Abstract
Helicobacter pylori infection plays an important role in the pathogenesis of peptic ulcer disease (PUD). Several factors have been proposed as possible H. pylori virulence determinants; for example, bacterial adhesins and gastric inflammation factors are associated with an increased risk of PUD. However, differences in bacterial virulence factors alone cannot explain the opposite ends of the PUD disease spectrum, i.e., duodenal and gastric ulcers; presumably, both bacterial and host factors contribute to the differential response. Carriers of the high-producer alleles of the pro-inflammatory cytokines interleukin (IL)-1B, IL-6, IL-8, IL-10, and tumor necrosis factor-α who also carry low-producer allele carriers of anti-inflammatory cytokines have severe gastric mucosal inflammation, whereas carriers of the alternative alleles have mild inflammation. Recent reports have suggested that the PSCA and CYP2C19 ultra-rapid metabolizer genotypes are also associated with PUD.
Keywords: Helicobacter pylori, Peptic ulcer disease, Virulence factors, Host genetic polymorphism, CYP2C19, PSCA
Introduction
Helicobacter pylori is a spiral-shaped, gram-negative bacterium that establishes chronic colonization in the human stomach and is a causative pathogen of various gastroduodenal diseases, including gastritis, peptic ulcer disease (PUD), gastric cancer, and mucosa-associated lymphoid tissue lymphoma [1]. Several conditions facilitate the survival of bacteria and its colonization of the stomach. Acute infections due to the bacterium cause marked inflammation of the stomach and lead to transient hypochlorhydria; they elicit the secretion of interleukin (IL)-1β and tumor necrosis factor (TNF)-α and/or accompanying inflammation, inhibiting parietal cell function, either directly or indirectly (i.e., via hormonal, paracrine, and neural regulatory mechanisms). Moreover, many of these bacteria adhere superficially to the epithelial cell layer where immune effectors are not easily accessible [2].
Gastric ulcer (GU) and duodenal ulcer (DU), commonly referred to as PUD, are defined as the loss of continuity in part of the gastrointestinal tract wall penetrating the muscularis mucosa with a diameter of at least 0.5 cm [2]. PUD is a common disease worldwide with a lifetime prevalence in the adult population of ~10%. H. pylori infection plays an important role in the pathogenesis of PUD, and is present in 90–100% and 60–90% of GU and DU patients, respectively, depending on geographic location and socioeconomic status [3]. Several studies have shown that H. pylori infection is associated with a 3–4-fold increased risk of PUD and that 10–15% of H. pylori-infected individuals will have PUD in their lifetime [4]. This observation suggests that the classic aphorism “no acid no ulcer” which still remains valid today, can be extended to “no H. pylori no ulcer”. The decline in the prevalence of H. pylori infection in the last several decades has paralleled, but cannot be entirely explained by, the increase in the proportion of H. pylori-negative ulcers based on endoscopic series from around the world [5]. It is suggested that the greater use of cyclooxygenase (Cox)-2 specific non-steroidal anti-inflammatory drugs (NSAIDs) represents the most frequently identifiable cause in H. pylori negative-DU, including Western countries. Nonetheless, several hypotheses suggested that H. pylori negative-DU related to false negative results because of diagnostic methods, the use of NSAIDs and concomitant prescription of proton pump inhibitors (PPIs) [5]. Although there is still controversy about the ability of H. pylori infection as the initial or primary cause of the PUD, there is no doubt regarding the value of H. pylori eradication leading to long-term healing of PUD. Eradication of this bacterium improves PUD recovery and is a primary and secondary prophylaxis to reduce the risk of recurrent ulcer bleeding. A meta-analysis suggested a remission rate of 97% and 98% for GU and DU, respectively, after successfully eradicating infection compared to 61% and 65% in patients with persistent infection. In addition, treatment of H. pylori infection is superior to ulcer healing drugs and reduces recurrent bleeding by 17% compared with ranitidine or omeprazole. Similar results have been found in a Cochrane Database meta-analysis, which showed that eradication therapy is superior to ulcer healing drugs and no DU treatment [6].
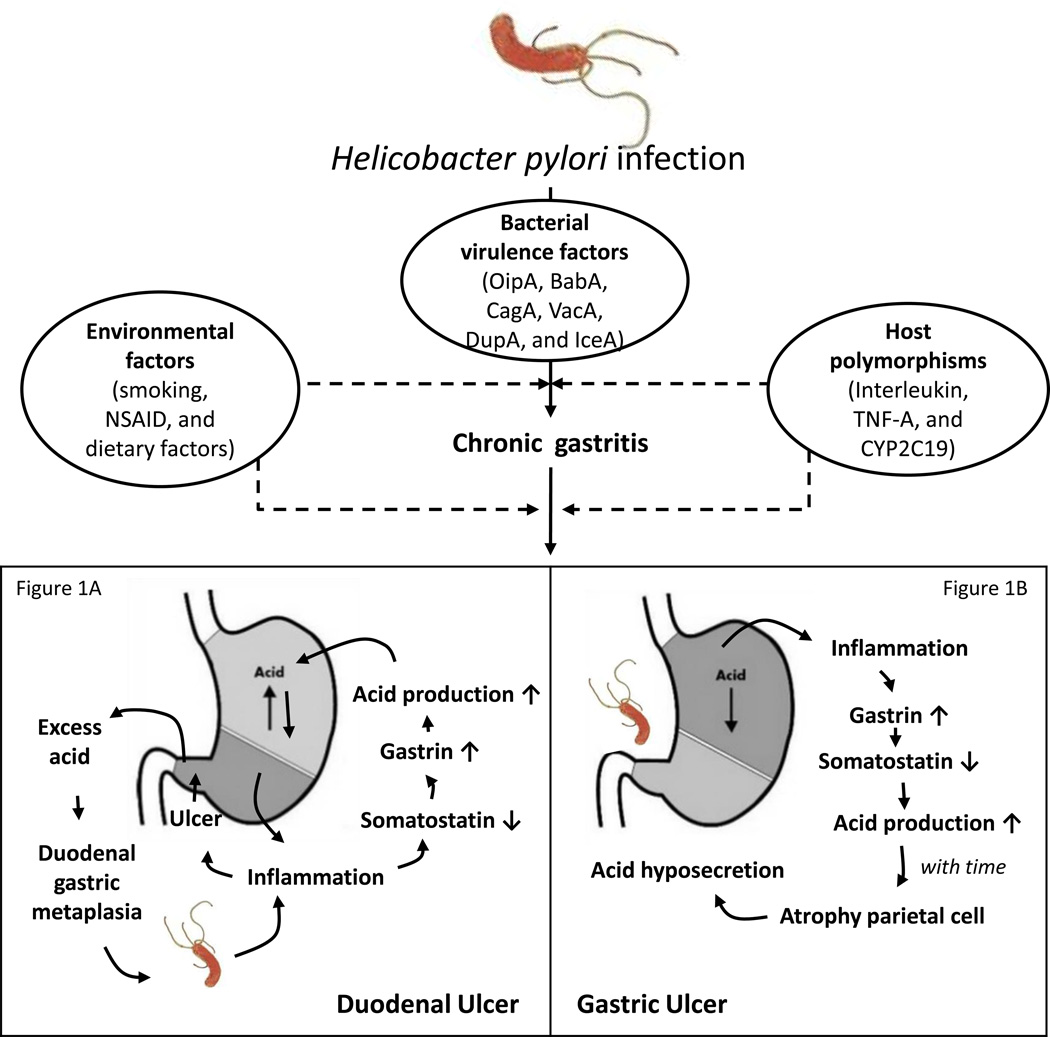
Interestingly, PUD has contrary effects on opposite ends of the disease spectrum, which may be related to differences in the severity and distribution of gastritis (Figure 1). DU is usually diagnosed at a young age, in males, and in patients with high antral inflammatory scores and high acid secretion. GU occurs more frequently in older patients, is not sex-biased, and occurs in patients with corporal gastritis or pangastritis and normal or decreased acid secretion [7]. Although H. pylori cannot normally establish infections in the duodenum because it is inhibited by bile, antral-predominant gastritis induces hyperacidity as a result of reduced antral somatostatin content and elevated basal and stimulated gastrin secretion (Figure 1A). This leads to an increased acid load in the duodenum, which subsequently results in protective gastric metaplasia as the defense response or adaptation. The appearance of gastric-type epithelium over the duodenal villi probably results from substitution by cells migrating from Brunner`s gland ducts. Although still debatable, the involvement of gastric metaplastic tissue may provide sites for H. pylori colonization and the resulting local inflammation and damage further promote DU [8].The mechanism by which somatostatin secretion is decreased is not known but may involve three potential mechanisms. First, H. pylori raises mucosal surface pH by virtue of its high urease activity and ammonia synthesis. Low antral pH is an important physiological stimulus to the synthesis and release of antral somatostatin [9]. Second, H. pylori antral gastritis might alter Gastrin and D cell function via the local production of specific cytokines. H. pylori infection results in severe antral gastritis via infiltration of the mucosa with acute and chronic inflammatory cells. There is also upregulation of local production of various cytokines (e.g. TNF, IL-6, IL-8, etc) [9]. Third, cytokines are induced by the inflammation and/or the production of N-methyl histamine, a selective H3-receptor agonist, by H. pylori. One may speculate that the H3-receptor agonist could diffuse across the antral mucosa to interact with H3 receptors on antral somatostatin cells, causing inhibition of somatostatin secretion and thus, stimulation of gastrin secretion. Gastrin, in turn, stimulates histamine secretion from Enterochromaffin-like (ECL) cells leading to enhanced acid secretion [7].
