Abstract
Genetic and environmental factors are believed to influence development of systemic lupus erythematosus (SLE). Endogenous retroviruses (ERV) correspond to the integrated proviral form of infectious retroviruses, which are trapped within the genome due to mutations. ERV represent a key molecular link between the host genome and infectious viral particles. ERV-encoded proteins are recognized by antiviral immune responses and become targets of autoreactivity. Alternatively, ERV protein may influence cellular processes and the life cycle of infectious viruses. As examples, the HRES-1 human ERV encodes a 28-kDa nuclear autoantigen and a 24-kDa small GTP-ase, termed HRES-1/Rab4. HRES-1/p28 is a nuclear autoantigen recognized by cross-reactive antiviral antibodies, while HRES-1/Rab4 regulates surface expression of CD4 and the transferrin receptor (TFR) through endosome recycling. Expression of HRES-1/Rab4 is induced by the tat gene of HIV-1, which in turn down-regulates expression of CD4 and susceptibility to re-infection by HIV-1. CD4 and the TFR play essential roles in formation of the immunological synapse (IS) during normal T-cell activation by a cognate MHC class II peptide complex. The key intracellular transducer of T-cell activation, Lck, is brought to the IS via binding to CD4. T-cell receptorζ (TCRζ) chain binds to the TFR. Abnormal T-cell responses in SLE have been associated with reduced lck and TCRζ chain levels. HRES-1 is centrally located on chromosome 1 at q42 relative to lupus-linked microsatellite markers and polymorphic HRES-1 alleles have been linked to the development of SLE. 1q42 is one of the three most common fragile sites in the human genome, and is inducible by DNA demethylation, a known mechanism of retroviral gene activation. Molecular mimicry and immunomodulation by a ERV, such as HRES-1, may contribute to self-reactivity and abnormal Tand B-cell functions in SLE.
Keywords: Systemic lupus erythematosus, cross-reactivity, HRES, endogenous retroviral sequences, immunodulation, receptor recycling
Genetic and environmental factors in SLE
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease characterized by dysfunction of T and B lymphocytes and autoantibody production. Independent lines of evidence have implicated genetic and environmental factors in the causation of lupus [1]. On the one hand, significant familial aggregation of SLE was demonstrated by epidemiologic studies [2]. The disease occurs in relatives of SLE patients with a frequency between 0.4 and 5%, several 100-fold greater than in the general population [3]. The concordance rate of SLE among monozygotic twins may be as high as 25–60% [3,4]. On the other hand, discordance rates of SLE are as high as 70% among monozygotic twins [3], suggesting a significant role for exogenous factors. Initially, findings of virion-like tubuloreticular structures in endothelial cells and lymphocytes as well as demonstration of elevated serum levels of type I interferon (IFN) raised the possibility of a viral etiology in lupus [5]. An increased prevalence of the nearly ubiquitous Epstein–Barr virus (EBV) has been demonstrated in adolescent lupus patients [6] which could contribute to disease pathogenesis via B-cell activation and triggering of antinuclear antibodies [7]. Retroviruses were implicated by detection of retroviral p30 gag protein in renal glomeruli and serum reactivities towards p30 gag antigen in patients with SLE [8]. Indeed, many features of human retroviral infections caused by HTLV-I and HIV-1 resemble those of SLE, and viral proteins have profound effects on both antigen presentation and effector functions of the immune system [9]. Dysregulation of programmed cell death has been documented in HIV-infected [10] and lupus patients as well [11–13]. Similar to SLE, anemia [14], leukopenia [15], thrombocytopenia [16], polymyositis [17] and vasculitis have been widely reported in patients with AIDS [18]. Direct virus isolation attempts from tissues of SLE patients have not been successful [19]. Nevertheless, it is possible that a (retro)virus, responsible for provoking an immune response cross-reactive with self-antigens, has been cleared from the host, so the absence of lupus-specific viral particles is not conclusive. An alternative (retro) viral etiology, i.e. activation of endogenous retroviral sequences (ERS) was initially proposed by a study of the New Zealand mouse model of SLE [20]. Endogenous retroviral envelope glycoprotein, gp70, was found in immunecomplex deposits of autoimmune lupus-prone NZB/NZW mice [20]. Abnormal expression of an ERS was noted in the thymus of lupusprone mouse strains [21,22]. More recently, expression and autoantigenicity of human ERS has been demonstrated in patients with SLE [23–27].
Molecular biology of ERV
Endogenous retroviruses (ERV) and other retroviral elements have been found in all vertebrates investigated. They belong to the larger family of retrotransposable elements that make up as much as 40% of the human genome [28]. These elements include short interspersed nucleotide elements such as ~300 bp Alu repeats and ~1 kb transaldolase-associated repetitive elements (TAREs), and long interspersed nucleotide elements (LINEs), such as L1. Alus and TAREs can be transcribed into nonpolyadenylated RNA by RNA polymerase III [29]. LINEs are polyadenylated and transcribed by RNA polymerase II. Their reintegration is dependent on reverse transcription. Most retroelements, Alus and truncated ERVs, lack reverse transcriptase (RT) which can be provided in-trans by other retroelements, such as L1 [30]. Occasionally, mRNA transcripts of functional genes can be reverse transcribed and reintegrated into the genome thus giving rise to retropseudogenes. These sequences lack introns and contain a poly-A tail at their 3′ end. As an example, the human genome contains an intronless and poly-adenylated transaldolase pseudogene on human chromosome 1 [31].
Human ERVs (HERVs) have the basic structures of the integrated proviral form of infectious retroviruses with long terminal repeats (LTRs) of several hundred nucleotides flanking sequences homologous to gag, pol, and env genes [32]. The gag gene codes for inner structural core proteins, such as matrix and capsid. The pol gene encodes RT, which copies viral RNA into DNA as well as protease and integrase allowing for integration of proviral DNA into the host genome. The env gene codes for transmembrane and outer envelop proteins, the latter playing key roles in binding to cell surface receptors. Sequence homologies between the pol genes have been used to divide ERVs into two classes: class I with homologies to mammalian type C retroviruses and class II with homologies to mammalian type A, B, and D retroviruses and avian type C retroviruses (Table I). HERVs are commonly designated as HERV followed by a single letter amino acid code corresponding to a tRNA. The 3′ terminus of tRNA is predicted to initiate reverse transcription by annealing to an 18 nucleotide long primer-binding site (PBS) at the 5′ LTR. HERVs have generally been found to be defective proviruses having accumulated deletions or stop codons in gag, pol, and/or env open reading frames (ORFs; [33]).
Table I.
ERV families in the human genome.
| Designation | Organization | Length (kb) | Copy number * | Reference |
|---|---|---|---|---|
| Class I | ||||
| HERV-E | LTR-gag-pol-env-LTR | 8.8 | 35–85 | [32,127] |
| HERV-F | LTR-gag-pol-env | 7.1 | 1 | [128] |
| HERV-H | LTR-gag-pol-env-LTR | 8.7 | 660 | [129,130] |
| HERV-I | LTR-gag-pol-env-LTR | 9.0 | 25–85 | [32,130] |
| HERV-P | LTR-gag-pol-env-LTR | 8.2 | 20–100 | [32,131] |
| HERV-R | LTR-gag-pol-env-LTR | 9.9 | 10–15 | [130,132] |
| HERV-W | LTR-gag-pol-env-LTR | 7.6 | 15–115 | [81,130,133] |
| ERV-1 | gag-pol-env-LTR | 3–4 | 1–15 | [134]{1358} |
| ERV-9 | LTR-gag-pol-env-LTR | 9.6 | 40–70 | [32,130,135] |
| HRES-1 | LTR-gag-Δpol | 6 | 1 | [32] |
| RRHERV-I | LTR-gag-Δpol-LTR | 3.3 | 15–20 | [32,91] |
| S71 | gag-Δpol-env-LTR | 5.4 | 1–20 | [32,136,137] |
| Class II | ||||
| HERV-K | LTR-gag-pol-env-LTR | 9.2 | 170 | [32,130,138] |
| HERV-L | LTR-gag-pol-ΔLTR | 6.5 | 200–575 | [130,139] |
| HERV.HML6 | LTR-gag-Δpol-env-LTR | 7.5 | 30–45 | [130,140] |
Approximation based on hybridizations and frequency in the human genome sequence.
