Abstract
Background
Whether olanzapine/fluoxetine combination (OFC) is superior to olanzapine or fluoxetine monotherapy in patients with treatment-resistant depression (TRD) remains controversial. Thus, we conducted this meta-analysis of randomized controlled trials (RCTs) to compare the efficacy and safety of OFC with olanzapine or fluoxetine monotherapy for patients with TRD.
Materials and methods
RCTs published in PubMed, Embase, Web of Science, and the ClinicalTrials.gov registry were systematically reviewed to assess the efficacy and safety of OFC. Outcomes included mean changes from baseline in Montgomery–Asberg Depression Rating Scale (MADRS), Clinical Global Impression-Severity (CGI-S), Hamilton Rating Scale for Anxiety (HAM-A), Brief Psychiatric Rating Scale (BPRS) scores, response rate, remission rate, and adverse events. Results were expressed with weighted mean difference (WMD) with 95% confidence intervals (CIs) and risk ratio (RR) with 95% CIs.
Results
A total of five RCTs with 3,020 patients met the inclusion criteria and were included in this meta-analysis. Compared with olanzapine or fluoxetine monotherapy, OFC was associated with greater changes from baseline in MADRS (WMD =−3.37, 95% CI: −4.76, −1.99; P<0.001), HAM-A (WMD =−1.82, 95% CI: −2.25, −1.40; P<0.001), CGI-S (WMD =−0.37, 95% CI: −0.45, −0.28; P<0.001), and BPRS scores (WMD =−1.46, 95% CI: −2.16, −0.76; P<0.001). Moreover, OFC had significantly higher response rate (RR =1.35, 95% CI: 1.12, 1.63; P=0.001) and remission rate (RR =1.71, 95% CI: 1.31, 2.23; P<0.001). The incidence of treatment-related adverse events was similar between the OFC and monotherapy groups (RR =1.01, 95% CI: 0.94, 1.08; P=0.834).
Conclusion
OFC is more effective than olanzapine or fluoxetine monotherapy in the treatment of patients with TRD. Our results provided supporting evidence for the use of OFC in TRD. However, considering the limitations in this study, more large-scale, well-designed RCTs are needed to confirm these findings.
Keywords: treatment-resistant depression, olanzapine, fluoxetine, meta-analysis
Introduction
Treatment-resistant depression (TRD) is one of the greatest clinical challenges that psychiatrists face.1 Although there are an increased number of medications approved for major depressive disorder (MDD), approximately one-third of patients with MDD do not respond satisfactorily to the first antidepressant trial,2,3 and half of the patients have only partial response.4,5 Even after multiple interventions, approximately one-quarter of patients still remain depressed,4 and the likelihood of response to antidepressants decreases with the number of failed treatment trials.2 Moreover, patients with TRD appear to be at significantly higher risk of subsequent relapse,6 as well as heavy use of medical services.
Relapse in patients with TRD would result in high medical, social, and economic costs.7 Therefore, most clinicians attempt to prevent patients with MDD from relapsing. It is assumed that difficult-to-treat patients have a higher relapse rate than those who are resistant to treatment. Sequenced Treatment Alternatives to Relieve Depression (STAR*D) analyses reported that almost 25% of patients with TRD relapsed within 6–12 months after they remitted on level 1 citalopram.8 Furthermore, they also found that those who required more treatment levels to achieve remission had higher relapse rates, which indicated that patients with TRD are at a higher risk of relapse.9
There are numerous treatment strategies that have been applied to manage TRD, including augmentation with lithium,10 or second-generation antipsychotics,11 switching antidepressants,11 and combining antidepressants.12 Among these treatments, olanzapine/fluoxetine combination (OFC) is a combination of the antipsychotic, olanzapine, and the antidepressant, fluoxetine. It has been reported in the preclinical trials that OFC could improve extracellular levels of serotonin and norepinephrine, as well as dopamine in the rat brain.13,14 Moreover, in a 76-week, open-label study, OFC showed beneficial effects in improving depressive symptoms in patients with MDD.15 For TRD, there are also several clinical trials that evaluated the effects of OFC; however, their results remain inconsistent. Thus, we conducted this meta-analysis of randomized controlled trials (RCTs) to compare the efficacy and safety of OFC with olanzapine or fluoxetine monotherapy in the treatment of patients with TRD.
Materials and methods
Literature search
We conducted this study according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.16 Relevant studies were identified by searching PubMed, Embase, Web of Science, and the ClinicalTrials.gov registry (up to September 22, 2016). The following search items were used: (Treatment-Resistant[All Fields] AND (“depressive disorder, major”[MeSH Terms] OR (“depressive”[All Fields] AND “disorder”[All Fields] AND “major”[All Fields]) OR “major depressive disorder”[All Fields] OR (“major”[All Fields] AND “depressive”[All Fields] AND “disorder”[All Fields]) OR “major depressive disorder”[All Fields] OR “depressive disorder”[MeSH Terms] OR (“depressive”[All Fields] AND “disorder”[All Fields]) OR “depressive disorder”[All Fields] OR (“major”[All Fields] AND “depressive”[All Fields] AND “disorder”[All Fields]))) AND ((“olanzapine”[Supplementary Concept] OR “olanzapine”[All Fields]) OR (“fluoxetine”[MeSH Terms] OR “fluoxetine”[All Fields])). The search was limited to human subjects, and no language restriction was imposed. In addition, we also manually searched the reference lists of the original studies and previous review articles to identify any other potentially studies.
Study selection
Two independent investigators conducted the initial search, removed duplicate records, screened the title/abstracts and full information for relevance, and identified records as included for meta-analysis. The studies met the following inclusive criteria included in this meta-analysis: 1) study design: RCT; 2) sample size: >100; 3) study population: adult patients were diagnosed with TRD who had one or more documented historical antidepressant treatment failures; 4) study intervention: patients were treated with OFC or olanzapine or fluoxetine monotherapy; 5) outcome measure: response rate, remission rate, mean change from baseline in Montgomery–Asberg Depression Rating Scale (MADRS), Clinical Global Impression-Severity (CGI-S), Hamilton Rating Scale for Anxiety (HAM-A), Brief Psychiatric Rating Scale (BPRS), and incidence of treatment-related adverse events.
