Abstract
Pigment epithelium-derived factor (PEDF) expression is down- regulated in the kidneys of diabetic rats and delivery of PEDF suppressed renal fibrotic factors in these animals. PEDF has multiple functions including anti-angiogenic, anti-inflammatory and anti-fibrotic activities. Since the mechanism underlying its anti-fibrotic effect remains unclear, we studied this in several murine models of renal disease. Renal PEDF levels were significantly reduced in genetic models of type 1 and type 2 diabetes (Akita and db/db, respectively), negatively correlating with Wnt signaling activity in the kidneys. In unilateral ureteral obstruction, an acute renal injury model, there were significant decreases of renal PEDF levels. The kidneys of PEDF knock-out mice with ureteral obstruction displayed exacerbated expression of fibrotic and inflammatory factors, oxidative stress, tubulointerstitial fibrosis and tubule epithelial cell apoptosis, compared to the kidneys of wild-type mice with obstruction. PEDF knock-out enhanced Wnt signaling activation induced by obstruction, while PEDF inhibited the Wnt pathway-mediated fibrosis in primary renal proximal tubule epithelial cells. Additionally, oxidative stress was aggravated in renal proximal tubule epithelial cells isolated from knock-out mice, and suppressed by PEDF treatment of renal proximal tubule epithelial cells. PEDF also reduced oxidation-induced apoptosis in renal proximal tubule epithelial cells. Thus, the renoprotective effects of PEDF are mediated, at least partially, by inhibition of the Wnt pathway. Hence, restoration of renal PEDF levels may have therapeutic potential for renal fibrosis.
Keywords: β-catenin, fibrosis, inflammation, kidney, oxidative stress, PEDF, reno-protective, renal tubule epithelial cells, Wnt pathway
INTRODUCTION
Renal fibrosis, mainly categorized as glomerulosclerosis and tubulointerstitial fibrosis, is an advanced pathological feature of many acute kidney injuries1 and chronic kidney diseases.2 This irreversible process causes progressive decline of renal functions, eventually leading to renal insufficiency. Currently, there are no effective treatments for the devastating renal fibrosis.3
Tubules play important roles in maintaining renal functions. In human glomerular nephritis4 and diabetic nephropathy,5 loss of renal functions correlates more closely with tubular damages than with glomerular changes. Proximal tubules are recognized as a significant part of tubules carrying out regulatory functions on water and salt balance, acid-base homeostasis and endocrine system.6 A variety of kidney diseases are related to proximal tubule disorders, such as acute kidney injuries7 and diabetic nephropathy.8 Moreover, injuries of proximal tubules alone trigger glomerulosclerosis and tubulointerstitial fibrosis.9 During the renal fibrotic process, renal proximal tubule epithelial cells produce extracellular matrix2 and act as immune cells by expressing and releasing adhesion molecules, cytokines and chemokines.10
Pigment epithelium-derived factor (PEDF) is a non-inhibitory member of the serine proteinase inhibitor family.11 It possesses multiple functions12 including anti-angiogenesis13 especially in retinal vascular diseases such as diabetic retinopathy,14 anti-inflammation15 and anti-fibrosis activities.16, 17, 18, 19 In human kidneys, PEDF protein is highly expressed in tubules and moderately in glomeruli.20 The PEDF mRNA is detected in the kidneys of both fetal and adult humans.21 Decreased PEDF expression has been demonstrated in type 1 diabetic rat kidneys with renal fibrosis.16 Delivery of PEDF into diabetic rats suppresses the renal levels of fibrotic markers,19 suggesting an anti-fibrotic activity of PEDF. However, PEDF’s function in the tubules and the mechanism underlying PEDF’s anti-fibrosis activity remain elusive.
Aberrant activation of the canonical Wnt pathway is reported in kidney diseases including obstructive nephropathy,22, 23 adriamycin-induced nephropathy,24 and type 1 and 2 diabetic nephropathy.25 In an obstructive nephropathy model, i.e. unilateral ureteral obstruction (UUO), 16 Wnt ligands and 6 frizzle receptors are up-regulated.22 The Wnt pathway promotes myofibroblast differentiation.26 Persistent activation of this pathway further drives the transition from acute kidney injury to chronic kidney disease.27 A number of Wnt target genes are also reported to contribute to renal fibrosis, including fibrotic factors such as fibronectin and fibroblast-specific protein 1.28 Plasminogen activator inhibitor-1, another target gene of Wnt signaling, stimulates the accumulation of immune cells and myofibroblasts in the kidney.29 On the other hand, inhibitors of the Wnt pathway, such as ICG-001,27 secreted frizzled-related protein 423 and dickkopf-1,22 attenuate renal fibrosis. Interestingly, levels of β-catenin are predominantly induced in the tubules of the kidneys with UUO,22 diabetic nephropathy,25 adriamycin-induced nephropathy30 and ischemia/reperfusion renal injury.27
Our previous study identified PEDF as an endogenous inhibitor of the Wnt signaling pathway;31 it is possible that the anti-fibrotic effect of PEDF is mediated via inhibition of the Wnt signaling pathway. The present study has addressed the following questions: 1) What is the role of PEDF in tubulointerstitial fibrosis? 2) Does PEDF knock-out (KO) affect renal tubule epithelial cells, a group of cell types crucial for the regulation of renal inflammatory processes and tubulointerstitial fibrosis? 3) What is the underlying molecular mechanism mediating the anti-fibrotic effect of PEDF?
RESULTS
Inverse correlations between PEDF levels and Wnt signaling activation in diabetic kidneys
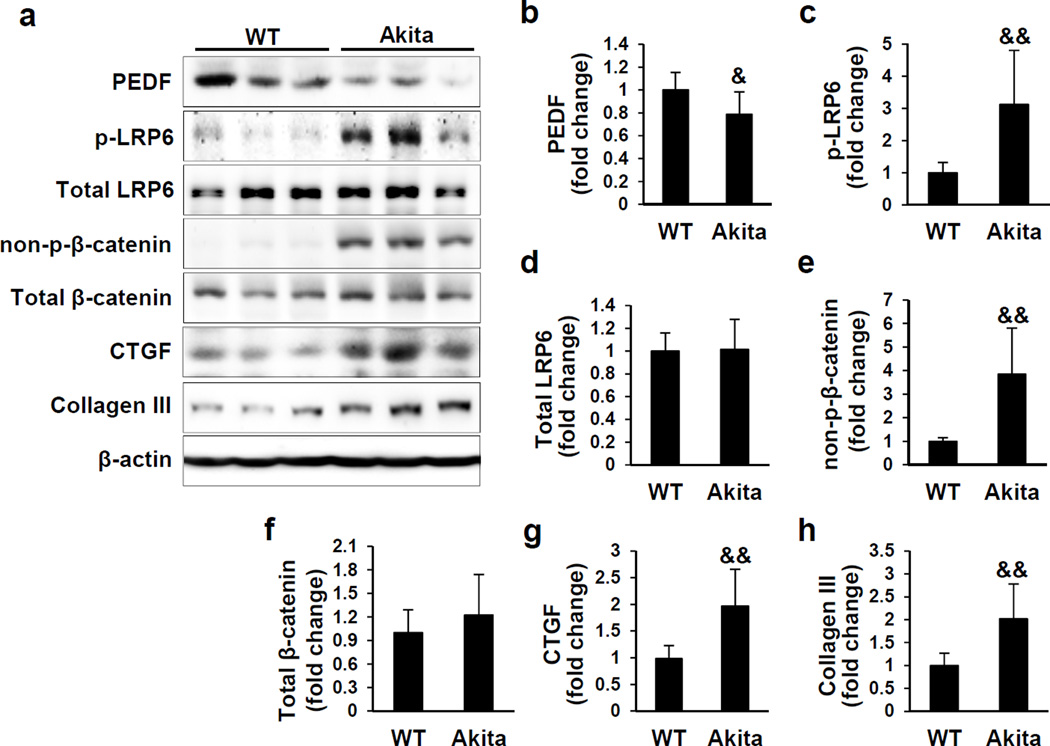
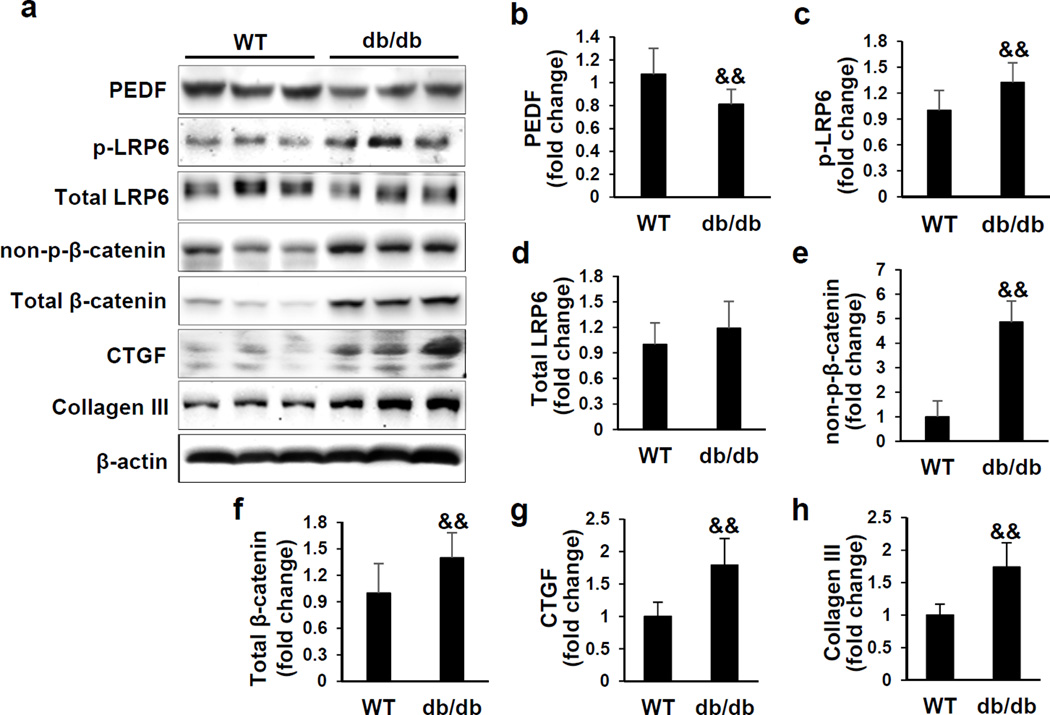
The renal PEDF levels have not been previously examined in genetic models of type 1 and type 2 diabetes mellitus. Thus, we examined PEDF protein levels in the kidneys of 3 month-old Akita mice, a type 1 diabetic mouse model, and 6-month-old db/db mice, a type 2 diabetic model. Renal PEDF was significantly down-regulated in these two diabetic models (Figures 1a, 1b and 2a, 2b), accompanied by increased levels of the fibrosis factors connective tissue growth factor (CTGF) and collagen III (Figures 1 and 2). To establish an inverse correlation between PEDF levels and Wnt signaling activation, we further evaluated phosphorylated low-density lipoprotein receptor-related protein 6 (p-LRP6) and total LRP6, a co-receptor of the Wnt pathway, and non-phosphorylated β-catenin (non-p-β-catenin) and total β-catenin, an effector of the canonical Wnt signaling pathway. As shown in Figures 1 and 2, renal p-LRP6 and non-p-β-catenin levels were significantly elevated in these two diabetic mouse models, compared to those in their respective age- and genetic background-matched non-diabetic controls, suggesting activated Wnt signaling in these diabetic kidneys. Taken together, these results demonstrated an inverse correlation between renal PEDF levels and Wnt signaling activation in these genetic diabetic models.
