Abstract
Background
Mosquito saliva is a complex cocktail whose pharmacological properties play an essential role in blood feeding by counteracting host physiological response to tissue injury. Moreover, vector borne pathogens are transmitted to vertebrates and exposed to their immune system in the context of mosquito saliva which, in virtue of its immunomodulatory properties, can modify the local environment at the feeding site and eventually affect pathogen transmission. In addition, the host antibody response to salivary proteins may be used to assess human exposure to mosquito vectors. Even though the role of quite a few mosquito salivary proteins has been clarified in the last decade, we still completely ignore the physiological role of many of them as well as the extent of their involvement in the complex interactions taking place between the mosquito vectors, the pathogens they transmit and the vertebrate host. The recent release of the genomes of 16 Anopheles species offered the opportunity to get insights into function and evolution of salivary protein families in anopheline mosquitoes.
Results
Orthologues of fifty three Anopheles gambiae salivary proteins were retrieved and annotated from 18 additional anopheline species belonging to the three subgenera Cellia, Anopheles, and Nyssorhynchus. Our analysis included 824 full-length salivary proteins from 24 different families and allowed the identification of 79 novel salivary genes and re-annotation of 379 wrong predictions. The comparative, structural and phylogenetic analyses yielded an unprecedented view of the anopheline salivary repertoires and of their evolution over 100 million years of anopheline radiation shedding light on mechanisms and evolutionary forces that contributed shaping the anopheline sialomes.
Conclusions
We provide here a comprehensive description, classification and evolutionary overview of the main anopheline salivary protein families and identify two novel candidate markers of human exposure to malaria vectors worldwide. This anopheline sialome catalogue, which is easily accessible as hyperlinked spreadsheet, is expected to be useful to the vector biology community and to improve the capacity to gain a deeper understanding of mosquito salivary proteins facilitating their possible exploitation for epidemiological and/or pathogen-vector-host interaction studies.
Electronic supplementary material
The online version of this article (doi:10.1186/s12864-017-3579-8) contains supplementary material, which is available to authorized users.
Keywords: Salivary glands, Salivary proteins, Anophelines, Mosquito saliva, Vector biology, Evolution, Salivary markers, Human exposure to malaria vectors, Positive selection
Background
Anopheline mosquitoes are responsible for the transmission of human malaria, a disease which despite a significant decline in the last 15 years still caused over two hundred million new cases and around half a million deaths in 2015 [1]. The malaria parasite Plasmodium is ingested by the mosquito vector along with the blood meal while feeding on an infected individual. After gametogenesis and fertilization, taking place in the midgut lumen, the ookynetes traverse the monolayer of midgut cells and lodge below the basal lamina, where they differentiate into oocysts [2]. Mature oocysts release into the hemolymph thousands of sporozoites that specifically invade the mosquito salivary glands reaching the secretory cavity [3]. In order to get its next blood meal the mosquito penetrates the skin of a new host with the mouth parts and, while probing and feeding, salivates releasing sporozoites and transmitting the disease.
The saliva of blood feeding arthropods is a complex cocktail whose antihemostatic, antiinflammatory and immunomodulatory properties play a crucial role in counterbalancing the physiological host response to tissue injury and in facilitating successful accomplishment of blood feeding [4–6]. Moreover, pathogens are deposited into the skin and exposed to the vertebrate host immune system in the context of arthropod saliva. These vector salivary components can modify the feeding site and may affect the transmission of pathogens as diverse as arboviruses, bacteria and protozoan parasites [7–12], pointing out the possible exploitation of vector salivary proteins as potential vaccine targets [13–16]. Finally, inoculation of arthropod salivary proteins triggers in vertebrate hosts an antibody response which can be used as a biomarker of host exposure to vector bites and may represent a useful tool for epidemiological studies and evaluation of efficacy of vector control interventions [7, 17].
As far as anopheline mosquitoes are concerned the salivary protein repertoires (sialomes) of relevant malaria vectors as Anopheles gambiae, An. funestus, An. stephensi and An. darlingi have been previously characterized by classical transcriptome analyses based on Sanger sequencing [18–23] and by a few proteomic studies [24–28]. The anopheline for which a more comprehensive sialome information is available is certainly An. gambiae where PCR-based tissue-specific expression profiling and transcriptome analyses of salivary glands of both sexes [18, 20] allowed to distinguish: (i) genes specifically expressed or highly enriched in female salivary glands (FSG) and, therefore, most likely involved in blood feeding; (ii) genes expressed in both FSG and male salivary glands (MSG) and presumably involved in sugar digestion, in containing microbial growth or in other more general organ-specific physiological functions. The An. gambiae sialome presently includes over 70 secreted proteins, a number that may be susceptible to increase using up-to-date next generation sequencing techniques, as suggested by previous studies on the culicine mosquito Aedes aegypti [29, 30]. Surprisingly, although the role of quite a few anopheline salivary proteins has been clarified [22] we still have no insights into the functions of approximately forty per cent of them.
The recent release of the genomes of 16 anopheles species [31] offered the unique opportunity to get insights into function and evolution of salivary genes and salivary protein families in anopheline mosquitoes. Based on the above mentioned transcriptomic and gene expression studies on the African malaria mosquito An. gambiae we selected 53 salivary proteins, whose expression is specific or highly enriched in the mosquito salivary glands, and identified/annotated orthologues from 18 additional anopheline species, which include malaria vectors from different geographic areas as well as two African non-vector species (An. quadriannulatus and An. christyi). All three Anopheles subgenera, that is Cellia (series Pyrethophorus, Myzomyia, Neocellia, Neomyzomyia), Anopheles and Nyssorhynchus (with the New World species An. albimanus and An. darlingi) are represented, providing the opportunity to look at the evolution of salivary genes in the time frame of anopheline radiation, which is estimated to have started approximately 100 million years ago. We used this information for sequence comparisons, phylogenetic analyses and for secondary structure prediction (when relevant) to evaluate divergence, evolution and gene gain/loss events which took place during anopheline radiation. We report here the results of our analysis, which provides detailed information and consistent classification on anopheline salivary proteins belonging to at least 24 different families. We are confident that this anopheline sialome catalogue will be useful to the vector biology community and it is expected to improve the capacity to gain a more accurate and deeper understanding of mosquito salivary proteins and to facilitate their possible exploitation for epidemiological and/or pathogen-vector-host interaction studies.
Results and discussion
Based on the previously assembled An. gambiae salivary gene catalogue [18], and excluding low complexity genes (e.g. salivary mucins), we selected 53 An. gambiae salivary proteins (Additional file 1) and searched the genomes of the eighteen anopheline species listed in the methods section using tblastn at the VectorBase web site [32]. Whenever possible, orthologous genes were retrieved through the genome browser and manually annotated using the Artemis tool [33]. The results of searches and annotation are summarized in Fig. 1, which shows for each species if the coding region: (i) could be identified (either as full-length, partial or with frameshifts); (ii) was not assessable (i.e. could not be identified but, at the same time, there was no convincing indication of gene loss, for example because of small genes and possible high divergence or due to highly fragmented or incomplete genome assembly); (iii) was absent (gene loss, as also evaluated from flanking genes); (iv) was duplicated (gene gain). Our analysis, which includes a total of 824 salivary proteins belonging to at least 24 different protein families from 19 anopheline mosquito species, allowed to re-annotate 379 wrongly predicted transcripts and to identify 79 novel salivary protein-coding genes not previously annotated in VectorBase (transcripts v1.00, June-October 2015 depending on the species). The coding sequences of the 824 full-length proteins were used to create an hyperlinked excel spreadsheet which carries sequences, accession numbers and several additional useful information (Additional file 2).
Fig. 1.
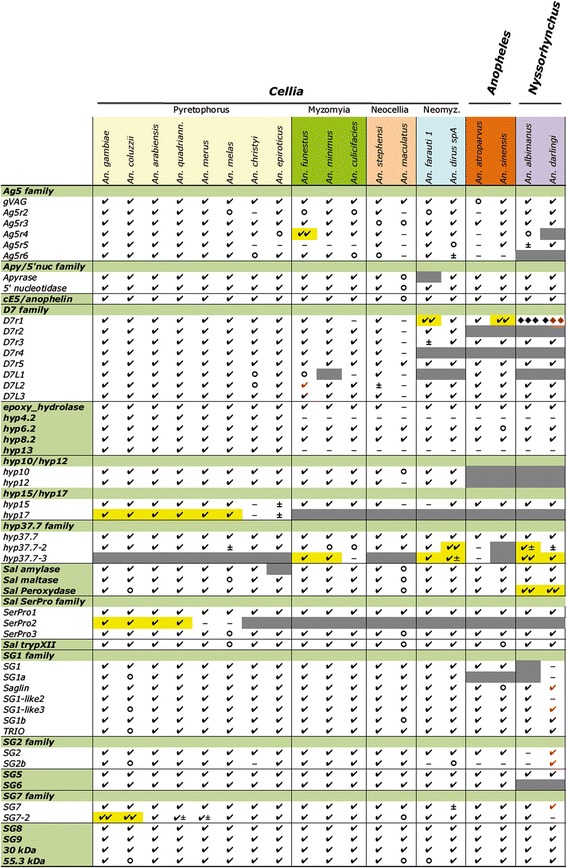
Distribution of the An. gambiae salivary proteins orthologues in anophelines. The selected An. gambiae proteins (left) were used to search orthologues in the genomes of the different anopheline species (top). Retrieval of full-length (✓), partial (○) or frameshift containing (±) coding sequences is reported. Red denotes genes not found but available from previous transcriptomes [19, 21]. Gene absence/loss and gene gain/duplication are highlighted in grey and yellow, respectively. Genes that were not found or not assessable (i.e. for which there was no clear evidence of gene loss) are indicated with a minus (−). Diamonds (♦) were used to indicate the shorter D7r typical of Nyssorhynchus species
The proteins were classified in four main categories: (1) Enzymes, (2) Widespread in blood feeding arthropods, (3) Conserved mosquito families (i.e. only found in mosquitoes) and (4) Anopheline-specific families (i.e. restricted to anopheline species). In the following paragraphs we will describe the main anopheline salivary protein families following this classification and will be referring often to Fig. 1 and Additional file 2. However, it is first useful clarifying that during the initial sialotranscriptome studies on An. gambiae [34–36] the suffix gSG (gambiae Salivary Gene) followed by a number was used to identify An. gambiae salivary gland genes/proteins with no similarity to known genes/proteins in databases. This has sometimes generated confusion in the following literature. Here we will use gSGX to refer to the An. gambiae gene/protein X and SGX to generically indicate the anopheline gene/protein family X; both the family name and species name will be used to identify a family member from a species different from An. gambiae. Moreover, in the following discussion on salivary proteins and protein families we will often report the range of percent identity among anophelines: in all cases the comparisons between species of the An. gambiae complex (An. gambiae s.s., An. coluzzii, An. arabiensis, An. quadriannulatus, An. merus and An. melas), which are very close to each other, were not considered.
Enzymes
Apyrase and 5′-nucleotidase
Apyrases (ATP diphosphohydrolases) are enzymes that catalyze the hydrolysis of ATP and ADP to AMP and inorganic phosphate. ATP and ADP, released in the extracellular environment by broken cells following tissue injury, play an important role as triggers of hemostasis and inflammation. ATP acts as pain mediator and activates neutrophils, which aggregate and degranulate at the site of injury, whereas ADP is a powerful inducer of platelet aggregation. Moreover, ATP and ADP are further released by degranulating neutrophils and platelets. Perhaps for these reasons a salivary apyrase activity has been found in every group of hematophagous arthropods analyzed so far [6, 22, 37, 38], except the bovine feeding Stomoxys calcitrans [39] and Haematobia irritans [40]. As a good example of convergent evolution at least three different classes of apyrases are found in blood feeding arthropods: the CD39 class of fleas [41], the Cimex-type of bed bugs and sand flies [42, 43] and the apyrases of the 5′-nucleotidase family, first found in the mosquito Ae. aegypti [44] and then identified in other mosquitoes as well as in black flies, tsetse flies, triatomine bugs and ticks [22, 37, 38].
5′-nucleotidases (5′-ribonucleotide phosphohydrolases) are ubiquitous enzymes that hydrolyze 5′-nucleotides to nucleosides. They are usually GPI anchored through the C-terminus and play an important role in nucleotide metabolism converting extracellular nucleotides to corresponding nucleosides, which can easily traverse cell membranes. Therefore, as first shown for Ae. aegypti [44] and then confirmed for An. gambiae [45] mosquito apyrases evolved from a member of the 5′-nucleotidase family by gene duplication, loss of the C-terminus involved in GPI anchoring and acquisition of tissue- and sex-specific expression: this way a membrane-bound molecule originally involved in general nucleotide metabolism evolved to a secreted salivary protein playing crucial roles in blood feeding.
Mosquitoes, as first shown in An. gambiae, carry in their saliva two secreted members of the 5′nucleotidase family [34, 45]. The specific enzymatic activity of these two different An. gambiae proteins has never been experimentally verified. However, according to both PCR- and microarray-based expression studies, AGAP011971 was found specifically expressed in adult FSG whereas AGAP011026, although enriched in female glands, showed a more promiscuous expression pattern [35, 46]. For this reason the former has been considered and named as the apyrase and the latter as the 5′-nucleotidase of this mosquito. The two enzymes could act sequentially in anophelines as first suggested for the sandfly Lutzomyia longipalpis [47]. In this scenario the apyrase would hydrolyze the ATP/ADP released from injured tissues to AMP, and the 5′-nucleotidase might further convert the AMP to adenosine, which is not only an antagonist of platelet recruitment, adhesion and aggregation but also a potent vasoactive agent [47, 48].
Full-length orthologues of the An. gambiae salivary apyrase and 5′-nucleotidase were found in all the anopheline species analyzed here with only two exceptions: An. maculatus, where only truncated sequences could be identified, and An. farauti, where the apyrase gene was apparently lost, as indicated by careful inspection of the genomic region and the flanking genes (Fig. 1). This observation is somehow surprising considering the widespread occurrence and the key role of salivary apyrases in blood sucking arthropods. It may be interesting to verify if an apyrase activity is present in the saliva of An. farauti, i.e. if after the gene loss some other gene was recruited to hydrolyze ATP and ADP to AMP. Sequence comparisons showed a minimal amino acid identity of 63% among anopheline apyrases and 67% among 5′-nucleotidases, whereas the identity between apyrases and 5′-nucleotidases is in the range of 46 to 50%. Phylogenetic analysis of the aligned mature proteins showed two independent clades for apyrases and 5′-nucleotidases with strongly supported subclades for Cellia, Anopheles and Nyssorhynchus species (Additional file 3).
Epoxy hydrolase
Epoxy hydrolases (also known as epoxy hydratates) are enzymes that metabolize compounds containing an epoxide (i.e. a cyclic ether with a three atom ring) giving the corresponding dihydroxy through the addition of water. A transcript encoding an epoxy hydrolase (AGAP011970) was found during a sialotranscriptome analysis in An. gambiae and full-length orthologues were identified in all anophelines analyzed here but An. maculatus (Fig. 1, Additional file 2). Multiple alignment showed an overall conserved structure with amino acid identity in the range of 48–87%. Among these eighteen hypoxy hydrolases only six seem to contain a canonical secretory signal according to prediction analysis and, therefore, their secretion in the saliva of anopheline mosquitoes is likely but not certain. However, we included this enzyme in our salivary list for at least two reasons. First, the An. gambiae epoxy hydrolase AGAP011970 is located in close proximity and in reverse orientation to the salivary apyrase (the two starting Met are separated by only 354 bp) and the two genes show a very similar expression profile, both being specific or highly enriched in adult FSG [18, 46]. Second, epoxy hydrolases play relevant roles in the metabolism of epoxy-fatty acids, which are known to be involved in inflammation, hemostasis and pain [49].
Salivary amylase and maltase
Transcripts encoding amylase and maltase were found in An. gambiae and shown to be over-expressed in adult salivary glands of both sexes [18, 46]. Their function is most likely associated with sugar feeding and they may help digestion in the mosquito crop and midgut. Members of the same families are expressed in the salivary glands of the culicine Ae. aegypti [50, 51] and were found in all blood feeding Nematocera sialomes done so far [22]. Orthologues of the An. gambiae salivary amylase and maltase were found, either as full-length or partial, in all anophelines (Fig. 1, Additional file 2) with the only notable exception of An. epiroticus, where the salivary amylase was lost. It is possible that in this species another member of the family, placed in a different genomic context, was recruited to salivary expression and sugar feeding function after the loss of the ancestral gene. Overall salivary maltase proteins show well conserved size in anophelines (593–598 aa) with 78% minimal identity. On the contrary salivary amylases showed a lower identity (range 51–77%) and are rather heterogeneous in size: 871–880 aa within the An. gambiae complex, 598–628 aa in the two Neomyzomyia species An. farauti and An. dirus, and 724–789 aa in the other anophelines. The divergence and fast evolution of these genes suggests they may be under host immune pressure if secreted by female anophelines while probing, as is the case with Ae. aegypti [52].
Salivary peroxidase
The first member of the family was identified in the mosquito An. albimanus by classical biochemical purification followed by cDNA cloning [53, 54]. It was shown to be a heme peroxidase with catechol oxidase/peroxidase activity acting as a vasodilator by inactivating vasoconstriction agents such as noradrenaline and serotonin. Transcripts coding for enzymes of the same family, and supposedly having the same function, were then found through sialotranscriptome studies in An. darlingi [55] and An. gambiae [18]. The An. gambiae salivary peroxidase (AGAP010735) appeared specifically expressed in adult FSG [18, 46]. Several members of the heme peroxidase family are found in the anopheline genomes, nevertheless full-length orthologues were identified with good confidence in all species with the only exceptions of An. coluzzii and An. maculatus where only partial sequences could be reconstructed (Fig. 1). In An. gambiae two additional peroxidase genes were found in the same genomic region coding for AGAP010735, approximately 9 kB upstream and 19 kB downstream. For the first no information on the expression profile is available, whereas the second (AGAP010734) was found expressed in several tissues and significantly upregulated in Malpighian tubes [46]. For this reason these two additional An. gambiae peroxidase family members will not be considered here. A similar situation was found in the other anophelines with the exception of the two species of the subgenus Nyssorhynchus which carry a cluster of five peroxidase genes in a region of ~15 kb. We named the An. albimanus gene encoding the salivary peroxidase biochemically characterized by Ribeiro & Valenzuela [54] as anoalb_Sal_Perox (Additional file 2). However, the true orthologue of AGAP010735 seems to be a close gene that shows the highest identity to the An. gambiae salivary peroxidase (75% identity, 85% similarity) and was named anoalb_Sal_Perox2 (Additional file 4) to indicate that this mosquito may express two salivary peroxidases. The possibility that also other members of the cluster may have salivary expression cannot be ruled out, however, in the absence of additional information we did not consider the other three peroxidases as salivary and indicated them as Perox3, Perox4 and Perox5 in Additional file 4. The other New World species An. darlingi also carries a cluster of multiple genes, although only one peroxidase transcript was found in a previous sialotranscriptome study [21]. We hypothesize here that An. albimanus and An. darlingi may express two salivary peroxidases and tentatively classify this as a gene gain in Fig. 1. Future sialome studies on these two New World species, preferably employing next generation sequencing techniques, should help clarify whether this hypothesis is correct.
