Abstract
Leukemia results from the expansion of self-renewing hematopoietic cells that are thought to contain mutations that contribute to disease initiation and progression. Studies of the gene expression profiles of human acute myeloid leukemia samples has allowed their classification based on the presence of translocations and French-American-British subtypes, but it is not yet clear whether their molecular signatures reflect the initiating mutations or mutations acquired during progression. To begin to address this question, we examined the expression profiles of normal murine promyelocyte-enriched samples, nontransformed murine promyelocytes expressing human promyelocytic leukemia-retinoic acid receptor alpha (PML-RARα) fusion gene, and primary acute promyelocytic leukemia cells. The expression profile of nontransformed cells expressing PML-RARα was remarkably similar to that of wild-type promyelocytes. In contrast, the expression profiles of fully transformed cells from three acute promyelocytic leukemia model systems were all different, suggesting that the expression signature of acute promyelocytic leukemia cells reflects the genetic changes that contributed to progression. To further evaluate these progression events, we compared two high-penetrance acute promyelocytic leukemia models that both commonly acquire an interstitial deletion of chromosome 2 during progression. The two models exhibited distinct gene expression profiles, suggesting that the dominant molecular signatures of murine acute promyelocytic leukemia can be influenced by several independent progression events.
Several groups have created transgenic mouse models of acute promyelocytic leukemia (also called M3 acute myeloid leukemia) with the human promyelocytic leukemia-retinoic acid receptor alpha (PML-RARα) fusion cDNA placed under the control of regulatory cassettes that direct expression to early myeloid cells (6, 8, 11). All of the models have long latent periods that precede the development of acute promyelocytic leukemia, and most have been only partially penetrant. These results suggested that PML-RARα expression is necessary for acute promyelocytic leukemia development but not sufficient to cause disease; the long latent period strongly suggests that additional genetic and/or epigenetic events must occur for leukemia to develop.
The genetic changes that accompany leukemia progression can be characterized directly, i.e., with cytogenetics, comparative genomic hybridization (27), and/or DNA sequencing (16), or indirectly, by studying the gene expression profiles of leukemia cells (7, 33); these expression profiles can serve as markers of specific mutations. For example, acute myeloid leukemia samples containing specific translocations, e.g., t(15;17), t(8;21), or inv(16), can be distinguished based on their distinct expression profiles (13, 26). Although this approach is useful for distinguishing different types of leukemia, it has been more difficult to characterize the consequences of mutations that contribute to disease initiation versus progression because human acute leukemias are usually detected in the fully transformed state.
In murine acute myeloid leukemia models, however, the initiation event can be engineered into the germ line, so that progression events can be studied prospectively. One cytogenetically defined progression event has been found to occur with high frequency in several mouse models of acute myeloid leukemia; this change is an interstitial deletion of one copy of mouse chromosome 2, del(2) (18, 28, 34). Importantly, some patients with relapsed acute promyelocytic leukemia develop an interstitial deletion of the syntenic region on human chromosome 11p11-13 (3). These two regions display nearly perfect synteny, which allows straightforward comparisons of potentially relevant genes between species. The deletion of genes on this interval may therefore be important for leukemia progression in both mice and humans.
Acute promyelocytic leukemia appears to be different from other types of acute myeloid leukemia in that the CD34+/CD38− population does not contain the leukemia-initiating cells (4, 5). In addition, the CD34+/CD38− population of acute promyelocytic leukemia cells does not contain the PML-RARα transcript that is present in CD34+/CD38+ cells, suggesting that more mature cells represent the transformed population in this disease (30). Since cells with promyelocytic morphology also accumulate in murine acute promyelocytic leukemia, we decided to examine the expression profiles of normal murine promyelocytes, nontransformed promyelocytes that express PML-RARα, and fully transformed promyelocytes in an attempt to understand the genetic events that contribute to disease initiation versus progression.
We report here that normal murine promyelocyte-enriched samples and promyelocyte-enriched cells expressing PML-RARα are developmentally similar and contain very similar gene expression profiles. In contrast, different events that facilitate the development of acute promyelocytic leukemia can ultimately yield distinct expression signatures, suggesting that the expression profiles observed in acute promyelocytic leukemia cells may be due to genetic changes that occurred during disease progression. Therefore, these acute promyelocytic leukemia signatures appear to mark the consequences of progression mutations and not the expression of PML-RARα per se.
MATERIALS AND METHODS
Generation of PML-RARα-expressing mice and irradiation parameters.
We have previously described the generation of the human cathepsin G-PML-RARα transgenic mice (in a C3H × C57BL/6 background, Taconic) (referred to as PR mice) (8). Age-matched wild-type littermate (WT+XRT), PR transgenic mice (PR+XRT), and control wild-type C3H, C57BL/6, and C3H × C57BL/6 F1 mice received 3 Gy of ionizing radiation (XRT; Mark 1 137Cs irradiator) at 8 to 10 weeks of age. The generation and characterization of the doubly transgenic PML-RARα/RARα-PML mice (referred to as PR+RP) have been described (22). The Washington University Animals Studies Committee approved all animal experiments.
Analysis of animals, leukemia cryopreservation, flow cytometry, and transfer of acute promyelocytic leukemia to genetically compatible recipients.
Leukemic mice and leukemic spleen cells from PR and PR+XRT transgenic animals were analyzed as previously described for PR+RP mice (22). Acute promyelocytic leukemia cells from 8 PR and 10 PR+RP leukemic mice were previously cryopreserved and used in subsequent experiments; 1 PR and 10 PR+XRT acute promyelocytic leukemia samples were harvested from the leukemic mice described in this report. Table S1 in the supplemental material contains complete blood counts, spleen weights, flow cytometry, and cytogenetic information (spectral karyotyping and fluorescence in situ hybridization) for all acute promyelocytic leukemia samples.
All-trans-retinoic acid treatment of acute promyelocytic leukemia cells in vitro.
Cryopreserved leukemic cells were thawed and incubated (106 cells/ml) for 72 h in RPMI 1640 with 10% fetal calf serum supplemented with either 1 μM all-trans-retinoic acid (Sigma, St. Louis, Mo.) dissolved in ethanol or ethanol alone (0.1% ethanol by volume). Cells were pelleted by centrifugation, and supernatants were assayed for pro- matrix metalloproteinase 9 levels with the Quantikine mouse pro- matrix metalloproteinase 9 immunoassay kit protocol (R&D Systems, Minneapolis, Minn.). Leukemia samples were assayed in at least two independent experiments.
Interphase fluorescence in situ hybridization.
DNA bacterial artificial chromosome probe labeling, hybridization, and posthybridization washes were carried out according to the manufacturer's recommendations for the nick translation kit (Vysis, Inc., Downers Grove, Ill.). Chromosome 2 bacterial artificial chromosome probes included RPCI23-6M16 (located at 7.7 megabases on the centromeric end of mouse chromosome 2), RPCI23-478G11 (92 megabases), RPCI23-163L4 (104 megabases), and RPCI23-83A14 (178 megabases on the telomeric end of chromosome 2).
Generation of an enriched promyelocyte population.
Bone marrow cells were harvested from five wild-type C3H × C57BL/6 mice 2 days after intraperitoneal injection with 5-fluorouracil (150 mg/kg) (Sigma, St. Louis, Mo.) (19). Total bone marrow was cultured in Dulbecco's modified Eagle's medium containing 20% fetal calf serum, recombinant murine stem cell factor (100 ng/ml) (R&D Systems, Minneapolis, Minn.), murine interleukin-3 (6 ng/ml) (R&D Systems, Minneapolis, Minn.), murine Flt ligand (50 ng/ml) (R&D Systems, Minneapolis, Minn.), and human thrombopoietin (10 ng/ml) (PeproTech Inc., Rocky Hill, N.J.). After 72 h, mononuclear cells were purified by centrifugation on Histopaque-1077 (Sigma, St. Louis, Mo.). Cells were stained with a lineage cocktail (B220, CD11b, Gr-1, Ter119, and CD3ɛ cocktail) (Becton Dickinson, San Jose, Calif.), and Sca+/lineage− cells were flow sorted (MoFlo; Cytomation) into medium that was supplemented with stem cell factor (100 ng/ml) and recombinant human granulocyte colony-stimulating factor (100 ng/ml) (Amgen, Thousand Oaks, Calif.), which induces myeloid differentiation. Total RNA was extracted from cells harvested on days 0 and 2 to 7 with the RNeasy reagent and used for quantitative reverse transcription-PCR (Qiagen, Valencia, Calif.). Cytospins were made with cells harvested on days 0 and 2 to 7, and differentials were performed after May-Grunwald-Giemsa staining (Sigma, St. Louis, Mo.). Two independent experiments were performed with five wild-type mice in each group (wild-type day 2 promyelocyte-enriched population). Two independent experiments were also performed with five PML-RARα transgenic mice in each group (PR day 2 promyelocyte-enriched population, referred to as PR day 2 promyelocytes).
