Abstract
Biological oscillations with an ultradian time scale of 1 to several hours include cycles in behavioral arousal, episodic glucocorticoid release, and gene expression. Ultradian rhythms are thought to have an extrinsic origin because of a perceived absence of ultradian rhythmicity in vitro and a lack of known molecular ultradian oscillators. We designed a novel, non–spectral-analysis method of separating ultradian from circadian components and applied it to a published gene expression dataset with an ultradian sampling resolution. Ultradian rhythms in mouse hepatocytes in vivo have been published, and we validated our approach using this control by confirming 175 of 323 ultradian genes identified in a prior study and found 862 additional ultradian genes. For the first time, we now report ultradian expression of >900 genes in vitro. Sixty genes exhibited ultradian transcriptional rhythmicity, both in vivo and in vitro, including 5 genes involved in the cell cycle. Within these 60 genes, we identified significant enrichment of specific DNA motifs in the 1000 bp proximal promotor, some of which associate with known transcriptional factors. These findings are in strong support of instrinsically driven ultradian rhythms and expose potential molecular mechanisms and functions underlying ultradian rhythms that remain unknown.—Van der Veen, D. R., Gerkema, M. P. Unmasking ultradian rhythms in gene expression.
Keywords: biological rhythm, circadian, metabolism, cell culture, transcriptome
Biological rhythms are widespread in behavior and physiology (1), and in past decades, the principal molecular mechanisms driving 24-h rhythms at the cellular level have been identified (2). From this work, it has emerged that these circadian rhythms play a critical role in human health and well-being and that the adverse effects of disrupting biological rhythms include obesity, diabetes, cancer, and mood disorders (3–5). We know considerably less about ultradian rhythmicity, which is a catch-all term for biological rhythms, with periods ranging from milliseconds to hours. Of particular interest to us are ultradian rhythms in the hourly range. Well-known examples of such rhythms include cycles in behavioral arousal (6–9), glucocorticoid level (10), rapid eye movement (REM)–non-REM sleep cycle (11), central monoamine release (12), cellular metabolism (13), and gene expression (14). Despite the broad recognition that these cycles exist, we know nothing about the biological mechanism driving these rhythms and hardly know their functional significance (15).
Ultradian rhythms are prevalent across species, and there are good arguments that they are intrinsically driven and not just imposed by external cycles. Ultradian behavioral locomotor patterns persist under constant environmental conditions (6–8). Experimental deprivation of sleep and food intake strongly suggests an ultradian clock regulation of activity onsets in voles (8). When investigated in mammals, ultradian locomotor rhythmicity is independent of the central circadian clock, and brain substrates such as the retrochiasmatic area of the hypothalamus (16) and the midbrain dopaminergic system (12) have been found to be involved in driving these ultradian patterns. Moreover, ultradian rhythms in in vitro cell cultures have been reported for glucocorticoid release (17, 18), single-cell firing (19), and protein synthesis (20) suggesting that these rhythms are intrinsically driven at the cellular level, but mechanisms driving them remain unknown.
So far, a fundamental obstacle in elucidating ultradian mechanisms seems to be the identification of cellular molecular correlates of ultradian rhythms in vitro, despite conducive attempts to measure these (14). One of the main reasons for this lack of success may be that ultradian rhythms are often coexpressed with circadian rhythms, which results in ultradian rhythms being overshadowed, or masked by the coexpressed circadian rhythms and their harmonics.
Biological masking of ultradian rhythms is a common phenomenon in behavioral activity: ultradian locomotor patterns, in rodents and Drosophila, for example, can be challenging to discern when the same animals also exhibit robust circadian timing (6, 7). When these circadian patterns in behavior are attenuated or removed by means of a surgical (16, 21) or genetic lesion (7, 22–27) of the circadian clock, robust ultradian locomotor rhythms appear. Coexpression of circadian and ultradian rhythms may also be prevalent in gene expression; this notion is supported by the recent finding that Per1, Per2, and Bmal1, all genes that are central to the circadian clock, exhibit both circadian and ultradian expression patterns in the hypothalamus of freely moving rats (28).
We have shown in a prior study that the hepatic expression of these clock genes is associated with both the ultradian and circadian timing system in the vole, a rodent in which the balance between ultradian and circadian timing of behavior can be altered (29). When the vole expresses strong ultradian behavioral patterns, liver expression of these clock genes is flat on a circadian time scale, whereas these same genes exhibit robust circadian expression patterns when the voles show strong circadian timing of behavior when they are housed with a running wheel, or food access is restricted to a 12 h period (30).
These findings confirm the presence of coexpression of ultradian and circadian rhythms in gene expression and suggest that these rhythms are more or less apparent (or more or less masked), depending on the relative contribution of the ultradian timing system to overall biological timing. Moreover, we tend to measure biological rhythms in our experiments on a circadian resolution (i.e., every 3 or 4 h), which captures only the circadian, not the ultradian, timing. We hypothesize that this “parallactic” (31) circadian view, at least in part, underlies our lack of success in detecting ultradian rhythms in gene expression under in vitro conditions. An added complication is the common practice in chronobiological signal processing to identify a single (circadian) rhythm and deem higher frequency signals as mathematical harmonics, rather than resolving them as a second (or more) coexpressed rhythm.
