Summary
Broadly-neutralizing antibodies (bNAbs) against human immunodeficiency virus (HIV) show great promise in HIV prevention as they are capable of potently neutralizing a considerable breadth of genetically diverse strains. Passive transfer of monoclonal bNAb proteins can confer protection in animal models of HIV infection at modest concentrations, inspiring efforts to develop an HIV vaccine capable of eliciting bNAb responses. However, these antibodies demonstrate high degrees of somatic mutation and other unique characteristics that may hinder the ability of conventional approaches to consistently and effectively produce bNAb analogues. As an alternative strategy, we and others have proposed vector-mediated gene transfer to generate long-term, systemic production of bNAbs in the absence of immunization. Herein, we review the use of adeno-associated virus (AAV) vectors for delivery of HIV bNAbs and antibody-like proteins and summarize both the advantages and disadvantages of this strategy as a method for HIV prevention.
Keywords: Adeno-associated virus, antibody gene transfer, broadly neutralizing antibodies, HIV, immunoprophylaxis
Introduction
The field of HIV vaccine research has been invigorated by the discovery of broadly-neutralizing antibodies (bNAbs). While natural infection predominantly elicits non-neutralizing or strain-specific antibodies, 10–30% of HIV-infected individuals generate bNAbs approximately 2–4 years after infection (1–3). These unusual antibodies are capable of neutralizing most circulating strains of HIV, making them promising candidates for HIV prevention. In fact, numerous in vivo studies in non-human primate (NHP) models have clearly demonstrated their potential to confer protection in models of HIV transmission. For example, passive transfer of bNAb proteins directed against various epitopes of the HIV envelope glycoprotein (Env) has effectively protected animals from intravenous challenge of SHIV, a chimeric SIV/HIV virus (4). Similar studies have extended these results to show that numerous bNAbs of varying epitope specificity can protect animals from both intravenous and mucosal SHIV transmission (5–8). Passive transfer of 2G12, an HIV bNAb recognizing a glycan-dependent Env epitope, led to reduced viral load in newborn macaques highlighting the potential of bNAbs to impact mother-to-child transmission (9).
In addition to animal models, several phase I clinical trials have demonstrated the efficacy of bNAbs against HIV infection. A single intravenous infusion of VRC01, a bNAb directed against the CD4 binding site (CD4bs) of HIV Env, reduced viral load by 1.1–1.8 log10 in 6 of 8 viremic subjects (10). Those who did not respond were found to have predominantly VRC01-resistant virus at the onset of the study. Similarly, a single intravenous infusion of 3BNC117, another CD4bs-directed bNAb, reduced viral load by 0.8–2.5 log10 at a dose of 30 mg/kg (11). Furthermore, passive transfer of 3BNC117 has been shown to delay viral rebound in HIV-infected individuals undergoing analytical treatment interruption (12). Two infusions of 30 mg/kg of antibody delayed viral rebound by 5–9 weeks whereas four infusions delayed for up to 19 weeks. This was significant compared to historical controls that typically rebounded less than 3 weeks after treatment interruption. Taken together, these studies highlight the safety and efficacy of passive transfer of HIV bNAbs for reduction of viremia and maintenance of viral suppression. Given this demonstrable potency against HIV in humans, bNAbs may also serve as effective tools in the context of HIV prophylaxis. In fact, a phase II clinical trial sponsored by the NIAID Vaccine Research Center (VRC) is currently recruiting participants to evaluate the safety and efficacy of VRC01 passive transfer in preventing HIV infection in high-risk, uninfected women in Sub-Saharan Africa (13). In addition, the VRC is recruiting participants for a phase I clinical trial to assess the safety of VRC01 passive transfer in infants less than 72 hours after birth at high risk for mother-to-child HIV transmission (14).
While these studies underscore the potential use for bNAbs in HIV therapy and prevention, passive transfer is likely an infeasible HIV vaccine approach. Given the short half-life (approximately 3 weeks) of antibodies in vivo, this approach would require continual readministration to maintain protective antibody concentrations. Previous clinical experience with preexposure prophylaxis indicates that consistent readministration generally reduces patient compliance (15). To lengthen the interval between doses, the fragment crystallizable (Fc) region of delivered antibodies can be engineered to exhibit enhanced affinity for neonatal Fc receptors (FcRn). This interaction facilitates antibody recycling through the endocytic salvage pathway in which endocytosed IgG binds FcRn within the early endosome and recycles back to the cell surface, thus preventing lysosomal degradation (16). Fc mutations that enhance interaction with FcRn, such as M428L/N434S (LS), T250Q/M428L (QL), and M252Y/S254T/T256E (YTE) have been shown to significantly improve antibody half-lives in vivo (17–19). In the NHP model, passive transfer of VRC01 harboring these mutations (VRC01-LS) resulted in a longer serum half-life, increased antibody concentration at mucosal surfaces, and enhanced protection against SHIV challenge compared to the wild-type antibody (20). A phase I clinical trial sponsored by the VRC is currently recruiting participants to assess the safety of passive transfer of VRC01-LS in healthy adults as the long-term immunogenicity of these engineered antibodies remains unknown (21). Even considering the potential for longer intervals between doses, additional logistical requirements such as continual antibody production, temperature-controlled storage, and complex distribution networks, will further hinder the implementation of a passive transfer vaccine approach, particularly in developing countries where intervention is most needed. Therefore, other strategies must be explored for the generation of bNAb responses against HIV.
