Abstract
Background
To report on imaging findings using 68Ga-PSMA-HBED-CC PET in a series of 19 breast carcinoma patients.
Methods
68Ga-PSMA-HBED-CC PET imaging results obtained were compared to routinely performed staging examinations and analyzed as to lesion location and progesterone receptor status.
Results
Out of 81 tumor lesions identified, 84% were identified on 68Ga-PSMA-HBED-CC PET. 68Ga-PSMA-HBED-CC SUVmean values of distant metastases proved significantly higher (mean, 6.86, SD, 5.68) when compared to those of primary or local recurrences (mean, 2.45, SD, 2.55, p = 0.04) or involved lymph nodes (mean, 3.18, SD, 1.79, p = 0.011). SUVmean values of progesterone receptor-positive lesions proved not significantly different from progesterone receptor-negative lesions. SUV values derived from FDG PET/CT, available in seven patients, and 68Ga-PSMA-HBED-CC PET/CT imaging proved weakly correlated (r = 0.407, p = 0.015).
Conclusions
68Ga-PSMA-HBED-CC PET/CT imaging in breast carcinoma confirms the reported considerable variation of PSMA expression on human solid tumors using immunohistochemistry.
Keywords: 68Ga-PSMA, PET/CT, Breast cancer
Introduction
Prostate-specific membrane antigen (PSMA) is an integral membrane protein, mapped to chromosome 11q14, which is over-expressed by a high number of prostate carcinomas; this expression is further increased in higher-grade carcinomas, in metastatic disease, and in hormone refractory prostate carcinomas, making it an interesting target for prostate carcinoma-specific imaging and therapy [1]. In this regard, the PSMA inhibitor Glu-NH-CO-NH-Lys(Ahx)-HBED-CC was labeled with 68Ga for positron emission tomography (PET) and shown to be more accurate for the detection of recurrent prostate carcinoma when compared to 18F-choline PET and, in combination with MRI, to be significantly more accurate for the detection of primary prostate carcinoma when compared to PET/CT [2–4]. Aside from prostate carcinoma, PSMA has also been reported to be selectively overexpressed in the tumor-associated neovasculature of a wide variety of solid tumors including breast carcinoma [5–8].
Sathekge et al. recently presented the first case of a patient with metastatic breast cancer, in whom PET/CT using the Glu-NH-CO-NH-Lys-(Ahx)-[68Ga(HBEDCC)] (68Ga-PSMA) ligand detected bone and liver metastases with essentially similar visual contrast to 18F-FDG PET/CT [6]. In this study, we built on these initial findings by reporting on imaging findings using 68Ga-PSMA-HBED-CC PET in a series of 19 breast carcinoma patients.
Patients and methods
Nineteen women (mean age, 45 years, range, 25-66 years) suffering from breast carcinoma were prospectively included in this study, approved by the Institutional Ethics Committee, following written informed consent. 68Ga-PSMA-HBED-CC PET imaging was performed in nine “de novo” diagnosed breast carcinoma patients, in five patients presenting with a loco-regional recurrence of breast carcinoma, and in a pre-treatment metastasized setting in another five patients. Six patients were progesterone receptor-positive and seven were progesterone receptor-negative. In the remaining six patients, progesterone receptor status was unknown. Seven of the 19 patients included additionally underwent FDG PET/CT imaging (three de novo patients, two loco-regional recurrent, and two metastasized patients). Both 68Ga-PSMA-HBED-CC and FDG PET/CT imaging was performed from the top of the pelvis to the skull following the injection of a body weight-adjusted dose, ((body weight/10) + 1) × 37 MBq for FDG PET imaging and 2 MBq/kg for 68Ga-PSMA-HBED-CC PET imaging. All 68Ga-PSMA-HBED-CC injections contained 2 mmol PSMA ligand, resulting in a median specific radioactivity of 66 GBq/µmol [9]. In all patients, available imaging data performed as part of the staging or restaging procedure, including contrast-enhanced CT imaging of the thoraco-abdominal region, echography, bone scintigraphy, and, when available, FDG-PET imaging (see also above, performed within 2 weeks from the 68Ga-PSMA-HBED-CC PET examination and prior to any treatment initiation), were used as gold standard to define the imaging potential of 68Ga-PSMA-HBED-CC PET imaging.