Figure 1. Down-regulated pigment epithelium-derived factor (PEDF) and activated Wnt signaling in the kidneys of Akita mice.
(a) Western blot analysis and (b–h) densitometry quantification of PEDF, phosphorylated low-density lipoprotein receptor-related protein 6 (p-LRP6), total LRP6, non-phosphorylated β-catenin (non-p-β-catenin), total β-catenin, connective tissue growth factor (CTGF) and collagen III in kidney homogenates from 3 month-old Akita mice and wild-type (WT) controls (n=10). Each lane represents an individual mouse. &p<0.05, &&p<0.01, Akita versus WT. All values are expressed as mean±SD.
Figure 2. Down-regulated pigment epithelium-derived factor (PEDF) and activated Wnt signaling in the kidneys of db/db mice.
(a) Western blot analysis and (b–h) densitometry quantification of PEDF, phosphorylated low-density lipoprotein receptor-related protein 6 (p-LRP6), total LRP6, non-phosphorylated β-catenin (non-p-β-catenin), total β-catenin, connective tissue growth factor (CTGF) and collagen III in kidney homogenates from 6 month-old db/db mice and wild-type (WT) controls (n=10). Each lane represents an individual mouse. &&p<0.01, db/db versus WT. All values are expressed as mean±SD.
PEDF levels were decreased in the kidney with tubulointerstitial fibrosis
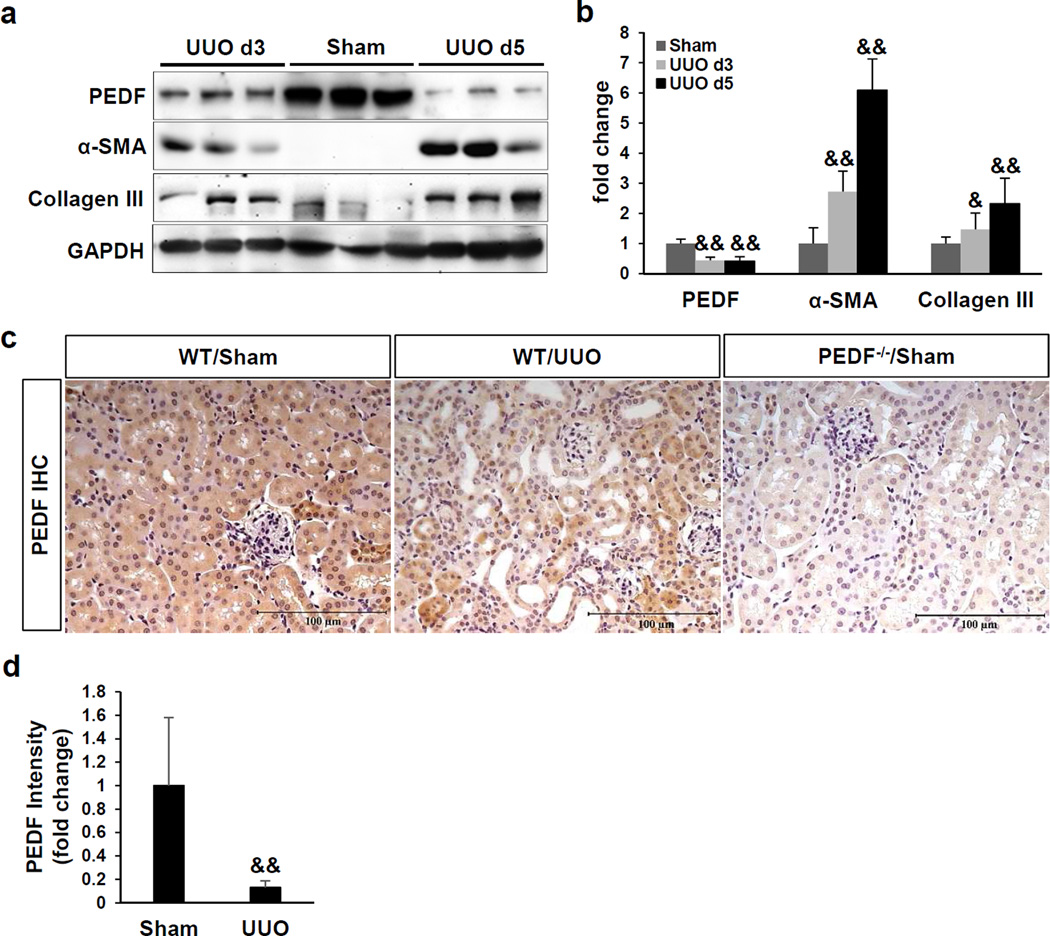
To understand the role of PEDF in non-diabetic kidneys with fibrosis, we utilized an UUO model which is characterized by tubulointerstitial fibrosis and tubular injury.32 As PEDF expression has not been previously examined in the kidneys with UUO, we plotted the time course of renal PEDF changes. At day 3 and day 5 post-surgery, renal PEDF levels were both significantly down-regulated in UUO kidneys, compared to those in sham control (Figure 3a and b). Declined PEDF levels were accompanied by increases of alpha smooth muscle actin (α-SMA) and collagen III (Figure 3a and b), suggesting a negative correlation between PEDF levels and fibrosis in the UUO kidneys. We further localized and compared PEDF expression in sham kidneys and UUO kidneys by immunohistochemistry. PEDF was abundantly expressed in the tubules of sham controls (Figure 3c). As shown by in situ hybridization, the PEDF mRNA was also predominantly detected in tubules (Supplemental Figure 2b). After 5 days of UUO, PEDF was down-regulated in renal tubules at the protein level (Figure 3c and d) and at the mRNA level (Supplemental Figure 1).
Figure 3. Down-regulation of pigment epithelium-derived factor (PEDF) in the kidney with tubulointerstitial fibrosis.
(a) Western blot analysis and (b) densitometry quantification of PEDF, alpha-smooth muscle actin (α-SMA) and collagen III in kidney homogenates of the sham group and unilateral ureteral obstruction (UUO) groups at day 3 and day 5 post-surgery. Each lane represents an individual mouse. (c) Representative images of immunohistochemical staining of PEDF and (d) quantification of PEDF signal in kidney sections at day 5 post-surgery. Scale bar=100 µm. n=5–6 per group. &p<0.05; &&p<0.01. All values are expressed as mean±SD.
Knock-out of PEDF exacerbated tubulointerstitial fibrosis
To further delineate the effect of PEDF on renal tubulointerstitial fibrosis, we utilized PEDF KO (PEDF−/−) mice. PEDF KO was verified in the kidney using Western blot analysis of PEDF (Supplemental Figure 2a) and immunohistochemistry (Figure 3c). Moreover, the PEDF mRNA was not detected in PEDF−/− kidneys by in situ hybridization (Supplemental Figure 2b). Under normal condition, 2-month-old PEDF−/− mice did not exhibit abnormal renal functions, as their 24-h urinary albumin excretion (UAE), urinary albumin/creatinine ratio (UACR) and glomerular filtration rate (GFR) were not significantly different from those of age-matched wild-type (WT) mice (Supplemental Figure 3a–c). Periodic acid-Schiff staining also showed normal glomerular and tubular structures in PEDF−/− kidneys (Supplemental Figure 3d).
