Abstract
Cells contain specialized DNA polymerases that are able to copy past lesions with an associated risk of generating mutations, the major cause of cancer. Here, we reconstitute translesion synthesis (TLS) using the replicative (Pol III) and major bypass (Pol V) DNA polymerases from Escherichia coli in the presence of accessory factors. When the replicative polymerase disconnects from the template in the vicinity of a lesion, Pol V binds the blocked replication intermediate and forms a stable complex by means of a dual interaction with the tip of the RecA filament and the β-clamp, the processivity factor donated by the blocked Pol III holoenzyme. Both interactions are required to confer to Pol V the processivity that will allow it synthesize, in a single binding event, a TLS patch long enough to support further extension by Pol III. In the absence of these accessory factors, the patch synthesized by Pol V is too short, being degraded by the Pol III-associated exonuclease activity that senses the distortion induced by the lesion, thus leading to an aborted bypass process.
Keywords: Pol III holoenzyme, Pol V, polymerase switch, processivity factor β-clamp, RecA filament
Introduction
In all living cells, DNA continuously incurs damages by endogenous and exogenous agents. Excision repair pathways remove lesions in an essentially error-free way (Friedberg et al, 1995). Despite these robust repair pathways, residual levels of lesions persist in DNA thus impairing the progression of replicative DNA polymerases. Escherichia coli cells respond to stress by inducing coregulated sets of genes, such as the SOS regulon (Radman, 1975). The initial molecular signal that causes activation of the SOS response is believed to be the single-stranded DNA that forms during replication of damaged DNA (Sassanfar and Roberts, 1990). Recently, it was shown that a replication block in one strands triggers the functional uncoupling of the coordinated replication of the leading and lagging strands in vivo, thus producing extended single-stranded DNA regions mostly when the lesion resides in the leading strand (Pages and Fuchs, 2003). Single-stranded DNA is then converted into the ‘SOS signal' via the formation of a RecA nucleofilament that facilitates autocleavage of the LexA repressor and consequently upregulates the transcription of at least 43 SOS genes (Fernandez De Henestrosa et al, 2000; Courcelle et al, 2001). Distinct cellular strategies usually referred to as tolerance mechanisms have evolved to cope with replication-blocking lesions. Among the tolerance mechanisms, translesion synthesis (TLS) involves copying the damage-containing template with the help of specialized DNA polymerases that transiently replace the replicative DNA polymerase (Cordonnier and Fuchs, 1999; Sutton and Walker, 2001; Goodman, 2002; Pages and Fuchs, 2002). Among the SOS-induced genes, three encode DNA polymerases (polB, dinB and umuDC), which have all recently been shown to be involved in lesion bypass in vivo (Napolitano et al, 2000; Wagner et al, 2002). Pol V, the major bypass polymerase (Reuven et al, 1999; Tang et al, 1999), is composed of one UmuC and a dimer of UmuD′ molecules, the RecA* catalyzed autocleaved form of UmuD (Burckhardt et al, 1988; Shinagawa et al, 1988). In addition, Pol II and Pol IV may also participate in TLS either alone or in combination with Pol V depending upon the nature of the lesion and its local sequence context (Napolitano et al, 2000; Wagner et al, 2002). The process of TLS, which is inevitably error-prone, is responsible for the majority of induced point mutations. In vivo, the process of TLS involves the recruitment of one or several specialized DNA polymerases (Napolitano et al, 2000; Bresson and Fuchs, 2002; Prakash and Prakash, 2002; Wagner et al, 2002) that will perform limited DNA synthesis in the vicinity of the damage, thus allowing the replicative polymerase to resume synthesis following bypass of the lesion. A key factor involved in these processes is the general replication processivity factor, the β-clamp, shown to interact with all E. coli DNA polymerases in vitro (Lopez de Saro et al, 2003) and in vivo (Dalrymple et al, 2001; Becherel et al, 2002; Lenne-Samuel et al, 2002). A key feature in the ‘DNA polymerase switch model' (Cordonnier and Fuchs, 1999) is the definition of the transition points between specialized and replicative polymerases. The size of the patch made by the bypass DNA polymerase (TLS patch) needs to be optimized: it should be sufficiently long to allow successful elongation upon rebinding of the replicative polymerase and at the same time it should not be too long, as the bypass polymerases exhibit low fidelity and would consequently be responsible for the induction of untargeted mutations.
In this paper we reconstituted the process of TLS using circular single-stranded DNA (ss-circular DNA) templates containing a single DNA lesion, the highly purified DNA polymerases from E. coli, namely the replicative Pol III holoenzyme (Pol III HE), Pol V and Pol II. The circular substrate used here allows stable loading of the necessary accessory factors, namely an ATP-activated RecA filament and the β-clamp. The present work highlights the key role of the β-clamp that is donated to Pol V by Pol III HE following its dissociation in the vicinity of the lesion site. The donated β-clamp specifically increases the processivity of Pol V, allowing it to synthesize, in a single binding event, a TLS patch long enough to escape Pol III-mediated exonuclease degradation. The minimal TLS assay described here recapitulates the major in vivo requirements of induced mutagenesis.
Results
Overall goals and strategy
The overall goal of the present investigation is to define the position of the switches between replicative and specialized DNA polymerases during lesion bypass. For this purpose, E. coli was chosen as a model system, as the genetics of induced mutagenesis in this organism has been well documented over the years (Friedberg et al, 1995). From the data gathered in vivo, it is clear that in order to reconstitute the whole process of TLS in vitro, one needs to set up an assay that not only includes the polymerases themselves but also some of the essential accessory factors that play a key role in the bypass process. Indeed, in addition to the replicative polymerase (Pol III HE; for a recent review, see McHenry, 2003) and the major bypass polymerase (Pol V), genetic data on umuDC-dependent mutagenesis clearly involve both an activated RecA-single-stranded DNA filament and the β-clamp, the general replication processivity factor (Dutreix et al, 1989; Becherel et al, 2002; Goodman, 2002). Following unsuccessful attempts with linear oligonucleotide substrates, we found that long circular single-stranded templates offer a simple experimental system to set up a minimal TLS assay. Indeed, these circular templates support both the stable loading of the β-clamp and the formation of extended RecA filaments in the presence of ATP.
The major objectives of the present work are (i) to define the position, with respect to the lesion site, of the switches between replicative and specialized DNA polymerases, (ii) to analyze the synthesis profiles when replicative and specialized polymerases are present either separately or together and (iii) to evaluate potential competition among polymerases during lesion bypass. We wanted to determine both the average length of the synthesis patch made by Pol V in a single binding event (defined here as the ‘TLS patch') and the minimal distance from the primer terminus a lesion needs to be located to escape degradation by the proofreading function upon Pol III rebinding.
Mixing replicative and specialized DNA polymerases: effect on lesion bypass profiles
In an attempt to reconstitute TLS, we performed a series of experiments involving replicative and bypass polymerases, either alone or in combination (Figure 1 and Table I). We purified DNA polymerase III* (Pol III*) (see Materials and methods), the dimeric form of the core DNA polymerase III that also includes the γ complex (McHenry, 2003). In the presence of the β-clamp, Pol III* forms E. coli's replicative enzyme, Pol III HE (McHenry, 2003). Pol V and Pol II were extensively purified and characterized as described (Becherel and Fuchs, 2001; Fujii et al, 2004). These experiments involve RecA-coated single-stranded circular templates in the presence of ATP and primers located 6 or 8 nucleotides (nt) upstream (running start conditions) from a single G-AAF adduct within the NarI (Figure 1A and B) or the 3G sequence context (Figure 1C), respectively. In E. coli, the NarI context is a hot spot for −2 frameshift mutagenesis induced by G-AAF adducts (Fuchs et al, 1981; Koffel-Schwartz et al, 1984). Extensive in vitro and in vivo studies have established that G-AAF adducts bound to the underlined guanine (5′-GGCGCC-) can be bypassed either by Pol V or Pol II in an error-free or a −2 frameshift pathway, respectively (Figure 1) (Napolitano et al, 2000; Becherel and Fuchs, 2001; Fujii et al, 2004). The 3G context is a comparatively weaker −1 frameshift hot spot that requires Pol V for both error-free and frameshift bypass (Napolitano et al, 2000).
