Abstract
Many bacterial pathogens employ the type III secretion system (T3SS) to translocate effector proteins into eukaryotic cells to overcome host defenses. To date, most of our knowledge about the T3SS molecular architecture comes from the studies on animal pathogens. In plant pathogens, nine Hrc proteins are believed to be structural components of the T3SS, of which HrcC and HrcJ form the outer and inner rings of the T3SS, respectively. Here, we demonstrated that a novel outer membrane-bound protein (HpaM) of Xanthomonas campestris pv. campestris is critical for the type III secretion and is structurally and functionally conserved in phytopathogenic Xanthomonas spp. We showed that the C-terminus of HpaM extends into the periplasm to interact physically with HrcJ and the middle part of HpaM interacts physically with HrcC. It is clear that the outer and inner rings compose the main basal body of the T3SS apparatus in animal pathogens. Therefore, we presume that HpaM may act as a T3SS structural component, or play a role in assisting assembling or affecting the stability of the T3SS apparatus. HpaM is a highly prevalent and specific protein in Xanthomonas spp., suggesting that the T3SS of Xanthomonas is distinctive in some aspects from other pathogens.
Many Gram-negative bacterial pathogens of plants and animals employ the type III secretion system (T3SS) to deliver effector proteins into host cells, where they manipulate host cellular pathways to benefit the pathogens and thus allow the bacteria to successfully multiply. The T3SS apparatus is a complex macromolecular nanomachine that is composed of more than 20 proteins1,2,3,4. A typical T3SS apparatus consists of three parts: an extracellular pilus-like (plant pathogens) or needle-like (animal pathogens) appendage, a membrane-spanning basal body and the peripheral inner membrane cytoplasmic components. The basal body supports the pilus or needle appendage by anchoring the appendage on the bacterial membranes. Normally, the T3SS needle from animal pathogens is about 40–80 nm in length and the pilus from plant pathogens is up to 2 μm. The basal body is built of stacked toroids: an outer membrane ring extends to the periplasm and associates with the inner membrane ring. The cytoplasmic components are the ATPase complex and predicted cytoplasmic ring (C-ring)5,6,7,8. To date, most of our knowledge about the T3SS molecular architecture comes from the studies on animal pathogens such as Shigella, Salmonella, and Yersinia. The T3SS of plant pathogenic bacteria is encoded by a cluster of more than 20 hrp (hypersensitive response and pathogenicity) genes. Inactivation of the T3SS abolished the ability of the pathogens to produce disease lesions in host plants and to elicit hypersensitive response (HR) in nonhost or resistant plants. Comparative sequence analyses revealed that nine hrp genes (termed hrc for hrp conserved) are conserved among different plant pathogens and the Hrc proteins are highly homologous to the proteins constituting the T3SS apparatus of animal pathogens. In addition, several studies have shown that the T3SS of plant pathogens can secrete effector proteins from animal pathogens and plant pathogen effectors can be secreted by the T3SS of animal pathogens9,10. Based on these facts, it is presumed that the Hrc proteins are the components of the T3SS in plant pathogens and the core T3SS apparatus may be conserved among plant and animal pathogens6,11. According to their homology to the T3SS components of animal pathogens, the function of the nine conserved Hrc proteins is believed to be: (1) HrcC is an outer membrane ring protein; (2) HrcJ is an inner membrane ring protein; (3) HrcR, S, T and U are integral inner membrane proteins with periplasmic extensions, taking part in the rod formation of the T3SS apparatus; and (4) HrcV, Q and N are inner membrane or peripheral cytoplasmic proteins engaged in initiation of effector secretion from the cytoplasm6,8,11,12,13,14.
Xanthomonas is a large genus of Gram-negative bacteria, which comprises 27 species and some of which include multiple pathovars. Many members of the genus are important plant pathogens, such as X. campestris pv. campestris (the crucifer black rot pathogen), X. citri subsp. citri (the citrus canker pathogen), X. euvesicatoria (the pepper and tomato bacterial spot pathogen), X. oryzae pv. oryzae (the rice bacterial blight pathogen), and X. oryzae pv. oryzicola (the rice bacterial leaf streak pathogen), and most of which rely on an efficient T3SS for their pathogenicity15,16. The T3SS-encoding hrp cluster of Xanthomonas spp. consists of six operons (hrpA to hrpF) which harbor more than 20 different genes including the nine conserved hrc genes17,18,19. Recently, we identified a novel outer membrane-bound protein that is involved in the HR and pathogenicity of X. campestris pv. campestris (Xcc), which was designated as HpaM (for Hrp-associated membrane-bound protein). Here, we present evidences showing that the protein is essential for type III secretion and conserved in Xanthomonas spp.
Results
HpaM is essential for the virulence and HR induction of Xcc
In our previous work, we isolated a large number of Xcc mutants from a library constructed by the transposon Tn5gusA5 insertion in the genome of Xcc wild-type strain 8004. One of the mutants, 083E12, was due to a Tn5gusA5 insertion in the ORF XC_2847 (named hpaM in this study). Plant tests showed that the mutant strain 083E12 almost completely lost virulence and hardly induced any disease or HR symptoms in the host plant Chinese radish or the non-host plant pepper (cultivar ECW-10R). The gene XC_2847 was annotated to be 1161 bp in length, locating at nucleotide (nt) positions from the 3426325th to the 3427485th nt, and predicted to encode a hypothetical protein20. Using a standard 5′-RACE method, the transcription initiation site (TIS) of XC_2847 was mapped at 89 nucleotides downstream of the predicted translational start codon GTG (Fig. S1). There is an in-frame ATG codon 22 bp downstream of the determined TIS (Fig. S1). Based on these data, we propose that the XC_2847 ORF should start with the ATG and consist of 1050 bp instead of 1161 bp.
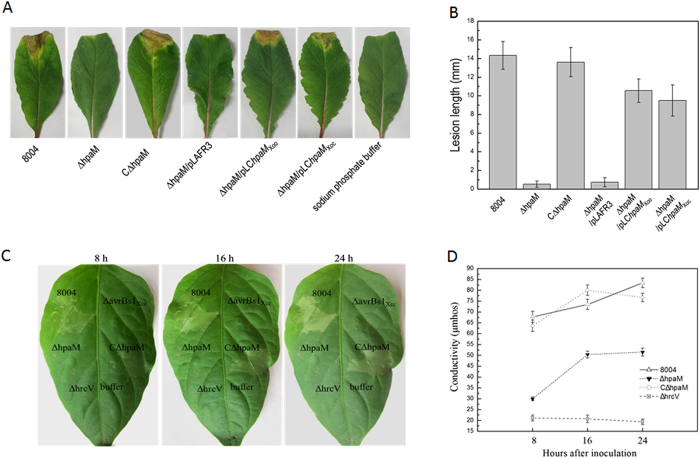
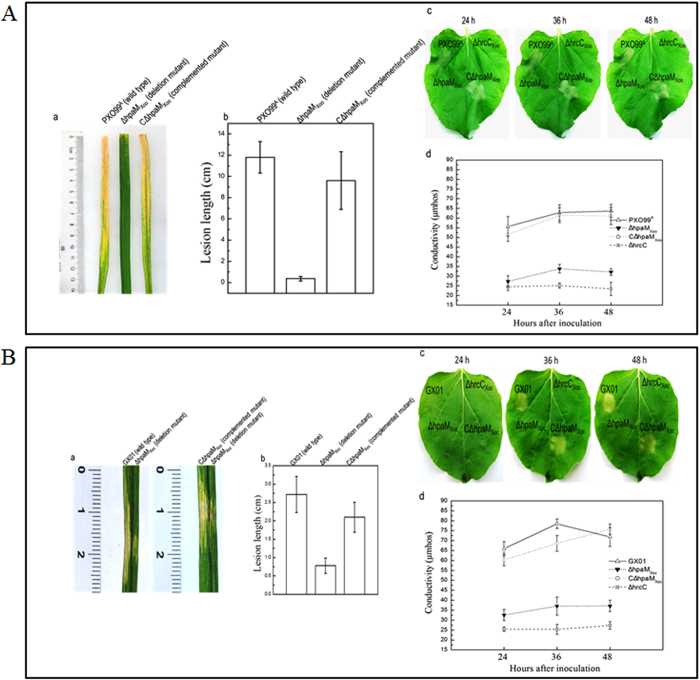
To facilitate further studies on the function of hpaM, a deletion mutant, named ΔhpaM, was constructed by using the suicide vector pK18mobsacB (Table S1). Simultaneously, a complemented strain was also constructed by introducing the recombinant plasmid pLChpaM, which carries an entire hpaM gene, into the mutant ΔhpaM. The resulting complemented strain was named as CΔhpaM (Table S1). As anticipated, the mutant ΔhpaM could hardly induce visible disease or HR symptoms (Fig. 1). However, the complemented strain CΔhpaM could produce wild-type disease and HR symptoms (Fig. 1), suggesting that the pathogenicity and HR of ΔhpaM could be restored by hpaM in trans. The growth in planta of the hpaM mutant was suppressed significantly, although its growth rate was not affected in minimal medium (Fig. S2), suggesting that mutation in hpaM decreased significantly fitness in planta. Taken together, the above data indicate that HpaM is essential for the virulence and HR induction of Xcc.
Figure 1. HpaM is essential for pathogenicity and HR induction of Xcc.
The Xcc wild-type strain 8004 and its derivatives from overnight culture were washed and resuspended in 10 mM SPB or sterile distilled water (for electrolyte leakage assay) to an OD600 of 0.1 (1 × 108 CFU ml−1). (A) Disease symptoms on Chinese radish (Raphanus sativus) leaves. Xcc strains were inoculated by cutting leaves with scissors dipped in the bacterial suspensions. (B) Lesion lengths were scored 10 days postinoculation. Values represent means and standard deviation from twenty inoculated leaves in one experiment. The experiment was repeated three times with similar results. (C) HR symptoms induced in pepper leaves (Capsicum annuum cv. ECW-10R) by Xcc strains. Approximately 5 μl bacterial resuspension (1 × 108 CFU ml−1) was infiltrated into the leaf mesophyll tissue with a blunt-end plastic syringe. Pictures were taken at 8, 16 and 24 h after infiltration. Three replications were done in each experiment, and the experiment was repeated three times. The results presented are from a representative experiment, and similar results were obtained in all other independent experiments. hrcV and avrBs1Xcc deletion mutants ΔhrcV and ΔavrBs1Xcc were used as negative controls. (D) Electrolyte leakage from pepper leaves inoculated with Xcc strains. For each sample, four 0.4 cm2 leaf disks were collected from the infiltrated area and incubated in 5 ml distilled water. Conductivity was measured with a DDS-307A conductometer. Three samples were taken for each measurement in each experiment. Results presented are from a representative experiment, and similar results were obtained in two other independent experiments. hrcV deletion mutant ΔhrcV was used as a negative control.
