Abstract
Membrane trafficking is central to construction of the cell plate during plant cytokinesis. Consequently, a detailed understanding of the process depends on the characterization of molecules that function in the formation, transport, targeting, and fusion of membrane vesicles to the developing plate, as well as those that participate in its consolidation and maturation into a fully functional partition. Here we report the initial biochemical and functional characterization of patellin1 (PATL1), a novel cell-plate-associated protein that is related in sequence to proteins involved in membrane trafficking in other eukaryotes. Analysis of the Arabidopsis genome indicated that PATL1 is one of a small family of Arabidopsis proteins, characterized by a variable N-terminal domain followed by two domains found in other membrane-trafficking proteins (Sec14 and Golgi dynamics domains). Results from immunolocalization and biochemical fractionation studies suggested that PATL1 is recruited from the cytoplasm to the expanding and maturing cell plate. In vesicle-binding assays, PATL1 bound to specific phosphoinositides, important regulators of membrane trafficking, with a preference for phosphatidylinositol(5)P, phosphatidylinositol(4,5)P2, and phosphatidylinositol(3)P. Taken together, these findings suggest a role for PATL1 in membrane-trafficking events associated with cell-plate expansion or maturation and point to the involvement of phosphoinositides in cell-plate biogenesis.
Plant cells partition their cytoplasm during cytokinesis by building a new cell wall from the inside-out between the two sets of daughter chromosomes (Staehelin and Hepler, 1996; Heese et al., 1998; Smith, 1999; Otegui and Staehelin, 2000a; Assaad, 2001). Construction of this new partition is dominated by membrane-trafficking events (Bednarek and Falbel, 2002). The process begins when the phragmoplast, a plant-specific cytoskeletal array, is assembled from the remnants of the mitotic spindle in late anaphase and guides Golgi-derived vesicles carrying cell wall materials to the plane of division. There they fuse to initiate formation of the cell plate, a transient disc-shaped membranous network that undergoes a complex series of transformations over the course of its development (Samuels et al., 1995; Segui-Simarro et al., 2004). The cell plate expands centrifugally by the addition of vesicles to its periphery, accompanied by depolymerization of phragmoplast microtubules from the center and their repolymerization at the edge (Zhang et al., 1990; Asada et al., 1991). An actomyosin-dependent mechanism guides the expanding cell plate to a previously determined cortical division site where it fuses with the parent cell membrane (Valster et al., 1997; Granger and Cyr, 2001; Molchan et al., 2002). Upon fusion, fenestrae in the plate are filled in by the addition of new vesicles. A complex maturation process, involving membrane recycling and cellulose biosynthesis, yields a fully functional and integrated partition (Samuels et al., 1995; Moore and Staehelin, 1998; Nickle and Meinke, 1998; Zuo et al., 2000; Segui-Simarro et al., 2004).
While the events of plant cytokinesis are known in detail at an ultrastructural level (Samuels et al., 1995; Otegui and Staehelin, 2000b, 2004; Otegui et al., 2001; Segui-Simarro et al., 2004), our understanding at the molecular level is quite limited. Significant progress has been made, however, by analysis of mutants that are defective in construction of the cell plate, typified by multinucleate cells and cell wall stubs and by studies of proteins that localize to the cell plate. Not surprisingly, many of the molecules that function in cell-plate biogenesis are related to proteins involved in membrane-trafficking in other eukaryotes (Bednarek and Falbel, 2002). Several kinesin-related proteins function specifically during cytokinesis. AtPAKRP2, which localizes to brefeldin A-sensitive puncta during early plate development, is a likely candidate for the motor that drives vesicle movement along the phragmoplast microtubules (Lee et al., 2001; Smith, 2002), and the kinesin-related protein HINKEL/NACK1 functions in microtubule dynamics during plate expansion in collaboration with a mitogen-activated protein kinase-signaling pathway (Nishihama et al., 2002; Strompen et al., 2002). Proteins implicated in vesicle formation and membrane remodeling during cytokinesis include dynamin-like proteins such as phragmoplastin in soybean (Glycine max; Gu and Verma, 1996, 1997) and ADL1A and ADL1E, which play an essential role in cytokinesis and polar cell growth in Arabidopsis (Kang et al., 2001, 2003). A growing number of SNAREs and other proteins involved in vesicle docking/fusion events localize to the cell plate and are also critical for normal cytokinesis. These include the SNAREs, KNOLLE (Lukowitz et al., 1996; Lauber et al., 1997), and AtSNAP33 (Heese et al., 2001) and KEULE, a Sec1 family protein likely to function in SNARE complex regulation (Assaad et al., 2001). In addition, the novel plant SNARE, NSPN11, localizes primarily to the developing cell plate and cofractionates with KNOLLE, evidence that it too is involved in vesicle fusion events at the cell plate (Zheng et al., 2002). AAA proteins (ATPases associated with various cellular activities) that regulate vesicle fusion events by disassembling the SNARE complexes to allow for subsequent rounds of fusion (May et al., 2001) also appear to be important for membrane traffic at the cell plate. The Arabidopsis ortholog of one of these, CDC48, colocalizes at the cell plate with KNOLLE and another plate-associated SNARE, SYP31. KNOLLE was also detected in a complex with a Sec18p/NSF AAA protein (Rancour et al., 2002). GTP-binding proteins of two families that function in membrane trafficking, Rho and Rab, have also been implicated in cell-plate biogenesis. Rho-related GTPases localize to the cell plate (Molendijk et al., 2001), and the scd1 mutant of Arabidopsis, which displays defects in guard cell cytokinesis, accompanied by the accumulation of secretory vesicles, contains a domain found in other proteins that interact with Rab proteins (Falbel et al., 2003).
We report here the identification and initial characterization of patellin1 (PATL1), a novel cell-plate-associated protein that is related in sequence to proteins involved in membrane trafficking in other eukaryotes. Based on its cell-plate localization we have chosen the name patellin from the Latin “patella,” which means “small plate.” PATL1 is one of a small family of Arabidopsis proteins characterized by two domains found in other membrane-trafficking proteins, a Sec14 lipid-binding domain and a Golgi dynamics (GOLD) domain. GOLD domains are found in a diverse group of proteins involved in Golgi function and vesicle traffic, where they are thought to participate in protein-protein interactions (Anantharaman and Aravind, 2002). Sec14p is the defining member of one of two families of eukaryotic proteins, originally defined by their ability to transfer phosphatidylinositol (PtdIns) and/or phosphatidylcholine (PtdCh) monomers between membrane bilayers (Allen-Baume et al., 2002). Sec14p, first identified in yeast (Saccharomyces cerevisiae), plays an essential role in the formation and exit of secretory vesicles from the trans-Golgi network (TGN; Bankaitis et al., 1989, 1990). The second family of these so-called PtdIns lipid transfer proteins (PITPs) is specific to animal cells and includes PITPα, the defective protein in mice with the vibrator neurodegenerative disorder (Hamilton et al., 1997), and the retinal degeneration protein B of Drosophila (Milligan et al., 1997). PITPs and related proteins of both families play diverse roles in membrane trafficking, the regulation of phospholipid metabolism, and phosphoinositide-signaling pathways (Kearns et al., 1998a; Li et al., 2000b; Hsuan and Cockcroft, 2001; Allen-Baume et al., 2002).
Proteins related to Sec14p have more recently been discovered in plants, mammals, and fungi. Yeast contains four partially redundant SEC14 homologs (SFH2, SFH3, SFH4, and SFH5) that play additional roles including the activation of phospholipase D and perhaps phosphoinositide and sterol production (van den Hazel et al., 1999; Li et al., 2000a; Wu et al., 2000). In mammalian cells the Sec14 family includes proteins that bind and/or transfer α-tocopherol- and cis-retinaldehyde and a supernatant protein factor that regulates cholesterol synthesis (Stocker and Baumann, 2003). In addition, mammalian cells contain a growing list of proteins that contain a Sec14 lipid-binding domain, including the human MEG2 protein tyrosine phosphatase that induces vesicle fusion in T cells and exchange factors and activating proteins for Rho family GTPases including members of the multifunctional Dbl family (Gu et al., 1992; Aravind et al., 1999; Huynh et al., 2003). In plants, several SEC14-like proteins have been described, and they all exhibit approximately 25% amino acid identity with Sec14p and complement the sec14-1ts mutant. The soybean proteins, Ssh1p and Ssh2p (soybean SEC14 homolog-1 and -2), are distinct from Sec14p in their lipid transfer and binding activities (Kearns et al., 1998b). Ssh1p functions during a hyperosmotic stress-induced signaling pathway and regulates the activity of PtdIns kinases, while the function of Ssh2p is yet to be determined (Monks et al., 2001). The Arabidopsis sec14-like protein, AtSEC14, which was cloned by screening for sequences that rescued the yeast sec14-1ts mutant, also transfers PtdIns (Jouannic et al., 1998). Although its biological function is unknown, recent studies are consistent with a role in vesicle trafficking; AtSEC14 was shown to copurify on a naphthylphthalmic acid affinity resin with proteins involved in vesicle traffic between the TGN, plasma membrane, and endocytic compartments, including ADL1 and β-adaptin (Murphy et al., 2002). Nitrogen-fixing root nodules also contain Sec14-like PITPs. A nodule-specific PITP gene family was identified in Lotus that is subject to elaborate transcriptional control during nodule development, where these proteins may function in lipid-signaling pathways that regulate membrane biogenesis during nodulation (Kapranov et al., 2001).
In this study we provide evidence for the involvement of a novel Sec14-like protein, PATL1, in plant cytokinesis. We show that PATL1 localizes to the somatic cell plate coincident with its expansion and maturation. We also show that PATL1 is a peripheral membrane protein that binds phosphoinositides with a preference for PtdIns(5)P, PtdIns(4,5)P2, and PtdIns3P. Taken together these results suggest a role for PATL1 in membrane-trafficking events associated with cell-plate expansion and maturation and point to the involvement of phosphoinositides in cell-plate biogenesis.
RESULTS
The Arabidopsis PATL Gene Family
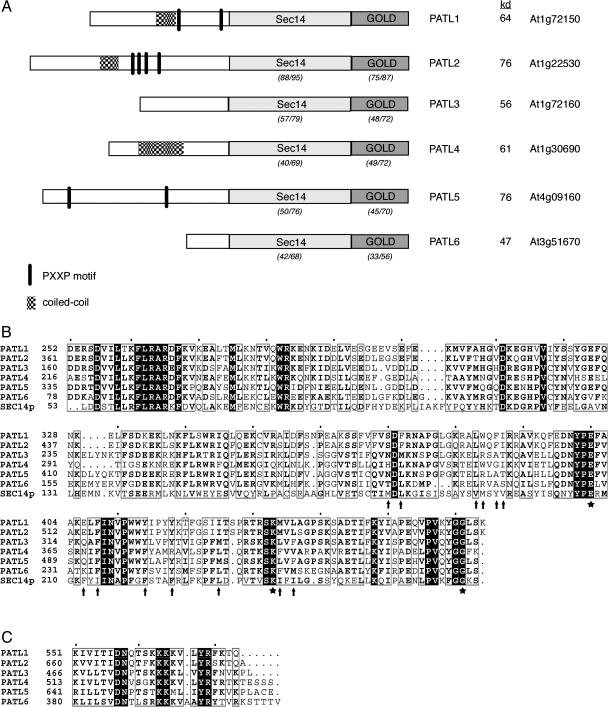
PATL1 was originally identified from a tryptic peptide sequence of a protein that copurified with a zucchini (Cucurbita pepo) membrane-associated F-actin-binding protein. Using this peptide sequence we cloned and sequenced the corresponding full-length PATL1 cDNA from Arabidopsis using standard reverse transcription-PCR methods. A search of the Arabidopsis genome database at the National Center for Biotechnology Information (NCBI) identified five additional proteins that show significant similarity in their overall amino acid sequence (27%–57% identity/44%–67% similarity) and protein domain structure (Fig. 1). Expressed sequence tag (EST)/cDNAs have been reported in the public databases for all of the PATLs, indicating that all members of the gene family are expressed. Furthermore, transcription of PATL1, PATL2, PATL3, PATL4, and PATL6 was confirmed by whole genome array analysis (Yamada et al., 2003). The predicted PATL proteins (PATL1, At1g72150; PATL2, At1g22530; PATL3, At1g72160; PATL4, At1g30690; PATL5, At4g09160; and PATL6, At3g51670) range in mass from 47 to 76 kD.