Figure 1.
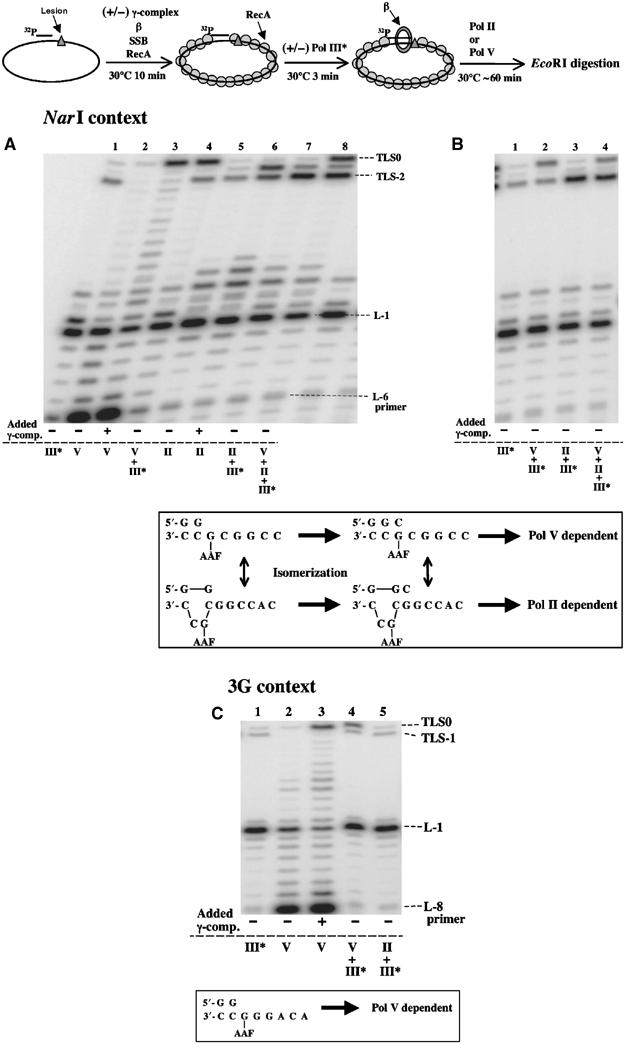
Interplay between polymerases during lesion bypass. All experiments involve circular single-stranded templates (≈2.7 kb) containing a G-AAF adduct located either within the NarI sequence primed with L-6 (A, B) or within the 3G sequence primed with L-8 (C). Standard reaction mixtures containing 50 nM β, 2 μM RecA and 10 nM SSB (see Materials and methods) were preincubated with or without γ complex. When indicated, reactions were initiated by adding Pol III* (III) for 3 min at 30°C, followed by the addition of Pol II and/or Pol V for an additional 60 min. Incubations with Pol V alone were performed for 15 min. The concentrations of enzymes used are as follows: (A) Pol III*: 4 nM, Pol II: 4 nM, Pol V: 100 nM; (B) Pol III*: 62.7 nM, Pol II: 4 nM, Pol V: 100 nM; (C) Pol III*: 20 nM, Pol II: 4 nM, Pol V: 100 nM. The reaction mixtures were digested by EcoRI located 11 and 14 nt downstream of the lesion in the 3G and NarI contexts, respectively. The reaction products are analyzed by 10% denaturing PAGE.
Table 1.
Efficiency of translesion synthesis (derived from Figure 1)
| A |
B |
C |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 5 | |
| Sample | |||||||||||||||||
| Pol IIIa | + | − | − | + | − | − | + | + | + | + | + | + | + | − | − | + | + |
| Pol V | − | + | + | + | − | − | − | + | − | + | − | + | − | + | + | + | − |
| Pol II | − | − | − | − | + | + | + | + | − | − | + | + | − | − | − | − | + |
| γ complex | − | − | + | − | − | + | − | − | − | − | − | − | − | − | + | − | − |
| Final product (%) | |||||||||||||||||
| TLS0 | 0 | <0.1a | 20a | 24 | <0.1 | <0.1 | <0.1 | 21 | 0 | 11 | <0.1 | 7.8 | 0 | <0.1a | 14 | 11 | <0.1 |
| TLS-1 | <0.1 | <0.1 | <0.1 | <0.1 | 0.5 | 19 | 4.2 | 3.7 | <0.1 | <0.1 | 0.3 | 0.2 | 1.7 | <0.1 | <0.1 | 1.5 | 2.0 |
| TLS-2 | 8.2 | <0.1 | <0.1 | 7.0 | 5.5 | 16 | 36 | 34 | 5.5 | 5.9 | 36 | 32 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Relative efficiencies of TLS (from Figure 1A–C with same lane numbers) were calculated by dividing the intensity of a given TLS band (TLS0, TLS-1 or TLS-2) by the summed intensities of the bands TLS0, TLS-1, TLS-2, L-1 and L0. TLS0 values have been corrected for the low amount of unmodified template as discussed in Results. | |||||||||||||||||
| aAdjusted for the extent of primer use in the absence (0.54 in (A) and 0.29 in (C)) and the presence (0.50 in (A) and 0.33 in (C) of the γ complex. | |||||||||||||||||
Activity of Pol III HE alone. In the NarI context, Pol III HE alone yields low amounts of bypass products, both a full-length product (TLS0) and a 2-nt shorter product (TLS-2) (Figure 1A, lane 1). The TLS0 band is not a genuine bypass product but results from a low contamination of our construction (≈0.5%) with adduct-free template, as the intensity of this band does not increase with time (data not shown). In contrast, the TLS-2 band slowly increases with incubation time (data not shown) and therefore reflects a weak capacity of Pol III HE to yield −2 frameshift bypass product within the NarI sequence context (5′-GGCGAAFCC-) in agreement with in vivo data (Napolitano et al, 2000). Similarly, in the 3G context, with Pol III HE alone (Figure 1C, lane 1), the minor TLS0 and TLS-1 bands result from a low contamination with unmodified template (0.2%) and a weak capacity of Pol III HE to yield −1 frameshift bypass product (0.5%), respectively. A weak band reflecting limited insertion of a nucleotide across G-AAF by Pol III HE (L0 band) is also seen in both NarI and 3G sequence contexts. In contrast, the intermediate bypass products migrating 1 and 2 nt past the lesion (bands L1 and L2; Figure 1A, lane 1) reflect NarI sequence-specific effects, as these bands are not seen in the 3G context (Figure 1C, lane 1).
