Abstract
Stromal cells of the tumor microenvironment have been shown to play important roles in both supporting and limiting cancer growth. The altered phenotype of tumor-associated stromal cells (fibroblasts, immune cells, endothelial cells etc.) is proposed to be mainly due to epigenetic dysregulation of gene expression; however, only limited studies have probed the roles of epigenetic mechanisms in the regulation of stromal cell function. We review recent studies demonstrating how specific epigenetic mechanisms (DNA methylation and histone post-translational modification-based gene expression regulation, and miRNA-mediated translational regulation) drive aspects of stromal cell phenotype, and discuss the implications of these findings for treatment of malignancies. We also summarize the effects of epigenetic mechanism-targeted drugs on stromal cells and discuss the consideration of the microenvironment response in attempts to use these drugs for cancer treatment.
Keywords: : cancer-associated fibroblasts, chromatin dynamics, DNA and histone methyltransferases, DNA methylation, endothelial cells, gene expression, histone deacetylases, histone marks, immune cells, noncoding RNAs
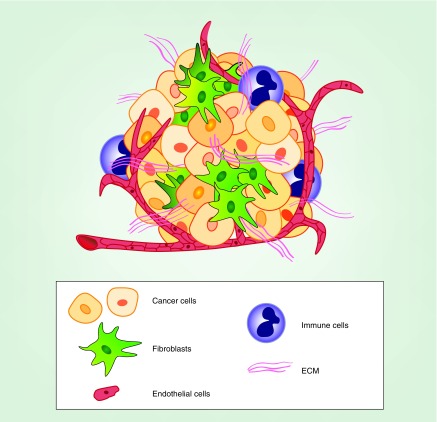
Tumors are an aggregation of cancer cells enmeshed in a tumor microenvironment consisting of nontumor (i.e., stromal) cells and accompanying extracellular matrix (ECM) proteins. Tumors typically contain a number of different stromal cell types, including fibroblasts, lymphocytes, macrophages and endothelial cells (see Figure 1) [1–5]. In some tumor types (e.g., pancreatic ductural adenocarcinoma [PDAC]), the number and mass of stromal cells in a tumor far exceeds that of the cancer cells [6,7]. In particular, fibroblast-type cells form a major structural component of many tumors. These cells have been variously referred to as cancer-associated fibroblasts (CAFs), myofibroblasts, stellate cells or mesenchymal cells. There is debate as to the origins of stromal fibroblasts in cancer; they may be derived from several cell types present in normal tissue, as well as recruited from bone marrow-derived mesenchymal cells [8–10]. However, given their similar phenotype in tumors, for the purpose of this review we will treat them as a single group and use the terms, fibroblasts or CAFs, to identify them. Fibroblasts are often quiescent in normal tissue but become activated in tumors as a result of tumor cell derived factors (e.g., TGF-β, PDGF and reactive oxygen species) in a process sometimes referred to as transdifferentiation [8,10]. In this process, fibroblasts become increasingly proliferative, secrete increased ECM proteins and various growth factors and alter their actin cytoskeleton to a more contractile configuration with increased stress fibers, often expressing α-smooth muscle actin (SMA) and fibroblast activation protein (FAP) [8,10]. The biology of activated fibroblasts is intimately linked with the tumor cells via their exchange of growth factors and cytokines, as well as other materials such as micro-RNAs (miRNAs) and metabolites (see Figure 2) [5,11–13]. Immune cells (e.g., infiltrating macrophages, lymphocytes, natural killer cells) are recruited to the tumor site by cytokines secreted from cancer cells, fibroblasts and resident macrophages [3,10,14]. These immune cells maintain the tumor microenvironment in a chronically inflammatory and immunosuppressive state [14]. Endothelial cells are recruited to form new blood vessels in the tumor by secretion of VEGF and other ligands from tumor cells and other cell types [4].
Figure 1. . Schematic representation of tumor and nontumor cells in the tumor microenvironment.
An idealized diagram is shown, demonstrating how tumors are composed of cancer cells, fibroblasts, multiple types of immune cells (lymphocytes, dendritic cells, macrophages etc.), vascular endothelial cells and ECM fibers.
ECM: Extracellular matrix.
Figure 2. . Epigenetic regulation of stromal cells in the tumor microenvironment.
Examples of epigenetic regulation in stromal cells are summarized. Cellular compartments of the microenvironment are labeled and highlighted with different background colors. White boxes describe examples of epigenetic mechanisms shown to occur in stromal cells and discussed in the text. Within the white boxes, up and down arrows indicate increased or decreased occurrence or expression, respectively. Right arrows indicate sequences of events leading to a final outcome. Line arrows running between cell type compartments indicate the direction of material transferred between cell types and point to white boxes describing epigenetic mechanisms initiated by this material transfer. Bold text alongside these arrows list the specific materials (e.g., ligands, miRNAs) transferred between the cell types.
Data taken with permission from [15–21].
Thus, as the tumor cells evolve and grow, the stromal cells also evolve, altering their phenotypes in ways to support and/or react to the tumor. In a sense, by secretion of cytokines, growth factors and metabolites, and manipulation of their surface proteins, cancer cells hijack ‘normal’ differentiation programs of stromal cell types, such as the activation of fibroblasts upon wounding and the stimulation of endothelial cell proliferation and migration during angiogenesis. The malignant behavior of cancer cells is driven by mutational events plus epigenetic alterations that together cause dysregulation of gene expression. A few studies reported mutations in stromal fibroblasts from breast cancer, and head and neck squamous carcinomas [22–24], while others found no genetic anomalies in stromal fibroblasts from cervical or breast cancer [25–27]. The current consensus is that mutations in stromal fibroblasts from epithelial-derived tumors are rare, and that some of the conflicting results could be a result of the difficulties in separating relatively pure pools of cancer cells and fibroblasts from tumors, or the inclusion in fibroblast pools of fibroblast-like cells derived from tumor cells by epithelial–mesenchymal transition [5,28–29]. Thus, the phenotype of the stromal cells is thought to be driven mainly by epigenetic mechanisms. However, surprisingly little is known concerning the role of epigenetics in tumor-associated stromal cells. In this review we present examples of epigenetic mechanisms that control stromal cell gene expression to illustrate what has been learned and where there are deficits in its understanding. We also point out the importance of understanding the epigenetics of stromal cells as epigenetic mechanism-targeted drugs are considered for treatment of specific cancers.
Evidence for epigenetic regulation in stromal cells
Epigenetics encompasses the mechanisms by which cells maintain or alter states of gene expression in a stable or heritable manner, independent of the DNA sequence [30,31]. For example, although all somatic cells of the human body possess the same DNA genome, each differentiated cell type expresses a different profile of mRNAs and proteins maintained by epigenetic mechanisms, thus resulting in specific phenotypes for each cell type. The three mechanisms by which these stable changes in gene expression are regulated are: DNA methylation, chromatin protein post-translational modification and expression of noncoding RNAs. Although these mechanisms have often been studied separately, it is likely that multiple epigenetic mechanisms function interactively in the regulation of expression of particular genes. For example, promoter DNA methylation and nucleosomal histone 3, lysine K27 trimethylation (H3K27me3) often occur together at repressed genes [32]. In addition, since miRNAs are produced by transcription from DNA templates, miRNA expression is subject to regulation by promoter DNA methylation and histone modification of associated nucleosomes. Figure 2 summarizes some of the examples discussed below to illustrate the different types of epigenetic regulation that have been described in tumor-associated stromal cells.
Regulation of gene expression in stromal cells by DNA methylation
DNA methylation is the longest studied epigenetic mechanism regulating gene expression and consists of the covalent modification of DNA cysteines by a methyl group. This methylation usually occurs at a cysteine followed by a guanine, denoted as CpG (5′cytosine-phosphate-guanine-3′). These CpGs are often found in clusters called CpG islands. Methylation of CpGs in the promoter region of genes is frequently associated with, and may be causal of, the silencing of those genes [33]. Newer studies indicate that CpG methylation within gene bodies can be associated with gene activation [34,35]. DNA methylation is regulated by the activities of DNA methyltransferases (DNMT1, DMNT3a and DMNT3b) and multiple DNA demethylases [36,37]. Numerous investigations have shown that CpG islands of DNA are globally hypomethylated in a number of cancer cell types, and that the aberrant expression of specific genes in cancer cells is in part due to demethylation of normally DNA methylated genes [34,38–39]. In addition, the local hypermethylation of DNA promoters of certain genes (e.g., tumor suppressors) and the associated silencing of these genes has been demonstrated in cancer cells, and shown to play a role in oncogenesis [40].
Several studies have investigated the role of DNA methylation in the regulation of CAF gene expression using genome-wide approaches (see Table 1). In one of the first studies of this type, Jiang et al. [41] used methylation-sensitive single nucleotide polymorphism array analysis to compare DNA methylation in stromal fibroblasts from gastric tumors versus normal stomach fibroblasts and reported global DNA hypomethylation with hypermethylation at only a few genes (e.g., HOXB6) in the tumor-associated fibroblasts. Using a methylation array approach to compare DNA methylation in genes from non-small-cell lung CAFs versus control fibroblasts from normal areas of lung, Vizoso et al. [42] also demonstrated global hypomethylation of lung cancer CAFs.
