Abstract
Calorie restriction (CR), a nutritional intervention of reduced energy intake but with adequate nutrition, has been shown to extend healthspan and lifespan in rodent and primate models. Accumulating data from observational and randomized clinical trials indicate that CR in humans results in some of the same metabolic and molecular adaptations that have been shown to improve health and retard the accumulation of molecular damage in animal models of longevity. In particular, moderate CR in humans ameliorates multiple metabolic and hormonal factors that are implicated in the pathogenesis of type 2 diabetes, cardiovascular diseases, and cancer, the leading causes of morbidity, disability and mortality. In this paper, we will discuss the effects of CR in non-obese humans on these physiological parameters. Special emphasis is committed to recent clinical intervention trials that have investigated the feasibility and effects of CR in young and middle-aged men and women on parameters of energy metabolism and metabolic risk factors of age-associated disease in great detail. Additionally, data from individuals who are either naturally exposed to CR or those who are self-practicing this dietary intervention allows us to speculate on longer-term effects of more severe CR in humans.
Keywords: Calorie restriction, Aging, Energy metabolism, Metabolic adaptation, CALERIE, Age-associated diseases
Introduction
Calorie restriction (CR) without malnutrition is the most studied and robust non-genetic non-pharmacological experimental intervention for extending healthspan and lifespan in multiple animal models. In budding yeast, fruit flies and worms, CR can extend lifespan dramatically (2–3 fold). A 20 to 50% reduction in caloric intake, without malnutrition, in some strains of rats and mice prolongs median and maximal lifespan up to 50%, and prevents or delays the onset of many chronic diseases, such as obesity, type 2 diabetes, cancer, nephropathy, cardiomyopathy, neurodegeneration and multiple autoimmune diseases (Heilbronn and Ravussin, 2003). The effects and mechanisms through which CR modulates health and lifespan in simple model organisms and rodents are discussed in more detail in two separate review articles of this special issue of Ageing Research Review (ref #1, ref #2).
During the past two decades, the biology of aging scientific community has kept a close watch on studies of prolonged CR in non-human primates. As discussed in more detail by De Cabo et al. (ref#) in this issue, accumulating data from two long-term ongoing primate studies clearly indicate that 30% CR drastically reduces the incidence of glucose intolerance/type 2 diabetes, cardiovascular disease and cancer (Colman et al., 2009; Colman et al., 2014; Mattison et al., 2012). Moreover, in the Wisconsin National Primate Research Center (WNPRC) study, CR has been shown to slow down age-related sarcopenia, hearing loss and brain atrophy in several subcortical regions (Colman et al., 2009; Colman et al., 2014). For the time being, data on longevity are still discordant, but differences in study design, husbandry and diet composition likely contribute to the controversial findings between the primate colonies studies (Cava and Fontana, 2013).
While studies to elucidate the CR-mediated effects on disease prevention and life extension continue in experimental animals, evidence for the benefit of CR on metabolic and molecular adaptations in humans is also growing. In this review, we discuss findings from the NIH funded CALERIE randomized clinical trials and ongoing longitudinal studies on the effects to date of CR on human health. We also consider the importance of diet quality, and meal frequency and timing in mediating some of the beneficial effects of CR. Finally, we highlight the importance of human studies in the context of aging research and its potential to advance our understanding of interventions that could prevent the accumulation of molecular damage leading to multiple chronic diseases in humans.
INSIGHTS FROM POPULATION STUDIES WITH CALORIE RESTRICTION IN HUMANS
Calorie restriction without malnutrition in World War I and II
Involuntary episodes of CR are not uncommon in human history, but only few of these events were not accompanied by malnutrition, because the local governments wisely enforced food restriction with an adequate consumption of essential nutrient-dense foods. During World War 1 in 1917, Danish men and women were forced to reduce food consumption for 2 years, but with a well-planned and adequate consumption of whole grain cereals, vegetables, and milk. The result of this undesired experiment was an impressive 34% reduction in death rates (Hindhede, 1920). Similarly, in Norway during World War 2, the citizens of Oslo underwent a forced 20% CR without malnutrition (i.e. Norwegians were provided with adequate intake of fresh vegetables, potatoes, fish and whole cereals) for approximately 4 years (1941–45). In this forced experiment, mortality dropped by 30% compared to the pre-war level in both men and women (Strom and Jensen, 1951).
The Centenarians of Okinawa
Another natural CR experiment took place in Okinawa, a beautiful island located 640 kilometers south of mainland Japan. On this island, an estimated 50 in every 100,000 people were 100 years of age or older; this is approximately 4–5 times higher than the number of centenarians residing in any other industrialized country (Japan Ministry of Health, 2005). All-cause mortality at age 60–64 for people living on Okinawa was half of that of other Japanese for the year 1995 (Willcox et al., 2006), and the mortality from ischemic heart disease and cancer (i.e. prostate, colon, breast, and lymphoma) was markedly lower than in the average mainland Japanese and US population (Kagawa, 1978). In the same year, both the average and maximum lifespan of people living on Okinawa (average 83.8 years, maximum: 104.9 years) was higher compared to Japanese living on mainland Japan (82.3 years, 101.1 years), and Americans residing in the USA (78.9 years, 101.3 years) (Willcox et al., 2007a).
Studies investigating the dietary intake of adults living on Okinawa suggest that Okinawans’ consumed approximately 17% fewer calories than the average adult in Japan (Suzuki et al., 2001), and 40% less than the average adult in the United States (Bureau of Agricultural Economics, 1950). Importantly, the lower energy intake was not (exclusively) explained by overeating (energy intake exceeding energy requirements) in the latter populations. For Okinawans, a 10–15% deficit in energy intake was estimated according to the Harris-Benedict-equation of the energy requirements (Frankenfield et al., 1998; Willcox et al., 2007b). The Okinawan diet is also reported to be lower in protein (9% of calories) and rich in fresh vegetables, fruits, sweet potatoes, soy and fish (Willcox et al., 2006). Sadly, because of an increase in fast food chains brought by US soldiers in the 1960’s, dietary habits of Okinawan’s have become more westernized and as a result, body mass index has increased and so has mortality (Kagawa, 1978). In 2010, life expectancy for newborns on Okinawa is no longer different than in mainland Japan (girls, 87.0 vs. 86.4; boys, 79.4 vs. 79.5), whereas life expectancy for Okinawans, aged 65 and older, was still higher (women, 89.9 vs. 88.9; men, 84.5 vs. 83.8) (System of Social and Demographic Statistics, 2016).
INSIGHTS FROM RANDOMIZED CONTROLLED TRIALS OF CALORIE RESTRICTION
Short duration CR clinical trials
In three independent studies of short-term CR in non-obese humans, energy expenditure assessed as resting metabolic rate was reduced by −100±27 kcal/day after 3 weeks of 20% CR (Heyman et al., 1992), by 12% after 6 weeks of 1000 kcal/d CR (Webb and Abrams, 1983) and by −255±151 kcal/day after 10 weeks of 20% CR (Velthuis-te Wierik et al., 1995c). Changes in total daily energy expenditure assessed by doubly labeled water after 3 weeks (−296±170 kcal/day) also were decreased, however did not reach statistical significance (Velthuis-te Wierik et al., 1995c). No change in sedentary energy expenditure with a 25% reduction in food intake has also been reported (Garby et al., 1988), however this may be due to poor compliance, since subjects were only asked to reduce food intake by 25% without any oversight or scientific control. Interestingly, after 20% CR, the resting metabolic rate per kg of FFM was reduced (Velthuis-te Wierik et al., 1995c), indicating that the energy expended per FFM is not fixed, and thus indicating metabolic adaptation (−7.4 kg body mass, −1.3 kg FFM). In this study, the reduction in energy expenditure after 10 weeks of 20% CR did not lead to a concomitant reduction of indicators of oxidative stress or insulin levels (Loft et al., 1995; Velthuis-te Wierik et al., 1995b). In the 10-week CR study of Velthuis et al. (Velthuis-te Wierik et al., 1994), blood pressure was significantly reduced (124 to 118 mmHg and 79 to 74 mmHg, for systolic and diastolic blood pressure, respectively). Impaired fibrinolytic activity has been shown to be predictive for developing CVD (Anand et al., 2003; Tofler et al., 2016) and the 10 week CR intervention reversed markers of these impairments and induced fibrinolytic activity, particularly in subjects that were more impaired at baseline (Loft et al., 1995; Velthuis-te Wierik et al., 1995a). Also in the study of Velthuis and Loft et al. (Loft et al., 1995), glucose concentrations were reduced significantly from 4.8 to 4.6 mmol/L in the CR-group (age, 42.9 years; BMI, 24.6 kg/m2). Of note, the subjects in this study, were likely still in active weight loss and had not reached new energy balance or weight maintenance on the CR diet. Thus, longer term studies of CR in humans were designed to elucidate the metabolic effect of prolonged CR in non-obese humans.