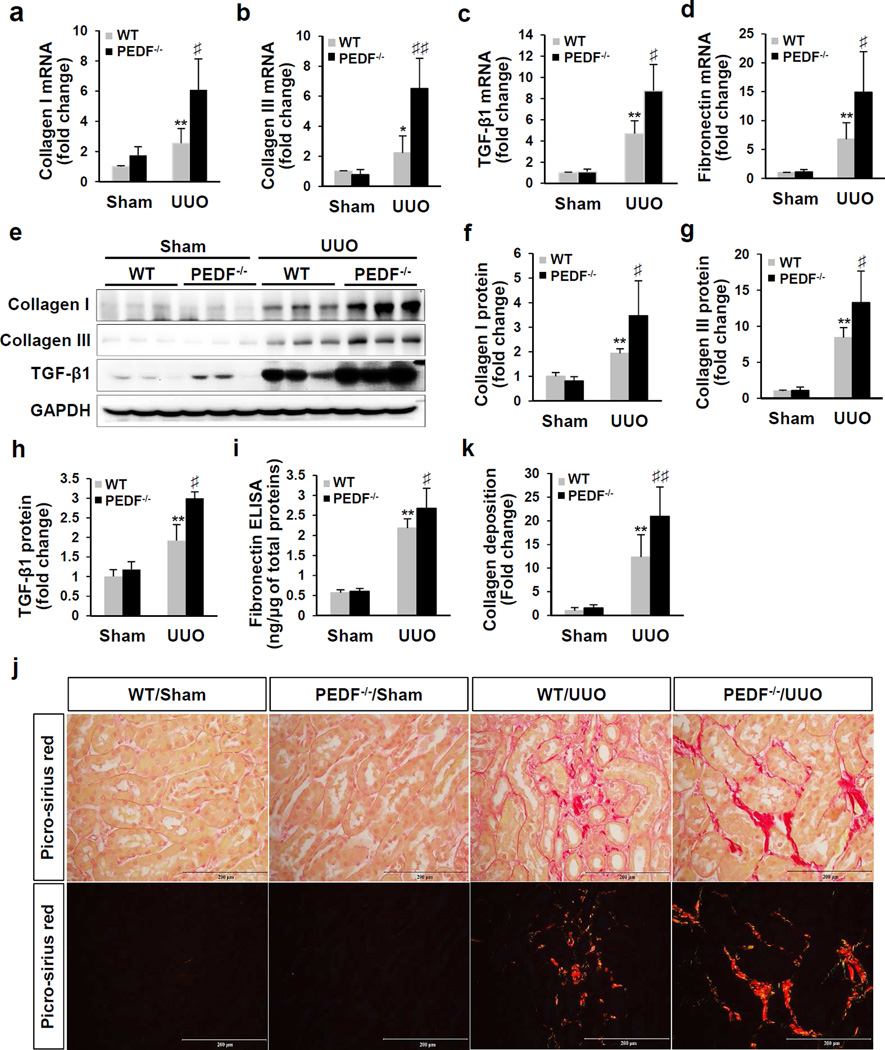
At day 5 post-UUO surgery, mRNA levels of collagen I, collagen III, transforming growth factor beta 1 (TGF-β1) and fibronectin were significantly higher in PEDF−/−/UUO kidneys than those in WT/UUO kidneys (Figure 4a–d). In agreement with the increased mRNA levels, significantly elevated protein levels of collagen I, collagen III and TGF-β1 were detected in PEDF−/−/UUO kidneys, compared to WT/UUO kidneys (Figure 4e–h). Interestingly, levels of TGF-β receptor type I (TβRI) and receptor type II (TβRII) were also significantly higher in PEDF−/−/UUO kidneys (Supplemental Figure 4). In agreement with renal fibronectin mRNA levels, kidney-derived fibronectin protein levels were significantly higher in PEDF−/−/UUO kidneys (2.68±0.49 ng/µg of total proteins) compared to WT/UUO kidneys (2.18±0.23 ng/µg) (Figure 4i). In the sham groups, however, KO of PEDF did not alter either the mRNA or protein levels of those fibrotic factors (Figure 4a–i). Collagen deposition was evaluated by picro-sirius red staining. Semi-quantification of images taken under a polarized microscope showed that UUO for 5 days induced dramatic increase of collagen deposition in the kidneys of PEDF−/− mice compared to WT kidneys (Figure 4j and k).
Figure 4. Exacerbated tubulointerstitial fibrosis in pigment epithelium-derived factor knockout (PEDF−/−) mice after unilateral ureteral obstruction (UUO).
(a–d) Real-time PCR measurement of mRNA levels of collagen I, collagen III, transforming growth factor beta 1 (TGF-β1) and fibronectin in kidneys from wild-type (WT) and PEDF−/− mice at day 5 post-surgery (n=5–6). (e) Western blot analysis and (f–h) densitometry quantification of collagen I, collagen III and TGF-β1 in kidney homogenates at day 5 post-surgery (n=5–6). Each lane represents an individual mouse. (i) Enzyme-linked immunosorbent assay (ELISA) measurement of fibronectin in kidney homogenates at day 5 post-surgery (n=8–12). (j) Representative images of picro-sirius red staining of kidney sections at day 5 post-surgery under a light microscope (upper panels) and under a polarized microscope (lower panels). Scale bar=200 µm. (k) Quantification of collagen deposition using a polarized microscope (n=5–6). *p<0.05, **p<0.01, WT/UUO versus WT/Sham; ♯p<0.05, ♯♯p<0.01, PEDF−/−/UUO versus WT/UUO. All values are expressed as mean±SD.
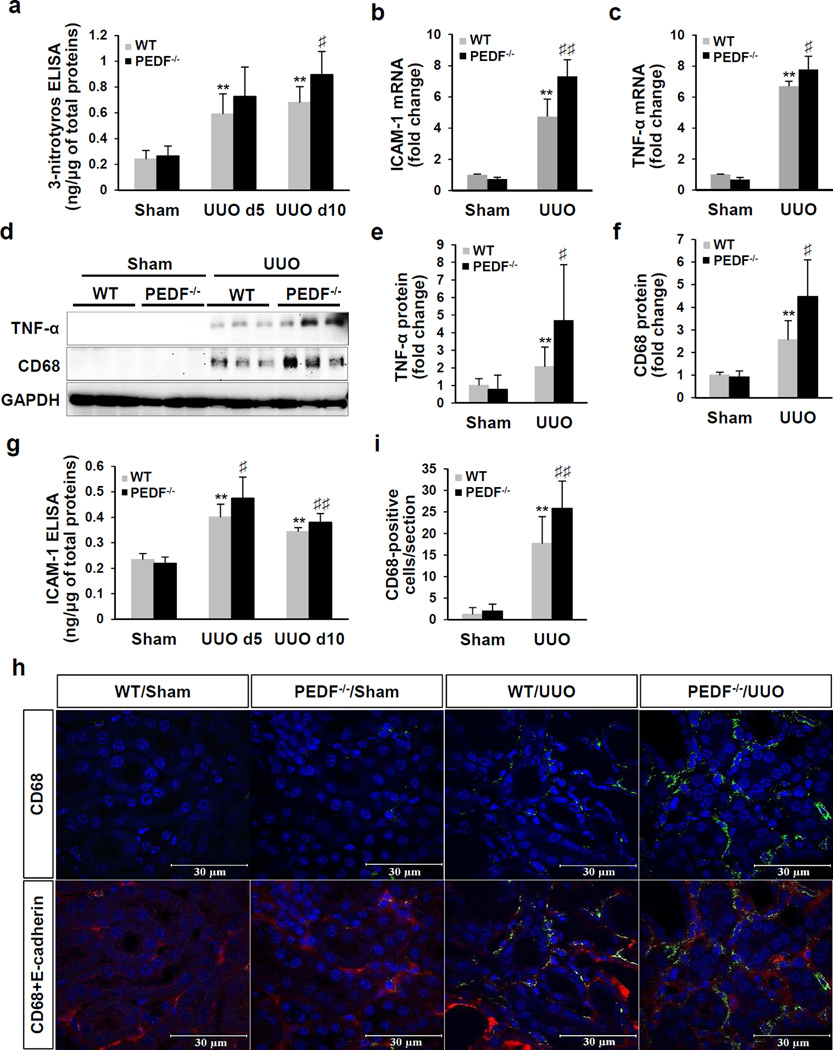
PEDF knock-out enhanced oxidative stress and inflammation in UUO kidneys
UUO is known to induce oxidative stress and inflammation, which promotes the progression of tubulointerstitial fibrosis.33 3-Nitrotyrosine (3-NT) is a product of tyrosine nitration and a marker of oxidative stress. At day 5 post-surgery, both WT/UUO and PEDF−/−/UUO mice showed significantly elevated 3-NT levels in the kidneys over the sham controls (0.591±0.155 ng/µg, 0.728±0. 226 ng/µg, respectively). At day 10 post-surgery, the PEDF−/−/UUO group displayed significantly higher 3-NT levels (0. 896±0.18 ng/µg) compared to WT/UUO group (0. 679±0.124 ng/µg) (Figure 5a).
Figure 5. Worsened oxidative stress and inflammation in the kidneys of pigment epithelium-derived factor knock-out (PEDF−/−) mice after unilateral ureteral obstruction (UUO).
(a) Enzyme-linked immunosorbent assay (ELISA) measurement of 3-nitrotyrosine in kidney homogenates from wild-type (WT) and PEDF−/− mice at day 5 and day 10 post-surgery (n=8–12). (b, c) Real-time PCR measurement of mRNA levels, (d) Western blot analysis and (e, f) densitometry quantification of intercellular adhesion molecule 1 (ICAM-1), tumor necrosis factor alpha (TNF-α) and CD68 (cluster of differentiation 68) in kidney homogenates at day 5 post-surgery (n=5–6). Each lane represents an individual mouse. (g) ELISA measurement of ICAM-1 in kidney homogenates from 5- and 10-day UUO (n=8–12). (h) Representative images of immunostaining of CD68 and (i) quantification of CD-68-positive cells in kidney sections at day 5 post-surgery. **p<0.01, WT/UUO versus WT/Sham; ♯p<0.05, ♯♯p<0.01, PEDF−/−/UUO versus WT/UUO. All values are expressed as mean±SD.