Statistical analysis
Differences in 68Ga-PSMA-HBED-CC SUVmean values between different subgroups were assessed using Student’s t test or ANOVA with post hoc Bonferroni correction where appropriate. Correlation analysis was performed using Pearson’s correlation or Spearman-rank correlation analysis where appropriate.
Results
Overall, in the 19 patients studied, 81 tumor lesions were identified: 13 primary tumors and/or local recurrences, 15 involving the lymph nodes, and 53 metastases (see Table 1 and Figs. 1 and 2). Out of these, six primary or recurrent lesions, two lymph nodes, and five metastases proved negative on 68Ga-PSMA-HBED-CC PET, yielding an overall detection rate of 84% for 68Ga-PSMA-HBED-CC PET.
Table 1.
Patient characteristics and PSMA imaging results (lesions identified on 68Ga-PSMA-HBED-CC PET/total number derived from routine examination procedures)
| Patient no. | Age | Carcinoma type | PR status | Clinical setting | Primary/local relapse | LN | M+ |
|---|---|---|---|---|---|---|---|
| 1 | 45 | Ductal | NA | Primary | 1/1 | 0/0 | 0/0 |
| 2 | 45 | NA | NA | M+ | 0/0 | 0/0 | 5/5 |
| 3 | 49 | Ductal | NA | M+ | 0/0 | 0/0 | 4/6 |
| 4 | 66 | Lobular | PR+ | Primary | 0/1 | 1/1 | 0/0 |
| 5 | 40 | NA | NA | Recurrence | 0/1 | 1/1 | 4/4 |
| 6 | 39 | Ductal | PR+ | Recurrence | 1/1 | 0/0 | 0/1 |
| 7 | 38 | Ductal | PR+ | Recurrence | 0/1 | 0/0 | 4/5 |
| 8 | 39 | Ductal | PR– | Primary | 0/1 | 0/0 | 0/1 |
| 9 | 25 | Ductal | PR– | Primary | 1/1 | 1/1 | 0/0 |
| 10 | 62 | Ductal | PR– | Recurrence | 0/1 | 0/0 | 0/0 |
