Abstract
The prevalence of aminoglycoside resistant enzymes has previously been reported and extended-spectrum β-lactamase among Acinetobacter baumannii. To track the risk of multidrug-resistant A. baumannii, the present study aimed to determine the prevalence of carbapenemases in high-level aminoglycoside resistant A. baumannii over two years. A total of 118 strains of A. baumannii were consecutively collected in the First Affiliated Hospital of Chengdu Medical College, Chengdu, China. These isolates were investigated on the genetic basis of their resistance to aminoglycosides. The results showed that 75 (63.56%) isolates were high-level resistant to aminoglycosides, including gentamicin and amikacin (minimum inhibitory concentration, ≥256 µg/ml). Aminoglycoside-resistant genes ant(2″)-Ia, aac(6′)-Ib, aph(3′)-Ia, aac(3)-Ia, aac(3)-IIa, armA, rmtA, rmtB, rmtC, rmtD, rmtE, rmtF, rmtG, rmtH and npmA, and carbapenem-resistant genes blaOXA-23, blaOXA-24, blaOXA-51, blaOXA-58, blaSIM, blaIMP, blaNDM-1 and blaKPC, were analyzed using polymerase chain reaction. The positive rate of ant(2″)-Ia, aac(6′)-Ib, aph(3′)-Ia, aac(3)-Ia and aac(3)-IIa was 66.95, 69.49, 42.37, 39.83 and 14.41%, respectively. armA was present in 72.0% (54/75) of A. baumannii isolates with high-level resistance to aminoglycosides. The remaining nine 16S ribosomal RNA methlyase genes (rmtA, rmtB, rmtC, rmtD, rmtE, rmtF, rmtG, rmtH and npmA) and aminoglycoside-modifying enzyme gene aac(6′)-Ib-cr were not detected. Among the 54 armA-positive isolates, the prevalence of the carbapenem resistant blaOXA-23 and blaOXA-51 genes was 79.63 and 100%, respectively. armA, ant(2″)-Ia and aac(6′)-Ib were positive in 43 isolates. The results of multilocus sequence typing revealed 31 sequence types (STs) in all clinical strains. Among these STs, the high-level aminoglycoside-resistant A. baumannii ST92, which mostly harbored blaOXA-23, was the predominant clone (29/75). In conclusion, A. baumannii harboring carbapenemases and aminoglycoside-resistant enzymes are extremely prevalent in western China, emphasizing the need to adopt surveillance programs to solve the therapeutic challenges that this presents.
Keywords: Acinetobacter baumannii, aminoglycosides, 16S ribosomal RNA methylase, carbapenemases, multilocus sequence typing
Introduction
Acinetobacter baumannii is an important opportunistic pathogen that causes various types of human infections and has become a primary cause of nosocomial infections because of its broad antimicrobial resistance (1–3). Aminoglycosides, a type of broad-spectrum antibiotics, continue to serve an important role in treating serious infections caused by gram-negative bacteria (4). However, aminoglycoside resistance of A. baumannii has rapidly increased and given rise to more challenges in the clinical treatment of infections (5).
A. baumannii shows resistance to aminoglycosides since functional aminoglycosides can be modified by various aminoglycoside-modifying enzymes, including acetyltransferases, phosphotransferases and nucleotidyltransferases, into non-functional forms in the bacteria (6). In addition, aminoglycoside antibiotics bind specifically to the A-site of 16S ribosomal (r)RNA in the 30S small subunit and interfere with the decoding of mRNA to inhibit protein synthesis (7). In addition, at least ten 16S rRNA methylase genes (armA, rmtA, rmtB, rmtC, rmtD, rmtE, rmtF, rmtG, rmtH and npmA) have been identified (8–12). These 16S rRNA methylases, which lead to the high-level resistance of various aminoglycosides, can easily transfer to other bacteria since their genes are typically present on plasmids (13). Therefore, the emergence and spread of such bacteria should be carefully monitored. Since the 16S rRNA methylases are key factors in the aminoglycoside resistance of A. baumannii, the investigation of the acquisition of 16S rRNA methylase genes by clinical isolates is important for the prevention and treatment of their infections (14).
Aminoglycosides and carbapenems represent the class of antimicrobials that are used to treat A. baumannii infections. Aminoglycoside antibiotics are frequently ineffective against strains of A. baumannii; however, combinations of aminoglycosides and carbapenems can produce synergistic effects to treat infected patients (15,16). Previously, it has become evident that the outgrowth of carbapenem-resistant isolates has resulted in it being difficult to treat A. baumannii infections. One of the most important mechanisms underlying the resistance of carbapenems is the production of carbapenemases in A. baumannii (17). Class D oxacillinases (OXA type) are the primary cause of prevalence in A. baumannii strains (18). In addition, causes stem from class B β-lactamases (metallo-β-lactamases) and Klebsiella pneumoniae Carbapenemase (KPC) producers. These carbapenemases are a diverse group of β-lactamases that are active against the carbapenems, resulting in their limited clinical use.
Several studies have documented the co-existence of blaOXA-23 and armA in multidrug resistant A. baumannii isolates (19–22). For example, Doi et al (19) first discovered that two of five A. baumannii isolates coproduced OXA-23 β-lactamase and ArmA in North America in 2007. In addition, further cases were reported in Korea (20,23), India (24), France (25), Bulgaria (26), Italy (27), Latvia (28), East Africa (29), Yemen (30), Japan (31), Brunei (32), Egypt (33) and China (21,34,35). The authors of the present study previously determined that extended-spectrum β-lactamase and 16S rRNA methylase are coproduced in A. baumannii (36). However, the high-level resistance to aminoglycosides, coupled with carbapenem resistance in A. baumannii, were not reported over the 4-year period in China, particularly in western China.
The aim of the present study was to explore the high-level resistance mechanisms against aminoglycosides, and to investigate the presence of carbapenemases among strains of A. baumannii. In addition, the relatedness of aminoglycoside- and carbapenem-resistant strains, determined through epidemiologic examination, is described. To the best of our knowledge, the present study is the first to document the emergence of A. baumannii producing blaOXA-23 and blaOXA-51 carbapenemase-encoding genes among armA 16S rRNA methylases at a university hospital in western China. Furthermore, the results aim to emphasize that the dearth of appropriate treatments remains a primary concern regarding multidrug-resistant infections.
Materials and methods
Clinical isolates
A total of 118 strains of A. baumannii were consecutively collected in a university hospital of western China between February 2012 and July 2013. Rapid species identification was performed by polymerase chain reaction (PCR), as reported within ‘Resistance gene amplification’ and previously described (37). A. baumannii was identified and confirmed if the following two PCR products were yielded: A 425-bp internal control amplicon corresponding to the recA gene of Acinetobacter spp., and a 208-bp fragment of the 16S rRNA intergenic spacer region of A. baumannii (38). Non-baumannii species of Acinetobacter, which yielded the 425-bp product alone, were excluded in this study. Isolates were obtained from specimens including sputum, secretion, lavage fluids, blood and other specimens. All strains were stored at −80°C. Bacteria were grown on tryptose agar or Mueller-Hinton broth. No amplicons were obtained with bacteria belonging to other genera.
