Abstract
Nutrient acquisition and energy storage are critical parts of achieving metabolic homeostasis. The foraging gene in Drosophila melanogaster has previously been implicated in multiple feeding-related and metabolic traits. Before foraging’s functions can be further dissected, we need a precise genetic null mutant to definitively map its amorphic phenotypes. We used homologous recombination to precisely delete foraging, generating the for0 null allele, and used recombineering to reintegrate a full copy of the gene, generating the {forBAC} rescue allele. We show that a total loss of foraging expression in larvae results in reduced larval path length and food intake behavior, while conversely showing an increase in triglyceride levels. Furthermore, varying foraging gene dosage demonstrates a linear dose-response on these phenotypes in relation to foraging gene expression levels. These experiments have unequivocally proven a causal, dose-dependent relationship between the foraging gene and its pleiotropic influence on these feeding-related traits. Our analysis of foraging’s transcription start sites, termination sites, and splicing patterns using rapid amplification of cDNA ends (RACE) and full-length cDNA sequencing, revealed four independent promoters, pr1–4, that produce 21 transcripts with nine distinct open reading frames (ORFs). The use of alternative promoters and alternative splicing at the foraging locus creates diversity and flexibility in the regulation of gene expression, and ultimately function. Future studies will exploit these genetic tools to precisely dissect the isoform- and tissue-specific requirements of foraging’s functions and shed light on the genetic control of feeding-related traits involved in energy homeostasis.
Keywords: foraging gene, behavior, fat, larva, null mutant
FEEDING is critical to the development and survival of all organisms. During development, Drosophila larvae eat almost continuously. They experience rapid growth and deposit large amounts of triglycerides as energy stores. Once sufficient energy stores are reached, larvae stop feeding and alter their behavior to find pupariation sites (Edgar 2006). The size that a larva reaches and the level of stored nutrients has profound effects on survivorship, adult body size, and reproductive success (Bakker 1962). Consequently, the coordination of larval movement, feeding, and energy storage is critical to the health of both the larva and adult. This coordination requires the communication of multiple tissue systems in Drosophila such as the brain, endocrine tissues, and fat body (Leopold and Perrimon 2007). As such, perturbations in a variety of tissue systems are sufficient to alter feeding behavior.
The Drosophila melanogaster foraging gene has become a classic model for the genetic influences on feeding-related behaviors (Sokolowski 2001). The differences in locomotion on a nutritive medium (path length) between the rover and sitter strains was mapped primarily to the foraging (for) gene, also known as dg2, which encodes a cGMP-dependent protein kinase (PKG) (de Belle et al. 1989; Kalderon and Rubin, 1989; Osborne et al. 1997). foraging is highly conserved at both the sequence and phenotypic levels (Manning et al. 2002; Fitzpatrick and Sokolowski 2004; Sokolowski 2010). The rover and sitter strains were subsequently shown to differ in a suite of other behavioral and physiological traits, such as food intake and metabolism (Kaun et al. 2007; Kent et al. 2009), and as such foraging appears to be pleiotropic.
Much of the work conducted to date on the foraging gene relied on the rover and sitter foraging allelic variants (de Belle et al. 1989, 1993; Kaun et al. 2007, 2008). In rover and sitter strains, the entirety of their second chromosomes, where foraging resides, differ (Bauer and Sokolowski 1984; Sokolowski 1980). There is thus the potential for other loci to contribute to the phenotypic differences between these strains. To understand the contributions of the allelic variants of the foraging gene in these two strains, we need a precise understanding of the gene structure, its products, and its amorphic phenotypes. Since a genetic null allele of foraging would facilitate an examination of its potential pleiotropic effects on feeding-related behaviors in the larvae, we generated a precise deletion of the foraging gene by homologous recombination (HR; Gong and Golic 2003). To further examine the connection between genotype and phenotype we manipulated gene dosage using recombination-mediated genetic engineering (recombineering; Warming et al. 2005; Venken et al. 2006). Using these engineered allelic combinations, our analyses revealed an unequivocal role for foraging in larval movement, food intake, and energy storage. These experiments provide the solid foundation required for future research into determining tissue- and isoform-specific functions of foraging, as well as determining the causal differences between the rover and sitter allelic variants.
Materials and Methods
Fly strains and rearing
The rover and sitter strains have been in the lab for over 30 years and share a common first and third chromosome. They have been isogenized multiple times over the years, most recently between 2010 and 2012. The strains differ in their second pair of chromosomes where foraging resides. The path lengths of these rover and sitter strains are comparable to those collected from the field (Sokolowski 1980; Sokolowski et al. 1997) and the relative path length differences remain regardless of how the design of the path length assay has changed over the years (Anreiter et al. 2016). Fly strains for HR included y1w*, hsFLP, hsI-SceI/Y, hs-his and y1w*; eyFLP5 and y1w*, eyFLP2; Pin/CyO. The y1, w67c23, P{Crey}1b; snaSco/CyO strain (Siegal and Hartl 1996; BDSC #766) was used to resolve the loxP sequences to remove w+mC, and delete foraging. The following foraging deletion strains were obtained from Bloomington Drosophila Stock Center: w1118; Df(2L)Exel7018/CyO (Parks et al. 2004; BDSC #7789), y1 w*; Df(2L)drm-P2, P{lacW}ND-PDSWk10101/SM6b (Green et al. 2002; BDSC #6507), and w1118; Df(2L)ED243, P{3′.RS5+3.3′}ED243/SM6a (Ryder et al. 2004; Belay et al. 2007; BDSC #24122). The foraging for0, fordup, and {forBAC} alleles were generated in this paper (see below); the for0 mutants were maintained over a CyO, act-GFP balancer chromosome. Strains were reared in 40-ml vials with 10 ml of food and 170-ml bottles with 40 ml of food at 25 ± 1° with a 12L:12D photoperiod with lights on at 0800 hr. The fly yeast–cornmeal–molasses–agar food recipe contained 1.5% sucrose, 1.4% agar, 3% glucose, 1.5% cornmeal, 1% wheat germ, 1% soy flour, 3% molasses, 3.5% yeast, 0.5% propionic acid, 0.2% Tegosept, and 1% ethanol in water. Mid third instar larvae were developmentally synchronized as described in Anreiter et al. (2016). Briefly, 5- to 7-day-old adults were allowed to oviposit on grape juice and agar media (45% grape juice, 2.5% ethanol, 2.5% acetic acid, 2% agar in water) for 20 hr. The following day, any hatched larvae were cleared from the media, followed by a 4-hr incubation; newly hatched larvae were seeded into a 100-mm diameter Petri dish containing 30 ml of food. The plates were incubated at 25 ± 1° in a 12L:12D photoperiod with lights on at 0800 hr until they reached mid third instar (72 ± 2 hr post hatch).
Gene model characterization
All primer design, sequence analysis, chromatograph editing, contig assembly, in-silico cloning, and digestion confirmation was performed using the Geneious 8.1.7 software package (Kearse et al. 2012). All primer sequences are listed in Supplemental Material, Table S1. Transcription start sites (TSSs) were identified with 5′-rapid amplification of cDNA ends (RACE) experiments that used homopolymeric tailing (Michelson and Orkin 1982; Sambrook and Russell 2001) and RNA ligase using GeneRacer (Thermo Fisher Scientific, cat# L150202). Total RNA was extracted from pooled mid third instar larvae and adult flies from our rover and sitter strains with TriZOL Reagent (Thermo Fisher Scientific, cat# 15596018). RNA was reverse transcribed with Superscript III (Thermo Fisher Scientific, cat# 18080044) and primed with random hexamers and oligo dT primers. Terminal Transferase (New England Biolabs, Beverly, MA, cat# M0315S) was used to add a poly-guanosine tail to the 5′ end of the isolated cDNA. The 5′ ends of the transcripts were amplified with an oligo dC primer and gene-specific primer (comRT-R.3) targeting the coding sequence for the catalytic domain of the foraging gene. Transcription end sites (TESs) were identified by 3′-RACE using the GeneRacer Kit (ThermoFisher Scientific, cat# L150202) following the manufacturer’s instructions, using a gene-specific primer (43S) targeting the catalytic domain. Splice variants were identified by RT-PCR using forward primers targeting the exons near the TSSs (TSS1-F, TSS2-F, TSS3-F, TSS4-F) and a reverse primer targeting the terminal exon (comORF3′-R). The resulting amplicons were cloned into the pGEM-Teasy vector (Promega, Madison, WI, cat# A1360). 144 RACE clones and 240 splice variant clones were then sequenced by Sanger sequencing on an ABI 3130xl Capillary Sequencer using BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, cat# 4337454).