Table 1. . DNA methylation studies in cancer-associated stromal cells discussed in this review.
| Study (year) | Cell type | Gene | Methylation status | Ref. |
|---|---|---|---|---|
| Jiang et al. (2008) | Gastric cancer CAFs | Global hypomethylation | [41] | |
| |
|
HOXB6 |
Hypermethylated |
|
| Vizoso et al. (2015) | Lung cancer CAFs | Global hypomethylation | [42] | |
| |
|
SMAD3 |
Hypermethylated |
|
| Gotze et al. (2015) | Activated hepatic stellate cells | Global hypomethylation | [43] | |
| Spon2 | Hypomethylated | |||
| |
|
Cnr2, Mmrn2 |
Hypermethylated |
|
| Yu et al. (2012) | Pancreatic cancer CAFs | Little gene regulation by hypermethylation | [15] | |
| |
|
ADAM12 |
Hypomethylated |
|
| Xiao et al. (2016) | Pancreatic cancer CAFs | Many genes downregulated by hypermethylation | [44] | |
| |
|
SOCS1 |
Hypermethylated |
|
| Albrengues et al. (2015) |
Head and neck CAFs |
PTPN6 |
Hypermethylated |
[45] |
| Bian et al. (2012) |
Activated hepatic stellate cells |
Pten |
Hypermethylated |
[46] |
| Janson et al. (2008) |
Colon cancer lymphocytes |
IFNG |
Hypermethylated |
[47] |
| Chung et al. (2007) | Prostate cancer xenograft endothelial cells | Cyp24 | Hypermethylated | [16] |
Under ‘Ref.’, the first author year and reference number are listed. Under ‘Gene’, specific genes identified as altered in methylation status are listed.
CAF: Cancer-associated fibroblast.
Yu et al. [15] isolated and cultured CAFs from resected tumors from PDAC patients and normal human pancreatic fibroblasts. After several passages, the cultured cells were grown with or without the DNMT inhibitor, 5-aza-2′-dexoycytidine (5-AzaDC; also known as decitabine), and studies were performed to determine the gene expression changes and DNA methylation alterations occurring due to 5-AzaDC treatment. This study concluded that relatively few genes were regulated by DNA hypermethylation status in CAFs compared with tumor cells, as demonstrated by minimal effects of 5-AzaDC treatment on gene expression. In contrast, a recent study by Xiao et al. compared PDAC-derived CAFs cultured alone versus CAFs cocultured for 24 h with PDAC cells using combined gene methylation and expression arrays [44]. An immunoisolation technique was used to separate fibroblasts from cancer cells after coculture. This study showed that a large number of genes were downregulated and promoter DNA methylated in CAFs cocultured with PDAC cells versus CAFs cultured alone, indicating that many genes were regulated by DNA methylation in these CAFs. When normal bone marrow-derived mesenchymal cells were co-cultured with PDAC cells, a similar pattern of gene methylation and expression was observed, suggesting that the gene expression pattern in these fibroblasts is driven by the cancer cells. The differences between these two studies suggest that: culture of CAFs and normal fibroblasts through several passages may minimize detectable differences in gene expression and DNA methylation; and continued contact with tumor cells may simulate the tumor microenvironment sufficiently to allow the identification of differentially methylated and expressed genes. Additional approaches such as analysis of gene expression and DNA methylation in microdissected CAFs from PDAC versus fibroblasts from normal pancreas, and comparison of fibroblasts in tissue sections from PDAC versus normal pancreas for specific protein (by immunohistochemistry [IHC]) or gene expression (by in situ hybridization) may be useful to validate the roles of DNA methylation the regulation of specific gene expression in PDAC-associated CAFs in vivo.
Several studies have focused on specific genes that are regulated by DNA methylation and play important roles in the CAF phenotype. For example, in the study by Vizoso et al. [42], pathway analysis of differentially methylated genes in lung cancer-derived CAFs indicated that the TGF-β signaling pathway was affected by altered DNA methylation. Among genes in this pathway, the SMAD3 gene was found to exhibit hypermethylation-associated silencing in CAFs (see Figure 2). The authors further showed that CAFs treated with TGF-β exhibited increased mRNA expression of wound-associated genes (e.g., COL1A1 and SPARC) compared with normal fibroblasts, and suggested that the loss of SMAD3 expression elicits hyper-responsiveness to TGF-β in CAFs. While the genetic deletion of SMAD3 in mice leads to increased responsiveness to TGF-β in terms of wound healing [48], SMAD3 depletion in various fibroblasts has also been shown to decrease ECM secretion and contractility [49,50]. Thus, the significance of SMAD3 depletion by epigenetics mechanism in CAFs remains incompletely explored. In PDAC, Yu et al. [15] found that the ADAM12 gene promoter was hypomethylated in PDAC-derived CAFs and overexpressed in these cells versus normal fibroblasts (see Figure 2). The significance of these findings to tumor biology are this study identifies a mechanism by which ADAM12, a protein reported to play a role in tumorigenesis, is overexpressed in tumor-associated stromal cells [51,52]. Xiao et al. [44] identified SOCS1 as a gene that is silenced and promoter methylated in fibroblasts as a result of contact with PDAC cells. The potential relevance of SOCS1 repression to PDAC biology was demonstrated by showing that SOCS1 depletion increases the expression of IGF-1, a protumoral growth factor. IHC of PDAC samples exhibited decreased SOCS1 expression in tumor-associated stroma compared with normal stroma areas of tissue, validating their finding in vivo. Finally, Albrengues et al. [45] demonstrated that treatment of normal fibroblasts with leukemia inducible factor, an IL6 class cytokine secreted by cancer cells, causes hypermethylation and silencing of the SHP-1 tyrosine phosphatase gene, PTPN6. Silencing of gene causes constitutive activation of JAK1, thus promoting the fibroblast proinvasive phenotype [45]. Treatment of head and neck carcinoma-derived CAFs or leukemia inducible factor treated normal fibroblasts with the DNMT inhibitor, 5-AzaDC, restored the expression of SHP-1, decreased JAK1/STAT3 activation, and decreased the protumoral properties of the fibroblasts, thereby demonstrating the significance of DNA methylation for fibroblast activation. Together, these studies demonstrate that both increased DNA methylation and silencing (SMAD3, SOCS1 and PTPN6) and decreased DNA methylation and consequent gene expression (ADAM12) contribute to the activated phenotype of CAFs.
The process of CAF activation has been shown to be mechanistically similar to the transdifferentiation of fibroblasts during tissue fibrosis, a process associated with certain malignancies [8,53]. For example, in both cases fibroblasts develop increased contractility and increase expression of SMA and type 1 collagen [5,46,53–54]. A number of studies have explored epigenetic alterations involved in the transdifferentiation of quiescent fibroblasts to activated myofibroblasts in models of fibrosis. For example, global DNA hypomethylation has been reported during the activation of hepatic stellate cells by culturing in vitro [43], similar to the findings in CAFs [41,42]. In a study focused on PTEN, Bian et al. [46] utilized a hepatic stellate cell line activated by TGF-β to demonstrate that PTEN is silenced by promoter DNA hypermethylation upon stellate cell activation. Treatment of cells with siRNAs to decrease the expression of DNMT1, or by incubation with 5-AzaDC reduced PTEN promoter hypermethylation and increased PTEN mRNA and protein expression. In addition, these treatments inhibited TGF-β-mediated increases in SMA and COL1A1 expression. PTEN silencing in stromal fibroblasts has been shown to increase the initiation and progression of mammary cancer in a genetically engineered mouse model [55,56]. Thus, it is possible that PTEN expression could be repressed by promoter hypermethylation in breast cancer stromal fibroblasts but further studies are needed to prove this mode of regulation occurs in the tumor microenvironment.
Few studies have investigated the significance of DNA methylation for altered gene expression in immune or endothelial cells in the tumor microenvironment. Janson et al. [47], demonstrated that the differentiation of naive human CD4+ lymphocytes into mature Th1 lymphocytes requires the DNA demethylation of the IFNG gene promoter and upstream enhancer region for expression of IFN-γ. In colon cancer-derived tumor-infiltrating lymphocytes, however, the IFNG gene remains hypermethylated, preventing the maturation of CD4+ Th1 lymphocytes and thus identifying a mechanism for immunosuppression in cancer associated lymphocytes. This group further demonstrated that treatment of cultured tumor-infiltrating lymphocytes with the DNMT inhibitor, 5-azacytidine (5-AzaC), induced the expression of IFN-γ, supporting the possibility that DNMT inhibitors may be useful for stimulating the immune response in cancer. In a study exploring the mechanism of selective sensitivity of tumor-associated endothelial cells to the vitamin D analog, calcitriol, Chung et al. [16] used mouse xenograft models to show that the Cyp24 gene was DNA hypermethylated and transcriptionally silenced in tumor-associated endothelial cells, in contrast to the non-methylated status of Cyp24 and inducibility of CYP24 expression by calcitriol in normal endothelial cells (see Figure 2). Treatment of tumor-derived endothelial cells with 5-AzaDC evoked Cyp24 expression in these cells, and decreased their sensitivity to calcitriol-mediated growth inhibition. Thus, this study identifies a mechanism by which tumor-associated endothelial cells are selectively sensitive to calcitriol treatment. Together, these two investigations illustrate the complexity of using epigenetic mechanism targeted drugs for cancer treatment, as in one case a DNMT inhibitor caused a potentially antitumoral effect (IFN-γ), while in the other, a protumoral effect (resistance of tumor endothelial cells to calcitriol) was found.
Regulation of gene expression in stromal cells by histone modifications
Chromatin is a highly regulated molecular complex composed of DNA, histones and other proteins involved in gene transcription and the maintenance of chromatin structure. Certain chromatin complexes are involved in the post-translational modification (e.g., methylation, acetylation, ubiquitylation) of histones, leading to activation or suppression of transcription of specific genes [57,58]. The methylation of nucleosomal histones at specific lysine residues can either repress (e.g., H3K9me3, H3K27me3) or stimulate (e.g., H3K4me3) transcription. For example, polycomb complex 2 (PRC2), including the histone methyltransferase, enhancer of zeste homolog 2 (EZH2), trimethylates histone 3 at lysine 27 (H3K27me3) at nucleosomes associated with certain gene promoters, resulting in transcriptional repression, whereas the mixed lineage leukemia 1 (MLL1) complex and other related complexes di- and trimethylate histone 3, lysine 4 (H3K4me2, H3K4me3), leading to transcriptional activation [59,60]. Histone demethylases, which remove methyl groups from histones, also play active roles in regulating the epigenetic status of the cell [61]. Histone acetylation at specific lysines (e.g., H3K9, H3K36, H3K27) is accomplished by a large group of histone acetylases and is often associated with gene activation, whereas removal of these acetyl marks by histone deacetylases (HDACs) is linked to gene repression [57,62–63].