Antimicrobial susceptibility testing
The minimum inhibitory concentrations (MICs) of amikacin and gentamicin (Sangon Biotech Co., Ltd., Shanghai, China) for A. baumannii were determined on Mueller-Hinton agar plates by agar dilution according to the protocol recommended by the Clinical and Laboratory Standards Institute (39). MICs of meropenem and imipenem (Sangon Biotech Co., Ltd.) were tested in high-level aminoglycoside-resistant isolates. The results were interpreted according to the CLSI guidelines. Escherichia coli [American Type Culture Collection (ATCC) 25922] and A. baumannii (ATCC 19606) (ATCC, Manassas, VA, USA) were used as quality control strains.
Resistance gene amplification
The aminoglycoside-modifying enzyme genes and the 16S rRNA methylase genes were detected by PCR. Total DNA was extracted by boiling at 95°C for 15 min. After centrifugation at 13,000 × g for 10 min to pellet the debris, the supernatant was stored at −20°C for further assays. PCR was performed in a total volume of 50 µl containing 0.2 mM of each deoxynucleotide, 0.5 µM of each primer (Table I), 2.5 units Taq polymerase (Takara Bio, Inc., Dalian, China) and 5 µl 10X buffer (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Primers listed in Table I were synthesized by Sangon Biotech Co., Ltd.. The PCR thermal cycling conditions were as follows: Initial denaturation at 94°C for 5 min in order to obtain partial activation of Taq polymerase; then, the number of cycles was increased to 30, each consisting of a denaturation step for 30 sec (at 94°C), an annealing step for 30 sec (at 55°C for armA, rmtA, rmtB, rmtC, rmtD, rmtE, rmtF, rmtG, rmtH and npmA, at 53°C for ant(2″)-Ia, aph(3′)-1a, aac(3)-Ia and aac(3)-IIa, and at 56°C for aac(6′)-Ib) and an extension step for 30 sec (at 72°C). Each amplification experiment included a blank containing the reagent except for target DNA. The products were electrophoresed in 1% agarose gels and visualized under ultra-violet light (Bio-Rad Laboratories, Inc., Hercules, CA, USA). All aac(6′)-Ib PCR products were directly sequenced and compared with the published nucleotide (NC_005327.1).
Table I.
Primers used in the present study for polymerase chain reaction detection.
| Primer | Target | Oligonucletides (5′ to 3′) | Expected size (bp) |
|---|---|---|---|
| armA forward | armA | AGGTTGTTTCCATTTCTGAG | 591 |
| armA-R | TCTCTTCCATTCCCTTCTCC | ||
| rmtA forward | rmtA | CTAGCGTCCATCCTTTCCTC | 635 |
| rmtA-R | TTTGCTTCCATGCCCTTGCC | ||
| rmtB forward | rmtB- | CCCAAACAGACCGTAGAGGC | 585 |
| rmtB-R | CTCAAACTCGGCGGGCAAGC | ||
| rmtC forward | rmtC | CGAAGAAGTAACAGCCAAAG | 711 |
| rmtC-R | ATCCCAACATCTCTCCCACT | ||
| rmtD forward | rmtD | CGGCACGCGATTGGGAAGC | 401 |
| rmtD-R | CGGAAACGATGCGACGAT | ||
| rmtE forward | rmtE | ATGAATATTGATGAAATGGTTGC | 823 |
| rmtE-R | TGATTGATTTCCTCCGTTTTTG | ||
| rmtF forward | rmtF | GCGATACAGAAAACCGAAGG | 589 |
| rmtF-R | ACCAGTCGGCATAGTGCTTT | ||
| rmtG forward | rmtG | AAATACCGCGATGTGTGTCC | 250 |
| rmtG reverse | ACACGGCATCTGTTTCTTCC | ||
| rmtH forward | rmtH | GCTTAAACCCGCTGATGCT | 332 |
| rmtH reverse | AAACCAGGTGGCGTAGTGC | ||
| npmA forward | npmA | GGAGGGCTATCTAATGTGGT | 371 |
| npmA reverse | GCCCAAAGAGAATTAAACTG | ||
| ant(2″)-Ia forward | ant(2″)-Ia | GCTTACGTTGTCCCGCATTT | 215 |
| ant(2″)-Ia reverse | CCTTGGTGATCTCGCCTTTC | ||
| aph(3′)-Ia forward | aph(3′)-Ia | CGAGCATCAAATGAAACTGC | 623 |
| aph(3′)-Ia reverse | GCGTTGCCAATGATGTTACAG | ||
| aac(3)-Ia forward | aac(3)-Ia | GACATAAGCCTGTTCGGTT | 372 |
| aac(3)-Ia reverse | CTCCGAACTCACGACCGA | ||
| aac(3)-IIa forward | aac(3)-IIa | ATGCATACGCGGAAGGC | 822 |
| aac(3)-IIa reverse | TGCTGGCACGATCGGAG | ||
| aac(6′)-Ib forward | aac(6′)-Ib | AAGCGTTTTAGCGCAAGAGT | 366 |
| aac(6′)-Ib reverse | GCGTGTTTGAACCATGTACA | ||
| OXA-23 forward | OXA-23 | GATCGGATTGGAGAACCAGA | 501 |
| OXA-23 reverse | ATTTCTGACCGCATTTCCAT | ||
| OXA-24 forward | OXA-24 | CAAGAGCTTGCAAGACGGACT | 420 |
| OXA-24 reverse | TCCAAGATTTTCTAGCRACTTATA | ||
| OXA-51 forward | OXA-51 | TAATGCTTTGATCGGCCTTG | 353 |
| OXA-51 reverse | TGGATTGCACTTCATCTTGG | ||
| OXA-58 forward | OXA-58 | TCGATCAGAATGTTCAAGCGC | 530 |
| OXA-58 reverse | ACGATTCTCCCCTCTGCGC | ||
| NDM-1 forward | NDM-1 | TCTCGACATGCCGGGTTTCGG | 475 |
| NDM-1 reverse | ACCGAGATTGCCGAGCGACTT | ||
| KPC forward | KPC | GCTCAGGCGCAACTGTAAGT | 823 |
| KPC reverse | GTCCAGACGGAACGTGGTAT | ||
| IMP forward | IMP | CTACCGCAGAGTCTTTG | 587 |
| IMP reverse | AACCAGTTTTGCCTTACCAT | ||
| SIM forward | SIM | TACAAGGGATTCGGCATCG | 570 |
| SIM reverse | TAATGGCCTGTTCCCATGTG |
Genes coding for classes A, B and D carbapenemases were investigated among high-level aminoglycoside-resistant isolates by PCR. The genes encoding class A, such as Klebsiella pneumoniae carbapenemase gene (blaKPC) (40), class B, such as the metallo-β-lactamase genes [blaIMP (41), blaVIM-1 (42), blaSIM (43) and blaNDM-1 (44)] and class D, such as CHDLs [blaOXA-23 (45), blaOXA-24 (45), blaOXA-51 (46) and blaOXA-58 (47)], were also analyzed using PCR. Reaction conditions of PCR were as follows: 94°C for 5 min; and 30 cycles of 94°C for 30 sec, 56°C for 30 sec and 72°C for 30 sec; followed by a final extension at 72°C for 5 min.
