Abstract
Objective
Mercaptopurine (MP) and pro-drug azathioprine are ‘first-line’ oral therapies for maintaining remission in IBD. It is believed that their pharmacodynamic action is due to a slow cumulative decrease in activated lymphocytes homing to inflamed gut. We examined the role of host metabolism, lymphocytes and microbiome for the amelioration of colitis by the related thioguanine (TG).
Design
C57Bl/6 mice with or without specific genes altered to elucidate mechanisms responsible for TG's actions were treated daily with oral or intrarectal TG, MP or water. Disease activity was scored daily. At sacrifice, colonic histology, cytokine message, caecal luminal and mucosal microbiomes were analysed.
Results
Oral and intrarectal TG but not MP rapidly ameliorated spontaneous chronic colitis in Winnie mice (point mutation in Muc2 secretory mucin). TG ameliorated dextran sodium sulfate-induced chronic colitis in wild-type (WT) mice and in mice lacking T and B lymphocytes. Remarkably, colitis improved without immunosuppressive effects in the absence of host hypoxanthine (guanine) phosphoribosyltransferase (Hprt)-mediated conversion of TG to active drug, the thioguanine nucleotides (TGN). Colonic bacteria converted TG and less so MP to TGN, consistent with intestinal bacterial conversion of TG to so reduce inflammation in the mice lacking host Hprt. TG rapidly induced autophagic flux in epithelial, macrophage and WT but not Hprt−/− fibroblast cell lines and augmented epithelial intracellular bacterial killing.
Conclusions
Treatment by TG is not necessarily dependent on the adaptive immune system. TG is a more efficacious treatment than MP in Winnie spontaneous colitis. Rapid local bacterial conversion of TG correlated with decreased intestinal inflammation and immune activation.
Keywords: AZATHIOPRINE, IBD MODELS, DRUG METABOLISM, COLONIC BACTERIA
Significance of this study.
What is already known on this subject?
Azathioprine or mercaptopurine (MP) are pro-drugs that require liver and white blood cell conversion of the pro-drug to an active metabolite, to arrest proliferation of activated T lymphocytes and induce apoptosis.
These thiopurines are associated with many unwanted side effects, which limit their use, so necessitating other often more expensive treatments.
The side effects include off-target effects on the liver and bone marrow, as well as pancreatitis, drug fever and myalgias.
What are the new findings?
Mediation of the beneficial clinical action of one of the thiopurines, thioguanine (TG), can occur independently of lymphocytes.
The action can be mediated independently of host conversion to the active metabolite.
An alternative route of conversion of TG pro-drug is via bacteria. Full conversion of a pro-drug to an active drug by gut bacteria has not been previously reported.
Oral and intrarectal TG act more efficaciously than MP in our in vivo mouse models.
In vitro, TG effects a rapid augmentation of autophagy and intracellular bacterial killing by gut epithelial cells.
How might it impact on clinical practice in the foreseeable future?
Preferential delivery of TG to sites of intestinal inflammation in IBD would decrease inflammation and immune activation, leading to rapid treatment responses while avoiding the unwanted effects occurring with predominant portal and blood leucocyte metabolism. Thus, targeted delivery of TG to take advantage of local conversion could reshape oral therapy for Crohn's and ulcerative colitis.
Introduction
The pathogenesis of IBD is multifactorial, involving interplay between the intestinal luminal environment, the intestinal epithelial barrier and adaptive and innate immune responses. The immune response is the target of most medical treatments for IBD. Glucocorticosteroids, (anti-tumour necrosis factor-alpha (TNF-α)) drugs, thiopurines, methotrexate and calcineurin inhibitors all act by modulating the immune response, although other mechanisms of action are not excluded as recently shown for glucocorticosteroids.1
The commonly used oral treatments to maintain remission of moderate to severe IBD, the thiopurine pro-drugs, azathioprine and mercaptopurine (MP),2 3 are believed to act via (i) competitive binding of the nucleotide metabolite, thioguanosine triphosphate (thioGTP), to ras-related C3 botulinum toxin substrate 1 (RAC1) inducing apoptosis in activated CD4+ T lymphocytes,4 (ii) effects of thiopurine nucleotide (TGN) metabolites on lymphocytes to arrest proliferating T lymphocytes5 and (iii) generation of methyl-thioinosine monophosphate (methyl-thioIMP) by thiopurine methyltransferase to inhibit de novo synthesis of purines.6 Thioguanine (TG), another thiopurine pro-drug, is thought to work similarly via thioGTP but without generation of methyl-thioIMP. TG may also have a clinically faster onset of action7–9 than azathioprine or MP; however, it is used infrequently because of dose-related vascular liver toxicity that can arise from rapid generation of thioGTP or related metabolites in the portal circulation.10
It is now widely accepted that the microbial community resident in the gut is a key factor in the aetiology of IBD. Mucosal inflammation in IBD is associated with an altered gut microbiota that is characterised by decreased microbial diversity at a species level and ‘dysbiosis’ that may be due to preferential colonisation of the inflammatory niche.11 12 The microbiota may also play a causal role in the chronic inflammation of IBD as described in reports of transmissible colitis in some experimental models.13 Genome-wide association studies of IBD have identified multiple pathways related to microbial responses emphasising the importance of the host–microbiome interaction for the pathogenesis of IBD.14 In particular, the autophagy pathway is a highly conserved homeostatic pathway that has been implicated in a variety of cellular processes, including the control of intracellular bacteria and production of pro-inflammatory cytokines.15 Interest persists in the role of antibiotics for treatment of IBD, in particular Crohn's disease16 17 and recent studies have reported a possible role for manipulation of the microbiome through faecal microbial transplantation.18
In this paper, we show that TG can act independently of the adaptive immune system. This can occur via bacterial activation in the absence of host metabolism and/or local mucosal metabolism of TG, which may avoid the requirement for high concentrations of thioGTP in the systemic circulation for its action. Thus, local delivery of TG to the intestinal sites of active IBD could treat intestinal inflammation rapidly without adversely affecting the liver, the systemic immune system or haematopoiesis.
Materials and methods
Animal experiments and treatments
All animal experiments were approved by the University of Queensland Animal Ethics Committee. C57Bl/6 wild-type (WT) mice were purchased from the Animal Resource Authority, Western Australia, and hypoxanthine (guanine) phosphoribosyltransferase (Hprt)−/−, Winnie and RaW mice were bred in-house in a pathogen-free animal facility. Male and or female mice were intragastrically gavaged daily with either vehicle control or thiopurine drugs (TG or MP) for periods between 14 and 28 days. TG or MP was also administered intrarectally to Winnie mice. TG (molecular weight (MW) 167, Sigma) was prepared as a suspension in water and mixed thoroughly before administration. MP (MW 170, MP monohydrate Sigma) was dissolved in water. Vehicle control was water.
Dextran sodium sulfate (DSS) (36–50 kDa; MP Biochemicals) was administered in the drinking water chronically (0.5% (w/v) for four cycles of 5 days on, followed by seven (first two cycles) or 9 days off (last two cycles).
Histological colitis scoring
Histological assessment of spontaneous and DSS-induced colitis was performed blinded to mouse genotype and treatment as previously described.19
Diarrhoea scores
Diarrhoea scoring for both spontaneous and DSS-induced colitis was performed daily by unblinded multiple scorers for the duration of the experiments19 (see online supplementary data).
gutjnl-2015-310874supp.pdf (1.4MB, pdf)
Supplementary data
See online supplementary data for methods on RNA extraction, cDNA synthesis and gene expression, sample collection and microbiome analysis,20–26 fluorescent activated cell sorting (FACS) analysis, TGN measurement,27 cell cultures, autophagy and bacterial replication assays,28 organoid cultures and treatment,29 operational taxonomic unit (MTS) cell viability assay and primers (supplementary table 1).
