Abstract
Mycobacterium tuberculosis Rv0560c, a putative benzoquinone methyl transferase, is heavily induced in response to salicylate exposure. It has some similarity to Escherichia coli UbiG, although its role in ubiquinone or menaquinone synthesis is not clear, since M. tuberculosis is not known to produce ubiquinone. We constructed an unmarked in-frame deletion of Rv0560c in M. tuberculosis to determine its role in vitro. Deletion of Rv0560c in M. tuberculosis had no effect on growth in medium containing salicylate or in its ability to grow in macrophages. In addition, no change to compound sensitivity, as determined by minimum inhibitory concentrations, for a range of compounds targeting respiration was noted. Plumbagin, ethambutol and CCCP had the same minimum bactericidal concentration against the deletion and wild-type strains. Taken together these data show that Rv0560c is dispensable under in vitro conditions in both axenic and macrophage culture and suggest that the role of Rv0560c may be in an alternate biosynthetic pathway of menaquinone which is only used under specific growth conditions.
Keywords: menaquinone, non-essential genes, virulence, mycobacteria
1. Introduction
When the genome of M. tuberculosis strain H37Rv was sequenced in 1998, ~60% of the predicted 3924 proteins were listed as either hypothetical or assigned a suggested function based on similarity [1]. Since then, many new gene functions have been ascribed, but the function of a large proportion of genes remains unclear [2, 3].
Rv0560c encodes a putative S-adenosylmethionine-dependent methyl transferase [4] which is highly induced in response to salicylate [5] and a number of structural analogs [4, 6]. Rv0560c is transcribed from a highly inducible promoter as a bicistronic transcript with Rv0559c [6]. Based on its chromosomal location near other genes involved in menaquinone or isoprenoid biosynthesis, it is proposed to be a benzoquinone methyl transferase involved in the synthesis of the redox cycling agent menaquinone [1, 3].
Exposure to salicylate in Escherichia coli results in the multiple antibiotic resistant (Mar) phenotype [7]; M. tuberculosis has a similar phenotype showing decreased sensitivity to several antibiotics after salicylate exposure [8]. It is not clear if the M. tuberculosis Mar phenotype results from similar mechanisms, which involves induction of Mar proteins and other machinery that result in decreased antibiotic accumulation [7]. Rv0560c may have a role in the Mar phenotype since it is upregulated by salicylate as well as gemfibrozil, fenofibrate and clofibrate [4, 9]. Promoter analysis also demonstrated that Rv0560c was induced by aspirin and PAS [6].
Rv0560c is not predicted to be essential for in vitro growth or for survival in macrophages, but the role of Rv0560c is unclear [10, 11]. In this study, we constructed a deletion strain of Rv0560c with the goal of discerning its role. We characterized the deletion strain with respect to sensitivity to salicylate and several antibiotics and survival/growth in macrophages.
2. Results
We were interested in the role of Rv0560c in M. tuberculosis. We hypothesized that it would be involved in the synthesis of menaquinone or a related molecule under specific conditions which might be relevant to infection. To address this we constructed and phenotypically characterized a deletion strain in M. tuberculosis.
2.1 Rv0560c is not essential in axenic culture
We used a two-step homologous recombination method to construct an unmarked in-frame deletion strain of Rv0560c in H37Rv (Figure S1). We were able to isolate the strain with no difficulty, demonstrating that Rv0560c is not required for growth in axenic culture. The strain was confirmed by Southern analysis (Figure S1D). The deletion strain was complemented using a single copy, integrating vector in which Rv0560c was expressed from its native promoter, as previously identified [6].