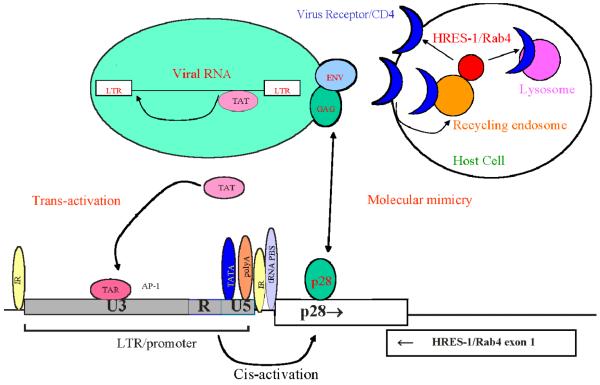
ERV represent a key molecular link between the host genome and infectious viral particles. ERVs may have originated from exogenous retroviruses that integrated into the genome and became trapped owing to mutations of essential genes [32]. They constitute a large reservoir of viral genes that may be activated by mutations caused by radiation or chemicals, or recombination with exogenous retroviruses. While exogenous retroviruses are infectious, with a replication cycle which requires integration of proviral DNA into host cell DNA, ERVs are transmitted genetically in a classical mendelian fashion through the germline as proviral DNA. Expression of ERVs can influence the outcome of infections in different ways both beneficial and detrimental to the host [32]. These include provision of genes for recombination with exogenous viruses, interference with virion assembly, blocking cellular receptors for viral entry, and modulation of immune responses to exogenous viruses (Figure 1). Recombination with murine ERV can expand cellular tropism of HIV-1 [34].
Figure 1.
Effect of ERVon viral immunity and autoimmunity via molecular mimicry, trans-activation, and receptor recycling. As an example, the HRES-1 LTR contains TATA nucleotide sequence-containing motif recognized by the RSA polymerase II transcription machinery (TATA) box, poly-adenylation (polyA) site, tRNA PBS, an HIV-1 trans-activation region, and inverted repeats at typical locations [66]. Transcription from the HRES-1 LTR may be stimulated by trans-acting factors, e.g. tat of HIV-1. ERV proteins may interfere with virion assembly or recycling of cell surface receptors, such as CD4, thus effecting replication, infectivity, and pathogenicity of exogenous viruses. Proteins encoded by an ERV may serve as cross-reactive targets of viral immune responses or may directly regulate signaling within the immune system, such as assembly of the IS via receptor recycling.
While expression of murine ERV can lead to production of infectious virus and cause viremia, no production of infectious virion has been documented by HERV. High copy number of most ERV families makes it difficult to distinguish which members of a group are expressed. Although, no single provirus with intact LTRs and uninterrupted gag, pol, and env ORFs has been identified, the HERV-K ERV, as a family, has been shown to encode gag [35], RT [36], integrase [37], and rev proteins [38,39]. The HERV-K rev protein, encoded by the LTR region, is functionally analogous to the HIV-1 rev and HTLV-I/II rex proteins. HERV-K rev binds to both the nuclear export factor Crm1 and to a cis-acting viral RNA to activate nuclear export of unspliced RNAs [39]. Alternatively, the HERV-K LTR is also recognized by HIV-1 rev, suggesting a potential interaction between these exogeneous and ERV.
The HERV-K class of HERV may be most potent in terms of its ability to form virions [38,40]. HERV show a characteristic pattern of tissue-specific expression. Most ERV are expressed in the placenta, teratocarcinomas, and other malignant tissues. Several ERV are expressed in normal peripheral blood lymphocytes [41–43], salivary gland [25], breast [44], and keratinocytes [45].
Mechanisms of endogenous retroviral involvement in autoimmunity
ERSs may lead to autoimmunity directly, by encoding autoantigens, or indirectly, by affecting the expression of genes regulating immune responses and tolerance [9]. Direct autoantigenicity of HRES-1 and ERV-3 has been documented in SLE (Table II). ERS, if expressed, are likely targets of cross-reactivity for virally induced immune responses. Such cross-reactivity, i.e. molecular mimicry between self-antigens and viral proteins has been proposed as a trigger of autoimmunity [46,47].
Table II.
Molecular mimicry between viral proteins and autoantigens in patients with SLE.
| Autoantigen | Prevalence * (%) | Viral protein | Reference | |
|---|---|---|---|---|
| 70k/U1 snRNP | 30 | gag | MoMLV, HRES-1 | [24,58] |
| HRES-1/p28 | 21–52 | gagp24 | HTLV-I | [23–27] |
| HRES-1/p28 | 21–52 | ORF2a, capsid 1 | TTV | [71] |
| La | 15 | gag | FSV | [141] |
| Sm B/B = | 30 | gagp24 | HIV-1 | [142] |
| C/U1 snRNP | 30 | ICP4 | HHV-1 | [143] |
| Sm D | 36 | Epstein-Barr virus nuclear antigen (EBNA)-1 |
EBV | [7] |
| p542 | 10–50 | EBNA-1 | EBV | [144,145] |
| ERV-3 | 32 | env | MoMLV | [146] |
Prevalence of antibodies in patients with SLE.
In addition to serving as cross-reactive targets of antiviral immunity, ERS may also have a direct role in regulating immune responses [9]. ERS and other retrotransposable elements possess a relatively high mobility and may cause immune dysregulation by insertional mutagenesis or cis or trans-regulation of cellular genes [48]. HERV-K10 has an integration site within the complement C2 gene [49]. Variable repeats of this element may have a role in C2 expression. Integration of a 5.3 kb ETn retrotransposon in the FasR gene locus resulted in dysruption of this apoptosis pathway in lupus prone MRL/lpr mice [50,51]. Expression of HERV-K18 is increased in juvenile rheumatoid arthritis [52]. A synthetic heptadecapeptide corresponding to the transmembrane domain of the env protein conserved among many exogenous and ERV has been shown to have potent immunosuppressive properties [53,54]. The tat gene of HIV-1 enhances expression of the HRES-1/Rab4 ERV protein which in turn inhibits recycling of CD4 and infection by HIV-1 [55].
Activation of ERV by DNA demethylation
Expression of HERV-E4-1 appears to be increased in patients with SLE due to DNA hypomethylation [56]. Interestingly, higher expression levels in SLE lymphocytes correlate with production of U1 snRNP [57] which includes a 70 kDa protein with a region of homology to gag antigens of infectious and ERV [24,58]. The 1q42 chromosomal region that has been associated with lupus susceptibility [59] and harbors the HRES-1 ERV [27,60] is genetically unstable [61]. 1q42 has been identified as one of the three most common fragile sites in the human genome [62,63]. In general, genomic loci harboring ERV has been associated with increased chromosomal fragility [64]. Fragility at 1q42 has shown an evolutionary conservation in man, gorilla, and chimpanzee [65], similar to the appearance of HRES-1 in old world monkeys [66]. Fragility at 1q42 can be triggered with 5-azacytidine (5AZA), a demethylating agent and relative inducer of endogenous retroviral genes in chicken cells [64]. This mechanism is particularly interesting with regards to induction of T-cell autoreactivity by 5AZA [67] and impaired DNA methylation in T cells of patients with SLE [68].
Molecular mimicry between ERV and infectious virus: Cross-reactivity of HRES-1/p28 with viral peptides and the 70 kDa protein of U1 snRNP in SLE
Immunological cross-reactivity between antigens of infectious viruses and self-proteins have long been documented (Table II). However, proving a causal role of the implicated viruses has proven challenging. EBV shows cross-reactivity to several lupus autoantigens [69,70] and appears to infect lupus patients earlier than control donors [6], yet its nearly ubiquitous presence in the normal adult population makes it difficult to prove a causal role in SLE. Nevertheless, EBV remains an attractive candidate both as an initiator of autoreactivity and stimulator of B-cell survival. Other viruses with strong cross-reactivity to self-antigens, particularly, the human retroviruses with homology to ERV-encoded antigens (Table II), are also difficult to implicate in disease pathogenesis, since these viruses rarely infect patients with SLE. Along this line, relatedness and cross-reactivity to HTLV-I and HIV 1 gag antigens, have been demonstrated [23,66]. However, HIV or HTLV-I provirus was absent in genomic DNA of lupus patients, thus, they could not have initiated immunore-activity to HRES-1/p28 [23]. Comprehensive epitope mapping with fourty-four 15 amino acid long peptides overlapping the entire protein HRES-1/p28 by 10 amino acids with antibodies of 16 HRES-1/p28 Western blot-seropositive SLE patients identified three immunodominant epitopes within residues 41–55, 121–13, and 156–170. Two newly identified immunodominant epitopes in peptides 41–55 and 156–170 showed significant homology to antigens of viruses commonly infecting humans [71]. The immunodominant HRES-1/p28 epitopes and viral peptides were synthesized on the same cellulose membrane and tested in parallel for binding by 16 HRES-1/p28 Western blot-reactive lupus sera. The highest prevalence of cross-reactivity was found with a ORF2a peptide of the newly discovered TT virus (TTV); 14/16 (87.5%) of lupus sera bound to this peptide. Further, antibodies from 11 patients recognized both HRES-1/p28 peptide 41–55 and TTV ORF2a peptide. All HRES-1/p28-reactive sera recognized at least one TTV peptide. In parallel, sera of four HRES-1/p28-seronegative lupus patients and four healthy donors failed to bind to the TTV peptides [71].
