Abstract
Hyperoxaluria is a major risk factor for kidney stones and has no specific therapy, although Oxalobacter formigenes colonization is associated with reduced stone risk. O. formigenes interacts with colonic epithelium and induces colonic oxalate secretion, thereby reducing urinary oxalate excretion, via an unknown secretagogue. The difficulties in sustaining O. formigenes colonization underscore the need to identify the derived factors inducing colonic oxalate secretion. We therefore evaluated the effects of O. formigenes culture conditioned medium (CM) on apical 14C-oxalate uptake by human intestinal Caco-2-BBE cells. Compared with control medium, O. formigenes CM significantly stimulated oxalate uptake (>2.4-fold), whereas CM from Lactobacillus acidophilus did not. Treating the O. formigenes CM with heat or pepsin completely abolished this bioactivity, and selective ultrafiltration of the CM revealed that the O. formigenes–derived factors have molecular masses of 10–30 kDa. Treatment with the protein kinase A inhibitor H89 or the anion exchange inhibitor 4,4'-diisothiocyano-2,2'-stilbenedisulfonic acid completely blocked the CM-induced oxalate transport. Knockdown of the oxalate transporter SLC26A6 also significantly restricted the induction of oxalate transport by CM. In a mouse model of primary hyperoxaluria type 1, rectal administration of O. formigenes CM significantly reduced (>32.5%) urinary oxalate excretion and stimulated (>42%) distal colonic oxalate secretion. We conclude that O. formigenes–derived bioactive factors stimulate oxalate transport in intestinal cells through mechanisms including PKA activation. The reduction in urinary oxalate excretion in hyperoxaluric mice treated with O. formigenes CM reflects the in vivo retention of biologic activity and the therapeutic potential of these factors.
Keywords: intestinal oxalate transport, secreted bioactive factors, Oxalobacter formigenes, PKA, SLC26A6
Nephrolithiasis is the second most prevalent kidney disease in the United States after hypertension, with a rising prevalence and complications including CKD and ESRD.1 Hyperoxaluria is a major risk factor for kidney stones, and 70%–80% of kidney stones are composed of calcium oxalate.2 Urinary oxalate is an important determinant of supersaturation, and the risk of stone formation is affected by small increases in urine oxalate.3,4 Mild-to-moderate hyperoxaluria is found in patients with diabetes and obesity and contributes to their increased risk of stone formation.5–9 More severe hyperoxaluria is seen in enteric hyperoxaluria (EH), most commonly observed in inflammatory bowel disease patients and after small-bowel resection and bariatric surgery for obesity.10–12 Primary hyperoxaluria (PH) is an inherited disease in which there is endogenous oxalate overproduction.13 Both EH and PH lead to recurrent calcium oxalate kidney stones (COKS), nephrocalcinosis, and ESRD.
The mammalian intestine plays a crucial role in oxalate homeostasis, by regulating the amount of absorbed dietary oxalate and providing an avenue for enteric oxalate excretion.14 Anion exchanger SLC26A6 (A6)-mediated intestinal oxalate secretion plays a critical role in preventing hyperoxaluria and COKS.15,16 Oxalobacter formigenes is an anaerobic bacterium and utilizes oxalate as its exclusive energy source.17 O. formigenes colonization correlates with reduced risk of COKS formation in a number of studies, presumably by reducing intestinal oxalate absorption and urinary oxalate excretion.18–21
In addition to degrading dietary oxalate, O. formigenes also interacts with colonic epithelium by inducing distal colonic oxalate secretion, leading to reduced urinary excretion via a potential secretagogue.22 This effect was demonstrated in rodent colon and whether similar regulation is also active in human colon remains to be determined. O. formigenes colonization of PH1 mice significantly reduced serum and urinary oxalate levels due to induction of colonic oxalate secretion.23 However, all PH1 mice lost colonization within 18 days when switched from a high oxalate/low calcium diet (1.5% oxalate/0.5% calcium) to regular mouse chow (0.25% oxalate/1% calcium).23 In addition, colonization cannot be maintained without reducing dietary calcium.22,24 Moreover, it has been suggested from studies in PH patients and PH1 mice that the intraluminal environment in PH is not supportive of sustained O. formigenes colonization.23,25 Collectively, maintaining O. formigenes colonization in the absence of high exogenous oxalate remains problematic, underscoring the need for identifying O. formigenes–derived bioactive factors exerting effects similar to live O. formigenes.
We therefore examined whether O. formigenes conditioned medium (CM) modulates intestinal oxalate transport by testing its effects on oxalate uptake by Caco2-BBE (C2) cells. We find that O. formigenes CM significantly stimulates oxalate uptake by C2 cells through PKA activation. small interfering RNA (siRNA) knockdown studies show that a significant component of the observed stimulation is A6-mediated. Importantly, O. formigenes CM also significantly reduces urinary oxalate excretion in PH1 mice by stimulating distal colonic oxalate secretion, reflecting its translational relevance. Of note is that A6 (mediates ≥50% of apical oxalate uptake by C2 cells26) operates in the direction of exchanging intracellular oxalate for mucosal Cl during the process of transepithelial intestinal oxalate secretion. However, A6 can operate in either direction,27 and we therefore measured its activity by the more convenient assay of cellular oxalate uptake.
Results
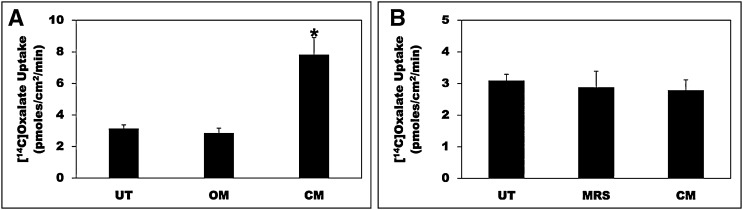
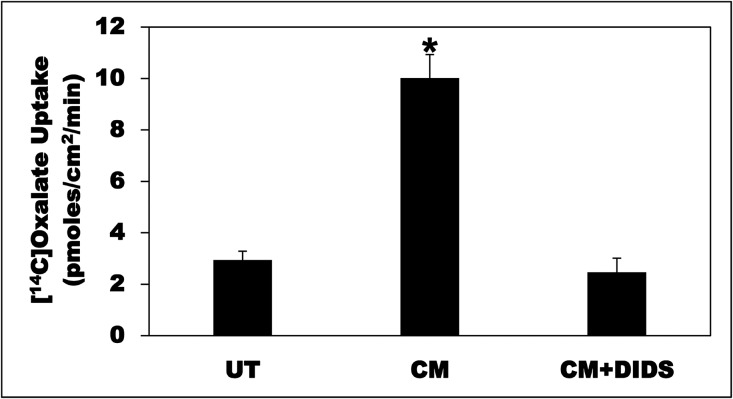
We previously established C2 cells as a model to study regulation of intestinal oxalate transport by extracellular nucleotides.28 We have now used C2 cells to examine whether intestinal oxalate transport is regulated by O. formigenes CM. As shown in Figure 1A, compared with untreated (UT) cells and cells treated with O. formigenes growth medium (OM), the CM significantly stimulated (>2.4-fold) oxalate uptake. Similar effects were also observed with 6-hour and 16-hour incubations, but no effect was seen at 1 hour (data not shown). The pH of the medium is not affected by OM or CM. Importantly, OM or CM has no effect on the transepithelial resistance (TER) (mean TER in Ω cm2, before and after treatment, respectively: UT=434.17±19.49 and 443.83±20.09; OM=417.45±7.24 and 430.11±11.74; CM=436.25±11.26 and 438.57±13.10), indicating that OM or CM did not affect the paracellular permeability. These results indicate that potential secreted factors are responsible for the observed stimulation, likely by modulating the activity of the involved transporter(s).
Figure 1.
O. formigenes CM stimulates oxalate uptake by C2 cells. (A) C2 cells were UT or were treated apically with OM or CM in the culture medium (1:50 dilution × 24 hours), and then 14C-oxalate uptake was measured as described in the Concise Methods. Values are means±SEM of 12 independent experiments each of which was done in triplicate. The CM significantly stimulated 14C-oxalate uptake (*P<0.001 for CM compared with UT and OM, by ANOVA). (B) C2 cells were UT or were treated apically with L. acidophilus growth medium (MRS) or CM in the culture medium (1:25 dilution × 24 hours), and then 14C-oxalate uptake was measured as described in the Concise Methods. Values are means±SEM of three independent experiments each of which was done in triplicate. L. acidophilus CM had no significant effect on 14C-oxalate uptake by C2 cells.
A crucial question is, how specific is the observed stimulation? To address specificity, we similarly evaluated the effect of Lactobacillus acidophilus (also degrades intraluminal oxalate,29 but it is unknown if it interacts with intestinal cells and modulates oxalate transport) CM. Compared with UT and cells treated with the control medium (MRS), L. acidophilus CM had no effect on oxalate uptake by C2 cells (Figure 1B). These results indicate that O. formigenes CM-mediated stimulation of oxalate transport is specific and is possibly mediated by one or more O. formigenes–derived factor(s).