Statistics and analysis
Mann-Whitney was used for non-parametric data that were graphed as box-and-whisker plots with median, quartiles and range. For the time and dose-related experiments, the significance was assessed by two-way analysis of variance (ANOVA) with Bonferroni post-test corrections. The significance of a result is shown by a * to indicate test versus WT TG 0 mg/kg and a # to indicate test versus Hprt−/−, Winnie or RaW TG 0 mg/kg. Statistics: * or # or &p<0.05, ** or ## or &&p<0.01, *** or ### or &&&p<0.001.
Results
High-dose TG improved the spontaneous colitis in the Winnie model of UC in 14 days
Winnie mice develop a spontaneous colitis resembling UC, which emerges by 4 weeks of age. The initiating factor is a defect in goblet cell mucin production due to a single nucleotide polymorphism in the D3 domain of Muc2, which leads to protein misfolding and endoplasmic reticulum (ER) stress in the mucin-secreting cells, resulting in a Th17 predominant colitis.1 19 30 31
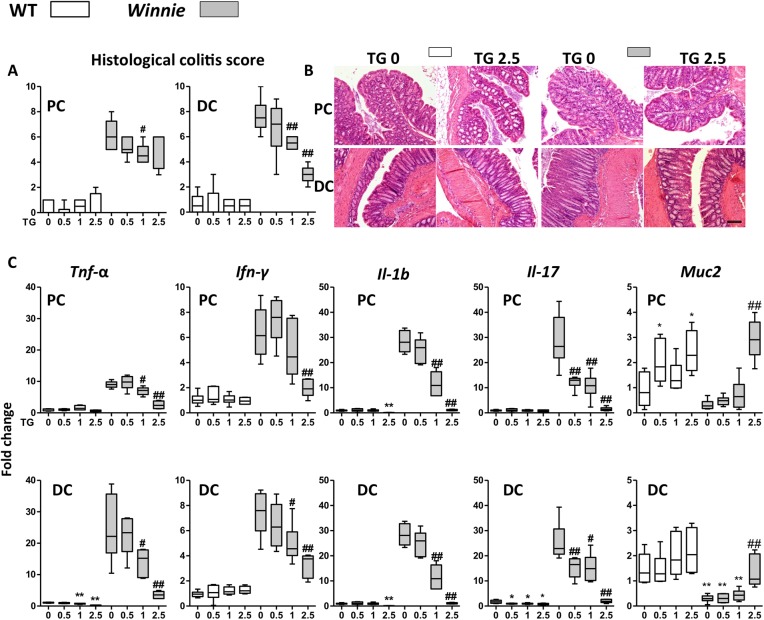
Juvenile Winnie and WT C57Bl/6 (WT) control mice were gavaged daily with either vehicle control or TG at concentrations of 0.5, 1 or 2.5 mg/kg for up to 14 days to investigate the effect of this thiopurine drug treatment on the spontaneous colitis. TG ameliorated the spontaneous colitis in a dose-dependent manner (figure 1A). Both the colitis and its improvement with treatment (figure 1A, B) were more marked in the distal colon (DC) of the Winnie mice consistent with previous reports.30 32 The Th1 and Th17 mRNA for inflammatory cytokines Tnf-α, interleukin (Il)-1b, interferon-gamma (Ifn-γ) and Il-17 characteristic for the immune response in this colitis model were decreased in the treatment groups in both proximal colon (PC) and DC (figure 1C). Conventional H&E histology and scoring revealed a decreased inflammatory infiltrate and a dose-dependent restoration of goblet cell morphology, crypt architecture and length and a decreased number of crypt abscesses and mucosal erosions (see online supplementary figure S1A). Mucin production was restored as evidenced by the H&E histology, increased expression of Muc2 (figure 1C) and the mucin-specific Agr2 chaperone (see online supplementary figure S2). ER stress and the unfolded protein response were diminished with treatment as shown by decreased transcription of Grp78 (see online supplementary figure S2) and sXbp1 (see online supplementary figure S2). There was an inverse correlation between amelioration of colitis and the peripheral white blood cell (WBC) count in TG-treated Winnie (see online supplementary figure S1B), consistent with a dose-dependent immunosuppression.
Figure 1.
Acute administration of thioguanine (TG) improved spontaneous Winnie murine colitis. C57Bl/6 (wild-type (WT), open symbols) and Winnie (grey symbols) were daily gavaged TG 0, 0.5, 1 or 2.5 mg/kg for up to 14 days. (A) Blinded scoring of histological colitis for proximal colon (PC) and distal colon (DC); (B) representative H&E for PC and DC of WT and Winnie treated with daily TG 0 or 2.5 mg/kg and (C) mRNA fold change normalised to β-actin gene and to WT control of Tnf-α, Ifn-γ, interleukin (Il)-1b, Il-17 and Muc2. Box-and-whisker plots of median, quartiles and range, N=4–6. Symbols: *versus WT TG 0 mg/kg; #versus Winnie TG 0 mg/kg. Statistical analysis: Mann-Whitney non-parametric test. Scale bar=100 μm.
Administration of clinically relevant low dose of TG (but not MP) over 28 days improved the spontaneous colitis in Winnie and also chronic DSS-induced colitis in WT mice
Low-dose TG (0.5 mg/kg/day) did not acutely improve the histological colitis score at 14 days of administration, but this dose was associated with decreased Il-17 message (figure 1C), suggesting that a longer administration of the lower dose could potentially improve the disease. Therefore, a clinically more relevant scenario for TG was tested by comparing 0.5 mg/kg/day TG for 28 days with a suprapharmacological dose (2.5 mg/kg/day) of the widely used thiopurine drug, MP, for 28 days. (The daily doses of TG and MP for IBD in patients with normal thiopurine methyltransferase activity are 0.4 and 1.5 mg/kg, respectively.)
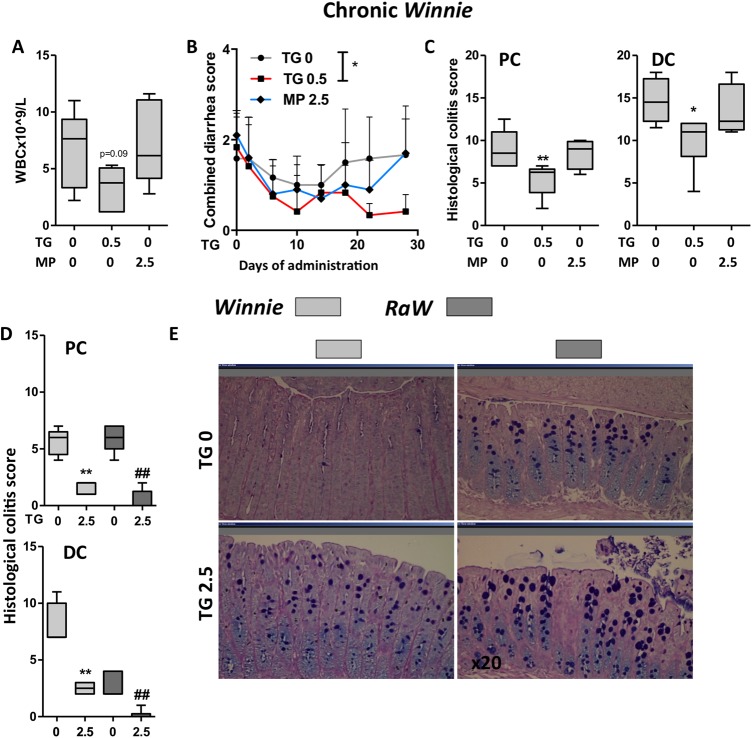
TG but not MP improved blinded histological colitis scores in both PC and DC (figure 2C). TG but not MP trended to reduce peripheral blood leucocytes (p=0.09, figure 2A). Winnie mice improved with TG but not with MP over the 28 days with respect to the combined diarrhoea and rectal bleeding score (figure 2B), but the colon weight/length ratio was unchanged (see online supplementary figure S3A).
Figure 2.