TTV is a recently discovered single-stranded circular DNA virus that has not been causally associated with any disease [72]. TTV was originally named after the initials of first patient TT, then transfusion-transmitted, and recently renamed “torque teno” virus [73]. Due to the high degree of genomic variability of the putative coding regions [74] and difficulties in expression of full-length TTV protein for antibody testing [75,76], the diagnosis of TTV infection has been dependent on PCR detection of viral DNA using primers specific for the noncoding regions [77]. TTV DNA was detected in 120/211 SLE patients and 66/199 healthy control donors (p < 0.0001). TTV DNA prevalence was also increased in SLE patients relative to RA patients (23/91; p < 0.0001). The prevalence of TTV DNA was increased in lupus patients (80/121) with respect to their first degree healthy relatives (40/78; p = 0.0184) and the prevalence of TTV DNA was also increased in first degree healthy relatives of lupus patients (40/78) with respect to unrelated healthy donors (66/199; p = 0.0026). Sera of all TTV PCR-positive patients recognized at least one TTV-derived peptide. HRES-1/p28 Western blot reactivity was observed in 12/23 TTV PCR-negative donors and 43/58 TTV PCR-positive lupus donors (p < 0.0281). In addition, TTV cross-reactive lupus sera showed high binding affinity to HRES-1/p28 peptide 121–135 (DRRREGPDRSPRQPP) harboring three consecutive highly charged amino acids (RRE). This RRE triplet is repeated three times in the retroviral gag-like region of 70K U1 snRNP lupus autoantigen and represent crossreactive epitopes between the two proteins [24].
HRES-1/p28 residues 41–55 (PRHRHPQDPRSPGPA) contain epitopes cross-reactive with EBV-LF3 and EBNA-3C antigens [71]. Co-infection of malignant B cells by EBV and TTV has recently been documented in nonHodgkin lymphomas and diffuse large B-cell lymphoma [78]. Expression of HRES-1/p28 is enhanced in EBV-transformed B cells [9]. These observations suggest that cooperativity between EBV and TTV may contribute to autoantigenicity of HRES-1. Thus, co-infection with EBV and TTV and molecular mimicry with immunodominant HRES-1/p28 epitopes may mediate epitope spreading to self-antigens such as the 70 kDa U1snRNP, and thus contribute to formation of antinuclear autoantibodies in SLE.
Immunomodulation by ERV
In addition to providing cross-reactive targets of antiviral immunity, ERV may also have a direct role in regulating immune responses. Changes in production of cytokines similar to those in patients with SLE, a shift from a Th1 to a Th2-type cytokine profile, have been described as a result of HIV-1 infection [79]. A synthetic heptadecapeptide (CKS-17) corresponding to the transmembrane domain of the env protein conserved among many exogenous and ERV has potent immunosuppressive properties [53], possibly via suppression of Th1 type cytokine production [54]. Recently, a full-length env protein of HERV-H was found to suppress anti-tumor immunity in the mouse [80]. The env protein of HERV-W, also called syncytin [81], stimulates expression of the type D mammalian retrovirus receptor in placenta [82]. HERV-W env can function as an envelope protein, form pseudotypes with human immunodeficiency virus type 1 (HIV-1) virions and confer tropism for CD4-negative cells [83]. HERV-W env may also act as a superantigen, causing Vβ16-specific T-cell expansions [84]. In turn, ERV expression may be induced by environmental signals and activation of the immune system. Interleukin 1 (IL-1) induces expression of xenotropic ERV in pancreatic β cells of NOD mice susceptible to IDDM [85]. IL-1 and tumor necrosis factor α (TNFα) stimulate, while IFN-γ inhibits transcription of HERV-R in human vascular endothelial cells [86]. IFN-α induces expression of HERV-K18.1 env, which acting as a superantigen, causes Vβ7- [87] or Vβ13-specific T-cell expansions [88]. Steroids have long been known to induce expression and virion formation from ERV in the mouse [89]. Promoter of HERV-K can be induced by treatment with estradiol and progesteron [90]. Unlike related mouse mammary tumor viruses, HERV-K is not sensitive to stimulation by dexamethasone. Expression of RRHERV-I [91] and HERV-R is enhanced by retinoic acid [92]. HERV-R can also be induced by vitamin D3, IFN-γ, and phorbol esters [92]. Transcription of ERV family members HERV-K, HERV-L, and ERV-9 was increased in ultraviolet B light (UVB)-irradiated skin and skin biopsies of lupus patients [45]. Expression of HRES-1/Rab4 is enhanced by the tat gene of HIV through trans-activation of the HRES-1 LTR [55]. In turn, HRES-1/Rab4 regulates expression of CD4, the cellular receptor of HIV, via endocytic recycling (Figure 1).
HRES-1/Rab4 regulates HIV infection via recycling of CD4
Several ORFs have been identified both in the sense and antisense strands of HRES-1.The 6 kb “sense” transcript encodes a 28 kDa nuclear protein, HRES-1/p28, which is expressed in T-cell lines, placenta, and epithelial cells but not in PBL [23,25,66]. We cloned a novel 2986 base long cDNA (Genbank accession number: AY585832) corresponding to the antisense strand of the HRES-1 locus [55]. This cDNA sequence has considerable homology to the 735 base long Rab4a gene (Genbank accession number: M28211.gb_pr1) and was termed HRES-1/Rab4. The 5′ and 3′ untranslated regions in the HRES-1/Rab4 cDNA were markedly different from those of Rab4a [55].
HRES-1/Rab4 codes for five additional amino acids and two discordant residues, 163 (D → N) and 209 (T → A) [55]. Since, the HRES-1 is a single copy sequence in the haploid genome [60], the previously identified Rab4a may originate from another chromosomal locus or correspond to an alternative translation product of the polymorphic HRES-1/Rab4 genomic locus. HRES-1 was previously mapped to human chromosome 1q42 [60]. All eight coding exons of the HRES-1/Rab4 cDNA were localized within contig NT 031728.1 mapped to the 1q42 genomic locus [55]. Bidirectional transcription has been previously documented at several genomic loci [93,94], including another ERS, HERV-H [95] and the 1q42 locus harboring HRES-1 [96].
The transcription start site of HRES-1/Rab4 was mapped to HRES-1 position 1611 [55]. HRES-1 (nucleotides 2151–1606) exhibited strong promoter activity when oriented in the direction of HRES-1/Rab4 transcription. The HRES-1 LTR enhanced the promoter activity in HeLa cells transfected with HIV-1 tat (HeLa-tat), while it diminished promoter activity in control HeLa cells. Moreover, HRES-1/Rab4 protein levels were elevated in HeLa-tat, Jurkat-tat, and HIV-infected H9 human T cells. Thus, HIV tat can increase expression of HRES-1/Rab4 via trans-activation of HRES-1 LTR [55]. Interestingly, higher expression levels of HRES-1/Rab4 abrogated production of HIV-1 gag p24 as determined by Western blot analysis and reduced the percentage of HIV-1-infected cells by flow cytometry of intracellular gag p24 staining and diminished HIV-induced apoptosis. HRES-1/Rab4S27N had the opposite effects [55].
Rab4a has been shown to regulate recycling of early endosomes carrying the TFR in epithelial cells [97] or GLUT4 in adipocytes [98]. Therefore, we examined whether the impact of HRES-1/Rab4 on HIV infection was mediated via recycling and expression of surface receptors. Expression of CD4 was markedly reduced on the surface of cells over-producing HRES-1/Rab4. By contrast, surface expression of CD4 was enhanced by dominant-negative HRES-1/Rab4S27N. As controls, HIV co-receptor fusin/CXCR4 and CD45RO were not influenced by HRES-1/Rab4 [55]. Coordinate suppression by HRES-1/Rab4 and upregulation by HRES-1/Rab4S27N indicated a specific role for HRES-1/Rab4 in regulation of CD4 expression.
CD4 undergoes protein kinase C (PKC)-mediated endocytosis following T-cell activation [99]. Thus, CD4 internalization was induced by activation of PKC with the phorbol ester PDBu (100 nM) for 1 h at 37°C. Surface expression and recycling of CD4 was profoundly reduced in cells overexpressing HRES-1/Rab4, while baseline expression and recycling of CD4 was markedly enhanced by HRES-1/Rab4S27N [55]. Following PDBu-induced internalization, CD4 co-localized with HRES-1/Rab4 to intracellular blebs [55]. CD4 recycled to the membrane of control and HRES-1/Rab4S27N-expressing cells and displayed a uniform ring pattern. CD4 failed to evenly recycle to the cell membrane and remained confined to discrete foci in cells overproducing HRES-1/Rab4. Interestingly, lysosomal inhibitors chloroquine and NH4Cl normalized CD4 levels diminished by HRES-1/Rab4. The abrogation of CD4 expression by HRES-1/Rab4 resulted from inhibition of endocytic recycling and from targeting of CD4 for lysosomal degradation [55].
Potential impact of HRES-1/Rab4 on immunological synapse formation and T-cell signaling in SLE
The role of receptor recycling and endosomal trafficking in the immune system is largely unknown, although, its likely to be significant based on a few established models. A dominant-negative form of Rab4, Rab4N121I, inhibited antigen-presentation by a B-cell line to a T-cell hybridoma [100]. In T cells, CD3-induced Ca2+ fluxing and proliferation are enhanced in transgenic mice expressing dominant-negative Rab5 [101]. Rab27, which is involved in granule exocytosis [102], is over-expressed 3-fold in effector memory T cells [103]. Patients with Griscelli syndrome and ashen mice exhibit albinism and hemophagocytosis due to macrophage and T-cell dysfunction. They carry inactivating mutations of Rab27 resulting in impaired melanosome transport melanocytes and defective T-cell cytotoxicity due to blocked cytotoxic granule exocytosis [102,104].
