Abstract
Background
ROS1 rearrangement is a novel molecular subgroup of non-small-cell lung cancer (NSCLC). This study aimed to investigate the efficacy of crizotinib and pemetrexed-based chemotherapy in Chinese NSCLC patients with ROS1 rearrangement.
Results
A total of 2309 patients received ROS1 fusion detection and 51(2.2%) patients had ROS1 rearrangement. There was no significant difference between ROS1 fusion-positive and fusion-negative cohorts in demographic data. For the ROS1 fusion-positive patients, crizotinb-treated group had a higher overall response rate (ORR, 80.0%), disease control rate (DCR, 90.0%) and longer progression-free survival (PFS, 294 days) compared with the rates in pemetrexed-treated group (ORR, 40.8%; DCR, 71.4%; PFS, 179 days) and non-pemetrexed-treated group (ORR, 25.0%; DCR, 47.7%; PFS, 110 days). Besides, ORR, DCR and PFS were similar in three major ROS1 fusion partners. For the first-line treatment, patients received pemetrexed had a significant longer PFS than those received non-pemetrexed chemotherapy (209 vs. 146 days, P = 0.0107). In pemetrexed-treated cohorts, ROS1-positive patients with low TS expression had a statistically significant longer PFS than those with high TS expression (184 vs. 110 days, P = 0.0105).
Materials and methods
We retrospectively identified patients with NSCLC who were screened for ROS1 fusion using multiplex reverse transcription-polymerase chain reaction (RT-PCR) from October 2013 to February 2016. The thymidylate synthase (TS) mRNA levels were tested using quantitative real-time RT-PCR.
Conclusions
Crizotinib was also highly active at treating Chinese NSCLC patients with ROS1 rearrangement. TS expression could predict the efficacy of pemetrexed-based therapy in ROS1 fusion-positive patients.
Keywords: non-small-cell lung cancer, ROS1 rearrangement, crizotinib, pemetrexed, thymidylate synthase
INTRODUCTION
Lung cancer is the most common malignant tumor and the leading cause of cancer death worldwide, with non-small-cell lung cancer (NSCLC) patients accounting for 80–85% of its cases [1]. In the past 10 years, with the identification of oncogenic drivers, such as epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangements, the quality of life and prognosis of NSCLC patients with these driver mutations have been significantly improved when they were treated with small molecular tyrosine kinase inhibitors (TKIs) [2–4].
The c-ros oncogene 1 (ROS1) rearrangements, detected in 1%~2% of NSCLC [5–9] and up to 3% in lung adenocarcinoma [10, 11], represent a novel molecular subgroup of NSCLC. Similar to ALK fusions, ROS1 rearrangement-positive patients had tendencies to be younger, never-smoker, and with adenocarcinoma histology [5–9]. ROS1 and ALK are receptor tyrosine kinases (RTK), and both of them belong to the insulin receptor superfamily. The kinase domains of ALK and ROS1 fusion proteins display highly homology, implying that ALK-TKI, such as crizotinib, will be also effective for ROS1 rearrangement-positive NSCLC patients [12]. Preliminary data from a phase 1 clinical trial showed that crizotinib was highly active in ROS1 fusion-positive patients [13]. Moreover, a retrospective study showed that ROS1 fusion-positive NSCLC patients were greatly sensitive to crizotinib [14]. On the basis of the demonstration of substantial efficacy in the above phase I study, crizotinib has recently been approved by the United States Food and Drug Administration (FDA) as a treatment for patients with ROS1 fusion-positive NSCLC. However, the studies above were carried out among Caucasian populations. It is still unknown about the efficacy of crizotinib in large-scale Chinese NSCLC patients with ROS1 rearrangement.
Furthermore, at some point in the course of their disease, most patients with ROS1 fusion-positive NSCLC will be treated with standard chemotherapeutic agents. Thus, establishing the efficacy of chemotherapeutic agents in this genetically defined subset of patients is clinically relevant. It has been previously shown that ROS1 fusion-positive patients are responsive to pemetrexed-based chemotherapy [15, 16]. Kim et al. reported that 5 NSCLC patients with ROS1 rearrangement had a better responsive to pemetrexed than thWose without ROS1/ALK rearrangement [15]. Another retrospective study showed that ROS1 fusion-positive patients who received pemetrexed-based regimens had a better ORR, DCR and longer PFS compared with patients harboring other driver mutations [16]. These findings have led to the notion that ROS1 rearrangement may serve as a predictive biomarker of enhanced pemetrexed sensitivity. In addition, many prior studies indicated that TS levels can predict the response of pemetrexed-based chemotherapy in NSCLC [17–22]. However, whether TS expression levels are correlated with the response of pemetrexed-based chemotherapy in ROS1 rearrangement NSCLC patients remains controversial.
Herein, we analyzed arguably the largest cohorts to assess the efficacy of crizotinib and pemetrexed-based regimen in Chinese NSCLC patients with ROS1 rearrangement in this study. Meanwhile, we also investigated whether TS mRNA levels were associated with the response of pemetrexed-based regimen in ROS1 rearrangement NSCLC patients.
