Abstract
Nephronophthisis-related ciliopathies (NPHP-RC) are a group of inherited diseases that affect genes encoding proteins that localize to primary cilia or centrosomes. With few exceptions, ciliopathies are inherited in an autosomal recessive manner, and affected individuals manifest early during childhood or adolescence. NPHP-RC are genetically very heterogeneous, and, currently, mutations in more than 90 genes have been described as single-gene causes. The phenotypes of NPHP-RC are very diverse, and include cystic-fibrotic kidney disease, brain developmental defects, retinal degeneration, skeletal deformities, facial dimorphism, and, in some cases, laterality defects, and congenital heart disease. Mutations in the same gene can give rise to diverse phenotypes depending on the mutated allele. At the same time, there is broad phenotypic overlap between different monogenic genes. The identification of monogenic causes of ciliopathies has furthered the understanding of molecular mechanism and cellular pathways involved in the pathogenesis.
Nephronophthisis and nephronophthisis-related ciliopathies are inherited disorders caused by mutations in genes involved in ciliary function. They are genetically and phenotypically very heterogeneous.
Cilia are organelles that are present on the apical surface of almost every cell type in various tissues and organs. They are involved in a variety of cellular functions such as planar cell polarity, cell-cycle regulation and mechanosensation. Furthermore, cilia integrate multiple signaling pathways that are of critical importance for vertebrate development and organ differentiation. Hence, ciliary dysfunction gives rise to a wide spectrum of human disease phenotypes involving various organ systems. Cilia can be categorized as motile cilia and immotile cilia, also known as primary cilia. Motile cilia are present on respiratory epithelial cells, ependymal cells of cerebrospinal fluid spaces, sperm cells, and cells of the embryonic node during development. Dysfunction of motile cilia results in the human phenotype of primary ciliary dyskinesia and Kartagener syndrome (OMIM #244400), which is characterized by impaired mucociliary clearance resulting in recurrent infections of the upper respiratory tract, recurrent pneumonia, and progressive destruction of functional respiratory tissue. Additional disease symptoms include heterotaxy, congenital heart disease, asplenia, infertility, and situs inversus in 50% of patients (Leigh et al. 2009). Disruption of primary cilia has first been linked to autosomal-recessive and autosomal-dominant polycystic kidney diseases, which are discussed in more detail in Ma et al. (2016). In contrast to polycystic kidney disease that typically presents with enlarged kidneys and massive cysts, the second group of cilia-related cystic kidney diseases, the group of nephronophthisis-related ciliopathies (NPHP-RC), rather presents with shrunken or normal-sized fibrotic kidneys and small cysts at the corticomedullary junction. The renal phenotype of NPHP frequently occurs in a syndromic manner and is accompanied by anomalies in other organ systems, specifically retinal degeneration, cerebellar vermis hypoplasia, hepatic fibrosis, skeletal anomalies, ectodermal dysplasia, brain malformations, and neurological impairment. Frequently, a broad phenotypic spectrum is caused by mutations in the same monogenic gene on an allelic basis (Table 1; Fig. 1).
Table 1.
Phenotypic spectrum of 92 monogenic genes of NPHP-RC
| OMIM disease | Gene symbol (first description) | Alias | Mode of inheritance | Human disease phenotype | Subcellular localization | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kidney | Eye | Brain | Liver | Skeletal anomalies | Laterality defects | Congenital heart disease | Other/syndromic | |||||
| Nephronopthisis (NPHP), Senior–Loken syndrome (SLS), Joubert syndrome (JBTS), Meckel–Gruber syndrome (MKS) | ||||||||||||
| NPHP1/SLS1/JBTS4 | NPHP1 (Hildebrandt et al. 1997a; Saunier et al. 1997) | Nephrocystin | AR | NPHP, juvenile | RP, OMA | CVH, rare | TZ | |||||
| NPHP2 | INVS (Otto et al. 2003b) | Inversin | AR | NPHP, infantile | RP | LF, BDP | SI | PH, OH | TZ | |||
| NPHP3/MKS7/RHPD1 | NPHP3 (Olbrich et al. 2003) | NPHP3 | AR | NPHP | RP | LF, LC | SI | CHD | MPD, MKS | TZ | ||
| NPHP4/SLS4 | NPHP4 (Mollet et al. 2002; Otto et al. 2002) | NPHP4, nephroretinin | AR | NPHP | RP, OMA | LF, BDP | HTX | CHD | BB, CA | |||
| SLS5 | IQCB1 (Otto et al. 2005) | NPHP5, IQCB1 | AR | NPHP | RP (all cases) | TZ, BB | ||||||
| NPHP6/SLS6/JBTS5/MKS4/BBS14 | CEP290 (Sayer et al. 2006; Valente et al. 2006) | CEP290, NPHP6, BBS14 | AR | NPHP | RP | CVH, CBD | LF, BDP | PD | VSD, rare | MKS, BBS | BB | |
| NPHP7 | GLIS2 (Attanasio et al. 2007) | NPHP7, GLIS2 | AR | NPHP | CA | |||||||
| NPHP8/JBTS7/MKS5/COACH | RPGRIP1L (Delous et al. 2007) | NPHP8, RPGRIP1L | AR | NPHP | RP, OMA, CB | CVH, CBD | LF, BDP | PD | MKS, BBS | TZ | ||
| NPHP9 | NEK8 (Otto et al. 2008) | NPHP9, NEK8 | AR | NPHP | LF | CHD | MKS | TZ | ||||
| SLS7/BBS16 | SDCCAG8 (Otto et al. 2010) | SDCCAG8, NPHP10 | AR | NPHP | RP | ID | BBS | BB | ||||
| NPHP11/JBTS6/MKS3/COACH | TMEM67 (Smith et al. 2006) | Meckelin, TMEM67 | AR | NPHP | RP, OMA, CB | CVH | LF, BDP | PD | MKS | TZ | ||
| NPHP12/JBTS11/SRTD4 | TTC21B (Davis et al. 2011) | TTC21B, NPHP12 | AR | NPHP | CVH | JATD | MKS | CA | ||||
| NPHP13/SLS8/CED4/SRTD5 | WDR19 (Bredrup et al. 2011) | WDR19, IFT144 | AR | NPHP | RP | LF | JATD, CED | CED | CA | |||
| NPHP14/JBTS19 | ZNF423 (Chaki et al. 2012) | NPHP14, ZNF423 | AR/AD | NPHP | RP | CVH | SI | |||||
| NPHP15 | CEP164 (Chaki et al. 2012) | NPHP15, CEP164 | AR | NPHP | RP | CVH, ID | LF | PD | OB | BB | ||
| NPHP16 | ANKS6 (Hoff et al. 2013) | NPHP16, ANKS6 | AR | NPHP | LF | SI | CHD | TZ | ||||
| NPHP17/SRTD10 | IFT172 (Halbritter et al. 2013a) | IFT172 | AR | NPHP | RP | CVH, ID | LF | SRTD, PD, CSE | VSD | OB | CA | |
| NPHP18 | CEP83 (Failler et al. 2014) | CEP83/CCDC41 | AR | NPHP | RP | ID, HC | LF | BB | ||||
| NPHP19 | DCDC2 (Schueler et al. 2015) | DCDC2 | AR | NPHP | LF | CA | ||||||
| IFT81 | IFT81 (Perrault et al. 2015) | IFT81 | AR | NPHP | RP | CVH | PD | CA | ||||
| SLS9 | IFT54 (Bizet et al. 2015) | TRAF3IP1 | AR | NPHP | RP | ID | BD | OB | CA, BB | |||
| JBTS1 | INPP5E (Bielas et al. 2009) | INPP5E | AR | NPHP, rare | RP, OMA, CB | CVH, CBD, ID | LF | PD, rare | MORM | CA | ||
| JBTS2/MKS2 | TMEM216 (Valente et al. 2010) | TMEM216 | AR | NPHP | RP, OMA, CB | CVH, ID, CBD, HC | PD | MKS | TZ | |||
| JBTS3 | AHI1 (Ferland et al. 2004) | AHI, Jouberin | AR | NPHP, rare | RP, OMA | CVH, ID | ||||||
| JBTS8 | ARL13B (Cantagrel et al. 2008) | ARL13B | AR | CVH, ID | CA | |||||||
| JBTS9/MKS6 | CC2D2A (Gorden et al. 2008) | CC2D2A | AR | RP | CVH, ID | LF | MKS | TZ | ||||
| JBTS12 | KIF7 (Putoux et al. 2011) | KIF7 | AR | CBD, ID, CVH | PD, FD | VSD | ACS, BBS, HLS | CA | ||||
| JBTS13 | TECT1 (Garcia-Gonzalo et al. 2011) | TECTONIC1 | AR | CVH | Abnormal limbs | TZ | ||||||
| JBTS14 | TMEM237 (Huang et al. 2011) | TMEM237 | AR | NPHP | OpA, CB, Nys | CVH, HC | TZ | |||||
| JBTS15 | CEP41 (Lee et al. 2012a) | CEP41 | AR | OMA, RP | CVH, ID | PD | BB | |||||
| JBTS16 | TMEM138 (Lee et al. 2012b) | TMEM138 | AR | NPHP, rare | OMA, CB | CVH | PD | TZ | ||||
| JBTS17/OFD6 | C5ORF42 (Srour et al. 2012b) | C5orf42 | AR | RC | OMA, RP | CVH, ID | PD, FD | - | ||||
| JBTS18/OFD4 | TCTN3 (Thomas et al. 2012) | TCTN3, C10orf61 | AR | RC | OMA | CVH, OE, ID | LF, BDP | PD, FD, Abnormal limbs | VSD | TZ | ||
| JBTS20/OFD3/MKS11 | TMEM231 (Srour et al. 2012a) | TMEM231 | AR | RC | OMA, RP | CVH, ID, OE | FD, PD | MKS | BB | |||
| JBTS21 | CSPP1 (Akizu et al. 2014) | CSPP1 | AR | NPHP | OMA, RP | CVH, CBD, ID | LF | JATD | SD, PH | BB | ||
| JBTS22 | PDE6D (Thomas et al. 2014) | PDE6D | AR | DK | CB, MO, RP | CVH, CBD, ID | FD, PD | BB | ||||
| JBTS23/SRTD14 | KIAA0586 (Alby et al. 2015; Bachmann-Gagescu et al. 2015) | KIAA0586, TALPID3 | AR | OMA, CB | CVH, HC, CBD | PD, FD, SRTD | ASD | HLS, PH | BB | |||
| JBTS24/MKS8 | TCTN2 (Sang et al. 2011) | TECTONIC2 | AR | DK | Nys, MO | CVH, ID, CBD | LF, BDP | PD | MKS | TZ | ||
| JBTS25 | CEP104 (Srour et al. 2015) | CEP104 | AR | NPHP | OMA, RP | CVH, ID, CBD | Abnormal limbs, PD | BB, ciliary tip | ||||
| JBTS26 | KIAA0556 (Sanders et al. 2015) | KIAA0556, KATNIP | AR | CVH, ID | FD | Ciliary base | ||||||
| MKS9 | B9D1 (Hopp et al. 2011) | B9D1 | AR | RC, DK | OE | Abnormal limbs | TZ | |||||
| MKS10 | B9D2 (Dowdle et al. 2011) | B9D2 | AR | RC | OE, AE | DPM, LF | PD | BB, TZ | ||||
| MKS12 | KIF14 (Filges et al. 2014) | KIF14 | AR | DK, RA | MiC, CVH, CBD | Abnormal limbs, FD | CA | |||||
| n/a | TMEM107 (Shaheen et al. 2015a; Lambacher et al. 2016; Shylo et al. 2016) | DK | RP | CVH, ID, OE | FD, PD | JBTS, OFD, MKS | TZ | |||||
| Bardet–Biedl syndrome (BBS) | ||||||||||||
| BBS1 | BBS1 (Mykytyn et al. 2002) | BBS1 | AR/DR | RC, DK | RP | ID | LF, rare | PD, BD, FD | OB | BBSome | ||
| BBS2 | BBS2 (Katsanis et al. 2001a; Nishimura et al. 2001) | BBS2 | AR | RC, DK | RP | ID | LF, rare | PD, BD, FD | OB | BBSome | ||
| BBS3 | ARL6 (Fan et al. 2004) | ARL6/BBS3 | AR/DR | RC, DK | RP | ID | LF, rare | PD, BD, FD | OB | BBSome | ||
| BBS4 | BBS4 (Katsanis et al. 2002) | BBS4 | AR | RC, DK | RP | ID | LF, rare | PD, BD, FD | OB | BBSome | ||
| BBS5 | BBS5 (Li et al. 2004) | BBS5 | AR | RC, DK | RP | ID | LF, rare | PD, BD, FD | OB | BBSome | ||
| BBS6/MKKS | MKKS (Katsanis et al. 2000; Slavotinek et al. 2000) | BBS6/MKKS | AR | RC, CAKUT | RP | ID | LF, rare | PD, BD, FD, congenital dislocation of hips | CHD | MKKS, OB, HL | BBSome | |
| BBS7 | BBS7 (Badano et al. 2003) | BBS7 | AR | RC, DK | RP | ID | LF, rare | PD, BD, FD | OB | BBSome | ||
| BBS8 | TTC8 (Ansley et al. 2003) | BBS8/TTC8 | AR | RC, DK | RP | ID | LF, rare | PD, BD, FD | OB | BBSome | ||
| BBS9 | PTHB1 (Nishimura et al. 2005) | PTHB1/BBS9 | AR | RC, DK | RP | ID | LF, rare | PD, BD, FD | OB | BBSome | ||
| BBS10 | BBS10 (Stoetzel et al. 2006) | BBS10/C12orf58 | AR | RC, DK | RP | ID | LF, rare | PD, BD, FD | OB | BBSome | ||
| BBS11 | TRIM32 (Chiang et al. 2006) | BBS11/TRIM32 | AR | RC, DK | RP | ID | LF, rare | PD, BD, FD | LGMD2H, OB | - | ||
| BBS12 | BBS12 (Stoetzel et al. 2007) | C4orf24 | AR | RC, DK | RP | ID | LF, rare | PD, BD, FD | OB | BBSome | ||
| BBS13/MKS1 | MKS1 (Kyttala et al. 2006; Leitch et al. 2008) | MKS1/BBS13 | AR | RC, DK, RA | RP, CB, MO | ID, MiC, OE, AA | LF, BDP | PD, BD, FD | CHD | OB, MKS, PH | BBSome | |
