petraityte-Burneikiene R, Zvirbliene A, Sasnauskas K. 2013. Production of human parvovirus 4 VP2 virus-like particles in yeast and their evaluation as an antigen for detection of virus-specific antibodies in human serum. Intervirology 56:271–277. doi: 10.1159/000353112. [DOI] [PubMed] [Google Scholar]
- 1170.Maple PA, Beard S, Parry RP, Brown KE. 2013. Testing UK blood donors for exposure to human parvovirus 4 using a time-resolved fluorescence immunoassay to screen sera and Western blot to confirm reactive samples. Transfusion 53:2575–2584. doi: 10.1111/trf.12278. [DOI] [PubMed] [Google Scholar]
- 1171.von Linstow ML, Rosenfeldt V, Lindberg E, Jensen L, Hedman L, Li X, Vaisanen E, Hedman K, Norja P. 2015. Absence of novel human parvovirus (PARV4) in Danish mothers and children. J Clin Virol 65:23–25. doi: 10.1016/j.jcv.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 1172.Yahiro T, Wangchuk S, Tshering K, Bandhari P, Zangmo S, Dorji T, Tshering K, Matsumoto T, Nishizono A, Söderlund-Venerm M, Ahmed K. 2014. Novel human bufavirus genotype 3 in children with severe diarrhea, Bhutan. Emerg Infect Dis 20:1037–1039. doi: 10.3201/eid2006.131430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1173.Smits SL, Schapendonk CM, van Beek J, Vennema H, Schurch AC, Schipper D, Bodewes R, Haagmans BL, Osterhaus AD, Koopmans MP. 2014. New viruses in idiopathic human diarrhea cases, the Netherlands. Emerg Infect Dis 20:1218–1222. doi: 10.3201/eid2007.140190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1174.Chieochansin T, Vutithanachot V, Theamboonlers A, Poovorawan Y. 2015. Bufavirus in fecal specimens of patients with and without diarrhea in Thailand. Arch Virol 160:1781–1784. doi: 10.1007/s00705-015-2441-z. [DOI] [PubMed] [Google Scholar]
- 1175.Huang DD, Wang W, Lu QB, Zhao J, Guo CT, Wang HY, Zhang XA, Tong YG, Liu W, Cao WC. 2015. Identification of bufavirus-1 and bufavirus-3 in feces of patients with acute diarrhea, China. Sci Rep 5:13272. doi: 10.1038/srep13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1176.Altay A, Yahiro T, Bozdayi G, Matsumoto T, Sahin F, Ozkan S, Nishizono A, Soderlund-Venermo M, Ahmed K. 2015. Bufavirus genotype 3 in Turkish children with severe diarrhoea. Clin Microbiol Infect 21:965.e1–965.e4. doi: 10.1016/j.cmi.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 1177.Vaisanen E, Kuisma I, Phan TG, Delwart E, Lappalainen M, Tarkka E, Hedman K, Söderlund-Venermo M. 2014. Bufavirus in feces of patients with gastroenteritis, Finland. Emerg Infect Dis 20:1077–1080. doi: 10.3201/eid2006.131674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1178.Phan TG, Sdiri-Loulizi K, Aouni M, Ambert-Balay K, Pothier P, Deng X, Delwart E. 2014. New parvovirus in child with unexplained diarrhea, Tunisia. Emerg Infect Dis 20:1911–1913. doi: 10.3201/eid2011.140428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1179.O'Sullivan MG, Anderson DC, Fikes JD, Bain FT, Carlson CS, Green SW, Young NS, Brown KE. 1994. Identification of a novel simian parvovirus in cynomolgus monkeys with severe anemia. A paradigm of human B19 parvovirus infection. J Clin Invest 93:1571–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1180.Gallinella G, Anderson SM, Young NS, Brown KE. 1995. Human parvovirus B19 can infect cynomolgus monkey marrow cells in tissue culture. J Virol 69:3897–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1181.Leon LA, Marchevsky RS, Gaspar AM, Garcia RC, Almeida AJ, Pelajo-Machado M, Castro TX, Nascimento JP, Brown KE, Pinto MA. 2016. Cynomolgus monkeys (Macaca fascicularis) experimentally infected with B19V and hepatitis A virus: no evidence of the co-infection as a cause of acute liver failure. Mem Inst Oswaldo Cruz 111:258–266. doi: 10.1590/0074-02760160013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1182.Duan D, Zhang Y, Engelhardt JF. 2001. Gene delivery to the airway. Curr Protoc Hum Genet Chapter 13:Unit 13.9. doi: 10.1002/0471142905.hg1309s23. [DOI] [PubMed] [Google Scholar]