Surprisingly, only a limited number of studies have addressed the role of histone marks in regulation of transcription in CAFs. Tyan et al. [17] demonstrated that coculture of fibroblasts derived from normal human breast tissue with breast cancer cells led to an increase in mRNA and protein levels of the ECM-modifying proteoglycanase, ADAMTS1, in the fibroblasts. No change in DNA methylation at the ADAMTS1 promoter was observed. However, a decrease in the repressive mark, H3K27me3, associated with the ADAMTS1 promoter was demonstrated. In addition, the presence of the methyltransferase responsible for the H3K27me3 mark, EZH2, was decreased at this promoter. This study suggests that the EZH2 is involved in the repression of the ADAMTS1 gene in normal fibroblasts, but unidentified factors from breast cancer cells initiate a removal of EZH2 from the ADAMTS1 promoter in fibroblasts causing increased transcription of this gene (see Figure 2). The clinical relevance of this study was supported by demonstration that ADAMTS1 expression levels in CAFs in breast cancer patients are positively correlated with features of the aggresiveness of this disease such as the extent of lymph node metastases in these patients.
Histone methyltransferases that add activating marks (e.g., H3K4me3, H3K36me3) to nucleosomal histones have been shown to play important roles in gene regulation in many contexts [60,64–65]. However, to our knowledge, no studies have been performed to investigate the roles of these enzymes or their marks in CAFs. In a study focused on liver fibrosis, Perugorria et al. [66] investigated the epigenetic mechanisms at play in hepatic stellate cells (SCs) activated by time in culture. They found that transcripts for the H3K4 trimethyl transferases, MLL1 and SET1, and the related H3K4 and H3K36 methyltransferase, ASH1, were increased in activated rat and human SCs. Total H3K4me2, H3K4me3 and H3K36me3 were dramatically increased in activated rat SCs, while total H3K9me3 and H3K27me2 were reduced, suggesting an overall increase in gene expression. ChIP studies showed that ASH1 presence and H3K4 trimethylation were increased upon rat SC activation at several profibrotic genes, including Col1a1, TIMP1 and TGF-β1. Finally, ASH1 knockdown caused a decrease in mRNA levels of these profibrotic genes, suggesting that ASH1 has a significant role in the activation of fibroblasts during fibrosis. It is likely that similar mechanisms are involved in the alterations in gene expression during CAF transdifferentiation, but future studies are necessary to determine the specific roles of histone methyltransferase-mediated gene activation in these fibroblasts, especially since inhibitors of these histone methyltransferases are being developed for use in cancer [65,67–68].
The role of chromatin changes within cancer cells themselves that promote the evasion of immunosurveillence (e.g., by altering their cell surface proteins or cytokine secretion) has been well documented. For example, transcription of T-helper 1-type chemokines, CXCL9 and CXCL10 is suppressed in colon and cancer cells via DNA methylation and PRC2 complex-mediated H3K27me3 marking of the promoters of these chemokines, thereby limiting T-cell tumor infiltration [69,70]. In addition, epigenetic processes have been shown to be essential in normal immune cell maturation and function [71–73], and similar processes are likely to regulate tumor-associated immune cells. However, only a few investigations of epigenetic mechanisms in immune cells have been conducted in the context of the tumor microenvironment. Examples showing the regulation of H3K27 trimethylase, EZH2, by miRNAs in tumor-associated T cells, and regulation of miR-146a transcription by promoter histone acetylation in macrophages will be discussed in a later section, in which we deal with the coordination between different epigenetic mechanisms
Endothelial cell regulation by chromatin-based epigenetic mechanisms in the tumor setting was explored by Hellebrekers et al. [18]. This group compared the gene expression of normal endothelial cells versus endothelial cells treated with cancer cell conditioned media. They identified genes for a number of proteins (e.g., clusterin, fibrillin 1, quiescin Q6) that were downregulated in the cancer cell conditioned state and found that each of these genes had reduced total acetylated H3 and H3K4me3 associated with their promoters in the cancer cell conditioned endothelial cells (see Figure 2). siRNA mediated reduction of clusterin, fibrillin 1 and quiescin Q6 in normal endothelial cells was shown to mediate an increase in endothelial cell sprouting and proliferation, suggesting that repression of these genes supports tumor angiogenesis. For each of these genes, treatment of cancer cell conditioned endothelial cells with the HDAC inhibitor, Trichostatin A, increased gene expression and increased the presence of H3-ac and H3K4me3. Repression of the same genes was demonstrated in endothelial cells isolated from human colon cancer samples, supporting the translational implications of these findings.
Intra- and intercellular regulation of stromal cells by miRNAs
Noncoding RNAs of different types and sizes have been shown to play various roles in gene regulation. We will focus on mi-RNAs, short (18–25 base) oligomers encoded in the genome that repress translation of target genes by complexing with mRNAs, leading to inhibition of translation and/or increased mRNA degradation [74], as this subgroup of noncoding RNAs has been significantly studied in stromal cells. A large number of miRNAs have been identified in the human genome and each species of miRNA may have multiple mRNA targets [75]. Many individual miRNA species have been shown to play roles in the regulation of stromal cells of the tumor microenvironment and this subject has recently been reviewed [76–79].
A number of groups have compared the miRNA profiles of CAFs versus normal fibroblasts. Aprelikova et al. [80] identified miRNAs that are altered in CAFs isolated from endometrial cancer versus normal endometrial fibroblasts. One of the most downregulated species in this study was miR-31, which found regulate the chromatin cofactor SATB2 gene, a protein significantly overexpressed in CAFs. SATB2 introduction into normal fibroblasts increased the expression of a number of several genes that play roles in cell migration and invasion, supporting the hypothesis that miR-31 downregulation contributes to the CAF phenotype. A key study by Mitra et al. [19] demonstrated that CAFs associated with metastatic serous ovarian cancer are regulated by miRNAs. By comparison of miRNA expression profiles of normal omental fibroblasts (NOFs) versus patient-derived CAFs, and a second comparison between NOFs cocultured with ovarian cancer cells (to induce CAF-like cells) versus NOFs without coculture, they found that miR-31 and miR-214 were downregulated, and miR-155 was upregulated, in both sets of CAFs versus NOFs. Transfection of normal fibroblasts with anti- miR-31, anti-miR-214 and pre-miR-155, recapitulated this miRNA profile and endowed these fibroblasts with an increased ability to support the invasiveness and colony formation of cocultured ovarian cancer cells. Further investigation showed that this miRNA profile upregulated the chemokine, CCL5 and that CCL5 secretion by CAFs contributed to the growth of tumor cells. Additional miRNA profiling studies [81–83] of fibroblasts from lung, prostate and gastric cancer tissue identified a number of miRNAs that are dysregulated in CAFs versus normal fibroblasts and play roles in the CAF phenotype (see Table 2). Perhaps surprisingly, the profile of significantly altered miRNA species discovered for each CAF is unique with little overlap, suggesting that there are many miRNA-modulated paths to becoming a CAF. Although these results are interesting and provocative, the significance of the unique miRNA profile associated with each CAF type is unknown? Are the different profiles intrinsic to the fibroblasts from each tissue or are they dictated by the tumor cells, as suggested by some studies (e.g., [19]). An informative experiment would be to incubate a single source of normal fibroblasts with different cancer cell types, and then assess the miRNA profile of the fibroblasts to determine if miRNA alterations are cancer cell type dependent.
Table 2. . miRNA studies in cancer-associated fibroblasts.
| Study (year) | Fibroblast origin | miRNAs | miRNA target | Relevance to oncogenesis | Ref. |
|---|---|---|---|---|---|
|
miRNA profile studies | |||||
| Aprelikova et al. (2010) |
Endometrial cancer |
miR-31↓ |
SATB2 |
↑Cancer cell migration |
[80] |
| Mitra et al. (2012) | Ovarian cancer | miR-31↓ | Colony formation | [19] | |
| miR-155↑ | |||||
| |
|
miR-214↓ |
CCL5 |
|
|
| Yang et al. (2014) | Gastric cancer | miR-93↑ | ↑Cancer cell | [84] | |
| miR-106b↑ | PTEN | Migration and invasiveness | |||
| |
|
miR-214↓ miR-424↓ |
|
|
|
| Doldi et al. (2015) | Prostate cancer | miR-1↑ miR-133b↑ | ↑CAF activation | [81] | |
| |
|
miR-143↑† miR-210↑ |
|
|
|
| Shen et al. (2016) | Lung cancer | miR-1↓ | CCL2 | ↑Cancer cell migration and colony formation | [82] |
| miR-31↑ | FOXO3a | ||||
| |
|
miR-206↓ |
CCL2 |
|
|
| Wang et al. (2016) |
Gastric cancer |
miR-181d↑ |
|
↑Cancer cell chemotaxis |
[83] |
|
Focused miRNA studies | |||||
| Musumeci et al. (2011) | Prostate cancer | miR-15a↓, miR-16↓ | FGF2 | ↑Cancer cell migration | [85] |
| |
|
|
FGFR1 |
|
|
| Aprelikova et al. (2013) |
Endometrial cancer |
miR-148a↓ |
WNT10B |
↑Cancer cell migration |
[86] |
| Bullock et al. (2013) | Colorectal cancer | miR-21↑ | RECK | ↑Cancer cell invasiveness | [87] |
| |
|
|
|
↑CAF activation |
|
| Ali et al. (2014) | Pancreatic cancer | miR-21↑ | ↑Cancer cell clonogenicity | [88] | |
| miR-221↑ | |||||
| |
|
|
|
↑CAF migration |
|
| Kuninty et al. (2016) | Pancreatic cancer | miR199-3p↑ | ↑Endothelial cell tube formation | [89] | |
| |
|
miR214-3p↑ |
|
|
|
| Chen et al. (2016) |
Liver cancer |
miR200a |
HGF |
↑Patient survival |
[90] |
| Baroni et al. (2016) | Triple negative | miR-9↑ | ↓HGF expression | [91] | |
| Breast cancer | ↑CAF motility | ||||
miRNA profile studies utilized miRNA array techniques to compare miRNAs in CAFs versus fibroblasts derived from normal tissue of the type (e.g., endometrium, pancreas) from which the cancer originated. Focused miRNA studies identified specific miRNAs from previous data and studied their function in CAFs. miRNA targets listed are transcripts that were verified as direct targets of specific miRNAs in these studies. Arrows indicate direction of change in expression or phenotype.
