Abstract
The purpose of this study was to determine mitochondrial changes in fast muscles from interleukin-15 receptor alpha knockout (IL-15RαKO) mice. We tested the hypothesis that fast muscles from IL-15RαKO mice would have a greater mitochondrial density and altered internal structure compared to muscles from control mice. In fast muscles from IL-15RαKO mice, mitochondrial density was 48% greater with a corresponding increase in mitochondrial DNA content. Although there were no differences in the relative size of isolated mitochondria, internal complexity was lower in mitochondria from IL-15RαKO mice. These data support an increase in mitochondrial biogenesis and provide direct evidence for a greater density and altered internal structure of mitochondria in EDL muscles deficient in IL-15Rα.
Keywords: interleukin, mitochondria, fast muscle, myokine
1. Introduction
Interleukin-15 (IL-15) is a cytokine that is highly expressed within skeletal muscles [1] and is included in the growing list of myokines, defined as cytokines that are expressed, produced, and/or released by muscle tissue with the ability to exert paracrine or endocrine effects [2]. The high amount of IL-15 mRNA within skeletal muscle, along with the alpha subunit of the IL-15 receptor (IL-15Rα) [1, 3], has led to the suggestion that IL-15-related signaling can extend beyond lymphoid cells of the immune system. Although the specific IL-15 signaling events within skeletal muscle have not been completely characterized, the proposed roles of IL-15 related to skeletal muscle have included: 1) stimulating contractile protein accretion in myogenic cultures [4]; sparing muscle mass during cachexia [5]; and altering body composition by secretion of IL-15 by skeletal muscle [6].
Recent work has demonstrated that genetic manipulation of IL-15 and IL-15Rα in vivo in the absence of exercise training results in a more oxidative muscle phenotype with functional changes similar to fatigue resistant skeletal muscles [6-8]. For example, in a transgenic mouse over-expressing IL-15 within skeletal muscle, increased expression of the slow isoform of troponin-c in the fast extensor digitotum longus (EDL) muscle along with greater expression of proteins associated with energy and metabolic homeostasis such as sirtuin1 (SIRT1), SIRT4, and uncoupling protein-2 (UCP2) were reported [8]. In the total tissue IL-15RαKO mouse, running wheel activity was 6-fold greater than control mice, and the fast EDL muscle displayed a resistance to contraction-induced fatigue. The mechanism for the phenotypic exercise and muscle function changes in IL-15RαKO mice was an increase in mitochondrial biogenesis through greater expression of peroxisome proliferator-activated receptor delta (PPARδ) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), along with greater levels of succinate dehydrogenase (SDH) and cytochrome oxidase subunit Va [7]. Therefore, the purpose of this study was to directly examine differences in the density and structure of mitochondria from fast muscles of IL-15RαKO mice relative to changes in myofiber size. We tested the hypothesis that fast EDL muscles from IL-15RαKO mice would have a greater mitochondrial density and altered internal structure when compared to EDL muscles from control mice.
2. Materials and Methods
2.1. Experimental Animals
IL-15RαKO mice (n=11) and B6129SF2/J control mice (n=11) were utilized for experiments between 9 and 11 weeks of age. Mice were maintained at 22°C under a 12-hour light/12-hour dark cycle and received standard rodent chow and water ab libitum. All experiments were approved by the Institutional Animal Care and Use Committee at West Virginia University (ACUC #: 11-0804).
2.2. Transmission Electron Microscopy (TEM) and Mitochondrial Density Quantification
Mice were sedated using 4% isoflurane, and the EDL muscles were dissected and immediately immersed into 3% glutaraldhyde in 0.1M phosphate buffer, pH 7.4. Electron micrographs were obtained through the West Virginia University Tissue Analysis and Processing Core Facility using standard methods [9]. Sections were viewed in a JEOL 1010 transmission electron microscope at magnifications ranging from 2000X to 20,000X. For mitochondrial quantification, micrographs were obtained at 2000X and mitochondrial density was quantified using a point counting system based on the methods by Weibel [10].
