Abstract
Objective
Complement-mediated vasculopathy of muscle and skin are clinical features of juvenile dermatomyositis (JDM). We assess gene copy-number variations (CNVs) for complement C4 and its isotypes, C4A and C4B, in genetic risks and pathogenesis of JDM.
Methods
The study population included 105 JDM patients and 500 healthy European Americans. Gene copy-numbers (GCNs) for total C4, C4A, C4B and HLA-DRB1 genotypes were determined by Southern blots and PCRs. Processed activation product C4d bound to erythrocytes (E-C4d) was measured by flow cytometry. Global gene-expression microarrays were performed in 19 JDM and 7 controls using PAXgene-blood RNA. Differential expression levels for selected genes were validated by qPCR.
Results
Significantly lower GCNs and differences in distribution of GCN groups for total C4 and C4A were observed between JDM and controls. Lower GCN of C4A in JDM remained among HLA DR3-positive subjects (p=0.015). Homozygous or heterozygous C4A-deficiency was present in 40.0% of JDM compared to 18.2% of controls [odds ratio (OR)=3.00 (1.87–4.79), p=8.2x10−6]. JDM had higher levels of E-C4d than controls (p=0.004). In JDM, C4A-deficient subjects had higher levels of E-C4d (p=0.0003) and higher frequency of elevated levels of multiple serum muscle enzymes at diagnosis (p=0.004). Microarray profiling of blood RNA revealed upregulation of type I Interferon-stimulated genes and lower abundance of transcripts for T-cell and chemokine function genes in JDM, but this was less prominent among C4A-deficient or DR3-positive patients.
Conclusions
Complement C4A-deficiency appears to be an important factor for the genetic risk and pathogenesis of JDM, particularly in patients with a DR3-positive background.
Keywords: erythrocyte-bound C4d (E-C4d), elevated serum muscle enzyme levels, HLA-DRB1*0301, HLA-DRB1*1501, gene expression profiles
INTRODUCTION
Juvenile dermatomyositis (JDM) is a rare, autoimmune, multi-system inflammatory disease affecting primarily muscle and skin in children. Characteristic clinical features and diagnostic criteria include proximal muscle weakness and inflammation, increased levels of serum muscle enzymes, distinct skin rashes such as Gottron’s papules or heliotrope rash, and pathological changes on muscle biopsy or magnetic resonance imaging (MRI) [1–7].
The HLA class II gene DRB1 allele *0301 (also known as DR3) has been identified as a major immunogenetic risk factor for JDM and was reaffirmed as the predominant risk locus of juvenile and adult dermatomyositis in a genome-wide association study [3–5 8]. HLA class I and class II genes are engaged in antigen presentation and processing. The class III genes are heterogeneous in structures and function, and include genes encoding for components of the complement system C4, C2 and factor B, and for cytokines such as tumor necrosis factor-α (TNF-α), and α and β lymphotoxins (Figure 1) [9]. Previous studies revealed that class II genes DRB1 allele *0301 and DQA1 allele *0501, class III gene TNFA–308A allele, and class I gene variants B*08 and A*01 are in strong linkage disequilibrium among human subjects of European descent and this is described as the ancestral haplotype AH8.1 [10–12]. Also present in AH8.1 is a single C4B gene but the absence of a C4A gene [10 13 14]. Remarkably, there are extensive inter-individual gene CNVs and gene-size dichotomy for complement C4. Briefly, two to eight copies of C4 genes can be present in a diploid genome [15 16]. Segmental duplications for complement C4 genes occur as RCCX modules, which always include the RP (STK19) gene upstream of C4, and the downstream genes CYP21 and TNX (RCCX). Each C4 gene can be a long gene of 20.6-kb or a short gene of 14.2-kb [17 18]. Each C4 gene either encodes for an acidic C4A or a basic C4B protein, with only four amino acid changes (PCPVLD 1101–1106 for C4A and LSPVIH for C4B), but these result in substantial differences in chemical reactivity for peptide and carbohydrate antigens [19–22].
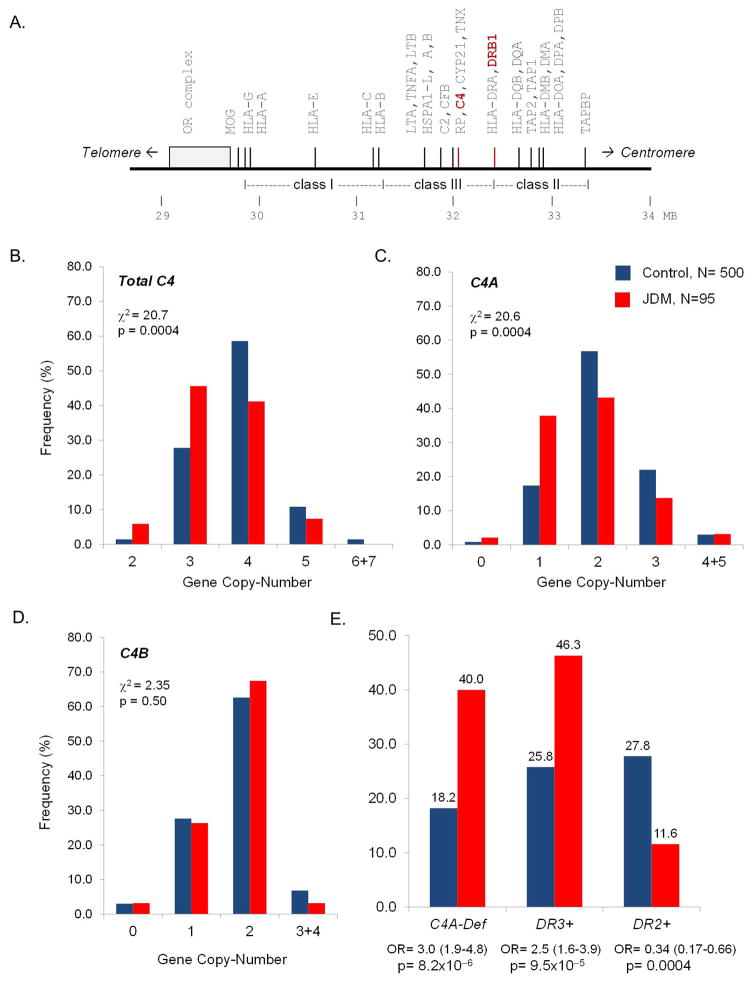
Figure 1.