RESULTS
Patients' characteristics
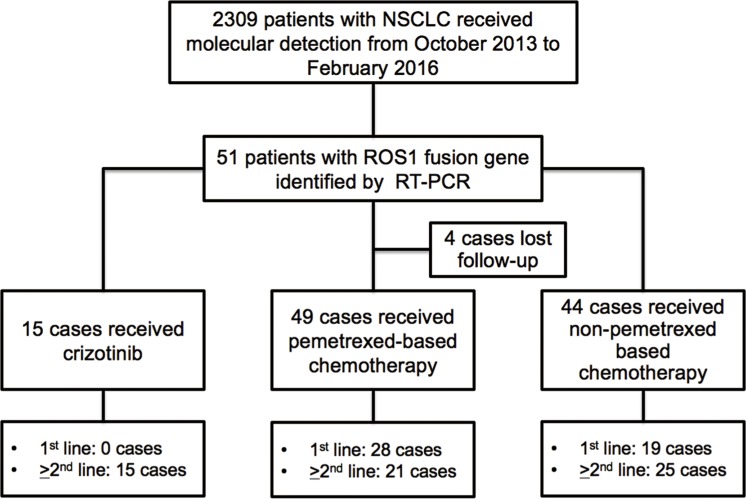
2309 patients with NSCLC received ROS1 rearrangement detection from October 2013 to February 2016 were included in this study. 51 patients (2.2%) were identified as ROS1 rearrangement-positive. Among those, 15 patients received crizotinib (1st line treatment, n = 0; and ≥ 2nd line treatment, n = 15), 49 patients received pemetrexed-based chemotherapy (1st line treatment, n = 28; and ≥ 2nd line treatment, n = 21), 44 patients received non-pemetrexed-based chemotherapy (1st line treatment, n = 19; and ≥ 2nd line treatment, n = 25), and 4 cases lost follow-up (Figure 1). Their baseline clinical characteristics are outlined in Table 1. No statistical significance was observed based on age (P = 0.248), sex (P = 0.146), smoking history (P = 0.882), pathology type (P = 0.961), and ECOG performance status (P = 0.431) between ROS1 fusion-positive and ROS1 fusion-negative patients.
Figure 1. Flow chart of the study design.
Table 1. Clinical characteristics of all included patients at baseline.
| Characteristic | Total (n = 2309) | ROS1 negative (n = 2262) | ROS1 positive (n = 47) | P value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age (years) | |||||||
| Median | 61 | 64 | 57 | ||||
| Range | 27–82 | 27–82 | 31–77 | ||||
| < 65 | 1486 | 64.4% | 1452 | 64.2% | 34 | 72.3% | 0.248 |
| ≥ 65 | 823 | 35.6% | 810 | 35.8% | 13 | 27.7% | |
| Sex | |||||||
| Male | 894 | 38.7% | 871 | 38.5% | 23 | 48.9% | 0.146 |
| Female | 1415 | 61.3% | 1391 | 61.5% | 24 | 51.1% | |
| Smoking History | |||||||
| Never-smoker | 1648 | 71.4% | 1614 | 71.4% | 34 | 72.3% | 0.882 |
| Former/current smoker | 661 | 28.6% | 648 | 28.6% | 13 | 27.7% | |
| Pathology Type | |||||||
| Adenocarcinoma | 1811 | 78.4% | 1774 | 78.4% | 37 | 78.7% | 0.961 |
| Non-adenocarcinoma | 498 | 21.6% | 488 | 21.6% | 10 | 21.3% | |
| ECOG Performance Status | |||||||
| 0–1 | 2055 | 89.0% | 2011 | 88.9% | 44 | 93.6% | 0.431 |
| ≥ 2 | 254 | 11.0% | 251 | 11.1% | 3 | 6.4% | |
EGFR, epidermal growth factor receptorr; ECOG, Eastern Cooperative Oncology Group.
Effect of crizotinib treatment in NSCLC patients with ROS1 fusion partners
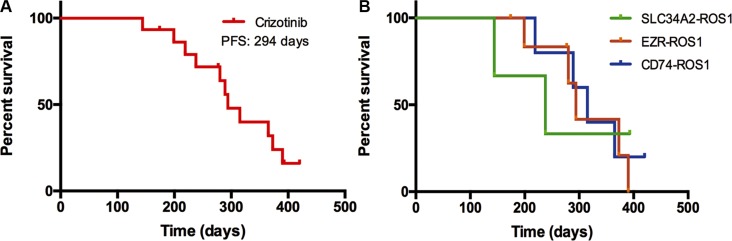
47 ROS1 fusion-positive samples were reconfirmed by means of direct sequencing. The most common ROS1 fusion partner was CD74, accounting for 40.4% (19 of 47); other partner genes included EZR in 13 (27.7%) patients, SLC34A2 in 8 (17.0%), SDC4 in 6 (12.8%), and GOPC in 1 (2.1%); the sequence of the ROS1 fusion-positive patients in our study were shown in Supplementary Figure S1. According to the frequency of ROS1 fusion partners, we classified these patients into 4 subgroups, patients with CD74-ROS1, SLC34A2-ROS1, EZR-ROS1, and other partner genes (including SDC4-ROS1, GOPC-ROS1). The clinical characteristics of the four subgroups are listed in Table 2. The tumor response was evaluated in 15 patients who received oral crizotinib with advanced NSCLC and ROS1 fusion-positive. Among them, five had CD74-ROS1 fusion, three had SLC34A2-ROS1 fusion, and seven had EZR-ROS1 fusion. For crizotinib treatment, one patient had a complete response, 11 had a partial response and three attained stable disease. However, there was no distinct correlation between the ROS1 fusion partners and the tumor response of crizotinib treatment (Supplementary Figure S2). The median PFS time was 294 days (Figure 2A), and no statistical significance was observed in PFS among the three different ROS1 fusion partners (Figure 2B).