Multilocus sequence typing (MLST)
MLST was performed according a the previously described A. baumannii MLST study (48). Briefly, internal fragments of seven housekeeping genes (gltA, gyrB, gdhB, recA, cpn60, gpi and rpoD) were amplified by PCR (49). The sequences of the seven housekeeping genes were compared with existing sequences in the MLST database (50) for the assignment of allelic numbers. Sequence types (STs) were assigned according to their allelic profiles. New allele sequences and STs were assigned in accordance with the PubMLST database (50). The eBURST program (http://eburst.mlst.net) was used to cluster STs into clonal complex (CC) and infer evolutionary descent (51).
Results
Antimicrobial susceptibility of aminoglycosides
All 118 clinical strains were identified as A. baumannii by 16S rRNA and recA amplification. Among these isolates, 73 (61.86%) and 72 (61.02%) strains were resistant to gentamicin and amikacin, respectively (Tables II and III). Thus, the resistance to amikacin and gentamicin was observed in 66 (55.93%, 66/118) A. baumannii isolates. A total of 78 (66.1%, 78/118) isolates were resistant to amikacin and gentamicin, and 75 (96.15%, 75/78) of the strains showed a high level of resistance (MIC, ≥256 µg/ml; Table III).
Table II.
Susceptibilities to two types of aminoglycosides of A. baumannii isolates.
| Drug name | Resistant isolates, n (%) | Intermediate isolates, n (%) | Sensitive isolates, n (%) | Total, n (%) |
|---|---|---|---|---|
| Gentamicin | 73 (61.86) | 3 (2.54) | 42 (35.60) | 118 (100) |
| Amikacin | 72 (61.02) | 0 (0.00) | 46 (38.98) | 118 (100) |
Table III.
Molecular resistance characteristics of 75 high level aminoglycoside resistance isolates.
| Susceptibility patter (MIC in µg/ml) | Resistance genes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolates | Gentamicin | Amikacin | Imipenem | Meropenem | armA | ant(2″)-Ia | aac(6′)-Ib | aph(3′)-Ia | aac(3)-Ia | aac(3)-IIa | blaOXA-23 | blaOXA-51 |
| 001 | >1024 | 1024 | 16 | 32 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 003 | 256 | 1024 | 16 | 32 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 005 | >1024 | 1024 | 32 | 64 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 006 | >1024 | 1024 | 32 | 64 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 007 | >1024 | 1024 | 32 | 64 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 008 | >1024 | 1024 | 32 | 64 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| 011 | >1024 | 1024 | 16 | 32 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 013 | 256 | 256 | 8 | 16 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 016 | >1024 | 1024 | 16 | 32 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 018 | 256 | 256 | 8 | 16 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| 020 | 2 | 256 | 8 | 16 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| 026 | >1024 | 1024 | 16 | 32 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 |
| 027 | >1024 | 1024 | 16 | 32 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| 028 | 1024 | 1024 | 8 | 16 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| 030 | >1024 | 1024 | 32 | 32 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| 031 | >1024 | 1024 | 32 | 16 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 |
| 034 | >1024 | 1024 | 32 | 64 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 035 | >1024 | 4 | 32 | 64 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| 036 | >1024 | 1024 | 16 | 32 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| 037 | >1024 | 1024 | 32 | 64 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| 039 | >1024 | 1024 | 32 | 64 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| 040 | >1024 | 1024 | 32 | 32 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| 041 | >1024 | 1024 | 32 | 64 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| 042 | >1024 | 512 | 32 | 64 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 043 | >1024 | 1024 | 32 | 64 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 044 | >1024 | 1024 | 64 | 128 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| 046 | >1024 | 1024 | 32 | 64 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 |
| 047 | >1024 | 1024 | 64 | 64 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| 048 | >1024 | 1024 | 32 | 64 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 |
| 049 | >1024 | 512 | 32 | 64 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 050 | >1024 | 512 | 32 | 32 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| 051 | >1024 | 1024 | 32 | 32 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| 052 | >1024 | 1024 | 64 | 128 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| 053 | >1024 | 512 | 32 | 32 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 054 | >1024 | 512 | 32 | 32 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| 057 | >1024 | 1024 | 32 | 64 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 058 | >1024 | 1024 | 32 | 64 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| 059 | >1024 | 512 | 32 | 32 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| 060 | >1024 | 1024 | 32 | 32 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| 061 | >1024 | 1024 | 16 | 32 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 |
| 062 | >1024 | 512 | 32 | 32 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| 063 | >1024 | 1024 | 32 | 32 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| 064 | 512 | 8 | 32 | 32 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| 065 | 256 | 8 | 32 | 16 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 066 | >1024 | 512 | 32 | 64 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 067 | 256 | 256 | 16 | 32 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 068 | >1024 | 512 | 32 | 32 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 069 | >1024 | 512 | 32 | 32 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 072 | 256 | 256 | 16 | 16 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 |
| 074 | 512 | 2 | 16 | 8 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
| 075 | 8 | 1024 | 32 | 32 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 |
| 076 | >1024 | >1024 | 64 | 64 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 |
| 079 | 512 | 512 | 32 | 64 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| 080 | 512 | 512 | 64 | 128 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| 082 | 512 | 512 | 32 | 32 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
| 085 | >1024 | 512 | 16 | 32 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| 087 | 512 | 512 | 32 | 32 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| 089 | 4 | 512 | 16 | 32 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| 090 | >1024 | 1024 | 32 | 32 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 |
| 093 | >1024 | 1024 | 32 | 128 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| 094 | >1024 | >1024 | 16 | 32 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| 095 | 512 | 512 | 16 | 32 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 |
| 096 | >1024 | >1024 | 32 | 32 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 097 | >1024 | >1024 | 16 | 32 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 098 | 512 | 512 | 16 | 32 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| 099 | 512 | 256 | 32 | 32 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| 100 | 8 | 512 | 0.5 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| 101 | >1024 | 512 | 32 | 32 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 |
| 102 | 512 | 256 | 32 | 32 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
| 104 | 512 | 256 | 32 | 32 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 106 | 1024 | 512 | 32 | 32 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| 107 | 512 | 256 | 16 | 32 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
| 109 | >1024 | 512 | 8 | 16 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
| 113 | >1024 | 1024 | 8 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 120 | >1024 | 2 | 8 | 32 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
MIC, minimum inhibitory concentration.
Co-occurrence of aminoglycoside-resistant enzymes and carbapenemases
To determine the role of the aminoglycoside-modifying enzymes in resistance and the 16S rRNA methylases, PCR was performed to detect the concomitant genes (Table III). The positive rates of ant(2″)-Ia, aac(6′)-Ib, aph(3′)-Ia, aac(3)-Ia and aac(3)-IIa were 66.95 (79/118), 69.49 (82/118), 42.37 (50/118), 39.83 (47/118) and 14.41% (17/118), respectively (Table IV). Fifty-four of 118 (45.76%) isolates harboring the 16S rRNA methyalse armA gene obtained high level of resistance to amikacin and gentamicin. rmtA, rmtB, rmtC, rmtD, rmtE, rmtF, rmtG and npmA genes were not detected in all of the isolates.
Table IV.
