Abstract
This review is on arsenic in agronomic systems, and covers processes that influence the entry of arsenic into the human food supply. The scope is from sources of arsenic (natural and anthropogenic) in soils, biogeochemical and rhizosphere processes that control arsenic speciation and availability, through to mechanisms of uptake by crop plants and potential mitigation strategies. This review makes a case for taking steps to prevent or limit crop uptake of arsenic, wherever possible, and to work toward a long-term solution to the presence of arsenic in agronomic systems. The past two decades have seen important advances in our understanding of how biogeochemical and physiological processes influence human exposure to soil arsenic, and this must now prompt an informed reconsideration and unification of regulations to protect the quality of agricultural and residential soils.
Keywords: arsenic, sources, soil, agriculture, plants, mitigation
Graphical abstract
1. Introduction
Consumption of staple foods such as rice, beverages such as apple juice, or vegetables grown in historically arsenic-contaminated soils are now recognized as tangible routes of arsenic exposure. The presence of elevated concentrations of arsenic in the soil is not a pre-requisite for dietary arsenic exposure; seen in the accumulation of arsenic by rice grown in uncontaminated soils1. When drinking-water arsenic concentrations are low, dietary arsenic can be a significant exposure2. Understanding the sources of arsenic to crop plants and the factors that influence them is key to reducing human exposure now and preventing exposure in future. In addition to the abundant natural sources of arsenic, there are a large number of industrial and agricultural sources of arsenic to the soil; from mining wastes, coal fly ash, glass manufacturing, pesticide application, wastewater sludge, pharmaceutical waste, livestock dips, smelting activities to phosphate fertilizers. Plant uptake of arsenic was previously assumed to be too low to merit setting limits for arsenic in food crops, but given that measurable biological effects occur in at arsenic levels below the current maximum contaminant level (MCL) for drinking water3, these low levels can still translate into significant exposures, particularly in children4 and presumably in adults who consume a lot of rice. In response, the World Health Organization (WHO) set an advisory MCL for inorganic arsenic in white (polished) rice of 0.2 mg/kg5 along with the limit of 10 μg/L in water, and the European Union set similar standards that included a lower MCL (0.1 mg/kg) for rice-containing baby foods6. Currently, dietary arsenic exposure is suspected to play a role in cardiovascular disease in adults7, and to disrupt the glucocorticoid system (involved in learning and memory) to those exposed in utero8. An in depth review of the current findings on the relationship between dietary arsenic exposure and human health is provided by Davis et al. (this issue).
In the United States, regulations on arsenic are distributed to several agencies. The Environmental Protection Agency (EPA) developed the MCL for arsenic in drinking water (10 μg/L) in 2006; a level supported by the World Health Organization, Canada and the European Union. In the state of New Jersey (USA) the limit is 5 μg/L, and in Australia, 7 μg/L. Many other nations still adopt a level of 50 μg/L (Bahrain, Bangladesh, Bolivia, China, Egypt, India, Indonesia, Oman, Philippines, Saudi Arabia, Sri Lanka, Vietnam, Zimbabwe)9, with the exception of Mexico (35 μg/L). In the USA, The Food and Drug Administration (FDA) is responsible for setting action levels for arsenic in food, which includes apple and pear juice at 10 μg/L, in line with EPA’s drinking water MCL. In Canada, the Canadian Food Inspection Agency issued alerts on excessive arsenic in rice and pear products in 2014. Consistent with the European Commission’s limit for arsenic in rice used in food production for infants and young children, the FDA is proposing an action level of 0.1 mg/kg for inorganic arsenic in infant rice cereal10. Foods in Australia and New Zealand may not contain more than 1 mg/kg dry mass of arsenic, and salt for food use must not contain more than 0.5 mg/kg. Japan has a limit of 15 mg/kg of arsenic in paddy soils11. Likewise, Thailand has an agricultural arsenic soil quality standard of 3.9 mg/kg. Within the USA, states differ widely in their action levels for arsenic in soil, for instance New Jersey has a cleanup criterion of 20 mg/kg and Florida has a cleanup target level of 2.1 mg/kg and 12 mg/kg for industrial sites12.
Arsenic occurs in food because it is present in soil and water and is taken up by plants. This review article brings together the latest scientific information on arsenic in agronomic systems, describing its sources in soils and the processes that influence the uptake of arsenic by crop plants. The intention of this review is to prompt a reconsideration and unification of government regulations on action levels for arsenic in agricultural soil; raise awareness of how both former and ongoing inputs of arsenic to soil can result in food contamination and impacts to human health and finally, to indicate the way forward for mitigation strategies that safeguard valuable soil resources.
2. Natural sources of arsenic in soil
Below toxic concentrations, the higher the total soil arsenic concentration (the sum of all arsenic species, regardless of bioavailability) the higher the crop uptake of arsenic. This is true of anaerobic cultivation systems such as rice13–15, aerobic horticultural systems16 as well as conventional (aerobic) agriculture15. The global average total soil arsenic concentration is 5 mg/kg, (equivalent to parts per million), but there is large variation between and within geographical regions17. Where soils have formed on arsenic-rich bedrock, or downstream of these bedrocks, very high concentrations of natural arsenic can result. Concentrations of up to 4000 mg/kg arsenic have been measured in soils from the arsenopyrite belt (iron arsenic sulphide, FeAsS) in Styria, Austria18, for instance. There are approximately 568 known minerals that contain arsenic as a critical component19. Arsenic is present in many rock-forming minerals because it can chemically substitute for phosphorus (V), silicate (IV), aluminum (III), iron (III) and titanium (IV) in mineral structures. Global mapping data of total arsenic concentrations in topsoil is not available, although large-scale regional maps are available for soil arsenic concentrations in Europe20 and the USA 21. European data predicts that most soils range < 7.5 – 20 mg/kg arsenic, with a median of 6 mg/kg20. This prediction comes from block regression-kriging; a spatial prediction technique based on regressing soil arsenic concentrations against auxiliary variables, and is useful because it uses a particularly high resolution (block size of 5 km2). On a continental scale, large zones of soils with approximately 30 mg/kg arsenic have been found in southern France, the north-eastern Iberian Peninsula and south-west England, with the two latter being zones of extensive natural mineralization associated with base and precious metal mining activities. The United State Geological Survey (USGS) soil sampling of the contiguous USA reports a mean soil arsenic concentration of approximately 5 mg/kg with 5 and 95 percentile values of approximately 1.3 and 13 mg/kg respectively22. Large regional patterns are apparent in the data, for example the soils of New Hampshire have soil arsenic concentrations of approximately 10 mg/kg arsenic, and Florida, 3.5 mg/kg. The sampling density goal for the USA surface soils and stream sediments database is 1 per 289 km2 23, but is currently at only 1 sample per 1600 km2. This contrasts with smaller regional surveys such as the recently published Tellus database for Northern Ireland that has a sampling density of 2 km2 24 (median total soil arsenic concentration 8.7 mg/kg). At this sampling density, fine-scale data for factors shown to affect soil arsenic, such as bedrock type, altitude and organic matter for instance, can be observed, providing the opportunity to make predictions about arsenic bioavailability and mobility.
Soil or sediment arsenic concentrations are the result of the complex and dynamic interplay between inputs and outputs25. Natural sources of arsenic to agronomic catchments are dominated by bedrock weathering (mechanical, chemical and biological) and depositional inputs, with the ultimate sinks at the base of catchments often being a significant distance from sources26. Outputs include leaching into water bodies (vertically and horizontally), soil erosion25 and biovolatilization27. In arid regions surface evaporation of water can lead to arsenic enrichment from the draw up of subsurface water25 and from waters used in crop irrigation28. Mass-balances (accounting for all inputs and outputs for a particular ecosystem) are rarely conducted for arsenic fluxes within catchment areas, but a good example is from a mining-impacted catchment area29, where chemical weathering, followed by mechanical weathering dominated arsenic inputs, which were primarily from arsenopyrite. Similarly, in a gold-mining region, weathering contributed an estimated 95% of the arsenic30. In a forested catchment area, where atmospheric arsenic inputs were the dominant source to highly organic soil (soils with more than 10% organic matter), inputs of arsenic via precipitation were ~6 g/ha/y31, and organic soils were a net source of arsenic, while mineral soils (less than 10% organic matter) were a sink. This agrees with depositional inputs of arsenic measured in the UK, which ranged from ~1 to ~10 g arsenic/ha/y32. UK regional scale maps show that arsenic deposition is highest at altitude and in the west of the country; the least polluted regions with air masses originating in the Atlantic. This suggests a marine source of arsenic. Depositional maps relate well to soil arsenic maps such as in maps of Northern Ireland and England33 that show highest arsenic concentrations in peat soils at higher altitude, along with bedrock geological anomalies. Peat soils at higher altitude are sinks for arsenic, and become sources if the peat is mineralized or eroded. The topic of upland organic soils acting as sinks and sources of arsenic is receiving more research attention34, and could be important on a regional scale as a source of arsenic to downstream sediments.
In large catchment areas of continental importance, such as the deltas that form to the south and east of the Himalayas, plate tectonic-derived mechanical weathering is thought to be the most important source of arsenic. One theory is that the mechanical weathering caused by Pleistocene tectonic uplift in the Himalayas is the key to understanding why arsenic is so elevated in Holocene aquifers, such as those of SE Asia, and in the glacial tills of Europe and North America26. Mechanical weathering of bedrock exposes previously inaccessible mineral surfaces, and the finer grinding leads to enhanced surface areas for chemical and microbial weathering to take place, causing greater solubilisation of arsenic25, 26, 35. Chemical and microbial weathering can take place at or near the source, or in sediment sinks. For instance, bacteria isolated from Bay of Bengal aquifers can mobilize arsenic from apatite35 (See Section 5). Invariably, the arsenic loadings into soil will be dependent on arsenic in the bedrock, and the extent of the weathering of that bedrock-derived material along the route from source to sink. Soils with basalt bedrock had the lowest median arsenic content, while those with psammite, semipelite, and lithic arsenite bedrocks had the highest. Interpretation of such fine-scale mapping can ultimately lead to predictions of soil arsenic concentrations where detailed maps are not available. Combined with an understanding of soil chemistry, this will enhance the ability to predict elevated concentrations of arsenic in crops36.
3. Anthropogenic sources of arsenic to soil
Many anthropogenic activities have increased soil arsenic concentrations above the natural, background levels mentioned in Section 2 above, and they have the potential to increase the arsenic concentration in food. This is especially the case in the USA where the widespread use of arsenic-based herbicides, pesticides and livestock antibiotics throughout the 20th century has ultimately increased the arsenic concentrations of current productive USA agricultural soils37–39.
3.1. Base and precious metal mining
The dominant mineral source of arsenic is thought to be pyrite (iron sulfide, FeS2)40, an economically important ore deposit. High arsenic concentrations are found in many oxide minerals and hydrous metal oxides, either part of their structure or as sorbed and occluded species41. Iron oxides accumulate arsenic up to concentrations of several weight percent (1 weight % being equivalent to 10,000 mg/kg), and arsenic tends to bind to iron (III) (hydr)oxides whenever they are present. Arsenic is found predominantly as arsenopyrite but also can occur as orpiment (arsenic trisulphide As2S3), realgar (α-As4S4) and other arsenic sulfide minerals42, 43. Arsenic is a byproduct of most mining operations and is present at high concentrations in the mine waste, and, because arsenic sulfides are particularly prone to oxidation in surface environments, in mining wastewaters42, 44. Arsenic can constitute 1% or more of the ore and solid waste, and wastewaters and impacted streams often contain dissolved arsenic concentrations ranging from 0.01 to over 10 mg/L. Because mining and smelting operations are localized, arsenic contamination of soils exists around the mine site with the concentration decreasing with distance from the source. Windblown dispersion of fine particulate material is a particular problem, spreading contamination greater distances from the mine site. This fine material - which is not completely removed by washing16 - can directly contaminate plant material; especially leafy material with high surface area. This presents a tangible risk to residents and home gardeners in the vicinity of areas with significant surface soil arsenic contamination. A comparison of arsenic concentrations in vegetables grown in SW England (the site of historic mining activities) with those from a pristine site in NW Scotland found a generally good correlation between total plant arsenic and soil arsenic concentrations. Increased arsenic concentrations were measured in produce from SW England where soil arsenic concentrations ranged from 120 – 1130 mg/kg. Arsenic concentrations were high in leafy greens (kale, spinach, lettuce) and some unpeeled vegetables (potatoes, swedes, carrots) were higher than when peeled, which, in both cases, points to contamination from windblown soil particles and soil adhesion to below ground biomass, rather than from root uptake. In this particular study, the majority of arsenic was present as the inorganic form16. Similar results were obtained from home gardens near the Iron King Mine Superfund Site in Arizona, USA45, 46. Here the tailings had arsenic concentrations of 3,710 mg/kg and residential soil sampled adjacent to the site ranged from 120 – 633 mg/kg. Edible plant tissue concentrations ranged from < 0.01 – 1.96 mg/kg (plant concentrations are expressed as dry weight throughout), and were generally positively correlated with soil arsenic concentrations. Leafy and high surface area vegetables such as lettuce, kale, broccoli and cabbage accumulated higher arsenic concentrations than beans, tomatoes, cucumbers and peppers. Arsenic in mine-affected vineyard soils in Italy ranged from 4 – 283 mg/kg and positive correlations were observed between soil concentrations and arsenic levels in vine leaves and grapes, however, levels in wine were low (< 1.62 μg/L)47. In the Hunan province, China, the high levels of inorganic arsenic in rice have been traced to mining activities in the area48–50.