CALERIE randomized clinical trials
The CALERIE (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy) trials were initiated by the US National Institute of Aging to provide the first controlled clinical trials of CR with adequate nutrient provision in healthy, non-obese humans. In CALERIE-1, three pilot studies were performed to evaluate the feasibility and effects of CR on metabolic health after 6 months (Das et al., 2007a; Heilbronn et al., 2006) or 12 months (Racette et al., 2006a). In the pilot studies, CR was studied by achieving a negative energy balance through different modalities 1) reduced calorie intake (CR), 2) increased exercise energy expenditure, or 3) CR and exercise in combination. The volunteers randomized in the CALERIE-1 trials were mostly overweight and their body mass index dropped to the upper limit of normality at the end of the study. The results of these studies were collated and thereafter a phase 2 multi-center trial was conducted to investigate the efficacy and safety of 2 years 25% CR in leaner and younger men and women. The CALERIE 2 study enrolled 220 healthy, young and middle-aged (21–51 years old), healthy non-obese (body mass index between 22 to 27.8 kg/m2) men and women and tested the effectiveness of the CR intervention on energy metabolism, metabolic adaptations, immune function, chronic disease risk factors and quality of life (Rickman et al., 2011; Rochon et al., 2011).
CALERIE-1 at the Pennington Center in Baton Rouge
At Pennington Biomedical Research Center (PBRC), a reduction of energy intake alone (25% CR) was compared to combined reduction in energy intake (12.5%) and a 12.5% increase in energy expenditure through exercise (−12.5% energy intake +12.5% energy expenditure = 25% CR), a positive weight loss control group that through a very low-calorie diet achieved a 15 kg weight loss and a weight-maintenance control group (Heilbronn et al., 2006; Redman et al., 2007). In the 6 month study the level of CR was estimated (by doubly labeled water) to be 18% (Redman et al., 2007). The CALERIE phase 1 trial conducted at Pennington Biomedical in Louisiana arguably provides the most compelling evidence for CR-induced metabolic adaptation. Energy expenditure was assessed by various independent gold-standard measurement techniques: doubly-labeled water for 14 days assessing daily free-living energy expenditure (Redman et al., 2009), metabolic chamber for 23 hours assessing sedentary and sleeping energy expenditure (Heilbronn et al., 2006), and ventilated hood system for assessing resting metabolic rate (Martin et al., 2007b). In this 6-month study, all components of energy expenditure, that is, sleeping (Heilbronn et al., 2006), resting (Martin et al., 2007b), 24h sedentary (Heilbronn et al., 2006) and free-living (Redman et al., 2009) were reduced below baseline levels. The metabolic adaptation in sedentary energy expenditures (sleep/24h) was ~6% based on a smaller energy expenditure than predicted on the basis of the metabolic size of the individuals at the end of the study (Heilbronn et al., 2006). The 24h sedentary energy expenditure was also significantly lower than 865 individuals matched for age and BMI studied in the NIDDK Chambers in Phoenix (Heilbronn et al., 2006; Weyer et al., 1999). Interestingly it does not appear that the metabolic cost of activity is affected by CR, as the relation between energy expenditure to spontaneous physical activity (simultaneously assessed in metabolic chamber, spontaneous physical activity as % activity by radar motion detector) was unchanged (Martin et al., 2007b). Since the metabolic cost of spontaneous activity was unchanged and the energy expenditures of free-living activity were reduced, the findings from this study suggest that individuals undergoing CR also make behavioral adjustments (conscious or unconscious) to decrease physical activity during CR (Redman et al., 2009).
In this pilot study, core body temperature was assessed over 24 hours on a metabolic ward and was reduced by 0.2° C after 6 months of 25% CR (Heilbronn et al., 2006). Also, fasting insulin concentrations declined after CR in CALERIE 1 and more sophisticated assessments of glucose homeostasis suggest a more pronounced benefit of CR on carbohydrate metabolism and potential risk for development of T2DM. Insulin sensitivity, assessed by an intravenous glucose tolerance test (with delayed insulin-infusion) was improved by 40% (p=0.08) (Larson-Meyer et al., 2006). Moreover, the acute insulin-response to glucose-infusion was increased by 29% which suggests an improvement in β-cell function (Larson-Meyer et al., 2006). Similarly, CR (−1000 kcal/d) increased insulin sensitivity (glucose infusion rate during hyperinsulinemic euglycemic clamp) by 46% (p<0.0001) after 16 weeks in older, obese subjects (55±2 yrs, 35.2±1.3kg/m2) (Johnson et al., 2016).
T2DM and insulin resistance are related to increased adipocyte size and ectopic fat deposition including the liver and skeletal muscle (Fox et al., 2007; Perry et al., 2014; Weyer et al., 2000). In line with the improvements in glucose homeostasis, 25% CR in CALERIE 1 resulted in a significant reduction in subcutaneous adipocyte size and deposition of lipid in the liver (intrahepatic lipid) but not in skeletal muscle (Larson-Meyer et al., 2006; Redman et al., 2007).
Surprisingly, in this CALERIE I trial, various factors that are associated with CVD (blood pressure, LDL, HDL, fibrinogen, homocysteine, CRP, TNFα, and Factor VIIc) were not affected by CR (Lefevre et al., 2009; Tam et al., 2012). Also, flow-mediated dilatation, a measure of endothelial function, was not improved and factors that have been associated with CR and aging such as IGF-1, DHEA-s, GH secretion (both GH pulse amplitude and pulse mass) were also unchanged in overweight subjects studied in CALERIE 1 (Heilbronn et al., 2006; Lefevre et al., 2009; Redman et al., 2010). This could be related to the young age of the study participants and their impeccable health status even before beginning the CR intervention (age, 39.0 years; BMI, 27.8 kg/m2, fasting glucose, 4.9 mmol/l). Nevertheless, based on values for total and HDL cholesterol (expressed as their ratio), systolic blood pressure, age and gender (Anderson et al., 1991), it was estimated that the 25% CR diet for 6 months induced a 29% reduction in the ten-year risk for CVD (Lefevre et al., 2009).
For the first time in humans undergoing CR, a decline in markers of oxidative stress was observed (DNA-damage and SOD-activity, indicating oxidative stress defense activity) (Civitarese et al., 2007; Heilbronn et al., 2006). The reduction in DNA damage to red blood cells was however not correlated to any measure of energy expenditure (Heilbronn et al., 2006). In line with previous results of the short-term CR-intervention studies discussed earlier (Velthuis-te Wierik et al., 1995b; Walford et al., 2002), thyroid hormone (T3 and T4) concentrations were reduced in CALERIE 1 after 6 months (Heilbronn et al., 2006). More important than the absolute changes in thyroid hormones which might be solely related to the energy deficit/weight loss, is that changes in thyroid hormone and leptin concentrations were significantly related to metabolic adaptation (Heilbronn et al., 2006; Lecoultre et al., 2011). Since the major contributor of metabolic rate is fat-free mass, molecular studies in the skeletal muscle of CR people may provide insight into the mechanisms of CR-induced metabolic adaptation or metabolic efficiency. In skeletal muscle biopsies collected from the CR participants, mitochondrial DNA content increased by 35%, suggesting an increase in mitochondrial mass. In line with this observation, expression of genes encoding proteins involved in mitochondrial function such as PGC1α, TFAM, eNOS, SIRT1, and PARL were also increased by CR (Civitarese et al., 2007). An increased number of mitochondria may enhance coupling due to a reduced mitochondrial membrane potential and contribute to reduce energy expenditure and oxidative stress. However, the activity of enzymes of the tricarboxylic acid cycle (citrate synthase), β-oxidation (β-hydroxyacyl-CoA dehydrogenase), and ETC (cytochrome C oxidase II) was unchanged. This lack of effect on mitochondrial enzyme activities questions whether skeletal muscle mitochondrial dynamics could affect whole-body energy expenditure.
