Abstract
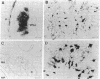
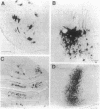
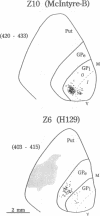
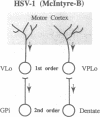
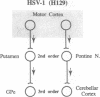
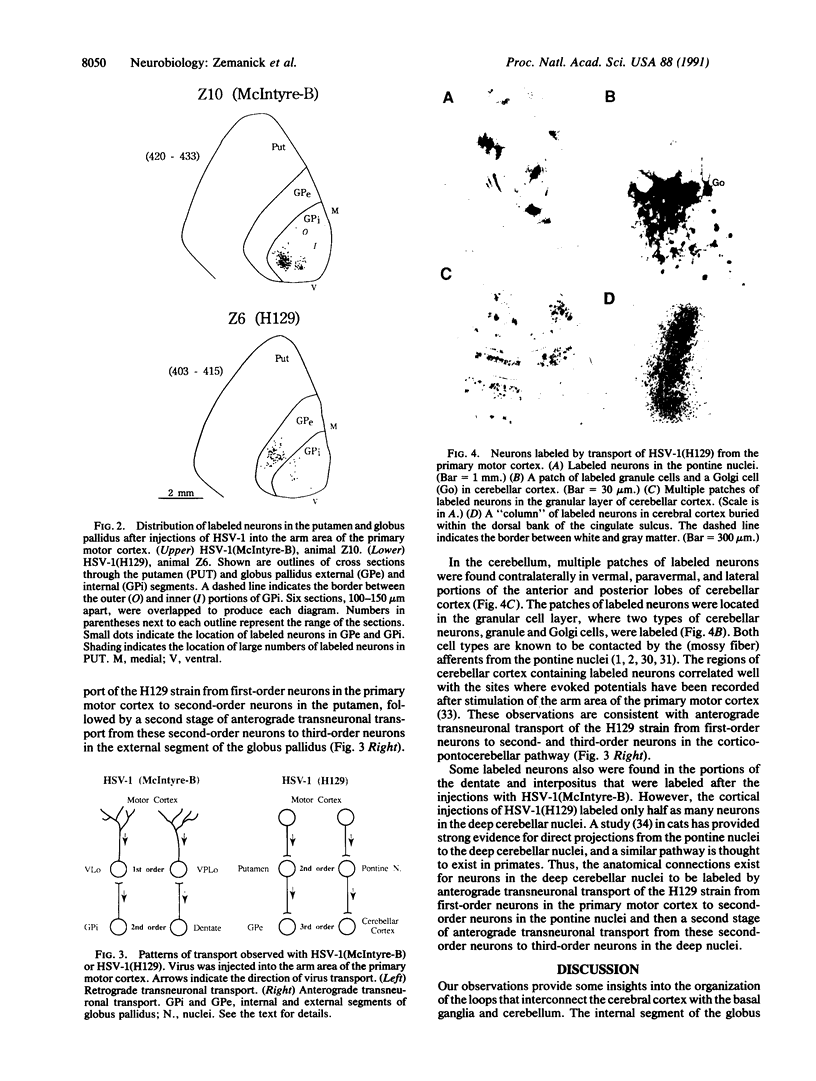
We examined the axonal transport of two strains of herpes simplex virus 1 (HSV-1) within the central nervous system of cebus monkeys. Each strain was injected into the "arm area" of the primary motor cortex. One strain, HSV-1(McIntyre-B), was transported transneuronally in the retrograde direction. It infected neurons at sites known to project to the arm area of the primary motor cortex (e.g., ventrolateral thalamus). In addition, "second-order" neurons were labeled in the deep cerebellar nuclei (dentate and interpositus) and in the globus pallidus (internal segment). This result supports the concept that the arm area of the primary motor cortex is a target of both cerebellar and basal ganglia output. In contrast, the other strain, HSV-1(H129), was transported transneuronally in the anterograde direction. It infected neurons at sites known to receive input from the arm area of the primary motor cortex (e.g., putamen, pontine nuclei). In addition, "third-order" neurons were labeled in the cerebellar cortex (granule and Golgi cells) and in the globus pallidus (largely the external segment). Our observations suggest that strain differences have an important impact on the direction of transneuronal transport of HSV-1. Furthermore, it should be possible to examine the organization of cerebellar and basal ganglia loops with cerebral cortex by exploiting transneuronal transport of HSV-1 and virus strain differences.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander G. E., DeLong M. R., Strick P. L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Allen G. I., Tsukahara N. Cerebrocerebellar communication systems. Physiol Rev. 1974 Oct;54(4):957–1006. doi: 10.1152/physrev.1974.54.4.957. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Horak F. B. Influence of the globus pallidus on arm movements in monkeys. III. Timing of movement-related information. J Neurophysiol. 1985 Aug;54(2):433–448. doi: 10.1152/jn.1985.54.2.433. [DOI] [PubMed] [Google Scholar]
- Asanuma H., Rosén I. Topographical organization of cortical efferent zones projecting to distal forelimb muscles in the monkey. Exp Brain Res. 1972;14(3):243–256. doi: 10.1007/BF00816161. [DOI] [PubMed] [Google Scholar]
- Brodal P. The corticopontine projection in the rhesus monkey. Origin and principles of organization. Brain. 1978 Jun;101(2):251–283. doi: 10.1093/brain/101.2.251. [DOI] [PubMed] [Google Scholar]
- Buchman T. G., Simpson T., Nosal C., Roizman B., Nahmias A. J. The structure of herpes simplex virus DNA and its application to molecular epidemiology. Ann N Y Acad Sci. 1980;354:279–290. doi: 10.1111/j.1749-6632.1980.tb27972.x. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C., Darian-Smith I., Cheema S. S. Thalamic projections to sensorimotor cortex in the macaque monkey: use of multiple retrograde fluorescent tracers. J Comp Neurol. 1990 Sep 1;299(1):17–46. doi: 10.1002/cne.902990103. [DOI] [PubMed] [Google Scholar]
- DeLong M. R., Crutcher M. D., Georgopoulos A. P. Primate globus pallidus and subthalamic nucleus: functional organization. J Neurophysiol. 1985 Feb;53(2):530–543. doi: 10.1152/jn.1985.53.2.530. [DOI] [PubMed] [Google Scholar]
