Abstract
Breathing is vital for survival but also interesting from the perspective of rhythm generation. This rhythmic behavior is generated within the brainstem and is thought to emerge through the interaction between independent oscillatory neuronal networks. In mammals, breathing is composed of three phases – inspiration, post-inspiration, and active expiration – and this article discusses the concept that each phase is generated by anatomically distinct rhythm-generating networks: the preBötzinger complex (preBötC), the post-inspiratory complex (PiCo), and the lateral parafacial nucleus (pF L), respectively. The preBötC was first discovered 25 years ago and was shown to be both necessary and sufficient for the generation of inspiration. More recently, networks have been described that are responsible for post-inspiration and active expiration. Here, we attempt to collate the current knowledge and hypotheses regarding how respiratory rhythms are generated, the role that inhibition plays, and the interactions between the medullary networks. Our considerations may have implications for rhythm generation in general.
Keywords: Respiration, breathing, rhythm generation, networks, pacemaker, preBotzinger complex, Oscillators, Postinspiration
Introduction
Rhythms and oscillations function at the core of many brain processes 1, 2. For example, rhythmic spinal circuits control locomotor gait 3, 4, thalamic oscillations detect attentional state 5, 6, cerebellar rhythms are important for motor coordination 7, 8, and circadian rhythms entrain our biological clocks to a 24-hour cycle 9, 10. Compared to these circuits, respiratory neural networks in the brainstem offer a uniquely advantageous system in which to study rhythm generation because of (1) the known anatomical location of respiratory rhythm generators 11– 15 and (2) the ability to reduce the breathing network into various levels in preparations that retain robust and autonomous rhythmic output 11, 15– 18. As a result, the control of respiration can be studied from the molecular to the systems level. Mammalian respiration consists of three phases: inspiration, post-inspiration, and active expiration 19, 20. The networks that collectively generate the three respiratory phases are distributed bilaterally in the ventral respiratory column (VRC) of the brainstem 21– 23.
Within the VRC, the first described respiratory neural network, the preBötzinger complex (preBötC), is both necessary and sufficient for the generation of inspiration 11, 24– 27. The preBötC can singularly reconfigure to produce the inspiratory phase of eupnea (normal breathing), gasps, and sighs 28. The respiratory rhythm generated within the preBötC is dependent on excitatory mechanisms, and the location of the network within the ventrolateral medulla has been identified in rodents 11, 29, cats 26, and humans 30. Rhythm-generating, glutamatergic, and bilaterally interconnected preBötC interneurons are derived from progenitors that express the homeobox gene Dbx1 25, 31. The preBötC can be isolated in an in vitro transverse slice that retains fictive inspiratory bursts in phase with inspiratory hypoglossal motor output 11. The transverse slice is amenable to rigorous electrophysiological, histochemical, and optogenetic manipulation. Recently, two distinct rhythm generators have been described that are hypothesized to control the other two phases of respiration: the post-inspiratory complex (PiCo) for the control of post-inspiration, and the lateral parafacial nucleus (pF L), a subpopulation within the retrotrapezoid nucleus parafacial respiratory group (RTN/pFRG), for the control of active expiration ( Figure 1). In addition to the previously mentioned transverse in vitro slice 12, 32, 33, en-bloc brainstem-spinal cord 31, 34, 35, in situ 36, sagittal slab 17, 37, and, most recently, horizontal slice 15 preparations offer further accessibility and tractability to begin to unravel how the three phases of breathing are generated and interconnected.
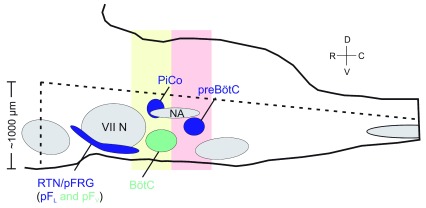
Figure 1. Anatomical map of oscillators in the ventral respiratory column.
Schematic of the brainstem from a sagittal view illustrating the approximate anatomical locations of the three respiratory rhythm generators. Shapes in blue represent the three distinct oscillators (preBötzinger complex [preBötC], post-inspiratory complex [PiCo], and retrotrapezoid nucleus parafacial respiratory group [RTN/pFRG]) that are thought to individually control the three phases of respiration. The RTN/pFRG is further segregated into the lateral parafacial nucleus (pF L), which is more lateral, dorsal, and rhythmogenic, and the ventral parafacial nucleus (pF V), which is more medial, ventral, and not considered rhythmogenic. Shapes in gray represent motor nuclei, specifically VII N = facial nucleus and NA = nucleus ambiguus. Green represents neuronal populations that contribute to the respiratory rhythm but are not thought to be independent rhythm generators. The dotted lines indicate the approximate boundaries of the horizontal slice, while the pink and yellow boxes illustrate the approximate boundaries of transverse slices isolating preBötC and PiCo, respectively.
Mammalian respiratory rhythmogenesis
Decades of research have revolved around the endeavor to unmask the underlying processes controlling inspiratory rhythm generation 11, 28, 38. Indeed, a long-standing question in the respiratory control field queries how rhythmic, inspiratory activity in the brainstem emerges from the interaction between intrinsic cellular properties and circuit-based synaptic properties. Amid many theories, the answer remains unresolved, but it is likely that multiple rhythmogenic mechanisms exist within the functionally and molecularly heterogeneous preBötC population, and these mechanisms may vary depending on the metabolic, behavioral, and environmental conditions of the organism 39.