Salivary serine proteases
Four secreted trypsin-like serine proteases with highly enriched or specific expression in salivary glands and named Sal_SerPro1-3 and Sal_trypXII were found in An. gambiae. Serine proteases are found expressed in the salivary glands of mosquitoes and other blood feeding Nematocera [22] but their function is presently unknown since no members of this family has been biochemically characterized so far. Their function may be related to immunity, for example as prophenoloxidase activators, or to blood feeding, by interfering with the inflammatory pathways or affecting hemostasis as in the case of the anticoagulant serine protease tabserin from the horsefly Tabanus yao [56].
Sal_SerPro1 (AGAP011912) and Sal_SerPro2 (AGAP011914) were identified in An. gambiae by previous transcriptomes and are located on 3 L:44C, being separated by a 4.2 kb intergenic region. Careful examination of the genomic locus showed that between these two genes there is a third member of the family (AGAP011913) not previously identified in sialome analyses. All three genes show identical expression pattern with significant upregulation in both FSG and MSG [46] and for this reason we included also this third gene in our analysis and named it Sal_SerPro3. The expression in salivary glands of both sexes suggests a more likely involvement in innate immunity rather than in blood feeding. A similar expression pattern in both FSG and MSG was also found during a recent RNAseq analysis for the Ae. aegypti salivary serine proteases [30]. All three Sal_SerPro proteins also contain an amino-terminal CUB domain (Pfam: PF00431), a module of approximately 110 amino acids with four conserved cysteine residues that can be involved in oligomerization and/or recognition of substrates and binding partners. CUB domain-containing serine proteases have also been identified in a sialotranscriptome analysis of the mosquito Ae. aegypti and the presence of the CUB domain interpreted as possibly involved in specialized substrate recognition [29]. Sal_SerPro1 and Sal_SerPro3 orthologues were found in all eighteen anopheline species analyzed here. On the contrary, Sal_SerPro2 was only found in some members of the An. gambiae species complex, but it was absent in all other anophelines (it should be noted that in An. merus and An. melas a third salivary serine protease was not found, most likely just because of the short contigs carrying the cluster) (Fig. 1). The An. gambiae Sal_SerPro2 is 49% identical to Sal_SerPro1 and 88% identical to Sal_SerPro3. Therefore, it it is likely that Sal_SerPro1 and Sal_SerPro3 were already present in the ancestral lineage of anophelines, which may have appeared >100 Mya before the complete splitting of Pangaea in Gondwana and Laurasia [57], whereas Sal_SerPro2 originated “recently” by gene duplication of Sal_SerPro3 in the progenitor of the An. gambiae species complex, i.e. around 2 Mya [58]. Multiple alignment of the forty Sal_SerPro polypeptides showed a well conserved overall structure (minimal identity 43.7%) and full conservation of the 16 cysteines and of the catalytic triad typical of serine proteases and consisting of histidine, aspartate and serine (H199, D249 and S341 in Sal_SerPro1, Additional file 5A). Phylogenetic analysis yielded two well supported independent clades, one including all anopheline Sal_SerPro1 and the other including all Sal_SerPro3 plus the Sal_SerPro2 from species of the An. gambiae complex (Additional file 5B).
A fourth FSG-specific trypsin-like serine protease was first identified in An. gambiae and named Sal_trypXII because of some similarity with Factor XII. Differently from the Sal_SerPro proteins it does not contain any additional conserved domain and, as previously reported, seems to undergo to a tissue- and sex-specific splicing that may play a role in tissue translation selectivity [18]. Orthologues, mostly full-length, were identified in all eighteen additional anophelines analyzed here (Fig. 1) and they share a minimal amino acid identity of 63%. Multiple alignment showed the presence and full conservation of the tripeptide motif (K/R)GD known for the ability to bind integrins. As other functional RGD motifs it is flanked by disulphide bonds able to form a peptide hairpin with the G at the apex [59]. If functional, it may affect platelet aggregation; however, the motif is immediately followed by the Serine that is part of the catalytic triad and, therefore, it may be buried and not available for the interaction (Additional file 6). Serine proteases containing RGD motifs are rather unusual, nevertheless it should be noted that thrombin is known to contain a buried RGD and has been suggested to be able to bind integrins through partial unfolding or after proteolitic digestion, which would expose the RGD to the solvent [60].
Widespread among blood feeding arthropods
This group includes at least three protein families, namely Antigen 5 (Ag5), D7 and 30 kDa. The D7 and Ag5 are multigene families organized in clusters and originated by multiple gene duplication and divergent evolution. In An. gambiae there are eight D7 and several Ag5 family members, of which six will be considered here. Identification of orthologues in the different anopheline species was sometime tricky for these multigene families. We were mainly based on the gene order in the clusters and sequence comparisons to solve ambiguous cases of gene gain/loss and for classification and naming (Fig. 1, Additional file 2).
Antigen 5 family
The first mosquito salivary member of the Ag5 family was identified in An. gambiae [35] and named gVAG (g ambiae Venom AllerGen) because of its similarity to allergens from the venom of ants and wasps [61]. Since then transcripts encoding proteins of the family were found in sialotranscriptomes of most or all blood feeding arthropods analyzed so far [6, 22]. Ag5 proteins are part of the widely spread CAP superfamily, which includes Cysteine-rich secretory proteins from mammals, Antigen 5 from insects, and Pathogenesis-related proteins from plants and whose functions are highly diversified [62, 63]. Members of this family from the venoms of snakes, lizards and Conus snails have been shown to function as toxins, ion channels inhibitors or proteases [64, 65], and Ag5 proteins in the venoms of ants and wasps are powerful allergens to mammals [66, 67]. Only more recently the function of a few Ag5 family members from the saliva of blood feeding insects has been clarified. This is the case of the RGD-containing platelet inhibitors tabinhibitins from Tabanus yao [56], of a 27 kDa immunoglobulin-binding protein from Stomoxys calcitrans [68] (which may function as an inhibitor of the classic pathway of complement) and of Ag5 proteins from the saliva of Triatomines, that are copper-dependent antioxidant enzymes inhibiting neutrophil oxidative burst and collagen-induced platelet aggregation [69]. No salivary Ag5 family members from mosquitoes have been functionally characterized so far.
Insect genomes encode a quite large number of rather divergent Ag5 family members and at least 19 are found in the genome of An. gambiae. Four Ag5 members were previously found expressed in the An. gambiae salivary glands: gVAG (AGAP006421), Ag5r2 (AGAP006419), Ag5r4 (AGAP006420) and Ag5r3 (AGAP003354). The first three form a cluster on 2 L-24D and are upregulated in both MSG and FSG; the fourth is located on 2R-15A, has lower level of expression in salivary glands and it is transcribed at similar level also in other tissues [18, 46]. Careful genome examination allowed to identify two additional family members, apparently the products of gene duplications of Ag5r2 and Ag5r3: (i) AGAP006418 which is close to Ag5r2 (~1.7 kb upstream), has similar expression pattern and high degree of amino acid identity (79%); (ii) AGAP013192 located ~3.2 kb downstream of Ag5r3 and sharing with it identical expression pattern and high identity (85%). For these reasons, although AGAP006418 and AGAP013192 were not described earlier, we included them in our analysis and named Ag5r5 and Ag5r6, respectively.
Full-length orthologues could be retrieved in most cases from the genomes of anophelines with a few exceptions, some of which representing events of gene gain or gene loss that occurred in single species. This may be the case for Ag5r4 that was duplicated in An. funestus and lost in An. darlingi. Moreover, the duplication originating the pair Ag5r2/Ag5r5 most likely took place before anopheline radiation, whereas Ag5r3 underwent a gene duplication giving rise to Ag5r6 after the separation of the New World Nyssorhynchus species from Old World anophelines, around 100 Mya (Fig. 1). Despite the very large divergence of family members (minimal aa identity 32.9%), the 82 full-length anopheline Ag5 proteins share a common structure, with full conservation of their ten cysteine residues and with Ag5r3/Ag5r6 carrying an additional cysteine pair. Multiple alignment also showed that Ag5r3 deduced proteins carry a conserved DPGR tetrapeptide, previously recognized as of crucial importance for thrombin recognition in an in vitro selection study [70] and shown to occupy the active site cleft of the enzyme in the crystal of the An. albimanus anophelin interacting with alpha-thrombin [71]. This tetrapeptide is conserved also in some Ag5r6 proteins, whereas in the remaining ones the R was replaced by a K (Additional file 7). The presence and the conservation of the DPGR motif is intriguing and a possible antithrombin function of Ag5r3 proteins cannot be ruled out, although the expression at similar levels in multiple tissues of both sexes (salivary glands, midgut, malpighian tubes) found by Baker et al. [46] in An. gambiae seems to make unlikely this hypothesis. Due to the variety of functions accomplished by members of this family it is difficult to predict or assign possible roles to these anopheline Ag5 salivary proteins. Nevertheless, as far as we know, the best candidates for a blood feeding role may be the gVAG proteins, due to the higher FSG/MSG ratio [20], followed by Ag5r2 and Ag5r4 that still reach relatively high expression in the FSG of An. gambiae [46]. Phylogenetic analysis including the 82 Ag5 anopheline proteins identified here yielded four very well supported clades including gVAG, Ag5r4 and the two pairs of duplicated genes Ag5r2/Ag5r5 and Ag5r3/Ag5r6 (Additional file 8).
D7 family
The D7 it is certainly one of the best-known salivary multigene families from blood sucking insects. The first D7 family member was identified 25 years ago in the mosquito Ae. aegypti as one of the most abundant proteins found in the saliva of adult females [72]. Following this initial observation the D7 was shown to be a multigene family in An. gambiae [18, 34, 36, 73, 74], to be part of the Odorant Binding Protein (OBP) superfamily [75] and to be widely spread among blood feeding nematocera, with representatives not only in anopheline and culicine mosquitoes [19, 21, 23, 29, 76, 77] but also in sand flies, black flies, frog biting flies and culicoides [22, 78–80]. We will mainly focus here on the D7 family in anophelines; a comprehensive discussion on the evolution of the D7 protein family in blood feeding insects has been provided elsewhere [6, 22].
Mosquito protein family members can be distinguished in short and long D7, possessing one and two OBP domains, respectively. These are atypical because they have seven alpha helices, two additional in comparison to canonical OBPs [81, 82]. The An. gambiae genome carries eight members of the D7 gene family clustered in a region of ~20 kb on 3R-30B. Three genes encode long D7 proteins of ~31–35 kDa (D7L1, D7L2 and D7L3) and five code for short D7 proteins of ~17 kDa (D7r1, D7r2, D7r3, D7r4 and D7r5) [18]. The eight genes are organized in two cassettes spaced by a region of ~1.4 kb. The long D7 cassette includes the three genes placed one after the other in the forward orientation, with D7L1 and D7L2 having four exons and D7L3 with only three exons. The short D7 cassette carries the five short-form genes in the reverse orientation, with the first four having three exons and the fifth, D7r5, having only two exons (Fig. 2). In An. gambiae the D7 proteins are among the most abundant salivary components and are specifically expressed in adult female salivary glands [18, 46, 73]. According to transcript representation in sialotranscriptome studies the first four short D7 (D7r1-D7r4) appear the most abundantly expressed, whereas D7L1 and D7L2 are expressed at lower levels. D7L3 and D7r5, which are the last gene in each cassette, are only poorly transcribed and it was proposed they may be turning into pseudogenes [18, 81, 83].
Fig. 2.

Genomic organization of the An. gambiae D7 cluster. Schematic representation of the ~20 kb genomic locus on 3R-30B carrying the three long D7 genes followed by the five short D7 in the reverse orientation. The red boxes represent exons. Introns and intergenic regions are shown as a black line. The red arrows point to the direction of transcription and numbers indicate the length in bp of the intergenic regions
The functional role of insect OBPs is to bind and carry small hydrophobic compounds such as odorants and pheromones. For this reason it was proposed that the mosquito D7 proteins may help blood feeding by capturing agonists of the hemostatic or inflammatory response of the host [73]. This prediction was confirmed by studies on the An. stephensi D7r1, which was named hamadarin [84], and on the An. gambiae D7r proteins [83]. Hamadarin was shown to inhibit activation of the plasma contact system and bradykinin release by binding factorXII and high molecular weight kininogen; it may facilitate blood feeding in virtue of its anticlotting and antiinflammatory action [84]. Afterwards, the five An. gambiae short D7 were expressed in recombinant form and, with the exception of D7r5, were all shown to bind serotonin (5-HT) and other biogenic amines as histamine (H), epinephrine (E) and norepinephrine (NE), although with some difference in preference and affinity. Biogenic amines, released by platelets and mast cells, elicit pain responses and trigger platelet aggregation, vasoconstriction and inflammation and their sequestration by salivary proteins of blood feeding arthropods seems to be a conserved mechanism of highly adaptive value in the evolution of hematophagy in insects [6]. The An. gambiae D7r1, as its orthologue in An. stephensi hamadarin, also showed anticlotting activity in the Activated Partial Thromboplastin Test, whereas no function could be assigned so far to the D7r5 protein [83]. Structural and functional analyses of mosquito long D7 proteins, which carry two OBP domains, indicated that they are bifunctional, being able to bind and neutralize two different classes of inflammatory mediators. In fact a long D7 protein from Ae. aegypti was shown to bind bioactive lipids, namely cysteinyl leukotrienes (cysLTs), with its N-terminal OBP domain and biogenic amines, as is the case for the An. gambiae D7r proteins, with the C-terminal domain [81–83]. CysLTs released by mast cells are potent vasodilators and additionally activate the endothelium inducing swelling, erythema, pain and itching, which may trigger defensive behaviors by the host; therefore, antagonizing their effects may be crucial to guarantee an efficient and uninterrupted blood feeding [6, 81]. Interestingly, the N-terminal OBP domain of the An. stephensi D7L1 retained the ability to bind cysLTs but also acquired the capacity to bind thromboxane A2 (TXA2), which stimulates platelet aggregation and it is a powerful vasoconstrictor. On the contrary the C-terminal domain is rearranged in comparison to the Ae. aegypti long D7 and to the An. gambiae D7r and lost the ability to bind biogenic amines, although it is not yet known if it acquired novel binding capacities [85]. Overall the mosquito D7 family is a very nice paradigm of how gene duplication and divergence, including domain duplications, played a pivotal role in evolution of novel functions and adaptation to hematophagy. This family also illustrates very well a recurrent strategy used by blood feeding arthropods, which is producing large amounts of salivary proteins with high affinity binding activity toward agonist of the host hemostatic and inflammatory responses. The name kratagonist has been proposed for this kind of inhibitors that include, besides the mosquito D7, also lipocalin family members from Rhodnius prolixus (binding H, nitric oxide and ADP) and from ticks (binding H, 5-HT, NE, TXA2, cysLTs) as well as sand fly salivary members of the Yellow family, that were also shown to bind 5-HT, E and NE [6, 86].
One hundred twenty seven full-length D7 family members were retrieved from the genomes of the 19 anophelines analyzed here, 83 being short D7 and 44 long D7 (Additional file 2). Identification of orthologues was guided mainly by the gene order in the cluster and partly by sequence similarity to the An. gambiae prototypes. However, in a few cases proper assignment was complicated: for this reason in Additional file 2 three short D7 from An. atroparvus, An. albimanus and An. darlingi were indicated as D7r2/D7r3 and one short D7 from An. farauti was named as D7r4/D7r2-like. Overall, with a few exceptions most likely due to incomplete genome assemblies, three short (D7r1, D7r3 and D7r5) and two long D7 genes (D7L2 and D7L3) were found in all anophelines analyzed (Fig. 1) suggesting that the progenitor of anophelines may have carried a cluster of 5 genes. D7r2 and D7r4 were absent in representatives of the Anopheles and Nyssorhynchus subgenera, and D7r4 was also absent in An. farauti and An. dirus (Cellia subgenus, Neomyzomyia series). It is possible that these two short D7 may have originated sometime in a progenitor of Cellia species by gene duplication of D7r3 and D7r1, respectively. D7L1 was absent in An. albimanus and An. darlingi as well as in a few additional anophelines: this distribution would be compatible with its appearance, most likely by duplication of D7L2, after the separation of the New World Nyssorhynchus species from Old World anophelines, around 100 Mya, followed by sporadic events of gene loss in species belonging to the Cellia subgenus (Fig. 1). An. albimanus and An. darlingi have orthologues of D7r3 and D7r5 and three copies each of shorter D7r genes typical of Nyssorhynchus that appear more closely related to D7r1 and are most likely the result of multiple gene duplication. We could find only one of these three genes in the genome of An. darlingi. However, two additional short D7 typical of Nyssorhynchus were previously found by transcriptome studies: GI: 208657479 (version ACI30036) and GI:208657495 (version ACI30044) [21]. These two additional genes are not included in Additional file 2 but were inserted in Fig. 1 as well as in the following phylogenetic analysis.
Multiple alignment of the 129 D7 family members indicated that D7r proteins align to the C-terminal region of long D7 with good conservation of the Cys framework (not shown). The phylogenetic analysis showed three main well supported clades (Fig. 3): (i) the first includes all D7r1 and D7r4 proteins, with the shorter D7 typical of Nyssorhynchus forming a strongly supported subclade; (ii) the second groups all D7 long proteins with two independent subclades, one comprising all D7L1 and D7L2 and the other the D7L3 proteins; (iii) the third clade includes D7r5 proteins, which are part of a separated and well supported subclade, as well as D7r2 and D7r3 proteins. This distribution fully agrees with the interpretation pointing to the pairs D7L1/D7L2, D7r1/D7r4 and D7r2/D7r3 as the products of gene duplication.
Fig. 3.
