Abstract
Alzheimer’s disease, Parkinson’s disease, traumatic brain and spinal cord injury and neuroinflammatory multiple sclerosis are diverse disorders of the central nervous system. However, they are all characterized by various levels of inappropriate inflammatory/immune response along with tissue destruction. In the gastrointestinal system, inflammatory bowel disease (IBD) is also a consequence of tissue destruction resulting from an uncontrolled inflammation. Interestingly, there are many similarities in the immunopathomechanisms of these CNS disorders and the various forms of IBD. Since it is very hard or impossible to cure them by conventional manner, novel therapeutic approaches such as the use of mesenchymal stem cells, are needed. Mesenchymal stem cells have already been isolated from various tissues including the dental pulp and periodontal ligament. Such cells possess transdifferentiating capabilities for different tissue specific cells to serve as new building blocks for regeneration. But more importantly, they are also potent immunomodulators inhibiting proinflammatory processes and stimulating anti-inflammatory mechanisms.
The present review was prepared to compare the immunopathomechanisms of the above mentioned neurodegenerative, neurotraumatic and neuroinflammatory diseases with IBD. Additionally, we considered the potential use of mesenchymal stem cells, especially those from dental origin to treat such disorders. We conceive that such efforts will yield considerable advance in treatment options for central and peripheral disorders related to inflammatory degeneration.
Keywords: Alzheimer’s disease, CNS damage, Crohn’s disease, Dental, Immunomodulation, Inflammation, Inflammatory bowel disease, Mesenchymal stem cells, Multiple sclerosis, Parkinson’s disease, Regeneration, Spinal cord injury, Traumatic brain injury, Ulcerative colitis
INTRODUCTION
Neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), traumatic disorders such as traumatic brain injury (TBI) and spinal cord injury (SCI) and neuroinflammatory diseases such as multiple sclerosis (MS) are very diverse illnesses of the central nervous system (CNS). The common features of these deteriorating disorders are the massive tissue destruction and various level of inflammation. It is very hard or impossible to treat them by conventional medical therapies since the nervous system has only a very limited ability to newly generate neurons and glial cells due to the limited number of necessary precursor cells, especially during inflammation when regenerative regulatory mechanisms are suppressed. Therefore, novel technologies such as application of stem cells hold a great promise for their cure in the nearby future. Present knowledge suggests that there are many similarities between CNS disorders and the various forms of inflammatory bowel disease (IBD). In fact, IBD is characterized by uncontrolled inflammation and damage to host tissues and its therapy constitutes a significant challenge for the physicians. Furthermore, both CNS disorders and IBD are becoming potential targets for stem cell therapy, especially that of mesenchymal origin.
Indeed, novel state-of-the-art therapeutic approaches for such disorders include the use of mesenchymal stem cells (MSCs), multipotent progenitors present in many tissues including bone marrow, dental pulp and periodontal ligament. MSCs of dental origin have high potential for transdifferentiating various tissue specific cells not only for mesenchymal regeneration but also for neuronal and epithelial reconstruction. These MSCs have also potent immunosuppressive properties inhibiting the proliferation and function of pro-inflammatory immune cells. They also modulate the activity of regenerative regulatory cells which is mediated by cell-cell contacts and by secreted factors.
In this review, we attempted to compare the immuno- patomechanisms of neurodegenerative, neurotraumatic and neuroinflammatory diseases with IBD, and described the potential use of MSCs, especially those that can be obtained from dental pulp and periodontal ligament. The startegic goal was to provide a common platform for MSC-dependent healing of central and peripheral disorders related to inflammatory degeneration.
NEURONAL DISORDERS AS POTENTIAL TARGETS FOR MSC THERAPY
Alzheimer’s Disease
AD is the most prevalent type of neurodegeneration. It is clinically defined as the onset of progressive deficits in memory and cognition [1-3]. The pathology of AD is characterized by the deposition of insoluble β-amyloid peptides in plaques, intracellular neurofibrillary tangles and also the loss of diverse neurons throughout the brain, particularly in basal forebrain, amygdala, hippocampus and cortical area [4, 5]. AD is multifactorial, heterogeneous and not well understood. But age, sex, apolipoprotein E genotype [6] and sequential proteolytic processing of amyloid precursor protein certainly belong to its risk factors [7]. Current treatment for AD usually includes targeting cholinergic and glutamatergic neurotransmission [8, 9], activation of γ-secretase to generate nontoxic β-amyloid fragments and delivering various growth factors into the brain. These treatments may improve the symptoms, but cannot stop or reverse the degeneration and loss of neurons [10, 11]. A possible avenue for slowing down the progression or even reverse it is the interference with ongoing neuronal cell destruction [12, 13]. In this respect novel approaches interfering with inflammatory cellular and molecular interactions are required. Although in the last decade our knowledge about these processes greatly expanded [14, 15], finding the key components and pharmacologically influence them is still challenging.
Parkinson’s Disease
PD is the second most prevalent neurodegenerative disorder after AD. This disease affects millions of individuals with increasing incidence worldwide, and is caused by the preferential degeneration of dopaminergic (DA) neurons of the substantia nigra pars compacta. Symptoms include resting tremor, muscle rigidity, bradykinesia, freezing, and postural instability as well as abnormalities in speech, mood, and cognition [16, 17]. Currently available pharmacological tools, including monoamine oxidase and catechol-O-methyl transferase inhibitors, dopamine receptor agonists, amantadine and L-DOPA, as well as various surgical interventions effectively ameliorate clinical symptoms in the early stages of the disease, but they cannot completely stop or reverse the degeneration of DA neurons, and the symptoms eventually become more pronounced by time [17-20]. Furthermore, the available medications have also considerable side effects. Thus, there is an urgent need to find novel drugs targeting DA and other neurotransmitter systems, growth factors and neurotrophic agents to ameliorate both symptoms and disease progression [19, 21]. New therapeutic avenues are offered by application of stem cells and other regulatory cells interfering with the interplay of cellular and molecular factors, thereby inhibiting accompanying inflammatory processes [16, 22].
Traumatic Brain Injury
TBI is an intracranial damage caused either by an external force that impacts the head breaking through the barriers of the brain (a bump, a violent blow or even a blast) or by extreme acceleration-deceleration and rotational forces. It contributes to 30% of deaths related to injury in the United States, and it is responsible for around 50 thousand death toll each year, 300 thousand hospitalizations and more than 1 million emergency treatments [23-25]. Similar ratios apply for the European Union as well [26]. Depending on the severity, TBI may lead to dizziness, headache, loss of consciousness, bruising, bleeding or even death, and it can be classified as mild, moderate and severe, according to Glasgow Coma Scale scores 3-8, 9-12 and 13-15, respectively [23-25]. Symptoms and neurological deficit caused by TBI may last temporarily, for days, weeks or even for lifetime [23, 27].
TBI consists of two phases. The primary injury is caused by the mechanical damage of neurons, axons, glia and blood vessels, leading to direct neural cell loss, which is followed by a secondary response of multiple biochemical events with various time frames. This secondary damage is characterized by excitotoxicity, oxidative stress, disturbed calcium homeostasis, blood–brain barrier disruption, mitochondrial dysfunction and inflammation, and leads to further neuronal damage mainly in the injury site and near-by tissue [23-25], although neuro- degeneration in brain areas located far from the primary impact has also been reported [28].
Neuroinflammation plays a pivotal role in the pathomechanism of TBI [28-30]. It involves various immune cells, microglia, cytokines, chemokines and other inflammatory mediators. After the primary injury, an endogenous inflammatory response is activated to defend the injury site from pathogens and to repair the damage. Invading neutrophils, monocytes and lymphocytes secrete prostaglandins, pro- inflammatory cytokines, and other inflammatory mediators [31]. The activation of microglia induces both beneficial and detrimental effects. These cells react to acute injury within a few hours, proliferate and migrate towards the site of injury to form a barrier between the damaged and healthy tissue [32]. But their excessive activation leads to increased production of numerous proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, IL-1β, IL-6 and IL-12, as well as to the release of other neurotoxic substances, including nitric oxide (NO) and superoxide free radicals [32, 33]. In addition, activated microglia increase the expression of major histocompatibility complex class II molecules [34], which is also directly correlated to neurodegeneration. However, it is important to note that although activated microglia often act as a major contributor in the inflammatory response, they may promote the opposite effect under appropriate conditions [35]. When exposed to IL-4 or IL-13, microglia can polarize into M2 (or alternatively activated) phenotype (in contrast to the classically activated M1 microglia), and suppress inflammation by reducing the production of proinflammatory cytokines and increasing anti-inflammatory ones, such as IL-10 and transforming growth factor (TGF)-β [32]. All these processes are supposed to be controlled not only by the immune system, but also by an immuno-neuro-stem cell interplay [36].
Spinal Cord Injury
SCI induces motor and sensory deficits impairing the functional activity of the spinal cord, and significantly affects expectancy and quality of life of exposed individuals. The estimated annual incidence of SCI is 2-4 cases per hundred thousand persons [37]. SCI, just like TBI, is followed by primary and secondary injury responses. Primary injury is due to the direct mechanical damage on axons, neuronal cells and blood vessels, while secondary injury is a response to the primary one [38, 39]. It involves extensive chemokine and cytokine liberation and disturbed blood flow, breakdown of the blood-brain barrier, apoptosis, excitotoxicity and production of free radicals [38, 39]. The neuroinflammatory process is very similar to that described above for TBI [40]. Besides surgical interventions, current treatments aim to prevent secondary injuries, promoting regeneration and replacing destroyed spinal cord tissue [40].
Multiple Sclerosis
MS is an immune-related neurodegenerative disorder of the CNS, affecting over 1.3 million people worldwide. It is characterized by the progressive demyelination of nerves in the brain and spinal cord [41, 42]. MS originates from blood-brain barrier disintegrity [43]. Histologically it shows lymphocyte infiltration, loss of oligodendrocytes, demyelination and widespread axonal damage [44, 45]. The consequence is the loss of neurological function with symptoms including pain, muscle spasms, loss of sensation and coordination, impaired vision and emotional distress. Current treatments for MS include the administration of interferon beta, glatiramer acetate, mitoxantrone [46], or dimethyl fumarate [47] but there is no real medical cure for MS for the time being.
During the course of MS, myelin-reactive CD4+ T cells, which secrete interferons and interleukins, play an important role [48]. The best current animal model for MS is experimental autoimmune encephalomyelitis (EAE) [49]. In EAE mice are immunized to induce an immune response to CNS associated antigens. EAE can also be induced by activated myelin-specific CD4+ T cell administration. EAE can follow distinct clinical courses depending upon the mouse strain used and the specific antigen against which the immune response is raised, each reflecting different aspects of MS in humans [49].
INFLAMMATORY BOWEL DISEASE AS POTENTIAL TARGET FOR MSC THERAPY
To eliminate pathogenic bacteria while tolerating harmless commensals, the host immune system must maintain a balance between inflammation and tolerance in the gastrointestinal tract. Disruption of this balance may cause a chronic inflammatory condition that ultimately damages the host tissues in the gut leading to IBD in the colon. Multiple factors are involved in the development of IBD but a common denominator is an initial microbial trigger, followed by an inappropriate polarization of both innate and adaptive immune mechanisms toward an inflammatory phenotype and the down-modulation of regulatory immune mechanisms [50].
IBD, an umbrella term for both Crohn’s disease (CD) and ulcerative colitis (UC), encompasses chronic inflammatory disorders of the gastrointestinal tract affecting 2.2 million people in Europe and 1.4 million people in the USA [51]. The chronic inflammation of the gut causes a wide range of clinical symptoms, including nausea, diarrhoea, abdominal pain/discomfort and bloating, even with absorption problems [52]. The course of the disease is intermittent with acute relapses. The chronic inflamed state of the gut may result in fibrosing strictures, fistulas and increased risk of malignancies [51, 53]. The high prevalence of IBDs (~ 0.4% worldwide) [54] and the costs of the long-term treatment of the patients place a significant burden on the healthcare system: the expenses exceed 1.7 billion dollars per year in the United States [55], and are in similar range in European countries.
Immunopathomechanism of Inflammatory Bowel Disease
The exact immunopathomechanism of IBD is unknown, but it is believed to result from an inappropriate immune response of the intestinal immune system to commensal bacteria and other luminal antigens [56] since the immune system falsely recognizes commensals as pathogens. Complex interactions between genetic, bacterial, and immunological factors are thought to contribute to this process [53, 57-59]. Certainly, an important element in the development of IBD is the barrier disruption leading to a change in the intestinal flora and a consequent aberrant activation of the mucosal immune system [60, 61]. In both CD and UC, isolated diseased intestinal epithelial cells activate CD4+ T cells in vitro [62] suggesting that non-immune cells are also able to contribute directly to the inappropriate immune behavior. The resulting inflammation is strongly dependent on CD4+ T helper cells in experimental animals [63]. CD is driven by increased Th1 and Th17 responses, with associated cytokines interferon (IFN)-γ, IL-12, IL-17 and IL-18 [64-66] UC is accompanied by a similar but not identical phenomenon: it is associated with a T cell profile skewed toward Th2 polarization compared to CD and higher mucosal levels of IL-13 [60, 65]. Thus, although T cell dysfunction is not the initiating factor in IBD [67], misregulated Th cell responses seem to play a central role in the progress of the chronic inflammatory process [68].
Different macrophage populations play distinct roles in the pathogenesis of IBD. In the lamina propria of healthy mouse intestine, CX3CR1+ macrophages were shown to express anti-inflammatory molecules such as IL-10 and to induce differentiation of Foxp3+ regulatory T (Treg) cells [69, 70]. On the other hand, lamina propria CD11b+ dendritic cells, which promote the differentiation of Th17 cells by increasing IL-17 production [69] were also identified. IL-17 production was suppressed by CX3CR1+ macrophages. Importantly, the existence of distinct macrophage populations has been shown under resting conditions and during inflammation in mouse colon [71]. In normal mice, a large number of F4/80+ macrophages were detected in colon lamina propria, expressing negligible amounts of TNF-α, negative for TLR2 and CCR2 but expressing high levels of CX3CR1 [71]. In ulcerative colitis F4/80+ macrophages express high levels of TLR2, CX3CR1, CCR2 and TNF-α [71]. The presence of two distinct macrophage populations has been shown in human intestine as well. Resident intestinal macrophages were negative for CD14 and produced no inflammatory cytokines [72]. Another inflammatory macro- phage subset was also identified from the intestine of CD patients [73]. These macrophages were positive for the CD14 bacterial LPS co-receptor and produced increased amounts of the proinflammatory cytokines such as IL-23, TNF-α and IL-6 compared to CD14- intestinal macrophages isolated either from healthy subjects or from UC patients [73]. Taken together, the above findings suggest that different macrophage populations exist in the intestine, each having a specific role either in maintaining immune tolerance in health or in perpetuating inflammation in IBD.
Dendritic cells (DC) are the key regulators of immunity against pathogens and tolerance toward commensals and therefore play important roles in intestinal immune homeostasis [74]. In healthy intestine DCs drive the differentiation of naive T cells into regulatory rather than effector T cells [75]. Conversely, an increased number of mature DCs was observed compared to controls in inflamed human colon mucosa [76]. Accordingly, two different populations of DCs have been identified in intestinal lamina propria that mediate either defense or tolerance [74]. One derives from common DC precursors via pre-classical dendritic cells and displays a CD103+ CX3CR1- phenotype [77, 78], while the other, CD14+ CX3CR1+ subset is derived from Ly6C+ circulating monocytes [77, 78]. When DCs were depleted and spontaneous self-reconstitution was allowed, mice developed only mild inflammation after a colitogenic challenge [78]. CD103+ DCs inhibit inflammation by potently inducing Treg cells via the production of IL-10, TGF-β and retinoic acid [79]. Others have also shown that CD11b+ CD103+ dendritic cells suppress intestinal inflammation by Treg cell activation [80, 81]. In summary, DCs tip the balance between a tolerogenic and a pro- inflammatory immune response by promoting a regulatory or an effector T cell response, respectively.