CALERIE-1 at Washington University in St. Louis
At Washington University, 48 overweight (BMI, 23.5–29.9 kg/m2) individuals, aged 50–60 years, were randomized for 1-year to 20% CR, or 20% increase in energy expenditure by means of endurance exercise or to a control group of healthy lifestyle (Racette et al., 2006a). At the end of the study, the CR group achieved only a 11.5±2.1% reduction in calorie intake (measured with doubly-labeled water), BMI dropped from 27.3±0.3 kg/m2 to 24.4±0.6 kg/m2, and visceral fat mass measured by MRI was reduced by 37% (Racette et al., 2006b). As in experimental animals, CR-induced weight loss improved insulin sensitivity, increased adiponectin and reduced the serum concentrations of leptin, insulin, LDL-cholesterol, and C-reactive protein (Fontana et al., 2007b; Villareal et al., 2006; Weiss et al., 2006). Plasma triiodothyronine levels were decreased in the CR, but not in the exercise groups (Weiss et al., 2008). In this study, both CR and exercise induced weight loss resulted in a significant reduction in oxidative damage to DNA and RNA measured ex-vivo in white blood cells, and in improvements of left ventricular diastolic function (Hofer et al., 2008; Riordan et al., 2008). CR decreased bone mass, muscle size and strength, and maximal aerobic capacity in proportion to the reduction in body weight (Villareal et al., 2006; Weiss et al., 2007). In this pilot trial, unlike in CR rodents, serum concentration of IGF-1, estradiol, testosterone and cortisol did not change with CR (Fontana et al., 2008; Villareal et al., 2006; Weiss et al., 2006).
CALERIE-1 at Tufts University in Boston
At Tufts University, 46 young (24–42 years old) overweight (BMI, 25–29.9 kg/m2) individuals were randomized to low versus high glycemic load during 30% CR (Das et al., 2007a). At 6 months, the individuals randomized to 30% CR experienced a significant reduction in body weight, and improvements in fasting insulin concentration, insulin sensitivity, first-phase acute insulin secretion, and lipid profile independent of the glycemic load of the diets (Das et al., 2007b; Pittas et al., 2006). Serum concentrations of CRP were reduced in the 30% low-glycemic CR group, but not in the 30% high-glycemic CR group (Pittas et al., 2006). Moreover, 30% CR significantly improved T-cell function (i.e. delayed-type hypersensitivity response and proliferative response of T cells to T-cell mitogens), and prostaglandin E2 production (Ahmed et al., 2009).
CALERIE-2 multicenter trial
The main goal of CALERIE-2 was to test the hypothesis that 2-years of sustained CR, involving a reduction in energy intake to 75% of baseline (25% CR) will result in the same adaptive changes that occur in rodents subjected to CR (Ravussin et al., 2015). One reason for 2-years of CR intervention is that the 6-mo and 1-yr in CALERIE-1 pilot trials were not sufficient to induce many of the metabolic and hormonal adaptations in humans that are thought to increase longevity in rodents. Overall, the intervention was safe and well-tolerated; only two participants were withdrawn from the study because of treatment-resistant anemia and one because of excessive bone mass loss (≥5% compared to baseline) (Romashkan et al., 2016). Unfortunately, in this large trial (CR: n=143, ad libitum: n=75), which required study subjects to self-select their own diets, compliance was not as good as expected, and average CR during the first 6-months was 19.5±0.8% and 9.1±0.7% over the next 18 months of the study.
In this study, food intake was monitored by self-reporting and estimated by the intake/balance method, which is described in Racette et al. (Racette et al., 2012). As imposed by the study design, the reduction in weight and body composition was achieved at 12 months; body weight at month 12 was reduced by 11.5% and was maintained throughout the study (9.9% at month 24 vs. baseline), FFM by 4.3% (4.2%) and FM by 23% (26%) (Ravussin et al., 2015; Villareal et al., 2016). Metabolic adaptation in resting metabolic rate was evident in the CR group at both 12 months and 24 months. However, it was only significantly different from the group adhering to the ad libitum-diet after the first period of 12 months (Ravussin et al., 2015). A declining level CR throughout the study might explain this lack of significance at the 24 month-assessment. The measurement of indirect calorimetry in the metabolic chamber is considered the gold-standard because all components of daily energy expenditure are assessed and the individual can properly equilibrate to sedentary activities over the course of 24 hour measurements. While we await official publication from an ancillary study of CALERIE-2 where additional measurements of 24h energy expenditure were performed at one center (ClinicalTrials.gov Identifier: NCT02695511) and in a subset of people, the first reported results indicate confirmation of metabolic slowing with a CR diet (Redman et al., 2014). In the ancillary, both 24h and sleeping energy expenditure were reduced to a larger extent than predicted based on their body composition, indicating metabolic adaptation which was significant after both one and two years of intervention in the CR group. Additional insight on the effect of CR on skeletal muscle metabolism will be provided by skeletal muscle biopsies that have been collected in CALERIE-2, yet these data have not been published. More advanced measurement techniques of mitochondrial respiration and oxidative stress by ex vivo respirometry may provide a clearer picture of mitochondrial adaptations to CR. Also, the involvement of other tissues in the reduced energy expenditure observed with CR remains to be investigated since skeletal muscle only accounts for ~20% of resting energy expenditure and metabolic adaptations are likely to occur in other tissues as well (Heymsfield et al., 2007; Muller et al., 2015).
The results of this large trial are important because they demonstrate that mild CR improves cardiometabolic risk factors, well below the conventional thresholds used in the clinical practice, even when implemented in healthy lean or overweight young and middle-aged men and women. Total cholesterol, LDL-cholesterol, triglycerides, C-reactive protein, TNFα and blood pressure decreased significantly and HDL-cholesterol increased in CR group, even in people who had normal risk factor at baseline (Ravussin et al., 2015). HOMA-IR, which is a measure of insulin resistance, was also reduced from baseline to 24 months (1.2 to 0.9), (Ravussin et al., 2015). Importantly, the baseline values of the participants in all studies are already within the normative ranges (<6.1 mmol/L for glucose and <2.2 for HOMA-IR), thus it remains arguable whether the observed reductions of these fasting parameters are physiologically relevant with respect to the development of T2DM.
Finally, in CALERIE-2 CR caused a significant increase in serum IGFBP-1 and a reduction in leptin, and T3, but unlike in rodents did not change IGF-1 and IGF-1:IGFBP-3 ratio or cortisol levels (Fontana et al., 2016). Moreover, in this study CR like the CALERIE 1 trials had no effect on core body temperature, and serum concentrations of DHEA-s, PDGF-AB and TGFβ-1 (Fontana et al., 2016). Most likely, the lack of the typical CR-induced adaptations on several important hormonal and physiological factors is due to insufficient reduction in calorie intake achieved in the CALERIE trials. Thus, the findings of these studies may be more relevant to the effects of weight loss than of chronic severe CR.
INSIGHTS FROM OBSERVATIONAL STUDIES OF SEVERE CALORIE RESTRICTION
Biosphere 2 Study
Biosphere 2 is a 3.15-acre ecological enclosure that was designed as a laboratory for ecological investigations (e.g., biogeochemical cycles, food web systems, and the population genetics of speciation), and unintentionally provided an opportunity to study the effects of severe CR in non-obese metabolically healthy human beings (Walford et al., 1992). A very small group of 8 non-obese individuals between the ages of 25 and 67 entered Biosphere 2 for 24 months. Due to unforeseen agricultural problems with growing foods, the crew underwent a forced ~29% CR for 18 months. The diet composition was diverse, largely vegetarian, very low in fat (~10% energy from fat) and provided adequate protein and high fiber. The CR diet coupled with high levels of physical activity (70–80 hours of high intensity work per week) resulted in a ~15% weight loss (BMI decreased from 23.9 to 19.7 kg/m2). The 8 members of this crew experienced several of the same adaptations previously reported in CR rodents, including marked reductions in levels of insulin, cholesterol, triglycerides and white blood cell count, and an increase in cortisol concentration (Walford et al., 1992; Walford et al., 2002). Furthermore, glucose concentrations (5.1 to 4.1 mmol/L), HDL (62 to 38 mg/dl), systolic (109 to 89 mmHg) and diastolic blood pressures (74 to 58 mmHg) were significantly decreased below values that are considered healthy.