Positive rates of genes in A. baumannii.
| Gene | Positive rate, % (n/118) | Gene | Positive rate, % (n/118) |
|---|---|---|---|
| armA | 45.76 (54/118) | aph(3′)-Ia | 42.37 (50/118) |
| aac(6′)-Ib | 69.45 (82/118) | aac(3)-Ia | 39.83 (47/118) |
| ant(2″)-la | 66.95 (79/118) | aac(3)-IIa | 14.41 (17/118) |
There was a marked difference in the distribution of aminoglycoside-resistant genes among the 75 high-level aminoglycoside-resistant A. baumannii (Tables III and IV). All 54 (72.0%, 54/75) armA-positive strains were confirmed to serve a primary role in high level aminoglycoside resistance. However, 21 (28%, 21/75) isolates harboring aminoglycoside-modifying enzymes without the armA gene served the same function (Table V).
Table V.
Distribution of aminoglycoside resistance genes in 75 high level aminoglycoside resistance clinical isolates of A. baumannii, expressed as positive (+) or negative (−).
| armA-positive aminoglycoside resistance gene profile (n=54) | blaOXA-23 (n=58) | blaOXA-51 (n=75) | No. of isolates |
|---|---|---|---|
| ant(2″)-Ia | +2 | + | 2 (2.67%) |
| aac(6)-Ib | +1 | + | 1 (1.33%) |
| ant(2)-Ia+aac(6′)-Ib | +4 | + | 4 (5.33%) |
| ant(2)-Ia+aph(3′)-Ia | +2 | + | 2 (2.67%) |
| aac(6′)-Ib+aac(3)-Ia | +3 | + | 3 (4.0%) |
| aac(6′)-Ib+aac(3)-IIa | +2 | + | 2 (2.67%) |
| ant(2″)-Ia+aac(6′)-Ib+aac(3)-Ia | +9/−3 | + | 12 (16%) |
| ant(2″)-Ia+aac(6′)-Ib+aac(3)-IIa | +1/−1 | + | 2 (2.67%) |
| ant(2″)-Ia+aac(6′)-Ib+aph(3′)-Ia | +9/−1 | + | 10 (13.3%) |
| ant(2″)-Ia+aph(3′)-Ia+aac(3)-IIa | +1 | + | 1 (1.33%) |
| ant(2″)-Ia+aac(6′)-Ib+aph(3′)-Ia+aac(3)-Ia | +8/−2 | + | 10 (13.3%) |
| ant(2″)-Ia+aac(6′)-Ib+aph(3′)-Ia+aac(3)-IIa | −1 | + | 1 (1.33%) |
| ant(2″)-Ia+aac(6′)-Ib+aac(3)-Ia+aac(3)-IIa | +1 | + | 1 (1.33%) |
| ant(2″)-Ia+aac(6′)-Ib+aph(3′)-Ia+aac(3)-Ia+aac(3)-IIa | −3 | + | 3 (4.0%) |
| None of armA genes (21) | |||
| ant(2″)-Ia | + | + | 1 (1.33%) |
| aac(6′)-Ib | − | + | 1 (1.33%) |
| aac(6′)-Ib+ant(2″)-Ia | + | + | 1 (1.33%) |
| aac(6′)-Ib+aac(3)-Ia | −2 | + | 2 (2.67%) |
| aac(6′)-Ib+aac(3)-IIa | + | + | 1 (1.33%) |
| ant(2″)-Ia+aac(6′)-Ib+aph(3′)-Ia | +5 | + | 5 (6.67%) |
| ant(2″)-Ia+aac(6′)-Ib+aac(3)-Ia | + | + | 1 (1.33%) |
| ant(2″)-Ia+aac(6′)-Ib+aac(3)-IIa | +2 | + | 2 (2.67%) |
| ant(2″)-Ia+aph(3′)-Ia+aac(3)-IIa | − | + | 1 (1.33%) |
| ant(2″)-Ia+aac(6′)-Ib+aph(3′)-Ia+aac(3)-Ia | +/−2 | + | 3 (4.0%) |
| ant(2″)-Ia+aac(6′)-Ib+aph(3′)-Ia+aac(3)-IIa | + | + | 1 (1.33%) |
| aac(6′)-Ib+aph(3′)-Ia+aac(3)-Ia+aac(3)-IIa | + | + | 1 (1.33%) |
| ant(2″)-Ia+aac(6′)-Ib+aph(3′)-Ia+aac(3)-Ia+aac(3)-IIa | + | + | 1 (1.33%) |
Among the 54 isolates that were armA-positive, the prevalence of blaOXA-23 and blaOXA-51 gene occurrences were 79.63 (43/54) and 100% (54/54), respectively. In addition, the prevalence of ant(2″)-Ia, aac(6′)-Ib, aph(3′)-Ia, aac(3)-Ia, and aac(3)-IIa positive rates of genes was distributed in the aminoglycoside-resistant and-susceptible strains (Table V). As described above, the present study demonstrated that aminoglycoside-modifying enzymes were mostly responsible for moderate level resistance (16 µg/ml<MIC<256 µg/ml) to aminoglycosides in A. baumannii, whereas armA was responsible for high-level resistance to aminoglycosides. All 75 isolates with high-level resistance to aminoglycosides harbored the carbapenemase genes blaOXA-23 (77.33%) or blaOXA-51 (100%; Tables III and V), which (except one isolate) showed resistance to the carbapenems, imipenem and meropenem. These data suggest that the resistance to carbapenems and aminoglysides poses a threat following combination treatment of A. baumannii infection.
Molecular genotyping analysis of drug-resistant isolates
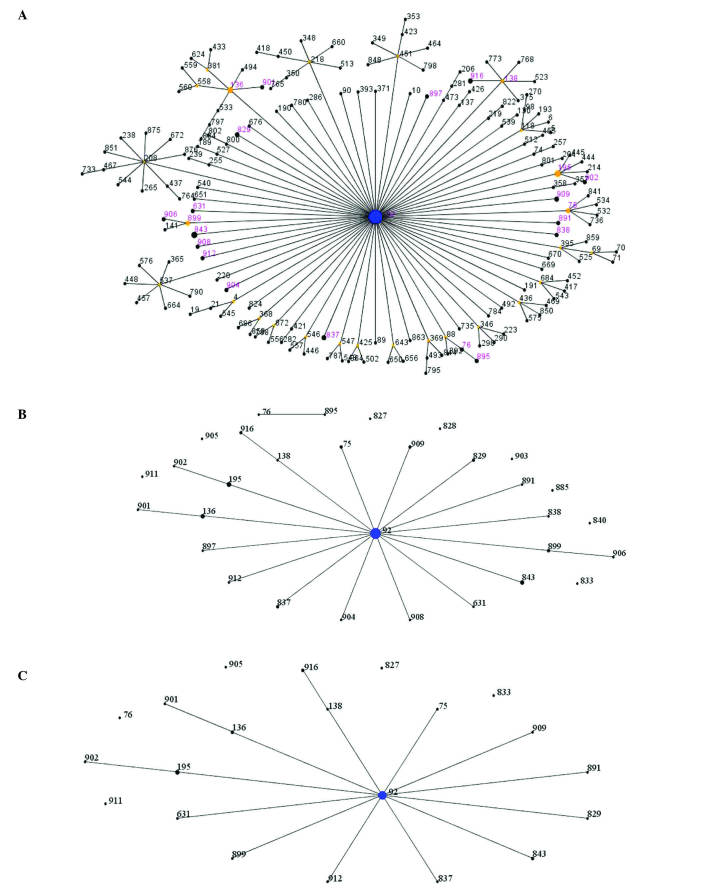
To better assess the A. baumannii clinical population epidemiology and the genetic background of these strains, a number of molecular typing systems were applied. By comparing the ST(s) of 75 high-level aminoglycoside resistant isolates with all identified ST(s) in A. baumannii in the MLST database by eBUSRT analysis, 29 strains were identified that belonged to ST92, which is a globally distributed strain (Fig. 1A). According to MLST analysis, a total of 31 different STs were assigned to the 75 high-level aminoglycoside resistant isolates, of which 21 STs were clustered into clonal complex 92 (CC92), and the remaining 10 STs were identified as singletons. The most common ST was ST92, which accounted for 38.67% (29/75) (Fig. 1A and B). ST195, followed by ST92, presented in 5 strains, whilst ST136 and ST843 were detected in 4 strains. ST75, ST829, ST837, ST899, ST909 and ST916 were represented by 2 isolates. Molecular analysis revealed that 37 (containing 6 different STs) of the 43 isolates, which produced carbapenemase OXA-23 and 16S rRNA methylase ArmA, were grouped into CC92, while the remaining 6 isolates, which had 6 different STs, could not be clustered into any known clonal complex (Fig. 1C). These data indicate that the prevalence of A. baumannii isolates was caused by CC92 dissemination.