Figure 1. The opposite ends of the peptic ulcer disease (PUD) spectrum.
Genetic polymorphisms in conjunction with bacterial and/or environmental factors including a combination of markers from the adaptive and innate immune systems are involved in the response to H. pylori infection. Duodenal ulcer (DU) is usually related to high antral inflammatory scores, and high acid secretion (Figure 1A). Gastric ulcer (GU) is related to corporal gastritis or pangastritis and normal or decreased acid secretion (Figure 1B).
In contrast, acid hypersecretion lasts at least 8 weeks and is due to hypergastrinemia- induced increases in parietal and ECL cell masses. Thus the stomach responds to the presence of inflammation by reducing somatostatin levels, thereby releasing the inhibition on the G and parietal cells to maximize gastric acid output [10]. With time, corpus-predominant gastritis in chronically infected patients decreases the amount of acid and causes atrophy of oxyntic glands with a loss of parietal cells [9]. This results in irreversible achlorhydria, which is related to GU (Figure 1B). The GU process is commonly observed at the transitional zone between the antrum and corpus on the lesser gastric curvature, which is likely related to the heavy colonization and consequent marked inflammation and epithelial damage at this site, leading directly to ulceration [7]. It is not clear why H. pylori-induced inflammation has a pan-gastritis or corpus-predominant pattern in some people, but is antral-predominant in others. One possibility is that, like autoimmune gastritis, it is caused by immune effectors with specificity for the gastric proton pump ATPase [11]. Interestingly, the involvement of acidity levels is supported by the observation that long-term gastric acid inhibition can result in a shift from antral-predominant to corpus-predominant inflammation [11].
Several factors have been proposed as possible H. pylori virulence determinants, which could be predictors of severe clinical outcomes as well as PUD. Strains that express multiple virulence factors are associated with more severe clinical outcomes [1]. Importantly, in conjunction with bacterial and/or environmental factors, genetic polymorphisms at a combination of markers related to the adaptive and innate immune systems are associated with H. pylori infection and promote chronic inflammation and reduced acidity. Following H. pylori infection, neutrophils and mononuclear cells are activated, and their products infiltrate the gastric mucosa and stimulate the transcription and synthesis of several pro-inflammatory and anti-inflammatory cytokines [12]. Most of these inflammatory cytokine genes have mutations that influence cytokine levels in the gastric mucosa. Carriers of low-producer alleles of pro-inflammatory cytokines who also carry high-producer alleles of anti-inflammatory cytokines have mild gastric mucosal inflammation, whereas carriers of the alternative alleles have extreme inflammation. In this review, we summarize the influence of several virulence genes and host polymorphisms on PUD pathogenesis.
Association between H. pylori virulence genes and PUD
H. pylori factors appear to reduce inflammation or recognition by the immune system. For example, H. pylori lipopolysaccharide (LPS) is 1,000 times less pyrogenic and 500-fold less toxic than LPS of gram-negative enteric bacteria. Moreover, modified H. pylori LPS may act as molecular mimics of human glycans to avoid immune recognition. H. pylori possesses several glycosyltransferase enzymes that have been implicated in LPS modifications with carbohydrate groups resembling the human Lewis blood group antigens Le(a), Le(b), Le(x), and Le(y) [13]. Putative H. pylori virulence factors, such as bacterial adhesins (oipA and babA) and gastric inflammation factors (cagA, vacA, dupA, and iceA), are associated with an increased risk of PUD.
Outer inflammatory protein: OipA
OipA is an outer membrane protein that functions in adhesion [14]. Its function is regulated by slipped-strand mispairing based on the number of CT dinucleotide repeats in the 5′ region (switch “on” = functional and switch “off” = nonfunctional) [14]. Most East Asian strains are classified as oipA status “on,” and the CT-repeat sequences in the signal region of oipA are half-collapsed (for example, the Japanese-derived strain JK51 contains a CTGCCTTTCT repeat sequence and its status is ‘on’); this suggests an intentional change in status in the evolution of the bacteria to prevent switching [14]. Interestingly, a recent study using whole-genome sequences found two oipA genes at different loci in strains from East Asian countries, but not in strains from Western countries [15].
Several mechanisms may explain the association between the oipA “on” status and PUD. Based on gene knockout models, the oipA “on” status has been linked to increased IL-8 production in gastric cancer cells [14]. In addition, oipA mutants do not induce gastric mucosal inflammation in mice infected for 12 weeks, whereas cagE mutants do induce mucosal inflammation, although the levels are lower than those observed in the parental strains (cagE is an important component of cag pathogenicity island [PAI]) [16]. Moreover, oipA mutations induce milder inflammation than the wild-type gene in a Mongolian gerbil model [17]. The mRNA levels of the genes encoding IL-1β, IL-17, IL-18, and TNF-α were significantly lower in gerbils infected with oipA mutant strains [17]. We also confirmed the role of the oipA “on” status in severe inflammation by inducing IL-8 expression in humans [18]. Human volunteers were challenged with oipA “on”/whole-cag PAI-negative clinical isolates (Baylor challenge strain 100; ATCC BAA-945). The IL-8 levels in the gastric mucosa increased by up to 20-fold within 2 weeks after inoculation [18]. We further determined that oipA is necessary for full activation of the IL-8 promoter and is involved in the STAT1-IRF1-ISRE pathway.
Our previous study showed that the oipA “on” status and the cag PAI-positive, vacA s1, and babA-positive genotypes are all related to the risk of DU. Importantly, a multiple logistic regression analysis showed that only the oipA “on” status is an independent predictor that differentiates DU from gastritis [1]. Moreover, when we examined four outer-membrane proteins (OipA, BabA, BabB, and SabA) by immunoblotting, a multiple logistic regression analysis showed that only the oipA-positive status was an independent predictor of DU vs. gastritis. A meta-analysis [19] also reported that the oipA “on,” but not “off,” status is significantly associated with an increased overall risk relative to controls (OR = 3.97). When GU and DU are analyzed separately, the oipA “on” status is significantly associated with an increased risk of DU relative to controls (OR = 3.83), but this was not observed for GU.
Blood group antigen-binding adhesion: BabA
The babA gene has been detected on the outer membrane of cells of the CCUG17875 bacterial strain, which contains one silent babA1 and one expressed babA2 gene. The sequences of these two genes differ only by the presence of a 10-bp deletion in the signal peptide sequence of babA1, which eliminates its translational initiation codon [20]. Several mechanisms for the regulation of BabA expression are predicted, including those acting at the transcriptional and translational levels. The transcription of babA appears to be regulated by the number of adenine [poly(A)] nucleotides within the −10 to −35 region of the babA promoter. The −10 and −35 region of the babA2 sequences are highly homologous to the consensus Escherichia coli σ70 promoter sequences [20]. A recent study reported that the babA mutant is less capable of inducing DNA double-strand breaks (DSBs) in primary and transformed murine and human epithelial and mesenchymal cells, suggesting that bacterial adhesion via babA is required to induce DSBs. The induction of DSBs contributes to genetic instability and frequent chromosomal aberrations, which are hallmarks of gastric cancer [21]. Moreover, BabA expression is a major determinant of the density of H. pylori colonization. The absence of Lewis B expression is inversely proportional to the density of colonization, and Lewis X or Lewis A expression and colonization density might adversely affect the efficacy of eradication and ulcer healing [22].
Our group showed that gerbils infected with BabA-expressing H. pylori exhibit higher levels of mucosal injury and inflammatory cell infiltration compared to strains with low levels or no expression of BabA [23]. Infections with BabA-positive strains are associated with a 2-fold increase in the diagnosis of gastric atrophy compared with infections with BabA-negative strains. In addition, an immunological analysis of inflammatory responses in the stomach revealed that BabA-positive strains colonize more densely and induce stronger IL-8 secretion in the mucosa compared to counterpart strains. Our group performed large-scale studies of 520 geographically diverse patients presenting with different clinical symptoms to evaluate BabA status by an immunoblot analysis [24]. Interestingly, all strains from East Asia expressed the BabA protein. Twenty-four (9.8%) Western strains were BabA-negative, and these strains were related to milder gastric injury and lower H. pylori density than BabA-positive strains. BabA expression is a major determinant of the density of H. pylori colonization. A meta-analysis demonstrated that the presence of babA is associated with an increased risk of PUD (OR = 2.069), especially the DU subgroup (OR = 1.588). However, a significant association between the babA gene and PUD and DU was observed only in Western countries and not in Asian countries [25].