2.3. Mitochondrial DNA Content
Total DNA was extracted from EDL muscles using a Blood and Tissue DNA kit (Qiagen, Valencia, CA). TaqMan primers for mitochondrial DNA-encoded cytochrome-c oxidase subunit II (COXII) and nuclear-encoded 18S ribosomal RNA were used to perform real-time qPCR. The wells of a 96-well optical reaction plate were loaded with a 20μl volume consisting of TaqMan 10X PCR Master Mix, a primer mix for either COXII or 18S, and 5ng.μl-1 DNA. Mitochondrial DNA content was expressed as the ratio of the threshold cycle (CT) values from the mitochondrial-encoded COXII gene and the CT values from the nuclear-encoded 18S gene.
2.4. Flow Cytometry
The relative size and internal complexity of intact mitochondrial were determined using the methods of Dabkowski et al. [11]. Mice were sedated using 4% isoflurane and the tibialis anterior muscles were dissected. Total mitochondria were isolated using a mitochondrial/cytosolic fractionation kit with modifications (BioVision, Mountain View, CA). Isolated mitochondria were incubated with 1.0μl MitoTracker Deep Red 633 (Invitrogen, Carlsbad, CA). Mitochondrial were analyzed in the West Virginia University Flow Cytometry Core Facility using a FACS Calibur flow cytometer (Becton and Dickinson, San Jose, CA). Up to 30,000 gated events were examined using forward scatter and side scatter detectors to indicate relative mitochondrial size and internal complexity, respectively.
2.5. Muscle Morphology
Gastrocnemius muscles from IL-15RαKO mice and B6129 control mice were dissected and frozen in isopentane cooled to the temperature of liquid nitrogen. Sections were fixed in methanol at -20°C, blocked in 5% FBS at room temperature, and incubated with a laminin polyclonal antibody (1:1000 dilution, Sigma, St. Louis, MO) followed by incubation with Alexa Fluor 488 fluorescent secondary antibody (1:1000 dilution, Invitrogen, Carlsbad, CA). Slides were visualized using a Nikon Eclipse E800 fluorescent microscope. A microscope mounted camera (Spot RT Diagnostic Instruments Inc) was used to obtain 5 random images of muscle sections at an objective magnification of 20X. Muscle fiber cross-sectional area was measured in 4000 individual muscle fibers in gastrocnemius muscles from IL-15RαKO mice and B6129 control mice.
2.6. Statistics
All data were analyzed using GraphPad Prism software and are expressed as Mean±SD. Independent t-test was used to determine differences of experimental parameters between IL-15RαKO mice and B6129 control mice. Statistical significance was set a p<0.05.
3. Results
3.1. Mitochondrial density and mitochondrial DNA content in EDL muscles from IL-15RαKO mice
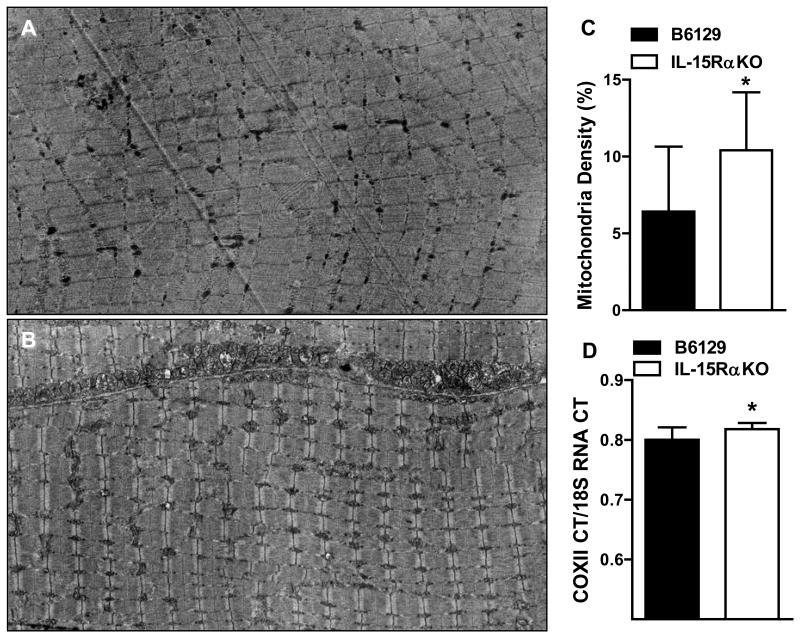
More mitochondria were visible in the fast EDL muscles from IL-15RαKO mice than in B6129 control mice (Figure 1A,B). Mitochondrial density was 48% greater in EDL muscles from IL-15RαKO mice compared to B6129 control mice (Figure 1C). Mitochondrial DNA content was greater in EDL muscles from IL-15RαKO mice (B6129: 0.80±0.007; IL-15RαKO: 0.82±0.004; +2.5%*) (Figure 1D). Collectively, these data provide direct evidence of alterations in skeletal muscle mitochondria and identify a different muscle phenotype in IL-15RαKO mice that may account for the functional differences in exercise and fatigue we previously reported [7].