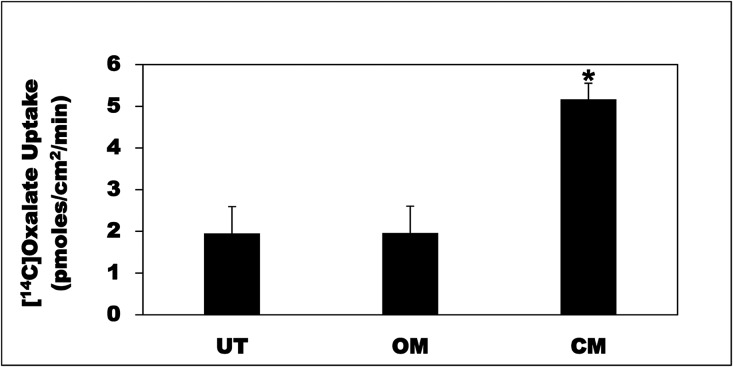
To ensure that the CM effects on intestinal oxalate transport are not cell-line specific, we evaluated the CM effects on oxalate uptake by the human colonic cell line T84. C2 and T84 cells are considered models for absorptive epithelial cells and secretory crypt cells, respectively. We previously demonstrated that A6 mediates most of oxalate transport in T84 cells.30 Compared with UT and OM, the CM significantly stimulated (>2.6-fold) oxalate uptake by T84 cells (Figure 2). These results indicate that the CM effects on oxalate transport by intestinal epithelial cells are not cell-line specific.
Figure 2.
O. formigenes CM stimulates oxalate uptake by T84 cells. T84 cells were UT or were treated apically with OM or CM in the culture medium (1:50 dilution × 24 hours), and then 14C-oxalate uptake was measured as described in the Concise Methods. Values are means±SEM of three independent experiments each of which was done in triplicate. The CM significantly stimulated 14C-oxalate uptake (*P<0.05 and 0.01 for CM compared with UT and OM, respectively, by ANOVA).
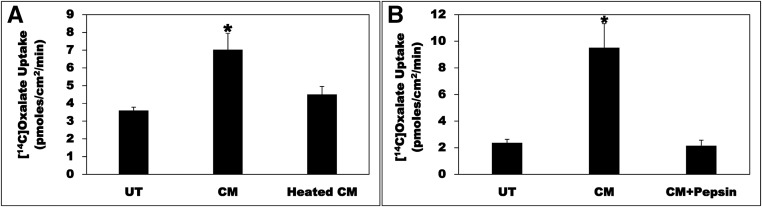
To test the possibility that the O. formigenes–derived bioactive factor(s) might be proteinaceous in nature, the CM was subjected to heat treatment. Compared with UT, the CM significantly stimulated (>1.9-fold) oxalate uptake by C2, an effect completely abolished by heat treatment (heated CM) (Figure 3A). These results indicate that the O. formigenes–derived factor(s) is/are likely to be protein(s) and/or peptide(s). To provide more evidence that the O. formigenes–derived bioactive factor(s) is/are protein(s) and/or peptide(s), the CM was treated with the protease pepsin. Compared with UT, the CM significantly stimulated (>4-fold) oxalate uptake by C2, an effect completely abolished by pepsin treatment (CM+Pepsin) (Figure 3B). Treatment of the CM with another protease, trypsin, also completely abolished its bioactivity (data not shown). These results provide further evidence that the O. formigenes–derived factor(s) is/are protein(s) and/or peptide(s).
Figure 3.
Heat and pepsin treatment abolish the CM bioactivity. (A) C2 cells were UT or were treated apically with CM or a CM that was subjected to heat treatment (heated CM) in the culture medium (1:50 dilution × 24 hours), and then 14C-oxalate uptake was measured as described in the Concise Methods. Values are means±SEM of four independent experiments each of which was done in triplicate. The CM significantly stimulated 14C-oxalate uptake, an effect completely abolished by heat treatment (*P<0.01 and <0.05 for CM compared with UT and heated CM, respectively, by ANOVA). (B) C2 cells were UT or were treated apically with CM or a CM that was treated with pepsin (CM+Pepsin) in the culture medium (1:50 dilution × 24 hours), and then 14C-oxalate uptake was measured as described in the Concise Methods. Values are means±SEM of four independent experiments each of which was done in triplicate. The CM significantly stimulated 14C-oxalate uptake, an effect completely abolished by pepsin treatment (* P<0.01 for CM compared with UT and CM+Pepsin, by ANOVA).
To determine the molecular mass(es) (MM) of the factor(s), the CM was subjected to selective ultrafiltration using 10 and 30 kDa cutoff spin columns. C2 cells were UT or treated with the CM, filtrate (F), retentate (R), or F+R. Using a 10 kDa column, the R and the F+R significantly stimulated oxalate uptake by C2 cells, whereas the F had no effect (Figure 4A). Using a 30 kDa column, the F and the F+R significantly stimulated oxalate uptake by C2 cells, whereas the R had no effect (Figure 4B). Collectively, these results indicate that the MMs of the factors are largely between 10 and 30 kDa. Because F+R had a better stimulatory effect than F, whereas R had no effect, such data suggest the possibility that these factors might exist as a multifunctional complex requiring a bacterial product of >30 kDa for optimal functioning.
Figure 4.
Selective ultrafiltration shows the O. formigenes–derived factors to have molecular masses of 10–30 kDa. (A) Selective ultrafiltration of the CM using 10 kDa cutoff spin column. C2 cells were UT or were treated with CM, F, R, or the combined fractions (F+R) in the culture medium (1:50 dilution × 24 hours), and then 14C-oxalate uptake was measured as described in the Concise Methods. Values are means±SEM of five independent experiments each of which was done in duplicate or triplicate. The CM, the R, and the F+R significantly stimulated 14C-oxalate uptake (*P<0.001, <0.001, and <0.01 for CM compared with UT, F, and R; **P<0.01 for R compared with UT and F; and ***P<0.001, <0.001, and <0.01 for F+R compared with UT, F, and R, respectively, by ANOVA). (B) Selective ultrafiltration of the CM using 30 kDa cutoff spin column. C2 cells were UT or were treated with CM, F, R, or F+R in the culture medium (1:50 dilution × 24 hours), and then 14C-oxalate uptake was measured as described in the Concise Methods. Values are means±SEM of eight independent experiments each of which was done in triplicate. The CM, the F, and the F+R significantly stimulated 14C-oxalate uptake (*P<0.001 and <0.01 for CM compared with UT and R, respectively; ** P<0.05 for F compared with UT and R; and ***P<0.001 and <0.05 for F+R compared with UT and R, respectively, by ANOVA).
O. formigenes was suggested to utilize a potential secretagogue as a survival strategy when dietary oxalate is limited.22 To test the hypothesis that lower growth medium oxalate concentration might lead to more secreted factor(s) and therefore to a CM with higher bioactivity, oxalate concentration was reduced from 37.5 mM to 18.8 and 9.4 mM, and conditioned media under these conditions were simultaneously prepared (CM37.5, CM18.8, and CM9.4, respectively). C2 cells were UT or treated with CM37.5, CM18.4, and CM9.4. Interestingly, the bioactivity of the CM was significantly higher (>2-fold) with CM9.4 compared with CM37.5 (Figure 5). These results strongly indicate that the secretion of these factors is inducible, which will significantly facilitate their characterization.
Figure 5.
Lowering O. formigenes growth medium oxalate concentration leads to a CM with higher bioactivity. C2 cells were UT or treated with CM37.5, CM18.8, and CM9.4 (conditioned media prepared with growth medium oxalate concentration of 37.5 mM, 18.8 mM, and 9.4 mM, respectively) in the culture medium (1:50 dilution × 24 hours), and then 14C-oxalate uptake was measured as described in the Concise Methods. Values are means±SEM of three independent experiments each of which was done in triplicate. CM9.4 had significantly higher bioactivity compared with CM37.5 (*P<0.01 and <0.05 for CM9.4 compared with UT and CM37.5, respectively, by ANOVA).