HpaM is required for T3Es secretion of Xcc
As mentioned above, the T3SS is critical for the pathogenicity and HR induction of Xcc. To gain an insight into the mechanisms by which HpaM affects the virulence and HR induction, we examined whether HpaM is involved in the T3SS. The T3SS of Xcc is encoded by six hrp operons (hrpA to hrpF) and the expression of the hrp operons is positively controlled by several key regulators including HrpG and HrpX21,22,23. To determine whether HpaM influences the expression of hrp genes, the plasmid-driven promoterless β-glucuronidase (gusA) transcriptional fusion reporters of hrpG and hrpX regulators as well as the six hrp operons, in which a DNA fragment containing the promoter region of each of the hrp operons (hrpA to hrpF) and hrpG and hrpX genes fused to the promoterless gusA gene with its ribosome binding site (RBS) was cloned into the vector pLAFR6 (Table S1), were introduced from E. coli JM109 by triparental conjugation into the hpaM mutant ΔhpaM and the wild-type strain 8004, and transconjugants (reporter strains) were screened on NYG medium as described previously22. As the expression of the hrp genes is induced in minimal media but inhibited in rich media23, β-glucuronidase (GUS) activities produced by the obtained reporter strains (Table S1) were measured after cultivation in MMX minimal medium. The results revealed that each of the reporters produced similar GUS activity in wild-type and hpaM deletion backgrounds (Fig. S3A), suggesting that mutation of hpaM did not affect the expression of the hrp genes. To clarify whether the expression of hpaM is subject to HrpG and HrpX regulation, the promoter-gusA transcriptional fusion reporter of hpaM was constructed. A 404-bp DNA fragment upstream of the hpaM ORF, amplified from the wild-type strain 8004, was fused with the coding region of promoterless gusA gene and cloned into pLAFR6, generating the reporter plasmid named pGUShpaM (Table S1). The GUS activities produced by the reporter plasmid in wild-type background and hrpG or hrpX mutation background were not significantly different (P = 0.05 by t test) (Fig. S3B), indicating that the expression of hpaM is not controlled by HrpG and HrpX. In addition, the reporter plasmid pGUShpaM in hpaM mutation background and wild-type background produced similar GUS activities (Fig. S3B), implying that HpaM plays no impact on its own expression.
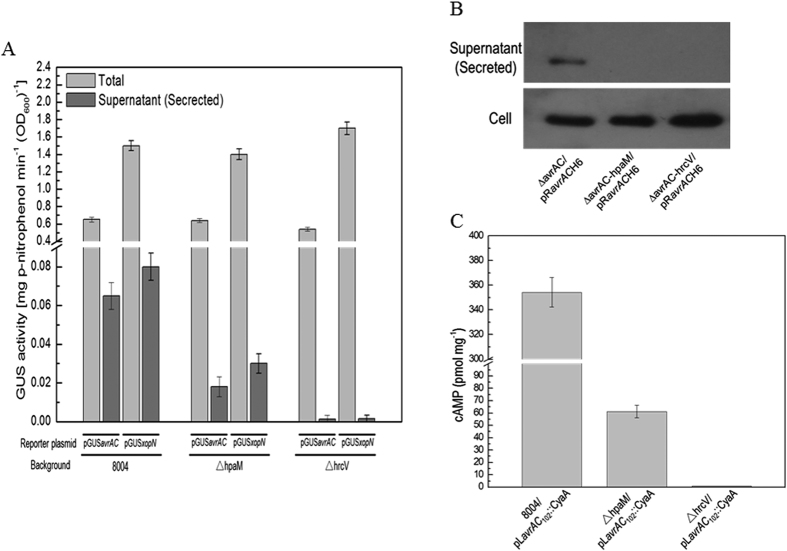
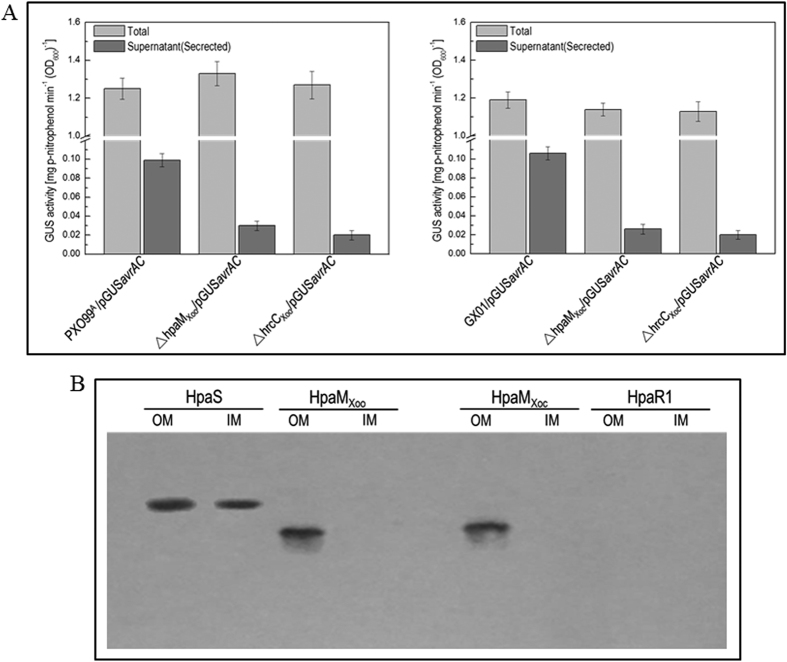
We further investigated whether HpaM is involved in T3Es secretion. It is well known that T3Es have a modular structure and the targeting signal generally resides in the N-terminal 50 or 100 amino acids (aa)24. Two reporter plasmids, pGUSavrAC and pGUSxopN (Table S1), were employed to study the secretion efficiency of Xcc T3SS. The reporters were previously constructed by fusing the promoterless gusA gene with a fragment including the promoter and targeting signal-encoding region of avrAC (XC_1553) or xopN (XC_0241), which encode the T3Es AvrAC and XopN, respectively25,26. pGUSavrAC and pGUSxopN were introduced into the hpaM mutant strain ΔhpaM and the wild-type strain 8004, respectively. The plasmids were also introduced into the hrcV-deficient mutant strain ΔhrcV as negative controls. HrcV is a conserved inner membrane protein of the core T3SS and the mutant ΔhrcV is defective in type III secretion13. The recombinant plasmid pL6gus, which was constructed by cloning a 1,832-bp promoterless gusA ORF into the promoterless cloning site of the plasmid pLAFR6, was introduced into the wild-type strain 8004 and the resulting strain 8004/pL6gus, which did not produced any significant GUS activity, was used as a negative control for the GUS assay. As shown in Fig. 2A,B, both reporters produced large amount of GUS activity in the wild-type and hpaM mutation backgrounds; however, the GUS activities in the cultural supernatants of the hpaM mutation background strains were significantly lower than those in the cultural supernatants of the wild-type background strains (P = 0.01 by t test), implying that mutation of hpaM significantly diminished the secretion of the T3Es AvrAC and XopN.
Figure 2. HpaM is essential for secretion of T3SS effectors in Xcc.
Type III secretion signal sequence-gusA fusion reporter plasmids pGUSavrAC and pGUSxopN were introduced into Xcc strains. The resulting recombinant strains were cultured in XVM2 medium for 12 h and the β-glucuronidase (GUS) activities were determined. Values are the means ± standard deviation from three repeats. (A) GUS activities in the cultural supernatant (Secreted) and the total culture (Total) produced by pGUSavrAC and pGUSxopN in different background strains. (B) Western blot assay. The recombinant plasmid pRavrACH6, which contains the T3E AvrAC encoding sequence fused with 6×His tag in its C-terminus, was introduced into Xcc strains. The resulting recombinant strains were cultured in XVM2 medium for 12 h and proteins in cultural supernatant (secreted protein) were collected by ultra-filtration using Amicon Ultra-15 centrifugal filter (Millipore Corporation, Billerica, MA, USA) and the total proteins in Xcc cells were prepared as previously described62. 30 μg of secreted or cell protein was electrophoresed in SDS-PAGE gel and transferred to a PVDF membrane. The presence of AvrAC was detected by anti-His6 monoclonal antibody. (C) Cya protein translocation assay. The pLavrAC102::CyaA fusion construct was transferred into Xcc strains and the resulting recombinant strains were then used to inoculate Chinese radish (Raphanus sativus) leaves. The cAMP level was determined 24 h post-inoculation. Values given are the means ± standard deviations of triplicate measurements from a representative experiment; similar results were obtained in two other independent experiments. 8004, wild type strain; ∆hpaM, hpaM deletion mutant; ∆hrcV, hrcV deletion mutant.
To further verify the effect of hpaM on the type III secretion, western blot assay was performed to examine the secretion of the T3E AvrAC in the hpaM mutation background. For this purpose, an avrAC deletion mutant (∆avrAC) and an avrAC/hpaM double deletion mutant (∆avrAC-hpaM) were constructed. Another double deletion mutant (∆avrAC-hrcV) that lacked avrAC and hrcV was also constructed and used as a negative control strain. The recombinant plasmid pRavrACH6, which was constructed by fusing 6×His-tag coding sequence to the 3′ end of the avrAC gene with its own promoter and cloning the fused fragment into the promoterless cloning site of the plasmid pLAFR6, was then introduced into the mutants. The resulting strains ∆avrAC/pRavrACH6, ∆avrAC-hpaM/pRavrACH6 and ∆avrAC-hrcV/pRavrACH6 (Table S1) were used to test the secretion of AvrAC protein by western blot assay. As shown in Fig. 2B, AvrAC protein was present in the cells of all the strains tested and the cultural supernatant of the strain ∆avrAC/pRavrACH6. Similar to the negative control strain ∆avrAC-hrcV/pRavrACH6, no AvrAC protein was detected in the cultural supernatant of the strain ∆avrAC-hpaM/pRavrACH6 under the test conditions (Fig. 2B), indicating that deletion of hpaM abolished the secretion of AvrAC. These data confirm that HpaM is indispensable for the type III secretion of Xcc.
To further estimate the effect of HpaM on T3Es translocation into plant cells, the N-terminal 102 aa of the T3E AvrAC were fused with the calmodulin-dependent reporter protein Cya27 and the resulting reporter plasmid, named pLavrAC102::CyaA (Table S1), was introduced into the hpaM mutant strain ΔhpaM, the wild-type strain 8004, and the T3SS-defective hrcV mutant ΔhrcV. The obtained recombinant strains were inoculated into radish leaves at 108 cfu ml−1 (OD600 = 0.1), and the cAMP levels were measured 24 h post-inoculation. Strain ΔhrcV/pLAFR6, which was constructed by introducing the vector pLAFR6 into the hrcV mutant strain ΔhrcV, was used as a negative control. As shown in Fig. 2C, the cAMP level in the leaves inoculated with the wild-type strain harboring the reporter plasmid was higher than that in the leaves inoculated with the mutants carrying the reporter plasmid. As the Cya protein produces a measurable cAMP level only in plant cells but not in bacterial cells or plant apoplasts28, the result reveals that HpaM is essential for T3Es translocation into plant cells.
Xcc secretes a series of extracellular enzymes including exoproteases by the type II secretion system (T2SS). To evaluate whether HpaM affects the T2SS, we compared the exoprotease activities produced by the hpaM mutant strain ΔhpaM and the wild type strain 8004. The result showed that the two strains produced similar enzyme activities (Fig. S4A), suggesting that HpaM is not involved in the T2SS. The extracellular polysaccharide (EPS) production and the motility of the mutant ΔhpaM were also determined. No significant difference on either EPS production or motility was observed between the mutant and the wild type (Fig. S4), indicating that HpaM does not affect EPS production and cell motility.
HpaM is located in the outer membrane of Xcc
The HpaM protein of Xcc consists of 349 aa. Domain analysis with the SMART (Simple Modular Architecture Research Tool) program (http://smart.embl-heidelberg.de) showed that HpaM contains a signal peptide (residues1–22), and 6 PbH1 domains (residues 120–163, 180–202, 203–225, 226–248, 249–271, and 288–311) which were annotated as “parallel β-helix repeats”. A prediction by the TMPRED program (http://www.ch.embnet.org/software/TMPRED_form.html) revealed that the residues from the 8th to the 29th aa in the N-terminal domain of HpaM constitute transmembrane helices (total score: 1405). These suggest that HpaM may be a membrane-bound protein.
