Abstract
The native cellulose of bacterial, algal, and animal origins has been well studied structurally using X-ray and neutron diffraction and solid-state NMR spectroscopy, and is known to consist of varying proportions of two allomorphs, Iα and Iβ, which differ in hydrogen bonding, chain packing, and local conformation. In comparison, cellulose structure in plant primary cell walls is much less understood because plant cellulose has lower crystallinity and extensive interactions with matrix polysaccharides. Here we have combined two-dimensional magic-angle-spinning (MAS) solid-state nuclear magnetic resonance (solid-state NMR) spectroscopy at high magnetic fields with density functional theory (DFT) calculations to obtain detailed information about the structural polymorphism and spatial distributions of plant primary-wall cellulose. 2D 13C-13C correlation spectra of uniformly 13C-labeled cell walls of several model plants resolved seven sets of cellulose chemical shifts. Among these, five sets (denoted a-e) belong to cellulose in the interior of the microfibril while two sets (f and g) can be assigned to surface cellulose. Importantly, most of the interior cellulose 13C chemical shifts differ significantly from the 13C chemical shifts of the Iα and Iβ allomorphs, indicating that plant primary-wall cellulose has different conformations, packing and hydrogen bonding from celluloses of other organisms. 2D 13C-13C correlation experiments with long mixing times and with water polarization transfer revealed the spatial distributions and matrix-polysaccharide interactions of these cellulose structures. Cellulose f and g are well mixed chains on the microfibril surface, cellulose a and b are interior chains that are in molecular contact with the surface chains, while cellulose c resides in the core of the microfibril, outside spin diffusion contact with the surface. Interestingly, cellulose d, whose chemical shifts differ most significantly from those of bacterial, algal and animal cellulose, interacts with hemicellulose, is poorly hydrated, and is targeted by the protein expansin during wall loosening. To obtain information about the C6 hydroxymethyl conformation of these plant celluloses, we carried out DFT calculations of 13C chemical shifts, using the Iα and Iβ crystal structures as templates and varying the C5-C6 torsion angle. Comparison with the experimental chemical shifts suggests that all interior cellulose favor the tg conformation, but cellulose d also has a similar propensity to adopt the gt conformation. These results indicate that cellulose in plant primary cell walls, due to their interactions with matrix polysaccharides, has polymorphic structures that are not a simple superposition of the Iα and Iβ allomorphs, thus distinguishing them from bacterial and animal celluloses.
Introduction
Cellulose is a linear chain of β(1–>4)-D-glucose units that hydrogen-bonds side by side in a parallel fashion to form microfibrils of varying thicknesses. Cellulose is produced by many organisms, including bacteria, algae, plants and some marine animals 1, but its largest source is plant cell walls, which are estimated to produce 120–140 billion tons of cellulose globally every year 2. 13C magic-angle-spinning (MAS) solid-state NMR (solid-state NMR) spectroscopy has been applied since the 1980’s to understand the principal structural features of native cellulose 3–5. Based on 13C NMR chemical shifts, cellulose from many sources was found to consist of two crystalline allomorphs, Iα and Iβ, in varying proportions 4. Cellulose of bacteria and algae such as Acetobacter xylinum and Valonia is dominated by the Iα allomorph, while cellulose of the secondary cell walls of higher plants such as cotton and ramie mainly contains the Iβ allomorph. These two allomorphs are distinguished by their C1, C4 and C6 chemical shifts: referenced to tetramethylsilane (TMS), the Iα allomorph exhibits a C1 chemical shift of 105 ppm and a C4 doublet at 90 ppm and 89 ppm, while the Iβ allomorph exhibits a C1 doublet at 106 ppm and 104 ppm and C4 chemical shifts of 89 ppm and 88 ppm. Recently, 2D 13C-13C and 13C-1H correlation NMR experiments were carried out on highly crystalline bacterial (Acetobacter xylinum), algal (Cladophora), and animal (tunicate) cellulose 6, 7 to definitively assign the chemical shifts of the Iα and Iβ allomorphs. These data resolved two sets of 13C chemical shifts for each allomorph with equimolar intensities, indicating two magnetically inequivalent glucopyranose rings in each allomorph. The A and A’ structures in Iα cellulose are spatially close together, as manifested by inter-residue cross peaks in 2D 13C-13C correlation spectra, while the B and B’ structures in Iβ cellulose are well separated 8. These results are in excellent agreement with crystal structures 9, 10, which showed that Iα cellulose contains identical chains with alternating glucose units with slightly different structures, while Iβ cellulose contains two types of chains that are separated in alternating sheets.
Compared to highly crystalline cellulose of bacteria, algae, and animals, plant primary cell wall cellulose is much less understood because of its extensive interactions with matrix polysaccharides, which give rise to low crystallinity and relatively small lateral dimensions (3–5 nm) 11, 12. These properties make primary-wall cellulose structure challenging to study at the molecular level. High-resolution structural information of primary-wall cellulose is important for understanding the physical and biochemical properties of plant cell walls and the mechanism of wall loosening during plant growth 13. Recently, we employed 13C isotopic labeling of whole plants and 2D and 3D correlation solid-state NMR techniques to investigate the structure and dynamics of primary-wall polysaccharides under near-native conditions 14–18. The multidimensional correlation approach allowed us to resolve and assign the 13C signals of wall polysaccharides and identify long-range cross peaks that are indicative of intermolecular interactions among wall polymers 19, 20. 13C labeling also facilitated the measurement of quantitative 13C NMR spectra to investigate the size of the cellulose microfibril. The number of glucan chains constituting the plant cellulose microfibrils has been a topic of active debate 13, 21. The original proposal was that the microfibril contains 36 chains, based on the hexameric organization of the cellulose synthase complex (CSC) in the plasma membrane 22. However, this number was recently revised to 18–24 based on joint analyses of 13C NMR spectra and X-ray scattering data 23–27, MD simulations 28 and biochemical analysis of the stoichiometry of different isoforms of cellulose synthase 29. Our recent quantitative 13C solid-state NMR spectra yielded surface-to-interior cellulose intensity ratios that correspond to at least 24 chains for primary-wall microfibrils 19. This number is consistent with long-mixing-time 2D correlation spectra that show that some interior cellulose chains are deeply embedded in the microfibril and are outside 13C spin diffusion reach of the surface chains. While these NMR data suggest more than 18–24 chains for primary-wall cellulose microfibrils, it remains to be determined whether this larger number reflects the size of isolated microfibrils or partly coalesced microfibrils, as seen, for example, in onion cell walls by AFM 30, 31.
In this work, we have resolved and identified five types of interior cellulose and two types of surface cellulose structures in the primary cell walls of both dicot and monocot plants. We resolved this multitude of cellulose structures from 2D 13C-13C correlation spectra measured at high magnetic fields of 18.8 and 21.1 Tesla, which correspond to 1H Larmor frequencies of 800 and 900 MHz. Interestingly, the 13C chemical shifts of most types of interior cellulose differ by more than 2 ppm from the chemical shifts of bacterial, algal and animal cellulose, indicating that the structures of plant primary-wall cellulose are not only polymorphic but also significantly different from the Iα and Iβ crystalline allomorphs. Using water polarization transfer experiments, long-range intermolecular correlation experiments, and comparison of wild-type and mutant cell walls, we obtained information about the spatial distribution and matrix-polysaccharide interactions of the different types of cellulose. Finally, using DFT calculations, we propose the hydroxymethyl conformation for the primary-wall cellulose.
Materials and Methods
Plant materials
We measured and compared six primary cell wall samples in this study, including two grass cell walls, Brachypodium 17 and Zea Mays, and four Arabidopsis cell walls, including a wild-type (WT) intact cell wall 18, an xxt1xxT2xxt5 mutant cell wall 14, a cesa1aegeus/cesa3ixr1-2 mutant cell wall 32, and a WT cell wall that has been digested with CDTA, Na2CO3, enzymes and 1M NaOH to remove most of the matrix polysaccharides 33. The Brachypodium, Zea Mays and wild-type Arabidopsis samples were never dried, while the xxt1xxT2xxt5 and cesa1aegeus/cesa3ixr1-2 mutants were air-dried and then rehydrated to ~40 wt%. Our recent study showed that polysaccharides in dried and rehydrated cell walls have the same molecular structure and dynamics as those in never-dried cell walls18. Detailed sample preparation procedures have been reported previously. Briefly, uniformly 13C-labeled plant materials were obtained by growing seedlings in the dark for 14 days in liquid culture containing 13C-labeled glucose as the sole carbon source. The seedlings were harvested and ground in liquid nitrogen. Starch and intracellular proteins were removed by treatment with α-amylase and pronase. The resulting insoluble cell wall materials were packed into 3.2 mm MAS rotors for solid-state NMR experiments.
Solid-State NMR experiments
1D 13C cross-polarization (CP) MAS spectra were measured on 800 MHz (18.8 Tesla) and 900 MHz (21.1 Tesla) Bruker NMR spectrometers using 3.2 mm MAS probes. Typical radiofrequency (rf) field strengths were 62.5 kHz for 13C and 62.5 - 83 kHz for 1H. All 13C chemical shifts were referenced to the adamantine CH2 peak at 38.48 ppm on the TMS scale. A 2D radio-frequency-driven recoupling (RFDR) correlation experiment 34 (Fig. S1a) was conducted on the Brachypodium cell wall to assign intra-residue cross peaks. The RFDR spectrum was measured under 16 kHz MAS and 83 kHz 1H decoupling. A train of π pulses in the middle of each rotor period recouples the 13C-13C dipolar couplings for longitudinal polarization transfer. A recoupling time of 1.5 ms was used to detect one-bond 13C-13C cross peaks. This mixing time was chosen to selectively detect one-bond cross peaks based on tests on the model peptide formyl-Met-Leu-Phe (MLF) 35, 36. At this mixing time, one-bond 13C-13C cross peaks have 17–32% of the intensities of diagonal peaks while two-bond cross peaks have less than 2% of the diagonal intensities. 2D proton-driven 13C spin diffusion (PDSD) experiments 37 were conducted on all cell walls using mixing times of 30 ms and 1.0 s, which have been shown previously to give multi-bond intramolecular cross peaks and long-range intermolecular cross peaks (Fig. S1b), respectively 16, 18, 20. All PDSD spectra were measured at 296 K under 10 kHz MAS. Most 2D spectra were processed using a QSINE window function with an SSB parameter of 2.5–3.0. The number of scans range from 32 to 96 and the signal-to-noise ratios are 10–70 for intermolecular cellulose cross peaks and 30–600 for intramolecular cross peaks. For the RFDR spectrum of the Brachypodium cell wall, the lowest contour level was 6% of the highest diagonal peak (75, 75 ppm), the level increment was 1.5, and 24 levels were plotted, so that the highest contour level is 67% of the highest peak. All PDSD spectra were plotted using a minimum contour level of 1% of the highest diagonal peak, which is the (72.5, 72.5 ppm) peak for most cell walls. The highest plotted contour level is ~35% of the highest peak for the 30 ms PDSD spectra and 70–80% of the highest peak for the 1.0 s PDSD spectra.