Data extraction and quality assessment
Two investigators independently extracted the following data: the first author’s name, year of publication, country, number of patients in each group, participant characteristics, duration of follow-up, dosage of olanzapine or fluoxetine, and main outcomes. If the data needed clarification or were not presented in the publication, we would contact the corresponding authors for the missing information. Any disagreement between the two investigators was resolved by discussion and consensus.
Risk of bias and evidence grade assessment
We used the Cochrane risk-of-bias tool to assess the risk of bias of the included study.17 The tool consists of the following criteria: random sequence generation, allocation concealment, blinding of participants and personnel to the study protocol, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. According to these criteria, each trial was considered to be at high, low, or unclear risk of bias.
We evaluated the quality of evidence for each outcome according to Grading of Recommendations Assessment, Development, and Evaluation (GRADE)18 methodology. In addition, each outcome was classified as very low, low, moderate, or high quality of evidence. GRADE profiler (version 3.6, GRADEpro) was used to construct the summary tables for these outcomes.
Statistical analysis
We assessed the efficacy and safety of OFC in TRD based on data from included studies. The mean changes from baseline in MADRS, CGI-S, HAM-A, and BPRS scores were treated as continuous variables; thus, they were expressed as weighted mean difference (WMD) with 95% confidence intervals (95% CIs). The response rate, remission rate, and incidence of adverse events were treated as dichotomous variables; thus, they were expressed as risk ratio (RR) with 95% CIs. Before the data were synthesized, we used the Cochrane Q chi-square test and I2 statistics to detect the heterogeneity among the included studies.19 A P-value <0.10 or I2>50% indicated significant heterogeneity.19 We used a random-effects model20 to pool the estimates when significant heterogeneity was identified; otherwise, a fixed-effects model was used.21 We also performed sensitivity analysis to explore the impact of a single study on the overall heterogeneity when significant heterogeneity was found. Since the number of included studies was <10, publication bias was not assessed. A P-value of <0.05 was considered statistically significant, except where otherwise specified. All analyses were performed using STATA version 12.0 (Stata Corporation, College Station, TX, USA).
Results
Study identification
The initial search yielded 1,109 records, and 476 were excluded because of duplicate records. After reviewing abstract and title, 615 were excluded because they were reviews, letters, case reports, or animal experiments. Then, 18 were left for full-text information review, and 13 of them were excluded because they were single-arm studies, did not provide outcomes of interest, or had sample size of <100. Finally, five RCTs met the inclusion criteria and were included in this meta-analysis.22–26 The search flowchart is shown in Figure 1.
Figure 1.
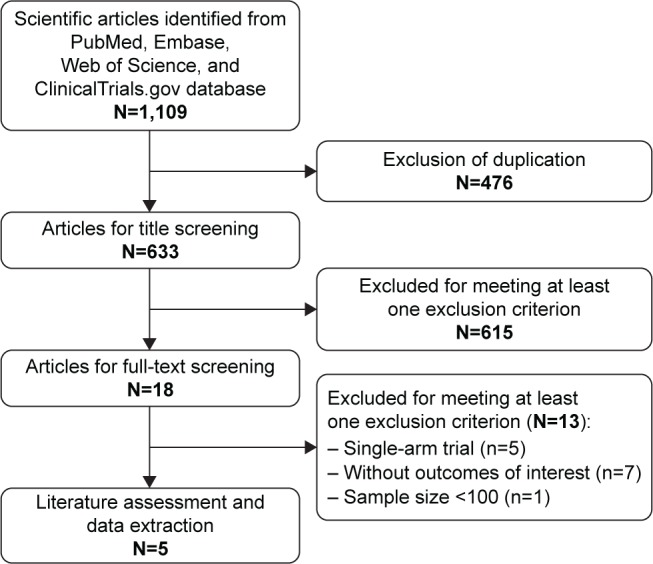
Eligibility of studies for inclusion in meta-analysis.
Study characteristics
The main characteristics of the included studies are presented in Table 1. These RCTs were published between 2005 and 2014. The total number of enrolled participants was 3,020, ranging from 365 to 1,174 patients per study. Of the patients with TRD, 33.9% were male and 66.1% were female. The duration of follow-up ranged from 8 to 12 weeks. All the studies were conducted in the USA. The dosage of OFC among these studies varied greatly, which ranged from 6/25 mg/day (olanzapine/fluoxetine) to 18/50 mg/day. The dosage of fluoxetine ranged from 25 to 50 mg/day, and the dosage of olanzapine ranged from 6 to 18 mg/day.
Table 1.
Baseline characteristics of patients in the trials included in the meta-analysis
| Study | Country | Treatment regimen | No of patients | Male/female | Age (mean ± SD, years) | BMI (mean ± SD, kg/m2) |
|---|---|---|---|---|---|---|
| Trivedi et al22 | USA | OFC: 6/50–18/50 mg/day | 473 | 155/318 | 44.8±10.6 | 29.7±7.4 |
| 50 mg/day fluoxetine | 352 | 112/240 | 44.2±10.3 | 30.3±7.8 | ||
| 6–18 mg/day olanzapine | 349 | 123/226 | 44.1±10.8 | 30.1±7.3 | ||
| Thase et al23 | USA | OFC: 6/50 mg/day | 200 | 68/132 | 33.3±10.2 | 30.5±7.6 |
| 50 mg/day fluoxetine | 206 | 78/128 | 44.6±10.0 | 29.4±7.1 | ||
| 6 mg/day olanzapine | 199 | 76/123 | 44.3±10.8 | 30.4±7.1 | ||
| Shelton et al24 | USA | OFC: 6/25–12/50 mg/day | 146 | 48/98 | 42.5±10.7 | 30.5±8.2 |
| 25–50 mg/day fluoxetine | 142 | 39/103 | 41.7±11.0 | 31.6±8.8 | ||
| 6–12 mg/day olanzapine | 144 | 51/93 | 43.3±11.0 | 30.3±7.6 | ||
| Corya et al25 | USA | OFC: 6/25–12/50 mg/day | 243 | NR | NR | NR |
| 25 mg/day fluoxetine | 60 | NR | NR | NR | ||
| 6–12 mg/day olanzapine | 62 | NR | NR | NR | ||
| Brunner et al26 | USA | OFC: 6/25–18/50 mg/day | 221 | NR | NR | NR |
| 25–50 mg/day fluoxetine | 223 | NR | NR | NR |
Abbreviations: BMI, body mass index; NR, not reported; OFC, olanzapine/fluoxetine combination; SD, standard deviation.