To evaluate the impact of PEDF ablation on kidney inflammation, we measured renal levels of inflammatory factors including intercellular adhesion molecule-1 (ICAM-1) and tumor necrosis factor alpha (TNF-α). At day 5 post-surgery, over-expression of ICAM-1 and TNF-α were significantly aggravated at the mRNA levels (Figure 5b and c) and protein levels (Figure 5d and e) in PEDF−/−/UUO kidneys relative to WT/UUO controls. As shown by ELISA, UUO for 5 days and 10 days induced significantly higher ICAM-1 levels in PEDF−/−/UUO kidneys (0.475±0.083 ng/µg, 0.381±0.034 ng/µg, respectively) relative to WT/UUO kidneys (0.404±0.05 ng/µg, 0.344±0.015 ng/µg, respectively) (Figure 5g). In addition, higher mRNA levels of interleukin-6 (IL-6) and macrophage colony-stimulating factor (M-CSF) were also detected in PEDF−/−/UUO kidneys, compared to WT/UUO controls (Supplemental Figure 5a and b). During kidney injuries, inflammatory monocytes infiltrate into the injury sites and differentiate into macrophages.34 We detected monocytes/macrophages by a specific marker CD68.35 As shown in Figure 5d, f, h and i, PEDF−/−/UUO kidneys at day 5 showed more prominent increases of monocytes/macrophages infiltration in the tubulointerstitial areas, relative to those of WT/UUO controls.
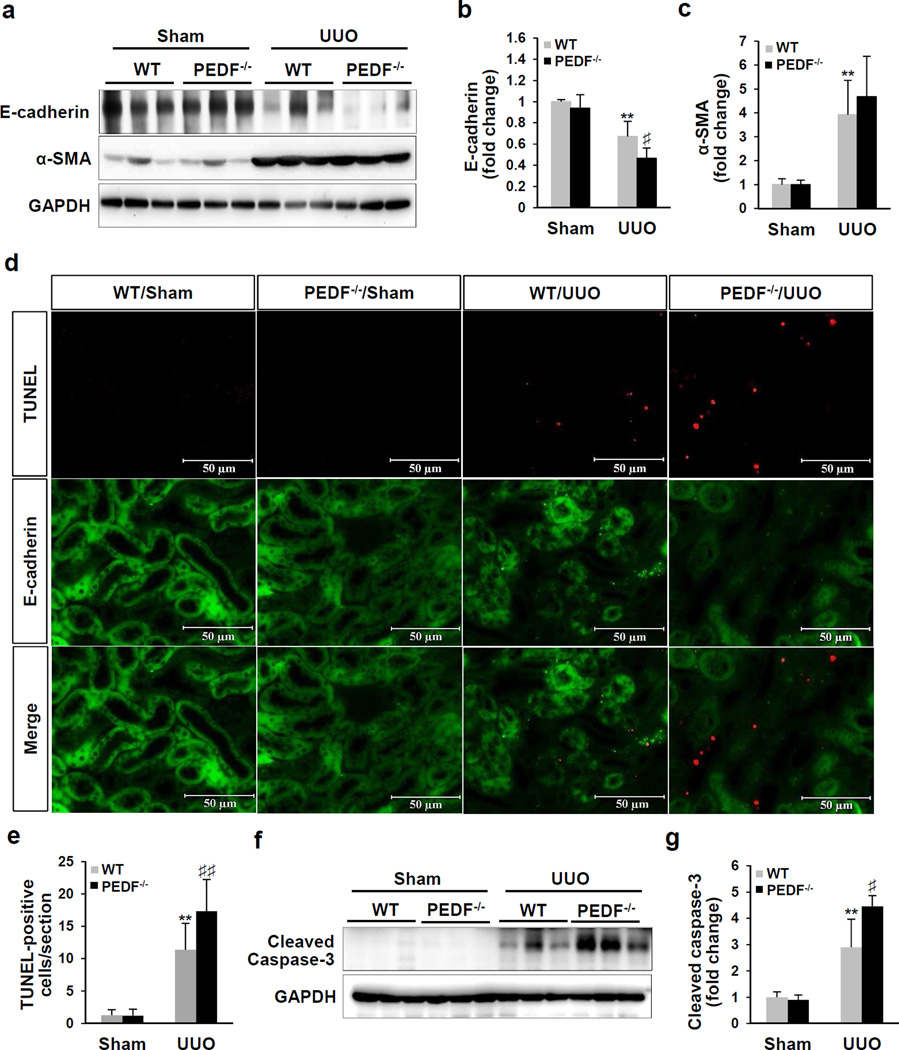
Aggravated renal tubule epithelial cell apoptosis in PEDF−/−/UUO kidneys
Increases of inflammation and oxidative stress in UUO models is known to result in progressive renal tubule epithelial cell death.33 At day 5 post-surgery, PEDF KO led to a significant decrease of the epithelial cell marker E-cadherin but unchanged myofibroblast marker α-SMA in UUO kidneys (Figure 6a–c), suggesting an aggravated loss of tubule epithelial cells and unaffected myofibroblast population in PEDF−/−/UUO kidneys relative to the WT/UUO group. Loss of tubule epithelial cells is primarily through the apoptosis pathway.32 Prevalent apoptosis was detected in UUO groups at day 10 post-surgery, while PEDF−/−/UUO group displaying significantly more TUNEL positive cells over the WT/UUO group (Figure 6d and e). There was no significant difference in the apoptotic cell numbers between PEDF−/−/Sham kidneys and WT/Sham kidneys. As apoptosis of tubule epithelial cells was caspase-3-dependent in the UUO model.36, we measured caspase-3 activation. At day 5 post-surgery, a significant increase of cleaved caspase-3 was detected in PEDF−/−/UUO kidneys, compared to WT/UUO controls (Figure 6f and g).
Figure 6. Aggravated renal tubule epithelial cell apoptosis in pigment epithelium-derived factor knock-out (PEDF−/−) kidneys after unilateral ureteral obstruction (UUO).
(a) Western blot analysis and (b, c) densitometry quantification of E-cadherin and alpha-smooth muscle actin (α-SMA) in kidney homogenates from wild-type (WT) mice and PEDF−/− mice at day 5 post-surgery (n=5–6). (d) Representative images of TUNEL (red) and E-cadherin (green) staining of kidney sections from mice as indicated at day 10 post-surgery, with nuclei counterstained with DAPI (blue). Scale bar=50 µm. (e) TUNEL-positive cells were counted and averaged in 8 fields/specimen (n=5–8). (f) Western blot analysis and (g) densitometry quantification of cleaved caspase-3 in kidney homogenates from WT mice and PEDF−/− mice at day 5 post-surgery (n=5–6). Each lane represents an individual mouse. **p<0.01, WT/UUO versus WT/Sham; ♯p<0.05, ♯♯p<0.01, PEDF−/−/UUO versus WT/UUO. All values are expressed as mean±SD.
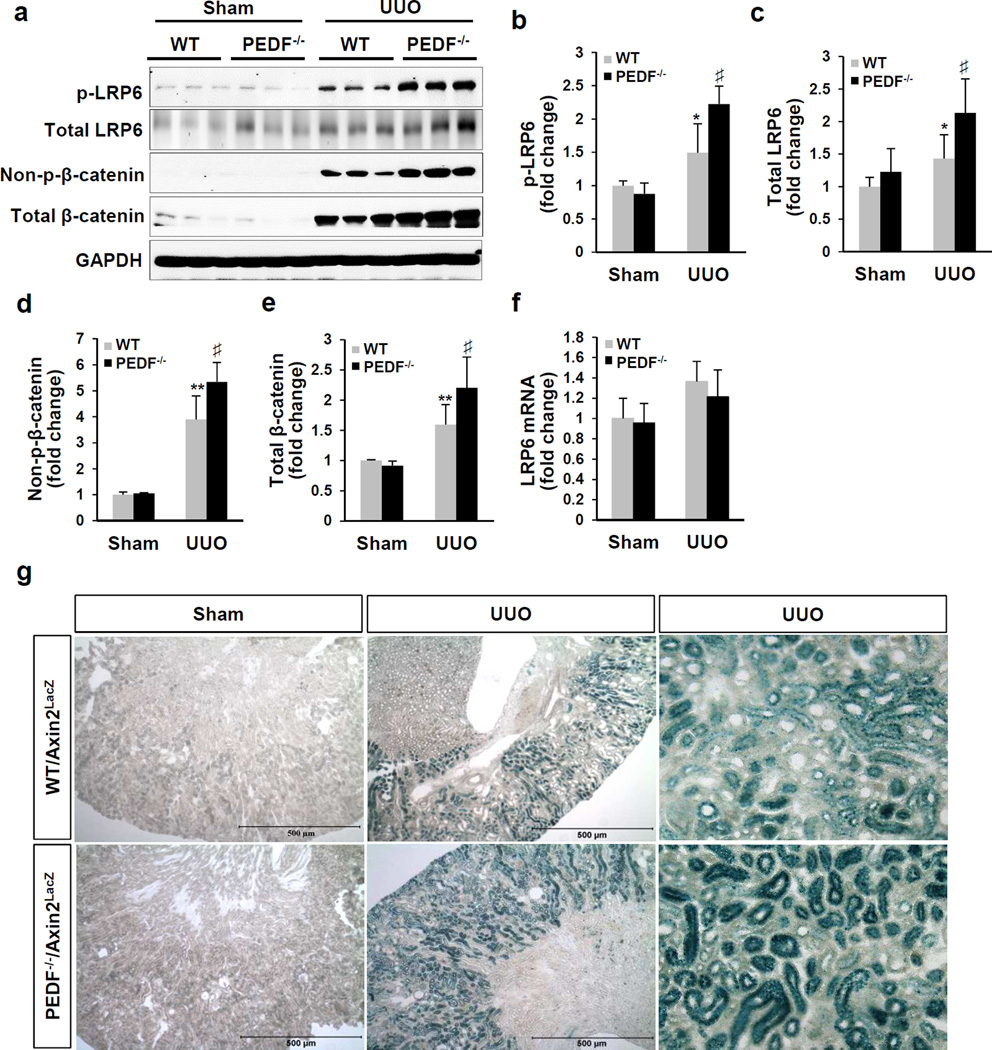
PEDF knock-out enhanced UUO-induced Wnt signaling activation
Our previous study established PEDF as an endogenous inhibitor of the Wnt pathway.31 Thus we further evaluated the impacts of PEDF KO on Wnt signaling activation in the UUO model. At day 5 post-UUO, renal levels of p-LRP6, total LRP6, non-p-β-catenin and total β-catenin were significantly higher in the PEDF−/−/UUO group, compared to the WT/UUO group (Figure 7a–e). PEDF KO did not affect LRP6 mRNA levels in both UUO groups and sham groups (Figure 7f), consistent with our previous report showing that PEDF regulated the LRP6 protein but not the LRP6 mRNA level.31 Moreover, PEDF KO did not affect p-LRP6 and non-p-β-catenin levels in the sham groups (Figure 7b and d), nor the levels of the canonical Wnt ligands (Wnt1, Wnt2, Wnt2b, Wnt3, Wnt3a, Wnt8a, Wnt9a, Wnt10b and Wnt16) in both sham and UUO groups (Supplemental Figure 6).To further confirm Wnt signaling activation in vivo, we utilized a transgenic Wnt signaling reporter mouse line, axis inhibition protein 2/LacZ (Axin2+/LacZ) mice, in which the β-galactosidase reporter gene is expressed in response to Wnt signaling activation.37 At day 5 post-surgery, X-gal staining showed that β-galactosidase was significantly induced in PEDF−/−/Axin2+/LacZ kidneys with UUO, compared to that in WT/Axin2+/LacZ kidneys with UUO (Figure 7g). Wnt signaling activation was detected predominantly in tubules, which was consistent with previous reports in the same model.22, 23 There was no significant difference in Wnt signaling activation in mice with sham surgery. Taken together, these results indicated that PEDF KO exacerbated Wnt signaling activation in UUO kidneys.