Bypass activity of Pol V alone or in combination with Pol III HE. Pol V alone, that is, in the absence of added γ complex, exhibits weak bypass activity as seen by a series of intermediate TLS bands (Figure 1A, lane 2, and Figure 1C, lane 2). As discussed above, the full-length TLS0 band seen in these lanes results from the low lesion-free template contamination (Table I). In contrast, upon addition of free γ complex, the β-clamp present in the reaction mixture can now be loaded upon the template, allowing efficient Pol V-mediated lesion bypass (Figure 1A and C, lane 3, and Table I) (Fujii et al, 2004). When Pol III* is first added to the reaction mixture, no additional γ complex is needed for efficient Pol V-mediated lesion bypass (Figure 1A and C, lane 4). We suggest that upon dissociation of Pol III HE in the vicinity of the lesion site, its associated β-clamp stimulates Pol V-mediated bypass. A strikingly different pattern of TLS intermediates is also observed whether Pol III* is added or not. Indeed, with Pol V alone, a complete ladder of all intermediates between L-1 and TLS0 is observed, while in the presence of Pol III*, these intermediates are not seen (compare lanes 3 and 4 in Figure 1A and C), suggesting that when Pol III* switches back to the template upon Pol V dissociation it will either elongate or degrade the TLS intermediates (see below). The remaining intermediate products (bands L1 and L2; Figure 1A, lane 4) reflect NarI sequence-specific effects, as these bands are not seen in the 3G context (Figure 1C, lane 4). Sequence-specific effects can be also noted in terms of bypass efficiency, as two-fold differences in Pol V-mediated TLS activity are observed between the 3G and the NarI context (Table I).
Interplay between DNA polymerases for the elongation of bypass intermediates. The NarI context constitutes an attractive model to study the interplay between Pol III and two specialized polymerases, Pol V and Pol II, that can bypass the G-AAF adduct via an error-free and a −2 frameshift pathway, respectively (see framed inset in Figure 1). In vitro, Pol II produces an additional TLS-1 product (Figure 1A, lane 6; Becherel and Fuchs, 2001) that is not formed in vivo (Napolitano et al, 2000; Pages and Fuchs, 2003). In the presence of Pol III*, we performed combination experiments with Pol V and/or Pol II to investigate the interplay between these polymerases during lesion bypass. The amounts of TLS0 and TLS-2 formed when both Pol V and Pol II are present simultaneously in the reaction mixture (lane 8 in Figure 1A) are approximately the same as the amount of TLS0 and TLS-2 formed when Pol V and Pol II are added in separate reaction mixtures, respectively (lanes 4 and 7 in Figure 1A). This observation means that Pol II and Pol V are not in strong competition under the present conditions. Experimental evidences further suggest that the two enzymes specifically use distinct intermediates, Pol V's major substrate being the L-1 intermediate while Pol II preferentially elongates the −2 frameshift intermediate formed from the L0 intermediate (data not shown).
Effect of Pol III HE concentration. We wanted to investigate the effect of Pol III HE concentration on the extent and kind of bypass products produced by Pol II and Pol V. A 15-fold increase in Pol III* concentration (from 4 to 62.7 nM) essentially suppresses Pol II-mediated −1 frameshift products (≈15-fold reduction), while it decreases less than three-fold Pol V-mediated TLS0 and does not affect Pol II-mediated TLS-2 pathways (Figure 1A, lane 8, and Figure 1B, lane 4; Table I). The significance of these observations will be discussed in terms of differential substrate competition in a paper describing the detailed interplay between Pol III*, Pol II and Pol V (S Fujii and RP Fuchs, unpublished). When the AAF adduct is located within the 3G sequence context (5′-CCCGAAFGG-), Pol V promotes TLS0 while Pol II no longer stimulates any bypass event (Figure 1C), in full agreement with in vivo data (Napolitano et al, 2000).
Effect of the β-clamp on the processivity of Pol V under single hit conditions
In vivo, the β-clamp was found to be essential for Pol V-mediated lesion bypass (Becherel et al, 2002). In the next set of experiments, we will show that the β-clamp donated by Pol III HE upon its dissociation in the vicinity of the lesion site will endow Pol V with enough processivity to synthesize a TLS patch that is sufficiently long to prevent nascent primer degradation upon rebinding of the replicative enzyme.
To measure the size distribution of the TLS patches synthesized by Pol V, we implemented single turnover experiments using heparin as a trap (Figure 2). The primer template is preincubated (10 min, 30°C) with stoichiometric amounts of RecA (2 μM) in the presence of ATP and low amounts of SSB (10 nM) under conditions found to be optimal for Pol V activity (Fujii et al, 2004) with or without the β-clamp and the clamp loader γ complex. Following an additional incubation period in the presence of Pol V (3 min, 30°C), the reaction is started by adding a mixture of dNTP and heparin. At various time points ranging from 0.5 to 8 min, the reaction is stopped and analyzed by PAGE. The efficiency of the heparin trap can be judged in lanes P where the heparin trap was added before Pol V. No Pol V activity is detected under these conditions, illustrating the capacity of heparin to trap Pol V. In contrast, when heparin is omitted, Pol V can perform multiple binding events during the 8 min incubation time, thus resulting in robust DNA synthesis (lanes -H). Under single hit conditions, we estimate the capacity of Pol V to form a productive initiation complex with its substrate by measuring the fraction of primer utilization. Primer utilization (%) is obtained by averaging the ratio of the summed intensities of all elongation products by the total amount of labeled material in each time track of a given experiment. In the absence of β-clamp, Pol V exhibits a very low capacity to form a productive initiation complex (primer utilization ≈0.17%). The average processivity is estimated to be ≈3 nt on undamaged template. In the presence of the β-clamp, the capacity of Pol V to form an initiation complex is greatly enhanced (primer utilization ≈3.6%) but still remains low despite the 50-fold excess of Pol V (100 nM) with respect to substrate (2 nM). Progression of Pol V along the template can be seen up to time point 4 min where a plateau is reached, no further activity being observed between 4 and 8 min (Figure 2). The average processivity is estimated to be ≈25 nt by intensity-weighed averaging (Table II). A slow elongation rate of ≈3 s per nucleotide incorporation event is estimated (≈0.29 nt/s), thus yielding an average residency time of ≈25/0.29=86 s for Pol V on its substrate (Table II). When Pol V is challenged to bypass a G-AAF adduct (standing start conditions), in the absence of the β-clamp, virtually no activity is detected except under multiple hit conditions (Figure 2). In contrast, in the presence of the β-clamp, Pol V exhibits an overall capacity to form an initiation complex that is about two- to three-fold lower than in the absence of lesion (primer utilization ≈1.2%), the average length of a single patch of synthesis being about 18 nt long (Table II).
Figure 2.
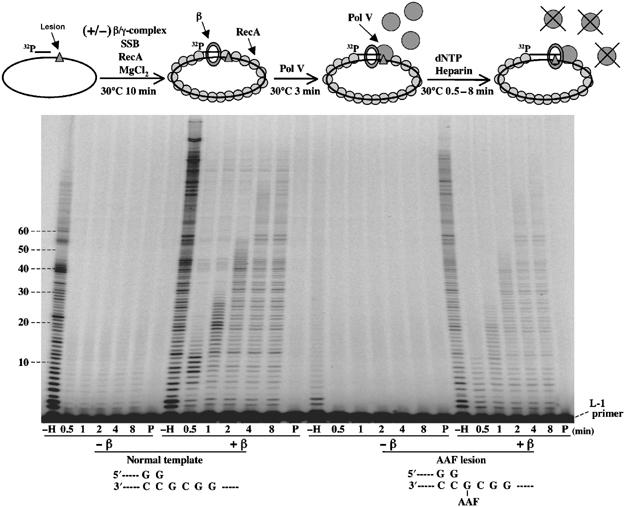
Processivity of Pol V in the presence of the β-clamp determined by single turnover experiments. Experiments are performed as outlined in the top of the figure. Briefly, Pol V is added to the preincubation mixture containing SSB, RecA, MgCl2 and (+/−) β/γ as indicated to allow formation of an initiation complex. The reaction is started by simultaneous addition of dNTP+heparin, and then incubated for 0.5–8 min before the reaction is stopped. Heparin is used to trap free Pol V molecules, as symbolized by the crossover free Pol V molecules. The lack of replication products in lanes ‘P', in which dNTP+heparin was added to the preincubation mixture before Pol V, proves the efficiency of the heparin trap (8 min of incubation). Lanes ‘-H', without heparin, show the activity of Pol V under conditions of multiple binding during an 8 min incubation period. With undamaged template in the presence of the β-clamp, low amounts of long elongation products (>40 nt) can be seen at all time points. These products result from a minor polymerase contamination, present in the γ complex preparation, that is stimulated by the β-clamp and inhibited by the G-AAF adduct.
