Abstract
Bone tissue engineering (BTE) is emerging as a possible solution for regeneration of bone in a number of applications. For effective utilization, BTE scaffolds often need modifications to impart biological cues that drive diverse cellular functions such as adhesion, migration, survival, proliferation, differentiation, and biomineralization. This review provides an outline of various approaches for building bioactive elements into synthetic scaffolds for BTE and classifies them broadly under two distinct schemes; namely, the top-down approach and the bottom-up approach. Synthetic and natural routes for top-down approaches to production of bioactive constructs for BTE, such as generation of scaffold-extracellular matrix (ECM) hybrid constructs or decellularized and demineralized scaffolds, are provided. Similarly, traditional scaffold-based bottom-up approaches, including growth factor immobilization or peptide-tethered scaffolds, are provided. Finally, a brief overview of emerging bottom-up approaches for generating biologically active constructs for BTE is given. A discussion of the key areas for further investigation, challenges, and opportunities is also presented.
Keywords: bone tissue engineering, synthetic scaffolds, biological modification, extracellular matrix, biomimetic scaffolds
Introduction
Bone formation occurs primarily through two processes, namely intramembranous ossification and endochondral ossification. 1,2 Whereas normal bone fracture healing and continuous bone remodeling occur throughout adult life, larger bone defects due to trauma (civilian and military injuries), tumor resection, congenital deformities, and surgical reconstruction often require intervention, as the natural regenerative response is either impaired or insufficient. 3,4,5,6
Additionally, the regenerative capacity of bone can be compromised by pathological conditions like osteoradionecrosis, 7,8,9 avascular necrosis, 10 atrophic non-unions, 11 and osteoporosis. 12 Current clinical approaches for treatment of bone defects include autologous, allogeneic, or xenogeneic bone grafts. 13 However, these clinical approaches present numerous drawbacks. For example, allogeneic and xenogeneic bone grafts carry a risk of pathogenicity and disease transmission, whereas autologous bone grafts suffer from secondary trauma (donor site morbidity) and limited availability. 13 Bone tissue engineering (BTE) is emerging as a promising avenue for regeneration of bone as it aims to address some of these limitations in current clinical practice. BTE employs stem/progenitor cells, biomaterial scaffolds, biologically active factors (e.g., growth factors), or their combinations to generate tissue-engineered bone grafts to facilitate bone regeneration.13 The ultimate challenge of BTE, however, is to produce a sufficient quality and quantity of functional and vascularized bone on a time frame suitable to meet the clinical need. 14,15
Scaffolds for BTE ideally need to facilitate and enhance vascularization, inhibit fibrous tissue formation, and present an ability to integrate with surrounding tissue, especially in the reconstruction of large orthopedic defects.16 Additionally, scaffolds for BTE need to mimic the mechanical properties, biological properties, and micro/nanostructure of native bone and be sterilizable to avoid infection at the site of implantation.17
Over the years, scaffolds for BTE have evolved to meet these requirements. Numerous scaffolds derived from natural and synthetic materials have been developed and investigated for BTE; however, these studies have also revealed several shortcomings.18 Whereas synthetic materials for generating BTE scaffolds generally can be produced reliably and reproducibly with minimal batch-to-batch variation, they often lack the biological cues required for engineering bone tissue. 19 On the other hand, scaffolds derived from natural materials have generally demonstrated better cell-instructive properties, but they also typically suffer from a lack of reliable and reproducible quality and greater batch-to-batch variation.20 More recently, hybrid scaffolds incorporating natural and synthetic materials have been employed for BTE to leverage their combined advantages.21,22, 23,24
There has been a concerted effort in recent years to functionalize synthetic scaffolds to impart biological cues, while still retaining their tunability to drive optimal cell-scaffold interactions for BTE. 25,26. The present review focuses on summarizing various approaches employed for building biomimetic elements into synthetic materials. While functionalization strategies do exist for metals, 27, 28,29 ceramics 30,31 and bioactive glass scaffolds 32,33,34,35 for BTE, this review primarily focuses on polymeric scaffolds for BTE. This review classifies these approaches broadly under two distinct schemes; namely, the top-down approach and the bottom-up approach. Various components used in each of these functionalization strategies, such as growth factors, cytokines, extracellular matrix (ECM) molecules, and peptides are discussed. Additionally, approaches to generation of scaffold-ECM hybrid constructs by deposition of ECM on scaffolds are highlighted. The present review also explores novel means for functional modification based on a combination of the scaffold-based and the emerging alternative bottom-up approaches. Key areas for further investigation, challenges, and opportunities in functionalization or biological modification of synthetic scaffolds are discussed. While the general principles are applicable to all tissue engineering strategies, relevant examples for BTE are given.
Top-down approaches
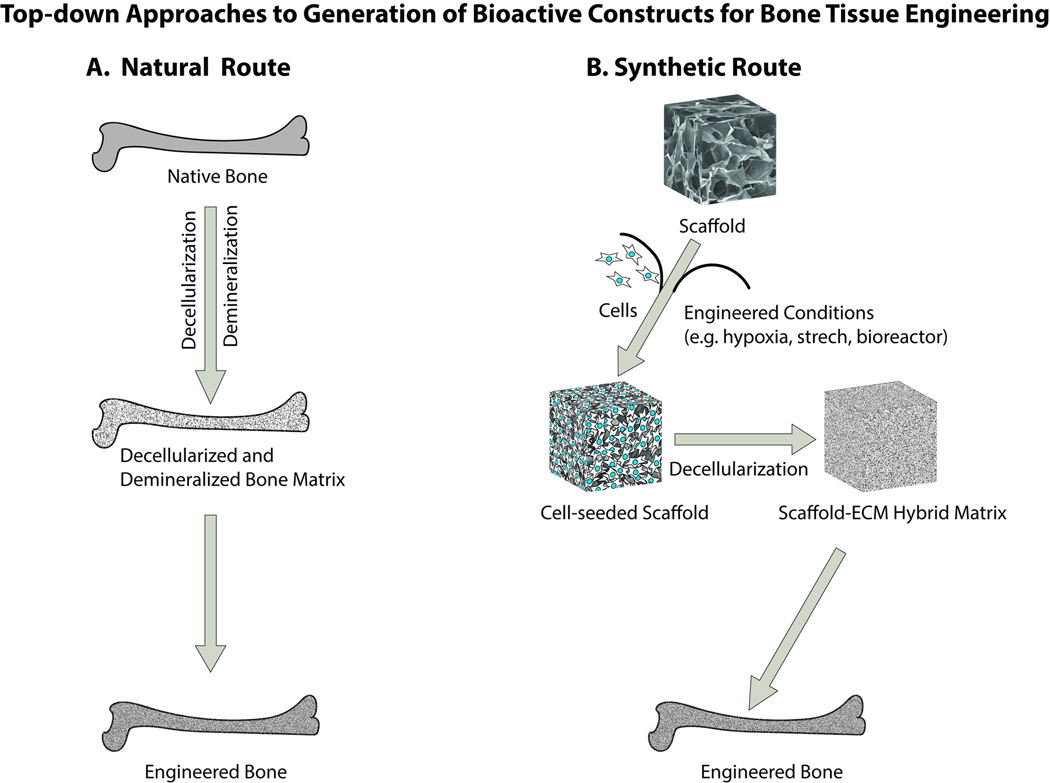
The top-down approach for biological modification of scaffolds relies on the concept that the natural tissue matrix provides a natural starting point for developing scaffolds with appropriate biological cues for a specific tissue of interest. In this respect, decellularized bone tissue and the remaining ECM after decellularization constitutes a natural choice for BTE scaffolds. Although these scaffolds are not synthetic, they provide insights that have guided the methods for modification of synthetic scaffolds and the development of hybrid scaffolds. Thus top-down approaches in the broadest sense may be further sub-classified based on the route taken for BTE construct generation into what will be termed in this review as a “natural route” and a “synthetic route” (Figure 1).
Figure 1.
Schematic representation of top-down approaches to generation of bioactive constructs for bone tissue engineering. In the natural route for the top-down approaches, a native bone can be decellularized and demineralized to obtain a natural matrix in various forms (e.g., powder, paste, putty, injectable; not shown) devoid of cells that can be utilized for generating engineered bone. For the synthetic route, scaffold-ECM hybrid matrices are developed by culturing of cell-seeded scaffolds for a limited duration of time often under engineered culture conditions and subsequently scaffold-ECM hybrid matrices are obtained after decellularization to produce a biologically active matrix that can be used for engineering bone tissue.
Natural routes for producing biologically active constructs
The natural routes for BTE construct production are primarily based on decellularization and demineralization of native bone. While these natural routes do not involve a synthetic substrate, they are discussed briefly here to highlight important similarities and differences with synthetic routes for top-down approaches for producing biologically active constructs for BTE.
Demineralized bone matrix
Demineralized bone matrix (DBM) comprises the bone matrix that is left over after minerals and cells have been removed from the bone. 36DBM still contains several active protein components and has been shown to be a source of bone morphogenetic proteins (BMPs). DBM is produced commercially by several companies from cadaver bones and used as an implant for bony defects. 37 DBM is available in several forms such as a powder, putty, paste, and an injectable form, often with a hydrogel as a carrier.38 DBM is normally used for filling voids and gaps and in filling osseous defects created either by surgery or due to traumatic injury. DBM is not generally indicated for providing structural support to bone during the healing process. Cancellous bone chips may be mixed with DBM to fill bony defects. DBM is often combined with natural bone or synthetic bone graft extenders such as hydroxyapatiteor tricalcium phosphate to fill osseous defects. DBM displays excellent osteoinductive and osteoconductive properties by activating cells involved in bone regeneration. 38
Decellularized tissue matrix
Several decellularized tissue-based materials are emerging as scaffolds for BTE (e.g., decellularized periosteum, cartilage, and goat lung). 39,40,41Advantages of the decellularized scaffolds include the intact structural framework of the tissue and the presence of bioactive molecules that drive tissue homeostasis and regeneration.42 Decellularized scaffolds do not elicit adverse immune reaction upon removal of cellular material with appropriate protocols. However, potential problems with decellularized scaffolds may include limited cell adhesion and denaturation of proteins and inactivation of growth factors in the decellularized matrix by harsh detergents like sodium dodecyl sulfate. 42 Nonionic and mild detergents and modification of decellularization protocols have been employed to improve the quality of decellularized scaffolds. 42 Similarly, modification of decellularized scaffolds with organic and inorganic materials like chitosan and hydroxyapatite has also been explored for improved cell adhesion. 41 Decellularized scaffolds from xenogeneic sources carry a greater risk for pathogenicity and immune reaction. They are often expensive to produce in bulk quantities and need far greater handling and stringent storage requirements compared to synthetic scaffolds.