Ends-out gene targeting
A 5-kb homology arm 5′ to the foraging gene was amplified with PCR using the primers HA1-F and HA1-R, with BsiWI and AscI sites, respectively. The 5′-homology arm was cloned into the BsiWI and AscI sites in the pW25 ends-out gene targeting vector (Gong and Golic 2004). An attP sequence (Thorpe and Smith 1998) was cloned into the KpnI and NotI sites. The attP sequence was amplified with PCR (attP-F, attP-R) from the pCaryP vector (Groth et al. 2004) obtained from Addgene. A KpnI was added to the forward primer and NotI and SbfI sites were added to the reverse primer. A 5.2-kb homology arm 3′ to the foraging gene was amplified with PCR using primers HA2-F and HA2-R, and adding SbfI and NotI sites to the forward and reverse primers, respectively. The 3′-homology arm was cloned into the SbfI and NotI sites in the pW25-HA1-attP vector resulting in the the pW25EO-for-attP vector.
The pW25EO-for-attP vector was injected into a w1118 strain using P element transgenesis (performed by Genetic Services). This yielded an X-chromosome transformant. A series of crosses were conducted to mobilize the targeting construct and to allow HR (as in Demir and Dickson 2005). Briefly, ∼ 1000 females containing the element were crossed into an hs-Flp, hs-I-SceI-containing background, and embryos and L1 larvae were heat shocked at 38° for 2 hr on two consecutive days. Approximately 8000–10,000 progeny were then crossed into an ey-Flp background and were screened for red eye color. These crosses yielded eight integrations at the foraging locus; however, none of these had a deletion of the gene. The recombinants had integrated at either the 5′- or 3′-end of the locus, corresponding to the two homology arms. There are several reasons why this might have occurred, for one, foraging is a large gene and the size difference relative to the considerably smaller cloned construct might have impeded proper alignment. Alternatively, after sequencing the alleles where our construct had integrated (forHR1 and forHR4), we found that the I-SceI restriction sites remained uncut causing the cloned targeting construct to not linearize, which would have prevented spanning of the endogenous locus. This could have been due to inadequate heat shock, or a less than functional hs-I-SceI transgene. Fortunately, it was still possible to generate a complete deletion of the foraging gene using the loxP sites in pW25, which were intended for removal of the w+mC gene. To accomplish this, we crossed the 5′ and 3′ recombinants to make a trans-heterozygote in a hs-Cre background (BDSC #766). Embryos and L1 larvae were heat shocked at 38° for 2 hr on two consecutive days. Single male flies were then isolated and balanced. Twenty-four iso-male populations were established and screened for a deletion of the foraging locus. Four populations were identified as deletions, for0, and eight as duplications, fordup. The remainder showed no recombination.
Recombineering
The P[acman] vector was redesigned to be paired with the pW25-attP vector to have a minimal footprint upon reintegration of the engineered locus of interest. The BAC backbone was isolated by digesting the P[acman] cut with SalI and SphI. The w+mC was isolated by digesting the P[acman] vector with EcoRI and SphI. The attB sequence was amplified with PCR using the following primers, attB-F and attB-R. The attB-F primer included SalI, loxP, AscI, NotI, PacI, and AsiSI sequences. The attB PCR product was cloned into the pSC-A-amp/kan (Agilent’s Strataclone) and digested with SalI and EcoRI. The purified P[acman], w+mC, and the attB fragment were ligated together. This resulted in a vector with loxP, MCS, attB, w+mC syntony. We called the resulting vector P[attlox]. The P[acman] clone CH321-64J02 BAC from Children’s Hospital Oakland Research Institute (http://www.chori.org) was used as the source of the foraging gene sequence. A gap repair protocol (like Venken et al. 2006) was used to trim the larger BAC down to a 39.3-kb segment containing foraging. Left and right homology arms were amplified with PCR using the following primers: LA-F with an AscI, LA-R with a NotI, RA-F with a NotI, and RA-R with an AsiSI. The left and right arms were cloned into the AscI and AsiSI sites of the P[attlox] vector creating P[GAP]for. P[GAP]for was linearized with NotI and transformed into induced SW102 cells (Warming et al. 2005) containing the CH321-64J02 BAC. Recombination between the P[GAP]for and the CH321-64J02 BAC yielded a foraging-specific BAC in the reengineered P[acman] vector, P[attlox]for. As the BAC from the P[acman] library used here was generated using DNA isolated from the y1;cn1,bw1,sp1 strain, containing a naturally occurring copia transposable element in the foraging gene, we used galK selection (Warming et al. 2005) to remove the copia transposable element. A 579-bp left homology arm and a 580-bp right homology arm flanking the copia element were cloned into the XhoI/EcoRI and BamHI/XbaI sites of the pGalK vector. The left arm was amplified with L-copia-F, and L-copia-R with an EcoRI site. The PCR product contained an internal XhoI site. The right arm was amplified with R-copia-F with a BamHI site, and R-copia-R. The PCR product contained an internal XbaI site. The XhoI/XbaI LA-galK-RA fragment was transformed into SW102 cells containing the P[attlox]for vector. The for-copia-galK BAC-containing cells were then transformed with a linear oligo, for-copia (Sigma Genesys). The resulting “wild-type” BAC was transformed into the EPI300 cells (Wild et al. 2002, acquired from Epicentre) and incorporated into the fly’s genome using φC31 integration into the attP2 landing site on the third-chromosomes (Groth et al. 2004). Transgenesis was done by Genetic Services.
Western blot analysis
Twenty mid third instar larvae (72 ± 2 hr post hatch) were homogenized on ice in 400 μl of lysis buffer (50 mM Tris-HCl pH 7.5, 10% glycerol, 150 mM NaCL, 1% Triton-X 100, 5 mM EDTA, 1× Halt Protease Inhibitor Cocktail (cat# 1862209)). Samples were centrifuged at 16,000 RCF at 4° and the supernatant was transferred to a new tube and placed on ice. Protein quantification was performed with Pierce BCA Protein Assay Kit (cat# 23227). Twenty micrograms of protein were denatured for 5 min at 100°. The samples were run on a 4% stacking/7% resolving polyacrylamide and SDS gel at 150 V for 1 hr in running buffer (25 mM Tris-HCl, 200 mM glycine, 0.1% SDS). Proteins were transferred onto a nitrocellulose membrane (Pal, cat# 66485) at 100 V for 1 hr in transfer buffer (25 mM Tris-HCl, 200 mM glycine, 10% methanol). Blots were blocked for 2 hr in 5% nonfat milk in 0.1% Tween-20 in 1× TBS (0.1% TBST), and incubated with primary antibody for 1 hr, at a concentration of 1:10,000 for anti-FOR (Belay et al. 2007) and 1:5000 for anti-ACTIN (Sigma, St. Louis, MO). The blots were rinsed twice and washed 3× for 5 min with each rinse in 0.1% TBST. Blots were then incubated with HRP conjugated secondary (Jackson ImmunoResearch Laboratories; goat-anti-mouse-HRP cat# 115-035-146, goat-anti-guinea pig-HRP cat#106-035-003) at a concentration of 1:10,000 for 45 min. Finally, blots were rinsed twice and washed 3× as above, incubated for 5 min in General Electric Healthcare's Amersham ECL Prime Detection reagent (cat# RPN2232), exposed to X-ray film, and developed with Kodak developer and fixer.