| 11 | 53 | Lobular | PR+ | Primary | 1/1 | 2/2 | 4/4 |
| 12 | 54 | Neuroendocrine differentiation | PR+ | Primary | 1/1 | 1/2 | 5/5 |
| 13 | 42 | Ductal | PR+ | Primary | 1/1 | 1/1 | 4/4 |
| 14 | 39 | Ductal | PR– | Primary | 1/1 | 1/2 | 2/3 |
| 15 | 57 | Ductal | NA | Primary | 0/1 | 0/0 | 0/0 |
| 16 | 40 | Ductal | NA | M+ | 0/0 | 2/2 | 4/4 |
| 17 | 31 | Ductal | PR– | M+ | 0/0 | 0/0 | 4/4 |
| 18 | 44 | NA | PR– | Recurrence | 0/0 | 2/2 | 5/5 |
| 19 | 56 | Ductal | PR– | M+ | 0/0 | 1/1 | 2/2 |
NA not available, PR progesterone receptor, M+ metastasized
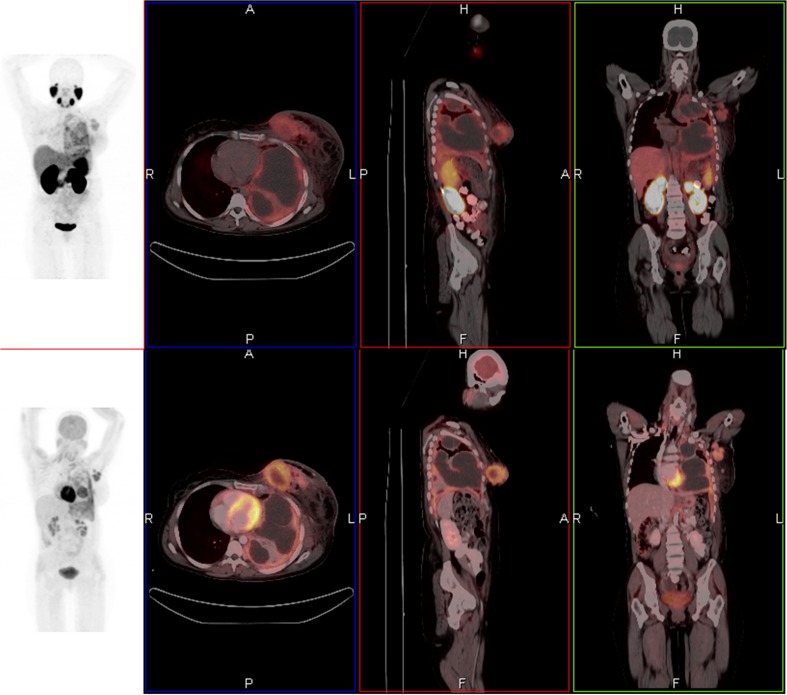
Fig. 1.
A 42-year-old female with metastatic breast carcinoma who underwent 68Ga-PSMA and 18F-FDG PET/CT. Axial, coronal, and sagittal fused 68Ga-PSMA PET/CT images demonstrated primary left breast cancer, axillary nodal and left pleural metastases (a). Avidity is slightly intense on 18F-FDG PET/CT images (b). Maximum-intensity-projection PET gives overview of all lesions (c, d)
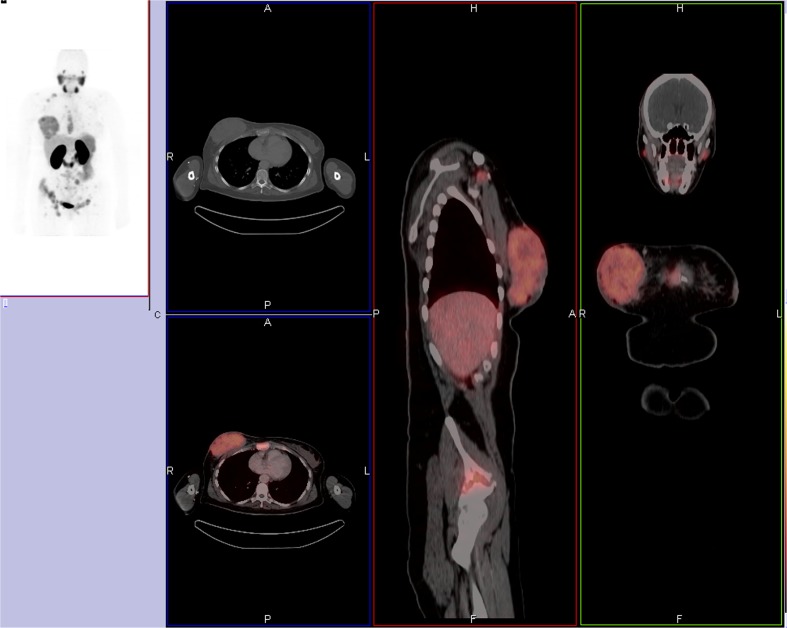
Fig. 2.
A 39-year-old woman with stage IV by 68Ga-PSMA PET/CT. a Maximum-intensity-projection PET demonstrated multiple osseous metastasis and a primary right breast cancer. Axial, coronal, and sagittal fused PET/CT confirms all the lesions (b)
68Ga-PSMA-HBED-CC SUVmean values of distant metastases proved significantly higher (mean, 6.86, SD, 5.68) when compared to those of primary or local recurrences (mean, 2.45, SD, 2.55, p = 0.04) or involved lymph nodes (mean, 3.18, SD, 1.79, p = 0.011).
