Abstract
Escherichia coli O157:H7 is a human pathogen that is carried and transmitted by cattle. Scotland is known to have one of the highest rates of E. coli O157 human infections in the world. Two hundred ninety-three isolates were obtained from naturally infected cattle and the environment on two farms in the Scottish Highlands. The isolates were typed by pulsed-field gel electrophoresis (PFGE) with XbaI restriction endonuclease enzyme, and 19 different variations in patterns were found. There was considerable genomic diversity within the E. coli O157 population on the two farms. The PFGE pattern of one of the observed subtypes matched exactly with that of a strain obtained from a Scottish patient with hemolytic-uremic syndrome. To examine the stability of an individual E. coli O157 strain, continuous subculturing of a strain was performed 110 times. No variation from the original PFGE pattern was observed. We found three indistinguishable subtypes of E. coli O157 on both study farms, suggesting common sources of infection. We also examined the antibiotic resistance of the isolated strains. Phenotypic studies demonstrated resistance of the strains to sulfamethoxazole (100%), chloramphenicol (3.07%), and at a lower rate, other antibiotics, indicating the preservation of antibiotic sensitivity in a rapidly changing population of E. coli O157.
Escherichia coli O157:H7 is an enterohemorrhagic E. coli strain that was first recognized as an important food-borne human pathogen in 1982 (25) in the United States, and since then, this serotype has been isolated in many countries around the world. Human infection with E. coli O157 has been particularly prevalent in Scotland, and over the past decade, there have been several major outbreaks, including a milk borne outbreak in West Lothian in 1994 and an outbreak in Lanark in November 1996, in which 496 cases of infection and 20 deaths were reported from contaminated meat (5).
E. coli O157:H7 causes diarrhea, severe abdominal pain, hemorrhagic colitis, and hemolytic-uremic syndrome. The pathogenic factors of enterohemorrhagic E. coli include Shiga toxins, the chromosomal LEE locus that carries factors (eaeA, tir) involved in the attaching and effacing process, and a large plasmid carrying the hemolysin genes (34).
The majority of food-borne outbreaks involving E. coli O157:H7 are caused by beef products, particularly ground beef, suggesting that cattle are one of the most important sources of E. coli O157:H7 infection. Cattle lack toxin receptors and therefore do not suffer from hemorrhagic colitis (23). Pulsed-field gel electrophoresis (PFGE) and phage typing have suggested that some E. coli O157 clones are common among bovine and human isolates (2).
Although some studies do not advise antibiotic treatment for infections caused by E. coli O157 in humans (37), others suggest that disease progression may be prevented by administrating antibiotics at an early stage (22, 29). It is important to determine whether E. coli O157:H7 develops resistance to antibiotics in the animal reservoir and to determine if it is a possible source for the spread of resistance factors to other microorganisms in the animal gut.
This paper examines, in detail, the characterization and population genetics of E. coli O157 in a series of longitudinal studies of cattle on two farms in Scotland.
MATERIALS AND METHODS
Study farms.
Studies of E. coli O157 shedding by cattle were conducted on two mixed beef and sheep farms, designated LE01 and LE02, located 22 km apart in northern Scotland. The number of cattle on farm LE01 ranged from approximately 150 when animals were housed to 250 when cattle were at grass. The number of cattle on farm LE02 ranged from approximately 75 during spring, summer, and autumn to 200 in winter. Three calves born on LE02 were transferred to farm LE01 in July 2001; however, they did not shed E. coli O157. We are not aware of any other cattle transfers between the two farms. The farms did not share a common water source, and the farmers grew their own silage. Both farms bought concentrates and may have had a common supplier. However, we do not know if they received concentrates from a common batch at any time before or during the study.
Bovine and environmental samples from farms LE01 and LE02.
On LE01, samples originated from three longitudinal studies. The primary longitudinal study began in May 2000 and continued until July 2002. Rectal fecal samples were taken from the entire herd every 3 months. Any cattle newly introduced to the farm (born or bought) were sampled monthly for 12 months following introduction to the farm and then sampled quarterly. Any animal found to be shedding E. coli O157 was sampled weekly for 12 weeks, then monthly for 9 months, and then quarterly. Environmental samples, shown in Table 1, were usually taken at the time of quarterly sampling. Sampling was suspended for 5 months from February 2001 because of the occurrence of a foot-and-mouth disease epidemic in the United Kingdom.
TABLE 1.
Environmental samples taken during quarterly sampling on farm LE01 from May 2000 to July 2002
| Sample source | No. of samples taken during month of:
|
|||||
|---|---|---|---|---|---|---|
| May 2000 | September 2000 | December 2000 | June 2001 | September 2001 | March 2002 | |
| Dog feces | 3 | 3 | ||||
| Feed (store) | 3 | 4 | 0 | 3 | 4 | 6 |
| Feed (trough) | 2 | 1 | 8 | 3 | 2 | 4 |
| Sheep feces | 7 | 2 | ||||
| Slurry | 6 | 6 | 2 | 4 | ||
| Straw bedding | 4 | 4 | ||||
| Stream | 1 | 3 | ||||
| Water trough water | 4 | 4 | 3 | 3 | ||
| Water trough sediment | 1 | 4 | 4 | 3 | ||
| Total | 20 | 26 | 22 | 6 | 15 | 22 |
A second longitudinal study, from August 2001 until March 2002, followed shedding patterns of a cohort of spring calves born in 2001. The calves were kept on pasture until they were housed on 25 October 2001, were sampled once in June 2001, and then were sampled every 1 to 2 weeks from July until housing on 25 October 2001. At the time of housing, the calves were separated from their dams and split into two groups according to size, one group with 32 calves and another with 33 calves. Both groups were housed on straw, and each had separate feed and water troughs. The dates, type, and number of samples taken following housing are given in Table 2.
TABLE 2.