Adaptive immune responses by T lymphocytes are mediated by interaction of the TCR with a specific peptide/major histocompatibility antigen complex on the antigen-presenting cell. The outcome of TCR engagement depends on concomitant signaling through the CD4 or CD8 co-receptor and costimulatory molecules (CD8, CD28, CD40L, LFA-1, CD2) and cytokines [105]. Intracellular signal transduction is mediated via protein tyrosine kinases (Lck, Syk) and phosphatases (CD45, SHP-1, phospholipase Cg1) leading to cleavage of phosphatidylinositol diphosphate (PIP2), and Ca2+ mobilization. Then, a secondary cascade of kinases, PKC and protein kinase A activate transcription factors, nuclear factor of activated T cells (NFAT), NFκB, activated protein 1 (AP-1), c-jun N-terminal kinase (JNK), Extracelluar signal-related kinase (ERK) and initiate cell proliferation and cytokine production [106].
Communication between TCR engagement by the peptide MHC complex and the intracellular machinery occurs via the TCR-associated CD3 chains. Each CD3 chain contains immune receptor tyrosine-based activation motifs (ITAMs) one each in γ, δ, and ε and three in ζ. Phosphorylation of ITAM by the Src-family kinase Lck is a critical event for initiating TCR signaling [106,107]. It has become increasingly clear that the macromolecular organization of the TCR, CD4, and signaling molecules with sphingolipid-rich membrane microdomains (lipid rafts) and formation of an IS, which has been also referred to as the central supramolecular activation complex (SMAC), plays an important role in determining functional outcomes of T-cell activation [108]. The central SMAC (cSMAC) harbors the TCR, adaptor proteins, CD4, and PKCθ, surrounded by a peripheral ring (pSMAC) of adhesion factors, such as LFA-1, and a distal ring (dSMAC) containing the tyrosine phoshatases CD148 and CD45 [108]. CD4 is critical for formation of the IS/SMAC. Because, a portion of Lck is constitutively associated with the CD4 co-receptor within GM1 ganglioside-positive lipid rafts [109], the peptide–MHC-induced co-localization of TCR with CD4 results in an increased local concentration of Lck around the TCR [110].
Abnormal T-cell responses in SLE have been associated with alterations in composition and dynamics of the IS [111–114]. The TCRζ chain and Lck levels are reduced [111] and replaced by the Fcε receptor type I γ chain and Syk in lipid rafts which may contribute to enhanced CD3-initiated Ca fluxing [112]. Interestingly, the TCRζ chain binds to the TFR [115]. While the plasma membrane-associated Lck is bound to CD4, an intracellular pool of Lck is associated with TFR-positive recycling endosomes [116,117]. Based on its homology to Rab4a, HRES-1/Rab4 is likely to be activated by p85-PI3K [118] and its GTP-bound active form associates with the CD2 adaptor protein [119] and the ubiquitin ligase Cbl [120] which are key components of the IS [108,121]. The existing data on direct binding of Rab4 to p85-PI3K and CD2 adaptor protein and regulation of CD4 and TFR recycling strongly suggest that HRES-1/Rab4 is involved in abnormal assembly and signaling through the IS. Polymorphic HRES-1 alleles have been associated with SLE [27,122] and HRES-1/Rab4 appears to be a prime candidate gene for conferring disease susceptibility at 1q42 [59,60,123–126].
Conclusions
ERV represent a key molecular link between the environmental and genetic factors that govern the development of SLE. HRES-1 is a transcriptionally active HERV that encodes a 28 kDa nuclear autoantigen, HRES-1/p28 and a small GTP-ase, HRES-1/Rab4. Autoantibodies to HRES-1/p28, which are found in half of the patients with SLE [23–27], are cross-reactive with antigens of viruses infecting patients with SLE, such as TTV and EBV [71] and the 70 kDa component of U1 snRNP [24] and may thus contribute to epitope spreading and development of anti-nuclear antibody (ANA). In turn, HRES-1/Rab4 regulates the recycling of CD4, a key component of the IS, which is abnormally assembled in T cells of patients with SLE. HRES-1 is centrally located relative to lupus-linked microsatellite markers on chromosome 1 at q42. Molecular mimicry and immunomodulation by HRES-1-encoded proteins support a role for this ERV in the pathogenesis of SLE.
Acknowledgements
This work was supported by grant RO1 AI 48079 from the National Institutes of Health and the Central New York Community Foundation.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- [1].Steinberg AD, Gourley MF, Klinman DM, Tsokos GC, Scott DE, Krieg AM. Systemic lupus erythematosus. Ann Intern Med. 1991;115:548–559. doi: 10.7326/0003-4819-115-7-548. [DOI] [PubMed] [Google Scholar]
- [2].Hochberg MC. Systemic lupus erythematosus. Rheum Dis Clin North Am. 1990;16:617–639. [PubMed] [Google Scholar]
- [3].Arnett FC, Reveille JD. Genetics of systemic lupus erythematosus. Rheum Dis Clin North Am. 1992;18:865–892. [PubMed] [Google Scholar]
- [4].Block SR, Winfield JB, Lochstein MC, D’Angelo WA, Christian CL. Studies of twins with systemic lupus erythematosus: A review of the literature and presentation of 12 additional sets. Am J Med. 1975;59:533–552. doi: 10.1016/0002-9343(75)90261-2. [DOI] [PubMed] [Google Scholar]
- [5].Rich SA. Human lupus inclusions and interferon. Science. 1981;213:772–775. doi: 10.1126/science.6166984. [DOI] [PubMed] [Google Scholar]
- [6].James JJ, Kaufman KM, Farris AD, Taylor-Albert E, Lehman TJA, Harley JB. An increased prevalence of Epstein–Barr virus infection in young patientts suggests a possible etiology for systemic lupus erythematosus. J Clin Invest. 1997;100:3019–3026. doi: 10.1172/JCI119856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sabbatini A, Bombardieri S, Migliorini P. Autoantibodies form patients with systemic lupus erythematosus bind a shared sequence of SmD and Epstein–Barr virus-encoded nuclear antigen EBNA I. Eur J Immunol. 1993;23:1146–1152. doi: 10.1002/eji.1830230525. [DOI] [PubMed] [Google Scholar]
- [8].Mellors RC, Mellors JW. Type C RNA virus-specific antibody in human SLE demonstrated by enzymoimmunoassay. Proc Natl Acad Sci USA. 1978;75:2463–2467. doi: 10.1073/pnas.75.5.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Perl A. Role of endogenous retroviruses in autoimmune diseases. [Review, 177 Refs] Rheum Dis Clin North Am. 2003;29(1):123–143. doi: 10.1016/s0889-857x(02)00098-4. [DOI] [PubMed] [Google Scholar]
- [10].Meyaard L, Otto SA, Jonker RR, Mijnster MJ, Keet RPM, Miedema F. Programmed death of T cells in HIV infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- [11].Emlen W, Niebur JA, Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1994;152:3685–3692. [PubMed] [Google Scholar]
- [12].Gergely PJ, Grossman C, Niland B, Puskas F, Neupane H, Allam F, et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Perl A, Gergely P, Jr., Nagy G, Koncz A, Banki K. Mitochondrial hyperpolarization: A checkpoint of T cell life, death, and autoimmunity. Trends Immunol. 2004;25:360–367. doi: 10.1016/j.it.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McGuinnis MH, Macher AH, Rook AH. Red cell autoantibodies in patients with AIDS. Transfusion. 1986;26:405–409. doi: 10.1046/j.1537-2995.1986.26587020112.x. [DOI] [PubMed] [Google Scholar]
- [15].Geissler RG, Rossol R, Mentzel U, Ottmann OG, Klein AS, Gute P, et al. Gamma delta-T cell-receptor positive lymphocytes inhibit human hematopoietic progenitor cell growth in HIV type I-infected patients. AIDS Res Hum Retrovirus. 1996;12:577–584. doi: 10.1089/aid.1996.12.577. [DOI] [PubMed] [Google Scholar]
- [16].Karpatkin S. HIV-1-related thrombocytopenia. Hematol Oncol Clin North Am. 1990;4:193–218. [PubMed] [Google Scholar]
- [17].Dalakas MC, Pezeshkpour GH, Gravell M, Sever JL. Polymyositis associated with AIDS retrovirus. JAMA. 1986;256:2381–2383. [PubMed] [Google Scholar]
- [18].Calabrese L. The rheumatic manifestations of infection with the HIV. Semin Arthritis Rheum. 1989;18:225–239. doi: 10.1016/0049-0172(89)90043-7. [DOI] [PubMed] [Google Scholar]