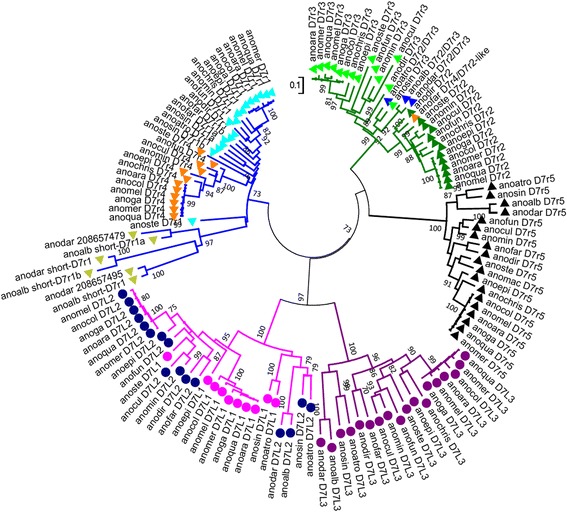
Phylogram of the salivary D7 proteins from anopheline mosquitoes. Numbers in the phylogram nodes show the percent bootstrap support for the phylogeny (≥70%). The bar at the bottom indicates 10% aminoacid divergence in the sequences. Coloured dots and triangles mark D7 long and D7 short proteins, respectively. Also the different clades are in colours: D7r1/D7r4 clade (light blue), D7r2/D7r3 (green), D7r5 (black), D7L1/D7L2 (pink), D7L3 (purple)
30 kDa family
Members of the 30 kDa family (also sometime indicated as 30 kDa allergens or GE-rich proteins) were first described as salivary allergens of Aedes mosquitoes [87]. With the growing of sialotranscriptome studies members of the family were found in culicine and anopheline mosquitoes [18, 19, 23, 29, 55, 74, 76, 77, 88, 89], but also in black flies [90], and more distantly related family members were recognized in sand flies [22]. 30 kDa proteins from Ae. aegypti (aegyptin) and An. stephensi (anopheline anti-platelet protein, aapp) were shown to have a conserved function and to inhibit collagen-induced platelet aggregation by binding to collagen and preventing its interaction with glycoprotein VI (GPVI), integrin α2β1 and von Willebrand Factor (vWf) [91, 92]. 30 kDa proteins are abundantly and specifically expressed in mosquito female salivary glands [18, 20, 30] and the promoter of the An. stephensi gene was shown to drive strong tissue-specific expression of exogenous genes in the female salivary glands of transgenic anophelines [93]. Noteworthy, anopheline mosquitoes, as also confirmed here, carry a single gene belonging to the family while culicine mosquitoes have multiple copies. As previously described, mature 30 kDa family proteins are characterized by two distinct domain: the N-terminal half, highly acidic and of low complexity (being rich in Gly, Glu and Asp residues), and the C-terminal domain, more complex and carrying four conserved cysteines [91, 92]. The C-terminal domain, which consists mainly of two alpha helices spaced by a short loop and connected by the two conserved disulfide bridges [94], has been shown to be the main region of the aegyptin involved in the binding to collagen [95].
In An. gambiae the 30 kDa protein is encoded by a four-exon gene (AGAP009974) located on 3R-36B. Full-length orthologues were retrieved in all anophelines (Fig. 1) and showed a degree of amino acid identity in the range of 46.7 to 81%. The mature proteins vary from 217 (An. darlingi) to 271 (An. farauti) amino acids in length, with isoelectric points between 3.9 and 4.3 (Additional file 2). Multiple alignment of the deduced proteins showed that the N-terminal low complexity domain is 96 to 153 amino acids in length and it is the region responsible both for the size heterogeneity and for the acidic nature of the protein, being highly enriched in Asp, Glu and Gly residues (54.6–71.5%) with 31–53 negatively charged amino acids against 1–6 positively charged (Additional file 9). The C-terminal domain carrying the four cysteines is conserved both in size (119–121 aa) and in sequence and it is essentially neutral with a difference in charged residues between −1 and +4. Phylogenetic analysis of anopheline 30 kDa family members showed a clustering fully consistent with known relationships between anopheline mosquitoes (not shown). A more comprehensive bootstrapped phylogram including Anopheline, Culicine, Simulium and Phlebotomus sequences has been reported previously [22].
Conserved mosquito families
Within this group are included protein families that are found in the saliva of both anopheline and culicine mosquitoes but were not detected so far in the saliva of other blood feeding arthropods. It consist mainly of single copy genes (SG5, SG8, SG9/41kDda, 55.3 kDa) but includes also the highly divergent multicopy 37.7 kDa family as well as the large SG1 family.
Hypothetical 37.7 kDa family
The first member of this family, encoding a protein of 37.3 kDa, was identified in An. stephensi [23], and a putative orthologue was found later in An. gambiae [18]. In the African malaria mosquito there are actually two family members: hyp37.7 (AGAP001988) and hyp37.7-2 (AGAP001989) located on 2R-10A and separated by a short intergenic region of approximately 0.5 kb. They are intronless and encode putative secreted proteins as indicated by signal peptide prediction analysis (Additional file 2). Considering their high divergence (the encoded proteins show 39% identity and 53% similarity) they are most likely the result of an ancient gene duplication and are highly or specifically expressed in female salivary glands [18, 46]. Blastp searches using the An. gambiae hyp37.7 or hyp37.7-2 retrieve proteins from other anophelines, two hyp37.3 salivary proteins from Culex quinquefasciatus (CPIJ018693, CPIJ018673), two hypothetical proteins from Aedes albopictus (AALF003062, AALF000758) but also an hypothetical protein from a Wolbachia endosimbiont of Drosophila simulans (WP 015589027.1). This Wolbachia protein shows 49% identity and 61% similarity to hyp37.7 (43% query cover, E-value 5e-24) and 36% identity and 56% similarity to hyp37.7-2 (88% query cover, E-value 1e-42). This similarity to a Wolbachia protein, along with the single exon structure of hyp37.7 family members, suggest that this gene family may have originated by horizontal transfer from the genome of a mosquito endosymbiont, as previously proposed for other An. gambiae salivary transcribed genes [18]. Multiple alignment of the mosquito and Wolbachia proteins (excluding the Ae. albopictus AALF003062 and AALF000758 for which there is no evidence of salivary glands expression) shows a well conserved region of 115 amino acids in length (Fig. 4).
Fig. 4.

Alignment of mosquito hyp37.7 kDa family members to an hypothetical protein from Wolbachia. Multiple alignment of hyp37.7 family members from An. gambiae, An. stephensi, An. darlingi and Cx. quinquefasciatus to an hypothetical protein from a Wolbachia endosimbiont of Drosophila simulans. Only the conserved 115 amino acid region is shown. Fully conserved residues (yellow), cysteins (red) and residues conserved in at least 2/3 of the aligned sequences (green) are highlighted. Numbers indicate aminoacid positions. Mosquito species names are abbreviated with the first letters of the generic name and the first three letters of the specific name; wolb indicates the Wolbachia sequence. Accession numbers follow (when available)
A total of 40 complete hyp37.7 family members were found in the nineteen anopheline species considered here, with at least one representative per species and with most of the family members (33/40) predicted to encode a secretory signal peptide (Fig. 1, Additional file 2). The hyp37.7 appears to be both a highly divergent and a highly dynamic gene family. The amino acid identity among different family members is in the range of 16 to 88% (hyp37.7, 38–72%; hyp37.7-2, 16–78%; hyp37.7-3, 23–88%). In addition, there is a large variation in gene copy numbers, indicative of multiple events of gene gain/loss with a few species carrying just one family member (An. atroparvus and An. sinensis, subgenus Anopheles), most species having two (all species of the series Pyrethophorus and Neocellia, subgenus Cellia) while the remaining possess three to five copies (Fig. 1). The function of members of this family is presently unknown.
SG1 family
In An. gambiae 7 members of the SG1 family have been recognized [18, 35, 36, 74]. Five of them, named SG1 (AGAP000612), SG1a (AGAP000611), Saglin (AGAP000610), SG1-like2 (AGAP000609) and SG1-like3 (AGAP000607), are closely clustered in a ~10 kb region on the X chromosome, division 1D (Additional file 10A). A sixth member, SG1b (AGAP000549), is located on the same chromosomal division at a distance of approximately 1.2 Mb, whereas the last gene, named TRIO (AGAP001374), is located on 2R-8A. Remarkably, gene family members are all intronless, unusual for eukaryotic genes coding for these relatively large proteins (~45 kDa), suggesting possible acquisition by horizontal transfer. They all share a very similar expression profile with strong upregulation in FSG [18, 20, 35, 36, 46] and a likely physiological role connected to blood feeding. An. gambiae SG1 family members are very divergent with a minimum amino acid identity of ~14% and a maximum identity of ~30–31% among SG1, SG1a and Saglin. PSIblast search with the An. gambiae SG1 protein against the non redundant database allowed to retrieve all anopheline SG1 family members but in addition, after a few iteration, also the salivary 62 kDa proteins of Aedes mosquitoes [22]. The Anopheles SG1 and the Aedes 62 kDa families are very distantly related (protein identity in the range of 11–17%) but should be considered as part of the same superfamily. According to these considerations the SG1 family, initially indicated as unique to anophelines [18], was reclassified as part of the SG1/62 kDa superfamily of mosquitoes [22] and it is included here among the group of the conserved mosquito family.
The SG1 family appeared well conserved among species of the subgenus Cellia, where seven family members, organized similarly to An. gambiae, could be easily recognized. The SG1a gene was missing in An. atroparvus and An. sinensis (subgenus Anopheles) as well as in An. albimanus (subgenus Nyssorhynchous), who also lacked SG1. Therefore, these species carry a cluster of four and three genes, respectively, rather than five genes as in the Cellia species (Figs. 1 and 5). An. darlingi was not readily assessable since the SG1 cluster, consisting of three to five genes in the other anophelines, could not be retrieved from the genome of this species and only SG1b and TRIO were identified. However, An. darlingi possesses at least two additional family members, i.e. Saglin (ACI30180) and SG1-like3 (ACI30121, ACI30123) as indicated by a previous transcriptome analysis [21]. Overall 119 full-length SG1 family members were identified in the genomes of the 19 anophelines studied here, and most (109/119) are predicted to encode proteins carrying a signal peptide at their N-terminus (Additional file 2), which is in agreement with the evidence for secretion found for the An. gambiae SG1b, SG1 and SG1-like3 by Edman degradation of SDS-PAGE protein bands [74]. According to the distribution of family members among anophelines, to sequence comparison and phylogenetic analysis a possible scenario is that Saglin, SG1-like2, SG1-like3, SG1b and TRIO were already present more than 100 Mya when anopheline radiation is supposed to have started [31], as indicated by their presence in the genome of all species of the three subgenera considered here (Fig. 1). SG1 may have evolved from Saglin by gene duplication after separation of Old World anophelines from New World species. A second gene duplication, which may have taken place in the progenitor of Cellia, gave rise to SG1a (either from Saglin or from SG1). An alternative, less conservative explanation of the situation observed today would be independent gene loss events in An. albimanus, An. atroparvus and An. sinensis.
Fig. 5.
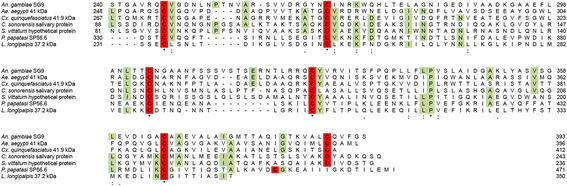
Alignment of the C-terminal region of selected members of the expanded SG9/41 kDa family. Multiple alignment of the C-terminal region encompassing ~200 amino acids of members of the SG9/41 kDa family from a few representative species. Residues conserved in at > 50% of the aligned sequences are highlighted in green, cysteines are shown in red. Accession numbers: Anopheles gambiae gi:347971052, Aedes aegypti gi:94468848, Culex quinquefasciatus gi:38350631, Culicoides sonorensis gi:51557691, Simulium vittatum gi:197260858, Phlebotomus papatasi gi:449060677, Lutzomyia longipalpis gi:42491533
Due to the absence of significant similarity to known proteins the function of members of the SG1 family is still unknown. However, Saglin was suggested to be involved in Anopheles salivary gland invasion by Plasmodium sporozoites [96] and its downregulation by RNAi drastically reduced the number of P. falciparum sporozoites in the salivary glands of infected An. gambiae [97]. Notably, Saglin was found in the genome of all nineteen anopheline species analyzed here, many of which are good malaria vectors. The functional significance of the expansion of the SG1 family and the possible involvement of other family members in pathogen-vector interaction stays as an open question. However, the ability of the SM1 peptide to interact not only to Saglin but also to SG1, as shown by cross-linking experiments [97], raises the possibility that other family members may play some role in Plasmodium salivary gland invasion. A phylogram of the anopheline SG1 protein family is included as supplemental material (Additional file 10B).
SG5, SG8 and SG9/41 kDa families
Founders of the SG5, SG8 and SG9 families of anophelines were identified during a signal sequence trap screening in An. gambiae [36]. Full-length orthologues of gSG5, gSG8 and gSG9 were retrieved in all anophelines (Fig. 1, Additional file 2) and members of the same families were also identified in sialotranscriptomes of culicine mosquitoes, where the SG9 was named as 41 kDa family [29, 76]. In An. gambiae and Ae. aegypti SG5 and SG8 transcripts were found specifically expressed in FSG whereas SG9/41 kDa family members were expressed in both FSG and MSG [18, 20, 30, 46, 76]. Conservation of the SG5 and SG8 protein families among anophelines is in the range of 48–87% and 50–78%, respectively. When the An. gambiae and Ae. aegypti proteins are compared conservation drops (SG5: 27% id., 54% sim.; SG8: 34% id., 66% sim.), nevertheless, multiple alignments show preservation of the overall structure with full conservation of the 8 (SG5) and 7 cysteine residues (SG8) (not shown). The function of SG5 and SG8 family members is presently unknown, but considering their overexpression in FSG they are expected to affect blood feeding/host physiology.
Also the SG9/41 kDa protein family appears well conserved among anophelines (50–78% identity). Comparison with the NR protein database only retrieves mosquito family members from Ae. aegypti, Cx. quinquefasciatus and Ae. albopictus; moreover a member of the family was also reported in the non-blood feeding mosquito Toxorhinchites amboinensis [98]. However, use of PSI-BLAST allowed to retrieve after a few iterations, first an unknown salivary protein from Culicoides sonorensis, then an hypothetical protein from Simulium vittatum and finally a few salivary proteins from sand flies (39 kDa protein from Phlebotomus ariasi, SP56.6 from P. papatasi, 37.2 kDa from Lutzomyia longipalpis and SP19 from P. perniciosus). Despite the different sizes (from the 236 aa of the S. vittatum protein to the 471 aa of the P. papatasi SP56.6) and the divergence in the N-terminal region, multiple alignment of SG9/41 kDa family members from a few representative species shows a good conservation of the C-terminal region encompassing ~200 amino acids and carrying six fully conserved cysteine residues (Fig. 5). These observations suggest that this protein family, still classified here as conserved mosquito family, may be more widely spread among blood feeding Nematocera than previously thought. The function of members of the SG9/41 kDa family is presently unknown. A phylogram including this expanded SG9/41 kDa protein family has been previously reported [21].
55.3 kDa family
The An. gambiae 55.3 kDa salivary protein is encoded by AGAP005822, an intronless gene located on 2 L-23A. Both according to RT-PCR and microarray data it is specifically expressed in adult salivary glands of both sexes [18, 20, 46]. Orthologues are also present in culicine mosquitoes, where they are slightly larger in size and, therefore, were classified as 56.5 kDa proteins. They have an expression pattern similar to the anopheline 55.3 kDa proteins [29, 76, 77], although a recent RNA-Seq analysis in Ae. aegypti showed significantly higher expression in male salivary glands as compared to female glands [30]. Database searches using the An. gambiae protein only retrieve mosquito proteins; however, after a few iterations of PSIblast also bacterial proteins start to appear. This observation, joined to the uniexonic structure, led to the suggestion that this gene family may have been acquired by mosquitoes through horizontal transfer from some bacterial genome [22]. Orthologues, mostly full-length, were retrieved from all anopheline genomes (Fig. 1, Additional file 2). Multiple alignment of anopheline and culicine members of the 55.3 kDa/56.5 kDa family shows a highly conserved block of 27 aminoacids at the N-terminus (pattern: RxV[LM]DSLVE[STVA]GSPIFQ[GAS]L[SA]N[AV]A[RK][LI]S[ST]G) and six fully conserved Cys residues at the C-terminus (Additional file 11), with amino acid identities in the range of 55–86% between anopheline family members and 31–37% between anophelines and culicines. Secondary structure prediction suggests that proteins of this family, whose function is presently unknown, may have a high alpha helical content.
Anopheline-specific families
We describe here a few genes that have been found so far only in the saliva of anopheline mosquitoes and, therefore, should have evolved in the Anopheles genus not earlier than ~145 Mya, after that ancestral anophelines diverged from ancestral culicines [57]. Some of them, as the SG2 and SG6 families, are absent in the Neotropical species An. albimanus and An. darlingi (Fig. 1) and therefore should have appeared not earlier than ~100 Mya, when South America started to separate from Africa. Other evolved even later during anopheline radiation as is the case of the hyp10/hyp12 family, that is absent in species of the genera Anopheles and Nyssorhynchus and most likely originated sometime in a progenitor of the Cellia subgenus.
cE5/anophelin family
The first member of the cE5/anophelin family was identified in An. gambiae as a secreted salivary component with no similarity to other known polypeptides and named cE5 [35]. Shortly later, a salivary inhibitor of thrombin from the South American malaria vector An. albimanus was biochemically identified and named anophelin. cDNA cloning and sequencing indicated the orthology relationships between these two salivary proteins [99]. Kinetic and structural studies showed that cE5/anophelin family members are intrinsically disordered, tight-binding reversible inhibitors with a unique mechanism of thrombin binding [71, 100, 101]. In An. gambiae the cE5 transcript appeared expressed at high levels in female salivary glands but also, and surprisingly for a thrombin inhibitor, in several additional tissues [35, 46]. However, the corresponding protein product was only detected in adult female salivary glands suggesting that some post-transcriptional mechanism of gene regulation is involved in the sex- and tissue-specific protein translation [101]. Members of the family were present in all species of the three subgenera Cellia, Anopheles and Nyssorhynchus included in the 16 anopheline genome project (Fig. 1, Additional file 2). Alignment of the different family members shows a largely conserved acidic N-terminal block of sixteen amino acids, with a consensus APQY[AST]xG[DE]xP[ST]YD[DE][DE][DET], and a highly conserved DPGR tetrapeptide toward the C-terminus (only exceptions the An. epiroticus and An. atroparvus proteins where Pro is replaced by Ala). Intriguingly, this tetrapeptide had been previously recognized in an in vitro selection study as crucial for αlpha-thrombin recognition [70]. The two conserved blocks are spaced by a more divergent central region of 31–40 aminoacids made up for approximately one third of its length of acidic residues (D or E). The alignment also shows that family members from the New World Nyssorhynchus species An. albimanus and An. darlingi are slightly shorter than in other anopheline species, which carry an additional stretch of 7 to 21 amino acids enriched in serine (Additional file 12). Moreover, most members of the An. gambiae complex carry at the N-terminus the RGD tripeptide known for the ability to bind integrins, although it is not flanked by the typical pair of cysteines involved in a disulphide bond [59]. The cE5/anophelin family looks to be quite variable with an identity range of 31.5 to 65.7% among all anophelines (44.9 to 65.7% within the subgenus Cellia). The An. gambiae cE5 protein was shown to be antigenic to humans and there is evidence it may be useful as a tool to evaluate efficacy of insecticide-treated bednets in reducing human-vector contact [102].