Expression profiling.
Total cellular RNA was purified from two wild-type day 2 promyelocyte samples, two PR day 2 promyelocyte samples, and 27 cryopreserved leukemia samples with Trizol reagent (Invitrogen, Carlsbad, Calif.) (9 PR, 10 PR+RP, and 8 PR+XRT acute promyelocytic leukemia samples). Total RNA was quantitated with a NanoDrop (NanoDrop technologies, Rockland, Del.) and analyzed for degradation with the RNA LabChip and 2100 Bioanalyzer (Agilent Technologies, Palo Alto, Calif.). For 9 PR, 10 PR+RP, and 8 PR+XRT acute promyelocytic leukemia samples, the Siteman Cancer Center Microarray Core Facility at Washington University labeled total RNA and hybridized 15 μg of labeled cRNA to the murine Affymetrix U74Av2 array according to the manufacturer's instructions (see http://pathbox.wustl.edu/∼mgacore/genechip.htm for a complete list of protocols). For two wild-type day 2 promyelocyte samples and two PR day 2 promyelocyte samples, 6 μg of labeled cRNA was hybridized to the murine Affymetrix U74Av2 arrays. Arrays were generated with 6 μg of cRNA because the promyelocyte RNA samples were extracted from approximately 1 × 105 to 2 × 105 cells, yielding smaller amounts of total labeled cRNA available to hybridize to the arrays. Washes and scanning were carried out according to the manufacturer's instructions. In order to perform interarray comparisons, the data for each array were scaled to a target intensity of 1,500 with Affymetrix Microarray Suite software (see the supplemental material for MIAME description and http://bioinformatics.wustl.edu for full access to the primary array data). To address array reproducibility, two arrays were hybridized with cRNA from the same labeling reaction for acute promyelocytic leukemia sample 10822.
Chromosomal localization and gene ontology categorization of probe sets on the U74Av2 array.
The accession numbers of all probe sets were used to query the NCBI Locus Link database for their genomic location and the Gene Ontology database for their gene ontology annotation.
Quantitative reverse transcription-PCR.
Total RNA was isolated from 27 acute promyelocytic leukemia samples described above. Quantitative reverse transcription-PCR was performed with SYBR Green (Molecular Probes, Eugene, Oreg.) as previously described (17). All samples were run in duplicate. Individual cDNA samples were normalized according to their levels of glyceraldehhyde-3-phosphate dehydrogenase transcript.
Statistical analysis.
Hierarchical clustering was performed with the DecisionSite for Functional Genomics Spotfire software (Spotfire, Somerville, Mass.). For this analysis, raw intensity values were normalized and converted to a z-score. Starting with 12,488 probe sets on the murine U74Av2 array, we excluded probe sets that were absent in all samples being compared as well as the Affymetrix control probe sets. For all gene expression profiling comparisons, we first performed unsupervised hierarchical clustering to investigate potential underlying relationships between samples with an unbiased approach. Subsequent supervised analysis was performed in order to generate a smaller list of genes and expressed sequence tags (ESTs) that could be annotated with gene ontology. Similar sample clustering was preserved for all supervised heat maps compared to unsupervised hierarchical clustering methods, suggesting that supervised methods did not falsely create or bias underlying sample clusters.
Probe sets included on supervised lists were selected solely based on statistical significance (not experimenter hand picking) for inclusion. Unsupervised hierarchical clustering heat maps were generated with an unweighted average (unweighted pairwise group mean average) method with a correlation metric. Unsupervised clustering results were validated with multiple hierarchical methods (unweighted pairwise group mean average and complete) and metrics (correlation and cosine correlation), as well as principal component analysis. A probe set was included in a supervised gene list only if it was identified with both a nonparametric test (either the Wilcoxon rank sum test for two-group comparisons or the Kruskal-Wallis test for three-group comparisons) and the permutation-based significance analysis of microarrays (SAM) algorithm (31). SAM was run with the two-class, unpaired parameter or the multiclass parameter, with 100 permutation cycles for all genotypes compared, and the average false discovery rate for comparisons was 1.84% (0.49 to 4.48%). Heat maps were generated for supervised gene lists as described for unsupervised hierarchical clustering.
RESULTS
Promyelocyte-enriched samples from wild-type and PML-RARα-expressing mice are developmentally similar.
To determine whether PML-RARα directly causes alterations in the gene expression profiles of primary promyelocytes, we first needed to enrich these cells. Wild-type promyelocyte-enriched samples (wild-type day 2 promyelocytes) were generated by culturing Sca+/lineage− bone marrow cells from wild-type C3H × C57BL/6 F1 mice in medium supplemented with stem cell factor and granulocyte colony-stimulating factor. During the 7 days following cytokine stimulation, there was a synchronized and orderly expansion of myeloid cells. Cells progressed from predominant myeloblasts on day 0 to promyelocytes on days 2 to 3, myelocytes and metamyelocytes on days 4 to 5, and bands and segmented neutrophils by days 6 to 7 (13a, 19).
Total RNA was extracted from cultured wild-type cells on days 0 and 2 to 7. Quantitative reverse transcription-PCR was performed for cathepsin G and neutrophil elastase, two primary granule genes that are highly expressed in promyelocytes. Cells removed on day 2 of culture contained the highest levels of cathepsin G and neutrophil elastase mRNA (13a). The morphology and primary granule gene expression pattern of day 2 cultured cells confirmed that the predominant cells in day 2 cultures are promyelocytes.
Next, we isolated promyelocyte-enriched samples from 8- to 12-week-old nonleukemic PML-RARα-expressing mice (PR day 2 promyelocytes). The morphology of PR day 2 promyelocytes was similar to that of wild-type day 2 promyelocytes (Fig. 1A and B). With quantitative reverse transcription-PCR, we determined that the fusion PML-RARα transcript increased significantly in cells from day 0 to day 2 of culture. Importantly, PML-RARα protein is present and functional in PR day 2 promyelocytes, since PML is distributed in a microspeckled pattern in the nuclei of these cells (13a). A major reduction in cell number occurred in PR cultures on day 3 of culture, while wild-type cells continued to proliferate (13a). This observation, in addition to the aforementioned data, necessitated the harvesting of PR promyelocytes on day 2.
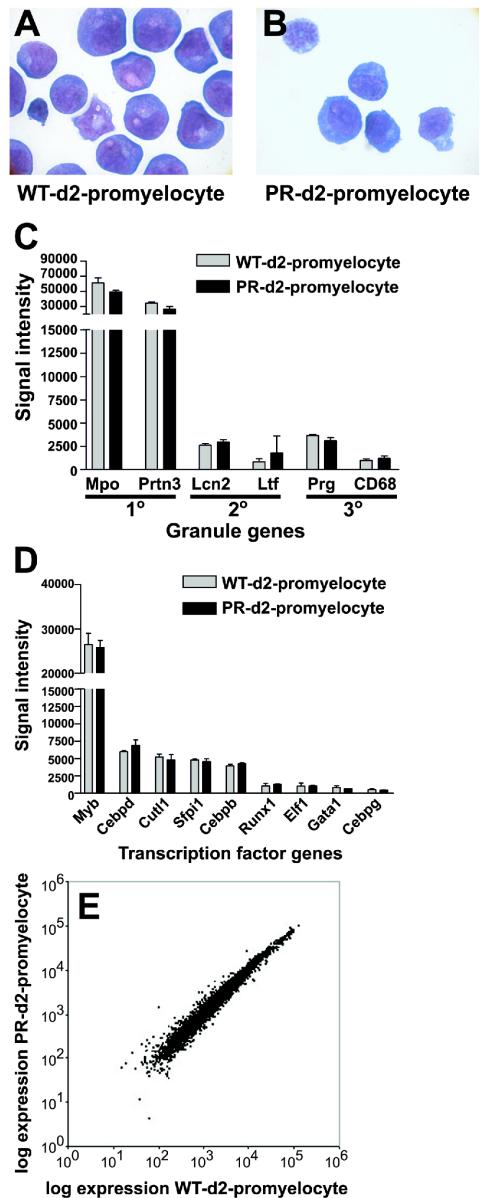
FIG. 1.