Figure 7. Enhanced Wnt signaling activation in the kidneys of pigment epithelium-derived factor knock-out (PEDF−/−) kidneys after unilateral ureteral obstruction (UUO).
(a) Western blot analysis and (b–e) densitometry quantification of phosphorylated low-density lipoprotein receptor-related protein 6 (p-LRP6), total LRP6, non-phosphorylated β-catenin (non-p-β-catenin) and total β-catenin in kidney homogenates from wild-type (WT) and PEDF−/− mice at day 5 post- surgery (n=5–6). Each lane represents an individual mouse. (f) Real-time PCR measurement of mRNA levels of LRP6 in 5-day sham and UUO kidneys (n=5–6). (g) Representative images of X-gal staining reflecting Wnt signaling activation (blue) in kidney sections from the WT/Axin2LacZ and PEDF−/−/Axin2LacZ mice at day 5 post-surgery. Scale bar=500 µm. *p<0.05, **p<0.01, WT/UUO versus WT/Sham; ♯p<0.05, PEDF−/−/UUO versus WT/UUO. All values are expressed as mean±SD.
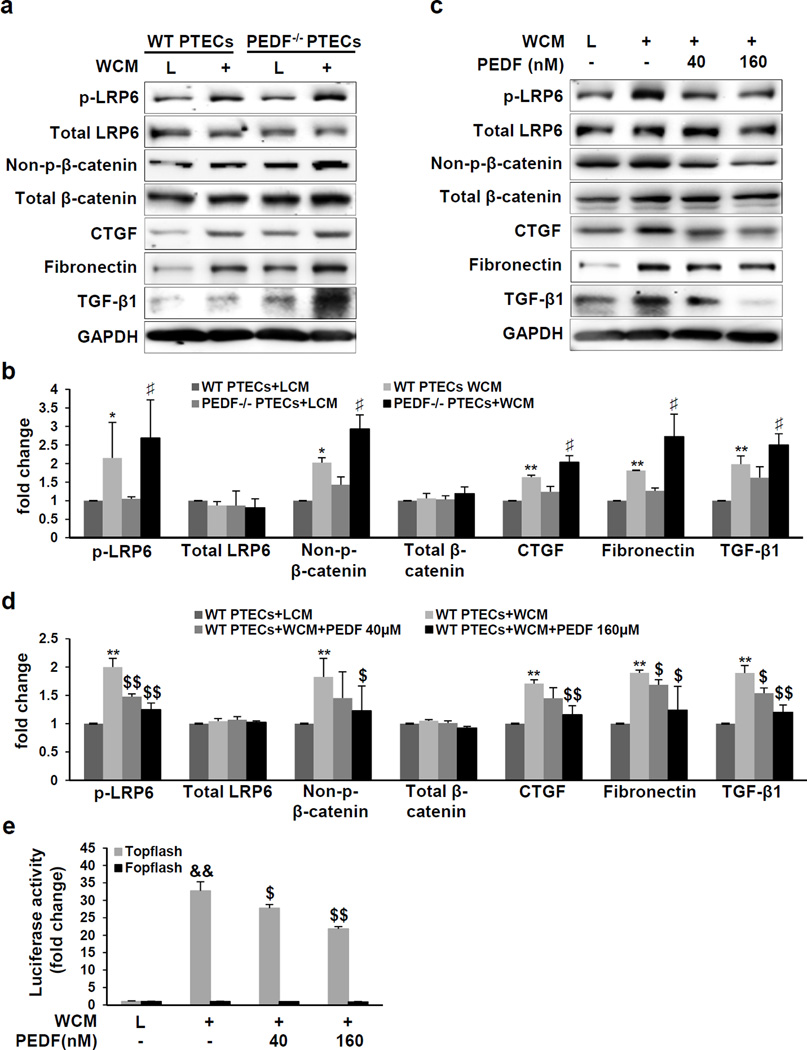
PEDF inhibited the Wnt pathway-mediated fibrosis in renal proximal tubule epithelial cells
Next, we evaluated PEDF’s effect on Wnt-mediated fibrosis in mouse primary renal proximal tubule epithelial cells (PTECs). PTECs were intensely immunostained by an antibody for the proximal tubule marker sodium-dependent glucose co-transporter 1 (SGLT-1) and very weakly by antibody for the distal convoluted tubule marker sodium-chloride symporter (NCC) (Supplemental Figure 7). In PEDF−/− PTECs, Wnt3a conditioned medium (WCM) induced more prominent increases of p-LRP6 and non-p-β-catenin, compared to those in WT PTECs (Figure 8a and b), consistent with the in vivo observations of Wnt signaling in PEDF−/−/UUO kidneys. Moreover, WCM induced more prominent increases of Wnt-targeted genes fibronectin38 and CTGF39 in PEDF−/− PTECs (Figure 8a and b). Interestingly, TGF-β1, a master regulator of tubulointerstitial fibrosis, was also up-regulated by WCM, which was further enhanced by PEDF KO (Figure 8a and b). PEDF treatment significantly reduced levels of p-LRP6, non-p-β-catenin, fibronectin, CTGF and TGF-β1 in WT PTECs (Figure 8c and d). In addition, a luciferase-based promoter activity assay showed that PEDF treatment suppressed the WCM-induced β-catenin transcriptional activity in a human renal proximal tubule epithelial cell line (Figure 8e).
Figure 8. Inhibition of the Wnt-mediated fibrosis by pigment epithelium-derived factor (PEDF).
(a, c) Western blot analysis and (b, d) densitometry quantification of phosphorylated low-density lipoprotein receptor-related protein 6 (p-LRP6), total LRP6, non-phosphorylated β-catenin (non-p-β-catenin), total β-catenin, connective tissue growth factor (CTGF), fibronectin and transforming growth factor beta 1 (TGF-β1) (a, b) in primary proximal tubule epithelial cells (PTECs) from wild-type (WT) mice and PEDF knock-out (PEDF−/−) mice and (c, d) in WT PTECs treated with Wnt3a conditioned medium (WCM) and indicated concentrations of human PEDF for 24 h, with L cell medium (L) as control. (e) Luciferase activity in HKC-8/Topflash cells and HKC-8/Fopflash cells treated with WCM and indicated concentrations of human PEDF for 24 h (n=3). *p<0.05, **p<0.01, WT PTECs+WCM versus WT PTECs+LCM; ♯p<0.05, PEDF−/− PTECs+WCM versus WT PTECs+WCM; $p<0.05, $$p<0.01, WT PTECs+WCM+PEDF versus WT PTECs+WCM. All values are expressed as mean±SD.