Synthetic routes for producing biologically active constructs
Deposition of ECM on synthetic scaffolds
A top-down approach for building bioactive and cell instructive biomaterial scaffolds is to create scaffold-ECM hybrid constructs by depositing extracellular matrix secreted by tissue-specific/stem cells on bare biomaterial scaffolds. This approach has been explored recently for example by Thibault et al. in a series of studies. 43,44,45,46 We classified these approaches under the top-down schema, as they represent an approach of building a biologically active milieu on synthetic scaffolds by a lone building block, namely cultured cells 43 under static 44 or engineered conditions. 45,47,48
In contrast to some traditional bottom-up methods of incorporating a single or select few ECM components in the biomaterial scaffolds, this approach focuses on harnessing multiple components of bone ECM, such as fibrous collagen, hydroxyapatite, proteoglycans, and growth factors at once by generating scaffold/extracellular matrix hybrid constructs for BTE. 43
Parameters affecting the deposition of extracellular matrix on synthetic scaffolds
A number of parameters, such as the choice of cells used and the culture conditions (static vs bioreactor or hypoxia vs normoxia), affect the quality of the ECM produced by the cells on the synthetic scaffolds. In one study, Gentleman et al. 49 compared mineralized bone nodules formed from different sources of cells, such as mouse embryonic stem cells (ESCs), neonatal calvarial osteoblasts, and adult bone marrow derived mesenchymal stem cells (MSCs) against native bone. This study found that, while osteoblasts and adult stem cells exhibited bone-specific biological activities and material characteristics similar to native bone, ESCs produced softer bone nodules that are devoid of the nano-level architecture and complex biomolecular and mineral composition noted in the native tissue. In another interesting study, Thibault et al. 44 utilized a factorial design to determine the effect and interactions of four culture factors: (i) the presence of whole bone marrow cells, (ii) the presence of in vitro-generated mineralized ECM, (iii) the presence of dexamethasone, and (iv) variations in culture duration on the proliferation and osteogenic differentiation of MSCs cultured on an electrospun poly(ε-caprolactone) (PCL) scaffold. This study found that alkaline phosphatase (ALP) activity, an earlier marker, and calcium deposition, a late marker for osteogenic differentiation, were higher in the scaffold-ECM hybrid constructs. Similarly the presence of whole bone marrow and dexamethasone and longer culture duration further enhanced osteogenic differentiation of MSCs. In another study, Thibault et al. 46 determined the effect of devitalization and demineralization on the retention of ECM components and the osteogenicity of the scaffold-ECM hybrid constructs. For this study, they generated scaffold-ECM hybrid constructs by culturing osteogenically pre-differentiated MSCs on PCL fiber mesh scaffolds in osteogenic media for 12–16 days within a flow perfusion bioreactor. The resulting constructs were then either devitalized using a freeze-thaw or a detergent technique or devitalized and demineralized or left untreated. The constructs were characterized by DNA, glycosaminoglycan, collagen, and calcium contents. Further, the osteogenic capacity of each construct was determined by culturing MSCs on the constructs for 4, 8, and 12 days in osteogenic medium in a flow perfusion bioreactor. The study reported that, while devitalization by the freeze-thaw method retained the thickest ECM coating with maximum retention of ECM components, combined demineralization and devitalization resulted in lower retention of ECM components and a decrease in osteogenicity.
Culture conditions also significantly affected the quality of deposited ECM on the scaffolds in other scaffold-ECM hybrid constructs. For example, Yeatts et al. developed a tubular perfusion bioreactor system 50 that provided enhanced nutrient transport in three-dimensional scaffolds and simultaneously subjected cell-seeded scaffolds to physiologically relevant shear stress. The authors reported that early osteogenic markers like ALP activity and late osteogenic gene expression pattern of osteocalcin, osteopontin, and bone morphogenetic protein-2 (BMP-2) were increased in cultures of human MSCs in tubular perfusion bioreactors. Moreover, osteogenic marker expression was further increased with an increase in media flow rate. 51 Thibault et al. 45 investigated the temporal composition of an osteogenic ECM generated by MSCs on electrospun biodegradable PCL fiber mesh scaffolds within a flow perfusion bioreactor. Analysis of ECM constructs of different maturities revealed that ECM generated in cultures of shorter duration consisted of a minimal prerequisite protein network, but long term culture durations allowed the ECM to acquire several key components of bone matrix, such as collagen I, hydroxyapatite, matrix remodeling proteins, and regulatory proteins. 45
Generation of devitalized or cell-free scaffold-ECM hybrid constructs
It is often desirable to generate scaffold-ECM hybrid constructs free of living cells, also known as devitalized constructs, for BTE applications.46 Devitalized constructs may be pre-produced and implanted into an individual with minimal concern for immunogenicity. Devitalized constructs require minimal handling and generally need to cross fewer regulatory hurdles as compared to cell-scaffold-ECM constructs for clinical adaptation. Devitalization of cell-ECM constructs is usually achieved by a freeze-thaw method.46
Bottom-up approaches
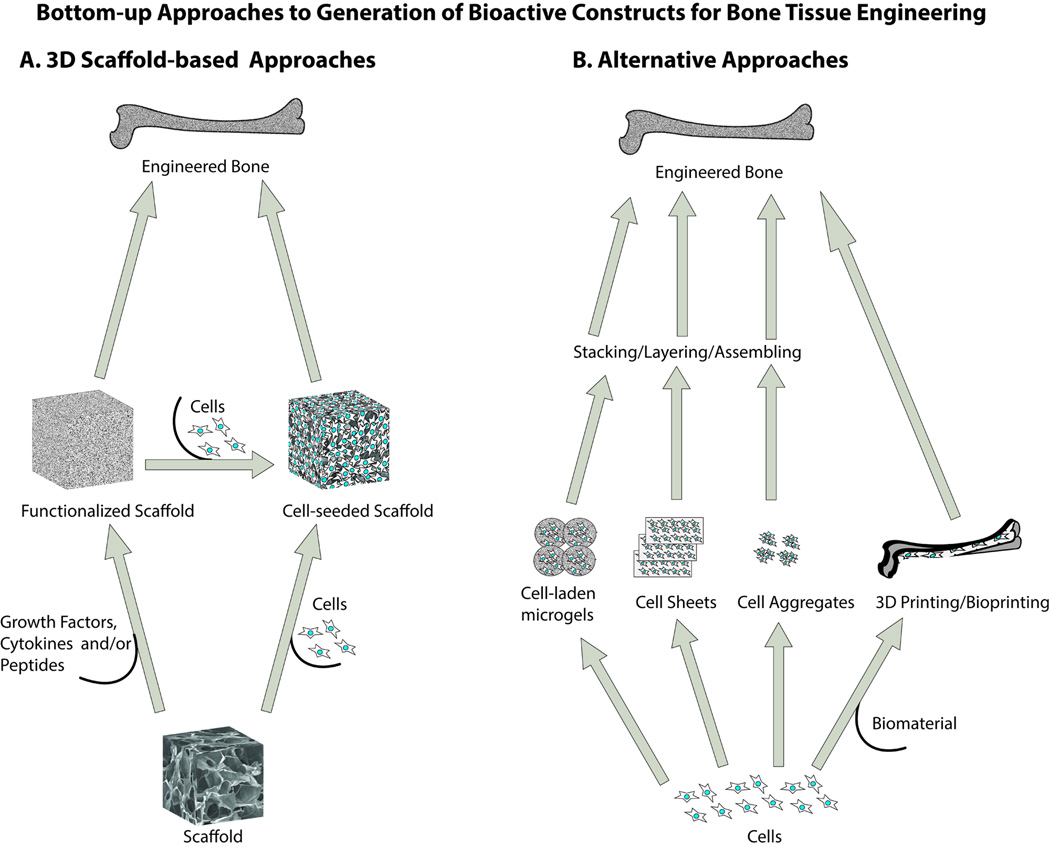
Although the top-down approaches discussed in the previous section enable production of biologically active constructs for bone tissue engineering, the complexity of the whole extracellular matrix component, be it derived from a decellularized tissue or produced by cells in culture, limits control over the composition of the construct. As an alternative, considerable effort has been invested in recent years to functionalize synthetic scaffolds with select ECM components or other biologically active factors, while still retaining the tunability of the synthetic scaffold material. These bottom-up approaches are represented broadly by layered or sequential building or incorporation of functional blocks of the ECM into synthetic scaffolds for bone tissue engineering. Bottom-up approaches can be further sub-classified into traditional scaffold-based approaches and the emerging alternative approaches (Figure 2). The traditional scaffold-based approaches involve strategies for functionalization of three-dimensional synthetic scaffolds with growth factor and/or cytokine immobilization 52,53 and covalent tethering of peptides.54 These local strategies are aimed largely at improving diverse cell functions, such as adhesion, survival, migration, proliferation, and differentiation. While the traditional scaffold-based bottom-up approaches to tissue engineering rely on optimal cell-scaffold interactions in three-dimensional porous scaffolds, it is often difficult to achieve desirable cell penetration, distribution, and tissue-like organization in these traditional approaches. A number of emerging methods like cell sheets, cell aggregates, cell-laden microgels, 3D printing, and self-assembly of peptide amphiphiles are being explored as novel means to micro-architecturally build 3D tissues.55,56 The latter alternative bottom-up approaches can potentially rectify some of the problems associated with traditional scaffold-based approaches. While we have included a brief discussion of the emerging routes for bottom-up approaches, we have omitted detailed discussions, as these techniques have been reviewed extensively elsewhere. 55,57
Figure 2.
Schematic representation of 3D scaffold-based and alternative bottom-up approaches to bone tissue engineering. In the traditional 3D scaffold-based bottom-up approaches, growth factors, peptides, cytokines and/or cells are utilized as building blocks to create a functionalized 3D scaffold that is ultimately used either with or without pre-seeded cells for engineering bone tissue. In the emerging alternative approaches, cell sheets, cell aggregates, cell laden microgels, or 3D/bioprinting technologies are used for assembling/stacking/layering components to generate 3D constructs.
Scaffold-based bottom-up approaches
The following bottom-up methods are primarily based on three-dimensional synthetic scaffolds and involve modification of the scaffolds by incorporation of biologically active agents, such as growth factors and peptides.