Adaptive immunity plays a key role in the development of IBD. The classical view held that the intestinal mucosa of patients with CD was dominated by Th1 cells, whereas Th2 cells were dominant in UC lesions [82]. However, certain characteristics of CD and UC were hard to explain based solely on the Th1/Th2 paradigm, and in the last years the involvement of Th17 cells also became evident in IBD both in humans and in animal models. Elevated numbers of Th17 cells are observed in the mucosa of both CD and UC patients compared to normal mucosa [83, 84] and IL-17 is upregulated [83, 84]. Furthermore, IL-17 receptor knockout mice are protected against experimental IBD [85]. The above results underline the role of Th17 cells and IL-17 signaling in IBD. In addition, IL-23, a key cytokine in Th17 development, was also shown to drive innate and T cell mediated intestinal inflammation [86, 87].
Regulatory T cells have been shown to be potent suppressors of intestinal inflammation. The cotransfer of natural Treg (nTreg) cells with naive CD4+ T cells prevents the development of colitis [88]. Moreover, nTregs can also suppress innate intestinal inflammation [89]. The mechanisms by which Treg cells suppress inflammation in the intestine are not entirely clear, but involve the immunoregulatory cytokines TGF-β and IL-10, and the co-stimulatory molecule CTLA-4 [90]. TGF-β inhibits immune responses by several mechanisms: inhibition of antigen-induced activation, pro- liferation and differentiation of T cells, inhibition of DC maturation and thus favoring the induction of Treg cells, and inhibiting the function of activated macrophages [91]. TGF-β is an important but not the solely factor for the Treg-mediated prevention of colitis [92, 93]. IL-10 has been shown to suppress inflammatory responses in both innate and adaptive models of intestinal inflammation [89, 94]. The role of CTLA-4 in nTreg function is not fully understood [90], but a CTLA-4 agonist has been shown to induce indoleamine 2,3-dioxygenase (IDO) in DCs [95], and IDO expression by antigen presenting cells inhibits T cell proliferation and promotes tolerance [96]. Furthermore, CTLA-4 promotes Foxp3 induction and Treg accumulation in the intestinal lamina propria [97].
STEM CELL THERAPY FOR INFLAMMATORY DISEASES
Potential Stem Cell Sources for Tissue Reconstruction
Stem cells by definition have capacity for unlimited self-renewal and ability to form differentiated cells. They can be classified into totipotent, pluripotent, multipotent and unipotent types by their developmental and differentiation potential or into embryonic, fetal, postnatal (also called adult) and induced stem cell categories by their origin. Embryonic stem cells (ESC) received great attention due to their pluripotency. Recent animal experiments provided evidence that ESC derivatives may provide functional cell replacements in various diseases such as PD [98], and clinical trials are currently in progress utilizing human ESC (hESC) based therapy for macular degeneration and SCI [99]. However, ethical and legal concerns also arise in conjunction with their application. Additionally, transplanted ESCs may induce immune response [100-102] and form teratomas in vivo [103]. Reprogramming of mature somatic cells to acquire ESC like characteristics is an alternative idea to avoid most concerns about ESCs. These induced pluripotent stem cells (iPSC) may be derived from the patient’s own tissue, bypassing most immunological and ethical problems associated with classical ESCs. Yamanaka received Nobel prize for inventing retroviral vector enforced expression of four key transcription factors, Oct4, Sox2, Klf4 and c-Myc to reprogram mouse fibroblasts to pluripotency and achieving similar developmental capability as ESCs [104]. Since retroviruses may induce cancer [105-107], the technology was further modified to produce iPSCs without any stable genomic modification of the target cells in both mouse [108, 109] and human [110, 111] models. Unquestionably, the fundamental reprogramming of postnatal cells has a great potential, but there are important problems to solve as well. The nature of the reprogramming process remains obscure, and the developmental potential of iPSCs derived by different methods from different tissues is unknown as yet [112]. Therefore, alternative cell sources for stem cell applications also have to be considered.
At present the most promising candidates among adult stem cells for clinical applications are the MSCs, a class of multipotent cells originally identified in bone marrow [113, 114]. Their high plasticity, in addition to their presence even in adult humans validate them attractive candidates for therapeutic use. Yet, standardized, reliable protocols are still to be developed for differentiating these cells into target tissues in large quantities [115, 116].
Mesenchymal Stem Cells
Since MSCs have been discovered in bone marrow, a number of other tissues such as brain, skin, skeletal muscle and the gastrointestinal tract have been shown to contain similar cell populations [117-119] including oral tissues [120]. It has also been demonstrated that MSCs have the potential to directly differentiate into multiple lineages of mesenchymal origin, including bone, cartilage, fat, connective tissue, smooth muscle and hematopoietic supportive structures [121].
An important selection criterion for MSCs is their plastic adherence and capacity to differentiate into osteoblasts, adipocytes and chondrocytes in vitro [122]. These cells express a variety of cell surface marker proteins such as CD73, CD90 and CD105. Additionally, MSCs do not express major histocompatibility complex class II surface molecules, endothelial CD31, and hematopoietic-specific markers CD34, CD45 and CD14 [121, 122]. Isolated MSC cultures are heterogeneous. Various research groups proposed specific cell surface markers to identify MSCs such as STRO1 (stromal precursor antigen 1) [123], GD2 (ganglioside 2) [124], SSEA4 (stage-specific embryonic antigen-4) [125], and CD146 [126], but the optimal combination of selection factors still needs to be identified.
Mesenchymal Stem Cells as Immunomodulators
One of the most intriguing features of MSCs is that they are not only able to facilitate tissue regeneration by differentiating into several cell types, but also able to escape immune recognition and to inhibit immune response. Therefore, MSCs may serve as immunosuppressive agents in inflammatory diseases, and other autoimmune diseases [127, 128]. These immunomodulatory effects of MSCs seem to be achieved by multiple mechanisms inhibiting pro-inflammatory cells and at the same time activating other immune cells that shift the balance toward regeneration.
First of all, MSCs inhibit T cell proliferation in mixed lymphocyte cultures in vitro [129-133]. The effect is primarily mediated by soluble factors [127, 134] such as TGF-β [130], hepatocyte growth factor [130], IL-10 [135] and prostaglandin E2 (PGE2) [136]. MSCs also block T cell proliferation by the secretion of IDO [137, 138]. The production of nitric oxide (NO) is also a potential mechanism by which MSCs inhibit T cell proliferation [139]. MSCs can also block pathogenic B cell response [140, 141]. Natural killer (NK) cells are parts of the innate immune system, having antitumor and antiviral effects due to their cytotoxic potential and secretion of pro-inflammatory cytokines such as TNF-α and IFN-γ. They contribute to inflammatory conditions in IBD [142]. MSCs suppress proliferation and secretory activity of NK cells [143, 144].
MSCs also modulate the immune system by acting on antigen presenting cells. The differentiation of CD34+ hematopoietic stem cells into DCs was prevented by MSCs [145]. MSCs also inhibit the differentiation of CD14+ human monocytes into dendritic cells and reduce their IL-12 secretion [146]. Another study essentially confirmed these results, reporting in addition that the inhibition of DC maturation but not differentiation was dependent on soluble factors [147]. MSCs alter the cytokine secretion profile of DCs as well, converting them into a regulatory phenotype [135, 136]. In an experimental cecal ligation and puncture sepsis model, PGE2 secreted by exogenously administered MSCs was reported to reprogram macrophages to produce IL-10, a potent anti-inflammatory cytokine, thereby sparing mice from dying of sepsis [148]. These findings show that MSCs suppress the differentiation and activation of antigen presenting cells and shift their development toward a regulatory phenotype.
MSCs may modulate the induction of CD4+ CD25+ Treg cells, too [136, 149]. MSCs prevent diabetes mellitus in NOD mice via the induction of IL-10 producing Treg cells [150]. The induction of CD4+ CD25+ FoxP3+ Treg cells by MSCs results in increased allograft tolerance in kidney transplantation [151]. Other studies have also shown that the effects of MSCs in autoimmune diseases at least partly due to the generation of antigen-specific CD4+ CD25+ FoxP3+ Treg cells [152, 153]. MSCs inhibit the differentiation and maturation of monocyte-derived dendritic cells and augment the generation of Treg cells [154].
The immunosuppressive effects of MSCs are partly mediated by cell–cell contact [154], however, soluble factors such as PGE2 and TGF-β1 also play a role [155]. Human gingiva-derived MSCs suppress peripheral blood lymphocyte proliferation by inducing IL-10, IDO, inducible NO synthase (iNOS) and cyclooxygenase 2 (COX-2) in experimental colitis [156]. Another study showed that the immuno- suppressive effect of MSCs is mediated by the secretion of galectin-3, a protein known to modulate T cell proliferation, gene expression, cell adhesion and migration [157].
Activation and Polarization of MSCs
Under resting conditions MSCs possess antiapoptotic and supporting properties toward different cells types such as hematopoietic stem cells, T cells, B cell precursors, plasma cells and neoplastic cells. Also, MSCs may act as antigen presenting and pro-inflammatory cells. Thus, the immuno- modulatory activity is not constitutively exerted by MSCs, but depends on a process of ‘licensing’ to be acquired. This involves a functional polarization depending on the cytokine milieu and toll-like receptor stimulation. Initial priming of human MSCs with either IFN-α or IFN-γ synergizes with downstream TLR3 or TLR4 stimulation to enhance the production of pro-inflammatory cytokines. The concentration of inflammatory cytokines has also been postulated to regulate MSC polarization. IFN-γ, IL-1 and TNF-α are key elements of the activatory milieu. Their induction of iNOS and NO production have been shown to be an effector mechanism inhibiting T cell proliferation [158].
As the proper activation of the MSCs is crucial to achieve the anti-inflammatory phenotype, the failure of some MSC-based protocols for immune modulation in animal models and in human clinical trials may be explained by either lack of a proper licensing by inflammatory microenvironment or incorrect timing in MSC administration [159]. Thus, optimization of MSC administration for immune-regulating purposes is required to maximize their beneficial effects.
MSCS FOR HEALING AND REGENERATION OF NEURONAL DISEASES
Alzheimer’s Disease as MSC Target
The potentially beneficial mechanisms of MSCs in the treatment of AD are not well understood, but certainly multifactorial. MSC-derived neurons have the capability to integrate into the existing neural networks of the host brain [160]. The release of neurotrophic and immunomodulatory factors from MSCs may improve the host environment [22]. MSC-based therapy may have neuroprotective effect by secretion of neurotrophic factors such as NGF and brain-derived neurotrophic factor (BDNF) [161-164]. Since AD is accompanied by chronic inflammation, MSCs exert anti-inflammatory effects by reducing pro-inflammatory cytokine release and upregulating expression of anti-inflammatory factors including IL-10 [165-167]. Abnormal accumulation of β-amyloid peptide is regarded to be an important pathological sign in AD, and its inhibition has been implied to delay AD progression. Engrafted MSCs have been demonstrated to eliminate deposited β-amyloid plaques brain through differentiation into microglia or recruitment of activated endogenous microglia in AD [12, 168].
Microglia cells exist in a quiescent state, but they prevent and/or clear β-amyloid peptide deposition when activated [169, 170]. MSCs activate and induce migration of microglia when exposed to β-amyloid peptide in vivo [171]. An alternative activation of microglia in AD mice is associated with elevated chemokine expression by MSCs [168]. MSCs can induce a neuroprotective effect in harmful microglia by increasing expression and release of CX3CR1, thus contributing to their beneficial effects [172, 173]. MSCs themselves can also differentiate into microglia and eliminate β-amyloid peptide deposition [166]. Additionally, MSCs could turn on neuroregeneration inducing neurogenesis, neuroplasticity, and neurorestoration in AD [1, 174-176].
Parkinson’s Disease as MSC Target
Several preclinical studies suggest that MSCs may have a great therapeutic value in PD [177, 178]. Various types of MSCs, including bone marrow-derived MSCs [179], adipocyte- [180] and umbilical cord-derived SCs [181] were shown to induce neuroprotection both in vitro and in vivo. Moreover, this neuroprotection can be achieved not only by engrafting MSCs directly into the striatum [179], but also by applying them via other routes, such as intranasally [182] or intravenously [183].
In the last years several mechanisms were implicated in the regenerative effect of MSCs in PD [22]. Although transdifferentiation of MSCs into DA neurons or their fusion with host neurons may also contribute to their beneficial effect [184, 185], several lines of evidence indicate that the neurological improvement observed after MSC-treatment is mainly due to secretion of bioactive factors [178]. MSCs secrete a wide array of growth factors; many of which, such as BDNF, glial-derived neurotrophic factor (GDNF), nerve growth factor (NGF), fibroblast growth factor 20 (FGF20) and basic fibroblast growth factor (bFGF) elicit potent trophic and neuroprotective effects on nigrostriatal DA neurons [180, 186]. MSCs transplanted into the striatum of 6-OHDA-lesioned rats differentiated toward an astroglial phenotype and increased GDNF expression, which resulted in neuroprotection [179]. There is evidence that neurotrophic factors released by grafted MSCs not only promote neuronal regeneration, but also induce proliferation and differentiation of endogenous neural stem cells, which thereafter release various endogenous neurotrophins [187]. Furthermore, MSCs secrete a variety of neuroregulatory molecules, cytokines and chemokines, which also promote neuronal survival and differentiation [187, 188].
Neuroinflammation has been recognized recently as an important contributor to the pathogenesis of PD. In fact, anti-inflammatory drugs are emerging as novel therapeutic tools to suppress neuronal degeneration [189, 190]. Therefore, it is of interest that MSCs are able to induce immunomodulatory and anti-inflammatory actions. For instance, MSC treatment has been demonstrated to decrease LPS-induced microglial activation and TNF-α and iNOS production, resulting in a significant reduction of DA neuronal loss [191]. Other mouse studies showed that MSCs promote the recovery of blood-brain barrier integrity and protected DA neurons from pharmacological toxicity through decreased microglial activation [192]. Thus, these studies underline the importance of MSC-induced immunomodulatory and anti-inflammatory effects in treatments for PD.
Traumatic Brain Injury as MSC Target
As depicted above, several biochemical events take part in the secondary phase of TBI, and considerable effort has been invested to find novel approaches to inhibit these processes and reduce the neuronal damage. A large number of preclinical studies suggest that various stem cells, including MSCs may promote the regeneration in TBI [24]. Similar to other neurodegenerative diseases, the beneficial effect of MSCs in TBI was originally attributed to their ability to differentiate into neurons and replace the damaged cells [193, 194]. However, currently it is believed that secretion of cytokines, chemokines and growth factors, inhibition of inflammation and enhancement of endogenous neurogenesis and angiogenesis are equally if not more important factors in the therapeutic effect of stem cells [36, 195].
MSCs are able to influence several processes during the secondary phase of tissue response during the course of TBI. They effectively inhibit neuroinflammation due to their potent immunomodulatory properties. Stereotactic or intravenous injection of MSCs was shown to decrease the level of various pro-inflammatory cytokines, such as IL-1β, IL-6, IL-17, IFN-γ or TNF-α both in the serum and brain after cerebral injury [36, 195]. MSCs migrate to the injury site and modulate the inflammatory response activating T lymphocytes, neutrophils, and microglial cells. The immunomodulatory effect of MSCs is at least partly due to the secretion of the TNF-α-stimulated gene/protein 6 (TSG-6), which possess potent anti-inflammatory action by suppressing the NF-κB signaling pathway [36, 196] and inactivating the pro-inflammatory hyaluronan [197]. Additionally, MSCs reduce the production of chemokines CXCL2 (or MIP-2) and CCL2 (or MCP-1) secreted by microglia and neurons. These are important chemoattractants for various immune cells and play significant role in the secondary brain damage [36].