To resolve the issues surrounding the hypothesized masked ultradian rhythmicity, we set out to develop a novel analysis pipeline of gene expression that filters out low-frequency, circadian, and stochastic variation in time series of gene expression and relies on analysis methods in the time-, rather than frequency-domain. Using this method on the only publicly available time series of gene expression on an ultradian resolution (14), we for the first time identified expression of bona fide ultradian rhythms in gene expression in vitro. We showed that both in vivo and in vitro ultradian gene expression is significantly enriched for metabolic processes and that 60 genes exhibit ultradian expression both in vivo and in vitro. These 60 genes include genes involved in the cell cycle and are significantly enriched with several DNA motifs in their proximal promotor, which could hold the first clues to unraveling the mechanism that drives ultradian gene expression.
MATERIALS AND METHODS
We developed a novel 3–criteria-based, non–spectral-analysis pipeline for detecting ultradian rhythmicity, which is based on autocorrelation—a method that lies within the time domain and does not fit harmonics (32, 33). Our approach was used to interrogate data for rhythmicity within a period range of 3–14 h that satisfied 3 a priori criteria: the rhythmicity had to be expressed with similar periods throughout the whole dataset; the rhythmicity had to persist after the removal of a low-frequency fundamental signal; and the rhythmic waveform had to be uniformly expressed over all cycles.
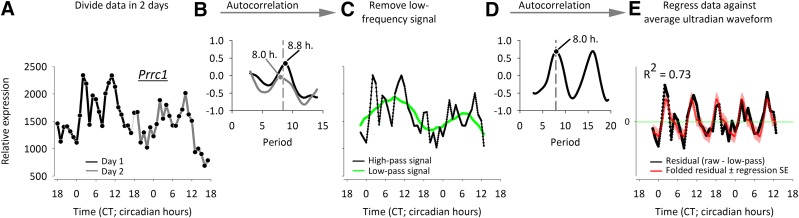
Our method is graphically described in Fig. 1, in the 48-h mRNA expression profile of the proline-rich coiled–coil 1 (Prrc1) gene, as reported in the mouse liver by Hughes et al. (14). The resolution of the autocorrelation method is limited by the sampling frequency, and, as a first step, we linearly interpolated the dataset to a 0.1-h resolution, for the purpose of obtaining this period resolution.
Figure 1.
Method of detection of ultradian rhythms in gene expression, with the 48-h expression profile of Prrc1 used as an example. A, B) An expression pattern is considered potentially ultradian when both the first and second half of the data (A) exhibit similar periodicity in an autocorrelation analysis (B). C, D) The second criterion, that an ultradian rhythm is not an artifact of a low-frequency fundamental signal, is tested by applying a low-pass filter (C), and retesting for ultradian periods using autocorrelation analysis (D). E) Finally, the ultradian waveform must consistently be expressed throughout the dataset, as evidenced by observing a value of R2 ≥ 0.6 in the regression of the average ultradian waveform against the actual signal.
To test the first criterion that ultradian rhythmicity be consistently evident throughout the time series, we divided the 48-h time series into 2 equal 24-h periods (d 1 and 2; Fig. 1A) and used autocorrelation to establish the potential ultradian period (Fig. 1B). Any probe that did not exhibit autocorrelation periods in d 1 and 2 that were within 2 h of each other were rejected and not considered for further analysis.
To satisfy the second criterion, that the ultradian rhythmicity be insensitive to removal of low-frequency signals, we applied a low-pass filter on the entire 48-h dataset through boxcar smoothing, with a window size of the average ultradian period of d 1 and 2 (Fig. 1C). This step unmasks optional ultradian rhythms and prohibits the occurrence of harmonics of a fundamental circadian signal. The residual signal was reanalyzed by using autocorrelation analysis of the average ultradian waveforms against the data for the whole period (Fig. 1D) and was rejected if the most significant period was over 13.5 h. The probe was only accepted if the autocorrelation period of d 1 and 2 and that of the ultradian residual were within 2 h of each other.
The third criterion, which was that the ultradian waveform be consistently expressed throughout the dataset, was tested by establishing the average ultradian waveform by folding the data on that ultradian period (29, 34). This average waveform was nonlinearly regressed against the residual ultradian data, and a cutoff value for the regression value was set. This cutoff was investigated by finding the 95% confidence limit (1.96 sd) of the normal distribution of the regression coefficients (R2). For the biological data from Hughes et al. (14), all potential ultradian signals in the in vivo liver and in vitro fibroblast datasets are shown separately (Supplemental Fig. S1; 0.64 and 0.57 for the liver and fibroblast datasets, respectively), and a combined 95% cutoff of those distributions was set at R2 ≥ 0.6.