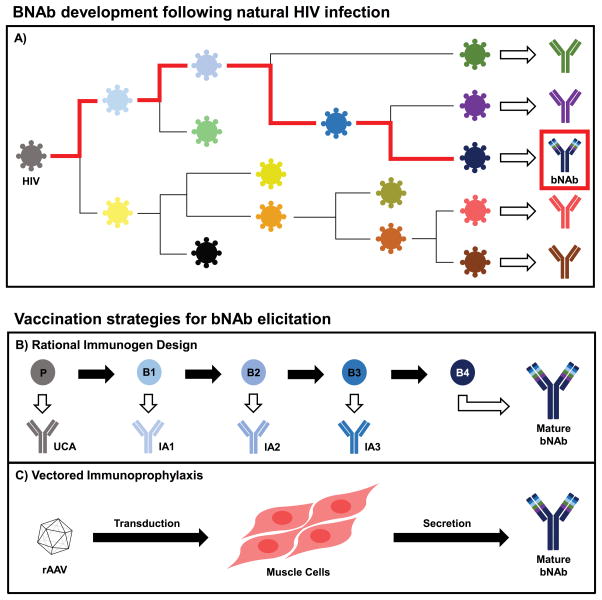
Historically, single- or limited-dose vaccines, such as for smallpox or polio, have represented the most effective eradication approaches against infectious diseases. Encouragingly, the bNAb-mediated protection observed in the aforementioned NHP studies and clinical trials occurred at modest antibody concentrations representing only about 1% of total circulating IgG1 (22). Therefore, substantial effort has been focused on the design of immunogens capable of eliciting HIV bNAb responses (23–25). Structural studies of bNAbs bound to their viral antigens have helped to elucidate major sites of Env vulnerability including the CD4bs, glycan-dependent epitopes in variable loops 1 and 2 (V1/V2) or variable loop 3 (V3), the gp120/gp41 interface, and the membrane proximal external region (MPER) (26–29). However, unique characteristics of bNAbs, such as high somatic mutation and a long heavy chain complementarity determining region 3 (CDRH3), make the use of existing vaccination strategies very challenging. For example, several studies have shown that vaccination against influenza elicits antibodies with mutation rates in the V-gene of approximately 5% (30,31); however, VRC01 demonstrates a mutation rate closer to 30% (32). This high level of affinity maturation is likely the result of continuous exposure to an evolving antigen which would be difficult to mimic with current immunogens (Fig. 1a). Therefore, many studies have examined the co-evolution of HIV with bNAbs in order to identify unmutated common and early ancestor antibodies and understand the antigens that direct bNAb development (33–36). This could inform potential prime-boost vaccination strategies involving sequential immunogens to direct bNAb elicitation (Fig. 1b). While such studies represent important conceptual advances for the field of immunogen design, these approaches would necessitate repeated administration, presenting challenges for patient compliance. In addition, the stochastic nature of antibody evolution and selection in each vaccine recipient makes it difficult to design regimens that will consistently and efficiently elicit bNAb responses in the vast majority of genetically diverse subjects.
Figure 1.
Elicitation of bNAb responses for prevention of HIV infection. A) Following natural infection, HIV replication leads to viral diversification and development of mostly strain-specific humoral responses. In rare instances, however, bNAbs capable of cross-clade neutralization develop after several years of infection through continual affinity maturation with evolving Env epitopes (red path). B) By mimicking key intermediates of the natural bNAb developmental pathway, prime-boost vaccination strategies are hypothesized to elicit bNAb responses. In this approach, an initial prime (P) consisting of recombinant Env protein is first administered to interact with the unmutated common ancestor (UCA) of the desired bNAb. Subsequent booster doses (B1-4) would elicit intermediate ancestors (IA1-3) and direct antibody development toward the mature bNAb. However, this approach would require several doses and may not consistently produce the desired responses. C) Vectored immunoprophylaxis requires only a single intramuscular injection of recombinant adeno-associated virus (rAAV) vector encoding the antibody gene to bypass humoral immunity and elicit long-term, systemic expression of mature bNAbs. Following administration, the encapsidated rAAV vector transduces muscle cells and forms a stable double-stranded episome within the host cell nucleus. The transcriptionally-active episome drives expression of both the heavy and light chain antibody genes which self-assemble to form full-length bNAbs that are secreted into circulation.
Antibody Gene Transfer with Adeno-Associated Viral Vectors
As an alternative to traditional vaccination approaches, gene transfer has been used to produce HIV bNAbs in vivo. In this method, transgenes encoding the desired antibody are delivered to tissues, resulting in secretion and systemic expression of biologically active antibodies without the need for immunization (Fig. 1c). A variety of vector systems have been developed to deliver antibody transgenes including naked DNA and lentiviral vectors (37–39). Herein, we will focus on the use of adeno-associated virus (AAV) vectors for the delivery of bNAbs as they constitute the best-studied gene therapy vector for use in humans. Recently, Glybera, an AAV vector expressing lipoprotein lipase enzyme, was the first gene therapy to be approved for clinical use in Europe as treatment for familial lipoprotein lipase deficiency. In addition, AAV vectors have been employed to conduct over 100 gene therapy clinical trials for diseases ranging from cystic fibrosis and blindness to hemophila B. These trials have proceeded in the absence of significant adverse events and some have yielded dramatic therapeutic efficacy (40). Beyond safety, AAV-based antibody gene transfer boasts many potential benefits as it bypasses dependence on humoral immunity and requires only a single dose for sustained expression of mature bNAbs. Moreover, AAV particles are resistant to high temperatures as well as wide-ranging pHs and can be stored in stable form for long periods of time without much loss of infectivity, enabling simpler distribution and implementation in resource-limited settings (41–43).
AAV is a small, icosahedral, non-enveloped virus belonging to the family Parvoviridae in the genus Dependovirus which depends on the presence of co-infecting helper viruses, such as adenovirus or herpesvirus, for productive infection. AAV packages a single-stranded DNA genome of approximately 4.7 kilobases (44) and is capable of infecting a variety of both non-dividing and dividing cell types including muscle, liver, and brain. Cellular tropism is defined by the identity of the viral capsid, of which hundreds have been described (45) and that fall into at least 9 serotypes. Upon infection, AAV escapes the endosomal network and traffics to the nucleus by a yet unknown mechanism where the 145 base pair AAV inverted terminal repeats (ITRs) located on either end of the genome self-prime second-strand synthesis by cellular polymerases. In the absence of helper virus, natural AAV infection utilizes the virally-encoded Rep proteins to facilitate site-specific integration at a locus on human chromosome 19 and establish latent infection (46–48).
Although over 70% of the human population are seropositive for AAV serotype 2 (AAV2) (49), natural AAV infection has not been definitively linked with any symptoms or disease. In 2015, Nault et al (50) identified AAV2 integrations in known cancer driver genes in a small subset (roughly 5%) of hepatocellular carcinomas, suggesting a potential pathogenic role for AAV2 in cancer. However, these results remain highly controversial as a second group re-analyzed the presented data and reached contradictory conclusions (51). While it is important to understand the risks associated with AAV, the insertional mutagenesis in question is likely dependent on viral Rep proteins which are excluded in AAV-based gene therapy vectors. The ITR sequences are the only cis-acting viral DNA elements necessary for replication and packaging; therefore, rAAV vectors consist only of the transgene of interest flanked by AAV2 ITR sequences. Rep and Cap proteins as well as necessary helper proteins are then provided in trans to produce encapsidated replication-incompetent rAAV in cell lines. Upon administration, the rAAV vector lacking Rep gene function can transduce cells where it persists as an episome composed of a series of head-to-tail concatemers of the recombinant genome in the nucleus of infected cells (52). Previous studies have demonstrated sustained expression following intramuscular (IM) injection of rAAV, with one study observing transgene expression in human skeletal muscle 10 years post administration (53–56). As a result of the highly vascularized nature of muscle tissue, transgenes introduced by IM injection can also be released into systemic circulation (57–59).