Administration of a clinically relevant low dose of thioguanine (TG) improved spontaneous Winnie colitis. (A–C): Winnie mice were daily gavaged with either TG 0, 0.5 or mercaptopurine (MP) 2.5 mg/kg/day for 28 days. (A) Peripheral white blood cell (WBC) count; (B) combined diarrhoea score one-way ANOVA p<0.05 at 28 days and (C) histological colitis scores for proximal colon (PC) and distal colon (DC) in Winnie. (D and E) Acute administration of TG in Winnie and RaW (Rag−/−×Winnie) mice daily gavaged TG 0 or 2.5 mg/kg/day for up to 14 days; (D) histological colitis scores for PC and DC in Winnie (light grey symbols) and RaW (dark symbols); (E) representative H&E/Alcian blue staining for DC of Winnie and RaW mice treated with daily TG 0 or 2.5 mg/kg for 12 days. Statistical analysis: Mann-Whitney non-parametric test. Symbols:*versus Winnie TG 0 mg/kg; #versus RaW TG 0 mg/kg.
Low-dose TG also ameliorated chronic colitis induced in C57Bl/6 WT by 0.5% DSS administration in drinking water for four cycles. These mice were daily gavaged TG 0.5 mg/kg from day 25 for a total of 28 days (last two on–off DSS cycles). The DSS colitis was improved with TG as evidenced by total colitis scores (see online supplementary figure S3B–E) and immune and epithelial damage subscores (see online supplementary figure S3F). The TG treatment was associated with decreased peripheral WBCs in both DSS-treated and untreated WT mice (see online supplementary figure S3G). The combined diarrhoea score and colon weight were significantly improved with TG, but not total body weight or colon length (see online supplementary figure S3B–D).
The results of this section overall confirm (i) that TG acts more efficaciously than MP in these murine models and (ii) that there is an inverse association between cumulative dose and leucocyte counts, consistent with the clinical experience with these immunomodulatory drugs in IBD. Thus, total mesenteric lymph node (MLN) cell numbers expectedly increased with DSS treatment in association with the DSS-induced colitis in WT, but were reduced by TG treatment (see online supplementary figure S3H). Interestingly, this was related to decreased numbers of MLN CD3e+CD4+ lymphocytes, as well as decreased MLN CD11b+ myeloid cells and CD11c+major histocompatibility complex class II (MHCII+) dendritic cells, which were significantly reduced by the low-dose TG treatment (see online supplementary figure S3I). MLN CD19+ and CD8+ lymphocytes were not altered (data not shown).
TG had a therapeutic effect independent of T lymphocytes
To further explore the immunomodulatory effect of TG on different leucocyte subtypes and compartments, the impact of daily TG on the bone marrow (BM) and spleen was assessed in WT animals treated with TG doses between 0.05 and 5 mg/kg for up to 28 days. Unsurprisingly, TG dose inversely correlated with BM leucocyte number (see online supplementary figure S4A) but interestingly, while FACS analysis showed that there were proportionate decreases in CD3e−CD19+ B lymphocytes and CD3e−CD11b+ myeloid cells, the CD3e+CD4+ and CD3e+CD8+ T lymphocyte fractions were not proportionately decreased in BM (see online supplementary figure S4A, B). In the spleen, CD3e+CD4+ and CD3e+CD4+CD69+ T helper cell numbers were decreased following administration of TG for 28 days, but there was also a reduction in CD3e−CD19+ B lymphocytes (see online supplementary figure S4C, D).
Our group previously published that T lymphocytes and B lymphocytes are not required for the occurrence of spontaneous colitis in Winnie mice. Winnie×Rag1−/− (RaW) mice are Winnie mice that lack T lymphocytes and B lymphocytes.30 RaW mice spontaneously develop a colitis phenotype with more proximal disease than Winnie. In an experiment with Winnie and RaW mice strains, TG 2.5 mg/kg administration for up to 14 days improved colon weight/length ratio (see online supplementary figure S5A), improved blinded histological PC and DC colitis scores (figure 2D) and restored goblet cell morphology in both RaW and Winnie mice (figure 2E). The TG treatment was associated with decreased inflammation (improved Ifn-γ, Il-1β) and increased Muc2 expression in both the PC and DC of RaW mice and the Winnie controls (see online supplementary figure S5B).
Taken altogether, these data show that TG ameliorates colitis through adaptive T lymphocyte-mediated immune responses4 5 and innate immunity.
Low-dose TG improved chronic DSS-induced colitis in Hprt−/− mice
ThioGTP, the most abundant of the TGN (thioGMP, thioGDP, thioGTP) within leucocytes,33 34 is required for the action of TG. We hypothesised that TG would not improve chronic DSS colitis in Hprt−/− mice because of the absolute requirement for HPRT enzyme for the synthesis of thioguanine nucleotides (TGN) from TG pro-drug.10
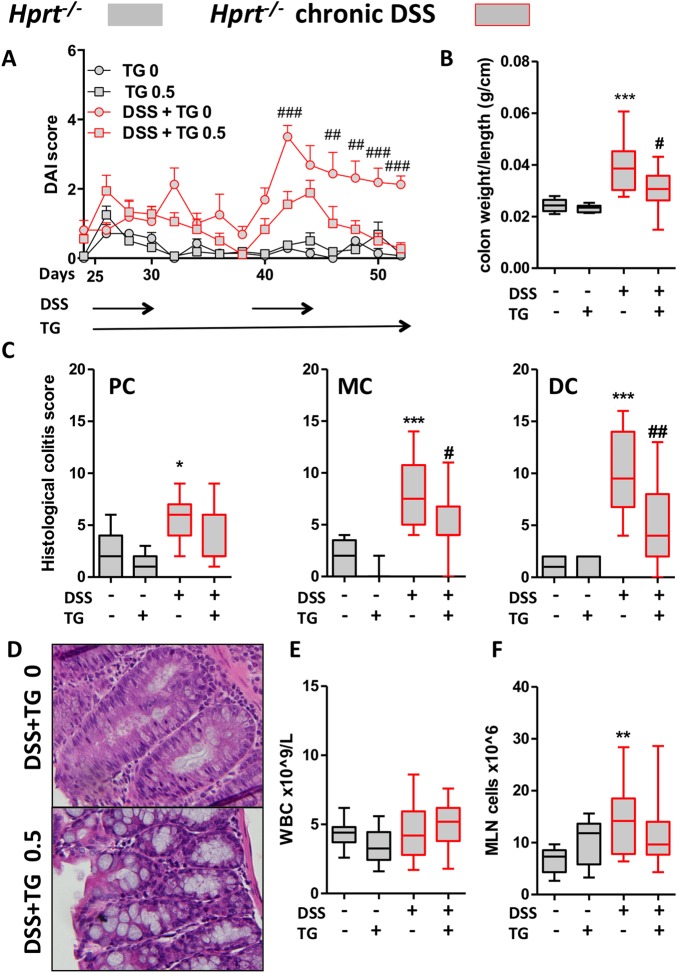
Unexpectedly, in the DSS-induced colitis in Hprt−/− mice, TG treatment was associated with improvement in all of the outcome measures: the disease activity index that includes diarrhoea, body weight change and rectal bleeding scores, colon weight/length ratio (figure 3A, B), colon weight (see online supplementary figure S6A) and blinded histological colitis scores of the different colonic segments (figure 3C, see online supplementary figure S6B). Representative H&E histology showed a more normal epithelium with intact goblet cells (figure 3D). As expected, TG treatment was not associated with immunosuppression in Hprt−/− mice since immunosuppression is related to generation of thioGTP (figure 3E). In addition, MLN cell counts in the Hprt−/− mice were expectedly increased by DSS in association with colitis but not depressed by TG (figure 3F). CD3e+CD4+, CD11b+ and CD11c+MHCII+ subset numbers in the MLN were not decreased by TG administration in untreated or DSS-exposed Hprt−/− mice (see online supplementary figure S6C).
Figure 3.