3.2. Coal combustion for energy
The concentration of arsenic in USA coal ranges from 1 – 71 mg/kg with an average concentration of 24 mg/kg51. Fly ash, the major byproduct of the coal combustion process, consists of fine particles that are driven out with the flue gases, and is a major source of arsenic to the wider environment. Coal ash is one of the most abundant of industrial wastes; close to 130 million tons52 of coal fly was generated in the USA in 2014, with 100 million tons estimated from the European Union in 201153. Arsenic concentrates in the fly ash during combustion of coal for energy; the median arsenic concentration in USA fly ash is 71 mg/kg54. Fly ash is often sluiced into settling basins, and because arsenic in fresh ash is quite soluble, wastewater arsenic concentrations can consequently be quite high. Arsenic can build up in the sediments of coal fly ash settling basins and reach concentrations of over 1000 mg/kg. Catastrophic failures of these setting basins have caused severe environmental problems and contaminated surface waters with arsenic55. There is a well-founded concern that arsenic from coal combustion wastes can contaminate soil and enter the food supply. The use of coal fly ash as a soil amendment can lead to elevated arsenic concentrations in crops (as well as boron, selenium and molybdenum), although its lack of soil macronutrients and the potential for arsenic toxicity prevents the sole application of coal fly ash as a soil amendment56, 57. Formulating ash/organic waste mixtures that conform to USEPA regulations for total arsenic application and meet soil and plant fertility requirements has been shown to safe and effective for agronomic use58.
3.3. Pesticides
Perhaps the largest anthropogenic input of arsenic to agricultural soils in the USA is from the agricultural use of arsenic-based pesticides and herbicides for most of the 20th century. Calcium arsenate and lead arsenate were used extensively up to the 1950s, mostly on orchard soils to combat the codling moth. At peak, 132,000 metric tons of each pesticide compound was applied annually between 1930–194037. In addition to apples, inorganic arsenic pesticides were used on a range of crops including essentially all fruit trees, vine berries, sweet potatoes, white potatoes, most vegetables and cotton37. Both lead and arsenate have long residence times in soils and high concentrations (often >100 mg/kg) of these two elements have been reported in old orchard soils in Washington59, North Carolina60, New Hampshire61, New Jersey62 and Virginia63. There is some evidence of greater mobility for arsenic (than lead)61, 64, and retention of both elements depends on soil type and other environmental factors but most of this legacy contaminant remains in the soil62. Use of lead arsenate decreased after 1950s and was finally banned in 1988. The organic arsenic compounds dimethylarsinic acid (DMA) and monomethylarsonic acid (MMA) were used as pesticides on cotton and herbicides for golf courses and right-of-ways until they too were withdrawn from use in 2013. High levels of MMA were reported in transient surface waters adjacent to a crop sprayer operation65. Legacy soil arsenic contamination resulting from organic arsenical pesticides plays a major role in straighthead disease of rice66 (See Section 6.1). It may be that arsenical pesticides have leached to groundwater, as has been suggested for the Texas High Plains Aquifer67, although a study of the Ogallala aquifer in the High Plains in Texas found no evidence of anthropogenic arsenic in the groundwater68. Similarly, there was no relationship between groundwater arsenic and past (inorganic) arsenic pesticide usage in a comprehensive study of New Hampshire groundwater sources69. About 10%, depending on soil substrate, of monosodium methyl arsenate applied to sandy soils (simulated golf course greens) leached into percolating water. Demethylation and methylation occurred because both inorganic arsenic species and DMA were also detected in the percolating water70. As with mining-impacted soils, plants grown on soils that are high in arsenic from arsenical pesticide contamination take up higher levels of arsenic into their edible tissues, observed for example in potatoes71, carrots72 and leafy green vegetables73, 74.
Former pesticide application has been suggested be a factor in the presence of higher levels of total arsenic found in rice grown in the south-central regions of the USA75, 76 compared to other areas of the USA and to other countries, such as Bangladesh77. Evidence on varietal differences in arsenic uptake, speciation and distribution within rice grain (See also Section 6) strongly suggest that soil arsenic concentration is not the sole, nor particularly the main driver of this phenomenon. Factors likely to be influential include the differences in the soil microbial community composition between geographical regions that affect arsenic methylation, considering that plants themselves cannot methylate arsenic78.
3.4. Wood preservatives
Chromated copper arsenate (CCA) is used as a wood preservative and was extensively used on decking and other residential usages until a voluntary manufacturer withdrawal in 2003. The primary health concern is for young children in direct contact with CCA-treated wood, but localized leaching of arsenic (as well as chromium and copper) also occurs to surrounding soil. Soil arsenic concentrations of 37 – 250 mg/kg have been reported for soils sampled near CCA-treated utility poles (N=12)79 and mean arsenic concentrations for soils collected below decks and footbridges in Florida, USA was reported to be 28.5 mg/kg compared with a control concentration of 1.3 mg/kg (N= 65)80. Arsenic from CCA contaminated soils appears to be more bioavailable than from other anthropogenic sources to soil81.
3.4. Organic manures
Land application of sewage sludge (biosolids) in the USA is regulated by Environmental Protection Agency Part 503 Biosolids rule; which set the maximum arsenic concentration of the sludge at 75 mg/kg, an annual pollutant-loading rate of 2.0 kilograms arsenic/hectare (kg/ha) and a cumulative pollution-loading rate of 41 kg/ha over the lifetime of applications. Assuming a plow layer of 17 cm, application at the maximum annual rate implies an approximate 1.2 mg/kg maximum increase in soil arsenic, while the cumulative maximum loading rate could increase soil arsenic concentrations by approximately 24 mg/kg over the lifetime of application and assuming no loss from the soil profile. This cumulative loading rate of 24 mg/kg is significant when considered against an average soil arsenic concentration of 5 mg/kg (See Section 2), however, relative to mine-impacted or inorganic arsenic pesticide impacted soils where arsenic concentrations are frequently > 100 mg/kg, it is of lesser concern. Also, sewage sludge is often high in aluminum and iron oxide phases, used in the flocculation process, which are efficient scavengers of inorganic arsenic thus lowering the arsenic bioavailability 82.
Arsenic occurs in animal wastes primarily because of the former use of arsenic antibiotics in poultry and turkey feed; until 2015 four drugs, roxarsone, p-arsanilic acid, carbarsone and nitrosone, were regulated for use, with roxarsone being the most prevalent. As of 2016 all four of these compounds have all been withdrawn from use83. All four are organic arsenic compounds with an arsenate functional group attached to a benzene ring, and differ by other substituents on the ring. The compounds are not readily adsorbed or metabolized and so occur at concentrations up to 40 mg/kg in animal manures. This provides three points of entry to the human food chain; directly through arsenic in chicken and turkey meat38, 39, 84, from plant uptake after land application of manure, and runoff to surface water or groundwater. A number of studies have shown that these organic arsenic compounds can be degraded by both photolytic85 and microbial86 processes and that this degradation happens both during composting of stockpiled litter87, 88 and after land application89, 90. Long term application of poultry litter to Upper Coastal Plain soils increased soil arsenic concentrations from 2.7 to 8.4 mg/kg after 25 years of application91. Similar increases have been reported for other southern states of the USA92. There is evidence to suggest that other soluble constituents of the litter, for example phosphate and dissolved organic carbon compounds, facilitate arsenic solubility and leaching89, 92, 93.
3.5. Seaweed fertilizers
Seaweeds can contain far higher concentrations of arsenic than crop plants: up to 100 mg/kg (Taylor et al, this issue). In most cases the arsenic is present as arsenosugars, which are of low toxicity to humans (Taylor et al, this issue). However, as in the case of poultry litter, these compounds degrade (ultimately) to inorganic arsenic after land application94. Although seaweeds are a ‘niche’ soil amendment, their use agriculture is increasing and has been adopted by many organic farms as a soil fertilizer as well as a feed additive in organic dairy farming95.
4. Biogeochemical cycling within terrestrial agronomic ecosystems
Arsenic cycles within the soil surface and near-surface environment96, influenced by mineralogy, abiotic factors such as pH and redox potential (EH), and biotic factors such as microbially-mediated biomethylation.
4.1. Redox regulation
The most important biogeochemical step in the exposure of humans to arsenic is its release from soils and sediments into pore water; the water contained within soil pores and/or rock40. With the exception of extreme pH conditions (<4 or >9), or high concentrations of competing ions (e.g. phosphate, silicic acid or silicate97) the release of arsenic from its strong bonds with soil particles depends upon redox potential (EH); the extent of aeration of the soil40. As EH falls, electron acceptors are depleted and anoxic conditions develop, causing iron oxides and oxyhydroxides to be reduced and dissolve, releasing sorbed arsenic into the soil solution98 where it can be taken up by plant roots, or leached into groundwater.
Agronomic cropping systems can be divided with respect to arsenic mobilization on the basis of their redox status. Dominant biogeochemical processes influencing aerobic systems, specifically cereals, upland rice, fruit tree orchards, and community gardens, differ from those that dominate in anaerobic systems, predominantly in flooded rice paddies. In aerobic soils, arsenic speciation is predominantly arsenate (arsenic (V)), and is tightly bound to soil particles. Under anaerobic or flooded conditions, arsenic is reduced, and arsenite (arsenic (III)) is the dominant species40. Arsenite is less stably bound to aluminum hydroxides and aluminosilicate clay minerals in the soil than arsenate, for which they exhibit a much stronger binding preference40. With few exceptions (such as under conditions of sulfur release), transition of arsenic speciation from arsenate to arsenite is the most influential factor to arsenic bioavailability; and it is under anaerobic conditions where arsenic becomes an imminent human health concern. Influential biogeochemical processes in aerobic systems are ageing and accumulation of arsenic in soil, and in anaerobic systems reductive dissolution of iron-bearing minerals is the dominant process.
4.2. Biotransformation to methylated and volatile species
Volatile arsenicals are arsenic species with a boiling point below 150°C; the mostvolatile of which is arsine gas (AsH3), followed by monomethylarsine (MeAsH2), dimethylarsine (Me2AsH2) and finally completely methylated trimethylarsine (TMA). Volatile arsenic species can be formed either biotically – by fungi, bacteria and algae27, 99, 100 or abiotically99. In natural systems arsines readily react with oxygen to form non-volatile oxidation products, with AsH3 most rapidly oxidized and challenging to detect in environmental samples. Oxidation of the arsine gases to inorganic arsenic species completes the arsenic cycle, with arsenic returned to the soil by rain or dry deposition101.