PEDF’s protective effect on renal proximal tubule epithelial cells
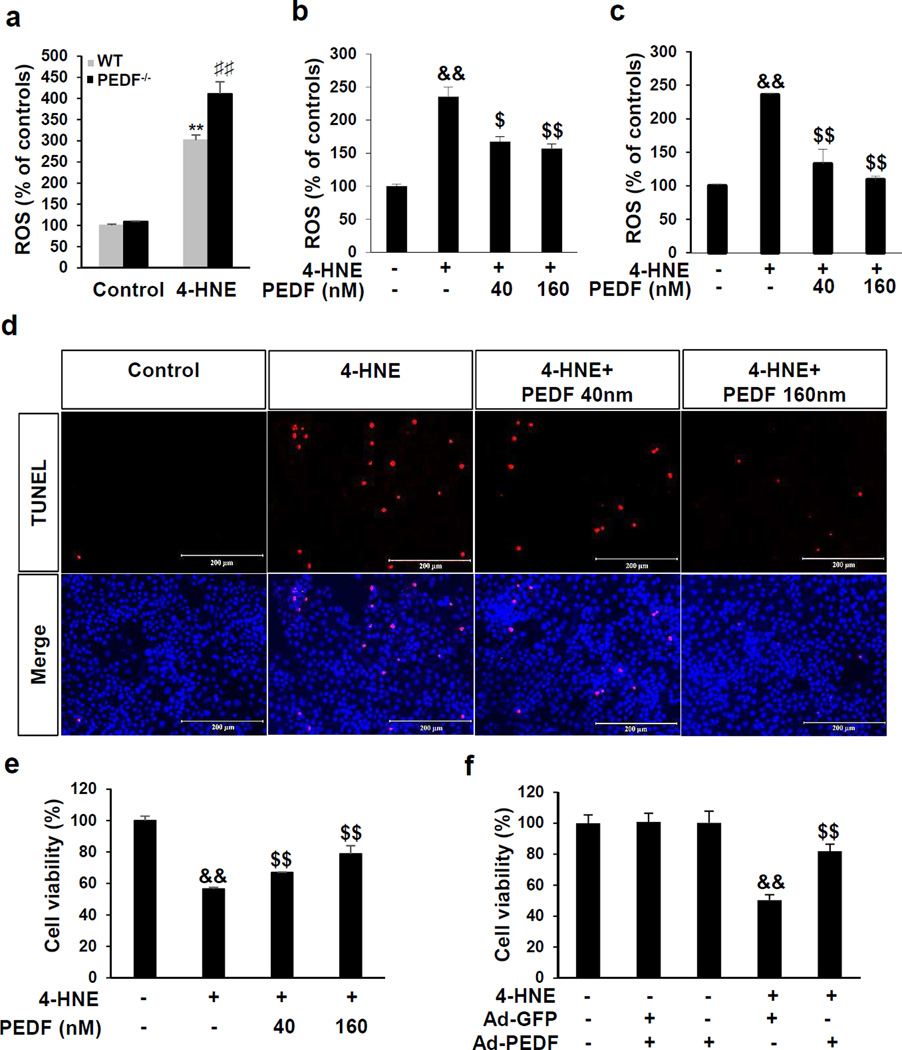
Increase of oxidative stress in the UUO model was reported to cause renal tubule cell impairment.33 Therefore, we examined PEDF’s effect on oxidative stress. WT PTECs and PEDF−/− PTECs were exposed to 4-hydroxynonenal (4-HNE), to mimic oxidative stress in the UUO model. 4-HNE induced a 4-fold increase of reactive oxygen species (ROS) generation in PEDF−/− PTECs comparing to the 3-fold ROS increase in WT PTECs (Figure 9a). Administration of PEDF significantly suppressed 4-HNE-induced ROS generation in WT PTECs (Figure 9b) and in HKC-8 cells (Figure 9c). Oxidative stressed-induced tubule epithelial cell death was further evaluated by TUNEL staining and Trypan blue exclusion assay. As shown in Figure 9d–f, PEDF protein and adenovirus expressing PEDF (Ad-PEDF) significantly protected HKC-8 cells from 4-HNE-induced cell death.
Figure 9. Protective effect of pigment epithelium-derived factor (PEDF) on renal tubule epithelial cells under oxidative stress.
(a–c) Measurement of reactive oxygen species (ROS) generation (a) in primary renal proximal tubule epithelial cells (PTECs) from wild-type (WT) and pigment epithelium-derived factor knock-out (PEDF−/−) mice treated with 30 µM 4-hydroxynonenal (4-HNE) for 3 h, (b) in WT PTECs treated with 30 µM 4-HNE and indicated concentrations of human PEDF for 3 h and (c) in HKC-8 cells treated with 10 µM 4-HNE and indicated concentrations of PEDF for 1 h. (d) TUNEL staining of apoptosis in HKC-8 cells (red), with the nuclei counterstained by DAPI (blue). Scale bar=200 µm. (e, f) Cell viability measured by trypan blue exclusion assay (e) in HKC-8 cells treated with 10 µM 4-HNE and indicated concentrations of human PEDF for 24 h and (f) in HKC-8 cells treated with 10 µm 4-HNE and adenovirus expressing green fluorescence protein (Ad-GFP) or adenovirus expressing PEDF (Ad-PEDF) for 24 h. **p<0.01, WT PTECs+4-HNE versus WT PTECs; ♯♯p<0.01, PEDF−/− PTECs+4-HNE versus WT PTECs+4-HNE. &&p<0.01, 4-HNE versus control; $p<0.05, $$p<0.01, 4-HNE+PEDF versus 4-HNE. All values are expressed as mean±SD.
DISCUSSION
The present study demonstrated that PEDF was significantly down-regulated in the kidneys of the UUO and type 1 and type 2 diabetic models. Utilizing PEDF−/− mice and PTECs from PEDF−/− mice, this study further demonstrated that PEDF possesses anti-fibrotic and anti-oxidant activities in the kidney. Furthermore, using Wnt signaling reporter mice, this study showed that PEDF KO aggravated Wnt signaling activation induced by UUO. Also, our results showed an inverse correlation between Wnt signaling activation and PEDF levels in the kidneys of UUO and type 1 and 2 diabetic models. PEDF protein suppressed Wnt ligand-induced expression of fibrotic factors in renal tubule epithelial cells. These observations suggest that PEDF’s inhibition of Wnt signaling represents a mechanism responsible for its anti-fibrotic effect.
In renal disease models characterized by tubulointerstitial fibrosis, such as UUO 22, 23 adriamycin-induced nephropathy30 and ischemia/reperfusion renal injury,27 increased β-catenin was predominantly detected in renal tubules, suggesting that tubular epithelial cells are the major sites for Wnt signaling activation. To verify Wnt signaling activation in vivo, the present study used Wnt signaling reporter mice, showing that UUO induced higher Wnt signaling activities in PEDF−/−kidneys, primarily in the tubules, compared to that in WT kidneys. Our previous study demonstrated that PEDF bound to the Wnt signaling co-receptor LRP6 and down-regulated p-LRP6 and total LRP6 protein levels.31 Consistently, higher p-LRP6 and total LRP6 protein levels were detected in PEDF−/−/UUO kidneys, compared to WT/UUO kidneys. However, PEDF KO did not affect LRP6 mRNA levels in either the sham or UUO group, further confirming that PEDF regulates LRP6 at the protein levels. In agreement, knocking out another Wnt inhibitor, very low-density lipoprotein receptor, resulted in increased LRP6 protein levels but unchanged LRP6 mRNA levels.40 Taken together, these results indicate that PEDF is indeed an endogenous inhibitor of Wnt signaling in the kidney.
Previously, we reported that Wnt signaling promoted inflammation and oxidative stress.41 In this study, PEDF−/−/UUO mice displayed enhanced Wnt signaling activation, correlating with exacerbated monocytes/macrophages infiltration and higher levels of ICAM-1, TNF-α and 3-NT, compared to WT/UUO kidneys. In addition, PEDF protein potently suppressed ROS generation and attenuated the expression of Wnt-target genes fibronectin25 and CTGF.42 These observations suggest that the anti-fibrotic and anti-oxidant effects of PEDF are mediated, at least in part, through inhibition of Wnt signaling.
Our results further demonstrated that PEDF KO exacerbated tubule epithelial cell apoptosis in UUO kidneys, while PEDF protein attenuated renal proximal tubule epithelial cell apoptosis, suggesting a novel protective effect of PEDF on tubule epithelial cells. However, Wnt signaling was reported to promote tubule epithelial cell survival;43 thus the anti-apoptotic effect of PEDF cannot be directly attributed to the inhibition of Wnt signaling. It is possible that PEDF inhibits Wnt signaling, resulting in attenuated Wnt-regulated inflammation and oxidative stress, which subsequently ameliorates tubule epithelial cell apoptosis. Moreover, an enhanced Wnt signaling activation is also supported by over-expression of the Wnt target genes Snail-1 and Twist,44 suppressors of epithelial cell marker E-cadherin,45 which might also explain the further decrease of E-cadherin in PEDF−/−/UUO kidneys. Interestingly, we did not detect higher levels of myofibroblast marker α-SMA in PEDF−/−/UUO kidneys, suggesting that PEDF KO does not affect the myofibroblast population in the UUO model.
Others46 and our group16, 19 have independently reported that PEDF down-regulated TGF-β1 levels in diabetic kidneys, human renal proximal tubule epithelial cells and human glomerular mesangial cells. In the present study, we found increased TGF-β1 expression in PEDF−/−/UUO kidneys and PEDF−/− PTECs, further confirming an inhibitory effect of PEDF on TGF-β1 expression. We also observed that Wnt3a alone was sufficient to up-regulate TGF-β1, accompanied by Wnt signaling activation in PTECs, and PEDF counteracted Wnt3a’s induction of TGF-β1. It is possible that PEDF suppresses TGF-β1 expression through inhibiting the Wnt pathway. However, this study does not exclude the possibility that PEDF might regulate the cross-talks between the Wnt and TGF-β pathways to affect TGF-β1 expression. This study also showed increases of TβRI and TβRII in PEDF−/−/UUO kidneys. It remains to be elucidated how TGF-β1 and its receptors are up-regulated in PEDF−/− kidneys.
Being a multi-functional protein that interacts with multiple cell surface proteins,12 PEDF was also reported to regulate other fibrotic signaling pathways. A PEDF-derived peptide down-regulated the expression of platelet-derived growth factor receptor (PDGF) in fibrotic livers and hepatic stellate cells.47 We also observed that PEDF suppresses TGF-β1-induced fibronectin and CTGF expression in human mesangial cells19 and in human renal tubule epithelial cells (Supplemental Figure 8). It remains to be studied whether PEDF regulates the PDGF and TGF-β signaling pathways in renal fibrosis models.