Besides their direct immunomodulatory properties, MSCs have additional beneficial effects in TBI. Intravenous administration of MSCs significantly improved blood-brain barrier function by reducing endothelial permeability, increasing VE-cadherin expression and VE-cadherin/β-catenin interaction [198]. Furthermore, hMSC-conditioned medium reduced superoxide production in an TBI model in vitro, suggesting that MSCs are also able to regulate the oxidative stress [199]. These studies clearly demonstrate that MSC-treatment induces numerous favorable effects in TBI and effectively reduces the secondary damage.
Spinal Cord Injury as MSC Target
Just like in other neuronal disorders, MSCs could be beneficial in SCI primarily because of their anti-inflammatory, immunomodulatory [200] and neuroprotective [201] effects. Moreover, the trophic effect of MSC-secreting pro-survival factors such as insulin-like growth factor (IGF), BDNF, vascular endothelial growth factor (VEGF), granulocyte-macrophage colony stimulating factor (GMCSF), FGF2 and TGF-β [202-206] could be of importance. Indeed, the healing actions of MSCs were confirmed by several investigations in SCI. The effects were attributed to secretion of neurotrophic factors [207, 208] and anti-inflammatory cytokines [209-211]. Furthermore, MSCs promoted axonal growth and sprouting in multiple species [212, 213].
Despite the large number of studies, very little is known about the exact mechanisms by which MSCs ameliorate SCI [214]. A recent study has shown that MSCs could alleviate spinal cord inflammation by weakening TLR4-mediated signaling pathways and reducing tissue levels of IL-1β and TNF-α [215]. In addition, MSCs exhibit marked anti-proliferative and anti-apoptotic effects leading to the reduction of inflammation in the site of SCI [209, 216-220]. Importantly, following SCI, MSC grafts modify the inflammatory environment by shifting macrophages from pro-inflammatory to anti-inflammatory phenotype, sparing axons and myelin [221]. MSCs also provide a permissive environment by stimulating angiogenesis [222]. All these factors contribute to healing, but probably the synergism of the above mechanisms is even more important.
Multiple Sclerosis as MSC Target
As described above, EAE is the best known and most commonly used model for MS showing both immunological and clinical manifestations of the human disease [45]. Transplantation of MSCs into EAE mice resulted in rapid and sustained functional recovery due to the reduced number of inflammatory myelin-specific Th1 cells and astrocytes as well as an increased number of inflammatory-inhibiting Th2 cells, oligodendrocytes and neurons. The effect was strong only if MSCs were administered prior the onset of the disease but not afterwards [223]. Human MSCs were similarly effective in EAE [224] suppressing the disease. The mechanisms of the MSC-mediated immunosuppression in EAE are not well understood. The reduction of inflammatory cytokine secreting T cells [225], and diminished T cell response to antigenic stimulation have been observed [223]. MSCs appear to mediate immune suppression at least partly by CCL2 chemokine [225] since CCL2-/- MSCs are unable to suppress disease [225]. These data along with other observations demonstrate that a defective MSC function may be important in the pathogenesis of EAE, because MSCs deriving from mice with ongoing disease were unable to suppress disease upon transfer to autologous recipients [42, 226]. Human clinical trials have also reported promising preliminary results for the use of MSCs. Besides beneficial clinical measures, MSCs increased the proportion of CD4+/CD25+ Treg cells, whereas the proliferative responses of lymphocytes decreased [227-229].
INFLAMMATORY BOWEL DISEASE AS MSC TARGET
In the past years a several animal studies were conducted to analyze the effect of various types of MSCs in colitis models; Table 1 provides a non-exhaustive list. MSCs isolated from human or murine adipose tissue, for instance, were shown to alleviate colonic inflammation by decreasing the level of inflammatory cytokines and chemokines, increasing IL-10 levels, inhibiting Th1 cell expansion and inducing Treg cells both in trinitrobenzene sulfonic acid (TNBS)-induced colitis [152] and in dextran sulfate sodium (DSS)-induced colitis [153] in mice. In rats, topical application of MSCs was reported to accelerate healing in TNBS-induced colitis, an effect thought to be mediated by increased production of TGF-β1 and VEGF [230]. In a DSS-induced rat experimental colitis model, intravenously administered MSCs reduced clinical signs of colitis and the expression of inflammatory cytokines in the rectum and accumulated within the lamina propria in inflamed regions [231].
Clinical trials suggest that MSCs may be a promising therapeutic options in IBD. In a phase I trial, the intravenous use of expanded autologous MSCs was reported to be safe in the treatment of refractory CD, even though only a third of the patients showed favorable clinical response [232]. Allogenic MSCs administered intravenously to 10 CD and 39 UC patients, however, resulted a beneficial effect on the clinical and morphological presentation of the inflammatory process in 80% of the patients [233]. Local/intrafistular use of MSCs was also shown to be effective in fistulizing CD: in a human phase II clinical trial, local injection of ASCs dramatically increased the healing of perianal fistulas both in CD and non-Crohn’s patients [234]. Likewise, intrafistular autologous MSC injections resulted in the closure of fistula tracks (70% complete, 30% incomplete), rectal mucosal healing and increased numbers of mucosal and circulating Treg cells with no side effects in CD patients [235].
MSCS OF DENTAL ORIGIN - NEW SOURCES FOR REGENERATIVE MEDICINE
Stem cells of dental origin, such as those isolated from the dental pulp (DPSC), exhibit mesenchymal stem cell
characteristics [120, 236, 237]. These cells have multiple differentiation potential towards osteogenic and odontodenic directions as well as neurogenic, adipogenic and chondrogenic lineages [120, 236-241]. Previous studies provided evidence that not only the human and rat dental pulp stem cell cultures, but also those originating from the periodontal ligament (PDLSC) may serve as MSC sources for regenerative purposes [50, 238, 242].
Potential for MSCs of Dental Origin in the Therapy of Neuronal Diseases and IBD
In addition to their potential for self-renewal and multi-lineage differentiation capacities, dental MSCs have strong anti-inflammatory and immunomodulatory properties comparable to other MSCs [243, 244]. In this respect it is important to note that oral MSCs derive from neural crest cells, whereas bone marrow MSCs originate from the mesoderm. Therefore, some differences in their behavior are predictable [245, 246].
DPSCs were shown to inhibit the proliferation of stimulated T cells [131, 247]. This immunomodulatory activity indicates that DPSCs could be suitable to suppress T cell-mediated graft-versus-host reaction in bone marrow transplantation [133]. Additionally, DPSCs inhibit peripheral blood mononuclear cells (PBMCs) proliferation induced either by mitogenic signals or allogeneic mixed lymphocytes. This effect is most likely mediated at least in part by TGF-β, acting on its receptors on PBMCs [248]. Toll-like receptors may be able to trigger the immunosuppression by DPSCs through the increased expression of TGF-β and IL-6 [249]. Indeed, immature human DPSCs were shown to induce immunosuppression in a canine model of Duchenne muscular dystrophy leading to the relief of symptoms [250]. Recent investigations have demonstrated that human and rat DPSCs are able to induce apoptosis of activated T cells in vitro [251]. In addition, in DSS-induced colitis in mice, rat DPSCs exhibited a very strong ameliorating effect when infused systemically [251].
The DSS-induced induced colitis in mouse is a well-established model of ulcerative colitis. Although the exact mechanism is not known, it is likely that the mucosal damage results from the complex maladaptive immune response triggered by the proinflammatory content of the bowel disseminating through the DSS caused epithelial barrier destruction [156, 252, 253]. The transmembrane protein Fas ligand (FasL) plays an important role in inducing the “Fas/FasL” apoptotic pathway. FasL silencing by siRNA in DPSCs resulted in the reduction of T cell apoptosis in cell culture and also strongly inhibited the deteriorating effect of DSS in the mouse colon [251]. The therapeutic mechanism may be related to the reduced colonic infiltration of inflammatory cells and down-regulation of inflammatory cytokines [251]. Other mechanisms may involve increased infiltration of Treg cells and the expression of anti-inflammatory cytokine IL-10 at the colonic sites [156]. Interestingly, FasL knockdown in rat DPSCs resulted in a reduced capacity to ameliorate colitis phenotypes, indicating that FasL is required for DPSC-mediated immunoregulation. Moreover, in vitro co-culture assays demonstrated that DPSCs induced apoptosis of Th17 but not Treg cells, suggesting that such FasL-mediated apoptosis was specific for T-helper cells. This finding is consistent with previous observations using bone marrow MSCs [252]. Furthermore, FasL knockdown did not affect the multipotent differentiation capacity of DPSCs, suggesting that the role of FasL may be limited to controlling the interaction between DPSCs and immune cells [251].
In co-culture studies, PDLSCs suppressed the proliferation of PBMCs without promoting their apoptosis [254]. In another study, Wada and colleagues found that PDLSCs, DPSCs and BMSCs induced the production of TGFβ1, hepatocyte growth factor and IDO, which in turn suppressed PBMC proliferation [248]. When PDLSC cell sheets were applied in a minipig periodontitis model in vivo, no immunological rejection was observed. Instead, PDLSCs ameliorated the experimental periodontitis in this model by PGE2-dependent manner, suppressing the activation of T cells [255]. The inhibitory effect on T cell proliferation was diminished using PDLSCs isolated from inflamed PDL. It was also shown that activated PBMCs exhibited significantly less induction of CD4+ CD25+ Foxp3+ Treg cells and IL-10 secretion in the presence of PDLSCs from inflamed tissue, than those co-cultured with normal PDLSCs. Furthermore, suppression of Th17 differentiation and IL-17 production were significantly less by these cells than normal PDLSCs [256]. Taken together, these data show that PDLSCs exert their immunomodulatory effects via similar mechanisms as BMSCs or DPSCs.
At present, information on the beneficial effects of dental originated MSC on neuronal disorders are scarce, but very promising. Certainly, DPSCs are able to differentiate into neurons in vitro when appropriate environment is formed and the right combination of neurodifferentiation agents are applied [238, 240, 257]. These neuro-differentiated cells are able to survive and integrate into the damaged brain [258]. Furthermore, DPSCs were shown to enhance post-stroke functional recovery, probably at least in part through non-neural replacement mechanisms [259]. DPSCs also promoted locomotor recovery after complete transection of the rat spinal cord by activating multiple mechanisms [260]. One of the identified potential mechanisms is the direct induction of endogenous axon guidance, a process which is very important for complex neuronal regeneration [261].
Taken together, MSCs of dental sources represent a high, but a yet not fully explored potential for treating neurodegenerative traumatic, and inflammatory diseases independent whether they appear in the central nervous system, the gut or other organs. However, human therapeutic use requires far more preclinical and clinical investigations.
TREATMENT OF DSS-INDUCED COLITIS WITH HDPSCS IN MICE – OUR OWN EXPERIENCE
To investigate the effect of human DPSCs on IBD, our group used the DSS-induced mouse colitis model. Human DPSCs were obtained from impacted wisdom teeth, the isolation procedure was carried out as previously reported [239, 240]. In brief, DPSCs were cultured in αMEM supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin under standard conditions (37 ˚C, 5% CO2). Cells from early passages (up to P3) were used for experiments.
Acute colitis was induced in 8 weeks old female C57BL/6 mice by adding DSS (3%, molecular weight: 35 000-55 000 Da; TdB Consultancy, Uppsala, Sweden) to the drinking water (tap water) for 7 days (see protocol, Fig. (1A). The DSS-containing drinking water was made up fresh every second day to avoid bacterial contamination. Food and water were available ad libitum; the consumption of water and food was monitored in all groups. Body weight was measured daily during the course of the treatment. The development of colitis was evaluated by recording disease activity index (DAI) covering general appearance (score: 0-1), stool consistency (score: 0-3) and presence of faecal blood (score: 0-3) summed to a total score ranging 0 to 7 (modified from Reichmann et al. [262]). Human DPSCs were injected intravenously at day 3 after colitis induction, at a dose of 1 x 105 cells/10 g body weight in 100 μL saline (based on the protocol of Zhao et al. [251]). At day 9 after colitis induction mice were sacrificed by decapitation and their whole colon were removed and the reduction of colon length measured to assess colonic inflammation [263]. All experimental procedures were carried out according to ethical guidelines issued by the Ethical Board of Semmelweis University, based on EC Directive 86/609/EEC and were approved by the National Scientific Ethical Committee on Animal Experimentation and permitted by the government (Food Chain Safety and Animal Health Directorate of the Central Agricultural Office, Permit no. PEI/001/1493-4/2015). Data are expressed as means ± S.E.M. Statistical analysis of the data was performed either with one-way ANOVA followed by Bonferroni post-hoc test, or with two-way repeated measures ANOVA in order to compare the time course of disease activity indices and weight losses between different groups. A probability of p<0.05 was considered statistically significant.
Fig. (1).
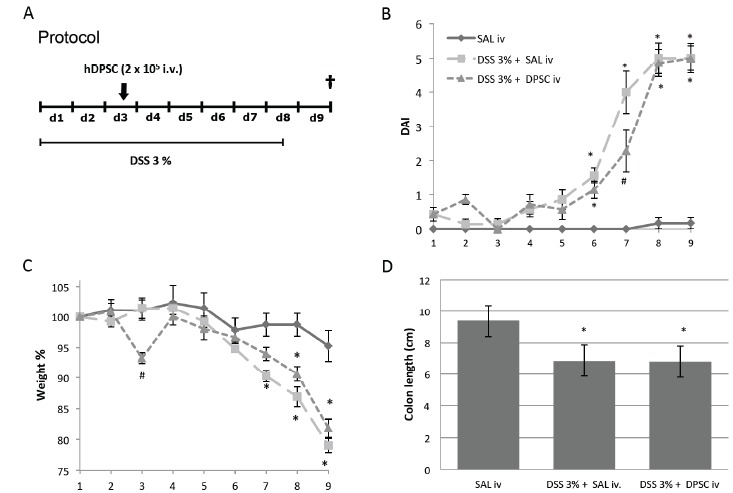
Effect of intravenously administered human DPSCs on acute experimental colitis in mice. Animals were treated with DSS (dextrane sulfate sodium) for 7 consecutive days, and sacrificed at day 9. DPSCs were administered at day 2 Fig. (1A). DSS-induced a moderate to severe colitis resulting in an increased disease activity index (DAI; significant elevation from day 6, Fig. (1B), marked weight loss (significant from day 7, Fig. (1C) and shortened colon on day 9 (upon sacrifice) Fig. (1D). Treatment with hDPSCs delayed the development of colitis, indicated by a lower DAI value (2.3 ± 0.6 vs. 4.0 ± 0.6, p<0.001) and the lack of significant weight loss on day 7. The severity of the colitis, however, did not differ between the saline-treated (SAL) and hDPSC-treated animals at day 9, as comparable DAI values, weight reductions and colon lengths were measured. *p<0.05 vs saline-treated (SAL) group, #p<0.05 vs saline+DSS-treated group. n=6-7/group.
Beneficial Effect of HDPSCs on DSS-induced Colitis in Mice
Administration of 3% DSS-induced a moderate to severe inflammatory response in the animals, characterized by elevated DAI (significant elevation from day 6, Fig. (1B), marked weight loss (significant from day 7, Fig. (1C) and shortened colon on day 9 (upon sacrifice) Fig. (1D). Treatment with hDPSCs considerably delayed the development of colitis, as manifested in lower DAI value (2.3 ± 0.6 vs 4.0 ± 0.6, p<0.001) and the lack of significant weight loss on day 7. At the end of the experiment, however, the severity of colitis did not differ between the saline-treated and hDPSC-treated animals, and comparable DAI values, weight reductions and colon lengths were measured.