A 1H T2 filtered PDSD experiment (Fig. S1c) was conducted on the Brachypodium cell wall to detect well-hydrated cellulose. The 1H T2 filter time was 1.2 ms, which suppressed the polysaccharide 1H magnetization to ~2% while retaining ~55% of the water 1H magnetization. A 1H mixing time (tm1) of 1 ms was used to transfer the water 1H polarization to polysaccharides, and a 30 ms 13C mixing time (tm2) was applied to detect intra-residue polysaccharide cross peaks. The resulting 2D spectra exhibit the signals of hydrated polysaccharides. The experiment was carried out at 263 K under 10 kHz MAS.
A 13C T1 filtered PDSD experiment was carried out to compare nanosecond-timescale motions of different celluloses. A variable 13C longitudinal mixing period was inserted before the evolution period of the PDSD experiment (Fig. S1d). Polysaccharides with long 13C T1 relaxation times retain the signals while polysaccharides with short T1 relaxation times show weaker signals. The spectra were measured at 296 K under 10 kHz MAS and the 13C spin diffusion mixing time was 30 ms.
DFT calculations
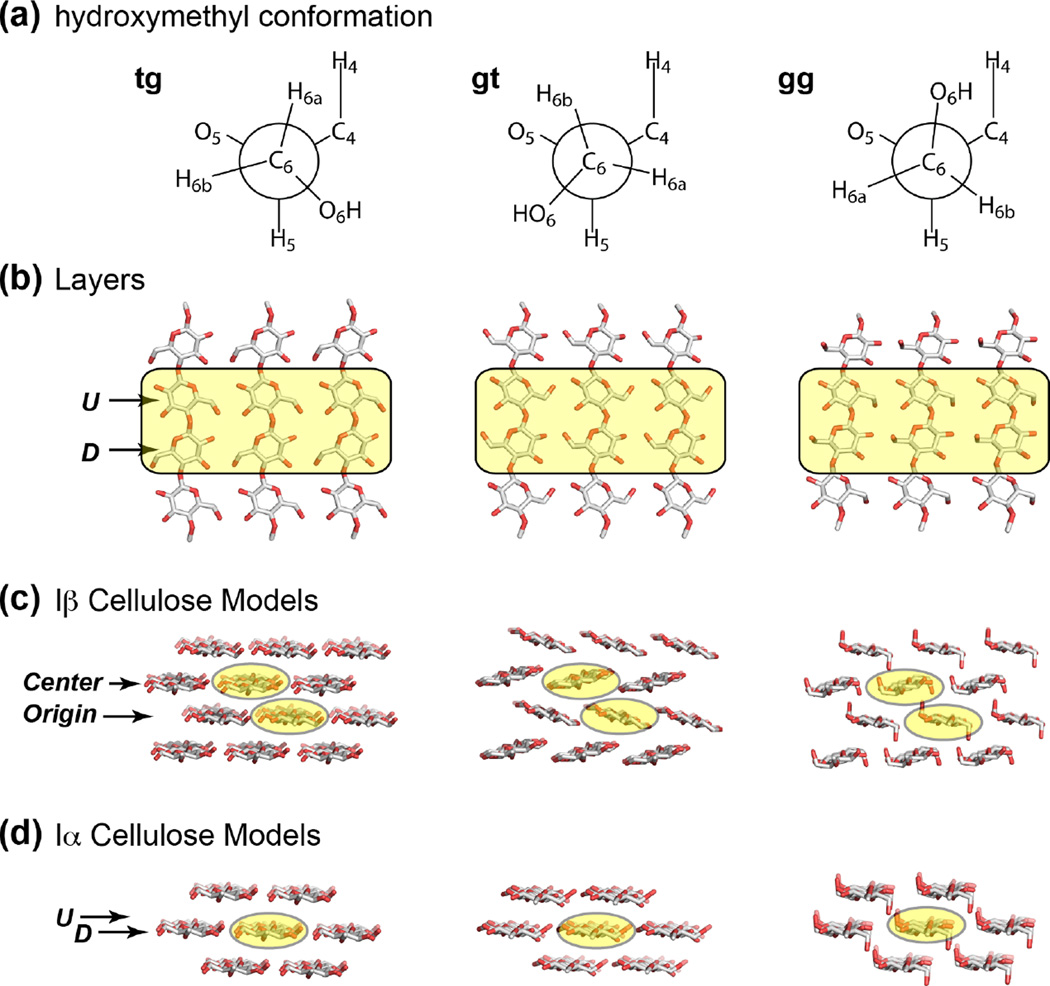
Cellulose Iα and Iβ models with three different conformations of the C6 exocyclic group (gg, gt and tg) were created based on X-ray and neutron diffraction structures 9, 10. The atomic positions of these models have been previously energy-minimized with periodic DFT-D2 calculations 38, 39. Clusters consist of four monomer units per chain, three chains wide and four layers high. This allows for four monomers to have at least one other unit next to them in all three dimensions to represent the atomic environment in cellulose. The ends of tetramer chains were terminated with a methyl group to satisfy the bonding of the terminal atoms. The atoms were then held fixed into the positions determined by periodic energy minimizations for calculating isotropic 13C chemical shifts based on the cluster model. Structural parameters such as the inter-atomic distances and torsion angles are listed in Table S1.
NMR shielding tensors were calculated using the modified Perdue-Wang exchange-correlation functional mPW1PW91 40 and the 6-31G(d) basis set 41 using the Gauge-independent atomic orbitals (GIAO) 42–47 method in Gaussian 09 48. Chemical shifts were calculated using the multi-reference method. Methanol was the secondary chemical shift standard, because it produces δ13C in better agreement with experiments 38, 39, 49, 50. An empirical correction of 49.5 ppm 51 was used for the difference between the δ13C of methanol and TMS 49. This is added to the calculated shielding of methanol to give a corrected isotropic chemical shielding of 193.0 ppm, which is then used to convert the computed cellulose chemical shielding σ13Ci for atom i to chemical shift δ13Ci.
Results and Discussion
Structural polymorphism of plant primary wall cellulose
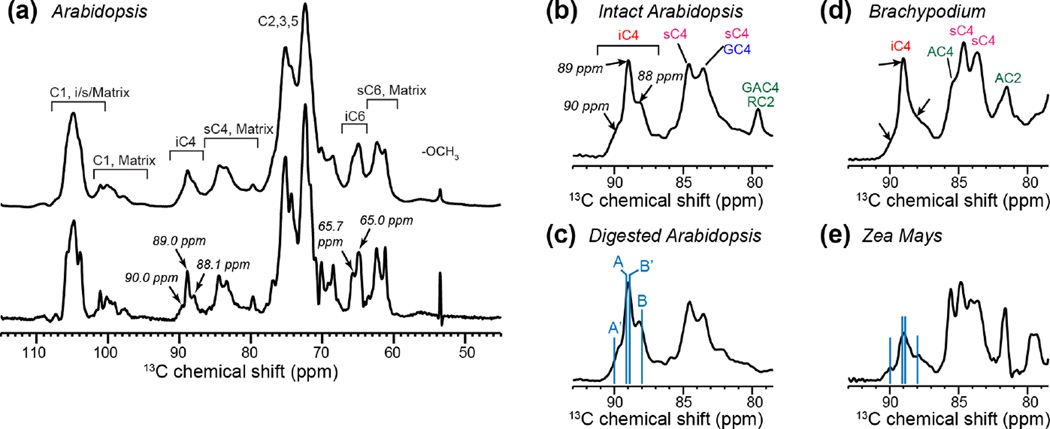
To better resolve the chemical shifts of cellulose in intact plant cell walls, we measured 13C NMR spectra at high magnetic fields corresponding to 1H Larmor frequencies of 800 and 900 MHz. Fig. 1a shows the 1D 13C CP-MAS spectrum of Arabidopsis primary cell walls at 900 MHz. Despite the high field, most cellulose signals still overlap with the matrix polysaccharide signals at common C1 chemical shifts of ~105 ppm and at C2, C3, C5 chemical shifts of 70-75 ppm (Fig. 1a). The interior cellulose C4 and C6 peaks (iC4 and iC6) are resolved from matrix polysaccharide signals, but the 2-3 ppm linewidths are still insufficient for resolving multiple structures of cellulose. The fact that multiple structures exist can be seen when window functions that increase the intensities of the middle part of the time-domain signals are applied 23, 52. With such a window function, the iC4 peak at ~89 ppm reveals three partially resolved peaks while the iC6 peak at ~65 ppm contains at least two peaks. The iC4 fine features resemble a superposition of the Iα allomorph’s C4 chemical shifts (90 and 89 ppm) and the Iβ C4 chemical shifts (89 and 88 ppm) 4, 53. These fine features are present in multiple plants, including Arabidopsis, Brachypodium and Zea Mays (Fig. 1b-e), suggesting that this structural polymorphism is common to plant primary-wall cellulose. Chemical and enzymatic digestion of matrix polysaccharides in the Arabidopsis primary walls did not remove these fine features (Fig. 1c), indicating that the cellulose structural polymorphism is robust.
Figure 1.
1D 13C CP-MAS spectra of primary cell walls at high magnetic fields. (a) Never-dried Arabidopsis cell walls without (top) and with (bottom) resolution-enhanced window function. The spectrum was measured at 277 K on a 900 MHz spectrometer. The bottom spectrum is processed with a Gaussian function using LB=−140 and GB=0.6. (b-e) C4 region of the 13C spectra of various cell walls. The spectra were measured at 296 K on an 800 MHz NMR, and were processed with LB=−100 and GB=0.6. (b) Intact Arabidopsis cell wall. (c) Partially digested Arabidopsis cell wall. (d) Brachypodium cell wall. (e) Zea Mays cell wall. Blue lines in (c) and (e) guide the eye for the literature crystalline cellulose C4 chemical shifts in Iα (A and A’) and Iβ (B and B’) allomorphs.