Reverse transcription quantitative PCR
Total RNA was extracted from 72-hr-old larvae using the RNeasy Mini Kit (Qiagen Cat# 74104) and the corresponding RNase-free DNase set (QIAGEN, Valencia, CA, Cat# 79254), following the manufacturer’s instructions for purification of total RNA from animal tissues. RNA was extracted from three biological replicates with n = 20 larvae per replicate. Following extraction RNA was quantified using a Nanodrop 2000c (Thermo Scientific), and RNA integrity was accessed by gel electrophoresis. Complementary DNA (cDNA) was synthesized with the iScript Advanced cDNA synthesis kit for RT-qPCR (Bio-Rad, Hercules, CA, Cat# 1725037), using 1 µg of RNA per sample, and following the manufacturer’s instructions. RT-qPCR was performed on a CFX384 Touch Real-Time PCR Detection System (Bio-Rad), using SsoAdvanced Universal SYBR Green Supermix and gene-specific primers (Table S1). Primer efficiency was calculated and only primers with efficiencies between 99 and 105% were used (a-tub – 99.8%; act5C – 99%; for_com2–99.5%; for_pr1–100.08%; for_pr2–102.6%; for_pr3–101.8%; for_pr4–100.5%). Cycling conditions followed the manufacturer’s protocol. Reference genes were initially selected based on stability values found in other studies (Ling and Salvaterra 2011; Ponton et al. 2011). Two reference genes were run (α-Tub84B and Act5c) and both had robust stability values (mean coefficient variance = 0.0296, mean M value = 0.0854). α-Tub84B had the lowest coefficient variance (0.0291) and was used to calculate relative expression values (Eff(Target)ΔCt/Eff(Ref)ΔCt) to determine differences between genotypes. Sample collection and data processing followed abbreviated MIQE recommendations (Taylor et al. 2010).
Path length
A detailed description of the foraging path length protocol is given in Anreiter et al. (2016). Briefly, foraging path length was measured using custom black rectangular Plexiglas plates (37 cm width, 60 cm length, 0.5 cm height) with 10 wells (0.5 mm depth, 9.5 cm diameter) arranged in a 2-by-5 pattern (Sokolowski et al. 1997). In the present study, mid third instar larvae (72 ± 2 hr post hatch) were randomly selected from the food plates and washed in a few drops of water. A homogenous yeast suspension (2:1 w/w) was spread across the wells creating a thin even layer in each well. Individual larvae were placed in the center of each well and covered with the lid of a 10-cm Petri dish. Larvae could move for 5 min after which the path length of each larva was traced onto the Petri dish lid. Path lengths were digitized using Fiji (Schindelin et al. 2012).
Food intake
We measured larval food intake using a new assay described here. Becton, Dickinson Falcon cell strainers (cat# 352350) were placed in 35-mm diameter Petri dishes and used to isolate and selectively feed groups of larvae. Fluorescent liquid food was prepared with 0.5% fluorescein (Sigma), 5% sucrose, and 5% yeast extraction New sentence. Third instar larvae (72 ± 2 hr post hatch) were removed from food plates, washed, and transferred in groups of 10 to cell strainers with 800 μl of liquid food. The above-mentioned Petri dish size and food volume resulted in the food being able to drain into the space below the mesh of the cell strainer, but remaining in contact with the mesh, flowing up by capillary action when it was eaten by the larvae. Once placed in the strainer, larvae were left to feed for 10 min, after which the strainers were lifted out of the food and rinsed with water, followed by three more washes with water. Washed individual larvae were placed into 0.2-ml wells of 96-well PCR plates and frozen at −20°. Frozen larvae were homogenized in 150 μl of 1× PBS with a 5/32′′ stainless steel ball bearing (OPS Diagnostics) and agitation using a Qiagen Tissue Lyser. Samples were centrifuged at 3500 RCF for 15 min. Twenty microliters of the supernatant was then mixed with 180 μl of 1× PBS in a fluorimeter plate (Corning, cat# 3631) and excited at 488 nm. Emission was measured at 562 nm. Homogenates from larvae fed with food containing no fluorescein were used as controls.
Triglyceride analysis
Groups of 20 larvae (72 ± 2 hr post hatch) were homogenized in 1 ml of 0.1% Tween 20 in 1× PBS. Samples were incubated at 70° for 5 min, chilled on ice for 2 min, and used for triglyceride and total protein quantification. To measure triglyceride content, 20 μl of the above supernatant was mixed with 200 μl Infinity TAG Reagent (Thermo Scientific, cat# TR22421) in a 96-well spectrophotometer plate (Corning, cat# 351172). Samples were incubated at 37° for 10 min, and absorbance was measured at 540 nm. To quantify total protein levels for standardization of the triglyceride measures, 20 μl of the above supernatant was quantified for protein content using the Pierce BCA Protein Assay Kit (cat# 23227), following the manufacturer’s instructions. BCA samples were incubated at 37° for 30 min, and absorbance was measured at 562 nm. Standard curves for triglycerides and albumin protein were used to calculate triglyceride and protein concentration from the obtained absorbance values. Lipid levels are displayed as microgram glycerol per milligram protein.
Statistical analysis
Statistical analysis was performed in R (R Core Team 2013). The effects of genotype as well as blocking factors such as the date of the experiment were modeled with general linear models using the lm function, and post hoc t-tests were run to compute pairwise statistical significances. Multiple comparisons were corrected using the Holm method (Holm 1979). α of P = 0.05 was considered significant. Results of statistical analyses are provided in the figure captions. Graphs were plotted in R and edited in Inkscape.
Data availability
Strains are available upon request. Information in supplemental material: Table S1 contains primer names and sequences used in this study. Figure S1 describes the forHR alleles, and the generation of the for0 null and fordup alleles. Figure S2 shows verification of the for0 allele. Figure S3 shows construction and verification of the forBAC construct. Figure S4 shows path length, food intake and fat levels of hemizygous rover and sitter larvae. Figure S5 shows foraging gene dosage and allelic contributions to foraging gene expression in whole larvae. Figure S6 provides foraging transcript sequences. File S1 provides the figure legends for Figures S1-S6.
Results
Characterizing the transcriptional complexity of the foraging gene
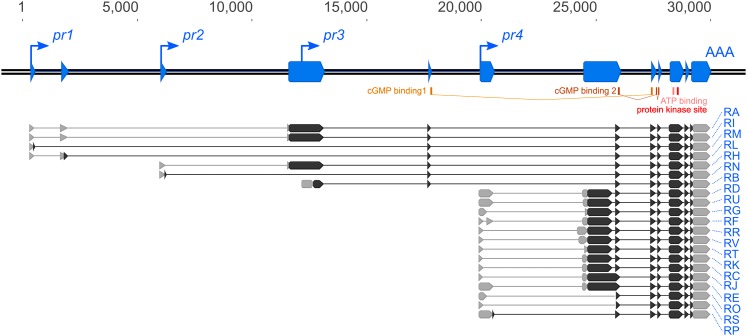
Previous studies have differed in the number and variety of foraging transcripts reported (Kalderon and Rubin 1989; Osborne et al. 1997; Stapleton et al. 2002). To produce an accurate model of the foraging gene, we first exhaustively identified the transcription start sites (TSSs) and transcription end sites (TESs) represented in mRNA transcripts produced by sequencing 144 RACE clones. We identified four separate TSSs and one TES, supporting a gene model for foraging that contains four independent minimal promoters (pr1, pr2, pr3, pr4, Figure 1) whose RNA products all splice into a common 3′-exon. A model containing multiple TSSs with one shared TES has also been shown for foraging’s orthologs in other organisms (Ørstavik et al. 1997; Stansberry et al. 2001). These promoters fall into two categories: pr1, 2 and 4 are peaked, whereas pr3 is broad. Peaked TSSs are typically found in regulated promoters, whereas broad TSSs are found in constitutive promoters (Hoskins et al. 2011). All four promoters contain clear consensus core promoter Initiator (Inr) and Downstream Promoter Elements.
Figure 1.
Schematic of the foraging gene and associated features. The D. melanogaster foraging gene has four promoters that produce 21 transcripts and nine open reading frames (ORFs). The transcription start sites (pr1–4, up-and-right arrows) and transcription end site (AAA) were identified with rapid amplification of cDNA ends (RACE). The splicing patterns of the transcripts were identified by sequencing full-length cDNAs. Exons (blue boxes) are annotated along the locus (double black line) with the transcripts below. UTRs (gray boxes), ORFs (black boxes), cGMP-binding domains (yellow), ATP-binding domain (pink), and kinase domains (red) are also annotated. RA through RK were previously annotated on FlyBase.
We next surveyed the alternative splicing complexity found in foraging mRNA. We isolated and sequenced 240 full-length cDNA clones which represented 21 distinct mRNA transcripts, encoding nine distinct open reading frames (ORFs; Figure 1). Due to the shared 3′-end of the transcripts, these variants differ in their 5′-UTR and the corresponding N-terminal coding sequences of their ORFs. The coding sequences for the cGMP-binding domains as well as the kinase domain of the protein products were all located in the shared exons. Ten of the 21 transcripts are currently annotated in Flybase (Attrill et al. 2016) making 11 transcripts novel. Six of these 11 transcripts exhibit a novel instance of exon skipping in the foraging gene (RL, RM, RN, RO, RP, RS), all of which reduce N-terminal coding sequence. Although these results suggest a more complex gene structure compared to previous reports, all the new variants identified here include the same originally described 12 exons (Kalderon and Rubin 1989).