Cytotoxin-associated gene A: CagA
The cagA gene is located at one end of the cag PAI, in an approximately 40-kb region that is thought to have been incorporated into the H. pylori genome by horizontal transfer from an unknown source [26]. CagA activates a number of signal transduction pathways that resemble signaling via growth factor receptors; simultaneously, it is involved in binding and perturbing the function of epithelial junctions, resulting in aberrations in tight junction function, cell polarity, and cellular differentiation [13]. There are two types of clinical H. pylori isolate: CagA-producing (cagA-positive) strains and CagA-non-producing (cagA-negative) strains. In Western countries, it has been reported that individuals infected with cagA-positive strains of H. pylori are at a higher risk of PUD or gastric cancer than those infected with cagA-negative strains [27]. Nomura et al. [28] reported that subjects with seropositivity for both H. pylori and CagA had an odds ratio of 4.4 (95% CI: 1.8, 10.5) for GU and 5.8 (95% CI: 1.1, 30.0) for DU. Our meta-analysis showed that cagA-positive strains are associated with an increased risk of PUD in Southeast Asian populations (OR 2.83) [29]. However, it is difficult to determine the importance of cagA in clinical outcomes in East Asian populations because nearly all H. pylori strains possess cagA. Interestingly, our previous study [30] showed that the presence of a 12-bp insertion in cagA in the Okinawa population is inversely associated with the presence of GU, and the prevalence of the 12-bp insertion was highest in DU subjects, followed by in subjects with gastritis, gastric cancer, and GU. This suggests that the low biological activity of cagA with the 12-bp insertion is associated with the presence of DU and might have a suppressive effect on GU and gastric cancer.
Following an injection of CagA into epithelial cells, EPIYA motifs are tyrosine-phosphorylated by Src and Abl family kinases, which results in the impairment of a variety of intracellular signaling systems [30]. Interestingly, cagA with an EPIYA-D segment (East-Asian-type cagA) has a higher binding affinity to Src homology-2 domain-containing phosphatase 2 (SHP2) than cagA with an EPIYA-C (Western-type cagA). Based on transfection experiments, IL-8 levels induced by East-Asian-type cagA are higher than those induced by Western-type cagA. Moreover, individuals infected with East-Asian-type cagA strains reportedly have an increased risk of PUD compared with those with Western-type cagA strains [31].
A conserved motif sequence of 16 amino acids (FPLXRXXXVXDLSKVG) in the C-terminal region of CagA is a prerequisite for the CagA–SHP-2 interaction and CagA multimerization, and plays a vital role in H. pylori pathogenesis [32]. It has been designated the CRPIA (conserved repeat responsible for phosphorylation-independent activity) or CagA multimerization (CM) motif. Western-type cagA strains possess multiple CM motifs, located within each EPIYA-C segment, plus one CM motif distal to the last EPIYA-C, whereas East-Asian cagA strains possess a single CM motif located distal to the EPIYA-D segment [32]. The type and number of CM motifs may influence the potential for individual CagA proteins to multimerize in host cells, and this may affect the ability of CagA to disturb host cell function via SHP-2 deregulation [33]. The EPIYT is the second most common EPIYA-B motif in Western-type cagA, but is very rarely observed in East-Asian-type-cagA [34]. Zhang et al. analyzed 364 Western-type-cagA and reported that gastric cancer is more significantly associated with EPIYA sequences in the EPIYA-B motif compared with gastritis alone, whereas EPIYT sequences are significantly associated with DU [34].
Vacuolating cytotoxin: VacA
VacA is a pore-forming cytotoxin; it is a large, 140-kilodalton polypeptide that is trimmed at both ends during secretion from bacterial cells [13]. It enters host cells via endocytosis and induces multiple cellular activities, including membrane-channel formation, cytochrome c release from mitochondria leading to apoptosis, and binding to cell membrane receptors followed by the initiation of a proinflammatory response [2]. In addition, VacA induces host-cell death via apoptosis. This is thought to occur by pore formation in mitochondrial membranes and indirectly via the activation of proapoptotic signaling molecules. Purified VacA can cause epithelial erosion when applied directly to the mouse gastric mucosa [13]. An in vitro study has shown VacA specifically inhibits gastric epithelial cell proliferation [35]. In addition, VacA in combination with heparan sulfate–binding proteins, released by H. pylori, bind host growth factors and may impair mucosal repair thus contributing to ulcer pathogenesis [36].
The gene encoding vacA displays allelic diversity, including the signal (s) regions s1 and s2 and middle (m) regions m1 and m2. Based on in vitro experiments, s1m1 strains have the highest cytotoxicity because they consistently induce cell vacuolation, followed by s1m2 strains (in which cell vacuolation is not consistently induced) and s2m2 strains, which have no cytotoxic activity due to a failure to induce cell vacuolation [29]. In agreement with in vitro data, many studies examining populations in Western countries [37–39] have shown that individuals infected with vacA s1 or m1 H. pylori strains have an increased risk of PUD compared with those with s2 or m2 strains. Our study in Okinawa [40] also found that the vacA s1m1 genotype is highly prevalent in strains from patients with GU (79.2%) than in those from patients with gastritis (59.2%); the vacA s1m2 genotype is more common in strains from gastritis patients than in those from GU patients (17.3% vs. 7.9%, P = 0.04). The prevalence of the vacA s2m2 genotype is significantly higher in strains from patients with gastritis than those from patients with GU and DU (22.4% vs. 11.9% and 10.5%, P = 0.04 and 0.01, respectively).
The intermediate (i) region is a third disease-related region of vacA. It is located between the s region and the m region and was first described in an Iranian population, in which the frequency of vacA s1/m2 strains was high [41]. Rhead et al. proposed that the i region of s1m1 and s2m2 be classified as i1 and i2, respectively. The i1 strains are more pathogenic. The s1m2 strains are classified as either type i1 or i2 [41]. A study in Western countries found that the vacA i1 prevalence was significantly higher (75.0%) in H. pylori strains from PUD patients than in strains isolated from patients with non-ulcer gastric diseases (58.6%) (P = 0.022) [42]. Basso et al. also reported that only i1, and not s1 or m1, strains are significantly associated with PUD. When they examined the association considering GU and DU strains separately, only DU (69%), but not GU (50%), patients had vacA i1 strains, significantly more than those with gastritis (44%, P = 0.01) [43]. However, we did not find an association between genotype and outcome when considering the vacA i region in 314 strains isolated from East Asian and Southeast Asian populations [44]. A true H. pylori virulence factor should have epidemiologic consistency across populations and regions [44]. The deletion (d) region located between the i region and the m region remains poorly studied. One study reported that the frequency of vacA in d1 is 43.4%, and is significantly higher in H. pylori isolates from patients with PUD (71.4%) than in those with gastritis (27.4%) [45].
Duodenal ulcer promoting gene: DupA
DupA is an H. pylori virulence factor that is located in the plasticity region of the H. pylori genome. Lu et al. reported an H. pylori gene (jhp0917–0918: dupA) whose presence is related to an increased risk of DU, neutrophil infiltration, and protection against atrophy, intestinal metaplasia, and gastric cancer, regardless of nationality (Japan, Korea, and Colombia) [46]. A meta-analysis indicated that the prevalence of dupA in patients with gastritis worldwide is 44.88% and differs significantly with respect to nationality and ethnicity; the highest prevalence was recorded in South America [47], and dupA-positive isolates are associated with DU (P = 0.001, OR = 1.4, CI = 1.1–1.7) [47]. Our meta-analysis [48] including 17 studies and a total of 2,466 patients revealed that the overall prevalence of the dupA gene is 31.0% (496/1,600) in Asian countries and 64.1% (526/820) in Western countries. An infection with dupA-positive H. pylori increases the risk for DU (OR 1.41, 95% confidence interval [CI] 1.12–1.76), particularly in Asian countries (OR 1.57, 95% CI 1.19–2.06), but not in Western countries (OR 1.09, 95% CI 0.73–1.62) [48].
Gomes et al. [49] reported a frameshift mutation in 16.28% (14/86) of dupA genes, which created a premature stop codon and had potentially considerable effects on protein expression or function. Intriguingly, the presence of dupA without a stop codon was more frequently observed in strains from patients with DU than in strains from those with gastritis and gastric cancer [50]. In our study, we found that the intact long-type dupA without a frameshift mutation, but not the short-type dupA, is significantly related to GU, but not to gastritis. Even after adjusting for age, gender, and cagA, the presence of the intact long-type dupA is more significantly associated with GU than gastritis (OR 3.35, 95% CI 1.55–7.24), suggesting that an intact long-type dupA might produce a functional DupA protein and an intact long-type dupA can be an effective virulence marker for severe outcomes [51].
Induced by contact with epithelium: IceA
The iceA gene has two main allelic variants, iceA1 and iceA2 [27]. Based on a sequence analysis, iceA1, but not iceA2, has strong homology to nlaIIIR, which encodes a CATG-specific endonuclease in Neisseria lactamica, and iceA1 appears to be a degenerated gene that was once part of a restriction-modification system [52]. The expression of iceA1 is upregulated in response to contact between H. pylori and human epithelial cells, and this has been linked with enhanced mucosal IL-8 expression and acute antral inflammation, and is regarded as a marker for PUD [52].
Van Doorn et al. [27] reported that the iceA allelic type is independent of the cagA and vacA status, and there is a significant association between the presence of the iceA1 allele and PUD. The significant association between DU and the iceA1 allele has also been reported by several studies [53,54]. Our meta-analysis that included 50 studies with a total of 5,357 patients revealed that the overall prevalence of iceA1 is significantly higher in Asian countries than in Western countries (64.6% vs. 42.1%), whereas the prevalence of iceA2 is more prevalent in Western countries than in Asian countries (45.1% vs. 25.8%). A sensitivity analysis showed that iceA1 is significantly associated with PUD (OR 1.25, 95% CI 1.08–1.44); however, iceA2 is inversely related to PUD (OR 0.76, 95% CI 0.65–0.89) [54].