Figure 1.
Arabidopsis PATL protein structures. A, Diagram of the domain structure of Arabidopsis PATLs. The percentage amino acid identity/similarity between the Sec14 and GOLD domains of PATL1 and the other patellin family members is shown in parentheses below the domains. Pairwise analysis was performed using ClustalW with a gap opening penalty of 10 and a gap extension penalty of 0.2. The molecular masses predicted from the open reading frame sequences are shown. B, Amino acid sequence alignments of the Sec14 domain of the Arabidopsis PATLs and yeast Sec14p. Sec14p residues involved in PtdIns transfer activity are marked with a star, while hydrophobic residues that line the lipid-binding pocket are indicated by arrows. C, Amino acid sequence alignments of the C-terminal Lys-rich motif of the PATLs.
The domain structure of the Arabidopsis PATLs is shown in Figure 1A. The PATL proteins possess an N-terminal region that is variable in length (81–357 amino acids) and sequence composition. In all of the family members, except for PATL6, this domain is very acidic (pI approximately 4) with an unusually high percentage of charged amino acids (30%–48%), especially Glu (19%–27%). The N termini of PATL1, PATL2, and PATL4 contain numerous EEK repeats, reminiscent of those found in the neurofilament triplet H proteins (Herrmann and Aebi, 2000). Their N-terminal sequences also predict a coiled coil, a common protein oligomerization-folding motif (Burkhard et al., 2001). In addition, the N termini of PATL1 and PATL5 contain several PXXP sequences consistent with binding to SH3 protein interaction domains (Feng et al., 1994). SH3 domains participate in protein-protein interactions involved in cytoskeletal dynamics, tyrosine kinase-signaling pathways, and vesicular trafficking (Kay et al., 2000).
Analysis with InterProScan (http://www.ebi.ac.uk/InterProScan/) revealed that the PATLs belong to the Sec14 family of PITPs. The variable N-terminal domain of the PATLs is followed by a region of similarity to the yeast PITP, Sec14p (approximately 26%/48% amino acid similarity/identity; Fig. 1B). The SEC14 domain of the PATLs includes a region of similarity to the last 74 residues of the N terminus of Sec14p followed by its C-terminal phospholipid-binding pocket. The small α-helical N-terminal domain of Sec14p contains most of the first 129 residues known to be sufficient for its stable association with Golgi membranes (Sha et al., 1998). The PATLs, however, are missing the first 55 residues of this region. The SEC14 domains of PATL1 and PATL2 are the most closely related to each other, with 88% amino acid sequence identity and 95% similarity. In contrast, the SEC14 domains of the other PATLs show only 40% to 57% identity and 69% to 79% similarity when compared to PATL1.
An alignment of the PATL SEC14 domain sequences with the C-terminal lipid-binding pocket of Sec14p suggested that the PATLs may interact with phospholipids (Fig. 1B). The Sec14p residues that have been shown by mutational analysis to be critical for PtdIns-binding and/or transfer (E207, K239, and G266) are perfectly conserved in all of the PATLs. The molecular basis of the sec14-1ts allele is a G266D substitution, which results in destabilization of the hydrophobic pocket (Cleves et al., 1989), while E207 and K239 form a salt bridge that has been conserved in all Sec14 proteins that either bind or transfer PtdIns (Li et al., 2000b). Moreover, many of the hydrophobic amino acids that line the hydrophobic pocket of Sec14p (M177, L179, V190, M191, Y193, V194, F212, I214, F221, F225, L232, I240, and L242) are also conserved in the PATLs (Sha et al., 1998; Sha and Luo, 1999). This is particularly striking for PATL1 and PATL2, where all but two of these sites are occupied by hydrophobic residues.
Analysis of the PATL sequences with the ScanProsite program (http://ca.expasy.org/tools/scanprosite) revealed that all members of the family also contain a conserved C-terminal GOLD domain. GOLD domains are found in a number of eukaryotic proteins involved in Golgi function and lipid traffic including the p24 family of proteins involved in traffic between the endoplasmic reticulum and Golgi, the yeast cytoplasmic oxysterol-binding protein, Osh3p, the human Golgi protein, GCP60, and animal Sec14-like proteins (Anantharaman and Aravind, 2002). The very C-terminal end of the GOLD domain of the PATLs is Lys rich and contains a conserved motif, K(X10)(K/R) KKK/M(φ2–3) YR, that is reminiscent of the PtdIns(4,5)P2-binding motif, K(X9) KX(K/R)(H/Y), found in two proteins involved in clathrin-coated vesicle formation, AP180 and μ2-adaptin (Fig. 1C; Ford et al., 2001; Mao et al., 2001; Rohde et al., 2002). As is true for their Sec14 domains, the GOLD domains of PATL1 and PATL2 are most closely related with 75% identity and 87% similarity in this region. In contrast, the GOLD domains of the other PATLs show only 33% to 49% identity and 56% to 72% similarity when compared to PATL1.
Production and Characterization of PATL1 Protein and Antibodies
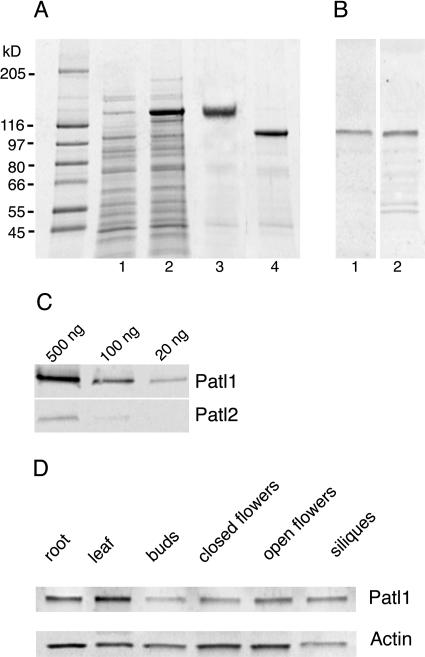
Recombinant PATL1 was produced in Escherichia coli for use in lipid-binding assays and the production of polyclonal antisera. PATL1 was expressed in E. coli as a glutathione S-transferase (GST) fusion protein in the vector pGEX6P-1 (Amersham Biosciences, Piscataway, NJ; Fig. 2A, lanes 1 and 2). The resulting fusion protein was affinity purified on GST-Sepharose (Amersham Biosciences; Fig. 2A, lane 3), cleaved with PreScission protease (Amersham Biosciences), and affinity purified again to remove the GST tag (Fig. 2A, lane 4). Surprisingly, the purified PATL1 has an apparent molecular mass of 105 kD on SDS-PAGE, while its predicted mass is only 64 kD. This discrepancy is most likely due to poor binding of SDS by the acidic N-terminal domain. Anomalous migration of acidic proteins in SDS gels is common (Armstrong and Roman, 1993). Furthermore, a truncated PATL1 that lacks the acidic N-terminal domain does not show this anomalous migration (data not shown). A protein that consists only of the C-terminal 190 amino acids of PATL1 was also expressed and purified (data not shown).
Figure 2.
PATL1 antibody production and characterization. The Arabidopsis PATL1 coding region was expressed as a GST-fusion in E. coli and purified for biochemical analysis and antibody production. A, SDS-PAGE of uninduced (lane 1) and induced (lane 2) E. coli extracts, purified GST-patellin (lane 3), and cleaved fusion protein (lane 4). Molecular mass markers are shown at left. B, Immunoblots of Arabidopsis root total protein extracts (10 μg/lane) probed with PATL1 antisera raised against full-length protein (lane 1) or the GOLD domain (lane 2). C, Immunoblots of PATL1 and PATL2-GST fusion proteins probed with PATL1 antibody. D, Immunoblots of Arabidopsis organ extracts (10 μg/lane) probed with PATL1 or actin antibodies.
Both the full-length and GOLD domain proteins were used for antibody production in rabbits. The resulting antibodies to the full-length protein reacted with a single band of 105 kD in immunoblots of Arabidopsis root extracts (Fig. 1B, lane 1), which comigrated with the bacterially expressed PATL1 (Fig. 1A, lane 4). The antiserum raised against the GOLD domain also reacted with a 105-kD protein; however, significant cross reaction to several smaller proteins was also seen (Fig. 1B, lane 2).
Occasionally, an additional very faint band with an apparent molecular mass of 125 kD was detected in tissue extracts with the antiserum to full-length PATL1, most likely due to a weak cross reaction with PATL2 (data not shown). To test directly for cross reaction with PATL2 (the PATL family member most similar in sequence to PATL1), immunoblots of PATL1- and PATL2-GST fusion proteins were probed with anti-PATL1. The results, shown in Figure 2C, revealed a very weak cross reaction; the signal obtained with only 20 ng of PATL1 was similar to that seen with 500 ng of PATL2, indicating an approximately 25-fold difference in sensitivity. We believe it is highly unlikely that the PATL1 antiserum cross-reacts with PATL3, 4, 5, and 6, given the high degree of sequence divergence between these antigens. Given its high degree of specificity, the antiserum to the full-length PATL1 was used for the remaining experiments.
PATL1 Is Ubiquitously Expressed in Arabidopsis Organs
Immunoblots of protein extracts of Arabidopsis organs were performed to determine the distribution of the PATL1 throughout the plant (Fig. 2D). An actin antibody was used as a positive control. PATL1 was detected in all tissues examined, including young roots and leaves, flowers at different stages of development, and green siliques. PATL1 was most abundant in expanding leaves and roots. PATL1 levels were low in flower buds but increased severalfold during flower development. The protein was also detected in developing siliques.