Calves born in spring 2001 on farm LE01
| Sampling date | Sampling:
|
No. of:
|
||
|---|---|---|---|---|
| Location (group) | Type | Cattle | Samples | |
| 30 October 2001 | N | Fresh pat | 32 | 10 |
| S | Fresh pat | 33 | 10 | |
| 05 November 2001 | N | Fresh pat | 32 | 10 |
| S | Fresh pat | 33 | 10 | |
| 12 November 2001 | N | Fresh pat | 32 | 10 |
| S | Fresh pat | 33 | 10 | |
| 19 November 2001 | N | Fresh pat | 32 | 30 |
| S | Fresh pat | 33 | 10 | |
| 27 November 2001 | N | Fresh pat | 32 | 10 |
| S | Fresh pat | 33 | 30 | |
| 02 December 2001 | N | Fresh pat | 32 | 10 |
| S | Fresh pat | 33 | 30 | |
| 10 December 2001 | N | Fresh pat | 32 | 20 |
| S | Fresh pat | 33 | 20 | |
| 04 February 2002 | N | Fresh pat | 32 | 20 |
| S | Fresh pat | 33 | 20 | |
| 11 February 2002 | N | Fresh pat | 32 | 20 |
| S | Fresh pat | 33 | 20 | |
| 20 February 2002 | N | Fresh pat | 32 | 20 |
| S | Fresh pat | 33 | 20 | |
| 25 February 2002 | N | Fresh pat | 32 | 20 |
| S | Fresh pat | 33 | 20 | |
| 04 March 2002 | N | Fresh pat | 32 | 20 |
| S | Fresh pat | 33 | 20 | |
| 18 March 2002 | N | Fresh pat | 32 | 20 |
| S | Fresh pat | 33 | 36 | |
| 25 March 2002 | N | Fresh pat | 32 | 20 |
| S | Fresh pat | 33 | 20 | |
| 08 April 2002 | N | Fresh pat | 29 | 20 |
| S | Fresh pat | 12 | 16 | |
| 14 April 2002 | N | Fresh pat | 10 | 20 |
| S | Fresh pat | 13 | 20 | |
The third longitudinal study, from August 2001 to January 2002, studied shedding patterns in calves by examining rectal fecal samples taken within 24 h of birth and then weekly from all calves born in autumn 2001 until 14 January 2002. Rectal samples were also taken from the dams of these calves at the time of birth and again on 14 January 2002.
Samples from farm LE02 resulted from a number of unrelated investigations. Rectal fecal samples from 56 cows and calves and fresh fecal pats from eight enclosures containing the remaining cattle on the farm were sampled on 9 October 2000. The numbers of samples taken from each enclosure were 1, 9, 23, 27, and 35 from five fields, and 11, 16, and 34 from three straw pens. All 132 cattle on the farm were sampled rectally over the course of 4 days in November and December 2000. Seventy-six cows and heifers were tested once rectally on 30 January 2001 and by examining 57 fresh fecal pats in their enclosure on 30 July 2001. A group of 24 cows and calves housed on straw bedding were tested on 30 October 2001 and 5 November 2001 by sampling 21 and 31 fresh fecal pats from their pen, respectively, on these dates. Cattle born in autumn 2000 were sampled rectally on 15 December 2000, 30 July 2001, and 14 September 2001. Subsequently, cattle born in autumn 2000 were tested by sampling fresh fecal pats in their enclosure. The sampling schedule for these animals is given in Table 3. An additional four yearlings were added to this group in early September 2001 while they were at pasture. The group was then reduced to 34 head at the time of housing on 12 October 2001.
TABLE 3.
Yearlings born in autumn 2000 on farm LE02a
| Date | No. of:
|
|
|---|---|---|
| Calves | Samples | |
| 09 August 2001 | 41 | 60 |
| 13 August 2001 | 41 | 61 |
| 20 August 2001 | 41 | 60 |
| 27 August 2001 | 41 | 93 |
| 26 September 2001 | 45 | 65 |
| 01 October 2003 | 45 | 68 |
| 09 October 2001 | 45 | 63 |
| 15 October 2001 | 45 | 63 |
| 23 October 2001 | 34 | 60 |
| 30 October 2001 | 34 | 19 |
| 05 November 2001 | 34 | 20 |
| 12 November 2001 | 34 | 20 |
Animals were at pasture until housed on 12 October 2001.
The sampling procedure for fecal pats did not require identification of the source animal for each pat, and it is possible that during each sampling more than one pat from any particular animal was sampled. All samples, both fecal and environmental, were refrigerated within 2 h of sampling and held at 4°C until testing, which occurred within 48 h of sampling.
E. coli O157 isolates from other sources.
Ten E. coli O157 strains isolated in 2000 and 2001 from humans, a goat, and cattle in various locations around Scotland were obtained from the Department of Medical Microbiology, Grampian University Hospital, Aberdeen, Scotland (see Table 6). We are not aware of any epidemiological connection between these isolates and the farms investigated in this study.
TABLE 6.
List of E. coli O157:H7 strains isolated from different sources in Scotland after restriction with XbaIa
| Location | Origin | PFGE type | Isolate no.b |
|---|---|---|---|
| Edinburgh | Human | 14A | 516 |
| Thurso | Bovine | NDc | 726 |
| Thurso | Human | ND | 1307 |
| Aberdeen | Human | 13A | 5731 |
| Aberdeen | Human | 13A | 5732 |
| Aberdeen | Goat | 13A | 5786 |
| Dumfries | Human | 12A | 5980 |
| Aberdeen | Human | 14B | 5995 |
| Glasgow | Human | ND | 6004 |
| Aberdeen | Human | ND | 6177 |
PFGE patterns were compared to the patterns of isolates studied here.
Reference Laboratory (Foresterhill) isolate number.
ND, PFGE pattern does not match any isolate from this study.
IMS.
