Abstract
Age-related macular degeneration (AMD) is one of the leading causes of vision loss in the elderly. With an increasingly aged population worldwide, the need for the prevention of AMD is rising. Multiple studies investigating AMD with the use of animal models and cell culture have identified oxidative stress–related retinal damage as an important contributing factor. In general, diet is an excellent source of the antioxidants, vitamins, and minerals necessary for healthy living; moreover, the general public is often receptive to recommendations made by physicians and health care workers regarding diet and supplements as a means of empowering themselves to avoid common and worrisome ailments such as AMD, which has made epidemiologists and clinicians enthusiastic about dietary intervention studies. A wide variety of nutrients, such as minerals, vitamins, ω-3 (n–3) fatty acids, and various carotenoids, have been associated with reducing the risk of AMD. Initial results from the Age-Related Eye Disease Study (AREDS) indicated that supplementation with antioxidants (β-carotene and vitamins C and E) and zinc was associated with a reduced risk of AMD progression. The AREDS2 follow-up study, designed to improve upon the earlier formulation, tested the addition of lutein, zeaxanthin, and ω-3 fatty acids. In this review, we examine the science behind the nutritional factors included in these interventional studies and the reasons for considering their inclusion to lower the rate of AMD progression.
Keywords: AMD, n–3 LC-PUFAs, micronutrients, xanthophylls, β-carotene, AREDS2
Introduction
Age-related macular degeneration (AMD)4 is the leading cause of irreversible blindness in the elderly in industrialized nations (1). It affects the macula, the central area of the retina responsible for high-acuity daylight vision. Although understanding of the molecular events underlying AMD has grown in recent decades, its pathogenesis remains puzzling, and our therapeutic resources for some forms of AMD continue to be limited. AMD, an aging disease that was once ignored and scarcely understood, is now consuming billions of health care dollars, not only in developed countries but in developing countries as well; moreover, with increasing life spans, its prevalence is also rising (2, 3). AMD has no single cause, and it results from variable contributions of age, genetic predisposition, environment, and oxidative stress. Research suggests a positive association between the use of vitamin, mineral, and micronutrient supplements and decreases in the progression of degenerative disorders such as AMD (4, 5). In addition, preventative interventions through dietary modification are attractive and affordable strategies that do not require specialists for administration.
Current Status of Knowledge
AMD.
The clinical hallmark of AMD is the appearance of drusen (hard or soft) (6), which are localized yellowish deposits of oxidized lipids, proteins, and inflammatory debris lying between the basement membrane of the retinal pigment epithelium (RPE) and Bruch’s membrane. Although the presence of small hard drusen (<62 μm) is not considered to be a risk factor for AMD development (7), the presence of numerous large drusen is a risk factor for vision loss (8). With increasing age, drusen can become calcified or filled with cholesterol, appearing crystalline or polychromatic. Soft drusen can coalesce to form serous detachments of the RPE.
Advanced forms of AMD include geographic atrophy (GA) and choroidal neovascularization (CNV). GA refers to confluent areas of RPE cell death accompanied by overlying photoreceptor atrophy (9). GA can develop after the fading of drusen in an area of RPE attenuation, after the flattening of an RPE detachment, or after involution of CNV. GA leads to a gradual, progressive visual decline, mostly because of photoreceptor loss (10). CNV refers to the growth of new blood vessels from the choroid (11); these vessels may remain beneath the RPE, breach the RPE, or even enter the subretinal space. CNV is the leading cause of wet AMD, which by repeated leakage of blood, serum, and lipids can stimulate fibroglial organization and lead to disciform scarring (10).
The most devastating manifestation of AMD is the wet or exudative form of AMD, because of its sudden onset and imminent vision loss, leading to legal blindness (12). Ten years ago, the introduction of effective antivascular endothelial growth factors provided hope that vision can be improved with treatment. Antivascular endothelial growth factor compounds, when injected into the vitreous, stop abnormal blood vessel growth, often stabilizing or even improving vision; however, these medications can cost thousands of dollars per injection and may require monthly revisits to the ophthalmologist’s office for additional intravitreal injections (13, 14). In contrast, GA, the advanced dry form of AMD, has proven to be far more resistant to treatments and therapy. Even though its advancement rate is much slower (≥10 times slower than exudative AMD), it still results in central vision loss, and the FDA has not yet approved any medicine for dry AMD. At this point, efforts made to identify and modify risk factors for AMD to delay the onset of this devastating blinding condition are drawing considerable interest and attention.
In 2004, the Eye Diseases Prevalence Group studied the prevalence of AMD with the use of a meta-analysis of regional population-based studies in the United States, Australia, and Europe. This group estimated that the prevalence of AMD is >1.75 million individuals in the United States. It is expected that this number will increase to almost 3 million by the year 2020 (15). Klein et al. (16) observed that the prevalence of any AMD in members of the US population aged ≥40 y was 6.5%, and the prevalence of late AMD was 0.8%. The projection of a large increase in the prevalence of blindness and low vision in the United States during the next 2 decades is driven primarily by the fact that the prevalence of visual impairment increases sharply in persons >65 y of age (17). Persons aged ≥80 y are at higher risk of AMD blindness, and this age group currently represents the fastest-growing segment of the US population.
AMD risk factors.
The prevalence, incidence, and progression of all forms of AMD increase with age. Although women are statistically associated with a slightly higher prevalence of AMD in many studies, the fact that they also have longer life spans and use health care resources at higher rates than men should be taken into consideration (10, 18). Pooled data from the Beaver Dam Eye Study, the Blue Mountains Eye Study, and the Rotterdam Study revealed no sex-related differences in AMD risk (19–22), whereas analysis from the Blue Mountains Eye Study suggests that the 5-y incidence of neovascular AMD in women is double that of men (23).
All forms of AMD are more prevalent in the Caucasian population than in those races with more darkly pigmented skin, hair, and eye color (24, 25). According to recent meta-analysis data, Europeans had a higher prevalence of the GA subtype than did Africans, Asians, and Hispanics (3). Light iris pigmentation is associated with more extensive retinal disease in patients with unilateral neovascular AMD (26–28). Furthermore, subjects with lighter iris pigmentation and CNV in one eye may tend to have more extensive atrophic disease in their other eye. The Blue Mountains Eye study suggested that blue iris color was associated with an increased risk of both late AMD and early maculopathy (27). Abnormal skin sensitivity to sunlight is also associated with an increased risk of AMD (29).
AMD is a complex disorder with multiple inherited risk factors, including one major genetic risk locus on chromosome 1 in the complement factor H region (30, 31) and in the high temperature requirement a serine peptidase 1/age-related maculopathy susceptibility 2 region on chromosome 10 (32, 33), along with a very large number of minor genetic risk factors identified through genome-wide association studies (34). Although many of the inheritable and nonmodifiable risk factors for AMD have been identified and can direct us toward the development of novel treatment strategies, such as pharmacologic, gene therapy, growth factor, and stem cell treatments, we must still focus attention on modifiable risk factors.
Cigarette smoking has been noted to be a frequent risk factor for AMD, and it is undisputed that subjects with AMD risk should quit smoking or never begin smoking at all (35). Excessive light exposure could reasonably be expected to be a substantial AMD risk factor based on the possibility that it aggravates oxidative damage to lipid membranes and proteins in chronic and acute conditions; however, it has been surprisingly difficult to demonstrate epidemiologically, and only a handful of studies have reported associations between long-term light exposure and AMD (29, 36, 37). Quantification of light exposure in participating cohorts is difficult, so most epidemiologic research has been restricted to studying long-term sun exposure conditions experienced by outdoor workers such as fishermen (38), in whom the association was determined by straightforward comparisons between participants who regularly wear hats and sunglasses and subjects who do not use any sun protection strategies (37, 39).
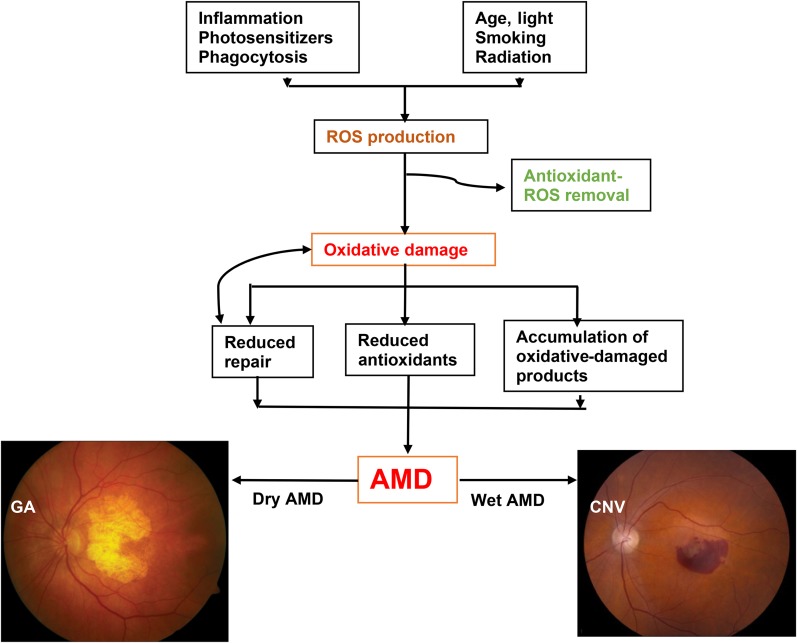
A substantial contributing factor for macular degeneration is oxidative stress, which refers to cellular damage caused by reactive oxygen species (ROS), a process that has also been implicated in many other degenerative diseases. ROS include free radicals, peroxides, singlet oxygen, and other by-products of oxygen metabolism. With age, RPE gradually accumulates lipofuscin, a heterogeneous fluorescent mixture rich in lipid-protein complexes, which is also composed of by-products of vitamin A metabolism and lipid peroxidation (40–42). Lipofuscin is undegradable and also acts as a plausible photosensitizer, generating ROS (43). The retina is particularly susceptible to oxidative stress because of its high oxygen consumption, high PUFA content (5, 44), and exposure to light. Unfortunately, as we age, oxidative damage increases and antioxidant capacity decreases, as does the efficiency of reparative systems (45). It appears that these age-related oxidative changes are characteristic indicators of early AMD, which, in combination with hereditary susceptibility and other retinal modifiers, can progress to the pathology and visual morbidity associated with advanced AMD (Figure 1). A broad range of antioxidants known to have chemical properties that can reduce oxidative damage have been proposed to decrease the risk of AMD (46–48).