Frequently, models of neural rhythmogenesis include autonomously bursting neurons (pacemakers, or endogenous bursters) as contributors to rhythmogenesis 40– 43. Endogenous bursting neurons have been described in numerous rhythm-generating networks and the respiratory network is not an exception 43, 44. Approximately 20% of preBötC neurons can be classified as pacemakers, as defined by their tendency to burst in the absence of synaptic input at a period and burst duration similar to the duty cycle of the in vitro respiratory rhythm 38, 45– 47. Pacemaker neurons in the preBötC can be either glutamatergic 38, 46 or glycinergic 48. The “pacemaker hypothesis”, in its strictest interpretation, is the idea that excitatory pacemaker cells play an obligatory role in driving the inspiratory rhythm. It is supported by studies in which antagonists of the persistent sodium current (I NaP; riluzole) and the calcium-activated nonspecific cationic current (I CAN; flufenamic acid [FFA]), the two mechanisms underlying bursting in preBötC neurons, block fictive inspiration in vitro 49 and inspiration in vivo 50. Moreover, regions such as the preBötC and the RTN/pFRG, that are known to have rhythmogenic functions, are rich in endogenous bursters 37. The exact role of endogenously bursting neurons in respiratory rhythm generation is still a matter of debate 38, 40, 43– 45, 47, 51, 52. However, it is generally agreed that these bursting neurons do not act as simple “pacemakers” that drive the rhythm. Instead, these neurons are well integrated within the respiratory network, and synaptic and other ionic mechanisms contribute to their timing and discharge properties 39, 40, 53, 54.
Although cellular properties have been identified that differentiate pacemaker from non-pacemaker neurons 55, 56, we shouldn’t think of these in a binary manner. Instead, bursting and non-bursting lie on a continuum of firing characteristics from weak tonic firing to strong bursting 53, consistent with the hypothesis that preBötC neurons exhibit a continuous distribution of membrane conductances 57– 59. For example, I CAN and I NaP currents are not exclusive to endogenous burster neurons but are present on many, if not the entire population of, preBötC inspiratory neurons in vitro 45, 46, 59, 60. The “group pacemaker” theory posits that activity of tonically firing, glutamatergic preBötC neurons can percolate and increase in activity by means of positive feedback 47, 61. The pre-inspiratory phase occurs when the positive feedback has surpassed other network constituents and recurrent excitation leads to the initiation of a synchronized inspiratory burst 62.
This idea was further tested by using in vitro physiological data and modeling techniques to hypothesize that each individual population burst is driven by a dynamic, stochastic, and flexible assembly of preBötC neurons within a sparsely connected network 63. Insights into the physiology of the sparsely connected network can be performed by multi-array recordings 64. Using this technique, Carroll et al. estimated a 1% functional connectivity between preBötC neurons 63, a figure much lower than another study that estimated a 13% probability of one-way excitatory connectivity from dual whole-cell patch recordings of visualized, closely located preBötC neurons 65. Reasons for the order of magnitude discrepancy in connectivity estimates have yet to be reconciled other than obvious differences in approach and preparation.
Rhythm generation and pattern generation have been suggested to be separable phenomena 66– 68. Rhythm generation refers to the generation of timing signals; however, the control of the timing and coordination of muscle activity is referred to as pattern generation 66, 69, 70. Intracellular burst activity and motor outputs can exhibit a variety of shapes such as decrementing, augmenting, or bell-shaped 16, 71. Under conventional perfusion conditions in vitro, preBötC bursts follow a 1:1 ratio with hypoglossal motor output 16. However, when excitability is lowered with decreased concentrations of extracellular potassium, burst frequency decreases 27, 72. When a burst is expected, Feldman and colleagues instead observe “burstlets” that are small in amplitude and do not produce a motor output signal. Burstlets appear at multiples of the shortest interburst interval (i.e. are quantized) and can also be observed under specific conditions in vivo 66. The authors hypothesize that these burstlets represent pre-inspiratory activity that triggers inspiratory bursts when a certain, undefined threshold is reached.
In addition to the preBötC, two other respiratory microcircuits have been identified that function as independent oscillators controlling the other two phases of breathing: post-inspiration and active expiration 14, 15, 73. Under physiological conditions, expiration is a passive process and mammals largely alternate their breathing between inspiration and post-inspiration 74. Located rostral to the preBötC and dorsomedial to the nucleus ambiguus, the PiCo was recently identified as the putative site for the generation of post-inspiratory activity 15. Similar to the preBötC, PiCo rhythms are also dependent on non-NMDA, excitatory mechanisms 15. Thus, it is likely that the two populations employ similar rhythm-generating mechanisms. Interestingly, one study completed in goats showed that the gradual ablation of the preBötC over the course of two weeks does not result in breathing abnormalities, at least in this species, suggesting that plasticity mechanisms are able to compensate if time is allowed for brainstem networks to reconfigure 75. Perhaps PiCo neurons are logical candidates for assuming the preBötC’s role?
During periods of higher metabolic activity, for example during exercise, a third phase of breathing is recruited during late expiration, called active expiration, that is required to breathe air out more forcibly than under rest conditions. The active expiratory rhythm reportedly originates in the pF L 13, 14. This area is defined as a conditional but independent oscillator owing to the observation that it is active only under certain conditions 73 but can generate rhythmic motor output from facial motor roots in the presence of an opioid agonist, DAMGO 76. Similar to the preBötC and PiCo, the pF L is dependent on excitatory mechanisms 35, 77. Further studies are required to fully elucidate the rhythmogenic mechanisms of these three excitatory oscillatory networks.
Role of inhibition
While it is generally accepted that the preBötC can burst autonomously in vitro, even when inhibition is blocked pharmacologically 11, the role of inhibition within the intact respiratory network is still debated. Originally, it was proposed that inspiration and expiration were generated by “half-centered oscillators” in which one population of neurons reciprocally inhibits the other population to generate an alternating two-phase breathing rhythm 78. However, these hypotheses have not been rigorously tested by specifically manipulating identified populations of neurons.