68Ga-PSMA-HBED-CC SUVmean values of progesterone receptor-positive lesions (n, number of lesions = 31) proved not significantly different from those obtained in progesterone receptor-negative lesions (n = 31), respectively 5.62 ± 5.40 (mean/SD) versus 4.19 ± 2.63 (p = 0.188).
FDG PET/CT imaging performed in seven patients identified 35 lesions. Of the 35 FDG-positive lesions, six proved PSMA-negative and FDG PET/CT was clearly more intense than 68Ga-PSMA with regards to primary lesions (Fig. 1). Inversely, one lesion identified on 68Ga-PSMA-HBED-CC PET proved FDG PET/CT-negative. In those patients that underwent both examinations,68Ga-PSMA values proved not significantly different from those obtained using FDG [mean 4.58, SD, 3.94) versus 6.1 (SD, 2.82), p = 0104].
Of interest, a weak but significant relationship was identified between SUV values derived from FDG PET/CT (mean, 6.1, SD, 2.82) and 68Ga-PSMA-HBED-CC PET/CT imaging (r = 0.407, p = 0.015).
Discussion
PSMA has been previously shown to be universally up-regulated on tumor-associated vascular endothelial cells in solid tumors and to participate in matrix degradation and facilitate integrin signaling and p21-activated kinase 1 (PAK-1) activation leading to productive tumor invasion [10]. Since PSMA is found in the neovasculature of many tumors, it is thought to regulate angiogenesis, however, the precise mechanism by which PSMA exerts its effect is unknown [10, 11]. To this effect some groups suggest that PSMA plays a number of roles in angiogenesis, some involving vascular endothelial factor (VEGF), others not [11, 12]. In a study by Wernicke et al. on breast carcinoma patients, tumor-associated vasculature was shown to be PSMA-positive in 68 out of 92 primary breast cancers (74%) and in 14 out of 14 of breast cancers metastatic to the brain [7]. Likewise, in a study by Natsuko et al., five breast cancer brain metastases showed PSMA expression on tumor blood vessels [8], and recently our manuscript demonstrated intense uptake by 68Ga-PSMA-HBED-CC in metastatic breast cancer [6]. In line with these findings, out of 81 tumor lesions identified, 84% were proven to be 68Ga-PSMA PET-positive in the series that presented with distant metastases displaying significantly higher 68Ga-PSMA-HBED-CC SUV values. Furthermore, 68Ga-PSMA-HBED-CC SUV values of tumor lesions were shown to vary significantly from one patient to another as well as from one lesion to another within one patient. These findings concur with the reported considerable variation of PSMA expression on human solid tumors using immunohistochemistry, thus further supporting the fact that breast cancer is a heterogeneous disease [13].
The hormonal receptor (estradiol receptor (ER)/progesterone receptor (PR) status is a strong prognostic factor for breast cancer. The progesterone receptor (PR) is an estrogen response element that is transcribed after effective binding of the estradiol-estradiol receptor (ER) complex to DNA in ER-positive, estradiol-responsive breast cancers [14]. In the study by Wernicke et al., patients with PR-negative tumors were more likely to present with a more extensive PSMA staining (PSMA-expression in > 50% of microvessels) when compared to PR-positive tumors [7]. In our series presented, no significant difference in 68Ga-PSMA SUV values between PR-positive and PR-negative tumors could be identified. However, in some patients under study, a considerable time interval existed between characterization of the PR-status on the primary tumor and subsequent imaging, performed in a metastasized setting. Accordingly, the tumor biology of some of these tumors may have changed due to ongoing mutations resulting in a loss of PR expression, thereby flawing the existence of a possible relationship between both variables.
Furthermore, there is increasing evidence of temporal and spatial heterogeneity in breast cancer receptor overexpression. Patients with negative test results at diagnosis can have positive test results later in the disease course and vice versa, a fact that explains why biopsy of metastatic disease is a strong recommendation of many clinical treatment guidelines [13]. Hence, heterogeneity in biomarker expression at metastatic sites is only beginning to be recognized, with growing appreciation for molecular imaging.