FIGURE 1.
Schematic representation of risk factors of AMD causing ROS production and oxidative stress, thereby leading to AMD with either GA or CNV. AMD, age-related macular degeneration; CNV, choroidal neovascularization; GA, geographic atrophy; ROS, reactive oxygen species.
Epidemiologic studies and biochemical investigations of nutrient roles in complex degenerative diseases of aging such as AMD are very helpful in formulating clinical recommendations, yet many clinicians demand definitive results from ≥1 adequately powered clinical trial before recommending such nutrients to patients. Once the associations between diet and AMD have been identified, they can be of great help in leading future research and intervention studies and in generating scientific data to design public health interventions. Epidemiologic studies, as well as small randomized clinical trials, show various associations between the intake of antioxidants and carotenoids or zinc and the risk of AMD (Table 1) (48–73). While these epidemiologic studies are crucial, it must also be considered that nutritional surveys and blood analyses are indirect measures of the status of the tissue of interest, the macula of the human eye. It is thus currently appropriate to review the basic and clinical science supporting the inclusion of micronutrients in interventions against AMD.
TABLE 1.
Summary of studies screening the relation between micronutrients and AMD1
| Author (reference) | Participants | Study design | Mode of assessment | Summary |
| Newsome et al. (49) | 151 | P (2 y), double-blind | Ophthalmic examinations, photographic grading, oral supplementation, serum zinc concentrations | Supplementation with zinc sulfate was associated with a retardation of vision loss (P < 0.05) |
| Eye Disease Case-Control Study Group (46) | 1072 | R, case-control study | Serum antioxidant measurements, FFQ, photographic grading | High serum concentrations of micronutrients (carotenoids in particular) may be associated with a decreased risk of AMD |
| Seddon et al. (50) | 876 | R, case-control study, EDCC study | FFQ, photographic grading | Consumption of carotenoid-rich vegetables, especially those rich in lutein and zeaxanthin, reduced the risk of AMD |
| West et al. (51) | 976 | R, BLSA study | Serum vitamin E and β-carotene measurement, photographic grading | High serum concentrations of vitamin E reduced the risk of AMD |
| Teikari et al. (52) | 941 | P (8 y), ATBC study, clinical trial | Fundus photographs, FFQ, supplementation and serum measurements of vitamin E and β-carotene | No beneficial effect of supplemental vitamin E or β-carotene was observed on the occurrence of ARM |
| VandenLangenberg et al. (53) | 1709 | R, cross-sectional, Beaver Dam Eye Study | Photograph grading, dietary intake assessment | Significant modest associations were observed between provitamin A carotenoid and vitamin E intake and incidence of large drusen (P < 0.05) |
| Christen et al. (54) | 279 | P (12.5 y), Physicians Health Study I | Medical records, supplement administration | Supplemental use of antioxidant vitamins C and E or multivitamins did result in nonsignificant reductions in AMD risk (13% and 10%, RR factor = 1.0) |
| Smith et al. (55) | 2900 | R, cross-sectional, Blue Mountains Eye Study | Fundus photographic grading, FFQ | No association was observed between increasing intake of foods high in antioxidant vitamins and decreasing prevalence of either late or early AMD (P ≥ 0.1) |
| Age-Related Eye Disease Study Research Group (56) | 3609 | P (6.3 y), clinical trial | Photographic grading, supplement administration and serum concentrations | Supplementation with antioxidants and zinc resulted in lowering AMD progression (P < 0.05) |
| Cho et al. (57) | 567 | P (10 y), follow-up study of NHS | FFQ, ophthalmology records | Dietary fat intake was positively associated with AMD (P < 0.05), whereas fish intake was inversely associated with AMD progression (P = 0.009). |
| Mares-Perlman et al. (58) | 8622 | R, NHANES III | Photographic grading, FFQ, serum measurements | No association between carotenoids in diet or serum with any form of AMD, except for soft drusen |
| Seddon et al. (59) | 853 | R, EDCC study | Semi-quantitative FFQ, serum measurements, ophthalmic measurements | Diets rich in n–3 FAs and fish were inversely associated with risk of AMD (P < 0.05) |
| Gale et al. (60) | 380 | R, cross-sectional eye study | Photographic grading, serum carotenoid measures | Risk of AMD (early or late) was significantly higher in subjects with lower plasma concentrations of zeaxanthin (P < 0.05) |
| Richer et al. (61) | 90 | P (1 y), Veterans LAST study | Supplementation with lutein and antioxidant vitamins, ophthalmic testing | Visual function was improved with lutein supplementation |
| van Leeuwen et al. (62) | 4175 | P (8 y), Rotterdam study | Photographic grading, FFQ | High dietary intake of vitamin E and zinc was associated with a reduced risk of AMD (P < 0.05) |
| Delcourt et al. (63) | 899 | R, cross-sectional, POLA study | Photographic grading, plasma carotenoid analysis | Plasma zeaxanthin was significantly associated with a reduced risk of ARM (P < 0.05) |
| Moeller et al. (64) | 1797 | P (7 y), CAREDS study | FFQ, serum analysis, stereoscopic color fundus photographic grading | Diets rich in lutein+zeaxanthin protect against intermediate AMD in healthy women |
| Seddon et al. (65) | 681 twins | R, US twin study of AMD | Color photographic grading, FFQ | Cigarette smoking increases AMD risk, whereas fish and n–3 PUFA intake reduces risk |
| SanGiovanni et al. (66) | 4519 | R, cross-sectional, AREDS study | FFQ, fundus photographic grading, serum analysis | Higher dietary intake of lutein+zeaxanthin was independently associated with a decreased likelihood of having neovascular AMD, GA, and large or extensive intermediate drusen (P < 0.05) |
| Trieschmann et al. (67) | 136 | P (0.25 y), LUNA study | Supplementation with lutein and zeaxanthin ester, MPOD and serum carotenoid measures, retinal angiography | Supplementation with lutein and zeaxanthin combined with coantioxidants resulted in an increase in MPOD in majority of subjects, including those with AMD (92%) |
| Cho et al. (68) | 71494 F 41564 M |
P (18 y), follow-up NHS and HPFS study | FFQ, photographic grading, ophthalmic records | No association was observed with lutein+zeaxanthin intake and risk of neovascular AMD (P > 0.05) |
| Tan et al. (69) | 5454 | P (10 y), follow-up of Blue Mountains Eye study | FFQ, retinal exams, fundus photograph grading | Increased dietary lutein and zeaxanthin intake reduced the risk of long-term incident AMD |
| Parekh et al. (70) | 1787 | P (4 y), ancillary CAREDS, Women’s Health Initiative observational study | Fundus photographic grading, FFQ | In women, diets high in total fat (n–6 PUFAs) were associated with increased AMD prevalence (P = 0.01) |
| Merle et al. (71) | 963 | P (2.8 y), Alienor study | Blood sample analysis, retinal photographs, optical coherence tomography | A potential role of n–3 LC-PUFAs was observed in prevention of late AMD |
| Souied et al. (72) | 263 | P (3 y), NAT-2 study | Fundus photography fluorescein angiography, serum and RBC lipid analysis, supplementation with EPA and DHA | Choroidal neovascularization was significantly reduced in DHA-supplemented subjects |
| Dawczynski et al. (73) | 172 | P (1 y), clinical trial | Supplementation with lutein and zeaxanthin, ω-3 PUFAs, MPOD, VA measurements | Supplementation with lutein, zeaxanthin, and n–3 PUFAs increased visual acuity and MPOD in nonexudative AMD subjects |
AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study; ARM, age-related maculopathy; ATBC, alpha-tocopherol beta-carotene; BLSA, Baltimore Longitudinal Study of Aging; CAREDS, Carotenoids in Age-Related Eye Disease Study; EDCC, Eye Disease Case-Control; GA, geographic atrophy; HPFS, Health Professionals Follow-Up Study; LAST, Lutein Antioxidant Supplementation Trial; LC-PUFA, long-chain PUFA; LUNA, lutein nutrition effects measured by autoflourescence; MPOD, macular pigment optical density; NAT-2, nutritional AMD treatment 2; NHS, Nurses' Health Study; P, prospective study; POLA, Pathologies Occulaires Lieesa L Age; R, retrospective study; VA, visual acuity.
Minerals.
Iron, zinc, copper, and selenium are essential trace elements that play key roles in retinal physiology. There is a higher concentration of iron in AMD retinas than there is in age-matched control retinas. Although these results do not demonstrate that iron overload is a reason for AMD, iron may contribute to oxidative stress, which might lead to AMD (74). An increase in iron deposition was also reported to lead to photoreceptor loss (75), but deficiency was not associated with adverse effects. Zinc is the second most abundant metal after iron in the human retina, suggesting an important physiologic role (76). Zinc depletion results in poor dark adaptation and reduced photopic and scotopic responses. Copper is necessary for the synthesis of melanin, a storage protein for iron, zinc, and copper in RPE and melanocytes (77, 78). The cellular homeostasis of iron, zinc, and copper is tightly interlinked. If one of these metals becomes deficient, another metal may accumulate (76). Concentrations of zinc affect the progression of AMD, and its concentrations in human retina and RPE also decrease with age (79). Copper deficiency has been associated with optic neuropathy, but there was no change in retinal function (80, 81). One report suggested an inverse association of manganese with AMD (82), whereas in other studies, no significant association was observed (83, 84). No significant benefit was associated with supplementation with selenium for AMD (85, 69).
Vitamins.