We examined the false-positive rate of our approach by feeding 3 noise datasets, consisting of 45,000 synthetic probes, through our pipeline. All 48 time points for a given probe varied in value within the bounds of an actual biological probe. The 3 noise datasets were dataset 1, white noise: time point values that were randomly varied between the minimum and maximum of a real probe; dataset 2, gaussian white noise: 48 random values that were normally distributed around the mean of a real probe, with a standard deviation of that real probe; and dataset 3, circadian sine combined with 50% gaussian white noise: 48 values describing a circadian sine with random phase and period between 20 and 28 h around the mean and standard deviation of a real probe, where 50% of the variability was gaussian white noise, as in dataset 2.
Our analysis pipeline found 168, 202, and 33 false positives in 45,000 probes in noise datasets 1, 2, and 3, respectively, corresponding to false-positive percentages of 0.37, 0.45, and 0.07%.
The now-established analysis pipeline was then applied to the publicly available 48-h dataset of hourly transcriptome measurements in mouse liver tissue in vivo and NIH 3T3 cells in vitro that was published by Hughes et al. (14). Transcriptome data were downloaded from the Gene Expression Omnibus data repository (35) (GSE11923 and GSE11922, respectively). The in vivo mouse liver data were originally acquired by pooling samples of 3 to 5 C57Bl/6J mouse livers on Mouse Genome 430 2.0 Arrays (Affymetrix, Santa Clara, CA, USA). Mice were entrained to a 12 h light, 12 h dark cycle and then released into constant darkness with the first sample taken 18 h after the light–dark cycles was discontinued (which is circadian time 18). The in vitro U.S. National Institutes of Health (NIH) 3T3 data were originally acquired from NIH 3T3 cells run on Affymetrix Mouse Genome 430 2.0 Arrays. Circadian rhythms in the cells were synchronized by application of forskolin, and sampling was started 20 h later.
Gene Ontology analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of probes that exhibit ultradian expression patterns were performed in WebGestalt (36) using the affy_mouse430_2 array as the background distribution. Motif discovery on the 1000 bp proximal promotor sequences was performed in Meme Suite 4.10.2 (37), in normal mode, searching for motifs between 6 and 50 bp in length.
RESULTS
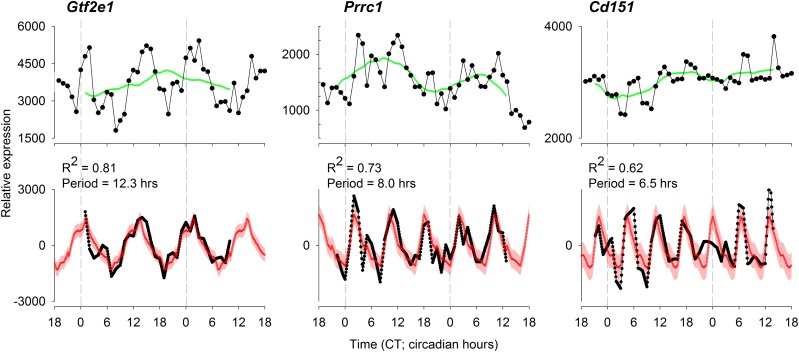
The application of our analysis pipeline on the 48 h time series of gene expression of the mouse liver in vivo resulted in a list of 1037 probes that passed all criteria for ultradian gene expression. Of the 323 probes that were identified as ultradian probes in the original publication (14), our method confirmed ultradian expression patterns in 175, exposing a large overlap between both methods, which adds to the validation of our analysis method. Figure 2 shows ultradian mRNA expression profiles of 3 probes (targeting the murine genes Gtf2e1, Prrc1, and Cd151) as examples of expression profiles that were not previously detected as exhibiting ultradian patterns. We chose 3 examples that exhibit robust ultradian expression patterns with periods of 12.3, 8.0, and 6.5 h—3 periods that are often observed as part of the harmonics of a fundamental circadian signal in frequency domain analysis. These harmonics have been removed in our analysis. Prrc1 was identified as a circadian probe in the original publication, suggesting that this probe exhibits temporal transcriptional dynamics in both the ultradian and circadian time scale.
Figure 2.
Three examples of probes detected as ultradian by our method. Top: the solid lines show the original expression data obtained from Hughes et al. (14), which were not identified as ultradian in their publication. Green line: the low-frequency signal that was removed in our method. Bottom: the achieved residual ultradian signal (plotted in black) and the average ± se ultradian waveform (plotted in red). All 3 probes passed all criteria for ultradian gene expression.