In 2002, Lewis et al were the first to demonstrate the feasibility of rAAV-vectored gene transfer to generate systemic expression of biologically active, full-length HIV bNAbs (60). Their vector consisted of two distinct promoters, each driving the expression of either the heavy or light chain gene of b12, an early-generation CD4bs-directed bNAb. Immunodeficient Rag1 mice given a single IM injection of rAAV1/b12 achieved serum antibody concentrations as high as 8 μg/mL which persisted for the 6 months of the study. The b12 produced in these animals retained its biological activity against HIV-1 as shown in in vitro neutralization assays against primary and T-cell line adapted HIV-1 isolates.
Fang et al (61) were able to achieve superior full-length antibody concentrations following intra-hepatic AAV8 administration by engineering a bicistronic vector in which both the heavy and light chain genes were expressed from a single open reading frame. This vector utilized the 2A self-processing peptide derived from the foot-and-mouth disease virus which promotes ribosomal skipping of a glycyl-prolyl peptide bond at the C-terminus of the 2A peptide (62). This results in release of the polypeptide from the ribosome while allowing synthesis of the downstream peptide to continue. Incorporation of an optimized furin cleavage site upstream of the 2A sequence ensured that no residual amino acids from the 2A peptide remained on the expressed antibody (63). When the anti-VEGFR2 antibody DC101 was expressed from this vector, functional antibody was produced which maintained antigen binding in vitro and achieved highly efficient expression in vivo. A single dose of rAAV8/DC101 administered through the portal vein to nude mice led to serum antibody concentrations greater than 1000 μg/mL for 4 months (61,63). Given the limited carrying capacity of AAV vectors, the use of 2A peptide is substantially more space-efficient than dual-promoter configurations and was fundamental to the development of vectors capable of efficient full-length HIV bNAb expression.
AAV-Delivered Antibodies Confer Protection from HIV Infection
In 2009, Johnson et al (64) were the first to demonstrate that AAV-delivered antibody-like proteins conferred protection against a challenge infection. They engineered immunoadhesin molecules consisting of SIV gp120-specific variable heavy and variable light chains connected by a linker and joined to the constant heavy region of the rhesus macaque IgG2 molecule. These antibody-like proteins exhibited potent neutralization against SIV strains in vitro and were used to test protection following gene transfer with a self-complementary AAV (scAAV) vector. The genome of scAAV vectors is engineered to form an intramolecular double-stranded DNA template, allowing for more rapid expression with the drawback of reduced carrying capacity. Rhesus macaques given an intramuscular injection of scAAV1 expressing SIV-specific immunoadhesins achieved serum concentrations between 40 and 190 μg/mL 4 weeks after AAV administration. At this time, animals were challenged intravenously with a single dose of the SIV molecular clone SIVmac316 that was sufficient to infect all control animals. Six of the 9 macaques previously given scAAV encoding immunoadhesins were completely protected from infection as assessed by the lack of both Gag-specific antibodies and SIV RNA in the plasma. Those macaques expressing immunoadhesins that went on to become infected were found to have significant anti-transgene responses in the form of immunoadhesin-specific antibodies, which correlated with a sharp decline in immunoadhesin serum concentrations and loss of protection. This was the first study to demonstrate the efficacy of an AAV-based vaccine approach in a model of HIV transmission. However, their AAV vector configuration exhibited limited carrying capacity that was unable to accommodate full-length antibody sequences necessitating the use of non-natural immunoadhesin molecules.
Our lab has previously described the development of an optimized expression vector capable of inducing long-term, systemic expression of full-length, naturally-occurring human bNAbs (65,66). This vector utilizes the aforementioned 2A peptide to enable bicistronic expression of bNAb heavy and light chains. IM injection of rAAV8 expressing b12 into both immunocompetent and immunodeficient mouse strains led to antibody serum concentrations greater than 100 μg/mL for at least 52 weeks. In humanized mice, rAAV delivery of HIV bNAbs protected animals from a single intravenous challenge dose of the HIV molecular clone NL4-3. Control mice exhibited significant CD4 cell depletion whereas mice expressing b12 did not. Other HIV bNAbs including 2G12, 4E10 and 2F5 exhibited partial protection in which some mice maintained high CD4 cell counts and others experienced delayed depletion. To assess the concentration necessary to afford protection from intravenous challenge, we performed a titration experiment in which we administered decreasing doses of rAAV expressing b12 or VRC01 which ultimately required serum antibody concentrations of 34 μg/mL and 8.3 μg/mL respectively to afford protection (65). This was the first study to demonstrate highly efficient, sustained expression of full-length HIV bNAbs from a single intramuscular injection that resulted in protection from intravenous HIV challenge.
The majority of human HIV transmission occurs through sexual contact. Therefore, we utilized the same approach, termed vectored immunoprophylaxis (VIP), in the bone marrow-liver-thymus (BLT) humanized mouse model to assess the ability of rAAV-delivered HIV bNAbs to protect against mucosal transmission. When rAAV8 expressing VRC01 was administered intramuscularly to BLT mice, antibody concentrations reached 100 μg/mL in the serum and approximately 100 ng/mL in the vaginal mucosa. Four weeks after AAV administration, mice were subjected to weekly, nonabrasive, low-dose, intravaginal challenge of JR-CSF, a CCR5-tropic HIV molecular clone. Control mice became infected after an average of 4 to 5 challenges as measured by viral RNA in the plasma. Conversely, 5 of 8 BLT mice expressing VRC01 were completely protected following 15 consecutive challenges. The 3 mice that became infected required 13–15 challenges before plasma viremia could be detected. Interestingly, two of these infected mice exhibited escape from VRC01 using the identical amino acid substitutions within envelope. This result argues against absolute sterilizing immunity as the mechanism of bNAb mediated protection in this model given the requirement for viral replication to acquire escape mutations. We favor a model in which bNAbs protect by blunting viral replication sufficiently to extinguish most but not all infection events. Heterosexual transmission of HIV between humans is a fairly inefficient process occurring between 1 in 100 and 1 in 1000 exposures (67,68). Most often, successful HIV infection is the result of propagation of one viral strain, termed the transmitted/founder (T/F) virus. In order to determine if T/F strains were more resistant to antibody neutralization compared to lab-adapted strains, we performed a second repetitive mucosal challenge study using REJO.c, a CCR5-tropic T/F molecular clone. IM injection of rAAV expressing VRC07G54W, a more potent CD4bs-directed bNAb, led to antibody concentrations in the serum and vaginal mucosa comparable to that observed for VRC01, but conferred complete protection against 21 consecutive intravaginal challenges of REJO.c (66).