Arsenic methylation in soils increases with decreasing redox potential102, and addition of organic matter. Increased arsenic volatilization was measured in soil after the addition of rice straw103, and animal waste products104. Inoculation of fungi (Penicillium and Ulocladium spp.) increased arsenic volatilization up to 8 fold in heavily contaminated and spiked soils105. Microbially mediated arsenic volatilization remains very inefficient, which hinders attempts to use it in soil remediation. Gaseous arsines are volatilized from arsenic contaminated soils into the atmosphere at very low rates: a microcosm study found 0.5 – 70 μg of arsenic kg−1 soil year−1 was volatilized from a range of soils and a range of arsenic levels27, and field measurements of arsenic volatilization are 1–2 orders of magnitude lower than those made in laboratory mesocosms 98. Genetic transformation of bacteria, using genes encoding for the protein product arsenite S-adenosyl methyltransferase (arsM) is an attempt to enhance arsenic methylation and volatilization. The arsM from Rhodopseudomonas palustris was expressed in Sphingomonas desiccabilis and Bacillus idriensis grown in an aqueous system, resulting in a 10-fold increase in arsenic volatilization compared to the wild type strains. In a soil-based system, 2.2 – 4.5% of arsenic was removed via microbially-mediated volatilization over an incubation period of 30 days106 (See also section 5.3).
4.3. Changes in soil arsenic bioavailability due to ageing
Although arsenic in aerobic soils has a lower bioavailability and presents less of an immediate concern for crop uptake, aerobic soil can accumulate arsenic from human inputs, retain them for long periods of time, and release them when redox conditions change (See Section 3.3). Human inputs of arsenic, as discussed in Section 3, are diverse; biosolids, sewage sludge, coal fly ash, poultry litter, industrial waste, arsenical pesticides and from irrigation with naturally arsenic-enriched groundwater. For aerobic soils, ageing – where binding stability of arsenic to soil particles increases over time, is a particularly important part of arsenic cycling. Factors controlling ageing of arsenic include soil type, organic matter content and arsenic species. Both inorganic and organic arsenic species are subject to ageing, with studies indicating a slow oxidation process from arsenite to arsenate over time107.
5. Rhizosphere processes
Processes occurring in the rhizosphere (the boundary layer of soil under the influence of plant roots) dramatically influence arsenic concentrations and bioavailability because they involve local alterations in redox potential, pH and organic matter content. Rhizosphere acidification occurs during iron uptake by all plant species during cation uptake and charge balance, when protons are released into the rhizosphere. Plants release anywhere from 10 to 250 mg of carbon per gram of root tissue into the rhizosphere; about 10–40% of their total photosynthetically fixed carbon108, making the rhizosphere particularly rich in organic carbon compared to bulk soil, which in turn exerts an influence on arsenic solubility by stimulating microbially-mediated reductive dissolution of soil minerals. Large differences have been found in the arsenic concentration of rhizosphere soils compared with bulk soils in highly arsenic-contaminated areas, with higher concentrations of arsenic in rhizosphere soils compared to bulk soils109.
In anaerobic soils, the iron plaque that develops on the submerged stem and roots of rice plants dominates rhizosphere dynamics of arsenic. In flooded environments such as paddy fields, plants oxygenate the rhizosphere through specialized tissues called aerenchyma, which are found in many aquatic plants and emergent macrophysics such as rice. This radial oxygen loss creates an oxidized layer around plant tissue that stimulates aerobic microbial activity and the oxidation of iron, which precipitates and forms a visible iron plaque on the root surface 110–114. Formation of an iron (oxyhydr)oxide plaque on root surfaces can alter the uptake of arsenic by rice, acting as a sorbent for excess nutrients such as ferrous iron (reduced iron) as well as arsenic and aluminum115. Rates of oxygen loss influence iron plaque formation 115, and vary between rice cultivars116, 117. Studies conducted over the last forty years are inconsistent on whether iron plaque prevents or enhances arsenic uptake by plants111, and the hypothesis that arsenic influences the quality and amount of the iron plaque113. Profound differences in mineral composition and quantity of laboratory-created iron plaques has been demonstrated experimentally111, which may have contributed to these inconsistencies.
5.1. Microbial activity
Microbes directly and indirectly influence arsenic speciation in rhizosphere soil, and are widely considered to play a key role in arsenic biogeochemistry118. Under certain nutrient-limited conditions, microbes actively weather minerals to access nutrients for cellular growth, which releases arsenic35, as well as creating abiotic conditions that induce changes in arsenic speciation via production of organic acids, polysaccharides and ligands. Soil microorganisms can strongly affect soil redox, regulating arsenic release into pore water119. A number of strains of bacteria have also been shown to contribute to the formation of arsenic minerals by using arsenic as a terminal electron acceptor, such as Desulfosporosinus auripigmentum120, Desulfovibrio strain Ben-RB121, Shewanella oneidensis122 and S. putrefaciences CN32123. These microorganisms also differ in their capabilities for liberating arsenic from specific arsenate-bearing minerals119.
Microbial transformation can mobilize arsenic by converting inorganic to organic forms, including MMA and DMA124, 125. Plants translocate organic arsenicals from roots to the (frequently edible) above-ground parts more efficiently than inorganic arsenic126–128 (See Section 6), therefore microbial transformation to organic arsenicals can increase human dietary exposure.
Plants, green algae and microbes can all enzymatically transform arsenic species124, 129, but methylated forms of arsenic detected in plants are a product of rhizosphere bacteria; plants cannot methylate arsenic78, 124, 130, 131. The genomes of more than 85 arsenic-metabolizing archaea and bacteria have been sequenced for genes involved in arsenic metabolism132. In bacteria, archea and fungi, arsenic methylation is catalyzed by homologs of arsM, (See Section 4)124. Resistance to arsenite and arsenate exists in nearly all microbes, which also confers the ability to transform arsenate into volatile arsine gases133, a particularly effective way of removing arsenic.
Profiling the transcriptome, proteome and metabolome of arsenic contaminated soils offers way of understanding microbially-mediated rhizosphere arsenic processes132. This approach measures the presence and expression of specific genes, rather than attempting to isolate and study the microbes that carry them, 98% of which – it is estimated - do not grow in culture134. Microbially mediated arsenic metabolic processes that play a major role in arsenic cycling in agronomic systems include arsenite oxidation (via the aio genes), arsenate respiration (via the arr genes), arsenate reduction (via the ars genes) and arsenite methylation (via the arsM genes)135. Interested readers are referred to the recent excellent work of Andres and Bertin132 for a comprehensive review of this subject. Microbially mediated redox processes strongly influence arsenic uptake in rice, involving aioA, arsC and arrA 124, with pH emerging as an important factor in the distribution of microbes in paddy soils. Testing a variety of soils has shown that bacteria possessing the arsM gene for methylating arsenic are widespread and phylogenetically diverse, and even in paddy soils with low concentrations of arsenic, genes for arsenic metabolism are abundant124.
6. Arsenic and crop plants
Much of our understanding about the physiological mechanisms of arsenic uptake in plants comes from the study of a limited number of species. Called model plant species, they are extensively studied, well described, easy to grow, and the results can be compared between studies. The understanding is that the information gained from studying model plants is applicable to other plant species. From a genetic perspective, orthologous genes exist in different plant species that have evolved from a common ancestral gene, and they usually retain the same function. Characterization of arsenic-related genes in a model plant strongly suggests that they exist and perform similar functions in other species. Caveats to this are their levels of expression, which makes some plants more adept at accumulating arsenic than others. In this section, much of the knowledge gained on arsenic uptake and metabolism of plants comes from the study of mouse-eared pennycress (also called thale cress or rockcress) (Arabidopsis thaliana Heynh.) and rice (Oryza sativa L.); model plants with fully sequenced genomes. These species represent dicotyledonous (e.g. flowers, vegetables, deciduous trees) and monocotyledonous plant species (e.g. grasses, palm trees) respectively, thereby representing much of the edible crop species. An exception to this is the study of the arsenic hyperaccumulating fern (Chinese Brake fern, Pteris vittata), a seedless plant that is able to accumulate up to 22,630 mg/kg (dry weight) arsenic in its fronds136.
6.1. Phytotoxicity of arsenicals
Arsenic is toxic to plants137. Despite lower acute human toxicity of the organic arsenicals (median lethal dose is 700–1,600 mg/kg and 700–2,600 mg/kg for MMA and DMA respectively compared to 10–20 mg/kg for inorganic forms)138 no one form of arsenic is consistently more toxic to plants139. Soybean yields are affected when tissue arsenic levels exceed 1 mg/kg, and 4 mg/kg limits cotton yields140, whereas in barley tissue concentrations of 20 mg/kg inhibited growth141. Higher yield-limiting arsenic levels have been recorded in rice: 20–100 mg/kg in above ground biomass, and 1000 mg/kg in root tissue142. By contrast, potatoes (Solanum tuberosum L.) suffered no growth inhibition in soils containing 290 mg/kg arsenic71. In some plants species, organic forms are more toxic than inorganic, for example in rice (order of toxicity: MMA > arsenite > arsenate = DMA)143, and in smooth cordgrass (Spartina alterniflora Loisel) (DMA = MMA > arsenite > arsenate)144.
Plants vary in their tolerance to arsenic, and the stress response differs for each arsenic species145–147. The chemical similarities between arsenate and phosphate means that arsenic can replace phosphate in biomolecules like ATP (adenosine triphosphate, a molecule used for intercellular energy transfer), with negative impacts on growth and metabolism148. In rice in particular, DMA and MMA induce straighthead disease (arsenic-associated straighthead disease), significantly lowering yield of certain rice varieties66. Straighthead is a physiological disorder of rice characterized by sterile florets, which remain upright at maturity instead of bending over under the weight of the filled grain. The exact cause of straighthead is unknown, but consistent flooding, low soil pH, high iron availability and high organic matter content have all been implicated in naturally-occurring straighthead disease66. Arsenic’s suspected role in straighthead comes from observations of more frequent outbreaks in rice grown in soil where arsenical herbicides such as monosodium methanearsonate (MSMA) – used in cotton production in the USA – have been historically applied.
6.2. Arsenic uptake mechanisms
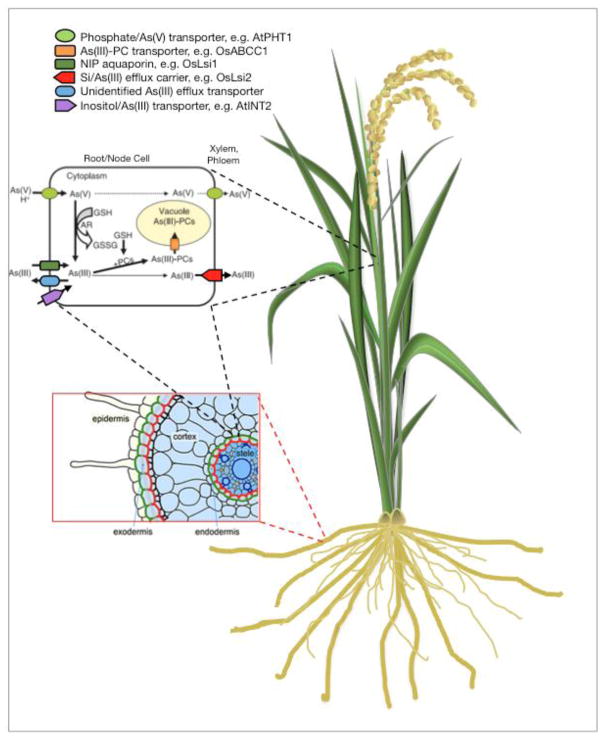
In magnitude, plants take up arsenicals from the soil in the order arsenite > arsenate > DMA > MMA149, 150), with the various arsenic species entering via different root membrane transport proteins in the root plasma membrane that allow ions and molecules to cross with varying levels of selectivity, or target specificity. Similarities in chemical structure between arsenate and phosphate, and between arsenite and silicic acid, govern their entry into root cells. Arsenate enters root cells through phosphate transporters (the Phosphate Transporter 1 family of proteins; PHT1) in both the model plant Arabidopsis thaliana151, 152 and in rice153–155 (Figure 1). In rice, Low Silicon 1 (OsLsi1) and OsLsi2 are silicic acid transporters and arsenite, MMAV, and DMAV are among their unintended targets156, 157. These Nodulin 26-like Intrinsic Proteins (NIPs)158, which are members of the aquaporin water channel superfamily of proteins159 embedded in the exodermal cell membranes of rice roots, move arsenic from the soil into the vascular system for distribution to the stem and leaves. OsLsi2 works in tandem with OsLsi1 to transport arsenite inward toward the xylem160, 161 (vascular tissue that conducts water and dissolved nutrients up from the roots). The arsenic uptake specificity of OsLsi1 is arsenite ≫ MMA > DMA158. These bidirectional NIP transport proteins also efflux arsenite back in to the soil, but since OsLsi1 effluxes only 15–20% of the arsenite in roots cells162, there may be other unidentified arsenite efflux transporters contributing to this process.