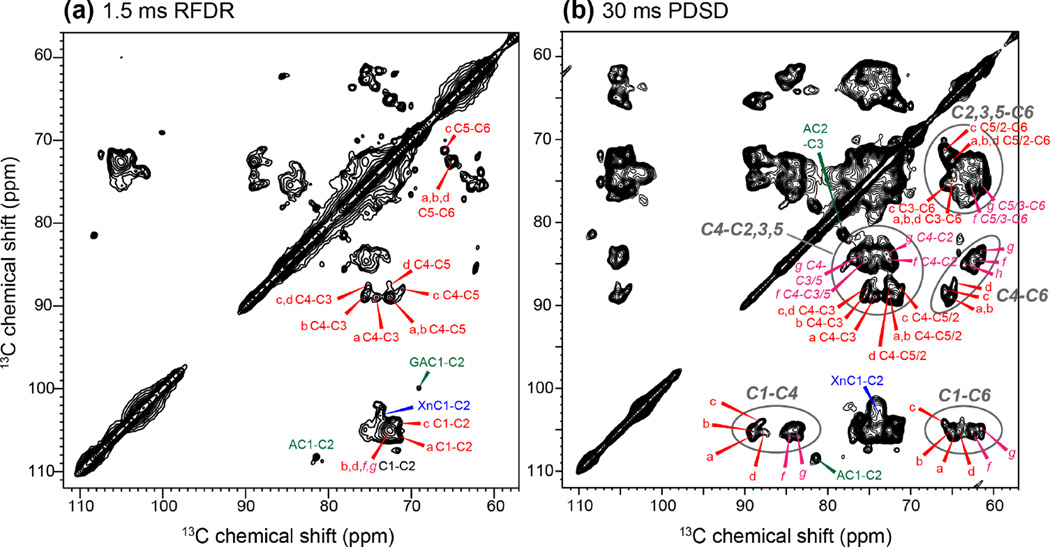
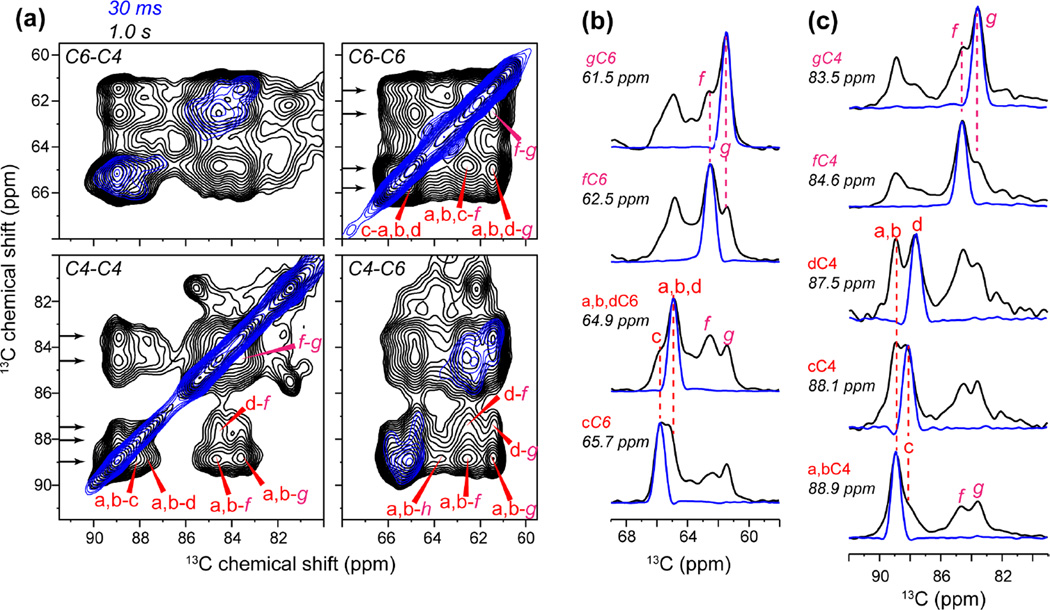
Extending the experiments to two dimensions at high fields resolved multiple structures of cellulose. Fig. 2 shows 2D 13C-13C RFDR and PDSD spectra of the Brachypodium cell wall at 800 MHz. A short RFDR mixing time of 1.5 ms was used to detect one-bond cross peaks while a 30 ms PDSD spin diffusion mixing time was used to measure multi-bond cross peaks 8, 16, 20. Comparison of the two spectra allowed us to assign multiple sets of cellulose chemical shifts. Although the C3 and C5 chemical shifts are similar (around 72 ppm), and both carbons are bonded to C4, the 2D RDFR spectrum yields unambiguous C5 chemical shifts from the C5-C6 cross peaks, thus distinguishing the C5 from the C3 chemical shift. The 2D PDSD spectrum exhibits all intra-residue cross peaks, the most characteristic ones being the C1-C4 and C4-C6 cross peaks. Fig. S2 shows examples of how the 2D RFDR and PDSD spectra are used together to resolve the signals of interior cellulose types a and d. Starting from the C1-C4 cross peaks in the PDSD spectrum, we can identify the C6-C4 cross peaks in the PDSD spectrum, followed by the C6-C5 and C4-C5 cross peaks in the RFDR spectrum. The C4-C5 cross peaks further correlate to C4-C3 cross peaks in the RFDR spectrum to give the C3 chemical shift. The C4-C5 cross peaks also connect to C4-C1, C4-C6 and other cross peaks in the PDSD spectrum to confirm the other chemical shifts. The starting C1-C4 cross peak in the PDSD spectrum can also be traced to the C1-C2 cross peak in the RFDR spectrum to obtain the C2 chemical shifts. It is noteworthy that a single C1-C2 cross peak at (105, 72.6) ppm is observed for cellulose types b, d, f, and g, but the narrow C2 linewidth of 1.2 ppm makes the C2 chemical shift assignment unambiguous. This situation differs from the resonance overlap often encountered in disordered proteins, where the broad linewidths lead to multiple possible assignments 54, 55. Consistent with the literature, the two types of surface cellulose (f and g) exhibit C4 and C6 chemical shifts that are 3-4 ppm smaller than (i.e. upfield from) the chemical shifts of interior cellulose. Similar 2D PDSD spectra were measured for the other primary cell walls, and the multiple sets of cellulose 13C chemical shifts of Brachypodium, Zea Mays and Arabidopsis are summarized in Table 1.
Figure 2.
2D 13C-13C correlation spectra of Brachypodium primary cell walls for resonance assignment of the polymorphic cellulose. (a) 1.5 ms RFDR spectrum showing one-bond cross peaks. (b) 30 ms PDSD spectrum showing intra-residue multi-bond cross peaks. The interior and surface cellulose signals are annotated in red and magenta, respectively. Ovals guide the eye for characteristic cross peak regions such as C1-C4, C4-C6 and C1-C6.
Table 1.
Cellulose 13C chemical shifts (ppm) of various plant primary cell walls. Weak signals are labeled as (w) and unresolved signals are underlined. Brachypodium, Arabidopsis and Zea Mays primary cell walls are measured in this study. Cladophora, tunicate 7, 8 and Arabidopsis secondary wall 66 cellulose chemical shifts are literature values.
| Arabidopsis primary wall cellulose | ||||||
|---|---|---|---|---|---|---|
| Type | C1 | C2 | C3 | C4 | C5 | C6 |
| type a | 105.8 | 71.6 | 74.4 | 89.0 | 72.6 | 65.0 |
| type b | 104.8 | 72.6 | 75.4 | 88.8 | 72.6 | 65.5/65.1 |
| type c | 104.1 | 71.8 | 75.2 | 88.1 | 71.2 | 65.9 |
| type d | 105.2 | 72.5 | 74.8 | 87.0 | 72.5 | 64.7 |
| type e (w) | 105.0 | 71.2/72.2 | 74.6 | 89.9 | 71.2/72.2 | 65.3 |
| type f | 105.1 | 72.5 | 75.3/74.1 | 84.5 | 75.3 | 62.5 |
| type g | 104.9 | 72.9 | 75.3 | 83.5 | 75.3 | 61.5 |
| Brachypodium primary wall cellulose | ||||||
| Type | C1 | C2 | C3 | C4 | C5 | C6 |
| type a | 105.8 | 71.8 | 74.4 | 89.1 | 72.6 | 64.9 |
| type b | 105.0 | 72.6 | 75.6 | 89.0 | 72.6 | 65.0/65.3 |
| type c | 104.1 | 71.8 | 75.2 | 88.1 | 71.1 | 65.9 |
| type d | 105.3 | 72.6 | 75.2/74.4 | 87.5/87.0 | 72.6 | 64.8/64.1(w) |
| type e (w) | 104.8 | ND | ND | 90.0 | ND | 65.4 |
| type f | 105.1 | 72.9 | 75.4 | 84.5 | 75.4 | 62.4 |
| type g | 105.0 | 72.6 | 75.3 | 83.7 | 75.3 | 61.6 |
| Zea Mays primary wall cellulose | ||||||
| Type | C1 | C2 | C3 | C4 | C5 | C6 |
| type a | 105.8 | 71.7 | 75.7 | 89.0 | 72.7 | 65.0 |
| type b | 105.0 | 72.7 | 75.7/74.0(w) | 89.0 | 72.7 | 65.3/65.5(w) |
| type c | 104.1 | 71.2 | ND | 88.0 | 71.2 | 66.0 |
| type d | 105.4 | 72.8 | 75.2/75.0/74.5 | 87.9/87.2 | 72.6 | 64.9 |
| type e (w) | 105.4 | 72.0 | ND | 90.1 | 72.0 | 65.4 |
| type f | 105.2/106.1 | 72.7 | 75.7 | 84.4/84.9 | 75.7 | 62.7/63.0 |
| type g | 105.2 | 74.2/73.1 | 74.2/73.1 | 83.6 | 75.5 | 61.6 |
| type h | 105.4/106.1 | 73.2 | 74.7/74.9(w) | 85.4 | 74.7 | 63.8 |
| Cladophora & tunicate cellulose 7, 8 | ||||||
| Type | C1 | C2 | C3 | C4 | C5 | C6 |
| Iα A | 105.0 | 70.1 | 73.9 | 89.1 | 72.6 | 65.2 |
| Iα A’ | 105.0 | 71.6 | 74.7 | 90.0 | 70.1 | 65.2 |
| Iβ B | 106.1 | 71.3 | 74.9 | 88.0 | 70.6 | 65.6 |
| Iβ B’ | 104.0 | 71.0 | 74.2 | 88.9 | 72.2 | 65.0 |
| Arabidopsis secondary-wall cellulose 66 | ||||||
| Type | C1 | C2 | C3 | C4 | C5 | C6 |
| Domain 1 | 105.2 | 72.4 | 74.5 | 88.9 | 72.7 | 65.0 |
| 104.1 | 72.1 | 74.8 | 88.1 | 71.3 | 65.7 | |
| Domain 2 | 105.2 | 72.4 | 74.5 | 84.2 | 75.5 | 62.7 |
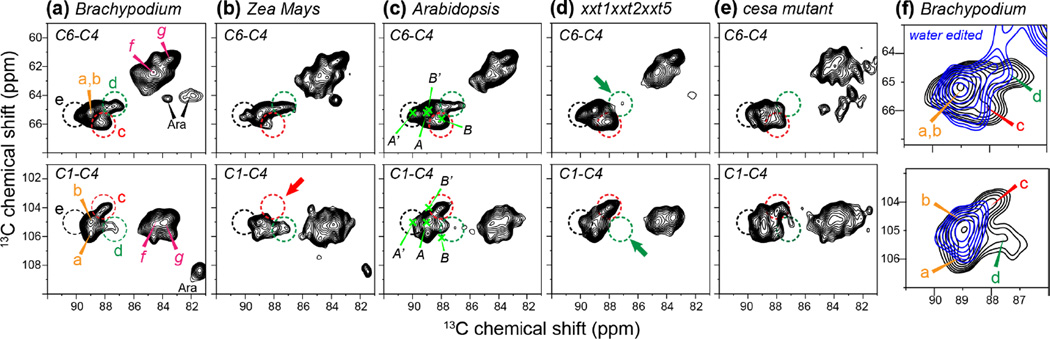
In total, the high-field 2D correlation spectra resolved five types of interior cellulose (a-e) and two types of surface cellulose (f and g), indicating significant structural heterogeneity of plant primary-wall cellulose (Fig. 3, S3). Fig. 3 compares the most important C6-C4 and C1-C4 regions of the five plant cell walls, including three Arabidopsis cell walls and two grass cell walls. The three Arabidopsis samples include a WT sample, a xyloglucan-deficient mutant, and a CESA mutant with reduced cellulose synthesis capability. As can be seen, the seven types of cellulose are observed in most cell walls but with varying intensities. For example, cellulose c signals are strong in Brachypodium and Arabidopsis cell walls, but much weaker in the Zea Mays cell wall. Table 2 lists the estimated percentages of the different cellulose subtypes in the cell walls investigated here. As we show below, cellulose c can be assigned to glucan chains that are more than one chain away from the microfibril surface 19, thus its lower intensity in Zea Mays suggests that the microfibrils of Zea Mays may have smaller lateral dimensions and fewer core chains. The Brachypodium cell wall has negligible amount of cellulose e, whose C4 chemical shift of 90 ppm matches that of the Iα crystalline allomorph. Importantly, cellulose d is absent in the xyloglucan-deficient xxt1xxT2xxt5 mutant, suggesting that cellulose d is responsible for contacting hemicellulose. The two grass cell walls, which contain negligible xyloglucan but have glucoarabinoxylan (GAX) as the major hemicellulose 17, also show clear cellulose d signals (Fig. 3a, b), indicating that GAX interacts with cellulose microfibrils in a similar fashion as xyloglucan in dicot cell walls. The CESA mutant was previously found to reduce the cellulose crystallinity 32. Consistently, the cellulose peaks of this mutant cell wall are broader and less defined (Fig. 3e).