Table 2.
Kinetic and processivity data of Pol V in the presence of the β-clamp
| Sample | Average processivity (nt) | Average velocity (nt/s) | Maximum product length up to (nt) |
|---|---|---|---|
| Nar0/−β | |||
| 0.5–8 min | 3 | >0.10 | 12 |
| Nar0/+β | |||
| 0.5 min | 8–9 | ≈0.28 | 18 |
| 1 min | 17–18 | ≈0.29 | 31 |
| 2 min | ≈25 | — | 55 |
| 4 min | ≈25 | — | >100a |
| 8 min | ≈25 | — | >100a |
| Nar3/+β | |||
| 0.5 min | 2–3 | ≈0.083 | 13 |
| 1 min | 10–11 | ≈0.18 | 26 |
| 2 min | ≈18 | — | 48 |
| 4 min | ≈18 | — | >60b |
| 8 min | ≈18 | — | >60b |
| These data are derived from the gel shown in Figure 2. Nar0 and Nar3 refer to undamaged and G-AAF-containing templates, respectively. Processivity data are determined by intensity-weighed averaging. | |||
| aProducts longer than >100 nt represent ≈9% of total products; a minor polymerase contamination in the γ complex preparation accounts for the low amount of long extension products (not seen in the presence of the adduct). | |||
| bProducts longer than >60 nt represent ≈1% of total products. No product is detected when Pol V is incubated with Nar3 in the absence of the β-clamp under single hit conditions (Figure 2). | |||
By comparing the progression of the enzyme on lesion-free and lesion-containing templates, the average delay for bypassing the G-AAF adduct with Pol V can be estimated to be in the order of 24 s (Figure 2). It should be noted that despite a broad distribution of patch sizes, that is, from 1 to ≈60 nt, most TLS patches (≈75%) exceed the minimal length of 5 nt beyond the lesion that is necessary for Pol III-mediated elongation (see below and model in Figure 5).
Figure 5.
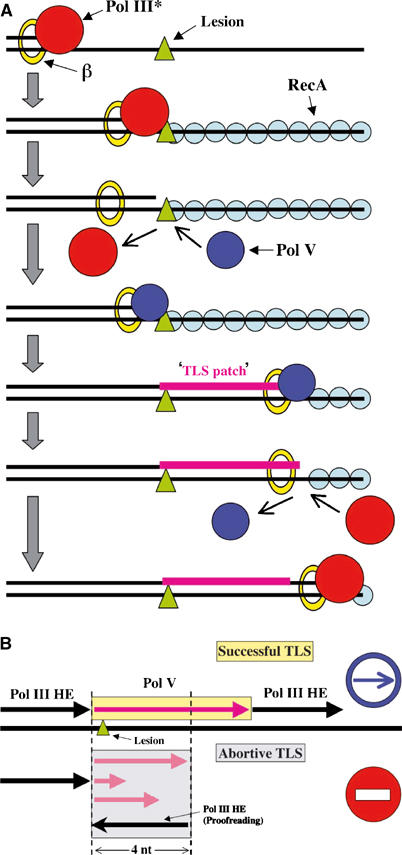
An integrated view of TLS. (A) Pol III* associated with its processivity factor the β-clamp encounters a noncoding lesion in the template and stops. A RecA filament forms on the single-stranded DNA region downstream the lesion site. This RecA filament together with the β-clamp forms a structure-specific template to which the bypass polymerase Pol V binds and mediates a synthesis patch 20 nt long on average. Pol V produces a large distribution of TLS patches ranging from 1 to 60 nt. (B) Successful lesion bypass requires the TLS patch to be ⩾5 nt long. When Pol III HE binds to the intermediate bypass products generated by Pol V, if the TLS patch is ⩾5 nt, the distortion due to the lesion is outside the ‘sensor domain' of Pol III, allowing efficient elongation to complete a successful bypass process. In contrast, if the TLS patch is <5nt, the distortion triggers primer degradation by the proofreading function leading to an aborted process. The ratio of successful to aborted events is ≈3:1 under the present experimental conditions.
Pol III HE efficiently resumes DNA synthesis on RecA-covered single-stranded DNA template
Following the dissociation of Pol V after lesion bypass, as the template is covered with RecA, we wanted to investigate the capacity of Pol III HE to use an ATP-activated RecA nucleofilament as a substrate for DNA synthesis. For this purpose, a primed ss-circular DNA template coated with RecA was used as a substrate for Pol III HE. Primer elongation proceeds efficiently whether the template is naked or covered with stoichiometric quantities of RecA protein in the presence of ATP (Figure 3). Similarly, low (10 nM) or high (300 nM) concentrations of SSB do not affect the activity of Pol III HE (Figure 3). The template used in these experiments contains a lesion 37 nt downstream from the 3′-end of the primer that makes Pol III stop at L-1.
Figure 3.
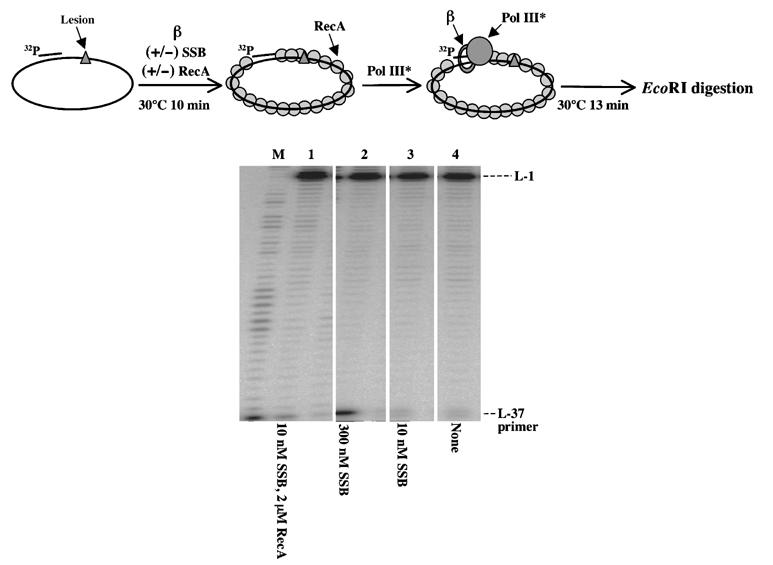
Pol III HE efficiently replicates an ATP-activated RecA filament. A circular single-stranded template (≈2.7 kb) containing a G-AAF adduct located within the NarI sequence primed with L-37 is used as a substrate for Pol III HE elongation experiments in the presence or absence of RecA and SSB. The DNA template was preincubated with SSB and RecA at the indicated concentrations in the presence of β-clamp (50 nM) for 10 min. Elongation reactions were initiated by adding 8 nM Pol III* and terminated after 13 min. All reaction products were digested by EcoRI and analyzed by a 10% denaturing PAGE. M indicates DNA size markers.
