Abstract
Reproductive transition, inflorescence architecture, meristem patterning, and floral organ identity have been studied as distinct research areas in plant science. By using the ornamental plant Gerbera, we demonstrate that all of these keystone aspects of reproductive meristematic fate are integrated genetically by a single SEPALLATA-like MADS-box gene from a functional class designated previously as “floral homeotic” or “organ identity.” This extended regulatory network has not been elaborated in the model plant systems, which have a floral design and inflorescence-determinacy state that obscures these relationships.
The vegetative and reproductive architecture of flowering plants (angiosperms) results from the continuous production of organs at apical meristems (AMs). The immense diversity of angiosperm body plans owes a great deal to simple combinations of alternative growth modes and organ identities at AMs. The genetic control of shoot AM identity and inflorescence architecture in Arabidopsis appears to be linked through the regulatory activities of several genes, including AGAMOUS (AG), APETALA 1 (AP1), LEAFY (LFY), and TERMINAL FLOWER 1 (1). Such coordinated control over meristematic development is critical to the fulfillment of individual phenotypic identity and, therefore, reproductive success. However, clear mechanistic connections between AM determinacy and identity, including the potential for identity reversion, have not been established.
Flowering involves stepwise conversion of vegetative into inflorescence and floral meristems. Vegetative shoots are typically indeterminate, producing organs continuously, whereas flowers are determinate, shutting down their growth after reproductive organs are initiated. Inflorescences can show either pattern, and like flowers, their AMs can revert to an earlier developmental identity under certain environmental or genetic conditions (1–3). Curiously, floral determinacy and reversion blend phenotypically in Arabidopsis mutants for either the AG or SEPALLATA (SEP) 1–3 organ identity functions (3–6).
Many of the genes that are known to control aspects of shoot AM development are members of the large, eukaryote-wide MADS-box gene family (7). MADS domain factors, which include the AM regulators AG and AP1, also comprise most of the elements of the ABC, extended ABCDE, and quartet models (8–11) for the determination of flower organ identity. Thus, they provide an outstanding example of the importance of duplication and divergence in the evolution of angiosperm gene function (12). Noteworthy examples within this developmental system are the so-called E-function factors (11), which are encoded by the phylogenetically related and functionally redundant SEP genes (5). Here, we dissect critical meristematic functions of two SEP-like genes from Gerbera hybrida [an ornamental plant in the sunflower family (Asteraceae)] that appear to be subfunctionalized duplication products. Splitting of functions among gene duplicates, which is one form of maintaining redundancy (13), can provide a powerful means by which to analyze complex gene activities.
Gerbera inflorescences, or capitula, are condensed and organized structures in which individual flowers play different roles in the reproductive biology of the plant. Because of differential elongation of the corolla (fused petals) in marginal versus central flowers, the entire inflorescence bears a resemblance to a single flower. Gerbera flowers differ also in sexuality, with the marginal flowers being female and the central flowers hermaphroditic (14). Reproductive specialization at the inflorescence level has been evolutionarily successful; Asteraceae are one of the largest families of flowering plants, with >20,000 species worldwide (15).
We have previously obtained and functionally characterized a number of MADS-box genes that are active during Gerbera inflorescence and flower development. Genes orthologous to B and C-function genes have conserved expression patterns in flower primordia, and transgenic alterations in their expression cause organ identity changes as predicted by the ABC model (14). We have also shown that the MADS-box gene Gerbera regulator of capitulum development 1 (GRCD1) is necessary for stamen development in whorl 3, and primarily in flowers at the margins of the inflorescence, where they form sterile staminodia (16).
In this article, we characterize GRCD2, a close homologue of GRCD1 and the Arabidopsis SEP genes. Like GRCD1, GRCD2 plays a role in reproductive organ determination. Down-regulation of both GRCD2 and the Gerbera AG homologues (14) results in loss of carpel identity, but only the latter causes indeterminacy in stylar and stigmatic tissues (14), and only the former results in floral reversion, which occurs in ovaries. Remarkably, loss of GRCD2 function also alters inflorescence architecture by switching off terminal, determinate growth. This integrated control over reproductive meristem fate by a single SEP-like factor has not been described from the model systems, which have a different pistil design and normally bear indeterminate inflorescences.
Materials and Methods
Plant Material and Transformation. G. hybrida var. Terra Regina was obtained from Terra Nigra (De Kwakel, The Netherlands). Transgenic Gerbera lines carrying GRCD2 in an antisense orientation under the Cauliflower mosaic virus 35S promoter were produced by using Agrobacterium-mediated transformation as described in ref. 17. Four independent lines, two lines with partially reduced and two lines with strongly reduced levels of endogenous GRCD2 expression, were obtained, and the floral structures of these plants were analyzed. Ectopic expression of GRCD2 under the 35S promoter did not lead to detectable phenotypic change.