Host polymorphisms and PUD
Genes encoding cytokines and related molecules harbor polymorphic regions that directly influence interindividual variation in the magnitude of the cytokine response, and this variation is clearly related to clinical outcomes. Activated neutrophils and mononuclear cells produce several pro-inflammatory cytokines. Several pro-inflammatory cytokines (e.g., IL-1B, IL-6, IL-8, and TNF-α) and anti-inflammatory cytokines (IL-10) are associated with PUD. A recent report also suggested that the PSCA and CYP2C19 ultra-rapid metabolizer genotype are associated with PUD.
Tumor necrosis factor-alpha (TNF-A)
TNF-A, which encodes TNF-α, is a polymorphic gene located in the central major histocompatibility complex; it is a key mediator in host responses against gram-negative bacteria [55]. TNF-α has many biological activities, including the stimulation of the expression of adhesion molecules, such as intercellular adhesion molecule 1, on endothelial cells (which facilitates the extravasation of neutrophils into the lamina propria of the mucosa), activation of leukocytes and T-lymphocytes, stimulation of the production of cytokines by macrophages and monocytes, and the induction of apoptosis. H. pylori infection elevates TNF-α in tissues and induces cytotoxicity and apoptosis of gastric epithelial cells [56]. The specific mechanisms between TNF-α and PUD has not been fully elucidated. However TNF-α plays a key role in regulating gastric acid secretion, which is one of the most important factors in the development of DU associated with H. pylori infection. It is supported by the levels of TNF-α being found to be significantly higher in antral biopsy specimens in DU patients than in those from H. pylori-negative subjects [57].
Kustmann et al. [58] reported that the G/G genotype of the TNF-A-308 polymorphism is a risk factor for DU in females with H. pylori (RR = 10.7). Subsequent studies have suggested that among five biallelic polymorphisms in the TNF-A promoter region, the TNF-A-238 G/A and -308 G/A polymorphisms are related to interindividual differences in transcriptional activity in Western countries, and these polymorphisms are associated with the development of GU and DU [59]. However, most Asian populations have low-producer alleles, TNF-A-238 G/G and −308 G/G [59]. Recently, three polymorphisms, TNF-A-1031 T/C, -863 C/A, and -857 C/T, which are related to high transcriptional promoter activity, have been identified in Japanese patients [60]. Subjects with TNF-A-857/-863/-1031 TAC polymorphisms have 1.8-fold higher TNF-α levels in peripheral mononuclear cells than subjects with dominant genotypes [60]. Lu et al. reported that among H. pylori-infected patients, -1031C or -863A carriers of the TNF-A promoter had more severe gastric neutrophil infiltration and TNF-α gastric staining than individuals with the -1031 TT or -863 CC genotype, respectively. Moreover, a multivariate logistic regression verified that both -1031C and −863A are independent risk factors for GU and DU without intestinal metaplasia in H. pylori-infected hosts [61]. A similar association also been found between TNF-A-857 TT and both GU and DU [62]. Sugimoto et al. reported that the alleles TNF-A-857 T (OR = 1.826), TNF-A-863 A (OR = 1.788), and TNF-A-1031 C (OR = 1.912) are associated with an increased risk of GU development [59].
IL-1B
The IL-1 gene cluster on chromosome 2q contains 3 related genes within a 430-kilobase (kb) region. IL-1A, IL-1B, and IL-1 receptor antagonist (IL-1RN), which encode the pro-inflammatory cytokines IL-1α and IL-1β as well as their endogenous receptor antagonist, respectively [63]. The IL-1B gene has three biallelic polymorphisms at positions −511, −31, and +3954 bp from the transcriptional start site, whilst the IL-RN gene has a variable number of identical tandem repeats of 86 bp in length in the intron [64]. Interestingly, mucosal IL-1β levels significantly differ among the IL-1B-511, −31, and IL-RN genotypes. In addition, IL-1B-511 T, −31 C, and IL-RN *2 carriers have increased IL-1β production compared with carriers of the alternative genotypes [12]. Together with TNF-α, IL-1β is important in initiating and amplifying inflammatory responses to H. pylori infection. IL-1β is also a potent inhibitor of gastric acid secretion; it is a 100-fold more potent inhibitor than proton pump inhibitors, and 6000-fold more potent than H2 receptor antagonists on a molar basis. Theoretically, increased production of IL-1β in the gastric mucosa would result in enhanced suppression of gastric acid secretion as well as enhanced inflammation, allowing the expansion of H. pylori colonization from the gastric antrum to the corpus, leading to the progression of severe atrophic gastritis in the corpus and further decreasing acid secretion [59].
Furuta et al. reported differences in the levels of fasting intra-gastric pH between H. pylori-infected individuals with the IL-1B-511 T allele and those with the IL-1B-511 C allele; the observed pH values were 6.5, 3.8, and 2.4 for patients with the IL-1B-511 T/T, C/T, and C/C genotypes, respectively. Moreover, inflammatory cell infiltration is significantly higher in the carriers of the IL-1B-511 T/T genotype than in those of the C/T or C/C genotype [65]. A similar finding with respect to the neutrophil infiltration score was also found between individuals with the IL-1RN*1/*1 and IL-1RN*1/*2 genotypes [65]. Several epidemiological studies have revealed that individuals with the combination of IL-1B-511 T and IL-1B-31 C or IL-1RN *2/*2 (2 repeats of 86 bp), which are categorized as high-producer alleles/genotypes, have a higher risk of gastric atrophy, PUD, and gastric cancer than individuals with the IL-1B-511 C, IL-1B-31 T, or non-IL-1RN*2 alleles [12,65,66]. Chakravorty et al. [67] reported a significantly higher frequency of IL-1B-511 T/T (OR = 4.22) and -31 C/C (OR = 2.16) genotypes in H. pylori-infected individuals with DU compared to infected individuals with normal mucosa. The C/T haplotype of the IL-1B-511 and IL-1B-31 loci is also present at a significantly higher frequency in H. pylori-infected DU patients than in infected controls (OR = 2.47, CI = 1.27–4.8). A quantitative analysis of mucosal IL-1B revealed that among H. pylori-infected individuals, carriers of the −31 C/C genotype had significantly lower IL-1B transcript levels than did carriers of the C/T and T/T genotypes. Moreover, an IL-1B promoter activity assay showed that the promoter with −31 T had a 10-fold increase in activity compared to the promoter with −31 C [67].
IL-6
IL-6, a multifunctional cytokine, plays an important role in host defense as a messenger between innate and adaptive systems by stimulating IFN-γ production, differentiation, and the maintenance of cytotoxic T-cells, and promoting immunoglobulin secretion in activated B cells [68]. The gastric mucosal levels of IL-6 are elevated in H. pylori-associated gastritis and diminished after infection is eradicated [69]. Three polymorphisms in the IL-6 gene, which is located on chromosome 7p21, have been identified, IL-6–174, −572, and -597 [70]. IL-6–174 G and IL-6–572 G allele carriers produce higher levels of IL-6 than those with the C/C genotype [64]. However, the association between this polymorphism and PUD remains unclear. A study reported that the frequencies of IL-6–572 G/G (OR 0.3, 95% CI: 0.1–0.9, P = 0.027) and of G carriers (OR 0.5, 95% CI: 0.4–0.8, P = 0.003) were lower in H. pylori-positive DU patients than in H. pylori-positive controls [71]. Moreover, the risk of GU was significantly higher for the G/G genotype (OR 58.86) and G allele carriers (OR = 33.10). In contrast, in our study, we failed to detect an association between IL-6 polymorphisms and PUD in Asian and Colombia populations [72].
IL-8
IL-8 is a pro-inflammatory chemokine that belongs to the CXC family and has an important role in the pathogenesis of H. pylori-induced diseases. High expression of IL-8 has been demonstrated for gastric epithelial cells during H. pylori infection, particularly in the cag-PAI-positive strain of H. pylori. It plays a key role in the initiation, modulation, and maintenance of gastrointestinal inflammatory responses [64]. Increased IL-8 levels may amplify the inflammatory response to H. pylori by recruiting neutrophils and monocytes, resulting in an advanced degree of gastritis [64]. Within the past few years, increasing evidence has indicated that the inflammatory response is an essential part of the pathogenesis of PUD, suggesting that the inflammatory cytokines, as well the variation in genes encoding the inflammatory mediators may play a significant role in the development of PUD [73]. There are three common polymorphisms in the IL-8 gene, -251 A/T, -396 T/G, and -781 C/T. A common single nucleotide polymorphism (SNP) at position -251 is associated with increased IL-8 production [74]. The T-to-A mutation in this promoter region could affect IL-8 transcription and secretion [75].
H. pylori-positive patients with the A/A genotype at position -251 of the IL-8 gene have an increased risk of PUD (OR = 2.08) [69]. Hofner et al. reported a higher frequency of the IL-8–251 A/T genotype (high IL-8 producers) among patients with DU than controls. Conversely, the prevalence of IL-8–251 T/T was significantly lower in the group of patients with DU (17%) than in the control group (40%) [76]. Similar results have also been found in European and Korean populations [57,71]. In the Japanese population, IL-8–251 A/A is associated with a higher risk of GU (OR 2.07) than T/T. Severe gastric atrophy is also substantially more common in the A/A or A/T genotype groups than in the T/T genotype group [75]. Although a subsequent study only found an association with gastric cancer [77], a meta-analysis confirmed that, only in Asians, PUD risk was higher for the IL-8–251 A/A polymorphism than T/T (OR = 1.40). Moreover, the IL-8-251 A/A polymorphism had higher GU and DU risks than T/T (OR = 1.24 and OR = 1.40, respectively) [73].