Figure 1. Mitochondrial density and mitochondrial DNA content in EDL muscles from IL-15RαKO mice.
(A) Representative electron micrograph of an EDL muscle from a B6129 control mouse. (B) Representative electron micrograph of an EDL muscle from an IL-15RαKO mouse. (C) Mitochondrial density was quantified through a point counting system, and was 48% greater in EDL muscles from IL-15RαKO mice compared to B6129 control mice. (D) Mitochondrial DNA content was significantly greater in EDL muscles from IL-15RαKO mice compared to B6129 control mice. *, p<0.05. Images were taken at 2000X.
3.2. Relative mitochondrial size and internal complexity
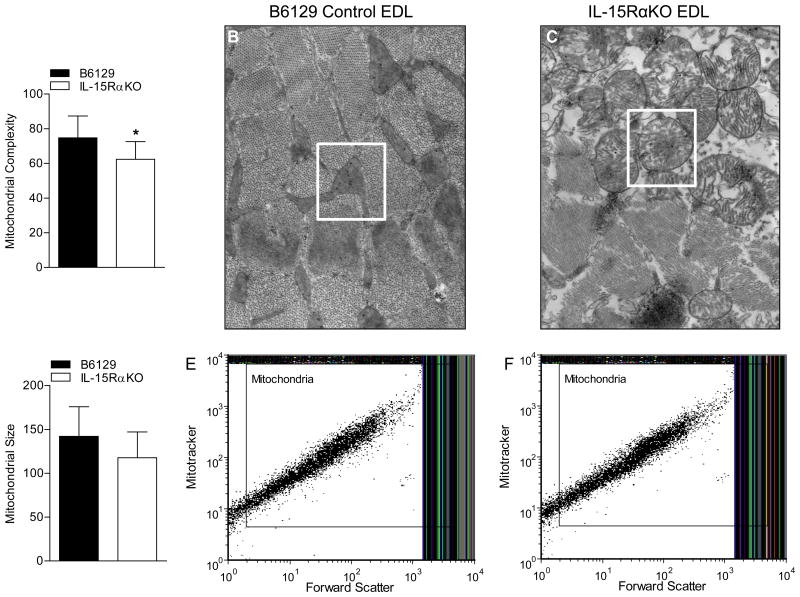
Flow cytometry was performed to analyze the relative size and internal complexity of isolated mitochondria based on forward scatter and side scatter. The degree of internal complexity was significantly less in mitochondria isolated from EDL muscles of IL-15RαKO mice compared to B6129 control mice (Figure 2A). These alterations in the internal structure of mitochondria were confirmed by TEM, revealing differences in the lamellar-like appearance of mitochondria from EDL muscles (Figure 2B, C) as well as soleus muscles (Supplemental Figure 1) from IL-15RαKO mice compared to B6129 control mice. There were no significant differences in the relative size of the total isolated mitochondrial pool from EDL muscles of IL-15RαKO mice and B6129 control mice (Figure 2D-F).
Figure 2. Relative mitochondrial size and internal complexity.