Variations of C4 haplotypes and gene copy-numbers (GCNs) of total C4, C4A and C4B in JDM subjects and race-matched healthy controls. A. A simplified map of the human major histocompatibility complex (MHC) showing genes of immunologic functions; RP-C4-CYP21-TNX (RCCX) and HLA-DRB are characterized by inter-individual copy-number variations. B, C and D. Gene copy-number variations of complement C4, C4A and C4B in JDM and controls. The p-values and chi-square values are indicated for each analysis. E. A summary of genetic risk factors in the MHC for JDM.
Complement-mediated destruction of perivascular endothelium and perifascicular ischemia of muscle fibers in biopsies from dermatomyositis patients have been demonstrated by multiple investigators [23–28]. Circulating immune complexes, immunoglobulins IgG and IgM, complement C3 and C5b-9 membrane attack complex were shown in dermatomyositis muscle and skin biopsies. However, the initiation for complement activation and the potential role of complement in the breakdown of immune tolerance in JDM remain unclear. Continuous CNVs with 1 to 4 copies of C4 genes on a haplotype with different combinations of C4A and C4B genes in human populations have only been established since 1999 [16 29 30]. Many earlier epidemiologic studies of complement C4A and C4B in rheumatic diseases, including JDM, were based on an incomplete or inaccurate model with two-locus (C4A-C4B) on a haplotype for data interpretation, and thus conclusions drawn became uncertain [31–34]. Here we perform a fresh and meticulous investigation of C4 genetic diversity and examine their effects on the risk and pathogenesis of JDM, with further considerations to the presence and absence of HLA-DRB1 risk and protective alleles.
PATIENTS AND METHODS
Study populations
IRB approval was obtained from Nationwide Children’s Hospital (NCH) and the National Institutes of Health (NIDDK/NIAMS, NIH). One hundred five JDM patients were enrolled, of which 45 were from NCH and 60 were from the NIH. Each patient met the diagnostic criteria for JDM according to the Bohan and Peter criteria [1 2]. Typical characteristic MRI abnormalities of muscle were applied in place of biopsy as a modification of the Bohan and Peter criteria for the NCH cohort [35]. The mean age (±SD) at recruitment was 10.8±7.6 years old, and at disease diagnosis was 7.4±4.2 years old. The self-reported racial distribution was 90.5% Caucasian, 6.7% African American, and 2.9% Hispanic. Complete demographics and disease characteristics are displayed in Table 1. Ten non-Caucasian JDM patients were not included in the genetic analysis. Race-matched healthy control subjects included 500 European Americans residing in Midwest-America.
Table 1.
Demographic Features and Clinical Characteristics of JDM Patients*
| Features | N (%) |
|---|---|
| Age at recruitment: mean ± SD, yrs. old | 10.8 ± 7.6 |
| Age at diagnosis: mean ± SD, yrs. old | 7.4 ± 4.2 |
| Sex: female / male | 67 (63.8%) / 38 (36.2%) |
| Race/Ethnicity: Caucasian / African / Hispanic | 95 (90.5%) / 7 (6.7%) / 3 (2.9%) |
| Calcinosis | 15 / 97 (15.5%) |
| Ulcerations | 17 / 97 (17.5%) |
| Lipodystrophy | 8 / 96 (8.3%) |
| Disease course† | |
| Monocyclic | 16 / 66 (24.2%) |
| Polycyclic | 12 / 66 (18.2%) |
| Chronic continuous | 38 / 66 (57.6%) |
| Positive ANA | 62 / 82 (75.6%) |
Values given indicate number of subjects (percentage) for which data was available.
Only patients who have been followed ≥2 years were categorized by disease courses.
Determination of total C4, C4A and C4B genotypes and phenotypes
Previously, we described protocols for genotyping and phenotyping of complement C4 by Southern blot analyses and immunofixation, respectively [36–38]. In cases of limited DNA quantities or ambiguous results, quantitative real-time PCR experiments for GCN of total C4, C4A, and C4B were performed as described [15]. All C4-CNV calls were validated rigorously by independent technology, or multiple amplicons in qPCR, and matched genotype and phenotype interpretations (Figures S1–S3, Tables S1 and S2).
Flow cytometric detection of erythrocyte-bound complement activation fragments
Erythrocytes from whole blood were used for antibody staining and flow cytometry. Mouse monoclonal antibodies specific for human C4d, for human C3d, or the isotype-matched control MOPC21 (Quidel, San Diego, CA) were used [39 40]. PE-conjugated goat anti-mouse IgG F(ab′)2 (Jackson ImmunoResearch, West Grove, PA) was used as a secondary antibody. FlowJo software (Tree Star Inc., Version 7.6) was used to electronically gate erythrocytes based on forward and sideward scatter properties. Among the gated cells, E-C4d or E-C3d was reported as median fluorescence intensity (MFI), which was calculated using C4d-specific (or C3d-specific) MFI minus the MOPC-isotype control MFI.
HLA-DRB1 Typing
Genotyping of HLA-DRB1 alleles for JDM and all control samples were determined using genomic DNA for sequence-specific primer PCR [41]. HLA-DRB1 frequency was calculated by the number of allele-positive subjects divided by the total number of subjects.
Gene expression profiling
RNA was extracted from whole blood using PAXgene collection tubes (PreAnalytiX, Becton, Dickinson and Company). Microarray analysis was performed by the Biomedical Genomics Core facility at the NCH. RNA samples passing quality control were labelled with Agilent’s One-Color microarray-based gene expression analysis labeling protocol and hybridized to the SurePrint G3 Human v2 GE 8x60K Microarray (AMADID 039494). Images were analyzed with Feature Extraction 10.9 (Agilent Technologies). Median foreground intensities were obtained for each spot and imported into the mathematical software package R. After pre-processing, the data were quantile normalized using the LIMMA package [42]. Statistical analysis was performed via Significance Analysis of Microarray (SAM) implemented using the Bioconductor Siggenes package to identify differentially expressed genes between JDM and control groups [43]. Changes in expression ≥1.5 fold and a 15% false discovery rate as estimated by SAM were considered significantly different. Selected genes were validated by SYBR-Green qPCR using PAXgene RNA.