- [19].Hicks JT, Aulakh GS, McGrath PP, Washington GC, Kim E, Alepa FP. Search for Epstein–Barr and type C oncorna-viruses in systemic lupus erythematosus. Arthritis Rheum. 1979;22:845–857. doi: 10.1002/art.1780220807. [DOI] [PubMed] [Google Scholar]
- [20].Yoshiki T, Mellors RC, Strand M, August JT. The viral envelope glycoprotein of murine leukemia virus and the pathoegenesis of immune complex glomerulonephritis of New Zealand mice. J Exp Med. 1974;140:1011–1025. doi: 10.1084/jem.140.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Krieg AM, Steinberg AD. Analysis of thymic endogenous retroviral expression in murine lupus. J Clin Invest. 1990;86:809–816. doi: 10.1172/JCI114778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Krieg AM, Gourley MF, Perl A. Endogenous retroviruses: Potential etiologic agents in autoimmunity. [Review] FASEB J. 1992;6:2537–2544. doi: 10.1096/fasebj.6.8.1592206. [DOI] [PubMed] [Google Scholar]
- [23].Banki K, Maceda J, Hurley E, Ablonczy E, Mattson DH, Szegedy L, et al. Human T-cell lymphotropic virus (HTLV)-related endogenous sequence, HRES-1, encodes a 28-kDa protein: A possible autoantigen for HTLV-I gag-reactive autoantibodies. Proc Natl Acad Sci USA. 1992;89:1939–1943. doi: 10.1073/pnas.89.5.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Perl A, Colombo E, Dai H, Agarwal RK, Mark KA, Banki K, et al. Antibody reactivity to the HRES-1 endogenous retroviral element identifies a subset of patients with systemic lupus erythematosus and overlap syndromes: Correlation with antinuclear antibodies and HLA class II alleles. Arthritis Rheum. 1995;38:1660–1671. doi: 10.1002/art.1780381119. [DOI] [PubMed] [Google Scholar]
- [25].Brookes SM, Pandolfino YA, Mitchell TJ, Venables TJW, Shattles WG, Clark DA, et al. The immune response to and expression of cross-reactive retroviral gag sequences in autoimmune disease. Br J Rheumatol. 1992;31:735–742. doi: 10.1093/rheumatology/31.11.735. [DOI] [PubMed] [Google Scholar]
- [26].Bengtsson A, Blomberg J, Nived O, Pipkorn R, Toth L, Sturfelt G. Selective antibody reactivity with peptides from human endogenous retroviruses and nonviral poly(amino acids) in patients with systemic lupus erythematosus. Arthritis Rheum. 1996;39:1654–1663. doi: 10.1002/art.1780391007. [DOI] [PubMed] [Google Scholar]
- [27].Magistrelli C, Samoilova E, Agarwal RK, Banki K, Ferrante P, Vladutiu A, et al. Polymorphic genotypes of the HRES-1 human endogenous retrovirus locus correlate with systemic lupus erythematosus and autoreactivity. Immunogenetics. 1999;49:829–834. doi: 10.1007/s002510050561. [DOI] [PubMed] [Google Scholar]
- [28].Kazazian HH., Jr. L1 retrotransposons shape the mammalian genome. Science. 2000;289:1152–1153. doi: 10.1126/science.289.5482.1152. [DOI] [PubMed] [Google Scholar]
- [29].Perl A, Colombo E, Samoilova E, Butler MC, Banki K. Human transaldolase-associated repetitive elements are transcribed by RNA polymerase III. J Biol Chem. 2000;275:7261–7272. doi: 10.1074/jbc.275.10.7261. [DOI] [PubMed] [Google Scholar]
- [30].Mathias SL, Scott AF, Kazazian HH, Jr., Boeke JD, Gabriel A. Reverse transcriptase encoded by a human transposable element. Science. 1991;254:1808–1810. doi: 10.1126/science.1722352. [DOI] [PubMed] [Google Scholar]
- [31].Banki K, Eddy RL, Shows TB, Halladay DL, Bullrich F, Croce CM, et al. The human transaldolase gene (TALDO1) is located on chromosome 11 at p15.4–p15.5. Genomics. 1997;45:233–238. doi: 10.1006/geno.1997.4932. [DOI] [PubMed] [Google Scholar]
- [32].Coffin JM, Hughes SH, Varmus HE. Retrotransposons, endogenous retroviruses, and the evolution of retroelements. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1997. pp. 343–435. [PubMed] [Google Scholar]
- [33].Wilkinson DA, Mager DL, Leong J-AC. Endogenous human retroviruses. In: Levy JA, editor. The retroviridae. Plenum Press; New York: 1994. pp. 465–535. [Google Scholar]
- [34].Lusso P, Di Marzo Veronese F, Ensoli B, Franchini G, Jemma C, DeRocco SE, et al. Expanded HIV-1 cellular tropism by phenotypic mixing with murine endogenous retroviruses. Science. 1990;247:848–852. doi: 10.1126/science.2305256. [DOI] [PubMed] [Google Scholar]
- [35].Mueller-Lantzsch N, Sauter M, Weiskircher A, Kramer K, Best B, Buck M, et al. Human endogenous retroviral element K10 (HERV-K10) encodes a full-length gag homologous 73-kDa protein and a functional protease. AIDS Res Hum Retroviruses. 1993;9(4):343–350. doi: 10.1089/aid.1993.9.343. [DOI] [PubMed] [Google Scholar]
- [36].Hashimoto W, Osaki T, Okamura H, Robbins PD, Kurimoto M, Nagata S, et al. Differential antitumor effects of administration of recombinant IL-18 or recombinant IL-12 are mediated primarily by Fas–Fas ligand- and perforin-induced tumor apoptosis, respectively. J Immunol. 1999;163:583–589. [PubMed] [Google Scholar]
- [37].Kitamura Y, Ayukawa T, Ishikawa T, Kanda T, Yoshiike K. Human endogenous retrovirus K10 encodes a functional integrase. J Virol. 1996;70(5):3302–3306. doi: 10.1128/jvi.70.5.3302-3306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lower R, Tonjes RR, Korbmacher C, Kurth R, Lower J. Identification of a Rev-related protein by analysis of spliced transcripts of the human endogenous retroviruses HTDV/HERV-K. J Virol. 1995;69(1):141–149. doi: 10.1128/jvi.69.1.141-149.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yang J, Bogerd HP, Peng S, Wiegand H, Truant R, Cullen BR. An ancient family of human endogenous retroviruses encodes a functional homolog of the HIV-1 Rev protein. Proc Natl Acad Sci USA. 1999;96(23):13404–13408. doi: 10.1073/pnas.96.23.13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lower R, Boller K, Hasenmaier B, Korbmacher C, Muller-Lantzsch N, Lower J, et al. Identification of human endogenous retroviruses with complex mRNA expression and particle formation. Proc Natl Acad Sci USA. 1993;90(10):4480–4484. doi: 10.1073/pnas.90.10.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Medstrand P, Lindeskog M, Blomberg J. Expression of human endogenous retroviral sequences in peripheral blood mononuclear cells of healthy individuals. J Gen Virol. 1992;73:2463–2466. doi: 10.1099/0022-1317-73-9-2463. [DOI] [PubMed] [Google Scholar]
- [42].Krieg AM, Gourley MF, Klinman DM, Perl A, Steinberg AD. Heterogeneous expression and coordinate regulation of endogenous retroviral sequences in human peripheral blood mononuclear cells. AIDS Res Hum Retrovirus. 1992;8:1991–1998. doi: 10.1089/aid.1992.8.1991. [DOI] [PubMed] [Google Scholar]
- [43].Lindeskog M, Medstrand P, Cunningham AA, Blomberg J. Coamplification and dispersion of adjacent human endogenous retroviral HERV-H and HERV-E elements; presence of spliced hybrid transcripts in normal leukocytes. Virology. 1998;244:219–229. doi: 10.1006/viro.1998.9106. [DOI] [PubMed] [Google Scholar]
- [44].Yin H, Medstrand P, Andersson ML, Borg A, Olsson H, Blomberg J. Transcription of human endogenous retroviral sequences related to mouse mammary tumor virus in human breast and placenta: Similar pattern in most malignant and nonmalignant breast tissues. AIDS Res Hum Retroviruses. 1997;13(6):507–516. doi: 10.1089/aid.1997.13.507. [DOI] [PubMed] [Google Scholar]
- [45].Hohenadl C, Germaier H, Walchner M, Hagenhofer M, Herrmann M, Sturzl M, et al. Transcriptional activation of endogenous retroviral sequences in human epidermal keratinocytes by UVB irradiation. J Invest Dermatol. 1999;113:587–594. doi: 10.1046/j.1523-1747.1999.00728.x. [DOI] [PubMed] [Google Scholar]
- [46].Oldstone MBA. Molecular mimicry and autoimmune disease. Cell. 1987;50:819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- [47].Perl A. Mechanisms of viral pathogenesis in rheumatic diseases (Invited Review) Ann Rheum Dis. 1999;58:454–461. doi: 10.1136/ard.58.8.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Perl A, Banki K. Human endogenous retroviral elements and autoimmunity: Data and concepts. Trends Microbiol. 1993;1:153–156. doi: 10.1016/0966-842x(93)90131-a. [DOI] [PubMed] [Google Scholar]
