Abstract
A projected doubling in the global population of people aged ≥60 y by the year 2050 has major health and economic implications, especially in developing regions. Burdens of unhealthy aging associated with chronic noncommunicable and other age-related diseases may be largely preventable with lifestyle modification, including diet. However, as adults age they become at risk of “nutritional frailty,” which can compromise their ability to meet nutritional requirements at a time when specific nutrient needs may be high. This review highlights the role of nutrition science in promoting healthy aging and in improving the prognosis in cases of age-related diseases. It serves to identify key knowledge gaps and implementation challenges to support adequate nutrition for healthy aging, including applicability of metrics used in body-composition and diet adequacy for older adults and mechanisms to reduce nutritional frailty and to promote diet resilience. This review also discusses management recommendations for several leading chronic conditions common in aging populations, including cognitive decline and dementia, sarcopenia, and compromised immunity to infectious disease. The role of health systems in incorporating nutrition care routinely for those aged ≥60 y and living independently and current actions to address nutritional status before hospitalization and the development of disease are discussed.
Keywords: nutrition, aging, chronic disease, cognitive decline, health care, risk factors, sarcopenia, age-related disease
Background
By the year 2050, the global population of older adults (defined as those aged ≥60 y) is projected to double from 841 million (2013) to 2 billion, or 21% of the world’s population (1). Moreover, the elderly population is living longer: by 2050, the number of individuals aged ≥80 y will be 3 times the 2013 population, reaching 392 million (1). This change in demography is a global challenge that can affect economics, politics, labor, and public health. Not only will older adults outnumber young children for the first time in history, but most of this population increase will occur in developing countries (2). During 2013–2100, half of the increase in the world’s population will occur in Nigeria, India, the United Republic of Tanzania, the Democratic Republic of Congo, Niger, Uganda, Ethiopia, and the United States (3). Because developing countries experience demographic and epidemiologic transitions more rapidly, many regions are not equipped to meet the demands of an aging population.
The prevalence of obesity is also increasing globally, especially in urban settings within developing countries, with older adults being no exception (4). As people live longer and age distribution shifts toward a greater number of older adults, the number of obese older adults will also grow (5).
Age is a major risk factor for noncommunicable chronic diseases (NCDs)15 such as chronic obstructive pulmonary disease, cardiovascular disease (CVD), type 2 diabetes, cognitive decline, dementia, and cancer (6), all of which have high associated costs of diagnosis, treatment, and care. Therefore, the aging of the population raises serious concerns about the fiscal integrity of health care systems (7). Estimates also suggest that the economic burden is currently skewed toward wealthy industrial countries, due to their relatively high incomes, high levels of health care spending, and rapidly aging population over the past half century. Meanwhile, middle-income countries in the near future will experience the sharpest increase in the economic burden of NCDs compared with other income groups (7).
To collectively quantify the burden of age-related NCDs and other age-related disease is, however, difficult. Many NCDs apparent in industrialized countries by middle age only become clinically identified once an individual reaches an advanced age, having taken many years to develop. Furthermore, the overall economic burden of NCDs and specific age-related chronic disease is magnified by disabilities and death, reducing labor contribution and potentially reducing productivity due to time taken to care for ill family or parents (8).
Although much of the resources necessary to support the aging population are economic, a reorganization of health systems and an increased emphasis on preventive, lifestyle-choice approaches to NCDs and other age-related diseases and care are needed for a sustainable, feasible, and affordable health care system (9). Therefore, we review current evidence in nutrition science to identify promoters of healthy aging, defined as increased healthy active years of life, and focus on nutrition-related disease in the older population (Figure 1). Although not a comprehensive review of age-related chronic disease, this review focuses on several specific nutrition-related conditions with advancing age. Nutrition experts from a variety of disciplines were part of discussions to narrow topics for review, and the authors contributed individual sections on the basis of their expertise, which were then revised by the full group. Approaches to preventing age-related nutritional frailty, as defined below, and chronic disease are imperative to healthy aging. The identification of research priorities (10) will move the aging nutrition and public health fields forward to prepare for the global “silver tsunami” expected by 2050 (Table 1).
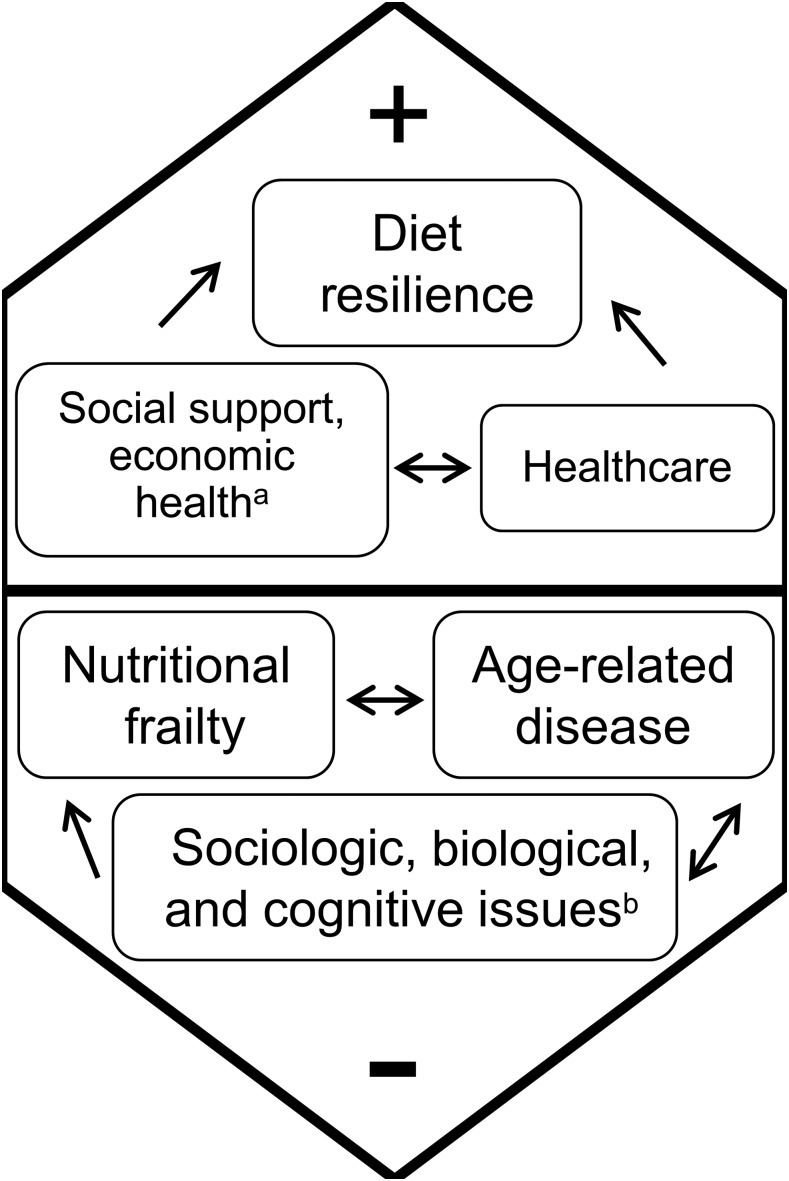
FIGURE 1.