Generation of a novel and precise foraging null allele (for0)
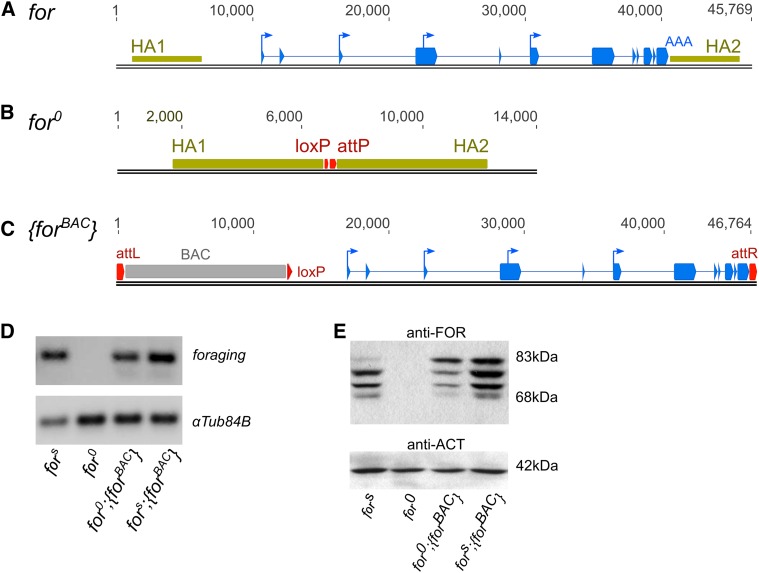
To generate a null mutation of the foraging gene we used the ends-out gene targeting system (Gong and Golic 2004). Regions of homology on either side of the foraging gene (Figure 2A) were cloned into the pW25 vector. The intended recombination event would delete all of foraging, leaving a loxP and attP site (Figure 2B). Mobilization of this targeting construct yielded eight second chromosome integrations, none of which deleted the foraging locus as expected. However, we did find separate integration events at both the 5′- and 3′-end of the locus (Figure S1). These recombination events allowed us to generate the intended null mutation of foraging (Figure 2B) by using the loxP site-specific recombination sequences in the pW25 vector, which were intended for removal of the white+mC gene used to identify integration events (Figure S1). To accomplish this, we induced a recombination event resulting in the deletion of the entire locus by crossing the 5′-loxP element (integrant forHR4) to the 3′-loxP element (integrant forHR1), generating a trans-heterozygous mutant in the presence of a Cre recombinase. This approach successfully generated a null allele of foraging, which we called for0, and a duplication allele, which we called fordup. PCR, Southern blotting, sequencing, and restriction enzyme analysis (Figure S2 and data not shown) confirmed that the Cre recombinants were indeed deletions of the foraging locus. Critically, RT-PCR and western blot experiments of homozygous for0 larvae did not detect any RNA or protein (Figure 2, D and E). Thus, our newly generated for0 mutant is a true null allele of the foraging gene.
Figure 2.
Generation of for0 and {forBAC} alleles. (A) Schematic of the D. melanogaster foraging locus with 5-kb homology arms (HA1, HA2, tan boxes) that were cloned into the pW25-attP vector. (B) Schematic of the for0 allele following recombination. The foraging gene was replaced with a loxP and attP site. (C) Schematic of the {forBAC} allele following φC31 integration at the attP2 site. (D) RT-PCR of whole larval homogenates of homozygous fors, for0, for0;{forBAC}, and fors;{forBAC} allelic combinations. Primers common to all annotated foraging transcripts, com2-F and com2-R, were used to amplify foraging. (E) Western blot of whole larval homogenates of homozygous fors, for0, for0;{forBAC}, and fors;{forBAC} individuals. Size markings are listed to the right of the blots, and antibodies are listed above each blot.
Generation and genomic integration of a foraging BAC
To manipulate foraging gene dosage, we used recombineering to generate a novel transgenic copy of the entire foraging locus that we named {forBAC}. The P[acman] library was used as a source of our {forBAC} allele (Venken et al. 2006). This BAC library was generated from the y1;cn1,bw1,sp1 strain that contained a copia transposable element within the foraging gene. Extensive DNA sequencing has shown that this copia element does not occur in any of our laboratory strains, nor have we detected it in any allele of the foraging gene other than the reference genome line (data not shown). We successfully removed this copia element using the galK-selection method (Warming et al. 2005; Figure S3). Furthermore, the gap repair method (Venken et al. 2006) we used here yielded a BAC spanning the 35-kb foraging locus, removing the rest of the large genomic region included in BAC CHORI-64J02 (Figure S3). The resulting P[attlox]for construct (Figure S3) was successfully incorporated into the fly’s genome using φC31 integrase into the attP2 landing site (Groth et al. 2004), generating the {forBAC} allele (Figure 2C). RT-PCR and western blot analyses of the {forBAC} allele showed similar expression patterns to the wild-type sitter strain (Figure 2, D and E).
foraging BAC rescue of for0 pupal lethality
The generation of a for0 allele allowed us to determine whether foraging is an essential gene. We found that homozygous for0 larvae died in the late pupal (pharate adult) stage. The recessive pupal lethality of homozygous for0 flies was fully rescued by a single copy of the {forBAC} allele, indicating that the 35-kb genomic fragment of the foraging gene in the BAC contained sufficient regulatory elements to fully restore the lethality of foraging null to wild-type; all adults were viable and fertile (data not shown). The survivorship of the for0 null mutants from first larval instar into the pupal stage was comparable to that of wild-type animals. The fact that late pharate homozygous for0 animals dissected from their pupal cases did not survive (data not shown) suggested that the lethality did not result from an inability to eclose from their pupal cases. Several previously published genomic deletions spanning all or part of the foraging gene were unable to complement the pupal lethality of the for0 mutant. We tested the Df(2L)Exel7018 (Parks et al. 2004), Df(2L)drmP2 (Green et al. 2002), and Df(2L)ED243 (Ryder et al. 2004) deletions, all of which failed to complement the for0 allele (data not shown). Like the for0 homozygous null, all three heterozygous combinations of these deletions with for0 were pharate adult lethal.
foraging gene dosage and allelic contributions significantly affect larval foraging path length
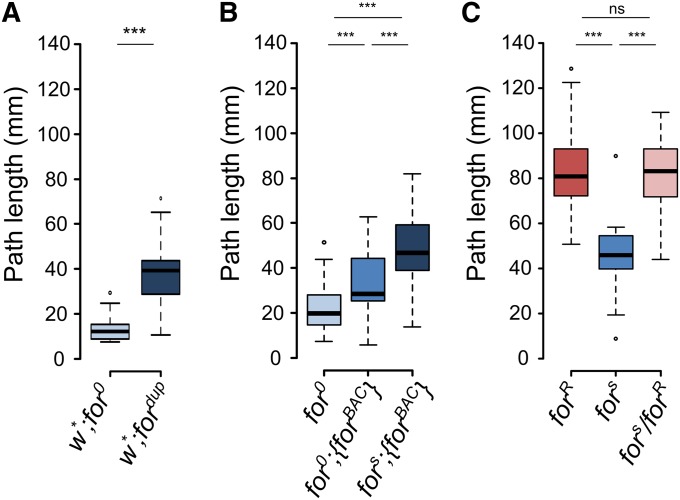
Metabolic homeostasis requires a balance of energy expenditure, energy acquisition, and energy storage. Food search and navigation of a nutritional environment is vital to this process. We first examined the larval path length phenotypes of the newly generated strains that differed in the dosage of the foraging gene. As mentioned previously, the recombination event that generated the for0 null allele also generated an allele with a duplication of the foraging gene locus, fordup (Figure S1). Larvae homozygous for the fordup allele showed longer path lengths than larvae with homozygous for0 null alleles (Figure 3A). We next examined the larval path length for the null (for0), rescue (for0;{forBAC}), and over expresser (fors;{forBAC}). The insertion of a {forBAC} into the for0 genetic background, which we call our rescue strain (for0;{forBAC}), significantly increased mean larval path lengths on yeast (Figure 3A). Furthermore, insertion of a {forBAC} into the fors genetic background (fors;{forBAC}) increased mean larval path lengths significantly more, relative to both for0;{forBAC} and for0. These results showed that increasing foraging gene expression by increasing gene dosage resulted in an increase in larval path length on yeast. We then investigated the allelic relationships between the wild-type rover (forR) and sitter (fors) alleles on larval path lengths, and asked if our gene dosage experiments can inform on the nature of rovers and sitters. As previously shown (Sokolowski 1980; de Belle et al. 1989; Osborne et al. 1997; Kaun et al. 2007), rover larvae had longer mean path lengths on yeast than sitters, and rover/sitter heterozygotes were indistinguishable from rover homozygotes (Figure 3C) confirming the dominance of the rover allele over the sitter allele. The rover/sitter difference in path length was maintained when the alleles were hemizygous with the for0 allele (Figure S4A).