Certain vitamins, such as vitamins C, E, B-6, and B-12 and folic acid, are hypothesized to decrease the risk of AMD progression. Vitamin C (ascorbic acid) is found throughout the whole retina (86) and may be protective against AMD. A previous study demonstrated that low concentrations of vitamin C in the plasma were associated with an increased risk of AMD, but that high concentrations were not protective (46); however, dietary consumption of vitamin C did not significantly affect the progression of AMD (51, 55, 50). Vitamin E is an essential micronutrient and is the most efficient scavenger of free radicals. Wiegand et al. (87) reported that there is upregulation of vitamin E concentrations upon induction of oxidative stress. Deprivation of vitamin E could lead to lipofuscin accumulation (88), retinal damage (89), and loss of photoreceptors (90, 91). Vitamin E deficiency accelerates with age (89), and epidemiologic studies also suggest a beneficial effect of vitamin E on the progression of AMD (92); however, there is conflicting evidence with regard to vitamin E use in nutritional treatment for AMD, because some studies have shown that Vitamin E has no adverse or protective effect on early AMD incidence and progression (52, 93). A 2009 study (94) indicated that daily supplementation with folic acid and vitamins B-6 and B-12 decreased the risk of AMD in women; this was supported by a 10-y follow-up in the Blue Mountains Eye Study (95), suggesting that deficiencies of folic acid and vitamin B-12 increase AMD risk.
Carotenes: the hydrocarbon carotenoids.
Carotenoids are phytochemicals with a conjugated polyene backbone chain commonly found in many organisms of the food chain. They are responsible for the colorful plumage of birds and bright coloration in fish and shrimp, are essential components of the plant photosynthetic apparatus, and are also responsible for the diverse colors in fruits and vegetables. Vertebrates and invertebrates cannot biosynthesize carotenoids; hence, they must be obtained through dietary consumption. Carotenoids are mostly hydrophobic, and they are therefore positioned in the lipophilic regions of the cell membranes or else are bound to specific proteins.
The primary proposed mechanisms for the protective action of carotenoids against AMD are the reduction of oxidative stress–inducers and blue light–mediated damage. Carotenoids neutralize ROS generated by different free radical reactions in the body. They are efficient absorbers of transmitted energy, and they can release the absorbed energy as heat without chemical degradation (96). They also act as potent scavengers of free radicals (e.g., hydroxyl radicals and superoxide anions), and are exceptionally effective at nullifying singlet oxygen species (97).
β-Carotene is among the most commonly occurring serum carotenoids, and it is abundantly present in leafy green vegetables and fruits. It is a provitamin A carotenoid with a molecular formula of C40H56 and a polyprenoid chain attached to 2 ionone rings. The role of β-carotene as a free radical scavenger has been well documented (98). Early epidemiologic studies suggested a link between carotenoid-rich diets and a lower incidence of AMD, but subsequent epidemiologic findings have suggested nonsignificant health benefits from dietary supplementation with β-carotene (P > 0.05) (50, 51, 62). Burton and Ingold (98) suggested that β-carotene acts as an efficient chain-breaking antioxidant, leading to a reduction in lipid peroxidation, which, under high oxygen pressures, may serve as a pro-oxidant. Intervention studies have suggested that β-carotene supplements offer no protection against cancer and other oxidative stress–related diseases such as AMD. In addition, among smokers, such supplements may increase these risks; it is therefore no longer recommended (4, 52, 99, 100). Investigators have sought to explain these findings by proposing that, under certain conditions, β-carotene can have pro-oxidant activity and act as a tumor promoter (101).
Age-Related Eye Disease Study.
Based on the knowledge of antioxidant vitamins, minerals, and carotenoids discussed above, the National Eye Institute initiated the Age-Related Eye Disease Study (AREDS) in 1990. It was an 11-center, double-masked AMD clinical trial that enrolled participants if they had extensive small drusen, intermediate drusen, large drusen, GA, or pigment abnormalities in one or both eyes, or advanced AMD or vision loss from AMD in one eye. In this study, the progression of AMD and loss of visual acuity were compared between groups receiving antioxidant supplementation or placebo, as well as those receiving zinc treatment or placebo. The mean follow-up of the 3640 enrolled participants, who were aged 55–80 y, was 6.3 y. This randomized clinical trial was designed to evaluate the effect of high doses of zinc and selected antioxidant vitamins (5–15 times the RDA) in the development of advanced AMD in a cohort of older individuals. The AREDS formulation was chosen on the basis of recommendations from expert nutritionists, ophthalmologists, and biochemists who reviewed basic science, clinical trials, and epidemiologic data at a series of meetings sponsored by the National Eye Institute. The macular carotenoids lutein and zeaxanthin, known to be present in the central retina of the eye, were strong candidates for inclusion in the AREDS formulation, but they were not commercially available when the AREDS was proposed. β-Carotene [15 mg (25,000 IU)/d] was chosen because it was a well-known carotenoid with systemic antioxidant properties that had been promoted for its effectiveness in ophthalmic nutritional supplements, was commercially available at the time, and was part of several other clinical trials related to heart disease and cancer. It was also decided to include pharmacologic doses of the antioxidant vitamins C (500 mg/d) and E (400 IU/d). The triple vitamin and antioxidant formulation was expected to affect the progression of both cataract and AMD development. Eighty milligrams of zinc oxide per day was chosen for inclusion in the mineral arm of the AREDS because one small randomized, 2-y, placebo-controlled clinical trial of zinc supplementation found a significant reduction in visual acuity loss in the zinc-treated group (P < 0.05) (49), and the use of antioxidants and zinc in eye-targeted formulations had already become a common practice. Copper oxide (2 mg/d) was added to offset potential zinc-induced copper-deficiency anemia.
The 3 active treatment groups receiving zinc, antioxidants, or the combination of both showed a decrease in the rate of progression to advanced AMD. The combined intervention group that received both zinc and antioxidants achieved a 25% reduction of progression to advanced AMD relative to placebo (56). After 5 y of follow up, the estimated probability of progression to advanced AMD was 28% of participants assigned to placebo, 23% and 22% for those assigned to antioxidants and zinc, respectively, and 20% for those assigned to antioxidants plus zinc (56). A single-arm comparison of treatment groups with placebo found statistically significant risk reductions for the antioxidants plus zinc arm (P < 0.05) and borderline significance in the zinc arm (P = 0.05), but not in the antioxidants arm. The decreased percentage in visual acuity scores from baseline were 29% for those assigned to placebo, 26% for those assigned to antioxidants, 25% for those assigned to zinc, and 23% for those assigned to antioxidants plus zinc. Comparisons of visual acuities between the zinc-supplemented group and the placebo group, as well as between the antioxidant-supplemented group and the placebo group, showed no statistically significant differences (P > 0.05) (56). The AREDS results demonstrate a statistically significant association between the incidence of AMD and risk factors such as smoking, BMI, race, education, and age (P < 0.05) (102). The strengths of the AREDS include having standardized protocols for obtaining data, standardized definitions for risk exposure, photographic assessment of the outcomes and retinal risk categories, a large cohort size, and a large number of secondary outcomes (103).
In 2001, the AREDS results were published, and the AREDS formulation quickly became the standard of care for AMD, but the AREDS researchers acknowledged that because the nutritional understanding of AMD development had progressed noticeably since the study had begun, a next-generation AREDS2 supplementation trial was necessary. A new concern had also been raised with regard to β-carotene’s safety and for AMD. First, 2 large randomized clinical trials with high-dose β-carotene supplementation that were conducted while the AREDS was in progress reported an unexpected increase in lung cancer risk in smokers, which raised substantial concerns regarding AREDS supplement use, because nearly one-half of the population with AMD risk are smokers or former smokers (104, 105). Second, the Eye Disease Case-Control epidemiologic study (50) results suggested that lutein and zeaxanthin might be the more physiologically applicable carotenoids for AMD supplementation. It is also widely known that β-carotene is undetectable in the human retina, whereas lutein and zeaxanthin are abundantly present as the macular pigment. Third, commercial sources of lutein and zeaxanthin suitable for clinical trials were now available. Thus, although the AREDS was a crucial first step in guiding nutritional interventions against AMD, during the interval between its initiation and publication of its results, it had become clear that the formulation potentially could be improved upon, so at this point, it is appropriate to review why the xanthophyll carotenoids lutein and zeaxanthin and the n–3 FAs EPA and DHA were chosen for the next-generation AREDS2.
The xanthophyll carotenoids: lutein and zeaxanthin.
Approximately 600 carotenoids exist in nature, 30–40 of which are found in the human diet, and ∼15 carotenoids are present in human serum, but only lutein, zeaxanthin, and meso-zeaxanthin are present in the human macula. These nonprovitamin A carotenoids are collectively known as the macular pigment. The presence of polar hydroxyl groups attached to the terminal ionone rings of the polyene chain makes them members of xanthophyll family. In the central retina, equal concentrations of lutein, zeaxanthin, and meso-zeaxanthin are present, whereas the ratio of meso-zeaxanthin to zeaxanthin decreases with increased eccentricity from the fovea (106). Studies from our laboratory and others have expanded this repertoire and identified the presence of various metabolites of lutein and zeaxanthin, such as meso-zeaxanthin, 3′-epilutein, and 3′-oxolutein in the primate retina, lens, and uveal tract (41, 107). These macular pigment carotenoids are concentrated near the fovea (∼13.3 ng/mm2), and their concentration decreases as we proceed to the peripheral retina (0.047 ng/mm2) (108). The presence of oxidation products of lutein and zeaxanthin in the eye indicates that these eye-protective nutrients undergo oxidation and a series of transformations to protect the macula (109). 3′-Oxolutein was identified as a direct oxidation product of lutein present in monkey retinas and human eye tissues (41). The presence of lutein and zeaxanthin in cultured RPE cells and in vivo has been shown to reduce the amount of lipofuscin formed (5, 42, 110). Although the aging process decreases scotopic and shortwave sensitivity, higher concentrations of macular carotenoids seem to preserve shortwave and scotopic function (111). The macular carotenoids are strongly related to improvements in glare disability and photo-stress recovery in a manner consistent with their spectral absorption and spatial profile (112). Lutein is present in many human tissues, such as the liver and serum, but it reaches maximum concentration in the retina of the eye, where its concentration is 500 times higher than that of other tissues. The presence of lutein and zeaxanthin in the macula of the human eye (41) and lutein- and zeaxanthin-specific binding proteins (STARD3, [StAR-related lipid transfer domain 3 (StARD3) and glutathione S-transferase pi 1 (GSTP1)] in the human macula (113, 114) indicate the importance of these carotenoids in the human eye.