Cloning of GRCD2. The full-length cDNA clone of GRCD2 was obtained from a Gerbera petal cDNA library (16) by screening with a degenerate oligonucleotide specific for AG MADS-box sequence (18).
RNA Gel Blot Analysis and in Situ Hybridization. Total RNA was extracted by using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. We loaded 15 μg of total RNA per lane. Electrophoresis was performed according to standard methods. For hybridization, the Ultrahyb reagent (Ambion, Austin, TX) was applied according to the manufacturer's instructions. Samples for in situ hybridization were fixed, dehydrated, cleared, and paraffin-embedded as described by Cox et al. (19). We pretreated and hybridized 10-μm-thick sections essentially as described by Di Laurenzio et al. (20), except that proteinase K was used at 10 μg/ml. We used full-length GRCD2 cDNA as probe, cloned into the pBluescript II SK(+) vector (Stratagene). The full-length probe is specific for GRCD2 in the hybridization conditions that were used. By using RNA gel blot hybridization, we carefully tested that it does not hybridize to GRCD1. For RNA gel blot hybridization, the fragment was labeled with 32P according to standard methods. For in situ hybridization, 1 μg of digested plasmid was used as a template to produce labeled RNA probes by using the DIG RNA labeling kit (SP6/T7; Roche Diagnostics). After synthesis, probes were hydrolyzed to represent a population of RNA fragments with an average size of 200 bp. The probes were used at a concentration of 50 ng·ml-1·kb-1. Detection of hybridization was performed by using a DIG nucleic acid detection kit (Roche Diagnostics).
Scanning Electron Microscopy (SEM). Plant material was fixed overnight in a buffer containing 50% ethanol, 5% acetic acid, and 2% formaldehyde, and it was transferred through an ethanol series into 100% ethanol. Samples were dried by using a critical point drying unit (BAL-TEC, Balzers, Principality of Liechtenstein), mounted on aluminum stubs with a tape and coated with platinum/palladium (AGAR sputter device, AGAR, Stansted, England). Samples were examined by SEM with a DSM 962 (Zeiss) in the Electron Microscopy Laboratory of the Institute of Biotechnology at the University of Helsinki.
Results
GRCD2 Is a SEP-Like MADS-Box Gene. We have used several strategies to obtain MADS-box genes expressed in the developing flowers and inflorescences (14, 16). Library screening with degenerate oligonucleotides led to isolation of a full-length cDNA for GRDC2. In addition to the ORF encoding GRCD2, the cDNA contains a second upstream ORF, possibly limiting translation of the GRCD2 ORF (21). The gene most similar to GRCD2 is the Gerbera organ identity gene GRCD1, with which it shares a 64% identity at nucleotide level (86% identity in the MADS-box).
Expression of GRCD2 Is Flower-Abundant. GRCD2 is expressed in young capitulum tissue, which suggests that GRCD2 is active during formative (i.e., prefloral) stages of inflorescence and flower development (Fig. 1). Early expression is detected equally in all cells of very young flower primordia (Fig. 2A). Later expression concentrates to the primordium center. When the identities of floral organs have been determined, GRCD2 expression is strongest in stamens, carpels, and developing ovules (Fig. 2C). Given this expression pattern and the similarity of GRCD2 to other MADS-box genes, it was reasonable to postulate that the gene plays a role in flower development. To study this possibility, transgenic Gerbera lines carrying 35S::GRCD2 in the antisense orientation were produced.
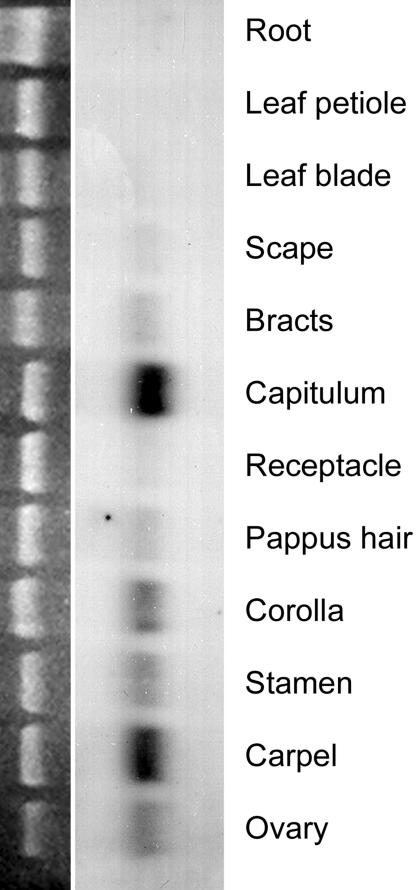
Fig. 1.