Defining the minimal size of the TLS patch that is required for efficient elongation by DNA Pol III HE
For a series of common replication-blocking DNA lesions, we wanted to measure the effect of the position of the 3′-end of the primer, with respect to the lesion site, on the capacity of the replicative polymerase to bind and extend the nascent strand. Either linear oligonucleotides (130-mer) or circular single-stranded vectors (2700 nt) were used as substrates following annealing with a series of complementary primers. For the linear templates, the primer annealing site was centrally located leaving both 3′ and 5′ single-stranded template overhangs. The templates were preincubated with SSB, followed by the addition of Pol III* (McHenry, 2003) and β-clamp in the presence of a mixture of all four dNTPs. Under these conditions, if Pol III HE cannot elongate a primer given the distortion imposed by the lesion in the template strand, we will observe primer degradation by its associated exonuclease activity despite the presence of dNTPs. The relative efficiencies of polymerization (% pol) and degradation (% exo) for a given primer Ln (listed above each panel in Figure 4) were quantified as follows: % pol, the sum of band intensities above position Ln divided by the sum of all bands except Ln; % exo, the sum of band intensities below position Ln divided by the sum of all bands except Ln.
Figure 4.
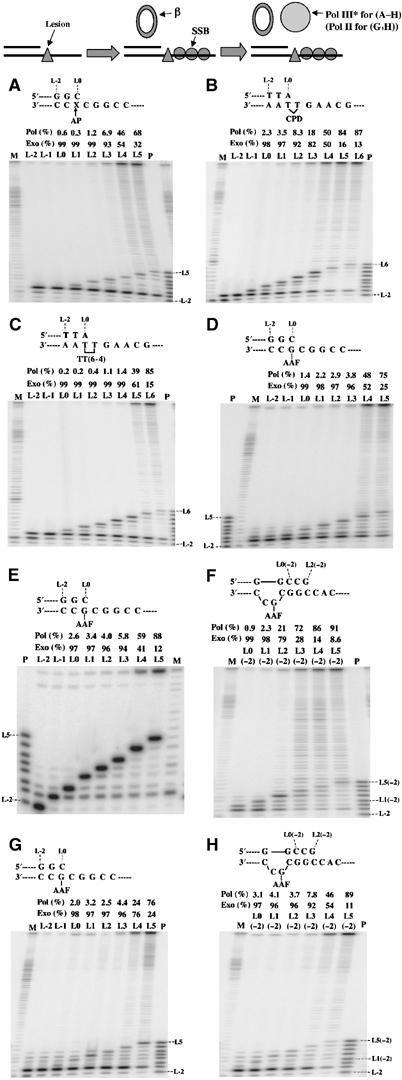
Pol III HE and Pol II require a minimum of 4 or 5 base pairs beyond the lesion to resume efficient DNA synthesis. Substrates are either linear oligonucleotides or circular single-stranded plasmids onto which 5′-end-labeled primers are annealed. For linear oligonucleotides (130-mer), the lesion is located about halfway from both ends of the template (details in Materials and methods). Lesions are G-AAF (D–H), AP site (A), TT cyclobutane dimer (B) and TT (6-4) photoproduct (C). Each panel shows the reaction products using a series of primers a various length ranging from L-2 (24-mer) to positions up to L6 (32-mer). Primers are named according to the position their 3′-end anneals to the template with respect to the lesion site (i.e., the 3′-end nucleotide of primer L2 ends 2 nt beyond the lesion site that is referred to as L0). In (F, H), the G-AAF adduct located within the NarI frameshift hot spot forms a −2 frameshift intermediate (Fuchs et al, 1981; Burnouf et al, 1989); ‘−2 frameshift' primers are named as follows: primer Ln(−2) designates a primer that ends at position n (with respect to the lesion site) in the absence of misalignment. When a −2 nt misalignment intermediate forms, primer Ln(−2) ends at position n+2 as shown in the top of (F, H). All Pol III reactions (A–F) were carried out under the standard conditions containing 50 nM β-clamp and 300 nM SSB (see Materials and methods). Pol II reactions (G, H) did not contain the β-clamp. Reactions were preincubated (10 min at 30°C), initiated by adding 2 nM Pol III* (A–F) or 4 nM Pol II (G, H) and terminated after 15 min. The reaction products were analyzed by 10% denaturing PAGE. (E) G-AAF in the NarI context is located in a circular template to evaluate the potential effect of circular versus linear templates. Reaction products in (E) were digested with EcoRI before electrophoresis. P and M indicate DNA size markers. For each reaction, the efficiencies of polymerization (% pol) and degradation (% exo) (listed above each panel) were calculated as follows: % pol, the sum of band intensities above position Ln divided by the sum of all bands except Ln; % exo, the sum of band intensities below position Ln divided by the sum of all bands except Ln.
In addition to the G-AAF adduct, we employed three commonly studied replication-blocking DNA lesions (Friedberg et al, 1995), namely the two major UV-induced lesions, TT_CPD and TT (6-4), and an abasic site (AP). A series of experiments involving primer/templates with primers of increasing length, the 3′-end of the primer being located either upstream (L-2, L-1), across (L0) or several nucleotides downstream (L1, L2, L3….) the lesion site, were set up (Figure 4).
Primer located upstream from the lesion site. For AP site, TT_CPD and TT (6-4) lesions, using primer L-2, Pol III HE adds 1 nt to reach position L-1 and then stops (Figure 4A–C). For the G-AAF adduct in the NarI context (Figure 4D and E), limited synthesis across and beyond the lesion site is observed (as already noted above; Figure 1). Similar results are observed with the G-AAF adduct located either in linear 130-mer oligonucleotides or in (2700 nt) ss-circular DNA templates (Figure 4D and E). These data show that, in general, Pol III HE synthesizes up to the position preceding the lesion site and then stops. With G-AAF, limited synthesis across the lesion site is observed in both 3G and NarI sequence contexts (Figures 4D and 1).
Primers located across or downstream from the lesion site. For all lesions tested, efficient elongation to the end of the substrate only occurs when the 3′-end of the primer is located 4 or 5 nt downstream from the lesion site (L4 or L5) (Figure 4A–E). With primers ranging from L0 to L3 or L4, the 3′ → 5′ exonuclease (proofreading) activity of Pol III dominates over its 5′ → 3′ polymerase activity. Quite remarkably, a sharp transition between the exonuclease and polymerase activities of Pol III is observed, between L3 and L4, for a variety of different lesions such as an AP site (Figure 4A), TT_CPD (Figure 4B) and G-AAF adducts (Figure 4D). For the more distorting TT (6-4) photoproduct, the transition point occurs between L4 and L5 (Figure 4C). The same transition point is observed whether the lesion is located in a linear or a circular template (compare Figure 4D and E).
Primers forming frameshift intermediates. We also investigated the position of the exo/pol transition point for frameshift intermediates. Frameshift mutations occur as a consequence of a primer/template misalignment process. Frameshift mutations usually occur within short repeats, the primer strand reannealing downstream from the lesion site thus creating a bulge in the template strand (Lambert et al, 1992). The NarI sequence context (5′-GGCGAAFCC) is known in vivo to be a strong −2 frameshift hot spot for AAF adducts (Fuchs et al, 1981; Burnouf et al, 1989). It turns out that despite the 2 nt -CGAAF bulge in the template strand, Pol III efficiently elongates the slipped replication intermediate provided the 3′-end of the primer forms 4 or 5 correct base pairs with the template (Figure 4F).