Leafy green vegetables, fruits, and other vegetables, such as kale, avocado, and corn, are good sources of lutein and zeaxanthin. In general, carotenoid absorption is affected by the same factors involved in fat absorption. Competitive absorption of carotenoid transport and tissue concentrations exists between hydrocarbon carotenoids and xanthophyll carotenoids (115–117). Bioavailability studies have suggested that interactions between different groups of carotenoids may affect each other’s bioavailability, either during micellization or during competition for gastrointestinal uptake into enterocytes, for chylomicron incorporation, or for tissue release from lipoproteins (118–120). High-dose dietary supplementation with a single carotenoid may alter the assimilation of other carotenoids (121). Chicken feeding experiments have suggested that accumulation of lutein and zeaxanthin in the retina is diminished when intake of β-carotene is high (121).
FAs.
n–3 Fatty acids belong to the group of long-chain PUFAs (LC-PUFAs) that are characterized by the presence of >1 double bond. Studies have demonstrated that an intake of dietary n–3 LC-PUFAs (EPA plus DHA) can alter inflammation-based processes and degenerative diseases such as AMD, which are mediated by n–6 LC-PUFAs such as arachidonic acid (20:4n–6) (122, 123). Current dietary estimates over the last 2 decades suggest that changes in dietary habits have increased the n–6:n–3 FA ratio to 25:1 from a healthy 2:1 ratio (124). High linoleic acid (18:2 n–6) consumption was associated with a 49% increased risk of AMD, and high DHA consumption was associated with a 30% reduced risk (57). A balance between dietary n–3 and n–6 PUFAs is essential for the optimal function of cell membranes, enzyme activity, gene expression, and intracellular communication. Hence, increased consumption of food-based sources of EPA, DHA, and their precursors has been recommended in most nutritional guidelines, with the recommended daily intake of DHA and EPA ranging from 250 to 1000 mg/d (125, 126). The n–3 LC-PUFAs have important structural and protective functions in the retina (127, 128). DHA reaches its highest concentration in the photoreceptor membranes, and is important in differentiation and survival of photoreceptors, as well as in retinal function (122). The anti-inflammatory properties of n–3 LC-PUFAs (127, 129) are of particular interest in AMD, because inflammation appears to play a pivotal role in this disease (130). n–3 LC-PUFAs may increase the macular pigment density, which alters blue light penetration and has local antioxidant and anti-inflammatory activities (131). Increased fish and n–3 FA consumption has also been correlated with decreased AMD risk (50, 132–135). As the primary FA in the retina, DHA helps regenerate rhodopsin, thereby reducing oxidative stress. Koto et al. (136) suggested that an EPA-rich diet resulted in significant suppression of neovascularization, although some studies reported no such relation (127). Studies from our laboratory indicate that serum and retinal EPA:arachidonic acid and n–3:n–6 LC-PUFA ratios were significantly lower in AMD donor eyes than they were in age-matched control subjects (137). Studies support the potential role of n–3 LC-PUFAs in the prevention of late AMD and highlight the necessity of clinical studies to determine more accurately the value of n–3 LC-PUFAs as a means of reducing the incidence of AMD (71, 72, 122, 134, 138).
AREDS2.
Based on the evidence discussed above, the AREDS2 was initiated in 2006 to evaluate the effect of the inclusion of lutein, zeaxanthin, and n–3 FAs into the original AREDS formulation on progression to advanced AMD over a period of 5 y. AREDS2 was designed to test whether the original AREDS supplement formulation could be made safer and more efficacious. In this study, 4203 participants aged ≥50 y with either bilateral large drusen or large drusen in one eye and advanced AMD in the other eye were enrolled and followed for 5 y. It was decided to mimic a typical dietary lutein-to-zeaxanthin ratio of 5:1, and a dose of 10 mg lutein plus 2 mg zeaxanthin was chosen for the carotenoid arm. This dose was based on a small pilot-scale, dose-ranging study, which observed a 4-time (400%) increase in serum lutein with the 10 mg lutein/d treatment (139). This dosage of lutein and zeaxanthin was much higher than that in a typical American diet (1–2 mg/d range for lutein and 0.2 mg/d for zeaxanthin); however, many vegetarian populations consume these carotenoid amounts regularly and safely. The amount of zinc in the original AREDS formulation was 80 mg/d, but the tolerable upper amount of zinc is 40 mg/d. A few studies had observed that high levels of zinc supplementation lead to genitourinary complications and self-reported anemia, which increased hospital admissions (56, 140); therefore, a subgroup was chosen to receive an AREDS formulation with lower zinc content. β-Carotene had been included in the original AREDS formulation, but because some clinical studies had shown that supplementation with β-carotene is associated with an increased risk of lung cancer in smokers, all current smokers and those who were recent previous smokers were assigned to subgroups that received an AREDS supplement without β-carotene.
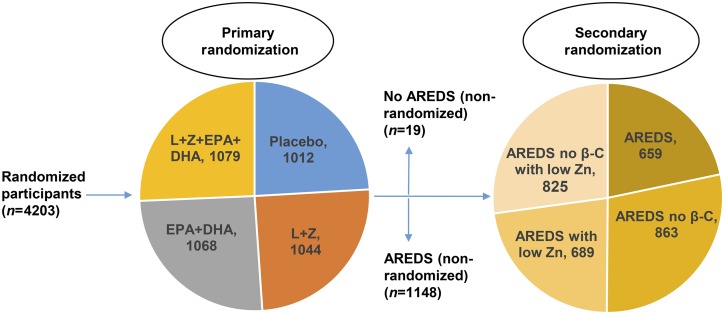
The primary randomization in AREDS2 was into 4 groups: 1) 10 mg lutein/d plus 2 mg zeaxanthin/d, 2) fish oil containing EPA plus DHA ethyl ester form (650 and 350 mg/d, respectively), 3) lutein plus zeaxanthin plus EPA plus DHA, and 4) placebo (Figure 2) (141). All nonsmoking enrolled subjects were offered secondary random assignment and were assigned to the original AREDS formula or to a modified AREDS formula with no β-carotene and/or lower concentrations of zinc. Secondary randomly assigned participants were divided into 4 groups: 1) AREDS formulation, 2) AREDS formulation (low zinc), 3) AREDS formulation (no β-carotene), or 4) AREDS formulation (no β-carotene and low zinc). Enrolled subjects who were smokers were randomly assigned to the AREDS supplement with no β-carotene with or without low concentrations of zinc. Subject retention and compliance were excellent, especially for a 5-y study with >4000 subjects. The participants were well nourished, with a 97% white and 1% black population. At every annual visit, AMD status and visual acuity were measured in a standardized manner, and fundus photographs of the macula and optic nerve and dilated eye examinations were performed. The primary purpose of the study was to compare the progression of advanced AMD between the 3 active treatment groups (lutein+zeaxanthin, lutein+zeaxanthin+EPA+DHA, and EPA+DHA) with placebo, based on fundus photographs in study eyes. Secondary analyses included the comparison of visual acuity, progression of lens opacities, and improvements in patients with AMD. Ancillary studies at different centers were also conducted to examine the effects of AREDS2 supplements on cognitive function and cardiovascular diseases. No macular pigment optical density (MPOD) measurements were included in the AREDS2 protocol, because the image-based instrumentation had not been sufficiently standardized at that time. Moreover, the psychophysical methods were thought to require too much time and subject training during the very busy study visits, but an ancillary study conducted at the Utah site had the equipment and experience to measure macular pigment with dual-wavelength autofluorescence imaging (142).
FIGURE 2.
AREDS2 study design. Participants were divided into 4 basic groups in primary randomization with supplements of macular carotenoids (L+Z) and/or n–3 FAs (EPA+DHA) or placebo. Patients who were willing to participate in secondary randomization were divided into 4 groups to test the original AREDS supplements with modifications [no β-carotene and/or low zinc (25 mg ZnO/d) instead of the 80 mg ZnO/d in the original AREDS supplement]. The secondary randomization groups were as follows: 1) original AREDS formulation, 2) AREDS formulation without β-carotene, 3) AREDS formulation with low zinc, and 4) AREDS formulation without β-carotene with low zinc. Participants who were smokers were distributed to either the AREDS formulation without β-carotene group or the AREDS formulation without β-carotene with low zinc group because of the increased risk of lung cancer posed by β-carotene. AREDS, Age-Related Eye Disease Study; L, lutein; Z, zeaxanthin; β-C, β-carotene.
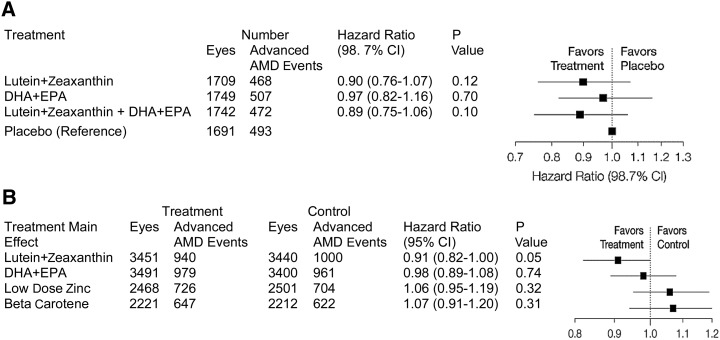
The median serum concentrations of lutein in participants randomly assigned to receive lutein supplements increased 2 times during the follow-up in comparison with baseline, whereas participants randomly assigned to receive placebo showed little change. Participants randomly assigned to receive EPA+DHA demonstrated a 30% increase in median serum DHA concentration, and median serum EPA concentrations doubled during the study (143). AREDS2 participants had high serum concentrations of lutein, zeaxanthin, DHA, and EPA at their baseline visits, suggesting that they were a particularly well-nourished population. The primary analyses indicated no significant reduction in progression to advanced AMD in the lutein+zeaxanthin, EPA+DHA, and lutein+zeaxanthin+EPA+DHA groups in comparison with placebo (Figure 3) (143). Although AREDS2 did not achieve its ambitious primary endpoint of a 25% incremental improvement beyond the already effective AREDS formula (143), an evaluation of the main effects of lutein+zeaxanthin in the entire study cohort that received the lutein+zeaxanthin supplement compared with the cohort that did not receive lutein+zeaxanthin showed a statistically significant reduction of progression to advanced AMD (P < 0.05) and a nonsignificant reduction in progression to central GA (P > 0.05) in those who received combined carotenoid supplementation (144). Furthermore, lutein+zeaxanthin supplementation decreased the progression of advanced AMD by 10% and GA by 11%, respectively, in comparison with no lutein+zeaxanthin supplementation.