As described above, Zhao et al. reported that systemic infusion of rat DPSCs protected mice from DSS-induced colitis via FasL-induced T cell apoptosis [251]. They observed similar FasL-dependent apoptotic effect in the case of human DPSCs in vitro, which, together with other studies demonstrating the immunomodulatory effect of hDPSCs [248, 252, 264], foreshadow that these cells are also able to ameliorate colonic inflammation [265]. However, to our best knowledge this issue has not been directly addressed yet. Our results indicate that a single intravenous injection of hDPSCs beneficially alters the development of acute colonic inflammation in vivo. On the other hand, the anti-inflammatory action of hDPSCs in our experimental setting was less pronounced than the protective effect of rDPSCs in the study by Zhao and co-investigators [251]. Although hDPSCs delayed the onset of the disease in our experiment, the beneficial effect was not strong enough to alleviate to full-blown symptoms.
The difference between the efficacy of DPSC in our study and in others could be due to a number of factors. First, the severity and course of the inflammation shows considerable variability across different studies, even if mice with similar strain, sex, age, and weight were used. This phenomenon may partly be explained by the different composition of the gut microbiota, resulting from slightly different environmental conditions in the different laboratories. It is well established that differences in the gut microbiota substantially alter the immune responses of the host [266], including the susceptibility of mice to DSS [267] and the microbiota strongly depends on housing conditions and diet [267]. Considering this, it is possible that our mice were more susceptible to DSS, and its damaging effect could not be so effectively abrogated by hDPSCs. This assumption is also supported by the fact that in our experiment DSS-induced weight loss was much more pronounced (3.9 g vs. around 2.5 g on day 9). It is also important that although we used DSS with the same molecular weight (~ 35-55 kDa), it came from a different source, which may also contribute to the observed difference.
It is also important to note that the heterogeneity of MSC preparations originating from different tissue sources, isolated by slightly different protocols and cultivated by similar, but somewhat variable methods may result in different biological activities. Various MSC preparations were successfully applied in the mouse colitis model to alleviate the inflammatory process [156, 251, 268-271], but their relative potency is still unknown. Moreover, several studies reported that MSCs without prestimulation had no or very mild effect on experimental colitis [269, 272, 273] and cytokine inducible functional maturation was needed to earn full anti-inflammatory MSC phenotype [159]. Indeed, pre-stimulation with INF-γ and TNF-α significantly enhanced the protective action of MSCs in murine colitis [268, 274-277]. Accordingly, the efficacy of MSCs may rely on the exogenous and endogenous cytokine levels and on the timing of MSC administration [159].
Our data showing the protective effect of human DPSCs in experimental colitis are in accordance with the available literature about the beneficial effects of other MSCs in this model. Nonetheless, the efficiency of stem cell treatment even in a well-defined disease model could be variable, depending on the preconditioning, timing, administration, species and probably by a number of presently unidentified factors.
CONCLUSIONS
Although Alzheimer’s disease, Parkinson’s disease, traumatic brain and spinal cord injuries and multiple sclerosis, are classically categorized as rather different diseases of the CNS, recent data indicate that they are all characterized by tissue damage due to an inappropriate inflammatory/immune response. This shows remarkable similarities with the known pathomechanisms of the inflammatory bowel diseases. Conventional pharmacological and surgical therapies are not sufficient to prevent, slow down or stop these processes, or to promote the regeneration of damaged tissue. Since mesenchymal stem cells potentially offer solution for both problems, the treatment of neuronal and gastrointestinal inflammatory diseases with MSCs is an emerging area of great interest. Importantly, MSCs can be obtained from a different of sources including dental tissues. These cells can be expanded to generate a sufficient number of cells for therapeutic purposes ex vivo. To our current knowledge, the beneficial effects of MSCs can be mainly accounted for their immunomodulatory and anti-inflammatory effects. Thus, the use of MSCs from different sources (including the dental pulp and the periodontal ligament) represent a very promising approach to treat diseases involving immune-mediated tissue destruction, either in the gastrointestinal or in the central nervous system.
Table 1. Examples for the protective effects of MSCs in animal colitis models.
| Animal | Colitis Model | Type of Stem Cells | Amount, Route and Time of Appl. | In Vivo Effects | Refs. |
|---|---|---|---|---|---|
| Sprague-Dawley rat | TNBS | rat BMSC | 1 x 107 topically (submucosal injection) and i.v., immediately after TNBS | - i.v. no effect, accumulation in lungs - topically protection, acceleration of healing, expression of VEGF and TGF-β1 |
Hayashi et al., 2008 [230] |
| Lewis rat | DSS | rat BMSC | 2 x 104/g i.v. on day 2 | - no effect on inductive phase, modest promotion of recovery - most growth factors and cytokines remained unchanged, but TGFα was increased and Notch signaling was inhibited |
Tanaka et al., 2011 [272] |
| C57BL/6J mouse | DSS | human BMSC and GMSC | 2 x 106 i.p., on day 2 | - amelioration of colitis, suppression of CD4+ T lymphocyte infiltration, reduction of pro-inflammatory cytokines IFN-γ, IL-6 and IL-17, elevation of IL-10 | Zhang et al., 2009 [156] |
| Balb/c mouse | TNBS | human and mouse ASC | 1x105 – 106 i.p., 12 h after TNBS (hASCs and mASCs) and 106 i.p., 6 and 7 days after TNBS (hASCs) | - inhibition of colitis development, partial reversal of already established colitis, decreased MPO activity, reduction of pro-inflammatory cytokines (TNF-α, IFN- γ, IL-6, IL-1β, IL-12) and chemokines (RANTES and macrophage inhibitory protein 2), elevation of IL-10 |
Gonzalez et al., 2009 [152] |
| C57BL/6 mouse | DSS | human and mouse ASC | 1x105 – 5x106 i.p, on day 2, or in the case of cyclic DSS administration on day 7 of each cycle | - amelioration of colitis, decreased MPO activity, reduction of pro-inflammatory cytokines (TNF-α, IFN- γ, IL-6, IL-1β, IL-12) and chemokines (RANTES and macrophage inhibitory protein 2), elevation of IL-10 |
Gonzalez-Rey et al., 2009 [153] |
| C57BL/6J Ico mouse Balb/c mouse |
DSS TNBS |
untreated and IFN- γ-prestimulated human and mouse BMSC | 0.5x106 i.p, on day 0 1x106 i.p, 6 h after TNBS |
- IFN- γ-prestimulated cells, but not untreated cells induced protection, decreased serum amyloid A levels, reduced TNF-α, IL-6 and IL-17A, increased the level of IL-10 |
Duijvestein et al., 2011 [268] |
| Balb/c mouse | DSS | mouse BMSC | 1x106 i.v, on days 2, 5 and 8 | - alleviation of colitis, reduction of pro-inflammatory cytokines TNF-α and IL-1β | He et al., 2012 [270] |
| C57BL/6J mouse | DSS | rat DPSC | 1x105/10g i.v, on day 3 | - protection from colitis via Fas ligand-induced T cell apoptosis | Zhao et al., 2012 [251] |
| Wistar rat | TNBS | rat ASC and BMSC | 2x106 i.p., 4 days after TNBS | - i.p., but not i.v. injected stem cells migrated to the inflamed colon - alleviation of colitis, reduction of epithelial apoptosis and pro-inflammatory cytokine production (TNF-α, IL-1β), elevation of IL-10 |
Castelo-Branco et al., 2012 [278] |
| Balb/c mouse C57BL/6 mouse |
TNBS DSS |
mouse ASC and ASC-induced regulatory macrophages | 1x106 i.p, immediately after TNBS, or in the case of chronic colitis on days 8 and 16 1x106 i.p, on day 2, or in the case of chronic colitis on days 4 and 14 |
- inhibition of colitis development and progression, decreased MPO activity, reduction of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) and chemokines (RANTES) |
Anderson et al., 2013 [279] |
| C57BL/6 mouse | DSS | mouse ASC | 1x106 i.v or i.p., on days 2 and 5 | - i.p. no effect - amelioration of colitis only in the case of i.v. administration |
Goncalves et al., 2014 [269] |
| Balb/c mouse | DSS | mouse BMSC | 2.5x106 i.v, on day 7 | - accelerated recovery, improved epithelial barrier function via restoration of E-cadherin expression, reduction of oxidative stress |
Sun et al., 2015 [280] |
| C57BL/6 mouse | DSS | untreated and IFN- γ-overexpressed human UCSC | 1x106 i.v., on day 2 | - IFN- γ-overexpressed stem cells ameliorated colitis, reduced the number of Th1/Th17 cells and increased that of Treg/Th2 cells, decreased TNF-α, IL-6 and IL-1β, upregulated indoleamine 2, 3-dioxygenase expression | Chen et al., 2015 [281] |
Abbreviations for MSCs by origin: BMSC – bone marrow-derived mesenchymal stem cell, GMSC – gingival mesenchymal stem cell, ASC – adipose tissue-derived mesenchymal stem cell, DPSC – dental pulp mesenchymal stem cell, UCSC – umbilical cord-derived stem cell.
ACKNOWLEDGEMENTS
Our research was supported by Hungarian National Development Agency (TÁMOP-4.2.1/B-09/1/KMR-2010-0001, TÁMOP-4.2.2/B-10/1-2010-0013) and by the Hungarian Scientific Research Fund (OTKA PD 109602). The authors thank to Dr. Viktória E. Tóth and Dr. Ágnes Fehér for their help during the colitis experiments, to Dr. Gábor Zoltán Rácz for his valuable suggestions during the preparation of this manuscript and to Ms. Dorottya Tamási for her contribution compiling the references.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Fan X., Sun D., Tang X., Cai Y., Yin Z.Q., Xu H. Stem-cell challenges in the treatment of Alzheimers disease: a long way from bench to bedside. Med. Res. Rev. 2014;34(5):957–978. doi: 10.1002/med.21309. [http://dx.doi.org/10.1002/med.21309]. [PMID: 24500883]. [DOI] [PubMed] [Google Scholar]
- 2.Berchtold N.C., Cotman C.W. Evolution in the conceptualization of dementia and Alzheimers disease: Greco-Roman period to the 1960s. Neurobiol. Aging. 1998;19(3):173–189. doi: 10.1016/S0197-4580(98)00052-9. [http://dx.doi.org/ 10.1016/S0197-4580(98)00052-9]. [PMID: 9661992]. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe D.J. SnapShot: pathobiology of Alzheimer's disease. 2013. [DOI] [PubMed]
- 4.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [http:// dx.doi.org/10.1007/BF00308809]. [PMID: 1759558]. [DOI] [PubMed] [Google Scholar]
- 5.Wenk G.L. Neuropathologic changes in Alzheimers disease: potential targets for treatment. J. Clin. Psychiatry. 2006;67(Suppl. 3):3–7. [PubMed] [Google Scholar]
- 6.Goedert M., Strittmatter W.J., Roses A.D. Alzheimers disease. Risky apolipoprotein in brain. Nature. 1994;372(6501):45–46. doi: 10.1038/372045a0. [DOI] [PubMed] [Google Scholar]
- 7.Waring S.C., Rosenberg R.N. Genome-wide association studies in Alzheimer disease. Arch. Neurol. 2008;65(3):329–334. doi: 10.1001/archneur.65.3.329. [DOI] [PubMed] [Google Scholar]
- 8.Geula C., Mesulam M.M., Saroff D.M., Wu C.K. Relationship between plaques, tangles, and loss of cortical cholinergic fibers in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1998;57(1):63–75. doi: 10.1097/00005072-199801000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Ondrejcak T., Klyubin I., Hu N.W., Barry A.E., Cullen W.K., Rowan M.J. Alzheimers disease amyloid beta-protein and synaptic function. Neuromolecular Med. 2010;12(1):13–26. doi: 10.1007/s12017-009-8091-0. [DOI] [PubMed] [Google Scholar]
- 10.Selkoe D.J. The therapeutics of Alzheimers disease: where we stand and where we are heading. Ann. Neurol. 2013;74(3):328–336. doi: 10.1002/ana.24001. [DOI] [PubMed] [Google Scholar]
- 11.Haas C. Strategies, development, and pitfalls of therapeutic options for Alzheimers disease. J. Alzheimers Dis. 2012;28(2):241–281. doi: 10.3233/JAD-2011-110986. [DOI] [PubMed] [Google Scholar]
- 12.Shin J.Y., Park H.J., Kim H.N., Oh S.H., Bae J.S., Ha H.J., Lee P.H. Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models. Autophagy. 2014;10(1):32–44. doi: 10.4161/auto.26508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barage S.H., Sonawane K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimers disease. Neuropeptides. 2015;52:1–18. doi: 10.1016/j.npep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Agis-Torres A., Sölhuber M., Fernandez M., Sanchez-Montero J.M. Multi-Target-Directed Ligands and other Therapeutic Strategies in the Search of a Real Solution for Alzheimers Disease. Curr. Neuropharmacol. 2014;12(1):2–36. doi: 10.2174/1570159X113116660047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heneka M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., Herrup K., Frautschy S.A., Finsen B., Brown G.C., Verkhratsky A., Yamanaka K., Koistinaho J., Latz E., Halle A., Petzold G.C., Town T., Morgan D., Shinohara M.L., Perry V.H., Holmes C., Bazan N.G., Brooks D.J., Hunot S., Joseph B., Deigendesch N., Garaschuk O., Boddeke E., Dinarello C.A., Breitner J.C., Cole G.M., Golenbock D.T., Kummer M.P. Neuroinflammation in Alzheimers disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao P., Luo Z., Tian W., Yang J., Ibáñez D.P., Huang Z., Tortorella M.D., Esteban M.A., Fan W. Solving the puzzle of Parkinsons disease using induced pluripotent stem cells. Exp. Biol. Med. (Maywood) 2014;239(11):1421–1432. doi: 10.1177/1535370214538588. [DOI] [PubMed] [Google Scholar]
- 17.OConnor D.M., Boulis N.M. Gene therapy for neurodegenerative diseases. Trends Mol. Med. 2015;21(8):504–512. doi: 10.1016/j.molmed.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Racette B.A., Willis A.W. Time to change the blind men and the elephant approach to Parkinson disease? Neurology. 2015;85(2):190–196. doi: 10.1212/WNL.0000000000001739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esposito E., Cuzzocrea S. New therapeutic strategy for Parkinsons and Alzheimers disease. Curr. Med. Chem. 2010;17(25):2764–2774. doi: 10.2174/092986710791859324. [DOI] [PubMed] [Google Scholar]
- 20.Wu X., Cai H., Ge R., Li L., Jia Z. Recent progress of imaging agents for Parkinsons disease. Curr. Neuropharmacol. 2014;12(6):551–563. doi: 10.2174/1570159X13666141204221238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hölscher C. New drug treatments show neuroprotective effects in Alzheimers and Parkinsons diseases. Neural Regen. Res. 2014;9(21):1870–1873. doi: 10.4103/1673-5374.145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanna T., Sachan V. Mesenchymal stem cells: potential in treatment of neurodegenerative diseases. Curr. Stem Cell Res. Ther. 2014;9(6):513–521. doi: 10.2174/1574888X09666140923101110. [DOI] [PubMed] [Google Scholar]