Low-dose thioguanine (TG) improved chronic dextran sodium sulfate (DSS)-induced colitis in hypoxanthine (guanine) phosphoribosyltransferase (Hprt)−/− mice. Hprt−/− mice were treated with ±DSS 0.5% in drinking water for four cycles, ±daily gavaged TG 0.5 mg/kg for the last two cycles (28 days from D25): (A) Disease activity indices (DAIs) two-way ANOVA p<0.05, p<0.001 from day 42; (B) colon weight/length ratio; (C) histological colitis scores for proximal colon (PC), mid-colon (MC) and distal colon (DC) in Hprt−/− treated with or without DSS or TG; (D) representative H&E from MC of Hprt−/− treated with 0.5% DSS, ±TG 0.5 mg/kg; (E) peripheral blood white blood cell (WBC) counts and (F) mesenteric lymph node (MLN) total cell number. Statistical analysis: Mann-Whitney non-parametric test. Symbols: *versus Hprt−/− TG 0 mg/kg; #versus Hprt−/−DSS TG 0 mg/kg. Scale bar=100 μm.
In summary, these data indicate that the therapeutic action of TG in ameliorating a chronic DSS colitis in Hprt−/− mice neither requires host thioGTP production, nor is associated with systemic immunosuppression.
TG shifted the host mucosal microbiome
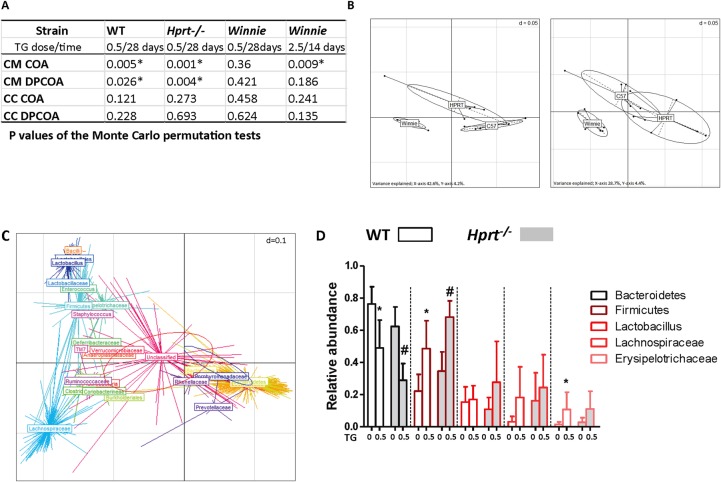
TG is an antimetabolite. To explore the possibility that the amelioration of colitis by TG might be due to the biotic effect of TG on the intestine's microbial community, age-matched and sex-matched WT, Hprt−/− and Winnie mice were gavaged daily with 0.5 mg/kg TG for 28 days. While treatment with TG had no detectable effect on the luminal caecal contents (CC) of the three mice strains (figure 4A), the structures of the caecal mucosa (CM)-associated communities were altered in both C57Bl/6 WT and Hprt−/− as shown by both phylogeny-dependent (double principal coordinates analysis (DPCOA)) and phylogeny-independent (correspondence analysis (COA)) methods. The CM microbiome in Winnie was not significantly altered by TG (p>0.05) suggesting that it is the impaired Winnie mucosal barrier and not the effect of low-dose TG, which was the dominant factor affecting the composition of the microbiota in the Winnie CM niche.
Figure 4.
The caecal mucosa (CM)-associated microbiome shifted with thioguanine (TG) treatment. The effect of once daily gavage TG 0 or 0.5 for 28 days in wild-type (WT), hypoxanthine (guanine) phosphoribosyltransferase (Hprt)−/− and Winnie, or TG 2.5 for 14 days in Winnie, on caecal contents (CC) and CM microbiomes was investigated by correspondence analysis (COA) and double principal coordinates analysis (DPCOA). (A) p Values of the Monte Carlo permutation tests indicating whether or not the resident communities of treatment and control groups differed significantly; (B) DPCOA based on the bacterial community profiles of the CM samples from all three strains of mice. Left—control samples, right—TG-treated samples. The panels represent similarities between sample profiles, with samples from the same strain grouped together; (C) DPCOA based on the bacterial community profiles of the CM samples from the WT mice. There are two ellipses, one red and one blue, which represent the collective variance of each treatment (blue control; red TG). The position of each centroid point (with a radiating star-like structure) represents the association of each OTU with each sample. Points are coloured and collectively labelled based on their taxonomy (obtained from the Ribosomal Database Project algorithm, rank level chosen to facilitate visualisation). The analysis indicates a strong partitioning of the samples based on treatment type driven by the relative abundances of Bacteroidetes (but possibly not Rikenellaceae and Prevotellaceae within this clade) and several groups within the Firmicutes and (D) relative abundances of chosen phylogenetic groups from WT and Hprt−/− CM samples (observed differences between control and TG treatments were analysed using a Wilcoxon test (a=0.05, false discovery rate (FDR)). N=6. Statistical analysis: *p<0.05).
We performed a second experiment in Winnie mice using a fivefold larger daily dose of TG (2.5 mg/kg). The coverage of this experiment was greater in terms of sequences retrieved per sample so that more subtle effects on the community structure of the microbiota could be assessed. Differences were detected using non-phylogenetic (COA)-based methods, showing changes in particular OTU (species) abundances, but overall the genetic structure of the community was not significantly altered (figure 4A). As with the low-dose TG, no differences were observed in the luminal caecal samples.
DPCOA based on the bacterial community profiles of the CM samples from all three strains of mice showed that community profiles were strongly influenced by strain origin (figure 4B left panel) and that treatment with TG mainly produced a similar effect on Hprt−/− and WT C57Bl/6 mice principally due to a decrease in Bacteroidetes and an increase in Firmicutes (figure 4C, D, p<0.05). This led to an increased similarity as evidenced by the increased overlap between the community profiles of Hprt−/− and WT (figure 4B right panel as opposed to left panel).
In summary, these data show significant shifts in the CM microbiota of WT and Hprt−/− mice treated with TG but only minor alterations to the CM microbiota of Winnie mice with TG treatment. There was no detectable effect on the luminal CC of the three mice strains. Taken together, alterations in the structure of the gut microbiota are unlikely to be a major factor that is contributing to TG's beneficial effect in improving the Winnie spontaneous colitis.
Intrarectal TG ameliorated spontaneous colitis within 14 days
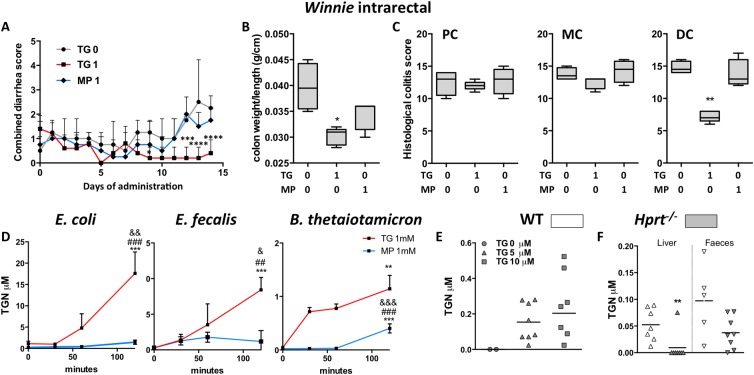
To determine if thiopurines can act locally in the colon, Winnie mice were treated daily with intrarectal TG 1 mg/kg, MP 1 mg/kg or vehicle control. WBC counts were not significantly altered by this treatment with median WBC count (IQR) for TG 9.8 (6.4–10.6), MP 12.3 (11.6–12.8) and control 11.0 (9.7–12.1). Only TG-treated mice had statistically significant improvement in the combined diarrhoea score from day 11 (figure 5A) and colon weight/length ratio (figure 5B). Blinded histological scoring of the DC confirmed a striking improvement with TG but not with MP in the DC (figure 5C, p<0.01, see online supplementary figure S7). There was no improvement in colitis in mid-colon or PC indicating that the beneficial effect on colitis was limited to regions of the colon in direct contact with administered TG (figure 5C, see online supplementary figure S7).