The details of the risk-of-bias assessment are shown in Figure 2. Among the five RCTs, two were judged to be at low risk of bias and three at unclear risk of bias. The main reason for the study with unclear risk of bias was that they did not describe the method used to generate the allocation sequence. The GRADE evidence profiles for outcomes are shown in Table 2. The GRADE level of evidence was moderate for response rate, remission rate, and mean changes from baseline in MADRS, CGI-S, HAM-A, and BPRS scores. The main reason for the outcomes with moderate evidence was that there was substantial heterogeneity among the included studies.
Figure 2.
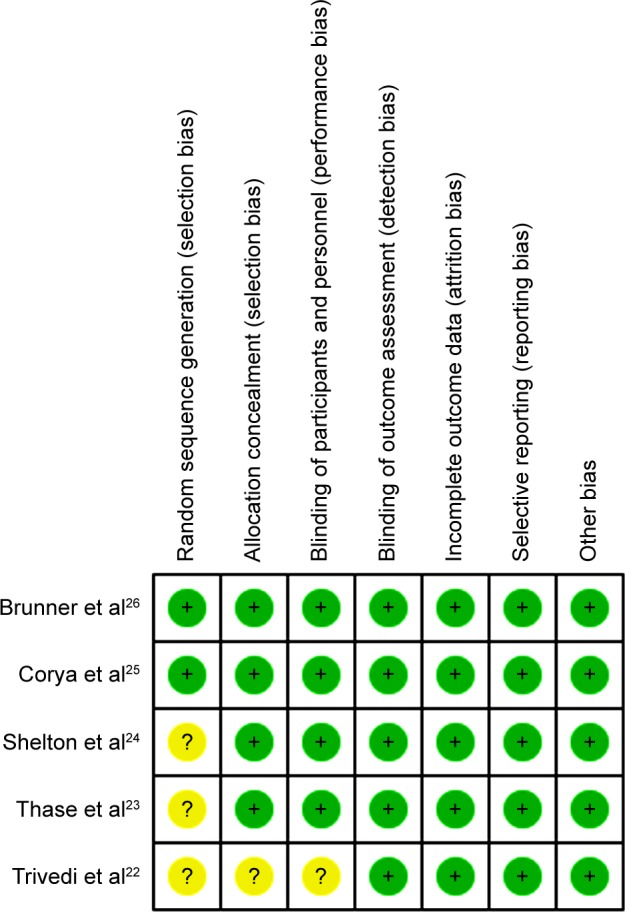
Risk of bias summary.
Table 2.
GRADE evidence profile
| Quality assessment
|
No of patients | Effect
|
Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Relative (95% CI) |
Absolute | ||||
| Mean changes from baseline in MADRS score (range of scores: −4.75 to −0.2; better indicated by lower values) | ||||||||||||
| 5 | Randomized trials | Seriousa | Seriousb | No serious indirectness | No serious imprecision | Strong associationc | 2,345 | 1,737 | – | WMD −3.37 lower (−4.76 higher to −1.99 lower) |
⊕⊕⊕O Moderate |
Critical |
| Mean change from baseline in CGI-S score (range of scores: −0.6 to −0.25; better indicated by lower values) | ||||||||||||
| 4 | Randomized trials | Seriousa | Seriousd | No serious indirectness | No serious imprecision | Strong associatione | 1,399 | 1,036 | – | WMD −0.37 lower (−0.45 higher to −0.28 lower) |
⊕⊕⊕O Moderate |
Important |
| Response rate | ||||||||||||
| 5 | Randomized trials | Seriousa | Seriousf | No serious indirectness |
No serious imprecision |
Strong associationc | 1,037/2,341 (44.3%) |
603/1,732 (34.8%) 27.8% |
RR 1.35 (1.12–1.63) |
122 more per 1,000 (from 42 more to 219 more) 97 more per 1,000 (from 33 more to 175 more) |
⊕⊕⊕O Moderate |
Critical |
| Mean change from baseline in HAM-A score (range of scores: −2.57 to −1.2; better indicated by lower values) | ||||||||||||
| 3 | Randomized trials | Seriousg | Serioush | No serious indirectness |
No serious imprecision |
Strong associationi | 1,178 | 813 | – | WMD −1.82 lower (−2.25 higher to −1.40 lower) |
⊕⊕⊕O Moderate |
Important |
| Remission rate | ||||||||||||
| 5 | Randomized trials |
Seriousa | Seriousj | No serious indirectness |
No serious imprecision |
Strong associationc | 808/2,538 (31.8%) |
475/1,948 (24.4%) 17.8% |
RR 1.71 (1.31–2.23) |
173 more per 1,000 (from 76 more to 300 more) 126 more per 1,000 (from 55 more to 219 more) |
⊕⊕⊕O Moderate |
Important |
| Mean change from baseline in BPRS score (range of scores: −2.85 to −0.5; better indicated by lower values) | ||||||||||||
| 3 | Randomized trials | Seriousg | Seriousk | No serious indirectness |
No serious imprecision |
Strong associationi | 1,178 | 813 | – | WMD −1.46 lower (−2.16 higher to −0.76 lower) |
⊕⊕⊕O Moderate |
Important |
Notes:
Only two trials were judged to be at low risk of bias.
Substantial heterogeneity (I2=99.6%) was found.
A total of 3,020 patients were enrolled.
Substantial heterogeneity (I2=97.6%) was found.
A total of 2,435 patients were enrolled.
Substantial heterogeneity (I2=82.9%) was found.
Only one trial was judged to be at low risk of bias.
Substantial heterogeneity (I2=94.5%) was found.
A total of 1,991 patients were enrolled.
Substantial heterogeneity (I2=83.1%) was found.
Substantial heterogeneity (I2=97.9%) was found.
Abbreviations: BPRS, Brief Psychiatric Rating Scale; CGI-S, Clinical Global Impression-Severity; CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HAM-A, Hamilton Rating Scale for Anxiety; MADRS, Montgomery–Asberg Depression Rating Scale; RR, risk ratio; WMD, weighted mean difference.