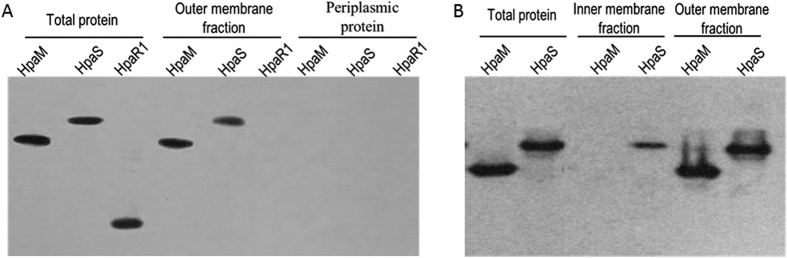
To validate whether HpaM is a membrane-bound protein, the cellular location of HpaM in Xcc was determined. We constructed a recombinant strain, ΔhpaM/pRhpaMH6, which expressed HpaM with a 6×His tag on its C-terminus in the hpaM deletion strain ΔhpaM. The total, periplasmic, and outer membrane protein fractions of the strain ΔhpaM/pRhpaMH6 grown at the late log phase were prepared. Western blot analysis revealed HpaM present in the total-protein and the outer membrane fractions but not in the periplasmic protein fraction (Fig. 3A). The cytoplasm protein HpaR129 and the outer and inner membrane protein HpaS21 were taken as controls (Fig. 3A). To further determine whether HpaM also locates in the inner membrane, the outer and inner membrane fraction proteins were prepared using the method as described by Chen and associates30. The result showed that HpaM was detected only in the outer membrane fraction but not in the inner membrane fraction, while the control protein HpaS was detected in both outer and inner membrane fractions (Fig. 3B). These combined data indicate that HpaM is an outer membrane protein in Xcc.
Figure 3. Subcellular localization of HpaM by western blot analysis.
Xcc strains were cultured to an OD600 of 1.0 and proteins were prepared using the method described by Feilmeier and associates (2000) (A) or the method described by Chen and associates (2010) (B). 30 (for total protein) or 10 μg of protein sample was separated by SDS-PAGE electrophoresis and transferred to a PVDF membrane. The presence of HpaM was detected by anti-His6 monoclonal antibody. The histidine sensor kinase HpaS and the transcription regulator HpaR1 were used as controls. HpaM, protein sample was prepared from strain ΔhpaM/pRhpaMH6; HpaS, protein sample was prepared from strain ∆hpaS/pRhpaSH6; HpaR1, protein sample was prepared from strain ∆hpaR1/pRhpaR1H6.
HpaM physically interacts with HrcC and HrcJ
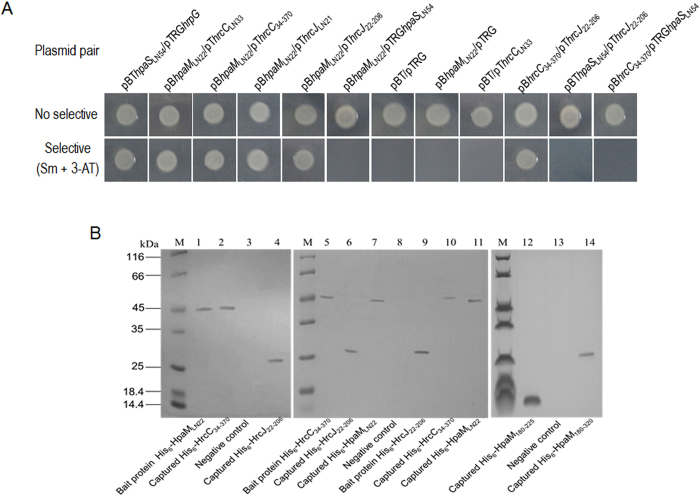
The above data demonstrate that HpaM locates in the bacterial outer membrane and contributes to T3Es secretion, but is not involved in the regulation of the T3SS expression. From these facts we presumed that HpaM may act as a component of T3SS apparatus or a factor affecting the assembly or stability of the T3SS apparatus. To verify these possibilities, we employed the BacterioMatch II two-hybrid system (Stratagene, La Jolla, CA, USA) to determine whether HpaM physically interacts with the T3SS apparatus outer and inner membrane ring proteins HrcC and HrcJ31. A truncated hpaM gene excluding the N-terminal 22-aa signal peptide coding sequence was cloned into the bait vector pBT, yielding a recombinant plasmid named pBhpaMLN22 (Table S1). DNA fragments of truncated hrcC and hrcJ (excluding the N-terminal 33- and 21-aa signal peptide encoding sequences of hrcC and hrcJ, respectively) were fused into the target vector pTRG, yielding recombinant plasmids named pThrcCLN33 and pThrcJLN21 (Table S1). The plasmids were introduced into the reporter strain XL1-Blue MRF′. The resulting recombinant strains, which harbor a pair of plasmids (Table S1) were tested for their growth ability on the double-selective indicator plate. In the reporter strain, if the HpaM and HrcC or HrcJ proteins interact with each other, the expression of HIS3 and addA reporter genes will be activated, leading to the growth of the bacterial cells in the presence of 3-amino-1, 2, 4-triazole (3-AT) and streptomycin; however, if no interaction between the proteins occurs, the bacteria cannot grow in the same conditions. As shown in Fig. 4A, like the positive control strain XL1-Blue MRF′/pBThpaSLN54/pTRGhrpG that showed an interaction between the histidine kinase HpaS and the response regulator HrpG of a two-component regulatory system21, the reporter strain XL1-Blue MRF′ harboring the plasmid pair pBhpaMLN22/pThrcCLN33 or pBhpaMLN22/pThrcJLN21 grew well in the selective agar plate, while the negative control strains (the reporter strain harboring the plasmid pair pBT/pTRG, pBhpaMLN22/pTRG, or pBT/pThrcCLN33) did not grow (Fig. 4A). These results indicate that HpaM interacts with HrcC as well as HrcJ in the reporter strain XL1-Blue MRF′. To evaluate whether the interaction between HpaM and HrcC or HrcJ is specific, the membrane-bound protein HpaS was included in the bacterial two-hybrid analysis. A truncated HpaS protein (lacking the N-terminal 54 aa transmembrane domain encoding sequence) was cloned into the target vector pTRG and the obtained plasmid pTRGhpaSLN54 was used in the analysis. The result showed that the reporter strain XL1-Blue MRF′ harboring the plasmid pair pBhpaMLN22/pTRGhpaSLN54 could not grow on the selective agar plate, indicating no interaction existed between HpaM and HpaS (Fig. 4A). It has been supposed that the periplasmic domains of the HrcC and HrcJ proteins interact with each other and compose the T3SS periplasmic rod of the T3SS apparatus32. We therefore tested whether HpaM interacts with the periplasmic domains of HrcC and HrcJ. For this purpose, a 1011 bp DNA fragment encoding the aa from the 34th to the 370th of HrcC and a 555-bp fragment encoding the aa from the 22th to the 206th of HrcJ were amplified and cloned into the target vector pTRG, yielding recombinant plasmids named pThrcC34–370 and pThrcJ22–206 (Table S1). As shown in Fig. 4A, the reporter strain XL1-Blue MRF′ harboring the plasmid pair pBhpaMLN22/pThrcC34–370 or pBhpaMLN22/pThrcJ22–206 was able to grow on the selective agar plate, indicating that HpaM interacts with the periplasmic domains of HrcC and HrcJ in the reporter strain.
Figure 4. HpaM interacts with HrcC and HrcJ.
(A) Bacterial two-hybrid assays. The BacterioMatch II two-hybrid system was used to test the interaction between HpaM and HrcC or HrcJ. The reporter strain XL1-Blue MRF′ harboring different plasmid pairs was grown on no selective plates and double-selective indicator plates containing 5 mM 3-amino-1,2,4-triazole (3-AT) and 12.5 μg ml−1 streptomycin, respectively. Protein-protein interaction activated the expression of the genes HIS3 and addA in the reporter strain, resulting in resistance to 3-AT and streptomycin. (B) Pull-down assays. His6-tagged fusion proteins were overexpressed and purified. Streptavidin sepharose beads were used to immobilize biotinylated His6-HpaMLN22, His6-HrcC34–370 or His6-HrcJ22–206, the potential prey protein was mixed with the bait protein and incubated. After elution, samples were separated on 12% SDS-PAGE and visualized by coomassie blue staining. Lanes: 1, biotinylated bait protein His6-HpaMLN22; 2, pull-down of His6-HrcC34–370 by His6-HpaMLN22; 3, bait protein His6-HpaMLN22 mixed with protein His6-HpaR1(negative control); 4, pull-down of protein His6-HrcJ22–206 by His6-HpaMLN22; 5, biotinylated bait protein His6-HrcC34–370; 6, pull-down of protein His6-HrcJ22–206 by His6-HrcC34–370; 7, pull-down of protein His6-HpaMLN22 by His6-HrcC34–370; 8, bait protein His6-HrcC34–370 mixed with protein His6-HpaR1(negative control); 9, biotinylated bait protein His6-HrcJ22–206; 10, pull-down of protein His6-HrcC34–370 by His6-HrcJ22–206; 11, pull-down of protein His6-HpaMLN22 by His6-HrcJ22–206; 12, pull-down of truncated protein His6-HpaMLN180–225 by His6-HrcC34–370; 13, biotinylated His6-HrcC34–370 was mixed with protein His6-HpaR1 (negative control); 14, pull-down of truncated protein His6-HpaMLN180–320 by His6-HrcJ22–206; M, molecular mass marker.
To confirm the interactions, pull-down biotinylated protein-protein assays were performed. For this purpose, an attempt was made to overproduce recombinant 6×His-tagged truncated HrcC and HrcJ proteins by cloning truncated hrcC and hrcJ excluding the N-terminal 33- and 21-aa signal peptide encoding sequences into the expression vector pET-30a. However, we failed to obtain soluble form of the fusion proteins. The periplasmic domains of HrcC and HrcJ, i.e. the 34th to the 370th aa of HrcC and the 22th to the 206th aa of HrcJ, were therefore overexpressed and soluble fusion proteins were obtained, which were named His6-HrcC34–370 and His6-HrcJ22–206 (Fig. S5). His6-HpaMLN22 was biotinylated and immobilized on streptavidin sepharose beads. Pull-down assays between His6-HpaMLN22 and His6-HrcC34–370 or His6-HrcJ22–206 were performed (see methods for details). As shown in Fig. 4B, the protein HpaMLN22 did capture both His6-HrcC34–370 (lane 2) and His6-HrcJ22–206 (lane 4) proteins. Overall, these combined data demonstrate that HpaM interacts directly with the periplasmic domains of HrcC and HrcJ.