PATL1 Is a Peripheral Membrane Protein
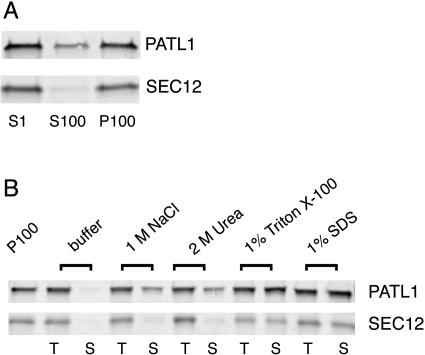
Biochemical fractionation studies were performed to determine the intracellular distribution of PATL1. A postnuclear supernatant (S1) prepared from Arabidopsis roots was ultracentrifuged at 100,000g to produce soluble cytosolic (S100) and microsomal membrane (P100) fractions. The resulting fractions were then analyzed by immunoblots using the antibodies to the full-length PATL1 as well as an integral membrane marker, AtSEC12 (Bar-Peled and Raikhel, 1997; Fig. 3A). While PATL1 was found in both the soluble and membrane fractions, the majority of the protein (approximately 80%) was associated with the membrane. AtSEC12, an integral membrane protein marker, was found in the P100 fraction as predicted. These results suggested that PATL1 exists in a cytoplasmic pool that can associate peripherally with intracellular membranes.
Figure 3.
PATL1 is a peripheral membrane protein. A, Immunoblots of Arabidopsis root subcellular fractions. Arabidopsis root extracts were centrifuged at 1,000g to yield a total cell extract (S1). The S1 fraction was centrifuged at 100,000g to yield soluble (S100) and membrane (P100) fractions. The S1, S100, and P100 fractions were analyzed by immunoblotting with antibodies to PATL1 and the integral membrane marker SEC12. Similar results were obtained in three independent experiments. B, Samples of the P100 fraction were washed in the indicated solutions. A total sample (T) was collected prior to centrifugation at 100,000g to yield the solubilized protein (S). Membrane equivalents of the T and S samples were analyzed by immunoblotting with antibodies to PATL1 or SEC12. Similar results were obtained in two independent experiments.
To determine the biochemical nature of the interaction of PATL1 with the membrane, we extracted samples of the pelleted membranes (P100) with buffers containing 1 m NaCl, 2 m urea, 1% (v/v) Triton X-100, or 1% (w/v) SDS (Fig. 3B). Both PATL1 and the integral membrane protein, AtSEC12, were completely solubilized by the detergent treatments. Approximately 50% of the PATL1 was also extracted by 1 m NaCl or 2 m urea, consistent with a peripheral membrane association. Also consistent with this conclusion is the observation that PATL1 was almost completely extracted by 0.1 m NaOH (data not shown). Partial extractability by either NaCl or urea suggests that both electrostatic interactions and hydrogen bonds stabilize the PATL1 interaction with the membrane.
PATL1 Localizes to the Cell Plate in Arabidopsis Roots
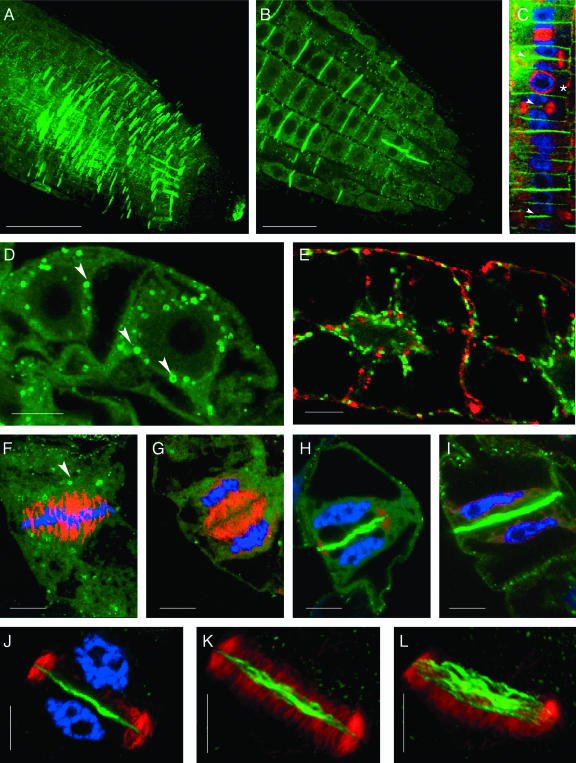
Immunolocalization studies of Arabidopsis roots were performed to provide insight into the cellular function of PATL1. Labeling was not detected with the preimmune serum (data not shown). Diffuse cytoplasmic staining for PATL1 was detected throughout the root tip, with the exception of the meristematic zone (Fig. 4A). Within the elongation zone, where newly born cells continue to divide and begin expansion, labeling was most intense on recently completed cell plates and punctate cytoplasmic structures (Fig. 4, A and B). PATL1 label was strongest on approximately every other transverse wall, consistent with persistence of PATL1 at the recently completed cell plates for a time following the completion of cytokinesis. Consequently, the PATL1 labeling pattern appears to provide a record of recently completed cell divisions.
Figure 4.
Immunolocalization of PATL1 in Arabidopsis roots and tobacco BY2 cells. Arabidopsis roots were immunolabeled and imaged by confocal microscopy. A, Confocal projection compiled from 1 μm sections through an Arabidopsis root tip shows cell-plate labeling for PATL1 (green) in the zone of cell division. B, Single optical section from the projection shown in A shows PATL1 staining of cell plates and punctate cytoplasmic structures. Bars in A and B = 20 μm. C, Colocalization of PATL1 (green) with tubulin (red) and DNA (blue) in a file of cortical cells. Arrowheads mark the sites of PATL1 staining of expanding cell plates. The asterisk marks a cell with a preprophase band. D, Interphase cells stained for PATL1 (green). E, Interphase cells expressing Golgi-associated GmMan1::GFP (green) and immunostained for PATL1 (red). F to L, Colocalization of PATL1 (green) with tubulin (red) and DNA (blue). Single optical sections midway through the division plane are shown in F to J. Interphase (D) and metaphase (F) cells show PATL1 labeling of 1 μm cytoplasmic structures (marked with arrowheads). Early- (G), late- (H), and post- (I) telophase cells show PATL1 staining of the developing cell plate. Three-dimensional computer reconstructions built from 0.2-μm sections through the top half of the phragmoplast shown in J are projected at 10° and 20° from the optical axis in K and L, respectively. These images show that PATL1 at the plate is fibrillar in structure. Bars in D to L = 10 μm.
To facilitate identification of PATL1's time of arrival at the cell plate, roots were triple labeled for PATL1, tubulin, and nuclei. In the file of rapidly dividing cortical cells shown in Figure 4C, PATL1 label was most pronounced at cell plates in which the microtubules had been displaced to the periphery of the centrifugally expanding phragmoplasts (marked with arrows). These results suggest a role for PATL1 in the later stages of cell-plate formation.
PATL1 Localization in Dividing Tobacco BY2 Cells
Immunolocalization studies of tobacco (Nicotiana tabacum) BY2 cells revealed that they too contain a cell-plate-associated PATL1 antigen. Logarithmically growing BY2 cells were chemically fixed and immunostained for PATL1 and tubulin and stained for DNA with TO-PRO-3. In interphase (Fig. 4D) and metaphase (Fig. 4F) cells, PATL1 was detected in the cytoplasm and in punctate Golgi-sized structures, many of which showed a doughnut-like pattern similar to that previously described for Golgi labeled with a Golgi-targeted green fluorescent protein (GFP; GmMan1::GFP; Nebenfuhr et al., 1999). In those experiments the “doughnuts” were thought to represent a face on view of Golgi stacks where the GmMan1::GFP fusion protein was restricted to the rim of the cisternae. During metaphase the PATL1-labeled structures accumulated at the spindle poles (Fig. 4F) as has been reported for Golgi (Nebenfuhr et al., 2000). To determine if the PATL1-labeled structures are in fact Golgi, we performed immunolocalization experiments using BY2 cells with a Golgi-targeted GFP (GmMan1::GFP; Nebenfuhr et al., 1999). The results, shown in Figure 4E, revealed that PATL1 does not localize to the Golgi; there was no colocalization of PATL1 with GmMan1::GFP. The PATL1-labeled structures may represent endosomal compartments. Ring-like structures have been reported for the early endosomal compartments labeled by the Rab GTPases, Ara6, and Ara7 (Ueda et al., 2001).
An examination of the cell plate at different stages of development (Fig. 4, G–I) revealed that PATL1 label first appeared at the plate during late telophase. PATL label was not detected at the cell plate of early telophase cells in which remnants of the spindle microtubules were still evident (Fig. 4G) but was observed in late telophase cells in which the phragmoplast was undergoing radial expansion with concomitant displacement of the microtubules to the periphery (Fig. 4, H and J). Many of the expanding phragmoplasts we observed, like the one shown in Figure 4H, were anchored at one side of the cell as has been previously described (Culter and Ehrhardt, 2002). Staining of the newly completed cell plate persisted for a time posttelophase and was typically observed in cells in which the two new daughter nuclei were still close to the recently completed partition (Fig. 4I). These results are similar to those observed in Arabidopsis roots and are consistent with a model in which PATL1 is recruited from the cytoplasm to the division plane at the time of cell-plate expansion.
To determine if the plate-associated PATL1 was evenly distributed throughout the plate or concentrated at the site of new vesicle addition at the periphery, we performed a three-dimensional computer reconstruction of a cell plate undergoing phragmoplast expansion (Fig. 4J). Projections of this reconstruction, shown in Figure 4, K and L, revealed that PATL1 is distributed across the plate and is fibrillar in nature. The signal was weakest at the periphery. Thus, PATL1 is not concentrated where new vesicles are being added to the expanding plate but rather in the region of the plate where the maturation process is about to begin (Samuels et al., 1995; Segui-Simarro et al., 2004). These results are in marked contrast to those observed for proteins, such as AtPAKRP2 (Lee et al., 2001) and KNOLLE (Lauber et al., 1997) involved in the initial vesicle traffic to the plate and early vesicle fusion events, respectively.
PATL1 Binds Phosphoinositides
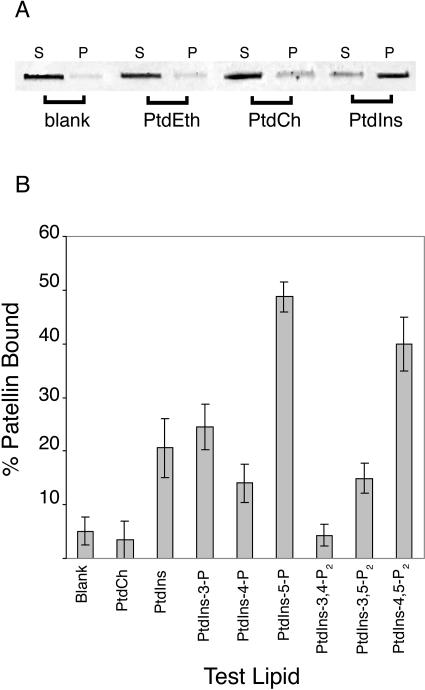
We were interested to explore the ability of PATL1 to interact with phospholipids, given the presence of the SEC14 phospholipid-binding domain and Lys-rich C-terminal motif. We first tested the ability of PATL1 to bind to unilamellar membrane vesicles composed of phosphatidylethanolamine (PtdEth), PtdCh, or PtdIns alone. Vesicle-bound PATL1 was separated from unbound protein by centrifugation of the reaction mixture at 164,000g. PATL1 in the supernatant (unbound) and the pellet (vesicle-bound) was then analyzed on SDS gels (Fig. 5A). To control for nonspecific pelleting of the protein, a blank with protein alone was run. PATL1 did not bind to the PtdEth vesicles but did bind to vesicles of PtdCh and PtdIns, both phospholipids transferred by yeast Sec14p. Under the conditions reported here, approximately 10% of the PATL1 bound to the PtdCh vesicles while over 90% bound to vesicles made of PtdIns.