A population termed the Bötzinger complex (BötC) was discovered to contain primarily inhibitory neurons including post-inspiratory and augmenting expiratory neurons 79– 81. Additionally, approximately 50% of the neurons that make up the preBötC are inhibitory, mostly glycinergic, interneurons 82. A contemporary model posits an “inhibitory connectome” or “inhibitory ring” hypothesis in which reciprocal inhibition between the preBötC and other brainstem circuits, such as the BötC, produce the three phases of breathing 83, 84. The theory states that glycinergic inhibition resets the activity of inspiratory, post-inspiratory, and expiratory neurons in the ventral respiratory network 84. These interpretations are derived mainly from intracellular recordings in vivo or in situ paired with computational modeling (for reviews see 18, 79, 84, 85).
However, some aspects of this theory have been considered controversial. The inhibitory ring model would predict that blocking inhibition in the preBötC or the BötC would result in apnea, or cessation of breathing. When Feldman and colleagues tested this by pharmacologically injecting glycinergic and GABA A receptor antagonists into the preBötC and BötC in vagotomized rats, they observed little to no effect on the breathing rhythm 86. They concluded that inhibition is not obligatory for rhythm generation but instead contributes to shaping the pattern of the rhythmic output. Of note, however, the injection of somatostatin, an inhibitory neuropeptide, into the BötC region resulted in the specific elimination of post-inspiratory vagal motor output 87.
These experiments were done under the assumption that the BötC was responsible for the generation of post-inspiration. However, as briefly mentioned above, it was recently discovered that the PiCo provides a necessary excitatory drive for the generation of post-inspiratory activity 15. The novel horizontal slice, described by Anderson et al., keeps the entire medullary VRC intact, and thus, using this preparation, one can simultaneously record fictive inspiratory bursts (from the preBötC) that are immediately followed by fictive post-inspiratory bursts (from the PiCo) 15 ( Figure 1). The PiCo rhythm persists in the absence of inhibition when the network is isolated in a transverse in vitro slice immediately rostral to the conventional transverse preBötC slice 15, 88 ( Figure 1). This is similar to the persistence of the preBötC rhythm in the absence of synaptic inhibition in vitro 89– 91. Similar to the in vivo experiment by Burke et al. 87, the PiCo rhythm was specifically abolished upon the application of somatostatin, with little to no change in the preBötC rhythm. Further experiments are necessary to fully elucidate the role of inhibition between respiratory rhythms in vivo.
Interactions between oscillators
To truly understand how respiration is generated, it is imperative to ascertain the interactions between the different rhythm generators. While this work is far from complete, some progress has been made studying the interactions between the preBötC and the pF L as well as interactions between the preBötC and the PiCo.
At embryonic day 14.5 (E14.5), before the preBötC is active, the pF L is rhythmic 35. A day later, at E15.5, the preBötC begins to oscillate and rhythmically couples to the pF L. In postnatal rats, glutamatergic pF L neurons provide excitatory drive to the preBötC, while the preBötC, in turn, provides inhibitory and excitatory influences on different subsets of pF L neurons 92, 93. In the in vivo adult rat, the preBötC can generate an inspiratory rhythm in the absence of pF L active expiratory activity 14, 94. However, in the converse situation, in order for the pF L to be active, a second low level of activity is simultaneously required: either activity from the preBötC or increased chemosensory drive 94. Thus, the pF L drives active expiration, but another source of excitation is required for the network to be rhythmically active.
Neurons in the pF L are excitatory 35, 76 and do not express inhibitory biomarkers 95, 96. Therefore, any inhibitory action associated with pF L activity must be occurring through an intermediate relay of neurons, perhaps from the preBötC 48. Even excitatory projections from the preBötC to the pF L appear to be indirect and require an intermediate relay. Neurons in the preBötC send projections rostrally to an area adjacent to the pF L, the ventral parafacial nucleus, or pF V 97, which has been shown to provide drive to expiration 14, 98, and could be functioning as the intermediate relay 94. While the preBötC and pF L are anatomically distinct and functionally separate oscillators, the preBötC appears to be dominant, while pF L activity is conditional and absent at rest.
In contrast, inspiration and post-inspiration are active at rest 74, suggesting that this activity may reflect the interaction between anatomically and functionally distinct oscillators, preBötC and PiCo 15. Horizontal slice population recordings of the preBötC and PiCo progressively synchronize when a GABA A receptor antagonist is applied to the slice. This observation suggests that GABAergic connections between the preBötC and PiCo help to coordinate the timing and phasing of the respiratory rhythms.
Light stimulation of channelrhodopsin-expressing Dbx1 neurons in the preBötC simultaneously evokes inspiratory population activity in the contralateral preBötC and hyperpolarizes a post-inspiratory PiCo neuron 15. However, when this experiment is repeated in the absence of inhibition, light stimulation now both activates an inspiratory population burst and depolarizes the PiCo neuron. Taken together, these results suggest that, under baseline conditions, the preBötC imparts an inhibitory influence on PiCo. However, when inhibition is blocked, it unmasks a concurrent excitatory influence of preBötC onto PiCo.
This work lays the foundation for beginning to understand the dynamic interplay between the three independent rhythm generators. In particular, further studies are needed that probe the interactions between the pF L and PiCo.
Conclusion
Reduced preparations that isolate respiratory microcircuits have led to a tremendous understanding of respiratory rhythm generation. Yet, with the availability of ever-more-advanced techniques such as computational modeling, access to transgenic animals, and the possibility of working in intact, alert animals, we will further progress in the unraveling of complex mechanisms.