| BBS15 | WDPCP (Kim et al. 2010) | WDPCP, BBS15 | AR | RC, DK | RP | ID | LF, rare | PD, BD, FD | CHD | OB | BBSome | |
| BBS17 | LZTFL1 (Marion et al. 2012) | LZTFL1/BBS17 | AR | RC, DK | RP | ID | LF, rare | PD, BD, FD | SI, HTX | OB | BBSome | |
| BBS18 | BBIP1 (Scheidecker et al. 2014) | BBIP1/BBS18 | AR | RC, DK | RP | ID | LF, rare | PD, BD, FD | OB | BBSome | ||
| BBS19 | IFT27 (Aldahmesh et al. 2014) | IFT27/RABL4 | AR | RC, DK | RP | ID | LF, rare | PD, BD, FD | OB | BBSome | ||
| Skeletal ciliopathies: oral-facial-digital syndrome (OFD), cranioectodermal dysplasia (CED), short-rib thoracic dysplasia (SRTD) | ||||||||||||
| OFD1/JBTS10/SGBS2 | OFD1 (Ferrante et al. 2001; Budny et al. 2006) | OFD1, CXorf5 | XD | RC | RP | ID, CBD, SZ, CVH | LC, LF | FD, OA, PD, BD | CHD | PC | BB | |
| OFD5 | DDX59 (Shamseldin et al. 2013) | DDX59 | AR | DK, rare | ID, CBD | PD, FD, OA | CHD, rare | - | ||||
| OFD9 (VUS) | SCLT1/TBC1D32 (Adly et al. 2014) | TBC1D32/C6orf170, SCLT1 | AR | CB, MO | MiC, CBD, CVH | PD, FD, OA | CHD | - | ||||
| OFD14 | C2CD3 (Thauvin-Robinet et al. 2014) | C2CD3 | AR | RP | ID, CBD, CVH | PD, FD, OA | BB | |||||
| CED1 | IFT122 (Walczak-Sztulpa et al. 2010) | AR | NPHP | RP, Nys | LF, LC | FD, OA, SRTD, BD | CHD | CA | ||||
| CED2/ SRTD7 | WDR35 (Gilissen et al. 2010; Mill et al. 2011) | WDR35/IFT121 | AR | NPHP | RP | LF, BDP, LC | FD, OA, SRTD, BD, PD | PH, PC | CA | |||
| CED3 | IFT43 (Arts et al. 2011) | IFT43 | AR | NPHP | LF | FD, OA, BD, PD, SRTD | CA | |||||
| SRTD2 | IFT80 (Beales et al. 2007) | IFT80 | AR | PD, BD, SRTD, TA | CA | |||||||
| SRTD3 | DYN2H1 (Dagoneau et al. 2009) | DYNC2H1 | AR/DR | CK, DK | RP, rare | CBD, rare | LF, rare | PD, BD, SRTD, FD | CHD, rare | JATD | CA | |
| SRTD6 | NEK1 (Thiel et al. 2011) | NEK1 | AR/DR | CK | HC, rare | LF, rare | PD, SRTD, FD, OA, Dw | CHD, rare | HF, PH | Ncl | ||
| SRTD8 | WDR60 (McInerney-Leo et al. 2013) | WDR60 | AR | CK | MaC, | DPM, LF | PD, SRTD | VSD | PH, PF | BB | ||
| SRTD9 / MZSDS | IFT140 (Perrault et al. 2012; Schmidts et al. 2013b) | IFT140 | AR | NPHP | RP, Nys | MiC, ID, DD | LF | SRTD, CSE, craniosynostosis | CA | |||
| SRTD11 | WDR34 (Huber et al. 2013; Schmidts et al. 2013c) | WDR34 | AR | BD, PD, SRTD, TA | PH | CA | ||||||
| SRTD13 | CEP120 (Shaheen et al. 2015b) | CEP120, CCDC100 | AR | NPHP | CVH | SRTD, PD, FD, OA | PH | BB | ||||
| EVC | EVC (Ruiz-Perez et al. 2000) | ID, DWM | PD, SRTD, CED, Dw, OA | CHD | CA + BB | |||||||
| EVC | EVC2 (Galdzicka et al. 2002) | ID, DWM | PD, SRTD, CED, Dw, OA | CHD | CA + BB | |||||||
| - | IFT57 (Thevenon et al. 2016) | IFT57 | AR | FD, PD | CA | |||||||
| - | IFT52 (Girisha et al. 2016) | IFT52 | AR | VL, Nys | PD, BD, FD, SRTD | CA | ||||||
| Alstrom and Usher syndrome | ||||||||||||
| ALMS | ALMS1 (Hearn et al. 2002) | ALMS1 | AR | NPHP, CAKUT | RP, Nys | LF | Skeletal anomalies | DCM | SD, OB, HT, HU, Dm | BB | ||
| USH1B | MYO7A (Weil et al. 1995) | MYO7A | AR | RP, VL | DD | SD | ||||||
| USH1C | USH1C (Verpy et al. 2000) | USH1C/HARMONIN | AR | RP, VL | SD | |||||||
| USH1D | CDH23 (Bolz et al. 2001) | CDH23 | AR/DR | RP, VL | SD | |||||||
| USH1E | USH1E (Chaib et al. 1997) | USH1E | AR | RP, VL | SD | |||||||
| USH1F | PCDH15 (Ahmed et al. 2001) | PCDH15 | AR | RP, VL | SD | |||||||
| USH1G | SANS (Weil et al. 2003) | SANS | AR | RP, VL | SD | |||||||
| USH1J/DFNB48 | CIB2 (Riazuddin et al. 2012) | AR | RP, VL | SD | ||||||||
| USH2A/RP39 | USH2A (Eudy et al. 1998) | USHERIN | AR | RP, VL | SD | |||||||
| USH2C | GPR98 (Weston et al. 2004) PDZD7, DR (Ebermann et al. 2010) | GPR98 | AR/DR | RP, VL | SD | |||||||
| USH2D/ DFNB31 | WHRN (Ebermann et al. 2007) | WHRN | AR | RP, VL | SD | |||||||
| USH3A | CLRN1 (Joensuu et al. 2001) | CLRN1 | AR | RP, VL | SD | |||||||
| USH3B | HARS (Puffenberger et al. 2012) | HARS | AR | RP, VL | SD | |||||||
Columns represent disease name, gene symbol, encoded protein/aliases, human disease phenotype, and subcellular localization. The first description of each gene is cited in the second column. Diseases are grouped as (I) Nephronophthisis (NPHP), Senior–Loken syndrome (SLS), Joubert syndrome (JBTS), Meckel–Gruber syndrome (MKS); (II) Bardet–Biedl syndrome (BBS); and (III) skeletal ciliopathies, oral-facial-digital syndrome (OFD), cranioectodermal dysplasia (CED), and short-rib thoracic dysplasia (SRTD). Grouping is in accordance with Figures 1 and 2.
ACS, acrocallosal syndrome; AE, anencephaly; AR, autosomal recessive; BB, basal body; BD, brachydactyly; BDP, bile duct proliferation; CA, ciliary axoneme; CAKUT, congenital anomalies of the kidney and urinary tract; CB, coloboma; CBD, congenital brain defects; CHD, congenital heart defect; COACH, COACH (cerebellar vermis defect, oligophrenia, ataxia, coloboma, hepatic fibrosis) syndrome; CSE, cone-shaped epiphyses; CVH, cerebellar vermis hypoplasia; DCM, dilated cardiomyopathy; DD, developmental delay; DK, dysplastic kidneys; Dm, diabetes mellitus; DPM, ductal plate malformations; DR, digenic recessive; Dw, dwarfism; DWM, Dandy–Walker malformation; EVC, Ellis–van Creveld syndrome; FD, facial dysmorphism; HC, hydrocephalus; HF, hydrops fetalis; HLS, hydrolethalus syndrome; HT, hypertension; HTX, heterotaxia; HU, hyperuricemia; ID, intellectual disability; JATD, Jeune asphyxiating thoracic dystrophy; LC, liver cysts; LF, liver fibrosis; MaC; macrocephaly; MiC; microcephaly; MKKS, McKusick–Kaufman syndrome; MO, microphthalmia, MORM, MORM (mental retardation, truncal obesity, retinal dystrophy, and micropenis) syndrome; MPD, multiorgan polycystic disease; Ncl, nuclear; Nys, nystagmus; OA, oral anomalies; OB, obesity; OE, occipital omphalocele; OH, oligohydramnios; OMA, oculomotor apraxia; OMIM, Online Mendelian Inheritance in Man, Johns Hopkins University; OpA, optic atrophy; PD, polydactyly; PF, pancreatic fibrosis; PC, pancreatic cysts; PH, pulmonary hypoplasia; RA, renal agenesis; RC, renal cysts; RF, renal failure; RHPD1, renal-hepatic-pancreatic dysplasia, type 1; RP, retinitis pigmentosa; SD, sensorineural deafness; SGBS2, SI, situs inversus; SZ, seizures; Simpson–Golabi–Behmel syndrome, type 2; TA, trident acetabulum; TZ, transition zone; VL, vision loss; VSD, ventricular septal defect; VUS, variant of unknown significance.
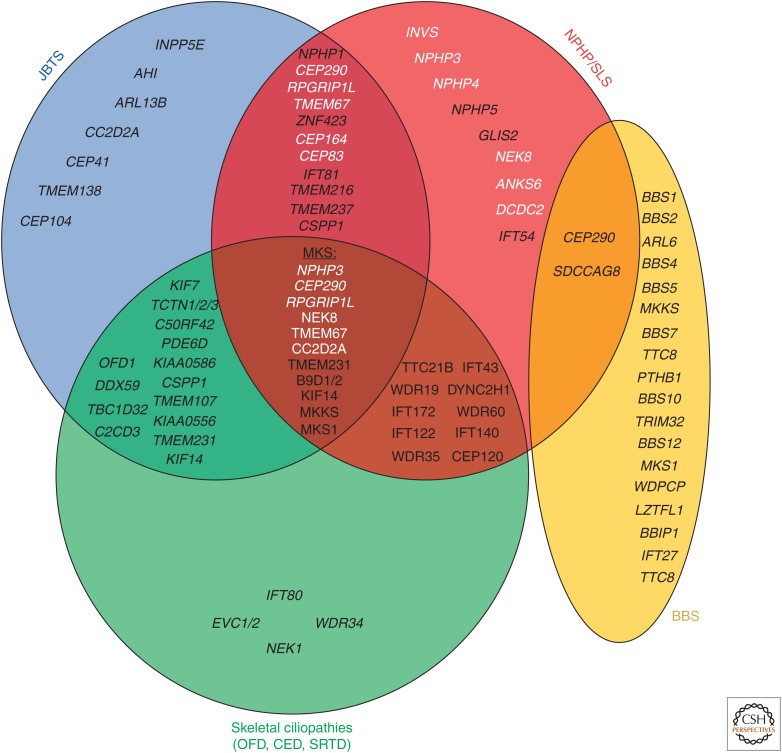
Figure 1.
Monogenic genes of nephronophthisis-related ciliopathies (NPHP-RC) cause distinct but widely overlapping phenotypes. Monogenic genes of NPHP-RC are categorized into four major phenotypes, namely, JBTS (blue, Joubert syndrome [JBTS]: congenital brain malformations, cerebellar vermis hypoplasia, and intellectual disability), NPHP/SLS (red, nephronophthisis/Senior–Loken syndrome [SLS]: nephronophthisis [NPHP], retinal degeneration, coloboma), BBS (yellow, Bardet–Biedl syndrome [BBS]: obesity, intellectual disability, retinal degeneration, cystic kidney disease, polydactyly, hypogonadisms), and skeletal ciliopathies (green, oral-facial-digital syndrome [OFD], cranioectodermal dysplasia (CED), short-rib thoracic dysplasia [SRTD]). As shown in a Venn diagram, numerous genes can give rise to overlapping phenotypes if mutated. Meckel–Gruber syndrome (MKS), the most severe clinical manifestation of NPHP-RC, can be caused by mutations in 10 monogenic genes. With the exception of the genes B9D1 and B9D2 that have not been described in association with other phenotypes, MKS is caused by mutations in monogenic genes of nephronophthisis, Joubert syndrome, and Bardet–Biedl syndrome on an allelic basis. White text indicates genes in which liver involvement has been reported.
NEPHRONOPHTHISIS AND RELATED DISORDERS
Nephronophthisis (NPHP) is an inherited disease that represents one of the most frequent monogenic causes of end-stage renal failure in children and young adults. NPHP is inherited in an autosomal-recessive manner, and currently mutations in up to 90 genes have been identified as disease causing. Depending on the composition of the examined cohort, mutations in these known monogenic genes account for up to 63% of cases (Braun et al. 2015). The first genetic cause of NPHP, the gene NPHP1, which encodes the protein nephrocystin was described in 1997 (Hildebrandt et al. 1997a; Saunier et al. 1997). It later became apparent that homozygous deletions in the NPHP1 gene are the most frequent cause of NPHP and that mutations in this gene alone account for 20%–25% of all cases (Halbritter et al. 2013b). Each of the subsequently identified monogenic genes accounts only for a small fraction of affected individuals (Halbritter et al. 2013b). The initial clinical presentation of NPHP is typically mild, and an increase in serum-creatinine, as an indicator of impaired renal function, is typically not noted before an average age of 9 years (Gretz 1989). Clinical symptoms are polyuria with secondary enuresis, polydipsia with regular fluid intake at night, anemia, and growth retardation (Kleinknecht 1989). Based on the age of onset, NPHP is further categorized into infantile (Gagnadoux et al. 1989), juvenile (Hildebrandt et al. 1992), and adolescent (Omran et al. 2000) NPHP, in which the median onset of clinical symptoms is at an age of 1, 13, and 15 years, respectively. In contrast to other renal diseases, patients usually do not develop arterial hypertension until the renal function is severely impaired. The clinical diagnosis of NPHP is typically based on renal ultrasound presentation with normal or small-sized kidneys, increased renal echogenicity, and loss of corticomedullary differentiation. Renal cysts in NPHP are not a necessary diagnostic criterion, and, if present, are rare, remain small, and are typically located in the corticomedullary junction region. This is in contrast to autosomal-dominant or -recessive polycystic kidney disease in which cysts are large and arise from all regions of the kidney. Hallmarks of NPHP in renal histology are thickening and disintegration of the tubular basement membrane, atrophy of renal tubular structure, and tubulointerstitial fibrosis. The prevalence of NPHP does not show regional clustering or gender predisposition (Kleinknecht 1989). Patients with NPHP have been described worldwide and in all ethnicities (Kleinknecht 1989). The incidence of NPHP has been reported as nine patients/8.3 million in the United States (Potter et al. 1980) or as one in 50,000 live births in Canada (Waldherr et al. 1982; Pistor et al. 1985). A North American study assessing causes of end-stage renal failure in pediatric patients estimated that NPHP-related ciliopathies account for ∼5% of all cases (Avner 1994; Warady et al. 1997).