Figure 5.
PATL1 binds phosphoinositides. Vesicle cosedimentation assays were used to test PATL1 protein for phospholipid-binding activity. A, PATL1 (2.5 μg) was mixed with unilamellar vesicles composed of 25 mm PtdEth, PtdCh, or PtdIns. Vesicle bound proteins (P) were separated from unbound proteins (S) by centrifugation at 164,000g and analyzed by fluorescence scanning of SYPRO red-stained SDS gels. PATL1 protein alone was used as a blank. B, PATL1 binding to phosphoinositides was tested as described above except that unilamellar vesicles were made from a mixture of 10:1 PtdEth:test phosphoinositide and the lipid concentration was 2.2 mm. The percentage of patellin bound was quantified using ImageQuant software. Values are the mean ± se from at least three different experiments.
PATL1's preference for PtdIns raised the possibility that it might bind to derivatives of PtdIns as well. The D-3, D-4, and D-5 hydroxyl groups of the inositol head group of PtdIns can be phosphorylated separately or in combination at each of these positions to produce seven possible derivatives (Fruman et al., 1998; Anderson et al., 1999). All of these, with the exception of PtdIns(3,4,5)P3, have been found in plant cells (Munnik et al., 1998; Stevenson et al., 2000; Mueller-Roeber and Pical, 2002). These so-called phosphoinositides have emerged as important regulators in a number of cellular activities including vesicle-trafficking and cytoskeletal dynamics, both processes that are central to cell-plate biogenesis. Consequently, we were very interested to test PATL1 for phosphoinositide-binding activity. For these experiments unilamellar vesicles were made from PtdEth spiked with the test phosphoinositide in a 10:1 molar ratio. A 10-fold lower amount of total lipid was employed. The relative amounts of PATL1 in pellets and supernatants were quantified and expressed as a percentage of the total PATL1 in the assay (Fig. 5B). Substantial binding (49%) was detected to PtdIns(5)P, a phosphoinositide just recently shown to exist in plants (Meijer et al., 2001). The function of PtdIns(5)P is relatively unexplored at this time although there is evidence that it is involved in osmotic stress responses in both mammalian cells and Chlamydomonas (Meijer et al., 2001; Sbrissa et al., 2002). In mammalian cells, PtdIns(5)P can also serve as a precursor to PtdIns(4,5)P2, a possibility that is yet to be explored in plant cells (Rameh et al., 1997).
A similar level of PATL1 binding (40%) was also detected to vesicles containing PtdIns(4,5)P2. PtdIns(4,5)P2 is a substrate for phospholipase C, which produces Ins(3,4,5)P3 and diacylgycerol, signals that release calcium intracellular stores and activate protein kinase C, respectively (Berridge, 1993). PtdIns(4,5)P2 is also an important regulator in its own right, directly regulating proteins involved in a diversity of cellular processes. Of particular interest here are its functions in actin cytoskeletal dynamics and membrane trafficking (Toker, 1998). An intermediate level of binding was observed for vesicles that contained PtdIns (21%) and PtdIns(3)P (25%). PtdIns(3)P functions in vesicle traffic from the TGN to the vacuole in both yeast and plants and has been implicated in traffic from the TGN to the lysosome in mammalian cells (Matsuoka et al., 1995; Corvera et al., 1999; Kim et al., 2001). Low, but significantly above background, binding was observed with PtdIns(4)P and PtdIns(3,5)P2. PATL1 did not bind to vesicles that contained PtdIns(3,4)P2.
DISCUSSION
We have reported here the identification and initial biochemical characterization of PATL1, one of a small family of novel Arabidopsis proteins characterized by the presence of a Sec14 and a GOLD domain, both domains found in membrane-trafficking proteins in other eukaryotes. We have shown that PATL1 localizes to the cell plate at the time of its expansion and maturation, a site of membrane traffic. In addition, we have demonstrated that PATL1 binds to specific phosphoinositides, important regulators of membrane-trafficking and cytoskeletal dynamics. Based on its conserved domain structure, localization patterns, and biochemical properties, we hypothesize that PATL1 plays a role in membrane-trafficking events during the later stages of cytokinesis.
The presence of the Sec14 and GOLD domains in PATL1, especially the striking conservation of the Sec14 lipid-binding pocket, strongly suggests a role in vesicle trafficking. In yeast, Sec14p catalyzes the transfer of PtdIns, and to a lesser extent PtdCho, between membrane compartments in vitro although the exact relationship between this activity and its in vivo role in protein secretion is unclear (Phillips et al., 1999). A wealth of in vivo studies, however, are consistent with a complex function for Sec14p in coordinating Golgi phospholipid metabolism with membrane traffic from the Golgi (Li et al., 2000b). Sec14p influences the lipid composition of the Golgi membrane in several ways including the following: (1) acting as a sensor of the PtdIns/PtdCho ratio in Golgi membranes (Cleves et al., 1991; Skinner et al., 1995); (2) regulating the levels of PtdIns(4)P (Whitters et al., 1993; Hama et al., 1999; Phillips et al., 1999; Stock et al., 1999); and (3) influencing phospholipase D activity, which in turn effects the phosphatidic acid and diacyglycerol levels in the membrane (Sreenivas et al., 1998; Xie et al., 1998). Recent studies suggest that Sec14p produces a Golgi membrane lipid environment required for activation of the Arf GTPase-activating proteins, Gcs1p, and Age2p, during vesicle biogenesis (Yanagisawa et al., 2002). The presence of the Sec14p-lipid-binding domain in PATL1 raises the intriguing possibility that PATL1, like Sec14p, may promote vesicle formation by influencing donor membrane lipid composition. PATL1's additional domains and distinctive phosphoinositide-binding specificities, however, also make novel functions likely.
The GOLD domain in the PATL1 C terminus also suggests a role in vesicle trafficking. GOLD domains, which are found in a number of proteins involved in Golgi dynamics and membrane traffic, are thought to mediate protein-protein interactions with a variety of target proteins. Proteins with GOLD domains have been classified into two groups. The first group, the p24 proteins, regulates cargo selection and packaging into the coated vesicles that traffic between the endoplasmic reticulum and Golgi (Anantharaman and Aravind, 2002). These proteins contain a central transmembrane domain with a C-terminal region that interacts with vesicle coat proteins and an N-terminal GOLD domain that extends into the lumen where it is thought to interact with cargo proteins (Dominguez et al., 1998; Kuehn et al., 1998). The second group of GOLD domain proteins, which includes the yeast Osh3p, the human Golgi protein GCP60, and some animal Sec14-like proteins, are peripheral proteins that interact with membranes through a second type of interaction domain (e.g. FYVE, Sec14, PH). These proteins may act as adaptors for recruiting GOLD-domain binding proteins to specific internal membrane sites (Anantharaman and Aravind, 2002). PATL1, which belongs to this second group, may bind to the cell-plate membrane through its Sec14 domain and then recruit other proteins required for the later stages of cytokinesis through interactions with its C-terminal GOLD and variable N-terminal domains. Identification of proteins that bind to these domains will likely provide valuable clues to PATL1's mode of action.
Our intracellular localization studies are consistent with a model in which PATL1 is recruited to the division plane at the time of cell-plate expansion. In Arabidopsis root cells, PATL1 staining was most pronounced at expanding cell plates, as identified by the ring of phragmoplast microtubules (Fig. 4C). This timing corresponds to Phase III of the somatic cell cytokinesis model recently proposed by Segui-Simarro et al. (2004). During Phase III, vesicle traffic continues at the periphery while the central region of the cell plate begins consolidation and maturation. Our studies of tobacco BY2 cells, which afforded the opportunity to more precisely pinpoint the appearance of the PATL antigen at the cell plate, yielded similar results (Fig. 4, G–I). Detection of both a cytosolic and peripheral membrane-associated pool of PATL1 in biochemical fractionation studies was also consistent with the recruitment of PATL1 from the cytosol to the membranes of the developing plate (Fig. 3). The exact nature of the interaction of PATL1 with the membrane, however, is unclear at this time. The PATL1 sequence does not contain myristoylation, prenylation, or obvious palmitoylation signals, suggesting that the protein is not attached to the membrane by a posttranslationally added lipid anchor. Further experimentation will be required to determine if PATL1 interacts directly with membrane phospholipids or through interactions with other membrane proteins.
A comparison of our results to localization studies of other cell-plate-associated proteins is also consistent with a role for PATL1 in completion and maturation of the cell plate, rather than its initial assembly. Our localization results are very similar to those reported for ADL1A and E, a pair of dynamin-like proteins that are functionally redundant with respect to cell-plate biogenesis and maintenance of plasma membrane integrity (Kang et al., 2001, 2003). ADL1A and E localize to the expanding cell plate and, like PATL1, persist there for a time upon completion of cytokinesis (Kang et al., 2003). Similar localization patterns were also reported for a plant myosin VIII, which is thought to be involved in the development of plasmodesmata during maturation of the cell plate (Reichelt et al., 1999). Our results are in marked contrast to those reported for proteins involved in early membrane-trafficking events such as translocation of vesicles to the division plane and vesicle fusion. AtPAKRP2, the kinesin-related protein thought to drive Golgi-derived vesicles to the plate, localizes to punctate structures that first appear at the division plane in early anaphase (Lee et al., 2001). Plate-associated PATL1 was not detected at this time. KNOLLE, the syntaxin that is required for early vesicle fusion events at the plate, associates with the plate in early telophase (Lauber et al., 1997) also before we first detected plate-associated PATL1. Furthermore, during cell-plate expansion both AtPAKRP2 and KNOLLE are most abundant at the periphery of the expanding plate, the site of continued vesicle addition, in contrast to PATL1, which was more concentrated in the central regions of the plate that are undergoing consolidation and maturation.
Although the majority of vesicle traffic is completed by Phase III, it continues to be important during the completion of cytokinesis (Segui-Simarro et al., 2004). The addition of vesicles to the periphery of the expanding plate continues along the remaining phragmoplast microtubules, although PATL1's distribution across the plate argues against its involvement in this activity (Fig. 4, J–L). PATL1 is more likely to function in other membrane-trafficking events that are occurring at this time. After fusion of the cell plate with the parent plasma membrane, vesicle traffic is required to close the remaining discontinuities in the membranous network. Membrane recycling from the membranous network stages is also extensive at this time, and the presence of clathrin-coated vesicles and endocytic multivesicular bodies in close proximity suggest that at least some fraction of this is clathrin dependent (Samuels et al., 1995; Battey et al., 1999; Otegui et al., 2001; Segui-Simarro et al., 2004). PATL1's localization at the cell plate, at a time of clathrin-dependent membrane recycling, is especially interesting in light of the presence of a motif (YGEFQ) that is recognized by γ-ear domains of clathrin-adaptor proteins (Mattera et al., 2004).
