All organisms, including bacteria, localize a fraction of all of their proteins partially or completely outside of the cytosol. Along the way, these proteins must cross at least one hydrophobic lipid membrane. The remarkable feat of delivering proteins across tightly sealed membranes is achieved largely by complex secretion machineries known as translocons. These machines recognize their substrates via signal sequences, which are required for proper targeting to the translocon. The bulk of protein transport across the inner cytoplasmic membrane is facilitated by the well-known general secretory (Sec) pathway, but additional categories for transport into or across the inner membrane, including the recently discovered twin-arginine translocation (Tat) pathway, exist. More-specialized mechanisms for targeting proteins to the inner membrane, the outer membrane, or the extracellular environment also exist and are reviewed elsewhere (19, 31, 71).
Proteins targeted to the Sec pathway achieve membrane translocation through the Sec translocon, a proteinaceous conduit formed by an oligomeric assembly of the heterotrimeric membrane protein complex SecYEG (7, 79) and the peripheral ATPase SecA as a molecular motor (26). Sec substrates traverse the membrane in a largely unfolded state and effectively thread their way through the pore. In stark contrast to the Sec-dependent threading of unstructured substrates, the Tat pathway has the unique ability to transport proteins that have attained a substantial degree of tertiary or even quaternary structure in the cytoplasm prior to membrane translocation (13, 22, 35, 66, 70). This process is enabled by a translocon consisting of the TatA, TatB, TatC, and TatE proteins, which share little homology with the components of the Sec translocon. Consistent with these distinct modes of translocation, both the Sec and Tat pathways have evolved unique measures for surveying the quality of their respective substrates.
This minireview will discuss how the proper structural integrity of proteins to be transported (hereinafter referred to as preproteins) is ensured during the early stages of Sec and Tat targeting so that these proteins remain compatible with their respective macromolecular transport machineries.
REQUIREMENTS FOR REMAINING COMPETENT WITH THE Sec AND Tat TRANSLOCONS
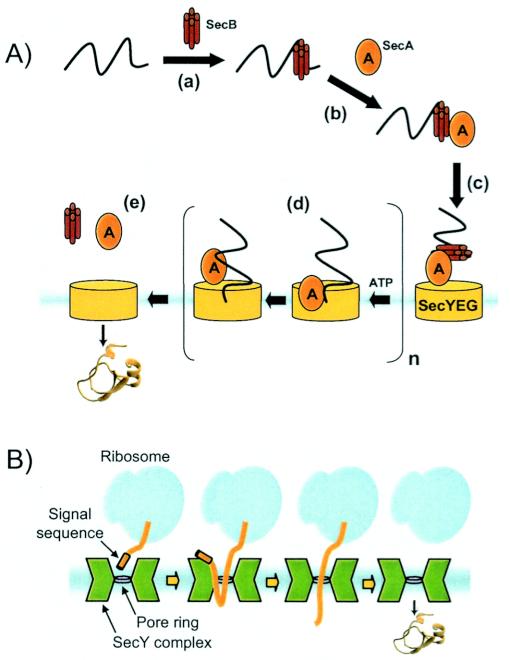
It is well established that the bacterial Sec system and its eukaryotic counterpart employ a threading mechanism for delivering preproteins across the cytoplasmic membrane (Fig. 1A) (26). In order for a productive threading event to occur, preproteins must be prohibited from attaining a well-ordered structure prior to transport by the Sec machinery (16, 17). This notion is well supported by experiments in which domain folding of a translocating polypeptide chain becomes possible only after the chain has emerged from the translocon pore (40). The requirement that preproteins be unstructured is mandated largely by physical constraints imposed by the translocon itself. Recent X-ray crystallography studies suggest that the Sec complex is an hourglass-shaped channel with aqueous funnels that taper to a 5- to 8-Å constriction in the middle of the membrane (Fig. 1B) (79). This constriction is created by a ring of 6 hydrophobic residues that may form a gasket-like seal around a translocating polypeptide. Slight expansion of this constriction, which could be envisioned to arise from shifts in the helices that line the channel, would be large enough to accommodate an α-helical sequence (anhydrous diameter of 10 to 12 Å) and would explain how α-helix-like structures could form inside the Sec translocon (52). However, the relatively small size of the pore and the absence of a large internal chamber indicate that polypeptide chains exhibiting significant tertiary structure are not tolerated within the Sec channel.
FIG. 1.
(A) Schematic of Sec translocation. Briefly, (a) SecB binding of a nascent polypeptide maintains export competence and assists in proper targeting to the Sec machinery. SecA serves several functions, including (b) preprotein binding; (c) targeting to the inner membrane; (not shown) maintaining quality control by assisting the cytoplasmic folding of nontransported polypeptides; and (d) driving preprotein translocation by repeated cycles of ATP-dependent membrane insertion-deinsertion. Finally, (e) translocation is completed and SecA and SecB are recycled. (B) Structural basis for Sec protein translocation adapted from the work of Van den Berg et al. (79) (see the text for a description).
More recently, a second pathway for delivering proteins across biological membranes was discovered first in plant thylakoid membranes and later in archaeal and bacterial inner membranes (3, 75, 81). This pathway was termed the Tat pathway because of the signature Arg-Arg dipeptide found in most of the leader peptides of proteins that utilize this mode of export (3). The hallmark of the Tat pathway that sets it apart from all other modes of protein translocation across lipid bilayer membranes is the ability to transport proteins of various dimensions that have already folded in the cytoplasm (Fig. 2). In many instances, substrates traverse the Tat pathway because they are inherently incompatible with the Sec machinery. This can occur if the substrate simply folds too rapidly to remain Sec export competent or if the substrate is unable to reach its native conformation in the compartment to which it is targeted. For instance, some transported proteins need to incorporate cofactors or assemble subunits in the cytoplasm prior to export (4, 33, 66). Others benefit from prefolding in the cytoplasmic compartment, which can provide a more favorable folding environment relative to certain extracytoplasmic locations (68).