Table 2. Clinical characteristics and comparison among 4 ROS1 fusion partners.
| Characteristics | CD74-ROS1 | SLC34A2-ROS1 | EZR-ROS1 | Others |
|---|---|---|---|---|
| Patients | 19 (40.4%) | 8 (17.0%) | 13 (27.7%) | 7 (14.9%) |
| Age (years) | ||||
| Median | 19 (38–73) | 8 (35–72) | 13 (31–77) | 7 (44–76) |
| < 65 | 15 (78.9%) | 4 (50.0%) | 11 (84.6%) | 4 (57.1%) |
| ≥ 65 | 4 (21.1%) | 4 (50.0%) | 2 (15.4%) | 3 (42.9%) |
| Sex | ||||
| Male | 5 (26.3%) | 8 (100%) | 6 (46.2%) | 4 (57.1%) |
| Female | 14 (73.7%) | 0 (0.0%) | 7 (53.8%) | 3 (42.9%) |
| Smoking History | ||||
| Never-smoker | 16 (84.2%) | 4 (50.0%) | 11 (84.6%) | 3 (42.9%) |
| Former/current smoker | 3 (15.8%) | 4 (50.0%) | 2 (15.4%) | 4 (57.1%) |
| Pathological Type | ||||
| Adenocarcinoma | 15 (78.9%) | 6 (75.0%) | 11 (84.6%) | 5 (71.4%) |
| Non-adenocarcinoma | 4 (21.1%) | 2 (25.0%) | 2 (15.4%) | 2 (28.6%) |
| ECOG Performance Status | ||||
| 0–1 | 19 (100%) | 8 (100%) | 12 (92.3%) | 5 (71.4%) |
| 2–3 | 0 (0.0%) | 0 (0.0%) | 1 (7.7%) | 2 (28.6%) |
Others included SDC4-ROS1 & GOPC-ROS1.
Figure 2.
(A) Progression-free survival (PFS) of ROS1 fusion-positive patients treated with crizotinib. (B) Comparison of PFS in three major ROS1 fusion patterns-positive patients treated with crizotinib.
Treatment response in different therapeutic group
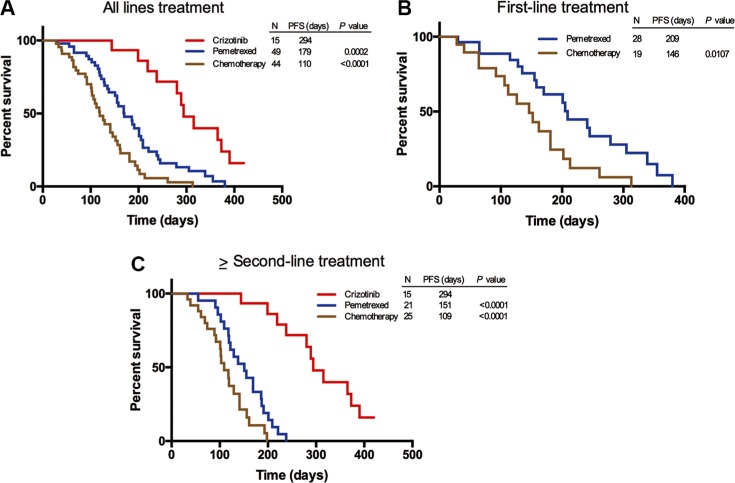
In the light of different therapeutic regimens that ROS1 fusion-positive patients received, we divided them into three groups: crizotinib-treated group, pemetrexed-treated group and non-pemetrexed-treated group. Among patients receiving cizotinib, pemetrexed-based chemotherapy and non-pemetrexed chemotherapy in any line treatment, both crizotinib-treated and pemetrexed-treated groups showed a longer PFS compared with the non-pemetrexed-treated group, with the mPFS of 294, 179, and 110 days, respectively (Figure 3A). There was a statistically significant difference in PFS among the crizotinib-treated group, pemetrexed-treated group (P = 0.0002) and non-pemetrexed-treated group (P < 0.0001). In the above-mentioned subgroups of patients who received pemetrexed-based and non-pemetrexed-treated as the first-line treatment, the difference in PFS was observed between the pemetrexed-treated group and non-pemetrexed-treated group (209 vs. 146 days, P = 0.0107) (Figure 3B). Similarly, among these patients who received three therapeutic regimens as the ≥ second-line treatment, a statistically significant difference was also observed in PFS among the crizotinib-treated group (294 days), pemetrexed-treated group (151 days, P < 0.0001) and non-pemetrexed-treated group (109 days, P < 0.0001) (Figure 3C). Taken together, these results showed that the efficacy of either crizotinib or pemetrexed-based chemotherapy was better than that of non-pemetrexed chemotherapy regimens in the ROS1 fusion-positive patients.