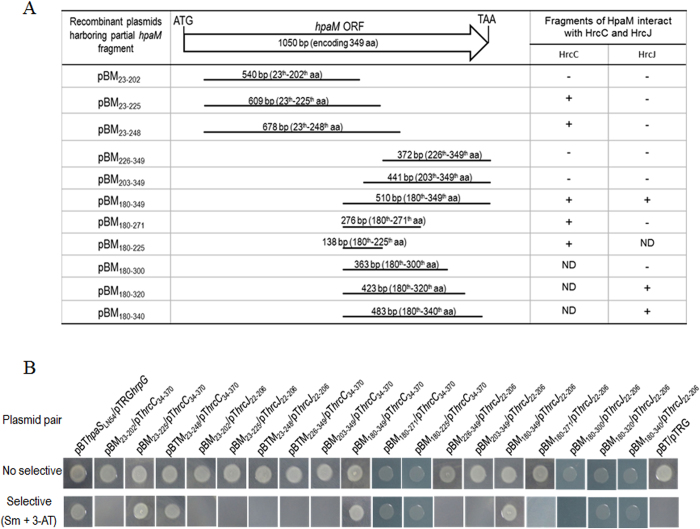
To gain a primary insight into the molecular interaction between HpaM and HrcC or HrcJ, we defined the peptides in HpaM required for the interaction. As described above, the first 22 aa in the N-terminus of HpaM was predicted to be a signal peptide. We therefore tested the N-terminal portion exclusive of the first 22 aa. 540, 609, and 678 bp DNA fragments encoding the peptides of the 23th–202th aa, 23th–225th aa, and 23th–248th aa, respectively, were amplified by using the corresponding primer sets listed in Table S2 and cloned into the vector pBT, respectively. 372, 441, 510, and 276 bp DNA fragments encoding the C-terminal peptides of the 226th–349th aa, 203th–349th aa, 180th–349th aa, and 180th–271th aa were also amplified and cloned into the vector pBT. The obtained recombinant plasmids (Table S1) as well as plasmid pThrcC34–370 or pThrcJ22–206 were introduced into the reporter strain XL1-Blue MRF′ and the growth of the resulting recombinant strains was examined. As shown in Fig. 5B, the recombinant strains that harbored the plasmid pair pBM23–225/pThrcC34–370, pBM23–248/pThrcC34–370, pBM180–349/pThrcC34–370, pBM180–271/pThrcC34–370 or pBM180–349/pThrcJ22–206 could grow on the selective plate but other strains could not, indicating that the peptide consisting of the 180th to the 225th aa of HpaM is essential for the interaction between HpaM and HrcC, and the C-terminus of HpaM from the 180th aa is involved in the interaction between HpaM and HrcJ. The 180th to the 225th aa of HpaM was further tested to see whether it suffices the interaction with HrcC, and the truncated proteins consisting of the 180th aa to the 340th, the 320th, or the 300th aa of HpaM were also further tested for their interactions with HrcJ, respectively. As shown in Fig. 5, the peptide from the 180th to the 225th aa of HpaM is sufficient for the interaction with HrcC (the strain containing pBM180–225/pThrcC34–370 could grow on the selective agar plate), and the peptide from the 180th to the 320th (but not to the 300th) aa of HpaM is sufficient for the interaction with HrcJ (the strain containing pBM180–320/pThrcJ22–206 could grow on the selective agar plate). The interactions were further confirmed by pull-down assays (Fig. 4B, lanes 12 and 14).
Figure 5. Determination of the peptides in HpaM required for the interaction with HrcC and HrcJ.
(A) Schematic representation of a set of HpaM fragments used to test the interaction with HrcC or HrcJ. The left part of the figure shows the PCR fragments used to clone into the vector pBT and the resulting recombinant pBM series plasmids which were used for bacterial two-hybrid assays. The numbers above each line represent the length of PCR fragments and the corresponding region in HpaM. The right part of the figure shows the interaction between each of the truncated HpaM fragments and the periplasmic domain of HrcC or HrcJ. +, interaction; −, no interaction. ND, not done. (B) The results of bacterial two-hybrid assays. The plasmid pair pBThpaSLN54/pTRGhrpG was used as a positive control.
To evaluate whether the interaction with HrcC or HrcJ is essential for HpaM function, the HpaM derivatives with deletion in 180th–202th aa consisting a PbH1 domain of parallel β-helix repeats and 288th–311th aa consisting a PbH1 domain of parallel β-helix repeats, respectively, were constructed, and the obtained hpaM partial deletion mutants were named ΔhpaM180–202 and ΔhpaM288–311, respectively. Plant assays revealed that the two mutant strains, similar to the hpaM full deletion mutant ΔhpaM, scarcely caused any disease or HR symptoms in the host plant Chinese radish or the non-host plant pepper (Fig. S6). Additionally, the recombinant plasmid pLChpaM carrying a full length hpaM gene was introduced into the mutants ΔhpaM180–202 and ΔhpaM288–311, respectively. The resulting complemented strains CΔhpaM180–202 and CΔhpaM288–311 showed wild-type virulence and HR phenotypes (Fig. S6).
Evidences that HrcC, HpaM and HrcJ are outer and inner membrane-bound proteins, respectively, and HrcC of Xcc interacts directly with HrcJ
In animal pathogens, the EscC/InvG/YscC family proteins compose of the outer membrane ring, and the EscJ/PrgK/YscJ family members are one of the inner membrane ring components. Periplasmic domains of EscC/InvG/YscC and EscJ/PrgK/YscJ proteins interact with each other and form the T3SS periplasmic rod32,33,34. HrcC and HrcJ in phytopathogens are isoforms of the EscC/InvG/YscC and EscJ/PrgK/YscJ families, respectively6. Deletion of hrcC or hrcJ abolished the virulence and HR induction of Xcc (Fig. S7). The N-termini of HrcC and HrcJ were predicted to be the periplasmic domains and their C-termini were supposed to integrate into the cell membranes. To verify the HrcC and HrcJ integration in Xcc cells, recombinant strains ∆hrcC/pRhrcCH6 and ∆hrcJ/pRhrcJH6 were constructed, which produced HrcC and HrcJ with a 6×His tag on the C-terminus in the mutants ∆hrcC and ∆hrcJ, respectively. The outer and inner membrane protein fractions of the two strains grown to the late-log phase were prepared and exposed to western blot analysis. As shown in Fig. 6, HrcC and HrcJ were present in the outer and inner membrane fractions, respectively, indicating that HrcC and HrcJ in Xcc, as speculated, are outer and inner membrane-bounded proteins, respectively.
Figure 6. Evidence from western blot analysis reveals that HpaM, HrcC and HrcJ are outer and inner membrane-bound proteins, respectively.
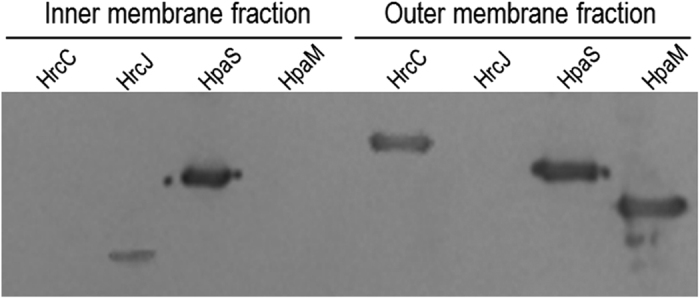
The outer and inner membrane fraction proteins from strain ∆hrcC/pRhrcCH6 (for HrcC detection), ∆hrcJ/pRhrcJH6 (for HrcJ detection), and ΔHpaM-HrcC/pRhpaMH6 (for HpaM detection) were prepared. 10 μg of protein for each sample was separated by SDS-PAGE electrophoresis and transferred to a PVDF membrane. The presence of HrcC, HrcJ, and HpaM was detected by anti-His6 monoclonal antibody. The histidine sensor kinase HpaS (from strain ∆hpaS/pRhpaSH6) was used as a control.
Our above data revealed that HpaM is an outer membrane-bound protein. As HrcC is believed to compose the outer membrane ring of the type III apparatus, we concerned that whether the outer membrane localization of HpaM depends on the presence of HrcC. We therefore detected the location of HpaM in the hrcC deletion mutant background. To do this, an hpaM and hrcC double deletion mutant named ∆hpaM-hrcC (Table S1) was constructed, and the recombinant plasmid pRhpaMH6 was introduced into the mutant. The resulting recombinant strain ∆hpaM-hrcC/pRhpaMH6 (Table S1) was used to locate HpaM protein. As shown in Fig. 6, HpaM protein was still present in the outer membrane fraction of the bacterial cells, indicating that the presence of HpaM in the outer membrane does not rely on HrcC, i.e. HpaM is in itself an outer membrane-bound protein.
To verify the Xcc HrcC and HrcJ proteins interact with each other, the truncated hrcC and hrcJ genes excluding the signal peptide coding sequence were cloned into the vector bait pBT and the prey pTRG, respectively, resulting the plasmids pBhrcC34–370 and pThrcJ22–206 (Table S1). The plasmids were introduced into the reporter strain XL1-Blue MRF′. As shown in Fig. 4A, the strain harboring the plasmid pair pBhrcC34–370/pThrcJ22–206 grew well on the selective indicator plate, while the strain harboring the plasmid pair pBThpaSLN54/pThrcJ22–206 or pBhrcC34–370/pTRGhpaSLN54 could not grow. These results indicate that the interaction between HrcC and HrcJ existed. Protein pull-down assay was carried out to further verify the bacterial two-hybrid assay result. His6-HrcC34–370 and His6-HrcJ22–206 were biotinylated with sulfo-NHS-LC-biotin and incubated with streptavidin sepharose™ beads, respectively, and then the protein His6-HrcJ22–206 or His6-HrcC34–370 was added. As shown in Fig. 4B, His6-HrcC34–370 and His6-HrcJ22–206 captured each other (lane 6 and 10). Additionally, both proteins His6-HrcC34–370 and His6-HrcJ22–206 were able to capture the protein His6-HpaMLN22 (Fig. 4B, lane 7 and 11). These combined data confirm that HrcC and HpaM are outer membrane-bound proteins, HrcJ is an inner membrane-bound protein, and HrcC and HrcJ interact with each other directly in Xcc.
HpaM is highly conserved in phytopathogenic Xanthomonads
To date, the whole genome sequences of more than one dozen Xanthomonas spp. or pathovars are available. A protein blast revealed that HpaM is conserved in all sequenced Xanthomonas spp. (Table S3). Although the rate of their amino acid sequence homology is varied among different species or pathovars, most of which share more than 90% similarity and 87% identity. Only three species, i.e., X. translucens, X. sacchari, and X. albilineans, share an HpaM homologue with lower similarity (71–74%) and identity (about 60%) to Xcc HpaM. Additionally, an HpaM homologue also exists in Pseudoxanthomonas spadix and Xylella fastidiosa, which shares ~55% identity and ~68% similarity with Xcc HpaM (Table S3). Transmembrane domain analysis using the TMPRED program (http://www.ch.embnet.org/software/TMPRED_form.html) revealed that the N-termini of all the HpaM homologues contain a transmembrane helice (Table S3).