2.2 Deletion of Rv0560c does not increase sensitivity to salicylate growth inhibition
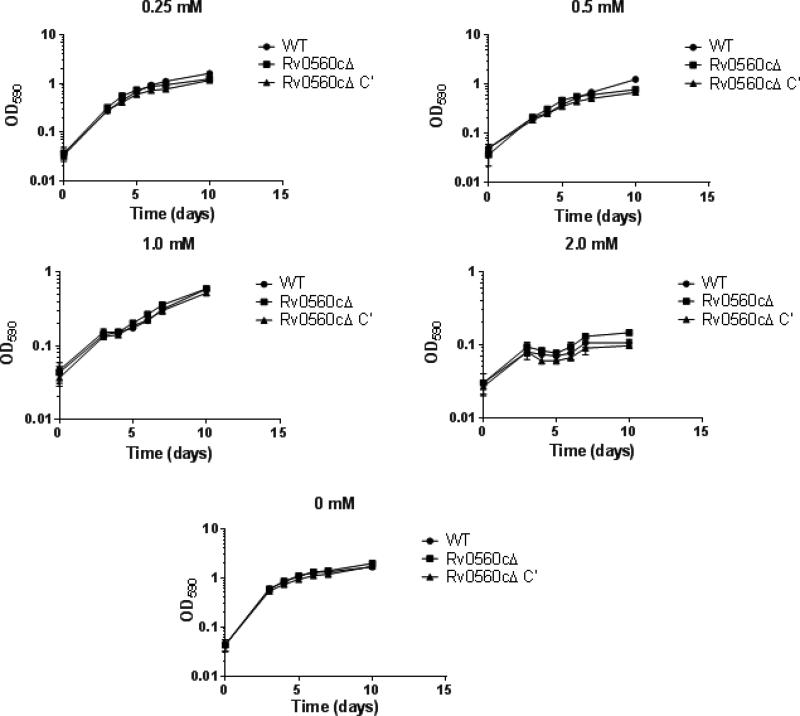
We reasoned that the high level of expression of Rv0560c after exposure to salicylate [5] might confer some protective effect to the bacteria under these conditions. We had previously noted that a high concentration of salicylate was inhibitory to growth. We tested the growth of the deletion and complemented strain in increasing concentrations of salicylate. All three strains grew well without salicylate, with no appreciable difference. Higher concentrations of salicylate progressively inhibited growth up to the highest concentration tested (2 mM) (Figure 1). There was no appreciable difference in growth rate or in final OD590 reached between the three strains at salicylate concentrations between 0.25 mM and 2 mM (Figure 1). These data demonstrate that Rv0560c is not needed for growth in the presence of low concentrations of salicylate, despite the massive upregulation of Rv0560c seen under this condition [5, 6, 12]. We also tested several other stress conditions, including low pH, hypoxia, and nutrient starvation, but saw no differences between the strains (data not shown).
Figure 1. Growth of recombinant strains in salicylate.
M. tuberculosis strains were grown in 5 mL 7H9-Tw-OADC plus salicylate at the indicated concentration in 16mm glass tubes with stirring. Data are the average ± standard deviation of three independent cultures.
2.3 Deletion of Rv0560c has no effect on intra-macrophage replication
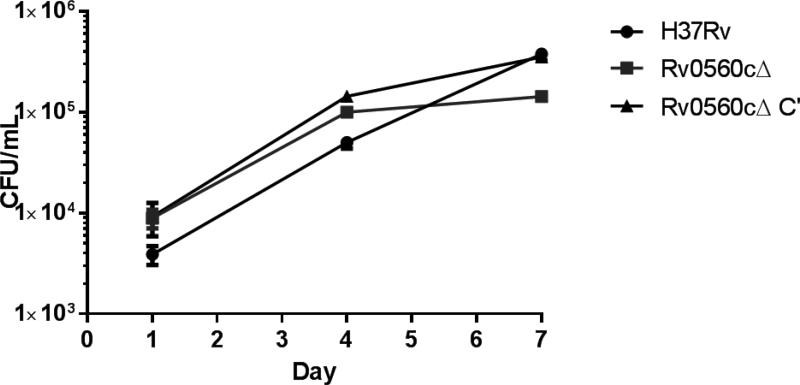
The metabolism of M. tuberculosis is altered during infection of macrophages. We saw no difference in the growth or survival of the deletion strain under various single stress conditions, but macrophage infection imposes several stresses simultaneously. We hypothesized that Rv0560c might be required under these conditions, particularly since remodeling of respiratory pathways is seen [13]. We tested the ability of the Rv0560cΔ strain to survive and replicate in the THP-1 human macrophage-like cell line over 7 days of infection. The proportion of bacteria taken up by macrophage phagocytosis was roughly equal (104 CFU/mL after overnight infection). The deletion strain was not significantly different from the wild-type or complemented strains, since all grew by ~1 log within the macrophages. These data confirm that Rv0560c is not required for survival in macrophages (Figure 2).
Figure 2. Intracellular bacterial replication in THP-1 macrophages.
M. tuberculosis strains were used to infect 5 × 105 THP-1 cells at a multiplicity of infection (MOI) of ~1 and the bacterial load was monitored by determining CFU. Data are the average ± standard deviation of triplicates. The inoculum for each strain was: wild-type 5.5 × 105 CFU; Rv0560cΔ 5.4 × 105 CFU; Rv0560cΔ C’ 6.0 × 105 CFU.