Since the use of 18F-FDG tumor uptake as a biomarker for predicting a pathologic response to treatment has been explored in the preclinical and clinical settings, with conflicting results [15], we also needed to demonstrate the role of 18F-FDG in advanced disease. More so, limited evidence supports the use of 18F-FDG PET to evaluate the extent of disease in selected patients with recurrent or metastatic disease [16, 17].
Although our case demonstrated concordance of 68Ga-PSMA and 18F-FDG lesions [6], of interest, we identified a weak but significant relationship between tumor metabolism as assessed by FDG uptake and tumor angiogenesis assessed by 68Ga-PSMA-HBED-CC PET imaging. This finding is in line with a previous report by Grobes et al. in a series of 20 consecutive newly diagnosed breast carcinoma patients in whom FDG uptake proved significantly associated with the degree of angiogenesis assessed using immunohistochemistry and CD105 staining [1, 18]. CD105 or endoglin is an accessory receptor for transforming growth factor beta (TGF-beta) of which the expression is up-regulated in actively proliferating endothelial cells. Most investigators, including Grobes et al., have reported a correlation between tumor angiogenesis and glucose metabolism [12, 19]. However, other studies failed to demonstrate a significant correlation between angiogenesis and FDG uptake. Avril et al. reported an inverse relationship between SUV and the number of microvessels in breast cancer patients [20]. This could be one of the reasons for the weak relationship between FDG uptake and 68Ga-PSMA-HBED-CC PET imaging.
The robust expression of PSMA by breast cancer lesions as evidenced using 68Ga-PSMA-HBED-CC PET imaging in this series and the absence of PSMA on normal vascular endothelium as well as its limited expression on the luminal side of the intestinal epithelium, which is not accessible via the vasculature, makes PSMA an interesting potential target for antiangiogenic therapy of breast carcinoma. More specifically, PSMA-targeting therapeutic agents may selectively destroy vessels perfusing tumor tissue and achieve high regional doses of drugs to overcome tumor resistance while sparing normal tissue, which typically lacks PSMA expression. In this regard, both the anti-PSMA monoclonal antibody J591 and 177Lu-PSMA-617 were shown to be well tolerated and to show considerable clinical efficacy, respectively in patients suffering from a variety of advanced solid tumors and prostate carcinoma [21, 22]. More recently, the results of a first-in human phase I trial to determine the safety, pharmacokinetics, and anti-tumor activity of BIND-014, a PSMA-targeting nanoparticle containing docetaxel were reported [23]. BIND-014 was shown to be generally well tolerated and clinical activity was noted in multiple tumor types.
Folkman characterized angiogenesis as being fundamental for tumor growth beyond 2 mm in 1971 [24]. Surprisingly, there is still no validated predictive biomarker for the selection of antiangiogenic therapy [25]. While angiogenesis is an important component in the progression of a number of diseases, it is clear that all angiogenic processes are not regulated by the same signals and are often distinct pathologies [26]. Hence 68Ga-PSMA-HBED-CC PET imaging as performed in the series presented may allow for selection of those patients most likely to benefit from these PSMA-targeting treatment modalities. Furthermore, it is not to be excluded that 68Ga-PSMA-HBED-CC PET imaging may also play a role in treatment response monitoring and selection of those patients suffering from breast carcinoma that may benefit from non-PSMA targeting antiangiogenic treatment strategies either given as monotherapy or in combination with chemotherapy, e.g., bevacizumab, aflibercept, integrin targeting antibodies, sunitinib, sorafenib, gamma-secretase inhibitors, angiopoietin inhibitors, and mTOR inhibitors [27].
The limitations of this study were the small number of patients included and lack of assessment of HER2 status of the metastatic lesions. This will be undertaken in a future large study. Although our study did not assess targeting antiangiogenic therapy for breast cancer; studies assessing the potential of 68Ga-PSAM-HBED-CC for predicting and monitoring response to antiangiogenic treatment in patients suffering from breast carcinoma could be helpful and thus warranted. In conclusion, 68Ga-PSMA-HBED-CC PET/CT imaging in breast carcinoma confirms the reported considerable variation of PSMA expression on human solid tumors using immunohistochemistry.
