Abstract
Endocrine disrupting chemicals (EDCs) may increase the risk of childhood diseases by disrupting hormonally mediated processes critical for growth and development during gestation, infancy, or childhood. The fetus, infant, and child may have enhanced sensitivity to environmental stressors like EDCs due to rapid development and greater exposure to some EDCs that results from their developmentally appropriate behavior, anatomy, and physiology. This review summarizes epidemiological studies examining the relations of early-life exposure to bisphenol A (BPA), phthalates, triclosan, and perfluoroalkyl substance (PFAS) with childhood neurobehavioral disorders and obesity. The available epidemiological evidence suggests that prenatal exposure to several of these ubiquitous EDCs is associated with adverse neurobehavior (BPA and phthalates) and excess adiposity or increased risk of obesity/overweight (PFAS). Quantifying the effects of EDC mixtures, improving EDC exposure assessment, reducing bias from confounding, identifying periods of heightened vulnerability, and elucidating the presence and nature of sexually dimorphic EDC effects would result in stronger inferences from epidemiological studies. Ultimately, better estimates of the causal effects of EDC exposures on child health could help identify susceptible sub-populations and lead to public health interventions to reduce these exposures.
Introduction
Accumulating research shows that environmental stressors during gestation, infancy, and early childhood are risk factors for diseases in childhood and adulthood.1,2 These studies demonstrate that perturbation of sensitive biological processes during distinct periods of development can increase the risk of adverse health outcomes years or decades after exposure to the stressor. For example, exposure to environmental chemicals, pharmaceuticals, tobacco smoke, alcohol, and stress increase the risk of obesity, type 2 diabetes, reproductive disorders, neurodevelopmental disorders/deficits, and cancer.3–9 Well-established examples include the increased risk of vaginal clear cell carcinoma following prenatal diethylstilbestrol exposure; cognitive decrements among children with prenatal or childhood exposure to lead or mercury; and childhood obesity among offspring born to smokers, despite lower birth weight.3–5,10
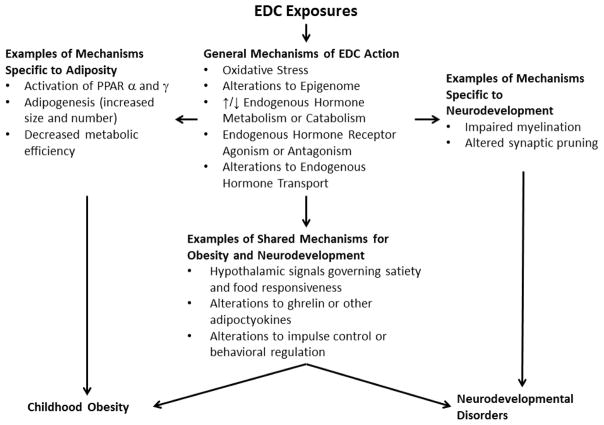
Environmental chemical exposures are one stressor that may adversely affect normal human development. Endocrine disrupting chemicals (EDCs) are a class of chemicals that could increase the risk of disease across the lifespan by altering the homeostasis or action of endogenous hormones or other signalling chemicals of the endocrine system.11 EDCs may increase the risk of disease by altering the production, release, transport, metabolism, binding, action, or elimination of endogenous hormones that program or maintain normal growth and development (Figure 1). There is particular concern that the fetus, infant, or child may have higher exposure to some EDCs or be more vulnerable compared to adults.
Figure 1.
Conceptual diagram illustrating general mechanisms of endocrine disrupting chemical (EDC) action and examples of specific biological targets relevant to childhood neurodevelopmental disorders and obesity
The fetus, infant, and child may have higher exposure to some EDCs than adults. because of developmentally appropriate differences in diet, behavior, physiology, anatomy, and toxicokinetics.12 For instance, infants and children may have higher exposure to some EDCs than adults because they consume more water and greater quantities of specific foods, and have higher ventilation rates, intestinal absorption, surface area to volume ratios, and hand-to-mouth activity.13 In addition, breastfed infants may have higher serum concentrations of some peristent EDCs than their mothers becaues of lactational exposure.14
In addition to higher exposure to some EDCs, the fetus, infant, and child may be more sensitive to the effects of EDCs than adults for two reasons. First, differences in toxicokinetics can result in higher circulating or tissue concentrations of an EDC for a given dose. For example, compared to adults, the fetus has lower levels of several cytochrome P450 enzymes that metabolize environmental chemicals and pharmaceuticals.15,16 Second, there are many time-dependent and synchronized processes that are programmed during early development that could increase the risk of childhood disease if perturbed. For instance, disruption of neurulation, neuronal differentiation/proliferation/migration, gliogenesis, synaptogenesis, dendritic growth, myelination, apoptosis, synaptic pruning, or neurotransmitter systems could increase the risk of behavioral disorders or cognitive deficits.17,18 In addition, epigenetic mechanisms, some of which are hormonally regulated, may mediate some of the effects of early life EDCs exposures on long term health outcomes.19 Thus, there is concern that early life exposure to EDCs may increase the risk of childhood diseases, including neurodevelopmental disorders and obesity. 20,21
EDCs may increase the risk of childhood neurodevelopmental disorders by interfering with early life thyroid hormone signaling or metabolism. Thyroid hormones play a critical role in neuronal migration, synaptogenesis, and myelination during gestation and childhood.22 Even clinically non-significant variations in maternal thyroxine or thyroid stimultaing hormone (TSH) levels during pregnancy are associated with reduced cognitive abilities, 23 attention-deficit hyperactivity disorder (ADHD) symptoms,24 and increased autism risk.23–26 Studies show that the timing of thyroid hormone availability influences the neurobehavioral phenotype. Gestational thyroxine reductions are associated with deficits in visual processing, visuomotor abilities, and motor skills, while postnatal reductions are associated with deficits in language, fine motor skills, attention, and memory.27–30 That both pre- and postnatal thyroid hormones are necessary for different aspects of neurodevelopment illustrates the potential time-dependent sensitivity of the developing brain to thyroid hormone disruptions.
Early life EDC exposures may perturb neuroendocrine systems involved in growth, energy metabolism, appetite, adipogenesis, and glucose-insulin homeostasis to promote childhood obesity, cardiometabolic dysfunction, and liver dysfunction.31–34 These perturbations may lead to a ‘thrifty phenotype’ that promotes more efficient energy storage, rapid early life weight gain, and excess adipose mass.35–41 Rapid growth and excess adiposity lead to increased circulating levels of free fatty acids, in turn causing a cascade of metabolic changes that reduce the capacity for liver and muscle to absorb, store, and metabolize glucose,42–44 which in turn casues increased pancreatic insulin secretion and resistance. In the setting of insulin resistance, excess adipose tissue lipolysis contributes to increased delivery of free fatty acids to the liver, de novo hepatic lipogenesis, accumulation of triglycerides in the liver vacuoles, and hepatic steatosis (i.e., non-alcoholic fatty liver disease).45
Despite their seemingly unrelated nature, shared neuroendocrine pathways could be disrupted by EDC exposures to influence the risk of both childhood obesity and neurodevelopment disorders (Figure 1).46–48 Indeed, the prevalence of excess adiposity is higher among children with behavioral disorders, like attention-deficit hyperactivity disorder (ADHD), and obese children have lower academic achievement, impaired attention and working memory, and reduced cortical thickness and white matter integrity compared to lean children.49–51 Moreover, up to 30% of the genes associated with adiposity are the same as those associated with processing speed.52 Finally, increased impulsivity, a key feature of ADHD, is related to food responsivity in adolescents53 and administration of the adipocytokine ghrelin to rodents increases impulsive behaviors.54
There is concern over the health effects of EDC mixtures.55 Despite biomonitoring studies documenting that humans are exposed to dozens of potential EDCs across the lifespan and that some EDC exposures are correlated with each other,56,57 most epidemiological studies have examined the health effects of EDCs as if they occur in isolation from one another. Without accounting for EDC mixtures, the available literature may fail to quanitfy the synergistic or cumulative healht effects of EDCs, as well as confounding due to correlated co-pollutants.
Given the above, this narrative review has three objectives. First, this review will discuss epidemiological studies examining associations between early-life exposure to several EDCs and childhood neurodevelopmental disorders and obesity. It will focus on select EDCs for which there is widespread general population exposure, specifically phthalates, bisphenol A (BPA), perfluoroalkyl substances (PFAS), and triclosan (Table 1). There are other excellent reviews for readers interested in chemicals with declining exposure that have been banned or phased out of production (e.g., organochlorine compounds).58 As a second objective, this review will describe some limitations to making stronger inferences from epidemiological studies about the impact of EDC exposures on child health and propose how researchers might address these limitations through better study designs and methods. Finally, this review closes with some guidance for clinicians to address patients’ concerns about EDC exposure reduction.
Table 1.