Growth Factors
Growth factors and cytokines play important roles in tissue regeneration and development 58,59 as well as adult and embryonic stem cell differentiation.60,61 Often cytokines are secreted in response to tissue injury or a pathological condition, and secreted cytokines then modulate several events as tissue repair occurs. 59,62 Thus, utilization of tissue engineering scaffolds encompassing growth factors in some form can potentially modulate stem cell differentiation and ultimately tissue regeneration and repair.63 Growth factors such as BMPs and TGF-β play prominent roles in orchestrating new bone formation by recapitulating different stages of bone development. 64 Growth factors in free form in solution usually lose their activity very quickly and become unavailable locally over time.52 Growth factors are often found naturally within an ECM microenvironment (e.g., bound to ECM moieties).65 Several strategies are employed to engineer growth factors into tissue engineering scaffolds.52
Presentation of growth factors in various forms
Growth factor adsorption onto synthetic scaffolds
Synthetic scaffolds can often be modified by simple protein adsorption. Adsorption of growth factors to synthetic scaffolds may be influenced by scaffold material properties, such as surface wettability, roughness, charge, charge density, and functional groups. 66 Similarly, growth factor solution properties, such as ionic strength and the presence of other proteins in the media, can significantly impact growth factor adsorption. 66 For example, recombinant human BMP-2 (rhBMP-2) adsorption could be increased by increasing the number of charged moieties on the scaffold and decreasing the ionic strength or increasing the pH of the growth factor solution and increasing the incubation time.67 Often synthetic scaffolds are coated with minerals like hydroxyapatite to enhance growth factor adsorption to the scaffolds.68 The binding strength by physical adsorption is generally lower compared to covalent immobilization, thus it is a limitation when aiming to produce functionalized implants because the binding strength may not be sufficient to keep adsorbed growth factors in place over longer durations. On the other hand, protein or growth factor release from scaffolds onto which it is physically adsorbed may be more efficient compared to immobilized systems in some situations. Coating is a cost-efficient and highly attractive method to deliver ECM proteins and growth factors.69
Direct incorporation of growth factors in synthetic scaffolds
Alternatively, growth factors can be incorporated directly into and released from scaffolds. However, for the purposes of this review, direct incorporation of growth factors into synthetic scaffolds for release will not be considered a biological modification of the scaffold itself. Nevertheless, it is worth mentioning briefly that there are several challenges associated with growth factor incorporation/release from synthetic scaffolds. Growth factor release from hydrogels, for example, is generally dependent on porosity, diffusion, and degradation characteristics of the gel.66 In addition to regular requirements for BTE scaffolds, growth factor-eluting scaffolds additionally need to have both high encapsulation efficiency as well as a controllable release rate allowing a sustained therapeutic dose. 70 It is often difficult to control release kinetics and titration of dosage response for growth factors released after direct incorporation into scaffolds. For example, dosage response of several growth factors like BMP-2 and VEGF is highly sensitive for tissue formation. Toxic effects can be observed due to higher concentration of growth factors released. For example, rhBMP-2 overdose can induce inflammatory and osteoclastic activity.71,72 The interested reader is kindly directed to numerous reviews that have been published on the topic of growth factor release from scaffolds for further information.52,66,70,73
Growth factor delivery via carriers suspended, glued, or tethered to scaffolds
Alternatively growth factors can be released from carriers such as microparticles 74 or nanoparticles 75 suspended (e.g., hydrogels) or glued into synthetic scaffolds (e.g., poly(lactic-co-glycolic acid) (PLGA)-fibrin glue incorporating microparticles). Sequential and timed release of growth factors can also be targeted to recapitulate in vivo conditions. Although, encapsulation of growth factors in micro- and nanoparticles facilitates controlled and sustained release, it is often necessary to load higher doses of growth factors, as the emulsification process used to prepare them can result in low loading efficiency. Indeed, growth factor stability and bioactivity may be severely affected by exposure to harsh processing conditions used for preparation of nanoparticles/microspheres. Another major drawback often associated with the encapsulation of growth factors in nanoparticles/microspheres is the exposure to harmful volatile organic solvents that are commonly employed in their preparation. Water miscible, non-volatile, and less toxic solvents as well as super critical fluids, such as CO2, have also been investigated as alternative solvents for their preparation. Non-toxic solvents such as glycofurol and isosorbide dimethyl ether (DMI) have also been explored. 76
Biodegradable polymers such as PLGA often produce acidic degradation products that reduce local pH, which in turn induces an inflammatory reaction and degradation of growth factors. Strategies such as addition of nanophase titania have been demonstrated to decrease harmful effects of PLGA degradation. 77 While nanoparticles/microspheres could be used in isolation for BTE applications, 52,78,79 for the purpose of this review we focused on composite scaffolds modified with nanoparticles/microspheres for BTE applications.80 For example, functionalized nanoparticles (NPs) with large surface area for grafting have been explored as a delivery platform for rhBMP-2. 81 In this approach, the grafted NPs suspended in the scaffold served as a platform for recruitment and differentiation of the osteoprogenitor bone marrow MSCs. rhBMP-2 was grafted to succinimide-functionalized degradable NPs. rhBMP-2-grafted NPs were as effective as the native protein in stimulating osteogenic differentiation of the osteoprogenitor bone marrow MSCs. 81 Furthermore, rhBMP-2-grafted NPs had higher expression of osteogenic markers osteopontin and osteocalcin compared to the native protein. Higher osteopontin and osteocalcin expression of rhBMP-2-grafted NP groups may also be related to other factors in the cascade of osteogenesis, such as differentiation of the MSCs to the vasculogenic lineage and formation of a vascularized/mineralized matrix.81
Tethering
Growth factors can be tethered to the scaffolds to improve their function over soluble free-form growth factors. 82,83 Growth factor delivery via tethering can be achieved by either tethering with a random orientation or tethering with specific orientation with or without a spacer arm. Tethering sequences with specific orientation or a spacer can confer cell specificity.84 Presentation of growth factors in a tethered manner has proven to permit greater control over temporal and spatial availability in the extracellular environment. Tethering growth factors facilitates ligand to retain significant mobility and active conformation.85 In one elegant study, tethering of TGF-β to poly(ethylene glycol) (PEG) hydrogels promoted chondrogenic differentiation of encapsulated human MSCs. 86
Indirect methods for growth factor immobilization
Growth factors can also be delivered using natural mechanisms of growth factor binding to ECM. For example, sequestration of growth factors by sulphated glycosaminoglycans (GAGs) in vivo not only protects them from degradation but also presents them to cell surface receptors. 87 The interactions between ECM and growth factors are often essential for physiological effects of growth factors. 88 For example, the presence of heparin-binding domains in certain growth factor molecules is crucial to mediate specific interactions with the ECM. FGF-2 requires heparin sulphate binding for dimerization and receptor activation. 89 Presentation of growth factors in a spatial manner can be regulated by ECM by controlling the extent of binding of growth factors to ECM. Growth factors that exhibit ECM-binding domains frequently are present in spatio-temporal gradients that provide essential cues to elicit specific cellular responses. 90 In contrast, growth factors lacking ECM binding capabilities are highly diffusible in tissues. To take advantage of this phenomenon, synthetic hydrogel scaffolds are often modified by chemical functionalization of heparin, chondroitin sulfate, or hyaluronic acid moieties to incorporate GAG-like functional domains by thiol-acrylate or thiol-maleimide Michael addition, specific binding, amine-carboxyl conjugation, and copolymerization.91,92,93,94,95,96,97,98
Growth factors for stem cell survival
Growth factors and growth factor-derived peptides have also been successfully employed for improving stem cell survival in a number of tissue engineering applications. The rationale for their use stems from the fact that they confer resistance to cell death at the implantation site caused by hypoxia, serum deprivation, and reactive oxygen species accumulation. Cell death is thought to be mediated via inflammatory cytokines such as FasL or via Caspase-3. Pretreatment of MSCs with growth factors such as VEGF and EGF or covalent tethering of peptides derived from these growth factors to biomaterial surfaces has demonstrated survival benefit to stem cells. For example, Fan et al. investigated the effect of covalent tethering of EGF to a biomaterial surface on MSC cell survival after implantation.99 These investigators reported that surface-tethered EGF promoted cell adhesion, cell spreading, and MSC survival compared to soluble EGF. Tethering of EGF conferred resistance to cell death, which was induced by the pro-inflammatory cytokine Fas ligand. They concluded that tethered EGF offered a protective advantage to MSCs in vivo during acute inflammatory reactions to tissue engineering scaffolds. 99
Growth factors for angiogenesis and chemotaxis
Multiple angiogenic factors can be delivered for some BTE applications. For example, Phipps et al. reported that PDGF-BB coated PCL/collagen/hydroxyl apatite scaffolds were able to induce significant MSC chemotaxis and recruitment thus facilitating new bone formation. Moreover, incorporation of native bone molecules, collagen I and nano-hydroxyl apatite into electrospun scaffolds enhanced both MSC adhesion and proliferation in addition to the amount of PDGF-BB that could be delivered from these scaffolds.100
In another example, Tengood et al. reported that bFGF and PDGF can be delivered sequentially to promote angiogenesis. While bFGF plays a significant role in the sprouting of new capillaries, PDGF plays a role in the recruitment of mural cells, which stabilize neovessels. Sequential delivery of growth factors was necessitated since these two growth factors have been demonstrated to inhibit each other when presented together. Sequential delivery promoted endothelial cell migration and co-localization of endothelial cells and vascular pericytes. More importantly, this delivery strategy was able to induce red blood cell-filled neovessels, suggesting integration of angiogenesis with the existing vasculature. 101
Park et al. utilized 3D printing technology to deliver dual growth factors (BMP-2 in peripheral zone and VEGF in central zone) with spatial and temporal control to achieve prevascularized bone tissue. Microvessels were newly formed in the central hypoxic area printed with VEGF, and angiogenesis from host tissue was also observed. It was shown that bone regeneration was faster in prevascularized structures than in nonvascularized structures.102
Gradients of ECM peptides and growth factors
Often gradients of growth factors and peptides are created in hydrogels to modulate cell-scaffold interactions for certain tissue engineering applications. Although hydrogels generally cannot replicate the load bearing capacity of bone, they are an attractive option for BTE scaffolds in some applications because of the wide choice of chemical and surface functionalizations that can be imparted and the capacity to support encapsulation of cells. 103,104,105 Molecular weights, cross linking density, and degradation kinetics can all be varied to obtain scaffolds tailored for different applications.104 However, in certain situations hydrophilicity and the biologically inert nature of the synthetic hydrogel scaffolds needs modifications to optimize cell-scaffold interactions. 106 Philippi et al. used a gradient of BMP-2 on fibrin films to demonstrate that growth factor can modulate differentiation lineage of muscle derived stem cells.107 Muscle derived stem cells could be differentiated under myogenic conditions to osteogenic lineage on gradient gels in the presence of rhBMP-2, whereas the lack of rhBMP-2 facilitated differentiation towards the myogenic lineage. 107 Hydrogels incorporating relevant gradient cues (mechanical, chemical, and biological) are often created for the regeneration of complex tissues such as interfacial zones (e.g., muscle-tendon junctions and the bone-cartilage interface) to create functional grafts with clinical applicability. Although fibrin does not represent a synthetic substrate, the study demonstrates the potential biological effects of presentation of a gradient of a growth factor toward inducing an osteogenic response.