- [49].Zhu ZB, Hsieh S-L, Bentley DR, Campbell D, Volanakis JE. A variable number of tandem repeat locus within the human complement C2 gene is associated with a retroposon derived from a human endogenous retrovirus. J Exp Med. 1992;175:1783–1787. doi: 10.1084/jem.175.6.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Watanabe-Fukunaga R, Brannan CL, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- [51].Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- [52].Sicat J, Sutkowski N, Huber BT. Expression of human endogenous retrovirus HERV-K18 superantigen is elevated in juvenile rheumatoid arthritis. J Rheumatol. 2005;32:1821–1831. [PubMed] [Google Scholar]
- [53].Cianciolo GJ, Copeland TD, Oroszlan S, Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985;230:453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- [54].Haraguchi S, Good RA, Day NK. Immunosuppressive retroviral peptides: cAMP and cytokine patterns. Immunol Today. 1995;16:595–603. doi: 10.1016/0167-5699(95)80083-2. [DOI] [PubMed] [Google Scholar]
- [55].Nagy G, Ward J, Mosser DD, Koncz A, Gergely P, Stancato C, et al. Regulation of CD4 expression via recycling by HRES-1/RAB4 controls susceptibility to HIV infection. J Biol Chem. 2006;281:34574–34591. doi: 10.1074/jbc.M606301200. [DOI] [PubMed] [Google Scholar]
- [56].Ogasawara H, Naito K, Kaneko H, Hishikawa T, Sekigawa I, Hashimoto H, et al. Quantitative analysis of messenger RNA of human endogenous retrovirus in systemic lupus erythematosus. J Rheumatol. 2001;29:1678–1682. [PubMed] [Google Scholar]
- [57].Piotrowski P, Duriagin S, Jagodzinski P. Expression of human endogenous retrovirus clone 4-1 may correlate with blood plasma concentration of anti-U1 RNP and anti-Sm nuclear antibodies. Clin Rheumatol. 2005;24(6):620–624. doi: 10.1007/s10067-005-1123-8. [DOI] [PubMed] [Google Scholar]
- [58].Query CC, Keene JD. A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell. 1987;51:211–220. doi: 10.1016/0092-8674(87)90148-6. [DOI] [PubMed] [Google Scholar]
- [59].Tsao BP. Lupus susceptibility genes on human chromosome 1. Int Rev Immunol. 2000;19:319–334. doi: 10.3109/08830180009055502. [DOI] [PubMed] [Google Scholar]
- [60].Perl A, Isaacs CM, Eddy RL, Byers MG, Sait SN, Shows TB. The human T-cell leukemia virus-related endogenous sequence (HRES1) is located on chromosome 1 at q42. Genomics. 1991;11:1172–1173. doi: 10.1016/0888-7543(91)90052-g. [DOI] [PubMed] [Google Scholar]
- [61].Murty VVVS, Li R-G, Mathew S, Reuter VE, Bronson DL, Bosl GJ, et al. Replication error-type genetic instability at 1q42-43 in human male germ cell tumors. Cancer Res. 1994;54:3983–3985. [PubMed] [Google Scholar]
- [62].Rocchi A, Pelliccia F. Synergistic effect of DAPI and thymidylate stress conditions on the induction of common fragile sites. Cytogenet Cell Genet. 1988;48(1):51–54. doi: 10.1159/000132585. [DOI] [PubMed] [Google Scholar]
- [63].Pelliccia F, Rocchi A. DAPI-inducible common fragile sites. Cytogenet Cell Genet. 1986;42(3):174–176. doi: 10.1159/000132272. [DOI] [PubMed] [Google Scholar]
- [64].Groudine M, Eisenman R, Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1985;292:311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- [65].Schmid M, Ott G, Haaf T, Scheres JM. Evolutionary conservation of fragile sites induced by 5-azacytidine and 5-azadeoxycytidine in man, gorilla, and chimpanzee. Hum Genet. 1985;71(4):342–350. doi: 10.1007/BF00388461. [DOI] [PubMed] [Google Scholar]
- [66].Perl A, Rosenblatt JD, Chen IS, DiVincenzo JP, Bever R, Poiesz BJ, et al. Detection and cloning of new HTLV-related endogenous sequences in man. Nucleic Acids Res. 1989;17:6841–6854. doi: 10.1093/nar/17.17.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Richardson BC, Strahler JR, Pivirotto TS, Quddus J, Bayliss GE, Gross LA, et al. Phenotypic and functional similarities between 5-azacytidine-treated T cells and a T-cell subset in patients with active systemic lupus erythematosus. Arthritis Rheum. 1992;35:647–662. doi: 10.1002/art.1780350608. [DOI] [PubMed] [Google Scholar]
- [68].Richardson B, Scheinbart L, Strahler J, Gross L, Hanash S, Johnson M. Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 1990;33:1665–1673. doi: 10.1002/art.1780331109. [DOI] [PubMed] [Google Scholar]
- [69].Poole BD, Scofield RH, Harley JB, James JA. Epstein–Barr virus and molecular mimicry in systemic lupus erythematosus. Autoimmunity. 2006;39(1):63–70. doi: 10.1080/08916930500484849. [DOI] [PubMed] [Google Scholar]
- [70].Harley JB, Harley IT, Guthridge JM, James JA. The curiously suspicious: A role for Epstein–Barr virus in lupus. [Review, 63 Refs] Lupus. 2006;15(11):768–777. doi: 10.1177/0961203306070009. [DOI] [PubMed] [Google Scholar]
- [71].Gergely P, Jr., Pullmann R, Jr., Stancato C, Otvos L, Koncz A, Blazsek A, et al. Increased prevalence of transfusion-transmitted virus and cross-reactivity with immunodominant epitopes of the HRES-1/p28 endogenous retroviral autoantigen in patients with systemic lupus erythematosus. Clin Immunol. 2005;116:124–134. doi: 10.1016/j.clim.2005.04.002. [DOI] [PubMed] [Google Scholar]
- [72].Nishizawa T, Okamoto H, Konishi K, Yoshizawa H, Miyakawa Y, Mayumi M. A novel DNA virus (TTV) associated with elevated transaminase levels in posttransfusion hepatitis of unknown etiology. Biochem Biophys Res Commun. 1997;241:92–97. doi: 10.1006/bbrc.1997.7765. [DOI] [PubMed] [Google Scholar]
- [73].Hino S, Miyata H. Torque teno virus (TTV): Current status. Rev Med Virol. 2007;17:45–57. doi: 10.1002/rmv.524. [DOI] [PubMed] [Google Scholar]
- [74].Bendinelli M, Pistello M, Maggi F, Fornai C, Freer G, Vatteroni ML. Molecular properties, biology, and clinical implications of TT virus, a recently identified widespread infectious agent of humans. [Review, 191 Refs] Clin Microbiol Rev. 2001;14:98–113. doi: 10.1128/CMR.14.1.98-113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kakkola L, Hedman K, Vanrobaeys H, Hedman L, Soderlund-Venermo M. Cloning and sequencing of TT virus genotype 6 and expression of antigenic open reading frame 2 proteins. J Gen Virol. 2002;83:979–990. doi: 10.1099/0022-1317-83-5-979. [DOI] [PubMed] [Google Scholar]
- [76].Okamoto H, Takahashi M, Nishizawa T, Tawara A, Sugai Y, Sai T, et al. Replicative forms of TT virus DNA in bone marrow cells. Biochem Biophys Res Commun. 2000;270:657–662. doi: 10.1006/bbrc.2000.2481. [DOI] [PubMed] [Google Scholar]
- [77].Abrams MT, Robertson NM, Yoon K, Wickstrom E. Inhibition of glucocorticoid-induced apoptosis by targeting the major splice variants of BIM mRNA with small interfering RNA and short hairpin RNA. J Biol Chem. 2004;279(53):55809–55817. doi: 10.1074/jbc.M411767200. [DOI] [PubMed] [Google Scholar]
- [78].Garbuglia AR, Iezzi T, Capobianchi MR, Pignoloni P, Pulsoni A, Sourdis J, et al. Detection of TT virus in lymph node biopsies of B-cell lymphoma and Hodgkin’s disease, and its association with EBV infection. Int J Immunopathol Pharmacol. 2003;16:109–118. doi: 10.1177/039463200301600204. [DOI] [PubMed] [Google Scholar]
- [79].Clerici M, Shearer GM. The Th1-Th2 hypothesis of HIV infection: New insights. Immunol Today. 1994;15:575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- [80].Mangeney M, de Parseval N, Thomas G, Heidmann T. The full-length envelope of an HERV-H human endogenous retrovirus has immunosuppressive properties. J Gen Virol. 2001;82(Pt 10):2515–2518. doi: 10.1099/0022-1317-82-10-2515. [DOI] [PubMed] [Google Scholar]
- [81].Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. [see comments] Nature. 2000;403(6771):785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- [82].Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, et al. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74(7):3321–3329. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].An DS, Xie Y, Chen IS. Envelope gene of the human endogenous retrovirus HERV-W encodes a functional retrovirus envelope. J Virol. 2001;75(7):3488–3489. doi: 10.1128/JVI.75.7.3488-3489.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Perron H, Jouvin-Marche E, Michel M, Ounanian-Paraz A, Camelo S, Dumon A, et al. Multiple sclerosis retrovirus particles and recombinant envelope trigger an abnormal immune response in vitro, by inducing polyclonal Vbeta16 T-lymphocyte activation. Virology. 2001;287(2):321–332. doi: 10.1006/viro.2001.1045. [DOI] [PubMed] [Google Scholar]