Expression of GRCD2 in various Gerbera tissues. (Right) Total RNA from different Gerbera organs, pooled from several developmental stages, was hybridized with GRCD2 full-length probe. The GRCD2 gene is expressed in all floral organs {pappus [i.e., sepals (14)], petals, stamens, and carpels} and other floral tissues, including ovary, bracts, scape, and receptacle. Expression was also detected in young capitula, suggesting that the GRCD2 gene is also active during early stages of inflorescence and flower development. Very weak expression was detected in the leaf blade, but there was no detectable expression in leaf petioles or roots. (Left) Equal loading of RNA was controlled with ethidium bromide staining (18S rRNA).
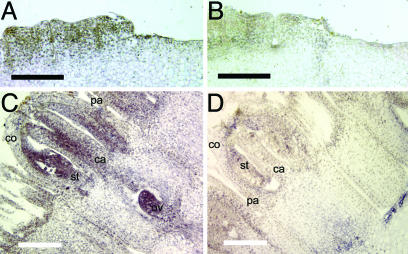
Fig. 2.
In situ hybridization analysis showing expression of GRCD2 in young Gerbera capitula. Longitudinal sections of wild-type capitulum [diameter, 8 mm (A and B) and 14 mm (C and D)] hybridized with antisense (A and C) and sense (B and D) GRCD2 probes. The initial expression of GRCD2 appears to be equal in all cells of young flower primordia from which flower organs have not yet emerged (A). When flowers reach the ring primordium stage (14), GRCD2 expression concentrates to the central part of the primordium, from which stamens and carpels will later emerge. In more mature flower primordia (C), in which the identities of floral organs have been determined, GRCD2 expression is strongest in stamens and carpels as well as in the developing ovule. In A and B, two flower primordia are visible. In C and D, a single flower primordium is shown, with pappus bristles (pa), corolla (co), stamens (st), carpels (ca), and the ovule (ov) indicated. [Scale bars indicate 200 μm (A and B) and 1 mm (C and D).]
Down-Regulation of GRCD2 Expression Affects Carpel Development. We observed that, in all down-regulated GRCD2 lines, the only affected flower organ was the carpel (whorl 4) (Fig. 3). The carpels were longer and broader than those of the wild type, and they were fused only partially. In plants with milder phenotypes, the whorl 4 organs were green and leaf-like (Fig. 3B), but in plants with the strongest phenotypes, the carpels were replaced by flattened, petal-like organs that had anthocyanin pigmentation (Fig. 3D). Corroborating their partial petal identity, we observed concomitant up-regulation of both Gerbera B function MADS-box genes, GERBERA GLOBOSA 1 and GERBERA DEFICIENS 2 (14) (see Table 1, which is published as supporting information on the PNAS web site). SEM also showed that the whorl 4 organs were mosaic structures, with cell types characteristic of several organs, including carpels and petals (Fig. 4). The changes caused by reduced GRCD2 function in whorl 4 resemble the homeotic conversions in Gerbera lines with reduced expression of the Gerbera AG homologues GERBERA AG 1 (GAGA1) or GAGA2. Partial reduction of the expression of these C-function genes converts whorl 4 organs into greenish structures (as phenocopied by anti-GRCD2; Fig. 3C), whereas in more severe plants, organs resembling pappus hairs and petals (whorl 1 and 2 organs) appear (14).
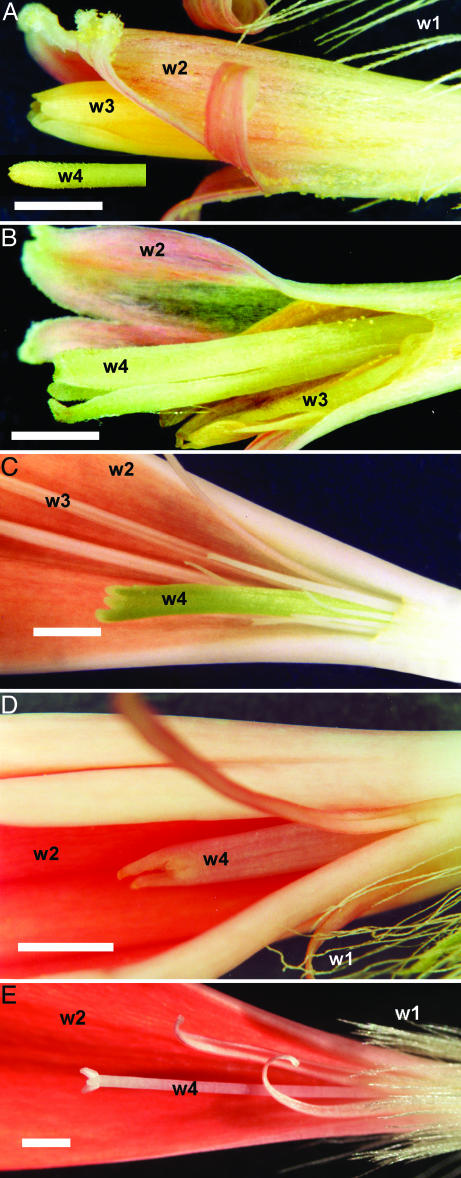
Fig. 3.
