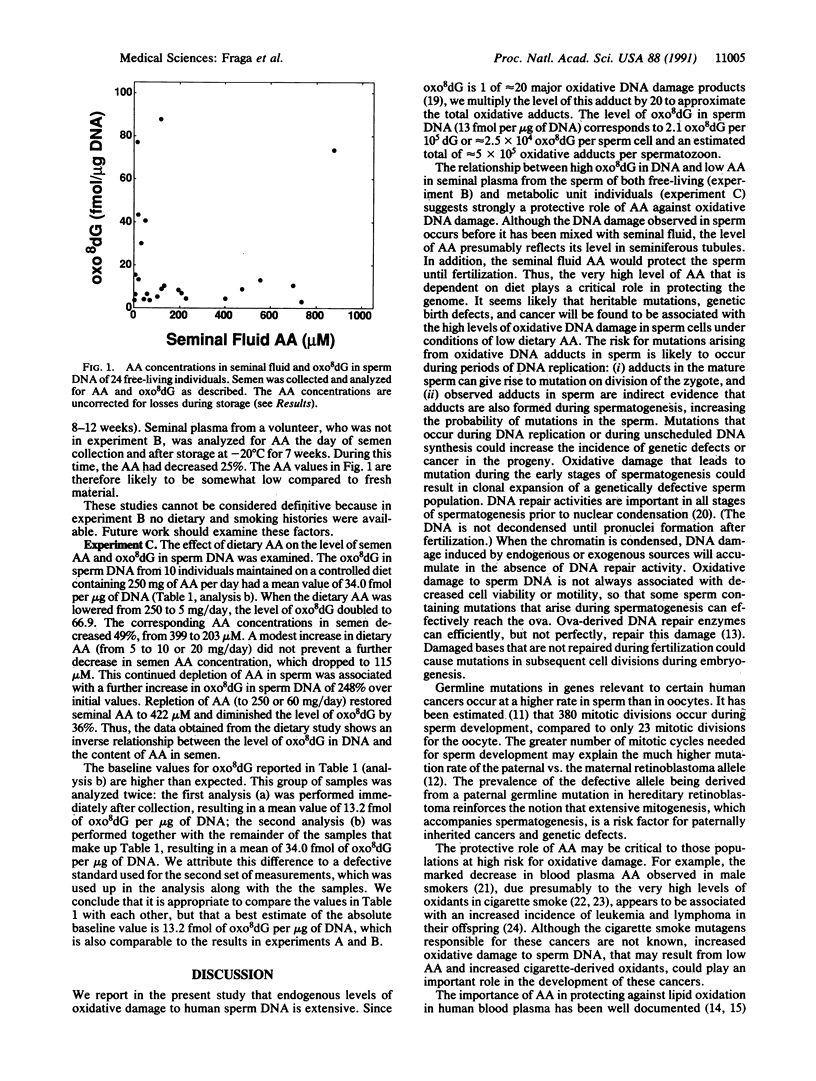
Abstract
Damage to the DNA of germ cells can lead to mutation, which may result in birth defects, genetic diseases, and cancer. The very high endogenous rate of oxidative DNA damage and the importance of dietary ascorbic acid (AA) in preventing this damage has prompted an examination of these factors in human sperm DNA. The oxidized nucleoside 8-hydroxy-2'-deoxyguanosine (8-oxo-7,8-dihydro-2'-deoxyguanosine; oxo8dG), 1 of approximately 20 major products of oxidative damage to DNA, was measured in DNA isolated from human sperm provided by healthy subjects and compared to the seminal fluid AA levels. This relationship was studied in two groups. In a group of 24 free-living individuals 20-50 years old high levels of oxo8dG were correlated with low seminal plasma AA. The endogenous level of oxo8dG in this group was 13 fmol per microgram of DNA or approximately 25,000 adducts per sperm cell. The second group of individuals was maintained on a controlled diet that varied only in AA content. When dietary AA was decreased from 250 to 5 mg/day, the seminal fluid AA decreased by half and the level of oxo8dG in sperm DNA increased 91%. Repletion of dietary AA for 28 days (from 5 mg/day to 250 or 60 mg/day) caused a doubling in seminal fluid AA and reduced oxo8dG by 36%. These results indicate that dietary AA protects human sperm from endogenous oxidative DNA damage that could affect sperm quality and increase risk of genetic defects, particularly in populations with low AA such as smokers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken R. J., Clarkson J. S., Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989 Jul;41(1):183–197. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- Alvarez J. G., Storey B. T. Role of glutathione peroxidase in protecting mammalian spermatozoa from loss of motility caused by spontaneous lipid peroxidation. Gamete Res. 1989 May;23(1):77–90. doi: 10.1002/mrd.1120230108. [DOI] [PubMed] [Google Scholar]
- Alvarez J. G., Touchstone J. C., Blasco L., Storey B. T. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987 Sep-Oct;8(5):338–348. doi: 10.1002/j.1939-4640.1987.tb00973.x. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun. 1989;7(3-6):121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- Carney J. M., Starke-Reed P. E., Oliver C. N., Landum R. W., Cheng M. S., Wu J. F., Floyd R. A. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Saul R. L., Ames B. N. Thymine glycol and thymidine glycol in human and rat urine: a possible assay for oxidative DNA damage. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5633–5637. doi: 10.1073/pnas.81.18.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Church D. F., Pryor W. A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985 Dec;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson E. B., Harris W. A., Rankin W. E., Charpentier L. A., McGanity W. J. Effect of ascorbic acid on male fertility. Ann N Y Acad Sci. 1987;498:312–323. doi: 10.1111/j.1749-6632.1987.tb23770.x. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Mukai S., Petersen R., Rapaport J. M., Walton D., Yandell D. W. Parental origin of mutations of the retinoblastoma gene. Nature. 1989 Jun 15;339(6225):556–558. doi: 10.1038/339556a0. [DOI] [PubMed] [Google Scholar]
- Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B., England L., Ames B. N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B., Forte T. M., Ames B. N., Cross C. E. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Protective effects of ascorbic acid. Biochem J. 1991 Jul 1;277(Pt 1):133–138. doi: 10.1042/bj2770133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B., Stocker R., Ames B. N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeulin C., Soufir J. C., Weber P., Laval-Martin D., Calvayrac R. Catalase activity in human spermatozoa and seminal plasma. Gamete Res. 1989 Oct;24(2):185–196. doi: 10.1002/mrd.1120240206. [DOI] [PubMed] [Google Scholar]
- John E. M., Savitz D. A., Sandler D. P. Prenatal exposure to parents' smoking and childhood cancer. Am J Epidemiol. 1991 Jan 15;133(2):123–132. doi: 10.1093/oxfordjournals.aje.a115851. [DOI] [PubMed] [Google Scholar]
- Jones R., Mann T. Lipid peroxides in spermatozoa; formation, rôle of plasmalogen, and physiological significance. Proc R Soc Lond B Biol Sci. 1976 Jun 30;193(1113):317–333. doi: 10.1098/rspb.1976.0050. [DOI] [PubMed] [Google Scholar]
- Kutnink M. A., Hawkes W. C., Schaus E. E., Omaye S. T. An internal standard method for the unattended high-performance liquid chromatographic analysis of ascorbic acid in blood components. Anal Biochem. 1987 Nov 1;166(2):424–430. doi: 10.1016/0003-2697(87)90594-x. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Seki N., Utsugi-Takeuchi T., Tobari I. X-ray- and mitomycin C (MMC)-induced chromosome aberrations in spermiogenic germ cells and the repair capacity of mouse eggs for the X-ray and MMC damage. Mutat Res. 1989 Mar;211(1):65–75. doi: 10.1016/0027-5107(89)90107-3. [DOI] [PubMed] [Google Scholar]
- Richter C., Park J. W., Ames B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schectman G., Byrd J. C., Gruchow H. W. The influence of smoking on vitamin C status in adults. Am J Public Health. 1989 Feb;79(2):158–162. doi: 10.2105/ajph.79.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga M. K., Park J. W., Cundy K. C., Gimeno C. J., Ames B. N. In vivo oxidative DNA damage: measurement of 8-hydroxy-2'-deoxyguanosine in DNA and urine by high-performance liquid chromatography with electrochemical detection. Methods Enzymol. 1990;186:521–530. doi: 10.1016/0076-6879(90)86146-m. [DOI] [PubMed] [Google Scholar]
- Van Loon A. A., Den Boer P. J., Van der Schans G. P., Mackenbach P., Grootegoed J. A., Baan R. A., Lohman P. H. Immunochemical detection of DNA damage induction and repair at different cellular stages of spermatogenesis of the hamster after in vitro or in vivo exposure to ionizing radiation. Exp Cell Res. 1991 Apr;193(2):303–309. doi: 10.1016/0014-4827(91)90101-y. [DOI] [PubMed] [Google Scholar]
- Vogel F., Rathenberg R. Spontaneous mutation in man. Adv Hum Genet. 1975;5:223–318. doi: 10.1007/978-1-4615-9068-2_4. [DOI] [PubMed] [Google Scholar]
- Yoganathan T., Eskild W., Hansson V. Investigation of detoxification capacity of rat testicular germ cells and Sertoli cells. Free Radic Biol Med. 1989;7(4):355–359. doi: 10.1016/0891-5849(89)90121-4. [DOI] [PubMed] [Google Scholar]