IL-10
IL-10 is an anti-inflammatory cytokine that downregulates IL-1B, TNF-α, Interferon-γ, and other proinflammatory cytokines. The effects of IL-10 on other cell types include the inhibition of proinflammatory cytokine production by activated monocytes/macrophages [68]. A relative deficiency in IL-10 may result in a Th-1-driven hyperinflammatory response to H. pylori with greater damage to the gastric mucosa. H. pylori can lead to IL-10 upregulation and the suppression of an efficient immune response, which favors infection and parasite survival [69]. Two SNPs that are associated with low IL-10 production have been detected in the promoter region of this gene: a C–T base transition at position −819 and a C–A base transition at position −592 [69]. Patient carriers of the T allele at position −819 of the IL-10 gene in the presence of H. pylori infection have an increased risk of PUD (OR = 1.24) [69]. However, one study reported a synergistic effect between IL-10–592 A/A and IL-8–251 A/A, which are associated with high IL-8 production, with respect to the development of GU [71].
Cytochrome P450 2C19 (CYP2C19)
CYP2C19, a CYP isoform, is a clinically important enzyme responsible for the metabolism of a number of therapeutic drugs (such as nonsteroidal anti-inflammatory drugs, proton pump inhibitors, antidepressants, benzodiazepines, and clopidogrel) [78]. CYP2C19 also plays a crucial role in either the detoxification or inactivation of potential carcinogens, and in the bioactivation of some environmental procarcinogens to reactive DNA-binding metabolites, such as nitrosamine. Therefore, CYP2C19 polymorphism is associated with differences among individuals in susceptibility to certain forms of cancer. Importantly, the CYP2C enzymes also metabolize endogenous substances, such as arachidonic acid and estrogens [79]. CYP2C19 efficiently metabolizes arachidonic acid to four kinds of epoxyeicosatrienoic acids (EETs; 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET) that have diverse physiological roles (including the control of vascular tone, angiogenesis, cellular migration, proliferation, and inflammation) [80]. With regards to the metabolic rate, individuals with the CYP2C19*1/*1 genotype are classified as extensive metabolizers (EMs). They carry two wild-type alleles and have no mutation, whereas those who have CYP2C19*2 or CYP2C19*3 mutation alleles are designated as intermediate metabolizers (IMs), if heterozygous, and poor metabolizers (PMs), if homozygous. IMs and especially PMs have superior acid suppression with conventional doses of proton pump inhibitors [81]. The CYP2C19*17 (−806 C.T) allele, a novel allele identified by Sim, et al. is associated with the ultra-rapid phenotype and provides decreased acid suppression with standard recommended doses of proton pump inhibitors. The CYP2C19*17 allele has a frequency of 18% in both Swedes and Ethiopians, but only 4% in Chinese populations [81].
Musumba et al. proposed that CYP2C19*17, associated with ultra-rapid metabolizers, alters arachidonic acid metabolism, resulting in impaired gastrointestinal mucosal defenses through a combination of reduced gastro-protective prostaglandin E2 production, enhanced vasoconstriction in the mucosal microcirculation, and promotion of the production of injurious reactive oxygen species [80]. Associations between eight functional SNPs in the CYP2C family of genes—CYP2C8*3 (rs11572080 and rs10509681), CYP2C8*4, CYP2C9*2, CYP2C9*3, CYP2C19*2, CYP2C19*3, and CYP2C19*17—and PUD have been detected in 1,239 Caucasian patients, irrespective of NSAID exposure or the presence of H. pylori infection [80]. A logistic regression analysis showed that only CYP2C19*17 was associated with PUD (OR = 1.47, CI 1.12 to 1.92). An additional analysis focusing on all CYP2C19 genotypes and PUD status (using *1/*1 as a baseline) showed that only *1/*17 was significantly associated with PUD. When they separated analysis between PUD type, a low association was found between CYP2C19*17 with GU and DU (P = 0.022 and P = 0.037, respectively), but was not significant after Bonferroni correction. The next analysis to find influence of PPI and gender found that the utilization of PPIs was significantly associated with the absence of PUD in all patients, both when those with evidence of H. pylori infection were included and when they were excluded (P < 0.0001). However, they failed to get association between CYP2C19*17 status and PPI use. A previous study also found that the prevalence of the rapid metabolizer genotype CYP2C19 (56%) was highest, followed by the intermediate metabolizers (32%) and poor metabolizers (12%). Furthermore, the prevalence of rapid metabolizers in was significantly greater in patients with PUD with bleeding than in gastritis patients (56% vs. 36%: OR = 2.3) [82]. In fact, the poor metabolizers with the CYP2C19 genotype appear to be at an increased risk for developing gastric cancer, especially the diffuse type, in the Japanese population [83].
Prostate stem cell antigen (PSCA)
Prostate stem cell antigen (PSCA), located on chromosome 8q24.2, encodes a protein consisted of 123 amino acid residues. PSCA was initially identified as a prostate-specific antigen, which is overexpressed in most prostate cancers and plays an important role in cell adhesion, proliferation and survival. PSCA was also found to be abnormally expressed in other types of malignancies, such as cancers of the bladder, pancreas, esophagus, and stomach [84]. A meta-analysis suggested the two loci of PSCA (rs2294008 and rs2976392) were both significantly associated with gastric cancer susceptibility [84].
Recently, genome-wide association studies (GWAS) are a powerful tool for high-risk population screening for many diseases in molecular epidemiology. Using GWAS, a study investigated the association of PSCA variations with GU in two Japanese case-control sample groups (4,291 GU cases and 22,665 controls) [85]. They found that the C-allele of rs2294008 at PSCA increased the risk of GU (OR =1.13). The same group also analyzed a total of 7,035 individuals with DU and 25,323 controls in Japanese population. The C allele of rs2294008 at PSCA was associated with increased risk of DU (OR = 1.84). In addition this allele also decreased risk of gastric cancer (OR = 0.79) [86].
Other risk factors for PUD
There are several other risk factors related to PUD, such as NSAID and polymorphism related to NSAID, smoking, alcohol, consumption of meat and fish, and a family history of PUD. These factors are not within the scope of this review.
Expert commentary
Although PUD is typically a non-fatal disease, it is the most important cause of upper gastrointestinal bleeding. Recurrent PUD, particularly in the pyloric and bulbar regions, can lead to scarring with stricture formation and gastric outlet obstruction. Patients infected with H. pylori have a 3–4-fold increased risk of PUD, and the eradication of H. pylori strongly reduces the otherwise high risk of renewed ulcer bleeding. In this review, we summarized several of the most likely virulence genes (adhesin bacterial and gastric inflammation factors) associated with an increased risk of PUD. Patients infected with H. pylori who have oipA “on,” cagA-positive, cagA with a 12-bp insertion, East-Asian-type cagA, Western-type-cagA with the EPIYT sequence, vacA s1, m1, or i1, babA-positive, dupA-positive (especially the intact long-type dupA), or iceA1-positive genotypes have a significantly increased risk of PUD (Table 1). However, it is possible that additional important pathogenic genes exist because the H. pylori genome consists of approximately 1,600 genes. Recently, a microarray analysis provided comprehensive information about the gene content of different strains and helped identify both strain-specific and shared genes. Salama et al. [87] examined the genomic content of 15 clinical isolates by using a whole-genome DNA microarray and defined 1,281 genes as functional core genes. They identified candidate virulence genes on the basis of coinheritance with the cag PAI [87]. In addition, whole-genome sequencing technology has become cheaper and is a powerful tool to study the pathogenicity of H. pylori. Massively parallel sequencing technology provides valuable information regarding candidates for novel virulence factors. Host polymorphisms directly influence variation among individuals in the magnitude of cytokine responses and result in a disequilibrium between aggressive and defensive factors acting in the mucosa, clearly contributing to an individual’s clinical outcome. Some TNF-A, IL-1B, IL-6, IL-8, IL-10 and PSCA polymorphisms are strongly associated with PUD, as are rapid and ultra-rapid metabolizer CYP2C19 genotypes. Importantly, there is geographical variation in bacterial genotypes and host polymorphisms, which influences clinical outcomes (Table 1). This observation indicated that the identification of the high-risk group for PUD with respect to both bacterial and host factors is important. Similar to the reason why H. pylori-induced inflammation has a pan-gastritis or corpus-predominant pattern in some people, but is antral-predominant in others has not been fully elucidated; the difference of bacterial virulence or host factors alone cannot be explained the end of spectrum PUD. Most of them become risk factors for both GU and DU. Presumably both bacterial and host factors contribute together with environmental factors to this differential response. More studies are needed to determine the mechanisms for the opposite ends of the PUD spectrum (GU and DU) and its association with H. pylori virulence and host genetic factors. Although this has become an ideal condition, we recommend intensive endoscopic screening and/or eradication therapy for patients with a high risk of PUD. Recently, a novel, fully automated rapid genetic analyzer was developed that is capable of determining clarithromycin resistance (e.g., the 23S rRNA point mutations A2143G and A2144G) within 60–120 min, whereas culture tests require 7–10 d, and the same method can likely be used for other screening tests. However, specialized laboratories and expensive equipment are required. At minimum, physicians should be aware of the predominant bacterial and host genotypes in their region. This would enable them to predict the risk of developing PUD or other gastrointestinal disease in patients infected with H. pylori.