- 23.Lozano D., Gonzales-Portillo G.S., Acosta S., de la Pena I., Tajiri N., Kaneko Y., Borlongan C.V. Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr. Dis. Treat. 2015;11:97–106. doi: 10.2147/NDT.S65815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gennai S., Monsel A., Hao Q., Liu J., Gudapati V., Barbier E.L., Lee J.W. Cell-based therapy for traumatic brain injury. Br. J. Anaesth. 2015;115(2):203–212. doi: 10.1093/bja/aev229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reis C., Wang Y., Akyol O., Ho W.M., Ii R.A., Stier G., Martin R., Zhang J.H. Whats New in Traumatic Brain Injury: Update on Tracking, Monitoring and Treatment. Int. J. Mol. Sci. 2015;16(6):11903–11965. doi: 10.3390/ijms160611903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stocchetti N., Taccone F.S., Citerio G., Pepe P.E., Le Roux P.D., Oddo M., Polderman K.H., Stevens R.D., Barsan W., Maas A.I., Meyfroidt G., Bell M.J., Silbergleit R., Vespa P.M., Faden A.I., Helbok R., Tisherman S., Zanier E.R., Valenzuela T., Wendon J., Menon D.K., Vincent J.L. Neuroprotection in acute brain injury: an up-to-date review. Crit. Care. 2015;19:186. doi: 10.1186/s13054-015-0887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tham S.W., Palermo T.M., Wang J., Jaffe K.M., Temkin N., Durbin D., Rivara F.P. Persistent pain in adolescents following traumatic brain injury. J. Pain. 2013;14(10):1242–1249. doi: 10.1016/j.jpain.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acosta S.A., Tajiri N., Shinozuka K., Ishikawa H., Sanberg P.R., Sanchez-Ramos J., Song S., Kaneko Y., Borlongan C.V. Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury. PLoS One. 2014;9(3):e90953. doi: 10.1371/journal.pone.0090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayeux J.P., Teng S.X., Katz P.S., Gilpin N.W., Molina P.E. Traumatic brain injury induces neuroinflammation and neuronal degeneration that is associated with escalated alcohol self-administration in rats. Behav. Brain Res. 2015;279:22–30. doi: 10.1016/j.bbr.2014.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz P.S., Sulzer J.K., Impastato R.A., Teng S.X., Rogers E.K., Molina P.E. Endocannabinoid degradation inhibition improves neurobehavioral function, blood-brain barrier integrity, and neuroinflammation following mild traumatic brain injury. J. Neurotrauma. 2015;32(5):297–306. doi: 10.1089/neu.2014.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Algattas H., Huang J.H. Traumatic Brain Injury pathophysiology and treatments: early, intermediate, and late phases post-injury. Int. J. Mol. Sci. 2013;15(1):309–341. doi: 10.3390/ijms15010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loane D.J., Byrnes K.R. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7(4):366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernandez-Ontiveros D.G., Tajiri N., Acosta S., Giunta B., Tan J., Borlongan C.V. Microglia activation as a biomarker for traumatic brain injury. Front. Neurol. 2013;4:30. doi: 10.3389/fneur.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pais T.F., Figueiredo C., Peixoto R., Braz M.H., Chatterjee S. Necrotic neurons enhance microglial neurotoxicity through induction of glutaminase by a MyD88-dependent pathway. J. Neuroinflammation. 2008;5:43. doi: 10.1186/1742-2094-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braun D., Madrigal J.L., Feinstein D.L. Noradrenergic regulation of glial activation: molecular mechanisms and therapeutic implications. Curr. Neuropharmacol. 2014;12(4):342–352. doi: 10.2174/1570159X12666140828220938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang R., Liu Y., Yan K., Chen L., Chen X.R., Li P., Chen F.F., Jiang X.D. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J. Neuroinflammation. 2013;10:106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vawda R., Fehlings M.G. Mesenchymal cells in the treatment of spinal cord injury: current & future perspectives. Curr. Stem Cell Res. Ther. 2013;8(1):25–38. doi: 10.2174/1574888X11308010005. [DOI] [PubMed] [Google Scholar]
- 38.Yiu G., He Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006;7(8):617–627. doi: 10.1038/nrn1956. [http://dx.doi.org/10.1038/ nrn1956]. [PMID: 16858390]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez A.M., Goulart C.O., Ramalho Bdos.S., Oliveira J.T., Almeida F.M. Neurotrauma and mesenchymal stem cells treatment: From experimental studies to clinical trials. World J. Stem Cells. 2014;6(2):179–194. doi: 10.4252/wjsc.v6.i2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forostyak S., Jendelova P., Sykova E. The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie. 2013;95(12):2257–2270. doi: 10.1016/j.biochi.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Mahad D.H., Trapp B.D., Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 42.Klinker M.W., Wei C.H. Mesenchymal stem cells in the treatment of inflammatory and autoimmune diseases in experimental animal models. World J. Stem Cells. 2015;7(3):556–567. doi: 10.4252/wjsc.v7.i3.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cramer S.P., Modvig S., Simonsen H.J., Frederiksen J.L., Larsson H.B. Permeability of the blood-brain barrier predicts conversion from optic neuritis to multiple sclerosis. Brain. 2015;138(Pt 9):2571–2583. doi: 10.1093/brain/awv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stadelmann C. Multiple sclerosis as a neurodegenerative disease: pathology, mechanisms and therapeutic implications. Curr. Opin. Neurol. 2011;24(3):224–229. doi: 10.1097/WCO.0b013e328346056f. [DOI] [PubMed] [Google Scholar]
- 45.Ng T.K., Fortino V.R., Pelaez D., Cheung H.S. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World J. Stem Cells. 2014;6(2):111–119. doi: 10.4252/wjsc.v6.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamm C.P., Uitdehaag B.M., Polman C.H. Multiple sclerosis: current knowledge and future outlook. Eur. Neurol. 2014;72(3-4):132–141. doi: 10.1159/000360528. [DOI] [PubMed] [Google Scholar]
- 47.Kawalec P., Mikrut A., Wiśniewska N., Pilc A. The effectiveness of dimethyl fumarate monotherapy in the treatment of relapsing-remitting multiple sclerosis: a systematic review and meta-analysis. Curr. Neuropharmacol. 2014;12(3):256–268. doi: 10.2174/1570159X12666140115214801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.OConnor K.C., Bar-Or A., Hafler D.A. The neuroimmunology of multiple sclerosis: possible roles of T and B lymphocytes in immunopathogenesis. J. Clin. Immunol. 2001;21(2):81–92. doi: 10.1023/A:1011064007686. [DOI] [PubMed] [Google Scholar]
- 49.Klaren R.E., Motl R.W., Woods J.A., Miller S.D. Effects of exercise in experimental autoimmune encephalomyelitis (an animal model of multiple sclerosis). J. Neuroimmunol. 2014;274(1-2):14–19. doi: 10.1016/j.jneuroim.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Racz G.Z., Kadar K., Foldes A., Kallo K., Perczel-Kovach K., Keremi B., Nagy A., Varga G. Immunomodulatory and potential therapeutic role of mesenchymal stem cells in periodontitis. J. Physiol. Pharmacol. 2014;65(3):327–339. [PubMed] [Google Scholar]
- 51.Loftus E.V., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 52.Hendrickson B.A., Gokhale R., Cho J.H. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin. Microbiol. Rev. 2002;15(1):79–94. doi: 10.1128/CMR.15.1.79-94.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baumgart D.C., Sandborn W.J. Crohns disease. Lancet. 2012;380(9853):1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 54.Lakatos P.L. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J. Gastroenterol. 2006;12(38):6102–6108. doi: 10.3748/wjg.v12.i38.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cannom R.R., Kaiser A.M., Ault G.T., Beart R.W., Jr, Etzioni D.A. Inflammatory bowel disease in the United States from 1998 to 2005: has infliximab affected surgical rates? Am. Surg. 2009;75(10):976–980. [PubMed] [Google Scholar]
- 56.Baumgart D.C., Carding S.R. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369(9573):1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 57.Murphy S.F., Kwon J.H., Boone D.L. Novel players in inflammatory bowel disease pathogenesis. Curr. Gastroenterol. Rep. 2012;14(2):146–152. doi: 10.1007/s11894-012-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ordás I., Eckmann L., Talamini M., Baumgart D.C., Sandborn W.J. Ulcerative colitis. Lancet. 2012;380(9853):1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 59.Gyires K., Tóth E.V., Zádori S.Z. Gut inflammation: current update on pathophysiology, molecular mechanism and pharmacological treatment modalities. Curr. Pharm. Des. 2014;20(7):1063–1081. doi: 10.2174/13816128113199990417. [DOI] [PubMed] [Google Scholar]
- 60.Danese S., Fiocchi C. Ulcerative colitis. N. Engl. J. Med. 2011;365(18):1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 61.Hardenberg G., Steiner T.S., Levings M.K. Environmental influences on T regulatory cells in inflammatory bowel disease. Semin. Immunol. 2011;23(2):130–138. doi: 10.1016/j.smim.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 62.Dotan I., Allez M., Nakazawa A., Brimnes J., Schulder-Katz M., Mayer L. Intestinal epithelial cells from inflammatory bowel disease patients preferentially stimulate CD4+ T cells to proliferate and secrete interferon-gamma. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292(6):G1630–G1640. doi: 10.1152/ajpgi.00294.2006. [DOI] [PubMed] [Google Scholar]
- 63.Powrie F., Correa-Oliveira R., Mauze S., Coffman R.L. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J. Exp. Med. 1994;179(2):589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 65.Brand S. Crohns disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohns disease. Gut. 2009;58(8):1152–1167. doi: 10.1136/gut.2008.163667. [DOI] [PubMed] [Google Scholar]
- 66.Pène J., Chevalier S., Preisser L., Vénéreau E., Guilleux M.H., Ghannam S., Molès J.P., Danger Y., Ravon E., Lesaux S., Yssel H., Gascan H. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J. Immunol. 2008;180(11):7423–7430. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]
- 67.Saleh M., Elson C.O. Experimental inflammatory bowel disease: insights into the host-microbiota dialog. Immunity. 2011;34(3):293–302. doi: 10.1016/j.immuni.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Himmel M.E., Yao Y., Orban P.C., Steiner T.S., Levings M.K. Regulatory T-cell therapy for inflammatory bowel disease: more questions than answers. Immunology. 2012;136(2):115–122. doi: 10.1111/j.1365-2567.2012.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Denning T.L., Wang Y.C., Patel S.R., Williams I.R., Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 2007;8(10):1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 70.Kamada N., Hisamatsu T., Okamoto S., Sato T., Matsuoka K., Arai K., Nakai T., Hasegawa A., Inoue N., Watanabe N., Akagawa K.S., Hibi T. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J. Immunol. 2005;175(10):6900–6908. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
- 71.Platt A.M., Bain C.C., Bordon Y., Sester D.P., Mowat A.M. An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J. Immunol. 2010;184(12):6843–6854. doi: 10.4049/jimmunol.0903987. [DOI] [PubMed] [Google Scholar]
- 72.Smythies L.E., Sellers M., Clements R.H., Mosteller-Barnum M., Meng G., Benjamin W.H., Orenstein J.M., Smith P.D. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J. Clin. Invest. 2005;115(1):66–75. doi: 10.1172/JCI200519229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kamada N., Hisamatsu T., Okamoto S., Chinen H., Kobayashi T., Sato T., Sakuraba A., Kitazume M.T., Sugita A., Koganei K., Akagawa K.S., Hibi T. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J. Clin. Invest. 2008;118(6):2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abraham C., Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140(6):1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 76.Hart A.L., Al-Hassi H.O., Rigby R.J., Bell S.J., Emmanuel A.V., Knight S.C., Kamm M.A., Stagg A.J. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129(1):50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 77.Geissmann F., Manz M.G., Jung S., Sieweke M.H., Merad M., Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Varol C., Vallon-Eberhard A., Elinav E., Aychek T., Shapira Y., Luche H., Fehling H.J., Hardt W.D., Shakhar G., Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31(3):502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 79.Coombes J.L., Siddiqui K.R., Arancibia-Cárcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204(8):1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bogunovic M., Ginhoux F., Helft J., Shang L., Hashimoto D., Greter M., Liu K., Jakubzick C., Ingersoll M.A., Leboeuf M., Stanley E.R., Nussenzweig M., Lira S.A., Randolph G.J., Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31(3):513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Annacker O., Coombes J.L., Malmstrom V., Uhlig H.H., Bourne T., Johansson-Lindbom B., Agace W.W., Parker C.M., Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J. Exp. Med. 2005;202(8):1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heller F., Florian P., Bojarski C., Richter J., Christ M., Hillenbrand B., Mankertz J., Gitter A.H., Bürgel N., Fromm M., Zeitz M., Fuss I., Strober W., Schulzke J.D. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129(2):550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 83.Fujino S., Andoh A., Bamba S., Ogawa A., Hata K., Araki Y., Bamba T., Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52(1):65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rovedatti L., Kudo T., Biancheri P., Sarra M., Knowles C.H., Rampton D.S., Corazza G.R., Monteleone G., Di Sabatino A., Macdonald T.T. Differential regulation of interleukin 17 and interferon gamma production in inflammatory bowel disease. Gut. 2009;58(12):1629–1636. doi: 10.1136/gut.2009.182170. [DOI] [PubMed] [Google Scholar]
- 85.Leppkes M., Becker C., Ivanov I.I., Hirth S., Wirtz S., Neufert C., Pouly S., Murphy A.J., Valenzuela D.M., Yancopoulos G.D., Becher B., Littman D.R., Neurath M.F. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136(1):257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 86.Hue S., Ahern P., Buonocore S., Kullberg M.C., Cua D.J., McKenzie B.S., Powrie F., Maloy K.J. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 2006;203(11):2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uhlig H.H., McKenzie B.S., Hue S., Thompson C., Joyce-Shaikh B., Stepankova R., Robinson N., Buonocore S., Tlaskalova-Hogenova H., Cua D.J., Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25(2):309–318. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 88.Morrissey P.J., Charrier K., Braddy S., Liggitt D., Watson J.D. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J. Exp. Med. 1993;178(1):237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maloy K.J., Salaun L., Cahill R., Dougan G., Saunders N.J., Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 2003;197(1):111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Izcue A., Coombes J.L., Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 91.Li M.O., Wan Y.Y., Sanjabi S., Robertson A.K., Flavell R.A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 92.Powrie F., Carlino J., Leach M.W., Mauze S., Coffman R.L. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J. Exp. Med. 1996;183(6):2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fahlén L., Read S., Gorelik L., Hurst S.D., Coffman R.L., Flavell R.A., Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 2005;201(5):737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kullberg M.C., Jankovic D., Gorelick P.L., Caspar P., Letterio J.J., Cheever A.W., Sher A. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J. Exp. Med. 2002;196(4):505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grohmann U., Orabona C., Fallarino F., Vacca C., Calcinaro F., Falorni A., Candeloro P., Belladonna M.L., Bianchi R., Fioretti M.C., Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat. Immunol. 2002;3(11):1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 96.Munn D.H., Sharma M.D., Lee J.R., Jhaver K.G., Johnson T.S., Keskin D.B., Marshall B., Chandler P., Antonia S.J., Burgess R., Slingluff C.L., Jr, Mellor A.L. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297(5588):1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 97.Barnes M.J., Griseri T., Johnson A.M., Young W., Powrie F., Izcue A. CTLA-4 promotes Foxp3 induction and regulatory T cell accumulation in the intestinal lamina propria. Mucosal Immunol. 2013;6(2):324–334. doi: 10.1038/mi.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bjorklund L.M., Sánchez-Pernaute R., Chung S., Andersson T., Chen I.Y., McNaught K.S., Brownell A.L., Jenkins B.G., Wahlestedt C., Kim K.S., Isacson O. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc. Natl. Acad. Sci. USA. 2002;99(4):2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aboody K., Capela A., Niazi N., Stern J.H., Temple S. Translating stem cell studies to the clinic for CNS repair: current state of the art and the need for a Rosetta stone. Neuron. 2011;70(4):597–613. doi: 10.1016/j.neuron.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 100.Blum B., Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv. Cancer Res. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- 101.Grinnemo K.H., Kumagai-Braesch M., Månsson-Broberg A., Skottman H., Hao X., Siddiqui A., Andersson A., Strömberg A.M., Lahesmaa R., Hovatta O., Sylven C., Corbascio M., Dellgren G. Human embryonic stem cells are immunogenic in allogeneic and xenogeneic settings. Reprod. Biomed. Online. 2006;13(5):712–724. doi: 10.1016/S1472-6483(10)60663-3. [DOI] [PubMed] [Google Scholar]