Figure 3.
foraging gene dosage and allelic contributions to larval path length. (A) Larval path length of homozygous for0 and fordup individuals. Increasing gene copy number increases path length (t = −8.24, df = 34.31, P = 1.2e−9). (B) Path length on yeast of homozygous for0, for0;{forBAC}, and fors;{forBAC} individuals. Increasing gene copy number increases path length (F(2,105) = 32.3, P = 1.2e−11). (C) Larval path length behavior of homozygous forR, fors and heterozygous forR/fors individuals (F(2,87) = 46.4, P = 1.9e−14). mm, millimeters; ns, nonsignificant; *** P < 0.001.
foraging gene dosage and allelic contributions significantly affect larval food intake
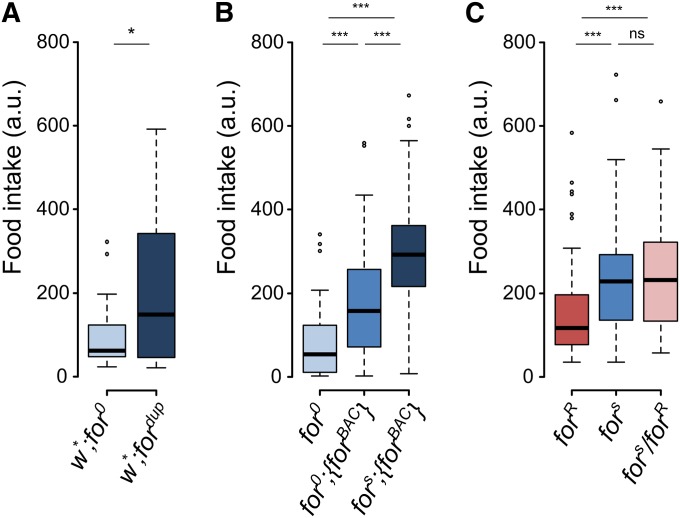
Once a larva has found a food source, it needs to ingest the food to acquire the nutrients needed for metamorphosis. Feeding rate can affect body size, which in turn can affect survival. We moved on to measure food intake, the next step in energy acquisition. We developed a novel fluorescence-based food intake assay. By using a liquid food medium and cell strainer dishes, larvae can be rapidly assayed and cleaned and frozen prior to quantification with a fluorometer. foraging null larvae (for0) had lower food intake than duplication allele (fordup) larvae (Figure 4A). The null allele also had lower mean food intake compared to the rescue (for0;{forBAC}) and over expresser (fors;{forBAC}) larvae (Figure 4B). Therefore, increasing foraging gene copy number increased food intake in a dose-dependent manner like that seen for the mean larval path length on yeast (Figure 3 and Figure 4). As with path length, we examined rovers and sitters for food intake in the light of the gene dosage experiments. Consistent with previous work that used a different food intake assay (Kaun et al. 2007, 2008), we found that rovers had lower food intake than sitters (Figure 4C). Previously, rover/sitter heterozygotes had not been tested for food intake. Interestingly, the food intake of rover/sitter heterozygotes was indistinguishable from that of homozygous sitter larvae (Figure 4C). This is the opposite pattern seen in our path length results. This rover/sitter difference was also seen when the alleles were hemizygous with the for0 allele (Figure S4B). This suggested that the pattern of dominance for food intake differed from that for larval path length, with the sitter allele being dominant to the rover allele for food intake.
Figure 4.
foraging gene dosage and allelic contributions to larval food intake. (A) Larval food intake of homozygous for0 and fordup individuals. Increasing gene copy number increases food intake (t = −2.65, df = 29.08, P = 0.012). (B) Food intake of homozygous for0, for0;{forBAC}, and fors;{forBAC} individuals. Increasing gene copy number increases food intake (F(2,249) = 70.4, P < 2.2e−16). (C) Larval food intake of homozygous forR, fors and heterozygous forR/fors individuals (F(2,213) = 10.0, P = 7.1e−5). a.u., arbitrary fluorescence units; ns, nonsignificant; * P < 0.05, *** P < 0.001.
Increased foraging gene dosage significantly decreases triglyceride levels
Fat stores are critical for survival during metamorphosis. After feeding, the acquired nutrients need to be digested and absorbed. Absorbed nutrients are primarily stored as triglycerides in the fat body of the larva. With our results showing that foraging plays a role in both energy expenditure and intake measures, we then moved on to measure fat levels, a component of energy storage. We used our foraging dosage-specific allelic series to analyze lipid storage. Whole larval homogenates were incubated with the Infinity TAG Reagent (Thermo Scientific), which contains a lipase, dissociating fatty acids from the glycerol backbone of triglycerides (as well as monoglycerides and diglycerides). Free glycerol is then colorimetrically quantified with a spectrophotometer. The for0 null larvae had higher triglyceride levels than the fordup duplication larvae (Figure 5A). Similarly, the for0 null mutants had significantly higher mean triglycerides than the rescue (for0;{forBAC}) and the over expresser (fors;{forBAC}) strains (Figure 5B), suggesting that increased foraging gene dosage decreased triglyceride levels. We then assayed rovers and sitters to see if they differed in triglyceride levels, and to see what their phenotype implies about their relative expression considering the gene dosage. We found that rovers had lower mean triglyceride levels than sitters and that rover/sitter heterozygotes had an intermediate level of mean triglycerides (Figure 5C). This rover/sitter difference was maintained when the alleles were hemizygous with the for0 allele (Figure S4C).
Figure 5.
foraging gene dosage and allelic contributions to larval fat levels. (A) Larval triglyceride levels of homozygous for0 and fordup individuals. Increasing gene copy number decreases fat levels (t = 91.03, df = 1, P = 4.4e−9). (B) triglyceride levels of homozygous for0, for0;{forBAC}, and fors;{forBAC}. Increasing gene copy number decreases triglyceride levels (F(2,21) = 8.4, P = 0.002). (C) Larval triglyceride levels of homozygous forR, fors and heterozygous forR/fors individuals (F(2,45) = 8.36, P = 0.00082). *** P < 0.001, ** P < 0.01, * P < 0.05; ns, nonsignificant.
foraging gene dosage and allelic contributions to foraging gene expression
We next set out to verify that our allelic combinations of foraging used to manipulate gene dosage do indeed confer differences in gene expression. We used reverse transcription quantitative PCR (RT-qPCR) to characterize the expression levels of the foraging gene in whole larval homogenates. We probed the common coding region of the gene to capture all known transcripts, as well as the four promoter regions (Figure 1 and Figure 6A). We identified clear differences in expression between our homozygous foraging null (for0), our homozygous genomic rescue (for0;{forBAC}), and homozygous over expresser (fors;{forBAC}) larvae for all regions assayed (Figure 6, B–F). As expected for0 showed no expression, for0;{forBAC} showed significantly higher expression than the null, and fors;{forBAC} showed significantly higher expression than both the null and the rescue. We saw no difference between homozygous rovers, sitters, and rover/sitter heterozygotes, but hemizygous rovers and sitters (forR/for0 and fors/for0) had half the expression of foraging (Figure S5). Osborne et al. (1997) showed using northern analyses that the foraging mRNA levels in whole adult rovers were higher than in sitters. In contrast, in the present study using RT-qPCR we assayed foraging mRNA levels from 72-hr post hatch whole larvae and found no differences in expression between rovers and sitters. The effects of the different allelic combinations were similar across the different promoters and were consistent with the pattern seen for the common coding region. The only exception to this pattern was the expression of pr1, which had lower expression in the {forBAC} allele relative to the fors allele, suggesting that the expression pattern of our rescue line differs in pr1 expression compared to wild type (Figure 6B). There were, however, large differences in the level of expression between the different promoters. Notably, pr1 and pr3 made up most of the foraging expression (Figure 6, B and D, respectively). Conversely, pr2 and pr4 only represent a small fraction of the total foraging expression (Figure 6, C and D, respectively). The signal seen with the pr3 primer set also captured some of the isoforms produced from pr1 and pr2 since the transcript RB has no unique element and is nested within RA, RH, and RI. These data would suggest that in the whole larva, pr1 and pr3 contribute roughly equally to the overall expression levels seen from foraging and they represent most of the expression, with pr2 and pr4 having overall low expression levels, or are expressed in only a small subset of foraging-expressing cells.