Table 1.
Summary of the associations between bacterial virulence genes, host genetic polymorphism, and PUD
| Gene | Region | GU | DU |
|---|---|---|---|
| Bacterial virulence | |||
| oipA | Asia and Western | ↑ | |
| babA | Western | ↑ | ↑ |
| cagA | Asian and Western | ↑ | ↑ |
| 12-bp insertion cagA | Asian | ↑ | |
| East-Asian type cagA | Asian | ↑ | ↑ |
| vacA s1m1 | Asian and Western | ↑ | ↑ |
| vacA i1 | Asian and Western | ↑ | ↑ |
| vacA d1 | Asian | ↑ | |
| dupA | Asian | ↑ | |
| intact long-type dupA | Asian | ↑ | |
| iceA1 | Asian and Western | ↑ | ↑ |
| Host genetic factors | |||
| TNF-A-238 G/A | Western | ↑ | ↑ |
| TNF-A-308 G/A | Western | ↑ | ↑ |
| TNF-A-857 T/T | Western | ↑ | ↑ |
| TNF-A-863 A carriers | Asian | ↑ | ↑ |
| TNF-A-1031 C carriers | Asian | ↑ | ↑ |
| IL-1B-31 C/C | Asian | ↑ | ↑ |
| IL-1B-511 T/T | Asian | ↑ | |
| IL-1RN*2/*2 | Asian | ↑ | ↑ |
| IL-6-572 G/G | Asian | ↑ | ↑ |
| IL-8-251 A/A | Asian | ↑ | ↑ |
| IL-10-819 T carriers | South American | ↑ | ↑ |
| IL-10-592 A/A | Asian | ↑ | |
| CYP2C19 URM* | Western | ↑ | ↑ |
| CYP2C19 RM* | Asian | ↑ | ↑ |
| PSCA rs2294008 C carriers | Asian | ↑ | ↑ |
URM: Ultra-rapid metabolizers, RM: Rapid metabolizers
Five-year view.
Important issues regarding the role of Helicobacter pylori virulence and host genetic factors in the development peptic ulcer disease (PUD) are summarized below.
H. pylori infection is still an important factor in the pathogenesis of PUD;
More studies are needed to determine the mechanisms for the opposite ends of the PUD spectrum (GU and DU) and its association with H. pylori virulence and host genetic factors;
Identification of the high-risk group based on bacterial and host factors is important to predict the outcomes of H. pylori infections;
Intensive endoscopic screening and/or eradication therapy is strongly recommended for patients with a high risk of PUD;
Whole-genome DNA microarray and whole-genome sequencing technology have become cheaper and are powerful tools to study the pathogenicity of H. pylori. Massively parallel sequencing technology provides valuable information regarding novel candidate virulence factors.
Key issues.
Helicobacter pylori PUD has contrary effects on opposite ends of the disease spectrum, which may be related to differences in the severity and distribution of gastritis.
In patients infected with H. pylori, the oipA “on,” cagA (especially East-Asian-type cagA or Western-type-cagA with the EPIYT sequence), vacA s1, m1, or i1, babA-positive, dupA-positive (especially the intact long-type dupA) or iceA1-positive genotypes were significantly associated with PUD.
Some TNF-A, IL-1B, IL-6, IL-8, IL-10 and PSCA polymorphisms are strongly associated with PUD, as are rapid and ultra-rapid metabolizer CYP2C19 genotypes.
There is geographical variation in the impact of bacterial genotypes and host polymorphisms on PUD pathogenesis.
It is important to identify the high-risk group for PUD with respect to bacterial and host factors. We recommend intensive endoscopic screening and/or eradication therapy for patients with a high risk of PUD. At minimum, physicians should be aware of the predominant bacterial and host genotypes in their region. This enables them to predict the risk of PUD or other gastrointestinal disease in patients infected with H. pylori.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (DK62813) and the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (24406015, 24659200, 25293104, 26640114, and 15H02657). It was also supported by the Japan Society for the Promotion of Science (JSPS) Institutional Program for Young Researcher Overseas Visits and the Strategic Funds for the Promotion of Science and Technology from the Japan Science and Technology Agency (JST).
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Yamaoka Y, Kikuchi S, el-Zimaity HM, Gutierrez O, Osato MS, Graham DY. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123(2):414–424. doi: 10.1053/gast.2002.34781. [DOI] [PubMed] [Google Scholar]
- 2. Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clinical microbiology reviews. 2006;19(3):449–490. doi: 10.1128/CMR.00054-05. A comprehensive review including microbiological, clinical, immunological, and biochemical aspects of the pathogenesis of H. pylori.
- 3.Tytgat GN. Treatment of peptic ulcer. Digestion. 1998;59(5):446–452. doi: 10.1159/000007522. [DOI] [PubMed] [Google Scholar]
- 4.Kuipers EJ, Thijs JC, Festen HP. The prevalence of Helicobacter pylori in peptic ulcer disease. Alimentary pharmacology & therapeutics. 1995;(9 Suppl 2):59–69. [PubMed] [Google Scholar]
- 5.Quan C, Talley NJ. Management of peptic ulcer disease not related to Helicobacter pylori or NSAIDs. The American journal of gastroenterology. 2002;97(12):2950–2961. doi: 10.1111/j.1572-0241.2002.07068.x. [DOI] [PubMed] [Google Scholar]
- 6.Sostres C, Gargallo CJ, Lanas A. Interaction between Helicobacter pylori infection, nonsteroidal anti-inflammatory drugs and/or low-dose aspirin use: old question new insights. World journal of gastroenterology : WJG. 2014;20(28):9439–9450. doi: 10.3748/wjg.v20.i28.9439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology. 2008;134(7):1842–1860. doi: 10.1053/j.gastro.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Walker MM, Dixon MF. Gastric metaplasia: its role in duodenal ulceration. Alimentary pharmacology & therapeutics. 1996;(10 Suppl 1):119–128. doi: 10.1046/j.1365-2036.1996.22164012.x. [DOI] [PubMed] [Google Scholar]
- 9.McColl KEL, El-Omar E, Gillen D. Interactions between H. pylori infection, gastric acid secretion and anti-secretory therapy. British Medical Bulletin. 1998;54(1):121–138. doi: 10.1093/oxfordjournals.bmb.a011663. [DOI] [PubMed] [Google Scholar]
- 10.Zavros Y, Rieder G, Ferguson A, Samuelson LC, Merchant JL. Hypergastrinemia in response to gastric inflammation suppresses somatostatin American journal of physiology. Gastrointestinal and liver physiology. 2002;282(1):G175–G183. doi: 10.1152/ajpgi.00287.2001. [DOI] [PubMed] [Google Scholar]
- 11.Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annual review of pathology. 2006;1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125. [DOI] [PubMed] [Google Scholar]
- 12. Hwang IR, Kodama T, Kikuchi S, et al. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123(6):1793–1803. doi: 10.1053/gast.2002.37043. Confirmed conflicting data about the effects of interleukin (IL)-1 genetic polymorphisms on IL-1β production.
- 13. Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134(1):306–323. doi: 10.1053/j.gastro.2007.11.009. Detail explanation about mechanism and how H. pylori interacts with its host.
- 14.Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori . Proceedings of the National Academy of Sciences of the United States of America. 2000;97(13):7533–7538. doi: 10.1073/pnas.130079797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai M, Furuta Y, Yahara K, et al. Evolution in an oncogenic bacterial species with extreme genome plasticity: Helicobacter pylori East Asian genomes. BMC microbiology. 2011;11:104. doi: 10.1186/1471-2180-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaoka Y, Kita M, Kodama T, et al. Helicobacter pylori infection in mice: Role of outer membrane proteins in colonization and inflammation. Gastroenterology. 2002;123(6):1992–2004. doi: 10.1053/gast.2002.37074. [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto M, Ohno T, Graham DY, Yamaoka Y. Gastric mucosal interleukin-17 and −18 mRNA expression in Helicobacter pylori-induced Mongolian gerbils. Cancer science. 2009;100(11):2152–2159. doi: 10.1111/j.1349-7006.2009.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham DY, Opekun AR, Osato MS, et al. Challenge model for Helicobacter pylori infection in human volunteers. Gut. 2004;53(9):1235–1243. doi: 10.1136/gut.2003.037499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, He C, Chen M, Wang Z, Xing C, Yuan Y. Association of presence/absence and on/off patterns of Helicobacter pylori oipA gene with peptic ulcer disease and gastric cancer risks: a meta-analysis. BMC infectious diseases. 2013;13:555. doi: 10.1186/1471-2334-13-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaoka Y. Roles of Helicobacter pylori BabA in gastroduodenal pathogenesis. World journal of gastroenterology : WJG. 2008;14(27):4265–4272. doi: 10.3748/wjg.14.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toller IM, Neelsen KJ, Steger M, et al. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(36):14944–14949. doi: 10.1073/pnas.1100959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai YC, Wang TH, Huang SH, et al. Density of Helicobacter pylori may affect the efficacy of eradication therapy and ulcer healing in patients with active duodenal ulcers. World journal of gastroenterology : WJG. 2003;9(7):1537–1540. doi: 10.3748/wjg.v9.i7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohno T, Vallstrom A, Rugge M, et al. Effects of blood group antigen-binding adhesin expression during Helicobacter pylori infection of Mongolian gerbils. The Journal of infectious diseases. 2011;203(5):726–735. doi: 10.1093/infdis/jiq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fujimoto S, Olaniyi Ojo O, Arnqvist A, et al. Helicobacter pylori BabA expression, gastric mucosal injury, and clinical outcome. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(1):49–58. doi: 10.1016/j.cgh.2006.09.015. Compared the ability of published PCR-based methods to assess BabA status with BabA immunoblotting and Lewis b (Le(b)) binding activity assays. They confirmed that currently used PCR-based methods must be interpreted with caution.