In 2015, Saunders et al (69) demonstrated that rAAV8 delivery of simianized broadly neutralizing antibodies protected non-human primates from mucosal SHIV infection. The study used the same rAAV expression vector we had previously developed. IM injection of AAV expressing simianized VRC07 resulted in serum antibody concentrations of 2.5 to 7.7 μg/mL 2 to 4 weeks following administration. However, antibody concentrations became undetectable by 9 weeks coinciding with an increase of anti-VRC07 responses. To circumvent this issue, the rhesus immune response was suppressed prior to rAAV administration using cyclosporine (CsA). This resulted in an average peak serum antibody concentration of 38.12 μg/mL across 6 macaques. Following cessation of CsA treatment, antibody concentrations decreased due to the appearance of anti-antibody responses. However, 3 of the 6 animals maintained serum antibody concentrations between 1 and 10 μg/mL for 16 weeks. Macaques given rAAV8/VRC07 and CsA treatment were challenged intrarectally with the CCR5-tropic SHIV-BaLP4 5.5 weeks after AAV administration. All control animals became infected, but 4 of the 6 macaques expressing VRC07 were protected. The 2 animals that became infected exhibited little to no VRC07 expression at the time of challenge.
A study by Fuchs et al (70) showed that long-term expression of anti-SIV antibodies could protect from successive intravenous challenges of SIVmac239. They used two AAV vector approaches to deliver full-length IgG1 antibodies derived from previously described SIV-specific immunoadhesin sequences. In the first approach, they used a traditional single-stranded rAAV vector to express the full-length antibody. In the second approach, they used scAAV in which the heavy and light chain genes were delivered on separate vectors. IM injection of AAV led to variable serum concentrations with no discernable difference between the two vector approaches. Most macaques exhibited a precipitous drop in antibody concentration that coincided with the development of anti-antibody responses. However, one animal achieved a serum concentration of 270 μg/mL which persisted for more than 2 years in the absence of anti-anti responses. Interestingly, this animal was the only one to resist 6 consecutive intravenous challenges with SIVmac239, the last of which was 10 intravenous infectious doses. While macaques with low-level antibody expression became infected, they did exhibit significantly lower SIV viral RNA during peak viremia and chronic phase infection. In addition, time from exposure to peak viremia was also significantly longer in these animals.
Lastly, Gardner et al (71) used a rAAV-based approach to deliver eCD4-Ig to NHPs in place of HIV bNAbs. This antibody-like protein consists of the immunoadhesin form of CD4 fused to a sulfopeptide mimic of CCR5 and demonstrates broad and potent neutralization of HIV-1 isolates in vitro. IM injection of rAAV expressing the rhesus macaque form of eCD4-Ig in conjunction with a second vector expressing a required sulfation enzyme resulted in serum concentrations that stabilized between 17–77 μg/mL for the last 10 weeks of the 40-week study. Beginning 8 weeks after rAAV administration, macaques were challenged intravenously with increasing doses of SHIV-AD8, none of which successfully infected macaques expressing eCD4-Ig. Interestingly, animals exhibited minimal anti-transgene responses indicating that eCD4-Ig may be less immunogenic than the simianized antibodies previously described. However, it remains to be seen whether the non-natural structure of this antibody-like protein may yet elicit anti-transgene responses over a longer period of observation. In addition, the need for co-administration of a second AAV encoding an enzyme for sulfation of the CCR5 mimetic peptide may hinder the clinical development of this approach as it relies on efficient transduction of both vectors for effective neutralization to occur and might necessitate an evaluation of the clinical safety of each separate component prior to testing their combination in human subjects.
Potential Pitfalls of AAV-Vectored Antibody Gene Transfer
While sustained expression of HIV broadly neutralizing antibodies and antibody-like proteins is a promising strategy for HIV prevention, there are still many obstacles to widespread use in humans. For one, AAV has a limited carrying capacity of approximately 5 kilobases which restricts the potential transgenes that can be expressed with these vectors. The introduction of the short 2A peptide sequence was an important advance as it maximized available vector space by abrogating the need for a second promoter. Despite this advance, efforts to further enhance transgene expression or engineer regulatable systems are still hindered by carrying capacity. Self-complementary AAV (scAAV) vectors may represent a method for more rapid and efficient transgene expression compared to traditional single-stranded rAAV vectors, though they can only accommodate half the natural carrying capacity, making them impractical for delivery of full-length antibodies in a single scAAV (32). The improved expression kinetics of scAAV vectors in vivo are thought to result from the ability of these vectors to form an intramolecular double-stranded DNA structure which circumvents the rate-limiting step of second-strand synthesis following transduction. However, it is not yet clear whether the improved expression characteristics are in fact a function of their unique structure or a result caused by difficulty in accurately quantifying scAAV vector preparations (72). Regardless, ongoing efforts will be necessary to further optimize rAAV vector configurations to maximize in vivo transgene expression in patients.
Another potential area of concern is the prevalence of pre-existing immunity to AAV. Approximately 60–70% of the human population is seropositive for antibodies against capsid for AAV1 and 2 (49). The presence of these antibodies would be predicted to significantly inhibit AAV transduction and thus reduce the efficacy of any AAV-based treatment. However, several strategies exist to address this issue. First, a wide variety of AAV serotypes could be employed which have exhibited limited circulation in human populations. The AAV8 serotype was isolated from rhesus macaques and is advantageous for its propensity for muscle transduction (73), reduced immunogenicity (74) and lower prevalence of seropositive individuals (about 38%) in the population (49). However, sufficient cross-reactivity between AAV capsids exist such that some individuals will still exhibit neutralizing activity against AAV8. While patient sera can be pre-screened for neutralizing factors prior to rAAV administration, new strategies will need to be developed to combat pre-existing immunity. Significant effort is being focused on the discovery of novel capsids that can improve tropism for tissues of interest (75) and reduce reactivity with existing human antibodies. For example, Zinn et al (76) utilized in silico reconstruction to infer the evolutionary intermediates of existing AAV serotypes. This lead to the identification of Anc80, the predicted ancestral capsid sequence, which maintained structural and functional integrity. Importantly, antibodies raised against current AAV serotypes demonstrated little to no cross-reactivity with Anc80. Regardless of the capsid used, antibodies will likely arise following administration thereby hindering repeated use in a single individual and highlighting the need to develop a repertoire of capsids for which pre-existing immunity is limited.