Statistical analysis
Statistical analyses were performed using Prism6 (GraphPad Software Inc., San Diego, CA) and JMP Genomics 6.0 (SAS Institute Inc., Cary, NC) software. Descriptive statistics are displayed as mean ± standard deviation (SD) for normally distributed data, and simple comparisons were made using Student’s t-test for continuous data, or by chi-square analysis for categorical data. Odds Ratios (OR) with 95% confidence intervals (CI) are reported. For non-normally distributed data, median with interquartile range (IQR) is reported, and Mann-Whitney test was used for comparisons. For all analyses, p≤0.05 was considered to be significant.
RESULTS
Gene CNVs of total C4, C4A, and C4B in JDM and race-matched healthy controls
Total C4 genes
In healthy controls (N=500), the variation of C4 GCN showed a normal distribution pattern, ranging from 2–7 total copies. In Caucasian JDM (N=95), total C4 genes ranged from 2–5 copies, with a shift of distribution to the lower copy-number compared to controls [χ2=20.7; degree of freedom (df)=4; p=0.0004, χ2 analysis) (Figure 1). JDM had a lower mean GCN than did controls (JDM: 3.49±0.71; controls: 3.83±0.69; p=1.8x10−5, t-test; Table 2). Low copy-number of total C4 (C4T, GCN≤3) was present in 50.5% of JDM and 29.2% of controls [OR=2.48 (1.59–3.87); p=7.5x10−5].
Table 2.
HLA-DRB1 Alleles and C4 Gene CNVs in JDM and Controls
| A. Analysis of single genetic risk factors in JDM | ||||
|---|---|---|---|---|
| JDM (N=95) | Control (N=500) | p-value | OR (95% CI) | |
| a. Distribution, N (%) | ||||
| C4T GCN ≤ 3 | 48 (50.5%) | 146 (29.2%) | 7.5x10−5 | 2.48 (1.59–3.87) |
| C4A deficiency, GCN ≤1 | 38 (40.0%) | 91 (18.2%) | 8.2x10−6 | 3.00 (1.87–4.79) |
| C4B deficiency, GCN ≤1 | 28 (29.5%) | 153 (30.6%) | 0.83† | |
| HLA DRB1*1501 | 11 (11.6%) | 139 (27.8%) | 0.0004 | 0.34 (0.18–0.66) |
| HLA DRB1*0301 (DR3+) | 44 (46.3%) | 129 (25.8%) | 9.5x10−5 | 2.48 (1.58–3.89) |
| C4A-deficiency with DR3+ | 35 (36.8%) | 77 (15.4%) | 4.8x10−6 | 3.20 (1.98–5.19) |
| b. Mean GCN ± SD | ||||
| Total C4 genes | 3.49 ± 0.71 | 3.83 ± 0.69 | 1.8x10−5 | |
| C4A genes | 1.79 ± 0.86 | 2.09 ± 0.75 | 0.0004 | |
| C4B genes | 1.71 ± 0.58 | 1.73 ± 0.63 | 0.68† | |
| B. Multiple logistic regression models for genetic factors of JDM | ||||
|---|---|---|---|---|
| Models and parameters | R2 | χ2 | p-value | OR (95% CI) |
| a. C4A-deficiency, DRB1*1501 and DR3+ | 0.058 | 30.2 | 1.2x10−6 | |
| DRB1*1501 | 8.95 | 0.0028 | 0.39 (0.19–0.74) | |
| C4A-deficiency | 6.21 | 0.013 | 2.27 (1.19–4.40) | |
| DR3+ | 0.70 | 0.40† | 1.31 (0.69–2.44) | |
| b. C4A-deficiency and DR3+ | 0.041 | 21.3 | 2.4x10−5 | |
| C4A-deficiency | 6.05 | 0.014 | 2.26 (1.18–4.39) | |
| DR3+ | 1.39 | 0.24† | 1.47 (0.77–2.74) | |
| c. C4A deficiency and DRB1*1501 | 0.057 | 29.5 | 3.9x10−7 | |
| DRB1*1501 | 9.64 | 0.0045 | 0.38 (0.19–0.71) | |
| C4A-deficiency | 16.8 | 2.7x10−5 | 2.76 (1.71–4.43) | |
| d. DR3+ and DRB1*1501 | 0.046 | 24.0 | 6.1x10−6 | |
| DRB1*1501 | 8.80 | 0.003 | 0.40 (0.19–0.74) | |
| DR3+ | 11.3 | 0.0008 | 2.21 (1.39–3.49) | |
| e. C4A-deficiency with DR3+ and DRB1*1501 | 0.059 | 30.6 | 2.2x10−7 | |
| C4A-deficiency with DR3+ | 17.9 | 2.4x10−5 | 2.96 (1.81–4.80) | |
| DRB1*1501 | 9.7 | 0.0018 | 0.34 (0.21–0.55) | |
| C. Subgroup analyses of C4A-GCN, C4A-deficiency and HLA-DR3 in JDM | ||||
|---|---|---|---|---|
| JDM | Control | p-value | OR (95% CI) | |
| Mean C4A GCN, DR3+ | 1.18±0.54 | 1.47±0.72 | 0.015 | |
| Mean C4A GCN, DR3− | 2.31±0.73 | 2.31±0.63 | 0.97† | |
| C4A-deficient, DR3+, N (%) | 35 (79.6) | 77 (59.7) | 0.014 | 2.63 (1.17–5.92) |
| C4A-proficient, DR3+, N (%) | 9 (20.5) | 52 (40.3) | ||
| C4A-deficient, DR3−, N (%) | 3 (5.9) | 14 (3.8) | 0.50† | |
| C4A-proficient, DR3−, N (%) | 48 (94.1) | 357 (96.2) | ||
| DR3+, C4A-deficient, N (%) | 35 (92.1) | 77 (84.6) | 0.23† | |
| DR3−, C4A-deficient, N (%) | 3 (7.9) | 14 (15.4) | ||
| DR3+, C4A-proficient, N (%) | 9 (15.8) | 52 (12.7) | 0.53† | |
| DR3−, C4A-proficient, N (%) | 48 (84.2) | 357 (87.3) | ||
Abbreviations: C4T, total copies of C4 genes; CI, confidence interval; GCN, gene copy-number; N, number; OR, odds ratio. C4A-deficient: C4A GCN =0 or 1; C4A-proficient: C4A GCN ≥2
not statistically significant; categorical data were compared by χ2 analyses; continuous data were compared by t-tests.