Figure 5.
Daily intrarectal thioguanine (TG), but not mercaptopurine (MP) at 1 mg/kg, effected a rapid local improvement in spontaneous colitis. (A) Combined diarrhoea score; (B) colon weight/length ratio and (C) histological scoring of colitis in distal colon (DC), mid-colon (MC) and proximal colon (PC) segments in the Winnie mice. Gut representative bacteria converted TG (and less so MP) to thioguanine nucleotides (TGN). In vitro: (D) mean (SEM) TGN in Escherichia coli (Gram negative), Enterococcus faecalis (Gram positive), Bacteroides thetaiotaomicron (Gram-negative anaerobe) cultures incubated with 1 mM TG or MP for up to 120 min, N=3–4, *versus T0, #versus T30 and &versus T60; (E) scatter plots, mean TGN at 6 hours in hypoxanthine (guanine) phosphoribosyltransferase (Hprt)−/− mouse faeces incubated with 0, 5 or 10 µM TG. In vivo: (F) wild-type (WT) and Hprt−/− mice gavaged 5 mg/kg TG: scatter plots, mean TGN in liver and faeces. Statistical analysis: Mann-Whitney non-parametric test. Symbols:*versus WT mice.
Bacteria converted TG but less so MP to TGN
Having shown in the Hprt−/− mice that TG improved colitis in the total absence of systemic immunosuppression, we explored whether the host's microbial metabolism could be generating thioGTP. HPRT: EC 2.4.2.8 and related purine salvage enzymes are highly conserved in bacteria.35
Bacterial metabolism of TG was first investigated in vitro using the representative gut bacteria Escherichia coli, Enterococcus faecalis and Bacteroides thetaiotaomicron. TGN (thioGMP, thioGDP and thioGTP) were detected in the bacterial pellets following incubation of log phase cultures of all three bacterial strains with 1 mM TG for 120 min (figure 5D), whereas TGN were minimal in cultures incubated with 1 mM MP. TGN were also detected by liquid chromatography–double mass spectrometry (LC-MS/MS) when faecal slurries derived from Hprt−/− mice were incubated with 5 or 10 μM TG for 6 hours (figure 5E). These data indicate that laboratory and gut bacteria are able to metabolise TG to the active TGN metabolites.
When WT and Hprt−/− mice were gavaged daily with TG 5 mg/kg for 10 days, a large dose which was expected to be incompletely absorbed by the small intestine, TGN were found in faeces of both mouse strains and the liver of WT but not in the liver of the Hprt−/− mice (figure 5F). Thus, a mechanism of action of TG may arise from bacterial Hprt conversion of TG to thioGTP.
TG enhanced autophagy in vitro in a TGN-dependent manner
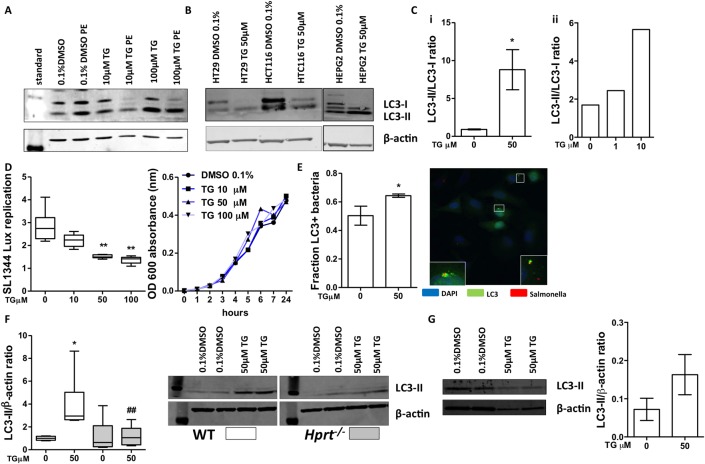
Based on the previous result sections, we further hypothesised that TG might rapidly ameliorate colitis by locally influencing the bacterial–epithelial interaction. Epithelial cells rely on autophagy to respond to luminal bacteria and maintain homeostasis. Impaired antibacterial autophagy in the gut has been hypothesised to be a contributor to IBD.15 Therefore, we analysed the effect of TG on bulk autophagy by monitoring the conversion of the autophagosome marker LC3-I to LC3-II. HeLa cells were incubated for 16 hours with either vehicle control or TG 1, 10 and 100 μM with or without Pepstatin A and E64D (PE) to examine autophagic flux. TG treatment increased the LC3-II to LC3-I ratio (figure 6A, Cii and see online supplementary figure S8A), indicative of increased autophagosome accumulation. Treatment with PE, which blocks autophagosome maturation and recycling of LC3-II back to LC3-I, further augmented the effect indicating that the effect of TG is due to increased autophagy induction rather than inhibition of maturation. This effect was also observed in human gut epithelial cell lines (HT29, HCT116) and hepatocytes (HepG2) (figure 6B and see online supplementary figure S8B) and in murine Ralph and William's cell line (RAW) macrophage-like cells (figure 6Ci and see online supplementary figure S8C). To determine if TG treatment had an effect on intracellular bacterial handling, we used a Salmonella intracellular replication model, which is known to be sensitive to effects on antibacterial autophagy. HeLa cells were pretreated with vehicle control or TG and infected with bioluminescent Salmonella typhimurium (SL1344) and replication monitored by measuring luminescence. Treatment with either 50 or 100 µM TG significantly decreased intracellular replication of the bacteria (figure 6D). We further quantified by fluorescence microscopy the autophagosome encapsulation of bacteria with or without treatment with TG 50 µM in HeLa cells expressing (LC3-green fluorescent protein (GFP)). This dose of TG significantly increased LC3 colocalisation with intracellular bacteria compared with control consistent with increased antibacterial autophagy (figure 6E).
Figure 6.
Thioguanine (TG) promoted autophagy. TG administration enhanced autophagy in vitro: western blots for LC3-I, LC3-II and β-actin. (A) HeLa cells treated with dimethyl sulfoxide (DMSO) 0.1%,TG (10 or 100 µM)±Pepstatin A and E64D (PE) for 16 hours; (B) HT29, HCT116 and HepG2 cell lines treated with TG 50 µM for 16 hours; (C) LC3-II:LC3-I ratio in TG-treated (i) RAW cells and (ii) in HeLa+PE. Bacterial replication assay: (D) HeLa pretreated DMSO 0.1% or TG (10, 50 and 100 µM) infected with Salmonella (SL1344): luminescence readings 12 hours post-infection; SL1344 growth in media containing TG was not altered over that time period. N=4–8 experiments; (E) fluorescence microscopy quantification and representative image of LC3-II colocalisation with SL1344 bacteria-infected HeLa cells+TG 50 µM. Blue—4′,6-diamidino-2-phenylindole (DAPI) nuclear stain; red—Salmonella; green—LC3. Bars, mean±SD, N=3. Statistical analysis: unpaired t-test *p<0.05. The TG effect on autophagy was hypoxanthine (guanine) phosphoribosyltransferase (Hprt) dependent: (F) LC3-II to β-actin ratio quantification. Wild-type (WT) and Hprt−/−-derived primary murine fibroblasts treated with DMSO 0.1% or 50 µM TG for 16 hours. N=5–6. Western blot image. (G) WT colon organoids differentiated for 72 hours and then treated with DMSO 0.1% or TG 50 µM for 16 hours. Western blot image. LC3-II to β-actin ratio quantification, bars, mean±SD, N=2 from 6 wells/condition/replicate. Statistical analyses: Mann-Whitney non-parametric test: *p<0.05 WT TG 50 versus 0 µM; ##p<0.01 TG 50 µM Hprt−/− versus WT TG 50 µM.