One of the most established theories for the generation of respiratory rhythms is the dual oscillator hypothesis, which posits that inspiration and expiration are generated by alternating activity between preBötC and RTN/pFRG oscillators and post-inspiration is merely a motor subcomponent of expiration 62, 73. We propose a triple oscillator hypothesis or that the three phases of breathing in mammals – inspiration, post-inspiration, and active expiration – are generated by anatomically distinct excitatory rhythm generators: the preBötC, PiCo, and the pF L, respectively ( Figure 2). It is interesting to note that three rhythm-generating networks have been hypothesized in the bullfrog 2, 99.
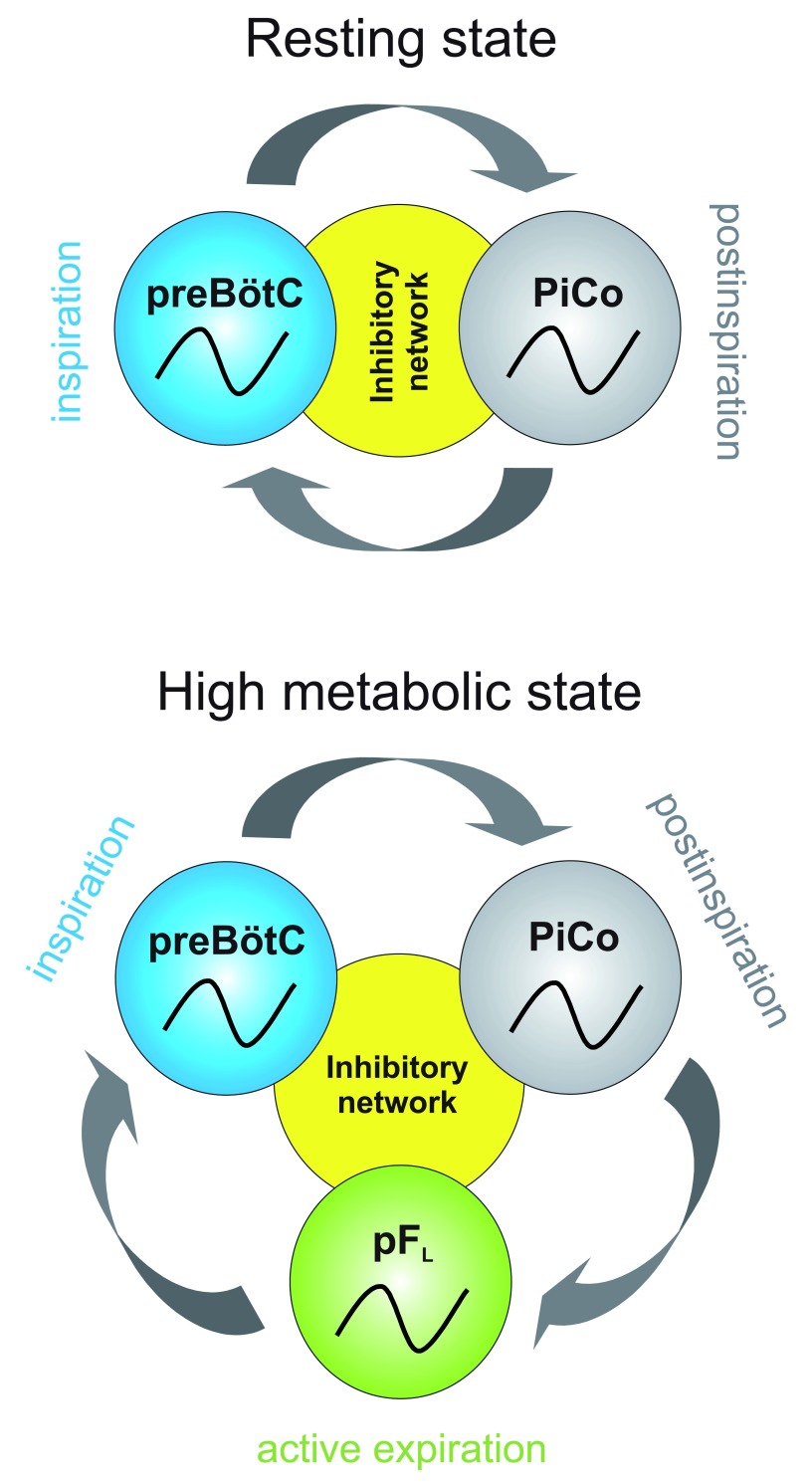
Figure 2. Illustration of triple-oscillator hypothesis.
We propose that, at rest, the preBötzinger complex (preBötC) and post-inspiratory complex (PiCo) alternate activity to generate a two-phase rhythm, inspiration and post-inspiration. Under periods of high metabolic demand, for instance during exercise, a third oscillator is incorporated to create a three-phase rhythm. We propose that each of the three phases – inspiration, post-inspiration, and active expiration – are controlled by independent oscillators: the preBötC, PiCo, and lateral parafacial nucleus (pF L), respectively. We further postulate that inhibition between these networks coordinates the phasing and timing of the rhythms.