This study demonstrated that in adult mouse kidneys, the PEDF protein and mRNA were abundantly expressed in tubules and moderately in glomeruli, consistent with previous observations of renal distribution of PEDF.20, 21 After UUO, both the PEDF mRNA and protein levels were significantly decreased. The decreased mRNA and protein levels of PEDF in UUO kidneys might be a result of tubule epithelial cell loss, as these cells express abundant amount of PEDF. However, it is not known if epithelial cell loss is a plausible explanation of decreased PEDF levels in diabetic kidneys. PEDF is a substrate of matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9).18, 48 As MMP-2 and MMP-9 are activated in UUO49 and diabetes,50 it is possible that MMP-2 and MMP-9 activation is a potential mechanism for decreased PEDF levels in these models.
In summary, the present study showed that decreased renal PEDF levels in UUO and diabetic models may account for the progression of renal tubulointerstitial fibrosis through the mediation of Wnt signaling. These findings provide a new pathogenic mechanism for tubulointerstitial fibrosis and suggest therapeutic potential of PEDF for renal fibrosis.
METHODS
Animal models and measurements of renal functions
PEDF−/− mice in C57BL/6J background was kindly provided by Regeneron Pharmaceuticals (Tarrytown, NY). PEDF−/− mice were crossbred with a Wnt reporter strain Axin2+/LacZ in B6.129 background;37 F2 homozygous PEDF−/−/Axin2+/LacZ and littermates WT/Axin2+/LacZ mice were used for this study. Eight-week-old mice were randomly assigned to sham surgery or UUO group. Briefly, UUO was achieved in anesthetized mice by double ligations at the left ureter using 4-0 silk. Ureters of sham mice were exposed, manipulated without ligations.22 Left kidneys were collected for analyses at day 5 or day 10 post-surgery. Twenty-four-hour urine was collected and creatinine was measured by HPLC as previously described.25 Urinary albumin was measured using a murine microalbuminuria ELISA kit (Exocell, Philadelphia, PA). GFR was measured as previously described.51
Cell culture, luciferase, ROS and trypan blue exclusion assay
Human proximal renal tubule epithelial cell line HKC-8 cells (Dr. L. Racusen, the Johns Hopkins University, Baltimore, MD) were maintained as previously described.52 Pretreatment of PEDF proteins was always performed in cell assays, using bovine serum albumin as the control protein. Ad-GFP or Ad-PEDF (1.25×107 pfu/ml) was used as previously described.52 Topflash activity was performed as previously described.53 ROS generation was measured by CM-H2DCFDA (ThermoFisher, Grand Island, NY) according to the manufacturer’s instruction. Cell viability was determined by trypan blue exclusion assay (ThermoFisher, Grand Island, NY). Briefly, cell pellets were collected and washed with PBS for three times, and then re-suspended in PBS at a concentration of 105–106/ml. After incubation with trypan blue dye (0.4%) at 1:1 ratio (v/v) for 5 minutes, viable and dead cells were counted using the Cellometer (Nexcelom, Lawrence, MA).
Primary culture of renal tubule epithelial cells
Isolation of mouse PTECs was performed as previously described.54 Briefly, the renal cortex was dissected, minced, and digested at 37°C with agitation for 30 min. The solution was then filtered sequentially through 80 µm and 200 µm sieves (Sigma-Aldrich, St. Louis, MO). The cells with size between 80–200 µm were collected and maintained in renal epithelial culture medium (ATCC, Manassas, VA). Cells of passages 2–5 were used for experiments.
Real-time RT-PCR
Real-time PCR was performed as described previously.25 Sequences of primers for genes are obtained from the qPrimerDepot website (Supplemental Table 1).
Western blot analysis and ELISA
Western blot analysis was performed as previously described.31 Antibodies used were listed in Supplemental Table 2. Individual band was semi-quantified by densitometry using the AlphaView software (AlphaView, Santa Clara, CA). Levels of fibronectin were determined by a mouse fibronectin ELISA kit (Assaypro, Charles, MO), 3-NT by a mouse 3-NT ELISA kit (EMD Millipore, Temecula, CA) and ICAM-1 by a mouse ICAM-1 ELISA kit (R&D Systems, Minneapolis, MN). ELISA results were normalized to total amount of proteins.
Immunohistochemistry, picro-sirius red staining, TUNEL and X-gal staining
Paraffin-embedded kidney sections were used for immunostaining of PEDF, picro-sirius red staining and TUNEL. The anti-PEDF antibody (Chemicon, Temecula, CA) was used for immunohistochemistry as described previously.22 Picro-sirius staining (Sigma-Aldrich, St. Louis, MO) and TUNEL were performed according to the manufacturer’s instruction (Roche Life Science, Indianapolis, IN). Kidney cryosections were kept in 24-well plate containing 1 ml of PBS with 0.02% NP-40 and 0.1% sodium deoxycholate for 1–2 hr at room temperature, and then in 0.5ml of X-gal staining solution (Promega, Madison, WI) at 37°C overnight.40 Quantification of immunohistochemical and picro-sirius red staining images was performed by measuring the intensity of staining using the Image Pro Plus 6.0. Values were means of 6–8 pictures of each sample.
In situ hybridization
The kidneys were fixed in 4% paraformaldehyde and cryosectioned at 14 µm. Dig-labelled PEDF anti-sense probe and sense probe (1.2kb) were synthesized using the DIG RNA labeling kit (Roche Life Science, Indianapolis, IN) from pBKS-PEDF plasmid. Dig-labelled probes were detected by AP-conjugated anti-Dig antibody (Roche Life Science, Indianapolis, IN) and developed with NBT/BCIP solution (Roche Life Science, Indianapolis, IN).55
Statistical analyses
Experiments were performed at least three times, and representative data were presented. All values were expressed as mean±SD. Statistical comparisons among groups were tested by one-way ANNOVA with Tukey’s multiple comparisons. Statistical significance was set at p<0.05.
Supplementary Material
Acknowledgments
We appreciate Dr. Xiang Wang (Director of the Cell Biology Imaging Facility) and the Diabetes COBRE Imaging Facility at the University of Oklahoma Health Science Center and COBRE Diabetic Animal Core for the technical supports. This study was supported by NIH, EY012231, EY018659, EY019309, EY016507 and GM104934, a fellowship from American Heart Association (AHA), 14PRE20460229 and a grant from Oklahoma Center for the Advancement of Science & Technology (OCAST), HR13-076 and HR16-041 and Rui Cheng’s Juvenile Diabetes Research Foundation fellowship (3-PDF-2014-107-A-N).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
All the authors declared no conflict of interests.
SUPPLEMENTARY INFORMATION IS AVAILABLE AT KIDNEY INTERNATIONAL’S WEBSITEREFERENCES
REFERENCES
- 1.Leung KC, Tonelli M, James MT. Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol. 2013;9:77–85. doi: 10.1038/nrneph.2012.280. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shihab FS. Do we have a pill for renal fibrosis? Clin J Am Soc Nephrol. 2007;2:876–878. doi: 10.2215/CJN.02660707. [DOI] [PubMed] [Google Scholar]
- 4.Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2:363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- 5.Bohle A, Wehrmann M, Bogenschutz O, et al. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract. 1991;187:251–259. doi: 10.1016/s0344-0338(11)80780-6. [DOI] [PubMed] [Google Scholar]
- 6.Nakhoul N, Batuman V. Role of proximal tubules in the pathogenesis of kidney disease. Contrib Nephrol. 2011;169:37–50. doi: 10.1159/000313944. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Humphreys BD, Bonventre JV. Pathophysiology of acute kidney injury to chronic kidney disease: maladaptive repair. Contrib Nephrol. 2011;174:149–155. doi: 10.1159/000329385. [DOI] [PubMed] [Google Scholar]
- 8.Vallon V. The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol Regul Integr Comp Physiol. 2012;300:R1009–R1022. doi: 10.1152/ajpregu.00809.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Phillips AO, Steadman R. Diabetic nephropathy: the central role of renal proximal tubular cells in tubulointerstitial injury. Histol Histopathol. 2012;17:247–252. doi: 10.14670/HH-17.247. [DOI] [PubMed] [Google Scholar]; Bonventre JV. Can we target tubular damage to prevent renal function decline in diabetes? Semin Nephrol. 2012;32:452–462. doi: 10.1016/j.semnephrol.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grgic I, Campanholle G, Bijol V, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Kooten C, Daha MR, van Es LA. Tubular epithelial cells: A critical cell type in the regulation of renal inflammatory processes. Exp Nephrol. 1999;7:429–437. doi: 10.1159/000020622. [DOI] [PubMed] [Google Scholar]
- 11.Steele FR, Chader GJ, Johnson LV, et al. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci U S A. 1993;90:1526–1530. doi: 10.1073/pnas.90.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He X, Cheng R, Benyajati S, et al. PEDF and its roles in physiological and pathological conditions: implication in diabetic and hypoxia-induced angiogenic diseases. Clin Sci (Lond) 2015;128:805–823. doi: 10.1042/CS20130463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 14.Spranger J, Osterhoff M, Reimann M, et al. Loss of the antiangiogenic pigment epithelium-derived factor in patients with angiogenic eye disease. Diabetes. 2001;50:2641–2645. doi: 10.2337/diabetes.50.12.2641. [DOI] [PubMed] [Google Scholar]