Bioactive peptides
Bioactive peptides derived from growth factors and ECM molecules may be used in place of the whole molecules to functionalize synthetic scaffolds. Scaffolds functionalized with peptides can transduce intracellular signals on stem and progenitor cells and promote osteogenic differentiation by inducing differentiation marker genes in osteoblasts. The utilization of peptides over growth factors in regenerative therapy has several advantages in terms of overcoming possible immunogenicity, lowering susceptibility to degradation, and tumor-related side effects.108
Peptides derived from ECM molecules
While early work used long chains of ECM molecules such as collagen, bone sialoprotein, fibronectin, and vitronectin to coat biomaterial surfaces, 109 more recent work relied on peptides derived from ECM molecules for functionalization of BTE scaffolds. Some of the common peptides used for functionalization of BTE scaffolds include arginine-glycine-aspartic acid (RGD) sequence,110 tyrosine-isoleucine-glycine-serine-arginine (YIGSR) and isoleucine-lysine-valine- alanine-valine (IKVAV) in laminin,111 arginine-glutamate-aspartate-arginine-valine (REDRV) and leucine-aspartic acid-valine (LDV) in fibronectin 112, aspartate-glycine-glutamate-alanine (DGEA) in collagen I, and various heparin-binding domains. 113
Signaling domains from ECM protein chains primarily interact with cell membrane receptors. Their short peptide fragments have been used for surface modification in numerous studies. 114 For example, Bhatnagar et al. identified a potent cell-binding domain P15 (766GTPGPQGIAGQRGVV780) from collagen I, that supported ECM synthesis.115 Similarly GFOGER (another collagen I-derived peptide)-modified surfaces supported the expression of multiple osteoblast specific genes, including osteocalcin and bone sialoprotein, and induced matrix mineralization in a manner similar to type I collagen. 116,117 Osteopontin, a major constituent of non-collagenous proteins in bones and teeth, has also been used to derive peptides that play an important role in binding to collagen and inducing biomineralization processes similar to osteopontin.118,119 Lee et al. showed that a collagen-binding motif (CBM, GLRSKSKKFRRPDIQYPDATDEDITSHM) identified from osteopontin was able to specifically bind collagen without chemical conjugation and presented apatite forming capability in vitro and in vivo. 120
Bone sialoprotein (BSP) another major non-collagenous protein in bone has been used to generate BSP fragments. BSP fragments (RGD and non-RGD-containing) have mediated the attachment of primary human osteoblast-like cells. 121 Another peptide sequence derived from BSP, phenylalaninehistidine-arginine-arginine-isoleucine-lysine-alanine (FHRRIKA), was recently identified as interacting with transmembrane proteoglycans and the heparin binding domain.122,123 Rezania et al. showed that the combination of two different peptide sequences, RGD and FHRRIKA, could potentially result in enhanced osteoblast adhesion, spreading, and amount of mineralized tissue formed. The authors have demonstrated that utilizing peptide sequences incorporating both cell- and heparin-adhesive motifs can enhance the degree of cell-scaffold interactions and enhance mineralization of ECM in vitro.123 A motif in the KRSR tetra-peptide, found in different ECM proteins, has been proposed as a heparin-sulfate binding peptide that promotes the adhesion of osteoblasts.124 The immobilized KRSR included a terminal glycine spacer (KRSRGGG) that was shown to mediate osteoblast adhesion. Osteoblast adhesion to the RGDS peptide, which lacked the terminal glycine spacer, was demonstrated to be sterically hindered.
Literature evidence suggests that biomaterial surfaces comprising RGD not only promote cell attachment but may also enhance other fundamental cellular functions. For example, mineralization was enhanced in osteoblasts cultured on an integrin binding surface composed of RGD. 124 Mertz et al. have demonstrated that heterogeneous mimetic peptide surfaces containing both the RGD and the FHRRIKA (putative heparin-binding) peptides at a ratio of 75:25 or 50:50 were biologically relevant for rat calvarial osteoblast cell function. The RGD signal was required to promote formation of focal contacts and cytoskeletal organization. 125
Peptides derived from growth factors
Peptides derived from growth factors such as BMP-2 and FGF-2 have also demonstrated beneficial effects in BTE applications. Saito et al. demonstrated that a BMP-2 peptide derived from 73–92 amino acid residues of the knuckle epitope of rhBMP-2 promoted matrix mineralization.126 Lee et al. demonstrated the existence of two heparin-binding domains in FGF-2. Both the residues, 105 to 111 (F105, YKRSRYT) and 119 to 135 (F119, KRTGQYKLGSKTGPGQK) interacted with cell-surface heparin sulfate proteoglycans. 127 Cell binding activity of heparin binding peptides was proven to have significant applications in cell and tissue engineering research.
Combinations of peptide and growth factor tethering
To mimic the complexity of the tissue microenvironment, smart biomaterial scaffolds often include multiple growth factors, cytokines, and other important tissue-specific factors presented in a spatially and temporally controlled manner. Components of the multiple delivery systems may promote and accelerate vascularization and tissue regeneration. For example He et al. generated auto-inductive bone grafts by grafting the integrin-binding cell adhesion peptide RGD and the osteoinductive BMP peptide sequence to an “inert” degradable hydrogel. 128 Hydrogels used in this study were made of poly(lactide-co-ethylene oxide fumarate) (PLEOF) macromer, low-molecular-weight poly(L-lactide) (LMW PLA) and PEG blocks linked by unsaturated fumarate units. 128,129 RGD peptide was coupled to the scaffold by the reaction between the acrylate functional group of the peptide and the fumarate groups of the scaffold. The BMP peptide sequence was grafted to the scaffold by the click reaction between the azide functional group of the peptide and the propargyl groups of the scaffold. This study also compared the effect of RGD conjugation and the BMP peptide sequence grafting on the osteogenic potential by measuring ALP activity and calcium content with incubation time. MSCs cultured on RGD-conjugated, BMP peptide sequence-grafted, and RGD+BMP peptide-modified hydrogels displayed 3, 2.5, and 5-fold increase in ALP activity, respectively, after 14 days of incubation. Similarly, MSCs seeded on RGD+BMP peptide-modified hydrogels displayed 4.9- and 11.8-fold increase in calcium content after 14 and 21 days, respectively, which was significantly higher than RGD-conjugated or BMP peptide sequence-grafted hydrogels. These results suggest that the BMP and the RGD peptides, grafted to the scaffold, synergistically enhanced osteogenic differentiation and mineralization of the MSCs derived from bone marrow compared to either one alone. 128Additionally auto-inductive scaffolds for BTE have also been generated by a combination of peptide tethering and protein grafted nanoparticle-mediated release of growth factors. 130 This can be considered as a multicomponent bottom-up approach.
In situ tissue regeneration by scaffolds incorporating growth factors, chemokines, and tissue-specific factors
A number of bioactive molecules including growth factors and chemotactic agents are used for in situ tissue engineering.131 In situ tissue regeneration works by recruitment of host stem cells and it eliminates the need to culture, expand, and handle stem and progenitor cells ex vivo. This concept takes advantage of the body’s own regenerative capacity and the host ability to recruit and mobilize endogenous stem cells to the injury site. Usually biomaterial implantation in the body leads to some tissue damage and infiltration of host cells into the implanted scaffold. This infiltration is generally assumed to be inflammatory and fibroblastic. Fibroblasts are the predominant cell population after the initial inflammation has subsided. However, a tissue-specific biomaterial scaffold can be employed to direct recruitment of tissue-specific stem cells. 131 In this situation, the patient’s body not only acts as a source of stem/progenitor cells but also provides an environment for differentiation of these cells. However, biological cues are often required from the biomaterial to direct cell recruitment and differentiation. Chemotaxis plays an important role in recruiting stem cells. Several classes of bioactive molecules like stem cell recruiting factors (e.g., SDF-1), collagen synthatase inhibitors (e.g., metalloproteinase inhibitor), tissue enhancing factors (e.g., TGF-β, IGFs, EGF), angiogenic factors (e.g., VEGF, FGF-2) and innervation factors (e.g., BDNF, GDNF, NGF, and Agrin) are employed to further functionalize scaffolds and to modulate cell-scaffold interactions and tissue regeneration.