In order to differentiate between TG effect and thioGTP effect, primary fibroblasts isolated from WT and Hprt−/− mice were incubated for 16 hours with either vehicle control or TG 50 μM. There was minimal LC3-I observed in fibroblasts but the LC3-II to β-actin ratio was significantly increased in the TG-treated WT-derived fibroblasts, consistent with induction of autophagy, but there was no effect in the absence of Hprt (figure 6F), although autophagy could be induced in Hprt−/− fibroblasts by the mTor inhibitor, Torin1, indicating an intact autophagy apparatus (see online supplementary figure S8D). Together these data show that TG treatment of epithelial cells induces autophagy in a thioGTP-dependent manner and improves restriction of intracellular bacterial growth, likely through induction of antibacterial autophagy.
To address the question of in vivo relevance of TG treatment, we cultured colonic organoids from WT mice. LC3-II to β-actin ratio was clearly increased by TG consistent with autophagy induction in the primary cultures (figure 6G). Attempts were also made to demonstrate increased LC3-II/LC3-I in our animal models. We discerned a trend for decreased autophagy (p=0.07, see online supplementary figure S8F) with the improvement in colitis with intrarectal TG treatment (figure 5A–C), which is consistent with a more healthy colonic epithelium, but we were unable to demonstrate any trend or association between autophagy flux in colonic mucosa and acute exposure to TG using our methods.
Discussion
There are important novel observations from this study. Most notable was the remarkable clinical and histological improvement in chronic DSS colitis in Hprt−/− mice. This was quite unexpected, because the action of thiopurines is widely held to involve thioGTP. ThioGTP is the most abundant of the TGN (thioGMP, thioGDP, thioGTP).33 34 Consistent with the inability of the Hprt-deficient murine host to convert TG to TGN, there was no reduction in leucocyte counts in either peripheral blood or MLN compartments. This led us to investigate the effect of TG on the microbiota: we hypothesised that TG would have both a biotic effect and be metabolised by the microbiota. The microbiome was altered by TG in the CM but not in the caecal lumen of C57Bl/6 WT and Hprt−/− mice. While these changes in the mucosal microbiome may have resulted in a less colitogenic (more tolerogenic) bacterial community because similar phyla and subphyla changes in Firmicutes and Bacteroidetes are reported in the mucosal microbiome between inflamed and non-inflamed samples from patients with IBD,36 the CM microbiome was only minimally altered in Winnie mice treated with TG. This indicated that the altered mucus barrier in Winnie mice dominated any effect of TG on this microbial niche. It was, thus, unlikely that the beneficial effect of TG in spontaneous Winnie colitis is due to the biotic effect of TG on the microbiota.
On the other hand, we demonstrated bacterial conversion of TG to TGN. This raises the possibility that intestinal bacteria locally generating TGN, including thioGTP, have the potential to locally alleviate inflammation by affecting either mucosal epithelial or immune cells. While recent literature emphasises the effect of thiopurines on the antigen-presenting cell–T lymphocyte synapse, with the action of TG having been shown to involve blockade of GTPase activation in T lymphocytes upon costimulation with CD28,4 37 there are many other cell types in addition to circulating T lymphocytes in the inflamed colonic mucosa, which could be affected by TG treatment. Thiopurine effects on other cell types have occasionally been reported.38–40 The improvement of colitis in RaW (Winnie×Rag1−/−) mice (figure 2) indicated that the therapeutic effect of TG did not require the presence of T lymphocytes or B lymphocytes. Moreover, while overall leucocyte counts in peripheral blood and BM compartments were decreased by TG therapy in (the Hprt-intact) WT and Winnie mice, it was myeloid/dendritic cell counts that were proportionately more diminished in the BM, spleen and MLN compartments in WT mice (see online supplementary figures S3 and S4).
The action of TG to ameliorate colitis was rapid. This is qualitatively consistent with the clinical impressions with oral immediate release TG, that TG has a faster action than conventional oral thiopurines,7 9 41 but the degree of rapidity of action in our experiments was unexpected. The pharmacodynamic effect of oral and intrarectal TG treatment in the Winnie mice was <2 weeks as manifested by the improved colonic histology with TG 1 or 2.5 mg/kg/day (figure 1), the fast symptomatic improvement in the DSS-induced colitis in WT and Hprt−/− mice treated with low-dose TG (figure 3, see online supplementary figure S3) and the rapid symptomatic and histological improvement in Winnie mice with low-dose intrarectal TG (figure 5A–C, see online supplementary figure S7). TG was also strikingly associated with restoration of mucin production by goblet cells as evidenced by the H&E histology, increased expression of Muc2 (figure 1) and of the mucin-specific Agr2 chaperone (see online supplementary figure S2). In contrast to direct effects of dexamethasone1 or IL-1032 on goblet cells where reduced ER stress and unfolded protein response leads to restitution of goblet cell mucin, we believe that restitution of goblet cell mucin with TG is due to a reduction in the inflammatory cytokine milieu (figure 1) rather than due to any direct effects of TG on epithelial cell ER stress and unfolded protein response (unpublished data).
The rapidity of the pharmacodynamic effect of TG but not MP in our murine colitis experiments is not completely explained by pharmacokinetic differences, since intravenous loading of azathioprine in IBD does not affect the time to onset of its therapeutic action,42 and TGN in peripheral blood reaches a steady state with oral MP treatment by 4 weeks, which is well before its pharmacodynamic action. However, TG effects a TGN steady state more rapidly,43–45 which is likely because the conversion of TG to active drug is not rate limited by inosine monophosphate dehydrogenase (IMPDH).33
We propose that the rapidity of the pharmacodynamic effect of gavaged TG in our murine models is due to local actions of TG that due to limited intestinal absorption reaches the inflamed distal intestine in the murine models to enhance mucosal barrier function, as well as to enhance innate immune function. (The peak gastric-anus transit time in mice is reported to be <7 hours and we demonstrated TGN in faeces with high-dose gavaged TG and rapid local improvement in distal colitis with intrarectal TG, figure 5.) We also demonstrated previously unappreciated effects of TG on epithelial cells: (i) TG treatment restored goblet cell morphology (figures 1B, 2E and 3D)1 32 and (ii) in vitro, TG also improved bulk autophagy and intracellular handling of bacteria (figure 6). Thiopurines have previously been reported to induce autophagy after prolonged exposure through the mismatch repair pathway, presumably due to incorporation into DNA synthesis.46 However, we demonstrated that TG induces autophagic flux relatively rapidly in both epithelial and macrophages cell lines as well as primary fibroblasts and organoids and that there is an augmented restriction of intracellular bacterial replication through an increase in antibacterial autophagy. The effect appeared to be TGN dependent (or a related metabolite) dependent because this effect was abolished in Hprt−/− fibroblasts (figure 6). These data—the TG-enhanced bactericidal function, the effect of TG on leucocyte subsets and the improvement in RaW murine colitis with TG—are consistent with the recent report which showed that RAC1 inhibition could boost innate immunity.47 Thus, compared with the slow pharmacodynamics action effected by loading of circulating effector lymphocytes, TG treatment of IBD could occur more rapidly through the local conversion of TG to thioGTP by mucosal or microbial HPRT.