Characterization of wild-type promyelocyte-enriched populations and PML-RARα (PR)-expressing promyelocyte-enriched populations. The morphology of (A) wild-type (WT) day 2 (d2) promyelocytes and (B) PR day 2 promyelocytes (magnification, 1 000×). (C) Affymetrix signal intensity for myeloid granule genes. The myeloperoxidase (Mpo), proteinase 3 (Prtn3), lipocalin 2 (Lcn2), lactotrans-ferrin (Ltf), and proteoglycan (Prg) genes are shown. (D) Affymetrix signal intensity for myeloid transcription factor genes. (E) Average expression (Affymetrix signal intensity) for 6,453 probe sets from wild-type day 2 promyelocytes (n = 2) versus PR day 2 promyelocytes (n = 2).
To perform valid comparisons of the profiles of the promyelocyte-enriched populations described above, the populations analyzed must be developmentally similar. All day 2 cultured samples contained 86 to 91% early myeloid cells (myeloblasts, promyelocytes, and myelocytes) (Fig. 1A and B). We also assessed developmental similarity by examining the expression patterns of myeloid-specific genes. Total RNA was extracted from two wild-type day 2 promyelocyte cultures and two PR day 2 promyelocyte cultures, 6 μg of labeled cRNA from each sample was hybridized with the Affymetrix U74Av2 oligonucleotide array, and signal intensity values were scaled to 1,500 to allow interarray comparisons.
The average signal intensity of primary, secondary, and tertiary granule genes from wild-type and PR day 2 promyelocytes samples was compared (Fig. 1C). The overall pattern of granule gene expression in PR day 2 promyelocytes was similar to that of wild-type day 2 promyelocytes. Myeloperoxidase was the most abundantly expressed granule gene in all samples (Fig. 1C). Transcripts from genes expressed in mature myeloid cells (matrix metalloproteinase 9 and CD11b) were called absent in all samples. The pattern of expression for myeloid transcription factor genes was also compared for wild-type day 2 promyelocyte and PR day 2 promyelocyte samples. Again, the pattern of gene expression of PR day 2 promyelocytes was similar to that of wild-type day 2 promyelocytes (Fig. 1D). The most abundant myeloid transcription factor mRNA in all samples was myb, a gene that is known to be expressed in early myeloid cells. Levels of the transcription factor mRNAs from the fos and jun genes, which are known to be expressed during late myeloid development, were called absent.
Finally, only 100 genes/ESTs (1.5% of eligible probe sets) demonstrated expression levels that changed by twofold or more between wild-type day 2 promyelocytes and PR day 2 promyelocytes (Fig. 1E; see also Table S2 in the supplemental material). The SAM algorithm was applied with 6,453 probe sets from wild-type and PR day 2 promyelocyte samples; 5,969 out of 12,422 probe sets were called absent in all samples and were excluded from the analysis. The SAM algorithm was unable to confidently identify probe sets that discriminated wild-type from PR day 2 promyelocyte samples (all false discovery rates exceeded 13%). These results suggest that PR day 2 promyelocytes are very similar in composition to wild-type day 2 promyelocytes, based on their similar morphology, similar expression patterns of several developmentally regulated genes, and similar gene expression profiles.
Gene ontology terms were assigned to 6,700 out of 12,422 probe sets on the U74Av2 array, which included 66 out of the 100 genes/ESTs described above. A gene can be classified by more than one gene ontology term, and therefore a gene may appear in multiple gene ontology categories. A gene ontology category was considered significantly overrepresented for a gene list if at least two genes (representing 10% or more of the annotated genes on the list) belonged to a category and if the observed gene frequency for a category occurred more than expected based on the number of annotated probe sets on the array. Gene ontology annotation revealed that PML-RARα expression was associated with the expression of genes active in the extracellular space (34%, 12 of 35 annotated genes) and genes that are involved in the inflammatory response (11%), compared to the expected frequency of these categories (P < 0.05). In contrast, PML-RARα expression was associated with lower expression of genes that encode cytoplasmic (29%, 9 of 31 annotated genes) or cytoskeleton proteins (13%) and genes that encode chaperone (10%) or ligase activities (10%) (P < 0.008) (see Table S2 in the supplemental material for a complete gene list and annotation).
Unique gene expression profile for murine acute promyelocytic leukemia cells that express RARα-PML and contain del(2).
We have previously shown that PR mice that also express an RARα-PML transgene (RP) have a higher penetrance of disease and usually contain a characteristic interstitial deletion on one copy of chromosome 2, del(2) (34). To determine whether the PML-RARα/RARα-PML (PR+RP) murine acute promyelocytic leukemia samples exhibit a gene expression profile similar to that of PR acute promyelocytic leukemia samples, we harvested RNA from nine acute promyelocytic leukemia samples without del(2) by spectral karyotyping or fluorescence in situ hybridization and 10 PR+RP acute promyelocytic leukemia samples that were known to contain del(2) by spectral karyotyping. We hybridized 15 μg of labeled cRNA to the Affymetrix U74Av2 oligonucleotide.
Differentials of leukemic spleens from PR and PR+RP acute promyelocytic leukemia mice contain at least 70% early myeloid cells (with a predominance of promyelocytes) (22). The morphology (see Fig. 4C and D) and cellular composition of leukemic PR and PR+RP spleens were similar (see Fig. 4B). In addition, the pattern of granule gene expression in PR acute promyelocytic leukemia and PR+RP acute promyelocytic leukemia samples was similar, with the primary granule gene for myeloperoxidase (Mpo) being the most highly expressed (see Fig. 4F). The myeloid transcription factor gene expression pattern was also similar in the PR acute promyelocytic leukemia and PR+RP acute promyelocytic leukemia samples, highlighting the similarities of these promyelocytic leukemias (see Fig. 4G).
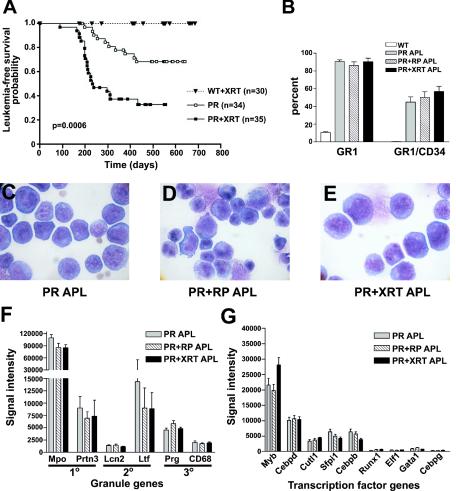
FIG. 4.
Characterization of leukemia arising in irradiated PML-RARα-expressing mice (PR+XRT) and comparison to PR+RP and PR acute promyelocytic leukemia samples. (A) Kaplan-Meier survival plot of irradiated wild-type littermate mice (WT+XRT), PR mice, and irradiated PR mice (PR+XRT). (B) Flow cytometric results for leukemic spleen cells stained with Gr-1 and CD34 antibodies. Wild-type (WT) (n = 8), PR acute promyelocytic leukemia (APL) (n = 9), PR+RP acute promyelocytic leukemia (n = 10), and PR+XRT acute promyelocytic leukemia (n = 8) samples. The morphology of (C) PR, (D) PR+RP, and (E) PR+XRT acute promyelocytic leukemia cells is shown (magnification, 1,000×). (F) Affymetrix signal intensity for myeloid granule genes. (G) Affymetrix signal intensity for myeloid transcription factor genes.