Within 48 h of sampling, 1 g of feces from each sample was suspended in 20 ml of buffered peptone water and incubated at 37°C for 6 h. Following incubation, 1 ml of buffered peptone water broth was added to 20 μl of serogroup O157-specific immunomagnetic separation (IMS) beads (Dynal Biotech Ltd., Bromborough, Wirral, United Kingdom) in a screw-cap microcentrifuge tube. The tube contents were mixed on a blood tube rotator for 30 min and then tubes were placed in IMS magnet racks for 5 min. Beads were then washed three times as follows. From each tube, supernatant was removed and beads were resuspended in 1 ml of phosphate-buffered saline with 0.05% Tween. Each tube was inverted gently four to five times and then placed in a magnet rack for 3 min. Following the final wash, the supernatant was removed and beads were resuspended in 50 μl of phosphate-buffered saline with 0.05% Tween. To ensure beads were thoroughly suspended, tubes were held upright and flicked gently several times. Fifty-microliter suspensions of serogroup O157 beads were plated on sorbitol MacConkey agar supplemented with cefixime (2.5 mg l−1) and potassium tellurite (0.05 mg l−1). Non-sorbitol-fermenting colonies were picked and plated onto Chromocult coliform agar (Merck, Poole, Dorset, United Kingdom) and incubated overnight at 37°C; distinctive hazy red-pink colonies were tested with anti-E. coli O157-coated latex reagent (Oxoid, Basingstoke, Hants, United Kingdom). All isolates were stored on cryobeads (Mast Diagnostics) at −80°C before further analysis.
PFGE.
PFGE analysis was based on techniques described elsewhere (7, 12). PFGE was performed on a CHEF DRII apparatus (Bio-Rad Laboratories, Hemel Hampstead, United Kingdom) with pulsed-field certified agarose (Bio-Rad Laboratories) in 0.5× Tris-borate-EDTA buffer. The electrophoresis conditions were a linearly ramped switching time from 2.1 to 54.2 s for 22 h at 14°C at 6.0 V/cm (200 V) for XbaI and 10 to 30 s for 18 h at 14°C at 6.0 V/cm (200 V) for NotI. After PFGE, the gels were stained with ethidium bromide, photographed, and recorded with the Bio-Rad diversity database software image capturing system. To rule out incomplete digestion, each isolate was digested and subjected to PFGE twice. To normalize bands from one gel to another, a midrange molecular weight lambda marker (New England Biolabs) was included in three lanes of each gel and an internal E. coli O157 control was included on every gel. The internal control (WX002277S01E) was selected from E. coli O157 strains isolated in this study. The PFGE profile of the internal control belongs to the most common pattern (14A). The analysis was performed with BioNumerics software, version 3.0 (Applied Maths, Ghent, Belgium). Fragments smaller than 48.5 kb in length were not used in analysis. This software facilitated the development of the algorithms necessary for further gel analysis, including the comparison of profiles of isolates based on the Dice coefficient, preparation of a phylogenetic tree, and cluster analysis with the hierarchic unweighted pair arithmetic average algorithm.
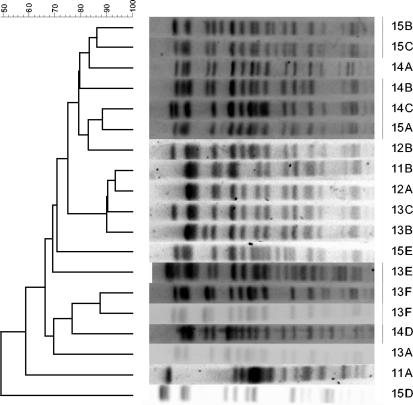
The most common PFGE pattern (14A; 65 of 293 isolates) in this study was designated as the pivotal baseline for the demonstration and comparison of the similarities and differences between each group in a simplified fashion.
Detection of Shiga toxins and intimin (eae) genes.
Multiplex PCR for identifying Shiga-like toxin and fimbrial adhesin intimin-producing isolates was as previously described (21, 35), except for the following modifications. Crude DNA was prepared by inoculating a colony into 50 μl of water and boiling the cultures for 10 min. HotStarTaq master mix kit (Qiagen GmbH, Hilden, Germany) was used in a total volume of 50 μl. The reaction was carried out in a Gene Amp PCR system 9700 (Perkin-Elmer) thermal cycler at 95°C for 15 min, with 30 cycles of 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min and a final extension at 72°C for 10 min. Each PCR product was visualized by electrophoresis on a 2% agarose gel and staining with ethidium bromide.
Repeated subculturing of E. coli O157 strain WF002277S01E.
Strain WF002277S01Ewas continuously subcultured on Luria-Bertani broth and Luria-Bertani agar plates five times per week for 22 weeks (110 times total) at 37°C overnight. One colony was randomly taken each week and subjected to PFGE analysis.
Multiple isolates from the same animal.
Twelve colonies of E. coli O157 were selected randomly from all of the available positive colonies on three different samples. PFGE was performed as mentioned above.
Antibiotic sensitivity testing.
Breakpoint sensitivity testing was performed on Iso-Sensitest agar (Oxoid) by a standard agar dilution method (6) at antibiotic concentrations of 4, 8, and 16 mg/liter for ampicillin, cefaclor, cefotaxime, cefuroxime, chloramphenicol, gentamicin, streptomycin, tetracycline, nalidixic acid, and trimethoprim and at 256 mg/liter for sulfamethoxazole. Bacterial strains were grown overnight and diluted to 104 CFU/ml. The isolates were inoculated on plates containing different concentrations of antibiotics with a multipoint inoculator (Denley, Billinghurst, United Kingdom) to give a final concentration of 2 × 102 CFU per spot. After inoculation, the plates were incubated overnight at 37°C.
RESULTS
Prevalence of shedding.
The crude prevalence of shedding among cattle on farm LE01 during quarterly sampling ranged from 0.0 to 18.7%. In December 2000 and in June 2001, following movement restrictions resulting from the United Kingdom foot-and-mouth disease outbreak, no shedding was detected. Shedding was highest in September 2000 (5.6%) and September 2001 (18.7%). At other quarterly samplings, the prevalence of shedding was 2.0 to 3.0%.