Figure 3. Progression-free survival (PFS) of ROS1 fusion-positive patients treated with cizotinib, pemetrexed-based chemotherapy and non-pemetrexed-based chemotherapy, respectively.
(A) Comparison of PFS in ROS1 fusion-positive patients who received cizotinib, pemetrexed-based chemotherapy or non-pemetrexed-based chemotherapy as their any line treatment. (B) Comparison of PFS in ROS1 fusion-positive patients who received pemetrexed-based chemotherapy or non-pemetrexed-based chemotherapy as their first line treatment. (C) Comparison of PFS in ROS1 fusion-positive patients who received cizotinib, pemetrexed-based chemotherapy or non-pemetrexed-based chemotherapy as their ≥ second-line treatment.
The tumor response of patients who received pemetrexed-based or non-pemetrexed-based chemotherapy was shown in Table 3. Patients received chemotherapy as a first-line treatment, in pemetrexed-treated group, none patient had CR, 15 achieved PR, eight had SD, five had PD; in non-pemetrexed-treated group, none patient had CR, seven achieved PR, five had SD, seven had PD. The ROS1 fusion-positive NSCLC patients who received pemetrexed-based chemotherapy had a relatively better ORR or DCR than those treated with non-pemetrexed-based chemotherapy; however, the difference did not reach the statistical significance (ORR, 53.6% vs. 36.8%, P = 0.259; DCR, 82.1% vs. 63.2%, P = 0.143). Patients received chemotherapy as a ≥ second-line treatment, in pemetrexed-treated group, none patient had CR, five achieved PR, seven had SD, seven had PD, and two had NE; in non-pemetrexed-treated group, none patient had CR, four achieved PR, five had SD, 13 had PD, and three had NE. The ROS1 fusion-positive NSCLC patients who received pemetrexed-based chemotherapy also had a relatively better ORR or DCR than those treated with non-pemetrexed-based chemotherapy; however, it did not have the statistically difference (ORR, 23.8% vs. 16.0%, P = 0.770; DCR, 57.1% vs. 36.0%, P = 0.152).
Table 3. Tumor response in patients received chemotherapy according to RECIST.
| Pemetrexed-based | Non-pemetrexed based | P value | ||
|---|---|---|---|---|
| First-line treatment | n = 28 | n = 19 | ||
| CR | 0 | 0 | ||
| PR | 15 | 7 | ||
| SD | 8 | 5 | ||
| PD | 5 | 7 | ||
| NE | 0 | 0 | ||
| ORR | 15 (53.6%) | 7 (36.8%) | 0.259 | |
| DCR | 23 (82.1%) | 12 (63.2%) | 0.143 | |
| ≥ Second-line treatment | n = 21 | n = 25 | ||
| CR | 0 | 0 | ||
| PR | 5 | 4 | ||
| SD | 7 | 5 | ||
| PD | 7 | 13 | ||
| NE | 2 | 3 | ||
| ORR | 5 (23.8%) | 4 (16.0%) | 0.770 | |
| DCR | 12 (57.1%) | 9 (36.0%) | 0.152 |
CR, complete response; PR, partial response; SD, stable disease; PD: progression disease; NE, Not evaluable; ORR: overall response rate; DCR: disease control response.
Correlation of TS RNA levels with PFS on pemetrexed-based chemotherapy in ROS1-positive NSCLC
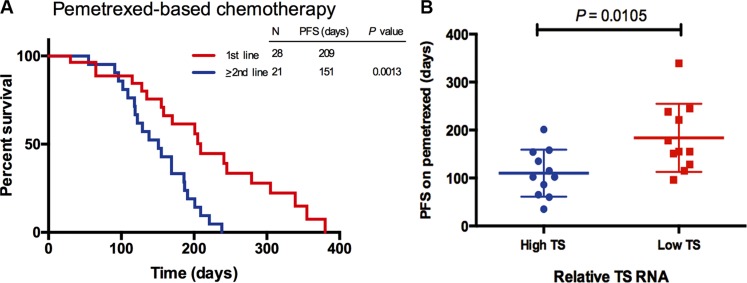
In the pemetrexed-treated group, a statistically significant difference was observed in PFS between the 1st line and ≥ 2nd line treatment (209 vs.151 days, P = 0.0013) (Figure 4A). In order to uncover the relation of TS levels and the efficacy of pemetrexed, we detected the TS mRNA levels in the pemetrexed-treated group of ROS1 fusion-positive patients. 22 of 49 ROS1 fusion-positive patients had sufficient tumor specimen for the RT-PCR, including 11 patients with low TS expression and 11 patients with high TS expression. From Figure 4B, the PFS of the ROS1 fusion-positive patients with low TS expression was statistically significant longer than those with high TS expression (184 vs. 110 days, P = 0.0105). This result suggested that TS levels can determine different response of ROS1-positive patients to pemetrexed.