Figure 1.
Generalized diagram of arsenic uptake, transport and metabolism in plants. GSH, glutathione; AR, arsenate reductase; GSSG, oxidized glutathione; PC, phytochelatin. Modified from Zhao et al.171 and Ma et al.172.
6.3. Arsenic transport and metabolism in plants
Transport of arsenite into the xylem for delivery to the shoot is less well characterized than its uptake from the soil. Arsenic is transported to the grain mainly via the phloem126–128 (vascular tissue that conducts sugars and metabolic products from the leaves), by transporters in the nodes163, but their characterization is still in the early stages. Transporters for myo-inositol (Inositol Transporter 2 and 4); an important sugar for developing rice grains, also transport arsenite into the phloem companion cells164, 165. In Arabidopsis, INT2 or AtINT4 load about 45–64% arsenite into the grain166. The identity of transporters that move arsenite out of the phloem and into the grain are also unknown, but manipulating the target specificity of the INT genes might show promise in molecular genetic or plant breeding mitigation efforts as a way to prevent arsenite from reaching the grain.
Despite having a lower affinity for transporters into the plant than the inorganic forms, organic arsenic species are more efficiently transported towards the shoot than inorganic forms149, 150 because they are not complexed by phytochelatins (PCs); sulphydryl-rich glutathione (GSH) polymers167, 168. Likewise, in broad beans (Vicia faba L.) grown in a soil containing 90% inorganic arsenic, DMA and MMA were the dominant arsenic forms in the bean (68%)169. In root vegetables, carrot (Daucus carota L.) and beet (Beta vulgaris L.) grown on arsenic-contaminated soils, arsenic forms were predominantly inorganic, but for beets in particular were not readily identified using the typical standards (arsenate, arsenite, MMA and DMA)170.
The arsenic species composition of rice grain is influenced by the arsenic transport rate of the particular cultivar173, 174. Rice cultivars currently grown in the USA have an arsenic speciation split approximately equally between inorganic arsenic and DMA, while cultivars grown in Bangladesh contain mostly inorganic arsenic174. While lower inorganic arsenic in rice grain seems favorable for avoiding human health effects, the assumed safety of DMA is contentious175, being based on acute toxicity data, and not on genotoxicity or carcinogenicity, which are equally relevant in long term safety considerations.
Arsenic detoxification inside cells uses a multi-step process beginning with reduction of arsenate to arsenite using an arsenate reductase enzyme176, 177. In Arabidopsis, the protein High Arsenic Content1 (HAC1; also called Arsenate Reductase QTL1; ARQ1) reduces arsenate177. Even though arsenite is more toxic than arsenate158, 178, 179, it is hypothesized that ancestral organisms to plants were exposed almost exclusively to arsenite before atmospheric oxygen enabled arsenate formation180, and this mechanism persisted through natural selection. Arsenite is then complexed by PCs, and transported in to the vacuole167 via ATP Binding Cassette tranporters181, 182. This process depletes glutathione availability, rendering the plant more susceptible to other oxidative stresses, which inhibits photosynthesis, pigment production, and the integrity of cell membranes183–186.
7. Limiting arsenic uptake by crops
7.1. Water management
Although the traditional method for cultivating rice involves flooding leveled, tilled fields before or shortly after planting germinated seedlings, flooded soil is not a biological requirement of rice plants. Flooding is used for weed and vermin control, for mobilization of key nutrients such as iron, phosphate and zinc, and importantly, flooding discourages the buildup of root nematodes over multiple years of rice growth. As mentioned earlier, flooded conditions mobilize soil-bound arsenic through reductive dissolution of Fe (oxyhydr)oxides, and the reduction of arsenate to the more mobile arsenite187. Water management strategies that involve periods of oxic soil conditions can decrease arsenic uptake in rice by limiting dissolution of arsenic. Rice grown in non-flooded or aerobic conditions has a lower yield than intermittently or constantly flooded rice188–190. Intermittent flooding (flooding maintained until full tillering, followed by intermittent irrigation) is a promising management technique to reduce arsenic levels, and can potentially produce higher grain yields than either non-flooded or constantly flooded conditions191. However, oxic conditions increase cadmium concentrations in the grain when grown in acidic soils191–193, and cadmium is also a highly toxic metal. The observed increases in cadmium were also a cultivar-specific trait, but the increase in cadmium uptake between rice grown under aerobic conditions were approximately an order of magnitude greater than their flooded counterparts. Pot experiments suggest that water management strategies implemented during the heading period of rice growth (when the rice panicle has emerged from the stem and is fully visible, just before flowering) can regulate both arsenic and cadmium concentration in the grain190, 192.
7.2. Amendment and fertilization practices
Soil amendment involves incorporating substances into the plow layer that either add missing nutrients, reduce the bioavailability of existing potentially toxic substances (to prevent crop uptake), or both. Soil amendments that have shown potential in reducing arsenic uptake by plants include iron-, and silica-based additives. The use of iron-based amendments increases in the concentration of free iron oxide in the soil, retarding the release of arsenite from the solid phase into soil solution, (mentioned in Section 4.1 and discussed in Section 5), whereas silica fertilization inhibits arsenic uptake by competitive inhibition at the plant root surface while adding an essential nutrient.
Zero valent iron powder (90% iron) and iron oxide (56% iron) incorporation prevented uptake of arsenic in to the grain of rice grown on soil containing 39.5 mg/kg total arsenic by approximately 45%, and corresponded with a reduction in bioavailable arsenic in the soil194. Amendment with iron oxides (at a rate of 2%) was also more effective at reducing grain arsenic than phosphate amendment195. Amendments have also been used in combination with water management strategies to try and reduce both arsenic and cadmium concentrations in rice simultaneously193, without success. Reduction of arsenic in the grain was achieved with iron oxide addition and constant flooding, whereas cadmium reduction was achieved with converter furnace slag addition and rain water management (no irrigation after midseason drainage until harvest).
A combination of ethylenediaminetetraceetic acid ferric sodium salt (iron EDTA) and calcium peroxide was effective for reducing arsenic uptake by vegetable crops (lettuce, Chinese cabbage and radish) from soils containing 14 mg/kg total arsenic196, again by increasing amorphous aluminum and iron oxides. It is likely that this this level of arsenic contamination would be deemed too high for commercial vegetable production, so these amendments may only be feasible for use in private vegetable gardens. Questions remain about whether iron oxide amendment application only temporarily reduces arsenic bioavailability197. In addition, the suitability for arsenic immobilization is highest at the lower soil pH range, and is strongly affected by soil phosphorus concentration, which strongly competes with arsenic.
Rice plants take up high concentrations of silica, constituting up to 10% of dry matter in the straw and husk of the plant198. As mentioned earlier (Section 6) the silicon membrane transporter (Lsi1) is the main route of arsenite entry in to rice root cells, and provision of silicon causes competitive inhibition of arsenite uptake. Increasing silicon availability in the soil also reduces the expression of the Lsi1 transporter in the plant, which further decreases the potential for arsenic uptake. Fertilization of rice paddy soils with silicon is a potential mitigation strategy for preventing or reducing arsenic uptake by rice through competitive inhibition of arsenite uptake199. The use of synthetic silicon fertilizers, such as calcium silicate or silica gel is prohibitively expensive for smallholder farmers in developing countries, however reusing the silicon-rich parts of the rice plant that remain after harvesting and grain processing may provide a sustainable solution that also addresses the ongoing issue of silicon depletion of the soil198. Soil incorporation of fresh rice husks, or the ash that remains after burning the husk and straw for energy (which is a common practice for smallholder farmers), can provide silicon without increasing methane production and decreases either total or inorganic arsenic in rice grain200. Despite the potential of soil amendment with iron oxides or silica to reduce arsenic bioavailability or prevent plant uptake of arsenic, the high cost of these amendments inevitably prevents their use, especially by smallholder farmers. Large rice producers in the US or Europe have not so far adopted widespread use of these soil amendments to reduce rice grain arsenic concentrations. It is also reasonable to assume that use of expensive soil amendments would drive up the cost of rice. Their lack of use may also be attributable to the fact that iron amendments are essentially untested in a diverse range of large-scale agricultural settings and their performance will vary between soil types. In non-rice agricultural systems arsenic is tightly bound to the solid phase; significant crop uptake from oxidized soil is likely to be a result of extreme contamination, in which case effective mitigation is restricted to redirecting land use away from edible crops. In systems subject to periodic flooding, improving drainage remains the best mitigation strategy.
7.3. Mitigation using plant breeding approaches
The development of crops that accumulate high levels of arsenic and yet remain healthy, while preventing arsenic from reaching the edible grain is thought to hold great potential as a strategy for reducing human exposure to dietary arsenic. The use of molecular genetics techniques such as alterations in gene expression characteristics, gene editing to alter target specificity, or alternately, using traditional plant breeding techniques are both tangible approaches. Both use knowledge of the arsenic uptake and tolerance characteristics of plants to develop varieties with desired characteristics. These characteristics include lower arsenic uptake201, higher arsenite efflux202 and increased vacuolar arsenic sequestration203. For instance, many rice cultivars have now been screened to identify those that accumulate lower levels of arsenic in their grain and efforts are underway to identify the genes underlying this trait201, 204. Overexpressing Arabidopsis ABC-type transporters that sequester arsenite-PC complexes in the cell vacuole results in plants able to grow in otherwise toxic concentrations of arsenic182. Conversely, knocking out the function of the related rice ABC transporter OsABCC1 results in higher levels of grain arsenic. The OsABCC1 transporter limits arsenic transport to grains by sequestering arsenic in the vacuoles of the phloem companion cells directly connected to the grain. By combining what we have learned from the overexpression studies in Arabidopsis and the loss-of-function study in rice, overexpression of OsABCC1 can be used as a strategy to breed arsenic tolerance and low-arsenic accumulating rice cultivars. Another promising strategy is based on expressing the arsenate efflux transporter from yeast (Saccharomyces cerevisiae) in rice, which can reduce arsenic accumulation in brown rice by 20%. A less successful idea to methylate sodium arsenite to DMA by expressing an algal arsM gene in Arabidopsis resulted in lethal phytotoxicity205, suggesting that arsenic methylation in plants can only be an effective detoxification strategy if volatile arsines are the end point of the methylation.
8. Conclusions
The discovery of arsenic in staple foods, beverages and other products has increased awareness and stimulated research on the sources and the processes involved. The information brought together here illustrates the numerous geochemical and biological processes that influence the movement of arsenic into the food supply. It is clear there must be strategies for preventing arsenic exposure, that operate in both the short term – to protect consumers from existing contamination – and in the long term, to prevent further contamination. This requires government regulation on the permissible levels of arsenic food, with lower levels for infant foods (see Nachman et al, this issue), which must work in tandem with long term goals to address arsenic in agricultural soils, actively prevent further inputs and identify contaminated areas for mitigation. Our recommendations are that the information in this review is used to inform a reconsideration and a unification of regulations on the action levels of agricultural soil arsenic, which in the USA for example, exist only at the state level, vary widely from state to state, and have no formal channels of enforcement. We recommend that educating the community and garnering their support and involvement for lowering exposure to arsenic through food is an approach already shown to hold enormous potential. Direct involvement of the commercial rice growing community in research and development of arsenic mitigation strategies and amendments is needed. Much effort has been given to short-term, greenhouse-scale testing of amendment formulations that will ultimately be too expensive, impractical, or ineffective in the long term. Community-based participatory research should extend to the agricultural community, leading to partnerships that will make longer-term field-scale testing of mitigation strategies accessible. Feasibility should be the first consideration in arsenic mitigation research. Community outreach efforts targeted to commercial growers or the home gardener specifically must raise awareness of the significance and potential impacts of former land uses, encouraging testing for the presence of arsenic in the soil and educating growers on crops shown to accumulate arsenic in their edible parts. Information gathering on former arsenic input into the soil from pesticides and from proximity to various waste sites is of paramount importance, and will allow monitoring and mitigation to be targeted to where it is needed most. Currently there is no readily available source of soil arsenic concentration information at a sufficient resolution to inform commercial producers or homeowners: this information needs to be accessible to everyone, everywhere. Going forward, management and remediation of arsenic contaminated soils is essential both for human health and food security, and innovative technologies are urgently needed that will expedite this process. Innovative solutions such as the use of rice husks to add silicon to the soil to offset arsenic uptake, and the use of cultivars with low-arsenic accumulating characteristics point the way forward for sustainable solutions.