While, to the best of our knowledge, this is the first report to provide evidence for the involvement of a SEC14-like protein in cytokinesis in plant cells, there is evidence that proteins related to Sec14p in structure and/or function play a role in membrane traffic during cytokinesis in other organisms. Spo20, a Schizosaccharomyces pombe homolog of Sec14p, is required for the completion of cytokinesis in vegetative cells and shows detects in completion of septation (Nakase et al., 2001). In wild-type cells, the spo20 protein localizes in an actin-dependent fashion to growing cell tips and the medial septation site, both areas of active membrane construction. Nir2, a multidomain mammalian protein that belongs to the animal-specific group of PITPs, is also essential for the completion of cytokinesis (Litvak et al., 2002; Tian et al., 2002). Nir2 is recruited from the cytoplasm to the midbody, a microtubule-based structure that is critical for cleavage furrow ingression and membrane trafficking to the site of cell cleavage. Although the exact roles of Spo20 and Nir2 in cytokinesis are unclear, a likely possibility is that their PtdIns transfer/binding domains function in some way in vesicle traffic to the cleavage plane during cytokinesis.
Lipid-binding studies confirmed our sequence-based prediction that PATL1 would bind phospholipids (Fig. 5). PATL1's preference for PtdIns and its phosphorylated derivatives, PtdIns(5)P, PtdIns(4,5)P2, and PtdIns(3)P, distinguish it from Sec14p, which preferentially binds to PtdCh and PtdIns. Conservation of key residues in the PATL1 Sec14 lipid-binding pocket suggests that this domain may bind phosphoinositides (Fig. 1B). Furthermore, there are several examples of proteins in which Sec14 domains serve as phosphoinositide-binding modules. The soybean Sec14-like proteins, Ssh1p and Ssh2p, both bind PtdIns(4,5)P2 with high affinity (Kearns et al., 1998b), and the human MEG protein tyrosine phosphatase, which regulates secretory vesicle fusion in T cells, binds PtdIns(3,4,5)P3, specifically via its Sec14 domain (Huynh et al., 2003). The PATL1 C-terminal Lys-rich motif, which is reminiscent of sequences found in AP180 and μ2-adaptin, is also an excellent candidate for a phosphoinositide-binding site (Ford et al., 2001; Mao et al., 2001; Rohde et al., 2002). Similar positively charged phosphoinositide-binding sites are found in the actin-binding proteins villin, gelsolin, and profilin (Janmey et al., 1992; Raghunathan et al., 1992; Yu et al., 1992; Sohn et al., 1995), synaptotagmin (Fukuda et al., 1995), and several clathrin-adaptor proteins (Gaidarov and Keen, 1999; Ford et al., 2001; Mao et al., 2001; Rohde et al., 2002). An important goal of future studies will be to determine if both the Sec14 domain and the C-terminal Lys-rich motif are functional lipid-binding sites and, if so, their specificity.
PATL1's ability to bind specific phosphoinositides and its localization at the cell plate suggest a role for these lipids in cell-plate biogenesis. Such a role is not surprising given the importance of phosphoinositides, particularly PtdIns(4,5)P2 and PtdIns(3)P, in regulating membrane traffic (Czech, 2000; Huijbregts et al., 2000; Cullen et al., 2001; Bankaitis and Morris, 2003; Gruenberg, 2003). Phosphoinositides typically exert their influence by directly regulating the activity of their target proteins upon binding to PtdIns-specific domains or by recruiting and assembling protein complexes to specific lipid microdomains. The phosphoinositide-binding activity of PATL1 may account for its recruitment to the cell plate, perhaps to a specific lipid microdomain. Once there, it could recruit other proteins involved in vesicle formation via its GOLD or N-terminal domains.
PATL1 may also function to establish phosphoinositide microdomains, required for vesicle formation during the late stages of cytokinesis, through regulation of phosphoinositide synthesis. PITPs of both the Sec14 and animal-specific families stimulate phosphoinositide synthesis. In fact, Sec14p and animal PITPs are interchangeable in the phosphoinositide synthesis-dependent reconstitution of secretion (Hay et al., 1995; Ohashi et al., 1995; Jones et al., 1998) and phospholipase C-signaling pathways in permeabilized mammalian cells (Cunningham et al., 1995, 1996; Kauffmann-Zeh et al., 1995). Although the mechanism is unknown, PITPs most likely stimulate phosphoinositide synthesis by serving as cofactors for PtdIns kinases. PITPs stimulate the activity of PtdIns kinases in vitro (Panaretou et al., 1997; Jones et al., 1998; Monks et al., 2001), and in some cases there is evidence that PITPs and PtdIns kinases are physically associated as well (Kauffmann-Zeh et al., 1995; Panaretou et al., 1997; Aikawa et al., 1999).
In conclusion, our results provide evidence that PATL1 is a novel phosphoinositide-binding protein that plays a role in membrane traffic during the expansion and maturation stages of cytokinesis. The presence of a family of related proteins in the Arabidopsis genome indicates that there may be redundancy of function, a likelihood if PATL1 plays an essential role in development of the cell plate. Sequence divergence, which is most pronounced in the N termini, may reflect specialization of function as well. The variable N-terminal domains are likely to confer upon each member of the family the ability to interact with different partners in the cell or to be recruited to distinct locations. Identification of binding partners and the characterization of PATL mutants is certain to lead to exciting advances in our understanding of the cellular functions of this novel group of proteins.
MATERIALS AND METHODS
Growth of Plant Material
Arabidopsis ecotype Columbia plants were grown in 1× Murashige and Skoog salts plus vitamins and 1% (w/v) Suc in liquid culture or on plates solidified with 0.7% plant tissue culture agar (Carolina Biological Supply, Burlington, NC). Seedlings were grown at 22°C with a 16-/8-h photoperiod or in continuous light with 150 μmol photon m−2 s−1. Tobacco Bright-Yellow 2 (BY2) cells were grown in suspension culture in modified Linsmaier-Skoog medium as described (Nagata et al., 1981).
PATL1 Cloning
PATL1 was identified from a tryptic peptide (ALQLFQDNYPEF) of a protein that copurified with a zucchini (Cucurbita pepo) membrane-associated F-actin-binding protein that will be described elsewhere. A BLAST search at the time identified four Arabidopsis EST sequences corresponding to two different mRNAs, now known to be PATL1 and PATL5 (Altschul et al., 1990). A full-length PATL1 cDNA was produced by 5′- and 3′-RACE PCR using gene-specific primers designed from the PATL1 EST sequences (Marathon-Ready cDNA kit, CLONTECH, Palo Alto, CA).
Sequence Analysis
BLAST algorithms were used to retrieve PATL sequences from public databases at NCBI (http://www.ncbi.nlm.nih.gov; Altschul et al., 1990). Alignments were performed using ClustalW (gap opening penalty = 10; gap extension penalty = 0.2; Thompson et al., 1994) and prepared for publication using ESPript (http://prodes.toulouse.inra.fr/ESPript/cgi-bin/ESPript.cgi). Domain analysis of the PATLs was performed with InterProScan (http://www.ebi.ac.uk/InterProScan/). The endpoints of the Sec14 domain of the PATLs were defined by pairwise alignments of the PATL sequences with yeast (Saccharomyces cerevisiae) Sec14p (GenBank accession no. P24280). GOLD domains (Prosite:PDOC50866) were identified by ScanProsite (http://ca.expasy.org/tools/scanprosite/; Gattiker et al., 2002). The position of potential coiled coils within the variable N-terminal domains was predicted by the Coils2 and Paircoil programs (Lupas et al., 1991; Berger et al., 1995).
PATL1 Antiserum Production
Sequences coding for full-length PATL1 and 2 as well as the GOLD domain of PATL1 (amino acid nos. 383–573) were cloned into the GST-fusion expression vector pGEX6P-1 (Amersham Biosciences). Inserts were produced by PCR using full-length cDNA as template and primers that created BamH1 and Sal1 sites at the 5′ and 3′ ends, respectively. The resulting constructs were verified by DNA sequence analysis before transformation into Escherichia coli BL21 pLysS cells for protein production. Protein production was induced in early log phase cultures by the addition of 0.5 mm isopropyl-β-d-thiogalactoside for 3 to 4 h at 37°C. The cells were harvested, washed with cold phosphate-buffered saline (PBS), and lysed in 1% (v/v) Triton X-100, 0.2 m NaCl, 20 mm sodium phosphate, pH 7.5, 5 mm dithiothreitol (DTT), and 1 mm phenylmethylsulfonyl fluoride. The lysate was cleared by centrifugation at 27,000g for 30 min and then incubated with GST-Sepharose beads (Amersham Biosciences) for 4 to 24 h. The beads were washed extensively with PBS and eluted with 20 mm reduced glutathione, 150 mm NaCl, and 100 mm Tris-Cl, pH 8. Purified PATL-GST fusion proteins were cleaved with PreScission Protease according to the manufacturer's instructions (Amersham Biosciences). GST-Sepharose chromatography was used to remove the free GST. Protein concentration was determined using the bicinchoninic acid assay (Pierce, Rockford, IL). Protein preparations were analyzed by SDS-PAGE followed by staining with SYPRO Red (Molecular Probes, Eugene, OR) and scanning with a Storm 860 fluorescence scanner (Amersham Biosciences). The purified PATL1 proteins were injected into rabbits for antiserum production.
Immunoblot Analysis
Arabidopsis organs, from seedlings grown on plates with a 16-/8-h photoperiod, were powdered in liquid N2 and stored at −80°C. Total proteins extracts were prepared by vortexing a sample of tissue powder in ice-cold extraction buffer (0.125 m Tris-Cl, pH 6.8, 4% [w/v] SDS, 1 mm DTT, and 10% [v/v] glycerol). The extracted proteins were denatured at 95°C for 15 min and then microfuged for 10 min at room temperature. Total protein concentration of the cleared extract was determined using the bicinchoninic acid assay. Proteins were separated by SDS-PAGE and transferred to Immobilon-P 0.45 μm polyvinylidene difluoride membranes (Millipore, Bedford, MA) by electroblotting. Blots were blocked for 1 h in 5% (w/v) powdered milk in Tris-buffered saline plus Tween 20 (10 mm Tris-Cl, pH 7.5, 150 mm NaCl, and 0.05% [v/v] Tween 20) and then probed with antibody diluted in blocking solution for 1 to 4 h. Primary antibodies were diluted as follows: anti-PATL1, 1:5,000 to 1:10,000; anti-SEC12, 1:500 (Secant Chemicals, Winchendon, MA), and C4 mouse anti-actin, 1:400 (ICN, Aurora, IL). The blots were then washed in Tris-buffered saline plus Tween 20 and probed with Cy5- or Alexa488-goat anti-rabbit secondary antibodies (2 μg/mL) for 1 h (Molecular Probes). The blots were washed and then scanned with a STORM 860 fluorescence scanner.
Biochemical Fractionation
Arabidopsis roots, from plants grown in liquid culture with continuous light, were powdered in liquid N2 and stored at −80°C. Powdered roots were homogenized using a mortar and pestle in 3 to 4 volumes of ice-cold homogenization buffer (25 mm Tris-Cl, pH 7.2, 0.25 m Suc, 3 mm EDTA, 1 mm DTT, 1 mm phenylmethylsulfonyl fluoride, and one complete protease inhibitor tablet/50 mL; Roche Applied Science, Indianapolis). The homogenate was filtered through a single layer of Miracloth (Calbiochem, San Diego) and centrifuged at 1,000g to remove nuclei and debris. The resulting S1 fraction was ultracentrifuged at 100,000g for 2 h at 4°C to yield cytosolic (S100) and microsomal membrane fractions (P100). The pellets (P100) were resuspended in homogenization buffer. Tissue equivalents of S1, S100, and P100 fractions were subjected to immunoblot analysis as described above. To determine the nature of PATL1's interaction with the membrane, samples of the P100 fraction were pelleted at 100,000g for 1 h, resuspended in homogenization buffer that contained 1 m NaCl, 2 m urea, 1% (v/v) Triton X-100, or 1% (w/v) SDS, and incubated on ice for 1 h. An aliquot was set aside for a total protein sample (T). The remainder was centrifuged at 100,000g for 1 h to produce the supernatant or solubilized protein fraction (S). S and T fractions were analyzed on immunoblots.