FIGURE 3.
AREDS2 primary and main effects results. Primary effects analyses of randomized groups for prevention of progression to advanced AMD (A). Main effects analyses of randomized groups for progression to advanced AMD (B). AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study. Adapted from reference 143 with permission.
The results were more impressive when the subgroup receiving the AREDS formulation with lutein+zeaxanthin was compared with the subgroup that received the AREDS formulation with β-carotene, as evidenced when taking the relative risk factors and AMD progression into consideration. A significant reduction in the risk of progression of advanced AMD and neovascular AMD was observed in the group assigned the AREDS formulation with lutein+zeaxanthin in comparison with the group assigned the AREDS formulation with β-carotene (P < 0.05) (Figure 3) (144). As discussed earlier in this review, it was hypothesized that, because of competitive absorption of carotenes over xanthophylls, the xanthophylls may not have been absorbed well (116, 145), and this was confirmed after analyzing the carotenoid concentrations in the serum (143). Lung cancer prevalence was not altered in participants assigned to the lutein+zeaxanthin arms, but its prevalence significantly increased in former smokers who consumed β-carotene. Hence, β-carotene appeared to be more risky than lutein+zeaxanthin supplementation in this clinical trial. Substituting 10 mg lutein/d and 2 mg zeaxanthin/d for 15 mg β-carotene/d was determined to be a safe and appropriate modification of the original AREDS formulation for all subjects, including smokers and former smokers. Clinicians and consumers have rapidly adopted these AREDS nutritional recommendations.
The group assigned to the AREDS formulation with low zinc was compared with the group assigned to the AREDS formulation with high zinc, and no significant progression of AMD or adverse side effects such as gastrointestinal disorders were observed (P = 0.31) (Figure 3). Also, no significant correlation was established for EPA+DHA supplementation with the progression of AMD (P = 0.74) (143). In contrast with the AREDS2 result, the Nutritional AMD Treatment-2 study, in which patients were administered 840 mg DHA/d and 270 mg EPA/d for 3 y, suggested that the CNV incidence was markedly reduced in DHA-supplemented patients (72).
The strengths of the AREDS2 are its rigorous statistical analysis, low rates of losses to follow-up, and good adherence to the treatment regimen. There are several limitations of the AREDS2, including its complex randomization scheme and lack of a true control arm. This resulted in more than a dozen preplanned subgroups, all of which had to be offered the previously proven effective original AREDS supplement or a suitable modification. This could account in part for the study’s failure to meet its primary endpoints and clinicians’ and the general public’s difficulties in appreciating the take-home message of the study. By necessity, the choice and dose of the AREDS2 supplement formulation tested had to be limited to the consensus of the organizing committee, so there will always be opportunities to second guess the results and suggest that other formulations (i.e., other fish oil preparations that might be more bioavailable or with different EPA:DHA ratios) and higher doses might have yielded superior outcomes. There is also a potential lack of generalizability to clinical practice in the United States and elsewhere, because the AREDS2 population appeared to be unusually well nourished, with an above average intake of many dietary nutrients. For example, a baseline study from Utah observed that its subjects had surprisingly high MPOD levels at the baseline visit of the study, probably because of an inadequate 30-d supplement-free washout period. This finding was consistent with the observation that 70% of Utah subjects had been taking lutein and zeaxanthin supplements regularly for many years before enrollment (142). Follow-up MPOD measurements of these subjects recorded at scheduled visits every 6 mo during the study period did not provide any convincing evidence of an effect of supplementation. This may be due to the relatively small number of subjects in this ancillary study and the challenges of reproducibly imaging macular pigment in patients with substantial macular pathology.
Even though both AREDS studies were designed to extend the idea of antioxidant supplementation in AMD subjects with intermediate or unilateral advanced AMD, it is still unclear whether lutein+zeaxanthin supplementation should be recommended to a younger, healthy, well-educated, and well-nourished population (the “worried well”), even if they carry high-risk alleles. Studies involving identification of risk alleles such as complement factor H and high temperature requirement A serine peptidase 1/age-related maculopathy susceptibility 2 and their association with AMD and gene-diet interactions have shown that dietary factors may play an important role in preventing AMD (146–148), but there has been considerable controversy regarding whether or not genotypes should actually guide supplementation options for AMD (149, 150). These studies, however, were all based on original AREDS data, so they provide no guidance on genotype relations with lutein and zeaxanthin supplementation and AMD. Such insights will be forthcoming when genotype data are released for the AREDS2 cohort.
Conclusions
The AREDS2 formulation with 10 mg lutein/d and 2 mg zeaxanthin/d is now the standard of care for reducing the probability of advanced AMD in patients with substantial risk factors for progression to severe visual loss, and there is even some evidence that subjects receiving AREDS2-type supplements could have stabilization and improvement of best-corrected visual acuity (151, 152). However, the AREDS2 failed to show that fish oil supplements had any benefits, and the β-carotene of the original AREDS formula is no longer generally recommended because of potential lung cancer risks.
The AREDS2 was not designed to determine whether or not individuals without signs or symptoms of AMD should take supplements, but clinicians should definitely advise everyone concerned about developing AMD to practice a healthy, vigorous lifestyle by avoiding smoking, getting regular physical activity, and maintaining a diet rich in colorful fruits and vegetables and oily fish. These nutritional and lifestyle recommendations are likely to be especially applicable to people with poor diets, high genetic risk profiles for AMD on the basis of family history or genotyping, or low concentrations of macular carotenoids.
Many clinic patients ask whether more nutritional interventions against AMD will be forthcoming. Supplements containing meso-zeaxanthin are already on the market, but it is unlikely that a study on the scale of the AREDS2 will be conducted to prove its efficacy. Herbal supplements and alternative medicines such as goji berries (153), saffron (154), anthocyanins (155), and flavonoids appear to decrease oxidative stress and are commonly consumed by people concerned about eye diseases such as AMD, but their efficacies and pharmacokinetics are unknown. On the other hand, there is a growing concern among clinicians that excessive intake not only of fat-soluble vitamins and micronutrients, but also minerals and water-soluble vitamins, may be associated with toxicity, including undesirable interactions with other drugs and vitamins. Therefore, it is important for practitioners to educate patients about the benefits of supplements, about why a certain supplement is being recommended for individual use, and that there can be risks when supplements are consumed in excess.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study; CNV, choroidal neovascularization; GA, geographic atrophy; LC-PUFA, long-chain PUFA; MPOD, macular pigment optical density; ROS, reactive oxygen species; RPE, retinal pigment epithelium.
References
- 1.Bourne RRA, Jonas JB, Flaxman SR, Keeffe J, Leasher J, Naidoo K, Parodi MB, Pesudovs K, Price H, White RA, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990–2010. Br J Ophthalmol 2014;98:629–38. [DOI] [PubMed] [Google Scholar]
- 2.Bourne RR, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, Jonas JB, Keeffe J, Leasher J, Naidoo K, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health 2013;1:e339–49. [DOI] [PubMed] [Google Scholar]
- 3.Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng C-Y, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–16. [DOI] [PubMed] [Google Scholar]
- 4.Mayne ST. Beta-carotene, carotenoids, and disease prevention in humans. FASEB J 1996;10:690–701. [PubMed] [Google Scholar]
- 5.Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 6.Gass JD. Drusen and disciform macular detachment and degeneration. Trans Am Ophthalmol Soc 1972;70:409–36. [PMC free article] [PubMed] [Google Scholar]
- 7.Sarks SH, Van Driel D, Maxwell L, Killingsworth M. Softening of drusen and subretinal neovascularization. Trans Ophthalmol Soc U K 1980;100:414–22. [PubMed] [Google Scholar]
- 8.Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation–induced regression. Surv Ophthalmol 1999;44:1–29. [DOI] [PubMed] [Google Scholar]
- 9.Green WR, Key SN 3rd. Senile macular degeneration: a histopathologic study. Trans Am Ophthalmol Soc 1977;75:180–254. [PMC free article] [PubMed] [Google Scholar]
- 10.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 2003;48:257–93. [DOI] [PubMed] [Google Scholar]
- 11.Green WR, Wilson DJ. Choroidal neovascularization. Ophthalmology 1986;93:1169–76. [DOI] [PubMed] [Google Scholar]
- 12.Ferris FL, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 1984;102:1640–2. [DOI] [PubMed] [Google Scholar]