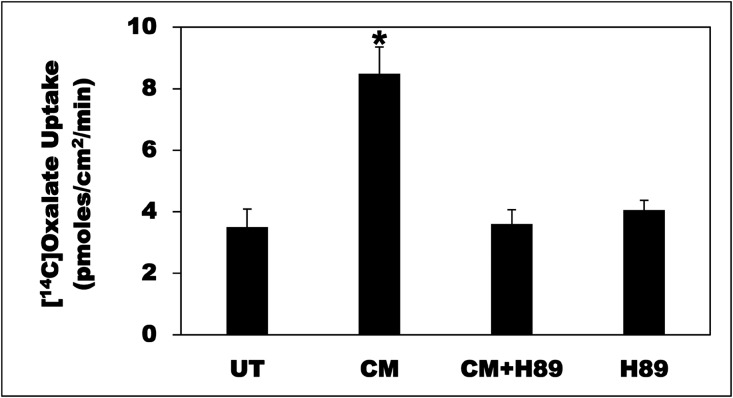
Probiotic-derived factors regulate intestinal epithelial homeostasis by modulating specific signaling pathways.31,32 To elucidate the involved signaling pathway(s), we tested the effects of several pharmacologic inhibitors. Preincubation of C2 cells with the PKA inhibitor H8933 before incubation with the CM (CM+H89) completely blocked the CM-induced stimulation, whereas it had no effect on baseline transport (H89) (Figure 6). We previously found PKC activation inhibits intestinal oxalate transport through a Gö6983-sensitive pathway28,30,34; however, this inhibitor had no effect on the observed stimulation (pmol/cm2 per minute: UT=3.12±0.32; CM=10.54±0.43; CM+Gö6983=9.64±0.67; Gö6983=3.60±0.77). On the other hand, Gö6976, U0126, SB 202190, LY 294002, genistein, and PP2 (described in the Concise Methods) had no significant effect on the observed stimulation (data not shown). Taken together, these results indicate that the CM stimulates oxalate transport in C2 cells by activating PKA, whereas PKC, MAPKs, and Src kinases are unlikely to be involved.
Figure 6.
The PKA inhibitor H89 completely blocked the CM-induced oxalate transport. C2 cells were UT or were treated apically with CM in the culture medium (1:50 dilution × 24 hours), and then 14C-oxalate uptake was measured as described in the Concise Methods. C2 cells were also treated apically with H89 (20 µM) for 30 minutes followed by the CM (1:50 dilution × 24 hours) with continued presence of H89 (CM+H89), or with H89 (20 µM) alone for 24.5 hours, and then 14C-oxalate uptake was similarly measured. Values are means±SEM of seven independent experiments each of which was done in triplicate. The CM significantly stimulated 14C-oxalate uptake, an effect completely blocked by H89 (*P<0.001 for CM compared with UT, CM+H89, and H89, by ANOVA).
To assess whether the CM-induced stimulation of oxalate uptake by C2 cells is due to an active transcellular transport process, the effects of the anion exchange inhibitor 4,4׳diisothiocyanostilbene-2,2׳-disulfonic acid (DIDS) were tested on the observed stimulation. Compared with UT cells, the CM significantly stimulated (>3.4-fold) oxalate uptake by C2, an effect completely abolished by DIDS (100 µM) treatment (CM+DIDS) (Figure 7). These results indicate that the observed stimulation is mediated by DIDS-sensitive transporters such as A6 and/or SLC26A2 (A2).26,30,35,36
Figure 7.
The CM-induced oxalate transport is DIDS-sensitive. C2 cells were UT or were treated apically with CM in the culture medium (1:50 dilution × 24 hours), and then 14C-oxalate uptake was measured as described in the Concise Methods. CM-treated cells were treated apically with DIDS (100 µM) for 36 minutes (30 minutes immediately before the flux as well as during the 6 minute flux period) (CM+DIDS). Values are means±SEM of seven independent experiments each of which was done in triplicate. The CM significantly stimulated 14C-oxalate uptake, an effect completely abolished by DIDS (*P<0.001 for CM compared with UT and CM+DIDS, by ANOVA).
To examine whether the CM stimulates oxalate transport in C2 cells by increasing the mRNA expression of the likely involved transporters (SLC26A1 [A1], A2, and/or A6), C2 cells were UT or similarly treated with the CM and then total RNA was isolated for qPCR analysis. Compared with UT, the CM did not significantly affect A1, 2, and 6 mRNA expression (Figure 8), indicating that the observed stimulation is unlikely to be through modulation of these transporters at a transcriptional level. In addition, the CM did not significantly affect A6 total and surface protein expression assessed by immunoblotting and surface biotinylation (Supplemental Figure 1). However, because of a lack of good antibodies, we did not test the CM effects on A1 or A2 total and surface protein expression.
Figure 8.
The CM has no effect on A1, A2, and A3 mRNA expression. C2 cells were UT or apically treated with CM in the culture medium (1:50 dilution × 24 hours), and then total RNA was isolated for real-time PCR analysis. Values are means±SEM of three independent experiments each of which was done in duplicate or triplicate. Relative A1, A2, and A6 mRNA expression levels were expressed as a percentage of UT normalized to GAPDH. The CM had no significant effect on A1, A2, and A6 mRNA expression levels.
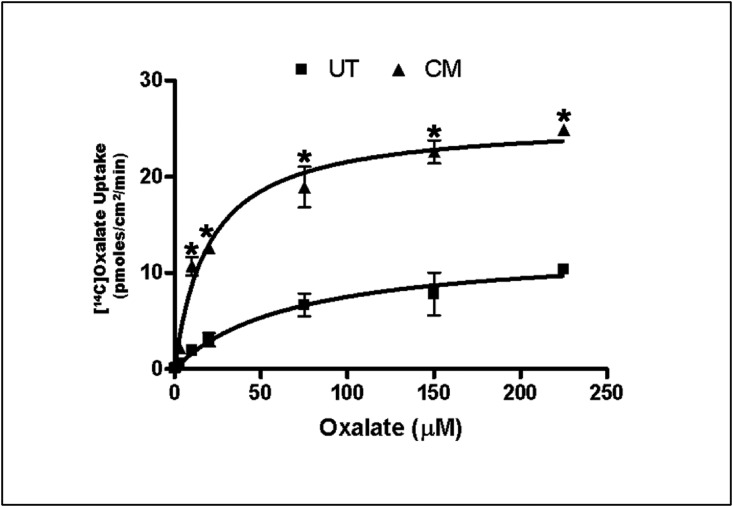
To examine whether the CM affects oxalate transport kinetic characteristics (i.e., the apparent affinity for oxalate [km] and maximal velocity [Vmax)]), 14C-oxalate uptake as a function of increasing 14C-oxalate concentration in the flux medium (0.3, 1, 3, 10, 20, 75, 150, and 225 µM) was assessed. Compared with UT cells, the CM significantly stimulated (>2.3-fold) oxalate uptake (which is saturable in the presence of increasing oxalate concentrations, reflecting a carrier-mediated transport process) at each concentration (Figure 9). Analysis of these results with a nonlinear regression Michaelis–Menten fit yielded a Km of 67.79±15.47 and 19.67±3.64 µM and a Vmax of 12.63±1.04 and 25.78±1.21 pmol/cm2 per minute for UT and CM, respectively. These data indicate that the observed stimulation is due to CM-induced increase (>2-fold) in Vmax (i.e., greater transport capacity) and reduction (>3.4-fold) in Km (i.e., greater affinity for oxalate) of the involved transporter(s).
Figure 9.
The CM increases the Vmax and reduces the Km. C2 cells were UT or apically treated with CM in the culture medium (1:50 dilution × 24 hours), and then14C-oxalate uptake as a function of increasing 14C-oxalate concentration in the flux medium (0.3, 1, 3, 10, 20, 75, 150, and 225 µM) was assessed. Values are means±SEM of three independent experiments each of which was done in triplicate. The CM significantly stimulated 14C-oxalate uptake at each oxalate concentration (*P<0.03, 0.003, 0.003, 0.01, 0.002, 0.01, 0.01, and 0.001 for CM compared with UT at the above corresponding 14C-oxalate concentrations, by unpaired t test).
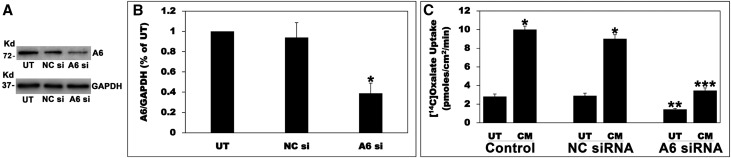
To investigate the A6 role in the observed stimulation, A6 expression in C2 cells was knocked down using siRNA. C2 cells were untransfected or transfected with a negative control siRNA (NC si) or an A6 specific siRNA (A6 si). A6 si significantly reduced A6 protein expression (approximately 61%) and baseline oxalate uptake (approximately 49%) by C2 cells (Figure 10), results which were similar to those previously reported,26 whereas NC si had no effect, indicating the observed reduction in A6 expression is not due to a general effect of the silencing procedure. Equal loading was verified by observing no difference in GAPDH. Silencing A6 greatly decreased the CM-induced stimulation of oxalate transport by C2 cells (Figure 10C), indicating that a significant component of the observed stimulation is mediated by A6.
Figure 10.