(A) Mitochondrial internal complexity, as determined using flow cytometric analysis of side-scatter, was significantly lower in mitochondrial isolated from IL-15RαKO muscles. (B) Representative high power electron micrograph depicting the internal structure of mitochondria located in EDL muscles from B6129 mice. (C) Representative high power electron micrograph depicting the altered internal structure of mitochondria located in EDL muscles from IL-15RαKO mice. (D) Relative mitochondrial size, as determined using flow cytometric analysis of forward scatter, was not different between mitochondria isolated B6129 control muscles and IL-15RαKO muscles. (E) Representative flow cytometry plot in which intact mitochondria were gated on forward scatter to determine relative size of mitochondria of muscles from B6129 mice. (F) Representative flow cytometry plot in which intact mitochondria were gated on forward scatter to determine relative size of mitochondria of muscles from IL-15RαKO mice. *, p<0.05. EM images were taken at 20,000X.
3.3. Muscle fiber area distribution and cumulative fiber area polygon
We have previously reported a significant leftward shift in the single fiber area distribution toward smaller fiber areas of the EDL and tibialis anterior muscles from IL-15RαKO mice, while there were no differences noted in the soleus muscle [7]. To determine whether a similar morphological change occurred in a muscle of differing contractile activity and mixed fiber composition, we determined the single fiber area distribution of laminin-stained gastrocnemius muscles from IL-15RαKO mice and B6129 control mice (Supplemental Figure 2). There was a significant leftward shift of the fiber area distribution in gastrocnemius muscles from IL-15RαKO mice compared to B6129 control mice, demonstrating a greater number of smaller sized muscle fibers (B6129: 4329.2μm ±1752.0; IL-15RαKO: 3804.3μm±1340.3; -12.1%***).
4. Discussion
This study extends our previous observations on the unique exercise and muscle fatigue phenotype in IL-15RαKO mice [7] and reveal a greater mitochondrial density and mitochondrial DNA content in EDL muscles from IL-15RαKO mice. These differences in mitochondria in skeletal muscles deficient in IL-15Rα appear to be responsible, in part, for the altered exercise capacity and function of isolated muscles [7, 12]. For example, a 6-fold greater amount of cage wheel running in 10-12 week old IL-15RαKO mice compared to age-matched control mice was accompanied by a greater spontaneous cage activity [7, 12]. The muscles from these mice also expressed greater mRNA and protein content of markers indicative of mitochondrial biogenesis (PPARδ, PGC-1α) and mitochondrial enzymes (SDH, CS) consistent with a greater mitochondrial number and/or activity as the mechanism for the increased exercise and fatigue-resistance [7]. The current study reveals that mitochondrial density in EDL muscles from IL-15RαKO mice was indeed increased, and this occurred in the absence of exercise training. Also, muscles from IL-15RαKO mice that contain fast type II myofibers such as the EDL, the tibialis anterior, and the gastrocnemius muscle have a greater number of smaller sized myofibers. However, the magnitude of the shift in myofiber area (decrease of 18%; [7]) cannot account for the dramatic increase in mitochondrial density (48%). Therefore, our data demonstrate that IL-15Rα deficient muscles have a greater mitochondrial density, that cannot be explained by smaller muscle fiber sizes.
Electron microscopy revealed an altered internal structure of mitochondria that included a swollen appearance with altered cristae. The altered internal structure of mitochondria was verified by flow cytometric analyses and was not an artifact of the muscle fixation used for electron microscopy preparation. Changes in mitochondrial appearance including rounding or swelling and changes in internal cristae have been reported following fatiguing exercise (close to 100% VO2max) [13]. Similarly, altered mitochondrial morphology was visible in muscle biopsy samples following a marathon. Samples taken before and for up to 3 days post-marathon showed the presence of mitochondria with a swollen appearance and altered cristae [14]. These data suggest that the altered mitochondrial structure was due to the extensive exercise training performed by marathon runners, since these alterations were evident prior to the marathon, and that these altered mitochondria remain functional [14].