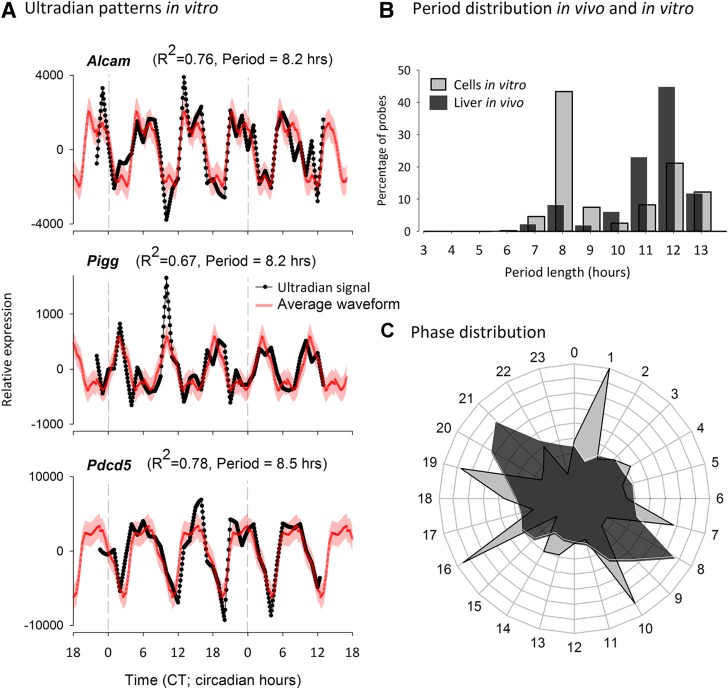
Given the confirmation of our method with previously identified ultradian rhythms in mRNA levels in liver cells in vivo and the identification of a substantial number of new ultradian rhythms in gene expression, we next applied our analysis to the ultradian time series of genome-wide gene expression in NIH 3T3 cells in vitro, which have been reported not to exhibit ultradian patterns in mRNA expression (14). By contrast to the previous findings, we identified 945 probes that passed all criteria for ultradian gene expression in vitro. Figure 3A depicts 3 examples of probes (targeting murine Alcam, Pigg, and Pdcd5), all exhibiting robust ultradian mRNA expression patterns in NIH 3T3 cells in vitro. These 3 examples exhibited rhythmicity within an 8-h period, and we generated a phase distribution plot (Fig 3B) that confirmed that a large cohort of our probes showed periods ∼8–9 h in NIH 3T3 cells in vitro. Ultradian periods in the in vivo murine liver transcriptome dataset also exhibited a cohort of periods of ∼7–8 h, but a larger fraction of probes exhibited periods of ∼12–13 h. This difference in period distribution between in vivo liver tissue and in vitro NIH 3T3 cells was also reflected in the phase distribution of peaks in mRNA expression profiles, as shown in Fig. 3C. Time courses of the low-pass residuals exhibited substantial variation between ultradian probes, with peak expression values occurring throughout the time span (Supplemental Fig. S2).
Figure 3.
A) Examples of ultradian residuals (black) and average ultradian waveform (red) for 3 probes classified as exhibiting ultradian expression patterns in NIH-3T3 cells in vitro. B) Comparison of the period distributions of ultradian rhythms in vivo and in vitro demonstrates that the largest group of in vitro ultradian rhythms oscillate with a period close to 8–9 h, whereas the largest group of ultradian rhythms in vivo exhibit a period close to 12–13 h. C) The difference in the period distribution between in vivo and in vitro ultradian rhythms is also reflected by the clustering of ultradian peak phases, which exhibit more clusters in vitro than in vivo.
We next used WebGestalt (36) to examine the Gene Ontology of the lists of probes that express ultradian gene expression in the mouse liver in vivo and the list of ultradian genes found in NIH 3T3 cells separately. Table 1 presents the top 10 categories of both lists and demonstrates a significant enrichment for metabolic process under both conditions, with 5 of 10 enriched processes identical between both conditions.
TABLE 1.
Top 10 Gene Ontology terms for ultradian genes in both the liver in vivo and NIH-3T3 cells in vitro
| Process | Gene (%) | P |
|---|---|---|
| In vivo liver | ||
| Organic substance metabolic process | 41.90 | 0.0364 |
| Primary metabolic process | 40.53 | 0.0316 |
| Cellular metabolic process | 40.21 | 0.0316 |
| Macromolecule metabolic process | 35.03 | 0.016 |
| Cellular macromolecule metabolic process | 32.28 | 0.0088 |
| Intracellular transport | 6.56 | 0.0316 |
| Protein catabolic process | 4.13 | 0.0316 |
| Proteolysis involved in cellular protein catabolic process | 3.07 | 0.0588 |
| Ubiquitin-dependent protein catabolic process | 2.86 | 0.0588 |
| Intrinsic apoptotic signaling pathway | 1.38 | 0.0588 |
| In vitro fibroblasts | ||
| Metabolic process | 43.60 | 5.07E-05 |
| Single-organism metabolic process | 40.74 | 0.0002 |
| Organic substance metabolic process | 39.05 | 0.0006 |
| Cellular metabolic process | 38.52 | 4.98E-05 |
| Primary metabolic process | 37.88 | 0.0003 |
| Macromolecule metabolic process | 32.49 | 0.0002 |
| Cellular macromolecule metabolic process | 30.26 | 5.07E-05 |
| Nucleic acid metabolic process | 20.21 | 0.0006 |
| Chromosome organization | 5.40 | 0.0002 |
| Protein modification by small protein conjugation or removal | 4.13 | 0.0006 |
With a view to determining common mechanisms and pathways of ultradian gene expression, we identified 28 unique probes that exhibit ultradian gene expression, both in the mouse liver in vivo and in NIH 3T3 cells in vitro. Furthermore, using the less stringent approach by looking at genes irrespective of probes led to identification of 60 genes that exhibited ultradian mRNA patterns in both in vivo and in vitro conditions. Although 60 genes is a low number for Gene Ontology analysis, KEGG pathway analysis revealed significant enrichments, which are presented in Table 2. Notably, KEGG analysis showed that 3 of 60 genes (Stag1, Ywhae, and E2f3) that are ultradian in vivo and in vitro are involved in the cell cycle, and we found that a further 2 of the 48 genes (Terf1 and Usp28) are involved in cell cycle checkpoints (38, 39).