Silencing A6 greatly reduced the CM-induced stimulation of oxalate transport. (A) A representative Western blot analysis of total A6 protein expression. A6 protein expression was evaluated in C2 cell lysate (10 µg protein/lane: untransfected cells; NC si, C2 cells transfected with the NC si; A6 si, C2 cells transfected with the siRNA targeting A6). The lower half of the same blot was probed with an anti-GAPDH antibody to normalize loading of protein in each lane (lower panel). (B) Densitometry of immunoblot results. Western blot band density was quantified using ImageJ software. Values are means±SEM for six independent experiments of relative total A6 abundance to GAPDH and are presented as a percentage of the UT value. A6 siRNA knockdown significantly reduced A6 protein expression (*P<0.001 for A6 si compared with UT and NC si, by ANOVA). (C) Effect of A6 silencing on the CM-induced stimulation of 14C-oxalate uptake by C2 cells. Untransfected (Control), NC siRNA (transfected with the NC si), and A6 siRNA (transfected with the siRNA targeting A6) C2 cells were UT or were treated apically with the CM in the culture medium (1:50 dilution × 24 hours), and then 14C-oxalate uptake was measured as described in the Concise Methods. Values are means±SEM of eight independent experiments each of which was done in triplicate. Silencing A6 significantly reduced the CM-induced stimulation of oxalate transport by C2 cells (*P<0.001 for CM compared with UT in the Control and NC siRNA; **P<0.01 for UT in A6 siRNA compared with UT in Control and NC siRNA; ***P<0.001 for CM in A6 siRNA compared with CM in Control and NC siRNA, by ANOVA).
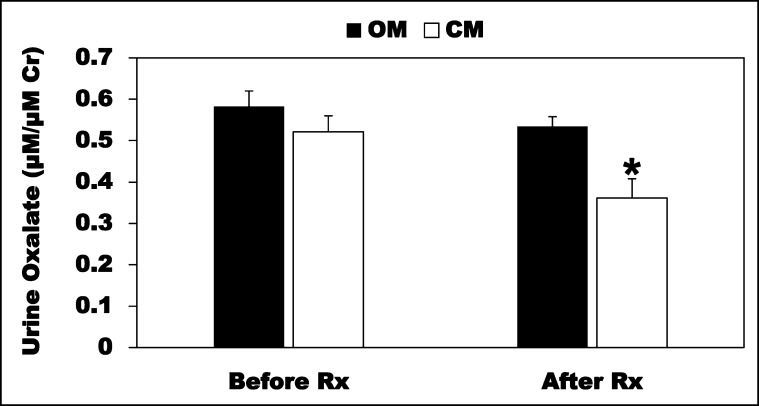
Although C2 cells closely resemble the native epithelium, confirmation of the in vitro findings in vivo is of paramount physiologic and translational interest. To this end, we evaluated the CM effects on the hyperoxaluria observed in PH1 mice.37 We first confirmed that these mice have significant (>3.6-fold) hyperoxaluria compared with their controls (C57BL/6J) (µM/µM creatinine: controls=0.16±0.01; PH1=0.57±0.03; n=6–10). PH1 mice (males; 38–42 weeks old) were given CM or OM (100 µl twice daily as rectal enemas × 21 days) and urine was collected before (Before Rx) and after treatment (After Rx) for oxalate measurements. Importantly, the CM significantly reduced (>32.5%) urinary oxalate excretion, whereas OM had no effect (Figure 11). These results indicate that the O. formigenes–derived factors retain their biologic activity in vivo and effectively lower urinary oxalate excretion. Of note is that distal colonic histology from OM - and CM-treated mice (compared with UT mice) looks normal, with no evidence of injury, irritation, or inflammation (Supplemental Figure 2).
Figure 11.
O. formigenes CM reduces urinary oxalate excretion in PH1 mice. The PH1 mice were given the CM or OM (100 µl twice daily as rectal enemas × 21 days; n=6–8) and urine was collected before (Before Rx) and after treatment (After Rx) for oxalate measurements. The CM significantly reduced urinary oxalate (adjusted for urinary creatinine [Cr]) excretion (*P<0.03 for CM compared with OM, by unpaired t test).
To test the possibility that the observed reduction in urinary oxalate excretion is due to CM-induced colonic oxalate secretion, distal colonic tissues from CM- and OM-treated PH1 mice were isolated and mounted in Ussing chambers at the end of treatment. Although a small net oxalate secretory flux (−4.71±2.44) is observed in distal colonic tissues from OM-treated PH1 mice, a >4.2-fold higher net oxalate secretory flux (−20.01±5.51) is seen in distal colonic tissues from CM-treated PH1 mice, which is due to significantly increased (>42%) secretory flux (JSM) (Table 1). CM or OM had no significant effect on the absorptive flux. These results indicate that the O. formigenes–derived factors reduce urinary oxalate excretion in PH1 mice through mechanisms including enhanced net distal colonic oxalate secretion. Although the transepithelial conductance is not affected, the CM significantly increased the short-circuit current (Table 1), suggesting that the CM also affects the movement of other ion(s), as observed with O. formigenes colonization of PH1 mice in some series, and rats treated with O. formigenes lysate.22,23 The latter was explained by concomitant induction of net chloride secretion, which could potentially stimulate transepithelial colonic oxalate secretion by providing a counter-ion gradient for exchanging intracellular oxalate for mucosal Cl.
Table 1.
Unidirectional (mucosa to serosa [JMS, absorptive flux], and serosa to mucosa [JSM, secretory flux]) and net (Jnet) transepithelial oxalate fluxes across distal colonic tissues (n=5 tissue pairs) isolated from OM- and CM-treated PH1 mice and mounted ex vivo in modified Ussing chambers
| PH1 Mice | JMS (pmol/h per cm2) | JSM (pmol/h per cm2) | Jnet (pmol/h per cm2) |
|---|---|---|---|
| OM | 37.84±5.92 | 42.56±5.48 | −4.71±2.44 |
| CM | 40.56±3.72 | 60.57±3.13a | −20.01±5.51b |
The CM significantly increased the JSM as well as the Jnet flux. OM or CM did not significantly affect the transepithelial conductance (GT) (OM = 20.94±1.39 mS/cm2; CM = 24.08±2.37 mS/cm2). The CM significantly increased the short-circuit current (Isc) (OM = 0.51±0.10 µEq/cm2 per hour; CM = 1.07±0.24 µEq/cm2 per hour; P<0.05, by unpaired t test). Data are expressed as means±SEM.
P<0.03 for CM compared with OM, with regards to JSM, by unpaired t test.
P<0.05 for CM compared with OM, with regards to Jnet, by unpaired t test.
Discussion
Probiotic bacteria have several health benefits; however, difficulties in determining intestinal bacterial bioavailability and biosafety concerns when administering live probiotics are potential problems facing current probiotics clinical application. Developing probiotics-derived factors as novel therapeutic agents is an alternative approach to address such concerns. Secreted factors from many probiotics stimulate several intestinal epithelial cell protective responses by activating different protein kinases.31,32,38–41 We now find that O. formigenes–derived factors significantly stimulate oxalate transport in C2 cells through a PKA-dependent signaling pathway. These factors retain their biologic activity in vivo and significantly reduce urinary oxalate excretion in PH1 mice by stimulating distal colonic oxalate secretion.
Hatch et al. reported that O. formigenes interacts with colonic epithelium by inducing distal colonic oxalate secretion, leading to reduced urinary excretion via a potential secretagogue.22 They also tested O. formigenes whole cells, cell membranes, and lysates on oxalate transport across rat distal colonic tissues mounted in Ussing chambers, and found no effect. Also, the effects of these products were tested for 45 minutes, which might be too short in view of the reported longer incubations (2–24 hours) for other probiotics,32,42 and the fact that the CM had no effect with 1 hour incubation. Feeding hyperoxaluric rats encapsulated O. formigenes lysate for 5 days significantly induced distal colonic oxalate secretion and reduced urinary oxalate excretion,22 which supports this assumption. In addition, the whole cells and lysates significantly degraded oxalate in the chamber, necessitating heat treatment of samples to eradicate this degradative effect.22 The authors acknowledged that such heat treatment might have destroyed any potential O. formigenes–derived factor(s), and our finding that heated CM lost its bioactivity supports this possibility.