We and others have demonstrated the dramatically greater amount of physical activity that occurs in IL-15RαKO mice [7, 12]. The alterations in internal structure of mitochondria from IL-15RαKO mice observed in the current study could result from extensive amounts of physical activity, although the mitochondria appear to be functional, resulting in increased fatigue-resistance of EDL muscles [7]. It is not clear if changes in phenotype resulted in altered mitochondria or if the high level of activity in these young mice induced muscle adaptations to exercise. Recently, IL-15 was identified as one of several candidate genes that regulate voluntary activity in mice [15] and it is not currently known if the greater amount of cage activity measured in these mice results from an increased central drive to be active or due to a direct effect of IL-15Rα-deficiency on peripheral skeletal muscles [12]. Therefore, additional experiments are currently being conducted to more fully explore this issue of central versus peripheral-based changes in IL-15RαKO mice.
In conclusion, a greater mitochondrial density and mitochondrial DNA content was found in fast muscles from IL-15RαKO mice that was not due to the presence of smaller muscle fibers. These mitochondria also had an altered internal structure, including a swollen appearance and altered cristae, perhaps as a result of excessive activity or altered mitochondrial biogenesis. Future studies should determine whether modulation of IL-15Rα and/or IL-15 can positively impact conditions where mitochondrial dysfunction is present in skeletal muscle, as our data clearly support our central hypothesis that IL-15Rα is a novel regulator of skeletal muscle mitochondria.
Supplementary Material
Supplemental Figure 1: Internal structure of mitochondria in soleus muscles from IL-15RαKO mice. (A) Representative electron micrograph of soleus muscle from B6129 control mouse, with mitochondria identified. (B) Representative electron micrograph of soleus muscle from IL-15RαKO mouse, with mitochondria identified. The altered internal structure is clearly evident in mitochondria within soleus muscles from IL-15RαKO mice. These structural changes occurred in muscles of differing fiber type composition and contractile activity (i.e. soleus and EDL), suggesting these changes are consistent among muscles from IL-15RαKO mice. EM images were taken at 20,000X.
Supplemental Figure 2: Muscle fiber area distribution and cumulative fiber area polygon. (A) Representative image of laminin-stained gastrocnemius skeletal muscle from B6129 mice. (B) Representative image of laminin-stained gastrocnemius skeletal muscle from IL-15RαKO mice. (C) The single fiber area distribution of the gastrocnemius muscle from IL-15RαKO mice was shifted to the left compared to the distribution from B6129 control mice, indicating a greater number of smaller sized muscle fibers. (D) The cumulative fiber area polygon, calculated from the fiber area distribution, verified the greater number of smaller sized muscle fibers throughout the gastrocnemius muscle from IL-15αKO mice. *, p<0.05. Images were taken at 20X.
Acknowledgments
The authors would like to acknowledge the West Virginia University Flow Cytometry Core facility (P30GM103488; P30RR032138), and the Tissue Processing and Analysis Core facility (P30RR031155). We would also like to acknowledge Dr. John Hollander and Andrew McElroy for assistance in the experiments contained in this manuscript.