Hypothetical 4.2 and hypothetical 13
Transcripts encoding hyp4.2 (AGAP003473) and hyp13 (AGAP003474) were identified in An. gambiae where the corresponding genes are located close to each other on 2R:15C at a distance of ~1.6 kb. They have a very similar, almost ubiquitous, expression pattern with higher levels in both male and female adult glands [18, 46]. Hyp4.2 and hyp13 code for mature peptides of ~4.3 kDa (40 aa) and ~3.8 kDa (34 aa), with 35% identity and 45% similarity. The similar structure, expression and close proximity suggest they may be the result of an old gene duplication. Clear orthologues of hyp4.2 and hyp13 could be identified only in the Pyretophora series (i.e. the An. gambiae species complex, An. christyi and An. epiroticus) where they share 39–62% (hyp4.2) and 41–59% (hyp13) amino acid identity. It is possible that orthologues are present in the other anophelines but difficult to reliably identify because of the combination of short size and wide divergence. Their function is presently unknown.
Hypothetical 6.2 and hypothetical 8.2
Hyp6.2 and hyp8.2 were found highly enriched in An. gambiae female salivary glands [18, 20, 46] and their salivary expression was confirmed by sialotranscriptome studies in a few additional anopheline species [19, 21]. In An. gambiae hyp6.2 and hyp8.2 are encoded by two intronless genes located at a distance of ~1.5 kb on 2 L-25A, a division where also other salivary genes are located (SG2, SG2b, SG3). Apparently they seem the result of a gene duplication, although these two small proteins do not share significant sequence similarity. Mature hyp6.2 and hyp8.2 have molecular weights of ~6.2 kDa (58 aa) and ~7.9 kDa (73 aa), do not carry any cysteine and are differently charged, with hyp6.2 being basic (pI 10.4) and hyp8.2 acidic (pI 4.2). Database searches do not show similarity to any known protein and, although their pattern of expression suggest a possible role in blood feeding, their function is presently unknown. Full-length orthologues of hyp6.2 and hyp8.2 were found in all anopheline species but An. sinensis where only a partial hyp6.2 could be retrieved (Fig. 1, Additional file 2). Multiple alignment of anopheline hyp6.2 proteins shows remarkable conservation of middle and C-terminal regions, which include 44 aminoacids with 16 invariant positions and it is predicted to structure forming two alpha helices. The aminoterminal region appears less preserved and, as previously noted [19], has three conserved prolines probably making two loops with variable lengths (Fig. 6). On the contrary alignment of hyp8.2 proteins showed an unusually large divergence between species with no invariant positions and amino acid identity varying in a wide range (12.2 to 68.1%) in pairwise comparisons.
Fig. 6.

Alignment of anopheline hyp6.2 proteins. Multiple alignment of hyp6.2 family members from 18 anopheline species. Invariant positions are highlighted in yellow and residues conserved in at least two thirds of the aligned sequences in green. Predicted alpha helices in the middle and C-terminal regions (blue cylinders) and conserved prolines in the amino-terminal region (red dots) are shown above the alignment. Species names are abbreviated with the first three letters of the generic name and the first three-four letters of the specific name. VectorBase accession numbers follow (when available)
Hypothetical 10 and hypothetical 12
In An. gambiae hyp10 and hyp12 are expressed in the salivary glands of both adult males and females [18, 20] and encode putative mature polypeptides of 67 (7.5 kDa) and 71 (7.9 kDa) amino acids in length, respectively. They are located on 3R-30B, the same chromosomal division where the D7 cluster maps, and are arranged in tandem and separated by a ~1.2 kb intergenic region. The two predicted polypeptides are 43% identical (60% similar) and are clearly the products of a gene duplication. They are absent in species of the subgenera Anopheles and Nyssorhynchus, but were found in all species of the subgenus Cellia analyzed in this study (only exception An. maculatus where, because of the short genomic contig, only a truncated hyp10 was found). The tandem arrangement observed in An. gambiae is conserved in the other Cellia where these two small genes are spaced by 0.5–1.8 kb intergenic regions. Alignment of putative mature polypeptides highlights the four highly conserved cysteines, which indicates they must share similar folding. Hyp10 shows 44–76% amino acid identity in the different species and similar values were found for hyp12 (41–77%); the two paralogues, as expected, are more distantly related with identities ranging within Cellia from 34 to 53%. Secondary structure prediction analysis indicated that hyp10/hyp12 family members have a secondary structure characterized by two alpha-helices (N-terminal and C-terminal) separated by a central loop 5–11 aa in length, suggesting a simple structure where the two helices may be hold together by disulfide bridges (Additional file 13A). These two proteins, as mentioned above, are restricted to anophelines of the subgenus Cellia, do not show similarity to any known protein and their function is presently unknown, although their presence in the saliva of both adult male and females may suggest a potential antimicrobial role. Phylogenetic analysis yielded two well-defined and supported clades for hyp10 and hyp12 (Additional file 13B).
Hypothetical 15 and hypothetical 17
The hyp15/17 protein family includes a group of small proteins specifically found in anopheline mosquitoes and whose expression in An. gambiae is highly enriched in adult female salivary glands [18, 46]. Gene family members encode putative mature polypeptides of 4.1–5.4 kDa (41–56 aa, pI 10–12) characterized by the highly conserved tetrapeptide PLPG at the N-terminus and by the fully conserved tetrapeptide HSLG spaced by a region (14–26 aa) enriched in positively charged residues (K, R). A glycin-rich carboxy terminus (27–36% in the last 21–22 residues) follows the HSLG motif (Fig. 7). Secondary structure prediction analysis suggests that these Cys-free proteins are largely disordered. The function of hyp15/hyp17 salivary proteins is unknown but the expression profile indicates they may be involved in blood feeding, perhaps binding some receptor or hemostasis mediator or containing microbial growth. Database searches only retrieve anopheline family members and do not show significant similarity to other known proteins. At least one member of the family was found in the different subgenera/series represented by the eighteen species studied here. Considering the small length of the gene(s), the inability to retrieve orthologs from the genomes of An. christyi, An. maculatus and An. farauti may be more likely due to incomplete genome assembly in these species rather than to events of gene loss. Noteworthy, full-length orthologues of both family members were found in species of the An. gambiae complex, with An. epiroticus carrying degenerated copies containing frameshifts, whereas only one member was found in the other anophelines. According to sequence similarity, the most likely scenario is that hyp15 was the ancestor gene and that a duplication took place in the lineage leading to Pyrethophorus originating the hyp17. Indeed in An. gambiae the two genes are located on chromosome X where they show a tandem arrangement and are separated by a very short intergenic region (~300 bp). Overall, outside the An. gambiae complex, hyp15 proteins share 34 to 86% identical amino acid residues among the different species.
Fig. 7.
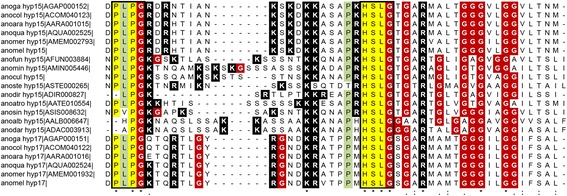
Alignment of the anopheline hyp15/hyp17 family members. Multiple alignment of the anopheline mature hyp15 and hyp17 proteins. Fully conserved residues are indicated by asterisks. Positively charged amino acids (K, R) and glycines (G) are shown in black and red background, respectively. Other residues are highlighted in yellow (fully conserved) or green (conserved in at least 2/3 of the aligned sequences). The consensus tetrapeptide PLPG at the N-terminus and the conserved tetrapeptide HSLG in the central region are boxed. Species names are abbreviated as in Fig. 6
SG2 family
The founder of this family was initially identified during a signal sequence trap screening in An. gambiae and named gSG2 [35], and a second family member was found during a second round of screening and named gSG2-like [36]. gSG2 (AGAP006506) and gSG2-like (AGAP006504) are located on 2 L-25A at a distance of ~5.1 kb; careful examination of the surrounding regions revealed the presence of a third member of the family (AGAP006505) located in between. SG2-like has been sometime also indicated as SG2a or SG2A, which created some confusion; however, following the physical order in the cluster we propose here to name AGAP006505 as gSG2a and indicate AGAP006504 as gSG2b or gSG2-like. gSG2 and gSG2b were found expressed in both male and female salivary glands [20, 35, 36, 46] and orthologues were identified in sialotranscriptomes of An. stephensi [23], An. funestus [19] and An. darlingi [21]. gSG2a most likely originated by gene duplication from gSG2b but was neither found during salivary transcriptome analyses nor differentially expressed in salivary glands [46] and for this reason will not be considered here.
The An. gambiae SG2 and SG2b encode putative secreted proteins of 9.7 kDa (94 aa, pI 3.5) and 15.6 kDa (155 aa, pI 7.02) with a limited similarity to each other. They are both low-complexity proteins enriched in Gly (SG2 21.3%, SG2b 27.1%), Phe (18.1%, 11.6%) and Gln (5.3%, 18.1%), and high similarity matches are only produced with other anopheline proteins. Orthologues of both SG2 and SG2b were retrieved from the genomes of most anopheline species analyzed here as reported in Fig. 1. The inability to identify orthologues in An. darlingi by genome tblastn searches, despite the fact that at least two SG2 family members were previously identified during sialotranscriptome analysis [21], suggest that this may be due perhaps to incomplete genome assembly or low-complexity and divergence rather than to gene loss events. The function of members of this family is presently unknown but their Gly-rich composition reminds of antimicrobial peptides isolated from insects [103, 104], suggesting they may be assisting feeding both in males and females acting as antimicrobials.
SG6 family
gSG6 is a small protein (mature polypeptide 87 aa) first identified in An. gambiae where it is specifically expressed in adult female salivary glands [36]. gSG6 must plays some relevant role in hematophagy since its depletion by RNAi affects mosquito blood feeding ability [105]; nevertheless, its specific function remained elusive so far. The search for orthologues among the anopheline species analyzed here allowed to retrieve SG6 family members in all species of the subgenera Cellia and Anopheles. However, notably, it was absent both in An. albimanus and An. darlingi suggesting that, as also previously suggested [105], SG6 was either lost in the progenitor of the New World Nyssorhynchus species or appeared later in Old World anophelines. Alignment of the seventeen family members available so far shows a full conservation of the ten Cys residues and an overall good degree of similarity among anophelines (Additional file 14), with a minimum of 52.5% identity and 72% similarity between An. gambiae and An. farauti. The restriction to anopheline mosquitoes, the absence of significant similarity to any known protein in databases and the antigenic properties of gSG6 allowed to exploit the IgG response to this An. gambiae protein as marker of human exposure to bites of Afrotropical malaria vectors (see below).
SG7 family
The founder of this family was first identified in An. gambiae and named gSG7 [36] and a second family member, named gSG7-2 was identified shortly later [18]. These two genes show a tandem arrangement on 3R-30A and are spaced by a short intergenic region (~0.8 kb); they encode mature proteins of approx. 13.5 kDa (118 and 116 amino acids, respectively) that are highly enriched or specifically expressed in the female salivary glands [18, 36, 46]. As summarized in Fig. 1 full-length orthologues of gSG7 and gSG7-2 were found in the genomes of most anophelines analyzed here. A third member of the family, which was named SG7-3, was found close by in the genomes of An. gambiae and An. coluzzii; degenerated copies containing frameshifts were also present in An. quadriannulatus and An. merus, whereas it could not be traced in other anophelines. Sequence comparison and phylogenetic analysis indicates that SG7-3 most likely originated from SG7-2 by gene duplication; in An. gambiae there is no evidence of SG7-3 salivary expression, both according to sialotranscriptomes and to microarray analyses, suggesting that either this gene is not expressed at all or may have acquired different tissue-specificity. Alignment of the 37 SG7 family proteins shows a common framework of four highly conserved Cys residues; most SG7-2 proteins and the SG7 of An. minimus and An. culicifacies carry an additional Cys (Additional file 15A). Sequence comparison and secondary structure prediction analysis suggests that SG7 and SG7-2 proteins have a high alpha helical content with four to five conserved helices. Phylogenetic analysis yielded two well distinct clades, suggesting that SG7 and SG7-2 are the products of a gene duplication that predated anopheline radiation (Additional file 15B). A more recent gene duplication may have originated SG7-3 in members of the An. gambiae complex. SG7 proteins share 42–85% identity and SG7-2 family members share 46 to 89% identical amino acid residues, with a minimum overall identity between family members of 29%.
The first member of the family whose function was clarified is the An. stephensi SG7, which was named anophensin and shown to inhibit the kallikrein-kinin system and bradykinin release [106]; it is supposed to help mosquito blood feeding in virtue of this antiinflammatory and anticoagulant action. Surprisingly, the SG7 proteins of An. albimanus (named albicin) and An. darlingi were recently shown to play a different role, being able to inhibit the alternative pathway of complement. On the contrary the An. albimanus SG7-2 and the saliva of a few representative anopheline species from the Old World did not, suggesting this is a specific function evolved in the saliva of New World species [107]. Anophensin binds factor XII (FXII) and high molecular weigth kininogen (HK) while albicin binds the C3 convertase enzymatic complex; it is likely that also other members of the SG7/SG7-2 gene family act by binding and inhibiting players of the hemostatic and/or inflammatory response.
Interestingly, the SG7 family offers a nice paradigm of how novel salivary gene functions may evolve. Indeed, blast searches using the An. gambiae SG7 protein retrieve with high significance (E-values < 6e-12) several anopheline family members but also, with a low significance (E-value 0.1, coverage 56%, identity 31%) the 30 kDa salivary protein from Cx. quinquefasciatus. Inclusion of this entry in a PSI-blast iteration allowed to retrieve 30 kDa family members from Ae. aegypti and Ae. albopictus and, after an additional iteration, also several anopheline 30 kDa proteins. As previously reported [31] a careful examination of the genomic loci encoding the An. gambiae 30 kDa and SG7 proteins suggested that the anopheline SG7 family of proteins most likely originated from the more ancient 30 kDa, an “older” gene already present in the blood feeding ancestor of mosquitoes and black flies. The degree of similarity between SG7/SG7-2/SG7-3 and the 30 kDa protein (exons 3–4) is rather low (in An. gambiae 23–28% identity, 44–47% similarity). However, the conserved Cys residues, predicted secondary structure and intron/exon boundaries support this scenario. The first member of the family was most likely SG7 that has a three-exon structure and may have arisen from the the 30 kDa protein by loss of the exon 2, which encodes the highly acidic and low complexity region enriched in Gly, Glu and Asp residues (Fig. 8, Additional file 15C). Therefore, the “novel” SG7 protein retained the region corresponding to the C-terminal half of the 30 kDa protein, that is encoded by exons three and four and it is the portion responsible for the binding to collagen [95]. The SG7 protein appeared sometime in the progenitor of anopheline mosquitoes after the separation from culicines and then could start to diverge acquiring new binding properties and novel functions, as shown by anophensin and albicin today (see above). Afterwards, duplication of SG7 with loss of the first intron generated SG7-2, an event that certainly took place around 100 mya or more, i.e. before separation of Old World mosquitoes from New World ones. This determined the tandem arrangement of SG7 and SG7-2 that we see today in anopheline species of the three different subgenera Cellia, Anopheles and Nyssorhynchus. SG7-2 underwent a more recent duplication within the An. gambiae species complex, most likely in the last 1.5–2.0 million years. As a consequence, in An. gambiae and An. coluzzii we found a cluster of three genes arranged in close proximity to each other, whereas in An. quadriannulatus and An. merus only degenerated copies containing frameshifts could be traced (Fig. 1). As mentioned earlier the mosquito 30 kDa protein possesses antihemostatic properties in virtue of its capacity to bind collagen, a function of crucial importance considering its conservation in both anophelines and culicines. Its duplication, followed by divergence, allowed the C-terminal domain to evolve new binding properties, as displayed by the An. stephensi anophensin and by the An. albimanus albicin, and provided anopheline mosquitoes with the opportunity to acquire new weapons to fight the inflammatory and hemostatic responses of their hosts.
Fig. 8.
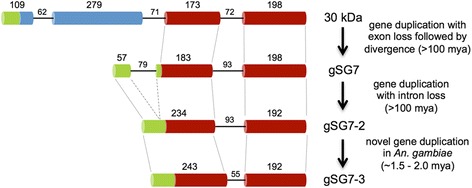
Origin of the SG7 family from the gene encoding the An. gambiae 30 kDa protein. The structure of the 30 kDa, gSG7, gSG7-2 and gSG7-3 genes of An. gambiae is shown. Exon and introns are represented by boxes and lines, respectively. Numbers indicates the length in base pairs. Conserved exon regions are marked in red and the portion encoding signal peptides in green. The likely scenario leading to the origin of the SG7 family as we can observe today in An. gambiae is shortly described on the right (see main text for additional details)
Anopheline salivary proteins as markers of host exposure
Salivary proteins injected into vertebrate hosts during blood feeding elicit an anti-saliva humoral response and, as a consequence, individuals repeatedly bitten by arthropods carry circulating antibodies directed against salivary proteins [7, 17]. This anti-saliva antibody response allows for the evaluation of host exposure to a wide range of arthropod disease vectors as diverse as ticks [108], sand flies [109], triatomines [110], tsetse flies [111] and mosquitoes [112–116]. Initial studies in the field were based on the development of immunoassays based on the use of whole saliva, which is difficult to reproducibly collect in large amounts and, being a complex cocktail, may cause problems with potential cross-reactivity. However, sialotranscriptome studies carried out in the last decade revealed both the complexity and the large diversification of blood feeding arthropod salivary repertoires, paving the way to the exploitation of individual recombinant salivary proteins for the development of immunoassays suitable to evaluate host exposure to specific disease vectors. As previously described the sialome of anopheline mosquitoes includes a group of genus-specific salivary proteins that, at least in principle, represent ideal candidates as markers of host exposure to malaria vectors.