Highlights.
Consumption of staple foods such as rice, apple juice and vegetables grown in contaminated soil is now recognized as a tangible route of human exposure to arsenic
Arsenic occurs in food because it is present in the soil and water and is taken up by crop plants.
Understanding the sources of arsenic to crop plants and influence the dynamics of the agronomic arsenic cycle are key to reducing crop uptake of arsenic now, and preventing exposure in future.
This review considers natural and anthropogenic sources of arsenic to the soil, biogeochemical cycling, rhizosphere processes, plant processes, and mitigation strategies
There must be strategies in place that protect human health from soil contamination by arsenic.
This review recommends: mobilizing existing soil data so that it is readily accessible to commercial and private growers; expanding detailed soil monitoring; reconsideration, unification and enforcement of action levels for agricultural soil arsenic based on updated science, community outreach and education about the potential for arsenic in the soil, as necessary steps to protecting valuable soil resources.
Acknowledgments
This paper, a product of the Collaborative on Food with Arsenic and associated Risk and Regulation (C-FARR), is supported by the Dartmouth College Toxic Metals Superfund Research Program through funds from the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number 1R13ES026493-01 to C. Chen and Award Number P42ES007373 to B. Stanton, and the Children’s Environmental Health and Disease Prevention Research Center at Dartmouth through funds from the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number P01ES022832 to M. Karagas. Although EPA contributed to this article, the research presented was not performed by or funded by EPA and was not subject to EPA’s quality system requirements. Consequently, the views, interpretations, and conclusions expressed in this article are solely those of the authors and do not necessarily reflect or represent EPA’s views or policies. TP was supported by funds from the National Institute of General Medical Sciences Center for Biomedical Research Excellence (P20GM104416). The authors disclose that there are no actual or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Norton GJ, et al. Variation in grain arsenic assessed in a diverse panel of rice (Oryza sativa) grown in multiple sites. New Phytologist. 2012;193(3):650–664. doi: 10.1111/j.1469-8137.2011.03983.x. [DOI] [PubMed] [Google Scholar]
- 2.Carlin DJ, et al. Arsenic and Environmental Health: State of the Science and Future Research Opportunities. Environ Health Perspect. 2015 doi: 10.1289/ehp.1510209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodwell JE, Kingsley LA, Hamilton JW. Arsenic at very low concentrations alters glucocorticoid receptor (GR)-mediated gene activation but not GR mediated gene repression; complex dose response effects are closely correlated with levels of activated GR and require a functional GR DNA binding domain. Chemical Research in Toxicology. 2004;17:1064–1076. doi: 10.1021/tx0499113. [DOI] [PubMed] [Google Scholar]
- 4.Davis MA, et al. Rice consumption and urinary arsenic concentrations in US children. Environmental Health Perpectives. 2012;120:1418–1424. doi: 10.1289/ehp.1205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. International Programme on Chemical Safety. Health Impacts of Chemicals. Arsenic. 2016 [cited 2016 November 22, 2016] Available from: http://www.who.int/ipcs/assessment/public_health/arsenic/en/
- 6.Union E. Free access to EU law (treaties, directives, regulations, decisions, consolidated legislation), preparatory acts, case-law, international agreements, EFTA focuments, summaries of EU legislation and other documents. Commission Regulation (EU) 2015/1006 of 25 June 2015 amending Regulation No. 1881/2006 as regards maximum levels of inorganic arsenic in foodstuffs. 2015 [cited 2016 November 22, 2016] Available from: http://eur-lex.europa.eu/eli/reg/2015/1006/oj.
- 7.Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep. 2012;14(6):542–55. doi: 10.1007/s11883-012-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caldwell KE, et al. Prenatal arsenic exposure alters the programming of the glucocorticoid signaling system during embryonic development. Neurotoxicol Teratol. 2014;47C:66–79. doi: 10.1016/j.ntt.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamura S, et al. United Nations Synthesis Report on Arsenic in Drinking water. United Nations; United Nation, Geneva: 2001. Drinking water guidelines and standards; p. 18. [Google Scholar]
- 10.Sucher L, editor. FDA, U. FDA proposes limit for inorganic arsenic in infant rice cereal. US Food and Drug Administration; 2016. [Google Scholar]
- 11.Japan, M.o.E.G.o. Water/Soil/Ground Environment. 2016. Environmental Quality Standards for Soil Pollution. [cited 2016 12 December] [Google Scholar]
- 12.Henke K. Appendix E: Regulation of Arsenic: A Brief Survey and Bibliography. In: Henke K, editor. Arsenic. John Wiley & Sons, Ltd; 2009. pp. 545–557. [Google Scholar]
- 13.Adomako EE, et al. Enhanced transfer of arsenic to grain for Bangladesh grown rice compared to US and EU. Environment International. 2009;35(3):476–479. doi: 10.1016/j.envint.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y, et al. Baseline Soil Variation Is a Major Factor in Arsenic Accumulation in Bengal Delta Paddy Rice. Environmental Science & Technology. 2009;43(6):1724–1729. doi: 10.1021/es802794w. [DOI] [PubMed] [Google Scholar]
- 15.Williams PN, et al. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environmental Science & Technology. 2007;41(19):6854–6859. doi: 10.1021/es070627i. [DOI] [PubMed] [Google Scholar]
- 16.Norton G, et al. Arsenic Speciation and Localization in Horticultural Produce Grown in a Historically Impacted Mining Region. Environmental Science & Technology. 2013;47(12):6164–6172. doi: 10.1021/es400720r. [DOI] [PubMed] [Google Scholar]
- 17.Koljonen T, et al. 12th International Geochemical Exploration Symposium and the 4th Symposium on Methods of Geochemical Prospecting Geochemical Atlas of Finland: preliminary aspects. Journal of Geochemical Exploration. 1989;32(1):231–242. [Google Scholar]
- 18.Geiszinger A, Goessler W, Kosmus W. Organoarsenic compounds in plants and soil on top of an ore vein. Applied Organometallic Chemistry. 2002;16(5):245–249. [Google Scholar]
- 19.IMA. The new International Mineralogical Association list of minerals. A work in progress. International Mineralogical Society; 2014. http://www.ima-mineralogy.org/Minlist.htm. [Google Scholar]
- 20.Lado LR, Hengl T, Reuter HI. Heavy metals in European soils: A geostatistical analysis of the FOREGS Geochemical database. Geoderma. 2008;148(2):189–199. [Google Scholar]
- 21.Shacklette HT, Boerngen JG. Element Concentrations in soils and other surficial materials of the Conterminous United States. US Geological Survey: US Government Printing Office; Washington, DC: 1984. p. 105. [Google Scholar]
- 22.Smith DB, et al. U.G. Survey, editor. Geochemical and mineralogical maps for soils of the conterminous United States: U.S. 2014. p. 386. [Google Scholar]
- 23.USGS. National scale geochemical analysis of stream sediments and soils in the US, from existing data, reanalysis of existing samples and new sampling. Mineral Resources On-Line Spatial Data: National Geochemical Survey database. [Website] 2016 September 3 2014 [cited 2016 July 18] Available from: http://mrdata.usgs.gov/geochemistry/ngs.html.
- 24.Young M, Donald A. A guide to the Tellus data. Belfast, UK: Geological Survey of Northern Ireland; 2013. p. 233. [Google Scholar]
- 25.Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry. 2002;17(5):517–568. [Google Scholar]
- 26.Saunders JA, et al. Natural arsenic contamination of Holocene alluvial aquifers by linked tectonic, weathering, and microbial processes. Geochemistry Geophysics Geosystems. 2005:6. [Google Scholar]
- 27.Mestrot A, et al. Field Fluxes and Speciation of Arsines Emanating from Soils. Environmental Science & Technology. 2011;45(5):1798–1804. doi: 10.1021/es103463d. [DOI] [PubMed] [Google Scholar]
- 28.Lawgali YF, Meharg AA. Levels of Arsenic and Other Trace Elements in Southern Libyan Agricultural Irrigated Soil and Non-irrigated Soil Projects. Water Quality Exposure and Health. 2011;3(2):79–90. [Google Scholar]
- 29.Melegy A, et al. Weathering fluxes of arsenic from a small catchment in Slovak Republic. Environmental Earth Sciences. 2011;64(2):549–555. [Google Scholar]
- 30.Drahota P, et al. Weathering and erosion fluxes of arsenic in watershed mass budgets. Science of the Total Environment. 2006;372(1):306–316. doi: 10.1016/j.scitotenv.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Huang JH, Matzner E. Biogeochemistry of organic and inorganic arsenic species in a forested catchment in Germany. Environmental Science & Technology. 2007;41(5):1564–1569. doi: 10.1021/es061586d. [DOI] [PubMed] [Google Scholar]
- 32.CEH. United Kingdom Pollutant Deposition Maps. 2008 2016 Database. Available from: http://www.pollutantdeposition.ceh.ac.uk/content/heavy-metals.
- 33.UKSO. Topsoil arsenic map of England and Wales. NSI topsoil Arsenic map. 2016 [cited 2016 August 11] Available from: http://www.ukso.org/nsi/Arsenic.html.
- 34.Mikutta C, Rothwell JJ. Peat Bogs as Hotspots for Organoarsenical Formation and Persistence. Environmental Science & Technology. 2016;50(8):4314–4323. doi: 10.1021/acs.est.5b06182. [DOI] [PubMed] [Google Scholar]
- 35.Mailloux BJ, et al. Microbial Mineral Weathering for Nutrient Acquisition Releases Arsenic. Applied and Environmental Microbiology. 2009;75(8):2558–2565. doi: 10.1128/AEM.02440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams PN, et al. Organic Matter-Solid Phase Interactions Are Critical for Predicting Arsenic Release and Plant Uptake in Bangladesh Paddy Soils. Environmental Science & Technology. 2011;45(14):6080–6087. doi: 10.1021/es2003765. [DOI] [PubMed] [Google Scholar]
- 37.Murphy EA, Aucott M. An assessment of the amounts of arsenical pesticides used historically in a geographical area. The Science of The Total Environment. 1998;218(2–3):89–101. [Google Scholar]
- 38.Lasky T, et al. Mean total arsenic concentrations in chicken 1989–2000 and estimated exposures for consumers of chicken. Environmental Health Perspectives. 2004;112(1):18–21. doi: 10.1289/ehp.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nachman KE, et al. Roxarsone, Inorganic Arsenic, and Other Arsenic Species in Chicken: A US-Based Market Basket Sample. Environmental Health Perspectives. 2013;121(7):818–824. doi: 10.1289/ehp.1206245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fendorf S, Kocar BD. Advances in Agronomy. Academic Press; 2009. Chapter 3 Biogeochemical Processes Controlling the Fate and Transport of Arsenic: Implications for South and Southeast Asia; pp. 137–164. [Google Scholar]
- 41.Bowell RJ, et al. The Environmental Geochemistry of Arsenic - An Overview - Arsenic: Environmental Geochemistry, Mineralogy, and Microbiology. 2014;79:1–16. [Google Scholar]
- 42.Craw D, Bowell RJ. The Characterization of Arsenic in Mine Waste. In: Bowell RJ, et al., editors. Arsenic: Environmental Geochemistry, Mineralogy, and Microbiology. 2014. pp. 473–505. [Google Scholar]
- 43.Bowell RJ, et al. Reviews in mineralogy and geochemistry. 2014;79:635. [Google Scholar]
- 44.Bowell RJ, et al. The Environmental Geochemistry of Arsenic - An Overview. In: Bowell RJ, et al., editors. Arsenic: Environmental Geochemistry, Mineralogy, and Microbiology. 2014. pp. 1–16. [Google Scholar]
- 45.Ramirez-Andreotta MD, et al. Home gardening near a mining site in an arsenic-endemic region of Arizona: Assessing arsenic exposure dose and risk via ingestion of home garden vegetables, soils, and water. Science of the Total Environment. 2013;454:373–382. doi: 10.1016/j.scitotenv.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramirez-Andreotta MD, et al. A greenhouse and field-based study to determine the accumulation of arsenic in common homegrown vegetables grown in mining-affected soils. Science of the Total Environment. 2013;443:299–306. doi: 10.1016/j.scitotenv.2012.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertoldi D, et al. Arsenic present in the soil-vine-wine chain in vineyards situated in an old mining area in Trentino, Italy. Environmental Toxicology and Chemistry. 2013;32(4):773–779. doi: 10.1002/etc.2119. [DOI] [PubMed] [Google Scholar]
- 48.Ma L, et al. Arsenic speciation in locally grown rice grains from Hunan Province, China: Spatial distribution and potential health risk. Science of The Total Environment. 2016;557–558:438–444. doi: 10.1016/j.scitotenv.2016.03.051. [DOI] [PubMed] [Google Scholar]
- 49.Williams PN, et al. Occurrence and Partitioning of Cadmium, Arsenic and Lead in Mine Impacted Paddy Rice: Hunan, China. Environmental Science & Technology. 2009;43(3):637–642. doi: 10.1021/es802412r. [DOI] [PubMed] [Google Scholar]
- 50.Zhu YG, et al. High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice. Environmental Science & Technology. 2008;42(13):5008–5013. doi: 10.1021/es8001103. [DOI] [PubMed] [Google Scholar]
- 51.Kolker A, et al. Arsenic in coal. Fact Sheet. 2006:4. [Google Scholar]
- 52.EPA. Coal Ash Basics [Web Page] EPA. 2016 Jun 7; 2016; Available from: https://www.epa.gov/coalash/coal-ash-basics.