Among calves in the second longitudinal study on farm LE01, the prevalence of shedding ranged from 0.0 to 48.0% while on pasture prior to housing. Following housing, waves of shedding were observed in both groups, and the shedding prevalence ranged from 0.0 to 90.0%. A total of 664 samples were taken from 49 calves in the third longitudinal study. Only 4 calves shed E. coli O157, each on one occasion only.
On farm LE02, E. coli O157 was detected in 12 of 156 (7.7%) fecal pat samples, but none was detected in any of the rectal samples from cows and calves during the initial screening on October 9 2000. With the exception of a group of cows and calves sampled on 30 October 2001 and 5 November 2001 and the calves born in autumn 2000, E. coli O157 was not subsequently detected in rectal samples or fecal pat samples from other cattle on the farm during the course of the study. Among fecal pats sampled in the enclosure holding 24 cows and calves on 30 October 2001 and 5 November 2001, E. coli O157 was detected in 7 of 21 (33.3%) and 5 of 31 (16.1%) fecal pats on these dates, respectively. Among the cattle born in autumn 2000, 2 of 40 (5.0%) were shedding E. coli O157 on 30 July 2001. Subsequently, while at pasture, the proportion of fecal pats testing positive for E. coli O157 in enclosures holding this group of cattle ranged from 4.4 to 44.1%. In the week following housing, the proportion of fecal pats testing positive for E. coli O157 rose to 68.8%, peaked at 98.3% the following week, and remained higher than 50% until 12 November 2001, when sampling of this group ceased.
E. coli O157 isolates.
A total of 293 isolates was obtained from the two farms. Forty-nine of these isolates were taken from 30 animals on LE01 before the foot-and-mouth disease outbreak (before February 2001), 45 isolates were fecal (i.e., taken direct from the ox), and 4 isolates were environmental samples obtained from a water trough, two fecal pats, and a dog. Eight cattle yielded at least 2 isolates. After the foot-and-mouth disease outbreak (from August 2001), 145 fecal isolates were obtained from farm LE01. Twenty-two of these animals yielded at least two isolates. Eighty-eight samples were obtained from farm LE02 after the foot-and-mouth disease outbreak: 19 fecal samples and 69 pat samples.
PFGE patterns of isolates.
We analyzed 293 E. coli O157 isolates and demonstrated 19 different PFGE patterns after genomic digestion with the restriction enzyme XbaI. The PFGE patterns of these groups are shown in Fig. 1. The PFGE groups detected differ from the baseline pattern, 14A, by 1 to 11 bands. Our results show that most pattern variations occurred in the region of 300 to 500 kb. As illustrated in Table 4, certain patterns were specific to an individual farm. For example, pattern 12A occurred only on LE01, whereas pattern 12B was only seen on LE02. Two patterns, 14A and 15A, were found on LE01 before but not after the foot-and-mouth disease epidemic in 2000. Variant 14A was detected continuously on LE02 from 2001. Some patterns (11A, 11C, and 14F) were only detected for short, discrete periods, but others were found on numerous sampling occasions (12A, 13B, 13F, and 14A).
FIG. 1.
Phylogenic analysis of E. coli O157 isolates by PFGE gel pattern following restriction with XbaI. Dice similarities were subjected to cluster analysis as unweighted matched-pair groups.
TABLE 4.
Classification of PFGE patterns obtained with XbaI restriction enzyme and comparison to pattern 14A
| Pattern | No. of bands similar to 14A | No. of bands different from 14A | No. of isolates from farm:
|
Total | |||
|---|---|---|---|---|---|---|---|
| LE01
|
LE02
|
||||||
| BFMa | AFMb | BFM | AFM | ||||
| 11A | 10 | 5 | 1 | 0 | 0 | 0 | 1 |
| 11B | 9 | 7 | 0 | 3 | 0 | 0 | 3 |
| 12A | 10 | 6 | 0 | 62 | 0 | 0 | 62 |
| 12B | 10 | 6 | 0 | 0 | 11 | 0 | 11 |
| 13A | 13 | 1 | 8 | 0 | 0 | 0 | 8 |
| 13B | 11 | 5 | 0 | 53 | 0 | 0 | 53 |
| 13C | 10 | 7 | 0 | 2 | 0 | 0 | 2 |
| 13E | 11 | 5 | 0 | 3 | 0 | 0 | 3 |
| 13F | 13 | 1 | 0 | 0 | 0 | 43 | 43 |
| 14A | 25 | 0 | 0 | 40 | 65 | ||
| 14B | 13 | 2 | 4 | 0 | 0 | 0 | 4 |
| 14C | 12 | 4 | 8 | 3 | 0 | 0 | 11 |
| 14D | 12 | 4 | 0 | 0 | 0 | 1 | 1 |
| 14F | 12 | 2 | 0 | 0 | 0 | 2 | 2 |
| 15A | 14 | 1 | 2 | 0 | 0 | 1 | 3 |
| 15B | 12 | 5 | 1 | 0 | 0 | 0 | 1 |
| 15C | 14 | 1 | 0 | 17 | 0 | 1 | 18 |
| 15D | 11 | 7 | 0 | 1 | 0 | 0 | 1 |
| 15E | 14 | 4 | 0 | 1 | 0 | 0 | 1 |
| Total | 49 | 145 | 11 | 88 | 293 | ||
BFM, before foot-and-mouth epidemic outbreak.
AFM, after foot-and-mouth epidemic outbreak.
At each sampling point there was usually more than one PFGE pattern of E. coli O157 present on the farms. We observed that one ox can shed E. coli O157 with different genotypic patterns at consecutive sampling times (Table 5). A particular example was an ox from farm LE01 (LE01.0647) which was sampled four times within a month and a different pattern was observed each time. Initially, this animal shed E. coli O157 with PFGE pattern 13B; at the following sampling, the band at 280 kb was not detected and, therefore, the pattern group changed to 13E. On the third occasion, this ox shed E. coli O157 with a different pattern (12A) missing two bands at 280 and 388 kb. On the fourth occasion, the original PFGE pattern (13B) was found once again. PFGE was performed on these isolates after restriction with NotI enzyme. Patterns 13B and 13E had similar profiles, whereas pattern 12A differed by at least three bands.