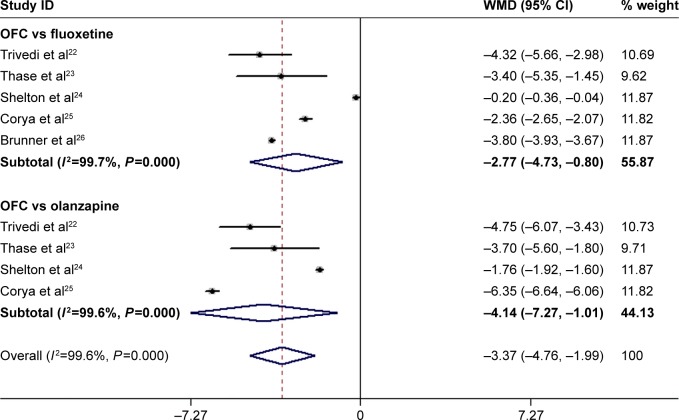
Effect of OFC on MADRS score
All the studies reported the data on MADRS scores.22–26 The aggregated results suggested that patients treated with OFC had a significantly greater change in MADRS score than those treated with fluoxetine or olanzapine monotherapy (WMD =−3.37, 95% CI: −4.76, −1.99; P<0.001; Figure 3). The test for heterogeneity was significant (P<0.001, I2=99.6%). Therefore, we conducted sensitivity analysis to explore the potential source of heterogeneity. When we excluded the trial conducted by Corya et al,25 which had the least number of patients (N=365), the pooled estimates changed slightly (WMD =−3.32, 95% CI: −4.92, −1.72; P<0.001), but the heterogeneity was still present (P<0.001, I2=99.5%). We also further excluded single trial once at a time, and the summarized results did not change substantially, which ranged from −4.12 (95% CI: −5.37, −2.88; P<0.001) to −3.05 (95% CI: −4.62, −1.49; P<0.001). However, the heterogeneity did not disappear.
Figure 3.
Forest plot showing the effect of OFC on MADRS score.
Note: Weights are from random-effects analysis.
Abbreviations: CI, confidence interval; MADRS, Montgomery–Asberg Depression Rating Scale; OFC, olanzapine/fluoxetine combination; WMD, weighted mean difference.
We also conducted subgroup analysis based on control therapy. The results showed that OFC was associated with a greater MADRS reduction than fluoxetine (WMD =−2.77, 95% CI: −4.73, −0.80; P=0.006) and olanzapine (WMD =−4.14, 95% CI: −7.27, −1.01; P=0.009; Figure 3).
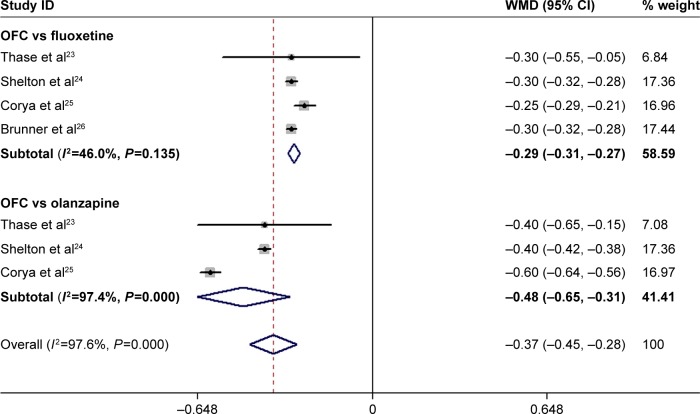
Effect of OFC on CGI-S, HAM-A, and BPRS scores
Four RCTs reported the data on CGI-S.23–26 The pooled estimates demonstrated that OFC was associated with a significantly greater reduction in CGI-S score than fluoxetine or olanzapine monotherapy (WMD =−0.37, 95% CI: −0.45, −0.28; P<0.001; Figure 4). Subgroup analysis based on control treatment suggested that patients treated with OFC had a significantly greater reduction in CGI-S than those treated with fluoxetine (WMD =−0.29, 95% CI: −0.31, −0.27; P<0.001) and olanzapine (WMD =−0.48, 95% CI: −0.65, −0.31; P<0.001; Figure 4). There was significant heterogeneity among the studies reporting the effect comparison between OFC and olanzapine (P<0.001, I2=97.4%). Then, we conducted sensitivity analysis by excluding the trial conducted by Corya et al.25 The pooled estimates of the remaining studies did not change substantially (WMD =−0.40, 95% CI: −0.42, −0.38; P<0.001), but the heterogeneity was not identified (P=1.00, I2=0.0%).
Figure 4.
Forest plot showing the effect of OFC on CGI-S score.
Note: Weights are from random-effects analysis.
Abbreviations: CGI-S, Clinical Global Impression-Severity; CI, confidence interval; OFC, olanzapine/fluoxetine combination; WMD, weighted mean difference.
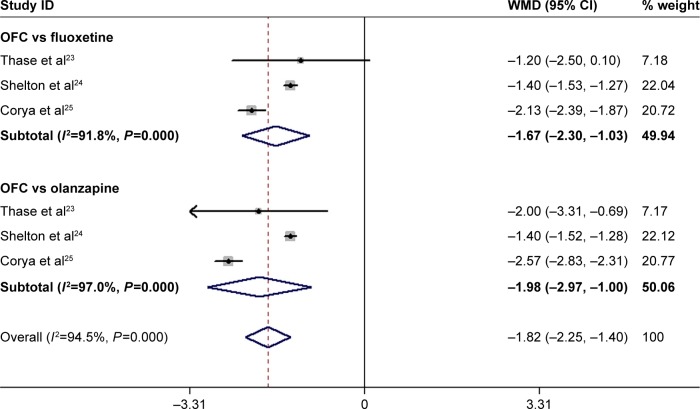
Three RCTs reported the data on HAM-A.23–25 The pooled estimates showed that OFC significantly decreased the HAM-A score as compared with fluoxetine or olanzapine monotherapy (WMD =−1.82, 95% CI: −2.25, −1.40; P<0.001; Figure 5). Subgroup analysis based on control treatment revealed that OFC was associated with a significantly greater change in HAM-A score than fluoxetine (WMD =−1.67, 95% CI: −2.30, −1.03; P<0.001) and olanzapine (WMD =−1.98, 95% CI: −2.97, −1.00; P<0.001; Figure 5). There was significant heterogeneity among the included studies. Exclusion of the trial conducted by Shelton et al24 showed that the pooled estimates altered slightly in the comparison between OFC and fluoxetine (WMD =−2.55, 95% CI: −2.80, −2.30, P<0.001), and olanzapine (WMD =−1.89, 95% CI: −2.69, −1.10, P<0.001). However, the heterogeneity was not significant (OFC vs fluoxetine: P=0.171, I2=46.8%; OFC vs olanzapine: P=1.0, I2=0.0%).