The Xanthomonas oryzae homologues of HpaM exhibit similar functions to Xcc HpaM
As described above, HpaM is highly conserved in Xanthomonas pathogens. To verify whether the HpaM homologues in other Xanthomonas spp. play similar roles to Xcc HpaM, we investigated the function of the HpaM homologues in the species Xanthomonas oryzae. X. oryzae consists of two pathovars, oryzae (Xoo) and oryzicola (Xoc), which are the causative agents for bacterial leaf blight and bacterial leaf streak of rice, respectively. The whole-genome sequences are available for Xoo strain PXO99A 35 and Xoc strain GX01 (our unpublished data), therefore, we used these strains in the study. The HpaM homologues in strain PXO99A and strain GX01 were designated as HpaMXoo and HpaMXoc, respectively. HpaMXoc is completely identical to its counterpart in the Xoc strain BLS25636. If HpaMXoo and HpaMXoc are entrusted with similar functions to Xcc HpaM, they should be able to replace Xcc HpaM and restore the virulence and HR induction of the Xcc hpaM deletion mutant. Therefore, we cloned the hpaM homologues of Xoo and Xoc into the vector pLAFR3 (Table S1) and introduced the resulting recombinant plasmids pLChpaMXoo and pLChpaMXoc (Table S1) into the Xcc hpaM deletion mutant strain ΔhpaM, respectively. Plant tests showed that either of pLChpaMXoo and pLChpaMXoc could restore the ability of the mutant to induce typical black rot symptoms in the host plant Chinese radish and HR in the non-host plant pepper leaves (Fig. 1A,B, Fig. S8), indicating that Xcc HpaM and its counterparts in Xoo and Xoc probably have similar functions.
To further investigate the function of HpaMXoo and HpaMXoc in Xoo and Xoc, hpaMXoo and hpaMXoc deletion mutants were constructed from strain PXO99A and strain GX01 by homologous recombination using the suicide vector pK18mobsacB37, and the resulting mutants, named ΔhpaMXoo and ΔhpaMXoc (Table S1), were tested for virulence in rice and HR in tobacco. As shown in Fig. 7, both mutants almost completely failed to stimulate disease symptoms in rice and HR in tobacco, while the complemented strains could induce wild-type disease symptoms and HR. As the T3SS is also essential for the pathogenicity and HR induction of both pathogens, the plant test result suggests that HpaM is probably indispensable for a functional T3SS of Xoo and Xoc. To verify this, the type III secretion efficiency of the mutants ΔhpaMXoo and ΔhpaMXoc was detected. To do this, the type III secretion reporter plasmid pLGUSavrAC (Table S1) was introduced into the mutants ΔhpaMXoo and ΔhpaMXoc as well as the wild type strains of Xoo and Xoc. The GUS activities of the resulting recombinant strains were then determined. As shown in Fig. 8A, both mutants harboring pLGUSavrAC produced significantly weaker GUS activity in cultural supernatants, compared to the wild type strains harboring pLGUSavrAC, suggesting that the type III secretion efficiency of the mutants was significantly weakened. These combined data demonstrate that the HpaM homologues of Xoo and Xoc are also critical for the type III secretion. The cellular location of HpaMXoo and HpaMXoc was also determined by western blot assay. The HpaMXoo and HpaMXoc encoding sequences fused with 6×His tag at their C-termini were cloned into pLAFR3 and the resulting recombinant plasmids named pRhpaMXooH6 and pRhpaMXocH6 (Table S1) were introduced into the mutant strains ΔhpaMXoo and ΔhpaMXoc, respectively. The outer and inner membrane proteins from the obtained recombinant strains ΔhpaMXoo/pRhpaMXooH6 and ΔhpaMXoc/pRhpaMXocH6 (Table S1) were prepared and analyzed by western blot assay. The result revealed that HpaMXoo and HpaMXoc were also located in the outer membrane of Xoo and Xoc (Fig. 8B). Taken together, the above combined data indicate that the Xoo and Xoc homologues of HpaM may have similar functions to Xcc HpaM.
Figure 7. HpaM homologues in Xoo and Xoc are critical for virulence and HR induction.
Bacterial cells were cultured in NB medium and resuspended in sterile distilled water to a concentration of OD600 of 0.3 (for virulence assay) or 0.5 (for HR assay). For virulence test, the bacterial resuspensions were inoculated onto 6-week-old leaves of rice plant (Oryza sativa L. ssp. Japonica cultivar Nipponbare) by the leaf-clipping method (for Xoo) or by infiltrating with needleless syringe (for Xoc). For HR induction, the bacterial resuspensions were infiltrated into tobacco (Nicotina benthamiana) leaf mesophyll tissue. (A) Xoo strains; (B) Xoc strains. (a) Disease symptoms 14 days after inoculation; (b) lesion lengths scored 14 days after inoculation. Values represent means and standard deviation from twenty inoculated leaves in one experiment. The experiment was repeated three times with similar results. (c) HR symptoms photographed at 24, 36 and 48 h after infiltration. The hrcC deletion mutant strains ΔhrcCXoo (derivative of Xoo) and ΔhrcCXoc (derivative of Xoc) were used as negative controls. Three replications were done in each experiment and the experiment was repeated three times. The results presented are from a representative experiment, and similar results were obtained in all other independent experiments. (d) Electrolyte leakage from tobacco leaves inoculated with Xoo or Xoc strains. For each sample, four 0.4 cm2 leaf disks were collected from the bacteria-inoculated area and incubated in 5 ml distilled water. Conductivity was measured with a DDS-307A conductometer. Three samples were taken for each measurement in each experiment. Results presented are from a representative experiment, and similar results were obtained in two other independent experiments.
Figure 8. HpaM homologues in Xoo and Xoc have similar functions to HpaM.
(A) HpaMXoo (HpaM homologue in Xoo) and HpaMXoc (HpaM homologue in Xoc) are essential for type III secretion. Type III secretion signal sequence-gusA fusion reporter plasmid pGUSavrAC was introduced into Xoo and Xoc strains. The resulting recombinant strains were cultured in XOM2 medium for 12 h and the β-glucuronidase (GUS) activities in the culture (Total) and the cultural supernatant (Secreted) were determined. Values are the means ± standard deviation from three repeats. Left and right elements, GUS activities produced by pGUSavrAC in Xoo and Xoc strains, respectively. (B) The HpaM homologues HpaMXoo and HpaMXoc are also located in the outer membrane. The outer and inner membrane fraction proteins from Xoo and Xoc strains were prepared and 10 μg of each protein sample was separated by SDS-PAGE electrophoresis and transferred to a PVDF membrane. The presence of tested proteins was detected by anti-His6 monoclonal antibody. The histidine sensor kinase HpaS and transcriptional regulator HpaR1 of Xcc were used as positive and negative controls. OM, outer membrane; IM, inner membrane.
Discussion
Here we have demonstrated that the novel outer membrane-bound protein HpaM is critical for the type III secretion of Xanthomonas spp. Mutation of hpaM did not alter the production of extracellular enzymes and polysaccharides as well as cell motility, suggesting that HpaM may specifically affect the T3SS. HpaM is not involved in the regulation of the expression of hrp genes that encode the components of the T3SS machinery, but interacts with HrcC and HrcJ, the homologues of the components that compose the outer and inner rings of the T3SS basal body of all bacterial pathogens that possess a T3SS. Mutation of hrcC or hrcJ almost completely broke the type III secretion of Xcc, resulting in loss of the ability to cause disease symptoms and HR. In animal pathogens, it has been shown that the outer and inner ring proteins are outer and inner membrane proteins, respectively, and they physically interact with each other directly5,6,7,8. In this work, we authenticated that Xcc HrcC and HrcJ, as expected, are located in the outer and inner membrane, respectively, and they interact with each other directly. These data provide supporting evidence to the inference that HrcC and HrcJ act as T3SS outer and inner ring proteins in Xanthomonas spp. The peptide consisting of 46 amino acids from the 180th to 225th aa of HpaM is sufficient for interaction with HrcC but not HrcJ, and the most portion of the C-terminus, containing the amino acids from the 180th to 320th aa, is indispensable for the interaction with HrcJ. Bioinformatics analysis revealed that transmembrane helices are present in the N-terminus of HpaM. Taken together, these data suggest that HpaM is integrated into the outer membrane with its N-terminal domain and extends into the periplasm, where its middle part interacts with the outer ring protein HrcC and the C-terminal portion including the middle part interacts with the inner ring protein HrcJ, forming a three protein complex. It is worth noting that HpaM is predicted to have six right-handed parallel β-helix repeats from the 120th to 311th residues (i.e. 120–163, 180–202, 203–225, 226–248, 249–271, and 288–311), five of which lie in the region related to its physical interaction with HrcC and HrcJ. The right-handed parallel β-helix repeats are most commonly associated with autotransporter proteins, many of which are extracellular enzymes. It is clear that the β-helix repeats are essential not only for protein folding but also for functions, such as forming an appropriate structure that recognizes the substrates38,39. The presence of the β-helix repeats within the region interacting with HrcC and HrcJ suggests that they may be critical for HpaM stability and the formation of the protein complex.
Comparative bioinformatics analysis revealed that HpaM is conserved in all sequenced Xanthomonas species. To expand our knowledge on the function of HpaM in other Xanthomonas spp., we also investigated the HpaM homologues in X. oryzae pathovars oryzae and oryzicola. The results demonstrated that the HpaM homologues in the two pathovars of X. oryzae also localize in the outer membrane and are critical for pathogenicity and HR as well as efficient type III secretion. Furthermore, they can replace HpaM in Xcc for the type III secretion. These results indicate that HpaM is conserved not only in structure but also in function in Xanthomonas spp. Interestingly, HpaM homologs are also present in the species Pseudoxanthomonas spadix and Xylella fastidiosa (Table S3). Like the genus Xanthomonas, Pseudoxanthomonas and Xylella genera also belong to the family Xanthomonadaceae. It is possible that HpaM homologues are also prevalent in the members of these genera. However, Pseudoxanthomonas spadix and Xylella fastidiosa do not seem to have a T3SS. To investigate the function of the HpaM homologues in these bacteria will be a valuable topic.
At this stage, the precise role of HpaM in the T3SS is not clear. However, given the facts that: 1) HpaM does not act as a regulator for hrp gene expression but is critical for type III secretion; 2) its N-terminus integrates in the outer membrane and C-terminus extends deeply into the periplasm to interact physically with the inner ring protein HrcJ; and 3) its middle part interacts physically with the outer ring protein HrcC, forming a HpaM-HrcC-HrcJ complex, we presume that HpaM is most likely to be a structural component of the T3SS in Xanthomonas spp., although it is not encoded by a gene within the hrp cluster. In general, T3SS structural components of animal and plant pathogens are encoded by chromosomal or plasmid-borne gene clusters that were probably acquired during evolution by horizontal gene transfer6,40. As described above, the cluster consists of more than 20 genes and nine of which are conserved among plant and animal pathogens. These conserved genes are believed to encode the core components of the T3SS machinery in both plant and animal pathogens. However, more than 50% of the genes in the clusters are varied, suggesting that the clusters have changed a lot in different pathogens during the long-term evolution. A phylogenetic tree analysis divides the T3SSs of plant and animal pathogens into at least six families including two families from plant pathogens41. Therefore, it is possible that although the architectures of the T3SS apparatuses in different pathogens are similar, some fittings may not be the same. As described above, the T3SS extracellular appendage (pilus-like) of plant pathogens is different from that (needle-like) of animal pathogens. In addition, the inner rod may be another case showing different fittings in the T3SSs between plant and animal pathogens. The inner rod is a part of the T3SS basal body found in animal pathogens, which is formed by a periplasmic protein that connects the outer and inner rings33,34. However, the inner rod homologous protein is missing in plant pathogens. A non-homologous protein, HrpB2, has been supposed to be a putative inner rod protein of X. euvesicatoria, based on the features that it contains a VxTLxK amino acid motif that is conserved in the inner rod proteins of animal pathogens, localizes to the periplasm and the outer membrane, and is essential for T3SS pilus formation6,42.