However, CR in these individuals did not result in reductions of serum concentration of IGF-1, testosterone and DHEA-s (Walford et al., 2002). Despite a significant reduction in serum T3 concentrations, this degree of CR produced only a subtle effect on core body temperature, but this may have been masked by technical limitations because the thermostats were not calibrated for temperatures <96 °F (Walford et al., 1999). Interestingly, 24-hr energy expenditure in the Biosphere inhabitants assessed one week and 6 months after exiting the Biosphere enclosure was lower when compared to non-CR subjects who were free-living and with appropriate statistical adjustment for age, sex, fat-free mass (FFM) and fat mass (FM) (Weyer et al., 1999). It should be noted that several years after the first publication, it was discovered that the 8 biospherians most likely endured chronic hypoxia (i.e. oxygen in the internal atmosphere recorded at only ~14%), which may have influenced the observed results and conclusions related to the potential role of the CR diet on some metabolic outcomes (Paglia and Walford, 2005). For example, chronic environmental (hypobaric) hypoxia has been reported to lead to weight loss and reduced appetite in obese men (Lippl et al., 2010). Furthermore, chronic hypoxia is also known to induce favorable effects on insulin sensitivity (Jain et al., 2016; Lecoultre et al., 2013).
CRON Study
Currently, the only direct evidence that CR may influence the biology of aging in humans comes from data collected in members of the Calorie Restriction Society, who follow a regimen of self-imposed severe CR with optimal nutrition (CRON) in the belief that this dietary lifestyle will prolong their healthy lifespan. These individuals are very lean (BMI 19.7±1.8 kg/m2) men and women, who had been voluntary restricting their caloric intake (~1800 kcal/d) for an average of 15 years, and consume approximately 30% less energy as compared to a group of individuals (matched for age, sex and socioeconomic status) consuming a regular Western diet. Importantly, their diet meets all dietary recommendations for essential nutrients, and is very high in vegetable fiber and low glycemic foods packed with a wide variety of phytochemicals, which may modulate metabolic health independently of caloric intake (Fontana et al., 2004).
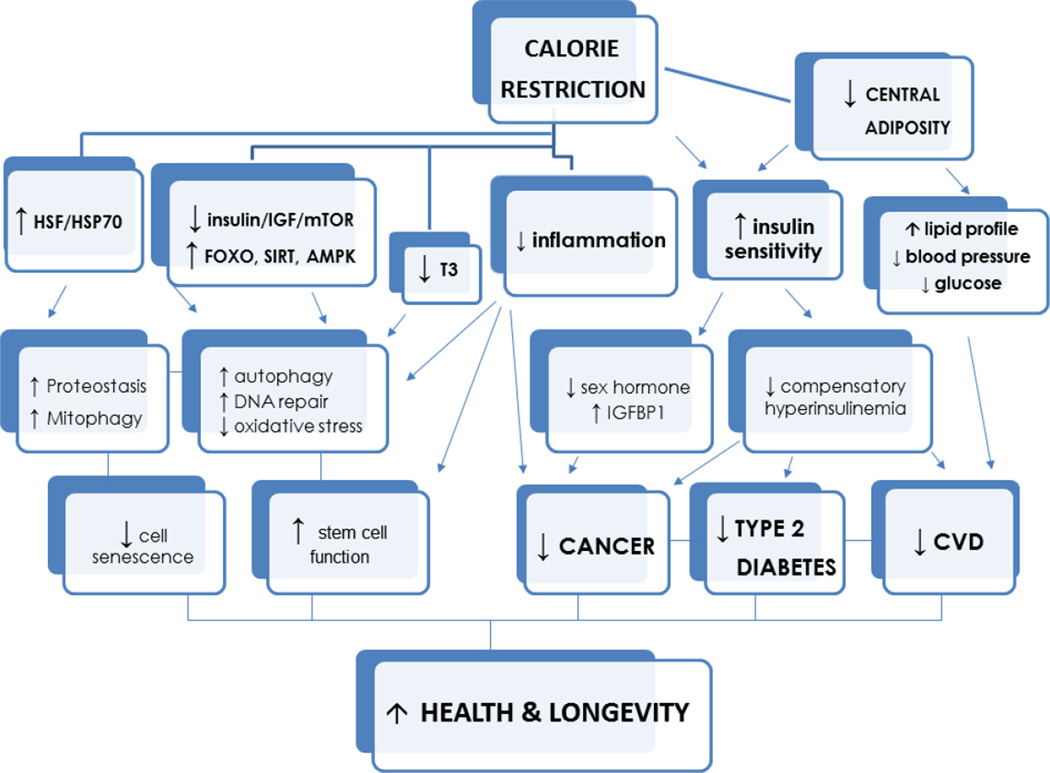
While data on longevity and mortality are not yet available, data indicate that moderate/severe CR in humans results in the same metabolic and molecular adaptations typical of long-lived CR animals (Fig.1). Moreover, CR with optimal intake of nutrients in these individuals reduces metabolic and hormonal risk factors implicated in the pathogenesis of type 2 diabetes, cardiovascular disease, stroke, cancer and vascular dementia. All cardiometabolic risk factors in the members of the CR Society are remarkably low (Fontana et al., 2004). The cholesterol-HDL ratio was 2.6 with triglycerides in the 50 mg/dl range. Systolic and diastolic blood pressure, even in people in their late 70s, was 110/70 mmHg, and C-reactive protein almost undetectable (Fontana et al., 2004; Meyer et al., 2006). Serum TNFα, IL6, fasting glucose and insulin were also remarkably low, and insulin sensitivity based on HOMA-IR and the Matsuda and DeFronzo indexes were improved (Fontana et al., 2010). Historical data from their medical records prior to starting CR strongly supports a cause-effect diet-induced beneficial adaptation on cardiovascular risk (Holloszy and Fontana, 2007). As a consequence of these cardiometabolic improvements, the intima-media thickness of the common carotid arteries was significantly lower in these CR individuals than in the age-matched controls eating usual Western diets (Fontana et al., 2004). Additionally, the CRONies had significantly better echocardiographic markers of left ventricular diastolic function (i.e. lower chamber stiffness and augmented viscoelasticity), and improved autonomic function with a reduction in sympathetic and increased in parasympathetic modulation of heart rate variability (Fontana et al., 2007a; Meyer et al., 2006; Stein et al., 2012). Heart rate variability in the CR practitioners was comparable with published norms for healthy men and women 20 years younger (Stein et al., 2012).
Fig.1.
A proposed hierarchical model for the effects of calorie restriction on health and longevity
Unlike in the CALERIE trials, the great majority of the hormonal adaptations that have been reported in long-lived CR rodents, and are also implicated in the pathogenesis of several common cancers (Longo and Fontana, 2010), occurred in these individuals practicing severe CR. Plasma triiodothyronine concentration, and as a consequence 24-hr core body temperature, were significantly lower in the CR society members (Fontana et al., 2006). Total and free testosterone and estradiol were also lower, and serum SHBG, adiponectin and cortisol concentration were higher in the CR group than in the age- and sex- matched control group. Consistent with the study of Biosphere 2 inhabitants, long-term CR in the CRONies did not affect DHEAS concentrations (Cangemi et al., 2010). However, unlike in rodents in which CR decreases circulating IGF-1 levels by ~20–40%, even in these individuals severe CR did not decrease total IGF-1 or IGF-1/IGFBP-3 ratio levels, unless protein intake was also reduced (Fontana et al., 2008; Sonntag et al., 1999).
At a molecular levels metabolic pathways that modulate the accumulation of molecular damage such as the PI3K/AKT and AMPK/SIRT pathway were changed by CR to an extent that resembles younger individuals, which again indicates the anti-aging potential of CR (Mercken et al., 2013). In particular, AKT mRNA expression and protein phosphorylation were significantly reduced by CR in the skeletal muscle, and transcription factors downstream of AKT, such as FOXO-3A and FOXO-4, were up-regulated. FOXO activation resulted in up-regulation of several anti-aging genes, including the antioxidant enzyme SOD2, the DNA repair transcript DDB1, and the autophagy genes beclin-1 and LC3 (Mercken et al., 2013). Consistent with some of these gene expression changes, beclin-1 and LC3 protein levels were significantly higher in the skeletal muscle of the CR volunteers (Yang et al., 2016). Moreover, key stress-induced cytosolic chaperones transcript and protein levels, such as HSP70 and GRP78, were significantly higher in the CR skeletal muscle (Yang et al., 2016). These data strongly suggest that CR in humans is associated with an increase in key molecular chaperones and autophagic mediators involved in cellular protein quality control and removal of dysfunctional proteins and organelles. To our knowledge, this is the first set of data showing that long-term CR in humans up-regulates the HSF/HSP pathway and down-regulates the activity of the insulin/IGF pathway, which have been shown to play a key roles in promoting health and longevity in several experimental model organisms (Hsu et al., 2003; Kenyon et al., 1993; Yokoyama et al., 2002).