Comparing Pol III and Pol II. The replication enzymes of many bacterial species, including Pol III of E. coli, belong to class C polymerases, in contrast to the replicative enzymes of eukaryotic cells that belong to class B. As E. coli also possesses a class B enzyme, namely Pol II, we checked the capacity of Pol II to elongate TLS intermediates. When Pol II is used here as a model replicative polymerase, it exhibits requirements similar to those of Pol III (compare Figure 4D–G and F–H). As discussed above (Figure 1), with the G-AAF adduct located within the NarI sequence context, Pol II functions as a TLS polymerase per se generating −2 frameshift mutations.
Discussion and conclusions
Pol V needs to interact with both the RecA filament and the β-clamp
As shown above, efficient elongation by Pol III requires the lesion to be located at least 5 base pairs upstream from the primer 3′-end. Therefore, to function in lesion bypass, Pol V needs to synthesize, in a single binding event, a patch of DNA of sufficient length to prevent degradation of the nascent primer upon rebinding of Pol III HE. Indeed, given the higher affinity of Pol III, compared to Pol V for the 3′-end of the primer, when Pol V dissociates, Pol III has a greater probability than Pol V to access the 3′ primer terminus. Upon rebinding, Pol III will either degrade or elongate the primer depending upon the distance to the lesion site. Therefore, in order to yield a productive bypass event, a sufficiently long TLS patch needs to be synthesized during a single Pol V binding event. During a single binding event, in the absence of β-clamp, Pol V has very little activity, both in terms of primer utilization and processivity (≈3 nt) (Table II) despite the presence of an activated ATP/RecA filament. In contrast, when the β-clamp is loaded onto the substrate, Pol V gains the capacity to synthesize a broad distribution of DNA patches ranging from 1 to ≈60 nt. From the single hit kinetic data, we estimate that the dual interaction of Pol V with the β-clamp and the RecA filament provides an average residency time of about 90 s (Table II). On nondamaged template, the average rate of nucleotide incorporation is about 0.3 nt/s. The delay for G-AAF bypass is about 24 s (Figure 2 and Table II). The average patch size made by Pol V on the G-AAF-containing template is estimated to be ≈18 nt long. Quantification of the bands in Figure 2 indicates that the proportion of TLS patches shorter or equal to 4 nt amounts to ≈25%. These intermediates will be degraded upon Pol III binding, leading thus to aborted bypass attempts (Figure 5). Conversely, the TLS patches that are equal or longer than 5 nt (≈75%) will yield successful bypass events upon Pol III elongation (Figure 5).
Pol III HE senses the distortion created by lesions 4–5 nt downstream from the damage site
Both misinserted bases opposite normal template bases (mismatches) or template lesions strongly inhibit the efficiency of subsequent nucleotide extensions by model DNA polymerases by several orders of magnitude (for a recent review, see Kunkel and Bebenek, 2000). The stalling of polymerases is not limited to the position following the mismatch but up to 4 base pairs away from the primer terminus (Carver et al, 1994). Similarly, with damage-containing templates, the polymerase activity of Pol I Klenow fragment is affected till 4 nt downstream from the lesion site (Miller and Grollman, 1997). In the present paper, we show that both Pol III and Pol II, members of class C and B enzymes respectively, require 4–5 correct base pairs downstream from the lesion to resume efficient synthesis. Similarly, frameshift intermediates such as those triggered by G-AAF adducts within specific hot spot sequences (Napolitano et al, 1997) also become extendable by the replicative enzyme provided the primer forms 4–6 correct base pairs with the template (Figure 4). Given the fact that all four lesions studied here (AP site, TT cyclobutane dimer, TT (6-4) photoproduct and G-AAF adduct) were shown to affect the thermodynamic stability and/or the overall structure of the duplex DNA quite dramatically (Koehl et al, 1989; Gelfand et al, 1998; Jing et al, 1998), it may not be surprising to observe that they trigger a similar response with respect to Pol III activity. Disruption of the minor-groove interactions between the lesion-containing double-stranded region of the primer template and the binding domain within polymerases (Johnson et al, 2003) is likely to cause stalling of DNA synthesis. Indeed, ‘replicative-type' polymerases possess a sensor domain (minor-groove recognition domain) that is able to discriminate between correctly and incorrectly paired nucleotides within the last 4–5 base pairs (Kiefer et al, 1998). Slowing down the extension kinetics will inevitably favor the degradation of the primer by the exonuclease that is associated with the replicative polymerase (proofreading function). When the perturbed region of the duplex DNA moves away from the polymerase sensor domain, an abrupt transition between the exonucleolytic and polymerizing modes is observed (Figure 4).
Possible TLS scenario
For the set of representative lesions studied here, Pol III HE is able to replicate at least till the nucleotide position preceding the damaged base. Following a replication block in one strand, transient uncoupling of concurrent leading and lagging strand syntheses occurs in vivo thus yielding a region of single-stranded DNA downstream from the lesion site (Pages and Fuchs, 2003). A filament of RecA builds up onto this single-stranded region of DNA with the aid of several other proteins, namely SSB and RecFOR (Morimatsu and Kowalczykowski, 2003). We suggest that the bypass polymerase Pol V binds transiently to the blocked primer terminus intermediate before locking down into a ‘stable' complex by means of its dual interaction with both the tip of the RecA filament (Bailone et al, 1991; Goodman, 2002) and the β-clamp, initially associated with the replicative enzyme. The stability of the complex is in the range of 1.5 min, allowing Pol V to synthesize, in a single binding event, a TLS patch 20 nt long on average (Table II). Following Pol V's dissociation, Pol III HE is able to efficiently resume synthesis on the ATP-activated RecA filament substrate thus completing the bypass pathway (Figure 3). It has been reported that neither Pol III nor Pol V can copy an ‘intact RecA filament' (Pham et al, 2001; Tippin et al, 2004). However, in these experiments, the authors used ATP-γS for RecA filament activation instead of the physiologically relevant ATP cofactor (see Fujii et al, 2004 for detailed discussion). The size of the average TLS patch is likely to be influenced by factors such as the local sequence context and the chemical nature of the template lesion. For instance, both UV-induced TT cyclobutane dimers or 6-4 photoproducts are bypassed by Pol V slightly less efficiently than the G-AAF adduct (Fujii et al, 2004). For the G-AAF adduct, it is estimated from the data in Figure 2 that ≈25% of the TLS patches are shorter than 5 nt and will consequently be degraded by Pol III (aborted bypass events), while 75% will produce a successful bypass event following Pol III elongation (Figure 5). The average patch size of ≈20 nt may appear as a reasonable trade-off between insuring efficient TLS (75%, in a single attempt) and preventing a heavy load of untargeted mutations. The level of untargeted mutation made by Pol V can be estimated as follows. Given its fidelity on undamaged DNA (10−3–10−4; Maor-Shoshani et al, 2000; Tang et al, 2000), Pol V will produce only one untargeted mutation per 50–500 TLS events, a low level of point mutations that will potentially be corrected by mismatch repair. We suggest that the process of TLS as unraveled here in E. coli represents a paradigm for lesion bypass in eukaryotes.