Figure 4.
(A) Comparison of progression-free survival (PFS) in ROS1 fusion-positive patients who received pemetrexed-based chemotherapy as 1st line or > 2nd line treatment. (B) Correlation of tumor thymidylate synthase (TS) RNA levels with PFS of ROS1 fusion-positive patients treated with pemetrexed-based chemotherapy. TS RNA levels were compared with the median TS value of control cases of NSCLC.
DISCUSSION
This is the first large-scale retrospective study to comprehensively investigate the efficacy of crizotinib, pemetrexed-based and non-pemetrexed-based chemotherapy in Chinese NSCLC patients with ROS1 fusion-positive. We discovered that there was no apparent correlation between the PFS of patients treated with crizotinib and the ROS1 fusion partners. The current study indicated that both crizotinib-treated and pemetrexed-treated groups had a significant longer PFS than non-pemetrexed-treated group, regardless of the lines of treatment. Besides that, in pemetrexed-treated group, a statistically significant difference was observed in PFS between the 1st and > 2nd regimens. Moreover, we found that ROS1 fusion-positive patients treated with pemetrexed-based chemotherapy with low TS expression had an obvious longer PFS than those with high TS expression.
The frequency of ROS1 rearrangement was 2.2% among unselected NSCLC patients in our study, which was a little bit higher than the records in previous studies [5–9]. In accordance with the previous study of Cai et al. [9], our results showed that ROS1 rearrangement was not prone to be younger, never-smoker, and with adenocarcinoma histology on the basis of the subgroup analysis; however, all of which were opposed to the other previous studies [5–8]. The discrepancy might be ascribed to that some patients in the present study were selected from wild-type EGFR and wide-type ALK population. Therefore, we found the incidence of ROS1 rearrangement was relatively higher than that in other studies [10, 11].
Several clinical data, including two Chinese clinical reports, have demonstrated the efficacy of crizotinib in NSCLC patients with ROS1 rearrangement [23–28]. Our results were compatible with the European retrospective study of Julien Mazières et al., who reported that ROS1 fusion patients treated with crizotinib showed a median PFS of 9.1 months [14], and the phase II study in 2016 ASCO, which reported that ROS1 fusion patients treated with crizotinib showed the ORR of 69.3% and a median PFS of 13.4 months. We also found that patients with diverse ROS1 fusions did not display significantly different efficacy, and no apparent correlation was detected between the specific ROS1 fusion partner and PFS of ROS1 fusion-positive patients treated with crizotinib in China, both of which were in line with a recently published prospective phase I study of crizotinib [13]. However, we should need large ROS1 fusion-positive NSCLC patients treated with crizotinib, cell line models and experimental animal models to further demonstrate these results. Moreover, our results further verify that the efficacy of crizotinib is definitive among Chinese ROS1 fusion-positive NSCLC patients.
Consistent with ALK-fusion NSCLC patients and with the previous studies, we found pemetrexed-based chemotherapy showed a better effect in ROS1 fusion-positive patients than non-pemetrexed-based chemotherapy [14–16, 29, 30]. We found that the PFS of patients who treated with pemetrexed-based chemotherapy was 179 days, which was shorter than that in a European prior retrospective study (7.2 months) [14], or in a recently published retrospective study (7.5 months) which was carried out in Taiwan [16]. The difference can be triggered by the study population, tumor heterogeneity and previous treatments of patients. Intriguingly, we observed pemetrexed-based chemotherapy can be preferentially used as the 1st line treatment in ROS1 fusion-positive NSCLC patients when they cannot receive crizotinib treatment.
As is known, TS is an important folate enzyme which can be mainly targeted by pemetrexed. Studies have demonstrated that the TS expression was associated with the treatment efficacy of pemetrexed in NSCLC patients, with low of TS expression conferring increased sensitivity to pemetrexed [17–22, 31]. Although a retrospective study suggested that H-scores of TS protein levels of ROS1 fusion-positive patients were not associated with the PFS of pemetrexed therapy [16], the immunohistochemical staining which was adopted to assess TS levels is less sensitive and semiquantitative than quantitative real-time RT-PCR. A host of studies manifested that TS RNA level can predict the sensitivity of pemetrexed in NSCLC patients [18, 19, 22, 31]. In addition, Alice Shaw et al. reported that TS RNA level was inversely associated with the efficacy of pemetrexed in ALK fusion-positive NSCLC [31]. Kim et al. discovered that the ROS1 fusion-positive HCC78 cell line had low TS expression and the most sensitivity to pemetrexed in comparison to other NSCLC cell lines [15]. By using quantitative real-time RT-PCR to measure the mRNA levels of TS, we discovered that ROS1-positive patients who had lower level of TS RNA showed better susceptibility to pemetrexed-based chemotherapy. Randomized clinical studies should be carried out to demonstrate these results.