- 1183.Filali M, Zhang Y, Ritchie TC, Engelhardt JF. 2002. Xenograft model of the CF airway. Methods Mol Med 70:537–550. [DOI] [PubMed] [Google Scholar]
- 1184.Yan Z, Zak R, Zhang Y, Ding W, Godwin S, Munson K, Peluso R, Engelhardt JF. 2004. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J Virol 78:2863–2874. doi: 10.1128/JVI.78.6.2863-2874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1185.Rudolf S, Neiger R, Konig M. 2016. Detection of bocavirus in 4-week-old puppies with acute diarrhea. Tierarztl Prax Ausg K Kleintiere Heimtiere 44:118–122. (In German.) doi: 10.15654/TPK-150484. [DOI] [PubMed] [Google Scholar]
- 1186.Sasaki M, Orba Y, Anindita PD, Ishii A, Ueno K, Hang'ombe BM, Mweene AS, Ito K, Sawa H. 2015. Distinct lineages of bufavirus in wild shrews and nonhuman primates. Emerg Infect Dis 21:1230–1233. doi: 10.3201/eid2107.141969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1187.Handley SA, Thackray LB, Zhao G, Presti R, Miller AD, Droit L, Abbink P, Maxfield LF, Kambal A, Duan E, Stanley K, Kramer J, Macri SC, Permar SR, Schmitz JE, Mansfield K, Brenchley JM, Veazey RS, Stappenbeck TS, Wang D, Barouch DH, Virgin HW. 2012. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell 151:253–266. doi: 10.1016/j.cell.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1188.Shen Q, Xu H, Cao Q, Zhou LJ, Xu J, Fang XY, Ge J. 2011. Long-term remission of recurrent severe anemia as a result of parvovirus B19 infection in a pediatric renal transplant recipient. Pediatr Transplant 15:E76–E79. doi: 10.1111/j.1399-3046.2010.01291.x. [DOI] [PubMed] [Google Scholar]
- 1189.Mrzljak A, Kardum-Skelin I, Cvrlje VC, Kanizaj TF, Sustercic D, Gustin D, Kocman B. 2010. Parvovirus B 19 (PVB19) induced pure red cell aplasia (PRCA) in immunocompromised patient after liver transplantation. Coll Antropol 34:271–274. [PubMed] [Google Scholar]
- 1190.Moudgil A, Shidban H, Nast CC, Bagga A, Aswad S, Graham SL, Mendez R, Jordan SC. 1997. Parvovirus B19 infection-related complications in renal transplant recipients: treatment with intravenous immunoglobulin. Transplantation 64:1847–1850. doi: 10.1097/00007890-199712270-00037. [DOI] [PubMed] [Google Scholar]
- 1191.Koduri PR, Kumapley R, Khokha ND, Patel AR. 1997. Red cell aplasia caused by parvovirus B19 in AIDS: use of i.v. immunoglobulin. Ann Hematol 75:67–68. [DOI] [PubMed] [Google Scholar]
- 1192.Koduri PR, Kumapley R, Valladares J, Teter C. 1999. Chronic pure red cell aplasia caused by parvovirus B19 in AIDS: use of intravenous immunoglobulin—a report of eight patients. Am J Hematol 61:16–20. [DOI] [PubMed] [Google Scholar]
- 1193.Hung CC, Lee KL, Chen MY. 2001. Increase in B19 viral load prior to relapse of anaemia in an AIDS patient with persistent B19 infection. J Infect 43:150–152. doi: 10.1053/jinf.2001.0882. [DOI] [PubMed] [Google Scholar]
- 1194.De Clercq E. 2007. The acyclic nucleoside phosphonates from inception to clinical use: historical perspective. Antiviral Res 75:1–13. doi: 10.1016/j.antiviral.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 1195.Bonvicini F, Bua G, Manaresi E, Gallinella G. 2015. Antiviral effect of cidofovir on parvovirus B19 replication. Antiviral Res 113:11–18. doi: 10.1016/j.antiviral.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 1196.Bonvicini F, Bua G, Manaresi E, Gallinella G. 2016. Enhanced inhibition of parvovirus B19 replication by cidofovir in extendedly exposed erythroid progenitor cells. Virus Res 220:47–51. doi: 10.1016/j.virusres.2016.04.002. [DOI] [PubMed] [Google Scholar]