†Smaller arrows indicate a change of lessor magnitude compared with full sized arrows.
CAF: Cancer-associated fibroblast; CCL2: Chemokine (c-c motif) ligand 2; FGFR: Fibroblast growth factor receptor; FOXO3a: Forkhead box 03a; HGF: Hepatocyte growth factor; PTEN: Phosphatase and tension homolog; RECK: Reversion inducing cysteine-rich protein with Kazal motifs; SATB2: Special AT-rich sequence-binding protein 2; WNT10: Wingless-related integration site 10.
Additional investigations have focused on particular miRNAs known or predicted to be involved in oncogenesis or cellular behavior, and studied the function of these miRNAs in CAFs (see Table 2). For example, Musumeci et al. [85] showed that miR-15a and miR-16 are decreased in prostate cancer CAFs leading to the upregulation of their direct targets, FGF2 and FGFR1. Restoration of miR-15a and miR-16 expression of CAFs led to decreased CAF cell growth, and a decreased ability of the CAF conditioned medium to support prostate cancer cell proliferation and migration. An example of an increase in miRNA expression was demonstrated by Bullock et al. [87], who showed that CAFs from colorectal cancer (CRC) exhibited increased expression of miR-21. This increased miRNA expression was shown to decrease expression of its target, RECK, an inhibitor of the matrix metalloprotease (MMP2). Thus, elevated miR-21 imparts CAFs with increased MMP2 activity, which contributes to CRC cell invasiveness. Additional examples of miRNAs studied in CAFs are described in Table 2 and reviewed elsewhere [77,78].
In addition to the cell-autonomous regulation of stromal cells by miRNAs, miRNAs are reported to be transferred from cancer cells to other cell type via extracellular vesicles (EVs, e.g., exosomes, microvesicles) [92]. For example, Pang et al. [93] demonstrated that EVs released from human PDAC cells contained miR-155, which was transferred to mouse primary pancreatic fibroblasts. Treatment of these fibroblasts with miR-155-containing EVs increased SMA and FAP expression, and increased cell proliferation. The EV-mediated transfer of miRNAs (miR-146a, miR-150 and miR-155) from chronic lymphocytic leukemia cells to mesenchymal cells has also been reported [94]. Endothelial cells are also regulated by miRNAs delivered via EVs secreted from cancer cells of various types, as reviewed recently [74]. For example, Yamada et al. [20] recently showed that miR-1246 secreted in EVs by human CRC cells is taken up by cultured endothelial cells. miR-1246 was shown to downregulate endothelial cell expression of its direct target, the promyelocytic leukemia protein, a known regulator of the TGF-β signaling pathway [95]. Promyelocytic leukemia protein depletion increased SMAD 1/5/8 signaling and suppressing SMAD 2/3 signaling (see Figure 2), which promoted endothelial cell migration and tube formation. Similarly, the exchange of miRNAs from tumor cells to immune cells has been investigated [96–98]. miRNAs can also be transferred from stromal cells to tumor cells. For example, miR-409 produced in prostate cancer CAFs is transferred via EVs to prostate cancer cells, where this miRNA contributes to increased tumor cell growth and EMT [99]. EV-mediated transfer of miRNAs from CAF to cancer cells has also been reported in breast cancer [100]. Yang et al. [21] found that IL4-stimulated human macrophages, which are similar to macrophages found in breast cancer infiltrate, released miR-223-containing exosomes that were taken up by breast cancer cells. Uptake of this miRNA was shown to increase the invasiveness of the breast cancer cells via downregulation of the miR-223 target, myocyte enhancer factor (Mef2c).
The application of technologies for miRNA profiling and manipulation of miRNA levels using mimics and anti-miRs has led to an explosion of research and publications. It is clear that miRNA synthesis can be dysregulated in stromal cells as a result of conditions in the tumor setting. In addition, miRNAs can be transferred bidirectionally between tumor and stromal cells, thereby orchestrating complex effects on the tumor microenvironment. However, these studies often raise as many questions as they answer. For example, what factors from tumor cells initiate the observed changes in the fibroblast miRNAs, and what chromatin-based epigenetic mechanisms in fibroblasts lead to the reregulation of miRNA expression. In only a few studies have specific materials released by tumor cells been proven to initiate changes in stromal cell miRNA expression. For example, Doldi et al. [81] found that normal fibroblasts treated with IL6 develop a similar miRNA profile to prostate cancer-derived CAFs. The ability of miRNAs to slip from one cell type to another only increases the complexity of these issues. In some studies, it is uncertain if the miRNAs are produced endogenously in stromal cells or are transcribed in another cell type and transferred to the cell type under study. Greater use of PCR techniques that amplify the pri-form of miRNAs (e.g., see [91]), rather than the mature form, would allow more certainty of the cell of origin for the miRNA being studied. Second, do the results of these studies reflect the levels of miRNA in vivo? Most studies have used tumor-associated stromal cells and their normal counterparts isolated from tissues and then cultured through several passages before miRNA analysis, which could certainly alter the miRNA profile. Some investigators have used in situ hybridization techniques on tumor sections or laser-capture microdissection of fixed tumor tissue to verify alterations of miRNA species in CAFs versus normal fibroblasts in vivo and also distinguish if the miRNAs are only present in CAFs or are also expressed in adjacent tumor cells. Another useful approach to gain a better understanding of miRNA alterations in vivo is to compare miRNA profiles of stromal cells (e.g., fibroblasts) isolated from tumors with induced tumor-associated-like cells prepared by coculturing normal cells with cancer cells or their conditioned medium, as has been performed in some studies [19,82].
Epigenetic regulation at multiple levels in stromal cells
As mentioned earlier, epigenetic regulation may occur at multiple levels simultaneously, although few studies in tumor-associated stromal cells have included such data. Indeed, it is likely that most alterations in gene expression are a net result of multiple epigenetic mechanisms working in concert, including, for example, changes in DNA methylation, altered binding of histone modifying enzymes and their cofactors, and alterations in histone marks. In addition, transcripts for histone modifying enzymes (methyltransferases, demethylases, acetyltransferases, HDACs etc.) can be direct targets of miRNAs. Thus, changes in the expression of these miRNAs can have a broad impact on gene expression by controlling these chromatin regulators. A few examples are presented to demonstrate such multilevel control of stromal cell behavior. In a study showing how miRNAs can modulate histone methylation, Zhao et al. [101] found that in the ovarian cancer tumor microenvironment, reduced glucose caused an elevation of miR-101 and miR-26a in T cells, which directly targeted the histone methyltransferase, EZH2, preventing its increased expression upon T-cell activation. This loss of EZH2 inhibited the transcriptional repression and promoter-associated H3K27 trimethylation of the genes for NUMB and FBXW7, two known suppressors of NOTCH 1–4 (see Figure 2). The retained expression of NUMB and FBXW7 inhibited the activation of NOTCH signaling, which is required for normal T-cell function [102]. This study also demonstrated that human ovarian cancer cell specific T cells infused into mice could inhibit the growth of human ovarian cancer xenografts, but that inhibition of EZH2 with DZNep in these cells suppressed T-cell antitumor activity. Finally, investigation of human ovarian cancer samples by immunohistochemistry showed that the number of EZH2+ T cells in the sample was positively correlated with overall and disease-free survival, supporting the concept that EZH2+ T cells play an antitumor role.
An example showing the regulation of miRNAs by DNA methylation was presented by Li et al. [103], who studied the interaction between gastric cancer cells and CAFs. They found that prostaglandin PGE2 secreted by tumor cells triggered DNA hypermethylation of the miRNA-149 (miR-149) promoter in CAFs. This hypermethylation decreased miR-149 expression, leading to increased expression of the miR-149 target, IL6 (see Figure 2), an important protumoral cytokine released by fibroblasts into the tumor milieu [10,104]. Finally, the regulation of miRNAs by histone acetylation has also been demonstrated. In macrophages, the TNF superfamily member, TNF-related apoptosis-inducing ligand, was shown to elicit an increase in miR-146a, which acts as a suppressor of proinflammatory cytokine production [105]. This burst of miR-146a transcription was mediated by increased H3 acetylation associated with the miR-146a promoter, at least in part resulting from transiently reduced expression and miR-146a promoter binding of HDAC1. These studies demonstrate that histone enzymes (e.g., EZH2) can be targeted for suppression by miRNAs, and that alterations in miRNA transcription in stromal cells can be mediated by DNA methylation or histone modification of miRNA genes; however, more investigations are needed to elucidate the coordinated actions of the different epigenetic mechanisms as they regulate gene expression in stromal cells.
Opportunities to epigenetically remodel stromal cells
A number of small molecule inhibitors have been developed to target epigenetic mechanisms (so-called epi-drugs) and there is increasing interest in using these compounds in solo or combination treatments for cancer [106–110]. Thus far, the DNMT inhibitors, 5-AzaC and 5-AzaDC, and the HDAC inhibitors, vorinostat and romidepsin, have been approved by the US FDA for use in humans [37]. HDAC inhibitors have been approved for the treatment of cutaneous T-cell lymphoma [109], and 5-AzaC and 5-AzaDC for myeloid cancers [111]. Additional epi-drugs have shown effectiveness in animal models and results from human trials are expected [108]. These drugs were developed or repurposed for cancer therapeutics based on their abilities to inhibit oncogenic properties of cancer cells. A few studies have described the effects of epi-drugs on stromal cells.