Epidemiological exposure assessment and commercial/industrial uses of phthalates, bisphenol A, polybrominated diphenyl ethers, and perfluoroalkyl substances
| EDC | Exposure Assessment | Uses in Commerce or Industry |
|---|---|---|
| Di-2-ethylhexyl phthalate (DEHP) | Urine concentrations of mono-2-ethylhexyl (MEHP), mono-2-ethyl-5-carboxypentyl (MECPP), mono-2-ethyl-5-hydroxyhexyl (MEHHP), and mono-2-ethyl-5-oxohexyl (MEOHP) phthalate | PVC plastics, food packaging, and plastic medical tubing and bags. |
| Butylbenzyl phthalate (BBzP) | Urine concentrations of monobenzyl phthalate (MBzP) | Vinyl flooring, adhesives, food packaging, synthetic leather, and toys. |
| Diethyl phthalate (DEP) | Urine concentrations of monoethyl phthalate (MEP) | Scent retainer in personal care products and medication excipient. |
| Di-n/i-butyl phthalate (DnBP and DiBP) | Urine concentrations of mono-n/i-butyl phthalates (MnBP and MiBP) | Scent retainer in personal care products, medication excipients, cellulose plastics, & adhesives. |
| Bisphenol A (BPA) | Urine concentrations of BPA | Polycarbonate plastics, resins, thermal receipts, food cans, dental fillings, and medical equipment. |
| Triclosan | Urine concentrations of triclosan | Antimicrobial soaps, personal care products, toothpaste, kitchen utensils, clothes, and cleaning products. |
| Perfluoroalkyl substances (PFAS) | Serum concentrations of individual perfluoroalkyl or perfluorinated chemicals | Stain/water resistant coatings, non-stick cookware, food container coatings, floor polish, fire-fighting foam, and industrial surfactants. |
Phthalates
Phthalate Uses, Exposure, and Measurement
Phthalates are a class of EDCs used in a multitude of consumer products, including personal care products, medications, and plastics (Table 1). Biomonitoring studies from around the world indicate that there is universal phthalate exposure among pregnant women, infants, and children.59–72 Phthalate exposure occurs through ingestion, inhalation, or dermal absorption.73–76 Additionally, phthalates can cross the placenta, resulting in exposure to the fetus.77
After entering the body, phthalates are rapidly hydrolyzed to their respective monoester metabolites (Table 1).78 Low molecular weight phthalates (di-ethyl phthalate [DEP], di-n-butyl phthalate, and di-iso-butyl phthalate) are excreted in the urine as glucuronide or sulfate conjugated hydrolytic monoesters, while mono-2-ethylhexyl phthalate, the hydrolytic metabolite of di-2-ethylhexyl phthalate (DEHP), undergoes additional enzymatic oxidation before being conjugated and excreted. Although phthalates do not persist in the body and have short biological half-lives (<24 hours), there is repeated, episodic, and long-term exposure. Phthalate exposure is assessed using urine biospecimens since phthalates are predominately excreted in the urine and blood levels, which are considerably lower, may be subject to exogenous contamination during sample collection, storage, or processing.79
Misclassification of phthalate exposure is a concern in epidemiological studies due to their short biological half-lives and the episodic nature of phthalate exposures from diet (e.g., DEHP) or personal care products (e.g., DEP). To address this concern, accurate phthalate exposure assessment necessitates the collection and analysis of multiple urine samples.80
Biological Mechanisms of Phthalate Action
Phthalates may interfere with the action or metabolism of androgens, thyroid hormones, and glucocorticoids. Some phthalates are anti-androgenic and reduce testicular testosterone production by decreasing the expression of genes involved in steroidogenesis and steroid trafficking.81,82 Animal and human studies show that some phthalates may reduce thyroxine and triiodothyronine concentration in pregnant women and children,83–85 antagonize T3 binding to thyroid receptor-β,86 reduce cellular T3 uptake,87 and affect transcription of the sodium-iodine transporter.88 Phthalates can also inhibit 11-β-hydroxysteroid dehydrogenase-2, which deactivates cortisol.89 In addition, phthalate exposure may affect offspring health by causing oxidative stress90 or via epigenetic re-programming of the fetus and placenta.91
There is concern about the health effects of phthalate mixtures since humans are exposed to multiple phthalates at once and rodent studies demonstrate that phthalates have concentration additive effects on fetal androgen production.82,92 Thus, the aggregate of individual phthalate exposures may have an additive impact on human health since individual phthalates share a common mechanism of action.
Phthalates and Neurodevelopment
Six publications from four prospective cohort studies report that prenatal exposure to several different phthalates is associated with ADHD behaviors,59,60,93,94 autistic behaviors,61 reduced mental and psychomotor development,60,62 emotional problems,60 and reduced IQ.63 In a prospective cohort of 328 mothers, the reductions in child IQ associated with increasing maternal urinary phthalate concentrations were as large as or larger than the cognitive decrements observed with childhood lead exposure (5th vs. 1st quintile: 7-points; 95% confidence interval [CI]: 2, 11).3,63 It is important to note that three other publications did not find associations between prenatal phthalate exposure and child neurobehavior.64–66
Three publications report that childhood exposure to some phthalates is associated with reduced cognitive abilities65 and behavioral problems.67,68 However, these studies were cross-sectional and reverse causality may explain these findings.
In summary, the epidemiological literature to date suggests that prenatal phthalate exposure may be associated with behavioral problems and cognitive decrements in children. Inconsistencies across studies may be due to differences in the timing of when phthalate exposure was assessed (e.g., early vs. late gestation), misclassification of phthalate exposure from studies using a single urine sample to assess exposure, or differences in child age at neurodevelopment assessment.
Phthalates and Adiposity/Obesity
Three publications from prospective cohorts examined prenatal phthalate exposure and childhood adiposity.70–72 One publication reported that prenatal DEHP exposure is associated with decreased body mass index (BMI) in boys and increased BMI in girls,70 whereas another publication found that non-DEHP phthalate exposures were associated with decreased BMI in boys, but not girls.71 A third publication did not report any association between prenatal phthalate exposure and childhood adiposity.72 In a pooled cohort including US participants from two of the previously mentioned cohort studies, increasing maternal urinary mono-3-carboxypropyl phthalate concentrations during pregnancy were associated with a doubling in the risk of being overweight or obese (95% CI: 1.2, 4.0).95
Four publications have examined childhood phthalate exposure and adiposity.71,96–98 Three of these reported that childhood exposure to DEP was associated with excess adiposity and increased prevalence of obesity or excess adiposity,98 but another did not.71 In a prospective cohort of over 1,000 US girls, higher DEP exposure at 6–8 years of age was associated with increased BMI scores and waist circumference at 7–13 years of age.97 In a cross-sectional study of US children, increasing urine concentrations of low molecular weight phthalates was associated with a 22% increase in the prevalence of obesity.99
In summary, the associations between early-life phthalate exposure and child adiposity or obesity risk have been inconsistent and do not support the hypothesis that phthalates are chemical obesogens. The one exception to this was the association between childhood DEP exposure and child adiposity or obesity prevalence. However, this association may be due to children with higher adiposity also having greater surface area, leading to the application of greater amounts of phthalate-containing personal care products to their skin, which in turn results in higher urinary MEP concentrations.99
BPA
BPA Uses, Exposure, and Measurement
BPA is used to produce polycarbonate plastics and resins that are used in a wide range of consumer products (Table 1). Oral ingestion is the predominant exposure route since BPA can leach into food and beverage containers; however, dermal absorption and inhalation may be additional routes of exposure among persons working with BPA-containing receipts.100–102 BPA is excreted in the urine as glucuronide/sulfate conjugates, does not persist in the body, and has an estimated biological half-life of ~6 hours.103 BPA exposure is assessed by measuring urine concentrations of free and conjugated BPA. Urine is used since BPA is almost exclusively excreted in the urine, and blood levels are considerably lower and subject to exogenous contamination.79 Biomonitoring studies around the world indicate nearly universal BPA exposure among pregnant women, infants, and children.56,64,104–120 Much like some phthalates, urinary BPA concentrations have considerable within-person variation due to diet being the predominant source of exposure. Thus, it is essential to collect multiple urine samples to ensure accurate exposure assessment.
Biological Mechanisms of BPA Action
BPA may interact with a variety of hormonal systems that affect growth, metabolism, and neurodevelopment. Dodds and Lawson recognized BPA as a weak estrogen in 1936.121 BPA is a weak agonist of the nuclear estrogen receptors α and β compared to estradiol.122 However, BPA may also act on plasma protein bound estrogen receptors allowing BPA to interfere with estrogenic signaling at nanomolar and picomolar levels.123,124 In vitro studies show that BPA may affect androgen/estrogen concentrations by inhibiting key enzymes involved in gonadal hormone synthesis and metabolism,125 but results from human studies are not consistent.126–128 Rodent studies have found that prenatal BPA exposure is associated with higher T4 levels in offspring129 and thyroid-specific gene expression.130 Some epidemiological studies show that BPA exposure is associated with altered maternal, neonatal, or adolescent thyroid parameters.131–133
BPA and Neurodevelopment
In 2008, the National Toxicology Program concluded that there was “some concern” over the effect of BPA on neurobehavioral endpoints based on findings in animal studies.134 Since then, there have been eight studies from five prospective cohorts examining prenatal BPA exposure and child neurobehavior.64,110–116 Four publications from three of these prospective cohorts reported that prenatal BPA exposure was associated with more internalizing behaviors in children, with stronger associations in boys than girls.110,113–115 Two publications from another cohort reported that prenatal BPA exposure was associated with more internalizing and externalizing behaviors in girls, but not boys.111,112 One publication reported that prenatal BPA concentrations were associated with increased risk of ADHD-related behaviors at 4 years of age, with stronger associations in boys.116 Two other publications have reported that prenatal urinary BPA concentrations were not associated with parent-reported reciprocal social behaviors.61,64 The association between prenatal BPA exposures and children’s cognitive abilities has not been thoroughly examined, with one study reporting that prenatal urinary BPA concentrations were associated with parent-reported executive function in 3-year old girls.112
Seven publications have examined the relation between childhood BPA exposures and behavior problems or ADHD-related behaviors.67,110,112–114,135,136 Generally, the results are inconsistent. Some report that childhood BPA exposures were associated with ADHD behaviors in boys,135 ADHD behaviors in both boys and girls,135 anxious/depressive/aggressive behaviors in girls,110,113,114 or learning problems.137 Some publications report null associations between childhood BPA exposures and neurobehavior.67,112
In summary, these studies suggest that both prenatal and postnatal BPA exposure is associated with parent-reported behavior problems in children, but there are inconsistencies across these studies with regard to the period of life with the greatest vulnerability to exposure (prenatal vs. infancy vs. childhood) and sex-specific effects. The heterogeneity of these findings could be due to the substantial within-person variation of urinary BPA concentrations that results in BPA exposure misclassification.
BPA and Adiposity/Obesity
Seven publications from prospective cohort studies have examined whether early-life BPA exposure is obesogenic.104–107,117,118,138 Three publications report that higher prenatal BPA exposure was associated with lower BMI and that these associations were stronger in girls.104–106 Two other publications reported that prenatal BPA exposure was associated with increased waist circumference, BMI, and risk of being overweight or obese.107,117 Two other publications reported no association between prenatal BPA exposure and child adiposity measures.118,138
Some of the aforementioned studies have prospectively examined the relation between infant or childhood BPA exposure and subsequent adiposity,104–107 and several cross-sectional studies have been conducted.119,120 Among publications with prospective measures of BPA exposure during infancy or childhood, there is little evidence to suggest that BPA exposure is associated with excess adiposity.104–107 However, cross-sectional studies show that BPA exposure is positively correlated with adiposity or the prevalence of being overweight or obese.105,106,120 For instance, in a nationally representative sample of US children, children in the top 3 quartiles of urinary BPA concentrations were ~25% more likely to be overweight and >2-fold more likely to be obese compared to children in the lowest quartile.119 However, the strength of this association did not monotonically increase across quartiles of BPA exposure, suggesting a threshold effect or potential residual confounding. Still, other publications report no association between infant or childhood BPA exposure and childhood obesity.104,107
The available epidemiological literature is equivocal about the obesogenic effects of early-life BPA exposure, with studies showing both increases and decreases in adiposity or risk of being overweight or obese with higher early-life BPA exposure. Cross-sectional associations between urinary BPA concentrations and childhood adiposity could be due to residual confounding from dietary factors that are associated with both BPA exposure and adiposity. In addition, reverse causality could be responsible for these correlations if persons with excess adiposity have different dietary patterns that increase their exposure to BPA.