Because of shared features in renal histology, namely, cysts that primarily arise from the corticomedullary region and tubulointerstitial fibrosis, NPHP was previously grouped with medullary–cystic kidney disease (MCKD) under the term “NPHP–MCKD complex.” However, they are now considered distinct disease entities because of differences in mode of inheritance, age of onset, extrarenal involvement, and molecular disease cause. MCKD is an autosomal-dominant disease that presents with adult-onset chronic kidney disease and renal salt wasting. In contrast to NPHP, end-stage renal failure typically occurs around the sixth decade of life (Wolf et al. 2004). MCKD does not present as a syndromic disease; the only described extrarenal associations are hyperuricemia and gout. By now, two monogenic causes of MCDK have been described, namely, the genes MUC1 (MCKD, type 1; OMIM #174000) and UMOD (MCKD, type 2; OMIM #603860). Interestingly, the UMOD protein localizes to renal primary ciliary and colocalizes with other ciliary proteins such as nephrocystin (Zaucke et al. 2010). Besides MCKD2, mutations of UMOD also give rise to the renal diseases glomerulocystic kidney disease with hyperuricemia and isosthenuria (OMIM #609886) and familial juvenile hyperuricemic nephropathy (OMIM #162000).
RENAL PHENOTYPE OF NPHP
Clinical Presentation of NPHP
The clinical presentation of NPHP was first described in 1951 by Guido Fanconi who introduced the term “nephronophthisis,” which translates as disappearance of nephrons to describe the renal histology of affected children (Fanconi et al. 1951). With the exception of infantile NPHP, caused by mutations in the gene IVSN (NPHP type 2), clinical symptoms of NPHP typically start at an average age of 4–6 years (Kleinknecht 1989). The major pathology in this early phase is an impaired ability of the kidney to concentrate urine and retain water. This results in reduced urine osmolality and explains the hallmark symptoms of NPHP, namely, polyuria and polydipsia, which are present in ∼80% of affected patients (Kleinknecht 1989). Secondary enuresis and regular fluid intake at night are characteristic features of NPHP and should be specifically investigated when taking the patient’s history. In contrast to other pediatric kidney diseases edema, hypertension, and recurrent infections of the urinary tract are uncommon in patients with NPHP. Later in the course of disease progression, anemia and growth retardation occur. It has been noticed that, when adjusted to the degree of renal failure, both symptoms are pronounced in NPHP as compared to other renal diseases (Ala-Mello et al. 1996). With further deterioration of renal function, the clinical presentation is dominated by classical symptoms of end-stage renal failure, such as fatigue, weakness, pruritus, pallor, electrolyte imbalance, and fluid overload. If the diagnosis is missed at this stage of disease progression, patients are at risk for cardiac arrhythmia and sudden death because of electrolyte imbalances, particularly hyperkalemia.
Therapy and Prognosis of NPHP
Despite intensive research, a curative or targeted therapy for NPHP is currently not available. Treatment of NPHP is therefore centered on correcting the secondary consequences of chronic kidney disease, such electrolyte imbalances, fluid overload, anemia, renal osteodystrophy, secondary hyperparathyroidism, and growth retardation. Psychosocial counseling and intense patient/family education are an integral part of successful treatment strategies as these patients have to cope with a chronic condition that requires intense livelong therapy. Once end-stage renal disease (ESRD) has occurred, renal replacement therapy with either dialysis or renal transplantation is the only remaining treatment option. Recurrence of NPHP after renal transplant has never been reported (Steel et al. 1980). Across all subtypes of NPHP, chronic renal failure typically develops within the first three decades of life (Hildebrandt et al. 1997b; Haider et al. 1998; Omran et al. 2000). Onset of ESRD later than 30 years of age is uncommon in all types of NPHP. Therefore, the clinical diagnosis should be reevaluated and other differential diagnoses should be considered if renal failure has not occurred by age 30 years. Interestingly, a high concordance in the rate of deterioration of renal function has been noticed between monozygotic twins, suggesting that the individual genotype determines the disease course and the development of ESRD (Mongeau and Worthen 1967; Makker et al. 1973).
Renal Pathology in NPHP
Macroscopic Pathology
The renal pathology of NPHP differs considerably from other cystic kidney diseases such as autosomal-dominant and autosomal-recessive polycystic kidney disease (ARPKD/ADPKD) (Waldherr et al. 1982). The kidney size is typically normal or slightly reduced. Renal echogenicity is characteristically enhanced as a result of renal fibrosis. Cysts are present in ∼70% of affected individuals, but are not a prerequisite for the diagnosis of NPHP. Furthermore, progressive intestinal fibrosis rather than cyst development represents the primary pathogenic mechanism driving disease progression (Bernstein and Gardner 1992). If cysts are present, they are typically small in size, arise from the corticomedullary border region of the kidney, (Sherman et al. 1971) and develop late in the course of disease (Sworn and Eisinger 1972). In contrast to this classical renal phenotype, mutations in some monogenic genes of NPHP, notably the genes INVS (Otto et al. 2003b) and ANKS6 (Hoff et al. 2013) (NPHP types 2 and 16), can cause a phenotype that resembles ARPKD and is characterized by enlarged, multicystic kidneys (Igarashi and Somlo 2002). Cysts in other organs have not been described in isolated NPHP. However, severe forms of renal ciliopathies can present as multicystic, dysplastic developmental phenotypes affecting all organ systems. In NPHP, there is always bilateral renal involvement.
Microscopic Pathology
Histologic hallmark findings of NPHP are summarized in a histological triad consisting of (a) disintegration and irregular thickening of the tubular basement membrane (TBM), (b) interstitial inflammation with round-cell infiltration and consecutive fibrosis, (c) tubular atrophy and cyst formation at the corticomedullary junction (Zollinger et al. 1980; Waldherr et al. 1982). Cyst formation in NPHP is thought to be secondary to degeneration and atrophy of kidney tissue rather than being an independent, primary process triggering disease progression. Early histological changes in NPHP are most prominent in distal tubules and, in contrast to other monogenic kidney disease, cells of the renal glomerulus are characteristically unaffected in NPHP. Transmission electron microscopy (TEM) in NPHP reveals characteristic changes of the tubular membrane, namely, thickening, attenuation, splitting, and granular disintegration. The presence of these alterations is discontinuous, and shows wide variability between different sections of an affected kidney. In end-stage NPHP, the histology is dominated by severe, diffuse sclerotic tubulointerstitial nephropathy.
Diagnostics and Differential Diagnosis of NPHP
Molecular genetic analysis represents the only method to diagnose NPHP-RC with certainty and thus provide patients and families with an unequivocal diagnosis. Because of an increasing number of potentially causative monogenic genes as well as advances in next-generation sequencing, whole-exome sequencing has mostly replaced targeted-sequencing panels in the diagnosis of NPHP-RC (Halbritter et al. 2012, 2013b; Gee et al. 2014). By applying this method, a causative single-gene mutation can be detected in up to 60% of cases depending on the composition of the cohort (Braun et al. 2015). However, if no mutation is detected, the diagnosis of NPHP is not excluded. Importantly, genetic testing should always be combined with thorough phenotyping as well as genetic counseling. Renal ultrasound imaging represents a very useful tool for the early diagnosis of otherwise asymptomatic renal ciliopathies. Characteristic findings include increased echogenicity, loss of corticomedullary differentiation and corticomedullary cysts (Blowey et al. 1996; Aguilera et al. 1997; Ala-Mello et al. 1998). Renal ultrasonography can help to distinguish NPHP from other inherited kidney diseases such as congenital anomalies of the kidney and urinary tract (CAKUT) or nephrocalcinosis. In some cases, magnetic resonance imaging (MRI) can provide additional insides. Other than molecular genetic testing, there is no laboratory test that specifically detects NPHP. However, serum chemistry analysis is very useful to monitor progressive renal impairment, as well as complications of chronic kidney disease. Unaffected siblings should be carefully examined and closely monitored to allow early diagnosis. However, it is generally not recommended to perform genetic testing in unaffected siblings to avoid stigmatization (Ross et al. 2013). In contrast to other monogenic kidney diseases, proteinuria, hematuria, or recurrent infections are characteristically absent in NPHP. Lack of hypertension, differences in kidney size, and distinct localization of cysts, can help to distinguish NPHP from other forms of cystic kidney disease. However, especially in late stages differential diagnosis can be challenging.
EXTRARENAL MANIFESTATIONS OF NPHP-RELATED CILIOPATHIES
Renal ciliopathies are frequently associated with additional clinical symptoms that affect other organ systems than the kidney. Typical extrarenal manifestations of renal ciliopathies include retina, central nervous system (CNS), liver, and bones. The association of NPHP with retinal degeneration (Senior–Loken syndrome) is most frequent and occurs in ∼10% of all patients with NPHP (Loken et al. 1961; Senior et al. 1961). Furthermore, NPHP can be accompanied by oculomotor apraxia (Cogan syndrome) (Betz et al. 2000), by cerebellar vermis hypoplasia and retinal coloboma (Joubert syndrome) (Saraiva and Baraitser 1992; Valente et al. 2006), by liver fibrosis (Boichis et al. 1973), by ectodermal dysplasia (Sensenbrenner syndrome, oral-facial-digital syndrome type I) (Bredrup et al. 2011; Fehrenbach et al. 2014), and in rare cases by congenital heart defects (Otto et al. 2003a; Rajagopalan et al. 2016). Skeletal malformations associated with renal ciliopathies include thoracic dystrophy (Jeune syndrome) (Halbritter et al. 2013a; Huber et al. 2013; Schmidts et al. 2013a), poly- and brachydactyly, and cone-shaped epiphysis (Mainzer-Saldino syndrome) (Perrault et al. 2012). In addition, patients with infantile NPHP can show heterotaxy, situs inversus and potentially lung involvement (Otto et al. 2003a). Because extrarenal symptoms can be discrete but result in severe complications, screening of affected patients is generally recommended. Therefore, in addition to thorough genotyping, all patients should undergo ophthalmoscopy to exclude retinal involvement, as well as liver ultrasound examination and laboratory test for liver function. Characteristic extrarenal phenotypes for monogenic genes of human ciliopathies are summarized in Table 1. As shown in Figure 1, there is broad phenotypic overlap between different monogenic disease genes. Advances in next-generation sequencing have drastically accelerated the identification of novel single-gene causes of human ciliopathies over the last years. With increasing numbers of identified disease genes, it has become apparent that the proteins encoded by genes responsible for different subtypes of ciliopathies cluster in protein complexes that localize to distinct subcellular localizations (Fig. 2).
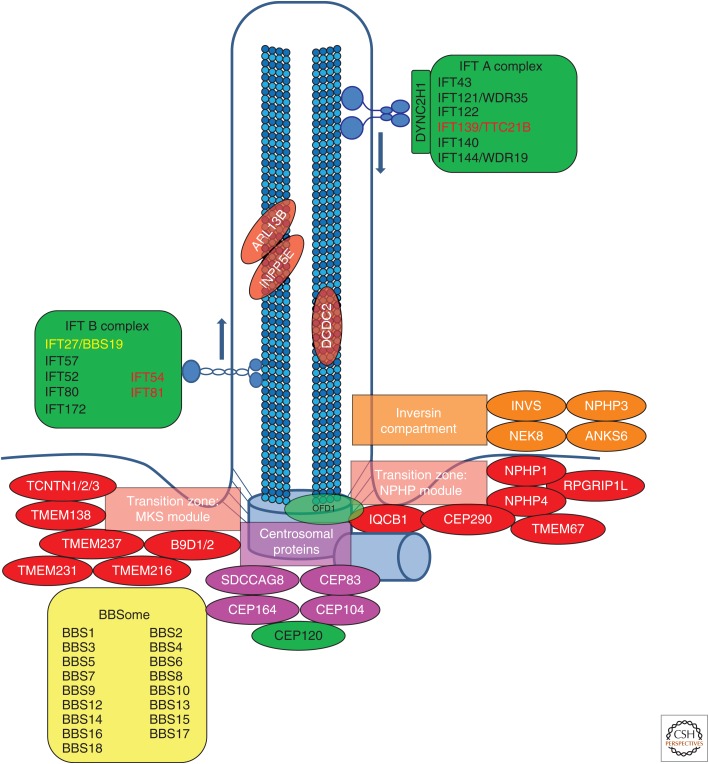
Figure 2.
Subcellular localization of proteins encoded by monogenic genes of nephronophthisis-related ciliopathies (NPHP-RC). Subcellular localization of proteins encoded by monogenic genes of NPHP-RC is depicted. Proteins are color-coded based on their respective disease group as shown in Table 1 (red/orange/pink: nephronophthisis [NPHP], Senior–Loken syndrome [SLS], Joubert syndrome [JBTS], Meckel–Gruber syndrome [MKS]; yellow: Bardet–Biedl syndrome [BBS]; green: skeletal ciliopathies). It becomes apparent that disease groups cluster to distinct subcellular localizations. IFT, Intraflagellar transport.
Retinal Involvement (Senior–Løken Syndrome)
Senior–Løken syndrome (SLS) or renal–retinal dysplasia describes the syndromic association of NPHP with retinal involvement. This association was first reported by Loken et al. (1961), Senior et al. (1961), and Fairley et al. (1963). The retinal pathology in SLS has been described as retinitis pigmentosa, tapetoretinal degeneration, and retinal aplasia, which likely represent a phenotypic spectrum ranging from dysplastic, developmental, to degenerative defects (Saraux et al. 1970). Similarly, early- and late-onset forms of SLS can be distinguished based on age of onset and clinical presentation. The early onset type (a form of Leber congenital amaurosis) typically manifests at birth with blindness, reduced or absent response in electroretinography, and nystagmus. If not present at birth, disease symptoms develop rapidly within the first 2 years of life (Medhioub et al. 1994). The late-onset form typically develops slowly, starting with night blindness and progressive vision loss during school age. The clinical diagnosis is based on electroretinography (ERG) that shows a constant and complete extinction of response to stimuli reflecting retinal degeneration, as well as characteristic findings on funduscopy. Typically, ophthalmoscopic alterations are present in all SLS patients by the age of 10 years. Specific findings in ophthalmoscopy in these patients include retinitis pigmentosa, characterized by increased retinal pigmentation, attenuation of retinal vessels, atrophy of retinal pigment epithelium, pallor of the optic disc, and taptoretinal degeneration. Additional eye symptoms include atrophy of the optic nerve, nystagmus, choroidal coloboma, and ametropia (myopia, hyperopia, amblyopia, and strabismus) (Adams et al. 2007). Retinitis pigmentosa is the most common extrarenal manifestation of NPHP-related ciliopathies. It occurs in ∼10% of affected individuals and affects predominantly patients with causative mutations in the genes IQCB1/NPHP5 (Otto et al. 2005) and CEP290/NPHP6 (Valente et al. 2006). Remarkably, to date, all described cases of mutations of IQCB1/NPHP5 showed retinal involvement (Otto et al. 2005). However, it can be present in virtually all types of NPHP. Interestingly, the renal phenotype of patients with and without retinal involvement is indistinguishable.