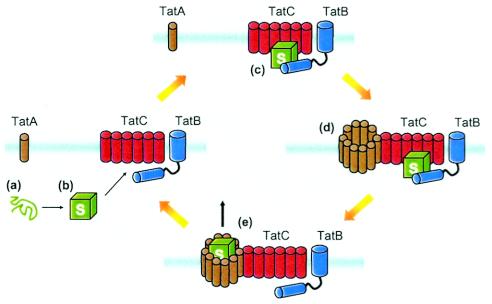
FIG. 2.
Working model for Tat transport of folded proteins. Following preprotein folding in the cytoplasm (a and b), Tat substrates (S) are recognized by the translocon (c) in a process that likely involves TatB, TatC, and the leader peptide. According to the cyclical assembly model of Mori and Cline (54), preprotein binding to the TatB-TatC complex triggers assembly of multiple TatA monomers that likely form a translocation pore (d) through which a folded substrate is able to pass (e). Following successful transport, the TatABC complex disassembles. This model of assembly-disassembly may explain how the translocon can accommodate proteins of various sizes and how the Tat system can be present within membranes without compromising permeability to ions and protons.
Processes which render proteins Sec incompatible, such as cofactor incorporation and the assembly of protein subunits, hinge on the formation of a secondary or tertiary structure. Therefore, the observation that Tat transport was abolished when cytoplasmic cofactor incorporation was blocked provided early evidence that Tat preproteins fold prior to transport (33). Consistent with these findings, in vitro experiments using the plant thylakoid system demonstrated that preproteins could be transported even after they were irreversibly cross-linked (13). Given that the Tat system accommodates folded proteins, it is reasonable to ask whether both folded and unfolded polypeptides can be accepted as substrates or whether only preproteins that have obtained a substantially native state in the cytoplasm are competent for translocation. In support of the latter model, Roffey and Theg showed that efficient in vitro translocation of a thylakoid Tat substrate requires the preprotein to be correctly folded (67). However, similar thylakoid assays demonstrated that malfolded dihydrofolate reductase can be translocated by the Tat system, as can physiological substrates that are severely malfolded by the incorporation of amino acid analogs (35). Thus, the thylakoidal Tat system apparently tolerates both folded and unfolded substrates in vitro; however, whether a strict folding requirement exists in vivo is an open question. In fact, in vivo genetic studies performed with Escherichia coli indicate that the bacterial Tat pathway exports only native-protein-like proteins (22, 66, 70). Those studies demonstrate a clear ability of the Tat system to selectively discriminate between properly folded and misfolded proteins in vivo and suggest the existence of a folding quality control mechanism intrinsic to the process. Since there is no current evidence for factors additional to TatABCE, it is plausible that this “proofreading” mechanism resides within the translocon itself, although the possibility of a yet-to-be-determined accessory protein that prescreens Tat substrates cannot be ruled out.
The Tat translocon must possess an amazing structural flexibility, especially considering the fact that Tat substrates can vary dramatically in size, surface properties, and three-dimensional structure and also that most bacterial genomes typically encode numerous Tat substrates (24). For instance, the Tat system can accommodate proteins with diameters ranging from 20 to 60 Å (9, 36). In agreement with these dimensions, low-resolution images of a detergent-solubilized TatAB complex appeared as a ring of macromolecular density surrounding a cavity of 65 to 70 Å (73), which has been postulated to be the substrate transport channel. Clearly, such a large pore would be sufficient to handle a folded polypeptide, but exactly how this pore tolerates proteins of various dimensions and still remains impermeable to ions and small molecules remains a mystery.
QUALITY CONTROL MECHANISMS THAT PRESERVE Sec AND Tat COMPETENCE
Since there is a distinct possibility that Sec preproteins exposed in the cytosol might fold into more highly ordered structures prior to the translocation process, clearly an important question to consider is how do cells prevent premature folding or at least delay the folding process of presecretory polypeptide chains prior to translocation? Similarly, since the Tat system transports proteins that have already folded, an equally important and inverse question is how do cells establish that a protein is sufficiently folded to be competent for transport? It turns out that cells have devised several ingenious surveillance strategies for ensuring that preproteins to be secreted are maintained in a translocation-competent state (Fig. 3). One elegant strategy is to couple translocation with ribosomal translation by bringing the site of preprotein synthesis into close proximity to the translocon, thus ensuring that no amount of secondary structure is formed in the cytoplasm. This process, known as cotranslational translocation, is utilized primarily by eukaryotes for delivery of Sec substrates into the endoplasmic reticulum, but emerging data suggest that a similar phenomenon occurs in bacteria via the signal recognition particle (SRP) pathway (61, 74). For proteins not transported in synergy with translation, some feature of the substrate protein or the transport process itself must actively ensure competence. For example, signal sequences themselves can act as intrapolypeptide chaperones to prevent rapid folding. Another common tactic is the use of cytosolic molecular chaperones that dynamically regulate folding (prevent tight folding or aggregation in the case of Sec and promote correct folding in the case of Tat) and, in some instances, guide the substrate from the ribosome to the translocon.
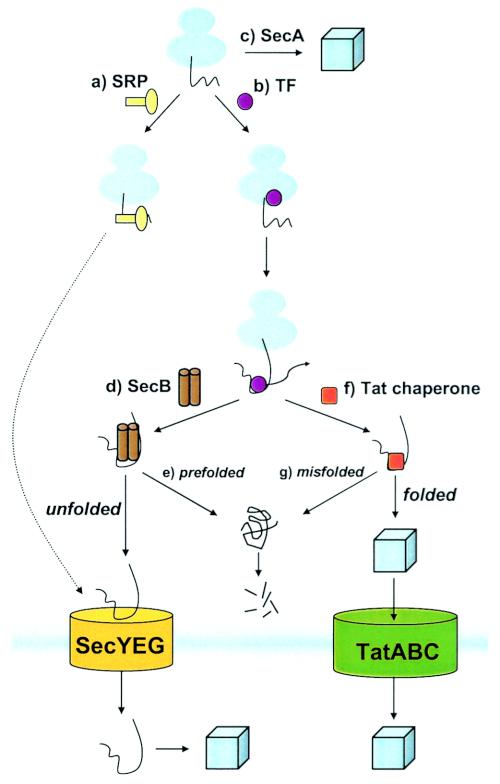
FIG. 3.