INSIGHTS FROM STUDIES OF EXTREME CALORIE RESTRICTION
It is well established that energy restriction lacking sufficient protein and micronutrients is associated with, but not limited to, short stature, late reproductive maturation (Eveleth and Tanner, 1990), lower baseline gonadal steroid production in adults (Ellison, 1996a, b), suppressed ovarian function (Ellison et al., 1993), impaired lactation performance (Roberts et al., 1982), impaired fecundity (Lee, 1979), and impaired immune function (Martorell, 1980; Ulijaszek, 1990). However, the health effects of extreme CR without protein and vitamin malnutrition in humans are less understood. Data from patients with anorexia nervosa - a psychiatric disease characterized by disturbed body image, heightened desire to lose more weight, and pervasive fear of fatness – indicate that extreme CR (i.e. weight <75% of the expected weight) may be associated with increased mortality, hypokalemia, hypophosphatemia, symptomatic hypoglycemia, dehydration, orthostatic hypotension, hypothermia (temperature <36.1°C), impaired menstrual and reproductive function, anemia, osteoporotic bone fractures, and cardiac arrhythmias (Fairburn and Harrison, 2003). The effects of extreme CR in metabolically and psychologically healthy individuals have been studied in the Minnesota Semi-Starvation Study.
The Minnesota Semi-Starvation Experiment
The pioneering ‘Minnesota Starvation Experiment’ by Ancel Keys (Kalm and Semba, 2005; Keys et al., 1950) is the only human study describing the physical and psychological effects of extreme CR in a clinical experiment. Designed to mimic dietary conditions during World War II, 32 lean and young 24 years old conscientious objectors were carefully studied during a 40% reduction in energy intake for 6 months. Participants were allowed to consume 1800 kcal/d, but were expected to walk 5 km/d and expend 3000 kcal/d. At the conclusion of the study, the men had lost ~25% body weight, of which 67% was fat mass (FM) and 17% fat-free mass. To properly reflect the diet during war, the quality of the diet was poor owing to the inadequate intake of protein (<0.8 g/kg body mass/d) as well as insufficient intake of fruits and vegetables. Therefore while this may be considered a study of extreme CR, the study diet was not indicative of true CR diets, which meet intake recommendations for macro- and micronutrients. The malnourished CR diet led to chronic weakness, reduced aerobic capacity, and severe painful lower limb edema (Keys et al., 1950). Abnormal psychological behaviors which emerged by 6 weeks included severe emotional distress, confusion, apathy, depression, hysteria, hypochondriasis, suicidal thoughts, and loss of sex drive. This study was concluded when these young men reached the 25% target weight loss by 6 months at average body mass index of 16. With refeeding, mood, behavior, and muscle mass very slowly normalized (Kalm and Semba, 2005). Interestingly, 50% of these objectors who survived to age 80 lived at least 8 years longer than expected for men born in 1920 (Kalm and Semba, 2005).
Psychological and mental capacity
In contrast to the Minnesota Semi-Starvation Experiment, well-controlled studies with a lesser degree of CR with adequate nutrition and interventions providing behavioral support to participants have reported no adverse effects of CR on psychological and mental capacity. For example, mood and mental performance were unchanged after 20% CR for 10 weeks (Velthuis-te Wierik et al., 1994) and cognitive performance, memory, attention and concentration were not affected by 6 months of 25% CR in CALERIE-1 (Martin et al., 2007a). Moreover, a 25% CR did not lead to the development of clinical signs or symptoms of eating disorders or binge eating (Williamson et al., 2008). Despite having higher dietary restraint scores the CR subjects also had reduced disinhibition regarding food and fewer concerns about body weight. Recent data from CALERIE-2 consistently showed, that mild CR for 2 years had no negative effects on health-related quality of life, based on assessments of vitality, mental health and bodily pain (SF-36) (Martin et al., 2016). Moreover, measures of mood were unaffected (anger, fatigue, confusion) or even improved (depression, less tension) in the CR-group as compared to the control group. Also, quality of sleep (assessed by PSQI) and sexual function (DISF-SR) were not affected or even improved (sexual drive and relationship). Nonetheless, more severe energy restriction such as in some members of the CR Society may result in reduction of libido, which seems to correlate with the reduction in circulating testosterone levels.
Bone mineral density and metabolism
No untoward effect of CR was observed on bone mass after 6 months of CR, but turnover increased, which may relate to the altered hormonal environment caused by the reduced body mass (Redman et al., 2008). In the longer term CALERIE-2 trial, bone mass significantly decreased at clinically important sites of osteoporotic fractures such as the hip and femoral neck and the lumbar spine (Villareal et al., 2016). Changes in body composition, macronutrient intake, physical activity and plasma hormones (Vitamin A and D, cortisol, IGF-1, adiponectin and leptin) explained 31% of these changes in a multiple regression analysis. These findings clearly represent a potential limitation of the implementation of CR perhaps in some older persons where accelerated bone loss is a cause for concern. Longer term studies should assess trabecular bone architecture (a major determinant of bone strength and a good predictor of the risk of developing fragility fracture) to ensure that bone quality is preserved with CR, and whether the observed reductions in bone mass indeed contribute to increased fracture risk. However, data from members of the CR Society have shown that trabecular bone microarchitecture of the distal radius (i.e. surface-to-curve ratio and erosion index) assessed by high resolution MRI was not significantly different between the CR and control groups, despite the CR-mediated low bone mineral density (Villareal et al., 2011). Serum CTX-1 and bone specific ALP, two well-accepted markers of bone resorption and formation, were also not significantly different between the two groups, which suggest that long-term CR with adequate micronutrient intake does not persistently increase the rate of bone turnover (Villareal et al., 2011). These results are consistent with the majority of studies published so far indicating that CR reduces bone mineral density in both rodents and monkeys, but improves bone quality and strength through a reduction of bone turnover and a prevention of secondary hyperparathyroidism (Kalu et al., 1984; Tatsumi et al., 2008). More studies are needed to understand the interactions between CR, dietary micronutrient content and resistance exercise in preserving bone health.
Physical functioning
In short-term CR studies, in which nutritional requirements are met, a reduced physical functioning was indicated by a reduced cycling time until exhaustion and reduced maximal power (Velthuis-te Wierik et al., 1995b). These findings are in line with CALERIE-1, which reported declines in muscle mass, strength and aerobic fitness, which were assessed by MRI of both thighs, concentric isokinetic and isometric knee flexor and knee extensor strength by dynamometer and absolute maximal aerobic capacity (VO2max and VO2peak) by a progressive treadmill test (Larson-Meyer et al., 2010; Weiss et al., 2007). However, VO2peak/VO2max expressed per kilogram of body mass were maintained or increased during CR, suggesting that the reduction in body weight may preserve or even beneficially affect physical functioning. A similar effect of CR on aerobic fitness has been observed in older and more obese subjects (>60 yrs, BMI≥30kg/m2) with heart failure with preserved ejection fraction (>50%) (Kitzman et al., 2016). Despite decreased body mass and lean body mass (−7 and −2%), CR by 400 up to 1000 kcal/d significantly increased VO2peak (expressed per kg body weight or per lean body mass, but not expressed without correction), 6-min walking distance and leg muscle quality (expressed in Watts per cm2 thigh muscle area). In addition, quality of life improved based on data on increased Short Form 36 Health survey physical component scores, reduced MEADS depression scores, and physical functioning improved after 4, 6 or 24 months of ~25% CR in metabolically compromised (Kitzman et al., 2016) and healthy participants (Ravussin et al., 2015; Williamson et al., 2008).