Triclosan
Triclosan Uses, Exposure, and Measurement
Triclosan is an antimicrobial chemical that disrupts bacterial lipid synthesis and cell membrane integrity and is used in numerous consumer products (Table 1).139 Exposure is predominately through oral and dermal routes.139 Triclosan is not persistent, has a biological half-life <24 hours, and is predominately excreted in the urine as a glucuronide or sulfate conjugate.140 Triclosan exposure is measured using urine biospecimens for the same reasons BPA and phthalates are measured using urine biospecimens.79 Biomonitoring studies indicate nearly universal triclosan exposure among pregnant women and children.56,108,141 While triclosan has a short biological half-life similar to that of BPA and some phthalates, unlike BPA and some phthalates, urinary triclosan concentrations are relatively stable over time within a person.
Biological Mechanisms of Triclosan Action
Triclosan can disrupt gonadal and thyroid hormone homeostasis. In rodents, triclosan reduces testosterone production by disrupting cholesterol biosynthesis in Leydig cells.142,143 In a systematic review and meta-analysis of eight rodent studies, triclosan exposure reduced thyroxine concentrations in the fetus, dam, neonate, and juvenile.144 Triclosan may reduce thyroxine concentrations by activating nuclear receptors to increase hepatic catabolism of thyroxine.145 Results from epidemiological studies of triclosan and thyroid function are not consistent.146,147 However, there are no prospective epidemiological studies examining the relation between triclosan and thyroid hormone biomarkers during gestation, infancy, or childhood. Finally, given its antimicrobial activity, triclosan may be capable of altering the composition or function of the microbiota, but there is little research examining this hypothesis.148
Triclosan and Neurodevelopment
Currently, there are no animal or epidemiological studies directly examining the neurotoxicity of early-life triclosan exposure. As noted, triclosan exposure may reduce serum thyroxine concentrations during pregnancy and this could cause adverse neurodevelopment given the important role that thyroxine plays in fetal brain development.23 Three epidemiological studies report suggestive inverse associations between prenatal triclosan exposure and neonatal anthropometry.118,149,150 Thus, given that head circumference is positively correlated with later life IQ, early-life triclosan exposure could adversely impact neurodevelopment.151
Triclosan and Adiposity/Obesity
Four publications have reported on the relation between triclosan exposure and childhood obesity or excess adiposity.152,153 Two cross-sectional studies using 6–19 year old children from the National Health and Nutrition Examination Survey report conflicting results. One publication (years: 2007–2010), found no association between urinary triclosan concentrations and BMI z-score, waist circumference, or prevalence of being overweight or obese.154 In the second publication (years: 2003–2010), urinary triclosan concentrations were associated with a monotonic decrease BMI and waist circumference.153 Another publication a reported that urinary triclosan concentrations were similar in lean and overweight/obese children.155 In a prospective cohort study, prenatal triclosan exposure was not associated with child adiposity at 4 to 9 years of age.138
In summary, there is insufficient evidence to determine if early-life triclosan exposure is associated with childhood obesity. The inconsistent results between studies using the NHANES speaks to the importance of replication when examining the potential health effects of EDCs.
Perfluoroalkyl Substances
PFAS Uses, Exposure, and Measurement
PFAS are a class of man-made fluorinated chemicals used in stain/water resistant coatings for textiles, non-stick cookware, food container coatings, floor polish, fire-fighting foam, and industrial surfactants (Table 1).156 The strong C-F chemical bond makes PFAS extremely resistant to thermal, chemical, and biological degradation, which results in bioaccumulation and persistence in human tissues for years.157 Four perfluoroalkyl acids - perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), perfluorononanoic acid (PFNA), and perfluorohexane sulfonate (PFHxS) - are almost universally detected in the serum of pregnant women, neonates, and children worldwide, indicating that exposure is ubiquitous and these chemicals can cross the placenta.56,64,158–176
Unlike phthalates, BPA, and triclosan, PFAS have long biological half-lives in humans, ranging from 3.8 to 7.3 years.177 Thus, a single serum or plasma concentration is sufficient to characterize exposure for epidemiological studies. The sources and relative contributions of different PFAS to human exposure vary according to age-related behavioral factors and dietary patterns, and PFAS exposures during infancy can equal or exceed prenatal exposures derived from the mother.156,178 For instance, breast milk and formula may contribute almost exclusively to infant’s exposure since PFAS are found in breast milk and water, and infants consume up to 6 times as much fluid as adults (150 vs. 26 ml/kg/day).179–181
Biological Mechanisms of PFAS Action
PFAS may act on a number of endocrine pathways to affect the risk of neurodevelopmental disorders and obesity. In epidemiological studies, PFOA and PFOS exposures are associated with lower global DNA cytosine methylation, higher Long Interspersed Nuclear Element-1 methylation, and changes in the expression of genes involved in cholesterol metabolism.182–184 PFOA and PFOS can bind to and activate the peroxisome proliferator activated receptor (PPAR)-α/γ to increase adipocyte differentiation and increase body fat.185–187 In addition, PFOA, PFOS, and PFHxS inhibit 11-β-hydroxysteroid dehydrogenase-2 to increase glucocorticoid concentrations in rodents, which might affect growth and brain development.89 Animal studies show that PFAS are capable of inducing changes in thyroid function,188 but results from human studies are not consistent.189
PFAS and Neurodevelopment
Eleven publications from prospective cohorts have examined the relations between prenatal PFAS exposure and cognitive abilities,165,166 attainment of developmental milestones,167 parent or teacher reported behaviors and executive function,64,167–170 psychomotor development,169 academic achievement,171 or risk of autism spectrum disorders, ADHD, or cerebral palsy.172–174 With regard to the most commonly detected PFAS (PFOA, PFOS, PFNA, and PFHxS), these publications report inconsistent results. In a prospective birth cohort of 218 mother-child pairs, higher prenatal PFOS exposure was associated with worse parent-reported executive function.170 In another prospective birth cohort, increasing prenatal PFOS and PFOA exposures were associated with 70 (95% CI: 1.0, 2.8) and 110% (95% CI: 1.2, 3.6) increased risk of cerebral palsy, respectively.172 Several studies report protective or null associations between prenatal PFAS exposure and child neurobehavior.64,165–167,169,173
Four publications have examined the relations between childhood PFAS exposure and neurodevelopment.165,168,175,176 Two publications from cross-sectional studies report that children’s serum PFAS concentrations were associated with increased prevalence of parent-reported ADHD or ADHD medication use.168,175 However, in a prospective cohort study with exceptionally high PFOA exposure, children’s serum PFOA concentrations were not consistently associated with parent- or teacher-reported ADHD-related behaviors or other neuropsychological measurements.165,172
The available body of evidence does not consistently suggest that early-life PFAS exposures are associated with neurodevelopment. While some studies suggest adverse neurobehavioral outcomes among children with elevated prenatal or childhood PFAS exposures, there are inconsistencies regarding which individual PFAS exposures may be associated with neurobehavior and whether there are heightened periods of vulnerability to PFAS exposures. The protective associations between early-life PFAS exposure and neurodevelopment is biologically plausible because in vitro studies report that PFOA and PFOS are agonists of PPAR-γ and activation of this receptor may be neuroprotective.190 Additional studies with longitudinal measures of exposure and comprehensive assessment of neurodevelopment are warranted given the ubiquity and persistence of PFAS exposure.
PFAS and Adiposity/Obesity
There is a compelling body of evidence suggesting that prenatal PFAS exposure could affect fetal growth and subsequent risk of childhood obesity. In a systematic review of 18 publications, and subsequent meta-analysis of nine of these, increasing prenatal PFOA exposure was associated with a 19 gram decrease in birth weight (95% CI: −30, −8).158 These results in humans are similar to those observed in 21 rodent studies where each 1 mg/kg/d increase in PFOA exposure was associated with a 0.023 gram decrease in pup birth weight (95% CI: −0.029, −0.016).191 Altered fetal growth patterns related to PFAS exposure may increase the risk of subsequent obesity and cardiometabolic disorders as prior studies show that fetal growth deceleration and infancy growth acceleration are associated with increased adiposity and cardiometabolic risk markers in later childhood.39,192,193
Consistent with this hypothesis, five publications from prospective cohort studies report that prenatal PFAS exposure is associated with alterations in infant or child growth,160,163 increased adiposity during childhood and adulthood,159–161 and higher waist-to-height ratio.162 Two publications from other prospective cohort studies, including one with exceptionally high PFOA exposure, did not observe an association between prenatal PFAS exposure and child or adult adiposity or risk of being overweight or obese.164,194 These two studies used self- or parent-reported anthropometry, which could be responsible for the null results since there are well-documented errors in self- and parent-reported anthropometry that could misclassify adiposity and attenuate associations towards the null.195,196
Two studies have examined childhood PFAS exposure and adiposity. In a cross-sectional study of US adolescents, PFOS exposure was associated with decreased BMI and waist circumference, while other PFAS were not associated with BMI or waist circumference.197 In a prospective cohort study, PFOS exposure at 9 years of age (and to a less precise extent PFOA exposure) was associated with increased adiposity at 15 and 21 years of age.198
Challenges to Making Stronger Inferences about EDCs and Child Health
Chemical mixtures, exposure misclassification, confounding, periods of heightened vulnerability, and sexually dimorphic associations are challenges to making stronger inferences about the causal links between early-life EDC exposures and the risk of childhood diseases. I discuss below some tractable solutions that if incorporated into study design or statistical analysis, could improve our confidence in making causal inferences.
Chemical Mixtures
Exposure to a mixture of EDCs occurs across the lifespan, yet researchers primarily examine exposures as if they occur in isolation from one another. This “one chemical at a time” approach has left us with insufficient knowledge about the individual, interactive, and cumulative health effects of EDC mixtures. Epidemiological studies can address three broad questions related to EDC mixtures.199 First, given the large number of environmental agents to which humans are exposed, there is a need to identify those most strongly associated with adverse child health outcomes, particularly when little data are available about the toxicity of individual exposures. Second, multiple EDCs may have a synergistic association with health outcomes by disrupting the homeostasis of compensatory mechanisms. Finally, cumulative exposure to certain classes of EDCs (e.g., phthalates) could adversely impact child health when individual components of the mixture act via common biological pathways and cumulative exposure to these individual agents is sufficient to induce an adverse effect.