Factors that positively and negatively influence nutritional health which affect healthy aging. aIncludes lack of social interaction, economic factors leading to food insecurity; bincludes age-associated biological and physiologic changes, such as loss of appetite, dentition, loss of smell, and changes in cognitive function.
TABLE 1.
Key knowledge gaps and research priorities
| Randomized controlled trials that include older adults with disease and medication use to make nutrient recommendations within these altered metabolic states |
| Randomized controlled trials in various life stages for prevention of mild cognitive decline and in different stages of Alzheimer disease with patient-tailored lifestyle nutrition treatments for evidence to support individual or broad recommendations on diet, lifestyle, or nutrient supplementation |
| Studies examining other biomarkers beyond nitrogen balance to fully understand the impact of advancing age on protein requirements and skeletal muscle protein turnover |
| Evidence for which nutritional factors (e.g., fruit and vegetable intake, vitamin D status, presence of obesity) may further modulate age-associated declines in skeletal muscle mass |
| Clinical trials to establish optimal nutrient requirements and to identify food components for older adults to improve immune function and reduce inflammatory diseases |
| Design of an effective, interoperable electronic medical record, integrated across health care settings, to promote improved documentation and communication across health care providers, enhance care coordination, and facilitate continuity in nutrition care as an older individual transitions between health care settings |
| Re-evaluation of how the current BMI guidelines are used in older adults and incorporation of nutrition screening and assessment into general practice and community settings |
Role of Nutrition in Healthy Aging
The goal of healthy aging is not only to increase years of life but also, and importantly, to extend healthy active years. Unfortunately, chronic diseases become increasingly common with age and are often considered an inevitable part of aging. However, accumulating research shows that the increasing prevalence of many of these conditions at younger ages is not a normal function of aging, but rather a consequence of inadequate practice of important health behaviors. The WHO estimates that the elimination of the major risk factors for chronic disease (smoking, lack of exercise, and poor diet) would reduce the risk of CVD, stroke, and type 2 diabetes by 80% (11).
Energy intake and body weight also have bearing on longevity and quality of old age. Caloric restriction, as a reduction in ad libitum energy intake, increases life span in many species (12), whereas implications for humans are less clear. Long-term randomized studies with caloric restriction in humans are unfeasible and unlikely based on ethical principles; however, studies spanning several years have been attempted with somewhat promising outcomes. For example, >2 y of caloric restriction in young- and middle-aged nonobese adults led to relative reductions in cardiometabolic risk factors (13). In contrast, however, there is also research that suggests that overweight and mild obesity are associated with the lowest all-cause mortality in older individuals (14). This controversial area is in need of additional research to clarify the roles of both energy intake patterns and weight status in optimal aging.
Key Nutrition Considerations
The term “nutritional frailty” has been used to describe a state commonly seen in vulnerable older adults, characterized by sudden significant weight loss and loss of muscle mass and strength, or an essential loss of physiologic reserves, making the individual susceptible to disability (15, 16). More recently, it has been recognized that increasing numbers of older adults are frail but also obese (17). Nutritional problems and increased risk of malnutrition, including obesity, contribute to frailty via the culmination of sociologic, biological, and cognitive issues.
Recent changes in the environment and in lifestyle make following dietary recommendations for many older adults difficult. Changing family dynamics means that older adults have less support, while facing substantial challenges in obtaining recommended nutrient-dense diets, because many experience changes in taste and smell (18), loss of appetite (19), dental and chewing problems (20), and limitations in mobility and access to high-quality fresh food (21). This is of particular concern because older individuals require even more nutrient-dense foods to meet their changing requirements. Aging-related inefficiencies in absorption and utilization mean that the requirement for some essential nutrients increases, despite lower energy needs (22–24). Furthermore, older adults commonly fall below recommendations for intake. National surveys and observational cohort studies have identified several nutrients that may be inadequately consumed in relation to health risk among older adults, including protein, n–3 FAs, dietary fiber, carotenoids (vitamin A precursors), calcium, magnesium, potassium, and vitamins B-6, B-12, D, and E (25). Furthermore, it is important to consider that even RDA intake levels may not always provide optimal intake for the older population because, generally, RDAs were determined on the basis of studies conducted in younger, healthy populations. Nutrients specifically identified by the 2015 Dietary Guidelines report as particular “shortfall nutrients” for US adults aged ≥70 y include calcium, vitamin D, dietary fiber, and potassium, with protein noted as a nutrient of concern (26). In addition, commonly used medications for chronic conditions can alter nutrient requirements by interacting in ways that may affect absorption or metabolism (27). For example, long-term use of acid-blocking medications may contribute to the development of vitamin B-12 deficiency (28). There is a potential need for adapted nutrient requirements with disease and medication use. Evidence from randomized controlled trials that include older adults with diseases is necessary to make nutrient recommendations within these altered metabolic states.
Although supplements may be helpful in the case of nutrient deficiencies, or when health conditions or medications interfere with absorption or effective nutrient utilization of specific nutrients, trials of specific nutrient supplements have generally been disappointing, emphasizing that whole foods are important (29). Exceptions include vitamin B-12, supplements of which may correct deficiency associated with atrophic gastritis and use of acid-blocking medication, metformin, or other interfering medications (30), and vitamin D for individuals who receive inadequate sun exposure and during the winter months in northern latitudes (31). Higher amounts of vitamin E might also be needed to maintain an optimal immune response and to enhance resistance to respiratory infections (32–35). The lack of rigorous assessment of individual nutritional status to identify those in need of specific supplementation might explain the failure of some of the nutrient supplementation trials. Still, although supplements are useful in the face of inadequate intakes of some nutrients, the promotion of food-based approaches to meeting nutrient requirements is needed as a first approach. Older adults tend to report inadequate intakes of fruit, vegetables, legumes, whole grains, nuts or seeds, fish, lean meat, poultry, and low-fat fluid dairy products but excess intakes of refined grain products, processed and fatty meats, fried foods, solid fats, and added sugars (36).
In contrast to the increased requirement for many nutrients, some nutrients may accumulate in the body and contribute to chronic disease through excess. The body is less able to excrete preformed vitamin A as retinol, and too much of this vitamin should be avoided (37). Iron, a shortfall nutrient in earlier life-cycle phases, accumulates with age, and high intakes of heme-iron (from meat or supplements) at older ages have been associated with the risk of heart disease; therefore, excess intakes of this nutrient should also be avoided (38). Furthermore, some nutrients, and especially minerals, have a small window between requirement and Tolerable Upper Intake Level, meaning that global supplementation is not a panacea to this issue due to the risk of toxicity; judicious use of supplements on the basis of their identified or likely deficit is required.
Longstanding poor food intake may result in undernutrition or malnutrition, defined as deficient energy and macro- and/or micronutrient status (due to inadequate food intake, malabsorption, and/or increased metabolism often associated with inflammation), which may result in measurable biological changes and loss of tissue and/or functional ability (39, 40). Undernutrition is diagnosed by identifying longstanding inadequate intake; loss of weight, body fat, and muscle mass; or loss of functional capacity (41). Thus, undernutrition occurs in older adults when they do not consume enough protein to retain skeletal muscle; have a lack of vitamin B-12, resulting in neurological deterioration; or have low vitamin D intakes resulting in decreased calcium storage in the bone. Nutritional risk is commonly defined as the presence of factors that impair food intake and thus eventually lead to malnutrition if not ameliorated (42, 43). Low appetite and inadequate intake contribute to nutritional frailty, sarcopenia, and physical frailty. Regardless of the terminology used (nutrition or malnutrition risk, mal- or undernutrition, or nutritional frailty), the key is that undernutrition is preventable (21); however, if left untreated, further negative functional, health, and quality-of-life outcomes occur (16, 44–48).