- [85].Tsumara H, Wang JZ, Ogawa S, Ohota H, Komada H, Ito Y, et al. IL-1 induces intracisternal type A virus and retrovirus type C in pancreatic beta-cells of NOD mice. J Exp Anim Sci. 1994;36(4–5):141–150. [PubMed] [Google Scholar]
- [86].Katsumata K, Ikeda H, Sato M, Ishizu A, Kawarada Y, Kato H, et al. Cytokine regulation of env gene expression of human endogenous retrovirus-R in human vascular endothelial cells. Clin Immunol. 1999;93(1):75–80. doi: 10.1006/clim.1999.4762. [DOI] [PubMed] [Google Scholar]
- [87].Stauffer Y, Marguerat S, Meylan F, Ucla C, Sutkowski N, Huber B, et al. Interferon-alpha-induced endogenous super-antigen. A model linking environment and autoimmunity. [see comments] Immunity. 2001;15(4):591–601. doi: 10.1016/s1074-7613(01)00212-6. [DOI] [PubMed] [Google Scholar]
- [88].Sutkowski N, Conrad B, Thorley-Lawson DA, Huber BT. Epstein–Barr virus transactivates the human endogenous retrovirus HERV-K18 that encodes a superantigen. [see comments] Immunity. 2001;15(4):579–589. doi: 10.1016/s1074-7613(01)00210-2. [DOI] [PubMed] [Google Scholar]
- [89].Dunn CY, Aaronson SA, Stephenson JR. Interactions of chemical inducers and steroid enhancers of endogenous mouse type-C RNA viruses. Virology. 1975;66:579–588. doi: 10.1016/0042-6822(75)90230-5. [DOI] [PubMed] [Google Scholar]
- [90].Ono M, Kawakami M, Ushikubo H. Stimulation of expression of the human endogenous retrovirus genome by female steroid hormones in human breast cancer cell line T47D. J Virol. 1987;61(6):2059–2062. doi: 10.1128/jvi.61.6.2059-2062.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kannan P, Buettner R, Pratt DR, Tainsky MA. Identification of a retinoic acid-inducible endogenous retroviral transcript in the human teratocarcinoma-derived cell line PA-1. J Virol. 1991;65(11):6343–6348. doi: 10.1128/jvi.65.11.6343-6348.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Larsson E, Venables PJ, Andersson AC, Fan W, Rigby S, Botling J, et al. Expression of the endogenous retrovirus ERV3 (HERV-R) during induced monocytic differentiation in the U-937 cell line. Int J Cancer. 1996;67(3):451–456. doi: 10.1002/(SICI)1097-0215(19960729)67:3<451::AID-IJC23>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- [93].Hastings ML, Milcarek C, Martincic K, Peterson ML, Munroe SH. Expression of the thyroid hormone receptor gene, erbAa, in B lymphocytes: Alternative mRNA processing is independent of differentiation but correlates with antisense RNA levels. Nucleic Acids Res. 1997;25:4296–4300. doi: 10.1093/nar/25.21.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Li AW, Too CKL, Knee R, Wilkinson M, Murphy PR. FGF-2 antisense RNA encodes a nuclear protein with MuT-like antimutator activity. Mol Cell Endocrinol. 1997;133:177–182. doi: 10.1016/s0303-7207(97)00148-2. [DOI] [PubMed] [Google Scholar]
- [95].Baban S, Freeman JD, Mager DL. Transcripts from a novel human KRAB zinc finger gene contain spliced Alu and endogenous retroviral segments. Genomics. 1996;33(3):463–472. doi: 10.1006/geno.1996.0221. [DOI] [PubMed] [Google Scholar]
- [96].Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. [see comment, erratum appears in Science 2001 Jun 5;292(5523): 1838] Science. 2001;291(5507):1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- [97].van der SP, Hull M, Webster P, Male P, Goud B, Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992;(70):729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- [98].Meusser B, Sommer T. Vpu-mediated degradation of CD4 reconstituted in yeast reveals mechanistic differences to cellular ER-associated protein degradation. Mol Cell. 2004;14(2):247–258. doi: 10.1016/s1097-2765(04)00212-6. [DOI] [PubMed] [Google Scholar]
- [99].Hoxie JA, Matthews DM, Callahan KJ, Cassel DL, Cooper RA. Transient modulation and internalization of T4 antigen induced by phorbol esters. J Immunol. 1986;137:1194–1201. [PubMed] [Google Scholar]
- [100].Lazzarino DA, Blier P, Mellman I. The monomeric guanosine triphosphatase rab4 controls an essential step on the pathway of receptor-mediated antigen processing in B cells. J Exp Med. 1998;188:1769–1774. doi: 10.1084/jem.188.10.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Andre P, Boretto J, Hueber AO, Regnier-Vigouroux A, Gorvel JP, Ferrier P, et al. A dominant-negative mutant of the Rab5 GTPase enhances T cell signaling by interfering with TCR down-modulation in transgenic mice. J Immunol. 1997;159(11):5253–5263. [PubMed] [Google Scholar]
- [102].Haddad EK, Wu X, Hammer JA, III, Henkart PA. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. [see comment] J Cell Biol. 2001;152:835–842. doi: 10.1083/jcb.152.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Riou C, Yassine-Diab B, Van grevenynghe J, Somogyi R, Greller LD, Gagnon D, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2006;204:79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. [Review, 65 Refs] Trends Mol Med. 2002;8(1):23–30. doi: 10.1016/s1471-4914(01)02227-4. [DOI] [PubMed] [Google Scholar]
- [105].Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, et al. Mature T lymphocyte apoptosis–immune regulation in a dynamic and unpredictable antigenic environment. [Review, 347 Refs] Ann Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- [106].Huang Y, Wange RL. T cell receptor signaling: Beyond complex complexes. J Biol Chem. 2004;279(28):28827–28830. doi: 10.1074/jbc.R400012200. [DOI] [PubMed] [Google Scholar]
- [107].Koretzky GA, Boerth NJ. The role of adapter proteins in T cell activation. [Review, 137 Refs] Cell Mol Life Sci. 1999;56:1048–1060. doi: 10.1007/s000180050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Lin J, Miller MJ, Shaw AS. The c-SMAC: Sorting it all out (or in) J Cell Biol. 2005;170(2):177–182. doi: 10.1083/jcb.200503032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Thomas S, Kumar R, Preda-Pais A, Casares S, Brumeanu TD. A model for antigen-specific T-Cell anergy: Displacement of CD4-p56lck Signalosome from the lipid rafts by a soluble, dimeric peptide-MHC class II chimera. J Immunol. 2003;170(12):5981–5992. doi: 10.4049/jimmunol.170.12.5981. [DOI] [PubMed] [Google Scholar]
- [110].Holdorf AD, Lee KH, Burack WR, Allen PM, Shaw AS. Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nat Immunol. 2002;3(3):259–264. doi: 10.1038/ni761. [DOI] [PubMed] [Google Scholar]
- [111].Enyedy EJ, Nambiar MP, Liossis SN, Dennis G, Kammer GM, Tsokos GC. Fc epsilon receptor type I gamma chain replaces the deficient T cell receptor zeta chain in T cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44(5):1114–1121. doi: 10.1002/1529-0131(200105)44:5<1114::AID-ANR192>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- [112].Krishnan S, Nambiar MP, Warke VG, Fisher CU, Mitchell J, Delaney N, et al. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J Immunol. 2004;172:7821–7831. doi: 10.4049/jimmunol.172.12.7821. [DOI] [PubMed] [Google Scholar]
- [113].Jury EC, Kabouridis PS, Abba A, Mageed RA, Isenberg DA. Increased ubiquitination and reduced expression of LCK in T lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48(5):1343–1354. doi: 10.1002/art.10978. [DOI] [PubMed] [Google Scholar]
- [114].Jury EC, Kabouridis PS, Flores-Borja F, Mageed RA, Isenberg DA. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 2004;113:1176–1187. doi: 10.1172/JCI20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Salmeron A, Borroto A, Fresno M, Crumpton MJ, Ley SC, Alarcon B. Transferrin receptor induces tyrosine phosphorylation in T cells and is physically associated with the TCR zeta-chain. J Immunol. 1995;154(4):1675–1683. [PubMed] [Google Scholar]
- [116].Ehrlich LIR, Ebert PJR, Krummel MF, Weiss A, Davis MM. Dynamics of p56lck translocation to the T Cell immuno-logical synapse following agonist and antagonist stimulation. Immunity. 2002;17(6):809–822. doi: 10.1016/s1074-7613(02)00481-8. [DOI] [PubMed] [Google Scholar]