A recent workshop at the National Institute of Environmental Health Sciences brought together leaders in the fields of epidemiology, biostatistics, and toxicology to develop, implement, and compare different methods of quantifying the health effects of environmental chemical mixtures.200,201 Several methods showed promise in addressing questions related to EDC mixtures. For instance, Least Absolute Shrinkage and Selection Operator and elastic net methods can identify individual EDCs associated with health outcomes and their interactions while controlling for co-pollutant confounding. Bayesian Kernel Machine Regression is another method that can estimate the health effects of individual EDCs of concern while also examining potential interactions, non-linear dose-response functions, and co-pollutant confounding.202
Weighted quantile sum regression is a method that shows promise for quantifying the cumulative effect of EDC exposures while also estimating the relative contribution of individual components of the mixture to the health outcome of interest.203,204 Other methods of estimating the cumulative effect of EDC mixtures include mathematically weighting the sum of individual components of the mixture by their biological potency (e.g., toxic equivalency factors for dioxins),205 or quantifying the biological activity in biospecimens.206 These summation methods of examining cumulative exposures are likely too simplistic since they assume a single mechanism of EDC action. Thus, there is a need to develop methods that incorporate the potency of EDCs on multiple biological pathways.
By implementing methods that account for chemical mixtures, we are likely to identify previously undocumented chemical risk factors for childhood disease, susceptible subgroups, or aggregate exposures that should be considered in the risk assessment process. However, the performance of these methods has not been fully evaluated and a simulation study showed that some of these methods do not have perfect sensitivity and in some situations, there may be a high rate of false positives. 207
Exposure Misclassification
The episodic nature of many EDC exposures and short biological half-lives of their biomarkers can cause exposure misclassification. Exposure misclassification represents a signal to noise problem, where the misclassification results in a less precise estimate of an individual’s exposure and this makes it more difficult to rank their exposure relative to other individuals in the study. If misclassification is not systematically different with respect to childhood health outcomes, then the study will have reduced statistical power to detect an association and observed associations will be attenuated towards the null.
A simulation study showed that at least 10 estimates of exposure per individual may be required to ensure adequate statistical power, especially for chemical exposures like BPA and DEHP.80 One solution is to collect multiple urine samples from the same individual during a specific developmental period (e.g., 2nd trimester) and then create a pooled specimen for each individual using an equal volume of sample from their repeated samples.79,80 One disadvantage to the pooling method is that it does not allow for the examination of discrete periods of heightened vulnerability unless multiple pools are created for different periods of development. When a single measurement of exposure is available, statistical techniques like regression calibration could correct non-differential measurement error.80 To date, studies have not used regression calibration methods to correct for non-differential EDC exposure misclassification, but studies of air pollutants have successfully used these methods to study a variety of health effects while accounting for exposure measurement error.208
Despite the potential for non-differential misclassification of BPA and phthalate exposures, several studies have observed that early life BPA or phthalate exposures are associated with adverse child neurodevelopment. If non-differential misclassification is present, then this suggests that observed associations may be attenuated towards the null and true associations may be much stronger. However, non-causal explanations should not be ruled out, including confounding by traditional risk factors for adverse neurodevelopment or correlated co-pollutants.
Confounding
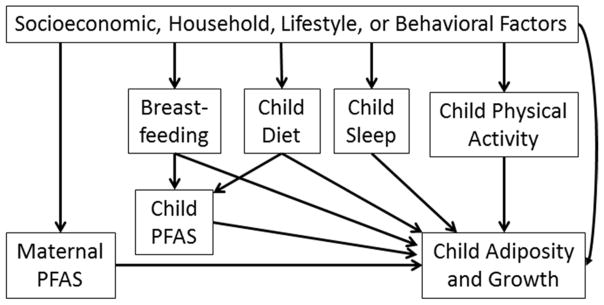
As is the case for all observational studies, there is the potential for confounding factors associated with both early-life EDC exposures and child health to bias study results. Socioeconomic factors are important determinants of childhood health and some chemical exposures. For instance, in the case of obesogens, many strong determinants of adiposity (e.g., diet) are correlated with lifestyle factors (e.g., maternal diet) that may also be associated with EDC exposures (Figure 2). Thus, to determine if a given EDC has obesogenic effects independent of other predictors of obesity, it is necessary to measure and control for factors like diet, physical activity, breast feeding, etc. When examining neurotoxic EDCs, many socioeconomic factors associated with exposure are also associated with parental IQ or behavior and the quality of caregiving environment, which are important determinants of child IQ and behavior. Finally, it is necessary to consider potential confounding from other EDC exposures since the effect of one EDC may be misattributed to another correlated co-pollutant.
Figure 2.
Directed acyclic graph for the relationship between early life PFAS exposure and child adiposity
Subject matter knowledge should guide the selection of potential confounders and various approaches can be used to identify a parsimonious statistical model (For an example, see the directed acyclic graphs and change in estimate approach development by Weng and colleagues).209 It is imperative to note that it is not appropriate to adjust for variables that are caused by EDC exposure and causes of poor childhood health (i.e., causal intermediates) since this ‘over-adjustment bias’ may mask causal associations.210 For instance, prenatal PFAS exposures are associated with lower birth weight and increased risk of childhood obesity. Moreover, birth weight is a determinant of childhood obesity risk.211 Thus, adjusting for birth weight might bias associations between PFAS and risk of childhood obesity.
Discrete Periods of Heightened Vulnerability
The potential effects of EDCs could be dependent on the timing of exposure given the possibility of unique periods of vulnerability to environmental stressors. For instance, the effect of EDC exposures on neurodevelopment could depend on different biological mechanisms specific to prenatal (e.g., neurulation) and postnatal (e.g., synaptic pruning) neurodevelopmental processes.18 This could be a reason for some of the heterogeneity in the results of the studies discussed above. This highlights the need for prospective studies with serial measures of EDC exposure across the lifespan, as well as appropriate statistical methods to identify periods of heightened vulnerability.199
Sexually Dimorphic Associations
Some associations between EDC exposures and childhood health are sexually dimorphic and EDCs may be capable of acting in a sex-specific manner given the important role that gonadal hormones play in shaping some sexually dimorphic traits.59,60,69,112,117 For instance, in a prospective cohort, prenatal exposure to anti-androgenic phthalates was associated with reduced masculine play behavior in boys, but not girls.69 The identification of sex-specific effects in epidemiological studies will require larger sample sizes than most studies conducted to date.
Advice for Clinicians and Concerned Patients
Presently, there are no evidence-based methods for reducing EDC exposures, but there are some general recommendations that clinicians could give to concerned patients. For EDCs found in the diet (e.g., BPA, DEHP, and PFAS), eating a balanced diet may be one way to avoid exposure from any one foodstuff, but this advice has not been empirically evaluated. Intervention studies show that decreasing or eliminating canned or packaged food consumption is effective at reducing BPA and DEHP exposure.73,100 An intervention study showed that handling BPA-containing thermal receipts was an important route of exposure and that wearing gloves could reduce BPA exposure from this route.102 Another study found some evidence that children who have handle thermal receipts may have higher BPA exposure.109
Individuals may be able to reduce their exposure to DEP and DnBP by reducing or eliminating the use of some lotions, cosmetics, and colognes/perfumes.76,212 However, there are no requirements for personal care products to include these phthalates in their ingredient list, making it difficult to avoid this source of exposure. Individuals can reduce triclosan exposure by avoiding triclosan-containing toothpastes. However, because triclosan-containing toothpastes are clinically indicated for some individuals, the benefits and risk of continued use should consider the specific conditions and susceptibilities of the individual (e.g., pregnancy). Finally, granular activated carbon water filtration systems may be effective at reducing PFAS exposure when consuming PFAS contaminated water,213 but this may have a minimal effect on total PFAS body burden when diet is the predominant source of PFAS exposure.214
Conclusions
Exposure to BPA, phthalates, triclosan, and PFAS is ubiquitous and occurs during potentially sensitive periods of development that are important in the etiology of childhood neurodevelopmental disorders and obesity. The available research suggests that prenatal BPA and phthalate exposures are related to adverse neurobehavioral outcomes in children. Furthermore, prenatal PFAS exposure is related to reduced fetal growth and excess childhood adiposity. While this review did not compare the findings of animal or laboratory studies to the results of epidemiological ones, future reviews or risk assessments of these chemicals should include this important feature of establishing causality.215
We can make stronger inferences about the role of EDCs in the etiology of childhood disease by quantifying the impact of chemical mixtures, reducing exposure misclassification, identifying sexually-dimorphic associations and periods of enhanced vulnerability, and collecting data on relevant potential confounders in prospective cohort studies. Ultimately, quantifying the impact of EDC exposures on child health could lead to the identification of susceptible sub-populations and reduction of EDC exposures via public health interventions.
Box 1. Neurodevelopmental Disorders of Childhood.
Many neurodevelopmental disorders develop during childhood, with prevalence in the United States of 1.5% for autism spectrum disorders (ASD), 6.7% for attention-deficit hyperactivity disorder (ADHD), 7.7% for specific learning disabilities (LD).216,217 The clinical presentation of these disorders varies between and within a diagnosis. For ASD, children have deficits in social communication and interactions, as well as repetitive, restricted, and stereotypic behaviors. Within the ASD diagnosis, deficits can be mild or severe and accompanied by intellectual disabilities (IQ<70) and substantial comorbidities.218 For ADHD, children present with hyperactivity, inattention, and poor impulse control, and display deficits in executive function (e.g., inhibition, behavioral regulation, and planning/organizing). ADHD diagnosis is classified into hyperactive/impulsive or inattentive subtypes.219 LD is characterized by difficulties learning and using specific academic skills (e.g., reading and mathematics) and is distinct from intellectual disability, which is defined by global impairments in cognitive function.