- 13.Olsen TW, Knobloch WH. Treatment of exudative age-related macular degeneration: many factors to consider. Am J Ophthalmol 2007;144:281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Channa R, Sophie R, Bagheri S, Shah SM, Wang J, Adeyemo O, Sodhi A, Wenick A, Ying HS, Campochiaro PA. Regression of choroidal neovascularization results in macular atrophy in anti-vascular endothelial growth factor-treated eyes. Am J Ophthalmol 2015;159:9–19. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA 2004;291:1900–1. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Chou C-F, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol 2011;129:75–80. [DOI] [PubMed] [Google Scholar]
- 17.Friedman DS, O’Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J; The Eye Diseases Prevalence Research G. Prevalence of age-related macular degeneration in the united states. Arch Ophthalmol 2004;122:564–72. [DOI] [PubMed] [Google Scholar]
- 18.Evans JR. Risk factors for age-related macular degeneration. Prog Retin Eye Res 2001;20:227–53. [DOI] [PubMed] [Google Scholar]
- 19.Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, Hofman A, Jensen S, Wang JJ, de Jong PT. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology 2001;108:697–704. [DOI] [PubMed] [Google Scholar]
- 20.Vinding T. Age-related macular degeneration. Macular changes, prevalence and sex ratio. An epidemiological study of 1000 aged individuals. Acta Ophthalmol (Copenh) 1989;67:609–16. [DOI] [PubMed] [Google Scholar]
- 21.Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, Kramer CFL, de Jong PTVM. The prevalence of age-related maculopathy in the Rotterdam study. Ophthalmology 1995;102:205–10. [DOI] [PubMed] [Google Scholar]
- 22.Klein R, Klein BEK, Linton KLP. Prevalence of age-related maculopathy. Ophthalmology 1992;99:933–43. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell P, Wang JJ, Foran S, Smith W. Five-year incidence of age-related maculopathy lesions: the Blue Mountains Eye Study. Ophthalmology 2002;109:1092–7. [DOI] [PubMed] [Google Scholar]
- 24.Klein R, Klein BEK, Marino EK, Kuller LH, Furberg C, Burke GL, Hubbard LD. Early age-related maculopathy in the cardiovascular health study. Ophthalmology 2003;110:25–33. [DOI] [PubMed] [Google Scholar]
- 25.Friedman DS, Katz J, Bressler NM, Rahmani B, Tielsch JM. Racial differences in the prevalence of age-related macular degeneration. Ophthalmology 1999;106:1049–55. [DOI] [PubMed] [Google Scholar]
- 26.Hyman LG, Lilienfeld AM, Ferris FL, Fine SL. Senile macular degeneration: a case-control study. Am J Epidemiol 1983;118:213–27. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell P, Smith W, Wang JJ. Iris color, skin sun sensitivity, and age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology 1998;105:1359–63. [DOI] [PubMed] [Google Scholar]
- 28.Klein R, Klein BE, Jensen SC, Cruickshanks KJ. The relationship of ocular factors to the incidence and progression of age-related maculopathy. Arch Ophthalmol 1998;116:506–13. [DOI] [PubMed] [Google Scholar]
- 29.Hirakawa M, Tanaka M, Tanaka Y, Okubo A, Koriyama C, Tsuji M, Akiba S, Miyamoto K, Hillebrand G, Yamashita T, et al. Age-related maculopathy and sunlight exposure evaluated by objective measurement. Br J Ophthalmol 2008;92:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein RJ, Zeiss C, Chew EY, Tsai J-Y, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, et al. Complement factor H polymorphism in age-related macular degeneration. Science 2005;308:385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donoso LA, Vrabec T, Kuivaniemi H. The role of complement Factor H in age-related macular degeneration: a review. Surv Ophthalmol 2010;55:227–46. [DOI] [PubMed] [Google Scholar]
- 32.Hadley D, Orlin A, Brown G, Brucker AJ, Ho AC, Regillo CD, Donoso LA, Tian L, Kaderli B, Stambolian D. Analysis of six genetic risk factors highly associated with AMD in the region surrounding ARMS2 and HTRA1 on chromosome 10, region q26. Invest Ophthalmol Vis Sci 2010;51:2191–6. [DOI] [PubMed] [Google Scholar]
- 33.Gibbs D, Yang Z, Constantine R, Ma X, Camp NJ, Yang X, Chen H, Jorgenson A, Hau V, DeWan A, et al. Further mapping of 10q26 supports strong association of HTRA1 polymorphisms with age-related macular degeneration. Vision Res 2008;48:685–9. [DOI] [PubMed] [Google Scholar]
- 34.van Lookeren Campagne M, Strauss EC, Yaspan BL. Age-related macular degeneration: complement in action. Immunobiology 2016;221:733–9. [DOI] [PubMed] [Google Scholar]
- 35.Khan JC, Thurlby DA, Shahid H, Clayton DG, Yates JR, Bradley M, Moore AT, Bird AC. Genetic Factors in AMDS. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol 2006;90:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delcourt C, Carriere I, Ponton-Sanchez A, Fourrey S, Lacroux A, Papoz L. Light exposure and the risk of age-related macular degeneration: the Pathologies Oculaires Liees a l’Age (POLA) study. Arch Ophthalmol 2001;119:1463–8. [DOI] [PubMed] [Google Scholar]
- 37.Fletcher AE, Bentham GC, Agnew M, Young IS, Augood C, Chakravarthy U, de Jong PT, Rahu M, Seland J, Soubrane G, et al. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch Ophthalmol 2008;126:1396–403. [DOI] [PubMed] [Google Scholar]
- 38.Bressler NM, Bressler SB, West SK, Fine SL, Taylor HR. The grading and prevalence of macular degeneration in Chesapeake Bay watermen. Arch Ophthalmol 1989;107:847–52. [DOI] [PubMed] [Google Scholar]
- 39.Cruickshanks KJ, Klein R, Klein BE. Sunlight and age-related macular degeneration. The Beaver Dam Eye Study. Arch Ophthalmol 1993;111:514–8. [DOI] [PubMed] [Google Scholar]
- 40.Boulton M, Docchio F, Dayhaw-Barker P, Ramponi R, Cubeddu R. Age-related changes in the morphology, absorption and fluorescence of melanosomes and lipofuscin granules of the retinal pigment epithelium. Vision Res 1990;30:1291–303. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res 2001;72:215–23. [DOI] [PubMed] [Google Scholar]
- 42.Bhosale P, Serban B, Bernstein PS. Retinal carotenoids can attenuate formation of A2E in the retinal pigment epithelium. Arch Biochem Biophys 2009;483:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci 2000;41:1981–9. [PubMed] [Google Scholar]
- 44.Conn PF, Schalch W, Truscott TG. The singlet oxygen and carotenoid interaction. J Photochem Photobiol B 1991;11:41–7. [DOI] [PubMed] [Google Scholar]
- 45.Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med 2012;33:399–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antioxidant status and neovascular age-related macular degeneration. Eye Disease Case-Control Study Group. Arch Ophthalmol 1993;111:104–9. Erratum in: Arch Ophthalmol 1993;111:1499; 111:1228; 111:1366. [DOI] [PubMed] [Google Scholar]
- 47.Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci 2001;42:235–40. [PubMed] [Google Scholar]
- 48.Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, Nolan JM. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res 2016;50:34–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newsome DA, Swartz M, Leone NC, Elston RC, Miller E. Oral zinc in macular degeneration. Arch Ophthalmol 1988;106:192–8. [DOI] [PubMed] [Google Scholar]
- 50.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA 1994;272:1413–20. [PubMed] [Google Scholar]
- 51.West S, Vitale S, Hallfrisch J, Muñoz B, Muller D, Bressler S, Bressler NM. Are antioxidants or supplements protective for age-related macular degeneration? Arch Ophthalmol 1994;112:222–7. [DOI] [PubMed] [Google Scholar]
- 52.Teikari JM, Laatikainen L, Virtamo J, Haukka J, Rautalahti M, Liesto K, Albanes D, Taylor P, Heinonen OP. Six-year supplementation with alpha-tocopherol and beta-carotene and age-related maculopathy. Acta Ophthalmol Scand 1998;76:224–9. [DOI] [PubMed] [Google Scholar]
- 53.VandenLangenberg GM, Mares-Perlman JA, Klein R, Klein BE, Brady WE, Palta M. Associations between antioxidant and zinc intake and the 5-year incidence of early age-related maculopathy in the Beaver Dam Eye Study. Am J Epidemiol 1998;148:204–14. [DOI] [PubMed] [Google Scholar]
- 54.Christen WG, Ajani UA, Glynn RJ, Manson JE, Schaumberg DA, Chew EC, Buring JE, Hennekens CH. Prospective cohort study of antioxidant vitamin supplement use and the risk of age-related maculopathy. Am J Epidemiol 1999;149:476–84. [DOI] [PubMed] [Google Scholar]
- 55.Smith W, Mitchell P, Webb K, Leeder SR. Dietary antioxidants and age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology 1999;106:761–7. [DOI] [PubMed] [Google Scholar]
- 56.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report No. 8. Arch Ophthalmol 2001;119:1417–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho E, Hung S, Willett WC, Spiegelman D, Rimm EB, Seddon JM, Colditz GA, Hankinson SE. Prospective study of dietary fat and the risk of age-related macular degeneration. Am J Clin Nutr 2001;73:209–18. [DOI] [PubMed] [Google Scholar]
- 58.Mares-Perlman JA, Fisher AI, Klein R, Palta M, Block G, Millen AE, Wright JD. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am J Epidemiol 2001;153:424–32. [DOI] [PubMed] [Google Scholar]
- 59.Seddon JM, Rosner B, Sperduto RD, Yannuzzi L, Haller JA, Blair NP, Willett W. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol 2001;119:1191–9. [DOI] [PubMed] [Google Scholar]
- 60.Gale CR, Hall NF, Phillips DIW, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci 2003;44:2461–5. [DOI] [PubMed] [Google Scholar]
- 61.Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, Pei K, Tsipursky M, Nyland J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004;75:216–30. [DOI] [PubMed] [Google Scholar]
- 62.van Leeuwen R, Boekhoorn S, Vingerling JR, Witteman JC, Klaver CC, Hofman A, de Jong PT. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA 2005;294:3101–7. [DOI] [PubMed] [Google Scholar]