Quality control of a nascent polypeptide during its voyage to the translocon. (a) The SRP targets nascent inner membrane proteins to the membrane by specifically recognizing transmembrane segments. On the other hand, (b) TF remains effectively bound to the mature region of nascent preproteins until a relatively late stage of translation. Following TF dissociation, cytosolic factors such as SecB and DnaK help to maintain preproteins in a loosely folded conformation. (c) SecA maintains quality control by assisting the cytoplasmic folding of nontransported polypeptides. Sec substrates that retain an extended conformation, such as through interaction with SecB (d), are efficiently transported. However, if prefolding of a Sec substrate occurs (e), the protein is degraded in the cytoplasm or else can become jammed in the translocon. For a subset of preproteins destined to the Tat translocon, association with a chaperone (f), such as DnaK or other Tat-specific factor, likely shields the signal sequence until folding is completed. This same factor or an additional factor may also promote correct folding and serve as a first layer of proofreading prior to translocation. Tat transport proceeds only if the Tat substrate is correctly folded; otherwise transport is aborted and the substrate is degraded by proteolytic machinery (g).
Signal sequence.
The first level of quality control is provided by the signal sequence. Indeed, the presence of a Sec leader peptide can retard the folding of its cognate substrate by as much as 15-fold relative to the speed of folding of the mature substrate alone (49). This appreciable destabilization is functionally significant because it enhances the likelihood that the preprotein will be in a translocation-competent form and it provides cytoplasmic chaperones (e.g., SecB [see below]) ample time to bind multiple regions of the polypeptide backbone, thereby minimizing premature folding. Interestingly, the quality control afforded by the signal sequence can be suppressed by mutations to the Sec machinery (e.g., prlA mutations), allowing the transport of Sec substrates which lack a signal sequence (23, 30, 63). This phenotype is likely due to a loosened SecYEG association, which may represent the “relaxed” state of the translocon (25, 55), but a disruption of translocon proofreading activity has also been postulated (57). It is noteworthy that bacterial strains that carry prl mutations can still accurately differentiate between cytoplasmic and secretory proteins. Therefore, entry into the export pathway must involve additional signals that compensate for the absence of a signal sequence, or there may exist one or more means of entry that do not require signal sequences at all.
Tat signal sequences are considerably less hydrophobic than their Sec and SRP counterparts, with Tat signals being the least and SRP signals being the most hydrophobic (15). In addition to playing a role in preventing mistargeting, the weaker hydrophobicity of Tat leader peptides is less likely to destabilize the passenger protein, as would be expected for a system that favors folding prior to transport. In fact, nuclear magnetic resonance data indicate that resonances from the mature protein are not significantly shifted in the presence of the signal sequence, arguing against a direct interaction of the signal with the mature domain in vitro (38). This conclusion rules out a leader peptide sequestration model whereby nonspecific protein-protein interactions with exposed hydrophobic residues of the substrate protein would sequester the signal sequence and prevent transport until folding was completed (4). Alternately, the binding of an accessory protein (e.g., chaperone) to the preprotein in a manner that “shelters” the signal sequence until folding is finished (72) could be envisioned to help maintain Tat transport competence. The chaperone DnaK is a plausible candidate based on the observations that virtually all Tat leader peptides contain putative DnaK binding sites (A. C. Fisher and M. P. DeLisa, unpublished observations, and reference 69) and also that DnaK exhibits affinity for at least one Tat leader peptide in vitro (56).
General molecular chaperones.
Bacteria possess numerous cytoplasmic chaperones which are known to lack substrate specificity, to recognize different structural motifs, and to survey the folding status of substrates. Owing to these properties, chaperones are well equipped to bind to nascent preproteins in order to maintain these chains in a conformation suitable for transport and to prevent illicit interactions between subunits of a polypeptide which lead to aggregation. Indeed, in vitro studies confirmed that GroEL, a member of the Hsp60 heat shock protein family and one of the best-studied of these chaperones, has a capacity for maintaining purified Sec preproteins in a translocation-competent state (44). A similar phenomenon was observed for another cytosolic molecular chaperone, trigger factor (TF) (18, 44). In addition, some of these chaperones are also involved in the specific targeting of the preprotein to Sec translocation sites at the membrane (6, 28).
However, while such chaperones apparently maintain preproteins in a Sec-permissible conformation in vitro, there does not appear to be a strict requirement for their involvement in vivo. For instance, deletion of TF has no effect on Sec protein transport (32) and in some instances its absence leads to an overall increase in transport efficiency (46). Similarly, the absence of GroEL or its cellular partner GroES (Hsp10) results in only a moderate decrease in the rate of Sec-mediated β-lactamase processing (43). Interestingly, GroEL and DnaK (Hsp70) were shown to promote transport of a normally translocation-incompetent β-galactosidase fusion protein, but this required that the chaperones be greatly overexpressed relative to their normal cellular levels (59). One explanation for why general chaperones play only a limited role in Sec transport might be the fact that many complex cytoplasmic chaperones actively promote correct folding, an outcome that is counterproductive for Sec translocation. Instead, the Sec system apparently favors chaperones that bind only to the unfolded or partially folded preprotein in order to prevent tight folding until contact is made with the translocon. Finally, should the tertiary structure be unavoidable, it appears that the translocation event itself can drive the unfolding of a substantial protein domain (2).