- 15.Yamagishi S, Inagaki Y, Nakamura K, et al. Pigment epithelium-derived factor inhibits TNF-alpha-induced interleukin-6 expression in endothelial cells by suppressing NADPH oxidase-mediated reactive oxygen species generation. J Mol Cell Cardiol. 2006;37:497–506. doi: 10.1016/j.yjmcc.2004.04.007. [DOI] [PubMed] [Google Scholar]; Zhang SX, Wang JJ, Gao G, et al. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. Faseb J. 2006;20:323–325. doi: 10.1096/fj.05-4313fje. [DOI] [PubMed] [Google Scholar]
- 16.Wang JJ, Zhang SX, Lu K, et al. Decreased expression of pigment epithelium-derived factor is involved in the pathogenesis of diabetic nephropathy. Diabetes. 2005;54:243–250. doi: 10.2337/diabetes.54.1.243. [DOI] [PubMed] [Google Scholar]
- 17.Chung C, Doll JA, Gattu AK, et al. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL) J Hepatol. 2011;48:471–478. doi: 10.1016/j.jhep.2007.10.012. [DOI] [PubMed] [Google Scholar]; Schmitz JC, Protiva P, Gattu AK, et al. Pigment epithelium-derived factor regulates early pancreatic fibrotic responses and suppresses the profibrotic cytokine thrombospondin-1. Am J Pathol. 2011;179:2990–2999. doi: 10.1016/j.ajpath.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung C, Shugrue C, Nagar A, et al. Ethanol exposure depletes hepatic pigment epithelium-derived factor, a novel lipid regulator. Gastroenterology. 2009;136:331–340. doi: 10.1053/j.gastro.2008.09.065. e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JJ, Zhang SX, Mott R, et al. Salutary effect of pigment epithelium-derived factor in diabetic nephropathy: evidence for antifibrogenic activities. Diabetes. 2006;55:1678–1685. doi: 10.2337/db05-1448. [DOI] [PubMed] [Google Scholar]
- 20.Abramson LP, Browne M, Stellmach V, et al. Pigment epithelium-derived factor targets endothelial and epithelial cells in Wilms’ tumor. J Pediatr Surg. 2006;41:1351–1356. doi: 10.1016/j.jpedsurg.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 21.Tombran-Tink J, Mazuruk K, Rodriguez IR, et al. Organization, evolutionary conservation, expression and unusual Alu density of the human gene for pigment epithelium-derived factor, a unique neurotrophic serpin. Mol Vis. 1996;2:11. [PubMed] [Google Scholar]
- 22.He W, Dai C, Li Y, et al. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 24.Dai C, Stolz DB, Kiss LP, et al. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20:1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou T, He X, Cheng R, et al. Implication of dysregulation of the canonical wingless-type MMTV integration site (WNT) pathway in diabetic nephropathy. Diabetologia. 2012;55:255–266. doi: 10.1007/s00125-011-2314-2. [DOI] [PubMed] [Google Scholar]
- 26.DiRocco DP, Kobayashi A, Taketo MM, et al. Wnt4/beta-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol. 2013;24:1399–1412. doi: 10.1681/ASN.2012050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao L, Zhou D, Tan RJ, et al. Sustained Activation of Wnt/beta-Catenin Signaling Drives AKI to CKD Progression. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan RJ, Zhou D, Zhou L, et al. Wnt/beta-catenin signaling and kidney fibrosis. Kidney Int Suppl (2011) 2014;4:84–90. doi: 10.1038/kisup.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eddy AA, Fogo AB. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol. 2009;17:2999–3012. doi: 10.1681/ASN.2006050503. [DOI] [PubMed] [Google Scholar]; Eddy AA. Serine proteases, inhibitors and receptors in renal fibrosis. Thromb Haemost. 2009;101:656–664. [PMC free article] [PubMed] [Google Scholar]
- 30.He W, Kang YS, Dai C, et al. Blockade of Wnt/beta-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park K, Lee K, Zhang B, et al. Identification of a novel inhibitor of the canonical Wnt pathway. Mol Cell Biol. 2011;31:3038–3051. doi: 10.1128/MCB.01211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1145–1152. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 33.Manucha W, Valles PG. Apoptosis modulated by oxidative stress and inflammation during obstructive nephropathy. Inflamm Allergy Drug Targets. 2012;11:303–312. doi: 10.2174/187152812800958997. [DOI] [PubMed] [Google Scholar]
- 34.Cao Q, Harris DC, Wang Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology (Bethesda) 2015;30:183–194. doi: 10.1152/physiol.00046.2014. [DOI] [PubMed] [Google Scholar]
- 35.Gottfried E, Kunz-Schughart LA, Weber A, et al. Expression of CD68 in non-myeloid cell types. Scand J Immunol. 2008;67:453–463. doi: 10.1111/j.1365-3083.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- 36.Power RE, Doyle BT, Higgins D, et al. Mechanical deformation induced apoptosis in human proximal renal tubular epithelial cells is caspase dependent. J Urol. 2004;171:457–461. doi: 10.1097/01.ju.0000091106.61065.e3. [DOI] [PubMed] [Google Scholar]
- 37.Lustig B, Jerchow B, Sachs M, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gradl D, Kuhl M, Wedlich D. The Wnt/Wg signal transducer beta-catenin controls fibronectin expression. Mol Cell Biol. 1999;19:5576–5587. doi: 10.1128/mcb.19.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo Q, Kang Q, Si W, et al. Connective tissue growth factor (CTGF) is regulated by Wnt and bone morphogenetic proteins signaling in osteoblast differentiation of mesenchymal stem cells. J Biol Chem. 2004;279:55958–55968. doi: 10.1074/jbc.M407810200. [DOI] [PubMed] [Google Scholar]
- 40.Lee K, Shin Y, Cheng R, et al. Receptor heterodimerization as a novel mechanism for the regulation of Wnt/beta-catenin signaling. J Cell Sci. 2014;127:4857–4869. doi: 10.1242/jcs.149302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou T, Hu Y, Chen Y, et al. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:4371–4379. doi: 10.1167/iovs.09-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang B, Zhou KK, Ma JX. Inhibition of connective tissue growth factor overexpression in diabetic retinopathy by SERPINA3K via blocking the WNT/beta-catenin pathway. Diabetes. 2010;59:1809–1816. doi: 10.2337/db09-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou D, Li Y, Lin L, et al. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney Int. 2012;82:537–547. doi: 10.1038/ki.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howe LR, Watanabe O, Leonard J, et al. Twist is up-regulated in response to Wnt1 and inhibits mouse mammary cell differentiation. Cancer Res. 2003;63:1906–1913. [PubMed] [Google Scholar]
- 45.Yook JI, Li XY, Ota I, et al. Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem. 2008;280:11740–11748. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]; Vesuna F, van Diest P, Chen JH, et al. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun. 2008;367:235–241. doi: 10.1016/j.bbrc.2007.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maeda S, Matsui T, Takeuchi M, et al. Pigment epithelium-derived factor (PEDF) inhibits proximal tubular cell injury in early diabetic nephropathy by suppressing advanced glycation end products (AGEs)-receptor (RAGE) axis. Pharmacol Res. 2013;63:241–248. doi: 10.1016/j.phrs.2010.11.008. [DOI] [PubMed] [Google Scholar]; Mao T, Chen H, Hong L, et al. Pigment epithelium-derived factor inhibits high glucose-induced JAK/STAT signalling pathway activation in human glomerular mesangial cells. Saudi Med J. 2013;34:793–800. [PubMed] [Google Scholar]
- 47.Tsai TH, Shih SC, Ho TC, et al. Pigment epithelium-derived factor 34-mer peptide prevents liver fibrosis and hepatic stellate cell activation through down-regulation of the PDGF receptor. PLoS One. 2014;9:e95443. doi: 10.1371/journal.pone.0095443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Notari L, Miller A, Martinez A, et al. Pigment epithelium-derived factor is a substrate for matrix metalloproteinase type 2 and type 9: implications for downregulation in hypoxia. Invest Ophthalmol Vis Sci. 2005;46:2736–2747. doi: 10.1167/iovs.04-1489. [DOI] [PubMed] [Google Scholar]
- 49.Tan TK, Zheng G, Hsu TT, et al. Matrix metalloproteinase-9 of tubular and macrophage origin contributes to the pathogenesis of renal fibrosis via macrophage recruitment through osteopontin cleavage. Lab Invest. 2012;93:434–449. doi: 10.1038/labinvest.2013.3. [DOI] [PubMed] [Google Scholar]; Du X, Shimizu A, Masuda Y, et al. Involvement of matrix metalloproteinase-2 in the development of renal interstitial fibrosis in mouse obstructive nephropathy. Lab Invest. 2012;92:1149–1160. doi: 10.1038/labinvest.2012.68. [DOI] [PubMed] [Google Scholar]
- 50.Signorelli SS, Malaponte G, Libra M, et al. Plasma levels and zymographic activities of matrix metalloproteinases 2 and 9 in type II diabetics with peripheral arterial disease. Vasc Med. 2005;10:1–6. doi: 10.1191/1358863x05vm582oa. [DOI] [PubMed] [Google Scholar]
- 51.Reiniger N, Lau K, McCalla D, et al. Deletion of the receptor for advanced glycation end products reduces glomerulosclerosis and preserves renal function in the diabetic OVE26 mouse. Diabetes. 2010;59:2043–2054. doi: 10.2337/db09-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J, Yang J, Liu Y. Role of Bcl-xL induction in HGF-mediated renal epithelial cell survival after oxidant stress. Int J Clin Exp Pathol. 2008;1:242–253. [PMC free article] [PubMed] [Google Scholar]
- 53.Lee K, Hu Y, Ding L, et al. Therapeutic potential of a monoclonal antibody blocking the Wnt pathway in diabetic retinopathy. Diabetes. 2012;61:2948–2957. doi: 10.2337/db11-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terryn S, Jouret F, Vandenabeele F, et al. A primary culture of mouse proximal tubular cells, established on collagen-coated membranes. Am J Physiol Renal Physiol. 2007;293:F476–F485. doi: 10.1152/ajprenal.00363.2006. [DOI] [PubMed] [Google Scholar]
- 55.Olson LE, Soriano P. Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell. 2009;16:303–313. doi: 10.1016/j.devcel.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.