Treatment of pancreatic CAFs with 5-AzaDC affected DNA methylation on the promoters of only a small number of genes compared with its effects on PDAC cells [15]. Using immortalized rat liver stellate cells stimulated with TGF-β1, a pathway known to promote/enhance the activation of these cells, Bian et al. [112] demonstrated that 5-AzaDC treatment decreased the TGF-β1-mediated induction of DNMT1, SMA and Col 1a1 and the TGF-β-driven suppression of Smad7, an inhibitor of TGF-β signaling. Similarly, Mann et al., [113] demonstrated that the activation of primary rat liver stellate cells was inhibited by 5-AzaDC. However, 5-AzaDC was shown to restore CPY24 expression to tumor-derived mouse endothelial cells, thus decreasing their sensitivity to calcitriol treatment [16]. This finding indicates how some treatments targeted for tumor cells may have unexpected or undesirable effects on other cell types.
A number of HDAC inhibitors have been demonstrated to inhibit hepatic stellate cell activation in vitro [114,115] and hepatic fibrosis in mouse models [116–118]. HDACs have the potential to both increase the susceptibility of tumor cells to immune recognition by upregulating surface proteins normally suppressed in these cells, and by altering gene expression in immune cells. For example, the HDAC inhibitor, etinostat increases the activating receptor, NKG2D on natural killer cells and also increases expression of the ligands for this receptor (MHC class I-related genes) on tumor cells [119]. The H3K27 methyltransferase, EZH2, plays roles in a number of cancers by suppressing the expression of certain genes, leading to the concept that EZH2 may be a useful therapeutic target in cancer [120]. Inhibitors of EZH2 (Tazemetostat, GSK2816126, CPI-1205) are currently in clinical trials [108,111,120]. One study showed that EZH2 expression is induced upon activation of rat hepatic stellate cells, and that the EZH2 inhibitor, 3-deazaneplanocin A, impairs stellate cell activation. EZH2 inhibitors have also been demonstrated to stimulate the effectiveness of natural killer cells [121]. Notably, EZH2 inhibitors can inhibit the antitumorigenic activities of T cells [101]. Thus, as with DNMT inhibitors, EZH2 inhibitors may have both pro- and antitumoral effects on the diverse cells of the tumor microenvironment. Surprisingly, although DNMT, HDAC and EZH2 inhibitors have been used in a number of cancer models, little has been reported of the effects of these drugs upon stromal cells in vivo during these studies.
Conclusion & future perspective
As described above, recent studies demonstrate that epigenetic mechanisms play critical roles in cells of the tumor microenvironment. Further understanding of these mechanisms may provide new approaches to treat cancer by affecting stromal cell behavior. However, the field of stromal cell epigenetics is still in its infancy. The study of epigenetic regulation in stromal cells is inherently difficult for several reasons. First, tumors occur as a mixture of different cell types that can be difficult to separate into relatively pure subfractions representing tumor cells, fibroblasts etc. Some studies can be performed by immunostaining or in situ hybridization of fixed tumor tissue to investigate expression (protein, miRNA or miRNA), or post-translational modifications (e.g., total acetylated histones) in individual cell types distinguished by morphology or marker proteins. However, for investigating the presence of DNA methylation on a specific promoter, histone marks associated with a particular gene or quantitative gene expression studies, novel methods have been developed (e.g., laser-capture microdissection, fluorescence-activated cell sorting, immuno-isolation, density gradient separation) to isolate specific cell types [41,122–124]. The separation of sufficient purified cells from a tissue can particularly daunting in the case of rare cell types such as endothelial cells, although several groups have reported successful methods [16,18,125]. Studies can also be performed using primary cultured cells or cell lines derived from particular cell types, often cultured with cancer cells or cancer cell derived conditioned media. While cultured stromal cells are invaluable tools in the study of stromal/tumor cell interaction, stromal cells may be altered during culture and stromal fibroblasts in particular become activated, and rapidly alter their transcriptome and epigenetic state upon even a few days in culture [43,66,126–128]. However, useful investigations have been performed using stromal cells isolated from tumors versus the normal tissue, or by comparing freshly isolated fibroblasts with cells activated by time in culture, as noted above.
Few studies have been performed to investigate the role of histone modifications in tumor-associated stromal cells but in contrast, there are a relatively large number of investigations of miRNA dysregulation in stromal cells. This imbalance may be partially driven by technology as it is relatively easier to generate miRNA data using available miRNA sequencing methodologies than to perform the ChIP studies needed to investigate histone marks (methylation or acetylation) and histone-modifying enzymes at specific gene promoters. Chromatin-based gene regulation of stromal cells needs to be further investigated, not only to promote basic understanding of epigenetic mechanisms in these cell types, but also to predict potential effects of epi-drugs (e.g., histone methyltransferase inhibitors, HDAC inhibitors) on stromal cells as these drugs are tested for use in cancer treatment. Nucleosome remodeling by protein complexes such as the SWI/SNF (SWItch/Sucrose Non-Fermentable) complex is an additional, important mode of chromatin-based gene modulation [129]. Studies of nucleosome remodeling mechanisms in stromal cells, which so far have been neglected, may also yield new insight into the regulation of stromal cell phenotypes.
Current models for studying stromal cell epigenetics in the tumor environment in vivo are limited. Great strides have been made in the development of genetic mouse models for different cancer types using tissue-specific promoters to drive the expression of oncogenes in epithelial cells. Transgenic models targeting specific stromal cell types via cell type-specific promoters should be developed so that epigenetic mechanisms in stromal cells can be probed in vivo, for example, by modifying the expression of histone modifying enzymes or DNMTs in the stromal cells. The promoter regions of SMA, FAP and COL1A1 have been utilized to target fibroblasts broadly in mice [130–132]. However, none of these promoters are specific to tumor stromal cells, and may only target certain populations of fibroblasts in the stromal compartment. Future gene expression or proteomic studies of tumor-associated stromal cells may identify new genes whose promoters could be useful for cell type specific targeting. In addition, studies of miRNAs in stromal cells indicate very cell-specific miRNA profiles in tumor-associated fibroblasts, immune cells and endothelial cells. Perhaps future studies could manipulate cell type-specific miRNA expression using transgenic methods, not only to learn the functions of these miRNAs, but also to target particular cell types in the tumor microenvironment.
The tumor microenvironment is of great interest because of the potential roles it plays in supporting or moderating tumor growth. In certain cancers with extensive stroma (e.g., PDAC and breast cancer), stroma-targeted therapies are being considered and tested [133–135]. Debate remains regarding whether stromal cells play a supportive role in tumorigenesis versus a host-defensive role, especially in PDAC [4,136–137], and a recent Phase Ib/II human trial of using the hedgehog inhibitor, vismodegib, to target stromal fibroblasts in PDAC was disappointing in its lack of effect [138]. Nonetheless, the concept of targeting the stroma remains viable, but should be guided by greater knowledge of the roles of each cell type in the tumor and the mechanisms that regulate their behavior. Efforts are ongoing to utilize epi-drugs for the treatment of various forms of cancer [37,111,120,139]. It will be important to have an understanding of the potential effects of these drugs on tumor cells and each stromal cell type in the tumor environment. These initiatives will require extensive studies because of the many variables involved. For example, when using an epigenetic targeted chemical as a codrug with a toxic chemotherapeutic agent, a low or moderate concentration of the epi-drug may be sufficient to reprogram stromal cells, while a maximum concentration of the epi-drug may present with additional side effects (e.g., cytotoxicity). Further, without testing, it is unknown if epi-drugs should be used in advance of the toxic compound, or if the two drugs should be given simultaneously. Such a pretreatment might have the effect of altering the stroma, making it less protective toward the tumor. Finally, in vivo investigations should carefully document the effects on stromal as well as tumor cells in order to fully understand the significance of any findings.
Executive summary.
In addition to cancer cells, tumors contain stromal cells (fibroblasts, endothelial and immune cells), which all interact together and contribute to the transformation process.
Epigenetic mechanisms (DNA methylation, histone modifications and miRNA regulation of mRNA stability) are involved in the development and maintenance the stromal cell phenotype.
Transcription of certain genes is controlled by promoter DNA methylation status in cancer-associated stomal cells.
Histone methylation and acetylation are known to play important roles in gene repression and activation but few studies of these mechanisms have been performed in cancer-associated stromal cells.
miRNAs regulate pro- and antitumoral behavior of stromal cells in the tumor microenvironment by targeting key mRNAs for translational repression or RNA degradation.
miRNAs can act intracellularly or be transferred to other cell types within tumors.
Drugs that inhibit epigenetic mechanisms, such as DNA methyltransferase or histone deacetylase inhibitors, have significant effects on stromal cells.
Futures studies are needed of regulation of stromal cells by epigenetic mechanisms to increase our understanding of the tumor microenvironment and to facilitate the development of novel therapeutic strategies.
Acknowledgements
We would like to acknowledge the contributions of the authors of the excellent research studies and comprehensive reviews that were cited herein. We apologize to any authors whose work we omitted due to space limitations.