Many questions remain, however. Is there a hierarchical relationship between the three oscillators, i.e. is the preBötC the “mother of all respiratory rhythms”? Similar to the reconfiguration of the preBötC network in the generation of eupnea, gasps, and sighs, does the PiCo reconfigure to help generate post-inspiratory behaviors such as vocalization, swallowing, breath-holding, and coughing? Are the preBötC and/or PiCo networks impaired when patients with neurodegenerative disorders fail to coordinate breathing and swallowing and subsequently develop aspiration pneumonia 100– 103? Do homologous networks for PiCo and pF L exist in humans? While substantial work remains to be accomplished, we hope that core concepts garnered from the study of the control of respiration could lead to the discovery of mechanisms that universally underlie other oscillatory networks and, ultimately, to therapies for patients with centrally derived respiratory disorders.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Richard Wilson, Hotchkiss Brain Institute and Alberta Children's Hospital Research Institute, Department of Physiology and Pharmacology, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada
Jose Fernando Pena-Ortega, Departamento de Neurobiología del Desarrollo y Neurofisiología, Instituto de Neurobiología, Universidad Nacional Autónoma de México-Campus Juriquilla, Querétaro, Mexico
Muriel Thoby-Brisson, Institut de Neurosciences Cognitives et Intégratives d'Aquitaine, CNRS UMR 5287, Université de Bordeaux, Bordeaux, France
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 3 approved]
References
- 1. Watson BO, Buzsáki G: Sleep, Memory & Brain Rhythms. Daedalus. 2015;144(1):67–82. 10.1162/DAED_a_00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramirez JM, Dashevskiy T, Marlin IA, et al. : Microcircuits in respiratory rhythm generation: commonalities with other rhythm generating networks and evolutionary perspectives. Curr Opin Neurobiol. 2016;41:53–61. 10.1016/j.conb.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kiehn O: Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci. 2016;17(4):224–38. 10.1038/nrn.2016.9 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Grillner S, El Manira A: The intrinsic operation of the networks that make us locomote. Curr Opin Neurobiol. 2015;31:244–9. 10.1016/j.conb.2015.01.003 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Destexhe A: Modelling corticothalamic feedback and the gating of the thalamus by the cerebral cortex. J Physiol Paris. 2000;94(5–6):391–410. 10.1016/S0928-4257(00)01093-7 [DOI] [PubMed] [Google Scholar]
- 6. Ketz NA, Jensen O, O'Reilly RC: Thalamic pathways underlying prefrontal cortex-medial temporal lobe oscillatory interactions. Trends Neurosci. 2015;38(1):3–12. 10.1016/j.tins.2014.09.007 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Cheron G, Márquez-Ruiz J, Dan B: Oscillations, Timing, Plasticity, and Learning in the Cerebellum. Cerebellum. 2016;15(2):122–38. 10.1007/s12311-015-0665-9 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Llinás RR: The olivo-cerebellar system: a key to understanding the functional significance of intrinsic oscillatory brain properties. Front Neural Circuits. 2014;7:96. 10.3389/fncir.2013.00096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robinson I, Reddy AB: Molecular mechanisms of the circadian clockwork in mammals. FEBS Lett. 2014;588(15):2477–83. 10.1016/j.febslet.2014.06.005 [DOI] [PubMed] [Google Scholar]
- 10. Rosenwasser AM, Turek FW: Neurobiology of Circadian Rhythm Regulation. Sleep Med Clin. 2015;10(4):403–12. 10.1016/j.jsmc.2015.08.003 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Smith JC, Ellenberger HH, Ballanyi K, et al. : Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254(5032):726–9. 10.1126/science.1683005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruangkittisakul A, Kottick A, Picardo MC, et al. : Identification of the pre-Bötzinger complex inspiratory center in calibrated "sandwich" slices from newborn mice with fluorescent Dbx1 interneurons. Physiol Rep. 2014;2(8): pii: e12111. 10.14814/phy2.12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pagliardini S, Janczewski WA, Tan W, et al. : Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci. 2011;31(8):2895–905. 10.1523/JNEUROSCI.5338-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Huckstepp RT, Cardoza KP, Henderson LE, et al. : Role of parafacial nuclei in control of breathing in adult rats. J Neurosci. 2015;35(3):1052–67. 10.1523/JNEUROSCI.2953-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson TM, Garcia AJ, 3rd, Baertsch NA, et al. : A novel excitatory network for the control of breathing. Nature. 2016;536(7614):76–80. 10.1038/nature18944 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Ramirez JM, Quellmalz UJ, Richter DW: Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. J Physiol. 1996;491(Pt 3):799–812. 10.1113/jphysiol.1996.sp021258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnes BJ, Tuong CM, Mellen NM: Functional imaging reveals respiratory network activity during hypoxic and opioid challenge in the neonate rat tilted sagittal slab preparation. J Neurophysiol. 2007;97(3):2283–92. 10.1152/jn.01056.2006 [DOI] [PubMed] [Google Scholar]
- 18. Smith JC, Abdala AP, Koizumi H, et al. : Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98(6):3370–87. 10.1152/jn.00985.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Richter DW: Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982;100:93–107. [DOI] [PubMed] [Google Scholar]
- 20. Richter DW, Ballanyi K, Schwarzacher S: Mechanisms of respiratory rhythm generation. Curr Opin Neurobiol. 1992;2(6):788–93. 10.1016/0959-4388(92)90135-8 [DOI] [PubMed] [Google Scholar]