Figure 1.
Relatedness of the STs of 75 high-level aminoglycoside resistance strains. Each circle represents a specific ST. The size of each circle corresponds to number of isolates, with larger sizes representing higher frequency of occurrence. The blue dot in the center corresponds to ST92, the most prevalent. Black numbers indicate existence of STs in the MLST database, and the numbers in purple indicate STs found in the present study. (A) eBURST population snapshot of CC92. Six was used as the minimum identical loci for the definition of CC and three was used as the minimum single locus variants. (B) Similar population snapshot pictures and superscript pictures. Similar population snapshot pictures were drawn by eBURST algorithm of Acinetobacter baumannii STs in the PubMLST database, and superscript pictures were analyzed through the University of Oxford database. (C) The relatedness of the STs of 43 A. baumannii strains carrying blaOXA-23 and armA genes. The radial diagram reflects the predicted evolutionary descent from the founder ST. The size of the circle corresponds to the number of isolates belonging to a ST. STs, sequence types; CC, clonal complex.
Discussion
A. baumannii are important hospital-acquired pathogens that cause various types of human infections (52). The present study demonstrated that 75 (63.56%) strains were high-level resistant to amikacin or gentamicin, determined by susceptibility testing (Table III), suggesting that these antibiotics can only be used for treating A. baumannii infections induced by susceptible strains.
As indicated above, at least one aminoglycoside resistance gene was detected in aminoglycoside-resistant A. baumannii strains, and different resistant genes were commonly present in the same isolates (Tables III and V). Among these strains, the dominant aminoglycoside-resistant genotypes are ant(2″)-Ia, armA and aac(6′)-Ib, which were present at 66.95, 45.76 and 69.49%, respectively (Table IV). These results indicated that the presence of armA and aminoglycoside-modifying enzmyes confers to the high level of aminoglycoside resistance.
The prevalence of armA genes in A. baumannii isolates has been described in several studies that showed 50% (52/104) in strains isolated in Lishui, eastern China (10), 60.4% (61/101) in clinical strains in Vietnam (53), and 59.54% (103/173) in hospitals in Beijing, China (54). In the present study, 45.76% (54/118, Table IV) of isolates harbored the armA gene, which is similar to the above cases reported in China. In addition, it was reported that armA was identified in 90% (97/107) of the multidrug-resistant strains in Shanghai, eastern China (55). In a previous study, however, 4 (8.5%) isolates were positive for the methylase enzyme ArmA in an Algerian hospital (56). In conclusion, armA is highly prevalent worldwide, particularly in China.
The emergence of high level aminoglycoside resistance may pose a question for the combination therapy of aminoglycoside with β-lactams, particularly carbapenems in treating A. baumannii infections. Previously, A. baumannii producing OXA-23 have been increasingly described in Shanghai, eastern China (38). Thus, the present study identified carbapenemase genes in 75 high-level aminoglycoside resistance strains. The positive ratios of blaOXA-51 and blaOXA-23 were 100 (75/75) and 77.33% (58/75), respectively (Table III), further demonstrating that the intrinsic OXA-51 family and the presence of OXA-23 are the most prevalent mechanisms for carbapenem resistance in A. baumannii (57). In addition, among 54 armA-positive isolates, the prevalence of blaOXA-23 and blaOXA-51 were 79.63 (43/54) and 100% (54/54) (Table V), which was similar to a previous study (27,56). Three hospital disseminations of A. baumannii co-producing OXA-23 and ArmA were reported in eastern China in 2009 and 2011 (21,34,35). To the best of our knowledge, the results in the present study are the first to demonstrate the co-occurrence of carbapenemases OXA-23, OXA-51 and 16S rRNA methylase ArmA with high level aminoglycoside resistance among clinical isolates of A. baumannii from Chengdu, western China.
Previously, it was reported that aminoglycosides with the aac/aad riboswitch control the expression of aminoglycoside modification enzymes (58), indicating that bacteria can survive in an energy saving way. Therefore, these efficient modification enzymes were responsible for aminoglycoside resistance (Table IV). In addition, it was identified that the aac(6′)-Ib enzyme is able to modify amikacin, even in phenotypically amikacin-susceptible isolates (59). Furthermore, the aac(6′)-Ib (69.49%) A. baumannii isolates were aminoglycoside-positive (Table IV), which is different from previous studies (10). The reason why these differences were observed may be due to the resistance level caused by aac(6′)-Ib, which was regional-dependent and host bacterium-dependent (59).
In the present study, a higher rate of aac(3)-IIa (14.41%) were detected. In addition, aac(3)-IIa genes were detected in 47.88% of E. coli isolated from an Iranian hospital (60). Miro et al (61) found 12.4% of strains possessing aac(3)-IIa genes. However, there is a paucity of data regarding the aac(3)-IIa gene distribution in A. baumannii. It was reported that only 4 strains (3.7%) carried aac(3)-IIa genes (62); aac(3)-IIa was not identified in any strains in a study by Nowak et al (63). Previous studies have reported that aac(3)-IIa modifies gentamicin, which explains the observed high rate of resistance to gentamicin in these A. baumannii strains (59). The increasing prevalence of aminoglycoside resistance is partly associated with the presence of aac(3′)-IIa.