- 25.Chen MY, He CY, Meng X, Yuan Y. Association of Helicobacter pylori babA2 with peptic ulcer disease and gastric cancer. World journal of gastroenterology : WJG. 2013;19(26):4242–4251. doi: 10.3748/wjg.v19.i26.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Censini S, Lange C, Xiang Z, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Doorn LJ, Figueiredo C, Sanna R, et al. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori . Gastroenterology. 1998;115(1):58–66. doi: 10.1016/s0016-5085(98)70365-8. [DOI] [PubMed] [Google Scholar]
- 28.Nomura AM, Perez-Perez GI, Lee J, Stemmermann G, Blaser MJ. Relation between Helicobacter pylori cagA status and risk of peptic ulcer disease. American journal of epidemiology. 2002;155(11):1054–1059. doi: 10.1093/aje/155.11.1054. [DOI] [PubMed] [Google Scholar]
- 29.Sahara S, Sugimoto M, Vilaichone RK, et al. Role of Helicobacter pylori cagA EPIYA motif and vacA genotypes for the development of gastrointestinal diseases in Southeast Asian countries: a meta-analysis. BMC infectious diseases. 2012;12:223. doi: 10.1186/1471-2334-12-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuo Y, Shiota S, Matsunari O, et al. Helicobacter pylori cagA 12-bp insertion can be a marker for duodenal ulcer in Okinawa, Japan. Journal of gastroenterology and hepatology. 2013;28(2):291–296. doi: 10.1111/jgh.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilaichone RK, Mahachai V, Tumwasorn S, Wu JY, Graham DY, Yamaoka Y. Molecular epidemiology and outcome of Helicobacter pylori infection in Thailand: a cultural cross roads. Helicobacter. 2004;9(5):453–459. doi: 10.1111/j.1083-4389.2004.00260.x. [DOI] [PubMed] [Google Scholar]
- 32.El-Etr SH, Mueller A, Tompkins LS, Falkow S, Merrell DS. Phosphorylation-independent effects of CagA during interaction between Helicobacter pylori and T84 polarized monolayers. J Infect Dis. 2004;190(8):1516–1523. doi: 10.1086/424526. [DOI] [PubMed] [Google Scholar]
- 33.Ren S, Higashi H, Lu H, Azuma T, Hatakeyama M. Structural basis and functional consequence of Helicobacter pylori CagA multimerization in cells. The Journal of biological chemistry. 2006;281(43):32344–32352. doi: 10.1074/jbc.M606172200. [DOI] [PubMed] [Google Scholar]
- 34.Zhang XS, Tegtmeyer N, Traube L, et al. A specific A/T polymorphism in Western tyrosine phosphorylation B-motifs regulates Helicobacter pylori CagA epithelial cell interactions. PLoS pathogens. 2015;11(2):e1004621. doi: 10.1371/journal.ppat.1004621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricci V, Ciacci C, Zarrilli R, et al. Effect of Helicobacter pylori on gastric epithelial cell migration and proliferation in vitro: role of VacA and CagA. Infection and immunity. 1996;64(7):2829–2833. doi: 10.1128/iai.64.7.2829-2833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ascencio F, Hansson HA, Larm O, Wadstrom T. Helicobacter pylori interacts with heparin and heparin-dependent growth factors. FEMS immunology and medical microbiology. 1995;12(3–4):265–272. doi: 10.1111/j.1574-695X.1995.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 37.Atherton JC, Cao P, Peek RM, Jr, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. The Journal of biological chemistry. 1995;270(30):17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 38.Sugimoto M, Yamaoka Y. The association of vacA genotype and Helicobacter pylori-related disease in Latin American and African populations. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2009;15(9):835–842. doi: 10.1111/j.1469-0691.2009.02769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugimoto M, Zali MR, Yamaoka Y. The association of vacA genotypes and Helicobacter pylori-related gastroduodenal diseases in the Middle East. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2009;28(10):1227–1236. doi: 10.1007/s10096-009-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsunari O, Shiota S, Suzuki R, et al. Association between Helicobacter pylori virulence factors and gastroduodenal diseases in Okinawa, Japan. Journal of clinical microbiology. 2012;50(3):876–883. doi: 10.1128/JCM.05562-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhead JL, Letley DP, Mohammadi M, et al. A new Helicobacter pylori vacuolating cytotoxin determinant, the intermediate region, is associated with gastric cancer. Gastroenterology. 2007;133(3):926–936. doi: 10.1053/j.gastro.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 42.Yordanov D, Boyanova L, Markovska R, Gergova G, Mitov I. Significance of Helicobacter pylori vacA intermediate region genotyping-a Bulgarian study. Diagnostic microbiology and infectious disease. 2012;74(3):253–257. doi: 10.1016/j.diagmicrobio.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 43. Basso D, Zambon CF, Letley DP, et al. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology. 2008;135(1):91–99. doi: 10.1053/j.gastro.2008.03.041. Confirmed the important associations of vacA intermediate region with PUD.
- 44.Ogiwara H, Graham DY, Yamaoka Y. vacA i-region subtyping. Gastroenterology. 2008;134(4):1267. doi: 10.1053/j.gastro.2007.11.062. author reply 1268. [DOI] [PubMed] [Google Scholar]
- 45.Basiri Z, Safaralizadeh R, Bonyadi MJ, Somi MH, Mahdavi M, Latifi-Navid S. Helicobacter pylori vacA d1 genotype predicts risk of gastric adenocarcinoma and peptic ulcers in northwestern Iran. Asian Pacific journal of cancer prevention : APJCP. 2014;15(4):1575–1579. doi: 10.7314/apjcp.2014.15.4.1575. [DOI] [PubMed] [Google Scholar]
- 46. Lu H, Hsu PI, Graham DY, Yamaoka Y. Duodenal ulcer promoting gene of Helicobacter pylori . Gastroenterology. 2005;128(4):833–848. doi: 10.1053/j.gastro.2005.01.009. Introduced a novel marker associated with an increased risk for DU and reduced risk for gastric atrophy and cancer.
- 47.Hussein NR. The association of dupA and Helicobacter pylori-related gastroduodenal diseases. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2010;29(7):817–821. doi: 10.1007/s10096-010-0933-z. [DOI] [PubMed] [Google Scholar]
- 48.Shiota S, Matsunari O, Watada M, Hanada K, Yamaoka Y. Systematic review and meta-analysis: the relationship between the Helicobacter pylori dupA gene and clinical outcomes. Gut pathogens. 2010;2(1):13. doi: 10.1186/1757-4749-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomes LI, Rocha GA, Rocha AM, et al. Lack of association between Helicobacter pylori infection with dupA-positive strains and gastroduodenal diseases in Brazilian patients. International journal of medical microbiology : IJMM. 2008;298(3–4):223–230. doi: 10.1016/j.ijmm.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Queiroz DM, Rocha GA, Rocha AM, et al. dupA polymorphisms and risk of Helicobacter pylori-associated diseases. International journal of medical microbiology : IJMM. 2011;301(3):225–228. doi: 10.1016/j.ijmm.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi A, Shiota S, Matsunari O, et al. Intact long-type dupA as a marker for gastroduodenal diseases in Okinawan subpopulation, Japan. Helicobacter. 2013;18(1):66–72. doi: 10.1111/j.1523-5378.2012.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Q, Morgan RD, Roberts RJ, et al. Functional analysis of iceA1, a CATG-recognizing restriction endonuclease gene in Helicobacter pylori . Nucleic acids research. 2002;30(17):3839–3847. doi: 10.1093/nar/gkf504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peek RM, Jr, Thompson SA, Donahue JP, et al. Adherence to gastric epithelial cells induces expression of a Helicobacter pylori gene, iceA, that is associated with clinical outcome. Proceedings of the Association of American Physicians. 1998;110(6):531–544. [PubMed] [Google Scholar]
- 54.Shiota S, Watada M, Matsunari O, Iwatani S, Suzuki R, Yamaoka Y. Helicobacter pylori iceA, clinical outcomes, and correlation with cagA: a meta-analysis. PloS one. 2012;7(1):e30354. doi: 10.1371/journal.pone.0030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kroeger KM, Carville KS, Abraham LJ. The −308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Molecular immunology. 1997;34(5):391–399. doi: 10.1016/s0161-5890(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 56.Shibata J, Goto H, Arisawa T, et al. Regulation of tumour necrosis factor (TNF) induced apoptosis by soluble TNF receptors in Helicobacter pylori infection. Gut. 1999;45(1):24–31. doi: 10.1136/gut.45.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gyulai Z, Klausz G, Tiszai A, et al. Genetic polymorphism of interleukin-8 (IL-8) is associated with Helicobacter pylori-induced duodenal ulcer. European cytokine network. 2004;15(4):353–358. [PubMed] [Google Scholar]
- 58.Kunstmann E, Epplen C, Elitok E, et al. Helicobacter pylori infection and polymorphisms in the tumor necrosis factor region. Electrophoresis. 1999;20(8):1756–1761. doi: 10.1002/(SICI)1522-2683(19990101)20:8<1756::AID-ELPS1756>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 59. Sugimoto M, Furuta T, Shirai N, et al. Different effects of polymorphisms of tumor necrosis factor-alpha and interleukin-1 beta on development of peptic ulcer and gastric cancer. Journal of gastroenterology and hepatology. 2007;22(1):51–59. doi: 10.1111/j.1440-1746.2006.04442.x. They clarified the association of polymorphisms of interleukin (IL)-1beta and tumor necrosis factor (TNF)-alpha with susceptibility to peptic ulcer diseases in Japan.