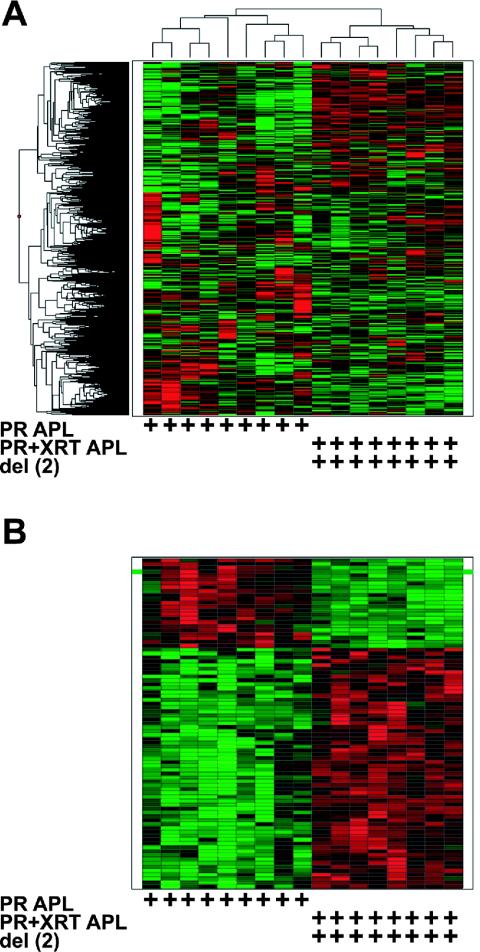
Unsupervised hierarchical clustering was performed with nine PR acute promyelocytic leukemia and 10 PR+RP acute promyelocytic leukemia samples with 7,125 probe sets (representing 4,759 unique genes and 1,757 ESTs); 5,297 out of 12,422 probe sets were called absent in all 19 samples and were excluded from the analysis. Three main clusters were apparent, one with PR acute promyelocytic leukemia samples only (at the far left of the column dendrogram), one with PR+RP acute promyelocytic leukemia samples only (at the far right), and a third with a mixture of two PR acute promyelocytic leukemia and five PR+RP acute promyelocytic leukemia samples (in the center, see Fig. 2A). Overall, the PR acute promyelocytic leukemia and PR+RP acute promyelocytic leukemia samples appear to segregate based on the presence or absence of RARα-PML expression and/or del(2). This suggests the presence of a distinct global gene expression profile in PR+RP samples.
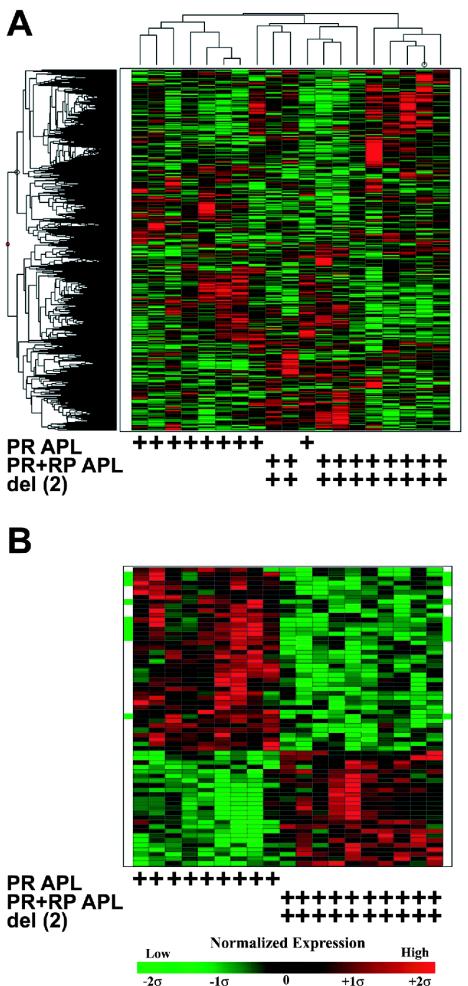
FIG. 2.
Expression profiles of PR versus PR+RP acute promyelocytic leukemia samples. (A) Unsupervised hierarchical clustering with 7,125 genes/ESTs (rows) and nine PR acute promyelocytic leukemia samples without del(2) and 10 PR+PR acute promyelocytic leukemia samples with del(2) (columns). Each row represents one gene/EST, and each column represents one sample. The genotype and the presence of an interstitial deletion on chromosome 2 [del(2)] are indicated at the bottom of each heat map by the presence of a + symbol below the corresponding sample column. The expression level of each gene was normalized across all samples and is represented by a color, with red representing expression above the mean and green representing expression below the mean, while the color intensity corresponds to the magnitude of deviation (σ) from the mean. The color-coded scale for normalized expression values for all heat maps is located at the bottom of the figure. (B) Heat map generated with 65 genes/ESTs (rows) and 19 samples (columns). Ten genes located on the del(2) interval, defined by spectral karyotyping (megabases 85 to 140 onchromosome 2), are highlighted by a green bar to the left and right of the corresponding row in the heat map. Each row represents one gene/EST, and each column represents one sample.
Next, a smaller gene list was generated with supervised methods in order to investigate potentially important gene expression patterns associated with PR+RP samples. The Wilcoxon rank-sum test and SAM algorithm were applied to the 7,125 probe sets, and the most highly significant set of genes common to both tests were identified based solely on the statistical algorithms. Sixty-five probe sets (representing 53 unique genes and 12 ESTs) were identified that distinguished PR from PR+RP acute promyelocytic leukemia samples. A heat map was generated with these 65 genes/ESTs in order to visualize the gene expression differences between the two acute promyelocytic leukemia models (Fig. 2B).
To test whether the molecular signature defined by the 65 genes/ESTs predicted the presence of del(2), we identified the genomic locations of the 65 defining genes and/or ESTs. We were able to identify the exact chromosomal locations of 9,154 probe sets out of 12,422 on the U74Av2 array; 745 of these mapped to chromosome 2, with 217 located between 85 and 140 megabases (encompassed by cytogenetic bands 2E and 2F, which was identified as the minimally deleted region by spectral karyotyping in PR+RP leukemias) (34); 77 were located between 85 and 106 megabases (which corresponds to the minimally deleted region on chromosome 2 in radiation-induced murine acute myeloid leukemia mapped by simple sequence length polymorphisms) (28). There were 40 genes/ESTs expressed at lower levels in PR+RP acute promyelocytic leukemia samples with del(2). Ten of these 40 genes/ESTs mapped to the megabase 85 to 140 region of chromosome 2, and 6 of these 10 were located between megabases 85 and 106. This frequency (10 of 40) was significantly greater than the expected frequency based on the number of del(2) genes present on the array (P < 0.0001). The green bars located at the left and right sides of the heat map highlight the 10 genes located on del(2) (Fig. 2B) (see Table S3 in the supplemental material for a complete gene list and annotation).
Validation of array results was performed with four genes located within the del(2) interval. Two of these genes were included in the 10 genes found to be underexpressed in the PR+RP acute promyelocytic leukemia samples (Api5 and Fbxo3), and two were not (Sfpi1 and Lmo2). Quantitative reverse transcription-PCR was performed with 27 acute promyelocytic leukemia samples (9 PR, 10 PR+RP, and 8 PR+XRT), and the results for the four genes correlated significantly with the oligonucleotide array results (P < 0.05) (Fig. S1 in the supplemental material).
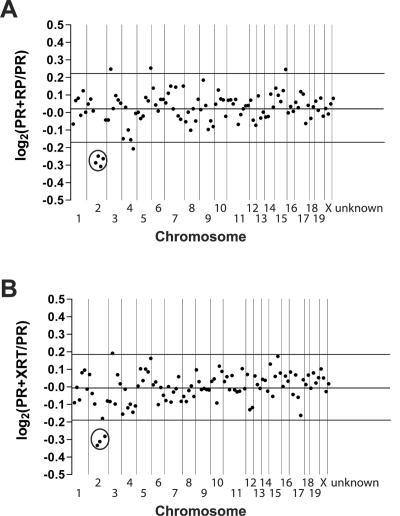
To determine whether the entire del(2) region was underexpressed in acute promyelocytic leukemia samples containing del(2), we calculated the expression ratio of genes across the entire genome in 10 PR+RP acute promyelocytic leukemia samples with del(2) versus 9 PR acute promyelocytic leukemia samples without del(2); 5,297 out of 12,422 probe sets were absent in all samples and excluded from the analysis. The locations of 5,491 probe sets out of 7,125 eligible probe sets were mapped to the genome. Proceeding from the centromere to the telomere of each chromosome, the signal intensity of 50 consecutive genes/ESTs was used to create a log2 PR+RP/PR acute promyelocytic leukemia sample expression ratio for each genomic interval (Fig. 3A). The average genomic size encompassed by 50 consecutive genes/ESTs was 22.7 megabases (2.4 to 49.6 megabases). The centromere of chromosome 1 is located at the left of the plot, and the telomere of chromosome X is at the right. In the center of chromosome 2, four data points (each point representing 50 consecutive genes/ESTs) display a decrease in expression in the del(2)-containing samples, which was more than two standard deviations below the mean ratio of all data points (Fig. 3A) (average log2 ratio for the four points decreased on chromosome 2 = −0.277, confidence interval = −0.251 to −0.303, P = 1.78 × 10−6 compared to the remaining data points). These genes map to the megabase 70 to 156 interval on chromosome 2, which corresponds to the minimally deleted region detected by spectral karyotyping in the PR+RP acute promyelocytic leukemia samples. There is no significant decrease in the average expression ratio for the corresponding genomic interval when comparing wild-type or PR day 2 promyelocytes versus PR acute promyelocytic leukemia samples (data not shown).