TABLE 5.
Different PFGE groups detected in E. coli O157-shedding animals on both farms during sampling
| Animal no. | PFGE group(s) detected on sampling date:
|
||||||
|---|---|---|---|---|---|---|---|
| 30 July 2001 | 18 August 2001 | 25 August 2001 | 1 September 2001 | 8 September 2001 | 15 September 2001 | 6 October 2001 | |
| LE01.0245 | 12A | 13B | |||||
| LE01.0567 | 13C | 13B | 13B | ||||
| LE01.0569 | 13B | ||||||
| LE01.0571 | 11B | 12A | |||||
| LE01.0573 | 13B | 15C | 15C | ||||
| LE01.0574 | 12A | 12A | 12A | 12A | |||
| LE01.0587 | 13B | 12A | |||||
| LE01.0590 | 13C | 12A | |||||
| LE01.0594 | 13B | 12A | 12A | ||||
| LE01.0597 | 12A | 12A | 12A | ||||
| LE01.0611 | 12A | 13B | 13B | ||||
| LE01.0631 | 12A | 12A | 13B | ||||
| LE01.0634 | 12A | 13B | |||||
| LE01.0636 | 13B | 13B | 12A | 13B | |||
| LE01.0647 | 13B | 13E | 12A | 13B | |||
| LE02.0014 | 14A | 13F | |||||
| LE02.0016 | 14A | 14A | |||||
| LE02.0026 | 14A | 14A | |||||
| LE02.0171 | 13F | 13F | |||||
PFGE analysis of multiple isolates from the same ox.
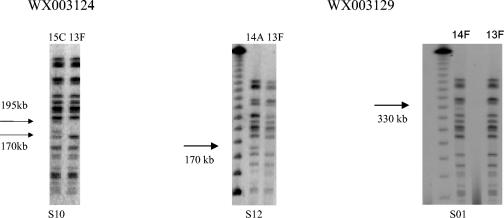
Thirty-six isolates were selected purposively from three positive samples. All 12 picks from one of the samples (WX003137) had indistinguishable PFGE patterns after digestion with XbaI. The other two samples (WX003124 and WX003129) contained strains represented by more than one PFGE group. Figure 2 shows the different PFGE variants after the genomic DNA was digested with XbaI. From the plating of sample WX003124, Twelve colonies were selected. All colonies had identical PFGE profiles (pattern 13F), except the 10th pick (S10) that was PFGE profile 15C.
FIG. 2.
PFGE patterns of isolates obtained from 12 colony picks from two plates. Twelve colonies (S01 to S12) were picked from plate WX003124 (left). Twelve colonies were picked from plate WX003129 (right).
From the plating of sample WX003129, 12 colonies were selected. Isolates from 10 picks (S02 to S11) had an indistinguishable pattern (13F), an isolate from another pick (S01) showed an extra band at 330 kb (pattern 14F), and an isolate (S12) from the remaining picks showed an extra band at 170 kb (pattern 14A).
Comparison of PFGE patterns of E. coli O157 from cattle with E. coli O157 from other sources.
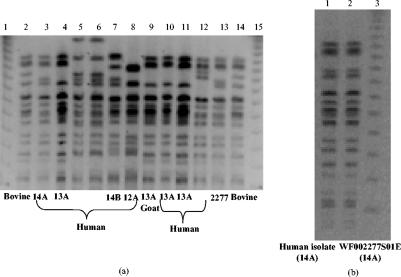
Table 6 shows the E. coli O157 strains isolated from different sources in Scotland. PFGE was performed on these strains, and the results were compared to the PFGE patterns of the strains isolated from the two study farms. Figure 3a displays these PFGE patterns. As shown, some of the isolates have indistinguishable patterns from the strains isolated from LE01 and LE02. Figure 3b shows the PFGE profile of one human isolate obtained from farm LE01. This isolate displays a PFGE profile identical to pattern 14A, which was the most common profile on LE01 before the foot-and-mouth disease outbreak. It is therefore possible that the patient was infected as a result of bovine shedding on the same farm. The E. coli O157 isolate recovered from the canine fecal sample from farm LE01 had PFGE pattern 15A, which was identical to a bovine sample recovered from that farm. The same pattern was later detected on farm LE02.
FIG. 3.
Comparison between PFGE patterns of E. coli O157:H7 obtained from different sources to the PFGE patterns of bovine strains in this study. (a) Lanes 1, 15: PFGE λ ladder marker. Lane 2: bovine isolate 726. Lane 3: human isolate 516. Lane 4: human isolate 5731. Lane 5: human isolate 6177. Lane 6: human isolate 6004. Lane 7: human isolate 5995. Lane 8: human isolate 5980. Lane 9: goat isolate 5786. Lane 10: human isolate 5732. Lane 11: human isolate 5731. Lane 12: human isolate 1307. Lane 13: internal control isolate WF002277S01E. Lane 14: bovine isolate 726. (b) Lane 1: PFGE pattern of E. coli O157:H7 isolate from patient on farm LE01. Lane 2: internal control E. coli O157:H7 from cattle on LE01. Lane 3: PFGE λ marker.
Analysis of PFGE patterns of E. coli O157 from calves and dams.
From 28 August 2001 to 4 January 2002, only four E. coli O157 strains were isolated from LE01, and they were from four different calves born to dams that did not shed E. coli O157. All had the same PFGE pattern (12A), the most common group on that farm. Ten dams shedding E. coli O157 with PFGE pattern 12A on 3 September 2001 gave birth to calves that did not shed E. coli O157 during this study.
Repeated subculturing of E. coli O157 WF002277S01E.
The PFGE pattern of our internal control E. coli O157 strain (WF002277S01E), selected from E. coli O157 strains isolated in this study, did not show any variation from the original pattern after it was continuously subcultured 110 times in broth and on solid media. This observation suggests that the genome of our internal standard was stable and that our experimental conditions had no affect on the stability of this strain.