2.4 Deletion of Rv0560c does not affect sensitivity to multiple compounds
In M. tuberculosis, salicylate increases oxygen consumption and reduces expression of a number of genes involved in energy metabolism. Therefore we wanted to determine if deletion of Rv0560c affected the sensitivity to a number of different agents which target respiration or membrane potential (SQ019, nigericin, CCCP, plumbagin) or that are salicylate analogs (menadione, PAS). We also included ethambutol, as an analog of SQ109, but with a different target. We also tested rifampicin, as a previous study suggested a link between Rv0560c expression and rifampicin resistance [9].
Since Rv0560c is induced by exposure to salicylate, we determined MICs in cultures that had been pre-exposed to 0.4 mM salicylate for 3 days. The MIC determination was run in the presence or absence of salicylate (Table 1). As noted with the growth curves, there was no difference between the strains with respect to salicylate sensitivity, with MICs of 0.5 - 0.9 mM. In addition no difference was seen in sensitivity to rifampicin (MICs 2-5 nM), suggesting that Rv0560c is not involved in rifampicin resistance. SQ109 and ethambutol had similar MICs, with no strain differences. We also ran MICs for 12 days, but saw no difference between the three strains (data not shown). Similarly there was no difference in MIC between the three strains for plumbagin, menadione, nigericin, PAS, or CCCP. Therefore the high level expression of Rv0560c is not protective against any of these agents.
Table 1.
Activity of compounds against recombinant strains.
| MIC (μM) | ||||||
|---|---|---|---|---|---|---|
| Wild-type | Rv0560cΔ | Rv0560cΔ C′ | ||||
| Untreated | Salicylate | Untreated | Salicylate | Untreated | Salicylate | |
| Plumbagin | 6.6 | 5.3 | 2.9 | 2.7 | 2.5 | 2.6 |
| Menadione | 48.9 | 44.3 | 58.7 | 51.5 | 56.4 | 51.2 |
| Nigericin | 2.3 | 1.6 | 2.4 | 1.9 | 1.6 | 1.3 |
| PAS | 7 | 6.4 | 6.8 | 8.1 | 7.3 | 8.9 |
| CCCP | 17.7 | 13.6 | 15.1 | 15.6 | 13.9 | 15.8 |
| SQ109 | 0.7 | 0.4 | 1.2 | 1 | 0.8 | 0.7 |
| Ethambutol | 2 | 1.6 | 1.7 | 1.9 | 2 | 1.8 |
| Salicylate | 555 | 626 | 641 | 924 | 594 | 598 |
| Rifampicin | 0.003 | 0.002 | 0.005 | 0.005 | 0.003 | 0.005 |
The minimum inhibitory concentration (μM) of selected compounds was determined against M. tuberculosis strains. Strains were inoculated in 96-well plates, grown for 5 days, and OD590 measured. The MIC was determined as the minimum concentration to inhibit growth completely from the dose response plot.
2.5 Rv0560c deletion does not affect the kill kinetics for several compounds
Although we saw no difference in growth inhibition, it was still possible that the Rv0560c deletion strain would be more susceptible to certain agents over a longer period of time and/or that kill kinetics would differ. For example, it had previously been shown that the kill kinetics of ethambutol and PAS were reduced when salicylate was added to the medium [8], if this were linked to Rv0560c up-regulation, we would expect to see differences in kill kinetics for the Rv0560c deletion strain. We selected four agents to test this, CCCP, plumbagin, ethambutol and SQ109. Bacterial viability was monitored over several weeks in varying concentrations of each compound (Figure S2). The minimum bactericidal concentration (defined as the minimum concentration required to effect a 3 log kill in 21 days) was determined We saw no difference in MBC between the three strains. SQ109 was not bactericidal, showing very little kill even at 10X MIC (10 M). In contrast plumbagin was bactericidal at all concentrations down to 1X MIC (7 M). Ethambutol MBC (2.5 μM) was 2.5 X MIC in all strains, and CCCP was bactericidal at 5X MIC and higher (75 μM). We also looked at kill kinetics with timepoints at 7, 14, and 21 days, but saw no significant differences (Figure S2). These data confirm that the deletion of Rv0560c had no effect on sensitivity to any of these agents.