Footnotes
Financial & competing interests disclosure
This work was supported by the NIH/NCI CA136526, Mayo Clinic Pancreatic SPORE P50 CA102701, and Mayo Clinic Center for Cell Signaling in Gastroenterology P30 DK84567 to ME Fernandez-Zapico. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Hansen JM, Coleman RL, Sood AK. Targeting the tumour microenvironment in ovarian cancer. Eur. J. Cancer. 2016;56:131–143. doi: 10.1016/j.ejca.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iijima J, Konno K, Itano N. Inflammatory alterations of the extracellular matrix in the tumor microenvironment. Cancers (Basel) 2011;3(3):3189–3205. doi: 10.3390/cancers3033189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neesse A, Algul H, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut. 2015;64(9):1476–1484. doi: 10.1136/gutjnl-2015-309304. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen MF, Mortensen MB, Detlefsen S. Key players in pancreatic cancer-stroma interaction: cancer-associated fibroblasts, endothelial and inflammatory cells. World J. Gastroenterol. 2016;22(9):2678–2700. doi: 10.3748/wjg.v22.i9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev. 2016;30(9):1002–1019. doi: 10.1101/gad.279737.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin. Cancer Res. 2012;18(16):4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houghton AN. LIGHTing the way for tumor immunity. Nat. Immunol. 2004;5(2):123–124. doi: 10.1038/ni0204-123. [DOI] [PubMed] [Google Scholar]
- 8.Hinz B, Phan SH, Thannickal VJ, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am. J. Pathol. 2012;180(4):1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schober M, Jesenofsky R, Faissner R, et al. Desmoplasia and chemoresistance in pancreatic cancer. Cancers (Basel) 2014;6(4):2137–2154. doi: 10.3390/cancers6042137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel) 2015;7(4):2443–2458. doi: 10.3390/cancers7040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchsbaum RJ, Oh SY. Breast cancer-associated fibroblasts: where we are and where we need to go. Cancers (Basel) 2016;8(2) doi: 10.3390/cancers8020019. pii: E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol. Cancer Res. 2012;10(11):1403–1418. doi: 10.1158/1541-7786.MCR-12-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing Y, Zhao S, Zhou BP, Mi J. Metabolic reprogramming of the tumour microenvironment. FEBS J. 2015;282(20):3892–3898. doi: 10.1111/febs.13402. [DOI] [PubMed] [Google Scholar]
- 14.Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann. Oncol. 2016;27(8):1482–1492. doi: 10.1093/annonc/mdw168. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Walter K, Omura N, et al. Unlike pancreatic cancer cells pancreatic cancer associated fibroblasts display minimal gene induction after 5-aza-2′-deoxycytidine. PLoS ONE. 2012;7(9):e43456. doi: 10.1371/journal.pone.0043456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung I, Karpf AR, Muindi JR, et al. Epigenetic silencing of CYP24 in tumor-derived endothelial cells contributes to selective growth inhibition by calcitriol. J. Biol. Chem. 2007;282(12):8704–8714. doi: 10.1074/jbc.M608894200. [DOI] [PubMed] [Google Scholar]; •• This investigation found that 5-AzaDC decreased the sensitivity of tumor-derived endothelial cells to calcitriol.
- 17.Tyan SW, Hsu CH, Peng KL, et al. Breast cancer cells induce stromal fibroblasts to secrete ADAMTS1 for cancer invasion through an epigenetic change. PLoS ONE. 2012;7(4):e35128. doi: 10.1371/journal.pone.0035128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellebrekers DM, Melotte V, Vire E, et al. Identification of epigenetically silenced genes in tumor endothelial cells. Cancer Res. 2007;67(9):4138–4148. doi: 10.1158/0008-5472.CAN-06-3032. [DOI] [PubMed] [Google Scholar]
- 19.Mitra AK, Zillhardt M, Hua Y, et al. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. 2012;2(12):1100–1108. doi: 10.1158/2159-8290.CD-12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Uses multiple models to show that altering the expression of three miRNAs in normal fibroblasts could reproduce the tumor-associated fibroblast phenotype.
- 20.Yamada N, Tsujimura N, Kumazaki M, et al. Colorectal cancer cell-derived microvesicles containing microRNA-1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down-regulation in endothelial cells. Biochim. Biophys. Acta. 2014;1839(11):1256–1272. doi: 10.1016/j.bbagrm.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Yang M, Chen J, Su F, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukino K, Shen L, Patocs A, Mutter GL, Eng C. Genomic instability within tumor stroma and clinicopathological characteristics of sporadic primary invasive breast carcinoma. JAMA. 2007;297(19):2103–2111. doi: 10.1001/jama.297.19.2103. [DOI] [PubMed] [Google Scholar]
- 23.Patocs A, Zhang L, Xu Y, et al. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N. Engl. J. Med. 2007;357(25):2543–2551. doi: 10.1056/NEJMoa071825. [DOI] [PubMed] [Google Scholar]
- 24.Weber F, Xu Y, Zhang L, et al. Microenvironmental genomic alterations and clinicopathological behavior in head and neck squamous cell carcinoma. JAMA. 2007;297(2):187–195. doi: 10.1001/jama.297.2.187. [DOI] [PubMed] [Google Scholar]
- 25.Corver WE, Ter Haar NT, Fleuren GJ, Oosting J. Cervical carcinoma-associated fibroblasts are DNA diploid and do not show evidence for somatic genetic alterations. Cell. Oncol. (Dordr.) 2011;34(6):553–563. doi: 10.1007/s13402-011-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosein AN, Wu M, Arcand SL, et al. Breast carcinoma-associated fibroblasts rarely contain p53 mutations or chromosomal aberrations. Cancer Res. 2010;70(14):5770–5777. doi: 10.1158/0008-5472.CAN-10-0673. [DOI] [PubMed] [Google Scholar]
- 27.Qiu W, Hu M, Sridhar A, et al. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat. Genet. 2008;40(5):650–655. doi: 10.1038/ng.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madar S, Goldstein I, Rotter V. ‘Cancer associated fibroblasts’ – more than meets the eye. Trends Mol. Med. 2013;19(8):447–453. doi: 10.1016/j.molmed.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth – bystanders turning into key players. Curr. Opin. Genet. Dev. 2009;19(1):67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 31.Easwaran H, Tsai HC, Baylin SB. Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol. Cell. 2014;54(5):716–727. doi: 10.1016/j.molcel.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlesinger Y, Straussman R, Keshet I, et al. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat. Genet. 2007;39(2):232–236. doi: 10.1038/ng1950. [DOI] [PubMed] [Google Scholar]
- 33.Jin B, Robertson KD. DNA methyltransferases, DNA damage repair, and cancer. Adv. Exp. Med. Biol. 2013;754:3–29. doi: 10.1007/978-1-4419-9967-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeschke J, Collignon E, Fuks F. DNA methylome profiling beyond promoters – taking an epigenetic snapshot of the breast tumor microenvironment. FEBS J. 2015;282(9):1801–1814. doi: 10.1111/febs.13125. [DOI] [PubMed] [Google Scholar]
- 35.Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell. 2014;26(4):577–590. doi: 10.1016/j.ccr.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheishvili D, Boureau L, Szyf M. DNA demethylation and invasive cancer: implications for therapeutics. Br. J. Pharmacol. 2015;172(11):2705–2715. doi: 10.1111/bph.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mummaneni P, Shord SS. Epigenetics and oncology. Pharmacotherapy. 2014;34(5):495–505. doi: 10.1002/phar.1408. [DOI] [PubMed] [Google Scholar]
- 38.Klutstein M, Nejman D, Greenfield R, Cedar H. DNA methylation in cancer and aging. Cancer Res. 2016;76(12):3446–3450. doi: 10.1158/0008-5472.CAN-15-3278. [DOI] [PubMed] [Google Scholar]
- 39.Vincent A, Omura N, Hong SM, Jaffe A, Eshleman J, Goggins M. Genome-wide analysis of promoter methylation associated with gene expression profile in pancreatic adenocarcinoma. Clin. Cancer Res. 2011;17(13):4341–4354. doi: 10.1158/1078-0432.CCR-10-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet. 2007;8(4):286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 41.Jiang L, Gonda TA, Gamble MV, et al. Global hypomethylation of genomic DNA in cancer-associated myofibroblasts. Cancer Res. 2008;68(23):9900–9908. doi: 10.1158/0008-5472.CAN-08-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]; • One of the first papers to investigate global DNA methylation in cancer-associated fibroblasts.
- 42.Vizoso M, Puig M, Carmona FJ, et al. Aberrant DNA methylation in non-small cell lung cancer-associated fibroblasts. Carcinogenesis. 2015;36(12):1453–1463. doi: 10.1093/carcin/bgv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gotze S, Schumacher EC, Kordes C, Haussinger D. Epigenetic changes during hepatic stellate cell activation. PLoS ONE. 2015;10(6):e0128745. doi: 10.1371/journal.pone.0128745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao Q, Zhou D, Rucki AA, et al. Cancer associated fibroblasts in pancreatic cancer are reprogrammed by tumor-induced alterations in genomic DNA methylation. Cancer Res. 2016;76(18):5395–5404. doi: 10.1158/0008-5472.CAN-15-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albrengues J, Bertero T, Grasset E, et al. Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nat. Commun. 2015;6:10204. doi: 10.1038/ncomms10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bian EB, Huang C, Ma TT, et al. DNMT1-mediated PTEN hypermethylation confers hepatic stellate cell activation and liver fibrogenesis in rats. Toxicol. Appl. Pharmacol. 2012;264(1):13–22. doi: 10.1016/j.taap.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 47.Janson PC, Marits P, Thorn M, Ohlsson R, Winqvist O. CpG methylation of the IFNG gene as a mechanism to induce immunosuppression [correction of immunosupression] in tumor-infiltrating lymphocytes. J. Immunol. 2008;181(4):2878–2886. doi: 10.4049/jimmunol.181.4.2878. [DOI] [PubMed] [Google Scholar]; •• Shows that IFN-γ production can be increased by 5-AzaC in tumor-associated lymphocytes.