In the case of the Tat system, it is tempting to speculate that this pathway would be a viable alternative for preproteins which require the assistance of ATP-dependent chaperone systems (e.g., GroELS) for correct folding, especially since the periplasm is devoid of such systems. In addition, such general folding catalysts may participate in the suspected proofreading of Tat substrates by sequestering misfolded proteins from the translocon until correct folding (or proteolytic degradation) had occurred. The strongest evidence that general molecular chaperones participate in Tat transport comes from plants, where the Tat-transported Rieske Fe/S protein has been found to interact with both Cpn60 (homologous to E. coli GroEL) and the DnaK/Hsp70 homolog prior to membrane insertion (50, 53). Currently, however, there is only limited and conflicting evidence for the involvement of such chaperones in bacterial Tat transport. For instance, both groEL and groES were essential for the in vivo processing and activity of the Tat-dependent E. coli hydrogenase-1 isoenzyme but not for the hydrogenase-2 isoenzyme, also a Tat substrate (65). Another ATP-dependent cytosolic chaperone, DnaK, displays affinity for Tat leader peptides in vitro (56) but is not required for the in vivo transport of the high-potential iron-sulfur protein Tat substrate (8). Finally, in a search for factors that, when overexpressed, confer enhanced Tat export of a short-lived version of the green fluorescent protein (green fluorescent protein-SsrA), DeLisa and coworkers identified the phage shock protein PspA, as well as the small heat shock chaperone IbpB (21). However, separate studies indicate that deletion of ibpAB improves Tat transport of long-lived green fluorescent protein in E. coli (Sang Yup Lee, personal communication). It is also noteworthy that, as was found for Sec transport, Tat translocation efficiency is largely unaffected by the loss of TF (Fisher and DeLisa, unpublished observations). Clearly, more experiments are needed to resolve the role of generalized molecular chaperones in Tat export.
Pathway-specific chaperones.
Unlike the general molecular chaperones discussed above, SecB has been classified as a translocation-specific molecular chaperone (14, 41, 80). Active SecB tetramers bind to numerous Sec preproteins but to only a few cytosolic proteins (41, 42). While early experiments suggested that SecB was primarily a signal sequence-specific recognition factor (80), it is now generally accepted that SecB exhibits a much broader selectivity that targets the mature portion of the preprotein. SecB has a high affinity in vitro for 9-residue sequence motifs enriched in aromatic and basic residues that occur statistically every 20 to 30 residues in the proteome (39) and helps explain why SecB substrates share no sequence homology. SecB appears to have a preference for those polypeptides, secretory and nonsecretory, that fold slowly, although this characteristic is not the sole factor in SecB selectivity, as simply retarding the folding of a nonsecretory protein is insufficient to allow SecB binding or membrane targeting (51). A close inspection of the high-resolution SecB structural data indicates that SecB recognition of unfolded preproteins is facilitated by two long channels that run along the side of SecB, defining a suitable environment for binding nonnative polypeptides (82). Based on these findings, an emerging interpretation is that SecB functions as a general chaperone that can mediate interactions between signal sequences of SecB-bound preproteins and the translocation apparatus. However, SecB can also perform chaperone activity independent of its role in translocation (78) and can even affect the transport efficiency of proteins that engage the Tat machinery (5, 12) or ABC transporters (20).
In the Tat pathway, a class of system-specific accessory proteins termed redox enzyme maturation proteins, which participate in the assembly of complex redox enzymes but do not constitute part of the final holoenzyme, have been identified (76). One of these, DmsD, binds specifically to the Tat-specific signal sequence of DmsA (56). Initially, it was proposed that DmsD was a bifunctional chaperone with one role in DmsA enzyme maturation and a second role in directing DmsA to the Tat translocon. However, more-recent data demonstrate that the DmsD protein, while essential for the attachment of the DmsA cofactor molybdopterin guanine dinucleotide, does not function as a guidance factor to target pre-DmsA to the translocon (64). Instead, it has been proposed that DmsD performs a “masking” function by binding to the DmsA signal sequence and rendering it unavailable to direct protein export until after DmsA cofactor attachment has been completed (72). In separate studies, Pop et al. present tantalizing evidence that Bacillus subtilis TatA interacts with the Tat-dependent prePhoD substrate prior to its own membrane integration (60), implying that cytoplasmic TatA might chaperone Tat preproteins directly to the site of translocation.
Given the involvement of molecular chaperones, a vital question is when do they become associated with preproteins? Cross-linking studies indicate that after emerging from the exit tunnel of the ribosome, the early mature region of a nascent preprotein is accessible to both SRP and TF, which are both cross-linked to protein L23 at the exit (10, 77). SRP and TF can bind simultaneously to ribosomes and ribosome-nascent chain complexes, exposing a highly hydrophobic SRP-type signal sequence, suggesting that SRP and TF sample nascent chains on the ribosome in a nonexclusive fashion (10). In the presence of a significantly hydrophobic targeting sequence, SRP binding is stabilized and excludes TF (10, 45), whereas in the absence of such hydrophobic sequences, TF remains bound to the nascent polypeptide in regions rich in aromatic and basic residues (58). Upon release of the polypeptide from the ribosome, TF dissociates from the preprotein, allowing access to SecA and SecB. While little is known about how Tat preproteins journey from the ribosome to the translocon, it seems likely that TF will also interact with Tat-specific nascent chains. The reduced hydrophobicity of Tat signal sequences might favor TF binding or otherwise alter the affinity of TF in a way that shunts a Tat preprotein into a productive folding pathway such as through DnaK association (Fig. 3).
Folding quality control.
Another Sec-specific factor, SecA, has multiple functions during the translocation process. In addition to its well-characterized roles in driving the translocation process (26) and in guiding preproteins to the translocon via binding to the inner membrane (28, 29), SecA also exhibits a chaperone activity that promotes the rapid folding of nonsecretory proteins (27). In this context, SecA performs a quality control function whereby it promotes the folding of signal sequenceless proteins, thereby excluding them from the Sec secretion process.