3. Discussion
Salicylate exposure has pleiotropic effects on gene expression and metabolism in M. tuberculosis, leading to increased oxygen consumption [12] suggestive of changes in basal metabolism. One of the most obvious consequences of salicylate treatment is the massive induction of Rv0560c, a potential benzoquinone methyl transferase [5, 6]. The role of Rv0560c is unknown, although we assumed it would be required for survival in high concentration of salicylate. However, our data show no phenotype for the Rv0560c deletion strain in salicylate-containing medium, nor under various stress conditions.
Previous work had suggested a link between Rv0560c up-regulation (or salicylate exposure) and the Mar phenotype, including resistance to rifampicin [9], ethambutol and PAS [8]. However, we saw no changes in drug sensitivity in the deletion strain, suggesting that the induction of Rv0560c is coincidental.
The role of Rv0560c in M. tuberculosis is not clear. It seems unlikely to play a role in normal menaquinone biosynthesis, since it is not essential, and there are annotated homologs of all the synthetic genes, except MenF. Base on homology it appears to be an S-adenosylmethionine-dependent methyl transferase which could be involved in biosynthesis of isoprenoids or ubiquinone [4]. Alternatively, it could be involved in an alternate route for menaquinone biosynthesis as in Streptomyces coelicolor [14]. Our data which show a lack of attenuation or phenotype under multiple conditions suggest that Rv0560c may be redundant with other gene products, or that it plays an essential role under very specific conditions, which have not yet been defined. Based on the energy required to synthesize such large quantities of protein after exposure to salicylate, it seems unlikely that it plays an unimportant role in metabolism. Future work, such as biochemical characterization or metabolite analysis should help elucidate the true function of Rv0560c.
4. Materials and Methods
4.1 Bacterial culture
E. coli was grown in Luria-Bertani broth or on Luria agar (LA). M. tuberculosis H37Rv (ATCC 25618) was grown in Middlebrook 7H9 (Difco) supplemented with 10% v/v oleic acid, albumin, dextrose and catalase supplement (OADC; Becton Dickinson) and 0.05% w/v Tween 80 (7H9-OADC-Tw) or on Middlebrook 7H10 (Difco) with 10% v/v OADC supplement. Where appropriate, the medium was supplemented with gentamicin (Gm) at 10 μg/mL for M. tuberculosis and 20 μg/mL for E. coli, kanamycin (kan) at 20 μg/mL for M. tuberculosis and 50 μg/mL for E. coli, hygromycin B (hyg) at 50 μg/mL for M. tuberculosis and 150 μg/mL for E. coli, and X-gal at 50 μg/mL.
4.2 Deletion Plasmid Construction
The 1 kb downstream region of Rv0560c, including the last 9 nucleotides of Rv0560c, was amplified by PCR from M. tuberculosis genomic DNA using RF560f and RF560r (Table S1), digested with XbaI and EcoRV, and cloned into pBackbone [15], a derivative of pBluescript KS(-). The 1 kb upstream region of Rv0560c, including nucleotides 1-59 of Rv0560c, was amplified using LF560f and LF560r, digested with PacI and XbaI, and cloned into pBackbone containing the downstream region. The hyg-sacB-lacZ cassette of pGOAL19 was added as a PacI fragment [16]. Blue colonies were selected and verified.
4.3 Generation of the Rv0560cΔ and complemented strains
Allelic exchange via homologous recombination was used to create an unmarked in-frame deletion of Rv0560c [16]. Briefly, the deletion vector was UV-irradiated, electroporated into M. tuberculosis, and single cross-over recombinants selected on hyg, kan and X-gal. Double crossover strains were generated by plating onto 2% sucrose and X-gal. White colonies were patched onto plates to check for kan sensitivity. KanS colonies were screened by PCR to distinguish the wild-type and deletion alleles using primers Rv0560cF1 and Rv0560cR1. Rv0560c was amplified with its native promoter [6] using primers Rv0560c-C’ F1 and Rv0560c-C’ R1 and cloned into pSC-B (Agilent Technologies). The L5 integrating cassette (containing GmR, L5 integrase, and attP) from pUC-Gm-Int [17] was inserted as a HindIII fragment. The plasmid was verified by sequencing and electroporated into the Rv0560cΔ strain. Recombinants were selected on Gm. The expected phenotype was confirmed by Southern analysis. Genomic DNA was isolated and digested with XmaI or ClaI. The 487bp probe was generated by PCR with primers Rv0560cF2 and Rv0560cR2. DNA detection was carried out using the AlkPhos system (GE).