BEYOND SEVERE CALORIE RESTRICTION
Data from the CALERIE trials, the Biosphere-2 and CRON studies clearly indicate that moderate CR with adequate nutrition improves human health and drastically reduces multiple metabolic factors implicated in the pathogenesis of the most common chronic diseases typical of Western countries, including type 2 diabetes, heart and cerebrovascular disease, and cancer. Accumulating data suggest that more severe CR may slow, or even revert, the accumulation of molecular damage with age and preserve some key physiological functions (i.e. left ventricular diastolic function and heart rate variability) at a younger stage (Mercken et al., 2013; Meyer et al., 2006; Stein et al., 2012; Yang et al., 2016). However, severe CR with optimal intake of vitamins and minerals is not feasible for most people because it is impractical and very difficult to sustain. Side effects such as extreme leanness, loss of sex drive, cold sensitivity and impaired menstrual cycles are also important deterrents to severe CR. Moreover, it is currently unknown which is the ideal caloric intake associated with optimal health. As studies in recombinant inbred mouse strains suggest (Harper et al., 2006; Liao et al., 2010), a definite degree of energy restriction that is ideal for some individuals might be excessive and detrimental in others. Interestingly, accumulating data suggest that severe CR possibly is not needed, other interventions such as intermittent fasting, restricted feeding, protein or selective amino acid restriction may recapture some of the beneficial effects of severe CR in modulating certain anti-aging pathways (Fontana and Partridge, 2015). The effects and mechanisms through which intermittent fasting, restricted feeding, protein or selective amino acid restriction modulates health and lifespan in model organisms will be discussed in more detail in two separate review articles of this special issue of Ageing Research Review (ref #3, ref #4).
CONCLUSION
Data from epidemiological, experimental and clinical studies strongly indicate that maintaining a healthy body weight and preventing the accumulation of abdominal fat is essential for the prevention of multiple chronic diseases and the promotion of healthy aging (Fontana and Hu, 2014). Hundreds of preclinical studies have shown that dietary restriction, by inhibiting key nutrient-sensing and inflammatory pathways, activates multiple molecular pathways that promote proteostasis, genome stability, stress resistance and stem cell function (Fontana and Partridge, 2015). Data collected in non-human primates indicate that CR in combination with diet quality modifications markedly decrease the incidence of cardiovascular disease, cancer and diabetes, and attenuates age-related neurodegeneration, sarcopenia, and auditory loss. Finally, data from human studies show that CR remains the cornerstone in the prevention and treatment of obesity and its complications. Moderate CR achieved through intermittent fasting or restricting feeding in combination with regular physical activity most likely exerts additional beneficial health effects even in non-obese individuals. More studies are warranted to elucidate the role of specific amino acid restriction with and without CR, and the effects of nutritional modulation of gut microbiome in promoting health and longevity in humans.
Highlights.
Well-nourished calorie restriction promotes metabolic and molecular health in non-obese humans
Calorie restriction reduces aging-associated biomarkers in humans
Calorie restriction induces metabolic adaptation and behavioral compensation in physical activity
Calorie restriction induces no adverse effects on quality of life or eating behavior
Acknowledgments
We apologize for the omission of relevant works due to space constraints. This work was funded in part by the National Institutes of Health in support of the CALERIE studies: U01-AG-020487, U01-AG-020480, U01-AG-022132 R01, AG029914 (Ravussin, E); U01 AG020478 (Ravussin, E). Dr. Fontana’s research was also supported by grants from the Bakewell Foundation, the Longer Life Foundation (an RGA/Washington University Partnership), the National Center for Research Resources (UL1 RR024992), and the European Union’s Seventh Framework Programme MOPACT (Mobilising the Potential of Active Ageing in Europe; FP7-SSH-2012-1 grant agreement No. 320333).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
No conflict of interest has to be disclosed.
References
- Ahmed T, Das SK, Golden JK, Saltzman E, Roberts SB, Meydani SN. Calorie restriction enhances T-cell-mediated immune response in adult overweight men and women. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2009;64:1107–1113. doi: 10.1093/gerona/glp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand SS, Yi Q, Gerstein H, Lonn E, Jacobs R, Vuksan V, Teo K, Davis B, Montague P, Yusuf S. Relationship of metabolic syndrome and fibrinolytic dysfunction to cardiovascular disease. Circulation. 2003;108:420–425. doi: 10.1161/01.CIR.0000080884.27358.49. [DOI] [PubMed] [Google Scholar]
- Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83:356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- Bureau of Agricultural Economics, U.S. Supplement for 1949 to Consumption of food in the United States, 1909–48. In: U.S. Dept. of Agriculture, B.o.A.E., editor. Miscellaneous publication (United States. Dept. of Agriculture) Washington, D.C.: 1950. p. 52. [Google Scholar]
- Cangemi R, Friedmann AJ, Holloszy JO, Fontana L. Long-term effects of calorie restriction on serum sex-hormone concentrations in men. Aging Cell. 2010;9:236–242. doi: 10.1111/j.1474-9726.2010.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cava E, Fontana L. Will calorie restriction work in humans? Aging. 2013;5:507–514. doi: 10.18632/aging.100581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nature communications. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, Dallal GE, Dutta C, Bhapkar MV, Delany JP, Saltzman E, Roberts SB. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007a;85:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, Dallal GE, Dutta C, Bhapkar MV, Delany JP, Saltzman E, Roberts SB. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. American Journal of Clinical Nutrition. 2007b;85:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- Ellison PT. Age and developmental effects on adult ovarian function. In: Rosetta L, Mascie-Taylor NCG, editors. Variability in Human Fertility: A Biological Anthropological Approach. New York: Cambridge University Press; 1996a. pp. 69–90. [Google Scholar]
- Ellison PT. Developmental influences on adult ovarian function. Am J Human Biol. 1996b:725–734. doi: 10.1002/(SICI)1520-6300(1996)8:6<725::AID-AJHB4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Ellison PT, Panter-Brick C, Lipson SF, O'Rourke MT. The ecological context of human ovarian function. Hum Reprod. 1993:2248–2258. doi: 10.1093/oxfordjournals.humrep.a138015. [DOI] [PubMed] [Google Scholar]
- Eveleth PB, Tanner JM. Worldwide Variation in Human Growth. 2nd. New York: Cambridge University Press; 1990. [Google Scholar]
- Fairburn CG, Harrison PJ. Eating disorders. Lancet. 2003;361:407–416. doi: 10.1016/S0140-6736(03)12378-1. [DOI] [PubMed] [Google Scholar]
- Fontana L, Hu FB. Optimal body weight for health and longevity: bridging basic, clinical, and population research. Aging Cell. 2014;13:391–400. doi: 10.1111/acel.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age. 2010;32:97–108. doi: 10.1007/s11357-009-9118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. The Journal of clinical endocrinology and metabolism. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161:106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Villareal DT, Das SK, Smith SR, Meydani SN, Pittas AG, Klein S, Bhapkar M, Rochon J, Ravussin E, Holloszy JO. Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell. 2016;15:22–27. doi: 10.1111/acel.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. American journal of physiology. Endocrinology and metabolism. 2007a;293:E197–E202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. American Journal of Physiology Endocrinology and Metabolism. 2007b;293:E197–E202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr, O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- Frankenfield DC, Muth ER, Rowe WA. The Harris-Benedict studies of human basal metabolism: history and limitations. Journal of the American Dietetic Association. 1998;98:439–445. doi: 10.1016/S0002-8223(98)00100-X. [DOI] [PubMed] [Google Scholar]
- Garby L, Kurzer MS, Lammert O, Nielsen E. Effect of 12 weeks' light-moderate underfeeding on 24-hour energy expenditure in normal male and female subjects. European Journal of Clinical Nutrition. 1988;42:295–300. [PubMed] [Google Scholar]