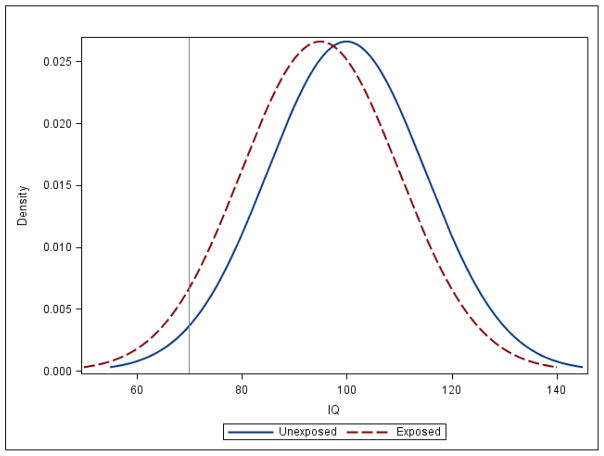
Many epidemiological studies assess continuously distributed measures of functional domains related to clinical phenotypes instead of clinical diagnoses.220,221 For instance, global measures of cognition (i.e., IQ) and academic performance can be used to study whether EDC exposures affect intellectual abilities and specific academic skills, respectively. Continuous outcomes are advantageous since they provide a relative ranking of an individual’s ability/behavior, which enhances statistical power. Moreover, clinical diagnosis could misclassify individuals since they may fail to detect earlier manifestations of disease and diagnostic thresholds are often set at arbitrary levels that change over time. By examining continuous neurobehavioral measures, epidemiological studies can determine if EDC exposures are associated with shifts in these traits at the population level, which could result in increased prevalence/incidence of clinical disorders (Figure B1).
Figure B1.
Density Distribution of IQ in EDC Unexposed and Exposed Populations
Figure B1 represents the distribution of IQ in two populations of one million individuals each. The grey line signifies the threshold for IQ scores consistent with intellectual disabilities (IQ<70).
In the unexposed population, the mean IQ is 100 (standard deviation=15), while in the exposed population the mean IQ is 95 (standard deviation=15). This 5-point shift in IQ results in nearly a doubling in the proportion of people with IQ scores consistent with intellectual disabilities (IQ<70) in the exposed population (4.48%) compared to the unexposed population (2.27%).
Box 2. Prevalence and Origins of Childhood Obesity.
Childhood obesity is a major threat to public health in the United States, with the prevalence rising from 7% in 1980 to 17% in 2012.222 Obesity is also a major public health problem globally, with 10–14% of adults worldwide being overweight or obese in 2008.223 Childhood obesity increases the risk of type-2 diabetes, cardiovascular disease, and metabolic syndrome, and has adverse effects on pulmonary, musculoskeletal, and psychosocial functioning.224
The principal cause of obesity is caloric imbalance from excess calorie intake and insufficient physical activity. However, there is considerable evidence that the in utero and neonatal environments program the developing fetus and infant for obesity risk.1,2 A non-optimal fetal or infant environment can lead to enduring functional and structural changes to the body that increase obesity risk by re-programming neuroendocrine systems involved in growth, energy metabolism, appetite, adipogenesis, and glucose-insulin homeostasis.32–34 These environmental stressors may program the fetus or infant towards a ‘thrifty phenotype’ that efficiently stores excess calories in a postnatal environment with abundant calories and reduced physical activity. Thus, children with this phenotype will efficiently store excess calories as fat, have altered insulin homeostasis, and ultimately develop a cardiometabolic disease profile.
Key Points.
Endocrine disrupting chemicals may increase the risk of childhood neurodevelopmental disorders or obesity by disrupting hormonally-mediated processes during critical periods of development.
The developing fetus, infant, and child may have enhanced sensitivity to environmental stressors like EDCs and greater exposure to some EDCs because of developmentally appropriate behavior, anatomy, and physiology.
The available epidemiological evidence suggests that prenatal bisphenol A and phthalate exposure is associated with adverse neurobehavioral outcomes in children, but not excess adiposity or risk of obesity/overweight.
Epidemiological studies show that prenatal PFAS exposure is associated with reduced fetal growth, excess adiposity, and risk of being overweight or obese, but not neurobehavioral outcomes.
Improving EDC exposure measurement, reducing confounding bias, identifying discrete periods of vulnerability and sexually dimorphic associations, and quantifying the effects of EDC mixtures will enhance inferences made from epidemiological studies.
Acknowledgments
Grant Funding: This work was support by NIH grants R00 ES020346, R01 ES025214, R01 ES024381, and R01 ES021357
I would like to thank Kerry L. Hanson and Russ Hauser for their helpful comments and edits on an earlier version of this manuscript.
Biography
Dr. Joseph M. Braun is an Assistant Professor in the Department of Epidemiology at the Brown University School of Public Health. He was formerly a school nurse in Milwaukee, WI before receiving his master’s and doctoral degrees in Epidemiology from the University of North Carolina-Chapel Hill. Dr. Braun studies the patterns, determinants, and health consequences of early-life chemical exposures in pregnant women, infants, and children. His research focuses primarily on childhood obesity, neurobehavioral disorders, and cardiometabolic outcomes.
Footnotes
Conflicts of Interest: The author has no conflicts of interest.
References
- 1.Barker DJ. Sir Richard Doll Lecture. Developmental origins of chronic disease. Public Health. 2012;126:185–189. doi: 10.1016/j.puhe.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Heindel JJ, et al. Developmental Origins of Health and Disease: Integrating Environmental Influences. Endocrinology. 2015;156:3416–3421. doi: 10.1210/EN.2015-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanphear BP, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axelrad DA, Bellinger DC, Ryan LM, Woodruff TJ. Dose-response relationship of prenatal mercury exposure and IQ: an integrative analysis of epidemiologic data. Environ Health Perspect. 2007;115:609–615. doi: 10.1289/ehp.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoover RN, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med. 2011;365:1304–1314. doi: 10.1056/NEJMoa1013961. [DOI] [PubMed] [Google Scholar]
- 6.Eubig PA, Aguiar A, Schantz SL. Lead and PCBs as risk factors for attention deficit/hyperactivity disorder. Environ Health Perspect. 2010;118:1654–1667. doi: 10.1289/ehp.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried PA, O’Connell CM, Watkinson B. 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: cognitive and language assessment. J Dev Behav Pediatr. 1992;13:383–391. [PubMed] [Google Scholar]
- 8.Tang-Peronard JL, Andersen HR, Jensen TK, Heitmann BL. Endocrine-disrupting chemicals and obesity development in humans: a review. Obes Rev. 2011;12:622–636. doi: 10.1111/j.1467-789X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- 9.Ronald A, Pennell CE, Whitehouse AJ. Prenatal Maternal Stress Associated with ADHD and Autistic Traits in early Childhood. Front Psychol. 2010;1:223. doi: 10.3389/fpsyg.2010.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) 2008;32:201–210. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zoeller RT, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153:4097–4110. doi: 10.1210/en.2012-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller MD, et al. Differences between children and adults: implications for risk assessment at California EPA. Int J Toxicol. 2002;21:403–418. doi: 10.1080/10915810290096630. [DOI] [PubMed] [Google Scholar]
- 13.Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health Perspect. 2000;108(Suppl 3):451–455. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grandjean P, Jensen AA. Breastfeeding and the weanling’s dilemma. Am J Public Health. 2004;94:1075. doi: 10.2105/ajph.94.7.1075. author reply 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cresteil T. Onset of xenobiotic metabolism in children: toxicological implications. Food Addit Contam. 1998;15(Suppl):45–51. doi: 10.1080/02652039809374614. [DOI] [PubMed] [Google Scholar]
- 16.Hakkola J, Tanaka E, Pelkonen O. Developmental expression of cytochrome P450 enzymes in human liver. Pharmacol Toxicol. 1998;82:209–217. doi: 10.1111/j.1600-0773.1998.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 17.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. sc271_5_1835 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev. 2006;82:257–266. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Opin Pediatr. 2009;21:243–251. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schug TT, Blawas AM, Gray K, Heindel JJ, Lawler CP. Elucidating the Links between Endocrine Disruptors and Neurodevelopment. Endocrinology. 2015 doi: 10.1210/en.2014-1734. en20141734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heindel JJ, Newbold R, Schug TT. Endocrine disruptors and obesity. Nat Rev Endocrinol. 2015;11:653–661. doi: 10.1038/nrendo.2015.163. [DOI] [PubMed] [Google Scholar]
- 22.Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16:809–818. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]
- 23.Henrichs J, Ghassabian A, Peeters RP, Tiemeier H. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin Endocrinol (Oxf) 2013;79:152–162. doi: 10.1111/cen.12227. [DOI] [PubMed] [Google Scholar]
- 24.Modesto T, et al. Maternal Mild Thyroid Hormone Insufficiency in Early Pregnancy and Attention-Deficit/Hyperactivity Disorder Symptoms in Children. JAMA Pediatr. 2015 doi: 10.1001/jamapediatrics.2015.0498. [DOI] [PubMed] [Google Scholar]
- 25.Roman GC, et al. Association of gestational maternal hypothyroxinemia and increased autism risk. Ann Neurol. 2013;74:733–742. doi: 10.1002/ana.23976. [DOI] [PubMed] [Google Scholar]
- 26.Ghassabian A, et al. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the generation R study. Pediatr Res. 2011;69:454–459. doi: 10.1203/PDR.0b013e3182125b0c. [DOI] [PubMed] [Google Scholar]
- 27.Rovet JF, Ehrlich RM, Sorbara DL. Neurodevelopment in infants and preschool children with congenital hypothyroidism: etiological and treatment factors affecting outcome. J Pediatr Psychol. 1992;17:187–213. doi: 10.1093/jpepsy/17.2.187. [DOI] [PubMed] [Google Scholar]
- 28.Rovet JF, Hepworth S. Attention problems in adolescents with congenital hypothyroidism: a multicomponential analysis. J Int Neuropsychol Soc. 2001;7:734–744. doi: 10.1017/s135561770176609x. [DOI] [PubMed] [Google Scholar]
- 29.Rovet JF, Hepworth SL. Dissociating attention deficits in children with ADHD and congenital hypothyroidism using multiple CPTs. J Child Psychol Psychiatry. 2001;42:1049–1056. doi: 10.1111/1469-7610.00804. [DOI] [PubMed] [Google Scholar]
- 30.Song SI, Daneman D, Rovet J. The influence of etiology and treatment factors on intellectual outcome in congenital hypothyroidism. J Dev Behav Pediatr. 2001;22:376–384. doi: 10.1097/00004703-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Gillman MW. Early infancy as a critical period for development of obesity and related conditions. Nestle Nutr Workshop Ser Pediatr Program. 2010;65:13–20. doi: 10.1159/000281141. discussion 20-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ornoy A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reproductive Toxicology (Elmsford, NY) 2011;32:205–212. doi: 10.1016/j.reprotox.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005;20:345–352. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Barker DJP. Developmental origins of chronic disease. Public Health. 2012;126:185–189. doi: 10.1016/j.puhe.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 36.Adair LS, et al. Size at birth, weight gain in infancy and childhood, and adult blood pressure in 5 low- and middle-income-country cohorts: when does weight gain matter? Am J Clin Nutr. 2009;89:1383–1392. doi: 10.3945/ajcn.2008.27139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Druet C, et al. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol. 2012;26:19–26. doi: 10.1111/j.1365-3016.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- 38.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life--a systematic review. Obes Rev. 2005;6:143–154. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 39.Gishti O, et al. Fetal and infant growth patterns associated with total and abdominal fat distribution in school-age children. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2013-4345. jc20134345. [DOI] [PubMed] [Google Scholar]