Copy-numbers of C4A and C4B genes
The reduction in copy-number of total C4 in JDM can be the result of a decrease in C4A, C4B, or both. We observed a significant shift to lower GCN of C4A in JDM patients (p=0.0004). The presence of homozygous or heterozygous deficiency of C4A genes (GCN=0 or 1) had a frequency of 40.0% in JDM, compared to 18.2% in controls [OR=3.00 (1.87–4.79); p=8.2x10−6]. The overall mean GCN of C4A observed in JDM was 1.79±0.86 compared to 2.09±0.75 in controls (p=0.0004).
As for C4B, no significant differences in the distribution of C4B-GCN or C4B-deficiency were observed between JDM and controls. Therefore, the basis for decreased GCN of total C4 in Caucasian JDM patients was attributable to lower GCN of C4A.
HLA-DRB1 alleles, C4A-GCN and C4A-deficiency on disease risks of JDM
The frequency of HLA-DRB1*0301 alleles (DR3) was 46.3% in Caucasian JDM patients (N=95), compared with 25.8% in a race-matched healthy population (N=500). HLA-DR3 was associated with JDM with an OR=2.48 (1.58–3.89) and a p-value of 9.5x10−5. The concurrence of C4A-deficiency and DR3 in a subject was present in 36.8% of JDM and 15.4% of controls, with an odds ratio of 3.20 (1.98–5.19) and a p-value of 4.8x10−6. By contrast, the frequency of HLA-DRB1*1501 (DR2) was 11.6% in Caucasian JDM compared to 27.8% in healthy controls. DRB1*1501 was a protective factor against JDM with OR=0.34 (0.18–0.66) and p=0.0004 (Figure 1E; Table 2A).
Multiple logistic regression analyses were performed to investigate if C4A-deficiency, the presence of DR3 and the presence of DRB1*1501 could serve as independent risk factors for JDM, conditional upon presence of other factor(s) in five different combinations of regression models (Table 2B). In models when DR3+ and C4A-deficiency were put together as individual factors (model a or b), the relevance of DR3+ as an independent parameter became insignificant. The presence of DRB1*1501 plus C4A-deficiency, or DR3+, or C4A-deficiency with DR3+ all yielded statistical significance to account for JDM genetic risks. The last model yielded the best fit: C4A-deficiency plus DR3+ was a strong risk factor with odds ratio of 2.96 (1.84–4.80) and DRB1*1501 was protective factor with odds ratio of 0.34 (0.21–0.55).
To further evaluate the relative roles of C4A-deficiency and DRB1*0301 on disease risk of JDM, we performed subgroup analyses (Table 2C). Among the DR3+ subjects, JDM patients had a significantly lower mean-GCN of C4A than controls (JDM: 1.18±0.54; controls: 1.47±0.72; p=0.015). Similarly among the DR3+ subjects, C4A-deficiency had a greater prevalence in JDM (79.6%) than controls (59.7%) [OR=2.63 (1.17–5.92), p=0.014]. Among DR3-negative subjects, however, there were no apparent differences in mean-GCN of C4A or the prevalence of C4A-deficiency between JDM and controls, suggesting the heightened risk of lower C4A-GCN or C4A-deficiency on JDM required a DR3+ background.
Reciprocal subgroup analyses to compare the prevalence of DR3+ between JDM and controls among C4A-deficient subjects (GCN≤1), or among C4A-proficient subjects (GCN≥2) revealed slight but insignificant increases in the frequency of DR3 in JDM (Table 2C).
Levels of erythrocyte-bound C4d (E-C4d) or C3d (E-C3d) in JDM and controls
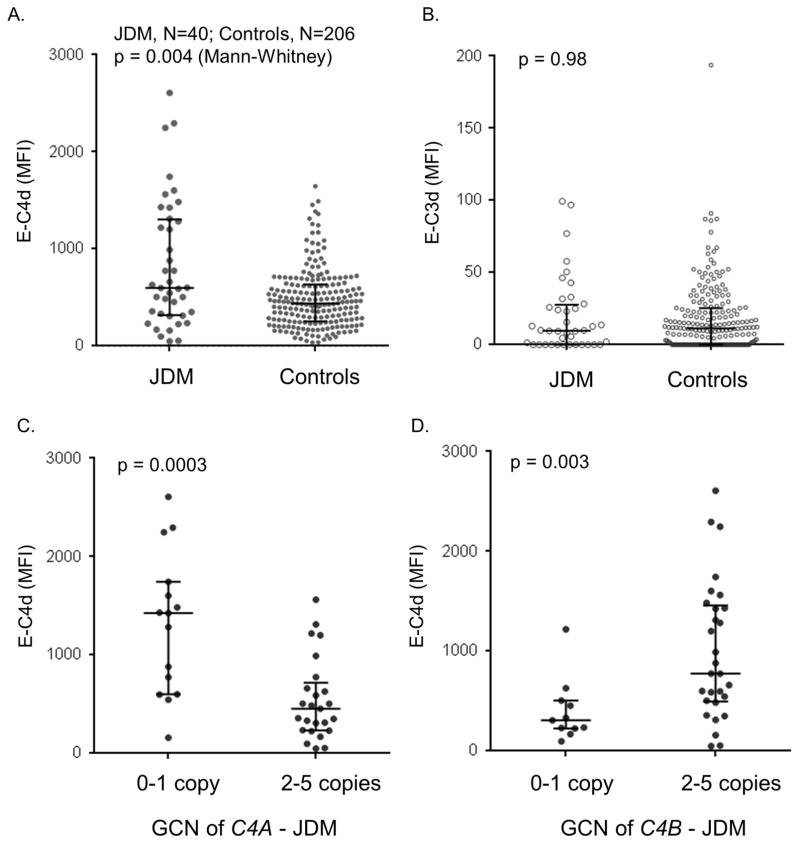
Cell-bound complement levels were determined in 40 Columbus JDM patients and 206 healthy subjects of European ancestry by flow cytometry. Comparing between JDM and healthy controls, significant elevation of E-C4d levels (p=0.004, Mann Whitney test, Figure 2A) but not E-C3d levels (Figure 2B) was observed in JDM. The mean fluorescent intensities (MFI) for E-C3d levels were substantially lower than those of E-C4d levels, which is consistent with the presence of complement regulation mechanisms on self cell surfaces.
Figure 2.