A periplasmic protein, named VrpA, encoded by a gene outside the hrp cluster of X. citri subsp. citri, was recently reported to contribute to the secretion efficiency of the T3SS43. Similar to HpaM, VrpA is conserved in Xanthomonas spp. and also physically interacts with HrcC and HrcJ but not HrpB2. It was presumed that VrpA may affect activation of secretion and assembly or stability of the T3SS apparatus via interacting with HrcC and HrcJ43. We cannot exclude the possibility that HpaM associates with the T3SS via assisting the apparatus assembling or affecting the apparatus stability rather than as a T3SS structural component. Nonetheless, given the fact that HpaM as well as VrpA are Xanthomonas genus-specific proteins which are absent in other bacterial pathogens that possess a T3SS, our results suggest that the T3SS of Xanthomonas is distinctive in some aspects from other pathogens. To further investigate the precise role of HpaM and VrpA will greatly facilitate our understanding of the T3SS biogenesis.
Materials and Methods
Bacterial strains, plasmids and growth conditions
The bacterial strains and plasmids used in this study are listed in Table S1. Escherichia coli strains were grown in Luria–Bertani medium44 at 37 °C. Xcc strains were grown at 28 °C in NYG medium45, the minimal medium MMX46 or XVM231. Xoo and Xoc strains were grown at 28 °C in OB medium47, NB medium48, or the minimal medium XOM249. Antibiotics were added at the following concentrations as required: kanamycin (Kan) 25 μg ml−1, rifampicin (Rif) 50 μg ml−1, ampicillin (Amp) 100 μg ml−1, spectinomycin (Spc) 50 μg ml−1, gentamicin (Gm) 5 μg ml−1, streptomycin (Sm) at 100 μg ml−1, and tetracycline (Tet) 5 μg ml−1 for Xanthomonas spp. and 15 μg ml−1 for E. coli.
DNA and RNA techniques, SDS-PAGE and western blotting
DNA manipulations followed the procedures described by Sambrook and associates50. Plasmids were transformed into cells of E. coli and Xanthomonas spp. by electroporation or conjugation described by Turner and associates51. The restriction endonucleases, T4 DNA ligase, and pfu polymerase were provided by Promega (Shanghai, China). The total RNAs from Xanthomonas spp. were extracted with a total-RNA extraction kit (Promega), and reverse transcription was performed using a cDNA synthesis kit (Fermentas Co., Vilnius, Lithuania). Each kit was used according to the manufacturer’s instructions.
Western blotting was carried out as previously described21. Briefly, bacterial proteins were separated by 12% (w/v) SDS-PAGE and transferred onto PVDF (polyvinylidene difluoride) membrane (Millipore Corporation, Billerica, MA, USA). After blocking, the 1:2500 diluted anti-His-tag mouse monoclonal antibody (Qiagen, Shanghai, China) was used as the primary antibody, and the 1:2500 diluted horseradish peroxidase conjugated goat antimouse IgG (Bio-Rad, Hercules, CA, USA) was used as secondary antibody.
Deletion mutant construction and complementation
The hpaM in Xcc and its homologues hpaMXoo (in Xoo) and hpaMXoc (in Xoc) were deleted by the method described by Schäfer and associates37. For construction of Xcc hpaM deletion mutant, 747-bp upstream and 726-bp downstream fragments flanking hpaM (XC_2847) were amplified with the primer sets LhpaM-F/R and RhpaM-F/R (Table S2), respectively, using the total DNA of the Xcc wild type strain 8004 as a template. Primers were modified to give EcoRI-, XbaI- or HindIII-compatible ends (underlined) (Table S2). The two fragments were cloned together into the vector pK18mobsacB37, and the resulting plasmid named pK18mobsacBhpaM was introduced into the Xcc strain 8004 by triparental conjugation. The transconjugants were screened on selective agar plates containing 5% sucrose. The obtained hpaM deletion mutant was further confirmed by PCR and named ΔhpaM.
The HpaM derivatives with deletion in 180th–202th aa or 288th–311th aa were constructed by using the same method. For the HpaM derivative with deletion in 180th–202th aa, a 767-bp fragment spanning the 230th nt upstream to the 537th nt downstream of the start codon ATG of hpaM ORF and a 560-bp fragment spanning the 607th nt to the 1166th nt downstream of the start codon ATG of hpaM ORF were amplified with the primer sets LhpaM-F180/R180 and RhpaM-F180/R180. The resulting hpaM partial deletion mutant was named ΔhpaM180–202. For the HpaM derivative with deletion in 288th–311th aa, a 715-bp DNA fragment spanning the 147th nt to the 861th nt downstream of the start codon ATG of hpaM ORF and a 588-bp DNA fragment spanning the 934th nt to the 1521th nt downstream of the start codon ATG of hpaM ORF were amplified with the primer sets LhpaM-F311/R311 and RhpaM-F311/R311. The resulting hpaM partial deletion mutant was named ΔhpaM288–311.
For deletion of hpaMXoo (PXO_01147) or hpaMXoc (XOC_3053 homologue), 879-bp upstream and 591-bp downstream fragments flanking hpaMXoo or hpaMXoc were amplified with the corresponding primer sets (Table S2) from the Xoo strain PXO99A and the Xoc strain GX01, respectively. The resulting deletion mutants were named ΔhpaMXoo and ΔhpaMXoc (Table S1). For complementation of the hpaM deletion mutant, a 1432-bp DNA fragment containing the hpaM coding region and extending from 352-bp upstream of the 5′ end to 30-bp downstream of the 3′ end of the ORF was amplified by PCR from the total DNA of the Xcc strain 8004 with the primer set ChpaM-F/R (Table S2). Primers were modified to give BamHI- or HindIII-compatible ends (underlined) (Table S2). The amplified fragment was confirmed by sequencing, and ligated into the promoterless cloning site of the plasmid pLAFR652, generating the recombinant plasmid named pLChpaM (Table S1). The plasmid was introduced into the hpaM deletion mutant or partial deletion mutants by triparental conjugation, generating complemented strains named CΔhpaM, CΔhpaM180–202 and CΔhpaM288–311, respectively (Table S1). 1053-bp DNA fragments of the hpaMXoo (PXO_01147) ORF and hpaMXoc (XOC_3053 homologue) ORF were also amplified by PCR from the Xoo strain PXO99A and the Xoc strain GX01, respectively, and cloned into the plasmid pLAFR353. The resulting recombinant plasmids named pLChpaMXoo and pLChpaMXoc (Table S1) were used to complement the mutant strains ΔhpaM, ΔhpaMXoo, and ΔhpaMXoc.
For construction of avrAC (XC_1553) deletion mutant, avrAC-hpaM double deletion mutant and avrAC-hrcV double deletion mutant, 577-bp upstream and 461-bp downstream fragments flanking the ORF XC_1553 (avrAC) were amplified with the primer sets LavrAC-F/R and RavrAC-F/R (Table S2). The two fragments were cloned together into the BamHI/HindIII sites of pK18mobsacB37. The resulting recombinant plasmid pK18mobsacBavrAC was introduced into the Xcc wild type strain 8004, the hpaM mutant strain ΔhpaM, and the hrcV mutant strain ΔhrcV, respectively. The obtained mutants were named ΔavrAC, ΔavrAC-hpaM, and ΔavrAC-hrcV, respectively. To complement these mutants, a 2196-bp DNA fragment of avrAC gene (including 588 bp upstream sequence and avrAC coding sequence) fused with 6×His tag encoding sequences was amplified with the primer set HavrAC-F/R (Table S2). The obtained PCR fragment was cloned into the promoterless BamH/PstI sites of pLAFR6. The resulting recombinant plasmid pRavrACH6 was introduced into the mutant strain ΔavrAC, ΔavrAC-hpaM, and ΔavrAC-hrcV, respectively, generating recombinant strains ∆avrAC/pRavrACH6, ∆avrAC-hpaM/pRavrACH6, and ∆avrAC-hrcV/pRavrACH6 (Table S1).
Determination of transcriptional start site
To determine the transcriptional start site of the hpaM gene, 5′-RACE (5′ rapid amplification of cDNA ends) method was carried out with the hpaM sequence-specific primers hpaM-RTP1-4 (Table S2). The assay was performed as previously described21. Briefly, total cellular RNA was extracted from the Xcc wild type strain 8004 grown in NYG medium to an OD600 of 1.0. cDNA fragments were obtained using the 5′-RACE kit (Invitrogen Life Technologies, San Diego, CA, USA), and PCR products were cloned into the vector pMD19-T and sequenced.
Construction of promoter reporter plasmid
A promoter reporter plasmid for hpaM was constructed by fusing a 404-bp DNA fragment upstream of hpaM ORF (including the translation start codon ATG) with the promoterless β-glucuronidase (GUS)-encoding ORF (excluding the translation start codon ATG). The hpaM promoter region was amplified from the total DNA of the Xcc wild type strain 8004 by using the primer set RP-hpaMF/R (Table S2). The gusA coding region was amplified by PCR with the primer set GusF/R (Table S2), using the transposon Tn5gusA5 DNA as template. Primers were modified to give EcoRI-, BamHI- or PstI- compatible ends (underlined) (Table S2). The two fragments obtained were cloned into the promoterless cloning sites of the plasmid pLAFR6 to generate the reporter plasmid named pGUShpaM (Table S1).
Bacterial two-hybrid assay
The BacterioMatch II two-hybrid system (Stratagene, La Jolla, CA, USA) was used to detect protein-protein interaction in vivo. The truncated (or full length) hpaM, hrcC and hrcJ were amplified by PCR using the total DNA of the Xcc wild type strain 8004 as template and corresponding oligonucleotide set as primers (Table S2), respectively. The 981 bp truncated hpaM gene (from the 67th to the 1047th nt of the hpaM gene coding sequence, excluding the signal peptide coding sequence) was cloned into the BamHI/XhoI sites of pBT (bait), generating the plasmid pBhpaMLN22 (Table S1). 1716- and 1011-bp fragments of truncated hrcC, and 699- and 555-bp fragments of truncated hrcJ were cloned into the BamHI/XhoI sites of the vector pTRG (prey), respectively, generating the plasmids named pThrcCLN33, pThrcC34–370, pThrcJLN21 and pThrcJ22–206 (Table S1). The bacterial two-hybrid assay was performed according to the manufacturer’s instructions. To test the interaction of a variety length of HpaM fragments with the periplasmic domain of HrcC and HrcJ, 540, 609, 678, 372, 441, 510, 276, 138, 363, 423 and 483-bp DNA fragments containing partial hpaM gene were amplified by PCR using the corresponding primer sets (Table S2), respectively, and the obtained DNA fragments were cloned into BamHI/XhoI sites of pBT, resulting a series of pBM recombinant plasmids (Table S2). The plasmid pairs (Fig. 5) were used to co-transform the reporter strain XL1-Blue MRF′ on M9 salt agar without 3-AT. Colonies were then restreaked on M9 salt agar containing 5 mM 3-AT and incubated at 37 °C for 24 h for the first detection of interaction. For confirmation, the colonies were cultured on dual selective medium containing 5 mM 3-AT and 12.5 μg ml−1 Sm, as described in the manual.