For community-living older adults, the inadequate intake of nutrient-dense foods is the primary mechanism for this undernutrition (43, 49). Prevalence estimates of undernutrition and nutrition risk depend on the measurements used and whether upstream approaches are considered in selecting risk factors to include in screening instruments (43, 50). Upward of 35% of community-living seniors have been found to have undernutrition (49, 50). Despite the considerable interest recently shown in nutrition risk and/or undernutrition, these conditions remain underdiagnosed and -treated in older adults living in the community (16, 43), potentially promoting the high prevalence of malnutrition identified at admission to the hospital (49, 51).
Although many nutrients are deficient in the diets of older adults (26), other nutrients are consumed in excess, contributing to overnutrition and the risk of obesity and chronic conditions. The latter include saturated fats from fatty meats, processed meat, and whole-fat dairy products; trans fats from margarine, shortening, and processed baked products; refined carbohydrates from soft drinks, white bread, and white rice; and sodium in canned and processed foods (26). An important approach to nutrition in healthy aging is to limit energy-dense foods while maximizing nutrient density. However, it is worth noting the appropriateness of current BMI guidelines because measures of body composition and adiposity in older adults have recently come into question, challenging the prudence of limiting energy intake to reduce body weight for otherwise healthy older adults. For example, some research (14) suggests that overweight and mild obesity are associated with the lowest all-cause mortality in older individuals. In addition, those older individuals with overweight and mild obesity who exhibit survival advantage do not manifest sarcopenia (52). Recent findings suggest that this observation may be partially explained by the inclusion of metabolically healthy overweight and obese older individuals, who do not have elevated mortality risk, in population studies of BMI and mortality (53).
Diet Resilience
More than 2 decades ago, Payette et al. (54) developed a conceptual model to describe the material, physical, psychological, and social factors that influence food purchase, preparation, and consumption to better understand why poor food intake occurs in older adults (54). A more recent model, called “Making the Most of Mealtimes,” is used to frame determinants of food intake with the domains of meal quality (e.g., cultural preferences, nutrient interactions with medications), meal access (e.g., lack of transportation), and mealtime experience (e.g., eating alone) (55). Epidemiologic studies focused on healthy and more vulnerable community-living older adults have identified various factors that show the utility of these and other models for understanding why nutritional frailty, undernutrition, and/or poor food intake occurs. These factors include disease states, self-reported health and medication use, widowhood, low income or food insecurity, food skills, isolation or low social connectedness, lack of transportation, inadequate community supports for food, cognition and dementia, depression, bereavement, anxiety or stress, poor appetite, dentition and oral health, swallowing problems, sensory and functional deficits, recent hospitalization, and polypharmacy (16, 49, 50, 54, 56, 57–59). Diet resilience is defined as having or developing adaptive strategies to maintain a nutrient-dense diet sufficient to meet requirements, despite challenges (60). Additional work in larger samples and with the use of a framework to identify, a priori, several potential determinants and mediators is needed to further clarify potential determinants of diet resilience and thus areas for intervention (50, 57).
In addition to further epidemiologic evidence to map the potential causal pathways for poor food intake and/or undernutrition and thus find solutions, identifying how older adults overcome challenges and remain diet resilient is required. It is proposed that the motivation to eat well is the foundation for diet resilience and that pleasure and health are key drivers of this motivation (60). Education programs that focus on good-tasting, easy-to-prepare food, that emphasize the importance of nutrition to health, and that help older adults understand their challenges to resiliency and how they can use community resources to promote food intake are needed. Evergreen Action Nutrition, which is provided in a recreation center for seniors, is a feasible and sustainable model for nutrition education in community-living older adults. This community-based program, developed with older adults at the center, included cooking groups, food workshops and demonstrations, and nutrition information in the form of newsletters, recipes, and tailored fact sheets. This program, initiated by research funding (2000–2003), continued activities for several years through senior champions, volunteers (students and seniors), and linking into and accessing other community and public health resources. The program showed that a modest investment can have an important impact with respect to changing nutrition knowledge, attitudes, and behaviors (61–63). Promoting intergenerational activities and family and friend gatherings around healthy food consumption will also go a long way to address both nutritional and mental well-being of older adults.
Nutrition in the Prognosis of Age-Related Disease
Sarcopenia.
Robust skeletal muscle mass is essential for maintaining whole body homeostasis and health (64). A characteristic hallmark of aging in humans is the well-described loss of skeletal muscle mass and function, which has been shown to contribute to functional limitation, disability, and mortality (65, 66). This age-associated muscle atrophy, termed “sarcopenia” (67), implicates a derangement of the equilibrium between muscle protein synthesis and muscle protein breakdown as a major contributor to sarcopenia etiology.
In particular, alterations in muscle protein synthesis during anabolic conditions in aging populations have been implicated as a significant contributor to this imbalance (68, 69). What remains unclear is the influence of aging and age-related changes in muscle mass on chronic protein turnover and effects on dietary protein requirements (70–73). In addition, the role of supplemental protein intake has yet to be fully clarified with respect to promoting skeletal muscle growth or attenuating the rate of skeletal muscle atrophy (74–78).
Although many older adults consume adequate protein on the basis of current standards, a subset of older individuals routinely have protein intakes below the current RDA (60), and thus, protein is considered a key shortfall nutrient for aging populations. In addition, some suggest that the current RDA for protein is inadequate for older adults (71, 72), although consensus from the available data on protein requirements for older adults is lacking (73). Furthermore, most information on dietary protein requirements for older adults derives from studies in healthy “disease-free” older individuals and thus the generalizability of these results to the large number of older adults with comorbid conditions, functional limitations, and disability may be limited.
Evidence from large epidemiologic cohort studies suggests that the loss of lean mass with advancing age is, in part, mediated by dietary consumption of protein. Houston et al. (79) reported from the Health, Aging, and Body Composition Study that a 3-y loss of lean mass was associated with average dietary protein intake and that this association persisted even when adjusted for daily energy intake and change in body mass. Subsequent analyses that used the InChianti and Women’s Health Initiative cohorts confirmed these findings and suggested a relation between protein intake and the development of the frailty syndrome, a condition typically associated with sarcopenia (80, 81).
Despite conflicting reports from carefully controlled nitrogen balance studies, observational studies in older people suggest a relation between lower dietary protein intake and loss of muscle mass (79–81). The discrepancy in findings may relate to limitations in the ability of the nitrogen balance technique to detect subtle alterations in whole-body protein metabolism and the selection of generally healthy individuals in these intensive metabolic studies. Future studies should carefully consider target populations and outcomes beyond nitrogen balance to fully understand the impact of advancing age on skeletal muscle protein turnover. In addition, other nutritional factors, such as fruit and vegetable intake (82), vitamin D status (83–86), and the presence of obesity (87–89), may further modulate age-associated declines in skeletal muscle mass.
Cognitive decline and Alzheimer disease.