FIG. 3.
Average expression levels for genes by chromosome in PR+RP and PR+XRT acute promyelocytic leukemia samples relative to PR acute promyelocytic leukemia samples. (A) We mapped 5,491 genes/ESTs to an exact genomic location. The average gene expression level was calculated for 10 PR+RP acute promyelocytic leukemia samples with del(2) and nine PR acute promyelocytic leukemia samples without del(2). A PR+RP/PR acute promyelocytic leukemia sample ratio was generated for each gene/EST. The log2 value of each ratio was calculated, and the log2 ratio for 50 consecutive genes on the same chromosome was averaged to generate one data point. The last data point at the right of the plot represents the average log2 expression ratio for all probe sets unable to be assigned a genomic location (unknown, n = 1,634). (B) We mapped 5,407 genes/ESTs to a genomic location. Each data point represents the average log2 ratio of 50 consecutive genes on a chromosome for 8 PR+XRT acute promyelocytic leukemia samples with del(2) versus nine PR acute promyelocytic leukemia samples without del(2). The last data point at the right of the plot represents the average log2 expression ratio for all probe sets unable to be assigned a genomic location (unknown, n = 1,586). Vertical lines separate chromosomal boundaries. Circles highlight the data points located on the del(2) interval that are expressed at significantly lower levels in PR+RP and PR+XRT acute promyelocytic leukemia samples compared to PR acute promyelocytic leukemia samples.
Acute promyelocytic leukemia arising in irradiated PML-RARα-expressing mice is associated with del(2) and is developmentally similar to PR and PR+RP acute promyelocytic leukemia.
Even though the presence of del(2) is associated with a striking gene dosage effect, our results do not explain whether the molecular signature found in PR+RP acute promyelocytic leukemia samples is influenced more heavily by del(2) or by RP expression. To address this question, we had to develop a second acute promyelocytic leukemia model that also contains a del(2) gene dosage effect at the genomic level. We generated a murine acute promyelocytic leukemia model associated with del(2) by irradiating young PML-RARα-expressing mice (PR+XRT acute promyelocytic leukemia). This approach was based on the fact that del(2) is the dominant cytogenetic abnormality associated with radiation-induced murine acute myeloid leukemia models (9, 10, 20, 25, 28, 29).
We found that 22 of 35 PR+XRT mice and 10 of 34 PR mice developed acute promyelocytic leukemia after a similar latency of 200 days (67% versus 32%, P = 0.0006) (Fig. 4A). No irradiated wild-type littermate mice in this experimental cohort developed leukemia. Additional controls were performed with wild-type animals to evaluate potential strain effects: 1 of 20 C3H, 1 of 20 C57BL/6, and 1 of 20 C3H × C57BL/6 F1 mice developed acute myeloid leukemia. Leukemic PR+XRT mice developed leukocytosis, anemia, thrombocytopenia, and splenomegaly (Table 1). The differential cell counts (79 ± 6% early myeloid cells, n = 6) of PR+XRT spleen cells was similar to that of PR acute promyelocytic leukemia and PR+RP acute promyelocytic leukemia samples (Fig. 4B to E; see also Fig. S2 in the supplemental material). Intraperitoneal injection of 3 × 105 to 10 × 105 cryopreserved leukemic spleen cells from two separate PR+XRT acute promyelocytic leukemia samples into syngeneic recipients caused uniformly lethal acute promyelocytic leukemia by 60 days. Cryopreserved leukemic spleen cells from five of five PR+XRT mice demonstrated in vitro differentiation in response to 1 μM all-trans-retinoic acid treatment (Table 1). These phenotypic results are highly similar to that observed with either PR or PR+RP leukemic mice (see Table S1 in the supplemental material). Although the average spleen weight of PR+XRT leukemic mice was less than the average spleen weight of PR and PR+RP leukemic mice (P = 0.042 and P = 0.003, respectively), all spleens from leukemic animals were greatly enlarged compared to age-matched controls (P < 0.002).
TABLE 1.
Characterization of PR + XRT mice and harvested spleen cells
| Animal no. | Peripheral white blood cell count (103 cells/μl) | Hemoglobin (g/dl) | Platelet count (103/μl) | Spleen wt (g) | No. of del(2) cells/no. counted | No. of tetraploid cells/no. counted | % of cells del(2) | Response to all-trans-retinoic acida |
|---|---|---|---|---|---|---|---|---|
| 15557 | 39.48 | 5.00 | 161 | 0.770 | 33/50 | 0/50 | 66 | ND |
| 15794 | 47.84 | 8.50 | 169 | 0.800 | 72/100 | 10/100 | 82 | ND |
| 16079 | 61.60 | 14.20 | 259 | 1.230 | 66/100 | 18/100 | 84 | + |
| 17194 | 28.08 | 8.30 | 91 | 0.490 | 37/50 | 3/50 | 80 | + |
| 17738 | 30.86 | 11.70 | 597 | 0.810 | 10/50 | 0/50 | 20 | + |
| 17749 | 21.22 | 7.80 | 344 | 1.060 | 42/50 | 2/50 | 88 | ND |
| 17755 | 76.28 | 6.20 | 296 | 0.770 | 39/50 | 0/50 | 78 | + |
| 17836 | 24.22 | 7.10 | 421 | 0.870 | 0/50 | 0/50 | 0 | ND |
| 17845 | 16.24 | 5.20 | 171 | 0.570 | 35/50 | 11/50 | 92 | ND |
| 17849 | 23.78 | 4.00 | 91 | 0.500 | 45/50 | 3/50 | 96 | + |
| Avg | 36.96 | 7.80 | 260 | 0.787 | 69 |
+, myeloid differentiation; ND, not done.
Nine out of 10 PR+XRT leukemias contained a deletion on one copy of chromosome 2 that was detected by fluorescence in situ hybridization (Table 1; see also Fig. S3 in the supplemental material); this frequency is similar to the 11 of 13 PR+RP leukemias that contained del(2) by spectral karyotyping (34). In four of four PR+XRT leukemias evaluated with two fluorescence in situ hybridization probes (in addition to centromeric and telomeric probes), the deletion was interstitial and spanned at least from megabases 92 to 104 (which is contained within the minimally deleted region reported for radiation-induced murine acute myeloid leukemia). One of six cryopreserved PR acute promyelocytic leukemia samples contained del(2) by fluorescence in situ hybridization, which is consistent with previously reported spectral karyotyping results showing that one of five PR acute promyelocytic leukemia samples contained del(2) (34); neither PR sample with del(2) was included in this study.
Acute promyelocytic leukemia cells from the two del(2)-associated models have distinct expression profiles.
To address whether the presence of del(2) dominantly influences the molecular signature found in del(2)-associated acute promyelocytic leukemia, we examined the expression signature in del(2)-associated PR+XRT samples. Total RNA was extracted from eight PR+XRT acute promyelocytic leukemia samples; 15 μg of labeled cRNA was hybridized to the Affymetrix U74Av2 oligonucleotide array. The average signal intensity of granule genes from PR+XRT acute promyelocytic leukemia samples was compared to PR acute promyelocytic leukemia and PR+RP acute promyelocytic leukemia samples and was similar (Fig. 4F); the myeloid transcription factor expression pattern was also similar to that of the PR and PR+RP acute promyelocytic leukemia samples (Fig. 4G). The log2 PR+XRT/PR acute promyelocytic leukemia sample expression ratio for 50 consecutive genes/ESTs located across the genome was plotted for eight del(2)-containing PR+XRT acute promyelocytic leukemia samples. In the center of chromosome 2, three out of four consecutive bins of genes/ESTs displayed a decrease in expression that was greater than two standard deviations below the mean (Fig. 3B) (average ratio for the four bins = −0.277, confidence interval = −0.211 to −0.344, and P = 0.003 compared to the remaining bins). This area maps to the same region identified in PR+RP samples with del(2).
Unsupervised hierarchical clustering with 6,993 probe sets (representing 4,691 unique genes and 1,689 ESTs) discriminated PR acute promyelocytic leukemia samples from PR+XRT acute promyelocytic leukemia samples (Fig. 5A); 5,429 out of 12,422 probe sets were absent in all samples and excluded from the analysis. To determine whether the most significant discriminating set of genes for PR+XRT acute promyelocytic leukemia samples was also related to a del(2) gene dosage effect, the top 85 probe sets (representing 68 unique genes and 14 ESTs) were identified by both the Wilcoxon rank sum test and SAM to be significantly associated with either PR or PR+XRT acute promyelocytic leukemia samples. Only one of 85 probe sets was also included in the 65-gene/EST supervised list used to discriminate PR versus PR+RP acute promyelocytic leukemia samples. A heat map was generated with these 85 genes/ESTs in order to visualize the gene expression differences between the two acute promyelocytic leukemia models (Fig. 5B).