Figure 3.
Comparison of the cellulose C6-C4 (top row) and C1-C4 (bottom row) of the 2D PDSD spectra of various primary cell walls. (a) Brachypodium cell wall. (b) Zea Mays cell wall. (c) Arabidopsis cell wall. (d) xxt1xxT2xxt5 mutant Arabidopsis cell wall. (e) CESA mutant Arabidopsis cell wall. Dashed circles guide the eye for the five types of interior cellulose and two types of surface cellulose chemical shifts. (f) Water-edited 2D PDSD spectrum (blue) of the Brachypodium cell wall, superimposed with the full PDSD spectrum (black). Cellulose a and b signals are retained while interior cellulose c and d signals are suppressed in the water-edited spectrum, indicating the different hydrations of these cellulose structures.
Table 2.
Estimated percentages of different cellulose types in plant primary cell walls, obtained by integrating the intensities of C1-C4 cross peaks in the 30 ms PDSD spectra.
| Cell walls | a | b | c | d | e | f | g |
|---|---|---|---|---|---|---|---|
| WT Arabidopsis | 14% | 13% | 10% | 6.0% | 3.2% | 30% | 23% |
| xxt1xxt2xxt5 Arabidopsis | 20% | 15% | 14% | 3.5% | 2.9% | 24% | 21% |
| Brachypodium | 14% | 14% | 7.6% | 6.9% | 1.7% | 26% | 31% |
| Zea Mays | 9.2% | 11% | 3.3% | 8.2% | 2.0% | 31% | 36% |
Differences between primary-wall cellulose and crystalline bacterial, algal and animal cellulose
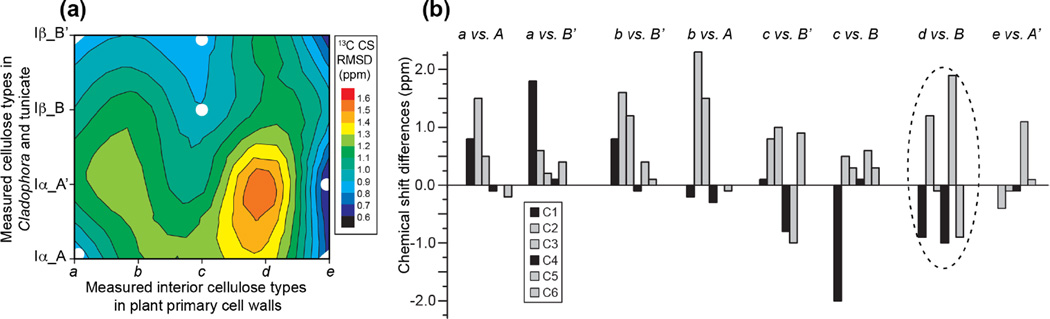
To gain insight into the structures underlying these chemical shifts, we compared the measured primary-wall cellulose chemical shifts with values reported for highly crystalline cellulose of Cladophora and tunicate 7 (Fig. 4). Cladophora is rich in Iα cellulose, which is formed by identical chains that each contains two alternating types of glucose units, A and A’ 8, 10. Tunicate cellulose is dominated by the Iβ allomorph, which contains two types of glucose residues (B and B’) separated into alternating sheets 8, 9. We show in Fig. 4 the chemical shift differences between the five primary wall interior cellulose a-e and the four types of crystalline Iα and Iβ cellulose. Both the average chemical shift RMSD values and the individual 13C chemical shift differences are shown to indicate the structural similarities and differences between different sources of cellulose. The chemical shifts of most carbons in the structurally known and distinct Iα and Iβ allomorphs (Table 1) differ by less than 1.0 ppm, with the exceptions of C5 of Iα A and A’ and C1 of Iα B and B’, whose chemical shifts differ by 2.0-2.5 ppm. Thus, we chose a cutoff of 1.5 ppm for an individual carbon as an indication that two structures are significantly different. In general, C1, C4 and C6 chemical shifts are sensitive to the glycosidic ϕ (O5-C1-O1-C4) and ψ (C1-O1-C4-C5) torsion angles and the χ’ (C4-C5-C6-O6) torsion angle, which dictates the C6 hydroxymethyl conformation 56.
Figure 4.
Comparison of the 13C chemical shifts of plant primary wall cellulose with crystalline cellulose of algae and animals. (a) 13C chemical shift RMSD values between the Arabidopsis primary wall interior cellulose a-e and literature Cladophora and tunicate cellulose, which represent the Iα and Iβ allomorphs, respectively. Each allomorph shows two types of chemical shifts. For ease of inspection, the twenty (5×4) discrete RMSD values are connected by contour lines, thus positions between the twenty points do not have physical meaning. (b) Differences of individual 13C chemical shifts between primary wall celluloses and crystalline Iα and Iβ celluloses. Two similarly good assignments are each found for cellulose a, b and c, but neither assignment is satisfactory, as shown by chemical shift differences of more than 1.5 ppm for some of the carbons. For cellulose e, good agreement with Iα-A’ is found. Cellulose d has large chemical shift differences from all known allomorphs, indicating that the cellulose d structure does not exist in the algal and animal celluloses.
Fig. 4 and Table 1 show that cellulose e matches the Iα allomorph glucose A’ very well, with a chemical shift RMSD of only 0.5 ppm. Moreover, no individual 13C chemical shift has large discrepancy with the literature values. Thus, cellulose e can be confidently assigned to a conformation and hydrogen-bonding pattern that are similar to the Iα A’ structure. However, the other four types of interior cellulose cannot be assigned satisfactorily to any Iα and Iβ structures. For cellulose a-c, even the best fits with the crystalline cellulose allomorphs have chemical shift RMSDs of ~1.0 ppm, and individual differences of more than 1.5 ppm are observed for some carbons. For example, cellulose a shows best matches with glucose A and B’ in the Iα and Iβ allomorphs, respectively, but the C2 chemical shift of cellulose a deviates by 1.7 ppm from the A C2 chemical shift, suggesting differences in hydrogen bonding near C2 (e.g. O2-O6), while the C1 chemical shift of cellulose a differs by 1.8 ppm from the C1 chemical shift of glucose B’ (Table 1). Similarly, cellulose b has significantly different C2 and C3 chemical shifts from those of B’ and A.
Interestingly, cellulose d, which interacts with xyloglucan, exhibits the worst agreement with any known crystalline cellulose structures. The chemical shift RMSDs range from 1.1 to 1.6 ppm (Fig. 4a). Five out of six carbons in cellulose d are more than 1.0 ppm different from the chemical shifts of cellulose B, which best matches cellulose d (Fig. 4b). This poor agreement is consistent with assignment of cellulose d to glucan chains that interact with hemicellulose, which do not exist in crystalline cellulose of non-plant organisms. We previously found that expansin, a wall-loosening protein 21, 57, 58, targeted these limited regions of cellulose microfibrils where xyloglucan was entrapped 59. The expansin-bound cellulose has a unique C4 chemical shift, 1.0 ppm upfield from the average cellulose C4 signal, and resembles the cellulose d C4 chemical shift seen here. Thus, cellulose d may play an important role in wall loosening.
These chemical shifts indicate that except for cellulose e, whose structure matches that of cellulose A’ well, the other four types of interior cellulose in plant primary walls do not resemble the glucose structures in crystalline bacterial and algal cellulose. Fig. 4b shows two best assignments each for cellulose a to c: a-A/B’, b-B’/A, and c-B/B’, while cellulose d is unique to plant primary cell walls. These results indicate that primary wall cellulose does not have the same structure as bacterial, algal and animal cellulose, and the long-held conceptual framework of Iα and Iβ allomorphs as the basic structural units of native cellulose do not adequately describe plant primary wall cellulose. In addition to chemical shift differences of individual carbons, the intensity distribution is not consistent with the equimolar ratio of A and A’ or B and B’ (Table S2), which are required for the Iα and Iβ allomorph. For example, while cellulose e chemical shifts match the cellulose A’ chemical shifts well, neither a or b cellulose, which have partial resemblance to the A structure, have similar intensities to the cellulose e peaks in the spectra. For all the cell walls examined here, spectral intensities indicate that the percentages of cellulose a and b are the highest among all interior cellulose types, followed by cellulose c, while cellulose d and e are present at low concentrations (Table 2). These results indicate that primary wall celluloses have distinct structures from the Iα and Iβ structures, and matrix polysaccharides have significant influences on the packing and conformation of the glucan chains.