E. coli O157 susceptibility to antibiotics.
Two hundred ninety-three E. coli O157 strains obtained from the two farms were tested. They were sensitive to ampicillin, nalidixic acid, cefaclor, cefuroxime, cefotaxime, and gentamicin at breakpoint concentrations of 4 and 8 mg/liter. All isolates were resistant to 256-mg/liter sulfamethoxazole. Thirty-four (11.6%) isolates were resistant to 8-mg/liter chloramphenicol, and only nine (3.07%) of those were resistant to 16-mg/liter chloramphenicol. Isolate WF006477S01E was obtained from farm LE02 (LE02.0005) and was found to be resistant to both 16-mg/liter chloramphenicol and 8-mg/liter tetracycline. A strain isolated from pat sample WX004092S01E in an enclosure with calves on farm LE02 was resistant to 8-mg/liter chloramphenicol and 8-mg/liter tetracycline.
DISCUSSION
Our results demonstrated a high variation among strains of E. coli O157 in just two farms in the same vicinity over a 2-year period. Diversity of subtypes among cattle has been reported elsewhere (2, 10), but isolates were obtained from a vast geographical area and included more than two farms. In one study, 20 PFGE subtypes were reported from 160 isolates from 29 dairy cattle and 3 water troughs (10), and in the other study, 81 subtypes from 376 isolates were found (24). Akiba et al. (2) obtained 50 PFGE profiles from E. coli O157:H7 isolates from 77 fecal samples isolated from 23 different regions in Japan. Rice et al. (24) have previously detected an average of 5.5 (3 to 11) XbaI subtypes per farm among feedlot farms in the United States. In England and Wales, up to three different verotoxigenic E. coli O157 strains were found among animals on a single farm (36). Our results demonstrated the presence of 19 PFGE subtypes in a localized cattle population on two beef farms in Scotland. Of these 19 PFGE subtypes, 15 and 7 different XbaI subtypes of E. coli O157 were present on farms LE01 and LE02, respectively, highlighting a high diversity over a 2-year period in a small geographic area. We observed that most of the bands distinguishing between the subtypes are in the region of 300 to 500 kb. The number of fragment differences between each pattern and the baseline group varied from 1 to 11 bands. The criteria applied for PFGE analysis in terms of numbers of band differences are important (30). We support the notion that the Tenover criteria may not be applicable to the subtyping of E. coli O157 and that one band difference is more important than indicated previously (32).
Iguchi and colleagues (14) observed that it is possible, by changing the storage environment, growth medium, and inoculating more than one colony for each experiment, that E. coli O157 strains may show differences of two or three fragments in their PFGE patterns. In our laboratory, the continuous subculturing of one fecal E. coli O157 isolate did not show any variation from the original PFGE pattern. Our finding indicates that this strain was genetically stable and that our experimental conditions had no affect on the stability of this strain. Therefore, we anticipated that under our experimental and storage conditions, study strains would remain stable. However, subculturing in the laboratory is not comparable to growth of the organisms inside the animals, and as Faith and colleagues (10) suggest, it may be possible that the turnover of E. coli O157 is faster in situ than in the laboratory.
To date, two mechanisms have been proposed to explain the diversity and strain variation in E. coli O157. Kudva et al. (18) reported that such differences are caused by discrete insertions or deletions of segments of DNA in the genome rather than point mutations in the XbaI sites. They also reported that the majority of genomic differences between E. coli O157:H7 strains occur in O island sequences, suggesting that phage-mediated events may be associated with diversity (16, 18). Diversity has also been proposed to occur in the E. coli O157:H7 genome through the transient appearance of mutator strains, which enhance the rate at which foreign DNA can be combined into the genome (19, 20).
There are a number of potential environmental sources of E. coli O157, including wild animals, farm vehicles, ingredients in animal feed (8, 9), and farm employees, which may transfer transient bacteria onto a farm (3, 11, 27, 28). Manure management, water trough sanitation, and feed management (13, 15) all contribute to preventing transient bacteria from becoming resident. In this study, there was only one strain isolated from water troughs. This strain had PFGE pattern 14A, the pattern most commonly found on farm LE01 prior to the outbreak of foot-and-mouth disease.
During the course of sampling, cattle movement was banned on both farms for several months to minimize the risk of foot-and-mouth disease infection. The PFGE profile of E. coli O157 strains contaminating the farms, especially LE01, was dramatically altered. The number of samples obtained from farm LE02 before the foot-and-mouth disease outbreak was not sufficient to enable us to draw similar conclusions for that farm. However, it is worth noting that two patterns that were detected on LE01 before the foot-and-mouth disease outbreak (14A and 15A) were found on LE02; this observation is not related to the transfer of the three calves from LE01 to LE02. The restriction of movement of animals during the foot-and-mouth disease epidemic may have played a role in the alteration of E. coli O157 subtypes transient or endemic to the farms. In general, the turnover of cattle on LE01 was relatively high, and therefore, the introduction of different strains of E. coli O157 may have occurred when new cattle arrived on the farm. The presence of indistinguishable subtypes on LE01 and LE02 suggests the possibility of one or more common sources of E. coli O157 strains for both farms.
At a single sampling point, more than one pattern of E. coli O157 was usually detected on each farm. There were also instances when an animal shed more than one subtype of E. coli O157 over a 4-week period on different sampling occasions. These observations may be a result of simultaneous shedding of more than one strain of E. coli O157 or caused by mutational events in the genome in vivo. Kudva et al. (17) demonstrated that multiple strains of E. coli O157 in sheep may be shed from a single animal simultaneously. On the other hand, Akiba et al. (4) observed a total of nine genetic subtypes from a total of 46 isolates from seven cattle over a 2-month period. Since most of the isolates were highly related, it was assumed that a single strain that colonized cattle on that farm could have mutated slightly during either carriage by cattle or in the farm environment.