The PubMLST database assigned A. baumannii strains to 920 different types. ST92, a globally distributed type, was the predicted founder of CC92 in the A. baumannii MLST database. CC92 is the largest and most geographically diverse clonal complex (64). Combined ST profiles from MLST and eBURST analyses showed that almost all isolates were clonally related and CC92 was responsible for the spread of disease (Fig. 1). The present study further suggests the possibility that A. baumannii carrying blaOXA-23 and armA genes contribute towards CC92 dissemination. In addition, the present study described the emergence and spread of a clonal strain of the high-level aminoglycoside-resistant A. baumannii. These findings support the hypothesis that certain restricted genetic backgrounds serve an important role in the emergence of aminoglycoside resistance, since some genetic backgrounds may be prone to acquire a foreign resistance gene and maintain its stability and expression (46). Further analysis of the epidemiology of A. baumannii is required in order to determine the prevalence of drug-resistant genes.
In conclusion, the present study demonstrated that 16S rRNA methylase ArmA and modifying enzyme occurrence confer high level resistance to aminoglycoside in A. baumannii. In addition, it was identified that the high level aminoglycoside resistance of A. baumannii strains, harboring high percentages of positive carbapenemases blaOXA-23 and blaOXA-51, strongly suggest that a better understanding of the global epidemiology and monitoring for the presence of resistance genes is urgently required.
Acknowledgments
The authors would like to thank members of the Key Laboratory of Non Coding RNA and Drug Discovery, the Education Department of Sichuan Province, Chengdu, China for their input. The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 81373454, 31300659, 31470246 and 31401099), Applied Basic Research Programs of Sichuan Province, China (grant no. 2013jy0065) Scientific Research and Innovation Team of Sichuan Province, China (grant no. 15TD0025) and the Preeminent Youth Fund of Sichuan Province, China (grant no. 2015JQO019).
References
- 1.Sheikh YA, Marie MA, John J, Krishnappa LG, Dabwab KH. Prevalence of 16S rRNA methylase genes among β-lactamase-producing Enterobacteriaceae clinical isolates in Saudi Arabia. Libyan J Med. 2014;9:24432. doi: 10.3402/ljm.v9.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belbel Z, Chettibi H, Dekhil M, Ladjama A, Nedjai S, Rolain JM. Outbreak of an armA Methyltransferase-Producing ST39 Klebsiella pneumoniae clone in a pediatric Algerian hospital. Microb Drug Resist. 2014;20:310–315. doi: 10.1089/mdr.2013.0193. [DOI] [PubMed] [Google Scholar]
- 3.Liu Z, Ling B, Zhou L. Prevalence of 16S rRNA methylase, modifying enzyme, and extended-spectrum beta-lactamase genes among Acinetobacter baumannii isolates. J Chemother. 2015;27:207–212. doi: 10.1179/1973947814Y.0000000190. [DOI] [PubMed] [Google Scholar]
- 4.Nemec A, Dolzani L, Brisse S, van den Broek P, Dijkshoorn L. Diversity of aminoglycoside-resistance genes and their association with class 1 integrons among strains of pan-European Acinetobacter baumannii clones. J Med Microbiol. 2004;53:1233–1240. doi: 10.1099/jmm.0.45716-0. [DOI] [PubMed] [Google Scholar]
- 5.Labby KJ, Garneau-Tsodikova S. Strategies to overcome the action of aminoglycoside-modifying enzymes for treating resistant bacterial infections. Future Med Chem. 2013;5:1285–1309. doi: 10.4155/fmc.13.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist Updat. 2010;13:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho YJ, Moon DC, Jin JS, Choi CH, Lee YC, Lee JC. Genetic basis of resistance to aminoglycosides in Acinetobacter spp. and spread of armA in Acinetobacter baumannii sequence group 1 in Korean hospitals. Diagn Microbiol Infect Dis. 2009;64:185–190. doi: 10.1016/j.diagmicrobio.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Bueno MF, Francisco GR, O'Hara JA, de Oliveira Garcia D, Doi Y. Coproduction of 16S rRNA methyltransferase RmtD or RmtG with KPC-2 and CTX-M group extended-spectrum β-lactamases in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2397–2400. doi: 10.1128/AAC.02108-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galimand M, Courvalin P, Lambert T. RmtF, a new member of the aminoglycoside resistance 16S rRNA N7 G1405 methyltransferase family. Antimicrob Agents Chemother. 2012;56:3960–3962. doi: 10.1128/AAC.00660-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J, Ye M, Jia X, Yu F, Wang M. Coexistence of armA and genes encoding aminoglycoside-modifying enzymes in Acinetobacter baumannii. Afr J Microbiol Res. 2012;6:5325–5330. [Google Scholar]
- 11.O'Hara JA, McGann P, Snesrud EC, Clifford RJ, Waterman PE, Lesho EP, Doi Y. Novel 16S rRNA methyltransferase RmtH produced by Klebsiella pneumoniae associated with war-related trauma. Antimicrob Agents Chemother. 2013;57:2413–2416. doi: 10.1128/AAC.00266-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wachino J, Arakawa Y. Exogenously acquired 16S rRNA methyltransferases found in aminoglycoside-resistant pathogenic Gram-negative bacteria: An update. Drug Resist Updat. 2012;15:133–148. doi: 10.1016/j.drup.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Wachino J, Shibayama K, Kurokawa H, Kimura K, Yamane K, Suzuki S, Shibata N, Ike Y, Arakawa Y. Novel plasmid-mediated 16S rRNA m1A1408 methyltransferase, NpmA, found in a clinically isolated Escherichia coli strain resistantto structurally diverse aminoglycosides. Antimicrob Agents Chemother. 2007;51:4401–4409. doi: 10.1128/AAC.00926-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagasawa M, Kaku M, Kamachi K, Shibayama K, Arakawa Y, Yamaguchi K, Ishii Y. Loop-mediated isothermal amplification assay for 16S rRNA methylase genes in Gram-negative bacteria. J Infect Chemother. 2014;20:635–638. doi: 10.1016/j.jiac.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Marques MB, Brookings ES, Moser SA, Sonke PB, Waites KB. Comparative in vitro antimicrobial susceptibilities of nosocomial isolates of Acinetobacter baumannii and synergistic activities of nine antimicrobial combinations. Antimicrob Agents Chemother. 1997;41:881–885. doi: 10.1128/aac.41.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen JT, Zhou Y, Yang L, Xu Y. Multidrug-resistantgenes of aminoglycoside-modifying enzymes and 16S rRNA methylases in Acinetobacter baumannii strains. Genet Mol Res. 2014;13:3842–3849. doi: 10.4238/2014.May.16.9. [DOI] [PubMed] [Google Scholar]