Comparison of plant primary wall cellulose with secondary wall cellulose
Cellulose in secondary cell walls has also been studied extensively using NMR; thus it is interesting to compare its chemical shifts with the primary-wall cellulose results here. In wood, cellulose microfibrils are estimated to be 10 - 20 nm across 60, and their order has been investigated by deconvolution of 1D 13C solid-state NMR spectra 61–63. The results indicate that pulping changes the cellulose structure, converting some of the Iα structure to the Iβ allomorph. 2D 13C-13C correlation spectra of extracted and regenerated wood cellulose showed a large distribution of chemical shifts 64, 65 and indicated the presence of not only crystalline cellulose Iα and Iβ allomorphs but also cellulose II, which consists of antiparallel packed glucan chains. This cellulose II structure is the result of the regeneration process and thus cannot be extended to native primary-wall cellulose. Recently, Dupree and coworkers used 2D and 3D 13C correlation solid-state NMR experiments to investigate the structure of intact dried Arabidopsis secondary walls. They resolved three sets of cellulose chemical shifts 66, two of which match those of interior cellulose types b and c found here, while the third set matches the surface cellulose f in our samples (Table 1). This suggests that a subset of the cellulose structure and packing may be conserved between the primary and secondary cell walls, and the conserved sub-types are evenly distributed in the core of the microfibril, between the core and the surface, and on the surface. Consistent with our current observations, these authors found no evidence of the Iβ allomorph in the secondary-wall cellulose. Taken together, these data suggest that cellulose in untreated and native plant primary and secondary cell walls share structural similarities, and both differ significantly from the crystalline cellulose found in bacteria, algae and animals.
DFT calculations of cellulose chemical shifts
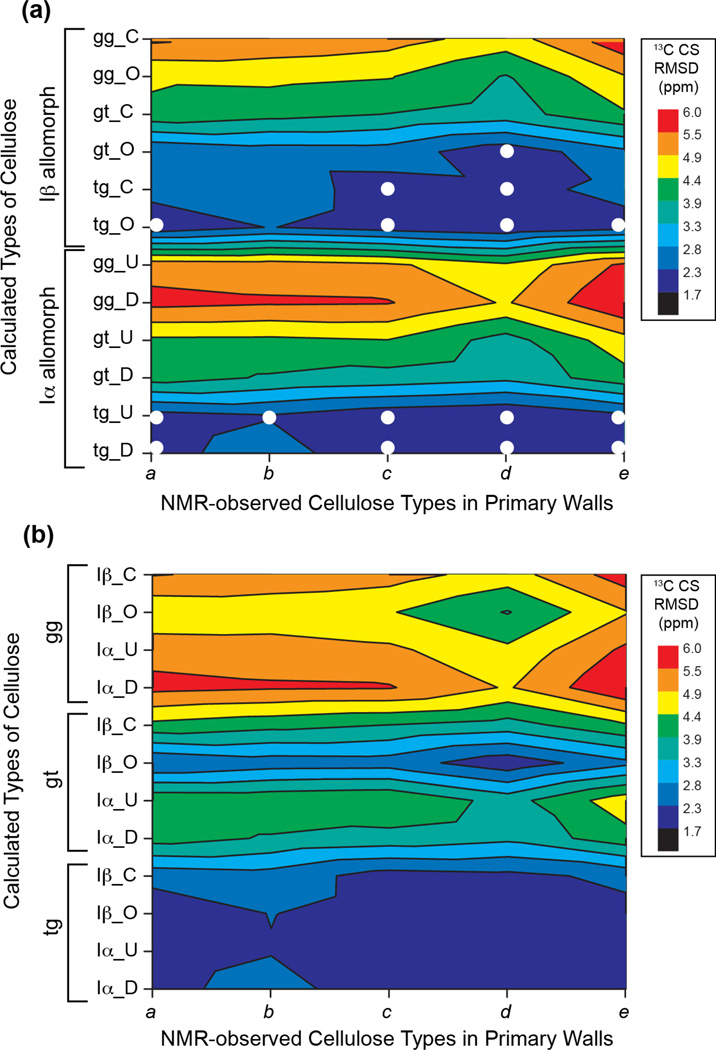
To gain insight into the structures underlying the multiple sets of cellulose 13C chemical shifts, we carried out DFT calculations of chemical shifts for cellulose with varying hydroxymethyl conformations. Density functional theory has been demonstrated to be a powerful tool for reproducing experimentally measured NMR chemical shifts of crystalline cellulose 38, 39 and for refining the structures of small molecules 67. We created energy-minimized structural models of Iα and Iβ cellulose 9, 10 with three hydroxymethyl conformations: tg, gt, gg (Table S1, Fig. 5). Following the crystal structure, the Iβ allomorph contains two non-equivalent glucan chains, the origin (O) and center (C) chains, which lie in alternating layers, while Iα cellulose comprises the same glucan chains, each containing alternating non-equivalent glucose residues U and D. To calculate the chemical shifts of the interior U, D, O, C residues, we used minimal numbers of 48 and 28 glucose residues for the Iβ and Iα allomorphs, respectively. Fig. 6 compares the measured and calculated (Table 3) 13C chemical shifts of these glucose residues. All measured interior cellulose (a-e) chemical shifts agree well with the calculated C6 chemical shifts for the tg conformation, with an RMSD of ~2.1 ppm (Table S3), which is close to the best agreement we have achieved when comparing calculated and observed δ13C values on known structures 38, 39, 68, 69. In addition, cellulose d, which interacts with matrix polysaccharides, shows good agreement with the calculated chemical shifts for the Iβ gt conformer (Fig. 6b), suggesting that tg and gt conformations may coexist in the plant-specific cellulose d. None of the measured chemical shifts agree with the calculated values for the gg conformation, indicating that the gg conformer is energetically unfavorable in interior cellulose.
Figure 5.
Cellulose Iα and Iβ structural models with three different C6 conformations: tg, gt and gg from left to right). The shaded glucose residues in each layer were treated as the interior cellulose and were analyzed for 13C and 1H NMR chemical shifts. (a) The three C6 conformations. (b) Face view of a layer of glucan chains in cellulose. U and D denote the two non-equivalent residues in the Iα allomorph. (c) Side view of the Iβ model used to calculate NMR chemical shifts. The center and origin chains are formed by two types of glucose residues in the unit cell of the Iβ crystal structure. (d) Side view of the Iα cellulose structure model. The U and D glucose units alternate along the same chain.
Figure 6.
13C chemical shift RMSDs between measured primary wall cellulose and DFT calculated chemical shifts for various crystalline allomorphs, hydroxymethyl conformations, and glucose residue locations. (a) Calculated cellulose chemical shifts are organized with the Iβ allomorph at the top and the Iα allomorph at the bottom. C and O denote center and origin sheets in the Iβ allomorph 9 while U and D denote the two non-equivalent glucose units in an Iα chain 10. (b) Calculated cellulose chemical shifts are organized according to the three C6 conformations. gg chemical shifts show poor agreement (high RMSD values) with experimental chemical shifts, while the tg conformations show good agreement with measured chemical shifts. The best-matched conformations are indicated as white circles in (a).
Table 3.
DFT calculated cellulose 13C chemical shifts (ppm). U and D denote the two non-equivalent glucose units in the same Iα chain while C and O denote center and origin chains in the Iβ allomorph.
| Form | Location | δ13C1 | δ13C2 | δ13C3 | δ13C4 | δ13C5 | δ13C6 |
|---|---|---|---|---|---|---|---|
| Iα gg | U D |
102.3 101.2 |
70.8 73.9 |
72.8 70.8 |
77.8 78.4 |
72.3 74.0 |
60.0 59.0 |
| Iα gt | U D |
103.9 98.5 |
72.1 72.6 |
73.0 73.6 |
81.3 83.5 |
74.0 72.5 |
58.4 61.1 |
| Iα tg | U D |
103.6 104.2 |
70.1 68.7 |
72.3 72.4 |
85.7 87.3 |
71.4 69.7 |
63.9 64.3 |
| Iβ gg | center (C) origin (O) |
100.8 102.9 |
72.9 71.3 |
72.7 70.8 |
77.9 79.8 |
71.6 73.8 |
59.8 61.3 |
| Iβ gt | center (C) origin (O) |
99.0 105.2 |
73.4 70.9 |
72.0 71.9 |
83.3 85.2 |
71.1 72.6 |
60.7 62.0 |
| Iβ tg | center (C) Origin (O) |
103.0 105.1 |
69.7 69.6 |
72.6 71.8 |
85.2 86.1 |
70.1 71.6 |
64.4 64.3 |
Spatial distribution and intermolecular contacts of different cellulose in plant primary cell walls
To obtain information about how the different cellulose structures interact with water and matrix polysaccharides and where they are located in the microfibril, we measured water-edited 2D correlation spectra and long-mixing-time 2D correlation spectra. A 1H T2 filtered PDSD experiment was used to select the water 1H magnetization and transfer it to neighboring polysaccharides 33. Fig. 3f shows the water-edited spectrum of the Brachypodium cell wall. The signals of cellulose a and b are well retained in the water-edited spectrum whereas the c and d signals are preferentially suppressed, indicating that cellulose a and b are close to the microfibril surface while cellulose c and d are more sequestered from water. The different distribution of cellulose with respect to water is consistent with recently reported 1H-13C 2D correlation spectra, which show much narrower 13C signals for water-proximal cellulose than the full 13C signals of all cellulose 18, 70, 71. The current water-edited 2D 13C-13C correlation spectrum shows the retention of the 89-ppm C4 chemical shift, which is the frequency of cellulose a and b, while the C4 peaks of cellulose c and d at 87 and 88 ppm have been suppressed.
2D PDSD correlation spectra measured with a long 13C spin diffusion mixing time of 1.0 s (Fig. 7a, Fig. S4) provided valuable information about the spatial contacts between different forms of cellulose. A large number of intermolecular cross peaks between the two types of surface cellulose, between surface and interior cellulose, and between different types of interior cellulose, are observed in the 1.0 s spectrum (Fig. 7b, c). For example, the 61.5 ppm cellulose-g C6 cross section shows a clear cross peak with cellulose f C6 at 62.5 ppm, indicating f-g contacts on the microfibril surface. Both gC6 and fC6 show cross peaks with interior cellulose C6 at 65 ppm. Interestingly, the surface-surface g-f cross peaks have similar intensities as the surface-interior cross peaks in both the 1.0 s PDSD spectrum and the 0.2 s PDSD spectrum (Fig. S5), strongly suggesting that g and f glucose rings do not reside in the same chain. The distances between adjacent glucose rings in the same chain are 2-3 Å, while the shortest distance between glucose in two different chains are 4-6 Å. Since spin diffusion rates are inversely proportional to the distance to the sixth power 72, if f and g are mixed in the same chain, then they would give rise to ~64-fold faster buildup and hence much stronger cross peaks than the surface-interior cross peaks, which is inconsistent with the data. It is also unlikely for g and f cellulose to be segregated onto different microfibril surfaces such as hydrophobic and hydrophilic surfaces, since this would make the distances too large to observe. Therefore, the 2D spectra indicate that cellulose g and f are well mixed chains on the same microfibril surface (Fig. 8). Further, these two types of surface chains have similar populations, since their intensities are similar in all spectra.