So far two An. gambiae salivary proteins have been tested as indicators of human exposure to malaria vectors: the gSG6 protein and the antithrombin cE5. The gSG6 protein was the first An. gambiae salivary protein shown to be immunogenic to humans [117] and, afterwards, it was validated as marker of human exposure to Afrotropical malaria vectors in different epidemiological settings in Senegal [118–121], Burkina Faso [122, 123], Tanzania [124, 125], Kenya [126] and Uganda [127]. Importantly, the human anti-gSG6 IgG response (i) was short term (i.e. decreased after a few months of absent or low exposure), (ii) it was sensitive enough also in conditions of relatively low vector density and (iii) it was a good marker of exposure to bites of the three major malaria vectors in tropical Africa: An. gambiae, An. arabiensis and An. funestus [128–130]. Moreover, the IgG response to gSG6 proved to be valuable to monitor the efficacy of vector control measures in reducing human-vector contact [131, 132], a precious tool for the implementation and evaluation of malaria control strategies. It should be emphasized that in these studies both the purified recombinant protein and a gSG6-based peptide (gSG6-P1) were employed, indicating that the careful selection of peptides designed on specific salivary proteins can be a reliable and effective strategy, although with the predictable drawback of a loss in marker sensitivity. Among the anophelines analyzed in this study the An. gambiae gSG6 shares good degree of identity with orthologues from the main African (An. arabiensis 98%, An. funestus 80%), Asian (An. stephensi and An. maculatus 79%, An. culicifacies 72%, An. sinensis 61%, An. dirus 54%) and European (An. atroparvus 66%) malaria vectors, whereas a more limited identity (52%) was found with An. farauti, which is known as a relevant vector in Solomon Islands, Vanuatu and part of Australia. Considering the fast evolutionary rate of salivary proteins from blood feeding arthropods (see below), and compared to other anopheline salivary proteins analyzed here, this degree of conservation is rather high and may support the additional exploitation of the An. gambiae gSG6 as indicator of human exposure to at least Asian anopheline vectors. The “high degree” of conservation found in the anophelines considered in this study is in agreement with the indications of strong purifying selection acting on gSG6 in an An. gambiae population from Burkina Faso [133]. The SG6 protein was not found during previous sialome studies in the Nyssorhynchus species An. albimanus and An. darlingi, the two main malaria vectors in Central and South America, and it was absent in the genome of these species, confirming the initial hypothesis that SG6 was either acquired from the progenitor of Old World species or lost in the ancestral lineage leading to Nyssorhynchus. These observations imply that the IgG response to the gSG6 protein/peptide cannot be a reliable indicator of exposure to anopheline vectors in America, at least not in areas where An. darlingi and An. albimanus are among the prevalent anopheline vectors. Therefore, serological data as those recently reported by Londono-Renteria and collaborators on the response to the gSG6-P1 peptide in the Americas [134] should be interpreted with caution and taking into consideration these novel anopheline genomic data.
A second An. gambiae salivary protein studied to a certain extent for its antigenic properties is the antithrombin polypeptide cE5, which was shown to be more immunogenic than gSG6 [135] and may represent a useful sensitive marker to evaluate efficacy of insecticide impregnated bednets in reducing human-vector contact [102]. Strikingly, the two salivary antigens gSG6 and cE5 evoked a substantially different response in naturally exposed individuals from a malaria hyperendemic area of Burkina Faso with (i) gSG6 inducing a short-lived IgG response, high levels of anti-gSG6 IgG4 antibodies and determining immune tolerance after prolonged exposure, whereas (ii) cE5 evoked a more persistent IgG response, dominated by the IgG1 subclass and not inducing tolerance mechanisms [135]. These observations suggest that beyond their exploitation as exposure markers these two antigens may be useful reagents to study the so far neglected role of Anopheles saliva and salivary proteins in the host early immune response to Plasmodium.
In order to identify potential additional candidates to be developed as general markers of human exposure to malaria vectors worldwide, we compared the An. gambiae genus-specific salivary proteins discussed in the previous section to their orthologues from major malaria vectors from Africa (An. arabiensis and An. funestus), Asia (An. stephensi, An. culicifacies, An. maculatus, An. dirus, An. sinensis), Oceania (An. farauti), Europe (An. atroparvus) and South/Central America (An. albimanus and An. darlingi) that were included in this study. The salivary proteins hyp4.2, hyp13 and hyp17 were not included in the analysis because only information on species of the series Pyretophorus were available. The SG6 protein, which may also be considered as a sort of internal control, ranked among the best candidates, despite the limitations mentioned above.
Our comparison also allowed to identify the SG7-2 and the hyp6.2 proteins, which are present in both Old World and New World anophelines, as novel potential candidates to be tested in future epidemiological studies as markers of human exposure to malaria vectors worldwide (Table 1). Furthermore, the information reported here (Table 1, Additional file 2, Fig. 1) may also guide studies aimed at identifying species-specific markers, which would allow for example to distinguish human exposure to members of the An. gambiae species complex from exposure to An. funestus [128].
Table 1.
Divergence of An. gambiae salivary proteins among major malaria vectors
| cE5 | hyp6.2 | hyp8.2 | hyp10 | hyp12 | hyp15 | SG2 | SG2b | SG6 | SG7 | SG7-2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Africa | 59–100 | 68–96 | 39–97 | 59–99 | 47–100 | 58–96 | 66–95 | 66–91 | 80–98 | 68–97 | 72–96 |
| Asia | 35–62 | 62–72 | 21–49 | 57–67 | 41–53 | 42–59 | 45–74 | 59–62 | 54–79 | 55–70 | 64–75 |
| Oceania | 54 | 64 | 44 | 53 | 42 | nf | 55 | nf | 52 | 60 | 67 |
| Europe | 36 | 55 | 32 | abs | abs | 59 | 49 | nf | 66 | 50 | 63 |
| America South-Cent. | 35–37 | 42–54 | 24–25 | abs | abs | 43–48 | NA | nf | abs. | 46–47 | 47 |
The An. gambiae genus-specific salivary proteins indicated on the top were compared to orthologues from malaria vectors in the different continents: Africa (An. arabiensis, An. funestus), Asia (An. stephensi, An. culicifacies, An. maculatus, An. dirus and An. sinensis), Oceania (An. farauti), Europe (An. atroparvus), South and Central America (An. albimanus, An. darlingi). Mature proteins were used for the comparison. Numbers indicate percent of identity or its range when multiple vectors from the same continent were available. Abs, gene not present; nf, gene not found but possibly present or not full-length. The three anopheline-specific salivary proteins more conserved among malaria vectors worldwide are highlighted in bold
Fast evolutionary rate of mosquito salivary proteins
Interspecific comparison of salivary proteins between anopheline mosquito species has previously shown that salivary proteins diverge at a significantly higher rate than housekeeping ones. This was the case for the pairs An. gambiae/An. stephensi, An. gambiae/An. funestus and An. gambiae/An. darlingi whose orthologous salivary proteins displayed an average identity of 62, 66 and 53%, respectively, vs the 93, 96 and 86% found for housekeeping proteins [19, 21, 23]. Similar results were also obtained for the culicines Ae. aegypti/Ae. albopictus [76]. These observations suggested that salivary genes of mosquitoes (and more generally of blood feeding arthropods) are at an accelerated pace of evolution, perhaps under the selective pressure of the host immune system [6]. Indeed signatures of positive selection were found in a limited set of salivary genes from An. gambiae [133]. In addition, over 1000 orthologous genes from species belonging to the subgenus Cellia were analyzed for the presence of signatures of positive selection on individual codons. Among the seven different classes analyzed the salivary genes, similarly to immune genes, appeared to have very high rate of positive selected codons [31].
In order to evaluate divergence rates of individual anopheline salivary proteins we used orthologs multiple alignments to perform pairwise species comparisons and calculate the average percent amino acid identity among all anopheline species and among those belonging to the three different subgenera. Only one member of the An. gambiae species complex, i.e. An. gambiae s.s., was included in the analysis which, therefore, involved up to 14 different species. Fifty orthologous salivary proteins were considered, due to the restriction to Pyretophorus of SerPro2, hyp4.2 and hyp13. In the subgenus Cellia four salivary proteins (hyp37.7-2, SG1a, SG1-like2, hyp8.2) displayed an average identity below 50% and other 23 were in the range of 50 to 70% identity (Additional file 16). Salivary proteins belonging to the category of Enzymes were among the most conserved, with average identity between 73 and 88% excluding epoxy hydrolase. We also selected for comparison 12 housekeeping proteins and found only 2 with percentage of identity below 96%, i.e. Integrin beta 1 (84%) and GSTT1 (glutathione S-transferase theta class 1, 86%). Only two salivary proteins showed identity >90% and comparable to housekeeping: Ag5r3 (95%) and Ag5r6 (94%). However, it should be noted that these two genes, according to Baker and collaborators (2001) and in comparison to other Ag5 family members, are not overexpressed in salivary glands and should not to be considered as typical salivary genes, although we included them in our list (mainly because a low number of Ag5r3 transcripts were found in a previous transcriptome study and because Ag5r6 is located close to and originated from Ag5r3 by gene duplication). Two anopheline salivary proteins, excluding those belonging to the Enzymes category, appeared more conserved as compared to the remaining ones, i.e. gVAG and D7r2 (83 and 82% identity among Cellia, respectively): both are members of multigene families and widespread among blood feeding arthropods. The “low” divergence of gVAG and D7r2 among anopheline mosquitoes found here is fully consistent with a previous polymorphism analysis of a few salivary genes in an An. gambiae population from Burkina Faso. In this study these genes showed the highest nucleotide diversity values in both coding and non-coding regions and the lowest dN/dS ratios, and they were suggested to be under strong evolutionary constraints negatively selecting replacement substitutions [133]. A situation similar to the one described above for Cellia species is found when average identities are calculated including all anophelines.
We also used multiple alignments of orthologous coding sequences to calculate a few additional parameters such as the number of amino acid substitution per site (d), the number of synonimous substitution per synonimous site (dS), the number of nonsynonimous substitution per nonsynonimous site (dN), the ratio dN/dS and the nucleotide diversity per site (Pi) (Additional file 16). Interestingly, members of the SG1 family showed a remarkably high diversity and appeared to be under strong selective pressure. In fact, when the salivary protein genes listed in Additional file 16 were sorted by the dN/dS ratio the seven SG1 family members ranked amongst the thirteen with the higher dN/dS ratio. It is worth pointing out that one of the SG1 family member, the saglin protein, was previously shown to be involved in salivary gland invasion by Plasmodium sporozoites [96, 97]. We do not know if other SG1 family members may play similar roles although, as previously mentioned, the SM1 peptide was shown by cross-linking experiments to interact not only with Saglin but also with SG1 [97]. Nevertheless, the very high diversity of saglin and other SG1 family members reported here, which may imply a similarly high inter- and intra-population diversity, raises a question on its possible connection with inter- and intra-specific variation in malaria parasite transmission capacity.
Conclusions
Exploiting the availability of the genome of sixteen anopheline species [31] we provided here a comprehensive overview of the major salivary protein families of anopheline mosquitoes. Overall, 824 full-length salivary proteins were included in our study, which allowed to identify 79 proteins not previously annotated and to correct 379 wrong predictions. This information was used for multiple alignments, phylogenetic analyses, secondary structure predictions and, importantly, to assemble an hyperlinked excel spreadsheet carrying additional documentation and made available as supplemental material. The anopheline species analyzed here span approximately 100 million years of evolution and the analysis of their salivary repertoires helped shedding some light on the main mechanisms driving the evolution of salivary proteins in anophelines and, more generally, in blood feeding arthropods. Gene duplication, followed by divergence, was one of the major driving forces and certainly a key mechanism, as clearly testified by the several duplicated salivary genes and the large multigene families (i.e. D7, Ag5 and SG1 families). The impact of gene duplication(s) in shaping the anopheline sialomes appears striking when the known An. gambiae salivary genes are mapped to their chromosomal location on polytene chromosomes as shown in Additional file 17. This extensive duplication of salivary genes may be not so surprising if one considers the redundant and multifaceted response of vertebrate hosts to tissue injury. Indeed redundant salivary repertoires (with proteins of the same family playing slightly different functions as for the short D7 proteins) may provide an opportunity to more efficiently face host hemostatic and inflammatory responses and, in addition, may contribute to a better adaption to feeding on a panel of different vertebrate hosts. However, gene duplication was not only important in the fine-tuning of salivary functions but also played a role on the evolution of novel salivary protein families and, therefore, of novel functions. This is nicely shown by the SG7 family which provides a paradigm of how salivary proteins with novel binding properties (inhibitors of complement and plasma contact system) evolved from the 30 kDa gene, encoding a platelet inhibitor binding collagen. Evolutionary pressure also must have played a relevant role and resulted in the very high rate of divergence of salivary proteins among anopheline mosquitoes: these observations further corroborate the idea that salivary genes from blood feeding arthropods should be added to the small number of gene classes under positive selection [31, 133]. Overall, we expect that this extended anopheline sialome catalogue will be useful to the vector biology community, both to clarify the role of the many “orphan” mosquito salivary proteins still without a function and to guide their possible exploitation for epidemiological and vector-host-pathogen interaction studies.
Methods
Anopheles species
The following Anopheles species and genome assemblies were used for the identification of orthologues of the 53 An. gambiae salivary proteins considered in this study: Anopheles gambiae (strain PEST, assembly AgamP4, gene set AgamP4.3), Anopheles coluzzii (strain Mali-NIH, assembly AcolM1, gene set AcolM1.2), Anopheles arabiensis (strain Dongola, assembly AaraD1, gene set AaraD1.3), Anopheles quadriannulatus A (strain SANGQUA, assembly AquaS1, gene set AquaS1.3), Anopheles merus (strain MAF, assembly AmerM2, gene set AmerM2.1), Anopheles melas (strain CM1001059_A, assembly AmelC2, gene set AmelC2.1), An christyi (strain ACHKN1017, assembly AchrA1, gene set AchrA1.3), Anopheles epiroticus (strain Epiroticus2, assembly AepiE1, gene set AepiE1.3), Anopheles funestus (strain FUMOZ, assembly AfunF1, gene set AfunF1.3), Anopheles minimus (strain MINIMUS1, assembly AminM1, gene set AminM1.3), Anopheles culicifacies (strain A-37, assembly AculA1, gene set AculA1.3), Anopheles stephensi (strain SDA-500, assembly AsteS1, gene set AsteS1.3), Anopheles maculatus (strain maculatus3, assembly AmacM1, gene set AmacM1.3), Anopheles farauti (strain FAR1, assembly AfarF2, gene set AfarF2.1), Anopheles dirus spA (strain WRAIR2, assembly AdirW1, gene set AdirW1.3), Anopheles atroparvus (strain EBRO, assembly AatrE1, gene set AatrE1.3), Anopheles sinensis (strain SINENSIS, assembly AsinS2, gene set AsinS2.1), Anopheles albimanus (strain STECLA, assembly AalbS1, gene set AalbS1.3), Anopheles darlingi (strain Coari, assembly AdarC3, gene set AdarC3.3).
Gene annotation
Protein sequences of the 53 An. gambiae salivary proteins listed in Additional file 1 were used to search the genomes of the eighteen anopheline species reported above using the blast tool [136] at the VectorBase web site [32]. Orthologous genes were retrieved through the genome browser and manually annotated using the Artemis tool [33, 137]. The An. funestus D7L2 and a few salivary genes from An. darlingi could not be found searching the genomes of these species but were previously reported among the genes identified through transcriptome analysis [19, 21] and were therefore included in Fig. 1 and marked in red. Fasta files including nucleotide coding sequences (from ATG to stop codon) and amino acid sequences of the 824 full-length anopheline salivary genes analyzed here were compiled and used for mapping to the hyperlinked spreadsheet. Among the 824 full-length sequences, 79 were not previously annotated and represent therefore novel protein coding sequences, whereas 379 represented wrong predictions. These sequences were sent to VectorBase curator for inclusion/correction in the next releases. Fasta files including the nucleotide coding sequences and corresponding polypeptides are included as supplemental material (Additional files 18 and 19).
Hyperlinked spreadsheet
Protein and their coding sequences were mapped to a hyperlinked spreadsheet as detailed previously [138], including the presence of signal peptide indicative of secretion [139], transmembrane domains [140], matches to the gene ontology database [141], to the NCBI set of proteins from Diptera, as well as rpsblast [142], matches to the Conserved Domains Database [143] and related motif databases. The data was also clustered as described in [77], facilitating organization of the database by sorting on clusters of related proteins.
Multiple alignments, secondary structure predictions and phylogenetic analysis
Multiple alignments were obtained by Clustal Omega [144] using as input, whenever possible, mature peptides as predicted by SignalP 4.0 [145]. Secondary structure predictions were performed using the PSIPRED server [146]. Alignments obtained by Clustal Omega were imported in MEGA 6 and used for evolutionary analyses [147]. Trees were constructed using the Neighbor-Joining method [148] and tested by 10000 bootstrap replications [149]. The evolutionary distances were computed using the Poisson correction method [150] and are in the units of the number of aminoacid substitutions per site. The pairwise deletion option was used to deal with gaps/missing data. Data used for the construction of phylogenetic trees are provided in Additional file 20.
Divergence of salivary proteins in anophelines
The diversity of salivary proteins among anophelines was evaluated for 50 salivary proteins (SerPro2, hyp4.2 and hyp13 were excluded because restricted to Pyretophorus species) in up to 14 different species (only An. gambiae was included as representative of the complex) using, whenever possible, the sequence of mature proteins. As a control the diversity among orthologues of 12 housekeeping proteins in the different species was also calculated (see Additional file 16). For each protein the mean percent identity among anophelines, Cellia, Anopheles and Nyssorhynchus was calculated as the average of the different pairwise comparisons of the orthologues aligned by Clustal Omega. The number of aminoacid substitution per site (d) was estimated by MEGA 6 (1000 bootstrap replications, Poisson model, uniform rates among sites, pairwise deletion option) [147]. Multiple alignments of the nucleotide coding sequences were constructed using the corresponding aligned aminoacid sequences as scaffold by the RevTrans 1.4 Server [151]. The number of synonimous substitution per synonimous site (dS) and the number of nonsynonimous substitution per nonsynonimous site (dN) were computed by MEGA 6 (1000 bootstrap replications, Nei-Gojobori method, pairwise deletion option). The number of variable sites (S) and the nucleotide diversity per site (Pi) were calculated using DnaSP 5.10 [152].