A rising prevalence of hyperoxaluria is seen in association with many conditions, including inflammatory bowel disease, bariatric surgery, and obesity. Significant hyperoxalemia is seen in PH and ESRD.13,43 Unfortunately, there is currently no specific therapy that effectively lowers urine and/or plasma oxalate level(s). Dietary oxalate restriction is ineffective in PH, and is only of modest benefit in EH. The risk of recurrent COKS, nephrocalcinosis, oxalate nephropathy, ESRD, and systemic oxalosis remains substantial in the absence of treatment. In those PH patients unresponsive to pyridoxine, liver transplantation is the only effective treatment.13 Cardiovascular diseases are the leading cause of morbidity and mortality in ESRD patients,44 and a recent report raised the possibility that the ESRD-associated hyperoxalemia might contribute to this increased risk.43 Lowering serum oxalate might improve cardiovascular outcomes in ESRD patients if these findings are confirmed. The difficulties in maintaining O. formigenes colonization and biosafety concerns with administering live probiotics necessitate the identification of the O. formigenes–derived factors. The fact that these factors are active in vivo provides compelling evidence to pursue their characterization, given their significant therapeutic potential. In addition, the observation that the secretion of these factors is inducible by lowering O. formigenes culture medium oxalate concentration will significantly facilitate their characterization. Of interest in this regard is that we identified several interesting O. formigenes secreted proteins by mass spectrometry, and the most promising candidates will be overexpressed in Escherichia coli to obtain the corresponding recombinant proteins in future studies. If this approach fails to identify the O. formigenes–derived bioactive factor(s), then an alternative future strategy will be to look at the O. formigenes transcriptome under low and high oxalate concentrations in the culture medium.
A6 plays a critical role in mouse duodenal and ileal oxalate secretion.15,16 A6 is also expressed apically in mouse colon,45 but its role in colonic oxalate transport remains unknown. Knockdown studies showed a significant component of the observed stimulation is A6-mediated. An increase in Vmax indicates greater transport capacity, which could result from more membrane transporters. However, because the CM did not affect A6 surface protein expression, and in view of the reduced Km (reflecting greater A6 affinity for oxalate), it is likely possible that the observed stimulation is due to mechanisms including CM-induced enhanced A6 transport activity (resulting from an increase in the intrinsic activity of the preexisting A6 membrane transporters). A2 is apically expressed in enterocytes (colon > small intestine).46–48 It mediates oxalate transport when expressed in heterologous systems and it is DIDS-sensitive35; however, its role in intestinal oxalate transport is unknown. The observed changes in Km and Vmax could also result from CM-mediated increased A2 transport activity. Although A2 mRNA expression is not affected, it is possible that the observed stimulation is due to CM-induced enhanced A2 total and/or surface protein expression, which will be tested in future studies. Because transcellular oxalate secretion requires oxalate influx into the enterocyte from the blood side, where A1 might be involved, and then its efflux from the luminal side, it is possible that CM-induced changes in A1 expression and/or activity also contribute to the observed enhanced distal colonic oxalate secretion.
SLC26A3 (A3) plays a critical role in transcellular mouse colonic oxalate absorption.49 However, its role in oxalate transport in C2 cells remains to be determined. Worthy of mentioning is that in contrast to the observed robust Cl-HCO3 exchange, human A3 transports oxalate poorly (both uptake and efflux, despite using a very high 14C-oxalate concentration of 1 mM) when expressed in Xenopus oocytes,50 strongly suggesting that Cl-HCO3 exchange is the main physiologic function of A3. In addition, A3-mediated Cl/OH exchange activity has been assessed as DIDS-sensitive (using 600 µM DIDS) 36Cl uptake by C2 cells,42,51 reflecting it is relatively DIDS-insensitive. It should be noted that A3 was proposed to serve as a DIDS-sensitive SO4/HCO3 exchanger, while it simultaneously operates as the DIDS-resistant Cl/HCO3 exchanger.52 At 0.5 mM, DIDS only weakly inhibited A3 activity (assessed as 36Cl efflux in A3 expressing Xenopus oocytes) in buffers containing >100 mM Cl,50,53 whereas 1 mM DIDS almost completely inhibited A3-mediated 36Cl uptake by A3 expressing Xenopus oocytes in buffers containing 1 mM Cl.54 On the basis of these observations, Whittamore et al. suggested that the observed differential sensitivity of A3-mediated Cl absorption and SO4 secretion to DIDS (0.5 mM) may be due to the extracellular concentrations of Cl (122 mM) and SO4 (0.5 mM), and that A3 may be sensitive to DIDS in the absence of Cl.52 Of note is that studies with the anion exchanger AE1 suggest that extracellular Cl allosterically lowers DIDS affinity for AE1.55 However, 36Cl uptake by A3 expressing HEK cells was found to be insensitive to 1 mM DIDS in Cl-free buffers.56 Therefore, the issue of A3 being DIDS-sensitive in Cl-free buffers is not yet resolved. In addition, it remains to be determined whether lower DIDS concentrations (e.g., 100 µM, which completely abolished the CM-induced stimulation by C2 cells) would have a similar effect as that observed with 0.5 mM DIDS (which could still reflect relative DIDS-insensitivity). Moreover, what was observed ex vivo in native mouse epithelium52 might not be operative in vitro in a human cell line like C2 cells. Importantly, we also observed that 100 µM DIDS in buffers containing >120 mM Cl also completely abolished the CM-induced stimulation by C2 cells (data not shown). Collectively, in the absence of clear data directly showing that A3 contributes to DIDS-sensitive oxalate transport in C2 cells, the previously reported L. acidophilus CM-induced increase in A3 activity42,51 should not necessarily result in enhanced oxalate uptake by C2 cells in Cl-free buffers as seen in Figure 1B. Furthermore, CM-mediated stimulation of A3 activity in native epithelium is expected to enhance transcellular colonic oxalate absorption, leading to increased urinary oxalate excretion. However, the CM has no effect on distal colonic oxalate absorption, as well as it significantly reduced urinary oxalate excretion in PH1 mice. Taken together, these observations strongly argue against an important role for A3 in the CM-induced stimulation of oxalate transport in C2 cells (which is very DIDS-sensitive) and native colonic epithelium. Of note is that the CM did not affect A3 mRNA level (data not shown).
Our finding that O. formigenes CM stimulates 14C-oxalate uptake by C2 cells through a PKA-dependent pathway fit with previous studies demonstrating cAMP stimulates oxalate secretion in rabbit proximal and distal colonic tissues.57,58 In view of these findings and because C2 cells closely resemble the native epithelium,59,60 we anticipate that the observed CM-induced stimulation of mouse distal colonic secretion might potentially be PKA-mediated in vivo. Of interest in this regard is that A1 and A2 are predicted to have highly conserved PKA phosphorylation sites (S467 and S693, respectively), raising their functional significance and potential involvement in PKA regulation of these transporters. However, A6 is not predicted to have a PKA phosphorylation site. Indirect PKA regulation of A6 is possible through its interaction with CFTR. CFTR stimulates A3 and A6 functions in cultured pancreatic duct cells.61 PKA-dependent phosphorylation of CFTR domain promotes its binding to SLC26 exchanger domain, resulting in significant mutual activation of CFTR and the SLC26 exchanger.62,63 Therefore, A6 might be involved in the observed CM-induced stimulation through mechanisms including PKA-dependent CFTR activation. We don’t know whether the O. formigenes–derived secretagogue directly modulates the activity of the involved transporter(s) or acts indirectly through other regulatory/accessory proteins, which might involve translational, post-translational, and/or other genomic changes. Future mechanistic studies will explore these possibilities, which will help explain why the CM requires >1 hour to stimulate oxalate transport in C2 cells. Worthy of mentioning is that we previously found that PKC activation (including cholinergic and purinergic signaling acting through PKC) negatively regulate A6-mediated oxalate transport in intestinal cells through a Gö6983-sensitive pathway.28,30,34 However, Gö6983 has no effect on the observed stimulation, which is expected because the CM is stimulating rather than inhibiting oxalate transport.
In summary, we have shown that O. formigenes CM significantly stimulates oxalate transport in C2 cells through mechanisms including PKA activation, with A6 being responsible for a significant component of the observed stimulation. The CM also significantly reduces urinary oxalate excretion in PH1 mice by stimulating distal colonic oxalate secretion. Given the significant potential for clinical application of the O. formigenes–derived bioactive factors as novel therapeutic agents for prevention and/or treatment of hyperoxaluria, hyperoxalemia, and related COKS, characterization of these factors is currently under active investigation.
Concise Methods
Cell Culture
Human intestinal C2 and T84 cells were grown and maintained (including monitoring the TER) as we reported previously.28,30 The oxalate flux and other studies described above were performed using confluent cells grown for 5–14 days postplating on 0.4 µm collagen-coated polystyrene transwell membrane filters (Corning, Corning, NY) in 12 mm inserts. The cells were switched from a DMEM-containing 8%–10% FBS to a DMEM with 0.1% FBS, and then incubated for 1 hour before treatment with the specified reagents (e.g., OM, CM, etc.) in the same 0.1% FBS medium.