Figure 5.
Forest plot showing the effect of OFC on HAM-A score.
Note: Weights are from random-effects analysis.
Abbreviations: CI, confidence interval; HAM-A, Hamilton Rating Scale for Anxiety; OFC, olanzapine/fluoxetine combination; WMD, weighted mean difference.
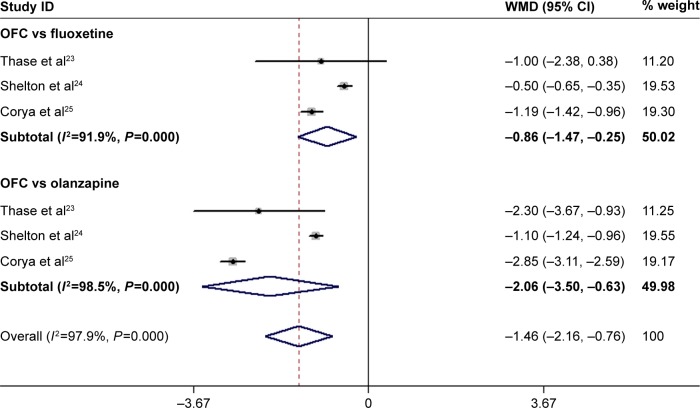
Three RCTs reported the data on BPRS.23–25 The pooled results showed that the mean change in BPRS score was significantly greater in the OFC group than that in the control group (WMD =−1.46, 95% CI: −2.16, −0.76; P<0.001; Figure 6). Subgroup analysis showed that OFC was associated with a significantly greater reduction in BPRS than fluoxetine (WMD =−0.86, 95% CI: −1.47, −0.25; P=0.006) and olanzapine (WMD =−2.06, 95% CI: −3.50, −0.63; P=0.005; Figure 6). There was substantial heterogeneity among the studies for subgroup analysis. However, when we deleted the trial conducted by Shelton et al,24 the pooled estimates in each subgroup did not change substantially (OFC vs fluoxetine: WMD =−1.18, 95% CI: −1.41, −0.96, P<0.001; OFC vs olanzapine: WMD =−2.83, 95% CI: −3.09, −2.57, P<0.001), and the heterogeneity disappeared (OFC vs fluoxetine: P=0.790, I2=0.0%; OFC vs olanzapine: P=0.441, I2=0.0%).
Figure 6.
Forest plot showing the effect of OFC on BPRS score.
Note: Weights are from random-effects analysis.
Abbreviations: BPRS, Brief Psychiatric Rating Scale; CI, confidence interval; OFC, olanzapine/fluoxetine combination; WMD, weighted mean difference.
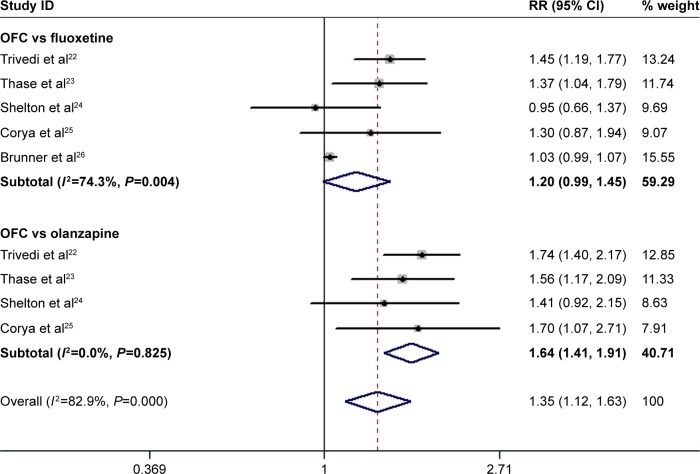
Effect of OFC on response rate and remission rate
All the studies reported the data on response rate.22–26 The response rates in the OFC and control groups were 44.3% and 34.8%, respectively. Pooled estimates showed that OFC had a significantly higher response rate than fluoxetine or olanzapine monotherapy (RR =1.35, 95% CI: 1.12, 1.63; P=0.001; Figure 7). However, when we conducted subgroup analysis based on the control therapy, we found that OFC had a comparable response rate with fluoxetine (RR =1.20, 95% CI: 0.99, 1.45; P=0.062), but a higher response rate than olanzapine (RR =1.64, 95% CI: 1.41, 1.91; P<0.001). There was significant heterogeneity among the studies reporting effect comparison between OFC and fluoxetine (P=0.004, I 2=74.3%). When we excluded the trial conducted by Trivedi et al,22 the pooled result did not change substantially (RR =1.11, 95% CI: 0.95, 1.31; P=0.184), but the heterogeneity turned to be not significant (P=0.143, I 2=44.7%).
Figure 7.
Forest plot showing the effect of OFC on response rate.
Note: Weights are from random-effects analysis.
Abbreviations: CI, confidence interval; OFC, olanzapine/fluoxetine combination; RR, risk ratio.
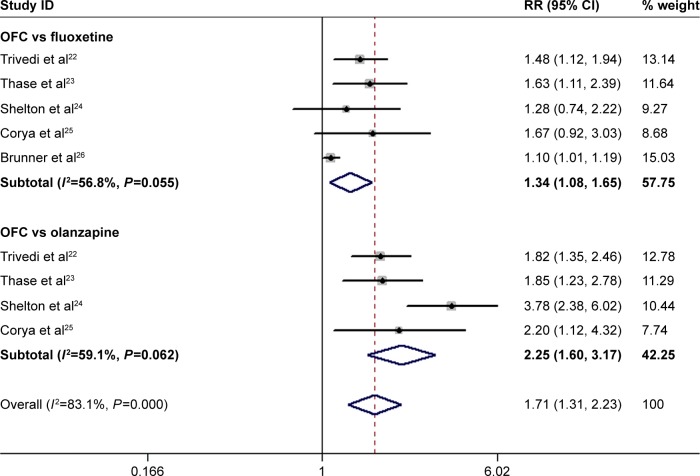
All the trials reported the data on remission rate.22–26 The remission rates in the OFC and control group were 31.8% and 24.4%, respectively. Pooled results showed that OFC resulted in a significantly higher remission rate than fluoxetine or olanzapine monotherapy (RR=1.71, 95% CI: 1.31, 2.23; P<0.001; Figure 8). Subgroup analysis suggested that OFC had a significantly higher remission rate than fluoxetine (RR=1.34, 95% CI: 1.08, 1.65; P=0.008) and olanzapine (RR=2.25, 95% CI: 1.60, 3.17; P<0.001; Figure 8). There was moderate heterogeneity among the studies for subgroup analysis. When we excluded the trial conducted by Thase et al,23 no heterogeneity was found among the studies comparing OFC with fluoxetine (P=0.112, I2=49.9%), and the pooled results changed slightly (RR=1.27, 95% CI: 1.02, 1.57; P=0.029). Similarly, when we excluded the trial conducted by Shelton et al,24 no heterogeneity was found among the studies comparing OFC with olanzapine (P=0.879, I2=0.0%), and the pooled estimate did not change substantially (RR=1.87, 95% CI: 1.49, 2.35; P<0.001).