In the case of the Tat system, it has been proposed that a folding quality control or proofreading mechanism monitors the “foldedness” of a Tat preprotein prior to transport, but it is unknown how such a process operates. One possibility is that a portion of the proofreading is handled by a cytoplasmic accessory factor(s). For instance, chaperone binding of a misfolded preprotein may shield it from the Tat transporter until it is either sufficiently folded for transport or shunted to the proteolytic machinery (e.g., ClpXP and FtsH). A second possibility is that proofreading is handled directly by the Tat machinery. In this scenario, one might envision Tat transport as a gated process that proceeds only in response to a competent substrate protein, i.e., a folded protein exhibiting low surface hydrophobicity. Exposed hydrophobic domains of a preprotein may form a binary complex with a sensor region present in one of the Tat proteins. One intriguing candidate is the large TatB cytoplasmic domain predicted by bioinformatics analysis to form a coiled coil in this region (47). Interaction with this sensor region would then prevent subsequent translocation steps. Some support for this model comes from recent cross-linking studies that show a protein-protein interaction between the mature portion of a Tat-specific preprotein and TatB but not to any of the other Tat proteins (1).
Finally, proteins that are deemed unfit for Tat transport are likely delivered to a salvage pathway to be refolded or else degraded. Indeed, mounting evidence indicates that accumulation of nontransported Tat preproteins that arise either from misfolding in the cytoplasm or from depletion of the tat genes often results in inactivation and degradation in the cytoplasm (11, 22). The precise players in this degradation process are not currently known, although likely candidates include the FtsH protease (8) and the Clp machinery.
Pathway cross talk.
An emerging question pertains to the notion of Sec and Tat pathway cross talk, both in terms of how it is prevented (i.e., pathway specificity) and in terms of cooperativity between the two pathways. At first glance, Sec and Tat signal sequences look very similar. Thus, it is not surprising that as few as two amino acid substitutions to a Tat signal can completely reroute the passenger protein to the Sec pathway (5, 15), although similar rerouting of a Sec signal towards the Tat pathway is significantly more difficult (B. Ribnicky, P. Lee, M. P. DeLisa, and G. Georgiou, unpublished observations). In addition, E. coli RbsB, a known Sec substrate, can engage both Sec and Tat machinery (62) and a number of canonical Tat leader peptides can direct preproteins to both the Sec and Tat pathways (22). The SecA protein has even been shown to bind weakly to a Tat-specific leader peptide (37). In plants, certain Tat substrates exhibit the innate ability to transit the Sec pathway, especially under conditions where the Tat system is inhibited (48, 53). Along similar lines, an artificial dual-targeting signal sequence, constructed by combining Tat and Sec domains, was used to simultaneously compare the transport capabilities of both pathways when confronted with different passenger proteins (34). Whereas Sec passengers were efficiently transported by both pathways, Tat passengers were arrested in translocation on the Sec pathway. Taken together, the above results clearly indicate a substantial level of pathway redundancy. Whether this redundancy is simply a remnant left over from the evolutionary divergence of these two pathways or is instead a programmed fail-safe mechanism to ensure function is currently unresolved and certainly warrants further investigation.
CONCLUDING REMARKS
We anticipate that many challenging aspects of Sec and Tat transport will be addressed in the next several years. Likely to take center stage will be the complete elucidation of the Tat mechanism, including how the quality control mechanism is integrated with translocation. Crystallographic structures of the Tat proteins should enable insights into the function of each of these proteins, but a full description of the Tat mechanism will also demand continued biochemical and genetic studies using both plant and bacterial Tat systems. Finally, the emerging notion that functional redundancy is programmed into these two systems highlights our lack of understanding of the structural determinants that dictate pathway-specific targeting, and a deeper consideration of this overlap will need to be exercised.
Acknowledgments
This work was supported by the New York State Office of Science, Technology and Academic Research in the form of a James D. Watson Young Investigator Award to M.P.D.
We thank Tracy Palmer and Joseph Peters for helpful discussions of the manuscript.
REFERENCES
- 1.Alami, M., I. Luke, S. Deitermann, G. Eisner, H. G. Koch, J. Brunner, and M. Muller. 2003. Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol. Cell 12:937-946. [DOI] [PubMed] [Google Scholar]
- 2.Arkowitz, R. A., J. C. Joly, and W. Wickner. 1993. Translocation can drive the unfolding of a preprotein domain. EMBO J. 12:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berks, B. C. 1996. A common export pathway for proteins binding complex redox cofactors? Mol. Microbiol. 22:393-404. [DOI] [PubMed] [Google Scholar]
- 4.Berks, B. C., F. Sargent, and T. Palmer. 2000. The Tat protein export pathway. Mol. Microbiol. 35:260-274. [DOI] [PubMed] [Google Scholar]
- 5.Blaudeck, N., P. Kreutzenbeck, R. Freudl, and G. A. Sprenger. 2003. Genetic analysis of pathway specificity during posttranslational protein translocation across the Escherichia coli plasma membrane. J. Bacteriol. 185:2811-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochkareva, E. S., M. E. Solovieva, and A. S. Girshovich. 1998. Targeting of GroEL to SecA on the cytoplasmic membrane of Escherichia coli. Proc. Natl. Acad. Sci. USA 95:478-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brundage, L., J. P. Hendrick, E. Schiebel, A. J. Driessen, and W. Wickner. 1990. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell 62:649-657. [DOI] [PubMed] [Google Scholar]