Erythrocyte-bound E-C4d and E-C3d in JDM patients and controls. A and B. A comparison of E-C4d and E-C3d, respectively, in JDM and controls. C. A comparison of E-C4d in C4A-deficient and C4A-proficient JDM patients. D. A comparison of E-C4d in C4B-deficient and C4B-proficient JDM patients. The median for each group is indicated by a horizontal bar, while the shorter bars represent interquartile ranges; the p-value for Mann Whitney test is indicated.
We investigated if there was a correlation of E-C4d levels with C4A or C4B gene dosages in JDM. The C4A-deficiency group (GCN≤1; N=15) had a median MFI of 1426 (IQR: 601–1744), which was significantly higher than that of the C4A-proficient group (GCN≥2; N=25; median MFI=454 (234–718); p=0.0003, Mann-Whitney test). On the other hand, the C4B-deficiency group (GCN≤1; N=11) had a median E-C4d MFI of 308 (226–505), which was significantly lower than that of the C4B-proficient group (GCN≥2, N=29; median MFI=775 (495–1458); p=0.003, Mann-Whitney test). Thus, C4A and C4B appeared to play opposite roles on the deposition of cell-bound E-C4d: high GCN of C4A dampened activation, while high GCN of C4B amplified activation.
JDM patients with elevated levels of multiple serum muscle enzymes had low GCN of C4A
JDM patients exhibited elevated levels of a variety of serum muscle enzymes. We performed intragroup comparisons to investigate if C4A-deficiency was related to elevated muscle enzyme levels at the time of disease diagnosis. Indeed, patients with C4A-deficiency had higher prevalence of abnormal serum muscle enzymes such as creatine kinase (C4A-deficient: 86.1%, C4A proficient: 58.2%; p=0.0034) and aldolase (C4A-deficient: 94.1%, C4A-proficient: 78.4%; p=0.038) and elevations of multiple serum muscle enzymes (C4A-deficient: 97.1%; C4A- proficient: 74.5%; p=0.0025) than C4A-proficient patients. The prevalence of elevated levels of serum aspartate aminotransferase and lactate dehydrogenase was not associated with C4A-deficiency (Table 3).
Table 3.
Elevation of serum muscle enzymes at disease diagnosis in JDM patients with and without C4A-deficiency
| Muscle Enzyme | N (%) with elevation of muscle enzyme levels | ||
|---|---|---|---|
| C4A-deficient | C4A-proficient | p-value* | |
| Aldolase | 32 (94.1) | 40 (78.4) | 0.038 |
| Aspartate aminotransferase | 27 (81.8) | 36 (67.9) | NS |
| Creatine kinase | 31 (86.1) | 32 (58.2) | 0.0034 |
| Lactate dehydrogenase | 24 (82.8) | 31 (77.5) | NS |
| ≥ 2 Muscle enzymes | 33 (97.1) | 38 (74.5) | 0.0025 |
by χ2 analysis;
NS, not significant
Differential gene expression profiling of JDM and controls
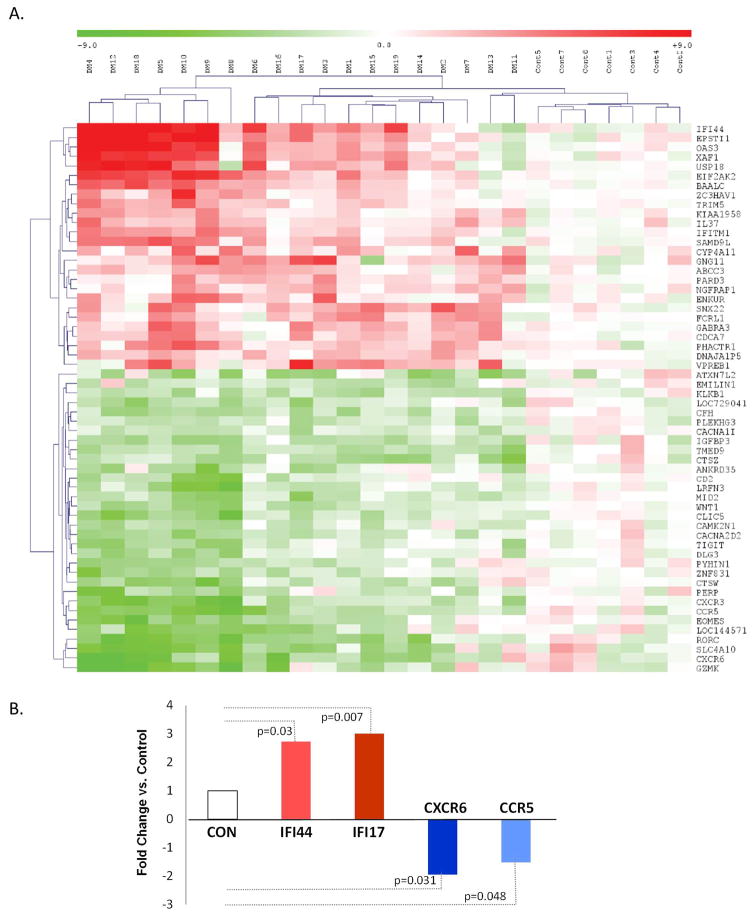
Global gene expression profiling was performed using PAXgene blood RNA from 19 consecutive JDM patients and 7 controls (Figure 3A and Tables S3 and S4). Expression profiles revealed differential expression of transcripts in JDM from 56 genes that were significantly different using SAM criteria. Differentially expressed genes included 24 upregulated and 32 downregulated genes. Of the upregulated genes, the most remarkable are nine type I interferon (IFN-I) response genes and three genes related to B-cell functions. Of the downregulated genes, the most distinct were genes related to T-cell functions, chemokines and chemokine receptors. Six JDM patients exhibited the most polarized upregulation of IFN-I genes and downregulation of genes for chemokine/chemokine receptor and T-cell functions (Figure 3A). Of interest, five of these six JDM patients were C4A-proficient (GCN≥2), and did not carry the HLA-DR3 allele.
Figure 3.