Several limitations cannot be avoided completely in this study. First, the number of patients who had particular ROS1 fusion partner treated with crizotinib was relatively small. Second, NSCLC patients enrolled in our study were drawn from the single-institution series. However, considering the relatively larger sample size, the current data can lay the foundation to further large-scale prospective studies of pemetrexed-based chemotherapy in Chinese ROS1 fusion-positive patients. Third, this study was a retrospective study, which might have induced selection bias. Therefore, the findings in this study need to be validated in prospective trials with large scale.
In conclusion, our study suggests that crizotinib and pemetrexed-based chemotherapy are effective to patients with ROS1 rearrangement-positive, and the relative expression of TS RNA can predict the sensitivity of ROS1-positive patients to pemetrexed.
MATERIALS AND METHODS
Study population and data collection
This study was carried out in a group of NSCLC patients received ROS1 rearrangement detection by reverse transcriptase polymerase chain reaction (RT-PCR) assay between October 2013 and February 2016 at Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China. Pathological diagnosis and staging was carried out according to the staging system of the 2009 International Association for the Study of Lung Cancer (version 7). As for the paraffin-embedded and formalin-fixed samples, all samples were reviewed by pathologists to confirm the tumor histology and the tumor cells over 30% of the samples. As for the cytological samples, once the operator acquired the tumor biopsy tissue, it was separated into two parts: one part was for pathologic analysis, and another part, which was preserved for extracting mRNA, was directly put in an RNase-free Eppendorf tube containing 500 μl of RNAlater (Cat No.AM 7021, life technologies) and stored at −80°C until the analyses were performed.
Patients' medical records were reviewed to evaluating the clinicopathological features and treatment regimens. All the eligible patients' clinical data included the age, gender, smoking status, histological type, Eastern Cooperative Oncology Group (ECOG) performance status (PS) and previous treatment regimens. Nonsmokers were defined as patients with the smoking dose of < 100 cigarettes in their lifetime. Clinical responses were evaluated according to the response evaluation criteria in solid tumors (RECIST) version 1.1. PFS was measured from the first day of treatment until either tumor progression or death. This study was approved by Shanghai Pulmonary Hospital Ethics Committee. Each patient had signed a written informed consent before the study started.
RNA preparation and reverse transcription
Total RNA was extracted from tissue samples using either RNeasy Mini Kit (Qiagen, Hilden, Germany) or AmoyDx RNA Kit (Amoy Diagnostics Co, Xiamen, China). The quantity and quality of extracted RNA was measured by NanoDrop 2000 Spectrophotometer (Thermo Scientific, Waltham, USA). Then, the extracted RNA was reversetranscribed to complementary DNA (cDNA) at 42°C for 1 hour, followed by 95°C for 5 min.
ROS1 rearrangements detection
ROS1 rearrangements were identified by an AmoyDx® ROS1 fusion gene detection kit (Amoy Diagnostics Co., Ltd, Xiamen, China). The patterns of ROS1 rearrangements were detected in our study as previously described [9]. The RT-PCR conditions of cDNA was as follows: one cycle of 95°C for 5 min; followed by 15 cycles of denaturation at 95°C for 25 s, annealing at 64°C for 20 s and elongation at 72°C for 20 s to ensure the specificity; and up to 31 cycles of 93°C for 25 s, 60°C for 35 s (data collection) and 72°C for 20 s. β-actin was used as an internal reference gene to ensure the quality of the extracted RNA and ROS1-rearranged DNA was used as positive control.
Measurement of TS RNA levels in tumor tissues
The TS RNA level was measured by quantitative RT-PCR methodology using SYBR Premix Ex Taq (TaKaRa) and an MX3000P instrument. The sequences of primers for TS and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) reference gene were as follows:
TS forward 5′-GGCCTCGGTGTGCCTTT-3′, TS reverse 5′-GATGTGCGCAATCATGTACGT-3′; GAPDH forward 5′-AGGGCTGCTTTTAACTCTGGT-3′;GAPDH reverse5′-CCCCACTTGATTTTGGAGGG A-3′; Control cases of ROS1 fusion-negative, EGFR and KRAS wild type NSCLC were also assessed to establish a median TS mRNA level.