Bacterial Cultivation and Preparation of Conditioned Media
O. formigenes was obtained from the American Type Culture Collection (ATCC) (35274; Manassas, VA) and grown under a 95% CO2, 5% H2 atmosphere at 37°C in Oxalobacter medium64 with varying concentrations of sodium oxalate (1 g/L=9.4 mM; 2.5 g/L=18.8 mM; and the standard 5 g/L=37.5 mM). Cultivation medium was dispensed in 50 ml aliquots per 250 ml anaerobic bottles in an anaerobic chamber (Coy Laboratories, Grass Lake, MI). After autoclaving, a solution of filter-sterilized sodium oxalate (using 0.22 µm filter) was then aseptically added to each bottle to achieve the desired final concentration of sodium oxalate. Batches of media were prepared in 50 ml volumes for each sodium oxalate concentration in duplicate and inoculated with 1:10 volume of actively growing culture. Cultures were then harvested after a 12 hour incubation for one set and after an 18 hour incubation for the second set (OD at 600 nm of 0.03, 0.075, and 0.150, respective to sodium oxalate concentration). O. formigenes cultures were centrifuged (3000 × g at 4°C for 10 minutes) and the supernatant (CM) was filtered through a 0.22 µm filter to sterilize and remove all bacterial cells and stored at −80°C. Of note is that we found CM9.4 (harvested at 12 hours from cultures containing 1g/L of sodium oxalate) had the highest bioactivity compared with CM18.8 or CM37.5 harvested at 12 or 18 hours (Figure 5). Therefore, most of the studies were done using CM9.4. Earlier in the study, we had used CM37.5 harvested at 12 hours before comparing the three different oxalate concentrations described above.
For the studies with heated CM, the CM was subjected to heat treatment (boiling at 100°C for 20–30 minutes) in a thermoregulatory water bath and then allowed to cool to room temperature before use.
For the studies with protease-treated CM, the CM was digested with pepsin. Because pepsin is active at acidic pH, the CM was first made slightly acidic by titrating with concentrated HCl to a pH of 4 before adding pepsin. The CM was incubated with pepsin (0.4% final concentration) at 37°C for 60 minutes, and then pepsin was irreversibly inactivated by altering the pH to 8.0 with concentrated NaOH and incubation for 10 minutes. Alternatively, after the 60 minute incubation, pepsin (MM=34.7 kDa) was removed by subjecting the pepsin-treated CM to sizing filtration using a 30 kDa cutoff spin column. The pH was adjusted to 7.4 before experimental use. Of note is that pepsin treatment completely abolished the CM bioactivity under both conditions (i.e., pepsin inactivation by pH 8.0 or pepsin removal by sizing filtration).
To determine the molecular weight(s) of the O. formigenes–derived secreted bioactive factor(s) present in the CM, the CM was subjected to selective ultrafiltration using 10 and 30 kDa molecular mass cutoff spin columns (Ultracel YM-10 and YM-30 membranes; EMD Millipore, Billerica, MA), per manufacturer’s protocols. The CM samples were spun until 10%–15% of the overall volume was retained upon concentration. These concentrated fractions were then used in the experiments and referred to as the filtrate (F; containing molecules <10 or 30 kDa) and the retentate (R; containing molecules >10 or 30 kDa).
L. acidophilus was obtained from ATCC (4357) and grown as previously reported.51 Briefly, L. acidophilus was grown overnight in MRS broth at 37°C in a 5% CO2, 95% O2 incubator. L. acidophilus CM was similarly prepared as described with O. formigenes CM.
Radioactive Flux Studies
Apical 14C-oxalate flux studies in C2 and T84 cells were performed following our previously published methods.28,30 Briefly, an outward Cl gradient is imposed by removing extracellular Cl (Cli>Clo, by incubating in a Cl-free solution containing 20 μM 14C-oxalate for 6 minutes) and the influx of 14C-oxalate in exchange for intracellular Cl (i.e., apical Cl-oxalate exchange activity, which we previously found to be inhibited [>91%] by 100 µM luminal DIDS [anion exchange inhibitor]28) is measured. The influx of 14C-oxalate was terminated by two to three rapid washes of the cell monolayers with ice-cold Cl-free solution and the transwells were then placed upside down and were allowed to dry for several minutes. Membrane filters containing the cells were cut from the support and placed into vials with scintillation fluid (Opti-Fluor, Packard), and the radioactivity was measured by scintillation spectrometry after overnight solubilization.
Animals
A mouse model of PH type 1 (called PH1 mice in this study), due to deficiency in liver alanine-glyoxylate aminotransferase,37 was kindly provided by Dr. Roy-Chowdhury (Albert Einstein College of Medicine). PH1 mice exhibit hyperoxalemia and hyperoxaluria.37 Mice were bred and maintained in an specific pathogen free facility, with free access to water and standard mouse chow. All animal studies were approved by the University of Chicago Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Rectal Administration of O. formigenes OM or CM
O. formigenes OM or CM was administered to PH1 mice as rectal enemas (100 µl twice daily × 21 days) using disposable animal feeding needles (catalog no. 01–208–87; Fisher Scientific, Waltham, MA). The needles were introduced approximately 2 cm from the anus, and the mice were then held from their tails with the heads down and kept in that position for approximately 1 minute. This protocol was developed in pilot studies, where CM or OM was given to PH1 mice for up to 28 days, and the CM was found to significantly reduce urine oxalate excretion, whereas OM had no effect.
Urine Collection and Oxalate Measurements
Mice were placed individually in well ventilated boxes containing sterile 96-well plates (in which the urine get collected) and baseline urine samples were collected over 1 hour. This method was approved by our Institutional Animal Care and Use Committee. Urine samples from the OM- and CM-treated PH1 mice were directly collected from the bladders at the end of treatment. The urine samples were acidified with 4N HCL (50 µl per ml of urine) and stored at −80°C. Urine oxalate was measured enzymatically using oxalate oxidase (Trinity Biotech, Bray, Ireland), employing sodium nitrite65 to prevent interference by ascorbic acid. Oxalate measurements in OM and CM were performed by ion chromatography. Urine creatinine was measured by a modified Jaffe alkaline picrate method.
Measurement of Oxalate Fluxes across Distal Colon Tissues
Immediately after euthanasia, distal colonic tissues were removed, opened longitudinally along the mesenteric border, thoroughly cleansed by flushing with ice-cold saline, and then mounted (one pair of tissues per mouse) as intact sheets in modified Ussing chambers (Physiologic Instruments, San Diego, CA) (exposed tissue surface area of 0.30 cm2). Two unidirectional oxalate fluxes (mucosa to serosa [JMS]; and serosa to mucosa [JSM]) were assessed and the magnitude and direction of the net flux (Jnet) across conductance-matched tissues was determined by calculating the difference between the two measured unidirectional fluxes. The mucosal and serosal surfaces of the tissue were bathed with 4 ml of warmed (37°C) Ringer buffer (140 mM Na+, 119.8 mM Cl−, 5.2 mM K+, 1.2 mM Mg2+, 1.2 mM Ca2+, 25 mM HCO3−, 2.4 mM HPO4−, 0.4 mM H2PO42−, pH 7.4, gassed with 95% O2-5% CO2), containing 10 mM mannitol or glucose on the mucosal or serosal side, respectively. Two micromolar 14C-oxalate was added either to the mucosal or the serosal bath, and 2 µM unlabeled oxalate was added to the opposite bath. All buffers contained 5 µM indomethacin to inhibit endogenous prostanoid production. After a 15 minute equilibration period, unidirectional 14C-oxalate fluxes were measured for 60 minutes at 15 minute intervals under short-circuit conditions. The electrical parameters of the tissues were continuously recorded (at 10–20 second intervals) using Acquire and Analyze Software (Physiologic Instruments) attached to the chamber. The viability of the tissue was ensured by treating the tissue with carbachol (100 µM, serosally) at the end of the experiment and observing an immediate increase in short-circuit current.66
A6 Knockdown in C2 Cells
A6 expression in C2 cells was knocked down as previously reported,26 using the same A6 and negative control siRNAs, with minor modifications as described below. C2 cells were untransfected or transfected with the NC si or the A6 si. We observed approximately 61% reduction in A6 total protein expression 2–3 days after transfection, and most of the studies were performed 3 days after transfection.
SDS-PAGE, Western Blotting, and Surface Biotinylation
C2 cells were scraped and lysed in lysis buffer (in mM: NaCl=150, TRIS-HCl=50 [pH 7.4], EDTA=2, Sodium Fluoride=50, NP-40=1%, Sodium Deoxycholate=0.5%, SDS=0.1%) supplemented with Complete Protease Inhibitor Cocktail (Roche Diagnostics, Indianapolis, IN). The lysate was then centrifuged (14,000 × g, 4°C, 10 minutes), and the supernatant was saved for gel electrophoresis. Total protein levels were determined and equal amounts of protein lysates were separated by SDS-PAGE using 7.5% polyacrylamide gels, with subsequent electro-transfer to polyvinylidene difluoride (Immobilon-P; EMD Millipore). All immunoblots were stained with Ponceau S Solution (0.1% Ponceau S [w/v] in 5% acetic acid [v/v]) after transfer. For Western blotting and to block nonspecific binding, polyvinylidene difluoride strips were first incubated in blotto (5% nonfat dry milk and 0.2% Tween 20 in tris buffered saline) for 1 hour. Immunoblots were then incubated overnight at 4°C with anti-A6 antibody (1:100 dilution) (catalog no. sc-26728; Santa Cruz Biotechnology, Santa Cruz, CA) and anti-GAPDH antibody (1:5000 dilution) (catalog no. sc-32233; Santa Cruz Biotechnology). The strips then were washed in blotto and incubated for 1 hour with horseradish peroxidase–conjugated secondary antibodies (donkey anti-mouse or mouse anti-goat IgG, 1:10,000 dilution). Antibody reactivity was detected with the enhanced chemiluminescence system (SuperSignal West; Thermo Fisher Scientific, Vernon Hills, IL) according to the manufacturer’s protocol.