- 40.Chomtho S, et al. Infant growth and later body composition: evidence from the 4-component model. Am J Clin Nutr. 2008;87:1776–1784. doi: 10.1093/ajcn/87.6.1776. [DOI] [PubMed] [Google Scholar]
- 41.de Rolfe EL, et al. Association between birth weight and visceral fat in adults. Am J Clin Nutr. 2010;92:347–352. doi: 10.3945/ajcn.2010.29247. [DOI] [PubMed] [Google Scholar]
- 42.Grundy SM, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 43.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75:473–486. doi: 10.1152/physrev.1995.75.3.473. [DOI] [PubMed] [Google Scholar]
- 45.Alberti KG, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 46.Llewellyn CH, van Jaarsveld CH, Plomin R, Fisher A, Wardle J. Inherited behavioral susceptibility to adiposity in infancy: a multivariate genetic analysis of appetite and weight in the Gemini birth cohort. Am J Clin Nutr. 2012;95:633–639. doi: 10.3945/ajcn.111.023671. [DOI] [PubMed] [Google Scholar]
- 47.Goldstone AP. The hypothalamus, hormones, and hunger: alterations in human obesity and illness. Prog Brain Res. 2006;153:57–73. doi: 10.1016/S0079-6123(06)53003-1. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57:359–372. doi: 10.1507/endocrj.k10e-077. [DOI] [PubMed] [Google Scholar]
- 49.Yau PL, Kang EH, Javier DC, Convit A. Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity (Silver Spring) 2014;22:1865–1871. doi: 10.1002/oby.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanc T, et al. Attention-Deficit/Hyperactive Disorder is Related to Decreased Weight in the Preschool Period and to Increased Rate of Overweight in School-Age Boys. J Child Adolesc Psychopharmacol. 2015 doi: 10.1089/cap.2014.0157. [DOI] [PubMed] [Google Scholar]
- 51.Racicka E, Hanc T, Giertuga K, Brynska A, Wolanczyk T. Prevalence of Overweight and Obesity in Children and Adolescents With ADHD: The Significance of Comorbidities and Pharmacotherapy. J Atten Disord. 2015 doi: 10.1177/1087054715578272. [DOI] [PubMed] [Google Scholar]
- 52.Frazier-Wood AC, et al. Cognitive performance and BMI in childhood: Shared genetic influences between reaction time but not response inhibition. Obesity (Silver Spring) 2014;22:2312–2318. doi: 10.1002/oby.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hofmann J, Ardelt-Gattinger E, Paulmichl K, Weghuber D, Blechert J. Dietary restraint and impulsivity modulate neural responses to food in adolescents with obesity and healthy adolescents. Obesity (Silver Spring) 2015;23:2183–2189. doi: 10.1002/oby.21254. [DOI] [PubMed] [Google Scholar]
- 54.Anderberg RH, et al. The Stomach-Derived Hormone Ghrelin Increases Impulsive Behavior. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braun JM, Gennings C, Hauser R, Webster TF. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environ Health Perspect. 2016;124:A6–9. doi: 10.1289/ehp.1510569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119:878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robinson O, et al. The Pregnancy Exposome: Multiple Environmental Exposures in the INMA-Sabadell Birth Cohort. Environ Sci Technol. 2015;49:10632–10641. doi: 10.1021/acs.est.5b01782. [DOI] [PubMed] [Google Scholar]
- 58.Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M. Environmental pollutants and child health-A review of recent concerns. Int J Hyg Environ Health. 2016 doi: 10.1016/j.ijheh.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Engel SM, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010;118:565–571. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whyatt RM, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect. 2012;120:290–295. doi: 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miodovnik A, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32:261–267. doi: 10.1016/j.neuro.2010.12.009. S0161-813X(10)00235-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim Y, et al. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children’s Environmental Health (MOCEH) study. Environ Health Perspect. 2011;119:1495–1500. doi: 10.1289/ehp.1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Factor-Litvak P, et al. Persistent Associations between Maternal Prenatal Exposure to Phthalates on Child IQ at Age 7 Years. PLoS One. 2014;9:e114003. doi: 10.1371/journal.pone.0114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braun JM, et al. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environ Health Perspect. 2014;122:513–520. doi: 10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang HB, et al. Fetal and Childhood Exposure to Phthalate Diesters and Cognitive Function in Children Up to 12 Years of Age: Taiwanese Maternal and Infant Cohort Study. PLoS One. 2015;10:e0131910. doi: 10.1371/journal.pone.0131910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gascon M, et al. Prenatal exposure to phthalates and neuropsychological development during childhood. Int J Hyg Environ Health. 2015 doi: 10.1016/j.ijheh.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 67.Arbuckle TE, Davis K, Boylan K, Fisher M, Fu J. Bisphenol A, Phthalates and Lead and Learning and Behavioral Problems in Canadian Children 6–11 Years of Age: CHMS 2007–2009. Neurotoxicology. 2016 doi: 10.1016/j.neuro.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 68.Kim BN, et al. Phthalates exposure and attention-deficit/hyperactivity disorder in school-age children. Biol Psychiatry. 2009;66:958–963. doi: 10.1016/j.biopsych.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 69.Swan SH, et al. Prenatal phthalate exposure and reduced masculine play in boys. Int J Androl. 2010;33:259–269. doi: 10.1111/j.1365-2605.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valvi D, et al. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect. 2015;123:1022–1029. doi: 10.1289/ehp.1408887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maresca MM, et al. Prenatal Exposure to Phthalates and Childhood Body Size in an Urban Cohort. Environ Health Perspect. 2015 doi: 10.1289/ehp.1408750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buckley JP, et al. Prenatal Phthalate Exposures and Childhood Fat Mass in a New York City Cohort. Environ Health Perspect. 2015 doi: 10.1289/ehp.1509788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rudel RA, et al. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect. 2011;119:914–920. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bornehag CG, et al. Phthalates in indoor dust and their association with building characteristics. Environ Health Perspect. 2005;113:1399–1404. doi: 10.1289/ehp.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Langer S, et al. Phthalate metabolites in urine samples from Danish children and correlations with phthalates in dust samples from their homes and daycare centers. Int J Hyg Environ Health. 2013 doi: 10.1016/j.ijheh.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 76.Braun JM, et al. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2014;24:459–466. doi: 10.1038/jes.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singh AR, Lawrence WH, Autian J. Maternal-fetal transfer of 14C-di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats. Journal of pharmaceutical sciences. 1975;64:1347–1350. doi: 10.1002/jps.2600640819. [DOI] [PubMed] [Google Scholar]
- 78.Gray TJ, Beamand JA. Effect of some phthalate esters and other testicular toxins on primary cultures of testicular cells. Food Chem Toxicol. 1984;22:123–131. doi: 10.1016/0278-6915(84)90092-9. [DOI] [PubMed] [Google Scholar]
- 79.Calafat AM. Contemporary Issues in Exposure Assessment Using Biomonitoring. Current Epidemiology Reports. 2016;3:145–153. doi: 10.1007/s40471-016-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perrier F, Giorgis-Allemand L, Slama R, Philippat C. Within-subject Pooling of Biological Samples to Reduce Exposure Misclassification in Biomarker-based Studies. Epidemiology. 2016;27:378–388. doi: 10.1097/EDE.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hannas BR, et al. Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate and diisononyl phthalate. Toxicol Sci. 2011 doi: 10.1093/toxsci/kfr146. kfr146 [pii] [DOI] [PubMed] [Google Scholar]
- 82.Howdeshell KL, et al. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008;105:153–165. doi: 10.1093/toxsci/kfn077. kfn077 [pii] [DOI] [PubMed] [Google Scholar]
- 83.Yao HY, et al. Maternal phthalate exposure during the first trimester and serum thyroid hormones in pregnant women and their newborns. Chemosphere. 2016;157:42–48. doi: 10.1016/j.chemosphere.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 84.Boas M, et al. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect. 2010;118:1458–1464. doi: 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johns LE, et al. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol. 2015;13:4. doi: 10.1186/1477-7827-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghisari M, Bonefeld-Jorgensen EC. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol Lett. 2009;189:67–77. doi: 10.1016/j.toxlet.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 87.Shimada N, Yamauchi K. Characteristics of 3,5,3′-triiodothyronine (T3)-uptake system of tadpole red blood cells: effect of endocrine-disrupting chemicals on cellular T3 response. J Endocrinol. 2004;183:627–637. doi: 10.1677/joe.1.05893. [DOI] [PubMed] [Google Scholar]
- 88.Breous E, Wenzel A, Loos U. The promoter of the human sodium/iodide symporter responds to certain phthalate plasticisers. Mol Cell Endocrinol. 2005;244:75–78. doi: 10.1016/j.mce.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 89.Ye L, Guo J, Ge RS. Environmental pollutants and hydroxysteroid dehydrogenases. Vitam Horm. 2014;94:349–390. doi: 10.1016/B978-0-12-800095-3.00013-4. [DOI] [PubMed] [Google Scholar]
- 90.Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ Health Perspect. 2015;123:210–216. doi: 10.1289/ehp.1307996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.LaRocca J, Binder AM, McElrath TF, Michels KB. First-Trimester Urine Concentrations of Phthalate Metabolites and Phenols and Placenta miRNA Expression in a Cohort of U.S. Women. Environ Health Perspect. 2015 doi: 10.1289/ehp.1408409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.NRC. Phthalates and Cumulative Risk Assessment The Task Ahead. National Academies Press; Washington, DC: 2008. [PubMed] [Google Scholar]