- 102.Grinnemo K.H., Sylvén C., Hovatta O., Dellgren G., Corbascio M. Immunogenicity of human embryonic stem cells. Cell Tissue Res. 2008;331(1):67–78. doi: 10.1007/s00441-007-0486-3. [DOI] [PubMed] [Google Scholar]
- 103.Blum B., Bar-Nur O., Golan-Lev T., Benvenisty N. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat. Biotechnol. 2009;27(3):281–287. doi: 10.1038/nbt.1527. [DOI] [PubMed] [Google Scholar]
- 104.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 105.Hacein-Bey-Abina S., Garrigue A., Wang G.P., Soulier J., Lim A., Morillon E., Clappier E., Caccavelli L., Delabesse E., Beldjord K., Asnafi V., MacIntyre E., Dal Cortivo L., Radford I., Brousse N., Sigaux F., Moshous D., Hauer J., Borkhardt A., Belohradsky B.H., Wintergerst U., Velez M.C., Leiva L., Sorensen R., Wulffraat N., Blanche S., Bushman F.D., Fischer A., Cavazzana-Calvo M. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118(9):3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Modlich U., Baum C. Preventing and exploiting the oncogenic potential of integrating gene vectors. J. Clin. Invest. 2009;119(4):755–758. doi: 10.1172/JCI38831. [http://dx.doi.org/10.1172/JCI38831]. [PMID: 19348042]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Okita K., Nakagawa M., Hyenjong H., Ichisaka T., Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 108.Stadtfeld M., Nagaya M., Utikal J., Weir G., Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu J., Hu K., Smuga-Otto K., Tian S., Stewart R., Slukvin I.I., Thomson J.A. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim D., Kim C.H., Moon J.I., Chung Y.G., Chang M.Y., Han B.S., Ko S., Yang E., Cha K.Y., Lanza R., Kim K.S. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vierbuchen T., Ostermeier A., Pang Z.P., Kokubu Y., Südhof T.C., Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Glowacki J., Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89(5):338–344. doi: 10.1002/bip.20871. [http://dx.doi.org/10.1002/ bip.20871]. [PMID: 17941007]. [DOI] [PubMed] [Google Scholar]
- 113.Bianco P., Riminucci M., Gronthos S., Robey P.G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19(3):180–192. doi: 10.1634/stemcells.19-3-180. [http://dx.doi.org/10.1634/stemcells. 19-3-180]. [PMID: 11359943]. [DOI] [PubMed] [Google Scholar]
- 114.Prockop D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 115.Hipp J., Atala A. Sources of stem cells for regenerative medicine. Stem Cell Rev. 2008;4(1):3–11. doi: 10.1007/s12015-008-9010-8. [DOI] [PubMed] [Google Scholar]
- 116.Vemuri M.C., Chase L.G., Rao M.S. Mesenchymal stem cell assays and applications. Methods Mol. Biol. 2011;698:3–8. doi: 10.1007/978-1-60761-999-4_1. [DOI] [PubMed] [Google Scholar]
- 117.Kuehnle I., Goodell M.A. The therapeutic potential of stem cells from adults. BMJ. 2002;325(7360):372–376. doi: 10.1136/bmj.325.7360.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Javazon E.H., Beggs K.J., Flake A.W. Mesenchymal stem cells: paradoxes of passaging. Exp. Hematol. 2004;32(5):414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 119.Le Blanc K., Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7(1):36–45. doi: 10.1016/S1465-3249(05)70787-8. [DOI] [PubMed] [Google Scholar]
- 120.Gronthos S., Mankani M., Brahim J., Robey P.G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 122.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop Dj., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 123.Gronthos S., Zannettino A.C., Hay S.J., Shi S., Graves S.E., Kortesidis A., Simmons P.J. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J. Cell Sci. 2003;116(Pt 9):1827–1835. doi: 10.1242/jcs.00369. [http://dx.doi.org/10.1242/jcs.00369]. [PMID: 12665563]. [DOI] [PubMed] [Google Scholar]
- 124.Martinez C., Hofmann T.J., Marino R., Dominici M., Horwitz E.M. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109(10):4245–4248. doi: 10.1182/blood-2006-08-039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gang E.J., Bosnakovski D., Figueiredo C.A., Visser J.W., Perlingeiro R.C. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109(4):1743–1751. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 126.Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., Tagliafico E., Ferrari S., Robey P.G., Riminucci M., Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 127.Rasmusson I., Ringdén O., Sundberg B., Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76(8):1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 128.Taddio A., Biondi A., Piscianz E., Valencic E., Biagi E., Badolato R. From bone marrow transplantation to cellular therapies: possible therapeutic strategies in managing autoimmune disorders. Curr. Pharm. Des. 2012;18(35):5776–5781. doi: 10.2174/138161212803530754. [DOI] [PubMed] [Google Scholar]
- 129.Bartholomew A., Sturgeon C., Siatskas M., Ferrer K., McIntosh K., Patil S., Hardy W., Devine S., Ucker D., Deans R., Moseley A., Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002;30(1):42–48. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- 130.Di Nicola M., Carlo-Stella C., Magni M., Milanesi M., Longoni P.D., Matteucci P., Grisanti S., Gianni A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- 131.Krampera M., Glennie S., Dyson J., Scott D., Laylor R., Simpson E., Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 132.Le Blanc K., Tammik L., Sundberg B., Haynesworth S.E., Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand. J. Immunol. 2003;57(1):11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 133.Pierdomenico L., Bonsi L., Calvitti M., Rondelli D., Arpinati M., Chirumbolo G., Becchetti E., Marchionni C., Alviano F., Fossati V., Staffolani N., Franchina M., Grossi A., Bagnara G.P. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80(6):836–842. doi: 10.1097/01.tp.0000173794.72151.88. [DOI] [PubMed] [Google Scholar]
- 134.Tse W.T., Pendleton J.D., Beyer W.M., Egalka M.C., Guinan E.C. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 135.Beyth S., Borovsky Z., Mevorach D., Liebergall M., Gazit Z., Aslan H., Galun E., Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 136.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 137.Krampera M., Cosmi L., Angeli R., Pasini A., Liotta F., Andreini A., Santarlasci V., Mazzinghi B., Pizzolo G., Vinante F., Romagnani P., Maggi E., Romagnani S., Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 138.Meisel R., Zibert A., Laryea M., Göbel U., Däubener W., Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 139.Sato K., Ozaki K., Oh I., Meguro A., Hatanaka K., Nagai T., Muroi K., Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 140.Gerdoni E., Gallo B., Casazza S., Musio S., Bonanni I., Pedemonte E., Mantegazza R., Frassoni F., Mancardi G., Pedotti R., Uccelli A. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann. Neurol. 2007;61(3):219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- 141.Asari S., Itakura S., Ferreri K., Liu C.P., Kuroda Y., Kandeel F., Mullen Y. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp. Hematol. 2009;37(5):604–615. doi: 10.1016/j.exphem.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yadav P.K., Chen C., Liu Z. Potential role of NK cells in the pathogenesis of inflammatory bowel disease. J. Biomed. Biotechnol. 2011. 2011, 348530. [DOI] [PMC free article] [PubMed]
- 143.Sotiropoulou P.A., Perez S.A., Gritzapis A.D., Baxevanis C.N., Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 144.Spaggiari G.M., Capobianco A., Becchetti S., Mingari M.C., Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107(4):1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 145.Shi M., Liu Z.W., Wang F.S. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin. Exp. Immunol. 2011;164(1):1–8. doi: 10.1111/j.1365-2249.2011.04327.x. [http://dx.doi.org/10.1111/j.1365-2249.2011.04327.x]. [PMID: 21352202]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jiang X.X., Zhang Y., Liu B., Zhang S.X., Wu Y., Yu X.D., Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 147.Zhang W., Ge W., Li C., You S., Liao L., Han Q., Deng W., Zhao R.C. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13(3):263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 148.Németh K., Leelahavanichkul A., Yuen P.S., Mayer B., Parmelee A., Doi K., Robey P.G., Leelahavanichkul K., Koller B.H., Brown J.M., Hu X., Jelinek I., Star R.A., Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Del Papa B., Sportoletti P., Cecchini D., Rosati E., Balucani C., Baldoni S., Fettucciari K., Marconi P., Martelli M.F., Falzetti F., Di Ianni M. Notch1 modulates mesenchymal stem cells mediated regulatory T-cell induction. Eur. J. Immunol. 2013;43(1):182–187. doi: 10.1002/eji.201242643. [DOI] [PubMed] [Google Scholar]
- 150.Madec A.M., Mallone R., Afonso G., Abou Mrad E., Mesnier A., Eljaafari A., Thivolet C. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia. 2009;52(7):1391–1399. doi: 10.1007/s00125-009-1374-z. [DOI] [PubMed] [Google Scholar]
- 151.Ge W., Jiang J., Arp J., Liu W., Garcia B., Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90(12):1312–1320. doi: 10.1097/TP.0b013e3181fed001. [DOI] [PubMed] [Google Scholar]
- 152.González M.A., Gonzalez-Rey E., Rico L., Büscher D., Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60(4):1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 153.Gonzalez-Rey E., Anderson P., González M.A., Rico L., Büscher D., Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58(7):929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 154.Tipnis S., Viswanathan C., Majumdar A.S. Immunosuppressive properties of human umbilical cord-derived mesenchymal stem cells: role of B7-H1 and IDO. Immunol. Cell Biol. 2010;88(8):795–806. doi: 10.1038/icb.2010.47. [DOI] [PubMed] [Google Scholar]
- 155.English K., Ryan J.M., Tobin L., Murphy M.J., Barry F.P., Mahon B.P. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009;156(1):149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang Q., Shi S., Liu Y., Uyanne J., Shi Y., Shi S., Le A.D. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 2009;183(12):7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sioud M., Mobergslien A., Boudabous A., Fløisand Y. Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand. J. Immunol. 2010;71(4):267–274. doi: 10.1111/j.1365-3083.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 158.Glenn J.D., Whartenby K.A. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J. Stem Cells. 2014;6(5):526–539. doi: 10.4252/wjsc.v6.i5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Krampera M. Mesenchymal stromal cell licensing: a multistep process. Leukemia. 2011;25(9):1408–1414. doi: 10.1038/leu.2011.108. [DOI] [PubMed] [Google Scholar]
- 160.Pluchino S., Cossetti C. How stem cells speak with host immune cells in inflammatory brain diseases. Glia. 2013;61(9):1379–1401. doi: 10.1002/glia.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Blurton-Jones M., Kitazawa M., Martinez-Coria H., Castello N.A., Müller F.J., Loring J.F., Yamasaki T.R., Poon W.W., Green K.N., LaFerla F.M. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. USA. 2009;106(32):13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li L.Y., Li J.T., Wu Q.Y., Li J., Feng Z.T., Liu S., Wang T.H. Transplantation of NGF-gene-modified bone marrow stromal cells into a rat model of Alzheimer disease. J. Mol. Neurosci. 2008;34(2):157–163. doi: 10.1007/s12031-007-9022-x. [DOI] [PubMed] [Google Scholar]
- 163.Park D., Yang G., Bae D.K., Lee S.H., Yang Y.H., Kyung J., Kim D., Choi E.K., Choi K.C., Kim S.U., Kang S.K., Ra J.C., Kim Y.B. Human adipose tissue-derived mesenchymal stem cells improve cognitive function and physical activity in ageing mice. J. Neurosci. Res. 2013;91(5):660–670. doi: 10.1002/jnr.23182. [DOI] [PubMed] [Google Scholar]
- 164.Ryu J.K., Cho T., Wang Y.T., McLarnon J.G. Neural progenitor cells attenuate inflammatory reactivity and neuronal loss in an animal model of inflamed AD brain. J. Neuroinflammation. 2009;6:39. doi: 10.1186/1742-2094-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Martino G., Pluchino S., Bonfanti L., Schwartz M. Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol. Rev. 2011;91(4):1281–1304. doi: 10.1152/physrev.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee J.K., Jin H.K., Endo S., Schuchman E.H., Carter J.E., Bae J.S. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimers disease mice by modulation of immune responses. Stem Cells. 2010;28(2):329–343. doi: 10.1002/stem.277. [DOI] [PubMed] [Google Scholar]
- 167.Lee H.J., Lee J.K., Lee H., Carter J.E., Chang J.W., Oh W., Yang Y.S., Suh J.G., Lee B.H., Jin H.K., Bae J.S. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimers disease mouse model through modulation of neuroinflammation. Neurobiol. Aging. 2012;33(3):588–602. doi: 10.1016/j.neurobiolaging.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 168.Lee J.K., Schuchman E.H., Jin H.K., Bae J.S. Soluble CCL5 derived from bone marrow-derived mesenchymal stem cells and activated by amyloid β ameliorates Alzheimers disease in mice by recruiting bone marrow-induced microglia immune responses. Stem Cells. 2012;30(7):1544–1555. doi: 10.1002/stem.1125. [DOI] [PubMed] [Google Scholar]
- 169.Takata K., Kitamura Y., Yanagisawa D., Morikawa S., Morita M., Inubushi T., Tsuchiya D., Chishiro S., Saeki M., Taniguchi T., Shimohama S., Tooyama I. Microglial transplantation increases amyloid-beta clearance in Alzheimer model rats. FEBS Lett. 2007;581(3):475–478. doi: 10.1016/j.febslet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 170.Hickman S.E., Allison E.K., El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimers disease mice. J. Neurosci. 2008;28(33):8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee J.K., Jin H.K., Bae J.S. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimers disease mouse model. Neurosci. Lett. 2009;450(2):136–141. doi: 10.1016/j.neulet.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 172.Giunti D., Parodi B., Usai C., Vergani L., Casazza S., Bruzzone S., Mancardi G., Uccelli A. Mesenchymal stem cells shape microglia effector functions through the release of CX3CL1. Stem Cells. 2012;30(9):2044–2053. doi: 10.1002/stem.1174. [DOI] [PubMed] [Google Scholar]