- 53.Feuerborn H-J. Coal Combustion Products in Europe - an update on Production and Ultilisation Standardisation and Regulation. World of Coal Ash (WOCA) Conference; 2011; Denver, CO, USA. [Google Scholar]
- 54.Yager JW, Greene T, Schoof RA. Arsenic relative bioavailability from diet and airborne exposures: Implications for risk assessment. Science of the Total Environment. 2015;536:368–381. doi: 10.1016/j.scitotenv.2015.05.141. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt C. COAL ASH SPILL STILL A PROBLEM. Chemical & Engineering News Archive. 2010;88(49):13. [Google Scholar]
- 56.Schumann AW, Sumner ME. Plant nutrient availability from mixtures of fly ashes and biosolids. Journal of Environmental Quality. 1999;28(5):1651–1657. [Google Scholar]
- 57.Jackson BP, et al. Trace element solubility from land application of fly ash/organic waste mixtures. Journal of Environmental Quality. 1999;28(2):639–647. [Google Scholar]
- 58.Schumann AW, Sumner ME. Formulation of environmentally soundwaste mixtures for land application. Water Air and Soil Pollution. 2004;152(1–4):195–217. [Google Scholar]
- 59.Yokel J, Delistraty DA. Arsenic, lead, and other trace elements in soils contaminated with pesticide residues at the Hanford site (USA) Environmental Toxicology. 2003;18(2):104–114. doi: 10.1002/tox.10106. [DOI] [PubMed] [Google Scholar]
- 60.Embrick LL, et al. Characterization of lead and arsenic contamination at Barber Orchard, Haywood County, NC. Microchemical Journal. 2005;81(1):117–121. [Google Scholar]
- 61.Renshaw CE, et al. Impact of land disturbance on the fate of arsenical pesticides. Journal of Environmental Quality. 2006;35(1):61–67. doi: 10.2134/jeq2005.0096. [DOI] [PubMed] [Google Scholar]
- 62.Peryea FJ, Creger TL. VERTICAL-DISTRIBUTION OF LEAD AND ARSENIC IN SOILS CONTAMINATED WITH LEAD ARSENATE PESTICIDE-RESIDUES. Water Air and Soil Pollution. 1994;78(3–4):297–306. [Google Scholar]
- 63.Robinson GR, et al. Assessment of contamination from arsenical pesticide use on orchards in the Great Valley region, Virginia and West Virginia, USA. Journal of Environmental Quality. 2007;36(3):654–663. doi: 10.2134/jeq2006.0413. [DOI] [PubMed] [Google Scholar]
- 64.Peryea FJ, Kammereck R. Phosphate-enhanced movement of arsenic out of lead arsenate-contaminated topsoil and through uncontaminated subsoil. Water Air and Soil Pollution. 1997;93(1–4):243–254. [Google Scholar]
- 65.Bednar AJ, et al. Presence of organoarsenicals used in cotton production in agricultural water and soil of the southern United States. Journal of Agricultural and Food Chemistry. 2002;50(25):7340–7344. doi: 10.1021/jf025672i. [DOI] [PubMed] [Google Scholar]
- 66.Rahman MA, et al. Straighthead disease of rice (Oryza sativa L.) induced by arsenic toxicity. Environmental and Experimental Botany. 2008;62(1):54–59. [Google Scholar]
- 67.Hudak PF. Distribution and sources of arsenic in the southern High Plains Aquifer, Texas, USA. Journal of Environmental Science and Health Part a-Toxic/Hazardous Substances & Environmental Engineering. 2000;35(6):899–913. [Google Scholar]
- 68.Reedy RC, et al. Unsaturated zone arsenic distribution and implications for groundwater contamination. Environmental Science & Technology. 2007;41(20):6914–6919. doi: 10.1021/es070281b. [DOI] [PubMed] [Google Scholar]
- 69.Ayotte JD, et al. Modeling the probability of arsenic in groundwater in New England as a tool for exposure assessment. Environmental Science & Technology. 2006;40(11):3578–3585. doi: 10.1021/es051972f. [DOI] [PubMed] [Google Scholar]
- 70.Feng M, et al. Arsenic transport and transformation associated with MSMA application on a golf course green. Journal of Agricultural and Food Chemistry. 2005;53(9):3556–3562. doi: 10.1021/jf047908j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Codling EE, Chaney RL, Green CE. Accumulation of Lead and Arsenic by Potato Grown on Lead-Arsenate-Contaminated Orchard Soils. Communications in Soil Science and Plant Analysis. 2016;47(6):799–807. [Google Scholar]
- 72.Codling EE, Chaney RL, Green CE. Accumulation of lead and arsenic by carrots grown on lead-arsenate contaminated orchard soils. Journal of Plant Nutrition. 2015;38(4):509–525. [Google Scholar]
- 73.McBride MB, et al. Arsenic and Lead Uptake by Vegetable Crops Grown on an Old Orchard Site Amended with Compost. Water Air and Soil Pollution. 2015;226(8) doi: 10.1007/s11270-015-2529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lim MP, McBride MB. Arsenic and lead uptake by Brassicas grown on an old orchard site. Journal of Hazardous Materials. 2015;299:656–663. doi: 10.1016/j.jhazmat.2015.07.082. [DOI] [PubMed] [Google Scholar]
- 75.Zavala YJ, Duxbury JM. Arsenic in rice: I. Estimating normal levels of total arsenic in rice grain. Environmental Science & Technology. 2008;42(10):3856–60. doi: 10.1021/es702747y. [DOI] [PubMed] [Google Scholar]
- 76.Zavala YJ, et al. Arsenic in rice: II. Arsenic speciation in USA grain and implications for human health. Environmental Science & Technology. 2008;42(10):3861–6. doi: 10.1021/es702748q. [DOI] [PubMed] [Google Scholar]
- 77.Williams PN, et al. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environmental Science and Technology. 2005;39(15):5531–5540. doi: 10.1021/es0502324. [DOI] [PubMed] [Google Scholar]
- 78.Lomax C, et al. Methylated arsenic species in plants originate from soil microorganisms. New Phytol. 2012;193(3):665–72. doi: 10.1111/j.1469-8137.2011.03956.x. [DOI] [PubMed] [Google Scholar]
- 79.Pouschat P, Zagury GJ. In vitro gastrointestinal bioavailability of arsenic in soils collected near CCA-treated utility poles. Environmental Science & Technology. 2006;40(13):4317–4323. doi: 10.1021/es0604156. [DOI] [PubMed] [Google Scholar]
- 80.Townsend TG, et al. Chromium, copper and arsenic concentrations in soil underneath CCA-treated wood structures. Soil & Sediment Contamination. 2003;12:779–798. [Google Scholar]
- 81.Juhasz AL, Weber J, Smith E. Predicting Arsenic Relative Bioavailability in Contaminated Soils Using Meta Analysis and Relative Bioavailability-Bioaccessibility Regression Models. Environmental Science & Technology. 2011;45(24):10676–10683. doi: 10.1021/es2018384. [DOI] [PubMed] [Google Scholar]
- 82.Carbonell-Barrachina AA, et al. Arsenic chemistry in municipal sewage sludge as affected by redox potential and pH. Water Research. 2000;34(1):216–224. [Google Scholar]
- 83.USFDA. Arsenic-based Animal Drugs and Poultry. Available from: http://www.fda.gov/AnimalVeterinary/SafetyHealth/ProductSafetyInformation/ucm257540.htm.