- Harper JM, Leathers CW, Austad SN. Does caloric restriction extend life in wild mice? Aging Cell. 2006;5:441–449. doi: 10.1111/j.1474-9726.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Heyman MB, Young VR, Fuss P, Tsay R, Joseph L, Roberts SB. Underfeeding and body weight regulation in normal-weight young men. Am J Physiol. 1992;263:R250–R257. doi: 10.1152/ajpregu.1992.263.2.R250. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Childers D, Beetsch J, Allison DB, Pietrobelli A. Body size and human energy requirements: reduced mass-specific resting energy expenditure in tall adults. Journal of applied physiology. 2007;103:1543–1550. doi: 10.1152/japplphysiol.00461.2007. [DOI] [PubMed] [Google Scholar]
- Hindhede M. The effect of food restriction during war on mortality in Copenhagen. Journal of the American Medical Association. 1920;74:381–382. [Google Scholar]
- Hofer T, Fontana L, Anton SD, Weiss EP, Villareal D, Malayappan B, Leeuwenburgh C. Long-term effects of caloric restriction or exercise on DNA and RNA oxidation levels in white blood cells and urine in humans. Rejuvenation research. 2008;11:793–799. doi: 10.1089/rej.2008.0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO, Fontana L. Caloric restriction in humans. Experimental gerontology. 2007;42:709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Jain IH, Zazzeron L, Goli R, Alexa K, Schatzman-Bone S, Dhillon H, Goldberger O, Peng J, Shalem O, Sanjana NE, Zhang F, Goessling W, Zapol WM, Mootha VK. Hypoxia as a therapy for mitochondrial disease. Science. 2016;352:54–61. doi: 10.1126/science.aad9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japan Ministry of Health, L.a.W. Journal of Health and Welfare Statistics. Tokyo: Health and Welfare Statistics Association; 2005. [Google Scholar]
- Johnson ML, Distelmaier K, Lanza IR, Irving BA, Robinson MM, Konopka AR, Shulman GI, Nair KS. Mechanism by Which Caloric Restriction Improves Insulin Sensitivity in Sedentary Obese Adults. Diabetes. 2016;65:74–84. doi: 10.2337/db15-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y. Impact of Westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians. Preventive medicine. 1978;7:205–217. doi: 10.1016/0091-7435(78)90246-3. [DOI] [PubMed] [Google Scholar]
- Kalm LM, Semba RD. They starved so that others be better fed: remembering Ancel Keys and the Minnesota experiment. The Journal of nutrition. 2005;135:1347–1352. doi: 10.1093/jn/135.6.1347. [DOI] [PubMed] [Google Scholar]
- Kalu DN, Hardin RR, Cockerham R, Yu BP, Norling BK, Egan JW. Lifelong food restriction prevents senile osteopenia and hyperparathyroidism in F344 rats. Mechanisms of ageing and development. 1984;26:103–112. doi: 10.1016/0047-6374(84)90169-6. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Keys AB, Brozek J, Henschel A, Mickelson O, Taylor A. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950. [Google Scholar]
- Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA : the journal of the American Medical Association. 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Redman L, Heilbronn LK, Martin CK, Ravussin E. Caloric restriction with or without exercise: the fitness versus fatness debate. Med Sci Sports Exerc. 2010;42:152–159. doi: 10.1249/MSS.0b013e3181ad7f17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoultre V, Peterson CM, Covington JD, Ebenezer PJ, Frost EA, Schwarz JM, Ravussin E. Ten nights of moderate hypoxia improves insulin sensitivity in obese humans. Diabetes Care. 2013;36:e197–e198. doi: 10.2337/dc13-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoultre V, Ravussin E, Redman LM. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. The Journal of clinical endocrinology and metabolism. 2011;96:E1512–E1516. doi: 10.1210/jc.2011-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RB. The !Kung San: Men, Women, and Work in a Foraging Society. Chicago, IL: University of Chicago Press; 1979. [Google Scholar]
- Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin E. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203:206–213. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippl FJ, Neubauer S, Schipfer S, Lichter N, Tufman A, Otto B, Fischer R. Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity. 2010;18:675–681. doi: 10.1038/oby.2009.509. [DOI] [PubMed] [Google Scholar]
- Loft S, Velthuis-te Wierik EJ, van den Berg H, Poulsen HE. Energy restriction and oxidative DNA damage in humans. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1995;4:515–519. [PubMed] [Google Scholar]
- Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends in pharmacological sciences. 2010;31:89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Anton SD, Han H, York-Crowe E, Redman LM, Ravussin E, Williamson DA. Examination of cognitive function during six months of calorie restriction: results of a randomized controlled trial. Rejuvenation research. 2007a;10:179–190. doi: 10.1089/rej.2006.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Bhapkar M, Pittas AG, Pieper CF, Das SK, Williamson DA, Scott T, Redman LM, Stein R, Gilhooly CH, Stewart T, Robinson L, Roberts SB. Effect of Calorie Restriction on Mood, Quality of Life, Sleep, and Sexual Function in Healthy Nonobese Adults: The CALERIE 2 Randomized Clinical Trial. JAMA internal medicine. 2016 doi: 10.1001/jamainternmed.2016.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Heilbronn LK, de Jonge L, DeLany JP, Volaufova J, Anton SD, Redman LM, Smith SR, Ravussin E. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity. 2007b;15:2964–2973. doi: 10.1038/oby.2007.354. [DOI] [PubMed] [Google Scholar]
- Martorell R. Interrelationships between diet, infectious disease, and nutritional status. In: Greene LS, Johnston FE, editors. Social and Biological Predictors of Nutritional Status, Physical Growth, and Neurological Development. New York: Academic Press; 1980. pp. 81–106. [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Crosby SD, Lamming DW, JeBailey L, Krzysik-Walker S, Villareal DT, Capri M, Franceschi C, Zhang Y, Becker K, Sabatini DM, de Cabo R, Fontana L. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12:645–651. doi: 10.1111/acel.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. Journal of the American College of Cardiology. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Muller MJ, Enderle J, Pourhassan M, Braun W, Eggeling B, Lagerpusch M, Gluer CC, Kehayias JJ, Kiosz D, Bosy-Westphal A. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. Am J Clin Nutr. 2015;102:807–819. doi: 10.3945/ajcn.115.109173. [DOI] [PubMed] [Google Scholar]
- Paglia DE, Walford RL. Atypical hematological response to combined calorie restriction and chronic hypoxia in Biosphere 2 crew: a possible link to latent features of hibernation capacity. Habitation. 2005;10:79–85. doi: 10.3727/154296605774791223. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas AG, Roberts SB, Das SK, Gilhooly CH, Saltzman E, Golden J, Stark PC, Greenberg AS. The effects of the dietary glycemic load on type 2 diabetes risk factors during weight loss. Obesity. 2006;14:2200–2209. doi: 10.1038/oby.2006.258. [DOI] [PubMed] [Google Scholar]
- Racette SB, Das SK, Bhapkar M, Hadley EC, Roberts SB, Ravussin E, Pieper C, DeLany JP, Kraus WE, Rochon J, Redman LM. Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. American journal of physiology. Endocrinology and metabolism. 2012;302:E441–E448. doi: 10.1152/ajpendo.00290.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. The journals of gerontology. Series A, Biological sciences and medical sciences. 2006a;61:943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006b;61:943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, Smith SR, Stein RI, Scott TM, Stewart TM, Saltzman E, Klein S, Bhapkar M, Martin CK, Gilhooly CH, Holloszy JO, Hadley EC, Roberts SB. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. The journals of gerontology. Series A, Biological sciences and medical sciences. 2015;70:1097–1104. doi: 10.1093/gerona/glv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. The Journal of clinical endocrinology and metabolism. 2007;92:865–872. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PloS one. 2009;4:e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Rood J, Anton SD, Champagne C, Smith SR, Ravussin E. Calorie restriction and bone health in young, overweight individuals. Archives of Internal Medicine. 2008;168:1859–1866. doi: 10.1001/archinte.168.17.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Smith SR, Dixit V, Ravussin E. Evidence of Metabolic Adaptation after 2 Years of 25% Calorie Restriction in Non-Obese Humans. The Obesity Week conference. 2014 Abstract T-3032-OR. [Google Scholar]