In our study, analyzing multiple isolates suggested that by far the majority of isolates shed by any given ox comprised indistinguishable PFGE patterns. However, we have observed different PFGE profiles of E. coli O157 shed by one ox. Although it is possible that the observed subtle PFGE variants are caused by genetic changes of the dominant variant within the animal, the results of our multiple-pick study strongly suggest the possibility of mixed infection in cattle. A study with more intensive sampling is required to examine this issue.
We could not detect any correlation between E. coli O157 shedding in dams and their calves. There were dams that gave birth to non-E. coli O157-shedding calves and E. coli O157:H7-shedding calves that were born from non-E. coli O157-shedding dams. This supports the notion that environmental spread is important in the dissemination of E. coli O157 among cattle.
The continued overwhelming sensitivity of E. coli O157 in this study to almost all antibiotics tested is astonishing considering the rapid increases in resistance found in other zoonotic bacteria such as Salmonella spp., Campylobacter spp. (1, 33), and other E. coli serotypes from the same farm (D. Hoyle, C. Yates, M. Pearce, H. Knight, G. Gunn, M. Woolhouse, and S. Amyes, Abstr. 12th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. P612, 2002). Interestingly, unlike other studies (11, 26, 31) where high rates of resistance to ampicillin (2 to 48%), tetracycline (4 to 27%), sulfamethoxazole (10 to 26%), streptomycin (2%), and chloramphenicol (<2%) were detected in E. coli O157 recovered from humans and cattle, we only found high-level resistance to sulfamethoxazole (100%) and chloramphenicol (3.6%) and a very low prevalence of resistance to other antibiotics. Temporal and geographical differences between these studies may be the reason for high discrepancies. According to the farmer's records on LE01, the sampled animals had not been treated with antibiotics. The isolates in our study were from untreated animals and therefore had not been exposed to elevated concentrations of antimicrobials as a result of treatment efforts. Most of the resistant isolates were obtained from farm LE02, and the antibiotic treatment records for animals were not available. Continued surveillance of antibiotic resistance among zoonotic E. coli O157 is important for determining the presence and prevalence of resistant strains.
Acknowledgments
This study is a part of the International Partnership Research Award in Veterinary Epidemiology (IPRAVE), “Epidemiology and Evolution of Enterobacteriaceae Infections in Humans and Domestic Animals,” funded by the Wellcome Trust (grant no. 073958/A/03/Z).
We thank the Department of Medical Microbiology, Grampian University Hospital, for the provision of E. coli O157 strains from animal and human patients for comparative purposes.
REFERENCES
- 1.Aarestrup, F. M., E. M. Nielsen, M. Madsen, and J. Engberg. 1997. Antimicrobial susceptibility patterns of thermophilic Campylobacter spp. From humans, pigs, cattle, and broilers in Denmark. Antimicrob. Agents Chemother. 41:2244-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiba, M., T. Masuda, T. Sameshima, K. Katsuda, and M. Nakazawa. 1999. Molecular typing of Escherichia coli O157:H7 (H−) isolates from cattle in Japan. Epidemiol. Infect. 122:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiba, M., D. H. Rice, M. A. Davis, T. Masuda, T. Sameshima, M. Nakazawa, and D. D. Hancock. 2000. A comparison of Escherichia coli O157 isolates from cattle in Japan and the USA by molecular biological methods. Epidemiol. Infect. 125:221-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiba, M., T. Sameshima, and M. Nakazawa. 1999. The shift of genetic subtypes of Escherichia coli O157:H7 isolates from cattle. Epidemiol. Infect. 122:343-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allison, L. J., P. E. Carter, and F. M. Thomson-Carter. 2000. Characterization of recurrent clonal type of Escherichia coli O157:H7 causing major outbreaks of infection in Scotland. J. Clin. Microbiol. 38:1632-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews, J. M. 2001. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 48:5-16. [DOI] [PubMed] [Google Scholar]
- 7.Barrett, T. J., H. Lior, J. H. Green, R. Khakharia, J. G. Wells, B. P. Bell, K. D. Greene, J. Lewis, and P. M. Griffin. 1994. Laboratory investigation of a multistate food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. J. Clin. Microbiol. 32:3013-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis, M. A., D. D. Hancock, D. H. Rice, D. R. Call, R. DiGiacomo, M. Samadpour, and T. E. Besser. 2003. Feedstuffs as a vehicle of cattle exposure to Escherichia coli O157:H7 and Salmonella enterica. Vet. Microbiol. 95:199-210. [DOI] [PubMed] [Google Scholar]
- 9.Dodd, C. C., M. W. Sanderson, J. M. Sargeant, T. G. Nagaraja, R. D. Oberst, R. A. Smith, and D. D. Griffin. 2003. Prevalence of Escherichia coli O157 in cattle feeds in Midwestern feedlots. Appl. Environ. Microbiol. 69:5243-5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faith, N. G., J. A. Shere, R. Brosch, K. W. Arnold, S. E. Ansay, M. S. Lee, J. B. Luchansky, and C. W. Kasper. 1996. Prevalence and clonal nature of Escherichia coli O157:H7 on dairy farms in Wisconsin. Appl. Environ. Microbiol. 62:1519-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galland, J. C., D. R. Hyatt, S. S. Crupper, and D. W. Acheson. 2001. Prevalence, antibiotic susceptibility, and diversity of Escherichia coli O157:H7 isolates from a longitudinal study of beef cattle feedlots. Appl. Environ. Microbiol. 67:1619-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goutom, R. K. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 35:2977-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hovde, C. J., P. R. Austin, K. A. Cloud, C. J. Williams, and C. W. Hunt. 1999. Effect of cattle diet on Escherichia coli O157:H7 acid resistance. Appl. Environ. Microbiol. 65:3233-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iguchi, A., R. Osawa, J. Kawano, A. Shimizu, J. Terajima, and H. Watanabe. 2002. Effects of repeated subculturing and prolonged storage at room temperature of enterohemorrhagic Escherichia coli O157:H7 on pulsed-field gel electrophoresis profiles. J. Clin. Microbiol. 40:3079-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph, S. W., D. T. Ingram, and J. B. Caper. 2002. The epidemiology, pathogenicity and microbiology of foodborne Escherichia coli O157:H7. Rev. Med. Microbiol. 13:53-62. [Google Scholar]