- 93.Kobrosly RW, et al. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environ Health Perspect. 2014;122:521–528. doi: 10.1289/ehp.1307063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lien YJ, et al. Prenatal Exposure to Phthalate Esters and Behavioral Syndromes in Children at Eight Years of Age: Taiwan Maternal and Infant Cohort Study. Environ Health Perspect. 2014 doi: 10.1289/ehp.1307154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buckley JP, et al. Prenatal phthalate exposures and body mass index among 4 to 7 year old children: A pooled analysis. Epidemiology. 2016 doi: 10.1097/EDE.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Teitelbaum SL, et al. Associations between phthalate metabolite urinary concentrations and body size measures in New York City children. Environmental research. 2012;112:186–193. doi: 10.1016/j.envres.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deierlein AL, et al. Longitudinal associations of phthalate exposures during childhood and body size measurements in young girls. Epidemiology. 2016 doi: 10.1097/EDE.0000000000000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trasande L, Attina TM, Sathyanarayana S, Spanier AJ, Blustein J. Race/ethnicity-specific associations of urinary phthalates with childhood body mass in a nationally representative sample. Environ Health Perspect. 2013;121:501–506. doi: 10.1289/ehp.1205526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hatch EE, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health. 2008;7:27–41. doi: 10.1186/1476-069X-7-27. 1476-069X-7-27 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carwile JL, Ye X, Zhou X, Calafat AM, Michels KB. Canned soup consumption and urinary bisphenol A: a randomized crossover trial. JAMA. 2011;306:2218–2220. doi: 10.1001/jama.2011.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.von Goetz N, Wormuth M, Scheringer M, Hungerbuhler K. Bisphenol a: how the most relevant exposure sources contribute to total consumer exposure. Risk Anal. 2010;30:473–487. doi: 10.1111/j.1539-6924.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 102.Ehrlich S, Calafat AM, Humblet O, Smith T, Hauser R. Handling of thermal receipts as a source of exposure to bisphenol A. JAMA. 2014;311:859–860. doi: 10.1001/jama.2013.283735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thayer KA, et al. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ Int. 2015;83:107–115. doi: 10.1016/j.envint.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Braun JM, et al. Early-life bisphenol a exposure and child body mass index: a prospective cohort study. Environ Health Perspect. 2014;122:1239–1245. doi: 10.1289/ehp.1408258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harley KG, et al. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ Health Perspect. 2013;121:514–520. 520e511–516. doi: 10.1289/ehp.1205548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vafeiadi M, et al. Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ Res. 2016;146:379–387. doi: 10.1016/j.envres.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 107.Hoepner LA, et al. Bisphenol A and Adiposity in an Inner-City Birth Cohort. Environ Health Perspect. 2016 doi: 10.1289/EHP205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Casas L, et al. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int. 2011;37:858–866. doi: 10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 109.Stacy SL, et al. Patterns, Variability, and Predictors of Urinary Bisphenol A Concentrations during Childhood. Environ Sci Technol. 2016 doi: 10.1021/acs.est.6b00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harley KG, et al. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ Res. 2013 doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Braun JM, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117:1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Braun JM, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128:873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roen EL, et al. Bisphenol A exposure and behavioral problems among inner city children at 7–9 years of age. Environ Res. 2015 doi: 10.1016/j.envres.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perera F, et al. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environ Health Perspect. 2012;120:1190–1194. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Evans SF, et al. Prenatal Bisphenol A Exposure and maternally reported behavior in boys and girls. Neurotoxicology. 2014 doi: 10.1016/j.neuro.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Casas M, et al. Exposure to bisphenol A during pregnancy and child neuropsychological development in the INMA-Sabadell cohort. Environ Res. 2015;142:671–679. doi: 10.1016/j.envres.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 117.Valvi D, et al. Prenatal bisphenol a urine concentrations and early rapid growth and overweight risk in the offspring. Epidemiology. 2013;24:791–799. doi: 10.1097/EDE.0b013e3182a67822. [DOI] [PubMed] [Google Scholar]
- 118.Philippat C, et al. Prenatal Exposure to Phenols and Growth in Boys. Epidemiology. 2014 doi: 10.1097/EDE.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308:1113–1121. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- 120.Wang HX, et al. Association between bisphenol A exposure and body mass index in Chinese school children: a cross-sectional study. Environ Health. 2012;11:79. doi: 10.1186/1476-069X-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.ECD, WL Synthetic oestrogenic agents without the phenanthrene nucleus. Nature. 1936;137:996. [Google Scholar]
- 122.Milligan SR, Balasubramanian AV, Kalita JC. Relative potency of xenobiotic estrogens in an acute in vivo mammalian assay. Environ Health Perspect. 1998;106:23–26. doi: 10.1289/ehp.9810623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wozniak AL, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect. 2005;113:431–439. doi: 10.1289/ehp.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wetherill YB, et al. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24:178–198. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 125.Zhang X, et al. Bisphenol A Disrupts Steroidogenesis in Human H295R Cells. Toxicol Sci. doi: 10.1093/toxsci/kfr061. [DOI] [PubMed] [Google Scholar]
- 126.Galloway T, et al. Daily Bisphenol A Excretion and Associations with Sex Hormone Concentrations: Results from the InCHIANTI Adult Population Study. Environ Health Perspect. 2010;118:1603–1608. doi: 10.1289/ehp.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Meeker JD, Calafat AM, Hauser R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ Sci Technol. 2010;44:1458–1463. doi: 10.1021/es9028292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mendiola J, et al. Are environmental levels of bisphenol a associated with reproductive function in fertile men? Environ Health Perspect. 2010;118:1286–1291. doi: 10.1289/ehp.1002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zoeller RT, Bansal R, Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146:607–612. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
- 130.Gentilcore D, et al. Bisphenol A interferes with thyroid specific gene expression. Toxicology. 2013;304:21–31. doi: 10.1016/j.tox.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 131.Chevrier J, et al. Maternal urinary bisphenol a during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environ Health Perspect. 2013;121:138–144. doi: 10.1289/ehp.1205092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ Health Perspect. 2011;119:1396–1402. doi: 10.1289/ehp.1103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Romano ME, et al. Gestational urinary bisphenol A and maternal and newborn thyroid hormone concentrations: The HOME Study. Environ Res. 2015;138:453–460. doi: 10.1016/j.envres.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chapin RE, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- 135.Tewar S, et al. Association of Bisphenol A exposure and Attention-Deficit/Hyperactivity Disorder in a national sample of U.S. children. Environ Res. 2016;150:112–118. doi: 10.1016/j.envres.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Perez-Lobato R, et al. Exposure to bisphenol A and behavior in school-age children. Neurotoxicology. 2016;53:12–19. doi: 10.1016/j.neuro.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 137.Hong SB, et al. Bisphenol A in relation to behavior and learning of school-age children. J Child Psychol Psychiatry. 2013 doi: 10.1111/jcpp.12050. [DOI] [PubMed] [Google Scholar]
- 138.Buckley JP, Herring AH, Wolff MS, Calafat AM, Engel SM. Prenatal exposure to environmental phenols and childhood fat mass in the Mount Sinai Children’s Environmental Health Study. Environ Int. 2016;91:350–356. doi: 10.1016/j.envint.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol. 2010;40:422–484. doi: 10.3109/10408441003667514. [DOI] [PubMed] [Google Scholar]
- 140.Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health A. 2006;69:1861–1873. doi: 10.1080/15287390600631706. [DOI] [PubMed] [Google Scholar]
- 141.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ Health Perspect. 2008;116:303–307. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kumar V, Balomajumder C, Roy P. Disruption of LH-induced testosterone biosynthesis in testicular Leydig cells by triclosan: probable mechanism of action. Toxicology. 2008;250:124–131. doi: 10.1016/j.tox.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 143.Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 144.Johnson PI, et al. Application of the Navigation Guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan. Environ Int. 2016 doi: 10.1016/j.envint.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Paul KB, Thompson JT, Simmons SO, Vanden Heuvel JP, Crofton KM. Evidence for triclosan-induced activation of human and rodent xenobiotic nuclear receptors. Toxicol In Vitro. 2013 doi: 10.1016/j.tiv.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 146.Koeppe ES, Ferguson KK, Colacino JA, Meeker JD. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci Total Environ. 2013;445–446:299–305. doi: 10.1016/j.scitotenv.2012.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cullinan MP, Palmer JE, Carle AD, West MJ, Seymour GJ. Long term use of triclosan toothpaste and thyroid function. Sci Total Environ. 2012;416:75–79. doi: 10.1016/j.scitotenv.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 148.Yee AL, Gilbert JA. Is triclosan harming your microbiome? Sci Gov Rep. 2016;353:348–349. doi: 10.1126/science.aag2698. [DOI] [PubMed] [Google Scholar]
- 149.Lassen TH, et al. Prenatal Triclosan Exposure and Anthropometric Measures including Anogenital Distance in Danish Infants. Environ Health Perspect. 2016 doi: 10.1289/ehp.1409637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wolff MS, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jensen RB, Juul A, Larsen T, Mortensen EL, Greisen G. Cognitive ability in adolescents born small for gestational age: Associations with fetal growth velocity, head circumference and postnatal growth. Early Hum Dev. 2015;91:755–760. doi: 10.1016/j.earlhumdev.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 152.Xue J, et al. Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ Res. 2015;137:120–128. doi: 10.1016/j.envres.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 153.Li S, et al. Urinary triclosan concentrations are inversely associated with body mass index and waist circumference in the US general population: Experience in NHANES 2003–2010. Int J Hyg Environ Health. 2015;218:401–406. doi: 10.1016/j.ijheh.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Buser MC, Murray HE, Scinicariello F. Association of urinary phenols with increased body weight measures and obesity in children and adolescents. J Pediatr. 2014;165:744–749. doi: 10.1016/j.jpeds.2014.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xue J, et al. Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environmental research. 2014;137:120–128. doi: 10.1016/j.envres.2014.12.007. Epub 2014 Dec 19. [DOI] [PubMed] [Google Scholar]
- 156.EFSA. Perfluoroctane sulfonate, perfluorooctanoic acid and their salts: Scientific opinion of the panel on contaminants in the food chain. EFSA Journal. 2008;653:1–131. doi: 10.2903/j.efsa.2008.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Buck RC, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011;7:513–541. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Johnson PI, et al. The Navigation Guide - evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014;122:1028–1039. doi: 10.1289/ehp.1307893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mora AM, et al. Prenatal Exposure to Perfluoroalkyl Substances and Adiposity in Early and Mid-Childhood. Environ Health Perspect. 2016 doi: 10.1289/EHP246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Braun JM, et al. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity (Silver Spring) 2016;24:231–237. doi: 10.1002/oby.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Halldorsson TI, et al. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect. 2012;120:668–673. doi: 10.1289/ehp.1104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hoyer BB, et al. Anthropometry in 5- to 9-Year-Old Greenlandic and Ukrainian Children in Relation to Prenatal Exposure to Perfluorinated Alkyl Substances. Environ Health Perspect. 2015;123:841–846. doi: 10.1289/ehp.1408881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maisonet M, et al. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Perspect. 2012;120:1432–1437. doi: 10.1289/ehp.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Andersen CS, et al. Prenatal exposures to perfluorinated chemicals and anthropometry at 7 years of age. Am J Epidemiol. 2013;178:921–927. doi: 10.1093/aje/kwt057. [DOI] [PubMed] [Google Scholar]