Gene expression profiling of PAXgene blood RNA in JDM and healthy controls. A. Hierarchical clustering analysis of PAXgene blood RNA gene expression microarray data in 19 JDM and 7 healthy subjects; red and green represent upregulated and downregulated genes, respectively; vertical columns represent the data for each subject, and rows indicate different genes (see Table S3 for details). B. Four genes from microarray results were selected for SYBR-green qPCR analysis using cDNA from PAXgene blood RNA to validate the upregulation and downregulation in JDM of the chosen genes. The white column shows normalized value to 1 for controls, while the fold-change in JDM for each respective gene is indicated by colored columns; p-values for Mann-Whitney tests are indicated. Number of subjects (N) used for SYBR-Green qPCR assays are: IFI44 - 28 JDM and 14 controls; IFI17 - 17 JDM and 15 controls; CXCR6 - 24 JDM and 19 controls; and CCR5 - 24 JDM and 19 controls.
To validate gene expression changes from microarray, we performed SYBR-Green qPCR using cDNA from PAXgene-blood RNA for IFI44, IFI17, CXCR6, and CCR5 transcripts. Results revealed in JDM upregulation of IFI44 (2.7-fold; p=0.028) and IFI17 (3.0-fold; p=0.0054), and downregulation of CXCR6 (1.9 fold; p=0.031) and CCR5 (1.5 fold; p=0.048) (Figure 3B). The housekeeping gene GAPDH was used as a normalization standard [44].
DISCUSSION
A great challenge for studying complex diseases associated with the HLA region, including JDM, is to determine which gene(s) or polymorphic variants contribute to disease development under the background of strong allelic associations or linkage disequilibrium. This is the first study to decipher the gene CNVs for complement C4 and its isotypes C4A and C4B in JDM with definitive techniques, and to dissect the relative roles of HLA-DRB1*0301 (DR3) and C4A-deficiency on JDM disease risk in subjects of European ancestry.
The carriage of DRB1*0301 was present in 46.3% of our JDM patients compared to 25.8% of race-matched healthy controls. DR3 is a medium effect-size risk factor for JDM (OR=2.48). Homozygous and heterozygous deficiency of complement C4A had a frequency of 40.0% in Caucasian JDM and 18.2% in healthy controls (OR=3.00). The co-existence of HLA-DR3 and C4A-deficiency confers higher risk than either individual risk factors, and such concurrence in a diploid genome was present in 36.8% of JDM and 15.4% of controls with an OR=3.20. The independent roles of DRB1 variants and C4A-deficiency in JDM were further validated by multiple logistic regression analyses. Moreover, among DR3-positive subjects, lower mean GCN of C4A or higher prevalence of C4A-deficiency persisted in JDM versus controls. An interpretation to this phenomenon is that DR3-positivity contributes to a permissive background and C4A-deficiency significantly elevated the vulnerability to an autoimmune disease including JDM. While HLA-DRB1 and complement C4 each is engaged in specific immunologic functions such as antigen presentation to effector T-cells, and complement-mediated cytolysis and immunoclearance, they are both involved in the recognition of self and non-self, and are key players for the process of archiving memory and tolerance in the immune system.
Destruction of perifascicular capillaries by complement and subsequent ischemia of muscle fibers in dermatomyositis have been demonstrated by multiple investigators over the past three decades [23–28]. Activation of complement can be initiated via the classical pathway that is triggered by immune complexes formed between myositis-associated or myositis-specific autoantibodies and self-antigens abundant in muscles and skin. Physiologically, low GCN or low production of C4A protein systemically may dampen immune complex clearance and therefore promote autoimmunity. Compared with controls, we observed a moderate but significant increase in the deposition of C4d on red-blood cells among JDM patients, which reiterates involvement of complement activation in the pathogenesis of JDM. Almost all JDM patients with two or more elevated muscle enzymes at disease diagnosis had a C4A-deficiency but normal mean GCN of C4B. In other words, immune-mediated tissue injuries in JDM might have been resulted through the activation of C4B. Consistent with this phenomenon, we observed increased deposition of processed complement activation product E-C4d in JDM patients than in controls. Interestingly, the levels of E-C4d were directly proportional to the GCN of C4B, and inversely proportional to the GCN of C4A. Physiologically, activated C4B protein is highly reactive and over-activation could lead to complement-mediated injuries. In addition to its role in immunoclearance and protection against autoimmunity, the presence of activated C4A may attenuate the activity of C4B and minimize its potential deleterious effect.
Among the JDM patients, 63.2% were not associated with C4A-deficiency on a DR3+ background, and the underlying genetic risk factors in this group of patients (C4A- proficient and DR3-negative) are yet to be identified. An emerging feature in JDM is the upregulation of type I interferon-stimulated gene expression in many patients [45–47]. Our microarray studies of peripheral blood samples revealed marked increase in transcripts in JDM for many IFN-I stimulated genes and B-cell specific genes, but diminished transcript levels of many genes related to chemokines and T-cell functions. Such differential levels of transcripts reflect both different gene expression levels and also compositions of leukocytes in the peripheral blood samples. The polarized upregulation of IFN-stimulated genes and B-cell function genes, and downregulation of chemokine receptor and T cell function genes were more marked among C4A-proficient or DR3-negative patients, implying the presence of additional or alternative mechanisms leading to the pathogenesis of JDM.
In conclusion, we report the novel finding of low GCN of complement C4 and C4A-deficiency associated with JDM. JDM patients with C4A-deficiency were more likely to have elevated levels of multiple serum muscle enzymes at diagnosis and high levels of E-C4d. Further in-depth studies through HLA-DRB1 and C4A genotypes, cell-bound C4d levels and differentially expressed genes including those engaged in muscle-cell functions and signaling, and characterization of clinical phenotypes [48] may help understanding the pathogenic mechanisms, enable patient stratification and facilitate genotype and gene expression guided therapies of JDM.
Supplementary Material
Acknowledgments
We are indebted to blood donors and juvenile dermatomyositis patients for their invaluable specimens and support, and to the following referring physicians for patient recruitment: Stacy Ardoin, Sharon Bout-Tabaku, Gloria Higgins, Bita Arabshahi, Ruy Carrasco, Victoria Cartwright, Anne Eberhardt, Barbara Edelheit, Harry Gewanter, Donald Goldsmith, Beth Gottlieb, Thomas Griffin, Melissa Hawkings-Holt, Roger Hollister, Yukiko Kimura, Patrick Knibbe, Lauren Pachman, Maria Perez, Abigail Smukler, Sangeeta Sule, Carol Wallace and Lawrence Zemel. We are grateful to Ms. Jeanie Shaw for help in recruiting healthy subjects, and Drs. Joe Ahearn and CC Liu for assistance in interpreting erythrocyte C4d data. We thank Drs. Elaine Remmers and Adriana Almeida de Jesus for critical review of the manuscript.