4.4 Growth curves
M. tuberculosis strains were grown standing in 10 mL cultures in 7H9-OADC-Tw until late log phase (OD590 of 0.6-1.0) then used to inoculate 5 mL of medium in glass culture tubes (16 mm diameter, 125 mm tall) with stir bars.to a theoretical OD590 of 0.04. Cultures were grown with stirring at 37° C. Growth was monitored by OD 590.
4.5 Minimum inhibitory concentration (MIC) determination
M. tuberculosis strains were grown to late log phase (OD590 of 0.6-1.0) in 7H9-OADC-Tw. Where required, salicylate was added to 0.4 mM and cultures were incubated for 3 days to induce expression of Rv0560c [6]. Late log phase cultures were filtered through a 5μM syringe filter and used to inoculate 96-well plates containing 2 -fold serial dilutions of compounds to a theoretical OD590 of 0.02. Cultures were incubated with humidification at 37° C for 5 days. Growth was measured by OD590. Dose response curves were generated using the Gompertz curve fit algorithm and the MIC was determined [18, 19].
4.6 Macrophage infection
THP-1 cells (ATCC TIB-202) were propagated in complete RPMI 1640 medium and diluted to 5 ×105 / mL. PMA was added to 80 nM, and 1 mL of cell suspension was inoculated into 24-well plates incubated overnight at 37°C, 5% CO2. Cells were washed three times in PBS and fresh medium added. Late log phase cultures of M. tuberculosis strains were resuspended in RPMI medium to ~5 × 106 bacteria / mL; 100 μL of bacterial culture was added to each well and incubated overnight at 37°C, 5% CO2. Macrophages were lysed with SDS, wells washed three times and bacteria harvested by centrifugation. Serial dilutions were plated to determine colony forming units (CFU) after 4 weeks of incubation at 37° C.
4.7 Kill kinetics
Compounds were prepared in 5 mL 7H9-OADC-Tw medium at 1 ×, 1.25 ×, 2.5 ×, 5 ×, and 10 × MIC (final concentration 2 % v/v DMSO). Late log phase cultures of M. tuberculosis strains were adjusted to OD590 0.1 and 105 CFU inoculated into each sample. Samples were incubated standing at 37° C and serial dilutions were plated for CFU determination.
Supplementary Material
Acknowledgements
We thank Dean Crick for useful discussion.
Funding: This work was funded by the European Union Project LSHP-CT-2005-018923 and by NIAID of the National Institutes of Health under award numbers R01AI049151 and R01AI0976550. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–44. doi: 10.1038/31159. doi: 10.1038/31159. PubMed PMID: 9634230. [DOI] [PubMed] [Google Scholar]
- 2.Slayden RA, Jackson M, Zucker J, Ramirez MV, Dawson CC, Crew R, et al. Updating and curating metabolic pathways of TB. Tuberculosis. 2013;93(1):47–59. doi: 10.1016/j.tube.2012.11.001. doi: 10.1016/j.tube.2012.11.001. PubMed PMID: 23375378; PubMed Central PMCID: PMC4121119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camus JC, Pryor MJ, Medigue C, Cole ST. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology. 2002;148(Pt 10):2967–73. doi: 10.1099/00221287-148-10-2967. PubMed PMID: 12368430. [DOI] [PubMed] [Google Scholar]
- 4.Garbe TR. Co-induction of methyltransferase Rv0560c by naphthoquinones and fibric acids suggests attenuation of isoprenoid quinone action in Mycobacterium tuberculosis. Can J Microbiol. 2004;50(10):771–8. doi: 10.1139/w04-067. doi: 10.1139/w04-067. PubMed PMID: 15644891. [DOI] [PubMed] [Google Scholar]
- 5.Sun Z, Cheng SJ, Zhang H, Zhang Y. Salicylate uniquely induces a 27-kDa protein in tubercle bacillus. FEMS microbiology letters. 2001;203(2):211–6. doi: 10.1111/j.1574-6968.2001.tb10843.x. PubMed PMID: 11583850. [DOI] [PubMed] [Google Scholar]