Figure 7.
Intermolecular cross peaks of Brachypodium primary wall cellulose from long-mixing-time 2D PDSD spectra. (a) Overlay of the 30 ms PDSD spectrum (blue) and the 1.0 s (black) PDSD spectrum. Long-range cross peaks are assigned. Arrows on the left indicate the extracted 1D cross sections in (b) and (c). (b) Cellulose C6 cross sections. (c) Cellulose C4 cross sections. Interior cellulose a, b, and d show stronger cross peaks with surface cellulose f and g compared to interior cellulose c.
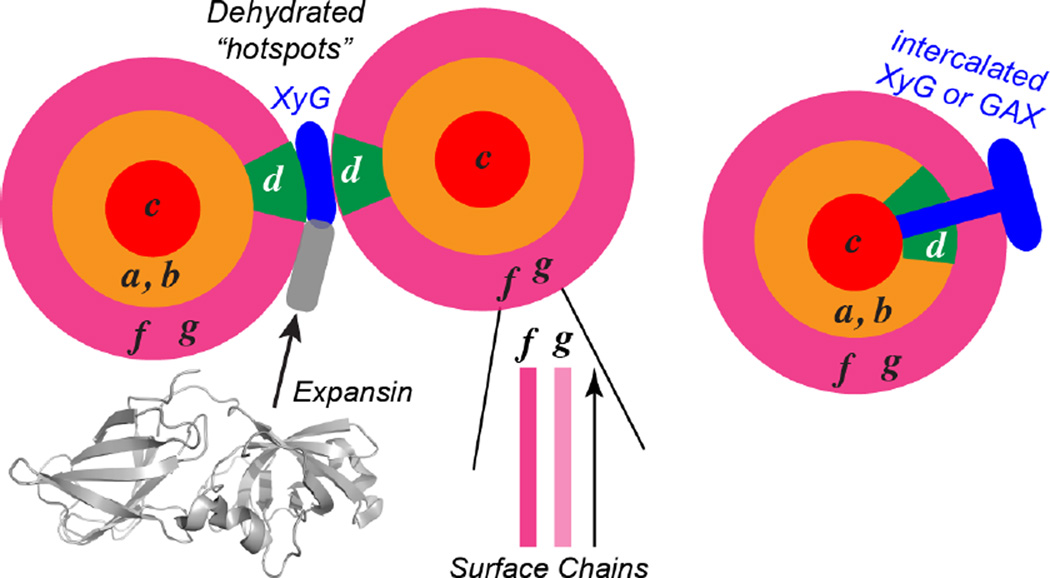
Figure 8.
Proposed model of the spatial distributions and intermolecular contacts of different celluloses in plant primary cell walls. Cellulose g and f reside on the surface of the microfibril and are well mixed with each other. Cellulose a and b are interior chains that are associated with the surface chains, while cellulose c is embedded in the core of the microfibril, inaccessible to water and not in molecular contact with the surface chains. Cellulose d contacts hemicellulose, is targeted by expansins, and is poorly hydrated. Cellulose d may lie on the microfibril surface in a hydrophobic assembly with hemicellulose and form part of the biomechanical “hotspots”, or may be embedded in the microfibril but disordered due to contact with inserted hemicellulose chains.
Multiple cross peaks are detected between surface and interior cellulose, but among interior cellulose a-d, cellulose c C6 has the weakest cross peak with the surface g/f C6 (Fig. 7b), indicating that cellulose c is the furthest away from the microfibril surface. This is consistent with the observation that cellulose c is inaccessible to water in the water-edited 2D spectra (Fig. 3f). In addition, the a/b C4 cross section at 88.9 ppm shows cross peaks with both the surface g/f C4 and with interior cellulose c C4 (Fig. 7c), suggesting that cellulose a and b are sandwiched between the core cellulose c and the surface cellulose. The presence of cross peaks between all significantly populated interior cellulose types (a-d) is important, as it indicates that the different cellulose structures are not separated into different microfibrils or along the long axis of the microfibril, but rather coexist laterally within each microfibril.
13C T1-filtered 2D PDSD spectra show that all interior cellulose 13C intensities decayed to ~62% after 4 s, indicating similar 13C T1 relaxation times of 8.4 s (Fig. S6), while the surface cellulose signals have faster relaxation, to ~ 55%, corresponding to a 13C T1 of ~6.7 s. The shorter T1 of surface cellulose indicates faster nanosecond motions than interior cellulose, while the similarity of the f and g cellulose T1 relaxation times is consistent with the intimate mixing of these chains. Similarly, the fact that all interior cellulose types have similar 13C T1 relaxation times indicates that they are tightly packed to each other, which is consistent with the strong cross peaks between different types of interior cellulose in the 1.0 s PDSD spectrum.
Combining the chemical shift information, the water-edited spectra, and the long-mixing-time spectra, we propose the following structural motifs and spatial locations of the various cellulose polymorphs in the plant primary-wall microfibril (Fig. 8). Surface cellulose f and g are well hydrated but separate chains that are well mixed on the microfibril surface. In the core of the microfibril lies cellulose c, with characteristic C1 and C4 chemical shifts of 104 ppm and 88 ppm. This cellulose does not contact the surface chains, does not interact with matrix polysaccharides, and is poorly hydrated. Between cellulose c and the surface cellulose lie cellulose conformations a and b, which are well hydrated and are in direct contact with the surface cellulose. Interior cellulose e is found at a low population in all cell walls, but is particularly depleted in grass cell walls. The most interesting cellulose in the primary cell walls is cellulose d. Multiple lines of evidence indicate that cellulose d is more disordered than other interior cellulose and interacts extensively with other polysaccharides. First, its C4 chemical shift of 87 ppm (Table 1) is smaller than that of other interior cellulose and is the closest to the surface cellulose C4 chemical shift, suggesting that cellulose d has structural similarity to the surface cellulose. Second, mutant cell wall spectra indicate that cellulose d interacts with hemicellulose in both dicot cell walls (xyloglucan) and grass cell walls (xylan). Third, cellulose d exhibits strong cross peaks with surface cellulose (Fig. 7) but is poorly hydrated (Fig. 3f). This paradox can be resolved if cellulose d, despite being on the microfibril surface, forms a sufficiently large hydrophobic assembly with hemicellulose that decreases its hydration. This hypothesis is supported by molecular dynamics simulations that suggested that xyloglucan can bind multiple cellulose microfibrils to form a sandwiched cellulose-hemicellulose-cellulose junction that provide mechanical strength to cell walls 73 (Fig. 8) as well as acting as biomechanical “hotspots” 74, 75 for wall loosening by expansin 59. Alternatively, cellulose d may reside inside the microfibril, but some hemicellulose chains may intercalate sufficiently into the microfibrils to restructure some of the glucan chains to form this structure. Fourth, while all interior cellulose types adopt the tg conformation for the exocyclic C6, cellulose d also has a significant propensity to form the gt conformer, which is absent in the Iα and Iβ structures 9, 10 but which has been attributed to exist in surface cellulose 76. Binding to hemicellulose may be partly responsible for this alternative C6 conformation. Thus, overall, cellulose d has mixed structural characteristics of interior cellulose and surface cellulose.
The observed structural polymorphism of primary-wall cellulose is more pronounced than that of crystalline microbial cellulose. While the Iα and Iβ allormorphs with a total of four resolvable sets of chemical shifts are well known for bacterial and algae cellulose, the Iα and Iβ conformations do not coexist in the same microfibril. This is supported by high-resolution synchrotron and neutron diffraction data 9, 10 as well as the lack of Iα to Iβ cross peaks in solid-state NMR spectra of bacterial and tunicate cellulose 8. The coexistence of as many as seven structures in each microfibril is thus unique to plant cell wall cellulose, and is consistent with the nature of the plant cell wall as a network of different polysaccharides together carrying out the mechanical and growth functions of the wall. In comparison, bacteria and algae secrete pure cellulose, in the absence of other polysaccharides, and the cellulose microfibrils assemble into large and highly water-absorbent sheets on the cell surface 77–79. The different molecular-level polymorphism of the plant and microbial celluloses thus likely underlie the distinct macroscopic physical and functional properties.
The structural polymorphism of the cellulose chains on the microfibril surface and in the interior indicates a lack of uniformity in the interactions of cellulose with matrix polysaccharides, thus cell wall structural models that depict cellulose as being uniformly coated by a layer of hemicellulose, such as the covalently crosslinked model and the diffuse layer model 58, are unlikely. In comparison, the tethered network model and the stratified layer model both posit xyloglucan to crosslink cellulose microfibrils at specific positions, thus they can in principle be consistent with the observed cellulose chemical shift multiplicity. However, these two models both consider pectins as separate from the cellulose-hemicellulose chains, which is not consistent with the reported intermolecular cross peaks between cellulose and pectins 14–16, 19. Thus the tethered network model and the stratified layer model must also be modified to include pectins into the cellulose-hemicellulose network. In comparison, recent solid-state NMR data, biochemical assays and atomic force microscopy data suggest that cellulose interacts with xyloglucan only in limited positions but contact pectins extensively 13, 14, 16, 19. This model is consistent with the structural polymorphism of cellulose in primary cell walls.
Conclusions
The above high-field 2D 13C-13C correlation solid-state NMR spectra of 13C-labeled plant primary cell walls resolved seven cellulose structures, five of which corresponding to interior cellulose. 13C chemical shifts indicate that most of these interior cellulose structures differ significantly from the Iα and Iβ structures found in the crystalline cellulose of bacteria, algae, and animals. DFT calculations indicate that interior cellulose types a, b and c adopt the tg hydroxymethyl conformation, while interior cellulose d may adopt a mixture of tg and gt conformations. Importantly, 2D correlation spectra detailing intermolecular water-polysaccharide and polysaccharide-polysaccharide contacts show that all interior celluloses are well mixed on the sub-nanometer scale. The surface cellulose f and g are separate chains that are adjacent to each other on the same microfibril surface, interior cellulose c is sequestered in the core of the microfibril and is dehydrated, while interior cellulose a and b are in contact with the surface. The most interesting cellulose, cellulose d, is poorly hydrated, interacts with hemicellulose, and is targeted by expansin during wall loosening. Cellulose d may either lie on the microfibril surface as part of a hydrophobic assembly with hemicellulose or reside in the microfibril but is disordered by embedded hemicellulose. Together, these results indicate that plant primary wall cellulose is much more polymorphic than microbial cellulose, and this structural difference is likely caused by different biosynthetic and assembly pathways of cellulose in the different organisms, as well as the intimate macromolecular interactions of the primary-wall cellulose with matrix polysaccharides, which do not exist in bacterial or algal cellulose 13.