- 48.Ashcroft GS, Yang X, Glick AB, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1999;1(5):260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 49.Flanders KC. Smad3 as a mediator of the fibrotic response. Int. J. Exp. Pathol. 2004;85(2):47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z, Gao Z, Shi Y, et al. Inhibition of Smad3 expression decreases collagen synthesis in keloid disease fibroblasts. J. Plast. Reconstr. Aesthet. Surg. 2007;60(11):1193–1199. doi: 10.1016/j.bjps.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Iba K, Albrechtsen R, Gilpin BJ, Loechel F, Wewer UM. Cysteine-rich domain of human ADAM 12 (meltrin alpha) supports tumor cell adhesion. Am. J. Pathol. 1999;154(5):1489–1501. doi: 10.1016/s0002-9440(10)65403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walter K, Omura N, Hong SM, et al. Overexpression of smoothened activates the sonic hedgehog signaling pathway in pancreatic cancer-associated fibroblasts. Clin. Cancer Res. 2010;16(6):1781–1789. doi: 10.1158/1078-0432.CCR-09-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int. J. Cancer. 2008;123(10):2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 54.Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Semin. Cell Dev. Biol. 2010;21(1):33–39. doi: 10.1016/j.semcdb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bronisz A, Godlewski J, Wallace JA, et al. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat. Cell Biol. 2012;14(2):159–167. doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trimboli AJ, Cantemir-Stone CZ, Li F, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461(7267):1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bedi U, Mishra VK, Wasilewski D, Scheel C, Johnsen SA. Epigenetic plasticity: a central regulator of epithelial-to-mesenchymal transition in cancer. Oncotarget. 2014;5(8):2016–2029. doi: 10.18632/oncotarget.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim KH, Roberts CW. Targeting EZH2 in cancer. Nat. Med. 2016;22(2):128–134. doi: 10.1038/nm.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shilatifard A. The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annu. Rev. Biochem. 2012;81:65–95. doi: 10.1146/annurev-biochem-051710-134100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lim S, Metzger E, Schule R, Kirfel J, Buettner R. Epigenetic regulation of cancer growth by histone demethylases. Int. J. Cancer. 2010;127(9):1991–1998. doi: 10.1002/ijc.25538. [DOI] [PubMed] [Google Scholar]
- 62.Simon RP, Robaa D, Alhalabi Z, Sippl W, Jung M. KATching-up on small molecule modulators of lysine acetyltransferases. J. Med. Chem. 2016;59(4):1249–1270. doi: 10.1021/acs.jmedchem.5b01502. [DOI] [PubMed] [Google Scholar]
- 63.Benedetti R, Conte M, Altucci L. Targeting histone deacetylases in diseases: where are we? Antioxid. Redox. Signal. 2015;23(1):99–126. doi: 10.1089/ars.2013.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagner EJ, Carpenter PB. Understanding the language of Lys36 methylation at histone H3. Nat. Rev. Mol. Cell Biol. 2012;13(2):115–126. doi: 10.1038/nrm3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yi X, Jiang XJ, Li XY, Jiang DS. Histone methyltransferases: novel targets for tumor and developmental defects. Am. J. Transl. Res. 2015;7(11):2159–2175. [PMC free article] [PubMed] [Google Scholar]
- 66.Perugorria MJ, Wilson CL, Zeybel M, et al. Histone methyltransferase ASH1 orchestrates fibrogenic gene transcription during myofibroblast transdifferentiation. Hepatology. 2012;56(3):1129–1139. doi: 10.1002/hep.25754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alicea-Velazquez NL, Shinsky SA, Loh DM, Lee JH, Skalnik DG, Cosgrove MS. Targeted disruption of the interaction between WD-40 repeat protein 5 (WDR5) and mixed lineage leukemia (MLL)/SET1 family proteins specifically inhibits MLL1 and SETd1A methyltransferase complexes. J. Biol. Chem. 2016 doi: 10.1074/jbc.M116.752626. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsai CT, So CW. Epigenetic therapies by targeting aberrant histone methylome in AML: molecular mechanisms, current preclinical and clinical development. Oncogene. 2016 doi: 10.1038/onc.2016.315. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagarsheth N, Peng D, Kryczek I, et al. PRC2 epigenetically silences Th1-Type chemokines to suppress effector T-cell trafficking in colon cancer. Cancer Res. 2016;76(2):275–282. doi: 10.1158/0008-5472.CAN-15-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng D, Kryczek I, Nagarsheth N, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527(7577):249–253. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alvarez-Errico D, Vento-Tormo R, Sieweke M, Ballestar E. Epigenetic control of myeloid cell differentiation, identity and function. Nat. Rev. Immunol. 2015;15(1):7–17. doi: 10.1038/nri3777. [DOI] [PubMed] [Google Scholar]
- 72.Mehta S, Jeffrey KL. Beyond receptors and signaling: epigenetic factors in the regulation of innate immunity. Immunol. Cell Biol. 2015;93(3):233–244. doi: 10.1038/icb.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suarez-Alvarez B, Baragano Raneros A, Ortega F, Lopez-Larrea C. Epigenetic modulation of the immune function: a potential target for tolerance. Epigenetics. 2013;8(7):694–702. doi: 10.4161/epi.25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frediani JN, Fabbri M. Essential role of miRNAs in orchestrating the biology of the tumor microenvironment. Mol. Cancer. 2016;15(1):42. doi: 10.1186/s12943-016-0525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gallach S, Calabuig-Farinas S, Jantus-Lewintre E, Camps C. MicroRNAs: promising new antiangiogenic targets in cancer. Biomed. Res. Int. 2014;2014:878450. doi: 10.1155/2014/878450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chou J, Shahi P, Werb Z. microRNA-mediated regulation of the tumor microenvironment. Cell Cycle. 2013;12(20):3262–3271. doi: 10.4161/cc.26087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kohlhapp FJ, Mitra AK, Lengyel E, Peter ME. MicroRNAs as mediators and communicators between cancer cells and the tumor microenvironment. Oncogene. 2015;34(48):5857–5868. doi: 10.1038/onc.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuninty PR, Schnittert J, Storm G, Prakash J. MicroRNA targeting to modulate tumor microenvironment. Front. Oncol. 2016;6:3. doi: 10.3389/fonc.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Svoronos AA, Engelman DM, Slack FJ. OncomiR or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res. 2016;76(13):3666–3670. doi: 10.1158/0008-5472.CAN-16-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aprelikova O, Yu X, Palla J, et al. The role of miR-31 and its target gene SATB2 in cancer-associated fibroblasts. Cell Cycle. 2010;9(21):4387–4398. doi: 10.4161/cc.9.21.13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doldi V, Callari M, Giannoni E, et al. Integrated gene and miRNA expression analysis of prostate cancer associated fibroblasts supports a prominent role for interleukin-6 in fibroblast activation. Oncotarget. 2015;6(31):31441–31460. doi: 10.18632/oncotarget.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen H, Yu X, Yang F, et al. Reprogramming of normal fibroblasts into cancer-associated fibroblasts by miRNAs-mediated CCL2/VEGFA signaling. PLoS Genet. 2016;12(8):e1006244. doi: 10.1371/journal.pgen.1006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L, Steele I, Kumar JD, et al. Distinct miRNA profiles in normal and gastric cancer myofibroblasts and significance in Wnt signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;310(9):G696–G704. doi: 10.1152/ajpgi.00443.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang TS, Yang XH, Chen X, et al. MicroRNA-106b in cancer-associated fibroblasts from gastric cancer promotes cell migration and invasion by targeting PTEN. FEBS Lett. 2014;588(13):2162–2169. doi: 10.1016/j.febslet.2014.04.050. [DOI] [PubMed] [Google Scholar]
- 85.Musumeci M, Coppola V, Addario A, et al. Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene. 2011;30(41):4231–4242. doi: 10.1038/onc.2011.140. [DOI] [PubMed] [Google Scholar]
- 86.Aprelikova O, Palla J, Hibler B, et al. Silencing of miR-148a in cancer-associated fibroblasts results in WNT10B-mediated stimulation of tumor cell motility. Oncogene. 2013;32(27):3246–3253. doi: 10.1038/onc.2012.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bullock MD, Pickard KM, Nielsen BS, et al. Pleiotropic actions of miR-21 highlight the critical role of deregulated stromal microRNAs during colorectal cancer progression. Cell Death Dis. 2013;4:e684. doi: 10.1038/cddis.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ali S, Suresh R, Banerjee S, et al. Contribution of microRNAs in understanding the pancreatic tumor microenvironment involving cancer associated stellate and fibroblast cells. Am. J. Cancer Res. 2015;5(3):1251–1264. [PMC free article] [PubMed] [Google Scholar]
- 89.Kuninty PR, Bojmar L, Tjomsland V, et al. MicroRNA-199a and -214 as potential therapeutic targets in pancreatic stellate cells in pancreatic tumor. Oncotarget. 2016;7(13):16396–16408. doi: 10.18632/oncotarget.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y, Du M, Wang J, et al. MiRNA-200a expression is inverse correlation with hepatocyte growth factor expression in stromal fibroblasts and its high expression predicts a good prognosis in patients with non-small cell lung cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.10302. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baroni S, Romero-Cordoba S, Plantamura I, et al. Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis. 2016;7(7):e2312. doi: 10.1038/cddis.2016.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lopatina T, Gai C, Deregibus MC, Kholia S, Camussi G. Cross talk between cancer and mesenchymal stem cells through extracellular vesicles carrying nucleic acids. Front. Oncol. 2016;6:125. doi: 10.3389/fonc.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pang W, Su J, Wang Y, et al. Pancreatic cancer-secreted miR-155 implicates in the conversion from normal fibroblasts to cancer-associated fibroblasts. Cancer Sci. 2015;106(10):1362–1369. doi: 10.1111/cas.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paggetti J, Haderk F, Seiffert M, et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015;126(9):1106–1117. doi: 10.1182/blood-2014-12-618025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin HK, Bergmann S, Pandolfi PP. Cytoplasmic PML function in TGF-beta signalling. Nature. 2004;431(7005):205–211. doi: 10.1038/nature02783. [DOI] [PubMed] [Google Scholar]
- 96.Kim J, Morley S, Le M, et al. Enhanced shedding of extracellular vesicles from amoeboid prostate cancer cells: potential effects on the tumor microenvironment. Cancer Biol. Ther. 2014;15(4):409–418. doi: 10.4161/cbt.27627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yin Y, Cai X, Chen X, et al. Tumor-secreted miR-214 induces regulatory T cells: a major link between immune evasion and tumor growth. Cell Res. 2014;24(10):1164–1180. doi: 10.1038/cr.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou M, Chen J, Zhou L, Chen W, Ding G, Cao L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell. Immunol. 2014;292(1–2):65–69. doi: 10.1016/j.cellimm.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 99.Josson S, Gururajan M, Sung SY, et al. Stromal fibroblast-derived miR-409 promotes epithelial-to-mesenchymal transition and prostate tumorigenesis. Oncogene. 2015;34(21):2690–2699. doi: 10.1038/onc.2014.212. [DOI] [PubMed] [Google Scholar]
- 100.Shah SH, Miller P, Garcia-Contreras M, et al. Hierarchical paracrine interaction of breast cancer associated fibroblasts with cancer cells via hMAPK-microRNAs to drive ER-negative breast cancer phenotype. Cancer Biol. Ther. 2015;16(11):1671–1681. doi: 10.1080/15384047.2015.1071742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao E, Maj T, Kryczek I, et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat. Immunol. 2016;17(1):95–103. doi: 10.1038/ni.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This highly mechanistic paper showed that EZH2 is repressed by miRNAs in tumor-associated T cells leading to inhibition of NOTCH signaling.