Figure 8.
Forest plot showing the effect of OFC on remission rate.
Note: Weights are from random-effects analysis.
Abbreviations: CI, confidence interval; OFC, olanzapine/fluoxetine combination; RR, risk ratio.
Adverse events
All the included RCTs reported the data on treatment-related adverse events.22–26 The incidences of adverse events in the OFC and control groups were 70.4% and 69.5%, respectively. There was no significant difference in the incidence of adverse events between the two groups (RR =1.01, 95% CI: 0.94, 1.08; P=0.834). Fewer patients (3.1%) in the fluoxetine group discontinued due to adverse events than in the OFC (10.9%) or olanzapine (13.1%) group. OFC resulted in a significantly higher rate of discontinuation than fluoxetine (RR =3.37, 95% CI: 2.30, 4.96; P<0.001), and a similar rate with olanzapine (RR =0.87, 95% CI: 0.67, 1.13; P=0.297).
Compared with fluoxetine, OFC induced a significantly higher incidence of somnolence (RR =1.97, 95% CI: 1.09, 3.56; P=0.026), fatigue (RR =1.57, 95% CI: 1.14, 2.16; P=0.006), peripheral edema (RR =10.16, 95% CI: 4.61, 22.38; P<0.001), sedation (RR =3.11, 95% CI: 1.60, 6.03; P=0.001), and hypersomnia (RR =3.57, 95% CI: 1.92, 6.63; P<0.001; Table 3). Besides, OFC had a higher incidence of tremor than olanzapine (RR =1.70, 95% CI: 1.18, 2.43; P=0.004; Table 3).
Table 3.
Summary of RRs with 95% CIs of adverse events
| Adverse events | OFC vs fluoxetine
|
OFC vs olanzapine
|
||
|---|---|---|---|---|
| RR (95% CI) | P-value | RR (95% CI) | P-value | |
| Weight increase | 2.53 (0.86–7.39) | 0.090 | 0.70 (0.41–1.20) | 0.197 |
| Increased appetite | 2.50 (0.92–6.77) | 0.072 | 0.66 (0.38–1.15) | 0.142 |
| Dry mouth | 1.58 (0.64–3.90) | 0.318 | 0.60 (0.33–1.11) | 0.105 |
| Somnolence | 1.97 (1.09–3.56) | 0.026 | 0.70 (0.34–1.46) | 0.346 |
| Fatigue | 1.57 (1.14–2.16) | 0.006 | 0.91 (0.69–1.19) | 0.482 |
| Headache | 0.53 (0.28–1.01) | 0.052 | 0.69 (0.35–1.37) | 0.294 |
| Peripheral edema | 10.16 (4.61–22.38) | <0.001 | 1.02 (0.46–2.24) | 0.968 |
| Tremor | 1.66 (0.91–3.01) | 0.096 | 1.70 (1.18–2.43) | 0.004 |
| Dizziness | 0.56 (0.18–1.75) | 0.319 | 0.66 (0.22–1.99) | 0.458 |
| Sedation | 3.11 (1.60–6.03) | 0.001 | 0.80 (0.52–1.22) | 0.297 |
| Hypersomnia | 3.57 (1.92–6.63) | <0.001 | 0.82 (0.57–1.20) | 0.307 |
Abbreviations: CI, confidence interval; OFC, olanzapine/fluoxetine combination; RR, risk ratio.
Discussion
In this meta-analysis, we summarized five RCTs to compare the efficacy and safety of OFC with fluoxetine or olanzapine monotherapy in the treatment of patients with TRD. Our pooled results showed that OFC had significantly greater changes from baseline in MADRS, HAM-A, CGI-S, and BPRS scores. Moreover, OFC was associated with higher response rate and remission rate than fluoxetine or olanzapine alone. The use of OFC in patients with TRD did not induce a higher incidence of adverse events than monotherapy, but it would result in a higher rate of discontinuation due to adverse events than fluoxetine.
There have been three systematic reviews27–29 and three meta-analyses,30–32 which assessed the effects and/or safety of OFC in the treatment of patients with TRD. Our study expends one, the prior meta-analysis, in providing more significant evidence for the use of OFC in TRD. 1) Our study is a meta-analysis using a fixed-effects or random-effects model to pool the results of included studies. Meta-analysis can increase the sample size to improve statistical power. Consequently, the quality of evidence from meta-analysis is higher than that from the original study. In addition, meta-analysis can systematically summarize the current original studies on a specific topic and provide some implications for future investigations and decision making, especially controversial topics. Therefore, the studies by Dodd and Berk,28 Bobo and Shelton,29 and Chen et al27 were systematic reviews rather than meta-analysis. The authors summarized eligible studies and introduced the main clinical outcomes of the published trials. However, it did not provide us the pooled estimates of these outcomes. Thus, we could not judge whether the combination was superior or inferior to the monotherapy. In this meta-analysis, we used the statistical methods to pool the data from included studies, and from the calculated values we can judge whether the two treatments were significantly different. 2) This study has an enlarged sample size, which provides a distinct advantage over the previous meta-analyses. The total sample size in this study was 3,020, whereas it was 1,146 in the Tohen et al’s30 study and 1,121 in the Spielmans et al’s31 study. 3) All the included studies are independent prospective RCTs, whereas in the trial conducted by Tohen et al,30 two RCTs were from the same study protocol. The overlapped data in the previous meta-analysis would reduce the reliability of the results. 4) In this study, we excluded studies with sample size <100, whereas in the previous studies30–32 they included one trial with a sample size of 20. Studies with small sample size would result in an overestimation of the treatment effect than the large trials. 5) In this study, two of the included studies were judged to be at low risk of bias, and three at unclear risk of bias. All the outcomes were regarded as moderate level of evidence. However, in the previous meta-analyses,30–32 the qualities of included studies and the outcomes were not evaluated. Thus, their results may not be reliable. The enlarged sample size and moderate study quality have enabled more accurate and reliable statistical analysis. Furthermore, we also assessed the treatment effects of OFC therapy in CGI-S, HAM-A, and BPRS scores, as well as remission rate and adverse events, which had not been discussed in the previous meta-analyses.