Acknowledgements
We thank Prof. Vincenzo Petrarca for providing the high resolution polytene map of the An. gambiae chromosomes and for the permission to use after modification.
Funding
BA was supported by funds from the European Union grant INFRAVEC (228421) and from the Ministry of Education, University and Research (MIUR) grant SKINFLAM (2010C2LKKJ_004). JMCR by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institute of Health, USA. CJS was supported by Brazilian Research Council (CNPq) and FAPERJ.
Availability of data and materials
Data generated or analysed during this study are already publicly available or included in this article as Additional files. Additional file 2 is an hyperlinked spreadsheed with links stored in Exon, which is a distribution server for software and genomic data resources developed by the NIAID research community (https://exon.niaid.nih.gov/). Links will also be provided by the authors upon request for local download/use.
Authors’ contributions
Conception and design of the study: BA JMCR. Gene annotation: BA FL JMCR. Bioinformatic analysis: BA JMCR. Data analysis and contribution to writing the manuscript: BA FL CJS JMCR. Writing of the first manuscript draft: BA. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- 5HT
Serotonin or 5-hydroxytryptamine
- ADP
Adenosine diphosphate
- Ag5
Antigen 5
- AMP
Adenosine monophosphate
- ATP
Adenosine triphosphate
- CAP
Cystein-rich secretory proteins, antigen 5, pathogenesis-related one proteins
- CUB
Complement C1r/C1s, Uegf, Bmp1
- CysLT
Cysteinyl leukotrienes
- dN
Number of nonsynonimous substitutions per nonsynonimous site
- dS
Number of synonimous substitutions per synonimous site
- E
Epinephrine or adrenalin
- FSG
Female salivary glands
- FXII
Factor XII
- GPI
Glycophosphatidylinositol
- H
Histamine
- HK
High molecular weight kininogen
- IgG
Immunoglobulin G
- MSG
Male salivary glands
- NE
Norepinephrine or noradrenalin
- OBP
Odorant binding proteins
- pI
Isoelectric point
- Pi
Nucleotide diversity per site
- PSI-BLAST
Position specific iterative basic local alignment search tool
- SDS-PAGE
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- TXA2
Thromboxane A2
- vWF
Von Willebrand factor
Additional files
List of An. gambiae salivary proteins used to search anopheline genomes. Protein names, VectorBase accession numbers, length, predicted molecular weight, location on the An. gambiae chromosomes and orientation (F, forward; R, reverse) are reported. Chromosomal locations in colours point to genes arranged in clusters. (PDF 42 kb)
Hyperlinked spreadsheet carrying 824 full-length salivary proteins from 19 anopheline species. Nucleotide coding sequences, peptide sequences, accession numbers (if available), status (novel or annotation needing correction) and several additional informations (protein length, molecular weight, pI, presence of secretory signal and potential glycosilation sites, results of blast searches, etc.) are reported. (XLSX 938 kb)
Phylogram of the anopheline apyrase and 5'-nucleotidase proteins. The numbers in the phylogram nodes show the percent bootstrap support for the phylogeny (≥70%). The bar at the bottom indicates 5% aminoacid divergence in the sequences. Species belonging to the subgenera Cellia, Anopheles and Nyssorhynchus are labelled with dots, triangles and diamonds, respectively. Within Cellia species belonging to the series Pyretophorus (black), Myzomyia (blue), Neocellia (pink) and Neomyzomyia (yellow) are shown. (PDF 24 kb)
Cluster of 5 peroxidase genes An. albimanus. (Top) Five peroxidase genes are clustered in a region of approximately 15 kb in An. albimanus. Sal_Perox is the heme peroxidase with catechol oxidase/peroxidase activity characterized by Ribeiro JM and Valenzuela J (1999) [54]. Sal_Perox2 is the orthologue of the An. gambiae AGAP010735 identified during a sialotranscriptome analysis [18] and it is indicated as a bona fide salivary peroxidase. The other genes of the cluster are indicated simply as Perox genes due to the absence of any evidence of expression in the salivary glands. (Bottom) Percentage of identity among the different putative proteins as indicated. Note the high identity af anoalb_Perox4 to anoalb_Sal_Perox (72.33%), which is suggestive of a relatively more recent gene duplication. (PDF 1284 kb)
Alignment and phylogram of anopheline salivary serine proteases Sal_SerPro1-3. (A) Multiple alignment of mature anopheline salivary serine proteases Sal_SerPro1-3. Conserved cysteines (red), fully conserved residues (yellow) and the catalytic triad H, D, S (orange) are highlighted. Residues identical in at least 75% of the aligned sequences are shown in green. Species names are abbreviated with the first three letters of the generic name and the first three-four letters of the specific name. VectorBase accession numbers follow (when available). (B) Phylogram of the anopheline Sal_SerPro 1–3 proteins. The numbers in the phylogram nodes indicate the percent bootstrap support (≥70%) for the phylogeny. The bar at the bottom indicates 5% aminoacid divergence in the sequences. The clades including Sal_SerPro1, Sal_SerPro2 and Sal_SerPro3 are shown with different colours. (PDF 1739 kb)
Alignment of the anopheline Sal_trypXII. Multiple alignment of the anopheline mature salivary serine protease Sal_trypXII. Conserved cysteines (red), fully conserved residues (yellow) and the catalytic triad H, D, S (orange) are highlighted. The conserved (K/R)GD motif is boxed. Residues identical in at least 75% of the aligned sequences are shown in green. Species names are abbreviated as in Additional file 5 and followed by VectorBase accession numbers (when available). (PDF 74 kb)
Alignment of the anopheline Antigen 5 family proteins. Multiple alignment of the anopheline mature Ag5 family members showing the fully conserved residues (yellow) and the cysteines (red). The DPGR/K tetrapeptide is boxed with the DPGR highlighted in pink. Species names are abbreviated as in Additional file 5 and followed by VectorBase accession numbers (when available). (PDF 181 kb)
Phylogram of the anopheline Antigen 5 family proteins. The numbers in the phylogram nodes show the percent bootstrap support for the phylogeny (≥70%). The bar at the bottom indicates 10% aminoacid divergence in the sequences. The four clades including gVAG, Ag5r4 and the two pairs of duplicated genes Ag5r2/Ag5r5 and Ag5r3/Ag5r6 are marked. Dots were used to label anopheline Ag5r2 (red), Ag5r5 (black), Ag5r3 (green) and Ag5r6 (light blue). (PDF 115 kb)
Alignment of the anopheline 30 kDa family members. Multiple alignment of the 30 kDa proteins from 19 anopheline species. Cysteins are highlighted in red, other fully conserved residues in yellow. Gly, Glu and Asp residues are shown in a dark blackground. The first two blocks represent the acidic N-terminal domain. The two alpha helices deduced from the X-ray-resolved crystal of the An. stephensi aapp in complex with a mouse Fab antibody are shown above the alignment as blue cilinders (http://www.rcsb.org/pdb/explore.do?structureId=4okv; [94]). Species names are abbreviated as in Additional file 5 and followed by VectorBase accession numbers (when available). (PDF 95 kb)
The anopheline SG1 protein family. (A) Schematic representation of the cluster including SG1, SG1a, Saglin, SG1-like2 and SG1-like3 on the An. gambiae X chromosome. The genes with the direction of transcription, accession numbers, length in nucleotide of coding regions and intervening sequences and the names of the encoded proteins are shown. (B) Phylogram including the 119 full-length SG1 family proteins from anophelines (Additional file 2) plus the An. darlingi Saglin and SG1-like3 from a previous transcriptome [21]. The numbers in the phylogram nodes indicate percent bootstrap support for the phylogeny (≥90%). The bar indicates 20% aminoacid divergence in the sequences. The different clades and corresponding family members are colour-coded as follows: SG1 (red), SG1a (green), Saglin (blue), SG1-like2 (pink), SG1-like3 (light blue), SG1b (yellow), TRIO (orange). (PDF 2039 kb)
Alignment of mosquito 55.3 kDa/56.5 kDa proteins. Multiple alignment of the 16 full-length anopheline 55.3 kDa family members and the three 56.5 kDa proteins from Cx. pipiens quinquefasciatus, Ae. aegypti and Ae. albopictus. Cysteins are highlighted in red and other fully conserved residues in yellow. Residues conserved in at least seventeen of the nineteen aligned sequences (~90) are highlighted in green. The Highly conserved block of 27 amino acids toward the N-terminus is boxed. Species names are abbreviated with the first three letters of the generic name and the first three-four letters of the specific name. Species names are abbreviated as in Additional file 5 and followed by VectorBase accession numbers (when available). (PDF 121 kb)
Alignment of the anopheline mature cE5/anophelin family members. Residues conserved in at least 2/3 of the sequences (green) or fully conserved (yellow) are highlighted. The amino-terminal conserved region, the DPGR tetrapeptide involved in trombin binding and the RGD tripeptide are boxed. Species names are abbreviated as in Additional file 5 and followed by VectorBase accession numbers (when available). (PDF 1384 kb)
Alignment and phylogram of the anopheline hyp10/hyp12 proteins. (A) Multiple alignment of the anopheline mature hyp10/hyp12 family members. Fully conserved residues (yellow), cysteins (red) and residues conserved in at least 2/3 of the aligned sequences (green) are highlighted. The predicted alpha helical regions are shown above the alignment as blue cilinders. Species names are abbreviated as in Additional file 5. (B) Phylogram including the 28 full-length hyp10 and hyp12 proteins from Cellia species. The numbers in the phylogram nodes indicate percent bootstrap support for the phylogeny (≥70%). The bar indicates 10% aminoacid divergence. Hyp10 and hyp12 family members are labelled by light blue and purple dots, respectively. (PDF 4131 kb)
Alignment of the anopheline SG6 family proteins. Fully conserved residues (yellow), cysteins (red) and residues conserved in at least 2/3 of the aligned sequences (green) are highlighted. Species names are abbreviated as in Additional file 5. (PDF 48 kb)
Anopheline SG7 family proteins: alignment, phylogram and comparison to the 30 kDa protein. (A) Multiple alignment of the 37 full-length anopheline SG7 family proteins. Fully conserved residues (yellow), cysteins (red) and residues conserved in at least 2/3 of the aligned sequences (green) are highlighted. Fully conserved residues in SG7 and SG7-2 proteins are also highlited in pink and light blue, respectively. Species names are abbreviated as in Additional file 5. (B) Phylogram of the SG7 anopheline proteins. The numbers in the phylogram nodes indicate percent bootstrap support for the phylogeny (≥70%). The bar indicates 10% aminoacid divergence. SG7, SG7-2 and SG7-3 proteins are labelled by orange triangles and by green and red dots, respectively. (C) Alignment of the An. gambiae 30 kDa and SG7 proteins. The different exons are marked with different colours to show exon junction conservation. Cysteins are highlighted in red and signal peptides are boxed. (PDF 1742 kb)
Divergence of orthologous salivary proteins among anophelines and in the subgenera Cellia, Anopheles and Nyssorhynchus. (Worksheet 1_salivary genes divergence) Protein name, corresponding An. gambiae accession number, size in amino acids, location on the An. gambiae chromosomes and average percent aminoacid identity ± standard deviation are reported. N, number of sequences; S, number of variable sites; d, number of amino acid substitution per site (average over all sequence pairs); dS, number of synonimous substitutions per synonimous site; dN, number of nonsynonimous substitutions per nonsynonimous site; Pi, nucleotide diversity per site; SE, standard error; SD, standard deviation. Average identities ± standard deviations were calculated for each protein from the pairwise comparisons between the different species. Only An. gambiae s.s. was included in the analysis as representative of the An. gambiae species complex. The grey shading indicates absence of the gene in the corresponding subgenus. NA, not applicable. Salivary proteins with percentage of identity below 50% (orange), between 50 and 60% (green), 60 and 70% (yellow) and above 90% (light blue) are highlighted. Identities in 12 orthologous housekeeping proteins are shown for comparison. EIF1A, Eukaryotic Initiation Factor 1A; G6PD, glucose-6-phosphate 1-dehydrogenase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GSTT1, glutathione S-transferase theta class 1; rpL5, ribosomal protein L5; rpS7, ribosomal protein S7; SDH, succinate dehydrogenase. (Worksheet 2_sorted by dN/dS) Salivary protein genes were sorted by dN/dS ratio. The thirteen with the higher dN/dS ratio and including all the seven SG1 family members are boxed. (XLSX 94 kb)
A polytene chromosome map of the An. gambiae salivary genes. Anopheles gambiae salivary genes mapped to their chromosomal location on the polytene chromosomes. Gene duplications are shown in bold and red; unrelated salivary genes located in the same chromosomal division are marked in bold and green. The twenty six additional genes previously identified [18] and not included in this study and their accession ID (when available) are: hyp1.2, AGAP005764, hypothetical 1.2 secreted peptide; hyp3.5, AGAP004836, hypothetical 3.5 putative secreted salivary peptide; hyp5.6, hypothetical 5.6 salivary basic secreted peptide; hyp6.3, AGAP007195, hypothetical 6.3 salivary protein; hyp11, AGAP001713, hypothetical salivary protein 11; hyp14.5, AGAP004883, hypothetical 14.5 similar to Culex 14.5 kDa salivary peptide; hyp14.5-1, AGAP001174, hypothetical 14.5-1 similar to Culex 14.5 kDa salivary peptide; hyp14.6, AGAP002085, hypothetical 14.6 putative secreted protein conserved in insects; hyp36, AGAP000911, hypothetical 36 kDa secreted peptide; C-rich, AGAP011183, cystein-rich repeat containing protein; mucin-like, AGAP002771, mucin-like protein; peroxinectin, peroxinectin precursor; Sal_C Lectin, AGAP006267, salivary c-type lectin; Sal_Calreticulin, AGAP004212, salivary calreticulin; Sal_Chymotryp, salivary chymotrypsin; Sal_cut, AGAP008450, salivary secreted protein – possible cuticle or duct protein; Sal_Galectin, AGAP001197, salivary galectin; Sal_Lyso1, AGAP007347, salivary lysozyme precursor – less abundant form; Sal_Lyso2, AGAP007385, salivary lysozyme precursor – abundant form; Sal-MMP1, matrix metalloproteinase 1 partial – may be secreted; Sal_PPOA1, AGAP004639, secreted serine protease possibly involved with prophenoloxidase activation; Sal_PPOA2, AGAP010968, secreted serine protease possibly involved with prophenoloxidase activation; Sal_ret, AGAP006148, putative secreted salivary protein similar to Drosophila retinin; Selenoprotein, AGAP004986, salivary selenoprotein; SG3, AGAP006507, SG3 protein – salivary mucin; SG10, AGAP003841, gSG10. Figure modified from Supplemental Fig. 1, Coluzzi M. et al., Science 298:1415 (2002) with author’s permission (V. Petrarca). Reprinted with permission from AAAS. (PDF 2400 kb)
Coding sequences in fasta format. This file includes the coding sequences of the 824 anopheline salivary proteins analyzed in this study and included in Additional file 2 (excel spreadsheet). The file is in fasta format (.fas) and can be viewed with a text editor. (FAS 736 kb)
Peptide sequences in fasta format. This file includes the amino acid sequences of the 824 anopheline salivary proteins analyzed in this study and included in Additional file 2 (excel spreadsheet). The file is in fasta format (.fas) and can be viewed with a text editor. (FAS 266 kb)
Phylogenetic data. The zipped file includes data used for the phylogenetic trees reported in Fig. 3 and Additional files 3, 5, 8, 10, 13 and 15. The.meg files are the protein alignments used for the construction of the phylogenetic trees (.mts files). All the files are in MEGA format and can be viewed using the software MEGA (Molecular Evolutionary Genetic Analysis). (ZIP 147 kb)
Contributor Information
Bruno Arcà, Email: bruno.arca@uniroma1.it.
Fabrizio Lombardo, Email: fabrizio.lombardo@uniroma1.it.
Claudio J. Struchiner, Email: stru@fiocruz.br
José M. C. Ribeiro, Email: JRIBEIRO@niaid.nih.gov
References
- 1.WHO. World Malaria Report 2015. 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/. Accessed 7 Feb 2017.