TABLE 2.
Significant KEGG pathways for genes that exhibit ultradian mRNA expression profiles in both in vivo and in vitro conditions
| KEGG pathway | P |
|---|---|
| Cell cycle | 0.0318 |
| Base excision repair | 0.0318 |
| Non–small-cell lung cancer | 0.0426 |
| Glioma | 0.0426 |
| Chronic myeloid leukemia | 0.0442 |
| ErbB signaling pathway | 0.0482 |
| Prostate cancer | 0.0482 |
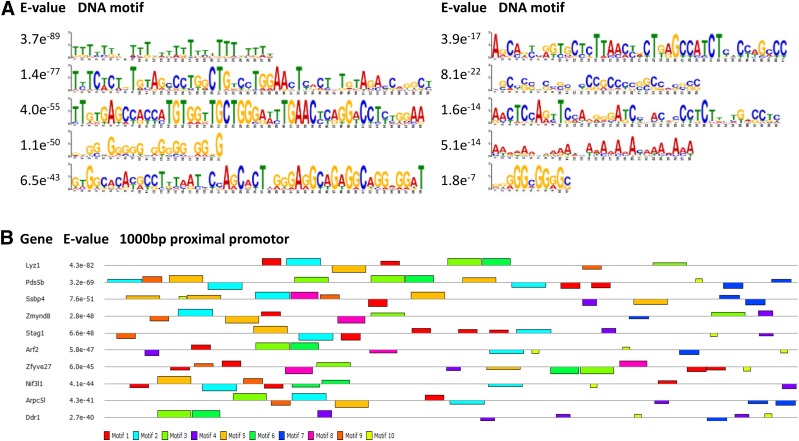
As a last step, we submitted the 1000 bp proximal promotor of these 60 genes that exhibit ultradian mRNA expression profiles in both conditions to Multiple EM for Motif Elicitation (MEME) analysis (37) to identify DNA motifs that may be enriched in these proximal promotors and serve as recognition sites for transcription factors. Figure 4A shows the 10 most significantly enriched DNA motifs, and many of the proximal promotors expressed several of these motifs, of which the most striking examples are given in Fig. 4B.
Figure 4.
MEME analysis of the 1000-bp proximal promotor of the 60 genes that exhibit ultradian mRNA expression profiles in both in vivo and in vitro conditions.
DISCUSSION
Our approach to unmasking ultradian rhythms for the first time exposed ultradian rhythms in in vitro gene expression, a critical line of evidence in strong support of intrinsically driven ultradian rhythmicity that previous attempts could not uncover.
We identified these intrinsically driven ultradian expression patterns in existing datasets generated and published by Hughes and colleagues (14), and the large overlap of ultradian genes identified by them and us in the mouse liver in vivo cross-validates our approaches. The identification of ultradian gene expression in vitro, where earlier approaches were unsuccessful, is in support of our hypothesis that coexpressed ultradian rhythms (with circadian or other long-term stochastic processes) can be unmasked by filter procedures before signal analysis. In these datasets, the time course trajectories of these long-term processes exhibited substantial variation in expression patterns, which testifies to the varied nature of these masking signals. This finding highlights that unmasking of ultradian rhythms cannot be achieved through application of a single static filter, but only by application of a dynamic filter, which may be a further reason that ultradian rhythms go unnoticed.
Because ultradian rhythms are so diverse, with periods ranging from milliseconds to hours, it is unlikely that they share a common molecular mechanism. Our current focus on rhythms within the hourly range resulted in a diversity of periods across only the 3- to 13-h range. We are acutely aware that the present sampling resolution precluded us from detecting faster rhythms, which for now remains an upcoming challenge. Within our current range, one way forward is to resolve several underlying mechanisms based on clusters of genes within the same period range. Within our period distribution, we saw clear clusters of genes at 4, 8, and 12 h, which cannot be perceived as harmonics of circadian rhythms because our detection methods ruled out mathematical harmonics. We thus showed true biological expression of these ultradian rhythms in gene expression. In terms of causative mechanisms, it is too early to say whether these clusters of rhythms are the result of unique novel mechanism, or even the result of specific coinciding circadian clocks (14). The latter is contrasted, however, by observations of ultradian rhythmicity when circadian clocks are excluded. The observation of these rhythms provides clear validity that some intrinsic mechanism is involved.