In this meta-analysis, we found that the mean reduction from baseline in MADRS score was 3.37 points more in the OFC group than that in the control group. This result was not consistent with the included studies. In the trial conducted by Trivedi et al,22 1,146 patients with a history of non-response during a 6- to 8-week antidepressant therapy were enrolled.22 Then, they were randomly assigned to receive OFC (n=462), fluoxetine (n=342), or olanzapine (n=342).22 After 8 weeks, patients with OFC achieved a greater reduction from baseline in MADRS score (−13.0 points) than fluoxetine (−8.6 points) and olanzapine (−8.2 points) patients.22 The mean reduction in MADRS score of OFC was 4.32 points more than fluoxetine and 4.75 points more than olanzapine. However, in another trial conducted by Shelton et al,24 they also observed a significant mean change in MADRS score, but the magnitude of reduction in MADRS score was less than that reported by Trivedi et al. In the study by Shelton et al,24 they selected 500 patients with TRD who had subsequently failed to respond to nortriptyline during an open-label lead-in phase. These patients were randomly assigned to receive olanzapine (6–12 mg/day) plus fluoxetine (25–50 mg/day), olanzapine (6–12 mg/day), or fluoxetine (25–50 mg/day).24 At the 8-week study end point, the MADRS scores decreased by 8.71 points from baseline with OFC, 6.95 points with olanzapine, and 8.51 points with fluoxetine.24 Compared with fluoxetine and olanzapine, OFC had 0.2 and 1.76 points more of reductions in MADRS score, respectively.
To explore the potential factors for the small advantage of OFC over fluoxetine and olanzapine, Shelton et al suggested the following possible reasons. 1) The treatment time was not enough since the full response for patients to an antidepressant can take 12 weeks or more.33 2) Patients and investigators were aware that there were no placebo group; thus, they may have an enhanced expectation for improvement for all the therapy groups.24 3) Patients randomized in the double-blind phase were not actually treatment resistant.24 This would account for the higher response in the fluoxetine and olanzapine groups.24
With regard to response rate, our results showed that OFC had a significantly higher response rate than olanzapine, but a similar response rate with fluoxetine. The response rate among the included studies ranged from 27.4% to 97.3% in the OFC group, 27.8% to 94.2% in the fluoxetine group, and 19.4% to 25.9% in the olanzapine group. The comparable response rate between OFC and fluoxetine was confirmed in three of the included studies. But in the remaining two studies,22,23 they reported a higher response rate of OFC. In the study by Trivedi et al,22 the clinical response rates in the OFC and fluoxetine groups were 40.3% and 27.8%, respectively, and the difference between the two groups was significant (P<0.001). Similarly, in the trial conducted by Thase et al,23 the response rate in the OFC group (40.4%) was significantly higher than that in the fluoxetine group (29.6%) (P=0.028).23 In contrast to these trials, Brunner et al26 reported that the response rates were similar between the two treatments. In that study, the response rate in the OFC group was 97.3% (215/221) compared with 94.2% (210/223) in the fluoxetine group (P=0.126).26 The authors speculated that the following various factors might account for these results: patients who have already been under open-label acute and stabilization treatment of OFC for ≤20 weeks; the response was required for patients before they were entering the double-blind, relapse-prevention period; fluoxetine is an established effective antidepressant.26
In the analysis of treatment-related adverse events, we found that OFC had similar incidence of adverse events with fluoxetine or olanzapine alone, which was consistent with the findings of previous studies. There were more patients in the OFC group than in the fluoxetine group who discontinued the study due to adverse events. The most common adverse events included weight increase, increased appetite, somnolence, fatigue, peripheral edema, sedation, and hypersomnia. Thus, health care professionals should consider the adverse event profile of OFC, other treatment choices, and the patient factors (severity of illness, quality of response to alternative medications, and urgency for rapid response) when making prescribing decisions for patients with TRD.
There are several potential limitations in this meta-analysis that should be considered with caution. 1) There was substantial heterogeneity among the included studies. However, it should not be surprising given the differences in characteristics of patients, duration of treatment, definitions of TRD and response, and dosage of fluoxetine and olanzapine. These differences may account for the heterogeneity and have a potential impact on the pooled estimates. To identify the potential sources of heterogeneity, we conducted sensitivity analysis and subgroup analysis. Our sensitivity analysis suggested that exclusion of one trial may reduce the level of heterogeneity, but the pooled estimates did not change substantially, which verified the robustness and reliability of our results. 2) Due to the limited data, we did not perform meta-analysis to assess the effects of OFC in time-to-relapse and time-to-remission. 3) Our data were abstracted from the published studies rather than individual patient; thus, the effect and safety of OFC in TRD may not be defined clearly.34 4) Due to the limited data, we were unable to assess the effect of OFC on the time to onset of response.
