Abstract
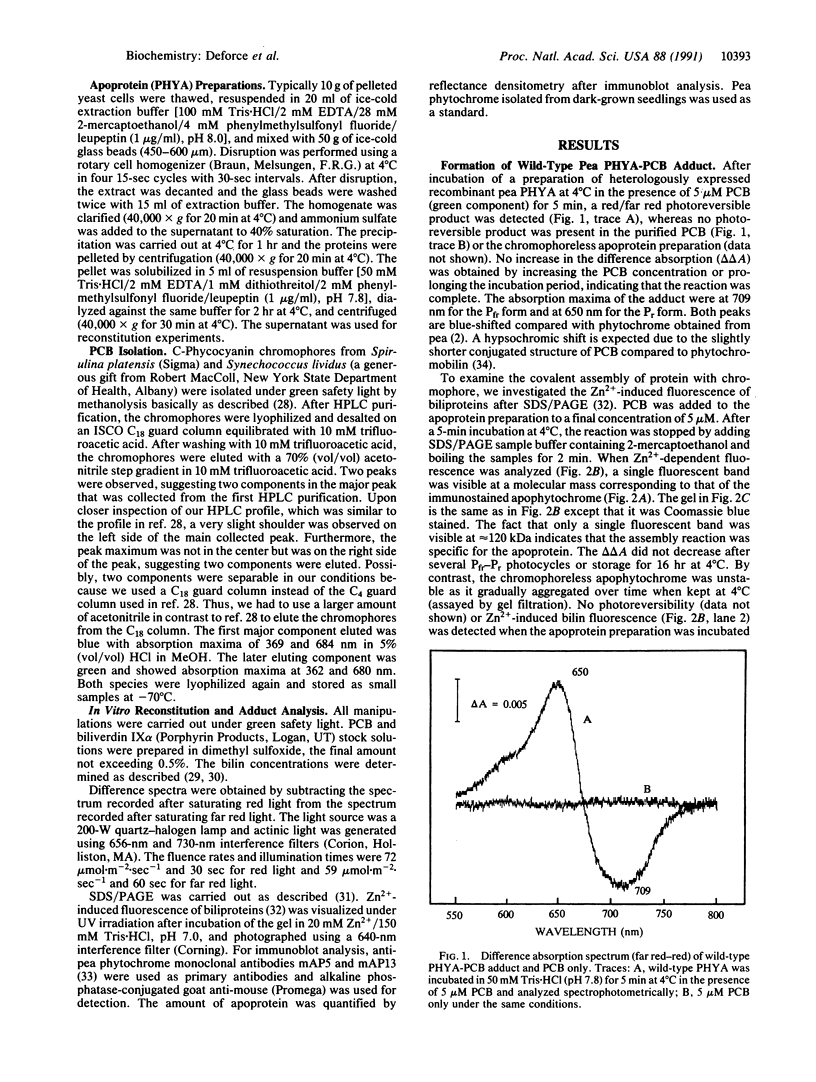
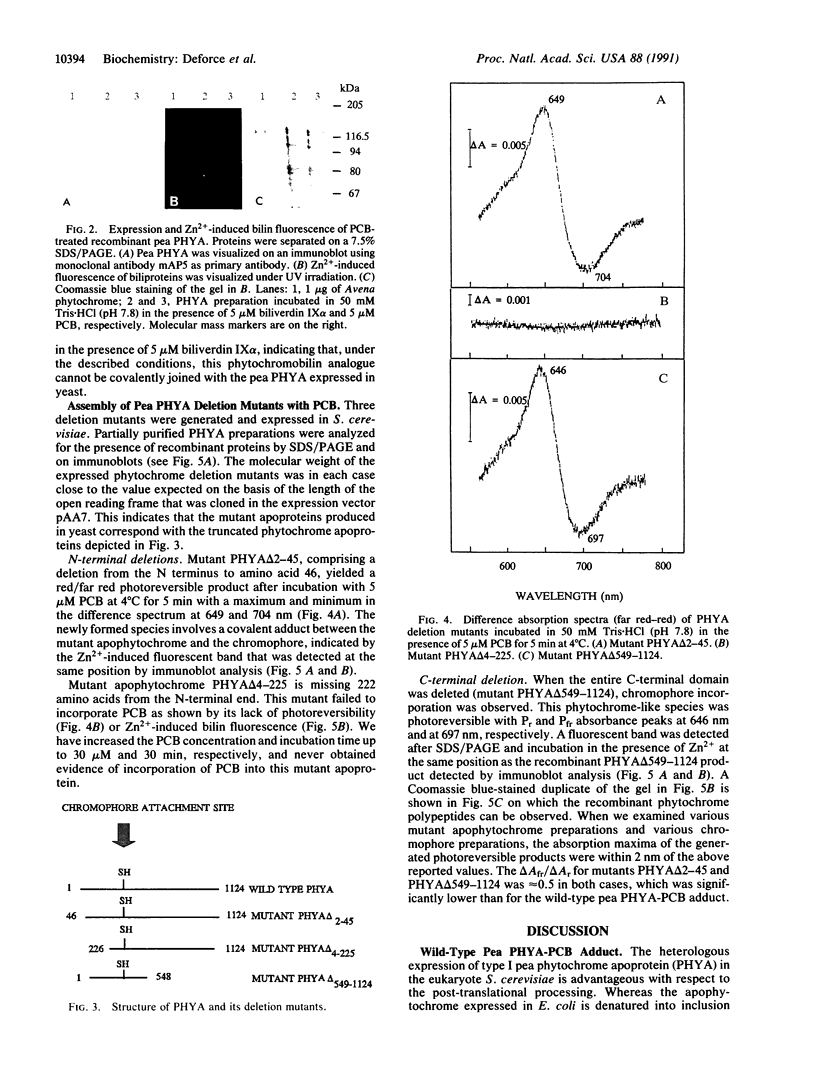
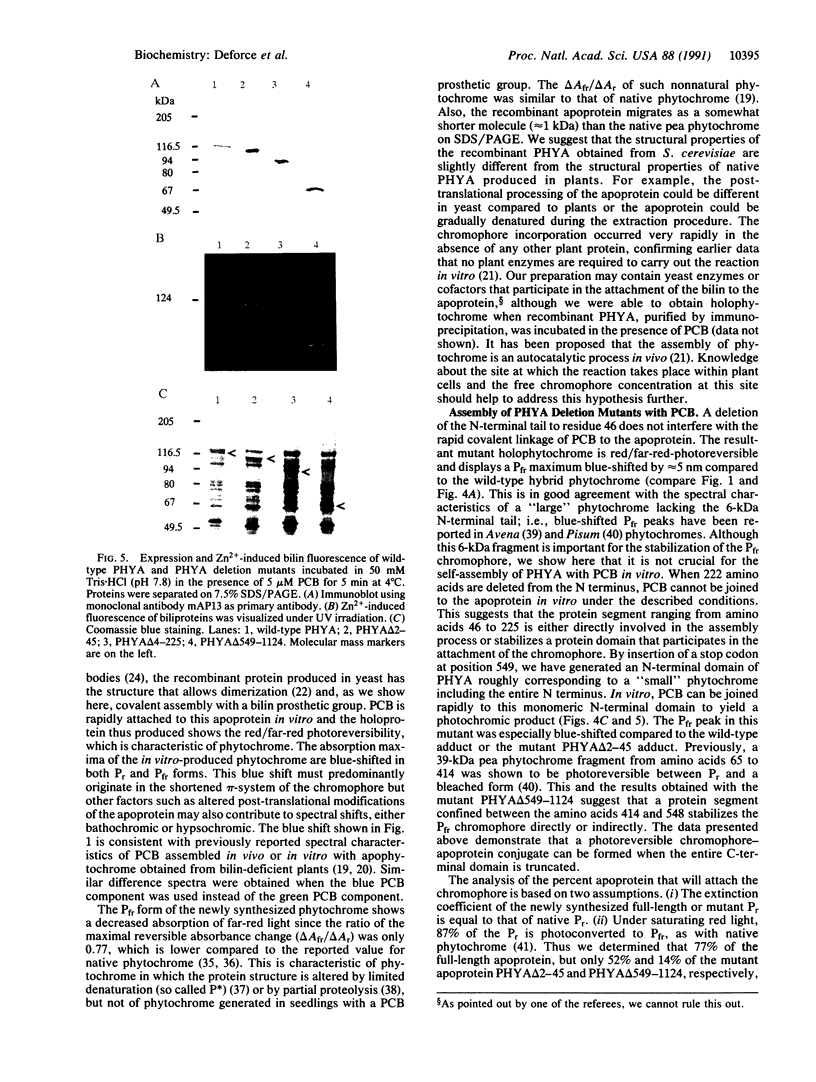
Recombinant pea type I phytochrome apoprotein expressed in yeast is shown to assemble in vitro with phycocyanobilin to produce a photoreversible phytochrome-like adduct. As an initial investigation of the amino acid sequence requirements for chromophore incorporation, three phyA gene product deletion mutants were produced in yeast. Truncation of the N-terminal tail to residue 46 demonstrates that this region is not critical to bilin attachment, but a deletion mutant lacking 222 amino acids from the N terminus failed to yield holophytochrome in vitro, under the same conditions. A mutant comprising a deletion of the C terminus to residue 548 showed bilin incorporation and red/far-red photoreversibility, indicating that bilin-apophytochrome assembly still occurred even when the entire C-terminal domain was truncated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arciero D. M., Bryant D. A., Glazer A. N. In vitro attachment of bilins to apophycocyanin. I. Specific covalent adduct formation at cysteinyl residues involved in phycocyanobilin binding in C-phycocyanin. J Biol Chem. 1988 Dec 5;263(34):18343–18349. [PubMed] [Google Scholar]
- BUTLER W. L., SIEGELMAN H. W., MILLER C. O. DENATURATION OF PHYTOCHROME. Biochemistry. 1964 Jun;3:851–857. doi: 10.1021/bi00894a022. [DOI] [PubMed] [Google Scholar]
- Berkelman T. R., Lagarias J. C. Visualization of bilin-linked peptides and proteins in polyacrylamide gels. Anal Biochem. 1986 Jul;156(1):194–201. doi: 10.1016/0003-2697(86)90173-9. [DOI] [PubMed] [Google Scholar]