- 8.Bruser, T., and C. Sanders. 2003. An alternative model of the twin arginine translocation system. Microbiol. Res. 158:7-17. [DOI] [PubMed] [Google Scholar]
- 9.Bruser, T., H. G. Truper, and C. Dahl. 1997. Cloning and sequencing of the gene encoding the high potential iron-sulfur protein (HiPIP) from the purple sulfur bacterium Chromatium vinosum. Biochim. Biophys. Acta 1352:18-22. [DOI] [PubMed] [Google Scholar]
- 10.Buskiewicz, I., E. Deuerling, S. Q. Gu, J. Jockel, M. V. Rodnina, B. Bukau, and W. Wintermeyer. 2004. Trigger factor binds to ribosome-signal-recognition particle (SRP) complexes and is excluded by binding of the SRP receptor. Proc. Natl. Acad. Sci. USA 101:7902-7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chanal, A., C. Santini, and L. Wu. 1998. Potential receptor function of three homologous components, TatA, TatB and TatE, of the twin-arginine signal sequence-dependent metalloenzyme translocation pathway in Escherichia coli. Mol. Microbiol. 30:674-676. [DOI] [PubMed] [Google Scholar]
- 12.Chou, C. P., J. H. Tseng, B. Y. Kuo, K. M. Lai, M. I. Lin, and H. K. Lin. 1999. Effect of SecB chaperone on production of periplasmic penicillin acylase in Escherichia coli. Biotechnol. Prog. 15:439-445. [DOI] [PubMed] [Google Scholar]
- 13.Clark, S. A., and S. M. Theg. 1997. A folded protein can be transported across the chloroplast envelope and thylakoid membranes. Mol. Biol. Cell 8:923-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collier, D. N., V. A. Bankaitis, J. B. Weiss, and P. J. Bassford, Jr. 1988. The antifolding activity of SecB promotes the export of the E. coli maltose-binding protein. Cell 53:273-283. [DOI] [PubMed] [Google Scholar]
- 15.Cristobal, S., J. W. de Gier, H. Nielsen, and G. von Heijne. 1999. Competition between Sec- and TAT-dependent protein translocation in Escherichia coli. EMBO J. 18:2982-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crooke, E., L. Brundage, M. Rice, and W. Wickner. 1988. ProOmpA spontaneously folds in a membrane assembly competent state which trigger factor stabilizes. EMBO J. 7:1831-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crooke, E., B. Guthrie, S. Lecker, R. Lill, and W. Wickner. 1988. ProOmpA is stabilized for membrane translocation by either purified E. coli trigger factor or canine signal recognition particle. Cell 54:1003-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crooke, E., and W. Wickner. 1987. Trigger factor: a soluble protein that folds pro-OmpA into a membrane-assembly-competent form. Proc. Natl. Acad. Sci. USA 84:5216-5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danese, P. N., and T. J. Silhavy. 1998. Targeting and assembly of periplasmic and outer-membrane proteins in Escherichia coli. Annu. Rev. Genet. 32:59-94. [DOI] [PubMed] [Google Scholar]
- 20.Delepelaire, P., and C. Wandersman. 1998. The SecB chaperone is involved in the secretion of the Serratia marcescens HasA protein through an ABC transporter. EMBO J. 17:936-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLisa, M. P., P. Lee, T. Palmer, and G. Georgiou. 2004. Phage shock protein PspA of Escherichia coli relieves saturation of protein export via the Tat pathway. J. Bacteriol. 186:366-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeLisa, M. P., D. Tullman, and G. Georgiou. 2003. Folding quality control in the export of proteins by the bacterial twin-arginine translocation pathway. Proc. Natl. Acad. Sci. USA 100:6115-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derman, A. I., J. W. Puziss, P. J. Bassford, Jr., and J. Beckwith. 1993. A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J. 12:879-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dilks, K., R. W. Rose, E. Hartmann, and M. Pohlschroder. 2003. Prokaryotic utilization of the twin-arginine translocation pathway: a genomic survey. J. Bacteriol. 185:1478-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duong, F., and W. Wickner. 1999. The PrlA and PrlG phenotypes are caused by a loosened association among the translocase SecYEG subunits. EMBO J. 18:3263-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Economou, A., and W. Wickner. 1994. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell 78:835-843. [DOI] [PubMed] [Google Scholar]
- 27.Eser, M., and M. Ehrmann. 2003. SecA-dependent quality control of intracellular protein localization. Proc. Natl. Acad. Sci. USA 100:13231-13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fekkes, P., and A. J. Driessen. 1999. Protein targeting to the bacterial cytoplasmic membrane. Microbiol. Mol. Biol. Rev. 63:161-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fekkes, P., C. van der Does, and A. J. Driessen. 1997. The molecular chaperone SecB is released from the carboxy-terminus of SecA during initiation of precursor protein translocation. EMBO J. 16:6105-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flower, A. M., R. C. Doebele, and T. J. Silhavy. 1994. PrlA and PrlG suppressors reduce the requirement for signal sequence recognition. J. Bacteriol. 176:5607-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galan, J. E., and A. Collmer. 1999. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284:1322-1328. [DOI] [PubMed] [Google Scholar]
- 32.Guthrie, B., and W. Wickner. 1990. Trigger factor depletion or overproduction causes defective cell division but does not block protein export. J. Bacteriol. 172:5555-5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halbig, D., T. Wiegert, N. Blaudeck, R. Freudl, and G. A. Sprenger. 1999. The efficient export of NADP-containing glucose-fructose oxidoreductase to the periplasm of Zymomonas mobilis depends both on an intact twin-arginine motif in the signal peptide and on the generation of a structural export signal induced by cofactor binding. Eur. J. Biochem. 263:543-551. [DOI] [PubMed] [Google Scholar]