Acknowledgments
Department of Nuclear Medicine at University Pretoria and NECSA.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was performed in accordance with the ethical standards of our institution and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.O’Keefe DS, Su SL, Bacich DJ, Horiguchi Y, Luo Y, Powell CT, et al. Mapping, genomic organization and promotor analysis of the human prostate-specific membrane antigen gene. Biochim Biophys Acta. 1998;1443(1-2):113–27. doi: 10.1016/S0167-4781(98)00200-0. [DOI] [PubMed] [Google Scholar]
- 2.Maurer T, Eiber M, Schwaiger M, Gschwend J. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13(4):226–35. doi: 10.1038/nrurol.2016.26. [DOI] [PubMed] [Google Scholar]
- 3.Evangelista L, Briganti A, Fanti S, Joniau S, Reske S, Schiavina R, et al. New clinical indications for 18F/11C-choline, new tracers for positron emission tomography and a promising hybrid device for prostate cancer staging: a systematic review of the literature. Eur Urol. 2016;70(1):161–75. doi: 10.1016/j.eururo.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 4.Rahbar K, Weckesser M, Huss S, Semjonow A, Breyholz HJ, Schrader AJ, et al. Correlation of intraprostatic tumor extent with 68Ga-PSMA distribution in patients with prostate cancer. J Nucl Med. 2016;57(4):563–7. doi: 10.2967/jnumed.115.169243. [DOI] [PubMed] [Google Scholar]
- 5.Chang S, Reuter V, Heston W, Bander N, Grauer L, Gaudin P. Five different anti-prostate-specific membrane antigen (PSMA) antibodies conform PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59(13):3192–8. [PubMed] [Google Scholar]
- 6.Sathekge M, Modiselle M, Vorster M, Mokgoro N, Nyakale N, Mokaleng B, et al. 68Ga-PSMA imaging of metastatic breast cancer. Eur J Nucl Med Mol Imaging. 2015;42(9):1482–3. doi: 10.1007/s00259-015-3066-x. [DOI] [PubMed] [Google Scholar]
- 7.Wernicke AG, Varma S, Greenwood EA, Christos PJ, Chao KS, Liu H, et al. Prostate-specific membrane antigen expression in tumor-associated vasculature of breast cancers. APMIS. 2014;122(6):482–9. doi: 10.1111/apm.12195. [DOI] [PubMed] [Google Scholar]
- 8.Nomura N, Pastorino S, Jiang P, Lambert G, Crawford JR, Gymnopoulos M, et al. Prostate-specific membrane antigen (PSMA) expression in primary gliomas and breast cancer brain metastases. Cancer Cell Int. 2014;14(1):26. doi: 10.1186/1475-2867-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebenhan T, Vorster M, Marjanovic-Painter B, Wagener J, Suthiram J, Modiselle M, et al. Development of a single vial kit solution for radiolabeling of 68Ga-DKFZ-PSMA-11 and its performance in prostate cancer patients. Molecules. 2015;20(8):14860–78. [DOI] [PMC free article] [PubMed]
- 10.Conway R, Petrovic N, Li Z, Heston W, Wu D, Shapiro L. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signaling transduction. Mol Cell Biol. 2006;26(14):5310–24. doi: 10.1128/MCB.00084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grant CL, Caromile LA, Ho V, Durrani K, Rahman MM, Claffey KP, et al. Prostate-specific membrane antigen (PSMA) regulates angiogenesis independently of VEGF during ocular neovascularization. PLoS One. 2012;7(7):e41285–9. doi: 10.1371/journal.pone.0041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsui P, Rubenstein M, Guinan P. Correlation between PSMA and VEGF expression as markers for LNCaP tumor angiogenesis. J Biomed Biotechnol. 