Undernutrition is particularly common among people with cognitive decline and Alzheimer disease (AD). Cognitive decline is progressive, with weight loss often preceding the onset of AD and increasing over the course of the disease. Epidemiologic studies showed that the Mediterranean diet, which includes intakes of fruit, fish, vegetables, and olive oil, may lower the risk of both mild cognitive decline and AD (90–94). However, no consistent evidence exists that nutritional supplements play a protective role (vitamins B-6, B-12, C, or E; folate; or n–3 polyunsaturated fats) in randomized controlled trials (95–97), which suggests that a variety of nutrients is important, likely including phytonutrients from foods, or that earlier intervention is needed for nutrient supplementation to be effective. Simple measurements, such as body weight over time, should be recorded in all patients with dementia at physical clinical visits. Special monitoring is needed, with a loss of >5 kg (10 pounds) over a 6-mo period in a person with dementia leading to intervention. Future research areas should include more randomized controlled trials in different life-stage periods of prevention for mild cognitive decline and in different stages of AD (mild, moderate, or severe) with patient-tailored treatments (98).
Infectious disease.
Older adults have an increased incidence of infectious and inflammatory diseases, with prolonged recovery time and higher mortality from these diseases. These changes are largely attributed to immune system dysregulation—that is, increased inflammation and reduced cell-mediated immune response, both of which are influenced by nutritional status and intake of particular bioactive dietary components. The interaction of nutrition, immune function, and infection is key in determining the risk of susceptibility to and morbidity from infectious disease (99–102).
As indicated earlier, older adults are at higher risk of inadequate consumption of nutrients and nutrient-rich foods than are younger adults, and both undernutrition (e.g., nutrient deficiencies) and overnutrition (obesity) are prevalent among older adults. Age-associated biological changes occur in multiple organs, tissues, and cell types, altering their ability to absorb or uptake and metabolize essential nutrients and other food components; thus, the current RDA may need adjustment for those aged >70 y. Furthermore, the RDAs are not based on specific requirements of the immune system and its ability to fight infection or control inflammation. Therefore, these requirements, in the case of several nutrients, might actually be higher for other body systems and cell types (33, 34, 99, 103–111). For example, higher-than-recommended intakes of vitamin E (200 mg/d), vitamin B-6, and zinc are required for optimal function of the immune system and, in some cases, resistance to infection in older adults (33, 108, 111–114). The dose of micronutrients is an important consideration: for example, dose-response studies indicate that there is no additional benefit from increasing vitamin E intakes >200 mg/d for immune response in older adults (35). Furthermore, zinc supplementation is most effective in those with low serum zinc concentrations, who represent ∼20–30% of older adults in the United States (113, 115).
Future studies, including adequate clinical trials, are required to determine the optimal level of nutrients and food components with immune-modulating effects—such as vitamins A, D, E, and C; selenium and zinc; and essential FAs—needed to support proper immune system function and leading to a reduction in the risk of, and morbidity and mortality from, infection. Results obtained on the basis of optimization of immune and inflammatory responses (key players in determining quality of life for older adults) could then be used to develop older adult–specific recommendations for these nutrients.
Although the immune system clearly plays an important role in the defense against infection, few established immunologic markers, such as delayed-type hypersensitivity skin response and vaccine efficacy, are readily available to use in clinical trials. Thus, a need exists for research into more defined immunologic markers of the aging immune system and their clinical value for assessing the risk of infections and the associated morbidity and mortality. Most studies related to nutrition and infection in older adults were conducted in animal models, whereas, with a few exceptions, human studies typically use immune function readouts as surrogate indicators for infection (116, 117). However, in both cases, depending on the model or the immune markers used, the applicability to clinical infection may be limited. Therefore, well-designed clinical trials are needed to establish the optimal requirement for nutrients in older adults and to identify food components that are particularly beneficial in improving immune function and reducing inflammatory diseases. The use of naturally occurring infection as the primary outcome would require a much larger sample size than that needed when established immunologic markers are used.
A Role for Health Systems
Health care systems play a key role in integrating nutrition care for older individuals across primary, acute, subacute, chronic care, and home settings. Primary care refers to the initial care contact with a health provider where the majority of health problems are addressed. As part of a primary care regimen, nutrition screening and dietary assessment are integral to the prevention and diagnosis of many conditions common in older adults, such as CVD, gastrointestinal conditions, diabetes, unexplained weight loss, and cancer. Due to the increased vulnerability of this life stage, a greater focus on dietary intake may be warranted during routine care (118).
In an acute care setting, the Joint Commission, a nonprofit organization that accredits and certifies health care organizations and programs in the United States (119), requires that nutrition screening be completed within 24 h of hospital admission (120). The screening process identifies risk factors, such as unintentional weight loss, low BMI, compromised dietary intake, alterations in swallowing ability, use of enteral or parenteral nutrition, and the presence of pressure ulcers (120). The finding that a patient is “at nutritional risk” triggers a nutrition consultation with a registered dietitian nutritionist (RDN), which includes a comprehensive nutrition assessment in accordance with the Nutrition Care Process that consists of 4 individual but interdependent steps: nutrition assessment, nutrition diagnosis, nutrition intervention, and nutrition monitoring and evaluation (121).
Adopted by the Academy of Nutrition and Dietetics in 2003, the Nutrition Care Process aims to improve both intra- and interorganizational communication and patient outcomes through application of a standardized process and language (122). The nutrition assessment is intended to be incorporated into the medical record (123), which is increasingly an electronic medical record. The use of electronic medical records should promote improved documentation and communication across health care providers to enhance care coordination and facilitate continuity in nutrition care as an older individual transitions between health care settings (124). However, implementing truly interoperable health information technology “ecosystems” remains a considerable challenge, because such systems are rarely integrated across diverse health care settings (125). The resulting poor communication and gaps in nutrition care often compromise transitions out of acute care.
As an older individual transitions back to the home setting, fully coordinating nutrition services with other services are necessary to promote improved outcomes and independent living. Krumholz (126) described “post-hospital syndrome” as an “acquired, transient condition of generalized risk.” Compromised nutritional status is a potent predictor of risk of early readmission to the hospital (127), with limited reimbursement for the RDN to provide services in the community setting considered to be a substantial barrier to obtaining nutrition services (128). Some innovative health system models, such as the community-based Care Transitions Program, mandated by section 3026 of the Affordable Care Act, and the patient-centered medical home, may contribute to potential solutions (129, 130). For example, community-based organizations, in partnership with hospitals, could include nutrition services by the RDN as part of their proposals to the Centers for Medicare and Medicaid Services.
In the community setting, title IIIC of the Older Americans Act provides congregate and home-delivered nutrition services for individuals >60 y of age (131). However, current funding for these programs is inadequate for the number of older individuals who need them (128). Moreover, as mentioned previously, some evidence casts doubt on the appropriateness of current BMI guidelines as measures of body composition and adiposity in older adults. Specifically, as currently designed, practitioners may use BMI guidelines for weight-loss interventions in older adults that may not be appropriate due to the presence of sarcopenia or sarcopenic obesity.
Conclusions
The risk of specific age-related disease may, in part, be mediated by dietary intervention in aging and in adulthood, although further research is needed to learn how dietary requirements change during the aging process. Furthermore, a more comprehensive nutritional assessment beyond anthropometric measurements needs to be incorporated into the routine health care of adults to identify specific nutrient needs. Increasing evidence suggests that a Mediterranean-style diet that is high in fruit, fish, and vegetables may reduce the risk of both mild cognitive impairment and AD. A diet high in fruit and vegetables, along with adequate dietary protein, also may support lean body mass maintenance. Moreover, intakes of specific micronutrients beyond the current recommendations for older adults may be required for optimal immune function and resistance to infection.