Immunofluorescence Microscopy
Immunostaining of Arabidopsis roots, grown on vertical plates or in liquid culture with continuous light, was as described (Harper et al., 1996). PATL1 antibody was diluted 1:250 to 1:500. Microtubules were stained using rat anti-α tubulin monoclonal YOL1/34 (1:100; Accurate Chemical and Scientific Corporation, Westbury, NY). TO-PRO-3, diluted 1:1,000 in the last 10 to 20 min of the final washes, was used as a nuclear counterstain (Molecular Probes). Alexa 488 and 568 goat secondary antibodies (2 μg/mL) were used to detect PATL1 and tubulin, respectively.
Early log phase BY2 cell cultures (wild type or the GmMan1::GFP line of Nebenfuhr et al., 1999) were transferred to polyethyleneimine-coated coverslips and allowed to settle for 10 min. The culture medium was removed, and the cells were prefixed for 15 min with 0.4 mm 3-maleimidobenzoyl-N-hydroxy-succinimide ester; Calbiochem) in 50 mm PIPES, 2 mm MgSO4, and 5 mm EGTA, pH 6.8) (1 × PME), followed by fixation with 4% (w/v) para-formaldehyde, 0.1% (w/v) glutaraldehyde, 1× PME, 0.05% (v/v) Triton X-100, and 1 mm 4-(2-aminoethyl)benzenesulfonyl fluoride for 1 h. After washing for 3 × 10 min with PBS (2.68 mm KCl, 1.47 mm KH2PO4, 13.69 mm NaCl, and 0.81 mm Na2HPO4, pH 7.4), the cell walls were digested for 2 to 3 min with 1× PME, 0.5% (w/v) bovine serum albumin, 0.01% (w/v) cellulase (Onozuka, Tokyo), 0.001% (w/v) Y23 pectolyase (Kikkomann, Tokyo), and 0.5% (v/v) Triton X-100. The coverslips were then submerged in −20°C methanol for 15 min. After air drying, the cells were rehydrated in PBS, blocked at reverse transcription for 1 h in antibody buffer (1% [w/v] bovine serum albumin in PBS with 0.05% [w/v] azide), incubated with primary antibodies in antibody buffer overnight at room temperature, and washed with PBS. The cells were incubated for 2 to 4 h with secondary antibodies, diluted as described above, in antibody buffer. Finally the cells were washed with PBS and counterstained with TO-PRO-3, as described above.
Images were collected using a Leica (Wetzlar, Germany) TCS NT/SP confocal microscope equipped with HC PL APO 20×/0.7, HCX PL APO 40×/1.25 to .75, and PL APO 100×/1.4 to .7 oil immersion objective lenses. Excitation was from argon (488 nm), krypton (568), and HeNe (633 nm) lasers. For triple labeling experiments, sequential scans were collected at each wavelength. The average size of the cytoplasmic puncta was determined using C-Imaging software (Compix, Cranberry Township, PA).
Vesicle-Binding Assays
Natural egg PtdEth, soybean (Glycine max) PtdCh, and PtdIns in chloroform were obtained from Avanti Polar Lipids (Alabaster, AL). Synthetic PtdIns and phosphoinositides (with 16 C chains) were obtained as lyophilized powders from Echelon Laboratories (Salt Lake City). Unilamellar vesicles were produced by multiple extrusions through a 0.1-μm polycarbonate filter using a LiposoFast microextruder (Avestin, Ottawa) as previously described (MacDonald et al., 1991). Recombinant PATL1 protein was precleared by spinning at 164,000g for 30 min in an Airfuge (Beckman Instruments, Palo Alto, CA) to remove insoluble protein. For binding experiments, precleared PATL1 (2.5 μg) was mixed with vesicles in lipid buffer (0.15 m NaCl, 10 mm MOPS, pH 7, 0.1 mm EDTA). The samples were incubated at room temperature for 1 h and centrifuged at 164,000g for 30 min to pellet the vesicles along with any bound protein. Equivalent amounts of supernatant (S) and pellet (P) were analyzed by SDS-PAGE gels stained with SYPRO Red. PATL1 was quantified using the ImageQuant software package (Amersham Biosciences).
Distribution of Materials
Upon request all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Nina S. Allen, Kevin Vaughn, and David Collings for their advice on immunocytochemistry and confocal micrsocopy and Wendy Boss and Amanda Davis for critical reading of the manuscript. We also thank Andreas Nebenfuhr for his generous gift of the GmMan1::GFP BY2 cells. We acknowledge use of the Wellesley College confocal microscope facility.
This work was supported by the United States Department of Agriculture (grant no. 2001–35304–10903 to T.K.P.) and benefited from support from the National Institutes of Health (grant no. GM33048 to E.J.L.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.045369.
References
- Aikawa Y, Kuraoka A, Kondo H, Kawabuchi M, Watanabe T (1999) Involvement of PITPnm, a mammalian homologue of Drosophila rdgB, in phosphoinositide synthesis on Golgi membranes. J Biol Chem 274: 20569–20577 [DOI] [PubMed] [Google Scholar]
- Allen-Baume V, Segui B, Cockcroft S (2002) Current thoughts on the phosphatidylinositol transfer protein family. FEBS Lett 531: 74–80 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410 [DOI] [PubMed] [Google Scholar]
- Anantharaman V, Aravind L (2002) The GOLD domain, a novel protein module involved in Golgi function and secretion. Genome Biol 3: 0023.0021–0023.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Boronenkov IV, Doughman SD, Kunz J, Loijens JC (1999) Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. J Biol Chem 274: 9907–9910 [DOI] [PubMed] [Google Scholar]
- Aravind L, Neuwald AF, Ponting CP (1999) Sec14p-like domains in NF1 and Dbl-like proteins indicate lipid regulation of Ras and Rho signaling. Curr Biol 9: R195–R197 [DOI] [PubMed] [Google Scholar]
- Armstrong DJ, Roman A (1993) The anomalous electrophoretic behavior of the human papillomavirus type 16 E7 protein is due to the high content of acidic amino acid residues. Biochem Biophys Res Commun 192: 1380–1387 [DOI] [PubMed] [Google Scholar]
- Asada T, Sonobe S, Shibaoka H (1991) Microtubule translocation in the cytokinetic apparatus of cultured tobacco cells. Nature 350: 238–241 [Google Scholar]
- Assaad FF (2001) Plant cytokinesis. Exploring the links. Plant Physiol 126: 509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaad FF, Huet Y, Mayer U, Jurgens G (2001) The cytokinesis gene KEULE encodes a Sec1 protein that binds the syntaxin KNOLLE. J Cell Biol 152: 531–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis VA, Aitken JR, Cleves AE, Dowhan W (1990) An essential role for a phospholipid transfer protein in yeast Golgi function. Nature 347: 561–562 [DOI] [PubMed] [Google Scholar]
- Bankaitis VA, Malehorn DE, Emr SD, Greene R (1989) The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol 108: 1271–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis VA, Morris AJ (2003) Lipids and the exocytotic machinery of eukaryotic cells. Curr Opin Cell Biol 15: 389–395 [DOI] [PubMed] [Google Scholar]
- Bar-Peled M, Raikhel NV (1997) Characterization of AtSEC12 and AtSAR1. Proteins likely involved in endoplasmic reticulum and Golgi transport. Plant Physiol 114: 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey NH, James NC, Greenland AJ, Brownlee C (1999) Exocytosis and endocytosis. Plant Cell 11: 643–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek SY, Falbel TG (2002) Membrane trafficking during plant cytokinesis. Traffic 3: 621–629 [DOI] [PubMed] [Google Scholar]
- Berger B, Wilson DB, Wolf E, Tonchev T, Milla M, Kim PS (1995) Predicting coiled coils by use of pairwise residue correlations. Proc Natl Acad Sci USA 92: 8259–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ (1993) Inositol triphosphate and calcium signalling. Nature 361: 315–325 [DOI] [PubMed] [Google Scholar]
- Burkhard P, Stetefeld J, Strelkov SV (2001) Coiled coils: a highly versatile protein folding motif. Trends Cell Biol 11: 82–88 [DOI] [PubMed] [Google Scholar]
- Cleves AE, McGee TP, Whitters EA, Champion KM, Aitken JR, Dowhan W, Goebl M, Bankaitis VA (1991) Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell 64: 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleves AE, Novick PJ, Bankaitis VA (1989) Mutations in the SAC1 gene suppress defects in yeast Golgi and yeast actin function. J Cell Biol 109: 2939–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera S, D'Arrigo A, Stenmark H (1999) Phosphoinositides in membrane traffic. Curr Opin Cell Biol 11: 460–465 [DOI] [PubMed] [Google Scholar]
- Cullen PJ, Cozier GE, Banting G, Mellor H (2001) Modular phosphoinositide-binding domains—their role in signalling and membrane trafficking. Curr Biol 11: R882–R893 [DOI] [PubMed] [Google Scholar]
- Culter SR, Ehrhardt DW (2002) Polarized cytokinesis in vacuolated cells of Arabidopsis. Proc Natl Acad Sci USA 99: 2812–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham E, Tan SK, Swigart P, Hsuan J, Bankaitis V, Cockcroft S (1996) The yeast and mammalian isoforms of phosphatidylinositol transfer protein can all restore phospholipase C-mediated inositol lipid signaling in cytosol-depleted RBL-2H3 and HL-60 cells. Proc Natl Acad Sci USA 93: 6589–6593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham E, Thomas GM, Ball A, Hiles I, Cockcroft S (1995) Phosphatidylinositol transfer protein dictates the rate of inositol trisphosphate production by promoting the synthesis of PIP2. Curr Biol 5: 775–783 [DOI] [PubMed] [Google Scholar]
- Czech MP (2000) PIP2 and PIP3: complex roles at the cell surface. Cell 100: 603–606 [DOI] [PubMed] [Google Scholar]
- Dominguez M, Dejgaard K, Fullekrug J, Dahan S, Fazel A, Paccaud JP, Thomas DY, Bergeron JJ, Nilsson T (1998) gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J Cell Biol 140: 751–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falbel TG, Koch LM, Nadeau JA, Segui-Simarro JM, Sack FD, Bednarek SY (2003) SCD1 is required for cytokinesis and polarized cell expansion in Arabidopsis thaliana. Development 130: 4011–4024 [DOI] [PubMed] [Google Scholar]
- Feng S, Chen JK, Yu H, Simon JA, Schreiber SL (1994) Two binding orientations for peptides to the Src SH3 domain: development of a general model for SH3-ligand interactions. Science 266: 1241–1247 [DOI] [PubMed] [Google Scholar]
- Ford MG, Pearse BM, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT (2001) Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science 291: 1051–1055 [DOI] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, Cantley LC (1998) Phosphoinositide kinases. Annu Rev Biochem 67: 481–507 [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kojima T, Aruga J, Niinobe M, Mikoshiba K (1995) Functional diversity of C2 domains of synaptotagmin family. Mutational analysis of inositol high polyphosphate binding domain. J Biol Chem 270: 26523–26527 [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Keen JH (1999) Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J Cell Biol 146: 755–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattiker A, Gasteiger E, Bairoch A (2002) ScanProsite: a reference implementation of a PROSITE scanning tool. Appl Bioinformatics 1: 107–108 [PubMed] [Google Scholar]