Coating BTE scaffolds with collagen and calcium phosphate
Since natural bone is made up of hydroxyapatite and collagen, hydroxyl apatite and other calcium phosphate salts have been used to modify both natural and synthetic scaffolds for BTE. 132,133,134 In one study, Zhao et al. reported a method for uniform coating of calcium phosphate onto electrospun keratin–PCL composite scaffolds (keratin–PCL). 135 Incorporation of keratin with the PCL decreased its solubility and facilitated homogeneous coating within a short time frame (~ 10 min) by immersing the scaffolds into Ca2+ and (PO4)3− solutions separately. The authors reported that incorporation of keratin into PCL scaffolds provided nucleation sites for Ca2+ adsorption and subsequent homogeneous calcium phosphate surface deposition. The mineralization rate was dependent on the amount of calcium phosphate available on scaffold surfaces. Additionally, the presence of keratin and calcium phosphate further increased the mechanical strength of the resultant scaffolds.135 Nanostructured calcium phosphate coatings and composites and with natural and synthetic polymeric scaffolds can influence stem cell interactions with the scaffolds and enhance osteogenesis and osteointegration.136
Alternative bottom-up approaches
Microfabrication methods for scaffold modification
Tissue regeneration usually involves interaction of multiple cell types and ECM components at the microscale and nanoscale. Thus modulation of the scaffold microarchitecture using microfabrication methods is a potent way of creating biomimetic tissues. The microfabrication methods for scaffold production often rely on recreating organ-specific tissue microarchitecture. However, given the complexity of 3D tissues and organs, these methods need to recapitulate spatio-temporal and microenvironmental factors such as physical forces and chemical cues. 55 Strategies for incorporation of micro features in engineered scaffolds in a controlled manner include photolithographic approaches, electrospinning, micromolding, embossing, and rapid prototyping methods.57,137,138
General limitations of traditional scaffold-based approaches include inhomogeneous distribution of cells and insufficient vasculature growth after implantation of the scaffolds. Novel alternative bottom-up approaches help to overcome these limitations by eliminating the need to seed pre-formed 3D porous scaffolds. Instead, they rely on stacking/assembling or layering cell-seeded constructs to generate 3D tissues and organs. 55 The alternative bottom-up approaches may be employed for the construction of multi-layer 3D scaffolds using microfabrication methods from 2D membranes. For example, Lima et al. demonstrated that PCL and starch-PCL (SPCL; 30 % starch) blended sheets could be used as substrates to produce the microfabricated membranes using micro hot embossing technology. 138Assembly of the microfabricated membranes was performed using successive stacking. These microfabricated membranes supported cell attachment and the cytoskeletal organization of human bone marrow stem cells (hBMSCs), macrovascular endothelial cells, and osteoblasts derived from hBMSCs. Furthermore, hBMSCs proliferated and maintained the expression of the stromal progenitor marker STRO-1 when cultured on both PCL and SPCL microfabricated membranes. 138
Directing stem cell fate using micro engineered platforms
Micro engineered platforms may enable regulation of the stem cell fate decisions and aid in investigation of cellular behaviors through interaction in different microenvironments. 139
Kachouie et al. described a number of approaches for using directed assembly to build tissue-like constructs with well-defined macroscale architectures from cell-laden microgels. 57 For example, the directed assembly of cell-laden microgels can be achieved by harnessing the surface tension characteristics of hydrophilic hydrogels in a two-phase oil-aqueous solution reactor.140 However, there are certain disadvantages in these techniques, such as the low efficiency of cell attachment to fibrous scaffolds and the weak strength of the gel system. Photopatterning of 3D cell-laden hydrogels is still another effective technique for creating microscale building blocks for constructing macroscale structures with microscale resolution and architecture. 137
Similarly, cell sheet-based techniques are also employed to successively stack and create 3D tissues that can improve the efficacy of cell seeding and reduce the biomaterial related inflammatory response. 141,142,143 However, cell sheets also suffer from certain disadvantages. In addition to low physical load bearing capacity, a multilayered cell sheet may lead to cell necrosis due to poor nutrition or hypoxia in the middle layers. Additionally, potential ischemia in vivo may limit cell sheet survival. Hypoxia pretreatment can potentially increase the survival rate of implanted MSCs and may promote angiogenesis in vivo. 142 Nevertheless, cell sheet techniques may be used either alone or in combination with scaffold materials to generate implants that exhibit osteogenic and vascularization capabilities. 144 For example, an MSC cell sheet technique may enhance cell-cell and cell-scaffold interactions by promoting osteoblasts attachment to the mineralized layered cell sheet and may mimic the in vivo deposition of bone matrix. 145
Self-assembling peptides
Scaffolds made from the self-assembling RAD16-I peptide have been employed as BTE scaffolds because of its nanostructure, biomechanical properties, and its commercial availability as Pura Matrix TM (3DM, Inc., Cambridge, MA, USA).146 The amino acid sequence of RAD16-I peptide is RADARADARADARADA (R, arginine; A, alanine; D, aspartic acid). Small (3 mm) bone defects in mice calvaria treated with application of RAD16-I promoted bone regeneration by inducing the expression of osteogenic genes such as alkaline phosphatase, Runx2, and Osterix in the cells of adjacent tissues. 147
Peptide amphiphiles
Recently, peptide amphiphile-based molecules have gained prominence as they are used as building blocks to create supramolecular nanostructures that can emulate both the architecture and the chemistry of the ECM and they are designed to degrade to harmless products.56,148 These bioactive matrices can either bind or mimic growth factors or other protein ligands to elicit a cellular response, promote specific mechano-biological responses, and also guide the migration of cells with programmed directionality. 56
Hartgerink et al. demonstrated that a nanostructured fibrous scaffold created by pH-induced self-assembly of peptide amphiphiles facilitated modulation of structural integrity of the matrix by reversible cross-linking of nanofibers. Cross-linking of the nanofibers facilitated direct mineralization of hydroxyapatite to form a composite material in which the crystallographic c axes of hydroxyapatite are aligned with the long axes of the fibers. This alignment was similar to that observed between collagen fibrils and hydroxyapatite crystals in bone. 149
Sargeant et al. reported that PA based self-assembled nanofibers incorporating phosphoserine residues promoted hydroxyapatite formation in calcium-supplemented osteogenic media. Similarly addition of RGDS-bearing PA nanofibers up to 5% by weight promoted cell adhesion without affecting mineral formation. The mineralized nanofibers also promoted osteogenic differentiation of mesenchymal stem cells. 150
Newcomb et al. reported the mineralization of supramolecular peptide amphiphile templates that varied in nanoscale morphology by altering the amino acid sequences. The authors found that the geometric constraints associated with the morphology of the nanostructures controlled hydroxyapatite nucleation and growth, and only aligned gel templates of cylindrical nanostructures lead to hierarchical control over hydroxyapatite orientation across multiple length scales as found in bone. 151
For example, self-assembling peptide nanostructures synthesized with a combination of BMP receptor binding peptides (also known as osteopromotive domains) and hydrophobic alkyl chains were investigated as three-dimensional scaffolding materials for osteoblastic differentiation. 152
Lee et al. reported that a supramolecular nanofiber network of a heparin-binding PA system could be used to enhance retention and amplification of the regenerative capacity of heparin-binding growth factors such as BMP-2, TGF-β, VEGF, and PDGF. They demonstrated the utility of the system by enhanced bone regeneration capability at a fraction of the BMP-2 dose required by emulating the role of syndecan and fibronectin of the ECM. 153
In a follow up study, Lee et al. demonstrated the utilization of bioactive PA nanofibers with binding affinity for BMP-2 to create a gel scaffold for osteogenesis in a rat posterolateral lumbar intertransverse spinal fusion model. Bioactive BMP-2-binding PA nanofibers exhibited superior spinal fusion rates relative to controls, and reduced clinical doses of BMP-2 by 10 to 100 times lower than that used in collagen sponges. Additionally bioactive nanofibers were able to recruit endogenous growth factor in the absence of exogenous growth factor and contributed to a 42% fusion rate. 154
3D/Bioprinting of biological elements into scaffolds
3D/Bioprinting is emerging as a novel platform technology for functionalization of synthetic scaffolds for BTE.155 Bioprinting was performed utilizing growth factors, peptides (peptide bioink), and ECM components, such as decellularized ECM powder as bioink.69 Bioprinting offers the possibility to control the orientation and differentiation of cells utilizing geometric cues that mimic the structural aspects of native ECM and biochemical cues that mimic ECM bound growth factors. In one study, Ker et al. recently reported that mouse C2C12 myoblasts or C3H10T1/2 mesenchymal fibroblasts seeded on oriented sub-micron fibers functionalized with printed growth factors (bFGF-2 or BMP-2) facilitated tenocyte or osteoblast phenotype, respectively, but promoted myocyte phenotype in the absence of printed growth factors. Additionally, the printed pattern allowed cell alignment along the fibers of the scaffold. 156
Also peptide-based bioinks are emerging as a novel means to synthesize or functionalize scaffolds. For example, lysine-containing hexapeptides that self-assemble into stable, nanofibrous three-dimensional hydrogels with stiffness values up to 40 kPa were described by Loo et al.157 These biocompatible scaffolds supported the three-dimensional culture of human stem cells and differentiation of primary cells into organotypic (gastrointestinal and skin) structures for high-throughput screening, diagnosis, and tissue engineering applications.
Functionalized synthetic scaffolds are also finding applications in drug delivery 69 and many tissue engineering applications. They also serve as potential in vitro model systems for screening drugs, predicting cancer metastasis 158,159 and for elucidation of biological mechanisms.
Hybrid approaches for BTE scaffold modification
Although we have classified approaches to functional modification of scaffolds primarily into distinct top-down and bottom-up approaches, often hybrid approaches combining aspects of both of these strategies are also employed in the tissue engineering research community. Given below are some examples of these approaches.
Modification of synthetic polymer scaffolds with components of decellularized tissues
Since decellularized tissue contains several proteins and minerals found in the biological tissue from which it is obtained, it is usually expected to promote tissue-specific cell-scaffold interactions. 160,161 To take advantage of this property, decellularized tissues can be powdered and mixed with synthetic polymeric materials during scaffold formation to confer tissue-specific bioactivity to synthetic scaffolds. This hybrid approach employs a combination of the top-down (decellularized tissue) and bottom-up (incorporating a bioactive component into a synthetic scaffold) approaches for the modification of synthetic scaffolds. However, processes such as decellularization and devitalization may denature proteins and significantly affect biological activity of growth factors. The interested reader is encouraged to consult review articles focused on the topic for additional detail.162, 163
MSC cell sheets for bone regeneration
Another example of combining top-down and bottom-up approaches is cell sheet-scaffold hybrid techniques to bone tissue regeneration that have recently been reviewed by Chen et al.142 For example, Ouyang et al. revitalized cryopreserved demineralized bone grafts (top-down approach) by wrapping them with human MSC cell sheets (a bottom-up approach) and culturing for 3 weeks. The MSC cell sheet in this set up was analogous to periosteum and could be differentiated into the osteochondral lineage. 164Additionally, Ouyang et al. used cell sheets of bMSCs to assemble on a knitted poly(L-lactide) (PLLA) scaffold for engineering ligament analogs. 165
Ma et al. 166demonstrated that demineralized bone matrix (DBM)/MSC cell sheet promoted greater bone formation and healing of critical-size rabbit calvarial defects at 6 and 12 weeks after implantation compared to DBM alone.
In another interesting example, Qi et al. 167combined a growth factor (rhBMP-2), an inorganic material (calcium sulfate), and an MSC cell sheets to repair ulnar segmental defects in rabbits. The defects treated with MSC sheet/rhBMP-2-loaded calcium sulfate showed significantly higher bone formation as determined by histology and microcomputed tomography.
Summary
Top-down approaches have the potential to recapitulate in vivo (tissue-like) conditions more closely than bottom-up approaches. However, it is often difficult to achieve uniform cell distribution in decellularized and demineralized scaffolds. Cell migration, proliferation, and differentiation may be suboptimal in decellularized/demineralized BTE scaffolds due to inactivation or reduction of biological activity due to processing conditions. In the case of scaffold-ECM hybrid constructs, complete characterization of cell-deposited ECM is challenging. For the purpose of this review article, we have considered cell-generated ECM on synthetic scaffolds as the top of the hierarchy for the top-down approach. However, a true top of the hierarchy could be the tissue itself that needs to be regenerated. In consideration of this point, a decellularized or a demineralized bone matrix could as well be considered the top of the hierarchy of a BTE scaffold. Additionally, consideration should be made of the differences in the ECM that is left over after the decellularization of bone and the cell-generated ECM, although recognizing that a decellularized or demineralized ECM derived from native bone tissue is not a synthetic scaffold. However, we mooted these points to give a holistic perspective on various approaches to functionalization of the scaffolds and the similarities and dissimilarities between decellularized and cell generated scaffold-ECM hybrid constructs.
Traditional growth factor/peptide-based modification of BTE scaffolds has shown great promise when used for functionalization of synthetic scaffolds in improving diverse cell functions, such as adhesion, migration, survival, proliferation, differentiation, and biomineralization in BTE applications. However, presentation of these factors in synthetic scaffolds to achieve controlled and user-defined concentration ranges, preserving their active conformation and thus bioactivity need careful consideration for the success of these approaches. Similarly, restricting the signaling of growth factors, cytokines, and peptides to the stem and progenitor cell compartment alone for an intended duration of time or until tissue regeneration has occurred and preventing adverse side effects due to non-specific interactions need further fine tuning to obtain desirable outcomes.