FIG. 5.
Expression profiles of PR versus PR+XRT acute promyelocytic leukemia samples. (A) Unsupervised hierarchical clustering with 6,993 genes/ESTs (rows) and nine PR acute promyelocytic leukemia samples without del(2) and eight PR+XRT acute promyelocytic leukemia samples with del(2) (columns). (B) Heat map generated with 85 genes/ESTs (rows) and 17 samples (columns). One gene located on the del(2) interval, defined by spectral karyotyping (megabases 85 to 140 on chromosome 2), is highlighted by a green bar at the left and right of the corresponding row in the heat map. Each row represents one gene/EST, and each column represents one sample.
Of the 23 probe sets that exhibited lower expression in PR+XRT acute promyelocytic leukemia samples, only one was located on chromosome 2 between megabases 85 and 140 (highlighted by a green bar in Fig. 5B), and none was located on chromosome 2 between megabases 85 to 106 (see Table S4 in the supplemental material for a complete gene list and annotations). This was not significantly different from the number of chromosome 2 genes that were expected to be included in a random list of 23 genes (P > 0.05). Furthermore, the 1 of 23 discriminatory genes located on the del(2) interval in the PR+XRT acute promyelocytic leukemia samples was significantly less than the 10 of 40 found in the PR+RP samples (P = 0.044). Therefore, the presence of del(2) in PR+XRT acute promyelocytic leukemia samples did not predict that the most significant set of defining genes would be del(2) related.
Expression profiles from acute promyelocytic leukemia tumors without del(2).
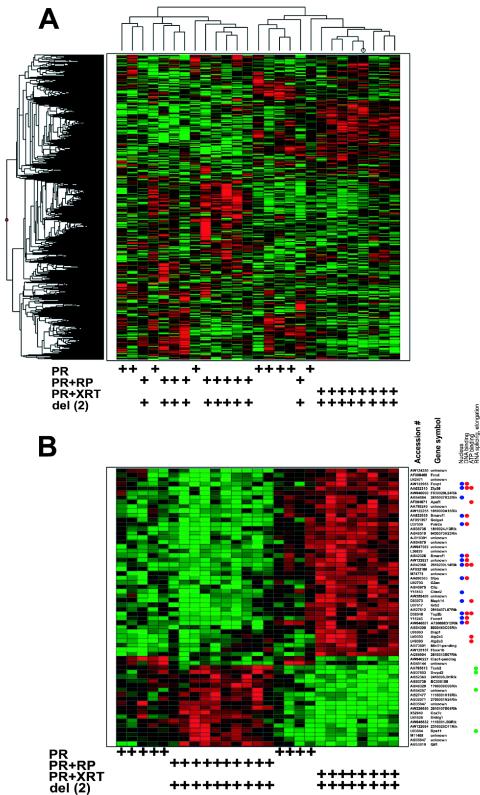
We wished to determine whether leukemias without del(2) had expression profiles that were distinct from del(2) leukemia samples. Unsupervised hierarchical clustering was performed with 9 PR acute promyelocytic leukemia samples without del(2), 10 PR+RP acute promyelocytic leukemia samples with del(2), and 8 PR+XRT acute promyelocytic leukemia samples with del(2), with 7,350 probe sets (representing 4,878 unique genes and 1,824 ESTs); 5,072 out of 12,422 probe sets were absent in all 27 samples and were excluded from the analysis. PR, PR+RP, and PR+XRT acute promyelocytic leukemia samples all contained unique gene signatures (Fig. 6A). In addition, the PR leukemias tended to cluster with either PR+RP or PR+XRT acute promyelocytic leukemia samples. Overall, the acute promyelocytic leukemia samples did not appear to segregate based on the presence or absence of del(2), implying that additional non-del(2) progression events also influence the global gene expression pattern in acute promyelocytic leukemia samples. A similar pattern of acute promyelocytic leukemia sample segregation was observed with a principal component analysis method (see Fig. S4 in the supplemental material). Collectively, these unsupervised clustering approaches suggested that del(2) status alone does not predict the global expression signature for acute promyelocytic leukemia samples.
FIG. 6.
Expression profiles of PR, PR+RP, and PR+XRT acute promyelocytic leukemia samples. (A) Unsupervised hierarchical clustering with 7,350 genes/ESTs (rows) and nine PR acute promyelocytic leukemia samples without del(2), 10 PR+RP acute promyelocytic leukemia samples with del(2), and eight PR+XRT acute promyelocytic leukemia samples with del(2) (columns). (B) Heat map generated with 62 genes/ESTs (rows) and 27 samples (columns). Each row represents one gene/EST, and each column represents one sample.
The acute promyelocytic leukemia sample clusters identified with unsupervised approaches were generated without a priori input or knowledge of a “correct” answer. These findings are further supported by results obtained with supervised methods (an approach that successfully discriminated PR from PR+RP and PR from PR+XRT acute promyelocytic leukemia samples). With the Kruskal-Wallis test and SAM algorithm, we identified the 62 most significant probe sets (representing 48 unique genes and 11 ESTs) that defined the 9 PR, 10 PR+RP, and 8 PR+XRT acute promyelocytic leukemia samples from each other; 5,072 probe sets were absent in all 27 samples and were excluded from the analysis. The heat map generated with these 62 genes/ESTs was remarkably similar to the unsupervised hierarchical clustering visualization (Fig. 6B).
Gene ontology annotation was available for 38 of the 62 defining probe sets. Twenty-nine of the 42 highly expressed genes in PR+XRT and 4 PR acute promyelocytic leukemia samples were classified, and 14 of 29 (48%) encode proteins active in the nucleus (Fig. 6B, blue dots) involved in DNA (38%) or ATP binding (24%) (Fig. 6B, red dots) (P < 0.05). Eight of the 18 genes that were expressed at higher levels in PR+RP and five PR acute promyelocytic leukemia samples were classified, and four of eight (50%) genes are involved in RNA splicing and elongation (Fig. 6B, green dots) (P < 0.05). One of the two genes expressed at higher levels in the nine PR acute promyelocytic leukemia samples was located on the del(2) interval. (see Table S5 in the supplemental material for a complete gene list and annotation).
DISCUSSION
In this report, we examined the gene expression profiles of wild-type murine promyelocyte-enriched populations, promyelocyte-enriched populations from nonleukemic PML-RARα (PR)-expressing mice, and leukemic promyelocytes from three murine acute promyelocytic leukemia models. The gene expression profiles present in acute promyelocytic leukemia models were dramatically different from each other, in contrast to the similar expression profiles seen for wild-type and nonleukemic, PR-expressing day 2 promyelocyte-enriched populations. Although the gene expression profile for del(2)-associated PR+RP acute promyelocytic leukemia samples was strongly associated with the loss of one copy of the genes on the del(2) interval, the presence of del(2) did not predict a similar expression profile in an irradiation-induced del(2) acute promyelocytic leukemia model. The molecular heterogeneity observed in these murine acute promyelocytic leukemia models strongly suggests that del(2) is one of several genetic events that can synergize with PML-RARα to cause progression.
Thorough cellular characterization of the samples being compared by gene expression profiling is necessary to determine whether any observed differences are simply a reflection of cellular differences between samples or preparation artifacts. The method used to enrich promyelocytes in this study represents the best currently available technique for the study of primary murine myeloid cells. We demonstrated that the composition of enriched promyelocytes was highly similar; based on morphology and the overall expression patterns of early versus late myeloid genes and transcription factor genes (Fig. 1). The same rigorous characterization parameters were used to compare the cellular composition of all of the acute promyelocytic leukemia models (Fig. 4). Therefore, our data strongly suggests that the expression profiling differences noted between the acute promyelocytic leukemia samples probably reflect genetic differences among these samples, not cellular ones.