Figure 6.
foraging gene dosage and allelic contributions to foraging gene expression in whole larvae. Expression of foraging mRNA of homozygous for0, for0;{forBAC}, fors;{forBAC}, and fors whole larvae homogenates amplifying each promoter region and the common coding region. (A) Schematic of the foraging (as in Figure 1). Promoter-specific regions targeted for qPCR identified by the upper vertex of the light blue triangles. (B) pr1-specific (F(3,8) =218.4, P = 5.2e−8), (C) pr2-specific (F(3,8) = 360.2, P = 7.2e−9), (D) pr (F(3,8) = 113.6, P = 6.7e−7), (E) pr4-specific (F(3,8) = 152.6, P = 2.1e−7), and (F) common coding (F(3,8) = 301.6, P = 1.4e−8) expression of regions of foraging quantified RT-qPCR. Due to the gene structure, pr3 is not specific and amplifies a subset of pr1 and pr2, as well. (B–F) Individual data points are plotted (n = 3/genotype; triangle, square, circle) with a bar representing the mean of the three samples. All the ΔΔCts were calculated relative to the mean fors ΔCt for the common coding region in F. *** P < 0.001, ** P < 0.01; P-values are relative to fors.
Modeling the effect of foraging gene dosage on path length, food intake, and triglyceride phenotypes
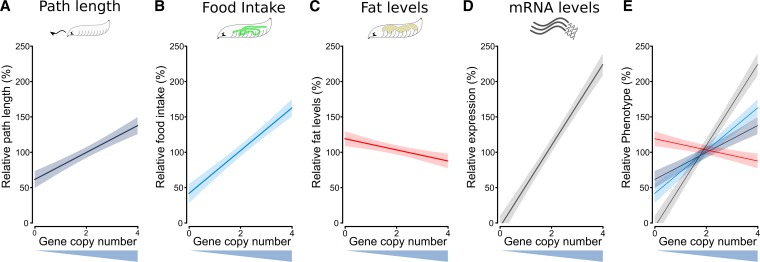
We then modeled the continuous effect of gene copy number on the path length, food intake, fat levels, and gene expression phenotypes. We performed linear regressions on the data (Figure 3B, Figure 4B, Figure 5B, and Figure 6F), but with the genotype variable exchanged for gene dosage number (null = 0, rescue = 2, over = 4). The phenotypes were normalized to rescue strain and are plotted as a percent. From these regression lines (solid lines) and their 95% confidence intervals (faded boxes), we can clearly see the linear gene dosage effect of the foraging gene on the phenotypes of path length, food intake, and triglyceride levels (Figure 7, A–C). The effect of gene dosage on foraging gene expression is added for comparison (Figure 7D). The regressions were highly significant, and had R2 values of between 0.3 and 0.4 for path length, food intake, and fat, but naturally much higher for gene expression, R2 = 0.98 (Figure 7, A–D). Aside from gene expression, food intake had the steepest slope and fat levels had the shallowest (Figure 7E). Therefore, increasing foraging gene expression had a larger relative effect on food intake than it did on path length (food intake vs. path length, t(320) = 3.21, P = 1.46e−3) or fat levels (food intake vs. fat, t(280) = 11.8, P = 1.99e−26).
Figure 7.
Modeled effects of foraging gene dosage on relative path length, food intake, fat, and expression. The fitted responses (solid lines) are plotted with 95% confidence intervals (greyed boxes). Raw data from Figure 3B, Figure 4B, Figure 5B, and Figure 6F were normalized to the mean of fors;{forBAC} for each phenotype; (A) path length (R2 = 0.374, slope = 19.05, intercept = 61.90, F(1,106) = 64.81, P = 1.3e−12), (B) food intake (R2 = 0.358, slope = 30.23, intercept = 42.17, F(1,250) = 141.0, P < 2.2e−16), (C) fat levels (R2 = 0.399, slope = −7.850, intercept = 119.5, F(1,22) = 16.29, P = 5.5e−4), (D) mRNA expression (R2 = 0.984, slope = 57.41, intercept = −5.227, F(1,7) =502.5, P = 8.9e−8). (E) path length, food intake, fat levels, and gene expression overlaid on each other for comparison.
Discussion
Meeting energy requirements and maintaining energy homeostasis is critical for every organism. The coordination of food searching, food acquisition, and nutrient storage is necessary for meeting the challenges of changing environments. Selecting for one of these traits has been shown to have knock-on effects on the others (Sewell et al. 1974; Burnet et al. 1977; Joshi and Mueller 1988; Sokolowski et al. 1997). The extensive behavioral literature on the foraging gene serves as an important example of how allelic variants can be used to infer the functions of a gene. Much of the work conducted to date on the foraging gene relied on the rover and sitter allelic variants (de Belle et al. 1989, 1993; Kaun et al. 2007) and studies using cDNA constructs driving expression beyond wild-type levels (Osborne et al. 1997; Mery et al. 2007; Kaun et al. 2008; Burns et al. 2012). However, it is important to note that this approach may only uncover a subset of foraging’s functions and does not allow for a genetic dissection of the relationship between them.
As a first step toward a comprehensive characterization of the foraging gene, we carried out an exhaustive molecular analysis, defining the transcriptional complexity of foraging expression in larvae. We have defined four independent promoters, producing 21 mRNA transcripts between them, 11 of which are novel. This complexity lends support to the idea that foraging’s gene products might be differentially regulated to produce its multiple phenotypes. All foraging transcripts encode ORFs with a common cGMP-binding and kinase domain; however, isoforms notably differ in their N-termini. Furthermore, among the novel transcripts, there is a class that exhibits exon skipping, relative to the other transcripts produced from the same promoter. This exon skipping results in a roughly 300–500 (depending on the transcript) amino acid truncation at the N-termini, which includes both the autoinhibitory domain and dimerization domain. Interestingly, the N-termini of PKGs are critical in determining PKG-substrate interaction (Pearce et al. 2010). So, foraging’s function not only depends on which cell a promoter drives expression, but also what substrate foraging’s proteins can bind.
A precise genetic null allele allows us to conclusively associate foraging’s phenotypes, and lay the foundation for future genetic dissection of foraging’s isoform- and tissue-specific functions. In the present study, we precisely deleted the entire 35-kb foraging gene using HR to generate a for0 null mutant, and we generated a transgenic {forBAC} allele using recombineering. We could study the resulting loss-of-function phenotypes in D. melanogaster larvae despite the pupal lethality of the homozygous for0 null mutant. We have shown that foraging plays a critical role in each of the feeding-related phenotypes studied here. Increasing foraging gene copy number, both by insertion of the {forBAC} allele in a wild-type background, as well as the duplication at the locus itself, increased both larval path length on yeast and food intake while reducing triglyceride levels.
We have provided compelling evidence for a causal relationship between the foraging gene and its three larval phenotypes, using gene dosage. The null mutant generated here also provides a basis for conditional rescue using transcript-specific cDNA lines, and tissue-specific GAL4 drivers. These tools will allow the rescue of the amorphic phenotype with spatial- and temporal-specific expression using the various foraging isoforms. Mapping the promoters and their transcripts involved in foraging-related phenotypes will allow us to narrow down DNA sequences in the locus that contain putative regulatory elements. This will in turn make it possible to select candidate SNPs between rovers and sitters that might be driving differences seen in expression and behavior. The fact that our {forBAC} construct rescues the null phenotypes, suggests that the 35-kb locus of the foraging gene contains the required regulatory elements to drive the expression necessary for viability, path length, food intake, and triglyceride levels. We have sequenced the foraging gene of rovers and sitters and found that there are >300 SNPs that differ between these lines. The vast majority of these differences lie in the noncoding region of the gene, suggesting key allelic differences are likely associated with the regulation of gene expression rather than the modification of the function of the kinase itself. Nevertheless, this number of SNPs is far too extensive to address each SNP as a candidate causal SNP. Thus, narrowing down putative regulatory regions will be the first step to address the underlying DNA differences that drive rover and sitter behavior.