Period clustering of different ultradian rhythms, opens the option of mutual coordination or resonance. In terms of functional significance, ultradian rhythmicity is often subjectively associated with metabolic homeostasis in vivo (15, 30) and cellular metabolism (13, 40), but these associations are complicated by the difference in ultradian period length. Such a metabolic relevance is objectively supported by the significant enrichment of metabolically relevant genes in our lists of ultradian genes, both in vivo and in vitro. One consequence of this may be that the plentiful metabolic environment of cells in culture reduces the strength, or robustness of ultradian rhythmicity in vitro, in effect causing them to be even more masked by circadian rhythms.
A further clue to the significance of ultradian rhythms in gene expression comes from the specific enrichment of genes associated with the cell cycle. Molecular interactions between the circadian clock and cell cycle checkpoints have been known for some time (41), and although the connection has been made in a completely different species, yeast ultradian cycles have been linked with the cell cycle and metabolism (42). The mammalian cell cycle exhibits a 24-h rhythm, and the molecular circadian clock is proposed to govern daily gating and phase-locking of the cell cycle (43, 44). It has been hypothesized that this circadian timing of the cell cycle serves to protect DNA replication against UV- and ROS-mediated damage (45) and that such rhythms at the cellular level of ROS are strongly associated with circadian rhythms in metabolism (3). The periods of the ultradian genes that we report to be associated with the cell cycle were within the 8.2- to13.2-h range, and one hypothesis may be that they govern the ultradian gating of the cell cycle. Given that we observed a comprehensive enrichment of ultradian gene expression for genes involved in metabolism, such ultradian gating may represent temporal segregation of the DNA replication and metabolism on an ultradian scale, as has also been hypothesized for circadian gating of the cell cycle.
As part of our autocorrelation analysis, we must consider the potential presence of nondeterministic peaks at 1/f or 1/f2. For circadian analysis we often assume that the frequency closest to 24 h is the fundamental signal and discard other frequencies, but for ultradian analysis, the fundamental period is unknown, and we cannot perform such an analysis. For this reason, we reverted to the original time series data, to test our hypothesis based on the autocorrelation analysis. If the hypothesized ultradian period is in fact a spectral alias rather than a deterministic peak in the autocorrelation, the nonlinear regression of the time series data against the ultradian mask should fail to reach a regression coefficient that falls outside the 95% confidence limits of the average regression coefficient. This statistical assumption is corroborated by the results of our (oscillatory) noise models, where the vast majority of the false-positive probes based on autocorrelation analysis failed to reach an R2 value in the time domain that significantly differed from noise.
If a molecular mechanism can drive biological rhythm, it can be hypothesized that it would do so in both in vivo and in vitro conditions. Analogous to the circadian timing system, in which it has been shown that, of the only 10 genes that are transcribed with a circadian rhythm in all analyzed tissues, 7 are central to the cellular circadian clock mechanism (46), we found that 60 genes exhibited ultradian gene expression in both in vivo and in vitro conditions, of which 5 were associated with the cell cycle.
Many analytical tools currently used in the field, such as those that lie within the frequency domain, implicitly assume sinusoidal waveforms. It is important to state that not all our ultradian gene expression patterns exhibited such a sinusoidal waveform. Indeed, examples of nonsinusoidal pulsatile ultradian rhythms have been extensively reported in behavioral activity (6, 7, 16, 21–27), hormone secretion (10), and expression of circadian clock genes, such as Per1, Per2, and Bmal1 (28). Such overt, physiological, and molecular pulsatile ultradian rhythmicity is in line with the functional validity of nonsinusoidal ultradian rhythms at the level of gene expression.
It is well established that rhythmic gene expression does not necessarily lead to rhythmic protein abundance (47), and, given the time scale of ultradian rhythmicity and protein stability, it should be established to what extent ultradian rhythms in protein concentration are present and with which physiological and behavioral processes these molecular ultradian rhythms are associated. However, bona fide ultradian rhythms in gene expression in vitro provide motivation to pursue such links between the molecular generation of ultradian rhythms and the well-known ultradian rhythms in behavior and physiology.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Hughes and colleagues (14) for the generation and publication of the data used in this study and for making the data publicly available, allowing us to test our hypothesis and analysis pipeline, and the valuable review comments received that were helpful in improving the manuscript. The authors declare no conflicts of interest.
Glossary
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- NIH
U.S. National Institutes of Health
- REM
rapid eye movement
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. R. van der Veen and M. P. Gerkema conceived of the hypothesis, designed, and completed the experimental approach, analyzed the data, and wrote the paper.