Wild-type day 2 promyelocytes, PR day 2 promyelocytes, and PR acute promyelocytic leukemia cells are morphologically similar and have similar expression patterns of myeloid transcription factors and granule genes (Fig. 1 and 4). Despite this, direct comparison of the expression profiles from these cells is difficult to interpret because the culture conditions required to generate the promyelocyte-enriched samples could potentially alter their global gene expression pattern. Aware of this limitation, we directly compared promyelocyte-enriched samples and PR acute promyelocytic leukemia samples. With unsupervised hierarchical clustering, the expression profiles of wild-type and PR day 2 promyelocytes were highly similar, and both were very different from that of the PR acute promyelocytic leukemia samples (see Fig. S5 in the supplemental material). This finding may reflect cellular differences between samples, or the expression profiles may reflect real genetic differences among these samples. Direct evidence supporting this hypothesis will require novel experimental methods (yet to be described) that allow the isolation of cells from all samples with identical conditions.
To address the direct effects of PML-RARα expression in primary early myeloid cells, we compared expression profiles from wild-type versus PR day 2 promyelocytes. Importantly, PML oncogenic domains are intact in day 0 cells but are fully disrupted in PR day 2 promyelocytes (13a), which shows that PML-RARα is activated and functional on day 2 of culture. Despite this, the expression of only 1.5% of genes/ESTs (100 total) was altered by twofold or more between wild-type day 2 promyelocytes and PR day 2 promyelocytes, which shows that PML-RARα expression does not cause widespread gene expression changes in primary cells. Although a difference in 100 genes/ESTs was slightly higher than the number of changed genes observed for duplicate array experiments (1.1%, P = 0.02), only 22 of 100 genes/ESTs changed threefold or more. In addition, these 100 probe sets do not overlap with the probe sets that discriminate the acute promyelocytic leukemia samples from each other (0 of 65 PR versus PR+RP, 0 of 85 PR versus PR+XRT, and 1 of 62 PR versus PR+RP versus PR+XRT discriminatory probe sets were included in the list of 100 genes/ESTs, proportions not significantly different than chance alone, P > 0.05).
The small gene expression changes observed in primary cells are supported by the modest changes recently reported for parental U937 cells compared to PR-9 cells induced to express PR for 8 or 16 h (1, 21). Perhaps not surprisingly, our list of 100 potential target genes does not overlap those reported previously. This may reflect the differences in the cellular compartment used to express PR (primary early myeloid cells versus adapted cell lines), the timing and level of PR expression, species differences (mouse versus human), array probe set representation, and experimental design. Further evaluation of potential PR target genes will require extensive experimentation and in vivo validation. Regardless, these results suggest that expression of PR in early myeloid cells does not cause large changes in gene expression and imply that the altered expression profile of acute promyelocytic leukemia cells is more likely due to additional changes acquired during progression.
Although del(2) may facilitate acute promyelocytic leukemia progression, it is clearly not required, since most acute promyelocytic leukemia tumors arising in PR-expressing mice do not have it (12, 14, 15, 34). Del(2) can occur in murine bone marrow cells within days of sublethal irradiation (2, 24), but only 25% (or less) of mice develop acute myeloid leukemia after a latency of approximately 12 months (9, 10, 20, 25, 28, 29). These observations suggest that leukemic progression can be influenced by the presence of del(2), but that additional events are also necessary—and these events may be the ones that predict the gene expression profile.
To test this possibility, we used unsupervised hierarchical clustering and principal component analysis methods to examine the clustering of acute promyelocytic leukemia samples with or without del(2). The results suggested that specific expression signatures were associated with each genotype (PR, PR+RP, and PR+XRT), but the clusters were not associated only with del(2) status (Fig. 6A). This was confirmed with a smaller list of genes identified by a supervised approach (Fig. 6B). The results suggest that the gene expression profiles of PR-expressing cells are influenced by facilitators of del(2) formation (RP and XRT) and also by other genetic events that are apparently shared between del(2) and non-del(2) leukemias (Fig. 6A and B). Myeloid cells expressing PML-RARα can therefore progress towards acute promyelocytic leukemia via one of several routes. Even in the presence of del(2), our data suggest that additional mutations are required for full transformation to occur (34). PR-expressing cells may acquire similar mutations in the absence of del(2), but they are less likely to occur (based on the lower penetrance of disease in unmanipulated PR mice).
Gene ontology annotation suggests that the facilitated pathway mutations lead to changes in nuclear (DNA and ATP binding) or RNA splicing and elongation gene expression, but the data also suggests that these facilitators are not required for these pathways to be engaged. We do not yet know whether these defining gene signatures are causally linked to progression or simply markers of it. Likewise, we do not yet know whether del(2) alone can facilitate acute promyelocytic leukemia progression in PR-expressing mice. The generation of a genetically engineered mouse lacking the del(2) interval will be required to address this question; our results predict that it would indeed synergize with PR and cause increased penetrance of murine acute promyelocytic leukemia.
In summary, our results show that the heterogeneity in gene expression profiles for various murine acute promyelocytic leukemia models is influenced by known secondary genetic events, i.e., del(2), and additional (as yet undefined) genetic events. The del(2) gene dosage effect associated with murine acute promyelocytic leukemia is similar to the gene dosage effects observed for trisomy 8 and deletions of 5q associated with human acute myeloid leukemia, suggesting that leukemia progression may be directly related to the gain or loss of a gene(s) on these intervals (23, 32). Although the presence of del(2) does not predict a single, dominant gene expression profile in murine acute promyelocytic leukemia, these findings warrant further investigation of genes on the del(2) interval. Insights gained from the study of murine del(2) may also help us to understand human acute promyelocytic leukemia cells that contain the syntenic 11p11-13 deletion.
Supplementary Material
Acknowledgments
We thank Mieke Hoock for excellent colony management and the Siteman Cancer Center's Multiplexed Gene Array, High Speed Cell Sorter, and Bioinformatics cores. We are grateful to Dan Link for helpful comments and critical reading of this manuscript. Nancy Reidelberger provided expert editorial assistance.
This work was supported by National Institutes of Health grants CA83962 and CA101937, the Bakewell Cancer Research Fund, the Buder Charitable Foundation (T.J.L.), and a Fellowship grant from the Leukemia Research Foundation (M.J.W.).
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org.
REFERENCES
- 1.Alcalay, M., N. Meani, V. Gelmetti, A. Fantozzi, M. Fagioli, A. Orleth, D. Riganelli, C. Sebastiani, E. Cappelli, C. Casciari, M. T. Sciurpi, A. R. Mariano, S. P. Minardi, L. Luzi, H. Muller, P. P. Di Fiore, G. Frosina, and P. G. Pelicci. 2003. Acute myeloid leukemia fusion proteins deregulate genes involved in stem cell maintenance and DNA repair. J. Clin. Investig. 112:1751-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ban, N., M. Kai, and T. Kusama. 1997. Chromosome aberrations in bone marrow cells of C3H/He mice at an early stage after whole-body irradiation. J. Radiat. Res. (Tokyo) 38:219-231. [DOI] [PubMed] [Google Scholar]
- 3.Berger, R., M. Le Coniat, J. Derre, D. Vecchione, and P. Jonveaux. 1991. Cytogenetic studies in acute promyelocytic leukemia: a survey of secondary chromosomal abnormalities. Genes Chromosomes Cancer 3:332-337. [DOI] [PubMed] [Google Scholar]
- 4.Blair, A., D. E. Hogge, L. E. Ailles, P. M. Lansdorp, and H. J. Sutherland. 1997. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood 89:3104-3112. [PubMed] [Google Scholar]