One of the major issues that has arisen from AAV studies in NHP models has been the elicitation of anti-transgene immune responses that in some cases abolish efficacy against viral challenge (64,69,70,77). It is important to note, however, that none of the transgenes delivered in these studies were native macaque proteins, despite substantial effort to optimize the molecules for use in NHP. For example, simianization of VRC07 was performed by transferring the complementarity-determining regions of human VRC07 onto homologous macaque germ line genes and engineering key somatic mutations within the framework regions. The heavy and light chain variable regions were then appended to macaque constant regions (69). While this simianized antibody maintained specificity and potency, it is uncertain how well it mimics native rhesus antibodies as it did not undergo immunological selection in macaques. Similarly, antibodies used by Fuchs et al (70) were originally isolated by phage display and then converted into full-length simian antibodies, again skipping the key negative selection process. There is recent evidence that naturally elicited SIV-specific macaque antibodies isolated from NHP by means similar to human bNAbs exhibit substantially better expression characteristics in the absence of detectable anti-antibody responses following rAAV administration (HIV Keystone meeting 2016, Mario Roederer). As AAV-vectored antibody gene transfer will be used to deliver only fully human antibodies that underwent selection by the human immune system, we hypothesize that they will exhibit reduced immunogeniticy than might have been predicted by published NHP studies. Importantly, individuals receiving infusions of VRC01 protein did not generate any detectable anti-antibody responses as long as 112 days post infusion (10). Therefore, it is unclear whether anti-antibody responses will be an area of concern in human patients.
While we do not believe that immunogenicity against the transgene will be widespread if rAAV/bNAb vectors are used at large, it is entirely possible that rare individuals may experience interactions that could not be predicted a priori. In these instances, it would be highly desirable to have a means of reversing antibody gene expression. Inducible rAAV vector systems may provide one such method for regulatable expression as transgene transcription is dependent upon the presence of a small molecule such as tetracycline, mifepristone, or rapamycin (78–82). In these systems, the small molecule allows for transcriptional activation domains to be recruited to a basal promoter driving transgene expression. In 2007, Fang et al (63) demonstrated the ability of a rapamycin-inducible rAAV vector to drive high-level expression of full-length antibodies in vivo. Using a dimerization-regulated system, they attained serum antibody concentrations greater than 1 mg/mL which rapidly returned to baseline after discontinuation of rapamycin treatment. However, these inducible systems present several challenges for clinical application in the context of VIP. First, they require expression of transcriptional activators that would be difficult to accommodate into a single rAAV vector along with a full-length antibody gene. Second, long-term expression of these heterologous proteins may be immunogenic in patients. In 2006, Murphy et al (83) described a drug-dependent inducible system that circumvented these issues as it did not depend on expression of heterologous transcriptional activation domains. Instead, they engineered the transgene of interest with a stop codon just downstream of the site of translation initiation, thus preventing transgene expression. Subsequent treatment with aminoglycoside antibiotics and other nonantibiotic compounds resulted in stop codon read-through and a 60-fold induction of transgene expression. In a similar approach, Yen et al (84) engineered transgenes to contain the hammerhead Sm1 ribozyme sequence, a cis-acting RNA motif derived from Schistosoma mansoni that mediates self-cleavage of the mRNA transcript and prevents transgene expression. Upon administration of antisense oligonucleotides, they observed anywhere from 20–2000-fold induction of transgene expression depending on the inducer molecule used. As with the stop codon read-through approach, this system is more amenable to rAAV vectors as only short nucleic acid sequences are required. However, all of the inducible systems described necessitate continual adherence to a daily pill regimen and therefore pose no advantage to current anti-retroviral treatments. We favor a system in which transgene expression is on by default unless intervention is required. Analogous to inducible systems, drug-dependent repressible rAAV vectors have been engineered in which small molecules block transgene expression (85) and are only necessary in the rare subset of patients in which a negative reaction occurs. In a similar approach, lentiviral vectors encoding the Herpes Simplex thymidine kinase (HSVTK) gene have been used to allow for specific destruction of transduced cells upon ganciclovir administration (86). However, these drug-dependent repressible systems would share the drawbacks of limited carrying capacity and potential immunogenicity of necessary heterologous proteins. Additionally, destruction of skeletal muscle with HSVTK-encoding vectors would likely have devastating effects. A more advanced approach currently under development by our lab utilizes Cre/loxP recombination to specifically elicit transgene destruction (unpublished data). In this method, the initial rAAV vector used for antibody gene delivery contains several loxP sites flanking the antibody gene and other regulatory elements. Upon Cre administration to transduced cells, recombination of these sites will lead to degradation of the transgene and a drastic reduction in expression. These regulated approaches will be important to ensure the safety of antibody gene transfer in widespread use.
Improving Expression and Efficacy of AAV-Delivered Broadly Neutralizing Antibodies
We and others are continually working to improve the efficacy of AAV-mediated antibody gene transfer. One strategy is to improve the potency and breadth of the antibodies delivered. Since the characterization of the first major bNAbs (b12, 2G12, 2F5, 4E10, and Z13) in the early 90s, significant effort has been invested in the isolation and engineering of newer bNAbs with improved neutralization profiles. For example, PGDM1400, an HIV bNAb specific to the Env trimer apex, has exhibited a cross-clade neutralization coverage of 83% at a median half-maximal inhibitory concentration (IC50) of 0.003 μg/mL according to in vitro neutralization panels (87). Similarly, Mark Connors’ lab at NIAID has recently identified N6, a new CD4bs-directed bNAb more potent than VRC07 and broader in the range of envelope sequences it recognizes (unpublished data). These antibodies represent a significant improvement over early generation bNAbs such as the CD4bs-directed bNAb, b12 which demonstrated an approximate 34% cross-clade neutralization coverage at a median IC50 of 2.82 μg/mL (3). Previous studies indicate that bNAbs with better in vitro neutralization characteristics can confer better protection in vivo. For example, the potent glycan-dependent bNAb, PGT121, was able to protect at an average serum antibody concentration approximately 100-fold lower than b12 in NHP models of mucosal SHIV transmission (88). However, in vitro neutralization potency is not always an accurate predictor of efficacy in vivo as evidenced by our experience with 2G12. Despite being well over the IC50 value of 2G12 for NL4-3, we observed only partial protection, perhaps suggesting a factor of “escapability” (i.e. the ease in which HIV can become resistant to a given antibody) as a new feature to consider when selecting bNAbs. As a consequence, newly discovered antibodies should be tested for their ability to confer protection in vivo in order to identify the best candidates for use in humans.