Oculomotor Apraxia (Cogan Syndrome)
Oculomotor apraxia, type Cogan, is a disorder of the CNS that affects the coordination of voluntary eye movements. Affected individuals are unable to execute purposeful horizontal eye movement to focus on or follow an object of interest. The clinical presentation is dominated by jerking head movements or compensatory head turning for side vision, as well as opticokinetic nystagmus. This symptom has been described in patients with mutations in the genes NPHP1 (Betz et al. 2000) and NPHP4 (Mollet et al. 2002). Frequently, the defect is transient and improves after the first years of life. In contrast to retinitis pigmentosa, in which the defect lies in the eye itself, the underlying defect in Cogan syndrome affects central nervous regions responsible for motor control of eye movements, such as the nuclei of the abducens or oculomotoric nerves as well as supranuclear control regions.
Cerebellar Vermis Hypoplasia (Joubert Syndrome)
Joubert syndrome is a developmental disorder that manifests with a complex phenotype and involves various organ systems. Most prominent are developmental defects of the CNS, particularly the brain stem region and the cerebellum (Romani et al. 2013). The hallmark symptom of Joubert syndrome on radiology imaging of the CNS, namely, the so-called molar tooth sign, is explained by agenesis or hypoplasia of the cerebellar vermis. Furthermore, occipital meningo- or meningomyocele can be present in some cases. Clinically affected patients present with various degrees of psychomotor retardation, intellectual disability, and disrupted motor coordination causing hypotonia, ataxia, and oculomotor apraxia. Severe cases can manifest with defects of neonatal breathing regulation and newborn tachypnea. Outside the CNS, Joubert syndrome can involve facial dysmorphism, retinal dysplasia, coloboma of the optic nerve, and, less frequently, polydactyly, renal cysts, and hepatic fibrosis. Genetically, Joubert syndrome is remarkably heterogeneous and, by now, 27 monogenic genes have been identified as causative when mutated. Among the first monogenic genes to be described in patients with Joubert syndrome were the genes NPHP1 (Parisi et al. 2004) and AHI (Dixon-Salazar et al. 2004), which encode for a protein called Jouberin. Jouberin interacts with NPHP1, NPHP3, and NPHP4 and, interestingly, Jouberin as well as other proteins that are altered in Joubert syndrome localizes to a defined region of primary cilia, the so-called transition zone (Fig. 2) (Omran 2010). With increasing numbers of molecularly diagnosed patients, specific genotype–phenotype correlations have been observed for specific genes (Hildebrandt et al. 2011). For example, mutations in NPHP1/JBTS4 typically cause isolated nephronophthisis with Joubert syndrome (Parisi et al. 2004), whereas mutations in CEP290/JBTS5 (Sayer et al. 2006; Valente et al. 2006) and RPGRIP1L/JBTS7 (Arts et al. 2007; Delous et al. 2007) frequently show retinal–renal syndrome, although mutations of TMEM67/JBTS6 characteristically involve liver fibrosis (Table 1; Fig. 1) (Otto et al. 2009).
Renal Ciliopathies with Liver Involvement
In patients with mutations in the genes NPHP3 (Olbrich et al. 2003), TMEM67 (Otto et al. 2009), ANKS6 (Hoff et al. 2013), and most recently DCDC2 (Schueler et al. 2015), renal ciliopathies can be associated with liver involvement. The liver phenotype in these patients is characterized by hepatomegaly, periportal liver fibrosis, ductal plate malformations, and bile duct proliferation. The phenotype of renal–hepatic ciliopathies is characteristically different from congenital liver fibrosis, which is dominated by dysplasia and agenesis of bile ducts. The association of cerebellar vermis hypoplasia, oligophrenia, congenital ataxia, ocular coloboma, and hepatic fibrosis is known as COACH syndrome and is most frequently caused by mutations of TMEM67 (NPHP11/JBTS6/MKS3) (Brancati et al. 2009) or in rare cases by mutations of CC2D2A or RPGRIP1L (Doherty et al. 2010). In patients with infantile nephronophthisis (caused by mutations of NPHP2), a transient elevation of liver enzymes without histopathologic changes or disturbed liver architecture has been described (Haider et al. 1998). Interestingly, in some cases, different phenotypes can be caused by mutations of the same gene on an allelic basis. Among others, this is true for mutations of the gene ANKS6 in which protein-truncating nonsense mutations results in a severe, syndromic phenotype that involves liver fibrosis, whereas hypomorphic mutations results in isolated nephronophthisis only (Hoff et al. 2013).
Skeletal Phenotypes and Polydactyly
Short-rib thoracic dysplasia with and without polydactyly (SRTD) describes a group of ciliopathies with skeletal involvement, including poly-/brachydactyly, shortening of the long bones, cone-shaped epiphysis, and malformations of the ribs and thoracic cage. The most severe form, which is known as Jeune syndrome or asphyxiating thoracic dystrophy (ATD), is characterized by severely shortened ribs resulting in respiratory distress after birth (Jeune et al. 1955; Donaldson et al. 1985). Other diseases summarized in this group are Ellis–van Creveld syndrome (chondroectodermal dysplasia, OMIM #225500) (Moudgil et al. 1998) and Mainzer–Saldino syndrome (Mortellaro et al. 2010). The phenotypes observed in cranioectodermal dysplasia (Sensenbrenner syndrome, OMIM #218330) partially overlap with those of SRTD (Gilissen et al. 2010). Interestingly, many of the proteins encoded by genes that are mutated in patients with skeletal ciliopathies play a role in ciliary transport, such as the components of the intraflaggellar transport (IFT) modules or dynein motor components (Fig. 2) (Perrault et al. 2012; Halbritter et al. 2013a; Schmidts et al. 2013a). It has been postulated that disruption of sonic hedgehog signaling, a pathway that is particularly important for bone morphogenesis and that depends on intact primary cilia for signal transduction, represents the molecular pathogenic link between ciliary dysfunction and skeletal anomalies (Huangfu et al. 2003; Qin et al. 2011).
Laterality Defects and Congenital Heart Disease
Although laterality defects such as situs inversus and situs solitus are frequently observed in patients with defects of motile cilia, they have only been described in very limited subgroups of patients with NPHP-RC. The most prominent example for this combination of phenotypes is represented by patients with mutations in the gene NPHP2/inversin, in which the association between infantile nephronophthisis and situs inversus was first described in 2003 (Otto et al. 2003a). Similar to what had been observed in the inv/inv knockout mouse (Mochizuki et al. 1998; Morgan et al. 1998), patients also showed congenital heart defects, such as heterotaxy, ventricular septum defects, and vascular malformations (Otto et al. 2003a). Comparably, knockdown of the ortholog of the inversin gene in zebrafish results in randomization of heart looping (Otto et al. 2003a). With growing numbers of detected mutations, there is increasing evidence that also proteins encoded by other monogenic genes of NPHP-RC play a role in the determination of left–right asymmetry. For example, nonsense mutations in the gene NPHP3 cause a severe developmental phenotype resulting in embryonic lethality, which among other features also includes situs inversus as well as congenital heart defects (Bergmann et al. 2008). Similarly, situs inversus and congenital heart defects have recently been described in patients with mutations of ANKS6 (Hoff et al. 2013) and NEK8 (Frank et al. 2013). Interestingly, the encoded proteins of all four genes interact and colocalize to a specific compartment of primary cilia, the so-called transition zone (Czarnecki et al. 2015). This finding underlines that specific “proteins modules” may give rise to distinct clinical phenotypes.
Other Syndromic Ciliopathies
Meckel–Gruber syndrome (MKS, OMIM #249000) represents the most severe clinical manifestation of human ciliopathies. It is an autosomal-recessive developmental disorder that can affect various organ systems and shows vast phenotypic pleiotropy. Frequently, the combination of severe developmental defects results in embryonic or perinatal lethality. Characteristic symptoms include malformations of the CNS, such as hydrocephalus, microcephaly, occipital encephalocele, and, most severely, complete anencephaly. In addition, most patient show cystic–dysplastic kidney disease, liver involvement, congenital heart defects, dysmorphic features, and skeletal malformation, predominantly polydactyly. MKS presents with oligohydramnios caused by impaired kidney function, and can be diagnosed on perinatal ultrasound. With the exception of the genes B9D1 (Hopp et al. 2011) and B9D2 (Dowdle et al. 2011) that have not been described in association with other phenotypes, MKS is caused by mutations in monogenic genes of nephronophthisis, Joubert syndrome, and Bardet–Biedl syndrome on an allelic basis. Typically, truncating mutations give rise to the severe developmental phenotype of MKS, whereas hypomorphic mutations result in limited, organ specific disease that is degenerative rather than developmental (Hildebrandt et al. 2011).
Oral-facial-digital syndrome, type 1 (OFD1, OMIM #311200) is an X-linked dominant ciliopathy with prominent dysmorphic and external features. Because the disease is embryonic lethal in males, all surviving affected patients are female. The syndrome is characterized by oral anomalies (i.e., defective dentation, cleft lip and palate, lobulated tongue), facial dysmorphism, and polydactyly, as well as other malformations of fingers and toes. About 50% of affected individuals show structural defects of the CNS, including microcephaly, defective gyration, agenesis of the corpus callosum, and arachnoidal cysts. Seizures and variable degrees of intellectual disability can be present (Macca and Franco 2009). In contrast to other subtypes of oral-facial-digital syndrome, adult-onset cystic kidney disease has only been described in patients with OFD type 1 in which it is present in ∼50% of cases. Furthermore, fibrocystic liver disease with elevated liver enzymes can be present (Chetty-John et al. 2010). The syndrome is caused by mutations in the gene OFD1, which on an allelic basis can also give rise to Joubert syndrome (JBTS10, OMIM #300804) as well as Simpson–Golabi–Behmel syndrome type 2 (GBS2, OMIM #300209). Interestingly, the gene product of OFD1 localizes to the centrosome and ciliary basal body (Chetty-John et al. 2010).
Bardet–Biedl syndrome (BBS, OMIM #209900) is a complex, syndromic ciliopathy that affects numerous organ systems. Classical symptoms include intellectual disability and behavioral anomalies, as well obesity, cystic kidney disease, retinitis pigmentosa, hypogonadism, and polydactyly. BBS is genetically very heterogeneous and by now, mutations in 19 monogenic genes have been described as causative. Although BBS is typically inherited in an autosomal-recessive manner, recent publications have suggested that digenic inheritance may also be possible (Katsanis et al. 2001b; Fan et al. 2004; Katsanis 2004). Thus far, BBS is the only ciliopathy for which a mode of inheritance different from strictly recessive (autosomal or X-linked) has been described. Interestingly, the protein products encoded by known monogenic genes of BBS interact with each other to form a tight protein complex, the so-called BBSome, which carries important functions for ciliary trafficking (Loktev et al. 2008; Jin and Nachury 2009; Zhang et al. 2013).
Alstrom syndrome (OMIM #203800) is an autosomal-recessive disorder that involves multiple organ systems and resembles BBS in some aspects of its clinical presentation. Prominent symptoms include progressive sensineural deafness and vision loss caused by cone-rod degeneration, as well dilated cardiomyopathy with congestive heart failure. Affected patients show childhood-onset obesity and can have endocrine disorders, predominantly diabetes mellitus type 2. In some cases, kidney involvement with progressive renal failure, recurrent pulmonary infections, and hepatomegaly have been described. To date, the gene ALMS1 (Collin et al. 2002; Hearn et al. 2002) is the only described single-gene cause of Alstrom syndrome.
Usher syndrome (OMIM #276901) is a ciliopathy that is characterized by sensorineural deafness and progressive vision loss caused by retinal degeneration. Based on severity and age of onset, Usher syndrome is further classified in subtypes 1, 2, and 3. In the most severe type 1, children are predominantly born deaf and progressive vision loss begins in childhood. The syndrome is genetically heterogeneous and, by now, mutations in 14 genes have been identified as causative in humans (Table 1). Although the mode of inheritance is classically autosomal-recessive, digenic inheritance has been described for some genes.
MOLECULAR GENETICS OF NEPHRONOPHTHISIS-RELATED CILIOPATHIES
Identification of monogenic causes of NPHP-RC has furthered the understanding of its pathogenesis. The observation that the protein products of most NPHP-RC genes localizes to primary cilia, basal bodies, or centrosomes has led to the term ciliopathies to classify this group of diseases (Hildebrandt et al. 2011). Mutational analysis allows an etiologic classification of different subtypes of NPHP-RC, and enables physicians to provide patients and families with an unequivocal diagnosis. For the identification of the first single-gene causes of NPHP, namely, NPHP1-GLIS2/NPHP7, researchers relied on linkage analysis and positional cloning. Because of recent progress in next-generation sequencing, gene discovery has exponentially increased and by now, in up to 63% of affected individuals, a causative single gene mutation can be identified (Halbritter et al. 2012, 2013b; Braun et al. 2015). The combination of whole-exome sequencing with homozygosity mapping in consanguineous kindred, or with linkage analysis in familial cases, has proven to be a powerful tool for the identification of novel ciliopathy genes (Otto et al. 2010; Chaki et al. 2012; Schueler et al. 2015). By now, 19 classical NPHP genes and more than 90 additional ciliopathy genes have been identified as mutated in affected individuals. With the exception of the gene NPHP1, which explains about 25% of all cases with NPHP, mutations in other subsequently identified genes each account for only small percentages of affected individuals.