- 102.Amsen D, Helbig C, Backer RA. Notch in T cell differentiation: all things considered. Trends Immunol. 2015;36(12):802–814. doi: 10.1016/j.it.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 103.Li P, Shan JX, Chen XH, et al. Epigenetic silencing of microRNA-149 in cancer-associated fibroblasts mediates prostaglandin E2/interleukin-6 signaling in the tumor microenvironment. Cell Res. 2015;25(5):588–603. doi: 10.1038/cr.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates the repression of an miRNA by DNA methylation of its gene.
- 104.Erez N, Glanz S, Raz Y, Avivi C, Barshack I. Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem. Biophys. Res. Commun. 2013;437(3):397–402. doi: 10.1016/j.bbrc.2013.06.089. [DOI] [PubMed] [Google Scholar]
- 105.Gao J, Wang D, Liu D, et al. Tumor necrosis factor-related apoptosis-inducing ligand induces the expression of proinflammatory cytokines in macrophages and re-educates tumor-associated macrophages to an antitumor phenotype. Mol. Biol. Cell. 2015;26(18):3178–3189. doi: 10.1091/mbc.E15-04-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates the regulation of miRNA expression by histone acetylation.
- 106.Chiappinelli KB, Zahnow CA, Ahuja N, Baylin SB. Combining Epigenetic and Immunotherapy to Combat Cancer. Cancer Res. 2016;76(7):1683–1689. doi: 10.1158/0008-5472.CAN-15-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Falahi F, Van Kruchten M, Martinet N, Hospers GA, Rots MG. Current and upcoming approaches to exploit the reversibility of epigenetic mutations in breast cancer. Breast Cancer Res. 2014;16(4):412. doi: 10.1186/s13058-014-0412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Simo-Riudalbas L, Esteller M. Targeting the histone orthography of cancer: drugs for writers, erasers and readers. Br. J. Pharmacol. 2015;172(11):2716–2732. doi: 10.1111/bph.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Strauss J, Figg WD. Using epigenetic therapy to overcome chemotherapy resistance. Anticancer Res. 2016;36(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- 110.Van Kampen JG, Marijnissen-Van Zanten MA, Simmer F, Van Der Graaf WT, Ligtenberg MJ, Nagtegaal ID. Epigenetic targeting in pancreatic cancer. Cancer Treat. Rev. 2014;40(5):656–664. doi: 10.1016/j.ctrv.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 111.Pleyer L, Greil R. Digging deep into “dirty” drugs – modulation of the methylation machinery. Drug Metab. Rev. 2015;47(2):252–279. doi: 10.3109/03602532.2014.995379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bian EB, Huang C, Wang H, et al. Repression of Smad7 mediated by DNMT1 determines hepatic stellate cell activation and liver fibrosis in rats. Toxicol. Lett. 2014;224(2):175–185. doi: 10.1016/j.toxlet.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 113.Mann J, Oakley F, Akiboye F, Elsharkawy A, Thorne AW, Mann DA. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: implications for wound healing and fibrogenesis. Cell Death Differ. 2007;14(2):275–285. doi: 10.1038/sj.cdd.4401979. [DOI] [PubMed] [Google Scholar]
- 114.Niki T, Rombouts K, De Bleser P, et al. A histone deacetylase inhibitor, trichostatin A, suppresses myofibroblastic differentiation of rat hepatic stellate cells in primary culture. Hepatology. 1999;29(3):858–867. doi: 10.1002/hep.510290328. [DOI] [PubMed] [Google Scholar]
- 115.Rombouts K, Niki T, Wielant A, Hellemans K, Geerts A. Trichostatin A, lead compound for development of antifibrogenic drugs. Acta Gastroenterol. Belg. 2001;64(3):239–246. [PubMed] [Google Scholar]
- 116.Bulow R, Fitzner B, Sparmann G, Emmrich J, Liebe S, Jaster R. Antifibrogenic effects of histone deacetylase inhibitors on pancreatic stellate cells. Biochem. Pharmacol. 2007;74(12):1747–1757. doi: 10.1016/j.bcp.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 117.Liu Y, Wang Z, Wang J, et al. A histone deacetylase inhibitor, largazole, decreases liver fibrosis and angiogenesis by inhibiting transforming growth factor-beta and vascular endothelial growth factor signalling. Liver Int. 2013;33(4):504–515. doi: 10.1111/liv.12034. [DOI] [PubMed] [Google Scholar]
- 118.Park KC, Park JH, Jeon JY, et al. A new histone deacetylase inhibitor improves liver fibrosis in BDL rats through suppression of hepatic stellate cells. Br. J. Pharmacol. 2014;171(21):4820–4830. doi: 10.1111/bph.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhu S, Denman CJ, Cobanoglu ZS, et al. The narrow-spectrum HDAC inhibitor entinostat enhances NKG2D expression without NK cell toxicity, leading to enhanced recognition of cancer cells. Pharm. Res. 2015;32(3):779–792. doi: 10.1007/s11095-013-1231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Italiano A. Role of the EZH2 histone methyltransferase as a therapeutic target in cancer. Pharmacol. Ther. 2016;165:26–31. doi: 10.1016/j.pharmthera.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 121.Yin J, Leavenworth JW, Li Y, et al. Ezh2 regulates differentiation and function of natural killer cells through histone methyltransferase activity. Proc. Natl Acad. Sci. USA. 2015;112(52):15988–15993. doi: 10.1073/pnas.1521740112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bantikassegn A, Song X, Politi K. Isolation of epithelial, endothelial, and immune cells from lungs of transgenic mice with oncogene-induced lung adenocarcinomas. Am. J. Respir. Cell Mol. Biol. 2015;52(4):409–417. doi: 10.1165/rcmb.2014-0312MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Galvan JA, Helbling M, Koelzer VH, et al. TWIST1 and TWIST2 promoter methylation and protein expression in tumor stroma influence the epithelial-mesenchymal transition-like tumor budding phenotype in colorectal cancer. Oncotarget. 2015;6(2):874–885. doi: 10.18632/oncotarget.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Massani M, Stecca T, Fabris L, et al. Isolation and characterization of biliary epithelial and stromal cells from resected human cholangiocarcinoma: a novel in vitro model to study tumor-stroma interactions. Oncol. Rep. 2013;30(3):1143–1148. doi: 10.3892/or.2013.2568. [DOI] [PubMed] [Google Scholar]
- 125.Luo W, Hu Q, Wang D, et al. Isolation and genome-wide expression and methylation characterization of CD31+ cells from normal and malignant human prostate tissue. Oncotarget. 2013;4(9):1472–1483. doi: 10.18632/oncotarget.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43(1):128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008;88(1):125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grupp C, Troche I, Klass C, Kohler M, Muller GA. A novel model to study renal myofibroblast formation in vitro . Kidney Int. 2001;59(2):543–553. doi: 10.1046/j.1523-1755.2001.059002543.x. [DOI] [PubMed] [Google Scholar]
- 129.Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat. Rev. Cancer. 2011;11(7):481–492. doi: 10.1038/nrc3068. [DOI] [PubMed] [Google Scholar]
- 130.Arina A, Idel C, Hyjek EM, et al. Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc. Natl Acad. Sci. USA. 2016;113(27):7551–7556. doi: 10.1073/pnas.1600363113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lund PK. The alpha-smooth muscle actin promoter: a useful tool to analyse autocrine and paracrine roles of mesenchymal cells in normal and diseased bowel. Gut. 1998;42(3):320–322. doi: 10.1136/gut.42.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Santos AM, Jung J, Aziz N, Kissil JL, Pure E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J. Clin. Invest. 2009;119(12):3613–3625. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Banerjee S, Modi S, Mcginn O, et al. Impaired synthesis of stromal components in response to minnelide improves vascular function, drug delivery, and survival in pancreatic cancer. Clin. Cancer Res. 2016;22(2):415–425. doi: 10.1158/1078-0432.CCR-15-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Murakami M, Ernsting MJ, Undzys E, Holwell N, Foltz WD, Li SD. Docetaxel conjugate nanoparticles that target alpha-smooth muscle actin-expressing stromal cells suppress breast cancer metastasis. Cancer Res. 2013;73(15):4862–4871. doi: 10.1158/0008-5472.CAN-13-0062. [DOI] [PubMed] [Google Scholar]
- 135.Sumida T, Kitadai Y, Shinagawa K, et al. Anti-stromal therapy with imatinib inhibits growth and metastasis of gastric carcinoma in an orthotopic nude mouse model. Int. J. Cancer. 2011;128(9):2050–2062. doi: 10.1002/ijc.25812. [DOI] [PubMed] [Google Scholar]
- 136.Carr RM, Fernandez-Zapico ME. Pancreatic cancer microenvironment, to target or not to target? EMBO Mol. Med. 2016;8(2):80–82. doi: 10.15252/emmm.201505948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Javle M, Golan T, Maitra A. Changing the course of pancreatic cancer–Focus on recent translational advances. Cancer Treat. Rev. 2016;44:17–25. doi: 10.1016/j.ctrv.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 138.Catenacci DV, Junttila MR, Karrison T, et al. Randomized Phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J. Clin. Oncol. 2015;33(36):4284–4292. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu K, Liu Y, Lau JL, Min J. Epigenetic targets and drug discovery part 2: histone demethylation and DNA methylation. Pharmacol. Ther. 2015;151:121–140. doi: 10.1016/j.pharmthera.2015.04.001. [DOI] [PubMed] [Google Scholar]