- 21. Muere C, Neumueller S, Olesiak S, et al. : Combined unilateral blockade of cholinergic, peptidergic, and serotonergic receptors in the ventral respiratory column does not affect breathing in awake or sleeping goats. J Appl Physiol (1985). 2015;119(3):308–20. 10.1152/japplphysiol.00145.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Gourévitch B, Mellen N: The preBötzinger complex as a hub for network activity along the ventral respiratory column in the neonate rat. Neuroimage. 2014;98:460–74. 10.1016/j.neuroimage.2014.04.073 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Raghuraman S, Garcia AJ, Anderson TM, et al. : Defining modulatory inputs into CNS neuronal subclasses by functional pharmacological profiling. Proc Natl Acad Sci U S A. 2014;111(17):6449–54. 10.1073/pnas.1404421111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tan W, Janczewski WA, Yang P, et al. : Silencing preBötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11(5):538–40. 10.1038/nn.2104 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Gray PA, Hayes JA, Ling GY, et al. : Developmental origin of preBötzinger complex respiratory neurons. J Neurosci. 2010;30(44):14883–95. 10.1523/JNEUROSCI.4031-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramirez JM, Schwarzacher SW, Pierrefiche O, et al. : Selective lesioning of the cat pre-Bötzinger complex in vivo eliminates breathing but not gasping. J Physiol. 1998;507(Pt 3):895–907. 10.1111/j.1469-7793.1998.895bs.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson SM, Koshiya N, Smith JC: Isolation of the kernel for respiratory rhythm generation in a novel preparation: the pre-Bötzinger complex "island". J Neurophysiol. 2001;85(4):1772–6. [DOI] [PubMed] [Google Scholar]
- 28. Lieske SP, Thoby-Brisson M, Telgkamp P, et al. : Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps [see comment]. Nat Neurosci. 2000;3(6):600–7. 10.1038/75776 [DOI] [PubMed] [Google Scholar]
- 29. Ramirez JM, Richter DW: The neuronal mechanisms of respiratory rhythm generation. Curr Opin Neurobiol. 1996;6(6):817–25. 10.1016/S0959-4388(96)80033-X [DOI] [PubMed] [Google Scholar]
- 30. Schwarzacher SW, Rüb U, Deller T: Neuroanatomical characteristics of the human pre-Bötzinger complex and its involvement in neurodegenerative brainstem diseases. Brain. 2011;134(Pt 1):24–35. 10.1093/brain/awq327 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Bouvier J, Thoby-Brisson M, Renier N, et al. : Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci. 2010;13(9):1066–74. 10.1038/nn.2622 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Ruangkittisakul A, Schwarzacher SW, Secchia L, et al. : High sensitivity to neuromodulator-activated signaling pathways at physiological [K +] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26(46):11870–80. 10.1523/JNEUROSCI.3357-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ruangkittisakul A, Panaitescu B, Ballanyi K: K + and Ca 2+ dependence of inspiratory-related rhythm in novel "calibrated" mouse brainstem slices. Respir Physiol Neurobiol. 2011;175(1):37–48. 10.1016/j.resp.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 34. Smith JC, Feldman JL: In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods. 1987;21(2–4):321–33. 10.1016/0165-0270(87)90126-9 [DOI] [PubMed] [Google Scholar]
- 35. Thoby-Brisson M, Karlén M, Wu N, et al. : Genetic identification of an embryonic parafacial oscillator coupling to the preBötzinger complex. Nat Neurosci. 2009;12(8):1028–35. 10.1038/nn.2354 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Paton JF: A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65(1):63–8. 10.1016/0165-0270(95)00147-6 [DOI] [PubMed] [Google Scholar]
- 37. Mellen NM, Mishra D: Functional anatomical evidence for respiratory rhythmogenic function of endogenous bursters in rat medulla. J Neurosci. 2010;30(25):8383–92. 10.1523/JNEUROSCI.5510-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peña F, Parkis MA, Tryba AK, et al. : Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43(1):105–17. 10.1016/j.neuron.2004.06.023 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Ramirez JM, Doi A, Garcia AJ, 3rd, et al. : The cellular building blocks of breathing. Compr Physiol. 2012;2(4):2683–731. 10.1002/cphy.c110033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramirez JM, Koch H, Garcia AJ, 3rd, et al. : The role of spiking and bursting pacemakers in the neuronal control of breathing. J Biol Phys. 2011;37(3):241–61. 10.1007/s10867-011-9214-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koch H, Garcia AJ, 3rd, Ramirez JM: Network reconfiguration and neuronal plasticity in rhythm-generating networks. Integr Comp Biol. 2011;51(6):856–68. 10.1093/icb/icr099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garcia AJ, 3rd, Zanella S, Koch H, et al. : Chapter 3--networks within networks: the neuronal control of breathing. Prog Brain Res. 2011;188:31–50. 10.1016/B978-0-444-53825-3.00008-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peña F: Contribution of pacemaker neurons to respiratory rhythms generation in vitro. Adv Exp Med Biol. 2008;605:114–8. 10.1007/978-0-387-73693-8_20 [DOI] [PubMed] [Google Scholar]
- 44. Ramirez JM, Tryba AK, Peña F: Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol. 2004;14(6):665–74. 10.1016/j.conb.2004.10.011 [DOI] [PubMed] [Google Scholar]
- 45. Del Negro CA, Morgado-Valle C, Hayes JA, et al. : Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci. 2005;25(2):446–53. 10.1523/JNEUROSCI.2237-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thoby-Brisson M, Ramirez JM: Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J Neurophysiol. 2001;86(1):104–12. [DOI] [PubMed] [Google Scholar]
- 47. Feldman JL, Del Negro CA, Gray PA: Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol. 2013;75:423–52. 10.1146/annurev-physiol-040510-130049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morgado-Valle C, Baca SM, Feldman JL: Glycinergic pacemaker neurons in preBötzinger complex of neonatal mouse. J Neurosci. 2010;30(10):3634–9. 10.1523/JNEUROSCI.3040-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peña F, Ramirez JM: Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci. 2004;24(34):7549–56. 10.1523/JNEUROSCI.1871-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peña F, Aguileta MA: Effects of riluzole and flufenamic acid on eupnea and gasping of neonatal mice in vivo. Neurosci Lett. 2007;415(3):288–93. 10.1016/j.neulet.2007.01.032 [DOI] [PubMed] [Google Scholar]