We quantified the expression of the individual promoters of foraging in whole larvae and saw striking differences in expression levels. These different expression levels show that the isoforms of foraging are differentially regulated. This differential regulation may result in different isoforms being expressed in different tissues at different times, and at different levels. Microarray data from Fly Atlas and RNA-Seq data from modENCODE support this possibility. foraging is expressed in many tissues (Chintapalli et al. 2007) throughout development (Graveley et al. 2011). Furthermore, isoform-specific exons are differentially expressed in different tissues (Brown et al. 2014). foraging’s function may be tissue-specific or distributed across many tissue systems for any given phenotype. foraging’s tissue-specific requirements for the larval phenotypes studied here are currently not known.
Whether foraging’s role in these phenotypes is due to acute or developmental functions of its gene products is also unknown. Formation of the proper circuits for feeding behavior are developmentally regulated (Friedrich et al. 2016), and foraging mutants have been shown to alter nervous system development in the embryo (Peng et al. 2016). It is possible that a portion of foraging’s function in the larva may be due to such a role in embryo development. Alternatively, foraging may be functioning acutely during the behavior to elicit its phenotypic effects. hugin neurons, or their targets, are candidate cells for foraging function since acute manipulation of these cells can alter larval locomotion and feeding (Schoofs et al. 2014). A lack of foraging in D. melanogaster could also affect larval path length or feeding due to altered muscle function, making the muscles less sensitive to incoming neuronal stimuli. foraging’s ortholog in mammals has been characterized for its role in muscle function (Pfeifer et al. 1998; Weber et al. 2007). As for the triglyceride level phenotype, foraging is highly expressed in the fat body of the larva (Chintipalli et al. 2007; Brown et al. 2014), and may function locally in the fat body to alter triglyceride levels. foraging’s orthologs have previously been shown to affect fat levels (Raizen et al. 2006; Miyashita et al. 2009). Future studies will unravel foraging’s tissue-specific requirements for larval path length, food intake, and triglyceride levels.
The use of a kinase such as foraging that can regulate the activity of many targets might allow the coregulation of physiologically related phenotypes such as path length, food intake, and fat in response to external inputs or internal physiological state. This would be important since behavioral responses are complex, originating from the interaction of different cellular processes in different tissues (e.g., changes in movement on food might be brought on by an interaction of fat metabolism, nervous system, and muscle activation), and need to be coordinated. Without knowing the precise tissues or cells where foraging expression is required to alter its associated behaviors, it is difficult to speculate on the nature of the gene networks in which it resides. That being said, previous genetic evidence has suggested possible interaction with insulin-signaling and foxo-signaling (Kent et al. 2009; Kanao et al. 2012).
foraging has been implicated in a variety of adult phenotypes (Sokolowski 2010). Given the pupal lethality seen in the foraging null mutant, it is possible that some of the previously associated adult phenotypes may be developmental in origin, possibly resulting from differential neurogenesis. In the present study, the pupal lethality of the foraging null restricted its use to larval rather than adult phenotypes. We have recently, however, overcome this limitation by using temperature-sensitive foraging RNAi conditional-knockdown, specifically during adulthood (data not shown). These results along with the results of the present study show that foraging is not required for viability specifically in the larval and adult stages, but is required for viability during pupal development.
In the present study, we described and characterized the structure of the foraging gene. We generated a foraging null mutant, for0, using HR. This mutant was pupal lethal, showing that foraging gene products are not required for viability during larval development. This allowed us to evaluate the feeding-related behavior and metabolic state of the for0 null larvae. We have shown that foraging influences how larvae navigate their food environment, how they ingest nutrients, and further deposits those nutrients into fat. Our deletion study combined with the genomic rescue experiments have unequivocally proven a causal, dose-dependent relationship between the foraging gene and its pleiotropic influence on these feeding-related traits. Future studies will shed further light onto foraging’s regulation and the nature of the rover and sitter alleles.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.197939/-/DC1.
Acknowledgments
We thank Scott Douglas, Jean-Christophe Billeter, and Joel Levine for guidance and advice; the Sokolowski Lab for helpful discussions; Jeff Dason, Stephen Goodwin, and the two anonymous reviewers for comments on the manuscript; and the Bloomington Drosophila Stock Center for fly lines. This research was supported by grants from the Natural Sciences and Engineering Council of Canada (NSERC) and the Canadian Institutes for Health Research (CIHR) to M.B.S.
Footnotes
Communicating editor: M. F. Wolfner
Literature Cited
- Anreiter I., Vasquez O. E., Allen A. M., Sokolowski M. B., 2016. Foraging path-length protocol for Drosophila melanogaster larvae. J. Vis. Exp. DOI: 10.3791/53980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attrill H., Falls K., Goodman J. L., Millburn G. H., Antonazzo G., et al. 2016. FlyBase: establishing a gene group resource for Drosophila melanogaster. Nucleic Acids Res. 44: D786–D792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker K., 1962. An analysis of factors which determine success in competition for food among larvae of Drosophila melanogaster. Arch. Néerl. Zool. 14: 200–281. [Google Scholar]
- Bauer S. J., Sokolowski M. B., 1984. Larval foraging behavior in isofemale lines of Drosophila melanogaster and D. pseudoobscura. J. Hered. 75: 131–134. [Google Scholar]
- Belay A. T., Scheiner R., So A. K.-C., Douglas S. J., Chakaborty-Chatterjee M., et al. , 2007. The foraging gene of Drosophila melanogaster: spatial-expression analysis and sucrose responsiveness. J. Comp. Neurol. 504: 570–582. [DOI] [PubMed] [Google Scholar]
- Brown J. B., Boley N., Eisman R., May G. E., Stoiber M. H., et al. , 2014. Diversity and dynamics of the Drosophila transcriptome. Nature 512: 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet B., Sewell D., Bos M., Bakker K., Birch L. C., et al. , 1977. Genetic analysis of larval feeding behaviour in Drosophila melanogaster: II. Growth relations and competition between selected lines. Genet. Res. 30: 149. [DOI] [PubMed] [Google Scholar]
- Burns J. G., Svetec N., Rowe L., Mery F., Dolan M. J., et al. , 2012. Gene-environment interplay in Drosophila melanogaster: chronic food deprivation in early life affects adult exploratory and fitness traits. Proc. Natl. Acad. Sci. USA 109: 17239–17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli V. R., Wang J., Dow J. A., 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39: 715–720. [DOI] [PubMed] [Google Scholar]
- de Belle J. S., Hilliker A. J., Sokolowski M. B., 1989. Genetic localization of foraging (for): a major gene for larval behavior in Drosophila melanogaster. Genetics 123: 157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Belle J. S., Sokolowski M. B., Hilliker A. J., 1993. Genetic analysis of the foraging microregion of Drosophila melanogaster. Genome 36: 94–101. [DOI] [PubMed] [Google Scholar]
- Demir E., Dickson B. J., 2005. fruitless splicing specifies male courtship behavior in Drosophila. Cell 121: 785–794. [DOI] [PubMed] [Google Scholar]
- Edgar B. A., 2006. How flies get their size: genetics meets physiology. Nat. Rev. Genet. 7: 907–916. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick M. J., Sokolowski M. B., 2004. In search of food: exploring the evolutionary link between cGMP-dependent protein kinase (PKG) and behaviour. Integr. Comp. Biol. 44: 28–36. [DOI] [PubMed] [Google Scholar]
- Friedrich J., Sorge S., Bujupi F., Eichenlaub M. P., Schulz N. G., et al. , 2016. Hox function is required for the development and maintenance of the Drosophila feeding motor unit. Cell Rep. 14: 850–860. [DOI] [PubMed] [Google Scholar]