- 6.Schuessler DL, Parish T. The promoter of Rv0560c is induced by salicylate and structurally-related compounds in Mycobacterium tuberculosis. PloS one. 2012;7(4):e34471. doi: 10.1371/journal.pone.0034471. doi: 10.1371/journal.pone.0034471. PubMed PMID: 22485172; PubMed Central PMCID: PMC3317779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen SP, Levy SB, Foulds J, Rosner JL. Salicylate induction of antibiotic resistance in Escherichia coli: activation of the mar operon and a mar-independent pathway. Journal of bacteriology. 1993;175(24):7856–62. doi: 10.1128/jb.175.24.7856-7862.1993. PubMed PMID: 7504664; PubMed Central PMCID: PMCPMC206962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaller A, Sun Z, Yang Y, Somoskovi A, Zhang Y. Salicylate reduces susceptibility of Mycobacterium tuberculosis to multiple antituberculosis drugs. Antimicrobial agents and chemotherapy. 2002;46(8):2636–9. doi: 10.1128/AAC.46.8.2636-2639.2002. PubMed PMID: 12121945; PubMed Central PMCID: PMC127383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Knegt GJ, Bruning O, ten Kate MT, de Jong M, van Belkum A, Endtz HP, et al. Rifampicin-induced transcriptome response in rifampicin-resistant Mycobacterium tuberculosis. Tuberculosis. 2013;93(1):96–101. doi: 10.1016/j.tube.2012.10.013. doi: 10.1016/j.tube.2012.10.013. PubMed PMID: 23182912. [DOI] [PubMed] [Google Scholar]
- 10.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Molecular microbiology. 2003;48(1):77–84. doi: 10.1046/j.1365-2958.2003.03425.x. PubMed PMID: 12657046. [DOI] [PubMed] [Google Scholar]
- 11.Rengarajan J, Bloom BR, Rubin EJ. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(23):8327–32. doi: 10.1073/pnas.0503272102. doi: 10.1073/pnas.0503272102. PubMed PMID: 15928073; PubMed Central PMCID: PMCPMC1142121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denkin S, Byrne S, Jie C, Zhang Y. Gene expression profiling analysis of Mycobacterium tuberculosis genes in response to salicylate. Arch Microbiol. 2005;184(3):152–7. doi: 10.1007/s00203-005-0037-9. doi: 10.1007/s00203-005-0037-9. PubMed PMID: 16175359. [DOI] [PubMed] [Google Scholar]
- 13.Shi LB, Sohaskey CD, Kana BD, Dawes S, North RJ, Mizrahi V, et al. Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2005;102(43):15629–34. doi: 10.1073/pnas.0507850102. PubMed PMID: ISI:000232929400065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiratsuka T, Furihata K, Ishikawa J, Yamashita H, Itoh N, Seto H, et al. An alternative menaquinone biosynthetic pathway operating in microorganisms. Science. 2008;321(5896):1670–3. doi: 10.1126/science.1160446. doi: 10.1126/science.1160446. PubMed PMID: 18801996. [DOI] [PubMed] [Google Scholar]
- 15.Gopaul KK. Transcription of the Mycobacterium tuberculosis recA gene. University College London; London, United Kingdom: 2002. [Google Scholar]
- 16.Parish T, Stoker NG. Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology. 2000;146(Pt 8):1969–75. doi: 10.1099/00221287-146-8-1969. doi: 10.1099/00221287-146-8-1969. PubMed PMID: 10931901. [DOI] [PubMed] [Google Scholar]
- 17.Mahenthiralingam E, Marklund BI, Brooks LA, Smith DA, Bancroft GJ, Stokes RW. Site-directed mutagenesis of the 19-kilodalton lipoprotein antigen reveals No essential role for the protein in the growth and virulence of Mycobacterium intracellulare. Infection and immunity. 1998;66(8):3626–34. doi: 10.1128/iai.66.8.3626-3634.1998. PubMed PMID: 9673242; PubMed Central PMCID: PMCPMC108395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ollinger J, Bailey MA, Moraski GC, Casey A, Florio S, Alling T, et al. A dual read-out assay to evaluate the potency of compounds active against Mycobacterium tuberculosis. PLoS One. 2013;8(4):e60531. doi: 10.1371/journal.pone.0060531. Epub 2013/04/18. doi: 10.1371/journal.pone.0060531. PubMed PMID: 23593234; PubMed Central PMCID: PMC3617142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert RJ, Pearson J. Susceptibility testing: accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. Journal of applied microbiology. 2000;88(5):784–90. doi: 10.1046/j.1365-2672.2000.01017.x. PubMed PMID: 10792538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.