Although, these traditional methods for BTE modification have the advantage that they can be utilized for investigating the effect of individual components of ECM on bone tissue regeneration, they often fail to replicate fully the intricate signaling that a complete ECM may be able to provide in driving the desired cell-scaffold interactions for BTE. The use of combinations of growth factors, peptides, and their presentation in a temporal and spatial manner, and approaches for building layered or sequential addition of individual components of an ECM progressively, until one achieves a minimal essential matrix may be a more appropriate strategy to achieve optimal cell-scaffold interactions and ultimately tissue regeneration response.
Emerging alternative bottom-up approaches such as cell sheets, cell aggregates, cell-laden microgels, 3D printing, and self-assembly of peptide amphiphiles are being explored as novel means to micro-architecturally build 3D tissues. The latter bottom-up approaches can potentially rectify some of the problems associated with traditional scaffold-based approaches. Hybrid approaches employing traditional and emerging methods such as cell sheet-scaffold hybrids have shown great potential for BTE scaffold modification. Nevertheless, several issues related to cell survival and optimal delivery needs further improvement.
Challenges and opportunities
The key challenges in biological modification or functionalization of synthetic scaffolds include modularization, standardization, and integration of these biological parts into scaffolds with desired functions.168 Additionally, challenges are manifested at every step in the process of adding or improving an existing biological functionality. Often times biological modifications are not modular, in that components cannot be added or removed at will. Also differences in function can occur based on the sequence of addition of functional blocks to a synthetic system. Definition of what constitutes a standardized system is often necessary, and systems need to be generated with reproducible quality to predict responses on a consistent basis. For example, cell-generated scaffold-ECM hybrid constructs can vary significantly based on the conditions utilized to generate these constructs.
In spite of the many challenges, innovations in chemical engineering, protein biochemistry, and materials science are providing exciting opportunities for modification of synthetic scaffolds to more closely mimic native bone tissue from a material and biological standpoint. Self-assembling peptide amphiphiles and many more such innovations will drive the field in the years to come. A fruitful collaboration between materials scientists, chemical engineers, and biologists will be the key to success and novel inventions in this field.
Supplementary Material
Acknowledgments
This work was supported in part by a grant from the National Cancer Institute of the National Institutes of Health under Award Number R01 CA180279. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Clarke B. CJASN. 2008;3:S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sims NA, Martin TJ. BoneKEy Rep. 2014;3:481. doi: 10.1038/bonekey.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oryan A, Alidadi S, Moshiri A. Hard Tissue. 2013:2. [Google Scholar]
- 4.Salgado AJ, Coutinho OP, Reis RL. Macromol Biosci. 2004;4:743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 5.Dimitriou R, Jones E, McGonagle D, Giannoudis PV. BMC Medicine. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aronson J. J Bone Joint Surg Am. 1997;79:1243–1258. doi: 10.2106/00004623-199708000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Mendenhall WM. JCO. 2004;22:4867–4868. doi: 10.1200/JCO.2004.09.959. [DOI] [PubMed] [Google Scholar]
- 8.Lyons A, Ghazali N. British Journal of Oral and Maxillofacial Surgery. 2008;46:653–660. doi: 10.1016/j.bjoms.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Rayatt SS, Mureau MAM, Hofer SOP. Indian Journal of Plastic Surgery. 2007;40:65. [Google Scholar]
- 10.DiGiovanni CW, Patel A, Calfee R, Nickisch F. J Am Acad Orthop Surg. 2007;15:208–217. doi: 10.5435/00124635-200704000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Reed Aa. C, Joyner CJ, Brownlow HC, Simpson AHRW. J. Orthop. Res. 2002;20:593–599. doi: 10.1016/S0736-0266(01)00142-5. [DOI] [PubMed] [Google Scholar]
- 12.Giannoudis P, Tzioupis C, Almalki T, Buckley R. Injury. 2007;38:S90–S99. doi: 10.1016/j.injury.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Amini AR, Laurencin CT, Nukavarapu SP. Crit Rev Biomed Eng. 2012;40:363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. PLoS Med. 2007:4. doi: 10.1371/journal.pmed.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercado-Pagán ÁE, Stahl AM, Shanjani Y, Yang Y. Ann Biomed Eng. 2015;43:718–729. doi: 10.1007/s10439-015-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biomaterials. 2006;27:3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 17.Szpalski C, Wetterau M, Barr J, Warren SM. Tissue Engineering Part B: Reviews. 2011;18:246–257. doi: 10.1089/ten.TEB.2011.0427. [DOI] [PubMed] [Google Scholar]
- 18.Bose S, Roy M, Bandyopadhyay A. Trends Biotechnol. 2012;30:546–554. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Place ES, George JH, Williams CK, Stevens MM. Chem. Soc. Rev. 2009;38:1139–1151. doi: 10.1039/b811392k. [DOI] [PubMed] [Google Scholar]
- 20.Mano J, Silva G, Azevedo H, Malafaya P, Sousa R, Silva S, Boesel L, Oliveira J, Santos T, Marques A, Neves N, Reis R. Journal of the Royal Society Interface. 2007;4:999–1030. doi: 10.1098/rsif.2007.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khang G, Lee SJ, Han CW, Rhee JM, Lee HB. In: Tissue Engineering, Stem Cells, and Gene Therapies. Elçin YM, editor. US: Springer; 2003. pp. 235–245. [Google Scholar]
- 22.Oliveira, Miyazaki T, Lopes MA, Ohtsuki C, Santos JD. Journal of Materials Science: Materials in MedicineJ. Mater. Sci. Mater. Med. 2005;16:253–259. doi: 10.1007/s10856-005-6687-y. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Stewart RJ, Kopecek J. Nature. 1999;397:417–420. doi: 10.1038/17092. [DOI] [PubMed] [Google Scholar]
- 24.Sheikh FA, Ju HW, Moon BM, Lee OJ, Kim J-H, Park HJ, Kim DW, Kim D-K, Jang JE, Khang G, Park CH. J Tissue Eng Regen Med. 2015 doi: 10.1002/term.1989. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Holzwarth JM, Ma PX. Macromol. Biosci. 2012;12:911–919. doi: 10.1002/mabi.201100466. [DOI] [PubMed] [Google Scholar]
- 26.Guo B, Lei B, Li P, Ma PX. Regenerative Biomaterials. 2015;2:47–57. doi: 10.1093/rb/rbu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan KH, Zhuo S, Ni M. International Journal of Medical Sciences. 2015;12:701–707. doi: 10.7150/ijms.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Damborenea JJ, Larosa MA, Arenas MA, Hernández-López JM, Jardini AL, Ierardi MCF, Zavaglia CAC, Filho RM, Conde A. Materials & Design. 2015;83:6–13. [Google Scholar]
- 29.Yeo GC, Santos M, Kondyurin A, Liskova J, Weiss AS, Bilek MMM. ACS Biomater. Sci. Eng. 2016 doi: 10.1021/acsbiomaterials.6b00049. [DOI] [PubMed] [Google Scholar]
- 30.Yun H, Kim, Khang D, Choi Kim. International Journal of Nanomedicine. 2011:2521. doi: 10.2147/IJN.S25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pilia M, Guda T, Appleford M, Pilia M, Guda T, Appleford M. BioMed Research International, BioMed Research International. 2013;2013:e458253. doi: 10.1155/2013/458253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerhardt L-C, Boccaccini AR. Materials. 2010;3:3867–3910. doi: 10.3390/ma3073867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen QZ, Thompson ID, Boccaccini AR. Biomaterials. 2006;27:2414–2425. doi: 10.1016/j.biomaterials.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 34.Haimi S, Suuriniemi N, Haaparanta A-M, Ellä V, Lindroos B, Huhtala H, Räty S, Kuokkanen H, Sándor GK, Kellomäki M, Miettinen S, Suuronen R. Tissue Eng Part A. 2009;15:1473–1480. doi: 10.1089/ten.tea.2008.0241. [DOI] [PubMed] [Google Scholar]
- 35.Jones JR, Lin S, Yue S, Lee PD, Hanna JV, Smith ME, Newport RJ. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine. 2010;224:1373–1387. doi: 10.1243/09544119JEIM836. [DOI] [PubMed] [Google Scholar]
- 36.Einhorn TA, Lane JM, Burstein AH, Kopman CR, Vigorita VJ. J Bone Joint Surg Am. 1984;66:274–279. [PubMed] [Google Scholar]
- 37.Damien CJ, Parsons JR. J Appl Biomater. 1991;2:187–208. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 38.Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Adv. Drug Deliv. Rev. 2012;64:1063–1077. doi: 10.1016/j.addr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen K, Lin X, Zhang Q, Ni J, Li J, Xiao J, Wang Y, Ye Y, Chen L, Jin K, Chen L. Acta Biomater. 2015;19:46–55. doi: 10.1016/j.actbio.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 40.Cunniffe GM, Vinardell T, Murphy JM, Thompson EM, Matsiko A, O’Brien FJ, Kelly DJ. Acta Biomaterialia. 2015;23:82–90. doi: 10.1016/j.actbio.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 41.Gupta SK, Dinda AK, Potdar PD, Mishra NC, Gupta SK, Dinda AK, Potdar PD, Mishra NC. BioMed Research International, BioMed Research International. 2013;2013:e651945. [Google Scholar]