- Gong W. J., Golic K. G., 2003. Ends-out, or replacement, gene targeting in Drosophila. Proc. Natl. Acad. Sci. USA 100: 2556–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W. J., Golic K. G., 2004. Genomic deletions of the Drosophila melanogaster Hsp70 genes. Genetics 168: 1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley B. R., Brooks A. N., Carlson J. W., Duff M. O., Landolin J. M., et al. , 2011. The developmental transcriptome of Drosophila melanogaster. Nature 471: 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. B., Hatini V., Johansen K. A., Liu X.-J., Lengyel J. A., 2002. Drumstick is a zinc finger protein that antagonizes Lines to control patterning and morphogenesis of the Drosophila hindgut. Development 129: 3645–3656. [DOI] [PubMed] [Google Scholar]
- Groth A. C., Fish M., Nusse R., Calos M. P., 2004. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics 166: 1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6: 65–70. [Google Scholar]
- Hoskins R. A., Landolin J. M., Brown J. B., Sandler J. E., Takahashi H., et al. , 2011. Genome-wide analysis of promoter architecture in Drosophila melanogaster. Genome Res. 21: 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A., Mueller L. D., 1988. Evolution of higher feeding rate in Drosophila due to density-dependent natural selection. Evolution 42: 1090. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Rubin M., 1989. cGMP-dependent protein kinase genes in Drosophila. J. Biol. Chem. 264: 10738–10748. [PubMed] [Google Scholar]
- Kanao T., Sawada T., Davies S.-A., Ichinose H., Hasegawa K., et al. , 2012. The nitric oxide-cyclic GMP pathway regulates FoxO and alters dopaminergic neuron survival in Drosophila. PLoS One 7: e30958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun K. R., Riedl C. A. L., Chakaborty-Chatterjee M., Belay A. T., Douglas S. J., et al. , 2007. Natural variation in food acquisition mediated via a Drosophila cGMP-dependent protein kinase. J. Exp. Biol. 210: 3547–3558. [DOI] [PubMed] [Google Scholar]
- Kaun K. R., Chakaborty-Chatterjee M., Sokolowski M. B., 2008. Natural variation in plasticity of glucose homeostasis and food intake. J. Exp. Biol. 211: 3160–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., et al. , 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent C. F., Daskalchuk T., Cook L., Sokolowski M. B., Greenspan R. J., 2009. The Drosophila foraging gene mediates adult plasticity and gene-environment interactions in behaviour, metabolites, and gene expression in response to food deprivation. PLoS Genet. 5: e1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold P., Perrimon N., 2007. Drosophila and the genetics of the internal milieu. Nature 450: 186–188. [DOI] [PubMed] [Google Scholar]
- Ling D., Salvaterra P. M., 2011. Robust RT-qPCR data normalization: Validation and selection of internal reference genes during post-experimental data analysis. PLoS One 6: e17762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G., Plowman G. D., Hunter T., Sudarsanam S., 2002. Evolution of protein kinase signaling from yeast to man. Trends Biochem. Sci. 27: 514–520. [DOI] [PubMed] [Google Scholar]
- Mery F., Belay A. T., So A. K.-C., Sokolowski M. B., Kawecki T. J., 2007. Natural polymorphism affecting learning and memory in Drosophila. Proc. Natl. Acad. Sci. USA 104: 13051–13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Orkin S. H., 1982. Characterization of the homopolymer tailing reaction catalyzed by terminal deoxynucleotidyl transferase. Implications for the cloning of cDNA. J. Biol. Chem. 257: 14773–14782. [PubMed] [Google Scholar]
- Miyashita K., Itoh H., Tsujimoto H., Tamura N., Fukunaga Y., et al. , 2009. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes 58: 2880–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørstavik S., Natarajan V., Taskén K., Jahnsen T., Sandberg M., 1997. Characterization of the human gene encoding the type I alpha and type I beta cGMP-dependent protein kinase (PRKG1). Genomics 42: 311–318. [DOI] [PubMed] [Google Scholar]
- Osborne K. A., Robichon A., Burgess E., Butland S., Shaw R. A., et al. , 1997. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science 277: 834–836. [DOI] [PubMed] [Google Scholar]
- Parks A. L., Cook K. R., Belvin M., Dompe N. A., Fawcett R., et al. , 2004. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36: 288–292. [DOI] [PubMed] [Google Scholar]
- Pearce L. R., Komander D., Alessi D. R., 2010. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 11: 9–22. [DOI] [PubMed] [Google Scholar]
- Peng Q., Wang Y., Li M., Yuan D., Xu M., et al. , 2016. cGMP-dependent protein kinase encoded by foraging regulates motor axon guidance in Drosophila by suppressing lola function. J. Neurosci. 36: 4635–4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer A., Klatt P., Massberg S., Ny L., Sausbier M., et al. , 1998. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J. 17: 3045–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton F., Chapuis M. P., Pernice M., Sword G. A., Simpson S. J., 2011. Evaluation of potential reference genes for reverse transcription-qPCR studies of physiological responses in Drosophila melanogaster. J. Insect Physiol. 57: 840–850. [DOI] [PubMed] [Google Scholar]
- R Core Team 2016 R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/.
- Raizen D. M., Cullison K. M., Pack A. I., Sundaram M. V., 2006. A novel gain-of-function mutant of the cyclic GMP-dependent protein kinase egl-4 affects multiple physiological processes in Caenorhabditis elegans. Genetics 173: 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder E., Blows F., Ashburner M., Bautista-Llacer R., Coulson D., et al. , 2004. The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics 167: 797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Russell D. W., 2001. Molecular Cloning: A Laboratory Manual, Ed. 3 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., et al. , 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoofs A., Hückesfeld S., Schlegel P., Miroschnikow A., Peters M., et al. , 2014. Selection of motor programs for suppressing food intake and inducing locomotion in the Drosophila brain. PLoS Biol. 12: e1001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell D., Burnet B., Connolly K., 1974. Genetic analysis of larval feeding behaviour in Drosophila melanogaster. Genet. Res. 24: 163. [DOI] [PubMed] [Google Scholar]
- Siegal M. L., Hartl D. L., 1996. Transgene coplacement and high efficiency site-specific recombination with the Cre/loxP system in Drosophila. Genetics 144: 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski M. B., 1980. Foraging strategies of Drosophila melanogaster: a chromosomal analysis. Behav. Genet. 10: 291–302. [DOI] [PubMed] [Google Scholar]
- Sokolowski M. B., 2001. Drosophila: genetics meets behaviour. Nat. Rev. Genet. 2: 879–890. [DOI] [PubMed] [Google Scholar]
- Sokolowski M. B., 2010. Social interactions in “simple” model systems. Neuron 65: 780–794. [DOI] [PubMed] [Google Scholar]
- Sokolowski M. B., Pereira H. S., Hughes K., 1997. Evolution of foraging behavior in Drosophila by density-dependent selection. Proc. Natl. Acad. Sci. USA 94: 7373–7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansberry J., Baude E. J., Taylor M. K., Chen P. J., Jin S. W., et al. , 2001. A cGMP-dependent protein kinase is implicated in wild-type motility in C. elegans. J. Neurochem. 76: 1177–1187. [DOI] [PubMed] [Google Scholar]
- Stapleton M., Liao G., Brokstein P., Hong L., Carninci P., et al. , 2002. The Drosophila gene collection: identification of putative full-length cDNAs for 70% of D. melanogaster genes. Genome Res. 12: 1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S., Wakem M., Dijkman G., Alsarraj M., Nguyen M., 2010. A practical approach to RT-qPCR-publishing data that conform to the MIQE guidelines. Methods 50: S1–S5. [DOI] [PubMed] [Google Scholar]
- Thorpe H. M., Smith M. C., 1998. In vitro site-specific integration of bacteriophage DNA catalyzed by a recombinase of the resolvase/invertase family. Proc. Natl. Acad. Sci. USA 95: 5505–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken K. J. T., He Y., Hoskins R. A., Bellen H. J., 2006. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science 314: 1747–1751. [DOI] [PubMed] [Google Scholar]
- Warming S., Costantino N., Court D. L., Jenkins N. A., Copeland N. G., 2005. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33: e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S., Bernhard D., Lukowski R., Weinmeister P., Wörner R., et al. , 2007. Rescue of cGMP kinase I knockout mice by smooth muscle specific expression of either isozyme. Circ. Res. 101: 1096–1103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains are available upon request. Information in supplemental material: Table S1 contains primer names and sequences used in this study. Figure S1 describes the forHR alleles, and the generation of the for0 null and fordup alleles. Figure S2 shows verification of the for0 allele. Figure S3 shows construction and verification of the forBAC construct. Figure S4 shows path length, food intake and fat levels of hemizygous rover and sitter larvae. Figure S5 shows foraging gene dosage and allelic contributions to foraging gene expression in whole larvae. Figure S6 provides foraging transcript sequences. File S1 provides the figure legends for Figures S1-S6.