- Granger C, Cyr R (2001) Use of abnormal preprophase bands to decipher division plane determination. J Cell Sci 114: 599–607 [DOI] [PubMed] [Google Scholar]
- Gruenberg J (2003) Lipids in endocytic membrane transport and sorting. Curr Opin Cell Biol 15: 382–388 [DOI] [PubMed] [Google Scholar]
- Gu M, Warshawsky I, Majerus PW (1992) Cloning and expression of a cytosolic megakaryocyte protein-tyrosine-phosphatase with sequence homology to retinaldehyde-binding protein and yeast SEC14p. Proc Natl Acad Sci USA 89: 2980–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Verma DP (1996) Phragmoplastin, a dynamin-like protein associated with cell plate formation in plants. EMBO J 15: 695–704 [PMC free article] [PubMed] [Google Scholar]
- Gu X, Verma DP (1997) Dynamics of phragmoplastin in living cells during cell plate formation and uncoupling of cell elongation from the plane of cell division. Plant Cell 9: 157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB (1999) Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J Biol Chem 274: 34294–34300 [DOI] [PubMed] [Google Scholar]
- Hamilton BA, Smith DJ, Mueller KL, Kerrebrock AW, Bronson RT, van Berkel V, Daly MJ, Kruglyak L, Reeve MP, Nemhauser JL, et al (1997) The vibrator mutation causes neurodegeneration via reduced expression of PITP alpha: positional complementation cloning and extragenic suppression. Neuron 18: 711–722 [DOI] [PubMed] [Google Scholar]
- Harper JD, Holdaway NJ, Brecknock SL, Busby CH, Overall RL (1996) A simple and rapid technique for the immunofluorescence confocal microscopy of intact Arabidopsis root tips. Cytobios 87: 71–78 [PubMed] [Google Scholar]
- Hay JC, Fisette PL, Jenkins GH, Fukami K, Takenawa T, Anderson RA, Martin TF (1995) ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature 374: 173–177 [DOI] [PubMed] [Google Scholar]
- Heese M, Gansel X, Sticher L, Wick P, Grebe M, Granier F, Jurgens G (2001) Functional characterization of the KNOLLE-interacting t-SNARE AtSNAP33 and its role in plant cytokinesis. J Cell Biol 155: 239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese M, Ulrike M, Jurgens G (1998) Cytokinesis in flowering plants: cellular process and developmental regulation. Curr Opin Plant Biol 1: 486–491 [DOI] [PubMed] [Google Scholar]
- Herrmann H, Aebi U (2000) Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol 12: 79–90 [DOI] [PubMed] [Google Scholar]
- Hsuan J, Cockcroft S (2001) The PITP family of phosphatidylinositol transfer proteins. Genome Biol 2: REVIEWS3011 [DOI] [PMC free article] [PubMed]
- Huijbregts RP, Topalof L, Bankaitis VA (2000) Lipid metabolism and regulation of membrane trafficking. Traffic 1: 195–202 [DOI] [PubMed] [Google Scholar]
- Huynh H, Wang X, Li W, Bottini N, Williams S, Nika K, Ishihara H, Godzik A, Mustelin T (2003) Homotypic secretory vesicle fusion induced by the protein tyrosine phosphatase MEG2 depends on polyphosphoinositides in T cells. J Immunol 171: 6661–6671 [DOI] [PubMed] [Google Scholar]
- Janmey PA, Lamb J, Allen PG, Matsudaira PT (1992) Phosphoinositide-binding peptides derived from the sequences of gelsolin and villin. J Biol Chem 267: 11818–11823 [PubMed] [Google Scholar]
- Jones SM, Alb JG Jr, Phillips SE, Bankaitis VA, Howell KE (1998) A phosphatidylinositol 3-kinase and phosphatidylinositol transfer protein act synergistically in formation of constitutive transport vesicles from the trans-Golgi network. J Biol Chem 273: 10349–10354 [DOI] [PubMed] [Google Scholar]
- Jouannic N, Lepetit M, Vergnolle C, Cantrel C, Gardies AM, Kader JC, Arondel V (1998) Isolation of a cDNA from Arabidopsis thaliana that complements the sec14 mutant of yeast. Eur J Biochem 258: 402–410 [DOI] [PubMed] [Google Scholar]
- Kang BH, Busse JS, Bednarek SY (2003) Members of the Arabidopsis dynamin-like gene family, ADL1, are essential for plant cytokinesis and polarized cell growth. Plant Cell 15: 899–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BH, Busse JS, Dickey C, Rancour DM, Bednarek SY (2001) The Arabidopsis cell plate-associated dynamin-like protein, ADL1Ap, is required for multiple stages of plant growth and development. Plant Physiol 126: 47–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapranov P, Routt SM, Bankaitis VA, de Bruijn FJ, Szczyglowski K (2001) Nodule-specific regulation of phosphatidylinositol transfer protein expression in Lotus japonicus. Plant Cell 13: 1369–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann-Zeh A, Thomas GM, Ball A, Prosser S, Cunningham E, Cockcroft S, Hsuan JJ (1995) Requirement for phosphatidylinositol transfer protein in epidermal growth factor signaling. Science 268: 1188–1190 [DOI] [PubMed] [Google Scholar]
- Kay BK, Williamson MP, Sudol M (2000) The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J 14: 231–241 [PubMed] [Google Scholar]
- Kearns BG, Alb JG Jr, Bankaitis V (1998. a) Phosphatidylinositol transfer proteins: the long and winding road to physiological function. Trends Cell Biol 8: 276–282 [DOI] [PubMed] [Google Scholar]
- Kearns MA, Monks DE, Fang M, Rivas MP, Courtney PD, Chen J, Prestwich GD, Theibert AB, Dewey RE, Bankaitis VA (1998. b) Novel developmentally regulated phosphoinositide binding proteins from soybean whose expression bypasses the requirement for an essential phosphatidylinositol transfer protein in yeast. EMBO J 17: 4004–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Eu YJ, Yoo CM, Kim YW, Pih KT, Jin JB, Kim SJ, Stenmark H, Hwang II (2001) Trafficking of phosphatidylinositol 3-phosphate from the trans-Golgi network to the lumen of the central vacuole in plant cells. Plant Cell 13: 287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MJ, Herrmann JM, Schekman R (1998) COPII-cargo interactions direct protein sorting into ER-derived transport vesicles. Nature 391: 187–190 [DOI] [PubMed] [Google Scholar]
- Lauber MH, Waizenegger I, Steinmann T, Schwarz H, Mayer U, Hwang I, Lukowitz W, Jurgens G (1997) The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J Cell Biol 139: 1485–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YR, Giang HM, Liu B (2001) A novel plant kinesin-related protein specifically associates with the phragmoplast organelles. Plant Cell 13: 2427–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Routt SM, Xie Z, Cui X, Fang M, Kearns MA, Bard M, Kirsch DR, Bankaitis VA (2000. a) Identification of a novel family of nonclassic yeast phosphatidylinositol transfer proteins whose function modulates phospholipase D activity and Sec14p-independent cell growth. Mol Biol Cell 11: 1989–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xie Z, Bankaitis VA (2000. b) Phosphatidylinositol/phosphatidylcholine transfer proteins in yeast. Biochim Biophys Acta 1486: 55–71 [DOI] [PubMed] [Google Scholar]
- Litvak V, Tian D, Carmon S, Lev S (2002) Nir2, a human homolog of Drosophila melanogaster retinal degeneration B protein, is essential for cytokinesis. Mol Cell Biol 22: 5064–5075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Mayer U, Jurgens G (1996) Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell 84: 61–71 [DOI] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J (1991) Predicting coiled coils from protein sequences. Science 252: 1162–1164 [DOI] [PubMed] [Google Scholar]
- MacDonald RC, MacDonald RI, Menco BP, Takeshita K, Subbarao NK, Hu LR (1991) Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta 1061: 297–303 [DOI] [PubMed] [Google Scholar]
- Mao Y, Chen J, Maynard JA, Zhang B, Quiocho FA (2001) A novel all helix fold of the AP180 amino-terminal domain for phosphoinositide binding and clathrin assembly in synaptic vesicle endocytosis. Cell 104: 433–440 [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Bassham DC, Raikhel NV, Nakamura K (1995) Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J Cell Biol 130: 1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera R, Ritter B, Sidhu SS, McPherson PS, Bonifacino JS (2004) Definition of the consensus motif recognized by gamma-adaptin ear domains. J Biol Chem 279: 8018–8028 [DOI] [PubMed] [Google Scholar]
- May AP, Whiteheart SW, Weis WI (2001) Unraveling the mechanism of the vesicle transport ATPase NSF, the N-ethylmaleimide-sensitive factor. J Biol Chem 276: 21991–21994 [DOI] [PubMed] [Google Scholar]
- Meijer HJ, Berrie CP, Iurisci C, Divecha N, Musgrave A, Munnik T (2001) Identification of a new polyphosphoinositide in plants, phosphatidylinositol 5-monophosphate (PtdIns5P), and its accumulation upon osmotic stress. Biochem J 360: 491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan SC, Alb JG Jr, Elagina RB, Bankaitis VA, Hyde DR (1997) The phosphatidylinositol transfer protein domain of Drosophila retinal degeneration B protein is essential for photoreceptor cell survival and recovery from light stimulation. J Cell Biol 139: 351–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molchan TM, Valster AH, Hepler PK (2002) Actomyosin promotes cell plate alignment and late lateral expansion in Tradescantia stamen hair cells. Planta 214: 683–693 [DOI] [PubMed] [Google Scholar]
- Molendijk AJ, Bischoff F, Rajendrakumar CS, Friml J, Braun M, Gilroy S, Palme K (2001) Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J 20: 2779–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks DE, Aghoram K, Courtney PD, DeWald DB, Dewey RE (2001) Hyperosmotic stress induces the rapid phosphorylation of a soybean phosphatidylinositol transfer protein homolog through activation of the protein kinases SPK1 and SPK2. Plant Cell 13: 1205–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PJ, Staehelin LA (1998) Immunogold localization of the cell-wall-matrix polysaccharides rhamnogalacturonan I and xyloglucan during cell expansion and cytokinesis in Trifolium pratense L.: implication of secretory pathways. Planta 174: 433–445 [DOI] [PubMed] [Google Scholar]
- Mueller-Roeber B, Pical C (2002) Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol 130: 22–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T, Irvine RF, Musgrave A (1998) Phospholipid signalling in plants. Biochim Biophys Acta 1389: 222–272 [DOI] [PubMed] [Google Scholar]