Supplementary Material
Acknowledgments
This work is supported by the Center for Lignocellulose Structure and Formation, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award # DE-SC0001090. Portions of this research were conducted with Advanced CyberInfrastructure computational resources provided by the Institute for CyberScience at The Pennsylvania State University (http://ics.psu.edu). The 900 MHz spectra were measured at the MIT/Harvard Center for Magnetic Resonance, which is supported by NIH grant EB002026. The authors thank Prof. Karl Mueller and Brittney Nagle for providing the Zea Mays sample.
Abbreviations
- Rha, R
rhamnose
- Ara, A
arabinose
- I
interior crystalline cellulose
- s
surface amorphous cellulose
- GalA, GA
galacturonic acid
- G
glucose in xyloglucan
- GAX
glucuronoarabinoxylan
- Xyl, x
xylose
- solid-state NMR
Solid-State nuclear magnetic resonance
- CP
cross polarization
- MAS
magic-angle spinning
- PDSD
protondriven spin diffusion
- RFDR
radio frequency-driven recoupling
- INADEQUATE
incredible natural-abundance double-quantum transfer experiment
Footnotes
Supporting Information
The structural parameters of glucose units in DFT calculation are shown in Table S1. The estimated fractions of glucose conformers in Iα and Iβ allomorphs are shown in Table S2. The 13C RMSDs between measured and calculated 13C chemical shifts are shown in Table S3. NMR pulse sequences are shown in Fig. S1. The assignment of Brachypodium cellulose signals is shown in Fig. S2. The 2D 13C-13C 30 ms PDSD spectrum of Arabidopsis cell walls is shown in Fig. S3. The 2D 13C- 13C long-mixing PDSD spectra of Brachypodium samples are shown in Fig. S4, 5. The 13C-T1 filtered PDSD spectra of Brachypodium cell walls are shown in Fig. S6.
References
- 1.Jarvis M. Chemistry: cellulose stacks up. Nature. 2003;426:611–612. doi: 10.1038/426611a. [DOI] [PubMed] [Google Scholar]
- 2.Pauly M, Keegstra K. Cell-wall carbohydrates and their modification as a resource for biofuels. The Plant journal : for cell and molecular biology. 2008;54:559–568. doi: 10.1111/j.1365-313X.2008.03463.x. [DOI] [PubMed] [Google Scholar]
- 3.Horii F, Hirai A, Kitamaru R. Solid-state high-resolution C-13 NMR studies of regenerated cellulose samples with different crystallites. Polymer Bulletin. 1982;8:163–170. [Google Scholar]
- 4.Atalla RH, Vanderhart DL. Native cellulose: a composite of two distinct crystalline forms. Science. 1984;223:283–285. doi: 10.1126/science.223.4633.283. [DOI] [PubMed] [Google Scholar]
- 5.Vanderhart DL, Atalla RH. Further 13C NMR evidence for the coexistence of two crystalline forms in native celluloses. ACS Symposium Series. 1987;340:88–118. [Google Scholar]
- 6.Kono H, Yunoki S, Shikano T, Fujiwara M, Erata T, Takai M. CP/MAS 13C NMR study of cellulose and cellulose derivatives. 1. Complete assignment of the CP/MAS 13C NMR spectrum of the native cellulose. J. Am. Chem. Soc. 2002;124:7506–7511. doi: 10.1021/ja010704o. [DOI] [PubMed] [Google Scholar]
- 7.Kono H, Erata T, Takai M. Determination of the through-bond carbon-carbon and carbonproton connectivities of the native celluloses in the solid state. Macromolecules. 2003;36:5131–5138. [Google Scholar]
- 8.Kono H, Numata Y. Structural investigation of cellulose Iα and Iβ by 2D RFDR NMR spectroscopy: determination of sequence of magnetically inequivalent D-glucose units along cellulose chain. Cellulose. 2006;13:317–326. [Google Scholar]
- 9.Nishiyama Y, Langan P, Chanzy H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002;124:9074–9082. doi: 10.1021/ja0257319. [DOI] [PubMed] [Google Scholar]
- 10.Nishiyama Y, Sugiyama J, Chanzy H, Langan P. Crystal structure and hydrogen bonding system in cellulose Iβ, from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2003;125:14300–14306. doi: 10.1021/ja037055w. [DOI] [PubMed] [Google Scholar]
- 11.Carpita NC. Structure and biogenesis of the cell walls of grasses. Annu. Rev. Plant Phys. 1996;47:445–476. doi: 10.1146/annurev.arplant.47.1.445. [DOI] [PubMed] [Google Scholar]
- 12.Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. The Plant journal : for cell and molecular biology. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 13.Cosgrove DJ. Re-constructing our models of cellulose and primary cell wall assembly. Curr. Opin. Plant Biol. 2014;22C:122–131. doi: 10.1016/j.pbi.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dick-Perez M, Zhang YA, Hayes J, Salazar A, Zabotina OA, Hong M. Structure and interactions of plant cell wall polysaccharides by two- and three-dimensional magic-angle-spinning solid-state NMR. Biochemistry-Us. 2011;50:989–1000. doi: 10.1021/bi101795q. [DOI] [PubMed] [Google Scholar]
- 15.Dick-Perez M, Wang T, Salazar A, Zabotina OA, Hong M. Multidimensional solidstate NMR studies of the structure and dynamics of pectic polysaccharides in uniformly 13C-labeled Arabidopsis primary cell walls. Magn. Reson. Chem. 2012;50:539–550. doi: 10.1002/mrc.3836. [DOI] [PubMed] [Google Scholar]
- 16.Wang T, Zabotina O, Hong M. Pectin-cellulose interactions in the Arabidopsis primary cell wall from two-dimensional magic-angle-spinning solid-state nuclear magnetic resonance. Biochemistry-Us. 2012;51:9846–9856. doi: 10.1021/bi3015532. [DOI] [PubMed] [Google Scholar]
- 17.Wang T, Salazar A, Zabotina OA, Hong M. Structure and dynamics of Brachypodium primary cell wall polysaccharides from two-dimensional 13C solid-state nuclear magnetic resonance spectroscopy. Biochemistry-Us. 2014;53:2840–2854. doi: 10.1021/bi500231b. [DOI] [PubMed] [Google Scholar]
- 18.Wang T, Park YB, Cosgrove DJ, Hong M. Cellulose-Pectin Spatial Contacts Are Inherent to Never-Dried Arabidopsis thaliana Primary Cell Walls: Evidence from Solid-State NMR. Plant Physiol. 2015;168:871. doi: 10.1104/pp.15.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang T, Hong M. Solid-state NMR investigations of cellulose structure and interactions with matrix polysaccharides in plant primary cell walls. J. Exp. Bot. 2016;67:503–514. doi: 10.1093/jxb/erv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang T, Williams JK, Schmidt-Rohr K, Hong M. Relaxation-compensated difference spin diffusion NMR for detecting 13C-13C long-range correlations in proteins and polysaccharides. J. Biomol. NMR. 2015;61:97–107. doi: 10.1007/s10858-014-9889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosgrove DJ. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005;6:850–861. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- 22.Kimura S, Laosinchai W, Itoh T, Cui X, Linder CR, Brown RMJ. Immunogold labeling of rosette terminal cellulose-synthesizing complexes in the vascular plant vigna angularis. Plant Cell. 1999;11:2075–2086. doi: 10.1105/tpc.11.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman RH, Davies LM, Harris PJ. Solid-state 13C nuclear magnetic resonance characterization of cellulose in the cell walls of Arabidopsis thaliana leaves. Plant Physiol. 1996;111:475–485. doi: 10.1104/pp.111.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy CJ, Cameron GJ, Sturcova A, Apperley DC, Altaner C, Wess TJ, Jarvis MC. Microfibril diameter in celery collenchyma cellulose: X-ray scattering and NMR evidence. Cellulose. 2007;14:235–246. [Google Scholar]
- 25.Fernandes AN, Thomas LH, Altaner CM, Callow P, Forsyth VT, Apperley DC, Kennedy CJ, Jarvis MC. Nanostructure of cellulose microfibrils in spruce wood. Proc. Natl. Acad. Sci. U.S.A. 2011;108:E1195–-E1203. doi: 10.1073/pnas.1108942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman RH, Hill SJ, Harris PJ. Wide-angle x-ray scattering and solid-state nuclear magnetic resonance data combined to test models for cellulose microfibrils in mung bean cell walls. Plant Physiol. 2013;163:1558–1567. doi: 10.1104/pp.113.228262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas LH, Forsyth VT, Sturcova A, Kennedy CJ, May RP, Altaner CM, Apperley DC, Wess TJ, Jarvis MC. Structure of cellulose microfibrils in primary cell walls from Collenchyma. Plant Physiol. 2013;161:465–476. doi: 10.1104/pp.112.206359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oehme DP, Downton MT, Doblin MS, Wagner J, Gidley MJ, Bacic A. Unique Aspects of the Structure and Dynamics of Elementary Iβ Cellulose Microfibrils Revealed by Computational Simulations. Plant Physiol. 2015;168:3-U654. doi: 10.1104/pp.114.254664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill JL, Hammudi MB, Tien M. The Arabidopsis Cellulose Synthase Complex: A Proposed Hexamer of CESA Trimers in an Equimolar Stoichiometry. Plant Cell. 2014;26:4834–4842. doi: 10.1105/tpc.114.131193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang T, Mahgsoudy-Louyeh S, Tittmann B, Cosgrove DJ. Visualization of the nanoscale pattern of recently-deposited cellulose microfibrils and matrix materials in never-dried primary walls of the onion epidermis. Cellulose. 2014;21:853–862. [Google Scholar]
- 31.Zhang T, Zheng Y, Cosgrove DJ. Spatial Organization of Cellulose Microfibrils and Matrix Polysaccharides in Primary Plant Cell Walls as Imaged by Multi-Channel Atomic Force Microscopy. The Plant journal : for cell and molecular biology. 2015;85:179–192. doi: 10.1111/tpj.13102. [DOI] [PubMed] [Google Scholar]