- 42.Benders KEM, van Weeren PR, Badylak SF, Saris DBF, Dhert WJA, Malda J. Trends Biotechnol. 2013;31:169–176. doi: 10.1016/j.tibtech.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Thibault RA, Mikos AG, Kasper FK. Adv Healthc Mater. 2013;2:13–24. doi: 10.1002/adhm.201200209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thibault RA, Scott Baggett L, Mikos AG, Kasper FK. Tissue Eng Part A. 2010;16:431–440. doi: 10.1089/ten.tea.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thibault RA, Mikos AG, Kasper FK. Biomacromolecules. 2011;12:4204–4212. doi: 10.1021/bm200975a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thibault RA, Mikos AG, Kasper FK. J Biomed Mater Res A. 2013;101:1225–1236. doi: 10.1002/jbm.a.34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeatts AB, Fisher JP. Tissue Eng Part C Methods. 2011;17:337–348. doi: 10.1089/ten.TEC.2010.0172. [DOI] [PubMed] [Google Scholar]
- 48.Yeatts AB, Geibel EM, Fears FF, Fisher JP. Biotechnol Bioeng. 2012;109:2381–2391. doi: 10.1002/bit.24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gentleman E, Swain RJ, Evans ND, Boonrungsiman S, Jell G, Ball MD, Shean TAV, Oyen ML, Porter A, Stevens MM. Nat Mater. 2009;8:763–770. doi: 10.1038/nmat2505. [DOI] [PubMed] [Google Scholar]
- 50.Yeatts AB, Fisher JP. Tissue Eng Part C Methods. 2011;17:337–348. doi: 10.1089/ten.TEC.2010.0172. [DOI] [PubMed] [Google Scholar]
- 51.Yeatts AB, Both SK, Yang W, Alghamdi HS, Yang F, Fisher JP, Jansen JA. Tissue Eng Part A. 2014;20:139–146. doi: 10.1089/ten.tea.2013.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee K, Silva EA, Mooney DJ. J R Soc Interface. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vo TN, Kasper FK, Mikos AG. Adv. Drug Deliv. Rev. 2012;64:1292–1309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu J. Biomaterials. 2010;31:4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nichol JW, Khademhosseini A. Soft Matter. 2009;5:1312–1319. doi: 10.1039/b814285h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubert Pérez CM, Stephanopoulos N, Sur S, Lee SS, Newcomb C, Stupp SI. Ann Biomed Eng. 2015;43:501–514. doi: 10.1007/s10439-014-1166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kachouie NN, Du Y, Bae H, Khabiry M, Ahari AF, Zamanian B, Fukuda J, Khademhosseini A. Organogenesis. 2010;6:234–244. doi: 10.4161/org.6.4.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerstenfeld LC, Edgar CM, Kakar S, Jacobsen KA, Einhorn TA. In: Engineering of functional skeletal tissues. Bronner F, Farach-Carson MC, Mikos AG, editors. Vol. 3. London, London: Springer; 2007. pp. 17–45. [Google Scholar]
- 59.Canalis E, McCarthy TL, Centrella M. Annual Review of Medicine. 1991;42:17–24. doi: 10.1146/annurev.me.42.020191.000313. [DOI] [PubMed] [Google Scholar]
- 60.Martin GR. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pittenger MF. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 62.Krafts KP. Organogenesis. 2010;6:225–233. doi: 10.4161/org.6.4.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Discher DE, Mooney DJ, Zandstra PW. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson EE, Urist MR, Finerman GA. Clin. Orthop. Relat. Res. 1988:249–257. [PubMed] [Google Scholar]
- 65.Liu HW, Chen CH, Tsai CL, Hsiue GH. Bone. 2006;39:825–836. doi: 10.1016/j.bone.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 66.King WJ, Krebsbach PH. Adv Drug Deliv Rev. 2012;64:1239–1256. doi: 10.1016/j.addr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friess W, Uludag H, Foskett S, Biron R, Sargeant C. Int J Pharm. 1999;187:91–99. doi: 10.1016/s0378-5173(99)00174-x. [DOI] [PubMed] [Google Scholar]
- 68.McKay WF, Peckham SM, Badura JM. In: Bone morphogenetic proteins: from local to systemic therapeutics. Vukicevic S, Sampath KT, editors. Basel: Birkhäuser; 2008. pp. 7–23. [Google Scholar]
- 69.Hinderer S, Layland SL, Schenke-Layland K. Advanced Drug Delivery Reviews. 2016;97:260–269. doi: 10.1016/j.addr.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 70.Blackwood KA, Bock N, Dargaville TR, Ann Woodruff M, Blackwood KA, Bock N, Dargaville TR, Ann Woodruff M. International Journal of Polymer Science, International Journal of Polymer Science. 2012;2012:e174942. [Google Scholar]
- 71.Carragee EJ, Hurwitz EL, Weiner BK. Spine J. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 72.Toth JM, Boden SD, Burkus JK, Badura JM, Peckham SM, McKay WF. Spine. 2009;34:539–550. doi: 10.1097/BRS.0b013e3181952695. [DOI] [PubMed] [Google Scholar]
- 73.Whitaker MJ, Quirk RA, Howdle SM, Shakesheff KM. J. Pharm. Pharmacol. 2001;53:1427–1437. doi: 10.1211/0022357011777963. [DOI] [PubMed] [Google Scholar]
- 74.King WJ, Toepke MW, Murphy WL. Acta Biomater. 2011;7:975–985. doi: 10.1016/j.actbio.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Panyam J, Labhasetwar V. Adv. Drug Deliv. Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 76.Swed A, Cordonnier T, Fleury F, Boury F. Journal of Nanomedicine & Nanotechnology. 2014:5. [Google Scholar]
- 77.Liu H, Slamovich EB, Webster TJ. Int J Nanomedicine. 2006;1:541–545. doi: 10.2147/nano.2006.1.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gittens SA, Uludag H. J Drug Target. 2001;9:407–429. doi: 10.3109/10611860108998776. [DOI] [PubMed] [Google Scholar]
- 79.Makadia HK, Siegel SJ. Polymers (Basel) 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buket Basmanav F, Kose GT, Hasirci V. Biomaterials. 2008;29:4195–4204. doi: 10.1016/j.biomaterials.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 81.Mercado AE, He X, Xu W, Jabbari E. Nanotechnology. 2008;19:325609. doi: 10.1088/0957-4484/19/32/325609. [DOI] [PubMed] [Google Scholar]
- 82.Suttinont C, Mashimo Y, Mie M, Kobatake E. BioMed Research International. 2015;2015:e208089. doi: 10.1155/2015/208089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Assal Y, Mizuguchi Y, Mie M, Kobatake E. Bioconjugate Chem. 2015;26:1672–1677. doi: 10.1021/acs.bioconjchem.5b00266. [DOI] [PubMed] [Google Scholar]
- 84.Mann BK, Schmedlen RH, West JL. Biomaterials. 2001;22:439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 85.Backer MV, Patel V, Jehning BT, Claffey KP, Backer JM. Biomaterials. 2006;27:5452–5458. doi: 10.1016/j.biomaterials.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 86.McCall JD, Luoma JE, Anseth KS. Drug Deliv. and Transl. Res. 2012;2:305–312. doi: 10.1007/s13346-012-0090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raman R, Sasisekharan V, Sasisekharan R. Chem. Biol. 2005;12:267–277. doi: 10.1016/j.chembiol.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 88.Gama CI. Nature Chem. Biol. 2006;2:467–473. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- 89.Pellegrini L, Burke DF, von Delft F, Mulloy B, Blundell TL. Nature. 2000;407:1029–1034. doi: 10.1038/35039551. [DOI] [PubMed] [Google Scholar]
- 90.Cao L, Mooney DJ. Adv Drug Deliv Rev. 2007;59:1340–1350. doi: 10.1016/j.addr.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benoit DSW, Durney AR, Anseth KS. Biomaterials. 2007;28:66–77. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 92.Tae G, Kim Y-J, Choi W-I, Kim M, Stayton PS, Hoffman AS. Biomacromolecules. 2007;8:1979–1986. doi: 10.1021/bm0701189. [DOI] [PubMed] [Google Scholar]
- 93.Zhang L, Furst EM, Kiick KL. J Control Release. 2006;114:130–142. doi: 10.1016/j.jconrel.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nie T, Akins RE, Jr, Kiick KL. Acta Biomaterialia. 2009;5:865–875. doi: 10.1016/j.actbio.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamaguchi N, Zhang L, Chae B-S, Palla CS, Furst EM, Kiick KL. J. Am. Chem. Soc. 2007;129:3040–3041. doi: 10.1021/ja0680358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cai S, Liu Y, Zheng Shu X, Prestwich GD. Biomaterials. 2005;26:6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 97.Rajangam K, Arnold MS, Rocco MA, Stupp SI. Biomaterials. 2008;29:3298–3305. doi: 10.1016/j.biomaterials.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin C-C, Anseth KS. Adv. Funct. Mater. 2009;19:2325–2331. doi: 10.1002/adfm.200900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fan VH, Tamama K, Au A, Littrell R, Richardson LB, Wright JW, Wells A, Griffith LG. Stem Cells. 2007;25:1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 100.Phipps MC, Xu Y, Bellis SL. PLOS ONE. 2012;7:e40831. doi: 10.1371/journal.pone.0040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tengood JE, Ridenour R, Brodsky R, Russell AJ, Little SR. Tissue Engineering Part A. 2010;17:1181–1189. doi: 10.1089/ten.tea.2010.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park JY, Shim J-H, Choi S-A, Jang J, Kim M, Lee SH, Cho D-W. J. Mater. Chem. B. 2015;3:5415–5425. doi: 10.1039/c5tb00637f. [DOI] [PubMed] [Google Scholar]
- 103.Hoffman AS. Adv. Drug Deliv. Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 104.Drury JL, Mooney DJ. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 105.Park J-B. Med Oral Patol Oral Cir Bucal. 2011;16:e115–e118. doi: 10.4317/medoral.16.e115. [DOI] [PubMed] [Google Scholar]
- 106.Seidi Azadeh, Ostrovidov Serge, Ramalingam Murugan. Biomaterials and stem cells in regenerative medicine. CRC Press; 2012. pp. 55–78. [Google Scholar]
- 107.Phillippi JA, Miller E, Weiss L, Huard J, Waggoner A, Campbell P. Stem Cells. 2008;26:127–134. doi: 10.1634/stemcells.2007-0520. [DOI] [PubMed] [Google Scholar]
- 108.Lee J-Y, Choi Y-S, Lee S-J, Chung C-P, Park Y-J. Current Pharmaceutical Design. 2011;17:2663–2676. doi: 10.2174/138161211797416011. [DOI] [PubMed] [Google Scholar]