- 63.Delcourt C, Carrière I, Delage M, Barberger-Gateau P, Schalch W. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA study. Invest Ophthalmol Vis Sci 2006;47:2329–35. [DOI] [PubMed] [Google Scholar]
- 64.Moeller SM, Parekh N, Tinker L, Ritenbaugh C, Blodi B, Wallace RB, Mares JA; CAREDS Research Study Group. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the carotenoids in Age-Related Eye Disease Study (CAREDS): ancillary study of the Women’s Health Initiative. Arch Ophthalmol 2006;124:1151–62. [DOI] [PubMed] [Google Scholar]
- 65.Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: The US twin study of age-related macular degeneration. Arch Ophthalmol 2006;124:995–1001. [DOI] [PubMed] [Google Scholar]
- 66.Age-Related Eye Disease Study Research Group; SanGiovanni JP, Chew EY, Clemons TE, Ferris FL 3rd, Gensler G, Lindblad AS, Milton RC, Seddon JM, Sperduto RD. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol 2007;125:1225–32. [DOI] [PubMed] [Google Scholar]
- 67.Trieschmann M, Beatty S, Nolan JM, Hense HW, Heimes B, Austermann U, Fobker M, Pauleikhoff D. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: the LUNA study. Exp Eye Res 2007;84:718–28. [DOI] [PubMed] [Google Scholar]
- 68.Cho E, Hankinson SE, Rosner B, Willett WC, Colditz GA. Prospective study of lutein/zeaxanthin intake and risk of age-related macular degeneration. Am J Clin Nutr 2008;87:1837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan JS, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology 2008;115:334–41. [DOI] [PubMed] [Google Scholar]
- 70.Parekh N, Voland RP, Moeller SM, Blodi BA, Ritenbaugh C, Chappell RJ, Wallace RB, Mares JA; CAREDS Research Study Group. Association between dietary fat intake and age-related macular degeneration in the carotenoids in Age-Related Eye Disease Study (CAREDS): An ancillary study of the women’s health initiative. Arch Ophthalmol 2009;127:1483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merle BM, Delyfer M-N, Korobelnik J-F, Rougier M-B, Malet F, Féart C, Le Goff M, Peuchant E, Letenneur L, Dartigues J-F. High concentrations of plasma n3 fatty acids are associated with decreased risk for late age-related macular degeneration. J Nutr 2013;143:505–11. [DOI] [PubMed] [Google Scholar]
- 72.Souied EH, Delcourt C, Querques G, Bassols A, Merle B, Zourdani A, Smith T, Benlian P. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: the Nutritional AMD Treatment 2 Study. Ophthalmology 2013;120:1619–31. [DOI] [PubMed] [Google Scholar]
- 73.Dawczynski J, Jentsch S, Schweitzer D, Hammer M, Lang GE, Strobel J. Long term effects of lutein, zeaxanthin and omega-3-LCPUFAs supplementation on optical density of macular pigment in AMD patients: the LUTEGA study. Graefes Arch Clin Exp Ophthalmol 2013;251:2711–23. [DOI] [PubMed] [Google Scholar]
- 74.Blasiak J, Szaflik J, Szaflik JP. Implications of altered iron homeostasis for age-related macular degeneration. Front Biosci (Landmark Ed) 2011;16:1551–9. [DOI] [PubMed] [Google Scholar]
- 75.Chen H, Liu B, Lukas TJ, Suyeoka G, Wu G, Neufeld AH. Changes in iron-regulatory proteins in the aged rodent neural retina. Neurobiol Aging 2009;30:1865–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ugarte M, Osborne NN, Brown LA, Bishop PN. Iron, zinc, and copper in retinal physiology and disease. Surv Ophthalmol 2013;58:585–609. [DOI] [PubMed] [Google Scholar]
- 77.Andrzejczyk J, Buszman E. Interaction of Fe3+, Cu2+ and Zn2+ with melanin and melanoproteins from bovine eyes. Acta Biochim Pol 1992;39:85–8. [PubMed] [Google Scholar]
- 78.Bowness JM, Morton RA, Shakir MH, Stubbs AL. Distribution of copper and zinc in mammalian eyes. Occurrence of metals in melanin fractions from eye tissues. Biochem J 1952;51:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Erie JC, Good JA, Butz JA, Pulido JS. Reduced zinc and copper in the retinal pigment epithelium and choroid in age-related macular degeneration. Am J Ophthalmol 2009;147:276–82.e1. [DOI] [PubMed] [Google Scholar]
- 80.Naismith RT, Shepherd JB, Weihl CC, Tutlam NT, Cross AH. Acute and bilateral blindness due to optic neuropathy associated with copper deficiency. Arch Neurol 2009;66:1025–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pineles SL, Wilson CA, Balcer LJ, Slater R, Galetta SL. Combined optic neuropathy and myelopathy secondary to copper deficiency. Surv Ophthalmol 2010;55:386–92. [DOI] [PubMed] [Google Scholar]
- 82.Park SJ, Lee JH, Woo SJ, Kang SW, Park KH. Five heavy metallic elements and age-related macular degeneration. Ophthalmology 2015;122:129–37. [DOI] [PubMed] [Google Scholar]
- 83.Tokarz P, Kaarniranta K, Blasiak J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013;14:461–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gotoh N, Yamada R, Matsuda F, Yoshimura N, Iida T. Manganese superoxide dismutase gene (SOD2) polymorphism and exudative age-related macular degeneration in the Japanese population. Am J Ophthalmol 2008;146:author reply146–7. [DOI] [PubMed] [Google Scholar]
- 85.Mayer MJ, van Kuijk FJ, Ward B, Glucs A. Whole blood selenium in exudative age-related maculopathy. Acta Ophthalmol Scand 1998;76:62–7. [DOI] [PubMed] [Google Scholar]
- 86.Nielsen JC, Naash MI, Anderson RE. The regional distribution of vitamins E and C in mature and premature human retinas. Invest Ophthalmol Vis Sci 1988;29:22–6. [PubMed] [Google Scholar]
- 87.Wiegand RD, Joel CD, Rapp LM, Nielsen JC, Maude MB, Anderson RE. Polyunsaturated fatty acids and vitamin E in rat rod outer segments during light damage. Invest Ophthalmol Vis Sci 1986;27:727–33. [PubMed] [Google Scholar]
- 88.Robison WG Jr, Kuwabara T, Bieri JG. The roles of vitamin E and unsaturated fatty acids in the visual process. Retina 1982;2:263–81. [PubMed] [Google Scholar]
- 89.Tanito M, Yoshida Y, Kaidzu S, Chen Z-H, Cynshi O, Jishage K-i, Niki E, Ohira A. Acceleration of age-related changes in the retina in α-tocopherol transfer protein null mice fed a vitamin E–deficient diet. Invest Ophthalmol Vis Sci 2007;48:396–404. [DOI] [PubMed] [Google Scholar]
- 90.Katz ML, Eldred GE. Failure of vitamin E to protect the retina against damage resulting from bright cyclic light exposure. Invest Ophthalmol Vis Sci 1989;30:29–36. [PubMed] [Google Scholar]
- 91.Katz ML, Eldred GE. Retinal light damage reduces autofluorescent pigment deposition in the retinal pigment epithelium. Invest Ophthalmol Vis Sci 1989;30:37–43. [PubMed] [Google Scholar]
- 92.Belda JI, Romá J, Vilela C, Puertas FJ, Díaz-Llopis M, Bosch-Morell F, Romero FJ. Serum vitamin E levels negatively correlate with severity of age-related macular degeneration. Mech Ageing Dev 1999;107:159–64. [DOI] [PubMed] [Google Scholar]
- 93.Taylor HR, Tikellis G, Robman LD, McCarty CA, McNeil JJ. Vitamin E supplementation and macular degeneration: randomised controlled trial. BMJ 2002;325:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Christen WG, Glynn RJ, Chew EY, Albert CM, Manson JE. Folic acid, pyridoxine, and cyanocobalamin combination treatment and age-related macular degeneration in women: the Women’s Antioxidant and Folic Acid Cardiovascular Study. Arch Intern Med 2009;169:335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gopinath B, Flood VM, Rochtchina E, Wang JJ, Mitchell P. Homocysteine, folate, vitamin B-12, and 10-y incidence of age-related macular degeneration. Am J Clin Nutr 2013;98:129–35. [DOI] [PubMed] [Google Scholar]
- 96.Britton G. Structure and properties of carotenoids in relation to function. FASEB J 1995;9:1551–8. [PubMed] [Google Scholar]
- 97.Stahl W, Nicolai S, Briviba K, Hanusch M, Broszeit G, Peters M, Martin HD, Sies H. Biological activities of natural and synthetic carotenoids: induction of gap junctional communication and singlet oxygen quenching. Carcinogenesis 1997;18:89–92. [DOI] [PubMed] [Google Scholar]
- 98.Burton GW, Ingold KU. beta-Carotene: an unusual type of lipid antioxidant. Science 1984;224:569–73. [DOI] [PubMed] [Google Scholar]
- 99.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL Jr, Valanis B, Williams JH Jr, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst 1996;88:1550–9. [DOI] [PubMed] [Google Scholar]
- 100.Albanes D, Heinonen OP, Huttunen JK, Taylor PR, Virtamo J, Edwards B, Haapakoski J, Rautalahti M, Hartman A, Palmgren J. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am J Clin Nutr 1995;62:1427S–30S. [DOI] [PubMed] [Google Scholar]
- 101.Cerutti PA. Oxy-radicals and cancer. Lancet 1994;344:862–3. [DOI] [PubMed] [Google Scholar]
- 102.Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL 3rd; Age-Related Eye Disease Study Research Group. Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology 2005;112:533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol 2001;119:1439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, et al. Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst 1996;88:1560–70. [DOI] [PubMed] [Google Scholar]
- 105.The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 1994;330:1029–35. [DOI] [PubMed] [Google Scholar]
- 106.Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci 1993;34:2033–40. [PubMed] [Google Scholar]
- 107.Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci 1988;29:843–9. [PubMed] [Google Scholar]
- 108.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr 1995;62:1448S–61S. [DOI] [PubMed] [Google Scholar]
- 109.Khachik F, de Moura FF, Zhao DY, Aebischer CP, Bernstein PS. Transformations of selected carotenoids in plasma, liver, and ocular tissues of humans and in nonprimate animal models. Invest Ophthalmol Vis Sci 2002;43:3383–92. [PubMed] [Google Scholar]
- 110.Sundelin SP, Nilsson SE. Lipofuscin-formation in retinal pigment epithelial cells is reduced by antioxidants. Free Radic Biol Med 2001;31:217–25. [DOI] [PubMed] [Google Scholar]