This work was supported in part by National Institute of Arthritis, Musculoskeletal Diseases, the National Institutes of Health grant 5R01 AR054459, the CureJM Foundation, an institutional grant from the Research Institute of the Nationwide Children’s Hospital, and the intramural research program of the National Institute of Environmental Health Sciences, National Institutes of Health.
Footnotes
CONTRIBUTORS
CHS, AP, KEL, LGR, LP and CYY conceived and designed the study. AP, RAA, LGR, FWM and CHS recruited JDM patients and investigated patients’ clinical presentations. KJ, YLW, KEL and CYY recruited healthy controls. KEL, YLW, BZ, EL, AA, DN, TPO and CYY performed experiments. KEL, RAA, CHS, PW, LP, LGR and CYY performed data interpretation and analyses. KEL, CHS, LGR and CYY wrote the manuscript. All authors (except DN who is deceased) revised and approved the manuscript as written.
References
- 1.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) The New England journal of medicine. 1975;292(7):344–7. doi: 10.1056/NEJM197502132920706. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) The New England journal of medicine. 1975;292(8):403–7. doi: 10.1056/NEJM197502202920807. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 3.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971–82. doi: 10.1016/S0140-6736(03)14368-1. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 4.Feldman BM, Rider LG, Reed AM, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371(9631):2201–12. doi: 10.1016/S0140-6736(08)60955-1. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 5.Robinson AB, Reed AM. Clinical features, pathogenesis and treatment of juvenile and adult dermatomyositis. Nature reviews Rheumatology. 2011;7(11):664–75. doi: 10.1038/nrrheum.2011.139. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 6.Rider LG, Miller FW. Deciphering the clinical presentations, pathogenesis, and treatment of the idiopathic inflammatory myopathies. JAMA : the journal of the American Medical Association. 2011;305(2):183–90. doi: 10.1001/jama.2010.1977. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalakas MC. Inflammatory Muscle Diseases. The New England journal of medicine. 2015;373(4):393–4. doi: 10.1056/NEJMc1506827. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 8.Miller FW, Cooper RG, Vencovsky J, et al. Genome-wide association study of dermatomyositis reveals genetic overlap with other autoimmune disorders. Arthritis and rheumatism. 2013;65(12):3239–47. doi: 10.1002/art.38137. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horton R, Wilming L, Rand V, et al. Gene map of the extended human MHC. Nature reviews Genetics. 2004;5(12):889–99. doi: 10.1038/nrg1489. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 10.Dawkins R, Leelayuwat C, Gaudieri S, et al. Genomics of the major histocompatibility complex: haplotypes, duplication, retroviruses and disease. Immunological reviews. 1999;167:275–304. doi: 10.1111/j.1600-065x.1999.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 11.Friedman JM, Pachman LM, Maryjowski ML, et al. Immunogenetic studies of juvenile dermatomyositis: HLA-DR antigen frequencies. Arthritis and rheumatism. 1983;26(2):214–6. doi: 10.1002/art.1780260216. [DOI] [PubMed] [Google Scholar]
- 12.Pachman LM, Fedczyna TO, Lechman TS, Lutz J. Juvenile dermatomyositis: the association of the TNF alpha-308A allele and disease chronicity. Current rheumatology reports. 2001;3(5):379–86. doi: 10.1007/s11926-996-0007-5. [DOI] [PubMed] [Google Scholar]
- 13.Yu CY, Whitacre CC. Sex, MHC and complement C4 in autoimmune diseases. Trends in immunology. 2004;25(12):694–9. doi: 10.1016/j.it.2004.10.006. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 14.Horton R, Gibson R, Coggill P, et al. Variation analysis and gene annotation of eight MHC haplotypes: the MHC Haplotype Project. Immunogenetics. 2008;60(1):1–18. doi: 10.1007/s00251-007-0262-2. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu YL, Savelli SL, Yang Y, et al. Sensitive and specific real-time polymerase chain reaction assays to accurately determine copy number variations (CNVs) of human complement C4A, C4B, C4-long, C4-short, and RCCX modules: elucidation of C4 CNVs in 50 consanguineous subjects with defined HLA genotypes. Journal of immunology. 2007;179(5):3012–25. doi: 10.4049/jimmunol.179.5.3012. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Mendoza AR, Welch TR, Zipf WB, Yu CY. Modular variations of the human major histocompatibility complex class III genes for serine/threonine kinase RP, complement component C4, steroid 21-hydroxylase CYP21, and tenascin TNX (the RCCX module). A mechanism for gene deletions and disease associations. The Journal of biological chemistry. 1999;274(17):12147–56. doi: 10.1074/jbc.274.17.12147. [DOI] [PubMed] [Google Scholar]
- 17.Dangel AW, Mendoza AR, Baker BJ, et al. The dichotomous size variation of human complement C4 genes is mediated by a novel family of endogenous retroviruses, which also establishes species-specific genomic patterns among Old World primates. Immunogenetics. 1994;40(6):425–36. doi: 10.1007/BF00177825. [DOI] [PubMed] [Google Scholar]
- 18.Yu CY. The complete exon-intron structure of a human complement component C4A gene. DNA sequences, polymorphism, and linkage to the 21-hydroxylase gene. Journal of immunology. 1991;146(3):1057–66. [PubMed] [Google Scholar]
- 19.Yu CY, Belt KT, Giles CM, Campbell RD, Porter RR. Structural basis of the polymorphism of human complement components C4A and C4B: gene size, reactivity and antigenicity. The EMBO journal. 1986;5(11):2873–81. doi: 10.1002/j.1460-2075.1986.tb04582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isenman DE, Young JR. The molecular basis for the difference in immune hemolysis activity of the Chido and Rodgers isotypes of human complement component C4. Journal of immunology. 1984;132(6):3019–27. [PubMed] [Google Scholar]