REFERENCES
- 1.Aschoff J., Meyer-Lohmann J. (1954) Die Schubfolge der lokomotorischen Aktivität bei Nagern [Burst sequence of locomotoric activity in rodents]. Pflugers Arch. 260, 81–86 [DOI] [PubMed] [Google Scholar]
- 2.Partch C. L., Green C. B., Takahashi J. S. (2014) Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 24, 90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bass J. (2012) Circadian topology of metabolism. Nature 491, 348–356 [DOI] [PubMed] [Google Scholar]
- 4.Fu L., Kettner N. M. (2013) The circadian clock in cancer development and therapy. Prog. Mol. Biol. Transl. Sci. 119, 221–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bechtel W. (2015) Circadian rhythms and mood disorders: are the phenomena and mechanisms causally related? Front. Psychiatry 6, 118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowse H., Umemori J., Koide T. (2010) Ultradian components in the locomotor activity rhythms of the genetically normal mouse, Mus musculus. J. Exp. Biol. 213, 1788–1795 [DOI] [PubMed] [Google Scholar]
- 7.Dowse H. B., Hall J. C., Ringo J. M. (1987) Circadian and ultradian rhythms in period mutants of Drosophila melanogaster. Behav. Genet. 17, 19–35 [DOI] [PubMed] [Google Scholar]
- 8.Gerkema M. P., van der Leest F. (1991) Ongoing ultradian activity rhythms in the common vole, Microtus arvalis, during deprivations of food, water and rest. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 168, 591–597 [DOI] [PubMed] [Google Scholar]
- 9.Van Oort B. E., Tyler N. J., Gerkema M. P., Folkow L., Stokkan K. A. (2007) Where clocks are redundant: weak circadian mechanisms in reindeer living under polar photic conditions. Naturwissenschaften 94, 183–194 [DOI] [PubMed] [Google Scholar]
- 10.Lightman S. L., Conway-Campbell B. L. (2010) The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat. Rev. Neurosci. 11, 710–718 [DOI] [PubMed] [Google Scholar]
- 11.Aserinsky E., Kleitman N. (1953) Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 118, 273–274 [DOI] [PubMed] [Google Scholar]
- 12.Blum I. D., Zhu L., Moquin L., Kokoeva M. V., Gratton A., Giros B., Storch K. F. (2014) A highly-tunable dopaminergic oscillator generates ultradian rhythms of behavioral arousal. eLife 3, 05105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brodsky V. Y. (2014) Circahoralian (ultradian) metabolic rhythms. Biochemistry (Mosc.) 79, 483–495 [DOI] [PubMed] [Google Scholar]
- 14.Hughes M. E., DiTacchio L., Hayes K. R., Vollmers C., Pulivarthy S., Baggs J. E., Panda S., Hogenesch J. B. (2009) Harmonics of circadian gene transcription in mammals. PLoS Genet. 5, e1000442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aschoff, J., Gerkema, M. P. (1985) On diversity and uniformity of ultradian rhythms. In Ultradian Rhythms in Physiology and Behaviour (Schulz, H., Lavie, P., eds), pp. 321–334, Springer Verlag, New York doi:10.1007/978-3-642-70483-3_21. [Google Scholar]
- 16.Gerkema M. P., Groos G. A., Daan S. (1990) Differential elimination of circadian and ultradian rhythmicity by hypothalamic lesions in the common vole, Microtus arvalis. J. Biol. Rhythms 5, 81–95 [DOI] [PubMed] [Google Scholar]
- 17.Ixart G., Barbanel G., Nouguier-Soulé J., Assenmacher I. (1991) A quantitative study of the pulsatile parameters of CRH-41 secretion in unanesthetized free-moving rats. Exp. Brain Res. 87, 153–158 [DOI] [PubMed] [Google Scholar]
- 18.Mershon J. L., Sehlhorst C. S., Rebar R. W., Liu J. H. (1992) Evidence of a corticotropin-releasing hormone pulse generator in the macaque hypothalamus. Endocrinology 130, 2991–2996 [DOI] [PubMed] [Google Scholar]
- 19.Schenda J., Vollrath L. (2000) Single-cell recordings from chick pineal glands in vitro reveal ultradian and circadian oscillations. Cell. Mol. Life Sci. 57, 1785–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brodsky V. Y., Zvezdina N. D. (2010) Melatonin as the most effective organizer of the rhythm of protein synthesis in hepatocytes in vitro and in vivo. Cell Biol. Int. 34, 1199–1204 [DOI] [PubMed] [Google Scholar]
- 21.Schwartz W. J., Zimmerman P. (1991) Lesions of the suprachiasmatic nucleus disrupt circadian locomotor rhythms in the mouse. Physiol. Behav. 49, 1283–1287 [DOI] [PubMed] [Google Scholar]
- 22.Bunger M. K., Wilsbacher L. D., Moran S. M., Clendenin C., Radcliffe L. A., Hogenesch J. B., Simon M. C., Takahashi J. S., Bradfield C. A. (2000) Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seki Y., Tanimura T. (2014) Ultradian rhythm unmasked in the Pdf clock mutant of Drosophila. J. Biosci. 39, 585–594 [DOI] [PubMed] [Google Scholar]
- 24.Van der Horst G. T., Muijtjens M., Kobayashi K., Takano R., Kanno S., Takao M., de Wit J., Verkerk A., Eker A. P., van Leenen D., Buijs R., Bootsma D., Hoeijmakers J. H., Yasui A. (1999) Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627–630 [DOI] [PubMed] [Google Scholar]