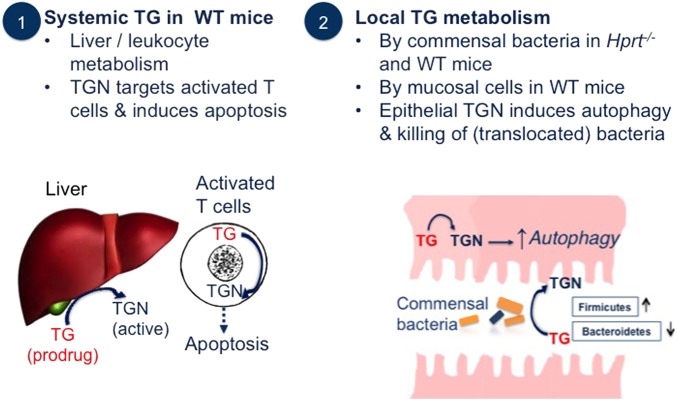
Our current model of TG action in murine colitis is summarised in figure 7. In WT mice, TG entering the portal circulation undergoes first-pass portal metabolism to TGN, which has myelosuppressive effects resulting in decreased activated circulating T lymphocyte numbers. On the other hand, TG reaching the colonic lumen has a mild biotic effect on mucosal bacteria and is converted by the microbiota and by the colonic mucosa in Hprt-intact mice to TGN. The microbiome metabolism of drugs is relatively unexplored and it is thought that its capacity to metabolise drugs to inactive or toxic products equals that of the liver.48 While there are well-known examples in IBD of bacterial enzymes releasing a drug from an inert carrier, we believe that this is the first demonstration of the microbiome converting a pro-drug to an active drug metabolite. Mucosa-associated bacteria are increased at sites of inflammation in IBD,49 and there is diminished mucosal barrier integrity which facilitates increased translocation of bacteria. Therefore, TGN could accumulate in the inflamed distal intestinal mucosa via local luminal or mucosal metabolism of TG or via autophagy of TGN-laden bacteria. TGN-augmented autophagy would result in rapid improvement of bacterial handling, decreased immune activation, decreased intestinal inflammation and potentially improved secretory function with restoration of the mucus layer. Unlike MP or azathioprine, TG conversion to active drug by the host or bacteria is not rate limited by IMPDH. Thus, local delivery of TG to sites of intestinal inflammation in IBD would have clinically significant advantages over current oral immunomodulating therapies. In particular, a local delivery of TG could permit a more rapid therapeutic action in IBD, which would also avoid unwanted adverse effects on liver or haematopoiesis.
Figure 7.
Cartoon illustrating (1) systemic and (2) local effects of conversion of thioguanine (TG) pro-drug to thioguanine nucleotides (TGN) drug. WT, wild-type.
Acknowledgments
We are grateful to Professor Michael McGuckin, Deputy Director Research MR-UQ, and Immunity Infection Inflammation and Cancer Care & Biology Programs; Professor Mark Morrison, Chair, Microbial Biology and Metagenomics, the UQ Diamantina Institute and Professor Deon Venter, MR-UQ Cancer Care & Biology Program, for their support of this project.
Footnotes
Contributors: IO had major roles in experimental design, data acquisition analysis and interpretation and writing of the manuscript; RM performed with direction from IO experiments in figures 3–6; ID was involved with acquisition and analysis of data in figures 1 and 2; DAdC produced the bacterial community profiles and performed the microbiome analyses and led interpretation in figure 4; VS and BH contributed to data acquisition and analysis for figure 6; YY performed with IO the experiments in figure 3; MP contributed to data acquisition in figures 2–7; RW contributed to data acquisition in figures 3, 5 and 6; YS contributed to data acquisition in figures 2–4; AP contributed along with IO to the organoid cultures and intrarectal experiments; RL instigated the organoid culture systems in our lab; ML contributed to method development, figure 5; PÓC provided oversight of the microbiology and contributed to the writing of the manuscript; JAD provided expertise throughout concerning thiopurine biochemistry measurement and intermediate metabolism; JB contributed to experimental design, method development and data analysis for figure 6 and contributed to manuscript writing; THJF conceived the overall project and experimental design, is responsible overall for the integrity of the data, data analysis and interpretation and the writing and final manuscript.
Funding: THJF gratefully acknowledges funding from the Gutsy Charity (Incorporated Victoria, Australia). IO and THJF also received funding from NHMRC project grant 1064440. IO and JB have had UQ Reginald Ferguson Fellowships. JB and THJF receive funding from the Mater Foundation.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Provisional Patent filed 29 September 2015. PAT-02211-AU-01 “Novel Formulation and Treatment Methods”. Data and Materials availability: Accession number PRJEB10595 (deposited at European Nucleotide Archive).
References
- 1.Das I, Png CW, Oancea I, et al. . Glucocorticoids alleviate intestinal ER stress by enhancing protein folding and degradation of misfolded proteins. J Exp Med 2013;210:1201–16. 10.1084/jem.20121268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gisbert JP, Niño P, Cara C, et al. . Comparative effectiveness of azathioprine in Crohn's disease and ulcerative colitis: prospective, long-term, follow-up study of 394 patients. Aliment Pharmacol Ther 2008;28:228–38. 10.1111/j.1365-2036.2008.03732.x [DOI] [PubMed] [Google Scholar]
- 3.Colombel JF, Sandborn WJ, Reinisch W, et al. . Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 2010;362:1383–95. 10.1056/NEJMoa0904492 [DOI] [PubMed] [Google Scholar]
- 4.Tiede I, Fritz G, Strand S, et al. . CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest 2003;111:1133–45. 10.1172/JCI16432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Horin S, Goldstein I, Fudim E, et al. . Early preservation of effector functions followed by eventual T cell memory depletion: a model for the delayed onset of the effect of thiopurines. Gut 2009;58:396–403. 10.1136/gut.2008.157339 [DOI] [PubMed] [Google Scholar]
- 6.Dervieux T, Brenner TL, Hon YY, et al. . De novo purine synthesis inhibition and antileukemic effects of mercaptopurine alone or in combination with methotrexate in vivo. Blood 2002;100:1240–7. 10.1182/blood-2002-02-0495 [DOI] [PubMed] [Google Scholar]
- 7.Dubinsky MC, Hassard PV, Seidman EG, et al. . An open-label pilot study using thioguanine as a therapeutic alternative in Crohn's disease patients resistant to 6-mercaptopurine therapy. Inflamm Bowel Dis 2001;7:181–9. 10.1097/00054725-200108000-00001 [DOI] [PubMed] [Google Scholar]
- 8.Derijks LJ, de Jong DJ, Gilissen LP, et al. . 6-Thioguanine seems promising in azathioprine- or 6-mercaptopurine-intolerant inflammatory bowel disease patients: a short-term safety assessment. Eur J Gastroenterol Hepatol 2003; 15:63–7. 10.1097/00042737-200301000-00011 [DOI] [PubMed] [Google Scholar]
- 9.Herrlinger KR, Kreisel W, Schwab M, et al. . 6-thioguanine—efficacy and safety in chronic active Crohn's disease. Aliment Pharmacol Ther 2003;17:503–8. 10.1046/j.1365-2036.2003.01440.x [DOI] [PubMed] [Google Scholar]
- 10.Oancea I, Png CW, Das I, et al. . A novel mouse model of veno-occlusive disease provides strategies to prevent thioguanine-induced hepatic toxicity. Gut 2013;62:594–605. 10.1136/gutjnl-2012-302274 [DOI] [PubMed] [Google Scholar]
- 11.Elinav E, Strowig T, Kau AL, et al. . NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011;145:745–57. 10.1016/j.cell.2011.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang MS, Poles J, Leung JM, et al. . Inferred metagenomic comparison of mucosal and fecal microbiota from individuals undergoing routine screening colonoscopy reveals similar differences observed during active inflammation. Gut Microbes 2015;6:48–56. 10.1080/19490976.2014.1000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zenewicz LA, Yin X, Wang G, et al. . IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J Immunol 2013;190:5306–12. 10.4049/jimmunol.1300016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jostins L, Ripke S, Weersma RK, et al. . Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491: 119–24. 10.1038/nature11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begun J, Xavier RJ. Autophagy at the crossroads of metabolism and cellular defense. Curr Opin Gastroenterol 2013;29:588–96. 10.1097/MOG.0b013e328365d34d [DOI] [PubMed] [Google Scholar]