Materials and methods
DNA substrates
ss-circular DNA constructions 2.7 kb long containing a single AAF adduct within the 3G or the NarI sequence context were described previously (Napolitano and Fuchs, 1997). Linear DNA templates (∼130-mer) were assembled by ligation of a lesion-containing oligonuleotide (13- or 15-mer, see below) to a 60-mer (5′-side) and a 55-mer (3′-side), the three oligonucleotides being held together by a complementary scaffold oligonucleotide (Seeberg and Fuchs, 1990). The sequences of the 60- and 55-mer are (5′-TTGTGAGCGGATAACAATTTCACACAGGAAA CAGCTATGACCATGATTACGAATTCAGTC-3′), an oligo (13 or 15-mer), and a 55-mer (5′-GACTAAGCTTGGCACTGGCCGTCGTTTTACA ACGTCGTGACTGGGAAAACCCTGG-3′), respectively. A single G-AAF adduct within the NarI sequence context is located either within a 15-mer (5′-ATCACCGGCGAAFCCACA-3′, the NarI sequence is underlined) or a 14-mer (5′-ATACCCGAAFGGACATC-3′), the 3G context. A single abasic (N=AP) site is located in a 15-mer (5′-ATCACCGGCNCCACA-3′). TT_CPD or TT (6-4) lesion is located in 13-mer (5′-GCAAGTTAACACG-3′, the TT is TT_CPD or TT (6-4)). The assembled ligation products, which consist of 5′-60-mer-(13-mer or 15-mer)-55-mer-3′, were purified by denaturing PAGE as described previously (Seeberg and Fuchs, 1990). The following 5′-32P-labeled primers were purified by denaturing PAGE and annealed to the 2.7-kb ss-circular or linear DNA templates: 23-mer L-37, 25-mer L-8, 25-mer L-6, 24-mer L-2, ∼ 32-mer L6 primers.
Proteins
Pol III*, which is a form of Pol III HE without the β-clamp, was purified from the E. coli strain MGC1030 (mcrA mcrB lamBDA(−) in (RRND-RRNE)1 lexA3 uvrD::Tc ompT::Km). The strain was grown at 37°C in 100- l of LB medium, and then cells were harvested by centrifugation. Until the Bio-Rex 70 column step, the purification procedure was carried out as described (Cull and McHenry, 1995). Thereafter, Heparin, MonoQ and Superose 6 columns were used in that order. Pol V, the β-clamp and SSB were prepared as described elsewhere (Fujii et al, 2004). RecA was purchased from Pharmacia. The clamp loader γ complex is a generous gift from CS McHenry. Pol II was prepared as described previously (Becherel and Fuchs, 2001).
DNA replication assays
The standard reaction contained 20 mM Tris–HCl (pH 7.5), 4% glycerol, 8 mM DTT, 80 μg/ml BSA, 2.5 mM ATP, 8 mM MgCl2, dATP, dTTP, dGTP and dCTP (each 0.1 mM). When indicated, 50 nM β (as a dimer), 2 μM RecA and 10 nM γ complex were mixed in the reaction. SSB (as a tetramer) concentration is indicated in the figures or figure legends. A 2 nM portion of primed template DNA was preincubated with all components except for DNA polymerase(s) for 10 min at 30°C. Reactions were initiated by mixing various concentrations of DNA polymerase, and incubated at 30°C (the details are described in the figure legends). Reactions were terminated by adding one volume of formamide containing 25 mM EDTA and bromophenol blue. If an EcoRI digestion step is necessary, replication reactions are stopped by adding one volume of 50 mM EDTA. Thereafter, the products were purified by phenol/chloroform extraction, followed by ethanol precipitation and digested by EcoRI. Reactions were terminated by adding one volume of formamide containing 25 mM EDTA and bromophenol blue. The products were heat denatured, separated on a 10% denaturing PAGE and visualized on a Molecular Imager (Bio-Rad).
Processivity measurements
The 2.7-kb ss-circular DNAs containing a single AAF adduct within the NarI sequence context or without lesion (normal template) were used as templates. Templates were annealed with primer L-1 (2 nM), preincubated with 10 nM SSB (as a tetramer) and 2 μM RecA in the standard reaction mixture without dNTP for 10 min at 30°C. When indicated, 50 nM β (as a dimer) and 10 nM γ complex were added to the reaction. Subsequently, Pol V (100 nM) was added to form an initiation complex. After 3 min of incubation, reactions were initiated by mixing 0.1 mM dNTPs and 0.2 mg/ml heparin. Reactions were terminated at the indicated times (0.5–8 min) by adding two volumes of formamide containing 25 mM EDTA and bromophenol blue. In order to assess the efficiency of the heparin trap, dNTP+heparin was added to the preincubation mixture before Pol V (8 min of incubation, lanes ‘P') or reaction mixtures were incubated without heparin (8 min of incubation, lanes ‘-H'). The products were heat denatured, separated on a 10% denaturing PAGE and visualized on a Molecular Imager (Bio-Rad).
Acknowledgments
We thank R Napolitano, J Wagner and N Koffel-Schwartz for discussions and critical reading. This work was partly funded by a HFSP grant RG 0351/1998-M to RPF and a fellowship from the FRM to SF. C McHenry, R Woodgate and T Lohman are acknowledged for their generous gift of materials.
References
- Bailone A, Sommer S, Knezevic J, Dutreix M, Devoret R (1991) A RecA protein mutant deficient in its interaction with the UmuDC complex. Biochimie 73: 479–484 [DOI] [PubMed] [Google Scholar]
- Becherel OJ, Fuchs RP (2001) Mechanism of DNA polymerase II-mediated frameshift mutagenesis. Proc Natl Acad Sci USA 98: 8566–8571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becherel OJ, Fuchs RPP, Wagner J (2002) Pivotal role of the β-clamp in translesion DNA synthesis and mutagenesis in E. coli cells. DNA Repair 1: 703–708 [DOI] [PubMed] [Google Scholar]
- Bresson A, Fuchs RP (2002) Lesion bypass in yeast cells: Pol eta participates in a multi-DNA polymerase process. EMBO J 21: 3881–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt SE, Woodgate R, Scheuermann RH, Echols H (1988) UmuD mutagenesis protein of Escherichia coli: overproduction, purification, and cleavage by RecA. Proc Natl Acad Sci USA 85: 1811–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnouf D, Koehl P, Fuchs RPP (1989) Single adduct mutagenesis: strong effect of the position of a single acetylaminofluorene adduct within a mutation hot spot. Proc Natl Acad Sci USA 86: 4147–4151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver TE Jr, Hochstrasser RA, Millar DP. (1994) Proofreading DNA: recognition of aberrant DNA termini by the Klenow fragment of DNA polymerase I. Proc Natl Acad Sci USA 91: 10670–10674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier AM, Fuchs RP (1999) Replication of damaged DNA: molecular defect in xeroderma pigmentosum variant cells. Mutat Res 435: 111–119 [DOI] [PubMed] [Google Scholar]
- Courcelle J, Khodursky A, Peter B, Brown PO, Hanawalt PC (2001) Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics 158: 41–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull MG, McHenry CS (1995) Purification of Escherichia coli DNA polymerase III holoenzyme. Methods Enzymol 262: 22–35 [DOI] [PubMed] [Google Scholar]
- Dalrymple BP, Kongsuwan K, Wijffels G, Dixon NE, Jennings PA (2001) A universal protein–protein interaction motif in the eubacterial DNA replication and repair systems. Proc Natl Acad Sci USA 98: 11627–11632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutreix M, Moreau PL, Bailone A, Galibert F, Battista JR, Walker GC, Devoret R (1989) New recA mutations that dissociate the various RecA protein activities in Escherichia coli provide evidence for an additional role for RecA protein in UV mutagenesis. J Bacteriol 171: 2415–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez De Henestrosa AR, Ogi T, Aoyagi S, Chafin D, Hayes JJ, Ohmori H, Woodgate R (2000) Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol Microbiol 35: 1560–1572 [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W (1995) DNA Repair and Mutagenesis. Washington, DC: American Society for Microbiology [Google Scholar]