Nephronophthisis Type 1 (NPHP1)
In 1997, using total genome search and linkage analysis, a gene locus for juvenile nephronophthisis was mapped to chromosome 2q12-q13 (Antignac et al. 1993; Hildebrandt et al. 1993). Molecular cloning subsequently allowed the identification of the gene NPHP1 within that region (Hildebrandt et al. 1997a; Saunier et al. 2000). Future studies showed that about 25% of all patients with NPHP harbor causative mutations in this gene. About 85% of the mutations in NPHP1 are large homozygous deletions of the complete NPHP1 gene (Konrad et al. 1996; Saunier et al. 2000; Hildebrandt et al. 2001). The high degree of genomic rearrangements is caused by two large inverted duplications that flank the NPHP1 locus on both sites (Saunier et al. 2000). The NPHP1 locus was later described as one of 24 regions within the human genome in which sequence duplications results in hot spots of genomic instability that subsequently give rise to human disease (Bailey et al. 2002). In patients with mutations of NPHP, end-stage renal disease is reached at a median age of 13 years. About 10% show a combined phenotype of retinitis pigmentosa and NPHP (Caridi et al. 2000). The occurrence of oculomotor apraxia (Betz et al. 2000) and cerebellar vermis hypoplasia (Parisi et al. 2004) in patients with NPHP1 mutations has been described but is very rare. Interestingly, there is no recognizable correlation between genotype and phenotype.
EVOLUTIONARY CONSERVATION AND MODEL ORGANISMS OF NPHP-RC
Numerous ciliary proteins and their functions are highly conserved throughout evolution. Most strikingly, the ciliary intraflagellar transport system is conserved down to the green algae chlamydomonas rheinhardii, and this model system has proven very useful for the identification of novel proteins and mechanisms that are part of this machinery (Pedersen et al. 2006; Engel et al. 2012; Taschner et al. 2016). Orthologs of ciliary proteins are expressed in sensory neurons of the nematode Caenorhabditis elegans. Expression of green fluorescent protein (GFP)-tagged fusion proteins containing the promotor regions of the orthologs of the human ciliopathy genes NPHP1 and NPHP4, showed that these genes are specifically expressed in ciliated sensory neurons in the head and tail regions (Wolf et al. 2005). Interestingly, on RNAi knockdown of nph-1 and nph-4 male animals showed abnormal mating behavior (Wolf et al. 2005), which was similar to the phenotype observed on knockdown of the orthologs of PKD1 and PKD2 (Barr et al. 2001). Mutations in nphp-8, the C. elegans ortholog of RPGRIP1L, resulted in reduced ciliary length and functional impairment of ciliated neuron (abnormal dye filling and reduced chemotaxis) (Liu et al. 2011). C. elegans ciliated neurons are a unique tool for in vivo microscopy, and, as one example, this system has helped to unravel the evolutionary conserved role of the BBSome for the assembly of intraflagellar transport particles (Wei et al. 2012). Furthermore, the strong evolutionary conservation of ciliary genes allows, using the C. elegans system, to study newly identified human disease genes, and test for pathogenicity of specific allelic mutations in these genes (Roberson et al. 2015). Genetic mouse and zebrafish models, as well as Xenopus tropicalis are established vertebrate organisms to model and study gene defects that give rise to human ciliopathies. The inv/inv mouse, in which the murine ortholog of NPHP2 is mutated, shows phenotypic features compatible with the one described in affected patients, in particular, cystic kidney disease, disrupted left–right determination, congenital heart defects, and liver involvement (Phillips et al. 2004). Similarly, the pcy mouse, in which the ortholog of human NPHP3 is disrupted, shows tubulointerstitial renal fibrosis and cysts. The genetic zebrafish model scorpionhi459 (Duldulao et al. 2009) carries a zygotic null allele for the ortholog of the human ciliopathy gene ALR13b. Mutant zebrafish show a characteristic ciliopathy phenotype including body axis curvature and pronephic renal cysts (Duldulao et al. 2009). Furthermore, a canine model of NPHP (Finco et al. 1970, 1977; Finco 1976) and a chicken with a mutation of Talpid3 (Izpisua-Belmonte et al. 1992; Davey et al. 2006), the ortholog of the human Joubert gene KIAA0586 (Alby et al. 2015; Bachmann-Gagescu et al. 2015; Malicdan et al. 2015; Roosing et al. 2015), have been described.
CONCLUDING REMARKS
Identification of monogenic causes of human ciliopathies has furthered the understanding of underlying pathogenic mechanisms, and has implicated numerous cellular pathways in pathogenesis and disease progression. To date, no treatment for affected children is available, and further research will be needed to better understand the molecular causes of disease, and thereby identify future therapeutic strategies and potential drug targets.
Footnotes
Editors: Wallace Marshall and Renata Basto
Additional Perspectives on Cilia available at www.cshperspectives.org
REFERENCES
- Adams NA, Awadein A, Toma HS. 2007. The retinal ciliopathies. Ophthalmic Genet 28: 113–125. [DOI] [PubMed] [Google Scholar]
- Adly N, Alhashem A, Ammari A, Alkuraya FS. 2014. Ciliary genes TBC1D32/C6orf170 and SCLT1 are mutated in patients with OFD type IX. Hum Mutat 35: 36–40. [DOI] [PubMed] [Google Scholar]
- Aguilera A, Rivera M, Gallego N, Nogueira J, Ortuno J. 1997. Sonographic appearance of the juvenile nephronophthisis-cystic renal medulla complex. Nephrol Dial Transplant 12: 625–626. [DOI] [PubMed] [Google Scholar]
- Ahmed ZM, Riazuddin S, Bernstein SL, Ahmed Z, Khan S, Griffith AJ, Morell RJ, Friedman TB, Wilcox ER. 2001. Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am J Hum Genet 69: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akizu N, Silhavy JL, Rosti RO, Scott E, Fenstermaker AG, Schroth J, Zaki MS, Sanchez H, Gupta N, Kabra M, et al. 2014. Mutations in CSPP1 lead to classical Joubert syndrome. Am J Hum Genet 94: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala-Mello S, Kivivuori SM, Ronnholm KA, Koskimies O, Siimes MA. 1996. Mechanism underlying early anaemia in children with familial juvenile nephronophthisis. Pediatr Nephrol 10: 578–581. [DOI] [PubMed] [Google Scholar]
- Ala-Mello S, Jaaskelainen J, Koskimies O. 1998. Familial juvenile nephronophthisis. An ultrasonographic follow-up of seven patients. Acta Radiol 39: 84–89. [DOI] [PubMed] [Google Scholar]
- Alby C, Piquand K, Huber C, Megarbane A, Ichkou A, Legendre M, Pelluard F, Encha-Ravazi F, Abi-Tayeh G, Bessieres B, et al. 2015. Mutations in KIAA0586 cause lethal ciliopathies ranging from a hydrolethalus phenotype to short-rib polydactyly syndrome. Am J Hum Genet 97: 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldahmesh MA, Li Y, Alhashem A, Anazi S, Alkuraya H, Hashem M, Awaji AA, Sogaty S, Alkharashi A, Alzahrani S, et al. 2014. IFT27, encoding a small GTPase component of IFT particles, is mutated in a consanguineous family with Bardet–Biedl syndrome. Hum Mol Genet 23: 3307–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, et al. 2003. Basal body dysfunction is a likely cause of pleiotropic Bardet–Biedl syndrome. Nature 425: 628–633. [DOI] [PubMed] [Google Scholar]
- Antignac C, Arduy CH, Beckmann JS, Benessy F, Gros F, Medhioub M, Hildebrandt F, Dufier JL, Kleinknecht C, Broyer M, et al. 1993. A gene for familial juvenile nephronophthisis (recessive medullary cystic kidney disease) maps to chromosome 2p. Nat Genet 3: 342–345. [DOI] [PubMed] [Google Scholar]
- Arts HH, Doherty D, van Beersum SE, Parisi MA, Letteboer SJ, Gorden NT, Peters TA, Marker T, Voesenek K, Kartono A, et al. 2007. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet 39: 882–888. [DOI] [PubMed] [Google Scholar]
- Arts HH, Bongers EM, Mans DA, van Beersum SE, Oud MM, Bolat E, Spruijt L, Cornelissen EA, Schuurs-Hoeijmakers JH, de Leeuw N, et al. 2011. C14ORF179 encoding IFT43 is mutated in Sensenbrenner syndrome. J Med Genet 48: 390–395. [DOI] [PubMed] [Google Scholar]
- Attanasio M, Uhlenhaut NH, Sousa VH, O’Toole JF, Otto E, Anlag K, Klugmann C, Treier AC, Helou J, Sayer JA, et al. 2007. Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat Genet 39: 1018–1024. [DOI] [PubMed] [Google Scholar]
- Avner ED. 1994. Medullary cystic disease and medullary sponge kidney. In Primer on kidney diseases (ed. Greenberg A). Academic, Boston. [Google Scholar]
- Bachmann-Gagescu R, Phelps IG, Dempsey JC, Sharma VA, Ishak GE, Boyle EA, Wilson M, Marques Lourenco C, Arslan M, Shendure J, et al. 2015. KIAA0586 is mutated in Joubert syndrome. Hum Mutat 36: 831–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano JL, Ansley SJ, Leitch CC, Lewis RA, Lupski JR, Katsanis N. 2003. Identification of a novel Bardet–Biedl syndrome protein, BBS7, that shares structural features with BBS1 and BBS2. Am J Hum Genet 72: 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JA, Gu Z, Clark RA, Reinert K, Samonte RV, Schwartz S, Adams MD, Myers EW, Li PW, Eichler EE. 2002. Recent segmental duplications in the human genome. Science 297: 1003–1007. [DOI] [PubMed] [Google Scholar]
- Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. 2001. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol 11: 1341–1346. [DOI] [PubMed] [Google Scholar]
- Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J, et al. 2007. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet 39: 727–729. [DOI] [PubMed] [Google Scholar]
- Bergmann C, Fliegauf M, Bruchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kranzlin B, et al. 2008. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel–Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet 82: 959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein J, Gardner KD. 1992. Hereditary tubulo interstitial nephritis. In Oxford textbook of clinical nephrology (ed. Cotran RS, Brenner BM, Stein JH). Oxford University Press, Oxford. [Google Scholar]
- Betz R, Rensing C, Otto E, Mincheva A, Zehnder D, Lichter P, Hildebrandt F. 2000. Children with ocular motor apraxia type Cogan carry deletions in the gene (NPHP1) for juvenile nephronophthisis. J Pediatr 136: 828–831. [PubMed] [Google Scholar]
- Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, et al. 2009. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet 41: 1032–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizet AA, Becker-Heck A, Ryan R, Weber K, Filhol E, Krug P, Halbritter J, Delous M, Lasbennes MC, Linghu B, et al. 2015. Mutations in TRAF3IP1/IFT54 reveal a new role for IFT proteins in microtubule stabilization. Nat Commun 6: 8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blowey DL, Querfeld U, Geary D, Warady BA, Alon U. 1996. Ultrasound findings in juvenile nephronophthisis. Pediatr Nephrol 10: 22–24. [DOI] [PubMed] [Google Scholar]
- Boichis H, Passwell J, David R, Miller H. 1973. Congenital hepatic fibrosis and nephronophthisis. A family study. Q J Med 42: 221–233. [PubMed] [Google Scholar]
- Bolz H, von Brederlow B, Ramirez A, Bryda EC, Kutsche K, Nothwang HG, Seeliger M, del CSCM, Vila MC, Molina OP, et al. 2001. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat Genet 27: 108–112. [DOI] [PubMed] [Google Scholar]
- Brancati F, Iannicelli M, Travaglini L, Mazzotta A, Bertini E, Boltshauser E, D’Arrigo S, Emma F, Fazzi E, Gallizzi R, et al. 2009. MKS3/TMEM67 mutations are a major cause of COACH syndrome, a Joubert syndrome related disorder with liver involvement. Hum Mutat 30: E432–E442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DA, Schueler M, Halbritter J, Gee HY, Porath JD, Lawson JA, Airik R, Shril S, Allen SJ, Stein D, et al. 2015. Whole exome sequencing identifies causative mutations in the majority of consanguineous or familial cases with childhood-onset increased renal echogenicity. Kidney Int 89: 468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredrup C, Saunier S, Oud MM, Fiskerstrand T, Hoischen A, Brackman D, Leh SM, Midtbo M, Filhol E, Bole-Feysot C, et al. 2011. Ciliopathies with skeletal anomalies and renal insufficiency due to mutations in the IFT-A gene WDR19. Am J Hum Genet 89: 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budny B, Chen W, Omran H, Fliegauf M, Tzschach A, Wisniewska M, Jensen LR, Raynaud M, Shoichet SA, Badura M, et al. 2006. A novel X-linked recessive mental retardation syndrome comprising macrocephaly and ciliary dysfunction is allelic to oral-facial-digital type I syndrome. Hum Genet 120: 171–178. [DOI] [PubMed] [Google Scholar]
- Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attie-Bitach T, Holden KR, Dobyns WB, et al. 2008. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet 83: 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caridi G, Dagnino M, Gusmano R, Ginevri F, Murer L, Ghio L, Piaggio G, Ciardi MR, Perfumo F, Ghiggeri GM. 2000. Clinical and molecular heterogeneity of juvenile nephronophthisis in Italy: Insights from molecular screening. Am J Kidney Dis 35: 44–51. [DOI] [PubMed] [Google Scholar]
- Chaib H, Kaplan J, Gerber S, Vincent C, Ayadi H, Slim R, Munnich A, Weissenbach J, Petit C. 1997. A newly identified locus for Usher syndrome type I, USH1E, maps to chromosome 21q21. Hum Mol Genet 6: 27–31. [DOI] [PubMed] [Google Scholar]