- 34.Henry, R., M. Carrigan, M. McCaffrey, X. Ma, and K. Cline. 1997. Targeting determinants and proposed evolutionary basis for the Sec and the Delta pH protein transport systems in chloroplast thylakoid membranes. J. Cell Biol. 136:823-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hynds, P. J., D. Robinson, and C. Robinson. 1998. The sec-independent twin-arginine translocation system can transport both tightly folded and malfolded proteins across the thylakoid membrane. J. Biol. Chem. 273:34868-34874. [DOI] [PubMed] [Google Scholar]
- 36.Jormakka, M., S. Tornroth, B. Byrne, and S. Iwata. 2002. Molecular basis of proton motive force generation: structure of formate dehydrogenase-N. Science 295:1863-1868. [DOI] [PubMed] [Google Scholar]
- 37.Kebir, M. O., and D. A. Kendall. 2002. SecA specificity for different signal peptides. Biochemistry 41:5573-5580. [DOI] [PubMed] [Google Scholar]
- 38.Kipping, M., H. Lilie, U. Lindenstrauss, J. R. Andreesen, C. Griesinger, T. Carlomagno, and T. Bruser. 2003. Structural studies on a twin-arginine signal sequence. FEBS Lett. 550:18-22. [DOI] [PubMed] [Google Scholar]
- 39.Knoblauch, N. T., S. Rudiger, H. J. Schonfeld, A. J. Driessen, J. Schneider-Mergener, and B. Bukau. 1999. Substrate specificity of the SecB chaperone. J. Biol. Chem. 274:34219-34225. [DOI] [PubMed] [Google Scholar]
- 40.Kowarik, M., S. Kung, B. Martoglio, and A. Helenius. 2002. Protein folding during cotranslational translocation in the endoplasmic reticulum. Mol. Cell 10:769-778. [DOI] [PubMed] [Google Scholar]
- 41.Kumamoto, C. A. 1989. Escherichia coli SecB protein associates with exported protein precursors in vivo. Proc. Natl. Acad. Sci. USA 86:5320-5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumamoto, C. A., and J. Beckwith. 1983. Mutations in a new gene, secB, cause defective protein localization in Escherichia coli. J. Bacteriol. 154:253-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kusukawa, N., T. Yura, C. Ueguchi, Y. Akiyama, and K. Ito. 1989. Effects of mutations in heat-shock genes groES and groEL on protein export in Escherichia coli. EMBO J. 8:3517-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lecker, S., R. Lill, T. Ziegelhoffer, C. Georgopoulos, P. J. Bassford, Jr., C. A. Kumamoto, and W. Wickner. 1989. Three pure chaperone proteins of Escherichia coli—SecB, trigger factor and GroEL—form soluble complexes with precursor proteins in vitro. EMBO J. 8:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee, H. C., and H. D. Bernstein. 2001. The targeting pathway of Escherichia coli presecretory and integral membrane proteins is specified by the hydrophobicity of the targeting signal. Proc. Natl. Acad. Sci. USA 98:3471-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee, H. C., and H. D. Bernstein. 2002. Trigger factor retards protein export in Escherichia coli. J. Biol. Chem. 277:43527-43535. [DOI] [PubMed] [Google Scholar]
- 47.Lee, P. A., G. Buchanan, N. R. Stanley, B. C. Berks, and T. Palmer. 2002. Truncation analysis of TatA and TatB defines the minimal functional units required for protein translocation. J. Bacteriol. 184:5871-5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leheny, E. A., S. A. Teter, and S. M. Theg. 1998. Identification of a role for an azide-sensitive factor in the thylakoid transport of the 17-kilodalton subunit of the photosynthetic oxygen-evolving complex. Plant Physiol. 116:805-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu, G., T. B. Topping, and L. L. Randall. 1989. Physiological role during export for the retardation of folding by the leader peptide of maltose-binding protein. Proc. Natl. Acad. Sci. USA 86:9213-9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madueno, F., J. A. Napier, and J. C. Gray. 1993. Newly imported Rieske iron-sulfur protein associates with both Cpn60 and Hsp70 in the chloroplast stroma. Plant Cell 5:1865-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mallik, I., M. A. Smith, and A. M. Flower. 2002. Recognition of secretory proteins in Escherichia coli requires signals in addition to the signal sequence and slow folding. BMC Microbiol. 2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mingarro, I., I. Nilsson, P. Whitley, and G. von Heijne. 2000. Different conformations of nascent polypeptides during translocation across the ER membrane. BMC Cell Biol. 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molik, S., I. Karnauchov, C. Weidlich, R. G. Herrmann, and R. B. Klosgen. 2001. The Rieske Fe/S protein of the cytochrome b6/f complex in chloroplasts: missing link in the evolution of protein transport pathways in chloroplasts? J. Biol. Chem. 276:42761-42766. [DOI] [PubMed] [Google Scholar]
- 54.Mori, H., and K. Cline. 2002. A twin arginine signal peptide and the pH gradient trigger reversible assembly of the thylakoid [Delta]pH/Tat translocase. J. Cell Biol. 157:205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nouwen, N., B. de Kruijff, and J. Tommassen. 1996. prlA suppressors in Escherichia coli relieve the proton electrochemical gradient dependency of translocation of wild-type precursors. Proc. Natl. Acad. Sci. USA 93:5953-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oresnik, I. J., C. L. Ladner, and R. J. Turner. 2001. Identification of a twin-arginine leader-binding protein. Mol. Microbiol. 40:323-331. [DOI] [PubMed] [Google Scholar]
- 57.Osborne, R. S., and T. J. Silhavy. 1993. PrlA suppressor mutations cluster in regions corresponding to three distinct topological domains. EMBO J. 12:3391-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patzelt, H., S. Rudiger, D. Brehmer, G. Kramer, S. Vorderwulbecke, E. Schaffitzel, A. Waitz, T. Hesterkamp, L. Dong, J. Schneider-Mergener, B. Bukau, and E. Deuerling. 2001. Binding specificity of Escherichia coli trigger factor. Proc. Natl. Acad. Sci. USA 98:14244-14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phillips, G. J., and T. J. Silhavy. 1990. Heat-shock proteins DnaK and GroEL facilitate export of LacZ hybrid proteins in E. coli. Nature 344:882-884. [DOI] [PubMed] [Google Scholar]