- Fuchs RPP, Schwartz N, Daune MP (1981) Hot spots of frameshift mutations induced by the ultimate carcinogen N-acetoxy-N-2-acetylaminofluorene. Nature 294: 657–659 [DOI] [PubMed] [Google Scholar]
- Fujii S, Gasser V, Fuchs RP (2004) The biochemical requirements of DNA polymerase V-mediated translesion synthesis revisited. J Mol Biol 341: 405–417 [DOI] [PubMed] [Google Scholar]
- Gelfand CA, Plum GE, Grollman AP, Johnson F, Breslauer KJ (1998) Thermodynamic consequences of an abasic lesion in duplex DNA are strongly dependent on base sequence. Biochemistry 37: 7321–7327 [DOI] [PubMed] [Google Scholar]
- Goodman MF (2002) Error-prone repair DNA polymerases in prokaryotes and eukaryotes. Annu Rev Biochem 71: 17–50 [DOI] [PubMed] [Google Scholar]
- Jing Y, Kao JF, Taylor JS (1998) Thermodynamic and base-pairing studies of matched and mismatched DNA dodecamer duplexes containing cis-syn, (6-4) and Dewar photoproducts of TT. Nucleic Acids Res 26: 3845–3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SJ, Taylor JS, Beese LS (2003) Processive DNA synthesis observed in a polymerase crystal suggests a mechanism for the prevention of frameshift mutations. Proc Natl Acad Sci USA 100: 3895–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer JR, Mao C, Braman JC, Beese LS (1998) Visualizing DNA replication in a catalytically active Bacillus DNA polymerase crystal. Nature 391: 304–307 [DOI] [PubMed] [Google Scholar]
- Koehl P, Valladier P, Lefevre JF, Fuchs RP (1989) Strong structural effect of the position of a single acetylaminofluorene adduct within a mutation hot spot. Nucleic Acids Res 17: 9531–9541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffel-Schwartz N, Verdier JM, Bichara M, Freund AM, Daune MP, Fuchs RPP (1984) Carcinogen induced mutation spectrum in wild type, uvrA and umuC strains in E. coli: strain specificity and mutation prone sequences. J Mol Biol 177: 33–51 [DOI] [PubMed] [Google Scholar]
- Kunkel TA, Bebenek K (2000) DNA replication fidelity. Annu Rev Biochem 69: 497–529 [DOI] [PubMed] [Google Scholar]
- Lambert IB, Napolitano RL, Fuchs RPP (1992) Carcinogen-induced frameshift mutagenesis in repetitive sequences. Proc Natl Acad Sci USA 89: 1310–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenne-Samuel N, Wagner J, Etienne H, Fuchs RPP (2002) The processivity factor β controls DNA polymerase IV traffic during spontaneous mutagenesis and translesion synthesis in vivo. EMBO Reports 3: 45–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Saro FJ, Georgescu RE, Goodman MF, O'Donnell M (2003) Competitive processivity-clamp usage by DNA polymerases during DNA replication and repair. EMBO J 22: 6408–6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maor-Shoshani A, Bacher Reuven N, Tomer G, Livneh Z (2000) Highly mutagenic replication by DNA polymerase V (UmuC) provides a mechanistic basis for SOS untargeted mutagenesis. Proc Natl Acad Sci USA 97: 565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry CS (2003) Chromosomal replicases as asymmetric dimers: studies of subunit arrangement and functional consequences. Mol Microbiol 49: 1157–1165 [DOI] [PubMed] [Google Scholar]
- Miller H, Grollman AP (1997) Kinetics of DNA polymerase I (Klenow fragment exo-) activity on damaged DNA templates: effect of proximal and distal template damage on DNA synthesis. Biochemistry 36: 15336–15342 [DOI] [PubMed] [Google Scholar]
- Morimatsu K, Kowalczykowski S (2003) RecFOR proteins load RecA protein onto gapped DNA to accelerate strand exchange: a universal step of recombinational repair. Mol Cell 11: 1137–1147 [DOI] [PubMed] [Google Scholar]
- Napolitano R, Janel-Bintz R, Wagner J, Fuchs RP (2000) All three SOS-inducible DNA polymerases (Pol II, Pol IV and Pol V) are involved in induced mutagenesis. EMBO J 19: 6259–6265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano RL, Fuchs RPP (1997) New strategy for the construction of single stranded plasmids with single mutagenic lesions. Chem Res Toxicol 10: 667–671 [DOI] [PubMed] [Google Scholar]
- Napolitano RL, Lambert IB, Fuchs RP (1997) SOS factors involved in translesion synthesis. Proc Natl Acad Sci USA 94: 5733–5738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages V, Fuchs RP (2002) How DNA lesions are turned into mutations within cells? Oncogene 21: 8957–8966 [DOI] [PubMed] [Google Scholar]
- Pages V, Fuchs RP (2003) Uncoupling of leading- and lagging-strand DNA replication during lesion bypass in vivo. Science 300: 1300–1303 [DOI] [PubMed] [Google Scholar]
- Pham P, Bertram JG, O'Donnell M, Woodgate R, Goodman MF (2001) A model for SOS-lesion-targeted mutations in Escherichia coli. Nature 409: 366–370 [DOI] [PubMed] [Google Scholar]
- Prakash S, Prakash L (2002) Translesion DNA synthesis in eukaryotes: a one- or two-polymerase affair. Genes Dev 16: 1872–1883 [DOI] [PubMed] [Google Scholar]
- Radman M (1975) SOS Repair Hypothesis: Phenomenology of an Inducible DNA Repair which is Accompanied by Mutagenesis. New York: Plenum Publishing Corp. [DOI] [PubMed] [Google Scholar]
- Reuven NB, Arad G, Maor-Shoshani A, Livneh Z (1999) The mutagenesis protein UmuC is a DNA polymerase activated by UmuD′, RecA, and SSB and is specialized for translesion replication. J Biol Chem 274: 31763–31766 [DOI] [PubMed] [Google Scholar]
- Sassanfar M, Roberts JW (1990) Nature of the SOS-inducing signal in Escherichia coli: the involvement of DNA replication. J Mol Biol 212: 79–96 [DOI] [PubMed] [Google Scholar]
- Seeberg E, Fuchs RPP (1990) Acetylaminofluorene bound to different guanines of the sequence -GGCGCC- is excised with different efficiencies by the UvrABC excision nuclease in a pattern not correlated to the potency of mutation induction. Proc Natl Acad Sci USA 87: 191–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa H, Iwasaki H, Kato T, Nakata A (1988) RecA protein-dependent cleavage of UmuD protein and SOS mutagenesis. Proc Natl Acad Sci USA 85: 1806–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MD, Walker GC (2001) Managing DNA polymerases: coordinating DNA replication, DNA repair, and DNA recombination. Proc Natl Acad Sci USA 98: 8342–8349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Pham P, Shen X, Taylor JS, O'Donnell M, Woodgate R, Goodman MF (2000) Roles of E. coli DNA polymerases IV and V in lesion-targeted and untargeted SOS mutagenesis. Nature 404: 1014–1018 [DOI] [PubMed] [Google Scholar]
- Tang M, Shen X, Frank EG, O'Donnell M, Woodgate R, Goodman MF (1999) UmuD′(2)C is an error-prone DNA polymerase, Escherichia coli pol V. Proc Natl Acad Sci USA 96: 8919–8924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippin B, Pham P, Goodman MF (2004) Error-prone replication for better or worse. Trends Microbiol 12: 288–295 [DOI] [PubMed] [Google Scholar]
- Wagner J, Etienne H, Janel-Bintz R, Fuchs RPP (2002) Genetics of mutagenesis in E. coli: various combinations of translesion polymerases (Pol II, IV and V) deal with lesion/sequence context diversity. DNA Repair 1: 159–167 [DOI] [PubMed] [Google Scholar]