Conclusion
This meta-analysis of five RCTs indicated that OFC had better effects than fluoxetine or olanzapine monotherapy, including higher response rate and remission rate and greater changes from baseline in MADRS, HAM-A, CGI-S, and BPRS scores. Moreover, OFC was associated with similar incidence of adverse events with monotherapy. These findings provided supporting evidence for the use of OFC in the treatment of patients with TRD. However, considering the potential limitations in the study, more large-scale RCTs are needed to verify our findings.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- 2.Amsterdam JD, Hornig-Rohan M. Treatment algorithms in treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):371–386. doi: 10.1016/s0193-953x(05)70293-8. [DOI] [PubMed] [Google Scholar]
- 3.Fawcett J. Progression in treatment-resistant depression and treatment- refractory depression: we still have a long way to go. Psychiatr Ann. 1994;24:214–216. [Google Scholar]
- 4.Nierenberg A, Amsterdam JD. Treatment-resistant depression: definition and treatment approaches. J Clin Psychiatry. 1990;51(6 suppl):39–47. [PubMed] [Google Scholar]
- 5.Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 6.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi MH. The link between depression and physical symptoms. Prim Care Companion J Clin Psychiatry. 2004;6(suppl 1):12–16. [PMC free article] [PubMed] [Google Scholar]
- 8.Ishak WW, Greenberg JM, Cohen RM. Predicting relapse in major depressive disorder using patient-reported outcomes of depressive symptom severity, functioning, and quality of life in the individual burden of illness index for depression (IBI-D) J Affect Disord. 2013;151(1):59–65. doi: 10.1016/j.jad.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaynes BN, Rush AJ, Trivedi MH, Wisniewski SR, Spencer D, Fava M. The STAR*D study: treating depression in the real world. Cleve Clin J Med. 2008;75(1):57–66. doi: 10.3949/ccjm.75.1.57. [DOI] [PubMed] [Google Scholar]
- 10.Berwaerts J, Lane R, Nuamah IF, Lim P, Remmerie B, Hough DW. Paliperidone extended-release as adjunctive therapy to lithium or valproate in the treatment of acute mania: a randomized, placebo-controlled study. J Affect Disord. 2011;129(1–3):252–260. doi: 10.1016/j.jad.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 11.de Bartolomeis A, Perugi G. Combination of aripiprazole with mood stabilizers for the treatment of bipolar disorder: from acute mania to long-term maintenance. Expert Opin Pharmacother. 2012;13(14):2027–2036. doi: 10.1517/14656566.2012.719876. [DOI] [PubMed] [Google Scholar]
- 12.Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol. 2009;19(7):457–465. doi: 10.1016/j.euroneuro.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Perry KW, Wong DT, et al. Synergistic effects of olanzapine and other antipsychotic agents in combination with fluoxetine on norepinephrine and dopamine release in rat prefrontal cortex. Neuropsychopharmacology. 2000;23(3):250–262. doi: 10.1016/S0893-133X(00)00119-6. [DOI] [PubMed] [Google Scholar]
- 14.Koch S, Perry KW, Bymaster FP. Brain region and dose effects of an olanzapine/fluoxetine combination on extracellular monoamine concentrations in the rat. Neuropharmacology. 2004;46(2):232–242. doi: 10.1016/j.neuropharm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Corya SA, Andersen SW, Detke HC, et al. Long-term antidepressant efficacy and safety of olanzapine/fluoxetine combination: a 76-week open-label study. J Clin Psychiatry. 2003;64(11):1349–1356. doi: 10.4088/jcp.v64n1111. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 22.Trivedi MH, Thase ME, Osuntokun O, et al. An integrated analysis of olanzapine/fluoxetine combination in clinical trials of treatment-resistant depression. J Clin Psychiatry. 2009;70(3):387–396. doi: 10.4088/jcp.08m04064. [DOI] [PubMed] [Google Scholar]
- 23.Thase ME, Corya SA, Osuntokun O, et al. A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorder. J Clin Psychiatry. 2007;68(2):224–236. doi: 10.4088/jcp.v68n0207. [DOI] [PubMed] [Google Scholar]
- 24.Shelton RC, Williamson DJ, Corya SA, et al. Olanzapine/fluoxetine combination for treatment-resistant depression: a controlled study of SSRI and nortriptyline resistance. J Clin Psychiatry. 2005;66(10):1289–1297. doi: 10.4088/jcp.v66n1012. [DOI] [PubMed] [Google Scholar]
- 25.Corya SA, Williamson D, Sanger TM, Briggs SD, Case M, Tollefson G. A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, fluoxetine, and venlafaxine in treatment-resistant depression. Depress Anxiety. 2006;23(6):364–372. doi: 10.1002/da.20130. [DOI] [PubMed] [Google Scholar]
- 26.Brunner E, Tohen M, Osuntokun O, Landry J, Thase ME. Efficacy and safety of olanzapine/fluoxetine combination vs fluoxetine mono-therapy following successful combination therapy of treatment-resistant major depressive disorder. Neuropsychopharmacology. 2014;39(11):2549–2559. doi: 10.1038/npp.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Gao K, Kemp DE. Second-generation antipsychotics in major depressive disorder: update and clinical perspective. Curr Opin Psychiatry. 2011;24(1):10–17. doi: 10.1097/YCO.0b013e3283413505. [DOI] [PubMed] [Google Scholar]
- 28.Dodd S, Berk M. Olanzapine/fluoxetine combination for treatment-resistant depression: efficacy and clinical utility. Expert Rev Neurother. 2008;8(9):1299–1306. doi: 10.1586/14737175.8.9.1299. [DOI] [PubMed] [Google Scholar]
- 29.Bobo WV, Shelton RC. Olanzapine and fluoxetine combination therapy for treatment-resistant depression: review of efficacy, safety, and study design issues. Neuropsychiatr Dis Treat. 2009;5:369–383. doi: 10.2147/ndt.s5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tohen M, Case M, Trivedi MH, Thase ME, Burke SJ, Durell TM. Olanzapine/fluoxetine combination in patients with treatment-resistant depression: rapid onset of therapeutic response and its predictive value for subsequent overall response in a pooled analysis of 5 studies. J Clin Psychiatry. 2010;71(4):451–462. doi: 10.4088/JCP.08m04984gre. [DOI] [PubMed] [Google Scholar]
- 31.Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10(3):e1001403. doi: 10.1371/journal.pmed.1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quoix E, Ramlau R, Westeel V, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 2011;12(12):1125–1133. doi: 10.1016/S1470-2045(11)70259-5. [DOI] [PubMed] [Google Scholar]
- 33.Georgotas A, McCue RE, Cooper TB, Nagachandran N, Friedhoff A. Factors affecting the delay of antidepressant effect in responders to nortriptyline and phenelzine. Psychiatry Res. 1989;28(1):1–9. doi: 10.1016/0165-1781(89)90192-3. [DOI] [PubMed] [Google Scholar]
- 34.Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet. 1993;341(8842):418–422. doi: 10.1016/0140-6736(93)93004-k. [DOI] [PubMed] [Google Scholar]