- Chai Y. G., Song P. S., Cordonnier M. M., Pratt L. H. A photoreversible circular dichroism spectral change in oat phytochrome is suppressed by a monoclonal antibody that binds near its N-terminus and by chromophore modification. Biochemistry. 1987 Aug 11;26(16):4947–4952. doi: 10.1021/bi00390a010. [DOI] [PubMed] [Google Scholar]
- Elich T. D., Lagarias J. C. Formation of a photoreversible phycocyanobilin-apophytochrome adduct in vitro. J Biol Chem. 1989 Aug 5;264(22):12902–12908. [PubMed] [Google Scholar]
- Elich T. D., Lagarias J. C. Phytochrome Chromophore Biosynthesis : Both 5-Aminolevulinic Acid and Biliverdin Overcome Inhibition by Gabaculine in Etiolated Avena sativa L. Seedlings. Plant Physiol. 1987 Jun;84(2):304–310. doi: 10.1104/pp.84.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elich T. D., McDonagh A. F., Palma L. A., Lagarias J. C. Phytochrome chromophore biosynthesis. Treatment of tetrapyrrole-deficient Avena explants with natural and non-natural bilatrienes leads to formation of spectrally active holoproteins. J Biol Chem. 1989 Jan 5;264(1):183–189. [PubMed] [Google Scholar]
- Fodor S. P., Lagarias J. C., Mathies R. A. Resonance Raman analysis of the Pr and Pfr forms of phytochrome. Biochemistry. 1990 Dec 18;29(50):11141–11146. doi: 10.1021/bi00502a018. [DOI] [PubMed] [Google Scholar]
- Gardner G., Gorton H. L. Inhibition of phytochrome synthesis by gabaculine. Plant Physiol. 1985 Mar;77(3):540–543. doi: 10.1104/pp.77.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey H. P., Barker R. F., Idler K. B., Lissemore J. L., Quail P. H. Analysis of cloned cDNA and genomic sequences for phytochrome: complete amino acid sequences for two gene products expressed in etiolated Avena. Nucleic Acids Res. 1985 Dec 9;13(23):8543–8559. doi: 10.1093/nar/13.23.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S. A., Nagatani A., Keith B., Deak M., Furuya M., Chua N. H. Rice Phytochrome Is Biologically Active in Transgenic Tobacco. Plant Cell. 1989 Aug;1(8):775–782. doi: 10.1105/tpc.1.8.