Homeotic changes in transgenic Gerbera. (A) Disk flowers of non-transgenic Gerbera show pappus hairs in whorl 1 (w1), fused petals (corolla) in whorl 2 (w2), and fused anthers in whorl 3 (w3). The disk flower whorl 4 (w4) organs, carpels, are covered by the fused anthers in nontransgenic flowers. (B) In transgenic Gerbera in which GRCD2 is partially down regulated, green leaf-like organs replace carpels in w4. (C) In transgenic Gerbera in which GAGA1 is partially down regulated (14), w4 organs are similarly replaced by green structures, and concomitantly, w3 organs by petal-like structures. (D) Transgenic Gerbera with strongly reduced GRCD2 expression shows petal-like organs in w4. (E) Nontransgenic ray flower showing normal fertile carpels in w4. (Scale bars indicate 1 mm.)
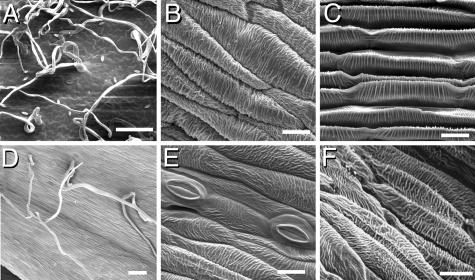
Fig. 4.
Epidermal characterization of the whorl 4 organs. SEM of the surface of a wild-type leaf (A), carpel (B), and petal (C). In GRCD2 down-regulated plants the whorl 4 organs show presence of multicellular hair-like structures (D) and stomata (E). Neither stomata nor hairs are present on normal carpels, but stomata are found on the abaxial surface of petals and, like hairs, on the adaxial and abaxial sides of leaves and bracts, as well as on the surfaces of scapes. In stronger phenotypes, the anti-GRCD2 whorl 4 surface (F) resembles the ridges typical to wild-type petals (C). The carpels of the transgenic plants were not functional, as confirmed by crossing tests with nontransgenic plants. [Scale bars indicate 100 μm (A and D) and 20 μm (B, C, E, and F).]
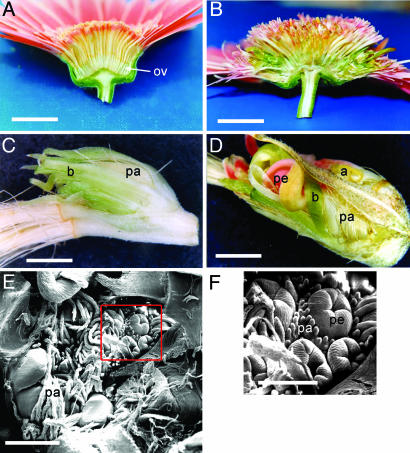
In addition to alterations in the styles and stigmata, ovary development in anti-GRCD2 flowers was altered dramatically. In the transgenic lines, ovule-like structures developed inside otherwise normal-appearing ovaries. However, at later stages, growth of these “ovules” was disturbed, and as a result, a secondary inflorescence comprising bracts, feminized outer and mid-radial flowers, and hermaphroditic disk flowers was formed (Fig. 5). In the middle of this secondary inflorescence, a meristematic region was found to comprise a number of floral primordia (Fig. 5 E and F). The carpels of the flowers in the secondary inflorescence showed the same phenotypic changes as those of the primary inflorescence (data not shown).
Fig. 5.
The ovaries in anti-GRCD2 Gerbera revert to inflorescences. (A) Wild-type inflorescence of Gerbera, which were cut in half to show inferior ovaries (ov) below the whorls of floral organs. (B) Transgenic Gerbera with reduced GRCD2 expression shows disrupted ovaries in which bracts (b), pappus hairs (pa), petals (pe), and anthers (a) develop instead of the ovule (C and D). (E and F) SEM micrograph of the inner meristematic regions replacing the ovary in anti-GRCD2 Gerbera shows several emerging flower primordia, each surrounded by its own pappus bristles (whorl 1) (F). [Scale bars indicate 2 cm (A and B), 2 mm (C and D), 500 μm (E), and 250 μm (F).]