- 51. Ramirez JM, Garcia A, 3rd: Point: Medullary pacemaker neurons are essential for both eupnea and gasping in mammals. J Appl Physiol (1985). 2007;103(2):717–8; discussion 722. 10.1152/japplphysiol.00003.2007 [DOI] [PubMed] [Google Scholar]
- 52. Mellen NM, Thoby-Brisson M: Respiratory circuits: development, function and models. Curr Opin Neurobiol. 2012;22(4):676–85. 10.1016/j.conb.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 53. Carroll MS, Ramirez JM: Cycle-by-cycle assembly of respiratory network activity is dynamic and stochastic. J Neurophysiol. 2013;109(2):296–305. 10.1152/jn.00830.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Purvis LK, Smith JC, Koizumi H, et al. : Intrinsic bursters increase the robustness of rhythm generation in an excitatory network. J Neurophysiol. 2007;97(2):1515–26. 10.1152/jn.00908.2006 [DOI] [PubMed] [Google Scholar]
- 55. Tryba AK, Peña F, Ramirez JM: Stabilization of bursting in respiratory pacemaker neurons. J Neurosci. 2003;23(8):3538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tryba AK, Ramirez JM: Background sodium current stabilizes bursting in respiratory pacemaker neurons. J Neurobiol. 2004;60(4):481–9. 10.1002/neu.20050 [DOI] [PubMed] [Google Scholar]
- 57. Koizumi H, Smith JC: Persistent Na + and K +-dominated leak currents contribute to respiratory rhythm generation in the pre-Bötzinger complex in vitro. J Neurosci. 2008;28(7):1773–85. 10.1523/JNEUROSCI.3916-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Purvis LK, Butera RJ: Ionic current model of a hypoglossal motoneuron. J Neurophysiol. 2005;93(2):723–33. 10.1152/jn.00703.2004 [DOI] [PubMed] [Google Scholar]
- 59. Del Negro CA, Koshiya N, Butera RJ, Jr, et al. : Persistent sodium current, membrane properties and bursting behavior of pre-bötzinger complex inspiratory neurons in vitro. J Neurophysiol. 2002;88(5):2242–50. 10.1152/jn.00081.2002 [DOI] [PubMed] [Google Scholar]
- 60. Rybak IA, Ptak K, Shevtsova NA, et al. : Sodium currents in neurons from the rostroventrolateral medulla of the rat. J Neurophysiol. 2003;90(3):1635–42. 10.1152/jn.00150.2003 [DOI] [PubMed] [Google Scholar]
- 61. Rubin JE, Hayes JA, Mendenhall JL, et al. : Calcium-activated nonspecific cation current and synaptic depression promote network-dependent burst oscillations. Proc Natl Acad Sci U S A. 2009;106(8):2939–44. 10.1073/pnas.0808776106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Feldman JL, Del Negro CA: Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7(3):232–42. 10.1038/nrn1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Carroll MS, Viemari JC, Ramirez JM: Patterns of inspiratory phase-dependent activity in the in vitro respiratory network. J Neurophysiol. 2013;109(2):285–95. 10.1152/jn.00619.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nieto-Posadas A, Flores-Martínez E, Lorea-Hernández JJ, et al. : Change in network connectivity during fictive-gasping generation in hypoxia: prevention by a metabolic intermediate. Front Physiol. 2014;5:265. 10.3389/fphys.2014.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rekling JC, Shao XM, Feldman JL: Electrical coupling and excitatory synaptic transmission between rhythmogenic respiratory neurons in the preBötzinger complex. J Neurosci. 2000;20(23):RC113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kam K, Worrell JW, Janczewski WA, et al. : Distinct inspiratory rhythm and pattern generating mechanisms in the preBötzinger complex. J Neurosci. 2013;33(22):9235–45. 10.1523/JNEUROSCI.4143-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Cui Y, Kam K, Sherman D, et al. : Defining preBötzinger Complex Rhythm- and Pattern-Generating Neural Microcircuits In Vivo. Neuron. 2016;91(3):602–14. 10.1016/j.neuron.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McCrea DA, Rybak IA: Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57(1):134–46. 10.1016/j.brainresrev.2007.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dick TE, Dutschmann M, Feldman JL, et al. : Facts and challenges in respiratory neurobiology. Respir Physiol Neurobiol. 2015; pii: S1569-9048(15)00027-0. 10.1016/j.resp.2015.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Marchenko V, Koizumi H, Mosher B, et al. : Perturbations of Respiratory Rhythm and Pattern by Disrupting Synaptic Inhibition within Pre-Bötzinger and Bötzinger Complexes. eNeuro. 2016;3(2): pii: ENEURO.0011-16.2016. 10.1523/ENEURO.0011-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ezure K: Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol. 1990;35(6):429–50. 10.1016/0301-0082(90)90030-K [DOI] [PubMed] [Google Scholar]
- 72. Paton JF, Ramirez JM, Richter DW: Functionally intact in vitro preparation generating respiratory activity in neonatal and mature mammals. Pflugers Arch. 1994;428(3–4):250–60. 10.1007/BF00724504 [DOI] [PubMed] [Google Scholar]
- 73. Janczewski WA, Feldman JL: Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570(Pt 2):407–20. 10.1113/jphysiol.2005.098848 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Mörschel M, Dutschmann M: Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philos Trans R Soc Lond B Biol Sci. 2009;364(1529):2517–26. 10.1098/rstb.2009.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Krause KL, Forster HV, Kiner T, et al. : Normal breathing pattern and arterial blood gases in awake and sleeping goats after near total destruction of the presumed pre-Botzinger complex and the surrounding region. J Appl Physiol (1985). 2009;106(2):605–19. 10.1152/japplphysiol.90966.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Onimaru H, Homma I: Two modes of respiratory rhythm generation in the newborn rat brainstem-spinal cord preparation. Adv Exp Med Biol. 2008;605:104–8. 10.1007/978-0-387-73693-8_18 [DOI] [PubMed] [Google Scholar]