- Dix R. D., McKendall R. R., Baringer J. R. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun. 1983 Apr;40(1):103–112. doi: 10.1128/iai.40.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Brinkman C., Porter R. A quantitative study of the distribution of neurons projecting to the precentral motor cortex in the monkey (M. fascicularis). J Comp Neurol. 1987 May 15;259(3):424–444. doi: 10.1002/cne.902590309. [DOI] [PubMed] [Google Scholar]
- Hamada I., DeLong M. R., Mano N. Activity of identified wrist-related pallidal neurons during step and ramp wrist movements in the monkey. J Neurophysiol. 1990 Dec;64(6):1892–1906. doi: 10.1152/jn.1990.64.6.1892. [DOI] [PubMed] [Google Scholar]
- Hedreen J. C., DeLong M. R. Organization of striatopallidal, striatonigral, and nigrostriatal projections in the macaque. J Comp Neurol. 1991 Feb 22;304(4):569–595. doi: 10.1002/cne.903040406. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Coulter J. D., Burton H., Porter R. Cells of origin and terminal distribution of corticostriatal fibers arising in the sensory-motor cortex of monkeys. J Comp Neurol. 1977 May 1;173(1):53–80. doi: 10.1002/cne.901730105. [DOI] [PubMed] [Google Scholar]
- Künzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975 May 2;88(2):195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- Leichnetz G. R. Afferent and efferent connections of the dorsolateral precentral gyrus (area 4, hand/arm region) in the macaque monkey, with comparisons to area 8. J Comp Neurol. 1986 Dec 22;254(4):460–492. doi: 10.1002/cne.902540403. [DOI] [PubMed] [Google Scholar]
- Lewis M. L., Culbertson W. W., Post J. D., Miller D., Kokame G. T., Dix R. D. Herpes simplex virus type 1. A cause of the acute retinal necrosis syndrome. Ophthalmology. 1989 Jun;96(6):875–878. doi: 10.1016/s0161-6420(89)32823-5. [DOI] [PubMed] [Google Scholar]
- Liles S. L., Updyke B. V. Projection of the digit and wrist area of precentral gyrus to the putamen: relation between topography and physiological properties of neurons in the putamen. Brain Res. 1985 Jul 29;339(2):245–255. doi: 10.1016/0006-8993(85)90089-7. [DOI] [PubMed] [Google Scholar]
- Matelli M., Luppino G., Fogassi L., Rizzolatti G. Thalamic input to inferior area 6 and area 4 in the macaque monkey. J Comp Neurol. 1989 Feb 15;280(3):468–488. doi: 10.1002/cne.902800311. [DOI] [PubMed] [Google Scholar]
- McLean J. H., Shipley M. T., Bernstein D. I. Golgi-like, transneuronal retrograde labelling with CNS injections of herpes simplex virus type 1. Brain Res Bull. 1989 May;22(5):867–881. doi: 10.1016/0361-9230(89)90032-4. [DOI] [PubMed] [Google Scholar]
- Mink J. W., Thach W. T. Basal ganglia motor control. I. Nonexclusive relation of pallidal discharge to five movement modes. J Neurophysiol. 1991 Feb;65(2):273–300. doi: 10.1152/jn.1991.65.2.273. [DOI] [PubMed] [Google Scholar]
- Nambu A., Yoshida S., Jinnai K. Projection on the motor cortex of thalamic neurons with pallidal input in the monkey. Exp Brain Res. 1988;71(3):658–662. doi: 10.1007/BF00248759. [DOI] [PubMed] [Google Scholar]
- Orioli P. J., Strick P. L. Cerebellar connections with the motor cortex and the arcuate premotor area: an analysis employing retrograde transneuronal transport of WGA-HRP. J Comp Neurol. 1989 Oct 22;288(4):612–626. doi: 10.1002/cne.902880408. [DOI] [PubMed] [Google Scholar]
- Parent A., Smith Y., Filion M., Dumas J. Distinct afferents to internal and external pallidal segments in the squirrel monkey. Neurosci Lett. 1989 Jan 16;96(2):140–144. doi: 10.1016/0304-3940(89)90047-5. [DOI] [PubMed] [Google Scholar]