- 25.Vitaterna M. H., King D. P., Chang A. M., Kornhauser J. M., Lowrey P. L., McDonald J. D., Dove W. F., Pinto L. H., Turek F. W., Takahashi J. S. (1994) Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264, 719–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng B., Albrecht U., Kaasik K., Sage M., Lu W., Vaishnav S., Li Q., Sun Z. S., Eichele G., Bradley A., Lee C. C. (2001) Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell 105, 683–694 [DOI] [PubMed] [Google Scholar]
- 27.Zheng B., Larkin D. W., Albrecht U., Sun Z. S., Sage M., Eichele G., Lee C. C., Bradley A. (1999) The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400, 169–173 [DOI] [PubMed] [Google Scholar]
- 28.Ono D., Honma K., Honma S. (2015) Circadian and ultradian rhythms of clock gene expression in the suprachiasmatic nucleus of freely moving mice. Sci. Rep. 5, 12310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van der Veen D. R., Saaltink D. J., Gerkema M. P. (2011) Behavioral responses to combinations of timed light, food availability, and ultradian rhythms in the common vole (Microtus arvalis). Chronobiol. Int. 28, 563–571 [DOI] [PubMed] [Google Scholar]
- 30.Van der Veen D. R., Minh N. L., Gos P., Arneric M., Gerkema M. P., Schibler U. (2006) Impact of behavior on central and peripheral circadian clocks in the common vole Microtus arvalis, a mammal with ultradian rhythms. Proc. Natl. Acad. Sci. USA 103, 3393–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Enright J. T. (1989) The parallactic view, statistical testing, and circular reasoning. J. Biol. Rhythms 4, 295–304 [PubMed] [Google Scholar]
- 32.De Prins J., Waldura J. (1993) Sightseeing around the single cosinor. Chronobiol. Int. 10, 395–400 [DOI] [PubMed] [Google Scholar]
- 33.Dutilleul P. (1995) Rhythms and autocorrelation analysis. Biol. Rhythm Res. 26, 173–193 [Google Scholar]
- 34.Strijkstra A. M., Meerlo P., Beersma D. G. (1999) Forced desynchrony of circadian rhythms of body temperature and activity in rats. Chronobiol. Int. 16, 431–440 [DOI] [PubMed] [Google Scholar]
- 35.Barrett T., Wilhite S. E., Ledoux P., Evangelista C., Kim I. F., Tomashevsky M., Marshall K. A., Phillippy K. H., Sherman P. M., Holko M., Yefanov A., Lee H., Zhang N., Robertson C. L., Serova N., Davis S., Soboleva A. (2013) NCBI GEO: archive for functional genomics data sets: update. Nucleic Acids Res. 41, D991–D995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duncan D., Prodduturi N., Zhang B. (2010) WebGestalt2: an updated and expanded version of the Web-based Gene Set Analysis Toolkit. BMC Bioinformatics 11, P10 20053291 [Google Scholar]
- 37. Bailey, T. L., Elkan, C. (1994) Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 2, 28–36. [PubMed] [Google Scholar]
- 38.Shen M., Haggblom C., Vogt M., Hunter T., Lu K. P. (1997) Characterization and cell cycle regulation of the related human telomeric proteins Pin2 and TRF1 suggest a role in mitosis. Proc. Natl. Acad. Sci. USA 94, 13618–13623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reyes-Turcu F. E., Ventii K. H., Wilkinson K. D. (2009) Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 78, 363–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ventre S., Indrieri A., Fracassi C., Franco B., Conte I., Cardone L., di Bernardo D. (2015) Metabolic regulation of the ultradian oscillator Hes1 by reactive oxygen species. J. Mol. Biol. 427, 1887–1902 [DOI] [PubMed] [Google Scholar]
- 41.Hunt T., Sassone-Corsi P. (2007) Riding tandem: circadian clocks and the cell cycle. Cell 129, 461–464 [DOI] [PubMed] [Google Scholar]
- 42.Tu B. P., Kudlicki A., Rowicka M., McKnight S. L. (2005) Logic of the yeast metabolic cycle: temporal compartmentalization of cellular processes. Science 310, 1152–1158 [DOI] [PubMed] [Google Scholar]
- 43.Matsuo T., Yamaguchi S., Mitsui S., Emi A., Shimoda F., Okamura H. (2003) Control mechanism of the circadian clock for timing of cell division in vivo. Science 302, 255–259 [DOI] [PubMed] [Google Scholar]
- 44.Feillet C., Krusche P., Tamanini F., Janssens R. C., Downey M. J., Martin P., Teboul M., Saito S., Lévi F. A., Bretschneider T., van der Horst G. T. J., Delaunay F., Rand D. A. (2014) Phase locking and multiple oscillating attractors for the coupled mammalian clock and cell cycle. Proc. Natl. Acad. Sci. USA 111, 9828–9833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaix A., Zarrinpar A., Panda S. (2016) The circadian coordination of cell biology. J. Cell Biol. 215, 15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang R., Lahens N. F., Ballance H. I., Hughes M. E., Hogenesch J. B. (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl. Acad. Sci. USA 111, 16219–16224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mauvoisin D., Wang J., Jouffe C., Martin E., Atger F., Waridel P., Quadroni M., Gachon F., Naef F. (2014) Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc. Natl. Acad. Sci. USA 111, 167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.