- 84.Taylor DA. Funky chicken - Consumers exposed to arsenic in poultry. Environmental Health Perspectives. 2004;112(1):A50–A50. doi: 10.1289/ehp.112-a50a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bednar AJ, et al. Photodegradation of roxarsone in poultry litter leachates. The Science of The Total Environment. 2003;302(1–3):237–245. doi: 10.1016/s0048-9697(02)00322-4. [DOI] [PubMed] [Google Scholar]
- 86.Stolz JF, et al. Biotransformation of 3-nitro-4-hydroxybenzene arsonic acid (roxarsone) and release of inorganic arsenic by Clostridium species. Environmental Science & Technology. 2007;41(3):818–823. doi: 10.1021/es061802i. [DOI] [PubMed] [Google Scholar]
- 87.Garbarino JR, et al. Environmental fate of roxarsone in poultry litter. I. Degradation of roxarsone during composting. Environmental Science & Technology. 2003;37(8):1509–1514. doi: 10.1021/es026219q. [DOI] [PubMed] [Google Scholar]
- 88.Jackson BP, et al. Trace element speciation in poultry litter. J Environ Qual. 2003;32(2):535–40. doi: 10.2134/jeq2003.5350. [DOI] [PubMed] [Google Scholar]
- 89.Jackson BP, Seaman JC, Bertsch PM. Fate of arsenic compounds in poultry litter upon land application. Chemosphere. 2006;65(11):2028–2034. doi: 10.1016/j.chemosphere.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 90.Rutherford DW, et al. Environmental fate of roxarsone in poultry litter. part II. Mobility of arsenic in soils amended with poultry litter. Environmental Science & Technology. 2003;37(8):1515–1520. doi: 10.1021/es026222+. [DOI] [PubMed] [Google Scholar]
- 91.Han FX, et al. Arsenic solubility and distribution in poultry waste and long-term amended soil. Science of the Total Environment. 2004;320(1):51–61. doi: 10.1016/S0048-9697(03)00441-8. [DOI] [PubMed] [Google Scholar]
- 92.Fisher DJ, Yonkos LT, Staver KW. Environmental Concerns of Roxarsone in Broiler Poultry Feed and Litter in Maryland, USA. Environmental Science & Technology. 2015;49(4):1999–2012. doi: 10.1021/es504520w. [DOI] [PubMed] [Google Scholar]
- 93.Shah SB, et al. Leaching of Nutrients and Trace Elements from Stockpiled Turkey Litter into Soil. Journal of Environmental Quality. 2009;38(3):1053–1065. doi: 10.2134/jeq2007.0639. [DOI] [PubMed] [Google Scholar]
- 94.Castlehouse H, et al. Biotransformation and accumulation of arsenic in soil amended with seaweed. Environ Sci Technol. 2003;37(5):951–7. doi: 10.1021/es026110i. [DOI] [PubMed] [Google Scholar]
- 95.Antaya NT, et al. Incremental amounts of Ascophyllum nodosum meal do not improve animal performance but do increase milk iodine output in early lactation dairy cows fed high-forage diets1. Journal of Dairy Science. 2015;98(3):1991–2004. doi: 10.3168/jds.2014-8851. [DOI] [PubMed] [Google Scholar]
- 96.Bowell RJ. Sorption of Arsenic by Iron-Oxides and Oxyhydroxides in Soils. Applied Geochemistry. 1994;9(3):279–286. [Google Scholar]
- 97.Luxton TP, Tadanier CJ, Eick MJ. Mobilization of arsenite by competitive interaction with silicic acid. Soil Science Society of America Journal. 2006;70(1):204–214. [Google Scholar]
- 98.Meharg AA, Zhao FJ. Arsenic & rice. xi. Dordrecht: Springer Verlag; 2012. p. 171. [Google Scholar]
- 99.Wang P, et al. A review on completing arsenic biogeochemical cycle: Microbial volatilization of arsines in environment. Journal of Environmental Sciences. 2014;26(2):371–381. doi: 10.1016/s1001-0742(13)60432-5. [DOI] [PubMed] [Google Scholar]
- 100.Turpeinen R, Pantsar-Kallio M, Kairesalo T. Role of microbes in controlling the speciation of arsenic and production of arsines in contaminated soils. Science of The Total Environment. 2002;285(1–3):133–145. doi: 10.1016/s0048-9697(01)00903-2. [DOI] [PubMed] [Google Scholar]
- 101.Pongratz R. Arsenic speciation in environmental samples of contaminated soil. Science of the Total Environment. 1998;224(1–3):133–141. [Google Scholar]
- 102.Frohne T, et al. Controlled variation of redox conditions in a floodplain soil: Impact on metal mobilization and biomethylation of arsenic and antimony. Geoderma. 2011;160(3–4):414–424. [Google Scholar]
- 103.Jia Y, et al. Microbial Arsenic Methylation in Soil and Rice Rhizosphere. Environmental Science & Technology. 2013;47(7):3141–3148. doi: 10.1021/es303649v. [DOI] [PubMed] [Google Scholar]
- 104.Mestrot A, et al. Arsenic volatilization in model anaerobic biogas digesters. Applied Geochemistry. 2013;33:294–297. [Google Scholar]
- 105.Edvantoro BB, et al. Microbial formation of volatile arsenic in cattle dip site soils contaminated with arsenic and DDT. Applied Soil Ecology. 2004;25(3):207–217. [Google Scholar]
- 106.Liu S, et al. Arsenic removal from contaminated soil via biovolatilization by genetically engineered bacteria under laboratory conditions. Journal of Environmental Sciences. 2011;23(9):1544–1550. doi: 10.1016/s1001-0742(10)60570-0. [DOI] [PubMed] [Google Scholar]
- 107.Yang JK, et al. Adsorption, oxidation, and bioaccessibility of As(III) in soils. Environmental Science & Technology. 2005;39(18):7102–7110. doi: 10.1021/es0481474. [DOI] [PubMed] [Google Scholar]
- 108.Newman EI. The rhizosphere: carbon sources and microbial populations. In: Fitter AH, Atkinson D, Read DJ, editors. Ecological Interactions in Soil: Plants, Microbes and Animals. Blackwell Scientific; Oxford, UK: 1985. pp. 107–121. [Google Scholar]
- 109.Acosta JA, Arocena JM, Faz A. Speciation of arsenic in bulk and rhizosphere soils from artisanal cooperative mines in Bolivia. Chemosphere. 2015;138:1014–1020. doi: 10.1016/j.chemosphere.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 110.Wu C, et al. The effect of silicon on iron plaque formation and arsenic accumulation in rice genotypes with different radial oxygen loss (ROL) Environmental Pollution. 2016;212:27–33. doi: 10.1016/j.envpol.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 111.Seyfferth AL. Abiotic effects of dissolved oxyanions on iron plaque quantity and mineral composition in a simulated rhizosphere. Plant and Soil. 2015;397(1–2):43–61. [Google Scholar]
- 112.Yamaguchi N, et al. Arsenic Distribution and Speciation near Rice Roots Influenced by Iron Plaques and Redox Conditions of the Soil Matrix. Environmental Science & Technology. 2014;48(3):1549–1556. doi: 10.1021/es402739a. [DOI] [PubMed] [Google Scholar]
- 113.Lee C-H, et al. Iron plaque formation and its effect on arsenic uptake by different genotypes of paddy rice. Plant and Soil. 2013;363(1–2):231–241. [Google Scholar]
- 114.Hossain MB, et al. The effects of iron plaque and phosphorus on yield and arsenic accumulation in rice. Plant Soil. 2009;317(1–2):167–176. [Google Scholar]
- 115.Wu C, et al. Do radial oxygen loss and external aeration affect iron plaque formation and arsenic accumulation and speciation in rice? Journal of Experimental Botany. 2012;63(8):2961–2970. doi: 10.1093/jxb/ers017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li RY, et al. Uptake and Accumulation Characteristics of Arsenic and Iron Plaque in Rice at Different Growth Stages. Communications in Soil Science and Plant Analysis. 2015;46(19):2509–2522. [Google Scholar]
- 117.Mei XQ, et al. The effects of radial oxygen loss on arsenic tolerance and uptake in rice and on its rhizosphere. Environmental Pollution. 2012;165:109–117. doi: 10.1016/j.envpol.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 118.Gadd GM. Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology-Sgm. 2010;156:609–643. doi: 10.1099/mic.0.037143-0. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, et al. Release and transformation of arsenic from As-bearing iron minerals by Fe-reducing bacteria. Chemical Engineering Journal. 2016;295:29–38. [Google Scholar]
- 120.Newman DK, et al. Dissimilatory arsenate and sulfate reduction in Desulfotomaculum auripigmentum sp. nov. Archives of Microbiology. 1997;168(5):380–388. doi: 10.1007/s002030050512. [DOI] [PubMed] [Google Scholar]
- 121.Macy JM, et al. Two new arsenate/sulfate-reducing bacteria: mechanisms of arsenate reduction. Archives of Microbiology. 2000;173(1):49–57. doi: 10.1007/s002030050007. [DOI] [PubMed] [Google Scholar]
- 122.Wang J, et al. Biotransformation and biomethylation of arsenic by Shewanella oneidensis MR-1. Chemosphere. 2016;145:329–335. doi: 10.1016/j.chemosphere.2015.11.107. [DOI] [PubMed] [Google Scholar]
- 123.Smeaton CM, et al. Simultaneous Release of Fe and As during the Reductive Dissolution of Pb-As Jarosite by Shewanella putrefaciens CN32. Environmental Science & Technology. 2012;46(23):12823–12831. doi: 10.1021/es3021809. [DOI] [PubMed] [Google Scholar]
- 124.Jia Y, et al. Microbial Arsenic Methylation in Soil and Rice Rhizosphere. Environmental Science & Technology. 2013;47(7):3141–3148. doi: 10.1021/es303649v. [DOI] [PubMed] [Google Scholar]
- 125.Xu L, et al. Speciation change and redistribution of arsenic in soil under anaerobic microbial activities. Journal of Hazardous Materials. 2016;301:538–546. doi: 10.1016/j.jhazmat.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 126.Carey AM, et al. Phloem transport of arsenic species from flag leaf to grain during grain filling. New Phytologist. 2011;192(1):87–98. doi: 10.1111/j.1469-8137.2011.03789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carey A-M, et al. A review of recent developments in the speciation and location of arsenic and selenium in rice grain. Analytical and Bioanalytical Chemistry. 2011;402:3275–3286. doi: 10.1007/s00216-011-5579-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carey AM, et al. Grain unloading of arsenic species in rice. Plant Physiology. 2010;152(1):309–319. doi: 10.1104/pp.109.146126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baker MD, et al. Methylation of Arsenic by Fresh-Water Green-Algae. Canadian Journal of Fisheries and Aquatic Sciences. 1983;40(8):1254–1257. [Google Scholar]
- 130.Hansen HR, et al. Identification of tetramethylarsonium in rice grains with elevated arsenic content. Journal of Environmental Monitoring. 2011;13(1):32–34. doi: 10.1039/c0em00460j. [DOI] [PubMed] [Google Scholar]
- 131.Arao T, et al. Effects of Arsenic Compound Amendment on Arsenic Speciation in Rice Grain. Environmental Science & Technology. 2011;45(4):1291–1297. doi: 10.1021/es1033316. [DOI] [PubMed] [Google Scholar]
- 132.Andres J, Bertin PN. The microbial genomics of arsenic. Fems Microbiology Reviews. 2016;40(2):299–322. doi: 10.1093/femsre/fuv050. [DOI] [PubMed] [Google Scholar]
- 133.Zhang S, et al. Cometabolic biotransformation of fenpropathrin by Clostridium species strain ZP3. Biodegradation. 2011;22(5):869–75. doi: 10.1007/s10532-010-9444-y. [DOI] [PubMed] [Google Scholar]
- 134.Stewart EJ. Growing Unculturable Bacteria. Journal of Bacteriology. 2012;194(16):4151–4160. doi: 10.1128/JB.00345-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiao K-Q, et al. Metagenomic analysis revealed highly diverse microbial arsenic metabolism genes in paddy soils with low-arsenic contents. Environmental Pollution. 2016;211:1–8. doi: 10.1016/j.envpol.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 136.Ma LQ, et al. A fern that hyperaccumulates arsenic (vol 409, pg 579, 2001) Nature. 2001;411(6836):438-U3. doi: 10.1038/35054664. [DOI] [PubMed] [Google Scholar]
- 137.Sharma I. Arsenic induced oxidative stress in plants. Biologia. 2012;67(3):447–453. [Google Scholar]
- 138.Le XC, et al. Determination of monomethylarsonous acid, a key arsenic methylation intermediate, in human urine. Environmental Health Perspectives. 2000;108(11):1015–1018. doi: 10.1289/ehp.001081015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Finnegan PM, Chen W. Arsenic toxicity: the effects on plant metabolism. Front Physiol. 2012;3:182. doi: 10.3389/fphys.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Deuel LE, Swoboda AR. Arsenic toxicity to cotton and soybeans. Journal of Environmental Quality. 1972;1(3):317–320. [Google Scholar]
- 141.Davis RD, Beckett PHT, Wollan E. Critical levels of twenty potentially toxic elements in young spring barley. Plant Soil. 1978;49:395–408. [Google Scholar]
- 142.Adriano DC. Trace Elements in Terrestrial Environments. 2. New York: Springer Verlag; 2001. p. 867. [Google Scholar]
- 143.Marin AR, Masscheleyn PH, Patrick WH. The influence of chemical form and concentration of arsenic on rice growth and tissue arsenic concentration. Plant and Soil. 1992;139:175–183. [Google Scholar]
- 144.Carbonell-Barrachina AA, et al. The influence of arsenic chemical form and concentration on Spartina patens and Spartina alterniflora growth and tissue arsenic concentration. Plant and Soil. 1998;198:33–43. [Google Scholar]