- 17.Chang Y, Luan G, Xu Y, Wang Y, Shen M, Zhang C, Zheng W, Huang J, Yang J, Jia X, Ling B. Characterization of carbapenem-resistant Acinetobacter baumannii isolates in a Chinese teaching hospital. Front Microbiol. 2015;6:910. doi: 10.3389/fmicb.2015.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walther-Rasmussen J, Høiby N. OXA-type carbapenemases. J Antimicrob Chemother. 2006;57:373–383. doi: 10.1093/jac/dki482. [DOI] [PubMed] [Google Scholar]
- 19.Doi Y, Adams JM, Yamane K, Paterson DL. Identification of 16S rRNA methylase-producing Acinetobacter baumannii clinical strains in North America. Antimicrob Agents Chemother. 2007;51:4209–4210. doi: 10.1128/AAC.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JW, Heo ST, Jin JS, Choi CH, Lee YC, Jeong YG, Kim SJ, Lee JC. Characterization of Acinetobacter baumannii carrying bla(OXA-23), bla(PER-1) and armA in a Korean hospital. Clin Microbiol Infect. 2008;14:716–718. doi: 10.1111/j.1469-0691.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhou H, Du XX, Yang Q, Zhou JY, Yu YS, Li LJ. Study on carbapenemase and 16S rRNA methylase of imipenem-resistant Acinetobacter baumannii. Zhonghua Liu Xing Bing Xue Za Zhi. 2009;30:269–272. (In Chinese) [PubMed] [Google Scholar]
- 22.Adams-Haduch JM, Paterson DL, Sidjabat HE, Pasculle AW, Potoski BA, Muto CA, Harrison LH, Doi Y. Genetic basis of multidrug resistance in Acinetobacter baumannii clinical isolates at a tertiary medical center in Pennsylvania. Antimicrob Agents Chemother. 2008;52:3837–3843. doi: 10.1128/AAC.00570-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung JY, Kwon KC, Cho HH, Koo SH. Antimicrobial resistance determinants in imipenem-nonsusceptible Acinetobacter calcoaceticus-baumannii complex isolated in Daejeon, Korea. Korean J Lab Med. 2011;31:265–270. doi: 10.3343/kjlm.2011.31.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karthikeyan K, Thirunarayan MA, Krishnan P. Coexistence of blaOXA-23 with blaNDM-1 and armA in clinical isolates of Acinetobacter baumannii from India. J Antimicrob Chemother. 2010;65:2253–2254. doi: 10.1093/jac/dkq273. [DOI] [PubMed] [Google Scholar]
- 25.Bonnin RA, Potron A, Poirel L, Lecuyer H, Neri R, Nordmann P. PER-7, an extended-spectrum beta-lactamase with increased activity toward broad-spectrum cephalosporins in Acinetobacter baumannii. Antimicrob Agents Chemother. 2011;55:2424–2427. doi: 10.1128/AAC.01795-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strateva T, Markova B, Marteva-Proevska Y, Ivanova D, Mitov I. Widespread dissemination of multidrug-resistant Acinetobacter baumannii producing OXA-23 carbapenemase and ArmA 16S ribosomal RNA methylase in a Bulgarian university hospital. Braz J Infect Dis. 2012;16:307–310. doi: 10.1590/S1413-86702012000300020. [DOI] [PubMed] [Google Scholar]
- 27.Brigante G, Migliavacca R, Bramati S, Motta E, Nucleo E, Manenti M, Migliorino G, Pagani L, Luzzaro F, Viganò FE. Emergence and spread of a multidrug-resistant Acinetobacter baumannii clone producing both the carbapenemase OXA-23 and the 16S rRNA methylase ArmA. J Med Microbiol. 2012;61:653–661. doi: 10.1099/jmm.0.040980-0. [DOI] [PubMed] [Google Scholar]
- 28.Saule M, Samuelsen Ø, Dumpis U, Sundsfjord A, Karlsone A, Balode A, Miklasevics E, Karah N. Dissemination of a carbapenem-resistant Acinetobacter baumannii strain belonging to international clone II/sequence type 2 and harboring a novel AbaR4-like resistance island in Latvia. Antimicrob Agents Chemother. 2013;57:1069–1072. doi: 10.1128/AAC.01783-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Revathi G, Siu LK, Lu PL, Huang LY. First report of NDM-1-producing Acinetobacter baumannii in East Africa. Int J Infect Dis. 2013;17:e1255–e1258. doi: 10.1016/j.ijid.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Bakour S, Alsharapy SA, Touati A, Rolain JM. Characterization of Acinetobacter baumannii clinical isolates carrying bla(OXA-23) carbapenemase and 16S rRNA methylase armA genes in Yemen. Microb Drug Resist. 2014;20:604–609. doi: 10.1089/mdr.2014.0018. [DOI] [PubMed] [Google Scholar]
- 31.Tada T, Miyoshi-Akiyama T, Shimada K, Shimojima M, Kirikae T. Dissemination of 16S rRNA methylase ArmA-producing Acinetobacter baumannii and emergence of OXA-72 carbapenemase coproducers in Japan. Antimicrob Agents Chemother. 2014;58:2916–2920. doi: 10.1128/AAC.01212-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tojo M, Mawatari M, Hayakawa K, Nagamatsu M, Shimada K, Mezaki K, Sugiki Y, Kuroda E, Takeshita N, Kutsuna S, et al. Multidrug-resistant Acinetobacter baumannii isolated from a traveler returned from Brunei. J Infect Chemothe. 2015;21:212–214. doi: 10.1016/j.jiac.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 33.El-Sayed-Ahmed MA, Amin MA, Tawakol WM, Loucif L, Bakour S, Rolain JM. High prevalence of bla(NDM-1) carbapenemase-encoding gene and 16S rRNA armA methyltransferase among Acinetobacter baumannii clinical isolates, Egypt. Antimicrob Agents Chemother. 2015;59:3602–3605. doi: 10.1128/AAC.04412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao WS, Liu GY, Mi ZH, Zhang F. Coexistence of blaOXA-23 with armA and novel gyrA mutation in a pandrug-resistant Acinetobacter baumannii isolate from the blood of a patient with haematological disease in China. J Hosp Infect. 2011;77:278–279. doi: 10.1016/j.jhin.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Zhou H, Zhang T, Yu D, Pi B, Yang Q, Zhou J, Hu S, Yu Y. Genomic analysis of the multidrug-resistant Acinetobacter baumannii strain MDR-ZJ06 widely spread in China. Antimicrob Agents Chemother. 2011;55:4506–4512. doi: 10.1128/AAC.01134-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Z, Ling B, Zhou L. Prevalence of 16S rRNA methylase, modifying enzyme, and extended-spectrum beta-lactamase genes among Acinetobacter baumannii isolates. J Chemother. 2015;27:207–212. doi: 10.1179/1973947814Y.0000000190. [DOI] [PubMed] [Google Scholar]
- 37.Chen TL, Siu LK, Wu RC, Shaio MF, Huang LY, Fung CP, Lee CM, Cho WL. Comparison of one-tube multiplex PCR, automated ribotyping and intergenic spacer (ITS) sequencing for rapid identification of Acinetobacter baumannii. Clin Microbiol Infect. 2007;13:801–806. doi: 10.1111/j.1469-0691.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Guo Q, Wang P, Zhu D, Ye X, Wu S, Wang M. Clonal dissemination of extensively drug-resistant Acinetobacter baumannii producing an OXA-23 β-lactamase at a teaching hospital in Shanghai, China. J Microbiol Immunol Infect. 2015;48:101–108. doi: 10.1016/j.jmii.2015.02.357. [DOI] [PubMed] [Google Scholar]
- 39.Clinical and Laboratory Standards Institute, corp-author. 24th Informational Supplement. CLSI; Wayne, PA, USA: M100. 2014. Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- 40.Li Y, Guo Q, Wang P, Zhu D, Ye X, Wu S, Wang M. Clonal dissemination of extensively drug-resistant Acinetobacter baumannii producing an OXA-23 β-lactamase at a teaching hospital in Shanghai, China. J Microbiol Immunol Infect. 2015;48:101–108. doi: 10.1016/j.jmii.2015.02.357. [DOI] [PubMed] [Google Scholar]