- Chaki M, Airik R, Ghosh AK, Giles RH, Chen R, Slaats GG, Wang H, Hurd TW, Zhou W, Cluckey A, et al. 2012. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell 150: 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty-John S, Piwnica-Worms K, Bryant J, Bernardini I, Fischer RE, Heller T, Gahl WA, Gunay-Aygun M. 2010. Fibrocystic disease of liver and pancreas; under-recognized features of the X-linked ciliopathy oral-facial-digital syndrome type 1 (OFD I). Am J Med Genet Part A 152A: 2640–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang AP, Beck JS, Yen HJ, Tayeh MK, Scheetz TE, Swiderski RE, Nishimura DY, Braun TA, Kim KY, Huang J, et al. 2006. Homozygosity mapping with SNP arrays identifies TRIM32, an E3 ubiquitin ligase, as a Bardet–Biedl syndrome gene (BBS11). Proc Natl Acad Sci 103: 6287–6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin GB, Marshall JD, Ikeda A, So WV, Russell-Eggitt I, Maffei P, Beck S, Boerkoel CF, Sicolo N, Martin M, et al. 2002. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alstrom syndrome. Nat Genet 31: 74–78. [DOI] [PubMed] [Google Scholar]
- Czarnecki PG, Gabriel GC, Manning DK, Sergeev M, Lemke K, Klena NT, Liu X, Chen Y, Li Y, San Agustin JT, et al. 2015. ANKS6 is the critical activator of NEK8 kinase in embryonic situs determination and organ patterning. Nat Commun 6: 6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagoneau N, Goulet M, Genevieve D, Sznajer Y, Martinovic J, Smithson S, Huber C, Baujat G, Flori E, Tecco L, et al. 2009. DYNC2H1 mutations cause asphyxiating thoracic dystrophy and short rib-polydactyly syndrome, type III. Am J Hum Genet 84: 706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MG, Paton IR, Yin Y, Schmidt M, Bangs FK, Morrice DR, Smith TG, Buxton P, Stamataki D, Tanaka M, et al. 2006. The chicken talpid3 gene encodes a novel protein essential for Hedgehog signaling. Genes Dev 20: 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EE, Zhang Q, Liu Q, Diplas BH, Davey LM, Hartley J, Stoetzel C, Szymanska K, Ramaswami G, Logan CV, et al. 2011. TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat Genet 43: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, et al. 2007. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet 39: 875–881. [DOI] [PubMed] [Google Scholar]
- Dixon-Salazar T, Silhavy JL, Marsh SE, Louie CM, Scott LC, Gururaj A, Al-Gazali L, Al-Tawari AA, Kayserili H, Sztriha L, et al. 2004. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet 75: 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D, Parisi MA, Finn LS, Gunay-Aygun M, Al-Mateen M, Bates D, Clericuzio C, Demir H, Dorschner M, van Essen AJ, et al. 2010. Mutations in 3 genes (MKS3, CC2D2A and RPGRIP1L) cause COACH syndrome (Joubert syndrome with congenital hepatic fibrosis). J Med Genet 47: 8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson MD, Warner AA, Trompeter RS, Haycock GB, Chantler C. 1985. Familial juvenile nephronophthisis, Jeune’s syndrome, and associated disorders. Arch Dis Child 60: 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle WE, Robinson JF, Kneist A, Sirerol-Piquer MS, Frints SG, Corbit KC, Zaghloul NA, van Lijnschoten G, Mulders L, Verver DE, et al. 2011. Disruption of a ciliary B9 protein complex causes Meckel syndrome. Am J Hum Genet 89: 94–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duldulao NA, Lee S, Sun Z. 2009. Cilia localization is essential for in vivo functions of the Joubert syndrome protein Arl13b/Scorpion. Development 136: 4033–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebermann I, Scholl HP, Charbel Issa P, Becirovic E, Lamprecht J, Jurklies B, Millan JM, Aller E, Mitter D, Bolz H. 2007. A novel gene for Usher syndrome type 2: Mutations in the long isoform of whirlin are associated with retinitis pigmentosa and sensorineural hearing loss. Hum Genet 121: 203–211. [DOI] [PubMed] [Google Scholar]
- Ebermann I, Phillips JB, Liebau MC, Koenekoop RK, Schermer B, Lopez I, Schafer E, Roux AF, Dafinger C, Bernd A, et al. 2010. PDZD7 is a modifier of retinal disease and a contributor to digenic Usher syndrome. J Clin Invest 120: 1812–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel BD, Ishikawa H, Wemmer KA, Geimer S, Wakabayashi K, Hirono M, Craige B, Pazour GJ, Witman GB, Kamiya R, et al. 2012. The role of retrograde intraflagellar transport in flagellar assembly, maintenance, and function. J Cell Biol 199: 151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eudy JD, Weston MD, Yao S, Hoover DM, Rehm HL, Ma-Edmonds M, Yan D, Ahmad I, Cheng JJ, Ayuso C, et al. 1998. Mutation of a gene encoding a protein with extracellular matrix motifs in Usher syndrome type IIa. Science 280: 1753–1757. [DOI] [PubMed] [Google Scholar]
- Failler M, Gee HY, Krug P, Joo K, Halbritter J, Belkacem L, Filhol E, Porath JD, Braun DA, Schueler M, et al. 2014. Mutations of CEP83 cause infantile nephronophthisis and intellectual disability. Am J Hum Genet 94: 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairley KF, Leighton PW, Kincaid-Smith P. 1963. Familial visual defects associated with polycystic kidney and medullary sponge kidney. Br Med J 1: 1060–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Esmail MA, Ansley SJ, Blacque OE, Boroevich K, Ross AJ, Moore SJ, Badano JL, May-Simera H, Compton DS, et al. 2004. Mutations in a member of the Ras superfamily of small GTP-binding proteins causes Bardet–Biedl syndrome. Nat Genet 36: 989–993. [DOI] [PubMed] [Google Scholar]
- Fanconi G, Hanhart E, Albertini A, Uhlinger E, Dolivo G, Prader A. 1951. Die familiäre juvenile nephronophthise. Helv Paediatr Acta 6: 1–49. [PubMed] [Google Scholar]
- Fehrenbach H, Decker C, Eisenberger T, Frank V, Hampel T, Walden U, Amann KU, Kruger-Stollfuss I, Bolz HJ, Haffner K, et al. 2014. Mutations in WDR19 encoding the intraflagellar transport component IFT144 cause a broad spectrum of ciliopathies. Pediatr Nephrol 29: 1451–1456. [DOI] [PubMed] [Google Scholar]
- Ferland RJ, Eyaid W, Collura RV, Tully LD, Hill RS, Al-Nouri D, Al-Rumayyan A, Topcu M, Gascon G, Bodell A, et al. 2004. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat Genet 36: 1008–1013. [DOI] [PubMed] [Google Scholar]
- Ferrante MI, Giorgio G, Feather SA, Bulfone A, Wright V, Ghiani M, Selicorni A, Gammaro L, Scolari F, Woolf AS, et al. 2001. Identification of the gene for oral-facial-digital type I syndrome. Am J Hum Genet 68: 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filges I, Nosova E, Bruder E, Tercanli S, Townsend K, Gibson WT, Rothlisberger B, Heinimann K, Hall JG, Gregory-Evans CY, et al. 2014. Exome sequencing identifies mutations in KIF14 as a novel cause of an autosomal recessive lethal fetal ciliopathy phenotype. Clin Genet 86: 220–228. [DOI] [PubMed] [Google Scholar]
- Finco DR. 1976. Familial renal disease in Norwegian Elkhound dogs: Physiologic and biochemical examinations. Am J Vet Res 37: 87–91. [PubMed] [Google Scholar]
- Finco DR, Kurtz HJ, Low DG, Perman V. 1970. Familial renal disease in Norwegian Elkhound dogs. J Am Vet Med Assoc 156: 747–760. [PubMed] [Google Scholar]
- Finco DR, Duncan JD, Crowell WA, Hulsey ML. 1977. Familial renal disease in Norwegian Elkhound dogs: Morphologic examinations. Am J Vet Res 38: 941–947. [PubMed] [Google Scholar]
- Frank V, Habbig S, Bartram MP, Eisenberger T, Veenstra-Knol HE, Decker C, Boorsma RA, Gobel H, Nurnberg G, Griessmann A, et al. 2013. Mutations in NEK8 link multiple organ dysplasia with altered Hippo signalling and increased c-MYC expression. Hum mol genet 22: 2177–2185. [DOI] [PubMed] [Google Scholar]
- Gagnadoux MF, Bacri JL, Broyer M, Habib R. 1989. Infantile chronic tubulo-interstitial nephritis with cortical microcysts: Variant of nephronophthisis or new disease entity? Pediatr Nephrol 3: 50–55. [DOI] [PubMed] [Google Scholar]
- Galdzicka M, Patnala S, Hirshman MG, Cai JF, Nitowsky H, Egeland JA, Ginns EI. 2002. A new gene, EVC2, is mutated in Ellis–van Creveld syndrome. Mol Genet Metab 77: 291–295. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, et al. 2011. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet 43: 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee HY, Otto EA, Hurd TW, Ashraf S, Chaki M, Cluckey A, Vega-Warner V, Saisawat P, Diaz KA, Fang H, et al. 2014. Whole-exome resequencing distinguishes cystic kidney diseases from phenocopies in renal ciliopathies. Kidney Int 85: 880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilissen C, Arts HH, Hoischen A, Spruijt L, Mans DA, Arts P, van Lier B, Steehouwer M, van Reeuwijk J, Kant SG, et al. 2010. Exome sequencing identifies WDR35 variants involved in Sensenbrenner syndrome. Am J Hum Genet 87: 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girisha KM, Shukla A, Trujillano D, Bhavani GS, Hebbar M, Kadavigere R, Rolfs A. 2016. A homozygous nonsense variant in IFT52 is associated with a human skeletal ciliopathy. Clin Genet 10.1111/cge.12762. [DOI] [PubMed] [Google Scholar]
- Gorden NT, Arts HH, Parisi MA, Coene KL, Letteboer SJ, van Beersum SE, Mans DA, Hikida A, Eckert M, Knutzen D, et al. 2008. CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am J Hum Genet 83: 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretz N. 1989. Rate of deterioration of renal function in juvenile nephronophthisis. Pediatr Nephrol 3: 56–60. [DOI] [PubMed] [Google Scholar]
- Haider NB, Carmi R, Shalev H, Sheffield VC, Landau D. 1998. A Bedouin kindred with infantile nephronophthisis demonstrates linkage to chromosome 9 by homozygosity mapping. Am J Hum Genet 63: 1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbritter J, Diaz K, Chaki M, Porath JD, Tarrier B, Fu C, Innis JL, Allen SJ, Lyons RH, Stefanidis CJ, et al. 2012. High-throughput mutation analysis in patients with a nephronophthisis-associated ciliopathy applying multiplexed barcoded array-based PCR amplification and next-generation sequencing. J Med Genet 49: 756–767. [DOI] [PubMed] [Google Scholar]
- Halbritter J, Bizet AA, Schmidts M, Porath JD, Braun DA, Gee HY, McInerney-Leo AM, Krug P, Filhol E, Davis EE, et al. 2013a. Defects in the IFT-B component IFT172 cause Jeune and Mainzer-Saldino syndromes in humans. Am J Hum Genet 93: 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbritter J, Porath JD, Diaz KA, Braun DA, Kohl S, Chaki M, Allen SJ, Soliman NA, Hildebrandt F, Otto EA. 2013b. Identification of 99 novel mutations in a worldwide cohort of 1,056 patients with a nephronophthisis-related ciliopathy. Hum Genet 132: 865–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn T, Renforth GL, Spalluto C, Hanley NA, Piper K, Brickwood S, White C, Connolly V, Taylor JF, Russell-Eggitt I, et al. 2002. Mutation of ALMS1, a large gene with a tandem repeat encoding 47 amino acids, causes Alstrom syndrome. Nat Genet 31: 79–83. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Otto E, Rensing C, Nothwang HG, Vollmer M, Adolphs J, Hanusch H, Brandis M. 1997a. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet 17: 149–153. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Strahm B, Nothwang HG, Gretz N, Schnieders B, Singh-Sawhney I, Kutt R, Vollmer M, Brandis M. 1997b. Molecular genetic identification of families with juvenile nephronophthisis type 1: Rate of progression to renal failure. APN Study Group. Arbeitsgemeinschaft fur Padiatrische Nephrologie. Kidney Int 51: 261–269. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Rensing C, Betz R, Sommer U, Birnbaum S, Imm A, Omran H, Leipoldt M, Otto E. 2001. Establishing an algorithm for molecular genetic diagnostics in 127 families with juvenile nephronophthisis. Kidney Int 59: 434–445. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N. 2011. Ciliopathies. N Engl J Med 364: 1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff S, Halbritter J, Epting D, Frank V, Nguyen TM, van Reeuwijk J, Boehlke C, Schell C, Yasunaga T, Helmstadter M, et al. 2013. ANKS6 is a central component of a nephronophthisis module linking NEK8 to INVS and NPHP3. Nat Genet 45: 951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp K, Heyer CM, Hommerding CJ, Henke SA, Sundsbak JL, Patel S, Patel P, Consugar MB, Czarnecki PG, Gliem TJ, et al. 2011. B9D1 is revealed as a novel Meckel syndrome (MKS) gene by targeted exon-enriched next-generation sequencing and deletion analysis. Hum Mol Genet 20: 2524–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Szymanska K, Jensen VL, Janecke AR, Innes AM, Davis EE, Frosk P, Li C, Willer JR, Chodirker BN, et al. 2011. TMEM237 is mutated in individuals with a Joubert syndrome related disorder and expands the role of the TMEM family at the ciliary transition zone. Am J Hum Genet 89: 713–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. 2003. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426: 83–87. [DOI] [PubMed] [Google Scholar]
- Huber C, Wu S, Kim AS, Sigaudy S, Sarukhanov A, Serre V, Baujat G, Le Quan Sang KH, Rimoin DL, Cohn DH, et al. 2013. WDR34 mutations that cause short-rib polydactyly syndrome type III/severe asphyxiating thoracic dysplasia reveal a role for the NF-κB pathway in cilia. Am J Hum Genet 93: 926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi P, Somlo S. 2002. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol 13: 2384–2398. [DOI] [PubMed] [Google Scholar]
- Izpisua-Belmonte JC, Ede DA, Tickle C, Duboule D. 1992. The mis-expression of posterior Hox-4 genes in talpid (ta3) mutant wings correlates with the absence of anteroposterior polarity. Development 114: 959–963. [DOI] [PubMed] [Google Scholar]
- Jeune M, Beraud C, Carron R. 1955. Dystrophie thoracique asphyxiante de caractere familial [Asphyxiating thoracic dystrophy with familial characteristics]. Arch Fr Pediatr 12: 886–891. [PubMed] [Google Scholar]
- Jin H, Nachury MV. 2009. The BBSome. Curr Biol 19: R472–473. [DOI] [PubMed] [Google Scholar]