Statistical analysis
Categorical variables were compared using χ2 test or Fisher exact test when necessary. PFS was estimated by the Kaplan-Meier method, and the log-rank test was used to compare the difference between the groups. A Cox regression model was used to calculate hazard ratio (HR) and its 95% confidence interval (CI). Statistical analysis was performed using SPSS version 22.0 software (IBM, Armonk, NY). All P values were two-sided, and a P value of < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIALS FIGURES
Acknowledgments
Limin Zhang, Tao Jiang, Chao Zhao, Shengxiang Ren and Caicun Zhou designed this study; Limin Zhang, Tao Jiang, Chao Zhao, Wei Li, Xuefei Li, Sha Zhao, Xiaozhen Liu, Yijun Jia, Hui Yang collected the clinical data; Tao Jiang and Limin Zhang performed statistical analyses; Shengxiang Ren and Caicun Zhou gave critical comments and suggestions; Limin Zhang, Tao Jiang, and Chao Zhao drafted the manuscript; Limin Zhang, Tao Jiang, Shengxiang Ren and Caicun Zhou revised the paper; all authors approved the final version of the manuscript.
Footnotes
CONFLICTS OF INTEREST
None.
FUNDING
This study was supported in part by grants from the National Natural Science Foundation of China (No. 81372392), key project of Shanghai Municipal Commission of Health and Family Planning (No. 2013zyjb0401) and Outstanding Yong Doctor Program of Shanghai Municipal Commission of Health and Family Planning (No. XYQ2013097).
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 3.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 4.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, Ou SH, Dezube BJ, Janne PA, Costa DB, Varella-Garcia M, Kim WH, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, Asaka R, Hamanaka W, Ninomiya H, Uehara H, Choi YL, Satoh Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nature Medicine. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 6.Davies KD, Le AT, Theodoro MF, Skokan MC, Aisner DL, Berge EM, Terracciano LM, Cappuzzo F, Incarbone M, Roncalli M, Alloisio M, Santoro A, et al. Identifying and Targeting ROS1 Gene Fusions in Non-Small Cell Lung Cancer. Clinical Cancer Research. 2012;18:4570–4579. doi: 10.1158/1078-0432.CCR-12-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, Massion PP, Siwak-Tapp C, Gonzalez A, Fang R, Mark EJ, Batten JM, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheffler M, Schultheis A, Teixido C, Michels S, Morales-Espinosa D, Viteri S, Hartmann W, Merkelbach-Bruse S, Fischer R, Schildhaus HU, Fassunke J, Sebastian M, et al. ROS1 rearrangements in lung adenocarcinoma: prognostic impact, therapeutic options and genetic variability. Oncotarget. 2015;6:10577–10585. doi: 10.18632/oncotarget.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai W, Li X, Su C, Fan L, Zheng L, Fei K, Zhou C, Manegold C, Schmid-Bindert G. ROS1 fusions in Chinese patients with non-small-cell lung cancer. Ann Oncol. 2013;24:1822–1827. doi: 10.1093/annonc/mdt071. [DOI] [PubMed] [Google Scholar]
- 10.Rimkunas VM, Crosby KE, Li D, Hu Y, Kelly ME, Gu TL, Mack JS, Silver MR, Zhou X, Haack H. Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion. Clin Cancer Res. 2012;18:4449–4457. doi: 10.1158/1078-0432.CCR-11-3351. [DOI] [PubMed] [Google Scholar]
- 11.Chen YF, Hsieh MS, Wu SG, Chang YL, Shih JY, Liu YN, Tsai MF, Tsai TH, Yu CJ, Yang JC, Yang PC. Clinical and the prognostic characteristics of lung adenocarcinoma patients with ROS1 fusion in comparison with other driver mutations in East Asian populations. J Thorac Oncol. 2014;9:1171–1179. doi: 10.1097/JTO.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 12.McDermott U, Iafrate AJ, Gray NS, Shioda T, Classon M, Maheswaran S, Zhou W, Choi HG, Smith SL, Dowell L, Ulkus LE, Kuhlmann G, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res. 2008;68:3389–3395. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]
- 13.Shaw AT, Ou SH, Bang YJ, Camidge DR, Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa DB, Doebele RC, Le LP, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazieres J, Zalcman G, Crino L, Biondani P, Barlesi F, Filleron T, Dingemans AM, Lena H, Monnet I, Rothschild SI, Cappuzzo F, Besse B, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33:992–999. doi: 10.1200/JCO.2014.58.3302. [DOI] [PubMed] [Google Scholar]
- 15.Kim HR, Lim SM, Kim HJ, Hwang SK, Park JK, Shin E, Bae MK, Ou SH, Wang J, Jewell SS, Kang DR, Soo RA, et al. The frequency and impact of ROS1 rearrangement on clinical outcomes in never smokers with lung adenocarcinoma. Ann Oncol. 2013;24:2364–2370. doi: 10.1093/annonc/mdt220. [DOI] [PubMed] [Google Scholar]