Delivery of AAV cocktails expressing combinations of HIV bNAbs may be advantageous to extend clade coverage and reduce potential for viral escape. However, we believe that prevention of HIV infection and reduction in transmission may be achievable through expression of a single bNAb provided it is sufficiently potent against most circulating strains of HIV. Similarly, single bNAb administration may be sufficient to maintain viral suppression as evidenced by the recent phase II clinical trial in which administration of 3BNC117 alone delayed viral rebound in suppressed patients following treatment interruption (12). Therefore, bNAb combinations may be particularly important in the context of HIV therapy as on-going viral replication produces quasi-species that may be differentially susceptible to any single bNAb. When combinations of bNAbs with varying epitope specificity were given by passive transfer to humanized mice, plasma viral load exhibited prolonged suppression compared to monotherapy (89). Interestingly, several mice receiving tri-mix therapy exhibited viral rebound due to the acquisition of viral escape variants whereas mice receiving penta-mix therapy suppressed viremia for the duration of the study. In 2016, Wagh et al assessed 15 different single bNAbs for neutralization potency against a panel of HIV clade C viruses in order to inform mathematical models of potential combinations (90). Certain 3 and 4 bNAb combinations were found to exhibit superior potency, breadth, complete neutralization, and active coverage compared to 2 bNAb combinations or any one bNAb alone. However, further in vivo studies will be necessary to determine the optimal combination of bNAbs for HIV therapy.
We are currently investigating additional methods to enhance the effectiveness of HIV bNAbs delivered by rAAV vectors. As previously mentioned with passive transfer studies, antibodies can be engineered to contain Fc mutations such as LS, QL, and YTE to selectively enhance the affinity of Fc for FcRn and significantly improve antibody half-life (17–19). It is unknown the extent to which FcRn-mediated antibody recycling contributes to the steady-state serum antibody concentration achieved following IM rAAV administration in vivo. This is important to determine as it will inform the design of optimal strategies to enhance bNAb expression. If recycling contributes substantially to steady-state concentrations, Fc mutations that enhance FcRn interactions may provide an important tool for enhancing both serum and mucosal antibody concentrations following rAAV administration. Translating such improvements to humans could enable the use of substantially reduced vector dosage to attain clinically relevant antibody concentrations, thereby improving both the cost-effectiveness and safety of VIP.
In addition to the FcRn, the Fc region mediates interactions with complement proteins and Fc receptors on innate immune cells to facilitate antibody effector functions including complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), and antibody-dependent cellular phagocytosis (ADCP). Growing evidence suggests a role for these antibody-mediated effector functions, particularly ADCC, in HIV protection and control. For example, the RV144 phase III clinical trial in Thailand demonstrated a 31.2% reduction in HIV infection among participants receiving the vaccine; this protection is thought to correlate with high levels of ADCC (91,92). Additionally, HIV controllers have been shown to exhibit higher levels of ADCC-mediating antibodies compared to viremic subjects (93). This interest in antibody-mediated effector functions has led to investigations of their contribution to the mechanism of bNAb-mediated protection. In the NHP model, mutations abolishing effector function activity were engineered into the Fc region of b12. The mutated bNAb exhibited a significantly diminished capacity to prevent SHIV infection compared to wild-type b12 indicating a role for effector functions in protection (94). Similar results were observed using mutant human-mouse chimeric antibodies in a mouse model of HIV entry (95). The relative importance of each effector function to bNAb efficacy and whether this contribution is similar across bNAbs of differing epitope specificity remains poorly understood. If Fc-mediated functions are shown to be important, then rAAV vectors can be used to delivery mutant bNAbs with enhanced effector function activity, potentially conferring protection superior to that of wild-type bNAbs and other available treatments.
Conclusion
AAV-vectored delivery of HIV broadly neutralizing antibodies and antibody-like proteins has led to long-term, systemic transgene expression that conferred protection from viral challenge in a variety of animal models. Given the apparent difficulty in eliciting bNAb responses through traditional vaccine approaches, vectored gene transfer may be a practical, effective strategy to prevent human HIV transmission. With the isolation of broader and more potent neutralizing antibodies as well as the potential for antibody engineering to enhance expression and effector function activity, it may be possible to further improve the protective efficacy of existing bNAbs. Anti-transgene responses following AAV administration are a potential area of concern as they have abrogated protection in NHP models; however, given the non-native nature of the molecules delivered in these studies it is uncertain whether analogous humoral responses will be elicited in human subjects receiving native human antibodies. Two phase I clinical trials have been planned to evaluate the safety of AAV-delivered HIV bNAbs. The first trial sponsored by the International Aids Vaccine Initiative recruited healthy adult men in the United Kingdom to receive a rAAV1 vector expressing PG9. This trial was scheduled to conclude in early 2016, however, the findings have not yet been published. The second trial sponsored by the VRC will test a rAAV8 vector expressing VRC07 in HIV infected patients. Results from these studies will undoubtedly shed light on the present controversy surrounding anti-antibody responses and is likely to reveal other potential challenges that will need to be overcome to enable widespread use of antibody gene transfer in humans.
Acknowledgments
A.B.B. is supported by the National Institutes for Drug Abuse (NIDA) Avenir New Innovator Award DP2DA040254, the MGH Transformative Scholars Program as well as funding from the Charles H. Hood Foundation. This independent research was supported by the Gilead Sciences Research Scholars Program in HIV. D.B. and A.B.B were supported by the National Institutes of Health (HHSN266200500035C) through a contract from the NIAID.
Footnotes
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS. 2014;28(2):163–9. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray ES, et al. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol. 2011;85(10):4828–40. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker LM, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–70. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascola JR, et al. Protection of macaques against pathogenic simian/human immunodeficiency virus 89. 6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73(5):4009–18. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6(2):207–10. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 6.Parren PW, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75(17):8340–7. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hessell AJ, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15(8):951–4. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hessell AJ, et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 2010;84(3):1302–13. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng CT, et al. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat Med. 2010;16(10):1117–9. doi: 10.1038/nm.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lynch RM, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;7(319):319ra206–6. doi: 10.1126/scitranslmed.aad5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caskey M, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522(7557):487–91. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheid JF, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016 doi: 10.1038/nature18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NIAID. Evaluating the safety and efficacy of the VRC01 antibody in reducing acquisition of HIV-1 infection in women. Available from: https://clinicaltrials.gov/ct2/show/NCT02568215.