In general, it is possible that dietary requirements shift with the dysregulated metabolisms of many age-related chronic disease states beyond those discussed in this review. Many chronic, age-related disease states, including CVD, chronic obstructive pulmonary disease, type 2 diabetes, and cancer, which incur even greater challenges to nutritional management in older adults, were not specifically addressed in this review. However, evidence supports the idea that all age-related disease states may benefit from careful attention to nutritional adequacy and a healthful diet. Finally, an integrated health system infrastructure is crucial to ensure quality nutrition care for the aging population. Incorporating nutrition evaluations and services into preventative care for aging adults within standard wellness practices is central to avoiding and minimizing the effects of nutrition-related disease. Therefore, work to optimize nutrition screening and dietary assessment into the health care system and perhaps a re-evaluation of how the current BMI guidelines are used in older adults are warranted. Coordinated efforts between health care policy makers, health care providers, insurance companies, and nutrition experts are needed to develop comprehensive preventive strategies based on individualized nutritional needs for older adults. Incorporation of “nutrition physical” or screening into the yearly physical examination of older adults will provide the foundation for developing the preventive measures needed.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AD, Alzheimer disease; CVD, cardiovascular disease; NCD, noncommunicable chronic disease; RDN, registered dietitian nutritionist.
References
- 1.UN Department of Economic and Social Affairs, Population Division. World population ageing 2013 [Internet]. 2013. ST/ESA/SER.A/348. [cited 2016 Jun 24]. Available from: http://www.un.org/esa/socdev/documents/ageing/Data/WorldPopulationAgeingReport2013.pdf.
- 2.National Institute on Aging. Global health and aging [Internet]. Washington (DC): US Department of Health and Human Services; 2011. NIH Publication No.: 11-7737. [cited 2016 Jun 24]. Available from: https://www.nia.nih.gov/research/publication/global-health-and-aging/preface.
- 3.UN Department of Economic and Social Affairs, Population Division. World population prospects: the 2015 revision, key findings and advance tables. 2015. Working Paper No.: ESA/P/WP.241. New York: United Nations. [cited 2016 Jun 24]. Available from: https://esa.un.org/unpd/wpp/publications/files/key_findings_wpp_2015.pdf.
- 4.WHO. Obesity and overweight [Internet] [cited 2016 Sep 13]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/.
- 5.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief No.: 219 [Internet]. Hyattsville (MD): National Center for Health Statistics; 2015 [cited 2016 Sep 13]. Available from: http://c.ymcdn.com/sites/www.acutept.org/resource/resmgr/Critical_EdgEmail/0216-prevalence-of-obesity.pdf.
- 6.Beard JR, Biggs S, Bloom DE, Fried LP, Hogan P, Kalache A, Jay Olshansky S, editors. Global population ageing: peril or promise [Internet]. Geneva (Switzerland): World Economic Forum; 2011. [cited 2016 Jun 24]. Available from: http://www3.weforum.org/docs/WEF_GAC_GlobalPopulationAgeing_Report_2012.pdf.
- 7.World Economic Forum. The Human Capital Report [Internet]. Geneva (Switzerland): World Economic Forum; 2013. [cited 2016 Jun 24]. Available from: http://reports.weforum.org/human-capital-index-2013/. [Google Scholar]
- 8.Bloom DE, Eggleston KN. The economic implications of population ageing in China and India: introduction to the special issue. J Econ Ageing 2014;4:1–7. [Google Scholar]
- 9.Fried L, Paccaud F. The public health needs for an ageing society [editorial]. Public Health Rev [Internet] 2011;32(2):351–5. [cited 2016 Jun 24]. Available from: http://www.publichealthreviews.eu/upload/pdf_files/8/PHR_32_2_Editorial.pdf. [Google Scholar]
- 10.Fang EF, Scheibye-Knudsen M, Jahn HJ, Li J, Ling L, Guo H, Zhu X, Preedy V, Lu H, Bohr VA, et al. A research agenda for aging in China in the 21st century. Ageing Res Rev 2015;24:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Preventing chronic diseases: a vital investment [Internet]. Geneva (Switzerland): WHO; 2005. [cited 2016 Jun 24]. Available from: http://www.who.int/chp/chronic_disease_report/en/. [Google Scholar]
- 12.Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun 2014;5:3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, et al. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci 2015;70:1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng FW, Gao X, Mitchell DC, Wood C, Still CD, Rolston D, Jensen GL. Body mass index and all-cause mortality among older adults. Obesity (Silver Spring) 2016;24:2232–39. [DOI] [PubMed] [Google Scholar]
- 15.Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr 2002;22:309–23. [DOI] [PubMed] [Google Scholar]
- 16.Kinney JM. Nutritional frailty, sarcopenia and falls in the elderly. Curr Opin Clin Nutr Metab Care 2004;7:15–20. [DOI] [PubMed] [Google Scholar]
- 17.Porter Starr KN, McDonald SR, Bales CW. Obesity and physical frailty in older adults: a scoping review of intervention trials. J Am Med Dir Assoc 2014;15:240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Syed Q, Hendler KT, Koncilja K. The impact of aging and medical status on dysgeusia. Am J Med 2016;129:753.e1–6. [DOI] [PubMed] [Google Scholar]
- 19.Landi F, Calvani R, Tosato M, Martone AM, Bernabei R, Onder G, Marzetti E. Impact of physical function impairment and multimorbidity on mortality among community-living older persons with sarcopaenia: results from the ilSIRENTE prospective cohort study. BMJ Open 2016;6:e008281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semba RD, Blaum CS, Bartali B, Xue QL, Ricks MO, Guralnik JM, Fried LP. Denture use, malnutrition, frailty, and mortality among older women living in the community. J Nutr Health Aging 2006;10:161–7. [PubMed] [Google Scholar]
- 21.Huang DL, Rosenberg DE, Simonovich SD, Belza B. Food access patterns and barriers among midlife and older adults with mobility disabilities. J Aging Res. 2012;2012:231489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell RM. Micronutrient requirements of the elderly. Nutr Rev 1992;50:463–6. [DOI] [PubMed] [Google Scholar]
- 23.Cieslak KP, Baur O, Verheij J, Bennink RJ, van Gulik TM. Liver function declines with increased age. HPB 2016;18:691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rémond D, Shahar DR, Gille D, Pinto P, Kachal J, Peyron M-A, Dos Santos CN, Walther B, Bordoni A, Dupont D, et al. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget 2015;6:13858–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tucker KL. High risk nutrients in the aging population. In: Bales C, Locher J, Saltzman, editors. Handbook of clinical nutrition and aging. 3rd ed. New York: Springer; 2015. p. 335–53.