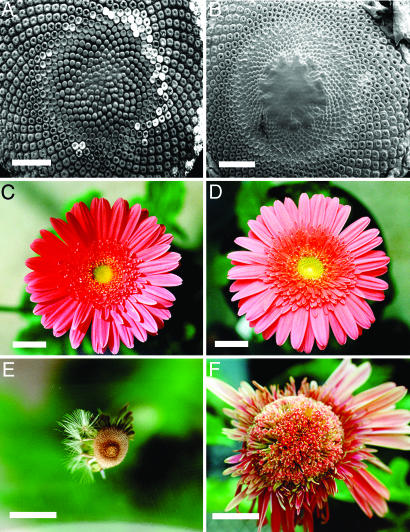
GRCD2 Controls Determinacy of the Inflorescence Meristem. In wild-type Gerbera, the inflorescence (capitulum) is a condensed structure that produces new flowers in a phyllotactic spiral from the AM located at its center (14, 16). Unexpectedly, transgenic lines with strongly reduced GRCD2 expression produced larger inflorescences and several-fold increases in numbers of flowers than wild type (Fig. 6). These inflorescences continued to produce new disk flowers until senescence (Fig. 6F). The inflorescence meristems were already altered in young capitula. In contrast to wild type, the central region of meristematic stem cells remained undifferentiated for an extended period and rose up from the surface of the capitulum (Fig. 6B).
Fig. 6.
Indeterminate growth of the inflorescence meristem in transgenic Gerbera. Development of wild-type (A, C, and E) and antisense GRCD2 (B, D, and F) inflorescence over time. The number of flower primordia is fixed at an early stage of development in wild-type Gerbera (A), whereas in the transgenic plants, the inflorescence meristem continues to proliferate (B). C and D show inflorescences at full flowering. At 35 days later, the wild-type inflorescence has matured and shed its seeds (E), whereas the transgenic inflorescences continue to produce flowers (F). The wild-type capitulum produces >600 florets (593 ± 56, n = 13), but the strong antisense GRCD2 lines produce up to 1,200 flowers (976 ± 98, n = 13). Transgenic plants in which GAGA2 was down-regulated (14) and that showed indeterminate flower meristems produced 594 ± 76 flowers (n = 6). The indeterminate growth mode of the anti-GRCD2 inflorescences was reflected both by continuously increasing production of disk flowers and by a concomitant enlargement of the receptacle. Although superficially unorganized, the spiral phyllotaxis of the transgenic inflorescences of the transgenic lines was not disturbed in terms of numbers of parastichies. [Scale bars indicate 1 mm (A and B) and 2 cm (C–F).]
GRCD2 Interacts with Gerbera C-Function MADS-Domain Proteins. MADS-domain transcription factors are known to function as dimers or as complexes of multiple proteins. It has been shown (9, 16, 22, 23) that SEP-like MADS-domain proteins interact with C-function and B-function proteins, as well as other MADS-domain proteins. Therefore, it was a reasonable hypothesis that GRDC2 could act by complexing with other MADS proteins. By using yeast two-hybrid assay, we showed that GRCD2 is able to form dimers with the Gerbera C-function proteins GAGA1 and GAGA2 (14) (see Fig. 7 and Supporting Methods, which are published as supporting information on the PNAS web site). These regulators also form heterodimers with GRCD1 in yeast, but they do not homodimerize or heterodimerize with each other (16). Interestingly, GRCD2 also heterodimerizes with GERBERA GLOBOSA 1, a Gerbera B-function MADS-domain protein. However, down-regulation of GRCD2 does not lead to phenotypic changes in whorls 2 or 3, where GERBERA GLOBOSA 1 is expressed (14).
Discussion
Subfunctionalized SEP-Like Genes Are Responsible for Gerbera Flower Organ Development. GRCD2 down-regulation in Gerbera provides strong evidence that this SEP-like gene has pleiotropic activities across at least three levels of reproductive development. First, a homeotic function in whorl 4 is necessary for determination of carpel identity. Second, GRCD2 is required for maintenance of flower meristem identity because flower AMs revert to inflorescence AMs when GRCD2 expression is reduced. Third, expression of GRCD2 is needed to acquire determinacy and a fixed number of flowers at the inflorescence level. It is possible to relate the first two functions to functions of orthologous genes in the model plant Arabidopsis. In Arabidopsis, SEP1, SEP2, and SEP3 are needed for the development of petals, stamens, and carpels. In a triple mutant for the SEP genes, all whorls of the Arabidopsis flower bear sepals (5). We have determined by using phylogenetic analysis (which is far more predictive of orthology than simple sequence identity or a blast search; ref. 24) that GRCD1 and GRCD2 group as a pair inside the SEP (or AGL2) clade (16). Recent phylogenetic studies of amino acid sequences suggest a similar partnership, with nesting among SEP proteins (V.A.A., unpublished data). Although the exact placement of the GRCD pair within the SEP clade is still tentative, our best estimate from DNA data is that the closest homologue in Arabidopsis for both genes may be SEP3 (16). This finding would suggest that GRCD1 and GRCD2 are gene duplicates that are orthologous to SEP3. SEP3 and its redundant homologues SEP1 and SEP2 are involved in determining organ identity in whorls 2, 3, and 4, together with the ABC MADS-box genes AP1, PISTILLATA, AP3, and AG (5). In Gerbera, our results indicate that the functions in whorls 3 and 4, corresponding to the SEP genes, are represented by whorl-specific counterparts, with GRCD2 acting on whorl 4 and GRCD1 acting on whorl 3. This finding provides a classic example of gene duplication and divergence by subfunctionalization.