References
- 1.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 3.Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, DuBose R, Cosman D, Park LS, Anderson DM. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. Embo J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinn LS, Haugk KL, Grabstein KH. Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology. 1995;136:3669–3672. doi: 10.1210/endo.136.8.7628408. [DOI] [PubMed] [Google Scholar]
- 5.Carbo N, Lopez-Soriano J, Costelli P, Busquets S, Alvarez B, Baccino FM, Quinn LS, Lopez-Soriano FJ, Argiles JM. Interleukin-15 antagonizes muscle protein waste in tumour-bearing rats. Br J Cancer. 2000;83:526–531. doi: 10.1054/bjoc.2000.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinn LS, Anderson BG, Strait-Bodey L, Stroud AM, Argiles JM. Oversecretion of interleukin-15 from skeletal muscle reduces adiposity. Am J Physiol Endocrinol Metab. 2009;296:E191–202. doi: 10.1152/ajpendo.90506.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pistilli EE, Bogdanovich S, Garton F, Yang N, Gulbin JP, Conner JD, Anderson BG, Quinn LS, North K, Ahima RS, Khurana TS. Loss of IL-15 receptor alpha alters the endurance, fatigability, and metabolic characteristics of mouse fast skeletal muscles. J Clin Invest. 2011;121:3120–3132. doi: 10.1172/JCI44945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn LS, Anderson BG, Conner JD, Pistilli EE, Wolden-Hanson T. Overexpression of interleukin-15 in mice promotes resistance to diet-induced obesity, increased insulin sensitivity, and markers of oxidative skeletal muscle metabolism. International Journal of Interferon, Cytokine, and Mediator Research. 2011;3:29–42. doi: 10.2147/IJICMR.S19007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spirou GA, Rowland KC, Berrebi AS. Ultrastructure of neurons and large synaptic terminals in the lateral nucleus of the trapezoid body of the cat. J Comp Neurol. 1998;398:257–272. doi: 10.1002/(sici)1096-9861(19980824)398:2<257::aid-cne7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Weibel ER. A stereological method for estimating volume and surface of sarcoplasmic reticulum. J Microsc. 1972;95:229–242. doi: 10.1111/j.1365-2818.1972.tb03722.x. [DOI] [PubMed] [Google Scholar]
- 11.Dabkowski ER, Williamson CL, Hollander JM. Mitochondria-specific transgenic overexpression of phospholipid hydroperoxide glutathione peroxidase (GPx4) attenuates ischemia/reperfusion-associated cardiac dysfunction. Free Radic Biol Med. 2008;45:855–865. doi: 10.1016/j.freeradbiomed.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 12.He Y, Wu X, Khan RS, Kastin AJ, Cornelissen-Guillaume GG, Hsuchou H, Robert B, Halberg F, Pan W. IL-15 receptor deletion results in circadian changes of locomotor and metabolic activity. J Mol Neurosci. 2010;41:315–321. doi: 10.1007/s12031-009-9319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCutcheon LJ, Byrd SK, Hodgson DR. Ultrastructural changes in skeletal muscle after fatiguing exercise. J Appl Physiol. 1992;72:1111–1117. doi: 10.1152/jappl.1992.72.3.1111. [DOI] [PubMed] [Google Scholar]
- 14.Hikida RS, Staron RS, Hagerman FC, Sherman WM, Costill DL. Muscle fiber necrosis associated with human marathon runners. J Neurol Sci. 1983;59:185–203. doi: 10.1016/0022-510x(83)90037-0. [DOI] [PubMed] [Google Scholar]
- 15.Kelly SA, Nehrenberg DL, Hua K, Garland T, Jr, Pomp D. Functional Genomic Architecture of Predisposition to Voluntary Exercise in Mice: Expression QTL in the Brain. Genetics. 2012;191:643–654. doi: 10.1534/genetics.112.140509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Internal structure of mitochondria in soleus muscles from IL-15RαKO mice. (A) Representative electron micrograph of soleus muscle from B6129 control mouse, with mitochondria identified. (B) Representative electron micrograph of soleus muscle from IL-15RαKO mouse, with mitochondria identified. The altered internal structure is clearly evident in mitochondria within soleus muscles from IL-15RαKO mice. These structural changes occurred in muscles of differing fiber type composition and contractile activity (i.e. soleus and EDL), suggesting these changes are consistent among muscles from IL-15RαKO mice. EM images were taken at 20,000X.
Supplemental Figure 2: Muscle fiber area distribution and cumulative fiber area polygon. (A) Representative image of laminin-stained gastrocnemius skeletal muscle from B6129 mice. (B) Representative image of laminin-stained gastrocnemius skeletal muscle from IL-15RαKO mice. (C) The single fiber area distribution of the gastrocnemius muscle from IL-15RαKO mice was shifted to the left compared to the distribution from B6129 control mice, indicating a greater number of smaller sized muscle fibers. (D) The cumulative fiber area polygon, calculated from the fiber area distribution, verified the greater number of smaller sized muscle fibers throughout the gastrocnemius muscle from IL-15αKO mice. *, p<0.05. Images were taken at 20X.