2005;2005(3):287–90. doi: 10.1155/JBB.2005.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aurilio G, Disalvatore D, Pruneri G, Bagnardi V, Viale G, Curigliano G, et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer. 2014;50(2):277–89. doi: 10.1016/j.ejca.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Vamesu S. Angiogenesis and progesterone receptor status in primary breast cancer patients: an analysis of 158 needle core biopsies. Rom J Morphol Embryol. 2007;48(3):267–74. [PubMed] [Google Scholar]
- 15.Gebhart G, Flamen P, De Vries EG, Jhaveri K, Wimana Z. Imaging diagnostic and therapeutic targets: human epidermal growth factor receptor 2. J Nucl Med. 2016;57(Suppl 1):81S–8. doi: 10.2967/jnumed.115.157941. [DOI] [PubMed] [Google Scholar]
- 16.Podoloff DA, Advani RH, Allred C, Benson AB, 3rd, Brown E, Burstein HJ, et al. NCCN task force report: positronemission tomography/computed tomography scanning in cancer. J Natl Compr Canc Netw. 2007;5(Suppl 1):S1–22. [PubMed] [Google Scholar]
- 17.Rosen EL, Eubank WB, Mankoff DA. FDG PET, PET/CT, and breast cancer imaging. Radiographics. 2007;27(suppl 1):S215–29. doi: 10.1148/rg.27si075517. [DOI] [PubMed] [Google Scholar]
- 18.Groves AM, Shastry M, Rodriguez-Justo M, Malhotra A, Endozo R, Davidson T, et al. 18F-FDG PET and biomarkers for tumor angiogenesis in early breast cancer. Eur J Nucl Med Mol Imaging. 2011;38(1):46–52. doi: 10.1007/s00259-010-1590-2. [DOI] [PubMed] [Google Scholar]
- 19.Bos R, van der Hoeven JJ, van der Wall E, van der Groep P, van Diest PJ, Comans EF, et al. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20(2):379–87. doi: 10.1200/JCO.20.2.379. [DOI] [PubMed] [Google Scholar]
- 20.Avril N, Menzel M, Dose J, Schelling M, Weber W, Jänicke F, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42(1):9–16. [PubMed] [Google Scholar]
- 21.Milowsky MI, Nanus DM, Kostakoglu L, Sheehan CE, Vallabhajosula S, Goldsmith SJ, et al. Vascular targeted therapy with anti-prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors. J Clin Oncol. 2007;25(5):540–7. doi: 10.1200/JCO.2006.07.8097. [DOI] [PubMed] [Google Scholar]
- 22.Rahbar K, Schmidt M, Heinzel A, Eppard E, Bode A, Yordanova A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. J Nucl Med. 2016;57(9):1334–8. doi: 10.2967/jnumed.116.173757. [DOI] [PubMed] [Google Scholar]
- 23.Von Hoff DD, Mita MM, Ramanathan RK, Weiss GJ, Mita AC, LoRusso PM, et al. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin Cancer Res. 2016;22(13):3157–63. doi: 10.1158/1078-0432.CCR-15-2548. [DOI] [PubMed] [Google Scholar]
- 24.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 25.Wilson PM, LaBonte MJ, Lenz HJ. Assessing the in vivo efficacy of biologic antiangiogenic therapies. Cancer Chemother Pharmacol. 2013;71(1):1–12. doi: 10.1007/s00280-012-1978-8. [DOI] [PubMed] [Google Scholar]
- 26.Friedlander M, Theesfeld CL, Sugita M, Fruttiger M, Thomas MA, Chang S, et al. Involvement of integrins alpha v beta 3 and alpha v beta 5 in ocular neovascular diseases. Proc Natl Acad Sci U S A. 1996;93(18):9764–9. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen D, Andersson M, Andersen J, Kamby C. Antiangiogenic therapy for breast cancer. Breast Cancer Res. 2010;12(5):209. doi: 10.1186/bcr2642. [DOI] [PMC free article] [PubMed] [Google Scholar]