Therefore, GRCD1 and GRCD2 must interact with the Gerbera C-function genes GAGA1 or GAGA2 in whorls 3 and 4. However, it is unlikely that these genes are transcriptionally dependent on each other. By using a set of transgenic plants, down-regulation of either GAGA1 or GAGA2 genes was shown not to affect steady-state mRNA levels of GRCD1 or GRCD2 transcripts, and vice versa (ref. 16 and data not shown). It is more likely that GRCD1 and GRCD2 act together with GAGA1 and GAGA2 in multimeric transcriptional complexes (11), as has been shown for related MADS-domain proteins in other plants (9, 10). In support of this hypothesis, we demonstrated by using two-hybrid assays that GRCD1 and GRCD2 interact with GAGA1 and GAGA2 in yeast (Fig. 7 and ref. 16).
Flower Meristems Revert to Inflorescences When GRCD2 Is Down-Regulated. Arabidopsis ag mutants defective in the C function lose determinacy at the flower meristem level, and sepal- and petal-like organs develop iteratively in the extra whorls that build up inside whorl 4 (8). This phenomenon also occurs in Gerbera flowers down-regulated for GAGA2, in which carpels are replaced by repeating whorl 1 and 2 organs (pappus hairs and petals) (14). Petal-like organs and hairs also develop in whorl 4 when GRCD2 is dramatically reduced, but intriguingly, no organ repetition occurs, indicating that the floral AM remains determinate.
In the Arabidopsis sep1/sep2/sep3 triple-mutant flowers, in addition to changes in floral organ identity, there seems to be a concomitant loss of floral meristem determinacy (5). In antisense 35S::GRCD2 Gerbera plants, floral reversion occurs instead of indeterminacy (i.e., the flower meristem converts back into an inflorescence meristem).
In Asteraceae, unlike Arabidopsis, the position of the ovary is inferior (i.e., below the whorls of floral organs). This structural distinction renders the positions at which repeating structures occur in anti-GAGA2 (stigma and style) (14), versus anti-GRCD2 (ovary), dramatically different. In Arabidopsis, loss of determinacy appears to blend with floral reversion. The repeating structures in the sep1/sep2/sep3 mutant appear to represent a mixture of primary sepalloid organs surrounding secondary, sepalloid flowers (5, 6). When grown under conditions unfavorable for floral induction, ag mutant plants also undergo floral reversion (3, 4), but it is not known whether this phenomenon involves down-regulation of the SEP genes. The situation in other plants is complex because of difficulties in interpreting transgenic phenotypes. In tomato, down-regulation of TAG1, an orthologue of the C-class gene AG, produces partial loss of floral determinacy but also features of floral reversion (25). Down-regulation of TM29, a SEP-like gene, causes reversion of the floral meristem into a shoot meristem (26). In Petunia, down-regulation of FBP2 (SEP homologue) causes floral reversion (6, 27), but down-regulation of the AG homologue pMADS3 also does, at least in whorl 3 (28). No simple loss of floral determinacy is known in Petunia. Therefore, reduction of AG or SEP homologue expression often causes floral reversion, with or without loss of floral determinacy.
Floral Determinacy and Floral Reversion Are Distinct Phenomena. In Gerbera, loss of flower meristem determinacy and floral reversion is separated spatially into different parts of the pistil and separated genetically by AG-like versus SEP-like gene control. This result strongly suggests that determinacy and identity of floral AMs are not part of a developmental continuum but are separate phenomena. Floral reversion occurs also when the activity of the master regulator of reproductive development, LFY, is limiting (29). LFY is a regulator of AG (29), but interestingly, LFY prevents floral reversion even when AG is nonfunctional (30). Recently, it was shown that in Arabidopsis LFY represses the MADS-box gene AGL24, which causes floral reversion when expressed ectopically (31). It is not known whether LFY or AGL24 also regulate the SEP genes, but our observations with Gerbera suggest this possibility.
Gerbera Inflorescences Are Determinate. The third function of GRCD2 in Gerbera flower development is in control of determinacy at the inflorescence level. In antisense 35S::GRCD2 plants, the inflorescence AM continuously produces new flower meristems, leading to an increased number of flowers in the capitulum.