- 16.Chen YF, Hsieh MS, Wu SG, Chang YL, Yu CJ, Yang JC, Yang PC, Shih JY. Efficacy of Pemetrexed-based Chemotherapy in Patients with ROS1 Fusion-positive Lung Adenocarcinoma Compared with Patients Harboring Other Driver Mutations in East Asian Populations. J Thorac Oncol. 2016 doi: 10.1016/j.jtho.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Chen CY, Chang YL, Shih JY, Lin JW, Chen KY, Yang CH, Yu CJ, Yang PC. Thymidylate synthase and dihydrofolate reductase expression in non-small cell lung carcinoma: the association with treatment efficacy of pemetrexed. Lung Cancer. 2011;74:132–138. doi: 10.1016/j.lungcan.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Ceppi P, Volante M, Saviozzi S, Rapa I, Novello S, Cambieri A, Lo Iacono M, Cappia S, Papotti M, Scagliotti GV. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer. 2006;107:1589–1596. doi: 10.1002/cncr.22208. [DOI] [PubMed] [Google Scholar]
- 19.Ceppi P, Volante M, Ferrero A, Righi L, Rapa I, Rosas R, Berruti A, Dogliotti L, Scagliotti GV, Papotti M. Thymidylate synthase expression in gastroenteropancreatic and pulmonary neuroendocrine tumors. Clin Cancer Res. 2008;14:1059–1064. doi: 10.1158/1078-0432.CCR-07-1513. [DOI] [PubMed] [Google Scholar]
- 20.Sun JM, Han J, Ahn JS, Park K, Ahn MJ. Significance of thymidylate synthase and thyroid transcription factor 1 expression in patients with nonsquamous non-small cell lung cancer treated with pemetrexed-based chemotherapy. J Thorac Oncol. 2011;6:1392–1399. doi: 10.1097/JTO.0b013e3182208ea8. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Noh KB, Lee JS, Lee EJ, Min KH, Hur GY, Lee SH, Lee SY, Kim JH, Lee SY, Shin C, Shim JJ, et al. Thymidylate synthase and ERCC1 as predictive markers in patients with pulmonary adenocarcinoma treated with pemetrexed and cisplatin. Lung Cancer. 2013;81:102–108. doi: 10.1016/j.lungcan.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Wang R, Pan Y, Sun Y, Zhang J, Chen H. The pemetrexed-containing treatments in the non-small cell lung cancer is −/low thymidylate synthase expression better than +/high thymidylate synthase expression: a meta-analysis. BMC Cancer. 2014;14:205. doi: 10.1186/1471-2407-14-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dziadziuszko K, Szurowska E, Pienkowska J, Jassem J, Dziadziuszko R. Miliary brain metastases in a patient with ROS1-rearranged lung adenocarcinoma: a case report. J Thorac Oncol. 2014;9:e34–36. doi: 10.1097/JTO.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 24.Bos M, Gardizi M, Schildhaus HU, Heukamp LC, Geist T, Kaminsky B, Zander T, Nogova L, Scheffler M, Dietlein M, Kobe C, Holstein A, et al. Complete metabolic response in a patient with repeatedly relapsed non-small cell lung cancer harboring ROS1 gene rearrangement after treatment with crizotinib. Lung Cancer. 2013;81:142–143. doi: 10.1016/j.lungcan.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Lu S, Azada MC, Ou SH. Choroidal metastasis response to crizotinib in a ROS1-rearranged NSCLC patient. Lung Cancer. 2015;87:207–209. doi: 10.1016/j.lungcan.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Remon J, Gazzah A, Besse B, Soria JC. Crizotinib Improves Osteoarthritis Symptoms in a ROS1-Fusion Advanced Non-Small Cell Lung Cancer Patient. J Thorac Oncol. 2015;10:e72–73. doi: 10.1097/JTO.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Pan Y, Wang R, Li Y, Sun Y, Chen H. Response to crizotinib observed in metastatic mediastinum lymph node from a non-small cell lung cancer patient harboring EZR-ROS1 fusion. J Cancer Res Clin Oncol. 2015;141:185–187. doi: 10.1007/s00432-014-1821-1. [DOI] [PubMed] [Google Scholar]
- 28.Zhang N, Yang JJ, Zhang XC, Xie Z, Wang BC, Tu HY, Jiang BY, Wu YL. Responses to crizotinib in a patient with c-ros oncogene 1, receptor tyrosine kinase-positive advanced lung adenocarcinoma: A case report. Oncol Lett. 2014;8:2624–2626. doi: 10.3892/ol.2014.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riess JW, Padda SK, Bangs CD, Das M, Neal JW, Adrouny AR, Cherry A, Wakelee HA. A case series of lengthy progression-free survival with pemetrexed-containing therapy in metastatic non--small-cell lung cancer patients harboring ROS1 gene rearrangements. Clin Lung Cancer. 2013;14:592–595. doi: 10.1016/j.cllc.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camidge DR, Kono SA, Lu X, Okuyama S, Baron AE, Oton AB, Davies AM, Varella-Garcia M, Franklin W, Doebele RC. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol. 2011;6:774–780. doi: 10.1097/JTO.0b013e31820cf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw AT, Varghese AM, Solomon BJ, Costa DB, Novello S, Mino-Kenudson M, Awad MM, Engelman JA, Riely GJ, Monica V, Yeap BY, Scagliotti GV. Pemetrexed-based chemotherapy in patients with advanced, ALK-positive non-small cell lung cancer. Ann Oncol. 2013;24:59–66. doi: 10.1093/annonc/mds242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.