For the surface biotinylation studies, C2 cells were grown in 0.4 µm collagen-coated polystyrene transwell membrane filters in 24 mm inserts and treated with the CM as described above. Surface biotinylation studies were performed as previously described.28
Real-Time PCR
RNA was extracted from UT and CM-treated C2 cells using E.Z.N.A. Total RNA Kit (Omega Bio-tek, Norcross, GA). RNA was reverse transcribed and amplified with Power SYBR Green PCR Master Mix kit (Applied Biosystems, Foster City, CA). Human A1 was amplified with gene-specific primers (forward primer, 5=-GGACTCCCTCAGCGTGCAAACA-3=; reverse primer, 5=-GCGTTAGGGAGGCGGTGCTTAG-3=). Human A2 was amplified with gene-specific primers (forward primer, 5=-CGCGGGCGTTTACACTGGCT-3=; reverse primer, 5=-GTCCCTTTCCCCGCCCATCG-3=). Human A6 was amplified with gene-specific primers (forward primer, 5=-GCAACACAAGCAATGGACCTG-3=; reverse primer, 5=-CCGGGGTAACCAGACCAAAAC-3=). Human GAPDH was amplified as an internal control using gene-specific primers (forward primer, 5=-TCCCTGAGCTGAACGGGAAG-3=; reverse primer, 5=-GGAGGAGTGGGTGTCGCTGT-3=). Relative A1, A2, and A6 mRNA levels were expressed as a percentage of UT normalized to GAPDH.
Histologic Examination
Distal colonic tissues were immediately placed in 10% neutral buffered formalin and were fixed at room temperature for 24 hours. After fixation, the tissues were transferred to 70% reagent alcohol and then submitted to our histology facility for processing and embedding. Hematoxylin and eosin-stained sections (4 μm thick) were blindly examined under a light microscope by a gastrointestinal pathologist.
Materials
14C-oxalate was purchased from Vitrax (specific activity: 54 mCi/mmol). H89, PP2, SB 202190, Gö6976, Gö6983, carbachol, genistein, and LY 294002 were purchased from Calbiochem (San Diego, CA). U0126 was purchased from Promega (Madison, WI). DIDS was purchased from Sigma-Aldrich (St. Louis, MO). H89, PP2, carbachol, Gö6983, SB 202190, LY 294002, U0126, and genistein were dissolved in DMSO and stored at −20°C. Gö6976 was dissolved in DMSO and stored at 4°C. Equivalent volumes of DMSO (0.1%–0.2%) were added to control media. Of note is that the PKC inhibitors Gö6983 and Gö6976, the ERK1/2 inhibitor U0126, the p38 inhibitor SB 202190, the PI3K inhibitor LY 294002, the general tyrosine kinase inhibitor genistein, and the specific Src family kinase inhibitor PP2 were used at concentrations known to effectively block the relevant signaling pathways in C2 and T84 intestinal cells.28,67–70
Statistical Analyses
Experimental data are presented as means±SEM. Data were analyzed by one-way ANOVA followed by Bonferroni or Student–Newman–Keuls post hoc test, or by t test for paired or unpaired samples when comparing two groups. P values <0.05 were considered statistically significant.
Disclosures
I.G. and J.A. are employed by LabCorp (Chicago, IL).
Supplementary Material
Acknowledgments
We would like to thank Ruhul Amin, Sapna Sharma, Sireesha Ratakonda, Ming Cheng, Sohee Jeon, Brad Hack, Wahaj Ahmed, and Mustafa Satti for technical assistance. We would also like to thank Dr. Christopher Weber (Department of Pathology, The University of Chicago) for blindly reviewing the mouse distal colonic histology.
This work was supported by National Institutes of Health grants K08-DK067245 (H.H.) and P30DK42086 (the Digestive Disease Research Center of the University of Chicago).
Part of this work was previously reported in abstract form (Arvans et al., J Am Soc Nephrol 26: 78A, 2015).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016020132/-/DCSupplemental.
References
- 1.Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Morgan C, Samuel S, Klarenbach SW, Curhan GC, Tonelli M; Alberta Kidney Disease Network : Kidney stones and kidney function loss: a cohort study. BMJ 345: e5287, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coe FL, Evan A, Worcester E: Kidney stone disease. J Clin Invest 115: 2598–2608, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curhan GC, Taylor EN: 24-h uric acid excretion and the risk of kidney stones. Kidney Int 73: 489–496, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Robertson WG, Peacock M: The cause of idiopathic calcium stone disease: hypercalciuria or hyperoxaluria? Nephron 26: 105–110, 1980 [DOI] [PubMed] [Google Scholar]
- 5.Kleinman JG: Bariatric surgery, hyperoxaluria, and nephrolithiasis: a plea for close postoperative management of risk factors. Kidney Int 72: 8–10, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Eisner BH, Porten SP, Bechis SK, Stoller ML: Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. J Urol 183: 2244–2248, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Sakhaee K, Capolongo G, Maalouf NM, Pasch A, Moe OW, Poindexter J, Adams-Huet B: Metabolic syndrome and the risk of calcium stones. Nephrol Dial Transplant 27: 3201–3209, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor EN, Curhan GC: Determinants of 24-hour urinary oxalate excretion. Clin J Am Soc Nephrol 3: 1453–1460, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemann J Jr , Pleuss JA, Worcester EM, Hornick L, Schrab D, Hoffmann RG: Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney Int 49: 200–208, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Caudarella R, Rizzoli E, Pironi L, Malavolta N, Martelli G, Poggioli G, Gozzetti G, Miglioli M: Renal stone formation in patients with inflammatory bowel disease. Scanning Microsc 7: 371–379, discussion 379–380, 1993 [PubMed] [Google Scholar]
- 11.Sinha MK, Collazo-Clavell ML, Rule A, Milliner DS, Nelson W, Sarr MG, Kumar R, Lieske JC: Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int 72: 100–107, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Mole DR, Tomson CR, Mortensen N, Winearls CG: Renal complications of jejuno-ileal bypass for obesity. QJM 94: 69–77, 2001 [DOI] [PubMed]
- 13.Danpure CJ: Molecular etiology of primary hyperoxaluria type 1: new directions for treatment. Am J Nephrol 25: 303–310, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Hatch M, Freel RW: The roles and mechanisms of intestinal oxalate transport in oxalate homeostasis. Semin Nephrol 28: 143–151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS: Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Freel RW, Hatch M, Green M, Soleimani M: Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290: G719–G728, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Stewart CS, Duncan SH, Cave DR: Oxalobacter formigenes and its role in oxalate metabolism in the human gut. FEMS Microbiol Lett 230: 1–7, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Siener R, Bangen U, Sidhu H, Hönow R, von Unruh G, Hesse A: The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int 83: 1144–1149, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Kaufman DW, Kelly JP, Curhan GC, Anderson TE, Dretler SP, Preminger GM, Cave DR: Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol 19: 1197–1203, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troxel SA, Sidhu H, Kaul P, Low RK: Intestinal Oxalobacter formigenes colonization in calcium oxalate stone formers and its relation to urinary oxalate. J Endourol 17: 173–176, 2003 [DOI] [PubMed]
- 21.Mikami K, Akakura K, Takei K, Ueda T, Mizoguchi K, Noda M, Miyake M, Ito H: Association of absence of intestinal oxalate degrading bacteria with urinary calcium oxalate stone formation. Int J Urol, 10: 293–296, 2003 [DOI] [PubMed]
- 22.Hatch M, Cornelius J, Allison M, Sidhu H, Peck A, Freel RW: Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int 69: 691–698, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Hatch M, Gjymishka A, Salido EC, Allison MJ, Freel RW: Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am J Physiol Gastrointest Liver Physiol 300: G461–G469, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidhu H, Schmidt ME, Cornelius JG, Thamilselvan S, Khan SR, Hesse A, Peck AB: Direct correlation between hyperoxaluria/oxalate stone disease and the absence of the gastrointestinal tract-dwelling bacterium Oxalobacter formigenes: possible prevention by gut recolonization or enzyme replacement therapy. J Am Soc Nephrol 10[Suppl 14]: S334–S340, 1999 [PubMed] [Google Scholar]