- Redman LM, Veldhuis JD, Rood J, Smith SR, Williamson D, Ravussin E, Pennington CT. The effect of caloric restriction interventions on growth hormone secretion in nonobese men and women. Aging Cell. 2010;9:32–39. doi: 10.1111/j.1474-9726.2009.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman AD, Williamson DA, Martin CK, Gilhooly CH, Stein RI, Bales CW, Roberts S, Das SK. The CALERIE Study: design and methods of an innovative 25% caloric restriction intervention. Contemporary clinical trials. 2011;32:874–881. doi: 10.1016/j.cct.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan MM, Weiss EP, Meyer TE, Ehsani AA, Racette SB, Villareal DT, Fontana L, Holloszy JO, Kovacs SJ. The effects of caloric restriction- and exercise-induced weight loss on left ventricular diastolic function. American Journal of Physiology Heart and Circulatory Physiology. 2008;294:H1174–H1182. doi: 10.1152/ajpheart.01236.2007. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Paul AA, Cole TJ, Whitehead RG. Seasonal changes in activity, birth weight and lactational performance in rural Gambian women. Trans R Soc Trop Med Hyg. 1982:668–678. doi: 10.1016/0035-9203(82)90239-5. [DOI] [PubMed] [Google Scholar]
- Rochon J, Bales CW, Ravussin E, Redman LM, Holloszy JO, Racette SB, Roberts SB, Das SK, Romashkan S, Galan KM, Hadley EC, Kraus WE. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. The journals of gerontology. Series A, Biological sciences and medical sciences. 2011;66:97–108. doi: 10.1093/gerona/glq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romashkan SV, Das SK, Villareal DT, Ravussin E, Redman LM, Rochon J, Bhapkar M, Kraus WE. Safety of two-year caloric restriction in non-obese healthy individuals. Oncotarget. 2016;7:19124–19133. doi: 10.18632/oncotarget.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cefalu WT, Ingram RL, Bennett SA, Thornton PL, Khan AS. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. The journals of gerontology. Series A, Biological sciences and medical sciences. 1999;54:B521–B538. doi: 10.1093/gerona/54.12.b521. [DOI] [PubMed] [Google Scholar]
- Stein PK, Soare A, Meyer TE, Cangemi R, Holloszy JO, Fontana L. Caloric restriction may reverse age-related autonomic decline in humans. Aging Cell. 2012;11:644–650. doi: 10.1111/j.1474-9726.2012.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom A, Jensen RA. Mortality from circulatory diseases in Norway 1940–1945. Lancet. 1951;1:126–129. doi: 10.1016/s0140-6736(51)91210-x. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Wilcox BJ, Wilcox CD. Implications from and for food cultures for cardiovascular disease: longevity. Asia Pac J Clin Nutr. 2001;10:165–171. doi: 10.1111/j.1440-6047.2001.00219.x. [DOI] [PubMed] [Google Scholar]
- System of Social and Demographic Statistics. Health and Medical Care. Ministry of Internal Affairs and Communications. 2016 [Google Scholar]
- Tam CS, Covington JD, Ravussin E, Redman LM. Little evidence of systemic and adipose tissue inflammation in overweight individuals(dagger) Frontiers in genetics. 2012;3:58. doi: 10.3389/fgene.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi S, Ito M, Asaba Y, Tsutsumi K, Ikeda K. Life-long caloric restriction reveals biphasic and dimorphic effects on bone metabolism in rodents. Endocrinology. 2008;149:634–641. doi: 10.1210/en.2007-1089. [DOI] [PubMed] [Google Scholar]
- Tofler GH, Massaro J, O'Donnell CJ, Wilson PW, Vasan RS, Sutherland PA, Meigs JB, Levy D, D'Agostino RB., Sr Plasminogen activator inhibitor and the risk of cardiovascular disease: The Framingham Heart Study. Thrombosis research. 2016;140:30–35. doi: 10.1016/j.thromres.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulijaszek SJ. Nutritional status and susceptibility to infectious disease. In: Harrison GA, Waterlow JC, editors. Diet and Disease. New York: Cambridge University Press; 1990. pp. 137–154. [Google Scholar]
- Velthuis-te Wierik EJ, Meijer P, Kluft C, van den Berg H. Beneficial effect of a moderately energy-restricted diet on fibrinolytic factors in non-obese men. Metabolism: clinical and experimental. 1995a;44:1548–1552. doi: 10.1016/0026-0495(95)90073-x. [DOI] [PubMed] [Google Scholar]
- Velthuis-te Wierik EJ, van den Berg H, Schaafsma G, Hendriks HF, Brouwer A. Energy restriction, a useful intervention to retard human ageing? Results of a feasibility study. European Journal of Clinical Nutrition. 1994;48:138–148. [PubMed] [Google Scholar]
- Velthuis-te Wierik EJ, van Leeuwen RE, Hendriks HF, Verhagen H, Loft S, Poulsen HE, Van den Berg H. Short-term moderate energy restriction does not affect indicators of oxidative stress and genotoxicity in humans. The Journal of nutrition. 1995b;125:2631–2639. doi: 10.1093/jn/125.10.2631. [DOI] [PubMed] [Google Scholar]
- Velthuis-te Wierik EJ, Westerterp KR, van den Berg H. Impact of a moderately energy-restricted diet on energy metabolism and body composition in non-obese men. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1995c;19:318–324. [PubMed] [Google Scholar]
- Villareal DT, Fontana L, Das SK, Redman L, Smith SR, Saltzman E, Bales C, Rochon J, Pieper C, Huang M, Lewis M, Schwartz AV. Effect of Two-Year Caloric Restriction on Bone Metabolism and Bone Mineral Density in Non-Obese Younger Adults: A Randomized Clinical Trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2016;31:40–51. doi: 10.1002/jbmr.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Archives of Internal Medicine. 2006;166:2502–2510. doi: 10.1001/archinte.166.22.2502. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Kotyk JJ, Armamento-Villareal RC, Kenguva V, Seaman P, Shahar A, Wald MJ, Kleerekoper M, Fontana L. Reduced bone mineral density is not associated with significantly reduced bone quality in men and women practicing long-term calorie restriction with adequate nutrition. Aging Cell. 2011;10:96–102. doi: 10.1111/j.1474-9726.2010.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford RL, Harris SB, Gunion MW. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proc Natl Acad Sci U S A. 1992;89:11533–11537. doi: 10.1073/pnas.89.23.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford RL, Mock D, MacCallum T, Laseter JL. Physiologic changes in humans subjected to severe, selective calorie restriction for two years in biosphere 2: health, aging, and toxicological perspectives. Toxicological sciences : an official journal of the Society of Toxicology. 1999;52:61–65. [PubMed] [Google Scholar]
- Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. The journals of gerontology. Series A, Biological sciences and medical sciences. 2002;57:B211–B224. doi: 10.1093/gerona/57.6.b211. [DOI] [PubMed] [Google Scholar]
- Webb P, Abrams T. Loss of fat stores and reduction in sedentary energy expenditure from undereating. Human nutrition. Clinical nutrition. 1983;37:271–282. [PubMed] [Google Scholar]
- Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Ehsani AA, Holloszy JO. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. Journal of applied physiology. 2007;102:634–640. doi: 10.1152/japplphysiol.00853.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. American Journal of Clinical Nutrition. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EP, Villareal DT, Racette SB, Steger-May K, Premachandra BN, Klein S, Fontana L. Caloric restriction but not exercise-induced reductions in fat mass decrease plasma triiodothyronine concentrations: a randomized controlled trial. Rejuvenation research. 2008;11:605–609. doi: 10.1089/rej.2007.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43:1498–1506. doi: 10.1007/s001250051560. [DOI] [PubMed] [Google Scholar]
- Weyer C, Snitker S, Rising R, Bogardus C, Ravussin E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1999;23:715–722. doi: 10.1038/sj.ijo.0800910. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Willcox DC, Todoriki H, Fujiyoshi A, Yano K, He Q, Curb JD, Suzuki M. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world's longest-lived people and its potential impact on morbidity and life span. Annals of the New York Academy of Sciences. 2007a;1114:434–455. doi: 10.1196/annals.1396.037. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Willcox DC, Todoriki H, Yano K, Curb D, Suzuki M. Caloric restriction, energy balance and healthy aging in Okinawans and Americans: Biomarker differences in Septuagenarians. The Okinawan Journal of American Studies. 2007b;4:62–74. [PMC free article] [PubMed] [Google Scholar]
- Willcox DC, Willcox BJ, Todoriki H, Curb JD, Suzuki M. Caloric restriction and human longevity: what can we learn from the Okinawans? Biogerontology. 2006;7:173–177. doi: 10.1007/s10522-006-9008-z. [DOI] [PubMed] [Google Scholar]
- Williamson DA, Martin CK, Anton SD, York-Crowe E, Han H, Redman L, Ravussin E. Is caloric restriction associated with development of eating-disorder symptoms? Results from the CALERIE trial. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2008;27:S32–S42. doi: 10.1037/0278-6133.27.1.S32. [DOI] [PubMed] [Google Scholar]
- Yang L, Licastro D, Cava E, Veronese N, Spelta F, Rizza W, Bertozzi B, Villareal DT, Hotamisligil GS, Holloszy JO, Fontana L. Long-Term Calorie Restriction Enhances Cellular Quality-Control Processes in Human Skeletal Muscle. Cell reports. 2016;14:422–428. doi: 10.1016/j.celrep.2015.12.042. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Fukumoto K, Murakami T, Harada S, Hosono R, Wadhwa R, Mitsui Y, Ohkuma S. Extended longevity of Caenorhabditis elegans by knocking in extra copies of hsp70F, a homolog of mot-2 (mortalin)/mthsp70/Grp75. FEBS letters. 2002;516:53–57. doi: 10.1016/s0014-5793(02)02470-5. [DOI] [PubMed] [Google Scholar]