- Pereira L., Cassai E., Honess R. W., Roizman B., Terni M., Nahmias A. Variability in the structural polypeptides of herpes simplex virus 1 strains: potential application in molecular epidemiology. Infect Immun. 1976 Jan;13(1):211–220. doi: 10.1128/iai.13.1.211-220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J. T., Kern E. R., Overall J. C., Jr, Glasgow L. A. Differences in neurovirulence among isolates of Herpes simplex virus types 1 and 2 in mice using four routes of infection. J Infect Dis. 1981 Nov;144(5):464–471. doi: 10.1093/infdis/144.5.464. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Oka H., Kawaguchi S., Jinnai K., Yasuda T. Mossy fibre and climbing fibre responses produced in the cerebeller cortex by stimulation of the cerebral cortex in monkeys. Exp Brain Res. 1977 Sep 28;29(3-4):419–428. doi: 10.1007/BF00236180. [DOI] [PubMed] [Google Scholar]
- Schell G. R., Strick P. L. The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J Neurosci. 1984 Feb;4(2):539–560. doi: 10.1523/JNEUROSCI.04-02-00539.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y., Sugiuchi Y., Futami T. Excitatory inputs to cerebellar dentate nucleus neurons from the cerebral cortex in the cat. Exp Brain Res. 1987;67(2):299–315. doi: 10.1007/BF00248551. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Cook M. L., Devi-Rao G. B., Wagner E. K., Stevens J. G. Functional and molecular analyses of the avirulent wild-type herpes simplex virus type 1 strain KOS. J Virol. 1986 Apr;58(1):203–211. doi: 10.1128/jvi.58.1.203-211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. L., Devi-Rao G. V., Stevens J. G., Wagner E. K. Rescue of a herpes simplex virus type 1 neurovirulence function with a cloned DNA fragment. J Virol. 1985 Aug;55(2):504–508. doi: 10.1128/jvi.55.2.504-508.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. L., Stevens J. G. Biological characterization of a herpes simplex virus intertypic recombinant which is completely and specifically non-neurovirulent. Virology. 1983 Nov;131(1):171–179. doi: 10.1016/0042-6822(83)90543-3. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Wagner E. K., Stevens J. G. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology. 1983 Nov;131(1):180–192. doi: 10.1016/0042-6822(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Ugolini G., Kuypers H. G., Simmons A. Retrograde transneuronal transfer of herpes simplex virus type 1 (HSV 1) from motoneurones. Brain Res. 1987 Oct 6;422(2):242–256. doi: 10.1016/0006-8993(87)90931-0. [DOI] [PubMed] [Google Scholar]
- Ugolini G., Kuypers H. G., Strick P. L. Transneuronal transfer of herpes virus from peripheral nerves to cortex and brainstem. Science. 1989 Jan 6;243(4887):89–91. doi: 10.1126/science.2536188. [DOI] [PubMed] [Google Scholar]
- Wiesendanger R., Wiesendanger M. Cerebello-cortical linkage in the monkey as revealed by transcellular labeling with the lectin wheat germ agglutinin conjugated to the marker horseradish peroxidase. Exp Brain Res. 1985;59(1):105–117. doi: 10.1007/BF00237671. [DOI] [PubMed] [Google Scholar]
- Wiesendanger R., Wiesendanger M., Rüegg D. G. An anatomical investigation of the corticopontaine projection in the primate (Macaca fascicularis and Saimiri sciureus)--II. The projection from frontal and parental association areas. Neuroscience. 1979;4(6):747–765. doi: 10.1016/0306-4522(79)90004-6. [DOI] [PubMed] [Google Scholar]
- Wiesendanger R., Wiesendanger M. The thalamic connections with medial area 6 (supplementary motor cortex) in the monkey (macaca fascicularis). Exp Brain Res. 1985;59(1):91–104. doi: 10.1007/BF00237670. [DOI] [PubMed] [Google Scholar]