- 60.Higuchi T, Seki N, Kamizono S, et al. Polymorphism of the 5’-flanking region of the human tumor necrosis factor (TNF)-alpha gene in Japanese. Tissue antigens. 1998;51(6):605–612. doi: 10.1111/j.1399-0039.1998.tb03002.x. [DOI] [PubMed] [Google Scholar]
- 61. Lu CC, Sheu BS, Chen TW, et al. Host TNF-alpha-1031 and −863 promoter single nucleotide polymorphisms determine the risk of benign ulceration after H. pylori infection. The American journal of gastroenterology. 2005;100(6):1274–1282. doi: 10.1111/j.1572-0241.2005.40852.x. They confirmed that TNF-alpha-1031 and −863 promoter SNP should be novel host factors to determine the gastric inflammation and risk of peptic ulceration upon H. pylori infection.
- 62. Zambon CF, Basso D, Navaglia F, et al. Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine. 2005;29(4):141–152. doi: 10.1016/j.cyto.2004.10.013. Evaluated several genetic polymorphisms and its correlation with Helicobacter pylori-associated diseases.
- 63. El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404(6776):398–402. doi: 10.1038/35006081. The first reported about the association between Interleukin-1 polymorphisms with gastric cancer.
- 64.Sugimoto M, Furuta T, Yamaoka Y. Influence of inflammatory cytokine polymorphisms on eradication rates of Helicobacter pylori . Journal of gastroenterology and hepatology. 2009;24(11):1725–1732. doi: 10.1111/j.1440-1746.2009.06047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Furuta T, El-Omar EM, Xiao F, Shirai N, Takashima M, Sugimura H. Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology. 2002;123(1):92–105. doi: 10.1053/gast.2002.34156. [DOI] [PubMed] [Google Scholar]
- 66.Furuta T, Shirai N, Takashima M, Xiao F, Sugimura H. Effect of genotypic differences in interleukin-1 beta on gastric acid secretion in Japanese patients infected with Helicobacter pylori . The American journal of medicine. 2002;112(2):141–143. doi: 10.1016/s0002-9343(01)01036-1. [DOI] [PubMed] [Google Scholar]
- 67. Chakravorty M, Ghosh A, Choudhury A, Santra A, Hembrum J, Roychoudhury S. Interaction between IL1B gene promoter polymorphisms in determining susceptibility to Helicobacter pylori associated duodenal ulcer. Human mutation. 2006;27(5):411–419. doi: 10.1002/humu.20299. A case-control study which determined the IL1B and IL1RN risk genotypes to H. pylori mediated duodenal ulcer.
- 68.Curfs JH, Meis JF, Hoogkamp-Korstanje JA. A primer on cytokines: sources, receptors, effects, and inducers. Clinical microbiology reviews. 1997;10(4):742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramis IB, Vianna JS, Goncalves CV, von Groll A, Dellagostin OA, da Silva PE. Polymorphisms of the IL-6, IL-8 and IL-10 genes and the risk of gastric pathology in patients infected with Helicobacter pylori . Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2015 doi: 10.1016/j.jmii.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. The Journal of biological chemistry. 2000;275(24):18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 71.Kang JM, Kim N, Lee DH, et al. The effects of genetic polymorphisms of IL-6, IL-8, and IL-10 on Helicobacter pylori-induced gastroduodenal diseases in Korea. Journal of clinical gastroenterology. 2009;43(5):420–428. doi: 10.1097/MCG.0b013e318178d1d3. [DOI] [PubMed] [Google Scholar]
- 72.Hwang IR, Hsu PI, Peterson LE, et al. Interleukin-6 genetic polymorphisms are not related to Helicobacter pylori-associated gastroduodenal diseases. Helicobacter. 2003;8(2):142–148. doi: 10.1046/j.1523-5378.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 73.Yin YW, Hu AM, Sun QQ, et al. Association between interleukin-8 gene −251 T/A polymorphism and the risk of peptic ulcer disease: a meta-analysis. Human immunology. 2013;74(1):125–130. doi: 10.1016/j.humimm.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Hull J, Thomson A, Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. 2000;55(12):1023–1027. doi: 10.1136/thorax.55.12.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ohyauchi M, Imatani A, Yonechi M, et al. The polymorphism interleukin 8 −251 A/T influences the susceptibility of Helicobacter pylori related gastric diseases in the Japanese population. Gut. 2005;54(3):330–335. doi: 10.1136/gut.2003.033050. Confirmed that the IL-8 −251A allele may be associated with progression of gastric atrophy and increase the risk of gastric ulcer in Japanese population.
- 76.Hofner P, Gyulai Z, Kiss ZF, et al. Genetic polymorphisms of NOD1 and IL-8, but not polymorphisms of TLR4 genes, are associated with Helicobacter pylori-induced duodenal ulcer and gastritis. Helicobacter. 2007;12(2):124–131. doi: 10.1111/j.1523-5378.2007.00481.x. [DOI] [PubMed] [Google Scholar]
- 77.Sugimoto M, Furuta T, Shirai N, et al. Effects of interleukin-10 gene polymorphism on the development of gastric cancer and peptic ulcer in Japanese subjects. Journal of gastroenterology and hepatology. 2007;22(9):1443–1449. doi: 10.1111/j.1440-1746.2006.04613.x. [DOI] [PubMed] [Google Scholar]
- 78.Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacology & therapeutics. 2007;116(3):496–526. doi: 10.1016/j.pharmthera.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Niwa T, Murayama N, Yamazaki H. Oxidation of endobiotics mediated by xenobiotic-metabolizing forms of human cytochrome. Current drug metabolism. 2009;10(7):700–712. doi: 10.2174/138920009789895525. [DOI] [PubMed] [Google Scholar]
- 80. Musumba CO, Jorgensen A, Sutton L, et al. CYP2C19*17 gain-of-function polymorphism is associated with peptic ulcer disease. Clinical pharmacology and therapeutics. 2013;93(2):195–203. doi: 10.1038/clpt.2012.215. Proposed that CYP2C19*17, a ultra rapid metabolizers is associated with PUD irrespective of etiology.
- 81.Baldwin RM, Ohlsson S, Pedersen RS, et al. Increased omeprazole metabolism in carriers of the CYP2C19*17 allele; a pharmacokinetic study in healthy volunteers. British journal of clinical pharmacology. 2008;65(5):767–774. doi: 10.1111/j.1365-2125.2008.03104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jainan W, Vilaichone RK. Effects of the CYP2C19 genetic polymorphism on gastritis, peptic ulcer disease, peptic ulcer bleeding and gastric cancer. Asian Pacific journal of cancer prevention : APJCP. 2014;15(24):10957–10960. doi: 10.7314/apjcp.2014.15.24.10957. [DOI] [PubMed] [Google Scholar]
- 83.Sugimoto M, Furuta T, Shirai N, et al. Poor metabolizer genotype status of CYP2C19 is a risk factor for developing gastric cancer in Japanese patients with Helicobacter pylori infection. Alimentary pharmacology & therapeutics. 2005;22(10):1033–1040. doi: 10.1111/j.1365-2036.2005.02678.x. [DOI] [PubMed] [Google Scholar]
- 84.Wang T, Zhang L, Li H, Wang B, Chen K. Prostate stem cell antigen polymorphisms and susceptibility to gastric cancer: a systematic review and meta-analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(5):843–850. doi: 10.1158/1055-9965.EPI-11-1176. [DOI] [PubMed] [Google Scholar]
- 85.Tanikawa C, Matsuo K, Kubo M, et al. Impact of PSCA variation on gastric ulcer susceptibility. PloS one. 2013;8(5):e63698. doi: 10.1371/journal.pone.0063698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tanikawa C, Urabe Y, Matsuo K, et al. A genome-wide association study identifies two susceptibility loci for duodenal ulcer in the Japanese population. Nature genetics. 2012;44(4):430–434. doi: 10.1038/ng.1109. S431-432. [DOI] [PubMed] [Google Scholar]
- 87.Salama N, Guillemin K, McDaniel TK, Sherlock G, Tompkins L, Falkow S. A whole-genome microarray reveals genetic diversity among Helicobacter pylori strains. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(26):14668–14673. doi: 10.1073/pnas.97.26.14668. [DOI] [PMC free article] [PubMed] [Google Scholar]