- 16.Kim, J., J. Neitfeldt, J. Ju, J. Wise, N. Fegan, P. Desmarchelier, and A. K. Benson. 2001. Ancestral divergence, genome diversification, and phylogeographic variation in subpopulations of sorbitol-negative, β-glucuronidase-negative enterohemorrhagic Escherichia coli O157. J. Bacteriol. 183:6885-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudva, I. T., C. W. Hunt, C. J. Williams, U. M. Nance, and C. J. Hovde. 1997. Evaluation of dietary influences on Escherichia coli O157:H7 shedding by sheep. Appl. Environ. Microbiol. 63:3878-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudva, I. T., P. S. Evans, N. T. Perna, T. J. Barrett, F. M. Ausubel, F. R. Blattner, and S. B. Calderwood. 2002. Strains of Escherichia coli O157:H7 differ primarily by insertions or deletions, not single nucleotide polymorphisms. J. Bacteriol. 184:1873-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeClerc, E. J., B. Li, W. L. Payne, and A. T. Cebula. 1996. High mutation frequencies among Escherichia coli and Salmonella pathogens. Science 274:1208-1211. [DOI] [PubMed] [Google Scholar]
- 20.Matic, I., M. Radman, F. Taddei, B. Picard, C. Doit, E. Bingen, E. Denamur, and J. Elion. 1997. Highly variable mutation rates in commensal and pathogenic Escherichia coli. Science 277:1833-1834. [DOI] [PubMed] [Google Scholar]
- 21.Meng, J., S. Zhao, M. P. Doyle, S. E. Mitchell, and S. Kresovich. 1997. A multiplex PCR for identifying Shiga-like toxin-producing Escherichia coli O157:H7. Lett. Appl. Microbiol. 24:172-176. [DOI] [PubMed] [Google Scholar]
- 22.Pickering, L. K. 2001. Antibiotic therapy of colitis. Pediatr. Infect. Dis. J. 20:465-466. [DOI] [PubMed] [Google Scholar]
- 23.Pruimboom-Brees, I. M., T. W. Morgan, M. R. Ackermann, E. D. Nystrom, J. E. Samuel, N. A. Cornick, and H. W. Moon. 2000. Cattle lack vascular receptors for Escherichia coli O157:H7 Shiga toxins. Proc. Natl. Acad. Sci. USA 97:10325-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice, D. H., K. M. McMenamin, L. C. Pritchett, D. D. Hancock, and T. E. Besser. 1999. Genetic subtyping of Escherichia coli O157 isolates from 41 pacific Northwest USA cattle farms. Epidemiol. Infect. 122:479-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Herbert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder, C. M., C. Zhao, C. DebRoy, J. Torcolini, S. Zhao, D. G. While, D. D. Wagner, P. F. McDermott, R. D. Walker, and J. Meng. 2002. Antimicrobial resistance of Escherichia coli O157 isolated from humans, cattle, swine, and food. Appl. Environ. Microbiol. 68:576-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shere, J. A., K. J. Bartlett, and C. W. Kaspar. 1998. Longitudinal study of Escherichia coli O157:H7 dissemination on four dairy farms in Wisconsin. Appl. Environ. Microbiol. 64:390-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shillam, P., A. Woo-Ming, L. Mascola, R. Bagby, C. Lohff, S. Bidol, M. G. Stobierski, C. Carlson, L. Schaefer, L. Kightlinger, S. Seys, K. Kubota, P. S. Mead, and P. Kalluri. 2002. Multistate outbreak of Escherichia coli O157:H7 infections associated with eating ground beef-United States, June-July 2002. Morb. Mortal. Wkly. Rep. 51:637-639. [PubMed] [Google Scholar]
- 29.Shiomi, M., M. Togawa, K. Fujita, and R. Murata. 1999. Effect of early oral fluoroquinolones in hemorrhagic colitis die to Escherichia coli O157:H7. Pediatr. Int. 41:228-232. [DOI] [PubMed] [Google Scholar]
- 30.Smith, D., G. Willshaw, J. Stanley, and C. Arnold. 2000. Genotyping of verocytotoxin-producing Escherichia coli O157: comparison of isolates of a prevalent phage type by fluorescent amplified-fragment length polymorphism analysis. J. Clin. Microbiol. 38:4616-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swartz, M. N. 2002. Human diseases caused by foodborne pathogens of animal origin. Clin. Infect. Dis. 34(Suppl. 3):S111-S122. [DOI] [PubMed] [Google Scholar]
- 32.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Threlfall, E. J., L. R. Ward, J. A. Frost, G. A. Willshaw. 2000. The emergence and spread of antibiotic resistance in food-borne bacteria. Int. J. Food Microbiol. 62:1-5. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe, H., J. Terajima, H. Izumiya, A. Wada, and K. Tamura. 1999. Molecular analysis of enterohemorrhagic Escherichia coli isolates in Japan and its application to epidemiological investigation. Pediatr. Int. 41:202-208. [DOI] [PubMed] [Google Scholar]
- 35.Willshaw, G. A., S. M. Scotland, H. R. Smith, T. Cheasty, A. Thomas, and B. Rowe. 1994. Hybridization of strains of Escherichia coli O157 with probes derived from the eaeA gene of enteropathogenic E. coli and the eaeA homolog from a Vero cytotoxin-producing strain of E. coli O157. J. Clin. Microbiol. 32:897-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willshaw, G. A., H. R. Smith, T. Cheasty, and S. J. O'Brien. 2001. Use of strain typing to provide evidence for specific interventions in the transmission of VTEC O157 infections. Int. J. Food Microbiol. 66:39-46. [DOI] [PubMed] [Google Scholar]
- 37.Wong, C. S., S. Jelacic, R. L. Habeeb, S. L. Watkins, and P. I. Tarr. 2000. The risk of hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Engl. J. Med. 342:1930-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]