- 14.NIAID. Evaluating the safety and pharmacokinetics of VRC01, a potent anti-HIV neutralizing monoclonal antibody, in HIV-1-exposed infants. Available from: https://clinicaltrials.gov/ct2/show/results/NCT02256631.
- 15.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26(7):F13–9. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 16.Goebl NA, Babbey CM, Datta-Mannan A, Witcher DR, Wroblewski VJ, Dunn KW. Neonatal Fc receptor mediates internalization of Fc in transfected human endothelial cells. Mol Biol Cell. 2008;19(12):5490–505. doi: 10.1091/mbc.E07-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zalevsky J, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28(2):157–9. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinton PR, et al. Engineered human IgG antibodies with longer serum half-lives in primates. J Biol Chem. 2004;279(8):6213–6. doi: 10.1074/jbc.C300470200. [DOI] [PubMed] [Google Scholar]
- 19.Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2006;281(33):23514–24. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 20.Ko S-Y, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514(7524):642–5. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NIAID. Safety and pharmacokinetics of a human monoclonal antibody, VRC-HIVMAB080-00-AB (VRC01LS), with broad HIV-1 neutralizing activity, administered intravenously or subcutaneously to healthy adults. Available from: https://clinicaltrials.gov/ct2/show/NCT02599896.
- 22.Vlug A, Nieuwenhuys EJ, van Eijk RV, Geertzen HG, van Houte AJ. Nephelometric measurements of human IgG subclasses and their reference ranges. Ann Biol Clin (Paris) 1994;52(7–8):561–7. [PubMed] [Google Scholar]
- 23.Kwong PD, Wilson IA. HIV-1 and influenza antibodies: seeing antigens in new ways. Nat Immunol. 2009;10(6):573–8. doi: 10.1038/ni.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burton DR, et al. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5(3):233–6. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 25.Dormitzer PR, Ulmer JB, Rappuoli R. Structure-based antigen design: a strategy for next generation vaccines. Trends Biotechnol. 2008;26(12):659–67. doi: 10.1016/j.tibtech.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou T, et al. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007;445(7129):732–7. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLellan JS, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480(7377):336–43. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pejchal R, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334(6059):1097–103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julien J-P, Lee PS, Wilson IA. Structural insights into key sites of vulnerability on HIV-1 Env and influenza HA. Immunol Rev. 2012;250(1):180–98. doi: 10.1111/imr.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moody MA, et al. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS ONE. 2011;6(10):e25797. doi: 10.1371/journal.pone.0025797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li G-M, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci USA. 2012;109(23):9047–52. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. 2011;333(6049):1593–602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao H-X, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496(7446):469–76. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doria-Rose NA, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 2014;509(7498):55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore PL, et al. Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat Med. 2012;18(11):1688–92. doi: 10.1038/nm.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wibmer CK, et al. Viral escape from HIV-1 neutralizing antibodies drives increased plasma neutralization breadth through sequential recognition of multiple epitopes and immunotypes. PLoS Pathog. 2013;9(10):e1003738. doi: 10.1371/journal.ppat.1003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muthumani K, Flingai S, Wise M, Tingey C, Ugen KE, Weiner DB. Optimized and enhanced DNA plasmid vector based in vivo construction of a neutralizing anti-HIV-1 envelope glycoprotein Fab. Hum Vaccin Immunother. 2013;9(10):2253–62. doi: 10.4161/hv.26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo XM, Maarschalk E, O’Connell RM, Wang P, Yang L, Baltimore D. Engineering human hematopoietic stem/progenitor cells to produce a broadly neutralizing anti-HIV antibody after in vitro maturation to human B lymphocytes. Blood. 2009;113(7):1422–31. doi: 10.1182/blood-2008-09-177139. [DOI] [PubMed] [Google Scholar]
- 39.Joseph A, et al. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. J Virol. 2010;84(13):6645–53. doi: 10.1128/JVI.02339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12(7):515–5. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 41.Croyle MA, Cheng X, Wilson JM. Development of formulations that enhance physical stability of viral vectors for gene therapy. Gene Ther. 2001;8(17):1281–90. doi: 10.1038/sj.gt.3301527. [DOI] [PubMed] [Google Scholar]
- 42.Rayaprolu V, et al. Comparative analysis of adeno-associated virus capsid stability and dynamics. J Virol. 2013;87(24):13150–60. doi: 10.1128/JVI.01415-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuck D, et al. Intranasal vaccination with recombinant adeno-associated virus type 5 against human papillomavirus type 16 L1. J Virol. 2006;80(6):2621–30. doi: 10.1128/JVI.80.6.2621-2630.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srivastava A, Lusby EW, Berns KI. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983;45(2):555–64. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5(3):285–97. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- 46.Kotin RM, et al. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87(6):2211–5. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samulski RJ, et al. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991;10(12):3941–50. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huser D, Weger S, Heilbronn R. Kinetics and frequency of adeno-associated virus site-specific integration into human chromosome 19 monitored by quantitative real-time PCR. J Virol. 2002;76(15):7554–9. doi: 10.1128/JVI.76.15.7554-7559.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boutin S, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704–12. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 50.Nault J-C, Datta S, Imbeaud S, Franconi A, Zucman-Rossi J. Adeno-associated virus type 2 as an oncogenic virus in human hepatocellular carcinoma. Mol Cell Oncol. 2016;3(2):e1095271. doi: 10.1080/23723556.2015.1095271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berns KI, et al. Adeno-associated virus type 2 and hepatocellular carcinoma? Hum Gene Ther. 2015;26(12):779–81. doi: 10.1089/hum.2015.29014.kib. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duan D, et al. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J Virol. 1998;72(11):8568–77. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark KR, Sferra TJ, Johnson PR. Recombinant adeno-associated viral vectors mediate long-term transgene expression in muscle. Hum Gene Ther. 1997;8(6):659–69. doi: 10.1089/hum.1997.8.6-659. [DOI] [PubMed] [Google Scholar]