- 5.Bonnet, D., and J. E. Dick. 1997. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3:730-737. [DOI] [PubMed] [Google Scholar]
- 6.Brown, D., S. Kogan, E. Lagasse, I. Weissman, M. Alcalay, P. G. Pelicci, S. Atwater, and J. M. Bishop. 1997. A PMLRARα transgene initiates murine acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 94:2551-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golub, T. R., D. K. Slonim, P. Tamayo, C. Huard, M. Gaasenbeek, J. P. Mesirov, H. Coller, M. L. Loh, J. R. Downing, M. A. Caligiuri, C. D. Bloomfield, and E. S. Lander. 1999. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science 286:531-537. [DOI] [PubMed] [Google Scholar]
- 8.Grisolano, J. L., R. L. Wesselschmidt, P. G. Pelicci, and T. J. Ley. 1997. Altered myeloid development and acute leukemia in transgenic mice expressing PML-RARα under control of cathepsin G regulatory sequences. Blood 89:376-387. [PubMed] [Google Scholar]
- 9.Haran-Ghera, N., A. Peled, R. Krautghamer, and P. Resnitzky. 1992. Initiation and promotion in radiation-induced myeloid leukemia. Leukemia 6:689-695. [PubMed] [Google Scholar]
- 10.Hayata, I., M. Seki, K. Yoshida, K. Hirashima, T. Sado, J. Yamagiwa, and T. Ishihara. 1983. Chromosomal aberrations observed in 52 mouse myeloid leukemias. Cancer Res. 43:367-373. [PubMed] [Google Scholar]
- 11.He, L. Z., C. Tribioli, R. Rivi, D. Peruzzi, P. G. Pelicci, V. Soares, G. Cattoretti, and P. P. Pandolfi. 1997. Acute leukemia with promyelocytic features in PML/RARα transgenic mice. Proc. Natl. Acad. Sci. USA 94:5302-5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kogan, S. C., D. E. Brown, D. B. Shultz, B. T. Truong, V. Lallemand-Breitenbach, M. C. Guillemin, E. Lagasse, I. L. Weissman, and J. M. Bishop. 2001. BCL-2 cooperates with promyelocytic leukemia retinoic acid receptor alpha chimeric protein (PMLRARα) to block neutrophil differentiation and initiate acute leukemia. J. Exp Med. 193:531-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohlmann, A., C. Schoch, S. Schnittger, M. Dugas, W. Hiddemann, W. Kern, and T. Haferlach. 2003. Molecular characterization of acute leukemias by use of microarray technology. Genes Chromosomes Cancer. 37:396-405. [DOI] [PubMed] [Google Scholar]
- 13a.Lane, A., and T. Ley. Neutrophil elastase is important for PML-retinoic acid receptor α activities in early myeloid cells. Mol. Cell. Biol., in press. [DOI] [PMC free article] [PubMed]
- 14.Le Beau, M. M., S. Bitts, E. M. Davis, and S. C. Kogan. 2002. Recurring chromosomal abnormalities in leukemia in PML-RARA transgenic mice parallel human acute promyelocytic leukemia. Blood 99:2985-2991. [DOI] [PubMed] [Google Scholar]
- 15.Le Beau, M. M., E. M. Davis, B. Patel, V. T. Phan, J. Sohal, and S. C. Kogan. 2003. Recurring chromosomal abnormalities in leukemia in PML-RARA transgenic mice identify cooperating events and genetic pathways to acute promyelocytic leukemia. Blood 102:1072-1074. [DOI] [PubMed] [Google Scholar]
- 16.Ley, T. J., P. J. Minx, M. J. Walter, R. E. Ries, H. Sun, M. McLellan, J. F. DiPersio, D. C. Link, M. H. Tomasson, T. A. Graubert, H. McLeod, H. Khoury, M. Watson, W. Shannon, K. Trinkaus, S. Heath, J. W. Vardiman, M. A. Caligiuri, C. D. Bloomfield, J. D. Milbrandt, E. R. Mardis, and R. K. Wilson. 2003. A pilot study of high-throughput, sequence-based mutational profiling of primary human acute myeloid leukemia cell genomes. Proc. Natl. Acad. Sci. USA 100:14275-14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magee, J. A., S. A. Abdulkadir, and J. Milbrandt. 2003. Haploinsufficiency at the Nkx3.1 locus. A paradigm for stochastic, dosage-sensitive gene regulation during tumor initiation. Cancer Cell 3:273-283. [DOI] [PubMed] [Google Scholar]
- 18.Matsushita, H., P. P. Scaglioni, A. C. Changou, M. A. Leversha, and P. P. Pandolfi. 2003. RARα inactivation antagonizes leukemogenesis in transgenic mouse models of acute promyelocytic leukemia. Blood 102:843-844a (abstract).12689938 [Google Scholar]
- 19.McLemore, M. L., S. Grewal, F. Liu, A. Archambault, J. Poursine-Laurent, J. Haug, and D. C. Link. 2001. STAT-3 activation is required for normal G-CSF-dependent proliferation and granulocytic differentiation. Immunity 14:193-204. [DOI] [PubMed] [Google Scholar]
- 20.Mole, R. H., D. G. Papworth, and M. J. Corp. 1983. The dose-response for x-ray induction of myeloid leukaemia in male CBA/H mice. Br. J. Cancer 47:285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park, D. J., P. T. Vuong, S. de Vos, D. Douer, and H. P. Koeffler. 2003. Comparative analysis of genes regulated by PML/RARα and PLZF/RARα in response to retinoic acid using oligonucleotide arrays. Blood 102:3727-3736. [DOI] [PubMed] [Google Scholar]
- 22.Pollock, J. L., P. Westervelt, A. K. Kurichety, P. G. Pelicci, J. L. Grisolano, and T. J. Ley. 1999. A bcr-3 isoform of RARα-PML potentiates the development of PML-RARα-driven acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA 96:15103-15108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian, Z., A. A. Fernald, L. A. Godley, R. A. Larson, and M. M. Le Beau. 2002. Expression profiling of CD34+ hematopoietic stem/ progenitor cells reveals distinct subtypes of therapy-related acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 99:14925-14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rithidech, K., V. P. Bond, E. P. Cronkite, M. H. Thompson, and J. E. Bullis. 1995. Hypermutability of mouse chromosome 2 during the development of x-ray-induced murine myeloid leukemia. Proc. Natl. Acad. Sci. USA 92:1152-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rithidech, K. N., E. P. Cronkite, and V. P. Bond. 1999. Advantages of the CBA mouse in leukemogenesis research. Blood Cells Mol. Dis. 25:38-45. [DOI] [PubMed] [Google Scholar]
- 26.Schoch, C., A. Kohlmann, S. Schnittger, B. Brors, M. Dugas, S. Mergenthaler, W. Kern, W. Hiddemann, R. Eils, and T. Haferlach. 2002. Acute myeloid leukemias with reciprocal rearrangements can be distinguished by specific gene expression profiles. Proc. Natl. Acad. Sci. USA 99:10008-10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwaenen, C., M. Nessling, S. Wessendorf, T. Salvi, G. Wrobel, B. Radlwimmer, H. A. Kestler, C. Haslinger, S. Stilgenbauer, H. Dohner, M. Bentz, and P. Lichter. 2004. Automated array-based genomic profiling in chronic lymphocytic leukemia: Development of a clinical tool and discovery of recurrent genomic alterations. Proc. Natl. Acad. Sci. USA 101:1039-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silver, A., J. Moody, R. Dunford, D. Clark, S. Ganz, R. Bulman, S. Bouffler, P. Finnon, E. Meijne, R. Huiskamp, and R. Cox. 1999. Molecular mapping of chromosome 2 deletions in murine radiation-induced AML localizes a putative tumor suppressor gene to a 1.0 cM region homologous to human chromosome segment 11p11-12. Genes Chromosomes Cancer 24:95-104. [PubMed] [Google Scholar]
- 29.Trakhtenbrot, L., R. Krauthgamer, P. Resnitzky, and N. Haran-Ghera. 1988. Deletion of chromosome 2 is an early event in the development of radiation-induced myeloid leukemia in SJL/J mice. Leukemia 2:545-550. [PubMed] [Google Scholar]
- 30.Turhan, A. G., F. M. Lemoine, C. Debert, M. L. Bonnet, C. Baillou, F. Picard, E. A. Macintyre, and B. Varet. 1995. Highly purified primitive hematopoietic stem cells are PML-RARα negative and generate nonclonal progenitors in acute promyelocytic leukemia. Blood 85:2154-2161. [PubMed] [Google Scholar]
- 31.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98:5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Virtaneva, K., F. A. Wright, S. M. Tanner, B. Yuan, W. J. Lemon, M. A. Caligiuri, C. D. Bloomfield, A. de La Chapelle, and R. Krahe. 2001. Expression profiling reveals fundamental biological differences in acute myeloid leukemia with isolated trisomy 8 and normal cytogenetics. Proc. Natl. Acad. Sci. USA 98:1124-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeoh, E. J., M. E. Ross, S. A. Shurtleff, W. K. Williams, D. Patel, R. Mahfouz, F. G. Behm, S. C. Raimondi, M. V. Relling, A. Patel, C. Cheng, D. Campana, D. Wilkins, X. Zhou, J. Li, H. Liu, C. H. Pui, W. E. Evans, C. Naeve, L. Wong, and J. R. Downing. 2002. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell 1:133-143. [DOI] [PubMed] [Google Scholar]
- 34.Zimonjic, D. B., J. L. Pollock, P. Westervelt, N. C. Popescu, and T. J. Ley. 2000. Acquired, nonrandom chromosomal abnormalities associated with the development of acute promyelocytic leukemia in transgenic mice. Proc. Natl. Acad. Sci. USA 97:13306-13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.