- Murphy A, Hoogner K, Peer WA, Taiz L (2002) Identification, purification and molecular cloning of N-1-napthylphthalmic acid-binding plasma membrane-associated aminopeptidases from Arabidopsis. Plant Physiol 128: 935–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T, Okada K, Takebe I, Matsui C (1981) Delivery of tobacco mosaic virus RNA into plant protoplasts mediated by reverse-phase evaporation vesicles (liposomes). Mol Gen Genet 184: 161–165 [Google Scholar]
- Nakase Y, Nakamura T, Hirata A, Routt SM, Skinner HB, Bankaitis VA, Shimoda C (2001) The Schizosaccharomyces pombe spo20(+) gene encoding a homologue of Saccharomyces cerevisiae Sec14 plays an important role in forespore membrane formation. Mol Biol Cell 12: 901–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenfuhr A, Frohlick JA, Staehelin LA (2000) Redistribution of Golgi stacks and other organelles during mitosis and cytokinesis in plant cells. Plant Physiol 124: 135–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenfuhr A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, Staehelin LA (1999) Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol 121: 1127–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickle TC, Meinke DW (1998) A cytokinesis-defective mutant of Arabidopsis (cyt1) characterized by embryonic lethality, incomplete cell walls, and excessive callose accumulation. Plant J 15: 321–332 [DOI] [PubMed] [Google Scholar]
- Nishihama R, Soyano T, Ishikawa M, Araki S, Tanaka H, Asada T, Irie K, Ito M, Terada M, Banno H, et al (2002) Expansion of the cell plate in plant cytokinesis requires a kinesin-like protein/MAPKKK complex. Cell 109: 87–99 [DOI] [PubMed] [Google Scholar]
- Ohashi M, Jan de Vries K, Frank R, Snoek G, Bankaitis V, Wirtz K, Huttner WB (1995) A role for phosphatidylinositol transfer protein in secretory vesicle formation. Nature 377: 544–547 [DOI] [PubMed] [Google Scholar]
- Otegui M, Staehelin LA (2000. a) Cytokinesis in flowering plants: more than one way to divide a cell. Curr Opin Plant Biol 3: 493–502 [DOI] [PubMed] [Google Scholar]
- Otegui M, Staehelin LA (2000. b) Syncytial-type cell plates: a novel kind of cell plate involved in endosperm cellularization of Arabidopsis. Plant Cell 12: 933–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui MS, Mastronarde DN, Kang BH, Bednarek SY, Staehelin LA (2001) Three-dimensional analysis of syncytial-type cell plates during endosperm cellularization visualized by high resolution electron tomography. Plant Cell 13: 2033–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otegui MS, Staehelin LA (2004) Electron tomographic analysis of post-meiotic cytokinesis during pollen development in Arabidopsis thaliana. Planta 218: 501–515 [DOI] [PubMed] [Google Scholar]
- Panaretou C, Domin J, Cockcroft S, Waterfield MD (1997) Characterization of p150, an adaptor protein for the human phosphatidylinositol (PtdIns) 3-kinase. Substrate presentation by phosphatidylinositol transfer protein to the p150. Ptdins 3-kinase complex. J Biol Chem 272: 2477–2485 [DOI] [PubMed] [Google Scholar]
- Phillips SE, Sha B, Topalof L, Xie Z, Alb JG, Klenchin VA, Swigart P, Cockcroft S, Martin TF, Luo M, et al (1999) Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Mol Cell 4: 187–197 [DOI] [PubMed] [Google Scholar]
- Raghunathan V, Mowery P, Rozycki M, Lindberg U, Schutt C (1992) Structural changes in profilin accompany its binding to phosphatidylinositol, 4,5-bisphosphate. FEBS Lett 297: 46–50 [DOI] [PubMed] [Google Scholar]
- Rameh LE, Tolias KF, Duckworth BC, Cantley LC (1997) A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature 390: 192–196 [DOI] [PubMed] [Google Scholar]
- Rancour DM, Dickey CE, Park S, Bednarek SY (2002) Characterization of AtCDC48. Evidence for multiple membrane fusion mechanisms at the plane of cell division in plants. Plant Physiol 130: 1241–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt S, Knight AE, Hodge TP, Baluska F, Samaj J, Volkmann D, Kendrick-Jones J (1999) Characterization of the unconventional myosin VIII in plant cells and its localization at the post-cytokinetic cell wall. Plant J 19: 555–567 [DOI] [PubMed] [Google Scholar]
- Rohde G, Wenzel D, Haucke V (2002) A phosphatidylinositol (4,5)-bisphosphate binding site within mu2-adaptin regulates clathrin-mediated endocytosis. J Cell Biol 158: 209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels AL, Giddings TH Jr, Staehelin LA (1995) Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol 130: 1345–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrissa D, Ikonomov OC, Deeb R, Shisheva A (2002) Phosphatidylinositol 5-phosphate biosynthesis is linked to PIKfyve and is involved in osmotic response pathway in mammalian cells. J Biol Chem 277: 47276–47284 [DOI] [PubMed] [Google Scholar]
- Segui-Simarro JM, Austin JR II, White EA, Staehelin LA (2004) Electron tomographic analysis of somatic cell plate formation in meristematic cells of Arabidopsis preserved by high-pressure freezing. Plant Cell 16: 836–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha B, Luo M (1999) PI transfer protein: the specific recognition of phospholipids and its functions. Biochim Biophys Acta 1441: 268–277 [DOI] [PubMed] [Google Scholar]
- Sha B, Phillips SE, Bankaitis VA, Luo M (1998) Crystal structure of the Saccharomyces cerevisiae phosphatidylinositol-transfer protein. Nature 391: 506–510 [DOI] [PubMed] [Google Scholar]
- Skinner HB, McGee TP, McMaster CR, Fry MR, Bell RM, Bankaitis VA (1995) The Saccharomyces cerevisiae phosphatidylinositol-transfer protein effects a ligand-dependent inhibition of choline-phosphate cytidylyltransferase activity. Proc Natl Acad Sci USA 92: 112–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LG (1999) Divide and conquer: cytokinesis in plant cells. Curr Opin Plant Biol 2: 447–453 [DOI] [PubMed] [Google Scholar]
- Smith LG (2002) Plant cytokinesis: motoring to the finish. Curr Biol 12: R206–R208 [DOI] [PubMed] [Google Scholar]
- Sohn RH, Chen J, Koblan KS, Bray PF, Goldschmidt-Clermont PJ (1995) Localization of a binding site for phosphatidylinositol 4,5-bisphosphate on human profilin. J Biol Chem 270: 21114–21120 [DOI] [PubMed] [Google Scholar]
- Sreenivas A, Patton-Vogt JL, Bruno V, Griac P, Henry SA (1998) A role for phospholipase D (Pld1p) in growth, secretion, and regulation of membrane lipid synthesis in yeast. J Biol Chem 273: 16635–16638 [DOI] [PubMed] [Google Scholar]
- Staehelin LA, Hepler PK (1996) Cytokinesis in higher plants. Cell 84: 821–824 [DOI] [PubMed] [Google Scholar]
- Stevenson JM, Perera IY, Heilmann I, Persson S, Boss WF (2000) Inositol signaling and plant growth. Trends Plant Sci 5: 252–258 [DOI] [PubMed] [Google Scholar]
- Stock SD, Hama H, DeWald DB, Takemoto JY (1999) SEC14-dependent secretion in Saccharomyces cerevisiae. Nondependence on sphingolipid synthesis-coupled diacylglycerol production. J Biol Chem 274: 12979–12983 [DOI] [PubMed] [Google Scholar]
- Stocker A, Baumann U (2003) Supernatant protein factor in complex with RRR-alpha-tocopherylquinone: a link between oxidized vitamin E and cholesterol biosynthesis. J Mol Biol 332: 759–765 [DOI] [PubMed] [Google Scholar]
- Strompen G, El Kasmi F, Richter S, Lukowitz W, Assaad FF, Jurgens G, Mayer U (2002) The Arabidopsis HINKEL gene encodes a kinesin-related protein involved in cytokinesis and is expressed in a cell cycle-dependent manner. Curr Biol 12: 153–158 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D, Litvak V, Toledo-Rodriguez M, Carmon S, Lev S (2002) Nir2, a novel regulator of cell morphogenesis. Mol Cell Biol 22: 2650–2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A (1998) The synthesis and cellular roles of phosphatidylinositol 4,5-bisphosphate. Curr Opin Cell Biol 10: 254–261 [DOI] [PubMed] [Google Scholar]
- Ueda T, Yamaguchi M, Uchimiya H, Nakano A (2001) Ara6, a plant-unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana. EMBO J 20: 4730–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valster AH, Pierson ES, Valenta R, Hepler PK, Emons A (1997) Probing the plant actin cytoskeleton during cytokinesis and interphase by profilin microinjection. Plant Cell 9: 1815–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hazel HB, Pichler H, do Valle Matta MA, Leitner E, Goffeau A, Daum G (1999) PDR16 and PDR17, two homologous genes of Saccharomyces cerevisiae, affect lipid biosynthesis and resistance to multiple drugs. J Biol Chem 274: 1934–1941 [DOI] [PubMed] [Google Scholar]
- Whitters EA, Cleves AE, McGee TP, Skinner HB, Bankaitis VA (1993) SAC1p is an integral membrane protein that influences the cellular requirement for phospholipid transfer protein function and inositol in yeast. J Cell Biol 122: 79–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WI, Routt S, Bankaitis VA, Voelker DR (2000) A new gene involved in the transport-dependent metabolism of phosphatidylserine, PSTB2/PDR17, shares sequence similarity with the gene encoding the phosphatidylinositol/phosphatidylcholine transfer protein, SEC14. J Biol Chem 275: 14446–14456 [DOI] [PubMed] [Google Scholar]
- Xie Z, Fang M, Rivas MP, Faulkner AJ, Sternweis PC, Engebrecht JA, Bankaitis VA (1998) Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc Natl Acad Sci USA 95: 12346–12351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Lim J, Dale JM, Chen H, Shinn P, Palm CJ, Southwick AM, Wu HC, Kim C, Nguyen M, et al (2003) Empirical analysis of transcriptional activity in the Arabidopsis genome. Science 302: 842–846 [DOI] [PubMed] [Google Scholar]
- Yanagisawa LL, Marchena J, Xie Z, Li X, Poon PP, Singer RA, Johnston GC, Randazzo PA, Bankaitis VA (2002) Activity of specific lipid-regulated ADP ribosylation factor-GTPase-activating proteins is required for Sec14p-dependent Golgi secretory function in yeast. Mol Biol Cell 13: 2193–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Sun HQ, Janmey PA, Yin HL (1992) Identification of a polyphosphoinositide-binding sequence in an actin monomer-binding domain of gelsolin. J Biol Chem 267: 14616–14621 [PubMed] [Google Scholar]
- Zhang D, Wadsworth P, Hepler PK (1990) Microtubule dynamics in living dividing plant cells: confocal imaging of microinjected fluorescent brain tubulin. Proc Natl Acad Sci USA 87: 8820–8824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Bednarek SY, Sanderfoot AA, Alonso J, Ecker JR, Raikhel NV (2002) NPSN11 is a cell plate-associated SNARE protein that interacts with the syntaxin KNOLLE. Plant Physiol 129: 530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Nishizawa N, Wu Y, Kost B, Chua NH (2000) KORRIGAN, an Arabidopsis endo-1,4-beta-glucanase, localizes to the cell plate by polarized targeting and is essential for cytokinesis. Plant Cell 12: 1137–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]