- [117].Luton F, Legendre V, Gorvel JP, Schmitt-Verhulst AM, Boyer C. Tyrosine and serine protein kinase activities associated with ligand-induced internalized TCR/CD3 complexes. J Immunol. 1997;158(7):3140–3147. [PubMed] [Google Scholar]
- [118].Chamberlain MD, Berry TR, Pastor MC, Anderson DH. The p85α subunit of phosphatidylinositol 3′-kinase binds to and stimulates the GTPase activity of Rab proteins. J Biol Chem. 2004;279(47):48607–48614. doi: 10.1074/jbc.M409769200. [DOI] [PubMed] [Google Scholar]
- [119].Cormont M, Meton I, Mari M, Monzo P, Keslair F, Gaskin C, et al. CD2AP/CMS regulates endosome morphology and traffic to the degradative pathway through its interaction with Rab4 and c-Cbl. Traffic. 2003;4(2):97–112. doi: 10.1034/j.1600-0854.2003.40205.x. [DOI] [PubMed] [Google Scholar]
- [120].Davanture S, Leignadier J, Milani P, Soubeyran P, Malissen B, Malissen M, et al. Selective defect in antigen-induced TCR internalization at the immune synapse of CD8 T cells bearing the ZAP-70(Y292F) mutation. J Immunol. 2005;175(5):3140–3149. doi: 10.4049/jimmunol.175.5.3140. [DOI] [PubMed] [Google Scholar]
- [121].Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302(5648):1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- [122].Magistrelli C, Banki K, Ferrante P, Perl A. Mapping and cloning of polymorphic genotypes of the HRES-1 LTR. Arthritis Rheum. 1994;37:S316. [Google Scholar]
- [123].Tsao BP, Cantor RM, Kalunian KC, Chen C-J, Badsha H, Singh R, et al. Evidence for linkage of a candidate chromosome 1 region to human systemic lupus erythematosus. J Clin Invest. 1997;99:725–731. doi: 10.1172/JCI119217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gaffney PM, Kearns GM, Shark KB, Ortmann WA, Selby SA, Malmgren ML, et al. A genome-wide search for susceptibility genes in human systemic lupus erythematosus sib-pair families. Proc Natl Acad Sci USA. 1998;95:14875–14879. doi: 10.1073/pnas.95.25.14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Moser KL, Neas BR, Salmon JE, Yu H, Gray-McGuire C, Asundi N, et al. Genome scan of human systemic lupus erythematosus: Evidence for linkage on chromosome 1q in African–American pedigrees. Proc Natl Acad Sci USA. 1998;95:14869–14874. doi: 10.1073/pnas.95.25.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Tsao BP, Cantor RM, Grossman JM, Shen N, Teophilov NT, Wallace DJ, et al. PARP alleles with the linked chromosomal region are associated with systemic lupus erythematosus. J Clin Invest. 1999;103:1135–1140. doi: 10.1172/JCI5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Repaske R, Steele PE, O’Neill RR, Rabson AB, Martin MA. Nucleotide sequence of a full-length human endogenous retroviral segment. J Virol. 1985;54:764–772. doi: 10.1128/jvi.54.3.764-772.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Kjellman C, Sjogren HO, Salford LG, Widegren B. HERV-F (XA34) is a full-length human endogenous retrovirus expressed in placental and fetal tissues. Gene. 1999;239(1):99–107. doi: 10.1016/s0378-1119(99)00372-8. [DOI] [PubMed] [Google Scholar]
- [129].Mager DL, Freeman JD. Human endogenous retrovirus like genome with type C pol sequences and gag sequences related to human T-cell lymphotropic viruses. J Virol. 1987;61(12):4060–4066. doi: 10.1128/jvi.61.12.4060-4066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Tristem M. Identification and characterization of novel human endogenous retrovirus families by phyligenetic screening of human genome mappng project database. J Virol. 2000;74:3715–3730. doi: 10.1128/jvi.74.8.3715-3730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Weber GF, Cantor H. Phosphatidylinositol synthesis is a proximal event in intracellular signaling coupled to T cell receptor ligation. Differential induction by conventional antigen and retroviral superantigen. J Immunol. 1994;152:4433–4443. [PubMed] [Google Scholar]
- [132].Kato N, Pfeifer-Ohlsson S, Kato M, Larsson E, Rydnert J, Ohlsson R, et al. Tissue-specific expression of human provirus ERV3 mRNA in human placenta: Two of the three ERV3 mRNAs contain human cellular sequences. J Virol. 1987;61(7):2182–2191. doi: 10.1128/jvi.61.7.2182-2191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Yi JM, Lee WH, Kim HM, Kim HS. Identification of new endogenous retroviral sequences belonging to the HERV-W family in human cancer cells. Intervirology. 2001;44(6):333–338. doi: 10.1159/000050067. [DOI] [PubMed] [Google Scholar]
- [134].Bonner TI, O’Connell C, Cohen M. Cloned endogenous retroviral sequences from human DNA. Proc Natl Acad Sci USA. 1982;79(15):4709–4713. doi: 10.1073/pnas.79.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].La Mantia G, Maglione D, Pengue G, Di Cristofano A, Simeone A, Lanfrancone L, et al. Identification and characterization of novel human endogenous retroviral sequences prefentially expressed in undifferentiated embryonal carcinoma cells. Nucleic Acids Res. 1991;19(7):1513–1520. doi: 10.1093/nar/19.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Leib-Mosch C, Brack R, Werner T, Erfle V, Hehlmann R. Isolation of an SSAV-related endogenous sequence from human DNA. Virology. 1986;155(2):666–677. doi: 10.1016/0042-6822(86)90226-6. [DOI] [PubMed] [Google Scholar]
- [137].Blusch JH, Haltmeier M, Frech K, Sander I, Leib-Mosch C, Brack-Werner R, et al. Identification of endogenous retroviral sequences based on modular organization: Proviral structure at the SSAV1 locus. Genomics. 1997;43(1):52–61. doi: 10.1006/geno.1997.4790. [DOI] [PubMed] [Google Scholar]
- [138].Ono M, Yasunaga T, Miyata T, Ushikubo H. Nucleotide sequence of human endogenous retrovirus genome related to the mouse mammary tumor virus genome. J Virol. 1986;60(2):589–598. doi: 10.1128/jvi.60.2.589-598.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Benit L, Lallemand JB, Casella JF, Philippe H, Heidmann T. ERV-L elements: A family of endogenous retrovirus-like elements active throughout the evolution of mammals. J Virol. 1999;73(4):3301–3308. doi: 10.1128/jvi.73.4.3301-3308.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Medstrand P, Mager DL, Yin H, Dietrich U, Blomberg J. Structure and genomic organization of a novel human endogenous retrovirus family: HERV-K (HML-6) J Gen Virol. 1997;78(Pt 7):1731–1744. doi: 10.1099/0022-1317-78-7-1731. [DOI] [PubMed] [Google Scholar]
- [141].Kohsaka H, Yamamoto K, Fujii H, Miura H, Miyasaka N, Nishioka K, et al. Fine epitope mapping of the human SSB/La protein. Identification of a distinct autoepitope homologous to a viral gag polyprotein. J Clin Invest. 1990;85:1566–1574. doi: 10.1172/JCI114606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Talal N, Garry RF, Schur PH, Alexander S, Dauphinee MJ, Livas IH, et al. A conserved idiotype and antibodies to retroviral proteins in systemic lupus erythematosus. J Clin Invest. 1990;85:1866–1871. doi: 10.1172/JCI114647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Misaki Y, Yamamoto K, Yanagi K, Miura H, Ichijo H, Kato T, et al. B cell epitope on the U1 snRNP-C autoantigen contains a sequence similar to that of the herpes simplex virus protein. Eur J Immunol. 1993;23:1064–1071. doi: 10.1002/eji.1830230513. [DOI] [PubMed] [Google Scholar]
- [144].Vaughan JH, Valbracht JR, Nguyen M-D, Handley HH, Smith RS, Patrick K, et al. Epstein–Barr virus-induced autoimmune responses I. Immunoglobulin M autoantibodies to mimicking and nonmimicking Epstein–Barr virus nuclear antigen-1. J Clin Invest. 1995;95:1306–1315. doi: 10.1172/JCI117781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Vaughan JH, Nguyen M-D, Valbracht JR, Patrick K, Rhodes GH. Epstein–Barr virus-induced autoimmune responses II. Immunoglobulin G autoantibodies to mimicking and nonmimicking epitopes. Presence in autoimmune disease. J Clin Invest. 1995;95:1316–1327. doi: 10.1172/JCI117782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Li J-M, Fan WS, Horsfall AC, Anderson AC, Rigby S, Larsson E, et al. The expression of human endogenous retrovirus-3 in fetal cardiac tissue and antibodies in congenital heart block. Clin Exp Immunol. 1996;104:388–393. [PubMed] [Google Scholar]