- 111.Hammond BR Jr, Wooten BR, Curran-Celentano J. Carotenoids in the retina and lens: possible acute and chronic effects on human visual performance. Arch Biochem Biophys 2001;385:41–6. [DOI] [PubMed] [Google Scholar]
- 112.Stringham JM, Hammond BR. Macular pigment and visual performance under glare conditions. Optom Vis Sci 2008;85:82–8. [DOI] [PubMed] [Google Scholar]
- 113.Li B, Vachali P, Frederick JM, Bernstein PS. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry 2011;50:2541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem 2004;279:49447–54. [DOI] [PubMed] [Google Scholar]
- 115.Reboul E, Richelle M, Perrot E, Desmoulins-Malezet C, Pirisi V, Borel P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J Agric Food Chem 2006;54:8749–55. [DOI] [PubMed] [Google Scholar]
- 116.Bohn T. Bioavailability of non-provitamin A carotenoids. Curr Nutr Food Sci 2008;4:240–58. [Google Scholar]
- 117.Wang W, Connor SL, Johnson EJ, Klein ML, Hughes S, Connor WE. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am J Clin Nutr 2007;85:762–9. [DOI] [PubMed] [Google Scholar]
- 118.Garrett DA, Failla ML, Sarama RJ, Craft N. Accumulation and retention of micellar β-carotene and lutein by Caco-2 human intestinal cells. J Nutr Biochem 1999;10:573–81. [DOI] [PubMed] [Google Scholar]
- 119.van den Berg H, van Vliet T. Effect of simultaneous, single oral doses of beta-carotene with lutein or lycopene on the beta-carotene and retinyl ester responses in the triacylglycerol-rich lipoprotein fraction of men. Am J Clin Nutr 1998;68:82–9. [DOI] [PubMed] [Google Scholar]
- 120.Faulks RM, Southon S. Challenges to understanding and measuring carotenoid bioavailability. Biochim Biophys Acta 2005;1740:95–100. [DOI] [PubMed] [Google Scholar]
- 121.Wang Y, Illingworth DR, Connor SL, Duell PB, Connor WE. Competitive inhibition of carotenoid transport and tissue concentrations by high dose supplements of lutein, zeaxanthin and beta-carotene. Eur J Nutr 2010;49:327–36. [DOI] [PubMed] [Google Scholar]
- 122.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res 2005;24:87–138. [DOI] [PubMed] [Google Scholar]
- 123.Lorente-Cebrián S, Costa AGV, Navas-Carretero S, Zabala M, Laiglesia LM, Martínez JA, Moreno-Aliaga MJ. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J Physiol Biochem 2015;71:341–9. [DOI] [PubMed] [Google Scholar]
- 124.Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr 1999;70:560S–9S. [DOI] [PubMed] [Google Scholar]
- 125.Nestel P, Clifton P, Colquhoun D, Noakes M, Mori TA, Sullivan D, Thomas B. Indications for omega-3 long chain polyunsaturated fatty acid in the prevention and treatment of cardiovascular disease. Heart Lung Circ 2015;24:769–79. [DOI] [PubMed] [Google Scholar]
- 126. EFSA Panel on Dietetic Products Nutrition, and Allergies. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J 2010;8:1461. [Google Scholar]
- 127.SanGiovanni JP, Chew EY, Agron E, Clemons TE, Ferris FL, Gensler G, Lindblad AS, Milton RC, Seddon JM, Klein R. The relationship of dietary ω-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol 2008;126:1274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.SanGiovanni J, Chew E, Clemons T, Seddon J, Klein R, AREDS Research. Dietary lipid intake and incident advanced age–related macular degeneration (AMD) in the Age–Related Eye Disease Study (AREDS). Invest Ophthalmol Vis Sci 2005;46:2382. [Google Scholar]
- 129.Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer’s disease. J Lipid Res 2009;50:S400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 2006;51:137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Delyfer M-N, Buaud B, Korobelnik J-F, Rougier M-B, Schalch W, Etheve S, Vaysse C, Combe N, Le Goff M, Wolf-Schnurrbusch UE. Association of macular pigment density with plasma omega-3 fatty acids: the PIMAVOSA study. Invest Ophthalmol Vis Sci 2012;53:1204–10. [DOI] [PubMed] [Google Scholar]
- 132.Wang JJ, Rochtchina E, Smith W, Klein R, Klein BE, Joshi T, Sivakumaran TA, Iyengar S, Mitchell P. Combined effects of complement factor H genotypes, fish consumption, and inflammatory markers on long-term risk for age-related macular degeneration in a cohort. Am J Epidemiol 2009;169:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Augood C, Chakravarthy U, Young I, Vioque J, de Jong PT, Bentham G, Rahu M, Seland J, Soubrane G, Tomazzoli L. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am J Clin Nutr 2008;88:398–406. [DOI] [PubMed] [Google Scholar]
- 134.Tan JS, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol 2009;127:656–65. [DOI] [PubMed] [Google Scholar]
- 135.SanGiovanni JP, Agrón E, Clemons TE, Chew EY. ω-3 long-chain polyunsaturated fatty acid intake inversely associated with 12-year progression to advanced age-related macular degeneration. Arch Ophthalmol 2009;127:110–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koto T, Nagai N, Mochimaru H, Kurihara T, Izumi-Nagai K, Satofuka S, Shinoda H, Noda K, Ozawa Y, Inoue M. Eicosapentaenoic acid is anti-inflammatory in preventing choroidal neovascularization in mice. Invest Ophthalmol Vis Sci 2007;48:4328–34. [DOI] [PubMed] [Google Scholar]
- 137.Gorusupudi A, Liu A, Hageman GS, Bernstein PS. Associations of human retinal very long-chain polyunsaturated fatty acids with dietary lipid biomarkers. J Lipid Res 2016;57:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Querques G, Benlian P, Chanu B, Portal C, Coscas G, Soubrane G, Souied E. Nutritional AMD treatment phase I (NAT-1): feasibility of oral DHA supplementation in age-related macular degeneration. Eur J Ophthalmol 2009;19:100. [DOI] [PubMed] [Google Scholar]
- 139.Rosenthal JM, Kim J, de Monasterio F, Thompson DJ, Bone RA, Landrum JT, de Moura FF, Khachik F, Chen H, Schleicher RL, et al. Dose-ranging study of lutein supplementation in persons aged 60 years or older. Invest Ophthalmol Vis Sci 2006;47:5227–33. Erratum in: Invest Ophthalmol Vis Sci 2007;48:14. [DOI] [PubMed] [Google Scholar]
- 140.Johnson AR, Munoz A, Gottlieb JL, Jarrard DF. High dose zinc increases hospital admissions due to genitourinary complications. J Urol 2007;177:639–43. [DOI] [PubMed] [Google Scholar]
- 141.AREDS2 Research Group, Chew EY, Clemons T, SanGiovanni JP, Danis R, Domalpally A, McBee W, Sperduto R, Ferris FL. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012;119:2282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bernstein PS, Ahmed F, Liu A, Allman S, Sheng X, Sharifzadeh M, Ermakov I, Gellermann W. Macular pigment imaging in AREDS2 participants: an ancillary study of AREDS2 subjects enrolled at the Moran Eye Center. Invest Ophthalmol Vis Sci 2012;53:6178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013;309:2005–15. Erratum in: JAMA 2013;310:208. [DOI] [PubMed] [Google Scholar]
- 144.Age-Related Eye Disease Study 2 Research Group, Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL III, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol 2014;132:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van Het Hof KH, West CE, Weststrate JA, Hautvast JG. Dietary factors that affect the bioavailability of carotenoids. J Nutr 2000;130:503–6. [DOI] [PubMed] [Google Scholar]
- 146.Meyers KJ, Liu Z, Millen AE, Iyengar SK, Blodi BA, Johnson E, Snodderly DM, Klein ML, Gehrs KM, Tinker L, et al. Joint associations of diet, lifestyle, and genes with age-related macular degeneration. Ophthalmology 2015;122:2286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang JJ, Buitendijk GHS, Rochtchina E, Lee KE, Klein BEK, van Duijn CM, Flood VM, Meuer SM, Attia J, Myers C, et al. Genetic susceptibility, dietary antioxidants, and long-term incidence of age-related macular degeneration in two populations. Ophthalmology 2014;121:667–75. [DOI] [PubMed] [Google Scholar]
- 148.Merle B, Delyfer MN, Korobelnik JF, Rougier MB, Colin J, Malet F, Feart C, Le Goff M, Dartigues JF, Barberger-Gateau P, et al. Dietary omega-3 fatty acids and the risk for age-related maculopathy: the Alienor Study. Invest Ophthalmol Vis Sci 2011;52:6004–11. [DOI] [PubMed] [Google Scholar]
- 149.Awh CC, Hawken S, Zanke BW. Treatment response to antioxidants and zinc based on CFH and ARMS2 genetic risk allele number in the Age-Related Eye Disease Study. Ophthalmology 2015;122:162–9. [DOI] [PubMed] [Google Scholar]
- 150.Chew EY, Klein ML, Clemons TE, Agron E, Abecasis GR. Genetic testing in persons with age-related macular degeneration and the use of the AREDS supplements: to test or not to test? Ophthalmology 2015;122:212–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.van der Made SM, Kelly ER, Kijlstra A, Plat J, Berendschot TTJM. Increased macular pigment optical density and visual acuity following consumption of a buttermilk drink containing lutein-enriched egg yolks: a randomized, double-blind, placebo-controlled trial. J Ophthalmol 2016;2016:9035745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ma L, Yan SF, Huang YM, Lu XR, Qian F, Pang HL, Xu XR, Zou ZY, Dong PC, Xiao X, et al. Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology 2012;119:2290–7. [DOI] [PubMed] [Google Scholar]
- 153.Cheng CY, Chung WY, Szeto YT, Benzie IF. Fasting plasma zeaxanthin response to Fructus barbarum L.(wolfberry; Kei Tze) in a food-based human supplementation trial. Br J Nutr 2005;93:123–30. [DOI] [PubMed] [Google Scholar]
- 154.Piccardi M, Marangoni D, Minnella A, Savastano MC, Valentini P, Ambrosio L, Capoluongo E, Maccarone R, Bisti S, Falsini B. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: sustained benefits to central retinal function. Evid Based Complement Alternat Med 2012;2012:429124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu Y, Song X, Zhang D, Zhou F, Wang D, Wei Y, Gao F, Xie L, Jia G, Wu W. Blueberry anthocyanins: protection against ageing and light-induced damage in retinal pigment epithelial cells. Br J Nutr 2012;108:16–27. [DOI] [PubMed] [Google Scholar]