- 109.J Cell Biol. 1986;103:2637–2647. doi: 10.1083/jcb.103.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Koivunen E, Wang B, Dickinson CD, Ruoslahti E. Meth. Enzymol. 1994;245:346–369. doi: 10.1016/0076-6879(94)45019-6. [DOI] [PubMed] [Google Scholar]
- 111.Vukicevic S, Luyten FP, Kleinman HK, Reddi AH. Cell. 1990;63:437–445. doi: 10.1016/0092-8674(90)90176-f. [DOI] [PubMed] [Google Scholar]
- 112.Aota S, Nagai T, Yamada KM. J. Biol. Chem. 1991;266:15938–15943. [PubMed] [Google Scholar]
- 113.Dalton BA, McFarland CD, Underwood PA, Steele JG. J. Cell. Sci. 1995;108(Pt 5):2083–2092. doi: 10.1242/jcs.108.5.2083. [DOI] [PubMed] [Google Scholar]
- 114.Shin H, Jo S, Mikos AG. Biomaterials. 2003;24:4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 115.Bhatnagar RS, Qian JJ, Wedrychowska A, Sadeghi M, Wu YM, Smith N. Tissue Eng. 1999;5:53–65. doi: 10.1089/ten.1999.5.53. [DOI] [PubMed] [Google Scholar]
- 116.Reyes CD, García AJ. J Biomed Mater Res A. 2004;69:591–600. doi: 10.1002/jbm.a.30034. [DOI] [PubMed] [Google Scholar]
- 117.Reyes CD, Petrie TA, Burns KL, Schwartz Z, García AJ. Biomaterials. 2007;28:3228–3235. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Denhardt DT, Guo X. FASEB J. 1993;7:1475–1482. [PubMed] [Google Scholar]
- 119.McKee MD, Nanci A. Microsc. Res. Tech. 1996;33:141–164. doi: 10.1002/(SICI)1097-0029(19960201)33:2<141::AID-JEMT5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 120.Lee S-H, Shin H. Advanced Drug Delivery Reviews. 2007;59:339–359. doi: 10.1016/j.addr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 121.Bianco P, Fisher LW, Young MF, Termine JD, Robey PG. Calcif. Tissue Int. 1991;49:421–426. doi: 10.1007/BF02555854. [DOI] [PubMed] [Google Scholar]
- 122.Rezania A, Thomas CH, Branger AB, Waters CM, Healy KE. J. Biomed. Mater. Res. 1997;37:9–19. doi: 10.1002/(sici)1097-4636(199710)37:1<9::aid-jbm2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 123.Rezania A, Healy KE. Biotechnol. Prog. 1999;15:19–32. doi: 10.1021/bp980083b. [DOI] [PubMed] [Google Scholar]
- 124.Dee KC, Andersen TT, Bizios R. J. Biomed. Mater. Res. 1998;40:371–377. doi: 10.1002/(sici)1097-4636(19980605)40:3<371::aid-jbm5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 125.Mertz PM, Davis SC, Franzen L, Uchima FD, Pickett MP, Pierschbacher MD, Polarek JW. J Burn Care Rehabil. 1996;17:199–206. doi: 10.1097/00004630-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 126.Saito A, Suzuki Y, Ogata S-I, Ohtsuki C, Tanihara M. J. Biomed. Mater. Res. 2005;72A:77–82. doi: 10.1002/jbm.a.30208. [DOI] [PubMed] [Google Scholar]
- 127.Lee J-Y, Choo J-E, Choi Y-S, Lee K-Y, Min D-S, Pi S-H, Seol Y-J, Lee S-J, Jo I-H, Chung C-P, Park Y-J. J Biomed Mater Res A. 2007;83:970–979. doi: 10.1002/jbm.a.31351. [DOI] [PubMed] [Google Scholar]
- 128.He X, Ma J, Jabbari E. Langmuir. 2008;24:12508–12516. doi: 10.1021/la802447v. [DOI] [PubMed] [Google Scholar]
- 129.He X, Jabbari E. Biomacromolecules. 2007;8:780–792. doi: 10.1021/bm060671a. [DOI] [PubMed] [Google Scholar]
- 130.Jabbari Esmaiel, Ramalingam Murugan. Biomaterials and stem cells in regenerative medicine. CRC Press; 2012. pp. 169–184. [Google Scholar]
- 131.Kim Jaehyun, Joo Sunyoung, Ko InKap, Atala Anthony, Yoo JamesJ, Lee SangJin. Biomaterials and Stem Cells in Regenerative Medicine. CRC Press; 2012. pp. 79–100. [Google Scholar]
- 132.GLIMCHER MJ. Rev. Mod. Phys. 1959;31:359–393. [Google Scholar]
- 133.Zhang R, Ma PX. J. Biomed. Mater. Res. 1999;44:446–455. doi: 10.1002/(sici)1097-4636(19990315)44:4<446::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 134.Prosecká E, Buzgo M, Rampichová M, Kocourek T, Kochová P, Vysloužilová L, Tvrdík D, Jelínek M, Lukáš D, Amler E, Prosecká E, Buzgo M, Rampichová M, Kocourek T, Kochová P, Vysloužilová L, Tvrdík D, Jelínek M, Lukáš D, Amler E. BioMed Research International, BioMed Research International. 2012;2012:e428503. doi: 10.1155/2012/428503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao X, Lui YS, Choo CKC, Sow WT, Huang CL, Ng KW, Tan LP, Loo JSC. Materials Science and Engineering: C. 2015;49:746–753. doi: 10.1016/j.msec.2015.01.084. [DOI] [PubMed] [Google Scholar]
- 136.Wang P, Zhao L, Liu J, Weir MD, Zhou X, Xu HHK. Bone Research. 2014;2:14017. doi: 10.1038/boneres.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zorlutuna P, Annabi N, Camci-Unal G, Nikkhah M, Cha JM, Nichol JW, Manbachi A, Bae H, Chen S, Khademhosseini A. Adv. Mater. Weinheim. 2012;24:1782–1804. doi: 10.1002/adma.201104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lima MJ, Pirraco RP, Sousa RA, Neves NM, Marques AP, Bhattacharya M, Correlo VM, Reis RL. Biomed Microdevices. 2014;16:69–78. doi: 10.1007/s10544-013-9806-4. [DOI] [PubMed] [Google Scholar]
- 139.Wheeldon I, Ahari AF, Khademhosseini A. Journal of Laboratory Automation. 2010;15:440–448. [Google Scholar]
- 140.Du Y, Lo E, Ali S, Khademhosseini A. PNAS. 2008;105:9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yamato M, Okano T. Materials Today. 2004;7:42–47. [Google Scholar]
- 142.Chen G, Qi Y, Niu L, Di T, Zhong J, Fang T, Yan W. Biomed Rep. 2015;3:749–757. doi: 10.3892/br.2015.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Matsuda N, Shimizu T, Yamato M, Okano T. Advanced Materials. 2007;19:3089–3099. [Google Scholar]
- 144.Zhou Y, Chen F, Ho ST, Woodruff MA, Lim TM, Hutmacher DW. Biomaterials. 2007;28:814–824. doi: 10.1016/j.biomaterials.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 145.Yang J, Yamato M, Kohno C, Nishimoto A, Sekine H, Fukai F, Okano T. Biomaterials. 2005;26:6415–6422. doi: 10.1016/j.biomaterials.2005.04.061. [DOI] [PubMed] [Google Scholar]
- 146.Zhang S, Lockshin C, Cook R, Rich A. Biopolymers. 1994;34:663–672. doi: 10.1002/bip.360340508. [DOI] [PubMed] [Google Scholar]
- 147.Misawa H, Kobayashi N, Soto-Gutierrez A, Chen Y, Yoshida A, Rivas-Carrillo JD, Navarro-Alvarez N, Tanaka K, Miki A, Takei J, Ueda T, Tanaka M, Endo H, Tanaka N, Ozaki T. Cell Transplant. 2006;15:903–910. doi: 10.3727/000000006783981369. [DOI] [PubMed] [Google Scholar]
- 148.Hartgerink JD, Beniash E, Stupp SI. PNAS. 2002;99:5133–5138. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hartgerink JD, Beniash E, Stupp SI. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 150.Sargeant TD, Aparicio C, Goldberger J, Cui H, Stupp SI. Acta Biomater. 2012;8:2456–2465. doi: 10.1016/j.actbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Newcomb CJ, Bitton R, Velichko YS, Snead ML, Stupp SI. Small. 2012;8:2195–2202. doi: 10.1002/smll.201102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee J-Y, Choo J-E, Choi Y-S, Suh J-S, Lee S-J, Chung C-P, Park Y-J. Biomaterials. 2009;30:3532–3541. doi: 10.1016/j.biomaterials.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 153.Lee SS, Huang BJ, Kaltz SR, Sur S, Newcomb CJ, Stock SR, Shah RN, Stupp SI. Biomaterials. 2013;34:452–459. doi: 10.1016/j.biomaterials.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee SS, Hsu EL, Mendoza M, Ghodasra J, Nickoli MS, Ashtekar A, Polavarapu M, Babu J, Riaz RM, Nicolas JD, Nelson D, Hashmi SZ, Kaltz SR, Earhart JS, Merk BR, McKee JS, Bairstow SF, Shah RN, Hsu WK, Stupp SI. Adv Healthc Mater. 2015;4:131–141. doi: 10.1002/adhm.201400129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chia HN, Wu BM. Journal of Biological Engineering. 2015;9:4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ker EDF, Nain AS, Weiss LE, Wang J, Suhan J, Amon CH, Campbell PG. Biomaterials. 2011;32:8097–8107. doi: 10.1016/j.biomaterials.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 157.Loo Y, Lakshmanan A, Ni M, Toh LL, Wang S, Hauser CAE. Nano Lett. 2015;15:6919–6925. doi: 10.1021/acs.nanolett.5b02859. [DOI] [PubMed] [Google Scholar]
- 158.Skardal A, Devarasetty M, Kang H-W, Mead I, Bishop C, Shupe T, Lee SJ, Jackson J, Yoo J, Soker S, Atala A. Acta Biomater. 2015;25:24–34. doi: 10.1016/j.actbio.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 159.Skardal A, Devarasetty M, Forsythe S, Atala A, Soker S. Biotechnol. Bioeng. 2016 doi: 10.1002/bit.25950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Thomas CB, Maxson S, Burg KJL. J Biomater Sci Polym Ed. 2011;22:589–610. doi: 10.1163/092050610X488232. [DOI] [PubMed] [Google Scholar]
- 161.Badylak SF, Park K, Peppas N, McCabe G, Yoder M. Experimental Hematology. 2001;29:1310–1318. doi: 10.1016/s0301-472x(01)00729-9. [DOI] [PubMed] [Google Scholar]
- 162.Crapo PM, Gilbert TW, Badylak SF. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Badylak SF, Taylor D, Uygun K. Annual Review of Biomedical Engineering. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ouyang HW, Cao T, Zou XH, Heng BC, Wang LL, Song XH, Huang HF. Transplantation. 2006;82:170–174. doi: 10.1097/01.tp.0000226232.79106.72. [DOI] [PubMed] [Google Scholar]
- 165.Ouyang HW, Toh SL, Goh J, Tay TE, Moe K. J. Biomed. Mater. Res. 2005;75B:264–271. doi: 10.1002/jbm.b.30281. [DOI] [PubMed] [Google Scholar]
- 166.Ma D, Ren L, Chen F, Liu Y, Zhang J, Xue Z, Mao T. Ann Plast Surg. 2010;65:259–265. doi: 10.1097/SAP.0b013e3181c9c3f5. [DOI] [PubMed] [Google Scholar]
- 167.Qi Y, Wang Y, Yan W, Li H, Shi Z, Pan Z. Cell Transplant. 2012;21:693–705. doi: 10.3727/096368911X623844. [DOI] [PubMed] [Google Scholar]
- 168.Luo Y, Lee J-K, Zhao H. Chemical Engineering Science. 2013;103:115–119. doi: 10.1016/j.ces.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.