- Joensuu T, Hamalainen R, Yuan B, Johnson C, Tegelberg S, Gasparini P, Zelante L, Pirvola U, Pakarinen L, Lehesjoki AE, et al. 2001. Mutations in a novel gene with transmembrane domains underlie Usher syndrome type 3. Am J Hum Genet 69: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsanis N. 2004. The oligogenic properties of Bardet–Biedl syndrome. Hum Mol Genet 13: R65–71. [DOI] [PubMed] [Google Scholar]
- Katsanis N, Beales PL, Woods MO, Lewis RA, Green JS, Parfrey PS, Ansley SJ, Davidson WS, Lupski JR. 2000. Mutations in MKKS cause obesity, retinal dystrophy and renal malformations associated with Bardet–Biedl syndrome. Nat Genet 26: 67–70. [DOI] [PubMed] [Google Scholar]
- Katsanis N, Ansley SJ, Badano JL, Eichers ER, Lewis RA, Hoskins BE, Scambler PJ, Davidson WS, Beales PL, Lupski JR. 2001a. Triallelic inheritance in Bardet–Biedl syndrome, a Mendelian recessive disorder. Science 293: 2256–2259. [DOI] [PubMed] [Google Scholar]
- Katsanis N, Lupski JR, Beales PL. 2001b. Exploring the molecular basis of Bardet–Biedl syndrome. Hum Mol Genet 10: 2293–2299. [DOI] [PubMed] [Google Scholar]
- Katsanis N, Eichers ER, Ansley SJ, Lewis RA, Kayserili H, Hoskins BE, Scambler PJ, Beales PL, Lupski JR. 2002. BBS4 is a minor contributor to Bardet–Biedl syndrome and may also participate in triallelic inheritance. Am J Hum Genet 71: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, et al. 2010. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science 329: 1337–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinknecht C. 1989. The inheritance of nephronophthisis. In Inheritance of kidney and urinary tract diseases (ed. Spitzer A, Avner ED), p. 464 Kluwer, Boston. [Google Scholar]
- Konrad M, Saunier S, Heidet L, Silbermann F, Benessy F, Calado J, Le Paslier D, Broyer M, Gubler MC, Antignac C. 1996. Large homozygous deletions of the 2q13 region are a major cause of juvenile nephronophthisis. Hum Mol Genet 5: 367–371. [DOI] [PubMed] [Google Scholar]
- Kyttala M, Tallila J, Salonen R, Kopra O, Kohlschmidt N, Paavola-Sakki P, Peltonen L, Kestila M. 2006. MKS1, encoding a component of the flagellar apparatus basal body proteome, is mutated in Meckel syndrome. Nat Genet 38: 155–157. [DOI] [PubMed] [Google Scholar]
- Lambacher NJ, Bruel AL, van Dam TJ, Szymanska K, Slaats GG, Kuhns S, McManus GJ, Kennedy JE, Gaff K, Wu KM, et al. 2016. TMEM107 recruits ciliopathy proteins to subdomains of the ciliary transition zone and causes Joubert syndrome. Nat Cell Biol 18: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Silhavy JL, Zaki MS, Schroth J, Bielas SL, Marsh SE, Olvera J, Brancati F, Iannicelli M, Ikegami K, et al. 2012a. CEP41 is mutated in Joubert syndrome and is required for tubulin glutamylation at the cilium. Nat Genet 44: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Silhavy JL, Lee JE, Al-Gazali L, Thomas S, Davis EE, Bielas SL, Hill KJ, Iannicelli M, Brancati F, et al. 2012b. Evolutionarily assembled cis-regulatory module at a human ciliopathy locus. Science 335: 966–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh MW, Pittman JE, Carson JL, Ferkol TW, Dell SD, Davis SD, Knowles MR, Zariwala MA. 2009. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet Med 11: 473–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch CC, Zaghloul NA, Davis EE, Stoetzel C, Diaz-Font A, Rix S, Alfadhel M, Lewis RA, Eyaid W, Banin E, et al. 2008. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet–Biedl syndrome. Nat Genet 40: 443–448. [DOI] [PubMed] [Google Scholar]
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, et al. 2004. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 117: 541–552. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang M, Xia Z, Xu P, Chen L, Xu T. 2011. Caenorhabditis elegans ciliary protein NPHP-8, the homologue of human RPGRIP1L, is required for ciliogenesis and chemosensation. Biochem Biophys Res Commun 410: 626–631. [DOI] [PubMed] [Google Scholar]
- Loken AC, Hanssen O, Halvorsen S, Jolster NJ. 1961. Hereditary renal dysplasia and blindness. Acta Paediatr 50: 177–184. [DOI] [PubMed] [Google Scholar]
- Loktev AV, Zhang Q, Beck JS, Searby CC, Scheetz TE, Bazan JF, Slusarski DC, Sheffield VC, Jackson PK, Nachury MV. 2008. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell 15: 854–865. [DOI] [PubMed] [Google Scholar]
- Ma M, Gallagher A-R, Somlo S. 2016. Ciliary mechanisms of cyst formation in polycystic kidney disease. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macca M, Franco B. 2009. The molecular basis of oral-facial-digital syndrome, type 1. Am J Med Genet C Semin Med Genet 151C: 318–325. [DOI] [PubMed] [Google Scholar]
- Makker SP, Grupe WE, Perrin E. 1973. Identical progression of juvenile hereditary nephronophthisis in monozygotic twins. J Pediatr 82: 773–779. [DOI] [PubMed] [Google Scholar]
- Malicdan MC, Vilboux T, Stephen J, Maglic D, Mian L, Konzman D, Guo J, Yildirimli D, Bryant J, Fischer R, et al. 2015. Mutations in human homologue of chicken talpid3 gene (KIAA0586) cause a hybrid ciliopathy with overlapping features of Jeune and Joubert syndromes. J Med Genet 52: 830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion V, Stutzmann F, Gerard M, De Melo C, Schaefer E, Claussmann A, Helle S, Delague V, Souied E, Barrey C, et al. 2012. Exome sequencing identifies mutations in LZTFL1, a BBSome and smoothened trafficking regulator, in a family with Bardet–Biedl syndrome with situs inversus and insertional polydactyly. J Med Genet 49: 317–321. [DOI] [PubMed] [Google Scholar]
- McInerney-Leo AM, Schmidts M, Cortes CR, Leo PJ, Gener B, Courtney AD, Gardiner B, Harris JA, Lu Y, Marshall M, et al. 2013. Short-rib polydactyly and Jeune syndromes are caused by mutations in WDR60. Am J Hum Genet 93: 515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhioub M, Cherif D, Benessy F, Silbermann F, Gubler MC, Le Paslier D, Cohen D, Weissenbach J, Beckmann J, Antignac C. 1994. Refined mapping of a gene (NPH1) causing familial juvenile nephronophthisis and evidence for genetic heterogeneity. Genomics 22: 296–301. [DOI] [PubMed] [Google Scholar]
- Mill P, Lockhart PJ, Fitzpatrick E, Mountford HS, Hall EA, Reijns MA, Keighren M, Bahlo M, Bromhead CJ, Budd P, et al. 2011. Human and mouse mutations in WDR35 cause short-rib polydactyly syndromes due to abnormal ciliogenesis. Am J Hum Genet 88: 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Saijoh Y, Tsuchiya K, Shirayoshi Y, Takai S, Taya C, Yonekawa H, Yamada K, Nihei H, Nakatsuji N, et al. 1998. Cloning of inv, a gene that controls left/right asymmetry and kidney development. Nature 395: 177–181. [DOI] [PubMed] [Google Scholar]
- Mollet G, Salomon R, Gribouval O, Silbermann F, Bacq D, Landthaler G, Milford D, Nayir A, Rizzoni G, Antignac C, et al. 2002. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat Genet 32: 300–305. [DOI] [PubMed] [Google Scholar]
- Mongeau JG, Worthen HG. 1967. Nephronophthisis and medullary cystic disease. Am J Med 43: 345–355. [DOI] [PubMed] [Google Scholar]
- Morgan D, Turnpenny L, Goodship J, Dai W, Majumder K, Matthews L, Gardner A, Schuster G, Vien L, Harrison W, et al. 1998. Inversin, a novel gene in the vertebrate left-right axis pathway, is partially deleted in the inv mouse. Nat Genet 20: 149–156. [DOI] [PubMed] [Google Scholar]
- Mortellaro C, Bello L, Pucci A, Lucchina AG, Migliario M. 2010. Saldino-Mainzer syndrome: Nephronophthisis, retinitis pigmentosa, and cone-shaped epiphyses. J Craniofac Surg 21: 1554–1556. [DOI] [PubMed] [Google Scholar]
- Moudgil A, Bagga A, Kamil ES, Rimoin DL, Lachman RS, Cohen AH, Jordan SC. 1998. Nephronophthisis associated with Ellis–van Creveld syndrome. Pediatr Nephrol 12: 20–22. [DOI] [PubMed] [Google Scholar]
- Mykytyn K, Nishimura DY, Searby CC, Shastri M, Yen HJ, Beck JS, Braun T, Streb LM, Cornier AS, Cox GF, et al. 2002. Identification of the gene (BBS1) most commonly involved in Bardet–Biedl syndrome, a complex human obesity syndrome. Nat Genet 31: 435–438. [DOI] [PubMed] [Google Scholar]
- Nishimura DY, Searby CC, Carmi R, Elbedour K, Van Maldergem L, Fulton AB, Lam BL, Powell BR, Swiderski RE, Bugge KE, et al. 2001. Positional cloning of a novel gene on chromosome 16q causing Bardet–Biedl syndrome (BBS2). Hum Mol Genet 10: 865–874. [DOI] [PubMed] [Google Scholar]
- Nishimura DY, Swiderski RE, Searby CC, Berg EM, Ferguson AL, Hennekam R, Merin S, Weleber RG, Biesecker LG, Stone EM, et al. 2005. Comparative genomics and gene expression analysis identifies BBS9, a new Bardet–Biedl syndrome gene. Am J Hum Genet 77: 1021–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich H, Fliegauf M, Hoefele J, Kispert A, Otto E, Volz A, Wolf MT, Sasmaz G, Trauer U, Reinhardt R, et al. 2003. Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet 34: 455–459. [DOI] [PubMed] [Google Scholar]
- Omran H. 2010. NPHP proteins: Gatekeepers of the ciliary compartment. J Cell Biol 190: 715–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omran H, Fernandez C, Jung M, Haffner K, Fargier B, Villaquiran A, Waldherr R, Gretz N, Brandis M, Ruschendorf F, et al. 2000. Identification of a new gene locus for adolescent nephronophthisis, on chromosome 3q22 in a large Venezuelan pedigree. Am J Hum Genet 66: 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto E, Hoefele J, Ruf R, Mueller AM, Hiller KS, Wolf MT, Schuermann MJ, Becker A, Birkenhager R, Sudbrak R, et al. 2002. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am J Hum Genet 71: 1167–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, et al. 2003a. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left–right axis determination. Nat Genet 34: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, et al. 2003b. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left–right axis determination. Nat Genet 34: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto E, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O’Toole JF, Helou J, Attanasio M, et al. 2005. A novel ciliary IQ domain protein, NPHP5, is mutated in Senior–Loken syndrome (nephronophthisis with retinitis pigmentosa), and interacts with RPGR and calmodulin. Nat Genet 37: 282–288. [DOI] [PubMed] [Google Scholar]
- Otto EA, Trapp ML, Schultheiss UT, Helou J, Quarmby LM, Hildebrandt F. 2008. NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. J Am Soc Nephrol 19: 587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Tory K, Attanasio M, Zhou W, Chaki M, Paruchuri Y, Wise EL, Wolf MT, Utsch B, Becker C, et al. 2009. Hypomorphic mutations in meckelin (MKS3/TMEM67) cause nephronophthisis with liver fibrosis (NPHP11). J Med Genet 46: 663–670. [DOI] [PubMed] [Google Scholar]
- Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S, Ghosh AK, Murga-Zamalloa CA, et al. 2010. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet 42: 840–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi MA, Bennett CL, Eckert ML, Dobyns WB, Gleeson JG, Shaw DW, McDonald R, Eddy A, Chance PF, Glass IA. 2004. The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome. Am J Hum Genet 75: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, Geimer S, Rosenbaum JL. 2006. Dissecting the molecular mechanisms of intraflagellar transport in chlamydomonas. Curr Biol 16: 450–459. [DOI] [PubMed] [Google Scholar]
- Perrault I, Saunier S, Hanein S, Filhol E, Bizet AA, Collins F, Salih MA, Gerber S, Delphin N, Bigot K, et al. 2012. Mainzer–Saldino syndrome is a ciliopathy caused by IFT140 mutations. Am J Hum Genet 90: 864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault I, Halbritter J, Porath JD, Gerard X, Braun DA, Gee HY, Fathy HM, Saunier S, Cormier-Daire V, Thomas S, et al. 2015. IFT81, encoding an IFT-B core protein, as a very rare cause of a ciliopathy phenotype. J Med Genet 52: 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CL, Miller KJ, Filson AJ, Nurnberger J, Clendenon JL, Cook GW, Dunn KW, Overbeek PA, Gattone VH II, Bacallao RL. 2004. Renal cysts of inv/inv mice resemble early infantile nephronophthisis. J Am Soc Nephrol 15: 1744–1755. [DOI] [PubMed] [Google Scholar]
- Pistor K, Schärer K, Olbing H, Tamminen-Möbius T. 1985. Children with chronic renal failure in the Federal Republic of Germany. II: Primary renal diseases, age and intervals from early renal failure to renal death. Clin Nephrol 23: 278–284. [PubMed] [Google Scholar]
- Potter DE, Holliday MA, Piel CF, Feduska NJ, Belzer FO, Salvatierra O Jr. 1980. Treatment of end-stage renal disease in children: A 15-year experience. Kidney Int 18: 103–109. [DOI] [PubMed] [Google Scholar]
- Puffenberger EG, Jinks RN, Sougnez C, Cibulskis K, Willert RA, Achilly NP, Cassidy RP, Fiorentini CJ, Heiken KF, Lawrence JJ, et al. 2012. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PloS ONE 7: e28936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putoux A, Thomas S, Coene KL, Davis EE, Alanay Y, Ogur G, Uz E, Buzas D, Gomes C, Patrier S, et al. 2011. KIF7 mutations cause fetal hydrolethalus and acrocallosal syndromes. Nat Genet 43: 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Lin Y, Norman RX, Ko HW, Eggenschwiler JT. 2011. Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components. Proc Natl Acad Sci 108: 1456–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan R, Grochowski CM, Gilbert MA, Falsey AM, Coleman K, Romero R, Loomes KM, Piccoli DA, Devoto M, Spinner NB. 2016. Compound heterozygous mutations in NEK8 in siblings with end-stage renal disease with hepatic and cardiac anomalies. Am J Hum Genet Part A 170: 750–753. [DOI] [PubMed] [Google Scholar]