- 26.Millen BE, Abrams S, Adams-Campbell L, Anderson CA, Brenna JT, Campbell WW, Clinton S, Hu F, Nelson M, Neuhouser ML, et al. The 2015 Dietary Guidelines Advisory Committee scientific report: development and major conclusions. Adv Nutr 2016;7:438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan L-N. Drug-nutrient interactions. JPEN J Parenter Enteral Nutr 2013;37:450–9. [DOI] [PubMed] [Google Scholar]
- 28.Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013;310:2435–42. [DOI] [PubMed] [Google Scholar]
- 29.Fortmann SP, Burda BU, Senger CA, Lin JS, Beil TL, O’Connor E, Whitlock EP. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: a systematic evidence review for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality; 2013 [cited 2016 Aug 31]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK173987/. [PubMed]
- 30.Parry-Strong A, Langdana F, Haeusler S, Weatherall M, Krebs J. Sublingual vitamin B12 compared to intramuscular injection in patients with type 2 diabetes treated with metformin: a randomised trial. N Z Med J 2016;129:67–75. [PubMed] [Google Scholar]
- 31.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev 2014;1; DOI: 10.1002/14651858.CD007470.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meydani SN, Barklund MP, Liu S, Meydani M, Miller RA, Cannon JG, Morrow FD, Rocklin R, Blumberg JB. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am J Clin Nutr 1990;52:557–63. [DOI] [PubMed] [Google Scholar]
- 33.Meydani SN, Han SN, Wu D. Vitamin E and immune response in the aged: molecular mechanisms and clinical implications. Immunol Rev 2005;205:269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF, Hamer DH. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA 2004;292:828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, Stollar BD. Vitamin E supplementation and in vivo immune response in healthy elderly subjects: a randomized controlled trial. JAMA 1997;277:1380–6. [DOI] [PubMed] [Google Scholar]
- 36.Ervin RB. Healthy Eating Index scores among adults, 60 years of age and over, by sociodemographic and health characteristics: United States, 1999–2002. Adv Data 2008;395:1–16. [PubMed] [Google Scholar]
- 37.Russell RM. The vitamin A spectrum: from deficiency to toxicity. Am J Clin Nutr 2000;71:878–84. [DOI] [PubMed] [Google Scholar]
- 38.Turnlund J, Costa F, Margen S. Zinc, copper, and iron balance in elderly men. Am J Clin Nutr 1981;34:2641–7. [DOI] [PubMed] [Google Scholar]
- 39.Jensen GL, Mirtallo J, Compher C, Dhaliwal R, Forbes A, Grijalba RF, Hardy G, Kondrup J, Labadarios D, Nyulasi I, et al. Adult starvation and disease-related malnutrition: a proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. JPEN J Parenter Enteral Nutr 2010;34:156–9. [DOI] [PubMed] [Google Scholar]
- 40.Elia M; British Association for Parenteral and Enteral Nutrition, Advisory Group on Malnutrition. Guidelines for detection and manageme nt of malnutrition. Maidenhead (United Kingdom): British Association for Parenteral and Enteral Nutrition; 2000. [Google Scholar]
- 41.White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group, A.S.P.E.N. Malnutrition Task Force, A.S.P.E.N. Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet 2012;112:730–8. [DOI] [PubMed] [Google Scholar]
- 42.Reuben DB, Greendale GA, Harrison GG. Nutrition screening in older persons. J Am Geriatr Soc 1995;43:415–25. [DOI] [PubMed] [Google Scholar]
- 43.Keller HH. Promoting food intake in older adults living in the community: a review. Appl Physiol Nutr Metab 2007;32:991–1000. [DOI] [PubMed] [Google Scholar]
- 44.Lee JS, Kritchevsky SB, Tylavsky F, Harris T, Simonsick EM, Rubin SM, Newman AB; Health ABC Study. Weight change, weight change intention, and the incidence of mobility limitation in well-functioning community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2005;60:1007–12. [DOI] [PubMed] [Google Scholar]
- 45.Keller HH, Østbye T. Body mass index (BMI), BMI change and mortality in community-dwelling seniors without dementia. J Nutr Health Aging 2005;9:316–20. [PubMed] [Google Scholar]
- 46.Luppa M, Luck T, Weyerer S, König H-H, Brähler E, Riedel-Heller SG. Prediction of institutionalization in the elderly: a systematic review. Age Ageing 2010;39:31–8. [DOI] [PubMed] [Google Scholar]
- 47.Keller HH, Østbye T, Goy R. Nutritional risk predicts quality of life in elderly community-living Canadians. J Gerontol A Biol Sci Med Sci 2004;59:68–74. [DOI] [PubMed] [Google Scholar]
- 48.Marshall S, Bauer J, Isenring E. The consequences of malnutrition following discharge from rehabilitation to the community: a systematic review of current evidence in older adults. J Hum Nutr Diet 2014;27:133–41. [DOI] [PubMed] [Google Scholar]
- 49.Agarwal E, Miller M, Yaxley A, Isenring E. Malnutrition in the elderly: a narrative review. Maturitas 2013;76:296–302. [DOI] [PubMed] [Google Scholar]
- 50.van der Pols-Vijlbrief R, Wijnhoven HAH, Schaap LA, Terwee CB, Visser M. Determinants of protein-energy malnutrition in community-dwelling older adults: a systematic review of observational studies. Ageing Res Rev 2014;18:112–31. [DOI] [PubMed] [Google Scholar]
- 51.Allard JP, Keller H, Jeejeebhoy KN, Laporte M, Duerksen DR, Gramlich L, Payette H, Bernier P, Vesnaver E, Davidson B, et al. Malnutrition at hospital admission—contributors and effect on length of stay: a prospective cohort study from the Canadian malnutrition task force. JPEN J Parenter Enteral Nutr 2016;40:487–97. [DOI] [PubMed] [Google Scholar]
- 52.Murphy RA, Reinders I, Garcia ME, Eiriksdottir G, Launer LJ, Benediktsson R, Gudnason V, Jonsson PV, Harris TB; Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-Reykjavik). Adipose tissue, muscle, and function: potential mediators of associations between body weight and mortality in older adults with type 2 diabetes. Diabetes Care 2014;37:3213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng FW, Gao X, Mitchell DC, Wood C, Rolston DDK, Still CD, Jensen GL. Metabolic health status and the obesity paradox in older adults. J Nutr Gerontol Geriatr 2016;35:161–76. [DOI] [PubMed] [Google Scholar]
- 54.Payette H, Gray-Donald K, Cyr R, Boutier V. Predictors of dietary intake in a functionally dependent elderly population in the community. Am J Public Health 1995;85:677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keller H, Carrier N, Duizer L, Lengyel C, Slaughter S, Steele C. Making the most of mealtimes (M3): grounding mealtime interventions with a conceptual model. J Am Med Dir Assoc 2014;15:158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hickson M. Malnutrition and ageing. Postgrad Med J 2006;82:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Payette H, Shatenstein B. Determinants of healthy eating in community-dwelling elderly people. Can J Public Health 2005;96(Suppl 3):S27–31. [PubMed] [Google Scholar]
- 58.Vesnaver E, Keller HH. Social influences and eating behavior in later life: a review. J Nutr Gerontol Geriatr 2011;30:2–23. [DOI] [PubMed] [Google Scholar]
- 59.Locher JL, Ritchie CS, Robinson CO, Roth DL, Smith West D, Burgio KL. A multidimensional approach to understanding under-eating in homebound older adults: the importance of social factors. Gerontologist 2008;48:223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vesnaver E, Keller HH, Payette H, Shatenstein B. Dietary resilience as described by older community-dwelling adults from the NuAge study “if there is a will—there is a way!”. Appetite 2012;58:730–8. [DOI] [PubMed] [Google Scholar]