Overproduction and purification of proteins
To overproduce 6×His-tagged truncated forms of HpaM, 981, 138 and 423-bp DNA fragments encoding 23th–349th (excluding signal peptide), 180th–225th and 180th–320th amino acids were amplified by using the primer set hpaM-OF/R, hpaMO-9F/R and hpaMO-11F/R (Table S2), respectively. The obtained DNA fragments were cloned into BamHI/HindIII sites of the expression vector pET-30a (Novagen), resulting the recombinant plasmid named pET-30a-HpaMLN22, pET-30a-HpaMLN180–225 and pET-30a-HpaMLN180–320, respectively (Table S1). The recombinant plasmids were then transformed into E. coli strain BL21 (DE3), resulting strains BL21/pET-30a-HpaMLN22, BL21/pET-30a-HpaMLN180–225 and BL21/pET-30a-HpaMLN180–320. After cultivation and induction by IPTG (isopropyl-thiogalactopyranoside), the cells were harvested and 6×His-tagged fused proteins were purified by Nickel-NTA resin (Qiagen). For overproduction of the periplasmic domain of HrcC and HrcJ, a 1011 bp DNA fragment encoding the 34th–370th amino acids of HrcC and a 555-bp fragment encoding the 22th–206th amino acids of HrcJ were amplified by using the primer sets hrcC-N2F/R and hrcJ-N2F/R (Table S2), respectively. The resulting fragments were cloned into the BamHI/HindIII sites of pET-30a (Novagen), generating the recombinant plasmids named pET-30a-HrcC34–370 and pET-30a-HrcJ22–206 (Table S1). The recombinant plasmids were transformed into E. coli strain BL21 (DE3), resulting recombinant strains BL21/pET-30a-HrcC34–370 and BL21/pET-30a-HrcJ22–206, respectively (Table S1).
Protein pull-down assay
Protein pull-down assay was performed as previously described21, with the ProFound pull-down biotinylated protein-protein interaction kit (Pierce, Rockford, IL, USA). The hpaM fusion protein His6-HpaMLN22 was biotinylated with sulfo-NHS-LC-biotin, and the labeled protein was purified by dialysis. 50 μl of the purified biotinylated His6-HpaMLN22 (0.5 mg ml−1) was incubated with 40 μl of streptavidin sepharose™ beads. After centrifugation, beads were washed three times with binding buffer containing 300 mM NaCl and 100 μl of sample containing 50 μg suspected prey protein (His6-HrcC34–370 or His6-HrcJ22–206) was added. After incubation at 4 °C for at least 60 min, beads were washed with wash buffer and prey protein was eluted in 150 μl elution buffer. 20 μl of the eluted sample was eletrophored on 12% SDS-PAGE gel and visualized by coomassie blue staining. For detection of HrcC-HrcJ interaction, His6-HrcC34–370 and His6-HrcJ22–206 were overproduced, purified and biotinylated with sulfo-NHS-LC-biotin, followed by incubation with streptavidin sepharose™ beads, respectively. His6-HrcC34–370 and His6-HrcJ22–206 proteins were then added into the above biotinylated His6-HrcJ22–206 and His6-HrcC34–370, respectively. For detection of the interaction between His6-HrcC34–370 (or His6-HrcJ22–206) and each of the truncated HpaM fragments, His6-HpaMLN22, His6-HpaMLN180–225, or His6-HpaMLN180–320 was added.
GUS activity assay
GUS activity was determined by measurement of the absorbance of OD415 using ρ-nitrophenyl-β-D-glucuronide as the substrate, as described by Henderson and associates54, after growth of bacterial cells in medium for a period of time. To determine the GUS activity of secreted proteins, the bacterial cells of 200 μl culture for each strain were separated by centrifugation and the cell-free supernatant was taken for GUS activity determination.
Plant assay
The virulence of Xcc to Chinese radish (Raphanus sativus) was tested by the leaf-clipping method55. Bacterial cells from overnight culture were collected, washed with 10 mM sodium phosphate buffer (SPB, 5.8 mM Na2HPO4 and 4.2 mM NaH2PO4, pH 7.0) and resuspended in the same buffer to an OD600 of 0.1 (1 × 108 CFU ml−1). Leaves were cut with scissors dipped in the bacterial suspensions. Lesion length was measured 10 days after inoculation, and data were analysed by t-test. The HR was tested on the pepper plant ECW-10R (Capsicumannuum cv. ECW-10R) as previously described21. For each Xcc strain tested, an approximately 5 μl bacterial resuspension (1 × 108 CFU ml−1) was infiltrated into the abaxial leaf surface of pepper plant. The inoculated plants were maintained in appropriated conditions, and HR symptoms were observed and photographed at 8, 16 and 24 h after inoculation. For the electrolyte leakage assay, bacterial cells were resuspended in sterile distilled water at a concentration of OD600 of 0.1. Four 0.4 cm2 leaf disks for each sample were collected from the bacteria-infiltrated area and incubated in 5 ml of distilled water. Conductivity was measured with a DDS-307A conductometer.
For Xoo virulence assay, the wild-type strain PXO99A 56 and its derivatives were tested on susceptible rice plant Oryza sativa L. ssp. Japonica cultivar Nipponbare using leaf clip inoculation method57. For Xoc, the wild type strain GX01 and its derivatives were infiltrated into rice leaves by needleless syringe58. Bacterial cells were grown for 72 h at 28 °C in NB medium with appropriate antibiotics. The cells were collected and resuspended in sterile distilled water to a concentration of OD600 = 0.3. Inoculation was carried out on 6-week-old rice plants under relevant conditions. Symptoms were recorded by photography and the disease lesion lengths were measured 14 days after inoculation. Twenty-five leaves were inoculated for each strain in each experiment. The experiment was repeated three times.
The HR test of Xoo and Xoc was conducted as described by Guo et al.59 and Zou et al.60, respectively. Briefly, Xoc or Xoo strains were cultured in NB medium to logarithmic phase, and cells were pelleted and suspended in water to a concentration of OD600 = 0.5. The suspensions were infiltrated into leaves of glass house-grown tobacco (Nicotina benthamiana), and the results were observed at 24, 36 and 48 h after infiltration. If the strain had the ability to trigger HR, the phenomenon of programmed cell death would be observed around the inoculation sites on tobacco leaves. The detection of the electrolyte leakage in tobacco leaves inoculated with Xoo or Xoc strains was similar to the method used to detect that in pepper leaves inoculated with Xcc strains.
Cya protein translocation assay
To determine Cya enzyme activity in vivo, a modification of the procedure described by Roden and associates61 was carried out. Briefly, a 894-bp DNA fragment spanning nucleotides 588-bp upstream to 306-bp downstream of the translation start codon ATG of avrAC (XC_1553) was fused with the ORF of cyaA (excluding the translational start codon ATG) and ligated into pLAFR6, resulting the plasmid pLavrAC102::CyaA (Table S1). The plasmid was then introduced into Xcc wild type, hpaM mutant, and hrcV mutant strains. The resulting recombinant strains were cultured in NYG medium, and bacterial cells from the cultures were resuspended in 10 mM MgCl2 to a concentration of OD600 of 0.1 and then infiltrated into 20 plant leaves. A direct cyclic AMP (cAMP) correlation enzyme immunoassay kit (Amersham) was used to process the leaf samples and measure the cAMP concentrations following the manufacturer’s instructions. The protein content of each sample was determined using the Bio-Rad protein assay (Bio-Rad). Cya enzyme activity was expressed as pmol of cAMP per mg of total protein.
Preparation of total, periplasmic, and outer membrane proteins
The bacterial total and periplasmic proteins were prepared using the method described previously62. The bacterial outer and inner membrane proteins were prepared as described by Chen and associates30. 100 ml of the bacterial culture for each strain was collected and disrupted by sonication, the unbroken cells and cell debris were removed by centrifugation at 14,000 g at 4 °C for 30 min. Supernatants were then centrifuged at 135,000 g at 4 °C for 1 h. The pellets, which contain membranes and ribosomes, were suspended in 1.0 ml cold TM buffer (10 mM Tris, pH 8.0, containing 8 mM MgSO4), and followed by centrifugation at 135,000 g. Pellets were then rinsed with 1.0 ml cold TM buffer, resuspended in 3.9 ml 0.25% (w/v) Sarkosyl and loaded into 3.9 ml ultracentrifuge tubes. After incubation at room temperature for 1 h, the tubes were centrifuged at 135,000 g for 1 h. Supernatants, containing the Sarkosyl-soluble inner membranes, were retained. The Sarkosyl-insoluble pellets, containing the outer membrane fraction, were washed twice with 1.0 ml 0.25% (w/v) Sarkosyl, incubated at room temperature for 1 h and centrifuged at 135,000 g. The pellets, containing the outer membrane fraction, were resuspended in 40 μl cold TM buffer.
The outer membrane proteins were also prepared as described by Leuzzi et al.63. Briefly, bacterial cells were disrupted by sonication and the supernatant containing the total membrane fraction was recovered and further centrifuged at 50,000 g for 90 min at 4 °C. The pellet containing the membranes was resuspended in 2% Sarkosyl in 20 mM Tris-HCl, pH 7.5 and 2 mM EDTA and incubated at room temperature to solubilize the inner membranes. To remove aggregates the suspension was first centrifuged at 10,000 g for 20 min at 4 °C and then centrifuged overnight at 75,000 g at 4 °C. The pellet containing the outer membranes was resuspended in SPB.
Additional Information
How to cite this article: Li, L. et al. Identification of a novel type III secretion-associated outer membrane-bound protein from Xanthomonas campestris pv. campestris. Sci. Rep. 7, 42724; doi: 10.1038/srep42724 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by the 973 Program of the Ministry of Science and Technology of China (2012CB114003), the National Natural Science Foundation of China (31371263; 31660506), and the Ba Gui Scholar Program of Guangxi Zhuang Autonomous Region of China (2014A002). We thank Professor Bo-Le Jiang for awarding the recombinant plasmid pLavrAC102::CyaA.
Footnotes
The authors declare no competing financial interests.
Author Contributions J.L.T. & G.T.L. conceived of and organized the study. L.L. & R.F.L. performed the experiments. J.L.T., G.T.L., Z.H.M., L.L. & R.F.L. performed the data analyses. J.L.T. & G.T.L. wrote the paper. All authors have read and approved the final manuscript.