- Riazuddin S, Belyantseva IA, Giese AP, Lee K, Indzhykulian AA, Nandamuri SP, Yousaf R, Sinha GP, Lee S, Terrell D, et al. 2012. Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat Genet 44: 1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson EC, Dowdle WE, Ozanturk A, Garcia-Gonzalo FR, Li C, Halbritter J, Elkhartoufi N, Porath JD, Cope H, Ashley-Koch A, et al. 2015. TMEM231, mutated in orofaciodigital and Meckel syndromes, organizes the ciliary transition zone. J Cell Biol 209: 129–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani M, Micalizzi A, Valente EM. 2013. Joubert syndrome: Congenital cerebellar ataxia with the molar tooth. Lancet Neurol 12: 894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosing S, Hofree M, Kim S, Scott E, Copeland B, Romani M, Silhavy JL, Rosti RO, Schroth J, Mazza T, et al. 2015. Functional genome-wide siRNA screen identifies KIAA0586 as mutated in Joubert syndrome. eLife 4: e06602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LF, Saal HM, David KL, Anderson RR. 2013. Technical report: Ethical and policy issues in genetic testing and screening of children. Genet Med 15: 234–245. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Ide SE, Strom TM, Lorenz B, Wilson D, Woods K, King L, Francomano C, Freisinger P, Spranger S, et al. 2000. Mutations in a new gene in Ellis–van Creveld syndrome and Weyers acrodental dysostosis. Nat Genet 24: 283–286. [DOI] [PubMed] [Google Scholar]
- Sanders AA, de Vrieze E, Alazami AM, Alzahrani F, Malarkey EB, Sorusch N, Tebbe L, Kuhns S, van Dam TJ, Alhashem A, et al. 2015. KIAA0556 is a novel ciliary basal body component mutated in Joubert syndrome. Genome Biol 16: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, et al. 2011. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell 145: 513–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva JM, Baraitser M. 1992. Joubert syndrome: A review. Am J Med Genet 43: 726–731. [DOI] [PubMed] [Google Scholar]
- Saraux H, Dhermy P, Fontaine JL, Boulesteix J, Lasfargue G, Grenet P, N’Ghiem M, Laplane R. 1970. La dégéneréscence rétino-tubulaire de Senior et Loken [Senior–Loken retino-tubular degeneration]. Arch Ophtalmol Rev Gen Ophtalmol 30: 683–696. [PubMed] [Google Scholar]
- Saunier S, Calado J, Heilig R, Silbermann F, Benessy F, Morin G, Konrad M, Broyer M, Gubler MC, Weissenbach J, et al. 1997. A novel gene that encodes a protein with a putative src homology 3 domain is a candidate gene for familial juvenile nephronophthisis. Hum Mol Genet 6: 2317–2323. [DOI] [PubMed] [Google Scholar]
- Saunier S, Calado J, Benessy F, Silbermann F, Heilig R, Weissenbach J, Antignac C. 2000. Characterization of the NPHP1 locus: Mutational mechanism involved in deletions in familial juvenile nephronophthisis. Am J Hum Genet 66: 778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, et al. 2006. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet 38: 674–681. [DOI] [PubMed] [Google Scholar]
- Scheidecker S, Etard C, Pierce NW, Geoffroy V, Schaefer E, Muller J, Chennen K, Flori E, Pelletier V, Poch O, et al. 2014. Exome sequencing of Bardet–Biedl syndrome patient identifies a null mutation in the BBSome subunit BBIP1 (BBS18). J Med Genet 51: 132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidts M, Arts HH, Bongers EM, Yap Z, Oud MM, Antony D, Duijkers L, Emes RD, Stalker J, Yntema JB, et al. 2013a. Exome sequencing identifies DYNC2H1 mutations as a common cause of asphyxiating thoracic dystrophy (Jeune syndrome) without major polydactyly, renal or retinal involvement. J Med Genet 50: 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidts M, Frank V, Eisenberger T, Al Turki S, Bizet AA, Antony D, Rix S, Decker C, Bachmann N, Bald M, et al. 2013b. Combined NGS approaches identify mutations in the intraflagellar transport gene IFT140 in skeletal ciliopathies with early progressive kidney disease. Hum Mutat 34: 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidts M, Vodopiutz J, Christou-Savina S, Cortes CR, McInerney-Leo AM, Emes RD, Arts HH, Tuysuz B, D’Silva J, Leo PJ, et al. 2013c. Mutations in the gene encoding IFT dynein complex component WDR34 cause Jeune asphyxiating thoracic dystrophy. Am J Hum Genet 93: 932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schueler M, Braun DA, Chandrasekar G, Gee HY, Klasson TD, Halbritter J, Bieder A, Porath JD, Airik R, Zhou W, et al. 2015. DCDC2 mutations cause a renal-hepatic ciliopathy by disrupting Wnt signaling. Am J Hum Genet 96: 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior B, Friedmann AI, Braudo JL. 1961. Juvenile familial nephropathy with tapetoretinal degeneration: A new oculorenal dystrophy. Am J Ophthalmol 52: 625–633. [DOI] [PubMed] [Google Scholar]
- Shaheen R, Almoisheer A, Faqeih E, Babay Z, Monies D, Tassan N, Abouelhoda M, Kurdi W, Al Mardawi E, Khalil MM, et al. 2015a. Identification of a novel MKS locus defined by TMEM107 mutation. Hum Mol Genet 24: 5211–5218. [DOI] [PubMed] [Google Scholar]
- Shaheen R, Schmidts M, Faqeih E, Hashem A, Lausch E, Holder I, Superti-Furga A, Mitchison HM, Almoisheer A, Alamro R, et al. 2015b. A founder CEP120 mutation in Jeune asphyxiating thoracic dystrophy expands the role of centriolar proteins in skeletal ciliopathies. Hum Mol Genet 24: 1410–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseldin HE, Rajab A, Alhashem A, Shaheen R, Al-Shidi T, Alamro R, Al Harassi S, Alkuraya FS. 2013. Mutations in DDX59 implicate RNA helicase in the pathogenesis of orofaciodigital syndrome. Am J Hum Genet 93: 555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman FE, Studnicki FM, Fetterman G. 1971. Renal lesions of familial juvenile nephronophthisis examined by microdissection. Am J Clin Pathol 55: 391–400. [DOI] [PubMed] [Google Scholar]
- Shylo NA, Christopher KJ, Iglesias A, Daluiski A, Weatherbee SD. 2016. TMEM107 is a critical regulator of ciliary protein composition and is mutated in orofaciodigital syndrome. Hum Mutat 37: 155–159. [DOI] [PubMed] [Google Scholar]
- Slavotinek AM, Stone EM, Mykytyn K, Heckenlively JR, Green JS, Heon E, Musarella MA, Parfrey PS, Sheffield VC, Biesecker LG. 2000. Mutations in MKKS cause Bardet–Biedl syndrome. Nat Genet 26: 15–16. [DOI] [PubMed] [Google Scholar]
- Smith UM, Consugar M, Tee LJ, McKee BM, Maina EN, Whelan S, Morgan NV, Goranson E, Gissen P, Lilliquist S, et al. 2006. The transmembrane protein meckelin (MKS3) is mutated in Meckel–Gruber syndrome and the wpk rat. Nat Genet 38: 191–196. [DOI] [PubMed] [Google Scholar]
- Srour M, Hamdan FF, Schwartzentruber JA, Patry L, Ospina LH, Shevell MI, Desilets V, Dobrzeniecka S, Mathonnet G, Lemyre E, et al. 2012a. Mutations in TMEM231 cause Joubert syndrome in French Canadians. J Med Genet 49: 636–641. [DOI] [PubMed] [Google Scholar]
- Srour M, Schwartzentruber J, Hamdan FF, Ospina LH, Patry L, Labuda D, Massicotte C, Dobrzeniecka S, Capo-Chichi JM, Papillon-Cavanagh S, et al. 2012b. Mutations in C5ORF42 cause Joubert syndrome in the French Canadian population. Am J Hum Genet 90: 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srour M, Hamdan FF, McKnight D, Davis E, Mandel H, Schwartzentruber J, Martin B, Patry L, Nassif C, Dionne-Laporte A, et al. 2015. Joubert syndrome in French Canadians and identification of mutations in CEP104. Am J Hum Genet 97: 744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel BT, Lirenman DS, Battie CW. 1980. Nephronophthisis. Am J Med 68: 531–538. [DOI] [PubMed] [Google Scholar]
- Stoetzel C, Laurier V, Davis EE, Muller J, Rix S, Badano JL, Leitch CC, Salem N, Chouery E, Corbani S, et al. 2006. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat Genet 38: 521–524. [DOI] [PubMed] [Google Scholar]
- Stoetzel C, Muller J, Laurier V, Davis EE, Zaghloul NA, Vicaire S, Jacquelin C, Plewniak F, Leitch CC, Sarda P, et al. 2007. Identification of a novel BBS gene (BBS12) highlights the major role of a vertebrate-specific branch of chaperonin-related proteins in Bardet–Biedl syndrome. Am J Hum Genet 80: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sworn MJ, Eisinger AJ. 1972. Medullary cystic disease and juvenile nephronophthisis in separate members of the same family. Arch Dis Child 47: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner M, Weber K, Mourao A, Vetter M, Awasthi M, Stiegler M, Bhogaraju S, Lorentzen E. 2016. Intraflagellar transport proteins 172, 80, 57, 54, 38, and 20 form a stable tubulin-binding IFT-B2 complex. EMBO J 35: 773–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauvin-Robinet C, Lee JS, Lopez E, Herranz-Perez V, Shida T, Franco B, Jego L, Ye F, Pasquier L, Loget P, et al. 2014. The oral-facial-digital syndrome gene C2CD3 encodes a positive regulator of centriole elongation. Nat Genet 46: 905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenon J, Duplomb L, Phadke S, Eguether T, Saunier A, Avila M, Carmignac V, Bruel AL, St-Onge J, Duffourd Y, et al. 2016. Autosomal recessive IFT57 hypomorphic mutation cause ciliary transport defect in unclassified oral-facial-digital syndrome with short stature and brachymesophalangia. Clin Genet 10.111/cge.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel C, Kessler K, Giessl A, Dimmler A, Shalev SA, von der Haar S, Zenker M, Zahnleiter D, Stoss H, Beinder E, et al. 2011. NEK1 mutations cause short-rib polydactyly syndrome type Majewski. Am J Hum Genet 88: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Legendre M, Saunier S, Bessieres B, Alby C, Bonniere M, Toutain A, Loeuillet L, Szymanska K, Jossic F, et al. 2012. TCTN3 mutations cause Mohr-Majewski syndrome. Am J Hum Genet 91: 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Wright KJ, Le Corre S, Micalizzi A, Romani M, Abhyankar A, Saada J, Perrault I, Amiel J, Litzler J, et al. 2014. A homozygous PDE6D mutation in Joubert syndrome impairs targeting of farnesylated INPP5E protein to the primary cilium. Hum Mutat 35: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Silhavy JL, Brancati F, Barrano G, Krishnaswami SR, Castori M, Lancaster MA, Boltshauser E, Boccone L, Al-Gazali L, et al. 2006. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat Genet 38: 623–625. [DOI] [PubMed] [Google Scholar]
- Valente EM, Logan CV, Mougou-Zerelli S, Lee JH, Silhavy JL, Brancati F, Iannicelli M, Travaglini L, Romani S, Illi B, et al. 2010. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat Genet 42: 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpy E, Leibovici M, Zwaenepoel I, Liu XZ, Gal A, Salem N, Mansour A, Blanchard S, Kobayashi I, Keats BJ, et al. 2000. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C. Nat Genet 26: 51–55. [DOI] [PubMed] [Google Scholar]
- Walczak-Sztulpa J, Eggenschwiler J, Osborn D, Brown DA, Emma F, Klingenberg C, Hennekam RC, Torre G, Garshasbi M, Tzschach A, et al. 2010. Cranioectodermal dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am J Hum Genet 86: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldherr R, Lennert T, Weber HP, Fodisch HJ, Scharer K. 1982. The nephronophthisis complex. A clinicopathologic study in children. Virchows Arch A Pathol Anat Histol 394: 235–254. [DOI] [PubMed] [Google Scholar]
- Warady BA, Hébert D, Sullivan EK, Alexander SR, Tejani A. 1997. Renal transplantation, chronic dialysis, and chronic renal insufficiency in children and adolescents. The 1995 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 11: 49–64. [DOI] [PubMed] [Google Scholar]
- Wei Q, Zhang Y, Li Y, Zhang Q, Ling K, Hu J. 2012. The BBSome controls IFT assembly and turnaround in cilia. Nat Cell Biol 14: 950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, et al. 1995. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature 374: 60–61. [DOI] [PubMed] [Google Scholar]
- Weil D, El-Amraoui A, Masmoudi S, Mustapha M, Kikkawa Y, Laine S, Delmaghani S, Adato A, Nadifi S, Zina ZB, et al. 2003. Usher syndrome type I G (USH1G) is caused by mutations in the gene encoding SANS, a protein that associates with the USH1C protein, harmonin. Hum Mol Genet 12: 463–471. [DOI] [PubMed] [Google Scholar]
- Weston MD, Luijendijk MW, Humphrey KD, Moller C, Kimberling WJ. 2004. Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am J Hum Genet 74: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MT, van Vlem B, Hennies HC, Zalewski I, Karle SM, Puetz M, Panther F, Otto E, Fuchshuber A, Lameire N, et al. 2004. Telomeric refinement of the MCKD1 locus on chromosome 1q21. Kidney Int 66: 580–585. [DOI] [PubMed] [Google Scholar]
- Wolf MT, Lee J, Panther F, Otto EA, Guan KL, Hildebrandt F. 2005. Expression and phenotype analysis of the nephrocystin-1 and nephrocystin-4 homologs in Caenorhabditis elegans. J Am Soc Nephrol 16: 676–687. [DOI] [PubMed] [Google Scholar]
- Zaucke F, Boehnlein JM, Steffens S, Polishchuk RS, Rampoldi L, Fischer A, Pasch A, Boehm CW, Baasner A, Attanasio M, et al. 2010. Uromodulin is expressed in renal primary cilia and UMOD mutations result in decreased ciliary uromodulin expression. Hum Mol Genet 19: 1985–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Nishimura D, Vogel T, Shao J, Swiderski R, Yin T, Searby C, Carter CS, Kim G, Bugge K, et al. 2013. BBS7 is required for BBSome formation and its absence in mice results in Bardet–Biedl syndrome phenotypes and selective abnormalities in membrane protein trafficking. J Cell Sci 126: 2372–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger HU, Mihatsch MJ, Edefonti A, Gaboardi F, Imbasciati E, Lennert T. 1980. Nephronophthisis (medullary cystic disease of the kidney). A study using electron microscopy, immunofluorescence, and a review of the morphological findings. Helv Paediatr Acta 35: 509–530. [PubMed] [Google Scholar]