- 60.Pop, O. I., M. Westermann, R. Volkmer-Engert, D. Schulz, C. Lemke, S. Schreiber, R. Gerlach, R. Wetzker, and J. P. Muller. 2003. Sequence-specific binding of prePhoD to soluble TatAd indicates protein-mediated targeting of the Tat export in Bacillus subtilis. J. Biol. Chem. 278:38428-38436. [DOI] [PubMed] [Google Scholar]
- 61.Powers, T., and P. Walter. 1997. Co-translational protein targeting catalyzed by the Escherichia coli signal recognition particle and its receptor. EMBO J. 16:4880-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pradel, N., C. L. Santini, C. Y. Ye, L. Fevat, F. Gerard, M. Alami, and L. F. Wu. 2003. Influence of tat mutations on the ribose-binding protein translocation in Escherichia coli. Biochem. Biophys. Res. Commun. 306:786-791. [DOI] [PubMed] [Google Scholar]
- 63.Prinz, W. A., C. Spiess, M. Ehrmann, C. Schierle, and J. Beckwith. 1996. Targeting of signal sequenceless proteins for export in Escherichia coli with altered protein translocase. EMBO J. 15:5209-5217. [PMC free article] [PubMed] [Google Scholar]
- 64.Ray, N., J. Oates, R. J. Turner, and C. Robinson. 2003. DmsD is required for the biogenesis of DMSO reductase in Escherichia coli but not for the interaction of the DmsA signal peptide with the Tat apparatus. FEBS Lett. 534:156-160. [DOI] [PubMed] [Google Scholar]
- 65.Rodrigue, A., N. Batia, M. Muller, O. Fayet, R. Bohm, M. A. Mandrand-Berthelot, and L. F. Wu. 1996. Involvement of the GroE chaperonins in the nickel-dependent anaerobic biosynthesis of NiFe-hydrogenases of Escherichia coli. J. Bacteriol. 178:4453-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodrigue, A., A. Chanal, K. Beck, M. Muller, and L. F. Wu. 1999. Co-translocation of a periplasmic enzyme complex by a hitchhiker mechanism through the bacterial tat pathway. J. Biol. Chem. 274:13223-13228. [DOI] [PubMed] [Google Scholar]
- 67.Roffey, R. A., and S. M. Theg. 1996. Analysis of the import of carboxyl-terminal truncations of the 23-kilodalton subunit of the oxygen-evolving complex suggests that its structure is an important determinant for thylakoid transport. Plant Physiol. 111:1329-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rose, R. W., T. Bruser, J. C. Kissinger, and M. Pohlschroder. 2002. Adaptation of protein secretion to extremely high-salt conditions by extensive use of the twin-arginine translocation pathway. Mol. Microbiol. 45:943-950. [DOI] [PubMed] [Google Scholar]
- 69.Rudiger, S., L. Germeroth, J. Schneider-Mergener, and B. Bukau. 1997. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 16:1501-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanders, C., N. Wethkamp, and H. Lill. 2001. Transport of cytochrome c derivatives by the bacterial Tat protein translocation system. Mol. Microbiol. 41:241-246. [DOI] [PubMed] [Google Scholar]
- 71.Sandkvist, M. 2001. Biology of type II secretion. Mol. Microbiol. 40:271-283. [DOI] [PubMed] [Google Scholar]
- 72.Santini, C. L., B. Ize, A. Chanal, M. Muller, G. Giordano, and L. F. Wu. 1998. A novel sec-independent periplasmic protein translocation pathway in Escherichia coli. EMBO J. 17:101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sargent, F., U. Gohlke, E. De Leeuw, N. R. Stanley, T. Palmer, H. R. Saibil, and B. C. Berks. 2001. Purified components of the Escherichia coli Tat protein transport system form a double-layered ring structure. Eur. J. Biochem. 268:3361-3367. [DOI] [PubMed] [Google Scholar]
- 74.Schierle, C. F., M. Berkmen, D. Huber, C. Kumamoto, D. Boyd, and J. Beckwith. 2003. The DsbA signal sequence directs efficient, cotranslational export of passenger proteins to the Escherichia coli periplasm via the signal recognition particle pathway. J. Bacteriol. 185:5706-5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Settles, A. M., A. Yonetani, A. Baron, D. R. Bush, K. Cline, and R. Martienssen. 1997. Sec-independent protein translocation by the maize Hcf106 protein. Science 278:1467-1470. [DOI] [PubMed] [Google Scholar]
- 76.Turner, R. J., A. L. Papish, and F. Sargent. 2004. Sequence analysis of bacterial redox enzyme maturation proteins (REMPs). Can. J. Microbiol. 50:225-238. [DOI] [PubMed] [Google Scholar]
- 77.Ullers, R. S., E. N. Houben, A. Raine, C. M. ten Hagen-Jongman, M. Ehrenberg, J. Brunner, B. Oudega, N. Harms, and J. Luirink. 2003. Interplay of signal recognition particle and trigger factor at L23 near the nascent chain exit site on the Escherichia coli ribosome. J. Cell Biol. 161:679-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ullers, R. S., J. Luirink, N. Harms, F. Schwager, C. Georgopoulos, and P. Genevaux. 2004. SecB is a bona fide generalized chaperone in Escherichia coli. Proc. Natl. Acad. Sci. USA 101:7853-7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van den Berg, B., W. M. Clemons, Jr., I. Collinson, Y. Modis, E. Hartmann, S. C. Harrison, and T. A. Rapoport. 2004. X-ray structure of a protein-conducting channel. Nature 427:36-44. [DOI] [PubMed] [Google Scholar]
- 80.Watanabe, M., and G. Blobel. 1989. Cytosolic factor purified from Escherichia coli is necessary and sufficient for the export of a preprotein and is a homotetramer of SecB. Proc. Natl. Acad. Sci. USA 86:2728-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weiner, J. H., P. T. Bilous, G. M. Shaw, S. P. Lubitz, L. Frost, G. H. Thomas, J. A. Cole, and R. J. Turner. 1998. A novel and ubiquitous system for membrane targeting and secretion of cofactor-containing proteins. Cell 93:93-101. [DOI] [PubMed] [Google Scholar]
- 82.Xu, Z., J. D. Knafels, and K. Yoshino. 2000. Crystal structure of the bacterial protein export chaperone secB. Nat. Struct. Biol. 7:1172-1177. [DOI] [PubMed] [Google Scholar]