- 32.Harris DM, Corbin K, Wang T, Gutierrez R, Bertolo AL, Petti C, Smilgies DM, Estevez JM, Bonetta D, Urbanowicz BR, Ehrhardt DW, Somerville CR, Rose JKC, Hong M, DeBolt S. Cellulose microfibril crystallinity is reduced by mutating C-terminal transmembrane region residues CESA1A903V and CESA3T942I of cellulose synthase. Proc. Natl. Acad. Sci. U.S.A. 2012;109:4098–4103. doi: 10.1073/pnas.1200352109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White PB, Wang T, Park YB, Cosgrove DJ, Hong M. Water-polysaccharide interactions in the primary cell wall of Arabidopsis thaliana from polarization transfer solid-state NMR. J. Am. Chem. Soc. 2014;136:10399–10409. doi: 10.1021/ja504108h. [DOI] [PubMed] [Google Scholar]
- 34.Bennett AE, Ok JH, Griffin RG, Vega S. Chemical shift correlation spectroscopy in rotating solids: radiofrequency-driven dipolar recoupling and longitudinal exchange. The Journal of chemical physics. 1992;96:8624–8627. [Google Scholar]
- 35.Hong M, Griffin RG. Resonance Assignment for Solid Peptides by Dipolar-Mediated 13C/15N Correlation Solid-State NMR. J. Am. Chem. Soc. 1998;120:7113–7114. [Google Scholar]
- 36.Rienstra CM, Hohwy M, Hong M, Griffin RG. 2D and 3D 15N-13C-13C NMR chemical shift correlation spectroscopy of solids: assignment of MAS spectra of peptides. J. Am. Chem. Soc. 2000;122:10979–10990. [Google Scholar]
- 37.Vanderhart DL. Natural-Abundance 13C-13C Spin Exchange in Rigid Crystalline Organic-Solids. J Magn Reson. 1987;72:13–47. [Google Scholar]
- 38.Kubicki JD, Mohamed MNA, Watts HD. Quantum mechanical modeling of the structures, energetics and spectral properties of I alpha and I beta cellulose. Cellulose. 2013;20:9–23. [Google Scholar]
- 39.Kubicki JD, Watts HD, Zhao Z, Zhong LH. Quantum mechanical calculations on cellulose-water interactions: structures, energetics, vibrational frequencies and NMR chemical shifts for surfaces of I alpha and I beta cellulose. Cellulose. 2014;21:909–926. [Google Scholar]
- 40.Adamo C, Barone V. Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The mPW and mPW1PW models. The Journal of chemical physics. 1998;108:664–675. [Google Scholar]
- 41.Rassolov VA, Ratner MA, Pople JA, Redfern PC, Curtiss LA. 6-31G* basis set for third-row atoms. J. Comput. Chem. 2001;22:976–984. [Google Scholar]
- 42.Wolinski K, Hinton JF, Pulay P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990;112:8251–8260. [Google Scholar]
- 43.Schreckenbach G, Ziegler T. Calculation of NMR Shielding Tensors Using Gauge-Including Atomic Orbitals and Modern Density Functional Theory. J. Phys. Chem. 1995;99:606–611. [Google Scholar]
- 44.Cheeseman JR, Trucks GW, Keith TA, Frisch MJ. A comparison of models for calculating nuclear magnetic resonance shielding tensors. The Journal of chemical physics. 1996;104:5497–5509. [Google Scholar]
- 45.Bühl M, Kaupp M, Malkina OL, Malkin VG. The DFT route to NMR chemical shifts. J. Comput. Chem. 1999;20:91–105. [Google Scholar]
- 46.Wiitala KW, Hoye TR, Cramer CJ. Hybrid Density Functional Methods Empirically Optimized for the Computation of 13C and 1H Chemical Shifts in Chloroform Solution. J. Chem. Theory Comput. 2006;2:1085–1092. doi: 10.1021/ct6001016. [DOI] [PubMed] [Google Scholar]
- 47.Lodewyk MW, Siebert MR, Tantillo DJ. Computational Prediction of 1H and 13C Chemical Shifts: A Useful Tool for Natural Product, Mechanistic, and Synthetic Organic Chemistry. Chem. Rev. 2012;112:1839–1862. doi: 10.1021/cr200106v. [DOI] [PubMed] [Google Scholar]
- 48.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC. Gaussian 09 Revision B.01. Wallingford, CT: 2009. [Google Scholar]
- 49.Sarotti AM, Pellegrinet SC. A Multi-standard Approach for GIAO 13C NMR Calculations. J. Org. Chem. 2009;74:7254–7260. doi: 10.1021/jo901234h. [DOI] [PubMed] [Google Scholar]
- 50.Watts HD, Mohamed MNA, Kubicki JD. Comparison of Multistandard and TMSStandard Calculated NMR Shifts for Coniferyl Alcohol and Application of the Multistandard Method to Lignin Dimers. The journal of physical chemistry. B. 2011;115:1958–1970. doi: 10.1021/jp110330q. [DOI] [PubMed] [Google Scholar]
- 51.Gottlieb HE, Kotlyar V, Nudelman A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997;62:7512–7515. doi: 10.1021/jo971176v. [DOI] [PubMed] [Google Scholar]
- 52.Newman RH, Hemmingson JA. 13C NMR distinction between categories of molecular order and disorder in cellulose. Cellulose. 1995;2:95–110. [Google Scholar]
- 53.Kono H, Erata T, Takai M. Complete assignment of the CP/MAS 13C NMR spectrum of cellulose IIII. Macromolecules. 2003;36:3589–3592. [Google Scholar]
- 54.Yang Y, Fritzsching KJ, Hong M. Resonance assignment of the NMR spectra of disordered proteins using a multi-objective non-dominated sorting genetic algorithm. J. Biomol. NMR. 2013;57:281–296. doi: 10.1007/s10858-013-9788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tycko R, Hu KN. A Monte Carlo/simulated annealing algorithm for sequential resonance assignment in solid state NMR of uniformly labeled proteins with magic-angle spinning. J Magn Reson. 2010;205:304–314. doi: 10.1016/j.jmr.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shklyaev OE, Kubicki JD, Watts HD, Crespi VH. Constraints on Iβ cellulose twist from DFT calculations of 13C NMR chemical shifts. Cellulose. 2014;21:3979–3991. [Google Scholar]
- 57.Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- 58.Cosgrove DJ. Wall structure and wall loosening. A look backwards and forwards. Plant Physiol. 2001;125:131–134. doi: 10.1104/pp.125.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang T, Park YB, Caporini MA, Rosay M, Zhong LH, Cosgrove DJ, Hong M. Sensitivity-enhanced solid-state NMR detection of expansin's target in plant cell walls. Proc. Natl. Acad. Sci. U.S.A. 2013;110:16444–16449. doi: 10.1073/pnas.1316290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Donaldson L. Cellulose microfibril aggregates and their size variation with cell wall type. Wood Sci. Technol. 2007;41:443–460. [Google Scholar]
- 61.Hult EL, Larsson PT, Iversen T. A comparative CP/MAS 13C NMR study of cellulose structure in spruce wood and kraft pulp. Cellulose. 2000;7:35–55. [Google Scholar]
- 62.Hult EL, Larsson PT, Iversen T. Cellulose fibril aggregation - an inherent property of kraft pulps. Polymer. 2001;42:3309–3314. [Google Scholar]
- 63.Wickholm K, Larsson PT, Iversen T. Assignment of non-crystalline forms in cellulose I by CP/MAS 13C NMR spectroscopy. Carbohydr. Res. 1998;312:123–129. [Google Scholar]
- 64.Cadars S, Lesage A, Emsley L. Chemical shift correlations in disordered solids. J. Am. Chem. Soc. 2005;127:4466–4476. doi: 10.1021/ja043698f. [DOI] [PubMed] [Google Scholar]
- 65.Lesage A, Bardet M, Emsley L. Through-bond carbon-carbon connectivities in disordered solids by NMR. J. Am. Chem. Soc. 1999;121:10987–10993. [Google Scholar]
- 66.Dupree R, Simmons TJ, Mortimer JC, Patel D, Iuga D, Brown SP, Dupree P. Probing the Molecular Architecture of Arabidopsis thaliana Secondary Cell Walls Using Two- and Three-Dimensional 13C Solid State Nuclear Magnetic Resonance Spectroscopy. Biochemistry-Us. 2015;54:2335–2345. doi: 10.1021/bi501552k. [DOI] [PubMed] [Google Scholar]
- 67.Salager E, Stein RS, Pickard CJ, Elena B, Emsley L. Powder NMR crystallography of thymol. Phys Chem Chem Phys. 2009;11:2610–2621. doi: 10.1039/b821018g. [DOI] [PubMed] [Google Scholar]
- 68.Watts HD, Mohamed MNA, Kubicki JD. A DFT study of vibrational frequencies and 13C NMR chemical shifts of model cellulosic fragments as a function of size. Cellulose. 2014;21:53–70. [Google Scholar]
- 69.Witter R, Sternberg U, Hesse S, Kondo T, Koch FT, Ulrich AS. 13C chemical shift constrained crystal structure refinement of cellulose Iα and its verification by NMR anisotropy experiments. Macromolecules. 2006;39:6125–6132. [Google Scholar]
- 70.Yao XL, Hong M. Dipolar filtered 1H-13C heteronuclear correlation spectroscopy for resonance assignment of proteins. J. Biomol. NMR. 2001;20:263–274. doi: 10.1023/a:1011251924874. [DOI] [PubMed] [Google Scholar]
- 71.Yao XL, Schmidt-Rohr K, Hong M. Medium- and long-distance 1H-13C heteronuclear correlation NMR in solids. J Magn Reson. 2001;149:139–143. [Google Scholar]
- 72.Dumez JN, Emsley L. A master-equation approach to the description of proton-driven spin diffusion from crystal geometry using simulated zero-quantum lineshapes. Phys Chem Chem Phys. 2011;13:7363–7370. doi: 10.1039/c1cp00004g. [DOI] [PubMed] [Google Scholar]
- 73.Zhao Z, Crespi VH, Kubicki JD, Cosgrove DJ, Zhong LH. Molecular dynamics simulation study of xyloglucan adsorption on cellulose surfaces: effects of surface hydrophobicity and side-chain variation. Cellulose. 2014;21:1025–1039. [Google Scholar]
- 74.Park YB, Cosgrove DJ. A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiol. 2012;158:1933–1943. doi: 10.1104/pp.111.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park YB, Cosgrove DJ. Changes in cell wall biomechanical properties in the xyloglucandeficient xxt1/xxt2 mutant of arabidopsis. Plant Physiol. 2012;158:465–475. doi: 10.1104/pp.111.189779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vietor RJ, Newman RH, Ha MA, Apperley DC, Jarvis MC. Conformational features of crystal-surface cellulose from higher plants. The Plant journal : for cell and molecular biology. 2002;30:721. doi: 10.1046/j.1365-313x.2002.01327.x. [DOI] [PubMed] [Google Scholar]
- 77.Czaja WK, Young DJ, Kawecki M, Brown RM. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules. 2007;8:1–12. doi: 10.1021/bm060620d. [DOI] [PubMed] [Google Scholar]
- 78.Ross P, Mayer R, Benziman M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991;55:35–58. doi: 10.1128/mr.55.1.35-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klemm D, Heublein B, Fink HP, Bohn A. Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed Engl. 2005;44:3358–3393. doi: 10.1002/anie.200460587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.