- 173.Fuhrmann M., Bittner T., Jung C.K., Burgold S., Page R.M., Mitteregger G., Haass C., LaFerla F.M., Kretzschmar H., Herms J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimers disease. Nat. Neurosci. 2010;13(4):411–413. doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kanno H. Regenerative therapy for neuronal diseases with transplantation of somatic stem cells. World J. Stem Cells. 2013;5(4):163–171. doi: 10.4252/wjsc.v5.i4.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mu Y., Gage F.H. Adult hippocampal neurogenesis and its role in Alzheimers disease. Mol. Neurodegener. 2011;6:85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Enciu A.M., Nicolescu M.I., Manole C.G., Mureşanu D.F., Popescu L.M., Popescu B.O. Neuroregeneration in neurodegenerative disorders. BMC Neurol. 2011;11:75. doi: 10.1186/1471-2377-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ganz J., Lev N., Melamed E., Offen D. Cell replacement therapy for Parkinsons disease: how close are we to the clinic? Expert Rev. Neurother. 2011;11(9):1325–1339. doi: 10.1586/ern.11.74. [DOI] [PubMed] [Google Scholar]
- 178.Teixeira F.G., Carvalho M.M., Sousa N., Salgado A.J. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell. Mol. Life Sci. 2013;70(20):3871–3882. doi: 10.1007/s00018-013-1290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Blandini F., Cova L., Armentero M.T., Zennaro E., Levandis G., Bossolasco P., Calzarossa C., Mellone M., Giuseppe B., Deliliers G.L., Polli E., Nappi G., Silani V. Transplantation of undifferentiated human mesenchymal stem cells protects against 6-hydroxydopamine neurotoxicity in the rat. Cell Transplant. 2010;19(2):203–217. doi: 10.3727/096368909X479839. [DOI] [PubMed] [Google Scholar]
- 180.McCoy M.K., Martinez T.N., Ruhn K.A., Wrage P.C., Keefer E.W., Botterman B.R., Tansey K.E., Tansey M.G. Autologous transplants of Adipose-Derived Adult Stromal (ADAS) cells afford dopaminergic neuroprotection in a model of Parkinsons disease. Exp. Neurol. 2008;210(1):14–29. doi: 10.1016/j.expneurol.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mathieu P., Roca V., Gamba C., Del Pozo A., Pitossi F. Neuroprotective effects of human umbilical cord mesenchymal stromal cells in an immunocompetent animal model of Parkinsons disease. J. Neuroimmunol. 2012;246(1-2):43–50. doi: 10.1016/j.jneuroim.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 182.Danielyan L., Schäfer R., von Ameln-Mayerhofer A., Bernhard F., Verleysdonk S., Buadze M., Lourhmati A., Klopfer T., Schaumann F., Schmid B., Koehle C., Proksch B., Weissert R., Reichardt H.M., van den Brandt J., Buniatian G.H., Schwab M., Gleiter C.H., Frey W.H., II Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation Res. 2011;14(1):3–16. doi: 10.1089/rej.2010.1130. [DOI] [PubMed] [Google Scholar]
- 183.Wang F., Yasuhara T., Shingo T., Kameda M., Tajiri N., Yuan W.J., Kondo A., Kadota T., Baba T., Tayra J.T., Kikuchi Y., Miyoshi Y., Date I. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci. 2010;11:52. doi: 10.1186/1471-2202-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Park H.J., Shin J.Y., Lee B.R., Kim H.O., Lee P.H. Mesenchymal stem cells augment neurogenesis in the subventricular zone and enhance differentiation of neural precursor cells into dopaminergic neurons in the substantia nigra of a parkinsonian model. Cell Transplant. 2012;21(8):1629–1640. doi: 10.3727/096368912X640556. [DOI] [PubMed] [Google Scholar]
- 185.Trzaska K.A., Kuzhikandathil E.V., Rameshwar P. Specification of a dopaminergic phenotype from adult human mesenchymal stem cells. Stem Cells. 2007;25(11):2797–2808. doi: 10.1634/stemcells.2007-0212. [DOI] [PubMed] [Google Scholar]
- 186.Somoza R., Juri C., Baes M., Wyneken U., Rubio F.J. Intranigral transplantation of epigenetically induced BDNF-secreting human mesenchymal stem cells: implications for cell-based therapies in Parkinsons disease. Biol. Blood Marrow Transplant. 2010;16(11):1530–1540. doi: 10.1016/j.bbmt.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 187.Munoz J.R., Stoutenger B.R., Robinson A.P., Spees J.L., Prockop D.J. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc. Natl. Acad. Sci. USA. 2005;102(50):18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xiao L., Tsutsui T. Human dental mesenchymal stem cells and neural regeneration. Hum. Cell. 2013;26(3):91–96. doi: 10.1007/s13577-013-0069-4. [DOI] [PubMed] [Google Scholar]
- 189.Chao Y., Wong S.C., Tan E.K. Evidence of inflammatory system involvement in Parkinson's disease. Biomed. Res. Int. 2014. 2014, 308654. [DOI] [PMC free article] [PubMed]
- 190.Yan J., Fu Q., Cheng L., Zhai M., Wu W., Huang L., Du G. Inflammatory response in Parkinsons disease (Review). Mol. Med. Rep. 2014;10(5):2223–2233. doi: 10.3892/mmr.2014.2563. [DOI] [PubMed] [Google Scholar]
- 191.Kim Y.J., Park H.J., Lee G., Bang O.Y., Ahn Y.H., Joe E., Kim H.O., Lee P.H. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009;57(1):13–23. doi: 10.1002/glia.20731. [DOI] [PubMed] [Google Scholar]
- 192.Chao Y.X., He B.P., Tay S.S. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinsons disease. J. Neuroimmunol. 2009;216(1-2):39–50. doi: 10.1016/j.jneuroim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 193.Mahmood A., Lu D., Wang L., Li Y., Lu M., Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49(1-2):1196–1203. discussion 1203-1194. [PubMed] [Google Scholar]
- 194.Mahmood A., Lu D., Lu M., Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.NEU.0000079333.61863.AA. discussion 702-693. [DOI] [PubMed] [Google Scholar]
- 195.Galindo L.T., Filippo T.R., Semedo P., Ariza C.B., Moreira C.M., Camara N.O., Porcionatto M.A. Mesenchymal stem cell therapy modulates the inflammatory response in experimental traumatic brain injury. Neurol. Res. Int. 2011 doi: 10.1155/2011/564089. 2011, 564089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chen M., Li X., Zhang X., He X., Lai L., Liu Y., Zhu G., Li W., Li H., Fang Q., Wang Z., Duan C. The inhibitory effect of mesenchymal stem cell on blood-brain barrier disruption following intracerebral hemorrhage in rats: contribution of TSG-6. J. Neuroinflammation. 2015;12:61. doi: 10.1186/s12974-015-0284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Baranova N.S., Nilebäck E., Haller F.M., Briggs D.C., Svedhem S., Day A.J., Richter R.P. The inflammation-associated protein TSG-6 cross-links hyaluronan via hyaluronan-induced TSG-6 oligomers. J. Biol. Chem. 2011;286(29):25675–25686. doi: 10.1074/jbc.M111.247395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pati S., Khakoo A.Y., Zhao J., Jimenez F., Gerber M.H., Harting M., Redell J.B., Grill R., Matsuo Y., Guha S., Cox C.S., Reitz M.S., Holcomb J.B., Dash P.K. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/β-catenin signaling. Stem Cells Dev. 2011;20(1):89–101. doi: 10.1089/scd.2010.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Torrente D., Avila M.F., Cabezas R., Morales L., Gonzalez J., Samudio I., Barreto G.E. Paracrine factors of human mesenchymal stem cells increase wound closure and reduce reactive oxygen species production in a traumatic brain injury in vitro model. Hum. Exp. Toxicol. 2014;33(7):673–684. doi: 10.1177/0960327113509659. [DOI] [PubMed] [Google Scholar]
- 200.Bai L., Lennon D.P., Eaton V., Maier K., Caplan A.I., Miller S.D., Miller R.H. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57(11):1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Torres-Espín A., Corona-Quintanilla D.L., Forés J., Allodi I., González F., Udina E., Navarro X. Neuroprotection and axonal regeneration after lumbar ventral root avulsion by re-implantation and mesenchymal stem cells transplant combined therapy. Neurotherapeutics. 2013;10(2):354–368. doi: 10.1007/s13311-013-0178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Azari M.F., Mathias L., Ozturk E., Cram D.S., Boyd R.L., Petratos S. Mesenchymal stem cells for treatment of CNS injury. Curr. Neuropharmacol. 2010;8(4):316–323. doi: 10.2174/157015910793358204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rehman J., Traktuev D., Li J., Merfeld-Clauss S., Temm-Grove C.J., Bovenkerk J.E., Pell C.L., Johnstone B.H., Considine R.V., March K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 204.Nakagami H., Maeda K., Morishita R., Iguchi S., Nishikawa T., Takami Y., Kikuchi Y., Saito Y., Tamai K., Ogihara T., Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler. Thromb. Vasc. Biol. 2005;25(12):2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 205.Wei X., Du Z., Zhao L., Feng D., Wei G., He Y., Tan J., Lee W.H., Hampel H., Dodel R., Johnstone B.H., March K.L., Farlow M.R., Du Y. IFATS collection: The conditioned media of adipose stromal cells protect against hypoxia-ischemia-induced brain damage in neonatal rats. Stem Cells. 2009;27(2):478–488. doi: 10.1634/stemcells.2008-0333. [DOI] [PubMed] [Google Scholar]
- 206.Hawryluk G.W., Mothe A., Wang J., Wang S., Tator C., Fehlings M.G. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012;21(12):2222–2238. doi: 10.1089/scd.2011.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lu P., Jones L.L., Tuszynski M.H. Axon regeneration through scars and into sites of chronic spinal cord injury. Exp. Neurol. 2007;203(1):8–21. doi: 10.1016/j.expneurol.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 208.Novikova L.N., Brohlin M., Kingham P.J., Novikov L.N., Wiberg M. Neuroprotective and growth-promoting effects of bone marrow stromal cells after cervical spinal cord injury in adult rats. Cytotherapy. 2011;13(7):873–887. doi: 10.3109/14653249.2011.574116. [DOI] [PubMed] [Google Scholar]
- 209.Ohta M., Suzuki Y., Noda T., Ejiri Y., Dezawa M., Kataoka K., Chou H., Ishikawa N., Matsumoto N., Iwashita Y., Mizuta E., Kuno S., Ide C. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp. Neurol. 2004;187(2):266–278. doi: 10.1016/j.expneurol.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 210.Cizkova D., Novotna I., Slovinska L., Vanicky I., Jergova S., Rosocha J., Radonak J. Repetitive intrathecal catheter delivery of bone marrow mesenchymal stromal cells improves functional recovery in a rat model of contusive spinal cord injury. J. Neurotrauma. 2011;28(9):1951–1961. doi: 10.1089/neu.2010.1413. [DOI] [PubMed] [Google Scholar]
- 211.Urdzíková L., Jendelová P., Glogarová K., Burian M., Hájek M., Syková E. Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J. Neurotrauma. 2006;23(9):1379–1391. doi: 10.1089/neu.2006.23.1379. [DOI] [PubMed] [Google Scholar]
- 212.Zurita M., Vaquero J., Bonilla C., Santos M., De Haro J. ; Zurita M., Vaquero J., Bonilla C., Santos M., De Haro J., Oya S., Aguayo C. Functional recovery of chronic paraplegic pigs after autologous transplantation of bone marrow stromal cells. Transplantation. 2008;86(6):845–853. doi: 10.1097/TP.0b013e318186198f. [DOI] [PubMed] [Google Scholar]
- 213.Deng Y.B., Liu X.G., Liu Z.G., Liu X.L., Liu Y., Zhou G.Q. Implantation of BM mesenchymal stem cells into injured spinal cord elicits de novo neurogenesis and functional recovery: evidence from a study in rhesus monkeys. Cytotherapy. 2006;8(3):210–214. doi: 10.1080/14653240600760808. [DOI] [PubMed] [Google Scholar]
- 214.Wright K.T., El Masri W., Osman A., Chowdhury J., Johnson W.E. Concise review: Bone marrow for the treatment of spinal cord injury: mechanisms and clinical applications. Stem Cells. 2011;29(2):169–178. doi: 10.1002/stem.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Han D., Wu C., Xiong Q., Zhou L., Tian Y. Anti-inflammatory Mechanism of Bone Marrow Mesenchymal Stem Cell Transplantation in Rat Model of Spinal Cord Injury. Cell Biochem. Biophys. 2014 doi: 10.1007/s12013-014-0354-1. [DOI] [PubMed] [Google Scholar]
- 216.Uccelli A., Benvenuto F., Laroni A., Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract. Res. Clin. Haematol. 2011;24(1):59–64. doi: 10.1016/j.beha.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 217.Neuhuber B., Timothy Himes B., Shumsky J.S., Gallo G., Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035(1):73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 218.Abrams M.B., Dominguez C., Pernold K., Reger R., Wiesenfeld-Hallin Z., Olson L., Prockop D. Multipotent mesenchymal stromal cells attenuate chronic inflammation and injury-induced sensitivity to mechanical stimuli in experimental spinal cord injury. Restor. Neurol. Neurosci. 2009;27(4):307–321. doi: 10.3233/RNN-2009-0480. [DOI] [PubMed] [Google Scholar]
- 219.Tran C.T., Gargiulo C., Thao H.D., Tuan H.M., Filgueira L., Michael Strong D. Culture and differentiation of osteoblasts on coral scaffold from human bone marrow mesenchymal stem cells. Cell Tissue Bank. 2011;12(4):247–261. doi: 10.1007/s10561-010-9208-2. [DOI] [PubMed] [Google Scholar]
- 220.Sakata N., Goto M., Yoshimatsu G., Egawa S., Unno M. Utility of co-transplanting mesenchymal stem cells in islet transplantation. World J. Gastroenterol. 2011;17(47):5150–5155. doi: 10.3748/wjg.v17.i47.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nakajima H., Uchida K., Guerrero A.R., Watanabe S., Sugita D., Takeura N., Yoshida A., Long G., Wright K.T., Johnson W.E., Baba H. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J. Neurotrauma. 2012;29(8):1614–1625. doi: 10.1089/neu.2011.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Quertainmont R., Cantinieaux D., Botman O., Sid S., Schoenen J., Franzen R. Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS One. 2012;7(6):e39500. doi: 10.1371/journal.pone.0039500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zappia E., Casazza S., Pedemonte E., Benvenuto F., Bonanni I., Gerdoni E., Giunti D., Ceravolo A., Cazzanti F., Frassoni F., Mancardi G., Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5):1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 224.Gharibi T., Ahmadi M., Seyfizadeh N., Jadidi-Niaragh F., Yousefi M. Immunomodulatory characteristics of mesenchymal stem cells and their role in the treatment of multiple sclerosis. Cell. Immunol. 2015;293(2):113–121. doi: 10.1016/j.cellimm.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 225.Rafei M., Campeau P.M., Aguilar-Mahecha A., Buchanan M., Williams P., Birman E., Yuan S., Young Y.K., Boivin M.N., Forner K., Basik M., Galipeau J. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J. Immunol. 2009;182(10):5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 226.Zhang X., Bowles A.C., Semon J.A., Scruggs B.A., Zhang S., Strong A.L., Gimble J.M., Bunnell B.A. Transplantation of autologous adipose stem cells lacks therapeutic efficacy in the experimental autoimmune encephalomyelitis model. PLoS One. 2014;9(1):e85007. doi: 10.1371/journal.pone.0085007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Karussis D., Karageorgiou C., Vaknin-Dembinsky A., Gowda-Kurkalli B., Gomori J.M., Kassis I., Bulte J.W., Petrou P., Ben-Hur T., Abramsky O., Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010;67(10):1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Connick P., Kolappan M., Patani R., Scott M.A., Crawley C., He X.L., Richardson K., Barber K., Webber D.J., Wheeler-Kingshott C.A., Tozer D.J., Samson R.S., Thomas D.L., Du M.Q., Luan S.L., Michell A.W., Altmann D.R., Thompson A.J., Miller D.H., Compston A., Chandran S. The mesenchymal stem cells in multiple sclerosis (MSCIMS) trial protocol and baseline cohort characteristics: an open-label pre-test: post-test study with blinded outcome assessments. Trials. 2011;12:62. doi: 10.1186/1745-6215-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Connick P., Kolappan M., Crawley C., Webber D.J., Patani R., Michell A.W., Du M.Q., Luan S.L., Altmann D.R., Thompson A.J., Compston A., Scott M.A., Miller D.H., Chandran S. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11(2):150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hayashi Y., Tsuji S., Tsujii M., Nishida T., Ishii S., Iijima H., Nakamura T., Eguchi H., Miyoshi E., Hayashi N., Kawano S. Topical implantation of mesenchymal stem cells has beneficial effects on healing of experimental colitis in rats. J. Pharmacol. Exp. Ther. 2008;326(2):523–531. doi: 10.1124/jpet.108.137083. [DOI] [PubMed] [Google Scholar]