- 165.Stein CR, Savitz DA, Bellinger DC. Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology. 2013;24:590–599. doi: 10.1097/EDE.0b013e3182944432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang Y, et al. Prenatal exposure to perfluroalkyl substances and children’s IQ: The Taiwan maternal and infant cohort study. Int J Hyg Environ Health. 2015;218:639–644. doi: 10.1016/j.ijheh.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Forns J, et al. Perfluoroalkyl substances measured in breast milk and child neuropsychological development in a Norwegian birth cohort study. Environ Int. 2015;83:176–182. doi: 10.1016/j.envint.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 168.Stein CR, Savitz DA. Serum perfluorinated compound concentration and attention deficit/hyperactivity disorder in children 5–18 years of age. Environ Health Perspect. 2011;119:1466–1471. doi: 10.1289/ehp.1003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fei C, Olsen J. Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7 years. Environ Health Perspect. 2011;119:573–578. doi: 10.1289/ehp.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vuong AM, et al. Prenatal polybrominated diphenyl ether and perfluoroalkyl substance exposures and executive function in school-age children. Environ Res. 2016;147:556–564. doi: 10.1016/j.envres.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lind PM, et al. Serum concentrations of phthalate metabolites are related to abdominal fat distribution two years later in elderly women. Environ Health. 2012;11:21. doi: 10.1186/1476-069X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liew Z, et al. Prenatal exposure to perfluoroalkyl substances and the risk of congenital cerebral palsy in children. Am J Epidemiol. 2014;180:574–581. doi: 10.1093/aje/kwu179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liew Z, et al. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: a nested case-control study in the Danish National Birth Cohort. Environ Health Perspect. 2015;123:367–373. doi: 10.1289/ehp.1408412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ode A, et al. Fetal exposure to perfluorinated compounds and attention deficit hyperactivity disorder in childhood. PLoS One. 2014;9:e95891. doi: 10.1371/journal.pone.0095891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in U.S. children 12–15 years of age. Environ Health Perspect. 2010;118:1762–1767. doi: 10.1289/ehp.1001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Stein CR, Savitz DA, Bellinger DC. Perfluorooctanoate exposure in a highly exposed community and parent and teacher reports of behaviour in 6–12-year-old children. Paediatr Perinat Epidemiol. 2014;28:146–156. doi: 10.1111/ppe.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Olsen GW, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Egeghy PP, Lorber M. An assessment of the exposure of Americans to perfluorooctane sulfonate: a comparison of estimated intake with values inferred from NHANES data. J Expo Sci Environ Epidemiol. 2011;21:150–168. doi: 10.1038/jes.2009.73. [DOI] [PubMed] [Google Scholar]
- 179.USEPA. United States Environmental Protection Agency, Child-Specific Exposure Factors Handbook. Washington, D.C: 2008. [Google Scholar]
- 180.Fromme H, et al. Pre- and postnatal exposure to perfluorinated compounds (PFCs) Environ Sci Technol. 2010;44:7123–7129. doi: 10.1021/es101184f. [DOI] [PubMed] [Google Scholar]
- 181.Post GB, Cohn PD, Cooper KR. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environ Res. 2012;116:93–117. doi: 10.1016/j.envres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 182.Guerrero-Preston R, et al. Global DNA hypomethylation is associated with in utero exposure to cotinine and perfluorinated alkyl compounds. Epigenetics. 2010;5:539–546. doi: 10.4161/epi.5.6.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Watkins DJ, et al. Associations between serum perfluoroalkyl acids and LINE-1 DNA methylation. Environ Int. 2014;63:71–76. doi: 10.1016/j.envint.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fletcher T, et al. Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environ Int. 2013;57–58:2–10. doi: 10.1016/j.envint.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 185.Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci. 2006;92:476–489. doi: 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- 186.Taxvig C, et al. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARgamma activation. Mol Cell Endocrinol. 2012;361:106–115. doi: 10.1016/j.mce.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 187.Bastos Sales L, et al. Effects of endocrine disrupting chemicals on in vitro global DNA methylation and adipocyte differentiation. Toxicol In Vitro. 2013;27:1634–1643. doi: 10.1016/j.tiv.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 188.Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol. 2012;355:240–248. doi: 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 189.Chan E, Burstyn I, Cherry N, Bamforth F, Martin JW. Perfluorinated acids and hypothyroxinemia in pregnant women. Environ Res. 2011;111:559–564. doi: 10.1016/j.envres.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 190.Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Koustas E, et al. The Navigation Guide-Evidence-Based Medicine Meets Environmental Health: Systematic Review of Nonhuman Evidence for PFOA Effects on Fetal Growth. Environ Health Perspect. 2014 doi: 10.1289/ehp.1307177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jaddoe VW, et al. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. 2014;348:g14. doi: 10.1136/bmj.g14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Perng W, et al. Birth Size, Early Life Weight Gain, and Midchildhood Cardiometabolic Health. J Pediatr. 2016 doi: 10.1016/j.jpeds.2016.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Barry V, Darrow LA, Klein M, Winquist A, Steenland K. Early life perfluorooctanoic acid (PFOA) exposure and overweight and obesity risk in adulthood in a community with elevated exposure. Environ Res. 2014;132C:62–69. doi: 10.1016/j.envres.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 195.Akinbami LJ, Ogden CL. Childhood Overweight Prevalence in the United States: The Impact of Parent-reported Height and Weight. Obesity. 2009;17:1574–1580. doi: 10.1038/Oby.2009.1. [DOI] [PubMed] [Google Scholar]
- 196.Hattori A, Sturm R. The obesity epidemic and changes in self-report biases in BMI. Obesity (Silver Spring) 2013;21:856–860. doi: 10.1002/oby.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect. 2010;118:197–202. doi: 10.1289/ehp.0901165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Domazet SL, Grontved A, Timmermann AG, Nielsen F, Jensen TK. Longitudinal Associations of Exposure to Perfluoroalkylated Substances in Childhood and Adolescence and Indicators of Adiposity and Glucose Metabolism 6 and 12 Years Later: The European Youth Heart Study. Diabetes Care. 2016 doi: 10.2337/dc16-0269. [DOI] [PubMed] [Google Scholar]
- 199.Sanchez BN, Hu H, Litman HJ, Tellez-Rojo MM. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect. 2011;119:409–415. doi: 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.NIEHS. Statistical Approaches for Assessing Health Effects of Environmental Chemical Mixtures in Epidemiology Studies. 2015 doi: 10.1289/EHP547. < http://www.niehs.nih.gov/about/visiting/events/pastmtg/2015/statistical/>. [DOI] [PMC free article] [PubMed]
- 201.Taylor K, et al. Statistical Approaches for Assessing Health Effects of Environmental Chemical Mixtures in Epidemiology. Environ Health Perspect. 2016 doi: 10.1289/EHP547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bobb JF, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16:493–508. doi: 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Carrico C, Gennings C, Wheeler D, Factor-Litvak P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. Journal of Agricultural, Biological and Environmental Statistics. 2014;20:100–120. doi: 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yorita Christensen KL, Carrico CK, Sanyal AJ, Gennings C. Multiple classes of environmental chemicals are associated with liver disease: NHANES 2003–2004. Int J Hyg Environ Health. 2013;216:703–709. doi: 10.1016/j.ijheh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Safe SH. Hazard and risk assessment of chemical mixtures using the toxic equivalency factor approach. Environ Health Perspect. 1998;106(Suppl 4):1051–1058. doi: 10.1289/ehp.98106s41051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vilahur N, et al. Male specific association between xenoestrogen levels in placenta and birthweight. Environ Int. 2013;51:174–181. doi: 10.1016/j.envint.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 207.Agier L, et al. A Systematic Comparison of Linear Regression-Based Statistical Methods to Assess Exposome-Health Associations. Environ Health Perspect. 2016 doi: 10.1289/EHP172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Alexeeff SE, Carroll RJ, Coull B. Spatial measurement error and correction by spatial SIMEX in linear regression models when using predicted air pollution exposures. Biostatistics. 2016;17:377–389. doi: 10.1093/biostatistics/kxv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169:1182–1190. doi: 10.1093/aje/kwp035. kwp035 [pii] [DOI] [PubMed] [Google Scholar]
- 210.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. 00001648-200907000-00004 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yu ZB, et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev. 2011;12:525–542. doi: 10.1111/j.1467-789X.2011.00867.x. [DOI] [PubMed] [Google Scholar]
- 212.Duty SM, Ackerman RM, Calafat AM, Hauser R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect. 2005;113:1530–1535. doi: 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bartell SM, et al. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect. 2010;118:222–228. doi: 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D. Perfluorinated compounds--exposure assessment for the general population in Western countries. Int J Hyg Environ Health. 2009;212:239–270. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 215.Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Boyle CA, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127:1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 217.Investigators AaDDMNSYP. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2010. MMWR. 2014;4:1–21. [PubMed] [Google Scholar]
- 218.Kohane IS, et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PLoS One. 2012;7:e33224. doi: 10.1371/journal.pone.0033224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Melegari MG, Sacco R, Manzi B, Vittori E, Persico AM. Deficient Emotional Self-Regulation in Preschoolers With ADHD: Identification, Comorbidity, and Interpersonal Functioning. J Atten Disord. 2016 doi: 10.1177/1087054715622015. [DOI] [PubMed] [Google Scholar]
- 220.Forns J, et al. A conceptual framework in the study of neuropsychological development in epidemiological studies. Neuroepidemiology. 2012;38:203–208. doi: 10.1159/000337169. [DOI] [PubMed] [Google Scholar]
- 221.Harris MH, et al. Prenatal and Childhood Traffic-Related Pollution Exposure and Childhood Cognition in the Project Viva Cohort (Massachusetts, USA) Environ Health Perspect. 2015 doi: 10.1289/ehp.1408803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.WHO. Global status report on noncommunicable diseases 2010. 2010 < http://www.who.int/nmh/publications/ncd_report2010/en/>.
- 224.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360:473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]