- 54.Kessler PD, et al. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93(24):14082–7. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70(11):8098–108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buchlis G, et al. Factor IX expression in skeletal muscle of a severe hemophilia B patient 10 years after AAV-mediated gene transfer. Blood. 2012;119(13):3038–41. doi: 10.1182/blood-2011-09-382317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herzog RW, et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94(11):5804–9. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monahan PE, et al. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther. 1998;5(1):40–9. doi: 10.1038/sj.gt.3300548. [DOI] [PubMed] [Google Scholar]
- 59.Murphy JE, Zhou S, Giese K, Williams LT, Escobedo JA, Dwarki VJ. Long-term correction of obesity and diabetes in genetically obese mice by a single intramuscular injection of recombinant adeno-associated virus encoding mouse leptin. Proc Natl Acad Sci USA. 1997;94(25):13921–6. doi: 10.1073/pnas.94.25.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis AD, Chen R, Montefiori DC, Johnson PR, Clark KR. Generation of neutralizing activity against human immunodeficiency virus type 1 in serum by antibody gene transfer. J Virol. 2002;76(17):8769–75. doi: 10.1128/JVI.76.17.8769-8775.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fang J, et al. Stable antibody expression at therapeutic levels using the 2A peptide. Nat Biotechnol. 2005;23(5):584–90. doi: 10.1038/nbt1087. [DOI] [PubMed] [Google Scholar]
- 62.Donnelly ML, et al. Analysis of the aphthovirus 2A/2B polyprotein ‘cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: a putative ribosomal ‘skip’. J Gen Virol. 2001;82(Pt 5):1013–25. doi: 10.1099/0022-1317-82-5-1013. [DOI] [PubMed] [Google Scholar]
- 63.Fang J, Yi S, Simmons A, Tu GH, Nguyen M, Harding TC, et al. An antibody delivery system for regulated expression of therapeutic levels of monoclonal antibodies in vivo. Mol Ther. 2007;15(6):1153–9. doi: 10.1038/sj.mt.6300142. [DOI] [PubMed] [Google Scholar]
- 64.Johnson PR, et al. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med. 2009;15(8):901–6. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481(7379):81–4. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balazs AB, et al. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat Med. 2014;20(3):296–300. doi: 10.1038/nm.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hughes JP, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis. 2012;205(3):358–65. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1(1):25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 69.Saunders KO, et al. Broadly neutralizing human immunodeficiency virus type 1 antibody gene transfer protects nonhuman primates from mucosal simian-human immunodeficiency virus infection. J Virol. 2015;89(16):8334–45. doi: 10.1128/JVI.00908-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fuchs SP, Martinez-Navio JM, Piatak M, Lifson JD, Gao G, Desrosiers RC. AAV-delivered antibody mediates significant protective effects against SIVmac239 challenge in the absence of neutralizing activity. PLoS Pathog. 2015;11(8):e1005090. doi: 10.1371/journal.ppat.1005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gardner MR, et al. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature. 2015;519(7541):87–91. doi: 10.1038/nature14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fagone P, Wright JF, Nathwani AC, Nienhuis AW, Davidoff AM, Gray JT. Systemic errors in quantitative polymerase chain reaction titration of self-complementary adeno-associated viral vectors and improved alternative methods. Hum Gene Ther Methods. 2012;23(1):1–7. doi: 10.1089/hgtb.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao G-P, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99(18):11854–9. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mays LE, et al. AAV8 induces tolerance in murine muscle as a result of poor APC transduction, T cell exhaustion, and minimal MHCI upregulation on target cells. Mol Ther. 2014;22(1):28–41. doi: 10.1038/mt.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lisowski L, et al. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature. 2014;506(7488):382–6. doi: 10.1038/nature12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zinn E, et al. In Silico reconstruction of the viral evolutionary lineage yields a potent gene therapy vector. Cell Rep. 2015;12(6):1056–68. doi: 10.1016/j.celrep.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinez-Navio JM, Fuchs SP, Pedreño-López S, Rakasz EG, Gao G, Desrosiers RC. Host anti-antibody responses following adeno-associated virus-mediated delivery of antibodies against HIV and SIV in rhesus monkeys. Mol Ther. 2016;24(1):76–86. doi: 10.1038/mt.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89(12):5547–51. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chtarto A, et al. Tetracycline-inducible transgene expression mediated by a single AAV vector. Gene Ther. 2003;10(1):84–94. doi: 10.1038/sj.gt.3301838. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, O’Malley BW, Tsai SY. A regulatory system for use in gene transfer. Proc Natl Acad Sci USA. 1994;91(17):8180–4. doi: 10.1073/pnas.91.17.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rivera VM, et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105(4):1424–30. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- 82.Clackson T. Regulated gene expression systems. Gene Ther. 2000;7(2):120–5. doi: 10.1038/sj.gt.3301120. [DOI] [PubMed] [Google Scholar]
- 83.Murphy GJ, Mostoslavsky G, Kotton DN, Mulligan RC. Exogenous control of mammalian gene expression via modulation of translational termination. Nat Med. 2006;12(9):1093–9. doi: 10.1038/nm1376. [DOI] [PubMed] [Google Scholar]
- 84.Yen L, et al. Exogenous control of mammalian gene expression through modulation of RNA self-cleavage. Nature. 2004;431(7007):471–6. doi: 10.1038/nature02844. [DOI] [PubMed] [Google Scholar]
- 85.Haberman RP, McCown TJ, Samulski RJ. Inducible long-term gene expression in brain with adeno-associated virus gene transfer. Gene Ther. 1998;5(12):1604–11. doi: 10.1038/sj.gt.3300782. [DOI] [PubMed] [Google Scholar]
- 86.Tiberghien P, et al. Ganciclovir treatment of herpes simplex thymidine kinase-transduced primary T lymphocytes: an approach for specific in vivo donor T-cell depletion after bone marrow transplantation? Blood. 1994;84(4):1333–41. [PubMed] [Google Scholar]
- 87.Sok D, et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc Natl Acad Sci USA. 2014;111(49):17624–9. doi: 10.1073/pnas.1415789111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moldt B, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci USA. 2012;109(46):18921–5. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klein F, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012;492(7427):118–22. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wagh K, et al. Optimal combinations of broadly neutralizing antibodies for prevention and treatment of HIV-1 clade C infection. PLoS Pathog. 2016;12(3):e1005520. doi: 10.1371/journal.ppat.1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bonsignori M, et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol. 2012;86(21):11521–32. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lambotte O, et al. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PLoS ONE. 2013;8(9):e74855. doi: 10.1371/journal.pone.0074855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449(7158):101–4. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 95.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158(6):1243–53. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]