The growth habit in species of the sunflower family varies, but in general, the inflorescence AM has been considered to be indeterminate (32). The number of flowers produced by the capitulum is fixed early in development (see Fig. 6A), but it has been rationalized that this pattern arises because of spatial constraints on the densely packed capitulum. Our observations are in clear contrast with this view; they demonstrate that the wild-type inflorescence meristem of Gerbera must be considered to be determinate and that GRCD2 is a requisite factor in regulation of its inflorescence determinacy.
SEP-Like Genes Act at the Infloral/Floral/Floral Organ Interface. The possibility that SEP1/SEP2/SEP3-like genes participate in the regulation of inflorescence determinacy in Arabidopsis, Petunia, or tomato has not been directly addressed previously, to our knowledge, probably because the inflorescences of these plants are indeterminate (1). Over-expression of SEP3 in Arabidopsis has been shown to produce terminally flowered inflorescences in some transformants (9). In Arabidopsis, mutations in the gene TERMINAL FLOWER 1 convert the normally indeterminate inflorescence into determinate (33). In snapdragons, a similar phenotype is caused by mutations in the TERMINAL FLOWER 1 relative CENTRORADIALIS (34). Based on our antisense 35S::GRCD2 phenotype with determinate Gerbera that is the converse of Arabidopsis ectopic 35S::SEP3 plants (9, 10), it is an attractive hypothesis that TERMINAL FLOWER 1/CENTRORADIALIS genes might be regulated by MADS-domain factors of the SEP (AGL2) clade in complex with other unidentified MADS proteins. Indeed, all of the pleiotropic functions of GRCD2 may represent an early-acting or even second tier of transcriptional regulation, lying near or directly past LFY floral induction and operating with alternative quaternary complex partners.
Furthermore, it can be stressed that all GRCD2 functions are in terminal AM activities, including identity determination of the innermost floral whorl. SEP-like proteins have been implicated also as partners in the control of ovule identity (35, 36), and ovules are terminal structures arising from multicellular AMs. These observations suggest that homologous (or paralogous) gene systems controlling meristematic stem cell proliferation (37) may have been evolutionarily co-opted for various terminal-meristematic functions in different plant lineages. Our results with GRCD2 indicate that further knowledge of SEP-like protein function in angiosperms may help to interconnect known genetic pathways governing shoot meristematic fate, including reproductive transition, inflorescence architecture, meristem patterning, and floral organ identity.
Supplementary Material
Acknowledgments
We thank Ykä Helariutta, Hong Ma, and Günter Theissen for comments on the manuscript. This research was supported by the Academy of Finland (T.H.T.) and the Swedish Research Council (V.A.A.).
Author contributions: A.U., M.K., P.E., and T.T. designed research; A.U., M.K., P.E., and D.Y. performed research; A.U., V.A.A., and T.T. analyzed the data; and A.U., V.A.A., and T.T. wrote the paper.
Abbreviations: AM, apical meristem; AG, AGAMOUS; AP, APETALA; LFY, LEAFY; SEP, SEPALLATA; GRCD1/2, Gerbera regulator of capitulum development 1/2; SEM, scanning electron microscopy; GAGA1/2, GERBERA AG 1/2.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AJ784156).
References
- 1.Battey, N. H. & Lyndon, R. F. (1990) Bot. Rev. 56, 162-189. [Google Scholar]
- 2.Leyser, O. & Day, S. (2003) Mechanisms in Plant Development (Blackwell, Oxford).
- 3.Okamuro, J. K., den Boer, B. G. W., Lotus-Prass, C., Szeto, W. & Jofuku, K.D. (1996) Proc. Natl. Acad. Sci. USA 93, 13831-13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizukami, Y. & Ma, H. (1997) Plant Cell 9, 393-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelaz, S., Ditta, G. S., Baumann, E., Wisman, E. & Yanofsky, M. F. (2000) Nature 405, 200-203. [DOI] [PubMed] [Google Scholar]
- 6.Ferrario, S., Immink, R. G. H., Shchennikova, A., Busscher-Lange, J. & Angenent, G. C. (2003) Plant Cell 15, 914-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theissen, G., Becker, A., Di Rosa, A., Kanno, A., Kim, J.T., Münster, T., Winter, K.-U. & Saedler, H. (2000) Plant Mol. Biol. 42, 115-149. [PubMed] [Google Scholar]
- 8.Coen, E. S. & Meyerowitz, E. M. (1991) Nature 353, 31-37. [DOI] [PubMed] [Google Scholar]
- 9.Honma, T. & Goto, K. (2001) Nature 409, 525-529. [DOI] [PubMed] [Google Scholar]
- 10.Pelaz, S., Tapia-López, R., Alvarez-Buylla, E. R. & Yanofsky, M. (2001) Curr. Biol. 11, 182-184. [DOI] [PubMed] [Google Scholar]
- 11.Theiβen, G. (2001) Curr. Opin. Plant Biol. 4, 75-85. [DOI] [PubMed] [Google Scholar]
- 12.Nam, J., Kim, J., Lee, S., An, G., Ma, H. & Nei, M. (2004) Proc. Natl. Acad. Sci. USA 101, 1910-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch, M. & Conery, J. S. (2000) Science 290, 1151-1155. [DOI] [PubMed] [Google Scholar]
- 14.Yu, D., Kotilainen, M., Pöllänen, E., Mehto, M., Elomaa, P., Helariutta, Y., Albert, V. A. & Teeri, T. H. (1999) Plant J. 17, 51-62. [DOI] [PubMed] [Google Scholar]
- 15.Bremer, K. (1994) Asteraceae: Cladistics and Classification (Timber, Portland, OR).