- 145.Choudhury B, Chowdhury S, Biswas AK. Regulation of growth and metabolism in rice (Oryza sativa L.) by arsenic and its possible reversal by phosphate. J Plant Interact. 2011;6(1):15–24. [Google Scholar]
- 146.Shri M, et al. Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Safety. 2009;72(4):1102–1110. doi: 10.1016/j.ecoenv.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 147.Chakrabarty D, et al. Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere. 2009;74(5):688–702. doi: 10.1016/j.chemosphere.2008.09.082. [DOI] [PubMed] [Google Scholar]
- 148.Ullrich-Eberius CI, Sanz A, Novacky AJ. Evaluation of arsenate-associated and vanadate-associated changes of electrical membrane potential and phosphate transport in Lemna gibba G1. J Exp Bot. 1989;40(210):119–128. [Google Scholar]
- 149.Raab A, et al. Uptake and translocation of inorganic and methylated arsenic species by plants. Environmental Chemistry. 2007;4(3):197–203. [Google Scholar]
- 150.Finnegan PM, Chen WH. Arsenic toxicity: the effects on plant metabolism. Frontiers in Physiology. 2012:3. doi: 10.3389/fphys.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shin H, et al. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 2004;39(4):629–42. doi: 10.1111/j.1365-313X.2004.02161.x. [DOI] [PubMed] [Google Scholar]
- 152.Remy E, et al. The Pht1;9 and Pht1;8 transporters mediate inorganic phosphate acquisition by the Arabidopsis thaliana root during phosphorus starvation. New Phytol. 2012;195(2):356–71. doi: 10.1111/j.1469-8137.2012.04167.x. [DOI] [PubMed] [Google Scholar]
- 153.Jia H, et al. The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol. 2011;156(3):1164–75. doi: 10.1104/pp.111.175240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ye Y, et al. The Phosphate Transporter Gene OsPht1;4 Is Involved in Phosphate Homeostasis in Rice. PLoS One. 2015;10(5):e0126186. doi: 10.1371/journal.pone.0126186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun S, et al. A constitutive expressed phosphate transporter, OsPht1;1, modulates phosphate uptake and translocation in phosphate-replete rice. Plant Physiol. 2012;159(4):1571–81. doi: 10.1104/pp.112.196345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li RY, et al. The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol. 2009;150(4):2071–80. doi: 10.1104/pp.109.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ma JF, et al. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci U S A. 2008;105(29):9931–5. doi: 10.1073/pnas.0802361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Abedin MJ, Feldmann J, Meharg AA. Uptake kinetics of arsenic species in rice plants. Plant Physiol. 2002;128(3):1120–8. doi: 10.1104/pp.010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pommerrenig B, Diehn TA, Bienert GP. Metalloido-porins: Essentiality of Nodulin 26-like intrinsic proteins in metalloid transport. Plant Sci. 2015;238:212–27. doi: 10.1016/j.plantsci.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 160.Mitani N, et al. Identification and Characterization of Maize and Barley Lsi2-Like Silicon Efflux Transporters Reveals a Distinct Silicon Uptake System from That in Rice. Plant Cell. 2009;21(7):2133–2142. doi: 10.1105/tpc.109.067884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ma JF, et al. An efflux transporter of silicon in rice. Nature. 2007;448(7150):209–12. doi: 10.1038/nature05964. [DOI] [PubMed] [Google Scholar]
- 162.Zhao FJ, et al. The role of the rice aquaporin Lsi1 in arsenite efflux from roots. New Phytol. 2010;186(2):392–9. doi: 10.1111/j.1469-8137.2010.03192.x. [DOI] [PubMed] [Google Scholar]
- 163.Song WY, et al. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc Natl Acad Sci U S A. 2014;111(44):15699–704. doi: 10.1073/pnas.1414968111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schneider S, et al. Arabidopsis INOSITOL TRANSPORTER4 Mediates High-Affinity H(+) Symport of Myoinositol across the Plasma Membrane. Plant Physiol. 2006;141(2):565–77. doi: 10.1104/pp.106.077123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schneider S, et al. Arabidopsis INOSITOL TRANSPORTER2 Mediates H+ Symport of Different Inositol Epimers and Derivatives across the Plasma Membrane. Plant Physiology. 2007;145(4):1395–1407. doi: 10.1104/pp.107.109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Duan G-L, et al. Inositol transporters AtINT2 and AtINT4 regulate arsenic accumulation in Arabidopsis seeds. Nature Plants. 2015;2:15202. doi: 10.1038/nplants.2015.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zenk MH. Heavy metal detoxification in higher plants--a review. Gene. 1996;179(1):21–30. doi: 10.1016/s0378-1119(96)00422-2. [DOI] [PubMed] [Google Scholar]
- 168.Schmoger ME, Oven M, Grill E. Detoxification of arsenic by phytochelatins in plants. Plant Physiol. 2000;122(3):793–801. doi: 10.1104/pp.122.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sadee BA, Foulkes ME, Hill SJ. A study of arsenic speciation in soil, irrigation water and plant tissue: A case study of the broad bean plant, Vicia faba. Food Chemistry. 2016;210:362–370. doi: 10.1016/j.foodchem.2016.04.066. [DOI] [PubMed] [Google Scholar]
- 170.Pizarro I, et al. Bioaccessibility and arsenic speciation in carrots, beets and quinoa from a contaminated area of Chile. Science of The Total Environment. 2016;565:557–563. doi: 10.1016/j.scitotenv.2016.04.199. [DOI] [PubMed] [Google Scholar]
- 171.Zhao FJ, et al. Arsenic uptake and metabolism in plants. New Phytologist. 2009;181:777–794. doi: 10.1111/j.1469-8137.2008.02716.x. [DOI] [PubMed] [Google Scholar]
- 172.Ma JF, et al. An efflux transporter of silicon in rice. Nature. 2007;448:209–12. doi: 10.1038/nature05964. [DOI] [PubMed] [Google Scholar]
- 173.Seyfferth A, et al. Defining the distribution of arsenic species and plant nutrients in rice (Oryza sativa L.) from the root to the grain. Geochim Cosmochim Acta. 2011;75:6655–6671. [Google Scholar]
- 174.Williams PN, et al. Variation in arsenic speciation and concentration in paddy rice related to dietary exposure. Environ Sci Technol. 2005;39(15):5531–5540. doi: 10.1021/es0502324. [DOI] [PubMed] [Google Scholar]
- 175.Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicology Letters. 2002;133(1):1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 176.Sanchez-Bermejo E, et al. Natural variation in arsenate tolerance identifies an arsenate reductase in Arabidopsis thaliana. Nat Commun. 2014;5:4617. doi: 10.1038/ncomms5617. [DOI] [PubMed] [Google Scholar]
- 177.Chao DY, et al. Genome-wide association mapping identifies a new arsenate reductase enzyme critical for limiting arsenic accumulation in plants. PLoS Biol. 2014;12(12):e1002009. doi: 10.1371/journal.pbio.1002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Styblo M, et al. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Archives of Toxicology. 2000;74(6):289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- 179.Marin AR, Masscheleyn PH, Patrick WH. The Influence of Chemical Form and Concentration of Arsenic on Rice Growth and Tissue Arsenic Concentration. Plant and Soil. 1992;139(2):175–183. [Google Scholar]
- 180.Rosen BP. Families of arsenic transporters. Trends Microbiol. 1999;7(5):207–12. doi: 10.1016/s0966-842x(99)01494-8. [DOI] [PubMed] [Google Scholar]
- 181.Song WY, et al. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc Natl Sci USA. 2014;111(44):15699–704. doi: 10.1073/pnas.1414968111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Song WY, et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci U S A. 2010;107(49):21187–92. doi: 10.1073/pnas.1013964107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hartley-Whitaker J, Ainsworth G, Meharg AA. Copper- and arsenate-induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ. 2001;24(7):713–722. [Google Scholar]
- 184.Lee KW, et al. Overexpression of alfalfa mitochondrial HSP23 in prokaryotic and eukaryotic model systems confers enhanced tolerance to salinity and arsenic stress. Biotechnol Lett. 2012;34(1):167–174. doi: 10.1007/s10529-011-0750-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mascher R, et al. Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants. Plant Sci. 2002;163(5):961–969. [Google Scholar]
- 186.Stoeva N, Berova M, Zlatev Z. Physiological response of maize to arsenic contamination. Biologia Plant. 2003;47(3):449–452. [Google Scholar]
- 187.Dixit S, Hering JG. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environmental Science & Technology. 2003;37(18):4182–4189. doi: 10.1021/es030309t. [DOI] [PubMed] [Google Scholar]
- 188.Li RY, et al. Mitigation of Arsenic Accumulation in Rice with Water Management and Silicon Fertilization. Environmental Science & Technology. 2009;43(10):3778–3783. doi: 10.1021/es803643v. [DOI] [PubMed] [Google Scholar]
- 189.Grassi C, et al. Arobic rice: crop performance and water use efficiency. Journal of Agriculture and Environment for International Development. 2009;103(4):259–270. [Google Scholar]
- 190.Arao T, et al. Effects of Water Management on Cadmium and Arsenic Accumulation and Dimethylarsinic Acid Concentrations in Japanese Rice. Environmental Science & Technology. 2009;43(24):9361–9367. doi: 10.1021/es9022738. [DOI] [PubMed] [Google Scholar]
- 191.Hu PJ, et al. Water management affects arsenic and cadmium accumulation in different rice cultivars. Environmental Geochemistry and Health. 2013;35(6):767–778. doi: 10.1007/s10653-013-9533-z. [DOI] [PubMed] [Google Scholar]
- 192.Hu PJ, et al. Effect of water management on cadmium and arsenic accumulation by rice (Oryza sativa L.) with different metal accumulation capacities. Journal of Soils and Sediments. 2013;13(5):916–924. [Google Scholar]
- 193.Honma T, et al. Effects of soil amendments on arsenic and cadmium uptake by rice plants (Oryza sativa L. cv. Koshihikari) under different water management practices. Soil Science and Plant Nutrition. 2016;62(4):349–356. [Google Scholar]
- 194.Matsumoto S, et al. Evaluation of the effects of application of iron materials on the accumulation and speciation of arsenic in rice grain grown on uncontaminated soil with relatively high levels of arsenic. Environmental and Experimental Botany. 2016;125:42–51. [Google Scholar]
- 195.Farrow EM, et al. Reducing arsenic accumulation in rice grain through iron oxide amendment. Ecotoxicology and Environmental Safety. 2015;118:55–61. doi: 10.1016/j.ecoenv.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 196.Chou ML, et al. Inhibition of ethylenediaminetetraacetic acid ferric sodium salt (EDTA-Fe) and calciumperoxide (CaO2) on arsenic uptake by vegetables in arsenic-rich agricultural soil. Journal of Geochemical Exploration. 2016;163:19–27. [Google Scholar]
- 197.Tiberg C, et al. Immobilization of Cu and As in two contaminated soils with zero-valent iron - Long-term performance and mechanisms. Applied Geochemistry. 2016;67:144–152. [Google Scholar]
- 198.Penido ES, et al. Biogeochemical impacts of silicon-rich rice residue incorporation into flooded soils: Implications for rice nutrition and cycling of arsenic. Plant and Soil. 2016;399(1–2):75–87. [Google Scholar]
- 199.Meharg C, Meharg AA. Silicon, the silver bullet for mitigating biotic and abiotic stress, and improving grain quality, in rice? Environmental and Experimental Botany. 2015;120:8–17. [Google Scholar]
- 200.Seyfferth AL, et al. Soil Incorporation of Silica-Rich Rice Husk Decreases Inorganic Arsenic in Rice Grain. Journal of Agricultural and Food Chemistry. 2016;64(19):3760–3766. doi: 10.1021/acs.jafc.6b01201. [DOI] [PubMed] [Google Scholar]
- 201.Norton GJ, et al. Identification of Low Inorganic and Total Grain Arsenic Rice Cultivars from Bangladesh. Environmental Science & Technology. 2009;43(15):6070–6075. doi: 10.1021/es901121j. [DOI] [PubMed] [Google Scholar]
- 202.Duan G, et al. Expressing ScACR3 in rice enhanced arsenite efflux and reduced arsenic accumulation in rice grains. Plant Cell Physiol. 2012;53(1):154–63. doi: 10.1093/pcp/pcr161. [DOI] [PubMed] [Google Scholar]
- 203.Song WY, et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA. 2010;107:21187–92. doi: 10.1073/pnas.1013964107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Norton GJ, et al. Genome wide association mapping of grain arsenic, copper, molybdenum and zinc in rice (Oryza sativa L.) grown at four international field sites. PLoS One. 2014;9(2):e89685. doi: 10.1371/journal.pone.0089685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tang Z, et al. Arsenic Methylation in Arabidopsis thaliana Expressing an Algal Arsenite Methyltransferase Gene Increases Arsenic Phytotoxicity. J Agric Food Chem. 2016;64(13):2674–81. doi: 10.1021/acs.jafc.6b00462. [DOI] [PMC free article] [PubMed] [Google Scholar]