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagarias J. C., Lagarias D. M. Self-assembly of synthetic phytochrome holoprotein in vitro. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5778–5780. doi: 10.1073/pnas.86.15.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima N., Nakagawa K., Yamamoto E., Shibata T. A subunit of yeast site-specific endonuclease SceI is a mitochondrial version of the 70-kDa heat shock protein. J Biol Chem. 1990 Sep 5;265(25):15189–15197. [PubMed] [Google Scholar]
- Rüdiger W., Thümmler F., Cmiel E., Schneider S. Chromophore structure of the physiologically active form (P(fr)) of phytochrome. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6244–6248. doi: 10.1073/pnas.80.20.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock R. A., Quail P. H. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989 Nov;3(11):1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Song P. S. The molecular topography of phytochrome: chromophore and apoprotein. J Photochem Photobiol B. 1988 Jul;2(1):43–57. doi: 10.1016/1011-1344(88)85036-x. [DOI] [PubMed] [Google Scholar]
- Vierstra R. D., Quail P. H., Hahn T. R., Song P. S. Comparison of the protein conformations between different forms (Pr and Pfr) of native (124 kDa) and degraded (118/114 kDa) phytochromes from Avena sativa. Photochem Photobiol. 1987 Mar;45(3):429–432. doi: 10.1111/j.1751-1097.1987.tb05398.x. [DOI] [PubMed] [Google Scholar]
- Vierstra R. D., Quail P. H. Spectral Characterization and Proteolytic Mapping of Native 120-Kilodalton Phytochrome from Cucurbita pepo L. Plant Physiol. 1985 Apr;77(4):990–998. doi: 10.1104/pp.77.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]