- 2.Aly AS, Vaughan AM, Kappe SH. Malaria parasite development in the mosquito and infection of the mammalian host. Annu Rev Microbiol. 2009;63:195–221. doi: 10.1146/annurev.micro.091208.073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pimenta PF, Touray M, Miller LH. The journey of malaria sporozoites in the mosquito salivary gland. J Euk Microbiol. 1994;41:608–24. doi: 10.1111/j.1550-7408.1994.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro JMC. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect Agents Dis. 1995;4:143–52. [PubMed] [Google Scholar]
- 5.Ribeiro JMC, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu Rev Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro JMC, Arcà B. From sialomes to the sialoverse: an insight into salivary potion of blood-feeding insects. Adv Insect Physiol. 2009;37:59–118. doi: 10.1016/S0065-2806(09)37002-2. [DOI] [Google Scholar]
- 7.Fontaine A, Diouf I, Bakkali N, Misse D, Pages F, Fusai T, et al. Implication of haematophagous arthropod salivary proteins in host-vector interactions. Parasit Vectors. 2011;4:187. doi: 10.1186/1756-3305-4-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science. 2000;290:1351–4. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- 9.Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436:573–7. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider BS, Higgs S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med Hyg. 2008;102:400–8. doi: 10.1016/j.trstmh.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Titus RG, Ribeiro JMC. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239:1306–8. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 12.Wikel S. Ticks and tick-borne pathogens at the cutaneous interface: host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front Microbiol. 2013;4:337. doi: 10.3389/fmicb.2013.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamhawi S, Aslan H, Valenzuela JG. Vector saliva in vaccines for visceral leishmaniasis: a brief encounter of high consequence? Front Public Health. 2014;2:99. doi: 10.3389/fpubh.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leitner WW, Wali T, Costero-Saint DA. Is arthropod saliva the achilles’ heel of vector-borne diseases? Front Immunol. 2013;4:255. doi: 10.3389/fimmu.2013.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDowell MA. Vector-transmitted disease vaccines: targeting salivary proteins in transmission (SPIT) Trends Parasitol. 2015;31:363–72. doi: 10.1016/j.pt.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Titus RG, Bishop JV, Mejia JS. The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunol. 2006;28:131–41. doi: 10.1111/j.1365-3024.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 17.Coutinho-Abreu IV, Guimaraes-Costa AB, Valenzuela JG. Impact of insect salivary proteins in blood feeding, host immunity, disease, and in the development of biomarkers for vector exposure. Curr Opin Insect Sci. 2015;10:98–103. doi: 10.1016/j.cois.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arcà B, Lombardo F, Valenzuela JG, Francischetti IM, Marinotti O, Coluzzi M, et al. An updated catalogue of salivary gland transcripts in the adult female mosquito, Anopheles gambiae. J Exp Biol. 2005;208:3971–86. doi: 10.1242/jeb.01849. [DOI] [PubMed] [Google Scholar]
- 19.Calvo E, Dao A, Pham VM, Ribeiro JM. An insight into the sialome of Anopheles funestus reveals an emerging pattern in anopheline salivary protein families. Insect Biochem Mol Biol. 2007;37:164–75. doi: 10.1016/j.ibmb.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calvo E, Pham VM, Lombardo F, Arcà B, Ribeiro JMC. The sialotranscriptome of adult male Anopheles gambiae mosquitoes. Insect Biochem Mol Biol. 2006;36:570–5. doi: 10.1016/j.ibmb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Calvo E, Pham VM, Marinotti O, Andersen JF, Ribeiro JM. The salivary gland transcriptome of the neotropical malaria vector Anopheles darlingi reveals accelerated evolution of genes relevant to hematophagy. BMC Genomics. 2009;10:57. doi: 10.1186/1471-2164-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro JM, Mans BJ, Arcà B. An insight into the sialome of blood-feeding Nematocera. Insect Biochem Mol Biol. 2010;40:767–84. doi: 10.1016/j.ibmb.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valenzuela JG, Francischetti IM, Pham VM, Garfield MK, Ribeiro JMC. Exploring the salivary gland transcriptome and proteome of the Anopheles stephensi mosquito. Insect Biochem Mol Biol. 2003;33:717–32. doi: 10.1016/S0965-1748(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 24.Chaerkady R, Kelkar DS, Muthusamy B, Kandasamy K, Dwivedi SB, Sahasrabuddhe NA, et al. A proteogenomic analysis of Anopheles gambiae using high-resolution fourier transform mass spectrometry. Genome Res. 2011;21:1872–81. doi: 10.1101/gr.127951.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choumet V, Attout T, Chartier L, Khun H, Sautereau J, Robbe-Vincent A, et al. Visualizing non infectious and infectious Anopheles gambiae blood feedings in naive and saliva-immunized mice. PLoS One. 2012;7:e50464. doi: 10.1371/journal.pone.0050464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choumet V, Carmi-Leroy A, Laurent C, Lenormand P, Rousselle JC, Namane A, et al. The salivary glands and saliva of Anopheles gambiae as an essential step in the Plasmodium life cycle: a global proteomic study. Proteomics. 2007;7:3384–94. doi: 10.1002/pmic.200700334. [DOI] [PubMed] [Google Scholar]
- 27.Fontaine A, Fusai T, Briolant S, Buffet S, Villard C, Baudelet E, et al. Anopheles salivary gland proteomes from major malaria vectors. BMC Genomics. 2012;13:614. doi: 10.1186/1471-2164-13-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalume DE, Okulate M, Zhong J, Reddy R, Suresh S, Deshpande N, et al. A proteomic analysis of salivary glands of female Anopheles gambiae mosquito. Proteomics. 2005;5:3765–77. doi: 10.1002/pmic.200401210. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro JM, Arcà B, Lombardo F, Calvo E, Phan VM, Chandra PK, et al. An annotated catalogue of salivary gland transcripts in the adult female mosquito, Aedes aegypti. BMC Genomics. 2007;8:6. doi: 10.1186/1471-2164-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribeiro JM, Martin-Martin I, Arca B, Calvo E. A deep insight into the sialome of male and female Aedes aegypti mosquitoes. PLoS One. 2016;11:e0151400. doi: 10.1371/journal.pone.0151400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neafsey DE, Waterhouse RM, Abai MR, Aganezov SS, Alekseyev MA, Allen JE, et al. Mosquito genomics. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes. Science. 2015;347:1258522. doi: 10.1126/science.1258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Megy K, Emrich SJ, Lawson D, Campbell D, Dialynas E, Hughes DS, et al. VectorBase: improvements to a bioinformatics resource for invertebrate vector genomics. Nucleic Acids Res. 2012;40:D729–34. doi: 10.1093/nar/gkr1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, Rajandream MA, et al. Artemis: sequence visualization and annotation. Bioinformatics. 2000;16:944–5. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- 34.Arcà B, Lombardo F, Capurro M, della Torre A, Spanos L, Dimopoulos G, et al. Salivary gland-specific gene expression in the malaria vector Anopheles gambiae. Parassitologia. 1999;41:483–7. [PubMed] [Google Scholar]
- 35.Arcà B, Lombardo F, de Lara Capurro M, della Torre A, Dimopoulos G, James AA, et al. Trapping cDNAs encoding secreted proteins from the salivary glands of the malaria vector Anopheles gambiae. Proc Natl Acad Sci U S A. 1999;96:1516–21. doi: 10.1073/pnas.96.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanfrancotti A, Lombardo F, Santolamazza F, Veneri M, Castrignano T, Coluzzi M, et al. Novel cDNAs encoding salivary proteins from the malaria vector Anopheles gambiae. FEBS Lett. 2002;517:67–71. doi: 10.1016/S0014-5793(02)02578-4. [DOI] [PubMed] [Google Scholar]
- 37.Francischetti IM. Platelet aggregation inhibitors from hematophagous animals. Toxicon. 2010;56:1130–44. doi: 10.1016/j.toxicon.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guiguet A, Dubreuil G, Harris MO, Appel HM, Schultz JC, Pereira MH, et al. Shared weapons of blood- and plant-feeding insects: Surprising commonalities for manipulating hosts. J Insect Physiol. 2016;84:4–21. doi: 10.1016/j.jinsphys.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Ribeiro JM, Broce AB, Wilkerson MJ, Kanost MR. An insight into the transcriptome and proteome of the salivary gland of the stable fly, Stomoxys calcitrans. Insect Biochem Mol Biol. 2009;39:607–14. doi: 10.1016/j.ibmb.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cupp EW, Cupp MS, Ribeiro JM, Kunz SE. Blood-feeding strategy of Haematobia irritans (Diptera: Muscidae) J Med Entomol. 1998;35:591–5. doi: 10.1093/jmedent/35.4.591. [DOI] [PubMed] [Google Scholar]
- 41.Andersen JF, Hinnebusch BJ, Lucas DA, Conrads TP, Veenstra TD, Pham VM, et al. An insight into the sialome of the oriental rat flea, Xenopsylla cheopis (Rots) BMC Genomics. 2007;8:102. doi: 10.1186/1471-2164-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charlab R, Valenzuela JG, Rowton ED, Ribeiro JMC. Toward an understanding of the biochemical and pharmacological complexity of the saliva of a hematophagous sand fly Lutzomyia longipalpis. Proc Natl Acad Sci U S A. 1999;96:15155–60. doi: 10.1073/pnas.96.26.15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valenzuela JG, Charlab R, Galperin MY, Ribeiro JMC. Purification, cloning, and expression of an Apyrase from the bed bug Cimex lectularius. J Biol Chem. 1998;273:30583–90. doi: 10.1074/jbc.273.46.30583. [DOI] [PubMed] [Google Scholar]
- 44.Champagne DE, Smartt CT, Ribeiro JMC, James AA. The salivary gland-specific apyrase of the mosquito Aedes aegypti is a member of the 5′-nucleotidase family. Proc Natl Acad Sci U S A. 1995;92:694–8. doi: 10.1073/pnas.92.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lombardo F, Di Cristina M, Spanos L, Louis C, Coluzzi M, Arcà B. Promoter sequences of the putative Anopheles gambiae apyrase confer salivary gland expression in Drosophila melanogaster. J Biol Chem. 2000;275:23861–8. doi: 10.1074/jbc.M909547199. [DOI] [PubMed] [Google Scholar]
- 46.Baker DA, Nolan T, Fischer B, Pinder A, Crisanti A, Russell S. A comprehensive gene expression atlas of sex- and tissue-specificity in the malaria vector, Anopheles gambiae. BMC Genomics. 2011;12:296. doi: 10.1186/1471-2164-12-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ribeiro JMC, Rowton ED, Charlab R. The salivary 5′-nucleotidase/phosphodiesterase of the hematophagous sand lutzomyia fly, Lutzomyia longipalpis. Insect Bioch Mol Biol. 2000;30:279–85. doi: 10.1016/S0965-1748(99)00123-X. [DOI] [PubMed] [Google Scholar]
- 48.Kaczmarek E, Koziak K, Sévigny J, Siegel JB, Anrather J, Beaudoin AR, et al. Identification and characterization of CD39/Vascular ATP diphosphohydrolase. J Biol Chem. 1996;271:33116–22. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 49.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grossman GL, James AA. The salivary glands of the vector mosquito, Aedes aegypti, express a novel member of the amylase gene family. Insect Mol Biol. 1993;1:223–32. doi: 10.1111/j.1365-2583.1993.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 51.James AA, Blackmer K, Racioppi JV. A salivary gland-specific, maltase-like gene of the vector mosquito, Aedes aegypti. Gene. 1989;75:73–83. doi: 10.1016/0378-1119(89)90384-3. [DOI] [PubMed] [Google Scholar]
- 52.Marinotti O, James AA, Ribeiro JC. Diet and salivation in female Aedes aegypti mosquitoes. J Insect Physiol. 1990;36:545–8. doi: 10.1016/0022-1910(90)90021-7. [DOI] [Google Scholar]
- 53.Ribeiro JMC, Nussenzveig RH. The salivary catechol oxidase/peroxidase activities of the mosquito Anopheles albimanus. J Exp Biol. 1993;179:273–87. doi: 10.1242/jeb.179.1.273. [DOI] [PubMed] [Google Scholar]
- 54.Ribeiro JMC, Valenzuela JG. Purification and cloning of the salivary peroxidase/catechol oxidase of the mosquito Anopheles albimanus. J Exp Biol. 1999;202:809–16. doi: 10.1242/jeb.202.7.809. [DOI] [PubMed] [Google Scholar]
- 55.Calvo E, Andersen J, Francischetti IM, De LCM, DeBianchi AG, James AA, et al. The transcriptome of adult female Anopheles darlingi salivary glands. Insect Mol Biol. 2004;13:73–88. doi: 10.1111/j.1365-2583.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- 56.Xu X, Yang H, Ma D, Wu J, Wang Y, Song Y, et al. Toward an understanding of the molecular mechanism for successful blood feeding by coupling proteomics analysis with pharmacological testing of horsefly salivary glands. Mol Cell Proteomics. 2008;7:582–90. doi: 10.1074/mcp.M700497-MCP200. [DOI] [PubMed] [Google Scholar]
- 57.Harbach RE. The Phylogeny and Classification of Anopheles. In: Anopheles mosquitoes - New insights into malaria vectors. Edited by Manguin S: INTECH. 2013. http://www.intechopen.com/books/anopheles-mosquitoes-new-insights-into-malaria-vectors/the-phylogeny-and-classification-of-anopheles. Accessed 7 Feb 2017.
- 58.Fontaine MC, Pease JB, Steele A, Waterhouse RM, Neafsey DE, Sharakhov IV, et al. Mosquito genomics. Extensive introgression in a malaria vector species complex revealed by phylogenomics. Science. 2015;347:1258524. doi: 10.1126/science.1258524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Assumpcao TC, Ribeiro JM, Francischetti IM. Disintegrins from hematophagous sources. Toxins (Basel) 2012;4:296–322. doi: 10.3390/toxins4050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papaconstantinou ME, Carrell CJ, Pineda AO, Bobofchak KM, Mathews FS, Flordellis CS, et al. Thrombin functions through its RGD sequence in a non-canonical conformation. J Biol Chem. 2005;280:29393–6. doi: 10.1074/jbc.C500248200. [DOI] [PubMed] [Google Scholar]
- 61.King TP, Spangfort MD. Structure and biology of stinging insect venom allergens. Int Arch Allergy Immunol. 2000;123:99–106. doi: 10.1159/000024440. [DOI] [PubMed] [Google Scholar]
- 62.Gibbs GM, Roelants K, O’Bryan MK. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins--roles in reproduction, cancer, and immune defense. Endocr Rev. 2008;29:865–97. doi: 10.1210/er.2008-0032. [DOI] [PubMed] [Google Scholar]
- 63.Schreiber MC, Karlo JC, Kovalick GE. A novel cDNA from Drosophila encoding a protein with similarity to mammalian cysteine-rich secretory proteins, wasp venom antigen 5, and plant group 1 pathogenesis-related proteins. Gene. 1997;191:135–41. doi: 10.1016/S0378-1119(97)00010-3. [DOI] [PubMed] [Google Scholar]
- 64.Milne TJ, Abbenante G, Tyndall JD, Halliday J, Lewis RJ. Isolation and characterization of a cone snail protease with homology to CRISP proteins of the pathogenesis-related protein superfamily. J Biol Chem. 2003;278:31105–10. doi: 10.1074/jbc.M304843200. [DOI] [PubMed] [Google Scholar]
- 65.Yamazaki Y, Morita T. Structure and function of snake venom cysteine-rich secretory proteins. Toxicon. 2004;44:227–31. doi: 10.1016/j.toxicon.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 66.Hoffman DR. Hymenoptera venom allergens. Clin Rev Allergy Immunol. 2006;30:109–28. doi: 10.1385/CRIAI:30:2:109. [DOI] [PubMed] [Google Scholar]
- 67.Hoffman DR. Ant venoms. Curr Opin Allergy Clin Immunol. 2010;10:342–6. doi: 10.1097/ACI.0b013e328339f325. [DOI] [PubMed] [Google Scholar]
- 68.Ameri M, Wang X, Wilkerson MJ, Kanost MR, Broce AB. An immunoglobulin binding protein (antigen 5) of the stable fly (Diptera: Muscidae) salivary gland stimulates bovine immune responses. J Med Entomol. 2008;45:94–101. doi: 10.1093/jmedent/45.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Assumpcao TC, Ma D, Schwarz A, Reiter K, Santana JM, Andersen JF, et al. Salivary antigen-5/CAP family members are Cu2 + −dependent antioxidant enzymes that scavenge O(2)(−). and inhibit collagen-induced platelet aggregation and neutrophil oxidative burst. J Biol Chem. 2013;288:14341–61. doi: 10.1074/jbc.M113.466995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raffler NA, Schneider-Mergener J, Famulok M. A novel class of small functional peptides that bind and inhibit human alpha-thrombin isolated by mRNA display. Chem Biol. 2003;10:69–79. doi: 10.1016/S1074-5521(02)00309-5. [DOI] [PubMed] [Google Scholar]
- 71.Figueiredo AC, de Sanctis D, Gutierrez-Gallego R, Cereija TB, Macedo-Ribeiro S, Fuentes-Prior P, et al. Unique thrombin inhibition mechanism by anophelin, an anticoagulant from the malaria vector. Proc Natl Acad Sci U S A. 2012;109:E3649–58. doi: 10.1073/pnas.1211614109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.James AA, Blackmer K, Marinotti O, Ghosn CR, Racioppi JV. Isolation and characterization of the gene expressing the major salivary gland protein of the female mosquito Aedes aegypti. Mol Biochem Parasitol. 1991;44:245–54. doi: 10.1016/0166-6851(91)90010-4. [DOI] [PubMed] [Google Scholar]
- 73.Arcà B, Lombardo F, Lanfrancotti A, Spanos L, Veneri M, Louis C, et al. A cluster of four D7-related genes is expressed in the salivary glands of the African malaria vector Anopheles gambiae. Insect Mol Biol. 2002;11:47–55. doi: 10.1046/j.0962-1075.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- 74.Francischetti IM, Valenzuela JG, Pham VM, Garfield MK, Ribeiro JMC. Toward a catalog for the transcripts and proteins (sialome) from the salivary gland of the malaria vector Anopheles gambiae. J Exp Biol. 2002;205:2429–51. doi: 10.1242/jeb.205.16.2429. [DOI] [PubMed] [Google Scholar]
- 75.Hekmat-Scafe DS, Dorit RL, Carlson JR. Molecular evolution of odorant-binding protein genes OS-E and OS-F in Drosophila. Genetics. 2000;155:117–27. doi: 10.1093/genetics/155.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arcà B, Lombardo F, Francischetti IM, Pham VM, Mestres-Simon M, Andersen JF, et al. An insight into the sialome of the adult female mosquito Aedes albopictus. Insect Biochem Mol Biol. 2007;37:107–27. doi: 10.1016/j.ibmb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 77.Ribeiro JMC, Charlab R, Pham VM, Garfield M, Valenzuela JG. An insight into the salivary transcriptome and proteome of the adult female mosquito Culex pipiens quinquefasciatus. Insect Biochem Mol Biol. 2004;34:543–63. doi: 10.1016/j.ibmb.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 78.Campbell CL, Vandyke KA, Letchworth GJ, Drolet BS, Hanekamp T, Wilson WC. Midgut and salivary gland transcriptomes of the arbovirus vector Culicoides sonorensis (Diptera: Ceratopogonidae) Insect Mol Biol. 2005;14:121–36. doi: 10.1111/j.1365-2583.2004.00537.x. [DOI] [PubMed] [Google Scholar]
- 79.Ribeiro JM, Chagas AC, Pham VM, Lounibos LP, Calvo E. An insight into the sialome of the frog biting fly, Corethrella appendiculata. Insect Biochem Mol Biol. 2014;44:23–32. [DOI] [PMC free article] [PubMed]