- 77. Onimaru H, Ikeda K, Kawakami K: CO 2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci. 2008;28(48):12845–50. 10.1523/JNEUROSCI.3625-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Burns BD: The central control of respiratory movements. Br Med Bull. 1963;19:7–9. [DOI] [PubMed] [Google Scholar]
- 79. Smith JC, Abdala AP, Rybak IA, et al. : Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci. 2009;364(1529):2577–87. 10.1098/rstb.2009.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ezure K, Tanaka I, Kondo M: Glycine is used as a transmitter by decrementing expiratory neurons of the ventrolateral medulla in the rat. J Neurosci. 2003;23(26):8941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Merrill EG, Fedorko L: Monosynaptic inhibition of phrenic motoneurons: a long descending projection from Bötzinger neurons. J Neurosci. 1984;4(9):2350–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Winter SM, Fresemann J, Schnell C, et al. : Glycinergic interneurons are functionally integrated into the inspiratory network of mouse medullary slices. Pflugers Arch. 2009;458(3):459–69. 10.1007/s00424-009-0647-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Richter A, Heyne K, Sagebiel J, et al. : Respiratorischer Notfall beim Neugeborenen: extreme laryngo-tracheo-oesophageale Spalte (Oesophago-Trachea). Monatsschr Kinderheilkd. 1986;134:874–7. [PubMed] [Google Scholar]
- 84. Richter DW, Smith JC: Respiratory rhythm generation in vivo. Physiology (Bethesda). 2014;29(1):58–71. 10.1152/physiol.00035.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Smith JC, Abdala AP, Borgmann A, et al. : Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013;36(3):152–62. 10.1016/j.tins.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Janczewski WA, Tashima A, Hsu P, et al. : Role of inhibition in respiratory pattern generation. J Neurosci. 2013;33(13):5454–65. 10.1523/JNEUROSCI.1595-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Burke PG, Abbott SB, McMullan S, et al. : Somatostatin selectively ablates post-inspiratory activity after injection into the Bötzinger complex. Neuroscience. 2010;167(2):528–39. 10.1016/j.neuroscience.2010.01.065 [DOI] [PubMed] [Google Scholar]
- 88. Ramirez JM, Quellmalz UJ, Wilken B, et al. : The hypoxic response of neurones within the in vitro mammalian respiratory network. J Physiol. 1998;507(Pt 2):571–82. 10.1111/j.1469-7793.1998.571bt.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brockhaus J, Ballanyi K: Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10(12):3823–39. 10.1046/j.1460-9568.1998.00396.x [DOI] [PubMed] [Google Scholar]
- 90. Ren J, Greer JJ: Modulation of respiratory rhythmogenesis by chloride-mediated conductances during the perinatal period. J Neurosci. 2006;26(14):3721–30. 10.1523/JNEUROSCI.0026-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shao XM, Feldman JL: Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Bötzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77(4):1853–60. [DOI] [PubMed] [Google Scholar]
- 92. Onimaru H, Ikeda K, Kawakami K: Defective interaction between dual oscillators for respiratory rhythm generation in Na +,K +-ATPase α2 subunit-deficient mice. J Physiol. 2007;584(pt 1):271–84. 10.1113/jphysiol.2007.136572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Takeda S, Eriksson LI, Yamamoto Y, et al. : Opioid action on respiratory neuron activity of the isolated respiratory network in newborn rats. Anesthesiology. 2001;95(3):740–9. 10.1097/00000542-200109000-00029 [DOI] [PubMed] [Google Scholar]
- 94. Huckstepp RT, Henderson LE, Cardoza KP, et al. : Interactions between respiratory oscillators in adult rats. eLife. 2016;5: pii:e14203. 10.7554/eLife.14203 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 95. Tanaka I, Ezure K, Kondo M: Distribution of glycine transporter 2 mRNA-containing neurons in relation to glutamic acid decarboxylase mRNA-containing neurons in rat medulla. Neurosci Res. 2003;47(2):139–51. 10.1016/S0168-0102(03)00192-5 [DOI] [PubMed] [Google Scholar]
- 96. Ellenberger HH: Distribution of bulbospinal gamma-aminobutyric acid-synthesizing neurons of the ventral respiratory group of the rat. J Comp Neurol. 1999;411(1):130–44. [DOI] [PubMed] [Google Scholar]
- 97. Tan W, Pagliardini S, Yang P, et al. : Projections of preBötzinger complex neurons in adult rats. J Comp Neurol. 2010;518(10):1862–78. 10.1002/cne.22308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Silva JN, Tanabe FM, Moreira TS, et al. : Neuroanatomical and physiological evidence that the retrotrapezoid nucleus/parafacial region regulates expiration in adult rats. Respir Physiol Neurobiol. 2016;227:9–22. 10.1016/j.resp.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 99. Baghdadwala MI, Duchcherer M, Paramonov J, et al. : Three brainstem areas involved in respiratory rhythm generation in bullfrogs. J Physiol. 2015;593(13):2941–54. 10.1113/JP270380 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 100. Alagiakrishnan K, Bhanji RA, Kurian M: Evaluation and management of oropharyngeal dysphagia in different types of dementia: a systematic review. Arch Gerontol Geriatr. 2013;56(1):1–9. 10.1016/j.archger.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 101. Easterling CS, Robbins E: Dementia and dysphagia. Geriatr Nurs. 2008;29(4):275–85. 10.1016/j.gerinurse.2007.10.015 [DOI] [PubMed] [Google Scholar]
- 102. Lin CW, Chang YC, Chen WS, et al. : Prolonged swallowing time in dysphagic Parkinsonism patients with aspiration pneumonia. Arch Phys Med Rehabil. 2012;93(11):2080–4. 10.1016/j.apmr.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 103. Kalia M: Dysphagia and aspiration pneumonia in patients with Alzheimer's disease. Metabolism. 2003;52(10 Suppl 2):36–8. 10.1016/S0026-0495(03)00300-7 [DOI] [PubMed] [Google Scholar]