- 61.Keller HH, Hedley MR, Wong SSL, Vanderkooy P, Tindale J, Norris J. Community organized food and nutrition education: participation, attitudes and nutritional risk in seniors. J Nutr Health Aging 2006;10:15–20. [PubMed] [Google Scholar]
- 62.Keller HH, Hedley M, Hadley T, Wong S, Vanderkooy P. Food workshops, nutrition education, and older adults: a process evaluation. J Nutr Elder 2005;24:5–23. [DOI] [PubMed] [Google Scholar]
- 63.Keller HH, Gibbs A, Wong S, Vanderkooy PD, Hedley M. Men can cook! Development, implementation, and evaluation of a senior men’s cooking group. J Nutr Elder 2004;24:71–87. [DOI] [PubMed] [Google Scholar]
- 64.Haran PH, Rivas DA, Fielding RA. Role and potential mechanisms of anabolic resistance in sarcopenia. J Cachexia Sarcopenia Muscle 2012;3:157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam T-TL, Kenny AM, Fragala MS, Harris TB, Kiel DP, Guralnik JM, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci 2014;69:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, Kenny AM, Peters KW, Ferrucci L, Guralnik JM, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol A Biol Sci Med Sci 2014;69:576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, et al. ; International Working Group on Sarcopenia. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. J Am Med Dir Assoc 2011;12:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422–4. [DOI] [PubMed] [Google Scholar]
- 69.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 2003;78:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fulgoni VL. Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr 2008;87(Suppl):1554S–7S. [DOI] [PubMed] [Google Scholar]
- 71.Campbell WW, Crim MC, Dallal GE, Young VR, Evans WJ. Increased protein requirements in elderly people: new data and retrospective reassessments. Am J Clin Nutr 1994;60:501–9. [DOI] [PubMed] [Google Scholar]
- 72.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The Recommended Dietary Allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci 2001;56:M373–80. [DOI] [PubMed] [Google Scholar]
- 73.Campbell WW, Johnson CA, McCabe GP, Carnell NS. Dietary protein requirements of younger and older adults. Am J Clin Nutr 2008;88:1322–9. [DOI] [PubMed] [Google Scholar]
- 74.Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, McMurdo MET, Mets T, Seal C, Wijers SL, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2015;16:740–7. [DOI] [PubMed] [Google Scholar]
- 75.Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LCPGM, van Loon LJC. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13:713–9. [DOI] [PubMed] [Google Scholar]
- 76.Tieland M, van de Rest O, Dirks ML, van der Zwaluw N, Mensink M, van Loon LJC, de Groot LCPGM. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 2012;13:720–6. [DOI] [PubMed] [Google Scholar]
- 77.Rondanelli M, Klersy C, Terracol G, Talluri J, Maugeri R, Guido D, Faliva MA, Solerte BS, Fioravanti M, Lukaski H, et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr 2016;103:830–40. [DOI] [PubMed] [Google Scholar]
- 78.Chalé A, Cloutier GJ, Hau C, Phillips EM, Dallal GE, Fielding RA. Efficacy of whey protein supplementation on resistance exercise-induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults. J Gerontol A Biol Sci Med Sci 2013;68:682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87:150–5. [DOI] [PubMed] [Google Scholar]
- 80.Beasley JM, LaCroix AZ, Neuhouser ML, Huang Y, Tinker L, Woods N, Michael Y, Curb JD, Prentice RL. Protein intake and incident frailty in the women’s health initiative observational study. J Am Geriatr Soc 2010;58:1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Isanejad M, Mursu J, Sirola J, Kröger H, Rikkonen T, Tuppurainen M, Erkkilä AT. Association of protein intake with the change of lean mass among elderly women: the Osteoporosis Risk Factor and Prevention–Fracture Prevention Study (OSTPRE-FPS). J Nutr Sci 2015;4:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dawson-Hughes B, Harris SS, Ceglia L. Alkaline diets favor lean tissue mass in older adults. Am J Clin Nutr 2008;87:662–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ceglia L, Niramitmahapanya S, da Silva Morais M, Rivas DA, Harris SS, Bischoff-Ferrari H, Fielding RA, Dawson-Hughes B. A randomized study on the effect of vitamin D3 supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab 2013;98:E1927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pojednic RM, Ceglia L. The emerging biomolecular role of vitamin D in skeletal muscle. Exerc Sport Sci Rev 2014;42:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pojednic RM, Ceglia L, Lichtenstein AH, Dawson-Hughes B, Fielding RA. Vitamin D receptor protein is associated with interleukin-6 in human skeletal muscle. Endocrine 2015;49:512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pojednic RM, Ceglia L, Olsson K, Gustafsson T, Lichtenstein AH, Dawson-Hughes B, Fielding RA. Effects of 1,25-dihydroxyvitamin D3 and vitamin D3 on the expression of the vitamin D receptor in human skeletal muscle cells. Calcif Tissue Int 2015;96:256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sitnick M, Bodine SC, Rutledge JC. Chronic high fat feeding attenuates load-induced hypertrophy in mice. J Physiol 2009;587:5753–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rivas DA, Morris EP, Fielding RA. Lipogenic regulators are elevated with age and chronic overload in rat skeletal muscle. Acta Physiol (Oxf) 2011;202:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rivas DA, Morris EP, Haran PH, Pasha EP, Morais M da S, Dolnikowski GG, Phillips EM, Fielding RA. Increased ceramide content and NFκB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. J Appl Physiol (1985) 2012:113;1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haring B, Wu C, Mossavar-Rahmani Y, Snetselaar L, Brunner R, Wallace RB, Neuhouser ML, Wassertheil-Smoller S. No association between dietary patterns and risk for cognitive decline in older women with 9-year follow-up: data from the Women’s Health Initiative memory study. J Acad Nutr Diet 2016;116:921–30, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Solfrizzi V, Panza F. Mediterranean diet and cognitive decline—a lesson from the whole-diet approach: what challenges lie ahead? J Alzheimers Dis 2014;39:283–6. [DOI] [PubMed] [Google Scholar]
- 92.Solfrizzi V, Colacicco AM, D’Introno A, Capurso C, Torres F, Rizzo C, Capurso A, Panza F. Dietary intake of unsaturated fatty acids and age-related cognitive decline: a 8.5-year follow-up of the Italian longitudinal study on aging. Neurobiol Aging 2006;27:1694–704. [DOI] [PubMed] [Google Scholar]
- 93.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement 2015;11:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr 2015;6:154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prince M, Albanese E, Guerchet M, Prina M. Nutrition and dementia: a review of available research [Internet]. [cited 2016 Jun 24]. Available from: https://www.alz.co.uk/sites/default/files/pdfs/nutrition-and-dementia.pdf.