- 41.Valenzuela JK, Thomas L, Partridge SR, van der Reijden T, Dijkshoorn L, Iredell J. Horizontal gene transfer in a polyclonal outbreak of carbapenem-resistant Acinetobacter baumannii. J Clin Microbiol. 2007;45:453–460. doi: 10.1128/JCM.01971-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsakris A, Pournaras S, Woodford N, Palepou MF, Babini GS, Douboyas J, Livermore DM. Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece. J Clin Microbiol. 2000;38:1290–1292. doi: 10.1128/jcm.38.3.1290-1292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellington MJ, Kistler J, Livermore DM, Woodford N. Multiplex PCR for rapid detection of genes encoding acquired metallo-beta-lactamases. J Antimicrob Chemother. 2007;59:321–322. doi: 10.1093/jac/dkl481. [DOI] [PubMed] [Google Scholar]
- 44.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1) and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, Amyes SG, Livermore DM. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006;27:351–353. doi: 10.1016/j.ijantimicag.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Fu Y, Zhou J, Zhou H, Yang Q, Wei Z, Yu Y, Li L. Wide dissemination of OXA-23-producing carbapenem-resistant Acinetobacter baumannii clonal complex 22 in multiple cities of China. J Antimicrob Chemother. 2010;65:644–650. doi: 10.1093/jac/dkq027. [DOI] [PubMed] [Google Scholar]
- 47.Netsvyetayeva I, Sikora M, Golas M, Swoboda-Kopec E, de Walthoffen SW, Dembicka O, Fraczek M, Mlynarczyk A, Pacholczyk M, Chmura A, Mlynarczyk G. Acinetobacter baumannii multidrug-resistant strain occurrence in liver recipients with reference to other high-risk groups. Transplant Proc. 2011;43:3116–3120. doi: 10.1016/j.transproceed.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S. The population structure of Acinetobacter baumannii: Expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One. 2010;5:e10034. doi: 10.1371/journal.pone.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartual SG, Seifert H, Hippler C, Luzon MA, Wisplinghoff H, Rodríguez-Valera F. Development of a multilocus sequence typing scheme for characterization of clinical isolates of Acinetobacter baumannii. J Clin Microbiol. 2005;43:4382–4390. doi: 10.1128/JCM.43.9.4382-4390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.PubMLST, corp-author. http://pubmlst.org/abaumannii/ Acinetobacter baumannii MLST Databases. [Google Scholar]
- 51.Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. eBURST: Inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J Bacteriol. 2004;186:1518–1530. doi: 10.1128/JB.186.5.1518-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agadzhanian VV, Semenikhin VA, Iu S Fedorov, Krasulina GP, Gaǐfulina IM, Mironova LA. Experience of health protection center on organization of medical care for coal miners in Kuzbass. Med Tr Prom Ekol. 2002:27–30. (In Russian) [PubMed] [Google Scholar]
- 53.Tada T, Miyoshi-Akiyama T, Kato Y, Ohmagari N, Takeshita N, Hung NV, Phuong DM, Thu TA, Binh NG, Anh NQ, et al. Emergence of 16S rRNA methylase-producing Acinetobacter baumannii and Pseudomonas aeruginosa isolates in hospitals in Vietnam. BMC Infect Dis. 2013;13:251. doi: 10.1186/1471-2334-13-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nie L, Lv Y, Yuan M, Hu X, Nie T, Yang X, Li G, Pang J, Zhang J, Li C, et al. Genetic basis of high level aminoglycoside resistance in Acinetobacter baumannii from Beijing, China. Acta Pharm Sin B. 2014;4:295–300. doi: 10.1016/j.apsb.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao SZ, Han LZ, Chu HQ, Zhao L, Chen X, Ni YX. Detection of aminoglycoside resistance related genes in multidrug-resistant Acinetobacter baumannii isolated from a single institute of Shanghai, China. Panminerva Med. 2015;57:49–53. [PubMed] [Google Scholar]
- 56.Bakour S, Touati A, Bachiri T, Sahli F, Tiouit D, Naim M, Azouaou M, Rolain JM. First report of 16S rRNA methylase ArmA-producing Acinetobacter baumannii and rapid spread of metallo-β-lactamase NDM-1 in Algerian hospitals. J Infect Chemother. 2014;20:696–701. doi: 10.1016/j.jiac.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Azimi L, Talebi M, Pourshafie MR, Owlia P, Lari A Rastegar. Characterization of carbapenemases in extensively drug resistance Acinetobacter baumannii in a burn care center in Iran. Int J Mol Cell Med. 2015;4:46–53. [PMC free article] [PubMed] [Google Scholar]
- 58.Jia X, Zhang J, Sun W, He W, Jiang H, Chen D, Murchie AI. Riboswitch control of aminoglycoside antibiotic resistance. Cell. 2013;152:68–81. doi: 10.1016/j.cell.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 59.Haldorsen BC, Simonsen GS, Sundsfjord A, Samuelsen Ø, Norwegian Study Group on Aminoglycoside Resistance Increased prevalence of aminoglycoside resistance in clinical isolates of Escherichia coli and Klebsiella spp. in Norway is associated with the acquisition of AAC(3)-II and AAC(6′)-Ib. Diagn Microbiol Infect Dis. 2014;78:66–69. doi: 10.1016/j.diagmicrobio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 60.Soleimani N, Aganj M, Ali L, Shokoohizadeh L, Sakinc T. Frequency distribution of genes encoding aminoglycoside modifying enzymes in uropathogenic E. coli isolated from Iranian hospital. BMC Res Notes. 2014;7:842. doi: 10.1186/1756-0500-7-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miro E, Grünbaum F, Gomez L, Rivera A, Mirelis B, Coll P, Navarro F. Characterization of aminoglycoside-modifying enzymes in enterobacteriaceae clinical strains and characterization of the plasmids implicated in their diffusion. Microb Drug Resist. 2013;19:94–99. doi: 10.1089/mdr.2012.0125. [DOI] [PubMed] [Google Scholar]
- 62.Akers KS, Chaney C, Barsoumian A, Beckius M, Zera W, Yu X, Guymon C, Keen EF, III, Robinson BJ, Mende K, Murray CK. Aminoglycoside resistance and susceptibility testing errors in Acinetobacter baumannii-calcoaceticus complex. J Clin Microbiol. 2010;48:1132–1138. doi: 10.1128/JCM.02006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nowak P, Paluchowska PM, Budak A. Co-occurrence of carbapenem and aminoglycoside resistance genes among multidrug-resistant clinical isolates of Acinetobacter baumannii from Cracow, Poland. Med Sci Monit Basic Res. 2014;20:9–14. doi: 10.12659/MSMBR.889811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamouda A, Evans BA, Towner KJ, Amyes SG. Characterization of epidemiologically unrelated Acinetobacter baumannii isolates from four continents by use of multilocus sequence typing, pulsed-field gel electrophoresis and sequence-based typing of bla(OXA-51-like) genes. J Clin Microbiol. 2010;48:2476–2483. doi: 10.1128/JCM.02431-09. [DOI] [PMC free article] [PubMed] [Google Scholar]