- 16.Prantera C, Lochs H, Grimaldi M, et al. . Rifaximin-extended intestinal release induces remission in patients with moderately active Crohn's disease. Gastroenterol 2012;142:473–81. 10.1053/j.gastro.2011.11.032 [DOI] [PubMed] [Google Scholar]
- 17.Selby W, Pavli P, Crotty B, et al. . Two-year combination antibiotic therapy with clarithromycin, rifabutin, and clofazimine for Crohn's disease. Gastroenterol 2007;132:2313–19. 10.1053/j.gastro.2007.03.031 [DOI] [PubMed] [Google Scholar]
- 18.Moayyedi P, Surette MG, Kim PT, et al. . Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterol 2015;149:102–9. 10.1053/j.gastro.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 19.Heazlewood CK, Cook MC, Eri R, et al. . Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med 2008;5:e54 10.1371/journal.pmed.0050054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ó Cuív P, Aguirre de Cárcer D, Jones M, et al. . The effects from DNA extraction methods on the evaluation of microbial diversity associated with human colonic tissue. Microb Ecol 2011;61:353–62. 10.1007/s00248-010-9771-x [DOI] [PubMed] [Google Scholar]
- 21.Yu Z, Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 2004;36:808–12. [DOI] [PubMed] [Google Scholar]
- 22.Caporaso JG, Kuczynski J, Stombaugh J, et al. . QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schloss PD, Westcott SL, Ryabin T, et al. . Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 2009;75:7537–41. 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cárcer DA, Denman SE, McSweeney C, et al. . Evaluation of subsampling-based normalization strategies for tagged high-throughput sequencing data sets from gut microbiomes. Appl Environ Microbiol 2011;77:8795–8. 10.1128/AEM.05491-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dray S, Pavoine S, Aguirre de Cárcer D. Considering external information to improve the phylogenetic comparison of microbial communities: a new approach based on constrained Double Principal Coordinates Analysis (cDPCoA). Mol Ecol Resour 2015;15:242–9. 10.1111/1755-0998.12300 [DOI] [PubMed] [Google Scholar]
- 26.Culhane AC, Perriere G, Considine EC, et al. . Between-group analysis of microarray data. Bioinform 2002;18:1600–8. 10.1093/bioinformatics/18.12.1600 [DOI] [PubMed] [Google Scholar]
- 27.Dervieux T, Meyer G, Barham R, et al. . Liquid chromatography-tandem mass spectrometry analysis of erythrocyte thiopurine nucleotides and effect of thiopurine methyltransferase gene variants on these metabolites in patients receiving azathioprine/6-mercaptopurine therapy. Clin Chem 2005;51:2074–84. 10.1373/clinchem.2005.050831 [DOI] [PubMed] [Google Scholar]
- 28.Huett A, Ng A, Cao Z, et al. . A novel hybrid yeast-human network analysis reveals an essential role for FNBP1L in antibacterial autophagy. J Immunol 2009;182:4917–30. 10.4049/jimmunol.0803050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.VanDussen KL, Marinshaw JM, Shaikh N, et al. . Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 2015;64:911–20. 10.1136/gutjnl-2013-306651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eri RD, Adams RJ, Tran TV, et al. . An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol 2011;4:354–64. 10.1038/mi.2010.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang R, Hasnain SZ, Tong H, et al. . Neutralizing IL-23 is superior to blocking IL-17 in suppressing intestinal inflammation in a spontaneous murine colitis model. Inflamm Bowel Dis 2015;21:973–84. 10.1097/MIB.0000000000000353 [DOI] [PubMed] [Google Scholar]
- 32.Hasnain SZ, Tauro S, Das I, et al. . IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterol 2013;144:357–68.e9. 10.1053/j.gastro.2012.10.043 [DOI] [PubMed] [Google Scholar]
- 33.Duley JA, Florin TH. Thiopurine therapies: problems, complexities, and progress with monitoring thioguanine nucleotides. Ther Drug Monit 2005;27:647–54. 10.1097/01.ftd.0000169061.52715.3e [DOI] [PubMed] [Google Scholar]
- 34.Karner S, Shi S, Fischer C, et al. . Determination of 6-thioguanosine diphosphate and triphosphate and nucleoside diphosphate kinase activity in erythrocytes: novel targets for thiopurine therapy? Ther Drug Monit 2010;32:119–28. 10.1097/FTD.0b013e3181d12f19 [DOI] [PubMed] [Google Scholar]
- 35.Levine RA, Taylor MW. Selection for purine regulatory mutants in an E. coli hypoxanthine phosphoribosyl transferase-guanine phosphoribosyl transferase double mutant. Mol Gen Genet 1981;181:313–18. 10.1007/BF00425604 [DOI] [PubMed] [Google Scholar]
- 36.Walker AW, Sanderson JD, Churcher C, et al. . High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol 2011;11:7 10.1186/1471-2180-11-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poppe D, Tiede I, Fritz G, et al. . Azathioprine suppresses ezrin-radixin-moesin-dependent T cell-APC conjugation through inhibition of Vav guanosine exchange activity on Rac proteins. J Immunol 2006;176:640–51. 10.4049/jimmunol.176.1.640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazmers IS, Daddona PE, Dalke AP, et al. . Effect of immunosuppressive agents on human T and B lymphoblasts. Biochem Pharmacol 1983;32:805–10. 10.1016/0006-2952(83)90580-4 [DOI] [PubMed] [Google Scholar]
- 39.Bhandaru M, Pasham V, Yang W, et al. . Effect of azathioprine on Na(+)/H(+) exchanger activity in dendritic cells. Cell Physiol Biochem 2012;29:533–42. 10.1159/000338507 [DOI] [PubMed] [Google Scholar]
- 40.Aldinucci A, Biagioli T, Manuelli C, et al. . Modulating dendritic cells (DC) from immunogenic to tolerogenic responses: a novel mechanism of AZA/6-MP. J Neuroimmunol 2010;218:28–35. 10.1016/j.jneuroim.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 41.Pavlidis P, Ansari A, Duley J, et al. . Splitting a therapeutic dose of thioguanine may avoid liver toxicity and be an efficacious treatment for severe inflammatory bowel disease: a 2-center observational cohort study. Inflamm Bowel Dis 2014;20:2239–46. 10.1097/MIB.0000000000000206 [DOI] [PubMed] [Google Scholar]
- 42.Sandborn WJ, Tremaine WJ, Wolf DC, et al. . Lack of effect of intravenous administration on time to respond to azathioprine for steroid-treated Crohn's disease. North American Azathioprine Study Group. Gastroenterol 1999;117:527–35. 10.1016/S0016-5085(99)70445-2 [DOI] [PubMed] [Google Scholar]
- 43.Lennard L, Rees CA, Lilleyman JS, et al. . Childhood leukaemia: a relationship between intracellular 6-mercaptopurine metabolites and neutropenia. Br J Clin Pharmacol 1983;16:359–63. 10.1111/j.1365-2125.1983.tb02178.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lancaster DL, Patel N, Lennard L, et al. . 6-Thioguanine in children with acute lymphoblastic leukaemia: influence of food on parent drug pharmacokinetics and 6-thioguanine nucleotide concentrations. Br J Clin Pharmacol 2001;51:531–9. 10.1046/j.0306-5251.2001.01391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lennard L, Davies HA, Lilleyman JS. Is 6-thioguanine more appropriate than 6-mercaptopurine for children with acute lymphoblastic leukaemia? Br J Cancer 1993;68:186–90. 10.1038/bjc.1993.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng X, Yan T, Schupp JE, et al. . DNA mismatch repair initiates 6-thioguanine--induced autophagy through p53 activation in human tumor cells. Clin Cancer Res 2007;13:1315–21. 10.1158/1078-0432.CCR-06-1517 [DOI] [PubMed] [Google Scholar]
- 47.Parikh K, Zhou L, Somasundaram R, et al. . Suppression of p21Rac signaling and increased innate immunity mediate remission in Crohn's disease. Sci Transl Med 2014;6:233ra53 10.1126/scitranslmed.3006763 [DOI] [PubMed] [Google Scholar]
- 48.Haiser HJ, Turnbaugh PJ. Is it time for a metagenomic basis of therapeutics? Science 2012;336:1253–5. 10.1126/science.1224396 [DOI] [PubMed] [Google Scholar]
- 49.Png CW, Lindén SK, Gilshenan KS, et al. . Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol 2010;105:2420–8. 10.1038/ajg.2010.281 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2015-310874supp.pdf (1.4MB, pdf)