- 25.Hoppe B, Beck B, Gatter N, von Unruh G, Tischer A, Hesse A, Laube N, Kaul P, Sidhu H: Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int 70: 1305–1311, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Freel RW, Morozumi M, Hatch M: Parsing apical oxalate exchange in Caco-2BBe1 monolayers: siRNA knockdown of SLC26A6 reveals the role and properties of PAT-1. Am J Physiol Gastrointest Liver Physiol 297: G918–G929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Z, Grichtchenko II, Boron WF, Aronson PS: Specificity of anion exchange mediated by mouse Slc26a6. J Biol Chem 277: 33963–33967, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Amin R, Sharma S, Ratakonda S, Hassan HA: Extracellular nucleotides inhibit oxalate transport by human intestinal Caco-2-BBe cells through PKC-δ activation. Am J Physiol Cell Physiol 305: C78–C89, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoppe B, von Unruh G, Laube N, Hesse A, Sidhu H: Oxalate degrading bacteria: new treatment option for patients with primary and secondary hyperoxaluria? Urol Res 33: 372–375, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Hassan HA, Cheng M, Aronson PS: Cholinergic signaling inhibits oxalate transport by human intestinal T84 cells. Am J Physiol Cell Physiol 302: C46–C58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB: Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132: 562–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO: Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol 290: C1018–C1030, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Nduati V, Yan Y, Dalmasso G, Driss A, Sitaraman S, Merlin D: Leptin transcriptionally enhances peptide transporter (hPepT1) expression and activity via the cAMP-response element-binding protein and Cdx2 transcription factors. J Biol Chem 282: 1359–1373, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Hassan HA, Mentone S, Karniski LP, Rajendran VM, Aronson PS: Regulation of anion exchanger Slc26a6 by protein kinase C. Am J Physiol Cell Physiol 292: C1485–C1492, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Heneghan JF, Akhavein A, Salas MJ, Shmukler BE, Karniski LP, Vandorpe DH, Alper SL: Regulated transport of sulfate and oxalate by SLC26A2/DTDST. Am J Physiol Cell Physiol 298: C1363–C1375, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS: Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci U S A 98: 9425–9430, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salido EC, Li XM, Lu Y, Wang X, Santana A, Roy-Chowdhury N, Torres A, Shapiro LJ, Roy-Chowdhury J: Alanine-glyoxylate aminotransferase-deficient mice, a model for primary hyperoxaluria that responds to adenoviral gene transfer. Proc Natl Acad Sci U S A 103: 18249–18254, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cicenia A, Scirocco A, Carabotti M, Pallotta L, Marignani M, Severi C: Postbiotic activities of lactobacilli-derived factors. J Clin Gastroenterol 48[Suppl 1]: S18–S22, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL: Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol 295: G1025–G1034, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W: Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol 303: G32–G41, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu L, Chaturvedi R, Peek RM Jr , Wilson KT, Polk DB: Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest 121: 2242–2253, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK: The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr 138: 1355–1359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gulhan B, Turkmen K, Aydin M, Gunay M, Cıkman A, Kara M: The Relationship between Serum Oxalic Acid, Central Hemodynamic Parameters and Colonization by Oxalobacter formigenes in Hemodialysis Patients. Cardiorenal Med 5: 164–174, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins AJ: Cardiovascular mortality in end-stage renal disease. Am J Med Sci 325: 163–167, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Petrovic S, Mann E, Soleimani M: Identification of an apical Cl(-)/HCO3(-) exchanger in the small intestine. Am J Physiol Gastrointest Liver Physiol 282: G573–G579, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Haila S, Hastbacka J, Bohling T, Karjalainen-Lindsberg ML, Kere J, Saarialho-Kere U: SLC26A2 (diastrophic dysplasia sulfate transporter) is expressed in developing and mature cartilage but also in other tissues and cell types. J Histochem Cytochem 49: 973–982, 2001 [DOI] [PubMed]
- 47.Satoh H, Susaki M, Shukunami C, Iyama K, Negoro T, Hiraki Y: Functional analysis of diastrophic dysplasia sulfate transporter. Its involvement in growth regulation of chondrocytes mediated by sulfated proteoglycans. J Biol Chem 273: 12307–12315, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Alper SL, Sharma AK: The SLC26 gene family of anion transporters and channels. Mol Aspects Med 34: 494–515, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freel RW, Whittamore JM, Hatch M: Transcellular oxalate and Cl- absorption in mouse intestine is mediated by the DRA anion exchanger Slc26a3, and DRA deletion decreases urinary oxalate. Am J Physiol Gastrointest Liver Physiol 305: G520–G527, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chernova MN, Jiang L, Shmukler BE, Schweinfest CW, Blanco P, Freedman SD, Stewart AK, Alper SL: Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes. J Physiol 549: 3–19, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK: Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol 298: G395–G401, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whittamore JM, Freel RW, Hatch M: Sulfate secretion and chloride absorption are mediated by the anion exchanger DRA (Slc26a3) in the mouse cecum. Am J Physiol Gastrointest Liver Physiol 305: G172–G184, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart AK, Shmukler BE, Vandorpe DH, Reimold F, Heneghan JF, Nakakuki M, Akhavein A, Ko S, Ishiguro H, Alper SL: SLC26 anion exchangers of guinea pig pancreatic duct: molecular cloning and functional characterization. Am J Physiol Cell Physiol 301: C289–C303, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moseley RH, Höglund P, Wu GD, Silberg DG, Haila S, de la Chapelle A, Holmberg C, Kere J: Downregulated in adenoma gene encodes a chloride transporter defective in congenital chloride diarrhea. Am J Physiol 276: G185–G192, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Salhany JM: Mechanism of competition between chloride and stilbenedisulfonates for binding to human erythrocyte band 3 (AE1). Biochem Cell Biol 76: 715–722, 1998 [DOI] [PubMed]
- 56.Barmeyer C, Ye JH, Sidani S, Geibel J, Binder HJ, Rajendran VM: Characteristics of rat downregulated in adenoma (rDRA) expressed in HEK 293 cells. Pflugers Arch 454: 441–450, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Hatch M, Freel RW, Vaziri ND: Mechanisms of oxalate absorption and secretion across the rabbit distal colon. Pflugers Arch 426: 101–109, 1994 [DOI] [PubMed] [Google Scholar]
- 58.Hatch M, Freel RW, Vaziri ND: Characteristics of the transport of oxalate and other ions across rabbit proximal colon. Pflugers Arch 423: 206–212, 1993 [DOI] [PubMed] [Google Scholar]
- 59.Peterson MD, Mooseker MS: Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci 102: 581–600, 1992 [DOI] [PubMed] [Google Scholar]
- 60.Merlin D, Steel A, Gewirtz AT, Si-Tahar M, Hediger MA, Madara JL: hPepT1-mediated epithelial transport of bacteria-derived chemotactic peptides enhances neutrophil-epithelial interactions. J Clin Invest 102: 2011–2018, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greeley T, Shumaker H, Wang Z, Schweinfest CW, Soleimani M: Downregulated in adenoma and putative anion transporter are regulated by CFTR in cultured pancreatic duct cells. Am J Physiol Gastrointest Liver Physiol 281: G1301–G1308, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S: Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol 6: 343–350, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shcheynikov N, Ko SB, Zeng W, Choi JY, Dorwart MR, Thomas PJ, Muallem S: Regulatory interaction between CFTR and the SLC26 transporters. Novartis Found Symp 273: 177–186; discussion 186–192, 261–174, 2006 [PubMed]
- 64.Atlas RM, editor: Handbook of Microbiological Media, 4th Ed., Boca Raton, FL, CRC Press, 2010, pp 1334
- 65.Kasidas GP, Rose GA: Continuous-flow assay for urinary oxalate using immobilised oxalate oxidase. Ann Clin Biochem 22: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 66.Chandan R, Megarry BH, O’Grady SM, Seybold VS, Brown DR: Muscarinic cholinergic regulation of electrogenic chloride secretion in porcine proximal jejunum. J Pharmacol Exp Ther 257: 908–917, 1991 [PubMed] [Google Scholar]
- 67.Ao M, Venkatasubramanian J, Boonkaewwan C, Ganesan N, Syed A, Benya RV, Rao MC: Lubiprostone activates Cl- secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci 56: 339–351, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Srivastava V, Dey I, Leung P, Chadee K: Prostaglandin E2 modulates IL-8 expression through formation of a multiprotein enhanceosome in human colonic epithelial cells. Eur J Immunol 42: 912–923, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Chaturvedi LS, Marsh HM, Shang X, Zheng Y, Basson MD: Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J Biol Chem 282: 14–28, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Yoo BK, He P, Lee SJ, Yun CC: Lysophosphatidic acid 5 receptor induces activation of Na(+)/H(+) exchanger 3 via apical epidermal growth factor receptor in intestinal epithelial cells. Am J Physiol Cell Physiol 301: C1008–C1016, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.