- 96.Irving GF, Freund-Levi Y, Eriksdotter-Jönhagen M, Basun H, Brismar K, Hjorth E, Palmblad J, Vessby B, Vedin I, Wahlund L-O, et al. Omega-3 fatty acid supplementation effects on weight and appetite in patients with Alzheimer’s disease: the Omega-3 Alzheimer’s Disease Study. J Am Geriatr Soc 2009;57:11–7. [DOI] [PubMed] [Google Scholar]
- 97.Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, Basun H, Faxén-Irving G, Garlind A, Vedin I, Vessby B, Wahlund L-O, Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease—OmegAD study: a randomized double-blind trial. Arch Neurol 2006;63:1402–8. [DOI] [PubMed] [Google Scholar]
- 98.Hackman RM, Aggarwal BB, Applebaum RS, deVere White RW, Dubick MA, Heber D, Ito T, Johnson GH, Keen CL, Winters BL, et al. Forecasting nutrition research in 2020. J Am Coll Nutr 2014;33:340–6. [DOI] [PubMed] [Google Scholar]
- 99.Pae M, Meydani SN, Wu D. The role of nutrition in enhancing immunity in aging. Aging Dis 2012;3:91–129. [PMC free article] [PubMed] [Google Scholar]
- 100.Calder PC. Feeding the immune system. Proc Nutr Soc 2013;72:299–309. [DOI] [PubMed] [Google Scholar]
- 101.Keusch GT. The history of nutrition: malnutrition, infection and immunity. J Nutr 2003;133(Suppl):336S–40S. [DOI] [PubMed] [Google Scholar]
- 102.High KP. Nutritional strategies to boost immunity and prevent infection in elderly individuals. Clin Infect Dis 2001;33:1892–900. [DOI] [PubMed] [Google Scholar]
- 103.Barnett JB, Hamer DH, Meydani SN. Low zinc status: a new risk factor for pneumonia in the elderly? Nutr Rev 2010;68:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bendich A. Criteria for determining Recommended Dietary Allowances for healthy older adults. Nutr Rev 1995;53:S105–8; discussion: S108–10. [DOI] [PubMed] [Google Scholar]
- 105.Kelley DS, Bendich A. Essential nutrients and immunologic functions. Am J Clin Nutr 1996;63(Suppl):994S–6S. [DOI] [PubMed] [Google Scholar]
- 106.Meydani SN. Micronutrients and immune function in the elderly. Ann N Y Acad Sci 1990;587:196–207. [DOI] [PubMed] [Google Scholar]
- 107.Meydani SN, Wu D, Santos MS, Hayek MG. Antioxidants and immune response in aged persons: overview of present evidence. Am J Clin Nutr 1995;62:1462S–76S. [DOI] [PubMed] [Google Scholar]
- 108.Rall LC, Meydani SN. Vitamin B6 and immune competence. Nutr Rev 1993;51:217–25. [DOI] [PubMed] [Google Scholar]
- 109.Weber P, Bendich A, Machlin LJ. Vitamin E and human health: rationale for determining recommended intake levels. Nutrition 1997;13:450–60. [DOI] [PubMed] [Google Scholar]
- 110.Weber P, Bendich A, Schalch W.. Vitamin C and human health—a review of recent data relevant to human requirements. Int J Vitam Nutr Res 1996;66:19–30. [PubMed] [Google Scholar]
- 111.Wu D, Meydani SN. Age-associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets 2014;14:283–9. [DOI] [PubMed] [Google Scholar]
- 112.Prasad AS, Beck FWJ, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, Cardozo LJ. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr 2007;85:837–44. [DOI] [PubMed] [Google Scholar]
- 113.Barnett JB, Dao MC, Hamer DH, Kandel R, Brandeis G, Wu D, Dallal GE, Jacques PF, Schreiber R, Kong E, et al. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2016;103:942–51. [DOI] [PubMed] [Google Scholar]
- 114.Haase H, Overbeck S, Rink L. Zinc supplementation for the treatment or prevention of disease: current status and future perspectives. Exp Gerontol 2008;43:394–408. [DOI] [PubMed] [Google Scholar]
- 115.Meydani SN, Barnett JB, Dallal GE, Fine BC, Jacques PF, Leka LS, Hamer DH. Serum zinc and pneumonia in nursing home elderly. Am J Clin Nutr 2007;86:1167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Folds JD, Schmitz JL. 24. Clinical and laboratory assessment of immunity. J Allergy Clin Immunol 2003;111:S702–11. [DOI] [PubMed] [Google Scholar]
- 117.Albers R, Bourdet-Sicard R, Braun D, Calder PC, Herz U, Lambert C, Lenoir-Wijnkoop I, Méheust A, Ouwehand A, Phothirath P, et al. Monitoring immune modulation by nutrition in the general population: identifying and substantiating effects on human health. Br J Nutr 2013;110(Suppl 2):S1–30. [DOI] [PubMed] [Google Scholar]
- 118.Bonilla C, Brauer P, Royall D, Keller H, Hanning RM, DiCenso A. Interprofessional dietary assessment practices in primary care: a mixed-methods study. J Interprof Care 2016;30:77–82. [DOI] [PubMed] [Google Scholar]
- 119.The Joint Commission. About the Joint Commission [Internet] [cited 2016 Sep 20]. Available from: https://www.jointcommission.org/about_us/about_the_joint_commission_main.aspx.
- 120.The Joint Commission. Standards FAQ. Details [Internet] [cited 2015 Feb 23]. Available from: http://www.jointcommission.org/mobile/standards_information/jcfaqdetails.aspx?StandardsFAQId=471&StandardsFAQChapterId=78.
- 121.Lacey K, Pritchett E. Nutrition Care Process and model: ADA adopts road map to quality care and outcomes management. J Am Diet Assoc 2003;103:1061–72. [DOI] [PubMed] [Google Scholar]
- 122.Hakel-Smith N, Lewis NM. A standardized nutrition care process and language are essential components of a conceptual model to guide and document nutrition care and patient outcomes. J Am Diet Assoc 2004;104:1878–84. [DOI] [PubMed] [Google Scholar]
- 123.HealthIT.gov. EHR incentive payment timeline: providers & professionals [Internet] [cited 2015 Feb 27]. Available from: http://www.healthit.gov/providers-professionals/ehr-incentive-payment-timeline.
- 124.HealthIT.gov. Improve care coordination using electronic health records: providers & professionals [Internet] [cited 2015 Feb 25]. Available from: http://www.healthit.gov/providers-professionals/improved-care-coordination.
- 125.Bouamrane M-M, Tao C, Sarkar IN. Managing interoperability and complexity in health systems. Methods Inf Med 2015;54:1–4. [DOI] [PubMed] [Google Scholar]
- 126.Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med 2013;368:100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Friedmann JM, Jensen GL, Smiciklas-Wright H, McCamish MA. Predicting early nonelective hospital readmission in nutritionally compromised older adults. Am J Clin Nutr 1997;65:1714–20. [DOI] [PubMed] [Google Scholar]
- 128.National Center for Biotechnology Information. Nutrition and healthy aging in the community. Workshop summary [Internet] [cited 2015 Feb 27]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22787687.
- 129.Centers for Medicare and Medicaid Services. Community-based care transitions program. [cited 2016 Oct 5]. Available from: https://innovation.cms.gov/initiatives/CCTP/. [Google Scholar]
- 130.Jortberg BT, Fleming MO. Registered dietitian nutritionists bring value to emerging health care delivery models. J Acad Nutr Diet 2014;114:2017–22. [DOI] [PubMed] [Google Scholar]
- 131.US Department of Health and Human Services. Nutrition services [Internet] [cited 2015 Feb 27]. Available from: http://www.aoa.acl.gov/AoA_Programs/HPW/Nutrition_Services/index.aspx.