- 16.Kotilainen, M., Elomaa, P., Uimari, A., Albert, V., Yu, D. & Teeri, T. H. (2000) Plant Cell 12, 1893-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elomaa, P., Honkanen, J., Puska, R., Seppänen, P., Helariutta, Y., Mehto, M., Kotilainen, M., Nevalainen, L. & Teeri, T.H. (1993) Bio/Technology 11, 508-511. [Google Scholar]
- 18.Tandre, K., Albert, V. A., Sundås, A. & Engström, P. (1995) Plant Mol. Biol. 27, 69-78. [DOI] [PubMed] [Google Scholar]
- 19.Cox, K. H. & Goldberg, R. B. (1988) in Plant Molecular Biology: A Practical Approach, ed. Shaw, C. H. (IRL, Oxford), pp. 1-34.
- 20.Di Laurenzio, L., Wysocka-Diller, J., Malamy, J. E., Pysh, L., Helariutta, Y., Freshour, G., Hahn, M. G., Feldmann, K. A. & Benfey, P. N. (1996) Cell 86, 423-433. [DOI] [PubMed] [Google Scholar]
- 21.Wang, L. & Wessler, S. R. (1998) Plant Cell 10, 1733-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan, H.-Y., Hu, Y., Tudor, M. & Ma H. (1997) Plant J. 12, 999-1010. [DOI] [PubMed] [Google Scholar]
- 23.Egea-Cortines, M., Saedler, H. & Sommer, H. (1999) EMBO J. 18, 5370-5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisen, J. A. (1998) Genome Res. 8, 163-167. [DOI] [PubMed] [Google Scholar]
- 25.Pnueli, L., Hareven, D., Rounsley, S. D., Yanofsky, M. F. & Lifschitz, E. (1994) Plant Cell 6, 163-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ampomah-Dwamena, C., Morris, B. A., Sutherland, P., Veit, B. & Yao, J.-L. (2002) Plant Physiol. 130, 605-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angenent, G. C., Franken, J., Busscher, M., Weiss, D. & van Tunen, A. J. (1994) Plant J. 5, 33-44. [DOI] [PubMed] [Google Scholar]
- 28.Kapoor, M., Tsuda, S., Tanaka, Y., Mayama, T., Okuyama, Y., Tsuchimoto, S. & Takatsui, H. (2002) Plant J. 32, 115-127. [DOI] [PubMed] [Google Scholar]
- 29.Parcy, F., Nilsson, O., Busch, M. A., Lee, I. & Weigel, D. (1998) Nature 395, 561-566. [DOI] [PubMed] [Google Scholar]
- 30.Parcy, F., Bomblies, K. & Weigel, D. (2002) Development (Cambridge, U.K.) 129, 2519-2527. [DOI] [PubMed] [Google Scholar]
- 31.Yu, H., Ito, T., Wellmer, F. & Meyerowitz, E. M. (2004) Nat. Genet. 36, 157-161. [DOI] [PubMed] [Google Scholar]
- 32.Harris, E. M. (1995) Bot. Rev. 61, 93-278. [Google Scholar]
- 33.Shannon, S. & Meeks-Wagner, D. R. (1991) Plant Cell 3, 877-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradley, D., Carpenter, R., Copsey, L., Vincent, C., Rothstein, S & Coen, E. (1996) Nature 379, 791-797. [DOI] [PubMed] [Google Scholar]
- 35.Favaro, R., Pinyopich, A., Battaglia, R., Kooiker, M., Borghi, L., Ditta, G., Yanofsky, M. F., Kater, M. M. & Colombo, L. (2003) Plant Cell 15, 2603-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandenbussche, M., Zethof, J., Souer, E., Koes, R., Tornielli, G. B., Pezzotti, M., Ferrario, S., Angenent, G.C. & Gerats, T. (2003) Plant Cell 15, 2680-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fletcher, J. C. (2002) Annu. Rev. Plant Biol. 53, 45-66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.