- 80.Valenzuela JG, Charlab R, Gonzalez EC, de Miranda-Santos IK, Marinotti O, Francischetti IM, et al. The D7 family of salivary proteins in blood sucking diptera. Insect Mol Biol. 2002;11:149–55. doi: 10.1046/j.1365-2583.2002.00319.x. [DOI] [PubMed] [Google Scholar]
- 81.Calvo E, Mans BJ, Ribeiro JM, Andersen JF. Multifunctionality and mechanism of ligand binding in a mosquito antiinflammatory protein. Proc Natl Acad Sci U S A. 2009;106:3728–33. doi: 10.1073/pnas.0813190106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mans BJ, Calvo E, Ribeiro JM, Andersen JF. The crystal structure of D7r4, a salivary biogenic amine-binding protein from the malaria mosquito Anopheles gambiae. J Biol Chem. 2007;282:36626–33. doi: 10.1074/jbc.M706410200. [DOI] [PubMed] [Google Scholar]
- 83.Calvo E, Mans BJ, Andersen JF, Ribeiro JMC. Function and evolution of a mosquito salivary protein family. J Biol Chem. 2006;281:1935–42. doi: 10.1074/jbc.M510359200. [DOI] [PubMed] [Google Scholar]
- 84.Isawa H, Yuda M, Orito Y, Chinzei Y. A mosquito salivary protein inhibits activation of the plasma contact system by binding to factor XII and high molecular weight kininogen. J Biol Chem. 2002;277:27651–8. doi: 10.1074/jbc.M203505200. [DOI] [PubMed] [Google Scholar]
- 85.Alvarenga PH, Francischetti IM, Calvo E, Sa-Nunes A, Ribeiro JM, Andersen JF. The function and three-dimensional structure of a thromboxane A2/cysteinyl leukotriene-binding protein from the saliva of a mosquito vector of the malaria parasite. PLoS Biol. 2010;8:e1000547. doi: 10.1371/journal.pbio.1000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu X, Oliveira F, Chang BW, Collin N, Gomes R, Teixeira C, et al. Structure and function of a “yellow” protein from saliva of the sand fly Lutzomyia longipalpis that confers protective immunity against Leishmania major infection. J Biol Chem. 2011;286:32383–93. doi: 10.1074/jbc.M111.268904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simons FE, Peng Z. Mosquito allergy: recombinant mosquito salivary antigens for new diagnostic tests. Int Arch Allergy Immunol. 2001;124:403–5. doi: 10.1159/000053771. [DOI] [PubMed] [Google Scholar]
- 88.Cazares-Raga FE, Gonzalez-Lazaro M, Montero-Solis C, Gonzalez-Ceron L, Zamudio F, Martinez-Barnetche J, et al. GP35 ANOAL, an abundant acidic glycoprotein of female Anopheles albimanus saliva. Insect Mol Biol. 2007;16:187–98. doi: 10.1111/j.1365-2583.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- 89.Jariyapan N, Choochote W, Jitpakdi A, Harnnoi T, Siriyasatein P, Wilkinson MC, et al. A glycine- and glutamate-rich protein is female salivary gland-specific and abundant in the malaria vector Anopheles dirus B (Diptera: Culicidae) J Med Entomol. 2006;43:867–74. doi: 10.1093/jmedent/43.5.867. [DOI] [PubMed] [Google Scholar]
- 90.Andersen JF, Pham VM, Meng Z, Champagne DE, Ribeiro JM. Insight into the sialome of the black Fly, Simulium vittatum. J Proteome Res. 2009;8:1474–88. doi: 10.1021/pr8008429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Calvo E, Tokumasu F, Marinotti O, Villeval JL, Ribeiro JM, Francischetti IM. Aegyptin, a novel mosquito salivary gland protein, specifically binds to collagen and prevents its interaction with platelet glycoprotein VI, integrin alpha2beta1, and von Willebrand factor. J Biol Chem. 2007;282:26928–38. doi: 10.1074/jbc.M705669200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoshida S, Sudo T, Niimi M, Tao L, Sun B, Kambayashi J, et al. Inhibition of collagen-induced platelet aggregation by anopheline antiplatelet protein, a saliva protein from a malaria vector mosquito. Blood. 2008;111:2007–14. doi: 10.1182/blood-2007-06-097824. [DOI] [PubMed] [Google Scholar]
- 93.Yoshida S, Watanabe H. Robust salivary gland-specific transgene expression in Anopheles stephensi mosquito. Insect Mol Biol. 2006;15:403–10. doi: 10.1111/j.1365-2583.2006.00645.x. [DOI] [PubMed] [Google Scholar]
- 94.Peng Z, Xu WW, Sham Y, Lam H, Sun D, Cheng L, et al. Mosquito salivary allergen Aed a 3: cloning, comprehensive molecular analysis, and clinical evaluation. Allergy. 2016;71:621–8. doi: 10.1111/all.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Calvo E, Tokumasu F, Mizurini DM, McPhie P, Narum DL, Ribeiro JM, et al. Aegyptin displays high-affinity for the von Willebrand factor binding site (RGQOGVMGF) in collagen and inhibits carotid thrombus formation in vivo. FEBS J. 2010;277:413–27. doi: 10.1111/j.1742-4658.2009.07494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Okulate MA, Kalume DE, Reddy R, Kristiansen T, Bhattacharyya M, Chaerkady R, et al. Identification and molecular characterization of a novel protein Saglin as a target of monoclonal antibodies affecting salivary gland infectivity of Plasmodium sporozoites. Insect Mol Biol. 2007;16:711–22. doi: 10.1111/j.1365-2583.2007.00765.x. [DOI] [PubMed] [Google Scholar]
- 97.Ghosh AK, Devenport M, Jethwaney D, Kalume DE, Pandey A, Anderson VE, et al. Malaria parasite invasion of the mosquito salivary gland requires interaction between the Plasmodium TRAP and the Anopheles saglin proteins. PLoS Pathog. 2009;5:e1000265. doi: 10.1371/journal.ppat.1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calvo E, Pham VM, Ribeiro JM. An insight into the sialotranscriptome of the non-blood feeding Toxorhynchites amboinensis mosquito. Insect Biochem Mol Biol. 2008;38:499–507. doi: 10.1016/j.ibmb.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Valenzuela JG, Francischetti IM, Ribeiro JMC. Purification, cloning, and synthesis of a novel salivary anti-thrombin from the mosquito Anopheles albimanus. Biochemistry. 1999;38:11209–15. doi: 10.1021/bi990761i. [DOI] [PubMed] [Google Scholar]
- 100.Francischetti IM, Valenzuela JG, Ribeiro JMC. Anophelin: kinetics and mechanism of thrombin inhibition. Biochemistry. 1999;38:16678–85. doi: 10.1021/bi991231p. [DOI] [PubMed] [Google Scholar]
- 101.Ronca R, Kotsyfakis M, Lombardo F, Rizzo C, Curra C, Ponzi M, et al. The Anopheles gambiae cE5, a tight- and fast-binding thrombin inhibitor with post-transcriptionally regulated salivary-restricted expression. Insect Biochem Mol Biol. 2012;42:610–20. doi: 10.1016/j.ibmb.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marie A, Ronca R, Poinsignon A, Lombardo F, Drame PM, Cornelie S et al. The Anopheles gambiae cE5 salivary protein: a sensitive biomarker to evaluate the efficacy of insecticide-treated nets in malaria vector control. Microbes Infect. 2015;17:409–16. [DOI] [PubMed]
- 103.Li Y, Xiang Q, Zhang Q, Huang Y, Su Z. Overview on the recent study of antimicrobial peptides: origins, functions, relative mechanisms and application. Peptides. 2012;37:207–15. doi: 10.1016/j.peptides.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 104.Otvos L., Jr Antibacterial peptides isolated from insects. J Pept Sci. 2000;6:497–511. doi: 10.1002/1099-1387(200010)6:10<497::AID-PSC277>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 105.Lombardo F, Ronca R, Rizzo C, Mestres-Simon M, Lanfrancotti A, Curra C, et al. The Anopheles gambiae salivary protein gSG6: an anopheline-specific protein with a blood-feeding role. Insect Biochem Mol Biol. 2009;39:457–66. doi: 10.1016/j.ibmb.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Isawa H, Orito Y, Iwanaga S, Jingushi N, Morita A, Chinzei Y, et al. Identification and characterization of a new kallikrein-kinin system inhibitor from the salivary glands of the malaria vector mosquito Anopheles stephensi. Insect Biochem Mol Biol. 2007;37:466–77. doi: 10.1016/j.ibmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 107.Mendes-Sousa AF, Queiroz DC, Vale VF, Ribeiro JM, Valenzuela JG, Gontijo NF, et al. An inhibitor of the alternative pathway of complement in saliva of New world anopheline mosquitoes. J Immunol. 2016;197:599–610. doi: 10.4049/jimmunol.1600020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schwartz BS, Ribeiro JM, Goldstein MD. Anti-tick antibodies: an epidemiologic tool in Lyme disease research. Am J Epidemiol. 1990;132:58–66. doi: 10.1093/oxfordjournals.aje.a115643. [DOI] [PubMed] [Google Scholar]
- 109.Barral A, Honda E, Caldas A, Costa J, Vinhas V, Rowton ED, et al. Human immune response to sand fly salivary gland antigens: a useful epidemiological marker? Am J Trop Med Hyg. 2000;62:740–5. doi: 10.4269/ajtmh.2000.62.740. [DOI] [PubMed] [Google Scholar]
- 110.Nascimento RJ, Santana JM, Lozzi SP, Araujo CN, Teixeira AR. Human IgG1 and IgG4: the main antibodies against Triatoma infestans (Hemiptera: Reduviidae) salivary gland proteins. Am J Trop Med Hyg. 2001;65:219–26. doi: 10.4269/ajtmh.2001.65.219. [DOI] [PubMed] [Google Scholar]
- 111.Poinsignon A, Remoue F, Rossignol M, Cornelie S, Courtin D, Grebaut P, et al. Human IgG antibody response to glossina saliva: an epidemiologic marker of exposure to glossina bites. Am J Trop Med Hyg. 2008;78:750–3. [PubMed] [Google Scholar]
- 112.Andrade BB, Rocha BC, Reis-Filho A, Camargo LM, Tadei WP, Moreira LA, et al. Anti-Anopheles darlingi saliva antibodies as marker of Plasmodium vivax infection and clinical immunity in the Brazilian Amazon. Malar J. 2009;8:121. doi: 10.1186/1475-2875-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Orlandi-Pradines E, Almeras L, Denis de Senneville L, Barbe S, Remoue F, Villard C, et al. Antibody response against saliva antigens of Anopheles gambiae and Aedes aegypti in travellers in tropical Africa. Microbes Infect. 2007;9:1454–62. doi: 10.1016/j.micinf.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 114.Remoue F, Cisse B, Ba F, Sokhna C, Herve JP, Boulanger D, et al. Evaluation of the antibody response to Anopheles salivary antigens as a potential marker of risk of malaria. Trans R Soc Trop Med Hyg. 2006;100:363–70. doi: 10.1016/j.trstmh.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 115.Trevejo RT, Reeves WC. Antibody response to Culex tarsalis salivary gland antigens among sentinel chickens in California. Am J Trop Med Hyg. 2005;72:481–7. [PubMed] [Google Scholar]
- 116.Waitayakul A, Somsri S, Sattabongkot J, Looareesuwan S, Cui L, Udomsangpetch R. Natural human humoral response to salivary gland proteins of Anopheles mosquitoes in Thailand. Acta Trop. 2006;98:66–73. doi: 10.1016/j.actatropica.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 117.Poinsignon A, Cornelie S, Mestres-Simon M, Lanfrancotti A, Rossignol M, Boulanger D, et al. Novel peptide marker corresponding to salivary protein gSG6 potentially identifies exposure to Anopheles bites. PLoS One. 2008;3:e2472. doi: 10.1371/journal.pone.0002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Drame PM, Machault V, Diallo A, Cornelie S, Poinsignon A, Lalou R, et al. IgG responses to the gSG6-P1 salivary peptide for evaluating human exposure to Anopheles bites in urban areas of Dakar region. Senegal Malar J. 2012;11:72. doi: 10.1186/1475-2875-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Poinsignon A, Cornelie S, Ba F, Boulanger D, Sow C, Rossignol M, et al. Human IgG response to a salivary peptide, gSG6-P1, as a new immuno-epidemiological tool for evaluating low-level exposure to Anopheles bites. Malar J. 2009;8:198. doi: 10.1186/1475-2875-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sagna AB, Gaayeb L, Sarr JB, Senghor S, Poinsignon A, Boutouaba-Combe S, et al. Plasmodium falciparum infection during dry season: IgG responses to Anopheles gambiae salivary gSG6-P1 peptide as sensitive biomarker for malaria risk in Northern Senegal. Malar J. 2013;12:301. doi: 10.1186/1475-2875-12-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sagna AB, Sarr JB, Gaayeb L, Drame PM, Ndiath MO, Senghor S, et al. gSG6-P1 salivary biomarker discriminates micro-geographical heterogeneity of human exposure to Anopheles bites in low and seasonal malaria areas. Parasit Vectors. 2013;6:68. doi: 10.1186/1756-3305-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rizzo C, Ronca R, Fiorentino G, Verra F, Mangano V, Poinsignon A, et al. Humoral response to the Anopheles gambiae salivary protein gSG6: a serological indicator of exposure to Afrotropical malaria vectors. PLoS One. 2011;6:e17980. doi: 10.1371/journal.pone.0017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rizzo C, Ronca R, Lombardo F, Mangano V, Sirima SB, Nebie I, et al. IgG1 and IgG4 antibody responses to the Anopheles gambiae salivary protein gSG6 in the sympatric ethnic groups Mossi and Fulani in a malaria hyperhendemic area of Burkina Faso. PLoS One. 2014;9:e96130. doi: 10.1371/journal.pone.0096130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stone W, Bousema T, Jones S, Gesase S, Hashim R, Gosling R, et al. IgG responses to Anopheles gambiae salivary antigen gSG6 detect variation in exposure to malaria vectors and disease risk. PLoS One. 2012;7:e40170. doi: 10.1371/journal.pone.0040170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yman V, White MT, Rono J, Arca B, Osier FH, Troye-Blomberg M, et al. Antibody acquisition models: a new tool for serological surveillance of malaria transmission intensity. Sci Rep. 2016;6:19472. doi: 10.1038/srep19472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Badu K, Siangla J, Larbi J, Lawson BW, Afrane Y, Ong’echa M, et al. Variation in exposure to Anopheles gambiae salivary gland peptide (gSG6-P1) across different malaria transmission settings in the western Kenya highlands. Malar J. 2012;11:318. doi: 10.1186/1475-2875-11-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Proietti C, Verra F, Bretscher MT, Stone W, Kanoi BN, Balikagala B, et al. Influence of infection on malaria-specific antibody dynamics in a cohort exposed to intense malaria transmission in northern Uganda. Parasite Immunol. 2013;35:164–73. doi: 10.1111/pim.12031. [DOI] [PubMed] [Google Scholar]
- 128.Ali ZM, Bakli M, Fontaine A, Bakkali N, Vu Hai V, Audebert S, et al. Assessment of Anopheles salivary antigens as individual exposure biomarkers to species-specific malaria vector bites. Malar J. 2012;11:439. doi: 10.1186/1475-2875-11-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Poinsignon A, Samb B, Doucoure S, Drame PM, Sarr JB, Sow C, et al. First attempt to validate the gSG6-P1 salivary peptide as an immuno-epidemiological tool for evaluating human exposure to Anopheles funestus bites. Trop Med Int Health. 2010;15:1198–203. doi: 10.1111/j.1365-3156.2010.02611.x. [DOI] [PubMed] [Google Scholar]
- 130.Rizzo C, Ronca R, Fiorentino G, Mangano VD, Sirima SB, Nebie I, et al. Wide cross-reactivity between Anopheles gambiae and Anopheles funestus SG6 salivary proteins supports exploitation of gSG6 as a marker of human exposure to major malaria vectors in tropical Africa. Malar J. 2011;10:206. doi: 10.1186/1475-2875-10-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Drame PM, Diallo A, Poinsignon A, Boussari O, Dos Santos S, Machault V, et al. Evaluation of the effectiveness of malaria vector control measures in urban settings of Dakar by a specific anopheles salivary biomarker. PLoS One. 2013;8:e66354. doi: 10.1371/journal.pone.0066354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Drame PM, Poinsignon A, Besnard P, Cornelie S, Le Mire J, Toto JC, et al. Human antibody responses to the Anopheles salivary gSG6-P1 peptide: a novel tool for evaluating the efficacy of ITNs in malaria vector control. PLoS One. 2010;5:e15596. doi: 10.1371/journal.pone.0015596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arcà B, Struchiner CJ, Pham VM, Sferra G, Lombardo F, Pombi M, et al. Positive selection drives accelerated evolution of mosquito salivary genes associated with blood-feeding. Insect Mol Biol. 2014;23:122–31. doi: 10.1111/imb.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Londono-Renteria B, Drame PM, Weitzel T, Rosas R, Gripping C, Cardenas JC, et al. An. gambiae gSG6-P1 evaluation as a proxy for human-vector contact in the Americas: a pilot study. Parasit Vectors. 2015;8:533. doi: 10.1186/s13071-015-1160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rizzo C, Lombardo F, Ronca R, Mangano V, Sirima S, Nebie I, et al. Differential antibody response to the Anopheles gambiae gSG6 and cE5 salivary proteins in individuals naturally exposed to bites of malaria vectors. Parasit Vectors. 2014;7:549. doi: 10.1186/s13071-014-0549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 137.Berriman M, Rutherford K. Viewing and annotating sequence data with Artemis. Brief Bioinform. 2003;4:124–32. doi: 10.1093/bib/4.2.124. [DOI] [PubMed] [Google Scholar]
- 138.Ribeiro JM, Topalis P, Louis C. AnoXcel: an Anopheles gambiae protein database. Insect Mol Biol. 2004;13:449–57. doi: 10.1111/j.0962-1075.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- 139.Nielsen H, Brunak S, von Heijne G. Machine learning approaches for the prediction of signal peptides and other protein sorting signals. Protein Eng. 1999;12:3–9. doi: 10.1093/protein/12.1.3. [DOI] [PubMed] [Google Scholar]
- 140.Sonnhammer EL, von Heijne G, Krogh A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc Int Conf Intell Syst Mol Biol. 1998;6:175–82. [PubMed] [Google Scholar]
- 141.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat Genet. 2000;25:25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marchler-Bauer A, Panchenko AR, Shoemaker BA, Thiessen PA, Geer LY, Bryant SH. CDD: a database of conserved domain alignments with links to domain three-dimensional structure. Nucleic Acids Res. 2002;30:281–3. doi: 10.1093/nar/30.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–6. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 146.Buchan DW, Minneci F, Nugent TC, Bryson K, Jones DT. Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic Acids Res. 2013;41:W349–57. doi: 10.1093/nar/gkt381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–9. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–25. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 149.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–91. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 150.Zuckerkandl E, Pauling L. Evolutionary divergence and convergence in proteins. In: Evolving Genes and Proteins. Academic Press; 1965. pp. 97–166. [Google Scholar]
- 151.Wernersson R, Pedersen AG. RevTrans: multiple alignment of coding DNA from aligned amino acid sequences. Nucleic Acids Res. 2003;31:3537–9. doi: 10.1093/nar/gkg609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Librado P, Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–2. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated or analysed during this study are already publicly available or included in this article as Additional files. Additional file 2 is an hyperlinked spreadsheed with links stored in Exon, which is a distribution server for software and genomic data resources developed by the NIAID research community (https://exon.niaid.nih.gov/). Links will also be provided by the authors upon request for local download/use.