- 231.Tanaka F., Tominaga K., Ochi M., Tanigawa T., Watanabe T., Fujiwara Y., Ohta K., Oshitani N., Higuchi K., Arakawa T. Exogenous administration of mesenchymal stem cells ameliorates dextran sulfate sodium-induced colitis via anti-inflammatory action in damaged tissue in rats. Life Sci. 2008;83(23-24):771–779. doi: 10.1016/j.lfs.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 232.Duijvestein M., Vos A.C., Roelofs H., Wildenberg M.E., Wendrich B.B., Verspaget H.W., Kooy-Winkelaar E.M., Koning F., Zwaginga J.J., Fidder H.H., Verhaar A.P., Fibbe W.E., van den Brink G.R., Hommes D.W. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohns disease: results of a phase I study. Gut. 2010;59(12):1662–1669. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 233.Lazebnik L.B., Konopliannikov A.G., Kniazev O.V., Parfenov A.I., Tsaregorodtseva T.M., Ruchkina I.N., Khomeriki S.G., Rogozina V.A., Konopliannikova O.A. [Use of allogeneic mesenchymal stem cells in the treatment of intestinal inflammatory diseases]. Ter. Arkh. 2010;82(2):38–43. [Use of allogeneic mesenchymal stem cells in the treatment of intestinal inflammatory diseases]. [Use of allogeneic mesenchymal stem cells in the treatment of intestinal inflammatory diseases]. [PubMed] [Google Scholar]
- 234.Garcia-Olmo D., Herreros D., Pascual I., Pascual J.A., Del-Valle E., Zorrilla J., De-La-Quintana P., Garcia-Arranz M., Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis. Colon Rectum. 2009;52(1):79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 235.Ciccocioppo R., Bernardo M.E., Sgarella A., Maccario R., Avanzini M.A., Ubezio C., Minelli A., Alvisi C., Vanoli A., Calliada F., Dionigi P., Perotti C., Locatelli F., Corazza G.R. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohns disease. Gut. 2011;60(6):788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 236.Miura M., Gronthos S., Zhao M., Lu B., Fisher L.W., Robey P.G., Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Seo B.M., Miura M., Gronthos S., Bartold P.M., Batouli S., Brahim J., Young M., Robey P.G., Wang C.Y., Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 238.Kadar K., Kiraly M., Porcsalmy B., Molnar B., Racz G.Z., Blazsek J., Kallo K., Szabo E.L., Gera I., Gerber G., Varga G. Differentiation potential of stem cells from human dental origin - promise for tissue engineering. J. Physiol. Pharmacol. 2009;60(Suppl. 7):167–175. [PubMed] [Google Scholar]
- 239.Kádár K., Porcsalmy B., Király M., Molnár B., Jobbágy-Ovári G., Somogyi E., Hermann P., Gera I., Varga G. [Isolating, culturing and characterizing stem cells of human dental pulp origin]. Fogorv. Sz. 2009;102(5):175–181. [Isolating, culturing and characterizing stem cells of human dental pulp origin]. [Isolating, culturing and characterizing stem cells of human dental pulp origin]. [PubMed] [Google Scholar]
- 240.Király M., Porcsalmy B., Pataki A., Kádár K., Jelitai M., Molnár B., Hermann P., Gera I., Grimm W.D., Ganss B., Zsembery A., Varga G. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem. Int. 2009;55(5):323–332. doi: 10.1016/j.neuint.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 241.Molnár B., Kádár K., Király M., Porcsalmy B., Somogyi E., Hermann P., Grimm W.D., Gera I., Varga G. [Isolation, cultivation and characterisation of stem cells in human periodontal ligament]. Fogorv. Sz. 2008;101(4):155–161. [Isolation, cultivation and characterisation of stem cells in human periodontal ligament]. [Isolation, cultivation and characterisation of stem cells in human periodontal ligament]. [PubMed] [Google Scholar]
- 242.Varga G., Bori E., Kállo K., Nagy K., Tarján I., Rácz G.Z. Novel possible pharmaceutical research tools: stem cells, gene delivery and their combination. Curr. Pharm. Des. 2013;19(1):133–141. doi: 10.2174/13816128130118. [DOI] [PubMed] [Google Scholar]
- 243.Li X.Y., Zheng Z.H., Li X.Y., Guo J., Zhang Y., Li H., Wang Y.W., Ren J., Wu Z.B. Treatment of foot disease in patients with type 2 diabetes mellitus using human umbilical cord blood mesenchymal stem cells: response and correction of immunological anomalies. Curr. Pharm. Des. 2013;19(27):4893–4899. doi: 10.2174/13816128113199990326. [DOI] [PubMed] [Google Scholar]
- 244.Tomar G.B., Srivastava R.K., Gupta N., Barhanpurkar A.P., Pote S.T., Jhaveri H.M., Mishra G.C., Wani M.R. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem. Biophys. Res. Commun. 2010;393(3):377–383. doi: 10.1016/j.bbrc.2010.01.126. [DOI] [PubMed] [Google Scholar]
- 245.Janebodin K., Horst O.V., Ieronimakis N., Balasundaram G., Reesukumal K., Pratumvinit B., Reyes M. Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS One. 2011;6(11):e27526. doi: 10.1371/journal.pone.0027526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Musina R.A., Bekchanova E.S., Belyavskii A.V., Sukhikh G.T. Differentiation potential of mesenchymal stem cells of different origin. Bull. Exp. Biol. Med. 2006;141(1):147–151. doi: 10.1007/s10517-006-0115-2. [DOI] [PubMed] [Google Scholar]
- 247.Sonoyama W., Liu Y., Yamaza T., Tuan R.S., Wang S., Shi S., Huang G.T. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J. Endod. 2008;34(2):166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wada N., Menicanin D., Shi S., Bartold P.M., Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J. Cell. Physiol. 2009;219(3):667–676. doi: 10.1002/jcp.21710. [DOI] [PubMed] [Google Scholar]
- 249.Iwasaki A., Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 250.Kerkis I., Ambrosio C.E., Kerkis A., Martins D.S., Zucconi E., Fonseca S.A., Cabral R.M., Maranduba C.M., Gaiad T.P., Morini A.C., Vieira N.M., Brolio M.P., SantAnna O.A., Miglino M.A., Zatz M. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: Local or systemic? J. Transl. Med. 2008;6:35. doi: 10.1186/1479-5876-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhao Y., Wang L., Jin Y., Shi S. Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. J. Dent. Res. 2012;91(10):948–954. doi: 10.1177/0022034512458690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Akiyama K., Chen C., Wang D., Xu X., Qu C., Yamaza T., Cai T., Chen W., Sun L., Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10(5):544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chassaing B., Aitken J.D., Malleshappa M., Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014 doi: 10.1002/0471142735.im1525s104. 104 Unit 15 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kim H.S., Kim K.H., Kim S.H., Kim Y.S., Koo K.T., Kim T.I., Seol Y.J., Ku Y., Rhyu I.C., Chung C.P., Lee Y.M. Immunomodulatory effect of canine periodontal ligament stem cells on allogenic and xenogenic peripheral blood mononuclear cells. J. Periodontal Implant Sci. 2010;40(6):265–270. doi: 10.5051/jpis.2010.40.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ding G., Liu Y., An Y., Zhang C., Shi S., Wang W., Wang S. Suppression of T cell proliferation by root apical papilla stem cells in vitro. Cells Tissues Organs (Print) 2010;191(5):357–364. doi: 10.1159/000276589. [DOI] [PubMed] [Google Scholar]
- 256.Liu D., Xu J., Liu O., Fan Z., Liu Y., Wang F., Ding G., Wei F., Zhang C., Wang S. Mesenchymal stem cells derived from inflamed periodontal ligaments exhibit impaired immunomodulation. J. Clin. Periodontol. 2012;39(12):1174–1182. doi: 10.1111/jcpe.12009. [DOI] [PubMed] [Google Scholar]
- 257.Arthur A., Rychkov G., Shi S., Koblar S.A., Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26(7):1787–1795. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 258.Király M., Kádár K., Horváthy D.B., Nardai P., Rácz G.Z., Lacza Z., Varga G., Gerber G. Integration of neuronally predifferentiated human dental pulp stem cells into rat brain in vivo. Neurochem. Int. 2011;59(3):371–381. doi: 10.1016/j.neuint.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 259.Leong W.K., Henshall T.L., Arthur A., Kremer K.L., Lewis M.D., Helps S.C., Field J., Hamilton-Bruce M.A., Warming S., Manavis J., Vink R., Gronthos S., Koblar S.A. Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. Stem Cells Transl. Med. 2012;1(3):177–187. doi: 10.5966/sctm.2011-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sakai K., Yamamoto A., Matsubara K., Nakamura S., Naruse M., Yamagata M., Sakamoto K., Tauchi R., Wakao N., Imagama S., Hibi H., Kadomatsu K., Ishiguro N., Ueda M. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Invest. 2012;122(1):80–90. doi: 10.1172/JCI59251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Arthur A., Shi S., Zannettino A.C., Fujii N., Gronthos S., Koblar S.A. Implanted adult human dental pulp stem cells induce endogenous axon guidance. Stem Cells. 2009;27(9):2229–2237. doi: 10.1002/stem.138. [DOI] [PubMed] [Google Scholar]
- 262.Reichmann F., Painsipp E., Holzer P. Environmental enrichment and gut inflammation modify stress-induced c-Fos expression in the mouse corticolimbic system. PLoS One. 2013;8(1):e54811. doi: 10.1371/journal.pone.0054811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Mitrovic M., Shahbazian A., Bock E., Pabst M.A., Holzer P. Chemo-nociceptive signalling from the colon is enhanced by mild colitis and blocked by inhibition of transient receptor potential ankyrin 1 channels. Br. J. Pharmacol. 2010;160(6):1430–1442. doi: 10.1111/j.1476-5381.2010.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Park J.Y., Jeon S.H., Choung P.H. Efficacy of periodontal stem cell transplantation in the treatment of advanced periodontitis. Cell Transplant. 2011;20(2):271–285. doi: 10.3727/096368910X519292. [DOI] [PubMed] [Google Scholar]
- 265.Wu T., Liu Y., Fan Z., Xu J., Jin L., Gao Z., Wu Z., Hu L., Wang J., Zhang C., Chen W., Wang S. miR-21 Modulates the Immunoregulatory Function of Bone Marrow Mesenchymal Stem Cells Through the PTEN/Akt/TGF-β1 Pathway. Stem Cells. 2015;33(11):3281–3290. doi: 10.1002/stem.2081. [DOI] [PubMed] [Google Scholar]
- 266.Aitken J.D., Gewirtz A.T. Gut microbiota in 2012: Toward understanding and manipulating the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2013;10(2):72–74. doi: 10.1038/nrgastro.2012.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ooi J.H., Waddell A., Lin Y.D., Albert I., Rust L.T., Holden V., Cantorna M.T. Dominant effects of the diet on the microbiome and the local and systemic immune response in mice. PLoS One. 2014;9(1):e86366. doi: 10.1371/journal.pone.0086366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Duijvestein M., Wildenberg M.E., Welling M.M., Hennink S., Molendijk I., van Zuylen V.L., Bosse T., Vos A.C., de Jonge-Muller E.S., Roelofs H., van der Weerd L., Verspaget H.W., Fibbe W.E., te Velde A.A., van den Brink G.R., Hommes D.W. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29(10):1549–1558. doi: 10.1002/stem.698. [DOI] [PubMed] [Google Scholar]
- 269.Gonçalves Fda.C., Schneider N., Pinto F.O., Meyer F.S., Visioli F., Pfaffenseller B., Lopez P.L., Passos E.P., Cirne-Lima E.O., Meurer L., Paz A.H. Intravenous vs intraperitoneal mesenchymal stem cells administration: what is the best route for treating experimental colitis? World J. Gastroenterol. 2014;20(48):18228–18239. doi: 10.3748/wjg.v20.i48.18228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.He X.W., He X.S., Lian L., Wu X.J., Lan P. Systemic infusion of bone marrow-derived mesenchymal stem cells for treatment of experimental colitis in mice. Dig. Dis. Sci. 2012;57(12):3136–3144. doi: 10.1007/s10620-012-2290-5. [DOI] [PubMed] [Google Scholar]
- 271.Martínez-Montiel Mdel.P., Gómez-Gómez G.J., Flores A.I. Therapy with stem cells in inflammatory bowel disease. World J. Gastroenterol. 2014;20(5):1211–1227. doi: 10.3748/wjg.v20.i5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tanaka H., Arimura Y., Yabana T., Goto A., Hosokawa M., Nagaishi K., Yamashita K., Yamamoto H., Sasaki Y., Fujimiya M., Imai K., Shinomura Y. Myogenic lineage differentiated mesenchymal stem cells enhance recovery from dextran sulfate sodium-induced colitis in the rat. J. Gastroenterol. 2011;46(2):143–152. doi: 10.1007/s00535-010-0320-7. [DOI] [PubMed] [Google Scholar]
- 273.Nam Y.S., Kim N., Im K.I., Lim J.Y., Lee E.S., Cho S.G. Negative impact of bone-marrow-derived mesenchymal stem cells on dextran sulfate sodium-induced colitis. World J. Gastroenterol. 2015;21(7):2030–2039. doi: 10.3748/wjg.v21.i7.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zhang Z., Han Y., Song J., Luo R., Jin X., Mu D., Su S., Ji X., Ren Y.F., Liu H. Interferon-γ regulates the function of mesenchymal stem cells from oral lichen planus via indoleamine 2,3-dioxygenase activity. J. Oral Pathol. Med. 2015;44(1):15–27. doi: 10.1111/jop.12224. [DOI] [PubMed] [Google Scholar]
- 275.Sivanathan K.N., Gronthos S., Rojas-Canales D., Thierry B., Coates P.T. Interferon-gamma modification of mesenchymal stem cells: implications of autologous and allogeneic mesenchymal stem cell therapy in allotransplantation. Stem Cell Rev. 2014;10(3):351–375. doi: 10.1007/s12015-014-9495-2. [DOI] [PubMed] [Google Scholar]
- 276.English K., Barry F.P., Field-Corbett C.P., Mahon B.P. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol. Lett. 2007;110(2):91–100. doi: 10.1016/j.imlet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 277.Cuerquis J., Romieu-Mourez R., François M., Routy J.P., Young Y.K., Zhao J., Eliopoulos N. Human mesenchymal stromal cells transiently increase cytokine production by activated T cells before suppressing T-cell proliferation: effect of interferon-γ and tumor necrosis factor-α stimulation. Cytotherapy. 2014;16(2):191–202. doi: 10.1016/j.jcyt.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 278.Castelo-Branco M.T., Soares I.D., Lopes D.V., Buongusto F., Martinusso C.A., do Rosario A., Jr, Souza S.A., Gutfilen B., Fonseca L.M., Elia C., Madi K., Schanaider A., Rossi M.I., Souza H.S. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012;7(3):e33360. doi: 10.1371/journal.pone.0033360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Anderson P., Souza-Moreira L., Morell M., Caro M., OValle F., Gonzalez-Rey E., Delgado M. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut. 2013;62(8):1131–1141. doi: 10.1136/gutjnl-2012-302152. [DOI] [PubMed] [Google Scholar]
- 280.Sun T., Gao G.Z., Li R.F., Li X., Li D.W., Wu S.S., Yeo A.E., Jin B. Bone marrow-derived mesenchymal stem cell transplantation ameliorates oxidative stress and restores intestinal mucosal permeability in chemically induced colitis in mice. Am. J. Transl. Res. 2015;7(5):891–901. [PMC free article] [PubMed] [Google Scholar]
- 281.Chen Y., Song Y., Miao H., Xu Y., Lv M., Wang T., Hou Y. Gene delivery with IFN-γ-expression plasmids enhances the therapeutic effects of MSCs on DSS-induced mouse colitis. Inflamm. Res. 2015;64(9):671–681. doi: 10.1007/s00011-015-0845-6. [DOI] [PubMed] [Google Scholar]