- 21.Law SK, Dodds AW, Porter RR. A comparison of the properties of two classes, C4A and C4B, of the human complement component C4. The EMBO journal. 1984;3(8):1819–23. doi: 10.1002/j.1460-2075.1984.tb02052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidmose RT, Laursen NS, Dobo J, et al. Structural basis for activation of the complement system by component C4 cleavage. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(38):15425–30. doi: 10.1073/pnas.1208031109. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitaker JN, Engel WK. Vascular deposits of immunoglobulin and complement in idiopathic inflammatory myopathy. The New England journal of medicine. 1972;286(7):333–8. doi: 10.1056/NEJM197202172860701. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 24.Kissel JT, Mendell JR, Rammohan KW. Microvascular deposition of complement membrane attack complex in dermatomyositis. The New England journal of medicine. 1986;314(6):329–34. doi: 10.1056/NEJM198602063140601. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 25.Emslie-Smith AM, Engel AG. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Annals of neurology. 1990;27(4):343–56. doi: 10.1002/ana.410270402. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 26.Kissel JT, Halterman RK, Rammohan KW, Mendell JR. The relationship of complement-mediated microvasculopathy to the histologic features and clinical duration of disease in dermatomyositis. Archives of neurology. 1991;48(1):26–30. doi: 10.1001/archneur.1991.00530130034016. [DOI] [PubMed] [Google Scholar]
- 27.Mascaro JM, Jr, Hausmann G, Herrero C, et al. Membrane attack complex deposits in cutaneous lesions of dermatomyositis. Archives of dermatology. 1995;131(12):1386–92. doi: 10.1001/archderm.1995.01690240040007. [DOI] [PubMed] [Google Scholar]
- 28.Burgin S, Stone JH, Shenoy-Bhangle AS, McGuone D. Case records of the Massachusetts General Hospital. Case 18-2014. A 32-Year-old man with a rash, myalgia, and weakness. The New England journal of medicine. 2014;370(24):2327–37. doi: 10.1056/NEJMcpc1304161. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 29.Blanchong CA, Zhou B, Rupert KL, et al. Deficiencies of human complement component C4A and C4B and heterozygosity in length variants of RP-C4-CYP21-TNX (RCCX) modules in caucasians. The load of RCCX genetic diversity on major histocompatibility complex-associated disease. The Journal of experimental medicine. 2000;191(12):2183–96. doi: 10.1084/jem.191.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung EK, Yang Y, Rennebohm RM, et al. Genetic sophistication of human complement components C4A and C4B and RP-C4-CYP21-TNX (RCCX) modules in the major histocompatibility complex. American journal of human genetics. 2002;71(4):823–37. doi: 10.1086/342777. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Neill GJ, Yang SY, Dupont B. Two HLA-linked loci controlling the fourth component of human complement. Proceedings of the National Academy of Sciences of the United States of America. 1978;75(10):5165–9. doi: 10.1073/pnas.75.10.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robb SA, Fielder AH, Saunders CE, et al. C4 complement allotypes in juvenile dermatomyositis. Human immunology. 1988;22(1):31–8. doi: 10.1016/0198-8859(88)90049-3. [DOI] [PubMed] [Google Scholar]
- 33.Reed AM, Pachman L, Ober C. Molecular genetic studies of major histocompatibility complex genes in children with juvenile dermatomyositis: increased risk associated with HLA-DQA1 *0501. Human immunology. 1991;32(4):235–40. doi: 10.1016/0198-8859(91)90085-n. [DOI] [PubMed] [Google Scholar]
- 34.Moulds JM, Rolih C, Goldstein R, et al. C4 null genes in American whites and blacks with myositis. The Journal of rheumatology. 1990;17(3):331–4. [PubMed] [Google Scholar]
- 35.Davis WR, Halls JE, Offiah AC, Pilkington C, Owens CM, Rosendahl K. Assessment of active inflammation in juvenile dermatomyositis: a novel magnetic resonance imaging-based scoring system. Rheumatology. 2011;50(12):2237–44. doi: 10.1093/rheumatology/ker262. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 36.Chung EK, Yang Y, Rupert KL, et al. Determining the one, two, three, or four long and short loci of human complement C4 in a major histocompatibility complex haplotype encoding C4A or C4B proteins. American journal of human genetics. 2002;71(4):810–22. doi: 10.1086/342778. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung EK, Wu YL, Yang Y, Zhou B, Yu CY. Human complement components C4A and C4B genetic diversities: complex genotypes and phenotypes. Current protocols in immunology / edited by John E. Coligan … [et al.] 2005;Chapter 13(Unit 13):8. doi: 10.1002/0471142735.im1308s68. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 38.Sim E, Cross SJ. Phenotyping of human complement component C4, a class-III HLA antigen. The Biochemical journal. 1986;239(3):763–7. doi: 10.1042/bj2390763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manzi S, Navratil JS, Ruffing MJ, et al. Measurement of erythrocyte C4d and complement receptor 1 in systemic lupus erythematosus. Arthritis and rheumatism. 2004;50(11):3596–604. doi: 10.1002/art.20561. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 40.Putterman C, Furie R, Ramsey-Goldman R, et al. Cell-bound complement activation products in systemic lupus erythematosus: comparison with anti-double-stranded DNA and standard complement measurements. Lupus science & medicine. 2014;1(1):e000056. doi: 10.1136/lupus-2014-000056. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hui K, Bidwell J. Handbook of HLA Typing Techniques. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- 42.Smyth GK, Speed T. Normalization of cDNA microarray data. Methods. 2003;31(4):265–73. doi: 10.1016/s1046-2023(03)00155-5. [DOI] [PubMed] [Google Scholar]
- 43.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(9):5116–21. doi: 10.1073/pnas.091062498. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willems E, Leyns L, Vandesompele J. Standardization of real-time PCR gene expression data from independent biological replicates. Analytical biochemistry. 2008;379(1):127–9. doi: 10.1016/j.ab.2008.04.036. [published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 45.Walsh RJ, Kong SW, Yao Y, et al. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis and rheumatism. 2007;56(11):3784–92. doi: 10.1002/art.22928. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tezak Z, Hoffman EP, Lutz JL, et al. Gene expression profiling in DQA1*0501+ children with untreated dermatomyositis: a novel model of pathogenesis. Journal of immunology. 2002;168(8):4154–63. doi: 10.4049/jimmunol.168.8.4154. [DOI] [PubMed] [Google Scholar]
- 47.Baechler EC, Bilgic H, Reed AM. Type I interferon pathway in adult and juvenile dermatomyositis. Arthritis research & therapy. 2011;13(6):249. doi: 10.1186/ar3531. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah M, Mamyrova G, Targoff IN, et al. The clinical phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine. 2013;92(1):25–41. doi: 10.1097/MD.0b013e31827f264d. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.