References
- Cornelis G. R. & Van Gijsegem F. Assembly and function of type III secretory systems. Annu Rev Microbiol 54, 735–774 (2000). [DOI] [PubMed] [Google Scholar]
- Galán J. E. & Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284, 1322 (1999). [DOI] [PubMed] [Google Scholar]
- Galán J. E. & Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444, 567–573 (2006). [DOI] [PubMed] [Google Scholar]
- He S. Y. Type III protein secretion systems in plant and animal pathogenic bacteria. Annu Rev Phytopathol 36, 363–392 (1998). [DOI] [PubMed] [Google Scholar]
- Abrusci P., McDowell M. A., Lea S. M. & Johnson S. Building a secreting nanomachine: a structural overview of the T3SS. Curr Opin Struct Biol 25, 111–117 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner D. Protein export according to schedule: architecture, assembly, and regulation of type III secretion systems from plant- and animal-pathogenic bacteria. Microbiol Mol Biol Rev 76, 262–310 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Lara-Tejero M., Marlovits T. C. & Wagner S. Bacterial type III secretion systems: specialized nanomachines for protein delivery into target cells. Annu Rev Microbiol 68, 415–438 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaliou A. G., Tsolis K. C., Loos M. S., Zorzini V. & Economou A. Type III secretion: building and operating a remarkable nanomachine. Trends Biochem Sci 41, 175–189 (2016). [DOI] [PubMed] [Google Scholar]
- Anderson D. M., Fouts D. E., Collmer A. & Schneewind O. Reciprocal secretion of proteins by the bacterial type III machines of plant and animal pathogens suggests universal recognition of mRNA targeting signals. Proc Natl Acad Sci USA 96, 12839–12843 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier O., Wengelnik K., Hahn K. & Bonas U. The Xanthomonas Hrp type III system secretes proteins from plant and mammalian bacterial pathogens. Proc Natl Acad Sci USA 96, 9368–9373 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampakaki A. P., Fadouloglou V. E., Gazi A. D., Panopoulos N. J. & Kokkinidis M. Conserved features of type III secretion. Cell Microbiol 6, 805–816 (2004). [DOI] [PubMed] [Google Scholar]
- Diepold A. & Wagner S. Assembly of the bacterial type III secretion machinery. FEMS Microbiol Rev 38, 802–822 (2014). [DOI] [PubMed] [Google Scholar]
- Hartmann N. & Büttner D. The inner membrane protein HrcV from Xanthomonas spp. is involved in substrate docking during type III secretion. Mol Plant Microbe Interact 26, 1176–1189 (2013). [DOI] [PubMed] [Google Scholar]
- He S. Y., Nomura K. & Whittam T. S. Type III protein secretion mechanism in mammalian and plant pathogens. Biochim Biophys Acta 1694, 181–206 (2004). [DOI] [PubMed] [Google Scholar]
- Büttner D. & Bonas U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol Rev 34, 107–133 (2010). [DOI] [PubMed] [Google Scholar]
- Ryan R. P. et al. Pathogenomics of Xanthomonas: understanding bacterium–plant interactions. Nat Rev Microbiol 9, 344–355 (2011). [DOI] [PubMed] [Google Scholar]
- Büttner D. & Bonas U. Port of entry—the type III secretion translocon. Trends Microbiol 10, 186–192 (2002). [DOI] [PubMed] [Google Scholar]
- Gürlebeck D., Thieme F. & Bonas U. Type III effector proteins from the plant pathogen Xanthomonas and their role in the interaction with the host plant. J Plant Physiol 163, 233–255 (2006). [DOI] [PubMed] [Google Scholar]
- Tampakaki A. P. et al. Playing the “Harp”: evolution of our understanding of hrp/hrc genes. Annu Rev Phytopathol 48, 347–370 (2010). [DOI] [PubMed] [Google Scholar]
- Qian W. et al. Comparative and functional genomic analyses of the pathogenicity of phytopathogen Xanthomonas campestris pv. campestris. Genome Res 15, 757–767 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. F. et al. Identification of a putative cognate sensor kinase for the two-component response regulator HrpG, a key regulator controlling the expression of the hrp genes in Xanthomonas campestris pv. campestris. Environ Microbiol 16, 2053–2071 (2014). [DOI] [PubMed] [Google Scholar]
- Huang D. L. et al. The Zur of Xanthomonas campestris is involved in hypersensitive response and positively regulates the expression of the hrp cluster via hrpX but not hrpG. Mol Plant Microbe Interact 22, 321–329 (2009). [DOI] [PubMed] [Google Scholar]
- Wengelnik K. & Bonas U. HrpXv, an AraC-type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria. J Bacteriol 178, 3462–3469 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett M. B. et al. Molecular signals required for type III secretion and translocation of the Xanthomonas campestris AvrBs2 protein to pepper plants. Proc Natl Acad Sci USA 97, 13324–13329 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B. L. et al. The type III secretion effector XopXccN of Xanthomonas campestris pv. campestris is required for full virulence. Res Microbiol 159, 216–220 (2008). [DOI] [PubMed] [Google Scholar]
- Xu R. Q. et al. AvrAC(Xcc8004), a type III effector with a leucine-rich repeat domain from Xanthomonas campestris pathovar campestris confers avirulence in vascular tissues of Arabidopsis thaliana ecotype Col-0. J Bacteriol 190, 343–355 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sory M. P. & Cornelis G. R. Translocation of a hybrid YopE adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol 14, 583–594 (1994). [DOI] [PubMed] [Google Scholar]
- Casper-Lindley C., Dahlbeck D., Clark E. T. & Staskawicz B. J. Direct biochemical evidence for type III secretion-dependent translocation of the AvrBs2 effector protein into plant cells. Proc Natl Acad Sci USA 99, 8336–8341 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S. Q. et al. Systematic mutagenesis of all predicted gntR genes in Xanthomonas campestris pv. campestris reveals a GntR family transcriptional regulator controlling hypersensitive response and virulence. Mol Plant Microbe Interact 24, 1027–1039 (2011). [DOI] [PubMed] [Google Scholar]
- Chen Y. Y., Wu C. H., Lin J. W., Weng S. F. & Tseng Y. H. Mutation of the gene encoding a major outer-membrane protein in Xanthomonas campestris pv. campestris causes pleiotropic effects, including loss of pathogenicity. Microbiology 156, 2842–2854 (2010). [DOI] [PubMed] [Google Scholar]
- Wengelnik K., Marie C., Russel M. & Bonas U. Expression and localization of HrpA1, a protein of Xanthomonas campestris pv. vesicatoria essential for pathogenicity and induction of the hypersensitive reaction. J Bacteriol 178, 1061–1069 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreter T. et al. A conserved structural motif mediates formation of the periplasmic rings in the type III secretion system. Nat Struct Mol Biol 16, 468–476 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlovits T. C. et al. Assembly of the inner rod determines needle length in the type III secretion injectisome. Nature 441, 637–640 (2006). [DOI] [PubMed] [Google Scholar]
- Marlovits T. C. et al. Structural insights into the assembly of the type III secretion needle complex. Science 306, 1040–1042 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg S. L. et al. Genome sequence and rapid evolution of the rice pathogen Xanthomonas oryzae pv. oryzae PXO99A. BMC Genomics 9, 204 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanove A. J. et al. Two new complete genome sequences offer insight into host and tissue specificity of plant pathogenic Xanthomonas spp. J Bacteriol 193, 5450–5464 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer A. et al. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmidspK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145, 69–73 (1994). [DOI] [PubMed] [Google Scholar]
- Jenkins J. & Pickersgill R. The architecture of parallel β-helices and related folds. Prog Biophys Mol Biol 77, 111–175 (2001). [DOI] [PubMed] [Google Scholar]
- Junker M. et al. Pertactin beta-helix folding mechanism suggests common themes for the secretion and folding of autotransporter proteins. Proc Natl Acad Sci USA 103, 4918–4923 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gophna U., Ron E. Z. & Graur D. Bacterial type III secretion systems are ancient and evolved by multiple horizontal-transfer events. Gene 312, 151–163 (2003). [DOI] [PubMed] [Google Scholar]
- Troisfontaines P. & Cornelis G. R. (2005) Type III secretion: more systems than you think. Physiology (Bethesda) 20, 326–339 (2003). [DOI] [PubMed] [Google Scholar]
- Hartmann N. et al. Characterization of HrpB2 from Xanthomonas campestris pv. vesicatoria identifies protein regions that are essential for type III secretion pilus formation. Microbiol 158, 1334–1349 (2012). [DOI] [PubMed] [Google Scholar]
- Zhou X., Hu X., Li J. & Wang N. A novel periplasmic protein, VrpA, contributes to efficient protein secretion by the type III secretion system in Xanthomonas spp. Mol Plant Microbe Interact 28, 143–153 (2015). [DOI] [PubMed] [Google Scholar]
- Miller J. H. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA (1972). [Google Scholar]
- Daniels M. J. et al. Cloning of genes involved in pathogenicity of Xanthomonas campestris pv. campestris using the broad host range cosmid pLAFR1. EMBO J. 3, 3323–3328 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. J., Barber C. E., Turner P. C., Cleary W. G. & Sawczyc M. K. Isolation of mutants of Xanthomonas campestris pathovar campestris showing altered pathogenicity. J Gen Microbiol 130, 2447–2455 (1984). [Google Scholar]
- Tang J. L. et al. Cloning and characterization of the rpfC gene of Xanthomonas oryzae pv. oryzae: involvement in exopolysaccharide production and virulence to rice. Mol Plant Microbe Interact 9, 664–666 (1996). [DOI] [PubMed] [Google Scholar]
- Li Y. R. et al. A novel regulatory role of HrpD6 in regulating hrp-hrc-hpa genes in Xanthomonas oryzae pv. oryzicola. Mol Plant Microbe Interact 24, 1086–1101 (2011). [DOI] [PubMed] [Google Scholar]
- Tsuge S. et al. Expression of Xanthomonas oryzae pv. oryzae hrp genes in a novel synthetic medium, XOM2. J Gen Plant Pathol 68, 363–371 (2002). [Google Scholar]
- Sambrook J., Fritsch E. F. & Maniatis T. Molecular Cloning: A Laboratory Manual. New York, USA: Cold Spring Harbor Laboratory Press (1989). [Google Scholar]
- Turner P., Barber C. E. & Daniels M. J. Evidence for clustered pathogenicity genes in Xanthomonas campestris pv. campestris. Mol Gen Genet 199, 338–343 (1985). [Google Scholar]
- Huynh T. V., Dahlbeck D. & Staskawicz B. J. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science 245, 1374–1377 (1989). [DOI] [PubMed] [Google Scholar]
- Staskawicz B., Dahlbeck D., Keen N. & Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol 169, 5789–5794 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. F. et al. New approaches for the evaluation of pulmonary toxicity: bronchoalveolar lavage fluid analysis. Fundam Appl Toxicol 5, 451–458 (1985). [DOI] [PubMed] [Google Scholar]
- Dow J. M. et al. Biofilm dispersal in Xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc Natl Acad Sci USA 100, 10995–11000 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C. M., White F. F., Choi S. H., Guo A. & Leach J. E. Identification of a family of avirulence genes from Xanthomonas oryzae pv. oryzae. Mol Plant Microbe Interact 5, 451–459 (1992). [DOI] [PubMed] [Google Scholar]
- Yang F. H. et al. A novel two-component system PdeK/PdeR regulates c-di-GMP turnover and virulence of Xanthomonas oryzae pv. oryzae. Mol Plant Microbe Interact 25, 1361–1369 (2012). [DOI] [PubMed] [Google Scholar]
- Qian G. et al. epv, encoding a hypothetical protein, is regulated by DSF-mediating quorum sensing as well as global regulator Clp and is required for optimal virulence in Xanthomonas oryzae pv. oryzicola. Phytopathol 102, 841–847 (2012). [DOI] [PubMed] [Google Scholar]
- Guo W. et al. Ketoglutarate transport protein KgtP is secreted through the type III secretion system and contributes to virulence in Xanthomonas oryzae pv. oryzae. Appl Environ Microbiol 78, 5672–5681 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L. F. et al. Elucidation of the hrp clusters of Xanthomonas oryzae pv. oryzicola that control the hypersensitive response in nonhost tobacco and pathogenicity in susceptible host rice. Appl Environ Microbiol 72, 6216–6224 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden J., Eardley L., Hotson A., Cao Y. Y. & Mudgett M. B. Characterization of the Xanthomonas AvrXv4 effector, a SUMO protease translocated into plant cells. Mol Plant Microbe Interact 17, 633–643 (2004). [DOI] [PubMed] [Google Scholar]
- Zang N. et al. Requirement of a mip-like gene for virulence in the phytopathogenic bacterium Xanthomonas campestris pv. campestris. Mol Plant Microbe Interact 20, 21–30 (2007). [DOI] [PubMed] [Google Scholar]
- Leuzzi R. et al. Ng-MIP, a surface-exposed lipoprotein of Neisseria gonorrhoeae, has a peptidyl-prolyl cis/trans isomerase (PPIase) activity and is involved in persistence in macrophages. Mol Microbiol 58, 669–681 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.